User login
Head CT for the Inpatient With Delirium
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
Delirium is a common and costly problem in hospitalized medical patients. It is present on admission in 10% to 31% of cases and develops in up to 56% of patients during hospitalization.[1, 2] Prompt identification and treatment of the cause of delirium is important, because delirium is associated with increased morbidity and mortality, long‐term cognitive impairment, higher cost of care, increased length of stay, and more frequent discharge to an extended care facility.[3, 4, 5, 6]
Delirium can be caused or worsened by a variety of factors including adverse drug events, metabolic abnormalities, infections, immobilization, the use of tethers (eg, physical restraints, bladder catheters, telemetry), and disruption of sleepwake cycle.[7] An appropriate history, medication review, physical examination, and tailored laboratory evaluation is sufficient workup in the majority of cases.[8] However, neurologic processes, such as intracranial mass, intracranial hemorrhage, or stroke, can also present as delirium and require head imaging for diagnosis.
Because head imaging is a costly limited resource, a number of studies have aimed to determine which patients with delirium require this evaluation. The majority of research has focused on head computed tomography (CT) in patients presenting for evaluation to the emergency department (ED). In ED patients presenting with delirium, acute confusion, or altered mental status, head imaging identifies acute intracranial pathologic findings in 14% to 39% of cases.[9, 10, 11, 12, 13, 14] Only 2 studies have evaluated patients with delirium who have already been admitted to the hospital. One study involved patients admitted to a neurology unit with acute confusion and found that the yield of head imaging (head CT and magnetic resonance imaging) was 14% for acute intracranial pathology.[15] Another study reviewed patients admitted to an acute delirium unit and found a similar rate of positive findings on head CT (14.5%).[16] Neither study specified whether the head imaging occurred during initial presentation or later in the hospitalization.
Factors that increase the likelihood that delirium is caused by acute intracranial pathology include acute neurologic deficit, recent history of fall or head trauma, and significantly impaired consciousness.[9, 10, 11, 12, 13, 14, 15, 16, 17] Based on these findings, current guidelines and expert clinical statements recommend head imaging for patients with acute neurologic deficit, recent head trauma, or recent fall.[18, 19, 20]
Expert clinical statements also recommend imaging in cases where the cause is unidentified after appropriate medical testing or where delirium continues despite treatment.[8, 21] Yet the utility of head CT performed for nonresolving delirium or delirium that develops during hospitalization in the absence of recent fall, head trauma, or new neurologic deficits is not known. Our study aimed to determine the diagnostic yield of performing a head CT in this patient population. We hypothesized that the diagnostic yield of head CT in this population would be low.
METHODS
Study Design
We conducted a retrospective medical record review of hospitalized general medicine patients with head CT imaging performed for the evaluation of delirium. The study was reviewed by the internal review board and determined to be exempt.
Setting and Eligibility Criteria
The study was conducted at a large academic medical center in Boston, Massachusetts. All patients admitted to general medicine, nephrology, hepatology, cardiology, or oncology services with head CT studies performed from January 1, 2010 through November 30, 2012 were included in this retrospective, observational cohort study. Data were extracted using a defined instrument developed for this study with outcome measures predefined. Head CT imaging acquired for patients in the intensive care unit were not included in the review. The medical records were evaluated to determine indication. To be included in the study, the indication for the scan had to be delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness. In addition, the patient must have been admitted for at least 24 hours prior to the completion of the head CT scan. Scans were excluded if there was documentation in the medical record of a fall, head trauma, or new neurologic deficit within the preceding 2 weeks, or an admitting diagnosis of intracranial pathology (eg, stroke or subdural hematoma). If a patient had multiple head CT studies completed for the indication of delirium, each study was included. However, once a head CT study returned positive or equivocal for an acute intracranial process, subsequent head CT studies for the indication of delirium were not included in the analysis.
Outcome Measures
A positive head CT was defined as an intracranial process that could explain delirium (eg, intracranial hemorrhage or stroke). An equivocal head CT was defined as the presence of a finding of unclear significance in relation to delirium (eg, hypodensity of unknown etiology or clinical significance). Chronic head CT findings were noted to be intracranial pathologic findings of a chronic nature that did not meet criteria for either a positive or equivocal image (eg, chronic small vessel ischemic disease or atrophy). A normal study was without positive, equivocal, or chronic findings.
Data Collection and Statistical Analysis
Using the medical center's clinical informatics infrastructure, an experienced clinical informaticist (R.A.) compiled a list of all head CT imaging studies performed during the study period in hospitalized medical patients. An experienced hospital medicine physician (J.T.) conducted the medical record review and determined if each head CT performed met eligibility criteria. For each included study, the following information was collected: date of admission, date of head CT, date of onset of delirium, indication for obtaining head CT scan, head CT results, age, gender, race/ethnicity (patient reported), presence of dementia (if documented in the medical record), active cancer, use of anticoagulants (defined as factor Xa inhibitors, low molecular weight heparin, direct thrombin inhibitor, or vitamin K antagonist) with documentation of internationalized normalized ratio (INR), partial thromboplastin time (PTT) prothrombin time and platelet count, active infection, history of stroke, and change in clinical management. Descriptive statistics were used to analyze data. Median and interquartile range were used to describe results for age and time from admission to head CT performed due to skewed distribution of results.
RESULTS
Of 1714 head CT studies performed on hospitalized medical patients from January 1, 2010 to November 30, 2012, 398 studies were performed for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence, or unresponsiveness in patients who were admitted for >24 hours. One hundred seventy‐eight studies were excluded (137 for admitting diagnosis of intracranial process, recent fall, or head trauma, and 41 for new neurologic deficit). There were 220 scans included in the study performed on 210 patients.
Table 1 displays characteristics of the 210 patients who underwent CT head imaging. Of the 42 patients on anticoagulation, 15 were potentially supratherapeutic; 10 were on warfarin (INR range, 3.37.7) and 5 were on intravenous heparin infusion (PTT range, 101>150 seconds). None of these individuals had positive or equivocal findings on head CT.
| Characteristic | N=210 |
|---|---|
| |
| Age, median (IQR) | 70 (5980) |
| Male, n (%) | 96 (45.7) |
| Race/ethnicity, n (%) | |
| White | 147 (70.0) |
| African American | 44 (21.0) |
| Hispanic | 4 (1.9) |
| Asian | 3 (1.4) |
| Unknown | 9 (4.3) |
| Other | 3 (1.4) |
| Comorbidities, n (%) | |
| Dementia | 30 (14.3) |
| Active cancer | 49 (23.3) |
| Anticoagulation | 42 (20.0) |
| Active infection | 105 (50.0) |
| History of stroke | 41 (19.5) |
| Days from admission to head CT, median (IQR) | 4 (38) |
| Days from delirium onset to head CT, median (IQR) | 2 (14) |
The main outcomes of the 220 included head CT scans and a separate analysis of the 60 head CT scans performed for indications of somnolence or unresponsiveness are shown in Table 2. The 6 (2.7%) positive and 4 (1.8%) equivocal head CT findings are listed in Table 3. Of the 3 positive results in patients on anticoagulation, 2 were on warfarin with an INR of 2.1 and 2.4, respectively, and another was on warfarin and therapeutic enoxaparin (dosed 1 mg/kg twice daily) with an INR of 1.6. The median time from admission to positive head CT was 8 days, with a range of 2 to 28 days. All of the positive head CT studies resulted in change of management. All equivocal head CT studies resulted in repeat imaging. None of these repeat head imaging studies diagnosed acute intracranial pathology. Chronic findings identified included 111 (50.5%) involution or atrophy, 95 (43.2%) small vessel ischemic disease, 31 (14.1%) prior stroke, and 18 (8.2%) other chronic abnormalities (eg, cyst or meningioma).
| Indication | Delirium, N=220 (100%)* | Somnolence or Unresponsiveness, N=60 (27.2%) |
|---|---|---|
| ||
| Outcome | ||
| Positive | 6 (2.7) | 0 |
| Equivocal | 4 (1.8) | 1 (1.6) |
| Chronic | 162 (73.6) | 41 (68.3) |
| Normal | 48 (21.8) | 18 (30.0) |
| CT Head Findings | Age (Sex) | Days From Onset | Change in Management | Outcome | |
|---|---|---|---|---|---|
| |||||
| Positive | |||||
| Case 1 | Subarachnoid hemorrhage in right frontal and temporal lobes | 64 (M) | 2 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 2 | Intraparenchymal hemorrhage with mild shift and vasogenic edema | 62 (M) | 1 | Neurosurgery consult, AC reversal | Discharged with outpatient follow‐up |
| Case 3 | Subacute subdural hematoma | 62 (M) | 5 | Neurosurgery consult | Discharged with outpatient follow‐up |
| Case 4 | Acute infarct or mass | 73 (F) | 2 | Neurology consult, palliative care consult | Transitioned to comfort‐focused care, discharged |
| Case 5 | 4 mm focus concerning for hemorrhagic metastatic focus | 50 (M) | 3 | Neurosurgery consult, MRI | Discharged with outpatient follow‐up |
| Case 6 | Left occipital lobe parenchymal hemorrhage | 81 (F) | 1 | Neurosurgery consult, neurology consult | Transitioned to comfort‐focused care, died 6 days later |
| Equivocal | |||||
| Case 1 | Several white matter hypodensities of uncertain etiology | 70 (F) | 1 | MRI | MRI with chronic small vessel ischemia |
| Case 2 | Colloid cyst likely although cannot rule out intraventricular hemorrhage | 59 (F) | 1 | Repeat head CT | Repeat imaging with equivocal findings, no additional evaluation |
| Case 3 | Questionable hypodensity, either hemorrhagic contusion or artifact | 52 (M) | 3 | Repeat head CT | Repeat imaging normal |
| Case 4 | Ill‐defined hypodensity in left basal ganglia, no clear acute process | 74 (F) | 0 | MRI | MRI with chronic small vessel ischemia |
DISCUSSION
In this retrospective review, we determined that there is a low diagnostic yield of head CT imaging for identifying the cause of nonresolving or new‐onset delirium in hospitalized medical patients. Only 2.7% of head CT scans resulted in identifying an acute intracranial process. Because of the low number of positive results, no risk factor associations could be made from our study.
The low diagnostic yield of head imaging in hospitalized patients with delirium is particularly important for clinicians who care for hospitalized medical patients. Prior to this study, the yield of head CT scans in hospitalized medical patients with nonresolving or new‐onset delirium was unknown. In cases with known risk factors, such as recent fall, head trauma, or acute neurologic deficit, the guidelines recommend head CT imaging.[18, 19, 20] However, in the absence of these findings, the guidelines do not make any recommendation regarding when and in whom to perform head imaging. Expert statements recommend considering head CT imaging when the cause is not identified after appropriate testing or delirium continues despite treatment.[8, 21] Given these recommendations and lack of data, there is no clear standard of care for ordering head CT imaging when hospitalized patients experience delirium in the absence of known risk factors. The low diagnostic yield in this study suggests that head CT imaging is unlikely to diagnose the cause of delirium in hospitalized patients with nonresolving or new‐onset delirium.
The diagnostic yield of head CT for diagnosis of acute intracranial process in delirium was lower in our study than prior studies, which found between 14.0 and 39.1%.[9, 10, 11, 12, 13, 14, 15, 16] This was expected, as our study excluded patients with new neurologic deficits, recent fall or trauma, or an admitting diagnosis of an intracranial process. Even with these exclusions, we still allowed for a number of findings that prior studies considered to be high risk for intracranial pathology, such as age over 73 years, use of anticoagulation, and deterioration in consciousness level or Glasgow coma score under 14.[10, 11, 16] The inclusion and exclusion criteria were designed to create a generalizable study population without a clear standard of care based on current guidelines and expert statements.
Though the rate of positive findings found in our study is low, it likely overestimates the overall yield of head CT in hospitalized patients with delirium. This is because most hospitalized patients with delirium never receive head imaging. Presumably, ordering clinicians have deemed these patients to be at higher risk for intracranial processes than the average hospitalized patient with delirium who does not receive a head CT. Thus, the true rate of positive findings in head CT imaging in delirious hospitalized medical patients is likely lower than what we identified.
Although head CT had a low diagnostic yield, the positive and equivocal studies had a high impact on clinical care. All of the positive and equivocal head CT results produced a change in management. The equivocal findings led to repeat head imaging; however, none of the repeat images identified the cause of delirium. The positive results produced a more significant change in management, ranging from a higher platelet transfusion target, reversal of anticoagulation, repeat advanced head imaging, neurosurgery consultation, and a change in goals of care to a focus on comfort. No patients in our study underwent neurosurgical intervention.
The challenge for inpatient clinicians is to weigh the low diagnostic yield of head CT with the consequences of a missed or delayed diagnosis of an acute intracranial process. The low diagnostic yield leads to unnecessary cost, resource utilization, radiation exposure, and downstream evaluation of insignificant or indeterminate results when head CT is performed. Alternatively, a missed or delayed diagnosis can lead to potentially reversible morbidity and mortality. Given this, we feel that the routine use of head CT in the evaluation of delirium in hospitalized patients is unnecessary. However, there may be a subset of patients with delirium with an increased risk of acute intracranial processes that would benefit from head imaging. Further research is needed to identify this high‐risk population.
There are a number of limitations to our study. It is a retrospective chart review, which introduces a possibility of bias and relies on proper and thorough documentation. In addition, the diagnosis of delirium was made by individual clinicians without the use of a standardized delirium assessment tool. Furthermore, it is possible there may have been CT scans that were not identified due to mischaracterization of indication, or studies may have been included in individuals with new neurologic deficit or recent fall or trauma that were not documented or clinically appreciated. Finally, the study was conducted on medicine and medical subspecialty patients at a single academic tertiary care institution, potentially limiting the generalizability to patients in other settings.
In conclusion, our study suggests that the diagnostic yield of head CT to evaluate delirium in hospitalized patients in the absence of recent fall, head trauma, or new neurologic deficit is low. The routine use of head CT in evaluation of these individuals is unnecessary. However, there may be a subset of high‐risk individuals in which head CT imaging would be indicated. Further research is needed to identify these high‐risk individuals.
Disclosures
Jesse Theisen‐Toupal, MD, has no conflicts of interest to disclose. Anthony Breu is a contributor to Practical Reviews in Hospital Medicine but has no conflicts of interest. Melissa Mattison, MD, is a contributor to UpToDate and Practical Reviews in Hospital Medicine but has no conflicts of interest. Ramy Arnaout, MD, has no conflicts of interest to disclose.
© 2014 Society of Hospital Medicine
Mechanical Ventilation in Hypoxemia
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
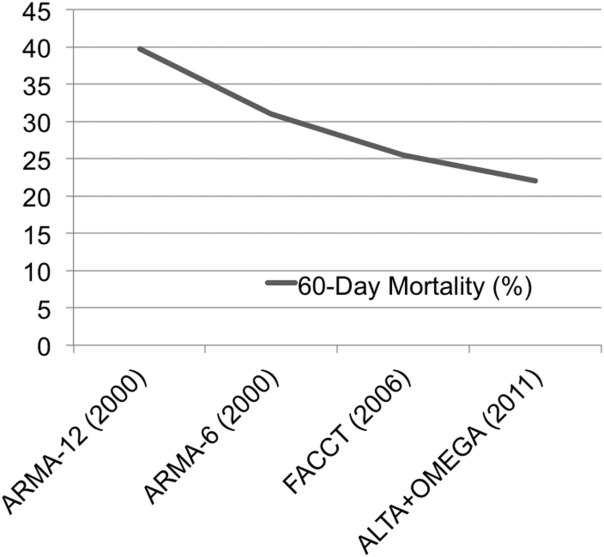
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]
Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of 150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]

Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of 150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]

Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of 150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
New guidelines address primary cutaneous T-cell lymphoproliferative disorders
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
HOLLYWOOD, FLA. – The treatment of patients with lymphomatoid papulosis depends on the presentation, according to new National Comprehensive Cancer Network guidelines for managing primary cutaneous CD30+ T-cell lymphoproliferative disorders.
No treatment is needed in patients with lymphomatoid papulosis (LyP) who present without symptoms because spontaneous remission is extremely common in this disease, and these patients typically won’t have problems with progressive disease, Dr. Andrew D. Zelenetz said at the annual conference of the National Comprehensive Cancer Network.
For those who are symptomatic, topical or systemic treatments are useful in some cases.
Topical steroids "are effective, but not great," commented Dr. Zelenetz, vice chairman of medical informatics at Memorial Sloan Kettering Cancer Center, New York; professor of medicine at Cornell University, New York; and chair of the NCCN Non-Hodgkin's Lymphomas Guidelines panel.
Reported response rates are in the 50%-60% range, he said.
Bexarotene is another treatment option, although experience with this drug is quite limited. The largest series included only 11 patients. Unpublished data from that series at Memorial Sloan Kettering Cancer Center show a response rate of 45% at a maximum oral dose of 600 mg daily, Dr. Zelenetz said.
However, where this is a response, it is "dramatic and quite obvious," he noted, adding that treatment duration needs to be adequate before a patient is considered a nonresponder; the median duration of treatment in the 11-patient series was 35.5 weeks.
In a series of 57 patients from Memorial Sloan Kettering Cancer Center (including the 11 treated systemically with bexarotene), 16 received no therapy; 19 received topical treatment with steroids (13 patients), bexarotene (2 patients), UVB (2 patients), cryotherapy (1 patient), or nitrogen mustard (1 patient); and 5 received systemic treatment with methotrexate.
At follow-up, 14% of patients had no evidence of disease, and, with the exception of one who died of another cause, the remaining patients were alive with disease, Dr. Zelenetz said.
LyP is a rare CD30+ cutaneous lymphoproliferative disorder characterized by self-healing cropped or generalized eruptions of papules that come and go on the trunk or proximal extremities. In rare cases they present as solitary lesions.
"Even though many patients actually have intermittent recurrent disease ... the death rate from LyP is zero. So this is a very manageable disease; don’t overtreat these tumors," he said.
At the other end of the spectrum of CD30+ lymphoproliferative disorders addressed in the new NCCN guidelines is anaplastic large cell lymphoma (ALCL).
Primary cutaneous ALCL is characterized by skin-only presentation that is often localized but which can be disseminated in some cases. Lesions also tend to be larger and "more piled up" than those seen with LyP.
"You can get clustering in a specific area, but we don’t tend to have these big crops of lesions that we see with LyP," Dr. Zelenetz said.
The pathology is also different, with diffuse infiltration of the subcutaneous tissue. The cells are large and anaplastic, and there is intense expression of CD30.
The course of disease is usually indolent, with progression to extracutaneous sites in about 10%-15% of cases.
Nodules or tumors in cases of ALCL are less likely than LyP lesions to regress spontaneously, Dr. Zelenetz said.
This disease must be distinguished from a skin presentation of systemic ALCL, he noted.
"So what’s the big difference? In anaplastic large cell lymphoma that’s systemic, you will have multiple nodules all over and happen to have skin disease. With primary cutaneous ALCL, you have skin only or skin and some regional lymph nodes but nothing beyond that," he said, adding that these primary cutaneous tumors do extremely well nevertheless, with cumulative survival rates above 90%.
Those with systemic ALCL, however, have much lower cumulative survival, in the 25% range.
As with LyP, treatment for primary cutaneous ALCL is based on presentation.
For solitary or grouped lesions, the preferred treatment is surgical excision if needed for diagnosis or radiation if the diagnosis is already established.
Methotrexate is the preferred treatment for multifocal lesions.
The subtype that includes regional lymph nodes is typically treated with very mild chemotherapy including methotrexate or pralatrexate. Radiation can be used for locoregional disease, Dr. Zelenetz said.
An exception to the rule that patients with primary cutaneous ALCL do well is in cases of extensive limb disease. Patients with involvement of a single limb – usually lower extremity, but not always – have poor survival, and their disease is refractory to chemotherapy and radiation. It is unclear why there is a distinction in this presentation, but it is important to be aware of it, he said.
Dr. Zelenetz is a scientific adviser for Cancer Genetics Inc. and Gilead and has received consulting fees, honoraria, and/or grant or other research support from Celgene Corp., Cephalon Inc., Genentech Inc., GlaxoSmithKline, Roche Laboratories Inc., sanofi-aventis U.S., and Seattle Genetics Inc.
AT THE NCCN ANNUAL CONFERENCE
Commentary: Performing clinical research as a CT trainee
"Why do I need to do research if I’m going into private practice anyway?"
I have heard this question multiple times throughout my career as a resident, fellow, and attending thoracic surgeon. The truth is, there are multiple reasons, any of which is sufficient to justify your participation in clinical research during training. First, and perhaps most importantly, it teaches you to critically appraise the literature. This is a skill that will serve you well throughout your career, guiding your clinical decision-making, regardless if you choose private practice or academic surgery. Another reason is that performing clinical research allows you to become a content expert on a specific topic early in your career. This knowledge base is something that will serve as a foundation for ongoing learning and may help in designing future studies. Once your project is complete, it will be your ticket to attend and present at regional, national, or international meetings. There is no better forum to gain public recognition for your investigative efforts and network with potential future partners than societal meetings. Formal and informal interviews routinely occur at these gatherings and you do not want to be left out because you chose not to participate in research as a trainee. Finally, it is your responsibility to the patients that you have sworn to treat. There are many ways to care for patients, and pushing back the frontiers of medical knowledge is as important as the day-to-day tasks that you perform on the ward or in the operating room.
So, now that you have decided that you want to participate in a research project as a trainee, how do you make it happen? Before you begin a project, you will have to choose a mentor, a topic, a clear, novel question, and the appropriate study design. Chances are that at some point, a mentor helped guide you toward a career in cardiothoracic surgery. A research mentor is just as important as a clinical mentor for a young surgeon.
The most important trait that you should seek out in a research mentor is the ability to delineate important questions. All too often, residents and fellows are approached by attending surgeons with good intentions, but bad research ideas. Trainees then feel obligated to take them up on the project (in order to not appear like a slacker) and for various reasons, it does not result in an abstract, presentation, or publication. In fact, all it results in is frustration, a distaste for investigation, and wasted time. The bottom line is that only you can protect your time, and as a surgical trainee, you must guard it ferociously. Look for a mentor who is an expert in your field of interest and who has a track record of publications.
He or she must be a logical thinker who can help you delineate a clear, novel question, choose the appropriate study design, guide your writing of the manuscript, and direct your submission to the appropriate meetings and journals. Finally, your mentor must be dedicated to your success. We are all busy, but if your mentor cannot find the time to routinely meet with you at every step of your project, you need to find a new mentor.
Choosing a clear, novel clinical question starts with choosing an appropriate topic (Table 1). With the right topic and question, the hypothesis is obvious, it is easy to define your endpoints, and your study design will fall into place. But with the wrong question, your study will lack focus, it will be difficult to explain the relevance of your study, and you will not want to present your data on the podium. An example of a good question is "Do patients with a given disease treated with operation X live longer than those treated with operation Y?" Stay away from the lure of "Let’s review our experience of operation X..." or "Why don’t I see how many of operation Y we’ve done over the past 10 years..." These topics are vague and do not ask a specific question. There must be a clear hypothesis for any study that is expected to produce meaningful results.
Once you have chosen an appropriate question, you must decide on a study design. Although case reports are marginally publishable, they will not answer your clinical questions. For many reasons, randomized, controlled trials, the gold standard of research, are difficult to design, carry out, and complete in your short time as a trainee. The good news is that well-designed and sufficiently powered observational studies often give similar results as randomized, controlled studies. Examples of common observational study designs include cohort studies, case control studies, and cross-sectional studies (Table 2). Each study design is different and your mentor should be able to help you decide which is the best to answer the question you want to ask.
When designing a study, one of the most important principles is defining a priori endpoints. Every study will have one primary endpoint that reflects the hypothesis. Secondary endpoints are interesting and potentially helpful, but are not the main message. It will be important to meet with a statistician before you start data collection. Understanding the statistics to be used will allow you to collect your data in the correct way (categorical vs. continuous, etc.). Reviewing charts is very time consuming and you have to do everything in your power to ensure you only do it once.
The next step is to create a research proposal. To do this, you will need to go to the literature, and see what published data relate to your study. Perhaps there are previous studies examining your question with conflicting results. Or if your question has not been previously investigated, what supporting literature suggests that yours is the next logical study? Your proposal should include a background section (1-2 paragraphs), hypothesis (1 sentence), the specific aims of the study (1-3 sentences), methods (2-4 paragraphs), anticipated results (1 paragraph), proposed timeline, and anticipated meeting to which it will be submitted. Your mentor will revise and critique the proposal and eventually give you a signature of approval.
This proposal serves many purposes. It will allow you to fully understand the study before you begin, some form of it is usually required for the Institutional Review Board (IRB) application, it will serve as the outline for your eventual manuscript, and it sets a timeline for completion of the project. Without an agreed upon deadline, too many good studies are left in various states of completion when the trainee moves on, and are never finished. The deadline should be based on the meeting that you and your mentor agree is appropriate for reporting your results.
Most would agree that data collection is the most painful part of doing clinical research. However, there are a few tricks to ease your pain. First, there are many databases available that you may be able to harvest data from to minimize your chart work (Table 3). Before you hit the charts, it is essential to think through every step of the project.
Anticipate problems (where in the chart will you locate each data point), do not collect unnecessary data points (postoperative data #3 serum [Na+] when looking at survival of thoracoscopic vs. open lobectomy), meet with your statistician beforehand to collect data for the correct analysis, collect the raw data (creatinine and weight, not presence of renal failure and obesity). Finally, be sure that your data are backed up in multiple places. Some prefer to collect data on paper then enter it later into a spreadsheet. This ensures a hard copy of the data regardless of whether the electronic version is lost.
After the data are collected and the statistics are done, you will be faced with interpreting your results and composing an abstract and manuscript. If your study is focused and hypothesis driven, this step should be fairly straightforward.
Schedule time with your mentor and discuss the results to ensure your interpretation of the data is correct. Next, using your proposal as an outline, put together a rough draft of a manuscript.
Remember that manuscripts are the currency of academia. If you do not present and publish your work, you have not fully capitalized on the hard work you have put in to your study. Your mentor will need to revise your manuscript repeatedly; use it as a learning experience for critiquing the literature and writing future manuscripts. He or she likely knows what editors and readers will be looking for in your finished product.
Remember, you will need multiple revisions of the abstract and manuscript, so plan adequate time prior to your deadline for writing. Most institutions have medical illustrators available for hire; consider including a drawing or photograph if it legitimately adds content to your manuscript.
The final step in the process is presenting your work in front of experts who likely know more about cardiothoracic surgery than you. Just remember, no one knows more about your data than you. Prepare relentlessly for your talk, take a deep breath before you walk on stage, speak with confidence, and if you don’t know the answer to a given question from the audience, admit it. Soon enough you will be the expert in the audience asking the tough questions.
Then spend as much time as possible after the session speaking with audience members about you and your study. You will meet lifelong colleagues, and maybe even your future partner. For many, research is a rewarding lifelong endeavor. For others, it is a means of learning to critically appraise the literature and landing a job. Either way, you cannot afford not to do research as a trainee.
Acknowledgement: I would like to thank my friend and colleague, Dr. Stephen H. McKellar (University of Utah), for his advice on performing research as a cardiothoracic trainee.
Dr. Seder is in the department of cardiovascular and thoracic surgery at Rush University Medical Center.
"Why do I need to do research if I’m going into private practice anyway?"
I have heard this question multiple times throughout my career as a resident, fellow, and attending thoracic surgeon. The truth is, there are multiple reasons, any of which is sufficient to justify your participation in clinical research during training. First, and perhaps most importantly, it teaches you to critically appraise the literature. This is a skill that will serve you well throughout your career, guiding your clinical decision-making, regardless if you choose private practice or academic surgery. Another reason is that performing clinical research allows you to become a content expert on a specific topic early in your career. This knowledge base is something that will serve as a foundation for ongoing learning and may help in designing future studies. Once your project is complete, it will be your ticket to attend and present at regional, national, or international meetings. There is no better forum to gain public recognition for your investigative efforts and network with potential future partners than societal meetings. Formal and informal interviews routinely occur at these gatherings and you do not want to be left out because you chose not to participate in research as a trainee. Finally, it is your responsibility to the patients that you have sworn to treat. There are many ways to care for patients, and pushing back the frontiers of medical knowledge is as important as the day-to-day tasks that you perform on the ward or in the operating room.
So, now that you have decided that you want to participate in a research project as a trainee, how do you make it happen? Before you begin a project, you will have to choose a mentor, a topic, a clear, novel question, and the appropriate study design. Chances are that at some point, a mentor helped guide you toward a career in cardiothoracic surgery. A research mentor is just as important as a clinical mentor for a young surgeon.
The most important trait that you should seek out in a research mentor is the ability to delineate important questions. All too often, residents and fellows are approached by attending surgeons with good intentions, but bad research ideas. Trainees then feel obligated to take them up on the project (in order to not appear like a slacker) and for various reasons, it does not result in an abstract, presentation, or publication. In fact, all it results in is frustration, a distaste for investigation, and wasted time. The bottom line is that only you can protect your time, and as a surgical trainee, you must guard it ferociously. Look for a mentor who is an expert in your field of interest and who has a track record of publications.
He or she must be a logical thinker who can help you delineate a clear, novel question, choose the appropriate study design, guide your writing of the manuscript, and direct your submission to the appropriate meetings and journals. Finally, your mentor must be dedicated to your success. We are all busy, but if your mentor cannot find the time to routinely meet with you at every step of your project, you need to find a new mentor.
Choosing a clear, novel clinical question starts with choosing an appropriate topic (Table 1). With the right topic and question, the hypothesis is obvious, it is easy to define your endpoints, and your study design will fall into place. But with the wrong question, your study will lack focus, it will be difficult to explain the relevance of your study, and you will not want to present your data on the podium. An example of a good question is "Do patients with a given disease treated with operation X live longer than those treated with operation Y?" Stay away from the lure of "Let’s review our experience of operation X..." or "Why don’t I see how many of operation Y we’ve done over the past 10 years..." These topics are vague and do not ask a specific question. There must be a clear hypothesis for any study that is expected to produce meaningful results.
Once you have chosen an appropriate question, you must decide on a study design. Although case reports are marginally publishable, they will not answer your clinical questions. For many reasons, randomized, controlled trials, the gold standard of research, are difficult to design, carry out, and complete in your short time as a trainee. The good news is that well-designed and sufficiently powered observational studies often give similar results as randomized, controlled studies. Examples of common observational study designs include cohort studies, case control studies, and cross-sectional studies (Table 2). Each study design is different and your mentor should be able to help you decide which is the best to answer the question you want to ask.
When designing a study, one of the most important principles is defining a priori endpoints. Every study will have one primary endpoint that reflects the hypothesis. Secondary endpoints are interesting and potentially helpful, but are not the main message. It will be important to meet with a statistician before you start data collection. Understanding the statistics to be used will allow you to collect your data in the correct way (categorical vs. continuous, etc.). Reviewing charts is very time consuming and you have to do everything in your power to ensure you only do it once.
The next step is to create a research proposal. To do this, you will need to go to the literature, and see what published data relate to your study. Perhaps there are previous studies examining your question with conflicting results. Or if your question has not been previously investigated, what supporting literature suggests that yours is the next logical study? Your proposal should include a background section (1-2 paragraphs), hypothesis (1 sentence), the specific aims of the study (1-3 sentences), methods (2-4 paragraphs), anticipated results (1 paragraph), proposed timeline, and anticipated meeting to which it will be submitted. Your mentor will revise and critique the proposal and eventually give you a signature of approval.
This proposal serves many purposes. It will allow you to fully understand the study before you begin, some form of it is usually required for the Institutional Review Board (IRB) application, it will serve as the outline for your eventual manuscript, and it sets a timeline for completion of the project. Without an agreed upon deadline, too many good studies are left in various states of completion when the trainee moves on, and are never finished. The deadline should be based on the meeting that you and your mentor agree is appropriate for reporting your results.
Most would agree that data collection is the most painful part of doing clinical research. However, there are a few tricks to ease your pain. First, there are many databases available that you may be able to harvest data from to minimize your chart work (Table 3). Before you hit the charts, it is essential to think through every step of the project.
Anticipate problems (where in the chart will you locate each data point), do not collect unnecessary data points (postoperative data #3 serum [Na+] when looking at survival of thoracoscopic vs. open lobectomy), meet with your statistician beforehand to collect data for the correct analysis, collect the raw data (creatinine and weight, not presence of renal failure and obesity). Finally, be sure that your data are backed up in multiple places. Some prefer to collect data on paper then enter it later into a spreadsheet. This ensures a hard copy of the data regardless of whether the electronic version is lost.
After the data are collected and the statistics are done, you will be faced with interpreting your results and composing an abstract and manuscript. If your study is focused and hypothesis driven, this step should be fairly straightforward.
Schedule time with your mentor and discuss the results to ensure your interpretation of the data is correct. Next, using your proposal as an outline, put together a rough draft of a manuscript.
Remember that manuscripts are the currency of academia. If you do not present and publish your work, you have not fully capitalized on the hard work you have put in to your study. Your mentor will need to revise your manuscript repeatedly; use it as a learning experience for critiquing the literature and writing future manuscripts. He or she likely knows what editors and readers will be looking for in your finished product.
Remember, you will need multiple revisions of the abstract and manuscript, so plan adequate time prior to your deadline for writing. Most institutions have medical illustrators available for hire; consider including a drawing or photograph if it legitimately adds content to your manuscript.
The final step in the process is presenting your work in front of experts who likely know more about cardiothoracic surgery than you. Just remember, no one knows more about your data than you. Prepare relentlessly for your talk, take a deep breath before you walk on stage, speak with confidence, and if you don’t know the answer to a given question from the audience, admit it. Soon enough you will be the expert in the audience asking the tough questions.
Then spend as much time as possible after the session speaking with audience members about you and your study. You will meet lifelong colleagues, and maybe even your future partner. For many, research is a rewarding lifelong endeavor. For others, it is a means of learning to critically appraise the literature and landing a job. Either way, you cannot afford not to do research as a trainee.
Acknowledgement: I would like to thank my friend and colleague, Dr. Stephen H. McKellar (University of Utah), for his advice on performing research as a cardiothoracic trainee.
Dr. Seder is in the department of cardiovascular and thoracic surgery at Rush University Medical Center.
"Why do I need to do research if I’m going into private practice anyway?"
I have heard this question multiple times throughout my career as a resident, fellow, and attending thoracic surgeon. The truth is, there are multiple reasons, any of which is sufficient to justify your participation in clinical research during training. First, and perhaps most importantly, it teaches you to critically appraise the literature. This is a skill that will serve you well throughout your career, guiding your clinical decision-making, regardless if you choose private practice or academic surgery. Another reason is that performing clinical research allows you to become a content expert on a specific topic early in your career. This knowledge base is something that will serve as a foundation for ongoing learning and may help in designing future studies. Once your project is complete, it will be your ticket to attend and present at regional, national, or international meetings. There is no better forum to gain public recognition for your investigative efforts and network with potential future partners than societal meetings. Formal and informal interviews routinely occur at these gatherings and you do not want to be left out because you chose not to participate in research as a trainee. Finally, it is your responsibility to the patients that you have sworn to treat. There are many ways to care for patients, and pushing back the frontiers of medical knowledge is as important as the day-to-day tasks that you perform on the ward or in the operating room.
So, now that you have decided that you want to participate in a research project as a trainee, how do you make it happen? Before you begin a project, you will have to choose a mentor, a topic, a clear, novel question, and the appropriate study design. Chances are that at some point, a mentor helped guide you toward a career in cardiothoracic surgery. A research mentor is just as important as a clinical mentor for a young surgeon.
The most important trait that you should seek out in a research mentor is the ability to delineate important questions. All too often, residents and fellows are approached by attending surgeons with good intentions, but bad research ideas. Trainees then feel obligated to take them up on the project (in order to not appear like a slacker) and for various reasons, it does not result in an abstract, presentation, or publication. In fact, all it results in is frustration, a distaste for investigation, and wasted time. The bottom line is that only you can protect your time, and as a surgical trainee, you must guard it ferociously. Look for a mentor who is an expert in your field of interest and who has a track record of publications.
He or she must be a logical thinker who can help you delineate a clear, novel question, choose the appropriate study design, guide your writing of the manuscript, and direct your submission to the appropriate meetings and journals. Finally, your mentor must be dedicated to your success. We are all busy, but if your mentor cannot find the time to routinely meet with you at every step of your project, you need to find a new mentor.
Choosing a clear, novel clinical question starts with choosing an appropriate topic (Table 1). With the right topic and question, the hypothesis is obvious, it is easy to define your endpoints, and your study design will fall into place. But with the wrong question, your study will lack focus, it will be difficult to explain the relevance of your study, and you will not want to present your data on the podium. An example of a good question is "Do patients with a given disease treated with operation X live longer than those treated with operation Y?" Stay away from the lure of "Let’s review our experience of operation X..." or "Why don’t I see how many of operation Y we’ve done over the past 10 years..." These topics are vague and do not ask a specific question. There must be a clear hypothesis for any study that is expected to produce meaningful results.
Once you have chosen an appropriate question, you must decide on a study design. Although case reports are marginally publishable, they will not answer your clinical questions. For many reasons, randomized, controlled trials, the gold standard of research, are difficult to design, carry out, and complete in your short time as a trainee. The good news is that well-designed and sufficiently powered observational studies often give similar results as randomized, controlled studies. Examples of common observational study designs include cohort studies, case control studies, and cross-sectional studies (Table 2). Each study design is different and your mentor should be able to help you decide which is the best to answer the question you want to ask.
When designing a study, one of the most important principles is defining a priori endpoints. Every study will have one primary endpoint that reflects the hypothesis. Secondary endpoints are interesting and potentially helpful, but are not the main message. It will be important to meet with a statistician before you start data collection. Understanding the statistics to be used will allow you to collect your data in the correct way (categorical vs. continuous, etc.). Reviewing charts is very time consuming and you have to do everything in your power to ensure you only do it once.
The next step is to create a research proposal. To do this, you will need to go to the literature, and see what published data relate to your study. Perhaps there are previous studies examining your question with conflicting results. Or if your question has not been previously investigated, what supporting literature suggests that yours is the next logical study? Your proposal should include a background section (1-2 paragraphs), hypothesis (1 sentence), the specific aims of the study (1-3 sentences), methods (2-4 paragraphs), anticipated results (1 paragraph), proposed timeline, and anticipated meeting to which it will be submitted. Your mentor will revise and critique the proposal and eventually give you a signature of approval.
This proposal serves many purposes. It will allow you to fully understand the study before you begin, some form of it is usually required for the Institutional Review Board (IRB) application, it will serve as the outline for your eventual manuscript, and it sets a timeline for completion of the project. Without an agreed upon deadline, too many good studies are left in various states of completion when the trainee moves on, and are never finished. The deadline should be based on the meeting that you and your mentor agree is appropriate for reporting your results.
Most would agree that data collection is the most painful part of doing clinical research. However, there are a few tricks to ease your pain. First, there are many databases available that you may be able to harvest data from to minimize your chart work (Table 3). Before you hit the charts, it is essential to think through every step of the project.
Anticipate problems (where in the chart will you locate each data point), do not collect unnecessary data points (postoperative data #3 serum [Na+] when looking at survival of thoracoscopic vs. open lobectomy), meet with your statistician beforehand to collect data for the correct analysis, collect the raw data (creatinine and weight, not presence of renal failure and obesity). Finally, be sure that your data are backed up in multiple places. Some prefer to collect data on paper then enter it later into a spreadsheet. This ensures a hard copy of the data regardless of whether the electronic version is lost.
After the data are collected and the statistics are done, you will be faced with interpreting your results and composing an abstract and manuscript. If your study is focused and hypothesis driven, this step should be fairly straightforward.
Schedule time with your mentor and discuss the results to ensure your interpretation of the data is correct. Next, using your proposal as an outline, put together a rough draft of a manuscript.
Remember that manuscripts are the currency of academia. If you do not present and publish your work, you have not fully capitalized on the hard work you have put in to your study. Your mentor will need to revise your manuscript repeatedly; use it as a learning experience for critiquing the literature and writing future manuscripts. He or she likely knows what editors and readers will be looking for in your finished product.
Remember, you will need multiple revisions of the abstract and manuscript, so plan adequate time prior to your deadline for writing. Most institutions have medical illustrators available for hire; consider including a drawing or photograph if it legitimately adds content to your manuscript.
The final step in the process is presenting your work in front of experts who likely know more about cardiothoracic surgery than you. Just remember, no one knows more about your data than you. Prepare relentlessly for your talk, take a deep breath before you walk on stage, speak with confidence, and if you don’t know the answer to a given question from the audience, admit it. Soon enough you will be the expert in the audience asking the tough questions.
Then spend as much time as possible after the session speaking with audience members about you and your study. You will meet lifelong colleagues, and maybe even your future partner. For many, research is a rewarding lifelong endeavor. For others, it is a means of learning to critically appraise the literature and landing a job. Either way, you cannot afford not to do research as a trainee.
Acknowledgement: I would like to thank my friend and colleague, Dr. Stephen H. McKellar (University of Utah), for his advice on performing research as a cardiothoracic trainee.
Dr. Seder is in the department of cardiovascular and thoracic surgery at Rush University Medical Center.
Botulinum toxin A tops list of nonsurgical cosmetic procedures in 2013
Injection of botulinum toxin type A continues to be the most popular form of minimally invasive cosmetic surgery, with a total of more than 6.3 million procedures performed in 2013, the American Society of Plastic Surgeons reported.
Overall, botulinum toxins such as Botox and Dysport accounted for 47% of the market for minimally invasive procedures, which totaled 13.4 million procedures in 2013, according to the ASPS.
The second most popular surgery was injection of soft tissue fillers, with 2.2 million procedures performed, followed by chemical peels (1.2 million procedures), laser hair removal (1.1 million), and microdermabrasion (970,000), the ASPS said.
The total number of minimally invasive procedures increased by 3% from 2012, as did the number of botulinum injections. The largest increase for a single type of procedure was seen for the soft tissue fillers, with hyaluronic acid injections up 18% from 2012 to 2013, the ASPS noted.
The estimates for 2013 are based on data from a national database and survey responses from 800 dermatologists, ENTs, and plastic surgeons.
Injection of botulinum toxin type A continues to be the most popular form of minimally invasive cosmetic surgery, with a total of more than 6.3 million procedures performed in 2013, the American Society of Plastic Surgeons reported.
Overall, botulinum toxins such as Botox and Dysport accounted for 47% of the market for minimally invasive procedures, which totaled 13.4 million procedures in 2013, according to the ASPS.
The second most popular surgery was injection of soft tissue fillers, with 2.2 million procedures performed, followed by chemical peels (1.2 million procedures), laser hair removal (1.1 million), and microdermabrasion (970,000), the ASPS said.
The total number of minimally invasive procedures increased by 3% from 2012, as did the number of botulinum injections. The largest increase for a single type of procedure was seen for the soft tissue fillers, with hyaluronic acid injections up 18% from 2012 to 2013, the ASPS noted.
The estimates for 2013 are based on data from a national database and survey responses from 800 dermatologists, ENTs, and plastic surgeons.

Injection of botulinum toxin type A continues to be the most popular form of minimally invasive cosmetic surgery, with a total of more than 6.3 million procedures performed in 2013, the American Society of Plastic Surgeons reported.
Overall, botulinum toxins such as Botox and Dysport accounted for 47% of the market for minimally invasive procedures, which totaled 13.4 million procedures in 2013, according to the ASPS.
The second most popular surgery was injection of soft tissue fillers, with 2.2 million procedures performed, followed by chemical peels (1.2 million procedures), laser hair removal (1.1 million), and microdermabrasion (970,000), the ASPS said.
The total number of minimally invasive procedures increased by 3% from 2012, as did the number of botulinum injections. The largest increase for a single type of procedure was seen for the soft tissue fillers, with hyaluronic acid injections up 18% from 2012 to 2013, the ASPS noted.
The estimates for 2013 are based on data from a national database and survey responses from 800 dermatologists, ENTs, and plastic surgeons.

They love me, they love me not ...
It was the worst of days. It was the best of days.
When I opened the mail one day last week, I found a letter from someone I’ll call Thelma. It read, in part:
"Last Monday you were kind enough to look at my rash, which you thought was just eczema. You gave me cream and asked me to e-mail you Thursday about my condition. When I did and said I was still itchy, you said I should stick with the same and that I could come back Monday, but I couldn’t wait because I itched so bad I couldn’t take it anymore. I saw another doctor Friday who said the patch was host to something called pityriasis rosea. He said the rash was so textbook it should have been picked up immediately. I had to be put on an oral steroid right away.
"I am so upset that I’m sending you back your bill [for a $15 co-pay] because I had to go to another doctor who could really help me."
I thought of a few choice words for my esteemed Friday colleague, but kept them to myself. A single scaly patch is a textbook case of pityriasis rosea? Oral steroids for pityriasis? Really?
As far as this patient is concerned, I must be a bum. Thirty-five years on the job, and I haven’t mastered the textbook yet.
Sunk in gloom, I opened an e-mail sent to my website by a patient I’ll call Louise:
"I suffer from psoriasis and have been to countless dermatologists since I was 8 years old. I recently had a terrible outbreak and was really hesitant to even go to a dermatologist because I’ve never been satisfied with any of them. Your associate is wonderful! I can’t say enough about her. She is warm, thorough, and really takes the time to sit with you and listen. You can tell she truly cares about her patients and loves her job."
I looked at the patient’s chart. What was the wonderful and satisfying treatment that my associate had prescribed to deal with this patient’s lifelong, recalcitrant psoriasis?
Betamethasone dipropionate cream 0.05%. Wow.
I e-mailed my associate at once and we shared a gratified chuckle. Guess no one ever thought of treating Louise’s psoriasis with a topical steroid before. We must be geniuses, right out there on the cutting edge.
So which are we, dear colleagues – geniuses or bums?
We’re neither, of course, which doesn’t stop our patients from forming firm opinions one way or the other. Which they can share by angry letter, fulsome e-mail, or, of course, any on-line reviews they can slip past the mysterious algorithms of the Yelps and Angie’s Lists of the world.
When I get messages like Thelma’s and Louise’s, I show them to my students and make three suggestions:
• Don’t try to look smart at someone else’s expense. Next time around a patient will be in somebody else’s office calling you a fool.
• Don’t respond to snippy patients’ complaints by contacting the complainer and trying to justify yourself. Learn something if you can, and move on.
• Be grateful for praise. Just don’t take it too seriously.
In the meantime, the insurers and assorted bureaucrats who run our lives these days are busy defining good care and claiming to measure it so they can reward quality and punish inefficiency. I’m sure they think they’re doing a fine job, although I remain deeply skeptical that what they choose to measure has much relevance to what actually goes on in offices like ours.
I could, of course, try to tell them why I think so. (I have tried, in fact.) Getting through to people with a completely different way of looking at things than yours is not very rewarding, even when large sums of money are not involved. I would have as good a chance of winning them over as I would of convincing Thelma that a scaly patch is not textbook pityriasis that needs prednisone and Louise that betamethasone cream is not the breakthrough that will change her life.
So: Not the best of times. Not the worst of times. Just another day at the office.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since 1997.
It was the worst of days. It was the best of days.
When I opened the mail one day last week, I found a letter from someone I’ll call Thelma. It read, in part:
"Last Monday you were kind enough to look at my rash, which you thought was just eczema. You gave me cream and asked me to e-mail you Thursday about my condition. When I did and said I was still itchy, you said I should stick with the same and that I could come back Monday, but I couldn’t wait because I itched so bad I couldn’t take it anymore. I saw another doctor Friday who said the patch was host to something called pityriasis rosea. He said the rash was so textbook it should have been picked up immediately. I had to be put on an oral steroid right away.
"I am so upset that I’m sending you back your bill [for a $15 co-pay] because I had to go to another doctor who could really help me."
I thought of a few choice words for my esteemed Friday colleague, but kept them to myself. A single scaly patch is a textbook case of pityriasis rosea? Oral steroids for pityriasis? Really?
As far as this patient is concerned, I must be a bum. Thirty-five years on the job, and I haven’t mastered the textbook yet.
Sunk in gloom, I opened an e-mail sent to my website by a patient I’ll call Louise:
"I suffer from psoriasis and have been to countless dermatologists since I was 8 years old. I recently had a terrible outbreak and was really hesitant to even go to a dermatologist because I’ve never been satisfied with any of them. Your associate is wonderful! I can’t say enough about her. She is warm, thorough, and really takes the time to sit with you and listen. You can tell she truly cares about her patients and loves her job."
I looked at the patient’s chart. What was the wonderful and satisfying treatment that my associate had prescribed to deal with this patient’s lifelong, recalcitrant psoriasis?
Betamethasone dipropionate cream 0.05%. Wow.
I e-mailed my associate at once and we shared a gratified chuckle. Guess no one ever thought of treating Louise’s psoriasis with a topical steroid before. We must be geniuses, right out there on the cutting edge.
So which are we, dear colleagues – geniuses or bums?
We’re neither, of course, which doesn’t stop our patients from forming firm opinions one way or the other. Which they can share by angry letter, fulsome e-mail, or, of course, any on-line reviews they can slip past the mysterious algorithms of the Yelps and Angie’s Lists of the world.
When I get messages like Thelma’s and Louise’s, I show them to my students and make three suggestions:
• Don’t try to look smart at someone else’s expense. Next time around a patient will be in somebody else’s office calling you a fool.
• Don’t respond to snippy patients’ complaints by contacting the complainer and trying to justify yourself. Learn something if you can, and move on.
• Be grateful for praise. Just don’t take it too seriously.
In the meantime, the insurers and assorted bureaucrats who run our lives these days are busy defining good care and claiming to measure it so they can reward quality and punish inefficiency. I’m sure they think they’re doing a fine job, although I remain deeply skeptical that what they choose to measure has much relevance to what actually goes on in offices like ours.
I could, of course, try to tell them why I think so. (I have tried, in fact.) Getting through to people with a completely different way of looking at things than yours is not very rewarding, even when large sums of money are not involved. I would have as good a chance of winning them over as I would of convincing Thelma that a scaly patch is not textbook pityriasis that needs prednisone and Louise that betamethasone cream is not the breakthrough that will change her life.
So: Not the best of times. Not the worst of times. Just another day at the office.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since 1997.
It was the worst of days. It was the best of days.
When I opened the mail one day last week, I found a letter from someone I’ll call Thelma. It read, in part:
"Last Monday you were kind enough to look at my rash, which you thought was just eczema. You gave me cream and asked me to e-mail you Thursday about my condition. When I did and said I was still itchy, you said I should stick with the same and that I could come back Monday, but I couldn’t wait because I itched so bad I couldn’t take it anymore. I saw another doctor Friday who said the patch was host to something called pityriasis rosea. He said the rash was so textbook it should have been picked up immediately. I had to be put on an oral steroid right away.
"I am so upset that I’m sending you back your bill [for a $15 co-pay] because I had to go to another doctor who could really help me."
I thought of a few choice words for my esteemed Friday colleague, but kept them to myself. A single scaly patch is a textbook case of pityriasis rosea? Oral steroids for pityriasis? Really?
As far as this patient is concerned, I must be a bum. Thirty-five years on the job, and I haven’t mastered the textbook yet.
Sunk in gloom, I opened an e-mail sent to my website by a patient I’ll call Louise:
"I suffer from psoriasis and have been to countless dermatologists since I was 8 years old. I recently had a terrible outbreak and was really hesitant to even go to a dermatologist because I’ve never been satisfied with any of them. Your associate is wonderful! I can’t say enough about her. She is warm, thorough, and really takes the time to sit with you and listen. You can tell she truly cares about her patients and loves her job."
I looked at the patient’s chart. What was the wonderful and satisfying treatment that my associate had prescribed to deal with this patient’s lifelong, recalcitrant psoriasis?
Betamethasone dipropionate cream 0.05%. Wow.
I e-mailed my associate at once and we shared a gratified chuckle. Guess no one ever thought of treating Louise’s psoriasis with a topical steroid before. We must be geniuses, right out there on the cutting edge.
So which are we, dear colleagues – geniuses or bums?
We’re neither, of course, which doesn’t stop our patients from forming firm opinions one way or the other. Which they can share by angry letter, fulsome e-mail, or, of course, any on-line reviews they can slip past the mysterious algorithms of the Yelps and Angie’s Lists of the world.
When I get messages like Thelma’s and Louise’s, I show them to my students and make three suggestions:
• Don’t try to look smart at someone else’s expense. Next time around a patient will be in somebody else’s office calling you a fool.
• Don’t respond to snippy patients’ complaints by contacting the complainer and trying to justify yourself. Learn something if you can, and move on.
• Be grateful for praise. Just don’t take it too seriously.
In the meantime, the insurers and assorted bureaucrats who run our lives these days are busy defining good care and claiming to measure it so they can reward quality and punish inefficiency. I’m sure they think they’re doing a fine job, although I remain deeply skeptical that what they choose to measure has much relevance to what actually goes on in offices like ours.
I could, of course, try to tell them why I think so. (I have tried, in fact.) Getting through to people with a completely different way of looking at things than yours is not very rewarding, even when large sums of money are not involved. I would have as good a chance of winning them over as I would of convincing Thelma that a scaly patch is not textbook pityriasis that needs prednisone and Louise that betamethasone cream is not the breakthrough that will change her life.
So: Not the best of times. Not the worst of times. Just another day at the office.
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since 1997.
ACC/AHA cardiovascular risk equations get a thumbs-up
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.
The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
AT ACC 14
Major finding: The controversial cardiovascular risk equations at the heart of the latest ACC/AHA risk-assessment guidelines turned in a moderate to good performance in a validation study involving nearly 11,000 participants in a large, observational, prospective study.
Data source: The REGARDS study is a population-based study in which more than 30,000 U.S. black and white patients are being followed prospectively.
Disclosures: The analysis was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
Heparanase regulates response to chemo in MM, team says
SAN DIEGO—Experiments conducted in the lab and the clinic suggest the enzyme heparanase enhances resistance to chemotherapy in multiple myeloma (MM).
Researchers first found that expression of heparanase, an endoglycosidase that cleaves heparan sulfate, is highly elevated in MM patients after chemotherapy.
The team then used MM cell lines to investigate the mechanism behind this phenomenon.
Their results indicate that, by inhibiting heparanase, we might be able to prevent or delay relapse in MM.
Vishnu Ramani, PhD, of the University of Alabama Birmingham, and his colleagues conducted this research and presented the results at the AACR Annual Meeting 2014 (abstract 1708).
Several years ago, Dr Ramani’s colleagues (in the lab of Ralph Sanderson, PhD) identified heparanase as a master regulator of aggressive MM. Since then, research has suggested that heparanase fuels aggressive MM by upregulating the expression of pro-angiogenic genes, driving osteolysis, upregulating prometastatic molecules, and controlling the tumor microenvironment.
“We have done a lot of work on the biology of how this molecule works in myeloma, but the one thing I was really interested in was its role in drug resistance,” Dr Ramani said.
So he and his colleagues decided to study heparanase levels in 9 MM patients undergoing chemotherapy. The team isolated tumor cells from patients before and after 2 rounds of chemotherapy and compared heparanase levels at the different time points.
“What we find—and this is really remarkable—is that the expression of heparanase over rounds of therapy goes up several thousand-fold, and this is in the majority of patients,” Dr Ramani said. “In 8 out of 9 patients that we studied, at the end of chemotherapy, the cells that survive have extremely high levels of heparanase.”
To gain more insight into this phenomenon, the researchers studied it in MM cell lines. The team introduced bortezomib to RPMI-8226 and CAG cells and found that heparanase levels increased “dramatically” after treatment.
“The treatment is not only increasing the heparanase expression inside the cell,” Dr Ramani explained. “What the cells do is that, if you continue the treatment, they die, but they don’t take the heparanase with them. They leave it out in the media, and this can be taken in by other cells. So this can activate other cells to promote aggressive tumor growth too.”
Additional investigation revealed that the NF-κB pathway plays a role—namely, chemotherapy activates the pathway to upregulate heparanase. But inhibiting NF-κB activity can prevent that increase in heparanase.
The researchers tested the NF-κB inhibitors BAY 11-7085 and BMS345541 in combination with bortezomib. And they found that both agents prevented bortezomib from elevating heparanase expression in CAG cells.
Dr Ramani and his colleagues also evaluated heparanase levels in chemoresistant MM cell lines. Heparanase levels were 4-fold higher in a doxorubicin-resistant MM cell line and 10-fold higher in a melphalan-resistant cell line, when compared to a wild-type MM cell line.
Next, the researchers compared MM cells with high heparanase expression to those with low heparanase expression. And they discovered that high heparanase levels protect cells from chemotherapy.
After treatment with bortezomib, cells with high heparanase expression were significantly more viable than those with low expression (P<0.05). And there was a significantly higher percentage of apoptotic cells among the low-heparanase population compared to the high-heparanase population (P<0.05).
“If you take cells that have high heparanase and another group of cells that have low heparanase and expose both of them to therapy, the cells with high heparanase always survive better because the heparanase upregulates certain pathways, like the MAP kinase pathway, which helps the cells to survive the onslaught of chemotherapy,” Dr Ramani said. “So myeloma cells are actually hijacking the heparanase pathway to survive better after therapy.”
Building upon that finding, the researchers decided to assess whether inhibiting ERK activity might help cells overcome heparanase-mediated chemoresistance. And experiments showed that the ERK inhibitor U0126 can sensitize cells with high heparanase levels to treatment with bortezomib.
To take this research to the next level, Dr Ramani and his colleagues are collaborating with a company called Sigma Tau, which is developing a heparanase inhibitor called SST-0001. A phase 1 study of the drug in MM patients has been completed, and phase 2 studies are currently recruiting patients in Europe.
Dr Ramani is now conducting experiments in mice to determine how the inhibitor might work in combination with chemotherapy and when it should be administered in order to overcome treatment resistance. He is also looking for other molecular pathways that could be involved in heparanase-related treatment resistance. ![]()
SAN DIEGO—Experiments conducted in the lab and the clinic suggest the enzyme heparanase enhances resistance to chemotherapy in multiple myeloma (MM).
Researchers first found that expression of heparanase, an endoglycosidase that cleaves heparan sulfate, is highly elevated in MM patients after chemotherapy.
The team then used MM cell lines to investigate the mechanism behind this phenomenon.
Their results indicate that, by inhibiting heparanase, we might be able to prevent or delay relapse in MM.
Vishnu Ramani, PhD, of the University of Alabama Birmingham, and his colleagues conducted this research and presented the results at the AACR Annual Meeting 2014 (abstract 1708).
Several years ago, Dr Ramani’s colleagues (in the lab of Ralph Sanderson, PhD) identified heparanase as a master regulator of aggressive MM. Since then, research has suggested that heparanase fuels aggressive MM by upregulating the expression of pro-angiogenic genes, driving osteolysis, upregulating prometastatic molecules, and controlling the tumor microenvironment.
“We have done a lot of work on the biology of how this molecule works in myeloma, but the one thing I was really interested in was its role in drug resistance,” Dr Ramani said.
So he and his colleagues decided to study heparanase levels in 9 MM patients undergoing chemotherapy. The team isolated tumor cells from patients before and after 2 rounds of chemotherapy and compared heparanase levels at the different time points.
“What we find—and this is really remarkable—is that the expression of heparanase over rounds of therapy goes up several thousand-fold, and this is in the majority of patients,” Dr Ramani said. “In 8 out of 9 patients that we studied, at the end of chemotherapy, the cells that survive have extremely high levels of heparanase.”
To gain more insight into this phenomenon, the researchers studied it in MM cell lines. The team introduced bortezomib to RPMI-8226 and CAG cells and found that heparanase levels increased “dramatically” after treatment.
“The treatment is not only increasing the heparanase expression inside the cell,” Dr Ramani explained. “What the cells do is that, if you continue the treatment, they die, but they don’t take the heparanase with them. They leave it out in the media, and this can be taken in by other cells. So this can activate other cells to promote aggressive tumor growth too.”
Additional investigation revealed that the NF-κB pathway plays a role—namely, chemotherapy activates the pathway to upregulate heparanase. But inhibiting NF-κB activity can prevent that increase in heparanase.
The researchers tested the NF-κB inhibitors BAY 11-7085 and BMS345541 in combination with bortezomib. And they found that both agents prevented bortezomib from elevating heparanase expression in CAG cells.
Dr Ramani and his colleagues also evaluated heparanase levels in chemoresistant MM cell lines. Heparanase levels were 4-fold higher in a doxorubicin-resistant MM cell line and 10-fold higher in a melphalan-resistant cell line, when compared to a wild-type MM cell line.
Next, the researchers compared MM cells with high heparanase expression to those with low heparanase expression. And they discovered that high heparanase levels protect cells from chemotherapy.
After treatment with bortezomib, cells with high heparanase expression were significantly more viable than those with low expression (P<0.05). And there was a significantly higher percentage of apoptotic cells among the low-heparanase population compared to the high-heparanase population (P<0.05).
“If you take cells that have high heparanase and another group of cells that have low heparanase and expose both of them to therapy, the cells with high heparanase always survive better because the heparanase upregulates certain pathways, like the MAP kinase pathway, which helps the cells to survive the onslaught of chemotherapy,” Dr Ramani said. “So myeloma cells are actually hijacking the heparanase pathway to survive better after therapy.”
Building upon that finding, the researchers decided to assess whether inhibiting ERK activity might help cells overcome heparanase-mediated chemoresistance. And experiments showed that the ERK inhibitor U0126 can sensitize cells with high heparanase levels to treatment with bortezomib.
To take this research to the next level, Dr Ramani and his colleagues are collaborating with a company called Sigma Tau, which is developing a heparanase inhibitor called SST-0001. A phase 1 study of the drug in MM patients has been completed, and phase 2 studies are currently recruiting patients in Europe.
Dr Ramani is now conducting experiments in mice to determine how the inhibitor might work in combination with chemotherapy and when it should be administered in order to overcome treatment resistance. He is also looking for other molecular pathways that could be involved in heparanase-related treatment resistance. ![]()
SAN DIEGO—Experiments conducted in the lab and the clinic suggest the enzyme heparanase enhances resistance to chemotherapy in multiple myeloma (MM).
Researchers first found that expression of heparanase, an endoglycosidase that cleaves heparan sulfate, is highly elevated in MM patients after chemotherapy.
The team then used MM cell lines to investigate the mechanism behind this phenomenon.
Their results indicate that, by inhibiting heparanase, we might be able to prevent or delay relapse in MM.
Vishnu Ramani, PhD, of the University of Alabama Birmingham, and his colleagues conducted this research and presented the results at the AACR Annual Meeting 2014 (abstract 1708).
Several years ago, Dr Ramani’s colleagues (in the lab of Ralph Sanderson, PhD) identified heparanase as a master regulator of aggressive MM. Since then, research has suggested that heparanase fuels aggressive MM by upregulating the expression of pro-angiogenic genes, driving osteolysis, upregulating prometastatic molecules, and controlling the tumor microenvironment.
“We have done a lot of work on the biology of how this molecule works in myeloma, but the one thing I was really interested in was its role in drug resistance,” Dr Ramani said.
So he and his colleagues decided to study heparanase levels in 9 MM patients undergoing chemotherapy. The team isolated tumor cells from patients before and after 2 rounds of chemotherapy and compared heparanase levels at the different time points.
“What we find—and this is really remarkable—is that the expression of heparanase over rounds of therapy goes up several thousand-fold, and this is in the majority of patients,” Dr Ramani said. “In 8 out of 9 patients that we studied, at the end of chemotherapy, the cells that survive have extremely high levels of heparanase.”
To gain more insight into this phenomenon, the researchers studied it in MM cell lines. The team introduced bortezomib to RPMI-8226 and CAG cells and found that heparanase levels increased “dramatically” after treatment.
“The treatment is not only increasing the heparanase expression inside the cell,” Dr Ramani explained. “What the cells do is that, if you continue the treatment, they die, but they don’t take the heparanase with them. They leave it out in the media, and this can be taken in by other cells. So this can activate other cells to promote aggressive tumor growth too.”
Additional investigation revealed that the NF-κB pathway plays a role—namely, chemotherapy activates the pathway to upregulate heparanase. But inhibiting NF-κB activity can prevent that increase in heparanase.
The researchers tested the NF-κB inhibitors BAY 11-7085 and BMS345541 in combination with bortezomib. And they found that both agents prevented bortezomib from elevating heparanase expression in CAG cells.
Dr Ramani and his colleagues also evaluated heparanase levels in chemoresistant MM cell lines. Heparanase levels were 4-fold higher in a doxorubicin-resistant MM cell line and 10-fold higher in a melphalan-resistant cell line, when compared to a wild-type MM cell line.
Next, the researchers compared MM cells with high heparanase expression to those with low heparanase expression. And they discovered that high heparanase levels protect cells from chemotherapy.
After treatment with bortezomib, cells with high heparanase expression were significantly more viable than those with low expression (P<0.05). And there was a significantly higher percentage of apoptotic cells among the low-heparanase population compared to the high-heparanase population (P<0.05).
“If you take cells that have high heparanase and another group of cells that have low heparanase and expose both of them to therapy, the cells with high heparanase always survive better because the heparanase upregulates certain pathways, like the MAP kinase pathway, which helps the cells to survive the onslaught of chemotherapy,” Dr Ramani said. “So myeloma cells are actually hijacking the heparanase pathway to survive better after therapy.”
Building upon that finding, the researchers decided to assess whether inhibiting ERK activity might help cells overcome heparanase-mediated chemoresistance. And experiments showed that the ERK inhibitor U0126 can sensitize cells with high heparanase levels to treatment with bortezomib.
To take this research to the next level, Dr Ramani and his colleagues are collaborating with a company called Sigma Tau, which is developing a heparanase inhibitor called SST-0001. A phase 1 study of the drug in MM patients has been completed, and phase 2 studies are currently recruiting patients in Europe.
Dr Ramani is now conducting experiments in mice to determine how the inhibitor might work in combination with chemotherapy and when it should be administered in order to overcome treatment resistance. He is also looking for other molecular pathways that could be involved in heparanase-related treatment resistance. ![]()
How mechanical forces affect T cells

used for T-cell force research,
with 3 glass micropipettes
shown under green light.
Georgia Tech/Rob Felt
Investigators say they’ve discovered how T-cell receptors (TCRs) use mechanical contact to decide if the cells they encounter
pose a threat to the body.
The team made their discovery using a sensor based on a red blood cell and a technique for detecting calcium ions emitted by T cells as part of the signaling process.
The researchers studied the binding of antigens to more than a hundred T cells, measuring the forces involved in the binding and the lifetimes of the bonds.
Results revealed that force prolongs TCR bonds for agonists but shortens them for antagonists. And the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“This is the first systematic study of how T-cell recognition is affected by mechanical force, and it shows that forces play an important role in the functions of T cells,” said study author Cheng Zhu, PhD, of Georgia Tech and Emory University in Atlanta. “We think that mechanical force plays a role in almost every step of T-cell biology.”
Dr Zhu and his colleagues described this research in Cell.
The team used a biomembrane force probe to measure the strength and longevity of bonds between T cells and antigens. The probe consists, in part, of a red blood cell aspirated to a micropipette.
Attached to the red blood cell is a bead on which the investigators placed the antigen under study. Using a delicate mechanism that precisely controls motion, they moved the bead into contact with a TCR, allowing binding to take place.
To test the strength of the bond formed between an antigen and the TCR, the researchers applied piconewton forces to separate the bead holding the antigen from the TCR. The red blood cell then acted as a spring, stretching and allowing a measurement of the forces needed to separate the TCR and antigen.
To assess the impact of the binding on intracellular signaling, the investigators injected a dye into the cells that fluoresces when exposed to calcium signaling ions. Detecting the fluorescence allowed the team to determine when the mechanical force triggered T-cell signaling.
In this way, the researchers learned that interactions between the TCRs and agonist peptide-major histocompatibility complexes (MHCs) form catch bonds that become stronger with the application of additional force to initiate intracellular signaling.
And less active MHC complexes form slip bonds that weaken with force and don’t initiate signaling.
Overall, the investigators found that the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“Force adds another dimension to interactions with T cells,” Dr Zhu explained. “Antigens that have a bond lifetime that is prolonged by force would have a higher likelihood of triggering signaling. Repeat engagements and lifetime accumulations play a role, and the decision to signal is usually made based on the accumulation of actions, not a single action.”
Researchers already have examples of how mechanical force can affect the operation of cellular systems. For instance, mechanical stress created by blood flow acting on the endothelial cells that line blood vessel walls plays a role in atherosclerosis.
So it isn’t surprising that mechanical forces play a role in the immune system, according to Dr Zhu.
“We now have a broader recognition that the physical environment and mechanical environment regulate many of the biological phenomena in the body,” he said. “When you exert a force on the TCR bonds, some of them dissociate faster, while others come off more slowly. This has an effect on the response of the T-cell receptor.”
As a next step, Dr Zhu’s team would like to explore the effects of force on T-cell development using the new experimental techniques. Evidence suggests the forces to which the cells are exposed while in a juvenile stage may affect the fates of their development. ![]()

used for T-cell force research,
with 3 glass micropipettes
shown under green light.
Georgia Tech/Rob Felt
Investigators say they’ve discovered how T-cell receptors (TCRs) use mechanical contact to decide if the cells they encounter
pose a threat to the body.
The team made their discovery using a sensor based on a red blood cell and a technique for detecting calcium ions emitted by T cells as part of the signaling process.
The researchers studied the binding of antigens to more than a hundred T cells, measuring the forces involved in the binding and the lifetimes of the bonds.
Results revealed that force prolongs TCR bonds for agonists but shortens them for antagonists. And the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“This is the first systematic study of how T-cell recognition is affected by mechanical force, and it shows that forces play an important role in the functions of T cells,” said study author Cheng Zhu, PhD, of Georgia Tech and Emory University in Atlanta. “We think that mechanical force plays a role in almost every step of T-cell biology.”
Dr Zhu and his colleagues described this research in Cell.
The team used a biomembrane force probe to measure the strength and longevity of bonds between T cells and antigens. The probe consists, in part, of a red blood cell aspirated to a micropipette.
Attached to the red blood cell is a bead on which the investigators placed the antigen under study. Using a delicate mechanism that precisely controls motion, they moved the bead into contact with a TCR, allowing binding to take place.
To test the strength of the bond formed between an antigen and the TCR, the researchers applied piconewton forces to separate the bead holding the antigen from the TCR. The red blood cell then acted as a spring, stretching and allowing a measurement of the forces needed to separate the TCR and antigen.
To assess the impact of the binding on intracellular signaling, the investigators injected a dye into the cells that fluoresces when exposed to calcium signaling ions. Detecting the fluorescence allowed the team to determine when the mechanical force triggered T-cell signaling.
In this way, the researchers learned that interactions between the TCRs and agonist peptide-major histocompatibility complexes (MHCs) form catch bonds that become stronger with the application of additional force to initiate intracellular signaling.
And less active MHC complexes form slip bonds that weaken with force and don’t initiate signaling.
Overall, the investigators found that the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“Force adds another dimension to interactions with T cells,” Dr Zhu explained. “Antigens that have a bond lifetime that is prolonged by force would have a higher likelihood of triggering signaling. Repeat engagements and lifetime accumulations play a role, and the decision to signal is usually made based on the accumulation of actions, not a single action.”
Researchers already have examples of how mechanical force can affect the operation of cellular systems. For instance, mechanical stress created by blood flow acting on the endothelial cells that line blood vessel walls plays a role in atherosclerosis.
So it isn’t surprising that mechanical forces play a role in the immune system, according to Dr Zhu.
“We now have a broader recognition that the physical environment and mechanical environment regulate many of the biological phenomena in the body,” he said. “When you exert a force on the TCR bonds, some of them dissociate faster, while others come off more slowly. This has an effect on the response of the T-cell receptor.”
As a next step, Dr Zhu’s team would like to explore the effects of force on T-cell development using the new experimental techniques. Evidence suggests the forces to which the cells are exposed while in a juvenile stage may affect the fates of their development. ![]()

used for T-cell force research,
with 3 glass micropipettes
shown under green light.
Georgia Tech/Rob Felt
Investigators say they’ve discovered how T-cell receptors (TCRs) use mechanical contact to decide if the cells they encounter
pose a threat to the body.
The team made their discovery using a sensor based on a red blood cell and a technique for detecting calcium ions emitted by T cells as part of the signaling process.
The researchers studied the binding of antigens to more than a hundred T cells, measuring the forces involved in the binding and the lifetimes of the bonds.
Results revealed that force prolongs TCR bonds for agonists but shortens them for antagonists. And the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“This is the first systematic study of how T-cell recognition is affected by mechanical force, and it shows that forces play an important role in the functions of T cells,” said study author Cheng Zhu, PhD, of Georgia Tech and Emory University in Atlanta. “We think that mechanical force plays a role in almost every step of T-cell biology.”
Dr Zhu and his colleagues described this research in Cell.
The team used a biomembrane force probe to measure the strength and longevity of bonds between T cells and antigens. The probe consists, in part, of a red blood cell aspirated to a micropipette.
Attached to the red blood cell is a bead on which the investigators placed the antigen under study. Using a delicate mechanism that precisely controls motion, they moved the bead into contact with a TCR, allowing binding to take place.
To test the strength of the bond formed between an antigen and the TCR, the researchers applied piconewton forces to separate the bead holding the antigen from the TCR. The red blood cell then acted as a spring, stretching and allowing a measurement of the forces needed to separate the TCR and antigen.
To assess the impact of the binding on intracellular signaling, the investigators injected a dye into the cells that fluoresces when exposed to calcium signaling ions. Detecting the fluorescence allowed the team to determine when the mechanical force triggered T-cell signaling.
In this way, the researchers learned that interactions between the TCRs and agonist peptide-major histocompatibility complexes (MHCs) form catch bonds that become stronger with the application of additional force to initiate intracellular signaling.
And less active MHC complexes form slip bonds that weaken with force and don’t initiate signaling.
Overall, the investigators found that the signaling outcome of an interaction between an antigen and a TCR depends on the magnitude, duration, frequency, and timing of the force application.
“Force adds another dimension to interactions with T cells,” Dr Zhu explained. “Antigens that have a bond lifetime that is prolonged by force would have a higher likelihood of triggering signaling. Repeat engagements and lifetime accumulations play a role, and the decision to signal is usually made based on the accumulation of actions, not a single action.”
Researchers already have examples of how mechanical force can affect the operation of cellular systems. For instance, mechanical stress created by blood flow acting on the endothelial cells that line blood vessel walls plays a role in atherosclerosis.
So it isn’t surprising that mechanical forces play a role in the immune system, according to Dr Zhu.
“We now have a broader recognition that the physical environment and mechanical environment regulate many of the biological phenomena in the body,” he said. “When you exert a force on the TCR bonds, some of them dissociate faster, while others come off more slowly. This has an effect on the response of the T-cell receptor.”
As a next step, Dr Zhu’s team would like to explore the effects of force on T-cell development using the new experimental techniques. Evidence suggests the forces to which the cells are exposed while in a juvenile stage may affect the fates of their development. ![]()
Beware of invasive H influenzae disease among pregnant women
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”