User login
What drugs are effective for periodic limb movement disorder?
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
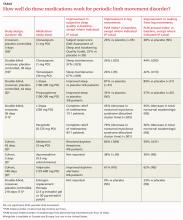
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
Evidence-based answers from the Family Physicians Inquiries Network
How does smoking in the home affect children with asthma?
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
Evidence-based answers from the Family Physicians Inquiries Network
Hearing loss: Help for the young and old
•Ensure that all the infants you care for underwent hearing screening shortly after birth and that those who tested positive are retested in ≤3 months. B
•Evaluate elderly patients for hearing loss during their initial visit and annually thereafter. A
•Speak clearly, maintain eye contact, and use nonverbal gestures when communicating with patients with hearing loss. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hearing impairment is a widespread problem, affecting approximately 36 million US adults1 and an increasing number of children.2 Yet it often goes undetected. The consequences of untreated or undertreated hearing loss can be severe.
Adverse effects are often age-dependent: In children, hearing loss is associated with a broad range of complications, including delays in language development, decreased reading comprehension, and poor academic performance, as well as social and emotional problems.2,3 In adults—particularly the elderly—hearing impairment can lead to social isolation, depression, and a diminished quality of life.4,5
Early detection and treatment can do much to alleviate these adverse effects. But many physicians received little training in the identification and treatment of hearing loss in medical school. What’s more, people with significant hearing loss tend to have fewer interactions with health care providers than their counterparts with no hearing impairment6—a finding that some attribute to fear, mistrust, and frustration.7
Physician awareness of the problems facing people with hearing loss, the importance of screening, and the need to improve communication with hearing-impaired patients (TABLE 1)8 can help change that. The strategies presented here were developed with this in mind.
TABLE 1
How to better communicate with patients who have hearing loss8
| Maintain eye contact and avoid covering your lips while speaking; avoid shouting |
| Use gestures and other nonverbal cues |
| Draw diagrams or use pictures to make a point |
| Reduce background noise (eg, by closing a door or finding a quiet corner) |
| Use the “teach-back” method to ensure understanding |
| Use sign language or provide a sign language interpreter or an oral transliterator* |
| *Ask whether the patient is comfortable with sign language or oral transliteration, which is sometimes used to facilitate oral communication with people who have hearing loss. |
The scope of hearing loss across the lifespan
Hearing loss affects 1 to 3 in every 1000 newborns.9 The prevalence increases to 2% among 5-year-olds, and to 10% to 20% by age 18.10,11 The risk accelerates in “early older life” (defined as ages 50-69 years), with men affected more often than women.12 Hearing loss is the fourth most common chronic condition among older adults, and it is estimated that ≥70% of nursing home residents have some degree of impairment.13
Hearing loss can be categorized as mild (a loss of 20-40 decibels [dB]), moderate (41-55 dB loss), moderate to severe (56-70 dB loss), severe (71-90 dB loss), or profound (>91 dB loss), but any degree of hearing loss should be considered noteworthy.
In children, the impact of mild impairment is often minimized by both professionals and parents, especially among those whose speech developed normally. Unfortunately, the failure to respond appropriately in such cases often increases the adverse effects of the hearing loss.14
In adults, even mild to moderate impairment can lead to significant functional impairment and, therefore, a decreased quality of life.5 And in elderly patients, any undetected hearing loss can adversely affect their performance on cognitive tests, leading to an incorrect diagnosis of cognitive impairment. Elderly patients often minimize hearing deficits, and many believe—incorrectly—that hearing loss due to aging is not amenable to treatment.15
Hearing loss in children may be congenital or acquired
In children, hearing loss can be divided into 2 main categories: congenital and acquired. Congenital etiologies include genetic diseases such as Down syndrome, Usher syndrome, and Alport syndrome—thought to account for 50% of pediatric hearing loss—and intrauterine infections. Causes of acquired hearing loss include recurrent otitis media—most common among infants and young children—and environmental noise (TABLE 2).16-18
TABLE 2
Common causes of hearing loss16-18
| Newborns, children, and adolescents |
|---|
| Childhood infection (eg, measles, mumps, meningitis) |
| Genetic syndrome (eg, Down syndrome, Usher syndrome, Alport syndrome) |
| Head trauma |
| In utero infection (eg, toxoplasmosis, rubella, HSV, CMV, syphilis) |
| Noise exposure |
| Otitis media (recurrent) |
| Ototoxic medication* |
| Premature delivery |
| Adults and the elderly |
| Acoustic neuroma |
| Head trauma |
| Impacted cerumen |
| Noise exposure |
| Otitis media (recurrent) |
| Otosclerosis |
| Ototoxic medication* |
| Presbycusis |
| *Includes aminoglycosides, cisplatin, and loop diuretics, among others. CMV, cytomegalovirus; HSV, herpes simplex virus. |
Adolescents and young adults often expose themselves to loud noises from personal electronic devices, and the use of hearing protection in this population is low.19 The results of one small study suggest that almost a third of adolescents regularly use the highest volume on their iPods or MP3 players, which can cause hearing damage over time.20 Noise levels at which hearing loss occurs can be found at http://www.cdc.gov/niosh/topics/noise/noisemeter.html.21 It is important for adolescents as well as adults to be aware of the risk of hearing loss from repeated exposure to loud noise, but evidence suggests that education about this danger is more likely to lead to behavior change in working-age adults than in teens.22
Screening parameters for infants and children
The US Preventive Services Task Force (USPSTF) recommends universal newborn hearing screening,23 but this does not always happen. That’s why it’s important to ask all new parents whether their baby underwent hearing screening shortly after birth. If the answer is No (or they’re not sure), you may want to order it at this time.
Infants at increased risk for hearing loss—those who spent >2 days on a neonatal intensive care unit; have a congenital syndrome, family history of hereditary childhood sensorineural hearing loss, or craniofacial abnormalities; or were exposed to certain intrauterine infections—should be screened again at 24 to 30 months of age.23 Those with positive results on a newborn hearing screen require repeat screening within 3 months.24,25 If the repeat screen is also positive, a full audiologic evaluation is necessary.
Testing newborns. The most common methods of screening newborns for hearing loss are otoacoustic emissions (OAE) and automated auditory brainstem response (AABR). The average age of detection of congenital hearing loss prior to the availability of these tests was 2 to 3 years. Earlier detection is associated with better developmental outcomes.26
OAE assesses cochlear integrity and measures outer hair cell function. AABR assesses auditory function from the eighth nerve through the auditory brainstem.
Testing toddlers and older children. Any child exhibiting signs of possible hearing loss, such as learning disabilities or speech delay, should be referred for audiometric testing, as should those who have had recurrent otitis media. Tympanograms can be used to diagnose conductive hearing loss, which often results from middle ear effusion. A parent’s expression of concern about a child’s hearing also warrants a referral, as parents can be 12 months ahead of physicians in identifying hearing loss.27
“Play audiometry,” a behavioral test of auditory thresholds in response to speech and frequency-specific stimuli, is commonly used for children between the ages of 2 and 4 years. In this test, the child is instructed to place a block into a box whenever he or she hears a sound.
Children >4 years are typically tested with conventional audiometry, and instructed to raise their hand in response to speech and frequency-specific stimuli. This technique may also be used in adolescents.
Consults, resources required after diagnosis
All children diagnosed with hearing loss after an audiologic evaluation require consultation with specialists in otolaryngology, ophthalmology, and genetics. They should also be offered special educational services, beginning with early intervention and continuing with appropriate monitoring and support throughout the school years. In addition, their parents should be given contact information for hearing loss resources ( TABLE 3). Adolescents and young adults with any degree of hearing loss should also receive counseling about noise exposure.28,29 We’ll review treatment options for hearing-impaired patients of all ages in a bit.
TABLE 3
Hearing loss resources for parents and patients
| Resource | What it offers |
|---|---|
| American Speech-Language-Hearing Association (ASHA) (www.asha.org) | Information about hearing loss in people of all ages |
| Beginnings for Parents of Children Who Are Deaf or Hard of Hearing (www.ncbegin.org) | Communication options for children with hearing loss |
| Better Hearing Institute (www.betterhearing.org) | Resources related to hearing loss for health care providers and patients |
| My Baby’s Hearing (www.babyhearing.org) | Information about newborn screening |
| National Institute on Deafness and Other Communication Disorders (www.nidcd.nih.gov ) | Information about hearing loss for the general public and health care providers |
In adults, most hearing loss is age-related
Advancing age is the single most important (and nonmodifiable) risk factor for hearing loss among older adults. Physiologic changes, including cerumen buildup, tympanic membrane thickening, degeneration of middle ear auditory structures, and decreased central auditory processing all may contribute to presbycusis—age-related sensorineural hearing impairment.30 High-frequency hearing loss is characteristic of presbycusis, and since consonants are high-frequency sounds, patients with this type of hearing loss often complain that they’re unable to understand speech.
Conductive hearing loss may be caused by cerumen buildup, foreign bodies, otosclerosis, cholesteatoma, or tympanic membrane perforation—all of which may be treatable. Potentially modifiable risk factors for hearing loss include smoking, diabetes, exposure to ototoxic medications, and occupational noise, as well as cerumen buildup.17
Maintain an index of suspicion
The most important factor in diagnosing hearing loss in older adults is simply remembering to screen. Elderly patients should be evaluated for hearing loss during their initial visit, and once a year thereafter.31 But all too often, that doesn’t happen. One study found that only 18% of patients between the ages of 65 and 74 years and 22% of patients ages 75 and older had undergone screening for hearing loss during their most recent physical examination.4
But what impact does screening actually have on patients’ quality of life? The evidence is mixed. One study in which asymptomatic individuals >50 years (mean age=61 years) underwent hearing screening found that, although screening increased hearing aid use at one year, it did not lead to an improvement in quality of life.32,33 Another study with a significantly older population (mean age=72 years) found that screening did positively affect quality of life.13
Screening tools—and a question
Several testing techniques have about the same accuracy rates in diagnosing hearing loss in adult patients. These include:
- the whisper test (which should be administered from a distance of 2 feet)
- handheld audiometry testing (with frequencies of 500-4000 Hertz [Hz] at 40 dB, these devices are 94% sensitive and 72% specific for detecting hearing loss)15
- the 10-question Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S), available at http://www.asha.org/docs/html/GL1997-00199-T19.html.
The HHIE-S takes <5 minutes to administer and can be used in conjunction with audiometry testing for increased accuracy in diagnosing hearing loss. An alternative is to ask just one question:
“Do you have a hearing problem now?” This question alone appears to be as effective as the HHIE-S in identifying older patients with hearing loss,34 and is likely to be the most efficient screening method for busy primary care physicians.
Consultation is needed when hearing loss is suspected
An audiology consultation should be considered when a patient’s caregiver or family member—or the patient himself—expresses concern about hearing loss. A positive result on a hearing screen, as well as clinical expression of hearing loss, also indicates a need for referral.
An otolaryngology consult is required for complicated presentations, including persistent cerumen impaction, foreign bodies, otosclerosis, cholesteatoma, tympanic membrane perforation, and asymmetrical hearing loss, which could be caused by a tumor.
Treating hearing loss in patients of all ages
Cerumen can contribute to hearing loss in children and adults alike, and can often be treated in an outpatient setting. A recent Cochrane review of various means of cerumen removal found the strongest evidence for irrigation, followed by cerumenolytic treatment and manual removal.35 The use of cerumenolytic agents appears to be more effective than no treatment, but there is no evidence favoring one product over another. (To learn more about self-removal, see “Wax removal: Help patients help themselves” (J Fam Pract. 2011;60:671-673).
Hearing aids are first-line treatment
Hearing aids should be considered as first-line treatment for children and adults with hearing loss in which easily treatable etiologies such as cerumen impaction have been excluded. They have been shown to improve the ability to understand speech and environmental sounds, as well as the quality of life, for patients of all ages.36 Even infants can be fitted with hearing aids, which are appropriate for mild, moderate, and severe hearing loss.37 But only about 20% of older patients who could benefit from hearing aids ever buy them—and an estimated 25% to 40% of those who have hearing aids use them only occasionally, stop using them completely, or continue to wear them despite receiving limited benefit.4
Cost is one potential barrier to greater use. Hearing aids range in price from about $1000 to $4000 or more for a pair. And, while insurance coverage varies from one health plan to another, hearing aids are not covered by Medicare.
What’s more, elderly patients sometimes have difficulty adjusting to hearing aids (see “Hearing aids don’t work if patients don’t wear them”).38 Cognitive deficits, difficulty manipulating hearing aids, and embarrassment often contribute to suboptimal use of hearing aids.
According to the National Institute on Deafness and Other Communication Disorders, only one out of 5 people who could benefit from hearing aids actually wears them. The use of hearing aids is relatively low even among those who own them: It is estimated that 25% to 40% of older people who have hearing aids wear them only occasionally—or not at all—or wear hearing aids that are of lim-ited benefit (eg, because they’re not adjusted properly, fit poorly, or do not provide adequate amplification).
Here’s some help in overcoming 6 common objections to their use:
- “They hurt my ears.” Explain that discomfort is not unusual at first but often resolves in time. Advise the patient to wear the hearing aids for short periods initially, and then use them for longer periods of time once he or she gets used to them.
- “My voice sounds too loud.” This is known as an “occlusion effect.” It occurs because of the trapping of bone-conducted sound vibrations between a hearing aid and tympanic membrane, and is usually self-limiting. If the problem persists, tell the patient to ask the audiologist to adjust the hearing aids.
- “The hearing aids whistle.” Feedback, such as a whistling noise, is an indication of a poorly fitting hearing aid, cerumen impaction, or fluid in the ear. If you inspect the patient’s ears and find no problem (and the whistling continues), recommend that the patient ask the audiologist for a hearing aid adjustment.
- “I’m bothered by background noise.” Explain that hearing aids may not be able to totally block background sounds, but that they can be adjusted to minimize this effect. Recommend a visit to the audiologist if the problem persists.
- “They don’t work with my cell phone.” Suggest that the patient bring the phone on the next visit to the audiologist and ask that the hearing aids be adjusted, as needed, to minimize interference.
- “I’m embarrassed to wear them.” Tell patients who are embarrassed by the need for hearing aids or don’t want to be seen wearing them that many hearing aids can be concealed, and advise them to discuss this with the audiologist. You might also point out that many people find it more embarrassing not to wear hearing aids, because they have to keep asking friends and family to repeat themselves. You might also refer them to “Guess who wears a hearing aid”—a blog with a lengthy list of actors, politicians, athletes, and even a former Miss America, who have worn hearing aids (http://newgenerationhearing.wordpress.com/2010/03/01/guess-who-uses-hearing-aids/).
Adapted from: National Institute on Deafness and Other Communication Disorders. Hearing aids.38
Is a cochlear implant a viable alternative?
For older adults for whom the cost of hearing aids is prohibitive, a less expensive pocket amplifier with headphones may be a good choice. Middle ear implants, which mechanically vibrate the middle ear structures to produce amplification, are another option for patients with presbycusis.
National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of cochlear implantation for children and adults after multidisciplinary team assessment.37 Cochlear implants are indicated for severe to profound hearing loss, and have been shown to improve speech recognition abilities equally in adolescents and older adults.39 And, unlike hearing aids, cochlear implants are covered by most health insurance plans.
CORRESPONDENCE Paul George, MD, Alpert Medical School of Brown University, 222 Richmond Street, Providence, RI 02912; [email protected]
1. National Institute on Deafness and Other Communication Disorders. Quick statistics. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx. Accessed March 30, 2012.
2. American Speech-Language-Hearing Association. The prevalence and incidence of hearing loss in children. Available at: http://www.asha.org/public/hearing/disorders/children.htm. Accessed March 26, 2012.
3. The Children’s Hearing Institute. Frequently asked questions about hearing loss. Available at: http://www.childrenshearing.org/custom/faq_hearing_loss.html. Accessed April 11, 2012.
4. Sprinzl GM, Riechelmann H. Current trends in hearing loss in elderly people: a review of the technology and treatment options-a mini-review. Gerontology. 2010;56:351-358.
5. Mulrow CD. Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc. 1990;38:45-50.
6. Meador HF, Zazove P. Health care interactions with deaf culture. J Am Board Fam Pract. 2005;18:218-222.
7. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility: experiences and perceptions of deaf people. J Gen Intern Med. 2006;21:260-266.
8. Scheier DB. Barriers to health care for people with hearing loss: a review of the literature. J NY State Nurses Assoc. 2009;40:4-10.
9. Nelson HD, Bougatsos C, Nygren P. Universal newborn hearing screening: systematic review to update the 2001 U.S. Preventive Services Task Force recommendation. Pediatrics. 2008;122:e266-e276.
10. National Institute for Deafness and Communication Disorders. Age at which hearing loss begins. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/begins.aspx. Accessed April 16, 2012.
11. Shargorodsky J, Curhan SG, Curhan GC, et al. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772-778.
12. Chao TK, Chen TH. Predictive model for progression of hearing loss: meta-analysis of multi-state outcome. J Eval Clin Pract. 2009;15:32-40.
13. Cohen-Mansfield J, Taylor JW. Hearing aid use in nursing homes. Part 1: prevalence rates of hearing impairment and hearing aid use. J Am Med Dir Assoc. 2004;5:283-288.
14. Wake M, Hughes EK, Collins CM, et al. Parent-reported health-related quality of life in children with congenital hearing loss: a population study. Ambul Pediatr. 2004;4:411-417.
15. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
16. Centers for Disease Control and Prevention. Adolescent and school health. About hearing loss. Available at: http://www.cdc.gov/healthyyouth/noise/signs.htm. Accessed March 20, 2012.
17. Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139-145.
18. American Speech-Language-Hearing Association. Causes of hearing loss. Available at: http://www.asha.org/public/hearing/Causes-of-Hearing-Loss. Accessed April 11, 2012.
19. Widen SE, Holmes AE, Johnson T, et al. Hearing, use of hearing protection, and attitudes towards noise among young American adults. Int J Audiol.. 2009;48:537-545.
20. Hoover A, Krishnamurti S. Survey of college students’ MP3 listening: habits, safety issues, attitudes, and education. Am J Audiol. 2010;19:73-83.
21. Centers for Disease Control and Prevention. Noise and hearing loss prevention. Noise meter. Available at: http://www.cdc.gov/niosh/topics/noise/noisemeter.html. Accessed April 11, 2012.
22. El Dib RP, Mathew JL. Interventions to promote the wearing of hearing protection. Cochrane Database Syst Rev. 2009;(4):CD005234.-
23. US Preventive Services Task Force Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics. 2008;122:143-148.
24. Hall JW, 3rd, Smith SD, Popelka GR. Newborn hearing screening with combined otoacoustic emissions and auditory brainstem responses. J Am Acad Audiol. 2004;15:414-425.
25. Norton SJ, Gorga MP, Widen JE, et al. Identification of neonatal hearing impairment: a multicenter investigation. Ear Hear. 2000;21:348-356.
26. Korver AM, Konings S, Dekker FW, et al. DECIBEL Collaborative Study Group. Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304:1701-1708.
27. American Academy of Pediatrics Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898-921.
28. Vogel I, Brug J, Hosli EJ, et al. MP3 players and hearing loss: adolescents’ perceptions of loud music and hearing conservation. J Pediatr. 2008;152:400-404.
29. Verbeek JH, Kateman E, Morata TC, et al. Interventions to prevent occupational noise induced hearing loss. Cochrane Database Syst Rev. 2009;(3):CD006396.-
30. Bade PF. Hearing impairment. In: Pacala JT, Sullivan GS, eds. Geriatrics Review Syllabus. 7th ed. New York, NY: American Geriatrics Society; 2010:197-206.
31. Yueh B, Shekelle P. Quality indicators for the care of hearing loss in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S335-S339.
32. Chou R, Dana T, Bougatsos C, et al. Screening adults aged 50 years or older for hearing loss: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;154:347-355.
33. Yueh B, Collins MP, Souza PE, et al. Long-term effectiveness of screening for hearing loss: the screening for auditory impairment—which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc. 2010;58:427-434.
34. Gates GA, Murphy M, Rees TS, et al. Screening for handicapped hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
35. Burton MJ, Doree C. Ear drops for the removal of ear wax. Cochrane Database Syst Rev. 2009;(1):CD004326.-
36. McDermott AL, Williams J, Kuo M, et al. Quality of life in children with a bone-anchored hearing aid. Otol Neurotol. 2009;30:344-349.
37. Fitzpatrick EM, Olds J, Gaboury I, et al. Comparison of outcomes in children with hearing aids and cochlear implants. Cochlear Implants Int. 2012;13:5-15.
38. National Institute on Deafness and Other Communication Disorders. Hearing aids. How can I adjust my hearing aid? Available at: http://www.nidcd.nih.gov/health/hearing/pages/hearingaid.aspx#hearingaid_08. Accessed March 27, 2012.
39. National Institute for Health and Clinical Excellence (NICE). Cochlear implants for children and adults with severe to profound deafness. London, UK: NICE; 2009:41.
•Ensure that all the infants you care for underwent hearing screening shortly after birth and that those who tested positive are retested in ≤3 months. B
•Evaluate elderly patients for hearing loss during their initial visit and annually thereafter. A
•Speak clearly, maintain eye contact, and use nonverbal gestures when communicating with patients with hearing loss. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hearing impairment is a widespread problem, affecting approximately 36 million US adults1 and an increasing number of children.2 Yet it often goes undetected. The consequences of untreated or undertreated hearing loss can be severe.
Adverse effects are often age-dependent: In children, hearing loss is associated with a broad range of complications, including delays in language development, decreased reading comprehension, and poor academic performance, as well as social and emotional problems.2,3 In adults—particularly the elderly—hearing impairment can lead to social isolation, depression, and a diminished quality of life.4,5
Early detection and treatment can do much to alleviate these adverse effects. But many physicians received little training in the identification and treatment of hearing loss in medical school. What’s more, people with significant hearing loss tend to have fewer interactions with health care providers than their counterparts with no hearing impairment6—a finding that some attribute to fear, mistrust, and frustration.7
Physician awareness of the problems facing people with hearing loss, the importance of screening, and the need to improve communication with hearing-impaired patients (TABLE 1)8 can help change that. The strategies presented here were developed with this in mind.
TABLE 1
How to better communicate with patients who have hearing loss8
| Maintain eye contact and avoid covering your lips while speaking; avoid shouting |
| Use gestures and other nonverbal cues |
| Draw diagrams or use pictures to make a point |
| Reduce background noise (eg, by closing a door or finding a quiet corner) |
| Use the “teach-back” method to ensure understanding |
| Use sign language or provide a sign language interpreter or an oral transliterator* |
| *Ask whether the patient is comfortable with sign language or oral transliteration, which is sometimes used to facilitate oral communication with people who have hearing loss. |
The scope of hearing loss across the lifespan
Hearing loss affects 1 to 3 in every 1000 newborns.9 The prevalence increases to 2% among 5-year-olds, and to 10% to 20% by age 18.10,11 The risk accelerates in “early older life” (defined as ages 50-69 years), with men affected more often than women.12 Hearing loss is the fourth most common chronic condition among older adults, and it is estimated that ≥70% of nursing home residents have some degree of impairment.13
Hearing loss can be categorized as mild (a loss of 20-40 decibels [dB]), moderate (41-55 dB loss), moderate to severe (56-70 dB loss), severe (71-90 dB loss), or profound (>91 dB loss), but any degree of hearing loss should be considered noteworthy.
In children, the impact of mild impairment is often minimized by both professionals and parents, especially among those whose speech developed normally. Unfortunately, the failure to respond appropriately in such cases often increases the adverse effects of the hearing loss.14
In adults, even mild to moderate impairment can lead to significant functional impairment and, therefore, a decreased quality of life.5 And in elderly patients, any undetected hearing loss can adversely affect their performance on cognitive tests, leading to an incorrect diagnosis of cognitive impairment. Elderly patients often minimize hearing deficits, and many believe—incorrectly—that hearing loss due to aging is not amenable to treatment.15
Hearing loss in children may be congenital or acquired
In children, hearing loss can be divided into 2 main categories: congenital and acquired. Congenital etiologies include genetic diseases such as Down syndrome, Usher syndrome, and Alport syndrome—thought to account for 50% of pediatric hearing loss—and intrauterine infections. Causes of acquired hearing loss include recurrent otitis media—most common among infants and young children—and environmental noise (TABLE 2).16-18
TABLE 2
Common causes of hearing loss16-18
| Newborns, children, and adolescents |
|---|
| Childhood infection (eg, measles, mumps, meningitis) |
| Genetic syndrome (eg, Down syndrome, Usher syndrome, Alport syndrome) |
| Head trauma |
| In utero infection (eg, toxoplasmosis, rubella, HSV, CMV, syphilis) |
| Noise exposure |
| Otitis media (recurrent) |
| Ototoxic medication* |
| Premature delivery |
| Adults and the elderly |
| Acoustic neuroma |
| Head trauma |
| Impacted cerumen |
| Noise exposure |
| Otitis media (recurrent) |
| Otosclerosis |
| Ototoxic medication* |
| Presbycusis |
| *Includes aminoglycosides, cisplatin, and loop diuretics, among others. CMV, cytomegalovirus; HSV, herpes simplex virus. |
Adolescents and young adults often expose themselves to loud noises from personal electronic devices, and the use of hearing protection in this population is low.19 The results of one small study suggest that almost a third of adolescents regularly use the highest volume on their iPods or MP3 players, which can cause hearing damage over time.20 Noise levels at which hearing loss occurs can be found at http://www.cdc.gov/niosh/topics/noise/noisemeter.html.21 It is important for adolescents as well as adults to be aware of the risk of hearing loss from repeated exposure to loud noise, but evidence suggests that education about this danger is more likely to lead to behavior change in working-age adults than in teens.22
Screening parameters for infants and children
The US Preventive Services Task Force (USPSTF) recommends universal newborn hearing screening,23 but this does not always happen. That’s why it’s important to ask all new parents whether their baby underwent hearing screening shortly after birth. If the answer is No (or they’re not sure), you may want to order it at this time.
Infants at increased risk for hearing loss—those who spent >2 days on a neonatal intensive care unit; have a congenital syndrome, family history of hereditary childhood sensorineural hearing loss, or craniofacial abnormalities; or were exposed to certain intrauterine infections—should be screened again at 24 to 30 months of age.23 Those with positive results on a newborn hearing screen require repeat screening within 3 months.24,25 If the repeat screen is also positive, a full audiologic evaluation is necessary.
Testing newborns. The most common methods of screening newborns for hearing loss are otoacoustic emissions (OAE) and automated auditory brainstem response (AABR). The average age of detection of congenital hearing loss prior to the availability of these tests was 2 to 3 years. Earlier detection is associated with better developmental outcomes.26
OAE assesses cochlear integrity and measures outer hair cell function. AABR assesses auditory function from the eighth nerve through the auditory brainstem.
Testing toddlers and older children. Any child exhibiting signs of possible hearing loss, such as learning disabilities or speech delay, should be referred for audiometric testing, as should those who have had recurrent otitis media. Tympanograms can be used to diagnose conductive hearing loss, which often results from middle ear effusion. A parent’s expression of concern about a child’s hearing also warrants a referral, as parents can be 12 months ahead of physicians in identifying hearing loss.27
“Play audiometry,” a behavioral test of auditory thresholds in response to speech and frequency-specific stimuli, is commonly used for children between the ages of 2 and 4 years. In this test, the child is instructed to place a block into a box whenever he or she hears a sound.
Children >4 years are typically tested with conventional audiometry, and instructed to raise their hand in response to speech and frequency-specific stimuli. This technique may also be used in adolescents.
Consults, resources required after diagnosis
All children diagnosed with hearing loss after an audiologic evaluation require consultation with specialists in otolaryngology, ophthalmology, and genetics. They should also be offered special educational services, beginning with early intervention and continuing with appropriate monitoring and support throughout the school years. In addition, their parents should be given contact information for hearing loss resources ( TABLE 3). Adolescents and young adults with any degree of hearing loss should also receive counseling about noise exposure.28,29 We’ll review treatment options for hearing-impaired patients of all ages in a bit.
TABLE 3
Hearing loss resources for parents and patients
| Resource | What it offers |
|---|---|
| American Speech-Language-Hearing Association (ASHA) (www.asha.org) | Information about hearing loss in people of all ages |
| Beginnings for Parents of Children Who Are Deaf or Hard of Hearing (www.ncbegin.org) | Communication options for children with hearing loss |
| Better Hearing Institute (www.betterhearing.org) | Resources related to hearing loss for health care providers and patients |
| My Baby’s Hearing (www.babyhearing.org) | Information about newborn screening |
| National Institute on Deafness and Other Communication Disorders (www.nidcd.nih.gov ) | Information about hearing loss for the general public and health care providers |
In adults, most hearing loss is age-related
Advancing age is the single most important (and nonmodifiable) risk factor for hearing loss among older adults. Physiologic changes, including cerumen buildup, tympanic membrane thickening, degeneration of middle ear auditory structures, and decreased central auditory processing all may contribute to presbycusis—age-related sensorineural hearing impairment.30 High-frequency hearing loss is characteristic of presbycusis, and since consonants are high-frequency sounds, patients with this type of hearing loss often complain that they’re unable to understand speech.
Conductive hearing loss may be caused by cerumen buildup, foreign bodies, otosclerosis, cholesteatoma, or tympanic membrane perforation—all of which may be treatable. Potentially modifiable risk factors for hearing loss include smoking, diabetes, exposure to ototoxic medications, and occupational noise, as well as cerumen buildup.17
Maintain an index of suspicion
The most important factor in diagnosing hearing loss in older adults is simply remembering to screen. Elderly patients should be evaluated for hearing loss during their initial visit, and once a year thereafter.31 But all too often, that doesn’t happen. One study found that only 18% of patients between the ages of 65 and 74 years and 22% of patients ages 75 and older had undergone screening for hearing loss during their most recent physical examination.4
But what impact does screening actually have on patients’ quality of life? The evidence is mixed. One study in which asymptomatic individuals >50 years (mean age=61 years) underwent hearing screening found that, although screening increased hearing aid use at one year, it did not lead to an improvement in quality of life.32,33 Another study with a significantly older population (mean age=72 years) found that screening did positively affect quality of life.13
Screening tools—and a question
Several testing techniques have about the same accuracy rates in diagnosing hearing loss in adult patients. These include:
- the whisper test (which should be administered from a distance of 2 feet)
- handheld audiometry testing (with frequencies of 500-4000 Hertz [Hz] at 40 dB, these devices are 94% sensitive and 72% specific for detecting hearing loss)15
- the 10-question Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S), available at http://www.asha.org/docs/html/GL1997-00199-T19.html.
The HHIE-S takes <5 minutes to administer and can be used in conjunction with audiometry testing for increased accuracy in diagnosing hearing loss. An alternative is to ask just one question:
“Do you have a hearing problem now?” This question alone appears to be as effective as the HHIE-S in identifying older patients with hearing loss,34 and is likely to be the most efficient screening method for busy primary care physicians.
Consultation is needed when hearing loss is suspected
An audiology consultation should be considered when a patient’s caregiver or family member—or the patient himself—expresses concern about hearing loss. A positive result on a hearing screen, as well as clinical expression of hearing loss, also indicates a need for referral.
An otolaryngology consult is required for complicated presentations, including persistent cerumen impaction, foreign bodies, otosclerosis, cholesteatoma, tympanic membrane perforation, and asymmetrical hearing loss, which could be caused by a tumor.
Treating hearing loss in patients of all ages
Cerumen can contribute to hearing loss in children and adults alike, and can often be treated in an outpatient setting. A recent Cochrane review of various means of cerumen removal found the strongest evidence for irrigation, followed by cerumenolytic treatment and manual removal.35 The use of cerumenolytic agents appears to be more effective than no treatment, but there is no evidence favoring one product over another. (To learn more about self-removal, see “Wax removal: Help patients help themselves” (J Fam Pract. 2011;60:671-673).
Hearing aids are first-line treatment
Hearing aids should be considered as first-line treatment for children and adults with hearing loss in which easily treatable etiologies such as cerumen impaction have been excluded. They have been shown to improve the ability to understand speech and environmental sounds, as well as the quality of life, for patients of all ages.36 Even infants can be fitted with hearing aids, which are appropriate for mild, moderate, and severe hearing loss.37 But only about 20% of older patients who could benefit from hearing aids ever buy them—and an estimated 25% to 40% of those who have hearing aids use them only occasionally, stop using them completely, or continue to wear them despite receiving limited benefit.4
Cost is one potential barrier to greater use. Hearing aids range in price from about $1000 to $4000 or more for a pair. And, while insurance coverage varies from one health plan to another, hearing aids are not covered by Medicare.
What’s more, elderly patients sometimes have difficulty adjusting to hearing aids (see “Hearing aids don’t work if patients don’t wear them”).38 Cognitive deficits, difficulty manipulating hearing aids, and embarrassment often contribute to suboptimal use of hearing aids.
According to the National Institute on Deafness and Other Communication Disorders, only one out of 5 people who could benefit from hearing aids actually wears them. The use of hearing aids is relatively low even among those who own them: It is estimated that 25% to 40% of older people who have hearing aids wear them only occasionally—or not at all—or wear hearing aids that are of lim-ited benefit (eg, because they’re not adjusted properly, fit poorly, or do not provide adequate amplification).
Here’s some help in overcoming 6 common objections to their use:
- “They hurt my ears.” Explain that discomfort is not unusual at first but often resolves in time. Advise the patient to wear the hearing aids for short periods initially, and then use them for longer periods of time once he or she gets used to them.
- “My voice sounds too loud.” This is known as an “occlusion effect.” It occurs because of the trapping of bone-conducted sound vibrations between a hearing aid and tympanic membrane, and is usually self-limiting. If the problem persists, tell the patient to ask the audiologist to adjust the hearing aids.
- “The hearing aids whistle.” Feedback, such as a whistling noise, is an indication of a poorly fitting hearing aid, cerumen impaction, or fluid in the ear. If you inspect the patient’s ears and find no problem (and the whistling continues), recommend that the patient ask the audiologist for a hearing aid adjustment.
- “I’m bothered by background noise.” Explain that hearing aids may not be able to totally block background sounds, but that they can be adjusted to minimize this effect. Recommend a visit to the audiologist if the problem persists.
- “They don’t work with my cell phone.” Suggest that the patient bring the phone on the next visit to the audiologist and ask that the hearing aids be adjusted, as needed, to minimize interference.
- “I’m embarrassed to wear them.” Tell patients who are embarrassed by the need for hearing aids or don’t want to be seen wearing them that many hearing aids can be concealed, and advise them to discuss this with the audiologist. You might also point out that many people find it more embarrassing not to wear hearing aids, because they have to keep asking friends and family to repeat themselves. You might also refer them to “Guess who wears a hearing aid”—a blog with a lengthy list of actors, politicians, athletes, and even a former Miss America, who have worn hearing aids (http://newgenerationhearing.wordpress.com/2010/03/01/guess-who-uses-hearing-aids/).
Adapted from: National Institute on Deafness and Other Communication Disorders. Hearing aids.38
Is a cochlear implant a viable alternative?
For older adults for whom the cost of hearing aids is prohibitive, a less expensive pocket amplifier with headphones may be a good choice. Middle ear implants, which mechanically vibrate the middle ear structures to produce amplification, are another option for patients with presbycusis.
National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of cochlear implantation for children and adults after multidisciplinary team assessment.37 Cochlear implants are indicated for severe to profound hearing loss, and have been shown to improve speech recognition abilities equally in adolescents and older adults.39 And, unlike hearing aids, cochlear implants are covered by most health insurance plans.
CORRESPONDENCE Paul George, MD, Alpert Medical School of Brown University, 222 Richmond Street, Providence, RI 02912; [email protected]
•Ensure that all the infants you care for underwent hearing screening shortly after birth and that those who tested positive are retested in ≤3 months. B
•Evaluate elderly patients for hearing loss during their initial visit and annually thereafter. A
•Speak clearly, maintain eye contact, and use nonverbal gestures when communicating with patients with hearing loss. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hearing impairment is a widespread problem, affecting approximately 36 million US adults1 and an increasing number of children.2 Yet it often goes undetected. The consequences of untreated or undertreated hearing loss can be severe.
Adverse effects are often age-dependent: In children, hearing loss is associated with a broad range of complications, including delays in language development, decreased reading comprehension, and poor academic performance, as well as social and emotional problems.2,3 In adults—particularly the elderly—hearing impairment can lead to social isolation, depression, and a diminished quality of life.4,5
Early detection and treatment can do much to alleviate these adverse effects. But many physicians received little training in the identification and treatment of hearing loss in medical school. What’s more, people with significant hearing loss tend to have fewer interactions with health care providers than their counterparts with no hearing impairment6—a finding that some attribute to fear, mistrust, and frustration.7
Physician awareness of the problems facing people with hearing loss, the importance of screening, and the need to improve communication with hearing-impaired patients (TABLE 1)8 can help change that. The strategies presented here were developed with this in mind.
TABLE 1
How to better communicate with patients who have hearing loss8
| Maintain eye contact and avoid covering your lips while speaking; avoid shouting |
| Use gestures and other nonverbal cues |
| Draw diagrams or use pictures to make a point |
| Reduce background noise (eg, by closing a door or finding a quiet corner) |
| Use the “teach-back” method to ensure understanding |
| Use sign language or provide a sign language interpreter or an oral transliterator* |
| *Ask whether the patient is comfortable with sign language or oral transliteration, which is sometimes used to facilitate oral communication with people who have hearing loss. |
The scope of hearing loss across the lifespan
Hearing loss affects 1 to 3 in every 1000 newborns.9 The prevalence increases to 2% among 5-year-olds, and to 10% to 20% by age 18.10,11 The risk accelerates in “early older life” (defined as ages 50-69 years), with men affected more often than women.12 Hearing loss is the fourth most common chronic condition among older adults, and it is estimated that ≥70% of nursing home residents have some degree of impairment.13
Hearing loss can be categorized as mild (a loss of 20-40 decibels [dB]), moderate (41-55 dB loss), moderate to severe (56-70 dB loss), severe (71-90 dB loss), or profound (>91 dB loss), but any degree of hearing loss should be considered noteworthy.
In children, the impact of mild impairment is often minimized by both professionals and parents, especially among those whose speech developed normally. Unfortunately, the failure to respond appropriately in such cases often increases the adverse effects of the hearing loss.14
In adults, even mild to moderate impairment can lead to significant functional impairment and, therefore, a decreased quality of life.5 And in elderly patients, any undetected hearing loss can adversely affect their performance on cognitive tests, leading to an incorrect diagnosis of cognitive impairment. Elderly patients often minimize hearing deficits, and many believe—incorrectly—that hearing loss due to aging is not amenable to treatment.15
Hearing loss in children may be congenital or acquired
In children, hearing loss can be divided into 2 main categories: congenital and acquired. Congenital etiologies include genetic diseases such as Down syndrome, Usher syndrome, and Alport syndrome—thought to account for 50% of pediatric hearing loss—and intrauterine infections. Causes of acquired hearing loss include recurrent otitis media—most common among infants and young children—and environmental noise (TABLE 2).16-18
TABLE 2
Common causes of hearing loss16-18
| Newborns, children, and adolescents |
|---|
| Childhood infection (eg, measles, mumps, meningitis) |
| Genetic syndrome (eg, Down syndrome, Usher syndrome, Alport syndrome) |
| Head trauma |
| In utero infection (eg, toxoplasmosis, rubella, HSV, CMV, syphilis) |
| Noise exposure |
| Otitis media (recurrent) |
| Ototoxic medication* |
| Premature delivery |
| Adults and the elderly |
| Acoustic neuroma |
| Head trauma |
| Impacted cerumen |
| Noise exposure |
| Otitis media (recurrent) |
| Otosclerosis |
| Ototoxic medication* |
| Presbycusis |
| *Includes aminoglycosides, cisplatin, and loop diuretics, among others. CMV, cytomegalovirus; HSV, herpes simplex virus. |
Adolescents and young adults often expose themselves to loud noises from personal electronic devices, and the use of hearing protection in this population is low.19 The results of one small study suggest that almost a third of adolescents regularly use the highest volume on their iPods or MP3 players, which can cause hearing damage over time.20 Noise levels at which hearing loss occurs can be found at http://www.cdc.gov/niosh/topics/noise/noisemeter.html.21 It is important for adolescents as well as adults to be aware of the risk of hearing loss from repeated exposure to loud noise, but evidence suggests that education about this danger is more likely to lead to behavior change in working-age adults than in teens.22
Screening parameters for infants and children
The US Preventive Services Task Force (USPSTF) recommends universal newborn hearing screening,23 but this does not always happen. That’s why it’s important to ask all new parents whether their baby underwent hearing screening shortly after birth. If the answer is No (or they’re not sure), you may want to order it at this time.
Infants at increased risk for hearing loss—those who spent >2 days on a neonatal intensive care unit; have a congenital syndrome, family history of hereditary childhood sensorineural hearing loss, or craniofacial abnormalities; or were exposed to certain intrauterine infections—should be screened again at 24 to 30 months of age.23 Those with positive results on a newborn hearing screen require repeat screening within 3 months.24,25 If the repeat screen is also positive, a full audiologic evaluation is necessary.
Testing newborns. The most common methods of screening newborns for hearing loss are otoacoustic emissions (OAE) and automated auditory brainstem response (AABR). The average age of detection of congenital hearing loss prior to the availability of these tests was 2 to 3 years. Earlier detection is associated with better developmental outcomes.26
OAE assesses cochlear integrity and measures outer hair cell function. AABR assesses auditory function from the eighth nerve through the auditory brainstem.
Testing toddlers and older children. Any child exhibiting signs of possible hearing loss, such as learning disabilities or speech delay, should be referred for audiometric testing, as should those who have had recurrent otitis media. Tympanograms can be used to diagnose conductive hearing loss, which often results from middle ear effusion. A parent’s expression of concern about a child’s hearing also warrants a referral, as parents can be 12 months ahead of physicians in identifying hearing loss.27
“Play audiometry,” a behavioral test of auditory thresholds in response to speech and frequency-specific stimuli, is commonly used for children between the ages of 2 and 4 years. In this test, the child is instructed to place a block into a box whenever he or she hears a sound.
Children >4 years are typically tested with conventional audiometry, and instructed to raise their hand in response to speech and frequency-specific stimuli. This technique may also be used in adolescents.
Consults, resources required after diagnosis
All children diagnosed with hearing loss after an audiologic evaluation require consultation with specialists in otolaryngology, ophthalmology, and genetics. They should also be offered special educational services, beginning with early intervention and continuing with appropriate monitoring and support throughout the school years. In addition, their parents should be given contact information for hearing loss resources ( TABLE 3). Adolescents and young adults with any degree of hearing loss should also receive counseling about noise exposure.28,29 We’ll review treatment options for hearing-impaired patients of all ages in a bit.
TABLE 3
Hearing loss resources for parents and patients
| Resource | What it offers |
|---|---|
| American Speech-Language-Hearing Association (ASHA) (www.asha.org) | Information about hearing loss in people of all ages |
| Beginnings for Parents of Children Who Are Deaf or Hard of Hearing (www.ncbegin.org) | Communication options for children with hearing loss |
| Better Hearing Institute (www.betterhearing.org) | Resources related to hearing loss for health care providers and patients |
| My Baby’s Hearing (www.babyhearing.org) | Information about newborn screening |
| National Institute on Deafness and Other Communication Disorders (www.nidcd.nih.gov ) | Information about hearing loss for the general public and health care providers |
In adults, most hearing loss is age-related
Advancing age is the single most important (and nonmodifiable) risk factor for hearing loss among older adults. Physiologic changes, including cerumen buildup, tympanic membrane thickening, degeneration of middle ear auditory structures, and decreased central auditory processing all may contribute to presbycusis—age-related sensorineural hearing impairment.30 High-frequency hearing loss is characteristic of presbycusis, and since consonants are high-frequency sounds, patients with this type of hearing loss often complain that they’re unable to understand speech.
Conductive hearing loss may be caused by cerumen buildup, foreign bodies, otosclerosis, cholesteatoma, or tympanic membrane perforation—all of which may be treatable. Potentially modifiable risk factors for hearing loss include smoking, diabetes, exposure to ototoxic medications, and occupational noise, as well as cerumen buildup.17
Maintain an index of suspicion
The most important factor in diagnosing hearing loss in older adults is simply remembering to screen. Elderly patients should be evaluated for hearing loss during their initial visit, and once a year thereafter.31 But all too often, that doesn’t happen. One study found that only 18% of patients between the ages of 65 and 74 years and 22% of patients ages 75 and older had undergone screening for hearing loss during their most recent physical examination.4
But what impact does screening actually have on patients’ quality of life? The evidence is mixed. One study in which asymptomatic individuals >50 years (mean age=61 years) underwent hearing screening found that, although screening increased hearing aid use at one year, it did not lead to an improvement in quality of life.32,33 Another study with a significantly older population (mean age=72 years) found that screening did positively affect quality of life.13
Screening tools—and a question
Several testing techniques have about the same accuracy rates in diagnosing hearing loss in adult patients. These include:
- the whisper test (which should be administered from a distance of 2 feet)
- handheld audiometry testing (with frequencies of 500-4000 Hertz [Hz] at 40 dB, these devices are 94% sensitive and 72% specific for detecting hearing loss)15
- the 10-question Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S), available at http://www.asha.org/docs/html/GL1997-00199-T19.html.
The HHIE-S takes <5 minutes to administer and can be used in conjunction with audiometry testing for increased accuracy in diagnosing hearing loss. An alternative is to ask just one question:
“Do you have a hearing problem now?” This question alone appears to be as effective as the HHIE-S in identifying older patients with hearing loss,34 and is likely to be the most efficient screening method for busy primary care physicians.
Consultation is needed when hearing loss is suspected
An audiology consultation should be considered when a patient’s caregiver or family member—or the patient himself—expresses concern about hearing loss. A positive result on a hearing screen, as well as clinical expression of hearing loss, also indicates a need for referral.
An otolaryngology consult is required for complicated presentations, including persistent cerumen impaction, foreign bodies, otosclerosis, cholesteatoma, tympanic membrane perforation, and asymmetrical hearing loss, which could be caused by a tumor.
Treating hearing loss in patients of all ages
Cerumen can contribute to hearing loss in children and adults alike, and can often be treated in an outpatient setting. A recent Cochrane review of various means of cerumen removal found the strongest evidence for irrigation, followed by cerumenolytic treatment and manual removal.35 The use of cerumenolytic agents appears to be more effective than no treatment, but there is no evidence favoring one product over another. (To learn more about self-removal, see “Wax removal: Help patients help themselves” (J Fam Pract. 2011;60:671-673).
Hearing aids are first-line treatment
Hearing aids should be considered as first-line treatment for children and adults with hearing loss in which easily treatable etiologies such as cerumen impaction have been excluded. They have been shown to improve the ability to understand speech and environmental sounds, as well as the quality of life, for patients of all ages.36 Even infants can be fitted with hearing aids, which are appropriate for mild, moderate, and severe hearing loss.37 But only about 20% of older patients who could benefit from hearing aids ever buy them—and an estimated 25% to 40% of those who have hearing aids use them only occasionally, stop using them completely, or continue to wear them despite receiving limited benefit.4
Cost is one potential barrier to greater use. Hearing aids range in price from about $1000 to $4000 or more for a pair. And, while insurance coverage varies from one health plan to another, hearing aids are not covered by Medicare.
What’s more, elderly patients sometimes have difficulty adjusting to hearing aids (see “Hearing aids don’t work if patients don’t wear them”).38 Cognitive deficits, difficulty manipulating hearing aids, and embarrassment often contribute to suboptimal use of hearing aids.
According to the National Institute on Deafness and Other Communication Disorders, only one out of 5 people who could benefit from hearing aids actually wears them. The use of hearing aids is relatively low even among those who own them: It is estimated that 25% to 40% of older people who have hearing aids wear them only occasionally—or not at all—or wear hearing aids that are of lim-ited benefit (eg, because they’re not adjusted properly, fit poorly, or do not provide adequate amplification).
Here’s some help in overcoming 6 common objections to their use:
- “They hurt my ears.” Explain that discomfort is not unusual at first but often resolves in time. Advise the patient to wear the hearing aids for short periods initially, and then use them for longer periods of time once he or she gets used to them.
- “My voice sounds too loud.” This is known as an “occlusion effect.” It occurs because of the trapping of bone-conducted sound vibrations between a hearing aid and tympanic membrane, and is usually self-limiting. If the problem persists, tell the patient to ask the audiologist to adjust the hearing aids.
- “The hearing aids whistle.” Feedback, such as a whistling noise, is an indication of a poorly fitting hearing aid, cerumen impaction, or fluid in the ear. If you inspect the patient’s ears and find no problem (and the whistling continues), recommend that the patient ask the audiologist for a hearing aid adjustment.
- “I’m bothered by background noise.” Explain that hearing aids may not be able to totally block background sounds, but that they can be adjusted to minimize this effect. Recommend a visit to the audiologist if the problem persists.
- “They don’t work with my cell phone.” Suggest that the patient bring the phone on the next visit to the audiologist and ask that the hearing aids be adjusted, as needed, to minimize interference.
- “I’m embarrassed to wear them.” Tell patients who are embarrassed by the need for hearing aids or don’t want to be seen wearing them that many hearing aids can be concealed, and advise them to discuss this with the audiologist. You might also point out that many people find it more embarrassing not to wear hearing aids, because they have to keep asking friends and family to repeat themselves. You might also refer them to “Guess who wears a hearing aid”—a blog with a lengthy list of actors, politicians, athletes, and even a former Miss America, who have worn hearing aids (http://newgenerationhearing.wordpress.com/2010/03/01/guess-who-uses-hearing-aids/).
Adapted from: National Institute on Deafness and Other Communication Disorders. Hearing aids.38
Is a cochlear implant a viable alternative?
For older adults for whom the cost of hearing aids is prohibitive, a less expensive pocket amplifier with headphones may be a good choice. Middle ear implants, which mechanically vibrate the middle ear structures to produce amplification, are another option for patients with presbycusis.
National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of cochlear implantation for children and adults after multidisciplinary team assessment.37 Cochlear implants are indicated for severe to profound hearing loss, and have been shown to improve speech recognition abilities equally in adolescents and older adults.39 And, unlike hearing aids, cochlear implants are covered by most health insurance plans.
CORRESPONDENCE Paul George, MD, Alpert Medical School of Brown University, 222 Richmond Street, Providence, RI 02912; [email protected]
1. National Institute on Deafness and Other Communication Disorders. Quick statistics. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx. Accessed March 30, 2012.
2. American Speech-Language-Hearing Association. The prevalence and incidence of hearing loss in children. Available at: http://www.asha.org/public/hearing/disorders/children.htm. Accessed March 26, 2012.
3. The Children’s Hearing Institute. Frequently asked questions about hearing loss. Available at: http://www.childrenshearing.org/custom/faq_hearing_loss.html. Accessed April 11, 2012.
4. Sprinzl GM, Riechelmann H. Current trends in hearing loss in elderly people: a review of the technology and treatment options-a mini-review. Gerontology. 2010;56:351-358.
5. Mulrow CD. Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc. 1990;38:45-50.
6. Meador HF, Zazove P. Health care interactions with deaf culture. J Am Board Fam Pract. 2005;18:218-222.
7. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility: experiences and perceptions of deaf people. J Gen Intern Med. 2006;21:260-266.
8. Scheier DB. Barriers to health care for people with hearing loss: a review of the literature. J NY State Nurses Assoc. 2009;40:4-10.
9. Nelson HD, Bougatsos C, Nygren P. Universal newborn hearing screening: systematic review to update the 2001 U.S. Preventive Services Task Force recommendation. Pediatrics. 2008;122:e266-e276.
10. National Institute for Deafness and Communication Disorders. Age at which hearing loss begins. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/begins.aspx. Accessed April 16, 2012.
11. Shargorodsky J, Curhan SG, Curhan GC, et al. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772-778.
12. Chao TK, Chen TH. Predictive model for progression of hearing loss: meta-analysis of multi-state outcome. J Eval Clin Pract. 2009;15:32-40.
13. Cohen-Mansfield J, Taylor JW. Hearing aid use in nursing homes. Part 1: prevalence rates of hearing impairment and hearing aid use. J Am Med Dir Assoc. 2004;5:283-288.
14. Wake M, Hughes EK, Collins CM, et al. Parent-reported health-related quality of life in children with congenital hearing loss: a population study. Ambul Pediatr. 2004;4:411-417.
15. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
16. Centers for Disease Control and Prevention. Adolescent and school health. About hearing loss. Available at: http://www.cdc.gov/healthyyouth/noise/signs.htm. Accessed March 20, 2012.
17. Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139-145.
18. American Speech-Language-Hearing Association. Causes of hearing loss. Available at: http://www.asha.org/public/hearing/Causes-of-Hearing-Loss. Accessed April 11, 2012.
19. Widen SE, Holmes AE, Johnson T, et al. Hearing, use of hearing protection, and attitudes towards noise among young American adults. Int J Audiol.. 2009;48:537-545.
20. Hoover A, Krishnamurti S. Survey of college students’ MP3 listening: habits, safety issues, attitudes, and education. Am J Audiol. 2010;19:73-83.
21. Centers for Disease Control and Prevention. Noise and hearing loss prevention. Noise meter. Available at: http://www.cdc.gov/niosh/topics/noise/noisemeter.html. Accessed April 11, 2012.
22. El Dib RP, Mathew JL. Interventions to promote the wearing of hearing protection. Cochrane Database Syst Rev. 2009;(4):CD005234.-
23. US Preventive Services Task Force Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics. 2008;122:143-148.
24. Hall JW, 3rd, Smith SD, Popelka GR. Newborn hearing screening with combined otoacoustic emissions and auditory brainstem responses. J Am Acad Audiol. 2004;15:414-425.
25. Norton SJ, Gorga MP, Widen JE, et al. Identification of neonatal hearing impairment: a multicenter investigation. Ear Hear. 2000;21:348-356.
26. Korver AM, Konings S, Dekker FW, et al. DECIBEL Collaborative Study Group. Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304:1701-1708.
27. American Academy of Pediatrics Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898-921.
28. Vogel I, Brug J, Hosli EJ, et al. MP3 players and hearing loss: adolescents’ perceptions of loud music and hearing conservation. J Pediatr. 2008;152:400-404.
29. Verbeek JH, Kateman E, Morata TC, et al. Interventions to prevent occupational noise induced hearing loss. Cochrane Database Syst Rev. 2009;(3):CD006396.-
30. Bade PF. Hearing impairment. In: Pacala JT, Sullivan GS, eds. Geriatrics Review Syllabus. 7th ed. New York, NY: American Geriatrics Society; 2010:197-206.
31. Yueh B, Shekelle P. Quality indicators for the care of hearing loss in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S335-S339.
32. Chou R, Dana T, Bougatsos C, et al. Screening adults aged 50 years or older for hearing loss: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;154:347-355.
33. Yueh B, Collins MP, Souza PE, et al. Long-term effectiveness of screening for hearing loss: the screening for auditory impairment—which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc. 2010;58:427-434.
34. Gates GA, Murphy M, Rees TS, et al. Screening for handicapped hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
35. Burton MJ, Doree C. Ear drops for the removal of ear wax. Cochrane Database Syst Rev. 2009;(1):CD004326.-
36. McDermott AL, Williams J, Kuo M, et al. Quality of life in children with a bone-anchored hearing aid. Otol Neurotol. 2009;30:344-349.
37. Fitzpatrick EM, Olds J, Gaboury I, et al. Comparison of outcomes in children with hearing aids and cochlear implants. Cochlear Implants Int. 2012;13:5-15.
38. National Institute on Deafness and Other Communication Disorders. Hearing aids. How can I adjust my hearing aid? Available at: http://www.nidcd.nih.gov/health/hearing/pages/hearingaid.aspx#hearingaid_08. Accessed March 27, 2012.
39. National Institute for Health and Clinical Excellence (NICE). Cochlear implants for children and adults with severe to profound deafness. London, UK: NICE; 2009:41.
1. National Institute on Deafness and Other Communication Disorders. Quick statistics. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx. Accessed March 30, 2012.
2. American Speech-Language-Hearing Association. The prevalence and incidence of hearing loss in children. Available at: http://www.asha.org/public/hearing/disorders/children.htm. Accessed March 26, 2012.
3. The Children’s Hearing Institute. Frequently asked questions about hearing loss. Available at: http://www.childrenshearing.org/custom/faq_hearing_loss.html. Accessed April 11, 2012.
4. Sprinzl GM, Riechelmann H. Current trends in hearing loss in elderly people: a review of the technology and treatment options-a mini-review. Gerontology. 2010;56:351-358.
5. Mulrow CD. Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc. 1990;38:45-50.
6. Meador HF, Zazove P. Health care interactions with deaf culture. J Am Board Fam Pract. 2005;18:218-222.
7. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility: experiences and perceptions of deaf people. J Gen Intern Med. 2006;21:260-266.
8. Scheier DB. Barriers to health care for people with hearing loss: a review of the literature. J NY State Nurses Assoc. 2009;40:4-10.
9. Nelson HD, Bougatsos C, Nygren P. Universal newborn hearing screening: systematic review to update the 2001 U.S. Preventive Services Task Force recommendation. Pediatrics. 2008;122:e266-e276.
10. National Institute for Deafness and Communication Disorders. Age at which hearing loss begins. Available at: http://www.nidcd.nih.gov/health/statistics/Pages/begins.aspx. Accessed April 16, 2012.
11. Shargorodsky J, Curhan SG, Curhan GC, et al. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772-778.
12. Chao TK, Chen TH. Predictive model for progression of hearing loss: meta-analysis of multi-state outcome. J Eval Clin Pract. 2009;15:32-40.
13. Cohen-Mansfield J, Taylor JW. Hearing aid use in nursing homes. Part 1: prevalence rates of hearing impairment and hearing aid use. J Am Med Dir Assoc. 2004;5:283-288.
14. Wake M, Hughes EK, Collins CM, et al. Parent-reported health-related quality of life in children with congenital hearing loss: a population study. Ambul Pediatr. 2004;4:411-417.
15. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
16. Centers for Disease Control and Prevention. Adolescent and school health. About hearing loss. Available at: http://www.cdc.gov/healthyyouth/noise/signs.htm. Accessed March 20, 2012.
17. Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139-145.
18. American Speech-Language-Hearing Association. Causes of hearing loss. Available at: http://www.asha.org/public/hearing/Causes-of-Hearing-Loss. Accessed April 11, 2012.
19. Widen SE, Holmes AE, Johnson T, et al. Hearing, use of hearing protection, and attitudes towards noise among young American adults. Int J Audiol.. 2009;48:537-545.
20. Hoover A, Krishnamurti S. Survey of college students’ MP3 listening: habits, safety issues, attitudes, and education. Am J Audiol. 2010;19:73-83.
21. Centers for Disease Control and Prevention. Noise and hearing loss prevention. Noise meter. Available at: http://www.cdc.gov/niosh/topics/noise/noisemeter.html. Accessed April 11, 2012.
22. El Dib RP, Mathew JL. Interventions to promote the wearing of hearing protection. Cochrane Database Syst Rev. 2009;(4):CD005234.-
23. US Preventive Services Task Force Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement. Pediatrics. 2008;122:143-148.
24. Hall JW, 3rd, Smith SD, Popelka GR. Newborn hearing screening with combined otoacoustic emissions and auditory brainstem responses. J Am Acad Audiol. 2004;15:414-425.
25. Norton SJ, Gorga MP, Widen JE, et al. Identification of neonatal hearing impairment: a multicenter investigation. Ear Hear. 2000;21:348-356.
26. Korver AM, Konings S, Dekker FW, et al. DECIBEL Collaborative Study Group. Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304:1701-1708.
27. American Academy of Pediatrics Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898-921.
28. Vogel I, Brug J, Hosli EJ, et al. MP3 players and hearing loss: adolescents’ perceptions of loud music and hearing conservation. J Pediatr. 2008;152:400-404.
29. Verbeek JH, Kateman E, Morata TC, et al. Interventions to prevent occupational noise induced hearing loss. Cochrane Database Syst Rev. 2009;(3):CD006396.-
30. Bade PF. Hearing impairment. In: Pacala JT, Sullivan GS, eds. Geriatrics Review Syllabus. 7th ed. New York, NY: American Geriatrics Society; 2010:197-206.
31. Yueh B, Shekelle P. Quality indicators for the care of hearing loss in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S335-S339.
32. Chou R, Dana T, Bougatsos C, et al. Screening adults aged 50 years or older for hearing loss: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;154:347-355.
33. Yueh B, Collins MP, Souza PE, et al. Long-term effectiveness of screening for hearing loss: the screening for auditory impairment—which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc. 2010;58:427-434.
34. Gates GA, Murphy M, Rees TS, et al. Screening for handicapped hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
35. Burton MJ, Doree C. Ear drops for the removal of ear wax. Cochrane Database Syst Rev. 2009;(1):CD004326.-
36. McDermott AL, Williams J, Kuo M, et al. Quality of life in children with a bone-anchored hearing aid. Otol Neurotol. 2009;30:344-349.
37. Fitzpatrick EM, Olds J, Gaboury I, et al. Comparison of outcomes in children with hearing aids and cochlear implants. Cochlear Implants Int. 2012;13:5-15.
38. National Institute on Deafness and Other Communication Disorders. Hearing aids. How can I adjust my hearing aid? Available at: http://www.nidcd.nih.gov/health/hearing/pages/hearingaid.aspx#hearingaid_08. Accessed March 27, 2012.
39. National Institute for Health and Clinical Excellence (NICE). Cochlear implants for children and adults with severe to profound deafness. London, UK: NICE; 2009:41.
Suicide and anxiety
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Suicide and anxiety
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
I found Dr. Scott Freeman’s article on suicide prevention (“Suicide assessment: Targeting acute risk factors,” Current Psychiatry, January 2012, p. 52-57) to be bold and courageous. Two of the 6 suicide risk factors he described are related to anxiety symptomatology: panic attacks and psychic anxiety. In the case study, Mr. L was prescribed clonazepam, a benzodiazepine, despite his history of comorbid alcohol abuse. Often, patients with substance abuse have related anxiety disorders—including posttraumatic stress disorder—and management with selective serotonin reuptake inhibitors (SSRIs) is not sufficient.
Because clinicians are hesitant to prescribe benzodiazepines to patients with a substance abuse history, patients often are forced to purchase these medications on the street or feel compelled to relapse to substance abuse in a frantic, albeit misguided, effort to contain their crippling symptoms. Even in inpatient drug rehabilitation settings, benzodiazepines often are not an option because they are not allowed. The “safer” SSRIs may be more dangerous when given to substance abusers in whom a comorbid mood disorder often is missed.
Current Psychiatry has never been shy in addressing the truth or uncomfortable issues in our complex field. Do we have the courage to open this up for dialogue and conversation?
Robert Barris, MD
Attending Psychiatrist
Nassau University Medical Center
East Meadow, NY
Binge eating disorder: Evidence-based treatments
Binge eating is consumption of an unusually large amount of food coupled with a feeling of loss of control over eating. Binge eating disorder (BED) is characterized by recurrent episodes of binge eating without inappropriate compensatory behaviors (eg, self-induced vomiting, misuse of laxatives, diuretics, or other agents, excessive exercise).1 It is the most common eating disorder in the United States, with a lifetime prevalence of approximately 3.5% in women and 2% in men.2 The diagnosis falls within the DSM-IV-TR category of eating disorders not otherwise specified,1 but clinicians often view it as a distinct clinical phenomenon. In DSM-IV-TR, an individual would meet criteria for BED if he or she engages in regular binge eating behavior in the absence of recurrent compensatory behaviors ≥2 days per week over 6 months.1 Proposed changes for DSM-5 recognize a distinct BED diagnosis, reduce the frequency criterion to once per week and the duration criterion to the past 3 months, and shift the focus from binge days to binge episodes (Table 1).3
Table 1
Proposed DSM-5 criteria for binge eating disorder
|
| Source:Reference 3 |
BED can occur in individuals of all body mass indices (BMI), but is common among individuals who are overweight or obese as well as those with depression or type 2 diabetes; BED can complicate treatment of these conditions.2,4,5 Primary treatment goals are:
- abstinence from binge eating
- improved psychological functioning
- appropriate weight regulation in overweight patients.
We report on 3 approaches to BED treatment: medication only, behavioral intervention only, and medication plus behavioral intervention. This article provides insights about emerging changes in diagnostic criteria for BED as well as evidence-informed treatment options and recommendations.
The evidence base
We conducted a review of 23 BED studies: 7 medication only, 5 medication plus behavioral, and 11 behavioral only. We focused on studies conducted since September 2005 that included binge frequency, weight, and depression as primary outcomes (see Berkman et al6 for a review of BED treatment studies before 2005). The studies included 2,527 participants (2,216 women and 311 men). Although the sex distribution of BED in the general population tends to slightly favor women,2 the proportion of women presenting for treatment generally is considerably higher than that of men. In studies that reported on race and/or ethnicity, 1,639 participants were identified as white, 191 as African American, 25 as Hispanic, 2 as Asian, 1 as Native American, and 25 as “other.” Ages ranged from 18 to 77.
Several medications are effective
In placebo-controlled studies, a high-dose selective serotonin reuptake inhibitor (escitalopram7), 2 anticonvulsants (zonisamide8 and topiramate9), a selective norepinephrine reuptake inhibitor (atomoxetine10), and an appetite suppressant (sibutramine11) were associated with significant decreases in binge eating frequency, weight, and BMI in overweight/obese patients diagnosed with BED (Table 2). In an open-label trial, memantine—a N-methyl-D-aspartate receptor antagonist often used to treat symptoms of Alzheimer’s disease—was associated with a significant reduction in binge eating but no change in weight.12 Lamotrigine was not significantly different from placebo in reducing binge eating or weight, but showed promise in reducing metabolic parameters such as glucose and triglyceride levels commonly associated with obesity and type 2 diabetes.13 Because BED often is comorbid with obesity and type 2 diabetes, lamotrigine augmentation when treating obese individuals with BED warrants further investigation. As with any pharmacologic agent, carefully consider potential side effects and interactions with other drugs before prescribing medications for BED. Informing patients of potential side effects is crucial for patient safety and accuracy of the data collected in well-controlled treatment studies.
Table 2
Pharmacotherapy for binge eating disorder
| Study | Drug/dosage | Comments |
|---|---|---|
| Guerdjikova et al, 20087 | Escitalopram, 10 to 30 mg/d, vs placebo for 12 weeks | Escitalopram was significantly better than placebo in reducing weight, BMI, and illness severity |
| McElroy et al, 20068 | Zonisamide, 100 to 600 mg/d, vs placebo for 16 weeks | Zonisamide was significantly better than placebo in reducing BE, weight, BMI, and various aspects of unhealthy eating behavior |
| McElroy et al, 20079 | Topiramate, 25 to 400 mg/d, vs placebo for 16 weeks | Topiramate was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| McElroy et al, 200710 | Atomoxetine, 40 to 120 mg/d, vs placebo for 10 weeks | Atomoxetine was significantly better than placebo in reducing BE, weight, BMI, and obsessive-compulsive features of BE, and in achieving remission |
| Wilfley et al, 200811 | Sibutramine, 15 mg/d, vs placebo for 24 weeks | Sibutramine was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| Brennan et al, 200812 | Open-label memantine, 5 to 20 mg/d, for 12 weeks | Memantine was associated with decreased binge frequency and related psychological features of BE |
| Guerdjikova et al, 200913 | Lamotrigine, 50 to 400 mg/d, vs placebo for 16 weeks | Lamotrigine was not significantly different from placebo |
| BE: binge eating; BMI: body mass index | ||
CBT vs other behavioral approaches
Cognitive-behavioral therapy (CBT), which focuses on identifying and modifying unhealthy thoughts that maintain disordered eating behaviors, is the most widely studied behavioral intervention for BED. Other studied treatments include interpersonal psychotherapy (IPT), motivational interviewing (MI), and structured behavioral weight loss (BWL) (Table 3).14-24 IPT is a psychodynamically based, time-limited treatment that focuses on the interpersonal context of the disorder and on building interpersonal skills. MI emphasizes exploring and resolving ambivalence about treatment, and works to facilitate change through motivational processes. BWL is centered on making dietary and physical activity changes to achieve weight loss. Behavioral treatments have been delivered in various formats, such as an individual or group setting, by electronic interface, and via self-help approaches. Most studies compared active treatment to a control group, but some compared active treatments head-to-head.
Table 3
CBT and other behavioral interventions for BED
| Study | Intervention | Comments |
|---|---|---|
| Annunziato et al, 200914 | 2 groups received CBT and hypocaloric diet for 8 weeks followed by 14 weeks of enhanced nutritional program (ie, reduced consumption of high energy density foods and once-daily liquid meal replacement) or control (normal diet) | Enhanced nutritional program was not significantly different from the control in reducing weight, BE, or psychological features of BE; variability in adherence to the enhanced nutritional program was identified as a significant effect modifier |
| Ashton et al, 200915 | 4 sessions of group CBT in an open trial | CBT was associated with significant reductions in BE and psychological features of BE in post-bariatric surgery patients |
| Dingemans et al, 200716 | CBT vs wait-list control | CBT significantly better than the wait-list control in reducing BE and psychological features of BE, and in achieving abstinence from BE |
| Friederich et al, 200717 | 15-session CBT blended with elements of interpersonal therapy (IPT), nutritional counseling, and supervised walking program; no control group | Treatment significantly reduced weight, BE, and related psychological features of BE in patients meeting sub-threshold and full criteria for BED |
| Grilo et al, 200518 | Guided self-help CBT (CBTgsh) vs guided self-help behavioral weight loss (BWLgsh) vs non-specific attention control for 12 weeks | CBTgsh significantly better than BWLgsh and control in BE remission; CBTgsh significantly better than BWLgsh, which was significantly better than control in reducing cognitive restraint; CBTgsh significantly better than control in reducing depression and eating-related psychopathology; no differences between groups in BMI change |
| Ricca et al, 201019 | Individual (I-CBT) vs group CBT (G-CBT) for 24 weeks in patients meeting subthreshold and full criteria for BED | BE and BMI were significantly reduced in both groups at 24 weeks and 3-year follow-up. I-CBT was not better than G-CBT in reducing BE or weight at 24 weeks or 3-year follow-up; I-CBT was significantly better than G-CBT in reducing eating-related psychopathology at 24 weeks and 3-year follow-up; I-CBT was significantly better than G-CBT in recovery (ie, no longer meeting full BED criteria) at 24 weeks but not at 3-year follow-up |
| Schlup et al, 200920 | 8 weekly sessions of group CBT vs wait-list control | CBT was significantly better than wait-list control in reducing BE and eating concerns and in achieving abstinence at end of treatment; CBT was not different than control in reducing BMI; treatment-related reductions in BE and eating concerns were maintained at 12-month follow-up |
| Shapiro et al, 200721 | 10 weekly sessions of group CBT (G-CBT) vs CD-ROM delivered CBT (CD-CBT) vs wait-list control | G-CBT and CD-CBT were not different from each other but both were significantly better than wait-list control in reducing BE |
| Tasca et al, 200622 | Group CBT (G-CBT) vs group psychodynamic interpersonal therapy (G-IPT) vs wait-list control for 16 weeks | G-CBT and G-IPT were not different from each other; G-CBT and G-IPT were significantly better than wait list in reducing BE and interpersonal problems (but not BMI) and increasing cognitive restraint post-treatment; depression was reduced in both groups at 6 months but only in G-IPT at 12 months; reductions in BE maintained at 12 months |
| Wilson et al, 201023 | 10 sessions of guided self-help CBT (CBTgsh) vs 19 sessions of IPT vs 20 sessions of behavioral weight loss (BWL) over 6 months | BWL was significantly better than IPT and CBTgsh in reducing BMI and in the number of patients achieving 5% weight loss at post-treatment but effects were not sustained over time; BWL was significantly better than CBTgsh in increasing dietary restraint |
| Cassin et al, 200824 | Self-help book + motivational interviewing (SH-MI) vs self-help book alone (SH) for 16 weeks | SH-MI was significantly better than SH in reducing BE and depression |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Studies found that CBT and IPT are effective in reducing the frequency of binge eating, whether measured by the number of binge eating episodes or days a patient reports having engaged in binge eating.14-23 However, some studies suggested that CBT can help a substantial number of patients achieve abstinence from binge eating.16,20 Adding MI to a self-help approach may improve binge eating outcomes,24 and binge eating can be successfully reduced using individual, group, and CD-ROM delivery formats.21 In direct comparisons, individual CBT outperformed group CBT in helping patients recover from BED (ie, no longer meeting diagnostic criteria),19 and CBT delivered via guided self-help outperformed BWL in helping patients achieve remission.18
Psychological features of BED typically include low levels of cognitive restraint and high levels of disinhibition, hunger, and shape and weight concerns. Improvements in these psychological measures were observed with CBT,15-20,22 IPT,22 and MI.24 In direct comparisons, self-help CBT demonstrated greater reductions in perceived hunger and disinhibition than self-help BWL,18 and individual CBT outperformed group CBT in reducing shape and weight concerns.19 Isolated studies reported improvements in depression after self-help CBT18 and MI,24 and sustained improvements22 after group CBT (6 months) and group IPT (12 months). Additional research is needed to determine whether CBT crafted specifically for BED improves self-rated depression or if enhancements targeting depressive symptoms are required.
The impact of behavioral interventions on weight in overweight patients has been mixed. Although some CBT studies reported a substantial decrease in weight,17,19 others suggested that weight loss among patients treated with CBT is not superior to those in a wait-list control group16 or is not significant over the course of treatment.20,21 The impact of BWL on weight outcomes in BED also has been unimpressive: after 12 weeks, self-help BWL was no better than self-help CBT in reducing BMI18; after 16 weeks, BWL was better than CBT and IPT in achieving clinically significant (≥5%) weight loss, but this advantage was not sustained at 1- and 2-year follow-up.23 It is difficult to determine why successfully treated BED patients fail to lose weight because one would expect decreases in binge eating to lead to weight loss. It is possible that calories previously consumed during binge eating episodes are distributed over non-binge meals or that patients label binges and non-binge meals differently as a result of treatment.
Combining treatments
BED patients often are treated with a combination of psychotherapy and pharmacotherapy (Table 4).25-29 When added to CBT, topiramate was associated with improvements in weight and some psychological outcomes,25,26 but fluoxetine was not.27,28 Direct comparisons also showed that CBT, alone or combined with fluoxetine, was better than fluoxetine alone in reducing binge eating.27 When combined with an individualized hypocaloric diet, the anti-obesity medication orlistat reduced weight in obese BED patients but had no appreciable effect on binge eating.29 Collectively, the studies we reviewed suggested that combining medication and CBT may improve binge eating and weight loss outcomes; however, additional trials are necessary to determine more definitively which medications combined with CBT are best at producing sustained weight loss while reducing binge eating frequency.
Table 4
Combining medication with behavioral interventions for BED
| Study | Drug/dosage | Comments |
|---|---|---|
| Brambilla et al, 200925 | 3 groups treated for 6 months: Group 1: CBT + setraline (50 to 150 mg/d) + topiramate (25 to 150 mg/d) + reduced calorie diet Group 2: CBT + sertraline (50 to 150 mg/d) + reduced calorie diet Group 3: CBT + nutritional counseling | Weight, BMI, and psychological features of BE reduced significantly only in group 1 |
| Claudino et al, 200726 | Group 1: CBT + topiramate (25 to 300 mg/d) Group 2: CBT + placebo 19 sessions over 21 weeks | Significant reductions in BE and depression in both groups; topiramate significantly better than placebo in reducing weight and in achieving BE remission |
| Devlin et al, 200527 | 4 groups, all received behavioral weight control intervention for 5 months (20 weeks) plus either: Group 1: CBT + fluoxetine Group 2: CBT + placebo Group 3: fluoxetineGroup 4 placebo (fluoxetine dose, 20 to 60 mg/d) | CBT groups (1 and 2) significantly better than non-CBT groups (3 and 4) in reducing BE and achieving abstinence from BE; fluoxetine significantly better than placebo in reducing depression |
| Molinari et al, 200528 | 3 groups, all received nutritional and diet counseling for 54 weeks (4 were inpatient) plus: Group 1: CBT Group 2: fluoxetine (20 to 60 mg/d) Group 3: CBT + fluoxetine | At 12 months, CBT (groups 1 and 3) associated with lower BE frequency and greater percentage of weight loss than fluoxetine |
| Golay et al, 200529 | Hypocaloric diet + orlistat (120 mg/d) vs hypocaloric diet + placebo for 24 weeks | Orlistat not different from placebo in reducing the number of patients classified with BED; orlistat significantly better than placebo in reducing weight and body fat |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Recommendations
Evidence suggests that pharmacotherapy and CBT—alone or in combination—are effective in reducing binge eating, and pharmacotherapy is effective in reducing weight in overweight individuals with BED. More research is needed for IPT and MI. It is unclear which medications provide the greatest benefit in terms of binge eating remission; however, pharmacotherapy has a clear advantage in facilitating short-term weight loss. Also, all BED patients should receive medical management to address possible complications such as hypertension or type 2 diabetes. In addition to reducing binge eating, CBT can improve related psychological comorbidities (eg, eating-related psychopathology and depression) and may have additional benefit when combined with pharmacotherapy.
In light of these findings, we recommend augmenting psychotherapeutic care with pharmacotherapy and medical management to address all relevant psychological and medical domains. Future investigations should address the benefits of coordinated psychological and medical care and evaluate how to maintain treatment gains.
Related Resources
- Binge Eating Disorder Association. www.bedaonline.com.
- Brownley KA, Berkman ND, Sedway JA, et al. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):337-348.
Drug Brand Names
- Atomoxetine • Strattera
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Memantine • Namenda
- Orlistat • Alli, Xenical
- Sertraline • Zoloft
- Sibutramine • Meridia
- Topiramate • Topamax, Topiragen
- Zonisamide • Zonegram
Disclosures
Dr. Peat receives a post-doctoral trainee grant from the National Institutes of Health.
Drs. Brownley, Berkman, and Bulik report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Hudson JI, Hiripi E, Pope HG, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
3. American Psychiatric Association. Proposed revision to DSM-5: K-05 Feeding and eating disorders. http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=372. Updated January 31 2011. Accessed March 26, 2012.
4. Grucza RA, Pryzbeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry. 2007;48(2):124-131.
5. Meneghini LF, Spadola J, Florez H. Prevalence and associations of binge eating disorder in a multiethnic population with type 2 diabetes. Diabetes Care. 2006;29(12):2760.-
6. Berkman ND, Bulik CM, Brownley KA, et al. Management of eating disorders. Evidence report/technology assessment No. 135. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ Publication No. 06-E010.
7. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
8. McElroy SL, Kotwal R, Guerdjikova AL, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
9. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
10. McElroy SL, Guerdjikova A, Kotwal R, et al. Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry. 2007;68(3):390-398.
11. Wilfley DE, Crow SJ, Hudson JI, et al. Efficacy of sibutramine for the treatment of binge eating disorder: a randomized multicenter placebo-controlled double-blind study. Am J Psychiatry. 2008;165(1):51-58.
12. Brennan BP, Roberts JL, Fogarty KV, et al. Memantine in the treatment of binge eating disorder: an open-label, prospective trial. Int J Eat Disord. 2008;41(6):520-526.
13. Guerdjikova AI, McElroy SL, Welge JA, et al. Lamotrigine in the treatment of binge-eating disorder with obesity: a randomized, placebo-controlled monotherapy trial. Int Clin Psychopharmacol. 2009;24(3):150-158.
14. Annunziato RA, Timko CA, Crerand CE, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat Behav. 2009;10(3):176-183.
15. Ashton K, Drerup M, Windover A, et al. Brief, four-session group CBT reduces binge eating behaviors among bariatric surgery candidates. Surg Obes Relat Dis. 2009;5(2):257-262.
16. Dingemans AE, Spinhoven P, van Furth EF. Predictors and mediators of treatment outcome in patients with binge eating disorder. Behav Res Ther. 2007;45(11):2551-2562.
17. Friederich HC, Schild S, Wild B, et al. Treatment outcome in people with subthreshold compared with full-syndrome binge eating disorder. Obesity. 2007;15(2):283-287.
18. Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther. 2005;43(11):1509-1525.
19. Ricca V, Castellini G, Mannucci E, et al. Comparison of individual and group cognitive behavioral therapy for binge eating disorder. A randomized, three-year follow-up study. Appetite. 2010;55(3):656-665.
20. Schlup B, Munsch S, Meyer AH, et al. The efficacy of a short version of a cognitive-behavioral treatment followed by booster sessions for binge eating disorder. Behav Res Ther. 2009;47(7):628-635.
21. Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, et al. Feasibility and acceptability of CD-ROM-based cognitive-behavioral treatment for binge-eating disorder. Eur Eat Disord Rev. 2007;15(3):175-184.
22. Tasca GA, Ritchie K, Conrad G, et al. Attachment scales predict outcome in a randomized controlled trial of two group therapies for binge eating disorder: an aptitude by treatment interaction. Psychother Res. 2006;16(1):106-121.
23. Wilson GT, Wilfley DE, Agras WS, et al. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67(1):94-101.
24. Cassin SE, von Ranson KM, Heng K, et al. Adapted motivational interviewing for women with binge eating disorder: a randomized controlled trial. Psychol Addict Behav. 2008;22(3):417-425.
25. Brambilla F, Samek L, Company M, et al. Multivariate therapeutic approach to binge-eating disorder: combined nutritional, psychological and pharmacological treatment. Int Clin Psychopharmacol. 2009;24(6):312-317.
26. Claudino AM, de Oliveira IR, Appolinario JC, et al. Double-blind, randomized, placebo-controlled trial of topiramate plus cognitive-behavior therapy in binge-eating disorder. J Clin Psychiatry. 2007;68(9):1324-1332.
27. Devlin MJ, Goldfein JA, Petkova E, et al. Cognitive behavioral therapy and fluoxetine as adjuncts to group behavioral therapy for binge eating disorder. Obes Res. 2005;13(6):1077-1088.
28. Molinari E, Baruffi M, Croci M, et al. Binge eating disorder in obesity: comparison of different therapeutic strategies. Eat Weight Disord. 2005;10(3):154-161.
29. Golay A, Laurent-Jaccard A, Habicht F, et al. Effect of orlistat in obese patients with binge eating disorder. Obes Res. 2005;13(10):1701-1708.
Binge eating is consumption of an unusually large amount of food coupled with a feeling of loss of control over eating. Binge eating disorder (BED) is characterized by recurrent episodes of binge eating without inappropriate compensatory behaviors (eg, self-induced vomiting, misuse of laxatives, diuretics, or other agents, excessive exercise).1 It is the most common eating disorder in the United States, with a lifetime prevalence of approximately 3.5% in women and 2% in men.2 The diagnosis falls within the DSM-IV-TR category of eating disorders not otherwise specified,1 but clinicians often view it as a distinct clinical phenomenon. In DSM-IV-TR, an individual would meet criteria for BED if he or she engages in regular binge eating behavior in the absence of recurrent compensatory behaviors ≥2 days per week over 6 months.1 Proposed changes for DSM-5 recognize a distinct BED diagnosis, reduce the frequency criterion to once per week and the duration criterion to the past 3 months, and shift the focus from binge days to binge episodes (Table 1).3
Table 1
Proposed DSM-5 criteria for binge eating disorder
|
| Source:Reference 3 |
BED can occur in individuals of all body mass indices (BMI), but is common among individuals who are overweight or obese as well as those with depression or type 2 diabetes; BED can complicate treatment of these conditions.2,4,5 Primary treatment goals are:
- abstinence from binge eating
- improved psychological functioning
- appropriate weight regulation in overweight patients.
We report on 3 approaches to BED treatment: medication only, behavioral intervention only, and medication plus behavioral intervention. This article provides insights about emerging changes in diagnostic criteria for BED as well as evidence-informed treatment options and recommendations.
The evidence base
We conducted a review of 23 BED studies: 7 medication only, 5 medication plus behavioral, and 11 behavioral only. We focused on studies conducted since September 2005 that included binge frequency, weight, and depression as primary outcomes (see Berkman et al6 for a review of BED treatment studies before 2005). The studies included 2,527 participants (2,216 women and 311 men). Although the sex distribution of BED in the general population tends to slightly favor women,2 the proportion of women presenting for treatment generally is considerably higher than that of men. In studies that reported on race and/or ethnicity, 1,639 participants were identified as white, 191 as African American, 25 as Hispanic, 2 as Asian, 1 as Native American, and 25 as “other.” Ages ranged from 18 to 77.
Several medications are effective
In placebo-controlled studies, a high-dose selective serotonin reuptake inhibitor (escitalopram7), 2 anticonvulsants (zonisamide8 and topiramate9), a selective norepinephrine reuptake inhibitor (atomoxetine10), and an appetite suppressant (sibutramine11) were associated with significant decreases in binge eating frequency, weight, and BMI in overweight/obese patients diagnosed with BED (Table 2). In an open-label trial, memantine—a N-methyl-D-aspartate receptor antagonist often used to treat symptoms of Alzheimer’s disease—was associated with a significant reduction in binge eating but no change in weight.12 Lamotrigine was not significantly different from placebo in reducing binge eating or weight, but showed promise in reducing metabolic parameters such as glucose and triglyceride levels commonly associated with obesity and type 2 diabetes.13 Because BED often is comorbid with obesity and type 2 diabetes, lamotrigine augmentation when treating obese individuals with BED warrants further investigation. As with any pharmacologic agent, carefully consider potential side effects and interactions with other drugs before prescribing medications for BED. Informing patients of potential side effects is crucial for patient safety and accuracy of the data collected in well-controlled treatment studies.
Table 2
Pharmacotherapy for binge eating disorder
| Study | Drug/dosage | Comments |
|---|---|---|
| Guerdjikova et al, 20087 | Escitalopram, 10 to 30 mg/d, vs placebo for 12 weeks | Escitalopram was significantly better than placebo in reducing weight, BMI, and illness severity |
| McElroy et al, 20068 | Zonisamide, 100 to 600 mg/d, vs placebo for 16 weeks | Zonisamide was significantly better than placebo in reducing BE, weight, BMI, and various aspects of unhealthy eating behavior |
| McElroy et al, 20079 | Topiramate, 25 to 400 mg/d, vs placebo for 16 weeks | Topiramate was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| McElroy et al, 200710 | Atomoxetine, 40 to 120 mg/d, vs placebo for 10 weeks | Atomoxetine was significantly better than placebo in reducing BE, weight, BMI, and obsessive-compulsive features of BE, and in achieving remission |
| Wilfley et al, 200811 | Sibutramine, 15 mg/d, vs placebo for 24 weeks | Sibutramine was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| Brennan et al, 200812 | Open-label memantine, 5 to 20 mg/d, for 12 weeks | Memantine was associated with decreased binge frequency and related psychological features of BE |
| Guerdjikova et al, 200913 | Lamotrigine, 50 to 400 mg/d, vs placebo for 16 weeks | Lamotrigine was not significantly different from placebo |
| BE: binge eating; BMI: body mass index | ||
CBT vs other behavioral approaches
Cognitive-behavioral therapy (CBT), which focuses on identifying and modifying unhealthy thoughts that maintain disordered eating behaviors, is the most widely studied behavioral intervention for BED. Other studied treatments include interpersonal psychotherapy (IPT), motivational interviewing (MI), and structured behavioral weight loss (BWL) (Table 3).14-24 IPT is a psychodynamically based, time-limited treatment that focuses on the interpersonal context of the disorder and on building interpersonal skills. MI emphasizes exploring and resolving ambivalence about treatment, and works to facilitate change through motivational processes. BWL is centered on making dietary and physical activity changes to achieve weight loss. Behavioral treatments have been delivered in various formats, such as an individual or group setting, by electronic interface, and via self-help approaches. Most studies compared active treatment to a control group, but some compared active treatments head-to-head.
Table 3
CBT and other behavioral interventions for BED
| Study | Intervention | Comments |
|---|---|---|
| Annunziato et al, 200914 | 2 groups received CBT and hypocaloric diet for 8 weeks followed by 14 weeks of enhanced nutritional program (ie, reduced consumption of high energy density foods and once-daily liquid meal replacement) or control (normal diet) | Enhanced nutritional program was not significantly different from the control in reducing weight, BE, or psychological features of BE; variability in adherence to the enhanced nutritional program was identified as a significant effect modifier |
| Ashton et al, 200915 | 4 sessions of group CBT in an open trial | CBT was associated with significant reductions in BE and psychological features of BE in post-bariatric surgery patients |
| Dingemans et al, 200716 | CBT vs wait-list control | CBT significantly better than the wait-list control in reducing BE and psychological features of BE, and in achieving abstinence from BE |
| Friederich et al, 200717 | 15-session CBT blended with elements of interpersonal therapy (IPT), nutritional counseling, and supervised walking program; no control group | Treatment significantly reduced weight, BE, and related psychological features of BE in patients meeting sub-threshold and full criteria for BED |
| Grilo et al, 200518 | Guided self-help CBT (CBTgsh) vs guided self-help behavioral weight loss (BWLgsh) vs non-specific attention control for 12 weeks | CBTgsh significantly better than BWLgsh and control in BE remission; CBTgsh significantly better than BWLgsh, which was significantly better than control in reducing cognitive restraint; CBTgsh significantly better than control in reducing depression and eating-related psychopathology; no differences between groups in BMI change |
| Ricca et al, 201019 | Individual (I-CBT) vs group CBT (G-CBT) for 24 weeks in patients meeting subthreshold and full criteria for BED | BE and BMI were significantly reduced in both groups at 24 weeks and 3-year follow-up. I-CBT was not better than G-CBT in reducing BE or weight at 24 weeks or 3-year follow-up; I-CBT was significantly better than G-CBT in reducing eating-related psychopathology at 24 weeks and 3-year follow-up; I-CBT was significantly better than G-CBT in recovery (ie, no longer meeting full BED criteria) at 24 weeks but not at 3-year follow-up |
| Schlup et al, 200920 | 8 weekly sessions of group CBT vs wait-list control | CBT was significantly better than wait-list control in reducing BE and eating concerns and in achieving abstinence at end of treatment; CBT was not different than control in reducing BMI; treatment-related reductions in BE and eating concerns were maintained at 12-month follow-up |
| Shapiro et al, 200721 | 10 weekly sessions of group CBT (G-CBT) vs CD-ROM delivered CBT (CD-CBT) vs wait-list control | G-CBT and CD-CBT were not different from each other but both were significantly better than wait-list control in reducing BE |
| Tasca et al, 200622 | Group CBT (G-CBT) vs group psychodynamic interpersonal therapy (G-IPT) vs wait-list control for 16 weeks | G-CBT and G-IPT were not different from each other; G-CBT and G-IPT were significantly better than wait list in reducing BE and interpersonal problems (but not BMI) and increasing cognitive restraint post-treatment; depression was reduced in both groups at 6 months but only in G-IPT at 12 months; reductions in BE maintained at 12 months |
| Wilson et al, 201023 | 10 sessions of guided self-help CBT (CBTgsh) vs 19 sessions of IPT vs 20 sessions of behavioral weight loss (BWL) over 6 months | BWL was significantly better than IPT and CBTgsh in reducing BMI and in the number of patients achieving 5% weight loss at post-treatment but effects were not sustained over time; BWL was significantly better than CBTgsh in increasing dietary restraint |
| Cassin et al, 200824 | Self-help book + motivational interviewing (SH-MI) vs self-help book alone (SH) for 16 weeks | SH-MI was significantly better than SH in reducing BE and depression |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Studies found that CBT and IPT are effective in reducing the frequency of binge eating, whether measured by the number of binge eating episodes or days a patient reports having engaged in binge eating.14-23 However, some studies suggested that CBT can help a substantial number of patients achieve abstinence from binge eating.16,20 Adding MI to a self-help approach may improve binge eating outcomes,24 and binge eating can be successfully reduced using individual, group, and CD-ROM delivery formats.21 In direct comparisons, individual CBT outperformed group CBT in helping patients recover from BED (ie, no longer meeting diagnostic criteria),19 and CBT delivered via guided self-help outperformed BWL in helping patients achieve remission.18
Psychological features of BED typically include low levels of cognitive restraint and high levels of disinhibition, hunger, and shape and weight concerns. Improvements in these psychological measures were observed with CBT,15-20,22 IPT,22 and MI.24 In direct comparisons, self-help CBT demonstrated greater reductions in perceived hunger and disinhibition than self-help BWL,18 and individual CBT outperformed group CBT in reducing shape and weight concerns.19 Isolated studies reported improvements in depression after self-help CBT18 and MI,24 and sustained improvements22 after group CBT (6 months) and group IPT (12 months). Additional research is needed to determine whether CBT crafted specifically for BED improves self-rated depression or if enhancements targeting depressive symptoms are required.
The impact of behavioral interventions on weight in overweight patients has been mixed. Although some CBT studies reported a substantial decrease in weight,17,19 others suggested that weight loss among patients treated with CBT is not superior to those in a wait-list control group16 or is not significant over the course of treatment.20,21 The impact of BWL on weight outcomes in BED also has been unimpressive: after 12 weeks, self-help BWL was no better than self-help CBT in reducing BMI18; after 16 weeks, BWL was better than CBT and IPT in achieving clinically significant (≥5%) weight loss, but this advantage was not sustained at 1- and 2-year follow-up.23 It is difficult to determine why successfully treated BED patients fail to lose weight because one would expect decreases in binge eating to lead to weight loss. It is possible that calories previously consumed during binge eating episodes are distributed over non-binge meals or that patients label binges and non-binge meals differently as a result of treatment.
Combining treatments
BED patients often are treated with a combination of psychotherapy and pharmacotherapy (Table 4).25-29 When added to CBT, topiramate was associated with improvements in weight and some psychological outcomes,25,26 but fluoxetine was not.27,28 Direct comparisons also showed that CBT, alone or combined with fluoxetine, was better than fluoxetine alone in reducing binge eating.27 When combined with an individualized hypocaloric diet, the anti-obesity medication orlistat reduced weight in obese BED patients but had no appreciable effect on binge eating.29 Collectively, the studies we reviewed suggested that combining medication and CBT may improve binge eating and weight loss outcomes; however, additional trials are necessary to determine more definitively which medications combined with CBT are best at producing sustained weight loss while reducing binge eating frequency.
Table 4
Combining medication with behavioral interventions for BED
| Study | Drug/dosage | Comments |
|---|---|---|
| Brambilla et al, 200925 | 3 groups treated for 6 months: Group 1: CBT + setraline (50 to 150 mg/d) + topiramate (25 to 150 mg/d) + reduced calorie diet Group 2: CBT + sertraline (50 to 150 mg/d) + reduced calorie diet Group 3: CBT + nutritional counseling | Weight, BMI, and psychological features of BE reduced significantly only in group 1 |
| Claudino et al, 200726 | Group 1: CBT + topiramate (25 to 300 mg/d) Group 2: CBT + placebo 19 sessions over 21 weeks | Significant reductions in BE and depression in both groups; topiramate significantly better than placebo in reducing weight and in achieving BE remission |
| Devlin et al, 200527 | 4 groups, all received behavioral weight control intervention for 5 months (20 weeks) plus either: Group 1: CBT + fluoxetine Group 2: CBT + placebo Group 3: fluoxetineGroup 4 placebo (fluoxetine dose, 20 to 60 mg/d) | CBT groups (1 and 2) significantly better than non-CBT groups (3 and 4) in reducing BE and achieving abstinence from BE; fluoxetine significantly better than placebo in reducing depression |
| Molinari et al, 200528 | 3 groups, all received nutritional and diet counseling for 54 weeks (4 were inpatient) plus: Group 1: CBT Group 2: fluoxetine (20 to 60 mg/d) Group 3: CBT + fluoxetine | At 12 months, CBT (groups 1 and 3) associated with lower BE frequency and greater percentage of weight loss than fluoxetine |
| Golay et al, 200529 | Hypocaloric diet + orlistat (120 mg/d) vs hypocaloric diet + placebo for 24 weeks | Orlistat not different from placebo in reducing the number of patients classified with BED; orlistat significantly better than placebo in reducing weight and body fat |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Recommendations
Evidence suggests that pharmacotherapy and CBT—alone or in combination—are effective in reducing binge eating, and pharmacotherapy is effective in reducing weight in overweight individuals with BED. More research is needed for IPT and MI. It is unclear which medications provide the greatest benefit in terms of binge eating remission; however, pharmacotherapy has a clear advantage in facilitating short-term weight loss. Also, all BED patients should receive medical management to address possible complications such as hypertension or type 2 diabetes. In addition to reducing binge eating, CBT can improve related psychological comorbidities (eg, eating-related psychopathology and depression) and may have additional benefit when combined with pharmacotherapy.
In light of these findings, we recommend augmenting psychotherapeutic care with pharmacotherapy and medical management to address all relevant psychological and medical domains. Future investigations should address the benefits of coordinated psychological and medical care and evaluate how to maintain treatment gains.
Related Resources
- Binge Eating Disorder Association. www.bedaonline.com.
- Brownley KA, Berkman ND, Sedway JA, et al. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):337-348.
Drug Brand Names
- Atomoxetine • Strattera
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Memantine • Namenda
- Orlistat • Alli, Xenical
- Sertraline • Zoloft
- Sibutramine • Meridia
- Topiramate • Topamax, Topiragen
- Zonisamide • Zonegram
Disclosures
Dr. Peat receives a post-doctoral trainee grant from the National Institutes of Health.
Drs. Brownley, Berkman, and Bulik report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Binge eating is consumption of an unusually large amount of food coupled with a feeling of loss of control over eating. Binge eating disorder (BED) is characterized by recurrent episodes of binge eating without inappropriate compensatory behaviors (eg, self-induced vomiting, misuse of laxatives, diuretics, or other agents, excessive exercise).1 It is the most common eating disorder in the United States, with a lifetime prevalence of approximately 3.5% in women and 2% in men.2 The diagnosis falls within the DSM-IV-TR category of eating disorders not otherwise specified,1 but clinicians often view it as a distinct clinical phenomenon. In DSM-IV-TR, an individual would meet criteria for BED if he or she engages in regular binge eating behavior in the absence of recurrent compensatory behaviors ≥2 days per week over 6 months.1 Proposed changes for DSM-5 recognize a distinct BED diagnosis, reduce the frequency criterion to once per week and the duration criterion to the past 3 months, and shift the focus from binge days to binge episodes (Table 1).3
Table 1
Proposed DSM-5 criteria for binge eating disorder
|
| Source:Reference 3 |
BED can occur in individuals of all body mass indices (BMI), but is common among individuals who are overweight or obese as well as those with depression or type 2 diabetes; BED can complicate treatment of these conditions.2,4,5 Primary treatment goals are:
- abstinence from binge eating
- improved psychological functioning
- appropriate weight regulation in overweight patients.
We report on 3 approaches to BED treatment: medication only, behavioral intervention only, and medication plus behavioral intervention. This article provides insights about emerging changes in diagnostic criteria for BED as well as evidence-informed treatment options and recommendations.
The evidence base
We conducted a review of 23 BED studies: 7 medication only, 5 medication plus behavioral, and 11 behavioral only. We focused on studies conducted since September 2005 that included binge frequency, weight, and depression as primary outcomes (see Berkman et al6 for a review of BED treatment studies before 2005). The studies included 2,527 participants (2,216 women and 311 men). Although the sex distribution of BED in the general population tends to slightly favor women,2 the proportion of women presenting for treatment generally is considerably higher than that of men. In studies that reported on race and/or ethnicity, 1,639 participants were identified as white, 191 as African American, 25 as Hispanic, 2 as Asian, 1 as Native American, and 25 as “other.” Ages ranged from 18 to 77.
Several medications are effective
In placebo-controlled studies, a high-dose selective serotonin reuptake inhibitor (escitalopram7), 2 anticonvulsants (zonisamide8 and topiramate9), a selective norepinephrine reuptake inhibitor (atomoxetine10), and an appetite suppressant (sibutramine11) were associated with significant decreases in binge eating frequency, weight, and BMI in overweight/obese patients diagnosed with BED (Table 2). In an open-label trial, memantine—a N-methyl-D-aspartate receptor antagonist often used to treat symptoms of Alzheimer’s disease—was associated with a significant reduction in binge eating but no change in weight.12 Lamotrigine was not significantly different from placebo in reducing binge eating or weight, but showed promise in reducing metabolic parameters such as glucose and triglyceride levels commonly associated with obesity and type 2 diabetes.13 Because BED often is comorbid with obesity and type 2 diabetes, lamotrigine augmentation when treating obese individuals with BED warrants further investigation. As with any pharmacologic agent, carefully consider potential side effects and interactions with other drugs before prescribing medications for BED. Informing patients of potential side effects is crucial for patient safety and accuracy of the data collected in well-controlled treatment studies.
Table 2
Pharmacotherapy for binge eating disorder
| Study | Drug/dosage | Comments |
|---|---|---|
| Guerdjikova et al, 20087 | Escitalopram, 10 to 30 mg/d, vs placebo for 12 weeks | Escitalopram was significantly better than placebo in reducing weight, BMI, and illness severity |
| McElroy et al, 20068 | Zonisamide, 100 to 600 mg/d, vs placebo for 16 weeks | Zonisamide was significantly better than placebo in reducing BE, weight, BMI, and various aspects of unhealthy eating behavior |
| McElroy et al, 20079 | Topiramate, 25 to 400 mg/d, vs placebo for 16 weeks | Topiramate was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| McElroy et al, 200710 | Atomoxetine, 40 to 120 mg/d, vs placebo for 10 weeks | Atomoxetine was significantly better than placebo in reducing BE, weight, BMI, and obsessive-compulsive features of BE, and in achieving remission |
| Wilfley et al, 200811 | Sibutramine, 15 mg/d, vs placebo for 24 weeks | Sibutramine was significantly better than placebo in reducing BE, weight, BMI, and related psychological features of BE |
| Brennan et al, 200812 | Open-label memantine, 5 to 20 mg/d, for 12 weeks | Memantine was associated with decreased binge frequency and related psychological features of BE |
| Guerdjikova et al, 200913 | Lamotrigine, 50 to 400 mg/d, vs placebo for 16 weeks | Lamotrigine was not significantly different from placebo |
| BE: binge eating; BMI: body mass index | ||
CBT vs other behavioral approaches
Cognitive-behavioral therapy (CBT), which focuses on identifying and modifying unhealthy thoughts that maintain disordered eating behaviors, is the most widely studied behavioral intervention for BED. Other studied treatments include interpersonal psychotherapy (IPT), motivational interviewing (MI), and structured behavioral weight loss (BWL) (Table 3).14-24 IPT is a psychodynamically based, time-limited treatment that focuses on the interpersonal context of the disorder and on building interpersonal skills. MI emphasizes exploring and resolving ambivalence about treatment, and works to facilitate change through motivational processes. BWL is centered on making dietary and physical activity changes to achieve weight loss. Behavioral treatments have been delivered in various formats, such as an individual or group setting, by electronic interface, and via self-help approaches. Most studies compared active treatment to a control group, but some compared active treatments head-to-head.
Table 3
CBT and other behavioral interventions for BED
| Study | Intervention | Comments |
|---|---|---|
| Annunziato et al, 200914 | 2 groups received CBT and hypocaloric diet for 8 weeks followed by 14 weeks of enhanced nutritional program (ie, reduced consumption of high energy density foods and once-daily liquid meal replacement) or control (normal diet) | Enhanced nutritional program was not significantly different from the control in reducing weight, BE, or psychological features of BE; variability in adherence to the enhanced nutritional program was identified as a significant effect modifier |
| Ashton et al, 200915 | 4 sessions of group CBT in an open trial | CBT was associated with significant reductions in BE and psychological features of BE in post-bariatric surgery patients |
| Dingemans et al, 200716 | CBT vs wait-list control | CBT significantly better than the wait-list control in reducing BE and psychological features of BE, and in achieving abstinence from BE |
| Friederich et al, 200717 | 15-session CBT blended with elements of interpersonal therapy (IPT), nutritional counseling, and supervised walking program; no control group | Treatment significantly reduced weight, BE, and related psychological features of BE in patients meeting sub-threshold and full criteria for BED |
| Grilo et al, 200518 | Guided self-help CBT (CBTgsh) vs guided self-help behavioral weight loss (BWLgsh) vs non-specific attention control for 12 weeks | CBTgsh significantly better than BWLgsh and control in BE remission; CBTgsh significantly better than BWLgsh, which was significantly better than control in reducing cognitive restraint; CBTgsh significantly better than control in reducing depression and eating-related psychopathology; no differences between groups in BMI change |
| Ricca et al, 201019 | Individual (I-CBT) vs group CBT (G-CBT) for 24 weeks in patients meeting subthreshold and full criteria for BED | BE and BMI were significantly reduced in both groups at 24 weeks and 3-year follow-up. I-CBT was not better than G-CBT in reducing BE or weight at 24 weeks or 3-year follow-up; I-CBT was significantly better than G-CBT in reducing eating-related psychopathology at 24 weeks and 3-year follow-up; I-CBT was significantly better than G-CBT in recovery (ie, no longer meeting full BED criteria) at 24 weeks but not at 3-year follow-up |
| Schlup et al, 200920 | 8 weekly sessions of group CBT vs wait-list control | CBT was significantly better than wait-list control in reducing BE and eating concerns and in achieving abstinence at end of treatment; CBT was not different than control in reducing BMI; treatment-related reductions in BE and eating concerns were maintained at 12-month follow-up |
| Shapiro et al, 200721 | 10 weekly sessions of group CBT (G-CBT) vs CD-ROM delivered CBT (CD-CBT) vs wait-list control | G-CBT and CD-CBT were not different from each other but both were significantly better than wait-list control in reducing BE |
| Tasca et al, 200622 | Group CBT (G-CBT) vs group psychodynamic interpersonal therapy (G-IPT) vs wait-list control for 16 weeks | G-CBT and G-IPT were not different from each other; G-CBT and G-IPT were significantly better than wait list in reducing BE and interpersonal problems (but not BMI) and increasing cognitive restraint post-treatment; depression was reduced in both groups at 6 months but only in G-IPT at 12 months; reductions in BE maintained at 12 months |
| Wilson et al, 201023 | 10 sessions of guided self-help CBT (CBTgsh) vs 19 sessions of IPT vs 20 sessions of behavioral weight loss (BWL) over 6 months | BWL was significantly better than IPT and CBTgsh in reducing BMI and in the number of patients achieving 5% weight loss at post-treatment but effects were not sustained over time; BWL was significantly better than CBTgsh in increasing dietary restraint |
| Cassin et al, 200824 | Self-help book + motivational interviewing (SH-MI) vs self-help book alone (SH) for 16 weeks | SH-MI was significantly better than SH in reducing BE and depression |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Studies found that CBT and IPT are effective in reducing the frequency of binge eating, whether measured by the number of binge eating episodes or days a patient reports having engaged in binge eating.14-23 However, some studies suggested that CBT can help a substantial number of patients achieve abstinence from binge eating.16,20 Adding MI to a self-help approach may improve binge eating outcomes,24 and binge eating can be successfully reduced using individual, group, and CD-ROM delivery formats.21 In direct comparisons, individual CBT outperformed group CBT in helping patients recover from BED (ie, no longer meeting diagnostic criteria),19 and CBT delivered via guided self-help outperformed BWL in helping patients achieve remission.18
Psychological features of BED typically include low levels of cognitive restraint and high levels of disinhibition, hunger, and shape and weight concerns. Improvements in these psychological measures were observed with CBT,15-20,22 IPT,22 and MI.24 In direct comparisons, self-help CBT demonstrated greater reductions in perceived hunger and disinhibition than self-help BWL,18 and individual CBT outperformed group CBT in reducing shape and weight concerns.19 Isolated studies reported improvements in depression after self-help CBT18 and MI,24 and sustained improvements22 after group CBT (6 months) and group IPT (12 months). Additional research is needed to determine whether CBT crafted specifically for BED improves self-rated depression or if enhancements targeting depressive symptoms are required.
The impact of behavioral interventions on weight in overweight patients has been mixed. Although some CBT studies reported a substantial decrease in weight,17,19 others suggested that weight loss among patients treated with CBT is not superior to those in a wait-list control group16 or is not significant over the course of treatment.20,21 The impact of BWL on weight outcomes in BED also has been unimpressive: after 12 weeks, self-help BWL was no better than self-help CBT in reducing BMI18; after 16 weeks, BWL was better than CBT and IPT in achieving clinically significant (≥5%) weight loss, but this advantage was not sustained at 1- and 2-year follow-up.23 It is difficult to determine why successfully treated BED patients fail to lose weight because one would expect decreases in binge eating to lead to weight loss. It is possible that calories previously consumed during binge eating episodes are distributed over non-binge meals or that patients label binges and non-binge meals differently as a result of treatment.
Combining treatments
BED patients often are treated with a combination of psychotherapy and pharmacotherapy (Table 4).25-29 When added to CBT, topiramate was associated with improvements in weight and some psychological outcomes,25,26 but fluoxetine was not.27,28 Direct comparisons also showed that CBT, alone or combined with fluoxetine, was better than fluoxetine alone in reducing binge eating.27 When combined with an individualized hypocaloric diet, the anti-obesity medication orlistat reduced weight in obese BED patients but had no appreciable effect on binge eating.29 Collectively, the studies we reviewed suggested that combining medication and CBT may improve binge eating and weight loss outcomes; however, additional trials are necessary to determine more definitively which medications combined with CBT are best at producing sustained weight loss while reducing binge eating frequency.
Table 4
Combining medication with behavioral interventions for BED
| Study | Drug/dosage | Comments |
|---|---|---|
| Brambilla et al, 200925 | 3 groups treated for 6 months: Group 1: CBT + setraline (50 to 150 mg/d) + topiramate (25 to 150 mg/d) + reduced calorie diet Group 2: CBT + sertraline (50 to 150 mg/d) + reduced calorie diet Group 3: CBT + nutritional counseling | Weight, BMI, and psychological features of BE reduced significantly only in group 1 |
| Claudino et al, 200726 | Group 1: CBT + topiramate (25 to 300 mg/d) Group 2: CBT + placebo 19 sessions over 21 weeks | Significant reductions in BE and depression in both groups; topiramate significantly better than placebo in reducing weight and in achieving BE remission |
| Devlin et al, 200527 | 4 groups, all received behavioral weight control intervention for 5 months (20 weeks) plus either: Group 1: CBT + fluoxetine Group 2: CBT + placebo Group 3: fluoxetineGroup 4 placebo (fluoxetine dose, 20 to 60 mg/d) | CBT groups (1 and 2) significantly better than non-CBT groups (3 and 4) in reducing BE and achieving abstinence from BE; fluoxetine significantly better than placebo in reducing depression |
| Molinari et al, 200528 | 3 groups, all received nutritional and diet counseling for 54 weeks (4 were inpatient) plus: Group 1: CBT Group 2: fluoxetine (20 to 60 mg/d) Group 3: CBT + fluoxetine | At 12 months, CBT (groups 1 and 3) associated with lower BE frequency and greater percentage of weight loss than fluoxetine |
| Golay et al, 200529 | Hypocaloric diet + orlistat (120 mg/d) vs hypocaloric diet + placebo for 24 weeks | Orlistat not different from placebo in reducing the number of patients classified with BED; orlistat significantly better than placebo in reducing weight and body fat |
| BE: binge eating; BED: binge eating disorder; BMI: body mass index; CBT: cognitive-behavioral therapy | ||
Recommendations
Evidence suggests that pharmacotherapy and CBT—alone or in combination—are effective in reducing binge eating, and pharmacotherapy is effective in reducing weight in overweight individuals with BED. More research is needed for IPT and MI. It is unclear which medications provide the greatest benefit in terms of binge eating remission; however, pharmacotherapy has a clear advantage in facilitating short-term weight loss. Also, all BED patients should receive medical management to address possible complications such as hypertension or type 2 diabetes. In addition to reducing binge eating, CBT can improve related psychological comorbidities (eg, eating-related psychopathology and depression) and may have additional benefit when combined with pharmacotherapy.
In light of these findings, we recommend augmenting psychotherapeutic care with pharmacotherapy and medical management to address all relevant psychological and medical domains. Future investigations should address the benefits of coordinated psychological and medical care and evaluate how to maintain treatment gains.
Related Resources
- Binge Eating Disorder Association. www.bedaonline.com.
- Brownley KA, Berkman ND, Sedway JA, et al. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40(4):337-348.
Drug Brand Names
- Atomoxetine • Strattera
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Memantine • Namenda
- Orlistat • Alli, Xenical
- Sertraline • Zoloft
- Sibutramine • Meridia
- Topiramate • Topamax, Topiragen
- Zonisamide • Zonegram
Disclosures
Dr. Peat receives a post-doctoral trainee grant from the National Institutes of Health.
Drs. Brownley, Berkman, and Bulik report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Hudson JI, Hiripi E, Pope HG, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
3. American Psychiatric Association. Proposed revision to DSM-5: K-05 Feeding and eating disorders. http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=372. Updated January 31 2011. Accessed March 26, 2012.
4. Grucza RA, Pryzbeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry. 2007;48(2):124-131.
5. Meneghini LF, Spadola J, Florez H. Prevalence and associations of binge eating disorder in a multiethnic population with type 2 diabetes. Diabetes Care. 2006;29(12):2760.-
6. Berkman ND, Bulik CM, Brownley KA, et al. Management of eating disorders. Evidence report/technology assessment No. 135. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ Publication No. 06-E010.
7. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
8. McElroy SL, Kotwal R, Guerdjikova AL, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
9. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
10. McElroy SL, Guerdjikova A, Kotwal R, et al. Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry. 2007;68(3):390-398.
11. Wilfley DE, Crow SJ, Hudson JI, et al. Efficacy of sibutramine for the treatment of binge eating disorder: a randomized multicenter placebo-controlled double-blind study. Am J Psychiatry. 2008;165(1):51-58.
12. Brennan BP, Roberts JL, Fogarty KV, et al. Memantine in the treatment of binge eating disorder: an open-label, prospective trial. Int J Eat Disord. 2008;41(6):520-526.
13. Guerdjikova AI, McElroy SL, Welge JA, et al. Lamotrigine in the treatment of binge-eating disorder with obesity: a randomized, placebo-controlled monotherapy trial. Int Clin Psychopharmacol. 2009;24(3):150-158.
14. Annunziato RA, Timko CA, Crerand CE, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat Behav. 2009;10(3):176-183.
15. Ashton K, Drerup M, Windover A, et al. Brief, four-session group CBT reduces binge eating behaviors among bariatric surgery candidates. Surg Obes Relat Dis. 2009;5(2):257-262.
16. Dingemans AE, Spinhoven P, van Furth EF. Predictors and mediators of treatment outcome in patients with binge eating disorder. Behav Res Ther. 2007;45(11):2551-2562.
17. Friederich HC, Schild S, Wild B, et al. Treatment outcome in people with subthreshold compared with full-syndrome binge eating disorder. Obesity. 2007;15(2):283-287.
18. Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther. 2005;43(11):1509-1525.
19. Ricca V, Castellini G, Mannucci E, et al. Comparison of individual and group cognitive behavioral therapy for binge eating disorder. A randomized, three-year follow-up study. Appetite. 2010;55(3):656-665.
20. Schlup B, Munsch S, Meyer AH, et al. The efficacy of a short version of a cognitive-behavioral treatment followed by booster sessions for binge eating disorder. Behav Res Ther. 2009;47(7):628-635.
21. Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, et al. Feasibility and acceptability of CD-ROM-based cognitive-behavioral treatment for binge-eating disorder. Eur Eat Disord Rev. 2007;15(3):175-184.
22. Tasca GA, Ritchie K, Conrad G, et al. Attachment scales predict outcome in a randomized controlled trial of two group therapies for binge eating disorder: an aptitude by treatment interaction. Psychother Res. 2006;16(1):106-121.
23. Wilson GT, Wilfley DE, Agras WS, et al. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67(1):94-101.
24. Cassin SE, von Ranson KM, Heng K, et al. Adapted motivational interviewing for women with binge eating disorder: a randomized controlled trial. Psychol Addict Behav. 2008;22(3):417-425.
25. Brambilla F, Samek L, Company M, et al. Multivariate therapeutic approach to binge-eating disorder: combined nutritional, psychological and pharmacological treatment. Int Clin Psychopharmacol. 2009;24(6):312-317.
26. Claudino AM, de Oliveira IR, Appolinario JC, et al. Double-blind, randomized, placebo-controlled trial of topiramate plus cognitive-behavior therapy in binge-eating disorder. J Clin Psychiatry. 2007;68(9):1324-1332.
27. Devlin MJ, Goldfein JA, Petkova E, et al. Cognitive behavioral therapy and fluoxetine as adjuncts to group behavioral therapy for binge eating disorder. Obes Res. 2005;13(6):1077-1088.
28. Molinari E, Baruffi M, Croci M, et al. Binge eating disorder in obesity: comparison of different therapeutic strategies. Eat Weight Disord. 2005;10(3):154-161.
29. Golay A, Laurent-Jaccard A, Habicht F, et al. Effect of orlistat in obese patients with binge eating disorder. Obes Res. 2005;13(10):1701-1708.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Hudson JI, Hiripi E, Pope HG, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348-358.
3. American Psychiatric Association. Proposed revision to DSM-5: K-05 Feeding and eating disorders. http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=372. Updated January 31 2011. Accessed March 26, 2012.
4. Grucza RA, Pryzbeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry. 2007;48(2):124-131.
5. Meneghini LF, Spadola J, Florez H. Prevalence and associations of binge eating disorder in a multiethnic population with type 2 diabetes. Diabetes Care. 2006;29(12):2760.-
6. Berkman ND, Bulik CM, Brownley KA, et al. Management of eating disorders. Evidence report/technology assessment No. 135. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ Publication No. 06-E010.
7. Guerdjikova AI, McElroy SL, Kotwal R, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23(1):1-11.
8. McElroy SL, Kotwal R, Guerdjikova AL, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
9. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
10. McElroy SL, Guerdjikova A, Kotwal R, et al. Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry. 2007;68(3):390-398.
11. Wilfley DE, Crow SJ, Hudson JI, et al. Efficacy of sibutramine for the treatment of binge eating disorder: a randomized multicenter placebo-controlled double-blind study. Am J Psychiatry. 2008;165(1):51-58.
12. Brennan BP, Roberts JL, Fogarty KV, et al. Memantine in the treatment of binge eating disorder: an open-label, prospective trial. Int J Eat Disord. 2008;41(6):520-526.
13. Guerdjikova AI, McElroy SL, Welge JA, et al. Lamotrigine in the treatment of binge-eating disorder with obesity: a randomized, placebo-controlled monotherapy trial. Int Clin Psychopharmacol. 2009;24(3):150-158.
14. Annunziato RA, Timko CA, Crerand CE, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat Behav. 2009;10(3):176-183.
15. Ashton K, Drerup M, Windover A, et al. Brief, four-session group CBT reduces binge eating behaviors among bariatric surgery candidates. Surg Obes Relat Dis. 2009;5(2):257-262.
16. Dingemans AE, Spinhoven P, van Furth EF. Predictors and mediators of treatment outcome in patients with binge eating disorder. Behav Res Ther. 2007;45(11):2551-2562.
17. Friederich HC, Schild S, Wild B, et al. Treatment outcome in people with subthreshold compared with full-syndrome binge eating disorder. Obesity. 2007;15(2):283-287.
18. Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther. 2005;43(11):1509-1525.
19. Ricca V, Castellini G, Mannucci E, et al. Comparison of individual and group cognitive behavioral therapy for binge eating disorder. A randomized, three-year follow-up study. Appetite. 2010;55(3):656-665.
20. Schlup B, Munsch S, Meyer AH, et al. The efficacy of a short version of a cognitive-behavioral treatment followed by booster sessions for binge eating disorder. Behav Res Ther. 2009;47(7):628-635.
21. Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, et al. Feasibility and acceptability of CD-ROM-based cognitive-behavioral treatment for binge-eating disorder. Eur Eat Disord Rev. 2007;15(3):175-184.
22. Tasca GA, Ritchie K, Conrad G, et al. Attachment scales predict outcome in a randomized controlled trial of two group therapies for binge eating disorder: an aptitude by treatment interaction. Psychother Res. 2006;16(1):106-121.
23. Wilson GT, Wilfley DE, Agras WS, et al. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67(1):94-101.
24. Cassin SE, von Ranson KM, Heng K, et al. Adapted motivational interviewing for women with binge eating disorder: a randomized controlled trial. Psychol Addict Behav. 2008;22(3):417-425.
25. Brambilla F, Samek L, Company M, et al. Multivariate therapeutic approach to binge-eating disorder: combined nutritional, psychological and pharmacological treatment. Int Clin Psychopharmacol. 2009;24(6):312-317.
26. Claudino AM, de Oliveira IR, Appolinario JC, et al. Double-blind, randomized, placebo-controlled trial of topiramate plus cognitive-behavior therapy in binge-eating disorder. J Clin Psychiatry. 2007;68(9):1324-1332.
27. Devlin MJ, Goldfein JA, Petkova E, et al. Cognitive behavioral therapy and fluoxetine as adjuncts to group behavioral therapy for binge eating disorder. Obes Res. 2005;13(6):1077-1088.
28. Molinari E, Baruffi M, Croci M, et al. Binge eating disorder in obesity: comparison of different therapeutic strategies. Eat Weight Disord. 2005;10(3):154-161.
29. Golay A, Laurent-Jaccard A, Habicht F, et al. Effect of orlistat in obese patients with binge eating disorder. Obes Res. 2005;13(10):1701-1708.
Generalized anxiety disorder: Helping patients overcome worry
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. M, age 44, is a married mother of 2 who presents to the psychiatric clinic with increased anxiety that recently has become intolerable, stating “I can’t stop my head.” She has experienced anxiety for “as long as I can remember.” She was a shy, anxious child who worried about her parents’ health. Her anxiety worsened at college, where she first sought care. She was prescribed diazepam as needed. The next semester, she had a depressive episode, treated with imipramine, 75 mg/d, which she tolerated poorly.
Mrs. M has received episodic supportive therapy since college. She has been plagued by bouts of anxiety and worry, with insomnia, tension, and fatigue. She worries about financial, career, family, and safety issues and has a phobia of spiders. Her family and friends often comment about her excessive worry, and it has strained her marriage and career; she was passed over for a promotion in part because of her anxiousness. Mrs. M also has experienced several depressive episodes.
Mrs. M has sought medical care for various non-specific somatic complaints; all laboratory tests were normal. Approximately 10 years ago, Mrs. M’s primary care physician prescribed fluoxetine, 20 mg/d, but Mrs. M stopped taking it after a few days, stating she felt “more anxious and jittery.”
To meet DSM-IV-TR diagnostic criteria for generalized anxiety disorder (GAD), patients must experience anxiety and worry that they find difficult to control. The worry and anxiety occur more days than not for at least 6 months and cause clinically significant distress and impairment (Table 1).1 These diagnostic criteria are being reevaluated—the DSM-5 Anxiety Work Group has proposed renaming the condition generalized worry disorder, specifying that only 2 domains need to be impacted by worry, shortening the required time frame of impairment from 6 months to 3 months, and including at least 1 behavioral change spawned by excessive worry.2 Although provisional, these recommendations suggest DSM-5 will include changes to GAD when it is published in 2013.
Table 1
DSM-IV-TR diagnostic criteria for generalized anxiety disorder
|
| OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder Source: Reference 1 |
A common, chronic condition
In the United States, the lifetime prevalence of GAD is 5.7%.3 It is twice as common in women. Although GAD can occur at any age, 75% of patients develop it before age 47; the median age is 31.3,4 Patients who present with GAD later in life have a better prognosis.3,4
Approximately 90% of GAD patients will meet criteria for another axis I disorder.3 When GAD patients present for treatment, social phobia and panic disorder are the most common comorbid psychiatric disorders. The lifetime prevalence of a mood disorder among GAD patients is 62%, but as few as 6% of GAD patients will meet criteria for a mood disorder at presentation.3 The onset of GAD usually precedes depression.5,6
Patients with GAD often first seek treatment from their primary care provider.7 A useful screening tool is the GAD-7 (Table 2).8 This instrument has a specificity of 92% and sensitivity of 76% for GAD for patients who score ≥8.7 Although the GAD-7 cannot confirm a GAD diagnosis, it can prompt clinicians to conduct a more structured interview. Because higher scores correlate with more severe symptoms, the GAD-7 can be used to measure progress.
Table 2
Screening for generalized anxiety disorder: The GAD-7
| Over the last 2 weeks, how often have you been bothered by any of the following problems? | Not at all | Several days | More than half the days | Nearly every day | |
|---|---|---|---|---|---|
| 1. | Feeling nervous, anxious, or on edge | 0 | 1 | 2 | 3 |
| 2. | Not being able to stop or control worrying | 0 | 1 | 2 | 3 |
| 3. | Worrying too much about different things | 0 | 1 | 2 | 3 |
| 4. | Trouble relaxing | 0 | 1 | 2 | 3 |
| 5. | Being so restless that it is hard to sit still | 0 | 1 | 2 | 3 |
| 6. | Becoming easily annoyed or irritable | 0 | 1 | 2 | 3 |
| 7. | Feeling afraid as if something awful might happen | 0 | 1 | 2 | 3 |
| GAD: generalized anxiety disorder Source: Reference 8 | |||||
Differential diagnosis
GAD typically has a chronic course with fluctuating symptom severity over the patient’s lifespan. Assess patients who present with anxiety for medical conditions that mimic GAD. These include:
- endocrine (hyperthyroidism), metabolic (electrolyte abnormalities), respiratory (asthma), neurologic (seizure disorder), or cardiac (arrhythmia) conditions
- nutritional deficiencies, especially of B vitamins and folate
- ingestion of substances or medications that may cause anxiety, such as caffeine or amphetamines.
A thorough history, medication review, and physical examination—as well as routine tests such as metabolic panel, complete blood count, thyroid function tests, urine drug screen, and electrocardiography—will capture most of these potential etiologies. In addition to ruling out medical causes, also assess for comorbid psychiatric conditions before reaching a diagnosis.
Evidence-based treatments
The treatment armamentarium for GAD includes psychotherapy and pharmacotherapy; complementary and alternative medicine (CAM) modalities may be useful adjunctive treatments. Which approach to use is determined by clinical judgment and the patient’s symptom severity and preferences. Combination therapy consisting of psychotherapy and medication often is appropriate.
Psychotherapy. Cognitive-behavioral therapy (CBT) is the preferred form of psychotherapy for GAD because it results in sustained improvements for patients with anxiety.5,9 Other modalities that may be effective include psychodynamic psychotherapy, mindfulness-based therapy, and interpersonal psychotherapy.10,11
Pharmacotherapy. As few as one-quarter of patients with GAD receive medications at appropriate dose and duration.12 Antidepressants are a first-line pharmacotherapy.5 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are highly effective for treating GAD.5 Paroxetine, escitalopram, duloxetine, and venlafaxine are FDA-approved for GAD, but other SSRIs also are used as primary treatment.5 Assuming the selected agent is tolerable and efficacious, a treatment course of 12 months is recommended.13
Benzodiazepines promote binding of the neuroinhibitory transmitter γ-aminobutyric acid and enhance chloride ion influx, thus reducing anxiety. Benzodiazepines have been widely used because of their rapid onset of action and effectiveness in managing anxiety, but their role in long-term management of GAD is unclear because these medications increase the risk of addiction, cognitive dulling, memory impairment, psychomotor retardation, and respiratory depression when combined with other CNS depressants such as alcohol and opiates. Before prescribing a benzodiazepine, conduct a thorough risk-benefit analysis and obtain informed consent. Long-term benzodiazepine monotherapy is not recommended.5,14
Hydroxyzine is an alternative to benzodiazepines.15 It works as an antihistamine and is FDA-approved for psychogenic neurosis, a Freudian distinction encompassing anxiety derived from psychological rather than physiological factors.
The azapirone buspirone is a nonaddictive, generally non-sedating 5-HT1A agonist. Although anecdotally some psychiatrists may report limited clinical utility, many analyses found azapirones, including buspirone, were effective for GAD,16,17 particularly for patients with comorbid depression.18
Tricyclic antidepressants are not a first-line choice because of their side effect profile and potential for drug-drug interactions. Nonetheless, some research suggests imipramine may be a reasonable option for GAD.14,19
Although not FDA-approved for GAD, anticonvulsant and antipsychotic medications may be reasonable adjunctive agents for patients with refractory GAD. Studies have suggested gabapentin20 and quetiapine21 as options.
Investigational treatments. Glutaminergic transmission is being investigated as a target for pharmacotherapy for GAD. In an 8-week, open-label trial, 12 of 15 GAD patients responded to the antiglutamatergic agent riluzole, 100 mg/d, and 8 patients achieved remission.22 In another study, pregabalin, which promotes calcium channel blockade, significantly reduced patients’ scores on the Hamilton Anxiety Rating Scale.23 However, this medication is a schedule V controlled substance and little is known about its long-term effects. Researchers had proposed that inhibition of corticotropin-releasing factor (CRF) may help reduce anxiety, but in a double-blind, placebo-controlled trial, they found that the CRF antagonist pexacerfont was no more effective than placebo.24
CAM treatments. A meta-analysis found that compared with placebo, kava extract (Piper methysticum) effectively reduced anxiety symptoms.25 However, considering its risk for hepatotoxicity, kava is not a recommended treatment.26 Although valerian, St. John’s wort, and passionflower have been used to manage GAD, there is insufficient evidence of their effectiveness and safety.26 No strong evidence supports nutritional supplements such as ginger, amino acids, and omega-3 fatty acids (fish oils) as treatment for GAD. Although there’s limited research on resistance training, aromatherapy, yoga, meditation, or acupuncture for treating anxiety, consider these treatments if your patient finds them helpful, because generally they are not contraindicated.
CASE CONTINUED: An antidepressant and CBT
Mrs. M reluctantly agrees to a trial of sertraline, 50 mg/d. She refuses a prescription for clonazepam because she is afraid of drug dependence but accepts a referral for CBT. Two days later, she calls the clinic and says she is more anxious and wants to stop the sertraline. The psychiatrist reassures her and reduces the dosage to 25 mg/d.
Mrs. M’s spike of anxiety resolves by her 2-week follow-up appointment and sertraline is titrated to 200 mg/d. Her irritability, anxiety, and mood improve within 2 months. The worry does not completely resolve, but she is much improved at 6 months, and the focus of her therapy shifts to her marriage.
Related Resources
- Goldberg D, Kendler KS, Sirvatka PJ, et al, eds. Diagnostic issues in depression and generalized anxiety disorder—refining the research agenda for DSM-V. Arlington, VA: American Psychiatric Publishing; 2010.
- Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
Drug Brand Names
- Buspirone • Buspar
- Clonazepam •Klonopin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin• Neurontin
- Hydroxyzine • Vistaril, Atarax
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Barry reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Andrews G, Hobbs MJ, Borkovec TD, et al. Generalized worry disorder: a review of DSM-IV generalized anxiety disorder and options for DSM-V. Depress Anxiety. 2010;27(2):134-147.
3. Turk CL, Mennin DS. Phenomenology of generalized anxiety disorder. Psychiatr Ann. 2011;41(2):72-78.
4. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
5. Ballenger JC, Davidson JR, Lecrubier Y, et al. Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2001;6(suppl 11):53-58.
6. Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):19-39.
7. Kavan MG, Elsasser G, Barone EJ. Generalized anxiety disorder: practical assessment and management. Am Fam Physician. 2009;79(9):785-791.
8. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092-1097.
9. Newman MG, Borkovec TD. Cognitive-behavioral treatment of generalized anxiety disorder. Clin Psychol. 1995;48(4):5-7.
10. Leichsenring F, Salzer S, Jaeger U, et al. Short-term psychodynamic psychotherapy and cognitive-behavioral therapy in generalized anxiety disorder: a randomized, controlled trial. Am J Psychiatry. 2009;166(8):875-881.
11. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
12. Stein MB, Sherbourne CD, Craske MG, et al. Quality of care for primary care patients with anxiety disorders. Am J Psychiatry. 2004;161(12):2230-2237.
13. Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
14. Rickels K, Downing R, Schweizer E, et al. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50(11):884-895.
15. Guaiana G, Barbui C, Cipriani A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst Rev. 2010;(12):CD006815.-
16. Gammans RE, Stringfellow JC, Hvizdos AJ, et al. Use of buspirone in patients with generalized anxiety disorder and coexisting depressive symptoms. A meta-analysis of eight randomized, controlled studies. Neuropsychobiology. 1992;25(4):193-201.
17. Chessick CA, Allen MH, Thase M, et al. Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev. 2006;(3):CD006115.-
18. Sramek JJ, Tansman M, Suri A, et al. Efficacy of buspirone in generalized anxiety disorder with coexisting mild depressive symptoms. J Clin Psychiatry. 1996;57(7):287-291.
19. Rickels K, DeMartinis N, García-España F, et al. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am J Psychiatry. 2000;157(12):1973-1979.
20. Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155(7):992-993.
21. Khan A, Joyce M, Atkinson S, et al. A randomized, double-blind study of once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2011;31(4):418-428.
22. Mathew SJ, Amiel JM, Coplan JD, et al. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162(12):2379-2381.
23. Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160(3):533-540.
24. Coric V, Feldman HH, Oren DA, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27(5):417-425.
25. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Database Syst Rev. 2003;(1):CD003383.-
26. Zoberi K, Pollard CA. Treating anxiety without SSRIs. J Fam Pract. 2010;59(3):148-154.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. M, age 44, is a married mother of 2 who presents to the psychiatric clinic with increased anxiety that recently has become intolerable, stating “I can’t stop my head.” She has experienced anxiety for “as long as I can remember.” She was a shy, anxious child who worried about her parents’ health. Her anxiety worsened at college, where she first sought care. She was prescribed diazepam as needed. The next semester, she had a depressive episode, treated with imipramine, 75 mg/d, which she tolerated poorly.
Mrs. M has received episodic supportive therapy since college. She has been plagued by bouts of anxiety and worry, with insomnia, tension, and fatigue. She worries about financial, career, family, and safety issues and has a phobia of spiders. Her family and friends often comment about her excessive worry, and it has strained her marriage and career; she was passed over for a promotion in part because of her anxiousness. Mrs. M also has experienced several depressive episodes.
Mrs. M has sought medical care for various non-specific somatic complaints; all laboratory tests were normal. Approximately 10 years ago, Mrs. M’s primary care physician prescribed fluoxetine, 20 mg/d, but Mrs. M stopped taking it after a few days, stating she felt “more anxious and jittery.”
To meet DSM-IV-TR diagnostic criteria for generalized anxiety disorder (GAD), patients must experience anxiety and worry that they find difficult to control. The worry and anxiety occur more days than not for at least 6 months and cause clinically significant distress and impairment (Table 1).1 These diagnostic criteria are being reevaluated—the DSM-5 Anxiety Work Group has proposed renaming the condition generalized worry disorder, specifying that only 2 domains need to be impacted by worry, shortening the required time frame of impairment from 6 months to 3 months, and including at least 1 behavioral change spawned by excessive worry.2 Although provisional, these recommendations suggest DSM-5 will include changes to GAD when it is published in 2013.
Table 1
DSM-IV-TR diagnostic criteria for generalized anxiety disorder
|
| OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder Source: Reference 1 |
A common, chronic condition
In the United States, the lifetime prevalence of GAD is 5.7%.3 It is twice as common in women. Although GAD can occur at any age, 75% of patients develop it before age 47; the median age is 31.3,4 Patients who present with GAD later in life have a better prognosis.3,4
Approximately 90% of GAD patients will meet criteria for another axis I disorder.3 When GAD patients present for treatment, social phobia and panic disorder are the most common comorbid psychiatric disorders. The lifetime prevalence of a mood disorder among GAD patients is 62%, but as few as 6% of GAD patients will meet criteria for a mood disorder at presentation.3 The onset of GAD usually precedes depression.5,6
Patients with GAD often first seek treatment from their primary care provider.7 A useful screening tool is the GAD-7 (Table 2).8 This instrument has a specificity of 92% and sensitivity of 76% for GAD for patients who score ≥8.7 Although the GAD-7 cannot confirm a GAD diagnosis, it can prompt clinicians to conduct a more structured interview. Because higher scores correlate with more severe symptoms, the GAD-7 can be used to measure progress.
Table 2
Screening for generalized anxiety disorder: The GAD-7
| Over the last 2 weeks, how often have you been bothered by any of the following problems? | Not at all | Several days | More than half the days | Nearly every day | |
|---|---|---|---|---|---|
| 1. | Feeling nervous, anxious, or on edge | 0 | 1 | 2 | 3 |
| 2. | Not being able to stop or control worrying | 0 | 1 | 2 | 3 |
| 3. | Worrying too much about different things | 0 | 1 | 2 | 3 |
| 4. | Trouble relaxing | 0 | 1 | 2 | 3 |
| 5. | Being so restless that it is hard to sit still | 0 | 1 | 2 | 3 |
| 6. | Becoming easily annoyed or irritable | 0 | 1 | 2 | 3 |
| 7. | Feeling afraid as if something awful might happen | 0 | 1 | 2 | 3 |
| GAD: generalized anxiety disorder Source: Reference 8 | |||||
Differential diagnosis
GAD typically has a chronic course with fluctuating symptom severity over the patient’s lifespan. Assess patients who present with anxiety for medical conditions that mimic GAD. These include:
- endocrine (hyperthyroidism), metabolic (electrolyte abnormalities), respiratory (asthma), neurologic (seizure disorder), or cardiac (arrhythmia) conditions
- nutritional deficiencies, especially of B vitamins and folate
- ingestion of substances or medications that may cause anxiety, such as caffeine or amphetamines.
A thorough history, medication review, and physical examination—as well as routine tests such as metabolic panel, complete blood count, thyroid function tests, urine drug screen, and electrocardiography—will capture most of these potential etiologies. In addition to ruling out medical causes, also assess for comorbid psychiatric conditions before reaching a diagnosis.
Evidence-based treatments
The treatment armamentarium for GAD includes psychotherapy and pharmacotherapy; complementary and alternative medicine (CAM) modalities may be useful adjunctive treatments. Which approach to use is determined by clinical judgment and the patient’s symptom severity and preferences. Combination therapy consisting of psychotherapy and medication often is appropriate.
Psychotherapy. Cognitive-behavioral therapy (CBT) is the preferred form of psychotherapy for GAD because it results in sustained improvements for patients with anxiety.5,9 Other modalities that may be effective include psychodynamic psychotherapy, mindfulness-based therapy, and interpersonal psychotherapy.10,11
Pharmacotherapy. As few as one-quarter of patients with GAD receive medications at appropriate dose and duration.12 Antidepressants are a first-line pharmacotherapy.5 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are highly effective for treating GAD.5 Paroxetine, escitalopram, duloxetine, and venlafaxine are FDA-approved for GAD, but other SSRIs also are used as primary treatment.5 Assuming the selected agent is tolerable and efficacious, a treatment course of 12 months is recommended.13
Benzodiazepines promote binding of the neuroinhibitory transmitter γ-aminobutyric acid and enhance chloride ion influx, thus reducing anxiety. Benzodiazepines have been widely used because of their rapid onset of action and effectiveness in managing anxiety, but their role in long-term management of GAD is unclear because these medications increase the risk of addiction, cognitive dulling, memory impairment, psychomotor retardation, and respiratory depression when combined with other CNS depressants such as alcohol and opiates. Before prescribing a benzodiazepine, conduct a thorough risk-benefit analysis and obtain informed consent. Long-term benzodiazepine monotherapy is not recommended.5,14
Hydroxyzine is an alternative to benzodiazepines.15 It works as an antihistamine and is FDA-approved for psychogenic neurosis, a Freudian distinction encompassing anxiety derived from psychological rather than physiological factors.
The azapirone buspirone is a nonaddictive, generally non-sedating 5-HT1A agonist. Although anecdotally some psychiatrists may report limited clinical utility, many analyses found azapirones, including buspirone, were effective for GAD,16,17 particularly for patients with comorbid depression.18
Tricyclic antidepressants are not a first-line choice because of their side effect profile and potential for drug-drug interactions. Nonetheless, some research suggests imipramine may be a reasonable option for GAD.14,19
Although not FDA-approved for GAD, anticonvulsant and antipsychotic medications may be reasonable adjunctive agents for patients with refractory GAD. Studies have suggested gabapentin20 and quetiapine21 as options.
Investigational treatments. Glutaminergic transmission is being investigated as a target for pharmacotherapy for GAD. In an 8-week, open-label trial, 12 of 15 GAD patients responded to the antiglutamatergic agent riluzole, 100 mg/d, and 8 patients achieved remission.22 In another study, pregabalin, which promotes calcium channel blockade, significantly reduced patients’ scores on the Hamilton Anxiety Rating Scale.23 However, this medication is a schedule V controlled substance and little is known about its long-term effects. Researchers had proposed that inhibition of corticotropin-releasing factor (CRF) may help reduce anxiety, but in a double-blind, placebo-controlled trial, they found that the CRF antagonist pexacerfont was no more effective than placebo.24
CAM treatments. A meta-analysis found that compared with placebo, kava extract (Piper methysticum) effectively reduced anxiety symptoms.25 However, considering its risk for hepatotoxicity, kava is not a recommended treatment.26 Although valerian, St. John’s wort, and passionflower have been used to manage GAD, there is insufficient evidence of their effectiveness and safety.26 No strong evidence supports nutritional supplements such as ginger, amino acids, and omega-3 fatty acids (fish oils) as treatment for GAD. Although there’s limited research on resistance training, aromatherapy, yoga, meditation, or acupuncture for treating anxiety, consider these treatments if your patient finds them helpful, because generally they are not contraindicated.
CASE CONTINUED: An antidepressant and CBT
Mrs. M reluctantly agrees to a trial of sertraline, 50 mg/d. She refuses a prescription for clonazepam because she is afraid of drug dependence but accepts a referral for CBT. Two days later, she calls the clinic and says she is more anxious and wants to stop the sertraline. The psychiatrist reassures her and reduces the dosage to 25 mg/d.
Mrs. M’s spike of anxiety resolves by her 2-week follow-up appointment and sertraline is titrated to 200 mg/d. Her irritability, anxiety, and mood improve within 2 months. The worry does not completely resolve, but she is much improved at 6 months, and the focus of her therapy shifts to her marriage.
Related Resources
- Goldberg D, Kendler KS, Sirvatka PJ, et al, eds. Diagnostic issues in depression and generalized anxiety disorder—refining the research agenda for DSM-V. Arlington, VA: American Psychiatric Publishing; 2010.
- Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
Drug Brand Names
- Buspirone • Buspar
- Clonazepam •Klonopin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin• Neurontin
- Hydroxyzine • Vistaril, Atarax
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Barry reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mrs. M, age 44, is a married mother of 2 who presents to the psychiatric clinic with increased anxiety that recently has become intolerable, stating “I can’t stop my head.” She has experienced anxiety for “as long as I can remember.” She was a shy, anxious child who worried about her parents’ health. Her anxiety worsened at college, where she first sought care. She was prescribed diazepam as needed. The next semester, she had a depressive episode, treated with imipramine, 75 mg/d, which she tolerated poorly.
Mrs. M has received episodic supportive therapy since college. She has been plagued by bouts of anxiety and worry, with insomnia, tension, and fatigue. She worries about financial, career, family, and safety issues and has a phobia of spiders. Her family and friends often comment about her excessive worry, and it has strained her marriage and career; she was passed over for a promotion in part because of her anxiousness. Mrs. M also has experienced several depressive episodes.
Mrs. M has sought medical care for various non-specific somatic complaints; all laboratory tests were normal. Approximately 10 years ago, Mrs. M’s primary care physician prescribed fluoxetine, 20 mg/d, but Mrs. M stopped taking it after a few days, stating she felt “more anxious and jittery.”
To meet DSM-IV-TR diagnostic criteria for generalized anxiety disorder (GAD), patients must experience anxiety and worry that they find difficult to control. The worry and anxiety occur more days than not for at least 6 months and cause clinically significant distress and impairment (Table 1).1 These diagnostic criteria are being reevaluated—the DSM-5 Anxiety Work Group has proposed renaming the condition generalized worry disorder, specifying that only 2 domains need to be impacted by worry, shortening the required time frame of impairment from 6 months to 3 months, and including at least 1 behavioral change spawned by excessive worry.2 Although provisional, these recommendations suggest DSM-5 will include changes to GAD when it is published in 2013.
Table 1
DSM-IV-TR diagnostic criteria for generalized anxiety disorder
|
| OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder Source: Reference 1 |
A common, chronic condition
In the United States, the lifetime prevalence of GAD is 5.7%.3 It is twice as common in women. Although GAD can occur at any age, 75% of patients develop it before age 47; the median age is 31.3,4 Patients who present with GAD later in life have a better prognosis.3,4
Approximately 90% of GAD patients will meet criteria for another axis I disorder.3 When GAD patients present for treatment, social phobia and panic disorder are the most common comorbid psychiatric disorders. The lifetime prevalence of a mood disorder among GAD patients is 62%, but as few as 6% of GAD patients will meet criteria for a mood disorder at presentation.3 The onset of GAD usually precedes depression.5,6
Patients with GAD often first seek treatment from their primary care provider.7 A useful screening tool is the GAD-7 (Table 2).8 This instrument has a specificity of 92% and sensitivity of 76% for GAD for patients who score ≥8.7 Although the GAD-7 cannot confirm a GAD diagnosis, it can prompt clinicians to conduct a more structured interview. Because higher scores correlate with more severe symptoms, the GAD-7 can be used to measure progress.
Table 2
Screening for generalized anxiety disorder: The GAD-7
| Over the last 2 weeks, how often have you been bothered by any of the following problems? | Not at all | Several days | More than half the days | Nearly every day | |
|---|---|---|---|---|---|
| 1. | Feeling nervous, anxious, or on edge | 0 | 1 | 2 | 3 |
| 2. | Not being able to stop or control worrying | 0 | 1 | 2 | 3 |
| 3. | Worrying too much about different things | 0 | 1 | 2 | 3 |
| 4. | Trouble relaxing | 0 | 1 | 2 | 3 |
| 5. | Being so restless that it is hard to sit still | 0 | 1 | 2 | 3 |
| 6. | Becoming easily annoyed or irritable | 0 | 1 | 2 | 3 |
| 7. | Feeling afraid as if something awful might happen | 0 | 1 | 2 | 3 |
| GAD: generalized anxiety disorder Source: Reference 8 | |||||
Differential diagnosis
GAD typically has a chronic course with fluctuating symptom severity over the patient’s lifespan. Assess patients who present with anxiety for medical conditions that mimic GAD. These include:
- endocrine (hyperthyroidism), metabolic (electrolyte abnormalities), respiratory (asthma), neurologic (seizure disorder), or cardiac (arrhythmia) conditions
- nutritional deficiencies, especially of B vitamins and folate
- ingestion of substances or medications that may cause anxiety, such as caffeine or amphetamines.
A thorough history, medication review, and physical examination—as well as routine tests such as metabolic panel, complete blood count, thyroid function tests, urine drug screen, and electrocardiography—will capture most of these potential etiologies. In addition to ruling out medical causes, also assess for comorbid psychiatric conditions before reaching a diagnosis.
Evidence-based treatments
The treatment armamentarium for GAD includes psychotherapy and pharmacotherapy; complementary and alternative medicine (CAM) modalities may be useful adjunctive treatments. Which approach to use is determined by clinical judgment and the patient’s symptom severity and preferences. Combination therapy consisting of psychotherapy and medication often is appropriate.
Psychotherapy. Cognitive-behavioral therapy (CBT) is the preferred form of psychotherapy for GAD because it results in sustained improvements for patients with anxiety.5,9 Other modalities that may be effective include psychodynamic psychotherapy, mindfulness-based therapy, and interpersonal psychotherapy.10,11
Pharmacotherapy. As few as one-quarter of patients with GAD receive medications at appropriate dose and duration.12 Antidepressants are a first-line pharmacotherapy.5 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are highly effective for treating GAD.5 Paroxetine, escitalopram, duloxetine, and venlafaxine are FDA-approved for GAD, but other SSRIs also are used as primary treatment.5 Assuming the selected agent is tolerable and efficacious, a treatment course of 12 months is recommended.13
Benzodiazepines promote binding of the neuroinhibitory transmitter γ-aminobutyric acid and enhance chloride ion influx, thus reducing anxiety. Benzodiazepines have been widely used because of their rapid onset of action and effectiveness in managing anxiety, but their role in long-term management of GAD is unclear because these medications increase the risk of addiction, cognitive dulling, memory impairment, psychomotor retardation, and respiratory depression when combined with other CNS depressants such as alcohol and opiates. Before prescribing a benzodiazepine, conduct a thorough risk-benefit analysis and obtain informed consent. Long-term benzodiazepine monotherapy is not recommended.5,14
Hydroxyzine is an alternative to benzodiazepines.15 It works as an antihistamine and is FDA-approved for psychogenic neurosis, a Freudian distinction encompassing anxiety derived from psychological rather than physiological factors.
The azapirone buspirone is a nonaddictive, generally non-sedating 5-HT1A agonist. Although anecdotally some psychiatrists may report limited clinical utility, many analyses found azapirones, including buspirone, were effective for GAD,16,17 particularly for patients with comorbid depression.18
Tricyclic antidepressants are not a first-line choice because of their side effect profile and potential for drug-drug interactions. Nonetheless, some research suggests imipramine may be a reasonable option for GAD.14,19
Although not FDA-approved for GAD, anticonvulsant and antipsychotic medications may be reasonable adjunctive agents for patients with refractory GAD. Studies have suggested gabapentin20 and quetiapine21 as options.
Investigational treatments. Glutaminergic transmission is being investigated as a target for pharmacotherapy for GAD. In an 8-week, open-label trial, 12 of 15 GAD patients responded to the antiglutamatergic agent riluzole, 100 mg/d, and 8 patients achieved remission.22 In another study, pregabalin, which promotes calcium channel blockade, significantly reduced patients’ scores on the Hamilton Anxiety Rating Scale.23 However, this medication is a schedule V controlled substance and little is known about its long-term effects. Researchers had proposed that inhibition of corticotropin-releasing factor (CRF) may help reduce anxiety, but in a double-blind, placebo-controlled trial, they found that the CRF antagonist pexacerfont was no more effective than placebo.24
CAM treatments. A meta-analysis found that compared with placebo, kava extract (Piper methysticum) effectively reduced anxiety symptoms.25 However, considering its risk for hepatotoxicity, kava is not a recommended treatment.26 Although valerian, St. John’s wort, and passionflower have been used to manage GAD, there is insufficient evidence of their effectiveness and safety.26 No strong evidence supports nutritional supplements such as ginger, amino acids, and omega-3 fatty acids (fish oils) as treatment for GAD. Although there’s limited research on resistance training, aromatherapy, yoga, meditation, or acupuncture for treating anxiety, consider these treatments if your patient finds them helpful, because generally they are not contraindicated.
CASE CONTINUED: An antidepressant and CBT
Mrs. M reluctantly agrees to a trial of sertraline, 50 mg/d. She refuses a prescription for clonazepam because she is afraid of drug dependence but accepts a referral for CBT. Two days later, she calls the clinic and says she is more anxious and wants to stop the sertraline. The psychiatrist reassures her and reduces the dosage to 25 mg/d.
Mrs. M’s spike of anxiety resolves by her 2-week follow-up appointment and sertraline is titrated to 200 mg/d. Her irritability, anxiety, and mood improve within 2 months. The worry does not completely resolve, but she is much improved at 6 months, and the focus of her therapy shifts to her marriage.
Related Resources
- Goldberg D, Kendler KS, Sirvatka PJ, et al, eds. Diagnostic issues in depression and generalized anxiety disorder—refining the research agenda for DSM-V. Arlington, VA: American Psychiatric Publishing; 2010.
- Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
Drug Brand Names
- Buspirone • Buspar
- Clonazepam •Klonopin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Gabapentin• Neurontin
- Hydroxyzine • Vistaril, Atarax
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pregabalin • Lyrica
- Quetiapine • Seroquel
- Riluzole • Rilutek
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Barry reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Andrews G, Hobbs MJ, Borkovec TD, et al. Generalized worry disorder: a review of DSM-IV generalized anxiety disorder and options for DSM-V. Depress Anxiety. 2010;27(2):134-147.
3. Turk CL, Mennin DS. Phenomenology of generalized anxiety disorder. Psychiatr Ann. 2011;41(2):72-78.
4. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
5. Ballenger JC, Davidson JR, Lecrubier Y, et al. Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2001;6(suppl 11):53-58.
6. Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):19-39.
7. Kavan MG, Elsasser G, Barone EJ. Generalized anxiety disorder: practical assessment and management. Am Fam Physician. 2009;79(9):785-791.
8. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092-1097.
9. Newman MG, Borkovec TD. Cognitive-behavioral treatment of generalized anxiety disorder. Clin Psychol. 1995;48(4):5-7.
10. Leichsenring F, Salzer S, Jaeger U, et al. Short-term psychodynamic psychotherapy and cognitive-behavioral therapy in generalized anxiety disorder: a randomized, controlled trial. Am J Psychiatry. 2009;166(8):875-881.
11. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
12. Stein MB, Sherbourne CD, Craske MG, et al. Quality of care for primary care patients with anxiety disorders. Am J Psychiatry. 2004;161(12):2230-2237.
13. Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
14. Rickels K, Downing R, Schweizer E, et al. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50(11):884-895.
15. Guaiana G, Barbui C, Cipriani A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst Rev. 2010;(12):CD006815.-
16. Gammans RE, Stringfellow JC, Hvizdos AJ, et al. Use of buspirone in patients with generalized anxiety disorder and coexisting depressive symptoms. A meta-analysis of eight randomized, controlled studies. Neuropsychobiology. 1992;25(4):193-201.
17. Chessick CA, Allen MH, Thase M, et al. Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev. 2006;(3):CD006115.-
18. Sramek JJ, Tansman M, Suri A, et al. Efficacy of buspirone in generalized anxiety disorder with coexisting mild depressive symptoms. J Clin Psychiatry. 1996;57(7):287-291.
19. Rickels K, DeMartinis N, García-España F, et al. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am J Psychiatry. 2000;157(12):1973-1979.
20. Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155(7):992-993.
21. Khan A, Joyce M, Atkinson S, et al. A randomized, double-blind study of once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2011;31(4):418-428.
22. Mathew SJ, Amiel JM, Coplan JD, et al. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162(12):2379-2381.
23. Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160(3):533-540.
24. Coric V, Feldman HH, Oren DA, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27(5):417-425.
25. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Database Syst Rev. 2003;(1):CD003383.-
26. Zoberi K, Pollard CA. Treating anxiety without SSRIs. J Fam Pract. 2010;59(3):148-154.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Andrews G, Hobbs MJ, Borkovec TD, et al. Generalized worry disorder: a review of DSM-IV generalized anxiety disorder and options for DSM-V. Depress Anxiety. 2010;27(2):134-147.
3. Turk CL, Mennin DS. Phenomenology of generalized anxiety disorder. Psychiatr Ann. 2011;41(2):72-78.
4. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
5. Ballenger JC, Davidson JR, Lecrubier Y, et al. Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry. 2001;6(suppl 11):53-58.
6. Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):19-39.
7. Kavan MG, Elsasser G, Barone EJ. Generalized anxiety disorder: practical assessment and management. Am Fam Physician. 2009;79(9):785-791.
8. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092-1097.
9. Newman MG, Borkovec TD. Cognitive-behavioral treatment of generalized anxiety disorder. Clin Psychol. 1995;48(4):5-7.
10. Leichsenring F, Salzer S, Jaeger U, et al. Short-term psychodynamic psychotherapy and cognitive-behavioral therapy in generalized anxiety disorder: a randomized, controlled trial. Am J Psychiatry. 2009;166(8):875-881.
11. Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22(4):716-721.
12. Stein MB, Sherbourne CD, Craske MG, et al. Quality of care for primary care patients with anxiety disorders. Am J Psychiatry. 2004;161(12):2230-2237.
13. Fricchione G. Generalized anxiety disorder. N Engl J Med. 2004;351(7):675-682.
14. Rickels K, Downing R, Schweizer E, et al. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry. 1993;50(11):884-895.
15. Guaiana G, Barbui C, Cipriani A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst Rev. 2010;(12):CD006815.-
16. Gammans RE, Stringfellow JC, Hvizdos AJ, et al. Use of buspirone in patients with generalized anxiety disorder and coexisting depressive symptoms. A meta-analysis of eight randomized, controlled studies. Neuropsychobiology. 1992;25(4):193-201.
17. Chessick CA, Allen MH, Thase M, et al. Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev. 2006;(3):CD006115.-
18. Sramek JJ, Tansman M, Suri A, et al. Efficacy of buspirone in generalized anxiety disorder with coexisting mild depressive symptoms. J Clin Psychiatry. 1996;57(7):287-291.
19. Rickels K, DeMartinis N, García-España F, et al. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am J Psychiatry. 2000;157(12):1973-1979.
20. Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155(7):992-993.
21. Khan A, Joyce M, Atkinson S, et al. A randomized, double-blind study of once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2011;31(4):418-428.
22. Mathew SJ, Amiel JM, Coplan JD, et al. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162(12):2379-2381.
23. Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160(3):533-540.
24. Coric V, Feldman HH, Oren DA, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27(5):417-425.
25. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Database Syst Rev. 2003;(1):CD003383.-
26. Zoberi K, Pollard CA. Treating anxiety without SSRIs. J Fam Pract. 2010;59(3):148-154.
Introduction
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
Introduction
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
The Evolution of Insulin Therapy in Diabetes Mellitus
The clinical milieu of type 2 diabetes mellitus (T2DM) is undoubtedly one of the most challenging faced by family physicians. The association of T2DM with other chronic diseases, such as hypertension, dyslipidemia, cardiovascular disease, and obesity, speaks to the complex issues that must be addressed. Considering the complexity of these issues, it is important to recognize that, as a chronic disease, T2DM is largely self-managed and patients mostly control their own DM-related health outcomes. To assist patients with T2DM to successfully take on this responsibility, family physicians should raise and discuss the treatment options available to achieve agreed upon goals, and, in consultation with the patient, recommend treatment options that best address the patient’s clinical issues and meet the patient’s needs. These steps are important to help motivate the patient and promote long-term treatment adherence. Among the treatment options available for T2DM, the challenges of self-management are perhaps greatest with insulin.
Insulin is the most physiologic and effective glucose-lowering agent available, and is recommended as glucose-lowering therapy over the spectrum of T2DM.1,2 Yet studies show that the initiation of insulin treatment is often delayed, sometimes for years, following loss of glycemic control with oral glucose-lowering agents.3,4 Once initiated, adherence to insulin tends to be moderate at best.5,6 It is crucial that family physicians address the issues that contribute to low levels of acceptance and adherence to insulin treatment. In addition, physicians need a firm understanding of how to initiate, modify, and intensify insulin therapy. The primary goal of this supplement is to provide the family physician with a detailed understanding of the current recommendations for, and advances in, insulin treatment.
This supplement includes three articles; the first of which is a historical review of the discovery of insulin. Also included in that article, by Michael Heile, MD, and Doron Schneider, MD, FACP, is a review of the evolution of insulin, including a comparison of the clinical pharmacology of human and analog insulins. The second article begins with a discussion of the conceptual strategies to address patient barriers that have a dramatic impact on the acceptance of, and self-management with, insulin. Building on that foundation, Luigi Meneghini, MD, MBA, and Timothy Reid, MD, present 4 case studies that detail how to assist patients in the implementation of these strategies when initiating or intensifying insulin therapy. The case studies also provide practical considerations with respect to dosing basal, basal-bolus, and premixed insulin. The third article examines advances in insulin, with a focus on the investigational agent, ultra–long-acting insulin degludec. Allen King, MD, provides a solid foundation of the clinical pharmacology of insulin degludec and the clinical experience to date regarding the use of insulin degludec in patients with type 1 DM or T2DM.
It is hoped that the information in this supplement will prove helpful for the practicing family physician in managing patients with this increasingly common disease and its associated clinical dilemmas.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association, European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
3. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418-e424.
4. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600-606.
5. Bonafede MM, Kalsekar A, Pawaskar M, et al. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-156.
6. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
Creating a community-based, patient-centered cancer survivorship program
A report from the Institute of Medicine in 20051 marked a watershed in the thinking about cancer patients and their long-term needs as survivors when it argued for oncology programs in the United States to address the unmet needs of cancer survivors at the community practice level. Cancer care in the United States has tended to focus on the more immediate aspects of the active treatment, limiting our long-term concerns for the patient’s well-being. Indeed, as a community oncologist in the early part of my career,2 decades ago, I considered cancer patients “finished” with their cancer and back to “normal” soon after their last acute toxicity ended.2 These days, our profession as well as patients and society as a whole have come to understand that the needs of cancer patients continue into well into survivorship, which can span years or even decades. These concerns should be addressed and accommodated through comprehensive, community-based survivorship programs.
*For a PDF of the full article, click on the link to the left of this introduction.
A report from the Institute of Medicine in 20051 marked a watershed in the thinking about cancer patients and their long-term needs as survivors when it argued for oncology programs in the United States to address the unmet needs of cancer survivors at the community practice level. Cancer care in the United States has tended to focus on the more immediate aspects of the active treatment, limiting our long-term concerns for the patient’s well-being. Indeed, as a community oncologist in the early part of my career,2 decades ago, I considered cancer patients “finished” with their cancer and back to “normal” soon after their last acute toxicity ended.2 These days, our profession as well as patients and society as a whole have come to understand that the needs of cancer patients continue into well into survivorship, which can span years or even decades. These concerns should be addressed and accommodated through comprehensive, community-based survivorship programs.
*For a PDF of the full article, click on the link to the left of this introduction.
A report from the Institute of Medicine in 20051 marked a watershed in the thinking about cancer patients and their long-term needs as survivors when it argued for oncology programs in the United States to address the unmet needs of cancer survivors at the community practice level. Cancer care in the United States has tended to focus on the more immediate aspects of the active treatment, limiting our long-term concerns for the patient’s well-being. Indeed, as a community oncologist in the early part of my career,2 decades ago, I considered cancer patients “finished” with their cancer and back to “normal” soon after their last acute toxicity ended.2 These days, our profession as well as patients and society as a whole have come to understand that the needs of cancer patients continue into well into survivorship, which can span years or even decades. These concerns should be addressed and accommodated through comprehensive, community-based survivorship programs.
*For a PDF of the full article, click on the link to the left of this introduction.