User login
Rehospitalization after Pneumonia
Pneumonia remains a significant problem in the United States, both in terms of mortality as well as cost to the healthcare system. Each year, over 1 million patients are hospitalized for pneumonia, with costs conservatively estimated at more than $7 billion in 2010.[1] One contributing factor for these costs is that pneumonia is the second most frequent reason for rehospitalization.[2] Rehospitalization after admission for pneumonia is now used as a marker for quality of care.
Given the cost and adverse outcomes of pneumonia, it is important to examine early rehospitalization to better understand what factors play a role. Studies have examined factors associated with readmission following an initial pneumonia‐related hospitalization. Adamuz et al. showed that additional healthcare visits and rehospitalizations within 30 days of discharge from the hospital were common and were associated with worsening of signs or symptoms of pneumonia and/or comorbidities.[3] Other studies demonstrated that instability on hospital discharge, as well as treatment failure, were associated with increased risk of rehospitalization.[4, 5] Jasti et al. found rehospitalizations following pneumonia were usually comorbidity related, primarily cardiopulmonary and/or neurologic disease, rather than due to the index pneumonia.[6] Many of these studies, and others looking at age, activity of daily living score, socioeconomic status, and comorbidity characteristics, were performed in relatively small cohorts. Predictors of rehospitalization have not been studied in a large cohort of patients in an integrated healthcare system.
Our study looks at factors not addressed in prior studies that have used administrative claims data to identify factors associated with early readmission. We also evaluated these admission risk factors in a veteran population, whereas prior studies have primarily focused on those who receive Medicare. The purpose of this study was to examine predictors of early (30 days) readmission in the Veterans Affairs (VA) healthcare system for patients age 65 years and older hospitalized for pneumonia. Our a priori hypothesis was that comorbid illnesses, such as congestive heart failure and chronic obstructive pulmonary disease, and patients with high medical complexity, such as high number of medications and/or prior hospitalizations and nursing home residence, are the primary factors associated with increased risk of rehospitalization.
METHODS
For this national cohort study, we used data from the VA healthcare system administrative and clinical databases that serve as repositories of clinical data from more than 150 VA hospitals and 850 outpatient clinics throughout the United States. The institutional review boards of the University of Texas Health Science Center at San Antonio and VA North Texas Health Care System approved this study. Details regarding the study design and methods were previously published.[7]
Inclusion Criteria
Patients included in this study were hospitalized between October 2001 and September 2007, had a primary diagnosis of pneumonia/emnfluenza (International Classification of Diseases, 9th Revision [ICD‐9] codes 480.0483.99 or 485487) or a secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (ICD‐9 code 518.81) or sepsis (ICD‐9 code 038.xx),[8] and were aged 65 years or older on the date of admission. To ensure patients obtained their care primarily at the VA, at least 1 VA outpatient clinic visit in the year preceding the index admission and at least 1 active and filled outpatient medication from a VA pharmacy within 90 days of admission were required for inclusion.
If a patient was admitted more than once during the study period, only the first hospitalization was included as the index admission.
Exclusion Criteria
Patients who died during the initial hospitalization were not included in this study. Patients admitted to hospitals with fewer than 25 reported hospitalizations during October 2001 to September 2007 were excluded, as these hospitals are neither representative nor generalizable. This resulted in the removal of 59 patients from 5 different hospitals.
Data Sources and Definitions
We used inpatient and outpatient demographic, utilization, and comorbidity data from the National Patient Care Database. Pharmacy data were extracted from the Decision Support System National Data Extracts and Pharmacy Benefits Management. Vital status information was obtained from the Vital Status file, which incorporates data from veterans' death benefits claims, inpatient deaths, Medicare Vital Status files, and the Social Security Administration's death master file. We used encrypted patient identifiers link to information across these databases.
We obtained demographic information (age, sex, race, marital status) from inpatient and outpatient data. We categorized race as white, black, Hispanic, and other/unknown. To infer active smoking and/or tobacco cessation attempts, we identified ICD‐9 codes for tobacco use (305.1, V15.82), smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence (Zyban, nicotine replacement, or varenicline). We used VA priority status as a proxy for socioeconomic and disability status. VA priority groups are a way for the VA to focus limited funds to those veterans most in need. The highest group (priority group 1) must have at least a 50% service‐connected disability. Priority groups 2 through 6 include veterans with up to 40% service‐connected disability, former prisoners of war, those awarded certain honors, veterans with lower incomes, and the catastrophically disabled. The lowest groups (priority groups 7 and 8) include veterans with relatively higher incomes who agree to pay copayments.[9]
We also obtained information on comorbid conditions from inpatient and outpatient administrative data. We defined alcohol abuse using ICD‐9 codes 291, 303, 305.0, and illicit drug use with ICD‐9 codes 292, 304, 305 excluding 305.0‐.1. We used the Charlson Comorbidity Index to quantify levels of preexisting comorbidity[10] adapted for administrative databases, using ICD‐9 codes for 19 comorbid conditions from prior outpatient and inpatient encounters. We defined cardiovascular events and lung cancer that were diagnosed during the hospitalization as previously described.[11, 12]
Outcomes
Our primary study outcome was readmission within 30 days of hospital discharge for pneumonia from any VA acute care hospital only. Medicare has used 30‐day readmission as a quality indicator, as readmissions that occur closer to discharge are believed to be more likely due to events during the index hospitalization.[13]
Statistical Analyses
We randomly divided patients from our initial cohort into equal derivation or validation cohorts. We assessed differences between the 2 groups using the Student t test for continuous variables and 2 test for categorical variables. We performed univariate logistic regression analyses in the derivation cohort to examine the relationship between 30‐day rehospitalization and each of our potential covariates. We entered covariates that were significant at P<0.10 in the univariate analyses into a multiple regression model. Significant covariates at P<0.10 were then entered into the final model. For this model, we used bootstrapping with replacement in 1000 replications to obtain standard errors of our coefficients and associated P values. Because some subjects had more than 1 index admission, we used robust variancecovariance matrix estimators to compute standard errors for model coefficients.
We used the C statistic to assess the discrimination of our model. Calibration of this model was measured using the Hosmer‐Lemeshow 2 goodness of fit test, using 10 quantiles to group the data. We evaluated differences in discrimination between the derivation and validation cohorts by comparing C statistics.
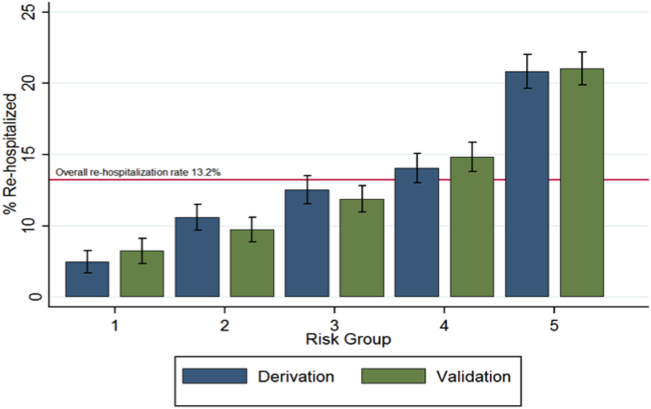
Last, using the final model in the derivation cohort, 5 categories of severity were created based on subjects' predicted risk values for readmission. Severity categories in both the derivation and validation cohorts were then graphically evaluated.
We defined statistical significance as a 2‐tailed P value of <0.05. We used Stata 12 (StataCorp, College Station, TX) for all analyses.
RESULTS
Patient Characteristics
Of the 45,134 eligible patients, 13.2% were rehospitalized within 30 days, and rates by hospital ranged from 1.6% to 20.7%. Table 1 presents the comparison of baseline difference between the derivation and validation cohorts. There were no statistically significant differences between the 2 groups. Overall, the mean age was 77 years, 98% were male, and 54% were married. Over 90% of the patients received guideline‐concordant antibiotics, and only 10% required a stay in the intensive care unit (ICU). The average length of stay was 7 days (standard deviation of 11 days).
| Characteristics | Derivation Cohort, N=22,567 | Validation Cohort, N=22,567 | P Value |
|---|---|---|---|
| |||
| Sociodemographics | |||
| Age, mean (SD) | 77.3 (6.5) | 77.2 (6.5) | 0.30 |
| Male, no (%) | 22,133 (98.1) | 22,175 (98.3) | 0.14 |
| White, no (%) | 18,858 (83.6) | 18,719 (83.0) | 0.08 |
| Black, no (%) | 2,562 (11.4) | 2,583 (11.5) | 0.76 |
| Hispanic, no (%) | 1,337 (5.9) | 1,414 (6.3) | 0.13 |
| Married, no (%) | 12,166 (53.9) | 12,258 (54.3) | 0.39 |
| VA priority group, no (%) | 0.28 | ||
| 1 | 4,286 (19.0) | 4,281 (19.0) | |
| 26 | 16,306 (72.3) | 16,399 (72.8) | |
| 78 | 1,954 (8.7) | 1,860 (8.3) | |
| Nursing home residence, no (%) | 199 (0.9) | 216 (1.0) | 0.41 |
| Smoker status, no (%) | 8,579 (38.0) | 8,677 (38.5) | 0.34 |
| Index hospitalization factors | |||
| Myocardial infarction during hospitalization, no (%) | 469 (2.1) | 452 (2.0) | 0.57 |
| Heart failure during hospitalization, no (%) | 4,817 (21.4) | 4,772 (21.2) | 0.61 |
| Arrhythmia during hospitalization, no (%) | 4,356 (19.3) | 4,287 (19.0) | 0.41 |
| Length of stay, mean (SD) | 7.2 (10.4) | 7.2 (11.7) | 0.76 |
| Lung cancer diagnosis during hospitalization, no (%) | 31 (0.1) | 31 (0.1) | 1.00 |
| ICU admission, no (%) | 2,282 (10.1) | 2,336 (10.4) | 0.40 |
| Guideline concordant antibiotics, no (%) | 20,724 (91.8) | 20,643 (91.5) | 0.17 |
| Invasive mechanical ventilation, no (%) | 793 (3.5) | 832 (3.7) | 0.32 |
| Vasopressor use, no (%) | 495 (2.2) | 489 (2.2) | 0.85 |
| Hospital complications, no (%) | |||
| Renal organ failure | 2,671 (11.8) | 2,640 (11.7) | 0.65 |
| Cardiac organ failure | 1,004 (4.5) | 959 (4.3) | 0.30 |
| Hepatic organ failure | 33 (0.2) | 40 (0.2) | 0.41 |
| Respiratory organ failure | 1,623 (7.2) | 1,583 (7.0) | 0.46 |
| Comorbid illnesses | |||
| Medical, no (%) | |||
| Myocardial infarction | 1,528 (6.8) | 1,542 (6.8) | 0.79 |
| Congestive heart failure | 5,815 (25.8) | 5,697 (25.2) | 0.20 |
| Peripheral vascular disease | 3,413 (15.1) | 3,454 (15.3) | 0.59 |
| Cerebrovascular disease | 3,993 (17.7) | 4,078 (18.1) | 0.30 |
| Dementia | 1,141 (5.1) | 1,110 (4.9) | 0.50 |
| COPD | 12,168 (53.9) | 12,076 (53.5) | 0.39 |
| Rheumatologic disease | 646 (2.9) | 660 (2.9) | 0.69 |
| Peptic ulcer disease | 795 (3.5) | 723 (3.2) | 0.06 |
| Severe liver disease | 169 (0.8) | 169 (0.8) | 1.00 |
| Mild liver disease | 78 (0.4) | 80 (0.4) | 0.87 |
| Diabetes | 7,310 (32.4) | 7,410 (32.8) | 0.32 |
| Diabetes with complications | 2,229 (9.9) | 2,248 (10.0) | 0.77 |
| Chronic renal disease | 2,836 (12.6) | 2,745 (12.2) | 0.19 |
| Hemi/paraplegia | 319 (1.4) | 338 (1.5) | 0.46 |
| Any prior malignancy | 5,226 (23.2) | 5,269 (23.4) | 0.63 |
| Metastatic solid tumor | 748 (3.3) | 795 (3.5) | 0.22 |
| AIDS | 56 (0.3) | 49 (0.2) | 0.49 |
| HIV | 23 (0.1) | 13 (0.1) | 0.10 |
| Alcohol abuse | 897 (4.0) | 934 (4.1) | 0.38 |
| Drug abuse | 254 (1.1) | 255 (1.1) | 0.96 |
| Psychiatric, no (%) | |||
| Anxiety disorder | 1,692 (7.5) | 1,722 (7.6) | 0.59 |
| Depression indicator | 3,655 (16.2) | 3,718 (16.5) | 0.42 |
| Bipolar disorder | 430 (1.9) | 433 (1.9) | 0.92 |
| Cataract indicator | 6,106 (27.1) | 6,180 (27.4) | 0.43 |
| Prostatitis indicator | 4,680 (20.7) | 4,593 (20.4) | 0.31 |
| Schizophrenia indicator | 685 (3.0) | 676 (3.0) | 0.80 |
| Post‐traumatic stress disorder | 844 (3.7) | 862 (3.8) | 0.66 |
| Medication history within 90 days | |||
| Cardiovascular drugs, mean (SD) | 1.8 (1.6) | 1.8 (1.6) | 0.65 |
| Diabetes drugs, mean (SD) | 0.3 (0.7) | 0.3 (0.7) | 0.60 |
| Inhaled corticosteroids, mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.28 |
| Pulmonary drug, mean (SD) | 1.3 (2.0) | 1.3 (2.0) | 0.36 |
| Oral corticosteroids, no (%) | 5,363 (23.8) | 5,505 (24.4) | 0.12 |
| Prior medical utilization | |||
| Number of primary care clinic visits within 1 year, mean (SD) | 4.9 (4.2) | 4.9 (4.2) | 0.67 |
| Number of emergency department visits within 1 year, mean (SD) | 1.2 (2.0) | 1.2 (2.0) | 0.86 |
| Number of outpatient clinic visits within 1 year, mean (SD) | 14.7 (13.1) | 14.6 (13.0) | 0.33 |
| Number of specialty clinic visits within 1 year, mean (SD) | 3.4 (5.5) | 3.3 (5.4) | 0.50 |
| Prior hospital admission within 90 days, no (%) | 5,062 (22.4) | 5,141 (22.8) | 0.37 |
Predictors of 30‐Day Readmission
All variables listed in Table 1 were evaluated. The derivation logistic regression model is shown in Table 2, and only includes predictors associated with rehospitalization. Antibiotic concordance and time in the ICU were evaluated and did not predict readmission. Variables that were significantly associated with rehospitalization in the models included age, marital status, the number of emergency department clinic visits a year prior, prior admission within 90 days, number of nonpharmacy clinic visits in a year prior, and hospital length of stay. The increasing age and number of emergency department and clinic visits were associated with higher odds of readmission. The longer the length of stay and those patients who were married had a lower odds of subsequent readmission. Other variables included were the presence of chronic renal disease, prior malignancy, nursing home residence, congestive heart failure, and prior use of oral corticosteroids. The C statistics for the derivation and validation models were 0.615 and 0.613, respectively. There were no significant differences in any of the variables between the derivation and validation cohorts.
| Predictors | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ||||
| Sociodemographics | ||||
| Age at admission | 1.01 | 1.00 | 1.02 | <0.01 |
| White | 1.14 | 1.02 | 1.28 | 0.02 |
| Nonwhiteb | 1.00 | |||
| Hispanic | 1.26 | 1.07 | 1.47 | 0.01 |
| Not Hispanicc | 1.00 | |||
| Married | 0.89 | 0.82 | 0.96 | <0.01 |
| Not marriedd | 1.00 | |||
| Nursing home residence | 1.68 | 1.18 | 2.39 | <0.01 |
| Comorbid illnesses | ||||
| Peptic ulcer disease | 1.24 | 1.02 | 1.50 | 0.03 |
| Chronic renal disease | 1.18 | 1.06 | 1.33 | <0.01 |
| Any prior malignancy | 1.17 | 1.06 | 1.28 | <0.01 |
| Metastatic solid tumor | 1.20 | 0.98 | 1.47 | 0.08 |
| AIDS | 1.99 | 1.06 | 3.74 | 0.03 |
| Index admission factors | ||||
| Heart failure on initial admission | 1.18 | 1.07 | 1.29 | <0.01 |
| Length of stay (per day) | 0.97 | 0.97 | 0.98 | <0.01 |
| Medication history | ||||
| Cardiovascular drug count 90 days prior | 1.03 | 1.00 | 1.06 | 0.02 |
| Oral corticosteroids 90 days prior | 1.16 | 1.06 | 1.27 | <0.01 |
| Prior medical utilization | ||||
| Number of emergency department visits within 1 year | 1.04 | 1.02 | 1.06 | <0.01 |
| Number of specialty clinic visits within 1 year | 1.01 | 1.00 | 1.02 | 0.01 |
| Prior hospital admission within 90 days | 1.60 | 1.46 | 1.75 | <0.01 |
| Number of nonpharmacy clinic visits within 1 year | 1.00 | 1.00 | 1.01 | <0.01 |
Figure 1 shows that the derivation and validation models are similar in percent rehospitalization group in all severity groups.
DISCUSSION
Sociodemographics and comorbidities, which are host factors, were significantly associated with rehospitalization of elderly patients admitted for pneumonia and length of hospital stay, the only pneumonia‐specific variable associated with readmission.[6, 14, 15, 16] Higher rates of all types of prior utilization (inpatient, outpatient, emergency department visits) were also associated with higher risk of rehospitalization. Our findings highlight the role that host factors play in rehospitalization of elderly patients with pneumonia and that there are few potentially modifiable targets to help reduce readmissions after pneumonia.
These findings may indicate 2 things: patients in this cohort are sicker than the general population at baseline and therefore require more medical care, and/or they are being evaluated by more providers increasing the chance they will be readmitted. For the former, an example is the association of prior use of oral corticosteroids with rehospitalization, which may demonstrate poorly controlled comorbidities (eg, chronic obstructive lung disease with recent exacerbation) that require steroid use. Given the VA's integrated healthcare system, greater access to primary care providers may lead to more opportunities for patients to voice their complaints, which can lead to more readmissions among severely ill patients. Weinberger et al. found an increase in hospital readmissions with increased access to primary care.[17] The association of readmission and length of stay is less likely related to pneumonia‐related clinical instabilities on discharge, but rather to the ability to address other potential issues related to the patient's underlying comorbidities.[5]
Our study presents a model looking at rehospitalization of patients with pneumonia, including factors not addressed in a prior publication by Lindenauer et al., the model Centers for Medicare and Medicaid Services (CMS) uses to predict early rehospitalization.[18] Lindenauer's model included 39 variables (of which 37 were clinical variables), including vertebral fractures, other injuries, other gastrointestinal disorders, other urinary tract disorders, as well as demographic and comorbidity factors that were similar to some of those included in our study. Their 30‐day rehospitalization medical record and administrative models had a C statistic of 0.59 and 0.63, respectively. A major difference between our presented model and the latter are the data on utilization of medical care. Despite these additional data, the resulting C statistics of 0.615 and 0.613 were not qualitatively better.
The similar C statistics demonstrates that factors playing a significant role in determining rehospitalization have yet to be identified, and it is not clear if researchers will be able to identify potentially modifiable risk factors for rehospitalization. Examples of other potential factors that should be examined in future studies include family/community support, quality of the transition of care back to their residence, and quality of inpatient care. Calvillo‐King et al. found a broad range of social factors that potentially affect the risk of postdischarge readmission for chronic heart failure.[19] For pneumonia, there were few studies that assessed the risk of social factors on readmission, and these were most commonly rather broad categories such as race/ethnicity or nursing home residence. In the publications that studied social factors and readmissions, there were inconsistent associations between hospital readmission with lower education, low income, and unemployment. Future articles may benefit from taking these additional factors into account.
Potential limitations to our study include the overwhelming majority of patients being male and in a VA population, which may limit generalizability. In addition, no data were available on the quality of the transition of care. Furthermore, limitations include the age of the data and the lack of evaluation of outpatient follow‐up and antibiotics on discharge. Though our data are up to 10 years old, this study evaluates risk factors that are still relevant. Finally, some variables noted in the CMS model were not evaluated in our study, such as protein‐calorie malnutrition, skin ulcers, and fracture history. Given these differences, we were unable to directly compare the performance of the 2 models.
Despite examining novel factors related to prior healthcare utilization, our model still performed suboptimally in identifying subjects at risk for rehospitalization after pneumonia. Future studies need to examine other social factors and patient frailty that may increase the risk of readmissions, as well as examine the impact of clinical stability on discharge. It remains to be determined if readmissions after pneumonia are truly preventable or mostly attributable to the patient's prior health status.
Disclosures: The project described was supported by grant number R01NR010828 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the VA North Texas Health Care System. Funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
- , , , et al. Incidence and cost of pneumonia in Medicare beneficiaries. Chest. 2012;142(4):973–981.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community‐acquired pneumonia. Respirology. 2011;16(7):1119–1126.
- , , , et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595–600.
- , , , et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278–1284.
- , , , et al. Causes and risk factors for rehospitalization of patients hospitalized with community‐acquired pneumonia. Clin Infect Dis. 2008;46(4):550–556.
- , , , et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55(11):1466–1473.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084.
- U.S. Department of Veterans Affairs. Health benefits. Priority groups table. Available at: http://www.va.gov/healthbenefits/resources/priority_groups.asp. Accessed July 1, 2013.
- , , , et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , , et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med. 2011;124(3):244–251.
- , , , et al. Diagnosis of pulmonary malignancy after hospitalization for pneumonia. Am J Med. 2010;123(1):66–71.
- MedPAC. 2013 [cited 2013 June 11]; Available from: http://www.hospitalcompare.gov.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- , , , et al. Predictors of short‐term rehospitalization following discharge of patients hospitalized with community‐acquired pneumonia. Chest. 2009;136(4):1079–1085.
- , , , et al. Effects of previous influenza vaccination on subsequent readmission and mortality in elderly patients hospitalized with pneumonia. Am J Med. 2003;115(6):454–461.
- , , . Does increased access to primary care reduce hospital readmissions? N Engl J Med. 1996;334(22):1441–1447.
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
Pneumonia remains a significant problem in the United States, both in terms of mortality as well as cost to the healthcare system. Each year, over 1 million patients are hospitalized for pneumonia, with costs conservatively estimated at more than $7 billion in 2010.[1] One contributing factor for these costs is that pneumonia is the second most frequent reason for rehospitalization.[2] Rehospitalization after admission for pneumonia is now used as a marker for quality of care.
Given the cost and adverse outcomes of pneumonia, it is important to examine early rehospitalization to better understand what factors play a role. Studies have examined factors associated with readmission following an initial pneumonia‐related hospitalization. Adamuz et al. showed that additional healthcare visits and rehospitalizations within 30 days of discharge from the hospital were common and were associated with worsening of signs or symptoms of pneumonia and/or comorbidities.[3] Other studies demonstrated that instability on hospital discharge, as well as treatment failure, were associated with increased risk of rehospitalization.[4, 5] Jasti et al. found rehospitalizations following pneumonia were usually comorbidity related, primarily cardiopulmonary and/or neurologic disease, rather than due to the index pneumonia.[6] Many of these studies, and others looking at age, activity of daily living score, socioeconomic status, and comorbidity characteristics, were performed in relatively small cohorts. Predictors of rehospitalization have not been studied in a large cohort of patients in an integrated healthcare system.
Our study looks at factors not addressed in prior studies that have used administrative claims data to identify factors associated with early readmission. We also evaluated these admission risk factors in a veteran population, whereas prior studies have primarily focused on those who receive Medicare. The purpose of this study was to examine predictors of early (30 days) readmission in the Veterans Affairs (VA) healthcare system for patients age 65 years and older hospitalized for pneumonia. Our a priori hypothesis was that comorbid illnesses, such as congestive heart failure and chronic obstructive pulmonary disease, and patients with high medical complexity, such as high number of medications and/or prior hospitalizations and nursing home residence, are the primary factors associated with increased risk of rehospitalization.
METHODS
For this national cohort study, we used data from the VA healthcare system administrative and clinical databases that serve as repositories of clinical data from more than 150 VA hospitals and 850 outpatient clinics throughout the United States. The institutional review boards of the University of Texas Health Science Center at San Antonio and VA North Texas Health Care System approved this study. Details regarding the study design and methods were previously published.[7]
Inclusion Criteria
Patients included in this study were hospitalized between October 2001 and September 2007, had a primary diagnosis of pneumonia/emnfluenza (International Classification of Diseases, 9th Revision [ICD‐9] codes 480.0483.99 or 485487) or a secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (ICD‐9 code 518.81) or sepsis (ICD‐9 code 038.xx),[8] and were aged 65 years or older on the date of admission. To ensure patients obtained their care primarily at the VA, at least 1 VA outpatient clinic visit in the year preceding the index admission and at least 1 active and filled outpatient medication from a VA pharmacy within 90 days of admission were required for inclusion.
If a patient was admitted more than once during the study period, only the first hospitalization was included as the index admission.
Exclusion Criteria
Patients who died during the initial hospitalization were not included in this study. Patients admitted to hospitals with fewer than 25 reported hospitalizations during October 2001 to September 2007 were excluded, as these hospitals are neither representative nor generalizable. This resulted in the removal of 59 patients from 5 different hospitals.
Data Sources and Definitions
We used inpatient and outpatient demographic, utilization, and comorbidity data from the National Patient Care Database. Pharmacy data were extracted from the Decision Support System National Data Extracts and Pharmacy Benefits Management. Vital status information was obtained from the Vital Status file, which incorporates data from veterans' death benefits claims, inpatient deaths, Medicare Vital Status files, and the Social Security Administration's death master file. We used encrypted patient identifiers link to information across these databases.
We obtained demographic information (age, sex, race, marital status) from inpatient and outpatient data. We categorized race as white, black, Hispanic, and other/unknown. To infer active smoking and/or tobacco cessation attempts, we identified ICD‐9 codes for tobacco use (305.1, V15.82), smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence (Zyban, nicotine replacement, or varenicline). We used VA priority status as a proxy for socioeconomic and disability status. VA priority groups are a way for the VA to focus limited funds to those veterans most in need. The highest group (priority group 1) must have at least a 50% service‐connected disability. Priority groups 2 through 6 include veterans with up to 40% service‐connected disability, former prisoners of war, those awarded certain honors, veterans with lower incomes, and the catastrophically disabled. The lowest groups (priority groups 7 and 8) include veterans with relatively higher incomes who agree to pay copayments.[9]
We also obtained information on comorbid conditions from inpatient and outpatient administrative data. We defined alcohol abuse using ICD‐9 codes 291, 303, 305.0, and illicit drug use with ICD‐9 codes 292, 304, 305 excluding 305.0‐.1. We used the Charlson Comorbidity Index to quantify levels of preexisting comorbidity[10] adapted for administrative databases, using ICD‐9 codes for 19 comorbid conditions from prior outpatient and inpatient encounters. We defined cardiovascular events and lung cancer that were diagnosed during the hospitalization as previously described.[11, 12]
Outcomes
Our primary study outcome was readmission within 30 days of hospital discharge for pneumonia from any VA acute care hospital only. Medicare has used 30‐day readmission as a quality indicator, as readmissions that occur closer to discharge are believed to be more likely due to events during the index hospitalization.[13]
Statistical Analyses
We randomly divided patients from our initial cohort into equal derivation or validation cohorts. We assessed differences between the 2 groups using the Student t test for continuous variables and 2 test for categorical variables. We performed univariate logistic regression analyses in the derivation cohort to examine the relationship between 30‐day rehospitalization and each of our potential covariates. We entered covariates that were significant at P<0.10 in the univariate analyses into a multiple regression model. Significant covariates at P<0.10 were then entered into the final model. For this model, we used bootstrapping with replacement in 1000 replications to obtain standard errors of our coefficients and associated P values. Because some subjects had more than 1 index admission, we used robust variancecovariance matrix estimators to compute standard errors for model coefficients.
We used the C statistic to assess the discrimination of our model. Calibration of this model was measured using the Hosmer‐Lemeshow 2 goodness of fit test, using 10 quantiles to group the data. We evaluated differences in discrimination between the derivation and validation cohorts by comparing C statistics.
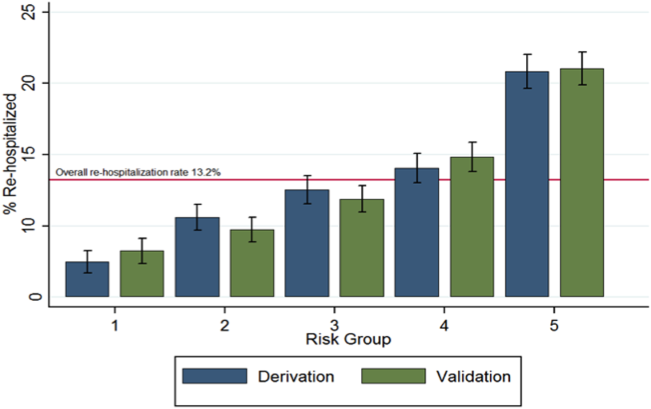
Last, using the final model in the derivation cohort, 5 categories of severity were created based on subjects' predicted risk values for readmission. Severity categories in both the derivation and validation cohorts were then graphically evaluated.
We defined statistical significance as a 2‐tailed P value of <0.05. We used Stata 12 (StataCorp, College Station, TX) for all analyses.
RESULTS
Patient Characteristics
Of the 45,134 eligible patients, 13.2% were rehospitalized within 30 days, and rates by hospital ranged from 1.6% to 20.7%. Table 1 presents the comparison of baseline difference between the derivation and validation cohorts. There were no statistically significant differences between the 2 groups. Overall, the mean age was 77 years, 98% were male, and 54% were married. Over 90% of the patients received guideline‐concordant antibiotics, and only 10% required a stay in the intensive care unit (ICU). The average length of stay was 7 days (standard deviation of 11 days).
| Characteristics | Derivation Cohort, N=22,567 | Validation Cohort, N=22,567 | P Value |
|---|---|---|---|
| |||
| Sociodemographics | |||
| Age, mean (SD) | 77.3 (6.5) | 77.2 (6.5) | 0.30 |
| Male, no (%) | 22,133 (98.1) | 22,175 (98.3) | 0.14 |
| White, no (%) | 18,858 (83.6) | 18,719 (83.0) | 0.08 |
| Black, no (%) | 2,562 (11.4) | 2,583 (11.5) | 0.76 |
| Hispanic, no (%) | 1,337 (5.9) | 1,414 (6.3) | 0.13 |
| Married, no (%) | 12,166 (53.9) | 12,258 (54.3) | 0.39 |
| VA priority group, no (%) | 0.28 | ||
| 1 | 4,286 (19.0) | 4,281 (19.0) | |
| 26 | 16,306 (72.3) | 16,399 (72.8) | |
| 78 | 1,954 (8.7) | 1,860 (8.3) | |
| Nursing home residence, no (%) | 199 (0.9) | 216 (1.0) | 0.41 |
| Smoker status, no (%) | 8,579 (38.0) | 8,677 (38.5) | 0.34 |
| Index hospitalization factors | |||
| Myocardial infarction during hospitalization, no (%) | 469 (2.1) | 452 (2.0) | 0.57 |
| Heart failure during hospitalization, no (%) | 4,817 (21.4) | 4,772 (21.2) | 0.61 |
| Arrhythmia during hospitalization, no (%) | 4,356 (19.3) | 4,287 (19.0) | 0.41 |
| Length of stay, mean (SD) | 7.2 (10.4) | 7.2 (11.7) | 0.76 |
| Lung cancer diagnosis during hospitalization, no (%) | 31 (0.1) | 31 (0.1) | 1.00 |
| ICU admission, no (%) | 2,282 (10.1) | 2,336 (10.4) | 0.40 |
| Guideline concordant antibiotics, no (%) | 20,724 (91.8) | 20,643 (91.5) | 0.17 |
| Invasive mechanical ventilation, no (%) | 793 (3.5) | 832 (3.7) | 0.32 |
| Vasopressor use, no (%) | 495 (2.2) | 489 (2.2) | 0.85 |
| Hospital complications, no (%) | |||
| Renal organ failure | 2,671 (11.8) | 2,640 (11.7) | 0.65 |
| Cardiac organ failure | 1,004 (4.5) | 959 (4.3) | 0.30 |
| Hepatic organ failure | 33 (0.2) | 40 (0.2) | 0.41 |
| Respiratory organ failure | 1,623 (7.2) | 1,583 (7.0) | 0.46 |
| Comorbid illnesses | |||
| Medical, no (%) | |||
| Myocardial infarction | 1,528 (6.8) | 1,542 (6.8) | 0.79 |
| Congestive heart failure | 5,815 (25.8) | 5,697 (25.2) | 0.20 |
| Peripheral vascular disease | 3,413 (15.1) | 3,454 (15.3) | 0.59 |
| Cerebrovascular disease | 3,993 (17.7) | 4,078 (18.1) | 0.30 |
| Dementia | 1,141 (5.1) | 1,110 (4.9) | 0.50 |
| COPD | 12,168 (53.9) | 12,076 (53.5) | 0.39 |
| Rheumatologic disease | 646 (2.9) | 660 (2.9) | 0.69 |
| Peptic ulcer disease | 795 (3.5) | 723 (3.2) | 0.06 |
| Severe liver disease | 169 (0.8) | 169 (0.8) | 1.00 |
| Mild liver disease | 78 (0.4) | 80 (0.4) | 0.87 |
| Diabetes | 7,310 (32.4) | 7,410 (32.8) | 0.32 |
| Diabetes with complications | 2,229 (9.9) | 2,248 (10.0) | 0.77 |
| Chronic renal disease | 2,836 (12.6) | 2,745 (12.2) | 0.19 |
| Hemi/paraplegia | 319 (1.4) | 338 (1.5) | 0.46 |
| Any prior malignancy | 5,226 (23.2) | 5,269 (23.4) | 0.63 |
| Metastatic solid tumor | 748 (3.3) | 795 (3.5) | 0.22 |
| AIDS | 56 (0.3) | 49 (0.2) | 0.49 |
| HIV | 23 (0.1) | 13 (0.1) | 0.10 |
| Alcohol abuse | 897 (4.0) | 934 (4.1) | 0.38 |
| Drug abuse | 254 (1.1) | 255 (1.1) | 0.96 |
| Psychiatric, no (%) | |||
| Anxiety disorder | 1,692 (7.5) | 1,722 (7.6) | 0.59 |
| Depression indicator | 3,655 (16.2) | 3,718 (16.5) | 0.42 |
| Bipolar disorder | 430 (1.9) | 433 (1.9) | 0.92 |
| Cataract indicator | 6,106 (27.1) | 6,180 (27.4) | 0.43 |
| Prostatitis indicator | 4,680 (20.7) | 4,593 (20.4) | 0.31 |
| Schizophrenia indicator | 685 (3.0) | 676 (3.0) | 0.80 |
| Post‐traumatic stress disorder | 844 (3.7) | 862 (3.8) | 0.66 |
| Medication history within 90 days | |||
| Cardiovascular drugs, mean (SD) | 1.8 (1.6) | 1.8 (1.6) | 0.65 |
| Diabetes drugs, mean (SD) | 0.3 (0.7) | 0.3 (0.7) | 0.60 |
| Inhaled corticosteroids, mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.28 |
| Pulmonary drug, mean (SD) | 1.3 (2.0) | 1.3 (2.0) | 0.36 |
| Oral corticosteroids, no (%) | 5,363 (23.8) | 5,505 (24.4) | 0.12 |
| Prior medical utilization | |||
| Number of primary care clinic visits within 1 year, mean (SD) | 4.9 (4.2) | 4.9 (4.2) | 0.67 |
| Number of emergency department visits within 1 year, mean (SD) | 1.2 (2.0) | 1.2 (2.0) | 0.86 |
| Number of outpatient clinic visits within 1 year, mean (SD) | 14.7 (13.1) | 14.6 (13.0) | 0.33 |
| Number of specialty clinic visits within 1 year, mean (SD) | 3.4 (5.5) | 3.3 (5.4) | 0.50 |
| Prior hospital admission within 90 days, no (%) | 5,062 (22.4) | 5,141 (22.8) | 0.37 |
Predictors of 30‐Day Readmission
All variables listed in Table 1 were evaluated. The derivation logistic regression model is shown in Table 2, and only includes predictors associated with rehospitalization. Antibiotic concordance and time in the ICU were evaluated and did not predict readmission. Variables that were significantly associated with rehospitalization in the models included age, marital status, the number of emergency department clinic visits a year prior, prior admission within 90 days, number of nonpharmacy clinic visits in a year prior, and hospital length of stay. The increasing age and number of emergency department and clinic visits were associated with higher odds of readmission. The longer the length of stay and those patients who were married had a lower odds of subsequent readmission. Other variables included were the presence of chronic renal disease, prior malignancy, nursing home residence, congestive heart failure, and prior use of oral corticosteroids. The C statistics for the derivation and validation models were 0.615 and 0.613, respectively. There were no significant differences in any of the variables between the derivation and validation cohorts.
| Predictors | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ||||
| Sociodemographics | ||||
| Age at admission | 1.01 | 1.00 | 1.02 | <0.01 |
| White | 1.14 | 1.02 | 1.28 | 0.02 |
| Nonwhiteb | 1.00 | |||
| Hispanic | 1.26 | 1.07 | 1.47 | 0.01 |
| Not Hispanicc | 1.00 | |||
| Married | 0.89 | 0.82 | 0.96 | <0.01 |
| Not marriedd | 1.00 | |||
| Nursing home residence | 1.68 | 1.18 | 2.39 | <0.01 |
| Comorbid illnesses | ||||
| Peptic ulcer disease | 1.24 | 1.02 | 1.50 | 0.03 |
| Chronic renal disease | 1.18 | 1.06 | 1.33 | <0.01 |
| Any prior malignancy | 1.17 | 1.06 | 1.28 | <0.01 |
| Metastatic solid tumor | 1.20 | 0.98 | 1.47 | 0.08 |
| AIDS | 1.99 | 1.06 | 3.74 | 0.03 |
| Index admission factors | ||||
| Heart failure on initial admission | 1.18 | 1.07 | 1.29 | <0.01 |
| Length of stay (per day) | 0.97 | 0.97 | 0.98 | <0.01 |
| Medication history | ||||
| Cardiovascular drug count 90 days prior | 1.03 | 1.00 | 1.06 | 0.02 |
| Oral corticosteroids 90 days prior | 1.16 | 1.06 | 1.27 | <0.01 |
| Prior medical utilization | ||||
| Number of emergency department visits within 1 year | 1.04 | 1.02 | 1.06 | <0.01 |
| Number of specialty clinic visits within 1 year | 1.01 | 1.00 | 1.02 | 0.01 |
| Prior hospital admission within 90 days | 1.60 | 1.46 | 1.75 | <0.01 |
| Number of nonpharmacy clinic visits within 1 year | 1.00 | 1.00 | 1.01 | <0.01 |
Figure 1 shows that the derivation and validation models are similar in percent rehospitalization group in all severity groups.

DISCUSSION
Sociodemographics and comorbidities, which are host factors, were significantly associated with rehospitalization of elderly patients admitted for pneumonia and length of hospital stay, the only pneumonia‐specific variable associated with readmission.[6, 14, 15, 16] Higher rates of all types of prior utilization (inpatient, outpatient, emergency department visits) were also associated with higher risk of rehospitalization. Our findings highlight the role that host factors play in rehospitalization of elderly patients with pneumonia and that there are few potentially modifiable targets to help reduce readmissions after pneumonia.
These findings may indicate 2 things: patients in this cohort are sicker than the general population at baseline and therefore require more medical care, and/or they are being evaluated by more providers increasing the chance they will be readmitted. For the former, an example is the association of prior use of oral corticosteroids with rehospitalization, which may demonstrate poorly controlled comorbidities (eg, chronic obstructive lung disease with recent exacerbation) that require steroid use. Given the VA's integrated healthcare system, greater access to primary care providers may lead to more opportunities for patients to voice their complaints, which can lead to more readmissions among severely ill patients. Weinberger et al. found an increase in hospital readmissions with increased access to primary care.[17] The association of readmission and length of stay is less likely related to pneumonia‐related clinical instabilities on discharge, but rather to the ability to address other potential issues related to the patient's underlying comorbidities.[5]
Our study presents a model looking at rehospitalization of patients with pneumonia, including factors not addressed in a prior publication by Lindenauer et al., the model Centers for Medicare and Medicaid Services (CMS) uses to predict early rehospitalization.[18] Lindenauer's model included 39 variables (of which 37 were clinical variables), including vertebral fractures, other injuries, other gastrointestinal disorders, other urinary tract disorders, as well as demographic and comorbidity factors that were similar to some of those included in our study. Their 30‐day rehospitalization medical record and administrative models had a C statistic of 0.59 and 0.63, respectively. A major difference between our presented model and the latter are the data on utilization of medical care. Despite these additional data, the resulting C statistics of 0.615 and 0.613 were not qualitatively better.
The similar C statistics demonstrates that factors playing a significant role in determining rehospitalization have yet to be identified, and it is not clear if researchers will be able to identify potentially modifiable risk factors for rehospitalization. Examples of other potential factors that should be examined in future studies include family/community support, quality of the transition of care back to their residence, and quality of inpatient care. Calvillo‐King et al. found a broad range of social factors that potentially affect the risk of postdischarge readmission for chronic heart failure.[19] For pneumonia, there were few studies that assessed the risk of social factors on readmission, and these were most commonly rather broad categories such as race/ethnicity or nursing home residence. In the publications that studied social factors and readmissions, there were inconsistent associations between hospital readmission with lower education, low income, and unemployment. Future articles may benefit from taking these additional factors into account.
Potential limitations to our study include the overwhelming majority of patients being male and in a VA population, which may limit generalizability. In addition, no data were available on the quality of the transition of care. Furthermore, limitations include the age of the data and the lack of evaluation of outpatient follow‐up and antibiotics on discharge. Though our data are up to 10 years old, this study evaluates risk factors that are still relevant. Finally, some variables noted in the CMS model were not evaluated in our study, such as protein‐calorie malnutrition, skin ulcers, and fracture history. Given these differences, we were unable to directly compare the performance of the 2 models.
Despite examining novel factors related to prior healthcare utilization, our model still performed suboptimally in identifying subjects at risk for rehospitalization after pneumonia. Future studies need to examine other social factors and patient frailty that may increase the risk of readmissions, as well as examine the impact of clinical stability on discharge. It remains to be determined if readmissions after pneumonia are truly preventable or mostly attributable to the patient's prior health status.
Disclosures: The project described was supported by grant number R01NR010828 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the VA North Texas Health Care System. Funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Pneumonia remains a significant problem in the United States, both in terms of mortality as well as cost to the healthcare system. Each year, over 1 million patients are hospitalized for pneumonia, with costs conservatively estimated at more than $7 billion in 2010.[1] One contributing factor for these costs is that pneumonia is the second most frequent reason for rehospitalization.[2] Rehospitalization after admission for pneumonia is now used as a marker for quality of care.
Given the cost and adverse outcomes of pneumonia, it is important to examine early rehospitalization to better understand what factors play a role. Studies have examined factors associated with readmission following an initial pneumonia‐related hospitalization. Adamuz et al. showed that additional healthcare visits and rehospitalizations within 30 days of discharge from the hospital were common and were associated with worsening of signs or symptoms of pneumonia and/or comorbidities.[3] Other studies demonstrated that instability on hospital discharge, as well as treatment failure, were associated with increased risk of rehospitalization.[4, 5] Jasti et al. found rehospitalizations following pneumonia were usually comorbidity related, primarily cardiopulmonary and/or neurologic disease, rather than due to the index pneumonia.[6] Many of these studies, and others looking at age, activity of daily living score, socioeconomic status, and comorbidity characteristics, were performed in relatively small cohorts. Predictors of rehospitalization have not been studied in a large cohort of patients in an integrated healthcare system.
Our study looks at factors not addressed in prior studies that have used administrative claims data to identify factors associated with early readmission. We also evaluated these admission risk factors in a veteran population, whereas prior studies have primarily focused on those who receive Medicare. The purpose of this study was to examine predictors of early (30 days) readmission in the Veterans Affairs (VA) healthcare system for patients age 65 years and older hospitalized for pneumonia. Our a priori hypothesis was that comorbid illnesses, such as congestive heart failure and chronic obstructive pulmonary disease, and patients with high medical complexity, such as high number of medications and/or prior hospitalizations and nursing home residence, are the primary factors associated with increased risk of rehospitalization.
METHODS
For this national cohort study, we used data from the VA healthcare system administrative and clinical databases that serve as repositories of clinical data from more than 150 VA hospitals and 850 outpatient clinics throughout the United States. The institutional review boards of the University of Texas Health Science Center at San Antonio and VA North Texas Health Care System approved this study. Details regarding the study design and methods were previously published.[7]
Inclusion Criteria
Patients included in this study were hospitalized between October 2001 and September 2007, had a primary diagnosis of pneumonia/emnfluenza (International Classification of Diseases, 9th Revision [ICD‐9] codes 480.0483.99 or 485487) or a secondary discharge diagnosis of pneumonia with a primary diagnosis of respiratory failure (ICD‐9 code 518.81) or sepsis (ICD‐9 code 038.xx),[8] and were aged 65 years or older on the date of admission. To ensure patients obtained their care primarily at the VA, at least 1 VA outpatient clinic visit in the year preceding the index admission and at least 1 active and filled outpatient medication from a VA pharmacy within 90 days of admission were required for inclusion.
If a patient was admitted more than once during the study period, only the first hospitalization was included as the index admission.
Exclusion Criteria
Patients who died during the initial hospitalization were not included in this study. Patients admitted to hospitals with fewer than 25 reported hospitalizations during October 2001 to September 2007 were excluded, as these hospitals are neither representative nor generalizable. This resulted in the removal of 59 patients from 5 different hospitals.
Data Sources and Definitions
We used inpatient and outpatient demographic, utilization, and comorbidity data from the National Patient Care Database. Pharmacy data were extracted from the Decision Support System National Data Extracts and Pharmacy Benefits Management. Vital status information was obtained from the Vital Status file, which incorporates data from veterans' death benefits claims, inpatient deaths, Medicare Vital Status files, and the Social Security Administration's death master file. We used encrypted patient identifiers link to information across these databases.
We obtained demographic information (age, sex, race, marital status) from inpatient and outpatient data. We categorized race as white, black, Hispanic, and other/unknown. To infer active smoking and/or tobacco cessation attempts, we identified ICD‐9 codes for tobacco use (305.1, V15.82), smoking cessation clinic use, and/or use of medications for the treatment of nicotine dependence (Zyban, nicotine replacement, or varenicline). We used VA priority status as a proxy for socioeconomic and disability status. VA priority groups are a way for the VA to focus limited funds to those veterans most in need. The highest group (priority group 1) must have at least a 50% service‐connected disability. Priority groups 2 through 6 include veterans with up to 40% service‐connected disability, former prisoners of war, those awarded certain honors, veterans with lower incomes, and the catastrophically disabled. The lowest groups (priority groups 7 and 8) include veterans with relatively higher incomes who agree to pay copayments.[9]
We also obtained information on comorbid conditions from inpatient and outpatient administrative data. We defined alcohol abuse using ICD‐9 codes 291, 303, 305.0, and illicit drug use with ICD‐9 codes 292, 304, 305 excluding 305.0‐.1. We used the Charlson Comorbidity Index to quantify levels of preexisting comorbidity[10] adapted for administrative databases, using ICD‐9 codes for 19 comorbid conditions from prior outpatient and inpatient encounters. We defined cardiovascular events and lung cancer that were diagnosed during the hospitalization as previously described.[11, 12]
Outcomes
Our primary study outcome was readmission within 30 days of hospital discharge for pneumonia from any VA acute care hospital only. Medicare has used 30‐day readmission as a quality indicator, as readmissions that occur closer to discharge are believed to be more likely due to events during the index hospitalization.[13]
Statistical Analyses
We randomly divided patients from our initial cohort into equal derivation or validation cohorts. We assessed differences between the 2 groups using the Student t test for continuous variables and 2 test for categorical variables. We performed univariate logistic regression analyses in the derivation cohort to examine the relationship between 30‐day rehospitalization and each of our potential covariates. We entered covariates that were significant at P<0.10 in the univariate analyses into a multiple regression model. Significant covariates at P<0.10 were then entered into the final model. For this model, we used bootstrapping with replacement in 1000 replications to obtain standard errors of our coefficients and associated P values. Because some subjects had more than 1 index admission, we used robust variancecovariance matrix estimators to compute standard errors for model coefficients.
We used the C statistic to assess the discrimination of our model. Calibration of this model was measured using the Hosmer‐Lemeshow 2 goodness of fit test, using 10 quantiles to group the data. We evaluated differences in discrimination between the derivation and validation cohorts by comparing C statistics.
Last, using the final model in the derivation cohort, 5 categories of severity were created based on subjects' predicted risk values for readmission. Severity categories in both the derivation and validation cohorts were then graphically evaluated.
We defined statistical significance as a 2‐tailed P value of <0.05. We used Stata 12 (StataCorp, College Station, TX) for all analyses.
RESULTS
Patient Characteristics
Of the 45,134 eligible patients, 13.2% were rehospitalized within 30 days, and rates by hospital ranged from 1.6% to 20.7%. Table 1 presents the comparison of baseline difference between the derivation and validation cohorts. There were no statistically significant differences between the 2 groups. Overall, the mean age was 77 years, 98% were male, and 54% were married. Over 90% of the patients received guideline‐concordant antibiotics, and only 10% required a stay in the intensive care unit (ICU). The average length of stay was 7 days (standard deviation of 11 days).
| Characteristics | Derivation Cohort, N=22,567 | Validation Cohort, N=22,567 | P Value |
|---|---|---|---|
| |||
| Sociodemographics | |||
| Age, mean (SD) | 77.3 (6.5) | 77.2 (6.5) | 0.30 |
| Male, no (%) | 22,133 (98.1) | 22,175 (98.3) | 0.14 |
| White, no (%) | 18,858 (83.6) | 18,719 (83.0) | 0.08 |
| Black, no (%) | 2,562 (11.4) | 2,583 (11.5) | 0.76 |
| Hispanic, no (%) | 1,337 (5.9) | 1,414 (6.3) | 0.13 |
| Married, no (%) | 12,166 (53.9) | 12,258 (54.3) | 0.39 |
| VA priority group, no (%) | 0.28 | ||
| 1 | 4,286 (19.0) | 4,281 (19.0) | |
| 26 | 16,306 (72.3) | 16,399 (72.8) | |
| 78 | 1,954 (8.7) | 1,860 (8.3) | |
| Nursing home residence, no (%) | 199 (0.9) | 216 (1.0) | 0.41 |
| Smoker status, no (%) | 8,579 (38.0) | 8,677 (38.5) | 0.34 |
| Index hospitalization factors | |||
| Myocardial infarction during hospitalization, no (%) | 469 (2.1) | 452 (2.0) | 0.57 |
| Heart failure during hospitalization, no (%) | 4,817 (21.4) | 4,772 (21.2) | 0.61 |
| Arrhythmia during hospitalization, no (%) | 4,356 (19.3) | 4,287 (19.0) | 0.41 |
| Length of stay, mean (SD) | 7.2 (10.4) | 7.2 (11.7) | 0.76 |
| Lung cancer diagnosis during hospitalization, no (%) | 31 (0.1) | 31 (0.1) | 1.00 |
| ICU admission, no (%) | 2,282 (10.1) | 2,336 (10.4) | 0.40 |
| Guideline concordant antibiotics, no (%) | 20,724 (91.8) | 20,643 (91.5) | 0.17 |
| Invasive mechanical ventilation, no (%) | 793 (3.5) | 832 (3.7) | 0.32 |
| Vasopressor use, no (%) | 495 (2.2) | 489 (2.2) | 0.85 |
| Hospital complications, no (%) | |||
| Renal organ failure | 2,671 (11.8) | 2,640 (11.7) | 0.65 |
| Cardiac organ failure | 1,004 (4.5) | 959 (4.3) | 0.30 |
| Hepatic organ failure | 33 (0.2) | 40 (0.2) | 0.41 |
| Respiratory organ failure | 1,623 (7.2) | 1,583 (7.0) | 0.46 |
| Comorbid illnesses | |||
| Medical, no (%) | |||
| Myocardial infarction | 1,528 (6.8) | 1,542 (6.8) | 0.79 |
| Congestive heart failure | 5,815 (25.8) | 5,697 (25.2) | 0.20 |
| Peripheral vascular disease | 3,413 (15.1) | 3,454 (15.3) | 0.59 |
| Cerebrovascular disease | 3,993 (17.7) | 4,078 (18.1) | 0.30 |
| Dementia | 1,141 (5.1) | 1,110 (4.9) | 0.50 |
| COPD | 12,168 (53.9) | 12,076 (53.5) | 0.39 |
| Rheumatologic disease | 646 (2.9) | 660 (2.9) | 0.69 |
| Peptic ulcer disease | 795 (3.5) | 723 (3.2) | 0.06 |
| Severe liver disease | 169 (0.8) | 169 (0.8) | 1.00 |
| Mild liver disease | 78 (0.4) | 80 (0.4) | 0.87 |
| Diabetes | 7,310 (32.4) | 7,410 (32.8) | 0.32 |
| Diabetes with complications | 2,229 (9.9) | 2,248 (10.0) | 0.77 |
| Chronic renal disease | 2,836 (12.6) | 2,745 (12.2) | 0.19 |
| Hemi/paraplegia | 319 (1.4) | 338 (1.5) | 0.46 |
| Any prior malignancy | 5,226 (23.2) | 5,269 (23.4) | 0.63 |
| Metastatic solid tumor | 748 (3.3) | 795 (3.5) | 0.22 |
| AIDS | 56 (0.3) | 49 (0.2) | 0.49 |
| HIV | 23 (0.1) | 13 (0.1) | 0.10 |
| Alcohol abuse | 897 (4.0) | 934 (4.1) | 0.38 |
| Drug abuse | 254 (1.1) | 255 (1.1) | 0.96 |
| Psychiatric, no (%) | |||
| Anxiety disorder | 1,692 (7.5) | 1,722 (7.6) | 0.59 |
| Depression indicator | 3,655 (16.2) | 3,718 (16.5) | 0.42 |
| Bipolar disorder | 430 (1.9) | 433 (1.9) | 0.92 |
| Cataract indicator | 6,106 (27.1) | 6,180 (27.4) | 0.43 |
| Prostatitis indicator | 4,680 (20.7) | 4,593 (20.4) | 0.31 |
| Schizophrenia indicator | 685 (3.0) | 676 (3.0) | 0.80 |
| Post‐traumatic stress disorder | 844 (3.7) | 862 (3.8) | 0.66 |
| Medication history within 90 days | |||
| Cardiovascular drugs, mean (SD) | 1.8 (1.6) | 1.8 (1.6) | 0.65 |
| Diabetes drugs, mean (SD) | 0.3 (0.7) | 0.3 (0.7) | 0.60 |
| Inhaled corticosteroids, mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.28 |
| Pulmonary drug, mean (SD) | 1.3 (2.0) | 1.3 (2.0) | 0.36 |
| Oral corticosteroids, no (%) | 5,363 (23.8) | 5,505 (24.4) | 0.12 |
| Prior medical utilization | |||
| Number of primary care clinic visits within 1 year, mean (SD) | 4.9 (4.2) | 4.9 (4.2) | 0.67 |
| Number of emergency department visits within 1 year, mean (SD) | 1.2 (2.0) | 1.2 (2.0) | 0.86 |
| Number of outpatient clinic visits within 1 year, mean (SD) | 14.7 (13.1) | 14.6 (13.0) | 0.33 |
| Number of specialty clinic visits within 1 year, mean (SD) | 3.4 (5.5) | 3.3 (5.4) | 0.50 |
| Prior hospital admission within 90 days, no (%) | 5,062 (22.4) | 5,141 (22.8) | 0.37 |
Predictors of 30‐Day Readmission
All variables listed in Table 1 were evaluated. The derivation logistic regression model is shown in Table 2, and only includes predictors associated with rehospitalization. Antibiotic concordance and time in the ICU were evaluated and did not predict readmission. Variables that were significantly associated with rehospitalization in the models included age, marital status, the number of emergency department clinic visits a year prior, prior admission within 90 days, number of nonpharmacy clinic visits in a year prior, and hospital length of stay. The increasing age and number of emergency department and clinic visits were associated with higher odds of readmission. The longer the length of stay and those patients who were married had a lower odds of subsequent readmission. Other variables included were the presence of chronic renal disease, prior malignancy, nursing home residence, congestive heart failure, and prior use of oral corticosteroids. The C statistics for the derivation and validation models were 0.615 and 0.613, respectively. There were no significant differences in any of the variables between the derivation and validation cohorts.
| Predictors | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ||||
| Sociodemographics | ||||
| Age at admission | 1.01 | 1.00 | 1.02 | <0.01 |
| White | 1.14 | 1.02 | 1.28 | 0.02 |
| Nonwhiteb | 1.00 | |||
| Hispanic | 1.26 | 1.07 | 1.47 | 0.01 |
| Not Hispanicc | 1.00 | |||
| Married | 0.89 | 0.82 | 0.96 | <0.01 |
| Not marriedd | 1.00 | |||
| Nursing home residence | 1.68 | 1.18 | 2.39 | <0.01 |
| Comorbid illnesses | ||||
| Peptic ulcer disease | 1.24 | 1.02 | 1.50 | 0.03 |
| Chronic renal disease | 1.18 | 1.06 | 1.33 | <0.01 |
| Any prior malignancy | 1.17 | 1.06 | 1.28 | <0.01 |
| Metastatic solid tumor | 1.20 | 0.98 | 1.47 | 0.08 |
| AIDS | 1.99 | 1.06 | 3.74 | 0.03 |
| Index admission factors | ||||
| Heart failure on initial admission | 1.18 | 1.07 | 1.29 | <0.01 |
| Length of stay (per day) | 0.97 | 0.97 | 0.98 | <0.01 |
| Medication history | ||||
| Cardiovascular drug count 90 days prior | 1.03 | 1.00 | 1.06 | 0.02 |
| Oral corticosteroids 90 days prior | 1.16 | 1.06 | 1.27 | <0.01 |
| Prior medical utilization | ||||
| Number of emergency department visits within 1 year | 1.04 | 1.02 | 1.06 | <0.01 |
| Number of specialty clinic visits within 1 year | 1.01 | 1.00 | 1.02 | 0.01 |
| Prior hospital admission within 90 days | 1.60 | 1.46 | 1.75 | <0.01 |
| Number of nonpharmacy clinic visits within 1 year | 1.00 | 1.00 | 1.01 | <0.01 |
Figure 1 shows that the derivation and validation models are similar in percent rehospitalization group in all severity groups.

DISCUSSION
Sociodemographics and comorbidities, which are host factors, were significantly associated with rehospitalization of elderly patients admitted for pneumonia and length of hospital stay, the only pneumonia‐specific variable associated with readmission.[6, 14, 15, 16] Higher rates of all types of prior utilization (inpatient, outpatient, emergency department visits) were also associated with higher risk of rehospitalization. Our findings highlight the role that host factors play in rehospitalization of elderly patients with pneumonia and that there are few potentially modifiable targets to help reduce readmissions after pneumonia.
These findings may indicate 2 things: patients in this cohort are sicker than the general population at baseline and therefore require more medical care, and/or they are being evaluated by more providers increasing the chance they will be readmitted. For the former, an example is the association of prior use of oral corticosteroids with rehospitalization, which may demonstrate poorly controlled comorbidities (eg, chronic obstructive lung disease with recent exacerbation) that require steroid use. Given the VA's integrated healthcare system, greater access to primary care providers may lead to more opportunities for patients to voice their complaints, which can lead to more readmissions among severely ill patients. Weinberger et al. found an increase in hospital readmissions with increased access to primary care.[17] The association of readmission and length of stay is less likely related to pneumonia‐related clinical instabilities on discharge, but rather to the ability to address other potential issues related to the patient's underlying comorbidities.[5]
Our study presents a model looking at rehospitalization of patients with pneumonia, including factors not addressed in a prior publication by Lindenauer et al., the model Centers for Medicare and Medicaid Services (CMS) uses to predict early rehospitalization.[18] Lindenauer's model included 39 variables (of which 37 were clinical variables), including vertebral fractures, other injuries, other gastrointestinal disorders, other urinary tract disorders, as well as demographic and comorbidity factors that were similar to some of those included in our study. Their 30‐day rehospitalization medical record and administrative models had a C statistic of 0.59 and 0.63, respectively. A major difference between our presented model and the latter are the data on utilization of medical care. Despite these additional data, the resulting C statistics of 0.615 and 0.613 were not qualitatively better.
The similar C statistics demonstrates that factors playing a significant role in determining rehospitalization have yet to be identified, and it is not clear if researchers will be able to identify potentially modifiable risk factors for rehospitalization. Examples of other potential factors that should be examined in future studies include family/community support, quality of the transition of care back to their residence, and quality of inpatient care. Calvillo‐King et al. found a broad range of social factors that potentially affect the risk of postdischarge readmission for chronic heart failure.[19] For pneumonia, there were few studies that assessed the risk of social factors on readmission, and these were most commonly rather broad categories such as race/ethnicity or nursing home residence. In the publications that studied social factors and readmissions, there were inconsistent associations between hospital readmission with lower education, low income, and unemployment. Future articles may benefit from taking these additional factors into account.
Potential limitations to our study include the overwhelming majority of patients being male and in a VA population, which may limit generalizability. In addition, no data were available on the quality of the transition of care. Furthermore, limitations include the age of the data and the lack of evaluation of outpatient follow‐up and antibiotics on discharge. Though our data are up to 10 years old, this study evaluates risk factors that are still relevant. Finally, some variables noted in the CMS model were not evaluated in our study, such as protein‐calorie malnutrition, skin ulcers, and fracture history. Given these differences, we were unable to directly compare the performance of the 2 models.
Despite examining novel factors related to prior healthcare utilization, our model still performed suboptimally in identifying subjects at risk for rehospitalization after pneumonia. Future studies need to examine other social factors and patient frailty that may increase the risk of readmissions, as well as examine the impact of clinical stability on discharge. It remains to be determined if readmissions after pneumonia are truly preventable or mostly attributable to the patient's prior health status.
Disclosures: The project described was supported by grant number R01NR010828 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the VA North Texas Health Care System. Funding agencies had no role in conducting the study, or role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
- , , , et al. Incidence and cost of pneumonia in Medicare beneficiaries. Chest. 2012;142(4):973–981.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community‐acquired pneumonia. Respirology. 2011;16(7):1119–1126.
- , , , et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595–600.
- , , , et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278–1284.
- , , , et al. Causes and risk factors for rehospitalization of patients hospitalized with community‐acquired pneumonia. Clin Infect Dis. 2008;46(4):550–556.
- , , , et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55(11):1466–1473.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084.
- U.S. Department of Veterans Affairs. Health benefits. Priority groups table. Available at: http://www.va.gov/healthbenefits/resources/priority_groups.asp. Accessed July 1, 2013.
- , , , et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , , et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med. 2011;124(3):244–251.
- , , , et al. Diagnosis of pulmonary malignancy after hospitalization for pneumonia. Am J Med. 2010;123(1):66–71.
- MedPAC. 2013 [cited 2013 June 11]; Available from: http://www.hospitalcompare.gov.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- , , , et al. Predictors of short‐term rehospitalization following discharge of patients hospitalized with community‐acquired pneumonia. Chest. 2009;136(4):1079–1085.
- , , , et al. Effects of previous influenza vaccination on subsequent readmission and mortality in elderly patients hospitalized with pneumonia. Am J Med. 2003;115(6):454–461.
- , , . Does increased access to primary care reduce hospital readmissions? N Engl J Med. 1996;334(22):1441–1447.
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. Incidence and cost of pneumonia in Medicare beneficiaries. Chest. 2012;142(4):973–981.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community‐acquired pneumonia. Respirology. 2011;16(7):1119–1126.
- , , , et al. Pneumonia: criteria for patient instability on hospital discharge. Chest. 2008;134(3):595–600.
- , , , et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162(11):1278–1284.
- , , , et al. Causes and risk factors for rehospitalization of patients hospitalized with community‐acquired pneumonia. Clin Infect Dis. 2008;46(4):550–556.
- , , , et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55(11):1466–1473.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084.
- U.S. Department of Veterans Affairs. Health benefits. Priority groups table. Available at: http://www.va.gov/healthbenefits/resources/priority_groups.asp. Accessed July 1, 2013.
- , , , et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , , et al. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med. 2011;124(3):244–251.
- , , , et al. Diagnosis of pulmonary malignancy after hospitalization for pneumonia. Am J Med. 2010;123(1):66–71.
- MedPAC. 2013 [cited 2013 June 11]; Available from: http://www.hospitalcompare.gov.
- , , . The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295.
- , , , et al. Predictors of short‐term rehospitalization following discharge of patients hospitalized with community‐acquired pneumonia. Chest. 2009;136(4):1079–1085.
- , , , et al. Effects of previous influenza vaccination on subsequent readmission and mortality in elderly patients hospitalized with pneumonia. Am J Med. 2003;115(6):454–461.
- , , . Does increased access to primary care reduce hospital readmissions? N Engl J Med. 1996;334(22):1441–1447.
- , , , et al. Development, validation, and results of a measure of 30‐day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
2014 Society of Hospital Medicine. This article is a US government work and, as such, is in the public domain in the United States of America.
Pazopanib helps significant minority of sarcoma patients
MILAN – Pazopanib, which received U.S. approval in 2012 for treating advanced soft-tissue sarcomas, can be very effective for durably halting tumor progression in a significant minority of sarcoma patients but requires close monitoring for adverse effects.
"Although the overall response rate is low, some patients experience important palliation of symptoms and prolonged disease control" from treatment with pazotinib (Votrient), Dr. Ian R. Judson said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"We see a number of adverse effects [from pazopanib treatment] that need careful monitoring, particularly fatigue, diarrhea, nausea, and weight loss," adverse effects that had previously been seen in patients with other tumor types treated with the drug, said Dr. Judson, professor of cancer pharmacology and head of the sarcoma unit at the Royal Marsden Hospital, London. Results from the phase III trial of pazopanib in patients with advanced soft-tissue sarcoma with a history of chemotherapy, also showed that the drug can cause other, previously unreported adverse effects: myocardial dysfunction, an increased risk for venous thromboembolism, and the possibility for some patients to develop pneumothorax (Lancet 2012;379:1879-86).
The upside of pazopanib treatment is that it can produce "clear and dramatic" increases in progression-free survival and "durable, stable disease" in certain patients, said Dr. Judson.
Pazopanib became the first tyrosine kinase inhibitor to receive approval from the Food and Drug Administration and other regulatory agencies for treating soft tissue sarcomas (STS), although it has not been proven effective for treating adipocyte STS and is also not indicated for gastrointestinal stromal tumors. But it remains unclear which patients with other types of STS will respond to pazopanib and which won’t. "I wish we knew what the molecular target for this drug really is," Dr. Judson said.
A recently published analysis retrospectively pooled data from 118 STS patients enrolled in a phase II study of pazopanib and 226 patients from the phase III study PALETTE (Pazopanib for Metastatic Soft Tissue Sarcoma). The analysis showed that 36% of the entire group of patients on pazopanib were long-term responders to the drug, defined as having progression-free survival for at least 6 months following the start of pazopanib treatment, and 34% of patients on the drug were long-term survivors on the drug, defined as living for at least 18 months on treatment, noted Dr. Shreyaskumar R. Patel in a talk at the conference (Ann. Oncol. 2014;25:719-24).
During an overall median follow-up of 2.3 years in the two studies, 76 patients (22%) were both long-term responders and long-term survivors. Twelve patients remained on pazopanib treatment for more than 2 years, with a median time on treatment of 2.4 years, and 1 patient from the combined groups stayed on pazopanib for as long as 3.7 years, said Dr. Patel, professor and deputy chair of the department of sarcoma medical oncology at M.D. Anderson Cancer Center, Houston.
"Pazopanib is probably my second-line choice" for treating advanced STS, "particularly synovial sarcomas" after treatment with doxorubicin (Adriamycin) and ifosfamide (Ifex) fails, said Dr. Robert S. Benjamin, professor and chair of sarcoma medical oncology at M.D. Anderson.
The pazopanib trials were sponsored by GlaxoSmithKline, which markets pazopanib. Dr. Judson said that he has received honoraria from GlaxoSmithKline and Novartis, and research support from GlaxoSmithKline, AstraZeneca, and other companies. Dr. Patel said that he has received honoraria or consulting fees from GlaxoSmithKline, Novartis, and Johnson & Johnson, and research support from Johnson & Johnson, PharmaMar, and other companies. Dr. Benjamin said that he has received research support from Johnson & Johnson, Merck, and Pfizer.
[email protected] Twitter: @mitchelzoler
MILAN – Pazopanib, which received U.S. approval in 2012 for treating advanced soft-tissue sarcomas, can be very effective for durably halting tumor progression in a significant minority of sarcoma patients but requires close monitoring for adverse effects.
"Although the overall response rate is low, some patients experience important palliation of symptoms and prolonged disease control" from treatment with pazotinib (Votrient), Dr. Ian R. Judson said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"We see a number of adverse effects [from pazopanib treatment] that need careful monitoring, particularly fatigue, diarrhea, nausea, and weight loss," adverse effects that had previously been seen in patients with other tumor types treated with the drug, said Dr. Judson, professor of cancer pharmacology and head of the sarcoma unit at the Royal Marsden Hospital, London. Results from the phase III trial of pazopanib in patients with advanced soft-tissue sarcoma with a history of chemotherapy, also showed that the drug can cause other, previously unreported adverse effects: myocardial dysfunction, an increased risk for venous thromboembolism, and the possibility for some patients to develop pneumothorax (Lancet 2012;379:1879-86).
The upside of pazopanib treatment is that it can produce "clear and dramatic" increases in progression-free survival and "durable, stable disease" in certain patients, said Dr. Judson.
Pazopanib became the first tyrosine kinase inhibitor to receive approval from the Food and Drug Administration and other regulatory agencies for treating soft tissue sarcomas (STS), although it has not been proven effective for treating adipocyte STS and is also not indicated for gastrointestinal stromal tumors. But it remains unclear which patients with other types of STS will respond to pazopanib and which won’t. "I wish we knew what the molecular target for this drug really is," Dr. Judson said.
A recently published analysis retrospectively pooled data from 118 STS patients enrolled in a phase II study of pazopanib and 226 patients from the phase III study PALETTE (Pazopanib for Metastatic Soft Tissue Sarcoma). The analysis showed that 36% of the entire group of patients on pazopanib were long-term responders to the drug, defined as having progression-free survival for at least 6 months following the start of pazopanib treatment, and 34% of patients on the drug were long-term survivors on the drug, defined as living for at least 18 months on treatment, noted Dr. Shreyaskumar R. Patel in a talk at the conference (Ann. Oncol. 2014;25:719-24).
During an overall median follow-up of 2.3 years in the two studies, 76 patients (22%) were both long-term responders and long-term survivors. Twelve patients remained on pazopanib treatment for more than 2 years, with a median time on treatment of 2.4 years, and 1 patient from the combined groups stayed on pazopanib for as long as 3.7 years, said Dr. Patel, professor and deputy chair of the department of sarcoma medical oncology at M.D. Anderson Cancer Center, Houston.
"Pazopanib is probably my second-line choice" for treating advanced STS, "particularly synovial sarcomas" after treatment with doxorubicin (Adriamycin) and ifosfamide (Ifex) fails, said Dr. Robert S. Benjamin, professor and chair of sarcoma medical oncology at M.D. Anderson.
The pazopanib trials were sponsored by GlaxoSmithKline, which markets pazopanib. Dr. Judson said that he has received honoraria from GlaxoSmithKline and Novartis, and research support from GlaxoSmithKline, AstraZeneca, and other companies. Dr. Patel said that he has received honoraria or consulting fees from GlaxoSmithKline, Novartis, and Johnson & Johnson, and research support from Johnson & Johnson, PharmaMar, and other companies. Dr. Benjamin said that he has received research support from Johnson & Johnson, Merck, and Pfizer.
[email protected] Twitter: @mitchelzoler
MILAN – Pazopanib, which received U.S. approval in 2012 for treating advanced soft-tissue sarcomas, can be very effective for durably halting tumor progression in a significant minority of sarcoma patients but requires close monitoring for adverse effects.
"Although the overall response rate is low, some patients experience important palliation of symptoms and prolonged disease control" from treatment with pazotinib (Votrient), Dr. Ian R. Judson said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"We see a number of adverse effects [from pazopanib treatment] that need careful monitoring, particularly fatigue, diarrhea, nausea, and weight loss," adverse effects that had previously been seen in patients with other tumor types treated with the drug, said Dr. Judson, professor of cancer pharmacology and head of the sarcoma unit at the Royal Marsden Hospital, London. Results from the phase III trial of pazopanib in patients with advanced soft-tissue sarcoma with a history of chemotherapy, also showed that the drug can cause other, previously unreported adverse effects: myocardial dysfunction, an increased risk for venous thromboembolism, and the possibility for some patients to develop pneumothorax (Lancet 2012;379:1879-86).
The upside of pazopanib treatment is that it can produce "clear and dramatic" increases in progression-free survival and "durable, stable disease" in certain patients, said Dr. Judson.
Pazopanib became the first tyrosine kinase inhibitor to receive approval from the Food and Drug Administration and other regulatory agencies for treating soft tissue sarcomas (STS), although it has not been proven effective for treating adipocyte STS and is also not indicated for gastrointestinal stromal tumors. But it remains unclear which patients with other types of STS will respond to pazopanib and which won’t. "I wish we knew what the molecular target for this drug really is," Dr. Judson said.
A recently published analysis retrospectively pooled data from 118 STS patients enrolled in a phase II study of pazopanib and 226 patients from the phase III study PALETTE (Pazopanib for Metastatic Soft Tissue Sarcoma). The analysis showed that 36% of the entire group of patients on pazopanib were long-term responders to the drug, defined as having progression-free survival for at least 6 months following the start of pazopanib treatment, and 34% of patients on the drug were long-term survivors on the drug, defined as living for at least 18 months on treatment, noted Dr. Shreyaskumar R. Patel in a talk at the conference (Ann. Oncol. 2014;25:719-24).
During an overall median follow-up of 2.3 years in the two studies, 76 patients (22%) were both long-term responders and long-term survivors. Twelve patients remained on pazopanib treatment for more than 2 years, with a median time on treatment of 2.4 years, and 1 patient from the combined groups stayed on pazopanib for as long as 3.7 years, said Dr. Patel, professor and deputy chair of the department of sarcoma medical oncology at M.D. Anderson Cancer Center, Houston.
"Pazopanib is probably my second-line choice" for treating advanced STS, "particularly synovial sarcomas" after treatment with doxorubicin (Adriamycin) and ifosfamide (Ifex) fails, said Dr. Robert S. Benjamin, professor and chair of sarcoma medical oncology at M.D. Anderson.
The pazopanib trials were sponsored by GlaxoSmithKline, which markets pazopanib. Dr. Judson said that he has received honoraria from GlaxoSmithKline and Novartis, and research support from GlaxoSmithKline, AstraZeneca, and other companies. Dr. Patel said that he has received honoraria or consulting fees from GlaxoSmithKline, Novartis, and Johnson & Johnson, and research support from Johnson & Johnson, PharmaMar, and other companies. Dr. Benjamin said that he has received research support from Johnson & Johnson, Merck, and Pfizer.
[email protected] Twitter: @mitchelzoler
EXPERT ANALYSIS FROM SARCOMA AND GIST 2014
Pseudobulbar affect: More common than you’d think
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.
He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
AT THE AAGP ANNUAL MEETING
Major finding: The prevalence of PBA symptoms among patients over age 65 years with any of six underlying neurologic disorders was 27.4%. That was significantly less than in younger patients with the same disorders, but the adverse effect of having PBA symptoms upon quality of life was markedly greater in the older group.
Data source: The PRISM study included 5,290 patients with Alzheimer’s disease or any of five other less common neurologic disorders, all of whom were screened for the presence of PBA symptoms using a brief validated measure.
Disclosures: The presenter serves on a scientific advisory board for Avanir Pharmaceuticals, which funded the PRISM study.
Why the ACA makes me appreciate hospital medicine more each day
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Online reviews
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
Cervical cancer screening
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Preventing Weight Loss in Patients With Dementia
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
FDA approves IV formulation of antifungal agent

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()
High cost of eculizumab needs explaining, NICE says

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()
Study may explain why targeted treatment falls short in angiosarcoma
Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()

Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()

Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()