User login
Is There a Link Between Diabetes and Bone Health?
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Diabetes can pose serious complications to bone health. “Clinical trials have revealed a startling elevation in fracture risk in diabetic patients,” says Liyun Wang, PhD, Associate Professor of Mechanical Engineering at the University of Delaware in Newark, Delaware. “Bone fractures can be life threatening — nearly 1 in 6 hip fracture patients dies within a year of injury.”
Because physical exercise is proven to improve bone properties and reduce fracture risk in non-diabetic people, Dr. Wang and colleagues tested its efficacy in type 1 diabetes. Their findings were published online ahead of print July 13 in Bone.
The researchers hypothesized that diabetic bone’s response to anabolic mechanical loading would be attenuated, partially due to impaired mechanosensing of osteocytes under hyperglycemia. For their study, heterozygous male and female diabetic mice and their age- and gender-matched wild-type controls were subjected to unilateral axial ulnar loading with a peak strain of 3500 με at 2 Hz and 3 minutes per day for 5 days.
Overall, the study demonstrated that exercise-induced bone formation was maintained in mildly diabetic mice at a similar level as non-diabetic controls, while the positive effects of exercise were nearly abolished in severely diabetic mice. At the cellular level, the researchers found that hyperglycemia reduced the sensitivity of osteocytes to mechanical stimulation and suppressed osteocytes’ secretion of proteins and signaling molecules that help build stronger bone.
“Our work demonstrates that diabetic bone can respond to exercise when the hyperglycemia is not severe, which suggests that mechanical interventions may be useful to improve bone health and reduce fracture risk in mildly affected diabetic patients,” said Dr. Wang. These results, along with previous findings showing adverse effects of hyperglycemia on osteoblasts and mesenchymal stem cells, suggest that failure to maintain normal glucose levels may impair bone’s responses to mechanical loading in diabetics.
To translate the findings of the study to patient care, Ms. Wang’s team has begun to collaborate with M. James Lenhard, MD, Director of the Center for Diabetes and Metabolic Diseases at Christiana Care Health System in Wilmington, Delaware.
“The plan for collaboration between the University of Delaware and Christiana Care is to evaluate these research findings in humans and expand the research to include other complications of diabetes, such as cardiovascular disease.
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Suggested Reading
Parajuli A, Liu C, Wen L, et al. Bone’s responses to mechanical loading are impaired in type 1 diabetes. Bone. 2015 July 13 [Epub ahead of print].
Generalized, well-dispersed rash • wheal development after tactile irritation • normal vital signs • Dx?
THE CASE
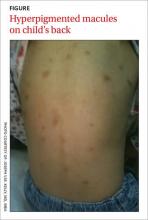
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
THE CASE
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
THE CASE
A 3-year-old girl was brought to our clinic with a generalized rash over her scalp, face, neck, chest, abdomen, back, perianal area, extremities, and the plantar surface of her right foot. On physical examination, we noted many round, hyperpigmented, brown and reddish pink, well-circumscribed macules on her body (FIGURE). Only a few of these macules had appeared on the girl’s trunk within the first 3 months of her life, but since then they’d increased in number and spread to other parts of her body as she’d aged. The lesions became edematous and erythematous with tactile irritation. Darier’s sign (the development of a hive or wheal when a lesion is stroked) was present.
The patient’s vitals at the time of examination included a temperature of 98.2°F, respiratory rate of 17 breaths/min, heart rate of 92 beats/min, blood pressure of 100/66 mm Hg, and oxygen saturation level of 100% on room air. The girl’s parents said they hadn’t traveled. There was no mucosal involvement and no systemic involvement. The patient had no past surgical or medical history, was not taking any medications, and had no significant birth history. A skin biopsy was performed.
THE DIAGNOSIS
Based on the presence of a positive Darier’s sign and the results of the skin biopsy (which showed increased mast cells), we diagnosed urticaria pigmentosa (UP), which is the most common form of cutaneous mastocytosis.1
The diagnosis had been delayed for almost 3 years because of several factors. For one thing, there had been few lesions present early in the child’s life, and as a result, the parents chalked them up to “beauty marks.” Then, as the lesions started to increase in number, the parents thought bed bugs were to blame.
As time went on, the parents attempted to treat their daughter’s hives with homeopathic remedies suggested by family members. When the lesions didn’t resolve with homeopathic remedies, the parents tried over-the-counter H1 and H2 antihistamines such as diphenhydramine, loratadine, and ranitidine. When these treatments failed, the parents brought their child to our office for medical evaluation.
DISCUSSION
UP is a chronic skin disorder in which there is an abnormal proliferation of mast cells in the dermis of the skin. It is considered an orphan disease.1 UP that presents in children is most often benign. Approximately 50% of cases occur before 6 months of age and 25% occur before puberty.2 The lesions are often self-limited and completely resolve in approximately 50% of patients by puberty.3 By adulthood, the lesions either resolve or some lightly colored non-urticating macules remain; however, some patients will continue to have a positive Darier’s sign.4
Dermatologic symptoms. A patient with UP may present with brown or reddish maculopapules, papules, nodules, pruritus, and flushing of the face. Darier’s sign is usually seen in cases of UP; in a study of mastocytosis in children, Darier’s sign was present in 94% of cases.5 Lesions are more prominent in areas where clothes can rub the skin, and they often vary in size and appearance. The presence of lesions can vary from localized and scant to hundreds located over the entire body. UP can be difficult to identify when the lesions are limited, which can lead to delayed diagnosis and treatment.6
Systemic involvement. UP also can affect the skeleton, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract, kidneys, cardiovascular system, and/or central nervous system. Skeletal involvement may manifest as osteoporosis or bone pain in 10% to 20% of patients with UP.7 Bone marrow involvement may progress to anemia or mast cell leukemia. UP can result in hepatomegaly or enlarged lymph nodes. Patients may experience nausea, diarrhea, or abdominal pain if the gastrointestinal tract is affected. Cardiovascular involvement may manifest as tachycardia and shock.8
Diagnosis of UP is made based on the physical exam findings noted earlier, as well as skin biopsy laboratory results. Skin biopsy will reveal increased mast cells. In up to two-thirds of patients who have systemic involvement, laboratory testing will show elevated urine histamine levels, as well as elevated serum concentrations of tryptase.9
UP can appear similar to many other skin conditions
The differential diagnosis of UP can include urticaria, atopic dermatitis, contact dermatitis, pityriasis rosea, an allergic reaction/drug eruption, Henoch-Schonlein purpura, erythema multiforme, fifth disease, folliculitis, guttate psoriasis, miliaria rubra, insect bites, viral exanthem, lichen planus, and scabies.10,11 In addition to the clinical appearance of the rash, these conditions can be distinguished from UP by skin biopsy and other relevant tests, as well as a thorough history.
Treatment options include antihistamines, corticosteroids, PUVA
Patients with UP should be instructed to avoid precipitating factors such as temperature changes, friction, alcohol ingestion, aspirin, physical exertion, or opiates. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA (psoralen plus ultraviolet A photochemotherapy).3 PUVA is normally avoided in pediatric patients because it is associated with an increased risk of skin cancer later in life.12
Our patient. We prescribed a topical corticosteroid, 0.05% betamethasone dipropionate cream, and oral cromolyn sodium 100 mg qid for our patient, but this failed to significantly improve the macules. The patient and parents grew increasingly anxious. Ultimately, the parents decided to have their daughter treated with PUVA in limited amounts. Topical psoralen was also used. After 2 months of treatment, the patient’s lesions substantially improved and many of them disappeared. In addition, the parents were educated on the importance of sunscreen and limiting their daughter’s exposure to the sun, when possible.
THE TAKEAWAY
UP can be diagnosed by taking a thorough history and conducting a physical examination; a skin biopsy that reveals increased mast cells will confirm the diagnosis. UP is usually self-limited and resolves in about one-half of patients by puberty. Treatment options include H1 and H2 antihistamines, cromolyn sodium, topical corticosteroids, and PUVA. Patients should be referred to a specialist if their symptoms become severe, systemic UP is suspected, or they do not respond to therapy.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
1. Lain EL, Hsu S. Photo quiz. Chronic, papular rash that develops a wheal when rubbed. Am Fam Physician. 2004;69:1493-1494.
2. Jain S. Dermatology: Illustrated Study Guide and Comprehensive Board Review. New York: Springer. 2012;47.
3. Soter NA. The skin in mastocytosis. J Invest Dermatol. 1991;96:32S-38S; discussion 38S-39S.
4. Caplan RM. Urticaria pigmentosa and systemic mastocytosis. JAMA. 1965;194:1077-1080.
5. Kiszewski AE, Durán-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
6. Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047-3054, 3059-3060.
7. Borenstein DG, Wiesel SW, Boden SD, eds. Low Back and Neck Pain: Comprehensive Diagnosis and Management. 3rd ed. Philadelphia, Pa: Elsevier; 2004.
8. Vigorita VJ. Metabolic bone disease: Part II. In: Vigorita VJ, Ghelman B, Mintz D, eds. Orthopaedic Pathology. 2nd ed. Philadelphia, PA: Walter Kluwer Lippincott Williams & Wilkins. 2008;197.
9. Rosenbaum RC, Frieri M, Metcalfe DD. Patterns of skeletal scintigraphy and their relationship to plasma and urinary histamine levels in systemic mastocytosis. J Nucl Med. 1984;25:859-864.
10. Islas AA, Penaranda E. Generalized brownish macules in infancy. Urticaria pigmentosa. Am Fam Physician. 2009;80:987.
11. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
12. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:22-31.
Infection prevention
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
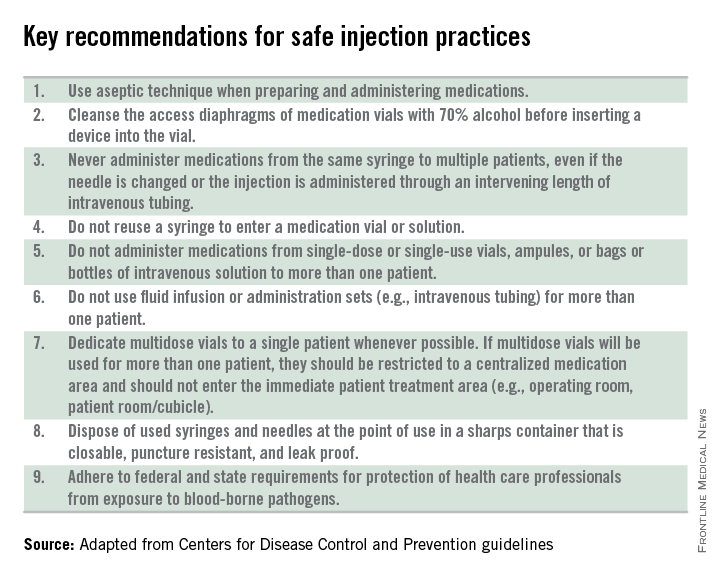
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.
Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Variations in blood cancer survival across Europe

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()

chemotherapy
Photo by Rhoda Baer
VIENNA—Results of the EUROCARE-5 study have revealed regional differences in survival for European patients with hematologic malignancies.
The data showed regional variations in 5-year relative survival rates for a number of cancers.
But the differences were particularly pronounced for leukemias, non-Hodgkin lymphomas (NHLs), and plasma cell neoplasms (PCNs).
Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, presented these results at the 2015 European Cancer Congress (LBA 1).
Data from this study have also been published in several articles in the October 2015 issue of the European Journal of Cancer.
EUROCARE-5 includes records from 22 million cancer patients diagnosed between 1978 and 2007. The latest data encompass more than 10 million patients (ages 15 and older) diagnosed from 1995 to 2007 and followed up to 2008.
The data came from 107 cancer registries in 29 countries. The researchers estimated 5-year relative survival and trends from 1999 to 2007 according to region—Ireland/UK, Northern Europe, Central Europe, Southern Europe, and Eastern Europe.
“In general, 5-year relative survival—survival that is adjusted for causes of death other than cancer—increased steadily over time in Europe, particularly in Eastern Europe, for most cancers,” Dr Sant said.
“However, the most dramatic geographical variations were observed for cancers of the blood where there have been recent advances in treatment, such as chronic myeloid and lymphocytic leukemias, non-Hodgkin lymphoma and 2 of its subtypes (follicular and diffuse large B-cell lymphoma), and multiple myeloma. Hodgkin lymphoma was the exception, with smaller regional variations and a fairly good prognosis in most countries.”
Hodgkin lymphoma and NHL
Of all the hematologic malignancies, 5-year relative survival was highest for Hodgkin lymphoma, at 80.8% (40,625 cases). Five-year survival was 79.4% in Ireland and the UK, 85% in Northern countries, and 74.3% in Eastern Europe, which was significantly below the European average (P<0.0001).
For NHL, the 5-year relative survival was 59.4% (329,204 cases). Survival rates for NHL patients ranged from 49.7% in Eastern Europe to 63.3% in Northern Europe.
CLL/SLL
For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), the 5-year relative survival was 70.4% (81,914 cases). CLL/SLL survival ranged from 58% in Eastern Europe to about 74% in Central and Northern Europe.
The researchers noted that between-country variations in CLL/SLL survival were high in all regions. Outliers that were significantly below the regional average were Austria (67%), Croatia (52%), and Bulgaria (45.5%).
PCNs
PCNs included multiple myeloma, plasmacytoma, and plasma cell leukemias. The 5-year relative survival for all PCNs was 39.2% (94,024 cases).
PCN survival rates were lowest in Eastern Europe (31.7%), slightly higher in the UK/Ireland (35.9%), and between 39.1% and 42% in the rest of Europe.
Myeloid leukemias
Of all the hematologic malignancies, 5-year relative survival was poorest for patients with acute myeloid leukemia (AML), at 17.1% (57,026 cases).
AML survival rates in Ireland/UK (15.0%) and Eastern Europe (13.0%) were significantly below the European average. But AML survival in Sweden, Belgium, France, and Germany was significantly higher than the average (P<0.005).
Five-year relative survival for chronic myeloid leukemia (CML) was 52.9% (17,713 cases).
Of all the hematologic malignancies, the survival gap between Eastern Europe and the rest of Europe was highest for CML. Five-year survival for CML patients was 33% in Eastern Europe and ranged from 51% to 58% in the rest of Europe.
The researchers also said there were striking survival variations by country in all areas. They found significant deviations from the regional average in Sweden (69.7%), Scotland (64.6%), France (71.7%), Austria (48.2%), Croatia (37.8%), Estonia (48.9%), Czech Republic (45.2%), and Latvia (22.1%).
“Results from EUROCARE can help to identify regions of low survival where action is needed to improve patients’ outcomes,” Dr Sant noted.
“Population-based survival information is essential for physicians, policy-makers, administrators, researchers, and patient organizations who deal with the needs of cancer patients, as well as with the issue of the growing expenditure on healthcare.” ![]()
mAb produces ‘encouraging’ results in MM

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()

multiple myeloma
ROME—Combination therapy incorporating a novel monoclonal antibody (mAb) can provide “encouraging, long-lasting tumor control” in heavily pretreated patients with relapsed/refractory multiple myeloma (MM), according to investigators.
The mAb, MOR202, was also considered to be well-tolerated in this ongoing phase 1/2a study.
Early results from this study were presented at the 15th International Myeloma Workshop (poster #156). The study was sponsored by MorphoSys AG, makers of MOR202. The poster is available on the company’s website.
MOR202 is a HuCAL-derived mAb directed against CD38. In the phase 1/2a study, 50 MM patients have received the drug thus far.
At baseline, the patients’ median age was 67. They had received a median of 4 prior therapies, including bortezomib (98%), lenalidomide (92%), melphalan (92%), cyclophosphamide (76%), doxorubicin (60%), thalidomide (32%), pomalidomide (14%), carfilzomib (6%), elotuzumab (4%), and panobinostat (4%). Seventy-six percent had received a stem cell transplant.
Study design
The study consists of several parts and dosing cohorts in which the investigators are assessing MOR202 alone or in combination with other agents.
Treatment in Part A consists of a 2-hour intravenous infusion of MOR202 once every 2 weeks at several different doses: 0.01 mg/kg , 0.04 mg/kg, 0.15 mg/kg, 0.5 mg/kg, 1.5 mg/kg, 4.0 mg/kg, 8.0 mg/kg, or 16.0 mg/kg.
Part B is a 2-hour intravenous infusion of MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part C is dexamethasone plus MOR202 once a week at 4 mg/kg, 8 mg/kg, or 16 mg/kg.
Part D is pomalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
Part E is lenalidomide, dexamethasone, and MOR202 once a week at 8 mg/kg or 16 mg/kg.
In the confirmatory cohorts, patients receive MOR202 with or without dexamethasone once a week or once every 2 weeks, MOR202 with pomalidomide and dexamethasone once a week, or MOR202 with lenalidomide and dexamethasone once a week.
Efficacy
Of the 50 patients treated thus far, 27 were evaluable for efficacy. One patient achieved a very good partial response, 2 had a partial response, and 2 had a minor response. Eleven patients had stable disease, and 11 progressed.
The very good partial response occurred in a patient receiving weekly MOR202 at 4 mg/kg plus dexamethasone.
One partial response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone. The other occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and pomalidomide.
One minor response occurred in a patient receiving weekly MOR202 at 8 mg/kg plus dexamethasone and lenalidomide. The other occurred in a patient receiving weekly MOR202 at 16 mg/kg plus dexamethasone.
“[T]he preliminary efficacy seen so far with MOR202 as single agent and in combinations is promising,” said investigator Marc-Steffen Raab, MD, of Heidelberg University Hospital and the German Cancer Research Center DKFZ in Heidelberg, Germany.
Safety
All 50 patients were evaluable for safety. Ninety-eight percent experienced an adverse event (AE), 66% of which were grade 3 or higher.
The most frequent AEs (overall and grade 3 or higher) were anemia (34%, 6%), leukopenia (30%, 10%), neutropenia (20%, 10%), thrombocytopenia (18%, 8%), fatigue (30%, 0%), nausea (22%, 0%), diarrhea (20%, 0%), and headache (16%, 0%).
Thirty-six percent of patients discontinued MOR202 due to treatment-emergent AEs. However, only 6% (n=3) of these AEs were considered possibly related to MOR202.
Infusion-related reactions occurred in 15 patients (30%). One of these patients received dexamethasone as well and experienced an infusion-related reaction (grade 1).
In the absence of dexamethasone, nearly all infusion reactions were grade 1-2. The exception was 1 patient with a grade 3 reaction that was mainly limited to the first infusion.
The maximum tolerated dose of MOR202 has not been reached.
“Considering the low rate of infusion reactions, even in cohorts without dexamethasone, the short infusion time, and other aspects, MOR202 may turn out to be an excellent choice in terms of safety and tolerability,” Dr Raab concluded. ![]()
Value‐Based Payment
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
Parent and Stakeholder Engagement
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).
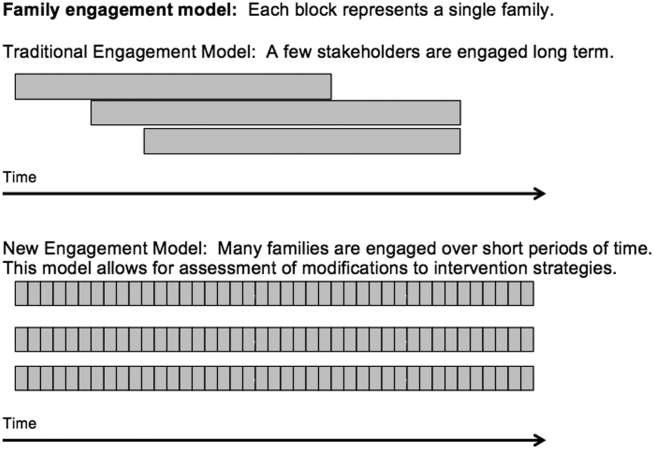
PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).

PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).

PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
Striving for Optimal Care/
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
SOAP‐V Method for Bending the Cost Curve
Today's medical students will enter practice over the next decade and inherit the escalating costs of the US healthcare system. Approximately 30% of healthcare costs, or $750 billion dollars annually, are spent on unnecessary tests or procedures.[1] High healthcare costs combined with calls to eliminate waste, improve patient safety, and increase quality[2] are driving our healthcare system to evolve from a fee‐based system to a value‐based system. Additionally, many patients are being harmed by overtesting and the stress associated with rising healthcare bills. Financial risk has increasingly shifted to patients in the form of higher deductibles and reduced caps, and medical indebtedness is the number 1 risk for bankruptcy.[3, 4] False positive results of low‐yield diagnostic tests lead to additional testing, anxiety, excess radiation exposure, and unnecessary invasive procedures.[5] To minimize harm to patients, evidence must guide physicians in their ordering behavior. In addition, any care plan a physician develops should be individualized to incorporate patients' values and preferences. Unfortunately, medical students, who are at an impressionable stage in their careers, frequently observe overtesting and unnecessary treatment behaviors in their clinical encounters.[6] Instead, our medical students and trainees must be prepared to deliver patient care that is evidence based, patient centered, and cost conscious. They must become effective stewards of limited healthcare resources.
To help prepare our students for this evolving healthcare paradigm, we created a new tool called SOAP‐V (Subjective‐Objective‐Assessment‐PlanValue), designed to embed discussion of healthcare value into medical student oral presentations and note writing. Students are encouraged to use this tool at the point of care to bring up value concepts with physicians and residents as part of medical decision making. In so doing, we propose that medical students can serve as change agents to shift physician practice at our academic medical centers to focus on healthcare value. This article describes the SOAP‐V tool, contains links to educational materials to help hospitalists and other clinician educators to implement this tool, and provides preliminary findings and reflections.
INNOVATION
SOAP‐V was conceived at the Millennium Conference on Teaching High‐Value Care, which was sponsored by the Beth Israel Deaconess Medical Center Shapiro Institute for Education and Research, the Association of American Medical Colleges, and the American College of Physicians. Educators from several medical schools decided to form a group to specifically consider ways to train medical students and residents in the concept of high‐value care (HVC), which is framed as improving patient outcomes while decreasing patient cost and harm.[7] Our group recognized several challenges in teaching HVC. First, physician practice habits are influenced by the way they are trained,[8] yet faculty who teach those future physicians frequently have not themselves been taught, nor do they consistently practice HVC.[9] Second, we needed to teach students the requisite HVC knowledge, attitudes, and skills, and therefore wanted to provide opportunities to not only learn, but practice, HVC, preferably in authentic patient experiences to optimize their learning.[10, 11] Third, we recognized that adding another teaching task to the already oversubscribed day of an attending might understandably be met with resistance. We envisioned a tool that could be used with minimal or no faculty training, could be attached to authentic patient experiences, and based on LEAN‐Six Sigma principles,[12] would be embedded in the normal workflow. Furthermore, we considered social networking principles, such as those described by Christakis and Fowler that describe how an individual's behavior impacts behaviors of those surrounding them,[13] and hoped to empower medical students to serve as change agents. Medical students could initiate discussions of value concepts at the point of care in a way that challenges a heavily entrenched test‐ordering culture and encourages other members of the team to balance potential benefit with harms and cost. Following the conference, the group held bimonthly phone conferences and subsequently developed the SOAP‐V tool, created teaching materials, and planned a research project on SOAP‐V.
SOAP‐V modifies the traditional SOAP (Subjective‐Objective‐Assessment‐Plan) oral presentation or medical note, to include value (V). It serves as a cognitive forcing function designed to create a pause and promote discussions of HVC during patient delivery. It prompts the student to ask 3 value questions: (1) Before choosing an intervention, have I considered whether the result would change management? (2) Have I incorporated the patient's goals and values, and considered the potential harm of the intervention compared to alternatives? (3) What is the known and potential cost of the intervention, both immediate and downstream? The student gathers information during the patient interview and brings back the information to the team during rounds where management decisions are made.
In the summer of 2014, we launched an institutional review boardapproved, multi‐institutional study to implement SOAP‐V at Penn State College of Medicine, Harvard Medical School, and Case Western Reserve University School of Medicine for third‐year medical students during their internal medicine clerkships. Students in the intervention arm participated in an interactive workshop on SOAP‐V. Authors S.F., S.G., and C.D.P., who serve as clerkship directors in internal medicine, provided student training for each cohort of intervention students at the beginning of each rotation on general medicine inpatient wards. The workshop began with trigger videos that demonstrate pressures encountered by a student on rounds that might lead to overuse.[14] Following a discussion on overuse and methods to avoid overuse, the students were introduced to the SOAP‐V framework, watched a video of a student modeling a SOAP‐V presentation on rounds,[15] and engaged in a SOAP‐V role play. They received a SOAP‐V pocket card as well as a Web link to Healthcare Bluebook[16] to research costs. An outline of the session and associated materials can be found in an online attachment.[17] The students then used the SOAP‐V tool during inpatient rounds. We advised supervising faculty that students might present using a SOAP‐V format, and provided them with a SOAP‐V card, but we did not provide faculty development on SOAP‐V. Students participating in the control arm did not receive training specific to SOAP‐V.
Students in intervention and control arms at each school were surveyed on their attitudes toward HVC at the beginning of the clerkship year and then again at the completion of the medicine clerkship via a 19‐item questionnaire soliciting perceptions and self‐reported practices in HVC. Intervention arm students received biweekly e‐mail links that allowed them to anonymously document their use of SOAP‐V, as well as an end‐of‐clerkship open‐ended question about the usefulness of SOAP‐V. We analyzed questionnaire results using McNemar's test for paired data.
PRELIMINARY FINDINGS
The preintervention attitudinal survey (n = 226) demonstrated that although 90% of medical students agreed on the importance of considering costs of treatments, only 50% felt comfortable bringing up cost considerations with their team, and 50% considered costs to the healthcare system in clinical decisions. An interim analysis of the available data at 6 months (response rate approximately 50% across sites) showed that students in the intervention arm reported increased agreement with the phrases, I have the power to address the economic healthcare crisis (pre‐37%, post‐65%, P = 0.046); I would be comfortable initiating a discussion about unnecessary tests or treatments with my team, (pre‐46%, post‐85%, P = 0.027); and In my clinical decisions, I consider the potential costs to the healthcare system (pre‐41%, post‐60%, P = 0.023) compared to control arm students, who showed no significant differences pre‐ versus postrotation in these 3 domains (Figure 1).
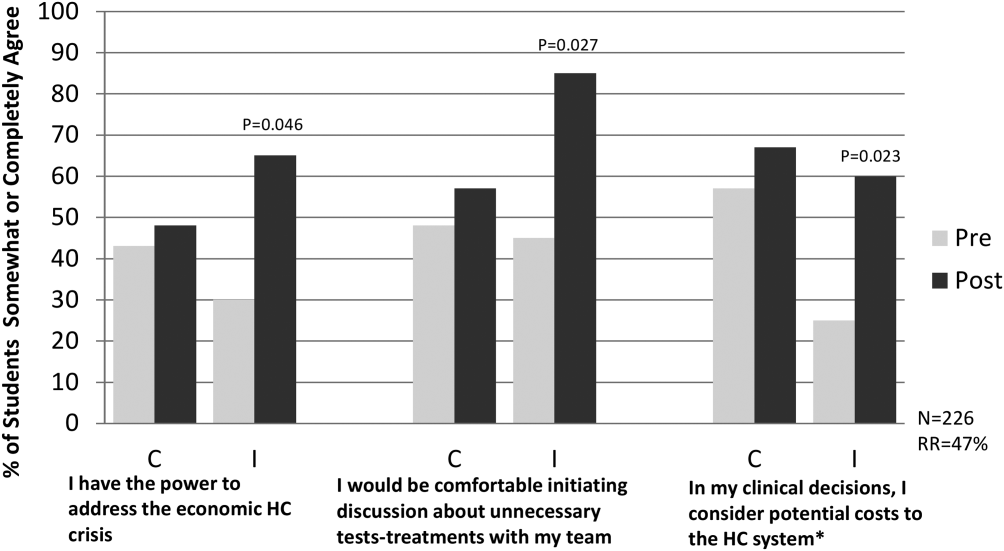
To date, biweekly surveys and direct observation of rounds have verified student use of SOAP‐V. Student comments have included: Allowed me the ability to raise important issues with the team while feeling like I was helping my patients and the healthcare system. A great principle that I used almost daily. Great to implement this at such a young stage in my med career. Broadened my perspective on the role of a physician.
SOAP‐V has inspired some of our medical students to consider value in healthcare more closely. In a notable example, a SOAP‐Vtrained student admitted a young man with lymphadenopathy, pulmonary infiltrates, and weight loss who underwent an extensive and costly workup including liver biopsy, bronchoscopy, and multiple computed tomography and positron emission tomography scans and was eventually diagnosed with sarcoidosis. The SOAP‐Vtrained student reviewed the patient's workup, estimated that the team spent more than $6000 to make the diagnosis, and recommended a more cost‐effective approach.
Common barriers experienced by the pilot sites included time constraints limiting discussion of value, variability in perceived receptivity depending on team leadership, and student confidence in initiating this dialogue. Solutions included underscoring the notion that value discussions can be brief, may be appropriately initiated by any member of the team, and may have an effect on choice of management and/or patient preference issues that can make medical care more efficient and effective. Resident and faculty physicians were made aware of the intervention, and encouraged to support students in using the SOAP‐V tool.
CONCLUSION
SOAP‐V was successfully implemented within the inpatient internal medicine clerkship at 3 academic institutions. Our preliminary results demonstrate that students can use this framework to apply considerations of high‐value, cost‐conscious care in their medical decision making and to promote discussion of these concepts during rounds with their inpatient teams. Students in the intervention arm report greater comfort discussing unnecessary tests and treatments with their team and a greater likelihood to consider potential costs to the healthcare system. Additionally, these students commented that the SOAP‐V framework broadened their perspective on their role as a physician in curbing costs, and that they felt more empowered to address the economic healthcare crisis. The next phase of our project will involve conducting end‐of‐year surveys to evaluate whether SOAP‐V has a persistent impact on the frequency and quality of value discussions on rounds, as well as students' attitudes about cost consciousness. We will also gauge whether resident and faculty attitudes about HVC have changed as a result of the intervention.
Our SOAP‐V student training was provided in a 1‐hour session. We believe that the ease of training and the simplicity of the SOAP‐V framework permit SOAP‐V to be easily transferred for use by residents, medical students in other clerkships, and other healthcare learners. Additional research is needed to demonstrate this expanded use and prove sustainability. An additional important question is whether use of SOAP‐V by students and residents results in reductions in unnecessary costs. Future educational efforts will include embedding the SOAP‐V tool in other clerkships and promoting the SOAP‐V tool within corresponding residencies in both hospital and outpatient clinic settings and analyzing potential reductions in wasteful spending.
It is generally conceived that medical students learn the information they are taught, and are impacted by the culture in which they reside; multiple studies bear this out.[18, 19] However, students may also be change agents. Our students will inherit the healthcare systems of the future. We must empower them to change the status quo. There can be tremendous utility in employing such a bottom up approach to process improvement. What a student discusses today may spark the resident (or faculty) to consider in their own workflow tomorrow. In this way, we envision that the SOAP‐V is a tool by which ideas concerning HVC can be generated and shared at the point of care. It is our hope that this straightforward intervention is one that may slowly change the culture and perhaps eventually the practice patterns of our academic medical centers.
Disclosure
Nothing to report.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes. Washington, DC: The National Academies Press; 2010.
- Institute for Healthcare Improvement. IHI triple aim initiative. Available at: http://www.ihi.org/Engage/Initiatives/TripleAim/pages/default.aspx. Accessed August 7, 2015.
- , , , . Medical bankruptcy in the United States, 2007. Am J Med. 2009;122(8):741–746.
- . Kaiser Family Foundation. Health care costs: a primer. Key information on health care costs and their impact. May 2012. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7670–03.pdf. Accessed August 7, 2015.
- , . Over‐testing: why more is not better. Am J Med. 2014;127:362–363.
- , , . Medical student perceptions of cost‐conscious care in an internal medicine clerkship: a thematic analysis [published online May 1, 2015]. J Gen Intern Med. doi: 10.1007/s11606‐015‐3324‐4.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155:386–388.
- , , . Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671–1672.
- , , . Theories of teaching. In: The Adult Learner. New York, NY: Routledge; 2012:72–114.
- . Medical education and the maintenance of incompetence. Med Teach. 2006;28:690–696.
- , , , , . Lean Six Sigma in healthcare. J Healthcare Qual. 2006;2:4–11
- , . Connected. New York, NY: Little, Brown 2009.
- Teaching Value Project. Costs of care. Available at: teachingvalue.org Available at: https://www.dropbox.com/s/tb8ysfjtzklwd8g/OverrunPart1.webm; https://www.dropbox.com/s/cxt9mvabj4re4g9/OverrunPart2.webm. Accessed August 7, 2015.
- , , . SOAP‐V [online video]. Available at: https://www.youtube.com/watch?v=goUgAzLuTzY47(2):134–143.
- , , , , , . How medical students learn from residents in the workplace: a qualitative study. Acad Med. 2014:89(3):490–496.
Today's medical students will enter practice over the next decade and inherit the escalating costs of the US healthcare system. Approximately 30% of healthcare costs, or $750 billion dollars annually, are spent on unnecessary tests or procedures.[1] High healthcare costs combined with calls to eliminate waste, improve patient safety, and increase quality[2] are driving our healthcare system to evolve from a fee‐based system to a value‐based system. Additionally, many patients are being harmed by overtesting and the stress associated with rising healthcare bills. Financial risk has increasingly shifted to patients in the form of higher deductibles and reduced caps, and medical indebtedness is the number 1 risk for bankruptcy.[3, 4] False positive results of low‐yield diagnostic tests lead to additional testing, anxiety, excess radiation exposure, and unnecessary invasive procedures.[5] To minimize harm to patients, evidence must guide physicians in their ordering behavior. In addition, any care plan a physician develops should be individualized to incorporate patients' values and preferences. Unfortunately, medical students, who are at an impressionable stage in their careers, frequently observe overtesting and unnecessary treatment behaviors in their clinical encounters.[6] Instead, our medical students and trainees must be prepared to deliver patient care that is evidence based, patient centered, and cost conscious. They must become effective stewards of limited healthcare resources.
To help prepare our students for this evolving healthcare paradigm, we created a new tool called SOAP‐V (Subjective‐Objective‐Assessment‐PlanValue), designed to embed discussion of healthcare value into medical student oral presentations and note writing. Students are encouraged to use this tool at the point of care to bring up value concepts with physicians and residents as part of medical decision making. In so doing, we propose that medical students can serve as change agents to shift physician practice at our academic medical centers to focus on healthcare value. This article describes the SOAP‐V tool, contains links to educational materials to help hospitalists and other clinician educators to implement this tool, and provides preliminary findings and reflections.
INNOVATION
SOAP‐V was conceived at the Millennium Conference on Teaching High‐Value Care, which was sponsored by the Beth Israel Deaconess Medical Center Shapiro Institute for Education and Research, the Association of American Medical Colleges, and the American College of Physicians. Educators from several medical schools decided to form a group to specifically consider ways to train medical students and residents in the concept of high‐value care (HVC), which is framed as improving patient outcomes while decreasing patient cost and harm.[7] Our group recognized several challenges in teaching HVC. First, physician practice habits are influenced by the way they are trained,[8] yet faculty who teach those future physicians frequently have not themselves been taught, nor do they consistently practice HVC.[9] Second, we needed to teach students the requisite HVC knowledge, attitudes, and skills, and therefore wanted to provide opportunities to not only learn, but practice, HVC, preferably in authentic patient experiences to optimize their learning.[10, 11] Third, we recognized that adding another teaching task to the already oversubscribed day of an attending might understandably be met with resistance. We envisioned a tool that could be used with minimal or no faculty training, could be attached to authentic patient experiences, and based on LEAN‐Six Sigma principles,[12] would be embedded in the normal workflow. Furthermore, we considered social networking principles, such as those described by Christakis and Fowler that describe how an individual's behavior impacts behaviors of those surrounding them,[13] and hoped to empower medical students to serve as change agents. Medical students could initiate discussions of value concepts at the point of care in a way that challenges a heavily entrenched test‐ordering culture and encourages other members of the team to balance potential benefit with harms and cost. Following the conference, the group held bimonthly phone conferences and subsequently developed the SOAP‐V tool, created teaching materials, and planned a research project on SOAP‐V.
SOAP‐V modifies the traditional SOAP (Subjective‐Objective‐Assessment‐Plan) oral presentation or medical note, to include value (V). It serves as a cognitive forcing function designed to create a pause and promote discussions of HVC during patient delivery. It prompts the student to ask 3 value questions: (1) Before choosing an intervention, have I considered whether the result would change management? (2) Have I incorporated the patient's goals and values, and considered the potential harm of the intervention compared to alternatives? (3) What is the known and potential cost of the intervention, both immediate and downstream? The student gathers information during the patient interview and brings back the information to the team during rounds where management decisions are made.
In the summer of 2014, we launched an institutional review boardapproved, multi‐institutional study to implement SOAP‐V at Penn State College of Medicine, Harvard Medical School, and Case Western Reserve University School of Medicine for third‐year medical students during their internal medicine clerkships. Students in the intervention arm participated in an interactive workshop on SOAP‐V. Authors S.F., S.G., and C.D.P., who serve as clerkship directors in internal medicine, provided student training for each cohort of intervention students at the beginning of each rotation on general medicine inpatient wards. The workshop began with trigger videos that demonstrate pressures encountered by a student on rounds that might lead to overuse.[14] Following a discussion on overuse and methods to avoid overuse, the students were introduced to the SOAP‐V framework, watched a video of a student modeling a SOAP‐V presentation on rounds,[15] and engaged in a SOAP‐V role play. They received a SOAP‐V pocket card as well as a Web link to Healthcare Bluebook[16] to research costs. An outline of the session and associated materials can be found in an online attachment.[17] The students then used the SOAP‐V tool during inpatient rounds. We advised supervising faculty that students might present using a SOAP‐V format, and provided them with a SOAP‐V card, but we did not provide faculty development on SOAP‐V. Students participating in the control arm did not receive training specific to SOAP‐V.
Students in intervention and control arms at each school were surveyed on their attitudes toward HVC at the beginning of the clerkship year and then again at the completion of the medicine clerkship via a 19‐item questionnaire soliciting perceptions and self‐reported practices in HVC. Intervention arm students received biweekly e‐mail links that allowed them to anonymously document their use of SOAP‐V, as well as an end‐of‐clerkship open‐ended question about the usefulness of SOAP‐V. We analyzed questionnaire results using McNemar's test for paired data.
PRELIMINARY FINDINGS
The preintervention attitudinal survey (n = 226) demonstrated that although 90% of medical students agreed on the importance of considering costs of treatments, only 50% felt comfortable bringing up cost considerations with their team, and 50% considered costs to the healthcare system in clinical decisions. An interim analysis of the available data at 6 months (response rate approximately 50% across sites) showed that students in the intervention arm reported increased agreement with the phrases, I have the power to address the economic healthcare crisis (pre‐37%, post‐65%, P = 0.046); I would be comfortable initiating a discussion about unnecessary tests or treatments with my team, (pre‐46%, post‐85%, P = 0.027); and In my clinical decisions, I consider the potential costs to the healthcare system (pre‐41%, post‐60%, P = 0.023) compared to control arm students, who showed no significant differences pre‐ versus postrotation in these 3 domains (Figure 1).

To date, biweekly surveys and direct observation of rounds have verified student use of SOAP‐V. Student comments have included: Allowed me the ability to raise important issues with the team while feeling like I was helping my patients and the healthcare system. A great principle that I used almost daily. Great to implement this at such a young stage in my med career. Broadened my perspective on the role of a physician.
SOAP‐V has inspired some of our medical students to consider value in healthcare more closely. In a notable example, a SOAP‐Vtrained student admitted a young man with lymphadenopathy, pulmonary infiltrates, and weight loss who underwent an extensive and costly workup including liver biopsy, bronchoscopy, and multiple computed tomography and positron emission tomography scans and was eventually diagnosed with sarcoidosis. The SOAP‐Vtrained student reviewed the patient's workup, estimated that the team spent more than $6000 to make the diagnosis, and recommended a more cost‐effective approach.
Common barriers experienced by the pilot sites included time constraints limiting discussion of value, variability in perceived receptivity depending on team leadership, and student confidence in initiating this dialogue. Solutions included underscoring the notion that value discussions can be brief, may be appropriately initiated by any member of the team, and may have an effect on choice of management and/or patient preference issues that can make medical care more efficient and effective. Resident and faculty physicians were made aware of the intervention, and encouraged to support students in using the SOAP‐V tool.
CONCLUSION
SOAP‐V was successfully implemented within the inpatient internal medicine clerkship at 3 academic institutions. Our preliminary results demonstrate that students can use this framework to apply considerations of high‐value, cost‐conscious care in their medical decision making and to promote discussion of these concepts during rounds with their inpatient teams. Students in the intervention arm report greater comfort discussing unnecessary tests and treatments with their team and a greater likelihood to consider potential costs to the healthcare system. Additionally, these students commented that the SOAP‐V framework broadened their perspective on their role as a physician in curbing costs, and that they felt more empowered to address the economic healthcare crisis. The next phase of our project will involve conducting end‐of‐year surveys to evaluate whether SOAP‐V has a persistent impact on the frequency and quality of value discussions on rounds, as well as students' attitudes about cost consciousness. We will also gauge whether resident and faculty attitudes about HVC have changed as a result of the intervention.
Our SOAP‐V student training was provided in a 1‐hour session. We believe that the ease of training and the simplicity of the SOAP‐V framework permit SOAP‐V to be easily transferred for use by residents, medical students in other clerkships, and other healthcare learners. Additional research is needed to demonstrate this expanded use and prove sustainability. An additional important question is whether use of SOAP‐V by students and residents results in reductions in unnecessary costs. Future educational efforts will include embedding the SOAP‐V tool in other clerkships and promoting the SOAP‐V tool within corresponding residencies in both hospital and outpatient clinic settings and analyzing potential reductions in wasteful spending.
It is generally conceived that medical students learn the information they are taught, and are impacted by the culture in which they reside; multiple studies bear this out.[18, 19] However, students may also be change agents. Our students will inherit the healthcare systems of the future. We must empower them to change the status quo. There can be tremendous utility in employing such a bottom up approach to process improvement. What a student discusses today may spark the resident (or faculty) to consider in their own workflow tomorrow. In this way, we envision that the SOAP‐V is a tool by which ideas concerning HVC can be generated and shared at the point of care. It is our hope that this straightforward intervention is one that may slowly change the culture and perhaps eventually the practice patterns of our academic medical centers.
Disclosure
Nothing to report.
Today's medical students will enter practice over the next decade and inherit the escalating costs of the US healthcare system. Approximately 30% of healthcare costs, or $750 billion dollars annually, are spent on unnecessary tests or procedures.[1] High healthcare costs combined with calls to eliminate waste, improve patient safety, and increase quality[2] are driving our healthcare system to evolve from a fee‐based system to a value‐based system. Additionally, many patients are being harmed by overtesting and the stress associated with rising healthcare bills. Financial risk has increasingly shifted to patients in the form of higher deductibles and reduced caps, and medical indebtedness is the number 1 risk for bankruptcy.[3, 4] False positive results of low‐yield diagnostic tests lead to additional testing, anxiety, excess radiation exposure, and unnecessary invasive procedures.[5] To minimize harm to patients, evidence must guide physicians in their ordering behavior. In addition, any care plan a physician develops should be individualized to incorporate patients' values and preferences. Unfortunately, medical students, who are at an impressionable stage in their careers, frequently observe overtesting and unnecessary treatment behaviors in their clinical encounters.[6] Instead, our medical students and trainees must be prepared to deliver patient care that is evidence based, patient centered, and cost conscious. They must become effective stewards of limited healthcare resources.
To help prepare our students for this evolving healthcare paradigm, we created a new tool called SOAP‐V (Subjective‐Objective‐Assessment‐PlanValue), designed to embed discussion of healthcare value into medical student oral presentations and note writing. Students are encouraged to use this tool at the point of care to bring up value concepts with physicians and residents as part of medical decision making. In so doing, we propose that medical students can serve as change agents to shift physician practice at our academic medical centers to focus on healthcare value. This article describes the SOAP‐V tool, contains links to educational materials to help hospitalists and other clinician educators to implement this tool, and provides preliminary findings and reflections.
INNOVATION
SOAP‐V was conceived at the Millennium Conference on Teaching High‐Value Care, which was sponsored by the Beth Israel Deaconess Medical Center Shapiro Institute for Education and Research, the Association of American Medical Colleges, and the American College of Physicians. Educators from several medical schools decided to form a group to specifically consider ways to train medical students and residents in the concept of high‐value care (HVC), which is framed as improving patient outcomes while decreasing patient cost and harm.[7] Our group recognized several challenges in teaching HVC. First, physician practice habits are influenced by the way they are trained,[8] yet faculty who teach those future physicians frequently have not themselves been taught, nor do they consistently practice HVC.[9] Second, we needed to teach students the requisite HVC knowledge, attitudes, and skills, and therefore wanted to provide opportunities to not only learn, but practice, HVC, preferably in authentic patient experiences to optimize their learning.[10, 11] Third, we recognized that adding another teaching task to the already oversubscribed day of an attending might understandably be met with resistance. We envisioned a tool that could be used with minimal or no faculty training, could be attached to authentic patient experiences, and based on LEAN‐Six Sigma principles,[12] would be embedded in the normal workflow. Furthermore, we considered social networking principles, such as those described by Christakis and Fowler that describe how an individual's behavior impacts behaviors of those surrounding them,[13] and hoped to empower medical students to serve as change agents. Medical students could initiate discussions of value concepts at the point of care in a way that challenges a heavily entrenched test‐ordering culture and encourages other members of the team to balance potential benefit with harms and cost. Following the conference, the group held bimonthly phone conferences and subsequently developed the SOAP‐V tool, created teaching materials, and planned a research project on SOAP‐V.
SOAP‐V modifies the traditional SOAP (Subjective‐Objective‐Assessment‐Plan) oral presentation or medical note, to include value (V). It serves as a cognitive forcing function designed to create a pause and promote discussions of HVC during patient delivery. It prompts the student to ask 3 value questions: (1) Before choosing an intervention, have I considered whether the result would change management? (2) Have I incorporated the patient's goals and values, and considered the potential harm of the intervention compared to alternatives? (3) What is the known and potential cost of the intervention, both immediate and downstream? The student gathers information during the patient interview and brings back the information to the team during rounds where management decisions are made.
In the summer of 2014, we launched an institutional review boardapproved, multi‐institutional study to implement SOAP‐V at Penn State College of Medicine, Harvard Medical School, and Case Western Reserve University School of Medicine for third‐year medical students during their internal medicine clerkships. Students in the intervention arm participated in an interactive workshop on SOAP‐V. Authors S.F., S.G., and C.D.P., who serve as clerkship directors in internal medicine, provided student training for each cohort of intervention students at the beginning of each rotation on general medicine inpatient wards. The workshop began with trigger videos that demonstrate pressures encountered by a student on rounds that might lead to overuse.[14] Following a discussion on overuse and methods to avoid overuse, the students were introduced to the SOAP‐V framework, watched a video of a student modeling a SOAP‐V presentation on rounds,[15] and engaged in a SOAP‐V role play. They received a SOAP‐V pocket card as well as a Web link to Healthcare Bluebook[16] to research costs. An outline of the session and associated materials can be found in an online attachment.[17] The students then used the SOAP‐V tool during inpatient rounds. We advised supervising faculty that students might present using a SOAP‐V format, and provided them with a SOAP‐V card, but we did not provide faculty development on SOAP‐V. Students participating in the control arm did not receive training specific to SOAP‐V.
Students in intervention and control arms at each school were surveyed on their attitudes toward HVC at the beginning of the clerkship year and then again at the completion of the medicine clerkship via a 19‐item questionnaire soliciting perceptions and self‐reported practices in HVC. Intervention arm students received biweekly e‐mail links that allowed them to anonymously document their use of SOAP‐V, as well as an end‐of‐clerkship open‐ended question about the usefulness of SOAP‐V. We analyzed questionnaire results using McNemar's test for paired data.
PRELIMINARY FINDINGS
The preintervention attitudinal survey (n = 226) demonstrated that although 90% of medical students agreed on the importance of considering costs of treatments, only 50% felt comfortable bringing up cost considerations with their team, and 50% considered costs to the healthcare system in clinical decisions. An interim analysis of the available data at 6 months (response rate approximately 50% across sites) showed that students in the intervention arm reported increased agreement with the phrases, I have the power to address the economic healthcare crisis (pre‐37%, post‐65%, P = 0.046); I would be comfortable initiating a discussion about unnecessary tests or treatments with my team, (pre‐46%, post‐85%, P = 0.027); and In my clinical decisions, I consider the potential costs to the healthcare system (pre‐41%, post‐60%, P = 0.023) compared to control arm students, who showed no significant differences pre‐ versus postrotation in these 3 domains (Figure 1).

To date, biweekly surveys and direct observation of rounds have verified student use of SOAP‐V. Student comments have included: Allowed me the ability to raise important issues with the team while feeling like I was helping my patients and the healthcare system. A great principle that I used almost daily. Great to implement this at such a young stage in my med career. Broadened my perspective on the role of a physician.
SOAP‐V has inspired some of our medical students to consider value in healthcare more closely. In a notable example, a SOAP‐Vtrained student admitted a young man with lymphadenopathy, pulmonary infiltrates, and weight loss who underwent an extensive and costly workup including liver biopsy, bronchoscopy, and multiple computed tomography and positron emission tomography scans and was eventually diagnosed with sarcoidosis. The SOAP‐Vtrained student reviewed the patient's workup, estimated that the team spent more than $6000 to make the diagnosis, and recommended a more cost‐effective approach.
Common barriers experienced by the pilot sites included time constraints limiting discussion of value, variability in perceived receptivity depending on team leadership, and student confidence in initiating this dialogue. Solutions included underscoring the notion that value discussions can be brief, may be appropriately initiated by any member of the team, and may have an effect on choice of management and/or patient preference issues that can make medical care more efficient and effective. Resident and faculty physicians were made aware of the intervention, and encouraged to support students in using the SOAP‐V tool.
CONCLUSION
SOAP‐V was successfully implemented within the inpatient internal medicine clerkship at 3 academic institutions. Our preliminary results demonstrate that students can use this framework to apply considerations of high‐value, cost‐conscious care in their medical decision making and to promote discussion of these concepts during rounds with their inpatient teams. Students in the intervention arm report greater comfort discussing unnecessary tests and treatments with their team and a greater likelihood to consider potential costs to the healthcare system. Additionally, these students commented that the SOAP‐V framework broadened their perspective on their role as a physician in curbing costs, and that they felt more empowered to address the economic healthcare crisis. The next phase of our project will involve conducting end‐of‐year surveys to evaluate whether SOAP‐V has a persistent impact on the frequency and quality of value discussions on rounds, as well as students' attitudes about cost consciousness. We will also gauge whether resident and faculty attitudes about HVC have changed as a result of the intervention.
Our SOAP‐V student training was provided in a 1‐hour session. We believe that the ease of training and the simplicity of the SOAP‐V framework permit SOAP‐V to be easily transferred for use by residents, medical students in other clerkships, and other healthcare learners. Additional research is needed to demonstrate this expanded use and prove sustainability. An additional important question is whether use of SOAP‐V by students and residents results in reductions in unnecessary costs. Future educational efforts will include embedding the SOAP‐V tool in other clerkships and promoting the SOAP‐V tool within corresponding residencies in both hospital and outpatient clinic settings and analyzing potential reductions in wasteful spending.
It is generally conceived that medical students learn the information they are taught, and are impacted by the culture in which they reside; multiple studies bear this out.[18, 19] However, students may also be change agents. Our students will inherit the healthcare systems of the future. We must empower them to change the status quo. There can be tremendous utility in employing such a bottom up approach to process improvement. What a student discusses today may spark the resident (or faculty) to consider in their own workflow tomorrow. In this way, we envision that the SOAP‐V is a tool by which ideas concerning HVC can be generated and shared at the point of care. It is our hope that this straightforward intervention is one that may slowly change the culture and perhaps eventually the practice patterns of our academic medical centers.
Disclosure
Nothing to report.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes. Washington, DC: The National Academies Press; 2010.
- Institute for Healthcare Improvement. IHI triple aim initiative. Available at: http://www.ihi.org/Engage/Initiatives/TripleAim/pages/default.aspx. Accessed August 7, 2015.
- , , , . Medical bankruptcy in the United States, 2007. Am J Med. 2009;122(8):741–746.
- . Kaiser Family Foundation. Health care costs: a primer. Key information on health care costs and their impact. May 2012. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7670–03.pdf. Accessed August 7, 2015.
- , . Over‐testing: why more is not better. Am J Med. 2014;127:362–363.
- , , . Medical student perceptions of cost‐conscious care in an internal medicine clerkship: a thematic analysis [published online May 1, 2015]. J Gen Intern Med. doi: 10.1007/s11606‐015‐3324‐4.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155:386–388.
- , , . Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671–1672.
- , , . Theories of teaching. In: The Adult Learner. New York, NY: Routledge; 2012:72–114.
- . Medical education and the maintenance of incompetence. Med Teach. 2006;28:690–696.
- , , , , . Lean Six Sigma in healthcare. J Healthcare Qual. 2006;2:4–11
- , . Connected. New York, NY: Little, Brown 2009.
- Teaching Value Project. Costs of care. Available at: teachingvalue.org Available at: https://www.dropbox.com/s/tb8ysfjtzklwd8g/OverrunPart1.webm; https://www.dropbox.com/s/cxt9mvabj4re4g9/OverrunPart2.webm. Accessed August 7, 2015.
- , , . SOAP‐V [online video]. Available at: https://www.youtube.com/watch?v=goUgAzLuTzY47(2):134–143.
- , , , , , . How medical students learn from residents in the workplace: a qualitative study. Acad Med. 2014:89(3):490–496.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes. Washington, DC: The National Academies Press; 2010.
- Institute for Healthcare Improvement. IHI triple aim initiative. Available at: http://www.ihi.org/Engage/Initiatives/TripleAim/pages/default.aspx. Accessed August 7, 2015.
- , , , . Medical bankruptcy in the United States, 2007. Am J Med. 2009;122(8):741–746.
- . Kaiser Family Foundation. Health care costs: a primer. Key information on health care costs and their impact. May 2012. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7670–03.pdf. Accessed August 7, 2015.
- , . Over‐testing: why more is not better. Am J Med. 2014;127:362–363.
- , , . Medical student perceptions of cost‐conscious care in an internal medicine clerkship: a thematic analysis [published online May 1, 2015]. J Gen Intern Med. doi: 10.1007/s11606‐015‐3324‐4.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155:386–388.
- , , . Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671–1672.
- , , . Theories of teaching. In: The Adult Learner. New York, NY: Routledge; 2012:72–114.
- . Medical education and the maintenance of incompetence. Med Teach. 2006;28:690–696.
- , , , , . Lean Six Sigma in healthcare. J Healthcare Qual. 2006;2:4–11
- , . Connected. New York, NY: Little, Brown 2009.
- Teaching Value Project. Costs of care. Available at: teachingvalue.org Available at: https://www.dropbox.com/s/tb8ysfjtzklwd8g/OverrunPart1.webm; https://www.dropbox.com/s/cxt9mvabj4re4g9/OverrunPart2.webm. Accessed August 7, 2015.
- , , . SOAP‐V [online video]. Available at: https://www.youtube.com/watch?v=goUgAzLuTzY47(2):134–143.
- , , , , , . How medical students learn from residents in the workplace: a qualitative study. Acad Med. 2014:89(3):490–496.
NICE backs discounted idelalisib for CLL

The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending that the PI3Kδ inhibitor idelalisib (Zydelig) be made available on the National Health Service (NHS) for some adults with chronic lymphocytic leukemia (CLL).
NICE is recommending idelalisib in combination with rituximab for adults with previously untreated CLL who have a 17p deletion or TP53 mutation and for adults with CLL who have relapsed within 24 months of their previous treatment.
This decision follows a preliminary decision earlier this year, when NICE asked Gilead Sciences, the company developing idelalisib, to provide further information on the cost-effectiveness of the drug.
Gilead responded by submitting new economic analyses and a simple discount agreement to the list price of idelalisib.
NICE’s recommendation for idelalisib is contingent upon the company providing the agreed upon discount.
NICE’s draft guidance is now with consultees, who have the opportunity to appeal against it. Until NICE issues a final guidance, NHS bodies should make decisions locally on the funding of specific treatments.
Patients whose treatment with idelalisib is not recommended in this NICE guidance but was started within the NHS before this guidance was published should be able to continue treatment until they and their NHS clinician consider it appropriate to stop.
Clinical effectiveness
The committee advising NICE concluded that idelalisib could not be recommended for patients whose disease had relapsed more than 24 months after previous treatment, as no evidence was submitted for this patient group.
For the other populations, the clinical effectiveness data from Study 116 showed that idelalisib plus rituximab produced a significant improvement in progression-free survival and overall survival, compared with rituximab alone, for patients with high-risk, relapsed or refractory CLL.
Cost-effectiveness
Idelalisib is priced at £3114.75 for sixty 150-mg tablets (British national formulary 2015). The mean cost of a 1-year treatment course is £37,922.
Gilead’s agreement provides a discount to the list price of idelalisib, but the level of the discount is currently confidential.
Analyses suggested that, at the discount agreement price, idelalisib plus rituximab was associated with higher costs and greater quality-adjusted life-year (QALY) gains when compared with rituximab alone.
The deterministic incremental cost-effectiveness ratio (ICER) for idelalisib plus rituximab compared with rituximab alone was £13,634 per QALY gained (incremental costs £26,128; incremental QALYs 1.92).
Compared with best supportive care, the ICER for idelalisib plus rituximab was £20,461 per QALY gained (incremental costs £39,211; incremental QALYs 1.92). And compared with ofatumumab, the ICER was £1527 per QALY gained (incremental costs £2926; incremental QALYs 1.92). ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending that the PI3Kδ inhibitor idelalisib (Zydelig) be made available on the National Health Service (NHS) for some adults with chronic lymphocytic leukemia (CLL).
NICE is recommending idelalisib in combination with rituximab for adults with previously untreated CLL who have a 17p deletion or TP53 mutation and for adults with CLL who have relapsed within 24 months of their previous treatment.
This decision follows a preliminary decision earlier this year, when NICE asked Gilead Sciences, the company developing idelalisib, to provide further information on the cost-effectiveness of the drug.
Gilead responded by submitting new economic analyses and a simple discount agreement to the list price of idelalisib.
NICE’s recommendation for idelalisib is contingent upon the company providing the agreed upon discount.
NICE’s draft guidance is now with consultees, who have the opportunity to appeal against it. Until NICE issues a final guidance, NHS bodies should make decisions locally on the funding of specific treatments.
Patients whose treatment with idelalisib is not recommended in this NICE guidance but was started within the NHS before this guidance was published should be able to continue treatment until they and their NHS clinician consider it appropriate to stop.
Clinical effectiveness
The committee advising NICE concluded that idelalisib could not be recommended for patients whose disease had relapsed more than 24 months after previous treatment, as no evidence was submitted for this patient group.
For the other populations, the clinical effectiveness data from Study 116 showed that idelalisib plus rituximab produced a significant improvement in progression-free survival and overall survival, compared with rituximab alone, for patients with high-risk, relapsed or refractory CLL.
Cost-effectiveness
Idelalisib is priced at £3114.75 for sixty 150-mg tablets (British national formulary 2015). The mean cost of a 1-year treatment course is £37,922.
Gilead’s agreement provides a discount to the list price of idelalisib, but the level of the discount is currently confidential.
Analyses suggested that, at the discount agreement price, idelalisib plus rituximab was associated with higher costs and greater quality-adjusted life-year (QALY) gains when compared with rituximab alone.
The deterministic incremental cost-effectiveness ratio (ICER) for idelalisib plus rituximab compared with rituximab alone was £13,634 per QALY gained (incremental costs £26,128; incremental QALYs 1.92).
Compared with best supportive care, the ICER for idelalisib plus rituximab was £20,461 per QALY gained (incremental costs £39,211; incremental QALYs 1.92). And compared with ofatumumab, the ICER was £1527 per QALY gained (incremental costs £2926; incremental QALYs 1.92). ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending that the PI3Kδ inhibitor idelalisib (Zydelig) be made available on the National Health Service (NHS) for some adults with chronic lymphocytic leukemia (CLL).
NICE is recommending idelalisib in combination with rituximab for adults with previously untreated CLL who have a 17p deletion or TP53 mutation and for adults with CLL who have relapsed within 24 months of their previous treatment.
This decision follows a preliminary decision earlier this year, when NICE asked Gilead Sciences, the company developing idelalisib, to provide further information on the cost-effectiveness of the drug.
Gilead responded by submitting new economic analyses and a simple discount agreement to the list price of idelalisib.
NICE’s recommendation for idelalisib is contingent upon the company providing the agreed upon discount.
NICE’s draft guidance is now with consultees, who have the opportunity to appeal against it. Until NICE issues a final guidance, NHS bodies should make decisions locally on the funding of specific treatments.
Patients whose treatment with idelalisib is not recommended in this NICE guidance but was started within the NHS before this guidance was published should be able to continue treatment until they and their NHS clinician consider it appropriate to stop.
Clinical effectiveness
The committee advising NICE concluded that idelalisib could not be recommended for patients whose disease had relapsed more than 24 months after previous treatment, as no evidence was submitted for this patient group.
For the other populations, the clinical effectiveness data from Study 116 showed that idelalisib plus rituximab produced a significant improvement in progression-free survival and overall survival, compared with rituximab alone, for patients with high-risk, relapsed or refractory CLL.
Cost-effectiveness
Idelalisib is priced at £3114.75 for sixty 150-mg tablets (British national formulary 2015). The mean cost of a 1-year treatment course is £37,922.
Gilead’s agreement provides a discount to the list price of idelalisib, but the level of the discount is currently confidential.
Analyses suggested that, at the discount agreement price, idelalisib plus rituximab was associated with higher costs and greater quality-adjusted life-year (QALY) gains when compared with rituximab alone.
The deterministic incremental cost-effectiveness ratio (ICER) for idelalisib plus rituximab compared with rituximab alone was £13,634 per QALY gained (incremental costs £26,128; incremental QALYs 1.92).
Compared with best supportive care, the ICER for idelalisib plus rituximab was £20,461 per QALY gained (incremental costs £39,211; incremental QALYs 1.92). And compared with ofatumumab, the ICER was £1527 per QALY gained (incremental costs £2926; incremental QALYs 1.92). ![]()