User login
Journal of hospital medicine in 2014 and beyond
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
2013 WAS A GREAT YEAR FOR JHM
As the field of hospital medicine continues to grow and prosper, so does the Journal of Hospital Medicine (JHM). For JHM, 2013 reflected the field's growth with continued excellence, as manifested in a number of ways.
First, submissions to JHM rose more than 25% over 2012, with the majority of this growth coming in the form of original research, a key indication of vigorous growth in hospital medicine. Growth in submissions was accommodated through a switch to monthly publication frequency, allowing the journal to keep acceptance rates equivalent over time.
Second, peer review time has markedly improved, with average times to first decision falling from more than 35 days in 2011 to fewer than 26 days in 2013. At the same time, the time to papers appearing in Early View fell from more than 3 months to under 2 months, and the time to appearance in print fell to 2 months. Time to decision and time to publication are important measures for the journal, as they represent JHM's service to authors while also ensuring timely publication of articles that may have relevant external context.
Third, the journal continues to garner attention from the press and frequent downloads by readers (Table 1). The most widely downloaded papers of the last 12 months provided evidence‐based guidelines for medication reconciliation and transitions programs, key features of hospital medicine practice. At least as importantly, clinical research articles were also frequently mentioned in the press and downloaded, and many of these important papers were published in the last year.
| Article | No. of Downloads |
|---|---|
| |
| Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists[1] | 4,010 |
| Making inpatient medication reconciliation patient centered, clinically relevant and implementable:A consensus statement on key principles and necessary first steps[2] | 3,580 |
| Hospital performance trends on national quality measures and the association with joint commission accreditation[3] | 3,357 |
| Zolpidem is independently associated with increased risk of inpatient falls[4] | 2,376 |
| Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization[5] | 2,271 |
| Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis[6] | 1,466 |
| BOOST and readmissions: Thinking beyond the walls of the hospital[7] | 1,182 |
| Nutrition in the hospitalized patient[8] | 1,181 |
| The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond?[9] | 1,003 |
| Nurse staffing ratios: Trends and policy implications for hospitalists and the safety net[10] | 1,003 |
Fourth, JHM implemented a social media strategy including Twitter and Facebook efforts that have resulted in rapid follower growth; the JHM twitter feed has more than 600 followers and a rapidly improving social media influence score.
Finally, the JHM editors remain deeply thankful to the many outstanding peer reviewers who contribute their time and expertise to the journal. Through their efforts, each article submitted to JHM is improved, whether published or not. Our peer reviewers help the journal, but also play a key role in ensuring the continued growth of the field of hospital medicine. We single out a select few of our most highly regarded reviewers in this editorial (Table 2), and all of our peer reviewers are acknowledged following this editorial.
| Gerry Barber, University of Colorado | Luke Hansen, Northwestern University | Jim Pile, Case Western ReserveUniversity |
| Joshua Baru, John Stroger Hospital of Cook County | Keiki Hinami Northwestern University | Jennifer Quartarolo, University of California San Diego |
| Arpi Bekmezian, University of California Los Angeles | Guibenson Hyppolite, Massachusetts General Hospital | Alvin Rajkomar, University of California San Francisco |
| Jacob Blazo, Virginia Tech Carilion School of Medicine and Research Institute | Devan Kansagara, Portland VA Medical Center | Maria Raven, University of California San Francisco |
| Christopher Bonafide, The Children's Hospital ofPhiladelphia | A. Scott Keller, Mayo Clinic | Allen Repp, Fletcher Allen Health Care |
| Elizabeth Cerceo, Cooper University Hospital | Scott Lorch, The Children's Hospital of Philadelphia and University of Pennsylvania | Stephen Schmaltz, The Joint Commission Health Services Research |
| Chayan Chakraborti, George Washington University Hospital | Henry Michtalik, Johns Hopkins University | Gregory Seymann, University of California San Diego |
| Chase Coffey, Henry Ford Health System | Hilary Mosher, University of Iowa Hospitals and Clinics | Ann Sheehy, University of Wisconsin |
| Lauren Doctoroff, Beth Israel Deaconess Medical Center | Stephanie Mueller, Brigham and Women's Hospital | Daniel Shine, New York University Langone Medical Center |
| Honora Englander, Oregon Health & Science University | Andrew Odden, University of Michigan | Kevin Smith, Loyola University Medical Center |
| Matt Garber, Palmetto Health | Vikas Parekh, University of Michigan | Brett Stauffer, Baylor University |
| Zachary Goldberger, University of Washington | Henry Perkins, University of Texas | Cecelia Theobald, VA Tennessee Valley Healthcare System |
| Paul Grant, University of Michigan | Jason Persoff, University of Colorado |
SO WHAT WILL 2014 BRING?
JHM continues to anticipate growth in submissions and will be working to accommodate need and maintain acceptance rates at a reasonable level. We feel this is a critical strategy for the journal as we seek to increase the level of academic discourse in hospital medicine. The editors will continue to work to ensure that authors receive a fair and expeditious review, one that will produce an article that is improved, whether or not it is accepted in JHM.
We are also pleased to continue to support the Clinical Cases and Conundrums (CCC) series in JHM. The CCC series is a highly respected part of the journal's offerings, and we have sought to improve JHM's ability to solicit and publish outstanding clinical cases by enlisting the help of a group of outstanding national correspondents who will work with the CCC series editor, Brian Harte, to turn fascinating clinical cases into outstanding publications.
JHM will continue to work to make as many articles open access as possible. Even though Society of Hospital Medicine members have free full‐text access to the journal, many other readers do not have direct access to the JHM articles; we will announce articles that are freely available through our Twitter (@JHospMedicine) and Facebook pages.
In addition, JHM will be announcing new criteria for reporting initial experiences with our evaluations of health system innovations. These criteria will help JHM authors and readers understand whether a quality improvement (or value improvement) program was innovative, whether it is implementable, and whether and how it has impact on patient outcomes.
Finally, JHM will be announcing a new series on healthcare value, to begin in the spring of 2014. More details about this series, which will include reviews of key topics in value improvement written by prominent authors, will be forthcoming. We view this as an incredible opportunity for JHM, and one that will confirm hospital medicine's role as a specialty focused on providing the highest quality and highest value care to its patients.
You should be proud of your journal, and we are pleased to have continued to shepherd its growth over the last 2 years. We look forward to your help in charting JHM's course in 2014 and to continuing to shape the future of hospital medicine.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5:477–485.
- , , , , . Hospital performance trends on national quality measures and the association with Ioint Commission accreditation. J Hosp Med. 2011;6:454–461.
- , , , . Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5:E12–E3.
- . BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471.
- , , , , . Nutrition in the hospitalized patient. J Hosp Med. 2013;8:52–58.
- , , , , . The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–E16.
- , , , , . Nurse staffing ratios: trends and policy implications for hospitalists and the safety net. J Hosp Med. 2008;3:193–199.
Ultrasound Screening for DVT
Hospital‐acquired venous thrombus embolism (VTE) is a pressing patient health and safety issue and has been identified as a causal factor in preventable deaths in the hospital setting.[1, 2] More than 540,000 hospitalizations with VTE occur each year among adults in the United States.[3] The number of adults with VTE is anticipated to increase from 0.95 million in 2006 to 1.82 million in 2050.[4] The Institute of Medicine has defined failure to provide adequate thromboprophylaxis to hospitalized, at‐risk patients as a medical error.[2, 5] The American College of Chest Physicians guidelines state that thromboprophylaxis is highly effective at preventing deep vein thrombosis (DVT) and proximal DVT, highly effective at preventing symptomatic VTE and fatal pulmonary emboli (PE), and that the prevention of DVT also prevents PE.[6] Where anticoagulation is contraindicated, mechanical methods of thromboprophylaxis are recommended as preferable to no thromboprophylaxis, with careful attention directed toward ensuring the proper use of, and optimal adherence with, mechanical prophylaxis.[7, 8] In our institution, concerns about the existence of asymptomatic clots being propagated into PEs by the placement of pneumatic compression boots (PCBs), led to routine performance of duplex Doppler ultrasound with compression (DUSC) before applying PCBs to those patients who were admitted and who were deemed to have a contraindication to anticoagulation prophylaxis. The recently released (April 2012) American College of Radiology Choosing Wisely list of practices specifically recommends forgoing imaging for DVT and PE in the absence of risk factors.[9] The recommendations do not specifically address screening for DVT prior to the initiation of prophylaxis. The goal of this prospective observational study, conducted prior to the Choosing Wisely campaign, was to verify our hypothesis that the prevalence of asymptomatic DVTs was very low, and provide our clinicians with evidence to allay concerns about placement of PCBs without imaging, allowing a practice pattern that would reduce costs without impacting patient safety.
METHODS
Study Population
We collected the records of all 1136 consecutive patients who underwent lower extremity DUSC within 48 hours of admission to the hospital, prior to PCB placement, between October 2005 and November 2006. The decision as to what type of prophylaxis was appropriate for each patient and if a DUSC was necessary prior to PCB placement was up to the individual attending physician. The study patient population included elective and emergent admissions from the medical, surgical, and obstetrical services.
Data Source
Study patients were identified at the time of the screening duplex study and entered into the database. A test was considered positive if a clot was detected at the level of the popliteal vein, or higher, in either leg. Patients' charts were reviewed for identification of DVT, defined as a positive (same criteria) DUSC during the hospitalization. Pulmonary emboli were defined as a positive computed tomography angiogram or high‐probability lung scan plus positive risk factors for DVT. A manual chart review (performed by J.U.), thoroughly examining all 1136 inpatient records, was completed to identify diagnoses and risk factors, which are defined as follows:
- Age >60 years.
- Cancer at time of admission or within 6 months of admission.
- Ambulatory dysfunction defined as diagnosis of ambulatory dysfunction stated in the electronic medical record (EMR), bedridden >3 days prior to admission, lower extremity cast or splinting, or major surgery (intra‐abdominal, neurosurgery, cardiac surgery, or orthopedic surgery requiring admission) within 8 weeks of admission.
- Obesity defined as diagnosis of obesity in EMR or body mass index (BMI) >30.
- Acute stroke (cerebrovascular accident) or transient ischemic attack.
- Acute myocardial infarction or acute coronary syndrome.
- Previous DVT/PE documented in EMR.
- Genetic predisposition defined as documented as history of, but not limited to, factor V Leiden syndrome, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia, or prothrombin 20210 mutation.
- Hormone replacement/birth control pills defined as hormone replacement therapy, birth control pills, including Nuva Ring and Ortho Evra, pregnancy, or <6 weeks postpartum.
Sociodemographic data (age, gender, race, weight, height, and status of healthcare insurance) and time from arrival at the emergency room to ultrasound (US) examination were extracted from the EMR database.
The study was conducted with the approval of the Christiana Care Health Services institutional review board, and procedures were conducted in accordance with institutional guidelines.
Statistical Analysis
A t test or Wilcoxon rank sum test for continuous variables, and [2] or Fisher exact test for categorical variables, were used to compare demographic and clinical data according to the presence or absence of DVT. Logistic regression was used to determine the relative importance of each risk factor on the risk of DVT. Because the variable time to US was not normally distributed, we transformed it into a categorical variable using the median as the cut point. All the tests were 2‐sided, and P values <0.05 were considered significant. We used Current Procedural Terminology (CPT) code 93970 and the associated 2012 Medicare National Average reimbursement of $261.07 to estimate the cost of DUSC that could be avoided. Data were analyzed using the Statistical Analysis System version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 1136 consecutive records were examined; 4 records were excluded from the analysis because they had a diagnosed PE prior to US, and 35 records were excluded because the US was performed beyond 48 hours after admission. The final dataset included 1097 hospital admissions for 1071 patients. Of the 1097 admissions, 759 (69.2%) originated from the emergency department (ED). It is important to note that 70,161 hospital admissions occurred during the same time period, of which 36,363 (51.8%) were admissions that started in the ED. The proportion of patients requiring mechanical DVT prophylaxis is therefore very small (<5%), assuming that a large number of the patients with unplanned admissions would require DVT prophylaxis.
Of the 1071 patients in the final analytical dataset, 544 (50.8%) were male, the mean age was 65.5 years, the mean BMI was 28.7 (median, 27.0) (Table 1), and the majority of the patients were white. US was performed within 24 hours in 712 (66.5%) patients, and 665 (62.1%) had Medicare. An asymptomatic DVT was detected by DUSC in 19 patients (1.8%). None of the clinical and demographic characteristics were statistically different between those with DVT and without (Table 1).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Male (%) | 544 (50.8) | 6 (31.6) | 538 (51.1) | 0.11 |
| Age, y, meanSD | 65.516.3 | 71.415.3 | 65.416.3 | 0.11 |
| BMI, kg/m2, meanSD | 28.77.6 | 30.112.9 | 28.77.5 | 0.52 |
| Time to US test from admission, h, median | 19.9 | 21.3 | 19.8 | 0.72 |
| Race | 0.74 | |||
| White (%) | 802 (74.9) | 15 (78.9) | 787 (74.8) | |
| Black (%) | 221 (20.6) | 3 (15.8) | 218 (20.7) | |
| Other (%) | 48 (4.5) | 1 (5.3) | 47 (4.5) | |
| Duplex US test <24 hours (%) | 712 (66.5) | 12 (63.2) | 700 (66.5) | 0.81 |
| DVT during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| PE during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| Medical insurance (%) | 0.79 | |||
| Self‐pay | 35 (3.3) | 0 (0.0) | 35 (3.3) | |
| Medicare | 665 (62.1) | 15 (78.9) | 650 (61.8) | |
| Medicaid | 44 (4.1) | 1 (5.3) | 43 (4.1) | |
| HMO | 49 (4.6) | 0 (0.0) | 49 (4.7) | |
| Blue Cross | 136 (12.7) | 2 (10.5) | 134 (12.7) | |
| Other | 142 (13.3) | 1 (5.3) | 141 (13.4) | |
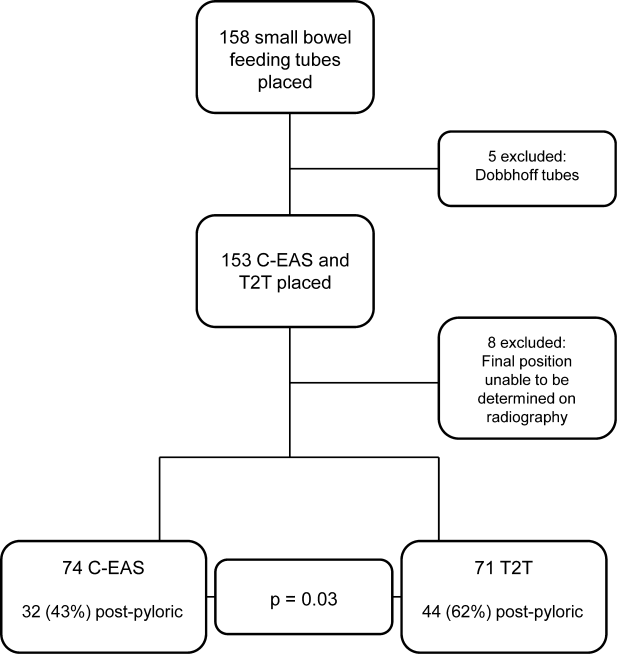
Patients with DVT had at least 1 risk factor; 16 (84.2%) of them had 2 or more risk factors. In addition, the presence of 2 or more risk factors was much more frequent among those with DVT than among those without (84.2% [16/19] vs 58.4% [614/1052], P=0.03).
As shown in Table 2, a history of DVT or PE and ambulatory dysfunction are the only risk factors associated with DVT at admission. In addition, the prevalence of DVT increases as the number of risk factors increases (Table 3). The prevalence is much higher in those who had 4 or more risk factors than among those with fewer than 4 risk factors (12.2% [6/49] vs 1.3% [13/1022], P=0.0001).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Age 60 years | 702 (65.6) | 15 (79.0) | 687 (65.3) | 0.33 |
| Previous DVT or PE | 80 (7.5) | 9 (47.4) | 71 (6.8) | <0.0001 |
| Ambulatory dysfunction | 228 (21.3) | 9 (47.4) | 219 (20.8) | 0.01 |
| Obesity | 372 (34.7) | 6 (31.6) | 366 (34.8) | 1.00 |
| Heart failure | 164 (15.3) | 4 (21.1) | 160 (15.2) | 0.52 |
| Stroke/TIA | 75 (7.0) | 3 (15.8) | 72 (6.8) | 0.14 |
| Acute coronary syndrome | 99 (9.2) | 1 (5.3) | 98 (9.3) | 1.00 |
| Active cancer | 124 (11.6) | 4 (21.1) | 120 (11.4) | 0.26 |
| Hormone | 30 (2.8) | 0 | 30 (2.9) | 1.00 |
| Genetic | 4 (0.4) | 0 | 4 (0.4) | 1.00 |
| No. of Risk Factors | Total, n=1071 | DVT, n=19 (1.8%) |
|---|---|---|
| ||
| 0 | 100 | 0 |
| 1 | 341 | 3 (0.9%) |
| 2 | 412 | 7 (1.7%) |
| 3 | 169 | 3 (1.8%) |
| 4 | 39 | 5 (12.8%) |
| 5 | 10 | 1 (10.0%) |
Results of the logistic regression, similar to those of the nonadjusted analysis, showed that the only risk factors independently associated with the discovery of a DVT upon DUSC were the presence of ambulatory dysfunction (odds ratio [OR]: 2.99, 95% confidence interval [CI]: 1.13‐7.90) and a history of DVT or PE (OR: 10.51, 95% CI: 3.90‐28.31) (Table 4).
| ORb | 95% CI | P | |
|---|---|---|---|
| |||
| Age 60 years | 1.76 | 0.535.84 | 0.353 |
| Active cancer | 2.12 | 0.637.17 | 0.227 |
| Ambulatory dysfunction | 2.99 | 1.137.90 | 0.027 |
| Obesity | 0.76 | 0.272.21 | 0.619 |
| Heart failure | 1.33 | 0.394.49 | 0.646 |
| Stroke/TIA | 3.00 | 0.7711.70 | 0.113 |
| Acute coronary syndrome | 1.06 | 0.138.66 | 0.957 |
| Previous DVT or PE | 10.51 | 3.9028.31 | <0.0001 |
| Time to duplex US (19.9 hours)c | 1.94 | 0.725.22 | 0.188 |
We estimated a savings for Medicare of approximately $266,000 to $280,000 ($261.07 1071 DUSC or $261.07 1022 [after excluding the patients with 4 or more risk factors]) over 13 months had the DUSC not being conducted.
DISCUSSION
This study shows that discovering an asymptomatic DVT is relatively rare (<2%) in patients arriving at the hospital for all causes of admission, even taking into account multiple risk factors that increase the risk for DVTs. The study strongly supports the practice of placing compression devices as soon as possible for those patients who have a contraindication to anticoagulant prophylaxis. Along with reducing the delay to placement while awaiting the test, there is significant cost reduction to the healthcare system by not doing DUSC. There appears to be no need for diagnostic studies prior to the placement of these devices unless the patient has more than 3 risk factors or there is a history of previous DVT or ambulatory dysfunction. This study strongly supports the premise that patients are not arriving with DVTs, but are developing them in the hospital.[1, 2, 10] The 1.8% prevalence of asymptomatic DVT in this study is somewhat lower than that found in other studies. The Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT) tested dalteparin vs unfractionated heparin on 3764 patients in the intensive care unit. Initial screening done to rule out DVT found that 3.5% of patients receiving dalteparin and 3.4% receiving unfractionated heparin had proximal DVTs.[8] Other Investigators used venous compression ultrasound examinations of the lower limbs to determine that 5.5% of patients hospitalized in a medical unit have an asymptomatic DVT of the lower limbs on admission.[5] A limitation of that study is the inclusion of all thrombo emboli, specifically those found in the calf (19 out of 21, or 90%). However, if one eliminates the calf venous thrombi, not considered risk factors for PE, the prevalence of DVT (0.85%) is about half that of our observed 1.8%.
In common with previous studies, a history of previous thromboembolic disease was clearly the most significant of many evaluated risk factors for DVT.[5, 6, 10] Ambulatory dysfunction was also a statistically significant risk factor that was likely under‐reported here because of the inexact documentation in many of the medical records. Interestingly, a history of active malignancy did not prove to be a significant risk factor, contrary to other study reports.[5, 6, 10]
The frequency of asymptomatic DVT appears to increase with the accumulation of risk factors. An asymptomatic DVT existed in 1.3% of the patients with 3 or fewer risk factors, compared with 12.2% of those with 4 or 5 risk factors. It is possible that a higher number of risk factors for DVT would be an indication for obtaining a DUSC prior to the placement of PCBs, although the small number of patients with more than 3 risk factors in our study population may limit the strength of this observation.
Limitations
As commented above, the number of patients in whom ambulatory dysfunction is present may be higher than is captured, due to insufficient recognition and poor documentation. Other studies have found a wide variety of risk factors associated with admission and the development of DVTs.[2, 5, 6, 10] Our study was not designed to establish an all‐inclusive list and/or prevalence of risk factors for thromboembolic disease. Another limitation is that only those patients who could not receive heparin prophylaxis received the DUSC evaluation. It is unclear if this could introduce bias inadvertently.
CONCLUSION
Our data strongly suggest, in alignment with recent recommendations, that there is no need to perform screening DUSC prior to the placement of prophylactic compression devices among hospital admissions who have contraindications to anticoagulation. Rather, efforts should be focused on implementing systems to ensure rapid placement of these compression devices at the time of admission for those patients who cannot receive anticoagulation prophylaxis. Evaluation for DVT may be of value if there is a history of previous DVT or PE, ambulatory dysfunction, or more than 3 risk factors, as the information may change the therapeutic approach. Current guidelines recommend the measurement of D‐dimers as a screening tool for DVT.[11]
Acknowledgements
The authors thank Michael Schnee and Alexandria Mapp for their assistance in editing and manuscript preparation.
Disclosure: Nothing to report.
- . The prevention of hospital‐acquired venous thromboembolism in the United Kingdom. Br J Haematol. 2009;144:642–652.
- .U.S. Department of Health and Human Services. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism 2008. Available at: http://www.surgeongeneral.gov/library/calls/deepvein/index.html. Accessed on October 14, 2013.
- , , , , . Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61:401–404.
- , , , et al. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2010;86:217–220.
- , , , et al. High prevalence of asymptomatic deep vein thrombosis on admission in a medical unit among elderly patients. Thromb Haemost. 2002;88:592–597.
- , , , et al. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141:e7S–e47S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e195S–e226S.
- , , , et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305–1314.
- American College of Radiology (2012). Five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/american‐college‐of‐radiology/. Accessed on October 11, 2013.
- , , , et al. Lack of prophylaxis before the onset of acute venous thromboembolism among hospitalized cancer patients: The SWIss Venous Thrombo Embolism Registry (SWIVTER). Ann Oncol. 2010;21:931–935.
- , , , et al. Diagnosis of DVT. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e351S–e418S.
Hospital‐acquired venous thrombus embolism (VTE) is a pressing patient health and safety issue and has been identified as a causal factor in preventable deaths in the hospital setting.[1, 2] More than 540,000 hospitalizations with VTE occur each year among adults in the United States.[3] The number of adults with VTE is anticipated to increase from 0.95 million in 2006 to 1.82 million in 2050.[4] The Institute of Medicine has defined failure to provide adequate thromboprophylaxis to hospitalized, at‐risk patients as a medical error.[2, 5] The American College of Chest Physicians guidelines state that thromboprophylaxis is highly effective at preventing deep vein thrombosis (DVT) and proximal DVT, highly effective at preventing symptomatic VTE and fatal pulmonary emboli (PE), and that the prevention of DVT also prevents PE.[6] Where anticoagulation is contraindicated, mechanical methods of thromboprophylaxis are recommended as preferable to no thromboprophylaxis, with careful attention directed toward ensuring the proper use of, and optimal adherence with, mechanical prophylaxis.[7, 8] In our institution, concerns about the existence of asymptomatic clots being propagated into PEs by the placement of pneumatic compression boots (PCBs), led to routine performance of duplex Doppler ultrasound with compression (DUSC) before applying PCBs to those patients who were admitted and who were deemed to have a contraindication to anticoagulation prophylaxis. The recently released (April 2012) American College of Radiology Choosing Wisely list of practices specifically recommends forgoing imaging for DVT and PE in the absence of risk factors.[9] The recommendations do not specifically address screening for DVT prior to the initiation of prophylaxis. The goal of this prospective observational study, conducted prior to the Choosing Wisely campaign, was to verify our hypothesis that the prevalence of asymptomatic DVTs was very low, and provide our clinicians with evidence to allay concerns about placement of PCBs without imaging, allowing a practice pattern that would reduce costs without impacting patient safety.
METHODS
Study Population
We collected the records of all 1136 consecutive patients who underwent lower extremity DUSC within 48 hours of admission to the hospital, prior to PCB placement, between October 2005 and November 2006. The decision as to what type of prophylaxis was appropriate for each patient and if a DUSC was necessary prior to PCB placement was up to the individual attending physician. The study patient population included elective and emergent admissions from the medical, surgical, and obstetrical services.
Data Source
Study patients were identified at the time of the screening duplex study and entered into the database. A test was considered positive if a clot was detected at the level of the popliteal vein, or higher, in either leg. Patients' charts were reviewed for identification of DVT, defined as a positive (same criteria) DUSC during the hospitalization. Pulmonary emboli were defined as a positive computed tomography angiogram or high‐probability lung scan plus positive risk factors for DVT. A manual chart review (performed by J.U.), thoroughly examining all 1136 inpatient records, was completed to identify diagnoses and risk factors, which are defined as follows:
- Age >60 years.
- Cancer at time of admission or within 6 months of admission.
- Ambulatory dysfunction defined as diagnosis of ambulatory dysfunction stated in the electronic medical record (EMR), bedridden >3 days prior to admission, lower extremity cast or splinting, or major surgery (intra‐abdominal, neurosurgery, cardiac surgery, or orthopedic surgery requiring admission) within 8 weeks of admission.
- Obesity defined as diagnosis of obesity in EMR or body mass index (BMI) >30.
- Acute stroke (cerebrovascular accident) or transient ischemic attack.
- Acute myocardial infarction or acute coronary syndrome.
- Previous DVT/PE documented in EMR.
- Genetic predisposition defined as documented as history of, but not limited to, factor V Leiden syndrome, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia, or prothrombin 20210 mutation.
- Hormone replacement/birth control pills defined as hormone replacement therapy, birth control pills, including Nuva Ring and Ortho Evra, pregnancy, or <6 weeks postpartum.
Sociodemographic data (age, gender, race, weight, height, and status of healthcare insurance) and time from arrival at the emergency room to ultrasound (US) examination were extracted from the EMR database.
The study was conducted with the approval of the Christiana Care Health Services institutional review board, and procedures were conducted in accordance with institutional guidelines.
Statistical Analysis
A t test or Wilcoxon rank sum test for continuous variables, and [2] or Fisher exact test for categorical variables, were used to compare demographic and clinical data according to the presence or absence of DVT. Logistic regression was used to determine the relative importance of each risk factor on the risk of DVT. Because the variable time to US was not normally distributed, we transformed it into a categorical variable using the median as the cut point. All the tests were 2‐sided, and P values <0.05 were considered significant. We used Current Procedural Terminology (CPT) code 93970 and the associated 2012 Medicare National Average reimbursement of $261.07 to estimate the cost of DUSC that could be avoided. Data were analyzed using the Statistical Analysis System version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 1136 consecutive records were examined; 4 records were excluded from the analysis because they had a diagnosed PE prior to US, and 35 records were excluded because the US was performed beyond 48 hours after admission. The final dataset included 1097 hospital admissions for 1071 patients. Of the 1097 admissions, 759 (69.2%) originated from the emergency department (ED). It is important to note that 70,161 hospital admissions occurred during the same time period, of which 36,363 (51.8%) were admissions that started in the ED. The proportion of patients requiring mechanical DVT prophylaxis is therefore very small (<5%), assuming that a large number of the patients with unplanned admissions would require DVT prophylaxis.
Of the 1071 patients in the final analytical dataset, 544 (50.8%) were male, the mean age was 65.5 years, the mean BMI was 28.7 (median, 27.0) (Table 1), and the majority of the patients were white. US was performed within 24 hours in 712 (66.5%) patients, and 665 (62.1%) had Medicare. An asymptomatic DVT was detected by DUSC in 19 patients (1.8%). None of the clinical and demographic characteristics were statistically different between those with DVT and without (Table 1).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Male (%) | 544 (50.8) | 6 (31.6) | 538 (51.1) | 0.11 |
| Age, y, meanSD | 65.516.3 | 71.415.3 | 65.416.3 | 0.11 |
| BMI, kg/m2, meanSD | 28.77.6 | 30.112.9 | 28.77.5 | 0.52 |
| Time to US test from admission, h, median | 19.9 | 21.3 | 19.8 | 0.72 |
| Race | 0.74 | |||
| White (%) | 802 (74.9) | 15 (78.9) | 787 (74.8) | |
| Black (%) | 221 (20.6) | 3 (15.8) | 218 (20.7) | |
| Other (%) | 48 (4.5) | 1 (5.3) | 47 (4.5) | |
| Duplex US test <24 hours (%) | 712 (66.5) | 12 (63.2) | 700 (66.5) | 0.81 |
| DVT during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| PE during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| Medical insurance (%) | 0.79 | |||
| Self‐pay | 35 (3.3) | 0 (0.0) | 35 (3.3) | |
| Medicare | 665 (62.1) | 15 (78.9) | 650 (61.8) | |
| Medicaid | 44 (4.1) | 1 (5.3) | 43 (4.1) | |
| HMO | 49 (4.6) | 0 (0.0) | 49 (4.7) | |
| Blue Cross | 136 (12.7) | 2 (10.5) | 134 (12.7) | |
| Other | 142 (13.3) | 1 (5.3) | 141 (13.4) | |
Patients with DVT had at least 1 risk factor; 16 (84.2%) of them had 2 or more risk factors. In addition, the presence of 2 or more risk factors was much more frequent among those with DVT than among those without (84.2% [16/19] vs 58.4% [614/1052], P=0.03).
As shown in Table 2, a history of DVT or PE and ambulatory dysfunction are the only risk factors associated with DVT at admission. In addition, the prevalence of DVT increases as the number of risk factors increases (Table 3). The prevalence is much higher in those who had 4 or more risk factors than among those with fewer than 4 risk factors (12.2% [6/49] vs 1.3% [13/1022], P=0.0001).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Age 60 years | 702 (65.6) | 15 (79.0) | 687 (65.3) | 0.33 |
| Previous DVT or PE | 80 (7.5) | 9 (47.4) | 71 (6.8) | <0.0001 |
| Ambulatory dysfunction | 228 (21.3) | 9 (47.4) | 219 (20.8) | 0.01 |
| Obesity | 372 (34.7) | 6 (31.6) | 366 (34.8) | 1.00 |
| Heart failure | 164 (15.3) | 4 (21.1) | 160 (15.2) | 0.52 |
| Stroke/TIA | 75 (7.0) | 3 (15.8) | 72 (6.8) | 0.14 |
| Acute coronary syndrome | 99 (9.2) | 1 (5.3) | 98 (9.3) | 1.00 |
| Active cancer | 124 (11.6) | 4 (21.1) | 120 (11.4) | 0.26 |
| Hormone | 30 (2.8) | 0 | 30 (2.9) | 1.00 |
| Genetic | 4 (0.4) | 0 | 4 (0.4) | 1.00 |
| No. of Risk Factors | Total, n=1071 | DVT, n=19 (1.8%) |
|---|---|---|
| ||
| 0 | 100 | 0 |
| 1 | 341 | 3 (0.9%) |
| 2 | 412 | 7 (1.7%) |
| 3 | 169 | 3 (1.8%) |
| 4 | 39 | 5 (12.8%) |
| 5 | 10 | 1 (10.0%) |
Results of the logistic regression, similar to those of the nonadjusted analysis, showed that the only risk factors independently associated with the discovery of a DVT upon DUSC were the presence of ambulatory dysfunction (odds ratio [OR]: 2.99, 95% confidence interval [CI]: 1.13‐7.90) and a history of DVT or PE (OR: 10.51, 95% CI: 3.90‐28.31) (Table 4).
| ORb | 95% CI | P | |
|---|---|---|---|
| |||
| Age 60 years | 1.76 | 0.535.84 | 0.353 |
| Active cancer | 2.12 | 0.637.17 | 0.227 |
| Ambulatory dysfunction | 2.99 | 1.137.90 | 0.027 |
| Obesity | 0.76 | 0.272.21 | 0.619 |
| Heart failure | 1.33 | 0.394.49 | 0.646 |
| Stroke/TIA | 3.00 | 0.7711.70 | 0.113 |
| Acute coronary syndrome | 1.06 | 0.138.66 | 0.957 |
| Previous DVT or PE | 10.51 | 3.9028.31 | <0.0001 |
| Time to duplex US (19.9 hours)c | 1.94 | 0.725.22 | 0.188 |
We estimated a savings for Medicare of approximately $266,000 to $280,000 ($261.07 1071 DUSC or $261.07 1022 [after excluding the patients with 4 or more risk factors]) over 13 months had the DUSC not being conducted.
DISCUSSION
This study shows that discovering an asymptomatic DVT is relatively rare (<2%) in patients arriving at the hospital for all causes of admission, even taking into account multiple risk factors that increase the risk for DVTs. The study strongly supports the practice of placing compression devices as soon as possible for those patients who have a contraindication to anticoagulant prophylaxis. Along with reducing the delay to placement while awaiting the test, there is significant cost reduction to the healthcare system by not doing DUSC. There appears to be no need for diagnostic studies prior to the placement of these devices unless the patient has more than 3 risk factors or there is a history of previous DVT or ambulatory dysfunction. This study strongly supports the premise that patients are not arriving with DVTs, but are developing them in the hospital.[1, 2, 10] The 1.8% prevalence of asymptomatic DVT in this study is somewhat lower than that found in other studies. The Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT) tested dalteparin vs unfractionated heparin on 3764 patients in the intensive care unit. Initial screening done to rule out DVT found that 3.5% of patients receiving dalteparin and 3.4% receiving unfractionated heparin had proximal DVTs.[8] Other Investigators used venous compression ultrasound examinations of the lower limbs to determine that 5.5% of patients hospitalized in a medical unit have an asymptomatic DVT of the lower limbs on admission.[5] A limitation of that study is the inclusion of all thrombo emboli, specifically those found in the calf (19 out of 21, or 90%). However, if one eliminates the calf venous thrombi, not considered risk factors for PE, the prevalence of DVT (0.85%) is about half that of our observed 1.8%.
In common with previous studies, a history of previous thromboembolic disease was clearly the most significant of many evaluated risk factors for DVT.[5, 6, 10] Ambulatory dysfunction was also a statistically significant risk factor that was likely under‐reported here because of the inexact documentation in many of the medical records. Interestingly, a history of active malignancy did not prove to be a significant risk factor, contrary to other study reports.[5, 6, 10]
The frequency of asymptomatic DVT appears to increase with the accumulation of risk factors. An asymptomatic DVT existed in 1.3% of the patients with 3 or fewer risk factors, compared with 12.2% of those with 4 or 5 risk factors. It is possible that a higher number of risk factors for DVT would be an indication for obtaining a DUSC prior to the placement of PCBs, although the small number of patients with more than 3 risk factors in our study population may limit the strength of this observation.
Limitations
As commented above, the number of patients in whom ambulatory dysfunction is present may be higher than is captured, due to insufficient recognition and poor documentation. Other studies have found a wide variety of risk factors associated with admission and the development of DVTs.[2, 5, 6, 10] Our study was not designed to establish an all‐inclusive list and/or prevalence of risk factors for thromboembolic disease. Another limitation is that only those patients who could not receive heparin prophylaxis received the DUSC evaluation. It is unclear if this could introduce bias inadvertently.
CONCLUSION
Our data strongly suggest, in alignment with recent recommendations, that there is no need to perform screening DUSC prior to the placement of prophylactic compression devices among hospital admissions who have contraindications to anticoagulation. Rather, efforts should be focused on implementing systems to ensure rapid placement of these compression devices at the time of admission for those patients who cannot receive anticoagulation prophylaxis. Evaluation for DVT may be of value if there is a history of previous DVT or PE, ambulatory dysfunction, or more than 3 risk factors, as the information may change the therapeutic approach. Current guidelines recommend the measurement of D‐dimers as a screening tool for DVT.[11]
Acknowledgements
The authors thank Michael Schnee and Alexandria Mapp for their assistance in editing and manuscript preparation.
Disclosure: Nothing to report.
Hospital‐acquired venous thrombus embolism (VTE) is a pressing patient health and safety issue and has been identified as a causal factor in preventable deaths in the hospital setting.[1, 2] More than 540,000 hospitalizations with VTE occur each year among adults in the United States.[3] The number of adults with VTE is anticipated to increase from 0.95 million in 2006 to 1.82 million in 2050.[4] The Institute of Medicine has defined failure to provide adequate thromboprophylaxis to hospitalized, at‐risk patients as a medical error.[2, 5] The American College of Chest Physicians guidelines state that thromboprophylaxis is highly effective at preventing deep vein thrombosis (DVT) and proximal DVT, highly effective at preventing symptomatic VTE and fatal pulmonary emboli (PE), and that the prevention of DVT also prevents PE.[6] Where anticoagulation is contraindicated, mechanical methods of thromboprophylaxis are recommended as preferable to no thromboprophylaxis, with careful attention directed toward ensuring the proper use of, and optimal adherence with, mechanical prophylaxis.[7, 8] In our institution, concerns about the existence of asymptomatic clots being propagated into PEs by the placement of pneumatic compression boots (PCBs), led to routine performance of duplex Doppler ultrasound with compression (DUSC) before applying PCBs to those patients who were admitted and who were deemed to have a contraindication to anticoagulation prophylaxis. The recently released (April 2012) American College of Radiology Choosing Wisely list of practices specifically recommends forgoing imaging for DVT and PE in the absence of risk factors.[9] The recommendations do not specifically address screening for DVT prior to the initiation of prophylaxis. The goal of this prospective observational study, conducted prior to the Choosing Wisely campaign, was to verify our hypothesis that the prevalence of asymptomatic DVTs was very low, and provide our clinicians with evidence to allay concerns about placement of PCBs without imaging, allowing a practice pattern that would reduce costs without impacting patient safety.
METHODS
Study Population
We collected the records of all 1136 consecutive patients who underwent lower extremity DUSC within 48 hours of admission to the hospital, prior to PCB placement, between October 2005 and November 2006. The decision as to what type of prophylaxis was appropriate for each patient and if a DUSC was necessary prior to PCB placement was up to the individual attending physician. The study patient population included elective and emergent admissions from the medical, surgical, and obstetrical services.
Data Source
Study patients were identified at the time of the screening duplex study and entered into the database. A test was considered positive if a clot was detected at the level of the popliteal vein, or higher, in either leg. Patients' charts were reviewed for identification of DVT, defined as a positive (same criteria) DUSC during the hospitalization. Pulmonary emboli were defined as a positive computed tomography angiogram or high‐probability lung scan plus positive risk factors for DVT. A manual chart review (performed by J.U.), thoroughly examining all 1136 inpatient records, was completed to identify diagnoses and risk factors, which are defined as follows:
- Age >60 years.
- Cancer at time of admission or within 6 months of admission.
- Ambulatory dysfunction defined as diagnosis of ambulatory dysfunction stated in the electronic medical record (EMR), bedridden >3 days prior to admission, lower extremity cast or splinting, or major surgery (intra‐abdominal, neurosurgery, cardiac surgery, or orthopedic surgery requiring admission) within 8 weeks of admission.
- Obesity defined as diagnosis of obesity in EMR or body mass index (BMI) >30.
- Acute stroke (cerebrovascular accident) or transient ischemic attack.
- Acute myocardial infarction or acute coronary syndrome.
- Previous DVT/PE documented in EMR.
- Genetic predisposition defined as documented as history of, but not limited to, factor V Leiden syndrome, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia, or prothrombin 20210 mutation.
- Hormone replacement/birth control pills defined as hormone replacement therapy, birth control pills, including Nuva Ring and Ortho Evra, pregnancy, or <6 weeks postpartum.
Sociodemographic data (age, gender, race, weight, height, and status of healthcare insurance) and time from arrival at the emergency room to ultrasound (US) examination were extracted from the EMR database.
The study was conducted with the approval of the Christiana Care Health Services institutional review board, and procedures were conducted in accordance with institutional guidelines.
Statistical Analysis
A t test or Wilcoxon rank sum test for continuous variables, and [2] or Fisher exact test for categorical variables, were used to compare demographic and clinical data according to the presence or absence of DVT. Logistic regression was used to determine the relative importance of each risk factor on the risk of DVT. Because the variable time to US was not normally distributed, we transformed it into a categorical variable using the median as the cut point. All the tests were 2‐sided, and P values <0.05 were considered significant. We used Current Procedural Terminology (CPT) code 93970 and the associated 2012 Medicare National Average reimbursement of $261.07 to estimate the cost of DUSC that could be avoided. Data were analyzed using the Statistical Analysis System version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 1136 consecutive records were examined; 4 records were excluded from the analysis because they had a diagnosed PE prior to US, and 35 records were excluded because the US was performed beyond 48 hours after admission. The final dataset included 1097 hospital admissions for 1071 patients. Of the 1097 admissions, 759 (69.2%) originated from the emergency department (ED). It is important to note that 70,161 hospital admissions occurred during the same time period, of which 36,363 (51.8%) were admissions that started in the ED. The proportion of patients requiring mechanical DVT prophylaxis is therefore very small (<5%), assuming that a large number of the patients with unplanned admissions would require DVT prophylaxis.
Of the 1071 patients in the final analytical dataset, 544 (50.8%) were male, the mean age was 65.5 years, the mean BMI was 28.7 (median, 27.0) (Table 1), and the majority of the patients were white. US was performed within 24 hours in 712 (66.5%) patients, and 665 (62.1%) had Medicare. An asymptomatic DVT was detected by DUSC in 19 patients (1.8%). None of the clinical and demographic characteristics were statistically different between those with DVT and without (Table 1).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Male (%) | 544 (50.8) | 6 (31.6) | 538 (51.1) | 0.11 |
| Age, y, meanSD | 65.516.3 | 71.415.3 | 65.416.3 | 0.11 |
| BMI, kg/m2, meanSD | 28.77.6 | 30.112.9 | 28.77.5 | 0.52 |
| Time to US test from admission, h, median | 19.9 | 21.3 | 19.8 | 0.72 |
| Race | 0.74 | |||
| White (%) | 802 (74.9) | 15 (78.9) | 787 (74.8) | |
| Black (%) | 221 (20.6) | 3 (15.8) | 218 (20.7) | |
| Other (%) | 48 (4.5) | 1 (5.3) | 47 (4.5) | |
| Duplex US test <24 hours (%) | 712 (66.5) | 12 (63.2) | 700 (66.5) | 0.81 |
| DVT during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| PE during admission (%) | 2 (0.19) | 0 | 2 (0.19) | 1.0 |
| Medical insurance (%) | 0.79 | |||
| Self‐pay | 35 (3.3) | 0 (0.0) | 35 (3.3) | |
| Medicare | 665 (62.1) | 15 (78.9) | 650 (61.8) | |
| Medicaid | 44 (4.1) | 1 (5.3) | 43 (4.1) | |
| HMO | 49 (4.6) | 0 (0.0) | 49 (4.7) | |
| Blue Cross | 136 (12.7) | 2 (10.5) | 134 (12.7) | |
| Other | 142 (13.3) | 1 (5.3) | 141 (13.4) | |
Patients with DVT had at least 1 risk factor; 16 (84.2%) of them had 2 or more risk factors. In addition, the presence of 2 or more risk factors was much more frequent among those with DVT than among those without (84.2% [16/19] vs 58.4% [614/1052], P=0.03).
As shown in Table 2, a history of DVT or PE and ambulatory dysfunction are the only risk factors associated with DVT at admission. In addition, the prevalence of DVT increases as the number of risk factors increases (Table 3). The prevalence is much higher in those who had 4 or more risk factors than among those with fewer than 4 risk factors (12.2% [6/49] vs 1.3% [13/1022], P=0.0001).
| Total, n=1071 | DVT, n=19 | Non‐DVT, n=1052 | P | |
|---|---|---|---|---|
| ||||
| Age 60 years | 702 (65.6) | 15 (79.0) | 687 (65.3) | 0.33 |
| Previous DVT or PE | 80 (7.5) | 9 (47.4) | 71 (6.8) | <0.0001 |
| Ambulatory dysfunction | 228 (21.3) | 9 (47.4) | 219 (20.8) | 0.01 |
| Obesity | 372 (34.7) | 6 (31.6) | 366 (34.8) | 1.00 |
| Heart failure | 164 (15.3) | 4 (21.1) | 160 (15.2) | 0.52 |
| Stroke/TIA | 75 (7.0) | 3 (15.8) | 72 (6.8) | 0.14 |
| Acute coronary syndrome | 99 (9.2) | 1 (5.3) | 98 (9.3) | 1.00 |
| Active cancer | 124 (11.6) | 4 (21.1) | 120 (11.4) | 0.26 |
| Hormone | 30 (2.8) | 0 | 30 (2.9) | 1.00 |
| Genetic | 4 (0.4) | 0 | 4 (0.4) | 1.00 |
| No. of Risk Factors | Total, n=1071 | DVT, n=19 (1.8%) |
|---|---|---|
| ||
| 0 | 100 | 0 |
| 1 | 341 | 3 (0.9%) |
| 2 | 412 | 7 (1.7%) |
| 3 | 169 | 3 (1.8%) |
| 4 | 39 | 5 (12.8%) |
| 5 | 10 | 1 (10.0%) |
Results of the logistic regression, similar to those of the nonadjusted analysis, showed that the only risk factors independently associated with the discovery of a DVT upon DUSC were the presence of ambulatory dysfunction (odds ratio [OR]: 2.99, 95% confidence interval [CI]: 1.13‐7.90) and a history of DVT or PE (OR: 10.51, 95% CI: 3.90‐28.31) (Table 4).
| ORb | 95% CI | P | |
|---|---|---|---|
| |||
| Age 60 years | 1.76 | 0.535.84 | 0.353 |
| Active cancer | 2.12 | 0.637.17 | 0.227 |
| Ambulatory dysfunction | 2.99 | 1.137.90 | 0.027 |
| Obesity | 0.76 | 0.272.21 | 0.619 |
| Heart failure | 1.33 | 0.394.49 | 0.646 |
| Stroke/TIA | 3.00 | 0.7711.70 | 0.113 |
| Acute coronary syndrome | 1.06 | 0.138.66 | 0.957 |
| Previous DVT or PE | 10.51 | 3.9028.31 | <0.0001 |
| Time to duplex US (19.9 hours)c | 1.94 | 0.725.22 | 0.188 |
We estimated a savings for Medicare of approximately $266,000 to $280,000 ($261.07 1071 DUSC or $261.07 1022 [after excluding the patients with 4 or more risk factors]) over 13 months had the DUSC not being conducted.
DISCUSSION
This study shows that discovering an asymptomatic DVT is relatively rare (<2%) in patients arriving at the hospital for all causes of admission, even taking into account multiple risk factors that increase the risk for DVTs. The study strongly supports the practice of placing compression devices as soon as possible for those patients who have a contraindication to anticoagulant prophylaxis. Along with reducing the delay to placement while awaiting the test, there is significant cost reduction to the healthcare system by not doing DUSC. There appears to be no need for diagnostic studies prior to the placement of these devices unless the patient has more than 3 risk factors or there is a history of previous DVT or ambulatory dysfunction. This study strongly supports the premise that patients are not arriving with DVTs, but are developing them in the hospital.[1, 2, 10] The 1.8% prevalence of asymptomatic DVT in this study is somewhat lower than that found in other studies. The Prophylaxis for Thromboembolism in Critical Care Trial (PROTECT) tested dalteparin vs unfractionated heparin on 3764 patients in the intensive care unit. Initial screening done to rule out DVT found that 3.5% of patients receiving dalteparin and 3.4% receiving unfractionated heparin had proximal DVTs.[8] Other Investigators used venous compression ultrasound examinations of the lower limbs to determine that 5.5% of patients hospitalized in a medical unit have an asymptomatic DVT of the lower limbs on admission.[5] A limitation of that study is the inclusion of all thrombo emboli, specifically those found in the calf (19 out of 21, or 90%). However, if one eliminates the calf venous thrombi, not considered risk factors for PE, the prevalence of DVT (0.85%) is about half that of our observed 1.8%.
In common with previous studies, a history of previous thromboembolic disease was clearly the most significant of many evaluated risk factors for DVT.[5, 6, 10] Ambulatory dysfunction was also a statistically significant risk factor that was likely under‐reported here because of the inexact documentation in many of the medical records. Interestingly, a history of active malignancy did not prove to be a significant risk factor, contrary to other study reports.[5, 6, 10]
The frequency of asymptomatic DVT appears to increase with the accumulation of risk factors. An asymptomatic DVT existed in 1.3% of the patients with 3 or fewer risk factors, compared with 12.2% of those with 4 or 5 risk factors. It is possible that a higher number of risk factors for DVT would be an indication for obtaining a DUSC prior to the placement of PCBs, although the small number of patients with more than 3 risk factors in our study population may limit the strength of this observation.
Limitations
As commented above, the number of patients in whom ambulatory dysfunction is present may be higher than is captured, due to insufficient recognition and poor documentation. Other studies have found a wide variety of risk factors associated with admission and the development of DVTs.[2, 5, 6, 10] Our study was not designed to establish an all‐inclusive list and/or prevalence of risk factors for thromboembolic disease. Another limitation is that only those patients who could not receive heparin prophylaxis received the DUSC evaluation. It is unclear if this could introduce bias inadvertently.
CONCLUSION
Our data strongly suggest, in alignment with recent recommendations, that there is no need to perform screening DUSC prior to the placement of prophylactic compression devices among hospital admissions who have contraindications to anticoagulation. Rather, efforts should be focused on implementing systems to ensure rapid placement of these compression devices at the time of admission for those patients who cannot receive anticoagulation prophylaxis. Evaluation for DVT may be of value if there is a history of previous DVT or PE, ambulatory dysfunction, or more than 3 risk factors, as the information may change the therapeutic approach. Current guidelines recommend the measurement of D‐dimers as a screening tool for DVT.[11]
Acknowledgements
The authors thank Michael Schnee and Alexandria Mapp for their assistance in editing and manuscript preparation.
Disclosure: Nothing to report.
- . The prevention of hospital‐acquired venous thromboembolism in the United Kingdom. Br J Haematol. 2009;144:642–652.
- .U.S. Department of Health and Human Services. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism 2008. Available at: http://www.surgeongeneral.gov/library/calls/deepvein/index.html. Accessed on October 14, 2013.
- , , , , . Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61:401–404.
- , , , et al. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2010;86:217–220.
- , , , et al. High prevalence of asymptomatic deep vein thrombosis on admission in a medical unit among elderly patients. Thromb Haemost. 2002;88:592–597.
- , , , et al. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141:e7S–e47S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e195S–e226S.
- , , , et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305–1314.
- American College of Radiology (2012). Five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/american‐college‐of‐radiology/. Accessed on October 11, 2013.
- , , , et al. Lack of prophylaxis before the onset of acute venous thromboembolism among hospitalized cancer patients: The SWIss Venous Thrombo Embolism Registry (SWIVTER). Ann Oncol. 2010;21:931–935.
- , , , et al. Diagnosis of DVT. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e351S–e418S.
- . The prevention of hospital‐acquired venous thromboembolism in the United Kingdom. Br J Haematol. 2009;144:642–652.
- .U.S. Department of Health and Human Services. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism 2008. Available at: http://www.surgeongeneral.gov/library/calls/deepvein/index.html. Accessed on October 14, 2013.
- , , , , . Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61:401–404.
- , , , et al. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2010;86:217–220.
- , , , et al. High prevalence of asymptomatic deep vein thrombosis on admission in a medical unit among elderly patients. Thromb Haemost. 2002;88:592–597.
- , , , et al. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141:e7S–e47S.
- , , , et al. Prevention of VTE in nonsurgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e195S–e226S.
- , , , et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305–1314.
- American College of Radiology (2012). Five things physicians and patients should question. Available at: http://www.choosingwisely.org/doctor‐patient‐lists/american‐college‐of‐radiology/. Accessed on October 11, 2013.
- , , , et al. Lack of prophylaxis before the onset of acute venous thromboembolism among hospitalized cancer patients: The SWIss Venous Thrombo Embolism Registry (SWIVTER). Ann Oncol. 2010;21:931–935.
- , , , et al. Diagnosis of DVT. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e351S–e418S.
© 2013 Society of Hospital Medicine
Electromagnetic vs Self‐Advancing Tube
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).
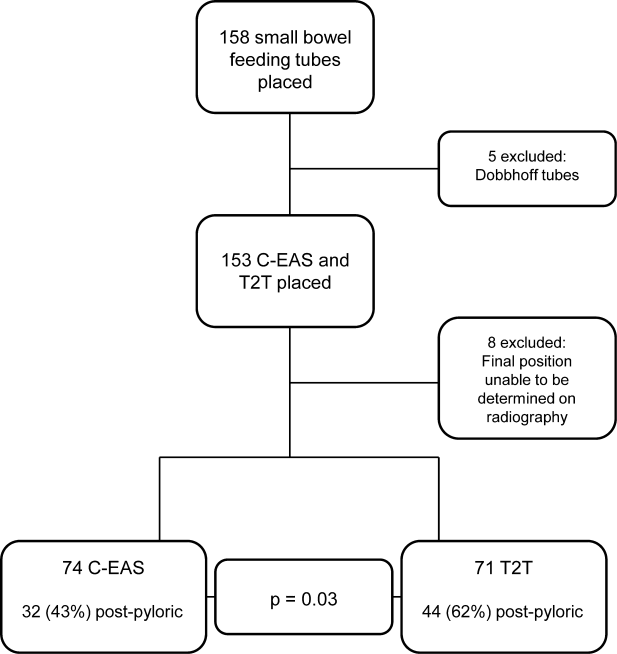
| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).
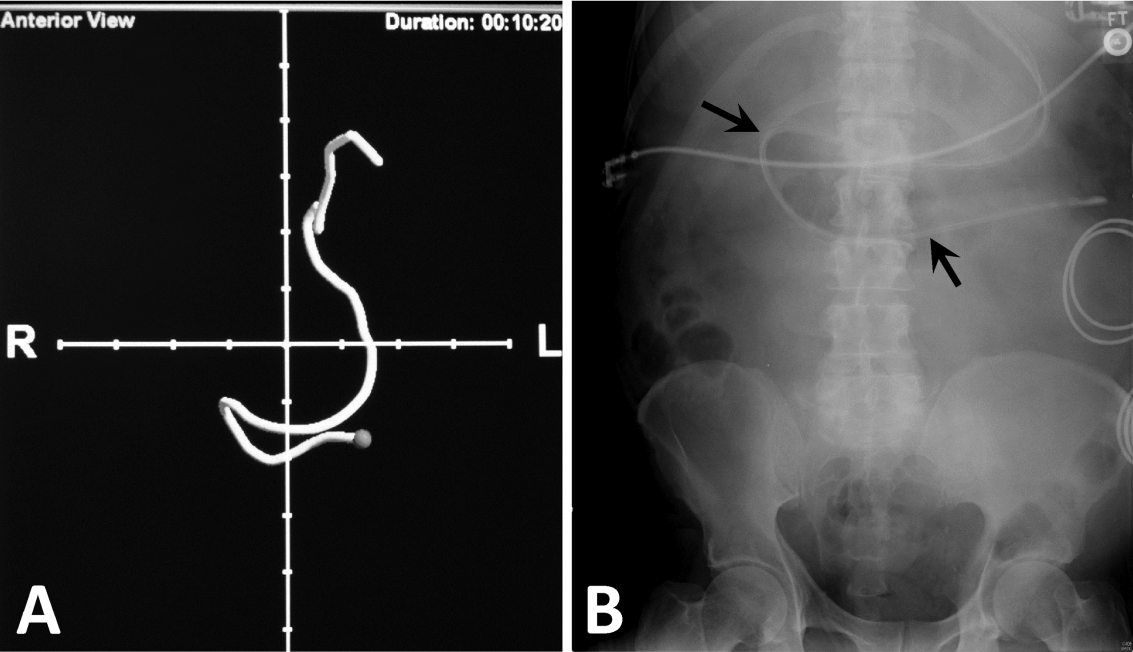
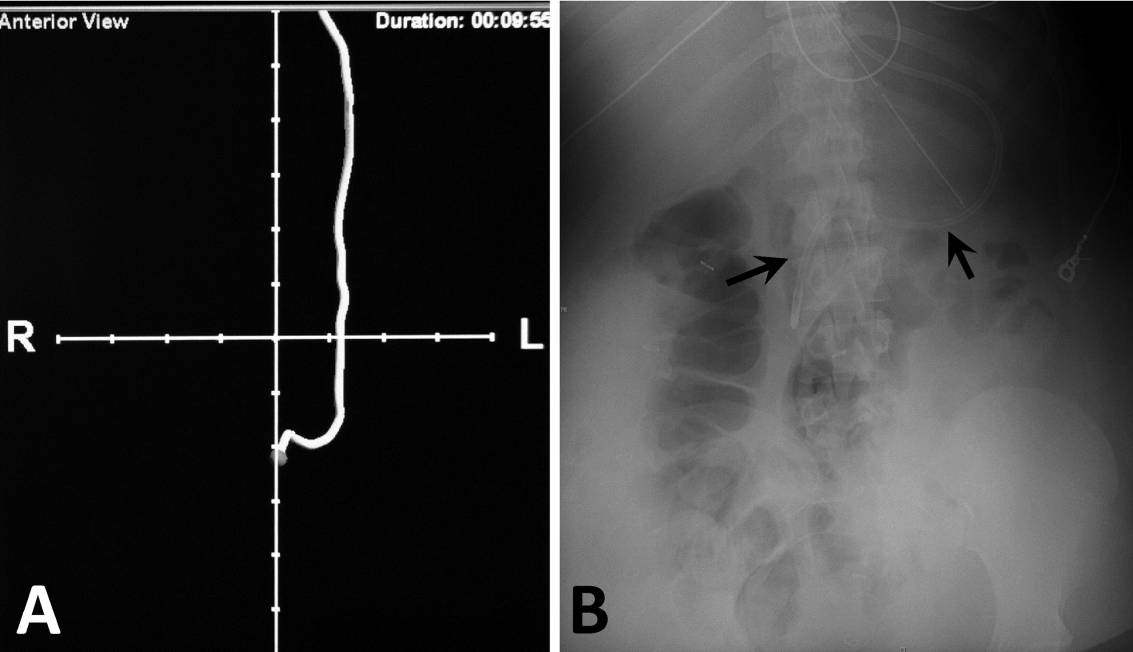
| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).

| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).


| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).

| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).


| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA
Lung cancer screening: USPSTF revises its recommendation
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
When pain persists, so shold investigation
When pain persists, so should investigation
TWO WEEKS OF ABDOMINAL PAIN brought a 63-year- old man to a group medical practice where an internist attributed the pain to gastritis and prescribed an over-the-counter medication.
The internist examined the man several times over the next 4 years, during which time the man complained periodically of nausea and abdominal pain and the doctor prescribed antacids. A different physician who examined the patient during this period recommended referral to a gastroenterologist. Although the internist was told of the recommendation, he didn’t make the referral.
Four years after the patient first reported abdominal pain to the internist, he was diagnosed with stage IV colon cancer. He died the following year at 68 years of age.
PLAINTIFF'S CLAIM The colon cancer should have been diagnosed when the patient initially complained of pain. His symptoms and age called for an immediate colonoscopy (which would have detected the cancer) or referral to a gastroenterologist.
THE DEFENSE The internist maintained that the pa- tient had been advised several times to undergo a colonoscopy and had refused to do so, although records didn’t support that claim. Earlier treatment wouldn’t have changed the outcome.
VERDICT $950,000 New York settlement.
COMMENT I do a fair amount of malpractice case reviews and find that most cases arise from diagnostic delays and missed diagnoses. This physician’s initial approach may have been sensible, but persistence of symptoms is always a reason to escalate the diagnostic approach, and early referral is necessary in the absence of a definitive diagnosis.
Failure to reconsider the initial evaluation
A 29-YEAR-OLD MAN complained of chronic constipation (3 years) and recent rectal bleeding at his first visit to an internist. The doctor performed a rectal examination and ordered a colonoscopy, which was negative and didn’t reveal the cause of the bleeding.
The following year, the patient returned to the internist, reporting new rectal bleeding. After a digital rectal examination, the doctor diagnosed internal hemorrhoids. She continued to treat the patient for the next 3 years. During that time, the patient reported rectal bleeding on 2 occasions; the physician diagnosed external hemorrhoids.
Almost 5 years after his first visit to the internist, the patient requested another colonoscopy, which revealed rectal cancer. After receiving radiation and chemotherapy, the patient underwent abdominoperineal resection with removal of the sphincter muscle, resulting in a permanent colostomy.
PLAINTIFF'S CLAIM The internist couldn’t have diagnosed internal hemorrhoids by digital exam alone unless the hemorrhoids were prolapsing. She was negligent in failing to perform an anoscopy or refer the patient to a gastroenterologist to confirm the cause of the rectal bleeding. Proper management would have enabled diagnosis of the cancer at a stage when radical surgery could have been avoided and the sphincter muscle preserved, eliminating the need for a permanent colostomy.
THE DEFENSE The internist claimed she had diagnosed prolapsing internal hemorrhoids, although the chart noted only internal hemorrhoids. Reliance on the initial negative colonoscopy was proper; earlier diagnosis wouldn’t have changed the patient’s treatment and outcome.
VERDICT $934,779 Illinois bench verdict.
COMMENT This is a difficult case. Colon and rectal cancer are very rare in 29-year-olds, and the initial evaluation was appropriate. At what point should the physician have re-evaluated with colonoscopy or anoscopy and biopsy? I don’t think any retrospectoscope will provide a definitive answer. If this case offers a take-away lesson, it is to reevaluate when potentially serious symptoms persist.
When pain persists, so should investigation
TWO WEEKS OF ABDOMINAL PAIN brought a 63-year- old man to a group medical practice where an internist attributed the pain to gastritis and prescribed an over-the-counter medication.
The internist examined the man several times over the next 4 years, during which time the man complained periodically of nausea and abdominal pain and the doctor prescribed antacids. A different physician who examined the patient during this period recommended referral to a gastroenterologist. Although the internist was told of the recommendation, he didn’t make the referral.
Four years after the patient first reported abdominal pain to the internist, he was diagnosed with stage IV colon cancer. He died the following year at 68 years of age.
PLAINTIFF'S CLAIM The colon cancer should have been diagnosed when the patient initially complained of pain. His symptoms and age called for an immediate colonoscopy (which would have detected the cancer) or referral to a gastroenterologist.
THE DEFENSE The internist maintained that the pa- tient had been advised several times to undergo a colonoscopy and had refused to do so, although records didn’t support that claim. Earlier treatment wouldn’t have changed the outcome.
VERDICT $950,000 New York settlement.
COMMENT I do a fair amount of malpractice case reviews and find that most cases arise from diagnostic delays and missed diagnoses. This physician’s initial approach may have been sensible, but persistence of symptoms is always a reason to escalate the diagnostic approach, and early referral is necessary in the absence of a definitive diagnosis.
Failure to reconsider the initial evaluation
A 29-YEAR-OLD MAN complained of chronic constipation (3 years) and recent rectal bleeding at his first visit to an internist. The doctor performed a rectal examination and ordered a colonoscopy, which was negative and didn’t reveal the cause of the bleeding.
The following year, the patient returned to the internist, reporting new rectal bleeding. After a digital rectal examination, the doctor diagnosed internal hemorrhoids. She continued to treat the patient for the next 3 years. During that time, the patient reported rectal bleeding on 2 occasions; the physician diagnosed external hemorrhoids.
Almost 5 years after his first visit to the internist, the patient requested another colonoscopy, which revealed rectal cancer. After receiving radiation and chemotherapy, the patient underwent abdominoperineal resection with removal of the sphincter muscle, resulting in a permanent colostomy.
PLAINTIFF'S CLAIM The internist couldn’t have diagnosed internal hemorrhoids by digital exam alone unless the hemorrhoids were prolapsing. She was negligent in failing to perform an anoscopy or refer the patient to a gastroenterologist to confirm the cause of the rectal bleeding. Proper management would have enabled diagnosis of the cancer at a stage when radical surgery could have been avoided and the sphincter muscle preserved, eliminating the need for a permanent colostomy.
THE DEFENSE The internist claimed she had diagnosed prolapsing internal hemorrhoids, although the chart noted only internal hemorrhoids. Reliance on the initial negative colonoscopy was proper; earlier diagnosis wouldn’t have changed the patient’s treatment and outcome.
VERDICT $934,779 Illinois bench verdict.
COMMENT This is a difficult case. Colon and rectal cancer are very rare in 29-year-olds, and the initial evaluation was appropriate. At what point should the physician have re-evaluated with colonoscopy or anoscopy and biopsy? I don’t think any retrospectoscope will provide a definitive answer. If this case offers a take-away lesson, it is to reevaluate when potentially serious symptoms persist.
When pain persists, so should investigation
TWO WEEKS OF ABDOMINAL PAIN brought a 63-year- old man to a group medical practice where an internist attributed the pain to gastritis and prescribed an over-the-counter medication.
The internist examined the man several times over the next 4 years, during which time the man complained periodically of nausea and abdominal pain and the doctor prescribed antacids. A different physician who examined the patient during this period recommended referral to a gastroenterologist. Although the internist was told of the recommendation, he didn’t make the referral.
Four years after the patient first reported abdominal pain to the internist, he was diagnosed with stage IV colon cancer. He died the following year at 68 years of age.
PLAINTIFF'S CLAIM The colon cancer should have been diagnosed when the patient initially complained of pain. His symptoms and age called for an immediate colonoscopy (which would have detected the cancer) or referral to a gastroenterologist.
THE DEFENSE The internist maintained that the pa- tient had been advised several times to undergo a colonoscopy and had refused to do so, although records didn’t support that claim. Earlier treatment wouldn’t have changed the outcome.
VERDICT $950,000 New York settlement.
COMMENT I do a fair amount of malpractice case reviews and find that most cases arise from diagnostic delays and missed diagnoses. This physician’s initial approach may have been sensible, but persistence of symptoms is always a reason to escalate the diagnostic approach, and early referral is necessary in the absence of a definitive diagnosis.
Failure to reconsider the initial evaluation
A 29-YEAR-OLD MAN complained of chronic constipation (3 years) and recent rectal bleeding at his first visit to an internist. The doctor performed a rectal examination and ordered a colonoscopy, which was negative and didn’t reveal the cause of the bleeding.
The following year, the patient returned to the internist, reporting new rectal bleeding. After a digital rectal examination, the doctor diagnosed internal hemorrhoids. She continued to treat the patient for the next 3 years. During that time, the patient reported rectal bleeding on 2 occasions; the physician diagnosed external hemorrhoids.
Almost 5 years after his first visit to the internist, the patient requested another colonoscopy, which revealed rectal cancer. After receiving radiation and chemotherapy, the patient underwent abdominoperineal resection with removal of the sphincter muscle, resulting in a permanent colostomy.
PLAINTIFF'S CLAIM The internist couldn’t have diagnosed internal hemorrhoids by digital exam alone unless the hemorrhoids were prolapsing. She was negligent in failing to perform an anoscopy or refer the patient to a gastroenterologist to confirm the cause of the rectal bleeding. Proper management would have enabled diagnosis of the cancer at a stage when radical surgery could have been avoided and the sphincter muscle preserved, eliminating the need for a permanent colostomy.
THE DEFENSE The internist claimed she had diagnosed prolapsing internal hemorrhoids, although the chart noted only internal hemorrhoids. Reliance on the initial negative colonoscopy was proper; earlier diagnosis wouldn’t have changed the patient’s treatment and outcome.
VERDICT $934,779 Illinois bench verdict.
COMMENT This is a difficult case. Colon and rectal cancer are very rare in 29-year-olds, and the initial evaluation was appropriate. At what point should the physician have re-evaluated with colonoscopy or anoscopy and biopsy? I don’t think any retrospectoscope will provide a definitive answer. If this case offers a take-away lesson, it is to reevaluate when potentially serious symptoms persist.
Breast biopsy delayed. $1.5M verdict
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Gastroesophageal reflux disease: The case for improving patient education in primary care
ABSTRACT
Purpose Gastroesophageal reflux disease (GERD) affects up to 25% of the western population, and the annual expenditure for managing GERD is estimated to be more than $14 billion. Most GERD patients do not consult a specialist, but rather rely on their primary care physician for symptom management. Research has shown that many patients—regardless of diagnosis—do not fully understand what their doctors tell them and remain uncertain as to what they are supposed to do to take care of themselves. To determine if patients are adequately educated in the management of GERD, we conducted a survey.
Method We administered a survey to patients with GERD in an outpatient setting and explored their knowledge of such management practices as modification of behavior and diet and use of medication.
Results Of 333 patients enrolled, 66% reported having an in-depth discussion with their primary care physician. Among patients taking a proton pump inhibitor, 85% of those who’d had an in-depth discussion were aware of the best time to take their medication, compared with only 18% of those who did not have an in-depth discussion. In addition, patients who’d had in-depth conversations were significantly more likely than those who didn’t to know some of the behavior modification measures that might improve their symptoms.
Conclusion Our study underscores the need for primary care providers to fully discuss GERD with their patients to improve overall management of the disease.
Gastroesophageal reflux disease (GERD) affects between 15% and 25% of populations in Western countries, and is estimated to account for health care costs totaling more than $14 billion.1 In North America, the prevalence of reflux symptoms is increasing, on average by 5% annually1—this despite significant improvements in the identification and treatment of the disorder. Could it be that improvement in physician-patient communication is also needed to ensure management success?
Most GERD patients are seen in the primary care (PC) setting. Although patient education is an important aspect of treating GERD, physicians often lack sufficient time to educate patients properly. Research has shown that many patients, regardless of diagnosis, do not fully understand what their doctors tell them and remain uncertain of what they are supposed to do to take care of themselves.2,3
In this article, we report the results of a simple survey administered in the PC setting to patients experiencing symptomatic GERD that necessitated the use of a medication. We hypothesized that patients were not adequately informed about their condition and that patient adherence was associated with the depth of dialogue with their physician.
METHODS
This study was approved by the Advocate Lutheran General Hospital Institutional Review Board. Fellows and faculty in the Advocate Lutheran General Hospital gastroenterology fellowship program developed the survey collaboratively and carried out the study at the Advocate Medical Group outpatient PC clinic and at local physicians’ offices. We opened participation to all patients >18 years of age with previously diagnosed GERD who visited affiliated outpatient clinics or offices during the data collection period between January 2009 and May 2010. There were no additional criteria or selection screens.
After obtaining a patient’s informed consent, an attending physician or resident handed the patient a multiple-choice survey (TABLE 1), but did not supervise the activity. A clerk collected the completed surveys and separated responses from personally identifiable information before entering results into a database.
Since one of the major goals of this study was to relate patient perception of the quality and clarity of education received from the physician to actual understanding of GERD, we intentionally avoided giving precise descriptions of the qualitative terms used in the survey, such as “in-depth,” “best,” and “likely.” We did, however, provide definitions and descriptions for nonqualitative words and terms, such as GERD and sleeping position. A clerk or administering physician fielded patients’ questions about the survey. We did not attempt to make comparisons between our survey and other available surveys.4,5
What we expected to find. Based on expert consultation with attending gastrointestinal faculty at our institution, we expected that approximately 30% of GERD patients would not have an in-depth discussion with their PC physician regarding lifestyle modifications and risk factors affecting GERD. We planned a study of independent cases (those not having an in-depth discussion) and controls (those having an in-depth discussion), with 2 controls per case. Our primary endpoint was the survey item that asked patients to specify the best time for taking their proton-pump inhibitor (PPI). We expected that 70% of the controls and 50% of independent cases would know the correct time to take their PPI medication. Our null hypothesis stated that the rates for case and control subjects would be equal with a probability (power) of 0.80. To reject the null hypothesis, we needed a minimum total sample of 207 patients taking a PPI (138 control subjects and 69 case subjects). The Type I error probability associated with this test of this null hypothesis is .05.6
We reported descriptive statistics for categorical and continuous data, and performed between-group statistical comparisons on survey items via the Chi-square test or Fisher’s Exact test when necessary. We performed age comparisons with the Independent t-test. We considered a 2-tailed value of .05 as statistically significant in all analyses, which we conducted with SPSS software (International Business Machines, Chicago, Ill).
RESULTS
All 333 patients invited to participate gave informed consent and completed the survey in its entirety. Patients were evenly distributed by gender. The median age of all patients was 44±13.1 years (range, 19-83). Approximately two-thirds of patients (66%) perceived that discussions with their PC physicians regarding lifestyle modifications and risk factors affecting GERD were “in-depth.”
We examined the gender and age of patients as functions of perceived discussion level. Men and women were equally likely to have in-depth discussions with their physicians (P>.05). On the other hand, younger patients were more likely than older ones to have in-depth discussions. The mean age of the patients who reported having an in-depth discussion was 42.6±13.1 years; of those who did not have an in-depth discussion, the mean age was 46.0±12.9 years (P<.05). However, for each individual survey question, the number of correct responses did not correlate with age, suggesting that age is not a true predictor of level of discussion.
Approximately one-third of patients (32%) were not aware that untreated GERD can be associated with increased risk of cancer of the esophagus, although there was no significant difference between discussion level groups (P>.05) (TABLE 2). Similar numbers of patients reported being aware of best sleeping position (33%), with those in the in-depth discussion group exhibiting a better understanding of the best position (36% vs 26%, respectively; P<.05). Patients perceiving they had an in-depth discussion with their PC physicians were significantly more likely than those in the other group to know the appropriate time to eat dinner (76% vs 24%, respectively; P<.001). Overall and without respect to discussion level, few patients understood dietary guidelines (18%, P>.05).
Patients having an in-depth discussion with their primary care physician were also more likely to know the best time to take antireflux medications (85% vs 18%, respectively, P<.001); that GERD symptoms can be reduced through lifestyle modifications (53% vs 22%, respectively; P<.001); and that GERD can manifest as chronic cough, hoarse voice, or sore throat (79% vs 36%, respectively; P<.001).
DISCUSSION
We conducted a simple, easy-to-use survey to gain an appreciation of patients’ levels of understanding of GERD after discussion with their physicians. Specifically, the survey related a patient’s perceived level of discussion to his or her knowledge of facts pertinent to GERD, including personal lifestyle choices. Importantly, the patient’s perception of the quality of discussion was surveyed, as perception and reality are not always in agreement.
Although the American Gastroenterological Association advocates that physicians should have in-depth discussions with their patients with respect to the disease process and lifestyle modifications, this is not typical.4,7
In our study, 66% of patients perceived that they had an in-depth discussion with their PC physician regarding factors affecting GERD. Of patients taking a PPI, 85% of those having in-depth discussions were aware of the correct time to take their medication. Of those not having an in-depth discussion, only 18% gave the correct answer, which is no better than a random guess (20%) and suggests that, essentially, none of these patients knew when to take their medication.
Study limitations. We did not validate our survey against other surveys or within our patient population; additional efforts are therefore warranted in this regard. Future studies might also explore how duration and severity of symptoms prior to patient-physician discussions, socioeconomic status, and education level influence the relationship between perception of care and understanding of GERD. A follow-up survey would help to define whether these discussions translate into improved disease management.
In spite of the limitations of our survey, our data clearly demonstrate that knowledge of GERD correlates with the perceived level of discussion that patients have with their physician, and that a large percentage of patients do not fully understand their condition and methods to manage it. Patients who did have an in-depth discussion with their primary care provider were likely to be better educated with regard to GERD. Because of the prevalence of GERD,1,7,8 its association with increased health care costs and reduced quality of life,1,8 and predominant management at the primary care level, we recommend that PC physicians place more emphasis on the education of patients diagnosed with GERD.
CORRESPONDENCE
Naser M. Khan, MD, Advanced Gastroenterology Associates, Doctors Office Building 3, Suite 2300b, 1555 Barrington Road, Hoffman Estates, IL 60169; [email protected]
1. Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:577-599, vii-ix.
2. Kessels RPC. Patients’ memory for medical information. J R Soc Med. 2003;96:219-222.
3. Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80:991-994.
4. American Gastroenterological Association. New Nationwide Survey Identifies Need for Increased Dialogue Between Gastroesophageal Reflux Disease or Frequent Heartburn Sufferers and Health Care Providers. Available at: http://www.gastro.org/news/articles/2011/03/23/new-nationwide-survey-identifiesneed-for-increased-dialogue-between-gastroesophageal-reflux-disease-or-frequent-heartburn-sufferers-and-health-careproviders. Accessed July 19, 2012.
5. ClinicalTrials.gov. National Survey on Gastroesophageal Reflux Disease (GERD) Patients (LINEA). Available at: http://clinicaltrials.gov/ct2/show/NCT00695838. Accessed July 19, 2012.
6. Dupont WD, Plummer WD. PS power and sample size program available for free on the internet. Control Clin Trials. 1997;18:274.
7. Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392-1413, 1413.e1-e5.
8. El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26.
ABSTRACT
Purpose Gastroesophageal reflux disease (GERD) affects up to 25% of the western population, and the annual expenditure for managing GERD is estimated to be more than $14 billion. Most GERD patients do not consult a specialist, but rather rely on their primary care physician for symptom management. Research has shown that many patients—regardless of diagnosis—do not fully understand what their doctors tell them and remain uncertain as to what they are supposed to do to take care of themselves. To determine if patients are adequately educated in the management of GERD, we conducted a survey.
Method We administered a survey to patients with GERD in an outpatient setting and explored their knowledge of such management practices as modification of behavior and diet and use of medication.
Results Of 333 patients enrolled, 66% reported having an in-depth discussion with their primary care physician. Among patients taking a proton pump inhibitor, 85% of those who’d had an in-depth discussion were aware of the best time to take their medication, compared with only 18% of those who did not have an in-depth discussion. In addition, patients who’d had in-depth conversations were significantly more likely than those who didn’t to know some of the behavior modification measures that might improve their symptoms.
Conclusion Our study underscores the need for primary care providers to fully discuss GERD with their patients to improve overall management of the disease.
Gastroesophageal reflux disease (GERD) affects between 15% and 25% of populations in Western countries, and is estimated to account for health care costs totaling more than $14 billion.1 In North America, the prevalence of reflux symptoms is increasing, on average by 5% annually1—this despite significant improvements in the identification and treatment of the disorder. Could it be that improvement in physician-patient communication is also needed to ensure management success?
Most GERD patients are seen in the primary care (PC) setting. Although patient education is an important aspect of treating GERD, physicians often lack sufficient time to educate patients properly. Research has shown that many patients, regardless of diagnosis, do not fully understand what their doctors tell them and remain uncertain of what they are supposed to do to take care of themselves.2,3
In this article, we report the results of a simple survey administered in the PC setting to patients experiencing symptomatic GERD that necessitated the use of a medication. We hypothesized that patients were not adequately informed about their condition and that patient adherence was associated with the depth of dialogue with their physician.
METHODS
This study was approved by the Advocate Lutheran General Hospital Institutional Review Board. Fellows and faculty in the Advocate Lutheran General Hospital gastroenterology fellowship program developed the survey collaboratively and carried out the study at the Advocate Medical Group outpatient PC clinic and at local physicians’ offices. We opened participation to all patients >18 years of age with previously diagnosed GERD who visited affiliated outpatient clinics or offices during the data collection period between January 2009 and May 2010. There were no additional criteria or selection screens.
After obtaining a patient’s informed consent, an attending physician or resident handed the patient a multiple-choice survey (TABLE 1), but did not supervise the activity. A clerk collected the completed surveys and separated responses from personally identifiable information before entering results into a database.
Since one of the major goals of this study was to relate patient perception of the quality and clarity of education received from the physician to actual understanding of GERD, we intentionally avoided giving precise descriptions of the qualitative terms used in the survey, such as “in-depth,” “best,” and “likely.” We did, however, provide definitions and descriptions for nonqualitative words and terms, such as GERD and sleeping position. A clerk or administering physician fielded patients’ questions about the survey. We did not attempt to make comparisons between our survey and other available surveys.4,5
What we expected to find. Based on expert consultation with attending gastrointestinal faculty at our institution, we expected that approximately 30% of GERD patients would not have an in-depth discussion with their PC physician regarding lifestyle modifications and risk factors affecting GERD. We planned a study of independent cases (those not having an in-depth discussion) and controls (those having an in-depth discussion), with 2 controls per case. Our primary endpoint was the survey item that asked patients to specify the best time for taking their proton-pump inhibitor (PPI). We expected that 70% of the controls and 50% of independent cases would know the correct time to take their PPI medication. Our null hypothesis stated that the rates for case and control subjects would be equal with a probability (power) of 0.80. To reject the null hypothesis, we needed a minimum total sample of 207 patients taking a PPI (138 control subjects and 69 case subjects). The Type I error probability associated with this test of this null hypothesis is .05.6
We reported descriptive statistics for categorical and continuous data, and performed between-group statistical comparisons on survey items via the Chi-square test or Fisher’s Exact test when necessary. We performed age comparisons with the Independent t-test. We considered a 2-tailed value of .05 as statistically significant in all analyses, which we conducted with SPSS software (International Business Machines, Chicago, Ill).
RESULTS
All 333 patients invited to participate gave informed consent and completed the survey in its entirety. Patients were evenly distributed by gender. The median age of all patients was 44±13.1 years (range, 19-83). Approximately two-thirds of patients (66%) perceived that discussions with their PC physicians regarding lifestyle modifications and risk factors affecting GERD were “in-depth.”
We examined the gender and age of patients as functions of perceived discussion level. Men and women were equally likely to have in-depth discussions with their physicians (P>.05). On the other hand, younger patients were more likely than older ones to have in-depth discussions. The mean age of the patients who reported having an in-depth discussion was 42.6±13.1 years; of those who did not have an in-depth discussion, the mean age was 46.0±12.9 years (P<.05). However, for each individual survey question, the number of correct responses did not correlate with age, suggesting that age is not a true predictor of level of discussion.
Approximately one-third of patients (32%) were not aware that untreated GERD can be associated with increased risk of cancer of the esophagus, although there was no significant difference between discussion level groups (P>.05) (TABLE 2). Similar numbers of patients reported being aware of best sleeping position (33%), with those in the in-depth discussion group exhibiting a better understanding of the best position (36% vs 26%, respectively; P<.05). Patients perceiving they had an in-depth discussion with their PC physicians were significantly more likely than those in the other group to know the appropriate time to eat dinner (76% vs 24%, respectively; P<.001). Overall and without respect to discussion level, few patients understood dietary guidelines (18%, P>.05).
Patients having an in-depth discussion with their primary care physician were also more likely to know the best time to take antireflux medications (85% vs 18%, respectively, P<.001); that GERD symptoms can be reduced through lifestyle modifications (53% vs 22%, respectively; P<.001); and that GERD can manifest as chronic cough, hoarse voice, or sore throat (79% vs 36%, respectively; P<.001).
DISCUSSION
We conducted a simple, easy-to-use survey to gain an appreciation of patients’ levels of understanding of GERD after discussion with their physicians. Specifically, the survey related a patient’s perceived level of discussion to his or her knowledge of facts pertinent to GERD, including personal lifestyle choices. Importantly, the patient’s perception of the quality of discussion was surveyed, as perception and reality are not always in agreement.
Although the American Gastroenterological Association advocates that physicians should have in-depth discussions with their patients with respect to the disease process and lifestyle modifications, this is not typical.4,7
In our study, 66% of patients perceived that they had an in-depth discussion with their PC physician regarding factors affecting GERD. Of patients taking a PPI, 85% of those having in-depth discussions were aware of the correct time to take their medication. Of those not having an in-depth discussion, only 18% gave the correct answer, which is no better than a random guess (20%) and suggests that, essentially, none of these patients knew when to take their medication.
Study limitations. We did not validate our survey against other surveys or within our patient population; additional efforts are therefore warranted in this regard. Future studies might also explore how duration and severity of symptoms prior to patient-physician discussions, socioeconomic status, and education level influence the relationship between perception of care and understanding of GERD. A follow-up survey would help to define whether these discussions translate into improved disease management.
In spite of the limitations of our survey, our data clearly demonstrate that knowledge of GERD correlates with the perceived level of discussion that patients have with their physician, and that a large percentage of patients do not fully understand their condition and methods to manage it. Patients who did have an in-depth discussion with their primary care provider were likely to be better educated with regard to GERD. Because of the prevalence of GERD,1,7,8 its association with increased health care costs and reduced quality of life,1,8 and predominant management at the primary care level, we recommend that PC physicians place more emphasis on the education of patients diagnosed with GERD.
CORRESPONDENCE
Naser M. Khan, MD, Advanced Gastroenterology Associates, Doctors Office Building 3, Suite 2300b, 1555 Barrington Road, Hoffman Estates, IL 60169; [email protected]
ABSTRACT
Purpose Gastroesophageal reflux disease (GERD) affects up to 25% of the western population, and the annual expenditure for managing GERD is estimated to be more than $14 billion. Most GERD patients do not consult a specialist, but rather rely on their primary care physician for symptom management. Research has shown that many patients—regardless of diagnosis—do not fully understand what their doctors tell them and remain uncertain as to what they are supposed to do to take care of themselves. To determine if patients are adequately educated in the management of GERD, we conducted a survey.
Method We administered a survey to patients with GERD in an outpatient setting and explored their knowledge of such management practices as modification of behavior and diet and use of medication.
Results Of 333 patients enrolled, 66% reported having an in-depth discussion with their primary care physician. Among patients taking a proton pump inhibitor, 85% of those who’d had an in-depth discussion were aware of the best time to take their medication, compared with only 18% of those who did not have an in-depth discussion. In addition, patients who’d had in-depth conversations were significantly more likely than those who didn’t to know some of the behavior modification measures that might improve their symptoms.
Conclusion Our study underscores the need for primary care providers to fully discuss GERD with their patients to improve overall management of the disease.
Gastroesophageal reflux disease (GERD) affects between 15% and 25% of populations in Western countries, and is estimated to account for health care costs totaling more than $14 billion.1 In North America, the prevalence of reflux symptoms is increasing, on average by 5% annually1—this despite significant improvements in the identification and treatment of the disorder. Could it be that improvement in physician-patient communication is also needed to ensure management success?
Most GERD patients are seen in the primary care (PC) setting. Although patient education is an important aspect of treating GERD, physicians often lack sufficient time to educate patients properly. Research has shown that many patients, regardless of diagnosis, do not fully understand what their doctors tell them and remain uncertain of what they are supposed to do to take care of themselves.2,3
In this article, we report the results of a simple survey administered in the PC setting to patients experiencing symptomatic GERD that necessitated the use of a medication. We hypothesized that patients were not adequately informed about their condition and that patient adherence was associated with the depth of dialogue with their physician.
METHODS
This study was approved by the Advocate Lutheran General Hospital Institutional Review Board. Fellows and faculty in the Advocate Lutheran General Hospital gastroenterology fellowship program developed the survey collaboratively and carried out the study at the Advocate Medical Group outpatient PC clinic and at local physicians’ offices. We opened participation to all patients >18 years of age with previously diagnosed GERD who visited affiliated outpatient clinics or offices during the data collection period between January 2009 and May 2010. There were no additional criteria or selection screens.
After obtaining a patient’s informed consent, an attending physician or resident handed the patient a multiple-choice survey (TABLE 1), but did not supervise the activity. A clerk collected the completed surveys and separated responses from personally identifiable information before entering results into a database.
Since one of the major goals of this study was to relate patient perception of the quality and clarity of education received from the physician to actual understanding of GERD, we intentionally avoided giving precise descriptions of the qualitative terms used in the survey, such as “in-depth,” “best,” and “likely.” We did, however, provide definitions and descriptions for nonqualitative words and terms, such as GERD and sleeping position. A clerk or administering physician fielded patients’ questions about the survey. We did not attempt to make comparisons between our survey and other available surveys.4,5
What we expected to find. Based on expert consultation with attending gastrointestinal faculty at our institution, we expected that approximately 30% of GERD patients would not have an in-depth discussion with their PC physician regarding lifestyle modifications and risk factors affecting GERD. We planned a study of independent cases (those not having an in-depth discussion) and controls (those having an in-depth discussion), with 2 controls per case. Our primary endpoint was the survey item that asked patients to specify the best time for taking their proton-pump inhibitor (PPI). We expected that 70% of the controls and 50% of independent cases would know the correct time to take their PPI medication. Our null hypothesis stated that the rates for case and control subjects would be equal with a probability (power) of 0.80. To reject the null hypothesis, we needed a minimum total sample of 207 patients taking a PPI (138 control subjects and 69 case subjects). The Type I error probability associated with this test of this null hypothesis is .05.6
We reported descriptive statistics for categorical and continuous data, and performed between-group statistical comparisons on survey items via the Chi-square test or Fisher’s Exact test when necessary. We performed age comparisons with the Independent t-test. We considered a 2-tailed value of .05 as statistically significant in all analyses, which we conducted with SPSS software (International Business Machines, Chicago, Ill).
RESULTS
All 333 patients invited to participate gave informed consent and completed the survey in its entirety. Patients were evenly distributed by gender. The median age of all patients was 44±13.1 years (range, 19-83). Approximately two-thirds of patients (66%) perceived that discussions with their PC physicians regarding lifestyle modifications and risk factors affecting GERD were “in-depth.”
We examined the gender and age of patients as functions of perceived discussion level. Men and women were equally likely to have in-depth discussions with their physicians (P>.05). On the other hand, younger patients were more likely than older ones to have in-depth discussions. The mean age of the patients who reported having an in-depth discussion was 42.6±13.1 years; of those who did not have an in-depth discussion, the mean age was 46.0±12.9 years (P<.05). However, for each individual survey question, the number of correct responses did not correlate with age, suggesting that age is not a true predictor of level of discussion.
Approximately one-third of patients (32%) were not aware that untreated GERD can be associated with increased risk of cancer of the esophagus, although there was no significant difference between discussion level groups (P>.05) (TABLE 2). Similar numbers of patients reported being aware of best sleeping position (33%), with those in the in-depth discussion group exhibiting a better understanding of the best position (36% vs 26%, respectively; P<.05). Patients perceiving they had an in-depth discussion with their PC physicians were significantly more likely than those in the other group to know the appropriate time to eat dinner (76% vs 24%, respectively; P<.001). Overall and without respect to discussion level, few patients understood dietary guidelines (18%, P>.05).
Patients having an in-depth discussion with their primary care physician were also more likely to know the best time to take antireflux medications (85% vs 18%, respectively, P<.001); that GERD symptoms can be reduced through lifestyle modifications (53% vs 22%, respectively; P<.001); and that GERD can manifest as chronic cough, hoarse voice, or sore throat (79% vs 36%, respectively; P<.001).
DISCUSSION
We conducted a simple, easy-to-use survey to gain an appreciation of patients’ levels of understanding of GERD after discussion with their physicians. Specifically, the survey related a patient’s perceived level of discussion to his or her knowledge of facts pertinent to GERD, including personal lifestyle choices. Importantly, the patient’s perception of the quality of discussion was surveyed, as perception and reality are not always in agreement.
Although the American Gastroenterological Association advocates that physicians should have in-depth discussions with their patients with respect to the disease process and lifestyle modifications, this is not typical.4,7
In our study, 66% of patients perceived that they had an in-depth discussion with their PC physician regarding factors affecting GERD. Of patients taking a PPI, 85% of those having in-depth discussions were aware of the correct time to take their medication. Of those not having an in-depth discussion, only 18% gave the correct answer, which is no better than a random guess (20%) and suggests that, essentially, none of these patients knew when to take their medication.
Study limitations. We did not validate our survey against other surveys or within our patient population; additional efforts are therefore warranted in this regard. Future studies might also explore how duration and severity of symptoms prior to patient-physician discussions, socioeconomic status, and education level influence the relationship between perception of care and understanding of GERD. A follow-up survey would help to define whether these discussions translate into improved disease management.
In spite of the limitations of our survey, our data clearly demonstrate that knowledge of GERD correlates with the perceived level of discussion that patients have with their physician, and that a large percentage of patients do not fully understand their condition and methods to manage it. Patients who did have an in-depth discussion with their primary care provider were likely to be better educated with regard to GERD. Because of the prevalence of GERD,1,7,8 its association with increased health care costs and reduced quality of life,1,8 and predominant management at the primary care level, we recommend that PC physicians place more emphasis on the education of patients diagnosed with GERD.
CORRESPONDENCE
Naser M. Khan, MD, Advanced Gastroenterology Associates, Doctors Office Building 3, Suite 2300b, 1555 Barrington Road, Hoffman Estates, IL 60169; [email protected]
1. Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:577-599, vii-ix.
2. Kessels RPC. Patients’ memory for medical information. J R Soc Med. 2003;96:219-222.
3. Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80:991-994.
4. American Gastroenterological Association. New Nationwide Survey Identifies Need for Increased Dialogue Between Gastroesophageal Reflux Disease or Frequent Heartburn Sufferers and Health Care Providers. Available at: http://www.gastro.org/news/articles/2011/03/23/new-nationwide-survey-identifiesneed-for-increased-dialogue-between-gastroesophageal-reflux-disease-or-frequent-heartburn-sufferers-and-health-careproviders. Accessed July 19, 2012.
5. ClinicalTrials.gov. National Survey on Gastroesophageal Reflux Disease (GERD) Patients (LINEA). Available at: http://clinicaltrials.gov/ct2/show/NCT00695838. Accessed July 19, 2012.
6. Dupont WD, Plummer WD. PS power and sample size program available for free on the internet. Control Clin Trials. 1997;18:274.
7. Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392-1413, 1413.e1-e5.
8. El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26.
1. Richter JE. The many manifestations of gastroesophageal reflux disease: presentation, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:577-599, vii-ix.
2. Kessels RPC. Patients’ memory for medical information. J R Soc Med. 2003;96:219-222.
3. Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80:991-994.
4. American Gastroenterological Association. New Nationwide Survey Identifies Need for Increased Dialogue Between Gastroesophageal Reflux Disease or Frequent Heartburn Sufferers and Health Care Providers. Available at: http://www.gastro.org/news/articles/2011/03/23/new-nationwide-survey-identifiesneed-for-increased-dialogue-between-gastroesophageal-reflux-disease-or-frequent-heartburn-sufferers-and-health-careproviders. Accessed July 19, 2012.
5. ClinicalTrials.gov. National Survey on Gastroesophageal Reflux Disease (GERD) Patients (LINEA). Available at: http://clinicaltrials.gov/ct2/show/NCT00695838. Accessed July 19, 2012.
6. Dupont WD, Plummer WD. PS power and sample size program available for free on the internet. Control Clin Trials. 1997;18:274.
7. Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392-1413, 1413.e1-e5.
8. El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17-26.
Upper Extremity DVT in Hospitalized Patients
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.
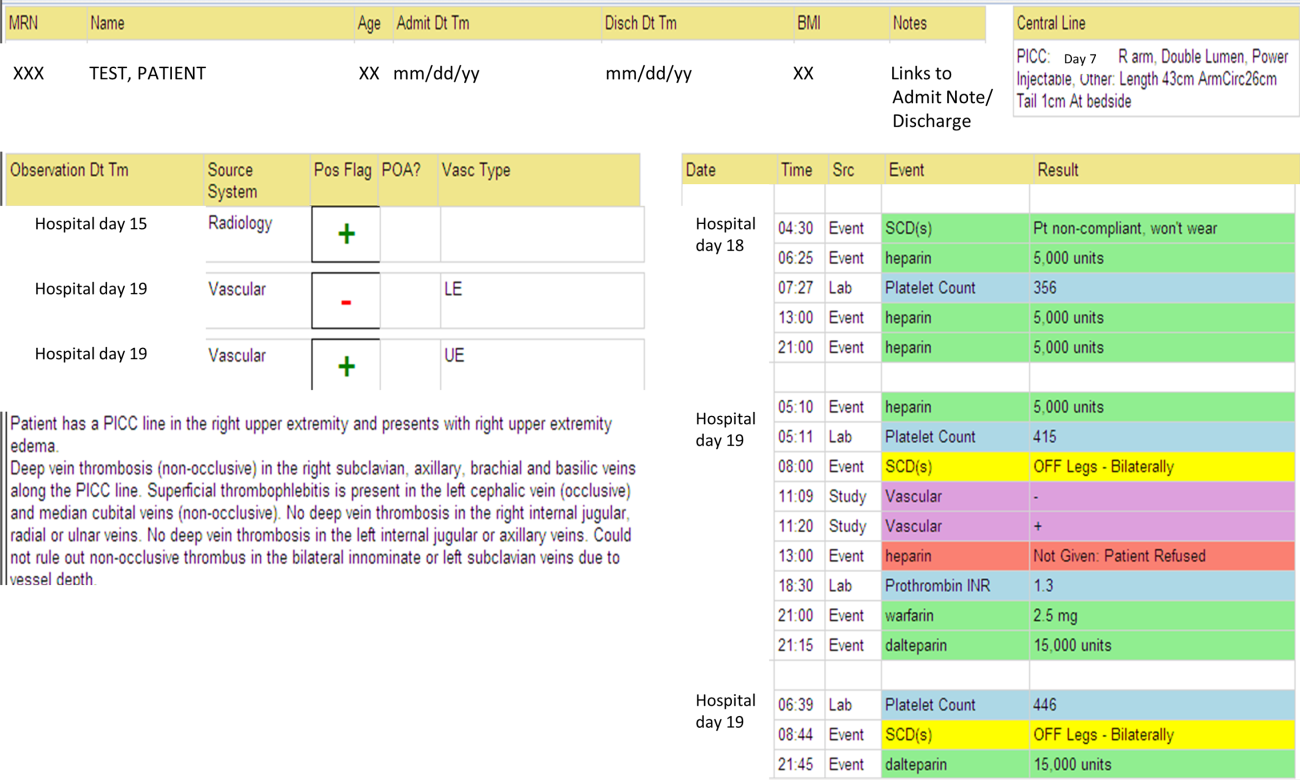
The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
Increasingly, there is a focus on prevention of hospital‐acquired conditions including venous thromboembolism (VTE). Many studies have evaluated pulmonary embolism (PE) and lower extremity deep vein thrombosis (LEDVT), but despite increasing recognition of upper extremity deep vein thrombosis (UEDVT),[1, 2, 3, 4] less is known about this condition in hospitalized patients.
UEDVTs may be classified as primary, including disorders such as Paget‐Schroetter syndrome or other structural abnormality, or may be idiopathic; the majority are secondary clots.[5] Conventional risk factors for LEDVT including older age and obesity have been found to be less commonly associated,[1, 2, 5, 6, 7] and patients with UEDVT are generally younger, leaner, and a higher proportion are men. They are more likely to have malignancy or history of VTE and have undergone recent surgery or intensive care unit stay.[1, 2, 6] Central venous catheters (CVCs), often used in hospitalized patients, remain among the biggest known risks for UEDVT[1, 2, 3, 7, 8, 9, 10]; concomitant malignancy, VTE history, severe infection, surgery lasting >1 hour, and length of stay (LOS) >10 days confer additional risks with CVCs.[6, 7, 8, 11]
UEDVTs, once thought to be relatively benign, are now recognized to result in complications including PE, progression, recurrence, and post‐thrombotic syndrome.[2, 4, 12, 13] Despite extensive efforts to increase appropriate VTE prophylaxis in inpatients,[14] the role of chemoprophylaxis to prevent UEDVT remains undefined. Current guidelines recommend anticoagulation for treatment and complication prevention,[13, 15] but to date the evidence derives largely from observational studies or is extrapolated from the LEDVT literature.[2, 13]
To improve understanding of UEDVT at our institution, we set out to (1) determine UEDVT incidence in hospitalized patients, (2) describe associated risks and outcomes, and (3) assess management during hospitalization and at discharge.
METHODS
We identified all consecutive adult patients diagnosed with Doppler ultrasound‐confirmed UEDVT during hospitalization at Harborview Medical Center between September 2011 and November 2012. For patients who were readmitted during the study period, the first of their hospitalizations was used to describe associated factors, management, and outcomes. We present characteristics of all other hospitalizations during this time period for comparison. Harborview is a 413‐bed academic tertiary referral center and the only level 1 trauma center in a 5‐state area. Patients with UEDVT were identified using an information technology (IT) tool (the Harborview VTE tool) (Figure 1), which captures VTE events from vascular laboratory and radiology studies using natural language processing. Doppler ultrasound to assess for deep vein thrombosis (DVT) and computed tomographic scans to diagnose PE were ordered by inpatient physicians for symptomatic patients. The reason for obtaining the study is included in the ultrasound reports. We do not routinely screen for UEDVT at our institution. UEDVT included clots in the deep veins of the upper extremities including internal jugular, subclavian, axillary, and brachial veins. Superficial thrombosis and thrombophlebitis were excluded. We previously compared VTE events captured by this tool with administrative billing data and found that all VTE events that were coded were captured with the tool.

The VTE tool (Figure 1) displays imaging results together with demographic, clinical, and medication data and links this information with admission, discharge, and death summaries as well as CVC insertion procedure notes from the electronic health record (EHR). Additional data, including comorbid conditions, primary reason for hospitalization, past medical history such as prior VTE events, and cause of death (if not available in the admission note or discharge/death summaries), were obtained from EHR abstraction by 1 of the investigators. A 10% random sample of charts was rereviewed by another investigator with complete concordance. Supplementary data about date of CVC insertion if placed at an outside facility, date of CVC removal if applicable, clinical assessments regarding whether a clot was CVC‐associated, and contraindications to therapeutic anticoagulation were also abstracted directly from the EHR. Administrative data were used to identify the case mix index, an indicator of severity of illness.
Pharmacologic VTE prophylaxis included all chemical prophylaxis specified on our institutional guideline, most commonly subcutaneous unfractionated heparin 5000 units every 8 hours or low molecular weight heparin (LMWH), either enoxaparin 40 mg every 12 or 24 hours or dalteparin 5000 units every 24 hours. Mechanical prophylaxis was defined as use of sequential compression devices (SCDs) when pharmacologic prophylaxis was contraindicated. Prophylaxis was considered to be appropriate if it was applied according to our guideline for >90% of hospital days prior to UEDVT diagnosis. Therapeutic anticoagulation included heparin bridging (most commonly continuous heparin infusion, LMWH 1 mg/kg or dalteparin) as well as oral vitamin K antagonists. The VTE tool (Figure 1) allows identification of pharmacologic prophylaxis and therapy that is actually administered (not just ordered) directly from our pharmacy IT system. SCD application (not just ordered SCDs) is electronically integrated into the tool from nursing documentation.
CVCs included internal jugular or subclavian triple lumen catheters, tunneled dialysis catheters, or peripherally inserted central catheters (PICCs), single or double lumen. Criteria used to identify that a UEDVT was CVC‐associated included temporal relationship (CVC was placed prior to clot diagnosis), plausibility (ipsilateral clot), evidence of clot surrounding CVC on ultrasound, and physician designation of association (as documented in progress notes or discharge summary).
Simple percentages of patient characteristics, associated factors, management, and outcomes were calculated using counts as the numerator and number of patients as the denominator. For information about UEDVTs, we used total number of UEDVTs as the denominator. Line days were day counts from insertion until removal if applicable. The CVC placement date was available in our mandated central line placement procedure notes (directly accessed from the VTE tool) for all lines placed at our institution; date of removal (if applicable) was determined from chart abstraction. For the vast majority of patients whose CVCs were placed at outside facilities, date of placement was available in the EHR (often in the admission note or in the ultrasound report/reason for study). If date of line placement at an outside facility was not known, date of admission was used. The University of Washington Human Subjects Board approved this review.
RESULTS
General Characteristics
Fifty inpatients were diagnosed with 76 UEDVTs during 53 hospitalizations. Three patients were admitted twice during the study period. Their first admission is used for the purposes of this review. None of these 3 patients had new UEDVTs diagnosed during their second admission.
The patients' mean age was 49 years (standard deviation [SD] 15.6; range, 2482 years) vs 50.9 years (SD 17.49; range, 18112 years) among all other hospitalizations during this time (Table 1). Seventy percent (35) of patients with UEDVT were men. Sixteen percent (8) of patients with UEDVT had known VTE history, 20% (10) of patients had malignancy, and 22% (11) of patients had stage V chronic kidney disease or were hemodialysis dependent.
| Characteristic | Patients With UEDVT, N=50 | All Hospitalizations, N=23,407a |
|---|---|---|
| ||
| Age, y, mean (range) | 49 (2482) | 51 (18112) |
| Sex, % male (no.) | 70% (35) | 63% (14,746) |
| Case mix index, mean (range) | 4.78 (0.6917.99) | 1.87 (0.1626.34) |
| Length of stay, d, mean (range) | 24.6 (291) | 7.2 (1178) |
| Transfer from outside hospital (no.) | 50% (25) | 25% (5,866) |
| Intensive care unit stay (no.) | 46% (23) | 36% (8,356) |
| Operative procedure (no.) | 46% (23) | 41% (9,706) |
| In‐hospital mortality (no.) | 10% (5) | 4% (842) |
| Discharge to skilled nursing facility or other hospital, n=45 surviving patients (no.) | 62% (28) | 13% (3,095) |
| 30‐day readmission, n=45 surviving patients (no.) | 18% (8) | 5% (1,167) |
Patients diagnosed with UEDVT had complex illness, long LOS, and were often transferred from outside hospitals relative to other hospitalizations during this time period (Table 1). Slightly more required intensive care and underwent surgery. Eighty‐four percent (42) of patients with UEDVT required CVCs during hospitalization. Among patients whose UEDVT was not present on admission, 94% received appropriate VTE prophylaxis prior to UEDVT diagnosis.
In patients with UEDVT, the most common reasons for hospitalization were sepsis/severe infection (43%), cerebral hemorrhage (16%), and trauma (8%). Primary service at diagnosis was medicine 56.9%, surgery 25.5%, and neurosciences 17.6%.
Upper Extremity Deep Vein Thromboses
Fifty patients were diagnosed with 76 UEDVTs during their hospitalizations. In 40% (20) of patients, UEDVTs were present in >1 upper extremity deep vein; concurrent LEDVT was present in 26% (13) and PE in 10% (5). The majority of UEDVTs were found in internal jugular veins, followed by brachial and axillary veins. Seventeen percent were present on admission. Upper extremity swelling was the most common sign/symptom and reason for study. Characteristics of UEDVTs diagnosed are listed in Table 2.
| Characteristic | % UEDVTs (No.), n=76 |
|---|---|
| |
| Anatomic site | |
| Internal jugular | 38% (29) |
| Axillary | 21% (16) |
| Subclavian/axillary | 9% (7) |
| Subclavian | 7% (5) |
| Brachial | 25% (19) |
| Hospital day of diagnosis, d, mean (range) | 9.2 (044) |
| Present on admission | 17% (13) |
| Diagnosed at outside hospital or within 24 hours of transfer | 54% (7) |
| Diagnosed during prior hospitalization at our institution | 15% (2) |
| Diagnosed within 24 hours of admission via our emergency department | 23% (3) |
| Patient‐reported chronic UEDVT | 8% (1) |
| Primary UEDVT/anatomic anomaly | 0% (0) |
| Signs and symptoms (reasons for obtaining study) | |
| Upper extremity swelling | 71% (54) |
| Presence of clot elsewhere (eg, pulmonary embolism) | 9% (7) |
| Inability to place central venous access | 8% (6) |
| Assessment of clot propagation (known clot) | 8% (6) |
| Pain | 3% (2) |
| Patient‐reported history | 1% (1) |
Of the 50 patients diagnosed with UEDVT during hospitalization, 44% (22) were found to have UEDVTs directly associated with a CVC. Forty‐two of the 50 patients had a CVC; 52% (22 of 42) had CVC‐associated UEDVTs. Fifty percent (11) of these CVCs were triple lumen catheters, 32% (7) were PICCs, and 18% (4) were tunneled dialysis lines. Three of 42 patients with CVCs and line‐associated clots were had a malignancy. For patients with CVC‐associated clot, lines were in place for an average of 14.3 days (range, 273 days) prior to UEDVT diagnosis.
Treatment and Management
Seventy‐eight percent (39) of patients with UEDVT received in‐hospital treatment with heparin/LMWH bridging and oral anticoagulation. Of the 45 patients who survived hospitalization, 75% (34) were prescribed anticoagulation for 3+ months at discharge; 23% (10) had documented contraindications to anticoagulation, most commonly recent gastrointestinal or intracranial bleeding. Two percent of patients (1) was not prescribed pharmacologic treatment at discharge and had no contraindications documented. No patients underwent thrombolysis or had superior vena cava filters placed. Sixty‐four percent (14 of 22) of CVCs that were thought to be directly associated with UEDVT were removed at diagnosis.
Outcomes
Five patients (10%) died during hospitalization, none because of VTE or complications thereof. Cause of death included septic shock, cancer, intracranial hemorrhage, heart failure, and recurrent gastrointestinal bleeding. Of the 45 surviving patients, only 38% (17) were discharged to self‐care; more than half (62%[28]) were discharged to skilled nursing facilities, other hospitals, or rehabilitation centers. Eight patients (18%) were readmitted to our institution within 30 days; none for recurrent or new DVT or PE. No additional patients died at our medical center within 30 days of discharge.
DISCUSSION
UEDVT is increasingly recognized in hospitalized patients.[3, 9] At our medical center, 0.2% of symptomatic inpatients were diagnosed with UEDVT over 14 months. These patients were predominantly men with high rates of CVCs, malignancy, VTE history, severe infection, and renal disease. Interestingly, although the literature suggests that some proportion of patients with UEDVT have anatomic abnormalities, such as Paget‐Schroetter syndrome,[15] none of the patients in our study were found to have these anomalies. In our review, hospitalized patients with UEDVT were critically ill, with a long LOS and high morbidity and mortality, suggesting that in addition to just being a complication of hospitalization,[1, 6] UEDVT may be a marker of severe illness.
In our institution, clinical presentation was consistent with what has been described with the majority of patients presenting with upper extremity swelling.[1, 3] The internal jugular veins were the most common anatomic UEDVT site, followed by brachial then axillary veins. In other series including both in‐ and outpatients, subclavian clots were most commonly diagnosed, reflecting in part higher rates of CVC association and CVC location in those studies.[3, 9] Concurrent DVT and PE rates were similar to those reported.[1, 3, 10]
Although many studies have focused on prevention of LEDVT and PE, few trials have specifically targeted UEDVT. Among our patients with UEDVTs that were not present on admission, VTE prophylaxis rates were considerably higher than what has been reported,[1, 6] suggesting that in these critically ill patients' prophylaxis may not prevent symptomatic UEDVT. It is unknown how many UEDVTs were prevented with prophylaxis, as only patients with symptomatic UEDVT were included. Adequacy of prophylaxis at outside hospitals for patients transferred in could not be assessed. Nonetheless, low numbers of UEDVT at a trauma referral center with many high‐risk patients raise the question of whether prophylaxis makes a difference. Additional study is needed to further define the role of chemoprophylaxis to prevent UEDVT in hospitalized patients.
In our inpatient group, 84% required CVCs; 44% of patients were thought to have CVC‐associated UEDVTs. Careful patient selection and attention to potentially modifiable risks, such as insertion site, catheter type, and tip position, may need further examination in this population.[3, 11, 16] Catheter duration was long; focus on removing CVCs when no longer necessary is important. Interestingly, almost 10% in our study underwent diagnostic ultrasound because a new CVC could not be successfully placed suggesting that UEDVT may develop in critically ill patients regardless of CVCs.
In our study, there were high rates of guideline‐recommended pharmacologic treatment; surprisingly the majority of CVCs with associated clot were removed. Guidelines currently support 3 months of anticoagulation for treatment of UEDVT[2, 13, 17]; evidence derives from observational trials or is largely extrapolated from LEDVT literature.[2, 13] Routine CVC removal is not specifically recommended for CVC‐associated UEDVT, particularly if lines remain functional and medically necessary; systemic anticoagulation should be provided.[13]
In our review, no hospitalized patients with UEDVT developed complications or were readmitted to our medical center within 30 days for clot progression, new PE, or post‐thrombotic syndrome, which is lower than rates reported over longer time periods.[2, 6, 10, 12] Ten percent died during hospitalization, all from their primary disease rather than from complications of VTE or VTE treatment, and no additional patients died at our institution within 30 days. Although these rates are lower than have been otherwise reported,[2, 10] the inpatient mortality rate is similar to a recent study that included inpatients; however, all patients who died in that study had cancer and CVCs.[3] In the latter study, 6.4% died within 30 days of discharge.
Limitations
There are several limitations to this study. It was conducted at a single academic referral center with a large and critically ill trauma and neurosciences population, thereby limiting generalizability. This study describes hospitalized patients at a tertiary care center who were diagnosed with UEDVT. For comparison, we obtained information regarding characteristics of hospitalization for all other inpatients during this time frame. Individuals may have had multiple hospitalizations during the study period, but because we were unable to identify information about individuals, direct statistical comparisons could not be made. However, in general, inpatients with UEDVT appeared to be sicker, with prolonged LOS and high in‐hospital mortality relative to other hospitalized patients.
Only symptomatic UEDVT events were captured, likely underestimating true UEDVT incidence. In addition, we defined UEDVTs as those diagnosed by Doppler ultrasound; therefore theoretically, UEDVTs that were more centrally located or diagnosed using another modality would not be represented here. However, in a prior internal review we found that all VTE events coded in billing data during this time period were identified using our operational definition.
In our study, VTE prophylaxis was administered in accordance with an institutional guideline. We did not have information regarding adequacy of prophylaxis at outside institutions for patients transferred in, and patients admitted through the emergency department likely were not on prophylaxis. Therefore, information about prophylaxis is limited to prophylaxis administered at our medical center for hospitalized patients who had UEDVTs not present on admission.
Information regarding CVC insertion date and CVC type for CVCs placed in our institution is accurate based on our internal reviews. Although we had reasonable capture of information about CVC placement at outside facilities, these data may be incomplete, thereby underestimating potential association of CVCs with UEDVTs identified in our hospitalized patients. Additionally, criteria used to assess association of a CVC with UEDVT may have led to underrepresentation of CVC‐associated UEDVT.
Management of UEDVT in this study was determined by the treating physicians, and patients were only followed for 30 days after discharge. Information about readmission or death within 30 days of discharge was limited to patient contact with our medical center only. Treatment at discharge was determined from the discharge summary. Therefore, compliance with treatment cannot be assessed. Although these factors may limit the nature of the conclusions, data reflect actual practice and experience in hospitalized patients with UEDVT and may be hypothesis generating.
CONCLUSIONS
Among hospitalized patients, UEDVT is increasingly recognized. In our medical center, hospitalized patients diagnosed with UEDVT were more likely to have CVCs, malignancy, renal disease, and severe infection. Many of these patients were transferred critically ill, had prolonged LOS, and had high in‐hospital mortality. Most developed UEDVT despite prophylaxis, and the majority of UEDVTs were treated even in the absence of concurrent LEDVT or PE. As we move toward an era of increasing accountability, with a focus on preventing hospital‐acquired conditions including VTE, additional research is needed to identify modifiable risks, explore opportunities for effective prevention, and optimize outcomes such as prevention of complications or readmissions, particularly in critically ill patients with UEDVT.
Acknowledgements
The authors would like to thank Ronald Pergamit and Kevin Middleton for their extraordinary creativity and expert programming.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
- , , , . Upper‐extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611.
- , , , et al. Clinical outcome of patients with upper‐extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148.
- , , . The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovascular Surg. 2012;46(2):139–144.
- , , , et al. Upper extremity versus lower extremity deep venous thrombosis. Am J Surg. 1997;174(2):214–217.
- , . Upper‐extremity deep vein thrombosis. Circulation. 2002;106(14):1874–1880.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e2.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , . Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg. 2004;18(5):552–557.
- , , , et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg. 2005;41(3):476–478.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , , . Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):48S–52S.
- . Clinical practice. Deep‐vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869.
- , , , et al. Diagnosis and management of upper extremity deep‐vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108.
- , , . Treatment of upper‐extremity deep vein thrombosis. J Thromb Haemost. 2011;9(10):1924–1930.
Hospitalists and Hospital‐Level Outcomes
Since Wachter and Goldman coined the term hospitalist in 1996,[1] the number of hospitalists in the United States has grown rapidly, to more than 30,000 in recent estimates, with at least 80% of hospitals with 200 beds or more having hospital medicine programs.[2] A number of factors have led to the growth of such programs. First, hospital‐level incentives to use hospitalists exist to improve patient flow and maximize bed use, thereby reducing length of stay (LOS) and improving efficiency. Hospitals also employ hospitalists to address limitations on the number of hours that medical residents can work. Second, the use of hospitalists allows primary care physicians (PCPs) to focus their practices on outpatient care, thus avoiding the complexity of hospital‐based medicine, which requires both hospital‐focused clinical skills as well as institutional knowledge. Supporters of the hospitalist movement claim that hospitalists can improve efficiency and quality of care because hospitalists (1) have more experience managing inpatient care, (2) are more available to patients, and (3) have greater commitment to hospital quality improvements than (nonemployed) community PCPs.[3, 4, 5] On the other hand, criticisms of hospitalists include concerns related to (1) discontinuity in care and patient handoffs, (2) patient dissatisfaction at being treated by someone other than their PCP, (3) loss of acute care skills by PCPs, and (4) hospitalist burnout due to large workloads and poor institutional support.[3, 4, 5]
Hospitalists have been shown to have an effect on lowering total patient costs through better resource utilization and reduced LOS.[6, 7, 8, 9] There is no clear agreement, however, that hospitalists more often implement guideline‐recommended care.[10, 11, 12] In fact, most evaluations have found no significant differences between mortality and readmission rates among hospitalist and nonhospitalist groups.[12, 13, 14, 15, 16] The majority of these studies, however, were conducted in individual institutions or with small sample sizes, thus limiting their generalizability.
As 1 of the fastest‐growing medical specialties, hospitalists have assumed a significant role in inpatient care. The Centers for Medicare and Medicaid Services (CMS) have identified heart failure (HF), acute myocardial infarction (AMI), and pneumonia (PN) as important inpatient conditions associated with substantial morbidity and mortality among the Medicare population. Further, Jencks et al.[17] found that nearly one‐fifth of Medicare beneficiaries discharged from a hospital were readmitted within 30 days, which incurred an estimated cost to Medicare of $17.4 billion in 2004. Hospital readmission is of particular importance under healthcare reform because CMS introduced financial penalties in 2013 for hospitals with excessive readmission rates. The reimbursement penalty related to readmissions is included in the Patient Protection and Affordable Care Act and will be gradually expanded across many other outcomes.[18]
METHODS
Data Sources
Using hierarchical, generalized, linear modeling with hospital‐specific random effects, CMS has developed and made publicly available national, hospital‐level data reporting case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission rates, as measured from the first day of the index inpatient admission. The models produce aggregate hospital‐level predictions of excess mortality and readmissions, as compared to other hospitals with the same case mix.[19, 20] Outcome measures in this study reflect these hospital‐specific, adjusted measures of mortality and readmission. Each of these measures is expressed as a continuous variable of the adjusted number of events within a 30‐day period, analogous to a ratio of observed‐to‐expected outcomes, multiplied by the national rate. Specifically, the numerator is the number of observed events in a 30‐day period based on the hospital's case mix‐adjusted performance, and the denominator is the number of expected events in a 30‐day period based on average national hospital performance with that hospital's case mix. CMS adjusts the measures for case mix to account for important patient‐level, clinically relevant variables such as age, sex, and comorbidities. However, the data do not allow the measures to be further adjusted for admission source, discharge destination, or patient socioeconomic status.[19] CMS also does not report rates for hospitals with fewer than 25 cases for a condition, which could limit the generalizability of our findings with regard to small hospitals or hospitals with only occasional patients discharged with a target condition. Details on specific inclusion/exclusion criteria, model adjustment, and statistical approach used by CMS can be found in their methodology reports.[21, 22]
The 2008 CMS risk‐standardized mortality and readmission measures described above were linked with the 2008 American Hospital Association (AHA) Annual Survey Database, using each hospital's 6‐digit Medicare provider identification number. The AHA Annual Survey Database provides comprehensive hospital‐level data for approximately 6500 US hospitals, including demographics, organizational structure, facilities and services, utilization data, community indicators, physician arrangements, managed care relationships, expenses, and staffing, including employment of hospitalists.[23]
Variables
We used the CMS case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission measures for HF, AMI, and PN as dependent variables. The primary independent variable was a dichotomous measure of whether or not hospitalists provided care within the hospital. Covariates identified from the literature[11, 23, 24, 25, 26, 27, 28] included hospital and community characteristics, organizational perspective, size, and resources. Models were adjusted for hospital ownership (government, nongovernment nonprofit, investor‐owned for profit), region (Northeast, South, Midwest, West), teaching status, bed size, number of nurses per hospital bed, intensive care unit (ICU) presence (medicalsurgical, cardiac), managed care contracts (health maintenance organization, preferred provider organization), urban/rural setting, and median household income in the hospital county.
Statistical Analysis
Descriptive statistics of the dependent and independent variables illustrated trends across hospitals with and without hospitalists, and bivariate statistics identified differences between the 2 groups. We employed multivariable ordinary least squares (OLS) regression to assess the association between the independent variables and risk‐standardized, 30‐day all‐cause excess mortality and readmission rates at the hospital level. OLS was used because the dependent variables were measured continuously; count models were not appropriate for our analyses, because we did not have access to patient‐level data that could provide person‐days at risk for mortality or readmission. This limitation is mitigated, however, because CMS had already used hierarchical, multivariate, patient‐level models to produce hospital‐specific predictions, which formed the basis of our outcome measures. Six OLS models were run reflecting each of the 6 outcomes of interest: AMI mortality, HF mortality, and PN mortality, and AMI readmission, HF readmission, and PN readmission. All statistical analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
RESULTS
Hospital Characteristics and Descriptive Measures
There were 3029 US hospitals in the final analysis dataset. Of these, 59.3% reported employing hospitalists on staff. Descriptive statistics are shown in Table 1.
| Hospitalist Presence, n=1,796, % or Mean (SD) | No Hospitalist Presence, n=1,233, % or Mean (SD) | P Value | |
|---|---|---|---|
| |||
| Hospital control | <0.001 | ||
| Government | 14.8% | 33.3% | |
| Nongovernment, nonprofit | 72.9% | 56.8% | |
| Investor owned, for profit | 12.4% | 10.0% | |
| Bed size | 257 (224) | 94 (106) | <0.001 |
| Nurses per inpatient bed | 1.5 (0.6) | 1.1 (0.7) | <0.001 |
| Urban | 75.3% | 32.7% | <0.001 |
| Rural | 24.7% | 67.3% | |
| Region | <0.001 | ||
| Northeast | 18.2% | 8.3% | |
| South | 40.1% | 33.1% | |
| Midwest | 24.4% | 46.3% | |
| West | 17.3% | 12.3% | |
| ICU presence | |||
| Medicalsurgical | 94.0% | 64.0% | <0.001 |
| Cardiac | 58.7% | 26.9% | <0.001 |
| Managed care contracts | |||
| HMO | 81.2% | 59.4% | <0.001 |
| PPO | 88.7% | 79.9% | <0.001 |
| Teaching hospital | 12.6% | 1.7% | <0.001 |
| Median household income in hospital county | $51,851 ($13,566) | $44,448 ($10,058) | <0.001 |
Table 2 presents bivariate analyses. Mortality for all 3 conditions and readmissions for AMI and HF were all significantly lower among hospitals employing hospitalists. Of the 3029 hospitals in the sample (both with and without hospitalist programs), over 93% had 25 or more cases per category for 4 of the 6 outcome variables, indicating only a minor risk of hospital selection bias due to small size or infrequent admissions for target conditions.
| Outcome Variable | Hospitalist Presence, Mean (SD) | No Hospitalist Presence, Mean (SD) | P Value | n |
|---|---|---|---|---|
| ||||
| AMI mortality | 16.3 (1.8) | 16.7 (1.7) | <0.001 | 2,007 |
| HF mortality | 11.1 (1.6) | 11.4 (1.5) | <0.001 | 2,625 |
| PN mortality | 11.4 (1.9) | 11.8 (1.8) | <0.001 | 2,746 |
| AMI readmission | 19.8 (1.4) | 20.1 (1.3) | 0.003 | 1,707 |
| HF readmission | 24.2 (2.1) | 24.8 (2.0) | <0.001 | 2,620 |
| PN readmission | 18.1 (1.7) | 18.1 (1.6) | 0.896 | 2,709 |
Multivariate Analyses: Mortality Outcomes
Multivariate analyses showed no significant relationship between hospitalist care and risk‐standardized mortality measures for any of the 3 target conditions (Table 3). Stated more precisely, the presence or absence of hospitalists was not associated with an increase or decrease in the case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality rates for these conditions. Covariates in the models generally performed as might be hypothesized. When stratified by ICU presence, urban/rural setting, and bed size, none of the hospitalist presence coefficients reached significance.
| Acute Myocardial Infarction (95% CI) | Heart Failure (95% CI) | Pneumonia (95% CI) | |
|---|---|---|---|
| |||
| Mortality | |||
| Hospitalist presence | 0.058 (0.132 to 0.247) | 0.104 (0.041 to 0.249) | 0.042 (0.132, 0.217) |
| Readmission | |||
| Hospitalist presence | 0.182 (0.343 to 0.022)a | 0.575 (0.763 to 0.387)c | 0.228 (0.380 to 0.075)b |
Multivariate Analyses: Readmission Outcomes
In contrast to the mortality measures, risk‐standardized readmission rates were significantly lower for all 3 conditions for hospitals employing hospitalists (Table 3). Specifically, hospitalist services within a hospital were associated with a decrease in case mix‐adjusted, risk‐standardized, 30‐day predicted excess readmissions for each of the 3 target conditions, as follows: 0.182 fewer predicted AMI readmissions per 100 people at risk (P<0.05), 0.575 fewer predicted HF readmissions per 100 people at risk (P<0.001), and 0.228 fewer predicted PN readmissions per 100 people at risk (P<0.01). Covariates in the models again generally performed as might be expected. When stratified, the presence of hospitalists tended to have a stronger negative association with medicalsurgical ICU presence, cardiac ICU presence, urban setting, and larger bed size.
Full results from the OLS regressions for mortality and readmission outcome variables, including significance levels and 95% confidence intervals, are available (see Supporting Information, Appendix Tables 1 and 2, in the online version of this article).
DISCUSSION
Most previous studies have used patient‐level data from single institutions, and have shown inconsistent association between hospitalist care and clinical outcomes. Only a few studies have been conducted at the national level, and we know of only 1 that uses the same types of clinical outcomes as in our approach. In particular, Goodrich et al. conducted an in‐depth survey of hospitalist programs, and found that hospitalist presence had a significant association with HF readmissions.[29] Our results, similar to those of Goodrich et al., showed that the presence of hospitalists was not associated with risk‐standardized, 30‐day, all‐cause predicted excess mortality rates for Medicare patients hospitalized for any of these 3 conditions. The presence of hospitalists was, however, associated with lower‐risk standardized, 30‐day, all‐cause predicted excess readmission rates in our study. Our analyses resulted in somewhat different coefficients than Goodrich et al., but that is most likely due to: (1) different sample sizes, (2) use of similar yet not identical control variables, and (3) reporting error, as we used different sets of self‐reported data to indicate hospitalist services. The presence of a hospital‐level association with inconclusive patient‐level evidence suggests that there may be a more nuanced relationship between hospitalists and quality of care than has been previously explored.
This result may be explained by a number of reasons, the first of which is that hospitalists generally have more experience in the increasingly specialized practice of hospital‐based medicine than PCPs or nonhospitalists. For example, Meltzer et al.[30] found that hospitalists have more experience than nonhospitalists in treating acute manifestations of cardiovascular and respiratory diseases. Even though we might expect that greater experience with hospital‐based medicine would be associated with lower mortality rates, this outcome may not be captured because mortality is a rare event in the reported 30‐day postdischarge period and may be less preventable than readmission. There are a number of other factors possibly affecting hospital readmission, such as inadequate information transfer by discharge planners, poor patient compliance, inadequate follow‐up, insufficient use of family caregivers, deterioration of a patient's clinical condition, and medical errors.[31]
Studies have found that hospitalists have had positive effects related to managing case complexity and navigating the discharge process, perhaps due to their increased availability to patients and commitment to hospital quality improvements.[16, 32] Some determinants of patient outcomes may be difficult for hospitalists to influence, however, such as poor patient compliance or lack of support by family caregivers. Hospitalists who have extensive discharge experience may understand key challenges and adopt strategies to ameliorate these negative effects, for instance by using appropriate motivational strategies to encourage compliance and capitalizing on family caregivers.[33] Being located in the hospital, hospitalists are more available to deal with emergencies that occur during the hospitalization, and may be more available and active in discharge planning. Benbassat and Taragin[34] found that between 9% and 48% of all readmissions were preventable because they were associated with indicators of substandard care during the index hospitalization. They further estimated between 12% and 75% more readmissions could have been prevented by implementing patient education, predischarge assessment, and at‐home aftercare programs. Hospitalists are in a unique position to use their specialized training to improve transitions from hospital to home, communicate needs with the family and caregivers during the index hospitalization, and ensure that adequate postdischarge care is received. Although the use of hospitalists creates another handoff in the transition between inpatient and outpatient settings, hospitalist care may have a positive effect on many of the determinants of readmission sufficient to overcome that discontinuity.
Quality of care may also be affected by tertiary factors such as hospital administration or organizational culture. Lower AMI mortality has been associated with factors beyond cardiologist care, including organizational behavior and the appointment of physician and nurse champions.[35] Although the exact mechanism is unclear, better patient outcomes may be a result of this combination of direct clinical care, care transition management, and administrative or organizational factors. The models showed several hospital and community characteristics having coefficients larger in magnitude than the hospitalist variable, including classification as a teaching hospital, region, and hospital county median income. Teaching hospitals have been shown to have varying effects on quality of care depending on the type of care being provided, and teaching status may also be a proxy for factors related to organizational culture or mission.[36] Community‐level contextual factors including poverty and income have been shown previously to be related to readmission rates, possibly due to lack of social support and financial resources in the community to help discharged patients manage their healthcare needs in community settings.
Research Limitations
Two important limitations of this study are assumptions made necessary using aggregated, hospital‐level data. These assumptions include: (1) that hospitalists regularly treat Medicare patients with HF, AMI, and PN, and (2) that patient exposure to hospitalists is consistent in amount and quality across all patients treated in the hospital. Due to the frequency of the 3 study conditions in the Medicare population, it is reasonable to assume that hospitalists treat these patients, but it is unlikely that all patients admitted to each hospital employing hospitalists are indeed treated by hospitalists or that they are all treated in a consistent manner. There is also significant variation among hospitalist services nationwide, from different types of hospitalists to varying responsibilities across settings. Differences in physician practice structure and hospital staffing could affect hospitalist care on individual patient outcomes between hospitals that employ hospitalists. Models also did not control for the extent to which hospitals have implemented specific interventions to prevent hospital readmissions; hospitals with hospitalists may more often implement other interventions potentially influencing readmissions. We further could not distinguish between effective and ineffective hospitalist programs. The inability to account for these factors would effectively weaken the indicator, most likely underestimating the association between hospitalist presence and the outcome variables. Finally, the AHA database is subject to some variability, as it utilizes self‐reported data from the hospitals, but the database is generally considered the industry standard.
Using OLS regression, this study reflects correlation, but cannot demonstrate causation between the presence of hospitalists and an increase or decrease in risk‐standardized predicted mortality or readmission rates. There is also controversy regarding the appropriateness of using risk‐standardized predicted mortality and readmission rates as measures of quality of care, because these rates represent outcomes that may be influenced by other factors beyond the care received during the inpatient stay. These rates will, however, be of increasing importance given emerging pay‐for‐performance initiatives.[35, 37, 38]
CONCLUSION
Reducing medical errors and improving patient outcomes are becoming more important in light of increased reporting of hospital performance and outcome measures. Post‐discharge 30‐day mortality and hospital readmission represent 2 major undesirable patient outcomes, and Medicare's new pay‐for‐performance initiatives only provide further incentives for hospitals to take action in reducing these rates. Because the likelihood of receiving inpatient care provided by a hospitalist has significantly increased among Medicare patients since the 1990s,[39] hospitalists have become important players in potentially reducing mortality and readmission for patients discharged from inpatient settings. This study has shown that use of hospitalists may be associated with lower hospital readmissions, a clear quality measure, but are not associated with any changes in 30‐day mortality.
Further studies are needed, however, to better characterize and validate the observed associations, as well as to determine how hospitalist programs can be enhanced to improve inpatient care quality. Case studies could be carried out within hospitals with high‐ and low‐performing hospitalist services to help identify key aspects of hospitalist care most closely associated with desirable outcomes. Discharge and transitional care processes could also be standardized according to best practices, with their implementation tailored to individual hospital settings. Finally, as patient‐level data become increasingly available, researchers should merge these data with hospital‐level data to assess more robustly the multilevel effect of hospitalists on inpatient quality of care and individual patient outcomes. Such information will be valuable to policymakers and health administrators alike in the ongoing and volatile economic and political environment surrounding healthcare.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- Society of Hospital Medicine. 2010. SHM fact sheet: about hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit130(4 pt 2):338–342.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2):382–387.
- . The hospitalist model: perspectives of the patient, the internist, and internal medicine. Ann Intern Med. 1999;130(4 pt 2):368–372.
- , . Economic and healthcare forces of hospitalist movement. Mt Sinai J Med. 2008;75(5):424–429.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs. nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , , , . Determinants of hospitalist efficiency: a qualitative and quantitative study. Med Care Res Rev. 2009;66(6):682–702.
- , , , , , . The value of a hospitalist service: efficient care for the aging population? Chest. 2001;119(2):580–589.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , , . Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869–1874.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act of 2010. P.L. 111–148, §3025 Stat. 328 (2010).
- Centers for Medicare and Medicaid Services. 2008. Hospital outcome of care measures [data file]. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/index.html?redirect=/HospitalQualityInits/11_HospitalCompare.asp. Accessed November 15, 2011.
- , . Public reporting of 30‐day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–1397.
- Quality Net. 2012. Measure methodology reports: mortality measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page35(3):22–34.
- , , , . Care in U.S. hospitals—the hospital quality alliance program. N Engl J Med. 2005;353(3):265–274.
- , , , et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511–2517.
- United States Census Bureau. 2008. Small area income and poverty estimates [data file]. Available at: http://www.census.gov/did/www/saipe/. Accessed March 5, 2012.
- United States Department of Agriculture Economic Research Service. 2004. Rural‐urban continuum codes [data file]. Available at: http://www.ers.usda.gov/data‐products/rural‐urban‐continuum‐codes.aspx. Accessed March 5, 2012.
- , , , , . Hospitalist utilization and hospital performance on 6 publicly reported patient outcomes. J Hosp Med. 2012;7(6):482–488.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , . Medicare hospital readmissions: issues, policy options, and PPACA (R40972). Congressional Research Service. Washington, DC: U.S. Government Printing Office; 2010.
- , , , . Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital. CMAJ. 2000;163(11):1477–1480.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160(8):1074–1081.
- , , , et al. Hospital strategies for reducing risk‐standardized mortality rates in acute myocardial infarction. Ann Intern Med. 2012;156(9):618–626.
- , . Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80(3):569–593.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , . Hospital readmissions as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
Since Wachter and Goldman coined the term hospitalist in 1996,[1] the number of hospitalists in the United States has grown rapidly, to more than 30,000 in recent estimates, with at least 80% of hospitals with 200 beds or more having hospital medicine programs.[2] A number of factors have led to the growth of such programs. First, hospital‐level incentives to use hospitalists exist to improve patient flow and maximize bed use, thereby reducing length of stay (LOS) and improving efficiency. Hospitals also employ hospitalists to address limitations on the number of hours that medical residents can work. Second, the use of hospitalists allows primary care physicians (PCPs) to focus their practices on outpatient care, thus avoiding the complexity of hospital‐based medicine, which requires both hospital‐focused clinical skills as well as institutional knowledge. Supporters of the hospitalist movement claim that hospitalists can improve efficiency and quality of care because hospitalists (1) have more experience managing inpatient care, (2) are more available to patients, and (3) have greater commitment to hospital quality improvements than (nonemployed) community PCPs.[3, 4, 5] On the other hand, criticisms of hospitalists include concerns related to (1) discontinuity in care and patient handoffs, (2) patient dissatisfaction at being treated by someone other than their PCP, (3) loss of acute care skills by PCPs, and (4) hospitalist burnout due to large workloads and poor institutional support.[3, 4, 5]
Hospitalists have been shown to have an effect on lowering total patient costs through better resource utilization and reduced LOS.[6, 7, 8, 9] There is no clear agreement, however, that hospitalists more often implement guideline‐recommended care.[10, 11, 12] In fact, most evaluations have found no significant differences between mortality and readmission rates among hospitalist and nonhospitalist groups.[12, 13, 14, 15, 16] The majority of these studies, however, were conducted in individual institutions or with small sample sizes, thus limiting their generalizability.
As 1 of the fastest‐growing medical specialties, hospitalists have assumed a significant role in inpatient care. The Centers for Medicare and Medicaid Services (CMS) have identified heart failure (HF), acute myocardial infarction (AMI), and pneumonia (PN) as important inpatient conditions associated with substantial morbidity and mortality among the Medicare population. Further, Jencks et al.[17] found that nearly one‐fifth of Medicare beneficiaries discharged from a hospital were readmitted within 30 days, which incurred an estimated cost to Medicare of $17.4 billion in 2004. Hospital readmission is of particular importance under healthcare reform because CMS introduced financial penalties in 2013 for hospitals with excessive readmission rates. The reimbursement penalty related to readmissions is included in the Patient Protection and Affordable Care Act and will be gradually expanded across many other outcomes.[18]
METHODS
Data Sources
Using hierarchical, generalized, linear modeling with hospital‐specific random effects, CMS has developed and made publicly available national, hospital‐level data reporting case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission rates, as measured from the first day of the index inpatient admission. The models produce aggregate hospital‐level predictions of excess mortality and readmissions, as compared to other hospitals with the same case mix.[19, 20] Outcome measures in this study reflect these hospital‐specific, adjusted measures of mortality and readmission. Each of these measures is expressed as a continuous variable of the adjusted number of events within a 30‐day period, analogous to a ratio of observed‐to‐expected outcomes, multiplied by the national rate. Specifically, the numerator is the number of observed events in a 30‐day period based on the hospital's case mix‐adjusted performance, and the denominator is the number of expected events in a 30‐day period based on average national hospital performance with that hospital's case mix. CMS adjusts the measures for case mix to account for important patient‐level, clinically relevant variables such as age, sex, and comorbidities. However, the data do not allow the measures to be further adjusted for admission source, discharge destination, or patient socioeconomic status.[19] CMS also does not report rates for hospitals with fewer than 25 cases for a condition, which could limit the generalizability of our findings with regard to small hospitals or hospitals with only occasional patients discharged with a target condition. Details on specific inclusion/exclusion criteria, model adjustment, and statistical approach used by CMS can be found in their methodology reports.[21, 22]
The 2008 CMS risk‐standardized mortality and readmission measures described above were linked with the 2008 American Hospital Association (AHA) Annual Survey Database, using each hospital's 6‐digit Medicare provider identification number. The AHA Annual Survey Database provides comprehensive hospital‐level data for approximately 6500 US hospitals, including demographics, organizational structure, facilities and services, utilization data, community indicators, physician arrangements, managed care relationships, expenses, and staffing, including employment of hospitalists.[23]
Variables
We used the CMS case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission measures for HF, AMI, and PN as dependent variables. The primary independent variable was a dichotomous measure of whether or not hospitalists provided care within the hospital. Covariates identified from the literature[11, 23, 24, 25, 26, 27, 28] included hospital and community characteristics, organizational perspective, size, and resources. Models were adjusted for hospital ownership (government, nongovernment nonprofit, investor‐owned for profit), region (Northeast, South, Midwest, West), teaching status, bed size, number of nurses per hospital bed, intensive care unit (ICU) presence (medicalsurgical, cardiac), managed care contracts (health maintenance organization, preferred provider organization), urban/rural setting, and median household income in the hospital county.
Statistical Analysis
Descriptive statistics of the dependent and independent variables illustrated trends across hospitals with and without hospitalists, and bivariate statistics identified differences between the 2 groups. We employed multivariable ordinary least squares (OLS) regression to assess the association between the independent variables and risk‐standardized, 30‐day all‐cause excess mortality and readmission rates at the hospital level. OLS was used because the dependent variables were measured continuously; count models were not appropriate for our analyses, because we did not have access to patient‐level data that could provide person‐days at risk for mortality or readmission. This limitation is mitigated, however, because CMS had already used hierarchical, multivariate, patient‐level models to produce hospital‐specific predictions, which formed the basis of our outcome measures. Six OLS models were run reflecting each of the 6 outcomes of interest: AMI mortality, HF mortality, and PN mortality, and AMI readmission, HF readmission, and PN readmission. All statistical analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
RESULTS
Hospital Characteristics and Descriptive Measures
There were 3029 US hospitals in the final analysis dataset. Of these, 59.3% reported employing hospitalists on staff. Descriptive statistics are shown in Table 1.
| Hospitalist Presence, n=1,796, % or Mean (SD) | No Hospitalist Presence, n=1,233, % or Mean (SD) | P Value | |
|---|---|---|---|
| |||
| Hospital control | <0.001 | ||
| Government | 14.8% | 33.3% | |
| Nongovernment, nonprofit | 72.9% | 56.8% | |
| Investor owned, for profit | 12.4% | 10.0% | |
| Bed size | 257 (224) | 94 (106) | <0.001 |
| Nurses per inpatient bed | 1.5 (0.6) | 1.1 (0.7) | <0.001 |
| Urban | 75.3% | 32.7% | <0.001 |
| Rural | 24.7% | 67.3% | |
| Region | <0.001 | ||
| Northeast | 18.2% | 8.3% | |
| South | 40.1% | 33.1% | |
| Midwest | 24.4% | 46.3% | |
| West | 17.3% | 12.3% | |
| ICU presence | |||
| Medicalsurgical | 94.0% | 64.0% | <0.001 |
| Cardiac | 58.7% | 26.9% | <0.001 |
| Managed care contracts | |||
| HMO | 81.2% | 59.4% | <0.001 |
| PPO | 88.7% | 79.9% | <0.001 |
| Teaching hospital | 12.6% | 1.7% | <0.001 |
| Median household income in hospital county | $51,851 ($13,566) | $44,448 ($10,058) | <0.001 |
Table 2 presents bivariate analyses. Mortality for all 3 conditions and readmissions for AMI and HF were all significantly lower among hospitals employing hospitalists. Of the 3029 hospitals in the sample (both with and without hospitalist programs), over 93% had 25 or more cases per category for 4 of the 6 outcome variables, indicating only a minor risk of hospital selection bias due to small size or infrequent admissions for target conditions.
| Outcome Variable | Hospitalist Presence, Mean (SD) | No Hospitalist Presence, Mean (SD) | P Value | n |
|---|---|---|---|---|
| ||||
| AMI mortality | 16.3 (1.8) | 16.7 (1.7) | <0.001 | 2,007 |
| HF mortality | 11.1 (1.6) | 11.4 (1.5) | <0.001 | 2,625 |
| PN mortality | 11.4 (1.9) | 11.8 (1.8) | <0.001 | 2,746 |
| AMI readmission | 19.8 (1.4) | 20.1 (1.3) | 0.003 | 1,707 |
| HF readmission | 24.2 (2.1) | 24.8 (2.0) | <0.001 | 2,620 |
| PN readmission | 18.1 (1.7) | 18.1 (1.6) | 0.896 | 2,709 |
Multivariate Analyses: Mortality Outcomes
Multivariate analyses showed no significant relationship between hospitalist care and risk‐standardized mortality measures for any of the 3 target conditions (Table 3). Stated more precisely, the presence or absence of hospitalists was not associated with an increase or decrease in the case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality rates for these conditions. Covariates in the models generally performed as might be hypothesized. When stratified by ICU presence, urban/rural setting, and bed size, none of the hospitalist presence coefficients reached significance.
| Acute Myocardial Infarction (95% CI) | Heart Failure (95% CI) | Pneumonia (95% CI) | |
|---|---|---|---|
| |||
| Mortality | |||
| Hospitalist presence | 0.058 (0.132 to 0.247) | 0.104 (0.041 to 0.249) | 0.042 (0.132, 0.217) |
| Readmission | |||
| Hospitalist presence | 0.182 (0.343 to 0.022)a | 0.575 (0.763 to 0.387)c | 0.228 (0.380 to 0.075)b |
Multivariate Analyses: Readmission Outcomes
In contrast to the mortality measures, risk‐standardized readmission rates were significantly lower for all 3 conditions for hospitals employing hospitalists (Table 3). Specifically, hospitalist services within a hospital were associated with a decrease in case mix‐adjusted, risk‐standardized, 30‐day predicted excess readmissions for each of the 3 target conditions, as follows: 0.182 fewer predicted AMI readmissions per 100 people at risk (P<0.05), 0.575 fewer predicted HF readmissions per 100 people at risk (P<0.001), and 0.228 fewer predicted PN readmissions per 100 people at risk (P<0.01). Covariates in the models again generally performed as might be expected. When stratified, the presence of hospitalists tended to have a stronger negative association with medicalsurgical ICU presence, cardiac ICU presence, urban setting, and larger bed size.
Full results from the OLS regressions for mortality and readmission outcome variables, including significance levels and 95% confidence intervals, are available (see Supporting Information, Appendix Tables 1 and 2, in the online version of this article).
DISCUSSION
Most previous studies have used patient‐level data from single institutions, and have shown inconsistent association between hospitalist care and clinical outcomes. Only a few studies have been conducted at the national level, and we know of only 1 that uses the same types of clinical outcomes as in our approach. In particular, Goodrich et al. conducted an in‐depth survey of hospitalist programs, and found that hospitalist presence had a significant association with HF readmissions.[29] Our results, similar to those of Goodrich et al., showed that the presence of hospitalists was not associated with risk‐standardized, 30‐day, all‐cause predicted excess mortality rates for Medicare patients hospitalized for any of these 3 conditions. The presence of hospitalists was, however, associated with lower‐risk standardized, 30‐day, all‐cause predicted excess readmission rates in our study. Our analyses resulted in somewhat different coefficients than Goodrich et al., but that is most likely due to: (1) different sample sizes, (2) use of similar yet not identical control variables, and (3) reporting error, as we used different sets of self‐reported data to indicate hospitalist services. The presence of a hospital‐level association with inconclusive patient‐level evidence suggests that there may be a more nuanced relationship between hospitalists and quality of care than has been previously explored.
This result may be explained by a number of reasons, the first of which is that hospitalists generally have more experience in the increasingly specialized practice of hospital‐based medicine than PCPs or nonhospitalists. For example, Meltzer et al.[30] found that hospitalists have more experience than nonhospitalists in treating acute manifestations of cardiovascular and respiratory diseases. Even though we might expect that greater experience with hospital‐based medicine would be associated with lower mortality rates, this outcome may not be captured because mortality is a rare event in the reported 30‐day postdischarge period and may be less preventable than readmission. There are a number of other factors possibly affecting hospital readmission, such as inadequate information transfer by discharge planners, poor patient compliance, inadequate follow‐up, insufficient use of family caregivers, deterioration of a patient's clinical condition, and medical errors.[31]
Studies have found that hospitalists have had positive effects related to managing case complexity and navigating the discharge process, perhaps due to their increased availability to patients and commitment to hospital quality improvements.[16, 32] Some determinants of patient outcomes may be difficult for hospitalists to influence, however, such as poor patient compliance or lack of support by family caregivers. Hospitalists who have extensive discharge experience may understand key challenges and adopt strategies to ameliorate these negative effects, for instance by using appropriate motivational strategies to encourage compliance and capitalizing on family caregivers.[33] Being located in the hospital, hospitalists are more available to deal with emergencies that occur during the hospitalization, and may be more available and active in discharge planning. Benbassat and Taragin[34] found that between 9% and 48% of all readmissions were preventable because they were associated with indicators of substandard care during the index hospitalization. They further estimated between 12% and 75% more readmissions could have been prevented by implementing patient education, predischarge assessment, and at‐home aftercare programs. Hospitalists are in a unique position to use their specialized training to improve transitions from hospital to home, communicate needs with the family and caregivers during the index hospitalization, and ensure that adequate postdischarge care is received. Although the use of hospitalists creates another handoff in the transition between inpatient and outpatient settings, hospitalist care may have a positive effect on many of the determinants of readmission sufficient to overcome that discontinuity.
Quality of care may also be affected by tertiary factors such as hospital administration or organizational culture. Lower AMI mortality has been associated with factors beyond cardiologist care, including organizational behavior and the appointment of physician and nurse champions.[35] Although the exact mechanism is unclear, better patient outcomes may be a result of this combination of direct clinical care, care transition management, and administrative or organizational factors. The models showed several hospital and community characteristics having coefficients larger in magnitude than the hospitalist variable, including classification as a teaching hospital, region, and hospital county median income. Teaching hospitals have been shown to have varying effects on quality of care depending on the type of care being provided, and teaching status may also be a proxy for factors related to organizational culture or mission.[36] Community‐level contextual factors including poverty and income have been shown previously to be related to readmission rates, possibly due to lack of social support and financial resources in the community to help discharged patients manage their healthcare needs in community settings.
Research Limitations
Two important limitations of this study are assumptions made necessary using aggregated, hospital‐level data. These assumptions include: (1) that hospitalists regularly treat Medicare patients with HF, AMI, and PN, and (2) that patient exposure to hospitalists is consistent in amount and quality across all patients treated in the hospital. Due to the frequency of the 3 study conditions in the Medicare population, it is reasonable to assume that hospitalists treat these patients, but it is unlikely that all patients admitted to each hospital employing hospitalists are indeed treated by hospitalists or that they are all treated in a consistent manner. There is also significant variation among hospitalist services nationwide, from different types of hospitalists to varying responsibilities across settings. Differences in physician practice structure and hospital staffing could affect hospitalist care on individual patient outcomes between hospitals that employ hospitalists. Models also did not control for the extent to which hospitals have implemented specific interventions to prevent hospital readmissions; hospitals with hospitalists may more often implement other interventions potentially influencing readmissions. We further could not distinguish between effective and ineffective hospitalist programs. The inability to account for these factors would effectively weaken the indicator, most likely underestimating the association between hospitalist presence and the outcome variables. Finally, the AHA database is subject to some variability, as it utilizes self‐reported data from the hospitals, but the database is generally considered the industry standard.
Using OLS regression, this study reflects correlation, but cannot demonstrate causation between the presence of hospitalists and an increase or decrease in risk‐standardized predicted mortality or readmission rates. There is also controversy regarding the appropriateness of using risk‐standardized predicted mortality and readmission rates as measures of quality of care, because these rates represent outcomes that may be influenced by other factors beyond the care received during the inpatient stay. These rates will, however, be of increasing importance given emerging pay‐for‐performance initiatives.[35, 37, 38]
CONCLUSION
Reducing medical errors and improving patient outcomes are becoming more important in light of increased reporting of hospital performance and outcome measures. Post‐discharge 30‐day mortality and hospital readmission represent 2 major undesirable patient outcomes, and Medicare's new pay‐for‐performance initiatives only provide further incentives for hospitals to take action in reducing these rates. Because the likelihood of receiving inpatient care provided by a hospitalist has significantly increased among Medicare patients since the 1990s,[39] hospitalists have become important players in potentially reducing mortality and readmission for patients discharged from inpatient settings. This study has shown that use of hospitalists may be associated with lower hospital readmissions, a clear quality measure, but are not associated with any changes in 30‐day mortality.
Further studies are needed, however, to better characterize and validate the observed associations, as well as to determine how hospitalist programs can be enhanced to improve inpatient care quality. Case studies could be carried out within hospitals with high‐ and low‐performing hospitalist services to help identify key aspects of hospitalist care most closely associated with desirable outcomes. Discharge and transitional care processes could also be standardized according to best practices, with their implementation tailored to individual hospital settings. Finally, as patient‐level data become increasingly available, researchers should merge these data with hospital‐level data to assess more robustly the multilevel effect of hospitalists on inpatient quality of care and individual patient outcomes. Such information will be valuable to policymakers and health administrators alike in the ongoing and volatile economic and political environment surrounding healthcare.
Since Wachter and Goldman coined the term hospitalist in 1996,[1] the number of hospitalists in the United States has grown rapidly, to more than 30,000 in recent estimates, with at least 80% of hospitals with 200 beds or more having hospital medicine programs.[2] A number of factors have led to the growth of such programs. First, hospital‐level incentives to use hospitalists exist to improve patient flow and maximize bed use, thereby reducing length of stay (LOS) and improving efficiency. Hospitals also employ hospitalists to address limitations on the number of hours that medical residents can work. Second, the use of hospitalists allows primary care physicians (PCPs) to focus their practices on outpatient care, thus avoiding the complexity of hospital‐based medicine, which requires both hospital‐focused clinical skills as well as institutional knowledge. Supporters of the hospitalist movement claim that hospitalists can improve efficiency and quality of care because hospitalists (1) have more experience managing inpatient care, (2) are more available to patients, and (3) have greater commitment to hospital quality improvements than (nonemployed) community PCPs.[3, 4, 5] On the other hand, criticisms of hospitalists include concerns related to (1) discontinuity in care and patient handoffs, (2) patient dissatisfaction at being treated by someone other than their PCP, (3) loss of acute care skills by PCPs, and (4) hospitalist burnout due to large workloads and poor institutional support.[3, 4, 5]
Hospitalists have been shown to have an effect on lowering total patient costs through better resource utilization and reduced LOS.[6, 7, 8, 9] There is no clear agreement, however, that hospitalists more often implement guideline‐recommended care.[10, 11, 12] In fact, most evaluations have found no significant differences between mortality and readmission rates among hospitalist and nonhospitalist groups.[12, 13, 14, 15, 16] The majority of these studies, however, were conducted in individual institutions or with small sample sizes, thus limiting their generalizability.
As 1 of the fastest‐growing medical specialties, hospitalists have assumed a significant role in inpatient care. The Centers for Medicare and Medicaid Services (CMS) have identified heart failure (HF), acute myocardial infarction (AMI), and pneumonia (PN) as important inpatient conditions associated with substantial morbidity and mortality among the Medicare population. Further, Jencks et al.[17] found that nearly one‐fifth of Medicare beneficiaries discharged from a hospital were readmitted within 30 days, which incurred an estimated cost to Medicare of $17.4 billion in 2004. Hospital readmission is of particular importance under healthcare reform because CMS introduced financial penalties in 2013 for hospitals with excessive readmission rates. The reimbursement penalty related to readmissions is included in the Patient Protection and Affordable Care Act and will be gradually expanded across many other outcomes.[18]
METHODS
Data Sources
Using hierarchical, generalized, linear modeling with hospital‐specific random effects, CMS has developed and made publicly available national, hospital‐level data reporting case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission rates, as measured from the first day of the index inpatient admission. The models produce aggregate hospital‐level predictions of excess mortality and readmissions, as compared to other hospitals with the same case mix.[19, 20] Outcome measures in this study reflect these hospital‐specific, adjusted measures of mortality and readmission. Each of these measures is expressed as a continuous variable of the adjusted number of events within a 30‐day period, analogous to a ratio of observed‐to‐expected outcomes, multiplied by the national rate. Specifically, the numerator is the number of observed events in a 30‐day period based on the hospital's case mix‐adjusted performance, and the denominator is the number of expected events in a 30‐day period based on average national hospital performance with that hospital's case mix. CMS adjusts the measures for case mix to account for important patient‐level, clinically relevant variables such as age, sex, and comorbidities. However, the data do not allow the measures to be further adjusted for admission source, discharge destination, or patient socioeconomic status.[19] CMS also does not report rates for hospitals with fewer than 25 cases for a condition, which could limit the generalizability of our findings with regard to small hospitals or hospitals with only occasional patients discharged with a target condition. Details on specific inclusion/exclusion criteria, model adjustment, and statistical approach used by CMS can be found in their methodology reports.[21, 22]
The 2008 CMS risk‐standardized mortality and readmission measures described above were linked with the 2008 American Hospital Association (AHA) Annual Survey Database, using each hospital's 6‐digit Medicare provider identification number. The AHA Annual Survey Database provides comprehensive hospital‐level data for approximately 6500 US hospitals, including demographics, organizational structure, facilities and services, utilization data, community indicators, physician arrangements, managed care relationships, expenses, and staffing, including employment of hospitalists.[23]
Variables
We used the CMS case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality and readmission measures for HF, AMI, and PN as dependent variables. The primary independent variable was a dichotomous measure of whether or not hospitalists provided care within the hospital. Covariates identified from the literature[11, 23, 24, 25, 26, 27, 28] included hospital and community characteristics, organizational perspective, size, and resources. Models were adjusted for hospital ownership (government, nongovernment nonprofit, investor‐owned for profit), region (Northeast, South, Midwest, West), teaching status, bed size, number of nurses per hospital bed, intensive care unit (ICU) presence (medicalsurgical, cardiac), managed care contracts (health maintenance organization, preferred provider organization), urban/rural setting, and median household income in the hospital county.
Statistical Analysis
Descriptive statistics of the dependent and independent variables illustrated trends across hospitals with and without hospitalists, and bivariate statistics identified differences between the 2 groups. We employed multivariable ordinary least squares (OLS) regression to assess the association between the independent variables and risk‐standardized, 30‐day all‐cause excess mortality and readmission rates at the hospital level. OLS was used because the dependent variables were measured continuously; count models were not appropriate for our analyses, because we did not have access to patient‐level data that could provide person‐days at risk for mortality or readmission. This limitation is mitigated, however, because CMS had already used hierarchical, multivariate, patient‐level models to produce hospital‐specific predictions, which formed the basis of our outcome measures. Six OLS models were run reflecting each of the 6 outcomes of interest: AMI mortality, HF mortality, and PN mortality, and AMI readmission, HF readmission, and PN readmission. All statistical analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
RESULTS
Hospital Characteristics and Descriptive Measures
There were 3029 US hospitals in the final analysis dataset. Of these, 59.3% reported employing hospitalists on staff. Descriptive statistics are shown in Table 1.
| Hospitalist Presence, n=1,796, % or Mean (SD) | No Hospitalist Presence, n=1,233, % or Mean (SD) | P Value | |
|---|---|---|---|
| |||
| Hospital control | <0.001 | ||
| Government | 14.8% | 33.3% | |
| Nongovernment, nonprofit | 72.9% | 56.8% | |
| Investor owned, for profit | 12.4% | 10.0% | |
| Bed size | 257 (224) | 94 (106) | <0.001 |
| Nurses per inpatient bed | 1.5 (0.6) | 1.1 (0.7) | <0.001 |
| Urban | 75.3% | 32.7% | <0.001 |
| Rural | 24.7% | 67.3% | |
| Region | <0.001 | ||
| Northeast | 18.2% | 8.3% | |
| South | 40.1% | 33.1% | |
| Midwest | 24.4% | 46.3% | |
| West | 17.3% | 12.3% | |
| ICU presence | |||
| Medicalsurgical | 94.0% | 64.0% | <0.001 |
| Cardiac | 58.7% | 26.9% | <0.001 |
| Managed care contracts | |||
| HMO | 81.2% | 59.4% | <0.001 |
| PPO | 88.7% | 79.9% | <0.001 |
| Teaching hospital | 12.6% | 1.7% | <0.001 |
| Median household income in hospital county | $51,851 ($13,566) | $44,448 ($10,058) | <0.001 |
Table 2 presents bivariate analyses. Mortality for all 3 conditions and readmissions for AMI and HF were all significantly lower among hospitals employing hospitalists. Of the 3029 hospitals in the sample (both with and without hospitalist programs), over 93% had 25 or more cases per category for 4 of the 6 outcome variables, indicating only a minor risk of hospital selection bias due to small size or infrequent admissions for target conditions.
| Outcome Variable | Hospitalist Presence, Mean (SD) | No Hospitalist Presence, Mean (SD) | P Value | n |
|---|---|---|---|---|
| ||||
| AMI mortality | 16.3 (1.8) | 16.7 (1.7) | <0.001 | 2,007 |
| HF mortality | 11.1 (1.6) | 11.4 (1.5) | <0.001 | 2,625 |
| PN mortality | 11.4 (1.9) | 11.8 (1.8) | <0.001 | 2,746 |
| AMI readmission | 19.8 (1.4) | 20.1 (1.3) | 0.003 | 1,707 |
| HF readmission | 24.2 (2.1) | 24.8 (2.0) | <0.001 | 2,620 |
| PN readmission | 18.1 (1.7) | 18.1 (1.6) | 0.896 | 2,709 |
Multivariate Analyses: Mortality Outcomes
Multivariate analyses showed no significant relationship between hospitalist care and risk‐standardized mortality measures for any of the 3 target conditions (Table 3). Stated more precisely, the presence or absence of hospitalists was not associated with an increase or decrease in the case mix‐adjusted, risk‐standardized, 30‐day all‐cause predicted excess mortality rates for these conditions. Covariates in the models generally performed as might be hypothesized. When stratified by ICU presence, urban/rural setting, and bed size, none of the hospitalist presence coefficients reached significance.
| Acute Myocardial Infarction (95% CI) | Heart Failure (95% CI) | Pneumonia (95% CI) | |
|---|---|---|---|
| |||
| Mortality | |||
| Hospitalist presence | 0.058 (0.132 to 0.247) | 0.104 (0.041 to 0.249) | 0.042 (0.132, 0.217) |
| Readmission | |||
| Hospitalist presence | 0.182 (0.343 to 0.022)a | 0.575 (0.763 to 0.387)c | 0.228 (0.380 to 0.075)b |
Multivariate Analyses: Readmission Outcomes
In contrast to the mortality measures, risk‐standardized readmission rates were significantly lower for all 3 conditions for hospitals employing hospitalists (Table 3). Specifically, hospitalist services within a hospital were associated with a decrease in case mix‐adjusted, risk‐standardized, 30‐day predicted excess readmissions for each of the 3 target conditions, as follows: 0.182 fewer predicted AMI readmissions per 100 people at risk (P<0.05), 0.575 fewer predicted HF readmissions per 100 people at risk (P<0.001), and 0.228 fewer predicted PN readmissions per 100 people at risk (P<0.01). Covariates in the models again generally performed as might be expected. When stratified, the presence of hospitalists tended to have a stronger negative association with medicalsurgical ICU presence, cardiac ICU presence, urban setting, and larger bed size.
Full results from the OLS regressions for mortality and readmission outcome variables, including significance levels and 95% confidence intervals, are available (see Supporting Information, Appendix Tables 1 and 2, in the online version of this article).
DISCUSSION
Most previous studies have used patient‐level data from single institutions, and have shown inconsistent association between hospitalist care and clinical outcomes. Only a few studies have been conducted at the national level, and we know of only 1 that uses the same types of clinical outcomes as in our approach. In particular, Goodrich et al. conducted an in‐depth survey of hospitalist programs, and found that hospitalist presence had a significant association with HF readmissions.[29] Our results, similar to those of Goodrich et al., showed that the presence of hospitalists was not associated with risk‐standardized, 30‐day, all‐cause predicted excess mortality rates for Medicare patients hospitalized for any of these 3 conditions. The presence of hospitalists was, however, associated with lower‐risk standardized, 30‐day, all‐cause predicted excess readmission rates in our study. Our analyses resulted in somewhat different coefficients than Goodrich et al., but that is most likely due to: (1) different sample sizes, (2) use of similar yet not identical control variables, and (3) reporting error, as we used different sets of self‐reported data to indicate hospitalist services. The presence of a hospital‐level association with inconclusive patient‐level evidence suggests that there may be a more nuanced relationship between hospitalists and quality of care than has been previously explored.
This result may be explained by a number of reasons, the first of which is that hospitalists generally have more experience in the increasingly specialized practice of hospital‐based medicine than PCPs or nonhospitalists. For example, Meltzer et al.[30] found that hospitalists have more experience than nonhospitalists in treating acute manifestations of cardiovascular and respiratory diseases. Even though we might expect that greater experience with hospital‐based medicine would be associated with lower mortality rates, this outcome may not be captured because mortality is a rare event in the reported 30‐day postdischarge period and may be less preventable than readmission. There are a number of other factors possibly affecting hospital readmission, such as inadequate information transfer by discharge planners, poor patient compliance, inadequate follow‐up, insufficient use of family caregivers, deterioration of a patient's clinical condition, and medical errors.[31]
Studies have found that hospitalists have had positive effects related to managing case complexity and navigating the discharge process, perhaps due to their increased availability to patients and commitment to hospital quality improvements.[16, 32] Some determinants of patient outcomes may be difficult for hospitalists to influence, however, such as poor patient compliance or lack of support by family caregivers. Hospitalists who have extensive discharge experience may understand key challenges and adopt strategies to ameliorate these negative effects, for instance by using appropriate motivational strategies to encourage compliance and capitalizing on family caregivers.[33] Being located in the hospital, hospitalists are more available to deal with emergencies that occur during the hospitalization, and may be more available and active in discharge planning. Benbassat and Taragin[34] found that between 9% and 48% of all readmissions were preventable because they were associated with indicators of substandard care during the index hospitalization. They further estimated between 12% and 75% more readmissions could have been prevented by implementing patient education, predischarge assessment, and at‐home aftercare programs. Hospitalists are in a unique position to use their specialized training to improve transitions from hospital to home, communicate needs with the family and caregivers during the index hospitalization, and ensure that adequate postdischarge care is received. Although the use of hospitalists creates another handoff in the transition between inpatient and outpatient settings, hospitalist care may have a positive effect on many of the determinants of readmission sufficient to overcome that discontinuity.
Quality of care may also be affected by tertiary factors such as hospital administration or organizational culture. Lower AMI mortality has been associated with factors beyond cardiologist care, including organizational behavior and the appointment of physician and nurse champions.[35] Although the exact mechanism is unclear, better patient outcomes may be a result of this combination of direct clinical care, care transition management, and administrative or organizational factors. The models showed several hospital and community characteristics having coefficients larger in magnitude than the hospitalist variable, including classification as a teaching hospital, region, and hospital county median income. Teaching hospitals have been shown to have varying effects on quality of care depending on the type of care being provided, and teaching status may also be a proxy for factors related to organizational culture or mission.[36] Community‐level contextual factors including poverty and income have been shown previously to be related to readmission rates, possibly due to lack of social support and financial resources in the community to help discharged patients manage their healthcare needs in community settings.
Research Limitations
Two important limitations of this study are assumptions made necessary using aggregated, hospital‐level data. These assumptions include: (1) that hospitalists regularly treat Medicare patients with HF, AMI, and PN, and (2) that patient exposure to hospitalists is consistent in amount and quality across all patients treated in the hospital. Due to the frequency of the 3 study conditions in the Medicare population, it is reasonable to assume that hospitalists treat these patients, but it is unlikely that all patients admitted to each hospital employing hospitalists are indeed treated by hospitalists or that they are all treated in a consistent manner. There is also significant variation among hospitalist services nationwide, from different types of hospitalists to varying responsibilities across settings. Differences in physician practice structure and hospital staffing could affect hospitalist care on individual patient outcomes between hospitals that employ hospitalists. Models also did not control for the extent to which hospitals have implemented specific interventions to prevent hospital readmissions; hospitals with hospitalists may more often implement other interventions potentially influencing readmissions. We further could not distinguish between effective and ineffective hospitalist programs. The inability to account for these factors would effectively weaken the indicator, most likely underestimating the association between hospitalist presence and the outcome variables. Finally, the AHA database is subject to some variability, as it utilizes self‐reported data from the hospitals, but the database is generally considered the industry standard.
Using OLS regression, this study reflects correlation, but cannot demonstrate causation between the presence of hospitalists and an increase or decrease in risk‐standardized predicted mortality or readmission rates. There is also controversy regarding the appropriateness of using risk‐standardized predicted mortality and readmission rates as measures of quality of care, because these rates represent outcomes that may be influenced by other factors beyond the care received during the inpatient stay. These rates will, however, be of increasing importance given emerging pay‐for‐performance initiatives.[35, 37, 38]
CONCLUSION
Reducing medical errors and improving patient outcomes are becoming more important in light of increased reporting of hospital performance and outcome measures. Post‐discharge 30‐day mortality and hospital readmission represent 2 major undesirable patient outcomes, and Medicare's new pay‐for‐performance initiatives only provide further incentives for hospitals to take action in reducing these rates. Because the likelihood of receiving inpatient care provided by a hospitalist has significantly increased among Medicare patients since the 1990s,[39] hospitalists have become important players in potentially reducing mortality and readmission for patients discharged from inpatient settings. This study has shown that use of hospitalists may be associated with lower hospital readmissions, a clear quality measure, but are not associated with any changes in 30‐day mortality.
Further studies are needed, however, to better characterize and validate the observed associations, as well as to determine how hospitalist programs can be enhanced to improve inpatient care quality. Case studies could be carried out within hospitals with high‐ and low‐performing hospitalist services to help identify key aspects of hospitalist care most closely associated with desirable outcomes. Discharge and transitional care processes could also be standardized according to best practices, with their implementation tailored to individual hospital settings. Finally, as patient‐level data become increasingly available, researchers should merge these data with hospital‐level data to assess more robustly the multilevel effect of hospitalists on inpatient quality of care and individual patient outcomes. Such information will be valuable to policymakers and health administrators alike in the ongoing and volatile economic and political environment surrounding healthcare.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- Society of Hospital Medicine. 2010. SHM fact sheet: about hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit130(4 pt 2):338–342.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2):382–387.
- . The hospitalist model: perspectives of the patient, the internist, and internal medicine. Ann Intern Med. 1999;130(4 pt 2):368–372.
- , . Economic and healthcare forces of hospitalist movement. Mt Sinai J Med. 2008;75(5):424–429.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs. nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , , , . Determinants of hospitalist efficiency: a qualitative and quantitative study. Med Care Res Rev. 2009;66(6):682–702.
- , , , , , . The value of a hospitalist service: efficient care for the aging population? Chest. 2001;119(2):580–589.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , , . Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869–1874.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act of 2010. P.L. 111–148, §3025 Stat. 328 (2010).
- Centers for Medicare and Medicaid Services. 2008. Hospital outcome of care measures [data file]. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/index.html?redirect=/HospitalQualityInits/11_HospitalCompare.asp. Accessed November 15, 2011.
- , . Public reporting of 30‐day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–1397.
- Quality Net. 2012. Measure methodology reports: mortality measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page35(3):22–34.
- , , , . Care in U.S. hospitals—the hospital quality alliance program. N Engl J Med. 2005;353(3):265–274.
- , , , et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511–2517.
- United States Census Bureau. 2008. Small area income and poverty estimates [data file]. Available at: http://www.census.gov/did/www/saipe/. Accessed March 5, 2012.
- United States Department of Agriculture Economic Research Service. 2004. Rural‐urban continuum codes [data file]. Available at: http://www.ers.usda.gov/data‐products/rural‐urban‐continuum‐codes.aspx. Accessed March 5, 2012.
- , , , , . Hospitalist utilization and hospital performance on 6 publicly reported patient outcomes. J Hosp Med. 2012;7(6):482–488.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , . Medicare hospital readmissions: issues, policy options, and PPACA (R40972). Congressional Research Service. Washington, DC: U.S. Government Printing Office; 2010.
- , , , . Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital. CMAJ. 2000;163(11):1477–1480.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160(8):1074–1081.
- , , , et al. Hospital strategies for reducing risk‐standardized mortality rates in acute myocardial infarction. Ann Intern Med. 2012;156(9):618–626.
- , . Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80(3):569–593.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , . Hospital readmissions as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- Society of Hospital Medicine. 2010. SHM fact sheet: about hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit130(4 pt 2):338–342.
- , . The hospitalist: new boon for internal medicine or retreat from primary care? Ann Intern Med. 1999;130(4 pt 2):382–387.
- . The hospitalist model: perspectives of the patient, the internist, and internal medicine. Ann Intern Med. 1999;130(4 pt 2):368–372.
- , . Economic and healthcare forces of hospitalist movement. Mt Sinai J Med. 2008;75(5):424–429.
- , . The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis. Med Care Res Rev. 2005;62(4):379–406.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs. nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , , . Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists. Arch Intern Med. 2002;162(11):1251–1256.
- , , , , . Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389–1394.
- , , , et al. Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists. J Gen Intern Med. 2008;23(9):1399–1406.
- , , , , , . Determinants of hospitalist efficiency: a qualitative and quantitative study. Med Care Res Rev. 2009;66(6):682–702.
- , , , , , . The value of a hospitalist service: efficient care for the aging population? Chest. 2001;119(2):580–589.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , , . Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869–1874.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- Patient Protection and Affordable Care Act of 2010. P.L. 111–148, §3025 Stat. 328 (2010).
- Centers for Medicare and Medicaid Services. 2008. Hospital outcome of care measures [data file]. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/index.html?redirect=/HospitalQualityInits/11_HospitalCompare.asp. Accessed November 15, 2011.
- , . Public reporting of 30‐day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–1397.
- Quality Net. 2012. Measure methodology reports: mortality measures. Available at: http://www.qualitynet.org/dcs/ContentServer?c=Page35(3):22–34.
- , , , . Care in U.S. hospitals—the hospital quality alliance program. N Engl J Med. 2005;353(3):265–274.
- , , , et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166(22):2511–2517.
- United States Census Bureau. 2008. Small area income and poverty estimates [data file]. Available at: http://www.census.gov/did/www/saipe/. Accessed March 5, 2012.
- United States Department of Agriculture Economic Research Service. 2004. Rural‐urban continuum codes [data file]. Available at: http://www.ers.usda.gov/data‐products/rural‐urban‐continuum‐codes.aspx. Accessed March 5, 2012.
- , , , , . Hospitalist utilization and hospital performance on 6 publicly reported patient outcomes. J Hosp Med. 2012;7(6):482–488.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , . Medicare hospital readmissions: issues, policy options, and PPACA (R40972). Congressional Research Service. Washington, DC: U.S. Government Printing Office; 2010.
- , , , . Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital. CMAJ. 2000;163(11):1477–1480.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160(8):1074–1081.
- , , , et al. Hospital strategies for reducing risk‐standardized mortality rates in acute myocardial infarction. Ann Intern Med. 2012;156(9):618–626.
- , . Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80(3):569–593.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , . Hospital readmissions as an accountability measure. JAMA. 2011;305(5):504–505.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
© 2013 Society of Hospital Medicine
Electronic Health Records Raise New Concerns for Hospitalists
Implementing an electronic health record (EHR) remains a major concern for hospitals and their hospitalists, with even successful “go live” EHR rollouts accompanied by a host of difficulties, says Russ Cucina, MD, MS, hospitalist and medical director of information technology at the University of California at San Francisco (UCSF) Medical Center.
Speaking at the 17th annual Management of the Hospitalized Patient conference in downtown San Francisco on Nov. 1, sponsored by UCSF and co-sponsored by SHM, Dr. Cucina said training of physicians should be mandatory—along with a test of their competency—before they use EHR and computerized physician order entry. But even more important is the “elbow-side” support provided during the rollout, while post-implementation training will have more impact.
Dr. Cucina urged hospitalists to look at EHR implementation as a process, not an event, and to develop their own goals for EHR, expecting that their hospital’s goals will only partially overlap with what they need from the system.
“Take a minute and forget the computers,” he said to participants. “How would you like to change your day-to-day practice to be more efficient, safer, with less paperwork, fewer redundancies, and processes that actually support your work?” An EHR system can impose new and unwanted structural requirements on physicians’ workflow if they don’t speak up about how they want it to be structured.
Dr. Cucina told attendees that UCSF spent a lot of money on its EHR, but still thinks the investment was worth it. With federal “meaningful use” incentives and penalties looming in 2014 for hospitals’ participation in health information technology, EHR will continue to become more important. Some hospitals may find it worthwhile to subscribe to an existing computerized system at another hospital in their region. UCSF will be making its system available by subscription to nearby Children’s Hospital of Oakland, Dr. Cucina said.
Barriers can be enormous for a dissatisfied hospital that wants to exit an unsatisfactory implemented EHR system, Dr. Cucina noted.
“Ask yourself: Does the software really stink, or is your implementation not so good?” he said. He recommended that dissatisfied hospitals ask their EHR vendor to name several top performing hospitals that use its system. “Go visit them, with all of the questions you didn’t know you needed to ask before you purchased the system,” he added. TH
Larry Beresford is a freelance writer in San Francisco.
Implementing an electronic health record (EHR) remains a major concern for hospitals and their hospitalists, with even successful “go live” EHR rollouts accompanied by a host of difficulties, says Russ Cucina, MD, MS, hospitalist and medical director of information technology at the University of California at San Francisco (UCSF) Medical Center.
Speaking at the 17th annual Management of the Hospitalized Patient conference in downtown San Francisco on Nov. 1, sponsored by UCSF and co-sponsored by SHM, Dr. Cucina said training of physicians should be mandatory—along with a test of their competency—before they use EHR and computerized physician order entry. But even more important is the “elbow-side” support provided during the rollout, while post-implementation training will have more impact.
Dr. Cucina urged hospitalists to look at EHR implementation as a process, not an event, and to develop their own goals for EHR, expecting that their hospital’s goals will only partially overlap with what they need from the system.
“Take a minute and forget the computers,” he said to participants. “How would you like to change your day-to-day practice to be more efficient, safer, with less paperwork, fewer redundancies, and processes that actually support your work?” An EHR system can impose new and unwanted structural requirements on physicians’ workflow if they don’t speak up about how they want it to be structured.
Dr. Cucina told attendees that UCSF spent a lot of money on its EHR, but still thinks the investment was worth it. With federal “meaningful use” incentives and penalties looming in 2014 for hospitals’ participation in health information technology, EHR will continue to become more important. Some hospitals may find it worthwhile to subscribe to an existing computerized system at another hospital in their region. UCSF will be making its system available by subscription to nearby Children’s Hospital of Oakland, Dr. Cucina said.
Barriers can be enormous for a dissatisfied hospital that wants to exit an unsatisfactory implemented EHR system, Dr. Cucina noted.
“Ask yourself: Does the software really stink, or is your implementation not so good?” he said. He recommended that dissatisfied hospitals ask their EHR vendor to name several top performing hospitals that use its system. “Go visit them, with all of the questions you didn’t know you needed to ask before you purchased the system,” he added. TH
Larry Beresford is a freelance writer in San Francisco.
Implementing an electronic health record (EHR) remains a major concern for hospitals and their hospitalists, with even successful “go live” EHR rollouts accompanied by a host of difficulties, says Russ Cucina, MD, MS, hospitalist and medical director of information technology at the University of California at San Francisco (UCSF) Medical Center.
Speaking at the 17th annual Management of the Hospitalized Patient conference in downtown San Francisco on Nov. 1, sponsored by UCSF and co-sponsored by SHM, Dr. Cucina said training of physicians should be mandatory—along with a test of their competency—before they use EHR and computerized physician order entry. But even more important is the “elbow-side” support provided during the rollout, while post-implementation training will have more impact.
Dr. Cucina urged hospitalists to look at EHR implementation as a process, not an event, and to develop their own goals for EHR, expecting that their hospital’s goals will only partially overlap with what they need from the system.
“Take a minute and forget the computers,” he said to participants. “How would you like to change your day-to-day practice to be more efficient, safer, with less paperwork, fewer redundancies, and processes that actually support your work?” An EHR system can impose new and unwanted structural requirements on physicians’ workflow if they don’t speak up about how they want it to be structured.
Dr. Cucina told attendees that UCSF spent a lot of money on its EHR, but still thinks the investment was worth it. With federal “meaningful use” incentives and penalties looming in 2014 for hospitals’ participation in health information technology, EHR will continue to become more important. Some hospitals may find it worthwhile to subscribe to an existing computerized system at another hospital in their region. UCSF will be making its system available by subscription to nearby Children’s Hospital of Oakland, Dr. Cucina said.
Barriers can be enormous for a dissatisfied hospital that wants to exit an unsatisfactory implemented EHR system, Dr. Cucina noted.
“Ask yourself: Does the software really stink, or is your implementation not so good?” he said. He recommended that dissatisfied hospitals ask their EHR vendor to name several top performing hospitals that use its system. “Go visit them, with all of the questions you didn’t know you needed to ask before you purchased the system,” he added. TH
Larry Beresford is a freelance writer in San Francisco.