User login
PHM15: Challenging Diagnoses, Ethical Dilemmas in Pediatric Immigrant, Refugee Patient Cases
Presenters: Nichole Chandler MD, Suresh Nagappan MD MSPH, Angela Hartsell MD MPH, and Emily Hodnett MD
This workshop focused on interactive cases to highlight healthcare issues specific to the population of refugee/immigrant children in the US. It was an interactive format with small groups to allow the participants to work through the diagnoses by discovery. The cases included a Burmese infant with macrocephaly due to congenital toxoplasmosis and a school-age Eritrean child with abdominal pain due to Plasmodium vivax malaria. In addition to discussing the clinical presentation and work up of the patients, there were accompanying ethical dilemmas that were a part of taking care these patients. The presenters emphasized the importance of culturally sensitive communication and seeking competent translation for key clinical discussions. At the end, they supplied a list of resources for more information on refugees in the US and refugee healthcare.
Bethany Hodge MD MPH
Just for Kids Pediatric Hospitalists, Kosair Children’s Hospital
Assistant professor, Department of Pediatrics
Director of the Distinction in Global Health Track
University of Louisville School of Medicine
Presenters: Nichole Chandler MD, Suresh Nagappan MD MSPH, Angela Hartsell MD MPH, and Emily Hodnett MD
This workshop focused on interactive cases to highlight healthcare issues specific to the population of refugee/immigrant children in the US. It was an interactive format with small groups to allow the participants to work through the diagnoses by discovery. The cases included a Burmese infant with macrocephaly due to congenital toxoplasmosis and a school-age Eritrean child with abdominal pain due to Plasmodium vivax malaria. In addition to discussing the clinical presentation and work up of the patients, there were accompanying ethical dilemmas that were a part of taking care these patients. The presenters emphasized the importance of culturally sensitive communication and seeking competent translation for key clinical discussions. At the end, they supplied a list of resources for more information on refugees in the US and refugee healthcare.
Bethany Hodge MD MPH
Just for Kids Pediatric Hospitalists, Kosair Children’s Hospital
Assistant professor, Department of Pediatrics
Director of the Distinction in Global Health Track
University of Louisville School of Medicine
Presenters: Nichole Chandler MD, Suresh Nagappan MD MSPH, Angela Hartsell MD MPH, and Emily Hodnett MD
This workshop focused on interactive cases to highlight healthcare issues specific to the population of refugee/immigrant children in the US. It was an interactive format with small groups to allow the participants to work through the diagnoses by discovery. The cases included a Burmese infant with macrocephaly due to congenital toxoplasmosis and a school-age Eritrean child with abdominal pain due to Plasmodium vivax malaria. In addition to discussing the clinical presentation and work up of the patients, there were accompanying ethical dilemmas that were a part of taking care these patients. The presenters emphasized the importance of culturally sensitive communication and seeking competent translation for key clinical discussions. At the end, they supplied a list of resources for more information on refugees in the US and refugee healthcare.
Bethany Hodge MD MPH
Just for Kids Pediatric Hospitalists, Kosair Children’s Hospital
Assistant professor, Department of Pediatrics
Director of the Distinction in Global Health Track
University of Louisville School of Medicine
New hope for treating fatal subtype of ALL

Photo by Aaron Logan
Preclinical research has revealed potential therapeutic options for TCF3-HLF-positive acute lymphoblastic leukemia (ALL).
Investigators discovered a range of mutations in this subtype of ALL and identified features that appear to contribute to treatment resistance.
However, the team also found that TCF3-HLF-positive ALL is sensitive to treatment with glucocorticoids, anthracyclines, and certain agents in clinical development.
The BCL2-specific inhibitor venetoclax (ABT-199) proved particularly active against the disease.
Jean-Pierre Bourquin, MD, PhD, of the University Children’s Hospital Zurich in Switzerland, and his colleagues reported these findings in Nature Genetics.
The investigators sequenced samples from patients with TCF3-HLF-positive ALL and found a range of mutations. Most samples (67%) had deletions in PAX5, and most of the samples without PAX5 deletions had deletions in VPREB1.
The team also found recurrent mutations of TCF3, a new fusion gene (KHDRBS1-LCK), and activating mutations in RAS signaling pathway genes (NRAS, KRAS, and PTPN11), among other mutations.
After additional investigation, Dr Bourquin and his colleagues hypothesized that the initiating TCF3-HLF fusion in this disease occurs in a B-cell progenitor, and the specific lineage context is constrained further in a restricted developmental stage by additional mutations.
The investigators also tested various treatments in mouse models of TCF3-HLF-positive ALL. They observed resistance to dasatinib, nucleotide analogs, mitotic spindle inhibitors, and polo-like and aurora kinase inhibitors.
On the other hand, the disease was sensitive to glucocorticoids, mTOR inhibitors, anthracyclines, bortezomib, the HSP90 inhibitor AUY922, and panobinostat.
The team also found evidence suggesting that BCL2 might promote leukemic cell survival and constitute a druggable target in TCF3-HLF-positive ALL. So they tested the BCL2 inhibitor venetoclax in the mice.
A 2-week course of daily venetoclax significantly delayed leukemia progression, and xenografts from relapsed patients and those with minimal residual disease remained sensitive to venetoclax. The drug also exhibited synergistic effects when combined with vincristine or dexamethasone.
“Further studies are now needed to test how the results of our study might be used for therapeutic possibilities,” Dr Bourquin concluded. ![]()

Photo by Aaron Logan
Preclinical research has revealed potential therapeutic options for TCF3-HLF-positive acute lymphoblastic leukemia (ALL).
Investigators discovered a range of mutations in this subtype of ALL and identified features that appear to contribute to treatment resistance.
However, the team also found that TCF3-HLF-positive ALL is sensitive to treatment with glucocorticoids, anthracyclines, and certain agents in clinical development.
The BCL2-specific inhibitor venetoclax (ABT-199) proved particularly active against the disease.
Jean-Pierre Bourquin, MD, PhD, of the University Children’s Hospital Zurich in Switzerland, and his colleagues reported these findings in Nature Genetics.
The investigators sequenced samples from patients with TCF3-HLF-positive ALL and found a range of mutations. Most samples (67%) had deletions in PAX5, and most of the samples without PAX5 deletions had deletions in VPREB1.
The team also found recurrent mutations of TCF3, a new fusion gene (KHDRBS1-LCK), and activating mutations in RAS signaling pathway genes (NRAS, KRAS, and PTPN11), among other mutations.
After additional investigation, Dr Bourquin and his colleagues hypothesized that the initiating TCF3-HLF fusion in this disease occurs in a B-cell progenitor, and the specific lineage context is constrained further in a restricted developmental stage by additional mutations.
The investigators also tested various treatments in mouse models of TCF3-HLF-positive ALL. They observed resistance to dasatinib, nucleotide analogs, mitotic spindle inhibitors, and polo-like and aurora kinase inhibitors.
On the other hand, the disease was sensitive to glucocorticoids, mTOR inhibitors, anthracyclines, bortezomib, the HSP90 inhibitor AUY922, and panobinostat.
The team also found evidence suggesting that BCL2 might promote leukemic cell survival and constitute a druggable target in TCF3-HLF-positive ALL. So they tested the BCL2 inhibitor venetoclax in the mice.
A 2-week course of daily venetoclax significantly delayed leukemia progression, and xenografts from relapsed patients and those with minimal residual disease remained sensitive to venetoclax. The drug also exhibited synergistic effects when combined with vincristine or dexamethasone.
“Further studies are now needed to test how the results of our study might be used for therapeutic possibilities,” Dr Bourquin concluded. ![]()

Photo by Aaron Logan
Preclinical research has revealed potential therapeutic options for TCF3-HLF-positive acute lymphoblastic leukemia (ALL).
Investigators discovered a range of mutations in this subtype of ALL and identified features that appear to contribute to treatment resistance.
However, the team also found that TCF3-HLF-positive ALL is sensitive to treatment with glucocorticoids, anthracyclines, and certain agents in clinical development.
The BCL2-specific inhibitor venetoclax (ABT-199) proved particularly active against the disease.
Jean-Pierre Bourquin, MD, PhD, of the University Children’s Hospital Zurich in Switzerland, and his colleagues reported these findings in Nature Genetics.
The investigators sequenced samples from patients with TCF3-HLF-positive ALL and found a range of mutations. Most samples (67%) had deletions in PAX5, and most of the samples without PAX5 deletions had deletions in VPREB1.
The team also found recurrent mutations of TCF3, a new fusion gene (KHDRBS1-LCK), and activating mutations in RAS signaling pathway genes (NRAS, KRAS, and PTPN11), among other mutations.
After additional investigation, Dr Bourquin and his colleagues hypothesized that the initiating TCF3-HLF fusion in this disease occurs in a B-cell progenitor, and the specific lineage context is constrained further in a restricted developmental stage by additional mutations.
The investigators also tested various treatments in mouse models of TCF3-HLF-positive ALL. They observed resistance to dasatinib, nucleotide analogs, mitotic spindle inhibitors, and polo-like and aurora kinase inhibitors.
On the other hand, the disease was sensitive to glucocorticoids, mTOR inhibitors, anthracyclines, bortezomib, the HSP90 inhibitor AUY922, and panobinostat.
The team also found evidence suggesting that BCL2 might promote leukemic cell survival and constitute a druggable target in TCF3-HLF-positive ALL. So they tested the BCL2 inhibitor venetoclax in the mice.
A 2-week course of daily venetoclax significantly delayed leukemia progression, and xenografts from relapsed patients and those with minimal residual disease remained sensitive to venetoclax. The drug also exhibited synergistic effects when combined with vincristine or dexamethasone.
“Further studies are now needed to test how the results of our study might be used for therapeutic possibilities,” Dr Bourquin concluded. ![]()
Corrected iPSCs fight hemophilia A in mice

Image from the Salk Institute
Researchers say they have found a way to correct the chromosomal inversions that can cause severe hemophilia A.
The team used CRISPR-Cas9 RNA-guided engineered nucleases (RGENs) to correct the inversions and reverse factor VIII (FVIII) deficiency in patient-specific induced pluripotent stem cells (iPSCs).
Once the iPSCs had matured into endothelial cells, the group transplanted those cells into mice with hemophilia A.
This increased FVIII activity in the mice without any off-target effects.
The researchers described this work in Cell Stem Cell.
“We used CRISPR RGENs to repair two recurrent, large chromosomal inversions responsible for almost half of all severe hemophilia A cases,” said Jin-Soo Kim, PhD, of the Institute for Basic Science in Seoul, Korea.
The inversions involve the FVIII intron 1 homolog (responsible for about 5% of severe hemophilia A cases) and the intron 22 homolog (responsible for about 40% of cases).
The researchers first collected urinary cells from patients with these inversions, using the cells to generate iPSCs. The team then applied CRISPR-Cas9 RGENs.
The gene-editing technique effectively repaired the FVIII gene in iPSCs that had harbored either inversion. The researchers then forced the corrected iPSCs to differentiate into mature endothelial cells and found these cells successfully expressed the FVIII protein.
To verify that the endothelial cells could reverse FVIII deficiency, the team transplanted the cells into mice with hemophilia A.
The mice soon began producing FVIII on their own, although the FVIII activity in these mice was 10% that of wild-type mice. The activity was higher than FVIII activity in control mice with hemophilia A (3.3% of wild-type mice), but the difference was not statistically significant.
“To the best of our knowledge, this report is the first demonstration that chromosomal inversions or other large rearrangements can be corrected using RGENs or any other programmable nuclease in patient iPSCs,” said Dong-Wook Kim, PhD, of Yonsei University College of Medicine in Seoul, Korea.
The researchers also noted that there was no evidence of off-target mutations with the 3 RGENS used in this study. ![]()

Image from the Salk Institute
Researchers say they have found a way to correct the chromosomal inversions that can cause severe hemophilia A.
The team used CRISPR-Cas9 RNA-guided engineered nucleases (RGENs) to correct the inversions and reverse factor VIII (FVIII) deficiency in patient-specific induced pluripotent stem cells (iPSCs).
Once the iPSCs had matured into endothelial cells, the group transplanted those cells into mice with hemophilia A.
This increased FVIII activity in the mice without any off-target effects.
The researchers described this work in Cell Stem Cell.
“We used CRISPR RGENs to repair two recurrent, large chromosomal inversions responsible for almost half of all severe hemophilia A cases,” said Jin-Soo Kim, PhD, of the Institute for Basic Science in Seoul, Korea.
The inversions involve the FVIII intron 1 homolog (responsible for about 5% of severe hemophilia A cases) and the intron 22 homolog (responsible for about 40% of cases).
The researchers first collected urinary cells from patients with these inversions, using the cells to generate iPSCs. The team then applied CRISPR-Cas9 RGENs.
The gene-editing technique effectively repaired the FVIII gene in iPSCs that had harbored either inversion. The researchers then forced the corrected iPSCs to differentiate into mature endothelial cells and found these cells successfully expressed the FVIII protein.
To verify that the endothelial cells could reverse FVIII deficiency, the team transplanted the cells into mice with hemophilia A.
The mice soon began producing FVIII on their own, although the FVIII activity in these mice was 10% that of wild-type mice. The activity was higher than FVIII activity in control mice with hemophilia A (3.3% of wild-type mice), but the difference was not statistically significant.
“To the best of our knowledge, this report is the first demonstration that chromosomal inversions or other large rearrangements can be corrected using RGENs or any other programmable nuclease in patient iPSCs,” said Dong-Wook Kim, PhD, of Yonsei University College of Medicine in Seoul, Korea.
The researchers also noted that there was no evidence of off-target mutations with the 3 RGENS used in this study. ![]()

Image from the Salk Institute
Researchers say they have found a way to correct the chromosomal inversions that can cause severe hemophilia A.
The team used CRISPR-Cas9 RNA-guided engineered nucleases (RGENs) to correct the inversions and reverse factor VIII (FVIII) deficiency in patient-specific induced pluripotent stem cells (iPSCs).
Once the iPSCs had matured into endothelial cells, the group transplanted those cells into mice with hemophilia A.
This increased FVIII activity in the mice without any off-target effects.
The researchers described this work in Cell Stem Cell.
“We used CRISPR RGENs to repair two recurrent, large chromosomal inversions responsible for almost half of all severe hemophilia A cases,” said Jin-Soo Kim, PhD, of the Institute for Basic Science in Seoul, Korea.
The inversions involve the FVIII intron 1 homolog (responsible for about 5% of severe hemophilia A cases) and the intron 22 homolog (responsible for about 40% of cases).
The researchers first collected urinary cells from patients with these inversions, using the cells to generate iPSCs. The team then applied CRISPR-Cas9 RGENs.
The gene-editing technique effectively repaired the FVIII gene in iPSCs that had harbored either inversion. The researchers then forced the corrected iPSCs to differentiate into mature endothelial cells and found these cells successfully expressed the FVIII protein.
To verify that the endothelial cells could reverse FVIII deficiency, the team transplanted the cells into mice with hemophilia A.
The mice soon began producing FVIII on their own, although the FVIII activity in these mice was 10% that of wild-type mice. The activity was higher than FVIII activity in control mice with hemophilia A (3.3% of wild-type mice), but the difference was not statistically significant.
“To the best of our knowledge, this report is the first demonstration that chromosomal inversions or other large rearrangements can be corrected using RGENs or any other programmable nuclease in patient iPSCs,” said Dong-Wook Kim, PhD, of Yonsei University College of Medicine in Seoul, Korea.
The researchers also noted that there was no evidence of off-target mutations with the 3 RGENS used in this study. ![]()
Some AEs not reported according to regulations

Photo courtesy of the FDA
A new study indicates that drug manufacturers fail to report about 10% of serious and unexpected adverse events (AEs) within the timeframe set out in federal regulations.
Manufacturers are required to report a serious AE (death, hospitalization, disability, etc.) or unexpected AE (anything not listed in the drug’s label) to the US Food and Drug Administration (FDA) within 15 calendar days of learning about the event.
But an analysis of more than 1.6 million AE reports showed that manufacturers failed to meet this requirement for nearly 10% of AEs.
Pinar Karaca-Mandic, PhD, of the University of Minnesota School of Public Health in Minneapolis, and her colleagues conducted the analysis and detailed the results in JAMA Internal Medicine.
The team examined data from the FDA Adverse Event Reporting System for AE reports made from January 2004 through June 2014. The final study sample included only reports that were subject to the regulation requiring submission within 15 days—a total of 1,613,079 reports.
The researchers found that 9.94% of reports were not received by the FDA within the 15-day timeframe. This was a total of 160,383 reports—40,464 cases in which patients died and 119,919 in which they did not.
So 90.06% of reports (n=1,452,696) were reported within 15 days, 5.28% (n=85,161) were reported within 16 to 90 days, 2.19% (n=35,392) were reported within 91 to 180 days, and 2.47% (n=39,830) were reported after 180 days.
Multivariate analysis revealed that patient death was positively associated with delayed AE reporting. About 91% (90.71%) of AEs that did not involve death were reported within 15 days, compared to 88.25% of AEs that did involve death.
The percentage of reports received within 16 to 90 days was 5.19% for AEs without death and 6.42% for AEs with death. The percentage of reports received within 91 to 180 days was 1.98% and 2.53%, respectively. And the percentage of reports received after 180 days was 2.12% and 2.80%, respectively.
The researchers said perhaps manufacturers spend additional time verifying reports concerning deaths, but this discretion is outside the scope of the current regulatory regime.
In a related Editor’s Note, Rita F. Redberg, MD, editor of JAMA Internal Medicine, wrote that delays in reporting AEs should never occur because they mean exposing patients to serious harm, including death, that could potentially be avoided.
“One improvement would be for AE reports to go directly to the FDA instead of via the manufacturer . . . ,” she wrote. “Physicians and their patients must be knowledgeable of benefits, harms, and alternatives for a wide choice of treatments, especially those recently approved for which clinical experience is limited.” ![]()

Photo courtesy of the FDA
A new study indicates that drug manufacturers fail to report about 10% of serious and unexpected adverse events (AEs) within the timeframe set out in federal regulations.
Manufacturers are required to report a serious AE (death, hospitalization, disability, etc.) or unexpected AE (anything not listed in the drug’s label) to the US Food and Drug Administration (FDA) within 15 calendar days of learning about the event.
But an analysis of more than 1.6 million AE reports showed that manufacturers failed to meet this requirement for nearly 10% of AEs.
Pinar Karaca-Mandic, PhD, of the University of Minnesota School of Public Health in Minneapolis, and her colleagues conducted the analysis and detailed the results in JAMA Internal Medicine.
The team examined data from the FDA Adverse Event Reporting System for AE reports made from January 2004 through June 2014. The final study sample included only reports that were subject to the regulation requiring submission within 15 days—a total of 1,613,079 reports.
The researchers found that 9.94% of reports were not received by the FDA within the 15-day timeframe. This was a total of 160,383 reports—40,464 cases in which patients died and 119,919 in which they did not.
So 90.06% of reports (n=1,452,696) were reported within 15 days, 5.28% (n=85,161) were reported within 16 to 90 days, 2.19% (n=35,392) were reported within 91 to 180 days, and 2.47% (n=39,830) were reported after 180 days.
Multivariate analysis revealed that patient death was positively associated with delayed AE reporting. About 91% (90.71%) of AEs that did not involve death were reported within 15 days, compared to 88.25% of AEs that did involve death.
The percentage of reports received within 16 to 90 days was 5.19% for AEs without death and 6.42% for AEs with death. The percentage of reports received within 91 to 180 days was 1.98% and 2.53%, respectively. And the percentage of reports received after 180 days was 2.12% and 2.80%, respectively.
The researchers said perhaps manufacturers spend additional time verifying reports concerning deaths, but this discretion is outside the scope of the current regulatory regime.
In a related Editor’s Note, Rita F. Redberg, MD, editor of JAMA Internal Medicine, wrote that delays in reporting AEs should never occur because they mean exposing patients to serious harm, including death, that could potentially be avoided.
“One improvement would be for AE reports to go directly to the FDA instead of via the manufacturer . . . ,” she wrote. “Physicians and their patients must be knowledgeable of benefits, harms, and alternatives for a wide choice of treatments, especially those recently approved for which clinical experience is limited.” ![]()

Photo courtesy of the FDA
A new study indicates that drug manufacturers fail to report about 10% of serious and unexpected adverse events (AEs) within the timeframe set out in federal regulations.
Manufacturers are required to report a serious AE (death, hospitalization, disability, etc.) or unexpected AE (anything not listed in the drug’s label) to the US Food and Drug Administration (FDA) within 15 calendar days of learning about the event.
But an analysis of more than 1.6 million AE reports showed that manufacturers failed to meet this requirement for nearly 10% of AEs.
Pinar Karaca-Mandic, PhD, of the University of Minnesota School of Public Health in Minneapolis, and her colleagues conducted the analysis and detailed the results in JAMA Internal Medicine.
The team examined data from the FDA Adverse Event Reporting System for AE reports made from January 2004 through June 2014. The final study sample included only reports that were subject to the regulation requiring submission within 15 days—a total of 1,613,079 reports.
The researchers found that 9.94% of reports were not received by the FDA within the 15-day timeframe. This was a total of 160,383 reports—40,464 cases in which patients died and 119,919 in which they did not.
So 90.06% of reports (n=1,452,696) were reported within 15 days, 5.28% (n=85,161) were reported within 16 to 90 days, 2.19% (n=35,392) were reported within 91 to 180 days, and 2.47% (n=39,830) were reported after 180 days.
Multivariate analysis revealed that patient death was positively associated with delayed AE reporting. About 91% (90.71%) of AEs that did not involve death were reported within 15 days, compared to 88.25% of AEs that did involve death.
The percentage of reports received within 16 to 90 days was 5.19% for AEs without death and 6.42% for AEs with death. The percentage of reports received within 91 to 180 days was 1.98% and 2.53%, respectively. And the percentage of reports received after 180 days was 2.12% and 2.80%, respectively.
The researchers said perhaps manufacturers spend additional time verifying reports concerning deaths, but this discretion is outside the scope of the current regulatory regime.
In a related Editor’s Note, Rita F. Redberg, MD, editor of JAMA Internal Medicine, wrote that delays in reporting AEs should never occur because they mean exposing patients to serious harm, including death, that could potentially be avoided.
“One improvement would be for AE reports to go directly to the FDA instead of via the manufacturer . . . ,” she wrote. “Physicians and their patients must be knowledgeable of benefits, harms, and alternatives for a wide choice of treatments, especially those recently approved for which clinical experience is limited.” ![]()
Improved HSCT outcomes due to conditioning or chemo?

Photo courtesy of NHS
Investigators have reported favorable results of allogeneic hematopoietic stem cell transplant (HSCT) in a small study of patients with juvenile myelomonocytic leukemia (JMML).
The team said the positive outcomes may be a result of conditioning with busulfan and melphalan (BuMel) or the conventional-dose chemotherapy some patients received before HSCT.
Regardless, all 7 patients studied are in remission at more than 1 year of follow-up.
“The lack of transplant-related mortality in the group of children we studied . . . suggests that BuMel may represent a successful HSCT high-dose chemotherapy regimen,” said study author Hisham Abdel-Azim, MD, of Children’s Hospital Los Angeles in California.
“It is also possible that administering conventional-dose chemotherapy before HSCT to patients with more progressive disease may have contributed to the improved outcomes.”
Dr Abdel-Azim and his colleagues described this research in a letter to Blood.
Conventional chemo and transplant
The investigators retrospectively analyzed 7 JMML patients with a median age of 2.6 years at HSCT.
Five patients received conventional-dose chemotherapy before transplant. All of these patients received mercaptopurine. One received hydroxyurea as well, and another patient received fludarabine, cytarabine, and cis-retinoic acid.
As for transplant, 2 patients received a 10/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched unrelated bone marrow graft, and 3 patients received cord blood grafts.
The median total nucleated cell count was 4.2 × 108 cells/kg, and the median CD34 cell dose was 3.3 × 106 cells/kg.
Conditioning and GVHD prophylaxis
All 7 patients received backbone conditioning with BuMel: Bu at 1 mg/kg dose every 6 hours intravenously on days −8 to −5 (with therapeutic drug monitoring to achieve overall concentration steady state [CSS] of 800-1000 ng/mL) and Mel at 45 mg/m2 per day intravenously on days −4 to −2.
The median Bu CSS and area under the curve were 884 µg/L (range, 560-1096) and 1293 µmol/L-minute (range, 819-1601), respectively.
The patient with a 9/10 HLA-matched related graft received BuMel and fludarabine at 35 mg/m2 per day intravenously on days −7 to −4.
The patient with the 9/10 HLA-matched unrelated graft received BuMel and alemtuzumab at 12 mg/m2 intravenously on day −10 and 20 mg/m2 on day −9, with methylprednisolone at 2 mg/kg per day in divided doses during the alemtuzumab infusion.
The patients who received cord blood grafts received BuMel and rabbit antithymocyte globulin at 2.5 mg/kg per day intravenously on days −4 to −1. They also received methylprednisolone at 2 mg/kg per day in divided doses during antithymocyte globulin infusion, then tapered over 6 weeks.
All patients received tacrolimus as graft-vs-host disease (GVHD) prophylaxis. Patients who received bone marrow grafts also received methotrexate at 5 mg/m2 on days 3, 6, and 11.
Outcomes
The median time to neutrophil engraftment (≥500/mm3) was 20 days, and the median time to platelet engraftment (≥20 000/mm3) was 36 days.
Six patients (85.7%) achieved predominant (>95%) donor hematopoietic stem cell engraftment.
One patient who received a cord blood graft had autologous recovery at day 54. She went on to receive a related haploidentical HSCT on day 105. One hundred days later, she is in remission, with predominant donor chimerism.
The patient who received the 9/10 HLA-matched related graft developed grade 4 acute GVHD, followed by severe chronic GVHD that required bowel resection.
This patient and one of the patients who received a 10/10 HLA-matched related graft developed severe sinusoidal obstructive syndrome, which resolved with supportive care.
At a median follow-up of 25.3 months (range, 6-99.3), all 7 patients are in remission.
The investigators said their target Bu CSS may have contributed to the improved outcomes they observed, or pre-HSCT chemotherapy may have been a contributing factor. A prospective clinical trial could provide answers. ![]()

Photo courtesy of NHS
Investigators have reported favorable results of allogeneic hematopoietic stem cell transplant (HSCT) in a small study of patients with juvenile myelomonocytic leukemia (JMML).
The team said the positive outcomes may be a result of conditioning with busulfan and melphalan (BuMel) or the conventional-dose chemotherapy some patients received before HSCT.
Regardless, all 7 patients studied are in remission at more than 1 year of follow-up.
“The lack of transplant-related mortality in the group of children we studied . . . suggests that BuMel may represent a successful HSCT high-dose chemotherapy regimen,” said study author Hisham Abdel-Azim, MD, of Children’s Hospital Los Angeles in California.
“It is also possible that administering conventional-dose chemotherapy before HSCT to patients with more progressive disease may have contributed to the improved outcomes.”
Dr Abdel-Azim and his colleagues described this research in a letter to Blood.
Conventional chemo and transplant
The investigators retrospectively analyzed 7 JMML patients with a median age of 2.6 years at HSCT.
Five patients received conventional-dose chemotherapy before transplant. All of these patients received mercaptopurine. One received hydroxyurea as well, and another patient received fludarabine, cytarabine, and cis-retinoic acid.
As for transplant, 2 patients received a 10/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched unrelated bone marrow graft, and 3 patients received cord blood grafts.
The median total nucleated cell count was 4.2 × 108 cells/kg, and the median CD34 cell dose was 3.3 × 106 cells/kg.
Conditioning and GVHD prophylaxis
All 7 patients received backbone conditioning with BuMel: Bu at 1 mg/kg dose every 6 hours intravenously on days −8 to −5 (with therapeutic drug monitoring to achieve overall concentration steady state [CSS] of 800-1000 ng/mL) and Mel at 45 mg/m2 per day intravenously on days −4 to −2.
The median Bu CSS and area under the curve were 884 µg/L (range, 560-1096) and 1293 µmol/L-minute (range, 819-1601), respectively.
The patient with a 9/10 HLA-matched related graft received BuMel and fludarabine at 35 mg/m2 per day intravenously on days −7 to −4.
The patient with the 9/10 HLA-matched unrelated graft received BuMel and alemtuzumab at 12 mg/m2 intravenously on day −10 and 20 mg/m2 on day −9, with methylprednisolone at 2 mg/kg per day in divided doses during the alemtuzumab infusion.
The patients who received cord blood grafts received BuMel and rabbit antithymocyte globulin at 2.5 mg/kg per day intravenously on days −4 to −1. They also received methylprednisolone at 2 mg/kg per day in divided doses during antithymocyte globulin infusion, then tapered over 6 weeks.
All patients received tacrolimus as graft-vs-host disease (GVHD) prophylaxis. Patients who received bone marrow grafts also received methotrexate at 5 mg/m2 on days 3, 6, and 11.
Outcomes
The median time to neutrophil engraftment (≥500/mm3) was 20 days, and the median time to platelet engraftment (≥20 000/mm3) was 36 days.
Six patients (85.7%) achieved predominant (>95%) donor hematopoietic stem cell engraftment.
One patient who received a cord blood graft had autologous recovery at day 54. She went on to receive a related haploidentical HSCT on day 105. One hundred days later, she is in remission, with predominant donor chimerism.
The patient who received the 9/10 HLA-matched related graft developed grade 4 acute GVHD, followed by severe chronic GVHD that required bowel resection.
This patient and one of the patients who received a 10/10 HLA-matched related graft developed severe sinusoidal obstructive syndrome, which resolved with supportive care.
At a median follow-up of 25.3 months (range, 6-99.3), all 7 patients are in remission.
The investigators said their target Bu CSS may have contributed to the improved outcomes they observed, or pre-HSCT chemotherapy may have been a contributing factor. A prospective clinical trial could provide answers. ![]()

Photo courtesy of NHS
Investigators have reported favorable results of allogeneic hematopoietic stem cell transplant (HSCT) in a small study of patients with juvenile myelomonocytic leukemia (JMML).
The team said the positive outcomes may be a result of conditioning with busulfan and melphalan (BuMel) or the conventional-dose chemotherapy some patients received before HSCT.
Regardless, all 7 patients studied are in remission at more than 1 year of follow-up.
“The lack of transplant-related mortality in the group of children we studied . . . suggests that BuMel may represent a successful HSCT high-dose chemotherapy regimen,” said study author Hisham Abdel-Azim, MD, of Children’s Hospital Los Angeles in California.
“It is also possible that administering conventional-dose chemotherapy before HSCT to patients with more progressive disease may have contributed to the improved outcomes.”
Dr Abdel-Azim and his colleagues described this research in a letter to Blood.
Conventional chemo and transplant
The investigators retrospectively analyzed 7 JMML patients with a median age of 2.6 years at HSCT.
Five patients received conventional-dose chemotherapy before transplant. All of these patients received mercaptopurine. One received hydroxyurea as well, and another patient received fludarabine, cytarabine, and cis-retinoic acid.
As for transplant, 2 patients received a 10/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched related bone marrow graft, 1 received a 9/10 HLA-matched unrelated bone marrow graft, and 3 patients received cord blood grafts.
The median total nucleated cell count was 4.2 × 108 cells/kg, and the median CD34 cell dose was 3.3 × 106 cells/kg.
Conditioning and GVHD prophylaxis
All 7 patients received backbone conditioning with BuMel: Bu at 1 mg/kg dose every 6 hours intravenously on days −8 to −5 (with therapeutic drug monitoring to achieve overall concentration steady state [CSS] of 800-1000 ng/mL) and Mel at 45 mg/m2 per day intravenously on days −4 to −2.
The median Bu CSS and area under the curve were 884 µg/L (range, 560-1096) and 1293 µmol/L-minute (range, 819-1601), respectively.
The patient with a 9/10 HLA-matched related graft received BuMel and fludarabine at 35 mg/m2 per day intravenously on days −7 to −4.
The patient with the 9/10 HLA-matched unrelated graft received BuMel and alemtuzumab at 12 mg/m2 intravenously on day −10 and 20 mg/m2 on day −9, with methylprednisolone at 2 mg/kg per day in divided doses during the alemtuzumab infusion.
The patients who received cord blood grafts received BuMel and rabbit antithymocyte globulin at 2.5 mg/kg per day intravenously on days −4 to −1. They also received methylprednisolone at 2 mg/kg per day in divided doses during antithymocyte globulin infusion, then tapered over 6 weeks.
All patients received tacrolimus as graft-vs-host disease (GVHD) prophylaxis. Patients who received bone marrow grafts also received methotrexate at 5 mg/m2 on days 3, 6, and 11.
Outcomes
The median time to neutrophil engraftment (≥500/mm3) was 20 days, and the median time to platelet engraftment (≥20 000/mm3) was 36 days.
Six patients (85.7%) achieved predominant (>95%) donor hematopoietic stem cell engraftment.
One patient who received a cord blood graft had autologous recovery at day 54. She went on to receive a related haploidentical HSCT on day 105. One hundred days later, she is in remission, with predominant donor chimerism.
The patient who received the 9/10 HLA-matched related graft developed grade 4 acute GVHD, followed by severe chronic GVHD that required bowel resection.
This patient and one of the patients who received a 10/10 HLA-matched related graft developed severe sinusoidal obstructive syndrome, which resolved with supportive care.
At a median follow-up of 25.3 months (range, 6-99.3), all 7 patients are in remission.
The investigators said their target Bu CSS may have contributed to the improved outcomes they observed, or pre-HSCT chemotherapy may have been a contributing factor. A prospective clinical trial could provide answers. ![]()
Small Bowel Block in Elderly Merits Full Hospitalization
NEW YORK (Reuters Health) - The "vast majority" of elderly patients admitted with small bowel obstruction (SBO) are hospitalized for more than two days, and the diagnosis alone should allow appropriate Medicare coverage, according to a new study.
In a paper online July 1 in Annals of Surgery, Dr. Zara Cooper, of Brigham and Women's Hospital, Boston, and colleagues noted that their study was prompted by the Two-Midnight Rule established by the Centers for Medicare & Medicaid Services (CMS) in 2013.
The authors explained that if a physician expects a patient to need a hospital stay that crosses two midnights and thus admits the patient, related costs may be covered. However, shorter stays are deemed as observational and can raise the possibility of non-reimbursement for hospitals.
For example, if someone is admitted as an inpatient, but discharged in less than two days, payment will be made only if it can be documented that a longer stay was reasonably expected and unforeseen circumstances led to the shorter stay. Hospital stays that are incorrectly classified or have improperly documented changes in admission status will not be paid.
However, Dr. Cooper told Reuters Health by email, "Older patients with SBO, a very common diagnosis, should be presumed to be admitted for more than two midnights and hospitals should not get penalized."
She and her colleagues pointed out that SBO accounts for about 15% of surgical admissions to U.S. hospitals and more than $1 billion in annual hospital charges. However, diagnosis requires surgeons to observe patients to determine if surgery is warranted.
Thus, the authors wrote, "It is critically important for surgeons to correctly assign admission status for patients with SBO to ensure that hospitals are reimbursed appropriately, and patients are not unduly burdened."
The investigators examined data on 855 older patients admitted with SBO from 2006 and 2013. Of these, 816 (95%) stayed for two midnights or longer. This was true of all patients aged 85 years or older (n=108, approximately 13%).
The only significant difference in clinical characteristics was the presence of inflammatory bowel disease. Of five such patients, only one stayed for less than two midnights.
"Based on our study and others," the investigators wrote, "we propose that hospital admission for SBO in elderly patients is sufficient justification for the reasonable expectation" of the required length of stay for reimbursement.
This also may be true of other conditions. Dr. Cooper concluded, "More studies like this are needed in surgical patients to better understand the impact of CMS admission guidelines. The rule may not make sense in certain populations, leading to heavy and unfair penalties for hospitals."
The authors reported no disclosures.
NEW YORK (Reuters Health) - The "vast majority" of elderly patients admitted with small bowel obstruction (SBO) are hospitalized for more than two days, and the diagnosis alone should allow appropriate Medicare coverage, according to a new study.
In a paper online July 1 in Annals of Surgery, Dr. Zara Cooper, of Brigham and Women's Hospital, Boston, and colleagues noted that their study was prompted by the Two-Midnight Rule established by the Centers for Medicare & Medicaid Services (CMS) in 2013.
The authors explained that if a physician expects a patient to need a hospital stay that crosses two midnights and thus admits the patient, related costs may be covered. However, shorter stays are deemed as observational and can raise the possibility of non-reimbursement for hospitals.
For example, if someone is admitted as an inpatient, but discharged in less than two days, payment will be made only if it can be documented that a longer stay was reasonably expected and unforeseen circumstances led to the shorter stay. Hospital stays that are incorrectly classified or have improperly documented changes in admission status will not be paid.
However, Dr. Cooper told Reuters Health by email, "Older patients with SBO, a very common diagnosis, should be presumed to be admitted for more than two midnights and hospitals should not get penalized."
She and her colleagues pointed out that SBO accounts for about 15% of surgical admissions to U.S. hospitals and more than $1 billion in annual hospital charges. However, diagnosis requires surgeons to observe patients to determine if surgery is warranted.
Thus, the authors wrote, "It is critically important for surgeons to correctly assign admission status for patients with SBO to ensure that hospitals are reimbursed appropriately, and patients are not unduly burdened."
The investigators examined data on 855 older patients admitted with SBO from 2006 and 2013. Of these, 816 (95%) stayed for two midnights or longer. This was true of all patients aged 85 years or older (n=108, approximately 13%).
The only significant difference in clinical characteristics was the presence of inflammatory bowel disease. Of five such patients, only one stayed for less than two midnights.
"Based on our study and others," the investigators wrote, "we propose that hospital admission for SBO in elderly patients is sufficient justification for the reasonable expectation" of the required length of stay for reimbursement.
This also may be true of other conditions. Dr. Cooper concluded, "More studies like this are needed in surgical patients to better understand the impact of CMS admission guidelines. The rule may not make sense in certain populations, leading to heavy and unfair penalties for hospitals."
The authors reported no disclosures.
NEW YORK (Reuters Health) - The "vast majority" of elderly patients admitted with small bowel obstruction (SBO) are hospitalized for more than two days, and the diagnosis alone should allow appropriate Medicare coverage, according to a new study.
In a paper online July 1 in Annals of Surgery, Dr. Zara Cooper, of Brigham and Women's Hospital, Boston, and colleagues noted that their study was prompted by the Two-Midnight Rule established by the Centers for Medicare & Medicaid Services (CMS) in 2013.
The authors explained that if a physician expects a patient to need a hospital stay that crosses two midnights and thus admits the patient, related costs may be covered. However, shorter stays are deemed as observational and can raise the possibility of non-reimbursement for hospitals.
For example, if someone is admitted as an inpatient, but discharged in less than two days, payment will be made only if it can be documented that a longer stay was reasonably expected and unforeseen circumstances led to the shorter stay. Hospital stays that are incorrectly classified or have improperly documented changes in admission status will not be paid.
However, Dr. Cooper told Reuters Health by email, "Older patients with SBO, a very common diagnosis, should be presumed to be admitted for more than two midnights and hospitals should not get penalized."
She and her colleagues pointed out that SBO accounts for about 15% of surgical admissions to U.S. hospitals and more than $1 billion in annual hospital charges. However, diagnosis requires surgeons to observe patients to determine if surgery is warranted.
Thus, the authors wrote, "It is critically important for surgeons to correctly assign admission status for patients with SBO to ensure that hospitals are reimbursed appropriately, and patients are not unduly burdened."
The investigators examined data on 855 older patients admitted with SBO from 2006 and 2013. Of these, 816 (95%) stayed for two midnights or longer. This was true of all patients aged 85 years or older (n=108, approximately 13%).
The only significant difference in clinical characteristics was the presence of inflammatory bowel disease. Of five such patients, only one stayed for less than two midnights.
"Based on our study and others," the investigators wrote, "we propose that hospital admission for SBO in elderly patients is sufficient justification for the reasonable expectation" of the required length of stay for reimbursement.
This also may be true of other conditions. Dr. Cooper concluded, "More studies like this are needed in surgical patients to better understand the impact of CMS admission guidelines. The rule may not make sense in certain populations, leading to heavy and unfair penalties for hospitals."
The authors reported no disclosures.
Vitamin D Assay May Give Misleading Results
NEW YORK (Reuters Health) - In certain circumstances one widely used test for vitamin D intoxication, the Diasorin radioimmunoassay, may not be entirely reliable, according to two case studies by U.S. and Irish investigators.
"Our study," Dr. Michael A. Levine told Reuters Health by email, "highlights the continuing challenge that we face when using current assay technologies to measure vitamin D metabolites." The patients involved "developed vitamin D toxicity from inadvertent overdosage using standard over-the-counter preparations of vitamin D."
In a June 22 online paper in the Journal of Clinical Endocrinology & Metabolism, Dr. Levine, of the University of Pennsylvania, Philadelphia, and colleagues note that vitamin D intoxication is characterized by elevated serum 25-hydroxyvitamin D (25(OH)D) and suppressed serum 1,25-dihydroxvitamin D (1,25(OH)2D).
The team used both the Diasorin radioimmunaossay test (RIA) and liquid chromatography and tandem mass spectrometry (LC-MS/MS) to evaluate samples from two retrospectively identified patients with hypercalcemia. One was a 15-year-old male with a two-week history of postprandial vomiting, abdominal pain and polyuria. The other, a 17-year old female, had a history of weight loss.
Both had elevated serum 1,25(OH)2D by RIA, but normal serum 1,25(OH)2D concentrations by LC-MS/MS. To help explain these surprising findings the team conducted further in vitro experiments on serum samples from a random set of inpatients and outpatients.
The team noted that concentrations of 25(OH)D2 or 25(OH)D3 increased as expected based on the amount of vitamin D metabolite added to pooled serum samples or artificial serum matrix in all experiments.
The addition of 100 ng/mL of 25(OH)D3 to pooled patient serum resulted in a median increase of 114% in measured 1,25(OH)D2 via RIA and a 21% increase via LC-MS/MS. At 700 ng/mL, the increase was 349% with RIA and 117% with LC-MS/MS.
Thus, wrote the researchers, "We recommend measurement of serum 24,25(OH)2D and use of LC-MS/MS, which appears less susceptible to this interference, to reassess serum levels of 1,25(OH)2D when the clinical scenario is confusing."
Summing up, Dr. Levine said, "Assessment of plasma levels of the most active vitamin D metabolite, 1,25(OH)2D, using a common laboratory immunoassay pointed away from nutritional vitamin D intoxication and suggested other more worrisome diagnoses. Repeating the testing with a mass spectrometer assay confirmed the clinical diagnosis of vitamin D intoxication."
He concluded, "Clinicians must remember that laboratory tests are not 100% reliable, and they must continue to rely upon their clinical judgment when confronted with test results that do not make sense."
Diasorin did not respond to a request for comment.
The authors reported no financial disclosures or competing interests.
NEW YORK (Reuters Health) - In certain circumstances one widely used test for vitamin D intoxication, the Diasorin radioimmunoassay, may not be entirely reliable, according to two case studies by U.S. and Irish investigators.
"Our study," Dr. Michael A. Levine told Reuters Health by email, "highlights the continuing challenge that we face when using current assay technologies to measure vitamin D metabolites." The patients involved "developed vitamin D toxicity from inadvertent overdosage using standard over-the-counter preparations of vitamin D."
In a June 22 online paper in the Journal of Clinical Endocrinology & Metabolism, Dr. Levine, of the University of Pennsylvania, Philadelphia, and colleagues note that vitamin D intoxication is characterized by elevated serum 25-hydroxyvitamin D (25(OH)D) and suppressed serum 1,25-dihydroxvitamin D (1,25(OH)2D).
The team used both the Diasorin radioimmunaossay test (RIA) and liquid chromatography and tandem mass spectrometry (LC-MS/MS) to evaluate samples from two retrospectively identified patients with hypercalcemia. One was a 15-year-old male with a two-week history of postprandial vomiting, abdominal pain and polyuria. The other, a 17-year old female, had a history of weight loss.
Both had elevated serum 1,25(OH)2D by RIA, but normal serum 1,25(OH)2D concentrations by LC-MS/MS. To help explain these surprising findings the team conducted further in vitro experiments on serum samples from a random set of inpatients and outpatients.
The team noted that concentrations of 25(OH)D2 or 25(OH)D3 increased as expected based on the amount of vitamin D metabolite added to pooled serum samples or artificial serum matrix in all experiments.
The addition of 100 ng/mL of 25(OH)D3 to pooled patient serum resulted in a median increase of 114% in measured 1,25(OH)D2 via RIA and a 21% increase via LC-MS/MS. At 700 ng/mL, the increase was 349% with RIA and 117% with LC-MS/MS.
Thus, wrote the researchers, "We recommend measurement of serum 24,25(OH)2D and use of LC-MS/MS, which appears less susceptible to this interference, to reassess serum levels of 1,25(OH)2D when the clinical scenario is confusing."
Summing up, Dr. Levine said, "Assessment of plasma levels of the most active vitamin D metabolite, 1,25(OH)2D, using a common laboratory immunoassay pointed away from nutritional vitamin D intoxication and suggested other more worrisome diagnoses. Repeating the testing with a mass spectrometer assay confirmed the clinical diagnosis of vitamin D intoxication."
He concluded, "Clinicians must remember that laboratory tests are not 100% reliable, and they must continue to rely upon their clinical judgment when confronted with test results that do not make sense."
Diasorin did not respond to a request for comment.
The authors reported no financial disclosures or competing interests.
NEW YORK (Reuters Health) - In certain circumstances one widely used test for vitamin D intoxication, the Diasorin radioimmunoassay, may not be entirely reliable, according to two case studies by U.S. and Irish investigators.
"Our study," Dr. Michael A. Levine told Reuters Health by email, "highlights the continuing challenge that we face when using current assay technologies to measure vitamin D metabolites." The patients involved "developed vitamin D toxicity from inadvertent overdosage using standard over-the-counter preparations of vitamin D."
In a June 22 online paper in the Journal of Clinical Endocrinology & Metabolism, Dr. Levine, of the University of Pennsylvania, Philadelphia, and colleagues note that vitamin D intoxication is characterized by elevated serum 25-hydroxyvitamin D (25(OH)D) and suppressed serum 1,25-dihydroxvitamin D (1,25(OH)2D).
The team used both the Diasorin radioimmunaossay test (RIA) and liquid chromatography and tandem mass spectrometry (LC-MS/MS) to evaluate samples from two retrospectively identified patients with hypercalcemia. One was a 15-year-old male with a two-week history of postprandial vomiting, abdominal pain and polyuria. The other, a 17-year old female, had a history of weight loss.
Both had elevated serum 1,25(OH)2D by RIA, but normal serum 1,25(OH)2D concentrations by LC-MS/MS. To help explain these surprising findings the team conducted further in vitro experiments on serum samples from a random set of inpatients and outpatients.
The team noted that concentrations of 25(OH)D2 or 25(OH)D3 increased as expected based on the amount of vitamin D metabolite added to pooled serum samples or artificial serum matrix in all experiments.
The addition of 100 ng/mL of 25(OH)D3 to pooled patient serum resulted in a median increase of 114% in measured 1,25(OH)D2 via RIA and a 21% increase via LC-MS/MS. At 700 ng/mL, the increase was 349% with RIA and 117% with LC-MS/MS.
Thus, wrote the researchers, "We recommend measurement of serum 24,25(OH)2D and use of LC-MS/MS, which appears less susceptible to this interference, to reassess serum levels of 1,25(OH)2D when the clinical scenario is confusing."
Summing up, Dr. Levine said, "Assessment of plasma levels of the most active vitamin D metabolite, 1,25(OH)2D, using a common laboratory immunoassay pointed away from nutritional vitamin D intoxication and suggested other more worrisome diagnoses. Repeating the testing with a mass spectrometer assay confirmed the clinical diagnosis of vitamin D intoxication."
He concluded, "Clinicians must remember that laboratory tests are not 100% reliable, and they must continue to rely upon their clinical judgment when confronted with test results that do not make sense."
Diasorin did not respond to a request for comment.
The authors reported no financial disclosures or competing interests.
Evidence‐Based Care for Cellulitis
Cellulitis is a common infection causing inflammation of the skin and subcutaneous tissues. Cellulitis has been attributed to gram‐positive organisms through historical evaluations including fine‐needle aspirates and punch biopsies of the infected tissue.[1] Neither of these diagnostic tests is currently used due to their invasiveness, poor diagnostic yield, and availability. Similarly, readily available tests such as blood cultures provide an etiology <5% of the time[1] and are not cost‐effective for most patients for diagnosing cellulitis.[2] In addition, the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) has steadily increased, complicating decisions about antibiotic selection.[3] The result of this uncertainty is a large variation in practice with respect to antibiotic and imaging selection for patients with a diagnosis of cellulitis.
University of Utah Health Care (UUHC) performed benchmarking for the management of cellulitis using the University HealthSystem Consortium (UHC) database and associated CareFx analytics tool. Benchmarking demonstrated that UUHC had a greater percentage of broad‐spectrum antibiotic use (defined as vancomycin, piperacillin/tazobactam, or carbapenems) than the top 5 performing UHC facilities for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses of cellulitis (vancomycin 83% vs 58% and carbapenem or piperacillin/tazobactam 44% vs 16%). Advanced imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) for the diagnosis of cellulitis was also found to be an opportunity for improvement (CT 27% vs 20% and MRI 8% vs 5%). The hospitalist group (most patients admitted with cellulitis were on this service) believed these data reflected current practice, as there was no standard of treatment for cellulitis despite an active order set. Therefore, cellulitis was considered an opportunity to improve value to our patients. A standardized clinical care pathway was created, as such pathways have demonstrated a reduction in variation in practice and improved efficiency and effectiveness of care for multiple disease states including cellulitis.[4, 5] We hypothesized that implementation of an evidence‐based care pathway would decrease broad‐spectrum antibiotic use, cost, and use of advanced imaging without having any adverse effects on clinical outcomes such as length of stay (LOS) or readmission.
METHODS
Study Setting and Population
UUHC is a 500‐bed academic medical center in Salt Lake City, Utah. All patients admitted to the emergency department observation unit (EDOU) or the hospital with a primary ICD‐9‐CM diagnosis of cellulitis between July 1, 2011 and December 31, 2013 were evaluated.
Intervention
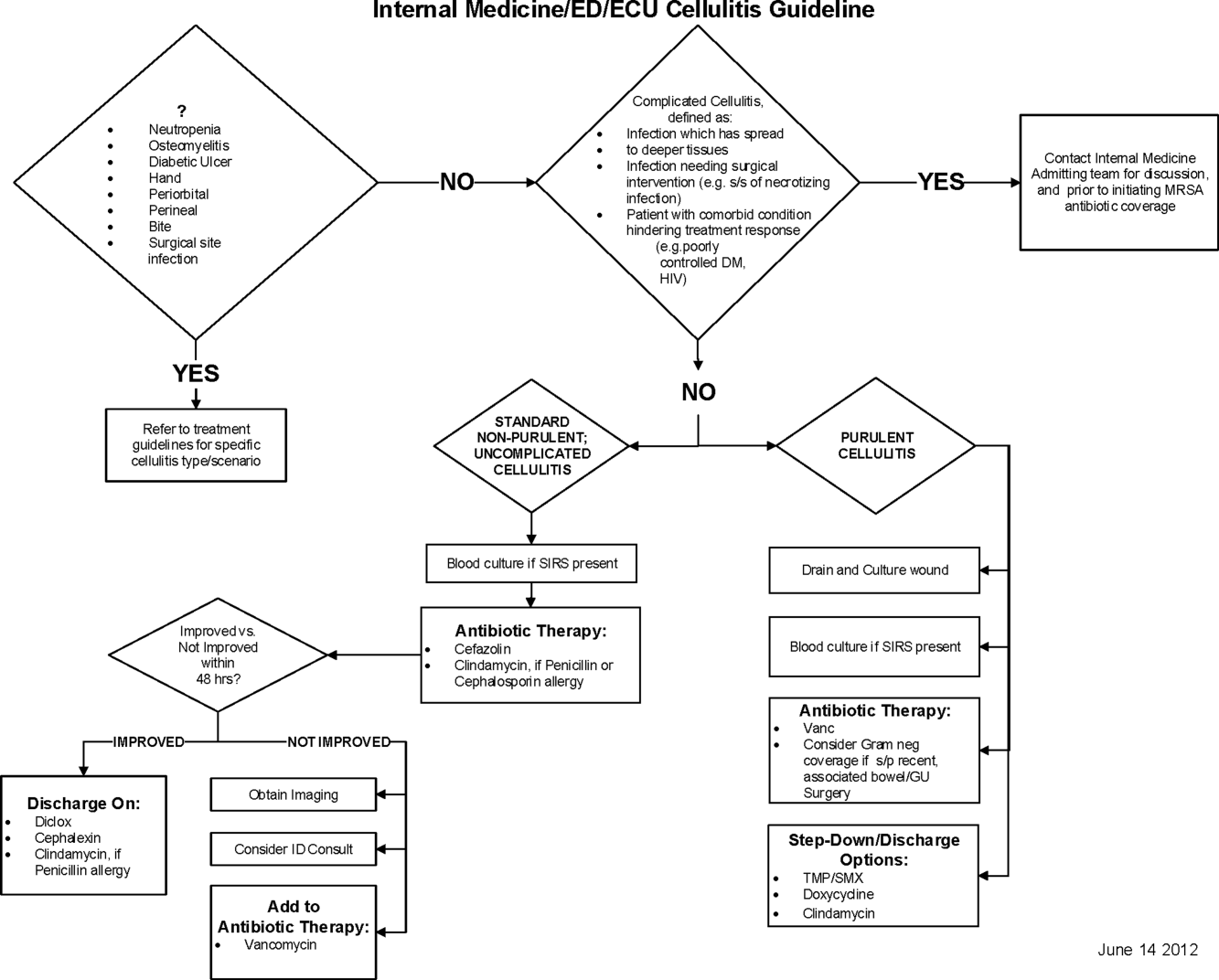
Initial steps involved the formation of a multidisciplinary team including key stakeholders from the hospitalist group, infectious diseases, the emergency department (ED), and nursing. This multidisciplinary team was charged with developing a clinical care pathway appropriate for local implementation. National guidance for the care pathway was mainly obtained from the Infectious Disease Society of America (IDSA) guidelines on skin and soft tissue infections (SSTIs)[6] and MRSA.[7] Specific attention was paid to recommendations on blood cultures (only when systemically ill), imaging (rarely needed), antibiotic selection (rarely gram‐negative coverage and consideration of MRSA coverage), and patient‐care principles that are often overlooked (elevation of the affected extremity). A distinction of purulent versus nonpurulent cellulitis was adopted based on the guidelines and a prospective evaluation of the care of patients with nonpurulent cellulitis.[8] The 2014 IDSA update on SSTIs incorporates this distinction more clearly in hopes of determining staphylococcal versus streptococcal infections.[9] After multiple iterations, an agreed‐upon care pathway was created that excluded patients with neutropenia, osteomyelitis, diabetic foot ulcerations; hand, perineal, periorbital, or surgical site infections; and human or animal bites (Figure 1). After the care pathway was determined, interventions were performed to implement this change.
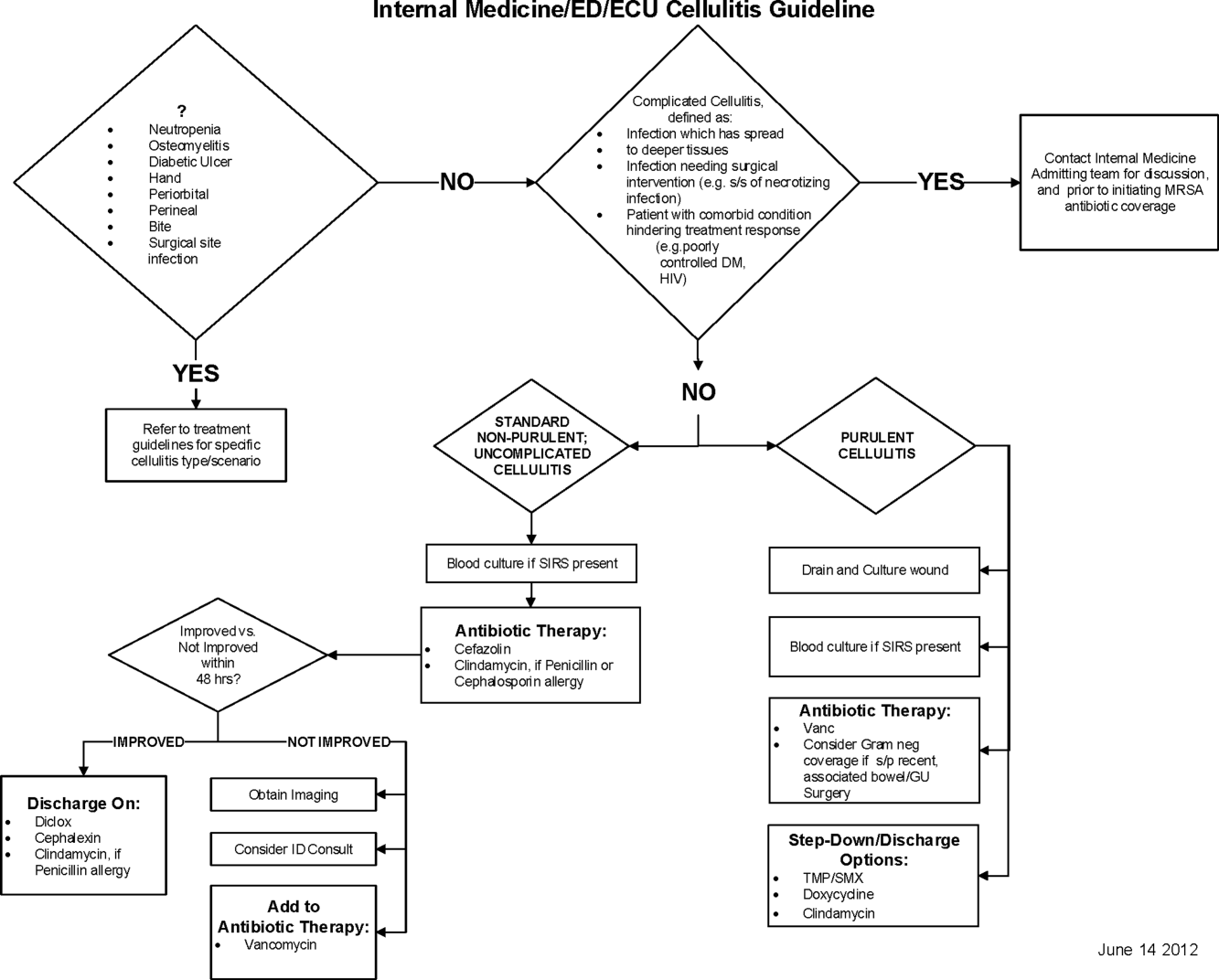
Education of all providers involved included discussion of cellulitis as a disease process, presentation of benchmarking data, dissemination of the care pathway to hospitalist and ED physicians, teaching conferences for internal medicine residents and ED residents, and reinforcement of these concepts at the beginning of resident rotations.
Incorporation of the care pathway into the existing electronic order sets for cellulitis care in the inpatient and ED settings, with links to the care pathway, links to excluded disease processes (eg, hand cellulitis), preselection of commonly needed items (eg, elevate leg), and recommendations for antibiotic selection based on categories of purulent or nonpurulent cellulitis. The electronic health record (EHR) did not allow for forced order set usage, so the order set required selection by the admitting physician if indicated. Additionally, an embedded 48‐hour order set could be accessed at any time by the ordering physician and included vancomycin dosing. Specific changes to the preexisting order set included the development of sections for purulent and nonpurulent cellulitis as well as recommended antibiotics. Piperacillin/tazobactam and nafcillin were both removed and vancomycin was limited to the purulent subheading. Additionally, elevation of the extremity was preselected, and orderables for imaging (chest x‐ray and duplex ultrasound), antiulcer prophylaxis, telemetry, and electrocardiograph were all removed.
Audit and feedback of cases of cellulitis and broad‐spectrum antibiotic usage was performed by a senior hospitalist.
Study Design
A retrospective before/after study was performed to assess overall impact of the intervention on the patient population. Additionally, a retrospective controlled pre‐/postintervention study was performed to compare changes in cellulitis management for visits where order sets were used with visits where order sets were not used. The intervention initiation date was July 9, 2012. The institutional review board classified this project as quality improvement and did not require review and oversight.
Study Population
We analyzed 2278 ED and inpatient visits for cellulitis, of which 677 met inclusion criteria. We partitioned visits into 2 groups: (1) those for which order sets were used (n = 370) and (2) control visits for which order sets were not used (n = 307). We analyzed outcomes for 2 subpopulations: hospitalized patients for whom the EDOU or admission order sets were used (n = 149) and patients not admitted and only seen in the EDOU for whom the EDOU order set was used (n = 262).
Inclusion Criteria
Inclusion criteria included hospital admission or admission to the EDOU between July 1, 2011 and December 31, 2013, age greater or equal to 18 years, and primary diagnosis of cellulitis as determined by ICD‐9‐CM billing codes 035, 457.2, 681, 681.0, 681.00, 681.01, 681.02, 681.1, 681.10, 681.11, 681.9, 680, 680.0‐9, 682.0‐9, 684, 685.0, 685.1, 686.00, 686.01, 686.09, 686.1, 686.8, 686.9, 910.1, 910.5, 910.7, 910.9, 911.1, 911.3, 911.5, 911.7, 911.9, 912.1, 912.3, 912.5, 912.7, 912.9, 913.1, 913.3, 913.5, 913.7, 913.9, 914.1, 914.3, 914.5, 914.7, 914.9, 915.1, 915.3, 915.5, 915.7, 915.9, 916.1, 916.3, 916.5, 916.7, 916.9, 917.1, 917.3, 917.5, 917.7, 917.9, 919.1, 919.3, 919.5, 919.7, or 919.9.
Data Collection and Preparation
Clinical data were collected in the inpatient EHR (Cerner Corp., Kansas City, MO) and later imported into the enterprise data warehouse (EDW) as part of the normal data flow. Billing data were imported into the EDW from the billing system. Cost data were estimated using the value‐driven outcomes (VDO) tool developed by the University of Utah to identify clinical costs to the UUHC system.[10] All data were extracted from the EDW on September 10, 2014.
Process Metrics, Clinical, and Cost Outcomes
We defined 1 primary outcome (use of broad‐spectrum antibiotics) and 8 secondary outcomes, including process metrics (MRI and CT orders), clinical outcomes (LOS and 30‐day readmissions), and cost outcomes (pharmacy, lab, imaging cost from radiology department, and total facility cost). Broad‐spectrum antibiotics were defined as any use of meropenem (UUHC's carbapenem), piperacillin/tazobactam, or vancomycin and were determined by orders. Thirty‐day readmissions included only inpatient encounters with the primary diagnosis of cellulitis.
Covariates
To control for patient demographics we included age at admission in years and gender into the statistical model. To control for background health state as well as cellulitis severity, we included Charlson Comorbidity Index (CCI) and hospitalization status. CCI was calculated according to the algorithm specified by Quan et al.[11]
Study Hypotheses
First, for all patients, we hypothesized that process metrics as well as clinical and cost outcomes would improve following the implementation of the care pathway. To evaluate this hypothesis, we estimated impact of the time interval (pre‐/postintervention) on all outcomes. Second, we hypothesized that among patients for whom order sets were used (which we deemed to be a proxy for following the agreed‐upon care pathway), there would be a greater improvement than in patients for whom order sets were not used. To evaluate this hypothesis, we estimated interactions between order set use and time period (pre‐/postintervention) for all outcomes.
Statistical Analysis
The variable time period was created to represent the time period before and after the intervention.
We provided unadjusted descriptive statistics for study outcomes and visit characteristics for all patients before and after intervention. Descriptive statistics were expressed as n (%) and mean standard deviation. Simple comparisons were performed based on 2 test of homogeneity for categorical variables and t test or Wilcoxon test for continuous variables.
For before/after analysis, we fitted generalized linear regression models to estimate the change in outcomes of interest before and after intervention for all patients simultaneously. Generalized linear model defined by a binomial distributional assumption and logit link function was used to estimate the effect of the intervention on antibiotic use, imaging orders, and readmission adjusting for effects of age, gender, CCI, and hospitalization status. A generalized linear model defined by a gamma distributional assumption and log link function was used to estimate effect of the intervention on clinical LOS and cost outcomes adjusting for the effects of the same covariates. Generalized linear models with gamma distributional assumptions were used because they are known to perform well even for zero‐inflated semicontinuous cost variables and are easier to interpret than 2‐part models.
For the controlled before/after analysis, the variable order set used was created to represent groups where order sets were used or not used. Similarly, generalized linear models were used to estimate differential effect of the intervention at 2 different order set use levels using an interaction term between order set use and the time period.
P values <0.05 were considered significant. We used SAS version 9.3 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
Descriptive Characteristics
Patient characteristics before and after intervention for 677 EDOU and inpatient visits for cellulitis by 618 patients are summarized in the first 4 columns of Table 1. Patient age at admission ranged from 18 to 98 years. Thirty‐eight percent of visits were by female patients. There were 274 visits before the intervention and 403 visits after. Four hundred thirty‐two (64%) were admitted, and 295 (44%) were seen in the EDOU. The admission order set alone was used for 104 visits, the EDOU order set alone was used for 242 visits, and both order sets were used for 24 visits.
| Characteristic | Overall | Order Sets Not Used | Order Sets Used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 274 | Intervention, N = 403 | P* | Baseline, N = 127 | Intervention, N = 180 | P* | Baseline, N = 147 | Intervention, N = 223 | P* | |
| |||||||||
| Patient Characteristics | |||||||||
| Age, y | 46.8 16.0 | 48.9 17.1 | 0.097 | 49.8 16.0 | 5.1 16.3 | 0.88 | 44.2 15.5 | 48.0 17.6 | 0.032 |
| Female gender | 105 (38%) | 155 (39%) | 0.93 | 50 (39%) | 74 (41%) | 0.73 | 55 (37%) | 81 (36%) | 0.86 |
| CCI | 2.6 3.2 | 2.6 3.0 | 0.69 | 3.2 3.5 | 3.2 3.2 | 0.82 | 2.0 2.8 | 2.1 2.7 | 0.68 |
| Clinical process characteristics | |||||||||
| EDOU admission | 122 (45%) | 173 (43%) | 0.68 | 12 (9%) | 19 (11%) | 0.75 | 110 (75%) | 154 (69%) | 0.23 |
| Hospital admission | 173 (63%) | 259 (64%) | 0.76 | 117 (92%) | 166 (92%) | 0.98 | 56 (38%) | 93 (42%) | 0.49 |
| EDOU order set used | 111 (41%) | 155 (38%) | 0.59 | NA | NA | NA | 111 (76%) | 155 (70%) | 0.21 |
| ADM order set used | 47 (17%) | 81 (20%) | 0.34 | NA | NA | NA | 47 (32%) | 81 (36%) | 0.39 |
| Process outcomes | |||||||||
| Broad‐spectrum antibiotics used | 205 (75%) | 230 (57%) | <0.001 | 90 (71%) | 121 (67%) | 0.50 | 115 (78%) | 109 (49%) | <0.001 |
| MRI done | 27 (10%) | 32 (8%) | 0.39 | 13 (10%) | 20 (11%) | 0.81 | 14 (10%) | 12 (5%) | 0.13 |
| CT done | 56 (20%) | 76 (19%) | 0.61 | 32 (25%) | 43 (24%) | 0.79 | 24 (16%) | 33 (15%) | 0.69 |
| Clinical outcomes | |||||||||
| Length of stay, d | 2.7 2.6 | 2.6 2.8 | 0.35 | 3.6 2.8 | 3.8 3.4 | 0.62 | 2.0 2.1 | 1.7 1.6 | 0.48 |
| 30‐day readmission | 14 (5%) | 17 (4%) | 0.59 | 7 (6%) | 9 (5%) | 0.84 | 7 (5%) | 8 (4%) | 0.58 |
| Cost outcomes | |||||||||
| Pharmacy cost ($) | 1 | 0.76 | 0.002 | 1 | 0.89 | 0.13 | 1 | 0.56 | 0.004 |
| Lab cost ($) | 1 | 0.52 | <0.001 | 1 | 0.53 | 0.001 | 1 | 0.51 | 0.055 |
| Imaging cost ($) | 1 | 0.82 | 0.11 | 1 | 0.95 | 0.52 | 1 | 0.67 | 0.13 |
| Total facility cost ($) | 1 | 0.85 | 0.027 | 1 | 0.91 | 0.042 | 1 | 0.77 | 0.26 |
Before/After Analysis
Among all patients, use of broad‐spectrum antibiotics decreased from 75% to 57% (Table 1). Analysis adjusted for gender, age at admission, CCI, and hospital admission status is provided in Table 2. Overall, there was a 59% decrease in the odds of ordering broad‐spectrum antibiotics (P < 0.001), a 23% decrease in pharmacy cost (P = 0.002), a 44% decrease in laboratory cost (P < 0.001), and a 13% decrease in total facility cost (P = 0.006).
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Antibiotics used | Time period | 0.41 (0.29, 0.59) | 59% (71% to 41%) | <0.001 |
| MRI done | Time period | 0.74 (0.43, 1.30) | 26% (57% to 30%) | 0.29 |
| CT done | Time period | 0.92 (0.62, 1.36) | 8% (38% to 36%) | 0.67 |
| 30‐day readmission | Time period | 0.86 (0.41, 1.80) | 14% (59% to 80%) | 0.69 |
| Length of stay, d | Time period | 0.97 (0.91, 1.03) | 3% (9% to 3%) | 0.34 |
| Pharmacy cost ($) | Time period | 0.77 (0.65, 0.91) | 23% (35% to 9%) | 0.002 |
| Lab cost ($) | Time period | 0.56 (0.48, 0.65) | 44% (52% to 35%) | <0.001 |
| Imaging cost($) | Time period | 0.90 (0.71, 1.14) | 10% (29% to 14%) | 0.38 |
| Total facility cost ($) | Time Period | 0.87 (0.79, 0.96) | 13% (21% to 4%) | 0.006 |
Order Set Use Groups Analysis
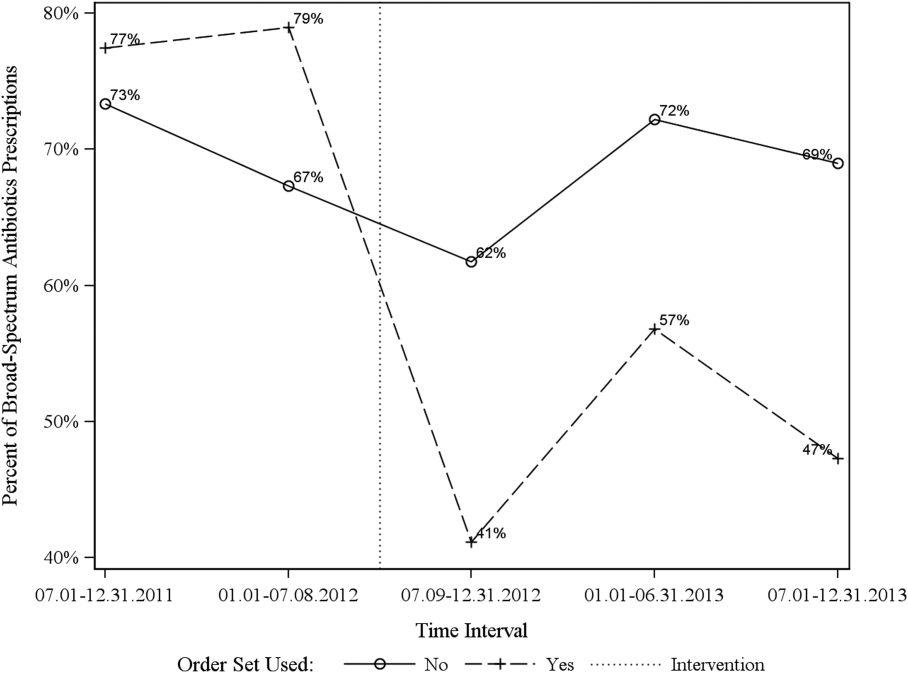
Descriptive statistics and simple comparison before/after the intervention for the 2 study groups are shown in the last 6 columns of Table 1. Among patients for whom order sets were used, broad‐spectrum antibiotic usage significantly decreased from 78% before the intervention to 49% after the intervention (P < 0.001). In contrast, among patients for whom order sets were not used, broad‐spectrum antibiotic usage remained relatively constant71% before the intervention versus 67% after the intervention (P = 0.50). Figure 2 shows semiannual changes in the prescription of broad‐spectrum antibiotics. There is a noticeable drop after the intervention among patients for whom order sets were used.
Analysis of the interaction between time period and order set usage is provided in Table 3. After the intervention, patients for whom the order sets were used had greater improvement in broad‐spectrum antibiotic selection (75% decrease, P < 0.001) and LOS (25% decrease, P = 0.041) than patients for whom order sets were not used. Pharmacy costs also decreased by 13% more among patients for whom the order sets were used, although the interaction was not statistically significant (P = 0.074). Laboratory costs decreased in both groups, but order set use did not demonstrate an interaction (P = 0.5). Similar results were found for the subgroups of admitted patients and patients seen in the EDOU.
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Broad spectrum antibiotics | Time period | 0.84 (0.50, 1.40) | 16% (50% to 40%) | 0.50 |
| Time periodorder set | 0.25 (0.12, 0.52) | 75% (88% to 48%) | <0.001 | |
| MRI done | Time period | 1.04 (0.49, 2.20) | 4% (51% to 120%) | 0.92 |
| Time periodorder set | 0.44 (0.14, 1.38) | 56% (86% to 38%) | 0.16 | |
| CT done | Time period | 0.94 (0.55, 1.60) | 6% (45% to 60%) | 0.81 |
| Time periodorder set | 0.96 (0.44, 2.12) | 4% (56% to 112%) | 0.93 | |
| 30‐day readmission | Time period | 0.91 (0.33, 2.53) | 9% (67% to 153%) | 0.86 |
| Time periodorder set | 0.88 (0.20, 3.93) | 12% (80% to 293%) | 0.87 | |
| Clinical length of stay | Time period | 1.04 (0.95, 1.14) | 4% (5% to 14%) | 0.41 |
| Time periodorder set | 0.87 (0.77, 0.99) | 13% (23% to 1%) | 0.041 | |
| Pharmacy cost ($) | Time period | 0.88 (0.70, 1.12) | 12% (30% to 12%) | 0.31 |
| Time periodorder set | 0.75 (0.54, 1.03) | 25% (46% to 3%) | 0.074 | |
| Lab cost ($) | Time period | 0.53 (0.42, 0.66) | 47% (58% to 34%) | <0.001 |
| Time periodorder set | 1.11 (0.82, 1.50) | 11% (18% to 50%) | 0.50 | |
| Imaging cost ($) | Time period | 1.00 (0.71, 1.40) | 0% (29% to 40%) | 0.98 |
| Time periodorder set | 0.82 (0.51, 1.30) | 18% (49% to 30%) | 0.39 | |
| Facility cost ($) | Time period | 0.92 (0.80, 1.05) | 8% (20% to 5%) | 0.22 |
| Time periodorder set | 0.90 (0.75, 1.09) | 10% (25% to 9%) | 0.29 | |
Audit and feedback was initially performed for cases of cellulitis using broad‐spectrum antibiotics. However, given the complexity of cellulitis as a disease process and the frequency of broad‐spectrum antibiotic usage, in all cases of review, it was deemed reasonable to use broad‐spectrum antibiotics. Therefore, the audit was not continued.
DISCUSSION
Care pathways have demonstrated improvement across multiple different disease states including cellulitis.[4, 5] They have been noted to reduce variation in practice and improve physician agreement about treatment options.[4] The best method for implementation is not clearly understood,[12] and there remains concern about maintaining flexibility for patient care.[13] Additionally, although implementation of pathways is often well described, evaluations of the processes are noted to frequently be weak.[12] UUHC felt that the literature supported implementing a care pathway for the diagnosis of cellulitis, but that a thorough evaluation was also needed to understand any resulting benefits or harms. Through this study, we found that the implementation of this pathway resulted in a significant decrease in broad‐spectrum antibiotic use, pharmacy costs, and total facility costs. There was also a trend to decrease in imaging cost, and there were no adverse effects on LOS or 30‐day readmissions. Our findings demonstrate that care‐pathway implementation accompanied by education, pathway‐compliant electronic order sets, and audit and feedback can help drive improvements in quality while reducing costs. This finding furthers the evidence supporting standard work through the creation of clinical care pathways for cellulitis as an effective intervention.[4] Additionally, although not measured in this study, reduction of antibiotic use is supported as a measure to help reduce Clostridium difficile infections, a further potential benefit.[14]
This study has several important strengths. First, we included accurate cost analyses using the VDO tool. Given the increasing importance of improving care value, we feel the inclusion of such cost analysis is an increasingly important aspect of health service intervention evaluations. Second, we used a formal benchmarking approach to identify a priority care improvement area and to monitor changes in practice following the rollout of the intervention. We feel this approach provides a useful example on how to systematically improve care quality and value in a broader health system context. Third, we evaluated not order set implementation per se, but rather changing an existing order set. Because studies in this area generally focus on initial order set implementation, our study contributes insights on what can be expected through modifications of existing order sets based on care pathways. Fourth, the analysis accounted for a variety of variables including the CCI. Of interest, our study found that the intervention group (patients for whom order sets were used) had a lower CCI, confirming Allen et al.'s findings that diseases with predictable trajectories are the most likely to benefit from care pathways.[4] As a final strength, the narrative‐based order set intervention was relatively simple, and the inclusion criteria were broad, making the process generalizable.
Limitations of this study include that it was a single center pre‐/postintervention study and not a randomized controlled trial. Related to this limitation, the control group for which order sets were not used reflected a different patient population compared to the intervention group for which order sets were used. Specifically, it was more common for order sets to be used in the EDOU than upon admission, resulting in the order set group consisting of patients with less comorbidities than patients in the nonorder set group. Additionally, patients in the order set intervention group were older than in the baseline group (48.0 vs 44.2 years). However, these differences in population remained relatively stable before and after the intervention, and relevant variables including demographic factors and CCI were accounted for in the regression models. Nevertheless, it remains possible that secular trends existed that we did not capture that affected the 2 populations differently. For example, there was a separate project that overlapped with the intervention period to reduce unnecessary laboratory usage at UUHC. This intervention could have influenced the trend to decreased laboratory utilization in the postintervention period. However, there were no concurrent initiatives to reduce antibiotic use during the study period. As a final limitation, the statistical analyses have not corrected for multiple testing for the secondary outcomes.
CONCLUSION
Using benchmark data from UHC, an academic medical center was able to identify an opportunity for improving the care of patients with cellulitis and subsequently develop an evidence‐based care pathway. The implementation of this pathway correlated with a significant reduction of broad‐spectrum antibiotic use, pharmacy costs, and total facility costs without adverse clinical affects. An important factor in the success of the intervention was the use of electronic order sets for cellulitis, which provided support for the implementation of the care pathway. This study demonstrates that the intervention was not only effective overall, but that it was more effective for those patients for whom the order set was used. This study adds to the growing body of literature suggesting that a well‐defined care pathway can improve outcomes and reduce costs for patients and institutions.
Acknowledgements
The authors thank Ms. Pam Proctor for her assistance in implementation of the care pathway and Ms. Selma Lopez for her editorial assistance.
Disclosures: K.K. is or has been a consultant on clinical decision support (CDS) or electronic clinical quality measurement to the US Office of the National Coordinator for Health Information Technology, ARUP Laboratories, McKesson InterQual, ESAC, Inc., JBS International, Inc., Inflexxion, Inc., Intelligent Automation, Inc., Partners HealthCare, Mayo Clinic, and the RAND Corporation. K.K. receives royalties for a Duke Universityowned CDS technology for infectious disease management known as CustomID that he helped develop. K.K. was formerly a consultant for Religent, Inc. and a co‐owner and consultant for Clinica Software, Inc., both of which provide commercial CDS services, including through use of a CDS technology known as SEBASTIAN that K.K. developed. K.K. no longer has a financial relationship with either Religent or Clinica Software. K.K. has no competing interest with any specific product or intervention evaluated in this manuscript. All other authors declare no competing interests.
- . Cellulitis. N Engl J Med. 2004;350(9):904–912.
- , , , , , . Cost‐effectiveness of blood cultures for adult patients with cellulitis. Clin Infect Dis. 1999;29(6):1483–1488.
- , , , et al. Methicillin‐resistant s. aureus infectious among patients in the emergency department. N Engl J Med. 2006;355:666–674.
- , , . Systematic review of the effectiveness of integrated care pathways: what works, for whom, in which circumstances? Int J Evid Based Healthc. 2009;7:61–74.
- . Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072–1079.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41:1373–1406.
- , , , et al. Clinical practice guidelines by the Infectious Disease Society of American for the treatment of methicillin‐resistant Staphylococcus aureus infectious in adults and children. Clin Infect Dis. 2011;42:1–38.
- , , , . The role of b‐hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;89:217–226.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Disease Society of America. Clin Infect Dis. 2014;59(2):147–159.
- , , , et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223–235.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:113–1139.
- , . Computerization of workflows, guidelines, and care pathways: a review of implementation challenges for process‐oriented health information systems. J Am Med Inform Assoc. 2011;18:738–748.
- , , , et al. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32(5):911–920.
- , , , et al. Clinical practice guidelines for clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455.
Cellulitis is a common infection causing inflammation of the skin and subcutaneous tissues. Cellulitis has been attributed to gram‐positive organisms through historical evaluations including fine‐needle aspirates and punch biopsies of the infected tissue.[1] Neither of these diagnostic tests is currently used due to their invasiveness, poor diagnostic yield, and availability. Similarly, readily available tests such as blood cultures provide an etiology <5% of the time[1] and are not cost‐effective for most patients for diagnosing cellulitis.[2] In addition, the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) has steadily increased, complicating decisions about antibiotic selection.[3] The result of this uncertainty is a large variation in practice with respect to antibiotic and imaging selection for patients with a diagnosis of cellulitis.
University of Utah Health Care (UUHC) performed benchmarking for the management of cellulitis using the University HealthSystem Consortium (UHC) database and associated CareFx analytics tool. Benchmarking demonstrated that UUHC had a greater percentage of broad‐spectrum antibiotic use (defined as vancomycin, piperacillin/tazobactam, or carbapenems) than the top 5 performing UHC facilities for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses of cellulitis (vancomycin 83% vs 58% and carbapenem or piperacillin/tazobactam 44% vs 16%). Advanced imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) for the diagnosis of cellulitis was also found to be an opportunity for improvement (CT 27% vs 20% and MRI 8% vs 5%). The hospitalist group (most patients admitted with cellulitis were on this service) believed these data reflected current practice, as there was no standard of treatment for cellulitis despite an active order set. Therefore, cellulitis was considered an opportunity to improve value to our patients. A standardized clinical care pathway was created, as such pathways have demonstrated a reduction in variation in practice and improved efficiency and effectiveness of care for multiple disease states including cellulitis.[4, 5] We hypothesized that implementation of an evidence‐based care pathway would decrease broad‐spectrum antibiotic use, cost, and use of advanced imaging without having any adverse effects on clinical outcomes such as length of stay (LOS) or readmission.
METHODS
Study Setting and Population
UUHC is a 500‐bed academic medical center in Salt Lake City, Utah. All patients admitted to the emergency department observation unit (EDOU) or the hospital with a primary ICD‐9‐CM diagnosis of cellulitis between July 1, 2011 and December 31, 2013 were evaluated.
Intervention
Initial steps involved the formation of a multidisciplinary team including key stakeholders from the hospitalist group, infectious diseases, the emergency department (ED), and nursing. This multidisciplinary team was charged with developing a clinical care pathway appropriate for local implementation. National guidance for the care pathway was mainly obtained from the Infectious Disease Society of America (IDSA) guidelines on skin and soft tissue infections (SSTIs)[6] and MRSA.[7] Specific attention was paid to recommendations on blood cultures (only when systemically ill), imaging (rarely needed), antibiotic selection (rarely gram‐negative coverage and consideration of MRSA coverage), and patient‐care principles that are often overlooked (elevation of the affected extremity). A distinction of purulent versus nonpurulent cellulitis was adopted based on the guidelines and a prospective evaluation of the care of patients with nonpurulent cellulitis.[8] The 2014 IDSA update on SSTIs incorporates this distinction more clearly in hopes of determining staphylococcal versus streptococcal infections.[9] After multiple iterations, an agreed‐upon care pathway was created that excluded patients with neutropenia, osteomyelitis, diabetic foot ulcerations; hand, perineal, periorbital, or surgical site infections; and human or animal bites (Figure 1). After the care pathway was determined, interventions were performed to implement this change.

Education of all providers involved included discussion of cellulitis as a disease process, presentation of benchmarking data, dissemination of the care pathway to hospitalist and ED physicians, teaching conferences for internal medicine residents and ED residents, and reinforcement of these concepts at the beginning of resident rotations.
Incorporation of the care pathway into the existing electronic order sets for cellulitis care in the inpatient and ED settings, with links to the care pathway, links to excluded disease processes (eg, hand cellulitis), preselection of commonly needed items (eg, elevate leg), and recommendations for antibiotic selection based on categories of purulent or nonpurulent cellulitis. The electronic health record (EHR) did not allow for forced order set usage, so the order set required selection by the admitting physician if indicated. Additionally, an embedded 48‐hour order set could be accessed at any time by the ordering physician and included vancomycin dosing. Specific changes to the preexisting order set included the development of sections for purulent and nonpurulent cellulitis as well as recommended antibiotics. Piperacillin/tazobactam and nafcillin were both removed and vancomycin was limited to the purulent subheading. Additionally, elevation of the extremity was preselected, and orderables for imaging (chest x‐ray and duplex ultrasound), antiulcer prophylaxis, telemetry, and electrocardiograph were all removed.
Audit and feedback of cases of cellulitis and broad‐spectrum antibiotic usage was performed by a senior hospitalist.
Study Design
A retrospective before/after study was performed to assess overall impact of the intervention on the patient population. Additionally, a retrospective controlled pre‐/postintervention study was performed to compare changes in cellulitis management for visits where order sets were used with visits where order sets were not used. The intervention initiation date was July 9, 2012. The institutional review board classified this project as quality improvement and did not require review and oversight.
Study Population
We analyzed 2278 ED and inpatient visits for cellulitis, of which 677 met inclusion criteria. We partitioned visits into 2 groups: (1) those for which order sets were used (n = 370) and (2) control visits for which order sets were not used (n = 307). We analyzed outcomes for 2 subpopulations: hospitalized patients for whom the EDOU or admission order sets were used (n = 149) and patients not admitted and only seen in the EDOU for whom the EDOU order set was used (n = 262).
Inclusion Criteria
Inclusion criteria included hospital admission or admission to the EDOU between July 1, 2011 and December 31, 2013, age greater or equal to 18 years, and primary diagnosis of cellulitis as determined by ICD‐9‐CM billing codes 035, 457.2, 681, 681.0, 681.00, 681.01, 681.02, 681.1, 681.10, 681.11, 681.9, 680, 680.0‐9, 682.0‐9, 684, 685.0, 685.1, 686.00, 686.01, 686.09, 686.1, 686.8, 686.9, 910.1, 910.5, 910.7, 910.9, 911.1, 911.3, 911.5, 911.7, 911.9, 912.1, 912.3, 912.5, 912.7, 912.9, 913.1, 913.3, 913.5, 913.7, 913.9, 914.1, 914.3, 914.5, 914.7, 914.9, 915.1, 915.3, 915.5, 915.7, 915.9, 916.1, 916.3, 916.5, 916.7, 916.9, 917.1, 917.3, 917.5, 917.7, 917.9, 919.1, 919.3, 919.5, 919.7, or 919.9.
Data Collection and Preparation
Clinical data were collected in the inpatient EHR (Cerner Corp., Kansas City, MO) and later imported into the enterprise data warehouse (EDW) as part of the normal data flow. Billing data were imported into the EDW from the billing system. Cost data were estimated using the value‐driven outcomes (VDO) tool developed by the University of Utah to identify clinical costs to the UUHC system.[10] All data were extracted from the EDW on September 10, 2014.
Process Metrics, Clinical, and Cost Outcomes
We defined 1 primary outcome (use of broad‐spectrum antibiotics) and 8 secondary outcomes, including process metrics (MRI and CT orders), clinical outcomes (LOS and 30‐day readmissions), and cost outcomes (pharmacy, lab, imaging cost from radiology department, and total facility cost). Broad‐spectrum antibiotics were defined as any use of meropenem (UUHC's carbapenem), piperacillin/tazobactam, or vancomycin and were determined by orders. Thirty‐day readmissions included only inpatient encounters with the primary diagnosis of cellulitis.
Covariates
To control for patient demographics we included age at admission in years and gender into the statistical model. To control for background health state as well as cellulitis severity, we included Charlson Comorbidity Index (CCI) and hospitalization status. CCI was calculated according to the algorithm specified by Quan et al.[11]
Study Hypotheses
First, for all patients, we hypothesized that process metrics as well as clinical and cost outcomes would improve following the implementation of the care pathway. To evaluate this hypothesis, we estimated impact of the time interval (pre‐/postintervention) on all outcomes. Second, we hypothesized that among patients for whom order sets were used (which we deemed to be a proxy for following the agreed‐upon care pathway), there would be a greater improvement than in patients for whom order sets were not used. To evaluate this hypothesis, we estimated interactions between order set use and time period (pre‐/postintervention) for all outcomes.
Statistical Analysis
The variable time period was created to represent the time period before and after the intervention.
We provided unadjusted descriptive statistics for study outcomes and visit characteristics for all patients before and after intervention. Descriptive statistics were expressed as n (%) and mean standard deviation. Simple comparisons were performed based on 2 test of homogeneity for categorical variables and t test or Wilcoxon test for continuous variables.
For before/after analysis, we fitted generalized linear regression models to estimate the change in outcomes of interest before and after intervention for all patients simultaneously. Generalized linear model defined by a binomial distributional assumption and logit link function was used to estimate the effect of the intervention on antibiotic use, imaging orders, and readmission adjusting for effects of age, gender, CCI, and hospitalization status. A generalized linear model defined by a gamma distributional assumption and log link function was used to estimate effect of the intervention on clinical LOS and cost outcomes adjusting for the effects of the same covariates. Generalized linear models with gamma distributional assumptions were used because they are known to perform well even for zero‐inflated semicontinuous cost variables and are easier to interpret than 2‐part models.
For the controlled before/after analysis, the variable order set used was created to represent groups where order sets were used or not used. Similarly, generalized linear models were used to estimate differential effect of the intervention at 2 different order set use levels using an interaction term between order set use and the time period.
P values <0.05 were considered significant. We used SAS version 9.3 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
Descriptive Characteristics
Patient characteristics before and after intervention for 677 EDOU and inpatient visits for cellulitis by 618 patients are summarized in the first 4 columns of Table 1. Patient age at admission ranged from 18 to 98 years. Thirty‐eight percent of visits were by female patients. There were 274 visits before the intervention and 403 visits after. Four hundred thirty‐two (64%) were admitted, and 295 (44%) were seen in the EDOU. The admission order set alone was used for 104 visits, the EDOU order set alone was used for 242 visits, and both order sets were used for 24 visits.
| Characteristic | Overall | Order Sets Not Used | Order Sets Used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 274 | Intervention, N = 403 | P* | Baseline, N = 127 | Intervention, N = 180 | P* | Baseline, N = 147 | Intervention, N = 223 | P* | |
| |||||||||
| Patient Characteristics | |||||||||
| Age, y | 46.8 16.0 | 48.9 17.1 | 0.097 | 49.8 16.0 | 5.1 16.3 | 0.88 | 44.2 15.5 | 48.0 17.6 | 0.032 |
| Female gender | 105 (38%) | 155 (39%) | 0.93 | 50 (39%) | 74 (41%) | 0.73 | 55 (37%) | 81 (36%) | 0.86 |
| CCI | 2.6 3.2 | 2.6 3.0 | 0.69 | 3.2 3.5 | 3.2 3.2 | 0.82 | 2.0 2.8 | 2.1 2.7 | 0.68 |
| Clinical process characteristics | |||||||||
| EDOU admission | 122 (45%) | 173 (43%) | 0.68 | 12 (9%) | 19 (11%) | 0.75 | 110 (75%) | 154 (69%) | 0.23 |
| Hospital admission | 173 (63%) | 259 (64%) | 0.76 | 117 (92%) | 166 (92%) | 0.98 | 56 (38%) | 93 (42%) | 0.49 |
| EDOU order set used | 111 (41%) | 155 (38%) | 0.59 | NA | NA | NA | 111 (76%) | 155 (70%) | 0.21 |
| ADM order set used | 47 (17%) | 81 (20%) | 0.34 | NA | NA | NA | 47 (32%) | 81 (36%) | 0.39 |
| Process outcomes | |||||||||
| Broad‐spectrum antibiotics used | 205 (75%) | 230 (57%) | <0.001 | 90 (71%) | 121 (67%) | 0.50 | 115 (78%) | 109 (49%) | <0.001 |
| MRI done | 27 (10%) | 32 (8%) | 0.39 | 13 (10%) | 20 (11%) | 0.81 | 14 (10%) | 12 (5%) | 0.13 |
| CT done | 56 (20%) | 76 (19%) | 0.61 | 32 (25%) | 43 (24%) | 0.79 | 24 (16%) | 33 (15%) | 0.69 |
| Clinical outcomes | |||||||||
| Length of stay, d | 2.7 2.6 | 2.6 2.8 | 0.35 | 3.6 2.8 | 3.8 3.4 | 0.62 | 2.0 2.1 | 1.7 1.6 | 0.48 |
| 30‐day readmission | 14 (5%) | 17 (4%) | 0.59 | 7 (6%) | 9 (5%) | 0.84 | 7 (5%) | 8 (4%) | 0.58 |
| Cost outcomes | |||||||||
| Pharmacy cost ($) | 1 | 0.76 | 0.002 | 1 | 0.89 | 0.13 | 1 | 0.56 | 0.004 |
| Lab cost ($) | 1 | 0.52 | <0.001 | 1 | 0.53 | 0.001 | 1 | 0.51 | 0.055 |
| Imaging cost ($) | 1 | 0.82 | 0.11 | 1 | 0.95 | 0.52 | 1 | 0.67 | 0.13 |
| Total facility cost ($) | 1 | 0.85 | 0.027 | 1 | 0.91 | 0.042 | 1 | 0.77 | 0.26 |
Before/After Analysis
Among all patients, use of broad‐spectrum antibiotics decreased from 75% to 57% (Table 1). Analysis adjusted for gender, age at admission, CCI, and hospital admission status is provided in Table 2. Overall, there was a 59% decrease in the odds of ordering broad‐spectrum antibiotics (P < 0.001), a 23% decrease in pharmacy cost (P = 0.002), a 44% decrease in laboratory cost (P < 0.001), and a 13% decrease in total facility cost (P = 0.006).
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Antibiotics used | Time period | 0.41 (0.29, 0.59) | 59% (71% to 41%) | <0.001 |
| MRI done | Time period | 0.74 (0.43, 1.30) | 26% (57% to 30%) | 0.29 |
| CT done | Time period | 0.92 (0.62, 1.36) | 8% (38% to 36%) | 0.67 |
| 30‐day readmission | Time period | 0.86 (0.41, 1.80) | 14% (59% to 80%) | 0.69 |
| Length of stay, d | Time period | 0.97 (0.91, 1.03) | 3% (9% to 3%) | 0.34 |
| Pharmacy cost ($) | Time period | 0.77 (0.65, 0.91) | 23% (35% to 9%) | 0.002 |
| Lab cost ($) | Time period | 0.56 (0.48, 0.65) | 44% (52% to 35%) | <0.001 |
| Imaging cost($) | Time period | 0.90 (0.71, 1.14) | 10% (29% to 14%) | 0.38 |
| Total facility cost ($) | Time Period | 0.87 (0.79, 0.96) | 13% (21% to 4%) | 0.006 |
Order Set Use Groups Analysis
Descriptive statistics and simple comparison before/after the intervention for the 2 study groups are shown in the last 6 columns of Table 1. Among patients for whom order sets were used, broad‐spectrum antibiotic usage significantly decreased from 78% before the intervention to 49% after the intervention (P < 0.001). In contrast, among patients for whom order sets were not used, broad‐spectrum antibiotic usage remained relatively constant71% before the intervention versus 67% after the intervention (P = 0.50). Figure 2 shows semiannual changes in the prescription of broad‐spectrum antibiotics. There is a noticeable drop after the intervention among patients for whom order sets were used.

Analysis of the interaction between time period and order set usage is provided in Table 3. After the intervention, patients for whom the order sets were used had greater improvement in broad‐spectrum antibiotic selection (75% decrease, P < 0.001) and LOS (25% decrease, P = 0.041) than patients for whom order sets were not used. Pharmacy costs also decreased by 13% more among patients for whom the order sets were used, although the interaction was not statistically significant (P = 0.074). Laboratory costs decreased in both groups, but order set use did not demonstrate an interaction (P = 0.5). Similar results were found for the subgroups of admitted patients and patients seen in the EDOU.
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Broad spectrum antibiotics | Time period | 0.84 (0.50, 1.40) | 16% (50% to 40%) | 0.50 |
| Time periodorder set | 0.25 (0.12, 0.52) | 75% (88% to 48%) | <0.001 | |
| MRI done | Time period | 1.04 (0.49, 2.20) | 4% (51% to 120%) | 0.92 |
| Time periodorder set | 0.44 (0.14, 1.38) | 56% (86% to 38%) | 0.16 | |
| CT done | Time period | 0.94 (0.55, 1.60) | 6% (45% to 60%) | 0.81 |
| Time periodorder set | 0.96 (0.44, 2.12) | 4% (56% to 112%) | 0.93 | |
| 30‐day readmission | Time period | 0.91 (0.33, 2.53) | 9% (67% to 153%) | 0.86 |
| Time periodorder set | 0.88 (0.20, 3.93) | 12% (80% to 293%) | 0.87 | |
| Clinical length of stay | Time period | 1.04 (0.95, 1.14) | 4% (5% to 14%) | 0.41 |
| Time periodorder set | 0.87 (0.77, 0.99) | 13% (23% to 1%) | 0.041 | |
| Pharmacy cost ($) | Time period | 0.88 (0.70, 1.12) | 12% (30% to 12%) | 0.31 |
| Time periodorder set | 0.75 (0.54, 1.03) | 25% (46% to 3%) | 0.074 | |
| Lab cost ($) | Time period | 0.53 (0.42, 0.66) | 47% (58% to 34%) | <0.001 |
| Time periodorder set | 1.11 (0.82, 1.50) | 11% (18% to 50%) | 0.50 | |
| Imaging cost ($) | Time period | 1.00 (0.71, 1.40) | 0% (29% to 40%) | 0.98 |
| Time periodorder set | 0.82 (0.51, 1.30) | 18% (49% to 30%) | 0.39 | |
| Facility cost ($) | Time period | 0.92 (0.80, 1.05) | 8% (20% to 5%) | 0.22 |
| Time periodorder set | 0.90 (0.75, 1.09) | 10% (25% to 9%) | 0.29 | |
Audit and feedback was initially performed for cases of cellulitis using broad‐spectrum antibiotics. However, given the complexity of cellulitis as a disease process and the frequency of broad‐spectrum antibiotic usage, in all cases of review, it was deemed reasonable to use broad‐spectrum antibiotics. Therefore, the audit was not continued.
DISCUSSION
Care pathways have demonstrated improvement across multiple different disease states including cellulitis.[4, 5] They have been noted to reduce variation in practice and improve physician agreement about treatment options.[4] The best method for implementation is not clearly understood,[12] and there remains concern about maintaining flexibility for patient care.[13] Additionally, although implementation of pathways is often well described, evaluations of the processes are noted to frequently be weak.[12] UUHC felt that the literature supported implementing a care pathway for the diagnosis of cellulitis, but that a thorough evaluation was also needed to understand any resulting benefits or harms. Through this study, we found that the implementation of this pathway resulted in a significant decrease in broad‐spectrum antibiotic use, pharmacy costs, and total facility costs. There was also a trend to decrease in imaging cost, and there were no adverse effects on LOS or 30‐day readmissions. Our findings demonstrate that care‐pathway implementation accompanied by education, pathway‐compliant electronic order sets, and audit and feedback can help drive improvements in quality while reducing costs. This finding furthers the evidence supporting standard work through the creation of clinical care pathways for cellulitis as an effective intervention.[4] Additionally, although not measured in this study, reduction of antibiotic use is supported as a measure to help reduce Clostridium difficile infections, a further potential benefit.[14]
This study has several important strengths. First, we included accurate cost analyses using the VDO tool. Given the increasing importance of improving care value, we feel the inclusion of such cost analysis is an increasingly important aspect of health service intervention evaluations. Second, we used a formal benchmarking approach to identify a priority care improvement area and to monitor changes in practice following the rollout of the intervention. We feel this approach provides a useful example on how to systematically improve care quality and value in a broader health system context. Third, we evaluated not order set implementation per se, but rather changing an existing order set. Because studies in this area generally focus on initial order set implementation, our study contributes insights on what can be expected through modifications of existing order sets based on care pathways. Fourth, the analysis accounted for a variety of variables including the CCI. Of interest, our study found that the intervention group (patients for whom order sets were used) had a lower CCI, confirming Allen et al.'s findings that diseases with predictable trajectories are the most likely to benefit from care pathways.[4] As a final strength, the narrative‐based order set intervention was relatively simple, and the inclusion criteria were broad, making the process generalizable.
Limitations of this study include that it was a single center pre‐/postintervention study and not a randomized controlled trial. Related to this limitation, the control group for which order sets were not used reflected a different patient population compared to the intervention group for which order sets were used. Specifically, it was more common for order sets to be used in the EDOU than upon admission, resulting in the order set group consisting of patients with less comorbidities than patients in the nonorder set group. Additionally, patients in the order set intervention group were older than in the baseline group (48.0 vs 44.2 years). However, these differences in population remained relatively stable before and after the intervention, and relevant variables including demographic factors and CCI were accounted for in the regression models. Nevertheless, it remains possible that secular trends existed that we did not capture that affected the 2 populations differently. For example, there was a separate project that overlapped with the intervention period to reduce unnecessary laboratory usage at UUHC. This intervention could have influenced the trend to decreased laboratory utilization in the postintervention period. However, there were no concurrent initiatives to reduce antibiotic use during the study period. As a final limitation, the statistical analyses have not corrected for multiple testing for the secondary outcomes.
CONCLUSION
Using benchmark data from UHC, an academic medical center was able to identify an opportunity for improving the care of patients with cellulitis and subsequently develop an evidence‐based care pathway. The implementation of this pathway correlated with a significant reduction of broad‐spectrum antibiotic use, pharmacy costs, and total facility costs without adverse clinical affects. An important factor in the success of the intervention was the use of electronic order sets for cellulitis, which provided support for the implementation of the care pathway. This study demonstrates that the intervention was not only effective overall, but that it was more effective for those patients for whom the order set was used. This study adds to the growing body of literature suggesting that a well‐defined care pathway can improve outcomes and reduce costs for patients and institutions.
Acknowledgements
The authors thank Ms. Pam Proctor for her assistance in implementation of the care pathway and Ms. Selma Lopez for her editorial assistance.
Disclosures: K.K. is or has been a consultant on clinical decision support (CDS) or electronic clinical quality measurement to the US Office of the National Coordinator for Health Information Technology, ARUP Laboratories, McKesson InterQual, ESAC, Inc., JBS International, Inc., Inflexxion, Inc., Intelligent Automation, Inc., Partners HealthCare, Mayo Clinic, and the RAND Corporation. K.K. receives royalties for a Duke Universityowned CDS technology for infectious disease management known as CustomID that he helped develop. K.K. was formerly a consultant for Religent, Inc. and a co‐owner and consultant for Clinica Software, Inc., both of which provide commercial CDS services, including through use of a CDS technology known as SEBASTIAN that K.K. developed. K.K. no longer has a financial relationship with either Religent or Clinica Software. K.K. has no competing interest with any specific product or intervention evaluated in this manuscript. All other authors declare no competing interests.
Cellulitis is a common infection causing inflammation of the skin and subcutaneous tissues. Cellulitis has been attributed to gram‐positive organisms through historical evaluations including fine‐needle aspirates and punch biopsies of the infected tissue.[1] Neither of these diagnostic tests is currently used due to their invasiveness, poor diagnostic yield, and availability. Similarly, readily available tests such as blood cultures provide an etiology <5% of the time[1] and are not cost‐effective for most patients for diagnosing cellulitis.[2] In addition, the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) has steadily increased, complicating decisions about antibiotic selection.[3] The result of this uncertainty is a large variation in practice with respect to antibiotic and imaging selection for patients with a diagnosis of cellulitis.
University of Utah Health Care (UUHC) performed benchmarking for the management of cellulitis using the University HealthSystem Consortium (UHC) database and associated CareFx analytics tool. Benchmarking demonstrated that UUHC had a greater percentage of broad‐spectrum antibiotic use (defined as vancomycin, piperacillin/tazobactam, or carbapenems) than the top 5 performing UHC facilities for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses of cellulitis (vancomycin 83% vs 58% and carbapenem or piperacillin/tazobactam 44% vs 16%). Advanced imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) for the diagnosis of cellulitis was also found to be an opportunity for improvement (CT 27% vs 20% and MRI 8% vs 5%). The hospitalist group (most patients admitted with cellulitis were on this service) believed these data reflected current practice, as there was no standard of treatment for cellulitis despite an active order set. Therefore, cellulitis was considered an opportunity to improve value to our patients. A standardized clinical care pathway was created, as such pathways have demonstrated a reduction in variation in practice and improved efficiency and effectiveness of care for multiple disease states including cellulitis.[4, 5] We hypothesized that implementation of an evidence‐based care pathway would decrease broad‐spectrum antibiotic use, cost, and use of advanced imaging without having any adverse effects on clinical outcomes such as length of stay (LOS) or readmission.
METHODS
Study Setting and Population
UUHC is a 500‐bed academic medical center in Salt Lake City, Utah. All patients admitted to the emergency department observation unit (EDOU) or the hospital with a primary ICD‐9‐CM diagnosis of cellulitis between July 1, 2011 and December 31, 2013 were evaluated.
Intervention
Initial steps involved the formation of a multidisciplinary team including key stakeholders from the hospitalist group, infectious diseases, the emergency department (ED), and nursing. This multidisciplinary team was charged with developing a clinical care pathway appropriate for local implementation. National guidance for the care pathway was mainly obtained from the Infectious Disease Society of America (IDSA) guidelines on skin and soft tissue infections (SSTIs)[6] and MRSA.[7] Specific attention was paid to recommendations on blood cultures (only when systemically ill), imaging (rarely needed), antibiotic selection (rarely gram‐negative coverage and consideration of MRSA coverage), and patient‐care principles that are often overlooked (elevation of the affected extremity). A distinction of purulent versus nonpurulent cellulitis was adopted based on the guidelines and a prospective evaluation of the care of patients with nonpurulent cellulitis.[8] The 2014 IDSA update on SSTIs incorporates this distinction more clearly in hopes of determining staphylococcal versus streptococcal infections.[9] After multiple iterations, an agreed‐upon care pathway was created that excluded patients with neutropenia, osteomyelitis, diabetic foot ulcerations; hand, perineal, periorbital, or surgical site infections; and human or animal bites (Figure 1). After the care pathway was determined, interventions were performed to implement this change.

Education of all providers involved included discussion of cellulitis as a disease process, presentation of benchmarking data, dissemination of the care pathway to hospitalist and ED physicians, teaching conferences for internal medicine residents and ED residents, and reinforcement of these concepts at the beginning of resident rotations.
Incorporation of the care pathway into the existing electronic order sets for cellulitis care in the inpatient and ED settings, with links to the care pathway, links to excluded disease processes (eg, hand cellulitis), preselection of commonly needed items (eg, elevate leg), and recommendations for antibiotic selection based on categories of purulent or nonpurulent cellulitis. The electronic health record (EHR) did not allow for forced order set usage, so the order set required selection by the admitting physician if indicated. Additionally, an embedded 48‐hour order set could be accessed at any time by the ordering physician and included vancomycin dosing. Specific changes to the preexisting order set included the development of sections for purulent and nonpurulent cellulitis as well as recommended antibiotics. Piperacillin/tazobactam and nafcillin were both removed and vancomycin was limited to the purulent subheading. Additionally, elevation of the extremity was preselected, and orderables for imaging (chest x‐ray and duplex ultrasound), antiulcer prophylaxis, telemetry, and electrocardiograph were all removed.
Audit and feedback of cases of cellulitis and broad‐spectrum antibiotic usage was performed by a senior hospitalist.
Study Design
A retrospective before/after study was performed to assess overall impact of the intervention on the patient population. Additionally, a retrospective controlled pre‐/postintervention study was performed to compare changes in cellulitis management for visits where order sets were used with visits where order sets were not used. The intervention initiation date was July 9, 2012. The institutional review board classified this project as quality improvement and did not require review and oversight.
Study Population
We analyzed 2278 ED and inpatient visits for cellulitis, of which 677 met inclusion criteria. We partitioned visits into 2 groups: (1) those for which order sets were used (n = 370) and (2) control visits for which order sets were not used (n = 307). We analyzed outcomes for 2 subpopulations: hospitalized patients for whom the EDOU or admission order sets were used (n = 149) and patients not admitted and only seen in the EDOU for whom the EDOU order set was used (n = 262).
Inclusion Criteria
Inclusion criteria included hospital admission or admission to the EDOU between July 1, 2011 and December 31, 2013, age greater or equal to 18 years, and primary diagnosis of cellulitis as determined by ICD‐9‐CM billing codes 035, 457.2, 681, 681.0, 681.00, 681.01, 681.02, 681.1, 681.10, 681.11, 681.9, 680, 680.0‐9, 682.0‐9, 684, 685.0, 685.1, 686.00, 686.01, 686.09, 686.1, 686.8, 686.9, 910.1, 910.5, 910.7, 910.9, 911.1, 911.3, 911.5, 911.7, 911.9, 912.1, 912.3, 912.5, 912.7, 912.9, 913.1, 913.3, 913.5, 913.7, 913.9, 914.1, 914.3, 914.5, 914.7, 914.9, 915.1, 915.3, 915.5, 915.7, 915.9, 916.1, 916.3, 916.5, 916.7, 916.9, 917.1, 917.3, 917.5, 917.7, 917.9, 919.1, 919.3, 919.5, 919.7, or 919.9.
Data Collection and Preparation
Clinical data were collected in the inpatient EHR (Cerner Corp., Kansas City, MO) and later imported into the enterprise data warehouse (EDW) as part of the normal data flow. Billing data were imported into the EDW from the billing system. Cost data were estimated using the value‐driven outcomes (VDO) tool developed by the University of Utah to identify clinical costs to the UUHC system.[10] All data were extracted from the EDW on September 10, 2014.
Process Metrics, Clinical, and Cost Outcomes
We defined 1 primary outcome (use of broad‐spectrum antibiotics) and 8 secondary outcomes, including process metrics (MRI and CT orders), clinical outcomes (LOS and 30‐day readmissions), and cost outcomes (pharmacy, lab, imaging cost from radiology department, and total facility cost). Broad‐spectrum antibiotics were defined as any use of meropenem (UUHC's carbapenem), piperacillin/tazobactam, or vancomycin and were determined by orders. Thirty‐day readmissions included only inpatient encounters with the primary diagnosis of cellulitis.
Covariates
To control for patient demographics we included age at admission in years and gender into the statistical model. To control for background health state as well as cellulitis severity, we included Charlson Comorbidity Index (CCI) and hospitalization status. CCI was calculated according to the algorithm specified by Quan et al.[11]
Study Hypotheses
First, for all patients, we hypothesized that process metrics as well as clinical and cost outcomes would improve following the implementation of the care pathway. To evaluate this hypothesis, we estimated impact of the time interval (pre‐/postintervention) on all outcomes. Second, we hypothesized that among patients for whom order sets were used (which we deemed to be a proxy for following the agreed‐upon care pathway), there would be a greater improvement than in patients for whom order sets were not used. To evaluate this hypothesis, we estimated interactions between order set use and time period (pre‐/postintervention) for all outcomes.
Statistical Analysis
The variable time period was created to represent the time period before and after the intervention.
We provided unadjusted descriptive statistics for study outcomes and visit characteristics for all patients before and after intervention. Descriptive statistics were expressed as n (%) and mean standard deviation. Simple comparisons were performed based on 2 test of homogeneity for categorical variables and t test or Wilcoxon test for continuous variables.
For before/after analysis, we fitted generalized linear regression models to estimate the change in outcomes of interest before and after intervention for all patients simultaneously. Generalized linear model defined by a binomial distributional assumption and logit link function was used to estimate the effect of the intervention on antibiotic use, imaging orders, and readmission adjusting for effects of age, gender, CCI, and hospitalization status. A generalized linear model defined by a gamma distributional assumption and log link function was used to estimate effect of the intervention on clinical LOS and cost outcomes adjusting for the effects of the same covariates. Generalized linear models with gamma distributional assumptions were used because they are known to perform well even for zero‐inflated semicontinuous cost variables and are easier to interpret than 2‐part models.
For the controlled before/after analysis, the variable order set used was created to represent groups where order sets were used or not used. Similarly, generalized linear models were used to estimate differential effect of the intervention at 2 different order set use levels using an interaction term between order set use and the time period.
P values <0.05 were considered significant. We used SAS version 9.3 statistical software (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
Descriptive Characteristics
Patient characteristics before and after intervention for 677 EDOU and inpatient visits for cellulitis by 618 patients are summarized in the first 4 columns of Table 1. Patient age at admission ranged from 18 to 98 years. Thirty‐eight percent of visits were by female patients. There were 274 visits before the intervention and 403 visits after. Four hundred thirty‐two (64%) were admitted, and 295 (44%) were seen in the EDOU. The admission order set alone was used for 104 visits, the EDOU order set alone was used for 242 visits, and both order sets were used for 24 visits.
| Characteristic | Overall | Order Sets Not Used | Order Sets Used | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 274 | Intervention, N = 403 | P* | Baseline, N = 127 | Intervention, N = 180 | P* | Baseline, N = 147 | Intervention, N = 223 | P* | |
| |||||||||
| Patient Characteristics | |||||||||
| Age, y | 46.8 16.0 | 48.9 17.1 | 0.097 | 49.8 16.0 | 5.1 16.3 | 0.88 | 44.2 15.5 | 48.0 17.6 | 0.032 |
| Female gender | 105 (38%) | 155 (39%) | 0.93 | 50 (39%) | 74 (41%) | 0.73 | 55 (37%) | 81 (36%) | 0.86 |
| CCI | 2.6 3.2 | 2.6 3.0 | 0.69 | 3.2 3.5 | 3.2 3.2 | 0.82 | 2.0 2.8 | 2.1 2.7 | 0.68 |
| Clinical process characteristics | |||||||||
| EDOU admission | 122 (45%) | 173 (43%) | 0.68 | 12 (9%) | 19 (11%) | 0.75 | 110 (75%) | 154 (69%) | 0.23 |
| Hospital admission | 173 (63%) | 259 (64%) | 0.76 | 117 (92%) | 166 (92%) | 0.98 | 56 (38%) | 93 (42%) | 0.49 |
| EDOU order set used | 111 (41%) | 155 (38%) | 0.59 | NA | NA | NA | 111 (76%) | 155 (70%) | 0.21 |
| ADM order set used | 47 (17%) | 81 (20%) | 0.34 | NA | NA | NA | 47 (32%) | 81 (36%) | 0.39 |
| Process outcomes | |||||||||
| Broad‐spectrum antibiotics used | 205 (75%) | 230 (57%) | <0.001 | 90 (71%) | 121 (67%) | 0.50 | 115 (78%) | 109 (49%) | <0.001 |
| MRI done | 27 (10%) | 32 (8%) | 0.39 | 13 (10%) | 20 (11%) | 0.81 | 14 (10%) | 12 (5%) | 0.13 |
| CT done | 56 (20%) | 76 (19%) | 0.61 | 32 (25%) | 43 (24%) | 0.79 | 24 (16%) | 33 (15%) | 0.69 |
| Clinical outcomes | |||||||||
| Length of stay, d | 2.7 2.6 | 2.6 2.8 | 0.35 | 3.6 2.8 | 3.8 3.4 | 0.62 | 2.0 2.1 | 1.7 1.6 | 0.48 |
| 30‐day readmission | 14 (5%) | 17 (4%) | 0.59 | 7 (6%) | 9 (5%) | 0.84 | 7 (5%) | 8 (4%) | 0.58 |
| Cost outcomes | |||||||||
| Pharmacy cost ($) | 1 | 0.76 | 0.002 | 1 | 0.89 | 0.13 | 1 | 0.56 | 0.004 |
| Lab cost ($) | 1 | 0.52 | <0.001 | 1 | 0.53 | 0.001 | 1 | 0.51 | 0.055 |
| Imaging cost ($) | 1 | 0.82 | 0.11 | 1 | 0.95 | 0.52 | 1 | 0.67 | 0.13 |
| Total facility cost ($) | 1 | 0.85 | 0.027 | 1 | 0.91 | 0.042 | 1 | 0.77 | 0.26 |
Before/After Analysis
Among all patients, use of broad‐spectrum antibiotics decreased from 75% to 57% (Table 1). Analysis adjusted for gender, age at admission, CCI, and hospital admission status is provided in Table 2. Overall, there was a 59% decrease in the odds of ordering broad‐spectrum antibiotics (P < 0.001), a 23% decrease in pharmacy cost (P = 0.002), a 44% decrease in laboratory cost (P < 0.001), and a 13% decrease in total facility cost (P = 0.006).
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Antibiotics used | Time period | 0.41 (0.29, 0.59) | 59% (71% to 41%) | <0.001 |
| MRI done | Time period | 0.74 (0.43, 1.30) | 26% (57% to 30%) | 0.29 |
| CT done | Time period | 0.92 (0.62, 1.36) | 8% (38% to 36%) | 0.67 |
| 30‐day readmission | Time period | 0.86 (0.41, 1.80) | 14% (59% to 80%) | 0.69 |
| Length of stay, d | Time period | 0.97 (0.91, 1.03) | 3% (9% to 3%) | 0.34 |
| Pharmacy cost ($) | Time period | 0.77 (0.65, 0.91) | 23% (35% to 9%) | 0.002 |
| Lab cost ($) | Time period | 0.56 (0.48, 0.65) | 44% (52% to 35%) | <0.001 |
| Imaging cost($) | Time period | 0.90 (0.71, 1.14) | 10% (29% to 14%) | 0.38 |
| Total facility cost ($) | Time Period | 0.87 (0.79, 0.96) | 13% (21% to 4%) | 0.006 |
Order Set Use Groups Analysis
Descriptive statistics and simple comparison before/after the intervention for the 2 study groups are shown in the last 6 columns of Table 1. Among patients for whom order sets were used, broad‐spectrum antibiotic usage significantly decreased from 78% before the intervention to 49% after the intervention (P < 0.001). In contrast, among patients for whom order sets were not used, broad‐spectrum antibiotic usage remained relatively constant71% before the intervention versus 67% after the intervention (P = 0.50). Figure 2 shows semiannual changes in the prescription of broad‐spectrum antibiotics. There is a noticeable drop after the intervention among patients for whom order sets were used.

Analysis of the interaction between time period and order set usage is provided in Table 3. After the intervention, patients for whom the order sets were used had greater improvement in broad‐spectrum antibiotic selection (75% decrease, P < 0.001) and LOS (25% decrease, P = 0.041) than patients for whom order sets were not used. Pharmacy costs also decreased by 13% more among patients for whom the order sets were used, although the interaction was not statistically significant (P = 0.074). Laboratory costs decreased in both groups, but order set use did not demonstrate an interaction (P = 0.5). Similar results were found for the subgroups of admitted patients and patients seen in the EDOU.
| Logistic Regression | ||||
|---|---|---|---|---|
| Outcome Variables | Selected Predictor Variables | Odds* | Percent Change | P |
| Gamma Regression | ||||
| Outcome Variables | Selected Predictor Variables | Fold Change* | Percent Change | P |
| ||||
| Broad spectrum antibiotics | Time period | 0.84 (0.50, 1.40) | 16% (50% to 40%) | 0.50 |
| Time periodorder set | 0.25 (0.12, 0.52) | 75% (88% to 48%) | <0.001 | |
| MRI done | Time period | 1.04 (0.49, 2.20) | 4% (51% to 120%) | 0.92 |
| Time periodorder set | 0.44 (0.14, 1.38) | 56% (86% to 38%) | 0.16 | |
| CT done | Time period | 0.94 (0.55, 1.60) | 6% (45% to 60%) | 0.81 |
| Time periodorder set | 0.96 (0.44, 2.12) | 4% (56% to 112%) | 0.93 | |
| 30‐day readmission | Time period | 0.91 (0.33, 2.53) | 9% (67% to 153%) | 0.86 |
| Time periodorder set | 0.88 (0.20, 3.93) | 12% (80% to 293%) | 0.87 | |
| Clinical length of stay | Time period | 1.04 (0.95, 1.14) | 4% (5% to 14%) | 0.41 |
| Time periodorder set | 0.87 (0.77, 0.99) | 13% (23% to 1%) | 0.041 | |
| Pharmacy cost ($) | Time period | 0.88 (0.70, 1.12) | 12% (30% to 12%) | 0.31 |
| Time periodorder set | 0.75 (0.54, 1.03) | 25% (46% to 3%) | 0.074 | |
| Lab cost ($) | Time period | 0.53 (0.42, 0.66) | 47% (58% to 34%) | <0.001 |
| Time periodorder set | 1.11 (0.82, 1.50) | 11% (18% to 50%) | 0.50 | |
| Imaging cost ($) | Time period | 1.00 (0.71, 1.40) | 0% (29% to 40%) | 0.98 |
| Time periodorder set | 0.82 (0.51, 1.30) | 18% (49% to 30%) | 0.39 | |
| Facility cost ($) | Time period | 0.92 (0.80, 1.05) | 8% (20% to 5%) | 0.22 |
| Time periodorder set | 0.90 (0.75, 1.09) | 10% (25% to 9%) | 0.29 | |
Audit and feedback was initially performed for cases of cellulitis using broad‐spectrum antibiotics. However, given the complexity of cellulitis as a disease process and the frequency of broad‐spectrum antibiotic usage, in all cases of review, it was deemed reasonable to use broad‐spectrum antibiotics. Therefore, the audit was not continued.
DISCUSSION
Care pathways have demonstrated improvement across multiple different disease states including cellulitis.[4, 5] They have been noted to reduce variation in practice and improve physician agreement about treatment options.[4] The best method for implementation is not clearly understood,[12] and there remains concern about maintaining flexibility for patient care.[13] Additionally, although implementation of pathways is often well described, evaluations of the processes are noted to frequently be weak.[12] UUHC felt that the literature supported implementing a care pathway for the diagnosis of cellulitis, but that a thorough evaluation was also needed to understand any resulting benefits or harms. Through this study, we found that the implementation of this pathway resulted in a significant decrease in broad‐spectrum antibiotic use, pharmacy costs, and total facility costs. There was also a trend to decrease in imaging cost, and there were no adverse effects on LOS or 30‐day readmissions. Our findings demonstrate that care‐pathway implementation accompanied by education, pathway‐compliant electronic order sets, and audit and feedback can help drive improvements in quality while reducing costs. This finding furthers the evidence supporting standard work through the creation of clinical care pathways for cellulitis as an effective intervention.[4] Additionally, although not measured in this study, reduction of antibiotic use is supported as a measure to help reduce Clostridium difficile infections, a further potential benefit.[14]
This study has several important strengths. First, we included accurate cost analyses using the VDO tool. Given the increasing importance of improving care value, we feel the inclusion of such cost analysis is an increasingly important aspect of health service intervention evaluations. Second, we used a formal benchmarking approach to identify a priority care improvement area and to monitor changes in practice following the rollout of the intervention. We feel this approach provides a useful example on how to systematically improve care quality and value in a broader health system context. Third, we evaluated not order set implementation per se, but rather changing an existing order set. Because studies in this area generally focus on initial order set implementation, our study contributes insights on what can be expected through modifications of existing order sets based on care pathways. Fourth, the analysis accounted for a variety of variables including the CCI. Of interest, our study found that the intervention group (patients for whom order sets were used) had a lower CCI, confirming Allen et al.'s findings that diseases with predictable trajectories are the most likely to benefit from care pathways.[4] As a final strength, the narrative‐based order set intervention was relatively simple, and the inclusion criteria were broad, making the process generalizable.
Limitations of this study include that it was a single center pre‐/postintervention study and not a randomized controlled trial. Related to this limitation, the control group for which order sets were not used reflected a different patient population compared to the intervention group for which order sets were used. Specifically, it was more common for order sets to be used in the EDOU than upon admission, resulting in the order set group consisting of patients with less comorbidities than patients in the nonorder set group. Additionally, patients in the order set intervention group were older than in the baseline group (48.0 vs 44.2 years). However, these differences in population remained relatively stable before and after the intervention, and relevant variables including demographic factors and CCI were accounted for in the regression models. Nevertheless, it remains possible that secular trends existed that we did not capture that affected the 2 populations differently. For example, there was a separate project that overlapped with the intervention period to reduce unnecessary laboratory usage at UUHC. This intervention could have influenced the trend to decreased laboratory utilization in the postintervention period. However, there were no concurrent initiatives to reduce antibiotic use during the study period. As a final limitation, the statistical analyses have not corrected for multiple testing for the secondary outcomes.
CONCLUSION
Using benchmark data from UHC, an academic medical center was able to identify an opportunity for improving the care of patients with cellulitis and subsequently develop an evidence‐based care pathway. The implementation of this pathway correlated with a significant reduction of broad‐spectrum antibiotic use, pharmacy costs, and total facility costs without adverse clinical affects. An important factor in the success of the intervention was the use of electronic order sets for cellulitis, which provided support for the implementation of the care pathway. This study demonstrates that the intervention was not only effective overall, but that it was more effective for those patients for whom the order set was used. This study adds to the growing body of literature suggesting that a well‐defined care pathway can improve outcomes and reduce costs for patients and institutions.
Acknowledgements
The authors thank Ms. Pam Proctor for her assistance in implementation of the care pathway and Ms. Selma Lopez for her editorial assistance.
Disclosures: K.K. is or has been a consultant on clinical decision support (CDS) or electronic clinical quality measurement to the US Office of the National Coordinator for Health Information Technology, ARUP Laboratories, McKesson InterQual, ESAC, Inc., JBS International, Inc., Inflexxion, Inc., Intelligent Automation, Inc., Partners HealthCare, Mayo Clinic, and the RAND Corporation. K.K. receives royalties for a Duke Universityowned CDS technology for infectious disease management known as CustomID that he helped develop. K.K. was formerly a consultant for Religent, Inc. and a co‐owner and consultant for Clinica Software, Inc., both of which provide commercial CDS services, including through use of a CDS technology known as SEBASTIAN that K.K. developed. K.K. no longer has a financial relationship with either Religent or Clinica Software. K.K. has no competing interest with any specific product or intervention evaluated in this manuscript. All other authors declare no competing interests.
- . Cellulitis. N Engl J Med. 2004;350(9):904–912.
- , , , , , . Cost‐effectiveness of blood cultures for adult patients with cellulitis. Clin Infect Dis. 1999;29(6):1483–1488.
- , , , et al. Methicillin‐resistant s. aureus infectious among patients in the emergency department. N Engl J Med. 2006;355:666–674.
- , , . Systematic review of the effectiveness of integrated care pathways: what works, for whom, in which circumstances? Int J Evid Based Healthc. 2009;7:61–74.
- . Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072–1079.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41:1373–1406.
- , , , et al. Clinical practice guidelines by the Infectious Disease Society of American for the treatment of methicillin‐resistant Staphylococcus aureus infectious in adults and children. Clin Infect Dis. 2011;42:1–38.
- , , , . The role of b‐hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;89:217–226.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Disease Society of America. Clin Infect Dis. 2014;59(2):147–159.
- , , , et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223–235.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:113–1139.
- , . Computerization of workflows, guidelines, and care pathways: a review of implementation challenges for process‐oriented health information systems. J Am Med Inform Assoc. 2011;18:738–748.
- , , , et al. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32(5):911–920.
- , , , et al. Clinical practice guidelines for clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455.
- . Cellulitis. N Engl J Med. 2004;350(9):904–912.
- , , , , , . Cost‐effectiveness of blood cultures for adult patients with cellulitis. Clin Infect Dis. 1999;29(6):1483–1488.
- , , , et al. Methicillin‐resistant s. aureus infectious among patients in the emergency department. N Engl J Med. 2006;355:666–674.
- , , . Systematic review of the effectiveness of integrated care pathways: what works, for whom, in which circumstances? Int J Evid Based Healthc. 2009;7:61–74.
- . Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072–1079.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41:1373–1406.
- , , , et al. Clinical practice guidelines by the Infectious Disease Society of American for the treatment of methicillin‐resistant Staphylococcus aureus infectious in adults and children. Clin Infect Dis. 2011;42:1–38.
- , , , . The role of b‐hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;89:217–226.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Disease Society of America. Clin Infect Dis. 2014;59(2):147–159.
- , , , et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223–235.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:113–1139.
- , . Computerization of workflows, guidelines, and care pathways: a review of implementation challenges for process‐oriented health information systems. J Am Med Inform Assoc. 2011;18:738–748.
- , , , et al. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32(5):911–920.
- , , , et al. Clinical practice guidelines for clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455.
© 2015 Society of Hospital Medicine
Ultrasound and Pleural Effusions
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions 300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]
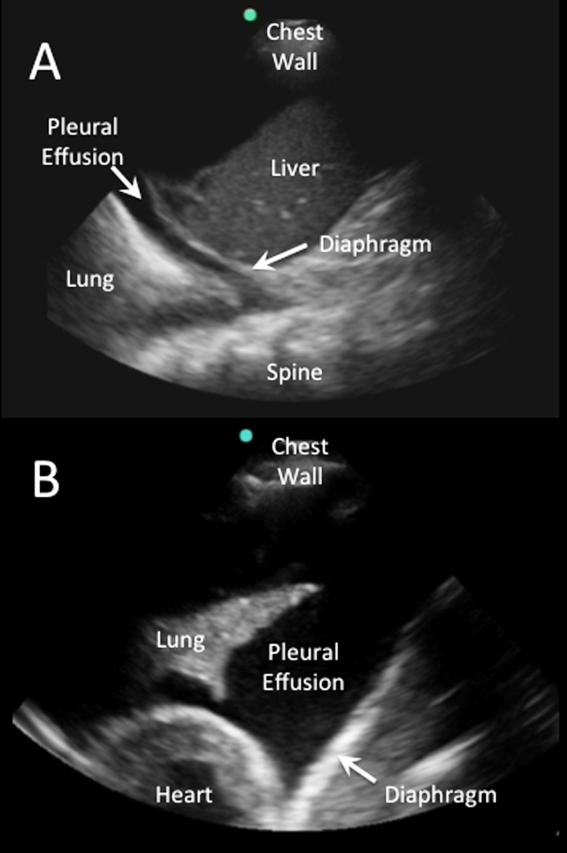
Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).
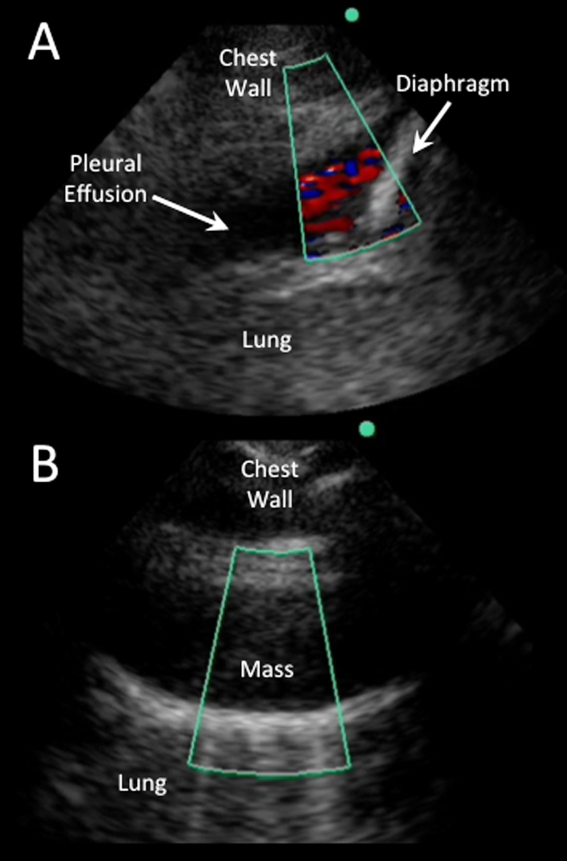
CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]
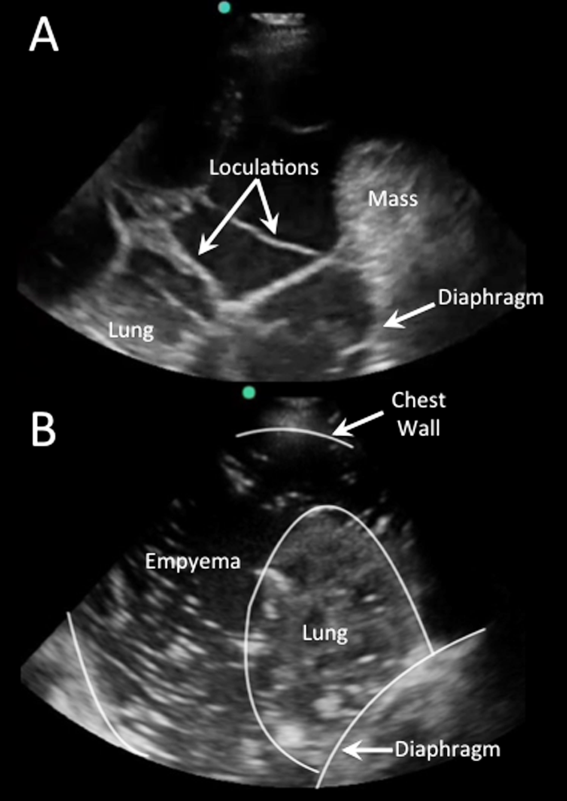
Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.
The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions 300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]


Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).

CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]

Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.

The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions 300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]


Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).

CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]

Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.

The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.
Update in Sepsis Management
Sepsis is one of the oldest and most elusive syndromes in medicine, and remains a significant contributor to morbidity, mortality, and healthcare expenditure.[1] A 1992 American College of Chest Physicians and Society of Critical Care Medicine consensus conference statement introduced the systemic inflammatory response syndrome (SIRS) into the medical lexicon, along with definitions of sepsis, severe sepsis, and septic shock.[2] A 2003 consensus panel expanded the list of signs and symptoms associated with sepsis, and warned that the findings of SIRS do not differentiate sepsis from other noninfectious conditions.[3] The terminology is important, as these definitions resulted in a shift of the label of the syndrome of infection complicated by end‐organ dysfunction from sepsis to severe sepsis or septic shock. Overlap of these terms has implications for categorizing such infections for the purpose of investigation, estimating epidemiology and outcome, and coding, billing, and reimbursement.[1]
Traditional definitions of the spectrum of sepsis disorders are outlined in Table 1,[2, 3] and it is important to note that an update to these definitions is anticipated in the near future. A recent publication has called into question the sensitivity and categorical requirement of at least 2 SIRS criteria to define severe sepsis.[4] This study of more than 1 million patients from 172 intensive care units (ICUs) in Australia and New Zealand from 2000 to 2013 found that the cutoff of 2 SIRS criteria to define severe sepsis excluded 1 in 8 patients with infection and end‐organ hypoperfusion. SIRS‐negative severe sepsis patients experienced the same mortality as SIRS‐positive patients. In addition, adjusted analysis determined a stepwise increase in mortality risk associated with each additional SIRS criterion without a transition point in risk noted at 2.[4]
| Definition | |
|---|---|
| |
| SIRS | The systemic inflammatory response to a variety of severe insults. Requires 2 of the following: |
| Temperature >38C or 36C | |
| Heart rate >90 beats/minute | |
| Respiratory rate >20 breaths/minute or partial pressure of carbon dioxide (PaCO2) 32 mm Hg | |
| White blood cell count >12,000 or 4,000 cells/L or 10% immature (band) forms | |
| Sepsis | The systemic response to infection, with SIRS criteria met in the setting of documented or strongly suspected infection |
| Severe sepsis | Sepsis associated with organ dysfunction, hypoperfusion (including but not limited to lactic acidosis, oliguria, or acute alteration in mental status), or hypotension (systolic blood pressure 90 mm Hg or >40 mm Hg below baseline). |
| Septic shock | Sepsis‐induced hypotension despite adequate volume resuscitation (2030 mL/kg) with perfusion abnormalities including but not limited to lactic acidosis, oliguria, or acute alteration in mental status |
From 1979 through 2000, there were over 10 million reported cases of sepsis, which accounted for 1.3% of all hospitalizations in the United States.[5] Normalized to the population distribution of the 2000 US Census, there was an annualized increase in sepsis cases of 8.7%. A 2011 report revealed rates of hospitalization for patients with septicemia or sepsis in the United States more than doubled from 2000 through 2008.[6] Patients with sepsis experienced longer length of stay than other inpatients and were 8 times more likely to die during hospitalization.[6] Estimates of severe sepsis incidence are complicated by how acute organ dysfunction is defined and whether it is related to infection. As of 2001, the number of severe sepsis cases in the United States was believed to exceed 750,000 and comprise approximately 10% of ICU admissions.[1] The incidence of severe sepsis cases in the United States continues to rise.[7, 8, 9] However, a more than doubling of the use of sepsis International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes from 2004 through 2009 has also been noted.[8] Based on ICD‐9‐CM codes indicating the presence of sepsis and organ system failure, the number of severe sepsis hospitalizations per 100,000 persons in the United States increased from 143 in 2000 to 343 in 2007.[7] Total hospital costs for patients with severe sepsis were estimated to increase 57%, from $15.4 billion in 2003 to $24.3 billion in 2007.[9] The Agency for Healthcare Research and Quality considered septicemia the most expensive medical condition in the United States in a 2011 data brief, with annual aggregate hospital costs exceeding $20 billion.[10]
Although many hospitalists care for patients in the ICU and other higher acuity or step‐down units, a significant proportion of patients with severe sepsis receive care on a general medical floor.[11, 12, 13] Sepsis is also clearly not an issue restricted to patients on internal medicine services. Of over 360,000 general surgery patients from 2005 to 2007, the incidences of sepsis (2.3%) and septic shock (1.6%) greatly exceeded those of pulmonary embolism (0.3%) and myocardial infarction (0.2%). In this cohort, the need for emergency surgery and the presence of any comorbidity increased the number of sepsis cases.[14]
Despite difficulties obtaining exact estimates of case numbers, the following appears true: the spectrum of sepsis disorders (including severe sepsis and septic shock) remains a common, costly, and increasing clinical entity that is encountered by hospital medicine physicians in a variety inpatient settings. This review will provide an update for hospitalists based on many important studies that have been published since the last review of this topic in this journal.[15] The expanding evidence base in sepsis includes early goal‐directed therapy (EGDT), clinical endpoints, and bundles of care for sepsis; antibiotics (choice and timing); volume resuscitation; ICU considerations, including the use of insulin and corticosteroids; and mortality, complications, and the advent of the condition of sepsis survivorship.
EARLY GOAL‐DIRECTED THERAPY
A 2001 prospective, randomized trial of EGDT initiated in the emergency department (ED) for patients with severe sepsis and septic shock resulted in an impressive 16% reduction of in‐hospital mortality compared to standard therapy.[16] The intervention protocol included central venous catheter placement and a 500‐mL bolus of crystalloid every 30 minutes to establish a central venous pressure (CVP) of 8 to 12 mm Hg. Vasopressors were used to maintain a mean arterial pressure (MAP) greater than 65 mm Hg, and patients with a MAP greater than 90 mm Hg were given vasodilators. Patients with a central venous oxygen saturation (Scv02) of less than 70% received red blood cell transfusion with a goal hematocrit of 30%. If central venous oxygen saturation remained less than 70% despite these interventions, dobutamine was used for inotropic effect until this goal was achieved or was limited by tachycardia or hypotension.[16]
These results prompted inclusion of the specific hemodynamic targets (CVP, MAP, and Scv02) into the original 2004 Surviving Sepsis Campaign guidelines and spurred a decade of interest worldwide.[17] The incremental importance of these individual components in managing severe sepsis and septic shock has since come under scrutiny. A recent randomized trial suggested that EGDT guided by venous lactate clearance of >10% was noninferior to the goal Scv02 of >70%. However, only 10% of the study population required transfusion or dobutamine.[18, 19] Prospective ICU data on lactate‐guided therapy[20] supported the revised 2012 Surviving Sepsis Campaign (SSC) guidelines to recommend lactate normalization as part of initial resuscitation efforts, particularly when Scv02 is not available.[21] Lactate measurement may assist in recognition of cases of severe sepsis or septic shock and provide valuable triage information, as serum lactate has been shown to predict mortality from severe sepsis independent of shock or organ failure.[22] In a retrospective study of patients presenting to the ED with sepsis, a lactate >4 mmol/L was associated with progression to septic shock within 4 to 48 hours.[23]
Our understanding of the specific benefits of EGDT is far from complete, as 3 recent large prospective, multicenter, randomized trials ProCESS (Protocolized Care for Early Septic Shock), ARISE (Australasian Resuscitation In Sepsis Evaluation), and ProMISe (Protocolised Management in Sepsis) did not show EGDT protocols to be superior to usual care.[24, 25, 26] Interpreted collectively, the benefit of EGDT may not be from targeting specific physiologic parameters, but rather from the early recognition of sepsis and the appropriate use of well‐supported interventions like aggressive fluid resuscitation and early/efficacious antibiotics.[27]
Although the precise benefit of EGDT as a package versus its individual components remains in question, we have a decade of experience in delivering this care as an integral component of the bundles put forth in the SSC guidelines.[28] Observational and retrospective studies have shown increased compliance with guidelines and improved mortality after implementing these protocols, although early bundles for severe sepsis included therapies that have subsequently been called into question on an individual basis like drotrecogin alfa (activated) and glucocorticoid therapy.[29, 30, 31] The mortality benefit from sepsis bundles deserves further explanation, although education and early recognition are likely contributory.[32]
Several studies evaluated individual components of EGDT. The TRISS (Transfusion Requirements in Septic Shock) trial randomized ICU patients with septic shock to 2 different red blood cell transfusion strategies, and found no mortality benefit or reduction in ischemic events for patients transfused at a hemoglobin of 9 g/dL compared to the 7 g/dL threshold.[33] The SEPSISPAM (Assessment of Two Levels of Arterial Pressure on Survival in Patients With Septic Shock) trial compared the MAP goal of 65 to 70 mm Hg to 80 to 85 mm Hg for patients with septic shock in a randomized, multicenter trial.[34] Although there was no difference in 28‐day mortality, more atrial fibrillation was diagnosed in the higher target group. For patients with chronic hypertension, targeting the higher MAP led to less renal injury and reduced the need for renal‐replacement therapy.[34, 35] Identifying specific subsets of patients with sepsis who benefit most from particular therapies should help clinicians set patient‐specific goals and targets.
Although we can expect additional studies to provide further guidance, it is reasonable at present to adhere to protocols designed to improve timely sepsis detection and management with aggressive volume resuscitation, early/efficacious antibiotic administration, and effective infection source control.
ANTIBIOTICS AND SOURCE CONTROL
Administration of broad‐spectrum antibiotics has long been the cornerstone of sepsis management. Timely antibiotic infusion is an integral part of the 2004 and 2012 SSC guidelines,[17, 21] with the caveat that blood cultures should be obtained prior to antibiotic therapy provided that no significant delay (>45 minutes) occurs.[21] Recent studies have begun to address fundamental clinical questions, including the timing of antibiotic administration and the efficacy of empiric antibiotic choice. A landmark retrospective cohort study of ICU patients with septic shock demonstrated survival to hospital discharge was highest in patients who received antibiotics within the first hour of hypotension.[36] Survival decreased on average by 7.6% with each hour that antibiotics were delayed. Only 50% of patients with septic shock in this study received effective antibiotic therapy within 6 hours of documented hypotension.[36] A subsequent retrospective, single‐center cohort study of ED patients with severe sepsis or septic shock undergoing EGDT showed a mortality benefit when antibiotics were administered within the first hour. However, it did not demonstrate a statistically significant decline in survival on an hourly basis thereafter.[37]
A prospective, multicenter ED trial that included patients with severe sepsis in addition to septic shock[38] did not show a mortality benefit to administration of antibiotics within the first hour. In‐hospital mortality risk for patients undergoing EGDT was similar across patients in whom time to antibiotics was delayed up to 6 hours after triage.[36, 38] However, patients with severe sepsis in whom antibiotics were delayed until shock was recognized faced a statistically significant increased risk of death (odds ratio = 2.35; 95% confidence interval = 1.12‐4.53).[38, 39] A retrospective study of 28,150 patients from the SSC database demonstrated a statistically significant increase in mortality for each hour that empiric antibiotics were delayed.[40] Importantly, this trend was preserved regardless of location of sepsis diagnosis (ED, ICU, and hospital ward) and across illness severity. Though there remains debate about the critical importance of the golden hour for antibiotic administration, overall current evidence supports early empiric antibiotics in severe sepsis and septic shock.
Choosing an empiric antibiotic regimen, based on infection source and host factors, also plays a key role in sepsis outcomes. A retrospective study of patients with septic shock from 1996 to 2005 showed that inappropriate initial antibiotics (based on eventual in vitro culture sensitivities or evaluation of clinical syndrome) were used 20% of the time and resulted in a 5‐fold reduction in survival.[41] A retrospective cohort study of patients with gram‐negative bacteremia and severe sepsis or septic shock found prior antibiotic exposure within 90 days to be an independent risk factor for drug resistance and in‐hospital mortality.[42] However, careful consideration of side effects should also influence choice of initial antibiotic therapy. A Cochrane review citing 69 trials and containing 7863 subjects with sepsis compared empiric ‐lactam therapy to ‐lactamaminoglycoside combination therapy.[43] All‐cause mortality and clinical failure was similar in both groups, as was the rate of resistance. Importantly, nephrotoxicity was significantly less in the ‐lactam monotherapy group.[43]
Infection source control is an essential component of sepsis management that should occur simultaneously with antibiotic administration. The 2012 SSC guidelines promote infection source control within 12 hours of diagnosis, with consideration of the risks and benefits therein and preference for interventions with the lowest associated physiologic insult.[21] Intravascular access devices should be recognized as a common source of infection, and should be removed after alternative access has been established.[21]
FLUID RESUSCITATION
Volume resuscitation is an essential component of sepsis management, regardless of algorithm or endpoint. Three main types of nonblood product fluid resuscitation have been used: crystalloid (saline and Ringer's solutions), colloid (typically an albumin‐containing solution), and synthetic volume expanders (hydroxyethyl starch [HES] and similar compounds).
Multiple large studies confirmed the lack of a favorable riskbenefit ratio with synthetic volume expanders. Among nearly 800 patients with severe sepsis who were randomized to receive either Ringer's acetate or HES 130/0.4, a significantly higher number of patients receiving HES died (51% vs 43%, relative risk [RR] = 1.17), and required renal‐replacement therapy (22% vs 16%, RR = 1.35). One patient in each group was dialysis dependent at 90 days.[44] An additional multicenter, prospective study of HES versus 0.9% (normal) saline for fluid resuscitation in the ICU found no significant difference in mortality (18% vs 17%, P = 0.26), but did note a higher need for renal‐replacement therapy in the HES group (7.0% vs 5.8%, RR = 1.21).[45] A systematic review incorporating 9 trials that randomized approximately 3400 patients with sepsis receiving either HES, crystalloid, or colloid showed no difference in mortality, although there was an excess risk for renal‐replacement therapy (RR = 1.36), serious adverse events (RR = 1.30), and red blood cell transfusion (RR = 1.29) in patients receiving HES.[46] A second, larger systematic review concluded that HES was associated with an increased mortality compared with crystalloids, albumin, or gelatin (RR = 1.09). Additionally, an increase in renal failure (RR = 1.27) and renal‐replacement therapy (RR = 1.32) was also noted.[47]
The debate between crystalloid and colloid (namely albumin) for fluid resuscitation is ongoing, with recent important additions to the literature. The SAFE (Saline Versus Albumin Fluid Evaluation) study investigators in 2004 randomized nearly 7000 patients to receive either 4% albumin solution or normal saline. At 28 days, no significant differences were found in mortality, new organ failure, ICU and hospital length of stay, days of mechanical ventilation, or days of renal‐replacement therapy.[48] In 2014, another multicenter prospective study of 1800 patients with severe sepsis or septic shock in 100 ICUs in Italy compared 20% albumin and crystalloid solution to crystalloid solution alone. Mortality, end‐organ dysfunction, and ICU length of stay did not differ between groups.[49] Two 2014 systematic reviews and meta‐analyses on fluid resuscitation produced somewhat differing conclusions. Patel et al. evaluated data from 16 randomized trials including more than 4000 patients receiving albumin for volume resuscitation in adults with sepsis. Albumin provided no significant survival advantage in total or in any subgroup, regardless of severity of illness or baseline albumin level, thus arguing against its routine use.[50] Rochwerg et al. evaluated 14 studies with approximately 19,000 patients using Bayesian network meta‐analysis technique. This study concluded that albumin is associated with reduced mortality compared with other fluids, and also that balanced crystalloids (eg, Ringer's lactate and similar) may have lower mortality than normal saline.[51] A chloride‐restrictive resuscitation approach has also been associated with a lower incidence of acute kidney injury in critically ill adults.[52]
The SSC confirmed its recommendations of a minimum of 30 mL/kg of crystalloids as the initial fluid of choice in sepsis in 2012, but added a suggestion for the addition of albumin in patients requiring substantial amounts of crystalloid.[21] The currently available data suggest crystalloid fluids to be the best‐supported initial fluid in the management of sepsis, and that synthetic colloids should be avoided. Prospective data are still required to answer questions regarding the potential advantages of albumin or balanced and/or chloride‐restricted crystalloids.
ICU CONSIDERATIONS
Appropriate management of patients with the syndrome of sepsis, severe sepsis, and septic shock on the hospital ward requires a working knowledge of recent research conducted in the ICU setting. Although conclusions based on data from patients with septic shock might not be generalizable to less severe cases of sepsis, recent trials on glucose control and corticosteroids deserve consideration.
Intensive insulin treatment in the medical ICU is no longer standard practice. In the NICE‐SUGAR (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) trial, a large, multicenter randomized controlled trial of ICU patients, close to 20% of patients had severe sepsis at the time of randomization.[53] This subgroup did not benefit from intensive glucose control (a target of 81108 mg/dL) in terms of 90‐day mortality.[53] In contrast to prior studies of glycemic control in the critically ill, the intensive treatment group overall suffered increased mortality.[54] The COIITSS (Combination of Corticotherapy and Intensive Insulin Therapy for Septic Shock) trial looked at intensive insulin therapy in patients with septic shock being treated with corticosteroids, a group particularly at risk for hyperglycemia. In this study, intensive insulin therapy did not improve in‐hospital mortality.[55] Based on these and other ICU data, the current SSC recommendation is to target a blood glucose of 180 mg/dL for patients with severe sepsis.[21, 56]
The use of corticosteroids to treat the host response in septic shock has been re‐evaluated.[57] The 2004 SSC guidelines recommended hydrocortisone therapy for 7 days in patients with septic shock requiring vasopressor support after fluid resuscitation.[17] This recommendation was based on data from a placebo‐controlled multicenter trial in France that showed improved shock reversal and reduced mortality in patients with septic shock who were treated with hydrocortisone and fludricortisone.[58] Of note, these patients were enrolled on the basis of hypotension despite intravenous fluids and the initiation of 1 vasopressor. The benefit of corticosteroids was seen only in patients deemed to have relative adrenal insufficiency based on response corticotropin testing.[58] However, the CORTICUS (Corticosteroid Therapy of Septic Shock) study, a subsequent multicenter, placebo‐controlled, randomized controlled trial failed to show a benefit to corticotropin testing in identifying patients with septic shock who would benefit from corticorsteroids.[59] The corticosteroid treatment arm similarly benefited from faster shock reversal, but at the expense of increased superinfection.[59] Although underpowered, CORTICUS did not show a survival benefit to corticosteroids in septic shock.[59] The most recent SSC guidelines do not recommend corticotropin (adrenocorticotropic hormone) stimulation testing and do not advise corticosteroids in septic shock if fluid resuscitation and vasopressor therapy restore hemodynamic stability during initial resuscitation.[21] Future studies may clarify subpopulations of patients with sepsis who benefit from corticosteroids.
OUTCOMES: MORTALITY AND COMPLICATIONS
An understanding of the currently available information regarding the morbidity and mortality associated with severe sepsis is essential for the practicing hospitalist. Whether transferring care of patients to or receiving patients from the ICU, hospitalists must lead discussions with patients and families regarding prognosis, especially as it informs disposition. Hospitalists are often asked to make projections on outcome as well as the timing and venue of disposition. Clarification of patient wishes and goals of care remains an essential first step in the care of septic patients. Recently published studies provide prognostic information, including mortality (both short and long term) as well as complications associated with severe sepsis.
The attributable mortality for severe sepsis has been predominantly reported to date as short‐term (usually in‐hospital). A meta‐analysis of US patients from 1991 to 2009 demonstrated a 3% annual decline in the short‐term (28 day) mortality from severe sepsis using 2 previously validated algorithms. Data from 36 trials (and approximately 14,000 patients) revealed a decrease in mortality from 47% in the period from 1991 to 1995 to 29% from 2006 to 2009.[60] Although the methods employed (sepsis definitions and ICD‐9‐CM codes) can have a significant impact on estimates of mortality, these results corroborate a progressive decline in short‐term mortality from severe sepsis in the United States between 2004 and 2009 using 4 validated algorithms.[8] Outside the United States, a recent retrospective analysis of more than 1 million patients with severe sepsis treated in the ICU in Australia and New Zealand from 2000 to 2012 also demonstrated a decrease in adjusted in‐hospital mortality. In this study, short‐term mortality declined yearly, with an odds ratio of death of 0.49 in 2012 compared with 2000.[61] Hospital case volume has also been shown to impact rates of inpatient death, with higher‐volume centers demonstrating lower mortality attributed to severe sepsis.[62, 63]
The sufficiency of short‐term mortality as the sole metric for severe sepsis outcome has been more recently questioned.[64, 65] The extent to which full recovery and significant morbidity are affected relative to the change in death rate is unknown, and as such, more data on morbidity and longer‐term mortality are necessary. A Danish study examined data from several registries of patients with severe sepsis. Compared with community‐matched controls, patients with severe sepsis had an increased risk of death at 30 days (hazard ratio [HR] = 90.8), from 30 days to 1 year (HR = 2.7), and 1 to 4 years (HR = 2.3) after discharge.[66] Older survivors of severe sepsis also appear to have higher healthcare utilization in the year following discharge. An analysis of older severe sepsis survivors showed a striking increase in healthcare use relative to their prior resource use, driven primarily by higher number of days in inpatient healthcare facilities. Survivors of severe sepsis also had a significantly higher 90‐day and 1‐year mortality than matched controls.[67]
Increased attention is currently being given to sepsis‐related complications, especially functional and cognitive impairments in older patients. Sepsis survivorship is a swiftly mounting public health issue for older Americans.[68] An 8‐year follow‐up of older sepsis survivors demonstrated a significant increase in the odds of both physical and cognitive dysfunction. During this period, moderate‐to‐severe cognitive dysfunction increased 3‐fold (6.1% before sepsis, 16.7% after).[69] The mechanism by which this dysfunction occurs is unknown, as are the relative contributions of infection site/etiology, ICU length of stay, and extent of organ dysfunction. New functional impairment has been demonstrated in patients with severe sepsis initially admitted to a general floor, even with good baseline function,[12] as well as decreased quality of life in sepsis survivors.[65, 70] Another study showed more admissions complicated by severe sepsis resulted in discharge to a long‐term care facility in 2007 compared to 2000.[7]
Additional organ‐specific consequences of severe sepsis have also been recently suggested. A retrospective analysis showed an increase in the incidence of new‐onset atrial fibrillation in severe sepsis, with an associated increase in risk of in‐hospital stroke and death. New‐onset atrial fibrillation was present in 5.9% of patients with severe sepsis, compared with 0.65% in patients without. Severe sepsis patients with new‐onset atrial fibrillation had an increased risk of in‐hospital stroke (adjusted odds ratio = 2.70) and mortality (adjusted RR = 1.07).[71] These findings suggest association only, and further investigation is warranted. It remains to be seen whether interventions to restore sinus rhythm or anticoagulation are warranted. Preoperative sepsis (within 48 hours) has also been proposed as a risk for postoperative (30 day) arterial (myocardial infarction, stroke) and venous (deep venous thrombosis, pulmonary embolism) thromboembolism. The authors of this study suggest deferral of elective surgery or specific attention to postoperative thromboprophylaxis in patients in whom procedures must occur.[72] This has particular relevance for those septic patients in whom surgical source control is indicated.
Estimates regarding mortality and specific complications attributable to severe sepsis are ongoing, though clearly with a new focus upon metrics other than short‐term mortality. Furthermore, recent data to suggest source of infection as a major driver of mortality in septic shock[73] may contribute to the evolution of the conceptualization of sepsis similar to that of cancer: a heterogeneous collection of disease, among which mortality is determined by specific subtypes. At present, this much appears clear: the previously held notion that survival of a septic insult is unlikely to have future implications is under siege.[74] The extent to which complications and increased longer‐term mortality may reflect generally poorer health at the time of infection versus a sequelae of the survived episode itself is not yet known.
CONCLUSIONS
The past decade of sepsis research has led to significant findings that will change clinical practice for hospital medicine practitioners. Although the incidence of severe sepsis in the United States has continued to rise, in‐hospital mortality has declined; in this context, the management of the spectrum of sepsis disorders is no longer restricted to the ICU, and the entity of sepsis survivorship has blossomed. Prompt recognition of sepsis and improvements in supportive care are likely responsible for improved patient outcomes. EGDT has been called into question as a protocol whose benefit lies not in specific targets or endpoints, but rather in the early recognition of sepsis, appropriate fluid resuscitation, and early/effective antibiotics. Synthetic volume expanders, intensive insulin therapy, and routine use of corticosteroids are no longer recommended.
Hospitalists are a critical link in providing timely, evidence‐based care for patients with sepsis from initial recognition to post‐ICU recovery. Specialized care for the survivors of septic shock is a burgeoning area, and hospitalists are integral in the management of the sequelae of multiorgan failure.
Disclosure: Nothing to report.
- , . Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al.; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , . Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS data brief, no 62. Hyattsville, MD: National Center for Health Statistics. 2011.
- , , , et al.; Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140(5):1223–1231.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the united states. Crit Care Med. 2013;41(5):1167–1174.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011. HCUP statistical brief #160. Rockville, MD: Agency for Healthcare Research and Quality, 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb160.pdf. Accessed March 15, 2015.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , , , . Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145(7):695–700.
- . Evidence‐based sepsis therapy: a hospitalist perspective. J Hosp Med. 2006;1(5):285–295.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873.
- , , , et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746.
- . Disassembling goal‐directed therapy for sepsis: a first step. JAMA. 2010;303(8):777–779.
- , , , et al. Early lactate‐guided therapy in intensive care unit patients: a multicenter, open‐label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761.
- , , , et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677.
- , , , et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43(5):983–988.
- ARISE Investigators; ANZICS Clinical Trials Group, , , , et al. Goal‐directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , , , et al.; ProMISe Trial Investigators. Trial of early, goal‐directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311.
- . The ProCESS trial—a new era of sepsis management. N Engl J Med. 2014;370(18):1750–1751.
- , . The role of bundles in sepsis care. Crit Care Clin. 2006;22(3):521–529, x.
- , , , et al. The surviving sepsis campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82.
- , , , et al. The GENESIS project (Generalized early sepsis intervention strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2013;28(6):355–368.
- , . Early recognition and treatment of severe sepsis. Am J Respir Crit Care Med. 2013;188(1):7–8.
- , , , et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391.
- , , , et al. High versus low blood‐pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–1593.
- . Is there a good MAP for septic shock? N Engl J Med. 2014;370(17):1649–1651.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal‐directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053.
- , , , et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–2071.
- , . Antibiotics in sepsis: timing, appropriateness, and (of course) timely recognition of appropriateness. Crit Care Med. 2011;39(9):2184–2186.
- , , , et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline‐based performance improvement program. Crit Care Med. 2014;42(8):1749–1755.
- , , , et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248.
- , , , , , . Impact of previous antibiotic therapy on outcome of gram‐negative severe sepsis. Crit Care Med. 2011;39(8):1859–1865.
- , , , . Beta lactam antibiotic monotherapy versus beta lactam‐aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344.
- , , , et al. Hydroxyethyl starch 130/0.42 versus ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134.
- , , , et al.; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–1911.
- , , , et al. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta‐analysis and trial sequential analysis. BMJ. 2013;346:f839.
- , , , et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta‐analysis. JAMA. 2013;309(7):678–688.
- , , , , , ; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256.
- , , , et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421.
- , , , . Randomised trials of human albumin for adults with sepsis: systematic review and meta‐analysis with trial sequential analysis of all‐cause mortality. BMJ. 2014;349:g4561.
- , , , et al. Fluid resuscitation in sepsis: a systematic review and network meta‐analysis. Ann Intern Med. 2014;161(5):347–355.
- , , , , , . Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572.
- NICE‐SUGAR Study Investigators, , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , . Glucose control in the ICU—how tight is too tight? N Engl J Med. 2009;360(13):1346–1349.
- COIITSS Study Investigators, , , , et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348.
- , , , et al. Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data. CMAJ. 2009;180(8):821–827.
- , , , et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362–2375.
- , , , et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871.
- , , , et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42(3):625–631.
- , , , , . Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316.
- , , , , , . The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665–674.
- , . Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189(5):548–555.
- , . Declining case fatality rates for severe sepsis: Good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295–1297.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , , , . Short‐ and long‐term mortality in patients with community‐acquired severe sepsis and septic shock. Scand J Infect Dis. 2013;45(8):577–583.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–69.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , ; Finnsepsis Study Group. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274.
- , , , , . Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254.
- , , , . Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334.
- , , , , , ; Co‐operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–1213.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.
Sepsis is one of the oldest and most elusive syndromes in medicine, and remains a significant contributor to morbidity, mortality, and healthcare expenditure.[1] A 1992 American College of Chest Physicians and Society of Critical Care Medicine consensus conference statement introduced the systemic inflammatory response syndrome (SIRS) into the medical lexicon, along with definitions of sepsis, severe sepsis, and septic shock.[2] A 2003 consensus panel expanded the list of signs and symptoms associated with sepsis, and warned that the findings of SIRS do not differentiate sepsis from other noninfectious conditions.[3] The terminology is important, as these definitions resulted in a shift of the label of the syndrome of infection complicated by end‐organ dysfunction from sepsis to severe sepsis or septic shock. Overlap of these terms has implications for categorizing such infections for the purpose of investigation, estimating epidemiology and outcome, and coding, billing, and reimbursement.[1]
Traditional definitions of the spectrum of sepsis disorders are outlined in Table 1,[2, 3] and it is important to note that an update to these definitions is anticipated in the near future. A recent publication has called into question the sensitivity and categorical requirement of at least 2 SIRS criteria to define severe sepsis.[4] This study of more than 1 million patients from 172 intensive care units (ICUs) in Australia and New Zealand from 2000 to 2013 found that the cutoff of 2 SIRS criteria to define severe sepsis excluded 1 in 8 patients with infection and end‐organ hypoperfusion. SIRS‐negative severe sepsis patients experienced the same mortality as SIRS‐positive patients. In addition, adjusted analysis determined a stepwise increase in mortality risk associated with each additional SIRS criterion without a transition point in risk noted at 2.[4]
| Definition | |
|---|---|
| |
| SIRS | The systemic inflammatory response to a variety of severe insults. Requires 2 of the following: |
| Temperature >38C or 36C | |
| Heart rate >90 beats/minute | |
| Respiratory rate >20 breaths/minute or partial pressure of carbon dioxide (PaCO2) 32 mm Hg | |
| White blood cell count >12,000 or 4,000 cells/L or 10% immature (band) forms | |
| Sepsis | The systemic response to infection, with SIRS criteria met in the setting of documented or strongly suspected infection |
| Severe sepsis | Sepsis associated with organ dysfunction, hypoperfusion (including but not limited to lactic acidosis, oliguria, or acute alteration in mental status), or hypotension (systolic blood pressure 90 mm Hg or >40 mm Hg below baseline). |
| Septic shock | Sepsis‐induced hypotension despite adequate volume resuscitation (2030 mL/kg) with perfusion abnormalities including but not limited to lactic acidosis, oliguria, or acute alteration in mental status |
From 1979 through 2000, there were over 10 million reported cases of sepsis, which accounted for 1.3% of all hospitalizations in the United States.[5] Normalized to the population distribution of the 2000 US Census, there was an annualized increase in sepsis cases of 8.7%. A 2011 report revealed rates of hospitalization for patients with septicemia or sepsis in the United States more than doubled from 2000 through 2008.[6] Patients with sepsis experienced longer length of stay than other inpatients and were 8 times more likely to die during hospitalization.[6] Estimates of severe sepsis incidence are complicated by how acute organ dysfunction is defined and whether it is related to infection. As of 2001, the number of severe sepsis cases in the United States was believed to exceed 750,000 and comprise approximately 10% of ICU admissions.[1] The incidence of severe sepsis cases in the United States continues to rise.[7, 8, 9] However, a more than doubling of the use of sepsis International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes from 2004 through 2009 has also been noted.[8] Based on ICD‐9‐CM codes indicating the presence of sepsis and organ system failure, the number of severe sepsis hospitalizations per 100,000 persons in the United States increased from 143 in 2000 to 343 in 2007.[7] Total hospital costs for patients with severe sepsis were estimated to increase 57%, from $15.4 billion in 2003 to $24.3 billion in 2007.[9] The Agency for Healthcare Research and Quality considered septicemia the most expensive medical condition in the United States in a 2011 data brief, with annual aggregate hospital costs exceeding $20 billion.[10]
Although many hospitalists care for patients in the ICU and other higher acuity or step‐down units, a significant proportion of patients with severe sepsis receive care on a general medical floor.[11, 12, 13] Sepsis is also clearly not an issue restricted to patients on internal medicine services. Of over 360,000 general surgery patients from 2005 to 2007, the incidences of sepsis (2.3%) and septic shock (1.6%) greatly exceeded those of pulmonary embolism (0.3%) and myocardial infarction (0.2%). In this cohort, the need for emergency surgery and the presence of any comorbidity increased the number of sepsis cases.[14]
Despite difficulties obtaining exact estimates of case numbers, the following appears true: the spectrum of sepsis disorders (including severe sepsis and septic shock) remains a common, costly, and increasing clinical entity that is encountered by hospital medicine physicians in a variety inpatient settings. This review will provide an update for hospitalists based on many important studies that have been published since the last review of this topic in this journal.[15] The expanding evidence base in sepsis includes early goal‐directed therapy (EGDT), clinical endpoints, and bundles of care for sepsis; antibiotics (choice and timing); volume resuscitation; ICU considerations, including the use of insulin and corticosteroids; and mortality, complications, and the advent of the condition of sepsis survivorship.
EARLY GOAL‐DIRECTED THERAPY
A 2001 prospective, randomized trial of EGDT initiated in the emergency department (ED) for patients with severe sepsis and septic shock resulted in an impressive 16% reduction of in‐hospital mortality compared to standard therapy.[16] The intervention protocol included central venous catheter placement and a 500‐mL bolus of crystalloid every 30 minutes to establish a central venous pressure (CVP) of 8 to 12 mm Hg. Vasopressors were used to maintain a mean arterial pressure (MAP) greater than 65 mm Hg, and patients with a MAP greater than 90 mm Hg were given vasodilators. Patients with a central venous oxygen saturation (Scv02) of less than 70% received red blood cell transfusion with a goal hematocrit of 30%. If central venous oxygen saturation remained less than 70% despite these interventions, dobutamine was used for inotropic effect until this goal was achieved or was limited by tachycardia or hypotension.[16]
These results prompted inclusion of the specific hemodynamic targets (CVP, MAP, and Scv02) into the original 2004 Surviving Sepsis Campaign guidelines and spurred a decade of interest worldwide.[17] The incremental importance of these individual components in managing severe sepsis and septic shock has since come under scrutiny. A recent randomized trial suggested that EGDT guided by venous lactate clearance of >10% was noninferior to the goal Scv02 of >70%. However, only 10% of the study population required transfusion or dobutamine.[18, 19] Prospective ICU data on lactate‐guided therapy[20] supported the revised 2012 Surviving Sepsis Campaign (SSC) guidelines to recommend lactate normalization as part of initial resuscitation efforts, particularly when Scv02 is not available.[21] Lactate measurement may assist in recognition of cases of severe sepsis or septic shock and provide valuable triage information, as serum lactate has been shown to predict mortality from severe sepsis independent of shock or organ failure.[22] In a retrospective study of patients presenting to the ED with sepsis, a lactate >4 mmol/L was associated with progression to septic shock within 4 to 48 hours.[23]
Our understanding of the specific benefits of EGDT is far from complete, as 3 recent large prospective, multicenter, randomized trials ProCESS (Protocolized Care for Early Septic Shock), ARISE (Australasian Resuscitation In Sepsis Evaluation), and ProMISe (Protocolised Management in Sepsis) did not show EGDT protocols to be superior to usual care.[24, 25, 26] Interpreted collectively, the benefit of EGDT may not be from targeting specific physiologic parameters, but rather from the early recognition of sepsis and the appropriate use of well‐supported interventions like aggressive fluid resuscitation and early/efficacious antibiotics.[27]
Although the precise benefit of EGDT as a package versus its individual components remains in question, we have a decade of experience in delivering this care as an integral component of the bundles put forth in the SSC guidelines.[28] Observational and retrospective studies have shown increased compliance with guidelines and improved mortality after implementing these protocols, although early bundles for severe sepsis included therapies that have subsequently been called into question on an individual basis like drotrecogin alfa (activated) and glucocorticoid therapy.[29, 30, 31] The mortality benefit from sepsis bundles deserves further explanation, although education and early recognition are likely contributory.[32]
Several studies evaluated individual components of EGDT. The TRISS (Transfusion Requirements in Septic Shock) trial randomized ICU patients with septic shock to 2 different red blood cell transfusion strategies, and found no mortality benefit or reduction in ischemic events for patients transfused at a hemoglobin of 9 g/dL compared to the 7 g/dL threshold.[33] The SEPSISPAM (Assessment of Two Levels of Arterial Pressure on Survival in Patients With Septic Shock) trial compared the MAP goal of 65 to 70 mm Hg to 80 to 85 mm Hg for patients with septic shock in a randomized, multicenter trial.[34] Although there was no difference in 28‐day mortality, more atrial fibrillation was diagnosed in the higher target group. For patients with chronic hypertension, targeting the higher MAP led to less renal injury and reduced the need for renal‐replacement therapy.[34, 35] Identifying specific subsets of patients with sepsis who benefit most from particular therapies should help clinicians set patient‐specific goals and targets.
Although we can expect additional studies to provide further guidance, it is reasonable at present to adhere to protocols designed to improve timely sepsis detection and management with aggressive volume resuscitation, early/efficacious antibiotic administration, and effective infection source control.
ANTIBIOTICS AND SOURCE CONTROL
Administration of broad‐spectrum antibiotics has long been the cornerstone of sepsis management. Timely antibiotic infusion is an integral part of the 2004 and 2012 SSC guidelines,[17, 21] with the caveat that blood cultures should be obtained prior to antibiotic therapy provided that no significant delay (>45 minutes) occurs.[21] Recent studies have begun to address fundamental clinical questions, including the timing of antibiotic administration and the efficacy of empiric antibiotic choice. A landmark retrospective cohort study of ICU patients with septic shock demonstrated survival to hospital discharge was highest in patients who received antibiotics within the first hour of hypotension.[36] Survival decreased on average by 7.6% with each hour that antibiotics were delayed. Only 50% of patients with septic shock in this study received effective antibiotic therapy within 6 hours of documented hypotension.[36] A subsequent retrospective, single‐center cohort study of ED patients with severe sepsis or septic shock undergoing EGDT showed a mortality benefit when antibiotics were administered within the first hour. However, it did not demonstrate a statistically significant decline in survival on an hourly basis thereafter.[37]
A prospective, multicenter ED trial that included patients with severe sepsis in addition to septic shock[38] did not show a mortality benefit to administration of antibiotics within the first hour. In‐hospital mortality risk for patients undergoing EGDT was similar across patients in whom time to antibiotics was delayed up to 6 hours after triage.[36, 38] However, patients with severe sepsis in whom antibiotics were delayed until shock was recognized faced a statistically significant increased risk of death (odds ratio = 2.35; 95% confidence interval = 1.12‐4.53).[38, 39] A retrospective study of 28,150 patients from the SSC database demonstrated a statistically significant increase in mortality for each hour that empiric antibiotics were delayed.[40] Importantly, this trend was preserved regardless of location of sepsis diagnosis (ED, ICU, and hospital ward) and across illness severity. Though there remains debate about the critical importance of the golden hour for antibiotic administration, overall current evidence supports early empiric antibiotics in severe sepsis and septic shock.
Choosing an empiric antibiotic regimen, based on infection source and host factors, also plays a key role in sepsis outcomes. A retrospective study of patients with septic shock from 1996 to 2005 showed that inappropriate initial antibiotics (based on eventual in vitro culture sensitivities or evaluation of clinical syndrome) were used 20% of the time and resulted in a 5‐fold reduction in survival.[41] A retrospective cohort study of patients with gram‐negative bacteremia and severe sepsis or septic shock found prior antibiotic exposure within 90 days to be an independent risk factor for drug resistance and in‐hospital mortality.[42] However, careful consideration of side effects should also influence choice of initial antibiotic therapy. A Cochrane review citing 69 trials and containing 7863 subjects with sepsis compared empiric ‐lactam therapy to ‐lactamaminoglycoside combination therapy.[43] All‐cause mortality and clinical failure was similar in both groups, as was the rate of resistance. Importantly, nephrotoxicity was significantly less in the ‐lactam monotherapy group.[43]
Infection source control is an essential component of sepsis management that should occur simultaneously with antibiotic administration. The 2012 SSC guidelines promote infection source control within 12 hours of diagnosis, with consideration of the risks and benefits therein and preference for interventions with the lowest associated physiologic insult.[21] Intravascular access devices should be recognized as a common source of infection, and should be removed after alternative access has been established.[21]
FLUID RESUSCITATION
Volume resuscitation is an essential component of sepsis management, regardless of algorithm or endpoint. Three main types of nonblood product fluid resuscitation have been used: crystalloid (saline and Ringer's solutions), colloid (typically an albumin‐containing solution), and synthetic volume expanders (hydroxyethyl starch [HES] and similar compounds).
Multiple large studies confirmed the lack of a favorable riskbenefit ratio with synthetic volume expanders. Among nearly 800 patients with severe sepsis who were randomized to receive either Ringer's acetate or HES 130/0.4, a significantly higher number of patients receiving HES died (51% vs 43%, relative risk [RR] = 1.17), and required renal‐replacement therapy (22% vs 16%, RR = 1.35). One patient in each group was dialysis dependent at 90 days.[44] An additional multicenter, prospective study of HES versus 0.9% (normal) saline for fluid resuscitation in the ICU found no significant difference in mortality (18% vs 17%, P = 0.26), but did note a higher need for renal‐replacement therapy in the HES group (7.0% vs 5.8%, RR = 1.21).[45] A systematic review incorporating 9 trials that randomized approximately 3400 patients with sepsis receiving either HES, crystalloid, or colloid showed no difference in mortality, although there was an excess risk for renal‐replacement therapy (RR = 1.36), serious adverse events (RR = 1.30), and red blood cell transfusion (RR = 1.29) in patients receiving HES.[46] A second, larger systematic review concluded that HES was associated with an increased mortality compared with crystalloids, albumin, or gelatin (RR = 1.09). Additionally, an increase in renal failure (RR = 1.27) and renal‐replacement therapy (RR = 1.32) was also noted.[47]
The debate between crystalloid and colloid (namely albumin) for fluid resuscitation is ongoing, with recent important additions to the literature. The SAFE (Saline Versus Albumin Fluid Evaluation) study investigators in 2004 randomized nearly 7000 patients to receive either 4% albumin solution or normal saline. At 28 days, no significant differences were found in mortality, new organ failure, ICU and hospital length of stay, days of mechanical ventilation, or days of renal‐replacement therapy.[48] In 2014, another multicenter prospective study of 1800 patients with severe sepsis or septic shock in 100 ICUs in Italy compared 20% albumin and crystalloid solution to crystalloid solution alone. Mortality, end‐organ dysfunction, and ICU length of stay did not differ between groups.[49] Two 2014 systematic reviews and meta‐analyses on fluid resuscitation produced somewhat differing conclusions. Patel et al. evaluated data from 16 randomized trials including more than 4000 patients receiving albumin for volume resuscitation in adults with sepsis. Albumin provided no significant survival advantage in total or in any subgroup, regardless of severity of illness or baseline albumin level, thus arguing against its routine use.[50] Rochwerg et al. evaluated 14 studies with approximately 19,000 patients using Bayesian network meta‐analysis technique. This study concluded that albumin is associated with reduced mortality compared with other fluids, and also that balanced crystalloids (eg, Ringer's lactate and similar) may have lower mortality than normal saline.[51] A chloride‐restrictive resuscitation approach has also been associated with a lower incidence of acute kidney injury in critically ill adults.[52]
The SSC confirmed its recommendations of a minimum of 30 mL/kg of crystalloids as the initial fluid of choice in sepsis in 2012, but added a suggestion for the addition of albumin in patients requiring substantial amounts of crystalloid.[21] The currently available data suggest crystalloid fluids to be the best‐supported initial fluid in the management of sepsis, and that synthetic colloids should be avoided. Prospective data are still required to answer questions regarding the potential advantages of albumin or balanced and/or chloride‐restricted crystalloids.
ICU CONSIDERATIONS
Appropriate management of patients with the syndrome of sepsis, severe sepsis, and septic shock on the hospital ward requires a working knowledge of recent research conducted in the ICU setting. Although conclusions based on data from patients with septic shock might not be generalizable to less severe cases of sepsis, recent trials on glucose control and corticosteroids deserve consideration.
Intensive insulin treatment in the medical ICU is no longer standard practice. In the NICE‐SUGAR (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) trial, a large, multicenter randomized controlled trial of ICU patients, close to 20% of patients had severe sepsis at the time of randomization.[53] This subgroup did not benefit from intensive glucose control (a target of 81108 mg/dL) in terms of 90‐day mortality.[53] In contrast to prior studies of glycemic control in the critically ill, the intensive treatment group overall suffered increased mortality.[54] The COIITSS (Combination of Corticotherapy and Intensive Insulin Therapy for Septic Shock) trial looked at intensive insulin therapy in patients with septic shock being treated with corticosteroids, a group particularly at risk for hyperglycemia. In this study, intensive insulin therapy did not improve in‐hospital mortality.[55] Based on these and other ICU data, the current SSC recommendation is to target a blood glucose of 180 mg/dL for patients with severe sepsis.[21, 56]
The use of corticosteroids to treat the host response in septic shock has been re‐evaluated.[57] The 2004 SSC guidelines recommended hydrocortisone therapy for 7 days in patients with septic shock requiring vasopressor support after fluid resuscitation.[17] This recommendation was based on data from a placebo‐controlled multicenter trial in France that showed improved shock reversal and reduced mortality in patients with septic shock who were treated with hydrocortisone and fludricortisone.[58] Of note, these patients were enrolled on the basis of hypotension despite intravenous fluids and the initiation of 1 vasopressor. The benefit of corticosteroids was seen only in patients deemed to have relative adrenal insufficiency based on response corticotropin testing.[58] However, the CORTICUS (Corticosteroid Therapy of Septic Shock) study, a subsequent multicenter, placebo‐controlled, randomized controlled trial failed to show a benefit to corticotropin testing in identifying patients with septic shock who would benefit from corticorsteroids.[59] The corticosteroid treatment arm similarly benefited from faster shock reversal, but at the expense of increased superinfection.[59] Although underpowered, CORTICUS did not show a survival benefit to corticosteroids in septic shock.[59] The most recent SSC guidelines do not recommend corticotropin (adrenocorticotropic hormone) stimulation testing and do not advise corticosteroids in septic shock if fluid resuscitation and vasopressor therapy restore hemodynamic stability during initial resuscitation.[21] Future studies may clarify subpopulations of patients with sepsis who benefit from corticosteroids.
OUTCOMES: MORTALITY AND COMPLICATIONS
An understanding of the currently available information regarding the morbidity and mortality associated with severe sepsis is essential for the practicing hospitalist. Whether transferring care of patients to or receiving patients from the ICU, hospitalists must lead discussions with patients and families regarding prognosis, especially as it informs disposition. Hospitalists are often asked to make projections on outcome as well as the timing and venue of disposition. Clarification of patient wishes and goals of care remains an essential first step in the care of septic patients. Recently published studies provide prognostic information, including mortality (both short and long term) as well as complications associated with severe sepsis.
The attributable mortality for severe sepsis has been predominantly reported to date as short‐term (usually in‐hospital). A meta‐analysis of US patients from 1991 to 2009 demonstrated a 3% annual decline in the short‐term (28 day) mortality from severe sepsis using 2 previously validated algorithms. Data from 36 trials (and approximately 14,000 patients) revealed a decrease in mortality from 47% in the period from 1991 to 1995 to 29% from 2006 to 2009.[60] Although the methods employed (sepsis definitions and ICD‐9‐CM codes) can have a significant impact on estimates of mortality, these results corroborate a progressive decline in short‐term mortality from severe sepsis in the United States between 2004 and 2009 using 4 validated algorithms.[8] Outside the United States, a recent retrospective analysis of more than 1 million patients with severe sepsis treated in the ICU in Australia and New Zealand from 2000 to 2012 also demonstrated a decrease in adjusted in‐hospital mortality. In this study, short‐term mortality declined yearly, with an odds ratio of death of 0.49 in 2012 compared with 2000.[61] Hospital case volume has also been shown to impact rates of inpatient death, with higher‐volume centers demonstrating lower mortality attributed to severe sepsis.[62, 63]
The sufficiency of short‐term mortality as the sole metric for severe sepsis outcome has been more recently questioned.[64, 65] The extent to which full recovery and significant morbidity are affected relative to the change in death rate is unknown, and as such, more data on morbidity and longer‐term mortality are necessary. A Danish study examined data from several registries of patients with severe sepsis. Compared with community‐matched controls, patients with severe sepsis had an increased risk of death at 30 days (hazard ratio [HR] = 90.8), from 30 days to 1 year (HR = 2.7), and 1 to 4 years (HR = 2.3) after discharge.[66] Older survivors of severe sepsis also appear to have higher healthcare utilization in the year following discharge. An analysis of older severe sepsis survivors showed a striking increase in healthcare use relative to their prior resource use, driven primarily by higher number of days in inpatient healthcare facilities. Survivors of severe sepsis also had a significantly higher 90‐day and 1‐year mortality than matched controls.[67]
Increased attention is currently being given to sepsis‐related complications, especially functional and cognitive impairments in older patients. Sepsis survivorship is a swiftly mounting public health issue for older Americans.[68] An 8‐year follow‐up of older sepsis survivors demonstrated a significant increase in the odds of both physical and cognitive dysfunction. During this period, moderate‐to‐severe cognitive dysfunction increased 3‐fold (6.1% before sepsis, 16.7% after).[69] The mechanism by which this dysfunction occurs is unknown, as are the relative contributions of infection site/etiology, ICU length of stay, and extent of organ dysfunction. New functional impairment has been demonstrated in patients with severe sepsis initially admitted to a general floor, even with good baseline function,[12] as well as decreased quality of life in sepsis survivors.[65, 70] Another study showed more admissions complicated by severe sepsis resulted in discharge to a long‐term care facility in 2007 compared to 2000.[7]
Additional organ‐specific consequences of severe sepsis have also been recently suggested. A retrospective analysis showed an increase in the incidence of new‐onset atrial fibrillation in severe sepsis, with an associated increase in risk of in‐hospital stroke and death. New‐onset atrial fibrillation was present in 5.9% of patients with severe sepsis, compared with 0.65% in patients without. Severe sepsis patients with new‐onset atrial fibrillation had an increased risk of in‐hospital stroke (adjusted odds ratio = 2.70) and mortality (adjusted RR = 1.07).[71] These findings suggest association only, and further investigation is warranted. It remains to be seen whether interventions to restore sinus rhythm or anticoagulation are warranted. Preoperative sepsis (within 48 hours) has also been proposed as a risk for postoperative (30 day) arterial (myocardial infarction, stroke) and venous (deep venous thrombosis, pulmonary embolism) thromboembolism. The authors of this study suggest deferral of elective surgery or specific attention to postoperative thromboprophylaxis in patients in whom procedures must occur.[72] This has particular relevance for those septic patients in whom surgical source control is indicated.
Estimates regarding mortality and specific complications attributable to severe sepsis are ongoing, though clearly with a new focus upon metrics other than short‐term mortality. Furthermore, recent data to suggest source of infection as a major driver of mortality in septic shock[73] may contribute to the evolution of the conceptualization of sepsis similar to that of cancer: a heterogeneous collection of disease, among which mortality is determined by specific subtypes. At present, this much appears clear: the previously held notion that survival of a septic insult is unlikely to have future implications is under siege.[74] The extent to which complications and increased longer‐term mortality may reflect generally poorer health at the time of infection versus a sequelae of the survived episode itself is not yet known.
CONCLUSIONS
The past decade of sepsis research has led to significant findings that will change clinical practice for hospital medicine practitioners. Although the incidence of severe sepsis in the United States has continued to rise, in‐hospital mortality has declined; in this context, the management of the spectrum of sepsis disorders is no longer restricted to the ICU, and the entity of sepsis survivorship has blossomed. Prompt recognition of sepsis and improvements in supportive care are likely responsible for improved patient outcomes. EGDT has been called into question as a protocol whose benefit lies not in specific targets or endpoints, but rather in the early recognition of sepsis, appropriate fluid resuscitation, and early/effective antibiotics. Synthetic volume expanders, intensive insulin therapy, and routine use of corticosteroids are no longer recommended.
Hospitalists are a critical link in providing timely, evidence‐based care for patients with sepsis from initial recognition to post‐ICU recovery. Specialized care for the survivors of septic shock is a burgeoning area, and hospitalists are integral in the management of the sequelae of multiorgan failure.
Disclosure: Nothing to report.
Sepsis is one of the oldest and most elusive syndromes in medicine, and remains a significant contributor to morbidity, mortality, and healthcare expenditure.[1] A 1992 American College of Chest Physicians and Society of Critical Care Medicine consensus conference statement introduced the systemic inflammatory response syndrome (SIRS) into the medical lexicon, along with definitions of sepsis, severe sepsis, and septic shock.[2] A 2003 consensus panel expanded the list of signs and symptoms associated with sepsis, and warned that the findings of SIRS do not differentiate sepsis from other noninfectious conditions.[3] The terminology is important, as these definitions resulted in a shift of the label of the syndrome of infection complicated by end‐organ dysfunction from sepsis to severe sepsis or septic shock. Overlap of these terms has implications for categorizing such infections for the purpose of investigation, estimating epidemiology and outcome, and coding, billing, and reimbursement.[1]
Traditional definitions of the spectrum of sepsis disorders are outlined in Table 1,[2, 3] and it is important to note that an update to these definitions is anticipated in the near future. A recent publication has called into question the sensitivity and categorical requirement of at least 2 SIRS criteria to define severe sepsis.[4] This study of more than 1 million patients from 172 intensive care units (ICUs) in Australia and New Zealand from 2000 to 2013 found that the cutoff of 2 SIRS criteria to define severe sepsis excluded 1 in 8 patients with infection and end‐organ hypoperfusion. SIRS‐negative severe sepsis patients experienced the same mortality as SIRS‐positive patients. In addition, adjusted analysis determined a stepwise increase in mortality risk associated with each additional SIRS criterion without a transition point in risk noted at 2.[4]
| Definition | |
|---|---|
| |
| SIRS | The systemic inflammatory response to a variety of severe insults. Requires 2 of the following: |
| Temperature >38C or 36C | |
| Heart rate >90 beats/minute | |
| Respiratory rate >20 breaths/minute or partial pressure of carbon dioxide (PaCO2) 32 mm Hg | |
| White blood cell count >12,000 or 4,000 cells/L or 10% immature (band) forms | |
| Sepsis | The systemic response to infection, with SIRS criteria met in the setting of documented or strongly suspected infection |
| Severe sepsis | Sepsis associated with organ dysfunction, hypoperfusion (including but not limited to lactic acidosis, oliguria, or acute alteration in mental status), or hypotension (systolic blood pressure 90 mm Hg or >40 mm Hg below baseline). |
| Septic shock | Sepsis‐induced hypotension despite adequate volume resuscitation (2030 mL/kg) with perfusion abnormalities including but not limited to lactic acidosis, oliguria, or acute alteration in mental status |
From 1979 through 2000, there were over 10 million reported cases of sepsis, which accounted for 1.3% of all hospitalizations in the United States.[5] Normalized to the population distribution of the 2000 US Census, there was an annualized increase in sepsis cases of 8.7%. A 2011 report revealed rates of hospitalization for patients with septicemia or sepsis in the United States more than doubled from 2000 through 2008.[6] Patients with sepsis experienced longer length of stay than other inpatients and were 8 times more likely to die during hospitalization.[6] Estimates of severe sepsis incidence are complicated by how acute organ dysfunction is defined and whether it is related to infection. As of 2001, the number of severe sepsis cases in the United States was believed to exceed 750,000 and comprise approximately 10% of ICU admissions.[1] The incidence of severe sepsis cases in the United States continues to rise.[7, 8, 9] However, a more than doubling of the use of sepsis International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes from 2004 through 2009 has also been noted.[8] Based on ICD‐9‐CM codes indicating the presence of sepsis and organ system failure, the number of severe sepsis hospitalizations per 100,000 persons in the United States increased from 143 in 2000 to 343 in 2007.[7] Total hospital costs for patients with severe sepsis were estimated to increase 57%, from $15.4 billion in 2003 to $24.3 billion in 2007.[9] The Agency for Healthcare Research and Quality considered septicemia the most expensive medical condition in the United States in a 2011 data brief, with annual aggregate hospital costs exceeding $20 billion.[10]
Although many hospitalists care for patients in the ICU and other higher acuity or step‐down units, a significant proportion of patients with severe sepsis receive care on a general medical floor.[11, 12, 13] Sepsis is also clearly not an issue restricted to patients on internal medicine services. Of over 360,000 general surgery patients from 2005 to 2007, the incidences of sepsis (2.3%) and septic shock (1.6%) greatly exceeded those of pulmonary embolism (0.3%) and myocardial infarction (0.2%). In this cohort, the need for emergency surgery and the presence of any comorbidity increased the number of sepsis cases.[14]
Despite difficulties obtaining exact estimates of case numbers, the following appears true: the spectrum of sepsis disorders (including severe sepsis and septic shock) remains a common, costly, and increasing clinical entity that is encountered by hospital medicine physicians in a variety inpatient settings. This review will provide an update for hospitalists based on many important studies that have been published since the last review of this topic in this journal.[15] The expanding evidence base in sepsis includes early goal‐directed therapy (EGDT), clinical endpoints, and bundles of care for sepsis; antibiotics (choice and timing); volume resuscitation; ICU considerations, including the use of insulin and corticosteroids; and mortality, complications, and the advent of the condition of sepsis survivorship.
EARLY GOAL‐DIRECTED THERAPY
A 2001 prospective, randomized trial of EGDT initiated in the emergency department (ED) for patients with severe sepsis and septic shock resulted in an impressive 16% reduction of in‐hospital mortality compared to standard therapy.[16] The intervention protocol included central venous catheter placement and a 500‐mL bolus of crystalloid every 30 minutes to establish a central venous pressure (CVP) of 8 to 12 mm Hg. Vasopressors were used to maintain a mean arterial pressure (MAP) greater than 65 mm Hg, and patients with a MAP greater than 90 mm Hg were given vasodilators. Patients with a central venous oxygen saturation (Scv02) of less than 70% received red blood cell transfusion with a goal hematocrit of 30%. If central venous oxygen saturation remained less than 70% despite these interventions, dobutamine was used for inotropic effect until this goal was achieved or was limited by tachycardia or hypotension.[16]
These results prompted inclusion of the specific hemodynamic targets (CVP, MAP, and Scv02) into the original 2004 Surviving Sepsis Campaign guidelines and spurred a decade of interest worldwide.[17] The incremental importance of these individual components in managing severe sepsis and septic shock has since come under scrutiny. A recent randomized trial suggested that EGDT guided by venous lactate clearance of >10% was noninferior to the goal Scv02 of >70%. However, only 10% of the study population required transfusion or dobutamine.[18, 19] Prospective ICU data on lactate‐guided therapy[20] supported the revised 2012 Surviving Sepsis Campaign (SSC) guidelines to recommend lactate normalization as part of initial resuscitation efforts, particularly when Scv02 is not available.[21] Lactate measurement may assist in recognition of cases of severe sepsis or septic shock and provide valuable triage information, as serum lactate has been shown to predict mortality from severe sepsis independent of shock or organ failure.[22] In a retrospective study of patients presenting to the ED with sepsis, a lactate >4 mmol/L was associated with progression to septic shock within 4 to 48 hours.[23]
Our understanding of the specific benefits of EGDT is far from complete, as 3 recent large prospective, multicenter, randomized trials ProCESS (Protocolized Care for Early Septic Shock), ARISE (Australasian Resuscitation In Sepsis Evaluation), and ProMISe (Protocolised Management in Sepsis) did not show EGDT protocols to be superior to usual care.[24, 25, 26] Interpreted collectively, the benefit of EGDT may not be from targeting specific physiologic parameters, but rather from the early recognition of sepsis and the appropriate use of well‐supported interventions like aggressive fluid resuscitation and early/efficacious antibiotics.[27]
Although the precise benefit of EGDT as a package versus its individual components remains in question, we have a decade of experience in delivering this care as an integral component of the bundles put forth in the SSC guidelines.[28] Observational and retrospective studies have shown increased compliance with guidelines and improved mortality after implementing these protocols, although early bundles for severe sepsis included therapies that have subsequently been called into question on an individual basis like drotrecogin alfa (activated) and glucocorticoid therapy.[29, 30, 31] The mortality benefit from sepsis bundles deserves further explanation, although education and early recognition are likely contributory.[32]
Several studies evaluated individual components of EGDT. The TRISS (Transfusion Requirements in Septic Shock) trial randomized ICU patients with septic shock to 2 different red blood cell transfusion strategies, and found no mortality benefit or reduction in ischemic events for patients transfused at a hemoglobin of 9 g/dL compared to the 7 g/dL threshold.[33] The SEPSISPAM (Assessment of Two Levels of Arterial Pressure on Survival in Patients With Septic Shock) trial compared the MAP goal of 65 to 70 mm Hg to 80 to 85 mm Hg for patients with septic shock in a randomized, multicenter trial.[34] Although there was no difference in 28‐day mortality, more atrial fibrillation was diagnosed in the higher target group. For patients with chronic hypertension, targeting the higher MAP led to less renal injury and reduced the need for renal‐replacement therapy.[34, 35] Identifying specific subsets of patients with sepsis who benefit most from particular therapies should help clinicians set patient‐specific goals and targets.
Although we can expect additional studies to provide further guidance, it is reasonable at present to adhere to protocols designed to improve timely sepsis detection and management with aggressive volume resuscitation, early/efficacious antibiotic administration, and effective infection source control.
ANTIBIOTICS AND SOURCE CONTROL
Administration of broad‐spectrum antibiotics has long been the cornerstone of sepsis management. Timely antibiotic infusion is an integral part of the 2004 and 2012 SSC guidelines,[17, 21] with the caveat that blood cultures should be obtained prior to antibiotic therapy provided that no significant delay (>45 minutes) occurs.[21] Recent studies have begun to address fundamental clinical questions, including the timing of antibiotic administration and the efficacy of empiric antibiotic choice. A landmark retrospective cohort study of ICU patients with septic shock demonstrated survival to hospital discharge was highest in patients who received antibiotics within the first hour of hypotension.[36] Survival decreased on average by 7.6% with each hour that antibiotics were delayed. Only 50% of patients with septic shock in this study received effective antibiotic therapy within 6 hours of documented hypotension.[36] A subsequent retrospective, single‐center cohort study of ED patients with severe sepsis or septic shock undergoing EGDT showed a mortality benefit when antibiotics were administered within the first hour. However, it did not demonstrate a statistically significant decline in survival on an hourly basis thereafter.[37]
A prospective, multicenter ED trial that included patients with severe sepsis in addition to septic shock[38] did not show a mortality benefit to administration of antibiotics within the first hour. In‐hospital mortality risk for patients undergoing EGDT was similar across patients in whom time to antibiotics was delayed up to 6 hours after triage.[36, 38] However, patients with severe sepsis in whom antibiotics were delayed until shock was recognized faced a statistically significant increased risk of death (odds ratio = 2.35; 95% confidence interval = 1.12‐4.53).[38, 39] A retrospective study of 28,150 patients from the SSC database demonstrated a statistically significant increase in mortality for each hour that empiric antibiotics were delayed.[40] Importantly, this trend was preserved regardless of location of sepsis diagnosis (ED, ICU, and hospital ward) and across illness severity. Though there remains debate about the critical importance of the golden hour for antibiotic administration, overall current evidence supports early empiric antibiotics in severe sepsis and septic shock.
Choosing an empiric antibiotic regimen, based on infection source and host factors, also plays a key role in sepsis outcomes. A retrospective study of patients with septic shock from 1996 to 2005 showed that inappropriate initial antibiotics (based on eventual in vitro culture sensitivities or evaluation of clinical syndrome) were used 20% of the time and resulted in a 5‐fold reduction in survival.[41] A retrospective cohort study of patients with gram‐negative bacteremia and severe sepsis or septic shock found prior antibiotic exposure within 90 days to be an independent risk factor for drug resistance and in‐hospital mortality.[42] However, careful consideration of side effects should also influence choice of initial antibiotic therapy. A Cochrane review citing 69 trials and containing 7863 subjects with sepsis compared empiric ‐lactam therapy to ‐lactamaminoglycoside combination therapy.[43] All‐cause mortality and clinical failure was similar in both groups, as was the rate of resistance. Importantly, nephrotoxicity was significantly less in the ‐lactam monotherapy group.[43]
Infection source control is an essential component of sepsis management that should occur simultaneously with antibiotic administration. The 2012 SSC guidelines promote infection source control within 12 hours of diagnosis, with consideration of the risks and benefits therein and preference for interventions with the lowest associated physiologic insult.[21] Intravascular access devices should be recognized as a common source of infection, and should be removed after alternative access has been established.[21]
FLUID RESUSCITATION
Volume resuscitation is an essential component of sepsis management, regardless of algorithm or endpoint. Three main types of nonblood product fluid resuscitation have been used: crystalloid (saline and Ringer's solutions), colloid (typically an albumin‐containing solution), and synthetic volume expanders (hydroxyethyl starch [HES] and similar compounds).
Multiple large studies confirmed the lack of a favorable riskbenefit ratio with synthetic volume expanders. Among nearly 800 patients with severe sepsis who were randomized to receive either Ringer's acetate or HES 130/0.4, a significantly higher number of patients receiving HES died (51% vs 43%, relative risk [RR] = 1.17), and required renal‐replacement therapy (22% vs 16%, RR = 1.35). One patient in each group was dialysis dependent at 90 days.[44] An additional multicenter, prospective study of HES versus 0.9% (normal) saline for fluid resuscitation in the ICU found no significant difference in mortality (18% vs 17%, P = 0.26), but did note a higher need for renal‐replacement therapy in the HES group (7.0% vs 5.8%, RR = 1.21).[45] A systematic review incorporating 9 trials that randomized approximately 3400 patients with sepsis receiving either HES, crystalloid, or colloid showed no difference in mortality, although there was an excess risk for renal‐replacement therapy (RR = 1.36), serious adverse events (RR = 1.30), and red blood cell transfusion (RR = 1.29) in patients receiving HES.[46] A second, larger systematic review concluded that HES was associated with an increased mortality compared with crystalloids, albumin, or gelatin (RR = 1.09). Additionally, an increase in renal failure (RR = 1.27) and renal‐replacement therapy (RR = 1.32) was also noted.[47]
The debate between crystalloid and colloid (namely albumin) for fluid resuscitation is ongoing, with recent important additions to the literature. The SAFE (Saline Versus Albumin Fluid Evaluation) study investigators in 2004 randomized nearly 7000 patients to receive either 4% albumin solution or normal saline. At 28 days, no significant differences were found in mortality, new organ failure, ICU and hospital length of stay, days of mechanical ventilation, or days of renal‐replacement therapy.[48] In 2014, another multicenter prospective study of 1800 patients with severe sepsis or septic shock in 100 ICUs in Italy compared 20% albumin and crystalloid solution to crystalloid solution alone. Mortality, end‐organ dysfunction, and ICU length of stay did not differ between groups.[49] Two 2014 systematic reviews and meta‐analyses on fluid resuscitation produced somewhat differing conclusions. Patel et al. evaluated data from 16 randomized trials including more than 4000 patients receiving albumin for volume resuscitation in adults with sepsis. Albumin provided no significant survival advantage in total or in any subgroup, regardless of severity of illness or baseline albumin level, thus arguing against its routine use.[50] Rochwerg et al. evaluated 14 studies with approximately 19,000 patients using Bayesian network meta‐analysis technique. This study concluded that albumin is associated with reduced mortality compared with other fluids, and also that balanced crystalloids (eg, Ringer's lactate and similar) may have lower mortality than normal saline.[51] A chloride‐restrictive resuscitation approach has also been associated with a lower incidence of acute kidney injury in critically ill adults.[52]
The SSC confirmed its recommendations of a minimum of 30 mL/kg of crystalloids as the initial fluid of choice in sepsis in 2012, but added a suggestion for the addition of albumin in patients requiring substantial amounts of crystalloid.[21] The currently available data suggest crystalloid fluids to be the best‐supported initial fluid in the management of sepsis, and that synthetic colloids should be avoided. Prospective data are still required to answer questions regarding the potential advantages of albumin or balanced and/or chloride‐restricted crystalloids.
ICU CONSIDERATIONS
Appropriate management of patients with the syndrome of sepsis, severe sepsis, and septic shock on the hospital ward requires a working knowledge of recent research conducted in the ICU setting. Although conclusions based on data from patients with septic shock might not be generalizable to less severe cases of sepsis, recent trials on glucose control and corticosteroids deserve consideration.
Intensive insulin treatment in the medical ICU is no longer standard practice. In the NICE‐SUGAR (Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) trial, a large, multicenter randomized controlled trial of ICU patients, close to 20% of patients had severe sepsis at the time of randomization.[53] This subgroup did not benefit from intensive glucose control (a target of 81108 mg/dL) in terms of 90‐day mortality.[53] In contrast to prior studies of glycemic control in the critically ill, the intensive treatment group overall suffered increased mortality.[54] The COIITSS (Combination of Corticotherapy and Intensive Insulin Therapy for Septic Shock) trial looked at intensive insulin therapy in patients with septic shock being treated with corticosteroids, a group particularly at risk for hyperglycemia. In this study, intensive insulin therapy did not improve in‐hospital mortality.[55] Based on these and other ICU data, the current SSC recommendation is to target a blood glucose of 180 mg/dL for patients with severe sepsis.[21, 56]
The use of corticosteroids to treat the host response in septic shock has been re‐evaluated.[57] The 2004 SSC guidelines recommended hydrocortisone therapy for 7 days in patients with septic shock requiring vasopressor support after fluid resuscitation.[17] This recommendation was based on data from a placebo‐controlled multicenter trial in France that showed improved shock reversal and reduced mortality in patients with septic shock who were treated with hydrocortisone and fludricortisone.[58] Of note, these patients were enrolled on the basis of hypotension despite intravenous fluids and the initiation of 1 vasopressor. The benefit of corticosteroids was seen only in patients deemed to have relative adrenal insufficiency based on response corticotropin testing.[58] However, the CORTICUS (Corticosteroid Therapy of Septic Shock) study, a subsequent multicenter, placebo‐controlled, randomized controlled trial failed to show a benefit to corticotropin testing in identifying patients with septic shock who would benefit from corticorsteroids.[59] The corticosteroid treatment arm similarly benefited from faster shock reversal, but at the expense of increased superinfection.[59] Although underpowered, CORTICUS did not show a survival benefit to corticosteroids in septic shock.[59] The most recent SSC guidelines do not recommend corticotropin (adrenocorticotropic hormone) stimulation testing and do not advise corticosteroids in septic shock if fluid resuscitation and vasopressor therapy restore hemodynamic stability during initial resuscitation.[21] Future studies may clarify subpopulations of patients with sepsis who benefit from corticosteroids.
OUTCOMES: MORTALITY AND COMPLICATIONS
An understanding of the currently available information regarding the morbidity and mortality associated with severe sepsis is essential for the practicing hospitalist. Whether transferring care of patients to or receiving patients from the ICU, hospitalists must lead discussions with patients and families regarding prognosis, especially as it informs disposition. Hospitalists are often asked to make projections on outcome as well as the timing and venue of disposition. Clarification of patient wishes and goals of care remains an essential first step in the care of septic patients. Recently published studies provide prognostic information, including mortality (both short and long term) as well as complications associated with severe sepsis.
The attributable mortality for severe sepsis has been predominantly reported to date as short‐term (usually in‐hospital). A meta‐analysis of US patients from 1991 to 2009 demonstrated a 3% annual decline in the short‐term (28 day) mortality from severe sepsis using 2 previously validated algorithms. Data from 36 trials (and approximately 14,000 patients) revealed a decrease in mortality from 47% in the period from 1991 to 1995 to 29% from 2006 to 2009.[60] Although the methods employed (sepsis definitions and ICD‐9‐CM codes) can have a significant impact on estimates of mortality, these results corroborate a progressive decline in short‐term mortality from severe sepsis in the United States between 2004 and 2009 using 4 validated algorithms.[8] Outside the United States, a recent retrospective analysis of more than 1 million patients with severe sepsis treated in the ICU in Australia and New Zealand from 2000 to 2012 also demonstrated a decrease in adjusted in‐hospital mortality. In this study, short‐term mortality declined yearly, with an odds ratio of death of 0.49 in 2012 compared with 2000.[61] Hospital case volume has also been shown to impact rates of inpatient death, with higher‐volume centers demonstrating lower mortality attributed to severe sepsis.[62, 63]
The sufficiency of short‐term mortality as the sole metric for severe sepsis outcome has been more recently questioned.[64, 65] The extent to which full recovery and significant morbidity are affected relative to the change in death rate is unknown, and as such, more data on morbidity and longer‐term mortality are necessary. A Danish study examined data from several registries of patients with severe sepsis. Compared with community‐matched controls, patients with severe sepsis had an increased risk of death at 30 days (hazard ratio [HR] = 90.8), from 30 days to 1 year (HR = 2.7), and 1 to 4 years (HR = 2.3) after discharge.[66] Older survivors of severe sepsis also appear to have higher healthcare utilization in the year following discharge. An analysis of older severe sepsis survivors showed a striking increase in healthcare use relative to their prior resource use, driven primarily by higher number of days in inpatient healthcare facilities. Survivors of severe sepsis also had a significantly higher 90‐day and 1‐year mortality than matched controls.[67]
Increased attention is currently being given to sepsis‐related complications, especially functional and cognitive impairments in older patients. Sepsis survivorship is a swiftly mounting public health issue for older Americans.[68] An 8‐year follow‐up of older sepsis survivors demonstrated a significant increase in the odds of both physical and cognitive dysfunction. During this period, moderate‐to‐severe cognitive dysfunction increased 3‐fold (6.1% before sepsis, 16.7% after).[69] The mechanism by which this dysfunction occurs is unknown, as are the relative contributions of infection site/etiology, ICU length of stay, and extent of organ dysfunction. New functional impairment has been demonstrated in patients with severe sepsis initially admitted to a general floor, even with good baseline function,[12] as well as decreased quality of life in sepsis survivors.[65, 70] Another study showed more admissions complicated by severe sepsis resulted in discharge to a long‐term care facility in 2007 compared to 2000.[7]
Additional organ‐specific consequences of severe sepsis have also been recently suggested. A retrospective analysis showed an increase in the incidence of new‐onset atrial fibrillation in severe sepsis, with an associated increase in risk of in‐hospital stroke and death. New‐onset atrial fibrillation was present in 5.9% of patients with severe sepsis, compared with 0.65% in patients without. Severe sepsis patients with new‐onset atrial fibrillation had an increased risk of in‐hospital stroke (adjusted odds ratio = 2.70) and mortality (adjusted RR = 1.07).[71] These findings suggest association only, and further investigation is warranted. It remains to be seen whether interventions to restore sinus rhythm or anticoagulation are warranted. Preoperative sepsis (within 48 hours) has also been proposed as a risk for postoperative (30 day) arterial (myocardial infarction, stroke) and venous (deep venous thrombosis, pulmonary embolism) thromboembolism. The authors of this study suggest deferral of elective surgery or specific attention to postoperative thromboprophylaxis in patients in whom procedures must occur.[72] This has particular relevance for those septic patients in whom surgical source control is indicated.
Estimates regarding mortality and specific complications attributable to severe sepsis are ongoing, though clearly with a new focus upon metrics other than short‐term mortality. Furthermore, recent data to suggest source of infection as a major driver of mortality in septic shock[73] may contribute to the evolution of the conceptualization of sepsis similar to that of cancer: a heterogeneous collection of disease, among which mortality is determined by specific subtypes. At present, this much appears clear: the previously held notion that survival of a septic insult is unlikely to have future implications is under siege.[74] The extent to which complications and increased longer‐term mortality may reflect generally poorer health at the time of infection versus a sequelae of the survived episode itself is not yet known.
CONCLUSIONS
The past decade of sepsis research has led to significant findings that will change clinical practice for hospital medicine practitioners. Although the incidence of severe sepsis in the United States has continued to rise, in‐hospital mortality has declined; in this context, the management of the spectrum of sepsis disorders is no longer restricted to the ICU, and the entity of sepsis survivorship has blossomed. Prompt recognition of sepsis and improvements in supportive care are likely responsible for improved patient outcomes. EGDT has been called into question as a protocol whose benefit lies not in specific targets or endpoints, but rather in the early recognition of sepsis, appropriate fluid resuscitation, and early/effective antibiotics. Synthetic volume expanders, intensive insulin therapy, and routine use of corticosteroids are no longer recommended.
Hospitalists are a critical link in providing timely, evidence‐based care for patients with sepsis from initial recognition to post‐ICU recovery. Specialized care for the survivors of septic shock is a burgeoning area, and hospitalists are integral in the management of the sequelae of multiorgan failure.
Disclosure: Nothing to report.
- , . Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al.; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , . Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS data brief, no 62. Hyattsville, MD: National Center for Health Statistics. 2011.
- , , , et al.; Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140(5):1223–1231.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the united states. Crit Care Med. 2013;41(5):1167–1174.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011. HCUP statistical brief #160. Rockville, MD: Agency for Healthcare Research and Quality, 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb160.pdf. Accessed March 15, 2015.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , , , . Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145(7):695–700.
- . Evidence‐based sepsis therapy: a hospitalist perspective. J Hosp Med. 2006;1(5):285–295.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873.
- , , , et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746.
- . Disassembling goal‐directed therapy for sepsis: a first step. JAMA. 2010;303(8):777–779.
- , , , et al. Early lactate‐guided therapy in intensive care unit patients: a multicenter, open‐label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761.
- , , , et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677.
- , , , et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43(5):983–988.
- ARISE Investigators; ANZICS Clinical Trials Group, , , , et al. Goal‐directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , , , et al.; ProMISe Trial Investigators. Trial of early, goal‐directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311.
- . The ProCESS trial—a new era of sepsis management. N Engl J Med. 2014;370(18):1750–1751.
- , . The role of bundles in sepsis care. Crit Care Clin. 2006;22(3):521–529, x.
- , , , et al. The surviving sepsis campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82.
- , , , et al. The GENESIS project (Generalized early sepsis intervention strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2013;28(6):355–368.
- , . Early recognition and treatment of severe sepsis. Am J Respir Crit Care Med. 2013;188(1):7–8.
- , , , et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391.
- , , , et al. High versus low blood‐pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–1593.
- . Is there a good MAP for septic shock? N Engl J Med. 2014;370(17):1649–1651.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal‐directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053.
- , , , et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–2071.
- , . Antibiotics in sepsis: timing, appropriateness, and (of course) timely recognition of appropriateness. Crit Care Med. 2011;39(9):2184–2186.
- , , , et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline‐based performance improvement program. Crit Care Med. 2014;42(8):1749–1755.
- , , , et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248.
- , , , , , . Impact of previous antibiotic therapy on outcome of gram‐negative severe sepsis. Crit Care Med. 2011;39(8):1859–1865.
- , , , . Beta lactam antibiotic monotherapy versus beta lactam‐aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344.
- , , , et al. Hydroxyethyl starch 130/0.42 versus ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134.
- , , , et al.; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–1911.
- , , , et al. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta‐analysis and trial sequential analysis. BMJ. 2013;346:f839.
- , , , et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta‐analysis. JAMA. 2013;309(7):678–688.
- , , , , , ; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256.
- , , , et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421.
- , , , . Randomised trials of human albumin for adults with sepsis: systematic review and meta‐analysis with trial sequential analysis of all‐cause mortality. BMJ. 2014;349:g4561.
- , , , et al. Fluid resuscitation in sepsis: a systematic review and network meta‐analysis. Ann Intern Med. 2014;161(5):347–355.
- , , , , , . Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572.
- NICE‐SUGAR Study Investigators, , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , . Glucose control in the ICU—how tight is too tight? N Engl J Med. 2009;360(13):1346–1349.
- COIITSS Study Investigators, , , , et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348.
- , , , et al. Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data. CMAJ. 2009;180(8):821–827.
- , , , et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362–2375.
- , , , et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871.
- , , , et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42(3):625–631.
- , , , , . Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316.
- , , , , , . The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665–674.
- , . Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189(5):548–555.
- , . Declining case fatality rates for severe sepsis: Good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295–1297.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , , , . Short‐ and long‐term mortality in patients with community‐acquired severe sepsis and septic shock. Scand J Infect Dis. 2013;45(8):577–583.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–69.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , ; Finnsepsis Study Group. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274.
- , , , , . Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254.
- , , , . Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334.
- , , , , , ; Co‐operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–1213.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.
- , . Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al.; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , . Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS data brief, no 62. Hyattsville, MD: National Center for Health Statistics. 2011.
- , , , et al.; Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140(5):1223–1231.
- , , , . Benchmarking the incidence and mortality of severe sepsis in the united states. Crit Care Med. 2013;41(5):1167–1174.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761.
- , . National inpatient hospital costs: the most expensive conditions by payer, 2011. HCUP statistical brief #160. Rockville, MD: Agency for Healthcare Research and Quality, 2013. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb160.pdf. Accessed March 15, 2015.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , , , . Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145(7):695–700.
- . Evidence‐based sepsis therapy: a hospitalist perspective. J Hosp Med. 2006;1(5):285–295.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
- , , , et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873.
- , , , et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746.
- . Disassembling goal‐directed therapy for sepsis: a first step. JAMA. 2010;303(8):777–779.
- , , , et al. Early lactate‐guided therapy in intensive care unit patients: a multicenter, open‐label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761.
- , , , et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
- , , , et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677.
- , , , et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43(5):983–988.
- ARISE Investigators; ANZICS Clinical Trials Group, , , , et al. Goal‐directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506.
- ProCESS Investigators, , , , et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693.
- , , , et al.; ProMISe Trial Investigators. Trial of early, goal‐directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311.
- . The ProCESS trial—a new era of sepsis management. N Engl J Med. 2014;370(18):1750–1751.
- , . The role of bundles in sepsis care. Crit Care Clin. 2006;22(3):521–529, x.
- , , , et al. The surviving sepsis campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82.
- , , , et al. The GENESIS project (Generalized early sepsis intervention strategies): a multicenter quality improvement collaborative. J Intensive Care Med. 2013;28(6):355–368.
- , . Early recognition and treatment of severe sepsis. Am J Respir Crit Care Med. 2013;188(1):7–8.
- , , , et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391.
- , , , et al. High versus low blood‐pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–1593.
- . Is there a good MAP for septic shock? N Engl J Med. 2014;370(17):1649–1651.
- , , , et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- , , , et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal‐directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053.
- , , , et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–2071.
- , . Antibiotics in sepsis: timing, appropriateness, and (of course) timely recognition of appropriateness. Crit Care Med. 2011;39(9):2184–2186.
- , , , et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline‐based performance improvement program. Crit Care Med. 2014;42(8):1749–1755.
- , , , et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248.
- , , , , , . Impact of previous antibiotic therapy on outcome of gram‐negative severe sepsis. Crit Care Med. 2011;39(8):1859–1865.
- , , , . Beta lactam antibiotic monotherapy versus beta lactam‐aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344.
- , , , et al. Hydroxyethyl starch 130/0.42 versus ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–134.
- , , , et al.; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–1911.
- , , , et al. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta‐analysis and trial sequential analysis. BMJ. 2013;346:f839.
- , , , et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta‐analysis. JAMA. 2013;309(7):678–688.
- , , , , , ; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256.
- , , , et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421.
- , , , . Randomised trials of human albumin for adults with sepsis: systematic review and meta‐analysis with trial sequential analysis of all‐cause mortality. BMJ. 2014;349:g4561.
- , , , et al. Fluid resuscitation in sepsis: a systematic review and network meta‐analysis. Ann Intern Med. 2014;161(5):347–355.
- , , , , , . Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572.
- NICE‐SUGAR Study Investigators, , , , et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297.
- , . Glucose control in the ICU—how tight is too tight? N Engl J Med. 2009;360(13):1346–1349.
- COIITSS Study Investigators, , , , et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348.
- , , , et al. Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data. CMAJ. 2009;180(8):821–827.
- , , , et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362–2375.
- , , , et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871.
- , , , et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42(3):625–631.
- , , , , . Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316.
- , , , , , . The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665–674.
- , . Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189(5):548–555.
- , . Declining case fatality rates for severe sepsis: Good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295–1297.
- , , , , , . Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283.
- , , , , , . Short‐ and long‐term mortality in patients with community‐acquired severe sepsis and septic shock. Scand J Infect Dis. 2013;45(8):577–583.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–69.
- , , , . Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , ; Finnsepsis Study Group. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274.
- , , , , . Incident stroke and mortality associated with new‐onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254.
- , , , . Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334.
- , , , , , ; Co‐operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–1213.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834.