User login
Hyponatremia in Cirrhosis
Cirrhosis is one of the main causes of hypervolemic hyponatremia, a dilutional form of hyponatremia that occurs when there is an increase in total body water but a relatively smaller increase in total serum sodium. Portal hypertension is the main precipitating factor in fluid retention that leads to the development of cirrhotic hyponatremia. In cirrhosis, portal hypertension is determined by 2 main factors: increased intrahepatic resistance and increased spanchnic blood flow. The increased intrahepatic resistance is due to both structural (fibrosis, conversion of low resistance fenestrated sinusoids into capillaries) and dynamic (vasoconstriction due to endothelial cell dysfunction) changes.1
The hepatic circulation normally is able to accommodate an increase in portal blood flow associated with postprandial hyperemia. The elevated intrahepatic resistance in cirrhosis results in an inability to accommodate the normal increase in portal blood flow that occurs in the postprandial hyperemia state.3 As a result, portal pressure increases during postprandial hyperemia, leading to reflex vasoconstriction, which creates a shear stress and increases splanchnic nitric oxide (NO) production.4 NO, one of the most important vasodilators in the splanchnic circulation, increases splanchnic blood flow and portal pressures. When this happens repeatedly, it leads to a progressive dilation of preexisting portosystemic vascular channels and the development of varices.5 At the same time, levels of vascular endothelial growth factor rise; this is a very important mediator for angiogenesis because it increases NO, further increasing splanchnic vasodilation.6
Progressive splanchnic vasodilation and increased blood flow into the splanchnic circulation leads to central hypovolemia, arterial underfilling, and decreased blood flow in renal afferent arterioles. Vasoconstrictor norepinephrine and antinatriuretic mechanisms are subsequently activated in an attempt to normalize renal perfusion pressures. Baroreceptor‐mediated nonosmotic release of arginine vasopressin (AVP) is triggered and renin angiotensin‐aldosterone system activity is increased, which increases sodium reabsorption and activates the stellate cells, causing fibrosis, vasoconstriction, and increased portal pressures.6, 7
AVP acts at vasopression‐1A (V1A) receptors to increase arterial vasoconstriction, and at V2 receptors in renal tubule cells for solute‐free water retention.1 The increased sodium and water reabsorption leads to fluid retention, increased central blood volume, venous return to the heart, and an increase in cardiac output to maintain arterial perfusion and create the hyperdynamic circulation that is characteristic of cirrhosis with advanced portal hypertension. Dilutional hyponatremia develops when free water retention is more pronounced than that of sodium retention.
CLINICAL FACTORS ASSOCIATED WITH CIRRHOTIC HYPONATREMIA
Diuretics lead to hyponatremia through several mechanisms.8 First, they induce a contraction of the central blood volume, leading to the nonosmotic release of AVP. In advanced cirrhosis, there is activation of the renin‐angiotensin system in addition to the nonosmotic release of AVP, leading to sodium and free water reabsorption. Diuretics block the sodium reabsorption. However, the water‐retaining effects persist, further contributing to dilutional hyponatremia.8 This cycle is made worse by low sodium intake and frequent thirst experienced by these patients.8 Other medications (eg, non‐steroidal anti‐inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors) commonly prescribed for cirrhotic patients may also contribute to the development or worsening of dilutional hyponatremia.8
Increased intrathoracic pressure in patients with tense ascites can also contribute to dilutional hyponatremia by increasing baroreceptor‐mediated release of AVP.9 Large volume paracentesis without the oncotic influence of albumin, an intervention commonly required in patients with cirrhosis and recurrent ascites, may also lead to significant increases in plasma renin activity and plasma aldosterone, which further worsen these pathophysiologic mechanisms, resulting in reduced serum sodium concentration.10 Following removal of excess peritoneal fluid, blood flow to the kidneys is initially improved, but ascitic fluid reaccumulates and the patient becomes intravascularly depleted.10
Infection is an important clinical mediator for the development of both portal hypertension as well as hyponatremia. Bacterial translocation leads to endotoxemia and increased tumor necrosis factor (TNF)‐alpha, resulting in increased splanchnic NO and splanchnic arterial vasodilatation. This process reduces cardiac output, which leads to increased AVP secretion.11, 12 Endotoxin‐mediated splanchnic vasodilatation, especially with spontaneous bacterial peritonitis (SBP), can adversely affect central blood volume status, especially in the presence of severe ascites.1 Clinicians providing care for patients with cirrhosis should be aware of these factors and closely monitor at‐risk patients for the onset or worsening of hyponatremia.1
PROGNOSTIC SIGNIFICANCE OF HYPONATREMIA IN CIRRHOSIS
Hyponatremia has several important clinical implications for patients with cirrhosis.13 Hyponatremia is associated with refractory ascites, greater fluid accumulation, the need for paracentesis, and, importantly, impaired renal function. In patients with ascites and cirrhosis, approximately 50% have some degree of hyponatremia.2 Moreover, the severity of hyponatremia associated with advanced cirrhosis correlates with the degree of cirrhosis complications, especially hyponatremia associated with hepatorenal syndrome, encephalopathy, and SBP (Table 1).2
| Serum [Na+] mEq/L | |||
|---|---|---|---|
| 130 | 131‐135 | >135 | |
| |||
| Hepatorenal syndrome | 3.45 | 1.75 | 1 (reference value) |
| Hepatic encephalopathy | 3.40 | 1.69 | 1 (reference value) |
| Gastrointestinal bleeding | 1.48 | 0.93 | 1 (reference value) |
| Spontaneous bacterial peritonitis | 2.36 | 1.44 | 1 (reference value) |
Similarly, hyponatremia is strongly associated with increasing Child‐Pugh and Model for End‐Stage Liver Disease (MELD) scores.14 In an analysis of data among candidates for liver transplantation from the Organ Procurement and Transplantation Network, the combination of MELD score and serum sodium concentration was a better predictor of death than the MELD score alone.14 In addition, the effect of hyponatremia on clinical outcomes was greater in patients with a low MELD score than those with a relatively high MELD score.. These results suggest that combining serum sodium concentrations with MELD scores to assign transplantation priority might reduce mortality among patients on the waiting list.14
Hyponatremia is also a marker for the development of overt hepatic encephalopathy in patients with cirrhosis.13 One of the proposed mechanisms for encephalopathy is low‐grade cerebral edema. This leads to the conversion of glutamate to glutamine by ammonia, which accumulates within astrocytes, causing astrocyte swelling and dysfunction. Because hyponatremia complicates the management of fluid overload, it increases the risk of developing or exacerbating hepatic encephalopathy.13
Hyponatremia is intimately involved with the development of renal failure in the patient with cirrhosis. It is an earlier and more sensitive marker of renal impairment and/or circulatory dysfunction than serum creatinine.15 It is often the precursor to the development of hepatorenal syndrome.16, 17
Hyponatremia is more common in hospitalized versus ambulatory patients with cirrhosis.1 In a study of 126 patients with cirrhosis admitted to an intensive care unit, patients with serum [Na+] 135 mEq/L had a greater frequency of ascites, illness severity scores, hepatic encephalopathy, sepsis, renal failure, and in‐hospital mortality than normonatremic patients (73.1% vs 55.9%).18 Persistent ascites and low serum sodium identified cirrhotic patients with a high mortality risk, despite low MELD scores, in a study of 507 veterans in the United States with cirrhosis.19 In a retrospective review of 127 patients, hyponatremia was predictive of the development of acute renal failure during hospitalization; among patients with hyponatremia who developed renal failure in the hospital, 72% died.20
Clinical assessment of a patient with cirrhosis who has hyponatremia can be difficult.1 These patients have too much salt and water in the wrong spaces (ie, in the peritoneal cavity and peripheral tissue). As a result, it is possible to have fluid overload with intravascular depletion. A further complication is that dilutional hyponatremia is associated with hepatorenal syndrome. Because these patients have elevated blood urea nitrogen (BUN) and creatinine, and decreased urine output and urine sodium concentration, they appear to be indistinguishable from a patient with prerenal azotemia prior to volume expansion.1 Many of these factors and concerns are illustrated in the following case we handled several years ago.
A 70‐YEAR‐OLD WOMAN WITH CIRRHOSIS
K.R. is a 70‐year‐old white woman recently discharged from the hospital following treatment of recurrent cellulitis. Her past medical history is positive for cirrhosis secondary to active alcohol use, chronic autoimmune hepatitis, and iron overload. Her hospital course was notable for tense ascites, asterixis, and a serum [Na+] of 126 mEq/L at admission. K.R. was managed with large volume paracentesis with 25% salt‐poor albumin, elevation of her lower extremities, discontinuation of diuretics, and 1 L fluid restriction. Her serum [Na+] increased to 128 mEq/L. Although her cellulitis and edema both improved, both persisted. In addition, her mental status also improved, but asterixis persisted. At this point in the hospitalization, effective management of the cellulitis was hindered by the persistent edema, and its treatment with diuretics was limited by the hyponatremia and hepatic encephalopathy.
Today, we have better treatment options for managing this patient. To effectively correct the hyponatremia and facilitate treatment of the other complications of cirrhosis, we can now initiate therapy with one of the vaptans currently available.
TREATMENT OF MILD ASYMPTOMATIC HYPERVOLEMIC HYPONATREMIA
The initial approach to treatment of patients with mild asymptomatic, hypervolemic hyponatremia consists of fluid restriction and a sodium‐restricted diet.1 Fluid restriction, however, has limited efficacy and is often not well tolerated by patients. For patients with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion. If patients have severe dilutional hyponatremia and tense ascites, therapeutic paracentesis with plasma expanders is safe.1
The pharmacologic approach to treating hyponatremia has advanced with the discovery of vaptans, drugs that inhibit V2 receptors in cells of the collecting ducts.21 In contrast to conventional diuretics, vaptans do not increase natriuresis. Administration of a vaptan agent for 1 to 2 weeks has been shown to significantly improve low serum sodium levels in patients with hyponatremia, and promote aquaresis without significantly altering renal or circulatory function or activity of the renin‐angiotensin‐aldosterone system. The most frequent side effect of vaptan therapy is thirst.21
Two vaptan agents are currently approved for use in the United States: conivaptan and tolvaptan. Conivaptan is administered intravenously, and is a nonselective vasopressin inhibitor, blocking both V1A and V2 receptors. The course of therapy for conivaptan is 4 days. Tolvaptan, on the other hand, selectively blocks V2 receptors, and is a once‐daily oral vaptan that can be given long‐term.21
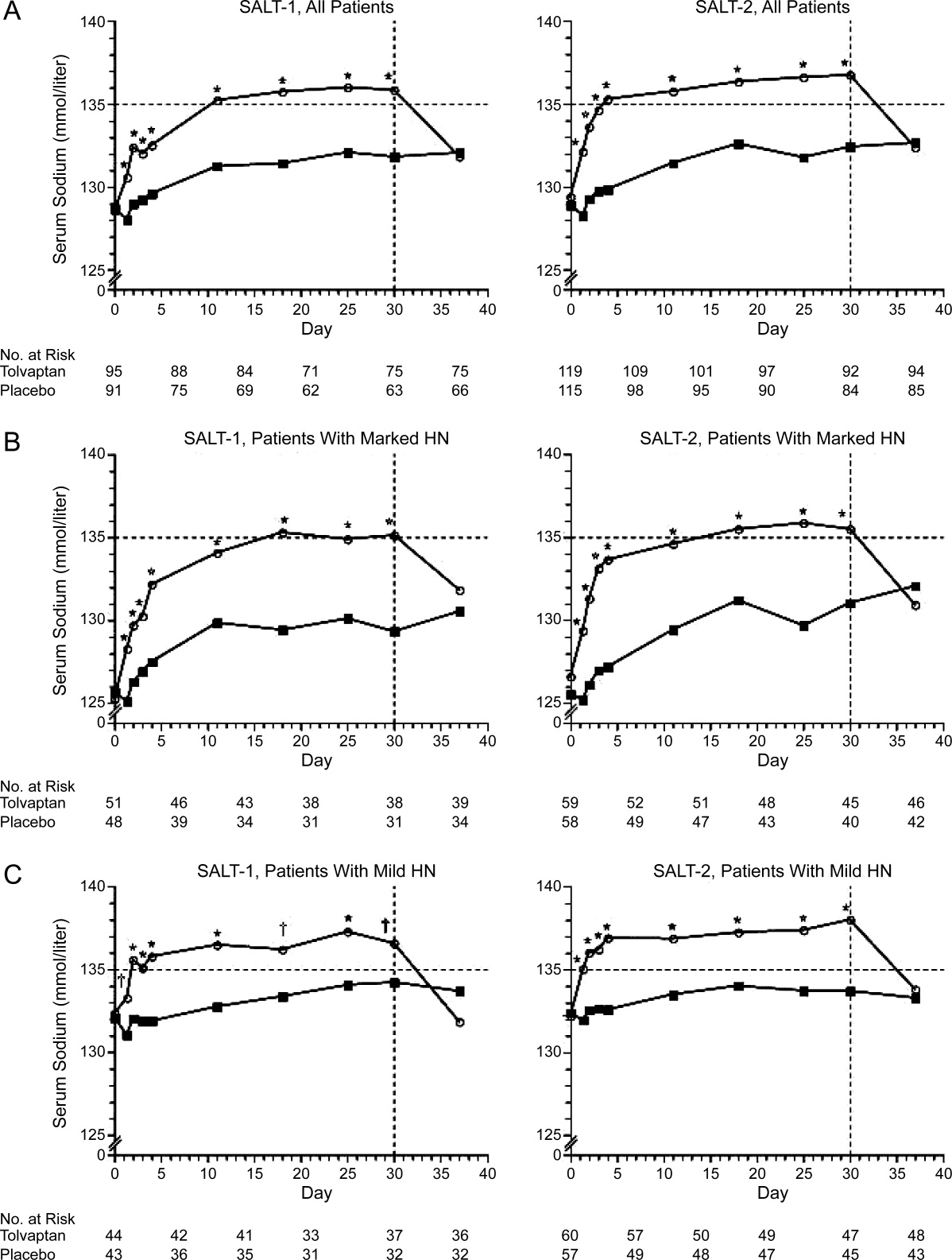
The efficacy of tolvaptan was evaluated in the Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).22 In these multicenter, prospective, randomized, placebo‐controlled trials, patients with dilutional hyponatremia (serum [Na+] 135 mEq/L) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), heart failure, or syndrome of inappropriate antidiuretic hormone (ADH) hypersecretion, and who were hospitalized and clinically stable, received tolvaptan 15 mg daily or placebo. Repeat serum sodium levels were obtained at 8 hours, 2, 3, and 4 days, and then weekly at days 11, 18, 25, and 30. The study drug was discontinued on day 30, with follow‐up serum sodium levels taken 7 days later. (In patients with persistent hyponatremia, the tolvaptan dose was adjusted to 30 mg and then 60 mg with the goal of achieving a serum [Na+] 135 mEq/L.) Increases in serum sodium concentration were seen as early as 8 hours after the first administration of tolvaptan and persisted throughout the study period. After tolvaptan was discontinued, serum sodium levels decreased to baseline within 1 week.22 Tolvaptan was well tolerated, with the most common side effects being increased thirst, dry mouth, and increased urination.22
Longer‐term administration of tolvaptan was shown to maintain a higher serum sodium concentration with an acceptable safety profile in SALTWATER, the open‐label extension of the SALT‐1 and SALT‐2 trials.23 The study included 111 patients with hyponatremia who received oral tolvaptan for a mean follow‐up of 701 days. The most common adverse effects potentially related to tolvaptan were thirst, dry mouth, polydipsia, and polyuria.22, 23 Overall, there were 9 possible and 1 probable serious adverse events, which represents an acceptable safety profile over 77,369 patient‐days of exposure. Over time, 64 patients discontinued tolvaptan, 30 due to adverse reactions or death.22 The results of SALTWATER indicated that most patients received benefit from treatment with tolvaptan, with a decreased need for fluid restriction.23
PATIENT CHARACTERISTICS FOR TOLVAPTAN
In the SALT trials, tolvaptan was administered to clinically stable patients. Based on recommendations by the US Food and Drug Administration (FDA), tolvaptan should be initiated or reinitiated in a hospital setting.1 Patients with severe neurologic symptoms due to hyponatremia should be treated with normal saline instead of tolvaptan; combination therapy with tolvaptan and normal saline should be avoided due to the potential for a too‐rapid correction of hyponatremia and the potential for central pontine myelinolysis. Saline should be discontinued and persistent hyponatremia confirmed before beginning tolvaptan therapy.1
Several additional factors should be considered before patients begin tolvaptan. First, tolvaptan increases thirst, as well as the frequency and volume of urination. Therefore, patients must be able to respond appropriately to thirst with increased water intake. Patients should not be fluid‐restricted during the first day of tolvaptan therapy; instead, they should be instructed to respond to their thirst with increased water ingestion. Because of these factors, caution should be exercised in administering tolvaptan to a confused, restrained patient. In addition, patients should have adequate toileting aids, such as a bedside urinal or commode.1
As with most new drugs, acquisition costs for tolvaptan should be considered in light of the clinical benefits of treatment outcomes. In a retrospective review, median hospital costs for patients with moderate‐to‐severe ($16,606) and mild‐to‐moderate hyponatremia ($14,266) were higher than matched patients without hyponatremia ($13,066).24 In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial, in which patients with severe congestive heart failure (including those with and without hyponatremia) were randomized to tolvaptan or placebo, the adjusted mean length of hospital stay for those with hyponatremia at baseline who received tolvaptan was 1.72 days shorter than those who received placebo.25 Although tolvaptan is somewhat expensive, the cost compares favorably with the daily cost of hospitalization.
SUMMARY
Portal hypertension plays a pivotal role in the development of hyponatremia in patients with cirrhosis. Reflex vasodilation in the splanchnic circulation compromises the effective central blood volume, triggering compensatory vasoconstrictor and antinatriuretic mechanisms. The net effect is greater free water accumulation than sodium retention, creating dilutional hyponatremia.
The severity of hyponatremia correlates with the severity of cirrhosis complications, such as hepatorenal syndrome, encephalopathy, SBP, and renal failure. The presence of hyponatremia is a marker for poor outcomes and shortened survival, regardless of MELD scores.
In a hospitalized, acutely ill patient with cirrhosis, such as the person in this case, therapy may involve discontinuation of diuretics, evaluation and treatment of infection, volume expansion with salt‐poor albumin, and tolvaptan for treatment of hyponatremia. Regarding tolvaptan, early morning administration is recommended. At initiation of therapy, fluid restriction should be discontinued, and off‐floor testing should be avoided. Concomitant medications should be reviewed to avoid potentially harmful interactions.
- ,.Managing hyponatremia in cirrhosis.J Hosp Med.2010;5(suppl 3):S8–S17.
- ,,,; for the CAPPS Investigators.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- .Molecular mechanisms of increased intrahepatic resistance in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S259–S261.
- ,,.The pathophysiology of portal hypertension.Dig Dis.2005;23:6–10.
- .The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S288–S294.
- ,.Vascular endothelial dysfunction in cirrhosis.J Hepatol.2007;46:927–934.
- ,, et al.Management of cirrhosis and ascites.N Engl J Med.2004;350(16):1646–1654.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52(1):144–153.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat.Gastroenterology.2002;122:85–93.
- ,,, et al.Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in in vivo in cirrhotic rats.Am J Physiol Heart Circ Physiol.2007;293:H1689–H1695.
- ,.Pathogenetic mechanisms of hepatic encephalopathy.Gut.2008;57:1156–1165.
- ,,, et al.Hyponatremia and mortality among patients on the liver‐transplant waiting list.N Engl J Med.2008;359:1018–1026.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44:220–226.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48(3):1002–1010.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al; for the SALTWATER Investigators.Oral tolvaptan is safe and effective in chronic hyponatremia.J Am Soc Nephrol.2010;21:705–712.
- ,,, et al.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Effect of serum sodium concentration and tolvaptan treatment on length of hospitalization in patients with heart failure.Am J Health Syst Pharm.2011;68(4):328–333.
Cirrhosis is one of the main causes of hypervolemic hyponatremia, a dilutional form of hyponatremia that occurs when there is an increase in total body water but a relatively smaller increase in total serum sodium. Portal hypertension is the main precipitating factor in fluid retention that leads to the development of cirrhotic hyponatremia. In cirrhosis, portal hypertension is determined by 2 main factors: increased intrahepatic resistance and increased spanchnic blood flow. The increased intrahepatic resistance is due to both structural (fibrosis, conversion of low resistance fenestrated sinusoids into capillaries) and dynamic (vasoconstriction due to endothelial cell dysfunction) changes.1
The hepatic circulation normally is able to accommodate an increase in portal blood flow associated with postprandial hyperemia. The elevated intrahepatic resistance in cirrhosis results in an inability to accommodate the normal increase in portal blood flow that occurs in the postprandial hyperemia state.3 As a result, portal pressure increases during postprandial hyperemia, leading to reflex vasoconstriction, which creates a shear stress and increases splanchnic nitric oxide (NO) production.4 NO, one of the most important vasodilators in the splanchnic circulation, increases splanchnic blood flow and portal pressures. When this happens repeatedly, it leads to a progressive dilation of preexisting portosystemic vascular channels and the development of varices.5 At the same time, levels of vascular endothelial growth factor rise; this is a very important mediator for angiogenesis because it increases NO, further increasing splanchnic vasodilation.6
Progressive splanchnic vasodilation and increased blood flow into the splanchnic circulation leads to central hypovolemia, arterial underfilling, and decreased blood flow in renal afferent arterioles. Vasoconstrictor norepinephrine and antinatriuretic mechanisms are subsequently activated in an attempt to normalize renal perfusion pressures. Baroreceptor‐mediated nonosmotic release of arginine vasopressin (AVP) is triggered and renin angiotensin‐aldosterone system activity is increased, which increases sodium reabsorption and activates the stellate cells, causing fibrosis, vasoconstriction, and increased portal pressures.6, 7
AVP acts at vasopression‐1A (V1A) receptors to increase arterial vasoconstriction, and at V2 receptors in renal tubule cells for solute‐free water retention.1 The increased sodium and water reabsorption leads to fluid retention, increased central blood volume, venous return to the heart, and an increase in cardiac output to maintain arterial perfusion and create the hyperdynamic circulation that is characteristic of cirrhosis with advanced portal hypertension. Dilutional hyponatremia develops when free water retention is more pronounced than that of sodium retention.
CLINICAL FACTORS ASSOCIATED WITH CIRRHOTIC HYPONATREMIA
Diuretics lead to hyponatremia through several mechanisms.8 First, they induce a contraction of the central blood volume, leading to the nonosmotic release of AVP. In advanced cirrhosis, there is activation of the renin‐angiotensin system in addition to the nonosmotic release of AVP, leading to sodium and free water reabsorption. Diuretics block the sodium reabsorption. However, the water‐retaining effects persist, further contributing to dilutional hyponatremia.8 This cycle is made worse by low sodium intake and frequent thirst experienced by these patients.8 Other medications (eg, non‐steroidal anti‐inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors) commonly prescribed for cirrhotic patients may also contribute to the development or worsening of dilutional hyponatremia.8
Increased intrathoracic pressure in patients with tense ascites can also contribute to dilutional hyponatremia by increasing baroreceptor‐mediated release of AVP.9 Large volume paracentesis without the oncotic influence of albumin, an intervention commonly required in patients with cirrhosis and recurrent ascites, may also lead to significant increases in plasma renin activity and plasma aldosterone, which further worsen these pathophysiologic mechanisms, resulting in reduced serum sodium concentration.10 Following removal of excess peritoneal fluid, blood flow to the kidneys is initially improved, but ascitic fluid reaccumulates and the patient becomes intravascularly depleted.10
Infection is an important clinical mediator for the development of both portal hypertension as well as hyponatremia. Bacterial translocation leads to endotoxemia and increased tumor necrosis factor (TNF)‐alpha, resulting in increased splanchnic NO and splanchnic arterial vasodilatation. This process reduces cardiac output, which leads to increased AVP secretion.11, 12 Endotoxin‐mediated splanchnic vasodilatation, especially with spontaneous bacterial peritonitis (SBP), can adversely affect central blood volume status, especially in the presence of severe ascites.1 Clinicians providing care for patients with cirrhosis should be aware of these factors and closely monitor at‐risk patients for the onset or worsening of hyponatremia.1
PROGNOSTIC SIGNIFICANCE OF HYPONATREMIA IN CIRRHOSIS
Hyponatremia has several important clinical implications for patients with cirrhosis.13 Hyponatremia is associated with refractory ascites, greater fluid accumulation, the need for paracentesis, and, importantly, impaired renal function. In patients with ascites and cirrhosis, approximately 50% have some degree of hyponatremia.2 Moreover, the severity of hyponatremia associated with advanced cirrhosis correlates with the degree of cirrhosis complications, especially hyponatremia associated with hepatorenal syndrome, encephalopathy, and SBP (Table 1).2
| Serum [Na+] mEq/L | |||
|---|---|---|---|
| 130 | 131‐135 | >135 | |
| |||
| Hepatorenal syndrome | 3.45 | 1.75 | 1 (reference value) |
| Hepatic encephalopathy | 3.40 | 1.69 | 1 (reference value) |
| Gastrointestinal bleeding | 1.48 | 0.93 | 1 (reference value) |
| Spontaneous bacterial peritonitis | 2.36 | 1.44 | 1 (reference value) |
Similarly, hyponatremia is strongly associated with increasing Child‐Pugh and Model for End‐Stage Liver Disease (MELD) scores.14 In an analysis of data among candidates for liver transplantation from the Organ Procurement and Transplantation Network, the combination of MELD score and serum sodium concentration was a better predictor of death than the MELD score alone.14 In addition, the effect of hyponatremia on clinical outcomes was greater in patients with a low MELD score than those with a relatively high MELD score.. These results suggest that combining serum sodium concentrations with MELD scores to assign transplantation priority might reduce mortality among patients on the waiting list.14
Hyponatremia is also a marker for the development of overt hepatic encephalopathy in patients with cirrhosis.13 One of the proposed mechanisms for encephalopathy is low‐grade cerebral edema. This leads to the conversion of glutamate to glutamine by ammonia, which accumulates within astrocytes, causing astrocyte swelling and dysfunction. Because hyponatremia complicates the management of fluid overload, it increases the risk of developing or exacerbating hepatic encephalopathy.13
Hyponatremia is intimately involved with the development of renal failure in the patient with cirrhosis. It is an earlier and more sensitive marker of renal impairment and/or circulatory dysfunction than serum creatinine.15 It is often the precursor to the development of hepatorenal syndrome.16, 17
Hyponatremia is more common in hospitalized versus ambulatory patients with cirrhosis.1 In a study of 126 patients with cirrhosis admitted to an intensive care unit, patients with serum [Na+] 135 mEq/L had a greater frequency of ascites, illness severity scores, hepatic encephalopathy, sepsis, renal failure, and in‐hospital mortality than normonatremic patients (73.1% vs 55.9%).18 Persistent ascites and low serum sodium identified cirrhotic patients with a high mortality risk, despite low MELD scores, in a study of 507 veterans in the United States with cirrhosis.19 In a retrospective review of 127 patients, hyponatremia was predictive of the development of acute renal failure during hospitalization; among patients with hyponatremia who developed renal failure in the hospital, 72% died.20
Clinical assessment of a patient with cirrhosis who has hyponatremia can be difficult.1 These patients have too much salt and water in the wrong spaces (ie, in the peritoneal cavity and peripheral tissue). As a result, it is possible to have fluid overload with intravascular depletion. A further complication is that dilutional hyponatremia is associated with hepatorenal syndrome. Because these patients have elevated blood urea nitrogen (BUN) and creatinine, and decreased urine output and urine sodium concentration, they appear to be indistinguishable from a patient with prerenal azotemia prior to volume expansion.1 Many of these factors and concerns are illustrated in the following case we handled several years ago.
A 70‐YEAR‐OLD WOMAN WITH CIRRHOSIS
K.R. is a 70‐year‐old white woman recently discharged from the hospital following treatment of recurrent cellulitis. Her past medical history is positive for cirrhosis secondary to active alcohol use, chronic autoimmune hepatitis, and iron overload. Her hospital course was notable for tense ascites, asterixis, and a serum [Na+] of 126 mEq/L at admission. K.R. was managed with large volume paracentesis with 25% salt‐poor albumin, elevation of her lower extremities, discontinuation of diuretics, and 1 L fluid restriction. Her serum [Na+] increased to 128 mEq/L. Although her cellulitis and edema both improved, both persisted. In addition, her mental status also improved, but asterixis persisted. At this point in the hospitalization, effective management of the cellulitis was hindered by the persistent edema, and its treatment with diuretics was limited by the hyponatremia and hepatic encephalopathy.
Today, we have better treatment options for managing this patient. To effectively correct the hyponatremia and facilitate treatment of the other complications of cirrhosis, we can now initiate therapy with one of the vaptans currently available.
TREATMENT OF MILD ASYMPTOMATIC HYPERVOLEMIC HYPONATREMIA
The initial approach to treatment of patients with mild asymptomatic, hypervolemic hyponatremia consists of fluid restriction and a sodium‐restricted diet.1 Fluid restriction, however, has limited efficacy and is often not well tolerated by patients. For patients with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion. If patients have severe dilutional hyponatremia and tense ascites, therapeutic paracentesis with plasma expanders is safe.1
The pharmacologic approach to treating hyponatremia has advanced with the discovery of vaptans, drugs that inhibit V2 receptors in cells of the collecting ducts.21 In contrast to conventional diuretics, vaptans do not increase natriuresis. Administration of a vaptan agent for 1 to 2 weeks has been shown to significantly improve low serum sodium levels in patients with hyponatremia, and promote aquaresis without significantly altering renal or circulatory function or activity of the renin‐angiotensin‐aldosterone system. The most frequent side effect of vaptan therapy is thirst.21
Two vaptan agents are currently approved for use in the United States: conivaptan and tolvaptan. Conivaptan is administered intravenously, and is a nonselective vasopressin inhibitor, blocking both V1A and V2 receptors. The course of therapy for conivaptan is 4 days. Tolvaptan, on the other hand, selectively blocks V2 receptors, and is a once‐daily oral vaptan that can be given long‐term.21
The efficacy of tolvaptan was evaluated in the Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).22 In these multicenter, prospective, randomized, placebo‐controlled trials, patients with dilutional hyponatremia (serum [Na+] 135 mEq/L) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), heart failure, or syndrome of inappropriate antidiuretic hormone (ADH) hypersecretion, and who were hospitalized and clinically stable, received tolvaptan 15 mg daily or placebo. Repeat serum sodium levels were obtained at 8 hours, 2, 3, and 4 days, and then weekly at days 11, 18, 25, and 30. The study drug was discontinued on day 30, with follow‐up serum sodium levels taken 7 days later. (In patients with persistent hyponatremia, the tolvaptan dose was adjusted to 30 mg and then 60 mg with the goal of achieving a serum [Na+] 135 mEq/L.) Increases in serum sodium concentration were seen as early as 8 hours after the first administration of tolvaptan and persisted throughout the study period. After tolvaptan was discontinued, serum sodium levels decreased to baseline within 1 week.22 Tolvaptan was well tolerated, with the most common side effects being increased thirst, dry mouth, and increased urination.22
Longer‐term administration of tolvaptan was shown to maintain a higher serum sodium concentration with an acceptable safety profile in SALTWATER, the open‐label extension of the SALT‐1 and SALT‐2 trials.23 The study included 111 patients with hyponatremia who received oral tolvaptan for a mean follow‐up of 701 days. The most common adverse effects potentially related to tolvaptan were thirst, dry mouth, polydipsia, and polyuria.22, 23 Overall, there were 9 possible and 1 probable serious adverse events, which represents an acceptable safety profile over 77,369 patient‐days of exposure. Over time, 64 patients discontinued tolvaptan, 30 due to adverse reactions or death.22 The results of SALTWATER indicated that most patients received benefit from treatment with tolvaptan, with a decreased need for fluid restriction.23
PATIENT CHARACTERISTICS FOR TOLVAPTAN
In the SALT trials, tolvaptan was administered to clinically stable patients. Based on recommendations by the US Food and Drug Administration (FDA), tolvaptan should be initiated or reinitiated in a hospital setting.1 Patients with severe neurologic symptoms due to hyponatremia should be treated with normal saline instead of tolvaptan; combination therapy with tolvaptan and normal saline should be avoided due to the potential for a too‐rapid correction of hyponatremia and the potential for central pontine myelinolysis. Saline should be discontinued and persistent hyponatremia confirmed before beginning tolvaptan therapy.1
Several additional factors should be considered before patients begin tolvaptan. First, tolvaptan increases thirst, as well as the frequency and volume of urination. Therefore, patients must be able to respond appropriately to thirst with increased water intake. Patients should not be fluid‐restricted during the first day of tolvaptan therapy; instead, they should be instructed to respond to their thirst with increased water ingestion. Because of these factors, caution should be exercised in administering tolvaptan to a confused, restrained patient. In addition, patients should have adequate toileting aids, such as a bedside urinal or commode.1
As with most new drugs, acquisition costs for tolvaptan should be considered in light of the clinical benefits of treatment outcomes. In a retrospective review, median hospital costs for patients with moderate‐to‐severe ($16,606) and mild‐to‐moderate hyponatremia ($14,266) were higher than matched patients without hyponatremia ($13,066).24 In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial, in which patients with severe congestive heart failure (including those with and without hyponatremia) were randomized to tolvaptan or placebo, the adjusted mean length of hospital stay for those with hyponatremia at baseline who received tolvaptan was 1.72 days shorter than those who received placebo.25 Although tolvaptan is somewhat expensive, the cost compares favorably with the daily cost of hospitalization.
SUMMARY
Portal hypertension plays a pivotal role in the development of hyponatremia in patients with cirrhosis. Reflex vasodilation in the splanchnic circulation compromises the effective central blood volume, triggering compensatory vasoconstrictor and antinatriuretic mechanisms. The net effect is greater free water accumulation than sodium retention, creating dilutional hyponatremia.
The severity of hyponatremia correlates with the severity of cirrhosis complications, such as hepatorenal syndrome, encephalopathy, SBP, and renal failure. The presence of hyponatremia is a marker for poor outcomes and shortened survival, regardless of MELD scores.
In a hospitalized, acutely ill patient with cirrhosis, such as the person in this case, therapy may involve discontinuation of diuretics, evaluation and treatment of infection, volume expansion with salt‐poor albumin, and tolvaptan for treatment of hyponatremia. Regarding tolvaptan, early morning administration is recommended. At initiation of therapy, fluid restriction should be discontinued, and off‐floor testing should be avoided. Concomitant medications should be reviewed to avoid potentially harmful interactions.
Cirrhosis is one of the main causes of hypervolemic hyponatremia, a dilutional form of hyponatremia that occurs when there is an increase in total body water but a relatively smaller increase in total serum sodium. Portal hypertension is the main precipitating factor in fluid retention that leads to the development of cirrhotic hyponatremia. In cirrhosis, portal hypertension is determined by 2 main factors: increased intrahepatic resistance and increased spanchnic blood flow. The increased intrahepatic resistance is due to both structural (fibrosis, conversion of low resistance fenestrated sinusoids into capillaries) and dynamic (vasoconstriction due to endothelial cell dysfunction) changes.1
The hepatic circulation normally is able to accommodate an increase in portal blood flow associated with postprandial hyperemia. The elevated intrahepatic resistance in cirrhosis results in an inability to accommodate the normal increase in portal blood flow that occurs in the postprandial hyperemia state.3 As a result, portal pressure increases during postprandial hyperemia, leading to reflex vasoconstriction, which creates a shear stress and increases splanchnic nitric oxide (NO) production.4 NO, one of the most important vasodilators in the splanchnic circulation, increases splanchnic blood flow and portal pressures. When this happens repeatedly, it leads to a progressive dilation of preexisting portosystemic vascular channels and the development of varices.5 At the same time, levels of vascular endothelial growth factor rise; this is a very important mediator for angiogenesis because it increases NO, further increasing splanchnic vasodilation.6
Progressive splanchnic vasodilation and increased blood flow into the splanchnic circulation leads to central hypovolemia, arterial underfilling, and decreased blood flow in renal afferent arterioles. Vasoconstrictor norepinephrine and antinatriuretic mechanisms are subsequently activated in an attempt to normalize renal perfusion pressures. Baroreceptor‐mediated nonosmotic release of arginine vasopressin (AVP) is triggered and renin angiotensin‐aldosterone system activity is increased, which increases sodium reabsorption and activates the stellate cells, causing fibrosis, vasoconstriction, and increased portal pressures.6, 7
AVP acts at vasopression‐1A (V1A) receptors to increase arterial vasoconstriction, and at V2 receptors in renal tubule cells for solute‐free water retention.1 The increased sodium and water reabsorption leads to fluid retention, increased central blood volume, venous return to the heart, and an increase in cardiac output to maintain arterial perfusion and create the hyperdynamic circulation that is characteristic of cirrhosis with advanced portal hypertension. Dilutional hyponatremia develops when free water retention is more pronounced than that of sodium retention.
CLINICAL FACTORS ASSOCIATED WITH CIRRHOTIC HYPONATREMIA
Diuretics lead to hyponatremia through several mechanisms.8 First, they induce a contraction of the central blood volume, leading to the nonosmotic release of AVP. In advanced cirrhosis, there is activation of the renin‐angiotensin system in addition to the nonosmotic release of AVP, leading to sodium and free water reabsorption. Diuretics block the sodium reabsorption. However, the water‐retaining effects persist, further contributing to dilutional hyponatremia.8 This cycle is made worse by low sodium intake and frequent thirst experienced by these patients.8 Other medications (eg, non‐steroidal anti‐inflammatory drugs, proton pump inhibitors, and selective serotonin reuptake inhibitors) commonly prescribed for cirrhotic patients may also contribute to the development or worsening of dilutional hyponatremia.8
Increased intrathoracic pressure in patients with tense ascites can also contribute to dilutional hyponatremia by increasing baroreceptor‐mediated release of AVP.9 Large volume paracentesis without the oncotic influence of albumin, an intervention commonly required in patients with cirrhosis and recurrent ascites, may also lead to significant increases in plasma renin activity and plasma aldosterone, which further worsen these pathophysiologic mechanisms, resulting in reduced serum sodium concentration.10 Following removal of excess peritoneal fluid, blood flow to the kidneys is initially improved, but ascitic fluid reaccumulates and the patient becomes intravascularly depleted.10
Infection is an important clinical mediator for the development of both portal hypertension as well as hyponatremia. Bacterial translocation leads to endotoxemia and increased tumor necrosis factor (TNF)‐alpha, resulting in increased splanchnic NO and splanchnic arterial vasodilatation. This process reduces cardiac output, which leads to increased AVP secretion.11, 12 Endotoxin‐mediated splanchnic vasodilatation, especially with spontaneous bacterial peritonitis (SBP), can adversely affect central blood volume status, especially in the presence of severe ascites.1 Clinicians providing care for patients with cirrhosis should be aware of these factors and closely monitor at‐risk patients for the onset or worsening of hyponatremia.1
PROGNOSTIC SIGNIFICANCE OF HYPONATREMIA IN CIRRHOSIS
Hyponatremia has several important clinical implications for patients with cirrhosis.13 Hyponatremia is associated with refractory ascites, greater fluid accumulation, the need for paracentesis, and, importantly, impaired renal function. In patients with ascites and cirrhosis, approximately 50% have some degree of hyponatremia.2 Moreover, the severity of hyponatremia associated with advanced cirrhosis correlates with the degree of cirrhosis complications, especially hyponatremia associated with hepatorenal syndrome, encephalopathy, and SBP (Table 1).2
| Serum [Na+] mEq/L | |||
|---|---|---|---|
| 130 | 131‐135 | >135 | |
| |||
| Hepatorenal syndrome | 3.45 | 1.75 | 1 (reference value) |
| Hepatic encephalopathy | 3.40 | 1.69 | 1 (reference value) |
| Gastrointestinal bleeding | 1.48 | 0.93 | 1 (reference value) |
| Spontaneous bacterial peritonitis | 2.36 | 1.44 | 1 (reference value) |
Similarly, hyponatremia is strongly associated with increasing Child‐Pugh and Model for End‐Stage Liver Disease (MELD) scores.14 In an analysis of data among candidates for liver transplantation from the Organ Procurement and Transplantation Network, the combination of MELD score and serum sodium concentration was a better predictor of death than the MELD score alone.14 In addition, the effect of hyponatremia on clinical outcomes was greater in patients with a low MELD score than those with a relatively high MELD score.. These results suggest that combining serum sodium concentrations with MELD scores to assign transplantation priority might reduce mortality among patients on the waiting list.14
Hyponatremia is also a marker for the development of overt hepatic encephalopathy in patients with cirrhosis.13 One of the proposed mechanisms for encephalopathy is low‐grade cerebral edema. This leads to the conversion of glutamate to glutamine by ammonia, which accumulates within astrocytes, causing astrocyte swelling and dysfunction. Because hyponatremia complicates the management of fluid overload, it increases the risk of developing or exacerbating hepatic encephalopathy.13
Hyponatremia is intimately involved with the development of renal failure in the patient with cirrhosis. It is an earlier and more sensitive marker of renal impairment and/or circulatory dysfunction than serum creatinine.15 It is often the precursor to the development of hepatorenal syndrome.16, 17
Hyponatremia is more common in hospitalized versus ambulatory patients with cirrhosis.1 In a study of 126 patients with cirrhosis admitted to an intensive care unit, patients with serum [Na+] 135 mEq/L had a greater frequency of ascites, illness severity scores, hepatic encephalopathy, sepsis, renal failure, and in‐hospital mortality than normonatremic patients (73.1% vs 55.9%).18 Persistent ascites and low serum sodium identified cirrhotic patients with a high mortality risk, despite low MELD scores, in a study of 507 veterans in the United States with cirrhosis.19 In a retrospective review of 127 patients, hyponatremia was predictive of the development of acute renal failure during hospitalization; among patients with hyponatremia who developed renal failure in the hospital, 72% died.20
Clinical assessment of a patient with cirrhosis who has hyponatremia can be difficult.1 These patients have too much salt and water in the wrong spaces (ie, in the peritoneal cavity and peripheral tissue). As a result, it is possible to have fluid overload with intravascular depletion. A further complication is that dilutional hyponatremia is associated with hepatorenal syndrome. Because these patients have elevated blood urea nitrogen (BUN) and creatinine, and decreased urine output and urine sodium concentration, they appear to be indistinguishable from a patient with prerenal azotemia prior to volume expansion.1 Many of these factors and concerns are illustrated in the following case we handled several years ago.
A 70‐YEAR‐OLD WOMAN WITH CIRRHOSIS
K.R. is a 70‐year‐old white woman recently discharged from the hospital following treatment of recurrent cellulitis. Her past medical history is positive for cirrhosis secondary to active alcohol use, chronic autoimmune hepatitis, and iron overload. Her hospital course was notable for tense ascites, asterixis, and a serum [Na+] of 126 mEq/L at admission. K.R. was managed with large volume paracentesis with 25% salt‐poor albumin, elevation of her lower extremities, discontinuation of diuretics, and 1 L fluid restriction. Her serum [Na+] increased to 128 mEq/L. Although her cellulitis and edema both improved, both persisted. In addition, her mental status also improved, but asterixis persisted. At this point in the hospitalization, effective management of the cellulitis was hindered by the persistent edema, and its treatment with diuretics was limited by the hyponatremia and hepatic encephalopathy.
Today, we have better treatment options for managing this patient. To effectively correct the hyponatremia and facilitate treatment of the other complications of cirrhosis, we can now initiate therapy with one of the vaptans currently available.
TREATMENT OF MILD ASYMPTOMATIC HYPERVOLEMIC HYPONATREMIA
The initial approach to treatment of patients with mild asymptomatic, hypervolemic hyponatremia consists of fluid restriction and a sodium‐restricted diet.1 Fluid restriction, however, has limited efficacy and is often not well tolerated by patients. For patients with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion. If patients have severe dilutional hyponatremia and tense ascites, therapeutic paracentesis with plasma expanders is safe.1
The pharmacologic approach to treating hyponatremia has advanced with the discovery of vaptans, drugs that inhibit V2 receptors in cells of the collecting ducts.21 In contrast to conventional diuretics, vaptans do not increase natriuresis. Administration of a vaptan agent for 1 to 2 weeks has been shown to significantly improve low serum sodium levels in patients with hyponatremia, and promote aquaresis without significantly altering renal or circulatory function or activity of the renin‐angiotensin‐aldosterone system. The most frequent side effect of vaptan therapy is thirst.21
Two vaptan agents are currently approved for use in the United States: conivaptan and tolvaptan. Conivaptan is administered intravenously, and is a nonselective vasopressin inhibitor, blocking both V1A and V2 receptors. The course of therapy for conivaptan is 4 days. Tolvaptan, on the other hand, selectively blocks V2 receptors, and is a once‐daily oral vaptan that can be given long‐term.21
The efficacy of tolvaptan was evaluated in the Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).22 In these multicenter, prospective, randomized, placebo‐controlled trials, patients with dilutional hyponatremia (serum [Na+] 135 mEq/L) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), heart failure, or syndrome of inappropriate antidiuretic hormone (ADH) hypersecretion, and who were hospitalized and clinically stable, received tolvaptan 15 mg daily or placebo. Repeat serum sodium levels were obtained at 8 hours, 2, 3, and 4 days, and then weekly at days 11, 18, 25, and 30. The study drug was discontinued on day 30, with follow‐up serum sodium levels taken 7 days later. (In patients with persistent hyponatremia, the tolvaptan dose was adjusted to 30 mg and then 60 mg with the goal of achieving a serum [Na+] 135 mEq/L.) Increases in serum sodium concentration were seen as early as 8 hours after the first administration of tolvaptan and persisted throughout the study period. After tolvaptan was discontinued, serum sodium levels decreased to baseline within 1 week.22 Tolvaptan was well tolerated, with the most common side effects being increased thirst, dry mouth, and increased urination.22
Longer‐term administration of tolvaptan was shown to maintain a higher serum sodium concentration with an acceptable safety profile in SALTWATER, the open‐label extension of the SALT‐1 and SALT‐2 trials.23 The study included 111 patients with hyponatremia who received oral tolvaptan for a mean follow‐up of 701 days. The most common adverse effects potentially related to tolvaptan were thirst, dry mouth, polydipsia, and polyuria.22, 23 Overall, there were 9 possible and 1 probable serious adverse events, which represents an acceptable safety profile over 77,369 patient‐days of exposure. Over time, 64 patients discontinued tolvaptan, 30 due to adverse reactions or death.22 The results of SALTWATER indicated that most patients received benefit from treatment with tolvaptan, with a decreased need for fluid restriction.23
PATIENT CHARACTERISTICS FOR TOLVAPTAN
In the SALT trials, tolvaptan was administered to clinically stable patients. Based on recommendations by the US Food and Drug Administration (FDA), tolvaptan should be initiated or reinitiated in a hospital setting.1 Patients with severe neurologic symptoms due to hyponatremia should be treated with normal saline instead of tolvaptan; combination therapy with tolvaptan and normal saline should be avoided due to the potential for a too‐rapid correction of hyponatremia and the potential for central pontine myelinolysis. Saline should be discontinued and persistent hyponatremia confirmed before beginning tolvaptan therapy.1
Several additional factors should be considered before patients begin tolvaptan. First, tolvaptan increases thirst, as well as the frequency and volume of urination. Therefore, patients must be able to respond appropriately to thirst with increased water intake. Patients should not be fluid‐restricted during the first day of tolvaptan therapy; instead, they should be instructed to respond to their thirst with increased water ingestion. Because of these factors, caution should be exercised in administering tolvaptan to a confused, restrained patient. In addition, patients should have adequate toileting aids, such as a bedside urinal or commode.1
As with most new drugs, acquisition costs for tolvaptan should be considered in light of the clinical benefits of treatment outcomes. In a retrospective review, median hospital costs for patients with moderate‐to‐severe ($16,606) and mild‐to‐moderate hyponatremia ($14,266) were higher than matched patients without hyponatremia ($13,066).24 In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial, in which patients with severe congestive heart failure (including those with and without hyponatremia) were randomized to tolvaptan or placebo, the adjusted mean length of hospital stay for those with hyponatremia at baseline who received tolvaptan was 1.72 days shorter than those who received placebo.25 Although tolvaptan is somewhat expensive, the cost compares favorably with the daily cost of hospitalization.
SUMMARY
Portal hypertension plays a pivotal role in the development of hyponatremia in patients with cirrhosis. Reflex vasodilation in the splanchnic circulation compromises the effective central blood volume, triggering compensatory vasoconstrictor and antinatriuretic mechanisms. The net effect is greater free water accumulation than sodium retention, creating dilutional hyponatremia.
The severity of hyponatremia correlates with the severity of cirrhosis complications, such as hepatorenal syndrome, encephalopathy, SBP, and renal failure. The presence of hyponatremia is a marker for poor outcomes and shortened survival, regardless of MELD scores.
In a hospitalized, acutely ill patient with cirrhosis, such as the person in this case, therapy may involve discontinuation of diuretics, evaluation and treatment of infection, volume expansion with salt‐poor albumin, and tolvaptan for treatment of hyponatremia. Regarding tolvaptan, early morning administration is recommended. At initiation of therapy, fluid restriction should be discontinued, and off‐floor testing should be avoided. Concomitant medications should be reviewed to avoid potentially harmful interactions.
- ,.Managing hyponatremia in cirrhosis.J Hosp Med.2010;5(suppl 3):S8–S17.
- ,,,; for the CAPPS Investigators.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- .Molecular mechanisms of increased intrahepatic resistance in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S259–S261.
- ,,.The pathophysiology of portal hypertension.Dig Dis.2005;23:6–10.
- .The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S288–S294.
- ,.Vascular endothelial dysfunction in cirrhosis.J Hepatol.2007;46:927–934.
- ,, et al.Management of cirrhosis and ascites.N Engl J Med.2004;350(16):1646–1654.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52(1):144–153.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat.Gastroenterology.2002;122:85–93.
- ,,, et al.Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in in vivo in cirrhotic rats.Am J Physiol Heart Circ Physiol.2007;293:H1689–H1695.
- ,.Pathogenetic mechanisms of hepatic encephalopathy.Gut.2008;57:1156–1165.
- ,,, et al.Hyponatremia and mortality among patients on the liver‐transplant waiting list.N Engl J Med.2008;359:1018–1026.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44:220–226.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48(3):1002–1010.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al; for the SALTWATER Investigators.Oral tolvaptan is safe and effective in chronic hyponatremia.J Am Soc Nephrol.2010;21:705–712.
- ,,, et al.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Effect of serum sodium concentration and tolvaptan treatment on length of hospitalization in patients with heart failure.Am J Health Syst Pharm.2011;68(4):328–333.
- ,.Managing hyponatremia in cirrhosis.J Hosp Med.2010;5(suppl 3):S8–S17.
- ,,,; for the CAPPS Investigators.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- .Molecular mechanisms of increased intrahepatic resistance in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S259–S261.
- ,,.The pathophysiology of portal hypertension.Dig Dis.2005;23:6–10.
- .The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension.J Clin Gastroenterol.2007;41(suppl 3):S288–S294.
- ,.Vascular endothelial dysfunction in cirrhosis.J Hepatol.2007;46:927–934.
- ,, et al.Management of cirrhosis and ascites.N Engl J Med.2004;350(16):1646–1654.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52(1):144–153.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat.Gastroenterology.2002;122:85–93.
- ,,, et al.Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in in vivo in cirrhotic rats.Am J Physiol Heart Circ Physiol.2007;293:H1689–H1695.
- ,.Pathogenetic mechanisms of hepatic encephalopathy.Gut.2008;57:1156–1165.
- ,,, et al.Hyponatremia and mortality among patients on the liver‐transplant waiting list.N Engl J Med.2008;359:1018–1026.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44:220–226.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48(3):1002–1010.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al; for the SALTWATER Investigators.Oral tolvaptan is safe and effective in chronic hyponatremia.J Am Soc Nephrol.2010;21:705–712.
- ,,, et al.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Effect of serum sodium concentration and tolvaptan treatment on length of hospitalization in patients with heart failure.Am J Health Syst Pharm.2011;68(4):328–333.
Managing Hyponatremia in Cirrhosis
The serum sodium (Na) level is the major determinant of serum osmolality. In normal physiologic states is tightly regulated between 135 mEq/L to 145 mEq/L despite variable intake of water and solute through the interaction of osmoreceptors in the hypothalamus where arginine vasopressin (AVP) is synthesized and then released by the posterior pituitary and the binding of AVP with V2 AVP receptors on the basolateral surface of the principal cells within the collecting duct of the kidney. Binding of AVP to the V2 receptors promotes the translocation and fusion of cytoplasmic vesicles which carry the water channel protein aquaporin 2 (AQP2) to the apical membrane of the cell and, in this manner, increases water permeability and absorption.1, 2, 3
Patients with hyponatremia, defined by a serum Na level 135 mEq/L, can be broadly classified by their volume status into those who are euvolemic, hypervolemic, and hypovolemic (Table 1). In patients with euvolemic hyponatremia such as those with Syndrome of Inappropriate Antidiuretic Hormone (SIADH), total body Na is nearly normal, but total body water is increased. In patients with hypervolemic hyponatremia, both total body Na and water are increased, but water to a much greater degree. These patients typically have increased extracellular fluid such as edema and/or ascites. The most common conditions associated with this condition are cirrhosis, congestive heart failure (CHF), and renal failure. In contrast, hypovolemic hyponatremia is associated with a reduction in both total body Na and water, but Na to a greater degree. This condition is encountered in patients with excessive fluid losses such as those with over‐diuresis, excessive gastrointestinal losses, burns, and pancreatitis.4
| Depletional Hyponatreima | Dilutional Hyponatremia | ||
|---|---|---|---|
| Euvolumic | Hypervolumic | ||
| Total body water | |||
| Total body Na | normal | ||
| Common etiologies | SIADH | cirrhosis/CHF | vomiting, diarrhea |
Hyponatremia is the most common electrolyte abnormality seen in general hospital patients.5 In a database of over 120,000 patients, a serum sodium level of 136mEq/L was observed in 28.2%.6 Hyponatremia is associated with selected medical conditions (especially cirrhosis and CHF), the extremes of age, and those receiving selected medications, including several that are commonly administered to cirrhotic patients (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors).7, 8 Hyponatremia is associated with increased total costs per hospital admission.5, 9 In an analysis of the effect of hyponatremia on length of stay in a retrospective cohort study of hospitalized patients derived from a large administrative database of 198,281 discharges from 39 US hospitals, mean length of stay was significantly greater among patients with hyponatremia than those with normal Na levels (8.6 8.0 vs. 7.2 8.2 days). After adjusting for confounders that may be associated with more severe disease and hyponatremia (age, gender, race, geographic region, teaching status of the hospital, admission source, principal payer, comorbidity index score and primary diagnosis), the presence of hyponatremia contributed an increase in length of stay of 1.0 day. Patients with hyponatremia are more frequently admitted to the intensive care unit (ICU) and require mechanical ventilation. In patients with CHF, the presence of hyponatremia at discharge is associated with increased risk for early mortality and rehospitalization.10
Although frequently asymptomatic, hyponatremia may be associated with a range of findings, from subtle and non‐specific complaints, including headache, fatigue, confusion, malaise, to severe and life‐threatening manifestations with lethargy, seizures, brainstem herniation, respiratory arrest and death.11 The most important complications are neurologic consequences related to cerebral edema. However, there is increased morbidity even in hyponatremic patients considered to be asymptomatic. Patients with low serum sodium have attention deficients, and falls are common. In a study of 122 patients who were considered to have chronic asymptomatic hyponatremia, the incidence of falls was significantly higher at 21.3% compared to only 5.3% in a control population.12
In hyponatremia, water enters into the cells to attain osmotic balance, resulting in cellular swelling.4 To avoid cerebral edema, the brain is capable of adapting to hyponatremia by regulating its volume to avoid swelling, especially when hyponatremia is chronic. In acute hyponatremia, astrocytes and neurons adapt through osmoregulatory mechanisms by extruding intracellular electrolytes such as potassium.13 Chronically, adaption occurs through the loss of low‐molecular weight organic compounds termed organic osmolytes including myoinsoitol, glutamine, choline and taurine. As a result, both the severity and the rate of its development are critical factors in determining the neurologic manifestation of hyponatremia in a given patient.14
Dilutional Hyponatremia and Cirrhosis
Patients with hyponatremia who are either euvolemic or hypervolemic are considered to have dilutional hyponatremia (DH). Management of these patients is distinct from those who are hypovolemic in whom appropriate therapy consists of the administration of normal saline. The remainder of this article addresses the pathogenesis, management and treatment of cirrhotic patients with DH.
Pathogenesis
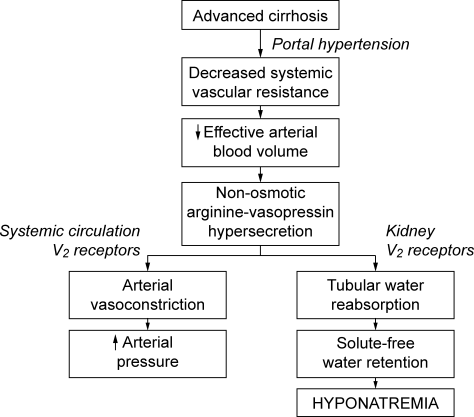
The development of hyponatremia in cirrhosis is intimately related to the pathophysiology of portal hypertension and the non‐osmotic release of AVP3, 15 (Figure 1). In the early phases of cirrhosis, portal hypertension is the result of an increase in intrahepatic resistance. With the development of porto‐systemic collaterals, a hyperdynamic splanchnic circulation develops as a result of splanchnic arterial vasodilatation and increased vascular capacity. Nitric oxide, an endothelial derived relaxing factor, is the critical mediator of this process, and upregulation of its expression is pivotal in the pathogenesis of portal hypertension.
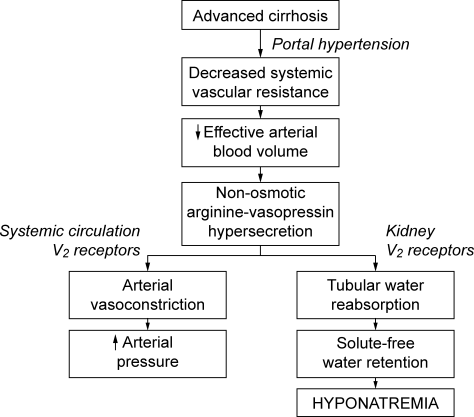
Multiple factors are related to the development of DH in cirrhosis. A reduction of effective central blood volume due to the development of porto‐venous collaterals and arterial splanchnic vasodilation, leading to baroreceptor‐mediated nonosmotic release of AVP, is considered the initiating and most important factor. Patients with cirrhosis and DH have higher plasma and urine vasopressin levels, higher plasma renin activity, and decreased plasma levels of atrial natriuretic factor than those with normal serum sodium concentrations, findings consistent with the presence of a decreased effective plasma volume.16 Arterial underfilling is sensed by baroreceptors located in the left ventricle, aortic arch, carotid sinus and renal afferent arterioles. Decreased activation leads to neurohumoral compensatory responses which include non‐osmotic release of vasopressin from the neurohypophysis and increased levels. Impaired catabolism of AVP that has been correlated with the severity of liver dysfunction may further contribute to increased levels.17 Initially, the increased AVP maintains arterial circulatory integrity by inducing splanchnic, peripheral and renal arterial vasoconstriction through its action on the V1a receptors and expansion of blood volume through renal water retention by its action on the V2 receptors located on the collecting ducts.
The initial adaptive response which leads to increased central blood volume can chronically result in detrimental effects, including the development of fluid overload with ascites, edema, and hyponatremia.16, 18 Additional factors that contribute to hyponatremia include decreased glomerular filtration rate (GFR) and/or increased proximal reabsorption of sodium (that reduce the distal delivery of filtrate and the potential for water reabsorption) and decreased cardiac function that further impairs effective central blood volume.19 In addition, urinary levels of AQP2 are increased in cirrhotic patients, especially those with decompensated disease with higher Child‐Pugh scores and ascites, and provide another potential mechanism to increase water reabsorption.20
Prevalence and Prognostic Significance
Hyponatremia in cirrhosis is a common finding. In a survey of 997 cirrhotic patients with ascites from 28 centers in Europe, North and South America, the prevalence of serum sodium concentration 135, 130, 125, 120 meq/L were 49.4%, 21.6%, 5.7%, and 1.2%, respectively.21 In a retrospective analysis of 188 inpatients, the prevalence of DH of 135, 130, and 125 were 20.8%, 14.9%, and 12.2%, respectively.22 The development of hyponatremia is a manifestation of increasing portal hypertension. In a natural history study of 263 patients hospitalized for first episode of significant ascites, 74 patients developed DH (Na level 130 mEq/L), including 11 patients in whom it appeared during the first episode and 63 cases during follow‐up (mean period of 40 3 months) with a 5‐year incidence of 37.1%.23
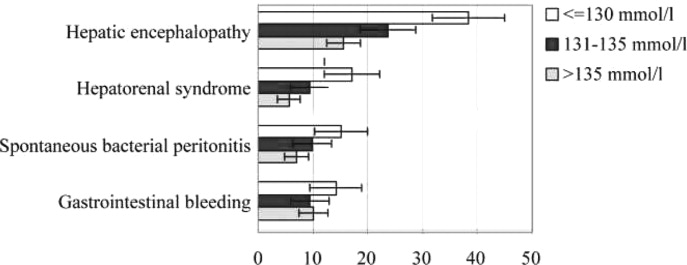
The presence of hyponatremia carries significant adverse prognostic significance. It is strongly associated with severity of liver function impairment as assessed by Child‐Pugh and model for end‐stage liver disease (MELD) scores.22 Even mild hyponatremia is associated with severe complications such as massive ascites, severe hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), and hepatic hydrothorax, and the severity of hyponatremia is directly related to the severity of these complications.21, 22 (Figure 2). In a natural history study of patients presenting with large volume ascites, 1‐year survival after its development was reduced to only 25.6%.230
Hyponatremia is an especially poor prognostic sign for a hospitalized cirrhotic patient. In a retrospective analysis of 156 cirrhotic patients, hyponatremiapresent in 57 (29.8%) of admissionswas associated with increased hospital mortality (26.3% vs. 8.9% among those with normal Na levels), and the mortality rate was even higher (48%) among the 25 patients who developed severe hyponatremia during the hospital stay.24 In hospitalized patients, hyponatremia is predictive of the development of acute renal failure which is associated with substantially increased mortality (73% vs. 13%).25 Similarly, a low serum sodium level in critically ill cirrhotic patients admitted to the ICU is associated with complications, in‐hospital mortality, and poor short‐term prognosis.26
Whether hyponatremia should impact liver transplant prioritization remains an area of controversy. The United Network for Organ Sharing (UNOS) contracted by the Organ Procurement and Transplant Network (OPTN) to optimize the efficient use of deceased organs through fair and timely allocation, currently uses the MELD score, a formula that calculates the risk of death within three months from the bilirubin, creatinine, and International Normalized Ratio (INR) levels. Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction in patients with advanced cirrhosis and adds to MELD in predicting waitlist mortality.2729 In patients with a MELD score of 21, only low serum sodium and persistent ascites are independent predictors of mortality.28 To account for the importance of hyponatremia on survival, both modification of the MELD score in which the Na level is incorporated (MELD‐Na model) and the MELD to serum sodium ratio (MESO) have been developed. Adding hyponatremia to the MELD score is a better predictor of death than MELD alone, particularly in patients with low MELD scores.27, 2931 The OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has discussed updating the liver allocation system to include the Na level. However, it was concluded that implementation of MELD‐Na would change the allocation status of only 4% of candidates. Further, based on the concerns about the ability to manipulate serum sodium levels and the utility of employing resources to change the system for a relatively small number of patients, it was decided to defer incorporating the Na level pending further analysis (Report of the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee To the Board of Directors, Los Angeles, California, September 17‐18, 2007). At this time, the use of Na is a regional decision.32 However, the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has recently solicited feedback from the transplant community about including Na in allocation for review at a forum in April 2010.
Precipitating Factors
The most important factor related to development of hyponatremia in cirrhosis is increasing severity of portal hypertension that is associated with impaired central blood volume as a result of progressive splanchnic vasodilatation. In a study in which 170 patients with decompensated alcoholic cirrhosis were prospectively followed for 33.9 27.9 months, the initial hepatic venous pressure gradient (HVPG) was an independent predictive factor for the 20 patients who developed hyponatremia.22
Cirrhotic patients with ascites with hyponatremia have increased AVP secretion, higher levels of plasma renin activity, and higher serum concentrations of aldosterone and norepinephrine compared to those with normal Na levels.33 Diuretic therapy is associated with the development of DH by inducing volume depletion and arterial underfilling, further activating the renin‐angiotensin system (RAS) and increasing the non‐osmotic release of AVP.34 Although diuretics block the salt retention associated with the RAS activation, the water‐retaining effects of AVP persist, and DH develops. The process is further exacerbated by a low sodium intake and a frequent uncontrollable thirst. As a result, diuretic therapy is commonly associated with the development of hyponatremia in patients with ascites.24, 35 Similarly, paracentesis (particularly when performed without albumin) is often associated with an increase in blood urea nitrogen and marked elevations in plasma renin activity and plasma aldosterone concentration, which may exacerbate this physiology, leading to further reduction in serum sodium concentration.36 Tense ascites can contribute to DH by increasing baroreceptor mediated AVP release by increasing intrathoracic pressure.37 Finally, non‐steroidal anti‐inflammatory drugs (NSAIDs) can cause DH by inhibiting the synthesis of renal prostaglandins (which normally function to antagonize the tubular action of AVP and are important in the maintenance of appropriate renal tubular transport of fluid and electrolytes in states of renal hypoperfusion).38
Medical Impact of Hyponatremia: Marker of Severe Disease or Direct Pathophysiologic Role?
Hyponatremia is associated with severe ascites, impaired renal function, hepatic encephalopathy, SBP, and hepatorenal syndrome.3, 20 Because hyponatremia is frequently present in advanced liver failure, it is unclear whether it is only a marker of advanced disease or whether it plays a direct pathophysiologic role, or both. Until recently, it has not been possible to address this issue due to the inability to easily and rapidly correct the hyponatremia. However, there is increasing evidence that hyponatremia has direct impact on the severity of hepatic encephalopathy (see Hepatic Encephalopathy section). The recent introduction of tolvaptan for the treatment of hyponatremia in cirrhosis (discussed below) will allow this question to be directly answered.
Fluid Management and Diuresis
The typical cirrhotic patient with DH is characterized by expanded extracellular fluid with ascites and edema. The profound vasodilation of the splanchnic arterial circulation is associated with decreased effective arterial blood volume, leading to the non‐osmotic release of AVP. Diuretic therapy can further exacerbate this process. In addition, the increased water permeability induced by AVP results in reduced urine volume and fluid retention. As a result, hyponatremia directly adversely affects severity of fluid overload and limits and/or precludes diuretic treatment.
Hepatorenal Syndrome
Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction and is frequently a precursor to overt hepatorenal syndrome.27 Hyponatremia is predictive of the development of acute renal failure during hospitalization, and in‐hospital development of acute renal failure portends a high mortality.25 In patients admitted with SBP, the presence of hyponatremia is significantly associated with higher mortality and renal failure.39
Hepatic Encephalopathy
The neurologic manifestations of cerebral edema associated with hyponatremia closely mirror those of hepatic encephalopathy. In fact, a recently proposed pathogenic mechanism for hepatic encephalopathy is the development of low‐grade cerebral edema associated with astrocyte swelling in response to ammonia and other precipitating factors.40 DH is associated with a further reduction in brain organic osmolytes that probably reflects a compensatory osmoregulatory mechanism against cell swelling triggered by a combination of high intracellular glutamine and low extracellular osmolality.41 As a result, it has been proposed that hyponatremia contributes to the development of hepatic encephalopathy through the development or exacerbation of low‐grade cerebral edema. In this manner, low serum sodium acts as a second hit to the swelling produced by increased intracellular glutamine created by ammonia metabolism.42
Clinically, hyponatremia is a major risk factor for hepatic encephalopathy. Serum sodium and ammonia levels are the major factors that predict electroencephalographic abnormalities in cirrhotics who do not have hepatic encephalopathy.43 In a prospective study of 61 patients, hyponatremia was associated with a low brain concentration of organic osmolytes as assessed by proton magnetic resonance spectroscopy (1H‐MRS) and magnetic resonance imaging, and both conditions were major risk factors for the development of overt hepatic encephalopathy.44 Finally, hyponatremia is a risk factor for hepatic encephalopathy in patients undergoing TIPS.45
Adverse Effect on Outcome After Liver Transplantation
Hyponatremia before liver transplantation is associated with adverse post‐transplant outcomes. Among patients undergoing liver transplantation, the presence of hyponatremia is associated with abnormal cardiac response in patients after reperfusion.46 Pre‐transplant hyponatremia is associated with longer ICU and hospital stay, higher rates of delirium and neurologic disorders, acute renal failure, acute cellular rejection, infection, and in one study a reduced 3‐month survival compared to normonatremic recipients.32, 47, 48 In 1 retrospective study that compared post‐transplant outcomes of patients with corrected vs. uncorrected pre‐transplant hyponatremia, patients with pre‐operative correction of hyponatremia had a lower risk of prolonged post‐transplant hospitalization than those with uncorrected hyponatremia.32 However, both hyponatremic groups had more complicated post‐transplant courses compared to those without a history of hyponatremia. However, given the small sample size, retrospective design, and the potential for confounding, the impact of correction of pre‐transplant hyponatremia remains to be determined.
Management
Most patients with mild hypervolemic hyponatremia are asymptomatic. The initial recommended approach is fluid restriction and an Na‐restricted diet. For those with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion.49 For patients with tense ascites and severe DH, therapeutic paracentesis with plasma expanders is safe.33 Unfortunately, fluid restriction is limited in efficacy and often poorly tolerated. The use of hypertonic saline is generally not recommended unless severe neurologic symptoms are present as it leads to increased ascites and edema. When administered, it is important to avoid a rapid correction of the hyponatremia to prevent the development of central pontine myelinolysis and the osmotic demyelination syndrome.
Due to the pivotal role of AVP in the pathogenesis of DH, antagonism of its action has long been proposed to be the most rational approach, but until recently, effective and specific antagonism of AVP has remained elusive. Approaches that have been attempted include interference with its secretion and actions. Intravenous albumin has been reported to improve hyponatremia in patients with cirrhosis, ascites, and hyponatremia, presumably by decreasing AVP release by plasma volume expansion.50 An attempt at inhibition of central AVP release with the use of a kappa‐opioid receptor agonist, niravoline, was limited by loss of efficacy and potential adverse effects.51 Use of demeclocycline and lithium (which induce renal resistance to AVP and lead to a modest increase in urine volume with decreased urine osmolality and a corresponding rise in serum sodium) is limited by nephrotoxicity and hepatotoxicity.7, 52 Because of the important role played by prostaglandins in the maintenance of renal hemodynamics and water excretion in cirrhosis, oral misoprostol has also been evaluated but determined to be ineffective in inducing significant changes in free water clearance in patients with functional renal failure and/or DH.53
The recent introduction of vaptans, vasopressin receptor antagonists that block the physiologic action of vasopressin, represents a revolutionary and highly effective approach to the treatment of hyponatremia. Vaptans are antagonists of the V2 receptors of AVP in the principal cells of the collecting ducts. In healthy subjects, vaptans cause a dose‐dependent increase in urine volume and produce a dilute urine without causing natriuresis. To date, 2 AVP antagonists, conivaptan and tolvaptan, have been Food and Drug Administration (FDA)‐approved for the treatment of DH. Conivaptan, the first to be approved in 2005, is a mixed vasopressin V1a and V2 receptor antagonist that is administered intravenously for up to 4 days. In a randomized placebo‐controlled study of patients with euvolemic or hypervolemic hyponatremia, intravenous conivaptan treatment increased serum Na levels by >6 mEq/l or to a serum Na >135 mEq/l in 69 to 88.5% of subjects compared to 20.7% of those receiving placebo (Zeltser D, Rosansky S, Van Rensburg H, et al. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. American J Nephrology 2007;27:447457). In a pilot study involving 24 patients with end‐stage liver disease, an infusion of conivaptan over 1 to 4 days was associated with an increase of serum sodium by >5 mmol/L in 60% of patients not receiving diuretics and 67% of patients on concomitant diuretic therapy by the end of treatment (O'Leary and Davis, 2009). Despite a concern about the potential for conivaptan to increase portal hypertension due to inhibition of splanchnic V1a receptors, the brief treatment appeared to be well tolerated without significant changes in systolic blood pressure, serum creatinine, variceal bleeding or worsening of ascites during the infusion period. However, approval for only 4 days of therapy and requirement for intravenous use eliminate any potential for chronic use.54
Tolvaptan is an orally available, selective V2 receptor antagonist whose efficacy was assessed in two multicenter, prospective, randomized, placebo‐controlled trials, Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).55 In these trials, clinically stable patients with DH (Na 135meq/l) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), CHF or SIADH were randomized in a hospital setting to receive tolvaptan 15mg daily or placebo. Repeat Na levels were obtained at 8 hours, 2, 3, and 4 days and then weekly at days 11, 18, 25 and 30 after which study drug was discontinued and follow‐up Na level was determined 7 days later. The dose was adjusted to 30 mg and then 60 mg in an attempt to achieve a Na level >135 in those in whom hyponatremia persisted. During the initial day of the titration phase, fluid restriction was not maintained, and the patients were encouraged to respond to thirst with increased water ingestion.
Tolvaptan use was associated with a prompt increase in Na level as early as 8 hours after administration of the first dose. Serum Na increased more among those receiving tolvaptan than among those receiving placebo during the first 4 days and throughout the study period regardless of baseline Na level but returned to baseline within 1 week after discontinuation (Figure 3). The main side effects were increased thirst, dry mouth and increased urination. Importantly, an increased incidence of renal failure was not observed. Based on these results, FDA approval for tolvaptan in patients with hyponatremia was obtained in May 2009 for patients with DH‐associated with cirrhosis, CHF or SIADH for patients with Na levels 125 or symptomatic patients with Na levels between 125 and 135 that have not responded to fluid restriction.
Management of the Hospitalized Cirrhotic Patient With Hyponatremia: Recommendations
Hyponatremia in hospitalized cirrhotic patients is a marker for severe disease and high risk of hospital mortality.24 As a result, prompt evaluation and treatment is imperative. The availability of tolvaptan potentially revolutionizes the manner in which these patients are treated. In the SALT trials, only clinically stable patients were enrolled. In this last section, a guideline for the evaluation and treatment of acutely ill, hospitalized cirrhotic patients with DH is presented.
Evaluation
Determination of volume status is paramount but frequently problematic in the hospitalized cirrhotic patient. Due to the vasodilated state present in severe portal hypertension that is characterized by a relative hypotension and resting tachycardia, the usual hemodynamic parameters of blood pressure and heart rate can be difficult to interpret. Although significant extravascular volume in the form of ascites and edema may be present, patients may be intravascularly depleted due to previous diuretic use and extra‐renal losses due to impaired oral intake, vomiting, lactulose‐induced diarrhea, and gastrointestinal bleeding. Infection is a commonly associated condition, and endotoxin mediated splanchnic vasodilatation, especially in the setting of SBP, can adversely effective central blood volume status in the presence of severe ascites. Also, due to the Na avidity of the kidney and previous diuretic use, renal electrolytes can be difficult to interpret.
For patients in whom there is strong clinical concern about intravascular depletion (history of impaired oral intake, excessive vomiting and/or diarrhea, rapid weight loss, small volume ascites with history of large volume, azotemia), administration of limited intravenous normal saline (0.5‐1 L) should be considered. Patients with severe neurological symptoms should receive normal saline or hypertonic saline. Unless severe neurologic symptoms associated with profound hyponatremia is present, however, intravenous normal saline should not be administered for the hyponatremia alone. Administration of salt poor albumin (25%), especially for those with marked fluid overload and ascites, is an effective means to expand the central blood volume without exacerbating ascites and edema. After evaluation for and/or treatment of hypovolemia, all patients should receive a Na restricted diet (2000 mg daily) and placed on fluid restriction (see below for liberalization of fluid restriction upon initiation of tolvaptan therapy).
Diagnostic paracentesis should be performed for those with ascites to rule out the presence of SBP, and antibiotics administered to those with evidence of infection. High dose intravenous salt poor albumin should also be administered, especially to those at high risk of renal failure as determined by the presence of azotemia (Cr > 1.0 mg/dL) or severe liver insufficiency (TBili > 4.0 mg/dL).56, 57 Finally, all medications should be reviewed, and those associated with hyponatremia (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors) discontinued if possible.
Tolvaptan for DH
Patient Selection
Appropriate patient selection for tolvaptan therapy is extremely important (Table 2). In the SALT trials, only clinically stable patients were enrolled. The presence of hyponatremia in a recently hospitalized cirrhotic patient, however, frequently indicates severe disease with a high risk of acute renal failure and hospital death. In the SALT trials, many received concomitant diuretic therapy. Because of the importance of avoiding tolvaptan administration to hypovolemic patients, discontinuation of diuretic therapy prior the initiation of tolvaptan therapy and/or reevaluation after limited volume expansion should be considered.
| Hospital setting |
| Euvolumia or hypervolumia |
| Absence of recent weight loss, decrease in ascites, edema |
| Absence of excessive vomiting, diarrhea |
| Consider discontinuation of diuretic therapy prior to initiation of tolvaptan |
| Consider evaluation after limited volume expansion, especially with salt poor albumin prior to initiation of tolvaptan |
| Presence of clinically significant hyponatremia: 125mEq/L or less severe but symptomatic hyponatremia (125 to 134 mEq/L) that has resisted fluid restriction |
| Absence of severe neurologic symptoms attributable to hyponatremia |
| No co‐administration with intravenous saline |
| Ability to respond to thirst |
| No co‐administration with strong CYP 3A inhibitors (ketoconazole) |
| Absence of kidney failure with anuria |
Tolvaptan is indicated for cirrhotic patients with DH in whom the serum sodium is 125 mEq/L and in those with less severe but symptomatic hyponatremia (125‐134 mEq/L) that has resisted fluid restriction. Although the definition of symptomatic was not specifically defined, possible considerations include symptoms of mild hepatic encephalopathy or inability to tolerate dieresis due to the presence of hyponatremia. According to FDA guidelines, tolvaptan therapy must be initiated and re‐initiated in a hospital setting. Patients with severe neurologic symptoms attributable to hyponatremia in whom rapid treatment is critical should not receive tolvaptan but should rather be treated with normal saline. Similarly, patients should not receive combination therapy with tolvaptan and normal saline due to potential for a too‐rapid correction of hyponatremia and the development of central pontine myelinolysis. If saline had been administered for treatment of possible hypovolemia, it should be discontinued and persistent hyponatremia confirmed before starting tolvaptan. Other factors that need to be considered before initiating tolvaptan include the ability of the patient to respond to thirst with increased water ingestion and recognition that the patient will experience increased urine volume and frequency, requiring easy access to toilet. Patients should not be fluid restricted during the first day of tolvaptan therapy, but should be instructed to respond to their thirst with increased water ingestion. As a result, caution should be exercised in administering tolvaptan to a confused, restrained, unresponsive and/or bed‐bound patient who is not able to respond appropriately to thirst or increased urination.
In the SALT trials, the incidence of hyperkalemia (5%) was similar in the tolvaptan and placebo treated patients.55 However, further analysis of all multiple‐dose, placebo‐controlled trials, demonstrated that the aggregate incidence of hyperkalemia was slightly higher for tolvaptan‐treated subjects compared with placebo‐treated subjects (Otsuka). Because treatment with tolvaptan is associated with an acute reduction of the extracellular fluid volume which could result in increased serum potassium through hemoconcentration, it is recommended that serum potassium levels be monitored after initiation of tolvaptan treatment in patients with a serum potassium > 5 mEq/L as well as those who are receiving drugs known to increase serum potassium levels such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or potassium sparing diuretics (Samsca Package Insert, Otsuka). Because tolvaptan is metabolized by the cytochrome P 3A system, patients receiving strong inhibitors such as ketoconazole should not receive tolvaptan. Anuric patients will not respond to tolvaptan. Finally, it is extremely important to administer tolvaptan only to patients with true hyponatremia and not to those with pseudohyponatremia in whom the plasma osmolality is normal but the measured serum sodium concentration artificially low due to marked elevations of other substances, such as can be seen in severe hyperglycemia, marked hyperlipidemia, or hyperproteinemia (as in multiple myeloma).
Tolvaptan Administration
The initial dose of tolvaptan is 15 mg daily. After receiving tolvaptan, many patients will develop an increased sense of thirst and need to urinate. As a result, patients should not be fluid restricted during the first day of therapy, and it is important to monitor the hemodynamics and Na level closely after initiating therapy with a repeat Na level at approximately 8 hours after the first dose. As a result, it should probably be administered early in the day and not at bedtime. The dose should be increased to 30 mg, then 60 mg in patients who do not respond by at least 5 mEq/L over the previous 24 hours and remain hyponatremic. In those with an excessive response (more than 8 meq/L during the first 8 hours or 12 meq/L on any subsequent day), the patient should be encourage to either drink more water, or the dose should be held or reduced. After the appropriate dose has been identified, the patient may be discharged and continued on tolvaptan long‐term.
With the advent of this exciting therapy, practical issues will need to be addressed, most important of which is its cost at $250 per day (Otsuka). In addition, the current recommendation to initiate tolvaptan only in a hospital further limits its widespread use. Most important, long‐term clinical benefit will need to be demonstrated. Although the SALT trials only involved treatment for up to 1 month, a multicenter, open‐label extension study for a mean duration of 701 days demonstrated that prolonged administration of tolvaptan maintains an increased serum sodium level.58 However, at this time, tolvaptan can only be considered as one of the promising drugs whose long‐term cost‐effectiveness is yet to be proven. Proof will require showing that correction of the hyponatremia leads to improved clinical outcomes, such as a reduction in length of stay or frequency of hospitalization, decreased renal failure, improved hepatic encephalopathy, deceased mortality, and improved post‐transplant outcomes.
Unanswered Questions
The vaptans provide an important opportunity to clarify the role that hyponatremia plays in the pathogenesis of cirrhosis. In the past, DH in a cirrhotic patient represented a sign of advanced disease. With the availability of safe and effective therapy, we can now determine whether it also plays an important role in the pathophysiology of end‐stage liver disease and whether its treatment will have a beneficial effect on patient outcomes.
Specific clinical questions that will inevitably be addressed over the next few years to determine whether DH is only a marker for advanced disease or whether it plays a direct but modifiable role in the pathophysiology of cirrhosis will include:
-
Role of vaptans in the management of ascites: In a 14‐day randomized, trial of a satavaptan, another selective vasopressin V(2) receptor antagonist, vs. placebo with spironolactone, combination therapy was associated with improved control of ascites and improvements in serum sodium levels in hyponatremic patients with ascites.59 If future similar studies demonstrate more prolonged benefits, this would constitute an important advance in the treatment of ascites in cirrhosis.
-
Effect on renal function: Prolonged use of tolvaptan leads to a compensatory increase in endogenous levels of AVP and, potentially, increased stimulation of V1a receptors, which might be helpful in the setting of portal hypertension. In patients with hepatorenal syndrome, vasopressin stimulation of splanchnic V1a receptors leads to improved renal function, presumably by decreasing splanchnic blood flow and improving central blood volume.60 As a result, tolvaptan may indirectly improve kidney function in patients with advanced cirrhosis and refractory ascites. Whether long‐term tolvaptan therapy will help to prevent hepatorenal syndrome through this mechanism remains to be determined but is an exciting possibility.61
-
Effect on hepatic encephalopathy: Hepatic encephalopathy is associated with poor quality of life in patients with cirrhosis. Although hepatic encephalopathy was not directly assessed in the SALT trials, the mean mental component summary of the Short Form General Health Survey, a quality of life measure, improved in cirrhotic patients receiving tolvaptan to a greater degree that those receiving placebo.62 A possible explanation for this finding is a beneficial effect of tolvaptan on hepatic encephalopathy. Confirmation of this hypothesis, however, will require prospective studies in which hepatic encephalopathy is directly assessed.
-
Effect on medical economics: Based on retrospective reviews, hyponatremia has an adverse impact on length of stay and outcomes following liver transplantation. It will be important to demonstrate in prospective studies that correction of hyponatremia with tolvaptan reduces length of stay, complications, and costs.
- ,,, et al.The aquaporin family of water channels in kidney: an update on physiology and pathophysiology of aquaporin‐2.Kidney Int.1996;49:1718–1723.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28:851–864.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48:1002–1010.
- ,.Hyponatremia.N Engl J Med.2000;342:1581–1589.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121:186–191.
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337:169–172.
- .Consequences of inadequate management of hyponatremia.Am J Nephrol.2005;25:240–249.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52:144–153.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24:1601–1608.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Supple 1):S30–S35.
- ,,.Disorders of sodium and water balance in hospitalized patients.Can J Anesth.2009;56:151–167.
- ,,, et al.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119;71.e1‐8.
- ,.Brain volume regulation in response to hypo‐osmolality and its correction.Am J Med.2006;119 (7 Suppl 1):S12–S16.
- ,,.Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes.Medicine.1976;55:121–129.
- ,.The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule.Hepatology.2006;43:S121–S131.
- ,,, et al.Hyponatremia of cirrhosis: role of vasopressin and decreased “effective” plasma volume.Scand J Gastroenterol.1997;32:829–834.
- ,,,.Metabolic clearance rate of arginine vasopressin in patients with cirrhosis.Hepatology.1992;16:974–979.
- .Water and sodium retention in edematous disorders: role of vasopressin and aldosterone.Am J Med.2006;119:S47–S53.
- ,,, et al.Circulatory function and hepatorenal syndrome in cirrhosis.Hepatology.2005;42:439–447.
- ,,, et al.Aquaporin‐2 urinary excretion in cirrhosis: relationship to vasopressin and nitric oxide.Dig Dis Sci.2010;55(4):1135–1141.
- ,,, et al.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- et al.Hyponatremia and mortality among patients on the liver‐transplant list.N Engl J Med.2009;359:1018–1026.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Clinical relevance of hyponatremia for the hospital outcome of cirrhotic patieints.Dig Liver Dis.2000;32:605–610.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol2010;44(3):220–226.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.,Validation of model for end‐stage liver disease score to serum sodium ratio index as a prognostic predictor in patients with cirrhosis.J Gastroenterol Hepatol.2009;24:1547–1553.
- ,,, et al.Hepatic venous pressure gradient can predict the development of hepatocellular carcinoma and hyponatremia in decompensated alcoholic cirrhosis.Eur J Gastroenterol Hepatol.2009;21:1241–1246.
- ,,, et al.Serum sodium predicts mortality in patients listed for liver transplantation.Hepatology.2005;41:32–39.
- ,,, et al.Effect of hyponatremia on outcomes following orthotopic liver transplantation.Liver Int.2009;29:1071–1077.
- ,,, et al.Total paracentesis in cirrhotic patients with tense ascites and dilutional hyponatremia.Am J Gastroenterol.1999;94:2219–2223.
- ,,, et al.Dilutional hyponatremia in patients with cirrhosis and ascites.Arch Intern Med.2002;162:323–328.
- ,.Therapeutic approaches to the treatment of edema and ascites: the use of diuretics.Am J Ther.2009;16:98–101.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- .Nephrotoxicities of nonsteroidal anti‐inflammatory drugs.J Formos Med Assoc.1997;96:157–171.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,.Pathogenic mechanisms of hepatic encephalopathy.Gut2008;57:1156–1165.
- ,,, et al.Effects of dilutional hyponatremia on brain organic osmolytes and water content in patients with cirrhosis.Hepatology.2004;39:1613–1622.
- .Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis.Hepatology.2006;43:1187–1190.
- ,,, et al.Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients.J Hepatol.2001;35:37–45.
- ,,, et al.Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a propective study with time‐dependent analysis.Am J Gastroenterol.2009;104:1382–1389.
- ,,, et al.Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystecim stent‐shunt for variceal haemorrhage.J Hepatol.1995;2:123–128.
- ,,, et al.Cardiac dysfunction during liver transplantation: incidence and preoperative predictors.Transplantation.2008;85:1766–1772.
- ,,, et al.,Impact of pretransplant hyponatremia on outcome following liver transplantation.Hepatology.2009;49:1610–1615.
- ,,, et al.Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation.Gastroenterology.2006;130:1135–1143.
- ,,.Hyponatremia in cirrhosis: clinical features and management.Gastroenterol Clin Biol.2006;30:1144–1151.
- ,,, et al.Intravenous albumin infusion is an effective therapy for hyponatremia in cirrhotic patients with ascites.Gut.1990;31:204–207.
- ,,, et al.Comparison of two aquaretic drugs (niravoline and OPC‐31260) in cirrhotic rats with ascites and water retention.J Pharmacol Exp Ther.1999;289:194–201.
- ,,.Plasma demeclocycline levels and nephrotoxicity. Correlation in hyponatremic cirrhotic patients.JAMA.1980;243:2513–2515.
- ,,, et al.Oral misoprostol or intravenous prostaglandin E2 do not improve renal function in patients with cirrhosis and ascites with hyponatremia and renal failure.J Hepatol.1993;17:220–226.
- ,,, et al.Effect of the V1a/V2‐AVP receptor antagonist, Conivaptan, on renal water metabolism and systemic hemodynamics in rats with cirrhosis and ascites.J Hepatol.2003;38:755–761.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al.Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis.N Engl J Med.1999;341:403–409.
- ,,, et al.Restricted use of albumin for spontaneous bacterial peritonitis.Gut.2007;56:597–599.
- ,,, et al.Oral tolvaptan is safe and effective in chronic hypyonatremia.J Am Soc Nephrol.2010;21(4):705–712.
- ,,, et al.Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial.Hepatology.2008;48:204–213.
- ,,, et al.A randomized, prospective, double‐blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome.Gastroenterology.2008;134:1360–1368.
- ,.Tolvapten and its potential in the treatment of hyponatremia.Ther Clin Risk Manag.2008;4:1149–11455.
- ,,, et al.The effects of vasopressin V2 receptor antagonist in the management of patients with cirrhosis and hyponatremia. Safety and efficacy of oral tolvaptan in the SALT trials.Hepatology.2009;50S:467A.
- ,,, et al.Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea.Nephron.1986;44:337–343.
- ,,, et al.Aquaretic effects of niravoline, a kappa‐opioid agonist, in patients with cirrhosis.J Hepatol.2000;32:38–42.
- ,, et al.The association between the serum sodium level and the severity of complications in liver cirrhosis.Korean J Intern Med.2009;24:106–112.
- ,,, et al.Nitric oxide in ascitic fluid is an independent predictor of the development of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis.Eur J Gastroenterol Hepatol.2004;16:571–577.
The serum sodium (Na) level is the major determinant of serum osmolality. In normal physiologic states is tightly regulated between 135 mEq/L to 145 mEq/L despite variable intake of water and solute through the interaction of osmoreceptors in the hypothalamus where arginine vasopressin (AVP) is synthesized and then released by the posterior pituitary and the binding of AVP with V2 AVP receptors on the basolateral surface of the principal cells within the collecting duct of the kidney. Binding of AVP to the V2 receptors promotes the translocation and fusion of cytoplasmic vesicles which carry the water channel protein aquaporin 2 (AQP2) to the apical membrane of the cell and, in this manner, increases water permeability and absorption.1, 2, 3
Patients with hyponatremia, defined by a serum Na level 135 mEq/L, can be broadly classified by their volume status into those who are euvolemic, hypervolemic, and hypovolemic (Table 1). In patients with euvolemic hyponatremia such as those with Syndrome of Inappropriate Antidiuretic Hormone (SIADH), total body Na is nearly normal, but total body water is increased. In patients with hypervolemic hyponatremia, both total body Na and water are increased, but water to a much greater degree. These patients typically have increased extracellular fluid such as edema and/or ascites. The most common conditions associated with this condition are cirrhosis, congestive heart failure (CHF), and renal failure. In contrast, hypovolemic hyponatremia is associated with a reduction in both total body Na and water, but Na to a greater degree. This condition is encountered in patients with excessive fluid losses such as those with over‐diuresis, excessive gastrointestinal losses, burns, and pancreatitis.4
| Depletional Hyponatreima | Dilutional Hyponatremia | ||
|---|---|---|---|
| Euvolumic | Hypervolumic | ||
| Total body water | |||
| Total body Na | normal | ||
| Common etiologies | SIADH | cirrhosis/CHF | vomiting, diarrhea |
Hyponatremia is the most common electrolyte abnormality seen in general hospital patients.5 In a database of over 120,000 patients, a serum sodium level of 136mEq/L was observed in 28.2%.6 Hyponatremia is associated with selected medical conditions (especially cirrhosis and CHF), the extremes of age, and those receiving selected medications, including several that are commonly administered to cirrhotic patients (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors).7, 8 Hyponatremia is associated with increased total costs per hospital admission.5, 9 In an analysis of the effect of hyponatremia on length of stay in a retrospective cohort study of hospitalized patients derived from a large administrative database of 198,281 discharges from 39 US hospitals, mean length of stay was significantly greater among patients with hyponatremia than those with normal Na levels (8.6 8.0 vs. 7.2 8.2 days). After adjusting for confounders that may be associated with more severe disease and hyponatremia (age, gender, race, geographic region, teaching status of the hospital, admission source, principal payer, comorbidity index score and primary diagnosis), the presence of hyponatremia contributed an increase in length of stay of 1.0 day. Patients with hyponatremia are more frequently admitted to the intensive care unit (ICU) and require mechanical ventilation. In patients with CHF, the presence of hyponatremia at discharge is associated with increased risk for early mortality and rehospitalization.10
Although frequently asymptomatic, hyponatremia may be associated with a range of findings, from subtle and non‐specific complaints, including headache, fatigue, confusion, malaise, to severe and life‐threatening manifestations with lethargy, seizures, brainstem herniation, respiratory arrest and death.11 The most important complications are neurologic consequences related to cerebral edema. However, there is increased morbidity even in hyponatremic patients considered to be asymptomatic. Patients with low serum sodium have attention deficients, and falls are common. In a study of 122 patients who were considered to have chronic asymptomatic hyponatremia, the incidence of falls was significantly higher at 21.3% compared to only 5.3% in a control population.12
In hyponatremia, water enters into the cells to attain osmotic balance, resulting in cellular swelling.4 To avoid cerebral edema, the brain is capable of adapting to hyponatremia by regulating its volume to avoid swelling, especially when hyponatremia is chronic. In acute hyponatremia, astrocytes and neurons adapt through osmoregulatory mechanisms by extruding intracellular electrolytes such as potassium.13 Chronically, adaption occurs through the loss of low‐molecular weight organic compounds termed organic osmolytes including myoinsoitol, glutamine, choline and taurine. As a result, both the severity and the rate of its development are critical factors in determining the neurologic manifestation of hyponatremia in a given patient.14
Dilutional Hyponatremia and Cirrhosis
Patients with hyponatremia who are either euvolemic or hypervolemic are considered to have dilutional hyponatremia (DH). Management of these patients is distinct from those who are hypovolemic in whom appropriate therapy consists of the administration of normal saline. The remainder of this article addresses the pathogenesis, management and treatment of cirrhotic patients with DH.
Pathogenesis
The development of hyponatremia in cirrhosis is intimately related to the pathophysiology of portal hypertension and the non‐osmotic release of AVP3, 15 (Figure 1). In the early phases of cirrhosis, portal hypertension is the result of an increase in intrahepatic resistance. With the development of porto‐systemic collaterals, a hyperdynamic splanchnic circulation develops as a result of splanchnic arterial vasodilatation and increased vascular capacity. Nitric oxide, an endothelial derived relaxing factor, is the critical mediator of this process, and upregulation of its expression is pivotal in the pathogenesis of portal hypertension.

Multiple factors are related to the development of DH in cirrhosis. A reduction of effective central blood volume due to the development of porto‐venous collaterals and arterial splanchnic vasodilation, leading to baroreceptor‐mediated nonosmotic release of AVP, is considered the initiating and most important factor. Patients with cirrhosis and DH have higher plasma and urine vasopressin levels, higher plasma renin activity, and decreased plasma levels of atrial natriuretic factor than those with normal serum sodium concentrations, findings consistent with the presence of a decreased effective plasma volume.16 Arterial underfilling is sensed by baroreceptors located in the left ventricle, aortic arch, carotid sinus and renal afferent arterioles. Decreased activation leads to neurohumoral compensatory responses which include non‐osmotic release of vasopressin from the neurohypophysis and increased levels. Impaired catabolism of AVP that has been correlated with the severity of liver dysfunction may further contribute to increased levels.17 Initially, the increased AVP maintains arterial circulatory integrity by inducing splanchnic, peripheral and renal arterial vasoconstriction through its action on the V1a receptors and expansion of blood volume through renal water retention by its action on the V2 receptors located on the collecting ducts.
The initial adaptive response which leads to increased central blood volume can chronically result in detrimental effects, including the development of fluid overload with ascites, edema, and hyponatremia.16, 18 Additional factors that contribute to hyponatremia include decreased glomerular filtration rate (GFR) and/or increased proximal reabsorption of sodium (that reduce the distal delivery of filtrate and the potential for water reabsorption) and decreased cardiac function that further impairs effective central blood volume.19 In addition, urinary levels of AQP2 are increased in cirrhotic patients, especially those with decompensated disease with higher Child‐Pugh scores and ascites, and provide another potential mechanism to increase water reabsorption.20
Prevalence and Prognostic Significance
Hyponatremia in cirrhosis is a common finding. In a survey of 997 cirrhotic patients with ascites from 28 centers in Europe, North and South America, the prevalence of serum sodium concentration 135, 130, 125, 120 meq/L were 49.4%, 21.6%, 5.7%, and 1.2%, respectively.21 In a retrospective analysis of 188 inpatients, the prevalence of DH of 135, 130, and 125 were 20.8%, 14.9%, and 12.2%, respectively.22 The development of hyponatremia is a manifestation of increasing portal hypertension. In a natural history study of 263 patients hospitalized for first episode of significant ascites, 74 patients developed DH (Na level 130 mEq/L), including 11 patients in whom it appeared during the first episode and 63 cases during follow‐up (mean period of 40 3 months) with a 5‐year incidence of 37.1%.23
The presence of hyponatremia carries significant adverse prognostic significance. It is strongly associated with severity of liver function impairment as assessed by Child‐Pugh and model for end‐stage liver disease (MELD) scores.22 Even mild hyponatremia is associated with severe complications such as massive ascites, severe hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), and hepatic hydrothorax, and the severity of hyponatremia is directly related to the severity of these complications.21, 22 (Figure 2). In a natural history study of patients presenting with large volume ascites, 1‐year survival after its development was reduced to only 25.6%.230


Hyponatremia is an especially poor prognostic sign for a hospitalized cirrhotic patient. In a retrospective analysis of 156 cirrhotic patients, hyponatremiapresent in 57 (29.8%) of admissionswas associated with increased hospital mortality (26.3% vs. 8.9% among those with normal Na levels), and the mortality rate was even higher (48%) among the 25 patients who developed severe hyponatremia during the hospital stay.24 In hospitalized patients, hyponatremia is predictive of the development of acute renal failure which is associated with substantially increased mortality (73% vs. 13%).25 Similarly, a low serum sodium level in critically ill cirrhotic patients admitted to the ICU is associated with complications, in‐hospital mortality, and poor short‐term prognosis.26
Whether hyponatremia should impact liver transplant prioritization remains an area of controversy. The United Network for Organ Sharing (UNOS) contracted by the Organ Procurement and Transplant Network (OPTN) to optimize the efficient use of deceased organs through fair and timely allocation, currently uses the MELD score, a formula that calculates the risk of death within three months from the bilirubin, creatinine, and International Normalized Ratio (INR) levels. Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction in patients with advanced cirrhosis and adds to MELD in predicting waitlist mortality.2729 In patients with a MELD score of 21, only low serum sodium and persistent ascites are independent predictors of mortality.28 To account for the importance of hyponatremia on survival, both modification of the MELD score in which the Na level is incorporated (MELD‐Na model) and the MELD to serum sodium ratio (MESO) have been developed. Adding hyponatremia to the MELD score is a better predictor of death than MELD alone, particularly in patients with low MELD scores.27, 2931 The OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has discussed updating the liver allocation system to include the Na level. However, it was concluded that implementation of MELD‐Na would change the allocation status of only 4% of candidates. Further, based on the concerns about the ability to manipulate serum sodium levels and the utility of employing resources to change the system for a relatively small number of patients, it was decided to defer incorporating the Na level pending further analysis (Report of the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee To the Board of Directors, Los Angeles, California, September 17‐18, 2007). At this time, the use of Na is a regional decision.32 However, the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has recently solicited feedback from the transplant community about including Na in allocation for review at a forum in April 2010.
Precipitating Factors
The most important factor related to development of hyponatremia in cirrhosis is increasing severity of portal hypertension that is associated with impaired central blood volume as a result of progressive splanchnic vasodilatation. In a study in which 170 patients with decompensated alcoholic cirrhosis were prospectively followed for 33.9 27.9 months, the initial hepatic venous pressure gradient (HVPG) was an independent predictive factor for the 20 patients who developed hyponatremia.22
Cirrhotic patients with ascites with hyponatremia have increased AVP secretion, higher levels of plasma renin activity, and higher serum concentrations of aldosterone and norepinephrine compared to those with normal Na levels.33 Diuretic therapy is associated with the development of DH by inducing volume depletion and arterial underfilling, further activating the renin‐angiotensin system (RAS) and increasing the non‐osmotic release of AVP.34 Although diuretics block the salt retention associated with the RAS activation, the water‐retaining effects of AVP persist, and DH develops. The process is further exacerbated by a low sodium intake and a frequent uncontrollable thirst. As a result, diuretic therapy is commonly associated with the development of hyponatremia in patients with ascites.24, 35 Similarly, paracentesis (particularly when performed without albumin) is often associated with an increase in blood urea nitrogen and marked elevations in plasma renin activity and plasma aldosterone concentration, which may exacerbate this physiology, leading to further reduction in serum sodium concentration.36 Tense ascites can contribute to DH by increasing baroreceptor mediated AVP release by increasing intrathoracic pressure.37 Finally, non‐steroidal anti‐inflammatory drugs (NSAIDs) can cause DH by inhibiting the synthesis of renal prostaglandins (which normally function to antagonize the tubular action of AVP and are important in the maintenance of appropriate renal tubular transport of fluid and electrolytes in states of renal hypoperfusion).38
Medical Impact of Hyponatremia: Marker of Severe Disease or Direct Pathophysiologic Role?
Hyponatremia is associated with severe ascites, impaired renal function, hepatic encephalopathy, SBP, and hepatorenal syndrome.3, 20 Because hyponatremia is frequently present in advanced liver failure, it is unclear whether it is only a marker of advanced disease or whether it plays a direct pathophysiologic role, or both. Until recently, it has not been possible to address this issue due to the inability to easily and rapidly correct the hyponatremia. However, there is increasing evidence that hyponatremia has direct impact on the severity of hepatic encephalopathy (see Hepatic Encephalopathy section). The recent introduction of tolvaptan for the treatment of hyponatremia in cirrhosis (discussed below) will allow this question to be directly answered.
Fluid Management and Diuresis
The typical cirrhotic patient with DH is characterized by expanded extracellular fluid with ascites and edema. The profound vasodilation of the splanchnic arterial circulation is associated with decreased effective arterial blood volume, leading to the non‐osmotic release of AVP. Diuretic therapy can further exacerbate this process. In addition, the increased water permeability induced by AVP results in reduced urine volume and fluid retention. As a result, hyponatremia directly adversely affects severity of fluid overload and limits and/or precludes diuretic treatment.
Hepatorenal Syndrome
Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction and is frequently a precursor to overt hepatorenal syndrome.27 Hyponatremia is predictive of the development of acute renal failure during hospitalization, and in‐hospital development of acute renal failure portends a high mortality.25 In patients admitted with SBP, the presence of hyponatremia is significantly associated with higher mortality and renal failure.39
Hepatic Encephalopathy
The neurologic manifestations of cerebral edema associated with hyponatremia closely mirror those of hepatic encephalopathy. In fact, a recently proposed pathogenic mechanism for hepatic encephalopathy is the development of low‐grade cerebral edema associated with astrocyte swelling in response to ammonia and other precipitating factors.40 DH is associated with a further reduction in brain organic osmolytes that probably reflects a compensatory osmoregulatory mechanism against cell swelling triggered by a combination of high intracellular glutamine and low extracellular osmolality.41 As a result, it has been proposed that hyponatremia contributes to the development of hepatic encephalopathy through the development or exacerbation of low‐grade cerebral edema. In this manner, low serum sodium acts as a second hit to the swelling produced by increased intracellular glutamine created by ammonia metabolism.42
Clinically, hyponatremia is a major risk factor for hepatic encephalopathy. Serum sodium and ammonia levels are the major factors that predict electroencephalographic abnormalities in cirrhotics who do not have hepatic encephalopathy.43 In a prospective study of 61 patients, hyponatremia was associated with a low brain concentration of organic osmolytes as assessed by proton magnetic resonance spectroscopy (1H‐MRS) and magnetic resonance imaging, and both conditions were major risk factors for the development of overt hepatic encephalopathy.44 Finally, hyponatremia is a risk factor for hepatic encephalopathy in patients undergoing TIPS.45
Adverse Effect on Outcome After Liver Transplantation
Hyponatremia before liver transplantation is associated with adverse post‐transplant outcomes. Among patients undergoing liver transplantation, the presence of hyponatremia is associated with abnormal cardiac response in patients after reperfusion.46 Pre‐transplant hyponatremia is associated with longer ICU and hospital stay, higher rates of delirium and neurologic disorders, acute renal failure, acute cellular rejection, infection, and in one study a reduced 3‐month survival compared to normonatremic recipients.32, 47, 48 In 1 retrospective study that compared post‐transplant outcomes of patients with corrected vs. uncorrected pre‐transplant hyponatremia, patients with pre‐operative correction of hyponatremia had a lower risk of prolonged post‐transplant hospitalization than those with uncorrected hyponatremia.32 However, both hyponatremic groups had more complicated post‐transplant courses compared to those without a history of hyponatremia. However, given the small sample size, retrospective design, and the potential for confounding, the impact of correction of pre‐transplant hyponatremia remains to be determined.
Management
Most patients with mild hypervolemic hyponatremia are asymptomatic. The initial recommended approach is fluid restriction and an Na‐restricted diet. For those with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion.49 For patients with tense ascites and severe DH, therapeutic paracentesis with plasma expanders is safe.33 Unfortunately, fluid restriction is limited in efficacy and often poorly tolerated. The use of hypertonic saline is generally not recommended unless severe neurologic symptoms are present as it leads to increased ascites and edema. When administered, it is important to avoid a rapid correction of the hyponatremia to prevent the development of central pontine myelinolysis and the osmotic demyelination syndrome.
Due to the pivotal role of AVP in the pathogenesis of DH, antagonism of its action has long been proposed to be the most rational approach, but until recently, effective and specific antagonism of AVP has remained elusive. Approaches that have been attempted include interference with its secretion and actions. Intravenous albumin has been reported to improve hyponatremia in patients with cirrhosis, ascites, and hyponatremia, presumably by decreasing AVP release by plasma volume expansion.50 An attempt at inhibition of central AVP release with the use of a kappa‐opioid receptor agonist, niravoline, was limited by loss of efficacy and potential adverse effects.51 Use of demeclocycline and lithium (which induce renal resistance to AVP and lead to a modest increase in urine volume with decreased urine osmolality and a corresponding rise in serum sodium) is limited by nephrotoxicity and hepatotoxicity.7, 52 Because of the important role played by prostaglandins in the maintenance of renal hemodynamics and water excretion in cirrhosis, oral misoprostol has also been evaluated but determined to be ineffective in inducing significant changes in free water clearance in patients with functional renal failure and/or DH.53
The recent introduction of vaptans, vasopressin receptor antagonists that block the physiologic action of vasopressin, represents a revolutionary and highly effective approach to the treatment of hyponatremia. Vaptans are antagonists of the V2 receptors of AVP in the principal cells of the collecting ducts. In healthy subjects, vaptans cause a dose‐dependent increase in urine volume and produce a dilute urine without causing natriuresis. To date, 2 AVP antagonists, conivaptan and tolvaptan, have been Food and Drug Administration (FDA)‐approved for the treatment of DH. Conivaptan, the first to be approved in 2005, is a mixed vasopressin V1a and V2 receptor antagonist that is administered intravenously for up to 4 days. In a randomized placebo‐controlled study of patients with euvolemic or hypervolemic hyponatremia, intravenous conivaptan treatment increased serum Na levels by >6 mEq/l or to a serum Na >135 mEq/l in 69 to 88.5% of subjects compared to 20.7% of those receiving placebo (Zeltser D, Rosansky S, Van Rensburg H, et al. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. American J Nephrology 2007;27:447457). In a pilot study involving 24 patients with end‐stage liver disease, an infusion of conivaptan over 1 to 4 days was associated with an increase of serum sodium by >5 mmol/L in 60% of patients not receiving diuretics and 67% of patients on concomitant diuretic therapy by the end of treatment (O'Leary and Davis, 2009). Despite a concern about the potential for conivaptan to increase portal hypertension due to inhibition of splanchnic V1a receptors, the brief treatment appeared to be well tolerated without significant changes in systolic blood pressure, serum creatinine, variceal bleeding or worsening of ascites during the infusion period. However, approval for only 4 days of therapy and requirement for intravenous use eliminate any potential for chronic use.54
Tolvaptan is an orally available, selective V2 receptor antagonist whose efficacy was assessed in two multicenter, prospective, randomized, placebo‐controlled trials, Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).55 In these trials, clinically stable patients with DH (Na 135meq/l) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), CHF or SIADH were randomized in a hospital setting to receive tolvaptan 15mg daily or placebo. Repeat Na levels were obtained at 8 hours, 2, 3, and 4 days and then weekly at days 11, 18, 25 and 30 after which study drug was discontinued and follow‐up Na level was determined 7 days later. The dose was adjusted to 30 mg and then 60 mg in an attempt to achieve a Na level >135 in those in whom hyponatremia persisted. During the initial day of the titration phase, fluid restriction was not maintained, and the patients were encouraged to respond to thirst with increased water ingestion.
Tolvaptan use was associated with a prompt increase in Na level as early as 8 hours after administration of the first dose. Serum Na increased more among those receiving tolvaptan than among those receiving placebo during the first 4 days and throughout the study period regardless of baseline Na level but returned to baseline within 1 week after discontinuation (Figure 3). The main side effects were increased thirst, dry mouth and increased urination. Importantly, an increased incidence of renal failure was not observed. Based on these results, FDA approval for tolvaptan in patients with hyponatremia was obtained in May 2009 for patients with DH‐associated with cirrhosis, CHF or SIADH for patients with Na levels 125 or symptomatic patients with Na levels between 125 and 135 that have not responded to fluid restriction.
Management of the Hospitalized Cirrhotic Patient With Hyponatremia: Recommendations
Hyponatremia in hospitalized cirrhotic patients is a marker for severe disease and high risk of hospital mortality.24 As a result, prompt evaluation and treatment is imperative. The availability of tolvaptan potentially revolutionizes the manner in which these patients are treated. In the SALT trials, only clinically stable patients were enrolled. In this last section, a guideline for the evaluation and treatment of acutely ill, hospitalized cirrhotic patients with DH is presented.
Evaluation
Determination of volume status is paramount but frequently problematic in the hospitalized cirrhotic patient. Due to the vasodilated state present in severe portal hypertension that is characterized by a relative hypotension and resting tachycardia, the usual hemodynamic parameters of blood pressure and heart rate can be difficult to interpret. Although significant extravascular volume in the form of ascites and edema may be present, patients may be intravascularly depleted due to previous diuretic use and extra‐renal losses due to impaired oral intake, vomiting, lactulose‐induced diarrhea, and gastrointestinal bleeding. Infection is a commonly associated condition, and endotoxin mediated splanchnic vasodilatation, especially in the setting of SBP, can adversely effective central blood volume status in the presence of severe ascites. Also, due to the Na avidity of the kidney and previous diuretic use, renal electrolytes can be difficult to interpret.
For patients in whom there is strong clinical concern about intravascular depletion (history of impaired oral intake, excessive vomiting and/or diarrhea, rapid weight loss, small volume ascites with history of large volume, azotemia), administration of limited intravenous normal saline (0.5‐1 L) should be considered. Patients with severe neurological symptoms should receive normal saline or hypertonic saline. Unless severe neurologic symptoms associated with profound hyponatremia is present, however, intravenous normal saline should not be administered for the hyponatremia alone. Administration of salt poor albumin (25%), especially for those with marked fluid overload and ascites, is an effective means to expand the central blood volume without exacerbating ascites and edema. After evaluation for and/or treatment of hypovolemia, all patients should receive a Na restricted diet (2000 mg daily) and placed on fluid restriction (see below for liberalization of fluid restriction upon initiation of tolvaptan therapy).
Diagnostic paracentesis should be performed for those with ascites to rule out the presence of SBP, and antibiotics administered to those with evidence of infection. High dose intravenous salt poor albumin should also be administered, especially to those at high risk of renal failure as determined by the presence of azotemia (Cr > 1.0 mg/dL) or severe liver insufficiency (TBili > 4.0 mg/dL).56, 57 Finally, all medications should be reviewed, and those associated with hyponatremia (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors) discontinued if possible.
Tolvaptan for DH
Patient Selection
Appropriate patient selection for tolvaptan therapy is extremely important (Table 2). In the SALT trials, only clinically stable patients were enrolled. The presence of hyponatremia in a recently hospitalized cirrhotic patient, however, frequently indicates severe disease with a high risk of acute renal failure and hospital death. In the SALT trials, many received concomitant diuretic therapy. Because of the importance of avoiding tolvaptan administration to hypovolemic patients, discontinuation of diuretic therapy prior the initiation of tolvaptan therapy and/or reevaluation after limited volume expansion should be considered.
| Hospital setting |
| Euvolumia or hypervolumia |
| Absence of recent weight loss, decrease in ascites, edema |
| Absence of excessive vomiting, diarrhea |
| Consider discontinuation of diuretic therapy prior to initiation of tolvaptan |
| Consider evaluation after limited volume expansion, especially with salt poor albumin prior to initiation of tolvaptan |
| Presence of clinically significant hyponatremia: 125mEq/L or less severe but symptomatic hyponatremia (125 to 134 mEq/L) that has resisted fluid restriction |
| Absence of severe neurologic symptoms attributable to hyponatremia |
| No co‐administration with intravenous saline |
| Ability to respond to thirst |
| No co‐administration with strong CYP 3A inhibitors (ketoconazole) |
| Absence of kidney failure with anuria |
Tolvaptan is indicated for cirrhotic patients with DH in whom the serum sodium is 125 mEq/L and in those with less severe but symptomatic hyponatremia (125‐134 mEq/L) that has resisted fluid restriction. Although the definition of symptomatic was not specifically defined, possible considerations include symptoms of mild hepatic encephalopathy or inability to tolerate dieresis due to the presence of hyponatremia. According to FDA guidelines, tolvaptan therapy must be initiated and re‐initiated in a hospital setting. Patients with severe neurologic symptoms attributable to hyponatremia in whom rapid treatment is critical should not receive tolvaptan but should rather be treated with normal saline. Similarly, patients should not receive combination therapy with tolvaptan and normal saline due to potential for a too‐rapid correction of hyponatremia and the development of central pontine myelinolysis. If saline had been administered for treatment of possible hypovolemia, it should be discontinued and persistent hyponatremia confirmed before starting tolvaptan. Other factors that need to be considered before initiating tolvaptan include the ability of the patient to respond to thirst with increased water ingestion and recognition that the patient will experience increased urine volume and frequency, requiring easy access to toilet. Patients should not be fluid restricted during the first day of tolvaptan therapy, but should be instructed to respond to their thirst with increased water ingestion. As a result, caution should be exercised in administering tolvaptan to a confused, restrained, unresponsive and/or bed‐bound patient who is not able to respond appropriately to thirst or increased urination.
In the SALT trials, the incidence of hyperkalemia (5%) was similar in the tolvaptan and placebo treated patients.55 However, further analysis of all multiple‐dose, placebo‐controlled trials, demonstrated that the aggregate incidence of hyperkalemia was slightly higher for tolvaptan‐treated subjects compared with placebo‐treated subjects (Otsuka). Because treatment with tolvaptan is associated with an acute reduction of the extracellular fluid volume which could result in increased serum potassium through hemoconcentration, it is recommended that serum potassium levels be monitored after initiation of tolvaptan treatment in patients with a serum potassium > 5 mEq/L as well as those who are receiving drugs known to increase serum potassium levels such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or potassium sparing diuretics (Samsca Package Insert, Otsuka). Because tolvaptan is metabolized by the cytochrome P 3A system, patients receiving strong inhibitors such as ketoconazole should not receive tolvaptan. Anuric patients will not respond to tolvaptan. Finally, it is extremely important to administer tolvaptan only to patients with true hyponatremia and not to those with pseudohyponatremia in whom the plasma osmolality is normal but the measured serum sodium concentration artificially low due to marked elevations of other substances, such as can be seen in severe hyperglycemia, marked hyperlipidemia, or hyperproteinemia (as in multiple myeloma).
Tolvaptan Administration
The initial dose of tolvaptan is 15 mg daily. After receiving tolvaptan, many patients will develop an increased sense of thirst and need to urinate. As a result, patients should not be fluid restricted during the first day of therapy, and it is important to monitor the hemodynamics and Na level closely after initiating therapy with a repeat Na level at approximately 8 hours after the first dose. As a result, it should probably be administered early in the day and not at bedtime. The dose should be increased to 30 mg, then 60 mg in patients who do not respond by at least 5 mEq/L over the previous 24 hours and remain hyponatremic. In those with an excessive response (more than 8 meq/L during the first 8 hours or 12 meq/L on any subsequent day), the patient should be encourage to either drink more water, or the dose should be held or reduced. After the appropriate dose has been identified, the patient may be discharged and continued on tolvaptan long‐term.
With the advent of this exciting therapy, practical issues will need to be addressed, most important of which is its cost at $250 per day (Otsuka). In addition, the current recommendation to initiate tolvaptan only in a hospital further limits its widespread use. Most important, long‐term clinical benefit will need to be demonstrated. Although the SALT trials only involved treatment for up to 1 month, a multicenter, open‐label extension study for a mean duration of 701 days demonstrated that prolonged administration of tolvaptan maintains an increased serum sodium level.58 However, at this time, tolvaptan can only be considered as one of the promising drugs whose long‐term cost‐effectiveness is yet to be proven. Proof will require showing that correction of the hyponatremia leads to improved clinical outcomes, such as a reduction in length of stay or frequency of hospitalization, decreased renal failure, improved hepatic encephalopathy, deceased mortality, and improved post‐transplant outcomes.
Unanswered Questions
The vaptans provide an important opportunity to clarify the role that hyponatremia plays in the pathogenesis of cirrhosis. In the past, DH in a cirrhotic patient represented a sign of advanced disease. With the availability of safe and effective therapy, we can now determine whether it also plays an important role in the pathophysiology of end‐stage liver disease and whether its treatment will have a beneficial effect on patient outcomes.
Specific clinical questions that will inevitably be addressed over the next few years to determine whether DH is only a marker for advanced disease or whether it plays a direct but modifiable role in the pathophysiology of cirrhosis will include:
-
Role of vaptans in the management of ascites: In a 14‐day randomized, trial of a satavaptan, another selective vasopressin V(2) receptor antagonist, vs. placebo with spironolactone, combination therapy was associated with improved control of ascites and improvements in serum sodium levels in hyponatremic patients with ascites.59 If future similar studies demonstrate more prolonged benefits, this would constitute an important advance in the treatment of ascites in cirrhosis.
-
Effect on renal function: Prolonged use of tolvaptan leads to a compensatory increase in endogenous levels of AVP and, potentially, increased stimulation of V1a receptors, which might be helpful in the setting of portal hypertension. In patients with hepatorenal syndrome, vasopressin stimulation of splanchnic V1a receptors leads to improved renal function, presumably by decreasing splanchnic blood flow and improving central blood volume.60 As a result, tolvaptan may indirectly improve kidney function in patients with advanced cirrhosis and refractory ascites. Whether long‐term tolvaptan therapy will help to prevent hepatorenal syndrome through this mechanism remains to be determined but is an exciting possibility.61
-
Effect on hepatic encephalopathy: Hepatic encephalopathy is associated with poor quality of life in patients with cirrhosis. Although hepatic encephalopathy was not directly assessed in the SALT trials, the mean mental component summary of the Short Form General Health Survey, a quality of life measure, improved in cirrhotic patients receiving tolvaptan to a greater degree that those receiving placebo.62 A possible explanation for this finding is a beneficial effect of tolvaptan on hepatic encephalopathy. Confirmation of this hypothesis, however, will require prospective studies in which hepatic encephalopathy is directly assessed.
-
Effect on medical economics: Based on retrospective reviews, hyponatremia has an adverse impact on length of stay and outcomes following liver transplantation. It will be important to demonstrate in prospective studies that correction of hyponatremia with tolvaptan reduces length of stay, complications, and costs.
The serum sodium (Na) level is the major determinant of serum osmolality. In normal physiologic states is tightly regulated between 135 mEq/L to 145 mEq/L despite variable intake of water and solute through the interaction of osmoreceptors in the hypothalamus where arginine vasopressin (AVP) is synthesized and then released by the posterior pituitary and the binding of AVP with V2 AVP receptors on the basolateral surface of the principal cells within the collecting duct of the kidney. Binding of AVP to the V2 receptors promotes the translocation and fusion of cytoplasmic vesicles which carry the water channel protein aquaporin 2 (AQP2) to the apical membrane of the cell and, in this manner, increases water permeability and absorption.1, 2, 3
Patients with hyponatremia, defined by a serum Na level 135 mEq/L, can be broadly classified by their volume status into those who are euvolemic, hypervolemic, and hypovolemic (Table 1). In patients with euvolemic hyponatremia such as those with Syndrome of Inappropriate Antidiuretic Hormone (SIADH), total body Na is nearly normal, but total body water is increased. In patients with hypervolemic hyponatremia, both total body Na and water are increased, but water to a much greater degree. These patients typically have increased extracellular fluid such as edema and/or ascites. The most common conditions associated with this condition are cirrhosis, congestive heart failure (CHF), and renal failure. In contrast, hypovolemic hyponatremia is associated with a reduction in both total body Na and water, but Na to a greater degree. This condition is encountered in patients with excessive fluid losses such as those with over‐diuresis, excessive gastrointestinal losses, burns, and pancreatitis.4
| Depletional Hyponatreima | Dilutional Hyponatremia | ||
|---|---|---|---|
| Euvolumic | Hypervolumic | ||
| Total body water | |||
| Total body Na | normal | ||
| Common etiologies | SIADH | cirrhosis/CHF | vomiting, diarrhea |
Hyponatremia is the most common electrolyte abnormality seen in general hospital patients.5 In a database of over 120,000 patients, a serum sodium level of 136mEq/L was observed in 28.2%.6 Hyponatremia is associated with selected medical conditions (especially cirrhosis and CHF), the extremes of age, and those receiving selected medications, including several that are commonly administered to cirrhotic patients (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors).7, 8 Hyponatremia is associated with increased total costs per hospital admission.5, 9 In an analysis of the effect of hyponatremia on length of stay in a retrospective cohort study of hospitalized patients derived from a large administrative database of 198,281 discharges from 39 US hospitals, mean length of stay was significantly greater among patients with hyponatremia than those with normal Na levels (8.6 8.0 vs. 7.2 8.2 days). After adjusting for confounders that may be associated with more severe disease and hyponatremia (age, gender, race, geographic region, teaching status of the hospital, admission source, principal payer, comorbidity index score and primary diagnosis), the presence of hyponatremia contributed an increase in length of stay of 1.0 day. Patients with hyponatremia are more frequently admitted to the intensive care unit (ICU) and require mechanical ventilation. In patients with CHF, the presence of hyponatremia at discharge is associated with increased risk for early mortality and rehospitalization.10
Although frequently asymptomatic, hyponatremia may be associated with a range of findings, from subtle and non‐specific complaints, including headache, fatigue, confusion, malaise, to severe and life‐threatening manifestations with lethargy, seizures, brainstem herniation, respiratory arrest and death.11 The most important complications are neurologic consequences related to cerebral edema. However, there is increased morbidity even in hyponatremic patients considered to be asymptomatic. Patients with low serum sodium have attention deficients, and falls are common. In a study of 122 patients who were considered to have chronic asymptomatic hyponatremia, the incidence of falls was significantly higher at 21.3% compared to only 5.3% in a control population.12
In hyponatremia, water enters into the cells to attain osmotic balance, resulting in cellular swelling.4 To avoid cerebral edema, the brain is capable of adapting to hyponatremia by regulating its volume to avoid swelling, especially when hyponatremia is chronic. In acute hyponatremia, astrocytes and neurons adapt through osmoregulatory mechanisms by extruding intracellular electrolytes such as potassium.13 Chronically, adaption occurs through the loss of low‐molecular weight organic compounds termed organic osmolytes including myoinsoitol, glutamine, choline and taurine. As a result, both the severity and the rate of its development are critical factors in determining the neurologic manifestation of hyponatremia in a given patient.14
Dilutional Hyponatremia and Cirrhosis
Patients with hyponatremia who are either euvolemic or hypervolemic are considered to have dilutional hyponatremia (DH). Management of these patients is distinct from those who are hypovolemic in whom appropriate therapy consists of the administration of normal saline. The remainder of this article addresses the pathogenesis, management and treatment of cirrhotic patients with DH.
Pathogenesis
The development of hyponatremia in cirrhosis is intimately related to the pathophysiology of portal hypertension and the non‐osmotic release of AVP3, 15 (Figure 1). In the early phases of cirrhosis, portal hypertension is the result of an increase in intrahepatic resistance. With the development of porto‐systemic collaterals, a hyperdynamic splanchnic circulation develops as a result of splanchnic arterial vasodilatation and increased vascular capacity. Nitric oxide, an endothelial derived relaxing factor, is the critical mediator of this process, and upregulation of its expression is pivotal in the pathogenesis of portal hypertension.

Multiple factors are related to the development of DH in cirrhosis. A reduction of effective central blood volume due to the development of porto‐venous collaterals and arterial splanchnic vasodilation, leading to baroreceptor‐mediated nonosmotic release of AVP, is considered the initiating and most important factor. Patients with cirrhosis and DH have higher plasma and urine vasopressin levels, higher plasma renin activity, and decreased plasma levels of atrial natriuretic factor than those with normal serum sodium concentrations, findings consistent with the presence of a decreased effective plasma volume.16 Arterial underfilling is sensed by baroreceptors located in the left ventricle, aortic arch, carotid sinus and renal afferent arterioles. Decreased activation leads to neurohumoral compensatory responses which include non‐osmotic release of vasopressin from the neurohypophysis and increased levels. Impaired catabolism of AVP that has been correlated with the severity of liver dysfunction may further contribute to increased levels.17 Initially, the increased AVP maintains arterial circulatory integrity by inducing splanchnic, peripheral and renal arterial vasoconstriction through its action on the V1a receptors and expansion of blood volume through renal water retention by its action on the V2 receptors located on the collecting ducts.
The initial adaptive response which leads to increased central blood volume can chronically result in detrimental effects, including the development of fluid overload with ascites, edema, and hyponatremia.16, 18 Additional factors that contribute to hyponatremia include decreased glomerular filtration rate (GFR) and/or increased proximal reabsorption of sodium (that reduce the distal delivery of filtrate and the potential for water reabsorption) and decreased cardiac function that further impairs effective central blood volume.19 In addition, urinary levels of AQP2 are increased in cirrhotic patients, especially those with decompensated disease with higher Child‐Pugh scores and ascites, and provide another potential mechanism to increase water reabsorption.20
Prevalence and Prognostic Significance
Hyponatremia in cirrhosis is a common finding. In a survey of 997 cirrhotic patients with ascites from 28 centers in Europe, North and South America, the prevalence of serum sodium concentration 135, 130, 125, 120 meq/L were 49.4%, 21.6%, 5.7%, and 1.2%, respectively.21 In a retrospective analysis of 188 inpatients, the prevalence of DH of 135, 130, and 125 were 20.8%, 14.9%, and 12.2%, respectively.22 The development of hyponatremia is a manifestation of increasing portal hypertension. In a natural history study of 263 patients hospitalized for first episode of significant ascites, 74 patients developed DH (Na level 130 mEq/L), including 11 patients in whom it appeared during the first episode and 63 cases during follow‐up (mean period of 40 3 months) with a 5‐year incidence of 37.1%.23
The presence of hyponatremia carries significant adverse prognostic significance. It is strongly associated with severity of liver function impairment as assessed by Child‐Pugh and model for end‐stage liver disease (MELD) scores.22 Even mild hyponatremia is associated with severe complications such as massive ascites, severe hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), and hepatic hydrothorax, and the severity of hyponatremia is directly related to the severity of these complications.21, 22 (Figure 2). In a natural history study of patients presenting with large volume ascites, 1‐year survival after its development was reduced to only 25.6%.230


Hyponatremia is an especially poor prognostic sign for a hospitalized cirrhotic patient. In a retrospective analysis of 156 cirrhotic patients, hyponatremiapresent in 57 (29.8%) of admissionswas associated with increased hospital mortality (26.3% vs. 8.9% among those with normal Na levels), and the mortality rate was even higher (48%) among the 25 patients who developed severe hyponatremia during the hospital stay.24 In hospitalized patients, hyponatremia is predictive of the development of acute renal failure which is associated with substantially increased mortality (73% vs. 13%).25 Similarly, a low serum sodium level in critically ill cirrhotic patients admitted to the ICU is associated with complications, in‐hospital mortality, and poor short‐term prognosis.26
Whether hyponatremia should impact liver transplant prioritization remains an area of controversy. The United Network for Organ Sharing (UNOS) contracted by the Organ Procurement and Transplant Network (OPTN) to optimize the efficient use of deceased organs through fair and timely allocation, currently uses the MELD score, a formula that calculates the risk of death within three months from the bilirubin, creatinine, and International Normalized Ratio (INR) levels. Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction in patients with advanced cirrhosis and adds to MELD in predicting waitlist mortality.2729 In patients with a MELD score of 21, only low serum sodium and persistent ascites are independent predictors of mortality.28 To account for the importance of hyponatremia on survival, both modification of the MELD score in which the Na level is incorporated (MELD‐Na model) and the MELD to serum sodium ratio (MESO) have been developed. Adding hyponatremia to the MELD score is a better predictor of death than MELD alone, particularly in patients with low MELD scores.27, 2931 The OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has discussed updating the liver allocation system to include the Na level. However, it was concluded that implementation of MELD‐Na would change the allocation status of only 4% of candidates. Further, based on the concerns about the ability to manipulate serum sodium levels and the utility of employing resources to change the system for a relatively small number of patients, it was decided to defer incorporating the Na level pending further analysis (Report of the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee To the Board of Directors, Los Angeles, California, September 17‐18, 2007). At this time, the use of Na is a regional decision.32 However, the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee has recently solicited feedback from the transplant community about including Na in allocation for review at a forum in April 2010.
Precipitating Factors
The most important factor related to development of hyponatremia in cirrhosis is increasing severity of portal hypertension that is associated with impaired central blood volume as a result of progressive splanchnic vasodilatation. In a study in which 170 patients with decompensated alcoholic cirrhosis were prospectively followed for 33.9 27.9 months, the initial hepatic venous pressure gradient (HVPG) was an independent predictive factor for the 20 patients who developed hyponatremia.22
Cirrhotic patients with ascites with hyponatremia have increased AVP secretion, higher levels of plasma renin activity, and higher serum concentrations of aldosterone and norepinephrine compared to those with normal Na levels.33 Diuretic therapy is associated with the development of DH by inducing volume depletion and arterial underfilling, further activating the renin‐angiotensin system (RAS) and increasing the non‐osmotic release of AVP.34 Although diuretics block the salt retention associated with the RAS activation, the water‐retaining effects of AVP persist, and DH develops. The process is further exacerbated by a low sodium intake and a frequent uncontrollable thirst. As a result, diuretic therapy is commonly associated with the development of hyponatremia in patients with ascites.24, 35 Similarly, paracentesis (particularly when performed without albumin) is often associated with an increase in blood urea nitrogen and marked elevations in plasma renin activity and plasma aldosterone concentration, which may exacerbate this physiology, leading to further reduction in serum sodium concentration.36 Tense ascites can contribute to DH by increasing baroreceptor mediated AVP release by increasing intrathoracic pressure.37 Finally, non‐steroidal anti‐inflammatory drugs (NSAIDs) can cause DH by inhibiting the synthesis of renal prostaglandins (which normally function to antagonize the tubular action of AVP and are important in the maintenance of appropriate renal tubular transport of fluid and electrolytes in states of renal hypoperfusion).38
Medical Impact of Hyponatremia: Marker of Severe Disease or Direct Pathophysiologic Role?
Hyponatremia is associated with severe ascites, impaired renal function, hepatic encephalopathy, SBP, and hepatorenal syndrome.3, 20 Because hyponatremia is frequently present in advanced liver failure, it is unclear whether it is only a marker of advanced disease or whether it plays a direct pathophysiologic role, or both. Until recently, it has not been possible to address this issue due to the inability to easily and rapidly correct the hyponatremia. However, there is increasing evidence that hyponatremia has direct impact on the severity of hepatic encephalopathy (see Hepatic Encephalopathy section). The recent introduction of tolvaptan for the treatment of hyponatremia in cirrhosis (discussed below) will allow this question to be directly answered.
Fluid Management and Diuresis
The typical cirrhotic patient with DH is characterized by expanded extracellular fluid with ascites and edema. The profound vasodilation of the splanchnic arterial circulation is associated with decreased effective arterial blood volume, leading to the non‐osmotic release of AVP. Diuretic therapy can further exacerbate this process. In addition, the increased water permeability induced by AVP results in reduced urine volume and fluid retention. As a result, hyponatremia directly adversely affects severity of fluid overload and limits and/or precludes diuretic treatment.
Hepatorenal Syndrome
Hyponatremia is an earlier and more sensitive marker than serum creatinine to detect renal impairment and/or circulatory dysfunction and is frequently a precursor to overt hepatorenal syndrome.27 Hyponatremia is predictive of the development of acute renal failure during hospitalization, and in‐hospital development of acute renal failure portends a high mortality.25 In patients admitted with SBP, the presence of hyponatremia is significantly associated with higher mortality and renal failure.39
Hepatic Encephalopathy
The neurologic manifestations of cerebral edema associated with hyponatremia closely mirror those of hepatic encephalopathy. In fact, a recently proposed pathogenic mechanism for hepatic encephalopathy is the development of low‐grade cerebral edema associated with astrocyte swelling in response to ammonia and other precipitating factors.40 DH is associated with a further reduction in brain organic osmolytes that probably reflects a compensatory osmoregulatory mechanism against cell swelling triggered by a combination of high intracellular glutamine and low extracellular osmolality.41 As a result, it has been proposed that hyponatremia contributes to the development of hepatic encephalopathy through the development or exacerbation of low‐grade cerebral edema. In this manner, low serum sodium acts as a second hit to the swelling produced by increased intracellular glutamine created by ammonia metabolism.42
Clinically, hyponatremia is a major risk factor for hepatic encephalopathy. Serum sodium and ammonia levels are the major factors that predict electroencephalographic abnormalities in cirrhotics who do not have hepatic encephalopathy.43 In a prospective study of 61 patients, hyponatremia was associated with a low brain concentration of organic osmolytes as assessed by proton magnetic resonance spectroscopy (1H‐MRS) and magnetic resonance imaging, and both conditions were major risk factors for the development of overt hepatic encephalopathy.44 Finally, hyponatremia is a risk factor for hepatic encephalopathy in patients undergoing TIPS.45
Adverse Effect on Outcome After Liver Transplantation
Hyponatremia before liver transplantation is associated with adverse post‐transplant outcomes. Among patients undergoing liver transplantation, the presence of hyponatremia is associated with abnormal cardiac response in patients after reperfusion.46 Pre‐transplant hyponatremia is associated with longer ICU and hospital stay, higher rates of delirium and neurologic disorders, acute renal failure, acute cellular rejection, infection, and in one study a reduced 3‐month survival compared to normonatremic recipients.32, 47, 48 In 1 retrospective study that compared post‐transplant outcomes of patients with corrected vs. uncorrected pre‐transplant hyponatremia, patients with pre‐operative correction of hyponatremia had a lower risk of prolonged post‐transplant hospitalization than those with uncorrected hyponatremia.32 However, both hyponatremic groups had more complicated post‐transplant courses compared to those without a history of hyponatremia. However, given the small sample size, retrospective design, and the potential for confounding, the impact of correction of pre‐transplant hyponatremia remains to be determined.
Management
Most patients with mild hypervolemic hyponatremia are asymptomatic. The initial recommended approach is fluid restriction and an Na‐restricted diet. For those with severe or progressive hyponatremia, diuretics should be minimized or discontinued to avoid intravascular volume depletion.49 For patients with tense ascites and severe DH, therapeutic paracentesis with plasma expanders is safe.33 Unfortunately, fluid restriction is limited in efficacy and often poorly tolerated. The use of hypertonic saline is generally not recommended unless severe neurologic symptoms are present as it leads to increased ascites and edema. When administered, it is important to avoid a rapid correction of the hyponatremia to prevent the development of central pontine myelinolysis and the osmotic demyelination syndrome.
Due to the pivotal role of AVP in the pathogenesis of DH, antagonism of its action has long been proposed to be the most rational approach, but until recently, effective and specific antagonism of AVP has remained elusive. Approaches that have been attempted include interference with its secretion and actions. Intravenous albumin has been reported to improve hyponatremia in patients with cirrhosis, ascites, and hyponatremia, presumably by decreasing AVP release by plasma volume expansion.50 An attempt at inhibition of central AVP release with the use of a kappa‐opioid receptor agonist, niravoline, was limited by loss of efficacy and potential adverse effects.51 Use of demeclocycline and lithium (which induce renal resistance to AVP and lead to a modest increase in urine volume with decreased urine osmolality and a corresponding rise in serum sodium) is limited by nephrotoxicity and hepatotoxicity.7, 52 Because of the important role played by prostaglandins in the maintenance of renal hemodynamics and water excretion in cirrhosis, oral misoprostol has also been evaluated but determined to be ineffective in inducing significant changes in free water clearance in patients with functional renal failure and/or DH.53
The recent introduction of vaptans, vasopressin receptor antagonists that block the physiologic action of vasopressin, represents a revolutionary and highly effective approach to the treatment of hyponatremia. Vaptans are antagonists of the V2 receptors of AVP in the principal cells of the collecting ducts. In healthy subjects, vaptans cause a dose‐dependent increase in urine volume and produce a dilute urine without causing natriuresis. To date, 2 AVP antagonists, conivaptan and tolvaptan, have been Food and Drug Administration (FDA)‐approved for the treatment of DH. Conivaptan, the first to be approved in 2005, is a mixed vasopressin V1a and V2 receptor antagonist that is administered intravenously for up to 4 days. In a randomized placebo‐controlled study of patients with euvolemic or hypervolemic hyponatremia, intravenous conivaptan treatment increased serum Na levels by >6 mEq/l or to a serum Na >135 mEq/l in 69 to 88.5% of subjects compared to 20.7% of those receiving placebo (Zeltser D, Rosansky S, Van Rensburg H, et al. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. American J Nephrology 2007;27:447457). In a pilot study involving 24 patients with end‐stage liver disease, an infusion of conivaptan over 1 to 4 days was associated with an increase of serum sodium by >5 mmol/L in 60% of patients not receiving diuretics and 67% of patients on concomitant diuretic therapy by the end of treatment (O'Leary and Davis, 2009). Despite a concern about the potential for conivaptan to increase portal hypertension due to inhibition of splanchnic V1a receptors, the brief treatment appeared to be well tolerated without significant changes in systolic blood pressure, serum creatinine, variceal bleeding or worsening of ascites during the infusion period. However, approval for only 4 days of therapy and requirement for intravenous use eliminate any potential for chronic use.54
Tolvaptan is an orally available, selective V2 receptor antagonist whose efficacy was assessed in two multicenter, prospective, randomized, placebo‐controlled trials, Study of Ascending Levels of Tolvaptan in Hyponatremia 1 and 2 (SALT‐1 and SALT‐2).55 In these trials, clinically stable patients with DH (Na 135meq/l) associated with cirrhosis (22.4% in SALT‐1, 30.5% in SALT‐2), CHF or SIADH were randomized in a hospital setting to receive tolvaptan 15mg daily or placebo. Repeat Na levels were obtained at 8 hours, 2, 3, and 4 days and then weekly at days 11, 18, 25 and 30 after which study drug was discontinued and follow‐up Na level was determined 7 days later. The dose was adjusted to 30 mg and then 60 mg in an attempt to achieve a Na level >135 in those in whom hyponatremia persisted. During the initial day of the titration phase, fluid restriction was not maintained, and the patients were encouraged to respond to thirst with increased water ingestion.
Tolvaptan use was associated with a prompt increase in Na level as early as 8 hours after administration of the first dose. Serum Na increased more among those receiving tolvaptan than among those receiving placebo during the first 4 days and throughout the study period regardless of baseline Na level but returned to baseline within 1 week after discontinuation (Figure 3). The main side effects were increased thirst, dry mouth and increased urination. Importantly, an increased incidence of renal failure was not observed. Based on these results, FDA approval for tolvaptan in patients with hyponatremia was obtained in May 2009 for patients with DH‐associated with cirrhosis, CHF or SIADH for patients with Na levels 125 or symptomatic patients with Na levels between 125 and 135 that have not responded to fluid restriction.
Management of the Hospitalized Cirrhotic Patient With Hyponatremia: Recommendations
Hyponatremia in hospitalized cirrhotic patients is a marker for severe disease and high risk of hospital mortality.24 As a result, prompt evaluation and treatment is imperative. The availability of tolvaptan potentially revolutionizes the manner in which these patients are treated. In the SALT trials, only clinically stable patients were enrolled. In this last section, a guideline for the evaluation and treatment of acutely ill, hospitalized cirrhotic patients with DH is presented.
Evaluation
Determination of volume status is paramount but frequently problematic in the hospitalized cirrhotic patient. Due to the vasodilated state present in severe portal hypertension that is characterized by a relative hypotension and resting tachycardia, the usual hemodynamic parameters of blood pressure and heart rate can be difficult to interpret. Although significant extravascular volume in the form of ascites and edema may be present, patients may be intravascularly depleted due to previous diuretic use and extra‐renal losses due to impaired oral intake, vomiting, lactulose‐induced diarrhea, and gastrointestinal bleeding. Infection is a commonly associated condition, and endotoxin mediated splanchnic vasodilatation, especially in the setting of SBP, can adversely effective central blood volume status in the presence of severe ascites. Also, due to the Na avidity of the kidney and previous diuretic use, renal electrolytes can be difficult to interpret.
For patients in whom there is strong clinical concern about intravascular depletion (history of impaired oral intake, excessive vomiting and/or diarrhea, rapid weight loss, small volume ascites with history of large volume, azotemia), administration of limited intravenous normal saline (0.5‐1 L) should be considered. Patients with severe neurological symptoms should receive normal saline or hypertonic saline. Unless severe neurologic symptoms associated with profound hyponatremia is present, however, intravenous normal saline should not be administered for the hyponatremia alone. Administration of salt poor albumin (25%), especially for those with marked fluid overload and ascites, is an effective means to expand the central blood volume without exacerbating ascites and edema. After evaluation for and/or treatment of hypovolemia, all patients should receive a Na restricted diet (2000 mg daily) and placed on fluid restriction (see below for liberalization of fluid restriction upon initiation of tolvaptan therapy).
Diagnostic paracentesis should be performed for those with ascites to rule out the presence of SBP, and antibiotics administered to those with evidence of infection. High dose intravenous salt poor albumin should also be administered, especially to those at high risk of renal failure as determined by the presence of azotemia (Cr > 1.0 mg/dL) or severe liver insufficiency (TBili > 4.0 mg/dL).56, 57 Finally, all medications should be reviewed, and those associated with hyponatremia (diuretics, selective serotonin reuptake inhibitors, opiates, proton‐pump inhibitors) discontinued if possible.
Tolvaptan for DH
Patient Selection
Appropriate patient selection for tolvaptan therapy is extremely important (Table 2). In the SALT trials, only clinically stable patients were enrolled. The presence of hyponatremia in a recently hospitalized cirrhotic patient, however, frequently indicates severe disease with a high risk of acute renal failure and hospital death. In the SALT trials, many received concomitant diuretic therapy. Because of the importance of avoiding tolvaptan administration to hypovolemic patients, discontinuation of diuretic therapy prior the initiation of tolvaptan therapy and/or reevaluation after limited volume expansion should be considered.
| Hospital setting |
| Euvolumia or hypervolumia |
| Absence of recent weight loss, decrease in ascites, edema |
| Absence of excessive vomiting, diarrhea |
| Consider discontinuation of diuretic therapy prior to initiation of tolvaptan |
| Consider evaluation after limited volume expansion, especially with salt poor albumin prior to initiation of tolvaptan |
| Presence of clinically significant hyponatremia: 125mEq/L or less severe but symptomatic hyponatremia (125 to 134 mEq/L) that has resisted fluid restriction |
| Absence of severe neurologic symptoms attributable to hyponatremia |
| No co‐administration with intravenous saline |
| Ability to respond to thirst |
| No co‐administration with strong CYP 3A inhibitors (ketoconazole) |
| Absence of kidney failure with anuria |
Tolvaptan is indicated for cirrhotic patients with DH in whom the serum sodium is 125 mEq/L and in those with less severe but symptomatic hyponatremia (125‐134 mEq/L) that has resisted fluid restriction. Although the definition of symptomatic was not specifically defined, possible considerations include symptoms of mild hepatic encephalopathy or inability to tolerate dieresis due to the presence of hyponatremia. According to FDA guidelines, tolvaptan therapy must be initiated and re‐initiated in a hospital setting. Patients with severe neurologic symptoms attributable to hyponatremia in whom rapid treatment is critical should not receive tolvaptan but should rather be treated with normal saline. Similarly, patients should not receive combination therapy with tolvaptan and normal saline due to potential for a too‐rapid correction of hyponatremia and the development of central pontine myelinolysis. If saline had been administered for treatment of possible hypovolemia, it should be discontinued and persistent hyponatremia confirmed before starting tolvaptan. Other factors that need to be considered before initiating tolvaptan include the ability of the patient to respond to thirst with increased water ingestion and recognition that the patient will experience increased urine volume and frequency, requiring easy access to toilet. Patients should not be fluid restricted during the first day of tolvaptan therapy, but should be instructed to respond to their thirst with increased water ingestion. As a result, caution should be exercised in administering tolvaptan to a confused, restrained, unresponsive and/or bed‐bound patient who is not able to respond appropriately to thirst or increased urination.
In the SALT trials, the incidence of hyperkalemia (5%) was similar in the tolvaptan and placebo treated patients.55 However, further analysis of all multiple‐dose, placebo‐controlled trials, demonstrated that the aggregate incidence of hyperkalemia was slightly higher for tolvaptan‐treated subjects compared with placebo‐treated subjects (Otsuka). Because treatment with tolvaptan is associated with an acute reduction of the extracellular fluid volume which could result in increased serum potassium through hemoconcentration, it is recommended that serum potassium levels be monitored after initiation of tolvaptan treatment in patients with a serum potassium > 5 mEq/L as well as those who are receiving drugs known to increase serum potassium levels such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or potassium sparing diuretics (Samsca Package Insert, Otsuka). Because tolvaptan is metabolized by the cytochrome P 3A system, patients receiving strong inhibitors such as ketoconazole should not receive tolvaptan. Anuric patients will not respond to tolvaptan. Finally, it is extremely important to administer tolvaptan only to patients with true hyponatremia and not to those with pseudohyponatremia in whom the plasma osmolality is normal but the measured serum sodium concentration artificially low due to marked elevations of other substances, such as can be seen in severe hyperglycemia, marked hyperlipidemia, or hyperproteinemia (as in multiple myeloma).
Tolvaptan Administration
The initial dose of tolvaptan is 15 mg daily. After receiving tolvaptan, many patients will develop an increased sense of thirst and need to urinate. As a result, patients should not be fluid restricted during the first day of therapy, and it is important to monitor the hemodynamics and Na level closely after initiating therapy with a repeat Na level at approximately 8 hours after the first dose. As a result, it should probably be administered early in the day and not at bedtime. The dose should be increased to 30 mg, then 60 mg in patients who do not respond by at least 5 mEq/L over the previous 24 hours and remain hyponatremic. In those with an excessive response (more than 8 meq/L during the first 8 hours or 12 meq/L on any subsequent day), the patient should be encourage to either drink more water, or the dose should be held or reduced. After the appropriate dose has been identified, the patient may be discharged and continued on tolvaptan long‐term.
With the advent of this exciting therapy, practical issues will need to be addressed, most important of which is its cost at $250 per day (Otsuka). In addition, the current recommendation to initiate tolvaptan only in a hospital further limits its widespread use. Most important, long‐term clinical benefit will need to be demonstrated. Although the SALT trials only involved treatment for up to 1 month, a multicenter, open‐label extension study for a mean duration of 701 days demonstrated that prolonged administration of tolvaptan maintains an increased serum sodium level.58 However, at this time, tolvaptan can only be considered as one of the promising drugs whose long‐term cost‐effectiveness is yet to be proven. Proof will require showing that correction of the hyponatremia leads to improved clinical outcomes, such as a reduction in length of stay or frequency of hospitalization, decreased renal failure, improved hepatic encephalopathy, deceased mortality, and improved post‐transplant outcomes.
Unanswered Questions
The vaptans provide an important opportunity to clarify the role that hyponatremia plays in the pathogenesis of cirrhosis. In the past, DH in a cirrhotic patient represented a sign of advanced disease. With the availability of safe and effective therapy, we can now determine whether it also plays an important role in the pathophysiology of end‐stage liver disease and whether its treatment will have a beneficial effect on patient outcomes.
Specific clinical questions that will inevitably be addressed over the next few years to determine whether DH is only a marker for advanced disease or whether it plays a direct but modifiable role in the pathophysiology of cirrhosis will include:
-
Role of vaptans in the management of ascites: In a 14‐day randomized, trial of a satavaptan, another selective vasopressin V(2) receptor antagonist, vs. placebo with spironolactone, combination therapy was associated with improved control of ascites and improvements in serum sodium levels in hyponatremic patients with ascites.59 If future similar studies demonstrate more prolonged benefits, this would constitute an important advance in the treatment of ascites in cirrhosis.
-
Effect on renal function: Prolonged use of tolvaptan leads to a compensatory increase in endogenous levels of AVP and, potentially, increased stimulation of V1a receptors, which might be helpful in the setting of portal hypertension. In patients with hepatorenal syndrome, vasopressin stimulation of splanchnic V1a receptors leads to improved renal function, presumably by decreasing splanchnic blood flow and improving central blood volume.60 As a result, tolvaptan may indirectly improve kidney function in patients with advanced cirrhosis and refractory ascites. Whether long‐term tolvaptan therapy will help to prevent hepatorenal syndrome through this mechanism remains to be determined but is an exciting possibility.61
-
Effect on hepatic encephalopathy: Hepatic encephalopathy is associated with poor quality of life in patients with cirrhosis. Although hepatic encephalopathy was not directly assessed in the SALT trials, the mean mental component summary of the Short Form General Health Survey, a quality of life measure, improved in cirrhotic patients receiving tolvaptan to a greater degree that those receiving placebo.62 A possible explanation for this finding is a beneficial effect of tolvaptan on hepatic encephalopathy. Confirmation of this hypothesis, however, will require prospective studies in which hepatic encephalopathy is directly assessed.
-
Effect on medical economics: Based on retrospective reviews, hyponatremia has an adverse impact on length of stay and outcomes following liver transplantation. It will be important to demonstrate in prospective studies that correction of hyponatremia with tolvaptan reduces length of stay, complications, and costs.
- ,,, et al.The aquaporin family of water channels in kidney: an update on physiology and pathophysiology of aquaporin‐2.Kidney Int.1996;49:1718–1723.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28:851–864.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48:1002–1010.
- ,.Hyponatremia.N Engl J Med.2000;342:1581–1589.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121:186–191.
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337:169–172.
- .Consequences of inadequate management of hyponatremia.Am J Nephrol.2005;25:240–249.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52:144–153.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24:1601–1608.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Supple 1):S30–S35.
- ,,.Disorders of sodium and water balance in hospitalized patients.Can J Anesth.2009;56:151–167.
- ,,, et al.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119;71.e1‐8.
- ,.Brain volume regulation in response to hypo‐osmolality and its correction.Am J Med.2006;119 (7 Suppl 1):S12–S16.
- ,,.Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes.Medicine.1976;55:121–129.
- ,.The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule.Hepatology.2006;43:S121–S131.
- ,,, et al.Hyponatremia of cirrhosis: role of vasopressin and decreased “effective” plasma volume.Scand J Gastroenterol.1997;32:829–834.
- ,,,.Metabolic clearance rate of arginine vasopressin in patients with cirrhosis.Hepatology.1992;16:974–979.
- .Water and sodium retention in edematous disorders: role of vasopressin and aldosterone.Am J Med.2006;119:S47–S53.
- ,,, et al.Circulatory function and hepatorenal syndrome in cirrhosis.Hepatology.2005;42:439–447.
- ,,, et al.Aquaporin‐2 urinary excretion in cirrhosis: relationship to vasopressin and nitric oxide.Dig Dis Sci.2010;55(4):1135–1141.
- ,,, et al.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- et al.Hyponatremia and mortality among patients on the liver‐transplant list.N Engl J Med.2009;359:1018–1026.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Clinical relevance of hyponatremia for the hospital outcome of cirrhotic patieints.Dig Liver Dis.2000;32:605–610.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol2010;44(3):220–226.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.,Validation of model for end‐stage liver disease score to serum sodium ratio index as a prognostic predictor in patients with cirrhosis.J Gastroenterol Hepatol.2009;24:1547–1553.
- ,,, et al.Hepatic venous pressure gradient can predict the development of hepatocellular carcinoma and hyponatremia in decompensated alcoholic cirrhosis.Eur J Gastroenterol Hepatol.2009;21:1241–1246.
- ,,, et al.Serum sodium predicts mortality in patients listed for liver transplantation.Hepatology.2005;41:32–39.
- ,,, et al.Effect of hyponatremia on outcomes following orthotopic liver transplantation.Liver Int.2009;29:1071–1077.
- ,,, et al.Total paracentesis in cirrhotic patients with tense ascites and dilutional hyponatremia.Am J Gastroenterol.1999;94:2219–2223.
- ,,, et al.Dilutional hyponatremia in patients with cirrhosis and ascites.Arch Intern Med.2002;162:323–328.
- ,.Therapeutic approaches to the treatment of edema and ascites: the use of diuretics.Am J Ther.2009;16:98–101.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- .Nephrotoxicities of nonsteroidal anti‐inflammatory drugs.J Formos Med Assoc.1997;96:157–171.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,.Pathogenic mechanisms of hepatic encephalopathy.Gut2008;57:1156–1165.
- ,,, et al.Effects of dilutional hyponatremia on brain organic osmolytes and water content in patients with cirrhosis.Hepatology.2004;39:1613–1622.
- .Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis.Hepatology.2006;43:1187–1190.
- ,,, et al.Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients.J Hepatol.2001;35:37–45.
- ,,, et al.Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a propective study with time‐dependent analysis.Am J Gastroenterol.2009;104:1382–1389.
- ,,, et al.Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystecim stent‐shunt for variceal haemorrhage.J Hepatol.1995;2:123–128.
- ,,, et al.Cardiac dysfunction during liver transplantation: incidence and preoperative predictors.Transplantation.2008;85:1766–1772.
- ,,, et al.,Impact of pretransplant hyponatremia on outcome following liver transplantation.Hepatology.2009;49:1610–1615.
- ,,, et al.Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation.Gastroenterology.2006;130:1135–1143.
- ,,.Hyponatremia in cirrhosis: clinical features and management.Gastroenterol Clin Biol.2006;30:1144–1151.
- ,,, et al.Intravenous albumin infusion is an effective therapy for hyponatremia in cirrhotic patients with ascites.Gut.1990;31:204–207.
- ,,, et al.Comparison of two aquaretic drugs (niravoline and OPC‐31260) in cirrhotic rats with ascites and water retention.J Pharmacol Exp Ther.1999;289:194–201.
- ,,.Plasma demeclocycline levels and nephrotoxicity. Correlation in hyponatremic cirrhotic patients.JAMA.1980;243:2513–2515.
- ,,, et al.Oral misoprostol or intravenous prostaglandin E2 do not improve renal function in patients with cirrhosis and ascites with hyponatremia and renal failure.J Hepatol.1993;17:220–226.
- ,,, et al.Effect of the V1a/V2‐AVP receptor antagonist, Conivaptan, on renal water metabolism and systemic hemodynamics in rats with cirrhosis and ascites.J Hepatol.2003;38:755–761.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al.Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis.N Engl J Med.1999;341:403–409.
- ,,, et al.Restricted use of albumin for spontaneous bacterial peritonitis.Gut.2007;56:597–599.
- ,,, et al.Oral tolvaptan is safe and effective in chronic hypyonatremia.J Am Soc Nephrol.2010;21(4):705–712.
- ,,, et al.Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial.Hepatology.2008;48:204–213.
- ,,, et al.A randomized, prospective, double‐blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome.Gastroenterology.2008;134:1360–1368.
- ,.Tolvapten and its potential in the treatment of hyponatremia.Ther Clin Risk Manag.2008;4:1149–11455.
- ,,, et al.The effects of vasopressin V2 receptor antagonist in the management of patients with cirrhosis and hyponatremia. Safety and efficacy of oral tolvaptan in the SALT trials.Hepatology.2009;50S:467A.
- ,,, et al.Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea.Nephron.1986;44:337–343.
- ,,, et al.Aquaretic effects of niravoline, a kappa‐opioid agonist, in patients with cirrhosis.J Hepatol.2000;32:38–42.
- ,, et al.The association between the serum sodium level and the severity of complications in liver cirrhosis.Korean J Intern Med.2009;24:106–112.
- ,,, et al.Nitric oxide in ascitic fluid is an independent predictor of the development of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis.Eur J Gastroenterol Hepatol.2004;16:571–577.
- ,,, et al.The aquaporin family of water channels in kidney: an update on physiology and pathophysiology of aquaporin‐2.Kidney Int.1996;49:1718–1723.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28:851–864.
- ,.Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management.Hepatology.2008;48:1002–1010.
- ,.Hyponatremia.N Engl J Med.2000;342:1581–1589.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121:186–191.
- .Age and gender as risk factors for hyponatremia and hypernatremia.Clin Chim Acta.2003;337:169–172.
- .Consequences of inadequate management of hyponatremia.Am J Nephrol.2005;25:240–249.
- ,,.A review of drug‐induced hyponatremia.Am J Kidney Dis.2008;52:144–153.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24:1601–1608.
- ,,.Incidence and prevalence of hyponatremia.Am J Med.2006;119(7 Supple 1):S30–S35.
- ,,.Disorders of sodium and water balance in hospitalized patients.Can J Anesth.2009;56:151–167.
- ,,, et al.Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits.Am J Med.2006;119;71.e1‐8.
- ,.Brain volume regulation in response to hypo‐osmolality and its correction.Am J Med.2006;119 (7 Suppl 1):S12–S16.
- ,,.Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes.Medicine.1976;55:121–129.
- ,.The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule.Hepatology.2006;43:S121–S131.
- ,,, et al.Hyponatremia of cirrhosis: role of vasopressin and decreased “effective” plasma volume.Scand J Gastroenterol.1997;32:829–834.
- ,,,.Metabolic clearance rate of arginine vasopressin in patients with cirrhosis.Hepatology.1992;16:974–979.
- .Water and sodium retention in edematous disorders: role of vasopressin and aldosterone.Am J Med.2006;119:S47–S53.
- ,,, et al.Circulatory function and hepatorenal syndrome in cirrhosis.Hepatology.2005;42:439–447.
- ,,, et al.Aquaporin‐2 urinary excretion in cirrhosis: relationship to vasopressin and nitric oxide.Dig Dis Sci.2010;55(4):1135–1141.
- ,,, et al.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44:1535–1542.
- et al.Hyponatremia and mortality among patients on the liver‐transplant list.N Engl J Med.2009;359:1018–1026.
- ,,, et al.Natural history of patients hospitalized for management of cirrhotic ascites.Clin Gastroenterol Hepatol.2006;4:1385–1394.
- ,,, et al.Clinical relevance of hyponatremia for the hospital outcome of cirrhotic patieints.Dig Liver Dis.2000;32:605–610.
- ,,, et al.Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis.Clin Nephrol.2006;65:28–33.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol2010;44(3):220–226.
- ,,, et al.Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone.Liver Transpl.2005;11:336–343.
- ,,, et al.Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death.Hepatology.2004;40:802–810.
- ,,, et al.,Validation of model for end‐stage liver disease score to serum sodium ratio index as a prognostic predictor in patients with cirrhosis.J Gastroenterol Hepatol.2009;24:1547–1553.
- ,,, et al.Hepatic venous pressure gradient can predict the development of hepatocellular carcinoma and hyponatremia in decompensated alcoholic cirrhosis.Eur J Gastroenterol Hepatol.2009;21:1241–1246.
- ,,, et al.Serum sodium predicts mortality in patients listed for liver transplantation.Hepatology.2005;41:32–39.
- ,,, et al.Effect of hyponatremia on outcomes following orthotopic liver transplantation.Liver Int.2009;29:1071–1077.
- ,,, et al.Total paracentesis in cirrhotic patients with tense ascites and dilutional hyponatremia.Am J Gastroenterol.1999;94:2219–2223.
- ,,, et al.Dilutional hyponatremia in patients with cirrhosis and ascites.Arch Intern Med.2002;162:323–328.
- ,.Therapeutic approaches to the treatment of edema and ascites: the use of diuretics.Am J Ther.2009;16:98–101.
- ,,, et al.Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis.Gastroenterol.1988;94:1493–1502.
- ,,, et al.Effect of intrathoracic pressure on plasma arginine vasopressin levels.Gastroenterology.1991;101:607–617.
- .Nephrotoxicities of nonsteroidal anti‐inflammatory drugs.J Formos Med Assoc.1997;96:157–171.
- ,,, et al.Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study.Liver Int.2009;29:415–419.
- ,.Pathogenic mechanisms of hepatic encephalopathy.Gut2008;57:1156–1165.
- ,,, et al.Effects of dilutional hyponatremia on brain organic osmolytes and water content in patients with cirrhosis.Hepatology.2004;39:1613–1622.
- .Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis.Hepatology.2006;43:1187–1190.
- ,,, et al.Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients.J Hepatol.2001;35:37–45.
- ,,, et al.Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a propective study with time‐dependent analysis.Am J Gastroenterol.2009;104:1382–1389.
- ,,, et al.Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystecim stent‐shunt for variceal haemorrhage.J Hepatol.1995;2:123–128.
- ,,, et al.Cardiac dysfunction during liver transplantation: incidence and preoperative predictors.Transplantation.2008;85:1766–1772.
- ,,, et al.,Impact of pretransplant hyponatremia on outcome following liver transplantation.Hepatology.2009;49:1610–1615.
- ,,, et al.Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation.Gastroenterology.2006;130:1135–1143.
- ,,.Hyponatremia in cirrhosis: clinical features and management.Gastroenterol Clin Biol.2006;30:1144–1151.
- ,,, et al.Intravenous albumin infusion is an effective therapy for hyponatremia in cirrhotic patients with ascites.Gut.1990;31:204–207.
- ,,, et al.Comparison of two aquaretic drugs (niravoline and OPC‐31260) in cirrhotic rats with ascites and water retention.J Pharmacol Exp Ther.1999;289:194–201.
- ,,.Plasma demeclocycline levels and nephrotoxicity. Correlation in hyponatremic cirrhotic patients.JAMA.1980;243:2513–2515.
- ,,, et al.Oral misoprostol or intravenous prostaglandin E2 do not improve renal function in patients with cirrhosis and ascites with hyponatremia and renal failure.J Hepatol.1993;17:220–226.
- ,,, et al.Effect of the V1a/V2‐AVP receptor antagonist, Conivaptan, on renal water metabolism and systemic hemodynamics in rats with cirrhosis and ascites.J Hepatol.2003;38:755–761.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355:2099–2112.
- ,,, et al.Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis.N Engl J Med.1999;341:403–409.
- ,,, et al.Restricted use of albumin for spontaneous bacterial peritonitis.Gut.2007;56:597–599.
- ,,, et al.Oral tolvaptan is safe and effective in chronic hypyonatremia.J Am Soc Nephrol.2010;21(4):705–712.
- ,,, et al.Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial.Hepatology.2008;48:204–213.
- ,,, et al.A randomized, prospective, double‐blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome.Gastroenterology.2008;134:1360–1368.
- ,.Tolvapten and its potential in the treatment of hyponatremia.Ther Clin Risk Manag.2008;4:1149–11455.
- ,,, et al.The effects of vasopressin V2 receptor antagonist in the management of patients with cirrhosis and hyponatremia. Safety and efficacy of oral tolvaptan in the SALT trials.Hepatology.2009;50S:467A.
- ,,, et al.Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea.Nephron.1986;44:337–343.
- ,,, et al.Aquaretic effects of niravoline, a kappa‐opioid agonist, in patients with cirrhosis.J Hepatol.2000;32:38–42.
- ,, et al.The association between the serum sodium level and the severity of complications in liver cirrhosis.Korean J Intern Med.2009;24:106–112.
- ,,, et al.Nitric oxide in ascitic fluid is an independent predictor of the development of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis.Eur J Gastroenterol Hepatol.2004;16:571–577.