User login
FDA approves elranatamab for multiple myeloma
The B-cell maturation antigen (BCMA) CD3-targeted bispecific antibody (BsAb) was given Priority Review in February and had previously received Breakthrough Therapy Designation for relapsed or refractory multiple myeloma (RRMM), according to Pfizer.
FDA approval was based on favorable response and duration of response rates in the single-arm, phase 2 MagnetisMM-3 trial. The trial showed meaningful responses in heavily pretreated patients with RRMM who received elranatamab as their first BCMA-directed therapy.
The overall response rate in 97 BCMA-naive patients (cohort A) who previously received at least four lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, was 58%, with an estimated 82% maintaining the response for 9 months or longer. Median time to first response was 1.2 months.
In 63 patients who received at least four prior lines of therapy, which also included a BCMA-directed therapy, the overall response rate was 33% after median follow-up of 10.2 months. An estimated 84% maintained a response for at least 9 months.
Elranatamab was given subcutaneously at a dose of 76 mg weekly on a 28-day cycle with a step-up priming dose regimen. The priming regimen included 12 mg and 32 mg doses on days 1 and 4, respectively, during cycle 1. Patients who received at least six cycles and showed at least a partial response for 2 or more months had a biweekly dosing interval.
Elranatamab carries a boxed warning for cytokine release syndrome (CRS) and neurologic toxicity, as well as warnings and precautions for infections, neutropenia, hepatotoxicity, and embryo–fetal toxicity. Therefore, the agent is available only through a restricted Risk Evaluation and Mitigation Strategy (REMS).
The boxed warning is included in the full prescribing information.
A confirmatory trial to gather additional safety and efficacy data was launched in 2022. Continued FDA approval is contingent on confirmed safety and efficacy data.
A version of this article first appeared on Medscape.com.
The B-cell maturation antigen (BCMA) CD3-targeted bispecific antibody (BsAb) was given Priority Review in February and had previously received Breakthrough Therapy Designation for relapsed or refractory multiple myeloma (RRMM), according to Pfizer.
FDA approval was based on favorable response and duration of response rates in the single-arm, phase 2 MagnetisMM-3 trial. The trial showed meaningful responses in heavily pretreated patients with RRMM who received elranatamab as their first BCMA-directed therapy.
The overall response rate in 97 BCMA-naive patients (cohort A) who previously received at least four lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, was 58%, with an estimated 82% maintaining the response for 9 months or longer. Median time to first response was 1.2 months.
In 63 patients who received at least four prior lines of therapy, which also included a BCMA-directed therapy, the overall response rate was 33% after median follow-up of 10.2 months. An estimated 84% maintained a response for at least 9 months.
Elranatamab was given subcutaneously at a dose of 76 mg weekly on a 28-day cycle with a step-up priming dose regimen. The priming regimen included 12 mg and 32 mg doses on days 1 and 4, respectively, during cycle 1. Patients who received at least six cycles and showed at least a partial response for 2 or more months had a biweekly dosing interval.
Elranatamab carries a boxed warning for cytokine release syndrome (CRS) and neurologic toxicity, as well as warnings and precautions for infections, neutropenia, hepatotoxicity, and embryo–fetal toxicity. Therefore, the agent is available only through a restricted Risk Evaluation and Mitigation Strategy (REMS).
The boxed warning is included in the full prescribing information.
A confirmatory trial to gather additional safety and efficacy data was launched in 2022. Continued FDA approval is contingent on confirmed safety and efficacy data.
A version of this article first appeared on Medscape.com.
The B-cell maturation antigen (BCMA) CD3-targeted bispecific antibody (BsAb) was given Priority Review in February and had previously received Breakthrough Therapy Designation for relapsed or refractory multiple myeloma (RRMM), according to Pfizer.
FDA approval was based on favorable response and duration of response rates in the single-arm, phase 2 MagnetisMM-3 trial. The trial showed meaningful responses in heavily pretreated patients with RRMM who received elranatamab as their first BCMA-directed therapy.
The overall response rate in 97 BCMA-naive patients (cohort A) who previously received at least four lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, was 58%, with an estimated 82% maintaining the response for 9 months or longer. Median time to first response was 1.2 months.
In 63 patients who received at least four prior lines of therapy, which also included a BCMA-directed therapy, the overall response rate was 33% after median follow-up of 10.2 months. An estimated 84% maintained a response for at least 9 months.
Elranatamab was given subcutaneously at a dose of 76 mg weekly on a 28-day cycle with a step-up priming dose regimen. The priming regimen included 12 mg and 32 mg doses on days 1 and 4, respectively, during cycle 1. Patients who received at least six cycles and showed at least a partial response for 2 or more months had a biweekly dosing interval.
Elranatamab carries a boxed warning for cytokine release syndrome (CRS) and neurologic toxicity, as well as warnings and precautions for infections, neutropenia, hepatotoxicity, and embryo–fetal toxicity. Therefore, the agent is available only through a restricted Risk Evaluation and Mitigation Strategy (REMS).
The boxed warning is included in the full prescribing information.
A confirmatory trial to gather additional safety and efficacy data was launched in 2022. Continued FDA approval is contingent on confirmed safety and efficacy data.
A version of this article first appeared on Medscape.com.
Advancements help guide achalasia management, experts say
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
16% of the U.S. population experience dysphagia, only half of whom seek medical care and the others manage their symptoms by modifying diet.
X-ray barium swallow and endoscopy with biopsy to exclude eosinophilic esophagitis are the initial tests for dysphagia diagnosis. If the above are normal, a high-resolution esophageal manometry impedance (HRMZ) is recommended to diagnose primary and secondary esophageal motility disorder.
However, only in a minority of patients is it likely to cause dysphagia because uncontrolled studies show that therapeutic strategies to address EGJOO (botox, dilation, and myotomy) relieve dysphagia symptoms in a minority of patients. Hence, in significant number of patients the cause of dysphagia symptoms remains obscure. It might be that our testing is inadequate, or possibly, patients have functional dysphagia (sensory dysfunction of the esophagus). My opinion is that it is the former.
The esophagus has only one simple function, that is, to transfer the pharyngeal pump driven, that is, swallowed contents to the stomach, for which its luminal cross-sectional area must be larger than that of the swallowed bolus and contraction (measured by manometry) behind the bolus must be of adequate strength. The latter is likely less relevant because humans eat in the upright position and gravity provides propulsion for the bolus. Stated simply, as long as esophagus can distend well and there is no resistance to the outflow at the EGJ, esophagus can achieve its goal. However, until recently, there was no single test to determine the distension and contraction, the two essential elements of primary esophageal peristalsis.
Endoscopy and x-ray barium swallow are tests to determine the luminal diameter but have limitations. Endoflip measures the opening function of the EGJ and is useful when the HRM is normal. However, pressures that are currently being used to measure the EGJ distensibility by Endoflip are not physiological. Furthermore, esophageal body motor function assessed by a bag that distends a long segment of the esophagus under high pressure is unphysiological. The distension-contraction plots, which determines the luminal CSA and contraction simultaneously during primary peristalsis is ideally suited to study the pathophysiology of esophageal motility disorders. Several studies from my laboratory show that in patients with nutcracker esophagus, EGJOO and normal HRM, the esophagus distends significantly less than that of normal subjects during primary peristalsis. I suspect that an esophageal contraction pushing bolus through a narrow lumen esophagus is the cause of dysphagia sensation in many patients that have been labeled as functional dysphagia.
The last 2 decades have seen significant progress in the diagnosis of esophageal motility disorders using HRM, Endoflip, and distension-contraction plots of peristalsis. Furthermore, endoscopic treatment of achalasia and “achalasia-like syndromes” is revolutionary. What is desperately needed is an understanding of the pathogenesis of esophageal motor disorders, pharmacotherapy of esophageal symptoms, such as chest pain, proton pump inhibitor–resistant heartburn, and others because dysfunctional esophagus is a huge burden on health care expenditures worldwide.
Ravinder K. Mittal, MD, is a professor of medicine and gastroenterologist with UC San Diego Health. He has patent application pending on the computer software Dplots.
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
at a pace that has left the line-tracing technology considered to have debatable merit just 15 years ago “now as obsolete as a typewriter,” experts said recently in a review in Gastro Hep Advances.
“We have come to conceptualize esophageal motility disorders by specific aspects of physiological dysfunction,” wrote a trio of experts – Peter Kahrilas, MD, professor of medicine; Dustin Carlson, MD, MS, assistant professor of medicine, and John Pandolfino, MD, chief of gastroenterology and hepatology, all at Northwestern University, Chicago. “A major implication of this approach is a shift in management strategy toward rendering treatment in a phenotype-specific manner.”
High-resolution manometry (HRM) was trail-blazing, they said, as it replaced line-tracing manometry in evaluating the motility of the esophagus. HRM led to the subtyping of achalasia based on the three patterns of pressurization in the esophagus that are associated with obstruction at the esophagogastric junction. But the field has continued to advance.
“It has since become clear that obstructive physiology also occurs in syndromes besides achalasia involving the esophagogastric junction and/or distal esophagus,” Dr. Kahrilas, Dr. Carlson, and Dr. Pandolfino said. “In fact, obstructive physiology is increasingly recognized as the fundamental abnormality leading to the perception of dysphagia with esophageal motility disorders. This concept of obstructive physiology as the fundamental abnormality has substantially morphed the clinical management of esophageal motility disorders.”
HRM, has many limitations, but in cases of an uncertain achalasia diagnosis, functional luminal imaging probe (FLIP) technology can help, they said. FLIP can also help surgeons tailor myotomy procedures.
In FLIP, a probe is carefully filled with fluid, causing distension of the esophagus. In the test, the distensibility of the esophagogastric junction is measured. The procedure allows a more refined assessment of the movement of the esophagus, and the subtypes of achalasia.
Identifying the achalasia subtype is crucial to choosing the right treatment, data suggests. There have been no randomized controlled trials on achalasia management that prospectively consider achalasia subtype, but retrospective analysis of RCT data “suggests that achalasia subtypes are of great relevance in forecasting treatment effectiveness,” they said.
In one trial, pneumatic dilation was effective in 100% of type II achalasia, which involves panesophageal pressurization, significantly better than laparoscopic Heller myotomy (LHM). But it was much less effective than LHM in type III achalasia, the spastic form, although a significance couldn’t be established because of the number of cases. Data from a meta-analysis showed that peroral endoscopic myotomy, which allows for a longer myotomy if needed, was better than LHM for classic achalasia and spastic achalasia and was most efficacious overall.
The writers said that the diagnostic classifications for achalasia are likely to continue to evolve, pointing to the dynamic nature of the Chicago Classification for the disorder.
“The fact that it has now gone through four iterations since 2008 emphasizes that this is a work in progress and that no classification scheme of esophageal motility disorders based on a single test will ever be perfect,” they said. “After all, there are no biomarkers of esophageal motility disorders and, in the absence of a biomarker, there can be no ‘gold standard’ for diagnosis.”
Dr. Pandolfino, Dr. Kahrilas, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP Panometry systems, methods, and apparatus with Medtronic. Dr. Kahrilas reported consulting with Ironwood, Reckitt, and Phathom. Dr. Carlson reported conflicts of interest with Medtronic and Phathom Pharmaceuticals. Dr. Pandolfino reported conflicts of interest with Sandhill Scientific/Diversatek, Takeda, AstraZeneca, Medtronic, Torax, and Ironwood.
FROM GASTRO HEP ADVANCES
News & Perspectives from Ob.Gyn. News
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
NEWS FROM THE FDA/CDC
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
https://www.mdedge.com/obgyn/news-fda/cdc
FEATURE
U.S. mammogram update sparks concern, reignites debates
A recent update to the U.S. recommendations for breast cancer screening is raising concerns about the costs associated with potential follow-up tests, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screen-ing mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
https://www.mdedge.com/obgyn/article/264198/breast-cancer/us-mammogram-update-sparks-concern-reignites-debates
Continue to: GENDER-AFFIRMING GYNECOLOGY...
GENDER-AFFIRMING GYNECOLOGY
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologistsand midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately, compared with the surgical, hormonal, and mental health aspects of gender-affirming care, fertility and pregnancy in transgender men is still a relatively new and under-researched topic.
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
REPRODUCTIVE ROUNDS
Affordable IVF—Are we there yet?
The price for an in vitro fertilization (IVF) cycle continues to increase annually by many clinics, particularly because of “add-ons” of dubious value.
The initial application of IVF was for tubal factor infertility. Over the decades since 1981, the year of the first successful live birth in the United States, indications for IVF have dramatically expanded—ovulation dysfunction, unexplained infertility, male factor, advanced stage endometriosis, unexplained infertility, embryo testing to avoid an inherited genetic disease from the intended parents carrying the same mutation, and family balancing for gender, along with fertility preservation, including before potentially gonadotoxic treatment and “elective” planned oocyte cryopreservation.
The cost of IVF remains a significant, and possibly leading, stumbling block for women, couples, and men who lack insurance coverage. From RESOLVE.org, the National Infertility Association: “As of June 2022, 20 states have passed fertility insurance coverage laws, 14 of those laws include IVF coverage, and 12 states have fertility preservation laws for iatrogenic (medically induced) infertility.” Consequently, “affordable IVF” is paramount to providing equal access for patients.
https://www.mdedge.com/obgyn/reproductive-rounds
CONFERENCE COVERAGE
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
The percentage of time spent in the pregnancy-specific target blood glucose range of 63-140 mg/dL (3.5-7.8 mmol/L) from 16 weeks’ gestation to delivery was significantly higher in women in the AID group.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
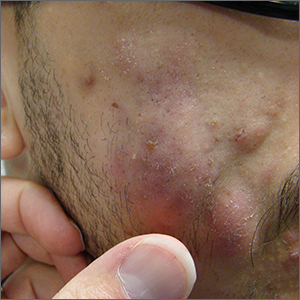
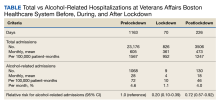
Fluctuant facial lesions
This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient had more than cystic acne; he had acne conglobata. AC is a severe form of inflammatory acne leading to coalescing lesions with purulent sinus tracts under the skin. It can be seen as part of the follicular tetrad syndrome of cystic acne, hidradenitis suppurativa, dissecting cellulitis, and pilonidal disease. AC is thought to be an elevated tumor necrosis factor (TNF)-alpha response to Propionibacterium acnes (now known as Cutibacterium acnes) that leads to excessive inflammation and sterile abscesses.1 Acne fulminans (AF) can also manifest as a purulent form of acne, but AF has associated systemic signs and symptoms that include fevers, chills, and malaise.
Due to the depth of the inflammation, AC is treated with systemic medications, most commonly isotretinoin. Isotretinoin can be started at 0.5 mg/kg (divided twice daily to enhance tolerability) and then increased to 1 mg/kg (divided twice daily) for 5 months. There is some variation in dosing regimens in practice; the target goal is 120 to 150 mg/kg over the course of treatment. In AF, the patient is pretreated with systemic steroids, and in AC, some clinicians will even prescribe systemic steroids (prednisone 0.5 mg/kg daily for the first month) along with isotretinoin.
Second-line medications include dapsone (50-150 mg/d).2 Case reports describe the successful use of the TNF-alpha antagonist adalimumab, although this is not a usual practice in AC treatment.1 Note that all of these medications have the potential for severe adverse effects and require laboratory evaluation prior to initiation.
This patient was counseled, prescribed isotretinoin (dose as above), and enrolled in the IPledge prescribing and monitoring system for isotretinoin. At 20 weeks of use, the purulent drainage ceased. The pus-filled sinus tracts and redness had resolved, although he still had thickened tissue and scarring where the tracts had been. In time, the scars will usually get flatter and softer.
If the patient’s AC were to flare, another 20-week course of isotretinoin could be prescribed after a 2-month hiatus or he could be switched to a second-line medication. Referral for any cosmetic therapy is typically delayed for another 6 months in case there is a need to treat a recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
1. Yiu ZZ, Madan V, Griffiths CE. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. 2015;40:383-386. doi: 10.1111/ced.12540
2. Hafsi W, Arnold DL, Kassardjian M. Acne Conglobata. StatPearls Publishing; 2023.
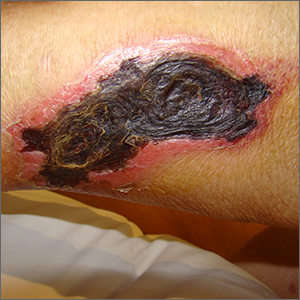
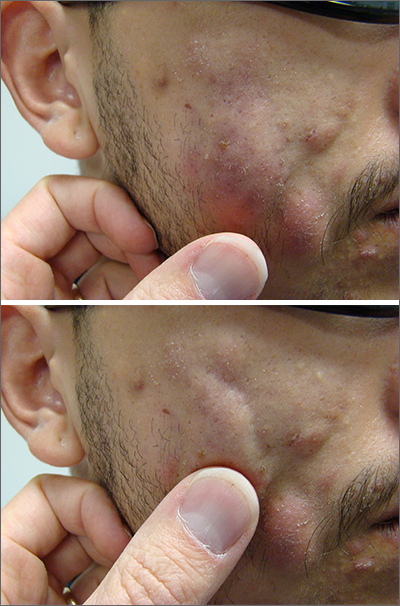
Leathery plaque on thigh
The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The necrotic eschar on this patient’s thigh is calciphylaxis, also known as calcific uremic arteriolopathy (CUA). Most cases are seen in ESRD and start as painful erythematous, firm lesions that progress to necrotic eschars. Up to 4% of patients with ESRD who are on dialysis develop CUA.1
The exact pathology of CUA is unknown. Calcification of the arterioles leads to ischemia and necrosis of tissue, which is not limited to the skin and can affect tissue elsewhere (eg, muscles, central nervous system, internal organs).2
Morbidity and mortality of CUA is often due to bacterial infections and sepsis related to the necrotic tissue. CUA can be treated with sodium thiosulfate (25 g in 100 mL of normal saline) infused intravenously during the last 30 minutes of dialysis treatment 3 times per week.3 Sodium thiosulfate (which acts as a calcium binder) and cinacalcet (a calcimimetic that leads to lower parathyroid hormone levels) have been used, but evidence of efficacy is limited. In a multicenter observational study involving 89 patients with chronic kidney disease and CUA, 17% of patients experienced complete wound healing, while 56% died over a median follow-up period of 5.8 months.1 (No cause of death data were available; sodium thiosulfate and a calcimimetic were the most widely used treatment strategies.) This extrapolated to a mortality rate of 72 patients per 100 individuals over the course of 1 year (the 100 patient-years rate).1
This patient continued her dialysis regimen and general care. She was seen by the wound care team and treated with topical wound care, including moist dressings for her open lesions. The eschars were not debrided because they showed no sign of active infection. Unfortunately, she was in extremely frail condition and died 1 month after evaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
1. Chinnadurai R, Huckle A, Hegarty J, et al. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34:1537-1545. doi: 10.1007/s40620-020-00908-9
2. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
3. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi: 10.1053/j.ajkd.2015.01.034
Gastrointestinal Bleeding Caused by Large Intestine Amyloidosis
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
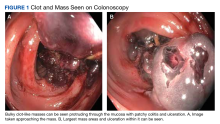
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
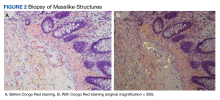
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
Implementing Smoking Cessation Telehealth Technologies Within the VHA: Lessons Learned
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
Health care systems need practical, scalable methods to reach patients and connect them to available, evidence-based resources. Ideally, these systems need to be resource nonintensive to deploy, maintain, and use. They should also be low cost, have a relative advantage to the organization, be sensitive to patient needs, use available resources, and have rigorous evidence regarding their effect on patient-centered outcomes.1,2 Phone service is one way to reach people that remains viable. More than 97% of Americans own a cellphone of some kind, and 40% still have a landline.3,4 One intervention that has been increasingly used in routine care settings is an interactive voice response (IVR) system that uses phones for connecting to patients.
IVR systems are a type of telehealth that provides information or adjunct health services through use of a telecommunication platform and information technologies.5 These systems are automated telephone systems that use prerecorded or text-to-speech–generated messages that allow respondents to provide and access information without a live agent.6 Text messaging (SMS) is another modality that can be used to asynchronously engage with participants. IVR systems have been used successfully for many health conditions and services, such as improving veterans’ adherence to continuous positive airway pressure, colorectal cancer screening, and cognitive behavioral therapy.7-10 By building on existing technology and infrastructure, IVR systems can be a cost-effective option for health care system services.
A 2016 Cochrane review of IVR systems for smoking cessation identified 7 studies.11 Although none used opt-out mechanisms (where individuals are automatically enrolled in programs until they decide not to participate) to engage people without an expressed motivation to quit, these interventions seemed safe and were promisingly effective. Among patients enrolled in primary care, a trial of an IVR system led to a higher quit rate: 18% vs 8%.12
In one study, patients in the emergency department, particularly older ones, preferred phone-based interventions over SMS.13 IVR-based proactive tobacco cessation systems are cost-effective and have been successfully used in the US Department of Veterans Affairs (VA).14,15 IVR systems using opt-out approaches are being studied, though their effectiveness in this setting has not been proven. The pros and cons of different interventions need to be explored since there is likely a tradeoff between feasibility and effectiveness. For example, intensive smoking cessation interventions are more effective but often require more resources to implement and sustain.16 Basing interventions that are not resource intensive within a reputable organizational system may amplify the effectiveness.17
This endeavor to establish an IVR system was initiated as part of our research study, a randomized trial of the Teachable Moment to Opt-Out of Tobacco (TeaM OUT) intervention at the VA Portland Health Care System in Oregon. We measured the reach and effectiveness of a novel, proactive, resource nonintensive, and pragmatic intervention to engage veterans with a recently diagnosed lung nodule who smoke cigarettes.18 Our research team extracted the contact information for patients currently smoking and found to a have a pulmonary nodule from the VA Corporate Data Warehouse.19 We then manually uploaded those data to an IVR website where the system contacted patients to connect them to smoking cessation resources on an opt-out basis. In the research study, we measured the acceptability and effectiveness of the TeaM OUT intervention using quantitative and qualitative methods.
We developed and implemented an IVR system for use at 4 facilities: VA Portland Health Care System, Minneapolis VA Health Care System, Ralph H. Johnson VA Medical Center (Charleston, NC), and the Baltimore VA Medical Center. Setting up any type of wide-scale technology within the VA can be challenging. Due to our experience in developing and implementing the IVR system in the VA, we share what we have learned about the process of finding, contracting, developing, and implementing an IVR system. We share our experiences with developing and implementing this system to provide guidance for those who may want to establish an IVR system (or similar technologies) within the VA.
Lessons Learned
During our development and implementation process, we learned several lessons about setting up an IVR system in the VA. It is important to note that VA facilities may have differing processes, and policies frequently change; thus coordination with departments (eg, contracting, finance, Office of Information and Technology [OIT], etc) to verify the following strategies is essential (Figure).
Vendor Selection
Check with the local OIT and contracting offices to see if the facility has previously used any vendors for these services and for advice on selection. We compiled a list of questions that may be helpful based on our discussions with 4 vendors, prior to selection of a vendor already VA-approved (Appendix). There are also questions to think about in parallel with choosing a vendor. Contact your OIT, contracting, and privacy (if necessary) offices before choosing a vendor.
Online Security
After selecting a vendor, if you want an online portal to view, upload, or downloaddata, then you will need to initiate the single sign-on internal (SSOI) process (www.data.va.gov/dataset/Single-Sign-On-Internal-SSOi-/cber-kxf9). Other benefits of a website are to identify call patterns (eg, no one picks up after the 10th call) and track respondents’ selections. The SSOI process can take up to 1 year. Notably, the website login at minimum needs to be created by the IVR vendor to start the process. After the SSOI is approved you can add more to the website beyond just the login capability. Note that the script needs to be finalized prior to SSOI initiation. You will need to initiate with the SSOI team, then the vendor will need to complete the process.
Contracting
Concurrent with the above steps, contact the contracting office to get a sense of the paperwork and timeline. Make sure you are comfortable with the vendor’s responses to the questions in the Appendix, and view their written proposal or scope of work (SOW) to ensure they can do what the project protocol demands.
If the vendor has previously worked with the VA, contact your local contract office (usually part of the Finance Office) for updated forms. We needed the 6500.6 Checklist, Document Checklist for Service Requests, Single Source Justification, Research & Development Order (if research-related), and Vendor File Request forms. The vendor can help complete these forms. Review the proposal/SOW and budget first, knowing that budgets have a wide range and depend on the length and complexity of the script, number of calls, number of respondents, etc. For example, our quote was $110,000 over 4 years, including development, training, hosting on a secure server, and maintenance. Our IVR system will call about 5000 patients across 4 sites. Each patient will receive up to 15 calls over 2 weeks if they do not answer. We created 2 IVR lines (1 inbound and 1 outbound). Next, contact the lead of the local OIT and contracting departments by email to justify sharing veteran information with a contracted entity via approved methods. Finally, contact the privacy officer and information security officer. Discuss where software would be installed, whether cloud storage would be used, and what information can be shared/stored. Remember that the rules may differ for research vs nonresearch projects. Also, determine whether a data-use agreement between the VA and the vendor is needed and how the institutional review board (if research) gets integrated.
If using an outside vendor who has never worked with the VA, submit form 6550.6. Note that contracting requires several months. First, contact OIT and contracting departments. Again, you will need to justify sharing veteran information with a contracted entity. Next, complete the Project Special Forces Software and Privacy Threshold Analysis process to purchase the system. Set up a meeting with OIT to determine other forms and next steps. Business need/case use form and data security categorization may be needed. If the software needs to be installed on a VA computer, you will need to submit a Technical Reference Model request if it does not have an entry.
Vendors can answer technical questions from the contracting office, especially about the SOW, but the VA team needs to write the contract and manage all documentation and communication. You will also need sole source documentation (receive from contracting office) with justification for why you want to use a specific vendor. If you do not have that justification, in cooperation with the contracts office, you must solicit bids from other companies. Importantly, understand the staff support needed for contracting and build into your timeline and budget. Not surprisingly, we found that in-person or phone meetings were invaluable compared with email correspondence. Meet with all parties involved early and often. Once the contract is clear, this begins the build process where the vendor can program and record the script. This process usually takes 1 to 2 months.
Patient Engagement, Tracking, and Long-term Support
The new Patient Engagement, Tracking, and Long-term Support (PETALS) initiative is an excellent place to start with any VA IVR-related questions. PETALS is used for research.20 We hoped to use this system for our study, but its implementation was delayed until 2022. The PETALS system is designed for VA investigators who conduct research studies and need a secure platform that is compliant with VA policies for deploying SMS and IVR systems for research.20 At this time, PETALS is for use only with veterans, so if research will occur outside the VA, you must use an outside vendor. Users who want to set up a new IVR system can ask their local contracting office whether any contracts have already been established for IVR development and support.
From our perspective as researchers who are not telehealth savvy, we encountered several delays from failing to ask the appropriate questions or inability to navigate complicated systems. For instance, there were several tasks that needed to be completed and were not included in the original timeline developed by the vendor and researcher. Therefore, it is important to have clear communication on both sides about who is doing what, when, and how. We tried to detail these unexpected steps to help researchers, administrators, or other VA employees in the future.
Conclusions
IVR systems, once they are developed and implemented, can be efficient, low-cost, resource-nonintensive solutions in a health care setting that can effectively connect patients with needed health care services. Our experience developing an IVR system within the VA was challenging and was a huge learning curve for our research team. We hope that our experience and lessons will help VA personnel in the future.
Acknowledgments
Thank you to everyone involved in this project and who answered questions about the process, especially Nicolle Marinec, MPH; Toan Tran, and Molly Delorit, BA. This study and Christopher Slatore, MD, are supported by an award from the US Department of Veterans Affairs (HSR&D IIR 19-425). It was also supported by resources from the Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon (VAPORHCS).
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp
1. Lewis CC, Mettert K, Lyon AR. Determining the influence of intervention characteristics on implementation success requires reliable and valid measures: results from a systematic review. Implement Res Pract. 2021;2:2633489521994197. doi:10.1177/2633489521994197
2. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. Published 2013 May 10. doi:10.1186/1748-5908-8-51
3. Pew Research Center. Mobile Fact Sheet. April 7, 2021. Accessed June 6, 2023. https://www.pewresearch.org/internet/fact-sheet/mobile/
4. Lieser EK. Study: Only 40 Percent of U.S. Households Have a Landline. The National Interest. March 20, 2020. Accessed June 6, 2023. https://nationalinterest.org/blog/buzz/study-only-40-percent-us-households-have-landline-135212
5. Lee H, Friedman ME, Cukor P, David Ahern. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51(6):277-283. doi:10.1016/S0029-6554(03)00161-1
6. IBM Cloud Education. What is interactive voice response (IVR)? IBM. March 15, 2021. Accessed June 6, 2023. https://www.ibm.com/cloud/learn/interactive-voice-response
7. Sparrow D, Aloia M, Demolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65(12):1061-1066. doi:10.1136/thx.2009.133215
8. Cohen-Cline H, Wernli KJ, Bradford SC, Boles-Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Med Care. 2014;52(6):496-499. doi:10.1097/MLR.0000000000000116
9. Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge Monitoring Using Interactive Voice Response System Reduces 30-Day Readmission Rates in a Case-managed Medicare Population. Med Care. 2012;50(1):50-57. doi:10.1097/MLR.0b013e318229433e
10. Piette JD, Newman S, Krein SL, et al. Patient-centered pain care using artificial intelligence and mobile health tools: a randomized comparative effectiveness trial. JAMA Intern Med. 2022;182(9):975-83. doi:10.1001/jamainternmed.2022.3178
11. Posadzki P, Mastellos N, Ryan R, et al. Automated telephone communication systems for preventive healthcare and management of long-term conditions. Cochrane Database Syst Rev. 2016;12(12):CD009921. Published 2016 Dec 14. doi:10.1002/14651858.CD009921.pub2
12. Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: A randomized clinical trial. JAMA Intern Med. 2015;175(2):218-226. doi:10.1001/jamainternmed.2014.6674
13. Fingrut W, Stewart L, Cheung KW. Choice of smoking cessation counselling via phone, text, or email in emergency department patients. Prev Med Rep. 2016;4:597-600. doi:10.1016/j.pmedr.2016.10.010
14. Levy DE, Klinger EV, Linder JA, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. 2017;19(12):1508-1515. doi:10.1093/ntr/ntw243
15. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord. 2016;17:85. doi:10.1186/s12891-016-0924-z
16. Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14-24. doi:10.1016/j.drugalcdep.2015.06.004
17. Bennett-Levy J, Richards D, Farrand P, et al. Oxford Guide to Low Intensity CBT Interventions. 1st ed. Oxford University Press; 2010.
18. Unger S, Golden SE, Melzer AC, et al. Study design for a proactive teachable moment tobacco treatment intervention among patients with pulmonary nodules. Contemp Clin Trials. 2022;121:106908. doi:10.1016/j.cct.2022.106908
19. US Department of Veterans Affairs. VA Information Resource Center [Internet]. VIReC Research User Guides. 2016. https://www.virec.research.va.gov/Resources/RUGs.asp
20. PETALS. US Department of Veteran Affairs. Updated June 14, 2021. Accessed June 6, 2023. https://www.annarbor.hsrd.research.va.gov/ANNARBORHSRDRESEARCH/PETALS.asp
Spider Bite Wound Care and Review of Traditional and Advanced Treatment Options
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
Alcohol-Related Hospitalizations During the Initial COVID-19 Lockdown in Massachusetts: An Interrupted Time-Series Analysis
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
The United States’ initial public health response to the COVID-19 pandemic included containment measures that varied by state but generally required closing or suspending schools, nonessential businesses, and travel (commonly called lockdown).1 During these periods, hospitalizations for serious and common conditions declined.2,3 In Massachusetts, a state of emergency was declared on March 10, 2020, which remained in place until May 18, 2020, when a phased reopening of businesses began.
Although the evidence on the mental health impact of containment periods has been mixed, it has been suggested that these measures could lead to increases in alcohol-related hospitalizations.4 Social isolation and increased psychosocial and financial stressors raise the risk of relapse among patients with substance use disorders.5-7 Marketing and survey data from the US and United Kingdom from the early months of the pandemic suggest that in-home alcohol consumption and sales of alcoholic beverages increased, while consumption of alcohol outside the home decreased.8-10 Other research has shown an increase in the percentage—but not necessarily the absolute number—of emergency department (ED) visits and hospitalizations for alcohol-related diagnoses during periods of containment.11,12 At least 1 study suggests that alcohol-related deaths increased beginning in the lockdown period and persisting into mid-2021.13
Because earlier studies suggest that lockdown periods are associated with increased alcohol consumption and relapse of alcohol use disorder, we hypothesized that the spring 2020 lockdown period in Massachusetts would be associated temporally with an increase in alcohol-related hospitalizations. To evaluate this hypothesis, we examined all hospitalizations in the US Department of Veterans Affairs (VA) Boston Healthcare System (VABHS) before, during, and after this lockdown period. VABHS includes a 160-bed acute care hospital and a 50-bed inpatient psychiatric facility.
Methods
We conducted an interrupted time-series analysis including all inpatient hospitalizations at VABHS from January 1, 2017, to December 31, 2020, to compare the daily number of alcohol-related hospitalizations across 3 exposure groups: prelockdown (the reference group, 1/1/2017-3/9/2020); lockdown (3/10/2020-5/18/2020); and postlockdown (5/19/2020-12/31/2020).
The VA Corporate Data Warehouse at VABHS was queried to identify all hospitalizations on the medical, psychiatry, and neurology services during the study period. Hospitalizations were considered alcohol-related if the International Statistical Classification of Diseases, Tenth Revision (ICD-10) primary diagnosis code (the main reason for hospitalization) was defined as an alcohol-related diagnosis by the VA Centralized Interactive Phenomics Resource (eAppendix 1, available online at doi:10.1278/fp.0404). This database, which has been previously used for COVID-19 research, is a catalog and knowledge-sharing platform of VA electronic health record–based phenotype algorithms, definitions, and metadata that builds on the Million Veteran Program and Cooperative Studies Program.14,15 Hospitalizations under observation status were excluded.
To examine whether alcohol-related hospitalizations could have been categorized as COVID-19 when the conditions were co-occurring, we identified 244 hospitalizations coded with a primary ICD-10 code for COVID-19 during the lockdown and postlockdown periods. At the time of admission, each hospitalization carries an initial (free text) diagnosis, of which 3 had an initial diagnosis related to alcohol use. The population at risk for alcohol-related hospitalizations was estimated as the number of patients actively engaged in care at the VABHS. This was defined as the number of patients enrolled in VA care who have previously received any VA care; patients who are enrolled but have never received VA care were excluded from the population-at-risk denominator. Population-at-risk data were available for each fiscal year (FY) of the study period (9/30-10/1); the following population-at-risk sizes were used: 38,057 for FY 2017, 38,527 for FY 2018, 39,472 for FY 2019, and 37,893 for FY 2020.
The primary outcome was the daily number of alcohol-related hospitalizations in the prelockdown, lockdown, and postlockdown periods. A sensitivity analysis was performed using an alternate definition of the primary outcome using a broader set of alcohol-related ICD-10 codes (eAppendix 2, available online at doi:10.1278/fp.0404).
Statistical Analysis
To visually examine hospitalization trends during the study period, we generated a smoothed time-series plot of the 7-day moving average of the daily number of all-cause hospitalizations and the daily number of alcohol-related hospitalizations from January 1, 2017, to December 31, 2020. We used multivariable regression to model the daily number of alcohol-related hospitalizations over prelockdown (the reference group), lockdown, and postlockdown. In addition to the exposure, we included the following covariates in our model: day of the week, calendar date (to account for secular trends), and harmonic polynomials of the day of the year (to account for seasonal variation).16
We also examined models that included the daily total number of hospitalizations to account for the reduced likelihood of hospital admission for any reason during the pandemic. We used generalized linear models with a Poisson link to generate rate ratios and corresponding 95% CIs for estimates of the daily number of alcohol-related hospitalizations. We estimated the population incidence of alcohol-related hospitalizations per 100,000 patient-months for the exposure periods using the population denominators previously described. All analyses were performed in Stata 16.1.
Results
During the study period, 27,508 hospitalizations were available for analysis. The 7-day moving average of total daily hospitalizations and total daily alcohol-related hospitalizations over time for the period January 1, 2017, to December 31, 2020, are shown in the Figure.
The incidence of alcohol-related hospitalizations in the population dropped from 72 per 100,000 patient-months to 10 per 100,000 patient-months during the lockdown period and increased to 46 per 100,000 patient-months during the postlockdown period (Table).
Our results were not substantially different when we ran a sensitivity analysis that excluded the total daily number of admissions from our model. Compared with the prelockdown period, the rate ratio for the number of alcohol-related hospitalizations during the lockdown period was 0.16 (95% CI, 0.08-0.30), and the rate ratio for the postlockdown period was 0.65 (95% CI, 0.52-0.82). We conducted an additional sensitivity analysis using a broader definition of the primary outcome to include all alcohol-related diagnosis codes; however, the results were unchanged.
Discussion
During the spring 2020 COVID-19 lockdown period in Massachusetts, the daily number of VABHS alcohol-related hospitalizations decreased by nearly 80% compared with the prelockdown period. During the postlockdown period, the daily number of alcohol-related hospitalizations increased but only to 72% of the prelockdown baseline by the end of December 2020. A similar trend was observed for all-cause hospitalizations for the same exposure periods.
These results differ from 2 related studies on the effect of the COVID-19 pandemic on alcohol-related hospitalizations.10,11 In a retrospective study of ED visits to 4 hospitals in New York City, Schimmel and colleagues reported that from March 1 to 31, 2020 (the initial COVID-19 peak), hospital visits for alcohol withdrawal increased while those for alcohol use decreased.10 However, these results are reported as a percentage of total ED visits rather than the total number of visits, which are vulnerable to spurious correlation because of concomitant changes in the total number of ED visits. In their study, the absolute number of alcohol-related ED visits did not increase during the initial 2020 COVID-19 peak, and the number of visits for alcohol withdrawal syndrome declined slightly (195 in 2019 and 180 in 2020). However, the percentage of visits increased from 7% to 10% because of a greater decline in total ED visits. This pattern of decline in the number of alcohol-related ED visits, accompanied by an increase in the percentage of alcohol-related ED visits, has been observed in at least 1 nationwide surveillance study.17 This apparent increase does not reflect an absolute increase in ED visits for alcohol withdrawal syndrome and represents a greater relative decline in visits for other causes during the study period.
Sharma and colleagues reported an increase in the percentage of patients who developed alcohol withdrawal syndrome while hospitalized in Delaware per 1000 hospitalizations during consecutive 2-week periods during the pandemic in 2020 compared with corresponding weeks in 2019.11 The greatest increase occurred during the last 2 weeks of the Delaware stay-at-home order. The Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA-Ar) score of > 8 was used to define alcohol withdrawal syndrome. The American Society of Addiction Medicine does not recommend using CIWA-Ar to diagnose alcohol withdrawal syndrome because the scale was developed to monitor response to treatment, not to establish a diagnosis.18
Although the true population incidence of alcohol-related hospitalizations is difficult to estimate because the size of the population at risk (ie, the denominator) often is not known, the total number of hospitalizations is not a reliable surrogate.19 Individuals hospitalized for nonalcohol causes are no longer at risk for alcohol-related hospitalization.
In our study, we assume the population at risk during the study period is constant and model changes in the absolute number—rather than percentage—of alcohol-related ED visits. These absolute estimates of alcohol-related hospitalizations better reflect the true burden on the health care system and avoid the confounding effect of declining total ED visits and hospitalizations that could lead to artificially increased percentages and spurious correlation.20 The absolute percentage of alcohol-related hospitalizations also decreased during this period; therefore, our results are not sensitive to this approach.
Several factors could have contributed to the decrease in alcohol-related hospitalizations. Our findings suggest that patient likelihood to seek care and clinician threshold to admit patients for alcohol-related conditions are influenced by external factors, in this case, a public health lockdown. Although our data do not inform why hospitalizations did not return to prelockdown levels, our experience suggests that limited bed capacity and longer length of stay might have contributed. Other hypotheses include a shift to outpatient care, increased use of telehealth (a significant focus early in the pandemic), and avoiding care for less severe alcohol-related complications because of lingering concerns about exposure to COVID-19 in health care settings reported early in the pandemic. Massachusetts experienced a particularly deadly outbreak of COVID-19 in the Soldiers’ Home, a long-term care facility for veterans in Holyoke.21
Evidence suggests that in-home consumption of alcohol increased during lockdowns.8-10 Our results show that during this period hospitalizations for alcohol-related conditions decreased at VABHS, a large urban VA medical system, while alcohol-related deaths increased nationally.13 Although this observation is not evidence of causality, these outcomes could be related.
In the 2 decades before the pandemic, alcohol-related deaths increased by about 2% per year.22 From 2019 to 2020, there was a 25% increase that continued through 2021.13 Death certificate data often are inaccurate, and it is difficult to determine whether COVID-19 had a substantial contributing role to these deaths, particularly during the initial period when testing was limited or unavailable. Nonetheless, deaths due to alcohol-associated liver disease, overdoses involving alcohol, and alcohol-related traffic fatalities increased by > 10%.13,23 These trends, along with a decrease in hospitalization for alcohol-related conditions, suggest missed opportunities for intervention with patients experiencing alcohol use disorder.
Limitations
In this study, hospitalizations under observation status were excluded, which could underestimate the total number of hospitalizations related to alcohol. We reasoned that this effect was likely to be small and not substantially different by year. ICD-10 codes were used to identify alcohol-related hospitalizations as any hospitalization with an included ICD-10 code listed as the primary discharge diagnosis code. This also likely underestimated the total number of alcohol-related hospitalizations. An ICD-10 code for COVID-19 was not in widespread use during our study period, which prohibited controlling explicitly for the volume of admissions due to COVID-19. The prelockdown period only contains data from the preceding 3 years, which might not be long enough for secular trends to become apparent. We assumed the population at risk remained constant when in reality, the net movement of patients into and out of VA care during the pandemic likely was more complex but not readily quantifiable. Nonetheless, the large drop in absolute number of alcohol-related hospitalizations is not likely to be sensitive to this change. In the absence of an objective measure of care-seeking behavior, we used the total daily number of hospitalizations as a surrogate for patient propensity to seek care. The total daily number of hospitalizations also reflects changes in physician admitting behavior over time. This allowed explicit modeling of care-seeking behavior as a covariate but does not capture other important determinants such as hospital capacity.
Conclusions
In this interrupted time-series analysis, the daily number of alcohol-related hospitalizations during the initial COVID-19 pandemic–associated lockdown period at VABHS decreased by 80% and remained 28% lower in the postlockdown period compared with the prepandemic baseline. In the context of evidence suggesting that alcohol-related mortality increased during the COVID-19 pandemic, alternate strategies to reach vulnerable individuals are needed. Because of high rates of relapse, hospitalization is an important opportunity to engage patients experiencing alcohol use disorder in treatment through referral to substance use treatment programs and medication-assisted therapy. Considering the reduction in alcohol-related hospitalizations during lockdown, other strategies are needed to ensure comprehensive and longitudinal care for this vulnerable population.
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266
1. Commonwealth of Massachussets, Executive Office of Health and Human Services, Department of Public Health. COVID-19 state of emergency. Accessed June 29, 2023. https://www.mass.gov/info-details/covid-19-state-of-emergency
2. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions-United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795-800. doi:10.15585/mmwr.mm6925e2
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. doi:10.1377/hlthaff.2020.00980
4. Prati G, Mancini AD. The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol Med. 2021;51(2):201-211. doi:10.1017/S0033291721000015
5. Yazdi K, Fuchs-Leitner I, Rosenleitner J, Gerstgrasser NW. Impact of the COVID-19 pandemic on patients with alcohol use disorder and associated risk factors for relapse. Front Psychiatry. 2020;11:620612. doi:10.3389/fpsyt.2020.620612
6. Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi:10.1016/j.psychres.2020.113096
7. Kim JU, Majid A, Judge R, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886-887. doi:10.1016/S2468-1253(20)30251-X
8. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi:10.1001/jamanetworkopen.2020.22942
9. Castaldelli-Maia JM, Segura LE, Martins SS. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol. 2021;96:37-42. doi:10.1016/j.alcohol.2021.06.004
10. Anderson P, O’Donnell A, Jané Llopis E, Kaner E. The COVID-19 alcohol paradox: British household purchases during 2020 compared with 2015-2019. PLoS One. 2022;17(1):e0261609. doi:10.1371/journal.pone.0261609
11. Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525-3530. doi:10.1111/add.15589
12. Sharma RA, Subedi K, Gbadebo BM, Wilson B, Jurkovitz C, Horton T. Alcohol withdrawal rates in hospitalized patients during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e210422. doi:10.1001/jamanetworkopen.2021.0422
13. White AM, Castle IP, Powell PA, Hingson RW, Koob, GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704-1706. doi:10.1001/jama.2022.4308
14. Dhond R, Acher R, Leatherman S, et al. Rapid implementation of a modular clinical trial informatics solution for COVID-19 research. Inform Med Unlocked. 2021;27:100788. doi:10.1016/j.imu.2021.100788
15. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. doi:10.1126/science.abm0620
16. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes. Circulation. 1998;98(1):31-39. doi:10.1161/01.cir.98.1.31
17. Esser MB, Idaikkadar N, Kite-Powell A, Thomas C, Greenlund KJ. Trends in emergency department visits related to acute alcohol consumption before and during the COVID-19 pandemic in the United States, 2018-2020. Drug Alcohol Depend Rep. 2022;3:100049. doi:10.1016/j.dadr.2022.100049
18. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S):1-72. doi:10.1097/ADM.0000000000000668
19. Council of State and Territorial Epidemiologists. Developmental indicator: hospitalizations related to alcohol in the United States using ICD-10-CM codes. Accessed June 29, 2023. https://cste.sharefile.com/share/view/s1ee0f8d039d54031bd7ee90462416bc0
20. Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc. 1993;156(3):379-392. doi:10.2307/2983064
21. Gullette MM. American eldercide. In: Sugrue TJ, Zaloom C, eds. The Long Year: A 2020 Reader. Columbia University Press; 2022: 237-244. http://www.jstor.org/stable/10.7312/sugr20452.26
22. White AM, Castle IP, Hingson RW, Powell PA. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44(1):178-187. doi:10.1111/acer.14239
23. National Highway Traffic Safety Administration. Overview of Motor Vehicle Crashes in 2020. US Department of Transportation; 2022. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/813266