User login
Supporting Primary Care Patient-Centered Medical Homes with Community Care Teams: Findings from a Pilot Study
From the Lehigh Valley Health Network, Allentown, PA.
Abstract
- Objective: With the growing recognition that team-based care might help meet the country’s primary care needs, this study’s objective was to evaluate the preliminary effectiveness of multidisciplinary community care teams (CCTs) deployed to primary care practices transforming into patient-centered medical homes (PCMHs).
- Methods: A nonrandomized longitudinal study design was used contrasting the CCT practices/patients with non-CCT comparison groups. Outcomes included utilization (ED/hospital use), quality indicators (QIs), practice joy, and patient satisfaction. Two CCTs (consisting of nurse care manager, behavioral health specialist, social worker, and pharmacist) were deployed to 6 primary care practices and provided services to 406 patients. Practice level analyses compared patients from the 6 CCT practices not receiving team services (29,881 patients) to 3 non-CCT practices (22,350 patients) that were also transforming toward PCMH. The comparison group for the patient level analyses (patients who received CCT services) was patients from the same CCT practice who did not receive CCT services.
- Results: At the practice level, there were significant improvements in QIs for practices both with and without CCTs. However, reductions in the probability of an admission and readmission occurred only for high-risk patients in CCT practices. At the patient level, the probability of an unplanned admission was reduced for CCT and non-CCT patients, but the probability of a readmission was only reduced in CCT patients receiving hospital discharge reconciliation calls from CCT staff.
- Conclusion: Preliminary results suggest possible added benefit of CCTs over PCMHs alone for reducing hospitalization.
As health care organizations move from a fee-for-service model to a value-based model in an accountable care environment, the transformation of primary care to patient-centered medical homes (PCMHs) is one of the fundamental strategies for achieving higher quality care at lower cost [1–3]. The core tenets of the PCMH are a commitment to high quality and safe care that is accessible, comprehensive, and coordinated across the health continuum, as well as patient-centered [4,5]. Newer to the PCMH model has been shifting the paradigm of care from individual encounters to using elements of population health management to proactively manage a panel of patients [1,3,6]. Given the array of patients seen in a primary care setting and the complexity of care required by many patients in a panel, particularly those with chronic conditions, a team approach to care capitalizes on multidisciplinary skills to collectively take responsibility for the ongoing care of the patients to improve health outcomes [7].
Multidisciplinary team-based care is considered a crucial strategy for meeting our society’s health care needs [8], especially in light of the expected shortage of primary care physicians coupled with the anticipated growth of the patient population due to the Affordable Care Act. Several different types of team-based care have been pursued. In 2003, for example, the State of Vermont pioneered health care reform through Vermont’s Blueprint for Health using principles of the PCMH that included team-based care [9]. The goal of the program was to deliver comprehensive and coordinated care to improve health outcomes for state residents. Vermont community health teams worked with primary care providers to manage short-term care for high-needs patients with an emphasis on better self-management, care coordination, chronic care management, and social and behavioral support services. An analysis of the first pilot program found significant decreases in hospital admissions and emergency department (ED) visits, and a per-person per-month reduction in costs [9].
The Lehigh Valley Health Network adapted the Vermont health improvement model to meet the unique needs of its practices that were transforming into PCMHs. When asked by leadership what would be most helpful in this transformational process, the practices cited additional staff resources to manage their high-risk patients. In response, the network enhanced support to the practices by implementing multidisciplinary teams called community care teams (CCTs), which were deployed to the practices to help manage their high-risk patients. The purpose of this study is to evaluate the effectiveness of CCTs within the PCMH model by examining key utilization, quality, and process measures. The CCTs were expected to increase the overall practice effectiveness and efficiency (practice level outcomes) by offsetting some of the workload regarding the management of high-risk patients and improving the outcomes of patients directly managed by the CCT (patient level outcomes).
Methods
Setting
Serving 5 counties, the Lehigh Valley Health Network is a large health care delivery system in southeastern PA that currently operates in a fee-for-service environment but is moving towards becoming an accountable care organization. The concept of using CCTs to support practices’ PCMH development originated with network leadership. Leadership approached 7 primary care practices with the most extensive PCMH involvement to pilot the CCT initiative (1 practice declined participation). More specifically, the practices were selected based upon their prior 3-year experience with practice transformation as a result of participating in the South Central PA Chronic Care Initiative [10] and having achieved National Committee for Quality Assurance level 3 PCMH recognition. Practice selection was also based on the results of a network-wide comprehensive practice assessment which included TransforMed’s MHIQ survey [11] of PCMH capabilities and in-house surveys to capture practice characteristics and readiness for change.
Program Design
The CCTs were designed to support 3 to 4 primary care practices in the short-term management of high-risk patients with chronic disease. Much like the Vermont community health team model, each team consisted of a RN care manager who functioned as the team lead, a behavioral health specialist, and a social worker. A clinical pharmacist was added to the CCT program shortly after implementing the project and was shared between 2 teams. The team engaged in population health management for patients identified as high-risk for poor outcomes by supporting the further development of disease self-management and goal setting skills, addressing behavioral health, social, and economic problems, and connecting the patient to other Network and community resources as needed. Furthermore, given the growing evidence demonstrating the positive impact of coordinated and continuity of care post hospital discharge on patient outcomes [12,13], the CCT also played a vital role in supporting the PCMH transition care program for high-risk patients, which involved contacting patients via the telephone within 48 business hours of discharge from the hospital to reconcile medications, assess and identify issues for follow-up, answer patient questions and coordinate appropriate appointments.
As a pilot program, 2 CCTs were deployed to 6 primary care practices (3 family medicine, 2 internal medicine, and 1 pediatric) in July 2012. Prior to engaging the practice and patients, each team member participated in an extensive orientation, which presented essential evidence-based knowledge on the CCT and PCMH models and provided application training and support in information systems, network resources, and care management.
Initially the CCT care managers forwarded the high-risk registry to each provider to review and identify the top patients for proactive care management outreach. CCT care managers then called the identified patients and/or coordinated outreach with patients through office appointments scheduled in the near future. At the same time as the registry outreach began, on-site clinician referrals to the CCT and hospital discharge reconciliation calls to patients by the CCT care manager commenced. Clinician referrals did not have to meet any formal criteria in order for that patient to receive CCT services. Ideally, the program was designed to service high-risk patients on the registry; however, the majority of patient contacts for care management emerged through day-to-day clinician referrals (not necessarily high-risk) and discharge reconciliation calls to high-risk patients for months 2 through 8 of the pilot phase. Patients' care was managed with continued, ongoing services until either patient goals were met or the patient was transferred to another practice or nursing home, expired, or declined further care management assistance. The CCT behavioral health specialist addressed short-term issues or bridged the gap or need until long-term services could be coordinated, sometimes requiring 6 to 8 meetings with a patient. CCT social worker services were assigned on a case-by-case basis and occasionally provided longer-term or intermittent need management, such as medication assistance.
Study Design and Sample
A naturalistic longitudinal study design was used to evaluate the CCT’s preliminary effectiveness. The CCTs were evaluated at 2 levels. First, based on the assumption that the CCT would off-set some of the practice workload and allow the practices to proactively manage more of their patient population, the effectiveness of the CCTs was evaluated at the practice level, ie, patients who did not receive CCT services but belonged to practices with CCTs. Second, the effectiveness of the CCTs was evaluated at the patient level, ie, patients receiving CCT services. At both levels, the CCT groups were contrasted with “non-CCT” comparison groups of convenience.
Measures
Primary outcome measures were utilization: ED visits, all-cause unplanned admissions, and 30-day readmissions. Secondary outcome measures included 2 types of quality indicators(QIs, see Appendix for scoring): (a) gaps in care measures that captured whether providers were following standards of care for diabetes, ischemic vascular disease, and prevention; and (b) patient composites which reflected patient illness severity for diabetes and cardiac disease. Higher scores indicated more gaps in care or greater disease severity. Both types of QI measures required at least a 12-month window and thus could not be computed for patients engaged with the CCT who had only a 6-month follow-up period (as their scores could reflect their pre-CCT status). In addition, comprehensive care was denoted by the provision of depression screening on the PHQ-2 [14], and whether HgA1c was greater or equal to 9.0 in diabetics served as an additional patient outcome measure. Other secondary outcome measures included practice joy via the Well-Being in the Workplace Questionnaire (WWQ) [15] and patient satisfaction from CAHPS-CG 12-month survey with PCMH items [16].
Data Collection
Utilization and quality data were extracted from the network’s hospital and outpatient electronic medical records (EMR). Practice staff were emailed every 6 months and asked to anonymously complete the WWQ via Survey Monkey [17]. At baseline, patient satisfaction surveys were distributed in the practice, and patients had the option of anonymously completing them during their visit or returning them in a prepaid envelop. While not recommended for CAHPS, this procedure had been internally used with success previously. At 12 months, the same survey was mailed to a random sample of patients with a prepaid return envelope.
At the practice level, utilization and QI data were only available for patients from 4 of the 6 practices: data were not available for 1 non-EMR practice and there was negligible variation in utilization from the pediatric practice. For the patient level analyses, utilization was available for patients in all 6 practices.
Statistical Analyses
To test whether outcomes were improved relative to a comparison group following the introduction of CCTs into the practices (practice level analyses) or CCT engagement (patient level analyses), mixed models analyses of variance with repeated measures on Time (pre- vs. post-CCT) were conducted with SAS [18] PROC MIXED and PROC GENMOD for continuous and dichotomous outcomes, respectively. To determine whether there was greater improvement in the CCT groups, all models included the interaction between Time and Group (CCT versus no CCT) in addition to their main effects. At the practice level, high-risk and non-high-risk patients were analyzed separately. And, at the patient level, CCT-MNGT and CCT-DCREC patients were analyzed separately with results adjusted for patient’s age. Some variables were not normally distributed. The quality variables were able to be normalized with natural log transformations, but utilization variables had to be dichotomized into “any” versus “none” due to severe skewness, inflated 0s and larger-valued counts. Practice joy and patient satisfaction can only be reported at the practice level (responses were aggregated within each practice because anonymous responses do not permit linking specific respondents over time and different patients were sampled across measurement occasions) and non-parametric tests (Wilcoxon signed ranked tests and tests of dependent proportions) were used to test for change over time given the small sample size (n = 6).
Results
Patient Characteristics
Practice samples had slightly more women than men (55.2%), were largely white (95.0%) and 50 years of age on average (mean = 49.53, SD = 20.18). The prevalence of diabetes (10.2%) and cardiac disease (10.4%) was relatively low. The percentage of women (56.3%) and whites (96.4%) for the patient level analytic samples was similar to the practice samples, whereas the prevalence of diabetes (33.6% ) and cardiac disease (44.6%) was notable higher and patients were more than 10 years older on average (mean = 61.62, SD = 23.96).
Practice Level Outcomes
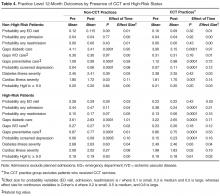
Given these group baseline differences and only one instance where the Time by Group interaction was significant where group baseline differences were absent (see below), simple pre-post analyses were conducted for each group separately (Table 4). The results for non-high-risk patients indicated that, in both CCT and non-CCT practices, there was no improvement in utilization, but there were significant reductions in gaps in diabetic and preventative care and cardiac illness severity and significant increases in depression screening, although effect sizes for the latter 2 outcomes were small to negligible.
Practice joy fell in the medium range [19] at all time-points, with no notable change over time (see Appendix in the online version of this article). There was also no change in patient satisfaction (see Appendix, online), although after 12 months the “always” response for following up on lab tests was no longer higher than the national comparison and helpfulness of staff rated “never/sometimes” was below the national comparison.
Patient Level Outcomes
Discussion
The empirical literature indicates that PCMH practice transformation is a long, effortful process, the effects of which are not quick to manifest [20–22]. In this context, the results of the preliminary evaluation of the CCT pilot were encouraging: team-based care in the form of CCTs can be effectively used to support population health management. Overall, the results at the practice level suggest that PCMH transformation alone may be effective in creating improvements in patient care and cardiac disease (there were improvements in 3 out of 4 care gap measures and 1 disease measure for both CCT and non-CCT practices), but the presence of CCT appears necessary to reduce unplanned admissions and readmissions, at least among high-risk patients. Of course, this reduced utilization at the practice level could also be due to selection bias (practices with the longest PCMH involvement were selected for the CCT pilot) and it awaits to be seen if this finding holds as CCTs are deployed to more practices. Still, similar evidence for the CCT was found at the patient level. The probability of an unplanned admission was reduced for all groups of patients from CCT practices; however, this effect was notably large only for patients who received hospital discharge reconciliation calls from the CCT. Moreover, the only group that had a significant reduction in the probability of a 30-day readmission was also patients who received hospital discharge reconciliation calls from the CCT. Both results suggest an added benefit of CCT engagement. Although there was no change in the probability of an ED visit for any group, the CCT staff indicated that there was a substantial minority of CCT-engaged patients who were not accessing the ED when they should have been and it might be that increased appropriate use by this minority due to CCT coaching therefore cancelled out expected reductions in ED use among other CCT patients.
The intent of the current endeavor was to perform a formative evaluation [23,24] of the CCTs effectiveness. We recognized there would be analytic challenges and limits to such early-stage analyses. Nonetheless, we believed it was vital, especially given the cost of the CCTs and the growing financial pressure on health care networks more globally, to determine the preliminary effectiveness of the CCTs’, care management interventions and, if possible, suggest improvements to the intervention. As intended in formative evaluations, the evaluation is ongoing. Future analyses will bring more rigor to the evaluation and solve the data and analytic obstacles that affected the results of this first round of analyses.
A major learning of the early-stage analyses was the difficulty in developing comparison groups that are equivalent to the intervention groups at baseline. A number of matching schemes were attempted at the patient level in addition to the one presented here, but they were equally problematic. Avenues for creating more valid comparison groups in the future include the use of propensity score matching as well as drawing comparison groups from data from other networks. In addition, as time passes and more than 1 follow-up point is available for analysis, multilevel modeling can be employed which can specify different intercepts (baseline values) for groups. Still, it’s worth noting that constructing appropriate comparison groups is challenging even with those approaches: most health networks do not collect data on the most relevant matching variables (eg, health literacy, social economic status, social isolation/support) due to their cost and burden to both practices and patients.
Another major gap revealed by the early-stage analyses was the need to improve the strategy used for selecting patients for CCT intervention. In addition to many physician referrals, there was a large number of patients on the high-risk registry who required intervention relative to the small CCT staff. Various strategies to prioritize the list were attempted, including cost-related analyses. As plagued the formation of comparisons groups, it seemed the variables most critical to risk stratification were unavailable in administrative datasets. Appreciating that data collection is costly and patients and busy practices already have survey fatigue, the evaluation team examined the empirical literature for a single useful tool for ranking patients as well as constructing better matched comparison groups. This search indicated that a measure of patient activation [25–27] would be particularly helpful not only for selecting the riskiest and costliest patients for CCT intervention but also for tailoring CCT services to different types of patients. Since the implementation of the CCTs the network has also contracted with a predictive analytics company to provide risk scores for the patients.
The current formative evaluation was an important learning journey, laying important ground work for better evaluating the CCTs’ effectiveness specifically and eventually becoming an accountable care provider more generally. If the network is to provide health care more effectively and efficiently, it will need to bring greater rigor to evaluations of its various interventions and other ACO endeavors. The current formative evaluation was a valuable demonstration to non-scientists of the weakness of single group pre-post designs and how more rigorous evaluations, which include comparison groups and address confounding variables, can enhance the validity of the analytic results. This learning journey also highlighted the limitations of administrative databases and the necessity of both primary data collection and mixed methods. For example, it seems that some practices may require educational interventions to take full advantage of the CCT and qualitative assessments on practice readiness seem a necessary addition to the quantitative practice assessment to identify the specific characteristics that need strengthening. In addition, the evaluation team also recently added a qualitative sub-study on high-risk patients’ experience with the CCT to overcome locally low CAHPS response rates and capture themes broader than patient satisfaction. Upcoming rounds of analyses will also tackle other aspects of formative evaluations including the study of the CCT implementation as more practices receive CCTs and determining if process and fidelity measures of the PCMH pillars are linked to better outcomes. Furthermore, future analytic plans include identifying the active ingredients and optimal doses of the CCT intervention as well as determine the most effective matches between different types of patients and different CCT interventions (eg, behavioral, care management, social, pharmacy). While we appreciate that barriers still remain and require solutions, we hope the current evaluation highlights the utility of performing such formative evaluations.
Corresponding author: Carol Foltz, PhD, Lehigh Valley Health Network, Allentown, PA, [email protected].
Funding/support: The evaluation was funded internally by Lehigh Valley Health Network and a close affiliate, the Lehigh Valley Physician Hospital Organization. The funding organizations had no role in any part of the study.
Financial disclosures: None.
1. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care—two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
2. American Academy of Family Physicians. Primary care for the 21st century: Ensuring a quality, physician-led team for every patient. September 2012. Accessed at www.aafp.org/dam/AAFP/documents/about_us/initiatives/AAFP-PCMHWhitePaper.pdf.
3. Agency for Healthcare Research and Quality. The patient-centered medical home: Strategies to put patients at the center of primary care, February 2011. Accessed at http://pcmh.ahrq.gov/sites/default/files/attachments/Strategies%20to%20Put%20Patients%20at%20the%20Center%20of%20Primary%20Care.pdf.
4. Agency for Healthcare Research and Quality. Defining the PCMH. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
5. Patient-Centered Primary Care Collaborative. Defining the medical home: A patient-centered philosophy that drives primary care excellence. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
6. Margolius D, Bodenheimer T. Transforming primary care: From past practice to the practice of the future. Health Aff 2010;29:779–84.
7. Robert Graham Center. The patient centered medical home: History, seven core features, evidence and transformational change, 2007. Accessed at www.graham-center.org/online/etc/medialib/graham/documents/publications/mongraphs-books/2007/rgcmo-medical-home.Par.0001.File.tmp/rgcmo-medical-home.pdf.
8. Goldberg A. It matters how we define health care equity. Institute of Medicine. Accessed at www.iom.edu/~/media/Files/Perspectives-Files/2013/Commentaries/BPH-It-Matters-How-we-define-Health-Equity.pdf.
9. Bielaszka-DuVernay C. Vermont’s blueprint for medical homes, community health teams, and better health at lower cost. Health Aff 2011;30:383–6.
10. Bricker PL, Baron RJ, Scheirer JJ. Collaboration in Pennsylvania: rapidly spreading improved chronic care for patients to practices. J Cont Educ Health Prof 2010;30:114–25.
11.TransforMed. What does your medical home look like? A jumble of unconnected pieces or a coherent structure? Accessed at www.transformed.com/mhiq/welcome.cfm .
12. Health policy brief: care transitions. Health Aff 2012. Accessed at http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_76.pdf.
13. Greenwald J, Denham C, Jack B. The hospital discharge: A review of a high-risk care transition with highlights of a reengineered discharge process. J Patient Saf 2007;3:97–106.
14. Kroenke K, Spitzer R, Williams W. The patient health questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–94.
15. Parker GB, Hyett MP. Measurement of well-being in the workplace: The development of the Work Well-Being Questionnaire. Nerv Ment Dis 2011;199:394-97.
16. Agency for Healthcare Research and Quality. Clinician & group expanded 12-month survey with CAHPS patient-centered medical home (PCMH) items. Accessed at https://cahps.ahrq.gov/surveys-guidance/cg/pcmh/index.html.
17. SurveyMonkey. Accessed at http://www.surveymonkey.com (last visited 06-23-2014). SurveyMonkey: Palo Alto, CA.
18. SAS [computer program]. Version 9.2. Cary, NC: SAS Institute; 2002-2008.
19. Black Dog Institute. Accessed at www.blackdoginstitute.org.au/docs/workplacewellbeingquestionnairepaperversion.pdf.
20. Jaén CR, Ferrer, RL, Miller WL, Palmer RF. Patient outcomes at 26 months in the patient-centered medical home national demonstration project. Ann Fam Med 2010;8(Suppl 1):s57–s67.
21. Solberg LI, Asche SE, Fontaine P, Flottemesch TJ. Trends in quality during medical home transformation. Ann Fam Med 2011;9:515–21.
22. Crabtree BF, Nutting PA, Miller WL, Stange KC. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med 2010;8(Suppl 1):s80–s90.
23. Stetler CB, Legro MW, Wallace CM et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21:S1–8.
24. Geonnotti K, Peikes D, Wang W, Smith J. Formative evaluation: fostering real-time adaptations and refinements to improve the effectiveness of patient-centered medical home models. Rockville, MD: Agency for Healthcare Research and Quality. February 2013. AHRQ Publication No. 13-0025-EF.
25. Insignia Health. Accessed at www.insigniahealth.com/solutions/patient-activation-measure.
26. Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients’ ‘scores’. Health Aff 2013;32:216–22.
27. Hibbard JH, Greene J. What The evidence shows about patient activation: better health outcomes and care experiences fewer data on costs. Health Aff 2013;32:207–14.
From the Lehigh Valley Health Network, Allentown, PA.
Abstract
- Objective: With the growing recognition that team-based care might help meet the country’s primary care needs, this study’s objective was to evaluate the preliminary effectiveness of multidisciplinary community care teams (CCTs) deployed to primary care practices transforming into patient-centered medical homes (PCMHs).
- Methods: A nonrandomized longitudinal study design was used contrasting the CCT practices/patients with non-CCT comparison groups. Outcomes included utilization (ED/hospital use), quality indicators (QIs), practice joy, and patient satisfaction. Two CCTs (consisting of nurse care manager, behavioral health specialist, social worker, and pharmacist) were deployed to 6 primary care practices and provided services to 406 patients. Practice level analyses compared patients from the 6 CCT practices not receiving team services (29,881 patients) to 3 non-CCT practices (22,350 patients) that were also transforming toward PCMH. The comparison group for the patient level analyses (patients who received CCT services) was patients from the same CCT practice who did not receive CCT services.
- Results: At the practice level, there were significant improvements in QIs for practices both with and without CCTs. However, reductions in the probability of an admission and readmission occurred only for high-risk patients in CCT practices. At the patient level, the probability of an unplanned admission was reduced for CCT and non-CCT patients, but the probability of a readmission was only reduced in CCT patients receiving hospital discharge reconciliation calls from CCT staff.
- Conclusion: Preliminary results suggest possible added benefit of CCTs over PCMHs alone for reducing hospitalization.
As health care organizations move from a fee-for-service model to a value-based model in an accountable care environment, the transformation of primary care to patient-centered medical homes (PCMHs) is one of the fundamental strategies for achieving higher quality care at lower cost [1–3]. The core tenets of the PCMH are a commitment to high quality and safe care that is accessible, comprehensive, and coordinated across the health continuum, as well as patient-centered [4,5]. Newer to the PCMH model has been shifting the paradigm of care from individual encounters to using elements of population health management to proactively manage a panel of patients [1,3,6]. Given the array of patients seen in a primary care setting and the complexity of care required by many patients in a panel, particularly those with chronic conditions, a team approach to care capitalizes on multidisciplinary skills to collectively take responsibility for the ongoing care of the patients to improve health outcomes [7].
Multidisciplinary team-based care is considered a crucial strategy for meeting our society’s health care needs [8], especially in light of the expected shortage of primary care physicians coupled with the anticipated growth of the patient population due to the Affordable Care Act. Several different types of team-based care have been pursued. In 2003, for example, the State of Vermont pioneered health care reform through Vermont’s Blueprint for Health using principles of the PCMH that included team-based care [9]. The goal of the program was to deliver comprehensive and coordinated care to improve health outcomes for state residents. Vermont community health teams worked with primary care providers to manage short-term care for high-needs patients with an emphasis on better self-management, care coordination, chronic care management, and social and behavioral support services. An analysis of the first pilot program found significant decreases in hospital admissions and emergency department (ED) visits, and a per-person per-month reduction in costs [9].
The Lehigh Valley Health Network adapted the Vermont health improvement model to meet the unique needs of its practices that were transforming into PCMHs. When asked by leadership what would be most helpful in this transformational process, the practices cited additional staff resources to manage their high-risk patients. In response, the network enhanced support to the practices by implementing multidisciplinary teams called community care teams (CCTs), which were deployed to the practices to help manage their high-risk patients. The purpose of this study is to evaluate the effectiveness of CCTs within the PCMH model by examining key utilization, quality, and process measures. The CCTs were expected to increase the overall practice effectiveness and efficiency (practice level outcomes) by offsetting some of the workload regarding the management of high-risk patients and improving the outcomes of patients directly managed by the CCT (patient level outcomes).
Methods
Setting
Serving 5 counties, the Lehigh Valley Health Network is a large health care delivery system in southeastern PA that currently operates in a fee-for-service environment but is moving towards becoming an accountable care organization. The concept of using CCTs to support practices’ PCMH development originated with network leadership. Leadership approached 7 primary care practices with the most extensive PCMH involvement to pilot the CCT initiative (1 practice declined participation). More specifically, the practices were selected based upon their prior 3-year experience with practice transformation as a result of participating in the South Central PA Chronic Care Initiative [10] and having achieved National Committee for Quality Assurance level 3 PCMH recognition. Practice selection was also based on the results of a network-wide comprehensive practice assessment which included TransforMed’s MHIQ survey [11] of PCMH capabilities and in-house surveys to capture practice characteristics and readiness for change.
Program Design
The CCTs were designed to support 3 to 4 primary care practices in the short-term management of high-risk patients with chronic disease. Much like the Vermont community health team model, each team consisted of a RN care manager who functioned as the team lead, a behavioral health specialist, and a social worker. A clinical pharmacist was added to the CCT program shortly after implementing the project and was shared between 2 teams. The team engaged in population health management for patients identified as high-risk for poor outcomes by supporting the further development of disease self-management and goal setting skills, addressing behavioral health, social, and economic problems, and connecting the patient to other Network and community resources as needed. Furthermore, given the growing evidence demonstrating the positive impact of coordinated and continuity of care post hospital discharge on patient outcomes [12,13], the CCT also played a vital role in supporting the PCMH transition care program for high-risk patients, which involved contacting patients via the telephone within 48 business hours of discharge from the hospital to reconcile medications, assess and identify issues for follow-up, answer patient questions and coordinate appropriate appointments.
As a pilot program, 2 CCTs were deployed to 6 primary care practices (3 family medicine, 2 internal medicine, and 1 pediatric) in July 2012. Prior to engaging the practice and patients, each team member participated in an extensive orientation, which presented essential evidence-based knowledge on the CCT and PCMH models and provided application training and support in information systems, network resources, and care management.
Initially the CCT care managers forwarded the high-risk registry to each provider to review and identify the top patients for proactive care management outreach. CCT care managers then called the identified patients and/or coordinated outreach with patients through office appointments scheduled in the near future. At the same time as the registry outreach began, on-site clinician referrals to the CCT and hospital discharge reconciliation calls to patients by the CCT care manager commenced. Clinician referrals did not have to meet any formal criteria in order for that patient to receive CCT services. Ideally, the program was designed to service high-risk patients on the registry; however, the majority of patient contacts for care management emerged through day-to-day clinician referrals (not necessarily high-risk) and discharge reconciliation calls to high-risk patients for months 2 through 8 of the pilot phase. Patients' care was managed with continued, ongoing services until either patient goals were met or the patient was transferred to another practice or nursing home, expired, or declined further care management assistance. The CCT behavioral health specialist addressed short-term issues or bridged the gap or need until long-term services could be coordinated, sometimes requiring 6 to 8 meetings with a patient. CCT social worker services were assigned on a case-by-case basis and occasionally provided longer-term or intermittent need management, such as medication assistance.
Study Design and Sample
A naturalistic longitudinal study design was used to evaluate the CCT’s preliminary effectiveness. The CCTs were evaluated at 2 levels. First, based on the assumption that the CCT would off-set some of the practice workload and allow the practices to proactively manage more of their patient population, the effectiveness of the CCTs was evaluated at the practice level, ie, patients who did not receive CCT services but belonged to practices with CCTs. Second, the effectiveness of the CCTs was evaluated at the patient level, ie, patients receiving CCT services. At both levels, the CCT groups were contrasted with “non-CCT” comparison groups of convenience.
Measures
Primary outcome measures were utilization: ED visits, all-cause unplanned admissions, and 30-day readmissions. Secondary outcome measures included 2 types of quality indicators(QIs, see Appendix for scoring): (a) gaps in care measures that captured whether providers were following standards of care for diabetes, ischemic vascular disease, and prevention; and (b) patient composites which reflected patient illness severity for diabetes and cardiac disease. Higher scores indicated more gaps in care or greater disease severity. Both types of QI measures required at least a 12-month window and thus could not be computed for patients engaged with the CCT who had only a 6-month follow-up period (as their scores could reflect their pre-CCT status). In addition, comprehensive care was denoted by the provision of depression screening on the PHQ-2 [14], and whether HgA1c was greater or equal to 9.0 in diabetics served as an additional patient outcome measure. Other secondary outcome measures included practice joy via the Well-Being in the Workplace Questionnaire (WWQ) [15] and patient satisfaction from CAHPS-CG 12-month survey with PCMH items [16].
Data Collection
Utilization and quality data were extracted from the network’s hospital and outpatient electronic medical records (EMR). Practice staff were emailed every 6 months and asked to anonymously complete the WWQ via Survey Monkey [17]. At baseline, patient satisfaction surveys were distributed in the practice, and patients had the option of anonymously completing them during their visit or returning them in a prepaid envelop. While not recommended for CAHPS, this procedure had been internally used with success previously. At 12 months, the same survey was mailed to a random sample of patients with a prepaid return envelope.
At the practice level, utilization and QI data were only available for patients from 4 of the 6 practices: data were not available for 1 non-EMR practice and there was negligible variation in utilization from the pediatric practice. For the patient level analyses, utilization was available for patients in all 6 practices.
Statistical Analyses
To test whether outcomes were improved relative to a comparison group following the introduction of CCTs into the practices (practice level analyses) or CCT engagement (patient level analyses), mixed models analyses of variance with repeated measures on Time (pre- vs. post-CCT) were conducted with SAS [18] PROC MIXED and PROC GENMOD for continuous and dichotomous outcomes, respectively. To determine whether there was greater improvement in the CCT groups, all models included the interaction between Time and Group (CCT versus no CCT) in addition to their main effects. At the practice level, high-risk and non-high-risk patients were analyzed separately. And, at the patient level, CCT-MNGT and CCT-DCREC patients were analyzed separately with results adjusted for patient’s age. Some variables were not normally distributed. The quality variables were able to be normalized with natural log transformations, but utilization variables had to be dichotomized into “any” versus “none” due to severe skewness, inflated 0s and larger-valued counts. Practice joy and patient satisfaction can only be reported at the practice level (responses were aggregated within each practice because anonymous responses do not permit linking specific respondents over time and different patients were sampled across measurement occasions) and non-parametric tests (Wilcoxon signed ranked tests and tests of dependent proportions) were used to test for change over time given the small sample size (n = 6).
Results
Patient Characteristics
Practice samples had slightly more women than men (55.2%), were largely white (95.0%) and 50 years of age on average (mean = 49.53, SD = 20.18). The prevalence of diabetes (10.2%) and cardiac disease (10.4%) was relatively low. The percentage of women (56.3%) and whites (96.4%) for the patient level analytic samples was similar to the practice samples, whereas the prevalence of diabetes (33.6% ) and cardiac disease (44.6%) was notable higher and patients were more than 10 years older on average (mean = 61.62, SD = 23.96).
Practice Level Outcomes
Given these group baseline differences and only one instance where the Time by Group interaction was significant where group baseline differences were absent (see below), simple pre-post analyses were conducted for each group separately (Table 4). The results for non-high-risk patients indicated that, in both CCT and non-CCT practices, there was no improvement in utilization, but there were significant reductions in gaps in diabetic and preventative care and cardiac illness severity and significant increases in depression screening, although effect sizes for the latter 2 outcomes were small to negligible.
Practice joy fell in the medium range [19] at all time-points, with no notable change over time (see Appendix in the online version of this article). There was also no change in patient satisfaction (see Appendix, online), although after 12 months the “always” response for following up on lab tests was no longer higher than the national comparison and helpfulness of staff rated “never/sometimes” was below the national comparison.
Patient Level Outcomes
Discussion
The empirical literature indicates that PCMH practice transformation is a long, effortful process, the effects of which are not quick to manifest [20–22]. In this context, the results of the preliminary evaluation of the CCT pilot were encouraging: team-based care in the form of CCTs can be effectively used to support population health management. Overall, the results at the practice level suggest that PCMH transformation alone may be effective in creating improvements in patient care and cardiac disease (there were improvements in 3 out of 4 care gap measures and 1 disease measure for both CCT and non-CCT practices), but the presence of CCT appears necessary to reduce unplanned admissions and readmissions, at least among high-risk patients. Of course, this reduced utilization at the practice level could also be due to selection bias (practices with the longest PCMH involvement were selected for the CCT pilot) and it awaits to be seen if this finding holds as CCTs are deployed to more practices. Still, similar evidence for the CCT was found at the patient level. The probability of an unplanned admission was reduced for all groups of patients from CCT practices; however, this effect was notably large only for patients who received hospital discharge reconciliation calls from the CCT. Moreover, the only group that had a significant reduction in the probability of a 30-day readmission was also patients who received hospital discharge reconciliation calls from the CCT. Both results suggest an added benefit of CCT engagement. Although there was no change in the probability of an ED visit for any group, the CCT staff indicated that there was a substantial minority of CCT-engaged patients who were not accessing the ED when they should have been and it might be that increased appropriate use by this minority due to CCT coaching therefore cancelled out expected reductions in ED use among other CCT patients.
The intent of the current endeavor was to perform a formative evaluation [23,24] of the CCTs effectiveness. We recognized there would be analytic challenges and limits to such early-stage analyses. Nonetheless, we believed it was vital, especially given the cost of the CCTs and the growing financial pressure on health care networks more globally, to determine the preliminary effectiveness of the CCTs’, care management interventions and, if possible, suggest improvements to the intervention. As intended in formative evaluations, the evaluation is ongoing. Future analyses will bring more rigor to the evaluation and solve the data and analytic obstacles that affected the results of this first round of analyses.
A major learning of the early-stage analyses was the difficulty in developing comparison groups that are equivalent to the intervention groups at baseline. A number of matching schemes were attempted at the patient level in addition to the one presented here, but they were equally problematic. Avenues for creating more valid comparison groups in the future include the use of propensity score matching as well as drawing comparison groups from data from other networks. In addition, as time passes and more than 1 follow-up point is available for analysis, multilevel modeling can be employed which can specify different intercepts (baseline values) for groups. Still, it’s worth noting that constructing appropriate comparison groups is challenging even with those approaches: most health networks do not collect data on the most relevant matching variables (eg, health literacy, social economic status, social isolation/support) due to their cost and burden to both practices and patients.
Another major gap revealed by the early-stage analyses was the need to improve the strategy used for selecting patients for CCT intervention. In addition to many physician referrals, there was a large number of patients on the high-risk registry who required intervention relative to the small CCT staff. Various strategies to prioritize the list were attempted, including cost-related analyses. As plagued the formation of comparisons groups, it seemed the variables most critical to risk stratification were unavailable in administrative datasets. Appreciating that data collection is costly and patients and busy practices already have survey fatigue, the evaluation team examined the empirical literature for a single useful tool for ranking patients as well as constructing better matched comparison groups. This search indicated that a measure of patient activation [25–27] would be particularly helpful not only for selecting the riskiest and costliest patients for CCT intervention but also for tailoring CCT services to different types of patients. Since the implementation of the CCTs the network has also contracted with a predictive analytics company to provide risk scores for the patients.
The current formative evaluation was an important learning journey, laying important ground work for better evaluating the CCTs’ effectiveness specifically and eventually becoming an accountable care provider more generally. If the network is to provide health care more effectively and efficiently, it will need to bring greater rigor to evaluations of its various interventions and other ACO endeavors. The current formative evaluation was a valuable demonstration to non-scientists of the weakness of single group pre-post designs and how more rigorous evaluations, which include comparison groups and address confounding variables, can enhance the validity of the analytic results. This learning journey also highlighted the limitations of administrative databases and the necessity of both primary data collection and mixed methods. For example, it seems that some practices may require educational interventions to take full advantage of the CCT and qualitative assessments on practice readiness seem a necessary addition to the quantitative practice assessment to identify the specific characteristics that need strengthening. In addition, the evaluation team also recently added a qualitative sub-study on high-risk patients’ experience with the CCT to overcome locally low CAHPS response rates and capture themes broader than patient satisfaction. Upcoming rounds of analyses will also tackle other aspects of formative evaluations including the study of the CCT implementation as more practices receive CCTs and determining if process and fidelity measures of the PCMH pillars are linked to better outcomes. Furthermore, future analytic plans include identifying the active ingredients and optimal doses of the CCT intervention as well as determine the most effective matches between different types of patients and different CCT interventions (eg, behavioral, care management, social, pharmacy). While we appreciate that barriers still remain and require solutions, we hope the current evaluation highlights the utility of performing such formative evaluations.
Corresponding author: Carol Foltz, PhD, Lehigh Valley Health Network, Allentown, PA, [email protected].
Funding/support: The evaluation was funded internally by Lehigh Valley Health Network and a close affiliate, the Lehigh Valley Physician Hospital Organization. The funding organizations had no role in any part of the study.
Financial disclosures: None.
From the Lehigh Valley Health Network, Allentown, PA.
Abstract
- Objective: With the growing recognition that team-based care might help meet the country’s primary care needs, this study’s objective was to evaluate the preliminary effectiveness of multidisciplinary community care teams (CCTs) deployed to primary care practices transforming into patient-centered medical homes (PCMHs).
- Methods: A nonrandomized longitudinal study design was used contrasting the CCT practices/patients with non-CCT comparison groups. Outcomes included utilization (ED/hospital use), quality indicators (QIs), practice joy, and patient satisfaction. Two CCTs (consisting of nurse care manager, behavioral health specialist, social worker, and pharmacist) were deployed to 6 primary care practices and provided services to 406 patients. Practice level analyses compared patients from the 6 CCT practices not receiving team services (29,881 patients) to 3 non-CCT practices (22,350 patients) that were also transforming toward PCMH. The comparison group for the patient level analyses (patients who received CCT services) was patients from the same CCT practice who did not receive CCT services.
- Results: At the practice level, there were significant improvements in QIs for practices both with and without CCTs. However, reductions in the probability of an admission and readmission occurred only for high-risk patients in CCT practices. At the patient level, the probability of an unplanned admission was reduced for CCT and non-CCT patients, but the probability of a readmission was only reduced in CCT patients receiving hospital discharge reconciliation calls from CCT staff.
- Conclusion: Preliminary results suggest possible added benefit of CCTs over PCMHs alone for reducing hospitalization.
As health care organizations move from a fee-for-service model to a value-based model in an accountable care environment, the transformation of primary care to patient-centered medical homes (PCMHs) is one of the fundamental strategies for achieving higher quality care at lower cost [1–3]. The core tenets of the PCMH are a commitment to high quality and safe care that is accessible, comprehensive, and coordinated across the health continuum, as well as patient-centered [4,5]. Newer to the PCMH model has been shifting the paradigm of care from individual encounters to using elements of population health management to proactively manage a panel of patients [1,3,6]. Given the array of patients seen in a primary care setting and the complexity of care required by many patients in a panel, particularly those with chronic conditions, a team approach to care capitalizes on multidisciplinary skills to collectively take responsibility for the ongoing care of the patients to improve health outcomes [7].
Multidisciplinary team-based care is considered a crucial strategy for meeting our society’s health care needs [8], especially in light of the expected shortage of primary care physicians coupled with the anticipated growth of the patient population due to the Affordable Care Act. Several different types of team-based care have been pursued. In 2003, for example, the State of Vermont pioneered health care reform through Vermont’s Blueprint for Health using principles of the PCMH that included team-based care [9]. The goal of the program was to deliver comprehensive and coordinated care to improve health outcomes for state residents. Vermont community health teams worked with primary care providers to manage short-term care for high-needs patients with an emphasis on better self-management, care coordination, chronic care management, and social and behavioral support services. An analysis of the first pilot program found significant decreases in hospital admissions and emergency department (ED) visits, and a per-person per-month reduction in costs [9].
The Lehigh Valley Health Network adapted the Vermont health improvement model to meet the unique needs of its practices that were transforming into PCMHs. When asked by leadership what would be most helpful in this transformational process, the practices cited additional staff resources to manage their high-risk patients. In response, the network enhanced support to the practices by implementing multidisciplinary teams called community care teams (CCTs), which were deployed to the practices to help manage their high-risk patients. The purpose of this study is to evaluate the effectiveness of CCTs within the PCMH model by examining key utilization, quality, and process measures. The CCTs were expected to increase the overall practice effectiveness and efficiency (practice level outcomes) by offsetting some of the workload regarding the management of high-risk patients and improving the outcomes of patients directly managed by the CCT (patient level outcomes).
Methods
Setting
Serving 5 counties, the Lehigh Valley Health Network is a large health care delivery system in southeastern PA that currently operates in a fee-for-service environment but is moving towards becoming an accountable care organization. The concept of using CCTs to support practices’ PCMH development originated with network leadership. Leadership approached 7 primary care practices with the most extensive PCMH involvement to pilot the CCT initiative (1 practice declined participation). More specifically, the practices were selected based upon their prior 3-year experience with practice transformation as a result of participating in the South Central PA Chronic Care Initiative [10] and having achieved National Committee for Quality Assurance level 3 PCMH recognition. Practice selection was also based on the results of a network-wide comprehensive practice assessment which included TransforMed’s MHIQ survey [11] of PCMH capabilities and in-house surveys to capture practice characteristics and readiness for change.
Program Design
The CCTs were designed to support 3 to 4 primary care practices in the short-term management of high-risk patients with chronic disease. Much like the Vermont community health team model, each team consisted of a RN care manager who functioned as the team lead, a behavioral health specialist, and a social worker. A clinical pharmacist was added to the CCT program shortly after implementing the project and was shared between 2 teams. The team engaged in population health management for patients identified as high-risk for poor outcomes by supporting the further development of disease self-management and goal setting skills, addressing behavioral health, social, and economic problems, and connecting the patient to other Network and community resources as needed. Furthermore, given the growing evidence demonstrating the positive impact of coordinated and continuity of care post hospital discharge on patient outcomes [12,13], the CCT also played a vital role in supporting the PCMH transition care program for high-risk patients, which involved contacting patients via the telephone within 48 business hours of discharge from the hospital to reconcile medications, assess and identify issues for follow-up, answer patient questions and coordinate appropriate appointments.
As a pilot program, 2 CCTs were deployed to 6 primary care practices (3 family medicine, 2 internal medicine, and 1 pediatric) in July 2012. Prior to engaging the practice and patients, each team member participated in an extensive orientation, which presented essential evidence-based knowledge on the CCT and PCMH models and provided application training and support in information systems, network resources, and care management.
Initially the CCT care managers forwarded the high-risk registry to each provider to review and identify the top patients for proactive care management outreach. CCT care managers then called the identified patients and/or coordinated outreach with patients through office appointments scheduled in the near future. At the same time as the registry outreach began, on-site clinician referrals to the CCT and hospital discharge reconciliation calls to patients by the CCT care manager commenced. Clinician referrals did not have to meet any formal criteria in order for that patient to receive CCT services. Ideally, the program was designed to service high-risk patients on the registry; however, the majority of patient contacts for care management emerged through day-to-day clinician referrals (not necessarily high-risk) and discharge reconciliation calls to high-risk patients for months 2 through 8 of the pilot phase. Patients' care was managed with continued, ongoing services until either patient goals were met or the patient was transferred to another practice or nursing home, expired, or declined further care management assistance. The CCT behavioral health specialist addressed short-term issues or bridged the gap or need until long-term services could be coordinated, sometimes requiring 6 to 8 meetings with a patient. CCT social worker services were assigned on a case-by-case basis and occasionally provided longer-term or intermittent need management, such as medication assistance.
Study Design and Sample
A naturalistic longitudinal study design was used to evaluate the CCT’s preliminary effectiveness. The CCTs were evaluated at 2 levels. First, based on the assumption that the CCT would off-set some of the practice workload and allow the practices to proactively manage more of their patient population, the effectiveness of the CCTs was evaluated at the practice level, ie, patients who did not receive CCT services but belonged to practices with CCTs. Second, the effectiveness of the CCTs was evaluated at the patient level, ie, patients receiving CCT services. At both levels, the CCT groups were contrasted with “non-CCT” comparison groups of convenience.
Measures
Primary outcome measures were utilization: ED visits, all-cause unplanned admissions, and 30-day readmissions. Secondary outcome measures included 2 types of quality indicators(QIs, see Appendix for scoring): (a) gaps in care measures that captured whether providers were following standards of care for diabetes, ischemic vascular disease, and prevention; and (b) patient composites which reflected patient illness severity for diabetes and cardiac disease. Higher scores indicated more gaps in care or greater disease severity. Both types of QI measures required at least a 12-month window and thus could not be computed for patients engaged with the CCT who had only a 6-month follow-up period (as their scores could reflect their pre-CCT status). In addition, comprehensive care was denoted by the provision of depression screening on the PHQ-2 [14], and whether HgA1c was greater or equal to 9.0 in diabetics served as an additional patient outcome measure. Other secondary outcome measures included practice joy via the Well-Being in the Workplace Questionnaire (WWQ) [15] and patient satisfaction from CAHPS-CG 12-month survey with PCMH items [16].
Data Collection
Utilization and quality data were extracted from the network’s hospital and outpatient electronic medical records (EMR). Practice staff were emailed every 6 months and asked to anonymously complete the WWQ via Survey Monkey [17]. At baseline, patient satisfaction surveys were distributed in the practice, and patients had the option of anonymously completing them during their visit or returning them in a prepaid envelop. While not recommended for CAHPS, this procedure had been internally used with success previously. At 12 months, the same survey was mailed to a random sample of patients with a prepaid return envelope.
At the practice level, utilization and QI data were only available for patients from 4 of the 6 practices: data were not available for 1 non-EMR practice and there was negligible variation in utilization from the pediatric practice. For the patient level analyses, utilization was available for patients in all 6 practices.
Statistical Analyses
To test whether outcomes were improved relative to a comparison group following the introduction of CCTs into the practices (practice level analyses) or CCT engagement (patient level analyses), mixed models analyses of variance with repeated measures on Time (pre- vs. post-CCT) were conducted with SAS [18] PROC MIXED and PROC GENMOD for continuous and dichotomous outcomes, respectively. To determine whether there was greater improvement in the CCT groups, all models included the interaction between Time and Group (CCT versus no CCT) in addition to their main effects. At the practice level, high-risk and non-high-risk patients were analyzed separately. And, at the patient level, CCT-MNGT and CCT-DCREC patients were analyzed separately with results adjusted for patient’s age. Some variables were not normally distributed. The quality variables were able to be normalized with natural log transformations, but utilization variables had to be dichotomized into “any” versus “none” due to severe skewness, inflated 0s and larger-valued counts. Practice joy and patient satisfaction can only be reported at the practice level (responses were aggregated within each practice because anonymous responses do not permit linking specific respondents over time and different patients were sampled across measurement occasions) and non-parametric tests (Wilcoxon signed ranked tests and tests of dependent proportions) were used to test for change over time given the small sample size (n = 6).
Results
Patient Characteristics
Practice samples had slightly more women than men (55.2%), were largely white (95.0%) and 50 years of age on average (mean = 49.53, SD = 20.18). The prevalence of diabetes (10.2%) and cardiac disease (10.4%) was relatively low. The percentage of women (56.3%) and whites (96.4%) for the patient level analytic samples was similar to the practice samples, whereas the prevalence of diabetes (33.6% ) and cardiac disease (44.6%) was notable higher and patients were more than 10 years older on average (mean = 61.62, SD = 23.96).
Practice Level Outcomes
Given these group baseline differences and only one instance where the Time by Group interaction was significant where group baseline differences were absent (see below), simple pre-post analyses were conducted for each group separately (Table 4). The results for non-high-risk patients indicated that, in both CCT and non-CCT practices, there was no improvement in utilization, but there were significant reductions in gaps in diabetic and preventative care and cardiac illness severity and significant increases in depression screening, although effect sizes for the latter 2 outcomes were small to negligible.
Practice joy fell in the medium range [19] at all time-points, with no notable change over time (see Appendix in the online version of this article). There was also no change in patient satisfaction (see Appendix, online), although after 12 months the “always” response for following up on lab tests was no longer higher than the national comparison and helpfulness of staff rated “never/sometimes” was below the national comparison.
Patient Level Outcomes
Discussion
The empirical literature indicates that PCMH practice transformation is a long, effortful process, the effects of which are not quick to manifest [20–22]. In this context, the results of the preliminary evaluation of the CCT pilot were encouraging: team-based care in the form of CCTs can be effectively used to support population health management. Overall, the results at the practice level suggest that PCMH transformation alone may be effective in creating improvements in patient care and cardiac disease (there were improvements in 3 out of 4 care gap measures and 1 disease measure for both CCT and non-CCT practices), but the presence of CCT appears necessary to reduce unplanned admissions and readmissions, at least among high-risk patients. Of course, this reduced utilization at the practice level could also be due to selection bias (practices with the longest PCMH involvement were selected for the CCT pilot) and it awaits to be seen if this finding holds as CCTs are deployed to more practices. Still, similar evidence for the CCT was found at the patient level. The probability of an unplanned admission was reduced for all groups of patients from CCT practices; however, this effect was notably large only for patients who received hospital discharge reconciliation calls from the CCT. Moreover, the only group that had a significant reduction in the probability of a 30-day readmission was also patients who received hospital discharge reconciliation calls from the CCT. Both results suggest an added benefit of CCT engagement. Although there was no change in the probability of an ED visit for any group, the CCT staff indicated that there was a substantial minority of CCT-engaged patients who were not accessing the ED when they should have been and it might be that increased appropriate use by this minority due to CCT coaching therefore cancelled out expected reductions in ED use among other CCT patients.
The intent of the current endeavor was to perform a formative evaluation [23,24] of the CCTs effectiveness. We recognized there would be analytic challenges and limits to such early-stage analyses. Nonetheless, we believed it was vital, especially given the cost of the CCTs and the growing financial pressure on health care networks more globally, to determine the preliminary effectiveness of the CCTs’, care management interventions and, if possible, suggest improvements to the intervention. As intended in formative evaluations, the evaluation is ongoing. Future analyses will bring more rigor to the evaluation and solve the data and analytic obstacles that affected the results of this first round of analyses.
A major learning of the early-stage analyses was the difficulty in developing comparison groups that are equivalent to the intervention groups at baseline. A number of matching schemes were attempted at the patient level in addition to the one presented here, but they were equally problematic. Avenues for creating more valid comparison groups in the future include the use of propensity score matching as well as drawing comparison groups from data from other networks. In addition, as time passes and more than 1 follow-up point is available for analysis, multilevel modeling can be employed which can specify different intercepts (baseline values) for groups. Still, it’s worth noting that constructing appropriate comparison groups is challenging even with those approaches: most health networks do not collect data on the most relevant matching variables (eg, health literacy, social economic status, social isolation/support) due to their cost and burden to both practices and patients.
Another major gap revealed by the early-stage analyses was the need to improve the strategy used for selecting patients for CCT intervention. In addition to many physician referrals, there was a large number of patients on the high-risk registry who required intervention relative to the small CCT staff. Various strategies to prioritize the list were attempted, including cost-related analyses. As plagued the formation of comparisons groups, it seemed the variables most critical to risk stratification were unavailable in administrative datasets. Appreciating that data collection is costly and patients and busy practices already have survey fatigue, the evaluation team examined the empirical literature for a single useful tool for ranking patients as well as constructing better matched comparison groups. This search indicated that a measure of patient activation [25–27] would be particularly helpful not only for selecting the riskiest and costliest patients for CCT intervention but also for tailoring CCT services to different types of patients. Since the implementation of the CCTs the network has also contracted with a predictive analytics company to provide risk scores for the patients.
The current formative evaluation was an important learning journey, laying important ground work for better evaluating the CCTs’ effectiveness specifically and eventually becoming an accountable care provider more generally. If the network is to provide health care more effectively and efficiently, it will need to bring greater rigor to evaluations of its various interventions and other ACO endeavors. The current formative evaluation was a valuable demonstration to non-scientists of the weakness of single group pre-post designs and how more rigorous evaluations, which include comparison groups and address confounding variables, can enhance the validity of the analytic results. This learning journey also highlighted the limitations of administrative databases and the necessity of both primary data collection and mixed methods. For example, it seems that some practices may require educational interventions to take full advantage of the CCT and qualitative assessments on practice readiness seem a necessary addition to the quantitative practice assessment to identify the specific characteristics that need strengthening. In addition, the evaluation team also recently added a qualitative sub-study on high-risk patients’ experience with the CCT to overcome locally low CAHPS response rates and capture themes broader than patient satisfaction. Upcoming rounds of analyses will also tackle other aspects of formative evaluations including the study of the CCT implementation as more practices receive CCTs and determining if process and fidelity measures of the PCMH pillars are linked to better outcomes. Furthermore, future analytic plans include identifying the active ingredients and optimal doses of the CCT intervention as well as determine the most effective matches between different types of patients and different CCT interventions (eg, behavioral, care management, social, pharmacy). While we appreciate that barriers still remain and require solutions, we hope the current evaluation highlights the utility of performing such formative evaluations.
Corresponding author: Carol Foltz, PhD, Lehigh Valley Health Network, Allentown, PA, [email protected].
Funding/support: The evaluation was funded internally by Lehigh Valley Health Network and a close affiliate, the Lehigh Valley Physician Hospital Organization. The funding organizations had no role in any part of the study.
Financial disclosures: None.
1. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care—two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
2. American Academy of Family Physicians. Primary care for the 21st century: Ensuring a quality, physician-led team for every patient. September 2012. Accessed at www.aafp.org/dam/AAFP/documents/about_us/initiatives/AAFP-PCMHWhitePaper.pdf.
3. Agency for Healthcare Research and Quality. The patient-centered medical home: Strategies to put patients at the center of primary care, February 2011. Accessed at http://pcmh.ahrq.gov/sites/default/files/attachments/Strategies%20to%20Put%20Patients%20at%20the%20Center%20of%20Primary%20Care.pdf.
4. Agency for Healthcare Research and Quality. Defining the PCMH. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
5. Patient-Centered Primary Care Collaborative. Defining the medical home: A patient-centered philosophy that drives primary care excellence. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
6. Margolius D, Bodenheimer T. Transforming primary care: From past practice to the practice of the future. Health Aff 2010;29:779–84.
7. Robert Graham Center. The patient centered medical home: History, seven core features, evidence and transformational change, 2007. Accessed at www.graham-center.org/online/etc/medialib/graham/documents/publications/mongraphs-books/2007/rgcmo-medical-home.Par.0001.File.tmp/rgcmo-medical-home.pdf.
8. Goldberg A. It matters how we define health care equity. Institute of Medicine. Accessed at www.iom.edu/~/media/Files/Perspectives-Files/2013/Commentaries/BPH-It-Matters-How-we-define-Health-Equity.pdf.
9. Bielaszka-DuVernay C. Vermont’s blueprint for medical homes, community health teams, and better health at lower cost. Health Aff 2011;30:383–6.
10. Bricker PL, Baron RJ, Scheirer JJ. Collaboration in Pennsylvania: rapidly spreading improved chronic care for patients to practices. J Cont Educ Health Prof 2010;30:114–25.
11.TransforMed. What does your medical home look like? A jumble of unconnected pieces or a coherent structure? Accessed at www.transformed.com/mhiq/welcome.cfm .
12. Health policy brief: care transitions. Health Aff 2012. Accessed at http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_76.pdf.
13. Greenwald J, Denham C, Jack B. The hospital discharge: A review of a high-risk care transition with highlights of a reengineered discharge process. J Patient Saf 2007;3:97–106.
14. Kroenke K, Spitzer R, Williams W. The patient health questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–94.
15. Parker GB, Hyett MP. Measurement of well-being in the workplace: The development of the Work Well-Being Questionnaire. Nerv Ment Dis 2011;199:394-97.
16. Agency for Healthcare Research and Quality. Clinician & group expanded 12-month survey with CAHPS patient-centered medical home (PCMH) items. Accessed at https://cahps.ahrq.gov/surveys-guidance/cg/pcmh/index.html.
17. SurveyMonkey. Accessed at http://www.surveymonkey.com (last visited 06-23-2014). SurveyMonkey: Palo Alto, CA.
18. SAS [computer program]. Version 9.2. Cary, NC: SAS Institute; 2002-2008.
19. Black Dog Institute. Accessed at www.blackdoginstitute.org.au/docs/workplacewellbeingquestionnairepaperversion.pdf.
20. Jaén CR, Ferrer, RL, Miller WL, Palmer RF. Patient outcomes at 26 months in the patient-centered medical home national demonstration project. Ann Fam Med 2010;8(Suppl 1):s57–s67.
21. Solberg LI, Asche SE, Fontaine P, Flottemesch TJ. Trends in quality during medical home transformation. Ann Fam Med 2011;9:515–21.
22. Crabtree BF, Nutting PA, Miller WL, Stange KC. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med 2010;8(Suppl 1):s80–s90.
23. Stetler CB, Legro MW, Wallace CM et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21:S1–8.
24. Geonnotti K, Peikes D, Wang W, Smith J. Formative evaluation: fostering real-time adaptations and refinements to improve the effectiveness of patient-centered medical home models. Rockville, MD: Agency for Healthcare Research and Quality. February 2013. AHRQ Publication No. 13-0025-EF.
25. Insignia Health. Accessed at www.insigniahealth.com/solutions/patient-activation-measure.
26. Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients’ ‘scores’. Health Aff 2013;32:216–22.
27. Hibbard JH, Greene J. What The evidence shows about patient activation: better health outcomes and care experiences fewer data on costs. Health Aff 2013;32:207–14.
1. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care—two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
2. American Academy of Family Physicians. Primary care for the 21st century: Ensuring a quality, physician-led team for every patient. September 2012. Accessed at www.aafp.org/dam/AAFP/documents/about_us/initiatives/AAFP-PCMHWhitePaper.pdf.
3. Agency for Healthcare Research and Quality. The patient-centered medical home: Strategies to put patients at the center of primary care, February 2011. Accessed at http://pcmh.ahrq.gov/sites/default/files/attachments/Strategies%20to%20Put%20Patients%20at%20the%20Center%20of%20Primary%20Care.pdf.
4. Agency for Healthcare Research and Quality. Defining the PCMH. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
5. Patient-Centered Primary Care Collaborative. Defining the medical home: A patient-centered philosophy that drives primary care excellence. Accessed at http://pcmh.ahrq.gov/page/defining-pcmh.
6. Margolius D, Bodenheimer T. Transforming primary care: From past practice to the practice of the future. Health Aff 2010;29:779–84.
7. Robert Graham Center. The patient centered medical home: History, seven core features, evidence and transformational change, 2007. Accessed at www.graham-center.org/online/etc/medialib/graham/documents/publications/mongraphs-books/2007/rgcmo-medical-home.Par.0001.File.tmp/rgcmo-medical-home.pdf.
8. Goldberg A. It matters how we define health care equity. Institute of Medicine. Accessed at www.iom.edu/~/media/Files/Perspectives-Files/2013/Commentaries/BPH-It-Matters-How-we-define-Health-Equity.pdf.
9. Bielaszka-DuVernay C. Vermont’s blueprint for medical homes, community health teams, and better health at lower cost. Health Aff 2011;30:383–6.
10. Bricker PL, Baron RJ, Scheirer JJ. Collaboration in Pennsylvania: rapidly spreading improved chronic care for patients to practices. J Cont Educ Health Prof 2010;30:114–25.
11.TransforMed. What does your medical home look like? A jumble of unconnected pieces or a coherent structure? Accessed at www.transformed.com/mhiq/welcome.cfm .
12. Health policy brief: care transitions. Health Aff 2012. Accessed at http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_76.pdf.
13. Greenwald J, Denham C, Jack B. The hospital discharge: A review of a high-risk care transition with highlights of a reengineered discharge process. J Patient Saf 2007;3:97–106.
14. Kroenke K, Spitzer R, Williams W. The patient health questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–94.
15. Parker GB, Hyett MP. Measurement of well-being in the workplace: The development of the Work Well-Being Questionnaire. Nerv Ment Dis 2011;199:394-97.
16. Agency for Healthcare Research and Quality. Clinician & group expanded 12-month survey with CAHPS patient-centered medical home (PCMH) items. Accessed at https://cahps.ahrq.gov/surveys-guidance/cg/pcmh/index.html.
17. SurveyMonkey. Accessed at http://www.surveymonkey.com (last visited 06-23-2014). SurveyMonkey: Palo Alto, CA.
18. SAS [computer program]. Version 9.2. Cary, NC: SAS Institute; 2002-2008.
19. Black Dog Institute. Accessed at www.blackdoginstitute.org.au/docs/workplacewellbeingquestionnairepaperversion.pdf.
20. Jaén CR, Ferrer, RL, Miller WL, Palmer RF. Patient outcomes at 26 months in the patient-centered medical home national demonstration project. Ann Fam Med 2010;8(Suppl 1):s57–s67.
21. Solberg LI, Asche SE, Fontaine P, Flottemesch TJ. Trends in quality during medical home transformation. Ann Fam Med 2011;9:515–21.
22. Crabtree BF, Nutting PA, Miller WL, Stange KC. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med 2010;8(Suppl 1):s80–s90.
23. Stetler CB, Legro MW, Wallace CM et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21:S1–8.
24. Geonnotti K, Peikes D, Wang W, Smith J. Formative evaluation: fostering real-time adaptations and refinements to improve the effectiveness of patient-centered medical home models. Rockville, MD: Agency for Healthcare Research and Quality. February 2013. AHRQ Publication No. 13-0025-EF.
25. Insignia Health. Accessed at www.insigniahealth.com/solutions/patient-activation-measure.
26. Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients’ ‘scores’. Health Aff 2013;32:216–22.
27. Hibbard JH, Greene J. What The evidence shows about patient activation: better health outcomes and care experiences fewer data on costs. Health Aff 2013;32:207–14.
Understanding the spectrum of multiport and single-site robotics for hysterectomy
We present this video with the objective of demonstrating a hysterectomy performed using the robotic single-site approach in juxtaposition with a robotic multiport hysterectomy. In the video, and briefly here, we review the benefits, disadvantages, and challenges of robotic single-site hysterectomy.
The advantages of single-site robotic hysterectomy include:
- possible improved aesthetics for the patient
- allowance for surgeon independence while minimizing the need for a bedside assistant
- automatic reassignment of the robotic arm controls
- circumvention of certain limitations seen in laparoscopic single-site procedures.
The disadvantages of single-site robotic hysterectomy include:
- instrumentation is nonwristed and less robust than that of multiport instrumentation
- decreased degrees of freedom
- longer suturing time
- restricted assistant port use
- decreased applicability to a wide range of procedures as the surgical approach is limited to less complex and smaller pathology.
Related articles:
The robot is broadly accessible less than 10 years after its introduction to gynecologic surgery. Janelle Yates (News for your Practice; December 2013)
The robot is gaining ground in gynecologic surgery. Should you be using it? Arnold P. Advincula MD; Cheryl B. Iglesia MD; Rosanne M. Kho MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; Jason D. Wright, MD (Roundtable; April 2013)
Identify your learning curve for robotic hysterectomy. Joshua L Woelk, MD, MS, and John B. Gebhart, MD, MS (Guest Editorial; April 2013)
In general, each step of the single-port procedure has been found to be equivalent in time to a multiport approach to robotic-assisted hysterectomy—except for the step of vaginal cuff closure. Since the initial experience, aside from overcoming the learning curve of a new surgical approach, various techniques have been modified in order to surmount this challenge, such as closing the vaginal cuff vertically, using a cutting needle versus a tapered needle, addition of a “plus one” wristed multiport robotic arm, or replacing the single-site robotic needle driver with a multiport 5-mm needle driver.
Nevertheless, widespread adoption of single-site robotic gynecologic surgery still requires further technological improvements, and further research and experience is needed to determine its role, benefits, and applications in gynecologic surgery.
--Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
WE WANT TO HEAR FROM YOU!Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
We present this video with the objective of demonstrating a hysterectomy performed using the robotic single-site approach in juxtaposition with a robotic multiport hysterectomy. In the video, and briefly here, we review the benefits, disadvantages, and challenges of robotic single-site hysterectomy.
The advantages of single-site robotic hysterectomy include:
- possible improved aesthetics for the patient
- allowance for surgeon independence while minimizing the need for a bedside assistant
- automatic reassignment of the robotic arm controls
- circumvention of certain limitations seen in laparoscopic single-site procedures.
The disadvantages of single-site robotic hysterectomy include:
- instrumentation is nonwristed and less robust than that of multiport instrumentation
- decreased degrees of freedom
- longer suturing time
- restricted assistant port use
- decreased applicability to a wide range of procedures as the surgical approach is limited to less complex and smaller pathology.
Related articles:
The robot is broadly accessible less than 10 years after its introduction to gynecologic surgery. Janelle Yates (News for your Practice; December 2013)
The robot is gaining ground in gynecologic surgery. Should you be using it? Arnold P. Advincula MD; Cheryl B. Iglesia MD; Rosanne M. Kho MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; Jason D. Wright, MD (Roundtable; April 2013)
Identify your learning curve for robotic hysterectomy. Joshua L Woelk, MD, MS, and John B. Gebhart, MD, MS (Guest Editorial; April 2013)
In general, each step of the single-port procedure has been found to be equivalent in time to a multiport approach to robotic-assisted hysterectomy—except for the step of vaginal cuff closure. Since the initial experience, aside from overcoming the learning curve of a new surgical approach, various techniques have been modified in order to surmount this challenge, such as closing the vaginal cuff vertically, using a cutting needle versus a tapered needle, addition of a “plus one” wristed multiport robotic arm, or replacing the single-site robotic needle driver with a multiport 5-mm needle driver.
Nevertheless, widespread adoption of single-site robotic gynecologic surgery still requires further technological improvements, and further research and experience is needed to determine its role, benefits, and applications in gynecologic surgery.
--Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
WE WANT TO HEAR FROM YOU!Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
We present this video with the objective of demonstrating a hysterectomy performed using the robotic single-site approach in juxtaposition with a robotic multiport hysterectomy. In the video, and briefly here, we review the benefits, disadvantages, and challenges of robotic single-site hysterectomy.
The advantages of single-site robotic hysterectomy include:
- possible improved aesthetics for the patient
- allowance for surgeon independence while minimizing the need for a bedside assistant
- automatic reassignment of the robotic arm controls
- circumvention of certain limitations seen in laparoscopic single-site procedures.
The disadvantages of single-site robotic hysterectomy include:
- instrumentation is nonwristed and less robust than that of multiport instrumentation
- decreased degrees of freedom
- longer suturing time
- restricted assistant port use
- decreased applicability to a wide range of procedures as the surgical approach is limited to less complex and smaller pathology.
Related articles:
The robot is broadly accessible less than 10 years after its introduction to gynecologic surgery. Janelle Yates (News for your Practice; December 2013)
The robot is gaining ground in gynecologic surgery. Should you be using it? Arnold P. Advincula MD; Cheryl B. Iglesia MD; Rosanne M. Kho MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; Jason D. Wright, MD (Roundtable; April 2013)
Identify your learning curve for robotic hysterectomy. Joshua L Woelk, MD, MS, and John B. Gebhart, MD, MS (Guest Editorial; April 2013)
In general, each step of the single-port procedure has been found to be equivalent in time to a multiport approach to robotic-assisted hysterectomy—except for the step of vaginal cuff closure. Since the initial experience, aside from overcoming the learning curve of a new surgical approach, various techniques have been modified in order to surmount this challenge, such as closing the vaginal cuff vertically, using a cutting needle versus a tapered needle, addition of a “plus one” wristed multiport robotic arm, or replacing the single-site robotic needle driver with a multiport 5-mm needle driver.
Nevertheless, widespread adoption of single-site robotic gynecologic surgery still requires further technological improvements, and further research and experience is needed to determine its role, benefits, and applications in gynecologic surgery.
--Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
WE WANT TO HEAR FROM YOU!Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Accountable Care Organizations: Theory and Practice
Since early 2012, a growing number of independent physician groups, physician-hospital organizations, hospitals and their employed physicians, and fully integrated health systems have entered into contracts with both CMS and commercial insurers to become accountable care organizations (ACOs). It is estimated that the health care of close to 20 million patients is now being provided under such arrangements [1].
ACOs are one manifestation of payment reform intended to slow the unsustainable cost of health care in the United States. While the details of the payment models vary widely, with many combinations of fee-for-service, bundled, and capitated arrangements, the underlying goal is consistent: ACOs are held accountable for both the cost and quality of care for a specific population. While some ACO models offer the promise of shared savings alone, others offer potential savings but also entail associated risk [2]. That pre-specified quality targets have to be met before savings can be accessed is presented as a safeguard against the perceived excesses of the managed care experience of the 1990s.
To succeed as an ACO, health care organizations face sobering structural, fiscal, and—perhaps most daunting—cultural challenges. Coordinating care between providers [3,4] and across episodes and sites of care—not a traditional strength of many provider entities—will become increasingly important. Creating equitable systems to distribute whatever savings are garnered may disrupt traditional relationships between primary care and specialist providers. Convincing organizations to make the necessary investment in an “evolved” primary care infra-structure—a prerequisite for accomplishing the goals of decreasing unnecessary and expensive health resource utilization—will be problematic in an era of shrinking overall reimbursement [5]. Finally, convincing clinicians that this model means that they are quite literally “in it together” will challenge long-standing and proud departmental and divisional identities and silos [6].
Recognizing that this grand experiment is still very much in its formative stages, we nonetheless thought that this was an opportune time to examine ACOs from several perspectives. Over the next few issues and beginning with this issue [7], we will sequentially hear from an expert in health care policy analysis, a clinician-leader working in a high-functioning patient-centered medical home practice, and a team in a large health care system charged with the overall success of population health management. We are confident that you will find these observations timely, interesting, and informative. We welcome your feedback.
1. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
2. Weissman JS, Bailit M, D’Andrea G, Rosenthal MB. The design and application of shared savings programs: lessons from early adopters. Health Affairs 2012;31:1959–68.
3. Greenberg JO, Barnett ML, Spinks MA, et al. The “medical neighborhood”: integrating primary and specialty care for ambulatory patients. JAMA Intern Med 2014;174:454–7.
4. Song Z, Sequist TD, Barnett ML. Patient referrals: a linchpin for increasing the value of care. JAMA. Published online July 03, 2014. Available at http://jama.jamanetwork.com/article.aspx?articleid=1886863.
5. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care – two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
6. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
7. Song Z. Accountable care organizations: early results and future challenges. J Clin Outcomes Manag 2014;8:364–71
Since early 2012, a growing number of independent physician groups, physician-hospital organizations, hospitals and their employed physicians, and fully integrated health systems have entered into contracts with both CMS and commercial insurers to become accountable care organizations (ACOs). It is estimated that the health care of close to 20 million patients is now being provided under such arrangements [1].
ACOs are one manifestation of payment reform intended to slow the unsustainable cost of health care in the United States. While the details of the payment models vary widely, with many combinations of fee-for-service, bundled, and capitated arrangements, the underlying goal is consistent: ACOs are held accountable for both the cost and quality of care for a specific population. While some ACO models offer the promise of shared savings alone, others offer potential savings but also entail associated risk [2]. That pre-specified quality targets have to be met before savings can be accessed is presented as a safeguard against the perceived excesses of the managed care experience of the 1990s.
To succeed as an ACO, health care organizations face sobering structural, fiscal, and—perhaps most daunting—cultural challenges. Coordinating care between providers [3,4] and across episodes and sites of care—not a traditional strength of many provider entities—will become increasingly important. Creating equitable systems to distribute whatever savings are garnered may disrupt traditional relationships between primary care and specialist providers. Convincing organizations to make the necessary investment in an “evolved” primary care infra-structure—a prerequisite for accomplishing the goals of decreasing unnecessary and expensive health resource utilization—will be problematic in an era of shrinking overall reimbursement [5]. Finally, convincing clinicians that this model means that they are quite literally “in it together” will challenge long-standing and proud departmental and divisional identities and silos [6].
Recognizing that this grand experiment is still very much in its formative stages, we nonetheless thought that this was an opportune time to examine ACOs from several perspectives. Over the next few issues and beginning with this issue [7], we will sequentially hear from an expert in health care policy analysis, a clinician-leader working in a high-functioning patient-centered medical home practice, and a team in a large health care system charged with the overall success of population health management. We are confident that you will find these observations timely, interesting, and informative. We welcome your feedback.
Since early 2012, a growing number of independent physician groups, physician-hospital organizations, hospitals and their employed physicians, and fully integrated health systems have entered into contracts with both CMS and commercial insurers to become accountable care organizations (ACOs). It is estimated that the health care of close to 20 million patients is now being provided under such arrangements [1].
ACOs are one manifestation of payment reform intended to slow the unsustainable cost of health care in the United States. While the details of the payment models vary widely, with many combinations of fee-for-service, bundled, and capitated arrangements, the underlying goal is consistent: ACOs are held accountable for both the cost and quality of care for a specific population. While some ACO models offer the promise of shared savings alone, others offer potential savings but also entail associated risk [2]. That pre-specified quality targets have to be met before savings can be accessed is presented as a safeguard against the perceived excesses of the managed care experience of the 1990s.
To succeed as an ACO, health care organizations face sobering structural, fiscal, and—perhaps most daunting—cultural challenges. Coordinating care between providers [3,4] and across episodes and sites of care—not a traditional strength of many provider entities—will become increasingly important. Creating equitable systems to distribute whatever savings are garnered may disrupt traditional relationships between primary care and specialist providers. Convincing organizations to make the necessary investment in an “evolved” primary care infra-structure—a prerequisite for accomplishing the goals of decreasing unnecessary and expensive health resource utilization—will be problematic in an era of shrinking overall reimbursement [5]. Finally, convincing clinicians that this model means that they are quite literally “in it together” will challenge long-standing and proud departmental and divisional identities and silos [6].
Recognizing that this grand experiment is still very much in its formative stages, we nonetheless thought that this was an opportune time to examine ACOs from several perspectives. Over the next few issues and beginning with this issue [7], we will sequentially hear from an expert in health care policy analysis, a clinician-leader working in a high-functioning patient-centered medical home practice, and a team in a large health care system charged with the overall success of population health management. We are confident that you will find these observations timely, interesting, and informative. We welcome your feedback.
1. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
2. Weissman JS, Bailit M, D’Andrea G, Rosenthal MB. The design and application of shared savings programs: lessons from early adopters. Health Affairs 2012;31:1959–68.
3. Greenberg JO, Barnett ML, Spinks MA, et al. The “medical neighborhood”: integrating primary and specialty care for ambulatory patients. JAMA Intern Med 2014;174:454–7.
4. Song Z, Sequist TD, Barnett ML. Patient referrals: a linchpin for increasing the value of care. JAMA. Published online July 03, 2014. Available at http://jama.jamanetwork.com/article.aspx?articleid=1886863.
5. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care – two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
6. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
7. Song Z. Accountable care organizations: early results and future challenges. J Clin Outcomes Manag 2014;8:364–71
1. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
2. Weissman JS, Bailit M, D’Andrea G, Rosenthal MB. The design and application of shared savings programs: lessons from early adopters. Health Affairs 2012;31:1959–68.
3. Greenberg JO, Barnett ML, Spinks MA, et al. The “medical neighborhood”: integrating primary and specialty care for ambulatory patients. JAMA Intern Med 2014;174:454–7.
4. Song Z, Sequist TD, Barnett ML. Patient referrals: a linchpin for increasing the value of care. JAMA. Published online July 03, 2014. Available at http://jama.jamanetwork.com/article.aspx?articleid=1886863.
5. Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care – two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–3.
6. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
7. Song Z. Accountable care organizations: early results and future challenges. J Clin Outcomes Manag 2014;8:364–71
An insider’s look at the 2014 atopic dermatitis guidelines
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
EXPERT OPINION FROM THE SPD ANNUAL MEETING
Accountable Care Organizations: Early Results and Future Challenges
From the Harvard Medical School and the Department of Medicine, Massachusetts General Hospital, Boston, MA.
In recent years, the growth of health care spending has climbed to the top of the domestic policy agenda. Medicare spending growth is now recognized as the biggest driver of the federal debt [1,2].Medicaid spending growth puts similar pressure on states. In the private sector, employee health care costs increasingly weigh on company balance sheets, affecting business operations and employee wages. All the while, individuals and families face insurance premium growth that far outpaces real income growth.
Out of this recent history emerged a broad recognition that health care spending growth is unsustainable at current rates. If Medicare spending continues to exceed gross domestic product (GDP) by 2.5 percentage points per year—the traditional gap over the past 4 decades—a greater than 160% increase in individual income taxes would be needed to pay for it [3].Even if the gap was 1 percentage point, the increase in income taxes needed would still be over 70%, with consequent contraction in GDP of 3% to 16% by 2015 [4,5].Other consequences, such as significant cuts in Medicare benefits or shifting of costs onto patients, are equally undesirable [6,7].
Policy options for slowing health care spending are varied. Some focus on changing the provider’s incentives, while others focus on changing the patient’s incentives. Some are based on federal solutions [8],while others are based on market solutions [9].In the current policy landscape, payment reform for physicians and hospitals has emerged as a leading candidate for addressing health care spending. Public and private payers are increasingly changing the way that providers are paid, moving away from fee-for-service towards bundled or global payments for populations of patients. Physicians and hospitals are in turn forming integrated provider organizations to take on these new payment systems. The pace of this change has been growing.
Key Features of the ACO Concept
An accountable care organization (ACO) is a group of providers—that can include both physicians and hospitals—that accepts joint responsibility for health care spending and quality for a defined population of patients. The ACO concept can be considered an extension of the staff-model health maintenance organization (HMO) [13,14].It also shares features with the patient-centered medical home (PCMH) model in its focus on a robust primary care nexus that serves to coordinate a patient’s care [15,16].Three key characteristics are embedded in this definition.
The first is joint accountability. In an ACO contract, incentives for providers are agreed upon at the organizational level. Physicians and hospitals bear the financial risks and rewards of the ACO contract together. Shared savings, quality bonuses, and other incentives are determined by how the organization performs as a whole rather than any individual physician, practice, or hospital. In this way, physicians across specialties and care settings are incentivized to approach patient care collectively and coordinate care more effectively.
Third, an ACO is responsible for the care of a population of people. Each year, spending and quality are measured for the population attributed, or assigned, to the ACO. Attribution of patients to organizations can take place in two ways. It can be prospective, meaning that before the start of a contract year, the ACO knows exactly the patients whose spending and quality it is responsible for. This is typically more feasible in commercial ACO contracts, especially in the HMO population, where patients designate a primary care physician at the beginning of the year. Otherwise, attribution is typically retrospective, such as in the Medicare ACO programs, where beneficiaries are assigned to organizations at the end of a contract year based on the organization which accounted for the plurality of a patient’s medical spending or primary care spending.
Evidence to Date
While formal results from most ACOs today are not yet available, several notable ACO experiments have been evaluated. These include early results from the Medicare ACO programs, previous evaluations of the Medicare Physician Group Practice Demonstration (a predecessor of today’s ACO contracting model), and early results from commercial ACO contracts, such as the Blue Cross Blue Shield of Massachusetts global budget contract.
In interpreting lessons from these evaluations, several questions are worth keeping in mind. If a new payment system is correlated with changes in medical spending, is this explained by underlying changes in prices or in quantities? Since medical spending is the product of prices of services and quantities (volume) of services, an intervention that affects spending must affect either the prices or the volume of care. In the Medicare program, where prices are standardized, a global budget contract that works off of the underlying physician fee schedule would only affect spending through volume. In the private insurance sector, however, an ACO contract may affect spending through both volume and prices, since variations in prices across providers creates an opportunity for savings if care is obtained through a less expensive provider.
Separate from its relationship to medical spending, which is measured through the claims submitted by providers, what is the connection between a new payment system and total payouts from the insurer to the provider? An ACO contract contains a variety of incentives to providers that may generate additional payments from the insurer (most notably shared savings and quality bonuses). These non-claims payments may partially or entirely offset savings obtained through medical claims, making them an important dimension in the evaluation of the contract. Yet they are also different from medical claim dollars in a meaningful way. Changes in medical spending reflect underlying physician (or patient) behavior—what care is delivered and how much of it is delivered—whereas non-claims payments reflect the incentive structure of the contract.
On the quality dimension, it is worth noting whether a new payment system has similar effects on process and outcome measures. Process measures, which have been widely used by health plans, are operationally similar to additional items on a fee schedule, whereby the delivery of a service effectively generates a payment. Clinical outcome measures, such as blood pressure or cholesterol, and patient experience measures, on the other hand, cannot be fulfilled by simply checking off a box. Therefore, these measures may represent quality in a more meaningful way.
Early Results from Medicare ACOs
Thirty-two provider organizations entered the Pioneer ACO program in 2012, with about 669,000 Medicare beneficiaries attributed to these organizations. According to CMS, medical spending growth for ACO beneficiaries was 0.3% in the first year, compared to 0.8% for similar beneficiaries outside of these organizations [18].This generated a gross savings of $87.6 million in 2012, of which $33 million were returned to the Medicare trust fund. These savings were driven by 13 ACOs, with another 17 ACOs not reporting statistically significant changes in spending and 2 ACOs garnering losses with spending above the target of about $4 million total. Lower rates of admissions and readmissions largely explained the savings. A separate analysis comparing these ACOs to their local markets estimated a higher year 1 savings of $147 million dollars, driven by 8 ACOs whose savings ranged from $396 to $1224 per beneficiary per year [19].
Pioneer ACOs also earned over $76 million for quality. In the first year, quality bonuses were awarded for the reporting of quality measures rather than for performance, and all 32 ACOs successfully reported. According to CMS, Pioneer ACOs on average performed better than fee-for-service Medicare beneficiaries on 15 clinical quality measures for which comparison data were available, including blood pressure control and cholesterol control for diabetic patients. A complete analysis of quality performance is not yet available.
In the Medicare Shared Savings Program, interim results from CMS for the first 2 cohorts of ACOs showed that 29 of the 114 organizations lowered spending sufficiently enough to generate shared savings while 2 organizations had shared losses [20].This suggests that the great majority of ACOs spent close to their target. Final results on spending and quality are pending.
Results from the Medicare Physician Group Practice Demonstration
Ten provider organizations entered one-sided ACO-type contracts with Medicare in 2005 via the Physician Group Practice Demonstration. In this contract, organizations shared in savings provided that their spending was at least 2% below target and they achieved threshold performance on certain quality measures, most of which were process metrics.
In year 1, only one organization decreased spending enough to earn a shared savings, but after 3 years, five organizations had generated shared savings, although half of the savings were awarded to one organization [21].A recent analysis showed that 4 organizations sustained shared savings by the end of the program, with savings concentrated in acute care, readmissions, and beneficiaries who are dually eligible for Medicaid. Across the 10 organizations, financial performance ranged from average savings of $866 to increased spending of $749 per beneficiary per year [22].In total, about $78 million in savings were generated by this demonstration. Although a positive finding, this is a relatively small amount in the context of total Medicare expenditures [23,24].
On quality, all organizations met threshold performance on at least 29 of the 32 measures by the end of 5 years [21]. Most of these were process measures focused on coronary artery disease, diabetes, heart failure, hypertension, and preventive care [25].
Early Results from Commercial ACO Contracts
One of the early commercial ACO contracts to be evaluated was the Blue Cross Blue Shield of Massachusetts Alternative Quality Contract (AQC) [26].Initially implemented in 2009, the AQC is a multi-year contract that pays provider organizations a risk-adjusted global budget over the continuum of care. Seven organizations in Massachusetts entered the contract in the first year, and 4 more entered in 2010. Enrollees in HMO plans were prospectively attributed to their ACO by their designation of a primary care physician. The AQC is a two-sided contract that offered an additional 10% of an organization’s budget as a bonus for performance on 64 quality measures, half outpatient measures and half inpatient. Budget growth rates were tied to inflation and terms of its growth were negotiated with the organizations [27].
Over the first 2 years, the contract was associated with a decrease in medical spending of about $90 per enrollee per year, a –2.8% change (–1.9% in year 1 and –3.3% in year 2) [28].These savings were concentrated in procedures, imaging, and tests in the outpatient facility setting, and were largely explained by lower prices achieved by referring patients to less expensive providers. They were also concentrated in organizations that entered the AQC from fee-for-service, rather than prior risk contracts, and driven by enrollees with the highest expected spending. Over the second year, decreases in volume for certain services, such as percutaneous coronary interventions, began to contribute more to the savings [29].However, medical savings in the first 2 years were exceeded by non-claims payments, including shared savings and quality bonuses [27,28].The AQC was also associated with improvements in outpatient quality, including chronic care management measures (3.7 percentage points increase per year), adult preventive measures (0.4 percentage points per year), and pediatric quality measures (1.3 percentage points per year). Outcome measures such as hemoglobin A1c, LDL cholesterol, and blood pressure also showed an upward trend in the early years [28].Inpatient quality measures have yet to be examined.
Elsewhere in the country, Cigna’s Collaborative Accountable Care model was rolled out in 2009 with provider organizations in New Hampshire, Texas, and Arizona. A one-sided shared savings contract, it features a care coordination fee that is counted towards a practice’s medical spending, helping fund registered nurses who work as care coordinators. Interim results in 2012 suggested cost savings and quality improvements, but they were not statistically significant [30].A two-sided contract between Blue Shield of California and the California Public Employees’ Retirement System slowed medical costs by shortening admission and reducing readmissions [31].Most recently, an accountable care partnership between Anthem Blue Cross and HealthCare Partners physician group in California claimed $4.7 million in savings in the first half of 2013 for 55,000 patients in preferred provider organization (PPO) plans [32].These savings were driven by an 18% reduction in inpatient days, 4% reduction in overall admissions, and a 4% reduction in visits for radiology and lab tests, although specific price and volume contributions are yet unknown. Similar to the AQC, the Anthem contract is a 5-year agreement. Unlike the initial AQC contract, shared savings were tied to meeting a quality threshold and HealthCare Partners did not bear downside risk in year 1 [33].
Lessons Learned
Strengths of the ACO Model
Evidence to date points to both the potential of ACOs to slow spending and improve quality, but also the significant obstacles that they face. One of the encouraging lessons so far is that quality of care need not be threatened by a contract that rewards savings, provided that meaningful incentives for quality are in place. In both public and private two-sided contracts, process quality seemed to consistently improve. However, less is known about performance on outcome measures, which is ultimately a more meaningful metric for patients.
Broadly speaking, ACO contracts may be able to induce changes in physician behavior that could lead to medical savings. The low hanging fruit in Medicare seems to be admissions and readmissions, while that in commercial contracts may be lower prices obtained by changing referral patterns. While these medical savings reveal changes in clinical decision-making, it is still poorly understood whether clinical choices geared towards higher value can extend to areas of utilization where wasteful care may be concentrated. Little is also known about whether an ACO’s physicians and hospitals are in agreement over these changes in utilization or referral patterns, given their consequences for referral business and admissions. Moreover, there has yet to be evidence suggesting that medical savings can be larger than non-claims payments (rewards and bonuses to the ACOs) in a given year, generating net savings to the health care system. This may well take time to materialize given the initial investment costs and inducements for provider participation embedded in the early year incentives, but it is an important metric of success.
ACOs serve as a vehicle for payment reform and organizational reform among providers. They bring physicians across specialties and hospitals together under the same contractual roof, allowing the organization to determine how it allocates its resources under the spending target. Historically, policies aimed to slow health care spending have focused on either cutting prices (provider fees) or constraining volume (gatekeeping, prior authorization, and utilization review, etc.), but both types of strategies have been complicated by unintended consequences. Medicare fee cuts have traditionally been followed by compensatory increases in utilization or intensity of coding, offsetting the intended savings to a significant degree [34–37].Managed care techniques have met resistance from both physicians and patients [38,39].A spending target or budget takes an alternative approach; rather than controlling prices or quantities directly, it seeks to control total spending. Although the underlying fee schedule is retained for accounting, a spending target or especially a global budget pushes the organization to decide what care is high or low value.
A two-sided contract imposes stronger incentives on the ACO than a one-sided contract, which is both a strength and a weakness. While ACOs facing downside risk may respond earlier to the incentives, as some of the evidence thus far suggests, this risk may also propel ACOs to abandon this contracting model. In the Medicare Pioneer ACO program, for example, 9 of the 32 organizations exited the contract after year 1, which was allowed given the voluntary nature of participation. Seven organizations opted for the one-sided Medicare Shared Savings Program in year 2 and two left the ACO programs altogether. Downside risk was thought to be a principal concern for these organizations [40].Massachusetts providers in the AQC have thus far remained in the contract, but the AQC was a multi-year agreement to begin with.
Spillovers are another potential strength of the ACO model. Given that organizations care for patients across multiple payers, strong payment incentives in one payer population may affect care broadly. In the AQC, for example, recent evidence showed slowing of spending for Medicare beneficiaries associated with the contract in similar settings and categories of care as for the Blue Cross Blue Shield patients [41].
Weaknesses of the ACO Model
Still in its nascent stages, the ACO paradigm faces a number of challenges. Some relate to inherent weaknesses of the model, while others relate to the institutions and economics of the broader health care economy. At a contractual level, a key challenge is setting the target growth rate of the budget. If too low, providers may be overly constrained; if too high, providers may not have enough incentive to change practice. In an extreme case, if the target is set above what spending would have been under the old arrangement, an ACO contract can in fact be cost increasing on claims spending alone. Financial rewards such as shared savings and quality bonuses can help offset the risk, but they also make it more difficult for the ACO contract to generate net savings.
Achieving the right balance of risks and rewards is difficult. As noted above, a one-sided contract may not be strong enough to induce behavior change [42,43],but a two-sided contract may be too risky, driving providers who are unable to align incentives and coordinate care to exit the model [44].Although the percentage of shared risk borne by payer versus provider can be negotiated, putting financial risk on providers in a palatable way will be a key challenge. Financial risk can be more daunting if ACOs do not know in advance which patients they are responsible for, as in contracts with retrospective attribution rules and enrollees in unmanaged plans. A payer can help providers handle risk by sharing data on spending and identifying potential areas of overuse and low value care. Payers can help further by implementing risk corridors, providing reinsurance, or improving risk adjustment of the organization’s global budget. But with all that said, it remains to be seen whether providers around the country will be willing to bear substantive risk.
Within the ACO, a primary challenge is dividing up risks and rewards among constituent providers. How much shared savings are given to the hospital, to primary care physicians, or to specialists? What share should each specialty receive? What about shared losses, should spending exceed the target? In a two-sided ACO contract, these questions are particularly salient as global budgets change the business model for providers. Revenue centers under fee-for-service become cost centers. Organizations are confronted with difficult tradeoffs. The ability of providers across specialties to find common ground will be crucial, and leadership from providers will be key [45].Physicians have established themselves as leaders of the majority of ACOs today [46].It remains to be seen whether these organizations can keep providers together through the tradeoffs.
Patient trust in the ACO model has yet to be established. The managed care backlash of the 1990s suggests that patient buy-in will be crucial for the sustainability of ACOs. ACOs can have similarities to the HMO that traditionally produce negative associations, including downside risk, gatekeeping, or managed care techniques. To earn patients’ trust, ACOs will need to prove their value, such as through delivering better preventive care, less expensive care, more holistic care through stronger teams of providers, or smoother transitions of care across settings. The task of primary care medical homes to provide patient-centered care and coordinate across specialists effectively will be crucial. While today’s ACOs may be better positioned because of risk sharing, quality bonuses, risk adjustment, electronic medical records, or other innovations, the patient’s experience may ultimately be the arbiter.
Broader Challenges
While clinical integration is a central tenet of ACOs, consolidation between providers is simultaneously a chief concern for policymakers. Consolidation generally reduces competition and drives up prices, which is at odds with the goals of cost containment [47,48].Across the nation, physicians are consolidating with hospitals and health systems at an increasing rate, with recent surveys reporting that the proportion of independent physicians has steadily declined to below 50% [49–52].Increasing the number of covered lives is a dominant growth strategy under risk contracts, and more covered lives also increases an ACO’s bargaining power during acquisitions of specialist practices, whose referrals are better protected by inclusion in the provider network. As this trend continues, its effect on commercial prices will likely be scrutinized [53,54].
The ACO paradigm may also have significant effects on the physician labor market. Over the past 4 decades, the rate of physician specialization has grown dramatically [55].Fee-for-service incentives were aligned with specialization, but a rapid transition to alternative payment systems may disrupt the more gradually evolving physician labor market. Most medical school graduates today choose to specialize, as do most graduates of general medicine training programs [56,57],yet it is unclear to what degree the demand for specialists will continue to grow in the accountable care era. Specialty services tend to be of higher cost than generalist services. In some situations, high-cost services are more likely to be of lower value [58–60].Yet having specialists allows an organization to integrate services across the continuum of care, for which they are now financially responsible. As a new generation of specialists prepares to enter practice, whether the health care system will be able to support them and fulfill their expectations about their practice environment may be in question.
Looking into the Future
The landscape of payment and organization in health care will likely continue to migrate towards the ACO concept [61,62]. As the federal government, states, and individual payers move in similar directions, physicians and hospitals will face increasing pressures to change and adapt to new incentives surrounding cost and quality. Whether ACOs succeed in slowing spending while improving quality may have important ramifications for future stages of health care reform. For example, the growing debate in Washington, DC, over the future of Medicare financing may be informed, in part, by whether ACOs succeed within the traditional Medicare program. Market-based reforms, such as converting Medicare into a premium support program whereby private insurers compete to insure Medicare beneficiaries for a pre-defined contribution from the federal government, have been gaining momentum in recent years. Although not without concerns, such proposals would expect to gain consideration if the ACO model does not succeed.
Perhaps the most meaningful contribution of the ACO model is that it gives providers a reason to change the culture of medicine. It asks providers across specialties to work together and coordinate care in a way that was not rewarded under fee-for-service. It asks organizations to stitch the separate pieces of the patient’s care trajectory together through teamwork. In the long run, this may be the most intangible but substantive legacy that the ACO model provides. Under a single, collective contract at the organizational level, providers are quite literally in it together. If providers can break down silos, improve care coordination, and manage population health with a collective vision towards keeping patients healthy, the ACO paradigm would be able to claim a profound achievement. Such changes, however, will take time and they are not guaranteed.
Corresponding author: Zirui Song, MD, PhD, Department of Medicine, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114, [email protected].
Funding/support: Supported by a grant from the National Institute on Aging F30 AG039175.
Financial disclosures: None.
1. Orszag PR, Ellis P. The challenge of rising health care costs—a view from the Congressional Budget Office. N Engl J Med 2007;357:1793–5.
2. Chernew ME, Hirth RA, Cutler DM. Increased spending on health care: long- term implications for the nation. Health Aff (Millwood) 2009;28:1253–5.
3. Congressional Budget Office. Financing projected spending in the long run. Washington, DC: Congressional Budget Office; 2007 Jul 9.
4. Financing projected spending in the long run: letter from Peter R. Orszag, director, Congressional Budget Office, to Senator Judd Gregg, 9 Jul 2007.
5. Baicker K, Chernew ME. The economics of financing Medicare. N Engl J Med 2011;28;365:e7.
6. Chernew ME, Baicker K, Hsu J. The specter of financial armageddon—health care and federal debt in the United States. N Engl J Med 2010;362:1166–8.
7. Newhouse JP. Assessing health reform’s impact on four key groups of Americans. Health Aff (Millwood). 2010;29:1714–24.
8. Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med 2012;367:949–54.
9. Antos JR, Pauly MV, Wilensky GR. Bending the cost curve through market-based incentives. N Engl J Med 2012;367:954–8.
10. Centers for Medicare and Medicaid Services. More partnerships between doctors and hospitals strengthen coordinated care for Medicare beneficiaries. 2013 Dec 23.
11. Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med 2011;365:2445–7.
12. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs Blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
13. Fisher ES, Shortell SM. Accountable care organizations: accountable for what, to whom, and how. JAMA 2010;304:1715–6.
14. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood) 2007;26:w44–w57.
15. Rittenhouse DR, Shortell SM. The patient-centered medical home: will it stand the test of health reform? JAMA 2009;301:2038–40.
16. Bodenheimer T, Grumbach K, Berenson RA. A lifeline for primary care. N Engl J Med 2009;360:2693–6.
17. Centers for Medicare and Medicaid Services. Improving quality of care for Medicare patients: accountable care organizations. 2011 Oct 20.
18. Centers for Medicare and Medicaid Services. Press release: Pioneer accountable care organizations succeed in improving care, lowering costs. 2013 Jul 16. Available at www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html.
19. L&M Policy Research. Effect of Pioneer ACOs on Medicare spending in the first year. 2013 Nov 3. Available at http://innovation.cms.gov/Files/reports/PioneerACOEvalReport1.pdf.
20. Centers for Medicare and Medicaid Services. Performance year 1 interim results for ACOs that started in April and July 2012. 2014 Jan 30. Available at www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/PY1-InterimResultsTable.pdf.
21. Wilensky GR. Lessons from the Physician Group Practice Demonstration — a sobering reflection. N Engl J Med 2011;365:1659–61.
22. Colla CH, Wennberg DE, Meara E, et al. Spending differences associated with the Medicare Physician Group Practice Demonstration. JAMA 2012;308:1015–23.
23. Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program N Engl J Med 2011;364:e32.
24. Haywood TT, Kosel KC. The ACO model—a three-year financial loss? N Engl J Med 2011;364.
25. Iglehart JK. Assessing an ACO prototype—Medicare’s Physician Group Practice Demonstration. N Engl J Med 2011;364:198–200.
26. Chernew ME, Mechanic RE, Landon BE, Safran DG. Private-payer innovation in Massachusetts: the “Alternative Quality Contract.” Health Aff (Millwood). 2011;30:51–61.
27. Song Z, Safran DG, Landon BE, et al. Health care spending and quality in year 1 of the alternative quality contract. N Engl J Med 2011;365:909–18.
28. Song Z, Safran DG, Landon BE, et al. The ‘Alternative Quality Contract,’ based on a global budget, lowered medical spending and improved quality. Health Aff (Millwood). 2012;31:1885–94.
29. Song Z, Fendrick AM, Safran DG, et al. Global budgets and technology-intensive medical services. Healthcare (Amst) 2013;1:15–21.
30. Salmon RB, Sanderson MI, Walters BA, et al. A collaborative accountable care model in three practices showed promising early results on costs and quality of care. Health Aff (Millwood). 2012;31:2379–87.
31. Markovich P. A global budget pilot project among provider partners and Blue Shield of California led to savings in first two years. Health Aff (Millwood) 2012;31:1969–76.
32. Terhune C. Anthem, HealthCare Partners save $4.7 million by coordinating care. Los Angeles Times. 2014 Jun 6. Available at www.latimes.com/business/money/la-fi-anthem-healthcare-partners-20140605-story.html.
33. Gbemudu JN, Larson BK, Van Citters AD, et al. HealthCare Partners: building on a foundation of global risk management to achieve accountable care. Commonwealth Fund Pub. 1572, Vol 2. 2012 Jan.
34. Rice TH. The impact of changing Medicare reimbursement rates on physician- induced demand. Med Care 1983;21:803–15.
35. Nguyen NX, Derrick FW. Physician behavioral response to a Medicare price reduction. Health Serv Res 1997;32:283–98.
36. Yip WC. Physician response to Medicare fee reductions:changes in the volume of coronary artery bypass graft (CABG) surgeries in the Medicare and private sectors. J Health Econ 1998;17:675–99.
37. Song Z, Ayanian JZ, Wallace J, et al. Unintended consequences of eliminating medicare payments for consultations. JAMA Intern Med 2013;173:15–21.
38. Marquis MS, Rogowski JA, Escarce JJ. The managed care backlash: did consumers vote with their feet? Inquiry 2004-2005 Winter;41:376–90.
39. Cooper PF, Simon KI, Vistnes J. A closer look at the managed care backlash. Med Care 2006 May;44(5 Suppl):I4–11.
40. Song Z. Pioneer accountable care organizations: lessons from year 1. In: Curfman G, Morrissey S, Morse G, Prokesch S, editors. Insight Center: leading health care innovation. N Engl J Med/Harv Bus Rev. 2013 Oct 8. Available at http://images.nejm.org/editorial/supplementary/2013/hbr06-song.pdf.
41. McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA 2013;310:829–36.
42. Berenson RA. Shared savings program for accountable care organizations: a bridge to nowhere? Am J Manag Care 2010;16:721–6.
43. Meyer H. Accountable care organization prototypes: winners and losers? Health Aff (Millwood) 2011;30:1227–31.
44. Burns LR, Pauly MV. Accountable care organizations may have difficulty avoiding the failures of integrated delivery networks of the 1990s. Health Aff (Millwood) 2012;31:2407–16.
45. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
46. Colla CH, Lewis VA, Shortell SM, Fisher ES. First national survey of ACOs finds that physicians are playing strong leadership and ownership roles. Health Aff (Millwood) 2014;33:964–71.
47. Baicker K, Levy H. Coordination versus competition in health care reform. N Engl J Med 2013;369:789–91.
48. Baker LC, Bundorf MK, Kessler DP. Vertical integration: hospital ownership of physician practices is associated with higher prices and spending. Health Aff (Millwood). 2014;33:756–63.
49. Isaacs SL, Jellinek PS, Ray WL. The independent physician--going, going.... N Engl J Med 2009;360:655–7.
50. Kocher R, Sahni NR. Hospitals’ race to employ physicians--the logic behind a money-losing proposition. N Engl J Med 2011;364:1790–3.
51. Gold J. Hospitals lure doctors away from private practice. Kaiser Health News, October 12, 2010.
52. Accenture. Clinical transformation: new business models for a new era in healthcare. 2012. Available at www.accenture.com/SiteCollectionDocuments/PDF/Accenture-Clinical-Transformation-New-Business-Models-for-a-New-Era-in-Healthcare.pdf.
53. Fraud and abuse: Leibenluft RF. ACOs and the enforcement of fraud, abuse, and antitrust laws. N Engl J Med 2011;364:99–101.
54. Kreindler SA, Larson BK, Wu FM, et al. Interpretations of integration in early accountable care organizations. Milbank Q 2012;90:457–83.
55. Cassel CK, Reuben DB. Specialization, subspecialization, and subsubspecialization in internal medicine. N Engl J Med 2011;364:1169–73.
56. West CP, Dupras DM. General medicine vs subspecialty career plans among internal medicine residents. JAMA 2012;308:2241–7.
57. Schwartz MD, Durning S, Linzer M, Hauer KE. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med 2011;171:744-749.
58. Schwartz AL, Landon BE, Elshaug AG, et al. Measuring low-value care in Medicare. JAMA Intern Med 2014 May 12. [Epub ahead of print]
59. American Board of Internal Medicine Foundation. Choosing wisely: lists of five things physicians and patients should question. Accessed 26 May 2013 at www.choosingwisely.org/doctor-patient-lists/.
60. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
61. Lewis VA, Colla CH, Carluzzo KL, et al. Accountable care organizations in the United States: market and demographic factors associated with formation. Health Serv Res 2013;48(6 Pt 1):1840–58.
62. Conrad D, Grembowski D, Gibbons C, et al. A report on eight early-stage state and regional projects testing value-based payment. Health Aff (Millwood) 2013;32:998–1006.
From the Harvard Medical School and the Department of Medicine, Massachusetts General Hospital, Boston, MA.
In recent years, the growth of health care spending has climbed to the top of the domestic policy agenda. Medicare spending growth is now recognized as the biggest driver of the federal debt [1,2].Medicaid spending growth puts similar pressure on states. In the private sector, employee health care costs increasingly weigh on company balance sheets, affecting business operations and employee wages. All the while, individuals and families face insurance premium growth that far outpaces real income growth.
Out of this recent history emerged a broad recognition that health care spending growth is unsustainable at current rates. If Medicare spending continues to exceed gross domestic product (GDP) by 2.5 percentage points per year—the traditional gap over the past 4 decades—a greater than 160% increase in individual income taxes would be needed to pay for it [3].Even if the gap was 1 percentage point, the increase in income taxes needed would still be over 70%, with consequent contraction in GDP of 3% to 16% by 2015 [4,5].Other consequences, such as significant cuts in Medicare benefits or shifting of costs onto patients, are equally undesirable [6,7].
Policy options for slowing health care spending are varied. Some focus on changing the provider’s incentives, while others focus on changing the patient’s incentives. Some are based on federal solutions [8],while others are based on market solutions [9].In the current policy landscape, payment reform for physicians and hospitals has emerged as a leading candidate for addressing health care spending. Public and private payers are increasingly changing the way that providers are paid, moving away from fee-for-service towards bundled or global payments for populations of patients. Physicians and hospitals are in turn forming integrated provider organizations to take on these new payment systems. The pace of this change has been growing.
Key Features of the ACO Concept
An accountable care organization (ACO) is a group of providers—that can include both physicians and hospitals—that accepts joint responsibility for health care spending and quality for a defined population of patients. The ACO concept can be considered an extension of the staff-model health maintenance organization (HMO) [13,14].It also shares features with the patient-centered medical home (PCMH) model in its focus on a robust primary care nexus that serves to coordinate a patient’s care [15,16].Three key characteristics are embedded in this definition.
The first is joint accountability. In an ACO contract, incentives for providers are agreed upon at the organizational level. Physicians and hospitals bear the financial risks and rewards of the ACO contract together. Shared savings, quality bonuses, and other incentives are determined by how the organization performs as a whole rather than any individual physician, practice, or hospital. In this way, physicians across specialties and care settings are incentivized to approach patient care collectively and coordinate care more effectively.
Third, an ACO is responsible for the care of a population of people. Each year, spending and quality are measured for the population attributed, or assigned, to the ACO. Attribution of patients to organizations can take place in two ways. It can be prospective, meaning that before the start of a contract year, the ACO knows exactly the patients whose spending and quality it is responsible for. This is typically more feasible in commercial ACO contracts, especially in the HMO population, where patients designate a primary care physician at the beginning of the year. Otherwise, attribution is typically retrospective, such as in the Medicare ACO programs, where beneficiaries are assigned to organizations at the end of a contract year based on the organization which accounted for the plurality of a patient’s medical spending or primary care spending.
Evidence to Date
While formal results from most ACOs today are not yet available, several notable ACO experiments have been evaluated. These include early results from the Medicare ACO programs, previous evaluations of the Medicare Physician Group Practice Demonstration (a predecessor of today’s ACO contracting model), and early results from commercial ACO contracts, such as the Blue Cross Blue Shield of Massachusetts global budget contract.
In interpreting lessons from these evaluations, several questions are worth keeping in mind. If a new payment system is correlated with changes in medical spending, is this explained by underlying changes in prices or in quantities? Since medical spending is the product of prices of services and quantities (volume) of services, an intervention that affects spending must affect either the prices or the volume of care. In the Medicare program, where prices are standardized, a global budget contract that works off of the underlying physician fee schedule would only affect spending through volume. In the private insurance sector, however, an ACO contract may affect spending through both volume and prices, since variations in prices across providers creates an opportunity for savings if care is obtained through a less expensive provider.
Separate from its relationship to medical spending, which is measured through the claims submitted by providers, what is the connection between a new payment system and total payouts from the insurer to the provider? An ACO contract contains a variety of incentives to providers that may generate additional payments from the insurer (most notably shared savings and quality bonuses). These non-claims payments may partially or entirely offset savings obtained through medical claims, making them an important dimension in the evaluation of the contract. Yet they are also different from medical claim dollars in a meaningful way. Changes in medical spending reflect underlying physician (or patient) behavior—what care is delivered and how much of it is delivered—whereas non-claims payments reflect the incentive structure of the contract.
On the quality dimension, it is worth noting whether a new payment system has similar effects on process and outcome measures. Process measures, which have been widely used by health plans, are operationally similar to additional items on a fee schedule, whereby the delivery of a service effectively generates a payment. Clinical outcome measures, such as blood pressure or cholesterol, and patient experience measures, on the other hand, cannot be fulfilled by simply checking off a box. Therefore, these measures may represent quality in a more meaningful way.
Early Results from Medicare ACOs
Thirty-two provider organizations entered the Pioneer ACO program in 2012, with about 669,000 Medicare beneficiaries attributed to these organizations. According to CMS, medical spending growth for ACO beneficiaries was 0.3% in the first year, compared to 0.8% for similar beneficiaries outside of these organizations [18].This generated a gross savings of $87.6 million in 2012, of which $33 million were returned to the Medicare trust fund. These savings were driven by 13 ACOs, with another 17 ACOs not reporting statistically significant changes in spending and 2 ACOs garnering losses with spending above the target of about $4 million total. Lower rates of admissions and readmissions largely explained the savings. A separate analysis comparing these ACOs to their local markets estimated a higher year 1 savings of $147 million dollars, driven by 8 ACOs whose savings ranged from $396 to $1224 per beneficiary per year [19].
Pioneer ACOs also earned over $76 million for quality. In the first year, quality bonuses were awarded for the reporting of quality measures rather than for performance, and all 32 ACOs successfully reported. According to CMS, Pioneer ACOs on average performed better than fee-for-service Medicare beneficiaries on 15 clinical quality measures for which comparison data were available, including blood pressure control and cholesterol control for diabetic patients. A complete analysis of quality performance is not yet available.
In the Medicare Shared Savings Program, interim results from CMS for the first 2 cohorts of ACOs showed that 29 of the 114 organizations lowered spending sufficiently enough to generate shared savings while 2 organizations had shared losses [20].This suggests that the great majority of ACOs spent close to their target. Final results on spending and quality are pending.
Results from the Medicare Physician Group Practice Demonstration
Ten provider organizations entered one-sided ACO-type contracts with Medicare in 2005 via the Physician Group Practice Demonstration. In this contract, organizations shared in savings provided that their spending was at least 2% below target and they achieved threshold performance on certain quality measures, most of which were process metrics.
In year 1, only one organization decreased spending enough to earn a shared savings, but after 3 years, five organizations had generated shared savings, although half of the savings were awarded to one organization [21].A recent analysis showed that 4 organizations sustained shared savings by the end of the program, with savings concentrated in acute care, readmissions, and beneficiaries who are dually eligible for Medicaid. Across the 10 organizations, financial performance ranged from average savings of $866 to increased spending of $749 per beneficiary per year [22].In total, about $78 million in savings were generated by this demonstration. Although a positive finding, this is a relatively small amount in the context of total Medicare expenditures [23,24].
On quality, all organizations met threshold performance on at least 29 of the 32 measures by the end of 5 years [21]. Most of these were process measures focused on coronary artery disease, diabetes, heart failure, hypertension, and preventive care [25].
Early Results from Commercial ACO Contracts
One of the early commercial ACO contracts to be evaluated was the Blue Cross Blue Shield of Massachusetts Alternative Quality Contract (AQC) [26].Initially implemented in 2009, the AQC is a multi-year contract that pays provider organizations a risk-adjusted global budget over the continuum of care. Seven organizations in Massachusetts entered the contract in the first year, and 4 more entered in 2010. Enrollees in HMO plans were prospectively attributed to their ACO by their designation of a primary care physician. The AQC is a two-sided contract that offered an additional 10% of an organization’s budget as a bonus for performance on 64 quality measures, half outpatient measures and half inpatient. Budget growth rates were tied to inflation and terms of its growth were negotiated with the organizations [27].
Over the first 2 years, the contract was associated with a decrease in medical spending of about $90 per enrollee per year, a –2.8% change (–1.9% in year 1 and –3.3% in year 2) [28].These savings were concentrated in procedures, imaging, and tests in the outpatient facility setting, and were largely explained by lower prices achieved by referring patients to less expensive providers. They were also concentrated in organizations that entered the AQC from fee-for-service, rather than prior risk contracts, and driven by enrollees with the highest expected spending. Over the second year, decreases in volume for certain services, such as percutaneous coronary interventions, began to contribute more to the savings [29].However, medical savings in the first 2 years were exceeded by non-claims payments, including shared savings and quality bonuses [27,28].The AQC was also associated with improvements in outpatient quality, including chronic care management measures (3.7 percentage points increase per year), adult preventive measures (0.4 percentage points per year), and pediatric quality measures (1.3 percentage points per year). Outcome measures such as hemoglobin A1c, LDL cholesterol, and blood pressure also showed an upward trend in the early years [28].Inpatient quality measures have yet to be examined.
Elsewhere in the country, Cigna’s Collaborative Accountable Care model was rolled out in 2009 with provider organizations in New Hampshire, Texas, and Arizona. A one-sided shared savings contract, it features a care coordination fee that is counted towards a practice’s medical spending, helping fund registered nurses who work as care coordinators. Interim results in 2012 suggested cost savings and quality improvements, but they were not statistically significant [30].A two-sided contract between Blue Shield of California and the California Public Employees’ Retirement System slowed medical costs by shortening admission and reducing readmissions [31].Most recently, an accountable care partnership between Anthem Blue Cross and HealthCare Partners physician group in California claimed $4.7 million in savings in the first half of 2013 for 55,000 patients in preferred provider organization (PPO) plans [32].These savings were driven by an 18% reduction in inpatient days, 4% reduction in overall admissions, and a 4% reduction in visits for radiology and lab tests, although specific price and volume contributions are yet unknown. Similar to the AQC, the Anthem contract is a 5-year agreement. Unlike the initial AQC contract, shared savings were tied to meeting a quality threshold and HealthCare Partners did not bear downside risk in year 1 [33].
Lessons Learned
Strengths of the ACO Model
Evidence to date points to both the potential of ACOs to slow spending and improve quality, but also the significant obstacles that they face. One of the encouraging lessons so far is that quality of care need not be threatened by a contract that rewards savings, provided that meaningful incentives for quality are in place. In both public and private two-sided contracts, process quality seemed to consistently improve. However, less is known about performance on outcome measures, which is ultimately a more meaningful metric for patients.
Broadly speaking, ACO contracts may be able to induce changes in physician behavior that could lead to medical savings. The low hanging fruit in Medicare seems to be admissions and readmissions, while that in commercial contracts may be lower prices obtained by changing referral patterns. While these medical savings reveal changes in clinical decision-making, it is still poorly understood whether clinical choices geared towards higher value can extend to areas of utilization where wasteful care may be concentrated. Little is also known about whether an ACO’s physicians and hospitals are in agreement over these changes in utilization or referral patterns, given their consequences for referral business and admissions. Moreover, there has yet to be evidence suggesting that medical savings can be larger than non-claims payments (rewards and bonuses to the ACOs) in a given year, generating net savings to the health care system. This may well take time to materialize given the initial investment costs and inducements for provider participation embedded in the early year incentives, but it is an important metric of success.
ACOs serve as a vehicle for payment reform and organizational reform among providers. They bring physicians across specialties and hospitals together under the same contractual roof, allowing the organization to determine how it allocates its resources under the spending target. Historically, policies aimed to slow health care spending have focused on either cutting prices (provider fees) or constraining volume (gatekeeping, prior authorization, and utilization review, etc.), but both types of strategies have been complicated by unintended consequences. Medicare fee cuts have traditionally been followed by compensatory increases in utilization or intensity of coding, offsetting the intended savings to a significant degree [34–37].Managed care techniques have met resistance from both physicians and patients [38,39].A spending target or budget takes an alternative approach; rather than controlling prices or quantities directly, it seeks to control total spending. Although the underlying fee schedule is retained for accounting, a spending target or especially a global budget pushes the organization to decide what care is high or low value.
A two-sided contract imposes stronger incentives on the ACO than a one-sided contract, which is both a strength and a weakness. While ACOs facing downside risk may respond earlier to the incentives, as some of the evidence thus far suggests, this risk may also propel ACOs to abandon this contracting model. In the Medicare Pioneer ACO program, for example, 9 of the 32 organizations exited the contract after year 1, which was allowed given the voluntary nature of participation. Seven organizations opted for the one-sided Medicare Shared Savings Program in year 2 and two left the ACO programs altogether. Downside risk was thought to be a principal concern for these organizations [40].Massachusetts providers in the AQC have thus far remained in the contract, but the AQC was a multi-year agreement to begin with.
Spillovers are another potential strength of the ACO model. Given that organizations care for patients across multiple payers, strong payment incentives in one payer population may affect care broadly. In the AQC, for example, recent evidence showed slowing of spending for Medicare beneficiaries associated with the contract in similar settings and categories of care as for the Blue Cross Blue Shield patients [41].
Weaknesses of the ACO Model
Still in its nascent stages, the ACO paradigm faces a number of challenges. Some relate to inherent weaknesses of the model, while others relate to the institutions and economics of the broader health care economy. At a contractual level, a key challenge is setting the target growth rate of the budget. If too low, providers may be overly constrained; if too high, providers may not have enough incentive to change practice. In an extreme case, if the target is set above what spending would have been under the old arrangement, an ACO contract can in fact be cost increasing on claims spending alone. Financial rewards such as shared savings and quality bonuses can help offset the risk, but they also make it more difficult for the ACO contract to generate net savings.
Achieving the right balance of risks and rewards is difficult. As noted above, a one-sided contract may not be strong enough to induce behavior change [42,43],but a two-sided contract may be too risky, driving providers who are unable to align incentives and coordinate care to exit the model [44].Although the percentage of shared risk borne by payer versus provider can be negotiated, putting financial risk on providers in a palatable way will be a key challenge. Financial risk can be more daunting if ACOs do not know in advance which patients they are responsible for, as in contracts with retrospective attribution rules and enrollees in unmanaged plans. A payer can help providers handle risk by sharing data on spending and identifying potential areas of overuse and low value care. Payers can help further by implementing risk corridors, providing reinsurance, or improving risk adjustment of the organization’s global budget. But with all that said, it remains to be seen whether providers around the country will be willing to bear substantive risk.
Within the ACO, a primary challenge is dividing up risks and rewards among constituent providers. How much shared savings are given to the hospital, to primary care physicians, or to specialists? What share should each specialty receive? What about shared losses, should spending exceed the target? In a two-sided ACO contract, these questions are particularly salient as global budgets change the business model for providers. Revenue centers under fee-for-service become cost centers. Organizations are confronted with difficult tradeoffs. The ability of providers across specialties to find common ground will be crucial, and leadership from providers will be key [45].Physicians have established themselves as leaders of the majority of ACOs today [46].It remains to be seen whether these organizations can keep providers together through the tradeoffs.
Patient trust in the ACO model has yet to be established. The managed care backlash of the 1990s suggests that patient buy-in will be crucial for the sustainability of ACOs. ACOs can have similarities to the HMO that traditionally produce negative associations, including downside risk, gatekeeping, or managed care techniques. To earn patients’ trust, ACOs will need to prove their value, such as through delivering better preventive care, less expensive care, more holistic care through stronger teams of providers, or smoother transitions of care across settings. The task of primary care medical homes to provide patient-centered care and coordinate across specialists effectively will be crucial. While today’s ACOs may be better positioned because of risk sharing, quality bonuses, risk adjustment, electronic medical records, or other innovations, the patient’s experience may ultimately be the arbiter.
Broader Challenges
While clinical integration is a central tenet of ACOs, consolidation between providers is simultaneously a chief concern for policymakers. Consolidation generally reduces competition and drives up prices, which is at odds with the goals of cost containment [47,48].Across the nation, physicians are consolidating with hospitals and health systems at an increasing rate, with recent surveys reporting that the proportion of independent physicians has steadily declined to below 50% [49–52].Increasing the number of covered lives is a dominant growth strategy under risk contracts, and more covered lives also increases an ACO’s bargaining power during acquisitions of specialist practices, whose referrals are better protected by inclusion in the provider network. As this trend continues, its effect on commercial prices will likely be scrutinized [53,54].
The ACO paradigm may also have significant effects on the physician labor market. Over the past 4 decades, the rate of physician specialization has grown dramatically [55].Fee-for-service incentives were aligned with specialization, but a rapid transition to alternative payment systems may disrupt the more gradually evolving physician labor market. Most medical school graduates today choose to specialize, as do most graduates of general medicine training programs [56,57],yet it is unclear to what degree the demand for specialists will continue to grow in the accountable care era. Specialty services tend to be of higher cost than generalist services. In some situations, high-cost services are more likely to be of lower value [58–60].Yet having specialists allows an organization to integrate services across the continuum of care, for which they are now financially responsible. As a new generation of specialists prepares to enter practice, whether the health care system will be able to support them and fulfill their expectations about their practice environment may be in question.
Looking into the Future
The landscape of payment and organization in health care will likely continue to migrate towards the ACO concept [61,62]. As the federal government, states, and individual payers move in similar directions, physicians and hospitals will face increasing pressures to change and adapt to new incentives surrounding cost and quality. Whether ACOs succeed in slowing spending while improving quality may have important ramifications for future stages of health care reform. For example, the growing debate in Washington, DC, over the future of Medicare financing may be informed, in part, by whether ACOs succeed within the traditional Medicare program. Market-based reforms, such as converting Medicare into a premium support program whereby private insurers compete to insure Medicare beneficiaries for a pre-defined contribution from the federal government, have been gaining momentum in recent years. Although not without concerns, such proposals would expect to gain consideration if the ACO model does not succeed.
Perhaps the most meaningful contribution of the ACO model is that it gives providers a reason to change the culture of medicine. It asks providers across specialties to work together and coordinate care in a way that was not rewarded under fee-for-service. It asks organizations to stitch the separate pieces of the patient’s care trajectory together through teamwork. In the long run, this may be the most intangible but substantive legacy that the ACO model provides. Under a single, collective contract at the organizational level, providers are quite literally in it together. If providers can break down silos, improve care coordination, and manage population health with a collective vision towards keeping patients healthy, the ACO paradigm would be able to claim a profound achievement. Such changes, however, will take time and they are not guaranteed.
Corresponding author: Zirui Song, MD, PhD, Department of Medicine, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114, [email protected].
Funding/support: Supported by a grant from the National Institute on Aging F30 AG039175.
Financial disclosures: None.
From the Harvard Medical School and the Department of Medicine, Massachusetts General Hospital, Boston, MA.
In recent years, the growth of health care spending has climbed to the top of the domestic policy agenda. Medicare spending growth is now recognized as the biggest driver of the federal debt [1,2].Medicaid spending growth puts similar pressure on states. In the private sector, employee health care costs increasingly weigh on company balance sheets, affecting business operations and employee wages. All the while, individuals and families face insurance premium growth that far outpaces real income growth.
Out of this recent history emerged a broad recognition that health care spending growth is unsustainable at current rates. If Medicare spending continues to exceed gross domestic product (GDP) by 2.5 percentage points per year—the traditional gap over the past 4 decades—a greater than 160% increase in individual income taxes would be needed to pay for it [3].Even if the gap was 1 percentage point, the increase in income taxes needed would still be over 70%, with consequent contraction in GDP of 3% to 16% by 2015 [4,5].Other consequences, such as significant cuts in Medicare benefits or shifting of costs onto patients, are equally undesirable [6,7].
Policy options for slowing health care spending are varied. Some focus on changing the provider’s incentives, while others focus on changing the patient’s incentives. Some are based on federal solutions [8],while others are based on market solutions [9].In the current policy landscape, payment reform for physicians and hospitals has emerged as a leading candidate for addressing health care spending. Public and private payers are increasingly changing the way that providers are paid, moving away from fee-for-service towards bundled or global payments for populations of patients. Physicians and hospitals are in turn forming integrated provider organizations to take on these new payment systems. The pace of this change has been growing.
Key Features of the ACO Concept
An accountable care organization (ACO) is a group of providers—that can include both physicians and hospitals—that accepts joint responsibility for health care spending and quality for a defined population of patients. The ACO concept can be considered an extension of the staff-model health maintenance organization (HMO) [13,14].It also shares features with the patient-centered medical home (PCMH) model in its focus on a robust primary care nexus that serves to coordinate a patient’s care [15,16].Three key characteristics are embedded in this definition.
The first is joint accountability. In an ACO contract, incentives for providers are agreed upon at the organizational level. Physicians and hospitals bear the financial risks and rewards of the ACO contract together. Shared savings, quality bonuses, and other incentives are determined by how the organization performs as a whole rather than any individual physician, practice, or hospital. In this way, physicians across specialties and care settings are incentivized to approach patient care collectively and coordinate care more effectively.
Third, an ACO is responsible for the care of a population of people. Each year, spending and quality are measured for the population attributed, or assigned, to the ACO. Attribution of patients to organizations can take place in two ways. It can be prospective, meaning that before the start of a contract year, the ACO knows exactly the patients whose spending and quality it is responsible for. This is typically more feasible in commercial ACO contracts, especially in the HMO population, where patients designate a primary care physician at the beginning of the year. Otherwise, attribution is typically retrospective, such as in the Medicare ACO programs, where beneficiaries are assigned to organizations at the end of a contract year based on the organization which accounted for the plurality of a patient’s medical spending or primary care spending.
Evidence to Date
While formal results from most ACOs today are not yet available, several notable ACO experiments have been evaluated. These include early results from the Medicare ACO programs, previous evaluations of the Medicare Physician Group Practice Demonstration (a predecessor of today’s ACO contracting model), and early results from commercial ACO contracts, such as the Blue Cross Blue Shield of Massachusetts global budget contract.
In interpreting lessons from these evaluations, several questions are worth keeping in mind. If a new payment system is correlated with changes in medical spending, is this explained by underlying changes in prices or in quantities? Since medical spending is the product of prices of services and quantities (volume) of services, an intervention that affects spending must affect either the prices or the volume of care. In the Medicare program, where prices are standardized, a global budget contract that works off of the underlying physician fee schedule would only affect spending through volume. In the private insurance sector, however, an ACO contract may affect spending through both volume and prices, since variations in prices across providers creates an opportunity for savings if care is obtained through a less expensive provider.
Separate from its relationship to medical spending, which is measured through the claims submitted by providers, what is the connection between a new payment system and total payouts from the insurer to the provider? An ACO contract contains a variety of incentives to providers that may generate additional payments from the insurer (most notably shared savings and quality bonuses). These non-claims payments may partially or entirely offset savings obtained through medical claims, making them an important dimension in the evaluation of the contract. Yet they are also different from medical claim dollars in a meaningful way. Changes in medical spending reflect underlying physician (or patient) behavior—what care is delivered and how much of it is delivered—whereas non-claims payments reflect the incentive structure of the contract.
On the quality dimension, it is worth noting whether a new payment system has similar effects on process and outcome measures. Process measures, which have been widely used by health plans, are operationally similar to additional items on a fee schedule, whereby the delivery of a service effectively generates a payment. Clinical outcome measures, such as blood pressure or cholesterol, and patient experience measures, on the other hand, cannot be fulfilled by simply checking off a box. Therefore, these measures may represent quality in a more meaningful way.
Early Results from Medicare ACOs
Thirty-two provider organizations entered the Pioneer ACO program in 2012, with about 669,000 Medicare beneficiaries attributed to these organizations. According to CMS, medical spending growth for ACO beneficiaries was 0.3% in the first year, compared to 0.8% for similar beneficiaries outside of these organizations [18].This generated a gross savings of $87.6 million in 2012, of which $33 million were returned to the Medicare trust fund. These savings were driven by 13 ACOs, with another 17 ACOs not reporting statistically significant changes in spending and 2 ACOs garnering losses with spending above the target of about $4 million total. Lower rates of admissions and readmissions largely explained the savings. A separate analysis comparing these ACOs to their local markets estimated a higher year 1 savings of $147 million dollars, driven by 8 ACOs whose savings ranged from $396 to $1224 per beneficiary per year [19].
Pioneer ACOs also earned over $76 million for quality. In the first year, quality bonuses were awarded for the reporting of quality measures rather than for performance, and all 32 ACOs successfully reported. According to CMS, Pioneer ACOs on average performed better than fee-for-service Medicare beneficiaries on 15 clinical quality measures for which comparison data were available, including blood pressure control and cholesterol control for diabetic patients. A complete analysis of quality performance is not yet available.
In the Medicare Shared Savings Program, interim results from CMS for the first 2 cohorts of ACOs showed that 29 of the 114 organizations lowered spending sufficiently enough to generate shared savings while 2 organizations had shared losses [20].This suggests that the great majority of ACOs spent close to their target. Final results on spending and quality are pending.
Results from the Medicare Physician Group Practice Demonstration
Ten provider organizations entered one-sided ACO-type contracts with Medicare in 2005 via the Physician Group Practice Demonstration. In this contract, organizations shared in savings provided that their spending was at least 2% below target and they achieved threshold performance on certain quality measures, most of which were process metrics.
In year 1, only one organization decreased spending enough to earn a shared savings, but after 3 years, five organizations had generated shared savings, although half of the savings were awarded to one organization [21].A recent analysis showed that 4 organizations sustained shared savings by the end of the program, with savings concentrated in acute care, readmissions, and beneficiaries who are dually eligible for Medicaid. Across the 10 organizations, financial performance ranged from average savings of $866 to increased spending of $749 per beneficiary per year [22].In total, about $78 million in savings were generated by this demonstration. Although a positive finding, this is a relatively small amount in the context of total Medicare expenditures [23,24].
On quality, all organizations met threshold performance on at least 29 of the 32 measures by the end of 5 years [21]. Most of these were process measures focused on coronary artery disease, diabetes, heart failure, hypertension, and preventive care [25].
Early Results from Commercial ACO Contracts
One of the early commercial ACO contracts to be evaluated was the Blue Cross Blue Shield of Massachusetts Alternative Quality Contract (AQC) [26].Initially implemented in 2009, the AQC is a multi-year contract that pays provider organizations a risk-adjusted global budget over the continuum of care. Seven organizations in Massachusetts entered the contract in the first year, and 4 more entered in 2010. Enrollees in HMO plans were prospectively attributed to their ACO by their designation of a primary care physician. The AQC is a two-sided contract that offered an additional 10% of an organization’s budget as a bonus for performance on 64 quality measures, half outpatient measures and half inpatient. Budget growth rates were tied to inflation and terms of its growth were negotiated with the organizations [27].
Over the first 2 years, the contract was associated with a decrease in medical spending of about $90 per enrollee per year, a –2.8% change (–1.9% in year 1 and –3.3% in year 2) [28].These savings were concentrated in procedures, imaging, and tests in the outpatient facility setting, and were largely explained by lower prices achieved by referring patients to less expensive providers. They were also concentrated in organizations that entered the AQC from fee-for-service, rather than prior risk contracts, and driven by enrollees with the highest expected spending. Over the second year, decreases in volume for certain services, such as percutaneous coronary interventions, began to contribute more to the savings [29].However, medical savings in the first 2 years were exceeded by non-claims payments, including shared savings and quality bonuses [27,28].The AQC was also associated with improvements in outpatient quality, including chronic care management measures (3.7 percentage points increase per year), adult preventive measures (0.4 percentage points per year), and pediatric quality measures (1.3 percentage points per year). Outcome measures such as hemoglobin A1c, LDL cholesterol, and blood pressure also showed an upward trend in the early years [28].Inpatient quality measures have yet to be examined.
Elsewhere in the country, Cigna’s Collaborative Accountable Care model was rolled out in 2009 with provider organizations in New Hampshire, Texas, and Arizona. A one-sided shared savings contract, it features a care coordination fee that is counted towards a practice’s medical spending, helping fund registered nurses who work as care coordinators. Interim results in 2012 suggested cost savings and quality improvements, but they were not statistically significant [30].A two-sided contract between Blue Shield of California and the California Public Employees’ Retirement System slowed medical costs by shortening admission and reducing readmissions [31].Most recently, an accountable care partnership between Anthem Blue Cross and HealthCare Partners physician group in California claimed $4.7 million in savings in the first half of 2013 for 55,000 patients in preferred provider organization (PPO) plans [32].These savings were driven by an 18% reduction in inpatient days, 4% reduction in overall admissions, and a 4% reduction in visits for radiology and lab tests, although specific price and volume contributions are yet unknown. Similar to the AQC, the Anthem contract is a 5-year agreement. Unlike the initial AQC contract, shared savings were tied to meeting a quality threshold and HealthCare Partners did not bear downside risk in year 1 [33].
Lessons Learned
Strengths of the ACO Model
Evidence to date points to both the potential of ACOs to slow spending and improve quality, but also the significant obstacles that they face. One of the encouraging lessons so far is that quality of care need not be threatened by a contract that rewards savings, provided that meaningful incentives for quality are in place. In both public and private two-sided contracts, process quality seemed to consistently improve. However, less is known about performance on outcome measures, which is ultimately a more meaningful metric for patients.
Broadly speaking, ACO contracts may be able to induce changes in physician behavior that could lead to medical savings. The low hanging fruit in Medicare seems to be admissions and readmissions, while that in commercial contracts may be lower prices obtained by changing referral patterns. While these medical savings reveal changes in clinical decision-making, it is still poorly understood whether clinical choices geared towards higher value can extend to areas of utilization where wasteful care may be concentrated. Little is also known about whether an ACO’s physicians and hospitals are in agreement over these changes in utilization or referral patterns, given their consequences for referral business and admissions. Moreover, there has yet to be evidence suggesting that medical savings can be larger than non-claims payments (rewards and bonuses to the ACOs) in a given year, generating net savings to the health care system. This may well take time to materialize given the initial investment costs and inducements for provider participation embedded in the early year incentives, but it is an important metric of success.
ACOs serve as a vehicle for payment reform and organizational reform among providers. They bring physicians across specialties and hospitals together under the same contractual roof, allowing the organization to determine how it allocates its resources under the spending target. Historically, policies aimed to slow health care spending have focused on either cutting prices (provider fees) or constraining volume (gatekeeping, prior authorization, and utilization review, etc.), but both types of strategies have been complicated by unintended consequences. Medicare fee cuts have traditionally been followed by compensatory increases in utilization or intensity of coding, offsetting the intended savings to a significant degree [34–37].Managed care techniques have met resistance from both physicians and patients [38,39].A spending target or budget takes an alternative approach; rather than controlling prices or quantities directly, it seeks to control total spending. Although the underlying fee schedule is retained for accounting, a spending target or especially a global budget pushes the organization to decide what care is high or low value.
A two-sided contract imposes stronger incentives on the ACO than a one-sided contract, which is both a strength and a weakness. While ACOs facing downside risk may respond earlier to the incentives, as some of the evidence thus far suggests, this risk may also propel ACOs to abandon this contracting model. In the Medicare Pioneer ACO program, for example, 9 of the 32 organizations exited the contract after year 1, which was allowed given the voluntary nature of participation. Seven organizations opted for the one-sided Medicare Shared Savings Program in year 2 and two left the ACO programs altogether. Downside risk was thought to be a principal concern for these organizations [40].Massachusetts providers in the AQC have thus far remained in the contract, but the AQC was a multi-year agreement to begin with.
Spillovers are another potential strength of the ACO model. Given that organizations care for patients across multiple payers, strong payment incentives in one payer population may affect care broadly. In the AQC, for example, recent evidence showed slowing of spending for Medicare beneficiaries associated with the contract in similar settings and categories of care as for the Blue Cross Blue Shield patients [41].
Weaknesses of the ACO Model
Still in its nascent stages, the ACO paradigm faces a number of challenges. Some relate to inherent weaknesses of the model, while others relate to the institutions and economics of the broader health care economy. At a contractual level, a key challenge is setting the target growth rate of the budget. If too low, providers may be overly constrained; if too high, providers may not have enough incentive to change practice. In an extreme case, if the target is set above what spending would have been under the old arrangement, an ACO contract can in fact be cost increasing on claims spending alone. Financial rewards such as shared savings and quality bonuses can help offset the risk, but they also make it more difficult for the ACO contract to generate net savings.
Achieving the right balance of risks and rewards is difficult. As noted above, a one-sided contract may not be strong enough to induce behavior change [42,43],but a two-sided contract may be too risky, driving providers who are unable to align incentives and coordinate care to exit the model [44].Although the percentage of shared risk borne by payer versus provider can be negotiated, putting financial risk on providers in a palatable way will be a key challenge. Financial risk can be more daunting if ACOs do not know in advance which patients they are responsible for, as in contracts with retrospective attribution rules and enrollees in unmanaged plans. A payer can help providers handle risk by sharing data on spending and identifying potential areas of overuse and low value care. Payers can help further by implementing risk corridors, providing reinsurance, or improving risk adjustment of the organization’s global budget. But with all that said, it remains to be seen whether providers around the country will be willing to bear substantive risk.
Within the ACO, a primary challenge is dividing up risks and rewards among constituent providers. How much shared savings are given to the hospital, to primary care physicians, or to specialists? What share should each specialty receive? What about shared losses, should spending exceed the target? In a two-sided ACO contract, these questions are particularly salient as global budgets change the business model for providers. Revenue centers under fee-for-service become cost centers. Organizations are confronted with difficult tradeoffs. The ability of providers across specialties to find common ground will be crucial, and leadership from providers will be key [45].Physicians have established themselves as leaders of the majority of ACOs today [46].It remains to be seen whether these organizations can keep providers together through the tradeoffs.
Patient trust in the ACO model has yet to be established. The managed care backlash of the 1990s suggests that patient buy-in will be crucial for the sustainability of ACOs. ACOs can have similarities to the HMO that traditionally produce negative associations, including downside risk, gatekeeping, or managed care techniques. To earn patients’ trust, ACOs will need to prove their value, such as through delivering better preventive care, less expensive care, more holistic care through stronger teams of providers, or smoother transitions of care across settings. The task of primary care medical homes to provide patient-centered care and coordinate across specialists effectively will be crucial. While today’s ACOs may be better positioned because of risk sharing, quality bonuses, risk adjustment, electronic medical records, or other innovations, the patient’s experience may ultimately be the arbiter.
Broader Challenges
While clinical integration is a central tenet of ACOs, consolidation between providers is simultaneously a chief concern for policymakers. Consolidation generally reduces competition and drives up prices, which is at odds with the goals of cost containment [47,48].Across the nation, physicians are consolidating with hospitals and health systems at an increasing rate, with recent surveys reporting that the proportion of independent physicians has steadily declined to below 50% [49–52].Increasing the number of covered lives is a dominant growth strategy under risk contracts, and more covered lives also increases an ACO’s bargaining power during acquisitions of specialist practices, whose referrals are better protected by inclusion in the provider network. As this trend continues, its effect on commercial prices will likely be scrutinized [53,54].
The ACO paradigm may also have significant effects on the physician labor market. Over the past 4 decades, the rate of physician specialization has grown dramatically [55].Fee-for-service incentives were aligned with specialization, but a rapid transition to alternative payment systems may disrupt the more gradually evolving physician labor market. Most medical school graduates today choose to specialize, as do most graduates of general medicine training programs [56,57],yet it is unclear to what degree the demand for specialists will continue to grow in the accountable care era. Specialty services tend to be of higher cost than generalist services. In some situations, high-cost services are more likely to be of lower value [58–60].Yet having specialists allows an organization to integrate services across the continuum of care, for which they are now financially responsible. As a new generation of specialists prepares to enter practice, whether the health care system will be able to support them and fulfill their expectations about their practice environment may be in question.
Looking into the Future
The landscape of payment and organization in health care will likely continue to migrate towards the ACO concept [61,62]. As the federal government, states, and individual payers move in similar directions, physicians and hospitals will face increasing pressures to change and adapt to new incentives surrounding cost and quality. Whether ACOs succeed in slowing spending while improving quality may have important ramifications for future stages of health care reform. For example, the growing debate in Washington, DC, over the future of Medicare financing may be informed, in part, by whether ACOs succeed within the traditional Medicare program. Market-based reforms, such as converting Medicare into a premium support program whereby private insurers compete to insure Medicare beneficiaries for a pre-defined contribution from the federal government, have been gaining momentum in recent years. Although not without concerns, such proposals would expect to gain consideration if the ACO model does not succeed.
Perhaps the most meaningful contribution of the ACO model is that it gives providers a reason to change the culture of medicine. It asks providers across specialties to work together and coordinate care in a way that was not rewarded under fee-for-service. It asks organizations to stitch the separate pieces of the patient’s care trajectory together through teamwork. In the long run, this may be the most intangible but substantive legacy that the ACO model provides. Under a single, collective contract at the organizational level, providers are quite literally in it together. If providers can break down silos, improve care coordination, and manage population health with a collective vision towards keeping patients healthy, the ACO paradigm would be able to claim a profound achievement. Such changes, however, will take time and they are not guaranteed.
Corresponding author: Zirui Song, MD, PhD, Department of Medicine, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114, [email protected].
Funding/support: Supported by a grant from the National Institute on Aging F30 AG039175.
Financial disclosures: None.
1. Orszag PR, Ellis P. The challenge of rising health care costs—a view from the Congressional Budget Office. N Engl J Med 2007;357:1793–5.
2. Chernew ME, Hirth RA, Cutler DM. Increased spending on health care: long- term implications for the nation. Health Aff (Millwood) 2009;28:1253–5.
3. Congressional Budget Office. Financing projected spending in the long run. Washington, DC: Congressional Budget Office; 2007 Jul 9.
4. Financing projected spending in the long run: letter from Peter R. Orszag, director, Congressional Budget Office, to Senator Judd Gregg, 9 Jul 2007.
5. Baicker K, Chernew ME. The economics of financing Medicare. N Engl J Med 2011;28;365:e7.
6. Chernew ME, Baicker K, Hsu J. The specter of financial armageddon—health care and federal debt in the United States. N Engl J Med 2010;362:1166–8.
7. Newhouse JP. Assessing health reform’s impact on four key groups of Americans. Health Aff (Millwood). 2010;29:1714–24.
8. Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med 2012;367:949–54.
9. Antos JR, Pauly MV, Wilensky GR. Bending the cost curve through market-based incentives. N Engl J Med 2012;367:954–8.
10. Centers for Medicare and Medicaid Services. More partnerships between doctors and hospitals strengthen coordinated care for Medicare beneficiaries. 2013 Dec 23.
11. Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med 2011;365:2445–7.
12. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs Blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
13. Fisher ES, Shortell SM. Accountable care organizations: accountable for what, to whom, and how. JAMA 2010;304:1715–6.
14. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood) 2007;26:w44–w57.
15. Rittenhouse DR, Shortell SM. The patient-centered medical home: will it stand the test of health reform? JAMA 2009;301:2038–40.
16. Bodenheimer T, Grumbach K, Berenson RA. A lifeline for primary care. N Engl J Med 2009;360:2693–6.
17. Centers for Medicare and Medicaid Services. Improving quality of care for Medicare patients: accountable care organizations. 2011 Oct 20.
18. Centers for Medicare and Medicaid Services. Press release: Pioneer accountable care organizations succeed in improving care, lowering costs. 2013 Jul 16. Available at www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html.
19. L&M Policy Research. Effect of Pioneer ACOs on Medicare spending in the first year. 2013 Nov 3. Available at http://innovation.cms.gov/Files/reports/PioneerACOEvalReport1.pdf.
20. Centers for Medicare and Medicaid Services. Performance year 1 interim results for ACOs that started in April and July 2012. 2014 Jan 30. Available at www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/PY1-InterimResultsTable.pdf.
21. Wilensky GR. Lessons from the Physician Group Practice Demonstration — a sobering reflection. N Engl J Med 2011;365:1659–61.
22. Colla CH, Wennberg DE, Meara E, et al. Spending differences associated with the Medicare Physician Group Practice Demonstration. JAMA 2012;308:1015–23.
23. Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program N Engl J Med 2011;364:e32.
24. Haywood TT, Kosel KC. The ACO model—a three-year financial loss? N Engl J Med 2011;364.
25. Iglehart JK. Assessing an ACO prototype—Medicare’s Physician Group Practice Demonstration. N Engl J Med 2011;364:198–200.
26. Chernew ME, Mechanic RE, Landon BE, Safran DG. Private-payer innovation in Massachusetts: the “Alternative Quality Contract.” Health Aff (Millwood). 2011;30:51–61.
27. Song Z, Safran DG, Landon BE, et al. Health care spending and quality in year 1 of the alternative quality contract. N Engl J Med 2011;365:909–18.
28. Song Z, Safran DG, Landon BE, et al. The ‘Alternative Quality Contract,’ based on a global budget, lowered medical spending and improved quality. Health Aff (Millwood). 2012;31:1885–94.
29. Song Z, Fendrick AM, Safran DG, et al. Global budgets and technology-intensive medical services. Healthcare (Amst) 2013;1:15–21.
30. Salmon RB, Sanderson MI, Walters BA, et al. A collaborative accountable care model in three practices showed promising early results on costs and quality of care. Health Aff (Millwood). 2012;31:2379–87.
31. Markovich P. A global budget pilot project among provider partners and Blue Shield of California led to savings in first two years. Health Aff (Millwood) 2012;31:1969–76.
32. Terhune C. Anthem, HealthCare Partners save $4.7 million by coordinating care. Los Angeles Times. 2014 Jun 6. Available at www.latimes.com/business/money/la-fi-anthem-healthcare-partners-20140605-story.html.
33. Gbemudu JN, Larson BK, Van Citters AD, et al. HealthCare Partners: building on a foundation of global risk management to achieve accountable care. Commonwealth Fund Pub. 1572, Vol 2. 2012 Jan.
34. Rice TH. The impact of changing Medicare reimbursement rates on physician- induced demand. Med Care 1983;21:803–15.
35. Nguyen NX, Derrick FW. Physician behavioral response to a Medicare price reduction. Health Serv Res 1997;32:283–98.
36. Yip WC. Physician response to Medicare fee reductions:changes in the volume of coronary artery bypass graft (CABG) surgeries in the Medicare and private sectors. J Health Econ 1998;17:675–99.
37. Song Z, Ayanian JZ, Wallace J, et al. Unintended consequences of eliminating medicare payments for consultations. JAMA Intern Med 2013;173:15–21.
38. Marquis MS, Rogowski JA, Escarce JJ. The managed care backlash: did consumers vote with their feet? Inquiry 2004-2005 Winter;41:376–90.
39. Cooper PF, Simon KI, Vistnes J. A closer look at the managed care backlash. Med Care 2006 May;44(5 Suppl):I4–11.
40. Song Z. Pioneer accountable care organizations: lessons from year 1. In: Curfman G, Morrissey S, Morse G, Prokesch S, editors. Insight Center: leading health care innovation. N Engl J Med/Harv Bus Rev. 2013 Oct 8. Available at http://images.nejm.org/editorial/supplementary/2013/hbr06-song.pdf.
41. McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA 2013;310:829–36.
42. Berenson RA. Shared savings program for accountable care organizations: a bridge to nowhere? Am J Manag Care 2010;16:721–6.
43. Meyer H. Accountable care organization prototypes: winners and losers? Health Aff (Millwood) 2011;30:1227–31.
44. Burns LR, Pauly MV. Accountable care organizations may have difficulty avoiding the failures of integrated delivery networks of the 1990s. Health Aff (Millwood) 2012;31:2407–16.
45. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
46. Colla CH, Lewis VA, Shortell SM, Fisher ES. First national survey of ACOs finds that physicians are playing strong leadership and ownership roles. Health Aff (Millwood) 2014;33:964–71.
47. Baicker K, Levy H. Coordination versus competition in health care reform. N Engl J Med 2013;369:789–91.
48. Baker LC, Bundorf MK, Kessler DP. Vertical integration: hospital ownership of physician practices is associated with higher prices and spending. Health Aff (Millwood). 2014;33:756–63.
49. Isaacs SL, Jellinek PS, Ray WL. The independent physician--going, going.... N Engl J Med 2009;360:655–7.
50. Kocher R, Sahni NR. Hospitals’ race to employ physicians--the logic behind a money-losing proposition. N Engl J Med 2011;364:1790–3.
51. Gold J. Hospitals lure doctors away from private practice. Kaiser Health News, October 12, 2010.
52. Accenture. Clinical transformation: new business models for a new era in healthcare. 2012. Available at www.accenture.com/SiteCollectionDocuments/PDF/Accenture-Clinical-Transformation-New-Business-Models-for-a-New-Era-in-Healthcare.pdf.
53. Fraud and abuse: Leibenluft RF. ACOs and the enforcement of fraud, abuse, and antitrust laws. N Engl J Med 2011;364:99–101.
54. Kreindler SA, Larson BK, Wu FM, et al. Interpretations of integration in early accountable care organizations. Milbank Q 2012;90:457–83.
55. Cassel CK, Reuben DB. Specialization, subspecialization, and subsubspecialization in internal medicine. N Engl J Med 2011;364:1169–73.
56. West CP, Dupras DM. General medicine vs subspecialty career plans among internal medicine residents. JAMA 2012;308:2241–7.
57. Schwartz MD, Durning S, Linzer M, Hauer KE. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med 2011;171:744-749.
58. Schwartz AL, Landon BE, Elshaug AG, et al. Measuring low-value care in Medicare. JAMA Intern Med 2014 May 12. [Epub ahead of print]
59. American Board of Internal Medicine Foundation. Choosing wisely: lists of five things physicians and patients should question. Accessed 26 May 2013 at www.choosingwisely.org/doctor-patient-lists/.
60. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
61. Lewis VA, Colla CH, Carluzzo KL, et al. Accountable care organizations in the United States: market and demographic factors associated with formation. Health Serv Res 2013;48(6 Pt 1):1840–58.
62. Conrad D, Grembowski D, Gibbons C, et al. A report on eight early-stage state and regional projects testing value-based payment. Health Aff (Millwood) 2013;32:998–1006.
1. Orszag PR, Ellis P. The challenge of rising health care costs—a view from the Congressional Budget Office. N Engl J Med 2007;357:1793–5.
2. Chernew ME, Hirth RA, Cutler DM. Increased spending on health care: long- term implications for the nation. Health Aff (Millwood) 2009;28:1253–5.
3. Congressional Budget Office. Financing projected spending in the long run. Washington, DC: Congressional Budget Office; 2007 Jul 9.
4. Financing projected spending in the long run: letter from Peter R. Orszag, director, Congressional Budget Office, to Senator Judd Gregg, 9 Jul 2007.
5. Baicker K, Chernew ME. The economics of financing Medicare. N Engl J Med 2011;28;365:e7.
6. Chernew ME, Baicker K, Hsu J. The specter of financial armageddon—health care and federal debt in the United States. N Engl J Med 2010;362:1166–8.
7. Newhouse JP. Assessing health reform’s impact on four key groups of Americans. Health Aff (Millwood). 2010;29:1714–24.
8. Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med 2012;367:949–54.
9. Antos JR, Pauly MV, Wilensky GR. Bending the cost curve through market-based incentives. N Engl J Med 2012;367:954–8.
10. Centers for Medicare and Medicaid Services. More partnerships between doctors and hospitals strengthen coordinated care for Medicare beneficiaries. 2013 Dec 23.
11. Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med 2011;365:2445–7.
12. Muhlestein D. Accountable care growth in 2014: a look ahead. Health Affairs Blog. 2014 Jan 29. Available at http://healthaffairs.org/blog/2014/01/29/accountable-care-growth-in-2014-a-look-ahead/.
13. Fisher ES, Shortell SM. Accountable care organizations: accountable for what, to whom, and how. JAMA 2010;304:1715–6.
14. Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood) 2007;26:w44–w57.
15. Rittenhouse DR, Shortell SM. The patient-centered medical home: will it stand the test of health reform? JAMA 2009;301:2038–40.
16. Bodenheimer T, Grumbach K, Berenson RA. A lifeline for primary care. N Engl J Med 2009;360:2693–6.
17. Centers for Medicare and Medicaid Services. Improving quality of care for Medicare patients: accountable care organizations. 2011 Oct 20.
18. Centers for Medicare and Medicaid Services. Press release: Pioneer accountable care organizations succeed in improving care, lowering costs. 2013 Jul 16. Available at www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html.
19. L&M Policy Research. Effect of Pioneer ACOs on Medicare spending in the first year. 2013 Nov 3. Available at http://innovation.cms.gov/Files/reports/PioneerACOEvalReport1.pdf.
20. Centers for Medicare and Medicaid Services. Performance year 1 interim results for ACOs that started in April and July 2012. 2014 Jan 30. Available at www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/PY1-InterimResultsTable.pdf.
21. Wilensky GR. Lessons from the Physician Group Practice Demonstration — a sobering reflection. N Engl J Med 2011;365:1659–61.
22. Colla CH, Wennberg DE, Meara E, et al. Spending differences associated with the Medicare Physician Group Practice Demonstration. JAMA 2012;308:1015–23.
23. Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program N Engl J Med 2011;364:e32.
24. Haywood TT, Kosel KC. The ACO model—a three-year financial loss? N Engl J Med 2011;364.
25. Iglehart JK. Assessing an ACO prototype—Medicare’s Physician Group Practice Demonstration. N Engl J Med 2011;364:198–200.
26. Chernew ME, Mechanic RE, Landon BE, Safran DG. Private-payer innovation in Massachusetts: the “Alternative Quality Contract.” Health Aff (Millwood). 2011;30:51–61.
27. Song Z, Safran DG, Landon BE, et al. Health care spending and quality in year 1 of the alternative quality contract. N Engl J Med 2011;365:909–18.
28. Song Z, Safran DG, Landon BE, et al. The ‘Alternative Quality Contract,’ based on a global budget, lowered medical spending and improved quality. Health Aff (Millwood). 2012;31:1885–94.
29. Song Z, Fendrick AM, Safran DG, et al. Global budgets and technology-intensive medical services. Healthcare (Amst) 2013;1:15–21.
30. Salmon RB, Sanderson MI, Walters BA, et al. A collaborative accountable care model in three practices showed promising early results on costs and quality of care. Health Aff (Millwood). 2012;31:2379–87.
31. Markovich P. A global budget pilot project among provider partners and Blue Shield of California led to savings in first two years. Health Aff (Millwood) 2012;31:1969–76.
32. Terhune C. Anthem, HealthCare Partners save $4.7 million by coordinating care. Los Angeles Times. 2014 Jun 6. Available at www.latimes.com/business/money/la-fi-anthem-healthcare-partners-20140605-story.html.
33. Gbemudu JN, Larson BK, Van Citters AD, et al. HealthCare Partners: building on a foundation of global risk management to achieve accountable care. Commonwealth Fund Pub. 1572, Vol 2. 2012 Jan.
34. Rice TH. The impact of changing Medicare reimbursement rates on physician- induced demand. Med Care 1983;21:803–15.
35. Nguyen NX, Derrick FW. Physician behavioral response to a Medicare price reduction. Health Serv Res 1997;32:283–98.
36. Yip WC. Physician response to Medicare fee reductions:changes in the volume of coronary artery bypass graft (CABG) surgeries in the Medicare and private sectors. J Health Econ 1998;17:675–99.
37. Song Z, Ayanian JZ, Wallace J, et al. Unintended consequences of eliminating medicare payments for consultations. JAMA Intern Med 2013;173:15–21.
38. Marquis MS, Rogowski JA, Escarce JJ. The managed care backlash: did consumers vote with their feet? Inquiry 2004-2005 Winter;41:376–90.
39. Cooper PF, Simon KI, Vistnes J. A closer look at the managed care backlash. Med Care 2006 May;44(5 Suppl):I4–11.
40. Song Z. Pioneer accountable care organizations: lessons from year 1. In: Curfman G, Morrissey S, Morse G, Prokesch S, editors. Insight Center: leading health care innovation. N Engl J Med/Harv Bus Rev. 2013 Oct 8. Available at http://images.nejm.org/editorial/supplementary/2013/hbr06-song.pdf.
41. McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA 2013;310:829–36.
42. Berenson RA. Shared savings program for accountable care organizations: a bridge to nowhere? Am J Manag Care 2010;16:721–6.
43. Meyer H. Accountable care organization prototypes: winners and losers? Health Aff (Millwood) 2011;30:1227–31.
44. Burns LR, Pauly MV. Accountable care organizations may have difficulty avoiding the failures of integrated delivery networks of the 1990s. Health Aff (Millwood) 2012;31:2407–16.
45. Song Z, Lee TH. The era of delivery system reform begins. JAMA 2013;309:35–6.
46. Colla CH, Lewis VA, Shortell SM, Fisher ES. First national survey of ACOs finds that physicians are playing strong leadership and ownership roles. Health Aff (Millwood) 2014;33:964–71.
47. Baicker K, Levy H. Coordination versus competition in health care reform. N Engl J Med 2013;369:789–91.
48. Baker LC, Bundorf MK, Kessler DP. Vertical integration: hospital ownership of physician practices is associated with higher prices and spending. Health Aff (Millwood). 2014;33:756–63.
49. Isaacs SL, Jellinek PS, Ray WL. The independent physician--going, going.... N Engl J Med 2009;360:655–7.
50. Kocher R, Sahni NR. Hospitals’ race to employ physicians--the logic behind a money-losing proposition. N Engl J Med 2011;364:1790–3.
51. Gold J. Hospitals lure doctors away from private practice. Kaiser Health News, October 12, 2010.
52. Accenture. Clinical transformation: new business models for a new era in healthcare. 2012. Available at www.accenture.com/SiteCollectionDocuments/PDF/Accenture-Clinical-Transformation-New-Business-Models-for-a-New-Era-in-Healthcare.pdf.
53. Fraud and abuse: Leibenluft RF. ACOs and the enforcement of fraud, abuse, and antitrust laws. N Engl J Med 2011;364:99–101.
54. Kreindler SA, Larson BK, Wu FM, et al. Interpretations of integration in early accountable care organizations. Milbank Q 2012;90:457–83.
55. Cassel CK, Reuben DB. Specialization, subspecialization, and subsubspecialization in internal medicine. N Engl J Med 2011;364:1169–73.
56. West CP, Dupras DM. General medicine vs subspecialty career plans among internal medicine residents. JAMA 2012;308:2241–7.
57. Schwartz MD, Durning S, Linzer M, Hauer KE. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med 2011;171:744-749.
58. Schwartz AL, Landon BE, Elshaug AG, et al. Measuring low-value care in Medicare. JAMA Intern Med 2014 May 12. [Epub ahead of print]
59. American Board of Internal Medicine Foundation. Choosing wisely: lists of five things physicians and patients should question. Accessed 26 May 2013 at www.choosingwisely.org/doctor-patient-lists/.
60. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
61. Lewis VA, Colla CH, Carluzzo KL, et al. Accountable care organizations in the United States: market and demographic factors associated with formation. Health Serv Res 2013;48(6 Pt 1):1840–58.
62. Conrad D, Grembowski D, Gibbons C, et al. A report on eight early-stage state and regional projects testing value-based payment. Health Aff (Millwood) 2013;32:998–1006.
Esophageal Cancer: Current Diagnosis and Management
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
Powered toothbrushes really are better than manual ones at plaque control
Maintaining close collaborative relationships with my dental colleagues is one of the many benefits of my primary care practice. I never cease to be amazed by how much my dental colleagues know about medicine and how little I know about dentistry. But I do ask my patients how frequently they see a dentist because it is a powerful marker for what I am going to find during the oral examination.
Many of my patients seem to have trouble maintaining their native teeth. This is surprising to me given the abundance of options for dental care; and yet, not surprising when I remember that caries is the most prevalent disease worldwide. Oral health has a huge potential impact on overall health, and the control of dental plaque is the key to oral health. I typically do not recommend toothbrushes to my patients who have identified dental disease, but I may start doing this now that I understand more about toothbrushes.
Powered toothbrushes clean teeth through a variety of mechanisms: side-to-side action, counter oscillation, rotation oscillation, circular, ultrasonic, and ionic, just to name a few. They are more expensive than regular toothbrushes, but are they better for removing plaque?
An updated systematic review of the literature has been published comparing powered versus manual toothbrushing for the maintenance of oral health. Trials were selected if they evaluated at least 4 weeks of unsupervised toothbrushing. Fifty-one trials involving 4,624 participants provided data for the meta-analysis (Cochrane Database Syst. Rev. 2014;6:CD002281 [doi:10.1002/14651858.CD002281.pub3]).
Powered toothbrushes provide a statistically significant benefit, compared with manual toothbrushes, for the reduction of plaque in both the short (1-3 months; 11% reduction) and long term (longer than 3 months; 21% reduction) over manual toothbrushes. Powered toothbrushes also provide a statistically significant benefit in the short and long term for reduction in gingivitis. Most of the evidence is for rotation oscillation brushes.
So now I can give my patients a useful tip for maintaining oral health. Does improved plaque removal translate into general health benefits? We are uncertain, but it will certainly make for more enjoyable oral examinations.
Dr. Ebbert is a professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. Dr. Ebbert reports no disclosures. The opinions expressed are his alone and should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Maintaining close collaborative relationships with my dental colleagues is one of the many benefits of my primary care practice. I never cease to be amazed by how much my dental colleagues know about medicine and how little I know about dentistry. But I do ask my patients how frequently they see a dentist because it is a powerful marker for what I am going to find during the oral examination.
Many of my patients seem to have trouble maintaining their native teeth. This is surprising to me given the abundance of options for dental care; and yet, not surprising when I remember that caries is the most prevalent disease worldwide. Oral health has a huge potential impact on overall health, and the control of dental plaque is the key to oral health. I typically do not recommend toothbrushes to my patients who have identified dental disease, but I may start doing this now that I understand more about toothbrushes.
Powered toothbrushes clean teeth through a variety of mechanisms: side-to-side action, counter oscillation, rotation oscillation, circular, ultrasonic, and ionic, just to name a few. They are more expensive than regular toothbrushes, but are they better for removing plaque?
An updated systematic review of the literature has been published comparing powered versus manual toothbrushing for the maintenance of oral health. Trials were selected if they evaluated at least 4 weeks of unsupervised toothbrushing. Fifty-one trials involving 4,624 participants provided data for the meta-analysis (Cochrane Database Syst. Rev. 2014;6:CD002281 [doi:10.1002/14651858.CD002281.pub3]).
Powered toothbrushes provide a statistically significant benefit, compared with manual toothbrushes, for the reduction of plaque in both the short (1-3 months; 11% reduction) and long term (longer than 3 months; 21% reduction) over manual toothbrushes. Powered toothbrushes also provide a statistically significant benefit in the short and long term for reduction in gingivitis. Most of the evidence is for rotation oscillation brushes.
So now I can give my patients a useful tip for maintaining oral health. Does improved plaque removal translate into general health benefits? We are uncertain, but it will certainly make for more enjoyable oral examinations.
Dr. Ebbert is a professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. Dr. Ebbert reports no disclosures. The opinions expressed are his alone and should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Maintaining close collaborative relationships with my dental colleagues is one of the many benefits of my primary care practice. I never cease to be amazed by how much my dental colleagues know about medicine and how little I know about dentistry. But I do ask my patients how frequently they see a dentist because it is a powerful marker for what I am going to find during the oral examination.
Many of my patients seem to have trouble maintaining their native teeth. This is surprising to me given the abundance of options for dental care; and yet, not surprising when I remember that caries is the most prevalent disease worldwide. Oral health has a huge potential impact on overall health, and the control of dental plaque is the key to oral health. I typically do not recommend toothbrushes to my patients who have identified dental disease, but I may start doing this now that I understand more about toothbrushes.
Powered toothbrushes clean teeth through a variety of mechanisms: side-to-side action, counter oscillation, rotation oscillation, circular, ultrasonic, and ionic, just to name a few. They are more expensive than regular toothbrushes, but are they better for removing plaque?
An updated systematic review of the literature has been published comparing powered versus manual toothbrushing for the maintenance of oral health. Trials were selected if they evaluated at least 4 weeks of unsupervised toothbrushing. Fifty-one trials involving 4,624 participants provided data for the meta-analysis (Cochrane Database Syst. Rev. 2014;6:CD002281 [doi:10.1002/14651858.CD002281.pub3]).
Powered toothbrushes provide a statistically significant benefit, compared with manual toothbrushes, for the reduction of plaque in both the short (1-3 months; 11% reduction) and long term (longer than 3 months; 21% reduction) over manual toothbrushes. Powered toothbrushes also provide a statistically significant benefit in the short and long term for reduction in gingivitis. Most of the evidence is for rotation oscillation brushes.
So now I can give my patients a useful tip for maintaining oral health. Does improved plaque removal translate into general health benefits? We are uncertain, but it will certainly make for more enjoyable oral examinations.
Dr. Ebbert is a professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. Dr. Ebbert reports no disclosures. The opinions expressed are his alone and should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Pediatric Hospital Medicine 2014: Over-Diagnosis Is Harming Children
Presenters
Eric Coon, Ricardo Quinonez, Alan Schroeder
Summary
One of PHM2014’s first breakout sessions, coming on the heels of Dr. Meuthing’s opening talk on reducing serious safety events, focused on the topic of over-diagnosis in pediatric HM and its contribution to patient harm. The first key point is the distinction between over-diagnosis and mis-diagnosis. Over-diagnosis is the identification of an abnormality where detection will not benefit the patient. This is different from mis-diagnosis or incorrect diagnosis. Over-diagnosis has grown over the years due to several causes, including our fear of missing a diagnosis and increasing use of screening tests.
The speakers outlined many, varied drivers of over-diagnosis, including physicians’ unawareness of over-diagnosis, physicians’ discomfort with uncertainty, system incentives such as fee for service which reimburses or rewards increased testing, quality measures based on usage and testing, a perceived imperative to use testing and technology because it is available, and physicians’ inherent belief in technology and its results.
The classic example of over-diagnosis in pediatrics is asymptomatic urinary screening for neuroblastomas, where studies showed an increase in testing, an increase in diagnosis, but no change in mortality. A current example is children receiving head CT scans for minor head trauma that can lead to a diagnosis of small asymptomatic head bleeds or non-displaced skull fractures, which can then lead to PICU admissions, transfers to higher level centers, prophylactic administration of anti-seizure medications, and repeat CT scans.
From the patient perspective, over-diagnosis can lead to unnecessary hospitalizations, inappropriate medications and treatments, and increased patient or parental anxiety secondary being given a label of a diagnosis or disease.
Key Takeaway
The bottom line for physicians to consider when ordering a test is not just does the patient benefit from detection or diagnosis, but also what is the potential harm?
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Presenters
Eric Coon, Ricardo Quinonez, Alan Schroeder
Summary
One of PHM2014’s first breakout sessions, coming on the heels of Dr. Meuthing’s opening talk on reducing serious safety events, focused on the topic of over-diagnosis in pediatric HM and its contribution to patient harm. The first key point is the distinction between over-diagnosis and mis-diagnosis. Over-diagnosis is the identification of an abnormality where detection will not benefit the patient. This is different from mis-diagnosis or incorrect diagnosis. Over-diagnosis has grown over the years due to several causes, including our fear of missing a diagnosis and increasing use of screening tests.
The speakers outlined many, varied drivers of over-diagnosis, including physicians’ unawareness of over-diagnosis, physicians’ discomfort with uncertainty, system incentives such as fee for service which reimburses or rewards increased testing, quality measures based on usage and testing, a perceived imperative to use testing and technology because it is available, and physicians’ inherent belief in technology and its results.
The classic example of over-diagnosis in pediatrics is asymptomatic urinary screening for neuroblastomas, where studies showed an increase in testing, an increase in diagnosis, but no change in mortality. A current example is children receiving head CT scans for minor head trauma that can lead to a diagnosis of small asymptomatic head bleeds or non-displaced skull fractures, which can then lead to PICU admissions, transfers to higher level centers, prophylactic administration of anti-seizure medications, and repeat CT scans.
From the patient perspective, over-diagnosis can lead to unnecessary hospitalizations, inappropriate medications and treatments, and increased patient or parental anxiety secondary being given a label of a diagnosis or disease.
Key Takeaway
The bottom line for physicians to consider when ordering a test is not just does the patient benefit from detection or diagnosis, but also what is the potential harm?
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Presenters
Eric Coon, Ricardo Quinonez, Alan Schroeder
Summary
One of PHM2014’s first breakout sessions, coming on the heels of Dr. Meuthing’s opening talk on reducing serious safety events, focused on the topic of over-diagnosis in pediatric HM and its contribution to patient harm. The first key point is the distinction between over-diagnosis and mis-diagnosis. Over-diagnosis is the identification of an abnormality where detection will not benefit the patient. This is different from mis-diagnosis or incorrect diagnosis. Over-diagnosis has grown over the years due to several causes, including our fear of missing a diagnosis and increasing use of screening tests.
The speakers outlined many, varied drivers of over-diagnosis, including physicians’ unawareness of over-diagnosis, physicians’ discomfort with uncertainty, system incentives such as fee for service which reimburses or rewards increased testing, quality measures based on usage and testing, a perceived imperative to use testing and technology because it is available, and physicians’ inherent belief in technology and its results.
The classic example of over-diagnosis in pediatrics is asymptomatic urinary screening for neuroblastomas, where studies showed an increase in testing, an increase in diagnosis, but no change in mortality. A current example is children receiving head CT scans for minor head trauma that can lead to a diagnosis of small asymptomatic head bleeds or non-displaced skull fractures, which can then lead to PICU admissions, transfers to higher level centers, prophylactic administration of anti-seizure medications, and repeat CT scans.
From the patient perspective, over-diagnosis can lead to unnecessary hospitalizations, inappropriate medications and treatments, and increased patient or parental anxiety secondary being given a label of a diagnosis or disease.
Key Takeaway
The bottom line for physicians to consider when ordering a test is not just does the patient benefit from detection or diagnosis, but also what is the potential harm?
James O’Callaghan is a clinical assistant professor of pediatrics at the University of Washington and a member of Team Hospitalist.
Pediatric Hospital Medicine 2014: Building Blocks in the Evolution of a Successful Distributed Hospitalist Program
Presenters
Dan Hale, MD, FAAP, and Elisabeth Schainker, MD, FAAP, The Floating Hospital for Children at Tufts Medical Center, Boston
Summary
"Master the basics of a good hospitalist program and keep revisiting your core values, and you will continue to have a high-quality and sustainable program,” said Dr. Dan Hale at the PHM14 workshop “Building Blocks in the Evolution of a Successful Distributed Hospitalist Program.”
Dr. Elisabeth Schainker, chief of hospitalist medicine at The Floating Hospital for Children at Tufts Medical Center in Boston, and Dr. Hale, a hospitalist at The Floating Hospital and site director of the Lawrence General Hospital affiliated pediatric hospitalist program, allowed participants to share their experiences in program development.
This workshop reviewed the fundamentals that programs should review before starting and also periodically after established. Program changes should be made as needed. The workshop used an assessment tool to evaluate the basic elements of the participants’ programs. The February 2014 article “Key Principles and Characteristics of an Effective Hospital Medicine Group” in the Journal of Hospital Medicine were used as a starting point for program self-evaluation. These “building blocks” include:
• Establish the rationale for the program and include all stakeholders;
• Financial expectations; • Define scope of practice;
• Nursing and referral physician collaboration;
• Assess staffing and workload expectations;
• Referral base; and
• Basic code and emergency preparedness.
Ongoing program development elements of a program were discussed as well. These components help further integrate a hospitalist program with the hospital as a whole and help add value. These ongoing “building blocks” include:
• Communication and collaboration with other hospital departments (emergency, radiology, surgery, etc.);
• Newborn medicine care;
• Internal group clinical practice guidelines;
• Co-management of surgical or specialty patients;
• Transfers from other hospitals or continuing care from tertiary care centers;
• Pediatric code teams and rapid response teams;
• Advanced code and emergency preparedness and mock code training; and
• Nursing education.
These additive features may be different at each program. Not all of these components are applicable or needed at all hospitals. Thoughtful approaches and thorough planning can create synergy with other components of a program. The essentials of a successful distributed network of multiple hospitalist program site were also described. After assuring the fundamentals are present at each site, transparency and institutional alignment are imperative.
Key Takeaways
1. It is important to understand several fundamental elements of hospitalist programs and address goals before starting a program.
2. For existing programs, it is important to review the fundamentals periodically and provide program maintenance.
3. After a program is established and fundamentals are in place, other important advance practices can be added on. These include ongoing collaboration, advanced emergency planning, staff education, and clinical practice guidelines.
4. For a multiple site or distributed program, high level collaboration and transparency is essential.
Dr. Hale is a past member of Team Hospitalist and is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Presenters
Dan Hale, MD, FAAP, and Elisabeth Schainker, MD, FAAP, The Floating Hospital for Children at Tufts Medical Center, Boston
Summary
"Master the basics of a good hospitalist program and keep revisiting your core values, and you will continue to have a high-quality and sustainable program,” said Dr. Dan Hale at the PHM14 workshop “Building Blocks in the Evolution of a Successful Distributed Hospitalist Program.”
Dr. Elisabeth Schainker, chief of hospitalist medicine at The Floating Hospital for Children at Tufts Medical Center in Boston, and Dr. Hale, a hospitalist at The Floating Hospital and site director of the Lawrence General Hospital affiliated pediatric hospitalist program, allowed participants to share their experiences in program development.
This workshop reviewed the fundamentals that programs should review before starting and also periodically after established. Program changes should be made as needed. The workshop used an assessment tool to evaluate the basic elements of the participants’ programs. The February 2014 article “Key Principles and Characteristics of an Effective Hospital Medicine Group” in the Journal of Hospital Medicine were used as a starting point for program self-evaluation. These “building blocks” include:
• Establish the rationale for the program and include all stakeholders;
• Financial expectations; • Define scope of practice;
• Nursing and referral physician collaboration;
• Assess staffing and workload expectations;
• Referral base; and
• Basic code and emergency preparedness.
Ongoing program development elements of a program were discussed as well. These components help further integrate a hospitalist program with the hospital as a whole and help add value. These ongoing “building blocks” include:
• Communication and collaboration with other hospital departments (emergency, radiology, surgery, etc.);
• Newborn medicine care;
• Internal group clinical practice guidelines;
• Co-management of surgical or specialty patients;
• Transfers from other hospitals or continuing care from tertiary care centers;
• Pediatric code teams and rapid response teams;
• Advanced code and emergency preparedness and mock code training; and
• Nursing education.
These additive features may be different at each program. Not all of these components are applicable or needed at all hospitals. Thoughtful approaches and thorough planning can create synergy with other components of a program. The essentials of a successful distributed network of multiple hospitalist program site were also described. After assuring the fundamentals are present at each site, transparency and institutional alignment are imperative.
Key Takeaways
1. It is important to understand several fundamental elements of hospitalist programs and address goals before starting a program.
2. For existing programs, it is important to review the fundamentals periodically and provide program maintenance.
3. After a program is established and fundamentals are in place, other important advance practices can be added on. These include ongoing collaboration, advanced emergency planning, staff education, and clinical practice guidelines.
4. For a multiple site or distributed program, high level collaboration and transparency is essential.
Dr. Hale is a past member of Team Hospitalist and is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Presenters
Dan Hale, MD, FAAP, and Elisabeth Schainker, MD, FAAP, The Floating Hospital for Children at Tufts Medical Center, Boston
Summary
"Master the basics of a good hospitalist program and keep revisiting your core values, and you will continue to have a high-quality and sustainable program,” said Dr. Dan Hale at the PHM14 workshop “Building Blocks in the Evolution of a Successful Distributed Hospitalist Program.”
Dr. Elisabeth Schainker, chief of hospitalist medicine at The Floating Hospital for Children at Tufts Medical Center in Boston, and Dr. Hale, a hospitalist at The Floating Hospital and site director of the Lawrence General Hospital affiliated pediatric hospitalist program, allowed participants to share their experiences in program development.
This workshop reviewed the fundamentals that programs should review before starting and also periodically after established. Program changes should be made as needed. The workshop used an assessment tool to evaluate the basic elements of the participants’ programs. The February 2014 article “Key Principles and Characteristics of an Effective Hospital Medicine Group” in the Journal of Hospital Medicine were used as a starting point for program self-evaluation. These “building blocks” include:
• Establish the rationale for the program and include all stakeholders;
• Financial expectations; • Define scope of practice;
• Nursing and referral physician collaboration;
• Assess staffing and workload expectations;
• Referral base; and
• Basic code and emergency preparedness.
Ongoing program development elements of a program were discussed as well. These components help further integrate a hospitalist program with the hospital as a whole and help add value. These ongoing “building blocks” include:
• Communication and collaboration with other hospital departments (emergency, radiology, surgery, etc.);
• Newborn medicine care;
• Internal group clinical practice guidelines;
• Co-management of surgical or specialty patients;
• Transfers from other hospitals or continuing care from tertiary care centers;
• Pediatric code teams and rapid response teams;
• Advanced code and emergency preparedness and mock code training; and
• Nursing education.
These additive features may be different at each program. Not all of these components are applicable or needed at all hospitals. Thoughtful approaches and thorough planning can create synergy with other components of a program. The essentials of a successful distributed network of multiple hospitalist program site were also described. After assuring the fundamentals are present at each site, transparency and institutional alignment are imperative.
Key Takeaways
1. It is important to understand several fundamental elements of hospitalist programs and address goals before starting a program.
2. For existing programs, it is important to review the fundamentals periodically and provide program maintenance.
3. After a program is established and fundamentals are in place, other important advance practices can be added on. These include ongoing collaboration, advanced emergency planning, staff education, and clinical practice guidelines.
4. For a multiple site or distributed program, high level collaboration and transparency is essential.
Dr. Hale is a past member of Team Hospitalist and is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Intergluteal Itching in Need of Relief
ANSWER
Admittedly, this is a bit of a trick question—but with a good teaching point to make. A course of oral fluconazole (choice “a”) is futile, since there’s no reason to think this problem is yeast-driven and since the patient has already demonstrated a lack of response to topical imidazoles.
Punch biopsy (choice “b”) would be a good choice, but not in this area, where it could quickly become a bigger problem than the one the patient presented with. Sutures would not likely hold the biopsy wound together, and resultant infection is all too likely.
A KOH test to detect fungal or yeast elements (choice “c”) is unlikely to shed any light on the problem, given the lack of response to antifungal creams. Finally, there’s no reason to suspect a bacterial origin, so oral antibiotics such as cephalexin (choice “d”) would be useless (and had already been tried unsuccessfully).
The correct answer is none of the above (choice “e”).
DISCUSSION
This case illustrates why dermatology seems so maddeningly difficult to the uninitiated. Any experienced derm provider would know the correct diagnosis, lichen sclerosus et atrophicus (LS&A), because it presents in such a distinctive way (in limited locations, predominantly in women) and because the differential is so limited. But if you’ve never heard of LS&A, you’re unlikely to diagnose it, let alone know how to treat it.
LS&A is an inflammatory condition of unknown origin that affects the upper epidermis. It can present in extragenital locations (particularly shoulders and legs) but is far more common in genital areas. As exhibited in this case, it presents with well-defined pigment loss, which is especially easy to see in patients with darker skin.
Although more commonly seen in women, LS&A can occur in men, usually manifesting on the penile glans and distal foreskin of uncircumcised patients. The dry atrophic changes seen on the glans can lead to stenosis of the urethral meatus and, proximally, to adhesions (phimosis) of the foreskin. (This condition was termed balanitis xerotica obliterans [BXO] long before its pathologic process was determined to be identical to LS&A’s. Tissue specimens obtained during circumcisions performed for chronic phimosis often yield evidence of BXO.)
In women, untreated chronic LS&A can lead to sclerotic changes in and around the urethra and labia minora and can cause introital stenosis. This case is a bit atypical; LS&A more often manifests in perivaginal and perirectal areas, where the intense hypopigmentation produces a classic “figure eight” appearance.
The differential includes lichen simplex chronicus, psoriasis, lichen planus, contact/irritant dermatitis, and seborrhea. Often, biopsy is necessary and appropriate to settle the issue, other factors being equal.
TREATMENT/PROGNOSIS
The patient was given a prescription for clobetasol 0.05% ointment for twice-daily application Monday through Friday (and no application for two consecutive days—in this case, the weekend—per week). Studies have established the efficacy and safety of this treatment regimen.
In a month or two, application can be reduced to once or twice a week to control the condition.
ANSWER
Admittedly, this is a bit of a trick question—but with a good teaching point to make. A course of oral fluconazole (choice “a”) is futile, since there’s no reason to think this problem is yeast-driven and since the patient has already demonstrated a lack of response to topical imidazoles.
Punch biopsy (choice “b”) would be a good choice, but not in this area, where it could quickly become a bigger problem than the one the patient presented with. Sutures would not likely hold the biopsy wound together, and resultant infection is all too likely.
A KOH test to detect fungal or yeast elements (choice “c”) is unlikely to shed any light on the problem, given the lack of response to antifungal creams. Finally, there’s no reason to suspect a bacterial origin, so oral antibiotics such as cephalexin (choice “d”) would be useless (and had already been tried unsuccessfully).
The correct answer is none of the above (choice “e”).
DISCUSSION
This case illustrates why dermatology seems so maddeningly difficult to the uninitiated. Any experienced derm provider would know the correct diagnosis, lichen sclerosus et atrophicus (LS&A), because it presents in such a distinctive way (in limited locations, predominantly in women) and because the differential is so limited. But if you’ve never heard of LS&A, you’re unlikely to diagnose it, let alone know how to treat it.
LS&A is an inflammatory condition of unknown origin that affects the upper epidermis. It can present in extragenital locations (particularly shoulders and legs) but is far more common in genital areas. As exhibited in this case, it presents with well-defined pigment loss, which is especially easy to see in patients with darker skin.
Although more commonly seen in women, LS&A can occur in men, usually manifesting on the penile glans and distal foreskin of uncircumcised patients. The dry atrophic changes seen on the glans can lead to stenosis of the urethral meatus and, proximally, to adhesions (phimosis) of the foreskin. (This condition was termed balanitis xerotica obliterans [BXO] long before its pathologic process was determined to be identical to LS&A’s. Tissue specimens obtained during circumcisions performed for chronic phimosis often yield evidence of BXO.)
In women, untreated chronic LS&A can lead to sclerotic changes in and around the urethra and labia minora and can cause introital stenosis. This case is a bit atypical; LS&A more often manifests in perivaginal and perirectal areas, where the intense hypopigmentation produces a classic “figure eight” appearance.
The differential includes lichen simplex chronicus, psoriasis, lichen planus, contact/irritant dermatitis, and seborrhea. Often, biopsy is necessary and appropriate to settle the issue, other factors being equal.
TREATMENT/PROGNOSIS
The patient was given a prescription for clobetasol 0.05% ointment for twice-daily application Monday through Friday (and no application for two consecutive days—in this case, the weekend—per week). Studies have established the efficacy and safety of this treatment regimen.
In a month or two, application can be reduced to once or twice a week to control the condition.
ANSWER
Admittedly, this is a bit of a trick question—but with a good teaching point to make. A course of oral fluconazole (choice “a”) is futile, since there’s no reason to think this problem is yeast-driven and since the patient has already demonstrated a lack of response to topical imidazoles.
Punch biopsy (choice “b”) would be a good choice, but not in this area, where it could quickly become a bigger problem than the one the patient presented with. Sutures would not likely hold the biopsy wound together, and resultant infection is all too likely.
A KOH test to detect fungal or yeast elements (choice “c”) is unlikely to shed any light on the problem, given the lack of response to antifungal creams. Finally, there’s no reason to suspect a bacterial origin, so oral antibiotics such as cephalexin (choice “d”) would be useless (and had already been tried unsuccessfully).
The correct answer is none of the above (choice “e”).
DISCUSSION
This case illustrates why dermatology seems so maddeningly difficult to the uninitiated. Any experienced derm provider would know the correct diagnosis, lichen sclerosus et atrophicus (LS&A), because it presents in such a distinctive way (in limited locations, predominantly in women) and because the differential is so limited. But if you’ve never heard of LS&A, you’re unlikely to diagnose it, let alone know how to treat it.
LS&A is an inflammatory condition of unknown origin that affects the upper epidermis. It can present in extragenital locations (particularly shoulders and legs) but is far more common in genital areas. As exhibited in this case, it presents with well-defined pigment loss, which is especially easy to see in patients with darker skin.
Although more commonly seen in women, LS&A can occur in men, usually manifesting on the penile glans and distal foreskin of uncircumcised patients. The dry atrophic changes seen on the glans can lead to stenosis of the urethral meatus and, proximally, to adhesions (phimosis) of the foreskin. (This condition was termed balanitis xerotica obliterans [BXO] long before its pathologic process was determined to be identical to LS&A’s. Tissue specimens obtained during circumcisions performed for chronic phimosis often yield evidence of BXO.)
In women, untreated chronic LS&A can lead to sclerotic changes in and around the urethra and labia minora and can cause introital stenosis. This case is a bit atypical; LS&A more often manifests in perivaginal and perirectal areas, where the intense hypopigmentation produces a classic “figure eight” appearance.
The differential includes lichen simplex chronicus, psoriasis, lichen planus, contact/irritant dermatitis, and seborrhea. Often, biopsy is necessary and appropriate to settle the issue, other factors being equal.
TREATMENT/PROGNOSIS
The patient was given a prescription for clobetasol 0.05% ointment for twice-daily application Monday through Friday (and no application for two consecutive days—in this case, the weekend—per week). Studies have established the efficacy and safety of this treatment regimen.
In a month or two, application can be reduced to once or twice a week to control the condition.

For almost a year, a 55-year-old African-American woman has experienced itchy skin changes in her perianal area. Treatment attempts with several topical creams—including clotrimazole, combination clotrimazole/betamethasone, and ketoconazole—have not helped. The patient has seen several primary care providers for the problem. All have told her that it was yeast-related, except the last clinician, who suspected psoriasis. When the topical medication prescribed by that provider did not yield a resolution, the patient decided to consult dermatology. Due to her lack of insurance, she had to wait four months to see a derm clinician, since her only option was a once-a-month free clinic in her community. Aside from mild hypertension, the patient claims to be in good health. Recent work-up indicated she does not have diabetes. She denies any family history of skin diseases, including psoriasis. She has had no previous complaints regarding her vaginal/perivaginal areas. The patient’s type V skin is free of notable changes except in the intergluteal and perianal areas. Specifically, no rash is noted on her extensor elbows or knees or in her scalp, and there are no changes in her fingernails. When the patient lies on her left side, extending her left leg and bringing her right knee toward her chest, the entire intergluteal and perianal areas can be visualized. Distinct loss of dark pigment is seen in the upper intergluteal/lower coccygeal areas. Closer inspection reveals that the pigment loss is complete, giving the affected skin a porcelain-like white appearance that also seems moderately atrophic. Palpation confirms this impression. No such changes are noted in the perianal or perineal areas. However, there is diffuse hyperpigmentation, as well as signs of mild chronic excoriation.