User login
Positive nodal status and stage T4 tied to worsened long-term prognosis in HER2+ breast cancer
Key clinical point: Pathologic regional lymph node stages I-III (pN1-3) and pathologic tumor size stage IV (pT4) were associated with worse long-term breast-cancer–specific survival (BCSS) outcomes in patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC).
Major finding: In patients who were followed up for more than 60 months, increasing vs no nodal involvement (pN1-3 vs pN0; all hazard ratios [HR] >3; all P ≤ .001) and a tumor size of pT4 vs pT1 (HR 4.528; P = .007) were associated with poor BCSS outcomes.
Study details: This study used data from a registry to analyze 20,672 patients with HER2+ stage I-III BC who underwent surgery.
Disclosures: This study was supported by the Korean Breast Cancer Society. The authors declared no conflicts of interest.
Source: Kang YJ et al. Predictive biological factors for late survival in patients with HER2-positive breast cancer. Sci Rep. 2023;13:11008 (Jul 7). Doi: 10.1038/s41598-023-38200-y
Key clinical point: Pathologic regional lymph node stages I-III (pN1-3) and pathologic tumor size stage IV (pT4) were associated with worse long-term breast-cancer–specific survival (BCSS) outcomes in patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC).
Major finding: In patients who were followed up for more than 60 months, increasing vs no nodal involvement (pN1-3 vs pN0; all hazard ratios [HR] >3; all P ≤ .001) and a tumor size of pT4 vs pT1 (HR 4.528; P = .007) were associated with poor BCSS outcomes.
Study details: This study used data from a registry to analyze 20,672 patients with HER2+ stage I-III BC who underwent surgery.
Disclosures: This study was supported by the Korean Breast Cancer Society. The authors declared no conflicts of interest.
Source: Kang YJ et al. Predictive biological factors for late survival in patients with HER2-positive breast cancer. Sci Rep. 2023;13:11008 (Jul 7). Doi: 10.1038/s41598-023-38200-y
Key clinical point: Pathologic regional lymph node stages I-III (pN1-3) and pathologic tumor size stage IV (pT4) were associated with worse long-term breast-cancer–specific survival (BCSS) outcomes in patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC).
Major finding: In patients who were followed up for more than 60 months, increasing vs no nodal involvement (pN1-3 vs pN0; all hazard ratios [HR] >3; all P ≤ .001) and a tumor size of pT4 vs pT1 (HR 4.528; P = .007) were associated with poor BCSS outcomes.
Study details: This study used data from a registry to analyze 20,672 patients with HER2+ stage I-III BC who underwent surgery.
Disclosures: This study was supported by the Korean Breast Cancer Society. The authors declared no conflicts of interest.
Source: Kang YJ et al. Predictive biological factors for late survival in patients with HER2-positive breast cancer. Sci Rep. 2023;13:11008 (Jul 7). Doi: 10.1038/s41598-023-38200-y
Receiving anthracyclines raises risk for myeloid neoplasms in BC patients
Key clinical point:Although the cumulative incidence of myeloid neoplasms, such as myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML), was low in breast cancer (BC) survivors, the receipt of anthracycline-based adjuvant therapy was associated with a long-term risk for both AML and MDS.
Major finding: The 10-year cumulative incidences of both AML and MDS were very low (~0.2%) in the anthracycline group and <0.2% in the chemotherapy without anthracycline and no chemotherapy groups. However, the risk for AML (hazard ratio [HR] 9.531; 95% CI 4.156-21.861) and MDS (HR 2.559; 95% CI 1.600-4.095) was significantly higher in the anthracycline group vs no chemotherapy group.
Study details: Findings are from a retrospective cohort study including 153,565 patients with BC who underwent surgery and received adjuvant treatment with anthracyclines (n = 79,321), chemotherapy without anthracyclines (n = 15,475), or no chemotherapy (n = 46,657).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lee JW et al. Therapy related myeloid neoplasm in early breast cancer patients treated with adjuvant chemotherapy. Eur J Cancer. 2023;191:112952 (Jun 22). Doi: 10.1016/j.ejca.2023.112952
Key clinical point:Although the cumulative incidence of myeloid neoplasms, such as myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML), was low in breast cancer (BC) survivors, the receipt of anthracycline-based adjuvant therapy was associated with a long-term risk for both AML and MDS.
Major finding: The 10-year cumulative incidences of both AML and MDS were very low (~0.2%) in the anthracycline group and <0.2% in the chemotherapy without anthracycline and no chemotherapy groups. However, the risk for AML (hazard ratio [HR] 9.531; 95% CI 4.156-21.861) and MDS (HR 2.559; 95% CI 1.600-4.095) was significantly higher in the anthracycline group vs no chemotherapy group.
Study details: Findings are from a retrospective cohort study including 153,565 patients with BC who underwent surgery and received adjuvant treatment with anthracyclines (n = 79,321), chemotherapy without anthracyclines (n = 15,475), or no chemotherapy (n = 46,657).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lee JW et al. Therapy related myeloid neoplasm in early breast cancer patients treated with adjuvant chemotherapy. Eur J Cancer. 2023;191:112952 (Jun 22). Doi: 10.1016/j.ejca.2023.112952
Key clinical point:Although the cumulative incidence of myeloid neoplasms, such as myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML), was low in breast cancer (BC) survivors, the receipt of anthracycline-based adjuvant therapy was associated with a long-term risk for both AML and MDS.
Major finding: The 10-year cumulative incidences of both AML and MDS were very low (~0.2%) in the anthracycline group and <0.2% in the chemotherapy without anthracycline and no chemotherapy groups. However, the risk for AML (hazard ratio [HR] 9.531; 95% CI 4.156-21.861) and MDS (HR 2.559; 95% CI 1.600-4.095) was significantly higher in the anthracycline group vs no chemotherapy group.
Study details: Findings are from a retrospective cohort study including 153,565 patients with BC who underwent surgery and received adjuvant treatment with anthracyclines (n = 79,321), chemotherapy without anthracyclines (n = 15,475), or no chemotherapy (n = 46,657).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lee JW et al. Therapy related myeloid neoplasm in early breast cancer patients treated with adjuvant chemotherapy. Eur J Cancer. 2023;191:112952 (Jun 22). Doi: 10.1016/j.ejca.2023.112952
Increased contralateral BC risk after adjuvant radiotherapy in germline-BRCA2 pathogenic variants
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Key clinical point: Patients with germline (g) BRCA1/2-associated primary breast cancer (BC), particularly those with gBRCA2 pathogenic mutations, faced a moderately increased risk of developing contralateral breast cancer (CBC) after receiving adjuvant radiotherapy.
Major finding: The risk for invasive and in situ CBC increased by 44% in patients with gBRCA1/2 mutations who did vs did not receive radiotherapy (adjusted hazard ratio [HR] 1.44; 95% CI 1.12-1.86), with the risk being even more prominent in gBRCA2 pathogenic variant carriers (adjusted HR 1.77; 95% CI 1.13-2.77).
Study details: Findings are from an analysis including 3602 patients with gBRCA1/2-associated primary BC from the prospective international BRCA1/2 Carrier Cohort Study, of whom 64% of patients received adjuvant radiotherapy.
Disclosures: This study did not receive any specific external funding. DG Evans reported ties with AstraZeneca and AmGen, K Kast declared ties with Roche Pharma AG, and J Simard declared holding patents related to BRCA1 and BRCA2.
Source: van Barele M et al. Contralateral breast cancer risk in irradiated breast cancer patients with a germline-BRCA1/2 pathogenic variant. J Natl Cancer Inst. 2023 (Jun 27). Doi: 10.1093/jnci/djad116
Benefit of regional nodal irradiation remains questionable in HR+/ERBB2− node-positive BC
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Key clinical point: Patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (ERBB2−, aka HER2-) breast cancer (BC) experienced very low locoregional recurrence (LRR) events and comparable invasive disease-free survival (iDFS) outcomes both with and without receiving regional nodal irradiation (RNI) after surgery.
Major finding: The cumulative incidence of LRR at 5 years was low among patients who underwent breast-conserving surgery and radiotherapy with RNI (0.85%), breast-conserving surgery and radiotherapy without RNI (0.55%), mastectomy followed by radiotherapy (0.11%), or mastectomy without radiotherapy (1.7%). Receiving RNI was not associated with better iDFS outcomes (P > .1 for both pre- and postmenopausal women).
Study details: Findings are from the secondary analysis of the SWOG S1007 trial including 4871 women with HR+/ERBB2− node-positive BC, of whom 2274 women received RNI.
Disclosures: This study was supported by the US National Institutes of Health (NIH) and other sources. Some authors declared serving as consultants or receiving grants and personal fees from various sources, including NIH.
Source: Jagsi R et al. Radiotherapy use and incidence of locoregional recurrence in patients with favorable-risk, node-positive breast cancer enrolled in the SWOG S1007 trial. JAMA Oncol. 2023 (Jul 6). Doi: 10.1001/jamaoncol.2023.1984
Number of cervical cancer screenings linked to higher preterm birth risk
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
FROM JAMA HEALTH FORUM
Subcutaneous ketamine for TRD practical, safe, and highly effective
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
“In this severely treatment-resistant population, of which 24% had failed to respond to treatment with electroconvulsive therapy, adequately dosed racemic ketamine produced benefits that were large, being both clinically and statistically superior to midazolam,” report the researchers, led by Colleen Loo, MD, MBBS, with Black Dog Institute, University of New South Wales, Sydney.
The study was published online in the British Journal of Psychiatry.
Individualized dosing
“Previously, most studies of racemic ketamine [administered] it by intravenous infusion over half an hour to several hours, which is a much more medically complex and expensive procedure,” Dr. Loo said in an interview.
The fact that subcutaneously administered ketamine was “highly effective” given by this practical and feasible route is a “major contribution to the field,” said Dr. Loo.
The Ketamine for Adult Depression trial assessed the acute efficacy and safety of a 4-week course of twice-weekly subcutaneous injections of racemic ketamine or midazolam (active control) in 174 adults with TRD.
Initially, the trial tested a fixed dose of 0.5 mg/kg ketamine vs. 0.025 mg/kg midazolam (cohort 1; 68 patients). Dosing was subsequently revised, after the data safety monitoring board recommended flexible-dose ketamine (0.5-0.9 mg/kg) or midazolam (0.025-0.045 mg/kg) with response-guided dosing increments (cohort 2; 106 patients).
The primary outcome was remission defined as Montgomery-Åsberg Rating Scale for Depression (MADRS) score ≤ 10 at week 4.
On this outcome, in the fixed-dose cohort, there was no statistically significant difference in remission rates between ketamine and midazolam (6.3% and 8.8%; odds ratio, 1.34; 95% CI, 0.22-8.21; P = .76).
However, there was a significant difference in remission in the flexible-dose cohort, with remission rates 19.6% for ketamine vs. just 2% for midazolam (OR, 12.11; 95% CI, 2.12-69.17; P = .005).
“The study showed that individualized dose adjustment, based on clinical response, was very important in optimizing the benefit of ketamine,” said Dr. Loo.
“It meant that one, you are more likely to respond as you receive a higher dose if needed, and two, you don’t receive a higher dose than needed, given that side effects are also dose-related,” she said.
Results also favored flexible-dose ketamine over midazolam for the secondary outcomes of response (≥ 50% reduction in MADRS: 29% vs. 4%; P = .001) and remission defined by a less rigid definition (MADRS ≤ 12: 22% vs. 4%; P = .007).
The results also confirm that the antidepressant effects of ketamine are not sustained when treatment stops.
“The study included careful follow-up for 4 weeks after the end of treatment. This is an important contribution to the literature, as it shows that ongoing treatment beyond the 4 weeks will be necessary for most people to maintain the benefits of ketamine treatment if you respond to the treatment. This study provided clear evidence of this, for racemic ketamine,” said Dr. Loo.
Overall, ketamine was well-tolerated, with the well-established acute effects of ketamine observed in both cohorts. The acute effects resolved or returned to pretreatment levels within the 2-hour observation period. No one required medical intervention, and there was no evidence of cognitive impairment.
Rigorous research, compelling data
Reached for comment, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said the data are “compelling with respect to efficacy and safety of subcutaneous ketamine in adults with major depression.”
Dr. McIntyre said the data are “highly relevant” for several reasons. “First, it is the most rigorous study conducted to date with subcutaneous administration of ketamine for adults living with treatment-resistant depression.”
Second, it “demonstrates the efficacy and safety of this route of delivery, which until now has not been studied with this level of rigor and which is a more scalable and accessible approach to administer ketamine to suitable candidates,” Dr. McIntyre said.
The study was funded by a competitive research grant from the Australian National Health and Medical Research Council. Dr. Loo has disclosed relationships with Douglas Pharmaceuticals and Janssen Cilag and is the medical director of neurostimulation and interventional psychiatry at Ramsay Health Care. Dr. McIntyre has received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF PSYCHIATRY
New cancer survival calculator focuses on oral cancer
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD AND NECK SURGERY
Biofeedback Training Is a Good Alternative to Preventive Medication for Migraine and Tension-Type Headache
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
What types of headache and other illnesses respond to biofeedback training?
Migraine affects about 12% of the US and most Western populations, and is 3 times more common in women than men. Migraine can be treated with a variety of strategies that include medications and nonpharmacologic therapies such as biofeedback, as well as other behavioral therapies and devices. Biofeedback refers to the use of instrumentation to monitor and display physiologic responses that the patient may not be aware of so that they can be “modified” in a more adaptive direction. Feedback gives immediate objective information and is usually combined with a relaxation-based therapy. The most common biofeedback treatments for migraine include feeding back muscle activity in the face and neck to help people relax contracted muscles and teach them a “low-arousal” response; they learn to increase finger temperature, which coincides with modifying the “stress” nervous system. Biofeedback has been shown to be helpful for migraine and tension-type headache. It is also helpful to decrease anxiety and stress levels and lower blood pressure.
Can patients use biofeedback treatments in conjunction with migraine medication?
Research has shown that migraine, in particular higher-frequency migraine, is best treated by a combination of medications and behavioral strategies. Biofeedback is a good option for use in conjunction with medication for migraine.
Who is trained to provide biofeedback treatments?
Biofeedback for migraine is something usually performed by psychologists and other professionals with specialized training. Many practitioners are certified by the Biofeedback Certification Institute of America (BCIA). It is important to find an experienced biofeedback practitioner who also has some expertise in treating headache disorders.
How are biofeedback treatments performed?
Biofeedback is a therapy that follows a learning model whereby the patient gradually learns a self-regulation skill that impacts their headaches.
Patients come to an office, typically once per week, where they are attached to instruments that measure physical responses associated with migraine. The patient typically sits in a comfortable chair or recliner, and numerous physiologic responses are monitored with surface electrodes.
When treating headache, sensors are typically placed on the head and neck to monitor muscle responses and a thermistor is placed on a finger to measure temperature. Feedback is given via visual cues (computer graphics) or a change in auditory tone as the patient is taught various relaxation techniques.
Patients use the feedback to learn a physiologic relaxation response that may be beneficial for their headache management. Most of the research on biofeedback is related to treatment to prevent migraine; however, these techniques can be helpful to use during an acute attack, ideally paired with an acute care migraine medication.
Can children with migraine have biofeedback treatments as well?
Most children typically have a lower frequency of migraine than adults, although some have frequent migraines with associated disability and are candidates for preventive medication (although no medications are yet approved for children by the US Food and Drug Association). Most children are good candidates for behavioral therapies such as biofeedback. There are many computer games utilized in biofeedback training that children easily learn to modify physical responses. There are some fairly recent data suggesting that behavioral treatments, some of which include biofeedback, are effective strategies for children and may be more effective than preventive medication.
What other types of illnesses could benefit from biofeedback training?
There are data showing that biofeedback therapy can be helpful for anxiety disorders, insomnia, and functional bowel disorders and may help in modifying high blood pressure. It can also be a very effective stress-management strategy.
Do you refer some of your patients with migraine to a psychologist for biofeedback treatments?
Yes, but it is always good for the referring physician to check on the credentials of the person performing the biofeedback treatment. Some headache specialists might do it themselves, but we do not. It takes a while—at least several sessions—and psychologists are better at it. Patients’ perspectives are also important. We communicate with the referred biofeedback treatment specialist to get more insight on the patient. For example, some patients are anxious and depressed, and they are not sleeping at night. The doctor we referred the patient to may recommend an antidepressant for the patient to help address those issues. It is a team effort.
What is the average cost per treatment?
The costs vary throughout the country and range from $75 to $400 per session. Insurance coverage varies.
How many times should a patient go in for treatment?
Most protocols are about 10 to 12 sessions, depending on patient response.
Have there been clinical trials on biofeedback treatments/devices?
There have been many clinical trials that have been positive, so there is good evidence that biofeedback can be an effective treatment for migraine and tension-type headache. There are many types of biofeedback devices that measure different modalities. The most common one used in migraine is measuring scalp muscle contraction with surface electromyography or measuring peripheral blood flow with a temperature gauge. The goals are to relax muscles and learn to increase finger temperature, which is related to decreased arousal of the sympathetic nervous system or stress system. Other modalities include learning to modulate brain waves (electroencephalography or neurofeedback) and certain cardiovascular measures that reduce the stress response by a different mechanism. The goal is for patients to learn a low arousal response that they can utilize in their natural environment. Certain breathing techniques and visualization exercises are also helpful, but biofeedback refers to using physiologic recording equipment to help learn to change physical responses related to headache disorder.
Over our years of experience, we have found that biofeedback can help a large percentage of our patients with migraine and tension-type headache, and it is associated with almost no adverse events.
Total cesarean delivery rates in the US, 2022
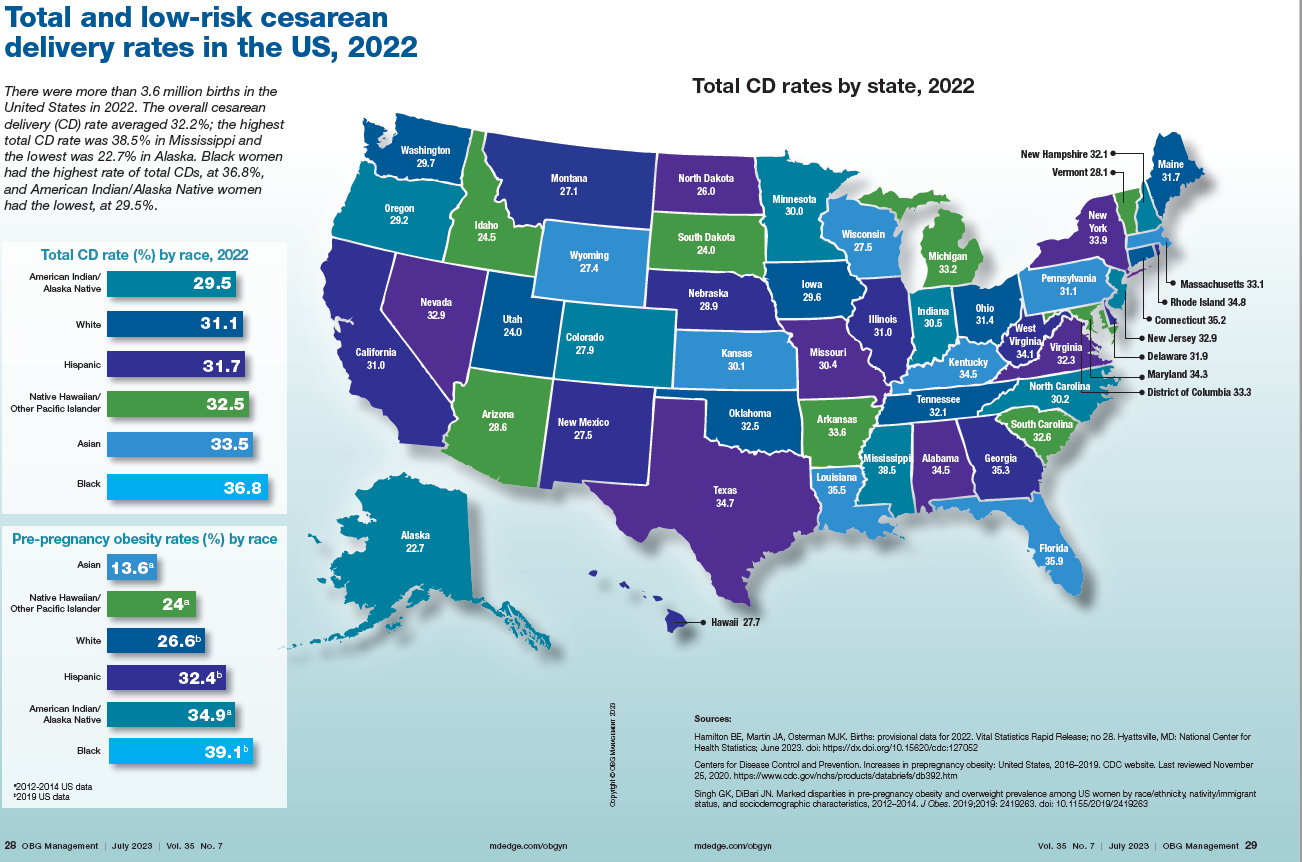


News & Perspectives from Ob.Gyn. News
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.







