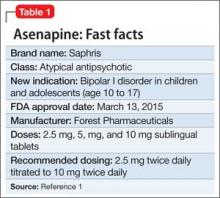
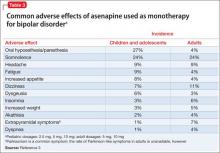
User login
Malaria vaccine may be on the way to approval

Photo by Caitlin Kleiboer
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of the malaria vaccine RTS,S (also known as RTS,S/AS01 or Mosquirix) for use in children ages 6 weeks to 17 months.
RTS,S is the first candidate vaccine for malaria to reach this milestone. The vaccine was designed to prevent malaria caused by the Plasmodium falciparum parasite.
The CHMP’s opinion on RTS,S is a final stage in the European Medicines Agency’s Article 58 procedure. This allows the CHMP, in cooperation with the World Health Organization (WHO), to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as those intended for use in the European Union.
Because the CHMP has issued a positive opinion of RTS,S, the WHO is now formulating a policy recommendation on use of the vaccine in national immunization programs.
The vaccine must also pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in countries in sub-Saharan Africa, where P falciparum malaria is most prevalent.
About RTS,S
RTS,S aims to trigger the body’s immune system to defend against P falciparum when it first enters the human host’s bloodstream and/or when the parasite infects liver cells.
The vaccine is designed to prevent the parasite from infecting, maturing, and multiplying in the liver, after which time the parasite would re-enter the bloodstream and infect red blood cells, leading to disease symptoms.
The safety and efficacy of RTS,S has been evaluated in a large-scale, phase 3 trial. The CHMP’s recommendation was based mainly on the results of this study. Updated results were published in The Lancet last April.
According to that account, the trial included 15,459 young infants (aged 6 weeks to 12 weeks at first vaccination) and children (5 months to 17 months at first vaccination) from 11 sites across 7 sub-Saharan African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and United Republic of Tanzania) with varying levels of malaria transmission.
The subjects received RTS,S in 3 doses, 1 month apart. Some subjects received an additional booster dose 18 months later. Researchers compared subjects receiving RTS,S to those receiving a control vaccine.
Children who received 3 doses of RTS,S plus a booster had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 3 doses of RTS,S plus a booster had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received the booster dose of RTS,S. The vaccine (with or without a booster dose) did not confer the same benefit in infants.
Subjects who received RTS,S had more adverse events than subjects in the control group. This included meningitis and convulsions.
The road to approval
Two of the WHO’s independent advisory groups, the Strategic Advisory Group of Experts (SAGE) on Immunization and the Malaria Policy Advisory Committee (MPAC), are reviewing the evidence base for RTS,S and will make a joint policy recommendation for how it might be used with other tools to prevent malaria if RTS,S is approved by national regulatory authorities in sub-Saharan Africa.
The WHO has indicated that such a policy recommendation may be possible by end of this year.
Once the WHO policy recommendation is complete, GSK (the company developing RTS,S in partnership with the PATH Malaria Vaccine Initiative) will submit an application to the WHO for pre-qualification of RTS,S.
WHO pre-qualification involves a scientific assessment of the quality, safety, and efficacy of any new vaccine proposed for introduction in the WHO Expanded Programme on Immunization. A pre-qualification decision is used by the United Nations agencies and other large-scale public procurement agencies to help inform vaccine purchasing decisions.
Once a WHO pre-qualification is granted, GSK would then apply for marketing authorization in sub-Saharan Africa on a country-by-country basis. These regulatory and policy decisions would, if positive, enable countries to begin using RTS,S through their universal immunization program.
Both a WHO policy recommendation and WHO pre-qualification are requirements for Gavi, the Vaccine Alliance, to support eligible African countries introducing RTS,S into local immunization programs supported by UNICEF.
GSK has committed to a not-for-profit price for RTS,S. If the vaccine is approved, the price would cover the cost of
manufacturing RTS,S and a return of around 5% to be reinvested in
research and development for second-generation malaria vaccines or
vaccines against other neglected tropical diseases. ![]()

Photo by Caitlin Kleiboer
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of the malaria vaccine RTS,S (also known as RTS,S/AS01 or Mosquirix) for use in children ages 6 weeks to 17 months.
RTS,S is the first candidate vaccine for malaria to reach this milestone. The vaccine was designed to prevent malaria caused by the Plasmodium falciparum parasite.
The CHMP’s opinion on RTS,S is a final stage in the European Medicines Agency’s Article 58 procedure. This allows the CHMP, in cooperation with the World Health Organization (WHO), to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as those intended for use in the European Union.
Because the CHMP has issued a positive opinion of RTS,S, the WHO is now formulating a policy recommendation on use of the vaccine in national immunization programs.
The vaccine must also pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in countries in sub-Saharan Africa, where P falciparum malaria is most prevalent.
About RTS,S
RTS,S aims to trigger the body’s immune system to defend against P falciparum when it first enters the human host’s bloodstream and/or when the parasite infects liver cells.
The vaccine is designed to prevent the parasite from infecting, maturing, and multiplying in the liver, after which time the parasite would re-enter the bloodstream and infect red blood cells, leading to disease symptoms.
The safety and efficacy of RTS,S has been evaluated in a large-scale, phase 3 trial. The CHMP’s recommendation was based mainly on the results of this study. Updated results were published in The Lancet last April.
According to that account, the trial included 15,459 young infants (aged 6 weeks to 12 weeks at first vaccination) and children (5 months to 17 months at first vaccination) from 11 sites across 7 sub-Saharan African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and United Republic of Tanzania) with varying levels of malaria transmission.
The subjects received RTS,S in 3 doses, 1 month apart. Some subjects received an additional booster dose 18 months later. Researchers compared subjects receiving RTS,S to those receiving a control vaccine.
Children who received 3 doses of RTS,S plus a booster had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 3 doses of RTS,S plus a booster had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received the booster dose of RTS,S. The vaccine (with or without a booster dose) did not confer the same benefit in infants.
Subjects who received RTS,S had more adverse events than subjects in the control group. This included meningitis and convulsions.
The road to approval
Two of the WHO’s independent advisory groups, the Strategic Advisory Group of Experts (SAGE) on Immunization and the Malaria Policy Advisory Committee (MPAC), are reviewing the evidence base for RTS,S and will make a joint policy recommendation for how it might be used with other tools to prevent malaria if RTS,S is approved by national regulatory authorities in sub-Saharan Africa.
The WHO has indicated that such a policy recommendation may be possible by end of this year.
Once the WHO policy recommendation is complete, GSK (the company developing RTS,S in partnership with the PATH Malaria Vaccine Initiative) will submit an application to the WHO for pre-qualification of RTS,S.
WHO pre-qualification involves a scientific assessment of the quality, safety, and efficacy of any new vaccine proposed for introduction in the WHO Expanded Programme on Immunization. A pre-qualification decision is used by the United Nations agencies and other large-scale public procurement agencies to help inform vaccine purchasing decisions.
Once a WHO pre-qualification is granted, GSK would then apply for marketing authorization in sub-Saharan Africa on a country-by-country basis. These regulatory and policy decisions would, if positive, enable countries to begin using RTS,S through their universal immunization program.
Both a WHO policy recommendation and WHO pre-qualification are requirements for Gavi, the Vaccine Alliance, to support eligible African countries introducing RTS,S into local immunization programs supported by UNICEF.
GSK has committed to a not-for-profit price for RTS,S. If the vaccine is approved, the price would cover the cost of
manufacturing RTS,S and a return of around 5% to be reinvested in
research and development for second-generation malaria vaccines or
vaccines against other neglected tropical diseases. ![]()

Photo by Caitlin Kleiboer
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of the malaria vaccine RTS,S (also known as RTS,S/AS01 or Mosquirix) for use in children ages 6 weeks to 17 months.
RTS,S is the first candidate vaccine for malaria to reach this milestone. The vaccine was designed to prevent malaria caused by the Plasmodium falciparum parasite.
The CHMP’s opinion on RTS,S is a final stage in the European Medicines Agency’s Article 58 procedure. This allows the CHMP, in cooperation with the World Health Organization (WHO), to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as those intended for use in the European Union.
Because the CHMP has issued a positive opinion of RTS,S, the WHO is now formulating a policy recommendation on use of the vaccine in national immunization programs.
The vaccine must also pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in countries in sub-Saharan Africa, where P falciparum malaria is most prevalent.
About RTS,S
RTS,S aims to trigger the body’s immune system to defend against P falciparum when it first enters the human host’s bloodstream and/or when the parasite infects liver cells.
The vaccine is designed to prevent the parasite from infecting, maturing, and multiplying in the liver, after which time the parasite would re-enter the bloodstream and infect red blood cells, leading to disease symptoms.
The safety and efficacy of RTS,S has been evaluated in a large-scale, phase 3 trial. The CHMP’s recommendation was based mainly on the results of this study. Updated results were published in The Lancet last April.
According to that account, the trial included 15,459 young infants (aged 6 weeks to 12 weeks at first vaccination) and children (5 months to 17 months at first vaccination) from 11 sites across 7 sub-Saharan African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and United Republic of Tanzania) with varying levels of malaria transmission.
The subjects received RTS,S in 3 doses, 1 month apart. Some subjects received an additional booster dose 18 months later. Researchers compared subjects receiving RTS,S to those receiving a control vaccine.
Children who received 3 doses of RTS,S plus a booster had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 3 doses of RTS,S plus a booster had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received the booster dose of RTS,S. The vaccine (with or without a booster dose) did not confer the same benefit in infants.
Subjects who received RTS,S had more adverse events than subjects in the control group. This included meningitis and convulsions.
The road to approval
Two of the WHO’s independent advisory groups, the Strategic Advisory Group of Experts (SAGE) on Immunization and the Malaria Policy Advisory Committee (MPAC), are reviewing the evidence base for RTS,S and will make a joint policy recommendation for how it might be used with other tools to prevent malaria if RTS,S is approved by national regulatory authorities in sub-Saharan Africa.
The WHO has indicated that such a policy recommendation may be possible by end of this year.
Once the WHO policy recommendation is complete, GSK (the company developing RTS,S in partnership with the PATH Malaria Vaccine Initiative) will submit an application to the WHO for pre-qualification of RTS,S.
WHO pre-qualification involves a scientific assessment of the quality, safety, and efficacy of any new vaccine proposed for introduction in the WHO Expanded Programme on Immunization. A pre-qualification decision is used by the United Nations agencies and other large-scale public procurement agencies to help inform vaccine purchasing decisions.
Once a WHO pre-qualification is granted, GSK would then apply for marketing authorization in sub-Saharan Africa on a country-by-country basis. These regulatory and policy decisions would, if positive, enable countries to begin using RTS,S through their universal immunization program.
Both a WHO policy recommendation and WHO pre-qualification are requirements for Gavi, the Vaccine Alliance, to support eligible African countries introducing RTS,S into local immunization programs supported by UNICEF.
GSK has committed to a not-for-profit price for RTS,S. If the vaccine is approved, the price would cover the cost of
manufacturing RTS,S and a return of around 5% to be reinvested in
research and development for second-generation malaria vaccines or
vaccines against other neglected tropical diseases. ![]()
Value-based care poses new legal risks for doctors
The government’s push toward value-based care aims to fix a broken reimbursement system and improve quality of care for patients. But the new payment models also bring new legal risks for physicians, experts and anti-fraud officials warn.
“Novel payment methodologies may present new program integrity vulnerabilities,” Dr. Shantanu Agrawal, director of the Center for Program Integrity at the Centers for Medicare & Medicaid Services, said at a recent American Bar Association meeting. “As they assume financial risk, providers are also assuming program integrity risk. Without adequate controls, provider-run systems may be relatively vulnerable.”
The Department of Health and Human Services plans to have 30% of Medicare payments in value-based payment structures by the end of 2016, and 50% by the end of 2018. The transition will be driven through investments in alternative payment models such as Accountable Care Organizations (ACOs), advanced primary care medical home models, bundled payments models, and integrated care demonstrations for Medicare and Medicaid patients.
At the end of 2014, value-based payments represented 20% of Medicare fee-for-service payments to providers, according to CMS data. The rate was fueled by government programs such as the Medicare Shared Savings Program (MSSP), Pioneer ACOs, the Bundled Payments for Care Improvement Initiative, and the Comprehensive Primary Care Initiative. Meanwhile, HHS is encouraging private payers, marketplace plans, Medicare Advantage plans, and state Medicaid programs to move in the same value-based direction.
With so many new regulations, mandates, and programs coming down the pipeline, physicians are likely not thinking about the legal dangers that may arise with alternative payment structures, said Mark S. Kopson, a health law attorney in Bloomfield Hills, Mich., and chair of the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group.
Fee-for-service models can involve claims “about excess treatments and unnecessary services to drive up reimbursement,” Mr. Kopson said in an interview. “When you get into these [value-based] types of programs, it’s the exact opposite. The real threat is the withholding of necessary care in order to reduce expenses and therefore drive up those margins for the providers.”
To avoid such claims, physicians should ensure that their charts include the reasoning behind treatment decisions and a thorough record of why certain treatments were chosen and diagnoses were made, Mr. Kopson advised.
“Going forward, your charting better be completely accurate and detailed so that you don’t leave room for the government to make an argument that you should have provided this or that additional treatment,” he said.
Inaccurate reporting of enrollment data or financial information within new payment models could also land doctors in legal trouble, according to CMS officials.
Problematic reports, enrollee data, or other information physicians are required to submit to the government could be considered falsification and lead to False Claims Act violations.
“Providers are responsible for the information reported and should ensure that the appropriate checks and balances are in place that verify data is reported timely and accurately,” Tony A. Salters, a CMS spokesman, said in an interview. “For some models, providers must attest to the accuracy of this data. [To] report inaccurately could result in violations of federal laws.”
Physician-run payment models, such as doctor-led ACOs, may also draw legal scrutiny if physicians fail to prevent bad behavior by de facto partners. Physicians must ensure that all costs claimed by subcontractors, other providers, and suppliers who are paid from or authorized by the provider-run system, have been validated, Mr. Salters said.
“Doctors need to be aware that other entities who become new partners should hold themselves to the same high standards,” he said. “Providers should have basic financial mechanisms in place, with more sophisticated systems requiring more sophisticated methods,” to ensure validation.
CMS officials recommend doctors conduct independent audits of their accounts, manual validation of record system accuracy, and periodic verification of subcontractor claims to confirm the accuracy of claims and costs within new payment models.
These are “all routine steps that practitioners can take in their own offices but which are even more important when the doctor assumes responsibility for a larger scope of services,” Mr. Salters said.
Gaps in documentation surrounding bundled payments can be another legal land mine, Mr. Kopson noted. Adequate records of the care spectrum are essential to prevent accusations that care was not provided during a single episode of care, or over a specific period of time.
“You have to capture and document all the services you are delivering, and have accurate tracking in place for the entire continuum of care,” Mr. Kopson said.
CMS recommends that physicians establish a strong compliance program to assist with anti-fraud controls of new payment systems. When creating or updating a compliance program, government officials said providers should consider the unique characteristics of the model in which they participate.
On Twitter @legal_med
The government’s push toward value-based care aims to fix a broken reimbursement system and improve quality of care for patients. But the new payment models also bring new legal risks for physicians, experts and anti-fraud officials warn.
“Novel payment methodologies may present new program integrity vulnerabilities,” Dr. Shantanu Agrawal, director of the Center for Program Integrity at the Centers for Medicare & Medicaid Services, said at a recent American Bar Association meeting. “As they assume financial risk, providers are also assuming program integrity risk. Without adequate controls, provider-run systems may be relatively vulnerable.”
The Department of Health and Human Services plans to have 30% of Medicare payments in value-based payment structures by the end of 2016, and 50% by the end of 2018. The transition will be driven through investments in alternative payment models such as Accountable Care Organizations (ACOs), advanced primary care medical home models, bundled payments models, and integrated care demonstrations for Medicare and Medicaid patients.
At the end of 2014, value-based payments represented 20% of Medicare fee-for-service payments to providers, according to CMS data. The rate was fueled by government programs such as the Medicare Shared Savings Program (MSSP), Pioneer ACOs, the Bundled Payments for Care Improvement Initiative, and the Comprehensive Primary Care Initiative. Meanwhile, HHS is encouraging private payers, marketplace plans, Medicare Advantage plans, and state Medicaid programs to move in the same value-based direction.
With so many new regulations, mandates, and programs coming down the pipeline, physicians are likely not thinking about the legal dangers that may arise with alternative payment structures, said Mark S. Kopson, a health law attorney in Bloomfield Hills, Mich., and chair of the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group.
Fee-for-service models can involve claims “about excess treatments and unnecessary services to drive up reimbursement,” Mr. Kopson said in an interview. “When you get into these [value-based] types of programs, it’s the exact opposite. The real threat is the withholding of necessary care in order to reduce expenses and therefore drive up those margins for the providers.”
To avoid such claims, physicians should ensure that their charts include the reasoning behind treatment decisions and a thorough record of why certain treatments were chosen and diagnoses were made, Mr. Kopson advised.
“Going forward, your charting better be completely accurate and detailed so that you don’t leave room for the government to make an argument that you should have provided this or that additional treatment,” he said.
Inaccurate reporting of enrollment data or financial information within new payment models could also land doctors in legal trouble, according to CMS officials.
Problematic reports, enrollee data, or other information physicians are required to submit to the government could be considered falsification and lead to False Claims Act violations.
“Providers are responsible for the information reported and should ensure that the appropriate checks and balances are in place that verify data is reported timely and accurately,” Tony A. Salters, a CMS spokesman, said in an interview. “For some models, providers must attest to the accuracy of this data. [To] report inaccurately could result in violations of federal laws.”
Physician-run payment models, such as doctor-led ACOs, may also draw legal scrutiny if physicians fail to prevent bad behavior by de facto partners. Physicians must ensure that all costs claimed by subcontractors, other providers, and suppliers who are paid from or authorized by the provider-run system, have been validated, Mr. Salters said.
“Doctors need to be aware that other entities who become new partners should hold themselves to the same high standards,” he said. “Providers should have basic financial mechanisms in place, with more sophisticated systems requiring more sophisticated methods,” to ensure validation.
CMS officials recommend doctors conduct independent audits of their accounts, manual validation of record system accuracy, and periodic verification of subcontractor claims to confirm the accuracy of claims and costs within new payment models.
These are “all routine steps that practitioners can take in their own offices but which are even more important when the doctor assumes responsibility for a larger scope of services,” Mr. Salters said.
Gaps in documentation surrounding bundled payments can be another legal land mine, Mr. Kopson noted. Adequate records of the care spectrum are essential to prevent accusations that care was not provided during a single episode of care, or over a specific period of time.
“You have to capture and document all the services you are delivering, and have accurate tracking in place for the entire continuum of care,” Mr. Kopson said.
CMS recommends that physicians establish a strong compliance program to assist with anti-fraud controls of new payment systems. When creating or updating a compliance program, government officials said providers should consider the unique characteristics of the model in which they participate.
On Twitter @legal_med
The government’s push toward value-based care aims to fix a broken reimbursement system and improve quality of care for patients. But the new payment models also bring new legal risks for physicians, experts and anti-fraud officials warn.
“Novel payment methodologies may present new program integrity vulnerabilities,” Dr. Shantanu Agrawal, director of the Center for Program Integrity at the Centers for Medicare & Medicaid Services, said at a recent American Bar Association meeting. “As they assume financial risk, providers are also assuming program integrity risk. Without adequate controls, provider-run systems may be relatively vulnerable.”
The Department of Health and Human Services plans to have 30% of Medicare payments in value-based payment structures by the end of 2016, and 50% by the end of 2018. The transition will be driven through investments in alternative payment models such as Accountable Care Organizations (ACOs), advanced primary care medical home models, bundled payments models, and integrated care demonstrations for Medicare and Medicaid patients.
At the end of 2014, value-based payments represented 20% of Medicare fee-for-service payments to providers, according to CMS data. The rate was fueled by government programs such as the Medicare Shared Savings Program (MSSP), Pioneer ACOs, the Bundled Payments for Care Improvement Initiative, and the Comprehensive Primary Care Initiative. Meanwhile, HHS is encouraging private payers, marketplace plans, Medicare Advantage plans, and state Medicaid programs to move in the same value-based direction.
With so many new regulations, mandates, and programs coming down the pipeline, physicians are likely not thinking about the legal dangers that may arise with alternative payment structures, said Mark S. Kopson, a health law attorney in Bloomfield Hills, Mich., and chair of the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group.
Fee-for-service models can involve claims “about excess treatments and unnecessary services to drive up reimbursement,” Mr. Kopson said in an interview. “When you get into these [value-based] types of programs, it’s the exact opposite. The real threat is the withholding of necessary care in order to reduce expenses and therefore drive up those margins for the providers.”
To avoid such claims, physicians should ensure that their charts include the reasoning behind treatment decisions and a thorough record of why certain treatments were chosen and diagnoses were made, Mr. Kopson advised.
“Going forward, your charting better be completely accurate and detailed so that you don’t leave room for the government to make an argument that you should have provided this or that additional treatment,” he said.
Inaccurate reporting of enrollment data or financial information within new payment models could also land doctors in legal trouble, according to CMS officials.
Problematic reports, enrollee data, or other information physicians are required to submit to the government could be considered falsification and lead to False Claims Act violations.
“Providers are responsible for the information reported and should ensure that the appropriate checks and balances are in place that verify data is reported timely and accurately,” Tony A. Salters, a CMS spokesman, said in an interview. “For some models, providers must attest to the accuracy of this data. [To] report inaccurately could result in violations of federal laws.”
Physician-run payment models, such as doctor-led ACOs, may also draw legal scrutiny if physicians fail to prevent bad behavior by de facto partners. Physicians must ensure that all costs claimed by subcontractors, other providers, and suppliers who are paid from or authorized by the provider-run system, have been validated, Mr. Salters said.
“Doctors need to be aware that other entities who become new partners should hold themselves to the same high standards,” he said. “Providers should have basic financial mechanisms in place, with more sophisticated systems requiring more sophisticated methods,” to ensure validation.
CMS officials recommend doctors conduct independent audits of their accounts, manual validation of record system accuracy, and periodic verification of subcontractor claims to confirm the accuracy of claims and costs within new payment models.
These are “all routine steps that practitioners can take in their own offices but which are even more important when the doctor assumes responsibility for a larger scope of services,” Mr. Salters said.
Gaps in documentation surrounding bundled payments can be another legal land mine, Mr. Kopson noted. Adequate records of the care spectrum are essential to prevent accusations that care was not provided during a single episode of care, or over a specific period of time.
“You have to capture and document all the services you are delivering, and have accurate tracking in place for the entire continuum of care,” Mr. Kopson said.
CMS recommends that physicians establish a strong compliance program to assist with anti-fraud controls of new payment systems. When creating or updating a compliance program, government officials said providers should consider the unique characteristics of the model in which they participate.
On Twitter @legal_med
CHMP recommends eltrombopag for SAA

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for eltrombopag (Revolade) as a treatment for adults with severe aplastic anemia (SAA) who have had an insufficient response to immunosuppressive therapy (IST) and are not eligible to receive a hematopoietic stem cell transplant (HSCT).
If approved by the European Commission, eltrombopag would be the first treatment option in its class for these patients.
The European Commission will review the CHMP recommendation and is expected to deliver its final decision within 3 months. The decision will be applicable to all 28 European Union member states plus Iceland, Norway, and Liechtenstein.
Phase 2 trial of eltrombopag in SAA
The CHMP’s positive opinion of eltrombopag is mainly based on results of a phase 2 trial (NCT00922883). Results from this ongoing study were published in NEJM in 2012 and Blood in 2013.
The trial has enrolled 43 patients with SAA who had an insufficient response to at least one prior IST and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) received at least 2 prior ISTs.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least one lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 6 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments. The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for eltrombopag (Revolade) as a treatment for adults with severe aplastic anemia (SAA) who have had an insufficient response to immunosuppressive therapy (IST) and are not eligible to receive a hematopoietic stem cell transplant (HSCT).
If approved by the European Commission, eltrombopag would be the first treatment option in its class for these patients.
The European Commission will review the CHMP recommendation and is expected to deliver its final decision within 3 months. The decision will be applicable to all 28 European Union member states plus Iceland, Norway, and Liechtenstein.
Phase 2 trial of eltrombopag in SAA
The CHMP’s positive opinion of eltrombopag is mainly based on results of a phase 2 trial (NCT00922883). Results from this ongoing study were published in NEJM in 2012 and Blood in 2013.
The trial has enrolled 43 patients with SAA who had an insufficient response to at least one prior IST and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) received at least 2 prior ISTs.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least one lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 6 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments. The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for eltrombopag (Revolade) as a treatment for adults with severe aplastic anemia (SAA) who have had an insufficient response to immunosuppressive therapy (IST) and are not eligible to receive a hematopoietic stem cell transplant (HSCT).
If approved by the European Commission, eltrombopag would be the first treatment option in its class for these patients.
The European Commission will review the CHMP recommendation and is expected to deliver its final decision within 3 months. The decision will be applicable to all 28 European Union member states plus Iceland, Norway, and Liechtenstein.
Phase 2 trial of eltrombopag in SAA
The CHMP’s positive opinion of eltrombopag is mainly based on results of a phase 2 trial (NCT00922883). Results from this ongoing study were published in NEJM in 2012 and Blood in 2013.
The trial has enrolled 43 patients with SAA who had an insufficient response to at least one prior IST and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) received at least 2 prior ISTs.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least one lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 6 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments. The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()
FDA expands approval of carfilzomib

Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) in combination with lenalidomide and dexamethasone to treat patients with relapsed multiple myeloma (MM) who have received 1 to 3 prior lines of therapy.
Carfilzomib already has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of completing their last treatment.
The FDA’s expanded approval was based on results of the phase 3 ASPIRE trial.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive lenalidomide and dexamethasone with or without carfilzomib for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
There was a statistically significant prolongation of progression-free survival (PFS), as determined by an independent review committee, in the carfilzomib arm (hazard ratio=0.69, P=0.0001). The median PFS was 26.3 months in the 3-drug arm and 17.6 months in the 2-drug arm.
A treatment effect was observed across all subgroups tested, but the magnitude of the treatment effect was reduced in patients with higher tumor burden at baseline. The improvement in median PFS was 11 months for patients with International Staging System (ISS) Stage I disease, 8 months for ISS Stage II, and 2 months for ISS Stage III.
An interim analysis of overall survival, the key secondary endpoint, was conducted at the same time. The difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate (partial response or better) was 87% in the 3-drug arm and 67% in the 2-drug arm.
The safety profile of carfilzomib in the 3-drug combination was similar to that described on the current label.
Cardiovascular events, venous thromboembolic events (VTE), and thrombocytopenia occurred more frequently in the 3-drug arm than in the 2-drug arm. In cycles 1 to 12, the VTE rate was 13% and 6%, respectively, despite protocol-mandated use of thromboprophylaxis.
The revised labeling for carfilzomib includes new Warnings and Precautions for VTE, cardiac toxicities, acute renal failure, pulmonary toxicities, and hypertension. The increased safety risks, including mortality, for elderly patients is described. Detailed safety information in the prescribing information was also updated for the use of carfilzomib monotherapy.
The recommended dose schedule for carfilzomib has been revised for both monotherapy and combination treatment. For details, see the full prescribing information.
Carfilzomib is marketed as Kyprolis in the US by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. ![]()

Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) in combination with lenalidomide and dexamethasone to treat patients with relapsed multiple myeloma (MM) who have received 1 to 3 prior lines of therapy.
Carfilzomib already has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of completing their last treatment.
The FDA’s expanded approval was based on results of the phase 3 ASPIRE trial.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive lenalidomide and dexamethasone with or without carfilzomib for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
There was a statistically significant prolongation of progression-free survival (PFS), as determined by an independent review committee, in the carfilzomib arm (hazard ratio=0.69, P=0.0001). The median PFS was 26.3 months in the 3-drug arm and 17.6 months in the 2-drug arm.
A treatment effect was observed across all subgroups tested, but the magnitude of the treatment effect was reduced in patients with higher tumor burden at baseline. The improvement in median PFS was 11 months for patients with International Staging System (ISS) Stage I disease, 8 months for ISS Stage II, and 2 months for ISS Stage III.
An interim analysis of overall survival, the key secondary endpoint, was conducted at the same time. The difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate (partial response or better) was 87% in the 3-drug arm and 67% in the 2-drug arm.
The safety profile of carfilzomib in the 3-drug combination was similar to that described on the current label.
Cardiovascular events, venous thromboembolic events (VTE), and thrombocytopenia occurred more frequently in the 3-drug arm than in the 2-drug arm. In cycles 1 to 12, the VTE rate was 13% and 6%, respectively, despite protocol-mandated use of thromboprophylaxis.
The revised labeling for carfilzomib includes new Warnings and Precautions for VTE, cardiac toxicities, acute renal failure, pulmonary toxicities, and hypertension. The increased safety risks, including mortality, for elderly patients is described. Detailed safety information in the prescribing information was also updated for the use of carfilzomib monotherapy.
The recommended dose schedule for carfilzomib has been revised for both monotherapy and combination treatment. For details, see the full prescribing information.
Carfilzomib is marketed as Kyprolis in the US by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. ![]()

Photo courtesy of Amgen
The US Food and Drug Administration (FDA) has approved carfilzomib (Kyprolis) in combination with lenalidomide and dexamethasone to treat patients with relapsed multiple myeloma (MM) who have received 1 to 3 prior lines of therapy.
Carfilzomib already has accelerated approval from the FDA as monotherapy for MM patients who have received at least 2 prior therapies, including bortezomib and an immunomodulatory agent, and have demonstrated disease progression on or within 60 days of completing their last treatment.
The FDA’s expanded approval was based on results of the phase 3 ASPIRE trial.
The trial enrolled 792 patients with relapsed or refractory MM who had received 1 to 3 prior lines of therapy. The patients were randomized (1:1) to receive lenalidomide and dexamethasone with or without carfilzomib for 18 cycles.
Lenalidomide and dexamethasone were continued thereafter until disease progression. There was no planned cross-over from the control arm to treatment with carfilzomib.
There was a statistically significant prolongation of progression-free survival (PFS), as determined by an independent review committee, in the carfilzomib arm (hazard ratio=0.69, P=0.0001). The median PFS was 26.3 months in the 3-drug arm and 17.6 months in the 2-drug arm.
A treatment effect was observed across all subgroups tested, but the magnitude of the treatment effect was reduced in patients with higher tumor burden at baseline. The improvement in median PFS was 11 months for patients with International Staging System (ISS) Stage I disease, 8 months for ISS Stage II, and 2 months for ISS Stage III.
An interim analysis of overall survival, the key secondary endpoint, was conducted at the same time. The difference in overall survival did not reach the prespecified boundary for statistical significance.
The overall response rate (partial response or better) was 87% in the 3-drug arm and 67% in the 2-drug arm.
The safety profile of carfilzomib in the 3-drug combination was similar to that described on the current label.
Cardiovascular events, venous thromboembolic events (VTE), and thrombocytopenia occurred more frequently in the 3-drug arm than in the 2-drug arm. In cycles 1 to 12, the VTE rate was 13% and 6%, respectively, despite protocol-mandated use of thromboprophylaxis.
The revised labeling for carfilzomib includes new Warnings and Precautions for VTE, cardiac toxicities, acute renal failure, pulmonary toxicities, and hypertension. The increased safety risks, including mortality, for elderly patients is described. Detailed safety information in the prescribing information was also updated for the use of carfilzomib monotherapy.
The recommended dose schedule for carfilzomib has been revised for both monotherapy and combination treatment. For details, see the full prescribing information.
Carfilzomib is marketed as Kyprolis in the US by Onyx Pharmaceuticals, Inc., an Amgen subsidiary. ![]()
Aromatase Inhibitors, Bisphosphonates Cut Postmenopausal Breast Cancer Recurrence
Aromatase inhibitors and bisphosphonates can improve survival in postmenopausal early-stage breast cancer, and combining the two drug classes can help negate their individual adverse effects, according to two studies published online in The Lancet.
The research offers “the best evidence yet for the effects of aromatase inhibitors and bisphosphonates on postmenopausal women with early breast cancer,” according to a news release by The Lancet that accompanied the reports.
Breast cancer typically occurs after menopause and is usually detected early enough to be operable, but can metastasize years later in bone or other sites if dormant malignant cells become activated, noted researchers from the Early Breast Cancer Trialists’ Collaborative Group, which conducted both meta-analyses.
For the aromatase inhibitor (AI) study, researchers analyzed data from almost 32,000 postmenopausal women with estrogen receptor–positive (ER-positive) early breast cancer who had participated in nine randomized, multiyear trials comparing AIs with standard tamoxifen-based endocrine therapy. Compared with tamoxifen, AI therapy cut the chances of breast cancer recurrence by about 30% during years 0-1 and 2-4 (P less than .001), they reported. “However, in the 2014 ASCO guidelines on endocrine treatment of postmenopausal women with ER-positive early breast cancer, three of the four recommended options start with tamoxifen; a review seems appropriate,” the investigators noted (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)61074-1]). Treatment with AIs also appeared to cut 10-year breast cancer mortality by about 15% compared with tamoxifen, and to lower the risk of dying of breast cancer by about 40% compared with no endocrine therapy, the researchers reported. “The impact of AIs is particularly remarkable given how specific these drugs are – removing only the tiny amount of estrogen that remains in the circulation of women after the menopause – and given the extraordinary molecular differences between ER-positive tumors,” Dr. Mitch Dowsett, the lead author, said in a statement.
“But AI treatment is not free of side effects, and it’s important to ensure that women with significant side effects are supported to try to continue to take treatment and fully benefit from it,” added Dr. Dowsett of The Royal Marsden and The Institute of Cancer Research, both in London.
Because AIs can increase fracture risk, clinicians need to monitor treated patients’ bone health and should consider using bisphosphonates when indicated, Dr. Dowsett and his associates added. The study also linked AIs to a slightly lower rate of endometrial cancer compared with tamoxifen therapy, helping offset the increased fracture risk, they said.
For the second study, investigators analyzed data from more than 18,700 women who had participated in 26 randomized controlled trials of bisphosphonates that assessed breast cancer recurrence, distant metastasis, and mortality. Bisphosphonate treatment did not seem to affect outcomes in premenopausal women, they found. But in postmenopausal patients, treatment led to “highly significant reductions” in local recurrence (risk ratio, 0.86, 95% confidence interval, 0.78 to 0.94; P = .002), distant recurrence (P = .0003), bone recurrence (P = .0002) and breast cancer mortality (P = .002), they reported (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)60908-4]).
Use of bisphosphonates also was tied to a small drop in fracture rates, which was probably real based on studies of other groups of patients, they noted. While bisphosphonates have been used primarily to help prevent bone loss and fractures in postmenopausal women with ER-positive disease who are receiving AIs, the findings show an additional oncological benefit “and suggest that adjuvant bisphosphonates should be considered in a broader range of postmenopausal women,” the researchers concluded. They were unable to assess rates of osteonecrosis of the jaw, but past reports point to rates of about 1% of patients on clodronate, ibandronate, or 6-monthly zoledronic acid therapy, and about 2% of those receiving more intensive zoledronic acid treatment, they noted.
Cancer Research UK and the UK Medical Research Council funded both studies. Ten authors from the AI study and eight authors from the bisphosphonate study reported financial relationships with a number of pharmaceutical companies.
Aromatase inhibitors and bisphosphonates can improve survival in postmenopausal early-stage breast cancer, and combining the two drug classes can help negate their individual adverse effects, according to two studies published online in The Lancet.
The research offers “the best evidence yet for the effects of aromatase inhibitors and bisphosphonates on postmenopausal women with early breast cancer,” according to a news release by The Lancet that accompanied the reports.
Breast cancer typically occurs after menopause and is usually detected early enough to be operable, but can metastasize years later in bone or other sites if dormant malignant cells become activated, noted researchers from the Early Breast Cancer Trialists’ Collaborative Group, which conducted both meta-analyses.
For the aromatase inhibitor (AI) study, researchers analyzed data from almost 32,000 postmenopausal women with estrogen receptor–positive (ER-positive) early breast cancer who had participated in nine randomized, multiyear trials comparing AIs with standard tamoxifen-based endocrine therapy. Compared with tamoxifen, AI therapy cut the chances of breast cancer recurrence by about 30% during years 0-1 and 2-4 (P less than .001), they reported. “However, in the 2014 ASCO guidelines on endocrine treatment of postmenopausal women with ER-positive early breast cancer, three of the four recommended options start with tamoxifen; a review seems appropriate,” the investigators noted (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)61074-1]). Treatment with AIs also appeared to cut 10-year breast cancer mortality by about 15% compared with tamoxifen, and to lower the risk of dying of breast cancer by about 40% compared with no endocrine therapy, the researchers reported. “The impact of AIs is particularly remarkable given how specific these drugs are – removing only the tiny amount of estrogen that remains in the circulation of women after the menopause – and given the extraordinary molecular differences between ER-positive tumors,” Dr. Mitch Dowsett, the lead author, said in a statement.
“But AI treatment is not free of side effects, and it’s important to ensure that women with significant side effects are supported to try to continue to take treatment and fully benefit from it,” added Dr. Dowsett of The Royal Marsden and The Institute of Cancer Research, both in London.
Because AIs can increase fracture risk, clinicians need to monitor treated patients’ bone health and should consider using bisphosphonates when indicated, Dr. Dowsett and his associates added. The study also linked AIs to a slightly lower rate of endometrial cancer compared with tamoxifen therapy, helping offset the increased fracture risk, they said.
For the second study, investigators analyzed data from more than 18,700 women who had participated in 26 randomized controlled trials of bisphosphonates that assessed breast cancer recurrence, distant metastasis, and mortality. Bisphosphonate treatment did not seem to affect outcomes in premenopausal women, they found. But in postmenopausal patients, treatment led to “highly significant reductions” in local recurrence (risk ratio, 0.86, 95% confidence interval, 0.78 to 0.94; P = .002), distant recurrence (P = .0003), bone recurrence (P = .0002) and breast cancer mortality (P = .002), they reported (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)60908-4]).
Use of bisphosphonates also was tied to a small drop in fracture rates, which was probably real based on studies of other groups of patients, they noted. While bisphosphonates have been used primarily to help prevent bone loss and fractures in postmenopausal women with ER-positive disease who are receiving AIs, the findings show an additional oncological benefit “and suggest that adjuvant bisphosphonates should be considered in a broader range of postmenopausal women,” the researchers concluded. They were unable to assess rates of osteonecrosis of the jaw, but past reports point to rates of about 1% of patients on clodronate, ibandronate, or 6-monthly zoledronic acid therapy, and about 2% of those receiving more intensive zoledronic acid treatment, they noted.
Cancer Research UK and the UK Medical Research Council funded both studies. Ten authors from the AI study and eight authors from the bisphosphonate study reported financial relationships with a number of pharmaceutical companies.
Aromatase inhibitors and bisphosphonates can improve survival in postmenopausal early-stage breast cancer, and combining the two drug classes can help negate their individual adverse effects, according to two studies published online in The Lancet.
The research offers “the best evidence yet for the effects of aromatase inhibitors and bisphosphonates on postmenopausal women with early breast cancer,” according to a news release by The Lancet that accompanied the reports.
Breast cancer typically occurs after menopause and is usually detected early enough to be operable, but can metastasize years later in bone or other sites if dormant malignant cells become activated, noted researchers from the Early Breast Cancer Trialists’ Collaborative Group, which conducted both meta-analyses.
For the aromatase inhibitor (AI) study, researchers analyzed data from almost 32,000 postmenopausal women with estrogen receptor–positive (ER-positive) early breast cancer who had participated in nine randomized, multiyear trials comparing AIs with standard tamoxifen-based endocrine therapy. Compared with tamoxifen, AI therapy cut the chances of breast cancer recurrence by about 30% during years 0-1 and 2-4 (P less than .001), they reported. “However, in the 2014 ASCO guidelines on endocrine treatment of postmenopausal women with ER-positive early breast cancer, three of the four recommended options start with tamoxifen; a review seems appropriate,” the investigators noted (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)61074-1]). Treatment with AIs also appeared to cut 10-year breast cancer mortality by about 15% compared with tamoxifen, and to lower the risk of dying of breast cancer by about 40% compared with no endocrine therapy, the researchers reported. “The impact of AIs is particularly remarkable given how specific these drugs are – removing only the tiny amount of estrogen that remains in the circulation of women after the menopause – and given the extraordinary molecular differences between ER-positive tumors,” Dr. Mitch Dowsett, the lead author, said in a statement.
“But AI treatment is not free of side effects, and it’s important to ensure that women with significant side effects are supported to try to continue to take treatment and fully benefit from it,” added Dr. Dowsett of The Royal Marsden and The Institute of Cancer Research, both in London.
Because AIs can increase fracture risk, clinicians need to monitor treated patients’ bone health and should consider using bisphosphonates when indicated, Dr. Dowsett and his associates added. The study also linked AIs to a slightly lower rate of endometrial cancer compared with tamoxifen therapy, helping offset the increased fracture risk, they said.
For the second study, investigators analyzed data from more than 18,700 women who had participated in 26 randomized controlled trials of bisphosphonates that assessed breast cancer recurrence, distant metastasis, and mortality. Bisphosphonate treatment did not seem to affect outcomes in premenopausal women, they found. But in postmenopausal patients, treatment led to “highly significant reductions” in local recurrence (risk ratio, 0.86, 95% confidence interval, 0.78 to 0.94; P = .002), distant recurrence (P = .0003), bone recurrence (P = .0002) and breast cancer mortality (P = .002), they reported (Lancet 2015 July 24 [doi:10.1016/S0140-6736(15)60908-4]).
Use of bisphosphonates also was tied to a small drop in fracture rates, which was probably real based on studies of other groups of patients, they noted. While bisphosphonates have been used primarily to help prevent bone loss and fractures in postmenopausal women with ER-positive disease who are receiving AIs, the findings show an additional oncological benefit “and suggest that adjuvant bisphosphonates should be considered in a broader range of postmenopausal women,” the researchers concluded. They were unable to assess rates of osteonecrosis of the jaw, but past reports point to rates of about 1% of patients on clodronate, ibandronate, or 6-monthly zoledronic acid therapy, and about 2% of those receiving more intensive zoledronic acid treatment, they noted.
Cancer Research UK and the UK Medical Research Council funded both studies. Ten authors from the AI study and eight authors from the bisphosphonate study reported financial relationships with a number of pharmaceutical companies.
FROM THE LANCET
Managing hospitalized methadone–maintained patients
Methadone maintenance therapy is widely used for helping patients recover from an opioid use disorder. When these patients develop an acute medical problem that requires hospitalization, there often is confusion among providers regarding methadone pharmacology, regulations, and general safety issues. We have observed that the lack of awareness of these practices can lead to poor medical and surgical outcomes, increased length of stay, and diminished patient satisfaction.
Consider the following common pitfalls—all of which we have encountered on our psychiatry consult service—and ways to avoid them when treating methadone-maintained patients.
Don’t give a full methadone maintenance dosage without verifying the dosage and the date when it was last administered. Methadone typically has a long, but variable, half-life, with ranges of 4 to 130 hours being reported.1 Do not rush to give the full dose without verification from the patient’s methadone maintenance treatment program (MMTP). Small doses—not to exceed 40 mg in 24 hours—can be administered until you verify the dosage. Multiple days of missed dosing result in decreased tolerance and will require a dosage reduction.
Consult with the MMTP when restarting methadone in a patient who has missed any days of outpatient dosing. Because methadone can take days to reach a serum steady state, it can cause oversedation or obtundation after it’s restarted in a person who has lost tolerance due to multiple consecutive days of missed doses.
Don’t automatically give the full, verified dose if the patient appears sedated. A variety of other substances (benzodiazepines, heroin, tricyclic antidepressants) can increase the effects of methadone. Even the verified methadone maintenance dosage may need to be reduced or held until these other substances are cleared from the patient’s system.
Don’t be afraid to adjust the methadone dosage if medically indicated. Medically hospitalized patients might be placed on medications that can alter methadone metabolism. The primary enzyme responsible for methadone metabolism is cytochrome P450 3A4, which can create significant drug-drug interactions with rifampin, carbamazepine, phenytoin, and barbiturates, among others.2
Don’t taper methadone just because the patient does not want to be on it any longer. A patient’s methadone dosage should be adjusted in the hospital only if there is an acute medical indication to do so. Otherwise, all dosage changes must be made on an outpatient basis at the MMTP.
Don’t be afraid to give opioids to treat acute pain. Methadone maintenance does not treat acute pain. In fact, compared with the general population, these patients likely will need a higher-than-expected opioid dosage to treat acute pain.3
Don’t initiate methadone maintenance in the hospital. Methadone maintenance can be initiated only at an MMTP that has been certified by appropriate federal and state agencies.4 Small doses of methadone can be given to treat or prevent opioid withdrawal in patients admitted to the hospital for conditions other than an opioid use disorder. An exception: A pregnant woman with an opioid use disorder who seeks methadone initiation in the hospital.
Don’t forget to monitor the QTc interval. Methadone can prolong the QTc interval. Although the overall rate of cardiac toxicity is low, it is reasonable to obtain an electrocardiogram in patients with heart disease, those predisposed to prolonged QTc, or those taking another QT-prolonging agent.5
Don’t let negative countertransference prevent you from giving quality care. Patients with a drug addiction can be challenging. They can elicit anger among members of their treatment team because of their character pathology or a provider’s discomfort and unfamiliarity. One might be tempted to spend less time with so-called “difficult” patients, but keep in mind that methadone-maintained patients often carry chaotic medical and social issues that require a thoughtful and thorough approach to treatment.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41(14):1153-1193.
2. Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73-83.
3. Athanasos P, Smith CS, White JM, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120(3):267-275.
4. Heit HA, Covington E, Good PM. Dear DEA. Pain Med. 2004;5(3):303-308.
5. Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306.
Methadone maintenance therapy is widely used for helping patients recover from an opioid use disorder. When these patients develop an acute medical problem that requires hospitalization, there often is confusion among providers regarding methadone pharmacology, regulations, and general safety issues. We have observed that the lack of awareness of these practices can lead to poor medical and surgical outcomes, increased length of stay, and diminished patient satisfaction.
Consider the following common pitfalls—all of which we have encountered on our psychiatry consult service—and ways to avoid them when treating methadone-maintained patients.
Don’t give a full methadone maintenance dosage without verifying the dosage and the date when it was last administered. Methadone typically has a long, but variable, half-life, with ranges of 4 to 130 hours being reported.1 Do not rush to give the full dose without verification from the patient’s methadone maintenance treatment program (MMTP). Small doses—not to exceed 40 mg in 24 hours—can be administered until you verify the dosage. Multiple days of missed dosing result in decreased tolerance and will require a dosage reduction.
Consult with the MMTP when restarting methadone in a patient who has missed any days of outpatient dosing. Because methadone can take days to reach a serum steady state, it can cause oversedation or obtundation after it’s restarted in a person who has lost tolerance due to multiple consecutive days of missed doses.
Don’t automatically give the full, verified dose if the patient appears sedated. A variety of other substances (benzodiazepines, heroin, tricyclic antidepressants) can increase the effects of methadone. Even the verified methadone maintenance dosage may need to be reduced or held until these other substances are cleared from the patient’s system.
Don’t be afraid to adjust the methadone dosage if medically indicated. Medically hospitalized patients might be placed on medications that can alter methadone metabolism. The primary enzyme responsible for methadone metabolism is cytochrome P450 3A4, which can create significant drug-drug interactions with rifampin, carbamazepine, phenytoin, and barbiturates, among others.2
Don’t taper methadone just because the patient does not want to be on it any longer. A patient’s methadone dosage should be adjusted in the hospital only if there is an acute medical indication to do so. Otherwise, all dosage changes must be made on an outpatient basis at the MMTP.
Don’t be afraid to give opioids to treat acute pain. Methadone maintenance does not treat acute pain. In fact, compared with the general population, these patients likely will need a higher-than-expected opioid dosage to treat acute pain.3
Don’t initiate methadone maintenance in the hospital. Methadone maintenance can be initiated only at an MMTP that has been certified by appropriate federal and state agencies.4 Small doses of methadone can be given to treat or prevent opioid withdrawal in patients admitted to the hospital for conditions other than an opioid use disorder. An exception: A pregnant woman with an opioid use disorder who seeks methadone initiation in the hospital.
Don’t forget to monitor the QTc interval. Methadone can prolong the QTc interval. Although the overall rate of cardiac toxicity is low, it is reasonable to obtain an electrocardiogram in patients with heart disease, those predisposed to prolonged QTc, or those taking another QT-prolonging agent.5
Don’t let negative countertransference prevent you from giving quality care. Patients with a drug addiction can be challenging. They can elicit anger among members of their treatment team because of their character pathology or a provider’s discomfort and unfamiliarity. One might be tempted to spend less time with so-called “difficult” patients, but keep in mind that methadone-maintained patients often carry chaotic medical and social issues that require a thoughtful and thorough approach to treatment.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Methadone maintenance therapy is widely used for helping patients recover from an opioid use disorder. When these patients develop an acute medical problem that requires hospitalization, there often is confusion among providers regarding methadone pharmacology, regulations, and general safety issues. We have observed that the lack of awareness of these practices can lead to poor medical and surgical outcomes, increased length of stay, and diminished patient satisfaction.
Consider the following common pitfalls—all of which we have encountered on our psychiatry consult service—and ways to avoid them when treating methadone-maintained patients.
Don’t give a full methadone maintenance dosage without verifying the dosage and the date when it was last administered. Methadone typically has a long, but variable, half-life, with ranges of 4 to 130 hours being reported.1 Do not rush to give the full dose without verification from the patient’s methadone maintenance treatment program (MMTP). Small doses—not to exceed 40 mg in 24 hours—can be administered until you verify the dosage. Multiple days of missed dosing result in decreased tolerance and will require a dosage reduction.
Consult with the MMTP when restarting methadone in a patient who has missed any days of outpatient dosing. Because methadone can take days to reach a serum steady state, it can cause oversedation or obtundation after it’s restarted in a person who has lost tolerance due to multiple consecutive days of missed doses.
Don’t automatically give the full, verified dose if the patient appears sedated. A variety of other substances (benzodiazepines, heroin, tricyclic antidepressants) can increase the effects of methadone. Even the verified methadone maintenance dosage may need to be reduced or held until these other substances are cleared from the patient’s system.
Don’t be afraid to adjust the methadone dosage if medically indicated. Medically hospitalized patients might be placed on medications that can alter methadone metabolism. The primary enzyme responsible for methadone metabolism is cytochrome P450 3A4, which can create significant drug-drug interactions with rifampin, carbamazepine, phenytoin, and barbiturates, among others.2
Don’t taper methadone just because the patient does not want to be on it any longer. A patient’s methadone dosage should be adjusted in the hospital only if there is an acute medical indication to do so. Otherwise, all dosage changes must be made on an outpatient basis at the MMTP.
Don’t be afraid to give opioids to treat acute pain. Methadone maintenance does not treat acute pain. In fact, compared with the general population, these patients likely will need a higher-than-expected opioid dosage to treat acute pain.3
Don’t initiate methadone maintenance in the hospital. Methadone maintenance can be initiated only at an MMTP that has been certified by appropriate federal and state agencies.4 Small doses of methadone can be given to treat or prevent opioid withdrawal in patients admitted to the hospital for conditions other than an opioid use disorder. An exception: A pregnant woman with an opioid use disorder who seeks methadone initiation in the hospital.
Don’t forget to monitor the QTc interval. Methadone can prolong the QTc interval. Although the overall rate of cardiac toxicity is low, it is reasonable to obtain an electrocardiogram in patients with heart disease, those predisposed to prolonged QTc, or those taking another QT-prolonging agent.5
Don’t let negative countertransference prevent you from giving quality care. Patients with a drug addiction can be challenging. They can elicit anger among members of their treatment team because of their character pathology or a provider’s discomfort and unfamiliarity. One might be tempted to spend less time with so-called “difficult” patients, but keep in mind that methadone-maintained patients often carry chaotic medical and social issues that require a thoughtful and thorough approach to treatment.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41(14):1153-1193.
2. Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73-83.
3. Athanasos P, Smith CS, White JM, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120(3):267-275.
4. Heit HA, Covington E, Good PM. Dear DEA. Pain Med. 2004;5(3):303-308.
5. Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306.
1. Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41(14):1153-1193.
2. Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73-83.
3. Athanasos P, Smith CS, White JM, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain. 2006;120(3):267-275.
4. Heit HA, Covington E, Good PM. Dear DEA. Pain Med. 2004;5(3):303-308.
5. Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306.
Give patients a workout in the ‘ego gym’ with mindfulness exercises
Mindfulness has become an important supportive psychotherapeutic intervention for a variety of psychiatric conditions,1-3 regardless of what other modalities the psychiatrist employs (eg, pharmacotherapy, other psychotherapeutic interventions). In general, mindfulness involves engaging in meditation exercises, analogous to working out in the gym, to strengthen “mindfulness muscles.” These exercises increase the patient’s ability to remain in the moment, “as is,” and without judgment.
I think of mindfulness exercises as an “ego gym” for the patient as he (she) gets to exercise the ego functions of agency, attention, awareness, acceptance, and empathy. Advising and helping patients to be present and exist with their thoughts is a psycho-educational approach and form of advice consistent with principles of supportive therapy. In this article, I provide a practical framework for doing and teaching mindfulness using the mnemonic BREATHE.
Flow is more important than sequence
The 7 elements of mindfulness exercises contained in BREATHE do not need to be done in order. Rather, mindfulness generally involves each of the following elements flowing, or tumbling, into each other, not standing as a distinct entity.
Being in the now, “as is,” without judgment (eg, being present/being vs doing; Buddhist origins; diaphragmatic breathing/body scans; “breathing-space” meditation exercises). In general, mindfulness meditation exercises focus on some sensory experience (eg, the physical sensation of breathing or of a difficult emotion, or sounds and smells in the environment). Some mindfulness meditations are called “body scans.”
A patient can shift his (her) focus during mindfulness meditation to a sound or some other stimulus intruding on his original meditative focus, such as an intense emotion or pain, that might arise and become the new focus of mindfulness meditation. Ideally, mindfulness exercises are done without the intention of achieving anything (ie, there is no “striving” for anything when being mindful). Striving, after all, is doing; mindfulness is being.
R(AIN). Mindfulness, as operationalized by Kabat-Zinn,4 starts with a focus on breathing similar to many meditation practices in Buddhism. When the patient wanders into intense emotions, such as suffering, that become the focus of mindfulness, use the mnemonic-within-a-mnemonic RAIN as a guide; typically, this involves first anchoring with a few deep breaths, and then becoming mindful by:
• Recognizing (and labeling, naming, “tagging”) the emotion (eg, sad, hurt, angry, embarrassed); this engages frontal lobe processes that diminishes amygdaloid limbic system overactivity1
• Allowing (ie, accepting suffering)
• Investigating, with an open and curious attitude, using one’s senses to experience, feel, and explore thoughts and emotions
• Non-identifying with one’s thoughts, feelings, emotions, or suffering (expressed in the important mindfulness refrain: “You are not your thoughts or emotions. You are the entity that simply is aware of them.”).
Experiencing. The patient stops at the perceived experience or sensation and does not automatically react with thoughts, emotions, distress, or judgments. Mindfulness is a psychotherapeutic intervention that is “more experiential than cognitive.” Encourage the patient to stop at the “door of experience” and not enter the doors of thinking, emotion, and feeling.
Accepting without judgment—also called “awarenessing” or “avoid avoiding.” This involves being aware of the experience regardless of what it entails, whether suffering, thoughts, emotions, or pain, and not trying to escape or avoid the difficult experience. Psychodynamic principles help us understand how psychological defenses designed to avoid the experience of the “unbearable affect” often lead to more problems for patients. In mindfulness, only avoiding is to be avoided.
Thoughts. People tend to over-identify with their thoughts and emotions. In mindfulness, you emphasize to the patient that (1) he is not his thoughts or emotions and (2) these cognitive processes do not represent facts.
Heartfulness—or, healthy, happy, free from harm. Mindfulness from the Buddhist tradition also includes “heartfulness” and “loving-kindness” and the development of compassion and kindness for one’s self and others. Mindfulness meditation therefore also involves development of loving-kindness/compassion toward oneself and others—even one’s enemies (eg, “May I be healthy, happy, and free from harm.”). I have found this aspect of mindfulness useful for patients who feel angry or entitled, with characterological problems.
Empathy for others. As an extension of, or further emphasis on, loving-kindness, meditation focuses on understanding the suffering of others. In certain monastic practices, this mindfulness meditation involves “taking on” the suffering of another.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lau MA, Grabovac AD. Mindfulness-based interventions: effective for depression and anxiety. Current Psychiatry. 2009;8(12):39,40,45-47,53-55.
2. Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
3. Varghese SP, Koola MM, Eiger RI, et al. Opioid use remits, depression remains. Current Psychiatry. 2014;13(8):45-50.
4. Kabat-Zinn J, Hanh TN. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York, NY: Delta; 1990.
Mindfulness has become an important supportive psychotherapeutic intervention for a variety of psychiatric conditions,1-3 regardless of what other modalities the psychiatrist employs (eg, pharmacotherapy, other psychotherapeutic interventions). In general, mindfulness involves engaging in meditation exercises, analogous to working out in the gym, to strengthen “mindfulness muscles.” These exercises increase the patient’s ability to remain in the moment, “as is,” and without judgment.
I think of mindfulness exercises as an “ego gym” for the patient as he (she) gets to exercise the ego functions of agency, attention, awareness, acceptance, and empathy. Advising and helping patients to be present and exist with their thoughts is a psycho-educational approach and form of advice consistent with principles of supportive therapy. In this article, I provide a practical framework for doing and teaching mindfulness using the mnemonic BREATHE.
Flow is more important than sequence
The 7 elements of mindfulness exercises contained in BREATHE do not need to be done in order. Rather, mindfulness generally involves each of the following elements flowing, or tumbling, into each other, not standing as a distinct entity.
Being in the now, “as is,” without judgment (eg, being present/being vs doing; Buddhist origins; diaphragmatic breathing/body scans; “breathing-space” meditation exercises). In general, mindfulness meditation exercises focus on some sensory experience (eg, the physical sensation of breathing or of a difficult emotion, or sounds and smells in the environment). Some mindfulness meditations are called “body scans.”
A patient can shift his (her) focus during mindfulness meditation to a sound or some other stimulus intruding on his original meditative focus, such as an intense emotion or pain, that might arise and become the new focus of mindfulness meditation. Ideally, mindfulness exercises are done without the intention of achieving anything (ie, there is no “striving” for anything when being mindful). Striving, after all, is doing; mindfulness is being.
R(AIN). Mindfulness, as operationalized by Kabat-Zinn,4 starts with a focus on breathing similar to many meditation practices in Buddhism. When the patient wanders into intense emotions, such as suffering, that become the focus of mindfulness, use the mnemonic-within-a-mnemonic RAIN as a guide; typically, this involves first anchoring with a few deep breaths, and then becoming mindful by:
• Recognizing (and labeling, naming, “tagging”) the emotion (eg, sad, hurt, angry, embarrassed); this engages frontal lobe processes that diminishes amygdaloid limbic system overactivity1
• Allowing (ie, accepting suffering)
• Investigating, with an open and curious attitude, using one’s senses to experience, feel, and explore thoughts and emotions
• Non-identifying with one’s thoughts, feelings, emotions, or suffering (expressed in the important mindfulness refrain: “You are not your thoughts or emotions. You are the entity that simply is aware of them.”).
Experiencing. The patient stops at the perceived experience or sensation and does not automatically react with thoughts, emotions, distress, or judgments. Mindfulness is a psychotherapeutic intervention that is “more experiential than cognitive.” Encourage the patient to stop at the “door of experience” and not enter the doors of thinking, emotion, and feeling.
Accepting without judgment—also called “awarenessing” or “avoid avoiding.” This involves being aware of the experience regardless of what it entails, whether suffering, thoughts, emotions, or pain, and not trying to escape or avoid the difficult experience. Psychodynamic principles help us understand how psychological defenses designed to avoid the experience of the “unbearable affect” often lead to more problems for patients. In mindfulness, only avoiding is to be avoided.
Thoughts. People tend to over-identify with their thoughts and emotions. In mindfulness, you emphasize to the patient that (1) he is not his thoughts or emotions and (2) these cognitive processes do not represent facts.
Heartfulness—or, healthy, happy, free from harm. Mindfulness from the Buddhist tradition also includes “heartfulness” and “loving-kindness” and the development of compassion and kindness for one’s self and others. Mindfulness meditation therefore also involves development of loving-kindness/compassion toward oneself and others—even one’s enemies (eg, “May I be healthy, happy, and free from harm.”). I have found this aspect of mindfulness useful for patients who feel angry or entitled, with characterological problems.
Empathy for others. As an extension of, or further emphasis on, loving-kindness, meditation focuses on understanding the suffering of others. In certain monastic practices, this mindfulness meditation involves “taking on” the suffering of another.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mindfulness has become an important supportive psychotherapeutic intervention for a variety of psychiatric conditions,1-3 regardless of what other modalities the psychiatrist employs (eg, pharmacotherapy, other psychotherapeutic interventions). In general, mindfulness involves engaging in meditation exercises, analogous to working out in the gym, to strengthen “mindfulness muscles.” These exercises increase the patient’s ability to remain in the moment, “as is,” and without judgment.
I think of mindfulness exercises as an “ego gym” for the patient as he (she) gets to exercise the ego functions of agency, attention, awareness, acceptance, and empathy. Advising and helping patients to be present and exist with their thoughts is a psycho-educational approach and form of advice consistent with principles of supportive therapy. In this article, I provide a practical framework for doing and teaching mindfulness using the mnemonic BREATHE.
Flow is more important than sequence
The 7 elements of mindfulness exercises contained in BREATHE do not need to be done in order. Rather, mindfulness generally involves each of the following elements flowing, or tumbling, into each other, not standing as a distinct entity.
Being in the now, “as is,” without judgment (eg, being present/being vs doing; Buddhist origins; diaphragmatic breathing/body scans; “breathing-space” meditation exercises). In general, mindfulness meditation exercises focus on some sensory experience (eg, the physical sensation of breathing or of a difficult emotion, or sounds and smells in the environment). Some mindfulness meditations are called “body scans.”
A patient can shift his (her) focus during mindfulness meditation to a sound or some other stimulus intruding on his original meditative focus, such as an intense emotion or pain, that might arise and become the new focus of mindfulness meditation. Ideally, mindfulness exercises are done without the intention of achieving anything (ie, there is no “striving” for anything when being mindful). Striving, after all, is doing; mindfulness is being.
R(AIN). Mindfulness, as operationalized by Kabat-Zinn,4 starts with a focus on breathing similar to many meditation practices in Buddhism. When the patient wanders into intense emotions, such as suffering, that become the focus of mindfulness, use the mnemonic-within-a-mnemonic RAIN as a guide; typically, this involves first anchoring with a few deep breaths, and then becoming mindful by:
• Recognizing (and labeling, naming, “tagging”) the emotion (eg, sad, hurt, angry, embarrassed); this engages frontal lobe processes that diminishes amygdaloid limbic system overactivity1
• Allowing (ie, accepting suffering)
• Investigating, with an open and curious attitude, using one’s senses to experience, feel, and explore thoughts and emotions
• Non-identifying with one’s thoughts, feelings, emotions, or suffering (expressed in the important mindfulness refrain: “You are not your thoughts or emotions. You are the entity that simply is aware of them.”).
Experiencing. The patient stops at the perceived experience or sensation and does not automatically react with thoughts, emotions, distress, or judgments. Mindfulness is a psychotherapeutic intervention that is “more experiential than cognitive.” Encourage the patient to stop at the “door of experience” and not enter the doors of thinking, emotion, and feeling.
Accepting without judgment—also called “awarenessing” or “avoid avoiding.” This involves being aware of the experience regardless of what it entails, whether suffering, thoughts, emotions, or pain, and not trying to escape or avoid the difficult experience. Psychodynamic principles help us understand how psychological defenses designed to avoid the experience of the “unbearable affect” often lead to more problems for patients. In mindfulness, only avoiding is to be avoided.
Thoughts. People tend to over-identify with their thoughts and emotions. In mindfulness, you emphasize to the patient that (1) he is not his thoughts or emotions and (2) these cognitive processes do not represent facts.
Heartfulness—or, healthy, happy, free from harm. Mindfulness from the Buddhist tradition also includes “heartfulness” and “loving-kindness” and the development of compassion and kindness for one’s self and others. Mindfulness meditation therefore also involves development of loving-kindness/compassion toward oneself and others—even one’s enemies (eg, “May I be healthy, happy, and free from harm.”). I have found this aspect of mindfulness useful for patients who feel angry or entitled, with characterological problems.
Empathy for others. As an extension of, or further emphasis on, loving-kindness, meditation focuses on understanding the suffering of others. In certain monastic practices, this mindfulness meditation involves “taking on” the suffering of another.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lau MA, Grabovac AD. Mindfulness-based interventions: effective for depression and anxiety. Current Psychiatry. 2009;8(12):39,40,45-47,53-55.
2. Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
3. Varghese SP, Koola MM, Eiger RI, et al. Opioid use remits, depression remains. Current Psychiatry. 2014;13(8):45-50.
4. Kabat-Zinn J, Hanh TN. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York, NY: Delta; 1990.
1. Lau MA, Grabovac AD. Mindfulness-based interventions: effective for depression and anxiety. Current Psychiatry. 2009;8(12):39,40,45-47,53-55.
2. Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
3. Varghese SP, Koola MM, Eiger RI, et al. Opioid use remits, depression remains. Current Psychiatry. 2014;13(8):45-50.
4. Kabat-Zinn J, Hanh TN. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York, NY: Delta; 1990.
Asenapine for pediatric bipolar disorder: New indication
Asenapine an atypical antipsychotic sold under the brand name Saphris, was granted a second, pediatric indication by the FDA in March 2015 as monotherapy for acute treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17 (Table 1).1 (Asenapine was first approved in August 2009 as monotherapy or adjunctive therapy to lithium or valproate in adults for schizophrenia and bipolar I disorder.1,2)
Dosage and administration
Asenapine is available as 2.5-, 5-, and 10-mg sublingual tablets, the only atypical antipsychotic with this formulation.1 The recommended dosage for the new indication is 2.5 mg twice daily for 3 days, titrated to 5 mg twice daily, titrated again to 10 mg twice daily after 3 days.3 In a phase I study, pediatric patients appeared to be more sensitive to dystonia when the recommended dosage escalation schedule was not followed.3
In clinical trials, drinking water 2 to 5 minutes after taking asenapine decreased exposure to the drug. Instruct patients not to swallow the tablet and to avoid eating and drinking for 10 minutes after administration.3
For full prescribing information for pediatric and adult patients, see Reference 3.
Safety and efficacy
In a 3-week, placebo-controlled, double-blind trial of 403 patients, 302 children and adolescents age 10 to 17 received asenapine at fixed dosages of 2.5 to 10 mg twice daily; the remainder were given placebo. The Young Mania Rating Scale (YMRS) total score and Clinical Global Impressions Severity of Illness scores of patients who received asenapine improved significantly compared with those who received placebo, as measured by change from baseline to week 3 (Table 2).1
The safety and efficacy of asenapine has not been evaluated in pediatric bipolar disorder patients age ≤10 or pediatric schizophrenia patients age ≤12, or as an adjunctive therapy in pediatric bipolar disorder patients.
Asenapine was not shown to be effective in pediatric patients with schizophrenia in an 8-week, placebo-controlled, double-blind trial.
The pharmacokinetics of asenapine in pediatric patients are similar to those seen in adults.
Adverse effects
In pediatric patients, the most common reported adverse effects of asenapine are:
• dizziness
• dysgeusia
• fatigue
• increased appetite
• increased weight
• nausea
• oral paresthesia
• somnolence.
Similar adverse effects were reported in the pediatric bipolar disorder and adult bipolar disorder clinical trials (Table 3).3 A complete list of reported adverse effects is given in the package insert.3
When treating pediatric patients, monitor the child’s weight gain against expected normal weight gain.
Asenapine is contraindicated in patients with hepatic impairment and those who have a hypersensitivity to asenapine or any components in its formulation.3
1. Actavis receives FDA approval of Saphris for pediatric patients with bipolar I disorder. Drugs.com. http://www.drugs.com/newdrugs/actavis-receivesfda-
approval-saphris-pediatric-patients-bipolardisorder-4188.html. Published March 2015. Accessed June 19, 2015.
2. Lincoln J, Preskon S. Asenapine for schizophrenia and bipolar I disorder. Current Psychiatry. 2009;12(8):75-76,83-85.
3. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2015.
Asenapine an atypical antipsychotic sold under the brand name Saphris, was granted a second, pediatric indication by the FDA in March 2015 as monotherapy for acute treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17 (Table 1).1 (Asenapine was first approved in August 2009 as monotherapy or adjunctive therapy to lithium or valproate in adults for schizophrenia and bipolar I disorder.1,2)
Dosage and administration
Asenapine is available as 2.5-, 5-, and 10-mg sublingual tablets, the only atypical antipsychotic with this formulation.1 The recommended dosage for the new indication is 2.5 mg twice daily for 3 days, titrated to 5 mg twice daily, titrated again to 10 mg twice daily after 3 days.3 In a phase I study, pediatric patients appeared to be more sensitive to dystonia when the recommended dosage escalation schedule was not followed.3
In clinical trials, drinking water 2 to 5 minutes after taking asenapine decreased exposure to the drug. Instruct patients not to swallow the tablet and to avoid eating and drinking for 10 minutes after administration.3
For full prescribing information for pediatric and adult patients, see Reference 3.
Safety and efficacy
In a 3-week, placebo-controlled, double-blind trial of 403 patients, 302 children and adolescents age 10 to 17 received asenapine at fixed dosages of 2.5 to 10 mg twice daily; the remainder were given placebo. The Young Mania Rating Scale (YMRS) total score and Clinical Global Impressions Severity of Illness scores of patients who received asenapine improved significantly compared with those who received placebo, as measured by change from baseline to week 3 (Table 2).1
The safety and efficacy of asenapine has not been evaluated in pediatric bipolar disorder patients age ≤10 or pediatric schizophrenia patients age ≤12, or as an adjunctive therapy in pediatric bipolar disorder patients.
Asenapine was not shown to be effective in pediatric patients with schizophrenia in an 8-week, placebo-controlled, double-blind trial.
The pharmacokinetics of asenapine in pediatric patients are similar to those seen in adults.
Adverse effects
In pediatric patients, the most common reported adverse effects of asenapine are:
• dizziness
• dysgeusia
• fatigue
• increased appetite
• increased weight
• nausea
• oral paresthesia
• somnolence.
Similar adverse effects were reported in the pediatric bipolar disorder and adult bipolar disorder clinical trials (Table 3).3 A complete list of reported adverse effects is given in the package insert.3
When treating pediatric patients, monitor the child’s weight gain against expected normal weight gain.
Asenapine is contraindicated in patients with hepatic impairment and those who have a hypersensitivity to asenapine or any components in its formulation.3
Asenapine an atypical antipsychotic sold under the brand name Saphris, was granted a second, pediatric indication by the FDA in March 2015 as monotherapy for acute treatment of manic or mixed episodes of bipolar I disorder in children and adolescents age 10 to 17 (Table 1).1 (Asenapine was first approved in August 2009 as monotherapy or adjunctive therapy to lithium or valproate in adults for schizophrenia and bipolar I disorder.1,2)
Dosage and administration
Asenapine is available as 2.5-, 5-, and 10-mg sublingual tablets, the only atypical antipsychotic with this formulation.1 The recommended dosage for the new indication is 2.5 mg twice daily for 3 days, titrated to 5 mg twice daily, titrated again to 10 mg twice daily after 3 days.3 In a phase I study, pediatric patients appeared to be more sensitive to dystonia when the recommended dosage escalation schedule was not followed.3
In clinical trials, drinking water 2 to 5 minutes after taking asenapine decreased exposure to the drug. Instruct patients not to swallow the tablet and to avoid eating and drinking for 10 minutes after administration.3
For full prescribing information for pediatric and adult patients, see Reference 3.
Safety and efficacy
In a 3-week, placebo-controlled, double-blind trial of 403 patients, 302 children and adolescents age 10 to 17 received asenapine at fixed dosages of 2.5 to 10 mg twice daily; the remainder were given placebo. The Young Mania Rating Scale (YMRS) total score and Clinical Global Impressions Severity of Illness scores of patients who received asenapine improved significantly compared with those who received placebo, as measured by change from baseline to week 3 (Table 2).1
The safety and efficacy of asenapine has not been evaluated in pediatric bipolar disorder patients age ≤10 or pediatric schizophrenia patients age ≤12, or as an adjunctive therapy in pediatric bipolar disorder patients.
Asenapine was not shown to be effective in pediatric patients with schizophrenia in an 8-week, placebo-controlled, double-blind trial.
The pharmacokinetics of asenapine in pediatric patients are similar to those seen in adults.
Adverse effects
In pediatric patients, the most common reported adverse effects of asenapine are:
• dizziness
• dysgeusia
• fatigue
• increased appetite
• increased weight
• nausea
• oral paresthesia
• somnolence.
Similar adverse effects were reported in the pediatric bipolar disorder and adult bipolar disorder clinical trials (Table 3).3 A complete list of reported adverse effects is given in the package insert.3
When treating pediatric patients, monitor the child’s weight gain against expected normal weight gain.
Asenapine is contraindicated in patients with hepatic impairment and those who have a hypersensitivity to asenapine or any components in its formulation.3
1. Actavis receives FDA approval of Saphris for pediatric patients with bipolar I disorder. Drugs.com. http://www.drugs.com/newdrugs/actavis-receivesfda-
approval-saphris-pediatric-patients-bipolardisorder-4188.html. Published March 2015. Accessed June 19, 2015.
2. Lincoln J, Preskon S. Asenapine for schizophrenia and bipolar I disorder. Current Psychiatry. 2009;12(8):75-76,83-85.
3. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2015.
1. Actavis receives FDA approval of Saphris for pediatric patients with bipolar I disorder. Drugs.com. http://www.drugs.com/newdrugs/actavis-receivesfda-
approval-saphris-pediatric-patients-bipolardisorder-4188.html. Published March 2015. Accessed June 19, 2015.
2. Lincoln J, Preskon S. Asenapine for schizophrenia and bipolar I disorder. Current Psychiatry. 2009;12(8):75-76,83-85.
3. Saphris [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2015.
Young Adult Cancer Survivors Have Higher Rates of Hospitalization
Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health via e-mail. The patients in this population-based study were treated from 1992-1999. "Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology. During this period, 34.3% had at least one hospitalization,
vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51. At all-time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (P<0.0001). Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to
11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20. Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management."
Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health via e-mail. The patients in this population-based study were treated from 1992-1999. "Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology. During this period, 34.3% had at least one hospitalization,
vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51. At all-time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (P<0.0001). Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to
11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20. Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management."
Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health via e-mail. The patients in this population-based study were treated from 1992-1999. "Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology. During this period, 34.3% had at least one hospitalization,
vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51. At all-time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (P<0.0001). Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to
11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20. Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management."
PHM15: A Closer Look at Quality Indicators, Evaluation Tools
Session: Let’s Measure Our Own Performance: Propose and Evaluate Pediatric Hospital Medicine Quality Indicators
Summary: During this workshop, a staff of multiple, nationally-recognized quality leaders led a group to review, help develop, and help validate quality measures. The workshop was facilitated via the use of interactive survey tools, didactic sessions, and small groups.
Presenters discussed why quality measures are important and relevant. These included:
- Improved quality of care,
- Demonstration of value,
- Third-party pay for performance indicators,
- Determining our own indicators (versus being chosen for us), and
- Performance incentives.
As part of the introduction to the workshop, the various quality measure validation methods were reviewed. These consisted of methods such as UCLA/RAND and Delphi Panel, as a means to determine validation and feasibility.
Validation was discussed in terms of what is being measured is the true outcome that was hoped to be achieved. The feasibility component used to make sure that the data used for quality measures, or process to be implemented for improvement, can easily be acquired to determine adherence, and that data is free of error. Facilitators reviewed various examples of validity and feasibility of quality measures with direct examples and discussions with attendees.
During the first breakout session, the groups were separated into teams focused on 1. care transitions, 2. safety, and 3. clinical care. The groups were asked to determine three quality indicators per individual, discuss the top five indicators voted on by the group, and than to review and discuss as a group the validity and feasibility of the measures using a scoring tool of 1-3: Not Valid/Feasible, 4-6: Equivocal, 7-9: Valid/Feasible. At the end, a delegated group speaker was asked to discuss either the pros/cons of one of their measures in regards to validity and feasibility to the total audience. Facilitators assisted on clarifying the reasons of why validity and feasibility metrics were appropriate.
During the final parts of workshop, positive and negatives of quality metrics determination methodology were discussed. The attendees reflected on the process of how quality measures are determined along with how these may be used within their settings.
Key Takeaways
Clearly determining the validity and feasibility of quality metrics for pediatrics has become an important topic. It not only has significant ramifications to the value we provide to our patients, but also the financial sustainability of programs and institutions, especially with the current changes in payment models. The workshop gave a clear and concise way of how to come up with quality metrics and the facilitators greatly added to the understanding of how we can “raise the floor” and “raise the ceiling” of pediatric care. TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.
Session: Let’s Measure Our Own Performance: Propose and Evaluate Pediatric Hospital Medicine Quality Indicators
Summary: During this workshop, a staff of multiple, nationally-recognized quality leaders led a group to review, help develop, and help validate quality measures. The workshop was facilitated via the use of interactive survey tools, didactic sessions, and small groups.
Presenters discussed why quality measures are important and relevant. These included:
- Improved quality of care,
- Demonstration of value,
- Third-party pay for performance indicators,
- Determining our own indicators (versus being chosen for us), and
- Performance incentives.
As part of the introduction to the workshop, the various quality measure validation methods were reviewed. These consisted of methods such as UCLA/RAND and Delphi Panel, as a means to determine validation and feasibility.
Validation was discussed in terms of what is being measured is the true outcome that was hoped to be achieved. The feasibility component used to make sure that the data used for quality measures, or process to be implemented for improvement, can easily be acquired to determine adherence, and that data is free of error. Facilitators reviewed various examples of validity and feasibility of quality measures with direct examples and discussions with attendees.
During the first breakout session, the groups were separated into teams focused on 1. care transitions, 2. safety, and 3. clinical care. The groups were asked to determine three quality indicators per individual, discuss the top five indicators voted on by the group, and than to review and discuss as a group the validity and feasibility of the measures using a scoring tool of 1-3: Not Valid/Feasible, 4-6: Equivocal, 7-9: Valid/Feasible. At the end, a delegated group speaker was asked to discuss either the pros/cons of one of their measures in regards to validity and feasibility to the total audience. Facilitators assisted on clarifying the reasons of why validity and feasibility metrics were appropriate.
During the final parts of workshop, positive and negatives of quality metrics determination methodology were discussed. The attendees reflected on the process of how quality measures are determined along with how these may be used within their settings.
Key Takeaways
Clearly determining the validity and feasibility of quality metrics for pediatrics has become an important topic. It not only has significant ramifications to the value we provide to our patients, but also the financial sustainability of programs and institutions, especially with the current changes in payment models. The workshop gave a clear and concise way of how to come up with quality metrics and the facilitators greatly added to the understanding of how we can “raise the floor” and “raise the ceiling” of pediatric care. TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.
Session: Let’s Measure Our Own Performance: Propose and Evaluate Pediatric Hospital Medicine Quality Indicators
Summary: During this workshop, a staff of multiple, nationally-recognized quality leaders led a group to review, help develop, and help validate quality measures. The workshop was facilitated via the use of interactive survey tools, didactic sessions, and small groups.
Presenters discussed why quality measures are important and relevant. These included:
- Improved quality of care,
- Demonstration of value,
- Third-party pay for performance indicators,
- Determining our own indicators (versus being chosen for us), and
- Performance incentives.
As part of the introduction to the workshop, the various quality measure validation methods were reviewed. These consisted of methods such as UCLA/RAND and Delphi Panel, as a means to determine validation and feasibility.
Validation was discussed in terms of what is being measured is the true outcome that was hoped to be achieved. The feasibility component used to make sure that the data used for quality measures, or process to be implemented for improvement, can easily be acquired to determine adherence, and that data is free of error. Facilitators reviewed various examples of validity and feasibility of quality measures with direct examples and discussions with attendees.
During the first breakout session, the groups were separated into teams focused on 1. care transitions, 2. safety, and 3. clinical care. The groups were asked to determine three quality indicators per individual, discuss the top five indicators voted on by the group, and than to review and discuss as a group the validity and feasibility of the measures using a scoring tool of 1-3: Not Valid/Feasible, 4-6: Equivocal, 7-9: Valid/Feasible. At the end, a delegated group speaker was asked to discuss either the pros/cons of one of their measures in regards to validity and feasibility to the total audience. Facilitators assisted on clarifying the reasons of why validity and feasibility metrics were appropriate.
During the final parts of workshop, positive and negatives of quality metrics determination methodology were discussed. The attendees reflected on the process of how quality measures are determined along with how these may be used within their settings.
Key Takeaways
Clearly determining the validity and feasibility of quality metrics for pediatrics has become an important topic. It not only has significant ramifications to the value we provide to our patients, but also the financial sustainability of programs and institutions, especially with the current changes in payment models. The workshop gave a clear and concise way of how to come up with quality metrics and the facilitators greatly added to the understanding of how we can “raise the floor” and “raise the ceiling” of pediatric care. TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.