User login
Team calls for change in warfarin dosing algorithms

Photo courtesy of NIGMS
Investigators have proposed the use of race-stratified algorithms to help clinicians better calculate the appropriate warfarin dose for a patient.
The team’s study, published in Blood, showed that clinical and genetic factors affecting warfarin dose requirements vary by race.
“As the outcomes of disease can vary by race, so can response to medications,” said Nita Limdi, PhD, PharmD, of the University of Alabama at Birmingham.
“Therefore, warfarin dosing equations that combine race groups for analysis (race-adjusted analysis) assume that the effect of variables—such as age and genetics—are the same across race groups, which may compromise dose prediction among patients of both races.”
To better understand how genetics and clinical factors influence warfarin dosing across race groups, Dr Limdi and her colleagues analyzed 1357 patients—595 African American and 762 European American—treated with warfarin.
The team calculated and compared dose recommendations according to both race-adjusted dosing models and race-stratified dosing models. They found that race-stratified analysis improved dose prediction in both racial groups, as compared to race- adjusted analysis.
Race-stratified analysis showed that European Americans with the CYP2C9*2 variant required less warfarin than European Americans with wild-type CYP2C9. But the same was not true for African Americans.
And although all participants who carried VKORC1 required lower doses, regardless of race, the proportional dose reduction was greater among European Americans.
The investigators therefore concluded that the influence of genetic and clinical factors on warfarin dose differs by race. So race-stratified algorithms, rather than race-adjusted algorithms, should be used to guide warfarin dosing.
“Our findings highlight the need for adequate racial representation in warfarin dosing studies to improve our understanding of how the factors that influence warfarin dose differ according to race,” Dr Limdi said. “This is the first step to developing race-specific algorithms to personalize therapy.” ![]()

Photo courtesy of NIGMS
Investigators have proposed the use of race-stratified algorithms to help clinicians better calculate the appropriate warfarin dose for a patient.
The team’s study, published in Blood, showed that clinical and genetic factors affecting warfarin dose requirements vary by race.
“As the outcomes of disease can vary by race, so can response to medications,” said Nita Limdi, PhD, PharmD, of the University of Alabama at Birmingham.
“Therefore, warfarin dosing equations that combine race groups for analysis (race-adjusted analysis) assume that the effect of variables—such as age and genetics—are the same across race groups, which may compromise dose prediction among patients of both races.”
To better understand how genetics and clinical factors influence warfarin dosing across race groups, Dr Limdi and her colleagues analyzed 1357 patients—595 African American and 762 European American—treated with warfarin.
The team calculated and compared dose recommendations according to both race-adjusted dosing models and race-stratified dosing models. They found that race-stratified analysis improved dose prediction in both racial groups, as compared to race- adjusted analysis.
Race-stratified analysis showed that European Americans with the CYP2C9*2 variant required less warfarin than European Americans with wild-type CYP2C9. But the same was not true for African Americans.
And although all participants who carried VKORC1 required lower doses, regardless of race, the proportional dose reduction was greater among European Americans.
The investigators therefore concluded that the influence of genetic and clinical factors on warfarin dose differs by race. So race-stratified algorithms, rather than race-adjusted algorithms, should be used to guide warfarin dosing.
“Our findings highlight the need for adequate racial representation in warfarin dosing studies to improve our understanding of how the factors that influence warfarin dose differ according to race,” Dr Limdi said. “This is the first step to developing race-specific algorithms to personalize therapy.” ![]()

Photo courtesy of NIGMS
Investigators have proposed the use of race-stratified algorithms to help clinicians better calculate the appropriate warfarin dose for a patient.
The team’s study, published in Blood, showed that clinical and genetic factors affecting warfarin dose requirements vary by race.
“As the outcomes of disease can vary by race, so can response to medications,” said Nita Limdi, PhD, PharmD, of the University of Alabama at Birmingham.
“Therefore, warfarin dosing equations that combine race groups for analysis (race-adjusted analysis) assume that the effect of variables—such as age and genetics—are the same across race groups, which may compromise dose prediction among patients of both races.”
To better understand how genetics and clinical factors influence warfarin dosing across race groups, Dr Limdi and her colleagues analyzed 1357 patients—595 African American and 762 European American—treated with warfarin.
The team calculated and compared dose recommendations according to both race-adjusted dosing models and race-stratified dosing models. They found that race-stratified analysis improved dose prediction in both racial groups, as compared to race- adjusted analysis.
Race-stratified analysis showed that European Americans with the CYP2C9*2 variant required less warfarin than European Americans with wild-type CYP2C9. But the same was not true for African Americans.
And although all participants who carried VKORC1 required lower doses, regardless of race, the proportional dose reduction was greater among European Americans.
The investigators therefore concluded that the influence of genetic and clinical factors on warfarin dose differs by race. So race-stratified algorithms, rather than race-adjusted algorithms, should be used to guide warfarin dosing.
“Our findings highlight the need for adequate racial representation in warfarin dosing studies to improve our understanding of how the factors that influence warfarin dose differ according to race,” Dr Limdi said. “This is the first step to developing race-specific algorithms to personalize therapy.” ![]()
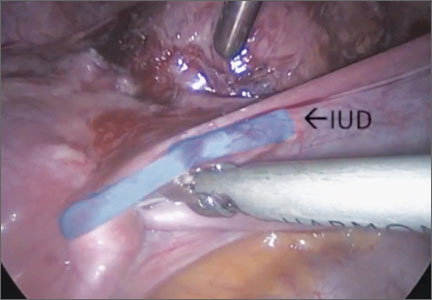
Surgical removal of malpositioned IUDs
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today’s intrauterine devices (IUDs) represent an excellent form of long-acting reversible contraception. Depending on the type of IUD, many also are used to help alleviate such gynecologic symptoms as abnormal uterine bleeding. Approximately 10% of IUD insertions are complicated by malpositioning, which can include embedding, translocation, or perforation. Malpositioned IUDs are often amenable to office removal but, occasionally, hysteroscopy or laparoscopy is necessary.
In this video, we begin by reviewing techniques for complicated office IUD removal. Then we present 4 cases of malpositioned IUDs that required surgical intervention; hysteroscopic, laparoscopic, or combined techniques were used in each case. This video highlights how preoperative imaging often is not sufficient to determine the necessary surgical approach. Therefore, patients should be counseled on the potential need for hysteroscopy or laparoscopy to surgically remove a malpositioned IUD.
Although risk factors for malpositioned IUDs are not well studied in the literature, understanding proper placement and identification of complications at the time of IUD placement are essential to malpositioning prevention.
My colleagues and I hope you enjoy this video.
—Dr. Arnold Advincula

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Knee Extensor Mechanism Reconstruction With Complete Extensor Allograft After Failure of Patellar Tendon Repair
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
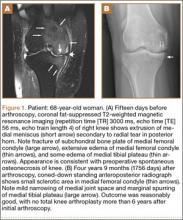
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
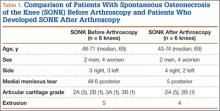
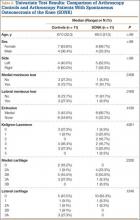
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
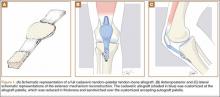
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
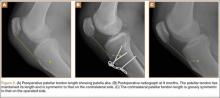
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
Intra-Articular Dislocation of the Patella With Associated Hoffa Fracture in a Skeletally Immature Patient
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
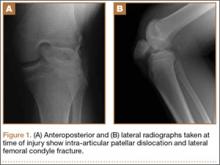
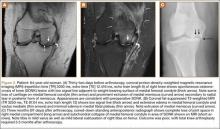
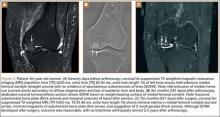
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
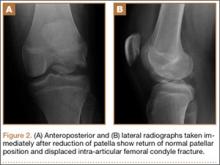
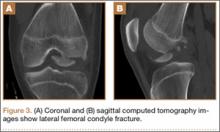
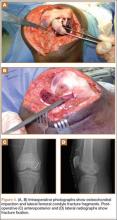
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
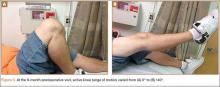
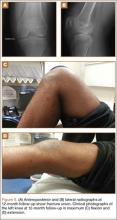
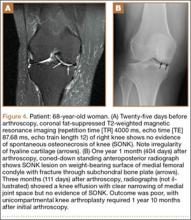
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
Precaution Guidelines Updated for Visitors of Inpatients with Infectious Diseases
Hospitalists may soon see changes in precaution protocols for some hospital visitors, thanks to a revised set of guidelines published by the Society for Healthcare Epidemiology of America (SHEA). The guidelines include recommendations for visitors to patients hospitalized with infectious diseases.
"Up until now, visitors have been wearing contact precautions just like healthcare providers—gowns, gloves, masks, sometimes respirators," says lead author L. Silvia Munoz-Price, MD, PhD, enterprise epidemiologist at the Institute for Health and Society of the Medical College of Wisconsin based in Milwaukee. "We looked at the evidence for these policies. Using our judgment, a literature review, and a survey of our membership, we came up with new guidelines."
Among SHEA's recommendations are two major changes:
- Visitors of patients diagnosed with methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) don't need gowns and gloves if those pathogens are endemic in the region and the institution;
- For cases involving Clostridium difficile infection and extensively drug-resistant gram-negative organisms, contact isolation precautions are still recommended; and
- Visitors of patients under airborne isolation precautions do not need N95 respirators. Healthcare workers still need the protective masks, but because the masks only function when fitted properly to an individual, the masks loaned to visitors are probably ineffective.
"We've been asking visitors to wear N95, even though we know most likely it’s doing nothing," Dr. Munoz-Price says. "Now we're saying, stop doing that."
If visitors have had enough exposure to the patient at home, they can wear surgical masks or maybe no precautions at all, she adds. If visitors have not seen the patient for weeks, visitation may have to be restricted.
Dr. Munoz-Price notes that hospitalists should be aware of these revised guidelines and know that they apply only to visitors.
"Even though family members are not wearing contact precaution gear, that doesn't mean that hospitalists shouldn't," Dr. Munoz-Price says. "It's extremely important that hospitalists be compliant with their protocols and not be influenced by what we're doing with visitors." TH
Suzanne Bopp is a freelance writer in New Jersey.
Visit our website for more information on managing patients with infectious diseases.
Hospitalists may soon see changes in precaution protocols for some hospital visitors, thanks to a revised set of guidelines published by the Society for Healthcare Epidemiology of America (SHEA). The guidelines include recommendations for visitors to patients hospitalized with infectious diseases.
"Up until now, visitors have been wearing contact precautions just like healthcare providers—gowns, gloves, masks, sometimes respirators," says lead author L. Silvia Munoz-Price, MD, PhD, enterprise epidemiologist at the Institute for Health and Society of the Medical College of Wisconsin based in Milwaukee. "We looked at the evidence for these policies. Using our judgment, a literature review, and a survey of our membership, we came up with new guidelines."
Among SHEA's recommendations are two major changes:
- Visitors of patients diagnosed with methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) don't need gowns and gloves if those pathogens are endemic in the region and the institution;
- For cases involving Clostridium difficile infection and extensively drug-resistant gram-negative organisms, contact isolation precautions are still recommended; and
- Visitors of patients under airborne isolation precautions do not need N95 respirators. Healthcare workers still need the protective masks, but because the masks only function when fitted properly to an individual, the masks loaned to visitors are probably ineffective.
"We've been asking visitors to wear N95, even though we know most likely it’s doing nothing," Dr. Munoz-Price says. "Now we're saying, stop doing that."
If visitors have had enough exposure to the patient at home, they can wear surgical masks or maybe no precautions at all, she adds. If visitors have not seen the patient for weeks, visitation may have to be restricted.
Dr. Munoz-Price notes that hospitalists should be aware of these revised guidelines and know that they apply only to visitors.
"Even though family members are not wearing contact precaution gear, that doesn't mean that hospitalists shouldn't," Dr. Munoz-Price says. "It's extremely important that hospitalists be compliant with their protocols and not be influenced by what we're doing with visitors." TH
Suzanne Bopp is a freelance writer in New Jersey.
Visit our website for more information on managing patients with infectious diseases.
Hospitalists may soon see changes in precaution protocols for some hospital visitors, thanks to a revised set of guidelines published by the Society for Healthcare Epidemiology of America (SHEA). The guidelines include recommendations for visitors to patients hospitalized with infectious diseases.
"Up until now, visitors have been wearing contact precautions just like healthcare providers—gowns, gloves, masks, sometimes respirators," says lead author L. Silvia Munoz-Price, MD, PhD, enterprise epidemiologist at the Institute for Health and Society of the Medical College of Wisconsin based in Milwaukee. "We looked at the evidence for these policies. Using our judgment, a literature review, and a survey of our membership, we came up with new guidelines."
Among SHEA's recommendations are two major changes:
- Visitors of patients diagnosed with methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) don't need gowns and gloves if those pathogens are endemic in the region and the institution;
- For cases involving Clostridium difficile infection and extensively drug-resistant gram-negative organisms, contact isolation precautions are still recommended; and
- Visitors of patients under airborne isolation precautions do not need N95 respirators. Healthcare workers still need the protective masks, but because the masks only function when fitted properly to an individual, the masks loaned to visitors are probably ineffective.
"We've been asking visitors to wear N95, even though we know most likely it’s doing nothing," Dr. Munoz-Price says. "Now we're saying, stop doing that."
If visitors have had enough exposure to the patient at home, they can wear surgical masks or maybe no precautions at all, she adds. If visitors have not seen the patient for weeks, visitation may have to be restricted.
Dr. Munoz-Price notes that hospitalists should be aware of these revised guidelines and know that they apply only to visitors.
"Even though family members are not wearing contact precaution gear, that doesn't mean that hospitalists shouldn't," Dr. Munoz-Price says. "It's extremely important that hospitalists be compliant with their protocols and not be influenced by what we're doing with visitors." TH
Suzanne Bopp is a freelance writer in New Jersey.
Visit our website for more information on managing patients with infectious diseases.
Most Hospitalists Not Eager to Screen Inpatients for Breast Cancer: JHM Study
A recent Journal of Hospital Medicine study found that most hospitalists do not believe they should be involved in breast cancer screening for their hospitalized patients who are overdue for a screening.
Study authors at Johns Hopkins Bayview (JHB) Medical Center in Baltimore surveyed nearly 100 hospitalists about their thoughts on ordering a mammography for hospitalized women and possible concerns for hospitalists ordering inpatient screenings. Only 38% of those surveyed believed that hospitalists should be involved with breast cancer screening. The main concerns, according to survey takers, were following up on the results of the screening and that the mammography might not be covered by patients’ insurance.
The Hospitalist caught up with lead author Waseem Khaliq MD, MPH, who is a hospitalist and assistant professor of medicine at Johns Hopkins School of Medicine and a member of the JHB Cancer Committee.
Question: What are the key takeaways from this study?
Answer: About three years ago, we looked up what the adherence rate is among women who are admitted to the hospital for breast cancer screenings, and what we found was that a lot of these women were nonadherent to the breast cancer screening. So we polled those women who were nonadherent to the breast cancer screening and asked, “What if we were able to offer you a mammogram while you were in the hospital for other issues?” About 76% said that they would like to have a mammogram while they were in the hospital.
Looking at that background, we polled this question to our hospitalists, too. What we found out was that a lot of the hospitalists were not willing to order a mammogram or were not too excited about getting a breast cancer screening done in the hospital setting. A majority told us that they’re more worried about how those results are going to be followed up, and it is possible that even if they order this mammogram that it may interfere with patient care or patient discharge. Then who would cover the cost of the mammogram if they do it in the inpatient setting?
So although a third of the hospitalists would still order a mammogram for those women who were high risk … a majority of them were not willing to because there were some perceived barriers to that.
Q: What is your reaction to the concerns with screening inpatients?
A: I can understand the concerns that most of the hospitalists have in regard to screening every patient that comes to the hospital. What I think we can do is, at the very least, we can be smart enough to figure out if a patient were at high risk for developing cancer and at least have those patients who were at high risk get screened.
Q: Where do you think hospitalists should go from here with regard to their patients who are overdue for breast cancer screenings?
A: We need to test for the feasibility and the financial issue of actually getting a screening mammogram in the hospital setting. I think down the road it should not matter what setting a patient [intersects] with the health system; it could be inpatient or outpatient. Patients should be provided the care and prevention needs that are recommended for their routine care. The next step should be doing a feasibility study, looking at whether or not these mammograms can be done in the hospital setting and do not interfere with the patient’s acute care. TH
Candace Mitchell is a freelance writer in New Jersey.
A recent Journal of Hospital Medicine study found that most hospitalists do not believe they should be involved in breast cancer screening for their hospitalized patients who are overdue for a screening.
Study authors at Johns Hopkins Bayview (JHB) Medical Center in Baltimore surveyed nearly 100 hospitalists about their thoughts on ordering a mammography for hospitalized women and possible concerns for hospitalists ordering inpatient screenings. Only 38% of those surveyed believed that hospitalists should be involved with breast cancer screening. The main concerns, according to survey takers, were following up on the results of the screening and that the mammography might not be covered by patients’ insurance.
The Hospitalist caught up with lead author Waseem Khaliq MD, MPH, who is a hospitalist and assistant professor of medicine at Johns Hopkins School of Medicine and a member of the JHB Cancer Committee.
Question: What are the key takeaways from this study?
Answer: About three years ago, we looked up what the adherence rate is among women who are admitted to the hospital for breast cancer screenings, and what we found was that a lot of these women were nonadherent to the breast cancer screening. So we polled those women who were nonadherent to the breast cancer screening and asked, “What if we were able to offer you a mammogram while you were in the hospital for other issues?” About 76% said that they would like to have a mammogram while they were in the hospital.
Looking at that background, we polled this question to our hospitalists, too. What we found out was that a lot of the hospitalists were not willing to order a mammogram or were not too excited about getting a breast cancer screening done in the hospital setting. A majority told us that they’re more worried about how those results are going to be followed up, and it is possible that even if they order this mammogram that it may interfere with patient care or patient discharge. Then who would cover the cost of the mammogram if they do it in the inpatient setting?
So although a third of the hospitalists would still order a mammogram for those women who were high risk … a majority of them were not willing to because there were some perceived barriers to that.
Q: What is your reaction to the concerns with screening inpatients?
A: I can understand the concerns that most of the hospitalists have in regard to screening every patient that comes to the hospital. What I think we can do is, at the very least, we can be smart enough to figure out if a patient were at high risk for developing cancer and at least have those patients who were at high risk get screened.
Q: Where do you think hospitalists should go from here with regard to their patients who are overdue for breast cancer screenings?
A: We need to test for the feasibility and the financial issue of actually getting a screening mammogram in the hospital setting. I think down the road it should not matter what setting a patient [intersects] with the health system; it could be inpatient or outpatient. Patients should be provided the care and prevention needs that are recommended for their routine care. The next step should be doing a feasibility study, looking at whether or not these mammograms can be done in the hospital setting and do not interfere with the patient’s acute care. TH
Candace Mitchell is a freelance writer in New Jersey.
A recent Journal of Hospital Medicine study found that most hospitalists do not believe they should be involved in breast cancer screening for their hospitalized patients who are overdue for a screening.
Study authors at Johns Hopkins Bayview (JHB) Medical Center in Baltimore surveyed nearly 100 hospitalists about their thoughts on ordering a mammography for hospitalized women and possible concerns for hospitalists ordering inpatient screenings. Only 38% of those surveyed believed that hospitalists should be involved with breast cancer screening. The main concerns, according to survey takers, were following up on the results of the screening and that the mammography might not be covered by patients’ insurance.
The Hospitalist caught up with lead author Waseem Khaliq MD, MPH, who is a hospitalist and assistant professor of medicine at Johns Hopkins School of Medicine and a member of the JHB Cancer Committee.
Question: What are the key takeaways from this study?
Answer: About three years ago, we looked up what the adherence rate is among women who are admitted to the hospital for breast cancer screenings, and what we found was that a lot of these women were nonadherent to the breast cancer screening. So we polled those women who were nonadherent to the breast cancer screening and asked, “What if we were able to offer you a mammogram while you were in the hospital for other issues?” About 76% said that they would like to have a mammogram while they were in the hospital.
Looking at that background, we polled this question to our hospitalists, too. What we found out was that a lot of the hospitalists were not willing to order a mammogram or were not too excited about getting a breast cancer screening done in the hospital setting. A majority told us that they’re more worried about how those results are going to be followed up, and it is possible that even if they order this mammogram that it may interfere with patient care or patient discharge. Then who would cover the cost of the mammogram if they do it in the inpatient setting?
So although a third of the hospitalists would still order a mammogram for those women who were high risk … a majority of them were not willing to because there were some perceived barriers to that.
Q: What is your reaction to the concerns with screening inpatients?
A: I can understand the concerns that most of the hospitalists have in regard to screening every patient that comes to the hospital. What I think we can do is, at the very least, we can be smart enough to figure out if a patient were at high risk for developing cancer and at least have those patients who were at high risk get screened.
Q: Where do you think hospitalists should go from here with regard to their patients who are overdue for breast cancer screenings?
A: We need to test for the feasibility and the financial issue of actually getting a screening mammogram in the hospital setting. I think down the road it should not matter what setting a patient [intersects] with the health system; it could be inpatient or outpatient. Patients should be provided the care and prevention needs that are recommended for their routine care. The next step should be doing a feasibility study, looking at whether or not these mammograms can be done in the hospital setting and do not interfere with the patient’s acute care. TH
Candace Mitchell is a freelance writer in New Jersey.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Spontaneous Osteonecrosis of Knee After Arthroscopy Is Not Necessarily Related to the Procedure
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
The use of aripiprazole in the management of bipolar disorder during pregnancy
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
More mental illness?
When you decided to go to medical school, did you expect that you would be seeing as many patients with mental health complaints as you are seeing now? If you have been practicing pediatrics for more than 15 years, has your patient mix significantly taken on a more behavioral flavor? Do you think that more of your patients are experiencing serious mental health issues?
If you answered yes to any or all of those questions, your perception of the mental health status of this country’s children agrees with mine and probably that of most other Americans. However, a recent study suggests that not all of our perceptions are reality based (N. Engl. J. Med. 2015;372:2029-38). The authors used a parent-scored scale of the children’s impairment and found that the rate of severe mental illness has fallen significantly over the last generation. Despite the decline in severe cases that they observed, the percentage of children receiving outpatient mental health services (including psychotherapy and psychotropic drugs) has increased. In other words, while we and other providers are indeed seeing more children and adolescents with mental health and behavioral complaints, the tip of the iceberg is shrinking.
Does that divergence make any sense? As the chief of the National Institute of Mental Health’s in-house genetic epidemiological research program observes, it is hard to make any sense of the results of this new study, or any study, because there is a plethora of agencies doing surveys often using different methodologies. In Kathleen Merikangas’ words, “It’s a nightmare” (“Severe Mental Illness Found to Drop in Young, Defying Perceptions” by Benedict Carey in the New York Times on May 20, 2015).
The situation seems to be a classic case of comparing apples and oranges. It is probably even worse because different agencies can’t even agree on whether McIntoshes and Granny Smiths should both be counted as apples. With this degree of uncertainty, the officials charged with making decisions about funding and allocating mental health services are flying blind much of the time.
When it comes to divining the trends in the prevalence of mental illness in children and adolescents, your guess is as good as mine. So ... because I happen to have the time, I’m going to give you mine.
From my lofty perch here on the rocky coast of Maine, it appears to me that the recent study in the New England Journal of Medicine is accurate in its observation that serious mental illness is not increasing and may in be decreasing. But why does it feel that our office schedules are bulging with the patients presenting with less serious behavioral problems? One answer is that many of the cases of serious physical illness that we once saw never make it to the waiting room. For example, most children with congenital heart disease are now diagnosed in utero and delivered and treated in tertiary centers. Serious infectious diseases such as meningitis and epiglottitis have been damped down by successful immunizations. The abundance of subspecialists, the tendency of some physicians to issue knee-jerk referrals, and the awareness by parents that they can self-refer has left a void in our schedules that in the blink of an eye has filled with the walking worried.
It is worry and anxiety that in my estimation is on the rise and generating a large percentage of visits. Whether this is a post 9-11 phenomenon or simply a reflection of too-much-news-too-quickly is unclear. But the bottom line is that parents are worried and as a result so are many of their children. I am less sure on whether there has been a true increase in depression. It may be that people are more willing to talk about their unhappiness or it may be a ripple effect from our national sleep deprivation.
Finally, there has been a tendency to narrow the definition of normal that goes hand in hand with the notion that if it isn’t “normal,” there must be some medication to fix the problem. Attention-deficit/hyperactivity disorder is the poster child for this schedule-filling duo.
So that’s what I think. I suspect you feel you are seeing more behavior-related problems. But is this because of a true increase in the level of mental health problems in this country? How do you explain it?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
When you decided to go to medical school, did you expect that you would be seeing as many patients with mental health complaints as you are seeing now? If you have been practicing pediatrics for more than 15 years, has your patient mix significantly taken on a more behavioral flavor? Do you think that more of your patients are experiencing serious mental health issues?
If you answered yes to any or all of those questions, your perception of the mental health status of this country’s children agrees with mine and probably that of most other Americans. However, a recent study suggests that not all of our perceptions are reality based (N. Engl. J. Med. 2015;372:2029-38). The authors used a parent-scored scale of the children’s impairment and found that the rate of severe mental illness has fallen significantly over the last generation. Despite the decline in severe cases that they observed, the percentage of children receiving outpatient mental health services (including psychotherapy and psychotropic drugs) has increased. In other words, while we and other providers are indeed seeing more children and adolescents with mental health and behavioral complaints, the tip of the iceberg is shrinking.
Does that divergence make any sense? As the chief of the National Institute of Mental Health’s in-house genetic epidemiological research program observes, it is hard to make any sense of the results of this new study, or any study, because there is a plethora of agencies doing surveys often using different methodologies. In Kathleen Merikangas’ words, “It’s a nightmare” (“Severe Mental Illness Found to Drop in Young, Defying Perceptions” by Benedict Carey in the New York Times on May 20, 2015).
The situation seems to be a classic case of comparing apples and oranges. It is probably even worse because different agencies can’t even agree on whether McIntoshes and Granny Smiths should both be counted as apples. With this degree of uncertainty, the officials charged with making decisions about funding and allocating mental health services are flying blind much of the time.
When it comes to divining the trends in the prevalence of mental illness in children and adolescents, your guess is as good as mine. So ... because I happen to have the time, I’m going to give you mine.
From my lofty perch here on the rocky coast of Maine, it appears to me that the recent study in the New England Journal of Medicine is accurate in its observation that serious mental illness is not increasing and may in be decreasing. But why does it feel that our office schedules are bulging with the patients presenting with less serious behavioral problems? One answer is that many of the cases of serious physical illness that we once saw never make it to the waiting room. For example, most children with congenital heart disease are now diagnosed in utero and delivered and treated in tertiary centers. Serious infectious diseases such as meningitis and epiglottitis have been damped down by successful immunizations. The abundance of subspecialists, the tendency of some physicians to issue knee-jerk referrals, and the awareness by parents that they can self-refer has left a void in our schedules that in the blink of an eye has filled with the walking worried.
It is worry and anxiety that in my estimation is on the rise and generating a large percentage of visits. Whether this is a post 9-11 phenomenon or simply a reflection of too-much-news-too-quickly is unclear. But the bottom line is that parents are worried and as a result so are many of their children. I am less sure on whether there has been a true increase in depression. It may be that people are more willing to talk about their unhappiness or it may be a ripple effect from our national sleep deprivation.
Finally, there has been a tendency to narrow the definition of normal that goes hand in hand with the notion that if it isn’t “normal,” there must be some medication to fix the problem. Attention-deficit/hyperactivity disorder is the poster child for this schedule-filling duo.
So that’s what I think. I suspect you feel you are seeing more behavior-related problems. But is this because of a true increase in the level of mental health problems in this country? How do you explain it?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
When you decided to go to medical school, did you expect that you would be seeing as many patients with mental health complaints as you are seeing now? If you have been practicing pediatrics for more than 15 years, has your patient mix significantly taken on a more behavioral flavor? Do you think that more of your patients are experiencing serious mental health issues?
If you answered yes to any or all of those questions, your perception of the mental health status of this country’s children agrees with mine and probably that of most other Americans. However, a recent study suggests that not all of our perceptions are reality based (N. Engl. J. Med. 2015;372:2029-38). The authors used a parent-scored scale of the children’s impairment and found that the rate of severe mental illness has fallen significantly over the last generation. Despite the decline in severe cases that they observed, the percentage of children receiving outpatient mental health services (including psychotherapy and psychotropic drugs) has increased. In other words, while we and other providers are indeed seeing more children and adolescents with mental health and behavioral complaints, the tip of the iceberg is shrinking.
Does that divergence make any sense? As the chief of the National Institute of Mental Health’s in-house genetic epidemiological research program observes, it is hard to make any sense of the results of this new study, or any study, because there is a plethora of agencies doing surveys often using different methodologies. In Kathleen Merikangas’ words, “It’s a nightmare” (“Severe Mental Illness Found to Drop in Young, Defying Perceptions” by Benedict Carey in the New York Times on May 20, 2015).
The situation seems to be a classic case of comparing apples and oranges. It is probably even worse because different agencies can’t even agree on whether McIntoshes and Granny Smiths should both be counted as apples. With this degree of uncertainty, the officials charged with making decisions about funding and allocating mental health services are flying blind much of the time.
When it comes to divining the trends in the prevalence of mental illness in children and adolescents, your guess is as good as mine. So ... because I happen to have the time, I’m going to give you mine.
From my lofty perch here on the rocky coast of Maine, it appears to me that the recent study in the New England Journal of Medicine is accurate in its observation that serious mental illness is not increasing and may in be decreasing. But why does it feel that our office schedules are bulging with the patients presenting with less serious behavioral problems? One answer is that many of the cases of serious physical illness that we once saw never make it to the waiting room. For example, most children with congenital heart disease are now diagnosed in utero and delivered and treated in tertiary centers. Serious infectious diseases such as meningitis and epiglottitis have been damped down by successful immunizations. The abundance of subspecialists, the tendency of some physicians to issue knee-jerk referrals, and the awareness by parents that they can self-refer has left a void in our schedules that in the blink of an eye has filled with the walking worried.
It is worry and anxiety that in my estimation is on the rise and generating a large percentage of visits. Whether this is a post 9-11 phenomenon or simply a reflection of too-much-news-too-quickly is unclear. But the bottom line is that parents are worried and as a result so are many of their children. I am less sure on whether there has been a true increase in depression. It may be that people are more willing to talk about their unhappiness or it may be a ripple effect from our national sleep deprivation.
Finally, there has been a tendency to narrow the definition of normal that goes hand in hand with the notion that if it isn’t “normal,” there must be some medication to fix the problem. Attention-deficit/hyperactivity disorder is the poster child for this schedule-filling duo.
So that’s what I think. I suspect you feel you are seeing more behavior-related problems. But is this because of a true increase in the level of mental health problems in this country? How do you explain it?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].