User login
Trio-CES produces higher molecular diagnostic yield

Calvin, who was diagnosed
with Pitt-Hopkins Syndrome
via trio-CES
Credit: Lapidus family
A 3-pronged approach to clinical exome sequencing (CES) can provide a higher diagnostic yield than traditional molecular diagnostic methods, results of a new study suggest.
Investigators found that sequencing a patient’s exome together with his or her parents’—a method known as trio-CES—greatly improved the ability to reach a firm diagnosis in children with suspected genetic conditions.
This research was published in JAMA. It was released to coincide with a presentation at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers performed CES on 814 patients with undiagnosed, suspected genetic conditions between January 2012 and August 2014. Sequencing was conducted as trio-CES or as proband-CES (only the affected individual sequenced) when parental samples were not available.
The team funneled the raw data through an informatics pipeline to identify variants from the standard human genome. Next, they applied a series of filters to the data based on the patient’s family history and other relevant aspects of his or her condition.
The investigators then hunted for all genes and mutations linked by medical literature to the patient’s symptoms. And a multidisciplinary team of experts reviewed the findings to reach a diagnosis.
Overall, 26% of patients (213/814) received a molecular diagnosis, with the causative variant(s) identified in a well-established clinical gene.
There was a significantly higher molecular diagnostic yield from cases performed as trio-CES relative to proband-CES—31% (127/410) and 22% (74/338), respectively.
In cases of developmental delay in children younger than 5 years (n=138), the molecular diagnosis rate was 41% (45/ 109) for trio-CES cases and 9% (2/23) for proband-CES cases.
The typical turnaround time for exome sequencing is less than 8 weeks, though test results have been returned to physicians within 10 days in medically urgent situations.
With preauthorization, many insurance providers cover the cost to sequence a child and both parents. If not, the out-of-pocket fee is about $6650.
“All families deserve a clear diagnosis of their child’s condition,” said study author Wayne Grody, MD, PhD, of the University of California, Los Angeles.
“Exome sequencing plays an important role in identifying the precise cause of a child’s illness. This is immediately useful to families and physicians in understanding how the disease occurred, preventing unnecessary testing, and developing the best strategies to treat it.”
The researchers noted, however, that the clinical implications of their findings should be better understood before trio- or proband-CES are routinely adopted. ![]()

Calvin, who was diagnosed
with Pitt-Hopkins Syndrome
via trio-CES
Credit: Lapidus family
A 3-pronged approach to clinical exome sequencing (CES) can provide a higher diagnostic yield than traditional molecular diagnostic methods, results of a new study suggest.
Investigators found that sequencing a patient’s exome together with his or her parents’—a method known as trio-CES—greatly improved the ability to reach a firm diagnosis in children with suspected genetic conditions.
This research was published in JAMA. It was released to coincide with a presentation at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers performed CES on 814 patients with undiagnosed, suspected genetic conditions between January 2012 and August 2014. Sequencing was conducted as trio-CES or as proband-CES (only the affected individual sequenced) when parental samples were not available.
The team funneled the raw data through an informatics pipeline to identify variants from the standard human genome. Next, they applied a series of filters to the data based on the patient’s family history and other relevant aspects of his or her condition.
The investigators then hunted for all genes and mutations linked by medical literature to the patient’s symptoms. And a multidisciplinary team of experts reviewed the findings to reach a diagnosis.
Overall, 26% of patients (213/814) received a molecular diagnosis, with the causative variant(s) identified in a well-established clinical gene.
There was a significantly higher molecular diagnostic yield from cases performed as trio-CES relative to proband-CES—31% (127/410) and 22% (74/338), respectively.
In cases of developmental delay in children younger than 5 years (n=138), the molecular diagnosis rate was 41% (45/ 109) for trio-CES cases and 9% (2/23) for proband-CES cases.
The typical turnaround time for exome sequencing is less than 8 weeks, though test results have been returned to physicians within 10 days in medically urgent situations.
With preauthorization, many insurance providers cover the cost to sequence a child and both parents. If not, the out-of-pocket fee is about $6650.
“All families deserve a clear diagnosis of their child’s condition,” said study author Wayne Grody, MD, PhD, of the University of California, Los Angeles.
“Exome sequencing plays an important role in identifying the precise cause of a child’s illness. This is immediately useful to families and physicians in understanding how the disease occurred, preventing unnecessary testing, and developing the best strategies to treat it.”
The researchers noted, however, that the clinical implications of their findings should be better understood before trio- or proband-CES are routinely adopted. ![]()

Calvin, who was diagnosed
with Pitt-Hopkins Syndrome
via trio-CES
Credit: Lapidus family
A 3-pronged approach to clinical exome sequencing (CES) can provide a higher diagnostic yield than traditional molecular diagnostic methods, results of a new study suggest.
Investigators found that sequencing a patient’s exome together with his or her parents’—a method known as trio-CES—greatly improved the ability to reach a firm diagnosis in children with suspected genetic conditions.
This research was published in JAMA. It was released to coincide with a presentation at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers performed CES on 814 patients with undiagnosed, suspected genetic conditions between January 2012 and August 2014. Sequencing was conducted as trio-CES or as proband-CES (only the affected individual sequenced) when parental samples were not available.
The team funneled the raw data through an informatics pipeline to identify variants from the standard human genome. Next, they applied a series of filters to the data based on the patient’s family history and other relevant aspects of his or her condition.
The investigators then hunted for all genes and mutations linked by medical literature to the patient’s symptoms. And a multidisciplinary team of experts reviewed the findings to reach a diagnosis.
Overall, 26% of patients (213/814) received a molecular diagnosis, with the causative variant(s) identified in a well-established clinical gene.
There was a significantly higher molecular diagnostic yield from cases performed as trio-CES relative to proband-CES—31% (127/410) and 22% (74/338), respectively.
In cases of developmental delay in children younger than 5 years (n=138), the molecular diagnosis rate was 41% (45/ 109) for trio-CES cases and 9% (2/23) for proband-CES cases.
The typical turnaround time for exome sequencing is less than 8 weeks, though test results have been returned to physicians within 10 days in medically urgent situations.
With preauthorization, many insurance providers cover the cost to sequence a child and both parents. If not, the out-of-pocket fee is about $6650.
“All families deserve a clear diagnosis of their child’s condition,” said study author Wayne Grody, MD, PhD, of the University of California, Los Angeles.
“Exome sequencing plays an important role in identifying the precise cause of a child’s illness. This is immediately useful to families and physicians in understanding how the disease occurred, preventing unnecessary testing, and developing the best strategies to treat it.”
The researchers noted, however, that the clinical implications of their findings should be better understood before trio- or proband-CES are routinely adopted. ![]()
AUDIO: Conjugated estrogen, bazedoxifene combo offers menopause treatment option
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE NAMS ANNUAL MEETING
Exome sequencing shows potential as diagnostic tool

Credit: Graham Colm
In a large study, whole-exome sequencing provided 25% of patients with a diagnosis related to a known genetic disease, giving young
patients and their parents some long-sought answers.
The technology was able to detect a number of rare genetic events and new mutations contributing to disease.
Among the medically actionable findings were mutations related to Fanconi anemia, erythrocytosis, hemolytic anemia, and von Willebrand disease.
“The findings in this report, I believe, will forever change the future practice of pediatrics and medicine as a whole,” said study author James R. Lupski, MD, PhD, of the Baylor College of Medicine in Houston.
“It is just a matter of time before genomics moves up on the physician’s list of things to do and is ordered before formulating a differential diagnosis. It will be the new ‘family history’ that, better yet, gets you both the important variants inherited from each parent and the new mutations that contribute to disease susceptibility.”
This research was published in JAMA and is set to be presented on October 21 at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers had previously conducted a pilot study of whole-exome sequencing that included 250 patients and revealed a 25% molecular diagnostic rate.
This current study included 2000 patients (88% pediatric) with clinical whole-exome sequencing analyzed between June 2012 and August 2014. The majority of patients—87.8%—had neurological disorders or a developmental delay, and 12.2% had non-neurological disorders.
The researchers collected peripheral blood, tissue, or extracted DNA samples from patients and their parents. The team sequenced patients’ DNA and compared those results to the normal reference. Any disease-associated mutations were then compared with the parent’s DNA to determine if the child inherited it from one or both parents.
In all, 504 patients (25.2%) received a molecular diagnosis, and 58% of the diagnostic mutations had not previously been reported. Two hundred and eighty patients had a single mutation that caused disease, 181 were autosomal recessive, 65 were X-linked, and 1 was presumed inherited through the mitochondria.
In 5 cases, the patient inherited 2 copies of the mutated gene from the same parent. Of the dominant mutations, 208 were de novo mutations not inherited from either parent, 32 were inherited, and 40 were not determined because parental samples were not available.
Among the de novo mutations, 5 demonstrated mosaicism, which suggested the mutation occurred after fertilization.
The researchers found 708 presumptive causative variant alleles in the 504 cases. Almost 30% of the diagnoses occurred in disease genes only identified by researchers in the last 3 years. In 65 cases, there was no available genetic test other than whole-exome sequencing to find the mutated gene at the time the test was ordered.
Twenty-three patients (about 5%) had mutations in 2 different genes, which could account for various aspects of the patient’s medical condition.
“Doctors generally try to find one diagnosis that explains all the issues a patient may have,” said study author Christine Eng, MD, of the Baylor College of Medicine.
“We have found that, in some cases, a patient may have a blended phenotype of 2 different conditions. That patients may have 2 different rare genetic diseases to explain their condition was an unexpected finding prior to the use of whole-exome sequencing.”
In the 2000 cases, incidental findings of medically actionable results that could result in early diagnosis, screening, or treatment were found in 92 patients. Three patients had more than 1 finding.
“Clinical exome sequencing can assist diagnosis in a wide range of disorders that are diagnostic dilemmas,” Dr Lupski said. “Rare variants and Mendelian disease are important contributors to disease populations. This is in sharp contrast to the thinking of population geneticists, who investigate how common variants contribute to disease susceptibility.”
“We find ‘rare variants’ in aggregate actually contribute to disease susceptibility in a big way. The individual diseases may be rare, but there are thousands of such diseases and many more being defined through genomics.” ![]()

Credit: Graham Colm
In a large study, whole-exome sequencing provided 25% of patients with a diagnosis related to a known genetic disease, giving young
patients and their parents some long-sought answers.
The technology was able to detect a number of rare genetic events and new mutations contributing to disease.
Among the medically actionable findings were mutations related to Fanconi anemia, erythrocytosis, hemolytic anemia, and von Willebrand disease.
“The findings in this report, I believe, will forever change the future practice of pediatrics and medicine as a whole,” said study author James R. Lupski, MD, PhD, of the Baylor College of Medicine in Houston.
“It is just a matter of time before genomics moves up on the physician’s list of things to do and is ordered before formulating a differential diagnosis. It will be the new ‘family history’ that, better yet, gets you both the important variants inherited from each parent and the new mutations that contribute to disease susceptibility.”
This research was published in JAMA and is set to be presented on October 21 at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers had previously conducted a pilot study of whole-exome sequencing that included 250 patients and revealed a 25% molecular diagnostic rate.
This current study included 2000 patients (88% pediatric) with clinical whole-exome sequencing analyzed between June 2012 and August 2014. The majority of patients—87.8%—had neurological disorders or a developmental delay, and 12.2% had non-neurological disorders.
The researchers collected peripheral blood, tissue, or extracted DNA samples from patients and their parents. The team sequenced patients’ DNA and compared those results to the normal reference. Any disease-associated mutations were then compared with the parent’s DNA to determine if the child inherited it from one or both parents.
In all, 504 patients (25.2%) received a molecular diagnosis, and 58% of the diagnostic mutations had not previously been reported. Two hundred and eighty patients had a single mutation that caused disease, 181 were autosomal recessive, 65 were X-linked, and 1 was presumed inherited through the mitochondria.
In 5 cases, the patient inherited 2 copies of the mutated gene from the same parent. Of the dominant mutations, 208 were de novo mutations not inherited from either parent, 32 were inherited, and 40 were not determined because parental samples were not available.
Among the de novo mutations, 5 demonstrated mosaicism, which suggested the mutation occurred after fertilization.
The researchers found 708 presumptive causative variant alleles in the 504 cases. Almost 30% of the diagnoses occurred in disease genes only identified by researchers in the last 3 years. In 65 cases, there was no available genetic test other than whole-exome sequencing to find the mutated gene at the time the test was ordered.
Twenty-three patients (about 5%) had mutations in 2 different genes, which could account for various aspects of the patient’s medical condition.
“Doctors generally try to find one diagnosis that explains all the issues a patient may have,” said study author Christine Eng, MD, of the Baylor College of Medicine.
“We have found that, in some cases, a patient may have a blended phenotype of 2 different conditions. That patients may have 2 different rare genetic diseases to explain their condition was an unexpected finding prior to the use of whole-exome sequencing.”
In the 2000 cases, incidental findings of medically actionable results that could result in early diagnosis, screening, or treatment were found in 92 patients. Three patients had more than 1 finding.
“Clinical exome sequencing can assist diagnosis in a wide range of disorders that are diagnostic dilemmas,” Dr Lupski said. “Rare variants and Mendelian disease are important contributors to disease populations. This is in sharp contrast to the thinking of population geneticists, who investigate how common variants contribute to disease susceptibility.”
“We find ‘rare variants’ in aggregate actually contribute to disease susceptibility in a big way. The individual diseases may be rare, but there are thousands of such diseases and many more being defined through genomics.” ![]()

Credit: Graham Colm
In a large study, whole-exome sequencing provided 25% of patients with a diagnosis related to a known genetic disease, giving young
patients and their parents some long-sought answers.
The technology was able to detect a number of rare genetic events and new mutations contributing to disease.
Among the medically actionable findings were mutations related to Fanconi anemia, erythrocytosis, hemolytic anemia, and von Willebrand disease.
“The findings in this report, I believe, will forever change the future practice of pediatrics and medicine as a whole,” said study author James R. Lupski, MD, PhD, of the Baylor College of Medicine in Houston.
“It is just a matter of time before genomics moves up on the physician’s list of things to do and is ordered before formulating a differential diagnosis. It will be the new ‘family history’ that, better yet, gets you both the important variants inherited from each parent and the new mutations that contribute to disease susceptibility.”
This research was published in JAMA and is set to be presented on October 21 at the American Society of Human Genetics Annual Meeting in San Diego.
The researchers had previously conducted a pilot study of whole-exome sequencing that included 250 patients and revealed a 25% molecular diagnostic rate.
This current study included 2000 patients (88% pediatric) with clinical whole-exome sequencing analyzed between June 2012 and August 2014. The majority of patients—87.8%—had neurological disorders or a developmental delay, and 12.2% had non-neurological disorders.
The researchers collected peripheral blood, tissue, or extracted DNA samples from patients and their parents. The team sequenced patients’ DNA and compared those results to the normal reference. Any disease-associated mutations were then compared with the parent’s DNA to determine if the child inherited it from one or both parents.
In all, 504 patients (25.2%) received a molecular diagnosis, and 58% of the diagnostic mutations had not previously been reported. Two hundred and eighty patients had a single mutation that caused disease, 181 were autosomal recessive, 65 were X-linked, and 1 was presumed inherited through the mitochondria.
In 5 cases, the patient inherited 2 copies of the mutated gene from the same parent. Of the dominant mutations, 208 were de novo mutations not inherited from either parent, 32 were inherited, and 40 were not determined because parental samples were not available.
Among the de novo mutations, 5 demonstrated mosaicism, which suggested the mutation occurred after fertilization.
The researchers found 708 presumptive causative variant alleles in the 504 cases. Almost 30% of the diagnoses occurred in disease genes only identified by researchers in the last 3 years. In 65 cases, there was no available genetic test other than whole-exome sequencing to find the mutated gene at the time the test was ordered.
Twenty-three patients (about 5%) had mutations in 2 different genes, which could account for various aspects of the patient’s medical condition.
“Doctors generally try to find one diagnosis that explains all the issues a patient may have,” said study author Christine Eng, MD, of the Baylor College of Medicine.
“We have found that, in some cases, a patient may have a blended phenotype of 2 different conditions. That patients may have 2 different rare genetic diseases to explain their condition was an unexpected finding prior to the use of whole-exome sequencing.”
In the 2000 cases, incidental findings of medically actionable results that could result in early diagnosis, screening, or treatment were found in 92 patients. Three patients had more than 1 finding.
“Clinical exome sequencing can assist diagnosis in a wide range of disorders that are diagnostic dilemmas,” Dr Lupski said. “Rare variants and Mendelian disease are important contributors to disease populations. This is in sharp contrast to the thinking of population geneticists, who investigate how common variants contribute to disease susceptibility.”
“We find ‘rare variants’ in aggregate actually contribute to disease susceptibility in a big way. The individual diseases may be rare, but there are thousands of such diseases and many more being defined through genomics.” ![]()
Ibrutinib gets EU approval for CLL, MCL

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()

Credit: Steven Harbour
The European Commission has granted marketing approval for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the European Union (EU).
The drug is now approved to treat adult patients with relapsed or refractory mantle cell lymphoma (MCL), adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy, and first-line CLL patients who have 17p deletion or TP53 mutation and are unsuitable for chemotherapy.
In the EU and all other countries except the US, ibrutinib is marketed by Janssen Pharmaceutical Companies. In the US, the drug is being jointly developed and commercialized by Pharmacyclics and Janssen Biotech, Inc.
The EU approval of ibrutinib was based on data from a phase 2 study (PCYC-1104) in patients with MCL, the phase 3 RESONATE trial (PCYC-1112-CA) in CLL and small lymphocytic lymphoma (SLL), and a phase 1b/2 study (PCYC-1102) in CLL/SLL.
PCYC-1104: Ibrutinib in MCL
Results of this trial were presented at ASH 2012 and published in NEJM in 2013. The NEJM data included 111 patients who received ibrutinib at 560 mg daily in continuous, 28-day cycles until disease progression.
The overall response rate was 68%, with a complete response rate of 21% and a partial response rate of 47%. With an estimated median follow-up of 15.3 months, the estimated median response duration was 17.5 months.
The estimated progression-free survival was 13.9 months, and the overall survival was not reached. The estimated rate of overall survival was 58% at 18 months.
Common nonhematologic adverse events included diarrhea (50%), fatigue (41%), nausea (31%), peripheral edema (28%), dyspnea (27%), constipation (25%), upper respiratory tract infection (23%), vomiting (23%), and decreased appetite (21%). The most common grade 3, 4, or 5 infection was pneumonia (6%).
Grade 3 and 4 hematologic adverse events included neutropenia (16%), thrombocytopenia (11%), and anemia (10%). Grade 3 bleeding events occurred in 5 patients.
RESONATE: Ibrutinib in CLL/SLL
Results of the RESONATE trial were reported at EHA 2014 and published in NEJM in July. This trial was stopped early after an interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
The trial included 391 patients with relapsed or refractory CLL or SLL who were randomized to receive ibrutinib (n=195) or ofatumumab (n=196). Patients in the ofatumumab arm were allowed to cross over to ibrutinib if they progressed (n=57). The median time on study was 9.4 months.
The best overall response rate was higher in the ibrutinib arm than the ofatumumab arm, at 78% and 11%, respectively. And ibrutinib significantly prolonged progression-free survival. The median was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001).
Ibrutinib significantly prolonged overall survival as well. The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39% of patients, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
PCYC-1102: Ibrutinib in CLL/SLL
Results of this phase 1b/2 trial were published in The Lancet Oncology in January. The trial enrolled 29 patients with previously untreated CLL and 2 with SLL.
They received 28-day cycles of once-daily ibrutinib at 420 mg or 840 mg. The 840 mg dose was discontinued after enrollment had begun because the doses showed comparable activity.
After a median follow-up of 22.1 months, 71% of patients achieved an objective response. Four patients (13%) had a complete response. The median time to response was 1.9 months.
Study investigators did not establish whether ibrutinib confers improvements in survival or disease-related symptoms.
Common adverse events included diarrhea (68%), nausea (48%), fatigue (32%), peripheral edema (29%), hypertension (29%), dizziness (26%), dyspepsia (26%), upper respiratory tract infection (26%), arthralgia (23%), constipation (23%), urinary tract infection (23%), and vomiting (23%).
Grade 3 adverse events included diarrhea (13%), fatigue (3%), hypertension (6%), dizziness (3%), urinary tract infection (3%), headache (3%), back pain (3%), and neutropenia (3%). One patient (3%) had grade 4 thrombocytopenia. ![]()
Bigger Than His Bite
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
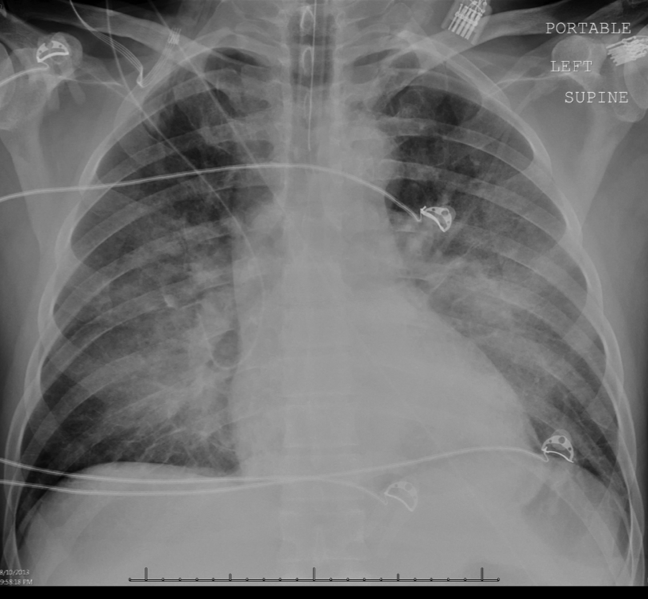
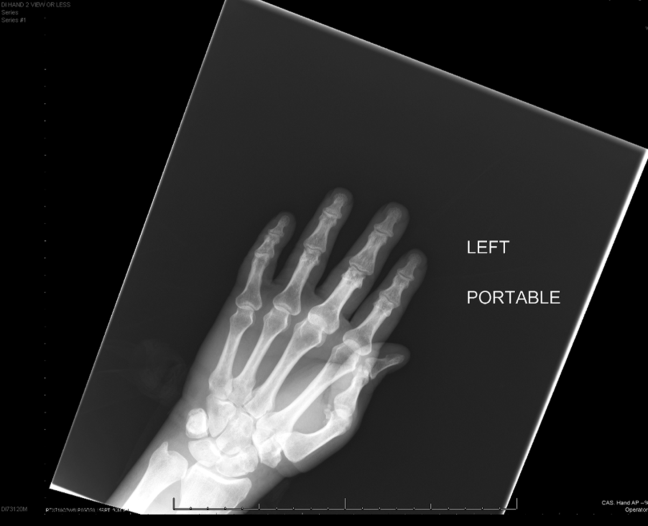
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.
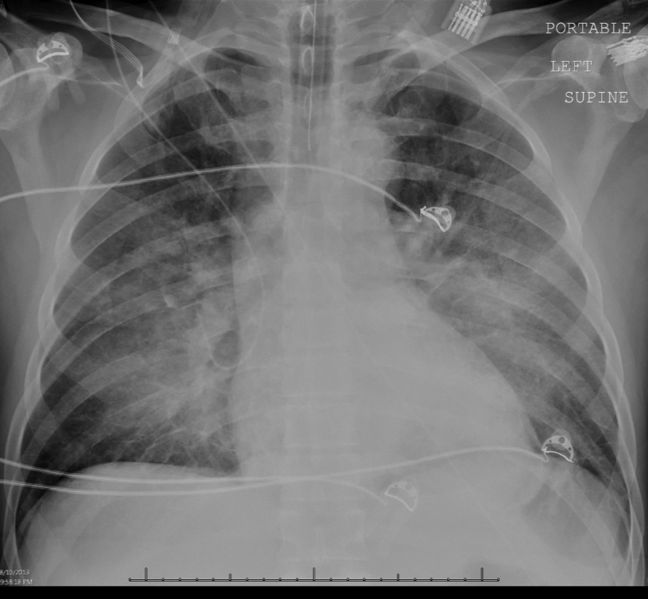
Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
A 58‐year‐old male presented to a local community hospital emergency department with fever and altered mental status. Earlier in the day he had complained of chills, swollen tongue, numbness and tingling in his extremities with associated burning pain, and generalized weakness. En route to the emergency department, he was extremely agitated and moving uncontrollably. On arrival, he was noted to be in respiratory distress and was intubated for hypoxic respiratory failure. He was subsequently transferred to an academic medical center, and in transit was noted to have sustained supraventricular tachycardia with a heart rate of 160 beats per minute.
Although the differential for altered mental status is broad, associated fever limits the main diagnostic considerations to infectious, toxic, and some inflammatory disorders. Confusion and fever are most concerning for a central nervous system infection, either meningitis or encephalitis. Sepsis from a broader range of infectious etiologies may also present with these symptoms. His respiratory failure could represent acute respiratory distress syndrome (ARDS) due to sepsis, aspiration, or a manifestation of a multisystem inflammatory disease.
He did not have any significant past medical or surgical history. Three days before his initial presentation, the patient was bitten on the left hand and forearm while breaking up a dogfight. The dogs that bit him belonged to his son, but were unvaccinated. He did not seek medical attention and it was unclear how he treated his wounds at home.
Dogs may serve as vectors for a number of zoonoses. Species of both Pasteurella and Capnocytophaga may cause sepsis and rarely meningitis as a consequence of dog bites. The incubation period of 3 days, though brief, does not exclude either infection. Rabies encephalitis is also possible, particularly given the dogs' unvaccinated status. However, the typical incubation period for rabies is on the order of months, and a 3‐day interval from inoculation to symptoms would be highly unusual. Although other explanations for his symptoms are more likely, he should still be considered for vaccination and rabies immune globulin. The dogs should be observed for clinical manifestations of rabies. Despite the patient's history of dog bite, a broad differential diagnosis must be maintained.
The patient lived in Michigan and worked in a chemical factory driving equipment without any hazardous exposures. He did not have any allergies. He drank 6 beers per day; he did not smoke cigarettes and had no history of illicit drug use. He was single and had 4 adult children.
His history of heavy alcohol consumption raises several additional possibilities. Delirium tremens, alcohol withdrawal seizures, or hepatic encephalopathy as a consequence of alcoholic cirrhosis are both potential contributors to his presentation. Furthermore, the physiologic signs of alcohol withdrawal are similar to many critical illnesses, which may present a diagnostic challenge. The patient's history of employment at a chemical factory is intriguing, though the details of any potential occupational exposures are unknown. Carbon monoxide poisoning can present with altered mental status and agitation, whereas anticholinergic toxicity can present with fever, tachycardia, and altered mental status; however, there is no obvious source of exposure to either.
On physical examination, the patient was intubated with a Glasgow Coma Scale of 11 without sedation; serial examinations revealed a fluctuating level of consciousness. His temperature was 38.1C, heart rate was 158 beats per minute, and blood pressure was 93/68 mm Hg. Mechanical ventilation was provided with assist control mode, a respiratory rate of 28 breaths per minute, tidal volume 466 mL, and positive end expiratory pressure of 20 cm of water. His oxygen saturation was 81% on 100% oxygen. Examination of his neck exhibited a large left neck hematoma from the unsuccessful placement of an external jugular intravenous catheter. Pupils were 4 mm in diameter and minimally reactive. There was no scleral icterus. Cardiac exam revealed tachycardia and regular rhythm without murmurs, rubs, or gallops. Lung exam was significant for bilateral rhonchi and minimal tracheal secretions. Extremity exam revealed 0.25 to 1.5 cm in diameter puncture bite marks with abrasions on his left third and fourth upper extremity digits as well as on his left forearm. Skin exam was diffusely cool with a mottled appearance. Neurologic exam revealed absent deep tendon reflexes throughout and apparent flaccid paralysis of all 4 extremities. Examination of the abdomen, lymph nodes, mouth, and throat were unremarkable.
The shock associated with sepsis is typically distributive, with intense vasodilation that classically leads to warm extremities. His mottled, cool extremities raise concern for disseminated intravascular coagulation (DIC), which can be seen in patients with septic shock, particularly cases caused by meningococcal disease and Capnocytophaga infections. His neurologic examination is typical of lower motor neuron disease, although acute upper motor neuron lesions can also be associated with hyporeflexia. Rabies can manifest as flaccid paralysis, but this would classically predate the mental status changes. Rabies remains a consideration, albeit a less likely one. Zoonoses, particularly Capnocytophaga and Pasteurella, are possible; however, a thorough search for other infections leading to sepsis is still indicated. His lung findings suggest severe ARDS.
The white blood cell count was 5,900/mm3, with 91% neutrophils, 6.6% lymphocytes, and 0.5% monocytes. The hemoglobin level was 13.0 g/dL, and the platelet count was 12,000/mm3. The fibrinogen level was 89 mg/dL (normal range 200400 mg/dL), international normalized ratio and partial‐thromboplastin time were 4.6 (normal range 0.8 to 1.1) and greater than 120.0 seconds (normal range 2535 seconds), respectively. Lactate dehydrogenase level was 698 IU/L (normal 120240 IU/L), and haptoglobin was 54 mg/dL (normal 41165 mg/dL). Serum sodium was 136 mmol/L, potassium 4.6 mmol/L, chloride 101 mmol/L, bicarbonate 16 mmol/L, blood urea nitrogen 29 mg/dL, creatinine 2.28 mg/dL, glucose 123 mg/dL, calcium 7.0 mg/dL, magnesium 1.7 mg/dL, and phosphorus 7.2 mg/dL. Total protein was 4.3 g/dL (normal 6.08.3 g/dL), albumin 2.5 g/dL (normal 3.54.9 g/dL), total bilirubin 2.3 mg/dL (normal 0.21.2 mg/dL), aspartate aminotransferase 71 IU/L (normal 830 IU/L), alanine aminotransferase 29 IU/L (normal 735 IU/L), and alkaline phosphatase 107 IU/L (normal 30130 IU/L). The serum troponin‐I level was 0.76 ng/mL, creatine phosphokinase 397 ng/mL, and creatine kinase‐myocardial band 3.5 ng/mL. Initial arterial blood gas analysis revealed a pH of 7.00, pCO2 57 mm Hg, pO2 98 mm Hg, and a lactic acid of 6.5 mmol/L (normal 0.52.2 mmol/L).
The patient has a normal absolute white blood cell count in the setting of septic shock. He has a relative neutrophilia and a marked leukopenia, both of which can be seen in overwhelming infections. The patient's arterial blood gas analysis indicates he has a mixed metabolic and respiratory acidosis. The normal physiologic response to metabolic acidosis is to increase minute ventilation and induce a compensatory respiratory alkalosis. His concomitant respiratory acidosis in the face of mechanical ventilation and presumed adequate minute ventilation suggests severely impaired alveolar gas exchange, most likely from ARDS. He has numerous other metabolic abnormalities, including acute kidney injury, DIC, and hemolytic anemia, all of which may be seen with severe bacterial infections or septic shock. Neisseria meningitidis and other gram‐negative infections would be of particular concern in this case. The combination of fever, altered mental status, thrombocytopenia, hemolytic anemia, and renal failure could be consistent with thrombotic thrombocytopenic purpura. However, the prolonged coagulation studies are much more consistent with DIC.
Intravenous antimicrobials were administered including ceftriaxone (initiated in the emergency department of the transferring hospital), ampicillin, vancomycin, piperacillin/tazobactam, clindamycin, metronidazole, doxycycline, and acyclovir. He received tetanus and rabies vaccines as well as tetanus and rabies immune globulin. The patient was given aggressive intravenous crystalloid fluids with minimal response in blood pressure. Intravenous norepinephrine was initiated to maintain a mean arterial pressure above 65 mm Hg. A plain chest radiograph (Figure 1) revealed perihilar airspace opacities. Head computed tomography without contrast revealed global cerebral volume loss greater than expected for the patient's age; no evidence of intracranial hemorrhage, mass effect, or edema; and proptosis of the eyes with adjacent preseptal soft tissue swelling without evidence of retrobulbar hemorrhage or vascular engorgement. Ultrasound of the left neck hematoma was negative for pulsatile mass. Electrocardiogram (ECG) revealed sinus tachycardia without evidence of ischemic changes. A bedside transthoracic echocardiogram showed hyperdynamic changes without evidence of hypokinesis but with inspiratory collapse of the inferior vena cava. Abdominal ultrasound was normal. Plain radiographs of the left hand (Figure 2) identified only mild soft tissue swelling over the dorsum of the hand. An ultrasound of the left hand and left forearm did not identify any abnormal fluid collection. A dialysis catheter was placed after the patient received platelets and fresh frozen plasma for initiation of continuous renal replacement therapy.


Given this patient's fulminant presentation, he was appropriately started on a very broad anti‐infective regimen. Although fungal infections are less likely, his current antimicrobial regimen lacks antifungal coverage. His finding of proptosis raises concern for mucormycosis, although the time course and clinical presentation are somewhat atypical. Because of the severity of his presentation, initiation of amphotericin B could be considered if he fails to quickly respond to the current regimen. There is no known effective treatment for rabies. Thus, if his presentation is due to rabies encephalitis, rabies vaccine and immunoglobulin will not be effective at treating active rabies infection. However, given his exposure history and the dogs' unvaccinated status, postexposure prophylaxis was appropriate to prevent future development of rabies. The inspiratory collapse and hyperdynamic ventricular response seen on his bedside echocardiogram is consistent with decreased effective circulating volume from sepsis or severe hypovolemia rather than acute heart failure.
Less than 36 hours after admission (60 hours after his symptoms began), the patient's oxygenation status had not improved. He developed diffuse cutaneous purpura with hemorrhagic bullae. Liver, renal, and cardiac function markers were all markedly abnormal. All cultures from the transferring hospital, collected before antibiotics were initiated, were negative to date. However, Gram stain of blood cultures performed at the academic medical center revealed possible gram‐negative rods. The patient remained unresponsive without sedation. ECG revealed evidence of inferior and anterolateral ischemia. The patient's family was informed of his persistently deteriorating condition and elected to pursue comfort measures. Two hours later the patient expired. The family agreed to an autopsy.
This patient succumbed to overwhelming sepsis and multiorgan failure. Although the etiologic pathogen is not immediately clear, several clues point to a likely unifying diagnosis. First, he has a history of a recent dog bite with minimal local evidence of infection. Second, he presented with fulminant sepsis with DIC, hemolytic anemia, and diffuse mottling that progressed to purpura fulminans. Third, a possible gram‐negative rod was isolated on blood Gram stain. Fourth, he has a history of heavy alcohol use. For these reasons, Capnocytophaga canimorsus is the most likely underlying etiology. C canimorsus is a fastidious gram‐negative coccobacillus that is an uncommon cause of fulminant sepsis in patients with dog bites. It is difficult to isolate due to culture growth requirements, which may explain the negative blood cultures in this case. Patients with alcoholism are predisposed to fulminant sepsis from C canimorsus, which often presents with hepatic and renal failure. The myocardial ischemia may be secondary to the metabolic and thrombotic complications of sepsis.
On autopsy, there was purpura fulminans involving over 90% of the total body surface area as well as skin slippage and loose bullae of greater than 75% of the total body surface area. There was infarction of the kidneys, liver, spleen, and adrenal glands as well as focal contraction bands of necrosis of the myocardium. The lungs showed diffuse alveolar damage. There was hemorrhage, edema, and necrosis seen in sections taken from the puncture wounds. Following the patient's death, it was reported by the transferring institution that C canimorsus was identified from 2 of 2 antemortem blood cultures, and pan‐sensitive Acinetobacter lwoffii in 1 of 2 blood cultures, though no sensitivities were performed on the C canimorsus isolate. In addition, antemortem cultures obtained at the academic medical center identified Capnocytophaga species in 1 of 2 peripheral blood culture specimens; sensitivities were not performed. Autopsy determined the cause of death in this patient to be septic complications of dog bite.
COMMENTARY
Dog bites are frequent, with over 12,000 occurring daily in the United States; of these, approximately 20% require medical attention.[1] Although most patients rapidly recover with conservative management, even initially benign‐appearing injuries can lead to long‐term morbidity or death. The hands are most often affected and are associated with more frequent need for both antibiotics and surgical intervention.[2, 3] The severity of injury does not correlate with subsequent infections.[3]
Management of dog bite injuries includes careful wound management. All patients with moderate to severe injury should be assessed within 48 hours by physical examination and radiography to assess the degree of injury and any associated nerve, tendon, joint, or bone damage. If there is concern for rabies based on history or vaccination status of the animal, prompt irrigation and debridement is crucial. Antimicrobial prophylaxis, typically with amoxicillinclavulanate, should be given to high‐risk patients, such as those with cirrhosis, asplenia, or other immunosuppressing conditions.[4] Most infections are caused by Pasteurella and Bacteroides, whereas Capnocytophaga may cause severe disease, particularly in patients with immunosuppression or excessive alcohol intake.[5] This patient was at increased risk of infection due to his late presentation following the initial bite and consequent delayed wound care, injury to the hand, and his history of alcoholism.[4]
Several members of the genus Capnocytophaga have been found in the oral cavities of both humans and canines. C canimorsus, found only in canine or feline oral cavities, is the only member of the genus known to cause human disease.[6] It is a fastidious gram‐negative rod requiring an environment enriched with carbon dioxide, making it notoriously difficult to isolate. Cultures typically do not show growth for 5 to 7 days; thus, it is not surprising all cultures were initially negative in this case.[4, 7] C canimorsus is a well‐described cause of sepsis related to dog bites, with some cases bearing similarity to fulminant meningococcal disease.[8] Severe illness typically occurs in immunosuppressed patients, particularly those with asplenia or cirrhosis.[9, 10] The pathophysiology of fulminant C canimorsus infections is not well described. It has been suggested that certain strains may produce a toxin that inhibits macrophages and inactivates tumor necrosis factor in humans, although this is not yet widely accepted.[11] Treatment of C canimorsus involves early administration of effective antimicrobials, supportive care, and standard management of the bite injury. C canimorsus is susceptible to several classes of antibiotics; ‐lactams, such as penicillin derivatives and cephalosporins, and potentiated sulphonamides, such as trimethoprim/sulfamethoxazole, typically have the best in vitro activity.[12] As illustrated in this case, even with prompt, effective antibiotic administration, C canimorsus infection can progress to DIC, multisystem organ failure, and death.[9]
A lwoffii was also identified, but was almost certainly a contaminant. It is a gram‐negative bacillus that is widely distributed throughout the environment. Commonly found on human skin and within the human oropharynx, it rarely causes human disease. Clinical manifestations of infection with A lwoffii are typically mild, and include superficial skin and soft tissue infection, urinary tract infection, and rarely bacteremia. Because of the severe presentation in this case and the compelling alternative explanation of C canimorsus, A lwoffii was almost certainly a contaminant.
Rabies was an intriguing possibility in this case given the unvaccinated status of the dogs and the patient's prominent neurologic findings. Clinicians must consider the possibility of rabies in any patient with a bite injury from an unvaccinated dog. However, rabies remains extremely rare in the developed world as a result of the overwhelming success of animal vaccination and postexposure prophylaxis. Furthermore, rabies typically has an incubation period of several months. If rabies had caused this patient's presentation, rabies immunoglobulin would have been ineffective. Nevertheless, rabies prophylaxis with rabies immunoglobulin and vaccination is appropriate to prevent subsequent disease unless rabies infection can be definitively excluded.[13]
This patient presented with septic shock, DIC, and multisystem organ failure after a dog bite. The discussant quickly recognized the propensity of Capnocytophaga to cause this constellation of findings in alcoholic patients after dog bites. This patient did not have cirrhosis or asplenia, both of which are known risk factors for C canimorsus infection; however, the fulminant presentation made C canimorsus a necessary consideration. Ultimately, the dramatic nature of the patient's presentation combined with his history of heavy alcohol intake led the discussant to the correct diagnosis of septic shock secondary to C canimorsus infection complicating a benign‐appearing dog bite. Clinicians caring for patients who present with sepsis after a recent dog bite should consider C canimorsus, remembering that on occasion, a dog's bark may not be bigger than his bite.
TEACHING POINTS
- The initial management of moderate or severe dog‐bite injuries includes careful wound assessment and radiography to exclude any associated bone, nerve, joint, or tendon injury.
- Immunosuppressed patients with dog bites, including those with cirrhosis or asplenia, should receive amoxicillin/clavulanate prophylaxis.
- C canimorsus is a fastidious gram‐negative bacillus that may cause fulminant sepsis after dog bites. It is associated with DIC, purpura fulminans, and multisystem organ failure.
- ‐lactam antibiotics, such as penicillin derivatives or cephalosporins, or sulphonamides, are the treatment of choice for C canimorsus.
Disclosure
Nothing to report.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
- , , , . Dog bites: still a problem? Injury Prev. 2008;14(5):296–301.
- , , , . Dog bite injuries: primary and secondary emergency department presentations—a retrospective cohort study. ScientificWorldJournal. 2013;2013:393176.
- , , , et al. Management of vascular trauma from dog bites. J Vascular Surg. 2013;58(5):1346–1352.
- , . Dog bites. BMJ. 2007;334(7590):413–417.
- , , , . Bacterial infections as complications of dog bites [in Danish]. Ugeskrift Laeger. 1998;160(34):4860–4863.
- , , , , . Bite‐related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439–447.
- , , , , . Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340(2):85–92.
- , , , . Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12(2):340–342.
- , , . Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clinical Infect Dis. 1996;23(1):71–75.
- . Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841.
- , , , , , . Molecular characterization of Capnocytophaga canimorsus and other canine Capnocytophaga spp. and assessment by PCR of their frequencies in dogs. J Clin Microbiol. 2009;47(10):3218–3225.
- , , , . The bacteriology and antimicrobial susceptibility of infected and non‐infected dog bite wounds: fifty cases. Vet Microbiol. 2008;127(3‐4):360–368.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Human rabies—Alabama, Tennessee, and Texas, 1994. Morbidity and Mortality Weekly Report; 1995. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00036736.htm. Accessed March 1, 2014.
PODCAST: Alternatives to hormone therapy exist for women with moderate to severe menopause symptoms
NATIONAL HARBOR, MD. – “The good news is that, if someone presents with hot flashes but isn’t a candidate for hormone therapy, you can offer them a range of options,” Dr. JoAnne Pinkerton, professor of ob.gyn. at the University of Virginia, Charlottesville, said at the annual meeting of the North American Menopause Society.
In addition to hypnosis or products such as phytoestrogens that have a demonstrated strong placebo effect, Dr. Pinkerton recommends in this audio interview that physicians familiarize themselves with a Food and Drug Administration–approved paroxetine salt, certain doses of a variety of antidepressants, and other off-label treatments that can help alleviate menopause symptoms, including hot flashes, insomnia, and night sweats.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – “The good news is that, if someone presents with hot flashes but isn’t a candidate for hormone therapy, you can offer them a range of options,” Dr. JoAnne Pinkerton, professor of ob.gyn. at the University of Virginia, Charlottesville, said at the annual meeting of the North American Menopause Society.
In addition to hypnosis or products such as phytoestrogens that have a demonstrated strong placebo effect, Dr. Pinkerton recommends in this audio interview that physicians familiarize themselves with a Food and Drug Administration–approved paroxetine salt, certain doses of a variety of antidepressants, and other off-label treatments that can help alleviate menopause symptoms, including hot flashes, insomnia, and night sweats.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – “The good news is that, if someone presents with hot flashes but isn’t a candidate for hormone therapy, you can offer them a range of options,” Dr. JoAnne Pinkerton, professor of ob.gyn. at the University of Virginia, Charlottesville, said at the annual meeting of the North American Menopause Society.
In addition to hypnosis or products such as phytoestrogens that have a demonstrated strong placebo effect, Dr. Pinkerton recommends in this audio interview that physicians familiarize themselves with a Food and Drug Administration–approved paroxetine salt, certain doses of a variety of antidepressants, and other off-label treatments that can help alleviate menopause symptoms, including hot flashes, insomnia, and night sweats.
On Twitter @whitneymcknight
Slowly Spreading Hand Lesions
Several months ago, lesions appeared on both of this 48-year-old woman’s hands. They have been completely unresponsive to any of the treatments prescribed by her primary care provider, including oral (terbinafine) and topical antifungal medications.
The lesions, which are asymptomatic, manifested slowly. They first appeared on the dorsa of her hands and then gradually spread until they covered the central portion of both hands. The lesions wax and wane in terms of thickness and extent but never disappear.
The patient denies similar lesions elsewhere. She also denies contact with animals or children. She has never been immunosuppressed and is quite healthy aside from her skin problems. She has a family history but no personal history of diabetes.
EXAMINATION
The condition affects both hands equally. It is composed of intradermal reddish brown papules and plaques that cover the metacarpal areas and extend onto the proximal interphalangeal skin and the distal dorsa. No epidermal component (scale or other broken skin surface) is seen.
The margins of the lesions are arciform, slightly raised, and smooth. The centers of several are concave.
What is the diagnosis?
DISCUSSION
This is the classic presentation of granuloma annulare (GA), in terms of morphology, distribution, and patient gender. Though this common condition can manifest in a number of locations and in men, it tends to favor the extensor surfaces of the extremities of women. The dorsum of the foot, anterior tibial skin, and elbows are typical locations that make it a relatively easy condition to recognize.
GA can be more difficult to identify, however, when it is generalized or deeper in the dermis. The latter, termed subcutaneous GA, is most common in children, who present with large dark annular patches of discoloration too deep in the dermis to produce any palpable surface changes. The more rare generalized GA can be difficult to diagnose and treat. Biopsy of suspected generalized or subcutaneous GA is necessary to differentiate it from its lookalikes, such as secondary syphilis, lichen planus, sarcoidosis, and tinea.
The annular borders of GA lesions are notorious for deceiving the unwary practitioner into diagnosing fungal infection. But the latter is, by definition, an infection of the outermost layers of the skin on which fungal organisms feed. That process almost invariably produces significant scaling, especially on the periphery of the lesions. Such scaling is completely absent on GA lesions, which also have a papularity and induration rarely seen with superficial fungal infections. In this particular case, the patient denied any potential source for a fungal infection (animals, children) and denied the itching we would expect with one.
The cause of GA is unknown, but it appears to represent a reaction to an unknown antigen. One previous hypothesis held that it was connected to diabetes, but this was proved false.
Ordinary GA resolves on its own (though this can take months)—which is fortunate, since there is no uniformly successful treatment. Many things have been tried, including topical or intralesional steroids and cryotherapy, with varying levels of success. Generalized GA is even more difficult to treat, can linger for years, and can be highly pruritic. Treatments have included hydroxychloroquine, dapsone, potassium iodide, isotretinoin, and PUVA. More recently, biologics are being used with some success.
The bigger problem with GA is simply to consider it in the differential for odd, annular, or generalized lesions.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is often mistaken for fungal infection, but it is an intradermal rather than epidermal process.
• The extensor surfaces of the extremities of women are favored sites for GA lesions.
• Biopsy of suspected GA lesions confirms the diagnosis while ruling out other items in the differential (eg, sarcoidosis and lichen planus).
• GA has no connection to diabetes.
Several months ago, lesions appeared on both of this 48-year-old woman’s hands. They have been completely unresponsive to any of the treatments prescribed by her primary care provider, including oral (terbinafine) and topical antifungal medications.
The lesions, which are asymptomatic, manifested slowly. They first appeared on the dorsa of her hands and then gradually spread until they covered the central portion of both hands. The lesions wax and wane in terms of thickness and extent but never disappear.
The patient denies similar lesions elsewhere. She also denies contact with animals or children. She has never been immunosuppressed and is quite healthy aside from her skin problems. She has a family history but no personal history of diabetes.
EXAMINATION
The condition affects both hands equally. It is composed of intradermal reddish brown papules and plaques that cover the metacarpal areas and extend onto the proximal interphalangeal skin and the distal dorsa. No epidermal component (scale or other broken skin surface) is seen.
The margins of the lesions are arciform, slightly raised, and smooth. The centers of several are concave.
What is the diagnosis?
DISCUSSION
This is the classic presentation of granuloma annulare (GA), in terms of morphology, distribution, and patient gender. Though this common condition can manifest in a number of locations and in men, it tends to favor the extensor surfaces of the extremities of women. The dorsum of the foot, anterior tibial skin, and elbows are typical locations that make it a relatively easy condition to recognize.
GA can be more difficult to identify, however, when it is generalized or deeper in the dermis. The latter, termed subcutaneous GA, is most common in children, who present with large dark annular patches of discoloration too deep in the dermis to produce any palpable surface changes. The more rare generalized GA can be difficult to diagnose and treat. Biopsy of suspected generalized or subcutaneous GA is necessary to differentiate it from its lookalikes, such as secondary syphilis, lichen planus, sarcoidosis, and tinea.
The annular borders of GA lesions are notorious for deceiving the unwary practitioner into diagnosing fungal infection. But the latter is, by definition, an infection of the outermost layers of the skin on which fungal organisms feed. That process almost invariably produces significant scaling, especially on the periphery of the lesions. Such scaling is completely absent on GA lesions, which also have a papularity and induration rarely seen with superficial fungal infections. In this particular case, the patient denied any potential source for a fungal infection (animals, children) and denied the itching we would expect with one.
The cause of GA is unknown, but it appears to represent a reaction to an unknown antigen. One previous hypothesis held that it was connected to diabetes, but this was proved false.
Ordinary GA resolves on its own (though this can take months)—which is fortunate, since there is no uniformly successful treatment. Many things have been tried, including topical or intralesional steroids and cryotherapy, with varying levels of success. Generalized GA is even more difficult to treat, can linger for years, and can be highly pruritic. Treatments have included hydroxychloroquine, dapsone, potassium iodide, isotretinoin, and PUVA. More recently, biologics are being used with some success.
The bigger problem with GA is simply to consider it in the differential for odd, annular, or generalized lesions.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is often mistaken for fungal infection, but it is an intradermal rather than epidermal process.
• The extensor surfaces of the extremities of women are favored sites for GA lesions.
• Biopsy of suspected GA lesions confirms the diagnosis while ruling out other items in the differential (eg, sarcoidosis and lichen planus).
• GA has no connection to diabetes.
Several months ago, lesions appeared on both of this 48-year-old woman’s hands. They have been completely unresponsive to any of the treatments prescribed by her primary care provider, including oral (terbinafine) and topical antifungal medications.
The lesions, which are asymptomatic, manifested slowly. They first appeared on the dorsa of her hands and then gradually spread until they covered the central portion of both hands. The lesions wax and wane in terms of thickness and extent but never disappear.
The patient denies similar lesions elsewhere. She also denies contact with animals or children. She has never been immunosuppressed and is quite healthy aside from her skin problems. She has a family history but no personal history of diabetes.
EXAMINATION
The condition affects both hands equally. It is composed of intradermal reddish brown papules and plaques that cover the metacarpal areas and extend onto the proximal interphalangeal skin and the distal dorsa. No epidermal component (scale or other broken skin surface) is seen.
The margins of the lesions are arciform, slightly raised, and smooth. The centers of several are concave.
What is the diagnosis?
DISCUSSION
This is the classic presentation of granuloma annulare (GA), in terms of morphology, distribution, and patient gender. Though this common condition can manifest in a number of locations and in men, it tends to favor the extensor surfaces of the extremities of women. The dorsum of the foot, anterior tibial skin, and elbows are typical locations that make it a relatively easy condition to recognize.
GA can be more difficult to identify, however, when it is generalized or deeper in the dermis. The latter, termed subcutaneous GA, is most common in children, who present with large dark annular patches of discoloration too deep in the dermis to produce any palpable surface changes. The more rare generalized GA can be difficult to diagnose and treat. Biopsy of suspected generalized or subcutaneous GA is necessary to differentiate it from its lookalikes, such as secondary syphilis, lichen planus, sarcoidosis, and tinea.
The annular borders of GA lesions are notorious for deceiving the unwary practitioner into diagnosing fungal infection. But the latter is, by definition, an infection of the outermost layers of the skin on which fungal organisms feed. That process almost invariably produces significant scaling, especially on the periphery of the lesions. Such scaling is completely absent on GA lesions, which also have a papularity and induration rarely seen with superficial fungal infections. In this particular case, the patient denied any potential source for a fungal infection (animals, children) and denied the itching we would expect with one.
The cause of GA is unknown, but it appears to represent a reaction to an unknown antigen. One previous hypothesis held that it was connected to diabetes, but this was proved false.
Ordinary GA resolves on its own (though this can take months)—which is fortunate, since there is no uniformly successful treatment. Many things have been tried, including topical or intralesional steroids and cryotherapy, with varying levels of success. Generalized GA is even more difficult to treat, can linger for years, and can be highly pruritic. Treatments have included hydroxychloroquine, dapsone, potassium iodide, isotretinoin, and PUVA. More recently, biologics are being used with some success.
The bigger problem with GA is simply to consider it in the differential for odd, annular, or generalized lesions.
TAKE-HOME LEARNING POINTS
• Granuloma annulare (GA) is often mistaken for fungal infection, but it is an intradermal rather than epidermal process.
• The extensor surfaces of the extremities of women are favored sites for GA lesions.
• Biopsy of suspected GA lesions confirms the diagnosis while ruling out other items in the differential (eg, sarcoidosis and lichen planus).
• GA has no connection to diabetes.
Vitamin D deficiency associated with Alzheimer’s
Our relationship with vitamins and supplements may be approach-avoidance. On one hand, if they are beneficial and patients are motivated to take them, we do not complain. This is likely a marker of motivated patient who may heed other health promotional advice that we proffer. On the other hand, it is difficult to keep up with the massive amount of good and bad literature about them. Patients can challenge us on our medical knowledge, pinging our opinions about the latest findings tweeted out while we struggle to keep up with all the wheelchair forms.
Vitamins are clearly not consistently beneficial. B vitamins may increase lung cancer risk in smokers. Vitamin D, however, seems to have some of the greatest “staying power” in the clinical realm and has a good reputation as far as vitamins go. Vitamin D is probably good for the heart, but how about the head? Could low D cause dementia? If so, how?
Previous studies of the relationship between vitamin D and dementia have not shown consistent results. Thomas Littlejohns, M.Sc., and colleagues have published a fantastic piece of work (Neurology 2014 Aug. 6 [doi: 10.1212/WNL.0000000000000755]) that sheds some light. They evaluated a prospective cohort of 1,658 elderly ambulatory adults with no history of dementia, CVD, or stroke who had baseline 25-hydroxyvitamin D [25(OH)D] concentrations at baseline. Severely low levels of 25(OH)D and deficiency (≥25 to <50 nmol/L) were associated with a significantly increased risk for all-cause dementia and Alzheimer’s dementia.
Several hypotheses exist as to why vitamin D helps the brain. Vitamin D may attenuate amyloid-induced cytotoxicity and neural apoptosis. It also may reduce the risk of strokes by promoting healthy cerebral vasculature.
The Institute of Medicine recommends a serum concentration of 25(OH)D at 50 nmol/L. This study would suggest that sufficiency to this level is neuroprotective. The next step is to see if supplementation can modify baseline risk, but many of my patients may wait for these data to come out before starting their vitamin D supplements.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Our relationship with vitamins and supplements may be approach-avoidance. On one hand, if they are beneficial and patients are motivated to take them, we do not complain. This is likely a marker of motivated patient who may heed other health promotional advice that we proffer. On the other hand, it is difficult to keep up with the massive amount of good and bad literature about them. Patients can challenge us on our medical knowledge, pinging our opinions about the latest findings tweeted out while we struggle to keep up with all the wheelchair forms.
Vitamins are clearly not consistently beneficial. B vitamins may increase lung cancer risk in smokers. Vitamin D, however, seems to have some of the greatest “staying power” in the clinical realm and has a good reputation as far as vitamins go. Vitamin D is probably good for the heart, but how about the head? Could low D cause dementia? If so, how?
Previous studies of the relationship between vitamin D and dementia have not shown consistent results. Thomas Littlejohns, M.Sc., and colleagues have published a fantastic piece of work (Neurology 2014 Aug. 6 [doi: 10.1212/WNL.0000000000000755]) that sheds some light. They evaluated a prospective cohort of 1,658 elderly ambulatory adults with no history of dementia, CVD, or stroke who had baseline 25-hydroxyvitamin D [25(OH)D] concentrations at baseline. Severely low levels of 25(OH)D and deficiency (≥25 to <50 nmol/L) were associated with a significantly increased risk for all-cause dementia and Alzheimer’s dementia.
Several hypotheses exist as to why vitamin D helps the brain. Vitamin D may attenuate amyloid-induced cytotoxicity and neural apoptosis. It also may reduce the risk of strokes by promoting healthy cerebral vasculature.
The Institute of Medicine recommends a serum concentration of 25(OH)D at 50 nmol/L. This study would suggest that sufficiency to this level is neuroprotective. The next step is to see if supplementation can modify baseline risk, but many of my patients may wait for these data to come out before starting their vitamin D supplements.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Our relationship with vitamins and supplements may be approach-avoidance. On one hand, if they are beneficial and patients are motivated to take them, we do not complain. This is likely a marker of motivated patient who may heed other health promotional advice that we proffer. On the other hand, it is difficult to keep up with the massive amount of good and bad literature about them. Patients can challenge us on our medical knowledge, pinging our opinions about the latest findings tweeted out while we struggle to keep up with all the wheelchair forms.
Vitamins are clearly not consistently beneficial. B vitamins may increase lung cancer risk in smokers. Vitamin D, however, seems to have some of the greatest “staying power” in the clinical realm and has a good reputation as far as vitamins go. Vitamin D is probably good for the heart, but how about the head? Could low D cause dementia? If so, how?
Previous studies of the relationship between vitamin D and dementia have not shown consistent results. Thomas Littlejohns, M.Sc., and colleagues have published a fantastic piece of work (Neurology 2014 Aug. 6 [doi: 10.1212/WNL.0000000000000755]) that sheds some light. They evaluated a prospective cohort of 1,658 elderly ambulatory adults with no history of dementia, CVD, or stroke who had baseline 25-hydroxyvitamin D [25(OH)D] concentrations at baseline. Severely low levels of 25(OH)D and deficiency (≥25 to <50 nmol/L) were associated with a significantly increased risk for all-cause dementia and Alzheimer’s dementia.
Several hypotheses exist as to why vitamin D helps the brain. Vitamin D may attenuate amyloid-induced cytotoxicity and neural apoptosis. It also may reduce the risk of strokes by promoting healthy cerebral vasculature.
The Institute of Medicine recommends a serum concentration of 25(OH)D at 50 nmol/L. This study would suggest that sufficiency to this level is neuroprotective. The next step is to see if supplementation can modify baseline risk, but many of my patients may wait for these data to come out before starting their vitamin D supplements.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
FDA panel supports retaining Chantix boxed warning for now
SILVER SPRING, MD. – The boxed warning about the risk of serious neuropsychiatric effects associated with the smoking cessation drug varenicline should remain on the drug’s label, and the need for this warning can be reevaluated when the results of a postmarketing safety study become available next year, according to the majority of a Food and Drug Administration advisory panel.
On Oct. 16, at a joint meeting of the FDA’s Psychopharmacologic Drugs and the Drug Safety and Risk Management Advisory committees, 11 of the 18 panelists voted to retain the boxed warning about neuropsychiatric symptoms and suicidality, agreeing that a decision about whether to retain the warning should not be made until the results of the study are available.
Pfizer, which manufactures varenicline, a nicotinic receptor partial agonist in a tablet formulation, as Chantix, is conducting the prospective, randomized, double-blind study comparing neuropsychiatric events in 8,000 smokers, with and without a psychiatric history, treated with varenicline, nicotine replacement therapy, bupropion, or placebo. Although the results are expected in 2015, the company maintained that the boxed warning is no longer justified and can be put in the warnings and precautions section, based on analyses of observational studies and randomized clinical trials. The company submitted those analyses to the FDA earlier this year.
The boxed warning in the drug’s prescribing information states that the serious events reported in patients taking the drug include but “are not limited to” depression, suicidal ideation, suicide attempts, and completed suicides; and that some cases “may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking.” The reason the boxed warning is used for this drug is that there is a “serious adverse reaction that can be prevented or reduced in frequency or severity,” the FDA said.
Because the precedent for removing a boxed warning from a drug label is limited and the FDA’s view that the decision should consider the final results of the postmarketing trial, the agency convened the joint meeting to review the analyses submitted by the company. Overall, the panelists were not convinced by the analyses. They cited various weaknesses in the observational studies and the meta-analysis, including questions about whether the correct outcomes were being captured.
Six panelists voted to modify (but not weaken) the language in the boxed warning, and most recommended that the last few lines about the health benefits of quitting smoking be removed and argued that the wording had a promotional tone and did not belong in a boxed warning. Several panelists recommended adding sleep disruption and disorders, and recognized adverse events of the drugs to the boxed warning.
Among those who voted to retain the boxed warning, Dr. Jeanmarie Perrone, director of the medical toxicology division in the emergency medicine department at the University of Pennsylvania, Philadelphia, said there was biologic plausibility for these events. Her biggest concern, she said, was that removing the boxed warning might be interpreted as “an endorsement of safety and that has not been demonstrated.” In the emergency department, she said, she sees many patients who have been prescribed varenicline “by a myriad of clinicians,” and the presence of a boxed warning helps keep this safety issue in mind.
Dr. Rajiv N. Rimal, professor and chair of the department of prevention and community health, George Washington University, Washington, who voted to retain the warning with modified wording, said: “I heard enough compelling evidence to suggest that even some of the more rare events were severe enough” that revisiting the labeling change would be necessary once the other data became available.
Varenicline, approved by the FDA in 2006 as an aid to smoking-cessation treatment, provides a “low level” of dopamine release as people try to quit smoking, according to Pfizer. The boxed warning was added to the label in July 2009, based on the FDA’s review of spontaneous adverse event reports, conducted after an alert about suicidality reports associated with the drug from European regulators about 1 year after FDA approval. About the same time, there was a widely publicized case of a Texas musician who was taking varenicline and was shot and killed by a neighbor while exhibiting uncharacteristic aggressive behavior in the fall of 2007.
The reports provided by the company at that time included 102 suicide-related cases, and 525 reports of aggressive/irrational behavior. A review of the reports in the FDA’s adverse events database included many cases with “hallmarks of drug relatedness,” patients reporting unusual symptoms with “similar distinctive language,” and positive dechallenges and rechallenges, said Dr. Celia Winchell, the medical team leader of addiction products in the FDA’s division of anesthesia, analgesia, and addiction products. Not all cases involved suicidality, depression, or aggression. Postmarketing reports have provided a “rich narrative with detail” about patient experiences, including descriptions that are relatively easy to describe or classify, such as suicide and aggression, while others “defy coding,” such as one person who reported feeling “like a zombie,” she told the panel.
“Based on a review of the cases, there appears to be a syndrome of debilitating symptoms that interfere with people’s ability to function in their daily lives that is associated with the use of Chantix,” Dr. Winchell said.
The company agreed that the spontaneous reports in late 2007 raised concerns about whether the drug was associated with an increase in serious neuropsychiatric events. But since 2009, this issue has been evaluated in controlled studies, which “consistently show no evidence of an increased risk,” Dr. Christopher Wohlberg, the head of Pfizer’s safety surveillance and risk management group, told the panel.
These include a meta-analysis of adverse events in 18 controlled studies of about 5,000 patients treated with varenicline and about 3,500 on placebo, which found no significant difference in the rate of neuropsychiatric events (with the exception of sleep disorders and disturbance) among those on the drug vs. those on placebo; and a meta-analysis of 5 placebo-controlled studies that used the Columbia-Suicide Severity Rating Scale to evaluate suicidal ideation and behavior, which also found no significant differences between the two groups.
In addition, four observational studies that included patients with psychiatric illnesses showed that the rates of serious neuropsychiatric events in patients treated with varenicline were not significantly different from those treated with nicotine replacement therapy or bupropion for smoking cessation. In individual studies of patients with psychiatric illnesses (schizophrenia, schizoaffective disorder, or major depressive disorder), no evidence was found of an increased risk among those treated with varenicline, compared with those on placebo, according to the company.
But the FDA reviewers raised issues with the meta-analysis of the 18 studies and the observational data. The observational data have limitations and “preclude” a conclusion that there is no association of varenicline and an increased risk of neuropsychiatric events, said Natasha Chen, Ph.D., an epidemiologist in the FDA’s office of surveillance and epidemiology. The ongoing postmarketing safety trial “is likely to offer better insights into varenicline’s neuropsychiatric risks,” she added.
The one panelist who voted to remove the warning, Dr. Andrew J. Saxon, said while there “may be some serious adverse or neuropsychiatric effects, the data, while not perfect,” do not show a signal in his view. He added that in his experience working daily with patients who are trying to quit smoking, “patients are afraid to take this medication because of the boxed warning, and it does deter use.” Moreover, in the Veterans Affairs system, there are restrictions on prescribing the drug because of the boxed warning, including a limitation on prescriptions to a 28-day supply, with no refills. Such restrictions increase hassles for patients and prescribers, and often result in patients stopping treatment after 4 weeks.
“If I’m doing a good job as a physician, I’m going to monitor the patient,” said Dr. Saxon, director of the addiction psychiatry residency program, University of Washington, Seattle.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Members of these two panels had no conflicts to disclose. Occasionally, panelists with a conflict are given a waiver, but not at this meeting.
In a statementissued by Pfizer after the meeting, Dr. Steven Romano, senior vice president and head of the medicines development group for Pfizer, said the “completion of our currently ongoing safety study will represent one more step forward in the process of accurately characterizing the neuropsychiatric safety of this important medication.”
The varenicline label is available at http://labeling.pfizer.com/ShowLabeling.aspx?id=557. Serious adverse events associated with this drug should be reported to the FDA’s MedWatch program at http://www.fda.gov/Safety/MedWatch/default.htm.
SILVER SPRING, MD. – The boxed warning about the risk of serious neuropsychiatric effects associated with the smoking cessation drug varenicline should remain on the drug’s label, and the need for this warning can be reevaluated when the results of a postmarketing safety study become available next year, according to the majority of a Food and Drug Administration advisory panel.
On Oct. 16, at a joint meeting of the FDA’s Psychopharmacologic Drugs and the Drug Safety and Risk Management Advisory committees, 11 of the 18 panelists voted to retain the boxed warning about neuropsychiatric symptoms and suicidality, agreeing that a decision about whether to retain the warning should not be made until the results of the study are available.
Pfizer, which manufactures varenicline, a nicotinic receptor partial agonist in a tablet formulation, as Chantix, is conducting the prospective, randomized, double-blind study comparing neuropsychiatric events in 8,000 smokers, with and without a psychiatric history, treated with varenicline, nicotine replacement therapy, bupropion, or placebo. Although the results are expected in 2015, the company maintained that the boxed warning is no longer justified and can be put in the warnings and precautions section, based on analyses of observational studies and randomized clinical trials. The company submitted those analyses to the FDA earlier this year.
The boxed warning in the drug’s prescribing information states that the serious events reported in patients taking the drug include but “are not limited to” depression, suicidal ideation, suicide attempts, and completed suicides; and that some cases “may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking.” The reason the boxed warning is used for this drug is that there is a “serious adverse reaction that can be prevented or reduced in frequency or severity,” the FDA said.
Because the precedent for removing a boxed warning from a drug label is limited and the FDA’s view that the decision should consider the final results of the postmarketing trial, the agency convened the joint meeting to review the analyses submitted by the company. Overall, the panelists were not convinced by the analyses. They cited various weaknesses in the observational studies and the meta-analysis, including questions about whether the correct outcomes were being captured.
Six panelists voted to modify (but not weaken) the language in the boxed warning, and most recommended that the last few lines about the health benefits of quitting smoking be removed and argued that the wording had a promotional tone and did not belong in a boxed warning. Several panelists recommended adding sleep disruption and disorders, and recognized adverse events of the drugs to the boxed warning.
Among those who voted to retain the boxed warning, Dr. Jeanmarie Perrone, director of the medical toxicology division in the emergency medicine department at the University of Pennsylvania, Philadelphia, said there was biologic plausibility for these events. Her biggest concern, she said, was that removing the boxed warning might be interpreted as “an endorsement of safety and that has not been demonstrated.” In the emergency department, she said, she sees many patients who have been prescribed varenicline “by a myriad of clinicians,” and the presence of a boxed warning helps keep this safety issue in mind.
Dr. Rajiv N. Rimal, professor and chair of the department of prevention and community health, George Washington University, Washington, who voted to retain the warning with modified wording, said: “I heard enough compelling evidence to suggest that even some of the more rare events were severe enough” that revisiting the labeling change would be necessary once the other data became available.
Varenicline, approved by the FDA in 2006 as an aid to smoking-cessation treatment, provides a “low level” of dopamine release as people try to quit smoking, according to Pfizer. The boxed warning was added to the label in July 2009, based on the FDA’s review of spontaneous adverse event reports, conducted after an alert about suicidality reports associated with the drug from European regulators about 1 year after FDA approval. About the same time, there was a widely publicized case of a Texas musician who was taking varenicline and was shot and killed by a neighbor while exhibiting uncharacteristic aggressive behavior in the fall of 2007.
The reports provided by the company at that time included 102 suicide-related cases, and 525 reports of aggressive/irrational behavior. A review of the reports in the FDA’s adverse events database included many cases with “hallmarks of drug relatedness,” patients reporting unusual symptoms with “similar distinctive language,” and positive dechallenges and rechallenges, said Dr. Celia Winchell, the medical team leader of addiction products in the FDA’s division of anesthesia, analgesia, and addiction products. Not all cases involved suicidality, depression, or aggression. Postmarketing reports have provided a “rich narrative with detail” about patient experiences, including descriptions that are relatively easy to describe or classify, such as suicide and aggression, while others “defy coding,” such as one person who reported feeling “like a zombie,” she told the panel.
“Based on a review of the cases, there appears to be a syndrome of debilitating symptoms that interfere with people’s ability to function in their daily lives that is associated with the use of Chantix,” Dr. Winchell said.
The company agreed that the spontaneous reports in late 2007 raised concerns about whether the drug was associated with an increase in serious neuropsychiatric events. But since 2009, this issue has been evaluated in controlled studies, which “consistently show no evidence of an increased risk,” Dr. Christopher Wohlberg, the head of Pfizer’s safety surveillance and risk management group, told the panel.
These include a meta-analysis of adverse events in 18 controlled studies of about 5,000 patients treated with varenicline and about 3,500 on placebo, which found no significant difference in the rate of neuropsychiatric events (with the exception of sleep disorders and disturbance) among those on the drug vs. those on placebo; and a meta-analysis of 5 placebo-controlled studies that used the Columbia-Suicide Severity Rating Scale to evaluate suicidal ideation and behavior, which also found no significant differences between the two groups.
In addition, four observational studies that included patients with psychiatric illnesses showed that the rates of serious neuropsychiatric events in patients treated with varenicline were not significantly different from those treated with nicotine replacement therapy or bupropion for smoking cessation. In individual studies of patients with psychiatric illnesses (schizophrenia, schizoaffective disorder, or major depressive disorder), no evidence was found of an increased risk among those treated with varenicline, compared with those on placebo, according to the company.
But the FDA reviewers raised issues with the meta-analysis of the 18 studies and the observational data. The observational data have limitations and “preclude” a conclusion that there is no association of varenicline and an increased risk of neuropsychiatric events, said Natasha Chen, Ph.D., an epidemiologist in the FDA’s office of surveillance and epidemiology. The ongoing postmarketing safety trial “is likely to offer better insights into varenicline’s neuropsychiatric risks,” she added.
The one panelist who voted to remove the warning, Dr. Andrew J. Saxon, said while there “may be some serious adverse or neuropsychiatric effects, the data, while not perfect,” do not show a signal in his view. He added that in his experience working daily with patients who are trying to quit smoking, “patients are afraid to take this medication because of the boxed warning, and it does deter use.” Moreover, in the Veterans Affairs system, there are restrictions on prescribing the drug because of the boxed warning, including a limitation on prescriptions to a 28-day supply, with no refills. Such restrictions increase hassles for patients and prescribers, and often result in patients stopping treatment after 4 weeks.
“If I’m doing a good job as a physician, I’m going to monitor the patient,” said Dr. Saxon, director of the addiction psychiatry residency program, University of Washington, Seattle.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Members of these two panels had no conflicts to disclose. Occasionally, panelists with a conflict are given a waiver, but not at this meeting.
In a statementissued by Pfizer after the meeting, Dr. Steven Romano, senior vice president and head of the medicines development group for Pfizer, said the “completion of our currently ongoing safety study will represent one more step forward in the process of accurately characterizing the neuropsychiatric safety of this important medication.”
The varenicline label is available at http://labeling.pfizer.com/ShowLabeling.aspx?id=557. Serious adverse events associated with this drug should be reported to the FDA’s MedWatch program at http://www.fda.gov/Safety/MedWatch/default.htm.
SILVER SPRING, MD. – The boxed warning about the risk of serious neuropsychiatric effects associated with the smoking cessation drug varenicline should remain on the drug’s label, and the need for this warning can be reevaluated when the results of a postmarketing safety study become available next year, according to the majority of a Food and Drug Administration advisory panel.
On Oct. 16, at a joint meeting of the FDA’s Psychopharmacologic Drugs and the Drug Safety and Risk Management Advisory committees, 11 of the 18 panelists voted to retain the boxed warning about neuropsychiatric symptoms and suicidality, agreeing that a decision about whether to retain the warning should not be made until the results of the study are available.
Pfizer, which manufactures varenicline, a nicotinic receptor partial agonist in a tablet formulation, as Chantix, is conducting the prospective, randomized, double-blind study comparing neuropsychiatric events in 8,000 smokers, with and without a psychiatric history, treated with varenicline, nicotine replacement therapy, bupropion, or placebo. Although the results are expected in 2015, the company maintained that the boxed warning is no longer justified and can be put in the warnings and precautions section, based on analyses of observational studies and randomized clinical trials. The company submitted those analyses to the FDA earlier this year.
The boxed warning in the drug’s prescribing information states that the serious events reported in patients taking the drug include but “are not limited to” depression, suicidal ideation, suicide attempts, and completed suicides; and that some cases “may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking.” The reason the boxed warning is used for this drug is that there is a “serious adverse reaction that can be prevented or reduced in frequency or severity,” the FDA said.
Because the precedent for removing a boxed warning from a drug label is limited and the FDA’s view that the decision should consider the final results of the postmarketing trial, the agency convened the joint meeting to review the analyses submitted by the company. Overall, the panelists were not convinced by the analyses. They cited various weaknesses in the observational studies and the meta-analysis, including questions about whether the correct outcomes were being captured.
Six panelists voted to modify (but not weaken) the language in the boxed warning, and most recommended that the last few lines about the health benefits of quitting smoking be removed and argued that the wording had a promotional tone and did not belong in a boxed warning. Several panelists recommended adding sleep disruption and disorders, and recognized adverse events of the drugs to the boxed warning.
Among those who voted to retain the boxed warning, Dr. Jeanmarie Perrone, director of the medical toxicology division in the emergency medicine department at the University of Pennsylvania, Philadelphia, said there was biologic plausibility for these events. Her biggest concern, she said, was that removing the boxed warning might be interpreted as “an endorsement of safety and that has not been demonstrated.” In the emergency department, she said, she sees many patients who have been prescribed varenicline “by a myriad of clinicians,” and the presence of a boxed warning helps keep this safety issue in mind.
Dr. Rajiv N. Rimal, professor and chair of the department of prevention and community health, George Washington University, Washington, who voted to retain the warning with modified wording, said: “I heard enough compelling evidence to suggest that even some of the more rare events were severe enough” that revisiting the labeling change would be necessary once the other data became available.
Varenicline, approved by the FDA in 2006 as an aid to smoking-cessation treatment, provides a “low level” of dopamine release as people try to quit smoking, according to Pfizer. The boxed warning was added to the label in July 2009, based on the FDA’s review of spontaneous adverse event reports, conducted after an alert about suicidality reports associated with the drug from European regulators about 1 year after FDA approval. About the same time, there was a widely publicized case of a Texas musician who was taking varenicline and was shot and killed by a neighbor while exhibiting uncharacteristic aggressive behavior in the fall of 2007.
The reports provided by the company at that time included 102 suicide-related cases, and 525 reports of aggressive/irrational behavior. A review of the reports in the FDA’s adverse events database included many cases with “hallmarks of drug relatedness,” patients reporting unusual symptoms with “similar distinctive language,” and positive dechallenges and rechallenges, said Dr. Celia Winchell, the medical team leader of addiction products in the FDA’s division of anesthesia, analgesia, and addiction products. Not all cases involved suicidality, depression, or aggression. Postmarketing reports have provided a “rich narrative with detail” about patient experiences, including descriptions that are relatively easy to describe or classify, such as suicide and aggression, while others “defy coding,” such as one person who reported feeling “like a zombie,” she told the panel.
“Based on a review of the cases, there appears to be a syndrome of debilitating symptoms that interfere with people’s ability to function in their daily lives that is associated with the use of Chantix,” Dr. Winchell said.
The company agreed that the spontaneous reports in late 2007 raised concerns about whether the drug was associated with an increase in serious neuropsychiatric events. But since 2009, this issue has been evaluated in controlled studies, which “consistently show no evidence of an increased risk,” Dr. Christopher Wohlberg, the head of Pfizer’s safety surveillance and risk management group, told the panel.
These include a meta-analysis of adverse events in 18 controlled studies of about 5,000 patients treated with varenicline and about 3,500 on placebo, which found no significant difference in the rate of neuropsychiatric events (with the exception of sleep disorders and disturbance) among those on the drug vs. those on placebo; and a meta-analysis of 5 placebo-controlled studies that used the Columbia-Suicide Severity Rating Scale to evaluate suicidal ideation and behavior, which also found no significant differences between the two groups.
In addition, four observational studies that included patients with psychiatric illnesses showed that the rates of serious neuropsychiatric events in patients treated with varenicline were not significantly different from those treated with nicotine replacement therapy or bupropion for smoking cessation. In individual studies of patients with psychiatric illnesses (schizophrenia, schizoaffective disorder, or major depressive disorder), no evidence was found of an increased risk among those treated with varenicline, compared with those on placebo, according to the company.
But the FDA reviewers raised issues with the meta-analysis of the 18 studies and the observational data. The observational data have limitations and “preclude” a conclusion that there is no association of varenicline and an increased risk of neuropsychiatric events, said Natasha Chen, Ph.D., an epidemiologist in the FDA’s office of surveillance and epidemiology. The ongoing postmarketing safety trial “is likely to offer better insights into varenicline’s neuropsychiatric risks,” she added.
The one panelist who voted to remove the warning, Dr. Andrew J. Saxon, said while there “may be some serious adverse or neuropsychiatric effects, the data, while not perfect,” do not show a signal in his view. He added that in his experience working daily with patients who are trying to quit smoking, “patients are afraid to take this medication because of the boxed warning, and it does deter use.” Moreover, in the Veterans Affairs system, there are restrictions on prescribing the drug because of the boxed warning, including a limitation on prescriptions to a 28-day supply, with no refills. Such restrictions increase hassles for patients and prescribers, and often result in patients stopping treatment after 4 weeks.
“If I’m doing a good job as a physician, I’m going to monitor the patient,” said Dr. Saxon, director of the addiction psychiatry residency program, University of Washington, Seattle.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Members of these two panels had no conflicts to disclose. Occasionally, panelists with a conflict are given a waiver, but not at this meeting.
In a statementissued by Pfizer after the meeting, Dr. Steven Romano, senior vice president and head of the medicines development group for Pfizer, said the “completion of our currently ongoing safety study will represent one more step forward in the process of accurately characterizing the neuropsychiatric safety of this important medication.”
The varenicline label is available at http://labeling.pfizer.com/ShowLabeling.aspx?id=557. Serious adverse events associated with this drug should be reported to the FDA’s MedWatch program at http://www.fda.gov/Safety/MedWatch/default.htm.
AT AN FDA ADVISORY COMMITTEE MEETING
Blocking STAT3 in NK cells to fight leukemia

Credit: Bjorn Onfelt/Dan Davis
Inhibiting STAT3 in natural killer (NK) cells can kill leukemia in two ways, according to research published in Blood.
In mouse models lacking STAT3, NK cells were still able to develop normally, and the loss of STAT3 prompted an increase in antileukemic activity.
Mice whose NK cells lacked STAT3 showed a reduction in tumor growth and an increase in survival compared to controls.
Similar results were observed in mouse models of melanoma.
“We were expecting the loss of STAT3 to make the NK cells less efficient,” said study author Dagmar Gotthardt, of the University of Veterinary Medicine, Vienna (Vetmeduni Vienna).
“Instead, it makes them even more potent killers. Inhibiting STAT3 could thus help cancer patients in two ways: both stopping the cancer cells from dividing and helping the patients’ NK cells to fight them more efficiently.”
Researchers are already attempting to develop STAT3 inhibitors for cancer therapy, but their effect on NK cells was not known.
So Gotthardt and her colleagues assessed the function of STAT3 in NK cells using Stat3Δ/ΔNcr1-iCreTg mice, whose NK cells lack STAT3.
The team discovered that NK cells lacking STAT3 still develop and mature normally, but they do have alterations in the kinetics of interferon-γ production. In addition, there is a consistent increase in levels of perforin, granzyme B, and DNAM-1 in the absence of STAT3.
The investigators also found the loss of STAT3 in NK cells improved tumor surveillance against leukemia.
They injected v-abl1 leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. After 12 days, the Stat3fl/fl mice had large tumors, but there was “a pronounced reduction of tumor mass” in the Stat3Δ/ΔNcr1-iCreTg mice.
The researchers then injected 2 individually derived leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. And the results were similar to those of the previous experiment. Stat3Δ/ΔNcr1-iCreTg mice survived significantly longer than Stat3fl/fl mice (P=0.0002).
Next, the investigators injected newborn mice with a replication-incompetent ecotropic retrovirus encoding for v-abl, as this model more closely mimics the development of human disease.
Again, there was a significant difference in survival between Stat3fl/fl mice and Stat3Δ/ΔNcr1-iCreTg mice (P=0.007). All Stat3fl/fl mice died, but 20% of Stat3Δ/ΔNcr1-iCreTg mice were still alive at 150 days post-injection, and their disease latency was significantly delayed.
The researchers also found that loss of STAT3 in NK cells led to an increase in killing activity against melanoma cells. They said the decrease in metastasis caused by melanoma cells was especially dramatic and confirmed that NK cells lacking STAT3 are extremely efficient killers of tumor cells. ![]()

Credit: Bjorn Onfelt/Dan Davis
Inhibiting STAT3 in natural killer (NK) cells can kill leukemia in two ways, according to research published in Blood.
In mouse models lacking STAT3, NK cells were still able to develop normally, and the loss of STAT3 prompted an increase in antileukemic activity.
Mice whose NK cells lacked STAT3 showed a reduction in tumor growth and an increase in survival compared to controls.
Similar results were observed in mouse models of melanoma.
“We were expecting the loss of STAT3 to make the NK cells less efficient,” said study author Dagmar Gotthardt, of the University of Veterinary Medicine, Vienna (Vetmeduni Vienna).
“Instead, it makes them even more potent killers. Inhibiting STAT3 could thus help cancer patients in two ways: both stopping the cancer cells from dividing and helping the patients’ NK cells to fight them more efficiently.”
Researchers are already attempting to develop STAT3 inhibitors for cancer therapy, but their effect on NK cells was not known.
So Gotthardt and her colleagues assessed the function of STAT3 in NK cells using Stat3Δ/ΔNcr1-iCreTg mice, whose NK cells lack STAT3.
The team discovered that NK cells lacking STAT3 still develop and mature normally, but they do have alterations in the kinetics of interferon-γ production. In addition, there is a consistent increase in levels of perforin, granzyme B, and DNAM-1 in the absence of STAT3.
The investigators also found the loss of STAT3 in NK cells improved tumor surveillance against leukemia.
They injected v-abl1 leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. After 12 days, the Stat3fl/fl mice had large tumors, but there was “a pronounced reduction of tumor mass” in the Stat3Δ/ΔNcr1-iCreTg mice.
The researchers then injected 2 individually derived leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. And the results were similar to those of the previous experiment. Stat3Δ/ΔNcr1-iCreTg mice survived significantly longer than Stat3fl/fl mice (P=0.0002).
Next, the investigators injected newborn mice with a replication-incompetent ecotropic retrovirus encoding for v-abl, as this model more closely mimics the development of human disease.
Again, there was a significant difference in survival between Stat3fl/fl mice and Stat3Δ/ΔNcr1-iCreTg mice (P=0.007). All Stat3fl/fl mice died, but 20% of Stat3Δ/ΔNcr1-iCreTg mice were still alive at 150 days post-injection, and their disease latency was significantly delayed.
The researchers also found that loss of STAT3 in NK cells led to an increase in killing activity against melanoma cells. They said the decrease in metastasis caused by melanoma cells was especially dramatic and confirmed that NK cells lacking STAT3 are extremely efficient killers of tumor cells. ![]()

Credit: Bjorn Onfelt/Dan Davis
Inhibiting STAT3 in natural killer (NK) cells can kill leukemia in two ways, according to research published in Blood.
In mouse models lacking STAT3, NK cells were still able to develop normally, and the loss of STAT3 prompted an increase in antileukemic activity.
Mice whose NK cells lacked STAT3 showed a reduction in tumor growth and an increase in survival compared to controls.
Similar results were observed in mouse models of melanoma.
“We were expecting the loss of STAT3 to make the NK cells less efficient,” said study author Dagmar Gotthardt, of the University of Veterinary Medicine, Vienna (Vetmeduni Vienna).
“Instead, it makes them even more potent killers. Inhibiting STAT3 could thus help cancer patients in two ways: both stopping the cancer cells from dividing and helping the patients’ NK cells to fight them more efficiently.”
Researchers are already attempting to develop STAT3 inhibitors for cancer therapy, but their effect on NK cells was not known.
So Gotthardt and her colleagues assessed the function of STAT3 in NK cells using Stat3Δ/ΔNcr1-iCreTg mice, whose NK cells lack STAT3.
The team discovered that NK cells lacking STAT3 still develop and mature normally, but they do have alterations in the kinetics of interferon-γ production. In addition, there is a consistent increase in levels of perforin, granzyme B, and DNAM-1 in the absence of STAT3.
The investigators also found the loss of STAT3 in NK cells improved tumor surveillance against leukemia.
They injected v-abl1 leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. After 12 days, the Stat3fl/fl mice had large tumors, but there was “a pronounced reduction of tumor mass” in the Stat3Δ/ΔNcr1-iCreTg mice.
The researchers then injected 2 individually derived leukemic cell lines into Stat3fl/fl and Stat3Δ/ΔNcr1-iCreTg mice. And the results were similar to those of the previous experiment. Stat3Δ/ΔNcr1-iCreTg mice survived significantly longer than Stat3fl/fl mice (P=0.0002).
Next, the investigators injected newborn mice with a replication-incompetent ecotropic retrovirus encoding for v-abl, as this model more closely mimics the development of human disease.
Again, there was a significant difference in survival between Stat3fl/fl mice and Stat3Δ/ΔNcr1-iCreTg mice (P=0.007). All Stat3fl/fl mice died, but 20% of Stat3Δ/ΔNcr1-iCreTg mice were still alive at 150 days post-injection, and their disease latency was significantly delayed.
The researchers also found that loss of STAT3 in NK cells led to an increase in killing activity against melanoma cells. They said the decrease in metastasis caused by melanoma cells was especially dramatic and confirmed that NK cells lacking STAT3 are extremely efficient killers of tumor cells. ![]()