User login
Cases of ‘misconduct’ were really mistakes, STAP cell researcher says

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year. ![]()

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year. ![]()

Associated Press
The scientist who led the research on STAP cells (stimulus-triggered acquisition of pluripotency cells) has apologized for the errors in her published work but maintains that she is not guilty of misconduct.
She offered additional explanations for the errors and argued that they do not change the conclusion of the research—that the STAP phenomenon is real.
Earlier this month, Haruko Obokata, PhD, was accused of research misconduct by her institution, the RIKEN Center for Developmental Biology in Kobe, Japan.
RIKEN had launched an investigation into the STAP cell research (published in an article and a letter to Nature) after members of the scientific community began questioning its validity.
The researchers had claimed they could induce pluripotency in somatic cells by introducing them to a low-pH environment.
RIKEN investigated 6 issues with the research and ruled that there were 2 cases of data mishandling, 2 unintentional errors, and 2 cases of misconduct in the form of data manipulation.
In the first case of data manipulation, Dr Obokata switched 1 lane of a diagram for another. In the second, she used an image of a teratoma from her doctoral thesis.
Dr Obokata said the first manipulation was for the purpose of image clarity and not made with an intent to deceive readers. And the second “manipulation” was the result of a mix up in Power Point slides.
Dr Obokata also maintained that STAP cells do exist, and she has produced them more than 200 times. She added that she is willing to help scientists trying to replicate her experiments.
Furthermore, the notes that RIKEN reviewed in their investigation—2 notebooks that detail the STAP cell experiments—are not the complete set of notes Dr Obokata made. She said she has at least 4 or 5 other notebooks in different labs.
Dr Obokata has filed an appeal with RIKEN, asking the institute to reconsider its judgment. If RIKEN upholds the allegations of misconduct, Dr Obokata and 2 of her co-authors—Yoshiki Sasai, MD, PhD, and Teruhiko Wakayama, PhD—will face disciplinary action.
Meanwhile, a group of RIKEN investigators is conducting research to determine if STAP cells can be created, but they expect the process to take up to a year. ![]()
Synthetic collagen can function as hemostatic agent
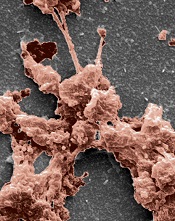
the clotting power of KOD
Hartgerink Lab/Rice University
Researchers have synthesized a collagen mimetic that may help wounds heal by directing the natural clotting of blood.
It was several years ago that the team developed KOD, a synthetic collagen mimetic made of 36 amino acids that self-assemble into triple-helix nanofibers and hydrogels.
With their latest research, the group showed that KOD collagen matrices adhere to platelets and activate them, thereby creating clots in blood and plasma.
An account of this research appears in Biomacromolecules.
“We showed we can make small peptides that we can easily synthesize chemically . . .,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“Those peptides self-assemble into fibers that, in turn, become a hydrogel. This hierarchy of assembly—from a peptide to a triple helix to a fiber to a hydrogel—mimics much of the hierarchy of assembly of natural collagen.”
Dr Hartgerink added that collagen’s importance goes beyond its role as a scaffold for cells.
“We’ve been thinking about KOD for hemostasis for a long time,” he said. “Natural collagen is already used in a variety of on-the-market products for hemostasis, but there are benefits to a synthetic system. We can avoid the immune problems associated with using collagen from cows, for example. The ability to synthesize KOD chemically gives us a pure product.”
Lab tests showed that KOD hydrogel traps red blood cells to stop bleeding and, unlike commercial barriers, binds and activates platelets that form clots to promote healing. The synthetic collagen matrices adhered to the platelets, and their activation was confirmed via the secretion of soluble P-selectin.
The tests also indicated that KOD does not promote inflammation. The researchers incubated THP-1 monocytes with KOD and observed “minimal” production of the proinflammatory cytokines TNF-α and IL-1β.
The team therefore believes KOD could improve upon commercial sponges or therapies based on naturally derived porcine or bovine-derived collagen that are now used to aid healing during or after surgery.
“We wouldn’t envision using KOD for major trauma, because there are conventional methods like tourniquets or using clay-based materials that are much more effective in that immediate situation,” said study author Vivek Kumar, PhD, also of Rice University.
“This is not going to be a battlefield dressing or something a first-responder is likely to use,” Dr Hartgerink added. “But when the goal is to promote delicate and natural healing where scarring is a concern, this can be more nuanced and effective.” ![]()

the clotting power of KOD
Hartgerink Lab/Rice University
Researchers have synthesized a collagen mimetic that may help wounds heal by directing the natural clotting of blood.
It was several years ago that the team developed KOD, a synthetic collagen mimetic made of 36 amino acids that self-assemble into triple-helix nanofibers and hydrogels.
With their latest research, the group showed that KOD collagen matrices adhere to platelets and activate them, thereby creating clots in blood and plasma.
An account of this research appears in Biomacromolecules.
“We showed we can make small peptides that we can easily synthesize chemically . . .,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“Those peptides self-assemble into fibers that, in turn, become a hydrogel. This hierarchy of assembly—from a peptide to a triple helix to a fiber to a hydrogel—mimics much of the hierarchy of assembly of natural collagen.”
Dr Hartgerink added that collagen’s importance goes beyond its role as a scaffold for cells.
“We’ve been thinking about KOD for hemostasis for a long time,” he said. “Natural collagen is already used in a variety of on-the-market products for hemostasis, but there are benefits to a synthetic system. We can avoid the immune problems associated with using collagen from cows, for example. The ability to synthesize KOD chemically gives us a pure product.”
Lab tests showed that KOD hydrogel traps red blood cells to stop bleeding and, unlike commercial barriers, binds and activates platelets that form clots to promote healing. The synthetic collagen matrices adhered to the platelets, and their activation was confirmed via the secretion of soluble P-selectin.
The tests also indicated that KOD does not promote inflammation. The researchers incubated THP-1 monocytes with KOD and observed “minimal” production of the proinflammatory cytokines TNF-α and IL-1β.
The team therefore believes KOD could improve upon commercial sponges or therapies based on naturally derived porcine or bovine-derived collagen that are now used to aid healing during or after surgery.
“We wouldn’t envision using KOD for major trauma, because there are conventional methods like tourniquets or using clay-based materials that are much more effective in that immediate situation,” said study author Vivek Kumar, PhD, also of Rice University.
“This is not going to be a battlefield dressing or something a first-responder is likely to use,” Dr Hartgerink added. “But when the goal is to promote delicate and natural healing where scarring is a concern, this can be more nuanced and effective.” ![]()

the clotting power of KOD
Hartgerink Lab/Rice University
Researchers have synthesized a collagen mimetic that may help wounds heal by directing the natural clotting of blood.
It was several years ago that the team developed KOD, a synthetic collagen mimetic made of 36 amino acids that self-assemble into triple-helix nanofibers and hydrogels.
With their latest research, the group showed that KOD collagen matrices adhere to platelets and activate them, thereby creating clots in blood and plasma.
An account of this research appears in Biomacromolecules.
“We showed we can make small peptides that we can easily synthesize chemically . . .,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“Those peptides self-assemble into fibers that, in turn, become a hydrogel. This hierarchy of assembly—from a peptide to a triple helix to a fiber to a hydrogel—mimics much of the hierarchy of assembly of natural collagen.”
Dr Hartgerink added that collagen’s importance goes beyond its role as a scaffold for cells.
“We’ve been thinking about KOD for hemostasis for a long time,” he said. “Natural collagen is already used in a variety of on-the-market products for hemostasis, but there are benefits to a synthetic system. We can avoid the immune problems associated with using collagen from cows, for example. The ability to synthesize KOD chemically gives us a pure product.”
Lab tests showed that KOD hydrogel traps red blood cells to stop bleeding and, unlike commercial barriers, binds and activates platelets that form clots to promote healing. The synthetic collagen matrices adhered to the platelets, and their activation was confirmed via the secretion of soluble P-selectin.
The tests also indicated that KOD does not promote inflammation. The researchers incubated THP-1 monocytes with KOD and observed “minimal” production of the proinflammatory cytokines TNF-α and IL-1β.
The team therefore believes KOD could improve upon commercial sponges or therapies based on naturally derived porcine or bovine-derived collagen that are now used to aid healing during or after surgery.
“We wouldn’t envision using KOD for major trauma, because there are conventional methods like tourniquets or using clay-based materials that are much more effective in that immediate situation,” said study author Vivek Kumar, PhD, also of Rice University.
“This is not going to be a battlefield dressing or something a first-responder is likely to use,” Dr Hartgerink added. “But when the goal is to promote delicate and natural healing where scarring is a concern, this can be more nuanced and effective.” ![]()
System allows blood group typing at the DNA level

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()
Draft recommendations back aspirin for preeclampsia prevention
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Doc groups say Medicare payment data need context

Credit: NIH
In an effort to make the US healthcare system more transparent, the Centers for Medicare & Medicaid Services (CMS) released data on Medicare payments made to healthcare providers in 2012.
The CMS said the data provide the public with new insight into healthcare spending and physician practice patterns.
But physician groups argued that releasing the data without context—such as specific drivers of cost—could lead to misinterpretation.
The data set includes information for more than 880,000 distinct healthcare providers who collectively received $77 billion in Medicare payments in 2012, under the Medicare Part B Fee-For-Service program.
The CMS said these data make it possible to conduct a wide range of analyses that compare 6000 different types of services and procedures provided, as well as payments received by individual healthcare providers.
The information allows comparisons by physician, specialty, location, the types of medical service and procedures delivered, Medicare payment, and submitted charges.
“Currently, consumers have limited information about how physicians and other healthcare professionals practice medicine,” said Health and Human Services Secretary Kathleen Sebelius. “This data will help fill that gap by offering insight into the Medicare portion of a physician’s practice.”
The presidents of the American Society of Hematology (ASH) and the American Medical Association (AMA) expressed less positive views about the data.
“While ASH supports greater transparency about Medicare physician payment and its potential to enhance the quality of the US healthcare system, the society strongly believes that this incredibly complex data must be released with appropriate disclosures and explanatory statements that will encourage and facilitate value-based consumer decision making,” said ASH President Linda J. Burns, MD.
“Specifically, the numbers alone will not explain quality of care or account for specific drivers of cost such as specialty, location, supply costs, and support staff. The release of data without placing these aspects of care and others into context may result in inaccurate and misleading information for consumers.”
For example, the data show that the highest-paid cardiologist (a physician in Ocala, Florida) received more than $18 million in Medicare payments, or nearly $23 million when totaling the amount Medicare pays, the deductible and co-insurance amounts the beneficiary pays, and any amounts a third party pays. And that $23 million figure is nearly 80 times higher than the average payment for a cardiologist in 2012.
While this high figure could be a result of improper billing, it might also be explained by a number of other factors. For instance, the physician might specialize in geriatric care and therefore receive nearly all his payments from Medicare, or the figure might include payments for staff, medical devices, tests, medications, and supplies.
“We believe that [CMS’s data set] has significant shortcomings regarding the accuracy and value of the medical services rendered by physicians,” said AMA President Ardis Dee Hoven, MD.
“Releasing the data without context will likely lead to inaccuracies, misinterpretations, false conclusions, and other unintended consequences. The AMA is disappointed that CMS did not include reasonable safeguards that would help the public understand the limitations of this data.”
The CMS did compile a document that lists the limitations of the data (eg, they might not be representative of a physician’s entire practice). This document and the complete data set are available for download from the CMS website. ![]()

Credit: NIH
In an effort to make the US healthcare system more transparent, the Centers for Medicare & Medicaid Services (CMS) released data on Medicare payments made to healthcare providers in 2012.
The CMS said the data provide the public with new insight into healthcare spending and physician practice patterns.
But physician groups argued that releasing the data without context—such as specific drivers of cost—could lead to misinterpretation.
The data set includes information for more than 880,000 distinct healthcare providers who collectively received $77 billion in Medicare payments in 2012, under the Medicare Part B Fee-For-Service program.
The CMS said these data make it possible to conduct a wide range of analyses that compare 6000 different types of services and procedures provided, as well as payments received by individual healthcare providers.
The information allows comparisons by physician, specialty, location, the types of medical service and procedures delivered, Medicare payment, and submitted charges.
“Currently, consumers have limited information about how physicians and other healthcare professionals practice medicine,” said Health and Human Services Secretary Kathleen Sebelius. “This data will help fill that gap by offering insight into the Medicare portion of a physician’s practice.”
The presidents of the American Society of Hematology (ASH) and the American Medical Association (AMA) expressed less positive views about the data.
“While ASH supports greater transparency about Medicare physician payment and its potential to enhance the quality of the US healthcare system, the society strongly believes that this incredibly complex data must be released with appropriate disclosures and explanatory statements that will encourage and facilitate value-based consumer decision making,” said ASH President Linda J. Burns, MD.
“Specifically, the numbers alone will not explain quality of care or account for specific drivers of cost such as specialty, location, supply costs, and support staff. The release of data without placing these aspects of care and others into context may result in inaccurate and misleading information for consumers.”
For example, the data show that the highest-paid cardiologist (a physician in Ocala, Florida) received more than $18 million in Medicare payments, or nearly $23 million when totaling the amount Medicare pays, the deductible and co-insurance amounts the beneficiary pays, and any amounts a third party pays. And that $23 million figure is nearly 80 times higher than the average payment for a cardiologist in 2012.
While this high figure could be a result of improper billing, it might also be explained by a number of other factors. For instance, the physician might specialize in geriatric care and therefore receive nearly all his payments from Medicare, or the figure might include payments for staff, medical devices, tests, medications, and supplies.
“We believe that [CMS’s data set] has significant shortcomings regarding the accuracy and value of the medical services rendered by physicians,” said AMA President Ardis Dee Hoven, MD.
“Releasing the data without context will likely lead to inaccuracies, misinterpretations, false conclusions, and other unintended consequences. The AMA is disappointed that CMS did not include reasonable safeguards that would help the public understand the limitations of this data.”
The CMS did compile a document that lists the limitations of the data (eg, they might not be representative of a physician’s entire practice). This document and the complete data set are available for download from the CMS website. ![]()

Credit: NIH
In an effort to make the US healthcare system more transparent, the Centers for Medicare & Medicaid Services (CMS) released data on Medicare payments made to healthcare providers in 2012.
The CMS said the data provide the public with new insight into healthcare spending and physician practice patterns.
But physician groups argued that releasing the data without context—such as specific drivers of cost—could lead to misinterpretation.
The data set includes information for more than 880,000 distinct healthcare providers who collectively received $77 billion in Medicare payments in 2012, under the Medicare Part B Fee-For-Service program.
The CMS said these data make it possible to conduct a wide range of analyses that compare 6000 different types of services and procedures provided, as well as payments received by individual healthcare providers.
The information allows comparisons by physician, specialty, location, the types of medical service and procedures delivered, Medicare payment, and submitted charges.
“Currently, consumers have limited information about how physicians and other healthcare professionals practice medicine,” said Health and Human Services Secretary Kathleen Sebelius. “This data will help fill that gap by offering insight into the Medicare portion of a physician’s practice.”
The presidents of the American Society of Hematology (ASH) and the American Medical Association (AMA) expressed less positive views about the data.
“While ASH supports greater transparency about Medicare physician payment and its potential to enhance the quality of the US healthcare system, the society strongly believes that this incredibly complex data must be released with appropriate disclosures and explanatory statements that will encourage and facilitate value-based consumer decision making,” said ASH President Linda J. Burns, MD.
“Specifically, the numbers alone will not explain quality of care or account for specific drivers of cost such as specialty, location, supply costs, and support staff. The release of data without placing these aspects of care and others into context may result in inaccurate and misleading information for consumers.”
For example, the data show that the highest-paid cardiologist (a physician in Ocala, Florida) received more than $18 million in Medicare payments, or nearly $23 million when totaling the amount Medicare pays, the deductible and co-insurance amounts the beneficiary pays, and any amounts a third party pays. And that $23 million figure is nearly 80 times higher than the average payment for a cardiologist in 2012.
While this high figure could be a result of improper billing, it might also be explained by a number of other factors. For instance, the physician might specialize in geriatric care and therefore receive nearly all his payments from Medicare, or the figure might include payments for staff, medical devices, tests, medications, and supplies.
“We believe that [CMS’s data set] has significant shortcomings regarding the accuracy and value of the medical services rendered by physicians,” said AMA President Ardis Dee Hoven, MD.
“Releasing the data without context will likely lead to inaccuracies, misinterpretations, false conclusions, and other unintended consequences. The AMA is disappointed that CMS did not include reasonable safeguards that would help the public understand the limitations of this data.”
The CMS did compile a document that lists the limitations of the data (eg, they might not be representative of a physician’s entire practice). This document and the complete data set are available for download from the CMS website. ![]()
Compound is potent FLT3 inhibitor, team says

Credit: Rhoda Baer
An experimental compound called TTT-3002 could be “one of the most potent FLT3 inhibitors to date,” according to researchers.
In preclinical experiments, TTT-3002 proved more active than the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 blocked FLT3 activity in human FLT3/ITD mutant leukemia cell lines, prolonged survival in a mouse model of FLT3-associated acute myeloid leukemia (AML), and proved toxic to leukemic cells from patients with AML.
Donald Small, MD, PhD, of Johns Hopkins University School of Medicine in Baltimore, and his colleagues reported these results in Blood.
“We’re very excited about TTT-3002, because it appears in our tests so far to be the most potent FLT3 inhibitor to date,” Dr Small said. “It showed activity against FLT3-mutated cells taken from patients and with minimal toxicity to normal bone marrow cells, making it a promising new candidate for the treatment of AML.”
In a series of experiments, the researchers found that the amount of TTT-3002 needed to block FLT3 activity in human leukemia cell lines was 6- to 7-fold lower than for ACC220, the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 also inhibited proteins made by genes further down the FLT3 signaling pathway, including STAT5, AKT, and MAPK. And it showed activity against the most frequently occurring FLT3 mutations, FLT3/ITD and FLT3/D835Y. (Many other inhibitors are ineffective against FLT3/D835Y mutations.)
When the researchers tested TTT-3002 in a mouse model of leukemia, they found the drug eliminated the presence of leukemic cells within 10 days of treatment.
Mice lived an average of more than 100 days after TTT-3002 treatment and resumed normal bone marrow activity. In comparison, mice treated with a placebo died an average of 18 days after treatment.
The researchers also found that TTT-3002 was toxic to leukemia samples taken from newly diagnosed and relapsed patients with AML, but it did not affect normal bone marrow cells taken from healthy donors.
A single dose of TTT-3002 caused more than 90% inhibition against FLT3 signaling that lasted for 12 hours. ![]()

Credit: Rhoda Baer
An experimental compound called TTT-3002 could be “one of the most potent FLT3 inhibitors to date,” according to researchers.
In preclinical experiments, TTT-3002 proved more active than the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 blocked FLT3 activity in human FLT3/ITD mutant leukemia cell lines, prolonged survival in a mouse model of FLT3-associated acute myeloid leukemia (AML), and proved toxic to leukemic cells from patients with AML.
Donald Small, MD, PhD, of Johns Hopkins University School of Medicine in Baltimore, and his colleagues reported these results in Blood.
“We’re very excited about TTT-3002, because it appears in our tests so far to be the most potent FLT3 inhibitor to date,” Dr Small said. “It showed activity against FLT3-mutated cells taken from patients and with minimal toxicity to normal bone marrow cells, making it a promising new candidate for the treatment of AML.”
In a series of experiments, the researchers found that the amount of TTT-3002 needed to block FLT3 activity in human leukemia cell lines was 6- to 7-fold lower than for ACC220, the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 also inhibited proteins made by genes further down the FLT3 signaling pathway, including STAT5, AKT, and MAPK. And it showed activity against the most frequently occurring FLT3 mutations, FLT3/ITD and FLT3/D835Y. (Many other inhibitors are ineffective against FLT3/D835Y mutations.)
When the researchers tested TTT-3002 in a mouse model of leukemia, they found the drug eliminated the presence of leukemic cells within 10 days of treatment.
Mice lived an average of more than 100 days after TTT-3002 treatment and resumed normal bone marrow activity. In comparison, mice treated with a placebo died an average of 18 days after treatment.
The researchers also found that TTT-3002 was toxic to leukemia samples taken from newly diagnosed and relapsed patients with AML, but it did not affect normal bone marrow cells taken from healthy donors.
A single dose of TTT-3002 caused more than 90% inhibition against FLT3 signaling that lasted for 12 hours. ![]()

Credit: Rhoda Baer
An experimental compound called TTT-3002 could be “one of the most potent FLT3 inhibitors to date,” according to researchers.
In preclinical experiments, TTT-3002 proved more active than the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 blocked FLT3 activity in human FLT3/ITD mutant leukemia cell lines, prolonged survival in a mouse model of FLT3-associated acute myeloid leukemia (AML), and proved toxic to leukemic cells from patients with AML.
Donald Small, MD, PhD, of Johns Hopkins University School of Medicine in Baltimore, and his colleagues reported these results in Blood.
“We’re very excited about TTT-3002, because it appears in our tests so far to be the most potent FLT3 inhibitor to date,” Dr Small said. “It showed activity against FLT3-mutated cells taken from patients and with minimal toxicity to normal bone marrow cells, making it a promising new candidate for the treatment of AML.”
In a series of experiments, the researchers found that the amount of TTT-3002 needed to block FLT3 activity in human leukemia cell lines was 6- to 7-fold lower than for ACC220, the most potent FLT3 inhibitor currently in clinical trials.
TTT-3002 also inhibited proteins made by genes further down the FLT3 signaling pathway, including STAT5, AKT, and MAPK. And it showed activity against the most frequently occurring FLT3 mutations, FLT3/ITD and FLT3/D835Y. (Many other inhibitors are ineffective against FLT3/D835Y mutations.)
When the researchers tested TTT-3002 in a mouse model of leukemia, they found the drug eliminated the presence of leukemic cells within 10 days of treatment.
Mice lived an average of more than 100 days after TTT-3002 treatment and resumed normal bone marrow activity. In comparison, mice treated with a placebo died an average of 18 days after treatment.
The researchers also found that TTT-3002 was toxic to leukemia samples taken from newly diagnosed and relapsed patients with AML, but it did not affect normal bone marrow cells taken from healthy donors.
A single dose of TTT-3002 caused more than 90% inhibition against FLT3 signaling that lasted for 12 hours. ![]()
How NK cells kill abnormal blood cells

Credit: Bjorn Onfelt/Dan Davis
New research provides additional insight into how natural killer (NK) cells eliminate abnormal hematopoietic cells.
The investigators evaluated 2 molecules that are known to play important roles in this process.
Ewing’s sarcoma-associated transcript 2 (EAT-2) and signaling lymphocytic activation molecule (SLAM)–associated protein (SAP) are expressed in NK cells, and their combined expression is essential for NK cells to kill abnormal hematopoietic cells.
“We knew that EAT-2 cooperates with SAP, and, with this research project, we wanted to better understand why they are both required for the proper functioning of NK cells,” said study author André Veillette, PhD, of the Institut de Recherches Cliniques de Montréal (IRCM) in Canada.
Dr Veillette and his colleagues described this research in the Journal of Experimental Medicine.
“We identified the molecular chain of events that occur and showed that EAT-2 and SAP perform different functions using distinct mechanisms,” Dr Veillette said. “These findings explain the cooperative and essential function of these 2 molecules in activating NK cells, thereby allowing them to kill abnormal blood cells.”
The investigators noted that SAP couples SLAM family receptors to the protein tyrosine kinase Fyn and the exchange factor Vav, thereby promoting conjugate formation between NK cells and target hematopoietic cells.
EAT-2, on the other hand, works by accelerating the polarization and exocytosis of cytotoxic granules toward hematopoietic cells.
EAT-2 mediates its effects in NK cells by linking SLAM family receptors to phospholipase Cγ, calcium fluxes, and Erk kinase. These signals are triggered by 1 or 2 tyrosines that are located in the carboxyl-terminal tail of EAT-2.
Dr Veillete pointed out that, although EAT-2 and SAP behave differently, both are linked to receptors of the SLAM family on the cell surface.
“Because they can make better drug targets, our future work will focus on these receptors,” he said, “which could eventually lead to identifying new potential treatment avenues for blood cancers such as leukemia and lymphoma.” ![]()

Credit: Bjorn Onfelt/Dan Davis
New research provides additional insight into how natural killer (NK) cells eliminate abnormal hematopoietic cells.
The investigators evaluated 2 molecules that are known to play important roles in this process.
Ewing’s sarcoma-associated transcript 2 (EAT-2) and signaling lymphocytic activation molecule (SLAM)–associated protein (SAP) are expressed in NK cells, and their combined expression is essential for NK cells to kill abnormal hematopoietic cells.
“We knew that EAT-2 cooperates with SAP, and, with this research project, we wanted to better understand why they are both required for the proper functioning of NK cells,” said study author André Veillette, PhD, of the Institut de Recherches Cliniques de Montréal (IRCM) in Canada.
Dr Veillette and his colleagues described this research in the Journal of Experimental Medicine.
“We identified the molecular chain of events that occur and showed that EAT-2 and SAP perform different functions using distinct mechanisms,” Dr Veillette said. “These findings explain the cooperative and essential function of these 2 molecules in activating NK cells, thereby allowing them to kill abnormal blood cells.”
The investigators noted that SAP couples SLAM family receptors to the protein tyrosine kinase Fyn and the exchange factor Vav, thereby promoting conjugate formation between NK cells and target hematopoietic cells.
EAT-2, on the other hand, works by accelerating the polarization and exocytosis of cytotoxic granules toward hematopoietic cells.
EAT-2 mediates its effects in NK cells by linking SLAM family receptors to phospholipase Cγ, calcium fluxes, and Erk kinase. These signals are triggered by 1 or 2 tyrosines that are located in the carboxyl-terminal tail of EAT-2.
Dr Veillete pointed out that, although EAT-2 and SAP behave differently, both are linked to receptors of the SLAM family on the cell surface.
“Because they can make better drug targets, our future work will focus on these receptors,” he said, “which could eventually lead to identifying new potential treatment avenues for blood cancers such as leukemia and lymphoma.” ![]()

Credit: Bjorn Onfelt/Dan Davis
New research provides additional insight into how natural killer (NK) cells eliminate abnormal hematopoietic cells.
The investigators evaluated 2 molecules that are known to play important roles in this process.
Ewing’s sarcoma-associated transcript 2 (EAT-2) and signaling lymphocytic activation molecule (SLAM)–associated protein (SAP) are expressed in NK cells, and their combined expression is essential for NK cells to kill abnormal hematopoietic cells.
“We knew that EAT-2 cooperates with SAP, and, with this research project, we wanted to better understand why they are both required for the proper functioning of NK cells,” said study author André Veillette, PhD, of the Institut de Recherches Cliniques de Montréal (IRCM) in Canada.
Dr Veillette and his colleagues described this research in the Journal of Experimental Medicine.
“We identified the molecular chain of events that occur and showed that EAT-2 and SAP perform different functions using distinct mechanisms,” Dr Veillette said. “These findings explain the cooperative and essential function of these 2 molecules in activating NK cells, thereby allowing them to kill abnormal blood cells.”
The investigators noted that SAP couples SLAM family receptors to the protein tyrosine kinase Fyn and the exchange factor Vav, thereby promoting conjugate formation between NK cells and target hematopoietic cells.
EAT-2, on the other hand, works by accelerating the polarization and exocytosis of cytotoxic granules toward hematopoietic cells.
EAT-2 mediates its effects in NK cells by linking SLAM family receptors to phospholipase Cγ, calcium fluxes, and Erk kinase. These signals are triggered by 1 or 2 tyrosines that are located in the carboxyl-terminal tail of EAT-2.
Dr Veillete pointed out that, although EAT-2 and SAP behave differently, both are linked to receptors of the SLAM family on the cell surface.
“Because they can make better drug targets, our future work will focus on these receptors,” he said, “which could eventually lead to identifying new potential treatment avenues for blood cancers such as leukemia and lymphoma.” ![]()
More ways to make the most of lasers in clinical practice
PHOENIX – What’s the latest in lasers? The most stubborn tattoos – those with blue or green ink – are now the easiest to remove with new laser technology and techniques. Microwave treatment for armpit hair is a real option, even on difficult-to-remove blond hair. Cutaneous laser expert Dr. Roy Geronemus, director of the Laser and Skin Surgery Center of New York, describes what dermatologists need to know about these and other innovative cosmetic treatments in an interview at the annual meeting of the American Society for Laser Medicine and Surgery.
But that’s not all. More data support the use of lasers for common medical conditions, says Dr. Geronemus. Hear his description of how the same new laser used for tattoo removal can be a noninvasive treatment to reduce either hypertrophic or atrophic scarring in any skin type, with practically no downtime. He also explains several new approaches that show promise as acne therapy.
On Twitter @sherryboschert
PHOENIX – What’s the latest in lasers? The most stubborn tattoos – those with blue or green ink – are now the easiest to remove with new laser technology and techniques. Microwave treatment for armpit hair is a real option, even on difficult-to-remove blond hair. Cutaneous laser expert Dr. Roy Geronemus, director of the Laser and Skin Surgery Center of New York, describes what dermatologists need to know about these and other innovative cosmetic treatments in an interview at the annual meeting of the American Society for Laser Medicine and Surgery.
But that’s not all. More data support the use of lasers for common medical conditions, says Dr. Geronemus. Hear his description of how the same new laser used for tattoo removal can be a noninvasive treatment to reduce either hypertrophic or atrophic scarring in any skin type, with practically no downtime. He also explains several new approaches that show promise as acne therapy.
On Twitter @sherryboschert
PHOENIX – What’s the latest in lasers? The most stubborn tattoos – those with blue or green ink – are now the easiest to remove with new laser technology and techniques. Microwave treatment for armpit hair is a real option, even on difficult-to-remove blond hair. Cutaneous laser expert Dr. Roy Geronemus, director of the Laser and Skin Surgery Center of New York, describes what dermatologists need to know about these and other innovative cosmetic treatments in an interview at the annual meeting of the American Society for Laser Medicine and Surgery.
But that’s not all. More data support the use of lasers for common medical conditions, says Dr. Geronemus. Hear his description of how the same new laser used for tattoo removal can be a noninvasive treatment to reduce either hypertrophic or atrophic scarring in any skin type, with practically no downtime. He also explains several new approaches that show promise as acne therapy.
On Twitter @sherryboschert
AT LASER 2014
HM 14 Special Report: Care of the Hospitalized Patient with Diabetes
Presenter:
Eric Felner, MD, Associate Professor of Pediatric Endocrinology and Director of the Pediatric Endocrine Fellowship Program at Emory University
Espousing a “3-Bag Theory of DKA management,” Dr. Eric Fellner presented an update of the inpatient management of diabetes mellitus at SHM 2014. This approach to dKA involves maintenance IV fluids based on BSA after fluid resuscitation, with variable proportions of ½ NS and D10 ½ NS with potassium chloride/potassium phosphate, and insulin given intravenously. This approach can reduce costs by avoiding multiple changes in IV fluid solution bags, and avoids multiple mistake-prone calculations. He recommends all patients with DKA under the age of 5 years be admitted to the PICU. Constant monitoring of lab values, glucose, vital signs, and clinical condition is also required. In general, insulin boluses do not provide a benefit over insulin drip alone, and use of bicarbonate remains controversial.
Although it is somewhat controversial as to whether all new type 1 diabetics need to be admitted, Dr. Felner favored admission due to improved teaching of patients/families, evaluation of the proposed insulin and carbohydrate regimen, and identification of potential insurance and social problems. Type 2 diabetics rarely get admitted for diabetic complications, but there are increasing numbers admitted for hyperglycemic hyperosmolar state.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego, and the pediatric editor for The Hospitalist.
Presenter:
Eric Felner, MD, Associate Professor of Pediatric Endocrinology and Director of the Pediatric Endocrine Fellowship Program at Emory University
Espousing a “3-Bag Theory of DKA management,” Dr. Eric Fellner presented an update of the inpatient management of diabetes mellitus at SHM 2014. This approach to dKA involves maintenance IV fluids based on BSA after fluid resuscitation, with variable proportions of ½ NS and D10 ½ NS with potassium chloride/potassium phosphate, and insulin given intravenously. This approach can reduce costs by avoiding multiple changes in IV fluid solution bags, and avoids multiple mistake-prone calculations. He recommends all patients with DKA under the age of 5 years be admitted to the PICU. Constant monitoring of lab values, glucose, vital signs, and clinical condition is also required. In general, insulin boluses do not provide a benefit over insulin drip alone, and use of bicarbonate remains controversial.
Although it is somewhat controversial as to whether all new type 1 diabetics need to be admitted, Dr. Felner favored admission due to improved teaching of patients/families, evaluation of the proposed insulin and carbohydrate regimen, and identification of potential insurance and social problems. Type 2 diabetics rarely get admitted for diabetic complications, but there are increasing numbers admitted for hyperglycemic hyperosmolar state.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego, and the pediatric editor for The Hospitalist.
Presenter:
Eric Felner, MD, Associate Professor of Pediatric Endocrinology and Director of the Pediatric Endocrine Fellowship Program at Emory University
Espousing a “3-Bag Theory of DKA management,” Dr. Eric Fellner presented an update of the inpatient management of diabetes mellitus at SHM 2014. This approach to dKA involves maintenance IV fluids based on BSA after fluid resuscitation, with variable proportions of ½ NS and D10 ½ NS with potassium chloride/potassium phosphate, and insulin given intravenously. This approach can reduce costs by avoiding multiple changes in IV fluid solution bags, and avoids multiple mistake-prone calculations. He recommends all patients with DKA under the age of 5 years be admitted to the PICU. Constant monitoring of lab values, glucose, vital signs, and clinical condition is also required. In general, insulin boluses do not provide a benefit over insulin drip alone, and use of bicarbonate remains controversial.
Although it is somewhat controversial as to whether all new type 1 diabetics need to be admitted, Dr. Felner favored admission due to improved teaching of patients/families, evaluation of the proposed insulin and carbohydrate regimen, and identification of potential insurance and social problems. Type 2 diabetics rarely get admitted for diabetic complications, but there are increasing numbers admitted for hyperglycemic hyperosmolar state.
Dr. Chang is a pediatric hospitalist with the University of San Diego Medical Center and Rady Children's Hospital, San Diego, and the pediatric editor for The Hospitalist.
Hormone therapy may decrease risk of NHL

SAN DIEGO—The use of hormone therapy may lower the risk of B-cell non-Hodgkin lymphoma (NHL) in menopausal women, according to a presentation at the AACR Annual Meeting 2014.
Researchers found that menopausal women who used hormone therapy were about 30% less likely than their untreated peers to develop NHL.
And the risk of NHL decreased further if a woman began receiving hormone therapy at a younger age and used it for a longer period of time.
Sophia Wang, PhD, of City of Hope National Medical Center in Duarte, California, presented these findings at the meeting as abstract 2918.
“The connection between lymphomas and menopausal hormone therapy use hinges on understanding the disease’s biology and the window of susceptibility,” Dr Wang said. “Hormone therapy is of interest because the loss of estrogen coupled with aging in women result in decreased immune function, which can elevate the risk of non-Hodgkin lymphoma.”
For this study, Dr Wang and her colleagues examined data from the Los Angeles Cancer Surveillance Program. They compared 685 postmenopausal women diagnosed with B-cell NHL to 685 postmenopausal women without lymphoma.
The researchers assessed the women’s use of menopausal hormone therapy, which included estrogen alone or estrogen with progestin in pill, patch, topical cream, or injected forms.
After controlling for factors such as age, race, and socioeconomic status, Dr Wang and her colleagues found that women who reported using any form of menopausal hormone therapy were approximately 30% less likely to be diagnosed with B-cell NHL, compared to women who reported never using hormone therapy.
An additional analysis showed that the risk reduction was even greater for women who initiated menopausal hormone therapy at 45 years of age or younger and used it for at least 5 years.
This group was approximately 40% less likely to be diagnosed with B-cell NHL compared to those who had never used hormone therapy.
Dr Wang said further research is needed to determine the exact biological mechanisms that might be linked to a lower NHL risk. These mechanisms could include supporting a healthy immune system or reducing inflammation.
She also cautioned that these findings are preliminary and should not change current recommendations and guidelines for menopausal hormone therapy use.
Due to well-established evidence tying menopausal hormone therapy to elevated risks of breast and endometrial cancers, the American Cancer Society recommends that women considering or using this therapy do so at the lowest effective dose for the shortest amount of time needed and that they discuss with their physicians other treatments to alleviate menopausal symptoms. ![]()

SAN DIEGO—The use of hormone therapy may lower the risk of B-cell non-Hodgkin lymphoma (NHL) in menopausal women, according to a presentation at the AACR Annual Meeting 2014.
Researchers found that menopausal women who used hormone therapy were about 30% less likely than their untreated peers to develop NHL.
And the risk of NHL decreased further if a woman began receiving hormone therapy at a younger age and used it for a longer period of time.
Sophia Wang, PhD, of City of Hope National Medical Center in Duarte, California, presented these findings at the meeting as abstract 2918.
“The connection between lymphomas and menopausal hormone therapy use hinges on understanding the disease’s biology and the window of susceptibility,” Dr Wang said. “Hormone therapy is of interest because the loss of estrogen coupled with aging in women result in decreased immune function, which can elevate the risk of non-Hodgkin lymphoma.”
For this study, Dr Wang and her colleagues examined data from the Los Angeles Cancer Surveillance Program. They compared 685 postmenopausal women diagnosed with B-cell NHL to 685 postmenopausal women without lymphoma.
The researchers assessed the women’s use of menopausal hormone therapy, which included estrogen alone or estrogen with progestin in pill, patch, topical cream, or injected forms.
After controlling for factors such as age, race, and socioeconomic status, Dr Wang and her colleagues found that women who reported using any form of menopausal hormone therapy were approximately 30% less likely to be diagnosed with B-cell NHL, compared to women who reported never using hormone therapy.
An additional analysis showed that the risk reduction was even greater for women who initiated menopausal hormone therapy at 45 years of age or younger and used it for at least 5 years.
This group was approximately 40% less likely to be diagnosed with B-cell NHL compared to those who had never used hormone therapy.
Dr Wang said further research is needed to determine the exact biological mechanisms that might be linked to a lower NHL risk. These mechanisms could include supporting a healthy immune system or reducing inflammation.
She also cautioned that these findings are preliminary and should not change current recommendations and guidelines for menopausal hormone therapy use.
Due to well-established evidence tying menopausal hormone therapy to elevated risks of breast and endometrial cancers, the American Cancer Society recommends that women considering or using this therapy do so at the lowest effective dose for the shortest amount of time needed and that they discuss with their physicians other treatments to alleviate menopausal symptoms. ![]()

SAN DIEGO—The use of hormone therapy may lower the risk of B-cell non-Hodgkin lymphoma (NHL) in menopausal women, according to a presentation at the AACR Annual Meeting 2014.
Researchers found that menopausal women who used hormone therapy were about 30% less likely than their untreated peers to develop NHL.
And the risk of NHL decreased further if a woman began receiving hormone therapy at a younger age and used it for a longer period of time.
Sophia Wang, PhD, of City of Hope National Medical Center in Duarte, California, presented these findings at the meeting as abstract 2918.
“The connection between lymphomas and menopausal hormone therapy use hinges on understanding the disease’s biology and the window of susceptibility,” Dr Wang said. “Hormone therapy is of interest because the loss of estrogen coupled with aging in women result in decreased immune function, which can elevate the risk of non-Hodgkin lymphoma.”
For this study, Dr Wang and her colleagues examined data from the Los Angeles Cancer Surveillance Program. They compared 685 postmenopausal women diagnosed with B-cell NHL to 685 postmenopausal women without lymphoma.
The researchers assessed the women’s use of menopausal hormone therapy, which included estrogen alone or estrogen with progestin in pill, patch, topical cream, or injected forms.
After controlling for factors such as age, race, and socioeconomic status, Dr Wang and her colleagues found that women who reported using any form of menopausal hormone therapy were approximately 30% less likely to be diagnosed with B-cell NHL, compared to women who reported never using hormone therapy.
An additional analysis showed that the risk reduction was even greater for women who initiated menopausal hormone therapy at 45 years of age or younger and used it for at least 5 years.
This group was approximately 40% less likely to be diagnosed with B-cell NHL compared to those who had never used hormone therapy.
Dr Wang said further research is needed to determine the exact biological mechanisms that might be linked to a lower NHL risk. These mechanisms could include supporting a healthy immune system or reducing inflammation.
She also cautioned that these findings are preliminary and should not change current recommendations and guidelines for menopausal hormone therapy use.
Due to well-established evidence tying menopausal hormone therapy to elevated risks of breast and endometrial cancers, the American Cancer Society recommends that women considering or using this therapy do so at the lowest effective dose for the shortest amount of time needed and that they discuss with their physicians other treatments to alleviate menopausal symptoms.

