User login
Tight Glycemic Control Leads to More Hypoglycemia in the Pediatric ICU Population
Question
Does tight control of hyperglycemia improve outcomes in the pediatric intensive care unit?
Bottom line
Tight glycemic control does not increase the number of days alive and free from mechanical ventilation for pediatric patients in the intensive care unit (ICU), but does increase the risk of severe hypoglycemia. Children in the ICU for reasons other than cardiac surgery and were treated with tight control had lower overall healthcare costs and reduced lengths of stay. However, these benefits must be weighed against the increased risk of hypoglycemia.
Reference
Macrae D, Grieve R, Allen E, et al, for the CHiP Investigators. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014;370(2):107-118. (LOE: 1b)
Allocation
(Concealed)
Design
Randomized controlled trial (nonblinded)
Setting
Inpatient (ICU only)
Synopsis
Using concealed allocation, these investigators randomized 1369 patients in the pediatric ICU to receive either tight glycemic control with a target blood glucose of 72 mg/dL to 126 mg/dL (4 - 7 mmol/L) or conventional glycemic control with a target of less than 216 mg/dL (12 mmol/L). Eligible patients were aged between 36 weeks and 16 years. They required mechanical ventilation and vasoactive drugs for an anticipated 12 hours following an injury or major surgery or to treat a critical illness. Children with diabetes were excluded. Analysis was by intention to treat. Baseline characteristics of the 2 groups were similar, and 60% of the patients in the total cohort had undergone cardiac surgery. There was no significant difference detected between the 2 groups for the primary outcome – the number of days alive and free from mechanical ventilation at 30 days. As expected, patients in the tight control group were more likely to have multiple severe hypoglycemic episodes (7.3% vs 1.5%; odds ratio = 5.27; 95% CI, 2.65-10.48). Although major clinical outcomes did not improve, there were some benefits associated with tight control, including reduced costs and reduced lengths of stay in the subgroup of patients who had not undergone cardiac surgery, as well as decreased need for renal replacement therapy in the overall group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Question
Does tight control of hyperglycemia improve outcomes in the pediatric intensive care unit?
Bottom line
Tight glycemic control does not increase the number of days alive and free from mechanical ventilation for pediatric patients in the intensive care unit (ICU), but does increase the risk of severe hypoglycemia. Children in the ICU for reasons other than cardiac surgery and were treated with tight control had lower overall healthcare costs and reduced lengths of stay. However, these benefits must be weighed against the increased risk of hypoglycemia.
Reference
Macrae D, Grieve R, Allen E, et al, for the CHiP Investigators. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014;370(2):107-118. (LOE: 1b)
Allocation
(Concealed)
Design
Randomized controlled trial (nonblinded)
Setting
Inpatient (ICU only)
Synopsis
Using concealed allocation, these investigators randomized 1369 patients in the pediatric ICU to receive either tight glycemic control with a target blood glucose of 72 mg/dL to 126 mg/dL (4 - 7 mmol/L) or conventional glycemic control with a target of less than 216 mg/dL (12 mmol/L). Eligible patients were aged between 36 weeks and 16 years. They required mechanical ventilation and vasoactive drugs for an anticipated 12 hours following an injury or major surgery or to treat a critical illness. Children with diabetes were excluded. Analysis was by intention to treat. Baseline characteristics of the 2 groups were similar, and 60% of the patients in the total cohort had undergone cardiac surgery. There was no significant difference detected between the 2 groups for the primary outcome – the number of days alive and free from mechanical ventilation at 30 days. As expected, patients in the tight control group were more likely to have multiple severe hypoglycemic episodes (7.3% vs 1.5%; odds ratio = 5.27; 95% CI, 2.65-10.48). Although major clinical outcomes did not improve, there were some benefits associated with tight control, including reduced costs and reduced lengths of stay in the subgroup of patients who had not undergone cardiac surgery, as well as decreased need for renal replacement therapy in the overall group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Question
Does tight control of hyperglycemia improve outcomes in the pediatric intensive care unit?
Bottom line
Tight glycemic control does not increase the number of days alive and free from mechanical ventilation for pediatric patients in the intensive care unit (ICU), but does increase the risk of severe hypoglycemia. Children in the ICU for reasons other than cardiac surgery and were treated with tight control had lower overall healthcare costs and reduced lengths of stay. However, these benefits must be weighed against the increased risk of hypoglycemia.
Reference
Macrae D, Grieve R, Allen E, et al, for the CHiP Investigators. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014;370(2):107-118. (LOE: 1b)
Allocation
(Concealed)
Design
Randomized controlled trial (nonblinded)
Setting
Inpatient (ICU only)
Synopsis
Using concealed allocation, these investigators randomized 1369 patients in the pediatric ICU to receive either tight glycemic control with a target blood glucose of 72 mg/dL to 126 mg/dL (4 - 7 mmol/L) or conventional glycemic control with a target of less than 216 mg/dL (12 mmol/L). Eligible patients were aged between 36 weeks and 16 years. They required mechanical ventilation and vasoactive drugs for an anticipated 12 hours following an injury or major surgery or to treat a critical illness. Children with diabetes were excluded. Analysis was by intention to treat. Baseline characteristics of the 2 groups were similar, and 60% of the patients in the total cohort had undergone cardiac surgery. There was no significant difference detected between the 2 groups for the primary outcome – the number of days alive and free from mechanical ventilation at 30 days. As expected, patients in the tight control group were more likely to have multiple severe hypoglycemic episodes (7.3% vs 1.5%; odds ratio = 5.27; 95% CI, 2.65-10.48). Although major clinical outcomes did not improve, there were some benefits associated with tight control, including reduced costs and reduced lengths of stay in the subgroup of patients who had not undergone cardiac surgery, as well as decreased need for renal replacement therapy in the overall group.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Corporate health care is here
Hospital mergers have been accelerating in the last few years, and doctors and other health care workers have been swept up in the process.
The last time merger mania took place in the 1990s, seemingly to provide efficiencies and savings, costs went up. At that time some doctors became interested in joining their local hospitals and became salaried employees. This time around multiple incentives are playing out, and the exodus from private practice has accelerated. Between 2007 and 2012 the number of cardiologists employed by hospitals has increased from 11% to 35% (N. Engl. J. Med. 2014;370;198-9).
The increased need for investment in financial infrastructure has led many private practitioners to seek the umbrella of the local hospital. Cardiology has seen a shift in federal reimbursement rates for imaging favoring hospital-based testing. At the same time, local hospitals have sought out mergers and acquisitions of varying sorts in order to become more competitive in the marketplace and to acquire more development capital. The number of hospital mergers increased almost twofold from 2009 to 2012 (N.Y. Times, Aug. 12, 2013, p. B1). Local hospitals have been anxious to solidify relationships within their local communities by creating referral networks. Others have looked nationally for the "quality branding" for their institution.
Merger mania has also moved from local to national control by both profit and nonprofit corporations. Entrepreneurism has driven financial incentives in order to develop large networks that have the potential to improve quality and efficiency. An unwritten motivation is the potential to generate large profits that have the potential of increasing health care costs in the pre-Medicare population that we saw in the last merger go-around. Several large medical groups, like the Mayo Clinic or the Cleveland Clinic, have expanded their network and instituted franchiselike arrangements with hospitals thousands of miles distant from their headquarters, to create referral networks for highly specialized and high-cost procedures.
Much of this is hardly news to any of us. This trend is a result of multiple forces that include the changes in imaging fees, which provided the potential for expanding sources of revenue to hospitals and hospital networks. Many physicians found that merging their practice with their local hospital, where they had been practicing, was not too wrenching. That is, until they woke up the next morning to learn that their local hospital had just merged with another system. They now found that they had to deal with unfamiliar administrators with different views on health care. The system was no longer sensitive to local health care but to the corporate bottom line. Suddenly, the familiarity with the local hospital administrator, whom they knew, had been replaced by a "corporate vice president for physician relations."
Recent press coverage has recounted tales of corporate initiatives that have driven up expenses in order to improve the bottom line. One recent report recounts the story of emergency department physicians who were financially rewarded or penalized based upon the statistics of their hospital admission rate (N.Y. Times, Jan. 23, 2014, p. A1).
According to the attorney who represented the doctors, "It’s not a doctor in there watching those statistics – it’s the finance people." The economics of cardiology provide many targets for finance people to improve the bottom line. Some examples are biannual or annual stress tests, multiple imaging procedures, and "tack-on" procedures during angiography, to name just a few. The most recent story (Bloomberg News, March 6, 2014) of how one of America’s most prestigious hospitals manipulated admissions for coronary angiography and trolled local communities with stress tests to increase the number of angiograms, raised shudders in this reader. In 2010, seven of the hospital-based cardiologists each averaged 301 referrals to the cath lab, which was "15 times the average by all 546 doctors who sent patients to the lab that year."
These events were not driven by "finance people" alone, but had complicity by doctors. They suggest that the process is endemic in cardiology today. It has been said before; the enemy is US.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Hospital mergers have been accelerating in the last few years, and doctors and other health care workers have been swept up in the process.
The last time merger mania took place in the 1990s, seemingly to provide efficiencies and savings, costs went up. At that time some doctors became interested in joining their local hospitals and became salaried employees. This time around multiple incentives are playing out, and the exodus from private practice has accelerated. Between 2007 and 2012 the number of cardiologists employed by hospitals has increased from 11% to 35% (N. Engl. J. Med. 2014;370;198-9).
The increased need for investment in financial infrastructure has led many private practitioners to seek the umbrella of the local hospital. Cardiology has seen a shift in federal reimbursement rates for imaging favoring hospital-based testing. At the same time, local hospitals have sought out mergers and acquisitions of varying sorts in order to become more competitive in the marketplace and to acquire more development capital. The number of hospital mergers increased almost twofold from 2009 to 2012 (N.Y. Times, Aug. 12, 2013, p. B1). Local hospitals have been anxious to solidify relationships within their local communities by creating referral networks. Others have looked nationally for the "quality branding" for their institution.
Merger mania has also moved from local to national control by both profit and nonprofit corporations. Entrepreneurism has driven financial incentives in order to develop large networks that have the potential to improve quality and efficiency. An unwritten motivation is the potential to generate large profits that have the potential of increasing health care costs in the pre-Medicare population that we saw in the last merger go-around. Several large medical groups, like the Mayo Clinic or the Cleveland Clinic, have expanded their network and instituted franchiselike arrangements with hospitals thousands of miles distant from their headquarters, to create referral networks for highly specialized and high-cost procedures.
Much of this is hardly news to any of us. This trend is a result of multiple forces that include the changes in imaging fees, which provided the potential for expanding sources of revenue to hospitals and hospital networks. Many physicians found that merging their practice with their local hospital, where they had been practicing, was not too wrenching. That is, until they woke up the next morning to learn that their local hospital had just merged with another system. They now found that they had to deal with unfamiliar administrators with different views on health care. The system was no longer sensitive to local health care but to the corporate bottom line. Suddenly, the familiarity with the local hospital administrator, whom they knew, had been replaced by a "corporate vice president for physician relations."
Recent press coverage has recounted tales of corporate initiatives that have driven up expenses in order to improve the bottom line. One recent report recounts the story of emergency department physicians who were financially rewarded or penalized based upon the statistics of their hospital admission rate (N.Y. Times, Jan. 23, 2014, p. A1).
According to the attorney who represented the doctors, "It’s not a doctor in there watching those statistics – it’s the finance people." The economics of cardiology provide many targets for finance people to improve the bottom line. Some examples are biannual or annual stress tests, multiple imaging procedures, and "tack-on" procedures during angiography, to name just a few. The most recent story (Bloomberg News, March 6, 2014) of how one of America’s most prestigious hospitals manipulated admissions for coronary angiography and trolled local communities with stress tests to increase the number of angiograms, raised shudders in this reader. In 2010, seven of the hospital-based cardiologists each averaged 301 referrals to the cath lab, which was "15 times the average by all 546 doctors who sent patients to the lab that year."
These events were not driven by "finance people" alone, but had complicity by doctors. They suggest that the process is endemic in cardiology today. It has been said before; the enemy is US.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Hospital mergers have been accelerating in the last few years, and doctors and other health care workers have been swept up in the process.
The last time merger mania took place in the 1990s, seemingly to provide efficiencies and savings, costs went up. At that time some doctors became interested in joining their local hospitals and became salaried employees. This time around multiple incentives are playing out, and the exodus from private practice has accelerated. Between 2007 and 2012 the number of cardiologists employed by hospitals has increased from 11% to 35% (N. Engl. J. Med. 2014;370;198-9).
The increased need for investment in financial infrastructure has led many private practitioners to seek the umbrella of the local hospital. Cardiology has seen a shift in federal reimbursement rates for imaging favoring hospital-based testing. At the same time, local hospitals have sought out mergers and acquisitions of varying sorts in order to become more competitive in the marketplace and to acquire more development capital. The number of hospital mergers increased almost twofold from 2009 to 2012 (N.Y. Times, Aug. 12, 2013, p. B1). Local hospitals have been anxious to solidify relationships within their local communities by creating referral networks. Others have looked nationally for the "quality branding" for their institution.
Merger mania has also moved from local to national control by both profit and nonprofit corporations. Entrepreneurism has driven financial incentives in order to develop large networks that have the potential to improve quality and efficiency. An unwritten motivation is the potential to generate large profits that have the potential of increasing health care costs in the pre-Medicare population that we saw in the last merger go-around. Several large medical groups, like the Mayo Clinic or the Cleveland Clinic, have expanded their network and instituted franchiselike arrangements with hospitals thousands of miles distant from their headquarters, to create referral networks for highly specialized and high-cost procedures.
Much of this is hardly news to any of us. This trend is a result of multiple forces that include the changes in imaging fees, which provided the potential for expanding sources of revenue to hospitals and hospital networks. Many physicians found that merging their practice with their local hospital, where they had been practicing, was not too wrenching. That is, until they woke up the next morning to learn that their local hospital had just merged with another system. They now found that they had to deal with unfamiliar administrators with different views on health care. The system was no longer sensitive to local health care but to the corporate bottom line. Suddenly, the familiarity with the local hospital administrator, whom they knew, had been replaced by a "corporate vice president for physician relations."
Recent press coverage has recounted tales of corporate initiatives that have driven up expenses in order to improve the bottom line. One recent report recounts the story of emergency department physicians who were financially rewarded or penalized based upon the statistics of their hospital admission rate (N.Y. Times, Jan. 23, 2014, p. A1).
According to the attorney who represented the doctors, "It’s not a doctor in there watching those statistics – it’s the finance people." The economics of cardiology provide many targets for finance people to improve the bottom line. Some examples are biannual or annual stress tests, multiple imaging procedures, and "tack-on" procedures during angiography, to name just a few. The most recent story (Bloomberg News, March 6, 2014) of how one of America’s most prestigious hospitals manipulated admissions for coronary angiography and trolled local communities with stress tests to increase the number of angiograms, raised shudders in this reader. In 2010, seven of the hospital-based cardiologists each averaged 301 referrals to the cath lab, which was "15 times the average by all 546 doctors who sent patients to the lab that year."
These events were not driven by "finance people" alone, but had complicity by doctors. They suggest that the process is endemic in cardiology today. It has been said before; the enemy is US.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Ode to my immune system
Our bodies are amazing feats of nature
Pathways that we understand through science
Among the most complex though, I would wager
Immunity, autoimmunity, and balance.
First there is the issue of barriers,
Skin, and gut, and membranes
Primary defense against invaders
Seems ordinary, but really far from mundane.
What comes next is not pure serendipity
Not chance but an evolutionary gift
We kill germs with innate immunity
Imprecise but efficient and swift.
Phagocytes, a fitting name for greed
Neutrophils, macrophages, dendritic cells
Summoned to areas of injury, they proceed
To ingest and digest and clear dead cells.
Complement, a cascade of proteases
Opsonize invading pathogens
Activated by three different pathways
Membrane attack complex a terminal engine.
Simultaneously, adaptive immunity
In special regions, lymph nodes and Peyer’s patches
B cells develop some memory
Immunoglobulins churned out in batches.
Helper Ts aid antibody production
Cytotoxic Ts kill the bugs hiding within
Regulatory Ts promote self toleration
MHCs on cell surfaces weigh in.
Many elements require orchestration
Helped along by a bevy of proteins
Chemokines, interleukins, growth factors, interferons
Enzymatic cascades form routine.
This cellular/molecular adventure
Fantastically intricate choreography
Self or non-self, intruder, interloper
Defense against microbial tomfoolery.
Dr. Chan practices rheumatology is Pawtucket, R.I.
Our bodies are amazing feats of nature
Pathways that we understand through science
Among the most complex though, I would wager
Immunity, autoimmunity, and balance.
First there is the issue of barriers,
Skin, and gut, and membranes
Primary defense against invaders
Seems ordinary, but really far from mundane.
What comes next is not pure serendipity
Not chance but an evolutionary gift
We kill germs with innate immunity
Imprecise but efficient and swift.
Phagocytes, a fitting name for greed
Neutrophils, macrophages, dendritic cells
Summoned to areas of injury, they proceed
To ingest and digest and clear dead cells.
Complement, a cascade of proteases
Opsonize invading pathogens
Activated by three different pathways
Membrane attack complex a terminal engine.
Simultaneously, adaptive immunity
In special regions, lymph nodes and Peyer’s patches
B cells develop some memory
Immunoglobulins churned out in batches.
Helper Ts aid antibody production
Cytotoxic Ts kill the bugs hiding within
Regulatory Ts promote self toleration
MHCs on cell surfaces weigh in.
Many elements require orchestration
Helped along by a bevy of proteins
Chemokines, interleukins, growth factors, interferons
Enzymatic cascades form routine.
This cellular/molecular adventure
Fantastically intricate choreography
Self or non-self, intruder, interloper
Defense against microbial tomfoolery.
Dr. Chan practices rheumatology is Pawtucket, R.I.
Our bodies are amazing feats of nature
Pathways that we understand through science
Among the most complex though, I would wager
Immunity, autoimmunity, and balance.
First there is the issue of barriers,
Skin, and gut, and membranes
Primary defense against invaders
Seems ordinary, but really far from mundane.
What comes next is not pure serendipity
Not chance but an evolutionary gift
We kill germs with innate immunity
Imprecise but efficient and swift.
Phagocytes, a fitting name for greed
Neutrophils, macrophages, dendritic cells
Summoned to areas of injury, they proceed
To ingest and digest and clear dead cells.
Complement, a cascade of proteases
Opsonize invading pathogens
Activated by three different pathways
Membrane attack complex a terminal engine.
Simultaneously, adaptive immunity
In special regions, lymph nodes and Peyer’s patches
B cells develop some memory
Immunoglobulins churned out in batches.
Helper Ts aid antibody production
Cytotoxic Ts kill the bugs hiding within
Regulatory Ts promote self toleration
MHCs on cell surfaces weigh in.
Many elements require orchestration
Helped along by a bevy of proteins
Chemokines, interleukins, growth factors, interferons
Enzymatic cascades form routine.
This cellular/molecular adventure
Fantastically intricate choreography
Self or non-self, intruder, interloper
Defense against microbial tomfoolery.
Dr. Chan practices rheumatology is Pawtucket, R.I.
Antibiotic Therapy and Bacterial Resistance in Patients With Spinal Cord Injury
Nosocomial urinary tract infections (UTIs) are often associated with significant morbidity, mortality, and health care costs.1,2 Patients with spinal cord injury (SCI) often have indwelling or intermittent urinary catheters and are prone to have asymptomatic bacteriuria and UTIs. As a result, they frequently receive antimicrobial therapy and have a higher prevalence of antibiotic resistant urinary tract isolates compared with patients without SCI.3-5 Unfortunately, data are lacking to provide guidance for optimal treatment and duration for UTIs in patients with SCI.
Many studies have evaluated patient propensity for development of antibiotic resistance in UTIs. Age > 65 years, use of a urinary catheter, previous hospitalization, and prior antimicrobial use have been identified as common risk factors.6-8 Waites and colleagues evaluated antimicrobial resistance of urinary tract organisms in outpatients with SCI and found that 33% of urinary cultures isolated multidrug-resistant microorganisms. The authors demonstrated a relationship between antimicrobial resistance and broad spectrum and prophylactic use of antibiotics.3,9
This study sought to determine the incidence of resistance acquisition by comparing susceptibility profiles of the same organisms isolated from the same patient in consecutive episodes of bacteriuria. Given that prior antimicrobial use was identified as a common risk factor for antibiotic resistance in previous reports, this study also sought to determine patterns of antibiotic use in patients with SCI at the VA North Texas Health Care System (VANTHCS) in Dallas, Texas, to evaluate whether any correlations between antibiotic use and resistance acquisition exist. A secondary objective included identification of other risk factors that may increase acquisition of resistance.
Study Design
This study was a retrospective chart review approved by the Institutional Review Board at the VANTHCS. Since computerized charting was available beginning July 2003, the VA Computerized Patient Record System was queried to identify male or female adult (aged ≥ 18 years) veterans admitted to the SCI inpatient unit between July 1, 2003, and December 31, 2009, for review. Patients who had an ICD-9 code consistent with paraplegia, tetraplegia, or quadriplegia and 2 consecutive urine cultures that isolated the same organism within 6 months of each other were included. Males with a diagnosis of epididymitis or prostatitis were excluded.
The following data were collected for analysis: gender, age, weight, height, American Spinal Injury Association (ASIA) Impairment Scale Grades (A-E), duration of hospitalization in the SCI unit, the presence and type of urinary catheter, microbiology and antibiotic regimen, past medical history, previous antibiotic history, comorbidities, and concomitant drug therapy. The presence and type of urinary catheter was determined by the primary investigator and verified by the physician who oversaw care of patients with SCI.
All antimicrobial sensitivity testing was performed via the Microscan (Microscan Systems, Inc., Renton, WA) automated testing system. Acquisition of antibiotic resistance was defined as an increase of at least 2 dilutions in the breakpoint or change on the susceptibility panel from Susceptible (S) to Resistant (R) on the repeat urine culture.
Analysis of Resistance
Continuous parameters were reported as mean (standard deviation [SD]), and discrete parameters were reported as a percentage. Analyses of variance (ANOVA) were computed to evaluate the difference in the mean of the continuous parameters. The Mann-Whitney U test replaced the ANOVA when a dependent variable was not normally distributed. Associations between pairs of discrete parameters were tested with the Pearson chi-square test. Logistic regression analyses were performed to determine the associations between potential risk factors (age, ASIA grade, antibiotic duration, class of antibiotic) and antibiotic resistance. The study alpha was α < .05. All analyses were performed with SPSS 20.0 for Windows.
Three hundred fifty-five veterans admitted to the SCI unit during the study period were initially identified. Of those, 269 did not meet inclusion criteria and were excluded. The most common reason for exclusion was absence of a second positive urine culture with isolation of the same organism. Other reasons for exclusion included no urine cultures completed while admitted to the SCI unit or no diagnosis of SCI.
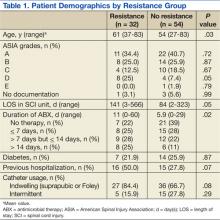
A total of 86 subjects, mean aged 56.7 years (SD, 14.2), were included in the study. Subjects were primarily men (93%) with a mean body mass index of 25.5 (SD, 7). Most of the subjects were classified Complete on the ASIA scale, meaning no motor strength or sensation below their neurologic level of injury (ASIA A; 38.4%), followed by Sensory Incomplete (ASIA B; 25.6%), Motor Incomplete-Low Muscle Strength (ASIA C; 16.3%), Motor Incomplete-High Muscle Strength (ASIA D; 14%), and Normal (ASIA E; 1.2%).
Both groups (resistance and no resistance) had similar baseline characteristics, and no differences were found for the following characteristics: ASIA grade, length of stay (LOS), presence of or control of diabetes, and presence of an indwelling urinary catheter (Table 1). However, veterans in the resistance group were significantly older than those in the no resistance group (aged 61 years vs aged 54 years; P = .03) and spent more time housed in the SCI unit with a mean LOS of 141 days vs 84 days (P = .049). Urinary pathogens developed resistance in 32 patients (37.2%, resistance group), and 54 patients (62.8%, no resistance group) did not.
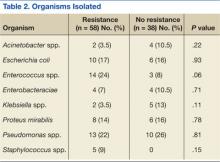
No significant differences in the types of organisms isolated were noted between the groups (Table 2). The most common pathogens isolated were Pseudomonas aeruginosa (24%), Enterococcus spp. (18%), Escherichia coli (17%), Proteus spp. (14%), Klebsiella spp. (7%), and Acinetobacter spp. (6%).
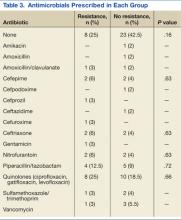
Thirty-six percent of the pathogens in the first cultures were not treated with any antibiotics, because they were considered as colonizers or contaminants. Only 61% of pathogens in the no resistance group vs 78% in the resistance group were exposed to antimicrobial treatment. In those veterans who were treated, antibiotic usage on the first urine culture was assessed to determine whether any relationship existed between receipt of a particular antimicrobial class and development of resistance. Fluoroquinolones were the most commonly prescribed antimicrobials in both the resistance and no resistance groups (Table 3).
Four risk factors (ASIA grade, antibiotic treatment duration, prior use of a cephalosporin, and prior use of penicillin) were initially identified by logistic regression analyses as being associated with resistance development. Since veterans in the resistance group were significantly older than those in the no resistance group, the analysis was repeated with age as a covariate to independently assess the association between the risk factors and resistance. After controlling for age, no significant association between the ASIA grade and resistance was identified (adjusted odds ratio [OR], 1.03; 95% confidence interval [CI]: 0.66 – 1.6). Median duration of antibiotic treatment was 6 days in all patients, 3.5 days in the no resistance group, and 9 days in the resistance group. Longer duration of treatment significantly predicted resistance (adjusted OR, 1.07; P = .03; 95% CI: 1.01 – 1.03). For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop resistance.
The incidence of resistant organisms after exposure to a cephalosporin was not statistically different between groups (adjusted OR, 1.74; P = .36; 95% CI: 1.0 – 1.2). In the resistance group, 28% of the antibiotics prescribed were cephalosporins (cefuroxime, ceftriaxone, ceftazidime, and cefepime), which were used for Proteus mirabilis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. In the no resistance group, 17% of the antibiotics prescribed were cephalosporins (cefepime only) and were used for Proteus mirabilis.
Organisms treated with penicillin were significantly less likely to become resistant (adjusted OR, 0.26; P = .04; 95% CI: 0.07 - 0.96). In the resistance group, 16% of the antibiotics were penicillins (piperacillin/tazobactam), which were used for Escherichia coli, Enterococcus faecalis, Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. In the no resistance group, 22% of the antibiotics were penicillins (amoxicillin, amoxicillin/clavulanate and piperacillin/tazobactam), which were used for Proteus mirabilis, Enterococcus faecalis, and Acinetobacter baumannii.
Discussion
Longer duration of treatment significantly increased resistance on the subsequent culture in this study. For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop a resistance. However, the potential impact of using a given antibiotic class on the acquisition of resistance in patients with SCI who had a UTI was not demonstrated. Surprisingly, the use of a cephalosporin was not associated with an increased incidence of resistance in this study, which was inconsistent with the findings from other studies.10 Weber and colleagues evaluated nosocomial infections in the intensive care unit. The authors suggested that restriction on the use of third-generation cephalosporins might decrease antibiotic resistance, especially in extended spectrum beta-lactamase producing gram-negative bacilli.11
The difference in this study may be explained by the lower incidence of Escherichia coli and Klebsiella pneumoniae, which are known to exhibit inducible resistance on exposure to third-generation cephalosporins. Conversely, it was found that patients treated with a penicillin were significantly less likely to develop resistant organisms from subsequent cultures. The most common penicillin used in this study’s patient population was piperacillin/tazobactam.
For complicated UTIs including pyelonephritis, the European Association of Urology (EAU) guidelines for the management of urinary and male genital tract infections recommend treatment for 3 to 5 days after defervescence or control of complicating factors.12 These recommendations could lead to much shorter treatment durations than the traditional 14-day “standard” course often prescribed. One meta-analysis recommends a 5-day course for UTIs without fever in patients with SCI vs a 14-day course for patients with fever.13 Due to the lack of data, care often varies based on the patient’s clinical status, provider experience, and opinions. The Pannek study surveyed 16 centers that specialized in SCI care. When compared with the recommendations in the EAU guidelines, the study found providers in > 50% of the responding facilities overtreated UTIs.14
Limitations
This study has several limitations. First, the sample size was much smaller than expected. Of the 355 charts reviewed, only 86 met all the criteria to be included, which limited analysis. Additionally, given the retrospective nature of the study, it was impossible to determine provider rationale for the treatment. Since a diagnosis of UTI in patients with SCI often cannot be done with conventional methods due to lack of symptoms, many investigators have emphasized the use of quantitative urinalysis to differentiate true infection vs contamination.15-17
According to the National Institute on Disability and Rehabilitation Research consensus conference recommendations, the definition of significant bacteriuria will vary, depending on the method of bladder drainage.18 While this study reviewed microbiologic cultures and the type of patient’s urinary catheter, the method of bladder drainage in the context of quantitative urinalysis was not evaluated, which limited the interpretation of microbiologic data.
It was also impossible to determine whether bacteria were cleared by the initial treatment, leading to new bacterial strains with a multidrug resistance, or whether patients relapsed. While antibiotic selection was appropriate for antimicrobial coverage, this study was not designed to detect potential inadequacies in dosing, which could also affect resistance. Last, since no genetic evaluation of the microorganisms was done, the authors cannot be sure whether the microorganisms noted on the first urine culture were of the same genetic makeup as those identified in the second urine culture.
Conclusion
Optimal duration of therapy for treatment of UTIs in patients with SCI is unclear. Despite its limitations, the study suggests exposure to longer antibiotic treatment courses may lead to increased antimicrobial resistance in the urinary tract organisms in this patient population. Further investigation with a larger sample size is required to confirm these findings.
Author disclosures
Dr. Bedimo received research grant funding from Janssen Pharmaceuticals and Merck and Company. He also serves as an ad hoc scientific advisor for Viiv Healthcare, Gilead Science, and BMD Science. All other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch Intern Med. 1999;159(8):800-808.
2. Laupland KB, Bagshaw SM, Gregson DB, Kirkpatrick AW, Ross T, Church DL. Intensive care unit-acquired urinary tract infections in a regional critical care system. Crit Care. 2005;9(2):R60-R65.
3. Girard R, Mazoyer MA, Plauchu MM, Rode G. High prevalence of nosocomial infections in rehabilitation units accounted for by urinary tract infections in patients with spinal cord injury. J Hosp Infect. 2006;62(4):473-479.
4. Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76(3):272-280.
5. Salomon J, Gory A, Bernard L, Ruffion A, Denys P, Chartier-Kastler E. [Urinary tract infection and neurogenic bladder]. Prog Urol. 2007;17(3):448-453.
6. Ena J, Amador C, Martinez C, Ortiz de la Tabla V. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J Urol. 1995;153(1):117-120.
7. Allen UD, MacDonald N, Fuite L, Chan F, Stephens D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ. 1999;160(10):1436-1440.
8. De Mouy D, Cavallo JD, Armengaud M, et al. [Urinary tract infection in an urban population: Etiology and antibiotic sensitivity as a function of patient history]. Presse Med. 1999;28(30):1624-1628.
9. Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81(6):764-769.
10. Shah PS, Cannon JP, Sullivan CL, Nemchausky B, Pachucki CT. Controlling antimicrobial use and decreasing microbiological laboratory tests for urinary tract infections in spinal-cord-injury patients with chronic indwelling catheters. Am J Health Syst Pharm. 2005;62(1):74-77.
11. Weber DJ, Raasch R, Rutala WA. Nosocomial infections in the ICU: The growing importance of antibiotic-resistant pathogens. Chest. 1999;115(suppl 3):34S-41S.
12. Naber KG, Bergman B, Bishop MC, et al; Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001;40(5):576-588.
13. Everaert K, Lumen N, Kerckhaert W, Willaert P, van Driel M. Urinary tract infections in spinal cord injury: Prevention and treatment guidelines. Acta Clin Belg. 2009;64(4):335-340.
14. Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: Guidelines, evidence, and clinical practice. A questionnaire-based survey and review of the literature. J Spinal Cord Med. 2011;34(1):11-15.
15. Musher DM, Thorsteinsson SB, Airola VM II. Quantitative urinalysis. Diagnosing urinary tract infection in men. JAMA. 1976;236(18):2069-2072.
16. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part two: Diagnostic value of symptoms and of quantitative urinalysis. J Am Paraplegia Soc. 1985;8(1):7-10.
17. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part one: Bacteriologic diagnosis. J Am Paraplegia Soc. 1985;8(1):4-6.
18. Garcia Leoni ME, Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9(8):780-785.
Nosocomial urinary tract infections (UTIs) are often associated with significant morbidity, mortality, and health care costs.1,2 Patients with spinal cord injury (SCI) often have indwelling or intermittent urinary catheters and are prone to have asymptomatic bacteriuria and UTIs. As a result, they frequently receive antimicrobial therapy and have a higher prevalence of antibiotic resistant urinary tract isolates compared with patients without SCI.3-5 Unfortunately, data are lacking to provide guidance for optimal treatment and duration for UTIs in patients with SCI.
Many studies have evaluated patient propensity for development of antibiotic resistance in UTIs. Age > 65 years, use of a urinary catheter, previous hospitalization, and prior antimicrobial use have been identified as common risk factors.6-8 Waites and colleagues evaluated antimicrobial resistance of urinary tract organisms in outpatients with SCI and found that 33% of urinary cultures isolated multidrug-resistant microorganisms. The authors demonstrated a relationship between antimicrobial resistance and broad spectrum and prophylactic use of antibiotics.3,9
This study sought to determine the incidence of resistance acquisition by comparing susceptibility profiles of the same organisms isolated from the same patient in consecutive episodes of bacteriuria. Given that prior antimicrobial use was identified as a common risk factor for antibiotic resistance in previous reports, this study also sought to determine patterns of antibiotic use in patients with SCI at the VA North Texas Health Care System (VANTHCS) in Dallas, Texas, to evaluate whether any correlations between antibiotic use and resistance acquisition exist. A secondary objective included identification of other risk factors that may increase acquisition of resistance.
Study Design
This study was a retrospective chart review approved by the Institutional Review Board at the VANTHCS. Since computerized charting was available beginning July 2003, the VA Computerized Patient Record System was queried to identify male or female adult (aged ≥ 18 years) veterans admitted to the SCI inpatient unit between July 1, 2003, and December 31, 2009, for review. Patients who had an ICD-9 code consistent with paraplegia, tetraplegia, or quadriplegia and 2 consecutive urine cultures that isolated the same organism within 6 months of each other were included. Males with a diagnosis of epididymitis or prostatitis were excluded.
The following data were collected for analysis: gender, age, weight, height, American Spinal Injury Association (ASIA) Impairment Scale Grades (A-E), duration of hospitalization in the SCI unit, the presence and type of urinary catheter, microbiology and antibiotic regimen, past medical history, previous antibiotic history, comorbidities, and concomitant drug therapy. The presence and type of urinary catheter was determined by the primary investigator and verified by the physician who oversaw care of patients with SCI.
All antimicrobial sensitivity testing was performed via the Microscan (Microscan Systems, Inc., Renton, WA) automated testing system. Acquisition of antibiotic resistance was defined as an increase of at least 2 dilutions in the breakpoint or change on the susceptibility panel from Susceptible (S) to Resistant (R) on the repeat urine culture.
Analysis of Resistance
Continuous parameters were reported as mean (standard deviation [SD]), and discrete parameters were reported as a percentage. Analyses of variance (ANOVA) were computed to evaluate the difference in the mean of the continuous parameters. The Mann-Whitney U test replaced the ANOVA when a dependent variable was not normally distributed. Associations between pairs of discrete parameters were tested with the Pearson chi-square test. Logistic regression analyses were performed to determine the associations between potential risk factors (age, ASIA grade, antibiotic duration, class of antibiotic) and antibiotic resistance. The study alpha was α < .05. All analyses were performed with SPSS 20.0 for Windows.
Three hundred fifty-five veterans admitted to the SCI unit during the study period were initially identified. Of those, 269 did not meet inclusion criteria and were excluded. The most common reason for exclusion was absence of a second positive urine culture with isolation of the same organism. Other reasons for exclusion included no urine cultures completed while admitted to the SCI unit or no diagnosis of SCI.
A total of 86 subjects, mean aged 56.7 years (SD, 14.2), were included in the study. Subjects were primarily men (93%) with a mean body mass index of 25.5 (SD, 7). Most of the subjects were classified Complete on the ASIA scale, meaning no motor strength or sensation below their neurologic level of injury (ASIA A; 38.4%), followed by Sensory Incomplete (ASIA B; 25.6%), Motor Incomplete-Low Muscle Strength (ASIA C; 16.3%), Motor Incomplete-High Muscle Strength (ASIA D; 14%), and Normal (ASIA E; 1.2%).
Both groups (resistance and no resistance) had similar baseline characteristics, and no differences were found for the following characteristics: ASIA grade, length of stay (LOS), presence of or control of diabetes, and presence of an indwelling urinary catheter (Table 1). However, veterans in the resistance group were significantly older than those in the no resistance group (aged 61 years vs aged 54 years; P = .03) and spent more time housed in the SCI unit with a mean LOS of 141 days vs 84 days (P = .049). Urinary pathogens developed resistance in 32 patients (37.2%, resistance group), and 54 patients (62.8%, no resistance group) did not.
No significant differences in the types of organisms isolated were noted between the groups (Table 2). The most common pathogens isolated were Pseudomonas aeruginosa (24%), Enterococcus spp. (18%), Escherichia coli (17%), Proteus spp. (14%), Klebsiella spp. (7%), and Acinetobacter spp. (6%).
Thirty-six percent of the pathogens in the first cultures were not treated with any antibiotics, because they were considered as colonizers or contaminants. Only 61% of pathogens in the no resistance group vs 78% in the resistance group were exposed to antimicrobial treatment. In those veterans who were treated, antibiotic usage on the first urine culture was assessed to determine whether any relationship existed between receipt of a particular antimicrobial class and development of resistance. Fluoroquinolones were the most commonly prescribed antimicrobials in both the resistance and no resistance groups (Table 3).
Four risk factors (ASIA grade, antibiotic treatment duration, prior use of a cephalosporin, and prior use of penicillin) were initially identified by logistic regression analyses as being associated with resistance development. Since veterans in the resistance group were significantly older than those in the no resistance group, the analysis was repeated with age as a covariate to independently assess the association between the risk factors and resistance. After controlling for age, no significant association between the ASIA grade and resistance was identified (adjusted odds ratio [OR], 1.03; 95% confidence interval [CI]: 0.66 – 1.6). Median duration of antibiotic treatment was 6 days in all patients, 3.5 days in the no resistance group, and 9 days in the resistance group. Longer duration of treatment significantly predicted resistance (adjusted OR, 1.07; P = .03; 95% CI: 1.01 – 1.03). For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop resistance.
The incidence of resistant organisms after exposure to a cephalosporin was not statistically different between groups (adjusted OR, 1.74; P = .36; 95% CI: 1.0 – 1.2). In the resistance group, 28% of the antibiotics prescribed were cephalosporins (cefuroxime, ceftriaxone, ceftazidime, and cefepime), which were used for Proteus mirabilis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. In the no resistance group, 17% of the antibiotics prescribed were cephalosporins (cefepime only) and were used for Proteus mirabilis.
Organisms treated with penicillin were significantly less likely to become resistant (adjusted OR, 0.26; P = .04; 95% CI: 0.07 - 0.96). In the resistance group, 16% of the antibiotics were penicillins (piperacillin/tazobactam), which were used for Escherichia coli, Enterococcus faecalis, Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. In the no resistance group, 22% of the antibiotics were penicillins (amoxicillin, amoxicillin/clavulanate and piperacillin/tazobactam), which were used for Proteus mirabilis, Enterococcus faecalis, and Acinetobacter baumannii.
Discussion
Longer duration of treatment significantly increased resistance on the subsequent culture in this study. For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop a resistance. However, the potential impact of using a given antibiotic class on the acquisition of resistance in patients with SCI who had a UTI was not demonstrated. Surprisingly, the use of a cephalosporin was not associated with an increased incidence of resistance in this study, which was inconsistent with the findings from other studies.10 Weber and colleagues evaluated nosocomial infections in the intensive care unit. The authors suggested that restriction on the use of third-generation cephalosporins might decrease antibiotic resistance, especially in extended spectrum beta-lactamase producing gram-negative bacilli.11
The difference in this study may be explained by the lower incidence of Escherichia coli and Klebsiella pneumoniae, which are known to exhibit inducible resistance on exposure to third-generation cephalosporins. Conversely, it was found that patients treated with a penicillin were significantly less likely to develop resistant organisms from subsequent cultures. The most common penicillin used in this study’s patient population was piperacillin/tazobactam.
For complicated UTIs including pyelonephritis, the European Association of Urology (EAU) guidelines for the management of urinary and male genital tract infections recommend treatment for 3 to 5 days after defervescence or control of complicating factors.12 These recommendations could lead to much shorter treatment durations than the traditional 14-day “standard” course often prescribed. One meta-analysis recommends a 5-day course for UTIs without fever in patients with SCI vs a 14-day course for patients with fever.13 Due to the lack of data, care often varies based on the patient’s clinical status, provider experience, and opinions. The Pannek study surveyed 16 centers that specialized in SCI care. When compared with the recommendations in the EAU guidelines, the study found providers in > 50% of the responding facilities overtreated UTIs.14
Limitations
This study has several limitations. First, the sample size was much smaller than expected. Of the 355 charts reviewed, only 86 met all the criteria to be included, which limited analysis. Additionally, given the retrospective nature of the study, it was impossible to determine provider rationale for the treatment. Since a diagnosis of UTI in patients with SCI often cannot be done with conventional methods due to lack of symptoms, many investigators have emphasized the use of quantitative urinalysis to differentiate true infection vs contamination.15-17
According to the National Institute on Disability and Rehabilitation Research consensus conference recommendations, the definition of significant bacteriuria will vary, depending on the method of bladder drainage.18 While this study reviewed microbiologic cultures and the type of patient’s urinary catheter, the method of bladder drainage in the context of quantitative urinalysis was not evaluated, which limited the interpretation of microbiologic data.
It was also impossible to determine whether bacteria were cleared by the initial treatment, leading to new bacterial strains with a multidrug resistance, or whether patients relapsed. While antibiotic selection was appropriate for antimicrobial coverage, this study was not designed to detect potential inadequacies in dosing, which could also affect resistance. Last, since no genetic evaluation of the microorganisms was done, the authors cannot be sure whether the microorganisms noted on the first urine culture were of the same genetic makeup as those identified in the second urine culture.
Conclusion
Optimal duration of therapy for treatment of UTIs in patients with SCI is unclear. Despite its limitations, the study suggests exposure to longer antibiotic treatment courses may lead to increased antimicrobial resistance in the urinary tract organisms in this patient population. Further investigation with a larger sample size is required to confirm these findings.
Author disclosures
Dr. Bedimo received research grant funding from Janssen Pharmaceuticals and Merck and Company. He also serves as an ad hoc scientific advisor for Viiv Healthcare, Gilead Science, and BMD Science. All other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch Intern Med. 1999;159(8):800-808.
2. Laupland KB, Bagshaw SM, Gregson DB, Kirkpatrick AW, Ross T, Church DL. Intensive care unit-acquired urinary tract infections in a regional critical care system. Crit Care. 2005;9(2):R60-R65.
3. Girard R, Mazoyer MA, Plauchu MM, Rode G. High prevalence of nosocomial infections in rehabilitation units accounted for by urinary tract infections in patients with spinal cord injury. J Hosp Infect. 2006;62(4):473-479.
4. Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76(3):272-280.
5. Salomon J, Gory A, Bernard L, Ruffion A, Denys P, Chartier-Kastler E. [Urinary tract infection and neurogenic bladder]. Prog Urol. 2007;17(3):448-453.
6. Ena J, Amador C, Martinez C, Ortiz de la Tabla V. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J Urol. 1995;153(1):117-120.
7. Allen UD, MacDonald N, Fuite L, Chan F, Stephens D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ. 1999;160(10):1436-1440.
8. De Mouy D, Cavallo JD, Armengaud M, et al. [Urinary tract infection in an urban population: Etiology and antibiotic sensitivity as a function of patient history]. Presse Med. 1999;28(30):1624-1628.
9. Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81(6):764-769.
10. Shah PS, Cannon JP, Sullivan CL, Nemchausky B, Pachucki CT. Controlling antimicrobial use and decreasing microbiological laboratory tests for urinary tract infections in spinal-cord-injury patients with chronic indwelling catheters. Am J Health Syst Pharm. 2005;62(1):74-77.
11. Weber DJ, Raasch R, Rutala WA. Nosocomial infections in the ICU: The growing importance of antibiotic-resistant pathogens. Chest. 1999;115(suppl 3):34S-41S.
12. Naber KG, Bergman B, Bishop MC, et al; Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001;40(5):576-588.
13. Everaert K, Lumen N, Kerckhaert W, Willaert P, van Driel M. Urinary tract infections in spinal cord injury: Prevention and treatment guidelines. Acta Clin Belg. 2009;64(4):335-340.
14. Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: Guidelines, evidence, and clinical practice. A questionnaire-based survey and review of the literature. J Spinal Cord Med. 2011;34(1):11-15.
15. Musher DM, Thorsteinsson SB, Airola VM II. Quantitative urinalysis. Diagnosing urinary tract infection in men. JAMA. 1976;236(18):2069-2072.
16. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part two: Diagnostic value of symptoms and of quantitative urinalysis. J Am Paraplegia Soc. 1985;8(1):7-10.
17. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part one: Bacteriologic diagnosis. J Am Paraplegia Soc. 1985;8(1):4-6.
18. Garcia Leoni ME, Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9(8):780-785.
Nosocomial urinary tract infections (UTIs) are often associated with significant morbidity, mortality, and health care costs.1,2 Patients with spinal cord injury (SCI) often have indwelling or intermittent urinary catheters and are prone to have asymptomatic bacteriuria and UTIs. As a result, they frequently receive antimicrobial therapy and have a higher prevalence of antibiotic resistant urinary tract isolates compared with patients without SCI.3-5 Unfortunately, data are lacking to provide guidance for optimal treatment and duration for UTIs in patients with SCI.
Many studies have evaluated patient propensity for development of antibiotic resistance in UTIs. Age > 65 years, use of a urinary catheter, previous hospitalization, and prior antimicrobial use have been identified as common risk factors.6-8 Waites and colleagues evaluated antimicrobial resistance of urinary tract organisms in outpatients with SCI and found that 33% of urinary cultures isolated multidrug-resistant microorganisms. The authors demonstrated a relationship between antimicrobial resistance and broad spectrum and prophylactic use of antibiotics.3,9
This study sought to determine the incidence of resistance acquisition by comparing susceptibility profiles of the same organisms isolated from the same patient in consecutive episodes of bacteriuria. Given that prior antimicrobial use was identified as a common risk factor for antibiotic resistance in previous reports, this study also sought to determine patterns of antibiotic use in patients with SCI at the VA North Texas Health Care System (VANTHCS) in Dallas, Texas, to evaluate whether any correlations between antibiotic use and resistance acquisition exist. A secondary objective included identification of other risk factors that may increase acquisition of resistance.
Study Design
This study was a retrospective chart review approved by the Institutional Review Board at the VANTHCS. Since computerized charting was available beginning July 2003, the VA Computerized Patient Record System was queried to identify male or female adult (aged ≥ 18 years) veterans admitted to the SCI inpatient unit between July 1, 2003, and December 31, 2009, for review. Patients who had an ICD-9 code consistent with paraplegia, tetraplegia, or quadriplegia and 2 consecutive urine cultures that isolated the same organism within 6 months of each other were included. Males with a diagnosis of epididymitis or prostatitis were excluded.
The following data were collected for analysis: gender, age, weight, height, American Spinal Injury Association (ASIA) Impairment Scale Grades (A-E), duration of hospitalization in the SCI unit, the presence and type of urinary catheter, microbiology and antibiotic regimen, past medical history, previous antibiotic history, comorbidities, and concomitant drug therapy. The presence and type of urinary catheter was determined by the primary investigator and verified by the physician who oversaw care of patients with SCI.
All antimicrobial sensitivity testing was performed via the Microscan (Microscan Systems, Inc., Renton, WA) automated testing system. Acquisition of antibiotic resistance was defined as an increase of at least 2 dilutions in the breakpoint or change on the susceptibility panel from Susceptible (S) to Resistant (R) on the repeat urine culture.
Analysis of Resistance
Continuous parameters were reported as mean (standard deviation [SD]), and discrete parameters were reported as a percentage. Analyses of variance (ANOVA) were computed to evaluate the difference in the mean of the continuous parameters. The Mann-Whitney U test replaced the ANOVA when a dependent variable was not normally distributed. Associations between pairs of discrete parameters were tested with the Pearson chi-square test. Logistic regression analyses were performed to determine the associations between potential risk factors (age, ASIA grade, antibiotic duration, class of antibiotic) and antibiotic resistance. The study alpha was α < .05. All analyses were performed with SPSS 20.0 for Windows.
Three hundred fifty-five veterans admitted to the SCI unit during the study period were initially identified. Of those, 269 did not meet inclusion criteria and were excluded. The most common reason for exclusion was absence of a second positive urine culture with isolation of the same organism. Other reasons for exclusion included no urine cultures completed while admitted to the SCI unit or no diagnosis of SCI.
A total of 86 subjects, mean aged 56.7 years (SD, 14.2), were included in the study. Subjects were primarily men (93%) with a mean body mass index of 25.5 (SD, 7). Most of the subjects were classified Complete on the ASIA scale, meaning no motor strength or sensation below their neurologic level of injury (ASIA A; 38.4%), followed by Sensory Incomplete (ASIA B; 25.6%), Motor Incomplete-Low Muscle Strength (ASIA C; 16.3%), Motor Incomplete-High Muscle Strength (ASIA D; 14%), and Normal (ASIA E; 1.2%).
Both groups (resistance and no resistance) had similar baseline characteristics, and no differences were found for the following characteristics: ASIA grade, length of stay (LOS), presence of or control of diabetes, and presence of an indwelling urinary catheter (Table 1). However, veterans in the resistance group were significantly older than those in the no resistance group (aged 61 years vs aged 54 years; P = .03) and spent more time housed in the SCI unit with a mean LOS of 141 days vs 84 days (P = .049). Urinary pathogens developed resistance in 32 patients (37.2%, resistance group), and 54 patients (62.8%, no resistance group) did not.
No significant differences in the types of organisms isolated were noted between the groups (Table 2). The most common pathogens isolated were Pseudomonas aeruginosa (24%), Enterococcus spp. (18%), Escherichia coli (17%), Proteus spp. (14%), Klebsiella spp. (7%), and Acinetobacter spp. (6%).
Thirty-six percent of the pathogens in the first cultures were not treated with any antibiotics, because they were considered as colonizers or contaminants. Only 61% of pathogens in the no resistance group vs 78% in the resistance group were exposed to antimicrobial treatment. In those veterans who were treated, antibiotic usage on the first urine culture was assessed to determine whether any relationship existed between receipt of a particular antimicrobial class and development of resistance. Fluoroquinolones were the most commonly prescribed antimicrobials in both the resistance and no resistance groups (Table 3).
Four risk factors (ASIA grade, antibiotic treatment duration, prior use of a cephalosporin, and prior use of penicillin) were initially identified by logistic regression analyses as being associated with resistance development. Since veterans in the resistance group were significantly older than those in the no resistance group, the analysis was repeated with age as a covariate to independently assess the association between the risk factors and resistance. After controlling for age, no significant association between the ASIA grade and resistance was identified (adjusted odds ratio [OR], 1.03; 95% confidence interval [CI]: 0.66 – 1.6). Median duration of antibiotic treatment was 6 days in all patients, 3.5 days in the no resistance group, and 9 days in the resistance group. Longer duration of treatment significantly predicted resistance (adjusted OR, 1.07; P = .03; 95% CI: 1.01 – 1.03). For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop resistance.
The incidence of resistant organisms after exposure to a cephalosporin was not statistically different between groups (adjusted OR, 1.74; P = .36; 95% CI: 1.0 – 1.2). In the resistance group, 28% of the antibiotics prescribed were cephalosporins (cefuroxime, ceftriaxone, ceftazidime, and cefepime), which were used for Proteus mirabilis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. In the no resistance group, 17% of the antibiotics prescribed were cephalosporins (cefepime only) and were used for Proteus mirabilis.
Organisms treated with penicillin were significantly less likely to become resistant (adjusted OR, 0.26; P = .04; 95% CI: 0.07 - 0.96). In the resistance group, 16% of the antibiotics were penicillins (piperacillin/tazobactam), which were used for Escherichia coli, Enterococcus faecalis, Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. In the no resistance group, 22% of the antibiotics were penicillins (amoxicillin, amoxicillin/clavulanate and piperacillin/tazobactam), which were used for Proteus mirabilis, Enterococcus faecalis, and Acinetobacter baumannii.
Discussion
Longer duration of treatment significantly increased resistance on the subsequent culture in this study. For every additional day the patient was on an antibiotic, he or she was 7% more likely to develop a resistance. However, the potential impact of using a given antibiotic class on the acquisition of resistance in patients with SCI who had a UTI was not demonstrated. Surprisingly, the use of a cephalosporin was not associated with an increased incidence of resistance in this study, which was inconsistent with the findings from other studies.10 Weber and colleagues evaluated nosocomial infections in the intensive care unit. The authors suggested that restriction on the use of third-generation cephalosporins might decrease antibiotic resistance, especially in extended spectrum beta-lactamase producing gram-negative bacilli.11
The difference in this study may be explained by the lower incidence of Escherichia coli and Klebsiella pneumoniae, which are known to exhibit inducible resistance on exposure to third-generation cephalosporins. Conversely, it was found that patients treated with a penicillin were significantly less likely to develop resistant organisms from subsequent cultures. The most common penicillin used in this study’s patient population was piperacillin/tazobactam.
For complicated UTIs including pyelonephritis, the European Association of Urology (EAU) guidelines for the management of urinary and male genital tract infections recommend treatment for 3 to 5 days after defervescence or control of complicating factors.12 These recommendations could lead to much shorter treatment durations than the traditional 14-day “standard” course often prescribed. One meta-analysis recommends a 5-day course for UTIs without fever in patients with SCI vs a 14-day course for patients with fever.13 Due to the lack of data, care often varies based on the patient’s clinical status, provider experience, and opinions. The Pannek study surveyed 16 centers that specialized in SCI care. When compared with the recommendations in the EAU guidelines, the study found providers in > 50% of the responding facilities overtreated UTIs.14
Limitations
This study has several limitations. First, the sample size was much smaller than expected. Of the 355 charts reviewed, only 86 met all the criteria to be included, which limited analysis. Additionally, given the retrospective nature of the study, it was impossible to determine provider rationale for the treatment. Since a diagnosis of UTI in patients with SCI often cannot be done with conventional methods due to lack of symptoms, many investigators have emphasized the use of quantitative urinalysis to differentiate true infection vs contamination.15-17
According to the National Institute on Disability and Rehabilitation Research consensus conference recommendations, the definition of significant bacteriuria will vary, depending on the method of bladder drainage.18 While this study reviewed microbiologic cultures and the type of patient’s urinary catheter, the method of bladder drainage in the context of quantitative urinalysis was not evaluated, which limited the interpretation of microbiologic data.
It was also impossible to determine whether bacteria were cleared by the initial treatment, leading to new bacterial strains with a multidrug resistance, or whether patients relapsed. While antibiotic selection was appropriate for antimicrobial coverage, this study was not designed to detect potential inadequacies in dosing, which could also affect resistance. Last, since no genetic evaluation of the microorganisms was done, the authors cannot be sure whether the microorganisms noted on the first urine culture were of the same genetic makeup as those identified in the second urine culture.
Conclusion
Optimal duration of therapy for treatment of UTIs in patients with SCI is unclear. Despite its limitations, the study suggests exposure to longer antibiotic treatment courses may lead to increased antimicrobial resistance in the urinary tract organisms in this patient population. Further investigation with a larger sample size is required to confirm these findings.
Author disclosures
Dr. Bedimo received research grant funding from Janssen Pharmaceuticals and Merck and Company. He also serves as an ad hoc scientific advisor for Viiv Healthcare, Gilead Science, and BMD Science. All other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Saint S, Lipsky BA. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch Intern Med. 1999;159(8):800-808.
2. Laupland KB, Bagshaw SM, Gregson DB, Kirkpatrick AW, Ross T, Church DL. Intensive care unit-acquired urinary tract infections in a regional critical care system. Crit Care. 2005;9(2):R60-R65.
3. Girard R, Mazoyer MA, Plauchu MM, Rode G. High prevalence of nosocomial infections in rehabilitation units accounted for by urinary tract infections in patients with spinal cord injury. J Hosp Infect. 2006;62(4):473-479.
4. Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76(3):272-280.
5. Salomon J, Gory A, Bernard L, Ruffion A, Denys P, Chartier-Kastler E. [Urinary tract infection and neurogenic bladder]. Prog Urol. 2007;17(3):448-453.
6. Ena J, Amador C, Martinez C, Ortiz de la Tabla V. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J Urol. 1995;153(1):117-120.
7. Allen UD, MacDonald N, Fuite L, Chan F, Stephens D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ. 1999;160(10):1436-1440.
8. De Mouy D, Cavallo JD, Armengaud M, et al. [Urinary tract infection in an urban population: Etiology and antibiotic sensitivity as a function of patient history]. Presse Med. 1999;28(30):1624-1628.
9. Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81(6):764-769.
10. Shah PS, Cannon JP, Sullivan CL, Nemchausky B, Pachucki CT. Controlling antimicrobial use and decreasing microbiological laboratory tests for urinary tract infections in spinal-cord-injury patients with chronic indwelling catheters. Am J Health Syst Pharm. 2005;62(1):74-77.
11. Weber DJ, Raasch R, Rutala WA. Nosocomial infections in the ICU: The growing importance of antibiotic-resistant pathogens. Chest. 1999;115(suppl 3):34S-41S.
12. Naber KG, Bergman B, Bishop MC, et al; Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001;40(5):576-588.
13. Everaert K, Lumen N, Kerckhaert W, Willaert P, van Driel M. Urinary tract infections in spinal cord injury: Prevention and treatment guidelines. Acta Clin Belg. 2009;64(4):335-340.
14. Pannek J. Treatment of urinary tract infection in persons with spinal cord injury: Guidelines, evidence, and clinical practice. A questionnaire-based survey and review of the literature. J Spinal Cord Med. 2011;34(1):11-15.
15. Musher DM, Thorsteinsson SB, Airola VM II. Quantitative urinalysis. Diagnosing urinary tract infection in men. JAMA. 1976;236(18):2069-2072.
16. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part two: Diagnostic value of symptoms and of quantitative urinalysis. J Am Paraplegia Soc. 1985;8(1):7-10.
17. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients. Part one: Bacteriologic diagnosis. J Am Paraplegia Soc. 1985;8(1):4-6.
18. Garcia Leoni ME, Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9(8):780-785.
Enhancing Patient Satisfaction Through the Use of Complementary Therapies
In October 1998, the National Center for Complementary and Alternative Medicine (NCCAM) was funded and established. This center is the federal government’s lead agency for scientific research on complementary and alternative medicine (CAM) and is 1 of the 27 institutes and centers that make up the National Institutes of Health. The mission of the NCCAM is to define, through rigorous scientific investigation, the usefulness and safety of CAM interventions and roles in improving health and health care.
Although a significant number of adults in the U.S. use some form of CAM, physicians rarely recommend these therapies to their patients, and their use is limited in conventional medical settings.1-3 This is often attributed to a lack of knowledge or scientific evidence, despite a belief by many providers of the potential positive effects.3
In an attempt to disseminate knowledge about various CAM therapies investigated by NCCAM, the Complementary and Alternative Resources to Enhance Satisfaction (CARES) program was organized as a resource center at the Louis Stokes Cleveland VA Medical Center (VAMC). It was anticipated that increasing knowledge about CAM and offering these therapies in conjunction with the conventional medical practices at the VAMC would lead to a more comprehensive, patient-centered system of care. In this way, the goal was to transform current thinking from a focus solely on treating the patient’s disease to a holistic approach, which encompassed comfort, family support, and quality of life (QOL).
Background
The number of veterans with chronic illnesses and pain continues to rise. While aggressive efforts have been aimed at safely decreasing pain and discomfort, many veterans report dissatisfaction with traditional treatment methods, which focus on drug therapy and have little emphasis on preventive or holistic care.4 Health care providers often share patients’ frustrations regarding the use of medications that have varying degrees of efficacy and multiple adverse effects. Innovative approaches to improving health and decreasing pain and stress have focused on more holistic and patient-centered philosophies of care. However, there have been few studies to assess feasibility, implementation, and outcomes within an established medical center.
As an ideal goal among patients, families, and HCPs in all care settings, patient-centered care has become a more prominent focus of the VA health care system (VAHCS). The incorporation of patient-centered care, along with an electronic medical record, structural transformation, and greater focus on performance accountability have contributed to dramatic improvements in care within the VAHCS in the past decade.5,6 Mounting evidence continues to validate the positive health outcomes of models of care that engage patients and families with valuable roles in the healing process.7,8 Professional caregiver satisfaction has also been linked to increased patient satisfaction.9
Integral to patient-centered care is the ability of caregivers to see the whole person—body, mind, and soul. The implementation of therapies or environments that complement traditional medicine and provide for physical comfort and pain management can be important in achieving this form of holistic medicine.1,10 By definition, CAM is any method used outside of and in addition to conventional medicine to prevent or treat disease.6 As CAM takes a holistic approach to healing, most therapies involve not only the treatment of the symptoms of the illness, but also the development of a method of healing that focuses on the spiritual and emotional origins from which the illness arises.11
According to the National Health Interview Survey, complementary and alternative therapies were used by one-third of adults in the U.S. in 2002 and by 4 in 10 adults in 2007.11 However, these estimates may be conservative, as other studies have found that at least the majority of adults had used some form of CAM at one time.1 The most common CAM therapies used by adults in 2007 were nonvitamin, nonmineral, natural products, such as fish oil or ginseng; deep breathing exercises; meditation; chiropractic or osteopathic manipulation; massage; and yoga.11 In 2007, adults most commonly used CAM to treat a variety of musculoskeletal problems (ie, back, neck, or joint pain).11
As a patient-centered philosophy, the most general benefit of the use of CAM involves the idea of patient empowerment and participation in the healing process. Many therapies, such as tai chi, meditation, and guided imagery, require active patient involvement, which can encourage feelings of self-control over the disease process. Complementary and alternative medicine has been shown to be effective in decreasing pain, anxiety, stress, and nausea.10,12-14 Increasing evidence supports an association between stress or negative emotions and health outcomes, such as hypertension, diabetes, and heart disease.15,16 When used in conjunction with traditional medical treatment, CAM can help patients cope with devastating symptoms of their disease processes or to avoid some symptoms altogether.
Despite the widespread use of CAM therapies by the public, HCPs rarely recommend CAM therapies to their patients.2,3 This has been attributed to a lack of scientific evidence, a lack of knowledge or comfort, and a lack of an available CAM provider.3 The basic philosophy of self-motivated stress and pain management, which is fundamental to most CAM therapies, is learned and embraced by most HCPs, but the implementation is not often seen in the real world of busy clinical practice. With its numerous benefits, CAM has the potential to significantly improve the health and QOL. Therefore, innovative programs that help HCPs become knowledgeable and competent in incorporating CAM into current systems of care are needed.
In 2010, the Cleveland VAMC was funded through the Innovations in Patient-Centered Care grant to design and implement a complementary therapy resource center. This project was the CARES program and was organized through the Cleveland Geriatric Research Education and Clinical Center (GRECC). The project team included researchers and clinicians within the GRECC as well as other clinical departments. A CAM coordinator was hired to organize lectures, order supplies, and network with various departments within the Cleveland VAMC. Additionally, a major focus of the CARES program was to encourage the involvement of family and friends in the care of the veteran. An integral goal of this project was to bring CAM resources to the bedside of veterans in acute and long-term care on a 24/7 basis.
The rationale for the implementation of a complementary therapy resource center was based on the Planetree model of patient-centered care, which encourages healing in all dimensions and the integration of complementary therapies with conventional medical practices.17 Offering such therapies in an established medical center with knowledgeable HCPs may increase the safety of such use.1 Providing workshops and lectures for HCPs about various complementary therapies would help educate them and provide them with a knowledge base to feel comfortable in recommending therapies to their patients. By opening workshops and lectures about CAM to the public, veterans would be given the opportunity to learn about the therapies available and their efficacy.
Advancing Patient QOL
The Cleveland VAMC has a history of research and policies to advance a culture of patient-centered care with an emphasis on QOL, customer service, and the use of CAM.In 2001, Anthony D’Eramo, a member of the Cleveland VAMC GRECC, developed a program to educate nursing assistants at the Cleveland and Chillicothe VAMCs on complementary therapies, including meditation, spirituality, therapeutic touch, and yoga. The overall response to the program was positive.18 The focus of the training was on the QOL of nursing assistants; most found participation in the training to be a valuable and worthwhile experience. They indicated their intent to use the techniques they learned for themselves, their families, and their patients.18
Also in 2001, researchers at the Cleveland and Pittsburgh VAMCs identified that older veterans with osteoarthritis perceived the use of prayer and meditation as more useful than medications or surgery for the treatment of pain associated with osteoarthritis.19 Since that time, the Cleveland VAMC has worked with the Pittsburgh VAMC to study the use of motivational interviewing—a communication technique that focuses on patient engagement to achieve changes in behavior—for patients with knee osteoarthritis to consider total knee replacement surgery.
In 2004, Antall and Kresevic implemented a program of guided imagery for patients undergoing joint replacement surgery.20 Although the sample size was small, results indicated positive trends for pain relief, decreased anxiety, and decreased length of stay following surgery. Due to the small sample size, statistical comparisons were not performed; however, the mean pain medication use in the 4 days following surgery was morphine 84.76 mg in the control group vs 36.7 mg in the guided imagery group.20 The overall response to the guided imagery tapes was positive, with 75% of the subjects indicating that use of the tapes made them feel more relaxed and decreased their pain.
More recently, the clinical nurse specialist group at the Cleveland VAMC began a study using music and education to decrease pain. In 2009, a Patient-Centered Care Council was established for the medical center to advance a culture of patient-centered care by analyzing the results of performance measures and satisfaction reports. Additionally, the nursing staff at the Cleveland VAMC Community Living Center (CLC) expressed an interest in expanding the use of CAM by creating a wellness center with exercise equipment and aromatherapy. This center was well-received but had only limited access to patients in acute and long-term care and was unable to be sustained due to insufficient staffing.
The CARES Program
The objectives of the CARES program were to (1) change the culture of the medical center to a more holistic approach, encouraging family and patient participation in care and emphasizing comfort and satisfaction; (2) increase knowledge of complementary therapies for relaxation; (3) improve patient and family satisfaction with nursing and medical care; and (4) build on preexisting medical center initiatives for patient-centered care.
The CARES program presented lectures and training workshops on various CAM therapies for all HCPs in order to provide useful information that may not otherwise have been available. Evidence has shown that those who receive training for complementary therapies respond positively and view the experience as valuable.18 It was hoped that these training sessions would empower nurses and other health care staff to provide care while recognizing the importance of treating the entire person. Programs were planned for various times of the day and evening in various patient care locations. (Aims and initiatives of the CARES program are further expanded in the Figure.)
Prior to any educational sessions, a survey was distributed to HCPs about their knowledge and experience with CAM. Though responses to the survey were limited, the results indicate interest in learning more about CAM therapies (Table 1).
Over the course of the yearlong grant, a total of 19 workshops were scheduled and held for HCPs and veterans for a total of 346 participants. This included 3 intensive training sessions for staff, 1 on Reiki and 2 on Healing Touch. All programs, including the intensive training sessions, were available free to participants. Some of the sessions were videotaped and archived for later viewing. (See Table 2 for a list of all training sessions provided by the CARES program.) The project was limited in both time and funds, so only a limited number of topics were able to be covered, and the topics were based mostly on the availability of experts in each field.
Resources
In addition to lectures, organizers of the CARES program purchased 20 comfort carts for inpatient units at the Cleveland VAMC. These were small rolling lockable wooden carts approved by Interior Design, who evaluated and designed previous work spaces at the Cleveland VAMC to make them functional, appealing, and well-suited for the veterans. The carts were stocked with various resources that focused on comfort and entertainment. Specifically related to CAM, these carts contained guided imagery CDs and Playaways. (Playaways are small audio players with included earbud headphones meant for individual use, which are preloaded with a specific guided imagery session.) Additionally, the comfort carts contained books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthy snacks, DVDs, and a portable DVD player. Other items purchased to be distributed to various inpatient and outpatient units included Nintendo Wii game consoles and small televisions. Mobile sleepers were purchased for inpatient units to encourage extended-family visitation. These sleepers have been widely adopted throughout the medical center.
Additional resources purchased by the CARES program included educational pamphlets on various health issues affecting veterans, such as the management of stress. In an effort to increase patient education about complementary therapies, the CARES program provided funding for 2 dedicated channels on the patient television system, broadcasting 24-hour, evidence-based relaxation and guided imagery programming. Finally, the CARES program enhanced the Wellness Center begun by the nurses in the CLC. This included the purchase of exercise equipment, computers, aromatherapy, massage tables, and massage cushions. The exercise equipment, including a recumbent stepper, recumbent bike, and treadmill, was provided by funds from the CARES project. The equipment was available 24/7 to veterans and could be accessed once the veteran was cleared by his primary care and admitting physician. Competencies were developed and completed by the staff. The competencies included orienting the patient on use of the equipment, observation and documentation of equipment used, and response. Veterans who had established home exercise routines were able to continue their programs while hospitalized in the CLC. This helped maintain and regain leisure activity and promoted wellness.
Program Outcomes
Evaluations of the training sessions were overwhelmingly positive (Table 3), and many individuals requested further education and training. A total of 204 participants (59%) completed posttraining evaluations. Some common themes identified through comments on program evaluations included requests for training in the evenings and on weekends. Of the 329 HCPs who participated, 36.5% were nurses or nurse practitioners, 13.7% were ancillary staff (eg, nursing assistants), 9.7% were social workers, 8.5% were students, 5.8% were physicians or physician assistants, 5.2% were psychiatry staff members, 4.9% were occupational/physical/recreational therapy staff members, and 15.7% were other/unknown. The remaining 17 individuals who participated were veterans and their family members.
Reiki and guided imagery classes for increasing relaxation and comfort are still offered to veterans. An attendee of the initial level 1 training offered from the first grant progressed in certifications and received Master status. This Master has trained 60% of the nurses in her unit in level 1 Reiki. Weekly sessions are being implemented for veterans. Guided imagery training provided by the initial CARES grant project is sustained via weekly groups. Reports of an increased sense of well-being and relaxation as well as relief from chronic pain have been reported.
Although evaluations were created for the comfort carts, they were not regularly completed by patients. However, direct subjective feedback from nursing staff who spoke to organizers of the project about both the beds and the carts was very positive. Additionally, members of the project were able to talk to some veterans and family members who agreed to discuss their use of these items. They expressed appreciation for the snacks, which helped “tide them over,” and the beds, which allowed them to stay and comfortably visit their sick loved ones. Utilization of the CARES comfort carts and mobile sleepers on the inpatient units continued after completion of this study. The GRECC has continued to function as a resource center by distributing educational materials, restocking the comfort carts, and providing educational programs on CAM.
Objectively measuring satisfaction related to the implementation of the program proved challenging. At program commencement, plans involved an evaluation of the CARES program using overall hospital satisfaction measures. However, different components of the program took effect at different times, and not all components affected all parts of the hospital. Satisfaction measures, such as the National Veteran’s Survey of Healthcare Experiences of Patients (SHEP) and the local Quick-Kards, which report aggregate scores for patient satisfaction, were analyzed prior, during, and after program implementation but could not be clearly correlated to program impact on patient and family satisfaction with health care. Additionally, the categories addressed in the surveys were very broad while the CARES program addressed only some aspects of hospital care. Despite the weak correlation, SHEP results of inpatient services were analyzed and evaluations did increase in the categories of inpatient overall quality and shared decision making from prior-to-program implementation to postprogram implementation. Quick-Kard results remained essentially the same related to patient-provider communication pre- and postprogram implementation. Additional quantitative and qualitative measures of satisfaction linked specifically to program components need to be created or further explored.
Limitations
This project was not able to address all aspects of the wide range of topics under the general term CAM. In a short time, many individuals taught courses in their areas of expertise. However, many areas, such as acupuncture, chiropractic manipulation, and massage therapy, were not included. Additionally, although herbal therapies are likely the most used CAM method, they also present many challenges when combined with medications and other common therapies among veteran patients.11 The study was not intended to provide any general information endorsing the safety of these herbal therapies when combined with medications, so this topic was avoided altogether. However, this is a topic that needs further exploration and medical involvement, as these therapies can have medical consequences despite their casual use and availability.
Conclusions
The most important lesson learned through this program was that CAM is a very “hot topic” at the Cleveland VAMC and many staff members are enthusiastic and open to integrating it into their practice. This was important throughout program implementation as staff buy-in is integral to a successful medical center initiative. Veterans and family members were receptive to learning about CAM and participating in programs. An abundance of local experts outside of the facility were also willing to share their knowledge about their particular therapy.
Securing continuing education (CE) credit hours was challenging, requiring applications and close work with presenters. However, the added benefit of CE credits helped to garner an audience. Marketing the programs in a time sensitive nature to allow staff or family members to arrange schedules was critical.
Multiple opportunities, including initiatives for patient-centered care, CLCs, and management of veterans with pain and delirium can be helpful for maintaining and expanding the CARES program. Most important, it was learned that a small group of clinicians who can think outside the box can make a big difference for veterans. Implementing a holistic and patient-centered program of CAM that brings resources to veterans 24/7 is both feasible and fun.
Future Directions
Plans for future educational programs on CAM will include the use of interactive audio/video technology to expand outreach, yet still allow the active participation of HCPs and possibly veterans. Cleveland VAMC GRECC staff members continue to work on various aspects of the CARES program, such as the use of audio tapes for relaxation and augmentation of pain treatment and to support the Wellness Center. The carts and mobile sleepers are still heavily used to support the “Care Partners” program at the Cleveland VAMC, and they continue to be stocked with items. These items helped meet the project’s goal of providing resources to be available 24/7.
The CARES program and aspects of CAM have continued to be marketed at professional educational activities and to veterans at health fairs at the medical center. Additional funding sources and small grants have helped to sustain the educational programs and restock the carts, particularly the current VA-funded T21 grant to manage patients with delirium. Future funding opportunities continue to be explored. Additionally future directions would include the incorporation of various other methods of CAM, which were unable to be explored in this time-limited project, including acupuncture, chiropractic manipulation, and massage therapy.
Though evaluations of educational programs were very positive and subjective feedback from the use of the carts and mobile sleepers was positive, it was not possible to establish a direct correlation between improved patient and family satisfaction and health care. Future directions of program evaluation should focus on objective measurements, which can be directly linked to program impact on satisfaction. It is hoped that the inclusion of CAM will contribute to continued improvements in quality and patient satisfaction throughout the entire VAHCS.
Acknowledgements
This manuscript and the program described are the results of work funded by the VHA Innovations for Patient Centered Care and supported by the use of resources and facilities at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, specifically, the Geriatric Research Education and Clinical Center (GRECC).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Vohra S, Feldman K, Johnston B, Waters K, Boon H. Integrating complementary and alternative medicine into academic medical centers: Experience and perceptions of nine leading centers in North America. BMC Health Serv Res. 2005;5:78-84.
2. Kurtz ME, Nolan RB, Rittinger WJ. Primary care physicians’ attitudes and practices regarding complementary and alternative medicine. J Am Osteopath Assoc. 2003;103(12):597-602.
3. Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physician’s attitudes toward complementary and alternative medicine and their knowledge of specific therapies: A survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501.
4. Kroesen K, Baldwin CM, Brooks AJ, Bell IR. U.S. military veterans’ perceptions of the conventional medical care system and their use of complementary and alternative medicine. Fam Pract. 2002;19(1):57-64.
5. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348(22):2218-2227.
6. Perlin JB, Kolodner RM, Roswell RH. The Veterans Health Administration: Quality, value, accountability, and information as transforming strategies for patient-centered care. Am J Manag Care. 2004;10(11, pt 2):828-836.
7. Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients’ families. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. JAMA. 1994;272(23):1839-1844.
8. Cullen L, Titler M, Drahozal R. Family and pet visitation in the critical care unit. Crit Care Nurse. 2003;23(5):62-67.
9. Haas JS, Cook EF, Puopolo AL, Burstin HR, Cleary PD, Brennan TA. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15(2):122-128.
10. Kreitzer MJ, Snyder M. Healing the heart: Integrating complementary therapies and healing practices into the care of cardiovascular patients. Prog Cardiovasc Nurs. 2002;17(2):73-80.
11. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
12. Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: A systematic review. Arch Intern Med. 2004;164(5):493-501.
13. Gregory S, Verdouw J. Therapeutic touch: Its application for residents in aged care. Aust Nurs J. 2005;12(7):23-25.
14. Hilliard RE. Music therapy in hospice and palliative care: A review of empirical data. Evid Based Complement Alternat Med. 2005;2(2):173-178.
15. Jonas BS, Lando JF. Negative affect as a prospective risk factor for hypertension. Psychosom Med. 2000;62(2):188-196.
16. Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn Emot. 1998;12(2):191-220.
17. Katz DL, Ali A. Integrating complementary and alternative practices into conventional care. In: Frampton SB, Charmel P, eds. Putting Patients First: Best Practices in Patient-Centered Care. 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
18. D’Eramo AL, Papp KK, Rose JH. A program on complementary therapies for long-term care nursing assistants. Geriatr Nurs. 2001;22(4):201-207.
19. Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: A comparison between African American and white patients. Arthritis Rheum. 2001;45(4):340-345.
20. Antall GF, Kresevic D. The use of guided imagery to manage pain in an elderly orthopaedic population. Orthop Nurs. 2004;23(5):335-340.
In October 1998, the National Center for Complementary and Alternative Medicine (NCCAM) was funded and established. This center is the federal government’s lead agency for scientific research on complementary and alternative medicine (CAM) and is 1 of the 27 institutes and centers that make up the National Institutes of Health. The mission of the NCCAM is to define, through rigorous scientific investigation, the usefulness and safety of CAM interventions and roles in improving health and health care.
Although a significant number of adults in the U.S. use some form of CAM, physicians rarely recommend these therapies to their patients, and their use is limited in conventional medical settings.1-3 This is often attributed to a lack of knowledge or scientific evidence, despite a belief by many providers of the potential positive effects.3
In an attempt to disseminate knowledge about various CAM therapies investigated by NCCAM, the Complementary and Alternative Resources to Enhance Satisfaction (CARES) program was organized as a resource center at the Louis Stokes Cleveland VA Medical Center (VAMC). It was anticipated that increasing knowledge about CAM and offering these therapies in conjunction with the conventional medical practices at the VAMC would lead to a more comprehensive, patient-centered system of care. In this way, the goal was to transform current thinking from a focus solely on treating the patient’s disease to a holistic approach, which encompassed comfort, family support, and quality of life (QOL).
Background
The number of veterans with chronic illnesses and pain continues to rise. While aggressive efforts have been aimed at safely decreasing pain and discomfort, many veterans report dissatisfaction with traditional treatment methods, which focus on drug therapy and have little emphasis on preventive or holistic care.4 Health care providers often share patients’ frustrations regarding the use of medications that have varying degrees of efficacy and multiple adverse effects. Innovative approaches to improving health and decreasing pain and stress have focused on more holistic and patient-centered philosophies of care. However, there have been few studies to assess feasibility, implementation, and outcomes within an established medical center.
As an ideal goal among patients, families, and HCPs in all care settings, patient-centered care has become a more prominent focus of the VA health care system (VAHCS). The incorporation of patient-centered care, along with an electronic medical record, structural transformation, and greater focus on performance accountability have contributed to dramatic improvements in care within the VAHCS in the past decade.5,6 Mounting evidence continues to validate the positive health outcomes of models of care that engage patients and families with valuable roles in the healing process.7,8 Professional caregiver satisfaction has also been linked to increased patient satisfaction.9
Integral to patient-centered care is the ability of caregivers to see the whole person—body, mind, and soul. The implementation of therapies or environments that complement traditional medicine and provide for physical comfort and pain management can be important in achieving this form of holistic medicine.1,10 By definition, CAM is any method used outside of and in addition to conventional medicine to prevent or treat disease.6 As CAM takes a holistic approach to healing, most therapies involve not only the treatment of the symptoms of the illness, but also the development of a method of healing that focuses on the spiritual and emotional origins from which the illness arises.11
According to the National Health Interview Survey, complementary and alternative therapies were used by one-third of adults in the U.S. in 2002 and by 4 in 10 adults in 2007.11 However, these estimates may be conservative, as other studies have found that at least the majority of adults had used some form of CAM at one time.1 The most common CAM therapies used by adults in 2007 were nonvitamin, nonmineral, natural products, such as fish oil or ginseng; deep breathing exercises; meditation; chiropractic or osteopathic manipulation; massage; and yoga.11 In 2007, adults most commonly used CAM to treat a variety of musculoskeletal problems (ie, back, neck, or joint pain).11
As a patient-centered philosophy, the most general benefit of the use of CAM involves the idea of patient empowerment and participation in the healing process. Many therapies, such as tai chi, meditation, and guided imagery, require active patient involvement, which can encourage feelings of self-control over the disease process. Complementary and alternative medicine has been shown to be effective in decreasing pain, anxiety, stress, and nausea.10,12-14 Increasing evidence supports an association between stress or negative emotions and health outcomes, such as hypertension, diabetes, and heart disease.15,16 When used in conjunction with traditional medical treatment, CAM can help patients cope with devastating symptoms of their disease processes or to avoid some symptoms altogether.
Despite the widespread use of CAM therapies by the public, HCPs rarely recommend CAM therapies to their patients.2,3 This has been attributed to a lack of scientific evidence, a lack of knowledge or comfort, and a lack of an available CAM provider.3 The basic philosophy of self-motivated stress and pain management, which is fundamental to most CAM therapies, is learned and embraced by most HCPs, but the implementation is not often seen in the real world of busy clinical practice. With its numerous benefits, CAM has the potential to significantly improve the health and QOL. Therefore, innovative programs that help HCPs become knowledgeable and competent in incorporating CAM into current systems of care are needed.
In 2010, the Cleveland VAMC was funded through the Innovations in Patient-Centered Care grant to design and implement a complementary therapy resource center. This project was the CARES program and was organized through the Cleveland Geriatric Research Education and Clinical Center (GRECC). The project team included researchers and clinicians within the GRECC as well as other clinical departments. A CAM coordinator was hired to organize lectures, order supplies, and network with various departments within the Cleveland VAMC. Additionally, a major focus of the CARES program was to encourage the involvement of family and friends in the care of the veteran. An integral goal of this project was to bring CAM resources to the bedside of veterans in acute and long-term care on a 24/7 basis.
The rationale for the implementation of a complementary therapy resource center was based on the Planetree model of patient-centered care, which encourages healing in all dimensions and the integration of complementary therapies with conventional medical practices.17 Offering such therapies in an established medical center with knowledgeable HCPs may increase the safety of such use.1 Providing workshops and lectures for HCPs about various complementary therapies would help educate them and provide them with a knowledge base to feel comfortable in recommending therapies to their patients. By opening workshops and lectures about CAM to the public, veterans would be given the opportunity to learn about the therapies available and their efficacy.
Advancing Patient QOL
The Cleveland VAMC has a history of research and policies to advance a culture of patient-centered care with an emphasis on QOL, customer service, and the use of CAM.In 2001, Anthony D’Eramo, a member of the Cleveland VAMC GRECC, developed a program to educate nursing assistants at the Cleveland and Chillicothe VAMCs on complementary therapies, including meditation, spirituality, therapeutic touch, and yoga. The overall response to the program was positive.18 The focus of the training was on the QOL of nursing assistants; most found participation in the training to be a valuable and worthwhile experience. They indicated their intent to use the techniques they learned for themselves, their families, and their patients.18
Also in 2001, researchers at the Cleveland and Pittsburgh VAMCs identified that older veterans with osteoarthritis perceived the use of prayer and meditation as more useful than medications or surgery for the treatment of pain associated with osteoarthritis.19 Since that time, the Cleveland VAMC has worked with the Pittsburgh VAMC to study the use of motivational interviewing—a communication technique that focuses on patient engagement to achieve changes in behavior—for patients with knee osteoarthritis to consider total knee replacement surgery.
In 2004, Antall and Kresevic implemented a program of guided imagery for patients undergoing joint replacement surgery.20 Although the sample size was small, results indicated positive trends for pain relief, decreased anxiety, and decreased length of stay following surgery. Due to the small sample size, statistical comparisons were not performed; however, the mean pain medication use in the 4 days following surgery was morphine 84.76 mg in the control group vs 36.7 mg in the guided imagery group.20 The overall response to the guided imagery tapes was positive, with 75% of the subjects indicating that use of the tapes made them feel more relaxed and decreased their pain.
More recently, the clinical nurse specialist group at the Cleveland VAMC began a study using music and education to decrease pain. In 2009, a Patient-Centered Care Council was established for the medical center to advance a culture of patient-centered care by analyzing the results of performance measures and satisfaction reports. Additionally, the nursing staff at the Cleveland VAMC Community Living Center (CLC) expressed an interest in expanding the use of CAM by creating a wellness center with exercise equipment and aromatherapy. This center was well-received but had only limited access to patients in acute and long-term care and was unable to be sustained due to insufficient staffing.
The CARES Program
The objectives of the CARES program were to (1) change the culture of the medical center to a more holistic approach, encouraging family and patient participation in care and emphasizing comfort and satisfaction; (2) increase knowledge of complementary therapies for relaxation; (3) improve patient and family satisfaction with nursing and medical care; and (4) build on preexisting medical center initiatives for patient-centered care.
The CARES program presented lectures and training workshops on various CAM therapies for all HCPs in order to provide useful information that may not otherwise have been available. Evidence has shown that those who receive training for complementary therapies respond positively and view the experience as valuable.18 It was hoped that these training sessions would empower nurses and other health care staff to provide care while recognizing the importance of treating the entire person. Programs were planned for various times of the day and evening in various patient care locations. (Aims and initiatives of the CARES program are further expanded in the Figure.)
Prior to any educational sessions, a survey was distributed to HCPs about their knowledge and experience with CAM. Though responses to the survey were limited, the results indicate interest in learning more about CAM therapies (Table 1).
Over the course of the yearlong grant, a total of 19 workshops were scheduled and held for HCPs and veterans for a total of 346 participants. This included 3 intensive training sessions for staff, 1 on Reiki and 2 on Healing Touch. All programs, including the intensive training sessions, were available free to participants. Some of the sessions were videotaped and archived for later viewing. (See Table 2 for a list of all training sessions provided by the CARES program.) The project was limited in both time and funds, so only a limited number of topics were able to be covered, and the topics were based mostly on the availability of experts in each field.
Resources
In addition to lectures, organizers of the CARES program purchased 20 comfort carts for inpatient units at the Cleveland VAMC. These were small rolling lockable wooden carts approved by Interior Design, who evaluated and designed previous work spaces at the Cleveland VAMC to make them functional, appealing, and well-suited for the veterans. The carts were stocked with various resources that focused on comfort and entertainment. Specifically related to CAM, these carts contained guided imagery CDs and Playaways. (Playaways are small audio players with included earbud headphones meant for individual use, which are preloaded with a specific guided imagery session.) Additionally, the comfort carts contained books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthy snacks, DVDs, and a portable DVD player. Other items purchased to be distributed to various inpatient and outpatient units included Nintendo Wii game consoles and small televisions. Mobile sleepers were purchased for inpatient units to encourage extended-family visitation. These sleepers have been widely adopted throughout the medical center.
Additional resources purchased by the CARES program included educational pamphlets on various health issues affecting veterans, such as the management of stress. In an effort to increase patient education about complementary therapies, the CARES program provided funding for 2 dedicated channels on the patient television system, broadcasting 24-hour, evidence-based relaxation and guided imagery programming. Finally, the CARES program enhanced the Wellness Center begun by the nurses in the CLC. This included the purchase of exercise equipment, computers, aromatherapy, massage tables, and massage cushions. The exercise equipment, including a recumbent stepper, recumbent bike, and treadmill, was provided by funds from the CARES project. The equipment was available 24/7 to veterans and could be accessed once the veteran was cleared by his primary care and admitting physician. Competencies were developed and completed by the staff. The competencies included orienting the patient on use of the equipment, observation and documentation of equipment used, and response. Veterans who had established home exercise routines were able to continue their programs while hospitalized in the CLC. This helped maintain and regain leisure activity and promoted wellness.
Program Outcomes
Evaluations of the training sessions were overwhelmingly positive (Table 3), and many individuals requested further education and training. A total of 204 participants (59%) completed posttraining evaluations. Some common themes identified through comments on program evaluations included requests for training in the evenings and on weekends. Of the 329 HCPs who participated, 36.5% were nurses or nurse practitioners, 13.7% were ancillary staff (eg, nursing assistants), 9.7% were social workers, 8.5% were students, 5.8% were physicians or physician assistants, 5.2% were psychiatry staff members, 4.9% were occupational/physical/recreational therapy staff members, and 15.7% were other/unknown. The remaining 17 individuals who participated were veterans and their family members.
Reiki and guided imagery classes for increasing relaxation and comfort are still offered to veterans. An attendee of the initial level 1 training offered from the first grant progressed in certifications and received Master status. This Master has trained 60% of the nurses in her unit in level 1 Reiki. Weekly sessions are being implemented for veterans. Guided imagery training provided by the initial CARES grant project is sustained via weekly groups. Reports of an increased sense of well-being and relaxation as well as relief from chronic pain have been reported.
Although evaluations were created for the comfort carts, they were not regularly completed by patients. However, direct subjective feedback from nursing staff who spoke to organizers of the project about both the beds and the carts was very positive. Additionally, members of the project were able to talk to some veterans and family members who agreed to discuss their use of these items. They expressed appreciation for the snacks, which helped “tide them over,” and the beds, which allowed them to stay and comfortably visit their sick loved ones. Utilization of the CARES comfort carts and mobile sleepers on the inpatient units continued after completion of this study. The GRECC has continued to function as a resource center by distributing educational materials, restocking the comfort carts, and providing educational programs on CAM.
Objectively measuring satisfaction related to the implementation of the program proved challenging. At program commencement, plans involved an evaluation of the CARES program using overall hospital satisfaction measures. However, different components of the program took effect at different times, and not all components affected all parts of the hospital. Satisfaction measures, such as the National Veteran’s Survey of Healthcare Experiences of Patients (SHEP) and the local Quick-Kards, which report aggregate scores for patient satisfaction, were analyzed prior, during, and after program implementation but could not be clearly correlated to program impact on patient and family satisfaction with health care. Additionally, the categories addressed in the surveys were very broad while the CARES program addressed only some aspects of hospital care. Despite the weak correlation, SHEP results of inpatient services were analyzed and evaluations did increase in the categories of inpatient overall quality and shared decision making from prior-to-program implementation to postprogram implementation. Quick-Kard results remained essentially the same related to patient-provider communication pre- and postprogram implementation. Additional quantitative and qualitative measures of satisfaction linked specifically to program components need to be created or further explored.
Limitations
This project was not able to address all aspects of the wide range of topics under the general term CAM. In a short time, many individuals taught courses in their areas of expertise. However, many areas, such as acupuncture, chiropractic manipulation, and massage therapy, were not included. Additionally, although herbal therapies are likely the most used CAM method, they also present many challenges when combined with medications and other common therapies among veteran patients.11 The study was not intended to provide any general information endorsing the safety of these herbal therapies when combined with medications, so this topic was avoided altogether. However, this is a topic that needs further exploration and medical involvement, as these therapies can have medical consequences despite their casual use and availability.
Conclusions
The most important lesson learned through this program was that CAM is a very “hot topic” at the Cleveland VAMC and many staff members are enthusiastic and open to integrating it into their practice. This was important throughout program implementation as staff buy-in is integral to a successful medical center initiative. Veterans and family members were receptive to learning about CAM and participating in programs. An abundance of local experts outside of the facility were also willing to share their knowledge about their particular therapy.
Securing continuing education (CE) credit hours was challenging, requiring applications and close work with presenters. However, the added benefit of CE credits helped to garner an audience. Marketing the programs in a time sensitive nature to allow staff or family members to arrange schedules was critical.
Multiple opportunities, including initiatives for patient-centered care, CLCs, and management of veterans with pain and delirium can be helpful for maintaining and expanding the CARES program. Most important, it was learned that a small group of clinicians who can think outside the box can make a big difference for veterans. Implementing a holistic and patient-centered program of CAM that brings resources to veterans 24/7 is both feasible and fun.
Future Directions
Plans for future educational programs on CAM will include the use of interactive audio/video technology to expand outreach, yet still allow the active participation of HCPs and possibly veterans. Cleveland VAMC GRECC staff members continue to work on various aspects of the CARES program, such as the use of audio tapes for relaxation and augmentation of pain treatment and to support the Wellness Center. The carts and mobile sleepers are still heavily used to support the “Care Partners” program at the Cleveland VAMC, and they continue to be stocked with items. These items helped meet the project’s goal of providing resources to be available 24/7.
The CARES program and aspects of CAM have continued to be marketed at professional educational activities and to veterans at health fairs at the medical center. Additional funding sources and small grants have helped to sustain the educational programs and restock the carts, particularly the current VA-funded T21 grant to manage patients with delirium. Future funding opportunities continue to be explored. Additionally future directions would include the incorporation of various other methods of CAM, which were unable to be explored in this time-limited project, including acupuncture, chiropractic manipulation, and massage therapy.
Though evaluations of educational programs were very positive and subjective feedback from the use of the carts and mobile sleepers was positive, it was not possible to establish a direct correlation between improved patient and family satisfaction and health care. Future directions of program evaluation should focus on objective measurements, which can be directly linked to program impact on satisfaction. It is hoped that the inclusion of CAM will contribute to continued improvements in quality and patient satisfaction throughout the entire VAHCS.
Acknowledgements
This manuscript and the program described are the results of work funded by the VHA Innovations for Patient Centered Care and supported by the use of resources and facilities at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, specifically, the Geriatric Research Education and Clinical Center (GRECC).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Vohra S, Feldman K, Johnston B, Waters K, Boon H. Integrating complementary and alternative medicine into academic medical centers: Experience and perceptions of nine leading centers in North America. BMC Health Serv Res. 2005;5:78-84.
2. Kurtz ME, Nolan RB, Rittinger WJ. Primary care physicians’ attitudes and practices regarding complementary and alternative medicine. J Am Osteopath Assoc. 2003;103(12):597-602.
3. Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physician’s attitudes toward complementary and alternative medicine and their knowledge of specific therapies: A survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501.
4. Kroesen K, Baldwin CM, Brooks AJ, Bell IR. U.S. military veterans’ perceptions of the conventional medical care system and their use of complementary and alternative medicine. Fam Pract. 2002;19(1):57-64.
5. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348(22):2218-2227.
6. Perlin JB, Kolodner RM, Roswell RH. The Veterans Health Administration: Quality, value, accountability, and information as transforming strategies for patient-centered care. Am J Manag Care. 2004;10(11, pt 2):828-836.
7. Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients’ families. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. JAMA. 1994;272(23):1839-1844.
8. Cullen L, Titler M, Drahozal R. Family and pet visitation in the critical care unit. Crit Care Nurse. 2003;23(5):62-67.
9. Haas JS, Cook EF, Puopolo AL, Burstin HR, Cleary PD, Brennan TA. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15(2):122-128.
10. Kreitzer MJ, Snyder M. Healing the heart: Integrating complementary therapies and healing practices into the care of cardiovascular patients. Prog Cardiovasc Nurs. 2002;17(2):73-80.
11. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
12. Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: A systematic review. Arch Intern Med. 2004;164(5):493-501.
13. Gregory S, Verdouw J. Therapeutic touch: Its application for residents in aged care. Aust Nurs J. 2005;12(7):23-25.
14. Hilliard RE. Music therapy in hospice and palliative care: A review of empirical data. Evid Based Complement Alternat Med. 2005;2(2):173-178.
15. Jonas BS, Lando JF. Negative affect as a prospective risk factor for hypertension. Psychosom Med. 2000;62(2):188-196.
16. Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn Emot. 1998;12(2):191-220.
17. Katz DL, Ali A. Integrating complementary and alternative practices into conventional care. In: Frampton SB, Charmel P, eds. Putting Patients First: Best Practices in Patient-Centered Care. 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
18. D’Eramo AL, Papp KK, Rose JH. A program on complementary therapies for long-term care nursing assistants. Geriatr Nurs. 2001;22(4):201-207.
19. Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: A comparison between African American and white patients. Arthritis Rheum. 2001;45(4):340-345.
20. Antall GF, Kresevic D. The use of guided imagery to manage pain in an elderly orthopaedic population. Orthop Nurs. 2004;23(5):335-340.
In October 1998, the National Center for Complementary and Alternative Medicine (NCCAM) was funded and established. This center is the federal government’s lead agency for scientific research on complementary and alternative medicine (CAM) and is 1 of the 27 institutes and centers that make up the National Institutes of Health. The mission of the NCCAM is to define, through rigorous scientific investigation, the usefulness and safety of CAM interventions and roles in improving health and health care.
Although a significant number of adults in the U.S. use some form of CAM, physicians rarely recommend these therapies to their patients, and their use is limited in conventional medical settings.1-3 This is often attributed to a lack of knowledge or scientific evidence, despite a belief by many providers of the potential positive effects.3
In an attempt to disseminate knowledge about various CAM therapies investigated by NCCAM, the Complementary and Alternative Resources to Enhance Satisfaction (CARES) program was organized as a resource center at the Louis Stokes Cleveland VA Medical Center (VAMC). It was anticipated that increasing knowledge about CAM and offering these therapies in conjunction with the conventional medical practices at the VAMC would lead to a more comprehensive, patient-centered system of care. In this way, the goal was to transform current thinking from a focus solely on treating the patient’s disease to a holistic approach, which encompassed comfort, family support, and quality of life (QOL).
Background
The number of veterans with chronic illnesses and pain continues to rise. While aggressive efforts have been aimed at safely decreasing pain and discomfort, many veterans report dissatisfaction with traditional treatment methods, which focus on drug therapy and have little emphasis on preventive or holistic care.4 Health care providers often share patients’ frustrations regarding the use of medications that have varying degrees of efficacy and multiple adverse effects. Innovative approaches to improving health and decreasing pain and stress have focused on more holistic and patient-centered philosophies of care. However, there have been few studies to assess feasibility, implementation, and outcomes within an established medical center.
As an ideal goal among patients, families, and HCPs in all care settings, patient-centered care has become a more prominent focus of the VA health care system (VAHCS). The incorporation of patient-centered care, along with an electronic medical record, structural transformation, and greater focus on performance accountability have contributed to dramatic improvements in care within the VAHCS in the past decade.5,6 Mounting evidence continues to validate the positive health outcomes of models of care that engage patients and families with valuable roles in the healing process.7,8 Professional caregiver satisfaction has also been linked to increased patient satisfaction.9
Integral to patient-centered care is the ability of caregivers to see the whole person—body, mind, and soul. The implementation of therapies or environments that complement traditional medicine and provide for physical comfort and pain management can be important in achieving this form of holistic medicine.1,10 By definition, CAM is any method used outside of and in addition to conventional medicine to prevent or treat disease.6 As CAM takes a holistic approach to healing, most therapies involve not only the treatment of the symptoms of the illness, but also the development of a method of healing that focuses on the spiritual and emotional origins from which the illness arises.11
According to the National Health Interview Survey, complementary and alternative therapies were used by one-third of adults in the U.S. in 2002 and by 4 in 10 adults in 2007.11 However, these estimates may be conservative, as other studies have found that at least the majority of adults had used some form of CAM at one time.1 The most common CAM therapies used by adults in 2007 were nonvitamin, nonmineral, natural products, such as fish oil or ginseng; deep breathing exercises; meditation; chiropractic or osteopathic manipulation; massage; and yoga.11 In 2007, adults most commonly used CAM to treat a variety of musculoskeletal problems (ie, back, neck, or joint pain).11
As a patient-centered philosophy, the most general benefit of the use of CAM involves the idea of patient empowerment and participation in the healing process. Many therapies, such as tai chi, meditation, and guided imagery, require active patient involvement, which can encourage feelings of self-control over the disease process. Complementary and alternative medicine has been shown to be effective in decreasing pain, anxiety, stress, and nausea.10,12-14 Increasing evidence supports an association between stress or negative emotions and health outcomes, such as hypertension, diabetes, and heart disease.15,16 When used in conjunction with traditional medical treatment, CAM can help patients cope with devastating symptoms of their disease processes or to avoid some symptoms altogether.
Despite the widespread use of CAM therapies by the public, HCPs rarely recommend CAM therapies to their patients.2,3 This has been attributed to a lack of scientific evidence, a lack of knowledge or comfort, and a lack of an available CAM provider.3 The basic philosophy of self-motivated stress and pain management, which is fundamental to most CAM therapies, is learned and embraced by most HCPs, but the implementation is not often seen in the real world of busy clinical practice. With its numerous benefits, CAM has the potential to significantly improve the health and QOL. Therefore, innovative programs that help HCPs become knowledgeable and competent in incorporating CAM into current systems of care are needed.
In 2010, the Cleveland VAMC was funded through the Innovations in Patient-Centered Care grant to design and implement a complementary therapy resource center. This project was the CARES program and was organized through the Cleveland Geriatric Research Education and Clinical Center (GRECC). The project team included researchers and clinicians within the GRECC as well as other clinical departments. A CAM coordinator was hired to organize lectures, order supplies, and network with various departments within the Cleveland VAMC. Additionally, a major focus of the CARES program was to encourage the involvement of family and friends in the care of the veteran. An integral goal of this project was to bring CAM resources to the bedside of veterans in acute and long-term care on a 24/7 basis.
The rationale for the implementation of a complementary therapy resource center was based on the Planetree model of patient-centered care, which encourages healing in all dimensions and the integration of complementary therapies with conventional medical practices.17 Offering such therapies in an established medical center with knowledgeable HCPs may increase the safety of such use.1 Providing workshops and lectures for HCPs about various complementary therapies would help educate them and provide them with a knowledge base to feel comfortable in recommending therapies to their patients. By opening workshops and lectures about CAM to the public, veterans would be given the opportunity to learn about the therapies available and their efficacy.
Advancing Patient QOL
The Cleveland VAMC has a history of research and policies to advance a culture of patient-centered care with an emphasis on QOL, customer service, and the use of CAM.In 2001, Anthony D’Eramo, a member of the Cleveland VAMC GRECC, developed a program to educate nursing assistants at the Cleveland and Chillicothe VAMCs on complementary therapies, including meditation, spirituality, therapeutic touch, and yoga. The overall response to the program was positive.18 The focus of the training was on the QOL of nursing assistants; most found participation in the training to be a valuable and worthwhile experience. They indicated their intent to use the techniques they learned for themselves, their families, and their patients.18
Also in 2001, researchers at the Cleveland and Pittsburgh VAMCs identified that older veterans with osteoarthritis perceived the use of prayer and meditation as more useful than medications or surgery for the treatment of pain associated with osteoarthritis.19 Since that time, the Cleveland VAMC has worked with the Pittsburgh VAMC to study the use of motivational interviewing—a communication technique that focuses on patient engagement to achieve changes in behavior—for patients with knee osteoarthritis to consider total knee replacement surgery.
In 2004, Antall and Kresevic implemented a program of guided imagery for patients undergoing joint replacement surgery.20 Although the sample size was small, results indicated positive trends for pain relief, decreased anxiety, and decreased length of stay following surgery. Due to the small sample size, statistical comparisons were not performed; however, the mean pain medication use in the 4 days following surgery was morphine 84.76 mg in the control group vs 36.7 mg in the guided imagery group.20 The overall response to the guided imagery tapes was positive, with 75% of the subjects indicating that use of the tapes made them feel more relaxed and decreased their pain.
More recently, the clinical nurse specialist group at the Cleveland VAMC began a study using music and education to decrease pain. In 2009, a Patient-Centered Care Council was established for the medical center to advance a culture of patient-centered care by analyzing the results of performance measures and satisfaction reports. Additionally, the nursing staff at the Cleveland VAMC Community Living Center (CLC) expressed an interest in expanding the use of CAM by creating a wellness center with exercise equipment and aromatherapy. This center was well-received but had only limited access to patients in acute and long-term care and was unable to be sustained due to insufficient staffing.
The CARES Program
The objectives of the CARES program were to (1) change the culture of the medical center to a more holistic approach, encouraging family and patient participation in care and emphasizing comfort and satisfaction; (2) increase knowledge of complementary therapies for relaxation; (3) improve patient and family satisfaction with nursing and medical care; and (4) build on preexisting medical center initiatives for patient-centered care.
The CARES program presented lectures and training workshops on various CAM therapies for all HCPs in order to provide useful information that may not otherwise have been available. Evidence has shown that those who receive training for complementary therapies respond positively and view the experience as valuable.18 It was hoped that these training sessions would empower nurses and other health care staff to provide care while recognizing the importance of treating the entire person. Programs were planned for various times of the day and evening in various patient care locations. (Aims and initiatives of the CARES program are further expanded in the Figure.)
Prior to any educational sessions, a survey was distributed to HCPs about their knowledge and experience with CAM. Though responses to the survey were limited, the results indicate interest in learning more about CAM therapies (Table 1).
Over the course of the yearlong grant, a total of 19 workshops were scheduled and held for HCPs and veterans for a total of 346 participants. This included 3 intensive training sessions for staff, 1 on Reiki and 2 on Healing Touch. All programs, including the intensive training sessions, were available free to participants. Some of the sessions were videotaped and archived for later viewing. (See Table 2 for a list of all training sessions provided by the CARES program.) The project was limited in both time and funds, so only a limited number of topics were able to be covered, and the topics were based mostly on the availability of experts in each field.
Resources
In addition to lectures, organizers of the CARES program purchased 20 comfort carts for inpatient units at the Cleveland VAMC. These were small rolling lockable wooden carts approved by Interior Design, who evaluated and designed previous work spaces at the Cleveland VAMC to make them functional, appealing, and well-suited for the veterans. The carts were stocked with various resources that focused on comfort and entertainment. Specifically related to CAM, these carts contained guided imagery CDs and Playaways. (Playaways are small audio players with included earbud headphones meant for individual use, which are preloaded with a specific guided imagery session.) Additionally, the comfort carts contained books, books on tape, magazines, portable CD players, music CDs, games, exercise bands, healthy snacks, DVDs, and a portable DVD player. Other items purchased to be distributed to various inpatient and outpatient units included Nintendo Wii game consoles and small televisions. Mobile sleepers were purchased for inpatient units to encourage extended-family visitation. These sleepers have been widely adopted throughout the medical center.
Additional resources purchased by the CARES program included educational pamphlets on various health issues affecting veterans, such as the management of stress. In an effort to increase patient education about complementary therapies, the CARES program provided funding for 2 dedicated channels on the patient television system, broadcasting 24-hour, evidence-based relaxation and guided imagery programming. Finally, the CARES program enhanced the Wellness Center begun by the nurses in the CLC. This included the purchase of exercise equipment, computers, aromatherapy, massage tables, and massage cushions. The exercise equipment, including a recumbent stepper, recumbent bike, and treadmill, was provided by funds from the CARES project. The equipment was available 24/7 to veterans and could be accessed once the veteran was cleared by his primary care and admitting physician. Competencies were developed and completed by the staff. The competencies included orienting the patient on use of the equipment, observation and documentation of equipment used, and response. Veterans who had established home exercise routines were able to continue their programs while hospitalized in the CLC. This helped maintain and regain leisure activity and promoted wellness.
Program Outcomes
Evaluations of the training sessions were overwhelmingly positive (Table 3), and many individuals requested further education and training. A total of 204 participants (59%) completed posttraining evaluations. Some common themes identified through comments on program evaluations included requests for training in the evenings and on weekends. Of the 329 HCPs who participated, 36.5% were nurses or nurse practitioners, 13.7% were ancillary staff (eg, nursing assistants), 9.7% were social workers, 8.5% were students, 5.8% were physicians or physician assistants, 5.2% were psychiatry staff members, 4.9% were occupational/physical/recreational therapy staff members, and 15.7% were other/unknown. The remaining 17 individuals who participated were veterans and their family members.
Reiki and guided imagery classes for increasing relaxation and comfort are still offered to veterans. An attendee of the initial level 1 training offered from the first grant progressed in certifications and received Master status. This Master has trained 60% of the nurses in her unit in level 1 Reiki. Weekly sessions are being implemented for veterans. Guided imagery training provided by the initial CARES grant project is sustained via weekly groups. Reports of an increased sense of well-being and relaxation as well as relief from chronic pain have been reported.
Although evaluations were created for the comfort carts, they were not regularly completed by patients. However, direct subjective feedback from nursing staff who spoke to organizers of the project about both the beds and the carts was very positive. Additionally, members of the project were able to talk to some veterans and family members who agreed to discuss their use of these items. They expressed appreciation for the snacks, which helped “tide them over,” and the beds, which allowed them to stay and comfortably visit their sick loved ones. Utilization of the CARES comfort carts and mobile sleepers on the inpatient units continued after completion of this study. The GRECC has continued to function as a resource center by distributing educational materials, restocking the comfort carts, and providing educational programs on CAM.
Objectively measuring satisfaction related to the implementation of the program proved challenging. At program commencement, plans involved an evaluation of the CARES program using overall hospital satisfaction measures. However, different components of the program took effect at different times, and not all components affected all parts of the hospital. Satisfaction measures, such as the National Veteran’s Survey of Healthcare Experiences of Patients (SHEP) and the local Quick-Kards, which report aggregate scores for patient satisfaction, were analyzed prior, during, and after program implementation but could not be clearly correlated to program impact on patient and family satisfaction with health care. Additionally, the categories addressed in the surveys were very broad while the CARES program addressed only some aspects of hospital care. Despite the weak correlation, SHEP results of inpatient services were analyzed and evaluations did increase in the categories of inpatient overall quality and shared decision making from prior-to-program implementation to postprogram implementation. Quick-Kard results remained essentially the same related to patient-provider communication pre- and postprogram implementation. Additional quantitative and qualitative measures of satisfaction linked specifically to program components need to be created or further explored.
Limitations
This project was not able to address all aspects of the wide range of topics under the general term CAM. In a short time, many individuals taught courses in their areas of expertise. However, many areas, such as acupuncture, chiropractic manipulation, and massage therapy, were not included. Additionally, although herbal therapies are likely the most used CAM method, they also present many challenges when combined with medications and other common therapies among veteran patients.11 The study was not intended to provide any general information endorsing the safety of these herbal therapies when combined with medications, so this topic was avoided altogether. However, this is a topic that needs further exploration and medical involvement, as these therapies can have medical consequences despite their casual use and availability.
Conclusions
The most important lesson learned through this program was that CAM is a very “hot topic” at the Cleveland VAMC and many staff members are enthusiastic and open to integrating it into their practice. This was important throughout program implementation as staff buy-in is integral to a successful medical center initiative. Veterans and family members were receptive to learning about CAM and participating in programs. An abundance of local experts outside of the facility were also willing to share their knowledge about their particular therapy.
Securing continuing education (CE) credit hours was challenging, requiring applications and close work with presenters. However, the added benefit of CE credits helped to garner an audience. Marketing the programs in a time sensitive nature to allow staff or family members to arrange schedules was critical.
Multiple opportunities, including initiatives for patient-centered care, CLCs, and management of veterans with pain and delirium can be helpful for maintaining and expanding the CARES program. Most important, it was learned that a small group of clinicians who can think outside the box can make a big difference for veterans. Implementing a holistic and patient-centered program of CAM that brings resources to veterans 24/7 is both feasible and fun.
Future Directions
Plans for future educational programs on CAM will include the use of interactive audio/video technology to expand outreach, yet still allow the active participation of HCPs and possibly veterans. Cleveland VAMC GRECC staff members continue to work on various aspects of the CARES program, such as the use of audio tapes for relaxation and augmentation of pain treatment and to support the Wellness Center. The carts and mobile sleepers are still heavily used to support the “Care Partners” program at the Cleveland VAMC, and they continue to be stocked with items. These items helped meet the project’s goal of providing resources to be available 24/7.
The CARES program and aspects of CAM have continued to be marketed at professional educational activities and to veterans at health fairs at the medical center. Additional funding sources and small grants have helped to sustain the educational programs and restock the carts, particularly the current VA-funded T21 grant to manage patients with delirium. Future funding opportunities continue to be explored. Additionally future directions would include the incorporation of various other methods of CAM, which were unable to be explored in this time-limited project, including acupuncture, chiropractic manipulation, and massage therapy.
Though evaluations of educational programs were very positive and subjective feedback from the use of the carts and mobile sleepers was positive, it was not possible to establish a direct correlation between improved patient and family satisfaction and health care. Future directions of program evaluation should focus on objective measurements, which can be directly linked to program impact on satisfaction. It is hoped that the inclusion of CAM will contribute to continued improvements in quality and patient satisfaction throughout the entire VAHCS.
Acknowledgements
This manuscript and the program described are the results of work funded by the VHA Innovations for Patient Centered Care and supported by the use of resources and facilities at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, specifically, the Geriatric Research Education and Clinical Center (GRECC).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Vohra S, Feldman K, Johnston B, Waters K, Boon H. Integrating complementary and alternative medicine into academic medical centers: Experience and perceptions of nine leading centers in North America. BMC Health Serv Res. 2005;5:78-84.
2. Kurtz ME, Nolan RB, Rittinger WJ. Primary care physicians’ attitudes and practices regarding complementary and alternative medicine. J Am Osteopath Assoc. 2003;103(12):597-602.
3. Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physician’s attitudes toward complementary and alternative medicine and their knowledge of specific therapies: A survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501.
4. Kroesen K, Baldwin CM, Brooks AJ, Bell IR. U.S. military veterans’ perceptions of the conventional medical care system and their use of complementary and alternative medicine. Fam Pract. 2002;19(1):57-64.
5. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348(22):2218-2227.
6. Perlin JB, Kolodner RM, Roswell RH. The Veterans Health Administration: Quality, value, accountability, and information as transforming strategies for patient-centered care. Am J Manag Care. 2004;10(11, pt 2):828-836.
7. Covinsky KE, Goldman L, Cook EF, et al. The impact of serious illness on patients’ families. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. JAMA. 1994;272(23):1839-1844.
8. Cullen L, Titler M, Drahozal R. Family and pet visitation in the critical care unit. Crit Care Nurse. 2003;23(5):62-67.
9. Haas JS, Cook EF, Puopolo AL, Burstin HR, Cleary PD, Brennan TA. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15(2):122-128.
10. Kreitzer MJ, Snyder M. Healing the heart: Integrating complementary therapies and healing practices into the care of cardiovascular patients. Prog Cardiovasc Nurs. 2002;17(2):73-80.
11. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
12. Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: A systematic review. Arch Intern Med. 2004;164(5):493-501.
13. Gregory S, Verdouw J. Therapeutic touch: Its application for residents in aged care. Aust Nurs J. 2005;12(7):23-25.
14. Hilliard RE. Music therapy in hospice and palliative care: A review of empirical data. Evid Based Complement Alternat Med. 2005;2(2):173-178.
15. Jonas BS, Lando JF. Negative affect as a prospective risk factor for hypertension. Psychosom Med. 2000;62(2):188-196.
16. Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn Emot. 1998;12(2):191-220.
17. Katz DL, Ali A. Integrating complementary and alternative practices into conventional care. In: Frampton SB, Charmel P, eds. Putting Patients First: Best Practices in Patient-Centered Care. 2nd ed. San Francisco, CA: Jossey-Bass; 2009.
18. D’Eramo AL, Papp KK, Rose JH. A program on complementary therapies for long-term care nursing assistants. Geriatr Nurs. 2001;22(4):201-207.
19. Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: A comparison between African American and white patients. Arthritis Rheum. 2001;45(4):340-345.
20. Antall GF, Kresevic D. The use of guided imagery to manage pain in an elderly orthopaedic population. Orthop Nurs. 2004;23(5):335-340.
The Clinical Impact of Electronic Consultation in Diabetes Care
In the U.S., the prevalence of diabetes continues to escalate at alarming rates. From 1980-2010 the incidence of diabetes increased by 200% for people aged < 45 years, 124% for people 45 to 64 years, and 126% for people 65 to 74 years. Furthermore, based on the National Institute of Health, diabetes currently affects 25.8 million people in the U.S.1,2 Glycemic control has been demonstrated to reduce the risk of microvascular disease in patients with diabetes.3 Most patients with diabetes are managed by primary care practitioners (PCPs), and as the number of patients with diabetes continues to increase, there is an increasing demand on PCPs to achieve recommended glycemic targets.4
The Veterans Health Administration (VHA) VISN 16 has a notably higher prevalence of diabetes compared with that of the national rate. Failure to achieve glycemic targets continues to impose an escalating economic burden.3 Endocrine assistance is often sought by PCPs, but due to a scarcity of endocrinologists, patients commonly wait weeks or months before being seen. Furthermore, rural patients often must travel for several hours before they can reach a specialty center.
The Office of Specialty Care Transformation has provided a unique opportunity for PCPs to gain greater access to specialty advice via electronic consultations. This initiative allows PCPs and specialists to communicate promptly, to institute definitive solutions for patient care, and to augment the clinical and academic aims of primary and specialty care providers. The Michael E. DeBakey VAMC (MEDVAMC) in Houston, Texas, was chosen to initiate a VISN 16-wide diabetes management e-consult service (DMECS).
Endocrinologists at MEDVAMC developed DMECS to serve as a multifunctional tool to reach as many veterans as possible throughout VISN 16, broaden the scope of the existing diabetes endocrine practice, and engender a collaborative spirit between PCPs and specialty care providers. Initiation of this service has been particularly useful for patients with physical disabilities or financial constraints whose care is managed at the community-based outpatient clinics (CBOCs). The purpose of this article is to discuss the concept and initiation of the DMECS, the structure of the consult note, the implementation process, early provider feedback on the project, and future plans.
The DMECS Process
The DMECS allows endocrinologists to provide advice to PCPs to help improve diabetes care while minimizing travel to specialty centers. The advice generated by the DMECS is electronically conveyed to the referring physician (usually the PCP), not to the patient. The requesting physician is then responsible for implementing the recommendations. The DMECS does not comanage patients with diabetes but rather provides support to the PCP in complex cases that would otherwise require an outside referral.
The diabetes management e-consult team has 1 administrator and 3 health care providers (HCPs). Promotion of the service consisted of electronic distribution of flyers to all primary care teams, posters in the main lobby of the hospital, and electronic distribution of a letter to all VISN 16 HCPs. The DMECS team contacted the chiefs of primary care and CBOC directors to promote the service. Communication was augmented by scheduling videoconferencing with all interested facilities. Presentations were given to the VISN-wide transformational care collaborative and women’s health groups.
Any specialties that assist in diabetes management, including ophthalmology and vascular medicine, were encouraged to refer consults to DMECS if clinically indicated. The recommendation is that PCPs submit an e-consult for any patient with a hemoglobin A1C (A1C) > 9%. The only prerequisite to placing the e-consult order is an A1C > 7.5% within the preceding 3 months. Any patient with reported or objective evidence of hypoglycemia is eligible for an e-consult, regardless of the A1C value. Women who are pregnant and patients on an insulin pump are excluded from the program.
All diabetes e-consults are supervised by a board-certified endocrinologist and are resolved within 2 to 3 business days. On receipt of the consult request, the DMECS provider reviews the chart, including active medication lists, blood glucose levels documented in progress notes, care and coordination of home telehealth data regarding blood glucose levels and changes in diabetes medication management, laboratory results and pharmacy refill patterns.
Recommendations are completed and the DMECS provider alerts the requesting physician by adding them as a cosigner to the note in the Computerized Patient Record System (CPRS). When possible, the patient’s nurse manager is also added to the note. For interfacility consults, the DMECS provider contacts the requesting provider directly via email or telephone. Consistent communication with the requesting physician ensures clarity of understanding between specialist and PCP.
The e-consult recommendations are consolidated into 3 distinct sections. The Impression section provides an explanation to the provider about the specialist’s impression of current diabetes control and the reasoning behind the recommendations. The Recommendation section lists in medical terminology the recommended changes to diabetes medications. A unique component to the e-consult is the Instructions to Patient section, which summarizes both oral and insulin medications that can be provided to the patient. Every note includes a Diabetes Surveillance section and several web links to diabetes education that can be downloaded through the MyHealtheVet website.
Current approaches to e-consult implementation are subject to the discretion of the requesting provider. The most commonly observed approach is that the requesting provider reviews the e-consult note and requests that the patient’s nurse manager instructs the patient on the recommendations. Some providers schedule the patient for a physician or nurse visit to discuss the diabetes management recommendations in a clinic setting. Other providers contact the patient by telephone and mail the instructions to the patient.
To streamline the e-consult implementation process, the DMECS team has the option of placing an e-consult 1 week before a patient’s scheduled clinic visit with the PCP. This helps ensure that the e-consult is completed within 2 to 3 business days before the patient’s scheduled primary care appointment, at which time the recommendations can be implemented. Using the option of a “pre-clinic e-consult” method expedites the implementation process.
Initial Results of DMECS
The first e-consult was completed on January 23, 2012. Since its inception, 3,703 e-consults have been completed. There has been a steady increase in the number of referrals, with an average of 154 e-consults completed monthly from January 2012 to December 2013 (Figure 1). Most e-consults have been completed based on requests submitted by providers in Houston, Texas and affiliated CBOCs. However, a growing number of interfacility consults have been completed for providers at VISN-16 facilities located in Louisiana, Mississippi, Arkansas, and Oklahoma (Figure 2).
The initial response to the e-consult service has been positive. One provider described DMECS as a means to “obtain faster access to an endocrinologist’s input for complex diabetics, which has resulted in faster intervention for patients, particularly those at high risk.”
Additionally, another provider noted, “Along with all the benefits of accelerated access to specialty care recommendations, the patients benefit because they do not have to travel to the VA to receive this care. In many cases, they don’t have to be scheduled to see the endocrinologist, if the treatment recommendations are successful.”
One of the nurse managers explained that “The e-consult service has given me a guide to manage each veteran’s diabetes…One veteran stated that he initially was seeing a private endocrinologist at an outside clinic for his diabetes, but when he lost his insurance and began to receive his care at the VA, he stated that he never realized how high the quality of services for diabetes is at the VA.” With regard to implementation, she noted that “the diabetes instructions as provided by the e-consult specialist enhance the patient’s sense of personalized care.”
Limitations
Another challenge observed by DMECS providers is the variation in the length of time for implementing DMECS recommendations by the requesting providers. Due to the novelty of this service, providers across the VISN are still becoming acquainted with the e-consult process.
In an effort to assist PCPs, DMECS providers perform an objective chart review about 3 months after the e-consult is completed. A note is placed in the CPRS that documents whether e-consult recommendations were implemented and the date of implementation. With time, it is anticipated that a standardized set of recommendations for requesting providers may be instituted to serve as a suggested algorithm for timely and efficient implementation of the e-consult recommendations.
DMECS Goals
In addition to supporting the MEDVAMC initiative to improve glycemic trends among all patients with diabetes within the facility, DMECS providers hope to share in VISN-wide efforts to improve diabetes control by broadening the interfacility referral base. The most successful methods of advertisement and consult recruitment include the recommendation that all patients with diabetes with an A1C > 9% receive an e-consult. Also, when any patient with an A1C > 9% is seen at any of the MEDVAMC eye clinics, an alert is sent to the PCP from the DMECS team, suggesting placement of an e-consult. These strategies have increased the number of referrals within the MEDVAMC, and the goal is to implement similar strategies in all primary care, geriatrics, and women’s health clinics across VISN 16.
There are many sites across the VISN that may not have ready access to certified diabetes educators. In support of the VHA goals to promote virtual health, the DMECS team plans to initiate diabetes patient education sessions through clinical videoconferencing with patients in groups or individually.
In addition to the continued growth of the e-consult service and their efforts at patient education, the DMECS providers are also initiating a CME-accredited course for PCPs and HCPs on outpatient management of diabetes, which will be led by 1 of the 4 endocrinology staff at MEDVAMC. The benefits of provider education have been demonstrated by the University of New Mexico Health Science Center’s Project ECHO, which not only improved the quality of care for hepatitis C in a rural territory, but also increased PCP awareness and capacity to treat and manage complex patients.5 Project ECHO was used as the model for the initiation of the Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) program at the VA. Accordingly, the DMECS providers envision that continued efforts at provider education should facilitate an improvement in clinical management strategies used by PCPs to optimize diabetes control.
Now that the diabetes management e-consult program has been set up and seems to play an additive role in the management of outpatient diabetes, the next step is to assess the effect of the diabetes e-consult service on patient clinical outcomes. Currently, DMECS is completing retrospective outcome studies to investigate the baseline characteristics of patients who are referred for the e-consult. These DMECS results will be compared with face-to-face diabetes care and management in a specialty clinic. In addition researchers will attempt to assess whether the time-to-implementation of recommendations has an impact on changes in glycemic parameters.
Conclusion
In support of the VHA goal of veteran-centered care, the diabetes e-consult service for VISN 16 is an innovative and creative addition to the armamentarium of outpatient diabetes management that has accelerated access to endocrine diabetes care. The service has reached > 1,000 veterans with diabetes since its inception and is set to continue expanding its referral base across VISN 16. Through DMECS, specialty care has become more readily accessible to providers and patients across a greater geographic area. The diabetes management e-consult service has been particularly useful for patients with physical disabilities or financial constraints and has been able to bridge the communication gap between primary and specialty care, with the goal of improving diabetes outcomes for veterans across the VISN.
Acknowledgments
The authors would like to express their appreciation for the assistance provided by the program analysts who extracted the number of completed diabetes e-consults from the VISN 16 data warehouse: Pamela Croston, Melody Darbe, and Andrew Spiegelman, PhD.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Centers for Disease Control and Prevention. Percentage of civilian non-institutionalized population with diagnosed diabetes, by age, United States, 1980-2010. Centers for Disease Control and Prevention Website. http://www.cdc.gov/Diabetes/statistics/prev/national/figbyage.htm. Accessed February 5, 2014.
2. National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Centers for Disease Control and Prevention Website. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 4, 2014.
3. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
4. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2010;364(23):2199-2207.
In the U.S., the prevalence of diabetes continues to escalate at alarming rates. From 1980-2010 the incidence of diabetes increased by 200% for people aged < 45 years, 124% for people 45 to 64 years, and 126% for people 65 to 74 years. Furthermore, based on the National Institute of Health, diabetes currently affects 25.8 million people in the U.S.1,2 Glycemic control has been demonstrated to reduce the risk of microvascular disease in patients with diabetes.3 Most patients with diabetes are managed by primary care practitioners (PCPs), and as the number of patients with diabetes continues to increase, there is an increasing demand on PCPs to achieve recommended glycemic targets.4
The Veterans Health Administration (VHA) VISN 16 has a notably higher prevalence of diabetes compared with that of the national rate. Failure to achieve glycemic targets continues to impose an escalating economic burden.3 Endocrine assistance is often sought by PCPs, but due to a scarcity of endocrinologists, patients commonly wait weeks or months before being seen. Furthermore, rural patients often must travel for several hours before they can reach a specialty center.
The Office of Specialty Care Transformation has provided a unique opportunity for PCPs to gain greater access to specialty advice via electronic consultations. This initiative allows PCPs and specialists to communicate promptly, to institute definitive solutions for patient care, and to augment the clinical and academic aims of primary and specialty care providers. The Michael E. DeBakey VAMC (MEDVAMC) in Houston, Texas, was chosen to initiate a VISN 16-wide diabetes management e-consult service (DMECS).
Endocrinologists at MEDVAMC developed DMECS to serve as a multifunctional tool to reach as many veterans as possible throughout VISN 16, broaden the scope of the existing diabetes endocrine practice, and engender a collaborative spirit between PCPs and specialty care providers. Initiation of this service has been particularly useful for patients with physical disabilities or financial constraints whose care is managed at the community-based outpatient clinics (CBOCs). The purpose of this article is to discuss the concept and initiation of the DMECS, the structure of the consult note, the implementation process, early provider feedback on the project, and future plans.
The DMECS Process
The DMECS allows endocrinologists to provide advice to PCPs to help improve diabetes care while minimizing travel to specialty centers. The advice generated by the DMECS is electronically conveyed to the referring physician (usually the PCP), not to the patient. The requesting physician is then responsible for implementing the recommendations. The DMECS does not comanage patients with diabetes but rather provides support to the PCP in complex cases that would otherwise require an outside referral.
The diabetes management e-consult team has 1 administrator and 3 health care providers (HCPs). Promotion of the service consisted of electronic distribution of flyers to all primary care teams, posters in the main lobby of the hospital, and electronic distribution of a letter to all VISN 16 HCPs. The DMECS team contacted the chiefs of primary care and CBOC directors to promote the service. Communication was augmented by scheduling videoconferencing with all interested facilities. Presentations were given to the VISN-wide transformational care collaborative and women’s health groups.
Any specialties that assist in diabetes management, including ophthalmology and vascular medicine, were encouraged to refer consults to DMECS if clinically indicated. The recommendation is that PCPs submit an e-consult for any patient with a hemoglobin A1C (A1C) > 9%. The only prerequisite to placing the e-consult order is an A1C > 7.5% within the preceding 3 months. Any patient with reported or objective evidence of hypoglycemia is eligible for an e-consult, regardless of the A1C value. Women who are pregnant and patients on an insulin pump are excluded from the program.
All diabetes e-consults are supervised by a board-certified endocrinologist and are resolved within 2 to 3 business days. On receipt of the consult request, the DMECS provider reviews the chart, including active medication lists, blood glucose levels documented in progress notes, care and coordination of home telehealth data regarding blood glucose levels and changes in diabetes medication management, laboratory results and pharmacy refill patterns.
Recommendations are completed and the DMECS provider alerts the requesting physician by adding them as a cosigner to the note in the Computerized Patient Record System (CPRS). When possible, the patient’s nurse manager is also added to the note. For interfacility consults, the DMECS provider contacts the requesting provider directly via email or telephone. Consistent communication with the requesting physician ensures clarity of understanding between specialist and PCP.
The e-consult recommendations are consolidated into 3 distinct sections. The Impression section provides an explanation to the provider about the specialist’s impression of current diabetes control and the reasoning behind the recommendations. The Recommendation section lists in medical terminology the recommended changes to diabetes medications. A unique component to the e-consult is the Instructions to Patient section, which summarizes both oral and insulin medications that can be provided to the patient. Every note includes a Diabetes Surveillance section and several web links to diabetes education that can be downloaded through the MyHealtheVet website.
Current approaches to e-consult implementation are subject to the discretion of the requesting provider. The most commonly observed approach is that the requesting provider reviews the e-consult note and requests that the patient’s nurse manager instructs the patient on the recommendations. Some providers schedule the patient for a physician or nurse visit to discuss the diabetes management recommendations in a clinic setting. Other providers contact the patient by telephone and mail the instructions to the patient.
To streamline the e-consult implementation process, the DMECS team has the option of placing an e-consult 1 week before a patient’s scheduled clinic visit with the PCP. This helps ensure that the e-consult is completed within 2 to 3 business days before the patient’s scheduled primary care appointment, at which time the recommendations can be implemented. Using the option of a “pre-clinic e-consult” method expedites the implementation process.
Initial Results of DMECS
The first e-consult was completed on January 23, 2012. Since its inception, 3,703 e-consults have been completed. There has been a steady increase in the number of referrals, with an average of 154 e-consults completed monthly from January 2012 to December 2013 (Figure 1). Most e-consults have been completed based on requests submitted by providers in Houston, Texas and affiliated CBOCs. However, a growing number of interfacility consults have been completed for providers at VISN-16 facilities located in Louisiana, Mississippi, Arkansas, and Oklahoma (Figure 2).
The initial response to the e-consult service has been positive. One provider described DMECS as a means to “obtain faster access to an endocrinologist’s input for complex diabetics, which has resulted in faster intervention for patients, particularly those at high risk.”
Additionally, another provider noted, “Along with all the benefits of accelerated access to specialty care recommendations, the patients benefit because they do not have to travel to the VA to receive this care. In many cases, they don’t have to be scheduled to see the endocrinologist, if the treatment recommendations are successful.”
One of the nurse managers explained that “The e-consult service has given me a guide to manage each veteran’s diabetes…One veteran stated that he initially was seeing a private endocrinologist at an outside clinic for his diabetes, but when he lost his insurance and began to receive his care at the VA, he stated that he never realized how high the quality of services for diabetes is at the VA.” With regard to implementation, she noted that “the diabetes instructions as provided by the e-consult specialist enhance the patient’s sense of personalized care.”
Limitations
Another challenge observed by DMECS providers is the variation in the length of time for implementing DMECS recommendations by the requesting providers. Due to the novelty of this service, providers across the VISN are still becoming acquainted with the e-consult process.
In an effort to assist PCPs, DMECS providers perform an objective chart review about 3 months after the e-consult is completed. A note is placed in the CPRS that documents whether e-consult recommendations were implemented and the date of implementation. With time, it is anticipated that a standardized set of recommendations for requesting providers may be instituted to serve as a suggested algorithm for timely and efficient implementation of the e-consult recommendations.
DMECS Goals
In addition to supporting the MEDVAMC initiative to improve glycemic trends among all patients with diabetes within the facility, DMECS providers hope to share in VISN-wide efforts to improve diabetes control by broadening the interfacility referral base. The most successful methods of advertisement and consult recruitment include the recommendation that all patients with diabetes with an A1C > 9% receive an e-consult. Also, when any patient with an A1C > 9% is seen at any of the MEDVAMC eye clinics, an alert is sent to the PCP from the DMECS team, suggesting placement of an e-consult. These strategies have increased the number of referrals within the MEDVAMC, and the goal is to implement similar strategies in all primary care, geriatrics, and women’s health clinics across VISN 16.
There are many sites across the VISN that may not have ready access to certified diabetes educators. In support of the VHA goals to promote virtual health, the DMECS team plans to initiate diabetes patient education sessions through clinical videoconferencing with patients in groups or individually.
In addition to the continued growth of the e-consult service and their efforts at patient education, the DMECS providers are also initiating a CME-accredited course for PCPs and HCPs on outpatient management of diabetes, which will be led by 1 of the 4 endocrinology staff at MEDVAMC. The benefits of provider education have been demonstrated by the University of New Mexico Health Science Center’s Project ECHO, which not only improved the quality of care for hepatitis C in a rural territory, but also increased PCP awareness and capacity to treat and manage complex patients.5 Project ECHO was used as the model for the initiation of the Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) program at the VA. Accordingly, the DMECS providers envision that continued efforts at provider education should facilitate an improvement in clinical management strategies used by PCPs to optimize diabetes control.
Now that the diabetes management e-consult program has been set up and seems to play an additive role in the management of outpatient diabetes, the next step is to assess the effect of the diabetes e-consult service on patient clinical outcomes. Currently, DMECS is completing retrospective outcome studies to investigate the baseline characteristics of patients who are referred for the e-consult. These DMECS results will be compared with face-to-face diabetes care and management in a specialty clinic. In addition researchers will attempt to assess whether the time-to-implementation of recommendations has an impact on changes in glycemic parameters.
Conclusion
In support of the VHA goal of veteran-centered care, the diabetes e-consult service for VISN 16 is an innovative and creative addition to the armamentarium of outpatient diabetes management that has accelerated access to endocrine diabetes care. The service has reached > 1,000 veterans with diabetes since its inception and is set to continue expanding its referral base across VISN 16. Through DMECS, specialty care has become more readily accessible to providers and patients across a greater geographic area. The diabetes management e-consult service has been particularly useful for patients with physical disabilities or financial constraints and has been able to bridge the communication gap between primary and specialty care, with the goal of improving diabetes outcomes for veterans across the VISN.
Acknowledgments
The authors would like to express their appreciation for the assistance provided by the program analysts who extracted the number of completed diabetes e-consults from the VISN 16 data warehouse: Pamela Croston, Melody Darbe, and Andrew Spiegelman, PhD.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Centers for Disease Control and Prevention. Percentage of civilian non-institutionalized population with diagnosed diabetes, by age, United States, 1980-2010. Centers for Disease Control and Prevention Website. http://www.cdc.gov/Diabetes/statistics/prev/national/figbyage.htm. Accessed February 5, 2014.
2. National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Centers for Disease Control and Prevention Website. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 4, 2014.
3. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
4. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2010;364(23):2199-2207.
In the U.S., the prevalence of diabetes continues to escalate at alarming rates. From 1980-2010 the incidence of diabetes increased by 200% for people aged < 45 years, 124% for people 45 to 64 years, and 126% for people 65 to 74 years. Furthermore, based on the National Institute of Health, diabetes currently affects 25.8 million people in the U.S.1,2 Glycemic control has been demonstrated to reduce the risk of microvascular disease in patients with diabetes.3 Most patients with diabetes are managed by primary care practitioners (PCPs), and as the number of patients with diabetes continues to increase, there is an increasing demand on PCPs to achieve recommended glycemic targets.4
The Veterans Health Administration (VHA) VISN 16 has a notably higher prevalence of diabetes compared with that of the national rate. Failure to achieve glycemic targets continues to impose an escalating economic burden.3 Endocrine assistance is often sought by PCPs, but due to a scarcity of endocrinologists, patients commonly wait weeks or months before being seen. Furthermore, rural patients often must travel for several hours before they can reach a specialty center.
The Office of Specialty Care Transformation has provided a unique opportunity for PCPs to gain greater access to specialty advice via electronic consultations. This initiative allows PCPs and specialists to communicate promptly, to institute definitive solutions for patient care, and to augment the clinical and academic aims of primary and specialty care providers. The Michael E. DeBakey VAMC (MEDVAMC) in Houston, Texas, was chosen to initiate a VISN 16-wide diabetes management e-consult service (DMECS).
Endocrinologists at MEDVAMC developed DMECS to serve as a multifunctional tool to reach as many veterans as possible throughout VISN 16, broaden the scope of the existing diabetes endocrine practice, and engender a collaborative spirit between PCPs and specialty care providers. Initiation of this service has been particularly useful for patients with physical disabilities or financial constraints whose care is managed at the community-based outpatient clinics (CBOCs). The purpose of this article is to discuss the concept and initiation of the DMECS, the structure of the consult note, the implementation process, early provider feedback on the project, and future plans.
The DMECS Process
The DMECS allows endocrinologists to provide advice to PCPs to help improve diabetes care while minimizing travel to specialty centers. The advice generated by the DMECS is electronically conveyed to the referring physician (usually the PCP), not to the patient. The requesting physician is then responsible for implementing the recommendations. The DMECS does not comanage patients with diabetes but rather provides support to the PCP in complex cases that would otherwise require an outside referral.
The diabetes management e-consult team has 1 administrator and 3 health care providers (HCPs). Promotion of the service consisted of electronic distribution of flyers to all primary care teams, posters in the main lobby of the hospital, and electronic distribution of a letter to all VISN 16 HCPs. The DMECS team contacted the chiefs of primary care and CBOC directors to promote the service. Communication was augmented by scheduling videoconferencing with all interested facilities. Presentations were given to the VISN-wide transformational care collaborative and women’s health groups.
Any specialties that assist in diabetes management, including ophthalmology and vascular medicine, were encouraged to refer consults to DMECS if clinically indicated. The recommendation is that PCPs submit an e-consult for any patient with a hemoglobin A1C (A1C) > 9%. The only prerequisite to placing the e-consult order is an A1C > 7.5% within the preceding 3 months. Any patient with reported or objective evidence of hypoglycemia is eligible for an e-consult, regardless of the A1C value. Women who are pregnant and patients on an insulin pump are excluded from the program.
All diabetes e-consults are supervised by a board-certified endocrinologist and are resolved within 2 to 3 business days. On receipt of the consult request, the DMECS provider reviews the chart, including active medication lists, blood glucose levels documented in progress notes, care and coordination of home telehealth data regarding blood glucose levels and changes in diabetes medication management, laboratory results and pharmacy refill patterns.
Recommendations are completed and the DMECS provider alerts the requesting physician by adding them as a cosigner to the note in the Computerized Patient Record System (CPRS). When possible, the patient’s nurse manager is also added to the note. For interfacility consults, the DMECS provider contacts the requesting provider directly via email or telephone. Consistent communication with the requesting physician ensures clarity of understanding between specialist and PCP.
The e-consult recommendations are consolidated into 3 distinct sections. The Impression section provides an explanation to the provider about the specialist’s impression of current diabetes control and the reasoning behind the recommendations. The Recommendation section lists in medical terminology the recommended changes to diabetes medications. A unique component to the e-consult is the Instructions to Patient section, which summarizes both oral and insulin medications that can be provided to the patient. Every note includes a Diabetes Surveillance section and several web links to diabetes education that can be downloaded through the MyHealtheVet website.
Current approaches to e-consult implementation are subject to the discretion of the requesting provider. The most commonly observed approach is that the requesting provider reviews the e-consult note and requests that the patient’s nurse manager instructs the patient on the recommendations. Some providers schedule the patient for a physician or nurse visit to discuss the diabetes management recommendations in a clinic setting. Other providers contact the patient by telephone and mail the instructions to the patient.
To streamline the e-consult implementation process, the DMECS team has the option of placing an e-consult 1 week before a patient’s scheduled clinic visit with the PCP. This helps ensure that the e-consult is completed within 2 to 3 business days before the patient’s scheduled primary care appointment, at which time the recommendations can be implemented. Using the option of a “pre-clinic e-consult” method expedites the implementation process.
Initial Results of DMECS
The first e-consult was completed on January 23, 2012. Since its inception, 3,703 e-consults have been completed. There has been a steady increase in the number of referrals, with an average of 154 e-consults completed monthly from January 2012 to December 2013 (Figure 1). Most e-consults have been completed based on requests submitted by providers in Houston, Texas and affiliated CBOCs. However, a growing number of interfacility consults have been completed for providers at VISN-16 facilities located in Louisiana, Mississippi, Arkansas, and Oklahoma (Figure 2).
The initial response to the e-consult service has been positive. One provider described DMECS as a means to “obtain faster access to an endocrinologist’s input for complex diabetics, which has resulted in faster intervention for patients, particularly those at high risk.”
Additionally, another provider noted, “Along with all the benefits of accelerated access to specialty care recommendations, the patients benefit because they do not have to travel to the VA to receive this care. In many cases, they don’t have to be scheduled to see the endocrinologist, if the treatment recommendations are successful.”
One of the nurse managers explained that “The e-consult service has given me a guide to manage each veteran’s diabetes…One veteran stated that he initially was seeing a private endocrinologist at an outside clinic for his diabetes, but when he lost his insurance and began to receive his care at the VA, he stated that he never realized how high the quality of services for diabetes is at the VA.” With regard to implementation, she noted that “the diabetes instructions as provided by the e-consult specialist enhance the patient’s sense of personalized care.”
Limitations
Another challenge observed by DMECS providers is the variation in the length of time for implementing DMECS recommendations by the requesting providers. Due to the novelty of this service, providers across the VISN are still becoming acquainted with the e-consult process.
In an effort to assist PCPs, DMECS providers perform an objective chart review about 3 months after the e-consult is completed. A note is placed in the CPRS that documents whether e-consult recommendations were implemented and the date of implementation. With time, it is anticipated that a standardized set of recommendations for requesting providers may be instituted to serve as a suggested algorithm for timely and efficient implementation of the e-consult recommendations.
DMECS Goals
In addition to supporting the MEDVAMC initiative to improve glycemic trends among all patients with diabetes within the facility, DMECS providers hope to share in VISN-wide efforts to improve diabetes control by broadening the interfacility referral base. The most successful methods of advertisement and consult recruitment include the recommendation that all patients with diabetes with an A1C > 9% receive an e-consult. Also, when any patient with an A1C > 9% is seen at any of the MEDVAMC eye clinics, an alert is sent to the PCP from the DMECS team, suggesting placement of an e-consult. These strategies have increased the number of referrals within the MEDVAMC, and the goal is to implement similar strategies in all primary care, geriatrics, and women’s health clinics across VISN 16.
There are many sites across the VISN that may not have ready access to certified diabetes educators. In support of the VHA goals to promote virtual health, the DMECS team plans to initiate diabetes patient education sessions through clinical videoconferencing with patients in groups or individually.
In addition to the continued growth of the e-consult service and their efforts at patient education, the DMECS providers are also initiating a CME-accredited course for PCPs and HCPs on outpatient management of diabetes, which will be led by 1 of the 4 endocrinology staff at MEDVAMC. The benefits of provider education have been demonstrated by the University of New Mexico Health Science Center’s Project ECHO, which not only improved the quality of care for hepatitis C in a rural territory, but also increased PCP awareness and capacity to treat and manage complex patients.5 Project ECHO was used as the model for the initiation of the Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) program at the VA. Accordingly, the DMECS providers envision that continued efforts at provider education should facilitate an improvement in clinical management strategies used by PCPs to optimize diabetes control.
Now that the diabetes management e-consult program has been set up and seems to play an additive role in the management of outpatient diabetes, the next step is to assess the effect of the diabetes e-consult service on patient clinical outcomes. Currently, DMECS is completing retrospective outcome studies to investigate the baseline characteristics of patients who are referred for the e-consult. These DMECS results will be compared with face-to-face diabetes care and management in a specialty clinic. In addition researchers will attempt to assess whether the time-to-implementation of recommendations has an impact on changes in glycemic parameters.
Conclusion
In support of the VHA goal of veteran-centered care, the diabetes e-consult service for VISN 16 is an innovative and creative addition to the armamentarium of outpatient diabetes management that has accelerated access to endocrine diabetes care. The service has reached > 1,000 veterans with diabetes since its inception and is set to continue expanding its referral base across VISN 16. Through DMECS, specialty care has become more readily accessible to providers and patients across a greater geographic area. The diabetes management e-consult service has been particularly useful for patients with physical disabilities or financial constraints and has been able to bridge the communication gap between primary and specialty care, with the goal of improving diabetes outcomes for veterans across the VISN.
Acknowledgments
The authors would like to express their appreciation for the assistance provided by the program analysts who extracted the number of completed diabetes e-consults from the VISN 16 data warehouse: Pamela Croston, Melody Darbe, and Andrew Spiegelman, PhD.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Centers for Disease Control and Prevention. Percentage of civilian non-institutionalized population with diagnosed diabetes, by age, United States, 1980-2010. Centers for Disease Control and Prevention Website. http://www.cdc.gov/Diabetes/statistics/prev/national/figbyage.htm. Accessed February 5, 2014.
2. National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Centers for Disease Control and Prevention Website. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 4, 2014.
3. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
4. American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
5. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2010;364(23):2199-2207.
Group calls for standardized data collection practices across cancer centers

Credit: CDC
Researchers have identified significant variations in how cancer centers gather data, particularly that pertaining to racial and ethnic minorities.
Although racial and ethnic categories were similar across the centers, those categories were defined differently.
And the centers’ definitions of “catchment area,” the geographic region they expect to influence with their programs, differed widely.
This research, published in Cancer, was part of a national effort to recruit more racial/ethnic minorities into clinical trials and, ultimately, reduce the disproportional incidence of many cancers among those populations.
Five National Cancer Institute-designated comprehensive cancer centers participated in the endeavor, known as EMPaCT—Enhancing Minority Participation in Clinical Trials. They were:
- University of Minnesota, Minneapolis, which represents the Midwest and targets the accrual of Native Americans and African Americans
- University of Alabama, Birmingham, representing the Southeast, targeting African Americans
- Johns Hopkins University, representing the East, targeting African Americans
- University of Texas MD Anderson, Houston, representing the Southwest, targeting Latinos
- University of California, Davis, representing the West, targeting Asian Americans.
Ernest T. Hawk, MD, of the MD Anderson Cancer Center, and his colleagues reviewed the collection and reporting of patient data and other practices by these 5 centers.
This revealed significant variation in the centers’ methods of data collection. For example, patients’ insurance status was routinely documented at 2 centers, collected for non-research patients only at a third center, collected for billing of researcher enrollees at a fourth center, and not documented at all at a fifth center.
There were differences in data collection according to race/ethnicity as well. Racial/ethnic categories were generally similar across the centers—white, black/African American, Asian, Native American, Hispanic/Latino, and “other/unknown.”
However, the means of race/ethnicity data collection differed. Each center collected self-reported data on race/ethnicity, but 2 centers included data from staff observations.
Two centers compared the proportions of racial/ethnic groups enrolled in trials with those of their catchment area(s). But the others did not.
The centers also differed in how they defined their patient catchment area, in terms of their cancer patient-vs-general-population specificity, levels of specificity, and geographic coverage.
That merits notice, according to the researchers, because National Cancer Institute cancer centers are required to accrue women and minorities to clinical trials in rough proportion to the cancer patient population of the center’s primary catchment area.
Given these findings, the researchers recommended better standardization of data definition, collection, and reporting as an essential first step toward expanding minority participation in clinical trials.
The team also advised that cancer centers collect socioeconomic data, including a patient’s income and education levels, given past evidence of the strong link between socioeconomic status and cancer outcomes.
Finally, the group recommended collecting patient zip codes and insurance status to allow researchers to assess differences in access to clinical trials that may be related to geography and the availability of health insurance coverage. ![]()

Credit: CDC
Researchers have identified significant variations in how cancer centers gather data, particularly that pertaining to racial and ethnic minorities.
Although racial and ethnic categories were similar across the centers, those categories were defined differently.
And the centers’ definitions of “catchment area,” the geographic region they expect to influence with their programs, differed widely.
This research, published in Cancer, was part of a national effort to recruit more racial/ethnic minorities into clinical trials and, ultimately, reduce the disproportional incidence of many cancers among those populations.
Five National Cancer Institute-designated comprehensive cancer centers participated in the endeavor, known as EMPaCT—Enhancing Minority Participation in Clinical Trials. They were:
- University of Minnesota, Minneapolis, which represents the Midwest and targets the accrual of Native Americans and African Americans
- University of Alabama, Birmingham, representing the Southeast, targeting African Americans
- Johns Hopkins University, representing the East, targeting African Americans
- University of Texas MD Anderson, Houston, representing the Southwest, targeting Latinos
- University of California, Davis, representing the West, targeting Asian Americans.
Ernest T. Hawk, MD, of the MD Anderson Cancer Center, and his colleagues reviewed the collection and reporting of patient data and other practices by these 5 centers.
This revealed significant variation in the centers’ methods of data collection. For example, patients’ insurance status was routinely documented at 2 centers, collected for non-research patients only at a third center, collected for billing of researcher enrollees at a fourth center, and not documented at all at a fifth center.
There were differences in data collection according to race/ethnicity as well. Racial/ethnic categories were generally similar across the centers—white, black/African American, Asian, Native American, Hispanic/Latino, and “other/unknown.”
However, the means of race/ethnicity data collection differed. Each center collected self-reported data on race/ethnicity, but 2 centers included data from staff observations.
Two centers compared the proportions of racial/ethnic groups enrolled in trials with those of their catchment area(s). But the others did not.
The centers also differed in how they defined their patient catchment area, in terms of their cancer patient-vs-general-population specificity, levels of specificity, and geographic coverage.
That merits notice, according to the researchers, because National Cancer Institute cancer centers are required to accrue women and minorities to clinical trials in rough proportion to the cancer patient population of the center’s primary catchment area.
Given these findings, the researchers recommended better standardization of data definition, collection, and reporting as an essential first step toward expanding minority participation in clinical trials.
The team also advised that cancer centers collect socioeconomic data, including a patient’s income and education levels, given past evidence of the strong link between socioeconomic status and cancer outcomes.
Finally, the group recommended collecting patient zip codes and insurance status to allow researchers to assess differences in access to clinical trials that may be related to geography and the availability of health insurance coverage. ![]()

Credit: CDC
Researchers have identified significant variations in how cancer centers gather data, particularly that pertaining to racial and ethnic minorities.
Although racial and ethnic categories were similar across the centers, those categories were defined differently.
And the centers’ definitions of “catchment area,” the geographic region they expect to influence with their programs, differed widely.
This research, published in Cancer, was part of a national effort to recruit more racial/ethnic minorities into clinical trials and, ultimately, reduce the disproportional incidence of many cancers among those populations.
Five National Cancer Institute-designated comprehensive cancer centers participated in the endeavor, known as EMPaCT—Enhancing Minority Participation in Clinical Trials. They were:
- University of Minnesota, Minneapolis, which represents the Midwest and targets the accrual of Native Americans and African Americans
- University of Alabama, Birmingham, representing the Southeast, targeting African Americans
- Johns Hopkins University, representing the East, targeting African Americans
- University of Texas MD Anderson, Houston, representing the Southwest, targeting Latinos
- University of California, Davis, representing the West, targeting Asian Americans.
Ernest T. Hawk, MD, of the MD Anderson Cancer Center, and his colleagues reviewed the collection and reporting of patient data and other practices by these 5 centers.
This revealed significant variation in the centers’ methods of data collection. For example, patients’ insurance status was routinely documented at 2 centers, collected for non-research patients only at a third center, collected for billing of researcher enrollees at a fourth center, and not documented at all at a fifth center.
There were differences in data collection according to race/ethnicity as well. Racial/ethnic categories were generally similar across the centers—white, black/African American, Asian, Native American, Hispanic/Latino, and “other/unknown.”
However, the means of race/ethnicity data collection differed. Each center collected self-reported data on race/ethnicity, but 2 centers included data from staff observations.
Two centers compared the proportions of racial/ethnic groups enrolled in trials with those of their catchment area(s). But the others did not.
The centers also differed in how they defined their patient catchment area, in terms of their cancer patient-vs-general-population specificity, levels of specificity, and geographic coverage.
That merits notice, according to the researchers, because National Cancer Institute cancer centers are required to accrue women and minorities to clinical trials in rough proportion to the cancer patient population of the center’s primary catchment area.
Given these findings, the researchers recommended better standardization of data definition, collection, and reporting as an essential first step toward expanding minority participation in clinical trials.
The team also advised that cancer centers collect socioeconomic data, including a patient’s income and education levels, given past evidence of the strong link between socioeconomic status and cancer outcomes.
Finally, the group recommended collecting patient zip codes and insurance status to allow researchers to assess differences in access to clinical trials that may be related to geography and the availability of health insurance coverage. ![]()
Cancer survivors’ risk of health problems increases with age

cancer patient and her father
Credit: Rhoda Baer
The “health gap” between childhood cancer survivors and their siblings widens with age, according to a study published in the Journal of Clinical Oncology.
Cancer survivors aged 20 to 34 years old were 3.8 times more likely than siblings of the same age to develop new cancers and other serious health conditions.
By age 35 and beyond, survivors had a 5-fold greater risk.
“Survivors remain at risk for serious health problems into their 40s and 50s, decades after they have completed treatment for childhood cancer,” said study author Gregory Armstrong, MD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
“In fact, for survivors, the risk of illness and death increases significantly beyond the age of 35. Their siblings don’t share these same risks.”
Dr Armstrong and his colleagues uncovered these results by analyzing data from the Childhood Cancer Survivor Study, which included 14,359 survivors and 4301 healthy siblings.
The patients had been diagnosed with leukemias, lymphomas, and other pediatric cancers before age 21 and were followed for a median of 24.5 years (range, 5 to 39.3 years).
The researchers compared survivors to age-matched siblings, evaluating the incidence of severe, disabling, life-threatening, or fatal health conditions. This included new malignancies as well as diseases of the heart, lungs, liver, kidneys, and hormones.
The team found a heightened risk of these health conditions among cancer survivors. And that risk increased as the survivors aged.
At 20 years of age, 16% of survivors had serious health conditions, compared to 3.3% of siblings. But by age 50, the incidence had increased to 53.6% among survivors and 19.8% among siblings. At 50, 22.5% of survivors had at least 2 serious health problems, and 10.1% had 3 or more.
In a multivariate analysis, the hazard ratio for developing serious health conditions was significantly higher among survivors aged 35 and older than for those aged 20 to 34 (P=0.03).
Among survivors who reached age 35 without serious health problems, 25.9% developed a significant health problem in the next decade. In comparison, 6% of siblings developed their first serious health condition between the ages of 35 and 45.
In addition to showing a health gap between childhood cancer survivors and their siblings, this research adds to evidence that survivors experience accelerated aging. The 24-year-old cancer survivors had roughly the same cumulative incidence of grade 3 to 5 health conditions (19.6%) as the 50-year-old siblings (19.8%).
Overall, these findings highlight the importance of lifelong, risk-based healthcare for childhood cancer survivors, Dr Armstrong said. Depending on their cancer treatment and other risk factors, follow-up care may include performing health checks at a younger age than is recommended for the general public.
This study involved survivors whose cancer was diagnosed between 1970 and 1986. The researchers are now studying the health of adult cancer survivors from a more recent treatment era. ![]()

cancer patient and her father
Credit: Rhoda Baer
The “health gap” between childhood cancer survivors and their siblings widens with age, according to a study published in the Journal of Clinical Oncology.
Cancer survivors aged 20 to 34 years old were 3.8 times more likely than siblings of the same age to develop new cancers and other serious health conditions.
By age 35 and beyond, survivors had a 5-fold greater risk.
“Survivors remain at risk for serious health problems into their 40s and 50s, decades after they have completed treatment for childhood cancer,” said study author Gregory Armstrong, MD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
“In fact, for survivors, the risk of illness and death increases significantly beyond the age of 35. Their siblings don’t share these same risks.”
Dr Armstrong and his colleagues uncovered these results by analyzing data from the Childhood Cancer Survivor Study, which included 14,359 survivors and 4301 healthy siblings.
The patients had been diagnosed with leukemias, lymphomas, and other pediatric cancers before age 21 and were followed for a median of 24.5 years (range, 5 to 39.3 years).
The researchers compared survivors to age-matched siblings, evaluating the incidence of severe, disabling, life-threatening, or fatal health conditions. This included new malignancies as well as diseases of the heart, lungs, liver, kidneys, and hormones.
The team found a heightened risk of these health conditions among cancer survivors. And that risk increased as the survivors aged.
At 20 years of age, 16% of survivors had serious health conditions, compared to 3.3% of siblings. But by age 50, the incidence had increased to 53.6% among survivors and 19.8% among siblings. At 50, 22.5% of survivors had at least 2 serious health problems, and 10.1% had 3 or more.
In a multivariate analysis, the hazard ratio for developing serious health conditions was significantly higher among survivors aged 35 and older than for those aged 20 to 34 (P=0.03).
Among survivors who reached age 35 without serious health problems, 25.9% developed a significant health problem in the next decade. In comparison, 6% of siblings developed their first serious health condition between the ages of 35 and 45.
In addition to showing a health gap between childhood cancer survivors and their siblings, this research adds to evidence that survivors experience accelerated aging. The 24-year-old cancer survivors had roughly the same cumulative incidence of grade 3 to 5 health conditions (19.6%) as the 50-year-old siblings (19.8%).
Overall, these findings highlight the importance of lifelong, risk-based healthcare for childhood cancer survivors, Dr Armstrong said. Depending on their cancer treatment and other risk factors, follow-up care may include performing health checks at a younger age than is recommended for the general public.
This study involved survivors whose cancer was diagnosed between 1970 and 1986. The researchers are now studying the health of adult cancer survivors from a more recent treatment era. ![]()

cancer patient and her father
Credit: Rhoda Baer
The “health gap” between childhood cancer survivors and their siblings widens with age, according to a study published in the Journal of Clinical Oncology.
Cancer survivors aged 20 to 34 years old were 3.8 times more likely than siblings of the same age to develop new cancers and other serious health conditions.
By age 35 and beyond, survivors had a 5-fold greater risk.
“Survivors remain at risk for serious health problems into their 40s and 50s, decades after they have completed treatment for childhood cancer,” said study author Gregory Armstrong, MD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
“In fact, for survivors, the risk of illness and death increases significantly beyond the age of 35. Their siblings don’t share these same risks.”
Dr Armstrong and his colleagues uncovered these results by analyzing data from the Childhood Cancer Survivor Study, which included 14,359 survivors and 4301 healthy siblings.
The patients had been diagnosed with leukemias, lymphomas, and other pediatric cancers before age 21 and were followed for a median of 24.5 years (range, 5 to 39.3 years).
The researchers compared survivors to age-matched siblings, evaluating the incidence of severe, disabling, life-threatening, or fatal health conditions. This included new malignancies as well as diseases of the heart, lungs, liver, kidneys, and hormones.
The team found a heightened risk of these health conditions among cancer survivors. And that risk increased as the survivors aged.
At 20 years of age, 16% of survivors had serious health conditions, compared to 3.3% of siblings. But by age 50, the incidence had increased to 53.6% among survivors and 19.8% among siblings. At 50, 22.5% of survivors had at least 2 serious health problems, and 10.1% had 3 or more.
In a multivariate analysis, the hazard ratio for developing serious health conditions was significantly higher among survivors aged 35 and older than for those aged 20 to 34 (P=0.03).
Among survivors who reached age 35 without serious health problems, 25.9% developed a significant health problem in the next decade. In comparison, 6% of siblings developed their first serious health condition between the ages of 35 and 45.
In addition to showing a health gap between childhood cancer survivors and their siblings, this research adds to evidence that survivors experience accelerated aging. The 24-year-old cancer survivors had roughly the same cumulative incidence of grade 3 to 5 health conditions (19.6%) as the 50-year-old siblings (19.8%).
Overall, these findings highlight the importance of lifelong, risk-based healthcare for childhood cancer survivors, Dr Armstrong said. Depending on their cancer treatment and other risk factors, follow-up care may include performing health checks at a younger age than is recommended for the general public.
This study involved survivors whose cancer was diagnosed between 1970 and 1986. The researchers are now studying the health of adult cancer survivors from a more recent treatment era. ![]()
Adult minorities underrepresented in cancer trials

Credit: Rhoda Baer
New research indicates that less than 2% of trials funded by the National Cancer Institute focus on racial and ethnic minorities, and minority participation in adult cancer trials is not representative of the US population.
The researchers said these findings suggest we must do more to promote minority-focused research and clinical trial recruitment, beyond the National Institutes of Health (NIH) Revitalization Act of 1993, which mandated the appropriate inclusion of minorities in all NIH-funded research.
“What is needed is deliberate effort,” said study author Moon Chen, Jr, PhD, of the University of California, Davis. “Minorities are not hard to reach. They are hardly reached.”
To assess minority inclusion in clinical trials, Dr Chen and his colleagues searched ClinicalTrials.gov, looking for trials sponsored by the National Cancer Institute that were available in January 2013.
They searched using terms for different minority groups, then counted the number of clinical trials with a primary focus on a particular ethnic or minority population. Roughly 150 trials out of 10,000—or less than 2%—met the criteria.
The researchers also reviewed abstracts and articles accessed from January through March 2013 on PubMed to find those that specifically examined minority accrual in clinical trials.
Of the 42 citations found, 5 included reports explicitly discussing participation levels by race and ethnicity. Those reports revealed an “encouraging but less than optimal” increase in specification of race or ethnicity in published results of clinical trials.
Dr Chen and his colleagues also reported that participation of adult minorities is not proportional to their representation in the US population.
For example, African Americans experience the highest cancer incidence of any racial group (593.7 cases per 100,000), but they have the lowest rates of cancer trial participation (tied with Hispanics), at 1.3%. It’s important to note, however, that clinical trial participation is low for all adult cancer patients, at 3% to 5%.
In contrast, the researchers pointed out that 60% of all patients under age 15 are enrolled in clinical trials. And minority representation among children is excellent, either equal to or greater than their proportion of the population.
To put the adult population on par with the pediatric population, researchers should design trials to include and focus on specific populations, Dr Chen said. Furthermore, scientific journals should insist on appropriate representation and analyses of NIH research by race and ethnicity.
“Whatever happens in the laboratory or in the clinic needs to be applied to solving real-world problems,” Dr Chen said. “And those relate to the disproportionate effects of cancer and other diseases on racial and ethnic minorities.”
Dr Chen and his colleagues reported this research in Cancer. ![]()

Credit: Rhoda Baer
New research indicates that less than 2% of trials funded by the National Cancer Institute focus on racial and ethnic minorities, and minority participation in adult cancer trials is not representative of the US population.
The researchers said these findings suggest we must do more to promote minority-focused research and clinical trial recruitment, beyond the National Institutes of Health (NIH) Revitalization Act of 1993, which mandated the appropriate inclusion of minorities in all NIH-funded research.
“What is needed is deliberate effort,” said study author Moon Chen, Jr, PhD, of the University of California, Davis. “Minorities are not hard to reach. They are hardly reached.”
To assess minority inclusion in clinical trials, Dr Chen and his colleagues searched ClinicalTrials.gov, looking for trials sponsored by the National Cancer Institute that were available in January 2013.
They searched using terms for different minority groups, then counted the number of clinical trials with a primary focus on a particular ethnic or minority population. Roughly 150 trials out of 10,000—or less than 2%—met the criteria.
The researchers also reviewed abstracts and articles accessed from January through March 2013 on PubMed to find those that specifically examined minority accrual in clinical trials.
Of the 42 citations found, 5 included reports explicitly discussing participation levels by race and ethnicity. Those reports revealed an “encouraging but less than optimal” increase in specification of race or ethnicity in published results of clinical trials.
Dr Chen and his colleagues also reported that participation of adult minorities is not proportional to their representation in the US population.
For example, African Americans experience the highest cancer incidence of any racial group (593.7 cases per 100,000), but they have the lowest rates of cancer trial participation (tied with Hispanics), at 1.3%. It’s important to note, however, that clinical trial participation is low for all adult cancer patients, at 3% to 5%.
In contrast, the researchers pointed out that 60% of all patients under age 15 are enrolled in clinical trials. And minority representation among children is excellent, either equal to or greater than their proportion of the population.
To put the adult population on par with the pediatric population, researchers should design trials to include and focus on specific populations, Dr Chen said. Furthermore, scientific journals should insist on appropriate representation and analyses of NIH research by race and ethnicity.
“Whatever happens in the laboratory or in the clinic needs to be applied to solving real-world problems,” Dr Chen said. “And those relate to the disproportionate effects of cancer and other diseases on racial and ethnic minorities.”
Dr Chen and his colleagues reported this research in Cancer. ![]()

Credit: Rhoda Baer
New research indicates that less than 2% of trials funded by the National Cancer Institute focus on racial and ethnic minorities, and minority participation in adult cancer trials is not representative of the US population.
The researchers said these findings suggest we must do more to promote minority-focused research and clinical trial recruitment, beyond the National Institutes of Health (NIH) Revitalization Act of 1993, which mandated the appropriate inclusion of minorities in all NIH-funded research.
“What is needed is deliberate effort,” said study author Moon Chen, Jr, PhD, of the University of California, Davis. “Minorities are not hard to reach. They are hardly reached.”
To assess minority inclusion in clinical trials, Dr Chen and his colleagues searched ClinicalTrials.gov, looking for trials sponsored by the National Cancer Institute that were available in January 2013.
They searched using terms for different minority groups, then counted the number of clinical trials with a primary focus on a particular ethnic or minority population. Roughly 150 trials out of 10,000—or less than 2%—met the criteria.
The researchers also reviewed abstracts and articles accessed from January through March 2013 on PubMed to find those that specifically examined minority accrual in clinical trials.
Of the 42 citations found, 5 included reports explicitly discussing participation levels by race and ethnicity. Those reports revealed an “encouraging but less than optimal” increase in specification of race or ethnicity in published results of clinical trials.
Dr Chen and his colleagues also reported that participation of adult minorities is not proportional to their representation in the US population.
For example, African Americans experience the highest cancer incidence of any racial group (593.7 cases per 100,000), but they have the lowest rates of cancer trial participation (tied with Hispanics), at 1.3%. It’s important to note, however, that clinical trial participation is low for all adult cancer patients, at 3% to 5%.
In contrast, the researchers pointed out that 60% of all patients under age 15 are enrolled in clinical trials. And minority representation among children is excellent, either equal to or greater than their proportion of the population.
To put the adult population on par with the pediatric population, researchers should design trials to include and focus on specific populations, Dr Chen said. Furthermore, scientific journals should insist on appropriate representation and analyses of NIH research by race and ethnicity.
“Whatever happens in the laboratory or in the clinic needs to be applied to solving real-world problems,” Dr Chen said. “And those relate to the disproportionate effects of cancer and other diseases on racial and ethnic minorities.”
Dr Chen and his colleagues reported this research in Cancer. ![]()
Company issues nationwide recall of blood sets

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()