User login
New and Noteworthy Information—June 2016
Aspects of spatial navigation may be particularly sensitive for detecting the earliest cognitive deficits of Alzheimer’s disease, according to a study published April 26 in the Journal of Alzheimer’s Disease. This study included 42 clinically normal people without preclinical Alzheimer’s disease, 13 clinically normal people with preclinical Alzheimer’s disease, and 16 people with early-stage symptomatic Alzheimer’s disease. Preclinical Alzheimer’s disease was defined based on CSF Aβ42 levels below 500 pg/mL. Preclinical Alzheimer’s disease was associated with deficits in the use of a wayfinding strategy, but not in the use of a route-learning strategy. In addition, post hoc analyses indicated that wayfinding performance had moderate sensitivity and specificity. Results also confirmed early-stage symptomatic Alzheimer’s disease-related deficits in the use of wayfinding and route-learning strategies.
One meal per week of seafood and long-chain omega-3 fatty acids protects against decline in multiple cognitive domains, according to a study published online ahead of print May 4 in Neurology. Researchers examined 915 participants who completed at least one follow-up cognitive assessment and provided dietary data. Scores for global cognitive function and five cognitive domains were assessed using 19 cognitive tests. Consumption of seafood was associated with slower decline in semantic memory and perceptual speed in separate models adjusted for age, sex, education, participation in cognitive activities, physical activity, alcohol consumption, smoking, and total energy intake. In secondary analyses, APOE ε4 carriers demonstrated slower rates of decline in global cognition and in multiple cognitive domains with weekly seafood consumption and with moderate to high long-chain omega-3 intake from food.
Persistent environmental pollutants measured in blood are significantly associated with amyotrophic lateral sclerosis (ALS) and may represent modifiable risk factors, according to a study published online ahead of print May 9 in JAMA Neurology. Participants included 156 cases and 128 controls. Complete demographic and pollutant data were available for 101 cases and 110 controls. Survey data revealed that reported pesticide exposure in the cumulative exposure windows was significantly associated with ALS. A multivariable model of measured persistent environmental pollutants in the blood, representing cumulative occupational and residential exposure, showed increased odds of ALS for two organochlorine pesticides, two polychlorinated biphenyls, and one brominated flame retardant. There was modest concordance between survey data and the measurements of persistent environmental pollutants in blood. Additionally, tau correlation coefficients ranged from –0.18 to 0.24.
Outcomes of sports-related concussion vary according to the level of competition, according to a study published online ahead of print May 2 in JAMA Pediatrics. Three injury surveillance programs recorded 1,429 sports-related concussions between 2012 and 2014 among youth, high school, and college-level football athletes. Across all levels, 15.3% of concussions resulted in return to play at least 30 days after the concussion, and 3.1% resulted in return to play less than 24 hours after the concussion. Compared with youth, a higher number of concussion symptoms were reported in high school athletes. Compared with college athletes, the odds of return to play at least 30 days after injury were larger in youth athletes and high school athletes.
A screening test for newborns identifies infants with Niemann-Pick type C, according to a study published May 4 in Science Translational Medicine. The bile acids most elevated in infants with Niemann-Pick type C were identified as 3β,5α,6β-trihydroxycholanic acid and its glycine conjugate, which were shown to be metabolites of cholestane-3β,5α,6β-triol. Analysis of dried blood spots from 4,992 controls, 134 Niemann-Pick type C carriers, and 44 subjects with Niemann-Pick type C provided 100% sensitivity and specificity in the study samples. The researchers found that infants with Niemann-Pick type C have about thirtyfold higher amounts of 3β,5α,6β-trihydroxycholanic acid in the blood than healthy individuals. In addition, levels of 3β,5α,6β-trihydroxycholanic acid also could distinguish between patients with Niemann-Pick type C and carriers of the disease who show no symptoms.
The FDA has approved pimavanserin for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. The approval is based on data from a phase III study and other supportive studies. In the phase III study, pimavanserin significantly reduced the frequency and severity of psychotic symptoms, compared with placebo, on the Scale for Assessment of Positive Symptoms—Parkinson’s Disease. This benefit was achieved without impairing motor function. The most common adverse reactions in this study were peripheral edema and confusional state. The drug has a boxed warning alerting health care professionals about an increased risk of death associated with the use of atypical antipsychotics to treat older people with dementia-related psychosis. Acadia Pharmaceuticals, headquartered in San Diego, markets the product under the name Nuplazid.
Compared with the standard dose of t-PA (0.9 mg/kg), a low dose (0.6 mg/kg) is associated with slightly reduced rates of bleeding and mortality, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Using a two-by-two quasi-factorial open-label design, researchers randomly assigned 3,310 patients who were eligible for thrombolytic therapy to low-dose IV t-PA or the standard dose. At 90 days, 53.2% of participants in the low-dose group had died or had disability, compared with 51.1% of participants in the standard-dose group. Major symptomatic intracerebral hemorrhage occurred in 1.0% of the participants in the low-dose group and in 2.1% of the participants in the standard-dose group. Fatal events occurred within seven days in 0.5% and 1.5% of participants, respectively.
Triptans and dihydroergotamine (DHE) are not associated with acute or subacute ischemic vascular events in the abortive treatment of basilar migraine or hemiplegic migraine, according to a study published online ahead of print April 8 in Headache. A retrospective chart review of 80 patients with basilar migraine or hemiplegic migraine who received acute abortive treatment with either triptans or DHE was conducted at four headache centers to assess the frequency of ischemic vascular events after administration. No stroke or myocardial infarction was reported. In the triptan group, five patients reported adverse effects that included gastrointestinal upset, rash, neck dystonia, nightmares, and flushing. In the DHE group, five patients had adverse events that included chest tightness, dystonic reaction, transient asymptomatic anterior T wave inversion, and agitation.
The FDA has approved Fycompa (perampanel) CIII oral suspension as adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures, and of primary generalized tonic-clonic seizures in patients with epilepsy age 12 and older. The oral suspension formulation is a bioequivalent, interchangeable alternative to the Fycompa tablet formulation. The approval of Fycompa CIII oral suspension was based on a study that demonstrated bioequivalence between a single dose of perampanel oral suspension and a single dose of perampanel tablet when administered under fasted conditions in healthy subjects. Fycompa is an oral medication and the only FDA-approved noncompetitive AMPA receptor antagonist. Its most common side effects include dizziness, sleepiness, headache, tiredness, and irritability. Eisai, headquartered in Woodcliff Lake, New Jersey, markets the drug.
In patients with acute ischemic stroke or transient ischemic attack, ticagrelor is not superior to aspirin in reducing the rate of stroke, myocardial infarction, or death at 90 days, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Included in this study were 13,199 patients with nonsevere ischemic stroke or high-risk transient ischemic attack who had not received IV or intra-arterial thrombolysis and who had not had a cardioembolic stroke. During 90 days of treatment, a primary end point event (ie, stroke, myocardial infarction, or death) occurred in 442 of the 6,589 patients treated with ticagrelor, versus 497 of the 6,610 patients treated with aspirin. Ischemic stroke occurred in 385 patients treated with ticagrelor and in 441 patients treated with aspirin.
Physicians differ substantially when giving prognoses and treatment recommendations for patients with intracerebral hemorrhage, according to a study published online ahead of print April 15 in Neurology. A written survey with two intracerebral hemorrhage scenarios was completed by 742 practicing neurologists and neurosurgeons. Physician predictions of 30-day mortality varied widely, as did treatment recommendations. Responses encompassed the full range of options for each case. No physician demographic or personality characteristics were associated with treatment recommendations. Providing a prognostic score changed treatment recommendations, and the effect differed across cases. When the prognostic score suggested a 0% chance of functional independence, the likelihood of treatment limitations was increased, compared with no prognostic score. Conversely, if the score suggested a 66% chance of independence, treatment limitations were less likely.
Mitoxantrone slightly increases the overall incidence of malignancies and significantly increases the risk of leukemia and colorectal cancer, according to a study published online ahead of print May 11 in Neurology. Researchers retrospectively examined all mitoxantrone-treated patients with multiple sclerosis seen between 1994 and 2007 at one hospital. They collected follow-up information on medically confirmed malignancies, life status, and cause of death, as of 2010. The incidence ratio of malignancy was 1.50. The standardized incidence ratio of colorectal cancer was 2.98, and that of acute myeloid leukemia, 10.44. Higher age at treatment initiation was a risk factor, but neither cumulative mitoxantrone dose, treatment with other immunosuppressive drugs, nor sex was. In all, 55 patients died. Twelve deaths resulted from a malignancy, and 43 from other causes.
Tau tangles provide a good indication of cognitive decline in the later stages of Alzheimer’s disease, according to a study published May 11 in Science Translational Medicine. The study suggests that while β-amyloid remains a critical marker for the early detection of Alzheimer’s disease, tau may be more useful for tracking disease progression and, potentially, patient response to therapies. Researchers analyzed PET imaging of tau and β-amyloid in 10 patients with mild Alzheimer’s disease and 36 healthy adults. Compared with amyloid plaques, tau tangles in the temporal lobe more closely correlated with cognitive deficits, as measured by a battery of memory tests. Tau deposits in the temporal lobe also were strongly associated with tau detected in the patients’ CSF. Tau therefore may better predict dementia during Alzheimer’s disease progression than β-amyloid.
—Kimberly Williams
Aspects of spatial navigation may be particularly sensitive for detecting the earliest cognitive deficits of Alzheimer’s disease, according to a study published April 26 in the Journal of Alzheimer’s Disease. This study included 42 clinically normal people without preclinical Alzheimer’s disease, 13 clinically normal people with preclinical Alzheimer’s disease, and 16 people with early-stage symptomatic Alzheimer’s disease. Preclinical Alzheimer’s disease was defined based on CSF Aβ42 levels below 500 pg/mL. Preclinical Alzheimer’s disease was associated with deficits in the use of a wayfinding strategy, but not in the use of a route-learning strategy. In addition, post hoc analyses indicated that wayfinding performance had moderate sensitivity and specificity. Results also confirmed early-stage symptomatic Alzheimer’s disease-related deficits in the use of wayfinding and route-learning strategies.
One meal per week of seafood and long-chain omega-3 fatty acids protects against decline in multiple cognitive domains, according to a study published online ahead of print May 4 in Neurology. Researchers examined 915 participants who completed at least one follow-up cognitive assessment and provided dietary data. Scores for global cognitive function and five cognitive domains were assessed using 19 cognitive tests. Consumption of seafood was associated with slower decline in semantic memory and perceptual speed in separate models adjusted for age, sex, education, participation in cognitive activities, physical activity, alcohol consumption, smoking, and total energy intake. In secondary analyses, APOE ε4 carriers demonstrated slower rates of decline in global cognition and in multiple cognitive domains with weekly seafood consumption and with moderate to high long-chain omega-3 intake from food.
Persistent environmental pollutants measured in blood are significantly associated with amyotrophic lateral sclerosis (ALS) and may represent modifiable risk factors, according to a study published online ahead of print May 9 in JAMA Neurology. Participants included 156 cases and 128 controls. Complete demographic and pollutant data were available for 101 cases and 110 controls. Survey data revealed that reported pesticide exposure in the cumulative exposure windows was significantly associated with ALS. A multivariable model of measured persistent environmental pollutants in the blood, representing cumulative occupational and residential exposure, showed increased odds of ALS for two organochlorine pesticides, two polychlorinated biphenyls, and one brominated flame retardant. There was modest concordance between survey data and the measurements of persistent environmental pollutants in blood. Additionally, tau correlation coefficients ranged from –0.18 to 0.24.
Outcomes of sports-related concussion vary according to the level of competition, according to a study published online ahead of print May 2 in JAMA Pediatrics. Three injury surveillance programs recorded 1,429 sports-related concussions between 2012 and 2014 among youth, high school, and college-level football athletes. Across all levels, 15.3% of concussions resulted in return to play at least 30 days after the concussion, and 3.1% resulted in return to play less than 24 hours after the concussion. Compared with youth, a higher number of concussion symptoms were reported in high school athletes. Compared with college athletes, the odds of return to play at least 30 days after injury were larger in youth athletes and high school athletes.
A screening test for newborns identifies infants with Niemann-Pick type C, according to a study published May 4 in Science Translational Medicine. The bile acids most elevated in infants with Niemann-Pick type C were identified as 3β,5α,6β-trihydroxycholanic acid and its glycine conjugate, which were shown to be metabolites of cholestane-3β,5α,6β-triol. Analysis of dried blood spots from 4,992 controls, 134 Niemann-Pick type C carriers, and 44 subjects with Niemann-Pick type C provided 100% sensitivity and specificity in the study samples. The researchers found that infants with Niemann-Pick type C have about thirtyfold higher amounts of 3β,5α,6β-trihydroxycholanic acid in the blood than healthy individuals. In addition, levels of 3β,5α,6β-trihydroxycholanic acid also could distinguish between patients with Niemann-Pick type C and carriers of the disease who show no symptoms.
The FDA has approved pimavanserin for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. The approval is based on data from a phase III study and other supportive studies. In the phase III study, pimavanserin significantly reduced the frequency and severity of psychotic symptoms, compared with placebo, on the Scale for Assessment of Positive Symptoms—Parkinson’s Disease. This benefit was achieved without impairing motor function. The most common adverse reactions in this study were peripheral edema and confusional state. The drug has a boxed warning alerting health care professionals about an increased risk of death associated with the use of atypical antipsychotics to treat older people with dementia-related psychosis. Acadia Pharmaceuticals, headquartered in San Diego, markets the product under the name Nuplazid.
Compared with the standard dose of t-PA (0.9 mg/kg), a low dose (0.6 mg/kg) is associated with slightly reduced rates of bleeding and mortality, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Using a two-by-two quasi-factorial open-label design, researchers randomly assigned 3,310 patients who were eligible for thrombolytic therapy to low-dose IV t-PA or the standard dose. At 90 days, 53.2% of participants in the low-dose group had died or had disability, compared with 51.1% of participants in the standard-dose group. Major symptomatic intracerebral hemorrhage occurred in 1.0% of the participants in the low-dose group and in 2.1% of the participants in the standard-dose group. Fatal events occurred within seven days in 0.5% and 1.5% of participants, respectively.
Triptans and dihydroergotamine (DHE) are not associated with acute or subacute ischemic vascular events in the abortive treatment of basilar migraine or hemiplegic migraine, according to a study published online ahead of print April 8 in Headache. A retrospective chart review of 80 patients with basilar migraine or hemiplegic migraine who received acute abortive treatment with either triptans or DHE was conducted at four headache centers to assess the frequency of ischemic vascular events after administration. No stroke or myocardial infarction was reported. In the triptan group, five patients reported adverse effects that included gastrointestinal upset, rash, neck dystonia, nightmares, and flushing. In the DHE group, five patients had adverse events that included chest tightness, dystonic reaction, transient asymptomatic anterior T wave inversion, and agitation.
The FDA has approved Fycompa (perampanel) CIII oral suspension as adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures, and of primary generalized tonic-clonic seizures in patients with epilepsy age 12 and older. The oral suspension formulation is a bioequivalent, interchangeable alternative to the Fycompa tablet formulation. The approval of Fycompa CIII oral suspension was based on a study that demonstrated bioequivalence between a single dose of perampanel oral suspension and a single dose of perampanel tablet when administered under fasted conditions in healthy subjects. Fycompa is an oral medication and the only FDA-approved noncompetitive AMPA receptor antagonist. Its most common side effects include dizziness, sleepiness, headache, tiredness, and irritability. Eisai, headquartered in Woodcliff Lake, New Jersey, markets the drug.
In patients with acute ischemic stroke or transient ischemic attack, ticagrelor is not superior to aspirin in reducing the rate of stroke, myocardial infarction, or death at 90 days, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Included in this study were 13,199 patients with nonsevere ischemic stroke or high-risk transient ischemic attack who had not received IV or intra-arterial thrombolysis and who had not had a cardioembolic stroke. During 90 days of treatment, a primary end point event (ie, stroke, myocardial infarction, or death) occurred in 442 of the 6,589 patients treated with ticagrelor, versus 497 of the 6,610 patients treated with aspirin. Ischemic stroke occurred in 385 patients treated with ticagrelor and in 441 patients treated with aspirin.
Physicians differ substantially when giving prognoses and treatment recommendations for patients with intracerebral hemorrhage, according to a study published online ahead of print April 15 in Neurology. A written survey with two intracerebral hemorrhage scenarios was completed by 742 practicing neurologists and neurosurgeons. Physician predictions of 30-day mortality varied widely, as did treatment recommendations. Responses encompassed the full range of options for each case. No physician demographic or personality characteristics were associated with treatment recommendations. Providing a prognostic score changed treatment recommendations, and the effect differed across cases. When the prognostic score suggested a 0% chance of functional independence, the likelihood of treatment limitations was increased, compared with no prognostic score. Conversely, if the score suggested a 66% chance of independence, treatment limitations were less likely.
Mitoxantrone slightly increases the overall incidence of malignancies and significantly increases the risk of leukemia and colorectal cancer, according to a study published online ahead of print May 11 in Neurology. Researchers retrospectively examined all mitoxantrone-treated patients with multiple sclerosis seen between 1994 and 2007 at one hospital. They collected follow-up information on medically confirmed malignancies, life status, and cause of death, as of 2010. The incidence ratio of malignancy was 1.50. The standardized incidence ratio of colorectal cancer was 2.98, and that of acute myeloid leukemia, 10.44. Higher age at treatment initiation was a risk factor, but neither cumulative mitoxantrone dose, treatment with other immunosuppressive drugs, nor sex was. In all, 55 patients died. Twelve deaths resulted from a malignancy, and 43 from other causes.
Tau tangles provide a good indication of cognitive decline in the later stages of Alzheimer’s disease, according to a study published May 11 in Science Translational Medicine. The study suggests that while β-amyloid remains a critical marker for the early detection of Alzheimer’s disease, tau may be more useful for tracking disease progression and, potentially, patient response to therapies. Researchers analyzed PET imaging of tau and β-amyloid in 10 patients with mild Alzheimer’s disease and 36 healthy adults. Compared with amyloid plaques, tau tangles in the temporal lobe more closely correlated with cognitive deficits, as measured by a battery of memory tests. Tau deposits in the temporal lobe also were strongly associated with tau detected in the patients’ CSF. Tau therefore may better predict dementia during Alzheimer’s disease progression than β-amyloid.
—Kimberly Williams
Aspects of spatial navigation may be particularly sensitive for detecting the earliest cognitive deficits of Alzheimer’s disease, according to a study published April 26 in the Journal of Alzheimer’s Disease. This study included 42 clinically normal people without preclinical Alzheimer’s disease, 13 clinically normal people with preclinical Alzheimer’s disease, and 16 people with early-stage symptomatic Alzheimer’s disease. Preclinical Alzheimer’s disease was defined based on CSF Aβ42 levels below 500 pg/mL. Preclinical Alzheimer’s disease was associated with deficits in the use of a wayfinding strategy, but not in the use of a route-learning strategy. In addition, post hoc analyses indicated that wayfinding performance had moderate sensitivity and specificity. Results also confirmed early-stage symptomatic Alzheimer’s disease-related deficits in the use of wayfinding and route-learning strategies.
One meal per week of seafood and long-chain omega-3 fatty acids protects against decline in multiple cognitive domains, according to a study published online ahead of print May 4 in Neurology. Researchers examined 915 participants who completed at least one follow-up cognitive assessment and provided dietary data. Scores for global cognitive function and five cognitive domains were assessed using 19 cognitive tests. Consumption of seafood was associated with slower decline in semantic memory and perceptual speed in separate models adjusted for age, sex, education, participation in cognitive activities, physical activity, alcohol consumption, smoking, and total energy intake. In secondary analyses, APOE ε4 carriers demonstrated slower rates of decline in global cognition and in multiple cognitive domains with weekly seafood consumption and with moderate to high long-chain omega-3 intake from food.
Persistent environmental pollutants measured in blood are significantly associated with amyotrophic lateral sclerosis (ALS) and may represent modifiable risk factors, according to a study published online ahead of print May 9 in JAMA Neurology. Participants included 156 cases and 128 controls. Complete demographic and pollutant data were available for 101 cases and 110 controls. Survey data revealed that reported pesticide exposure in the cumulative exposure windows was significantly associated with ALS. A multivariable model of measured persistent environmental pollutants in the blood, representing cumulative occupational and residential exposure, showed increased odds of ALS for two organochlorine pesticides, two polychlorinated biphenyls, and one brominated flame retardant. There was modest concordance between survey data and the measurements of persistent environmental pollutants in blood. Additionally, tau correlation coefficients ranged from –0.18 to 0.24.
Outcomes of sports-related concussion vary according to the level of competition, according to a study published online ahead of print May 2 in JAMA Pediatrics. Three injury surveillance programs recorded 1,429 sports-related concussions between 2012 and 2014 among youth, high school, and college-level football athletes. Across all levels, 15.3% of concussions resulted in return to play at least 30 days after the concussion, and 3.1% resulted in return to play less than 24 hours after the concussion. Compared with youth, a higher number of concussion symptoms were reported in high school athletes. Compared with college athletes, the odds of return to play at least 30 days after injury were larger in youth athletes and high school athletes.
A screening test for newborns identifies infants with Niemann-Pick type C, according to a study published May 4 in Science Translational Medicine. The bile acids most elevated in infants with Niemann-Pick type C were identified as 3β,5α,6β-trihydroxycholanic acid and its glycine conjugate, which were shown to be metabolites of cholestane-3β,5α,6β-triol. Analysis of dried blood spots from 4,992 controls, 134 Niemann-Pick type C carriers, and 44 subjects with Niemann-Pick type C provided 100% sensitivity and specificity in the study samples. The researchers found that infants with Niemann-Pick type C have about thirtyfold higher amounts of 3β,5α,6β-trihydroxycholanic acid in the blood than healthy individuals. In addition, levels of 3β,5α,6β-trihydroxycholanic acid also could distinguish between patients with Niemann-Pick type C and carriers of the disease who show no symptoms.
The FDA has approved pimavanserin for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. The approval is based on data from a phase III study and other supportive studies. In the phase III study, pimavanserin significantly reduced the frequency and severity of psychotic symptoms, compared with placebo, on the Scale for Assessment of Positive Symptoms—Parkinson’s Disease. This benefit was achieved without impairing motor function. The most common adverse reactions in this study were peripheral edema and confusional state. The drug has a boxed warning alerting health care professionals about an increased risk of death associated with the use of atypical antipsychotics to treat older people with dementia-related psychosis. Acadia Pharmaceuticals, headquartered in San Diego, markets the product under the name Nuplazid.
Compared with the standard dose of t-PA (0.9 mg/kg), a low dose (0.6 mg/kg) is associated with slightly reduced rates of bleeding and mortality, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Using a two-by-two quasi-factorial open-label design, researchers randomly assigned 3,310 patients who were eligible for thrombolytic therapy to low-dose IV t-PA or the standard dose. At 90 days, 53.2% of participants in the low-dose group had died or had disability, compared with 51.1% of participants in the standard-dose group. Major symptomatic intracerebral hemorrhage occurred in 1.0% of the participants in the low-dose group and in 2.1% of the participants in the standard-dose group. Fatal events occurred within seven days in 0.5% and 1.5% of participants, respectively.
Triptans and dihydroergotamine (DHE) are not associated with acute or subacute ischemic vascular events in the abortive treatment of basilar migraine or hemiplegic migraine, according to a study published online ahead of print April 8 in Headache. A retrospective chart review of 80 patients with basilar migraine or hemiplegic migraine who received acute abortive treatment with either triptans or DHE was conducted at four headache centers to assess the frequency of ischemic vascular events after administration. No stroke or myocardial infarction was reported. In the triptan group, five patients reported adverse effects that included gastrointestinal upset, rash, neck dystonia, nightmares, and flushing. In the DHE group, five patients had adverse events that included chest tightness, dystonic reaction, transient asymptomatic anterior T wave inversion, and agitation.
The FDA has approved Fycompa (perampanel) CIII oral suspension as adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures, and of primary generalized tonic-clonic seizures in patients with epilepsy age 12 and older. The oral suspension formulation is a bioequivalent, interchangeable alternative to the Fycompa tablet formulation. The approval of Fycompa CIII oral suspension was based on a study that demonstrated bioequivalence between a single dose of perampanel oral suspension and a single dose of perampanel tablet when administered under fasted conditions in healthy subjects. Fycompa is an oral medication and the only FDA-approved noncompetitive AMPA receptor antagonist. Its most common side effects include dizziness, sleepiness, headache, tiredness, and irritability. Eisai, headquartered in Woodcliff Lake, New Jersey, markets the drug.
In patients with acute ischemic stroke or transient ischemic attack, ticagrelor is not superior to aspirin in reducing the rate of stroke, myocardial infarction, or death at 90 days, according to a study published online ahead of print May 10 in the New England Journal of Medicine. Included in this study were 13,199 patients with nonsevere ischemic stroke or high-risk transient ischemic attack who had not received IV or intra-arterial thrombolysis and who had not had a cardioembolic stroke. During 90 days of treatment, a primary end point event (ie, stroke, myocardial infarction, or death) occurred in 442 of the 6,589 patients treated with ticagrelor, versus 497 of the 6,610 patients treated with aspirin. Ischemic stroke occurred in 385 patients treated with ticagrelor and in 441 patients treated with aspirin.
Physicians differ substantially when giving prognoses and treatment recommendations for patients with intracerebral hemorrhage, according to a study published online ahead of print April 15 in Neurology. A written survey with two intracerebral hemorrhage scenarios was completed by 742 practicing neurologists and neurosurgeons. Physician predictions of 30-day mortality varied widely, as did treatment recommendations. Responses encompassed the full range of options for each case. No physician demographic or personality characteristics were associated with treatment recommendations. Providing a prognostic score changed treatment recommendations, and the effect differed across cases. When the prognostic score suggested a 0% chance of functional independence, the likelihood of treatment limitations was increased, compared with no prognostic score. Conversely, if the score suggested a 66% chance of independence, treatment limitations were less likely.
Mitoxantrone slightly increases the overall incidence of malignancies and significantly increases the risk of leukemia and colorectal cancer, according to a study published online ahead of print May 11 in Neurology. Researchers retrospectively examined all mitoxantrone-treated patients with multiple sclerosis seen between 1994 and 2007 at one hospital. They collected follow-up information on medically confirmed malignancies, life status, and cause of death, as of 2010. The incidence ratio of malignancy was 1.50. The standardized incidence ratio of colorectal cancer was 2.98, and that of acute myeloid leukemia, 10.44. Higher age at treatment initiation was a risk factor, but neither cumulative mitoxantrone dose, treatment with other immunosuppressive drugs, nor sex was. In all, 55 patients died. Twelve deaths resulted from a malignancy, and 43 from other causes.
Tau tangles provide a good indication of cognitive decline in the later stages of Alzheimer’s disease, according to a study published May 11 in Science Translational Medicine. The study suggests that while β-amyloid remains a critical marker for the early detection of Alzheimer’s disease, tau may be more useful for tracking disease progression and, potentially, patient response to therapies. Researchers analyzed PET imaging of tau and β-amyloid in 10 patients with mild Alzheimer’s disease and 36 healthy adults. Compared with amyloid plaques, tau tangles in the temporal lobe more closely correlated with cognitive deficits, as measured by a battery of memory tests. Tau deposits in the temporal lobe also were strongly associated with tau detected in the patients’ CSF. Tau therefore may better predict dementia during Alzheimer’s disease progression than β-amyloid.
—Kimberly Williams
Itchy Lesions, Picking Patient
A 59-year-old woman presents urgently to dermatology for evaluation of very itchy lesions that manifested at least 20 years ago. They have been previously diagnosed as staph infection, fungal infection, psoriasis, and scabies but have not responded to a variety of topical steroid creams and topical antibacterials (eg, triple-antibiotic cream, mupirocin, and clindamycin solution). The patient has also been prescribed numerous oral antibiotics, the most recent of which was a 10-day course of clindamycin. This induced a raging case of diarrhea; her primary care provider subsequently referred her to dermatology.
She denies any family history of skin problems until her sister, who has accompanied her to this appointment, mentions that she and her mother have had similar problems for decades. More questioning reveals that all three women also have extensive psychiatric histories of chronic anxiety and depression.
When pressed, the patient and her sister admit to picking at their skin “constantly,” but especially when under stress.
EXAMINATION
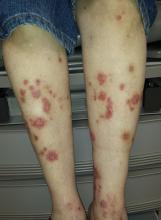
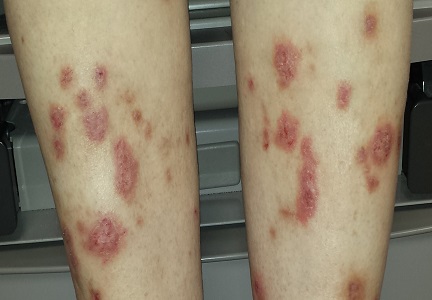
Most of the patient’s lesions—round to oval, orangish red, scaly plaques that range from 2 to 5 cm—are on her legs, below her knees. There are about 15 lesions total. They look and feel very thick but have no increased warmth or tenderness. The erythema is confined to the lesions and does not extend into the surrounding areas.
Punch biopsy of one leg lesion shows a hypertrophic epidermis with focal parakeratosis, elongation of rete ridges, and perhaps most importantly, no signs of other items in the differential (such as psoriasis or skin cancer).
Elsewhere on her skin, extensive punctate disciform scarring extends across her upper back, stopping abruptly at the T2 level. The skin below remains totally clear.
What is the diagnosis?
DISCUSSION
The correct diagnosis is prurigo nodularis (PN), which was first described in 1909 and has been extensively studied since. Though much remains unknown about PN, we know that, typically, the patient plays a major role in the genesis and perpetuation of the condition. In other words, these patients are pickers—but as they’ll tell you, they “wouldn’t pick if the lesions didn’t itch.” It does appear that they have a much lower threshold for itching than the general population.
Personal and family history of serious, ongoing bouts of depression and anxiety, as seen in this patient, are also typical among PN patients. Women appear to be far more likely to be diagnosed with PN, though there is no documentation of this in the literature.
These observations, while helpful for diagnosis, still don’t fully explain exactly why one person has PN while another doesn’t.
Besides the biopsy results, other keys to distinguishing PN include the characteristic lesions themselves, as well as the sparing of sites out of the patient’s reach. As is often the case, multiple old scars were seen on this patient’s upper back, confirming the chronicity of the condition.
In addition to educating patients about their role in the creation and perpetuation of PN, I find the most useful treatment to be intralesional triamcinolone in a 10-mg/cc strength (1/4 cc stock triamcinolone diluted in ¾ cc lidocaine), delivered directly into the lesion by 30-gauge needle Alternatively, a class 1 topical steroid cream (eg, clobetasol 0.05%) can be applied twice daily and then covered with a bandage. In cooler weather, when patients tend to bundle up, additional layers of clothing help to create a barrier that prevents patients from picking as much.
New cases of “picking” suggest the possibility of other diagnoses, including renal or hepatic failure, occult cancer, or other skin disease (eg, mastocytosis).
TAKE-HOME LEARNING POINTS
• Prurigo nodularis (PN) often presents with multiple papules, nodules, and/or plaques that develop primarily as a result of picking, rubbing, or scratching the area.
• PN patients appear to have a lowered threshold for itching, which may play a role in their disease.
• The differential for PN includes squamous cell carcinoma, psoriasis, atypical mycobacteria infection, and metastatic cancer.
• Biopsy plays a key role in establishing the correct diagnosis.
• Intralesional steroid injection with 10 mg/cc triamcinolone stops the itching and shrinks the lesion.
A 59-year-old woman presents urgently to dermatology for evaluation of very itchy lesions that manifested at least 20 years ago. They have been previously diagnosed as staph infection, fungal infection, psoriasis, and scabies but have not responded to a variety of topical steroid creams and topical antibacterials (eg, triple-antibiotic cream, mupirocin, and clindamycin solution). The patient has also been prescribed numerous oral antibiotics, the most recent of which was a 10-day course of clindamycin. This induced a raging case of diarrhea; her primary care provider subsequently referred her to dermatology.
She denies any family history of skin problems until her sister, who has accompanied her to this appointment, mentions that she and her mother have had similar problems for decades. More questioning reveals that all three women also have extensive psychiatric histories of chronic anxiety and depression.
When pressed, the patient and her sister admit to picking at their skin “constantly,” but especially when under stress.
EXAMINATION
Most of the patient’s lesions—round to oval, orangish red, scaly plaques that range from 2 to 5 cm—are on her legs, below her knees. There are about 15 lesions total. They look and feel very thick but have no increased warmth or tenderness. The erythema is confined to the lesions and does not extend into the surrounding areas.
Punch biopsy of one leg lesion shows a hypertrophic epidermis with focal parakeratosis, elongation of rete ridges, and perhaps most importantly, no signs of other items in the differential (such as psoriasis or skin cancer).
Elsewhere on her skin, extensive punctate disciform scarring extends across her upper back, stopping abruptly at the T2 level. The skin below remains totally clear.
What is the diagnosis?
DISCUSSION
The correct diagnosis is prurigo nodularis (PN), which was first described in 1909 and has been extensively studied since. Though much remains unknown about PN, we know that, typically, the patient plays a major role in the genesis and perpetuation of the condition. In other words, these patients are pickers—but as they’ll tell you, they “wouldn’t pick if the lesions didn’t itch.” It does appear that they have a much lower threshold for itching than the general population.
Personal and family history of serious, ongoing bouts of depression and anxiety, as seen in this patient, are also typical among PN patients. Women appear to be far more likely to be diagnosed with PN, though there is no documentation of this in the literature.
These observations, while helpful for diagnosis, still don’t fully explain exactly why one person has PN while another doesn’t.
Besides the biopsy results, other keys to distinguishing PN include the characteristic lesions themselves, as well as the sparing of sites out of the patient’s reach. As is often the case, multiple old scars were seen on this patient’s upper back, confirming the chronicity of the condition.
In addition to educating patients about their role in the creation and perpetuation of PN, I find the most useful treatment to be intralesional triamcinolone in a 10-mg/cc strength (1/4 cc stock triamcinolone diluted in ¾ cc lidocaine), delivered directly into the lesion by 30-gauge needle Alternatively, a class 1 topical steroid cream (eg, clobetasol 0.05%) can be applied twice daily and then covered with a bandage. In cooler weather, when patients tend to bundle up, additional layers of clothing help to create a barrier that prevents patients from picking as much.
New cases of “picking” suggest the possibility of other diagnoses, including renal or hepatic failure, occult cancer, or other skin disease (eg, mastocytosis).
TAKE-HOME LEARNING POINTS
• Prurigo nodularis (PN) often presents with multiple papules, nodules, and/or plaques that develop primarily as a result of picking, rubbing, or scratching the area.
• PN patients appear to have a lowered threshold for itching, which may play a role in their disease.
• The differential for PN includes squamous cell carcinoma, psoriasis, atypical mycobacteria infection, and metastatic cancer.
• Biopsy plays a key role in establishing the correct diagnosis.
• Intralesional steroid injection with 10 mg/cc triamcinolone stops the itching and shrinks the lesion.
A 59-year-old woman presents urgently to dermatology for evaluation of very itchy lesions that manifested at least 20 years ago. They have been previously diagnosed as staph infection, fungal infection, psoriasis, and scabies but have not responded to a variety of topical steroid creams and topical antibacterials (eg, triple-antibiotic cream, mupirocin, and clindamycin solution). The patient has also been prescribed numerous oral antibiotics, the most recent of which was a 10-day course of clindamycin. This induced a raging case of diarrhea; her primary care provider subsequently referred her to dermatology.
She denies any family history of skin problems until her sister, who has accompanied her to this appointment, mentions that she and her mother have had similar problems for decades. More questioning reveals that all three women also have extensive psychiatric histories of chronic anxiety and depression.
When pressed, the patient and her sister admit to picking at their skin “constantly,” but especially when under stress.
EXAMINATION
Most of the patient’s lesions—round to oval, orangish red, scaly plaques that range from 2 to 5 cm—are on her legs, below her knees. There are about 15 lesions total. They look and feel very thick but have no increased warmth or tenderness. The erythema is confined to the lesions and does not extend into the surrounding areas.
Punch biopsy of one leg lesion shows a hypertrophic epidermis with focal parakeratosis, elongation of rete ridges, and perhaps most importantly, no signs of other items in the differential (such as psoriasis or skin cancer).
Elsewhere on her skin, extensive punctate disciform scarring extends across her upper back, stopping abruptly at the T2 level. The skin below remains totally clear.
What is the diagnosis?
DISCUSSION
The correct diagnosis is prurigo nodularis (PN), which was first described in 1909 and has been extensively studied since. Though much remains unknown about PN, we know that, typically, the patient plays a major role in the genesis and perpetuation of the condition. In other words, these patients are pickers—but as they’ll tell you, they “wouldn’t pick if the lesions didn’t itch.” It does appear that they have a much lower threshold for itching than the general population.
Personal and family history of serious, ongoing bouts of depression and anxiety, as seen in this patient, are also typical among PN patients. Women appear to be far more likely to be diagnosed with PN, though there is no documentation of this in the literature.
These observations, while helpful for diagnosis, still don’t fully explain exactly why one person has PN while another doesn’t.
Besides the biopsy results, other keys to distinguishing PN include the characteristic lesions themselves, as well as the sparing of sites out of the patient’s reach. As is often the case, multiple old scars were seen on this patient’s upper back, confirming the chronicity of the condition.
In addition to educating patients about their role in the creation and perpetuation of PN, I find the most useful treatment to be intralesional triamcinolone in a 10-mg/cc strength (1/4 cc stock triamcinolone diluted in ¾ cc lidocaine), delivered directly into the lesion by 30-gauge needle Alternatively, a class 1 topical steroid cream (eg, clobetasol 0.05%) can be applied twice daily and then covered with a bandage. In cooler weather, when patients tend to bundle up, additional layers of clothing help to create a barrier that prevents patients from picking as much.
New cases of “picking” suggest the possibility of other diagnoses, including renal or hepatic failure, occult cancer, or other skin disease (eg, mastocytosis).
TAKE-HOME LEARNING POINTS
• Prurigo nodularis (PN) often presents with multiple papules, nodules, and/or plaques that develop primarily as a result of picking, rubbing, or scratching the area.
• PN patients appear to have a lowered threshold for itching, which may play a role in their disease.
• The differential for PN includes squamous cell carcinoma, psoriasis, atypical mycobacteria infection, and metastatic cancer.
• Biopsy plays a key role in establishing the correct diagnosis.
• Intralesional steroid injection with 10 mg/cc triamcinolone stops the itching and shrinks the lesion.
Venetoclax achieves responses in CLL refractory to ibrutinib, idelalisib
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
Venetoclax monotherapy was active, and even elicited minimal residual disease negativity in a proportion of patients with chronic lymphocytic leukemia (CLL) that was resistant or refractory to ibrutinib or idelalisib, Dr. Jeffrey Alan Jones of the Ohio State University Comprehensive Cancer Center, Columbus, and colleagues wrote in an abstract to be presented at the annual meeting of the American Society of Clinical Oncology.
The findings from their ongoing phase II study are the first prospective results to demonstrate efficacy in this poor-prognosis population, the researchers wrote. Earlier results from the study were reported at the American Society of Hematology meeting.
The 54 patients in the study, 25 of them refractory to ibrutinib and 6 to idelalisib, received venetoclax 20 mg daily followed by a 5-week ramp up in dosage to 400 mg daily. Over half of the patients had received more than five prior therapies; 83% did not have IGHV mutations, 20% had absolute lymphocyte counts exceeding 100 x 109, 35% had 17p deletions, and 24% had at least one node that was 10 cm or larger.
Of the patients who had previously received ibrutinib, four discontinued venetoclax because of progressive disease and four others had respiratory failure, multiorgan failure, death of unknown cause, or consent withdrawal. Of those who previously received idelalisib, four halted therapy because of progressive disease and failure to respond. Of 48 evaluable patients, 38 previously received ibrutinib, and 10 previously received idelalisib.
The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib.
Grade 3/4 adverse events were seen in over 10% of patients and included neutropenia (39%), thrombocytopenia (22%), anemia (20%), leukopenia (13%), and pneumonia (13%). Significant adverse events seen in at least two patients included pneumonia (9%), febrile neutropenia (7%), increased potassium, multiorgan failure, and septic shock (4% each).
The study is sponsored by AbbVie.
FROM ASCO 2016
Key clinical point: Venetoclax can achieve responses in patients who are refractory to ibrutinib.
Major finding: The overall response rate was 61% (23 of 38 patients) for patients refractory to ibrutinib and 50% (5 of 10 patients) for those refractory to idelalisib. A complete response was seen in three patients, all refractory to ibrutinib..
Data source: Data on 54 patients in an ongoing phase II study.
Disclosures: The study is sponsored by AbbVie.
What Is the Best Migraine Prevention Therapy?
STOWE, VERMONT—Many treament options are available for migraine prevention. Developing customized treatment strategies for patients is essential. At the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England, Peter McAllister, MD, discussed the most efficacious migraine prevention therapies. He also gave guidance on deciding who needs preventive therapy and how to choose the right preventive treatment for each patient.
Who Needs Migraine Prevention Therapy?
Migraine prevention therapy is underutilized. Thirty-eight percent of patients could benefit from migraine prevention therapy, but only 3% to 13% are actually receiving it, according to 2012 guidelines from the AAN and the American Headache Society. Frequency of headaches and functional disability are the main criteria for deciding which patients need preventive treatment. According to the 2012 US Headache Consortium Guidelines, migraineurs with six or more headache days per month, four or more headache days with functional disability, or three or more headache days per month resulting in disability requiring bed rest, should be offered migraine preventive medication. The Canadian Prophylactic Guidelines Development Group 2012 recommended that prophylactic therapy be considered for patients whose migraine attacks have a significant impact on their lives, despite appropriate use of acute medications, trigger management, and lifestyle modification strategies.
Disability may be the more important of the two criteria. “If [a patient is] disabled, we really have to push and be aggressive with preventive medications,” said Dr. McAllister, Medical Director of the New England Institute for Neurology and Headache in Stamford, Connecticut. A guiding principle to consider when deciding who should receive preventive therapy is that an accurate diagnosis follows the International Headache Society, third edition (ICHD-3) guidelines. Dr. McAllister also advised doctors to make sure that patients keep headache diaries and to set realistic treatment goals. He also emphasized the importance of understanding patients’ medical and psychiatric conditions. In general, drug treatment should start with a low dose and slowly be titrated to a therapeutic dose. In addition, preventive treatment should be included as part of an overall plan that encompasses lifestyle changes, trigger reduction, and a strategy for withdrawal of medication.
Pharmacologic Options
The FDA has approved several drugs for migraine prevention, including divalproex sodium, topiramate, propranolol, timolol, and methysergide. Of all classes of migraine preventive therapy, antiseizure medications have the most support in the data. Topiramate is “a top choice” for migraine prevention because Class I evidence supports its efficacy and it is fairly well tolerated, said Dr. McAllister. Topiramate was also approved for children based on a double-blind study of participants between ages 12 and 17. Divalproex, another antiseizure medication, has high efficacy, but side effects such as weight gain, hair loss, tremor, teratogenicity, increased liver function tests, and increased risk for pancreatitis make the drug unattractive to many.
Antihypertensive medicines also may be used for migraine prevention. Much research supports the efficacy of beta blockers in migraine prevention, and doctors can help patients manage their associated side effects. Metoprolol, a beta blocker, may be more tolerable for someone with asthma or glucose issues because it is selective for β1. Although physicians have used verapamil, a calcium channel blocker, for migraine prevention, the most recent guidelines give a Level U recommendation for its use because of conflicting or insufficient evidence. Lisinopril, an angiotensin-converting-enzyme inhibitor, and candesartan, an angiotensin receptor blocking agent, have good side effect profiles. These drugs are recommended for patients with mild hypertension. American guidelines provide a Level C recommendation of clonidine, but Canadian guidelines recommend against using the drug because of insufficient evidence and concern about the side effects.
Nonpharmacologic Options
Neurologists who seek to avoid pharmacologic treatments may choose options such as biologics or neurostimulators. OnabotulinumtoxinA injections are approved for patients with chronic migraine. Administered properly, the treatment has no systemic side effects and few local side effects.
In 2014, the FDA allowed the Cefaly device, which stimulates the trigeminal nerve, to be marketed for the prevention of migraine. In 2013, 67 patients with migraine were randomized to supraorbital stimulation with Cefaly or sham stimulation. The number of headache days per month decreased from seven to five among the participants randomized to Cefaly. Patients who received sham stimulation had no change in this end point.
Some patients may be interested in complementary or alternative treatment options like biofeedback. Biofeedback allows patients to monitor and change certain physiologic parameters (eg, muscle contraction and skin temperature) and is used to treat insomnia and anxiety. This therapy can be combined with a migraine preventive drug for the best results. Controlled studies indicate that biofeedback can result in a 45% to 60% headache reduction. When combined with medication, biofeedback reduces headache by more than 70%. Children and adolescents can also benefit from this treatment.
Selecting Individualized Migraine Prevention Treatment
Once a neurologist has given a proper diagnosis, he or she should thoroughly review the patient’s profile to select the proper migraine prevention therapy. Gender, age, childbearing potential, and BMI are factors to consider when finding the best treatment for a given patient. “When you know you have to put someone on a medicine, you should know the side effects and be able to discuss them,” said Dr. McAllister. “I like to come up with a scatter plot in my head looking at increasing safety and tolerability on the y axis and increasing efficacy on the x axis…. If someone is really sensitive, you might want to aim towards the more tolerable medications.” For a patient with exceptionally painful headaches, it’s better to treat with highly efficacious drugs, despite their side effects, he added.
No migraine preventive therapy is pregnancy category A, said Dr. McAllister. None has been demonstrated safe for the unborn child. Nonsteroidal anti-inflammatory drugs are pregnancy category B and are recommended only during the first two trimesters. Metoclopramide and acetaminophen are also pregnancy category B. Category C drugs include amitriptyline, propranolol, verapamil, and onabotulinumtoxinA injections. Topiramate should not be used during pregnancy because it increases the risk for cleft palate in the child. Clear evidence indicates that divalproex is a teratogen.
Every patient with migraine should work with his or her doctor to find the most efficacious strategy for treatment. “What is the ideal migraine preventive drug? It should decrease the number of headache days or attacks, attenuate the intensity—of not just the pain, but the associated symptoms—render acute treatment, be effective, and have low or no side effects,” Dr. McAllister concluded.
—Erica Robinson
Suggested Reading
Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52(6):930-945.
Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697-704.
Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345.
STOWE, VERMONT—Many treament options are available for migraine prevention. Developing customized treatment strategies for patients is essential. At the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England, Peter McAllister, MD, discussed the most efficacious migraine prevention therapies. He also gave guidance on deciding who needs preventive therapy and how to choose the right preventive treatment for each patient.
Who Needs Migraine Prevention Therapy?
Migraine prevention therapy is underutilized. Thirty-eight percent of patients could benefit from migraine prevention therapy, but only 3% to 13% are actually receiving it, according to 2012 guidelines from the AAN and the American Headache Society. Frequency of headaches and functional disability are the main criteria for deciding which patients need preventive treatment. According to the 2012 US Headache Consortium Guidelines, migraineurs with six or more headache days per month, four or more headache days with functional disability, or three or more headache days per month resulting in disability requiring bed rest, should be offered migraine preventive medication. The Canadian Prophylactic Guidelines Development Group 2012 recommended that prophylactic therapy be considered for patients whose migraine attacks have a significant impact on their lives, despite appropriate use of acute medications, trigger management, and lifestyle modification strategies.
Disability may be the more important of the two criteria. “If [a patient is] disabled, we really have to push and be aggressive with preventive medications,” said Dr. McAllister, Medical Director of the New England Institute for Neurology and Headache in Stamford, Connecticut. A guiding principle to consider when deciding who should receive preventive therapy is that an accurate diagnosis follows the International Headache Society, third edition (ICHD-3) guidelines. Dr. McAllister also advised doctors to make sure that patients keep headache diaries and to set realistic treatment goals. He also emphasized the importance of understanding patients’ medical and psychiatric conditions. In general, drug treatment should start with a low dose and slowly be titrated to a therapeutic dose. In addition, preventive treatment should be included as part of an overall plan that encompasses lifestyle changes, trigger reduction, and a strategy for withdrawal of medication.
Pharmacologic Options
The FDA has approved several drugs for migraine prevention, including divalproex sodium, topiramate, propranolol, timolol, and methysergide. Of all classes of migraine preventive therapy, antiseizure medications have the most support in the data. Topiramate is “a top choice” for migraine prevention because Class I evidence supports its efficacy and it is fairly well tolerated, said Dr. McAllister. Topiramate was also approved for children based on a double-blind study of participants between ages 12 and 17. Divalproex, another antiseizure medication, has high efficacy, but side effects such as weight gain, hair loss, tremor, teratogenicity, increased liver function tests, and increased risk for pancreatitis make the drug unattractive to many.
Antihypertensive medicines also may be used for migraine prevention. Much research supports the efficacy of beta blockers in migraine prevention, and doctors can help patients manage their associated side effects. Metoprolol, a beta blocker, may be more tolerable for someone with asthma or glucose issues because it is selective for β1. Although physicians have used verapamil, a calcium channel blocker, for migraine prevention, the most recent guidelines give a Level U recommendation for its use because of conflicting or insufficient evidence. Lisinopril, an angiotensin-converting-enzyme inhibitor, and candesartan, an angiotensin receptor blocking agent, have good side effect profiles. These drugs are recommended for patients with mild hypertension. American guidelines provide a Level C recommendation of clonidine, but Canadian guidelines recommend against using the drug because of insufficient evidence and concern about the side effects.
Nonpharmacologic Options
Neurologists who seek to avoid pharmacologic treatments may choose options such as biologics or neurostimulators. OnabotulinumtoxinA injections are approved for patients with chronic migraine. Administered properly, the treatment has no systemic side effects and few local side effects.
In 2014, the FDA allowed the Cefaly device, which stimulates the trigeminal nerve, to be marketed for the prevention of migraine. In 2013, 67 patients with migraine were randomized to supraorbital stimulation with Cefaly or sham stimulation. The number of headache days per month decreased from seven to five among the participants randomized to Cefaly. Patients who received sham stimulation had no change in this end point.
Some patients may be interested in complementary or alternative treatment options like biofeedback. Biofeedback allows patients to monitor and change certain physiologic parameters (eg, muscle contraction and skin temperature) and is used to treat insomnia and anxiety. This therapy can be combined with a migraine preventive drug for the best results. Controlled studies indicate that biofeedback can result in a 45% to 60% headache reduction. When combined with medication, biofeedback reduces headache by more than 70%. Children and adolescents can also benefit from this treatment.
Selecting Individualized Migraine Prevention Treatment
Once a neurologist has given a proper diagnosis, he or she should thoroughly review the patient’s profile to select the proper migraine prevention therapy. Gender, age, childbearing potential, and BMI are factors to consider when finding the best treatment for a given patient. “When you know you have to put someone on a medicine, you should know the side effects and be able to discuss them,” said Dr. McAllister. “I like to come up with a scatter plot in my head looking at increasing safety and tolerability on the y axis and increasing efficacy on the x axis…. If someone is really sensitive, you might want to aim towards the more tolerable medications.” For a patient with exceptionally painful headaches, it’s better to treat with highly efficacious drugs, despite their side effects, he added.
No migraine preventive therapy is pregnancy category A, said Dr. McAllister. None has been demonstrated safe for the unborn child. Nonsteroidal anti-inflammatory drugs are pregnancy category B and are recommended only during the first two trimesters. Metoclopramide and acetaminophen are also pregnancy category B. Category C drugs include amitriptyline, propranolol, verapamil, and onabotulinumtoxinA injections. Topiramate should not be used during pregnancy because it increases the risk for cleft palate in the child. Clear evidence indicates that divalproex is a teratogen.
Every patient with migraine should work with his or her doctor to find the most efficacious strategy for treatment. “What is the ideal migraine preventive drug? It should decrease the number of headache days or attacks, attenuate the intensity—of not just the pain, but the associated symptoms—render acute treatment, be effective, and have low or no side effects,” Dr. McAllister concluded.
—Erica Robinson
STOWE, VERMONT—Many treament options are available for migraine prevention. Developing customized treatment strategies for patients is essential. At the 26th Annual Stowe Headache Symposium of the Headache Cooperative of New England, Peter McAllister, MD, discussed the most efficacious migraine prevention therapies. He also gave guidance on deciding who needs preventive therapy and how to choose the right preventive treatment for each patient.
Who Needs Migraine Prevention Therapy?
Migraine prevention therapy is underutilized. Thirty-eight percent of patients could benefit from migraine prevention therapy, but only 3% to 13% are actually receiving it, according to 2012 guidelines from the AAN and the American Headache Society. Frequency of headaches and functional disability are the main criteria for deciding which patients need preventive treatment. According to the 2012 US Headache Consortium Guidelines, migraineurs with six or more headache days per month, four or more headache days with functional disability, or three or more headache days per month resulting in disability requiring bed rest, should be offered migraine preventive medication. The Canadian Prophylactic Guidelines Development Group 2012 recommended that prophylactic therapy be considered for patients whose migraine attacks have a significant impact on their lives, despite appropriate use of acute medications, trigger management, and lifestyle modification strategies.
Disability may be the more important of the two criteria. “If [a patient is] disabled, we really have to push and be aggressive with preventive medications,” said Dr. McAllister, Medical Director of the New England Institute for Neurology and Headache in Stamford, Connecticut. A guiding principle to consider when deciding who should receive preventive therapy is that an accurate diagnosis follows the International Headache Society, third edition (ICHD-3) guidelines. Dr. McAllister also advised doctors to make sure that patients keep headache diaries and to set realistic treatment goals. He also emphasized the importance of understanding patients’ medical and psychiatric conditions. In general, drug treatment should start with a low dose and slowly be titrated to a therapeutic dose. In addition, preventive treatment should be included as part of an overall plan that encompasses lifestyle changes, trigger reduction, and a strategy for withdrawal of medication.
Pharmacologic Options
The FDA has approved several drugs for migraine prevention, including divalproex sodium, topiramate, propranolol, timolol, and methysergide. Of all classes of migraine preventive therapy, antiseizure medications have the most support in the data. Topiramate is “a top choice” for migraine prevention because Class I evidence supports its efficacy and it is fairly well tolerated, said Dr. McAllister. Topiramate was also approved for children based on a double-blind study of participants between ages 12 and 17. Divalproex, another antiseizure medication, has high efficacy, but side effects such as weight gain, hair loss, tremor, teratogenicity, increased liver function tests, and increased risk for pancreatitis make the drug unattractive to many.
Antihypertensive medicines also may be used for migraine prevention. Much research supports the efficacy of beta blockers in migraine prevention, and doctors can help patients manage their associated side effects. Metoprolol, a beta blocker, may be more tolerable for someone with asthma or glucose issues because it is selective for β1. Although physicians have used verapamil, a calcium channel blocker, for migraine prevention, the most recent guidelines give a Level U recommendation for its use because of conflicting or insufficient evidence. Lisinopril, an angiotensin-converting-enzyme inhibitor, and candesartan, an angiotensin receptor blocking agent, have good side effect profiles. These drugs are recommended for patients with mild hypertension. American guidelines provide a Level C recommendation of clonidine, but Canadian guidelines recommend against using the drug because of insufficient evidence and concern about the side effects.
Nonpharmacologic Options
Neurologists who seek to avoid pharmacologic treatments may choose options such as biologics or neurostimulators. OnabotulinumtoxinA injections are approved for patients with chronic migraine. Administered properly, the treatment has no systemic side effects and few local side effects.
In 2014, the FDA allowed the Cefaly device, which stimulates the trigeminal nerve, to be marketed for the prevention of migraine. In 2013, 67 patients with migraine were randomized to supraorbital stimulation with Cefaly or sham stimulation. The number of headache days per month decreased from seven to five among the participants randomized to Cefaly. Patients who received sham stimulation had no change in this end point.
Some patients may be interested in complementary or alternative treatment options like biofeedback. Biofeedback allows patients to monitor and change certain physiologic parameters (eg, muscle contraction and skin temperature) and is used to treat insomnia and anxiety. This therapy can be combined with a migraine preventive drug for the best results. Controlled studies indicate that biofeedback can result in a 45% to 60% headache reduction. When combined with medication, biofeedback reduces headache by more than 70%. Children and adolescents can also benefit from this treatment.
Selecting Individualized Migraine Prevention Treatment
Once a neurologist has given a proper diagnosis, he or she should thoroughly review the patient’s profile to select the proper migraine prevention therapy. Gender, age, childbearing potential, and BMI are factors to consider when finding the best treatment for a given patient. “When you know you have to put someone on a medicine, you should know the side effects and be able to discuss them,” said Dr. McAllister. “I like to come up with a scatter plot in my head looking at increasing safety and tolerability on the y axis and increasing efficacy on the x axis…. If someone is really sensitive, you might want to aim towards the more tolerable medications.” For a patient with exceptionally painful headaches, it’s better to treat with highly efficacious drugs, despite their side effects, he added.
No migraine preventive therapy is pregnancy category A, said Dr. McAllister. None has been demonstrated safe for the unborn child. Nonsteroidal anti-inflammatory drugs are pregnancy category B and are recommended only during the first two trimesters. Metoclopramide and acetaminophen are also pregnancy category B. Category C drugs include amitriptyline, propranolol, verapamil, and onabotulinumtoxinA injections. Topiramate should not be used during pregnancy because it increases the risk for cleft palate in the child. Clear evidence indicates that divalproex is a teratogen.
Every patient with migraine should work with his or her doctor to find the most efficacious strategy for treatment. “What is the ideal migraine preventive drug? It should decrease the number of headache days or attacks, attenuate the intensity—of not just the pain, but the associated symptoms—render acute treatment, be effective, and have low or no side effects,” Dr. McAllister concluded.
—Erica Robinson
Suggested Reading
Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52(6):930-945.
Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697-704.
Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345.
Suggested Reading
Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52(6):930-945.
Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697-704.
Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345.
High Blood Pressure Predicts Motor Decline in Parkinson’s Disease
VANCOUVER—Elevated systolic blood pressure predicts worsening motor function among patients with Parkinson’s disease, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. Male gender and treatment with lower doses of dopaminergic medications also increase the risk of motor decline in these individuals, said Christina Lineback, a medical student at the University of Michigan in Ann Arbor.
Christina Lineback
Previous data have suggested that the presence and severity of comorbid brain pathologies may explain differences in disease progression between patients with Parkinson’s disease. In 2014, investigators at the University of Michigan found that cardiovascular risk factors such as hypertension, age, and diabetes influence the rate of motor disability accumulation in Parkinson’s disease.
Because high systolic blood pressure is a risk factor for the development of white matter hyperintensities, which in turn correlate with an increased rate of motor disability accumulation, Ms. Lineback and colleagues examined whether blood pressure predicted motor outcomes in patients with Parkinson’s disease. They performed a retrospective analysis of data from the CALM-PD trial, a two-year longitudinal study of 275 participants with Parkinson’s disease who were followed for 102 weeks. Each patient underwent several motor examinations using the Unified Parkinson’s Disease Rating Scale III (UPDRS III), as well as blood pressure measurements. The researchers examined mean systolic blood pressure using a multivariable linear-regression model that controlled for age, sex, disease duration, treatment arm, treatment dose, and open-label levodopa dose equivalence. Motor outcomes were determined by calculating the change in UPDRS III “on” scores from week 4 to week 102.
The study cohort of 275 patients with early Parkinson’s disease included 176 men and 99 women. Participants’ mean age was 60, and mean disease duration was 1.6 years. The overall mean systolic blood pressure was 130 mm Hg. Elevated mean systolic blood pressure was significantly associated with a greater decline in UPDRS III motor scores. In addition, male gender, randomization to pramipexole, and randomization to the lowest drug dose negatively affected motor performance.
Ms. Lineback offered several possible explanations for the association between elevated systolic blood pressure and worsening UPDRS III scores. High blood pressure may have a direct toxic effect on the brain that promotes the loss of neuronal integrity, she said. “Alternatively, elevated systolic blood pressure could serve as a trait marker for other unmeasured pathology … such as other cardiovascular risk factors.” In addition, the association may result from the autonomic dysfunction that is common in Parkinson’s disease. Lastly, the association could result from other unmeasured independent risk factors for motor decline, such as osteoarthritis or socioeconomic status.
One limitation of the study was the lack of information about other disease pathologies that could influence motor score, such as chronic conditions. In addition, the researchers did not account for the use of different classes of blood pressure medications.
“Identifying clinical features associated with more aggressive disease progression in Parkinson’s disease, such as blood pressure, may be important in future studies,” said Ms. Lineback. “Researchers may begin to incorporate these baseline prognostic markers into the randomization stage of future clinical trials.”
—Erik Greb
Suggested Reading
Kotagal V, Albin RL, Müller ML, et al. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology. 2014;82(17):1514-1520.
Parkinson Study Group CALM Cohort Investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol. 2009;66(5):563-570.
VANCOUVER—Elevated systolic blood pressure predicts worsening motor function among patients with Parkinson’s disease, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. Male gender and treatment with lower doses of dopaminergic medications also increase the risk of motor decline in these individuals, said Christina Lineback, a medical student at the University of Michigan in Ann Arbor.
Christina Lineback
Previous data have suggested that the presence and severity of comorbid brain pathologies may explain differences in disease progression between patients with Parkinson’s disease. In 2014, investigators at the University of Michigan found that cardiovascular risk factors such as hypertension, age, and diabetes influence the rate of motor disability accumulation in Parkinson’s disease.
Because high systolic blood pressure is a risk factor for the development of white matter hyperintensities, which in turn correlate with an increased rate of motor disability accumulation, Ms. Lineback and colleagues examined whether blood pressure predicted motor outcomes in patients with Parkinson’s disease. They performed a retrospective analysis of data from the CALM-PD trial, a two-year longitudinal study of 275 participants with Parkinson’s disease who were followed for 102 weeks. Each patient underwent several motor examinations using the Unified Parkinson’s Disease Rating Scale III (UPDRS III), as well as blood pressure measurements. The researchers examined mean systolic blood pressure using a multivariable linear-regression model that controlled for age, sex, disease duration, treatment arm, treatment dose, and open-label levodopa dose equivalence. Motor outcomes were determined by calculating the change in UPDRS III “on” scores from week 4 to week 102.
The study cohort of 275 patients with early Parkinson’s disease included 176 men and 99 women. Participants’ mean age was 60, and mean disease duration was 1.6 years. The overall mean systolic blood pressure was 130 mm Hg. Elevated mean systolic blood pressure was significantly associated with a greater decline in UPDRS III motor scores. In addition, male gender, randomization to pramipexole, and randomization to the lowest drug dose negatively affected motor performance.
Ms. Lineback offered several possible explanations for the association between elevated systolic blood pressure and worsening UPDRS III scores. High blood pressure may have a direct toxic effect on the brain that promotes the loss of neuronal integrity, she said. “Alternatively, elevated systolic blood pressure could serve as a trait marker for other unmeasured pathology … such as other cardiovascular risk factors.” In addition, the association may result from the autonomic dysfunction that is common in Parkinson’s disease. Lastly, the association could result from other unmeasured independent risk factors for motor decline, such as osteoarthritis or socioeconomic status.
One limitation of the study was the lack of information about other disease pathologies that could influence motor score, such as chronic conditions. In addition, the researchers did not account for the use of different classes of blood pressure medications.
“Identifying clinical features associated with more aggressive disease progression in Parkinson’s disease, such as blood pressure, may be important in future studies,” said Ms. Lineback. “Researchers may begin to incorporate these baseline prognostic markers into the randomization stage of future clinical trials.”
—Erik Greb
VANCOUVER—Elevated systolic blood pressure predicts worsening motor function among patients with Parkinson’s disease, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. Male gender and treatment with lower doses of dopaminergic medications also increase the risk of motor decline in these individuals, said Christina Lineback, a medical student at the University of Michigan in Ann Arbor.
Christina Lineback
Previous data have suggested that the presence and severity of comorbid brain pathologies may explain differences in disease progression between patients with Parkinson’s disease. In 2014, investigators at the University of Michigan found that cardiovascular risk factors such as hypertension, age, and diabetes influence the rate of motor disability accumulation in Parkinson’s disease.
Because high systolic blood pressure is a risk factor for the development of white matter hyperintensities, which in turn correlate with an increased rate of motor disability accumulation, Ms. Lineback and colleagues examined whether blood pressure predicted motor outcomes in patients with Parkinson’s disease. They performed a retrospective analysis of data from the CALM-PD trial, a two-year longitudinal study of 275 participants with Parkinson’s disease who were followed for 102 weeks. Each patient underwent several motor examinations using the Unified Parkinson’s Disease Rating Scale III (UPDRS III), as well as blood pressure measurements. The researchers examined mean systolic blood pressure using a multivariable linear-regression model that controlled for age, sex, disease duration, treatment arm, treatment dose, and open-label levodopa dose equivalence. Motor outcomes were determined by calculating the change in UPDRS III “on” scores from week 4 to week 102.
The study cohort of 275 patients with early Parkinson’s disease included 176 men and 99 women. Participants’ mean age was 60, and mean disease duration was 1.6 years. The overall mean systolic blood pressure was 130 mm Hg. Elevated mean systolic blood pressure was significantly associated with a greater decline in UPDRS III motor scores. In addition, male gender, randomization to pramipexole, and randomization to the lowest drug dose negatively affected motor performance.
Ms. Lineback offered several possible explanations for the association between elevated systolic blood pressure and worsening UPDRS III scores. High blood pressure may have a direct toxic effect on the brain that promotes the loss of neuronal integrity, she said. “Alternatively, elevated systolic blood pressure could serve as a trait marker for other unmeasured pathology … such as other cardiovascular risk factors.” In addition, the association may result from the autonomic dysfunction that is common in Parkinson’s disease. Lastly, the association could result from other unmeasured independent risk factors for motor decline, such as osteoarthritis or socioeconomic status.
One limitation of the study was the lack of information about other disease pathologies that could influence motor score, such as chronic conditions. In addition, the researchers did not account for the use of different classes of blood pressure medications.
“Identifying clinical features associated with more aggressive disease progression in Parkinson’s disease, such as blood pressure, may be important in future studies,” said Ms. Lineback. “Researchers may begin to incorporate these baseline prognostic markers into the randomization stage of future clinical trials.”
—Erik Greb
Suggested Reading
Kotagal V, Albin RL, Müller ML, et al. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology. 2014;82(17):1514-1520.
Parkinson Study Group CALM Cohort Investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol. 2009;66(5):563-570.
Suggested Reading
Kotagal V, Albin RL, Müller ML, et al. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology. 2014;82(17):1514-1520.
Parkinson Study Group CALM Cohort Investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol. 2009;66(5):563-570.
Investigational CDK4/6 inhibitor shows activity, less toxicity
Abemaciclib, a CDK4/6 inhibitor, showed durable clinical activity when given as continuous single-agent therapy to patients with advanced cancer, including breast cancer and non–small-cell lung cancer, according to investigators.
Neutropenia was rarely observed in patients treated with abemaciclib, the toxicity observed in some patients who receive the only Food and Drug Administration–approved CDK4/6 inhibitor, palbociclib.
“Abemaciclib is a small-molecule inhibitor of CDK4 and CDK6 that is structurally distinct from other dual inhibitors (such as palbociclib and ribociclib) and notably exhibits greater selectivity for CDK4 compared with CDK6,” wrote Dr. Amita Patnaik of South Texas Accelerated Research Therapeutics and her associates (Cancer Discov. 2016 May 23. doi: 10.1158/2159-8290.CD-16-0095).
Furthermore, preclinical models indicate that the drug can cross the blood-brain barrier, suggesting potential efficacy against primary and metastatic tumors involving the central nervous system, they said.
A total of 225 patients with various types of advanced cancers were enrolled in this multicohort phase I study (dose escalation, n = 33; single-agent abemaciclib therapy for breast cancer, n = 47; non–small-cell lung cancer, n = 68; glioblastoma, n = 17; melanoma, n = 26; colorectal cancer, n = 15; abemaciclib plus fulvestrant combination therapy for hormone receptor–positive breast cancer, n = 19). Abemaciclib was given orally to all patients.
Neither dose-limiting toxicity nor maximum tolerated dose was reached in patients treated at levels of 50 mg, 100 mg, 150 mg, or 225 mg once daily. The maximum tolerated dose was 200 mg for patients treated with abemaciclib twice daily.
In the single-agent breast cancer cohort, the disease control rate was 81% for hormone receptor–positive (HR-positive) tumors, 33% for HR-negative tumors, 100% for HR-positive HER2-positive tumors, 72% for HR-positive HER2-negative tumors, and 70% overall. The response rate was 31% for HR-positive tumors, 0% for HR-negative tumors, 36% for HR-positive HER2-positive tumors, 28% for HR-positive HER2-negative tumors, and 23% overall.
The overall response rate was 21% for breast cancer patients receiving abemaciclib plus fulvestrant.
Among the 68 patients with non–small-cell lung cancer, 2 had a partial response and 31 had stable disease. Of the 26 patients with melanoma, 1 had a partial response and 6 had stable disease. Of the 17 patients with glioblastoma, 3 had stable disease.
Overall, there were no study-related deaths. Diarrhea, nausea, and fatigue were the most common adverse events; all were reversible.
Neutropenia was observed in 39 patients (23% of 173 patients in the single-agent tumor-specific cohort) – 2 were grade 4 events. Grade 3 neutropenia occurred in 6 patients (32% of 19 patients with HR-positive breast cancer receiving combination therapy with abemaciclib plus fulvestrant).
“Previous reports have identified neutropenia as an adverse event associated with dual inhibition of CDK4 and CDK6. However, abemaciclib given as a single agent on a continuous schedule in the tumor-specific cohorts was associated with an acceptable incidence of investigator-reported grade 3 (9%, 16 of 173 patients) or grade 4 (1%, 2 of 173 patients) neutropenia,” wrote the investigators.
“In summary, the results of this clinical trial demonstrate the safety and antitumor activity of abemaciclib as a single agent and support its further development both as monotherapy and in rational combinations. Furthermore, these findings validate CDK4 and CDK6 as anticancer drug targets and translate preclinical predictions regarding therapeutic targeting of cell-cycle derangements in cancer into clinical efficacy,”they wrote.
Eli Lilly funded the study. Eight investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies including Eli Lilly.
On Twitter @JessCraig_OP
Abemaciclib, a CDK4/6 inhibitor, showed durable clinical activity when given as continuous single-agent therapy to patients with advanced cancer, including breast cancer and non–small-cell lung cancer, according to investigators.
Neutropenia was rarely observed in patients treated with abemaciclib, the toxicity observed in some patients who receive the only Food and Drug Administration–approved CDK4/6 inhibitor, palbociclib.
“Abemaciclib is a small-molecule inhibitor of CDK4 and CDK6 that is structurally distinct from other dual inhibitors (such as palbociclib and ribociclib) and notably exhibits greater selectivity for CDK4 compared with CDK6,” wrote Dr. Amita Patnaik of South Texas Accelerated Research Therapeutics and her associates (Cancer Discov. 2016 May 23. doi: 10.1158/2159-8290.CD-16-0095).
Furthermore, preclinical models indicate that the drug can cross the blood-brain barrier, suggesting potential efficacy against primary and metastatic tumors involving the central nervous system, they said.
A total of 225 patients with various types of advanced cancers were enrolled in this multicohort phase I study (dose escalation, n = 33; single-agent abemaciclib therapy for breast cancer, n = 47; non–small-cell lung cancer, n = 68; glioblastoma, n = 17; melanoma, n = 26; colorectal cancer, n = 15; abemaciclib plus fulvestrant combination therapy for hormone receptor–positive breast cancer, n = 19). Abemaciclib was given orally to all patients.
Neither dose-limiting toxicity nor maximum tolerated dose was reached in patients treated at levels of 50 mg, 100 mg, 150 mg, or 225 mg once daily. The maximum tolerated dose was 200 mg for patients treated with abemaciclib twice daily.
In the single-agent breast cancer cohort, the disease control rate was 81% for hormone receptor–positive (HR-positive) tumors, 33% for HR-negative tumors, 100% for HR-positive HER2-positive tumors, 72% for HR-positive HER2-negative tumors, and 70% overall. The response rate was 31% for HR-positive tumors, 0% for HR-negative tumors, 36% for HR-positive HER2-positive tumors, 28% for HR-positive HER2-negative tumors, and 23% overall.
The overall response rate was 21% for breast cancer patients receiving abemaciclib plus fulvestrant.
Among the 68 patients with non–small-cell lung cancer, 2 had a partial response and 31 had stable disease. Of the 26 patients with melanoma, 1 had a partial response and 6 had stable disease. Of the 17 patients with glioblastoma, 3 had stable disease.
Overall, there were no study-related deaths. Diarrhea, nausea, and fatigue were the most common adverse events; all were reversible.
Neutropenia was observed in 39 patients (23% of 173 patients in the single-agent tumor-specific cohort) – 2 were grade 4 events. Grade 3 neutropenia occurred in 6 patients (32% of 19 patients with HR-positive breast cancer receiving combination therapy with abemaciclib plus fulvestrant).
“Previous reports have identified neutropenia as an adverse event associated with dual inhibition of CDK4 and CDK6. However, abemaciclib given as a single agent on a continuous schedule in the tumor-specific cohorts was associated with an acceptable incidence of investigator-reported grade 3 (9%, 16 of 173 patients) or grade 4 (1%, 2 of 173 patients) neutropenia,” wrote the investigators.
“In summary, the results of this clinical trial demonstrate the safety and antitumor activity of abemaciclib as a single agent and support its further development both as monotherapy and in rational combinations. Furthermore, these findings validate CDK4 and CDK6 as anticancer drug targets and translate preclinical predictions regarding therapeutic targeting of cell-cycle derangements in cancer into clinical efficacy,”they wrote.
Eli Lilly funded the study. Eight investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies including Eli Lilly.
On Twitter @JessCraig_OP
Abemaciclib, a CDK4/6 inhibitor, showed durable clinical activity when given as continuous single-agent therapy to patients with advanced cancer, including breast cancer and non–small-cell lung cancer, according to investigators.
Neutropenia was rarely observed in patients treated with abemaciclib, the toxicity observed in some patients who receive the only Food and Drug Administration–approved CDK4/6 inhibitor, palbociclib.
“Abemaciclib is a small-molecule inhibitor of CDK4 and CDK6 that is structurally distinct from other dual inhibitors (such as palbociclib and ribociclib) and notably exhibits greater selectivity for CDK4 compared with CDK6,” wrote Dr. Amita Patnaik of South Texas Accelerated Research Therapeutics and her associates (Cancer Discov. 2016 May 23. doi: 10.1158/2159-8290.CD-16-0095).
Furthermore, preclinical models indicate that the drug can cross the blood-brain barrier, suggesting potential efficacy against primary and metastatic tumors involving the central nervous system, they said.
A total of 225 patients with various types of advanced cancers were enrolled in this multicohort phase I study (dose escalation, n = 33; single-agent abemaciclib therapy for breast cancer, n = 47; non–small-cell lung cancer, n = 68; glioblastoma, n = 17; melanoma, n = 26; colorectal cancer, n = 15; abemaciclib plus fulvestrant combination therapy for hormone receptor–positive breast cancer, n = 19). Abemaciclib was given orally to all patients.
Neither dose-limiting toxicity nor maximum tolerated dose was reached in patients treated at levels of 50 mg, 100 mg, 150 mg, or 225 mg once daily. The maximum tolerated dose was 200 mg for patients treated with abemaciclib twice daily.
In the single-agent breast cancer cohort, the disease control rate was 81% for hormone receptor–positive (HR-positive) tumors, 33% for HR-negative tumors, 100% for HR-positive HER2-positive tumors, 72% for HR-positive HER2-negative tumors, and 70% overall. The response rate was 31% for HR-positive tumors, 0% for HR-negative tumors, 36% for HR-positive HER2-positive tumors, 28% for HR-positive HER2-negative tumors, and 23% overall.
The overall response rate was 21% for breast cancer patients receiving abemaciclib plus fulvestrant.
Among the 68 patients with non–small-cell lung cancer, 2 had a partial response and 31 had stable disease. Of the 26 patients with melanoma, 1 had a partial response and 6 had stable disease. Of the 17 patients with glioblastoma, 3 had stable disease.
Overall, there were no study-related deaths. Diarrhea, nausea, and fatigue were the most common adverse events; all were reversible.
Neutropenia was observed in 39 patients (23% of 173 patients in the single-agent tumor-specific cohort) – 2 were grade 4 events. Grade 3 neutropenia occurred in 6 patients (32% of 19 patients with HR-positive breast cancer receiving combination therapy with abemaciclib plus fulvestrant).
“Previous reports have identified neutropenia as an adverse event associated with dual inhibition of CDK4 and CDK6. However, abemaciclib given as a single agent on a continuous schedule in the tumor-specific cohorts was associated with an acceptable incidence of investigator-reported grade 3 (9%, 16 of 173 patients) or grade 4 (1%, 2 of 173 patients) neutropenia,” wrote the investigators.
“In summary, the results of this clinical trial demonstrate the safety and antitumor activity of abemaciclib as a single agent and support its further development both as monotherapy and in rational combinations. Furthermore, these findings validate CDK4 and CDK6 as anticancer drug targets and translate preclinical predictions regarding therapeutic targeting of cell-cycle derangements in cancer into clinical efficacy,”they wrote.
Eli Lilly funded the study. Eight investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies including Eli Lilly.
On Twitter @JessCraig_OP
FROM CANCER DISCOVERY
Key clinical point: A phase I trial indicates that abemaciclib is safe and shows activity in treating patients with advanced breast and other cancers.
Major finding: In the single-agent breast cancer cohort, the overall disease control rate was 70%. Incidence of neutropenia was 9% for grade 3 and 1% for grade 4.
Data source: A multicenter phase I dose-escalation and tumor-specific cohort study of 225 patients with advanced cancers.
Disclosures: Eli Lilly funded the study. Eight investigators reported serving in advisory roles, having ownership or stock interest in, or receiving financial compensation from multiple companies including Eli Lilly.
Why access to public bathrooms matters
Going to the movies is something I have always enjoyed. What I don’t always enjoy is waiting in line to use the bathroom after the movie is over and invariably picking a stall that has run out of toilet paper or that is in need of cleaning. These minor inconveniences in no way compare to the experiences some of my transgender patients have shared with me. Many of my patients tell me that they avoid using bathrooms in public places because of the anxiety they feel at having to pick a bathroom. Do they use the one that matches their sex assigned at birth or the one that matches their gender identity? Will they be safe and free from harassment in either bathroom? Some of my patients tell me they avoid drinking water at school just so they do not have to deal with going to the bathroom there.
Recently there have been bills introduced in several states that seek to deny transgender youth access to sex-segregated spaces including restrooms and locker rooms. These bills stigmatize an already vulnerable population, potentially increasing their risk of negative health outcomes. In a survey of transgender people in Massachusetts, 65% of respondents reported being discriminated against in public accommodations, and this discrimination was associated with poorer mental and physical health outcomes.1
In February of 2016, the American Academy of Pediatrics and several other organizations dedicated to the health and welfare of children came out with a letter to state governors in opposition to these bills.2 It states: “Transgender kids are already at heightened risk for violence, bullying, and harassment, and these bills exacerbate those risks by creating a hostile environment. … In addition, students who would be affected by these bills are among our most vulnerable to experiencing depression and engaging in self-harm, including suicide.”
On May 13, 2016, the U.S. Department of Justice and the U.S. Department of Education jointly issued a letter directing public schools to allow transgender students to use bathrooms that correspond with their gender identity.3 The letter was accompanied by a 25-page document with examples of policies and emerging practices to support transgender students.4
Proponents of these bills state that their purpose is to increase public safety and protect privacy. There are concerns that individuals may take advantage of these policies to sexually harass people in sex-segregated spaces. To date, there are no data to support these claims. In interviews conducted with heads of state police departments in 12 states that have nondiscrimination laws to protect transgender people in public settings, not one of the participants indicated any increase in sexual harassment or abuse in connection with these laws.1 In addition, should any type of harassment occur, it would not be protected under antidiscrimination laws, and perpetrators would be subject to criminal penalties.
What can we do as health care providers to support our patients?
• Educate ourselves. Keep up to date with best practice guidelines and evidence on how to promote the health and well-being of all children. The National LGBT Health Education Center has many educational resources to help health care providers provide quality care to LGBT patients and families. It is important to be aware of resources to help patients and families be aware of their rights and advocate for themselves in other settings such as school and work. Two organizations that provide this support and information are Trans Youth Family Allies and Lambda Legal.
• Create safe spaces. Create spaces in our practice settings where children and youth can safely explore their gender identity and gender expression. This can be done by providing access to gender-neutral bathrooms, prominently displaying nondiscrimination policies that are inclusive of gender identity, and modeling recognition of the variety of ways gender can be experienced by asking and using patients’ preferred names and pronouns.
• Advocate. Advocate for gender-inclusive environments within local youth-serving organizations including schools, medical facilities, and child welfare agencies. Share available information about the potential negative health effects of stigmatization and discrimination in transgender youth.
Together we can work to promote the well-being of all children.
Resources
• The National LGBT Health Education Center (www.lgbthealtheducation.org/).
• Trans Youth Family Allies (www.imatyfa.org/).
• Lambda Legal (www.lambdalegal.org/know-your-rights/youth).
References
1. Policy Brief: State Anti-transgender Bathroom Bills Threaten Transgender People’s Health and Participation in Public Life. Fenway Institute and Center for American Progress, 2016.
2. American Academy of Pediatrics letter on sex-segregated spaces (www.aap.org/en-us/advocacy-and-policy/state-advocacy/Documents/AAP_HRCLetter.pdf).
3. Department of Justice and Department of Education Dear Colleague Letter on Transgender Students (www.justice.gov/opa/file/850996/download).
4. Department of Education Examples of Policies and Emerging Practices for Supporting Transgender Students (www2.ed.gov/about/offices/list/oese/oshs/emergingpractices.pdf).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Going to the movies is something I have always enjoyed. What I don’t always enjoy is waiting in line to use the bathroom after the movie is over and invariably picking a stall that has run out of toilet paper or that is in need of cleaning. These minor inconveniences in no way compare to the experiences some of my transgender patients have shared with me. Many of my patients tell me that they avoid using bathrooms in public places because of the anxiety they feel at having to pick a bathroom. Do they use the one that matches their sex assigned at birth or the one that matches their gender identity? Will they be safe and free from harassment in either bathroom? Some of my patients tell me they avoid drinking water at school just so they do not have to deal with going to the bathroom there.
Recently there have been bills introduced in several states that seek to deny transgender youth access to sex-segregated spaces including restrooms and locker rooms. These bills stigmatize an already vulnerable population, potentially increasing their risk of negative health outcomes. In a survey of transgender people in Massachusetts, 65% of respondents reported being discriminated against in public accommodations, and this discrimination was associated with poorer mental and physical health outcomes.1
In February of 2016, the American Academy of Pediatrics and several other organizations dedicated to the health and welfare of children came out with a letter to state governors in opposition to these bills.2 It states: “Transgender kids are already at heightened risk for violence, bullying, and harassment, and these bills exacerbate those risks by creating a hostile environment. … In addition, students who would be affected by these bills are among our most vulnerable to experiencing depression and engaging in self-harm, including suicide.”
On May 13, 2016, the U.S. Department of Justice and the U.S. Department of Education jointly issued a letter directing public schools to allow transgender students to use bathrooms that correspond with their gender identity.3 The letter was accompanied by a 25-page document with examples of policies and emerging practices to support transgender students.4
Proponents of these bills state that their purpose is to increase public safety and protect privacy. There are concerns that individuals may take advantage of these policies to sexually harass people in sex-segregated spaces. To date, there are no data to support these claims. In interviews conducted with heads of state police departments in 12 states that have nondiscrimination laws to protect transgender people in public settings, not one of the participants indicated any increase in sexual harassment or abuse in connection with these laws.1 In addition, should any type of harassment occur, it would not be protected under antidiscrimination laws, and perpetrators would be subject to criminal penalties.
What can we do as health care providers to support our patients?
• Educate ourselves. Keep up to date with best practice guidelines and evidence on how to promote the health and well-being of all children. The National LGBT Health Education Center has many educational resources to help health care providers provide quality care to LGBT patients and families. It is important to be aware of resources to help patients and families be aware of their rights and advocate for themselves in other settings such as school and work. Two organizations that provide this support and information are Trans Youth Family Allies and Lambda Legal.
• Create safe spaces. Create spaces in our practice settings where children and youth can safely explore their gender identity and gender expression. This can be done by providing access to gender-neutral bathrooms, prominently displaying nondiscrimination policies that are inclusive of gender identity, and modeling recognition of the variety of ways gender can be experienced by asking and using patients’ preferred names and pronouns.
• Advocate. Advocate for gender-inclusive environments within local youth-serving organizations including schools, medical facilities, and child welfare agencies. Share available information about the potential negative health effects of stigmatization and discrimination in transgender youth.
Together we can work to promote the well-being of all children.
Resources
• The National LGBT Health Education Center (www.lgbthealtheducation.org/).
• Trans Youth Family Allies (www.imatyfa.org/).
• Lambda Legal (www.lambdalegal.org/know-your-rights/youth).
References
1. Policy Brief: State Anti-transgender Bathroom Bills Threaten Transgender People’s Health and Participation in Public Life. Fenway Institute and Center for American Progress, 2016.
2. American Academy of Pediatrics letter on sex-segregated spaces (www.aap.org/en-us/advocacy-and-policy/state-advocacy/Documents/AAP_HRCLetter.pdf).
3. Department of Justice and Department of Education Dear Colleague Letter on Transgender Students (www.justice.gov/opa/file/850996/download).
4. Department of Education Examples of Policies and Emerging Practices for Supporting Transgender Students (www2.ed.gov/about/offices/list/oese/oshs/emergingpractices.pdf).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Going to the movies is something I have always enjoyed. What I don’t always enjoy is waiting in line to use the bathroom after the movie is over and invariably picking a stall that has run out of toilet paper or that is in need of cleaning. These minor inconveniences in no way compare to the experiences some of my transgender patients have shared with me. Many of my patients tell me that they avoid using bathrooms in public places because of the anxiety they feel at having to pick a bathroom. Do they use the one that matches their sex assigned at birth or the one that matches their gender identity? Will they be safe and free from harassment in either bathroom? Some of my patients tell me they avoid drinking water at school just so they do not have to deal with going to the bathroom there.
Recently there have been bills introduced in several states that seek to deny transgender youth access to sex-segregated spaces including restrooms and locker rooms. These bills stigmatize an already vulnerable population, potentially increasing their risk of negative health outcomes. In a survey of transgender people in Massachusetts, 65% of respondents reported being discriminated against in public accommodations, and this discrimination was associated with poorer mental and physical health outcomes.1
In February of 2016, the American Academy of Pediatrics and several other organizations dedicated to the health and welfare of children came out with a letter to state governors in opposition to these bills.2 It states: “Transgender kids are already at heightened risk for violence, bullying, and harassment, and these bills exacerbate those risks by creating a hostile environment. … In addition, students who would be affected by these bills are among our most vulnerable to experiencing depression and engaging in self-harm, including suicide.”
On May 13, 2016, the U.S. Department of Justice and the U.S. Department of Education jointly issued a letter directing public schools to allow transgender students to use bathrooms that correspond with their gender identity.3 The letter was accompanied by a 25-page document with examples of policies and emerging practices to support transgender students.4
Proponents of these bills state that their purpose is to increase public safety and protect privacy. There are concerns that individuals may take advantage of these policies to sexually harass people in sex-segregated spaces. To date, there are no data to support these claims. In interviews conducted with heads of state police departments in 12 states that have nondiscrimination laws to protect transgender people in public settings, not one of the participants indicated any increase in sexual harassment or abuse in connection with these laws.1 In addition, should any type of harassment occur, it would not be protected under antidiscrimination laws, and perpetrators would be subject to criminal penalties.
What can we do as health care providers to support our patients?
• Educate ourselves. Keep up to date with best practice guidelines and evidence on how to promote the health and well-being of all children. The National LGBT Health Education Center has many educational resources to help health care providers provide quality care to LGBT patients and families. It is important to be aware of resources to help patients and families be aware of their rights and advocate for themselves in other settings such as school and work. Two organizations that provide this support and information are Trans Youth Family Allies and Lambda Legal.
• Create safe spaces. Create spaces in our practice settings where children and youth can safely explore their gender identity and gender expression. This can be done by providing access to gender-neutral bathrooms, prominently displaying nondiscrimination policies that are inclusive of gender identity, and modeling recognition of the variety of ways gender can be experienced by asking and using patients’ preferred names and pronouns.
• Advocate. Advocate for gender-inclusive environments within local youth-serving organizations including schools, medical facilities, and child welfare agencies. Share available information about the potential negative health effects of stigmatization and discrimination in transgender youth.
Together we can work to promote the well-being of all children.
Resources
• The National LGBT Health Education Center (www.lgbthealtheducation.org/).
• Trans Youth Family Allies (www.imatyfa.org/).
• Lambda Legal (www.lambdalegal.org/know-your-rights/youth).
References
1. Policy Brief: State Anti-transgender Bathroom Bills Threaten Transgender People’s Health and Participation in Public Life. Fenway Institute and Center for American Progress, 2016.
2. American Academy of Pediatrics letter on sex-segregated spaces (www.aap.org/en-us/advocacy-and-policy/state-advocacy/Documents/AAP_HRCLetter.pdf).
3. Department of Justice and Department of Education Dear Colleague Letter on Transgender Students (www.justice.gov/opa/file/850996/download).
4. Department of Education Examples of Policies and Emerging Practices for Supporting Transgender Students (www2.ed.gov/about/offices/list/oese/oshs/emergingpractices.pdf).
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Exercise training cuts heart failure mortality
FLORENCE, ITALY – Exercise training boosts the longevity of patients with heart failure.
Although results from several prior randomized, controlled trials had already shown a mortality benefit from exercise training for heart failure patients, these findings have now been confirmed by a meta-analysis that used the original, individual patient raw data collected in 20 separate randomized, controlled trials that together involved more than 4,000 patients. The results showed that an exercise-training intervention run for at least 3 weeks produced a statistically significant, relative reduction in all-cause mortality of 18%, compared with similar patients who had been randomized to usual care without an exercise program, Oriana Ciani, Ph.D., reported at a meeting held by the Heart Failure Association of the European Society of Cardiology.
The individual patient data meta-analysis using results from randomized, controlled trials also showed a statistically significant 11% relative reduction in the incidence of all-cause hospitalization in heart failure patients during at least 6 months’ follow-up of exercise programs that lasted for at least 3 weeks, said Dr. Ciani, a health technology researcher at the University of Exeter (England).
Her analysis also showed no suggestion of heterogeneity for each of these two beneficial effects from exercise programs, regardless of patients’ age, sex, or baseline levels of left ventricular ejection fraction, heart failure etiology, functional status, or exercise capacity. “No evidence was found to support a differential treatment effect from exercise-based intervention across patient subgroups,” she said.
The Exercise Training for Chronic Heart Failure (ExTraMATCH II) meta-analysis used data collected in randomized trials published through 2014 that involved at least 50 patients, used an exercise intervention for at least 3 weeks, and had follow-up for at least 6 months. Dr. Ciani and her associates identified 20 studies that included a total of 4,043 heart failure patients who fulfilled these criteria and for whom the researchers from the studies were willing to share individual patient data.
The analysis also showed a median time to all-cause mortality of 605 days among patients who received exercise training and 615 days in the controls, and a median time to first all-cause hospitalization of 229 days with exercise training and 241 days in the controls. The percentage of patients who were hospitalized during follow-up was reduced by an absolute 3.8% for those in the exercise group, compared with the controls.
Although the type of exercise intervention used varied among the 20 studies, most involved aerobic training, and some also used resistance training, Dr. Ciani said. She said she plans additional analyses of the data she has collected to examine the impact of exercise in heart failure patients on cardiovascular mortality, heart failure hospitalization, and a combined endpoint of all-cause death and all-cause hospitalization.
On Twitter @mitchelzoler
These results are a step forward in confirming the safety and efficacy of exercise training for heart failure patients. It is a good addition to the literature. Its strength is its use of an analysis of individual patient data.

|
Dr. Theresa A. McDonagh |
The findings deliver the important message that exercise rehabilitation is important for all heart failure patients. Currently, uptake of such programs is low, involving about 20% of heart failure patients.
The analysis showed a consistent effect from exercise training across all subgroups examined. The actual reductions in adverse outcomes were meaningful, with a 2% absolute reduction in all-cause mortality and a nearly 4% absolute reduction in all-cause hospitalization. However, the findings do not tell us which type of exercise prescription works best. Future research needs to especially focus on patients with heart failure with preserved ejection fraction to determine whether exercise benefits this particular type of heart failure patient.
Dr. Theresa A. McDonagh is a professor of heart failure at King’s College, London. She made these comments as the designated discussant for the study. She had no relevant financial disclosures.
These results are a step forward in confirming the safety and efficacy of exercise training for heart failure patients. It is a good addition to the literature. Its strength is its use of an analysis of individual patient data.

|
Dr. Theresa A. McDonagh |
The findings deliver the important message that exercise rehabilitation is important for all heart failure patients. Currently, uptake of such programs is low, involving about 20% of heart failure patients.
The analysis showed a consistent effect from exercise training across all subgroups examined. The actual reductions in adverse outcomes were meaningful, with a 2% absolute reduction in all-cause mortality and a nearly 4% absolute reduction in all-cause hospitalization. However, the findings do not tell us which type of exercise prescription works best. Future research needs to especially focus on patients with heart failure with preserved ejection fraction to determine whether exercise benefits this particular type of heart failure patient.
Dr. Theresa A. McDonagh is a professor of heart failure at King’s College, London. She made these comments as the designated discussant for the study. She had no relevant financial disclosures.
These results are a step forward in confirming the safety and efficacy of exercise training for heart failure patients. It is a good addition to the literature. Its strength is its use of an analysis of individual patient data.

|
Dr. Theresa A. McDonagh |
The findings deliver the important message that exercise rehabilitation is important for all heart failure patients. Currently, uptake of such programs is low, involving about 20% of heart failure patients.
The analysis showed a consistent effect from exercise training across all subgroups examined. The actual reductions in adverse outcomes were meaningful, with a 2% absolute reduction in all-cause mortality and a nearly 4% absolute reduction in all-cause hospitalization. However, the findings do not tell us which type of exercise prescription works best. Future research needs to especially focus on patients with heart failure with preserved ejection fraction to determine whether exercise benefits this particular type of heart failure patient.
Dr. Theresa A. McDonagh is a professor of heart failure at King’s College, London. She made these comments as the designated discussant for the study. She had no relevant financial disclosures.
FLORENCE, ITALY – Exercise training boosts the longevity of patients with heart failure.
Although results from several prior randomized, controlled trials had already shown a mortality benefit from exercise training for heart failure patients, these findings have now been confirmed by a meta-analysis that used the original, individual patient raw data collected in 20 separate randomized, controlled trials that together involved more than 4,000 patients. The results showed that an exercise-training intervention run for at least 3 weeks produced a statistically significant, relative reduction in all-cause mortality of 18%, compared with similar patients who had been randomized to usual care without an exercise program, Oriana Ciani, Ph.D., reported at a meeting held by the Heart Failure Association of the European Society of Cardiology.
The individual patient data meta-analysis using results from randomized, controlled trials also showed a statistically significant 11% relative reduction in the incidence of all-cause hospitalization in heart failure patients during at least 6 months’ follow-up of exercise programs that lasted for at least 3 weeks, said Dr. Ciani, a health technology researcher at the University of Exeter (England).
Her analysis also showed no suggestion of heterogeneity for each of these two beneficial effects from exercise programs, regardless of patients’ age, sex, or baseline levels of left ventricular ejection fraction, heart failure etiology, functional status, or exercise capacity. “No evidence was found to support a differential treatment effect from exercise-based intervention across patient subgroups,” she said.
The Exercise Training for Chronic Heart Failure (ExTraMATCH II) meta-analysis used data collected in randomized trials published through 2014 that involved at least 50 patients, used an exercise intervention for at least 3 weeks, and had follow-up for at least 6 months. Dr. Ciani and her associates identified 20 studies that included a total of 4,043 heart failure patients who fulfilled these criteria and for whom the researchers from the studies were willing to share individual patient data.
The analysis also showed a median time to all-cause mortality of 605 days among patients who received exercise training and 615 days in the controls, and a median time to first all-cause hospitalization of 229 days with exercise training and 241 days in the controls. The percentage of patients who were hospitalized during follow-up was reduced by an absolute 3.8% for those in the exercise group, compared with the controls.
Although the type of exercise intervention used varied among the 20 studies, most involved aerobic training, and some also used resistance training, Dr. Ciani said. She said she plans additional analyses of the data she has collected to examine the impact of exercise in heart failure patients on cardiovascular mortality, heart failure hospitalization, and a combined endpoint of all-cause death and all-cause hospitalization.
On Twitter @mitchelzoler
FLORENCE, ITALY – Exercise training boosts the longevity of patients with heart failure.
Although results from several prior randomized, controlled trials had already shown a mortality benefit from exercise training for heart failure patients, these findings have now been confirmed by a meta-analysis that used the original, individual patient raw data collected in 20 separate randomized, controlled trials that together involved more than 4,000 patients. The results showed that an exercise-training intervention run for at least 3 weeks produced a statistically significant, relative reduction in all-cause mortality of 18%, compared with similar patients who had been randomized to usual care without an exercise program, Oriana Ciani, Ph.D., reported at a meeting held by the Heart Failure Association of the European Society of Cardiology.
The individual patient data meta-analysis using results from randomized, controlled trials also showed a statistically significant 11% relative reduction in the incidence of all-cause hospitalization in heart failure patients during at least 6 months’ follow-up of exercise programs that lasted for at least 3 weeks, said Dr. Ciani, a health technology researcher at the University of Exeter (England).
Her analysis also showed no suggestion of heterogeneity for each of these two beneficial effects from exercise programs, regardless of patients’ age, sex, or baseline levels of left ventricular ejection fraction, heart failure etiology, functional status, or exercise capacity. “No evidence was found to support a differential treatment effect from exercise-based intervention across patient subgroups,” she said.
The Exercise Training for Chronic Heart Failure (ExTraMATCH II) meta-analysis used data collected in randomized trials published through 2014 that involved at least 50 patients, used an exercise intervention for at least 3 weeks, and had follow-up for at least 6 months. Dr. Ciani and her associates identified 20 studies that included a total of 4,043 heart failure patients who fulfilled these criteria and for whom the researchers from the studies were willing to share individual patient data.
The analysis also showed a median time to all-cause mortality of 605 days among patients who received exercise training and 615 days in the controls, and a median time to first all-cause hospitalization of 229 days with exercise training and 241 days in the controls. The percentage of patients who were hospitalized during follow-up was reduced by an absolute 3.8% for those in the exercise group, compared with the controls.
Although the type of exercise intervention used varied among the 20 studies, most involved aerobic training, and some also used resistance training, Dr. Ciani said. She said she plans additional analyses of the data she has collected to examine the impact of exercise in heart failure patients on cardiovascular mortality, heart failure hospitalization, and a combined endpoint of all-cause death and all-cause hospitalization.
On Twitter @mitchelzoler
AT HEART FAILURE 2016
Key clinical point: A meta-analysis of 20 randomized controlled studies confirmed that an exercise training intervention in heart failure patients significantly reduces mortality and hospitalizations.
Major finding: All-cause mortality fell by a relative 18% in heart failure patients who underwent exercise training, compared with controls.
Data source: Individual patient data meta-analysis for 4,043 patients from 20 studies.
Disclosures: Dr. Ciani had no relevant financial disclosures.
Crossing your ‘t’s: Practice policies for the private practitioner
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Developing your practice policies and sharing them with your patients is essential to building long-term, trusting relationships. Having a clear starting point helps avert disagreement down the road and allows patients to feel comfortable knowing what they are getting in to, which will provide a foundation on which you and the patient can focus on clinical matters.
What’s in a policy?
Policies should cover administrative aspects of care, such as mandated disclosures; relevant Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act information; hospital privilege status; and fees and payment policies. Your policies also will touch on areas where business overlaps with patient care, such as confidentiality and its limits, communication methods outside of session, and the risks and benefits of treatment (Table).
Address communication and billing policies for complex scenarios. Although these scenarios might not come up often, if you wait until you are confronted with the situation, the patient might (rightly) feel that she (he) wasn’t properly informed before giving consent. For example:
- For college students. Do you try to build college students’ autonomy by sending them all billing statements directly? If not, how will you handle the diagnosis code that appears on the statement, which their parents could see? What if the student doesn’t act on the statements—will you start mailing them to the parents? Should you mandate that you be able to talk with their parents?
- For adolescents. Consider whether you will allow them to communicate with you directly. Will they be able to e-mail you? How will you communicate with her (his) parents if your relationship is primarily with the teenager? How will you handle medication changes when the teenager prefers you keep everything private, but the parents have the right to informed consent?
- Will you charge for the time it takes you to talk with other providers (CPT 90887); review reports (CPT 90885); for e-mails or phone calls that are only a minute, or 10 minutes (e-mail, CPT 99444; brief phone calls, CPT 99441); or out-of-session refills? What if an insurance company does, or doesn’t, cover these codes? Is it different for patients you see occasionally for medication checks and for those whom you see weekly for therapy?
Psychodynamics of policies
Nowhere does being both a business and a service intersect more than when discussing how much you charge, and for what services. Patients may have little understanding of all the time you spend on their care, and why you choose to bill or not to bill for certain services. They could naturally develop transference reactions based on your policies, or might not even read them and just sign off, which also can give you useful clinical data.
Patients should review and accept your policies before the first appointment is booked. However, it is still meaningful to extend the opportunity to discuss them with a patient at the first session—but if they do not want to ask questions or discuss administrative matters, then follow their lead. By at least offering, this conveys to the patient that you wish to develop a trusting relationship, and that you are open to addressing conflicts or confusion at the beginning.
A valuable investment in time
Spending a bit of time now to create or review your current policies will save a lot of time—and perhaps money or legal action—later. If you can’t think of every scenario or issue today, don’t fret. Your experience in practice will inevitably lead you to recalibrate and update your policies. What’s most important is that your patients know where you stand and that they can trust you over the long-term.
Atrial Fibrillation and Stroke May Be Temporally Related
CHICAGO—One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the previous 30 days, Rhea C. Pimentel, MD, said at the 65th Annual Meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) patients with this event died during their stroke hospitalization, compared with six of 83 (7%) patients whose strokes were not temporally related to atrial fibrillation, said Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients with atrial fibrillation entails about double the mortality rate of strokes in patients without atrial fibrillation. Mortality associated with atrial fibrillation–related stroke in the study was probably much higher because the hospital serves as a comprehensive stroke center and admits patients from across the Midwest, she said.
Dr. Pimentel reported data on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This study is described as the largest patient series ever reported. Patients’ mean age was 73, and 41% were women. The mean CHADS2 score was 3.96 and the mean CHA2DS2-VASc score was 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of atrial fibrillation, and a fifth were on an oral anticoagulant—warfarin, in 70% of cases—at the time of their stroke.
Investigators defined a stroke-related atrial fibrillation episode as a total of at least one hour spent in atrial fibrillation at 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal atrial fibrillation. They typically fulfilled the one-hour atrial fibrillation requirement with multiple short, self-terminated episodes rather than with an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality rate, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel presented the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into five-day segments to learn how soon after an atrial fibrillation episode the strokes occurred. Researchers at Stanford University have reported that the greatest stroke risk in patients with atrial fibrillation occurs during the first five days after an atrial fibrillation episode. Dr. Pimentel’s group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of atrial fibrillation is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of six minutes of atrial fibrillation rather than one hour during the 30 days prior to stroke.
—Bruce Jancin
CHICAGO—One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the previous 30 days, Rhea C. Pimentel, MD, said at the 65th Annual Meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) patients with this event died during their stroke hospitalization, compared with six of 83 (7%) patients whose strokes were not temporally related to atrial fibrillation, said Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients with atrial fibrillation entails about double the mortality rate of strokes in patients without atrial fibrillation. Mortality associated with atrial fibrillation–related stroke in the study was probably much higher because the hospital serves as a comprehensive stroke center and admits patients from across the Midwest, she said.
Dr. Pimentel reported data on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This study is described as the largest patient series ever reported. Patients’ mean age was 73, and 41% were women. The mean CHADS2 score was 3.96 and the mean CHA2DS2-VASc score was 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of atrial fibrillation, and a fifth were on an oral anticoagulant—warfarin, in 70% of cases—at the time of their stroke.
Investigators defined a stroke-related atrial fibrillation episode as a total of at least one hour spent in atrial fibrillation at 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal atrial fibrillation. They typically fulfilled the one-hour atrial fibrillation requirement with multiple short, self-terminated episodes rather than with an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality rate, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel presented the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into five-day segments to learn how soon after an atrial fibrillation episode the strokes occurred. Researchers at Stanford University have reported that the greatest stroke risk in patients with atrial fibrillation occurs during the first five days after an atrial fibrillation episode. Dr. Pimentel’s group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of atrial fibrillation is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of six minutes of atrial fibrillation rather than one hour during the 30 days prior to stroke.
—Bruce Jancin
CHICAGO—One-third of a large cohort of patients with an implantable cardiac device in place at the time of an ischemic stroke had one or more episodes of atrial fibrillation within the previous 30 days, Rhea C. Pimentel, MD, said at the 65th Annual Meeting of the American College of Cardiology.
The in-hospital mortality rate of these atrial fibrillation–related strokes was high: 11 of 42 (26%) patients with this event died during their stroke hospitalization, compared with six of 83 (7%) patients whose strokes were not temporally related to atrial fibrillation, said Dr. Pimentel, an electrophysiologist at the University of Kansas Medical Center in Kansas City.
Data from the Framingham Heart Study and other sources suggest that stroke in patients with atrial fibrillation entails about double the mortality rate of strokes in patients without atrial fibrillation. Mortality associated with atrial fibrillation–related stroke in the study was probably much higher because the hospital serves as a comprehensive stroke center and admits patients from across the Midwest, she said.
Dr. Pimentel reported data on 125 patients who presented with an ischemic stroke when a cardiac monitoring device was in place. This study is described as the largest patient series ever reported. Patients’ mean age was 73, and 41% were women. The mean CHADS2 score was 3.96 and the mean CHA2DS2-VASc score was 5.28. Of the patients, 62% had a pacemaker; the rest had an implantable cardioverter-defibrillator or cardiac resynchronization device. One-quarter of the group had a prior history of atrial fibrillation, and a fifth were on an oral anticoagulant—warfarin, in 70% of cases—at the time of their stroke.
Investigators defined a stroke-related atrial fibrillation episode as a total of at least one hour spent in atrial fibrillation at 30 days preceding the stroke. Eighty percent of affected patients had paroxysmal atrial fibrillation. They typically fulfilled the one-hour atrial fibrillation requirement with multiple short, self-terminated episodes rather than with an hour-long episode.
Being on an oral anticoagulant had no impact on in-hospital mortality rate, which was 14.2% in patients on warfarin or a newer anticoagulant and 14.3% in those who were not. Dr. Pimentel presented the results of the investigators’ initial look at the data. They are in the process of obtaining the patients’ international normalized ratio data, which “should be enlightening,” she said.
She and her coinvestigators also plan to subdivide their 30-day study period into five-day segments to learn how soon after an atrial fibrillation episode the strokes occurred. Researchers at Stanford University have reported that the greatest stroke risk in patients with atrial fibrillation occurs during the first five days after an atrial fibrillation episode. Dr. Pimentel’s group would like to confirm that observation.
In addition, because it remains an unresolved question whether any amount of atrial fibrillation is safe, Dr. Pimentel and her coworkers are considering reanalyzing their data using a cutoff of six minutes of atrial fibrillation rather than one hour during the 30 days prior to stroke.
—Bruce Jancin