User login
Surgery for Persistent Knee Pain? Not So Fast
PRACTICE CHANGER
Do not refer patients with a degenerative medial meniscus tear for arthroscopic partial meniscectomy, because surgery outcomes are no better than those of conservative treatment.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.1
Illustrative case
A 40-year-old man comes to your office for follow-up of medial left knee pain he’s had for three months that hasn’t responded to conservative treatment. The pain developed gradually, without a history of trauma. The patient has no signs of degenerative joint disease on x-ray, but MRI reveals a tear of the medial meniscus. Should you refer him for meniscectomy?
Study summary>>
Patients and doctors alike tend to look for a treatment that will “fix” the problem, which may be why we have continued to use arthroscopic partial meniscectomy to attempt to relieve symptoms of meniscal tears despite a lack of evidence to support the practice.
Guidelines from the American Academy of Orthopaedic Surgeons state that the evidence for medial meniscectomy in patients with a torn meniscus and osteoarthritis (OA) is inconclusive; the organization offers no guidelines for patients with a torn meniscus who don’t have OA.2 The American College of Occupational and Environmental Medicine states that there is insufficient evidence to support arthroscopic partial meniscectomy for symptomatic, torn medial menisci for select patients and “the vast majority of patients [with medial meniscal tears] do not require surgery.”3 Previous studies have concluded that arthroscopic surgery for OA of the knee provides no additional benefit to optimized physical and medical therapy.4 Furthermore, research by Katz et al5 shows that meniscectomy provides no benefit over conservative treatment in functional status at six months in patients with OA and a medial meniscal tear.
That said, arthroscopic partial meniscectomy is still the most common orthopedic procedure in the United States.1 Although its use has decreased in the past 15 years, it is performed nearly 700,000 times annually at a cost of approximately $4 billion.1,6,7 Like any surgical procedure, meniscectomy carries a risk for complications. In the double-blind, randomized trial reported on here, Sihvonen et al1 compared meniscectomy to a sham procedure for patients with knee pain but not OA.
STUDY SUMMARY
Meniscectomy and shamsurgery are equally effective
Sihvonen et al1 conducted a randomized, double-blind, sham-controlled trial at five orthopedic clinics in Finland. Patients ages 35 to 65 were enrolled if they had clinical findings of a medial meniscus tear and knee pain for more than three months that wasn’t relieved by conservative treatment. The trial excluded patients who had an obvious traumatic onset of symptoms; clinical or radiologic evidence of knee OA; a locked knee that could not be straightened; knee instability or decreased range of motion; previous surgery on the affected knee; fracture within the past 12 months on the affected limb; or other notable pathology on MRI or during arthroscopy.
Before randomization, 160 patients underwent diagnostic arthroscopy. Fourteen patients were excluded: six because they did not actually have a medial meniscal tear, one because he also had a lateral meniscus tear, three due to a major chondral flap, two who had already undergone meniscal repair, and two due to an osteochondral microfracture.
At the end of the diagnostic arthroscopy, each patient was blindly randomized to arthroscopic partial meniscectomy or sham surgery. To simulate the meniscectomy procedure, the surgeon similarly manipulated the knee, made comparable noise and vibration using tools and suction, and ensured that the patient was kept in the operating room (OR) for a comparable time. Only the orthopedic surgeon and OR staff were aware of which surgery the patient underwent, and these staff members were not included in further treatment or follow-up. After the procedure, all patients received the same walking aids and instructions for a graduated exercise program.
The 70 patients in the meniscectomy group and the 76 in the sham surgery group were similar in age (mean: 52 years), sex, BMI, and duration of pain (mean: 10 months). Patients in both groups also had similar tears noted on arthroscopy.
Three primary outcomes were measured before surgery and at 12 months: knee pain, knee symptoms and function, and quality of life (QoL). Knee pain after exercise was evaluated on a scale of 0 to 10, with 0 indicating no pain. The validated Lysholm knee score was used to assess knee symptoms and function, and the Western Ontario Meniscal Evaluation Tool (WOMET) was utilized to evaluate QoL; both are 100-point scales in which lower scores indicate more severe symptoms.
Both groups had marked improvement in pain and function from baseline to 12 months, and there was no significant difference between the two groups. Knee pain scores improved by 3.1 points in the meniscectomy group and 3.3 points in the sham surgery group. Lysholm symptom and function scores improved 21.7 points in the meniscectomy group and 23.3 points in the sham surgery group (a change of 11.5 points would have been considered clinically significant). The mean between-group difference was –1.6 points.
WOMET QoL scores improved 24.6 points in the meniscectomy group and 27.1 points in the sham surgery group (a change of 15.5 points would have been considered clinically significant). The mean between-group difference was –2.5 points.
There were no significant between-group differences in serious adverse events or number of patients who required subsequent knee surgery. Similar proportions in each group thought they had sham surgery, which confirmed the effectiveness of the blinding. Ninety-six percent of patients in the sham procedure group and 93% in the meniscectomy group reported they would be willing to repeat the procedure.
What's new and challenges to implementation >>
WHAT’S NEW
Recommend physical therapy, exercise instead of surgery
Previous studies of arthroscopic partial meniscectomy to treat degenerative meniscal tears in patients with knee OA found no benefit.6,8 This study specifically examined patients without OA and found arthroscopic partial meniscectomy offered no benefit over sham surgery.
In addition to fewer referrals for meniscectomy, these findings could lead to another change in practice: Clinicians may be less likely to order MRI to confirm the diagnosis of a medial meniscal tear, since doing so will not change their therapeutic approach. This approach centers on recommending that patients with a degenerative meniscal tear start and stick with physical therapy and their designated exercise regimen.
CAVEATS
Surgery might be effective for more active patients
This study, as well as previous research, did not look at surgery for an acute medial meniscus tear following a traumatic incident, such as a fall or direct blow. Additionally, these results are based on improved outcomes in activities of daily living, and may not extend to patients who engage in high-level functioning, such as sports or strenuous work. The sham surgery group received lavage, which could be considered an active treatment, although a previous trial found lavage had no benefit over conservative treatment in patients with knee OA.4
CHALLENGES TO IMPLEMENTATION
It might be hard to convince patients they don’t need surgery
Some patients expect immediate intervention with surgery. It may be difficult to convince such patients that active participation in physical therapy can lead to the same outcomes as surgery. Spending time with your patient to explain the injury, what happens during surgery, and the evidence that shows a lack of difference in outcomes can lead to fewer surgeries. Most patients and clinicians will want to do an MRI after three months of persistent pain to determine the diagnosis, although some may be comfortable with continuing conservative treatment.
References
1. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013; 369:2515-2524.
2. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-Based Guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2013.
3. Knee disorders. In: Hegmann KT, ed. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-503.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial for arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008; 359:1097-1107.
5. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
6. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health State Report. 2009;11:1-25.
7. Salzler MJ, Lin A, Miller CD, et al. Complications after arthroscopic knee surgery. Am J Sports Med. 2014;42:292-296.
8. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002; 347:81-88.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(9):534-536.
PRACTICE CHANGER
Do not refer patients with a degenerative medial meniscus tear for arthroscopic partial meniscectomy, because surgery outcomes are no better than those of conservative treatment.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.1
Illustrative case
A 40-year-old man comes to your office for follow-up of medial left knee pain he’s had for three months that hasn’t responded to conservative treatment. The pain developed gradually, without a history of trauma. The patient has no signs of degenerative joint disease on x-ray, but MRI reveals a tear of the medial meniscus. Should you refer him for meniscectomy?
Study summary>>
Patients and doctors alike tend to look for a treatment that will “fix” the problem, which may be why we have continued to use arthroscopic partial meniscectomy to attempt to relieve symptoms of meniscal tears despite a lack of evidence to support the practice.
Guidelines from the American Academy of Orthopaedic Surgeons state that the evidence for medial meniscectomy in patients with a torn meniscus and osteoarthritis (OA) is inconclusive; the organization offers no guidelines for patients with a torn meniscus who don’t have OA.2 The American College of Occupational and Environmental Medicine states that there is insufficient evidence to support arthroscopic partial meniscectomy for symptomatic, torn medial menisci for select patients and “the vast majority of patients [with medial meniscal tears] do not require surgery.”3 Previous studies have concluded that arthroscopic surgery for OA of the knee provides no additional benefit to optimized physical and medical therapy.4 Furthermore, research by Katz et al5 shows that meniscectomy provides no benefit over conservative treatment in functional status at six months in patients with OA and a medial meniscal tear.
That said, arthroscopic partial meniscectomy is still the most common orthopedic procedure in the United States.1 Although its use has decreased in the past 15 years, it is performed nearly 700,000 times annually at a cost of approximately $4 billion.1,6,7 Like any surgical procedure, meniscectomy carries a risk for complications. In the double-blind, randomized trial reported on here, Sihvonen et al1 compared meniscectomy to a sham procedure for patients with knee pain but not OA.
STUDY SUMMARY
Meniscectomy and shamsurgery are equally effective
Sihvonen et al1 conducted a randomized, double-blind, sham-controlled trial at five orthopedic clinics in Finland. Patients ages 35 to 65 were enrolled if they had clinical findings of a medial meniscus tear and knee pain for more than three months that wasn’t relieved by conservative treatment. The trial excluded patients who had an obvious traumatic onset of symptoms; clinical or radiologic evidence of knee OA; a locked knee that could not be straightened; knee instability or decreased range of motion; previous surgery on the affected knee; fracture within the past 12 months on the affected limb; or other notable pathology on MRI or during arthroscopy.
Before randomization, 160 patients underwent diagnostic arthroscopy. Fourteen patients were excluded: six because they did not actually have a medial meniscal tear, one because he also had a lateral meniscus tear, three due to a major chondral flap, two who had already undergone meniscal repair, and two due to an osteochondral microfracture.
At the end of the diagnostic arthroscopy, each patient was blindly randomized to arthroscopic partial meniscectomy or sham surgery. To simulate the meniscectomy procedure, the surgeon similarly manipulated the knee, made comparable noise and vibration using tools and suction, and ensured that the patient was kept in the operating room (OR) for a comparable time. Only the orthopedic surgeon and OR staff were aware of which surgery the patient underwent, and these staff members were not included in further treatment or follow-up. After the procedure, all patients received the same walking aids and instructions for a graduated exercise program.
The 70 patients in the meniscectomy group and the 76 in the sham surgery group were similar in age (mean: 52 years), sex, BMI, and duration of pain (mean: 10 months). Patients in both groups also had similar tears noted on arthroscopy.
Three primary outcomes were measured before surgery and at 12 months: knee pain, knee symptoms and function, and quality of life (QoL). Knee pain after exercise was evaluated on a scale of 0 to 10, with 0 indicating no pain. The validated Lysholm knee score was used to assess knee symptoms and function, and the Western Ontario Meniscal Evaluation Tool (WOMET) was utilized to evaluate QoL; both are 100-point scales in which lower scores indicate more severe symptoms.
Both groups had marked improvement in pain and function from baseline to 12 months, and there was no significant difference between the two groups. Knee pain scores improved by 3.1 points in the meniscectomy group and 3.3 points in the sham surgery group. Lysholm symptom and function scores improved 21.7 points in the meniscectomy group and 23.3 points in the sham surgery group (a change of 11.5 points would have been considered clinically significant). The mean between-group difference was –1.6 points.
WOMET QoL scores improved 24.6 points in the meniscectomy group and 27.1 points in the sham surgery group (a change of 15.5 points would have been considered clinically significant). The mean between-group difference was –2.5 points.
There were no significant between-group differences in serious adverse events or number of patients who required subsequent knee surgery. Similar proportions in each group thought they had sham surgery, which confirmed the effectiveness of the blinding. Ninety-six percent of patients in the sham procedure group and 93% in the meniscectomy group reported they would be willing to repeat the procedure.
What's new and challenges to implementation >>
WHAT’S NEW
Recommend physical therapy, exercise instead of surgery
Previous studies of arthroscopic partial meniscectomy to treat degenerative meniscal tears in patients with knee OA found no benefit.6,8 This study specifically examined patients without OA and found arthroscopic partial meniscectomy offered no benefit over sham surgery.
In addition to fewer referrals for meniscectomy, these findings could lead to another change in practice: Clinicians may be less likely to order MRI to confirm the diagnosis of a medial meniscal tear, since doing so will not change their therapeutic approach. This approach centers on recommending that patients with a degenerative meniscal tear start and stick with physical therapy and their designated exercise regimen.
CAVEATS
Surgery might be effective for more active patients
This study, as well as previous research, did not look at surgery for an acute medial meniscus tear following a traumatic incident, such as a fall or direct blow. Additionally, these results are based on improved outcomes in activities of daily living, and may not extend to patients who engage in high-level functioning, such as sports or strenuous work. The sham surgery group received lavage, which could be considered an active treatment, although a previous trial found lavage had no benefit over conservative treatment in patients with knee OA.4
CHALLENGES TO IMPLEMENTATION
It might be hard to convince patients they don’t need surgery
Some patients expect immediate intervention with surgery. It may be difficult to convince such patients that active participation in physical therapy can lead to the same outcomes as surgery. Spending time with your patient to explain the injury, what happens during surgery, and the evidence that shows a lack of difference in outcomes can lead to fewer surgeries. Most patients and clinicians will want to do an MRI after three months of persistent pain to determine the diagnosis, although some may be comfortable with continuing conservative treatment.
References
1. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013; 369:2515-2524.
2. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-Based Guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2013.
3. Knee disorders. In: Hegmann KT, ed. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-503.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial for arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008; 359:1097-1107.
5. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
6. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health State Report. 2009;11:1-25.
7. Salzler MJ, Lin A, Miller CD, et al. Complications after arthroscopic knee surgery. Am J Sports Med. 2014;42:292-296.
8. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002; 347:81-88.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(9):534-536.
PRACTICE CHANGER
Do not refer patients with a degenerative medial meniscus tear for arthroscopic partial meniscectomy, because surgery outcomes are no better than those of conservative treatment.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.1
Illustrative case
A 40-year-old man comes to your office for follow-up of medial left knee pain he’s had for three months that hasn’t responded to conservative treatment. The pain developed gradually, without a history of trauma. The patient has no signs of degenerative joint disease on x-ray, but MRI reveals a tear of the medial meniscus. Should you refer him for meniscectomy?
Study summary>>
Patients and doctors alike tend to look for a treatment that will “fix” the problem, which may be why we have continued to use arthroscopic partial meniscectomy to attempt to relieve symptoms of meniscal tears despite a lack of evidence to support the practice.
Guidelines from the American Academy of Orthopaedic Surgeons state that the evidence for medial meniscectomy in patients with a torn meniscus and osteoarthritis (OA) is inconclusive; the organization offers no guidelines for patients with a torn meniscus who don’t have OA.2 The American College of Occupational and Environmental Medicine states that there is insufficient evidence to support arthroscopic partial meniscectomy for symptomatic, torn medial menisci for select patients and “the vast majority of patients [with medial meniscal tears] do not require surgery.”3 Previous studies have concluded that arthroscopic surgery for OA of the knee provides no additional benefit to optimized physical and medical therapy.4 Furthermore, research by Katz et al5 shows that meniscectomy provides no benefit over conservative treatment in functional status at six months in patients with OA and a medial meniscal tear.
That said, arthroscopic partial meniscectomy is still the most common orthopedic procedure in the United States.1 Although its use has decreased in the past 15 years, it is performed nearly 700,000 times annually at a cost of approximately $4 billion.1,6,7 Like any surgical procedure, meniscectomy carries a risk for complications. In the double-blind, randomized trial reported on here, Sihvonen et al1 compared meniscectomy to a sham procedure for patients with knee pain but not OA.
STUDY SUMMARY
Meniscectomy and shamsurgery are equally effective
Sihvonen et al1 conducted a randomized, double-blind, sham-controlled trial at five orthopedic clinics in Finland. Patients ages 35 to 65 were enrolled if they had clinical findings of a medial meniscus tear and knee pain for more than three months that wasn’t relieved by conservative treatment. The trial excluded patients who had an obvious traumatic onset of symptoms; clinical or radiologic evidence of knee OA; a locked knee that could not be straightened; knee instability or decreased range of motion; previous surgery on the affected knee; fracture within the past 12 months on the affected limb; or other notable pathology on MRI or during arthroscopy.
Before randomization, 160 patients underwent diagnostic arthroscopy. Fourteen patients were excluded: six because they did not actually have a medial meniscal tear, one because he also had a lateral meniscus tear, three due to a major chondral flap, two who had already undergone meniscal repair, and two due to an osteochondral microfracture.
At the end of the diagnostic arthroscopy, each patient was blindly randomized to arthroscopic partial meniscectomy or sham surgery. To simulate the meniscectomy procedure, the surgeon similarly manipulated the knee, made comparable noise and vibration using tools and suction, and ensured that the patient was kept in the operating room (OR) for a comparable time. Only the orthopedic surgeon and OR staff were aware of which surgery the patient underwent, and these staff members were not included in further treatment or follow-up. After the procedure, all patients received the same walking aids and instructions for a graduated exercise program.
The 70 patients in the meniscectomy group and the 76 in the sham surgery group were similar in age (mean: 52 years), sex, BMI, and duration of pain (mean: 10 months). Patients in both groups also had similar tears noted on arthroscopy.
Three primary outcomes were measured before surgery and at 12 months: knee pain, knee symptoms and function, and quality of life (QoL). Knee pain after exercise was evaluated on a scale of 0 to 10, with 0 indicating no pain. The validated Lysholm knee score was used to assess knee symptoms and function, and the Western Ontario Meniscal Evaluation Tool (WOMET) was utilized to evaluate QoL; both are 100-point scales in which lower scores indicate more severe symptoms.
Both groups had marked improvement in pain and function from baseline to 12 months, and there was no significant difference between the two groups. Knee pain scores improved by 3.1 points in the meniscectomy group and 3.3 points in the sham surgery group. Lysholm symptom and function scores improved 21.7 points in the meniscectomy group and 23.3 points in the sham surgery group (a change of 11.5 points would have been considered clinically significant). The mean between-group difference was –1.6 points.
WOMET QoL scores improved 24.6 points in the meniscectomy group and 27.1 points in the sham surgery group (a change of 15.5 points would have been considered clinically significant). The mean between-group difference was –2.5 points.
There were no significant between-group differences in serious adverse events or number of patients who required subsequent knee surgery. Similar proportions in each group thought they had sham surgery, which confirmed the effectiveness of the blinding. Ninety-six percent of patients in the sham procedure group and 93% in the meniscectomy group reported they would be willing to repeat the procedure.
What's new and challenges to implementation >>
WHAT’S NEW
Recommend physical therapy, exercise instead of surgery
Previous studies of arthroscopic partial meniscectomy to treat degenerative meniscal tears in patients with knee OA found no benefit.6,8 This study specifically examined patients without OA and found arthroscopic partial meniscectomy offered no benefit over sham surgery.
In addition to fewer referrals for meniscectomy, these findings could lead to another change in practice: Clinicians may be less likely to order MRI to confirm the diagnosis of a medial meniscal tear, since doing so will not change their therapeutic approach. This approach centers on recommending that patients with a degenerative meniscal tear start and stick with physical therapy and their designated exercise regimen.
CAVEATS
Surgery might be effective for more active patients
This study, as well as previous research, did not look at surgery for an acute medial meniscus tear following a traumatic incident, such as a fall or direct blow. Additionally, these results are based on improved outcomes in activities of daily living, and may not extend to patients who engage in high-level functioning, such as sports or strenuous work. The sham surgery group received lavage, which could be considered an active treatment, although a previous trial found lavage had no benefit over conservative treatment in patients with knee OA.4
CHALLENGES TO IMPLEMENTATION
It might be hard to convince patients they don’t need surgery
Some patients expect immediate intervention with surgery. It may be difficult to convince such patients that active participation in physical therapy can lead to the same outcomes as surgery. Spending time with your patient to explain the injury, what happens during surgery, and the evidence that shows a lack of difference in outcomes can lead to fewer surgeries. Most patients and clinicians will want to do an MRI after three months of persistent pain to determine the diagnosis, although some may be comfortable with continuing conservative treatment.
References
1. Sihvonen R, Paavola M, Malmivaara A, et al; Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013; 369:2515-2524.
2. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee. Evidence-Based Guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2013.
3. Knee disorders. In: Hegmann KT, ed. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-503.
4. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial for arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008; 359:1097-1107.
5. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
6. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health State Report. 2009;11:1-25.
7. Salzler MJ, Lin A, Miller CD, et al. Complications after arthroscopic knee surgery. Am J Sports Med. 2014;42:292-296.
8. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002; 347:81-88.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(9):534-536.
Patient Experience Data Are Outcomes
From Press Ganey Associates, Inc., Wakefield, MA, and Harvard Medical School and Harvard School of Public Health, Boston, MA.
In fits and starts, but with increasing certainty, health care is changing its organizational focus from the activities of clinicians to meeting the needs of patients. That progress has been slowed and complicated, however, by lack of a performance framework that captures the extent to which patients’ needs are being met. In the absence of such data, “quality” has often been described in terms of the reliability of providers in complying with evidence-based guidelines.
Provider reliability is important, of course, but no substitute for the measurement and improvement of actual patient outcomes. After all, health care exists not to grade providers but to help patients. But clarity on that focus raises some important questions that we have not often discussed in the past.
What exactly are we trying to accomplish in health care? What is the goal? How can we tell how we are doing?
The answers cannot be found solely through measurement of “hard” clinical outcomes, such as death and disability. Yes, these are the most important focuses for improvement in health care, but we cannot deliver immortality, and we often can only delay complications of chronic disease. There is something else that our patients seek from health care, and it can only be measured by asking them directly.
Measuring "Peace of Mind"
That “something else” was described by one of my colleagues as “peace of mind that things are as good as they can be, given the cards that we have been dealt.” That may not be the most compact term in health policy, but I have yet to come up with something more concise— and it captures something immediately recognizable as important to anyone who has ever taken care of a patient with a serious disease, or spoken to that patient’s family. Of course, we should do our best to help patients live as long as possible, and of course we should optimize their health and relieve symptoms at every step of the way.
But there are other things that influence the degree of suffering that patients endure along that way, like hope, trust, anxiety, fear, and confusion. They are often considered part of the “art of medicine,” but I think we call them “art” because we are nervous about approaching them with discipline and rigor. In fact, these things can be measured, and managed—and the organizations that improve them are often rewarded with greater market share and professional pride.
Some of my clinician-colleagues do not immediately think of patients’ “peace of mind” as an important outcome, but when their family members or friends have medical problems, this issue immediately comes to the foreground. These clinicians do everything in their power to ensure that their special patients do not have to endure long delays or uncertainty about what is going to happen next, or reconcile conflicting advice, or wait for phone calls that will come…well, sometime soon. We know such experiences are part of what is often called the “disutility of care,” but when they happen to our intimates, we recognize it as something closer to torment.
The bad news is that the challenge of giving patients coordinated care gets harder every year, as medical progress introduces increasing complexity to health care. The good news is that we are in fact getting better at coordinating care every year, because we increasingly recognize the importance of the challenge, and are responding.
Data Collection Advances
One important first step in managing the coordination of care is measuring it. After all, only patients themselves can judge how adequately their needs have been met. And, until recently, the perspectives of patients could only be surmised, and clinicians were expected to improve their “bedside manners” on a case-by-case basis.
But that time has passed. Today, advances in health information systems and in communications technology allow data to be collected from patients at low costs; and for patients to be followed over time so that their outcomes can be measured, analyzed, and improved. Patients can be segmented into groups with similar shared needs, so that teams can be organized to meet those needs. Some of these needs are clearly clinical (eg, control of pain from diseases or treatments), while others are not directly related to patients’ medical problems but are instead driven by how well health care providers work together.
Evolution of the health care marketplace has made improvement of value for those patient segments a strategic imperative. Provider organizations have to meet the needs of patients if they are to maintain or increase their market share—and, not coincidentally, these organizations are discovering that measurement and improvement of patient experience is an important strategic priority.
In this context, innovations are rapidly being adopted in 4 areas:
- The data that are being collected—Data that reflect how well patients’ needs are being met (ie, actual outcomes) have taken center stage, while data on amenities (eg, food and parking) are increasingly peripheral.
- How the data are being collected—Paper and telephone surveys are been supplemented or replaced by electronic methods. Increasingly, health care organizations are seeking email or other electronic ways of communicating with their patients. The reliability of collecting data via older methods is increasingly problematic, and electronic data collection reach patients faster and more efficiently. On average, respondents to electronic surveys are slightly younger than respondents to paper surveys, but the goal of measuring patient experience is to drive improvement; thus, more data is essential (see below).
- How much data are collected—The clear trend is away from seeking data from small samples of patients to meet some regulatory requirement, and toward seeking data from large samples of patients (ideally, giving all patients a chance to provide information). Larger amounts of data are needed to analyze data at the levels where true accountability lies, and true improvement can occur, such as the individual clinician’s performance.
- How the data are being used—A growing number of organizations are taking bold steps to increase accountability for improving patient experience, up to and including public reporting at an individual physician level. Many organizations are placing a modest financial incentive on improvement, while others use internal peer pressure as their approach. For example, physicians may undergo performance reviews annually at which their patient experience data as well as other performance metrics are discussed. Or organizations may share data internally without blinding, so that clinicians’ colleagues can see the ratings and comments made by patients.
Some of the most dramatic improvements have been made by organizations that have adopted the approach pioneered by the University of Utah Health System, which began sharing all patient ratings and comments on the internet in December 2012. Although Utah’s physicians were of course leery of the risks involved, they have found that the vast majority of patient comments were in fact laudatory—patients want to believe that clinicians are doing a good job, and have a low threshold for praising them. And the small percentage of criticisms from patients have proved powerful drivers of improvement. As a result, Utah and other organizations who are pursuing the transparency approach have seen improvement in patient experience far beyond what one could ever expect using financial incentives.
Summary
In sum, measurement of patient experience is no longer focused upon amenities such as food and parking based on responses from a few hundred patients. Instead, the field is about capturing important outcomes from as many patients as possible, so that teams and individual clinicians can improve their actual patient care. The strategic imperative to measure and manage these outcomes has never been greater; it is the antidote to a major side effect of medical progress, which is the chaos that characterizes modern health care. By asking patients about how their care is really going, I am certain we will respond and improve.
Corresponding author: Thomas H. Lee, MD, [email protected]. Dr. Lee is Chief Medical Officer for Press Ganey Associates, Inc.
From Press Ganey Associates, Inc., Wakefield, MA, and Harvard Medical School and Harvard School of Public Health, Boston, MA.
In fits and starts, but with increasing certainty, health care is changing its organizational focus from the activities of clinicians to meeting the needs of patients. That progress has been slowed and complicated, however, by lack of a performance framework that captures the extent to which patients’ needs are being met. In the absence of such data, “quality” has often been described in terms of the reliability of providers in complying with evidence-based guidelines.
Provider reliability is important, of course, but no substitute for the measurement and improvement of actual patient outcomes. After all, health care exists not to grade providers but to help patients. But clarity on that focus raises some important questions that we have not often discussed in the past.
What exactly are we trying to accomplish in health care? What is the goal? How can we tell how we are doing?
The answers cannot be found solely through measurement of “hard” clinical outcomes, such as death and disability. Yes, these are the most important focuses for improvement in health care, but we cannot deliver immortality, and we often can only delay complications of chronic disease. There is something else that our patients seek from health care, and it can only be measured by asking them directly.
Measuring "Peace of Mind"
That “something else” was described by one of my colleagues as “peace of mind that things are as good as they can be, given the cards that we have been dealt.” That may not be the most compact term in health policy, but I have yet to come up with something more concise— and it captures something immediately recognizable as important to anyone who has ever taken care of a patient with a serious disease, or spoken to that patient’s family. Of course, we should do our best to help patients live as long as possible, and of course we should optimize their health and relieve symptoms at every step of the way.
But there are other things that influence the degree of suffering that patients endure along that way, like hope, trust, anxiety, fear, and confusion. They are often considered part of the “art of medicine,” but I think we call them “art” because we are nervous about approaching them with discipline and rigor. In fact, these things can be measured, and managed—and the organizations that improve them are often rewarded with greater market share and professional pride.
Some of my clinician-colleagues do not immediately think of patients’ “peace of mind” as an important outcome, but when their family members or friends have medical problems, this issue immediately comes to the foreground. These clinicians do everything in their power to ensure that their special patients do not have to endure long delays or uncertainty about what is going to happen next, or reconcile conflicting advice, or wait for phone calls that will come…well, sometime soon. We know such experiences are part of what is often called the “disutility of care,” but when they happen to our intimates, we recognize it as something closer to torment.
The bad news is that the challenge of giving patients coordinated care gets harder every year, as medical progress introduces increasing complexity to health care. The good news is that we are in fact getting better at coordinating care every year, because we increasingly recognize the importance of the challenge, and are responding.
Data Collection Advances
One important first step in managing the coordination of care is measuring it. After all, only patients themselves can judge how adequately their needs have been met. And, until recently, the perspectives of patients could only be surmised, and clinicians were expected to improve their “bedside manners” on a case-by-case basis.
But that time has passed. Today, advances in health information systems and in communications technology allow data to be collected from patients at low costs; and for patients to be followed over time so that their outcomes can be measured, analyzed, and improved. Patients can be segmented into groups with similar shared needs, so that teams can be organized to meet those needs. Some of these needs are clearly clinical (eg, control of pain from diseases or treatments), while others are not directly related to patients’ medical problems but are instead driven by how well health care providers work together.
Evolution of the health care marketplace has made improvement of value for those patient segments a strategic imperative. Provider organizations have to meet the needs of patients if they are to maintain or increase their market share—and, not coincidentally, these organizations are discovering that measurement and improvement of patient experience is an important strategic priority.
In this context, innovations are rapidly being adopted in 4 areas:
- The data that are being collected—Data that reflect how well patients’ needs are being met (ie, actual outcomes) have taken center stage, while data on amenities (eg, food and parking) are increasingly peripheral.
- How the data are being collected—Paper and telephone surveys are been supplemented or replaced by electronic methods. Increasingly, health care organizations are seeking email or other electronic ways of communicating with their patients. The reliability of collecting data via older methods is increasingly problematic, and electronic data collection reach patients faster and more efficiently. On average, respondents to electronic surveys are slightly younger than respondents to paper surveys, but the goal of measuring patient experience is to drive improvement; thus, more data is essential (see below).
- How much data are collected—The clear trend is away from seeking data from small samples of patients to meet some regulatory requirement, and toward seeking data from large samples of patients (ideally, giving all patients a chance to provide information). Larger amounts of data are needed to analyze data at the levels where true accountability lies, and true improvement can occur, such as the individual clinician’s performance.
- How the data are being used—A growing number of organizations are taking bold steps to increase accountability for improving patient experience, up to and including public reporting at an individual physician level. Many organizations are placing a modest financial incentive on improvement, while others use internal peer pressure as their approach. For example, physicians may undergo performance reviews annually at which their patient experience data as well as other performance metrics are discussed. Or organizations may share data internally without blinding, so that clinicians’ colleagues can see the ratings and comments made by patients.
Some of the most dramatic improvements have been made by organizations that have adopted the approach pioneered by the University of Utah Health System, which began sharing all patient ratings and comments on the internet in December 2012. Although Utah’s physicians were of course leery of the risks involved, they have found that the vast majority of patient comments were in fact laudatory—patients want to believe that clinicians are doing a good job, and have a low threshold for praising them. And the small percentage of criticisms from patients have proved powerful drivers of improvement. As a result, Utah and other organizations who are pursuing the transparency approach have seen improvement in patient experience far beyond what one could ever expect using financial incentives.
Summary
In sum, measurement of patient experience is no longer focused upon amenities such as food and parking based on responses from a few hundred patients. Instead, the field is about capturing important outcomes from as many patients as possible, so that teams and individual clinicians can improve their actual patient care. The strategic imperative to measure and manage these outcomes has never been greater; it is the antidote to a major side effect of medical progress, which is the chaos that characterizes modern health care. By asking patients about how their care is really going, I am certain we will respond and improve.
Corresponding author: Thomas H. Lee, MD, [email protected]. Dr. Lee is Chief Medical Officer for Press Ganey Associates, Inc.
From Press Ganey Associates, Inc., Wakefield, MA, and Harvard Medical School and Harvard School of Public Health, Boston, MA.
In fits and starts, but with increasing certainty, health care is changing its organizational focus from the activities of clinicians to meeting the needs of patients. That progress has been slowed and complicated, however, by lack of a performance framework that captures the extent to which patients’ needs are being met. In the absence of such data, “quality” has often been described in terms of the reliability of providers in complying with evidence-based guidelines.
Provider reliability is important, of course, but no substitute for the measurement and improvement of actual patient outcomes. After all, health care exists not to grade providers but to help patients. But clarity on that focus raises some important questions that we have not often discussed in the past.
What exactly are we trying to accomplish in health care? What is the goal? How can we tell how we are doing?
The answers cannot be found solely through measurement of “hard” clinical outcomes, such as death and disability. Yes, these are the most important focuses for improvement in health care, but we cannot deliver immortality, and we often can only delay complications of chronic disease. There is something else that our patients seek from health care, and it can only be measured by asking them directly.
Measuring "Peace of Mind"
That “something else” was described by one of my colleagues as “peace of mind that things are as good as they can be, given the cards that we have been dealt.” That may not be the most compact term in health policy, but I have yet to come up with something more concise— and it captures something immediately recognizable as important to anyone who has ever taken care of a patient with a serious disease, or spoken to that patient’s family. Of course, we should do our best to help patients live as long as possible, and of course we should optimize their health and relieve symptoms at every step of the way.
But there are other things that influence the degree of suffering that patients endure along that way, like hope, trust, anxiety, fear, and confusion. They are often considered part of the “art of medicine,” but I think we call them “art” because we are nervous about approaching them with discipline and rigor. In fact, these things can be measured, and managed—and the organizations that improve them are often rewarded with greater market share and professional pride.
Some of my clinician-colleagues do not immediately think of patients’ “peace of mind” as an important outcome, but when their family members or friends have medical problems, this issue immediately comes to the foreground. These clinicians do everything in their power to ensure that their special patients do not have to endure long delays or uncertainty about what is going to happen next, or reconcile conflicting advice, or wait for phone calls that will come…well, sometime soon. We know such experiences are part of what is often called the “disutility of care,” but when they happen to our intimates, we recognize it as something closer to torment.
The bad news is that the challenge of giving patients coordinated care gets harder every year, as medical progress introduces increasing complexity to health care. The good news is that we are in fact getting better at coordinating care every year, because we increasingly recognize the importance of the challenge, and are responding.
Data Collection Advances
One important first step in managing the coordination of care is measuring it. After all, only patients themselves can judge how adequately their needs have been met. And, until recently, the perspectives of patients could only be surmised, and clinicians were expected to improve their “bedside manners” on a case-by-case basis.
But that time has passed. Today, advances in health information systems and in communications technology allow data to be collected from patients at low costs; and for patients to be followed over time so that their outcomes can be measured, analyzed, and improved. Patients can be segmented into groups with similar shared needs, so that teams can be organized to meet those needs. Some of these needs are clearly clinical (eg, control of pain from diseases or treatments), while others are not directly related to patients’ medical problems but are instead driven by how well health care providers work together.
Evolution of the health care marketplace has made improvement of value for those patient segments a strategic imperative. Provider organizations have to meet the needs of patients if they are to maintain or increase their market share—and, not coincidentally, these organizations are discovering that measurement and improvement of patient experience is an important strategic priority.
In this context, innovations are rapidly being adopted in 4 areas:
- The data that are being collected—Data that reflect how well patients’ needs are being met (ie, actual outcomes) have taken center stage, while data on amenities (eg, food and parking) are increasingly peripheral.
- How the data are being collected—Paper and telephone surveys are been supplemented or replaced by electronic methods. Increasingly, health care organizations are seeking email or other electronic ways of communicating with their patients. The reliability of collecting data via older methods is increasingly problematic, and electronic data collection reach patients faster and more efficiently. On average, respondents to electronic surveys are slightly younger than respondents to paper surveys, but the goal of measuring patient experience is to drive improvement; thus, more data is essential (see below).
- How much data are collected—The clear trend is away from seeking data from small samples of patients to meet some regulatory requirement, and toward seeking data from large samples of patients (ideally, giving all patients a chance to provide information). Larger amounts of data are needed to analyze data at the levels where true accountability lies, and true improvement can occur, such as the individual clinician’s performance.
- How the data are being used—A growing number of organizations are taking bold steps to increase accountability for improving patient experience, up to and including public reporting at an individual physician level. Many organizations are placing a modest financial incentive on improvement, while others use internal peer pressure as their approach. For example, physicians may undergo performance reviews annually at which their patient experience data as well as other performance metrics are discussed. Or organizations may share data internally without blinding, so that clinicians’ colleagues can see the ratings and comments made by patients.
Some of the most dramatic improvements have been made by organizations that have adopted the approach pioneered by the University of Utah Health System, which began sharing all patient ratings and comments on the internet in December 2012. Although Utah’s physicians were of course leery of the risks involved, they have found that the vast majority of patient comments were in fact laudatory—patients want to believe that clinicians are doing a good job, and have a low threshold for praising them. And the small percentage of criticisms from patients have proved powerful drivers of improvement. As a result, Utah and other organizations who are pursuing the transparency approach have seen improvement in patient experience far beyond what one could ever expect using financial incentives.
Summary
In sum, measurement of patient experience is no longer focused upon amenities such as food and parking based on responses from a few hundred patients. Instead, the field is about capturing important outcomes from as many patients as possible, so that teams and individual clinicians can improve their actual patient care. The strategic imperative to measure and manage these outcomes has never been greater; it is the antidote to a major side effect of medical progress, which is the chaos that characterizes modern health care. By asking patients about how their care is really going, I am certain we will respond and improve.
Corresponding author: Thomas H. Lee, MD, [email protected]. Dr. Lee is Chief Medical Officer for Press Ganey Associates, Inc.
Defecation Disorders: Diagnosis and Treatment
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
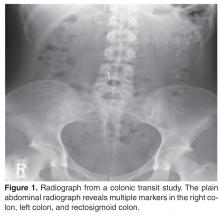
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
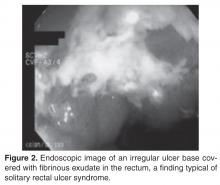
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
From the Digestive Health Center, Medical College of Georgia, Georgia Regents University, Augusta, GA
Defecation is a coordinated process that involves generation of sufficient propulsive forces in the abdomen and rectum together with relaxation of the puborectalis and external anal sphincter. Likewise, continence involves conscious retention of bowel contents until stool or gas can be voluntarily eliminated in an appropriate fashion. A failure of these processes leads to altered bowel function and disorders of defecation that are commonly encountered in clinical practice. They include a diverse group of maladies that result in altered defecation. Among them are functional disorders, such as dyssynergic defecation, and mechanical/structural disorders, such as rectocele, solitary rectal ulcer syndrome (SRUS), excessive perineal descent, and rectal prolapse. This article discusses 3 cases that illustrate the clinical features and management approaches to dyssynergic defecation, SRUS, and fecal incontinence.
Case Study 1
Presentation and History
A 26-year-old white woman with a 10-year history of constipation presents to a gastroenterologist after referral from her primary care physician. She reports spontaneous bowel movements once every 2 weeks, and often she has to induce stools by using enemas or suppositories. Stooling became progressively more difficult for her during her teenage years, with infrequent bowel movements and hard stools (type 1–2 on Bristol stool scale). She also reports having to strain excessively during bowel movements, and on average she spends 30 minutes in the bathroom. She denies experiencing any perianal pain or bleeding or using manual maneuvers to defecate, but she often feels a sense of incomplete evacuation. She also describes intermittent abdominal pain and bloating.
She has tried several over-the-counter laxatives, includ-ing milk of magnesia, senna, and magnesium citrate. Most recently, she tried lubiprostone and polyethylene glycol without improvement. Her past medical history is significant for endometriosis, exploratory laparotomy, and 1 vaginal delivery. There is no family history of colorectal cancer or inflammatory bowel disease. She works as a truck driver and does not use alcohol, illicit drugs, or tobacco. There is no history of physical or sexual abuse. Her current medications include lubiprostone 24 µg twice daily, polyethylene glycol 17 g twice daily, and a birth control pill.
Physical Examination
On physical examination, the patient appears healthy without any distress. Her body mass index is 26 kg/m2, and vital signs are normal. General examination is normal. Abdomen is flat, and bowel sounds are normal. Mild tenderness is noted in both lower quadrants. Rectal examination reveals normal anal skin folds. Digital exam-ination reveals a normal resting tone with pellet-like stool that is heme-negative. When asked to attempt defecation, she shows poor perineal descent and paradoxical contraction of the anal sphincter.
Laboratory Evaluation
Laboratory testing reveals normal levels of thyrotropin and thyroxine, no anemia on complete blood count, and normal levels of calcium, glucose, and electrolytes.
What are the possible causes for this patient’s altered bowel habits?
What is the approach to physical examination in patients with constipation?
Causes of Constipation
Constipation is a common digestive disorder, affecting up to 20% of the world’s population [1]. Primary or idiopathic constipation consists of 3 common overlapping subtypes: slow-transit constipation, dyssynergic defecation, and constipation-predominant irritable bowel syndrome. Slow-transit constipation involves the slow movement of stool through the colon. This is usually seen on a colonic transit study or with wireless motility capsule study. Dyssynergia in general is caused by functional outlet obstruction with or without normal colonic transit. Patients with dyssynergia often complain of incomplete evacuation, excessive straining, bloating, and blockage [2]. Often patients with dyssynergia resort to manual disimpaction/vaginal splinting and/or abdominal pressure to facilitate bowel movements. Secondary constipation may result from metabolic disorders (eg, hypercalcemia and hypokalemia, disorders associated with renal failure, hypothyroidism, and diabetes) as well as medications, including narcotics, anticholinergics, and antidepressants.
Rectal Examination
Physical examination in patients with constipation should include a detailed rectal examination. The perianal skin should be inspected closely for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with resting and squeeze anal tone. A study by Rao et al[3] showed that rectal examination could identify 76% of patients with dyssynergia. The sensitivity and positive predictive value for diagnosing dyssynergia with digital rectal examination was 81% and 99%, respectively, making it a good screening test for dyssynergia [3].
When is colonoscopy indicated in the workup of constipation?
What imaging studies may be useful?
Colonoscopy
Colonoscopic evaluation is only indicated in patients with alarming features such as rectal bleeding, weight loss, unex-plained abdominal pain, palpable mass in the abdomen or rectum, persistent and unexplained anal/rectal pain, or anemia, as well as in patients over age 50 years [4].
Colonic Transit Study
Two imaging studies can be useful in the evaluation of a patient with constipation: colonic transit study and defeco-graphy. A colonic transit study provides useful information regarding the rate at which stool travels through the colon. This test is performed by administering one capsule (Sitzmarks, Konsyl Pharmaceuticals, Fort Worth, TX) containing radiopaque markers. A plain radiograph of the abdomen is obtained on day 6 (120 hr after ingestion of capsule). A transit study is considered abnormal if more than 20% of markers (> 5) are present on a plain radiograph of the abdomen. Approximately two-thirds of patients with dyssynergia have an abnormal colonic transit study, with retention of markers either in the rectosigmoid region or throughout the colon [5]. Wireless motility capsule is a newer test that is comprised of ingesting a capsule and wearing a recorder for up to 5 days. This test measures regional transit (ie, gastric emptying, colonic transit time, and whole gut transit time), is standardized and validated, and avoids use of radiation [6].
Defecography
Defecography is conducted by instilling a barium paste in the rectum and monitoring evacuation of the barium radiologically. It can reveal poor activation of the levator ani muscles, prolonged retention of the barium, inability to expel the barium, absence of a striping wave, rectal mucosal intussusception, rectocele, abnormal perineal descent, or rectal prolapse [5]. Although abnormalities are frequently found on defecography, they may not translate into clinical dysfunction. In one study, 77% of women with complaints of defecation disorders had abnormalities on defecography, but there was no relationship between the abnormalities and the patients’ symptoms [7]. Hence, defecography is not recommended unless there is clinical suspicion of prolapse or excessive descent. Endoanal and dynamic pelvic magnetic resonance imaging (MRI) can evaluate global pelvic floor anatomy in dynamic function [8]. Dynamic MRI in the seated position provides the most physiologic approach.
What testing is needed to make a diagnosis of dyssynergic defecation?
Both an abnormal balloon expulsion test and an abnormal pattern of defecation on anal rectal manometry are required to diagnose dyssynergic defecation [9]. Anorectal manometry provides information regarding rectal and anal pressures at rest and during maneuvers of simulated defecation as well as information on rectal sensation, rectoanal reflexes, and compliance [2,10]. There are 4 patterns of dyssynergia found on anorectal manometry: type 1, normal push effort with paradoxical contraction of the anal sphincter; type 2, poor push effort with paradoxical contraction of the anal sphincter; type 3, normal push effort with incomplete or absent relaxation of the anal sphincter; and type 4, poor push with incomplete anal relaxation. The balloon expulsion test should be included in the work-up of dyssynergia.
Normal subjects can expel a 50-mL water-filled balloon in less than 1 minute. Although normal patients can show a dyssynergic pattern in the left lateral decubitus position, when seated on a commode and with a sensation of stooling most exhibit a normal pattern of defecation [9].
Diagnosis
What treatment options are available for dyssynergia?
The treatment of patients with dyssynergic defecation consists of standard therapies for constipation, including diet, laxatives, and timed toileting. Medical therapy includes laxatives, polyethylene glycol, and lubiprostone.
Case Study 2
Initial Presentation and History
A 39-year-old woman presents with a 5-year history of intermittent bright red blood with stooling. Most often, she notices blood on the toilet paper or when wiping and rarely in the commode. She reports having experienced difficulty with bowel movements since her teens. She does not have a daily urge but strains up to 30 minutes to pass stool that is hard in consistency (type 1–2 on the Bristol stool scale). Over the past year, she has started using fingers to remove stool.
The patient reports bloating and abdominal discomfort that is improved with stooling. Her weight has been stable. Current medications include polyethylene glycol 17 g twice daily, sodium docusate 100 mg twice daily, iron sulfate 325 mg 3 times daily, and a birth control pill. Her past medical history is significant for iron deficiency anemia. Family history is notable for her mother and sister with similar “bowel troubles,” but no family history of inflammatory bowel disease or colorectal cancer. She is a salesperson and has been married for 7 years. She does not use tobacco or alcohol. As a child, she was sexually abused. She did not receive any formal counseling for the abuse. Review of systems is negative.
Physical Examination
General and neurologic examinations are normal. The abdomen is mildly distended, bowel sounds are normal, there is mild tenderness, and stool is palpable in the left lower quadrant. Rectal examination reveals normal anal skin with no fissures, intact anocutaneous reflex, and hard stool in the rectal vault that is guaiac-positive. The resting anal sphincter tone is elevated, and when asked to attempt defecation, there is excessive perineal descent and rectal mucosal intussusception with paradoxical anal contraction.
Laboratory Evaluation and Endoscopy
What is SRUS and how is it diagnosed?
Evaluation and Diagnosis
SRUS is characterized by single or multiple ulcerations of the rectal mucosa along with distinct pathologic changes [17]. The term solitary rectal ulcer is a misnomer because many patients have more than 1 lesion, and it is not always an ulcer. Patients with SRUS present with several symptoms, but the most common is passage of blood or mucus, and up to 26% may be asymptomatic [18]. The pathophysiology of this condition is poorly understood. Multiple mechanisms have been implicated, including occult or overt rectal prolapse, dyssynergia, rectal mucosal intussusception, rectal hypersensitivity with a persistent feeling of a need to defecate, and reduced mucosal blood flow [19].
The diagnosis of SRUS is based on the patient’s clinical history combined with endoscopy and histopathology findings. Endoscopically, the lesions may vary in appearance. Shallow ulcerations on hyperemic surrounding mucosa located on the anterior wall is the most common finding [17]. Lesions vary in size, although most are 1 to 1.5 cm in diameter [17] and rarely involve more than half the circumference of the rectal wall. Polypoid lesions occur in approximately 25% of patients with SRUS, and multiple lesions occur in 30% [17].
Obtaining specimens for histology is an important step in the evaluation of SRUS. The differential diagnosis includes Crohn’s disease, ulcerative colitis, ischemic colitis, and malignancy. The typical histologic findings include fibromuscular hyperplasia with smooth muscle infiltration of the lamina propria, thickening of the muscularis mucosa, regenerative changes, and distortion of the crypt architecture [17].
Are physiologic or imaging studies helpful in the diagnosis of SRUS?
Two complementary physiologic tests for SRUS are anorectal manometry and defecography. Anorectal manometry often shows evidence of dyssynergia and rectal hypersensitivity in patients with SRUS [20,21]. Hyper-sensitivity may produce a sensation of incomplete evacuation, which in turn results in excessive straining. Defecography may reveal rectal mucosal intussusception or overt rectal prolapse. The patient in this case had evidence of rectal hypersensitivity on anorectal manometry along with excessive perineal descent on defecography.
What are treatment options for SRUS?
Treatment of SRUS is not standardized. The options include topical medical therapy, biofeedback, and surgery. Uncontrolled studies have suggested that 5-aminosalicylic acid enema [22], sucralfate enema [23], steroid enema [24], and fibrin glue [25] may improve symptoms. Patients who fail topical therapy and have evidence of dyssynergia on anorectal manometry should receive biofeedback therapy. A case-control study of biofeedback involving 11 patients with refractory SRUS and 15 healthy controls showed improvement in anorectal function, including dyssynergia [21]. At follow-up endoscopy, 36% had complete mucosal healing and more than 50% showed partial healing. In a study involving 16 patients with SRUS and 26 healthy controls, Jarrett et al [26] showed that 75% of patients who underwent biofeedback therapy had improved and 31% had ulcer resolution. Surgical therapy should be considered in rare patients who are refractory to medical therapy. The Delorme procedure is commonly performed with a success rate of 42% to 100% [27].
The case patient underwent biofeedback therapy, and after 5 sessions had complete healing of the lesion and resolution of rectal bleeding and bowel symptoms.
Case Study 3
Initial Presentation and History
A 75-year-old woman is referred to a gastroenterologist with complaints of incomplete stool evacuation and intermittent fecal seepage. She passes stools daily but sits on the toilet for 15 to 20 minutes, and after straining will pass only a small amount of stool. She describes stools as type 4 on the Bristol scale with no blood or mucus. One to 2 hours after a bowel movement, she experiences some wetness in the perineal region and upon checking often notices that a tablespoon full of stool material has leaked out. Sometimes, she will pass another large stool. She denies any leakage of stool while sleeping. Occasionally, she has urgency and leaks stool before reaching the toilet. In the past, she has used digital maneuvers to facilitate stooling. This problem has interfered with shopping, socializing, and taking vacations.
Her past medical history is significant for narcolepsy, hypertension, tubal ligation, appendectomy, and inguinal hernia repair. Obstetric history is significant for 6 vaginal deliveries, 1 requiring episiotomy but no forceps use. Her current medications include estradiol vaginal cream, hydrochlorothiazide, pilocarpine, and amitriptyline 10 mg 3 times daily. She also reports stress urinary incontinence, particularly with sneezing and coughing.
Physical Examination
Physical examination reveals a well-nourished woman with normal vital signs and a normal general examination. Abdominal examination is normal. A rectal examination shows no fissures, but the anocutaneous reflex is absent on the right side. Resting and squeeze sphincter tones are normal, with good perineal descent and normal anal relaxation.
Laboratory Evaluation
What are the mechanisms involved in fecal incontinence?
What are the 3 clinical subtypes of fecal incontinence?
Mechanisms and Subtypes
Fecal incontinence is often an unvoiced problem that causes significant social stigma. Approximately 2% of the US population suffers from fecal incontinence [28], with a higher prevalence among women and elderly persons. Several mechanisms are involved in the pathogenesis of fecal incontinence. A common cause is injury to the external or internal anal sphincter, puborectalis muscle, or pudendal nerves, often after obstetric trauma. Hence, a detailed obstetric history including number of vaginal deliveries, use of forceps, tears, and episiotomy is important. Sphincter disruption, most commonly after surgery for hemorrhoid or anal fissure, can result in incontinence. Likewise, reduced rectal compliance causes urgency and fecal incontinence. Impaired rectal sensation results in the accumulation of stool and overflow. Patients rarely have a single cause, with 80% having more than one factor that leads to incontinence [29].
Clinically, fecal incontinence can be classified into 3 categories. Urge incontinence is characterized by the inability to control stool discharge despite active attempts to retain contents. These patients often have disruption or injury to the external anal sphincter. Fecal seepage is the involuntary discharge of less than 2 tablespoons of stool matter without awareness. Seepage can result from impaired rectal evacuation and dyssynergia. Often patients with seepage complain of incomplete evacuation. Passive incontinence refers to the involuntary discharge of stool contents without awareness. These patients often have underlying neuropathy and sphincter weakness [30,31].
What is the approach to evaluation and diagnosis?
Evaluation and Diagnosis
Physical examination of patients with fecal incontinence should include a detailed rectal examination, similar to the exam performed in patients who present with constipation. It should include perineal inspection for fissures, fistulae, and skin excoriation. The anocutaneous reflex should be checked along with the resting and squeeze sphincter tone and sphincter relaxation. Further investigations should focus on determining the underlying mechanism in order to facilitate treatment.
Endoscopic investigation should be performed to exclude mucosal disease or malignancy. Anorectal manometry provides objective information regarding resting and squeeze anal sphincter tone, rectal compliance, rectal sensitivity, and rectoanal reflexes [29]. Some experts believe that anorectal manometry is not needed for diagnosis and emphasize the importance of rectal examination and history [32]. Proponents of anorectal manometry point out the importance of physiologic data that can be gained and how it may direct therapy. For example, anorectal manometry and sensory testing may reveal weak anal sphincters and impaired rectal sensation. The latter cannot be identified by clinical evaluation alone. These 2 pathophysiologic findings could enable the biofeedback therapist to focus on improving both anal sphincter tone and rectal sensation [33]. Defecography may reveal anterior rectocele, mucosal intussusception, or rectal prolapse. Anal ultrasound provides information on the structural integrity of the external and internal anal sphincters [34]. Ultrasound is widely available and is relatively inexpensive. Endoanal MRI may provide better information regarding the integrity of the external anal sphincter [35].
What are the treatment options?
The goal of treatment is to restore continence and quality of life. General considerations include stool bulking agents such as fiber supplements. Antidiarrheal agents, such as loperamide and diphenoxylate/atropine, are useful as they can decrease stool volume and increase and prolong sphincter pressure and colonic transit time [36,37]. Patients with diarrhea and functional incontinence may benefit from treatment with cholestyramine [38]. Biofeedback therapy improves sphincter tone and rectal sensation [39]. The number of biofeedback sessions is titrated to the patient’s needs, but often 6 sessions are required [40]. Generally, a 70% success rate has been described. Table 4 summarizes recent evidence supporting the use of biofeedback in the treatment of fecal incontinence [41–46].
Surgery for incontinence should be reserved for patients who have failed aggressive conservative management and biofeedback therapy. Overlapping sphincteroplasty is the most common surgery performed for fecal incontinence, with a success rate between 35% and 70% [47,48]. Creation of a neosphincter via dynamic graciloplasty or artificial sphincter has been tried in patients with an irreversibly damaged anal sphincter, but the success rate is low and the complication rate is high [49].
Sacral nerve stimulation (SNS) involves inserting electrodes in the lower back and connecting them to a pulse generator that produces pulses of electricity that innervate the nerves controlling the anal sphincters. Two double-blind crossover studies have reported a beneficial effect of SNS in fecal incontinence [50,51]. In 19 patients who preferred the periods when the stimulator was turned on, the median number of fecal incontinence episodes per week decreased from 1.7 to 0.7, and in the 5 patients who preferred the off period, the median number of fecal incontinence episodes per week increased from 1.7 to 3.7. SNS is now approved by FDA and insurance payers. Recently, hyaluronic acid/dextranomer injection (Solesta, Salix Pharmaceuticals, Raleigh, NC) has also been approved by FDA and has been shown to improve incontinence. A randomized controlled trial showed a 52% response rate to hyaluronic acid/dextranomer compared to a 31% response with placebo [52].
Conclusion
The 3 cases presented illustrate the complexities of several common anorectal disorders. A definitive diagnosis can be established in patients with defecation disorders through systematic evaluations and physiologic and imaging studies. Diagnosis in turn can pave the way for appropriate medical, behavioral, or surgical treatment. If facilities for appropriate testing are unavailable, it is important to refer these patients to appropriate specialists instead of embarking on empirical therapies which may prove futile. Treatment is often possible, and in a majority of patients their symptoms can be ameliorated.
Corresponding author: Satish S.C. Rao, MD, PhD, Section of Gastroenterology and Hepatology, Medical College of Georgia, Georgia Regents University, BB R2540, 1120 15th St., Augusta, GA 30912.
Funding/support: Portions of this work were supported by National Institutes of Health grant RO1 DK 57100-05.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
1. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–9.
2. Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:580–96.
3. Rao SS, Tantiphlachiva K, Rao PS, et al. How useful is digital rectal examination in the assessment of patients with dyssynergia? Am J Gastroenterol 2007;S268:419.
4. Pepin C, Ladabuam U. The yield of lower endoscopy in patients with constipation: survey of a university hospital, public county hospital, and a Veterans Administration medical center. Gastrointest Endosc 2002;56:325–32.
5. Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am 2003;32:659–83.
6. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil; 2011; 23: 8-23.
7. Savoye-Collet C, Savoye G, Koning E, et al. Defecography in symptomatic older women living at home. Age Ageing 2003;32:347–50.
8. Bolog N, Weishaupt D. Dynamic MR imaging of outlet obstruction. Rom J Gastroenterol 2005;14:293–302.
9. Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol 2006;101:2790–6.
10. Karlbom U, Lundin E, Graf W, Pahlman L. Anorectal physiology in relation to clinical subgroups of patients with severe constipation. Colorectal Dis 2004;6:343–9.
11. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657–64.
12. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331–8.
13. Chiarioni G, Saladini L, Whitehead W. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86–97.
14. Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to alternate treatments for patients with pelvic floor dyssynergia-type defecation [abstract]. Am J Gastroenterol 2005;100:S335.
15. Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 1988;2:714–7.
16. Boccasanta P, Venturi M, Stuto A, et al. Stapled transanal rectal resection for outlet obstruction: a prospective, multicenter trial. Dis Colon Rectum 2004;47:1285–97.
17. Sharara AI, Azar C, Amr SS, et al. Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755–62.
18. Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum 1992;35:227–34.
19. Felt-Bersma RJ, Cuesta A. Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin North Am 2001;30:199–222.
20. Vaizey CJ, van den Bogaerde JB, Emmanuel AV, et al. Solitary rectal ulcer syndrome. Br J Surg 1998;85:1617–23.
21. Rao SS, Ozturk R, De Ocampo S, Stessman M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am J Gastroenterol 2006;101:613–8.
22. Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer syndrome in a child treated with local sulfasalazine. Indian Pediatr 1994;31:1553–5.
23. Zargar SA, Khuroo MS, Mahajan R. Sucralfate retention enemas in solitary rectal ulcer. Dis Colon Rectum 1991;34:455–7.
24. Bishop PR, Nowicki MJ. Nonsurgical therapy for solitary rectal ulcer syndrome. Curr Treat Options Gastroenterol 2002;5:215–23.
25. Ederle A, Bulighin G, Orlandin PG, Pilati S. Endoscopic application of human fibrin sealant in the treatment of solitary rectal ulcer syndrome [letter]. Endoscopy 1992;24:736–7.
26. Jarrett ME, Emmanuel AV, Vaizey CJ, Kamm MA. Behavioural therapy (biofeedback) for solitary rectal ulcer syndrome improves symptoms and mucosal blood flow. Gut 2004;53:368–70.
27. Tweedie DJ, Varma JS. Long-term outcome of laparoscopic mesh rectopexy for solitary rectal ulcer syndrome. Colorectal Dis 2005;7:151–5.
28. Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastrenterol 2004;99:1585–604.
29. Rao SS, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol 1997;92:469–75.
30. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126(1 Suppl 1):S14–22.
31. Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 2007;102:351–61.
32. Wald A. Con: anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol 2006;101:2681–3.
33. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679–81.
34. Tuteja AK, Rao SS. Review article: recent trends in diagnosis and treatment of faecal incontinence. Aliment Pharmacol Ther 2004;19:829–40.
35. Terra MP, Beets-Tan RG, van der Hulst VP, et al. MRI in evaluating atrophy of the external anal sphincter in patients with fecal incontinence. AJR Am J Roentgenol 2006;187:991–9.
36. Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scan J Gastroenterol 1997;32:34–8.
37. Harford WV, Krejs GJ, Santa Ana CA, Fordtran JS. Acute effect of diphenoxylate with atropine (Lomotil) in patients with chronic diarrhea and fecal incontinence. Gastroenterol 1980;78:440–3.
38. Remes-Troche JM, Ozturk R, Philips C, et al. Cholesteryramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis 2008;23:189–99.
39. Rao SS. The technical aspects of biofeedback therapy for defecation disorders. Gastroenterologist 1998;6:96–103.
40. Rao SS, Welcher KD, Happel J. Can biofeedback therapy improve anorectal function in fecal incontinence? Am J Gastroenterol 1966;91:2360–6.
41. Byrne CM, Solomon MJ, Young JM, et al. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum 2007;50:417–27.
42. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenteroloy 2003;125:1320–9.
43. Heymen S, Whitehead W. EMG biofeedback vs. Kegel exercise for the treatment of fecal incontinence. Gastroenterology 2007;132:A–83.
44. Pager CK, Solomon MJ, Rex J, Roberts RA. Long-term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Dis Colon Rectum 2002;45:997–1003.
45. Ozturk R, Niazi S, Stessman M, Rao SS. Long-term and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther 2004;20:667–74.
46. Byrne CM, Solomon MJ, Rex J, et al. Telephone vs. face-to-face biofeedback for fecal incontinence: comparison of two techniques in 239 patients. Dis Colon Rectum 2005;48:2281–8.
47. Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum 2005;48:524–31.
48. Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology 2004;126(1 Suppl 1)S48–54.
49. Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis 2004;6:470–6.
50. Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–9.
51. Vaizey CJ, Kamm MA, Nicholls RJ. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2000;43:298–302.
52. Graf W, Mellgren A, Matzel KE, et al. . Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet;2011;377:997–1003.
Operational Lessons from a Large Accountable Care Organization
From Partners HealthCare, Boston, MA.
Abstract
- Objective: To describe operational lessons from a large accountable care organization (ACO).
- Methods: Description of an approach that includes the creation of a sustainable financing mechanism, new incentive structures, a high risk care management program, integrated mental health services, tools for specialist engagement, a post acute strategy, fostering patient engagement, and new clinical and analytic technologies.
- Results: Committed ACOs face challenges in enacting care delivery changes. Key challenges include educating boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
- Conclusion: A comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care.
After Massachusetts enacted legislation expanding health insurance to nearly all residents in 2006, additional legislation was enacted that focused on health cost containment. The goal of the many new regulations has been to hold the rate of health care cost growth to the rate of general inflation. Consistent with payment policy changes under the Accountable Care Act, Massachusetts regulatory efforts have emphasized putting health care providers at financial risk for some proportion of increases in costs of care. Providers who contract as accountable care organizations (ACOs) typically conduct their usual fee-for-service billing practices, but in addition the providers also agree to an annual total medical expenses (TME) spending target for an assigned population of patients. An annual reconciliation results in either penalties for exceeding targets or shared savings if spending remains below the target. The reconciliation incorporates a small number of commonly used primary care quality measures.
Partners HealthCare, an integrated health care delivery system in Massachusetts that includes 2 large academic medical centers—Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH)—began deploying new care models designed to reduce the growth in health care costs prior to these policy changes, but the new contracting environment has dramatically accelerated these efforts. Partners HealthCare signed accountable care risk contracts across all major payer categories—commercial, Medicare, and Medicaid—in 2011. Currently, Partners HealthCare has accountability for cost increases for nearly 500,000 lives, making it one of the largest providers of accountable care in the United States [1].
In this paper, we describe some of the initial lessons learned and share some of our concerns for the future success of risk-based contracting. We have organized the paper as we have organized our work—addressing different care services by site of care: primary care, specialty care, non-acute care, patient engagement, and necessary infrastructure. This framework has allowed us to engage the care providers throughout our organization with programs tailored to their specific circumstances. While practical, this framework is nonetheless somewhat artificial because much of our work could be characterized as building bridges between sites of care.
Organizing System-Wide ACO Programs
Focused efforts to lower cost trends and improve outcomes for a defined population began with MGH’s participation in a Medicare demonstration project in 2006. This successful program assigned specially trained nurse care managers to over 2000 of MGH’s highest cost Medicare beneficiaries [2]. The program was expanded in 2009 and then again in 2012, and now includes the entire Partners system. Building on this success, Partners’ providers evolved a broader set of tactics to include data, measurement and evidence-based methods of improving access, continuity, and care coordination to provide population-based health care [3,4]. To coordinate the system-wide work required by new risk contracting arrangements, Partners created the Division of Population Health Management (PHM). PHM works closely with organizational leadership at member institutions to collaboratively design and execute its system-wide accountable care strategy.
PHM has developed capacity, infrastructure, and expertise to implement and manage a clinical strategy for the entire integrated delivery system. This included some governance changes, new management processes, new investments in information technology and establishing system-wide incentives to promote care delivery innovation and improvement. Most importantly, through an extensive planning process Partners identified a comprehensive set of tactics and a multi-year plan for system-wide adoption of those tactics.
The majority of Partners information infrastructure to date was built internally, which allowed for rapid customization and flexibility, but also created significant interoperability problems. Moving forward, the majority of Partners systems will use a single IT platform. Partners has developed and implemented patient registries and care management decision support tools to help focus provider attention on patients most in need of interventions and to support reporting of quality metrics. In addition, Partners continues to expand a comprehensive data warehouse that incorporates a variety of clinical, administrative, and financial data sources to support advanced analytics for self-monitoring and continuous improvement. This extensive network-wide approach over the past several years has generated a number of lessons regarding successful accountable care organization implementation.
Implementing New Financing and Incentive Structures
Once an ACO is formed, the organization needs to restructure management to create organizational accountability for performance (as noted above), determine how to finance programmatic initiatives required to deliver the performance called for in the contracts, and create incentives for all the different providers within the ACO to drive performance towards the system’s goals. These latter two require the ACO to make specific design choices that include some trade-offs.
Partners chose to fund system-level population health management initiatives through a tax on net patient service revenue from its member providers—both hospitals and physicians. Alternative approaches include either setting aside a yearly allocation that is not proportional to a revenue stream or simply allocating the external risk to different operating units and allowing them to determine their own individual approaches (and investments) to managing the financial risk. By linking financing of PHM programs to clinical revenue (independent of risk contracts), and setting a uniform percentage tax, Partners has signaled that the wealthier parts of the system will contribute more to PHM (on an absolute basis) and more importantly that accountable care is a prioritized long-term investment. Allowing each entity within the organization to “sink or swim” based on its own performance was considered inconsistent with the interdependent nature of care delivery in a well functioning system. In addition, investments in the required infrastructure cannot be dependent on annual contract performance due to the volatility in contracted performance and the time it takes for an organization to get a return on their PHM investment.
Any organization involved in multiple performance based risk contracts faces the challenge of organizing the tactics and metrics for their providers. It is simply inconsistent with provider values and workflow to manage to different targets for different subpopulations of patients. In our attempts to promote the best possible care for all our patients and at the same time meet the demands of multiple external contract requirements, we have created an internal performance framework (IPF) that uses a single set of performance targets and a single incentive pool for all out contracts. The IPF rewards member institutions for (1) adopting programmatic initiatives (funded through the tax as described above), (2) meeting external quality measure targets, and (3) limiting the growth of cost-standardized medical expense trend (Figure).
Fixing Primary Care
Populations in risk contracts are typically defined by their primary care providers. In addition, the chronic underfunding of primary care in the US has resulted in unsustainable practice environments as well as well known access problems. Finally, the concentration of costs in a relatively small proportion of patients provides the greatest opportunity for ACOs to reduce costs through better care coordination for these patients. This core set of facts has guided our efforts to improve primary care. To address these issues, we have increased funding to primary care through our efforts to certify all 236 practices as patient-centered medical homes (PCMHs). In addition, we have begun transitioning our compensation models to include components based on risk-adjusted panel size and performance on quality metrics. As mentioned above, we have invested heavily in our complex care management program. Finally, we are working on a much tighter integration of mental health services with primary care. In this section we focus on lessons from our care management program and our efforts in mental health integration.
Complex Care Management
Provider-led high-risk care management has become the primary clinical lever for cost containment for most accountable care organizations [6]. The design and operational characteristics or our program have been described by Hong et al and are available on our website. Our decade of experience with this program has taught us a few lessons regarding the use of algorithms to identify patients, the management of program costs, and the difficulty of creating meaningful accountability.
Our analysis of commercially available risk prediction algorithms found minimal differences among the various products’ ability to predict high cost patients in the following year. A majority of the algorithms are exclusively claims based, though some include the ability to augment risk predictions with clinical data. Clinicians have played a critical role in improving our ability to identify high-risk patients. We have found that when physicians review a pre-selected set of their own patients, they have some ability to discriminate among patients who are likely to benefit from care management and those who are not. Clinicians are prone to overemphasizing recent events, but review of a list of patients who are predicted of becoming high cost mitigates this problem. Commercially available algorithms can help create an initial list, but physicians can add perspective on such important factors as social support and executive functioning. This additional information improves the specificity of the initial algorithm outputs, allowing clinicians to play an important role in refining the lists of patients eligible for high-risk care management.
The high cost of labor and space make high-risk care management programs among the most costly programs for an ACO. Care management requires a skilled nursing workforce (among others), which should be embedded into the primary care office for optimal effect [6,7].Given the high costs, there is understandable pressure to increase the ratio of patients per care manager. We have found that the optimal ratio is approximately 200 patients per care manager, with a third of the patients having active complex care management issues, a third being passively surveyed, and a third requiring modest care coordination. We continue with our attempts to refine how we manage this critical aspect of care management programs.
How can managers demonstrate that the investment in care coordination is impacting the ACO’s TME trend? Demonstrating a return on investment is difficult because a population of high-cost patients will inevitably show reduced costs in the following year (a phenomenon called regression to the mean). Isolating a well-matched control group to demonstrate program effectiveness would have the unintended consequence of reducing the potential effectiveness of the program. This situation is complicated by the different risk profiles of high-risk patients in different payer categories. For example, potentially avoidable Medicare costs are dominated by hospitalizations and end-of-life issues, Medicaid costs by mental illness and substance abuse, and commercial costs by specialty issues. In lieu of better management tools to assess the performance of our program, we have depended to date on process measures (eg., enrollment targets), patient surveys, and we are experimenting with some limited outcomes metrics (eg, admissions/1000).
Mental Health Integration
Another important lever for medical trend reduction within an ACO is the integration of mental health services into primary care. While our efforts in this complex area are only about a year old, some of our early lessons may prove valuable to others. First, we have worked hard to make the case for investment in mental health services, requiring assembling the evidence both for the magnitude of the problem as well as the effectiveness of available solutions.
A quarter of American adults suffer from diagnosable mental health disorders every year and it is estimated that PCPs manage between 40% and 80% of these patients [8,9].Rates of detection and adequate treatment in primary care settings are currently suboptimal, leading to poor disease management and driving excess utilization. Using claims data within the Partners’ primary care population, we have found medical expenditures are 45% higher for patients with a mental health diagnosis. Over 70% of mental health patients have additional illnesses, and the presence of a mental health disorder complicates overall clinical management [10].This results in a substantial increase in medical cost, independent of psychiatric medical spending [11].In addition, psychiatry shortage and access have become a major issue in mental health services [12].Over 70% of PCPs nationwide reported difficulty in finding high-quality outpatient mental health care for their patients [13].
The dominant clinical model for mental health integration is the collaborative care model (CCM), and evidence for its effectiveness is growing. Randomized controlled trials and meta-analyses have shown that CCMs are successful at improving detection and treatment of mental health disorders [14–16].Cost-savings analyses for many of these programs demonstrate considerable savings and favorable return on investment (ROI). Several CCMs that use nonmedical specialists and consulting psychiatrists to augment the management of mental health disorders for low- to moderate-risk primary care patients have been implemented. However, a majority of the CCMs are disease-specific—eg, integrating depression treatment resources into primary care. The challenge for ACOs is to determine how to build a comprehensive CCM that helps primary care manage the major primary care–based mental health conditions—depression, anxiety and substance abuse—in a coordinated, cost-efficient model. The ACO must consider how to implement both its high-risk program and its CCM programs in a way that is not disruptive but supportive to primary care practices.
Partners is implementing a multipronged strategy to address mental health issues within primary care. First, a universal screening program for mental health disorders using brief, well-validated screening tools (eg, Patient Health Questionnaire 2 and 9) will improve the identification of patients with mental health disorders. Second, consulting psychiatrist and mid-level health care providers, functioning as mental health specialists, will be virtually or physically integrated into our primary care teams. They will assist with issues such as initial clinical assessment; coordinate initiation of a mental health treatment plan; monitor the patient’s response to treatment; provide recommendations for treatment change based on evidence-based protocols and guidance from a consulting psychiatrist; provide therapy and mental health services to patients when indicated; and work closely with the patient to engage, activate, and educate him/her in order to promote disease management and treatment adherence. A unique feature of this integration program is the creation of a network-wide mental health access line for rapid mental health assessment and advice. Third, Partners is deploying sustained, network-wide educational programs that will train primary care personnel in brief interventions for improved disease management such as motivational interviewing, behavioral activation, problem-solving therapy, and other first-line interventions suitable for a primary care setting. Fourth, all primary care practices are developing and deploying standard workflows for the identification and treatment of mental health illnesses, starting with depression. Fifth, telehealth technologies will be used to improve access to specialty care and provide care in the most cost-effective setting. An initial focus is on online cognitive behavioral therapy, with virtual visit technologies to follow. Finally, registries will track mental health outcomes and provide prompts to ensure that follow-up screening tests are administered at periodic intervals and that treatment plans can be modified if progress is insufficient.
Implementing Prayer-Agnostic Programs For Specialty Services
Historically, cost containment by commercial payers focused on limiting access to specialist services. However, since costs are concentrated in a small portion of the population with complex chronic illnesses, considering the problems caused by gatekeeping in the 1990s, limiting access to specialists for the entire population may not be an appropriate lever for lowering TME trend. In addition, enhanced access to specialty services has the potential to reduce costs and improve quality through more efficient testing and treatment regimens. We have approached specialist services with the philosophy that early and coordinated access, through the application of tools such as bi-directional referral management systems and virtual visit capabilities, will have a greater ability to lower costs. The challenge for deploying these tools is that ACOs are built off of an aligned population of patients attributed to primary care physicians. Typically, specialists in ACOs are providing care to both a fee-for-service population as well as the ACO population. The costs of providing a nonbillable service such as virtual visits is not sustainable if a large portion of the patients are not in a risk contract. We have found that integrating virtual visits and e-referrals for a limited set of a specialist’s patients poses workflow and ethical challenges. As a system with 2 prominent academic medical centers, we have therefore focused our efforts on deploying these tools to specialists who have a high proportion of patients from our primary care physicians, and continue to work through these significant challenges.
Our specialist engagement tools are focused primarily on improving access and coordination or ensuring appropriateness and optimal outcomes. First, virtual visits (asynchronous and synchronous) between patients and providers or between providers help improve access and coordination. Second, referral management systems that allow for pre-consultative communications and review with key clinical data and messages allow for more thoughtful specialist consultations. This active management of referrals allows specialists to provide accelerated “curbside” consults without a formal consult for minor issues, appropriate pre-appointment testing for improved initial in-person consultation, or accelerated scheduling for initial consultation for urgent issues. Third, we are implementing technology and workflows to capture patient-reported outcomes in our specialty practices. We are collecting and reporting this data internally and externally to ensure we are monitoring the metrics that are most important to our patients. Monitoring patient-reported outcomes is especially important when a provider is concurrently implementing cost-containment measures. Fourth, we have developed technology to assess the appropriateness of surgical procedures. This technology combines analytics of both structured and unstructured data in an electronic platform and provide feedback to providers and patients regarding relative risks and benefits of certain procedures [17]. Lastly, we are implementing clinical bundles around select surgical procedures.
As an ACO that includes academic medical centers, we have a particular challenge of balancing the mission of fostering innovative and experimental technologies that may help advance human health and medical science, while ensuring we are stewards of limited financial resources. Academic medical center–led ACOs will need to thoughtfully balance these objectives [4].
Improving Non-Acute Services
We have included both improved access to emergency department alternatives as well as a focus on the appropriate and efficient use of post acute services in our approach to non-acute services. We have approached improving access to urgent care services by both implementing standards for access to primary care (through PCMH transformation) as well as partnering with urgent care providers.
The use of post-acute care services is the main driver of hospital referral region cost variation for both Medicare and Medicaid [18]. If an ACO is taking on risk in either of these payer categories, it is imperative that the organization has a strategy for managing post-acute care. We have been developing the following capabilities:
- Determine most appropriate level of post-acute care upon discharge from an acute facility (eg, home health vs. skilled nursing facility)
- Predict, to some level of reasonable confidence, the length of post-acute services required per episode of care
- Create a high performance post-acute referral network that can meet quality, efficiency and cost standards set forth by the ACO
The challenge in meeting these goals include the lack of high quality data required to execute on the first 2 objectives. In addition, development of a post-acute referral network is dependent on regional market characteristics. For example, if the region’s supply of post-acute facilities is limited, enforcing the ACO standards for high-quality post-acute care may be challenging. Executing a post-acute strategy that helps an ACO meet its financial and quality objectives may be one of the more challenging endeavors the ACO will undertake.
Engaging Patients in Accountable Care
Accountable care contracts provide a new imperative for providers to offer tools that help patients engage in their care outside of the traditional clinical encounter. Promoting shared decision making, where patients share preferences and clinicians incorporate these beliefs into clinical decision-making, is one of our primary patient engagement strategies. Systematic reviews have demonstrated the effectiveness of shared decision making in improving patient awareness and reducing variation in health care utilization [19–21]. In addition to shared decision making, we have invested in new video education tools and have updated our electronic patient portal to allow patients to access their clinical record, review educational materials, and communicate with their care team at their convenience. Patient engagement strategies are also embedded into other initiatives, such as our high-risk care management program. Keeping patient engagement integrated into all of an accountable care organization’s programs, instead of treating it as a distinct program, is critical for success.
Implementing Clinical Information Technology Tools
A majority of current clinical information technology tools have been created for synchronous, in-person delivery of health care, reflecting the dominant mode of care delivery in the US. However, under accountable care payment models, ACOs have the opportunity (and imperative) to deliver care either asynchronously and/or remotely. As we assessed our needs, we recognized several gaps between existing and desired technologies. Though many population health information technology frameworks exist, we have found three broad categories required for successful population health management: advanced data warehousing and analytics, next generation care delivery and coordination tools, and innovative clinical performance management tools.
Advanced data warehousing and analytics: ACOs must be able to integrate and analyze multiple data sources, including payer derived claims data (providing data on care rendered both inside and outside the ACO) as well as administrative data (scheduling, billing), and clinical (both structured and unstructured). This requires an ACO to invest in data warehousing technologies and analytical tools in order to take full advantage of the available information and provide guidance to providers and managers. We continue to build this set of solutions.
Next generation care delivery and coordination tools: The ACO must have new ways to connect various members of the patient care team including patient to provider, and provider to provider. These technologies include but are not limited to asynchronous and synchronous virtual visit capabilities, referral management software and high risk care management software, and remote monitoring for carefully selected patients. We are currently employing all of these types of health information technology.
Innovative clinical performance management tools: Third, the accountable care organization should have clinical performance management technologies that allow its providers to reduce care gaps and improve stewardship of resources. This includes, for example, advanced clinical decision support for radiology ordering and procedures and patient registries with clinical workflow integration.
In general, we have found a majority of accountable care information technology vendors are repurposing their existing assets for new applications towards population health management. For example, warehousing companies with strengths in the financial industry may convert their product for the health care market, or a company built for patient outreach and appointment reminders may convert their product into population health clinical registries. Given the relatively new nature of risk-based contracting, and uncertainty of the future of this payment model, it makes good sense from a vendor perspective to first try to repurpose existing assets, instead of creating built-for-purpose technologies that are more costly, and may not get to market fast enough to meet customer needs. The challenge for providers is that many of these repurposed technologies do not quite solve the particular challenges the provider is attempting to address. Accountable care organizations therefore are faced with challenging decisions regarding the purchase of a less than optimal product, waiting for the product segment to mature, or building the solution themselves.
Conclusion
We have described an approach to ACO success that includes the creation of a sustainable financing mechanism, new incentive structures, a high-risk care management program, integrated mental health services, tools for specialist engagement, a post-acute strategy, fostering patient engagement, and new clinical and analytic technologies. The breadth and depth of these changes to care delivery present numerous daunting challenges. Our experience suggests that partial approaches, implementing just a subset of the approaches listed above, will not constrain cost growth because costs are just shifted to a different part of the care delivery system. We conclude from this experience that a comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care. Nonetheless, the challenges associated with change on this scale are legion. Key challenges facing the committed ACOs include: educating their boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
Acknowledgment: The authors would like to thank the leadership of Partners Healthcare for their unambiguous support of the efforts described in this paper, as well as the clinicians, administrators, and support service workers that are committed to the achievement of the goals we have together set for the organization.
Corresponding author: Sreekanth K. Chaguturu, MD, 800 Boylston St., Ste. 1150, Boston, MA 02199, [email protected].
Financial disclosures: None.
1. Modern Healthcare’s 2014 accountable care organizations survey. Accessed 13 Aug 2014 at www.modernhealthcare.com/article/20140712/DATA/500032360/accountable-care-organizations-2014-excel-full-results.
2. McCall N, Cromwell J, Urato C. Evaluation of Medicare Care Management for High Cost Beneficiaries (CMHCB) Demonstration: Massachusetts General Hospital and Massachusetts General Physicians Organization (MGH). Centers for Medicare & Medicaid Services. 2010 Sept:1–171.
3. Milford CE, Ferris TG. A modified “golden rule” for health care organizations. Mayo Clin Proc 2012;87:717–20.
4. Nabel EG, Ferris TG, Slavin PL. Balancing AMCs’ missions and health care costs – mission impossible? N Engl J Med 2013;369:994–6.
5. Torchiana DF, Colton DG, Rao SK, et al. Massachusetts General Physicians Organization’s quality incentive program produces encouraging results. Health Aff 2013;32:1748–56.
6. Hong CS, Abrams MK, Ferris TG. Toward increased adoption of complex care management. N Engl J Med 2014;371:491–3.
7. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund) 2014;19:1–19.
8. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:617–27.
9. Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005;352:2515–23.
10. Druss BG, Walker ER. Mental disorders and medical comorbidity. Research synthesis report no. 21. Princeton, NJ: Robert Wood Johnson Foundation; 2011.
11. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Milliman Research Report. Seattle, WA: Milliman; 2008.
12. Leslie DL, Rosenbeck RA. Comparing quality of mental health care for public-sector and privately insured populations. Psychiatr Serv 2000; 51:650–5.
13. Goldman W. Economic grand rounds: is there a shortage of psychiatrists? Psychiatr Serv 2001;52:1587–9.
14. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314–21.
15. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry 2012;169:790–804.
16. Bower P, Gilbody S, Richards D, et al. Collaborative care for depression in primary care, making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484–93.
17. Milford CE, Hutter MM, Lillemoe KD, Ferris TG. Optimizing appropriate use of procedures in an era of payment reform. Ann Surg 2014;260:204–4.
18. Geographic variation in spending, utilization and quality: Medicare and Medicaid beneficiaries. May 2013. Accessed 13 Aug 2014 at http://iom.edu/~/media/Files/Report%20Files/2013/Geographic-Variation/Sub-Contractor/Acumen-Medicare-Medicaid.pdf
19. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011;(10)CD001431.
20. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6–8.
21. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood) 2012;31:2094–104.
From Partners HealthCare, Boston, MA.
Abstract
- Objective: To describe operational lessons from a large accountable care organization (ACO).
- Methods: Description of an approach that includes the creation of a sustainable financing mechanism, new incentive structures, a high risk care management program, integrated mental health services, tools for specialist engagement, a post acute strategy, fostering patient engagement, and new clinical and analytic technologies.
- Results: Committed ACOs face challenges in enacting care delivery changes. Key challenges include educating boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
- Conclusion: A comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care.
After Massachusetts enacted legislation expanding health insurance to nearly all residents in 2006, additional legislation was enacted that focused on health cost containment. The goal of the many new regulations has been to hold the rate of health care cost growth to the rate of general inflation. Consistent with payment policy changes under the Accountable Care Act, Massachusetts regulatory efforts have emphasized putting health care providers at financial risk for some proportion of increases in costs of care. Providers who contract as accountable care organizations (ACOs) typically conduct their usual fee-for-service billing practices, but in addition the providers also agree to an annual total medical expenses (TME) spending target for an assigned population of patients. An annual reconciliation results in either penalties for exceeding targets or shared savings if spending remains below the target. The reconciliation incorporates a small number of commonly used primary care quality measures.
Partners HealthCare, an integrated health care delivery system in Massachusetts that includes 2 large academic medical centers—Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH)—began deploying new care models designed to reduce the growth in health care costs prior to these policy changes, but the new contracting environment has dramatically accelerated these efforts. Partners HealthCare signed accountable care risk contracts across all major payer categories—commercial, Medicare, and Medicaid—in 2011. Currently, Partners HealthCare has accountability for cost increases for nearly 500,000 lives, making it one of the largest providers of accountable care in the United States [1].
In this paper, we describe some of the initial lessons learned and share some of our concerns for the future success of risk-based contracting. We have organized the paper as we have organized our work—addressing different care services by site of care: primary care, specialty care, non-acute care, patient engagement, and necessary infrastructure. This framework has allowed us to engage the care providers throughout our organization with programs tailored to their specific circumstances. While practical, this framework is nonetheless somewhat artificial because much of our work could be characterized as building bridges between sites of care.
Organizing System-Wide ACO Programs
Focused efforts to lower cost trends and improve outcomes for a defined population began with MGH’s participation in a Medicare demonstration project in 2006. This successful program assigned specially trained nurse care managers to over 2000 of MGH’s highest cost Medicare beneficiaries [2]. The program was expanded in 2009 and then again in 2012, and now includes the entire Partners system. Building on this success, Partners’ providers evolved a broader set of tactics to include data, measurement and evidence-based methods of improving access, continuity, and care coordination to provide population-based health care [3,4]. To coordinate the system-wide work required by new risk contracting arrangements, Partners created the Division of Population Health Management (PHM). PHM works closely with organizational leadership at member institutions to collaboratively design and execute its system-wide accountable care strategy.
PHM has developed capacity, infrastructure, and expertise to implement and manage a clinical strategy for the entire integrated delivery system. This included some governance changes, new management processes, new investments in information technology and establishing system-wide incentives to promote care delivery innovation and improvement. Most importantly, through an extensive planning process Partners identified a comprehensive set of tactics and a multi-year plan for system-wide adoption of those tactics.
The majority of Partners information infrastructure to date was built internally, which allowed for rapid customization and flexibility, but also created significant interoperability problems. Moving forward, the majority of Partners systems will use a single IT platform. Partners has developed and implemented patient registries and care management decision support tools to help focus provider attention on patients most in need of interventions and to support reporting of quality metrics. In addition, Partners continues to expand a comprehensive data warehouse that incorporates a variety of clinical, administrative, and financial data sources to support advanced analytics for self-monitoring and continuous improvement. This extensive network-wide approach over the past several years has generated a number of lessons regarding successful accountable care organization implementation.
Implementing New Financing and Incentive Structures
Once an ACO is formed, the organization needs to restructure management to create organizational accountability for performance (as noted above), determine how to finance programmatic initiatives required to deliver the performance called for in the contracts, and create incentives for all the different providers within the ACO to drive performance towards the system’s goals. These latter two require the ACO to make specific design choices that include some trade-offs.
Partners chose to fund system-level population health management initiatives through a tax on net patient service revenue from its member providers—both hospitals and physicians. Alternative approaches include either setting aside a yearly allocation that is not proportional to a revenue stream or simply allocating the external risk to different operating units and allowing them to determine their own individual approaches (and investments) to managing the financial risk. By linking financing of PHM programs to clinical revenue (independent of risk contracts), and setting a uniform percentage tax, Partners has signaled that the wealthier parts of the system will contribute more to PHM (on an absolute basis) and more importantly that accountable care is a prioritized long-term investment. Allowing each entity within the organization to “sink or swim” based on its own performance was considered inconsistent with the interdependent nature of care delivery in a well functioning system. In addition, investments in the required infrastructure cannot be dependent on annual contract performance due to the volatility in contracted performance and the time it takes for an organization to get a return on their PHM investment.
Any organization involved in multiple performance based risk contracts faces the challenge of organizing the tactics and metrics for their providers. It is simply inconsistent with provider values and workflow to manage to different targets for different subpopulations of patients. In our attempts to promote the best possible care for all our patients and at the same time meet the demands of multiple external contract requirements, we have created an internal performance framework (IPF) that uses a single set of performance targets and a single incentive pool for all out contracts. The IPF rewards member institutions for (1) adopting programmatic initiatives (funded through the tax as described above), (2) meeting external quality measure targets, and (3) limiting the growth of cost-standardized medical expense trend (Figure).
Fixing Primary Care
Populations in risk contracts are typically defined by their primary care providers. In addition, the chronic underfunding of primary care in the US has resulted in unsustainable practice environments as well as well known access problems. Finally, the concentration of costs in a relatively small proportion of patients provides the greatest opportunity for ACOs to reduce costs through better care coordination for these patients. This core set of facts has guided our efforts to improve primary care. To address these issues, we have increased funding to primary care through our efforts to certify all 236 practices as patient-centered medical homes (PCMHs). In addition, we have begun transitioning our compensation models to include components based on risk-adjusted panel size and performance on quality metrics. As mentioned above, we have invested heavily in our complex care management program. Finally, we are working on a much tighter integration of mental health services with primary care. In this section we focus on lessons from our care management program and our efforts in mental health integration.
Complex Care Management
Provider-led high-risk care management has become the primary clinical lever for cost containment for most accountable care organizations [6]. The design and operational characteristics or our program have been described by Hong et al and are available on our website. Our decade of experience with this program has taught us a few lessons regarding the use of algorithms to identify patients, the management of program costs, and the difficulty of creating meaningful accountability.
Our analysis of commercially available risk prediction algorithms found minimal differences among the various products’ ability to predict high cost patients in the following year. A majority of the algorithms are exclusively claims based, though some include the ability to augment risk predictions with clinical data. Clinicians have played a critical role in improving our ability to identify high-risk patients. We have found that when physicians review a pre-selected set of their own patients, they have some ability to discriminate among patients who are likely to benefit from care management and those who are not. Clinicians are prone to overemphasizing recent events, but review of a list of patients who are predicted of becoming high cost mitigates this problem. Commercially available algorithms can help create an initial list, but physicians can add perspective on such important factors as social support and executive functioning. This additional information improves the specificity of the initial algorithm outputs, allowing clinicians to play an important role in refining the lists of patients eligible for high-risk care management.
The high cost of labor and space make high-risk care management programs among the most costly programs for an ACO. Care management requires a skilled nursing workforce (among others), which should be embedded into the primary care office for optimal effect [6,7].Given the high costs, there is understandable pressure to increase the ratio of patients per care manager. We have found that the optimal ratio is approximately 200 patients per care manager, with a third of the patients having active complex care management issues, a third being passively surveyed, and a third requiring modest care coordination. We continue with our attempts to refine how we manage this critical aspect of care management programs.
How can managers demonstrate that the investment in care coordination is impacting the ACO’s TME trend? Demonstrating a return on investment is difficult because a population of high-cost patients will inevitably show reduced costs in the following year (a phenomenon called regression to the mean). Isolating a well-matched control group to demonstrate program effectiveness would have the unintended consequence of reducing the potential effectiveness of the program. This situation is complicated by the different risk profiles of high-risk patients in different payer categories. For example, potentially avoidable Medicare costs are dominated by hospitalizations and end-of-life issues, Medicaid costs by mental illness and substance abuse, and commercial costs by specialty issues. In lieu of better management tools to assess the performance of our program, we have depended to date on process measures (eg., enrollment targets), patient surveys, and we are experimenting with some limited outcomes metrics (eg, admissions/1000).
Mental Health Integration
Another important lever for medical trend reduction within an ACO is the integration of mental health services into primary care. While our efforts in this complex area are only about a year old, some of our early lessons may prove valuable to others. First, we have worked hard to make the case for investment in mental health services, requiring assembling the evidence both for the magnitude of the problem as well as the effectiveness of available solutions.
A quarter of American adults suffer from diagnosable mental health disorders every year and it is estimated that PCPs manage between 40% and 80% of these patients [8,9].Rates of detection and adequate treatment in primary care settings are currently suboptimal, leading to poor disease management and driving excess utilization. Using claims data within the Partners’ primary care population, we have found medical expenditures are 45% higher for patients with a mental health diagnosis. Over 70% of mental health patients have additional illnesses, and the presence of a mental health disorder complicates overall clinical management [10].This results in a substantial increase in medical cost, independent of psychiatric medical spending [11].In addition, psychiatry shortage and access have become a major issue in mental health services [12].Over 70% of PCPs nationwide reported difficulty in finding high-quality outpatient mental health care for their patients [13].
The dominant clinical model for mental health integration is the collaborative care model (CCM), and evidence for its effectiveness is growing. Randomized controlled trials and meta-analyses have shown that CCMs are successful at improving detection and treatment of mental health disorders [14–16].Cost-savings analyses for many of these programs demonstrate considerable savings and favorable return on investment (ROI). Several CCMs that use nonmedical specialists and consulting psychiatrists to augment the management of mental health disorders for low- to moderate-risk primary care patients have been implemented. However, a majority of the CCMs are disease-specific—eg, integrating depression treatment resources into primary care. The challenge for ACOs is to determine how to build a comprehensive CCM that helps primary care manage the major primary care–based mental health conditions—depression, anxiety and substance abuse—in a coordinated, cost-efficient model. The ACO must consider how to implement both its high-risk program and its CCM programs in a way that is not disruptive but supportive to primary care practices.
Partners is implementing a multipronged strategy to address mental health issues within primary care. First, a universal screening program for mental health disorders using brief, well-validated screening tools (eg, Patient Health Questionnaire 2 and 9) will improve the identification of patients with mental health disorders. Second, consulting psychiatrist and mid-level health care providers, functioning as mental health specialists, will be virtually or physically integrated into our primary care teams. They will assist with issues such as initial clinical assessment; coordinate initiation of a mental health treatment plan; monitor the patient’s response to treatment; provide recommendations for treatment change based on evidence-based protocols and guidance from a consulting psychiatrist; provide therapy and mental health services to patients when indicated; and work closely with the patient to engage, activate, and educate him/her in order to promote disease management and treatment adherence. A unique feature of this integration program is the creation of a network-wide mental health access line for rapid mental health assessment and advice. Third, Partners is deploying sustained, network-wide educational programs that will train primary care personnel in brief interventions for improved disease management such as motivational interviewing, behavioral activation, problem-solving therapy, and other first-line interventions suitable for a primary care setting. Fourth, all primary care practices are developing and deploying standard workflows for the identification and treatment of mental health illnesses, starting with depression. Fifth, telehealth technologies will be used to improve access to specialty care and provide care in the most cost-effective setting. An initial focus is on online cognitive behavioral therapy, with virtual visit technologies to follow. Finally, registries will track mental health outcomes and provide prompts to ensure that follow-up screening tests are administered at periodic intervals and that treatment plans can be modified if progress is insufficient.
Implementing Prayer-Agnostic Programs For Specialty Services
Historically, cost containment by commercial payers focused on limiting access to specialist services. However, since costs are concentrated in a small portion of the population with complex chronic illnesses, considering the problems caused by gatekeeping in the 1990s, limiting access to specialists for the entire population may not be an appropriate lever for lowering TME trend. In addition, enhanced access to specialty services has the potential to reduce costs and improve quality through more efficient testing and treatment regimens. We have approached specialist services with the philosophy that early and coordinated access, through the application of tools such as bi-directional referral management systems and virtual visit capabilities, will have a greater ability to lower costs. The challenge for deploying these tools is that ACOs are built off of an aligned population of patients attributed to primary care physicians. Typically, specialists in ACOs are providing care to both a fee-for-service population as well as the ACO population. The costs of providing a nonbillable service such as virtual visits is not sustainable if a large portion of the patients are not in a risk contract. We have found that integrating virtual visits and e-referrals for a limited set of a specialist’s patients poses workflow and ethical challenges. As a system with 2 prominent academic medical centers, we have therefore focused our efforts on deploying these tools to specialists who have a high proportion of patients from our primary care physicians, and continue to work through these significant challenges.
Our specialist engagement tools are focused primarily on improving access and coordination or ensuring appropriateness and optimal outcomes. First, virtual visits (asynchronous and synchronous) between patients and providers or between providers help improve access and coordination. Second, referral management systems that allow for pre-consultative communications and review with key clinical data and messages allow for more thoughtful specialist consultations. This active management of referrals allows specialists to provide accelerated “curbside” consults without a formal consult for minor issues, appropriate pre-appointment testing for improved initial in-person consultation, or accelerated scheduling for initial consultation for urgent issues. Third, we are implementing technology and workflows to capture patient-reported outcomes in our specialty practices. We are collecting and reporting this data internally and externally to ensure we are monitoring the metrics that are most important to our patients. Monitoring patient-reported outcomes is especially important when a provider is concurrently implementing cost-containment measures. Fourth, we have developed technology to assess the appropriateness of surgical procedures. This technology combines analytics of both structured and unstructured data in an electronic platform and provide feedback to providers and patients regarding relative risks and benefits of certain procedures [17]. Lastly, we are implementing clinical bundles around select surgical procedures.
As an ACO that includes academic medical centers, we have a particular challenge of balancing the mission of fostering innovative and experimental technologies that may help advance human health and medical science, while ensuring we are stewards of limited financial resources. Academic medical center–led ACOs will need to thoughtfully balance these objectives [4].
Improving Non-Acute Services
We have included both improved access to emergency department alternatives as well as a focus on the appropriate and efficient use of post acute services in our approach to non-acute services. We have approached improving access to urgent care services by both implementing standards for access to primary care (through PCMH transformation) as well as partnering with urgent care providers.
The use of post-acute care services is the main driver of hospital referral region cost variation for both Medicare and Medicaid [18]. If an ACO is taking on risk in either of these payer categories, it is imperative that the organization has a strategy for managing post-acute care. We have been developing the following capabilities:
- Determine most appropriate level of post-acute care upon discharge from an acute facility (eg, home health vs. skilled nursing facility)
- Predict, to some level of reasonable confidence, the length of post-acute services required per episode of care
- Create a high performance post-acute referral network that can meet quality, efficiency and cost standards set forth by the ACO
The challenge in meeting these goals include the lack of high quality data required to execute on the first 2 objectives. In addition, development of a post-acute referral network is dependent on regional market characteristics. For example, if the region’s supply of post-acute facilities is limited, enforcing the ACO standards for high-quality post-acute care may be challenging. Executing a post-acute strategy that helps an ACO meet its financial and quality objectives may be one of the more challenging endeavors the ACO will undertake.
Engaging Patients in Accountable Care
Accountable care contracts provide a new imperative for providers to offer tools that help patients engage in their care outside of the traditional clinical encounter. Promoting shared decision making, where patients share preferences and clinicians incorporate these beliefs into clinical decision-making, is one of our primary patient engagement strategies. Systematic reviews have demonstrated the effectiveness of shared decision making in improving patient awareness and reducing variation in health care utilization [19–21]. In addition to shared decision making, we have invested in new video education tools and have updated our electronic patient portal to allow patients to access their clinical record, review educational materials, and communicate with their care team at their convenience. Patient engagement strategies are also embedded into other initiatives, such as our high-risk care management program. Keeping patient engagement integrated into all of an accountable care organization’s programs, instead of treating it as a distinct program, is critical for success.
Implementing Clinical Information Technology Tools
A majority of current clinical information technology tools have been created for synchronous, in-person delivery of health care, reflecting the dominant mode of care delivery in the US. However, under accountable care payment models, ACOs have the opportunity (and imperative) to deliver care either asynchronously and/or remotely. As we assessed our needs, we recognized several gaps between existing and desired technologies. Though many population health information technology frameworks exist, we have found three broad categories required for successful population health management: advanced data warehousing and analytics, next generation care delivery and coordination tools, and innovative clinical performance management tools.
Advanced data warehousing and analytics: ACOs must be able to integrate and analyze multiple data sources, including payer derived claims data (providing data on care rendered both inside and outside the ACO) as well as administrative data (scheduling, billing), and clinical (both structured and unstructured). This requires an ACO to invest in data warehousing technologies and analytical tools in order to take full advantage of the available information and provide guidance to providers and managers. We continue to build this set of solutions.
Next generation care delivery and coordination tools: The ACO must have new ways to connect various members of the patient care team including patient to provider, and provider to provider. These technologies include but are not limited to asynchronous and synchronous virtual visit capabilities, referral management software and high risk care management software, and remote monitoring for carefully selected patients. We are currently employing all of these types of health information technology.
Innovative clinical performance management tools: Third, the accountable care organization should have clinical performance management technologies that allow its providers to reduce care gaps and improve stewardship of resources. This includes, for example, advanced clinical decision support for radiology ordering and procedures and patient registries with clinical workflow integration.
In general, we have found a majority of accountable care information technology vendors are repurposing their existing assets for new applications towards population health management. For example, warehousing companies with strengths in the financial industry may convert their product for the health care market, or a company built for patient outreach and appointment reminders may convert their product into population health clinical registries. Given the relatively new nature of risk-based contracting, and uncertainty of the future of this payment model, it makes good sense from a vendor perspective to first try to repurpose existing assets, instead of creating built-for-purpose technologies that are more costly, and may not get to market fast enough to meet customer needs. The challenge for providers is that many of these repurposed technologies do not quite solve the particular challenges the provider is attempting to address. Accountable care organizations therefore are faced with challenging decisions regarding the purchase of a less than optimal product, waiting for the product segment to mature, or building the solution themselves.
Conclusion
We have described an approach to ACO success that includes the creation of a sustainable financing mechanism, new incentive structures, a high-risk care management program, integrated mental health services, tools for specialist engagement, a post-acute strategy, fostering patient engagement, and new clinical and analytic technologies. The breadth and depth of these changes to care delivery present numerous daunting challenges. Our experience suggests that partial approaches, implementing just a subset of the approaches listed above, will not constrain cost growth because costs are just shifted to a different part of the care delivery system. We conclude from this experience that a comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care. Nonetheless, the challenges associated with change on this scale are legion. Key challenges facing the committed ACOs include: educating their boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
Acknowledgment: The authors would like to thank the leadership of Partners Healthcare for their unambiguous support of the efforts described in this paper, as well as the clinicians, administrators, and support service workers that are committed to the achievement of the goals we have together set for the organization.
Corresponding author: Sreekanth K. Chaguturu, MD, 800 Boylston St., Ste. 1150, Boston, MA 02199, [email protected].
Financial disclosures: None.
From Partners HealthCare, Boston, MA.
Abstract
- Objective: To describe operational lessons from a large accountable care organization (ACO).
- Methods: Description of an approach that includes the creation of a sustainable financing mechanism, new incentive structures, a high risk care management program, integrated mental health services, tools for specialist engagement, a post acute strategy, fostering patient engagement, and new clinical and analytic technologies.
- Results: Committed ACOs face challenges in enacting care delivery changes. Key challenges include educating boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
- Conclusion: A comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care.
After Massachusetts enacted legislation expanding health insurance to nearly all residents in 2006, additional legislation was enacted that focused on health cost containment. The goal of the many new regulations has been to hold the rate of health care cost growth to the rate of general inflation. Consistent with payment policy changes under the Accountable Care Act, Massachusetts regulatory efforts have emphasized putting health care providers at financial risk for some proportion of increases in costs of care. Providers who contract as accountable care organizations (ACOs) typically conduct their usual fee-for-service billing practices, but in addition the providers also agree to an annual total medical expenses (TME) spending target for an assigned population of patients. An annual reconciliation results in either penalties for exceeding targets or shared savings if spending remains below the target. The reconciliation incorporates a small number of commonly used primary care quality measures.
Partners HealthCare, an integrated health care delivery system in Massachusetts that includes 2 large academic medical centers—Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH)—began deploying new care models designed to reduce the growth in health care costs prior to these policy changes, but the new contracting environment has dramatically accelerated these efforts. Partners HealthCare signed accountable care risk contracts across all major payer categories—commercial, Medicare, and Medicaid—in 2011. Currently, Partners HealthCare has accountability for cost increases for nearly 500,000 lives, making it one of the largest providers of accountable care in the United States [1].
In this paper, we describe some of the initial lessons learned and share some of our concerns for the future success of risk-based contracting. We have organized the paper as we have organized our work—addressing different care services by site of care: primary care, specialty care, non-acute care, patient engagement, and necessary infrastructure. This framework has allowed us to engage the care providers throughout our organization with programs tailored to their specific circumstances. While practical, this framework is nonetheless somewhat artificial because much of our work could be characterized as building bridges between sites of care.
Organizing System-Wide ACO Programs
Focused efforts to lower cost trends and improve outcomes for a defined population began with MGH’s participation in a Medicare demonstration project in 2006. This successful program assigned specially trained nurse care managers to over 2000 of MGH’s highest cost Medicare beneficiaries [2]. The program was expanded in 2009 and then again in 2012, and now includes the entire Partners system. Building on this success, Partners’ providers evolved a broader set of tactics to include data, measurement and evidence-based methods of improving access, continuity, and care coordination to provide population-based health care [3,4]. To coordinate the system-wide work required by new risk contracting arrangements, Partners created the Division of Population Health Management (PHM). PHM works closely with organizational leadership at member institutions to collaboratively design and execute its system-wide accountable care strategy.
PHM has developed capacity, infrastructure, and expertise to implement and manage a clinical strategy for the entire integrated delivery system. This included some governance changes, new management processes, new investments in information technology and establishing system-wide incentives to promote care delivery innovation and improvement. Most importantly, through an extensive planning process Partners identified a comprehensive set of tactics and a multi-year plan for system-wide adoption of those tactics.
The majority of Partners information infrastructure to date was built internally, which allowed for rapid customization and flexibility, but also created significant interoperability problems. Moving forward, the majority of Partners systems will use a single IT platform. Partners has developed and implemented patient registries and care management decision support tools to help focus provider attention on patients most in need of interventions and to support reporting of quality metrics. In addition, Partners continues to expand a comprehensive data warehouse that incorporates a variety of clinical, administrative, and financial data sources to support advanced analytics for self-monitoring and continuous improvement. This extensive network-wide approach over the past several years has generated a number of lessons regarding successful accountable care organization implementation.
Implementing New Financing and Incentive Structures
Once an ACO is formed, the organization needs to restructure management to create organizational accountability for performance (as noted above), determine how to finance programmatic initiatives required to deliver the performance called for in the contracts, and create incentives for all the different providers within the ACO to drive performance towards the system’s goals. These latter two require the ACO to make specific design choices that include some trade-offs.
Partners chose to fund system-level population health management initiatives through a tax on net patient service revenue from its member providers—both hospitals and physicians. Alternative approaches include either setting aside a yearly allocation that is not proportional to a revenue stream or simply allocating the external risk to different operating units and allowing them to determine their own individual approaches (and investments) to managing the financial risk. By linking financing of PHM programs to clinical revenue (independent of risk contracts), and setting a uniform percentage tax, Partners has signaled that the wealthier parts of the system will contribute more to PHM (on an absolute basis) and more importantly that accountable care is a prioritized long-term investment. Allowing each entity within the organization to “sink or swim” based on its own performance was considered inconsistent with the interdependent nature of care delivery in a well functioning system. In addition, investments in the required infrastructure cannot be dependent on annual contract performance due to the volatility in contracted performance and the time it takes for an organization to get a return on their PHM investment.
Any organization involved in multiple performance based risk contracts faces the challenge of organizing the tactics and metrics for their providers. It is simply inconsistent with provider values and workflow to manage to different targets for different subpopulations of patients. In our attempts to promote the best possible care for all our patients and at the same time meet the demands of multiple external contract requirements, we have created an internal performance framework (IPF) that uses a single set of performance targets and a single incentive pool for all out contracts. The IPF rewards member institutions for (1) adopting programmatic initiatives (funded through the tax as described above), (2) meeting external quality measure targets, and (3) limiting the growth of cost-standardized medical expense trend (Figure).
Fixing Primary Care
Populations in risk contracts are typically defined by their primary care providers. In addition, the chronic underfunding of primary care in the US has resulted in unsustainable practice environments as well as well known access problems. Finally, the concentration of costs in a relatively small proportion of patients provides the greatest opportunity for ACOs to reduce costs through better care coordination for these patients. This core set of facts has guided our efforts to improve primary care. To address these issues, we have increased funding to primary care through our efforts to certify all 236 practices as patient-centered medical homes (PCMHs). In addition, we have begun transitioning our compensation models to include components based on risk-adjusted panel size and performance on quality metrics. As mentioned above, we have invested heavily in our complex care management program. Finally, we are working on a much tighter integration of mental health services with primary care. In this section we focus on lessons from our care management program and our efforts in mental health integration.
Complex Care Management
Provider-led high-risk care management has become the primary clinical lever for cost containment for most accountable care organizations [6]. The design and operational characteristics or our program have been described by Hong et al and are available on our website. Our decade of experience with this program has taught us a few lessons regarding the use of algorithms to identify patients, the management of program costs, and the difficulty of creating meaningful accountability.
Our analysis of commercially available risk prediction algorithms found minimal differences among the various products’ ability to predict high cost patients in the following year. A majority of the algorithms are exclusively claims based, though some include the ability to augment risk predictions with clinical data. Clinicians have played a critical role in improving our ability to identify high-risk patients. We have found that when physicians review a pre-selected set of their own patients, they have some ability to discriminate among patients who are likely to benefit from care management and those who are not. Clinicians are prone to overemphasizing recent events, but review of a list of patients who are predicted of becoming high cost mitigates this problem. Commercially available algorithms can help create an initial list, but physicians can add perspective on such important factors as social support and executive functioning. This additional information improves the specificity of the initial algorithm outputs, allowing clinicians to play an important role in refining the lists of patients eligible for high-risk care management.
The high cost of labor and space make high-risk care management programs among the most costly programs for an ACO. Care management requires a skilled nursing workforce (among others), which should be embedded into the primary care office for optimal effect [6,7].Given the high costs, there is understandable pressure to increase the ratio of patients per care manager. We have found that the optimal ratio is approximately 200 patients per care manager, with a third of the patients having active complex care management issues, a third being passively surveyed, and a third requiring modest care coordination. We continue with our attempts to refine how we manage this critical aspect of care management programs.
How can managers demonstrate that the investment in care coordination is impacting the ACO’s TME trend? Demonstrating a return on investment is difficult because a population of high-cost patients will inevitably show reduced costs in the following year (a phenomenon called regression to the mean). Isolating a well-matched control group to demonstrate program effectiveness would have the unintended consequence of reducing the potential effectiveness of the program. This situation is complicated by the different risk profiles of high-risk patients in different payer categories. For example, potentially avoidable Medicare costs are dominated by hospitalizations and end-of-life issues, Medicaid costs by mental illness and substance abuse, and commercial costs by specialty issues. In lieu of better management tools to assess the performance of our program, we have depended to date on process measures (eg., enrollment targets), patient surveys, and we are experimenting with some limited outcomes metrics (eg, admissions/1000).
Mental Health Integration
Another important lever for medical trend reduction within an ACO is the integration of mental health services into primary care. While our efforts in this complex area are only about a year old, some of our early lessons may prove valuable to others. First, we have worked hard to make the case for investment in mental health services, requiring assembling the evidence both for the magnitude of the problem as well as the effectiveness of available solutions.
A quarter of American adults suffer from diagnosable mental health disorders every year and it is estimated that PCPs manage between 40% and 80% of these patients [8,9].Rates of detection and adequate treatment in primary care settings are currently suboptimal, leading to poor disease management and driving excess utilization. Using claims data within the Partners’ primary care population, we have found medical expenditures are 45% higher for patients with a mental health diagnosis. Over 70% of mental health patients have additional illnesses, and the presence of a mental health disorder complicates overall clinical management [10].This results in a substantial increase in medical cost, independent of psychiatric medical spending [11].In addition, psychiatry shortage and access have become a major issue in mental health services [12].Over 70% of PCPs nationwide reported difficulty in finding high-quality outpatient mental health care for their patients [13].
The dominant clinical model for mental health integration is the collaborative care model (CCM), and evidence for its effectiveness is growing. Randomized controlled trials and meta-analyses have shown that CCMs are successful at improving detection and treatment of mental health disorders [14–16].Cost-savings analyses for many of these programs demonstrate considerable savings and favorable return on investment (ROI). Several CCMs that use nonmedical specialists and consulting psychiatrists to augment the management of mental health disorders for low- to moderate-risk primary care patients have been implemented. However, a majority of the CCMs are disease-specific—eg, integrating depression treatment resources into primary care. The challenge for ACOs is to determine how to build a comprehensive CCM that helps primary care manage the major primary care–based mental health conditions—depression, anxiety and substance abuse—in a coordinated, cost-efficient model. The ACO must consider how to implement both its high-risk program and its CCM programs in a way that is not disruptive but supportive to primary care practices.
Partners is implementing a multipronged strategy to address mental health issues within primary care. First, a universal screening program for mental health disorders using brief, well-validated screening tools (eg, Patient Health Questionnaire 2 and 9) will improve the identification of patients with mental health disorders. Second, consulting psychiatrist and mid-level health care providers, functioning as mental health specialists, will be virtually or physically integrated into our primary care teams. They will assist with issues such as initial clinical assessment; coordinate initiation of a mental health treatment plan; monitor the patient’s response to treatment; provide recommendations for treatment change based on evidence-based protocols and guidance from a consulting psychiatrist; provide therapy and mental health services to patients when indicated; and work closely with the patient to engage, activate, and educate him/her in order to promote disease management and treatment adherence. A unique feature of this integration program is the creation of a network-wide mental health access line for rapid mental health assessment and advice. Third, Partners is deploying sustained, network-wide educational programs that will train primary care personnel in brief interventions for improved disease management such as motivational interviewing, behavioral activation, problem-solving therapy, and other first-line interventions suitable for a primary care setting. Fourth, all primary care practices are developing and deploying standard workflows for the identification and treatment of mental health illnesses, starting with depression. Fifth, telehealth technologies will be used to improve access to specialty care and provide care in the most cost-effective setting. An initial focus is on online cognitive behavioral therapy, with virtual visit technologies to follow. Finally, registries will track mental health outcomes and provide prompts to ensure that follow-up screening tests are administered at periodic intervals and that treatment plans can be modified if progress is insufficient.
Implementing Prayer-Agnostic Programs For Specialty Services
Historically, cost containment by commercial payers focused on limiting access to specialist services. However, since costs are concentrated in a small portion of the population with complex chronic illnesses, considering the problems caused by gatekeeping in the 1990s, limiting access to specialists for the entire population may not be an appropriate lever for lowering TME trend. In addition, enhanced access to specialty services has the potential to reduce costs and improve quality through more efficient testing and treatment regimens. We have approached specialist services with the philosophy that early and coordinated access, through the application of tools such as bi-directional referral management systems and virtual visit capabilities, will have a greater ability to lower costs. The challenge for deploying these tools is that ACOs are built off of an aligned population of patients attributed to primary care physicians. Typically, specialists in ACOs are providing care to both a fee-for-service population as well as the ACO population. The costs of providing a nonbillable service such as virtual visits is not sustainable if a large portion of the patients are not in a risk contract. We have found that integrating virtual visits and e-referrals for a limited set of a specialist’s patients poses workflow and ethical challenges. As a system with 2 prominent academic medical centers, we have therefore focused our efforts on deploying these tools to specialists who have a high proportion of patients from our primary care physicians, and continue to work through these significant challenges.
Our specialist engagement tools are focused primarily on improving access and coordination or ensuring appropriateness and optimal outcomes. First, virtual visits (asynchronous and synchronous) between patients and providers or between providers help improve access and coordination. Second, referral management systems that allow for pre-consultative communications and review with key clinical data and messages allow for more thoughtful specialist consultations. This active management of referrals allows specialists to provide accelerated “curbside” consults without a formal consult for minor issues, appropriate pre-appointment testing for improved initial in-person consultation, or accelerated scheduling for initial consultation for urgent issues. Third, we are implementing technology and workflows to capture patient-reported outcomes in our specialty practices. We are collecting and reporting this data internally and externally to ensure we are monitoring the metrics that are most important to our patients. Monitoring patient-reported outcomes is especially important when a provider is concurrently implementing cost-containment measures. Fourth, we have developed technology to assess the appropriateness of surgical procedures. This technology combines analytics of both structured and unstructured data in an electronic platform and provide feedback to providers and patients regarding relative risks and benefits of certain procedures [17]. Lastly, we are implementing clinical bundles around select surgical procedures.
As an ACO that includes academic medical centers, we have a particular challenge of balancing the mission of fostering innovative and experimental technologies that may help advance human health and medical science, while ensuring we are stewards of limited financial resources. Academic medical center–led ACOs will need to thoughtfully balance these objectives [4].
Improving Non-Acute Services
We have included both improved access to emergency department alternatives as well as a focus on the appropriate and efficient use of post acute services in our approach to non-acute services. We have approached improving access to urgent care services by both implementing standards for access to primary care (through PCMH transformation) as well as partnering with urgent care providers.
The use of post-acute care services is the main driver of hospital referral region cost variation for both Medicare and Medicaid [18]. If an ACO is taking on risk in either of these payer categories, it is imperative that the organization has a strategy for managing post-acute care. We have been developing the following capabilities:
- Determine most appropriate level of post-acute care upon discharge from an acute facility (eg, home health vs. skilled nursing facility)
- Predict, to some level of reasonable confidence, the length of post-acute services required per episode of care
- Create a high performance post-acute referral network that can meet quality, efficiency and cost standards set forth by the ACO
The challenge in meeting these goals include the lack of high quality data required to execute on the first 2 objectives. In addition, development of a post-acute referral network is dependent on regional market characteristics. For example, if the region’s supply of post-acute facilities is limited, enforcing the ACO standards for high-quality post-acute care may be challenging. Executing a post-acute strategy that helps an ACO meet its financial and quality objectives may be one of the more challenging endeavors the ACO will undertake.
Engaging Patients in Accountable Care
Accountable care contracts provide a new imperative for providers to offer tools that help patients engage in their care outside of the traditional clinical encounter. Promoting shared decision making, where patients share preferences and clinicians incorporate these beliefs into clinical decision-making, is one of our primary patient engagement strategies. Systematic reviews have demonstrated the effectiveness of shared decision making in improving patient awareness and reducing variation in health care utilization [19–21]. In addition to shared decision making, we have invested in new video education tools and have updated our electronic patient portal to allow patients to access their clinical record, review educational materials, and communicate with their care team at their convenience. Patient engagement strategies are also embedded into other initiatives, such as our high-risk care management program. Keeping patient engagement integrated into all of an accountable care organization’s programs, instead of treating it as a distinct program, is critical for success.
Implementing Clinical Information Technology Tools
A majority of current clinical information technology tools have been created for synchronous, in-person delivery of health care, reflecting the dominant mode of care delivery in the US. However, under accountable care payment models, ACOs have the opportunity (and imperative) to deliver care either asynchronously and/or remotely. As we assessed our needs, we recognized several gaps between existing and desired technologies. Though many population health information technology frameworks exist, we have found three broad categories required for successful population health management: advanced data warehousing and analytics, next generation care delivery and coordination tools, and innovative clinical performance management tools.
Advanced data warehousing and analytics: ACOs must be able to integrate and analyze multiple data sources, including payer derived claims data (providing data on care rendered both inside and outside the ACO) as well as administrative data (scheduling, billing), and clinical (both structured and unstructured). This requires an ACO to invest in data warehousing technologies and analytical tools in order to take full advantage of the available information and provide guidance to providers and managers. We continue to build this set of solutions.
Next generation care delivery and coordination tools: The ACO must have new ways to connect various members of the patient care team including patient to provider, and provider to provider. These technologies include but are not limited to asynchronous and synchronous virtual visit capabilities, referral management software and high risk care management software, and remote monitoring for carefully selected patients. We are currently employing all of these types of health information technology.
Innovative clinical performance management tools: Third, the accountable care organization should have clinical performance management technologies that allow its providers to reduce care gaps and improve stewardship of resources. This includes, for example, advanced clinical decision support for radiology ordering and procedures and patient registries with clinical workflow integration.
In general, we have found a majority of accountable care information technology vendors are repurposing their existing assets for new applications towards population health management. For example, warehousing companies with strengths in the financial industry may convert their product for the health care market, or a company built for patient outreach and appointment reminders may convert their product into population health clinical registries. Given the relatively new nature of risk-based contracting, and uncertainty of the future of this payment model, it makes good sense from a vendor perspective to first try to repurpose existing assets, instead of creating built-for-purpose technologies that are more costly, and may not get to market fast enough to meet customer needs. The challenge for providers is that many of these repurposed technologies do not quite solve the particular challenges the provider is attempting to address. Accountable care organizations therefore are faced with challenging decisions regarding the purchase of a less than optimal product, waiting for the product segment to mature, or building the solution themselves.
Conclusion
We have described an approach to ACO success that includes the creation of a sustainable financing mechanism, new incentive structures, a high-risk care management program, integrated mental health services, tools for specialist engagement, a post-acute strategy, fostering patient engagement, and new clinical and analytic technologies. The breadth and depth of these changes to care delivery present numerous daunting challenges. Our experience suggests that partial approaches, implementing just a subset of the approaches listed above, will not constrain cost growth because costs are just shifted to a different part of the care delivery system. We conclude from this experience that a comprehensive approach undertaken within a system that is capable of integrating care across the full continuum of care delivery has the best chance for successfully managing costs and improving care. Nonetheless, the challenges associated with change on this scale are legion. Key challenges facing the committed ACOs include: educating their boards and management about requirements for success as an ACO; advocacy for state and federal regulations that support the success of ACOs; and engaging patients as active participants in the changes. Importantly, financial returns (as shared savings) on the investment required for these changes will not be available within short-term contract cycles, so committed organizations will need to plan for the long haul.
Acknowledgment: The authors would like to thank the leadership of Partners Healthcare for their unambiguous support of the efforts described in this paper, as well as the clinicians, administrators, and support service workers that are committed to the achievement of the goals we have together set for the organization.
Corresponding author: Sreekanth K. Chaguturu, MD, 800 Boylston St., Ste. 1150, Boston, MA 02199, [email protected].
Financial disclosures: None.
1. Modern Healthcare’s 2014 accountable care organizations survey. Accessed 13 Aug 2014 at www.modernhealthcare.com/article/20140712/DATA/500032360/accountable-care-organizations-2014-excel-full-results.
2. McCall N, Cromwell J, Urato C. Evaluation of Medicare Care Management for High Cost Beneficiaries (CMHCB) Demonstration: Massachusetts General Hospital and Massachusetts General Physicians Organization (MGH). Centers for Medicare & Medicaid Services. 2010 Sept:1–171.
3. Milford CE, Ferris TG. A modified “golden rule” for health care organizations. Mayo Clin Proc 2012;87:717–20.
4. Nabel EG, Ferris TG, Slavin PL. Balancing AMCs’ missions and health care costs – mission impossible? N Engl J Med 2013;369:994–6.
5. Torchiana DF, Colton DG, Rao SK, et al. Massachusetts General Physicians Organization’s quality incentive program produces encouraging results. Health Aff 2013;32:1748–56.
6. Hong CS, Abrams MK, Ferris TG. Toward increased adoption of complex care management. N Engl J Med 2014;371:491–3.
7. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund) 2014;19:1–19.
8. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:617–27.
9. Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005;352:2515–23.
10. Druss BG, Walker ER. Mental disorders and medical comorbidity. Research synthesis report no. 21. Princeton, NJ: Robert Wood Johnson Foundation; 2011.
11. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Milliman Research Report. Seattle, WA: Milliman; 2008.
12. Leslie DL, Rosenbeck RA. Comparing quality of mental health care for public-sector and privately insured populations. Psychiatr Serv 2000; 51:650–5.
13. Goldman W. Economic grand rounds: is there a shortage of psychiatrists? Psychiatr Serv 2001;52:1587–9.
14. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314–21.
15. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry 2012;169:790–804.
16. Bower P, Gilbody S, Richards D, et al. Collaborative care for depression in primary care, making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484–93.
17. Milford CE, Hutter MM, Lillemoe KD, Ferris TG. Optimizing appropriate use of procedures in an era of payment reform. Ann Surg 2014;260:204–4.
18. Geographic variation in spending, utilization and quality: Medicare and Medicaid beneficiaries. May 2013. Accessed 13 Aug 2014 at http://iom.edu/~/media/Files/Report%20Files/2013/Geographic-Variation/Sub-Contractor/Acumen-Medicare-Medicaid.pdf
19. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011;(10)CD001431.
20. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6–8.
21. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood) 2012;31:2094–104.
1. Modern Healthcare’s 2014 accountable care organizations survey. Accessed 13 Aug 2014 at www.modernhealthcare.com/article/20140712/DATA/500032360/accountable-care-organizations-2014-excel-full-results.
2. McCall N, Cromwell J, Urato C. Evaluation of Medicare Care Management for High Cost Beneficiaries (CMHCB) Demonstration: Massachusetts General Hospital and Massachusetts General Physicians Organization (MGH). Centers for Medicare & Medicaid Services. 2010 Sept:1–171.
3. Milford CE, Ferris TG. A modified “golden rule” for health care organizations. Mayo Clin Proc 2012;87:717–20.
4. Nabel EG, Ferris TG, Slavin PL. Balancing AMCs’ missions and health care costs – mission impossible? N Engl J Med 2013;369:994–6.
5. Torchiana DF, Colton DG, Rao SK, et al. Massachusetts General Physicians Organization’s quality incentive program produces encouraging results. Health Aff 2013;32:1748–56.
6. Hong CS, Abrams MK, Ferris TG. Toward increased adoption of complex care management. N Engl J Med 2014;371:491–3.
7. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund) 2014;19:1–19.
8. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:617–27.
9. Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005;352:2515–23.
10. Druss BG, Walker ER. Mental disorders and medical comorbidity. Research synthesis report no. 21. Princeton, NJ: Robert Wood Johnson Foundation; 2011.
11. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Milliman Research Report. Seattle, WA: Milliman; 2008.
12. Leslie DL, Rosenbeck RA. Comparing quality of mental health care for public-sector and privately insured populations. Psychiatr Serv 2000; 51:650–5.
13. Goldman W. Economic grand rounds: is there a shortage of psychiatrists? Psychiatr Serv 2001;52:1587–9.
14. Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314–21.
15. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry 2012;169:790–804.
16. Bower P, Gilbody S, Richards D, et al. Collaborative care for depression in primary care, making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484–93.
17. Milford CE, Hutter MM, Lillemoe KD, Ferris TG. Optimizing appropriate use of procedures in an era of payment reform. Ann Surg 2014;260:204–4.
18. Geographic variation in spending, utilization and quality: Medicare and Medicaid beneficiaries. May 2013. Accessed 13 Aug 2014 at http://iom.edu/~/media/Files/Report%20Files/2013/Geographic-Variation/Sub-Contractor/Acumen-Medicare-Medicaid.pdf
19. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011;(10)CD001431.
20. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6–8.
21. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood) 2012;31:2094–104.
Improved Coordination of Care for Patients with Abnormalities on Chest Imaging: The Rapid Access Chest and Lung Assessment Program
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
Postop Patient Reports “Wound Infection”
ANSWER
The correct answer is an allergic reaction to a contactant, most likely the triple-antibiotic ointment (choice “d”).
Irritant reactions to tape adhesive (choice “a”) are extremely common. However, the resultant rash would have been confined to the linear areas where the tape touched his skin.
Dissolving sutures, such as those used in this case, can provoke a “suture granuloma”—essentially a foreign body reaction to the suture material (choice “b”). But this would have caused a focal area of swelling and redness, and very possibly a show of pus.
Postop wound infections (choice “c”) are also quite common. However, they would not manifest solely with itching in a papulovesicular rash surrounding the wound. Had infection developed, the redness would have been broad-based, with ill-defined margins, and the patient’s complaint would have been of pain, not itching. No vesicles would have been seen with bacterial infection.
DISCUSSION
This case illustrates the phenomenon of “treatment as problem,” in which the medication the patient applies becomes more problematic than the condition being addressed. Reactions to the neomycin in triple-antibiotic ointment are common but still provoke considerable worry on the part of patients and providers alike, especially when mistaken for “infection.”
This patient, like many, was dubious of the diagnosis, pointing out that he had used this same topical medication on many occasions without incident (though not recently). What he didn’t know is that it takes repeated exposure to a given allergen to develop T-memory cells that eventually begin to react. This same phenomenon is seen with poison ivy; patients will recall the ability, as a child, to practically wallow in poison ivy with impunity, making them doubtful about being allergic to it as an adult.
Neomycin, an aminoglycoside with a fairly wide spectrum of antibacterial activity, was first noted as a contact allergen in 1952. It is such a notorious offender that it was named Allergen of the Year in 2010 by the American Contact Dermatology Society.
For the past 20 years, 7% to 13% of patch tests surveyed were positive for neomycin. For reasons not entirely clear, Americans older than 60 are 150% more likely to experience a reaction to neomycin than are younger patients. (It could simply be that they’ve had more chances for exposure.)
In another interesting twist, the ointment vehicle appears to play a role. A reaction to this preparation is considerably more likely than to the same drug in other forms (eg, powders, solutions, creams). This is true of most medications, such as topical steroids, which are effectively self-occluded by this vehicle.
Persons with impaired barrier function, such as those with atopic dermatitis or whose skin has been prepped for surgery, appear to be at increased risk for these types of contact dermatoses.
Though there are other items in the differential, the configuration of the papulovesicular rash and the sole symptom of itching are essentially pathognomic for contact dermatitis. Besides the use of potent topical steroids for a few days, the real “cure” for this problem is for the patient to switch to “double-antibiotic” creams or ointments that do not include neomycin.
ANSWER
The correct answer is an allergic reaction to a contactant, most likely the triple-antibiotic ointment (choice “d”).
Irritant reactions to tape adhesive (choice “a”) are extremely common. However, the resultant rash would have been confined to the linear areas where the tape touched his skin.
Dissolving sutures, such as those used in this case, can provoke a “suture granuloma”—essentially a foreign body reaction to the suture material (choice “b”). But this would have caused a focal area of swelling and redness, and very possibly a show of pus.
Postop wound infections (choice “c”) are also quite common. However, they would not manifest solely with itching in a papulovesicular rash surrounding the wound. Had infection developed, the redness would have been broad-based, with ill-defined margins, and the patient’s complaint would have been of pain, not itching. No vesicles would have been seen with bacterial infection.
DISCUSSION
This case illustrates the phenomenon of “treatment as problem,” in which the medication the patient applies becomes more problematic than the condition being addressed. Reactions to the neomycin in triple-antibiotic ointment are common but still provoke considerable worry on the part of patients and providers alike, especially when mistaken for “infection.”
This patient, like many, was dubious of the diagnosis, pointing out that he had used this same topical medication on many occasions without incident (though not recently). What he didn’t know is that it takes repeated exposure to a given allergen to develop T-memory cells that eventually begin to react. This same phenomenon is seen with poison ivy; patients will recall the ability, as a child, to practically wallow in poison ivy with impunity, making them doubtful about being allergic to it as an adult.
Neomycin, an aminoglycoside with a fairly wide spectrum of antibacterial activity, was first noted as a contact allergen in 1952. It is such a notorious offender that it was named Allergen of the Year in 2010 by the American Contact Dermatology Society.
For the past 20 years, 7% to 13% of patch tests surveyed were positive for neomycin. For reasons not entirely clear, Americans older than 60 are 150% more likely to experience a reaction to neomycin than are younger patients. (It could simply be that they’ve had more chances for exposure.)
In another interesting twist, the ointment vehicle appears to play a role. A reaction to this preparation is considerably more likely than to the same drug in other forms (eg, powders, solutions, creams). This is true of most medications, such as topical steroids, which are effectively self-occluded by this vehicle.
Persons with impaired barrier function, such as those with atopic dermatitis or whose skin has been prepped for surgery, appear to be at increased risk for these types of contact dermatoses.
Though there are other items in the differential, the configuration of the papulovesicular rash and the sole symptom of itching are essentially pathognomic for contact dermatitis. Besides the use of potent topical steroids for a few days, the real “cure” for this problem is for the patient to switch to “double-antibiotic” creams or ointments that do not include neomycin.
ANSWER
The correct answer is an allergic reaction to a contactant, most likely the triple-antibiotic ointment (choice “d”).
Irritant reactions to tape adhesive (choice “a”) are extremely common. However, the resultant rash would have been confined to the linear areas where the tape touched his skin.
Dissolving sutures, such as those used in this case, can provoke a “suture granuloma”—essentially a foreign body reaction to the suture material (choice “b”). But this would have caused a focal area of swelling and redness, and very possibly a show of pus.
Postop wound infections (choice “c”) are also quite common. However, they would not manifest solely with itching in a papulovesicular rash surrounding the wound. Had infection developed, the redness would have been broad-based, with ill-defined margins, and the patient’s complaint would have been of pain, not itching. No vesicles would have been seen with bacterial infection.
DISCUSSION
This case illustrates the phenomenon of “treatment as problem,” in which the medication the patient applies becomes more problematic than the condition being addressed. Reactions to the neomycin in triple-antibiotic ointment are common but still provoke considerable worry on the part of patients and providers alike, especially when mistaken for “infection.”
This patient, like many, was dubious of the diagnosis, pointing out that he had used this same topical medication on many occasions without incident (though not recently). What he didn’t know is that it takes repeated exposure to a given allergen to develop T-memory cells that eventually begin to react. This same phenomenon is seen with poison ivy; patients will recall the ability, as a child, to practically wallow in poison ivy with impunity, making them doubtful about being allergic to it as an adult.
Neomycin, an aminoglycoside with a fairly wide spectrum of antibacterial activity, was first noted as a contact allergen in 1952. It is such a notorious offender that it was named Allergen of the Year in 2010 by the American Contact Dermatology Society.
For the past 20 years, 7% to 13% of patch tests surveyed were positive for neomycin. For reasons not entirely clear, Americans older than 60 are 150% more likely to experience a reaction to neomycin than are younger patients. (It could simply be that they’ve had more chances for exposure.)
In another interesting twist, the ointment vehicle appears to play a role. A reaction to this preparation is considerably more likely than to the same drug in other forms (eg, powders, solutions, creams). This is true of most medications, such as topical steroids, which are effectively self-occluded by this vehicle.
Persons with impaired barrier function, such as those with atopic dermatitis or whose skin has been prepped for surgery, appear to be at increased risk for these types of contact dermatoses.
Though there are other items in the differential, the configuration of the papulovesicular rash and the sole symptom of itching are essentially pathognomic for contact dermatitis. Besides the use of potent topical steroids for a few days, the real “cure” for this problem is for the patient to switch to “double-antibiotic” creams or ointments that do not include neomycin.
A week ago, a 56-year-old man had a skin cancer surgically removed. Last night, he presented to an urgent care clinic for evaluation of a “wound infection” and received a prescription for double-strength trimethoprim/sulfa tablets (to be taken bid for 10 days). He is now in the dermatology office for follow-up. According to the patient, the problem manifested two days postop. There was no associated pain, only itching. The patient feels fine, with no fever or malaise, and there is no history of immunosuppression. He reports following his postop instructions well, changing his bandage daily and using triple-antibiotic ointment to dress the wound directly. The immediate peri-incisional area is indicated as the source of the problem. Surrounding the incision, which is healing well otherwise, is a sharply defined, bright pink, papulovesicular rash on a slightly edematous base. There is no tenderness on palpation, and no purulent material can be expressed from the wound. The area is only slightly warmer than the surrounding skin.
Optimal Wheelchair Service Provision for Children with Disabilities
Study Overview
Objective. To conduct a systematic review on the effectiveness, service user perspectives, policy intentions, and cost-effectiveness of wheelchairs for disabled children (< 18 years) for the purposes of developing a conceptual framework to inform future research and development.
Design. EPPI-Centre (eppi.ioe.ac.uk/cms/) mixed method systematic review with narrative summary and thematic and narrative synthesis.
Data. A search for relevant studies available in English and published in the last 15 years was performed. All identified study titles were assessed for relevance against the inclusion/exclusion criteria, and a second screening process was used to assess relevance of studies by their abstract. Studies deemed relevant were then obtained in full and underwent an additional review by a second researcher to reduce bias and reach consensus regarding inclusion. After data extraction, evidence was divided into 4 streams according to methodology and topic to enable separate syntheses by evidence type: (1) intervention evidence; (2) opinion evidence; (3) policy and not-for-profit organization (NFPO) literature; and (4) economic evidence. Intervention and economic streams were not synthesised due to vast differences in studies and lack of statistical evidence within each stream, thus narrative summary was conducted.
Main outcome. The primary outcome was to create a conceptual framework to inform future research and wheelchair service development in the UK, with international implications. To inform the searching, management, and interpretation of evidence, the review focused on the following 4 objectives regarding wheelchair interventions for disabled children and young people:
- to determine the effectiveness and cost-effectiveness of wheelchairs for said population;
- to better understand service users, parents, and professional perspectives regarding wheelchairs;
- to explore current UK policy, NFPO publication and clinical guideline recommendations and intentions regarding wheelchair provision; and
- to determine if disabled children’s desired outcomes match with existing policy aspirations and effectiveness evidence.
Main results. Synthesis of the integrated dataset elicited the following findings: (1) higher quality wheelchair services take into account the needs of the whole family; (2) disabled children benefit when psychosocial needs are considered along with health needs; (3) disabled children could benefit if policy recommendations focused on services meeting individual needs rather than following strict eligibility criteria; (4) without appropriate outcome measures the holistic benefits of powered wheelchair interventions cannot be evaluated; (5) disabled children may benefit more when physical outcomes of powered wheelchairs are seen as facilitators to wider holistic benefits, but lack of transition of evidence into practice hinders progress; and (6) disabled children would benefit from public buildings and spaces that promote inclusion of disabled people.
A key finding of this study is that wheelchairs offer disabled children independence and social integration and participation in age-appropriate activities. Secondary findings pertain to policy, specifically, the lack of effective translation of policy and evidence into practice, barriers to service delivery, lack of organization, and absence of knowledge application of what children desire from their wheelchairs. The resulting framework of this review lays out the interconnectedness of the problem areas, required actions, and overall development stages, which can lead to future cost-effective wheelchair services and interventions.
Conclusion. Wheelchairs offer children a variety of benefits, particularly with respect to health, development, and social inclusion. Given the barriers surrounding NHS wheelchair services in the UK, this review provides a solid research foundation for further research and has implications for wheelchair services globally. In particular, the lack of economic evidence found in the review process has implications for the need of appropriate methods to measure cost-effectiveness of interventions in order to promote more efficient service provision.
Commentary
Wheelchair access has compelling implications for improving children’s health, development, and social inclusion. It is this final benefit in particular that makes wheelchair interventions stand out from the rest due to the fact that the wheelchair goes beyond being a medical assistive device to being a gateway to societal participation.
Also related to social inclusion, an additional relevant observation made in this study is the lack of wheelchair access in public spaces. Such barriers can deem a wheelchair irrelevant if it cannot be used in the spaces where the subject needs to travel, such as schools, restaurants, parks, or government offices. Though this study was based in the UK, such barriers to inclusion remain all too common for persons with disability around the world [1], thus positioning the results of study as a starting framework for further global research.
That being said, the authors’ recommendations of resolving public space barriers with the simple addition of wheelchair access is an outdated approach towards inclusion that has been widely challenged by the community of persons with disabilities over the last decade. The promotion of “inclusive design” or “human-centered design” [2] to properly address the challenges of persons with disability is a growing trend, particularly in the United States, and takes into account the highest possible degree of permutation in the local demographics. A recommendation limited to mere wheelchair access stands to shut out other significant portions of the disabled community and exacerbates a “patchwork” approach toward resolving access that is not truly holistic.
Another observation pertains to the financial burden imposed on the family to modify the home when a child in the household has to use a wheelchair. While this is discussed in the article, it is treated separately as it occurs in the private sphere. However, construction regulations, permits, and other aspects pertaining to home-building reside on the government and policy side, even if a private independent entity does the home-building. Hence, policy changes towards inclusive design have implications for both public as well as private spaces.
With respect to the benefits of wheelchair interventions, the authors contend that appropriate interventions stand to “reduce disability discrimination and promote equality.” Concepts such as “discrimination” and “equality” cannot be discussed without political and cultural considerations [3]. The linkage between access and equality is a correlation worthy of discussion; however, the study was not designed to gather data to support sucha correlation.
Finally, while the overall findings of this study are relevant, it would be useful to know more about wheelchair service provision and the elderly, as it is they who comprise the majority of the disabled population [1,4]. The elderly disabled also need caretakers, need to make home modifications and travel to and from public spaces, and experience barriers and service delays Future research on wheelchair interventions would benefit from a comparative intra-population analysis.
Applications for Clinical Practice
This study outlines critical challenges and problems in the process of obtaining a wheelchair, such as poor evaluation methods for matching a wheelchair to patient needs, bureaucratic delays even after the order has been approved, physical accommodations that need to take place once the wheelchair has been acquired, and financial burdens assumed by the family and/or caretakers. Consideration needs to be given to addressing these problems given the importance of adequate wheelchairs for many disabled people.
—Molly A. Martínez, PhD, World Enabled, Berkeley, CA
1. World Health Organization. World report on disability 2011. Available at whqlibdoc.who.int/publications/2011/9789240685215_eng.pdf?ua–1.
2. Fletcher V. Driving innovation: universal design. Presented 14 Jan 2014 at IOM workshop. Available at www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/HearingLoss
Aging/2-7%20Fletcher.pdf .
3. United Nations Permanent Forum on Indigenous Issues. Study on the situation of indigenous persons with disabilities, with a particular focus on challenges faced with regard to the full enjoyment of human rights and inclusion in development. May 2013. Available at www.un.org/disabilities/documents/ecosoc/e.c.19.2013.6.pdf.
4. United Nations Department of Economic and Social Affairs. World population ageing 2013. Available at www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf.
Study Overview
Objective. To conduct a systematic review on the effectiveness, service user perspectives, policy intentions, and cost-effectiveness of wheelchairs for disabled children (< 18 years) for the purposes of developing a conceptual framework to inform future research and development.
Design. EPPI-Centre (eppi.ioe.ac.uk/cms/) mixed method systematic review with narrative summary and thematic and narrative synthesis.
Data. A search for relevant studies available in English and published in the last 15 years was performed. All identified study titles were assessed for relevance against the inclusion/exclusion criteria, and a second screening process was used to assess relevance of studies by their abstract. Studies deemed relevant were then obtained in full and underwent an additional review by a second researcher to reduce bias and reach consensus regarding inclusion. After data extraction, evidence was divided into 4 streams according to methodology and topic to enable separate syntheses by evidence type: (1) intervention evidence; (2) opinion evidence; (3) policy and not-for-profit organization (NFPO) literature; and (4) economic evidence. Intervention and economic streams were not synthesised due to vast differences in studies and lack of statistical evidence within each stream, thus narrative summary was conducted.
Main outcome. The primary outcome was to create a conceptual framework to inform future research and wheelchair service development in the UK, with international implications. To inform the searching, management, and interpretation of evidence, the review focused on the following 4 objectives regarding wheelchair interventions for disabled children and young people:
- to determine the effectiveness and cost-effectiveness of wheelchairs for said population;
- to better understand service users, parents, and professional perspectives regarding wheelchairs;
- to explore current UK policy, NFPO publication and clinical guideline recommendations and intentions regarding wheelchair provision; and
- to determine if disabled children’s desired outcomes match with existing policy aspirations and effectiveness evidence.
Main results. Synthesis of the integrated dataset elicited the following findings: (1) higher quality wheelchair services take into account the needs of the whole family; (2) disabled children benefit when psychosocial needs are considered along with health needs; (3) disabled children could benefit if policy recommendations focused on services meeting individual needs rather than following strict eligibility criteria; (4) without appropriate outcome measures the holistic benefits of powered wheelchair interventions cannot be evaluated; (5) disabled children may benefit more when physical outcomes of powered wheelchairs are seen as facilitators to wider holistic benefits, but lack of transition of evidence into practice hinders progress; and (6) disabled children would benefit from public buildings and spaces that promote inclusion of disabled people.
A key finding of this study is that wheelchairs offer disabled children independence and social integration and participation in age-appropriate activities. Secondary findings pertain to policy, specifically, the lack of effective translation of policy and evidence into practice, barriers to service delivery, lack of organization, and absence of knowledge application of what children desire from their wheelchairs. The resulting framework of this review lays out the interconnectedness of the problem areas, required actions, and overall development stages, which can lead to future cost-effective wheelchair services and interventions.
Conclusion. Wheelchairs offer children a variety of benefits, particularly with respect to health, development, and social inclusion. Given the barriers surrounding NHS wheelchair services in the UK, this review provides a solid research foundation for further research and has implications for wheelchair services globally. In particular, the lack of economic evidence found in the review process has implications for the need of appropriate methods to measure cost-effectiveness of interventions in order to promote more efficient service provision.
Commentary
Wheelchair access has compelling implications for improving children’s health, development, and social inclusion. It is this final benefit in particular that makes wheelchair interventions stand out from the rest due to the fact that the wheelchair goes beyond being a medical assistive device to being a gateway to societal participation.
Also related to social inclusion, an additional relevant observation made in this study is the lack of wheelchair access in public spaces. Such barriers can deem a wheelchair irrelevant if it cannot be used in the spaces where the subject needs to travel, such as schools, restaurants, parks, or government offices. Though this study was based in the UK, such barriers to inclusion remain all too common for persons with disability around the world [1], thus positioning the results of study as a starting framework for further global research.
That being said, the authors’ recommendations of resolving public space barriers with the simple addition of wheelchair access is an outdated approach towards inclusion that has been widely challenged by the community of persons with disabilities over the last decade. The promotion of “inclusive design” or “human-centered design” [2] to properly address the challenges of persons with disability is a growing trend, particularly in the United States, and takes into account the highest possible degree of permutation in the local demographics. A recommendation limited to mere wheelchair access stands to shut out other significant portions of the disabled community and exacerbates a “patchwork” approach toward resolving access that is not truly holistic.
Another observation pertains to the financial burden imposed on the family to modify the home when a child in the household has to use a wheelchair. While this is discussed in the article, it is treated separately as it occurs in the private sphere. However, construction regulations, permits, and other aspects pertaining to home-building reside on the government and policy side, even if a private independent entity does the home-building. Hence, policy changes towards inclusive design have implications for both public as well as private spaces.
With respect to the benefits of wheelchair interventions, the authors contend that appropriate interventions stand to “reduce disability discrimination and promote equality.” Concepts such as “discrimination” and “equality” cannot be discussed without political and cultural considerations [3]. The linkage between access and equality is a correlation worthy of discussion; however, the study was not designed to gather data to support sucha correlation.
Finally, while the overall findings of this study are relevant, it would be useful to know more about wheelchair service provision and the elderly, as it is they who comprise the majority of the disabled population [1,4]. The elderly disabled also need caretakers, need to make home modifications and travel to and from public spaces, and experience barriers and service delays Future research on wheelchair interventions would benefit from a comparative intra-population analysis.
Applications for Clinical Practice
This study outlines critical challenges and problems in the process of obtaining a wheelchair, such as poor evaluation methods for matching a wheelchair to patient needs, bureaucratic delays even after the order has been approved, physical accommodations that need to take place once the wheelchair has been acquired, and financial burdens assumed by the family and/or caretakers. Consideration needs to be given to addressing these problems given the importance of adequate wheelchairs for many disabled people.
—Molly A. Martínez, PhD, World Enabled, Berkeley, CA
Study Overview
Objective. To conduct a systematic review on the effectiveness, service user perspectives, policy intentions, and cost-effectiveness of wheelchairs for disabled children (< 18 years) for the purposes of developing a conceptual framework to inform future research and development.
Design. EPPI-Centre (eppi.ioe.ac.uk/cms/) mixed method systematic review with narrative summary and thematic and narrative synthesis.
Data. A search for relevant studies available in English and published in the last 15 years was performed. All identified study titles were assessed for relevance against the inclusion/exclusion criteria, and a second screening process was used to assess relevance of studies by their abstract. Studies deemed relevant were then obtained in full and underwent an additional review by a second researcher to reduce bias and reach consensus regarding inclusion. After data extraction, evidence was divided into 4 streams according to methodology and topic to enable separate syntheses by evidence type: (1) intervention evidence; (2) opinion evidence; (3) policy and not-for-profit organization (NFPO) literature; and (4) economic evidence. Intervention and economic streams were not synthesised due to vast differences in studies and lack of statistical evidence within each stream, thus narrative summary was conducted.
Main outcome. The primary outcome was to create a conceptual framework to inform future research and wheelchair service development in the UK, with international implications. To inform the searching, management, and interpretation of evidence, the review focused on the following 4 objectives regarding wheelchair interventions for disabled children and young people:
- to determine the effectiveness and cost-effectiveness of wheelchairs for said population;
- to better understand service users, parents, and professional perspectives regarding wheelchairs;
- to explore current UK policy, NFPO publication and clinical guideline recommendations and intentions regarding wheelchair provision; and
- to determine if disabled children’s desired outcomes match with existing policy aspirations and effectiveness evidence.
Main results. Synthesis of the integrated dataset elicited the following findings: (1) higher quality wheelchair services take into account the needs of the whole family; (2) disabled children benefit when psychosocial needs are considered along with health needs; (3) disabled children could benefit if policy recommendations focused on services meeting individual needs rather than following strict eligibility criteria; (4) without appropriate outcome measures the holistic benefits of powered wheelchair interventions cannot be evaluated; (5) disabled children may benefit more when physical outcomes of powered wheelchairs are seen as facilitators to wider holistic benefits, but lack of transition of evidence into practice hinders progress; and (6) disabled children would benefit from public buildings and spaces that promote inclusion of disabled people.
A key finding of this study is that wheelchairs offer disabled children independence and social integration and participation in age-appropriate activities. Secondary findings pertain to policy, specifically, the lack of effective translation of policy and evidence into practice, barriers to service delivery, lack of organization, and absence of knowledge application of what children desire from their wheelchairs. The resulting framework of this review lays out the interconnectedness of the problem areas, required actions, and overall development stages, which can lead to future cost-effective wheelchair services and interventions.
Conclusion. Wheelchairs offer children a variety of benefits, particularly with respect to health, development, and social inclusion. Given the barriers surrounding NHS wheelchair services in the UK, this review provides a solid research foundation for further research and has implications for wheelchair services globally. In particular, the lack of economic evidence found in the review process has implications for the need of appropriate methods to measure cost-effectiveness of interventions in order to promote more efficient service provision.
Commentary
Wheelchair access has compelling implications for improving children’s health, development, and social inclusion. It is this final benefit in particular that makes wheelchair interventions stand out from the rest due to the fact that the wheelchair goes beyond being a medical assistive device to being a gateway to societal participation.
Also related to social inclusion, an additional relevant observation made in this study is the lack of wheelchair access in public spaces. Such barriers can deem a wheelchair irrelevant if it cannot be used in the spaces where the subject needs to travel, such as schools, restaurants, parks, or government offices. Though this study was based in the UK, such barriers to inclusion remain all too common for persons with disability around the world [1], thus positioning the results of study as a starting framework for further global research.
That being said, the authors’ recommendations of resolving public space barriers with the simple addition of wheelchair access is an outdated approach towards inclusion that has been widely challenged by the community of persons with disabilities over the last decade. The promotion of “inclusive design” or “human-centered design” [2] to properly address the challenges of persons with disability is a growing trend, particularly in the United States, and takes into account the highest possible degree of permutation in the local demographics. A recommendation limited to mere wheelchair access stands to shut out other significant portions of the disabled community and exacerbates a “patchwork” approach toward resolving access that is not truly holistic.
Another observation pertains to the financial burden imposed on the family to modify the home when a child in the household has to use a wheelchair. While this is discussed in the article, it is treated separately as it occurs in the private sphere. However, construction regulations, permits, and other aspects pertaining to home-building reside on the government and policy side, even if a private independent entity does the home-building. Hence, policy changes towards inclusive design have implications for both public as well as private spaces.
With respect to the benefits of wheelchair interventions, the authors contend that appropriate interventions stand to “reduce disability discrimination and promote equality.” Concepts such as “discrimination” and “equality” cannot be discussed without political and cultural considerations [3]. The linkage between access and equality is a correlation worthy of discussion; however, the study was not designed to gather data to support sucha correlation.
Finally, while the overall findings of this study are relevant, it would be useful to know more about wheelchair service provision and the elderly, as it is they who comprise the majority of the disabled population [1,4]. The elderly disabled also need caretakers, need to make home modifications and travel to and from public spaces, and experience barriers and service delays Future research on wheelchair interventions would benefit from a comparative intra-population analysis.
Applications for Clinical Practice
This study outlines critical challenges and problems in the process of obtaining a wheelchair, such as poor evaluation methods for matching a wheelchair to patient needs, bureaucratic delays even after the order has been approved, physical accommodations that need to take place once the wheelchair has been acquired, and financial burdens assumed by the family and/or caretakers. Consideration needs to be given to addressing these problems given the importance of adequate wheelchairs for many disabled people.
—Molly A. Martínez, PhD, World Enabled, Berkeley, CA
1. World Health Organization. World report on disability 2011. Available at whqlibdoc.who.int/publications/2011/9789240685215_eng.pdf?ua–1.
2. Fletcher V. Driving innovation: universal design. Presented 14 Jan 2014 at IOM workshop. Available at www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/HearingLoss
Aging/2-7%20Fletcher.pdf .
3. United Nations Permanent Forum on Indigenous Issues. Study on the situation of indigenous persons with disabilities, with a particular focus on challenges faced with regard to the full enjoyment of human rights and inclusion in development. May 2013. Available at www.un.org/disabilities/documents/ecosoc/e.c.19.2013.6.pdf.
4. United Nations Department of Economic and Social Affairs. World population ageing 2013. Available at www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf.
1. World Health Organization. World report on disability 2011. Available at whqlibdoc.who.int/publications/2011/9789240685215_eng.pdf?ua–1.
2. Fletcher V. Driving innovation: universal design. Presented 14 Jan 2014 at IOM workshop. Available at www.iom.edu/~/media/Files/Activity%20Files/PublicHealth/HearingLoss
Aging/2-7%20Fletcher.pdf .
3. United Nations Permanent Forum on Indigenous Issues. Study on the situation of indigenous persons with disabilities, with a particular focus on challenges faced with regard to the full enjoyment of human rights and inclusion in development. May 2013. Available at www.un.org/disabilities/documents/ecosoc/e.c.19.2013.6.pdf.
4. United Nations Department of Economic and Social Affairs. World population ageing 2013. Available at www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf.
Quality of Life in Aging Multiple Sclerosis Patients
Study Overview
Objective. To evaluate the association between clinical and demographic factors and health-related quality of life (HRQOL) among older people with multiple sclerosis (MS).
Design. Cross-sectional survey-based study.
Setting and participants. Patients with MS aged 60 years or older were recruited from 4 MS centers in Long Island, NY. Patients with severe cognitive impairment as determined by the health care practitioner were excluded. Participants were asked to complete 3 surveys at 3 different time-points. In the first survey, participants completed the Morisky Medication Adherence Scale and the Patient Multiple Sclerosis Neuropsychological Screening Questionnaire (P-MSNQ). The second survey was the Multiple Sclerosis Quality of Life-54 (MSQOL-54), and the third survey included the Beck Depression Inventory-II (BDI-II) and a disability status self-assessment scale. Cognitive function was measured at the time of recruitment using the Symbol Digit Modalities Test (SDMT).
Analysis. The Andersen Healthcare Utilization model was used to structure the multivariate regression analysis. This model identifies multiple domains affecting quality of life, and the variables from the surveys were categorized according to domain: predisposing characteristics (demographic variables), enabling resources (caregiver support and living situation), needs (eg, health-related measures), and health behaviors (medication use, adherence).
Main results. A total of 211 completed the first survey, 188 the second, and 179 the third. 80% were female and 95% were white. Average age was 65.5 (SD 5.6) years. 56% of respondents’ self-reported scores on the SDMT classified them as cognitively impaired. Risk of neuropsychological impairment, depression, and disability status were significantly associated with a decreased mental and physical HRQOL. Significantly, there was a strong association between predisposing characteristics and QOL. Being widowed and remaining employed were the strongest predictors of better physical QOL and having an education level of high school or less was a predictor of lower mental HRQOL.
Conclusion. Clinicians should measure HRQOL in older MS patients regularly and assess for depression and cognitive impairment.
Commentary
Quality of life is an important marker of MS patients’ well-being as they cope with this chronic illness [1]. The progression of the disease and its symptomatology often negatively affect HRQOL. However, multiple psychosocial factors, such as coping, mood, self-efficacy, and perceived support, affect QOL of patients with MS more than biological variables such as weakness or burden of radiologic disease [2]. For example, many self-report HRQOL indices are strongly predicted by measures of depression [3]. In addition, many studies have found a positive association between physical disability and reduced QOL [4,5]. Further, while perceived HRQOL may be a meaningful outcome in itself, it may also be a predictor for outcomes such as disability-related changes [6].
MS leads to disability and loss of function in all age-groups, but only a few studies have focused on HRQOL among elderly patients with MS. As patients with MS age, they may develop comorbidities such as hypertension and diabetes that may affect HRQOL. However, in a previous study comparing QOL between older and younger patients with MS, elderly and younger patients with MS had similar QOL even though the elderly patients had more physical limitations [7].
The strength of the current study was using the Andersen Healthcare Utilization regression model in the analysis, since it factors in multiple influences on health status. The striking evidence that employment and being widowed were linked to better physical QOL suggest that older MS patients may have better adaptation and adjustment to their illness. Researchers have shown that the widowed elderly often take on more responsibilities and tasks when they lose their partner, which leads to increased self-esteem and QOL [8]. Another advantage of the study was the fact that the investigators evaluated the different exposure variables and their associations with mental and physical QOL while identifying multiple confounding variables. Additionally, the use of 2 cognitive assessment tools provided a stronger assessment of patients’ cognitive function.
The main weakness of the study was using a cross-sectional study design with convenience sampling. The convenience sample was based on voluntary participation, which may result in self-selection bias. In addition, the self-report design is subject to the usual limitations of self-reporting for data collection: participants may exaggerate symptoms in order to make their situation seem worse or may under-report the severity or frequency of symptoms in order to minimize their problems. While the overall sample size was 211, not all respondents completed all the surveys, and response rates varied by questions. Thus, missing data may have affected results, but which data are missing is not discernable from the paper. That patients were from a single geographic area and had relatively high education levels (44% with college or above) are among the factors that limit the generalizability of the study. Another limitation is the use of the Beck Depression Inventory, which was not specifically designed for use in the elderly. In addition, the results of this study might have been affected by unmeasured confounding variables, for example daily physical activity, which can be a factor that modifies between depression, cognition, and QOL.
Applications for Clinical Practice
This study reinforces the importance of monitoring older MS patients for factors that may influence their HRQOL. The presence of depression, disability, and cognitive impairment should be assessed for regularly. Clinicians should encourage and empower elderly patients to continue with activities, including employment, that promote their mental and physical well-being and help maintain their independence. Assessing patients with geriatric-specific tools may provide more reliable and accurate assessment data that better accounts for aging dynamics. In addition, comobidities must be managed appropriately.
—Aliza Bitton Ben-Zacharia, DNP, ANP, and Allison Squires, PhD, RN, New York University College of Nursing
1. Opara JA, Jaracz K, Brola W. Quality of life in multiple sclerosis. J Med Life 2010;3:352–8.
2. Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol 2005;4:556–66.
3. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 2005;231:29–34.
4. Göksel Karatepe A, Kaya T, Günaydn R, et al. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int J Rehabil Res 2011;34:290–8.
5. Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 1999;53:1098–103.
6. Visschedijk MA, Uitdehaag BM, Klein M, et al. Value of health-related quality of life to predict disability course in multiple sclerosis. Neurology 2004;63:2046–50.
7. Ploughman M, Austin MW, Murdoch M, et al. Factors influencing healthy aging with multiple sclerosis: a qualitative study. Disabil Rehabil 2012;34:26–33.
8. Minden SL, Frankel D, Hadden LS, et al. Disability in elderly people with multiple sclerosis: An analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. NeuroRehabilitation. 2004;19:55–67.
Study Overview
Objective. To evaluate the association between clinical and demographic factors and health-related quality of life (HRQOL) among older people with multiple sclerosis (MS).
Design. Cross-sectional survey-based study.
Setting and participants. Patients with MS aged 60 years or older were recruited from 4 MS centers in Long Island, NY. Patients with severe cognitive impairment as determined by the health care practitioner were excluded. Participants were asked to complete 3 surveys at 3 different time-points. In the first survey, participants completed the Morisky Medication Adherence Scale and the Patient Multiple Sclerosis Neuropsychological Screening Questionnaire (P-MSNQ). The second survey was the Multiple Sclerosis Quality of Life-54 (MSQOL-54), and the third survey included the Beck Depression Inventory-II (BDI-II) and a disability status self-assessment scale. Cognitive function was measured at the time of recruitment using the Symbol Digit Modalities Test (SDMT).
Analysis. The Andersen Healthcare Utilization model was used to structure the multivariate regression analysis. This model identifies multiple domains affecting quality of life, and the variables from the surveys were categorized according to domain: predisposing characteristics (demographic variables), enabling resources (caregiver support and living situation), needs (eg, health-related measures), and health behaviors (medication use, adherence).
Main results. A total of 211 completed the first survey, 188 the second, and 179 the third. 80% were female and 95% were white. Average age was 65.5 (SD 5.6) years. 56% of respondents’ self-reported scores on the SDMT classified them as cognitively impaired. Risk of neuropsychological impairment, depression, and disability status were significantly associated with a decreased mental and physical HRQOL. Significantly, there was a strong association between predisposing characteristics and QOL. Being widowed and remaining employed were the strongest predictors of better physical QOL and having an education level of high school or less was a predictor of lower mental HRQOL.
Conclusion. Clinicians should measure HRQOL in older MS patients regularly and assess for depression and cognitive impairment.
Commentary
Quality of life is an important marker of MS patients’ well-being as they cope with this chronic illness [1]. The progression of the disease and its symptomatology often negatively affect HRQOL. However, multiple psychosocial factors, such as coping, mood, self-efficacy, and perceived support, affect QOL of patients with MS more than biological variables such as weakness or burden of radiologic disease [2]. For example, many self-report HRQOL indices are strongly predicted by measures of depression [3]. In addition, many studies have found a positive association between physical disability and reduced QOL [4,5]. Further, while perceived HRQOL may be a meaningful outcome in itself, it may also be a predictor for outcomes such as disability-related changes [6].
MS leads to disability and loss of function in all age-groups, but only a few studies have focused on HRQOL among elderly patients with MS. As patients with MS age, they may develop comorbidities such as hypertension and diabetes that may affect HRQOL. However, in a previous study comparing QOL between older and younger patients with MS, elderly and younger patients with MS had similar QOL even though the elderly patients had more physical limitations [7].
The strength of the current study was using the Andersen Healthcare Utilization regression model in the analysis, since it factors in multiple influences on health status. The striking evidence that employment and being widowed were linked to better physical QOL suggest that older MS patients may have better adaptation and adjustment to their illness. Researchers have shown that the widowed elderly often take on more responsibilities and tasks when they lose their partner, which leads to increased self-esteem and QOL [8]. Another advantage of the study was the fact that the investigators evaluated the different exposure variables and their associations with mental and physical QOL while identifying multiple confounding variables. Additionally, the use of 2 cognitive assessment tools provided a stronger assessment of patients’ cognitive function.
The main weakness of the study was using a cross-sectional study design with convenience sampling. The convenience sample was based on voluntary participation, which may result in self-selection bias. In addition, the self-report design is subject to the usual limitations of self-reporting for data collection: participants may exaggerate symptoms in order to make their situation seem worse or may under-report the severity or frequency of symptoms in order to minimize their problems. While the overall sample size was 211, not all respondents completed all the surveys, and response rates varied by questions. Thus, missing data may have affected results, but which data are missing is not discernable from the paper. That patients were from a single geographic area and had relatively high education levels (44% with college or above) are among the factors that limit the generalizability of the study. Another limitation is the use of the Beck Depression Inventory, which was not specifically designed for use in the elderly. In addition, the results of this study might have been affected by unmeasured confounding variables, for example daily physical activity, which can be a factor that modifies between depression, cognition, and QOL.
Applications for Clinical Practice
This study reinforces the importance of monitoring older MS patients for factors that may influence their HRQOL. The presence of depression, disability, and cognitive impairment should be assessed for regularly. Clinicians should encourage and empower elderly patients to continue with activities, including employment, that promote their mental and physical well-being and help maintain their independence. Assessing patients with geriatric-specific tools may provide more reliable and accurate assessment data that better accounts for aging dynamics. In addition, comobidities must be managed appropriately.
—Aliza Bitton Ben-Zacharia, DNP, ANP, and Allison Squires, PhD, RN, New York University College of Nursing
Study Overview
Objective. To evaluate the association between clinical and demographic factors and health-related quality of life (HRQOL) among older people with multiple sclerosis (MS).
Design. Cross-sectional survey-based study.
Setting and participants. Patients with MS aged 60 years or older were recruited from 4 MS centers in Long Island, NY. Patients with severe cognitive impairment as determined by the health care practitioner were excluded. Participants were asked to complete 3 surveys at 3 different time-points. In the first survey, participants completed the Morisky Medication Adherence Scale and the Patient Multiple Sclerosis Neuropsychological Screening Questionnaire (P-MSNQ). The second survey was the Multiple Sclerosis Quality of Life-54 (MSQOL-54), and the third survey included the Beck Depression Inventory-II (BDI-II) and a disability status self-assessment scale. Cognitive function was measured at the time of recruitment using the Symbol Digit Modalities Test (SDMT).
Analysis. The Andersen Healthcare Utilization model was used to structure the multivariate regression analysis. This model identifies multiple domains affecting quality of life, and the variables from the surveys were categorized according to domain: predisposing characteristics (demographic variables), enabling resources (caregiver support and living situation), needs (eg, health-related measures), and health behaviors (medication use, adherence).
Main results. A total of 211 completed the first survey, 188 the second, and 179 the third. 80% were female and 95% were white. Average age was 65.5 (SD 5.6) years. 56% of respondents’ self-reported scores on the SDMT classified them as cognitively impaired. Risk of neuropsychological impairment, depression, and disability status were significantly associated with a decreased mental and physical HRQOL. Significantly, there was a strong association between predisposing characteristics and QOL. Being widowed and remaining employed were the strongest predictors of better physical QOL and having an education level of high school or less was a predictor of lower mental HRQOL.
Conclusion. Clinicians should measure HRQOL in older MS patients regularly and assess for depression and cognitive impairment.
Commentary
Quality of life is an important marker of MS patients’ well-being as they cope with this chronic illness [1]. The progression of the disease and its symptomatology often negatively affect HRQOL. However, multiple psychosocial factors, such as coping, mood, self-efficacy, and perceived support, affect QOL of patients with MS more than biological variables such as weakness or burden of radiologic disease [2]. For example, many self-report HRQOL indices are strongly predicted by measures of depression [3]. In addition, many studies have found a positive association between physical disability and reduced QOL [4,5]. Further, while perceived HRQOL may be a meaningful outcome in itself, it may also be a predictor for outcomes such as disability-related changes [6].
MS leads to disability and loss of function in all age-groups, but only a few studies have focused on HRQOL among elderly patients with MS. As patients with MS age, they may develop comorbidities such as hypertension and diabetes that may affect HRQOL. However, in a previous study comparing QOL between older and younger patients with MS, elderly and younger patients with MS had similar QOL even though the elderly patients had more physical limitations [7].
The strength of the current study was using the Andersen Healthcare Utilization regression model in the analysis, since it factors in multiple influences on health status. The striking evidence that employment and being widowed were linked to better physical QOL suggest that older MS patients may have better adaptation and adjustment to their illness. Researchers have shown that the widowed elderly often take on more responsibilities and tasks when they lose their partner, which leads to increased self-esteem and QOL [8]. Another advantage of the study was the fact that the investigators evaluated the different exposure variables and their associations with mental and physical QOL while identifying multiple confounding variables. Additionally, the use of 2 cognitive assessment tools provided a stronger assessment of patients’ cognitive function.
The main weakness of the study was using a cross-sectional study design with convenience sampling. The convenience sample was based on voluntary participation, which may result in self-selection bias. In addition, the self-report design is subject to the usual limitations of self-reporting for data collection: participants may exaggerate symptoms in order to make their situation seem worse or may under-report the severity or frequency of symptoms in order to minimize their problems. While the overall sample size was 211, not all respondents completed all the surveys, and response rates varied by questions. Thus, missing data may have affected results, but which data are missing is not discernable from the paper. That patients were from a single geographic area and had relatively high education levels (44% with college or above) are among the factors that limit the generalizability of the study. Another limitation is the use of the Beck Depression Inventory, which was not specifically designed for use in the elderly. In addition, the results of this study might have been affected by unmeasured confounding variables, for example daily physical activity, which can be a factor that modifies between depression, cognition, and QOL.
Applications for Clinical Practice
This study reinforces the importance of monitoring older MS patients for factors that may influence their HRQOL. The presence of depression, disability, and cognitive impairment should be assessed for regularly. Clinicians should encourage and empower elderly patients to continue with activities, including employment, that promote their mental and physical well-being and help maintain their independence. Assessing patients with geriatric-specific tools may provide more reliable and accurate assessment data that better accounts for aging dynamics. In addition, comobidities must be managed appropriately.
—Aliza Bitton Ben-Zacharia, DNP, ANP, and Allison Squires, PhD, RN, New York University College of Nursing
1. Opara JA, Jaracz K, Brola W. Quality of life in multiple sclerosis. J Med Life 2010;3:352–8.
2. Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol 2005;4:556–66.
3. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 2005;231:29–34.
4. Göksel Karatepe A, Kaya T, Günaydn R, et al. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int J Rehabil Res 2011;34:290–8.
5. Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 1999;53:1098–103.
6. Visschedijk MA, Uitdehaag BM, Klein M, et al. Value of health-related quality of life to predict disability course in multiple sclerosis. Neurology 2004;63:2046–50.
7. Ploughman M, Austin MW, Murdoch M, et al. Factors influencing healthy aging with multiple sclerosis: a qualitative study. Disabil Rehabil 2012;34:26–33.
8. Minden SL, Frankel D, Hadden LS, et al. Disability in elderly people with multiple sclerosis: An analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. NeuroRehabilitation. 2004;19:55–67.
1. Opara JA, Jaracz K, Brola W. Quality of life in multiple sclerosis. J Med Life 2010;3:352–8.
2. Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. Lancet Neurol 2005;4:556–66.
3. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 2005;231:29–34.
4. Göksel Karatepe A, Kaya T, Günaydn R, et al. Quality of life in patients with multiple sclerosis: the impact of depression, fatigue, and disability. Int J Rehabil Res 2011;34:290–8.
5. Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 1999;53:1098–103.
6. Visschedijk MA, Uitdehaag BM, Klein M, et al. Value of health-related quality of life to predict disability course in multiple sclerosis. Neurology 2004;63:2046–50.
7. Ploughman M, Austin MW, Murdoch M, et al. Factors influencing healthy aging with multiple sclerosis: a qualitative study. Disabil Rehabil 2012;34:26–33.
8. Minden SL, Frankel D, Hadden LS, et al. Disability in elderly people with multiple sclerosis: An analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. NeuroRehabilitation. 2004;19:55–67.
Self-Monitoring and Self-Titration of Antihypertensive Medications Result in Better Systolic Blood Pressure Control
Study Overview
Objective. To examine the effect of self-monitoring of blood pressure and self-titration of antihypertensive medications among hypertensive patients with cardiovascular disease, diabetes, or chronic kidney disease.
Design. Unblinded randomized controlled trial.
Setting and participants. The study was conducted in central and east England. Patients with poorly controlled blood pressure with a last recorded systolic blood pressure of at least 145 mm Hg at 59 UK primary care practices were invited to participate. Patients had to be at least 35 years old and have at least 1 of the following comorbidities: transient ischemic attack or stroke, stage 3 chronic kidney disease, or history of coronary artery bypass graft surgery, myocardial infarction, or angina. Patients were excluded if they could not self-monitor blood pressure, had dementia or failed a cognitive screen using the short-orientation memory concentration test, had postural hypotension, took more than 3 antihypertensive medications, had an acute cardiovascular event within the previous 3 months, were receiving care from a specialist for their hypertension, were pregnant, or had a terminal disease. Participants were randomized to the self-management intervention or usual care.
Intervention. Patients in the self-management group were asked to monitor their blood pressure using an automated blood pressure monitor and to titrate their blood pressure medications using an individualized 3-step plan devised by the patient with their family physician. They were trained to do these tasks in 2- or 3-hour sessions. Patients were instructed to take their blood pressure twice each morning for the first week of each month; if 4 or more blood pressure readings during the measurement week for 2 consecutive months were higher than the target blood pressure, patients were to follow their individualized plan to change their medications. The target blood pressure was 120/75 mm Hg, following British guidelines for patients with stroke, diabetes, chronic kidney disease, or coronary heart disease. If patients exhausted all 3 steps for medication titration, they were to return to their family physician for additional instructions. Patients in the usual care group had a routine blood pressure check and medication review appointment with their family physician, which was followed by follow-up care at the discretion of the family physician for blood pressure measurement, blood pressure targets, or adjustment of medication.
Main outcome measure. The primary outcome was systolic blood pressure at 12 months. The difference in outcomes between the intervention and usual care groups was examined while accounting for baseline blood pressure and other clinical factors. 6 blood pressures were taken at 1-minute intervals after an initial 5 minutes of rest. Blood pressure was taken by an electronic automated blood pressure machine. The mean of the second and third readings were used as primary outcome. Outcome assessor was not blinded to group assignment. The primary analysis included all cases with complete data, and a sensitivity analysis with multiple imputations was also performed. Preplanned subgroup analyses included older vs. younger age-groups, men vs. women, and other risk groups.
Main results. Among 10,764 patients assessed for eligibility, 3353 were excluded as they were considered by their family physician to be housebound, have a terminal illness, or not be a suitable candidate. Among the 7411 invited to participate, 4207 did not respond to the invitation and 2003 declined participation (with a third who did not want to alter their own medications, and a third who did not want to measure their own blood pressure). Among the 1201 who attended the baseline clinic, 138 withdrew their consent and 508 were deemed ineligible. A total of 555 were randomized, and 220 in the intervention group and 230 in the control group completed the study and provided outcome data (81%). Patients in the self-management group had a 9.2 mm Hg–lower systolic blood pressure at 12 months (95% CI, 5.7–12.7) compared with the usual care group. The self-management group also had a larger increase in the intake of antihypertensive drugs compared with controls, with an increase in both doses and number of medications. Although adverse symptoms were common in both groups, there were no significant differences in adverse symptoms between groups.
Conclusions. Self-management of hypertension among patients with stroke, cardiovascular disease, and other high-risk conditions is safe and effective in achieving better blood pressure control.
Commentary
Hypertension is a major public health problem. Significant resources have been devoted to advance hypertension management through research, practice improvements, and guideline developments; however, blood pressure control among those with hypertension in the United States remains suboptimal—with only about half achieving adequate control [1].
Advances in technology have made home blood pressure monitoring possible. It offers several advantages to traditional office-based blood pressure management [2], and several studies have shown home blood pressure telemonitoring and team care can achieve better outcomes than office-based management [3]. A significant contribution of the current study is that it demonstrated that the self-management approach is both safe and effective even in high-risk patients, who are perhaps the most likely to have adverse events from treatment but also the most likely to derive benefit from adequate treatment of hypertension.
Although the self-management approach has promise, it also has potential drawbacks. Specifically, as demonstrated by the low enrollment rate in this study, this intervention may not be suitable for all patients. About two-thirds of those who responded to the initial enrollment attempt ultimately declined participation because they did not want to modify their own medications or did not want to perform the tasks of home blood pressure monitoring. This perhaps is a realistic assessment of who may ultimately benefit from this approach—patients who wish to have an active role in managing their medical problems and have the ability to do so. For the clinician, it is important to identify patients who are able to manage the complex task of adjusting their medication regimen; otherwise, the potential for harm may be magnified.
Engaging patients in the management of their chronic disease is a growing trend in chronic disease management. Bringing management of hypertension to patients’ homes, as the accompanying editorial in the issue pointed out, reflects patient-centeredness at its best and represents an important step toward the adaptation of treatment for patients who want to actively take part in their own care [2].
Applications for Clinical Practice
Self-management of blood pressure in patients at high risk of cardiovascular disease appears feasible. As the editorialists note, this study is an important step toward adaptation of treatment for patients who want to actively take part in their own risk-factor control [2]. More research is needed to study the effects of self-titration on long-term outcomes and to identify the appropriate protocols that can be applied by clinicians in the community, both for patient selection and education and medication adjustment.
—William Hung, MD, MPH
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50.
2. Nilsson PM, Nystrom FH. Self-titration of antihypertensive therapy in high-risk patients. Bringing it home. JAMA 2014;312:795–6.
3. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control. a cluster randomized clinical trial. JAMA 2013;310:46–56.
Study Overview
Objective. To examine the effect of self-monitoring of blood pressure and self-titration of antihypertensive medications among hypertensive patients with cardiovascular disease, diabetes, or chronic kidney disease.
Design. Unblinded randomized controlled trial.
Setting and participants. The study was conducted in central and east England. Patients with poorly controlled blood pressure with a last recorded systolic blood pressure of at least 145 mm Hg at 59 UK primary care practices were invited to participate. Patients had to be at least 35 years old and have at least 1 of the following comorbidities: transient ischemic attack or stroke, stage 3 chronic kidney disease, or history of coronary artery bypass graft surgery, myocardial infarction, or angina. Patients were excluded if they could not self-monitor blood pressure, had dementia or failed a cognitive screen using the short-orientation memory concentration test, had postural hypotension, took more than 3 antihypertensive medications, had an acute cardiovascular event within the previous 3 months, were receiving care from a specialist for their hypertension, were pregnant, or had a terminal disease. Participants were randomized to the self-management intervention or usual care.
Intervention. Patients in the self-management group were asked to monitor their blood pressure using an automated blood pressure monitor and to titrate their blood pressure medications using an individualized 3-step plan devised by the patient with their family physician. They were trained to do these tasks in 2- or 3-hour sessions. Patients were instructed to take their blood pressure twice each morning for the first week of each month; if 4 or more blood pressure readings during the measurement week for 2 consecutive months were higher than the target blood pressure, patients were to follow their individualized plan to change their medications. The target blood pressure was 120/75 mm Hg, following British guidelines for patients with stroke, diabetes, chronic kidney disease, or coronary heart disease. If patients exhausted all 3 steps for medication titration, they were to return to their family physician for additional instructions. Patients in the usual care group had a routine blood pressure check and medication review appointment with their family physician, which was followed by follow-up care at the discretion of the family physician for blood pressure measurement, blood pressure targets, or adjustment of medication.
Main outcome measure. The primary outcome was systolic blood pressure at 12 months. The difference in outcomes between the intervention and usual care groups was examined while accounting for baseline blood pressure and other clinical factors. 6 blood pressures were taken at 1-minute intervals after an initial 5 minutes of rest. Blood pressure was taken by an electronic automated blood pressure machine. The mean of the second and third readings were used as primary outcome. Outcome assessor was not blinded to group assignment. The primary analysis included all cases with complete data, and a sensitivity analysis with multiple imputations was also performed. Preplanned subgroup analyses included older vs. younger age-groups, men vs. women, and other risk groups.
Main results. Among 10,764 patients assessed for eligibility, 3353 were excluded as they were considered by their family physician to be housebound, have a terminal illness, or not be a suitable candidate. Among the 7411 invited to participate, 4207 did not respond to the invitation and 2003 declined participation (with a third who did not want to alter their own medications, and a third who did not want to measure their own blood pressure). Among the 1201 who attended the baseline clinic, 138 withdrew their consent and 508 were deemed ineligible. A total of 555 were randomized, and 220 in the intervention group and 230 in the control group completed the study and provided outcome data (81%). Patients in the self-management group had a 9.2 mm Hg–lower systolic blood pressure at 12 months (95% CI, 5.7–12.7) compared with the usual care group. The self-management group also had a larger increase in the intake of antihypertensive drugs compared with controls, with an increase in both doses and number of medications. Although adverse symptoms were common in both groups, there were no significant differences in adverse symptoms between groups.
Conclusions. Self-management of hypertension among patients with stroke, cardiovascular disease, and other high-risk conditions is safe and effective in achieving better blood pressure control.
Commentary
Hypertension is a major public health problem. Significant resources have been devoted to advance hypertension management through research, practice improvements, and guideline developments; however, blood pressure control among those with hypertension in the United States remains suboptimal—with only about half achieving adequate control [1].
Advances in technology have made home blood pressure monitoring possible. It offers several advantages to traditional office-based blood pressure management [2], and several studies have shown home blood pressure telemonitoring and team care can achieve better outcomes than office-based management [3]. A significant contribution of the current study is that it demonstrated that the self-management approach is both safe and effective even in high-risk patients, who are perhaps the most likely to have adverse events from treatment but also the most likely to derive benefit from adequate treatment of hypertension.
Although the self-management approach has promise, it also has potential drawbacks. Specifically, as demonstrated by the low enrollment rate in this study, this intervention may not be suitable for all patients. About two-thirds of those who responded to the initial enrollment attempt ultimately declined participation because they did not want to modify their own medications or did not want to perform the tasks of home blood pressure monitoring. This perhaps is a realistic assessment of who may ultimately benefit from this approach—patients who wish to have an active role in managing their medical problems and have the ability to do so. For the clinician, it is important to identify patients who are able to manage the complex task of adjusting their medication regimen; otherwise, the potential for harm may be magnified.
Engaging patients in the management of their chronic disease is a growing trend in chronic disease management. Bringing management of hypertension to patients’ homes, as the accompanying editorial in the issue pointed out, reflects patient-centeredness at its best and represents an important step toward the adaptation of treatment for patients who want to actively take part in their own care [2].
Applications for Clinical Practice
Self-management of blood pressure in patients at high risk of cardiovascular disease appears feasible. As the editorialists note, this study is an important step toward adaptation of treatment for patients who want to actively take part in their own risk-factor control [2]. More research is needed to study the effects of self-titration on long-term outcomes and to identify the appropriate protocols that can be applied by clinicians in the community, both for patient selection and education and medication adjustment.
—William Hung, MD, MPH
Study Overview
Objective. To examine the effect of self-monitoring of blood pressure and self-titration of antihypertensive medications among hypertensive patients with cardiovascular disease, diabetes, or chronic kidney disease.
Design. Unblinded randomized controlled trial.
Setting and participants. The study was conducted in central and east England. Patients with poorly controlled blood pressure with a last recorded systolic blood pressure of at least 145 mm Hg at 59 UK primary care practices were invited to participate. Patients had to be at least 35 years old and have at least 1 of the following comorbidities: transient ischemic attack or stroke, stage 3 chronic kidney disease, or history of coronary artery bypass graft surgery, myocardial infarction, or angina. Patients were excluded if they could not self-monitor blood pressure, had dementia or failed a cognitive screen using the short-orientation memory concentration test, had postural hypotension, took more than 3 antihypertensive medications, had an acute cardiovascular event within the previous 3 months, were receiving care from a specialist for their hypertension, were pregnant, or had a terminal disease. Participants were randomized to the self-management intervention or usual care.
Intervention. Patients in the self-management group were asked to monitor their blood pressure using an automated blood pressure monitor and to titrate their blood pressure medications using an individualized 3-step plan devised by the patient with their family physician. They were trained to do these tasks in 2- or 3-hour sessions. Patients were instructed to take their blood pressure twice each morning for the first week of each month; if 4 or more blood pressure readings during the measurement week for 2 consecutive months were higher than the target blood pressure, patients were to follow their individualized plan to change their medications. The target blood pressure was 120/75 mm Hg, following British guidelines for patients with stroke, diabetes, chronic kidney disease, or coronary heart disease. If patients exhausted all 3 steps for medication titration, they were to return to their family physician for additional instructions. Patients in the usual care group had a routine blood pressure check and medication review appointment with their family physician, which was followed by follow-up care at the discretion of the family physician for blood pressure measurement, blood pressure targets, or adjustment of medication.
Main outcome measure. The primary outcome was systolic blood pressure at 12 months. The difference in outcomes between the intervention and usual care groups was examined while accounting for baseline blood pressure and other clinical factors. 6 blood pressures were taken at 1-minute intervals after an initial 5 minutes of rest. Blood pressure was taken by an electronic automated blood pressure machine. The mean of the second and third readings were used as primary outcome. Outcome assessor was not blinded to group assignment. The primary analysis included all cases with complete data, and a sensitivity analysis with multiple imputations was also performed. Preplanned subgroup analyses included older vs. younger age-groups, men vs. women, and other risk groups.
Main results. Among 10,764 patients assessed for eligibility, 3353 were excluded as they were considered by their family physician to be housebound, have a terminal illness, or not be a suitable candidate. Among the 7411 invited to participate, 4207 did not respond to the invitation and 2003 declined participation (with a third who did not want to alter their own medications, and a third who did not want to measure their own blood pressure). Among the 1201 who attended the baseline clinic, 138 withdrew their consent and 508 were deemed ineligible. A total of 555 were randomized, and 220 in the intervention group and 230 in the control group completed the study and provided outcome data (81%). Patients in the self-management group had a 9.2 mm Hg–lower systolic blood pressure at 12 months (95% CI, 5.7–12.7) compared with the usual care group. The self-management group also had a larger increase in the intake of antihypertensive drugs compared with controls, with an increase in both doses and number of medications. Although adverse symptoms were common in both groups, there were no significant differences in adverse symptoms between groups.
Conclusions. Self-management of hypertension among patients with stroke, cardiovascular disease, and other high-risk conditions is safe and effective in achieving better blood pressure control.
Commentary
Hypertension is a major public health problem. Significant resources have been devoted to advance hypertension management through research, practice improvements, and guideline developments; however, blood pressure control among those with hypertension in the United States remains suboptimal—with only about half achieving adequate control [1].
Advances in technology have made home blood pressure monitoring possible. It offers several advantages to traditional office-based blood pressure management [2], and several studies have shown home blood pressure telemonitoring and team care can achieve better outcomes than office-based management [3]. A significant contribution of the current study is that it demonstrated that the self-management approach is both safe and effective even in high-risk patients, who are perhaps the most likely to have adverse events from treatment but also the most likely to derive benefit from adequate treatment of hypertension.
Although the self-management approach has promise, it also has potential drawbacks. Specifically, as demonstrated by the low enrollment rate in this study, this intervention may not be suitable for all patients. About two-thirds of those who responded to the initial enrollment attempt ultimately declined participation because they did not want to modify their own medications or did not want to perform the tasks of home blood pressure monitoring. This perhaps is a realistic assessment of who may ultimately benefit from this approach—patients who wish to have an active role in managing their medical problems and have the ability to do so. For the clinician, it is important to identify patients who are able to manage the complex task of adjusting their medication regimen; otherwise, the potential for harm may be magnified.
Engaging patients in the management of their chronic disease is a growing trend in chronic disease management. Bringing management of hypertension to patients’ homes, as the accompanying editorial in the issue pointed out, reflects patient-centeredness at its best and represents an important step toward the adaptation of treatment for patients who want to actively take part in their own care [2].
Applications for Clinical Practice
Self-management of blood pressure in patients at high risk of cardiovascular disease appears feasible. As the editorialists note, this study is an important step toward adaptation of treatment for patients who want to actively take part in their own risk-factor control [2]. More research is needed to study the effects of self-titration on long-term outcomes and to identify the appropriate protocols that can be applied by clinicians in the community, both for patient selection and education and medication adjustment.
—William Hung, MD, MPH
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50.
2. Nilsson PM, Nystrom FH. Self-titration of antihypertensive therapy in high-risk patients. Bringing it home. JAMA 2014;312:795–6.
3. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control. a cluster randomized clinical trial. JAMA 2013;310:46–56.
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50.
2. Nilsson PM, Nystrom FH. Self-titration of antihypertensive therapy in high-risk patients. Bringing it home. JAMA 2014;312:795–6.
3. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control. a cluster randomized clinical trial. JAMA 2013;310:46–56.
CBT-i Coach
The VA National Center for PTSD, Stanford School of Medicine, and the DoD National Center for Telehealth and Technology collaborated to bring users the CBT-i (cognitive behavioral therapy for insomnia) Coach mobile app. Cognitive behavioral therapy is a nonmedication treatment that is based on scientific knowledge. The goals of CBT-i are “to help you fall asleep, stay asleep, and improve your daytime functioning as a result of better sleep at night.”
The CBT-i Coach is designed for patients engaged in CBT-i or who have experienced symptoms of insomnia and want to improve their sleep habits. Although the app is intended to augment face-to-face care with a health care professional, there are plenty of applications for users who are not enrolled in CBT-i and are not satisfied with the amount or quality of sleep they are getting.
SLEEP 101
Without knowing what or why sleep may need to be improved, it can be difficult to know what adjustments to make. To overcome a possible learning curve, users can navigate from the Learn option in the app menu to a section called “Sleep 101.” Here, the app lays out the fundamentals of sleep and why we need it, the stages of sleep and related brain-wave activity patterns, and what regulates our sleep and wakefulness.
The CBT-i approach is explained by its 2 parts: cognitive and behavioral aspects. The cognitive elements focus on “thoughts, feelings, and expectations about sleep and insomnia that may stand in the way of good sleep.” Behavioral aspects help people “adopt personal sleep habits that, based on the science of sleep, will help them sleep better.”
Important tips are provided in the “What is CBT-i,” section, including how dangerous it is to keep weapons within easy access of where an individual is sleeping and the important distinction between feeling sleepy and feeling tired. The app notes that sleepy refers to an actual need for sleep, whereas tired can mean that the person simply has low energy. Understanding this distinction, among many others, can aid us in determining when our bodies need sleep and how to enhance the process of falling asleep.
Also within the Learn menu is a section called “Habits and Sleep.” Here, users can scroll through a list of habits that may cause sleep issues and explore what they can do to make improvements. This list includes habits that might keep a person awake (exercise, worrying in bed, watching the clock, and napping), substances we might ingest that disrupt sleep (eating, caffeine, alcohol, and nicotine), and what can be done to improve sleep (winding down, limiting the bedroom to 2 activities, and getting comfortable).
TOOLS
“Create New Sleep Habits” is the first section within the Tools menu. Here, the app notes, “Incorporating new habits into your nighttime routine can help sleep come more easily.” Lists for middle of the night activities when you can’t sleep, setting up a proper sleep environment, and activities to keep you awake until your prescribed bedtime are all intended to lead to improved quality of sleep.
Another tool focuses on preventing insomnia, offering a self-administered quiz to determine personal areas that may need improvement. As the user grows more comfortable with the CBT-i Coach and uses it over a period of time, sleeping difficulties may still naturally return. The app reinforces “this is normal” and provides the quiz as a way for users to keep tabs on themselves and their habits to determine why their sleeping difficulties may have returned.
The app also includes a glossary to explain any language used in the app that the user doesn’t understand.
MY SLEEP
Tracking sleep patterns with the Sleep Diary can be useful for self-assessment and can provide a vital snapshot for the health care professional who is treating a patient for insomnia. All fields must be filled in, including how many times the user woke up in the middle of the night and how long it took him or her to fall asleep. If the app notices any inconsistencies the diary will prompt the user to correct any inaccurately entered field(s) before the entry can be saved. An optional push notification reminder to update the sleep diary is available, and the user can set this for any time of day.
Other optional reminders may be found in the primary app menu at the bottom of the screen, and the user may select from any of the following: Sleep Diary Entry, Wind Down Time, Prescribed Bed Time, Prescribed Wake Time, Update Sleep Prescription, Take Assessment, Stop Caffeine, and Worry Time.
FINAL THOUGHTS
There is enough information in this app to keep a person busy for hours. But once it becomes part of a daily routine, users will find that with a bit of guidance, exercises, and personal modifications, a good night’s sleep, or at least one that’s improved, may be within reach.
For someone who is experiencing difficulties sleeping, it is critical to work with a health care professional who can determine and help treat any underlying causes of the insomnia.
The VA National Center for PTSD, Stanford School of Medicine, and the DoD National Center for Telehealth and Technology collaborated to bring users the CBT-i (cognitive behavioral therapy for insomnia) Coach mobile app. Cognitive behavioral therapy is a nonmedication treatment that is based on scientific knowledge. The goals of CBT-i are “to help you fall asleep, stay asleep, and improve your daytime functioning as a result of better sleep at night.”
The CBT-i Coach is designed for patients engaged in CBT-i or who have experienced symptoms of insomnia and want to improve their sleep habits. Although the app is intended to augment face-to-face care with a health care professional, there are plenty of applications for users who are not enrolled in CBT-i and are not satisfied with the amount or quality of sleep they are getting.
SLEEP 101
Without knowing what or why sleep may need to be improved, it can be difficult to know what adjustments to make. To overcome a possible learning curve, users can navigate from the Learn option in the app menu to a section called “Sleep 101.” Here, the app lays out the fundamentals of sleep and why we need it, the stages of sleep and related brain-wave activity patterns, and what regulates our sleep and wakefulness.
The CBT-i approach is explained by its 2 parts: cognitive and behavioral aspects. The cognitive elements focus on “thoughts, feelings, and expectations about sleep and insomnia that may stand in the way of good sleep.” Behavioral aspects help people “adopt personal sleep habits that, based on the science of sleep, will help them sleep better.”
Important tips are provided in the “What is CBT-i,” section, including how dangerous it is to keep weapons within easy access of where an individual is sleeping and the important distinction between feeling sleepy and feeling tired. The app notes that sleepy refers to an actual need for sleep, whereas tired can mean that the person simply has low energy. Understanding this distinction, among many others, can aid us in determining when our bodies need sleep and how to enhance the process of falling asleep.
Also within the Learn menu is a section called “Habits and Sleep.” Here, users can scroll through a list of habits that may cause sleep issues and explore what they can do to make improvements. This list includes habits that might keep a person awake (exercise, worrying in bed, watching the clock, and napping), substances we might ingest that disrupt sleep (eating, caffeine, alcohol, and nicotine), and what can be done to improve sleep (winding down, limiting the bedroom to 2 activities, and getting comfortable).
TOOLS
“Create New Sleep Habits” is the first section within the Tools menu. Here, the app notes, “Incorporating new habits into your nighttime routine can help sleep come more easily.” Lists for middle of the night activities when you can’t sleep, setting up a proper sleep environment, and activities to keep you awake until your prescribed bedtime are all intended to lead to improved quality of sleep.
Another tool focuses on preventing insomnia, offering a self-administered quiz to determine personal areas that may need improvement. As the user grows more comfortable with the CBT-i Coach and uses it over a period of time, sleeping difficulties may still naturally return. The app reinforces “this is normal” and provides the quiz as a way for users to keep tabs on themselves and their habits to determine why their sleeping difficulties may have returned.
The app also includes a glossary to explain any language used in the app that the user doesn’t understand.
MY SLEEP
Tracking sleep patterns with the Sleep Diary can be useful for self-assessment and can provide a vital snapshot for the health care professional who is treating a patient for insomnia. All fields must be filled in, including how many times the user woke up in the middle of the night and how long it took him or her to fall asleep. If the app notices any inconsistencies the diary will prompt the user to correct any inaccurately entered field(s) before the entry can be saved. An optional push notification reminder to update the sleep diary is available, and the user can set this for any time of day.
Other optional reminders may be found in the primary app menu at the bottom of the screen, and the user may select from any of the following: Sleep Diary Entry, Wind Down Time, Prescribed Bed Time, Prescribed Wake Time, Update Sleep Prescription, Take Assessment, Stop Caffeine, and Worry Time.
FINAL THOUGHTS
There is enough information in this app to keep a person busy for hours. But once it becomes part of a daily routine, users will find that with a bit of guidance, exercises, and personal modifications, a good night’s sleep, or at least one that’s improved, may be within reach.
For someone who is experiencing difficulties sleeping, it is critical to work with a health care professional who can determine and help treat any underlying causes of the insomnia.
The VA National Center for PTSD, Stanford School of Medicine, and the DoD National Center for Telehealth and Technology collaborated to bring users the CBT-i (cognitive behavioral therapy for insomnia) Coach mobile app. Cognitive behavioral therapy is a nonmedication treatment that is based on scientific knowledge. The goals of CBT-i are “to help you fall asleep, stay asleep, and improve your daytime functioning as a result of better sleep at night.”
The CBT-i Coach is designed for patients engaged in CBT-i or who have experienced symptoms of insomnia and want to improve their sleep habits. Although the app is intended to augment face-to-face care with a health care professional, there are plenty of applications for users who are not enrolled in CBT-i and are not satisfied with the amount or quality of sleep they are getting.
SLEEP 101
Without knowing what or why sleep may need to be improved, it can be difficult to know what adjustments to make. To overcome a possible learning curve, users can navigate from the Learn option in the app menu to a section called “Sleep 101.” Here, the app lays out the fundamentals of sleep and why we need it, the stages of sleep and related brain-wave activity patterns, and what regulates our sleep and wakefulness.
The CBT-i approach is explained by its 2 parts: cognitive and behavioral aspects. The cognitive elements focus on “thoughts, feelings, and expectations about sleep and insomnia that may stand in the way of good sleep.” Behavioral aspects help people “adopt personal sleep habits that, based on the science of sleep, will help them sleep better.”
Important tips are provided in the “What is CBT-i,” section, including how dangerous it is to keep weapons within easy access of where an individual is sleeping and the important distinction between feeling sleepy and feeling tired. The app notes that sleepy refers to an actual need for sleep, whereas tired can mean that the person simply has low energy. Understanding this distinction, among many others, can aid us in determining when our bodies need sleep and how to enhance the process of falling asleep.
Also within the Learn menu is a section called “Habits and Sleep.” Here, users can scroll through a list of habits that may cause sleep issues and explore what they can do to make improvements. This list includes habits that might keep a person awake (exercise, worrying in bed, watching the clock, and napping), substances we might ingest that disrupt sleep (eating, caffeine, alcohol, and nicotine), and what can be done to improve sleep (winding down, limiting the bedroom to 2 activities, and getting comfortable).
TOOLS
“Create New Sleep Habits” is the first section within the Tools menu. Here, the app notes, “Incorporating new habits into your nighttime routine can help sleep come more easily.” Lists for middle of the night activities when you can’t sleep, setting up a proper sleep environment, and activities to keep you awake until your prescribed bedtime are all intended to lead to improved quality of sleep.
Another tool focuses on preventing insomnia, offering a self-administered quiz to determine personal areas that may need improvement. As the user grows more comfortable with the CBT-i Coach and uses it over a period of time, sleeping difficulties may still naturally return. The app reinforces “this is normal” and provides the quiz as a way for users to keep tabs on themselves and their habits to determine why their sleeping difficulties may have returned.
The app also includes a glossary to explain any language used in the app that the user doesn’t understand.
MY SLEEP
Tracking sleep patterns with the Sleep Diary can be useful for self-assessment and can provide a vital snapshot for the health care professional who is treating a patient for insomnia. All fields must be filled in, including how many times the user woke up in the middle of the night and how long it took him or her to fall asleep. If the app notices any inconsistencies the diary will prompt the user to correct any inaccurately entered field(s) before the entry can be saved. An optional push notification reminder to update the sleep diary is available, and the user can set this for any time of day.
Other optional reminders may be found in the primary app menu at the bottom of the screen, and the user may select from any of the following: Sleep Diary Entry, Wind Down Time, Prescribed Bed Time, Prescribed Wake Time, Update Sleep Prescription, Take Assessment, Stop Caffeine, and Worry Time.
FINAL THOUGHTS
There is enough information in this app to keep a person busy for hours. But once it becomes part of a daily routine, users will find that with a bit of guidance, exercises, and personal modifications, a good night’s sleep, or at least one that’s improved, may be within reach.
For someone who is experiencing difficulties sleeping, it is critical to work with a health care professional who can determine and help treat any underlying causes of the insomnia.