User login
Computerized checklist can reduce CLABSI rate
Staphylococcus infection
Credit: Bill Branson
A computerized safety checklist that pulls information from patients’ electronic medical records can reduce the incidence of central line-associated bloodstream infections (CLABSIs), according to a study published in Pediatrics.
The study was conducted among children admitted to the pediatric intensive care unit at Lucile Packard Children’s Hospital Stanford in California.
Researchers found the safety checklist increased overall staff compliance with best practices for CLABSI prevention and resulted in a 3-fold reduction in CLABSI incidence.
The automated checklist, and a dashboard-style interface used to interact with it, was designed to help caregivers follow national guidelines for CLABSI prevention. The system combed through data in a patient’s electronic medical record and pushed alerts to physicians and nurses when a patient’s central line was due for care.
The dashboard interface displayed real-time alerts on a large LCD screen in the nurses’ station. Alerts—shown as red, yellow, or green dots beside patients’ names—were generated if, for example, the dressing on a patient’s central line was due to be changed, or if it was time for caregivers to re-evaluate whether medications given in the central line could be switched to oral formulations instead.
“The information was visible and easy to digest,” said study author Deborah Franzon, MD. “We improved compliance with best-care practices and pulled information that otherwise would have been difficult to look for. It reduced busy work and made it possible for the healthcare team to perform their jobs more efficiently and effectively.”
The system was implemented on May 1, 2011, but the researchers considered the rollout period to extend to August 31, 2011. So this period was not included in the analysis.
The team compared data on CLABSI rates, compliance with bundle elements, and staff perceptions/knowledge before the intervention began—from June 1, 2009, to April 30, 2011—and after the system was fully implemented—September 1, 2011, to December 31, 2012.
CLABSI rates decreased from 2.6 per 1000 line-days before the intervention to 0.7 per 1000 line-days afterward (P=0.02). There were a total of 19 CLABSIs per 7322 line-days pre-intervention and 7 CLABSIs per 6155 line-days post-intervention.
The researchers estimated that the intervention saved approximately $260,000 per year in healthcare costs. Treating a single CLABSI costs approximately $39,000.
The team also found that daily documentation of line necessity increased from 30% before the intervention to 73% after (P<0.001). Compliance with dressing changes increased from 87% to 90% (P=0.003).
Compliance with cap changes increased from 87% to 93% (P<0.001). And compliance with port needle changes increased from 69% to 95% (P<0.001). However, compliance with insertion bundle documentation decreased from 67% to 62% (P=0.001).
After the system was implemented, there was a significant increase in staff perception that the medical team addressed central line necessity during rounds (P=0.02). But there was no significant difference in communication among team members (P=0.73) or knowledge regarding the components of the maintenance bundle (P=0.39).
Nevertheless, the researchers concluded that their system promotes compliance with best practices for CLABSI prevention, thereby reducing the risk of harm to patients.
The team hopes to use the system in other ways, such as monitoring the recovery of children who have received organ transplants.
“[The system] lets physicians focus on taking care of the patient while automating some of the background safety checks,” said study author Natalie Pageler, MD. “The nice thing about this tool is that it’s integrated into the electronic medical record, which we use every single day.” ![]()

Staphylococcus infection
Credit: Bill Branson
A computerized safety checklist that pulls information from patients’ electronic medical records can reduce the incidence of central line-associated bloodstream infections (CLABSIs), according to a study published in Pediatrics.
The study was conducted among children admitted to the pediatric intensive care unit at Lucile Packard Children’s Hospital Stanford in California.
Researchers found the safety checklist increased overall staff compliance with best practices for CLABSI prevention and resulted in a 3-fold reduction in CLABSI incidence.
The automated checklist, and a dashboard-style interface used to interact with it, was designed to help caregivers follow national guidelines for CLABSI prevention. The system combed through data in a patient’s electronic medical record and pushed alerts to physicians and nurses when a patient’s central line was due for care.
The dashboard interface displayed real-time alerts on a large LCD screen in the nurses’ station. Alerts—shown as red, yellow, or green dots beside patients’ names—were generated if, for example, the dressing on a patient’s central line was due to be changed, or if it was time for caregivers to re-evaluate whether medications given in the central line could be switched to oral formulations instead.
“The information was visible and easy to digest,” said study author Deborah Franzon, MD. “We improved compliance with best-care practices and pulled information that otherwise would have been difficult to look for. It reduced busy work and made it possible for the healthcare team to perform their jobs more efficiently and effectively.”
The system was implemented on May 1, 2011, but the researchers considered the rollout period to extend to August 31, 2011. So this period was not included in the analysis.
The team compared data on CLABSI rates, compliance with bundle elements, and staff perceptions/knowledge before the intervention began—from June 1, 2009, to April 30, 2011—and after the system was fully implemented—September 1, 2011, to December 31, 2012.
CLABSI rates decreased from 2.6 per 1000 line-days before the intervention to 0.7 per 1000 line-days afterward (P=0.02). There were a total of 19 CLABSIs per 7322 line-days pre-intervention and 7 CLABSIs per 6155 line-days post-intervention.
The researchers estimated that the intervention saved approximately $260,000 per year in healthcare costs. Treating a single CLABSI costs approximately $39,000.
The team also found that daily documentation of line necessity increased from 30% before the intervention to 73% after (P<0.001). Compliance with dressing changes increased from 87% to 90% (P=0.003).
Compliance with cap changes increased from 87% to 93% (P<0.001). And compliance with port needle changes increased from 69% to 95% (P<0.001). However, compliance with insertion bundle documentation decreased from 67% to 62% (P=0.001).
After the system was implemented, there was a significant increase in staff perception that the medical team addressed central line necessity during rounds (P=0.02). But there was no significant difference in communication among team members (P=0.73) or knowledge regarding the components of the maintenance bundle (P=0.39).
Nevertheless, the researchers concluded that their system promotes compliance with best practices for CLABSI prevention, thereby reducing the risk of harm to patients.
The team hopes to use the system in other ways, such as monitoring the recovery of children who have received organ transplants.
“[The system] lets physicians focus on taking care of the patient while automating some of the background safety checks,” said study author Natalie Pageler, MD. “The nice thing about this tool is that it’s integrated into the electronic medical record, which we use every single day.” ![]()

Staphylococcus infection
Credit: Bill Branson
A computerized safety checklist that pulls information from patients’ electronic medical records can reduce the incidence of central line-associated bloodstream infections (CLABSIs), according to a study published in Pediatrics.
The study was conducted among children admitted to the pediatric intensive care unit at Lucile Packard Children’s Hospital Stanford in California.
Researchers found the safety checklist increased overall staff compliance with best practices for CLABSI prevention and resulted in a 3-fold reduction in CLABSI incidence.
The automated checklist, and a dashboard-style interface used to interact with it, was designed to help caregivers follow national guidelines for CLABSI prevention. The system combed through data in a patient’s electronic medical record and pushed alerts to physicians and nurses when a patient’s central line was due for care.
The dashboard interface displayed real-time alerts on a large LCD screen in the nurses’ station. Alerts—shown as red, yellow, or green dots beside patients’ names—were generated if, for example, the dressing on a patient’s central line was due to be changed, or if it was time for caregivers to re-evaluate whether medications given in the central line could be switched to oral formulations instead.
“The information was visible and easy to digest,” said study author Deborah Franzon, MD. “We improved compliance with best-care practices and pulled information that otherwise would have been difficult to look for. It reduced busy work and made it possible for the healthcare team to perform their jobs more efficiently and effectively.”
The system was implemented on May 1, 2011, but the researchers considered the rollout period to extend to August 31, 2011. So this period was not included in the analysis.
The team compared data on CLABSI rates, compliance with bundle elements, and staff perceptions/knowledge before the intervention began—from June 1, 2009, to April 30, 2011—and after the system was fully implemented—September 1, 2011, to December 31, 2012.
CLABSI rates decreased from 2.6 per 1000 line-days before the intervention to 0.7 per 1000 line-days afterward (P=0.02). There were a total of 19 CLABSIs per 7322 line-days pre-intervention and 7 CLABSIs per 6155 line-days post-intervention.
The researchers estimated that the intervention saved approximately $260,000 per year in healthcare costs. Treating a single CLABSI costs approximately $39,000.
The team also found that daily documentation of line necessity increased from 30% before the intervention to 73% after (P<0.001). Compliance with dressing changes increased from 87% to 90% (P=0.003).
Compliance with cap changes increased from 87% to 93% (P<0.001). And compliance with port needle changes increased from 69% to 95% (P<0.001). However, compliance with insertion bundle documentation decreased from 67% to 62% (P=0.001).
After the system was implemented, there was a significant increase in staff perception that the medical team addressed central line necessity during rounds (P=0.02). But there was no significant difference in communication among team members (P=0.73) or knowledge regarding the components of the maintenance bundle (P=0.39).
Nevertheless, the researchers concluded that their system promotes compliance with best practices for CLABSI prevention, thereby reducing the risk of harm to patients.
The team hopes to use the system in other ways, such as monitoring the recovery of children who have received organ transplants.
“[The system] lets physicians focus on taking care of the patient while automating some of the background safety checks,” said study author Natalie Pageler, MD. “The nice thing about this tool is that it’s integrated into the electronic medical record, which we use every single day.” ![]()
Transfusion increases risks in PCI patients, study shows

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI. ![]()

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI. ![]()

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI. ![]()
Peer‐Reviewed Journals and Social Media
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
Only 20 years ago, science from peer‐reviewed journals was still distributed and consumed in the same fashion that evolved from the earliest days of medical science: in print at monthly or weekly intervals. The Internet radically accelerated this paradigm but left the essential processes intact; journals could publish the information and readers could read it more easily, but the basic forums for interaction and discussion over the content remained the same. Enter Web 2.0 and the era of social media. Authors, editors, and readers can now interact easily with each other over the content in real time and across great distances.
Social media may not have changed the way science is produced and reviewed, but it is certainly changing how people consume and use the science. Some have suggested that social media activity around particular articles or journals may be a more important measure of impact than traditional measures of citation,[1] and others have suggested that Twitter activity in particular has changed both the speed and quality of discussion about new studies within the scientific community.[2] In the face of these trends, the Journal of Hospital Medicine (JHM) has decided to develop a bold strategy for leadership in this emerging area, with an initial focus on increasing JHM's activity and visibility on Twitter.
As part of this initial focus, JHM has successfully developed and implemented a protocol for use by authors to compose 2 Tweets describing their publications: the first announces the article's publication (e.g., New evidence on white coats and risk for hospital‐acquired infections), and the second promotes a key point from the article (e.g., Does the doctor's white coat spread hospital infection?). These Tweets are encouraged (but not required) from the corresponding author for every article in every edition, and JHM's editorial staff works with individual authors to refine their message and maximize their impact. To help authors, we have developed several tips for effective tweeting (Table 1).
| 1. Make it short:The limit is 140 characters, but getting retweets requires additional room for others to add their 2 cents, so try to get it under 100 characters. |
| 2. Make it simple: If your tweet includes complex terminology or analytic methods, it is not likely to get picked up. Make it easy to read for the lay public. |
| 3. Make it clear: Your article may have several conclusions, but pick the most newsworthy for the general public. It is usually best to focus on the main finding. |
| 4. Pose a question: Raise interest by piquing the curiosity of potential readers. A good question can motivate readers to click on your article to find the answer. |
| 5. Add a hashtag: Hashtags index tweets on Twitter. It is best to pick 1 or 2 existing tags from the healthcare hashtag project that fit the focus of your article ( |
| 6. Build your following: Include your Twitter handle to alert current/prospective followers of your publication. |
Even after just 1 year of this Twitter‐focused strategy, we are already seeing noteworthy impact and have learned several lessons.
AUTHORS CAN AND WILL GENERATE TWEETS FOR THEIR ARTICLES
When we started asking authors to generate tweets for their articles, Twitter was relatively new, and we were unsure if authors would be willing and able to participate. Since we started, we have noticed a steady increase in the number of author‐generated tweets. Today, more than three‐quarters of tweets per issue are author generated. Anecdotal feedback has been very positive, and authors have expressed interest in the plan for tweeting as well as feedback on how well their tweets were written. If authors or institutions are on Twitter, we also encourage using the Twitter name or handle in the tweet, which serves as a way for others on Twitter to identify directly with the author or institution and often results in greater interest in a particular tweet. Of note, authors have no obligation to become regular users of Twitter or engage with followers of JHM's Twitter feed, but many find themselves following the journal's feed more closely (and responding to posts by other authors) once they have joined Twitter and tweeted about their own work via JHM.
#HASHTAGS MAKE IT HAPPEN
Because Twitter users are a very large crowd of people with diverse interests, it is important to target tweets to the groups that would be most interested in studies. The use of hashtags makes it easy to index tweets. One of the major edits of author‐generated tweets that we provide is to index the articles to the most popular hashtags. For example, medical education studies can be indexed under #meded, which is a popular hashtag for clinician educators. Other important hashtags for hospitalists include #ptsafety, #readmissions, #healthpolicy, #healthcosts, or #infectiousdisease. To select hashtags, we have found the healthcare hashtag directory maintained by Symplur (Upland, CA;
HIGH IMPACT STUDIES MAKE A BIGGER IMPACT ON TWITTER
We observed a high number of retweets and comments about articles that were the most viewed studies on JHM online, referring to Project BOOST (Better Outcomes for Older Adults Through Safe Transitions) and the Society of Hospital Medicine's Choosing Wisely campaign. This is not surprising given the national focus on readmissions as well as cost‐conscious care. Moreover, our experience is in line with observations that Twitter provides an additional source of page views and article downloads for medical journals[3] and research, which demonstrates that studies that are tweeted will eventually be cited more.[4, 5]
TECHNOLOGY STUDIES ARE ADORED BY TWITTER
Studies and research examining the use of smartphones, apps, or social media in healthcare draw a lot of attention on Twitter, particularly from other technophiles in healthcare who often use the #hscm healthcare social media hashtag. Such studies often resonate with Twitter users, who tend to be engaged in technology at a high level and are interested in how to advance the use of technology in the healthcare workplace.
JHM's social media strategy has already been very successful in its early implementation; the JHM twitter feed has >600 followers. Although most authors submit their own tweets (71/117 or 61% of articles over the last year), JHM has also created social media roles for editors to fill in tweets when missing and ensure timely and consistent output from the JHM feed. We have also started a Facebook page, with a rapidly growing number of followers, and we continue to see our social media influence scores rise. In the next year we hope to develop a JHM blog, with invited commentary as well as a process for unsolicited submissions from our readership.
Increasingly, a journal's impact (small i) is measured not only in the traditional metric of impact factor (a representation of the number of papers cited in a given journal publication year), but also by the journal's ability to disseminate knowledge and awareness of issues key to the field. Social media is a major element of the next phase of evidence dissemination, and JHM is pleased to be developing and growing its footprint in the digital world.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
- , . Exploring the use of social media to measure journal article impact. AMIA Annu Symp Proc. 2011;2011:374–381.
- . Peer review: trial by Twitter. Nature. 2011;469(7330):286–287.
- , , , . Social media release increases dissemination of original articles in the clinical pain sciences. PLoS One. 2013;8(7):e68914.
- . Can tweets predict citations? Metrics of social impact based on Twitter and correlation with traditional metrics of scientific impact. J Med Internet Res. 2011;13(4):e123.
- , , , . Do altmetrics work? Twitter and ten other social web services. PLoS One. 2013;8(5):e64841.
FIM at Discharge and Rehospitalization
Federally mandated pay‐for‐performance initiatives promote minimizing 30‐day hospital readmissions to improve healthcare quality and reduce costs. Although the reasons for readmissions are multifactorial, many patients are readmitted for a condition other than their initial hospital admitting diagnosis.[1] Impairments in functional status experienced during acute care hospitalization contribute to patients being discharged in a debilitated state and being vulnerable to postdischarge complications and potentially hospital readmission.[2] As such, decreased functional status may be an important and potentially modifiable risk factor for acute care hospital readmission.[3]
Previous studies have suggested that impaired functional status may be an important predictor of rehospitalization.[4, 5, 6, 7] However, inferences from existing studies are limited because they did not consider functional status as their primary focus, they only considered specific patient populations (eg, stroke) or readmissions occurring well beyond the 30‐day period defined by federal pay‐for‐performance standards.[4, 5, 6, 8, 9, 10] Our objective was to evaluate the association between functional status near the time of discharge from acute care hospital and 30‐day readmission for patients admitted to an acute inpatient rehabilitation facility. As a secondary objective, we sought to investigate the relationship between functional status and readmission by diagnostic category (medical, neurologic, or orthopedic).
METHODS
Study Population and Setting
We conducted a single‐center, retrospective study of patients admitted to an inpatient rehabilitation facility at a community hospital between July 1, 2006 and December 31, 2012. This facility provides intensive rehabilitation consisting of 3 hours of therapy per day, skilled nursing care on a 24‐hour basis, and medical care by a physiatrist. We excluded patients who died during inpatient rehabilitation (n=15, 0.2%) and patients not admitted directly from an acute care setting (n=178, 2.0%).
Data Source and Covariates
Data were derived from the Uniform Data System for Medical Rehabilitation (UDSMR), which is an administrative database providing the following data upon admission to an inpatient rehabilitation facility[11, 12, 13]: age, gender, race/ethnicity, marital status, the discharge setting, the admission Functional Independence Measure (FIM) score (details further below), and admission diagnostic category as defined by the primary discharge diagnosis from the acute care hospital and grouped by functional related groups (a case‐mix system for medical rehabilitation).[12, 14] The 3M ClinTrac management software (3M, St. Paul, MN), used for mandatory reporting to the State of Maryland, provided all‐payerrefined diagnosis related group (APRDRG) and severity of illness (SOI) combinations (a tool to group patients into clinically comparable disease and severity‐of‐illness categories expected to use similar resources and experience similar outcomes). The University HealthSystem Consortium (UHC) database provided national readmission rates for all APRDRG‐SOI combinations using a methodology that has been previously described.[15, 16] Expected readmission rates for APRDRG‐SOI combinations served as a patient risk stratification tool based on clinical logic that evaluates age, comorbidities, principal diagnosis during hospitalization, and procedures conducted during hospitalization.[17]
Primary Outcome: Acute Care Readmission
The primary outcome was all‐cause acute care readmission, defined as patient transfer to an acute care hospital during inpatient rehabilitation within 30 days from admission to inpatient rehabilitation. The care model for our inpatient rehabilitation unit is such that when patients become sick or develop a complication, they are admitted directly to a clinical unit (eg, intensive care unit) at the community hospital through a rapid‐response intervention, or the physiatrist arranges with an admitting inpatient attending to accept the patient directly to his or her service.
Primary Exposure: Functional Independence Measure
Functional status was measured using the FIM score.[18] The FIM score is an 18‐item measure of functional status, with each item scored on a scale from 1 to 7 (dependent to independent). Various aspects of motor function and cognitive function are assessed. The FIM has been validated and shown to be reliable and reproducible.[13, 19, 20] By definition for the FIM instrument, admission FIM scores are assessed by trained multidisciplinary personnel first over the 72 hours of the rehabilitation stay, and for this study served as a proxy for patient functional status upon discharge from the acute care setting in our analysis. This 72‐hour time window allows for full assessment by therapists and nurses; however, in clinical practice at the inpatient rehabilitation unit involved in this study, much of the FIM assessment occurs within the first 24 hours of the rehabilitation stay. For our analysis, we divided FIM scores into low, medium, and high functional groups. The thresholds for these groups were based on total FIM score tertiles from a prior study<60, 60 to 76, and >76.[16] As a secondary analysis we created 6 subscales of the overall FIM score based on previous research. These subscales included: transfers (transfer to chair/wheelchair, toilet, and tub/shower), locomotion (walking and stairs), self‐care (eating, grooming, bathing, dressing, and toileting), sphincter control (bladder and bowel management), communication (comprehension and expression), and social cognition (social interaction, problem solving, and memory).[21]
Statistical Analysis
To evaluate differences in patient characteristics by diagnostic category, analysis of variance and 2 tests were used for continuous and dichotomous variables, respectively. Logistic regression was used to evaluate the association between FIM score category and readmission status, adjusting for potentially confounding variables available from the UDSMR and UHC databases. We used interaction terms to test whether the association between the FIM score and readmissions varied significantly across diagnostic categories and by age. As a secondary analysis, we modeled FIM score as a continuous variable. We expressed the odds ratio in this analysis per 10‐point change in FIM, because this represents a clinically relevant change in function.[22] Logistic regression was also used to evaluate the association between FIM subscale scores (transfers, locomotion, self‐care, sphincter control, communication, and social cognition) and readmission status. Statistical significance was defined as a 2‐sided P<0.05. Data were analyzed with R (version 2.15.0;
RESULTS
Readmitted Patients and Diagnostic Categories
A total of 9405 consecutive eligible patients were admitted to the acute inpatient rehabilitation facility between July 1, 2006 and December 31, 2012. A total of 1182 (13%) patients were readmitted back to an acute care hospital from inpatient rehabilitation. Median (interquartile range) time to readmission from acute care hospital discharge was 6 days (310 days), and median length of stay for patients who were discharged to the community from inpatient rehabilitation was 8 days (612 days).
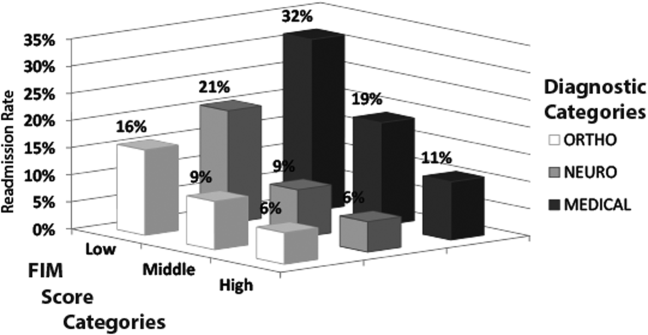
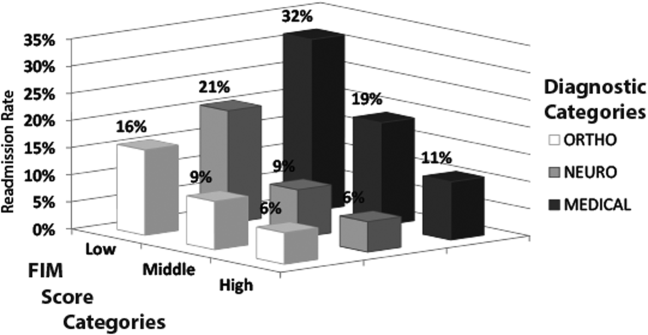
Table 1 shows characteristics of all inpatient rehabilitation patients by diagnostic category. For the neurologic category, the most common primary diagnoses were stroke and spinal cord injury; for the medical category, infection, renal failure, congestive heart failure, and chronic obstructive pulmonary disease; and for the orthopedic category, spinal arthrodesis, knee and hip replacements. Mean FIM scores were lowest and highest for patients admitted with a primarily neurologic and orthopedic diagnosis, respectively.
| Characteristic | All Patients, N=9405 | Diagnostic Category | |||
|---|---|---|---|---|---|
| Neurologic, n=3706 | Medical, n=2135 | Orthopedic, n=3564 | P Valueb | ||
| |||||
| Age, y | 67.8 (14.2) | 66.7 (15.3) | 67.0 (14.9) | 69.3 (12.4) | <0.001 |
| Male | 4,068 (43%) | 1,816 (49%) | 1,119 (52%) | 1,133 (32%) | <0.001 |
| Race | <0.001 | ||||
| Caucasian | 6,106 (65%) | 2344 (63%) | 1,320 (62%) | 2,442 (69%) | |
| African American | 2,501 (27%) | 984 (27%) | 658 (31%) | 859 (24%) | |
| Other | 798 (8%) | 378 (10%) | 157 (7%) | 263 (7%) | |
| Married | 4,330 (46%) | 1,683 (45%) | 931 (44%) | 1,716 (48%) | 0.002 |
| APRDRG‐SOI expected readmission rate | 18.0 (7.4) | 20.5 (6.8) | 21.3 (7.5) | 13.5 (5.6) | <0.001 |
| Total admission FIM score | 68.7 (17.2) | 60.4 (18.6) | 69.1 (15.5) | 77.2 (11.7) | <0.001 |
FIM Score Category and Risk of Readmission
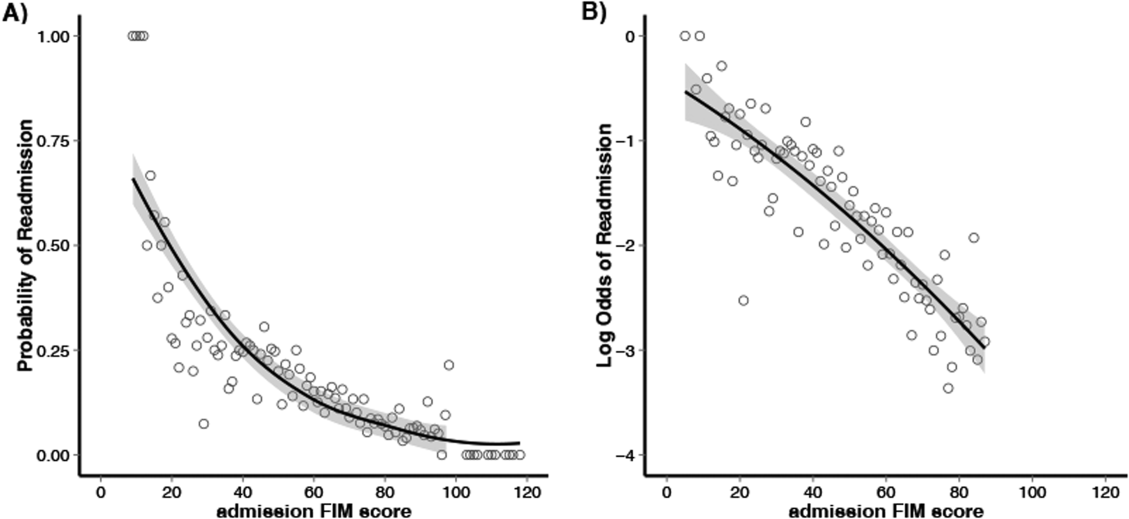
Figure 1 shows that patients in the low admission FIM score category had the highest unadjusted rate of readmission for each diagnostic category. In unadjusted analysis, Table 2 shows that younger age, male sex, APDRG‐SOI expected readmission rate, and orthopedic and medical diagnostic categories were associated with readmission. As a continuous variable, FIM scores were linearly associated with readmission (Figure 2), with an unadjusted odds ratio (OR) and 95% confidence interval (CI) of 1.4 (1.4‐1.4, P<0.001) for a 10‐point decrease in FIM. Compared to patients with high admission FIM scores, patients with low and middle FIM scores had higher unadjusted odds of readmission (OR: 4.0; 95% CI: 3.4‐4.7; P<0.001 and OR: 1.8; 95% CI: 1.5‐2.1; P<0.001, respectively). Mean FIM subscale scores for patients readmitted versus not readmitted were transfers (5.3 vs 7.0, P<0.001), locomotion (1.6 vs 2.3, P<0.001), self‐care (17.0 vs 20.8, P<0.001), communication (10.6 vs 11.5, P<0.001), and social cognition (15.1 vs 16.6, P<0.001).
| Bivariable Analysisb | Multivariable Analysisb | |||||
|---|---|---|---|---|---|---|
| Characteristic | All Patients, N=9405 | Readmitted, n=1,182 | OR (95% CI) | P Value | OR (95% CI) | P Value |
| ||||||
| Age, y | 68.0 (14.2) | 66.4 (14.5) | 0.9 (0.91.0) | <0.001 | 0.9 (0.91.0) | <0.001 |
| Male | 3,431 (42%) | 637 (54%) | 1.6 (1.41.8) | <0.001 | 1.3 (1.11.5) | < 0.001 |
| Race | ||||||
| Caucasian | 5,340 (65%) | 766 (65%) | 1.0 | 1.0 | ||
| African American | 2,177 (26%) | 324 (27%) | 1.0 (0.91.2) | 0.60 | 1.0 (0.81.1) | 0.75 |
| Other | 706 (9%) | 92 (8%) | 0.9 (0.71.1) | 0.41 | 0.8 (0.61.0) | 0.12 |
| Married | 3,775 (46%) | 555 (47%) | 1.0 (0.91.2) | 0.50 | 1.0 (0.91.2) | 0.67 |
| Admission diagnosis category | ||||||
| Neurologic | 3,205 (39%) | 501 (42%) | 1.0 | 1.0 | ||
| Medical | 1,726 (21%) | 409 (35%) | 1.5 (1.31.7) | <0.001 | 1.8 (1.62.1) | < 0.001 |
| Orthopedic | 3,292 (40%) | 272 (23%) | 0.5 (0.50.6) | <0.001 | 1.3 (1.11.6) | 0.005 |
| APDRG‐SOI expected readmission rate | 17.4 (7.1%) | 22.2 (8.0%) | 1.1 (1.11.1) | <0.001 | 1.1 (1.01.1) | < 0.001 |
| Total FIM score category | ||||||
| High FIM, >76 points | 3,517 (43%) | 257 (22%) | 1.0 | 1.0 | ||
| Middle FIM, 60points | 2,742 (33%) | 353 (30%) | 1.8 (1.52.1) | <0.001 | 1.5 (1.31.8) | < 0.001 |
| Low FIM, <60 points | 1,964 (24%) | 572 (48%) | 4.0 (3.44.7) | <0.001 | 3.0 (2.53.6) | < 0.001 |
Multivariable and Subset Analyses
Patients with a primary medical diagnosis had higher odds of readmission to the hospital, (OR: 1.8; 95% CI: 1.6‐2.1, P<0.001), relative to patients with a neurologic or orthopedic diagnosis (Table 2). Across all diagnoses, the adjusted odds ratios (95% CIs) for the low and middle versus high FIM score category were 3.0 (2.5‐3.6; P<0.001) and 1.5 (1.3‐1.8; P<0.001) respectively (Table 2). When modeled as a continuous variable, a 10‐point decrease in FIM score was associated with a significantly increased adjusted readmission rate (OR: 1.4; 95% CI: 1.3‐1.4; P<0.001). In adjusted analysis including all subscales of the FIM, only the physical subscales, transfers (P<0.001), locomotion (P=0.002), and self‐care (P<0.001), were significantly associated with readmission. For each diagnostic category, there were similar significant associations between admission FIM score group and readmission status (Table 3). The odds of readmission by FIM score did not differ significantly across the 3 major diagnostic categories (P=0.20 for interaction term), suggesting that the effect of functional status was similar across various types of patients. We also did not observe a statistical interaction between age and FIM score group in predicting readmission (P=0.58). Patients in the lowest FIM group with a medical diagnosis had the highest adjusted readmission rate of 28.7% (Table 3).
| Multivariable Analysisa | Adjusted Readmission Ratesb | |||
|---|---|---|---|---|
| No. | OR (95% CI) | P Value | % (95% CI) | |
| ||||
| Neurologic | ||||
| High FIM (>76 points) | 755 | 1.0 | 7.3 (4.710.0) | |
| Middle FIM (6076 points) | 1,283 | 1.4 (1.02.1) | 0.06 | 9.1 (7.011.1) |
| Low FIM (<60 points) | 1,668 | 3.3 (2.34.7) | <0.001 | 18.7 (16.820.6) |
| Medical | ||||
| High FIM (>76 points) | 807 | 1.0 | 11.2 (8.114.3) | |
| Middle FIM (6076 points) | 766 | 1.8 (1.32.4) | <0.001 | 17.7 (14.520.9) |
| Low FIM (<60 points) | 562 | 3.2 (2.44.3) | <0.001 | 28.7 (25.132.4) |
| Orthopedic | ||||
| High FIM (>76 points) | 2,212 | 1.0 | 6.1 (4.77.6) | |
| Middle FIM (6076 points) | 1,046 | 1.4 (1.11.9) | 0.02 | 8.3 (6.410.1) |
| Low FIM (<60 points) | 306 | 2.2 (1.53.3) | <0.001 | 13.5 (10.416.7) |
DISCUSSION
In this study of 9405 consecutive patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we investigated the association between functional status and readmission to an acute care hospital. We found that low functional status near the time of acute care hospital discharge was strongly associated with higher readmission rates. This relationship was consistently observed across major patient diagnostic categories, with low functioning medical patients having the highest rate of readmission (28.7%). Efforts to maintain or improve functional status during acute care hospitalization may be an important modifiable risk factor for acute care hospital readmission.
Previous studies have suggested that functional status may serve as an indicator of physiological reserve, and therefore vulnerability to medical complications and readmission.[6, 16, 23, 24, 25] Physiologic reserve refers to a person's ability to endure acute illness and is influenced by a number of factors, such as the adequacy of oxygen delivery to tissues, cardiovascular health, immune state, and nutritional status.[26] We found that motor subscales of the FIM score (transfers, locomotion, and self‐care), but not the other subscales, were independently associated with readmissions, which may suggest that lower motor scores are a stronger marker of physiologic reserve.[10, 16, 27] Although not our primary focus, we did note in our multivariable models that after adjusting for functional status, patients in a medical diagnostic category had higher readmission rates compared to patients with a primary neurologic or orthopedic diagnosis, but the impact of FIM score was consistent across all these diagnostic categories. We speculate that medical conditions that result in hospitalization, such as sepsis or acute kidney failure, may be more likely to result in multiorgan dysfunction that may impair physiological reserve and increase susceptibility to medical complications.[28, 29, 30, 31] In comparison, acute neurologic and orthopedic diagnoses, such as stroke or hip arthroplasty, directly impair gross motor function,[32, 33, 34, 35] with relative sparing of overall physiologic reserve.
The association between low functional status and readmissions is supported by previous studies across multiple hospital settings.[4, 5, 7, 8, 9, 27, 36] Despite this finding, routine inpatient medical practice may not fully address functional impairments. For instance, systematic measurement and documentation of functional status on admission and during hospitalization are not routine and may be a barrier to identifying medical patients at high risk for readmission.[37, 38, 39] Moreover, without recognition of functional impairment and its implications, current clinical practice may suboptimally prevent and treat physical impairments during inpatient care. However, such barriers can be surmounted. For example, in the medical intensive care unit setting, there is growing recognition that proactive and aggressive management of hospital‐acquired functional impairments through early rehabilitation is safe and feasible, improving patient outcomes while reducing hospital costs and readmissions.[3, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51] Moreover, 2 recent meta‐analyses have shown that physical therapy hospital‐based exercise programs can improve length of stay, overall hospital costs, and rates of discharge to home.[52, 53] Finally, a randomized trial has demonstrated that an individualized exercise regimen started in the acute hospital setting with long‐term telephone follow‐up can significantly reduce emergency hospital readmissions and improve quality of life in older adults.[54] Therefore, decreased functional status likely represents a modifiable risk factor for hospital readmission, and further research is necessary to more systematically identify low‐functioning patients and implement early mobility and activity programs to reduce hospital‐acquired functional impairment.[2, 49, 55]
Our analysis has potential limitations. First, this was an observational study and we are unable to demonstrate a direct cause‐and‐effect relationship between functional status and readmission. However, our results are consistent with prior literature in this field. Second, our cohort only included patients who were discharged from an acute hospital to a rehabilitation facility, which may limit its generalizability. However, we included a large patient sample size with a broad range of admission FIM scores, and our findings are consistent with other studies conducted in different clinical settings. Third, although 1 of our goals was to evaluate how readmission rates differed by diagnostic category, it is possible that individual diagnoses within each category may have different risks for readmission, and future larger studies could evaluate more detailed diagnostic grouping approaches. Fourth, we also recognize that although FIM score assessment has been validated, admission assessment occurs over a 72‐hour time period, during which patients' function could potentially change a clinically meaningful degree. Fifth, there may be residual confounding because of limitations in available data within our administrative dataset; however, we did account for severity of illness using a standardized measure, and prior research has demonstrated that the relationship between functional status and readmissions may be minimally confounded by demographic and clinical variables.[8, 16, 27, 56] Finally, we lacked readmission data following discharge from rehabilitation; it is possible that the association between FIM score at the time of rehabilitation initiation may have had limited predictive value among patients who successfully completed rehabilitation and were sent home.
CONCLUSION
In conclusion, in this study of patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we observed a strong association between decreased functional status and increased hospital readmission. In particular, medical patients with lower physical functioning exhibited an especially high rate of readmission. Incorporating functional status assessment into routine medical care may help identify patients at higher risk of readmission. Moreover, preventing and treating impaired functional status during inpatient admission, through early activity and mobility, should be evaluated as a way of improving patient outcomes and reducing hospital readmissions.
Disclosures: Erik Hoyer, MD, is supported by the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097). The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377.
- , . Association of physical functioning with same‐hospital readmission after stroke. Am J Phys Med Rehabil. 2004;83(6):434–438.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , , . Risk factors for nonelective hospital readmissions. J Gen Intern Med. 1996;11(12):762–764.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Hospital readmission in persons with stroke following postacute inpatient rehabilitation. J Gerontol A Biol Sci Med Sci. 2012;67(8):875–881.
- , , , , , . Hospital readmission of persons with hip fracture following medical rehabilitation. Arch Gerontol Geriatr. 2003;36(1):15–22.
- , , , , , . Characteristics of persons rehospitalized after stroke rehabilitation. Arch Phys Med Rehabil. 2001;82(10):1367–1374.
- , , , et al. A classification system for inpatient rehabilitation patients: a review and proposed revisions to the functional independence measure‐function related groups. PB98–105992, September. Washington, DC: US Department of Commerce, National Technical Information Services; 1997.
- , , , , , . A case‐mix classification system for medical rehabilitation. Med Care. 1994;32(4):366–379.
- , , , . The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77(12):1226–1232.
- , , , , , . Four methods for characterizing disability in the formation of function related groups. Arch Phys Med Rehabil. 1994;75(12):1277–1283.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA Intern Med. 2013;173(8):624–629.
- , , , , , . Functional status impairment is associated with unplanned readmissions. Arch Phys Med Rehabil. 2013;94(10):1951–1958.
- , , , et al. All patient refined diagnosis related groups (APR‐DRGs). Version 15.0. Report No.: 98‐054 Rev. 00. Wallingford, CT: 3M Health Information Systems; 1998.
- The inpatient rehabilitation facility–patient assessment instrument (IRF‐PAI) training manual. 2012. http://www.cms.gov/.
- , , , et al. Relationships between disability measures and nursing effort during medical rehabilitation for patients with traumatic brain and spinal cord injury. Arch Phys Med Rehabil. 1997;78(2):143–149.
- , , , . Interrater reliability of the 7‐level functional independence measure (FIM). Scand J Rehabil Med. 1994;26(3):115–119.
- , , , , , . Comparison of logistic regression and neural networks to predict rehospitalization in patients with stroke. J Clin Epidemiol. 2001;54(11):1159–1165.
- , , . Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: the impact of using different methods for measuring responsiveness. J Clin Epidemiol. 2002;55(9):922–928.
- , . Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. J Am Coll Cardiol. 1999;33(6):1560–1566.
- , , . Are all readmissions bad readmissions? N Engl J Med. 2010;363(3):297–298.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- . Susceptibility to critical illness: reserve, response and therapy. Intensive Care Med. 2000;26(suppl 1):S57–S63.
- , , , , . Predictors of discharge to acute care after inpatient rehabilitation in severely affected stroke patients. Am J Phys Med Rehabil. 2012;91(5):387–392.
- , , , et al. Clinical characteristics and outcomes of sepsis‐related vs non‐sepsis‐related ARDS. Chest. 2010;138(3):559–567.
- , . Long‐term outcomes from sepsis. Curr Infect Dis Rep. 2007;9(5):382–386.
- , . Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009;302(7):792–794.
- , , , , . Patients' self‐assessed functional status in heart failure by new york heart association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16(2):150–156.
- , , , et al. Knee arthroplasty: disabilities in comparison to the general population and to hip arthroplasty using a French national longitudinal survey. PLoS One. 2008;3(7):e2561.
- , , , et al. Gait asymmetry in community‐ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89(2):304–310.
- , , , . Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398.
- , , , , , . The effect of admission physiological variables on 30 day outcome after stroke. J Clin Neurosci. 2005;12(8):905–910.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- . Can hospitalization‐associated disability be prevented? JAMA. 2011;306(16):1800–1801.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , , , , , . Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690.
- , , . Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 suppl):S436–S441.
- , , , et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542.
- , , , et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717–724.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145.
- , . Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–281.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59(2):359–365.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304.
- , , , et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2):254–262.
- , , . Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;(1):CD005955.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabil. 2011;92(9):1490–1500.
- , , , , , . Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24‐week exercise and telephone follow‐up program. J Am Geriatr Soc. 2009;57(3):395–402.
- , , , , , . Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013:1–7.
- , , , , . Risks of acute hospital transfer and mortality during stroke rehabilitation. Arch Phys Med Rehabil. 2003;84(5):712–718.
Federally mandated pay‐for‐performance initiatives promote minimizing 30‐day hospital readmissions to improve healthcare quality and reduce costs. Although the reasons for readmissions are multifactorial, many patients are readmitted for a condition other than their initial hospital admitting diagnosis.[1] Impairments in functional status experienced during acute care hospitalization contribute to patients being discharged in a debilitated state and being vulnerable to postdischarge complications and potentially hospital readmission.[2] As such, decreased functional status may be an important and potentially modifiable risk factor for acute care hospital readmission.[3]
Previous studies have suggested that impaired functional status may be an important predictor of rehospitalization.[4, 5, 6, 7] However, inferences from existing studies are limited because they did not consider functional status as their primary focus, they only considered specific patient populations (eg, stroke) or readmissions occurring well beyond the 30‐day period defined by federal pay‐for‐performance standards.[4, 5, 6, 8, 9, 10] Our objective was to evaluate the association between functional status near the time of discharge from acute care hospital and 30‐day readmission for patients admitted to an acute inpatient rehabilitation facility. As a secondary objective, we sought to investigate the relationship between functional status and readmission by diagnostic category (medical, neurologic, or orthopedic).
METHODS
Study Population and Setting
We conducted a single‐center, retrospective study of patients admitted to an inpatient rehabilitation facility at a community hospital between July 1, 2006 and December 31, 2012. This facility provides intensive rehabilitation consisting of 3 hours of therapy per day, skilled nursing care on a 24‐hour basis, and medical care by a physiatrist. We excluded patients who died during inpatient rehabilitation (n=15, 0.2%) and patients not admitted directly from an acute care setting (n=178, 2.0%).
Data Source and Covariates
Data were derived from the Uniform Data System for Medical Rehabilitation (UDSMR), which is an administrative database providing the following data upon admission to an inpatient rehabilitation facility[11, 12, 13]: age, gender, race/ethnicity, marital status, the discharge setting, the admission Functional Independence Measure (FIM) score (details further below), and admission diagnostic category as defined by the primary discharge diagnosis from the acute care hospital and grouped by functional related groups (a case‐mix system for medical rehabilitation).[12, 14] The 3M ClinTrac management software (3M, St. Paul, MN), used for mandatory reporting to the State of Maryland, provided all‐payerrefined diagnosis related group (APRDRG) and severity of illness (SOI) combinations (a tool to group patients into clinically comparable disease and severity‐of‐illness categories expected to use similar resources and experience similar outcomes). The University HealthSystem Consortium (UHC) database provided national readmission rates for all APRDRG‐SOI combinations using a methodology that has been previously described.[15, 16] Expected readmission rates for APRDRG‐SOI combinations served as a patient risk stratification tool based on clinical logic that evaluates age, comorbidities, principal diagnosis during hospitalization, and procedures conducted during hospitalization.[17]
Primary Outcome: Acute Care Readmission
The primary outcome was all‐cause acute care readmission, defined as patient transfer to an acute care hospital during inpatient rehabilitation within 30 days from admission to inpatient rehabilitation. The care model for our inpatient rehabilitation unit is such that when patients become sick or develop a complication, they are admitted directly to a clinical unit (eg, intensive care unit) at the community hospital through a rapid‐response intervention, or the physiatrist arranges with an admitting inpatient attending to accept the patient directly to his or her service.
Primary Exposure: Functional Independence Measure
Functional status was measured using the FIM score.[18] The FIM score is an 18‐item measure of functional status, with each item scored on a scale from 1 to 7 (dependent to independent). Various aspects of motor function and cognitive function are assessed. The FIM has been validated and shown to be reliable and reproducible.[13, 19, 20] By definition for the FIM instrument, admission FIM scores are assessed by trained multidisciplinary personnel first over the 72 hours of the rehabilitation stay, and for this study served as a proxy for patient functional status upon discharge from the acute care setting in our analysis. This 72‐hour time window allows for full assessment by therapists and nurses; however, in clinical practice at the inpatient rehabilitation unit involved in this study, much of the FIM assessment occurs within the first 24 hours of the rehabilitation stay. For our analysis, we divided FIM scores into low, medium, and high functional groups. The thresholds for these groups were based on total FIM score tertiles from a prior study<60, 60 to 76, and >76.[16] As a secondary analysis we created 6 subscales of the overall FIM score based on previous research. These subscales included: transfers (transfer to chair/wheelchair, toilet, and tub/shower), locomotion (walking and stairs), self‐care (eating, grooming, bathing, dressing, and toileting), sphincter control (bladder and bowel management), communication (comprehension and expression), and social cognition (social interaction, problem solving, and memory).[21]
Statistical Analysis
To evaluate differences in patient characteristics by diagnostic category, analysis of variance and 2 tests were used for continuous and dichotomous variables, respectively. Logistic regression was used to evaluate the association between FIM score category and readmission status, adjusting for potentially confounding variables available from the UDSMR and UHC databases. We used interaction terms to test whether the association between the FIM score and readmissions varied significantly across diagnostic categories and by age. As a secondary analysis, we modeled FIM score as a continuous variable. We expressed the odds ratio in this analysis per 10‐point change in FIM, because this represents a clinically relevant change in function.[22] Logistic regression was also used to evaluate the association between FIM subscale scores (transfers, locomotion, self‐care, sphincter control, communication, and social cognition) and readmission status. Statistical significance was defined as a 2‐sided P<0.05. Data were analyzed with R (version 2.15.0;
RESULTS
Readmitted Patients and Diagnostic Categories
A total of 9405 consecutive eligible patients were admitted to the acute inpatient rehabilitation facility between July 1, 2006 and December 31, 2012. A total of 1182 (13%) patients were readmitted back to an acute care hospital from inpatient rehabilitation. Median (interquartile range) time to readmission from acute care hospital discharge was 6 days (310 days), and median length of stay for patients who were discharged to the community from inpatient rehabilitation was 8 days (612 days).
Table 1 shows characteristics of all inpatient rehabilitation patients by diagnostic category. For the neurologic category, the most common primary diagnoses were stroke and spinal cord injury; for the medical category, infection, renal failure, congestive heart failure, and chronic obstructive pulmonary disease; and for the orthopedic category, spinal arthrodesis, knee and hip replacements. Mean FIM scores were lowest and highest for patients admitted with a primarily neurologic and orthopedic diagnosis, respectively.
| Characteristic | All Patients, N=9405 | Diagnostic Category | |||
|---|---|---|---|---|---|
| Neurologic, n=3706 | Medical, n=2135 | Orthopedic, n=3564 | P Valueb | ||
| |||||
| Age, y | 67.8 (14.2) | 66.7 (15.3) | 67.0 (14.9) | 69.3 (12.4) | <0.001 |
| Male | 4,068 (43%) | 1,816 (49%) | 1,119 (52%) | 1,133 (32%) | <0.001 |
| Race | <0.001 | ||||
| Caucasian | 6,106 (65%) | 2344 (63%) | 1,320 (62%) | 2,442 (69%) | |
| African American | 2,501 (27%) | 984 (27%) | 658 (31%) | 859 (24%) | |
| Other | 798 (8%) | 378 (10%) | 157 (7%) | 263 (7%) | |
| Married | 4,330 (46%) | 1,683 (45%) | 931 (44%) | 1,716 (48%) | 0.002 |
| APRDRG‐SOI expected readmission rate | 18.0 (7.4) | 20.5 (6.8) | 21.3 (7.5) | 13.5 (5.6) | <0.001 |
| Total admission FIM score | 68.7 (17.2) | 60.4 (18.6) | 69.1 (15.5) | 77.2 (11.7) | <0.001 |
FIM Score Category and Risk of Readmission
Figure 1 shows that patients in the low admission FIM score category had the highest unadjusted rate of readmission for each diagnostic category. In unadjusted analysis, Table 2 shows that younger age, male sex, APDRG‐SOI expected readmission rate, and orthopedic and medical diagnostic categories were associated with readmission. As a continuous variable, FIM scores were linearly associated with readmission (Figure 2), with an unadjusted odds ratio (OR) and 95% confidence interval (CI) of 1.4 (1.4‐1.4, P<0.001) for a 10‐point decrease in FIM. Compared to patients with high admission FIM scores, patients with low and middle FIM scores had higher unadjusted odds of readmission (OR: 4.0; 95% CI: 3.4‐4.7; P<0.001 and OR: 1.8; 95% CI: 1.5‐2.1; P<0.001, respectively). Mean FIM subscale scores for patients readmitted versus not readmitted were transfers (5.3 vs 7.0, P<0.001), locomotion (1.6 vs 2.3, P<0.001), self‐care (17.0 vs 20.8, P<0.001), communication (10.6 vs 11.5, P<0.001), and social cognition (15.1 vs 16.6, P<0.001).

| Bivariable Analysisb | Multivariable Analysisb | |||||
|---|---|---|---|---|---|---|
| Characteristic | All Patients, N=9405 | Readmitted, n=1,182 | OR (95% CI) | P Value | OR (95% CI) | P Value |
| ||||||
| Age, y | 68.0 (14.2) | 66.4 (14.5) | 0.9 (0.91.0) | <0.001 | 0.9 (0.91.0) | <0.001 |
| Male | 3,431 (42%) | 637 (54%) | 1.6 (1.41.8) | <0.001 | 1.3 (1.11.5) | < 0.001 |
| Race | ||||||
| Caucasian | 5,340 (65%) | 766 (65%) | 1.0 | 1.0 | ||
| African American | 2,177 (26%) | 324 (27%) | 1.0 (0.91.2) | 0.60 | 1.0 (0.81.1) | 0.75 |
| Other | 706 (9%) | 92 (8%) | 0.9 (0.71.1) | 0.41 | 0.8 (0.61.0) | 0.12 |
| Married | 3,775 (46%) | 555 (47%) | 1.0 (0.91.2) | 0.50 | 1.0 (0.91.2) | 0.67 |
| Admission diagnosis category | ||||||
| Neurologic | 3,205 (39%) | 501 (42%) | 1.0 | 1.0 | ||
| Medical | 1,726 (21%) | 409 (35%) | 1.5 (1.31.7) | <0.001 | 1.8 (1.62.1) | < 0.001 |
| Orthopedic | 3,292 (40%) | 272 (23%) | 0.5 (0.50.6) | <0.001 | 1.3 (1.11.6) | 0.005 |
| APDRG‐SOI expected readmission rate | 17.4 (7.1%) | 22.2 (8.0%) | 1.1 (1.11.1) | <0.001 | 1.1 (1.01.1) | < 0.001 |
| Total FIM score category | ||||||
| High FIM, >76 points | 3,517 (43%) | 257 (22%) | 1.0 | 1.0 | ||
| Middle FIM, 60points | 2,742 (33%) | 353 (30%) | 1.8 (1.52.1) | <0.001 | 1.5 (1.31.8) | < 0.001 |
| Low FIM, <60 points | 1,964 (24%) | 572 (48%) | 4.0 (3.44.7) | <0.001 | 3.0 (2.53.6) | < 0.001 |

Multivariable and Subset Analyses
Patients with a primary medical diagnosis had higher odds of readmission to the hospital, (OR: 1.8; 95% CI: 1.6‐2.1, P<0.001), relative to patients with a neurologic or orthopedic diagnosis (Table 2). Across all diagnoses, the adjusted odds ratios (95% CIs) for the low and middle versus high FIM score category were 3.0 (2.5‐3.6; P<0.001) and 1.5 (1.3‐1.8; P<0.001) respectively (Table 2). When modeled as a continuous variable, a 10‐point decrease in FIM score was associated with a significantly increased adjusted readmission rate (OR: 1.4; 95% CI: 1.3‐1.4; P<0.001). In adjusted analysis including all subscales of the FIM, only the physical subscales, transfers (P<0.001), locomotion (P=0.002), and self‐care (P<0.001), were significantly associated with readmission. For each diagnostic category, there were similar significant associations between admission FIM score group and readmission status (Table 3). The odds of readmission by FIM score did not differ significantly across the 3 major diagnostic categories (P=0.20 for interaction term), suggesting that the effect of functional status was similar across various types of patients. We also did not observe a statistical interaction between age and FIM score group in predicting readmission (P=0.58). Patients in the lowest FIM group with a medical diagnosis had the highest adjusted readmission rate of 28.7% (Table 3).
| Multivariable Analysisa | Adjusted Readmission Ratesb | |||
|---|---|---|---|---|
| No. | OR (95% CI) | P Value | % (95% CI) | |
| ||||
| Neurologic | ||||
| High FIM (>76 points) | 755 | 1.0 | 7.3 (4.710.0) | |
| Middle FIM (6076 points) | 1,283 | 1.4 (1.02.1) | 0.06 | 9.1 (7.011.1) |
| Low FIM (<60 points) | 1,668 | 3.3 (2.34.7) | <0.001 | 18.7 (16.820.6) |
| Medical | ||||
| High FIM (>76 points) | 807 | 1.0 | 11.2 (8.114.3) | |
| Middle FIM (6076 points) | 766 | 1.8 (1.32.4) | <0.001 | 17.7 (14.520.9) |
| Low FIM (<60 points) | 562 | 3.2 (2.44.3) | <0.001 | 28.7 (25.132.4) |
| Orthopedic | ||||
| High FIM (>76 points) | 2,212 | 1.0 | 6.1 (4.77.6) | |
| Middle FIM (6076 points) | 1,046 | 1.4 (1.11.9) | 0.02 | 8.3 (6.410.1) |
| Low FIM (<60 points) | 306 | 2.2 (1.53.3) | <0.001 | 13.5 (10.416.7) |
DISCUSSION
In this study of 9405 consecutive patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we investigated the association between functional status and readmission to an acute care hospital. We found that low functional status near the time of acute care hospital discharge was strongly associated with higher readmission rates. This relationship was consistently observed across major patient diagnostic categories, with low functioning medical patients having the highest rate of readmission (28.7%). Efforts to maintain or improve functional status during acute care hospitalization may be an important modifiable risk factor for acute care hospital readmission.
Previous studies have suggested that functional status may serve as an indicator of physiological reserve, and therefore vulnerability to medical complications and readmission.[6, 16, 23, 24, 25] Physiologic reserve refers to a person's ability to endure acute illness and is influenced by a number of factors, such as the adequacy of oxygen delivery to tissues, cardiovascular health, immune state, and nutritional status.[26] We found that motor subscales of the FIM score (transfers, locomotion, and self‐care), but not the other subscales, were independently associated with readmissions, which may suggest that lower motor scores are a stronger marker of physiologic reserve.[10, 16, 27] Although not our primary focus, we did note in our multivariable models that after adjusting for functional status, patients in a medical diagnostic category had higher readmission rates compared to patients with a primary neurologic or orthopedic diagnosis, but the impact of FIM score was consistent across all these diagnostic categories. We speculate that medical conditions that result in hospitalization, such as sepsis or acute kidney failure, may be more likely to result in multiorgan dysfunction that may impair physiological reserve and increase susceptibility to medical complications.[28, 29, 30, 31] In comparison, acute neurologic and orthopedic diagnoses, such as stroke or hip arthroplasty, directly impair gross motor function,[32, 33, 34, 35] with relative sparing of overall physiologic reserve.
The association between low functional status and readmissions is supported by previous studies across multiple hospital settings.[4, 5, 7, 8, 9, 27, 36] Despite this finding, routine inpatient medical practice may not fully address functional impairments. For instance, systematic measurement and documentation of functional status on admission and during hospitalization are not routine and may be a barrier to identifying medical patients at high risk for readmission.[37, 38, 39] Moreover, without recognition of functional impairment and its implications, current clinical practice may suboptimally prevent and treat physical impairments during inpatient care. However, such barriers can be surmounted. For example, in the medical intensive care unit setting, there is growing recognition that proactive and aggressive management of hospital‐acquired functional impairments through early rehabilitation is safe and feasible, improving patient outcomes while reducing hospital costs and readmissions.[3, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51] Moreover, 2 recent meta‐analyses have shown that physical therapy hospital‐based exercise programs can improve length of stay, overall hospital costs, and rates of discharge to home.[52, 53] Finally, a randomized trial has demonstrated that an individualized exercise regimen started in the acute hospital setting with long‐term telephone follow‐up can significantly reduce emergency hospital readmissions and improve quality of life in older adults.[54] Therefore, decreased functional status likely represents a modifiable risk factor for hospital readmission, and further research is necessary to more systematically identify low‐functioning patients and implement early mobility and activity programs to reduce hospital‐acquired functional impairment.[2, 49, 55]
Our analysis has potential limitations. First, this was an observational study and we are unable to demonstrate a direct cause‐and‐effect relationship between functional status and readmission. However, our results are consistent with prior literature in this field. Second, our cohort only included patients who were discharged from an acute hospital to a rehabilitation facility, which may limit its generalizability. However, we included a large patient sample size with a broad range of admission FIM scores, and our findings are consistent with other studies conducted in different clinical settings. Third, although 1 of our goals was to evaluate how readmission rates differed by diagnostic category, it is possible that individual diagnoses within each category may have different risks for readmission, and future larger studies could evaluate more detailed diagnostic grouping approaches. Fourth, we also recognize that although FIM score assessment has been validated, admission assessment occurs over a 72‐hour time period, during which patients' function could potentially change a clinically meaningful degree. Fifth, there may be residual confounding because of limitations in available data within our administrative dataset; however, we did account for severity of illness using a standardized measure, and prior research has demonstrated that the relationship between functional status and readmissions may be minimally confounded by demographic and clinical variables.[8, 16, 27, 56] Finally, we lacked readmission data following discharge from rehabilitation; it is possible that the association between FIM score at the time of rehabilitation initiation may have had limited predictive value among patients who successfully completed rehabilitation and were sent home.
CONCLUSION
In conclusion, in this study of patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we observed a strong association between decreased functional status and increased hospital readmission. In particular, medical patients with lower physical functioning exhibited an especially high rate of readmission. Incorporating functional status assessment into routine medical care may help identify patients at higher risk of readmission. Moreover, preventing and treating impaired functional status during inpatient admission, through early activity and mobility, should be evaluated as a way of improving patient outcomes and reducing hospital readmissions.
Disclosures: Erik Hoyer, MD, is supported by the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097). The authors report no conflicts of interest.
Federally mandated pay‐for‐performance initiatives promote minimizing 30‐day hospital readmissions to improve healthcare quality and reduce costs. Although the reasons for readmissions are multifactorial, many patients are readmitted for a condition other than their initial hospital admitting diagnosis.[1] Impairments in functional status experienced during acute care hospitalization contribute to patients being discharged in a debilitated state and being vulnerable to postdischarge complications and potentially hospital readmission.[2] As such, decreased functional status may be an important and potentially modifiable risk factor for acute care hospital readmission.[3]
Previous studies have suggested that impaired functional status may be an important predictor of rehospitalization.[4, 5, 6, 7] However, inferences from existing studies are limited because they did not consider functional status as their primary focus, they only considered specific patient populations (eg, stroke) or readmissions occurring well beyond the 30‐day period defined by federal pay‐for‐performance standards.[4, 5, 6, 8, 9, 10] Our objective was to evaluate the association between functional status near the time of discharge from acute care hospital and 30‐day readmission for patients admitted to an acute inpatient rehabilitation facility. As a secondary objective, we sought to investigate the relationship between functional status and readmission by diagnostic category (medical, neurologic, or orthopedic).
METHODS
Study Population and Setting
We conducted a single‐center, retrospective study of patients admitted to an inpatient rehabilitation facility at a community hospital between July 1, 2006 and December 31, 2012. This facility provides intensive rehabilitation consisting of 3 hours of therapy per day, skilled nursing care on a 24‐hour basis, and medical care by a physiatrist. We excluded patients who died during inpatient rehabilitation (n=15, 0.2%) and patients not admitted directly from an acute care setting (n=178, 2.0%).
Data Source and Covariates
Data were derived from the Uniform Data System for Medical Rehabilitation (UDSMR), which is an administrative database providing the following data upon admission to an inpatient rehabilitation facility[11, 12, 13]: age, gender, race/ethnicity, marital status, the discharge setting, the admission Functional Independence Measure (FIM) score (details further below), and admission diagnostic category as defined by the primary discharge diagnosis from the acute care hospital and grouped by functional related groups (a case‐mix system for medical rehabilitation).[12, 14] The 3M ClinTrac management software (3M, St. Paul, MN), used for mandatory reporting to the State of Maryland, provided all‐payerrefined diagnosis related group (APRDRG) and severity of illness (SOI) combinations (a tool to group patients into clinically comparable disease and severity‐of‐illness categories expected to use similar resources and experience similar outcomes). The University HealthSystem Consortium (UHC) database provided national readmission rates for all APRDRG‐SOI combinations using a methodology that has been previously described.[15, 16] Expected readmission rates for APRDRG‐SOI combinations served as a patient risk stratification tool based on clinical logic that evaluates age, comorbidities, principal diagnosis during hospitalization, and procedures conducted during hospitalization.[17]
Primary Outcome: Acute Care Readmission
The primary outcome was all‐cause acute care readmission, defined as patient transfer to an acute care hospital during inpatient rehabilitation within 30 days from admission to inpatient rehabilitation. The care model for our inpatient rehabilitation unit is such that when patients become sick or develop a complication, they are admitted directly to a clinical unit (eg, intensive care unit) at the community hospital through a rapid‐response intervention, or the physiatrist arranges with an admitting inpatient attending to accept the patient directly to his or her service.
Primary Exposure: Functional Independence Measure
Functional status was measured using the FIM score.[18] The FIM score is an 18‐item measure of functional status, with each item scored on a scale from 1 to 7 (dependent to independent). Various aspects of motor function and cognitive function are assessed. The FIM has been validated and shown to be reliable and reproducible.[13, 19, 20] By definition for the FIM instrument, admission FIM scores are assessed by trained multidisciplinary personnel first over the 72 hours of the rehabilitation stay, and for this study served as a proxy for patient functional status upon discharge from the acute care setting in our analysis. This 72‐hour time window allows for full assessment by therapists and nurses; however, in clinical practice at the inpatient rehabilitation unit involved in this study, much of the FIM assessment occurs within the first 24 hours of the rehabilitation stay. For our analysis, we divided FIM scores into low, medium, and high functional groups. The thresholds for these groups were based on total FIM score tertiles from a prior study<60, 60 to 76, and >76.[16] As a secondary analysis we created 6 subscales of the overall FIM score based on previous research. These subscales included: transfers (transfer to chair/wheelchair, toilet, and tub/shower), locomotion (walking and stairs), self‐care (eating, grooming, bathing, dressing, and toileting), sphincter control (bladder and bowel management), communication (comprehension and expression), and social cognition (social interaction, problem solving, and memory).[21]
Statistical Analysis
To evaluate differences in patient characteristics by diagnostic category, analysis of variance and 2 tests were used for continuous and dichotomous variables, respectively. Logistic regression was used to evaluate the association between FIM score category and readmission status, adjusting for potentially confounding variables available from the UDSMR and UHC databases. We used interaction terms to test whether the association between the FIM score and readmissions varied significantly across diagnostic categories and by age. As a secondary analysis, we modeled FIM score as a continuous variable. We expressed the odds ratio in this analysis per 10‐point change in FIM, because this represents a clinically relevant change in function.[22] Logistic regression was also used to evaluate the association between FIM subscale scores (transfers, locomotion, self‐care, sphincter control, communication, and social cognition) and readmission status. Statistical significance was defined as a 2‐sided P<0.05. Data were analyzed with R (version 2.15.0;
RESULTS
Readmitted Patients and Diagnostic Categories
A total of 9405 consecutive eligible patients were admitted to the acute inpatient rehabilitation facility between July 1, 2006 and December 31, 2012. A total of 1182 (13%) patients were readmitted back to an acute care hospital from inpatient rehabilitation. Median (interquartile range) time to readmission from acute care hospital discharge was 6 days (310 days), and median length of stay for patients who were discharged to the community from inpatient rehabilitation was 8 days (612 days).
Table 1 shows characteristics of all inpatient rehabilitation patients by diagnostic category. For the neurologic category, the most common primary diagnoses were stroke and spinal cord injury; for the medical category, infection, renal failure, congestive heart failure, and chronic obstructive pulmonary disease; and for the orthopedic category, spinal arthrodesis, knee and hip replacements. Mean FIM scores were lowest and highest for patients admitted with a primarily neurologic and orthopedic diagnosis, respectively.
| Characteristic | All Patients, N=9405 | Diagnostic Category | |||
|---|---|---|---|---|---|
| Neurologic, n=3706 | Medical, n=2135 | Orthopedic, n=3564 | P Valueb | ||
| |||||
| Age, y | 67.8 (14.2) | 66.7 (15.3) | 67.0 (14.9) | 69.3 (12.4) | <0.001 |
| Male | 4,068 (43%) | 1,816 (49%) | 1,119 (52%) | 1,133 (32%) | <0.001 |
| Race | <0.001 | ||||
| Caucasian | 6,106 (65%) | 2344 (63%) | 1,320 (62%) | 2,442 (69%) | |
| African American | 2,501 (27%) | 984 (27%) | 658 (31%) | 859 (24%) | |
| Other | 798 (8%) | 378 (10%) | 157 (7%) | 263 (7%) | |
| Married | 4,330 (46%) | 1,683 (45%) | 931 (44%) | 1,716 (48%) | 0.002 |
| APRDRG‐SOI expected readmission rate | 18.0 (7.4) | 20.5 (6.8) | 21.3 (7.5) | 13.5 (5.6) | <0.001 |
| Total admission FIM score | 68.7 (17.2) | 60.4 (18.6) | 69.1 (15.5) | 77.2 (11.7) | <0.001 |
FIM Score Category and Risk of Readmission
Figure 1 shows that patients in the low admission FIM score category had the highest unadjusted rate of readmission for each diagnostic category. In unadjusted analysis, Table 2 shows that younger age, male sex, APDRG‐SOI expected readmission rate, and orthopedic and medical diagnostic categories were associated with readmission. As a continuous variable, FIM scores were linearly associated with readmission (Figure 2), with an unadjusted odds ratio (OR) and 95% confidence interval (CI) of 1.4 (1.4‐1.4, P<0.001) for a 10‐point decrease in FIM. Compared to patients with high admission FIM scores, patients with low and middle FIM scores had higher unadjusted odds of readmission (OR: 4.0; 95% CI: 3.4‐4.7; P<0.001 and OR: 1.8; 95% CI: 1.5‐2.1; P<0.001, respectively). Mean FIM subscale scores for patients readmitted versus not readmitted were transfers (5.3 vs 7.0, P<0.001), locomotion (1.6 vs 2.3, P<0.001), self‐care (17.0 vs 20.8, P<0.001), communication (10.6 vs 11.5, P<0.001), and social cognition (15.1 vs 16.6, P<0.001).

| Bivariable Analysisb | Multivariable Analysisb | |||||
|---|---|---|---|---|---|---|
| Characteristic | All Patients, N=9405 | Readmitted, n=1,182 | OR (95% CI) | P Value | OR (95% CI) | P Value |
| ||||||
| Age, y | 68.0 (14.2) | 66.4 (14.5) | 0.9 (0.91.0) | <0.001 | 0.9 (0.91.0) | <0.001 |
| Male | 3,431 (42%) | 637 (54%) | 1.6 (1.41.8) | <0.001 | 1.3 (1.11.5) | < 0.001 |
| Race | ||||||
| Caucasian | 5,340 (65%) | 766 (65%) | 1.0 | 1.0 | ||
| African American | 2,177 (26%) | 324 (27%) | 1.0 (0.91.2) | 0.60 | 1.0 (0.81.1) | 0.75 |
| Other | 706 (9%) | 92 (8%) | 0.9 (0.71.1) | 0.41 | 0.8 (0.61.0) | 0.12 |
| Married | 3,775 (46%) | 555 (47%) | 1.0 (0.91.2) | 0.50 | 1.0 (0.91.2) | 0.67 |
| Admission diagnosis category | ||||||
| Neurologic | 3,205 (39%) | 501 (42%) | 1.0 | 1.0 | ||
| Medical | 1,726 (21%) | 409 (35%) | 1.5 (1.31.7) | <0.001 | 1.8 (1.62.1) | < 0.001 |
| Orthopedic | 3,292 (40%) | 272 (23%) | 0.5 (0.50.6) | <0.001 | 1.3 (1.11.6) | 0.005 |
| APDRG‐SOI expected readmission rate | 17.4 (7.1%) | 22.2 (8.0%) | 1.1 (1.11.1) | <0.001 | 1.1 (1.01.1) | < 0.001 |
| Total FIM score category | ||||||
| High FIM, >76 points | 3,517 (43%) | 257 (22%) | 1.0 | 1.0 | ||
| Middle FIM, 60points | 2,742 (33%) | 353 (30%) | 1.8 (1.52.1) | <0.001 | 1.5 (1.31.8) | < 0.001 |
| Low FIM, <60 points | 1,964 (24%) | 572 (48%) | 4.0 (3.44.7) | <0.001 | 3.0 (2.53.6) | < 0.001 |

Multivariable and Subset Analyses
Patients with a primary medical diagnosis had higher odds of readmission to the hospital, (OR: 1.8; 95% CI: 1.6‐2.1, P<0.001), relative to patients with a neurologic or orthopedic diagnosis (Table 2). Across all diagnoses, the adjusted odds ratios (95% CIs) for the low and middle versus high FIM score category were 3.0 (2.5‐3.6; P<0.001) and 1.5 (1.3‐1.8; P<0.001) respectively (Table 2). When modeled as a continuous variable, a 10‐point decrease in FIM score was associated with a significantly increased adjusted readmission rate (OR: 1.4; 95% CI: 1.3‐1.4; P<0.001). In adjusted analysis including all subscales of the FIM, only the physical subscales, transfers (P<0.001), locomotion (P=0.002), and self‐care (P<0.001), were significantly associated with readmission. For each diagnostic category, there were similar significant associations between admission FIM score group and readmission status (Table 3). The odds of readmission by FIM score did not differ significantly across the 3 major diagnostic categories (P=0.20 for interaction term), suggesting that the effect of functional status was similar across various types of patients. We also did not observe a statistical interaction between age and FIM score group in predicting readmission (P=0.58). Patients in the lowest FIM group with a medical diagnosis had the highest adjusted readmission rate of 28.7% (Table 3).
| Multivariable Analysisa | Adjusted Readmission Ratesb | |||
|---|---|---|---|---|
| No. | OR (95% CI) | P Value | % (95% CI) | |
| ||||
| Neurologic | ||||
| High FIM (>76 points) | 755 | 1.0 | 7.3 (4.710.0) | |
| Middle FIM (6076 points) | 1,283 | 1.4 (1.02.1) | 0.06 | 9.1 (7.011.1) |
| Low FIM (<60 points) | 1,668 | 3.3 (2.34.7) | <0.001 | 18.7 (16.820.6) |
| Medical | ||||
| High FIM (>76 points) | 807 | 1.0 | 11.2 (8.114.3) | |
| Middle FIM (6076 points) | 766 | 1.8 (1.32.4) | <0.001 | 17.7 (14.520.9) |
| Low FIM (<60 points) | 562 | 3.2 (2.44.3) | <0.001 | 28.7 (25.132.4) |
| Orthopedic | ||||
| High FIM (>76 points) | 2,212 | 1.0 | 6.1 (4.77.6) | |
| Middle FIM (6076 points) | 1,046 | 1.4 (1.11.9) | 0.02 | 8.3 (6.410.1) |
| Low FIM (<60 points) | 306 | 2.2 (1.53.3) | <0.001 | 13.5 (10.416.7) |
DISCUSSION
In this study of 9405 consecutive patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we investigated the association between functional status and readmission to an acute care hospital. We found that low functional status near the time of acute care hospital discharge was strongly associated with higher readmission rates. This relationship was consistently observed across major patient diagnostic categories, with low functioning medical patients having the highest rate of readmission (28.7%). Efforts to maintain or improve functional status during acute care hospitalization may be an important modifiable risk factor for acute care hospital readmission.
Previous studies have suggested that functional status may serve as an indicator of physiological reserve, and therefore vulnerability to medical complications and readmission.[6, 16, 23, 24, 25] Physiologic reserve refers to a person's ability to endure acute illness and is influenced by a number of factors, such as the adequacy of oxygen delivery to tissues, cardiovascular health, immune state, and nutritional status.[26] We found that motor subscales of the FIM score (transfers, locomotion, and self‐care), but not the other subscales, were independently associated with readmissions, which may suggest that lower motor scores are a stronger marker of physiologic reserve.[10, 16, 27] Although not our primary focus, we did note in our multivariable models that after adjusting for functional status, patients in a medical diagnostic category had higher readmission rates compared to patients with a primary neurologic or orthopedic diagnosis, but the impact of FIM score was consistent across all these diagnostic categories. We speculate that medical conditions that result in hospitalization, such as sepsis or acute kidney failure, may be more likely to result in multiorgan dysfunction that may impair physiological reserve and increase susceptibility to medical complications.[28, 29, 30, 31] In comparison, acute neurologic and orthopedic diagnoses, such as stroke or hip arthroplasty, directly impair gross motor function,[32, 33, 34, 35] with relative sparing of overall physiologic reserve.
The association between low functional status and readmissions is supported by previous studies across multiple hospital settings.[4, 5, 7, 8, 9, 27, 36] Despite this finding, routine inpatient medical practice may not fully address functional impairments. For instance, systematic measurement and documentation of functional status on admission and during hospitalization are not routine and may be a barrier to identifying medical patients at high risk for readmission.[37, 38, 39] Moreover, without recognition of functional impairment and its implications, current clinical practice may suboptimally prevent and treat physical impairments during inpatient care. However, such barriers can be surmounted. For example, in the medical intensive care unit setting, there is growing recognition that proactive and aggressive management of hospital‐acquired functional impairments through early rehabilitation is safe and feasible, improving patient outcomes while reducing hospital costs and readmissions.[3, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51] Moreover, 2 recent meta‐analyses have shown that physical therapy hospital‐based exercise programs can improve length of stay, overall hospital costs, and rates of discharge to home.[52, 53] Finally, a randomized trial has demonstrated that an individualized exercise regimen started in the acute hospital setting with long‐term telephone follow‐up can significantly reduce emergency hospital readmissions and improve quality of life in older adults.[54] Therefore, decreased functional status likely represents a modifiable risk factor for hospital readmission, and further research is necessary to more systematically identify low‐functioning patients and implement early mobility and activity programs to reduce hospital‐acquired functional impairment.[2, 49, 55]
Our analysis has potential limitations. First, this was an observational study and we are unable to demonstrate a direct cause‐and‐effect relationship between functional status and readmission. However, our results are consistent with prior literature in this field. Second, our cohort only included patients who were discharged from an acute hospital to a rehabilitation facility, which may limit its generalizability. However, we included a large patient sample size with a broad range of admission FIM scores, and our findings are consistent with other studies conducted in different clinical settings. Third, although 1 of our goals was to evaluate how readmission rates differed by diagnostic category, it is possible that individual diagnoses within each category may have different risks for readmission, and future larger studies could evaluate more detailed diagnostic grouping approaches. Fourth, we also recognize that although FIM score assessment has been validated, admission assessment occurs over a 72‐hour time period, during which patients' function could potentially change a clinically meaningful degree. Fifth, there may be residual confounding because of limitations in available data within our administrative dataset; however, we did account for severity of illness using a standardized measure, and prior research has demonstrated that the relationship between functional status and readmissions may be minimally confounded by demographic and clinical variables.[8, 16, 27, 56] Finally, we lacked readmission data following discharge from rehabilitation; it is possible that the association between FIM score at the time of rehabilitation initiation may have had limited predictive value among patients who successfully completed rehabilitation and were sent home.
CONCLUSION
In conclusion, in this study of patients admitted from acute care hospitals to a single inpatient rehabilitation facility, we observed a strong association between decreased functional status and increased hospital readmission. In particular, medical patients with lower physical functioning exhibited an especially high rate of readmission. Incorporating functional status assessment into routine medical care may help identify patients at higher risk of readmission. Moreover, preventing and treating impaired functional status during inpatient admission, through early activity and mobility, should be evaluated as a way of improving patient outcomes and reducing hospital readmissions.
Disclosures: Erik Hoyer, MD, is supported by the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097). The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377.
- , . Association of physical functioning with same‐hospital readmission after stroke. Am J Phys Med Rehabil. 2004;83(6):434–438.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , , . Risk factors for nonelective hospital readmissions. J Gen Intern Med. 1996;11(12):762–764.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Hospital readmission in persons with stroke following postacute inpatient rehabilitation. J Gerontol A Biol Sci Med Sci. 2012;67(8):875–881.
- , , , , , . Hospital readmission of persons with hip fracture following medical rehabilitation. Arch Gerontol Geriatr. 2003;36(1):15–22.
- , , , , , . Characteristics of persons rehospitalized after stroke rehabilitation. Arch Phys Med Rehabil. 2001;82(10):1367–1374.
- , , , et al. A classification system for inpatient rehabilitation patients: a review and proposed revisions to the functional independence measure‐function related groups. PB98–105992, September. Washington, DC: US Department of Commerce, National Technical Information Services; 1997.
- , , , , , . A case‐mix classification system for medical rehabilitation. Med Care. 1994;32(4):366–379.
- , , , . The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77(12):1226–1232.
- , , , , , . Four methods for characterizing disability in the formation of function related groups. Arch Phys Med Rehabil. 1994;75(12):1277–1283.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA Intern Med. 2013;173(8):624–629.
- , , , , , . Functional status impairment is associated with unplanned readmissions. Arch Phys Med Rehabil. 2013;94(10):1951–1958.
- , , , et al. All patient refined diagnosis related groups (APR‐DRGs). Version 15.0. Report No.: 98‐054 Rev. 00. Wallingford, CT: 3M Health Information Systems; 1998.
- The inpatient rehabilitation facility–patient assessment instrument (IRF‐PAI) training manual. 2012. http://www.cms.gov/.
- , , , et al. Relationships between disability measures and nursing effort during medical rehabilitation for patients with traumatic brain and spinal cord injury. Arch Phys Med Rehabil. 1997;78(2):143–149.
- , , , . Interrater reliability of the 7‐level functional independence measure (FIM). Scand J Rehabil Med. 1994;26(3):115–119.
- , , , , , . Comparison of logistic regression and neural networks to predict rehospitalization in patients with stroke. J Clin Epidemiol. 2001;54(11):1159–1165.
- , , . Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: the impact of using different methods for measuring responsiveness. J Clin Epidemiol. 2002;55(9):922–928.
- , . Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. J Am Coll Cardiol. 1999;33(6):1560–1566.
- , , . Are all readmissions bad readmissions? N Engl J Med. 2010;363(3):297–298.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- . Susceptibility to critical illness: reserve, response and therapy. Intensive Care Med. 2000;26(suppl 1):S57–S63.
- , , , , . Predictors of discharge to acute care after inpatient rehabilitation in severely affected stroke patients. Am J Phys Med Rehabil. 2012;91(5):387–392.
- , , , et al. Clinical characteristics and outcomes of sepsis‐related vs non‐sepsis‐related ARDS. Chest. 2010;138(3):559–567.
- , . Long‐term outcomes from sepsis. Curr Infect Dis Rep. 2007;9(5):382–386.
- , . Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009;302(7):792–794.
- , , , , . Patients' self‐assessed functional status in heart failure by new york heart association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16(2):150–156.
- , , , et al. Knee arthroplasty: disabilities in comparison to the general population and to hip arthroplasty using a French national longitudinal survey. PLoS One. 2008;3(7):e2561.
- , , , et al. Gait asymmetry in community‐ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89(2):304–310.
- , , , . Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398.
- , , , , , . The effect of admission physiological variables on 30 day outcome after stroke. J Clin Neurosci. 2005;12(8):905–910.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- . Can hospitalization‐associated disability be prevented? JAMA. 2011;306(16):1800–1801.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , , , , , . Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690.
- , , . Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 suppl):S436–S441.
- , , , et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542.
- , , , et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717–724.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145.
- , . Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–281.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59(2):359–365.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304.
- , , , et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2):254–262.
- , , . Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;(1):CD005955.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabil. 2011;92(9):1490–1500.
- , , , , , . Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24‐week exercise and telephone follow‐up program. J Am Geriatr Soc. 2009;57(3):395–402.
- , , , , , . Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013:1–7.
- , , , , . Risks of acute hospital transfer and mortality during stroke rehabilitation. Arch Phys Med Rehabil. 2003;84(5):712–718.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377.
- , . Association of physical functioning with same‐hospital readmission after stroke. Am J Phys Med Rehabil. 2004;83(6):434–438.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , , . Risk factors for nonelective hospital readmissions. J Gen Intern Med. 1996;11(12):762–764.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Hospital readmission in persons with stroke following postacute inpatient rehabilitation. J Gerontol A Biol Sci Med Sci. 2012;67(8):875–881.
- , , , , , . Hospital readmission of persons with hip fracture following medical rehabilitation. Arch Gerontol Geriatr. 2003;36(1):15–22.
- , , , , , . Characteristics of persons rehospitalized after stroke rehabilitation. Arch Phys Med Rehabil. 2001;82(10):1367–1374.
- , , , et al. A classification system for inpatient rehabilitation patients: a review and proposed revisions to the functional independence measure‐function related groups. PB98–105992, September. Washington, DC: US Department of Commerce, National Technical Information Services; 1997.
- , , , , , . A case‐mix classification system for medical rehabilitation. Med Care. 1994;32(4):366–379.
- , , , . The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77(12):1226–1232.
- , , , , , . Four methods for characterizing disability in the formation of function related groups. Arch Phys Med Rehabil. 1994;75(12):1277–1283.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA Intern Med. 2013;173(8):624–629.
- , , , , , . Functional status impairment is associated with unplanned readmissions. Arch Phys Med Rehabil. 2013;94(10):1951–1958.
- , , , et al. All patient refined diagnosis related groups (APR‐DRGs). Version 15.0. Report No.: 98‐054 Rev. 00. Wallingford, CT: 3M Health Information Systems; 1998.
- The inpatient rehabilitation facility–patient assessment instrument (IRF‐PAI) training manual. 2012. http://www.cms.gov/.
- , , , et al. Relationships between disability measures and nursing effort during medical rehabilitation for patients with traumatic brain and spinal cord injury. Arch Phys Med Rehabil. 1997;78(2):143–149.
- , , , . Interrater reliability of the 7‐level functional independence measure (FIM). Scand J Rehabil Med. 1994;26(3):115–119.
- , , , , , . Comparison of logistic regression and neural networks to predict rehospitalization in patients with stroke. J Clin Epidemiol. 2001;54(11):1159–1165.
- , , . Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: the impact of using different methods for measuring responsiveness. J Clin Epidemiol. 2002;55(9):922–928.
- , . Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. J Am Coll Cardiol. 1999;33(6):1560–1566.
- , , . Are all readmissions bad readmissions? N Engl J Med. 2010;363(3):297–298.
- , . Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505.
- . Susceptibility to critical illness: reserve, response and therapy. Intensive Care Med. 2000;26(suppl 1):S57–S63.
- , , , , . Predictors of discharge to acute care after inpatient rehabilitation in severely affected stroke patients. Am J Phys Med Rehabil. 2012;91(5):387–392.
- , , , et al. Clinical characteristics and outcomes of sepsis‐related vs non‐sepsis‐related ARDS. Chest. 2010;138(3):559–567.
- , . Long‐term outcomes from sepsis. Curr Infect Dis Rep. 2007;9(5):382–386.
- , . Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009;302(7):792–794.
- , , , , . Patients' self‐assessed functional status in heart failure by new york heart association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16(2):150–156.
- , , , et al. Knee arthroplasty: disabilities in comparison to the general population and to hip arthroplasty using a French national longitudinal survey. PLoS One. 2008;3(7):e2561.
- , , , et al. Gait asymmetry in community‐ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89(2):304–310.
- , , , . Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398.
- , , , , , . The effect of admission physiological variables on 30 day outcome after stroke. J Clin Neurosci. 2005;12(8):905–910.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- . Can hospitalization‐associated disability be prevented? JAMA. 2011;306(16):1800–1801.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , , , , , . Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690.
- , , . Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 suppl):S436–S441.
- , , , et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542.
- , , , et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717–724.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145.
- , . Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–281.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59(2):359–365.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304.
- , , , et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2):254–262.
- , , . Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;(1):CD005955.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabil. 2011;92(9):1490–1500.
- , , , , , . Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24‐week exercise and telephone follow‐up program. J Am Geriatr Soc. 2009;57(3):395–402.
- , , , , , . Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013:1–7.
- , , , , . Risks of acute hospital transfer and mortality during stroke rehabilitation. Arch Phys Med Rehabil. 2003;84(5):712–718.
© 2014 Society of Hospital Medicine
Functional Status
Hospital readmission is not a new problem, but ever since the Centers for Medicaid and Medicare Services (CMS) announced that hospital reimbursement would be linked to readmission rates, the quest to understand drivers of this outcome has taken on new and remarkable vigor. Despite the avalanche of new studies on readmission factors[1] and transition interventions,[2, 3] surprisingly few have focused on conditions more prevalent in the aging Medicare population such as functional limitations. This trend in the literature reflects what is perhaps the greatest irony of the CMS readmission policy itself: while focused on improving care for a predominantly over 65‐year‐old population, it is agnostic to core geriatric vulnerabilities like function and cognition.
In this issue of the Journal of Hospital Medicine, Hoyer and colleagues take an important first step toward exploring such vulnerabilities.[4] Although it may not surprise many hospitalists that these play a role in complex outcomes such as readmission, the effects reported here are striking. The odds for readmission were 300% higher for patients with the lowest functional scores compared to those with highest scores after adjusting for other known factors such as comorbidities, age, and severity of illness. In terms of readmission rates, 29% of functionally impaired medical patients were readmitted compared to 11% of those with high function. Similar but less profound trends were seen in patients discharged from neurology and orthopedic services.
Although this was a single‐site study, and functional assessments were made on admission to an acute rehabilitation facility after hospital discharge, these findings are compelling and suggest many important areas for future research. First, these results suggest a need for replication in nationally representative data to better understand their scope and generalizability. Certainly, the number of participants (9405 patients) gives this study plenty of power; however, the sample is limited in that presumably all patients had some level of functional decline, but enough potential for functional recovery to warrant discharge to acute rehabilitation. We do not know what effects functional limitations might have on patients discharged to other settings (eg, community with home rehabilitation or skilled nursing facility with rehabilitation). Thus, future research should examine whether the impact of functional limitations described in this sample applies to the larger universe of hospital discharges.
We also do not know anything about the functional status of these patients at admission or their functional trajectory prior to hospitalization, which limits conclusions about whether the disabilities observed were hospital acquired. Functional ability, like vital signs, can be quite variable during the course of acute illness and should be interpreted in the context of each patient's baseline. The functional trajectory for a patient who was impaired at the time of hospital discharge, but independent before hospitalization, is likely very different than one who was chronically impaired at baseline. Thus, postdischarge is only half the story at best, and future research should explore the functional status and trajectory of patients before admission too.
Finally, to assess functional status, the authors of this study used the Functional Independence Measure (FIM) score, a well‐validated instrument used in rehabilitation facilities. One advantage of using this measure to predict readmission is that in addition to 12 items that assess physical domains overlapping with the Activities of Daily Living (ADL) measures commonly used in hospitals, it also includes 5 items about cognition and thus gives an overall view of both physical and mental status in context of functional ability. On the down side, the FIM score is less well known in the acute care setting and does not include instrumental ADLs, such as shopping, housekeeping, food preparation/cleanup, telephone, transportation, and technology like computers, that are often important for patients returning home. Given the interesting findings by Hoyer et al., future research should explore possible associations with these activities in patients discharged to community as well.
The results by Hoyer et al. also have important implications for policy and practice. At the level of national policy and ongoing healthcare reform, Medicare should consider ways to incentivize hospitals to collect data on functional status of patients more consistently. Currently, there is no International Classification of Diseases, 9th Revision code to capture functional limitation during hospitalization as a diagnosis or comorbidity (whether hospital acquired or not), which precludes any discussion about including functional status as an adjustor in the current CMS model for expected readmission rates for hospitals. Regardless of CMS policy and performance incentives or penalties, a lot more could be done at the level of hospital policy and practice to improve screening for functional vulnerabilities on admission and prior to discharge. Although this may require greater investment in standardizing physical therapy evaluation for most patients (especially those over 65 years old), the increased readmission rates found by Hoyer et al. in functionally impaired patients suggest it would be penny wise but pound foolish not to do so. In other words, if hospitals want to reduce their readmission rates by identifying and intervening on high‐risk patients, identifying functionally impaired patients seems to be the low‐hanging fruit.
In summary, Hoyer and colleagues have made an important contribution to the ever‐expanding literature on readmission risk factors, but they have likely just identified the tip of the iceberg. As Medicare enrollment continues to climb with the growth of baby boomers over 65 years old, the demand for acute care in older adults will continue to grow.[5] Moreover, as pressure mounts to improve the quality and reduce the costs of hospital care, greater understanding of geriatric vulnerabilities in this population will be increasingly important.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- et al. J Hosp Med. 2014;9(5):277–282.
- et al. US population aging and demand for inpatient services. J Hosp Med. 2014;9(3):193–196.
Hospital readmission is not a new problem, but ever since the Centers for Medicaid and Medicare Services (CMS) announced that hospital reimbursement would be linked to readmission rates, the quest to understand drivers of this outcome has taken on new and remarkable vigor. Despite the avalanche of new studies on readmission factors[1] and transition interventions,[2, 3] surprisingly few have focused on conditions more prevalent in the aging Medicare population such as functional limitations. This trend in the literature reflects what is perhaps the greatest irony of the CMS readmission policy itself: while focused on improving care for a predominantly over 65‐year‐old population, it is agnostic to core geriatric vulnerabilities like function and cognition.
In this issue of the Journal of Hospital Medicine, Hoyer and colleagues take an important first step toward exploring such vulnerabilities.[4] Although it may not surprise many hospitalists that these play a role in complex outcomes such as readmission, the effects reported here are striking. The odds for readmission were 300% higher for patients with the lowest functional scores compared to those with highest scores after adjusting for other known factors such as comorbidities, age, and severity of illness. In terms of readmission rates, 29% of functionally impaired medical patients were readmitted compared to 11% of those with high function. Similar but less profound trends were seen in patients discharged from neurology and orthopedic services.
Although this was a single‐site study, and functional assessments were made on admission to an acute rehabilitation facility after hospital discharge, these findings are compelling and suggest many important areas for future research. First, these results suggest a need for replication in nationally representative data to better understand their scope and generalizability. Certainly, the number of participants (9405 patients) gives this study plenty of power; however, the sample is limited in that presumably all patients had some level of functional decline, but enough potential for functional recovery to warrant discharge to acute rehabilitation. We do not know what effects functional limitations might have on patients discharged to other settings (eg, community with home rehabilitation or skilled nursing facility with rehabilitation). Thus, future research should examine whether the impact of functional limitations described in this sample applies to the larger universe of hospital discharges.
We also do not know anything about the functional status of these patients at admission or their functional trajectory prior to hospitalization, which limits conclusions about whether the disabilities observed were hospital acquired. Functional ability, like vital signs, can be quite variable during the course of acute illness and should be interpreted in the context of each patient's baseline. The functional trajectory for a patient who was impaired at the time of hospital discharge, but independent before hospitalization, is likely very different than one who was chronically impaired at baseline. Thus, postdischarge is only half the story at best, and future research should explore the functional status and trajectory of patients before admission too.
Finally, to assess functional status, the authors of this study used the Functional Independence Measure (FIM) score, a well‐validated instrument used in rehabilitation facilities. One advantage of using this measure to predict readmission is that in addition to 12 items that assess physical domains overlapping with the Activities of Daily Living (ADL) measures commonly used in hospitals, it also includes 5 items about cognition and thus gives an overall view of both physical and mental status in context of functional ability. On the down side, the FIM score is less well known in the acute care setting and does not include instrumental ADLs, such as shopping, housekeeping, food preparation/cleanup, telephone, transportation, and technology like computers, that are often important for patients returning home. Given the interesting findings by Hoyer et al., future research should explore possible associations with these activities in patients discharged to community as well.
The results by Hoyer et al. also have important implications for policy and practice. At the level of national policy and ongoing healthcare reform, Medicare should consider ways to incentivize hospitals to collect data on functional status of patients more consistently. Currently, there is no International Classification of Diseases, 9th Revision code to capture functional limitation during hospitalization as a diagnosis or comorbidity (whether hospital acquired or not), which precludes any discussion about including functional status as an adjustor in the current CMS model for expected readmission rates for hospitals. Regardless of CMS policy and performance incentives or penalties, a lot more could be done at the level of hospital policy and practice to improve screening for functional vulnerabilities on admission and prior to discharge. Although this may require greater investment in standardizing physical therapy evaluation for most patients (especially those over 65 years old), the increased readmission rates found by Hoyer et al. in functionally impaired patients suggest it would be penny wise but pound foolish not to do so. In other words, if hospitals want to reduce their readmission rates by identifying and intervening on high‐risk patients, identifying functionally impaired patients seems to be the low‐hanging fruit.
In summary, Hoyer and colleagues have made an important contribution to the ever‐expanding literature on readmission risk factors, but they have likely just identified the tip of the iceberg. As Medicare enrollment continues to climb with the growth of baby boomers over 65 years old, the demand for acute care in older adults will continue to grow.[5] Moreover, as pressure mounts to improve the quality and reduce the costs of hospital care, greater understanding of geriatric vulnerabilities in this population will be increasingly important.
Hospital readmission is not a new problem, but ever since the Centers for Medicaid and Medicare Services (CMS) announced that hospital reimbursement would be linked to readmission rates, the quest to understand drivers of this outcome has taken on new and remarkable vigor. Despite the avalanche of new studies on readmission factors[1] and transition interventions,[2, 3] surprisingly few have focused on conditions more prevalent in the aging Medicare population such as functional limitations. This trend in the literature reflects what is perhaps the greatest irony of the CMS readmission policy itself: while focused on improving care for a predominantly over 65‐year‐old population, it is agnostic to core geriatric vulnerabilities like function and cognition.
In this issue of the Journal of Hospital Medicine, Hoyer and colleagues take an important first step toward exploring such vulnerabilities.[4] Although it may not surprise many hospitalists that these play a role in complex outcomes such as readmission, the effects reported here are striking. The odds for readmission were 300% higher for patients with the lowest functional scores compared to those with highest scores after adjusting for other known factors such as comorbidities, age, and severity of illness. In terms of readmission rates, 29% of functionally impaired medical patients were readmitted compared to 11% of those with high function. Similar but less profound trends were seen in patients discharged from neurology and orthopedic services.
Although this was a single‐site study, and functional assessments were made on admission to an acute rehabilitation facility after hospital discharge, these findings are compelling and suggest many important areas for future research. First, these results suggest a need for replication in nationally representative data to better understand their scope and generalizability. Certainly, the number of participants (9405 patients) gives this study plenty of power; however, the sample is limited in that presumably all patients had some level of functional decline, but enough potential for functional recovery to warrant discharge to acute rehabilitation. We do not know what effects functional limitations might have on patients discharged to other settings (eg, community with home rehabilitation or skilled nursing facility with rehabilitation). Thus, future research should examine whether the impact of functional limitations described in this sample applies to the larger universe of hospital discharges.
We also do not know anything about the functional status of these patients at admission or their functional trajectory prior to hospitalization, which limits conclusions about whether the disabilities observed were hospital acquired. Functional ability, like vital signs, can be quite variable during the course of acute illness and should be interpreted in the context of each patient's baseline. The functional trajectory for a patient who was impaired at the time of hospital discharge, but independent before hospitalization, is likely very different than one who was chronically impaired at baseline. Thus, postdischarge is only half the story at best, and future research should explore the functional status and trajectory of patients before admission too.
Finally, to assess functional status, the authors of this study used the Functional Independence Measure (FIM) score, a well‐validated instrument used in rehabilitation facilities. One advantage of using this measure to predict readmission is that in addition to 12 items that assess physical domains overlapping with the Activities of Daily Living (ADL) measures commonly used in hospitals, it also includes 5 items about cognition and thus gives an overall view of both physical and mental status in context of functional ability. On the down side, the FIM score is less well known in the acute care setting and does not include instrumental ADLs, such as shopping, housekeeping, food preparation/cleanup, telephone, transportation, and technology like computers, that are often important for patients returning home. Given the interesting findings by Hoyer et al., future research should explore possible associations with these activities in patients discharged to community as well.
The results by Hoyer et al. also have important implications for policy and practice. At the level of national policy and ongoing healthcare reform, Medicare should consider ways to incentivize hospitals to collect data on functional status of patients more consistently. Currently, there is no International Classification of Diseases, 9th Revision code to capture functional limitation during hospitalization as a diagnosis or comorbidity (whether hospital acquired or not), which precludes any discussion about including functional status as an adjustor in the current CMS model for expected readmission rates for hospitals. Regardless of CMS policy and performance incentives or penalties, a lot more could be done at the level of hospital policy and practice to improve screening for functional vulnerabilities on admission and prior to discharge. Although this may require greater investment in standardizing physical therapy evaluation for most patients (especially those over 65 years old), the increased readmission rates found by Hoyer et al. in functionally impaired patients suggest it would be penny wise but pound foolish not to do so. In other words, if hospitals want to reduce their readmission rates by identifying and intervening on high‐risk patients, identifying functionally impaired patients seems to be the low‐hanging fruit.
In summary, Hoyer and colleagues have made an important contribution to the ever‐expanding literature on readmission risk factors, but they have likely just identified the tip of the iceberg. As Medicare enrollment continues to climb with the growth of baby boomers over 65 years old, the demand for acute care in older adults will continue to grow.[5] Moreover, as pressure mounts to improve the quality and reduce the costs of hospital care, greater understanding of geriatric vulnerabilities in this population will be increasingly important.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- et al. J Hosp Med. 2014;9(5):277–282.
- et al. US population aging and demand for inpatient services. J Hosp Med. 2014;9(3):193–196.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- et al. J Hosp Med. 2014;9(5):277–282.
- et al. US population aging and demand for inpatient services. J Hosp Med. 2014;9(3):193–196.
No Benefits to Therapeutic Hypothermia for Severe Bacterial Meningitis
Clinical question
Can therapeutic hypothermia improve functional outcomes in comatose patients with severe bacterial meningitis?
Bottom line
For critically ill patients with severe bacterial meningitis, induced hypothermia using intravascular cooling or other cooling techniques does not improve outcomes and may lead to increased mortality. This trial was stopped early and thus lacked the statistical power to make definitive conclusions about the potential harmful effects of this intervention. (LOE = 1b-)
Reference
Mourvillier B, Tubach F, van de Beek D, et al. Induced hypothermia in severe bacterial meningitis: A randomized clinical trial. JAMA 2013;310(20):2174-2183.
Study design
Randomized controlled trial (nonblinded)
Funding source
Industry + govt
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
Adult patients with suspected or confirmed bacterial meningitis who had a Glasgow Coma Scale (GCS) score of less than 8 for fewer than 12 hours were randomized, using concealed allocation, into the induced hypothermia group or to usual care. All patients received appropriate antimicrobial therapy. In the hypothermia group, intravascular cooling was achieved by a loading dose of 1500 mL 40C saline over 30 minutes, and additional 500 mL boluses over 10 minutes as needed, to achieve a temperature of 33.50C or lower. Other cooling techniques, including ice packs, cooling air, and cooling pads, were also used. Temperatures were maintained between 32C and 34C for 48 hours, and the rewarming phase was passive. Baseline characteristics in the intervention group and control group were similar: mean age was 59 years, median GCS score was 7, all patients were mechanically ventilated, and the causative organism was identified as Streptococcus pneumoniae in the majority of patients. Analysis was by intention to treat. The primary outcome was the score on the Glasgow Outcome Scale. A favorable outcome was a score of 5, indicating mild or no disability; an unfavorable outcome was any score 1 through 4, with 1 indicating death. At 3 months, there was a trend toward unfavorable outcomes in the hypothermia group (86% vs 73% in the control group; relative risk = 1.17; 0.95-1.43; P = .13), as well as a trend toward increased mortality (hazard ratio = 1.76; 0.89-3.45; P = .10). The trial was stopped early because of the higher mortality in the hypothermia group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Can therapeutic hypothermia improve functional outcomes in comatose patients with severe bacterial meningitis?
Bottom line
For critically ill patients with severe bacterial meningitis, induced hypothermia using intravascular cooling or other cooling techniques does not improve outcomes and may lead to increased mortality. This trial was stopped early and thus lacked the statistical power to make definitive conclusions about the potential harmful effects of this intervention. (LOE = 1b-)
Reference
Mourvillier B, Tubach F, van de Beek D, et al. Induced hypothermia in severe bacterial meningitis: A randomized clinical trial. JAMA 2013;310(20):2174-2183.
Study design
Randomized controlled trial (nonblinded)
Funding source
Industry + govt
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
Adult patients with suspected or confirmed bacterial meningitis who had a Glasgow Coma Scale (GCS) score of less than 8 for fewer than 12 hours were randomized, using concealed allocation, into the induced hypothermia group or to usual care. All patients received appropriate antimicrobial therapy. In the hypothermia group, intravascular cooling was achieved by a loading dose of 1500 mL 40C saline over 30 minutes, and additional 500 mL boluses over 10 minutes as needed, to achieve a temperature of 33.50C or lower. Other cooling techniques, including ice packs, cooling air, and cooling pads, were also used. Temperatures were maintained between 32C and 34C for 48 hours, and the rewarming phase was passive. Baseline characteristics in the intervention group and control group were similar: mean age was 59 years, median GCS score was 7, all patients were mechanically ventilated, and the causative organism was identified as Streptococcus pneumoniae in the majority of patients. Analysis was by intention to treat. The primary outcome was the score on the Glasgow Outcome Scale. A favorable outcome was a score of 5, indicating mild or no disability; an unfavorable outcome was any score 1 through 4, with 1 indicating death. At 3 months, there was a trend toward unfavorable outcomes in the hypothermia group (86% vs 73% in the control group; relative risk = 1.17; 0.95-1.43; P = .13), as well as a trend toward increased mortality (hazard ratio = 1.76; 0.89-3.45; P = .10). The trial was stopped early because of the higher mortality in the hypothermia group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Can therapeutic hypothermia improve functional outcomes in comatose patients with severe bacterial meningitis?
Bottom line
For critically ill patients with severe bacterial meningitis, induced hypothermia using intravascular cooling or other cooling techniques does not improve outcomes and may lead to increased mortality. This trial was stopped early and thus lacked the statistical power to make definitive conclusions about the potential harmful effects of this intervention. (LOE = 1b-)
Reference
Mourvillier B, Tubach F, van de Beek D, et al. Induced hypothermia in severe bacterial meningitis: A randomized clinical trial. JAMA 2013;310(20):2174-2183.
Study design
Randomized controlled trial (nonblinded)
Funding source
Industry + govt
Allocation
Concealed
Setting
Inpatient (ICU only)
Synopsis
Adult patients with suspected or confirmed bacterial meningitis who had a Glasgow Coma Scale (GCS) score of less than 8 for fewer than 12 hours were randomized, using concealed allocation, into the induced hypothermia group or to usual care. All patients received appropriate antimicrobial therapy. In the hypothermia group, intravascular cooling was achieved by a loading dose of 1500 mL 40C saline over 30 minutes, and additional 500 mL boluses over 10 minutes as needed, to achieve a temperature of 33.50C or lower. Other cooling techniques, including ice packs, cooling air, and cooling pads, were also used. Temperatures were maintained between 32C and 34C for 48 hours, and the rewarming phase was passive. Baseline characteristics in the intervention group and control group were similar: mean age was 59 years, median GCS score was 7, all patients were mechanically ventilated, and the causative organism was identified as Streptococcus pneumoniae in the majority of patients. Analysis was by intention to treat. The primary outcome was the score on the Glasgow Outcome Scale. A favorable outcome was a score of 5, indicating mild or no disability; an unfavorable outcome was any score 1 through 4, with 1 indicating death. At 3 months, there was a trend toward unfavorable outcomes in the hypothermia group (86% vs 73% in the control group; relative risk = 1.17; 0.95-1.43; P = .13), as well as a trend toward increased mortality (hazard ratio = 1.76; 0.89-3.45; P = .10). The trial was stopped early because of the higher mortality in the hypothermia group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
ACP Guidelines on Treatment of Anemia in Patients With Heart Disease
Clinical question
How should anemia and iron deficiency be treated in adults with heart disease?
Bottom line
For hospitalized patients with anemia and coronary heart disease, the American College of Physicians recommends a restrictive transfusion strategy and a trigger hemoglobin of 7 g/dL to 8 g/dL. Furthermore, erythropoiesis-stimulating agents (ESAs) should be avoided in patients with coronary heart disease or congestive heart failure and mild to moderate anemia. Evidence regarding intravenous iron for this patient population is inconclusive. (LOE = 1a)
Reference
Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: A systematic review. Ann Intern Med 2013;159(11):746-757. Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Treatment of anemia in patients with heart disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159(11):770-779.
Study design
Practice guideline
Funding source
Government
Allocation
Uncertain
Setting
Various (meta-analysis)
Synopsis
The American College of Physicians developed this guideline based on a systematic review of the literature that evaluated the benefits and harms of anemia treatment in adults with heart disease. The authors searched multiple databases including MEDLINE and the Cochrane Library, to identify trials that studied the effects of blood transfusions, ESAs, and iron in patients with anemia and congestive heart failure or coronary heart disease. Observational transfusion studies were also included. Two reviewers independently assessed studies for inclusion, extracted data, and assessed study quality. Data was combined for meta-analysis when possible. Although it was low-quality evidence, liberal transfusion strategies as compared with restrictive strategies in treating anemia showed no effect on mortality for patients with heart disease. Moderate-strength to high-strength evidence from the ESA studies also showed no benefit, but did show a potential for harm, including an increased risk of venous thromboembolism. Finally, although few studies evaluated intravenous iron therapy, one good-quality study showed that it increased short-term exercise tolerance and quality of life in patients with heart failure. Based on these findings, the American College of Physicians guideline committee makes the following recommendations: (1) Use a restrictive red blood cell transfusion strategy with a hemoglobin threshold of 7 g/dL to 8 g/dL in hospitalized patients with coronary heart disease; and (2) avoid ESAs in patients with mild to moderate anemia and congestive heart failure or coronary heart disease. Because of lack of evidence regarding long-term outcomes and possible harms, as well as limited overall data, there was no recommendation made regarding the use of intravenous iron.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
How should anemia and iron deficiency be treated in adults with heart disease?
Bottom line
For hospitalized patients with anemia and coronary heart disease, the American College of Physicians recommends a restrictive transfusion strategy and a trigger hemoglobin of 7 g/dL to 8 g/dL. Furthermore, erythropoiesis-stimulating agents (ESAs) should be avoided in patients with coronary heart disease or congestive heart failure and mild to moderate anemia. Evidence regarding intravenous iron for this patient population is inconclusive. (LOE = 1a)
Reference
Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: A systematic review. Ann Intern Med 2013;159(11):746-757. Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Treatment of anemia in patients with heart disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159(11):770-779.
Study design
Practice guideline
Funding source
Government
Allocation
Uncertain
Setting
Various (meta-analysis)
Synopsis
The American College of Physicians developed this guideline based on a systematic review of the literature that evaluated the benefits and harms of anemia treatment in adults with heart disease. The authors searched multiple databases including MEDLINE and the Cochrane Library, to identify trials that studied the effects of blood transfusions, ESAs, and iron in patients with anemia and congestive heart failure or coronary heart disease. Observational transfusion studies were also included. Two reviewers independently assessed studies for inclusion, extracted data, and assessed study quality. Data was combined for meta-analysis when possible. Although it was low-quality evidence, liberal transfusion strategies as compared with restrictive strategies in treating anemia showed no effect on mortality for patients with heart disease. Moderate-strength to high-strength evidence from the ESA studies also showed no benefit, but did show a potential for harm, including an increased risk of venous thromboembolism. Finally, although few studies evaluated intravenous iron therapy, one good-quality study showed that it increased short-term exercise tolerance and quality of life in patients with heart failure. Based on these findings, the American College of Physicians guideline committee makes the following recommendations: (1) Use a restrictive red blood cell transfusion strategy with a hemoglobin threshold of 7 g/dL to 8 g/dL in hospitalized patients with coronary heart disease; and (2) avoid ESAs in patients with mild to moderate anemia and congestive heart failure or coronary heart disease. Because of lack of evidence regarding long-term outcomes and possible harms, as well as limited overall data, there was no recommendation made regarding the use of intravenous iron.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
How should anemia and iron deficiency be treated in adults with heart disease?
Bottom line
For hospitalized patients with anemia and coronary heart disease, the American College of Physicians recommends a restrictive transfusion strategy and a trigger hemoglobin of 7 g/dL to 8 g/dL. Furthermore, erythropoiesis-stimulating agents (ESAs) should be avoided in patients with coronary heart disease or congestive heart failure and mild to moderate anemia. Evidence regarding intravenous iron for this patient population is inconclusive. (LOE = 1a)
Reference
Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: A systematic review. Ann Intern Med 2013;159(11):746-757. Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Treatment of anemia in patients with heart disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159(11):770-779.
Study design
Practice guideline
Funding source
Government
Allocation
Uncertain
Setting
Various (meta-analysis)
Synopsis
The American College of Physicians developed this guideline based on a systematic review of the literature that evaluated the benefits and harms of anemia treatment in adults with heart disease. The authors searched multiple databases including MEDLINE and the Cochrane Library, to identify trials that studied the effects of blood transfusions, ESAs, and iron in patients with anemia and congestive heart failure or coronary heart disease. Observational transfusion studies were also included. Two reviewers independently assessed studies for inclusion, extracted data, and assessed study quality. Data was combined for meta-analysis when possible. Although it was low-quality evidence, liberal transfusion strategies as compared with restrictive strategies in treating anemia showed no effect on mortality for patients with heart disease. Moderate-strength to high-strength evidence from the ESA studies also showed no benefit, but did show a potential for harm, including an increased risk of venous thromboembolism. Finally, although few studies evaluated intravenous iron therapy, one good-quality study showed that it increased short-term exercise tolerance and quality of life in patients with heart failure. Based on these findings, the American College of Physicians guideline committee makes the following recommendations: (1) Use a restrictive red blood cell transfusion strategy with a hemoglobin threshold of 7 g/dL to 8 g/dL in hospitalized patients with coronary heart disease; and (2) avoid ESAs in patients with mild to moderate anemia and congestive heart failure or coronary heart disease. Because of lack of evidence regarding long-term outcomes and possible harms, as well as limited overall data, there was no recommendation made regarding the use of intravenous iron.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
2014 Update on abnormal uterine bleeding
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
Targeting pathways can override resistance in ALL
Inhibiting two biosynthesis pathways can override treatment resistance in acute lymphoblastic leukemia (ALL), preclinical research suggests.
Researchers found that inhibiting only one pathway did not effectively kill ALL cells. The cells simply used another pathway to replicate their DNA and continue dividing.
But inhibiting both pathways induced apoptosis in human leukemia cells and reduced tumor burden in mouse models of T- and B-cell ALL.
“This new, dual-targeting approach shows that we can overcome the redundancy in DNA synthesis in ALL cells and identifies a potential target for metabolic intervention in ALL, and possibly in other hematological cancers,” said study author Caius Radu, MD, of the University of California, Los Angeles.
He and his colleagues described this approach in The Journal of Experimental Medicine.
The research began with the knowledge that deoxyribonucleotide triphosphates, including deoxycytidine triphosphate (dCTP), are required for DNA replication, which is necessary for cell division. And dCTP can be produced by the de novo pathway or the nucleoside salvage pathway.
Dr Radu and his colleagues discovered that inhibiting the de novo pathway with the compound thymidine caused leukemia cells to switch to the nucleoside salvage pathway for dCTP production.
However, inhibiting both the de novo and nucleoside salvage pathways prevented dCTP production and proved lethal for leukemia cells.
A number of experiments elicited these results. In one, the researchers knocked down the deoxycytidine kinase (dCK) in human T-ALL cells to inhibit the nucleoside salvage pathway. Then, they administered thymidine to inhibit the de novo pathway. This resulted in dCTP depletion, stalled DNA replication, replication stress, DNA damage, and apoptosis.
The researchers also used the small-molecule inhibitor DI-39 to target dCK. They found that co-administration of DI-39 and thymidine induced replication stress and apoptosis in several leukemia cell lines: CEM, Jurkat, MOLT-4, NALM-6 and RS4;116.
The team then tested DI-39 and thymidine in mice bearing CEM tumors. They found the combination reduced tumor growth in mice bearing established, subcutaneous CEM xenografts and in mice with systemic T-ALL.
In the systemic T-ALL model, treatment with thymidine alone resulted in a 7-fold reduction in tumor burden compared to vehicle control or DI-39 alone. But when thymidine and DI-39 were administered together, mice saw a 100-fold reduction in tumor burden compared to thymidine alone.
The thymidine-DI-39 combination also proved effective against B-ALL cells and in a mouse model of B-ALL. However, the effects were not as great as those observed in T-ALL.
Finally, the researchers evaluated the effects of thymidine and DI-39 in hematopoietic progenitor cells. They looked at the Lineage- Sca-1+ c-Kit+ hematopoietic stem cell population, as well as short-term, long-term, and multipotent progenitor hematopoietic progenitor cells.
There was a minor decrease in the percentage of Lineage- Sca-1+ c-Kit+ cells after thymidine treatment. However, there were no other significant changes in progenitor cells between the treatment and control groups. Why leukemic cells and normal hematopoietic progenitors respond so differently to this treatment requires further investigation, the researchers said.
But they also noted that this study advances our understanding of DNA synthesis in leukemic cells and suggests that targeted metabolic intervention could be a new therapeutic approach in ALL. ![]()

Inhibiting two biosynthesis pathways can override treatment resistance in acute lymphoblastic leukemia (ALL), preclinical research suggests.
Researchers found that inhibiting only one pathway did not effectively kill ALL cells. The cells simply used another pathway to replicate their DNA and continue dividing.
But inhibiting both pathways induced apoptosis in human leukemia cells and reduced tumor burden in mouse models of T- and B-cell ALL.
“This new, dual-targeting approach shows that we can overcome the redundancy in DNA synthesis in ALL cells and identifies a potential target for metabolic intervention in ALL, and possibly in other hematological cancers,” said study author Caius Radu, MD, of the University of California, Los Angeles.
He and his colleagues described this approach in The Journal of Experimental Medicine.
The research began with the knowledge that deoxyribonucleotide triphosphates, including deoxycytidine triphosphate (dCTP), are required for DNA replication, which is necessary for cell division. And dCTP can be produced by the de novo pathway or the nucleoside salvage pathway.
Dr Radu and his colleagues discovered that inhibiting the de novo pathway with the compound thymidine caused leukemia cells to switch to the nucleoside salvage pathway for dCTP production.
However, inhibiting both the de novo and nucleoside salvage pathways prevented dCTP production and proved lethal for leukemia cells.
A number of experiments elicited these results. In one, the researchers knocked down the deoxycytidine kinase (dCK) in human T-ALL cells to inhibit the nucleoside salvage pathway. Then, they administered thymidine to inhibit the de novo pathway. This resulted in dCTP depletion, stalled DNA replication, replication stress, DNA damage, and apoptosis.
The researchers also used the small-molecule inhibitor DI-39 to target dCK. They found that co-administration of DI-39 and thymidine induced replication stress and apoptosis in several leukemia cell lines: CEM, Jurkat, MOLT-4, NALM-6 and RS4;116.
The team then tested DI-39 and thymidine in mice bearing CEM tumors. They found the combination reduced tumor growth in mice bearing established, subcutaneous CEM xenografts and in mice with systemic T-ALL.
In the systemic T-ALL model, treatment with thymidine alone resulted in a 7-fold reduction in tumor burden compared to vehicle control or DI-39 alone. But when thymidine and DI-39 were administered together, mice saw a 100-fold reduction in tumor burden compared to thymidine alone.
The thymidine-DI-39 combination also proved effective against B-ALL cells and in a mouse model of B-ALL. However, the effects were not as great as those observed in T-ALL.
Finally, the researchers evaluated the effects of thymidine and DI-39 in hematopoietic progenitor cells. They looked at the Lineage- Sca-1+ c-Kit+ hematopoietic stem cell population, as well as short-term, long-term, and multipotent progenitor hematopoietic progenitor cells.
There was a minor decrease in the percentage of Lineage- Sca-1+ c-Kit+ cells after thymidine treatment. However, there were no other significant changes in progenitor cells between the treatment and control groups. Why leukemic cells and normal hematopoietic progenitors respond so differently to this treatment requires further investigation, the researchers said.
But they also noted that this study advances our understanding of DNA synthesis in leukemic cells and suggests that targeted metabolic intervention could be a new therapeutic approach in ALL. ![]()

Inhibiting two biosynthesis pathways can override treatment resistance in acute lymphoblastic leukemia (ALL), preclinical research suggests.
Researchers found that inhibiting only one pathway did not effectively kill ALL cells. The cells simply used another pathway to replicate their DNA and continue dividing.
But inhibiting both pathways induced apoptosis in human leukemia cells and reduced tumor burden in mouse models of T- and B-cell ALL.
“This new, dual-targeting approach shows that we can overcome the redundancy in DNA synthesis in ALL cells and identifies a potential target for metabolic intervention in ALL, and possibly in other hematological cancers,” said study author Caius Radu, MD, of the University of California, Los Angeles.
He and his colleagues described this approach in The Journal of Experimental Medicine.
The research began with the knowledge that deoxyribonucleotide triphosphates, including deoxycytidine triphosphate (dCTP), are required for DNA replication, which is necessary for cell division. And dCTP can be produced by the de novo pathway or the nucleoside salvage pathway.
Dr Radu and his colleagues discovered that inhibiting the de novo pathway with the compound thymidine caused leukemia cells to switch to the nucleoside salvage pathway for dCTP production.
However, inhibiting both the de novo and nucleoside salvage pathways prevented dCTP production and proved lethal for leukemia cells.
A number of experiments elicited these results. In one, the researchers knocked down the deoxycytidine kinase (dCK) in human T-ALL cells to inhibit the nucleoside salvage pathway. Then, they administered thymidine to inhibit the de novo pathway. This resulted in dCTP depletion, stalled DNA replication, replication stress, DNA damage, and apoptosis.
The researchers also used the small-molecule inhibitor DI-39 to target dCK. They found that co-administration of DI-39 and thymidine induced replication stress and apoptosis in several leukemia cell lines: CEM, Jurkat, MOLT-4, NALM-6 and RS4;116.
The team then tested DI-39 and thymidine in mice bearing CEM tumors. They found the combination reduced tumor growth in mice bearing established, subcutaneous CEM xenografts and in mice with systemic T-ALL.
In the systemic T-ALL model, treatment with thymidine alone resulted in a 7-fold reduction in tumor burden compared to vehicle control or DI-39 alone. But when thymidine and DI-39 were administered together, mice saw a 100-fold reduction in tumor burden compared to thymidine alone.
The thymidine-DI-39 combination also proved effective against B-ALL cells and in a mouse model of B-ALL. However, the effects were not as great as those observed in T-ALL.
Finally, the researchers evaluated the effects of thymidine and DI-39 in hematopoietic progenitor cells. They looked at the Lineage- Sca-1+ c-Kit+ hematopoietic stem cell population, as well as short-term, long-term, and multipotent progenitor hematopoietic progenitor cells.
There was a minor decrease in the percentage of Lineage- Sca-1+ c-Kit+ cells after thymidine treatment. However, there were no other significant changes in progenitor cells between the treatment and control groups. Why leukemic cells and normal hematopoietic progenitors respond so differently to this treatment requires further investigation, the researchers said.
But they also noted that this study advances our understanding of DNA synthesis in leukemic cells and suggests that targeted metabolic intervention could be a new therapeutic approach in ALL. ![]()
Malaria parasite originated in Africa, team says

David Morgan & Crickette Sanz
Investigators have found evidence suggesting the malaria parasite Plasmodium vivax originated in Africa.
Until recently, the closest genetic relatives of human P vivax were found only in Asian macaques, leading researchers to believe that P vivax originated in Asia.
The current study, published in Nature Communications, showed that wild apes in central Africa are widely infected with parasites that are, genetically, nearly identical to human P vivax.
This finding overturns the dogma that P vivax originated in Asia, despite being most prevalent in humans there now, and also solves other questions about P vivax infection.
For example, it explains why the Duffy-null phenotype, which confers resistance to P vivax, is common among people indigenous to Africa. And it explains how travelers returning from regions where most people are Duffy-negative can be infected with P vivax.
Paul Sharp, PhD, of the University of Edinburgh in the UK, and his colleagues conducted this research, testing more than 5000 ape fecal samples from dozens of field stations and sanctuaries in Africa for P vivax DNA.
They found P vivax-like sequences in chimpanzees, western gorillas, and eastern gorillas, but not in bonobos. Ape P vivax was highly prevalent in wild communities, exhibiting infection rates consistent with stable transmission of the parasite within the wild apes.
To examine the evolutionary relationships between ape and human parasites, the researchers generated parasite DNA sequences from wild and sanctuary apes, as well as from a global sampling of human P vivax infections.
They constructed a family tree of the sequences and found that ape and human parasites were very closely related. But ape parasites were more diverse than the human parasites and did not group according to their host species. The human parasites formed a single lineage that fell within the branches of ape parasite sequences.
From these evolutionary relationships, the investigators concluded that P vivax is of African—not Asian—origin and that all existing human P vivax parasites evolved from a single ancestor that spread out of Africa.
The high prevalence of P vivax in wild apes, along with the recent finding of ape P vivax in a European traveler, indicates the existence of a substantial natural reservoir of P vivax in Africa.
Resolving the Duffy-negative paradox
Of the 5 Plasmodium species known to cause malaria in humans, P vivax is the most widespread. Although highly prevalent in Asia and Latin America, P vivax was thought to be absent from west and central Africa due to a mutation that causes the Duffy-negative phenotype in most indigenous African people.
P vivax parasites enter human red blood cells via the Duffy protein receptor. Because the absence of the receptor on the surface of these cells confers protection against P vivax malaria, this parasite has long been suspected to be the agent that selected for this mutation. However, this hypothesis had been difficult to reconcile with the belief that P vivax originated in Asia.
“Our finding that wild-living apes in central Africa show widespread infection with diverse strains of P vivax provides new insight into the evolutionary history of human P vivax and resolves the paradox that a mutation conferring resistance to P vivax occurs with high frequency in the very region where this parasite is absent in humans,” said study author Beatrice Hahn, MD, of the University of Pennsylvania in Philadelphia.
“One interpretation of the relationships that we observed is that a single host switch from apes gave rise to human P vivax, analogous to the origin of human P falciparum,” Dr Sharp added. “However, this seems unlikely in this case, since ape P vivax does not divide into gorilla- and chimpanzee-specific lineages.”
A more plausible scenario, according to the researchers, is that an ancestral P vivax stock was able to infect humans, gorillas, and chimpanzees in Africa until the Duffy-negative mutation started to spread—around 30,000 years ago—and eliminated P vivax from humans there.
Under this scenario, existing human-infecting P vivax is a parasite that survived after spreading out of Africa.
“The existence of a P vivax reservoir within the forests of central Africa has public health implications,” said study author Martine Peeters, PhD, of the Institut de Recherche pour le Développement and the University of Montpellier in France.
“First, it solves the mystery of P vivax infections in travelers returning from regions where 99% of the human population is Duffy-negative. It also raises the possibility that Duffy-positive humans whose work may bring them in close proximity to chimpanzees and gorillas may become infected by ape P vivax. This has already happened once and may happen again, with unknown consequences.”
The investigators are also concerned about the possibility that ape P vivax may spread via international travel to countries where human P vivax is actively transmitted. Since ape P vivax is more genetically diverse than human P vivax, it may have more versatility to escape treatment and prevention measures, especially if human and ape parasites were able to recombine.
Given what biologists know about P vivax’s ability to switch hosts, the researchers suggest it is important to screen Duffy-positive and Duffy-negative humans in west central Africa, as well as transmitting mosquito vectors, for the presence of ape P vivax. The team believes this information is necessary to inform malaria control and eradication efforts of the propensity of ape P vivax to cross over to humans.
The investigators are also planning to compare and contrast the molecular and biological properties of human and ape parasites to identify host-specific interactions and transmission requirements, thereby uncovering vulnerabilities that can be exploited to combat human malaria. ![]()

David Morgan & Crickette Sanz
Investigators have found evidence suggesting the malaria parasite Plasmodium vivax originated in Africa.
Until recently, the closest genetic relatives of human P vivax were found only in Asian macaques, leading researchers to believe that P vivax originated in Asia.
The current study, published in Nature Communications, showed that wild apes in central Africa are widely infected with parasites that are, genetically, nearly identical to human P vivax.
This finding overturns the dogma that P vivax originated in Asia, despite being most prevalent in humans there now, and also solves other questions about P vivax infection.
For example, it explains why the Duffy-null phenotype, which confers resistance to P vivax, is common among people indigenous to Africa. And it explains how travelers returning from regions where most people are Duffy-negative can be infected with P vivax.
Paul Sharp, PhD, of the University of Edinburgh in the UK, and his colleagues conducted this research, testing more than 5000 ape fecal samples from dozens of field stations and sanctuaries in Africa for P vivax DNA.
They found P vivax-like sequences in chimpanzees, western gorillas, and eastern gorillas, but not in bonobos. Ape P vivax was highly prevalent in wild communities, exhibiting infection rates consistent with stable transmission of the parasite within the wild apes.
To examine the evolutionary relationships between ape and human parasites, the researchers generated parasite DNA sequences from wild and sanctuary apes, as well as from a global sampling of human P vivax infections.
They constructed a family tree of the sequences and found that ape and human parasites were very closely related. But ape parasites were more diverse than the human parasites and did not group according to their host species. The human parasites formed a single lineage that fell within the branches of ape parasite sequences.
From these evolutionary relationships, the investigators concluded that P vivax is of African—not Asian—origin and that all existing human P vivax parasites evolved from a single ancestor that spread out of Africa.
The high prevalence of P vivax in wild apes, along with the recent finding of ape P vivax in a European traveler, indicates the existence of a substantial natural reservoir of P vivax in Africa.
Resolving the Duffy-negative paradox
Of the 5 Plasmodium species known to cause malaria in humans, P vivax is the most widespread. Although highly prevalent in Asia and Latin America, P vivax was thought to be absent from west and central Africa due to a mutation that causes the Duffy-negative phenotype in most indigenous African people.
P vivax parasites enter human red blood cells via the Duffy protein receptor. Because the absence of the receptor on the surface of these cells confers protection against P vivax malaria, this parasite has long been suspected to be the agent that selected for this mutation. However, this hypothesis had been difficult to reconcile with the belief that P vivax originated in Asia.
“Our finding that wild-living apes in central Africa show widespread infection with diverse strains of P vivax provides new insight into the evolutionary history of human P vivax and resolves the paradox that a mutation conferring resistance to P vivax occurs with high frequency in the very region where this parasite is absent in humans,” said study author Beatrice Hahn, MD, of the University of Pennsylvania in Philadelphia.
“One interpretation of the relationships that we observed is that a single host switch from apes gave rise to human P vivax, analogous to the origin of human P falciparum,” Dr Sharp added. “However, this seems unlikely in this case, since ape P vivax does not divide into gorilla- and chimpanzee-specific lineages.”
A more plausible scenario, according to the researchers, is that an ancestral P vivax stock was able to infect humans, gorillas, and chimpanzees in Africa until the Duffy-negative mutation started to spread—around 30,000 years ago—and eliminated P vivax from humans there.
Under this scenario, existing human-infecting P vivax is a parasite that survived after spreading out of Africa.
“The existence of a P vivax reservoir within the forests of central Africa has public health implications,” said study author Martine Peeters, PhD, of the Institut de Recherche pour le Développement and the University of Montpellier in France.
“First, it solves the mystery of P vivax infections in travelers returning from regions where 99% of the human population is Duffy-negative. It also raises the possibility that Duffy-positive humans whose work may bring them in close proximity to chimpanzees and gorillas may become infected by ape P vivax. This has already happened once and may happen again, with unknown consequences.”
The investigators are also concerned about the possibility that ape P vivax may spread via international travel to countries where human P vivax is actively transmitted. Since ape P vivax is more genetically diverse than human P vivax, it may have more versatility to escape treatment and prevention measures, especially if human and ape parasites were able to recombine.
Given what biologists know about P vivax’s ability to switch hosts, the researchers suggest it is important to screen Duffy-positive and Duffy-negative humans in west central Africa, as well as transmitting mosquito vectors, for the presence of ape P vivax. The team believes this information is necessary to inform malaria control and eradication efforts of the propensity of ape P vivax to cross over to humans.
The investigators are also planning to compare and contrast the molecular and biological properties of human and ape parasites to identify host-specific interactions and transmission requirements, thereby uncovering vulnerabilities that can be exploited to combat human malaria. ![]()

David Morgan & Crickette Sanz
Investigators have found evidence suggesting the malaria parasite Plasmodium vivax originated in Africa.
Until recently, the closest genetic relatives of human P vivax were found only in Asian macaques, leading researchers to believe that P vivax originated in Asia.
The current study, published in Nature Communications, showed that wild apes in central Africa are widely infected with parasites that are, genetically, nearly identical to human P vivax.
This finding overturns the dogma that P vivax originated in Asia, despite being most prevalent in humans there now, and also solves other questions about P vivax infection.
For example, it explains why the Duffy-null phenotype, which confers resistance to P vivax, is common among people indigenous to Africa. And it explains how travelers returning from regions where most people are Duffy-negative can be infected with P vivax.
Paul Sharp, PhD, of the University of Edinburgh in the UK, and his colleagues conducted this research, testing more than 5000 ape fecal samples from dozens of field stations and sanctuaries in Africa for P vivax DNA.
They found P vivax-like sequences in chimpanzees, western gorillas, and eastern gorillas, but not in bonobos. Ape P vivax was highly prevalent in wild communities, exhibiting infection rates consistent with stable transmission of the parasite within the wild apes.
To examine the evolutionary relationships between ape and human parasites, the researchers generated parasite DNA sequences from wild and sanctuary apes, as well as from a global sampling of human P vivax infections.
They constructed a family tree of the sequences and found that ape and human parasites were very closely related. But ape parasites were more diverse than the human parasites and did not group according to their host species. The human parasites formed a single lineage that fell within the branches of ape parasite sequences.
From these evolutionary relationships, the investigators concluded that P vivax is of African—not Asian—origin and that all existing human P vivax parasites evolved from a single ancestor that spread out of Africa.
The high prevalence of P vivax in wild apes, along with the recent finding of ape P vivax in a European traveler, indicates the existence of a substantial natural reservoir of P vivax in Africa.
Resolving the Duffy-negative paradox
Of the 5 Plasmodium species known to cause malaria in humans, P vivax is the most widespread. Although highly prevalent in Asia and Latin America, P vivax was thought to be absent from west and central Africa due to a mutation that causes the Duffy-negative phenotype in most indigenous African people.
P vivax parasites enter human red blood cells via the Duffy protein receptor. Because the absence of the receptor on the surface of these cells confers protection against P vivax malaria, this parasite has long been suspected to be the agent that selected for this mutation. However, this hypothesis had been difficult to reconcile with the belief that P vivax originated in Asia.
“Our finding that wild-living apes in central Africa show widespread infection with diverse strains of P vivax provides new insight into the evolutionary history of human P vivax and resolves the paradox that a mutation conferring resistance to P vivax occurs with high frequency in the very region where this parasite is absent in humans,” said study author Beatrice Hahn, MD, of the University of Pennsylvania in Philadelphia.
“One interpretation of the relationships that we observed is that a single host switch from apes gave rise to human P vivax, analogous to the origin of human P falciparum,” Dr Sharp added. “However, this seems unlikely in this case, since ape P vivax does not divide into gorilla- and chimpanzee-specific lineages.”
A more plausible scenario, according to the researchers, is that an ancestral P vivax stock was able to infect humans, gorillas, and chimpanzees in Africa until the Duffy-negative mutation started to spread—around 30,000 years ago—and eliminated P vivax from humans there.
Under this scenario, existing human-infecting P vivax is a parasite that survived after spreading out of Africa.
“The existence of a P vivax reservoir within the forests of central Africa has public health implications,” said study author Martine Peeters, PhD, of the Institut de Recherche pour le Développement and the University of Montpellier in France.
“First, it solves the mystery of P vivax infections in travelers returning from regions where 99% of the human population is Duffy-negative. It also raises the possibility that Duffy-positive humans whose work may bring them in close proximity to chimpanzees and gorillas may become infected by ape P vivax. This has already happened once and may happen again, with unknown consequences.”
The investigators are also concerned about the possibility that ape P vivax may spread via international travel to countries where human P vivax is actively transmitted. Since ape P vivax is more genetically diverse than human P vivax, it may have more versatility to escape treatment and prevention measures, especially if human and ape parasites were able to recombine.
Given what biologists know about P vivax’s ability to switch hosts, the researchers suggest it is important to screen Duffy-positive and Duffy-negative humans in west central Africa, as well as transmitting mosquito vectors, for the presence of ape P vivax. The team believes this information is necessary to inform malaria control and eradication efforts of the propensity of ape P vivax to cross over to humans.
The investigators are also planning to compare and contrast the molecular and biological properties of human and ape parasites to identify host-specific interactions and transmission requirements, thereby uncovering vulnerabilities that can be exploited to combat human malaria. ![]()