User login
Naltrexone: a Novel Approach to Pruritus in Polycythemia Vera
P ruritus is a characteristic and often debilitating clinical manifestation reported by about 50% of patients with polycythemia vera (PV). The exact pathophysiology of PV-associated pruritus is poorly understood. The itch sensation may arise from a central phenomenon without skin itch receptor involvement, as is seen in opioid-induced pruritus, or peripherally via unmyelinated C fibers. Various interventions have been used with mixed results for symptom management in this patient population.1
Selective serotonin reuptake inhibitors (SSRIs), such as paroxetine and fluoxetine, have historically demonstrated some efficacy in treating PV-associated pruritus.2 Alongside SSRIs, phlebotomy, antihistamines, phototherapy, interferon a, and myelosuppressive medications also comprise the various current treatment options. In addition to lacking efficacy, antihistamines can cause somnolence, constipation, and xerostomia.3,4 Phlebotomy and cytoreductive therapy are often effective in controlling erythrocytosis but fail to alleviate the disabling pruritus.1,5,6 More recently, suboptimal symptom alleviation has prompted the discovery of agents that target the mammalian target of rapamycin (mTOR) and Janus kinase 2 (Jak2) pathways.1
Naltrexone is an opioid antagonist shown to suppress pruritus in various dermatologic pathologies involving histamine-independent pathways.3,7,8 A systematic search strategy identified 34 studies on PV-associated pruritus, its pathophysiology and interventions, and naltrexone as a therapeutic agent. Only 1 study in the literature has described the use of naltrexone for uremic and cholestatic pruritus.9 We describe the successful use of naltrexone monotherapy for the treatment of pruritus in a patient with PV.
Case Presentation
A 40-year-old man with Jak2-positive PV treated with ruxolitinib presented to the outpatient Michael E. DeBakey Veterans Affairs Medical Center Supportive Care Clinic in Houston, Texas, for severe refractory pruritus. Wheals manifested in pruritic regions of the patient’s skin without gross excoriations or erythema. Pruritus reportedly began diffusely across the posterior torso. Through the rapid progression of an episode lasting 30 to 45 minutes, the lesions and pruritus would spread to the anterior torso, extend to the upper extremities bilaterally, and finally descend to the lower extremities bilaterally. A persistent sensation of heat or warmth on the patient’s skin was present, and periodically, this would culminate in a burning sensation comparable to “lying flat on one’s back directly on a hornet’s nest…[followed by] a million stings” that was inconsistent with erythromelalgia given the absence of erythema. The intensity of the pruritic episodes was subjectively also described as “enough to make [him] want to jump off the roof of a building…[causing] moments of deep, deep frustration…[and] the worst of all the symptoms one may encounter because of [PV].”
Pruritus was exacerbated by sweating, heat, contact with any liquids on the skin, and sunburns, which doubled the intensity. The patient reported minimal, temporary relief with cannabidiol and cold fabric or air on his skin. His current regimen and nonpharmacologic efforts provided no relief and included oatmeal baths, cornstarch after showers, and patting instead of rubbing the skin with topical products. Trials with nonprescription diphenhydramine, loratadine, and calamine and zinc were not successful. He had not pursued phototherapy due to time limitations and travel constraints. He had a history of phlebotomies and hydroxyurea use, which he preferred to avoid and discontinued 1 year before presentation.
Despite improving hematocrit (< 45% goal) and platelet counts with ruxolitinib, the patient reported worsening pruritus that significantly impaired quality of life. His sleep and social and physical activities were hindered, preventing him from working. The patient’s active medications also included low-dose aspirin, sertraline, hydroxyzine, triamcinolone acetonide, and pregabalin for sciatica. Given persistent symptoms despite multimodal therapy and lifestyle modifications, the patient was started on naltrexone 25 mg daily, which provided immediate relief of symptoms. He continues to have adequate symptom control 2 years after naltrexone initiation.
Literature Review
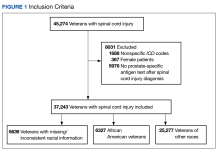
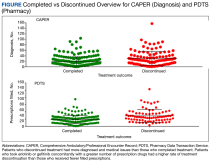
A systematic search strategy was developed with the assistance of a medical librarian in Medline Ovid, using both Medical Subject Heading (MeSH) terms and synonymous keywords. The strategy was then translated to Embase, Web of Science, and Cochrane to extract publications investigating PV, pruritus, and/or naltrexone therapy. All searches were conducted on July 18, 2022, and the results of the literature review were as follows: 2 results from Medline Ovid; 34 results from Embase (2 were duplicates of Medline Ovid results); 3 results from Web of Science (all of which were duplicates of Medline Ovid or Embase results); and 0 results from Cochrane (Figure).
Discussion
Although pruritus is a common and often excruciating manifestation of PV, its pathophysiology remains unclear. Some patients with decreasing or newly normal hematocrit and hemoglobin levels have paradoxically experienced an intensification of their pruritus, which introduces erythropoietin signaling pathways as a potential mechanism of the symptom.8 However, iron replacement therapy for patients with exacerbated pruritus after phlebotomies has not demonstrated consistent relief of pruritus.8 Normalization of platelet levels also has not been historically associated with improvement of pruritus.8,9 It has been hypothesized that cells harboring Jak2 mutations at any stage of the hematopoietic pathway mature and accumulate to cause pruritus in PV.9 This theory has been foundational in the development of drugs with activity against cells expressing Jak2 mutations and interventions targeting histamine-releasing mast cells.9-11
The effective use of naltrexone in our patient suggests that histamine may not be the most effective or sole therapeutic target against pruritus in PV. Naltrexone targets opioid receptors in all layers of the epidermis, affecting cell adhesion and keratinocyte production, and exhibits anti-inflammatory effects through interactions with nonopioid receptors, including Toll-like receptor 4.12 The efficacy of oral naltrexone has been documented in patients with pruritus associated with immune checkpoint inhibitors, psoriasis, eczema, lichen simplex chronicus, prurigo nodularis, cholestasis, uremia, and multiple rheumatologic diseases.3,4,7-9,12-14 Opioid pathways also may be involved in peripheral and/or central processing of pruritus associated with PV.
Importantly, patients who are potential candidates for naltrexone therapy should be notified and advised of the risk of drug interactions with opioids, which could lead to symptoms of opioid withdrawal. Other common adverse effects of naltrexone include hepatotoxicity (especially in patients with a history of significant alcohol consumption), abdominal pain, nausea, arthralgias, myalgias, insomnia, headaches, fatigue, and anxiety.12 Therefore, it is integral to screen patients for opioid dependence and determine their baseline liver function. Patients should be monitored following naltrexone initiation to determine whether the drug is an appropriate and effective intervention against PV-associated pruritus.
CONCLUSIONS
This case study demonstrates that naltrexone may be a safe, effective, nonsedating, and cost-efficient oral alternative for refractory PV-associated pruritus. Future directions involve consideration of case series or randomized clinical trials investigating the efficacy of naltrexone in treating PV-associated pruritus. Further research is also warranted to better understand the pathophysiology of this symptom of PV to enhance and potentially expand medical management for patients.
Acknowledgments
The authors thank Amy Sisson (The Texas Medical Center Library) for her guidance and support in the literature review methodology.
1. Saini KS, Patnaik MM, Tefferi A. Polycythemia vera-associated pruritus and its management. Eur J Clin Invest. 2010;40(9):828-834. doi:10.1111/j.1365-2362.2010.02334.x
2. Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99(7):2627. doi:10.1182/blood.v99.7.2627
3. Lee J, Shin JU, Noh S, Park CO, Lee KH. Clinical efficacy and safety of naltrexone combination therapy in older patients with severe pruritus. Ann Dermatol. 2016;28(2):159-163. doi:10.5021/ad.2016.28.2.159
4. Phan NQ, Bernhard JD, Luger TA, Stander S. Antipruritic treatment with systemic mu-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63(4):680-688. doi:10.1016/j.jaad.2009.08.052
5. Metze D, Reimann S, Beissert S, Luger T. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41(4):533-539.
6. Malekzad F, Arbabi M, Mohtasham N, et al. Efficacy of oral naltrexone on pruritus in atopic eczema: a double-blind, placebo-controlled study. J Eur Acad Dermatol Venereol. 2009;23(8):948-950. doi:10.1111/j.1468-3083.2009.03129.x
7. Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722. doi:10.1016/s0168-8278(02)00318-5
8. Lelonek E, Matusiak L, Wrobel T, Szepietowski JC. Aquagenic pruritus in polycythemia vera: clinical characteristics. Acta Derm Venereol. 2018;98(5):496-500. doi:10.2340/00015555-2906
9. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665-669. doi:10.1002/ajh.23474
10. Al-Mashdali AF, Kashgary WR, Yassin MA. Ruxolitinib (a JAK2 inhibitor) as an emerging therapy for refractory pruritis in a patient with low-risk polycythemia vera: a case report. Medicine (Baltimore). 2021;100(44):e27722. doi:10.1097/MD.0000000000027722
11. Benevolo G, Vassallo F, Urbino I, Giai V. Polycythemia vera (PV): update on emerging treatment options. Ther Clin Risk Manag. 2021;17:209-221. doi:10.2147/TCRM.S213020
12. Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. J Am Acad Dermatol. 2019;80(6):1746-1752. doi:10.1016/j.jaad.2018.12.031
13. de Carvalho JF, Skare T. Low-dose naltrexone in rheumatological diseases. Mediterr J Rheumatol. 2023;34(1):1-6. doi:10.31138/mjr.34.1.1
14. Singh R, Patel P, Thakker M, Sharma P, Barnes M, Montana S. Naloxone and maintenance naltrexone as novel and effective therapies for immunotherapy-induced pruritus: a case report and brief literature review. J Oncol Pract. 2019;15(6):347-348. doi:10.1200/JOP.18.00797
P ruritus is a characteristic and often debilitating clinical manifestation reported by about 50% of patients with polycythemia vera (PV). The exact pathophysiology of PV-associated pruritus is poorly understood. The itch sensation may arise from a central phenomenon without skin itch receptor involvement, as is seen in opioid-induced pruritus, or peripherally via unmyelinated C fibers. Various interventions have been used with mixed results for symptom management in this patient population.1
Selective serotonin reuptake inhibitors (SSRIs), such as paroxetine and fluoxetine, have historically demonstrated some efficacy in treating PV-associated pruritus.2 Alongside SSRIs, phlebotomy, antihistamines, phototherapy, interferon a, and myelosuppressive medications also comprise the various current treatment options. In addition to lacking efficacy, antihistamines can cause somnolence, constipation, and xerostomia.3,4 Phlebotomy and cytoreductive therapy are often effective in controlling erythrocytosis but fail to alleviate the disabling pruritus.1,5,6 More recently, suboptimal symptom alleviation has prompted the discovery of agents that target the mammalian target of rapamycin (mTOR) and Janus kinase 2 (Jak2) pathways.1
Naltrexone is an opioid antagonist shown to suppress pruritus in various dermatologic pathologies involving histamine-independent pathways.3,7,8 A systematic search strategy identified 34 studies on PV-associated pruritus, its pathophysiology and interventions, and naltrexone as a therapeutic agent. Only 1 study in the literature has described the use of naltrexone for uremic and cholestatic pruritus.9 We describe the successful use of naltrexone monotherapy for the treatment of pruritus in a patient with PV.
Case Presentation
A 40-year-old man with Jak2-positive PV treated with ruxolitinib presented to the outpatient Michael E. DeBakey Veterans Affairs Medical Center Supportive Care Clinic in Houston, Texas, for severe refractory pruritus. Wheals manifested in pruritic regions of the patient’s skin without gross excoriations or erythema. Pruritus reportedly began diffusely across the posterior torso. Through the rapid progression of an episode lasting 30 to 45 minutes, the lesions and pruritus would spread to the anterior torso, extend to the upper extremities bilaterally, and finally descend to the lower extremities bilaterally. A persistent sensation of heat or warmth on the patient’s skin was present, and periodically, this would culminate in a burning sensation comparable to “lying flat on one’s back directly on a hornet’s nest…[followed by] a million stings” that was inconsistent with erythromelalgia given the absence of erythema. The intensity of the pruritic episodes was subjectively also described as “enough to make [him] want to jump off the roof of a building…[causing] moments of deep, deep frustration…[and] the worst of all the symptoms one may encounter because of [PV].”
Pruritus was exacerbated by sweating, heat, contact with any liquids on the skin, and sunburns, which doubled the intensity. The patient reported minimal, temporary relief with cannabidiol and cold fabric or air on his skin. His current regimen and nonpharmacologic efforts provided no relief and included oatmeal baths, cornstarch after showers, and patting instead of rubbing the skin with topical products. Trials with nonprescription diphenhydramine, loratadine, and calamine and zinc were not successful. He had not pursued phototherapy due to time limitations and travel constraints. He had a history of phlebotomies and hydroxyurea use, which he preferred to avoid and discontinued 1 year before presentation.
Despite improving hematocrit (< 45% goal) and platelet counts with ruxolitinib, the patient reported worsening pruritus that significantly impaired quality of life. His sleep and social and physical activities were hindered, preventing him from working. The patient’s active medications also included low-dose aspirin, sertraline, hydroxyzine, triamcinolone acetonide, and pregabalin for sciatica. Given persistent symptoms despite multimodal therapy and lifestyle modifications, the patient was started on naltrexone 25 mg daily, which provided immediate relief of symptoms. He continues to have adequate symptom control 2 years after naltrexone initiation.
Literature Review
A systematic search strategy was developed with the assistance of a medical librarian in Medline Ovid, using both Medical Subject Heading (MeSH) terms and synonymous keywords. The strategy was then translated to Embase, Web of Science, and Cochrane to extract publications investigating PV, pruritus, and/or naltrexone therapy. All searches were conducted on July 18, 2022, and the results of the literature review were as follows: 2 results from Medline Ovid; 34 results from Embase (2 were duplicates of Medline Ovid results); 3 results from Web of Science (all of which were duplicates of Medline Ovid or Embase results); and 0 results from Cochrane (Figure).
Discussion
Although pruritus is a common and often excruciating manifestation of PV, its pathophysiology remains unclear. Some patients with decreasing or newly normal hematocrit and hemoglobin levels have paradoxically experienced an intensification of their pruritus, which introduces erythropoietin signaling pathways as a potential mechanism of the symptom.8 However, iron replacement therapy for patients with exacerbated pruritus after phlebotomies has not demonstrated consistent relief of pruritus.8 Normalization of platelet levels also has not been historically associated with improvement of pruritus.8,9 It has been hypothesized that cells harboring Jak2 mutations at any stage of the hematopoietic pathway mature and accumulate to cause pruritus in PV.9 This theory has been foundational in the development of drugs with activity against cells expressing Jak2 mutations and interventions targeting histamine-releasing mast cells.9-11
The effective use of naltrexone in our patient suggests that histamine may not be the most effective or sole therapeutic target against pruritus in PV. Naltrexone targets opioid receptors in all layers of the epidermis, affecting cell adhesion and keratinocyte production, and exhibits anti-inflammatory effects through interactions with nonopioid receptors, including Toll-like receptor 4.12 The efficacy of oral naltrexone has been documented in patients with pruritus associated with immune checkpoint inhibitors, psoriasis, eczema, lichen simplex chronicus, prurigo nodularis, cholestasis, uremia, and multiple rheumatologic diseases.3,4,7-9,12-14 Opioid pathways also may be involved in peripheral and/or central processing of pruritus associated with PV.
Importantly, patients who are potential candidates for naltrexone therapy should be notified and advised of the risk of drug interactions with opioids, which could lead to symptoms of opioid withdrawal. Other common adverse effects of naltrexone include hepatotoxicity (especially in patients with a history of significant alcohol consumption), abdominal pain, nausea, arthralgias, myalgias, insomnia, headaches, fatigue, and anxiety.12 Therefore, it is integral to screen patients for opioid dependence and determine their baseline liver function. Patients should be monitored following naltrexone initiation to determine whether the drug is an appropriate and effective intervention against PV-associated pruritus.
CONCLUSIONS
This case study demonstrates that naltrexone may be a safe, effective, nonsedating, and cost-efficient oral alternative for refractory PV-associated pruritus. Future directions involve consideration of case series or randomized clinical trials investigating the efficacy of naltrexone in treating PV-associated pruritus. Further research is also warranted to better understand the pathophysiology of this symptom of PV to enhance and potentially expand medical management for patients.
Acknowledgments
The authors thank Amy Sisson (The Texas Medical Center Library) for her guidance and support in the literature review methodology.
P ruritus is a characteristic and often debilitating clinical manifestation reported by about 50% of patients with polycythemia vera (PV). The exact pathophysiology of PV-associated pruritus is poorly understood. The itch sensation may arise from a central phenomenon without skin itch receptor involvement, as is seen in opioid-induced pruritus, or peripherally via unmyelinated C fibers. Various interventions have been used with mixed results for symptom management in this patient population.1
Selective serotonin reuptake inhibitors (SSRIs), such as paroxetine and fluoxetine, have historically demonstrated some efficacy in treating PV-associated pruritus.2 Alongside SSRIs, phlebotomy, antihistamines, phototherapy, interferon a, and myelosuppressive medications also comprise the various current treatment options. In addition to lacking efficacy, antihistamines can cause somnolence, constipation, and xerostomia.3,4 Phlebotomy and cytoreductive therapy are often effective in controlling erythrocytosis but fail to alleviate the disabling pruritus.1,5,6 More recently, suboptimal symptom alleviation has prompted the discovery of agents that target the mammalian target of rapamycin (mTOR) and Janus kinase 2 (Jak2) pathways.1
Naltrexone is an opioid antagonist shown to suppress pruritus in various dermatologic pathologies involving histamine-independent pathways.3,7,8 A systematic search strategy identified 34 studies on PV-associated pruritus, its pathophysiology and interventions, and naltrexone as a therapeutic agent. Only 1 study in the literature has described the use of naltrexone for uremic and cholestatic pruritus.9 We describe the successful use of naltrexone monotherapy for the treatment of pruritus in a patient with PV.
Case Presentation
A 40-year-old man with Jak2-positive PV treated with ruxolitinib presented to the outpatient Michael E. DeBakey Veterans Affairs Medical Center Supportive Care Clinic in Houston, Texas, for severe refractory pruritus. Wheals manifested in pruritic regions of the patient’s skin without gross excoriations or erythema. Pruritus reportedly began diffusely across the posterior torso. Through the rapid progression of an episode lasting 30 to 45 minutes, the lesions and pruritus would spread to the anterior torso, extend to the upper extremities bilaterally, and finally descend to the lower extremities bilaterally. A persistent sensation of heat or warmth on the patient’s skin was present, and periodically, this would culminate in a burning sensation comparable to “lying flat on one’s back directly on a hornet’s nest…[followed by] a million stings” that was inconsistent with erythromelalgia given the absence of erythema. The intensity of the pruritic episodes was subjectively also described as “enough to make [him] want to jump off the roof of a building…[causing] moments of deep, deep frustration…[and] the worst of all the symptoms one may encounter because of [PV].”
Pruritus was exacerbated by sweating, heat, contact with any liquids on the skin, and sunburns, which doubled the intensity. The patient reported minimal, temporary relief with cannabidiol and cold fabric or air on his skin. His current regimen and nonpharmacologic efforts provided no relief and included oatmeal baths, cornstarch after showers, and patting instead of rubbing the skin with topical products. Trials with nonprescription diphenhydramine, loratadine, and calamine and zinc were not successful. He had not pursued phototherapy due to time limitations and travel constraints. He had a history of phlebotomies and hydroxyurea use, which he preferred to avoid and discontinued 1 year before presentation.
Despite improving hematocrit (< 45% goal) and platelet counts with ruxolitinib, the patient reported worsening pruritus that significantly impaired quality of life. His sleep and social and physical activities were hindered, preventing him from working. The patient’s active medications also included low-dose aspirin, sertraline, hydroxyzine, triamcinolone acetonide, and pregabalin for sciatica. Given persistent symptoms despite multimodal therapy and lifestyle modifications, the patient was started on naltrexone 25 mg daily, which provided immediate relief of symptoms. He continues to have adequate symptom control 2 years after naltrexone initiation.
Literature Review
A systematic search strategy was developed with the assistance of a medical librarian in Medline Ovid, using both Medical Subject Heading (MeSH) terms and synonymous keywords. The strategy was then translated to Embase, Web of Science, and Cochrane to extract publications investigating PV, pruritus, and/or naltrexone therapy. All searches were conducted on July 18, 2022, and the results of the literature review were as follows: 2 results from Medline Ovid; 34 results from Embase (2 were duplicates of Medline Ovid results); 3 results from Web of Science (all of which were duplicates of Medline Ovid or Embase results); and 0 results from Cochrane (Figure).
Discussion
Although pruritus is a common and often excruciating manifestation of PV, its pathophysiology remains unclear. Some patients with decreasing or newly normal hematocrit and hemoglobin levels have paradoxically experienced an intensification of their pruritus, which introduces erythropoietin signaling pathways as a potential mechanism of the symptom.8 However, iron replacement therapy for patients with exacerbated pruritus after phlebotomies has not demonstrated consistent relief of pruritus.8 Normalization of platelet levels also has not been historically associated with improvement of pruritus.8,9 It has been hypothesized that cells harboring Jak2 mutations at any stage of the hematopoietic pathway mature and accumulate to cause pruritus in PV.9 This theory has been foundational in the development of drugs with activity against cells expressing Jak2 mutations and interventions targeting histamine-releasing mast cells.9-11
The effective use of naltrexone in our patient suggests that histamine may not be the most effective or sole therapeutic target against pruritus in PV. Naltrexone targets opioid receptors in all layers of the epidermis, affecting cell adhesion and keratinocyte production, and exhibits anti-inflammatory effects through interactions with nonopioid receptors, including Toll-like receptor 4.12 The efficacy of oral naltrexone has been documented in patients with pruritus associated with immune checkpoint inhibitors, psoriasis, eczema, lichen simplex chronicus, prurigo nodularis, cholestasis, uremia, and multiple rheumatologic diseases.3,4,7-9,12-14 Opioid pathways also may be involved in peripheral and/or central processing of pruritus associated with PV.
Importantly, patients who are potential candidates for naltrexone therapy should be notified and advised of the risk of drug interactions with opioids, which could lead to symptoms of opioid withdrawal. Other common adverse effects of naltrexone include hepatotoxicity (especially in patients with a history of significant alcohol consumption), abdominal pain, nausea, arthralgias, myalgias, insomnia, headaches, fatigue, and anxiety.12 Therefore, it is integral to screen patients for opioid dependence and determine their baseline liver function. Patients should be monitored following naltrexone initiation to determine whether the drug is an appropriate and effective intervention against PV-associated pruritus.
CONCLUSIONS
This case study demonstrates that naltrexone may be a safe, effective, nonsedating, and cost-efficient oral alternative for refractory PV-associated pruritus. Future directions involve consideration of case series or randomized clinical trials investigating the efficacy of naltrexone in treating PV-associated pruritus. Further research is also warranted to better understand the pathophysiology of this symptom of PV to enhance and potentially expand medical management for patients.
Acknowledgments
The authors thank Amy Sisson (The Texas Medical Center Library) for her guidance and support in the literature review methodology.
1. Saini KS, Patnaik MM, Tefferi A. Polycythemia vera-associated pruritus and its management. Eur J Clin Invest. 2010;40(9):828-834. doi:10.1111/j.1365-2362.2010.02334.x
2. Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99(7):2627. doi:10.1182/blood.v99.7.2627
3. Lee J, Shin JU, Noh S, Park CO, Lee KH. Clinical efficacy and safety of naltrexone combination therapy in older patients with severe pruritus. Ann Dermatol. 2016;28(2):159-163. doi:10.5021/ad.2016.28.2.159
4. Phan NQ, Bernhard JD, Luger TA, Stander S. Antipruritic treatment with systemic mu-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63(4):680-688. doi:10.1016/j.jaad.2009.08.052
5. Metze D, Reimann S, Beissert S, Luger T. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41(4):533-539.
6. Malekzad F, Arbabi M, Mohtasham N, et al. Efficacy of oral naltrexone on pruritus in atopic eczema: a double-blind, placebo-controlled study. J Eur Acad Dermatol Venereol. 2009;23(8):948-950. doi:10.1111/j.1468-3083.2009.03129.x
7. Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722. doi:10.1016/s0168-8278(02)00318-5
8. Lelonek E, Matusiak L, Wrobel T, Szepietowski JC. Aquagenic pruritus in polycythemia vera: clinical characteristics. Acta Derm Venereol. 2018;98(5):496-500. doi:10.2340/00015555-2906
9. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665-669. doi:10.1002/ajh.23474
10. Al-Mashdali AF, Kashgary WR, Yassin MA. Ruxolitinib (a JAK2 inhibitor) as an emerging therapy for refractory pruritis in a patient with low-risk polycythemia vera: a case report. Medicine (Baltimore). 2021;100(44):e27722. doi:10.1097/MD.0000000000027722
11. Benevolo G, Vassallo F, Urbino I, Giai V. Polycythemia vera (PV): update on emerging treatment options. Ther Clin Risk Manag. 2021;17:209-221. doi:10.2147/TCRM.S213020
12. Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. J Am Acad Dermatol. 2019;80(6):1746-1752. doi:10.1016/j.jaad.2018.12.031
13. de Carvalho JF, Skare T. Low-dose naltrexone in rheumatological diseases. Mediterr J Rheumatol. 2023;34(1):1-6. doi:10.31138/mjr.34.1.1
14. Singh R, Patel P, Thakker M, Sharma P, Barnes M, Montana S. Naloxone and maintenance naltrexone as novel and effective therapies for immunotherapy-induced pruritus: a case report and brief literature review. J Oncol Pract. 2019;15(6):347-348. doi:10.1200/JOP.18.00797
1. Saini KS, Patnaik MM, Tefferi A. Polycythemia vera-associated pruritus and its management. Eur J Clin Invest. 2010;40(9):828-834. doi:10.1111/j.1365-2362.2010.02334.x
2. Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99(7):2627. doi:10.1182/blood.v99.7.2627
3. Lee J, Shin JU, Noh S, Park CO, Lee KH. Clinical efficacy and safety of naltrexone combination therapy in older patients with severe pruritus. Ann Dermatol. 2016;28(2):159-163. doi:10.5021/ad.2016.28.2.159
4. Phan NQ, Bernhard JD, Luger TA, Stander S. Antipruritic treatment with systemic mu-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63(4):680-688. doi:10.1016/j.jaad.2009.08.052
5. Metze D, Reimann S, Beissert S, Luger T. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41(4):533-539.
6. Malekzad F, Arbabi M, Mohtasham N, et al. Efficacy of oral naltrexone on pruritus in atopic eczema: a double-blind, placebo-controlled study. J Eur Acad Dermatol Venereol. 2009;23(8):948-950. doi:10.1111/j.1468-3083.2009.03129.x
7. Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722. doi:10.1016/s0168-8278(02)00318-5
8. Lelonek E, Matusiak L, Wrobel T, Szepietowski JC. Aquagenic pruritus in polycythemia vera: clinical characteristics. Acta Derm Venereol. 2018;98(5):496-500. doi:10.2340/00015555-2906
9. Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665-669. doi:10.1002/ajh.23474
10. Al-Mashdali AF, Kashgary WR, Yassin MA. Ruxolitinib (a JAK2 inhibitor) as an emerging therapy for refractory pruritis in a patient with low-risk polycythemia vera: a case report. Medicine (Baltimore). 2021;100(44):e27722. doi:10.1097/MD.0000000000027722
11. Benevolo G, Vassallo F, Urbino I, Giai V. Polycythemia vera (PV): update on emerging treatment options. Ther Clin Risk Manag. 2021;17:209-221. doi:10.2147/TCRM.S213020
12. Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. J Am Acad Dermatol. 2019;80(6):1746-1752. doi:10.1016/j.jaad.2018.12.031
13. de Carvalho JF, Skare T. Low-dose naltrexone in rheumatological diseases. Mediterr J Rheumatol. 2023;34(1):1-6. doi:10.31138/mjr.34.1.1
14. Singh R, Patel P, Thakker M, Sharma P, Barnes M, Montana S. Naloxone and maintenance naltrexone as novel and effective therapies for immunotherapy-induced pruritus: a case report and brief literature review. J Oncol Pract. 2019;15(6):347-348. doi:10.1200/JOP.18.00797
Alcohol consumption may not influence breast cancer prognosis, study
, an analysis of data from a prospective cohort study suggests.
The study appears to show that drinking up to one serving of alcohol daily, including wine, beer, and liquor, was not associated with any specific outcomes after breast cancer diagnosis. The authors say these findings could have implications for developing more specific guidelines on alcohol use as it relates to the prevention of death and recurrence for cancer survivors.
Among 3,659 women followed for a mean of 11.2 years after a breast cancer diagnosis, overall alcohol consumption in the months before and up to 6 months after diagnosis was not associated with recurrence or mortality after adjusting for numerous factors such as age at diagnosis, cancer stage, socioeconomic details, smoking history, and preexisting conditions.
However, women with obesity (body mass index of 30 kg/m2 or greater) had a lower risk of mortality with increasing alcohol consumption for occasional drinking of 2 or more alcohol servings per week (hazard ratio, 0.71), and regular drinking of at least one alcohol serving daily (HR, 0.77), in a dose-response manner, Marilyn L. Kwan, PhD, and colleagues found.
Dr. Kwan is a senior research scientist at Kaiser Permanente Northern California Division of Research, Oakland.
Women with BMI less than 30 kg/m2 did not have a higher risk of mortality but a nonsignificant increase in the risk of recurrence was observed for those who consumed alcohol occasionally (HR, 1.29) and regularly (HR, 1.19), the investigators reported.
The findings were published online in Cancer.
Women included in the current study were participants in the Pathways Study and were diagnosed with stage I-IV breast cancer between 2003 and 2015. During follow-up, 524 recurrences and 834 deaths occurred, including 369 breast cancer-specific deaths, 314 cardiovascular disease-specific deaths, and 151 deaths from other health problems.
Alcohol consumption was assessed for the 6 months prior to cohort entry, which occurred at an average of about 2 months after diagnosis, as well as 6 months later – at an average of about 8 months after diagnosis – using a food-frequency questionnaire.
Compared with nondrinkers (36.9%), drinkers were more likely younger, more educated, and current or past smokers, the investigators noted.
“This profile appears counterintuitive yet might reflect a healthier lifestyle contributing to better overall survival. Furthermore, higher levels of alcohol consumption could lead to improvement in insulin sensitivity and reduction in insulin-like growth factor-1,” they speculated, noting that reduced fasting insulin concentrations and lower insulin-like growth factor-1 levels are linked with a decreased risk of type 2 diabetes, cardiovascular disease, and cancer.
“Many women with a history of breast cancer are interested in how to improve their prognosis and survival by making lifestyle changes after diagnosis,” they wrote, explaining the rationale for the study. “Current cancer prevention guidelines recommend avoiding alcohol intake or limiting consumption to no more than one drink per day for women. However, no specific guideline exists for cancer survivors other than following the cancer prevention guidelines to reduce the risk of a second cancer.”
High-quality studies on the impact of alcohol consumption on breast cancer prognosis are lacking, they added.
“Given that consuming alcohol is a potentially modifiable lifestyle factor after breast cancer diagnosis, further confirmation is warranted in other large prospective studies of breast cancer survivors with detailed exposure assessment and focus on body size,” they concluded.
The group is the first to report this finding in obese women, and they “strongly believe more research is needed to see if the same association is seen in other studies,” Dr. Kwan told this news organization.
“After a cancer diagnosis, many patients are motivated to make lifestyle changes,” she said. “That often includes adding exercise to their daily routine and eating a healthier diet. Our study findings suggest that doctors can tell patients that having up to a glass of alcohol a day is not likely to increase their risk of a breast cancer recurrence.”
This study was funded by the National Cancer Institute. The authors reported having no disclosures.
, an analysis of data from a prospective cohort study suggests.
The study appears to show that drinking up to one serving of alcohol daily, including wine, beer, and liquor, was not associated with any specific outcomes after breast cancer diagnosis. The authors say these findings could have implications for developing more specific guidelines on alcohol use as it relates to the prevention of death and recurrence for cancer survivors.
Among 3,659 women followed for a mean of 11.2 years after a breast cancer diagnosis, overall alcohol consumption in the months before and up to 6 months after diagnosis was not associated with recurrence or mortality after adjusting for numerous factors such as age at diagnosis, cancer stage, socioeconomic details, smoking history, and preexisting conditions.
However, women with obesity (body mass index of 30 kg/m2 or greater) had a lower risk of mortality with increasing alcohol consumption for occasional drinking of 2 or more alcohol servings per week (hazard ratio, 0.71), and regular drinking of at least one alcohol serving daily (HR, 0.77), in a dose-response manner, Marilyn L. Kwan, PhD, and colleagues found.
Dr. Kwan is a senior research scientist at Kaiser Permanente Northern California Division of Research, Oakland.
Women with BMI less than 30 kg/m2 did not have a higher risk of mortality but a nonsignificant increase in the risk of recurrence was observed for those who consumed alcohol occasionally (HR, 1.29) and regularly (HR, 1.19), the investigators reported.
The findings were published online in Cancer.
Women included in the current study were participants in the Pathways Study and were diagnosed with stage I-IV breast cancer between 2003 and 2015. During follow-up, 524 recurrences and 834 deaths occurred, including 369 breast cancer-specific deaths, 314 cardiovascular disease-specific deaths, and 151 deaths from other health problems.
Alcohol consumption was assessed for the 6 months prior to cohort entry, which occurred at an average of about 2 months after diagnosis, as well as 6 months later – at an average of about 8 months after diagnosis – using a food-frequency questionnaire.
Compared with nondrinkers (36.9%), drinkers were more likely younger, more educated, and current or past smokers, the investigators noted.
“This profile appears counterintuitive yet might reflect a healthier lifestyle contributing to better overall survival. Furthermore, higher levels of alcohol consumption could lead to improvement in insulin sensitivity and reduction in insulin-like growth factor-1,” they speculated, noting that reduced fasting insulin concentrations and lower insulin-like growth factor-1 levels are linked with a decreased risk of type 2 diabetes, cardiovascular disease, and cancer.
“Many women with a history of breast cancer are interested in how to improve their prognosis and survival by making lifestyle changes after diagnosis,” they wrote, explaining the rationale for the study. “Current cancer prevention guidelines recommend avoiding alcohol intake or limiting consumption to no more than one drink per day for women. However, no specific guideline exists for cancer survivors other than following the cancer prevention guidelines to reduce the risk of a second cancer.”
High-quality studies on the impact of alcohol consumption on breast cancer prognosis are lacking, they added.
“Given that consuming alcohol is a potentially modifiable lifestyle factor after breast cancer diagnosis, further confirmation is warranted in other large prospective studies of breast cancer survivors with detailed exposure assessment and focus on body size,” they concluded.
The group is the first to report this finding in obese women, and they “strongly believe more research is needed to see if the same association is seen in other studies,” Dr. Kwan told this news organization.
“After a cancer diagnosis, many patients are motivated to make lifestyle changes,” she said. “That often includes adding exercise to their daily routine and eating a healthier diet. Our study findings suggest that doctors can tell patients that having up to a glass of alcohol a day is not likely to increase their risk of a breast cancer recurrence.”
This study was funded by the National Cancer Institute. The authors reported having no disclosures.
, an analysis of data from a prospective cohort study suggests.
The study appears to show that drinking up to one serving of alcohol daily, including wine, beer, and liquor, was not associated with any specific outcomes after breast cancer diagnosis. The authors say these findings could have implications for developing more specific guidelines on alcohol use as it relates to the prevention of death and recurrence for cancer survivors.
Among 3,659 women followed for a mean of 11.2 years after a breast cancer diagnosis, overall alcohol consumption in the months before and up to 6 months after diagnosis was not associated with recurrence or mortality after adjusting for numerous factors such as age at diagnosis, cancer stage, socioeconomic details, smoking history, and preexisting conditions.
However, women with obesity (body mass index of 30 kg/m2 or greater) had a lower risk of mortality with increasing alcohol consumption for occasional drinking of 2 or more alcohol servings per week (hazard ratio, 0.71), and regular drinking of at least one alcohol serving daily (HR, 0.77), in a dose-response manner, Marilyn L. Kwan, PhD, and colleagues found.
Dr. Kwan is a senior research scientist at Kaiser Permanente Northern California Division of Research, Oakland.
Women with BMI less than 30 kg/m2 did not have a higher risk of mortality but a nonsignificant increase in the risk of recurrence was observed for those who consumed alcohol occasionally (HR, 1.29) and regularly (HR, 1.19), the investigators reported.
The findings were published online in Cancer.
Women included in the current study were participants in the Pathways Study and were diagnosed with stage I-IV breast cancer between 2003 and 2015. During follow-up, 524 recurrences and 834 deaths occurred, including 369 breast cancer-specific deaths, 314 cardiovascular disease-specific deaths, and 151 deaths from other health problems.
Alcohol consumption was assessed for the 6 months prior to cohort entry, which occurred at an average of about 2 months after diagnosis, as well as 6 months later – at an average of about 8 months after diagnosis – using a food-frequency questionnaire.
Compared with nondrinkers (36.9%), drinkers were more likely younger, more educated, and current or past smokers, the investigators noted.
“This profile appears counterintuitive yet might reflect a healthier lifestyle contributing to better overall survival. Furthermore, higher levels of alcohol consumption could lead to improvement in insulin sensitivity and reduction in insulin-like growth factor-1,” they speculated, noting that reduced fasting insulin concentrations and lower insulin-like growth factor-1 levels are linked with a decreased risk of type 2 diabetes, cardiovascular disease, and cancer.
“Many women with a history of breast cancer are interested in how to improve their prognosis and survival by making lifestyle changes after diagnosis,” they wrote, explaining the rationale for the study. “Current cancer prevention guidelines recommend avoiding alcohol intake or limiting consumption to no more than one drink per day for women. However, no specific guideline exists for cancer survivors other than following the cancer prevention guidelines to reduce the risk of a second cancer.”
High-quality studies on the impact of alcohol consumption on breast cancer prognosis are lacking, they added.
“Given that consuming alcohol is a potentially modifiable lifestyle factor after breast cancer diagnosis, further confirmation is warranted in other large prospective studies of breast cancer survivors with detailed exposure assessment and focus on body size,” they concluded.
The group is the first to report this finding in obese women, and they “strongly believe more research is needed to see if the same association is seen in other studies,” Dr. Kwan told this news organization.
“After a cancer diagnosis, many patients are motivated to make lifestyle changes,” she said. “That often includes adding exercise to their daily routine and eating a healthier diet. Our study findings suggest that doctors can tell patients that having up to a glass of alcohol a day is not likely to increase their risk of a breast cancer recurrence.”
This study was funded by the National Cancer Institute. The authors reported having no disclosures.
FROM CANCER
A Case Series of Rare Immune-Mediated Adverse Reactions at the New Mexico Veterans Affairs Medical Center
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
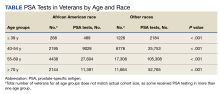
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
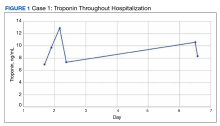
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
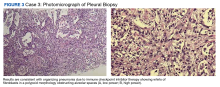
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Race and Age-Related PSA Testing Disparities in Spinal Cord Injured Men: Analysis of National Veterans Health Administration Data
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
Retrospective Evaluation of Drug-Drug Interactions With Erlotinib and Gefitinib Use in the Military Health System
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
Most cancer treatment regimens include the administration of several chemotherapeutic agents. Drug-drug interactions (DDIs) can increase the risk of fatal adverse events and reduce therapeutic efficacy.1,2 Erlotinib, gefitinib, afatinib, osimertinib, and icotinib are epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) that have proven efficacy for treating advanced non–small cell lung cancer (NSCLC). Erlotinib strongly inhibits cytochrome P450 (CYP) isoenzymes CYP 1A1, moderately inhibits CYP 3A4 and 2C8, and induces CYP 1A1 and 1A2.2 Gefitinib weakly inhibits CYP 2C19 and 2D6.2 CYP 3A4 inducers and inhibitors affect metabolism of both erlotinib and gefitinib.3,4
Erlotinib and gefitinib are first-generation EGFR-TKIs and have been approved for NSCLC treatment by the US Food and Drug Administration (FDA). These agents have been used since the early 2000s and increase the possibility of long-term response and survival.2,5,6 EGFR-TKIs have a range of potential DDIs, including interactions with CYP-dependent metabolism, uridine diphosphate-glucuronosyltransferase, and transporter proteins.2 Few retrospective studies have focused on the therapeutic efficacy of erlotinib, gefitinib, or the combination of these agents.7-14
DDIs from cancer and noncancer therapies could lead to treatment discontinuation and affect patient outcomes. The goals for this study were to perform a broad-scale retrospective analysis focused on investigating prescribed drugs used with erlotinib and gefitinib and determine patient outcomes as obtained through several Military Health System (MHS) databases. Our investigation focused on (1) the functions of these drugs; (2) identifying adverse effects (AEs) that patients experienced; (3) evaluating differences when these drugs are used alone vs concomitantly, and between the completed vs discontinued treatment groups; (4) identifying all drugs used during erlotinib or gefitinib treatment; and (5) evaluating DDIs with antidepressants.
This retrospective study was performed at the Department of Research Programs at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. The WRNMMC Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center of the US Department of Defense (DoD) Cancer Registry and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Methods
The DoD Cancer Registry Program was established in 1986 by the Assistant Secretary of Defense for Health Affairs. The registry currently contains data from 1998 to 2023. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2023.
Each observation in the PDTS record represents an outpatient prescription filled for an MHS beneficiary at MTFs through the TRICARE mail-order program or a retail pharmacy in the United States. Missing from this record are prescriptions filled at civilian pharmacies outside the United States and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2023. The Composite Health Care System—the legacy system—is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for erlotinib and gefitinib from 1998 to 2021. Data from the DoD Cancer Registry were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, while the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the Joint Pathology Center included cancer treatment (alone or concomitant), cancer information (cancer types and stages), demographics (sex, age at diagnosis), and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or a buffer of 6 months after the initial period). We used all collected data in this analysis. The only exclusion criterion was a provided physician’s note commenting that the patient did not use erlotinib or gefitinib.
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology (ICD-O) were used to decode disease and cancer types.15,16 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the total number of patients or data available within the gefitinib and erlotinib groups divided by total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to calculate P to determine statistical significance (P < .05) using a statistics website.17 Concomitant was defined as erlotinib or gefitinib taken with other medication(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with “.”, “,”, “/”, “;”, (period, comma, forward slash, semicolon) or space between medication names were interpreted as concurrent, while “+”, “-/+” (plus, minus/plus), or and between drug names were interpreted as combined. Completed treatment was defined as erlotinib or gefitinib as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
Results
Erlotinib
The Joint Pathology Center provided 387 entries for 382 patients aged 21 to 93 years (mean, 65 years) who were treated systemically with erlotinib from January 1, 2001, to December 31, 2020. Five patients had duplicate entries because they had different cancer sites. There were 287 patients (74%) with lung cancer, 61 (16%) with pancreatic cancer, and 39 (10%) with other cancers. For lung cancer, there were 118 patients (30%) for the upper lobe, 78 (20%) for the lower lobe, and 60 (16%) not otherwise specified (NOS). Other lung cancer sites had fewer patients: 21 (5%) middle lobe lung, 6 (2%) overlapping lung lesion(s), and 4 (1%) main bronchus of the lung. For pancreatic cancer, there were 27 patients (7%) for the head of the pancreas, 10 (3%) pancreas NOS, 9 (2%) body of the pancreas, 9 (2%) tail of the pancreas, 4 (1%) overlapping lesions of the pancreas, 1 (< 1%) pancreatic duct, and 1 (< 1%) other specified parts of the pancreas
There were 342 patients (88%) who were aged > 50 years; 186 male patients (48%) and 201 female patients (52%). There were 293 patients (76%) who had a cancer diagnosis of stage III or IV disease and 94 (24%) who had a cancer diagnosis of stage ≤ II (combination of data for stage 0, 1, and 2, not applicable, and unknown). For their systemic treatment, 161 patients (42%) were treated with erlotinib alone and 226 (58%) received erlotinib concomitantly with additional chemotherapy.
Patients were more likely to discontinue erlotinib for chemotherapy if they received concomitant treatment. Among the patients receiving erlotinib monotherapy, 5% stopped the treatment, whereas 51% of patients treated concomitantly discontinued (P < .001).
Among the 123 patients who discontinued their treatment, 101 switched treatment with no AEs notes, 22 died or experienced fatigue with blurry vision, constipation, nonspecific gastrointestinal effects, grade-4 diarrhea (as defined by the Common Terminology Criteria for Adverse Events), or developed a pleural fluid, pneumonitis, renal failure, skin swelling and facial rash, and unknown AEs of discontinuation. Patients who discontinued treatment because of unknown AEs had physicians’ notes that detailed emergency department visits, peripheral vascular disease, progressive disease, and treatment cessation, but did not specify the exact symptom(s) that led to discontinuation. The causes of death are unknown because they were not detailed in the available notes or databases. The overall results in this retrospective review cannot establish causality between taking erlotinib or gefitinib and death.
Gefitinib
In September 2021, the Joint Pathology Center provided 33 entries for 33 patients who were systemically treated with gefitinib from January 1, 2002, to December 31, 2017. The patient ages ranged from 49 to 89 years with a mean age of 66 years. There were 31 (94%) and 2 (6%) patients with lung and other cancers, respectively. The upper lobe, lower lobe, and lung NOS had the most patients: 14 (42%), 8 (24%), and 6 (18%), respectively.
There were 31 patients (94%) who were aged > 50 years; 15 were male (45%) and 18 were female (55%). There were 26 patients (79%) who had a cancer diagnosis of stage III or IV disease. Nineteen patients (58%) were treated with gefitinib alone, and 14 (42%) were treated with gefitinib concomitantly with additional chemotherapy. Thirty-one patients (94%) were treated for lung cancer (Table 2). Thirty-three patients are a small sample size to determine whether patients were likely to stop gefitinib if used concomitantly with other drugs. Among the patients treated with gefitinib monotherapy, 5% (n = 1) stopped treatment, whereas 29% (n = 4) of patients treated concomitantly discontinued treatment (P = .06). All comparisons for gefitinib yielded insignificant P values. Physicians’ notes indicated that the reasons for gefitinib discontinuation were life-altering pruritis and unknown (progressive disease outcome) (Table 3).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 348 patients among 415 submitted, with 232 and 112 patients completing and discontinuing erlotinib or gefitinib treatment, respectively. Each patient had 1 to 104 (completed treatment group) and 1 to 157 (discontinued treatment group) unique health conditions documented. The MHS reported 1319 unique-diagnosis conditions for the completed group and 1266 for the discontinued group. Patients with additional health issues stopped chemotherapy use more often than those without; P < .001 for the completed group (232 patients, 1319 diagnoses) vs the discontinued group (112 patients, 1266 diagnoses). The mean (SD) number of diagnoses was 19 (17) for the completed and 30 (22) for the discontinued treatment groups (Figure).
MHS data was provided for patients who filled erlotinib (n = 240) or gefitinib (n = 18). Among the 258 patients, there were 179 and 79 patients in the completed and discontinued treatment groups, respectively. Each patient filled 1 to 75 (for the completed treatment group) and 3 to 103 (for the discontinued treatment group) prescription drugs. There were 805 unique-filled prescriptions for the completed and 670 for the discontinued group. Patients in the discontinued group filled more prescriptions than those who completed treatment; P < .001 for the completed group (179 patients,805 drugs) vs the discontinued group (79 patients, 670 drugs).
The mean (SD) number of filled prescription drugs was 19 (11) for the completed group and 29 (18) for the discontinued treatment group. The 5 most filled prescriptions with erlotinib from 258 patients with PDTS data were ondansetron (151 prescriptions, 10 recorded AEs), dexamethasone (119 prescriptions, 9 recorded AEs), prochlorperazine (105 prescriptions, 15 recorded AEs), oxycodone (99 prescriptions, 1 AE), and docusate (96 prescriptions, 7 recorded AEs).
Discussion
The difference between erlotinib and gefitinib data can be attributed to the FDA approval date and gefitinib’s association with a higher frequency of hepatotoxicity.18-20 The FDA designated gefitinib as an orphan drug for EGFR mutation–positive NSCLC treatment. Gefitinib first received accelerated approval in 2003 for the treatment of locally advanced or metastatic NSCLC. Gefitinib then was voluntarily withdrawn from the market following confirmatory clinical trials that did not verify clinical benefit.
The current approval is for a different patient population—previously untreated, metastatic EGFR exon 19 or 21 L858R mutation—than the 2003 approval.4,6 There was no record of gefitinib use after 2017 in our study.
Erlotinib is a reversible EGFR-TKI that is approved by the FDA as first-line (maintenance) or second-line treatment (after progression following at least 1 earlier chemotherapy regimen) for patients with metastatic NSCLC who harbor EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.3 Since 2005, the FDA also approved erlotinib for first-line treatment of patients with locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine.3 Without FDA indication, erlotinib is used for colorectal, head and neck, ovarian carcinoma, pancreatic carcinoma, and breast cancer.21
Erlotinib and gefitinib are not considered first-line treatments in EGFR exon 19 or 21–mutated NSCLC because osimertinib was approved in 2018. Targeted therapies for EGFR mutation continue to advance at a fast pace, with amivantamab and mobocertinib now FDA approved for EGFR exon 20 insertion–mutated NSCLC.
Erlotinib Use
Thirty-nine patients (10%) in this study were prescribed erlotinib for off-label indications. Erlotinib was used alone or in combination with bevacizumab, capecitabine, cisplatin, denosumab, docetaxel, gemcitabine, and the MEK-inhibitor selumetinib. Erlotinib combined with cisplatin, denosumab, docetaxel, and gemcitabine had no recorded AEs, with 10 data entries for gemcitabine and 1 for other drugs. Three patients received bevacizumab and erlotinib, and 1 patient (diagnosed with kidney NOS) showed rash or facial swelling/erythema and diffuse body itching then stable disease after 2 cycles.
One patient (diagnosed with cancer located at the pancreas head) was bridged with capecitabine and erlotinib when going on a vacation, then received FOLFIRINOX (a combination chemotherapy regimen containing folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin), which led to significant fatigue, blurry vision, and constipation. One patient was treated for lung NOS with the MEK-inhibitor selumetinib plus erlotinib and developed pneumonitis following treatment.
Because oncologists followed guidelines and protocols in systemic treatment, DDIs of erlotinib concurrently (before or after) and in combination with cancer drugs were unlikely. Further investigation is needed for several 1:1:1 DDIs with noncancer drugs. A retrospective overview is not a randomized clinical study; therefore, analysis is limited. Data from the MHS were obtained solely from notes from physicians who treated the patients; therefore, exact information explaining whether a patient completed treatment or had to withdraw could not be extrapolated (ie, blood/plasma samples were not obtained to confirm).
Discontinued Treatment
The reasons for treatment discontinuation with erlotinib or gefitinib varied among patients, with no consistent AE or cause. Most data were for switching treatments after discontinuing treatment with erlotinib (101 of 123 patients) and gefitinib (2 of 5 patients). This is not surprising given the widely recognized pillars of therapy for NSCLC: chemotherapy, target therapy, and immunotherapy.22 From the MHS records, the reasons patients switched treatment of erlotinib or gefitinib were not listed or listed as due to negative EGFR testing, lack of responsiveness, or enrollment in a different treatment.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52
Conclusions
This retrospective study describes erlotinib and gefitinib use in the MHS and their potential for DDIs. Because of military service requirements, people who are qualified to serve must be healthy or have either controlled or nonactive medical diagnoses and be physically fit. Consequently, our patient population had fewer common medical illnesses, such as diabetes and obesity, compared with the general population. Most noncancer drugs mentioned in this study are not known CYP metabolizers; therefore, recorded AEs alone cannot conclusively determine whether there is a DDI among erlotinib or gefitinib and noncancer drugs. Antidepressants generally are safe but have boxed warnings in the US for increased risk of suicidal ideation in young people.53,54 This retrospective study did not find statistically significant DDIs for erlotinib or gefitinib with antidepressants. Based on this retrospective data analysis, future in vitro testing is needed to assess DDIs for erlotinib or gefitinib and cancer or noncancer drugs identified in this study.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Kimberly M. Greenfield, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Lee Ann Zarzabal, Brandon Jenkins, and Alex Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Wesley R. Campbell, CDR Ling Ye, Yaling Zhou, Elizabeth Schafer, Robert Roogow, Micah Stretch, Diane Beaner, Adrienne Woodard, David L. Evers, and Paula Amann.
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
1. van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG. Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315-e326. doi:10.1016/S1470-2045(13)70579-5
2. Xu ZY, Li JL. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019;12:5467-5484. doi:10.2147/OTT.S194870
3. Tarceva (erlotinib). Prescribing Information. Genetech, Astellas Pharma; 2016. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
4. Iressa (gefitinib). Prescribing Information. AstraZeneca; 2018. Accessed June 28, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
5. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303-306. doi:10.1634/theoncologist.8-4-303
6. Cohen MH, Williams GA, Sridhara R, Chen G, et al. United States Food and Drug Administration Drug Approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8. doi:10.1158/1078-0432.ccr-03-0564
7. Fiala O, Pesek M, Finek J, et al. Erlotinib in the treatment of advanced squamous cell NSCLC. Neoplasma. 2013;60(6):676-682. doi:10.4149/neo_2013_086
8. Platania M, Agustoni F, Formisano B, et al. Clinical retrospective analysis of erlotinib in the treatment of elderly patients with advanced non-small cell lung cancer. Target Oncol. 2011;6(3):181-186. doi:10.1007/s11523-011-0185-6
9. Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128-133. doi:10.1016/j.lungcan.2012.02.012
10. Sim EH, Yang IA, Wood-Baker R, Bowman RV, Fong KM. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1(1):CD006847. doi:10.1002/14651858.CD006847.pub2
11. Shrestha S, Joshi P. Gefitinib monotherapy in advanced non-small-cell lung cancer: a retrospective analysis. JNMA J Nepal Med Assoc. 2012;52(186):66-71.
12. Nakamura H, Azuma M, Namisato S, et al. A retrospective study of gefitinib effective cases in non-small cell lung cancer patients with poor performance status. J. Clin. Oncol. 2004 22:14_suppl, 8177-8177. doi:10.1200/jco.2004.22.90140.8177
13. Pui C, Gregory C, Lunqing Z, Long LJ, Tou CH, Hong CT. Retrospective analysis of gefitinib and erlotinib in EGFR-mutated non-small-cell lung cancer patients. J Lung Health Dis. 2017;1(1):16-24. doi:10.29245/2689-999X/2017/1.1105
14. Yoshida T, Yamada K, Azuma K, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol. 2013;30(1):349. doi:10.1007/s12032-012-0349-y
15. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed June 28, 2023. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
16. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 28, 2023. https://apps.who.int/iris/handle/10665/96612
17. Z Score Calculator for 2 population proportions. Social science statistics. Accessed April 25, 2023. https://www.socscistatistics.com/tests/ztest/default2.aspx
18. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74-79. doi:10.1016/j.lungcan.2015.01.026
19. Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist. 2015;20(4):400-410. doi:10.1634/theoncologist.2014-0154
20. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140(12):2805-2819. doi:10.1002/ijc.30691
21. Mack JT. Erlotinib. xPharm: The comprehensive pharmacology reference, 2007. Accessed June 28, 2023. https://www.sciencedirect.com/topics/chemistry/erlotinib
22. Melosky B. Rapidly changing treatment algorithms for metastatic nonsquamous non-small-cell lung cancer. Curr Oncol. 2018;25(suppl 1):S68-S76. doi:10.3747/co.25.3839
23. Xeloda (capecitabine). Prescribing Information. Hoffmann-La Roche, Genetech; 2015. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf
24. Paraplatin (carboplatin). Prescribing Information. Bristol-Myers Squibb; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020452s005lbl.pdf
25. Gemzar (gemcitabine). Prescribing Information. Eli Lilly and Company; 1996. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020509s064lbl.pdf
26. Megace (megestrol). Prescribing Information. Par Pharmaceutical, Bristol-Myers Squibb; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021778s016lbl.pdf
27. Taxol (paclitaxel). Prescribing Information. BASF Aktiengesellschaft, Bristol-Myers Squibb; 2011. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
28. Abraxane (paclitaxel). Prescribing Information. Celgene; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
29. Alima (pemetrexed). Prescribing Information. Sindan Pharma, Actavis Pharma; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208419s000lbl.pdf
30. Tagrisso (Osimertinib). Prescribing Information. AstraZeneca; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
31. Elavil (amitriptyline). Prescribing Information. Sandoz; 2014. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/085966s095,085969s084,085968s096,085971s075,085967s076,085970s072lbl.pdf
32. Lexapro (escitalopram). Prescribing Information. H. Lundbeck, Allergan; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf

33. Remeron (mirtazapine). Prescribing Information. Merck; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
34. Paxil (paroxetine). Prescribing Information. Apotex; 2021. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
35. Desyrel (trazodone). Prescribing Information. Pragma Pharmaceuticals; 2017. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
36. Effexor (venlafaxine). Prescribing Information. Norwich Pharmaceuticals, Almatica Pharma; 2022. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215429s000lbl.pdf
37. Sofran (ondansetron). Prescribing Information. GlaxoSmithKline; 2010. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020007s040,020403s018lbl.pdf
38. Hemady (dexamethasone). Prescribing Information. Dexcel Pharma; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
39. Levaquin (levofloxacin). Prescribing Information. Janssen Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020634s073lbl.pdf
40. Percocet (Oxycodone and Acetaminophen). Prescribing Information. Endo Pharmaceuticals; 2006. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf
41. Docusate Sodium usage information. Spirit Pharmaceuticals; 2010. Accessed June 29, 2023. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=84ee7230-0bf6-4107-b5fa-d6fa265139d0
42. Golytely (polyethylene glycol 3350). Prescribing Information. Sebela Pharmaceuticals; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019011s031lbl.pdf
43. Zithomax (azithromycin). Prescribing Information. Pliva, Pfizer; 2013. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
44. Acetaminophen. Prescribing Information. Fresenius Kabi; 2020. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204767s003lbl.pdf
45. Compazine (prochlorperazine). Prescribing Information. GlaxoSmithKline; 2004. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/010571s096lbl.pdf
46. Rayos (prednisone). Prescribing Information. Horizon Pharma; 2012. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf
47. Cortef (hydrocortisone). Prescribing Information. Pfizer; 2019. Accessed June 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/008697s036lbl.pdf
48. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS-3-HS-19. Accessed June 28, 2023. https://www.uspharmacist.com/article/overview-of-drugdrug-interactions-with-ssris
49. Jin X, Potter B, Luong TL, et al. Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J. 2016;15(1):280. doi:10.1186/s12936-016-1329-z
50. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. doi:10.2165/00003088-200544080-00003
51. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
52. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
53. Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. Published 2014 Jun 18. doi:10.1136/bmj.g359654. Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
Obesity Guidelines
‘Water fasting’ benefits don’t last
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
AGA invests in virtual care clinic Oshi Health
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
specializing in treating patients with gastrointestinal disorders that has been named a recipient of funding through the AGA Center for GI Innovation & Technology’s GI Opportunity Fund.
Launched in 2020, Oshi Health works with employers, health insurance partners, health systems, and community GI practices to scale access to multidisciplinary care, reduce health care costs, and help improve outcomes for patients.
Research shows that a whole-person, multidisciplinary GI care model – which for Oshi includes nutrition and diet support, health coaching, behavioral and mental health services – is highly effective in mitigating symptoms. For example, a 2020 literature review published in the Journal of the Canadian Association of Gastroenterology documented far more advantages with integrated care models, as compared with the GI specialist model of care. The study found that integrated care teams were better equipped to meet the needs of patients with inflammatory bowel disease (IBD), patient outcomes and satisfaction were better, overall direct and indirect costs were lower, and psychological health needs were better addressed.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition, and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process.
For more information about Oshi Health, visit https://oshihealth.com.
AGA president Barbara Jung asks UHC to cease advance notification
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at [email protected] at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at [email protected] at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
for gastrointestinal endoscopy procedures, which took effect June 1, and UnitedHealthcare’s proposed “Gold Card” prior authorization program planned for 2024.
Dr. Jung made three requests: A request for UnitedHealthcare’s deidentified aggregate data on which the Advance Notification program is based.
She asked for clarification in regards to gastroenterologists who opt not to participate in the Advance Notification program. Will they be automatically subject to prior authorization when UnitedHealthcare implements the 2024 Gold Card program?
And, how will information gathered through the Advance Notification program shape the GI Gold Card prior authorization program that UnitedHealthcare plans to implement in 2024?
Dr. Jung asked for a written response to each of these three issues and a meeting to discuss concerns and questions.
She stated that the Advance Notification Program was launched without adequate communication to gastroenterologists, plus the AGA, the American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy have had questions and concerns that haven’t yet been addressed.
“Despite multiple requests, you have not shared any UnitedHealthcare-specific overuse or variation data on a code-by-code basis that would warrant such a burdensome process. Please share the deidentified aggregate data. Absent data, there is no rationale for such a policy. The Advance Notification program directly contradicts UnitedHealthcare’s publicly stated goals of reducing administrative burden and streamlining access to care – goals we support and encourage you to work toward in the gastroenterological specialty. In contrast, Advance Notification is imposing significant administrative burdens on physician practices, which will negatively impact patient access to timely, medically necessary care,” she wrote.
Practice burden
“The chaotic rollout of the new data reporting requirements led to widespread confusion throughout the gastroenterological community and has since forced physicians and staff to spend multiple hours every day completing reporting requirements for data that UnitedHealthcare already has through claims forms. This is a serious drain on gastroenterology practices’ time, staff, and resources – which should be entirely focused on patient care, not reams of paperwork,” Dr. Jung wrote.
AGA members have stated that:
- Most local UHC representatives are unaware of the Advance Notification Program for GI endoscopy program and are unable to advise them regarding concerns or problems.
- Local UHC representatives have no information on the Gold Card program and how it might operate.
Many practices report they have not received any follow-up from UHC requesting additional records via the Advance Notification Program.
Some large GI practices report it takes their staff 5-7 minutes per patient to enter the required data. Others quantify the additional work of participating in the advance notification program as 25%-35% more work than before the program was implemented.
Practices with large UHC volume report having to divert multiple staff to work full-time on UHC accounts.
All practices report that they are required to input/upload the same clinical information as other UHC prior authorizations. Some practices additionally take a screenshot of the statement that the procedure does not require precertification and place it in the patient notes as a precaution in case issues arise in the future.
GI practices that have tried to use the telephone number to report a change in procedure report spending an average of an hour on hold per case.
Dr. Jung said that “given these challenges, many practices are not participating” in the advance notification program.
“AGA is troubled by the serious lack of specific details about the Gold Card prior authorization program to date. With less than 6 months until 2024, UnitedHealthcare has not issued any details about eligibility criteria, participation, or what new prior authorization requirements may be implemented for practices that do not qualify for a Gold Card. We resolutely oppose the implementation of any type of preauthorization requirements for colonoscopies and endoscopies. We are medical practitioners who have years of training and experience treating patients. Our medical decisions are evidence-based, which no prior authorization policy can claim.
“As you recognized when announcing UnitedHealthcare will slash prior authorization requirements by 20% earlier this spring, requiring physicians to apply for and receive preapproval before being able to deliver medically necessary care is not just frustrating – it is disruptive and dangerous for patients’ health. This is particularly true when it comes to performing colonoscopies and endoscopies, which are vital for detecting and monitoring diseases such as inflammatory bowel disease and colorectal cancer, the second deadliest form of cancer in the United States.
“AGA stands ready to partner with UnitedHealthcare on mutually beneficial educational initiatives to promote appropriate use of endoscopy procedures. However, we reiterate our call for UnitedHealthcare to halt the confusing and burdensome Advance Notification Program – and scrap plans to implement a Gold Card prior authorization program as planned in 2024. Instead, we invite UnitedHealthcare to work collaboratively with us to develop programs that improve quality of care without creating barriers to treatment for patients and unnecessary and inappropriate administrative burdens for physicians. We urge you to stop the Advance Notification and any prior authorization programs impacting GI endoscopy and directly engage with AGA to ensure patients’ continued access to high-value, patient-centered endoscopy care. Please contact Leslie Narramore at [email protected] at your earliest convenience so we can resume our dialogue.”
Dr. Jung closed the letter urging UHC to stop the advance notification program and planned prior authorization programs and instead engage in a dialogue with AGA about the issues.
To read Dr. Jung’s letter in full, see https://shorturl.at/dhjyH.
Intrauterine vacuum device treatment of postpartum hemorrhage
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
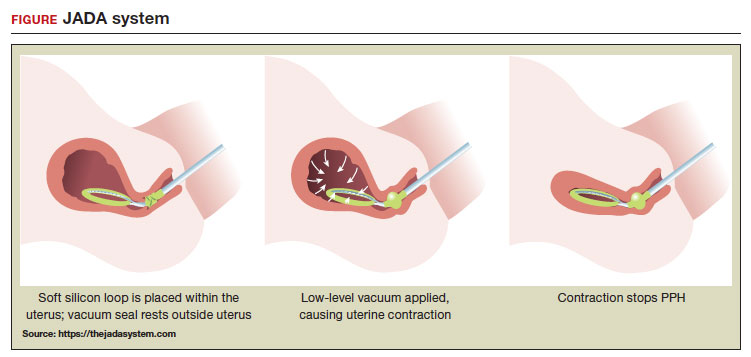 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.