User login
Active Surveillance is Safe Treatment Option for Low-Risk Prostate Cancer: PRIAS Study
NEW YORK - After a decade of follow-up in the Prostate Cancer Research International Active Surveillance (PRIAS) study, researchers have confirmed that active surveillance is a safe treatment option for men with low-risk prostate cancer.
"However," they say, "some changes could be made to the follow-up protocol to safely increase the number of men who remain on active surveillance."
Dr. Leonard P. Bokhorst of Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues analyzed data on more than 5,000 men in 18 countries followed in PRIAS, including 622 who were followed more than five years and 107 were followed more than 7.5 years.
The median age at diagnosis was 65.9 years.
Original inclusion criteria included Gleason score 3+3 or lower, stage cT2c or lower, and prostate-specific antigen (PSA) 10 ng/ml or lower. PSA tests were scheduled every three months and digital rectal examinations were scheduled every six months during the first two years of study, then less often later. Criteria to recommend starting active treatment include Gleason score higher than 3+3, more than two positive cores, and stage higher than cT2.
Median PSA for the study population was 5.7 ng/ml. Most men (69%) had one positive biopsy core, with Gleason score of 3+3 (99%) and a clinical stage of T1c (88%).
Almost 3,400 men received at least one repeat biopsy during follow-up and some received up to five biopsies.
Overall, 1,768 men discontinued active surveillance during follow-up, 1,102 due to protocol-based reclassification. Other reasons for discontinuations included anxiety, switch to watchful waiting, death, and loss to follow-up. Two-thirds (67%) of the men were treated with either radical prostatectomy or radiation therapy after discontinuation. Only 3% received hormonal therapy.
Almost half (48%) of the patients were still on active surveillance after five years and 27% were on active surveillance at 10 years, the researchers reported online June 19 in European Urology.
The team had pathology data on 360 men who had radical prostatectomy. Of the men who switched to treatment because of protocol-based reclassification, 82 (30%) had favorable pathological outcomes, 85 (34%) had intermediate outcomes, and 100 (36%) had unfavorable outcomes.
Mortality from prostate cancer was less than 1% during follow-up.
Because of the higher rate of unfavorable treatment outcomes, the researchers recommended a change in the PRIAS protocol.
"Instead of an immediate switch to active treatment if more than two cores are positive, men should receive further investigation to confirm higher risk disease," the researchers write. They recommend examination by magnetic resonance imaging because its negative predictive value for Gleason upgrading is near 100%.
They concluded, "Criteria used to recommend a switch to active treatment do not seem selective enough to avoid unnecessary switches to active treatment."
Dr. Bokhorst did not respond to a request for comment.
The Prostate Cancer Research Foundation, Rotterdam, supports the PRIAS study. The authors made no disclosures.
SOURCE: http://bit.ly/28Q5Cd3
Eur Urol 2016.
NEW YORK - After a decade of follow-up in the Prostate Cancer Research International Active Surveillance (PRIAS) study, researchers have confirmed that active surveillance is a safe treatment option for men with low-risk prostate cancer.
"However," they say, "some changes could be made to the follow-up protocol to safely increase the number of men who remain on active surveillance."
Dr. Leonard P. Bokhorst of Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues analyzed data on more than 5,000 men in 18 countries followed in PRIAS, including 622 who were followed more than five years and 107 were followed more than 7.5 years.
The median age at diagnosis was 65.9 years.
Original inclusion criteria included Gleason score 3+3 or lower, stage cT2c or lower, and prostate-specific antigen (PSA) 10 ng/ml or lower. PSA tests were scheduled every three months and digital rectal examinations were scheduled every six months during the first two years of study, then less often later. Criteria to recommend starting active treatment include Gleason score higher than 3+3, more than two positive cores, and stage higher than cT2.
Median PSA for the study population was 5.7 ng/ml. Most men (69%) had one positive biopsy core, with Gleason score of 3+3 (99%) and a clinical stage of T1c (88%).
Almost 3,400 men received at least one repeat biopsy during follow-up and some received up to five biopsies.
Overall, 1,768 men discontinued active surveillance during follow-up, 1,102 due to protocol-based reclassification. Other reasons for discontinuations included anxiety, switch to watchful waiting, death, and loss to follow-up. Two-thirds (67%) of the men were treated with either radical prostatectomy or radiation therapy after discontinuation. Only 3% received hormonal therapy.
Almost half (48%) of the patients were still on active surveillance after five years and 27% were on active surveillance at 10 years, the researchers reported online June 19 in European Urology.
The team had pathology data on 360 men who had radical prostatectomy. Of the men who switched to treatment because of protocol-based reclassification, 82 (30%) had favorable pathological outcomes, 85 (34%) had intermediate outcomes, and 100 (36%) had unfavorable outcomes.
Mortality from prostate cancer was less than 1% during follow-up.
Because of the higher rate of unfavorable treatment outcomes, the researchers recommended a change in the PRIAS protocol.
"Instead of an immediate switch to active treatment if more than two cores are positive, men should receive further investigation to confirm higher risk disease," the researchers write. They recommend examination by magnetic resonance imaging because its negative predictive value for Gleason upgrading is near 100%.
They concluded, "Criteria used to recommend a switch to active treatment do not seem selective enough to avoid unnecessary switches to active treatment."
Dr. Bokhorst did not respond to a request for comment.
The Prostate Cancer Research Foundation, Rotterdam, supports the PRIAS study. The authors made no disclosures.
SOURCE: http://bit.ly/28Q5Cd3
Eur Urol 2016.
NEW YORK - After a decade of follow-up in the Prostate Cancer Research International Active Surveillance (PRIAS) study, researchers have confirmed that active surveillance is a safe treatment option for men with low-risk prostate cancer.
"However," they say, "some changes could be made to the follow-up protocol to safely increase the number of men who remain on active surveillance."
Dr. Leonard P. Bokhorst of Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues analyzed data on more than 5,000 men in 18 countries followed in PRIAS, including 622 who were followed more than five years and 107 were followed more than 7.5 years.
The median age at diagnosis was 65.9 years.
Original inclusion criteria included Gleason score 3+3 or lower, stage cT2c or lower, and prostate-specific antigen (PSA) 10 ng/ml or lower. PSA tests were scheduled every three months and digital rectal examinations were scheduled every six months during the first two years of study, then less often later. Criteria to recommend starting active treatment include Gleason score higher than 3+3, more than two positive cores, and stage higher than cT2.
Median PSA for the study population was 5.7 ng/ml. Most men (69%) had one positive biopsy core, with Gleason score of 3+3 (99%) and a clinical stage of T1c (88%).
Almost 3,400 men received at least one repeat biopsy during follow-up and some received up to five biopsies.
Overall, 1,768 men discontinued active surveillance during follow-up, 1,102 due to protocol-based reclassification. Other reasons for discontinuations included anxiety, switch to watchful waiting, death, and loss to follow-up. Two-thirds (67%) of the men were treated with either radical prostatectomy or radiation therapy after discontinuation. Only 3% received hormonal therapy.
Almost half (48%) of the patients were still on active surveillance after five years and 27% were on active surveillance at 10 years, the researchers reported online June 19 in European Urology.
The team had pathology data on 360 men who had radical prostatectomy. Of the men who switched to treatment because of protocol-based reclassification, 82 (30%) had favorable pathological outcomes, 85 (34%) had intermediate outcomes, and 100 (36%) had unfavorable outcomes.
Mortality from prostate cancer was less than 1% during follow-up.
Because of the higher rate of unfavorable treatment outcomes, the researchers recommended a change in the PRIAS protocol.
"Instead of an immediate switch to active treatment if more than two cores are positive, men should receive further investigation to confirm higher risk disease," the researchers write. They recommend examination by magnetic resonance imaging because its negative predictive value for Gleason upgrading is near 100%.
They concluded, "Criteria used to recommend a switch to active treatment do not seem selective enough to avoid unnecessary switches to active treatment."
Dr. Bokhorst did not respond to a request for comment.
The Prostate Cancer Research Foundation, Rotterdam, supports the PRIAS study. The authors made no disclosures.
SOURCE: http://bit.ly/28Q5Cd3
Eur Urol 2016.
Most women conceive within 5 years of starting fertility treatment
HELSINKI, FINLAND – The overall birth rate within 5 years of initiating fertility treatment with homologous gametes was 71% among nearly 20,000 women in a Danish assisted reproductive technology registry.
Conception occurred after treatment in 57% of the women, and conception was spontaneous in 14%, Sara Malchau, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings allow physicians to give couples a comprehensible, age-stratified, long-term prognosis at the start of treatment, said Dr. Malchau of Copenhagen University Hospital, Hvidovre, Denmark.
To assess long-term outcomes, Dr. Malchau and her colleagues identified all women in the mandatory Danish ART Registry who initiated fertility treatments with homologous gametes in pubic and private clinics in Denmark between 1997 and 2010. The 19,884 subjects’ treatment cycles were cross-linked with the country’s Medical Birth Registry to identify live births after treatment and spontaneous conception. Follow-up was available for up to 2 years for all of the women, up to 3 years for 14,445 women, and up to 5 years for 5,165 women.
The total live birth rates at 2, 3, and 5 years after the first treatment using ART or intrauterine insemination (IUI) were 57%, 65% and 71%, respectively. Dr. Malchau reported.
In women who had ART as the first treatment, the corresponding ART-conception live birth rates were 46.1%, 51.1%, and 52.9%, and the birth rates with spontaneous conception and IUI after 5 years were 11.2% and 0.6%, respectively.
In those with IUI first, 34.2% delivered after IUI conceptions within 2 years, with no significant increase in birth rates after 3 and 5 years. Shifting to ART in these women resulted in birth rates for ART conceptions of 15.1%, 21.1%, and 23.7% after 2, 3, and 5 years, respectively. After 5 years, the birth rate from spontaneous conception in all women starting treatment with IUI was 16.6%, she noted.
Stratification by age showed that the birth rates at 5 years were 80% for women under age 35 years, 60.5% for those aged 35-40 years, and 26.2% for those aged 40 and older.
While the national ART registry in Denmark is compulsory, underreporting can occur. However, it is cycle based, and since most women have repeated treatments, is it unlikely that the number of women with no birth was underestimated in this study, Dr. Malchau noted.
The findings – which reflect the Danish treatment strategy of three to six cycles of IUI followed by ART, explaining why birth rates after IUI did not increase after 2 years – help answer important patient questions, she said.
“Infertility patients have two key questions: What are our chances of having a baby and when will it happen?” said Dr. Malchau. “Overall chances of a live birth are good, but successful treatment takes time. Couples will often need several treatment cycles. And even though the greatest chance of conception is following treatment, there is still a reasonable chance of spontaneous conception.”
Spontaneous conception is most common in women under age 35 starting IUI (18% vs. 8% in those over 35 starting IVF treatment).
The findings are “robust and realistic,” and since those undergoing treatment have no idea how many treatment cycles they will need or have, a “prognosis based on fixed points in time better reflects their prospect of conception and delivery than birth rates after different numbers of attempts,” Dr. Malchau said.
Copenhagen University Hospital Hvidovre and Ferring Pharmaceuticals funded the study.
HELSINKI, FINLAND – The overall birth rate within 5 years of initiating fertility treatment with homologous gametes was 71% among nearly 20,000 women in a Danish assisted reproductive technology registry.
Conception occurred after treatment in 57% of the women, and conception was spontaneous in 14%, Sara Malchau, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings allow physicians to give couples a comprehensible, age-stratified, long-term prognosis at the start of treatment, said Dr. Malchau of Copenhagen University Hospital, Hvidovre, Denmark.
To assess long-term outcomes, Dr. Malchau and her colleagues identified all women in the mandatory Danish ART Registry who initiated fertility treatments with homologous gametes in pubic and private clinics in Denmark between 1997 and 2010. The 19,884 subjects’ treatment cycles were cross-linked with the country’s Medical Birth Registry to identify live births after treatment and spontaneous conception. Follow-up was available for up to 2 years for all of the women, up to 3 years for 14,445 women, and up to 5 years for 5,165 women.
The total live birth rates at 2, 3, and 5 years after the first treatment using ART or intrauterine insemination (IUI) were 57%, 65% and 71%, respectively. Dr. Malchau reported.
In women who had ART as the first treatment, the corresponding ART-conception live birth rates were 46.1%, 51.1%, and 52.9%, and the birth rates with spontaneous conception and IUI after 5 years were 11.2% and 0.6%, respectively.
In those with IUI first, 34.2% delivered after IUI conceptions within 2 years, with no significant increase in birth rates after 3 and 5 years. Shifting to ART in these women resulted in birth rates for ART conceptions of 15.1%, 21.1%, and 23.7% after 2, 3, and 5 years, respectively. After 5 years, the birth rate from spontaneous conception in all women starting treatment with IUI was 16.6%, she noted.
Stratification by age showed that the birth rates at 5 years were 80% for women under age 35 years, 60.5% for those aged 35-40 years, and 26.2% for those aged 40 and older.
While the national ART registry in Denmark is compulsory, underreporting can occur. However, it is cycle based, and since most women have repeated treatments, is it unlikely that the number of women with no birth was underestimated in this study, Dr. Malchau noted.
The findings – which reflect the Danish treatment strategy of three to six cycles of IUI followed by ART, explaining why birth rates after IUI did not increase after 2 years – help answer important patient questions, she said.
“Infertility patients have two key questions: What are our chances of having a baby and when will it happen?” said Dr. Malchau. “Overall chances of a live birth are good, but successful treatment takes time. Couples will often need several treatment cycles. And even though the greatest chance of conception is following treatment, there is still a reasonable chance of spontaneous conception.”
Spontaneous conception is most common in women under age 35 starting IUI (18% vs. 8% in those over 35 starting IVF treatment).
The findings are “robust and realistic,” and since those undergoing treatment have no idea how many treatment cycles they will need or have, a “prognosis based on fixed points in time better reflects their prospect of conception and delivery than birth rates after different numbers of attempts,” Dr. Malchau said.
Copenhagen University Hospital Hvidovre and Ferring Pharmaceuticals funded the study.
HELSINKI, FINLAND – The overall birth rate within 5 years of initiating fertility treatment with homologous gametes was 71% among nearly 20,000 women in a Danish assisted reproductive technology registry.
Conception occurred after treatment in 57% of the women, and conception was spontaneous in 14%, Sara Malchau, MD, reported at the annual meeting of the European Society of Human Reproduction and Embryology.
The findings allow physicians to give couples a comprehensible, age-stratified, long-term prognosis at the start of treatment, said Dr. Malchau of Copenhagen University Hospital, Hvidovre, Denmark.
To assess long-term outcomes, Dr. Malchau and her colleagues identified all women in the mandatory Danish ART Registry who initiated fertility treatments with homologous gametes in pubic and private clinics in Denmark between 1997 and 2010. The 19,884 subjects’ treatment cycles were cross-linked with the country’s Medical Birth Registry to identify live births after treatment and spontaneous conception. Follow-up was available for up to 2 years for all of the women, up to 3 years for 14,445 women, and up to 5 years for 5,165 women.
The total live birth rates at 2, 3, and 5 years after the first treatment using ART or intrauterine insemination (IUI) were 57%, 65% and 71%, respectively. Dr. Malchau reported.
In women who had ART as the first treatment, the corresponding ART-conception live birth rates were 46.1%, 51.1%, and 52.9%, and the birth rates with spontaneous conception and IUI after 5 years were 11.2% and 0.6%, respectively.
In those with IUI first, 34.2% delivered after IUI conceptions within 2 years, with no significant increase in birth rates after 3 and 5 years. Shifting to ART in these women resulted in birth rates for ART conceptions of 15.1%, 21.1%, and 23.7% after 2, 3, and 5 years, respectively. After 5 years, the birth rate from spontaneous conception in all women starting treatment with IUI was 16.6%, she noted.
Stratification by age showed that the birth rates at 5 years were 80% for women under age 35 years, 60.5% for those aged 35-40 years, and 26.2% for those aged 40 and older.
While the national ART registry in Denmark is compulsory, underreporting can occur. However, it is cycle based, and since most women have repeated treatments, is it unlikely that the number of women with no birth was underestimated in this study, Dr. Malchau noted.
The findings – which reflect the Danish treatment strategy of three to six cycles of IUI followed by ART, explaining why birth rates after IUI did not increase after 2 years – help answer important patient questions, she said.
“Infertility patients have two key questions: What are our chances of having a baby and when will it happen?” said Dr. Malchau. “Overall chances of a live birth are good, but successful treatment takes time. Couples will often need several treatment cycles. And even though the greatest chance of conception is following treatment, there is still a reasonable chance of spontaneous conception.”
Spontaneous conception is most common in women under age 35 starting IUI (18% vs. 8% in those over 35 starting IVF treatment).
The findings are “robust and realistic,” and since those undergoing treatment have no idea how many treatment cycles they will need or have, a “prognosis based on fixed points in time better reflects their prospect of conception and delivery than birth rates after different numbers of attempts,” Dr. Malchau said.
Copenhagen University Hospital Hvidovre and Ferring Pharmaceuticals funded the study.
AT ESHRE 2016
Key clinical point: The overall birth rate within 5 years of starting fertility treatment with homologous gametes was more than 70%.
Major finding: Within 5 years, conception occurred after treatment in 57% of the women, and conception was spontaneous in 14%.
Data source: A population-based cohort study involving 19,884 Danish women.
Disclosures: Copenhagen University Hospital Hvidovre and Ferring Pharmaceuticals funded the study.
High rates of early complications seen in youth with diabetes
NEW ORLEANS – There is a high burden of early complications among young adults with type 1 or type 2 diabetes, results from an ongoing study demonstrated.
“We are witnessing a recent increase in type 2 diabetes in the pediatric U.S. population, paralleling a somewhat more established rise in type 1 diabetes among youth worldwide,” Dana Dabelea, MD, PhD, said at the annual scientific sessions of the American Diabetes Association. “In the face of this increasing disease burden, we are left with limited information on the consequences of having diabetes on these youth, specifically the burden of diabetes-related chronic complications. No study exists in the U.S. that is able to compare this burden in youth with type 2 versus those with type 1 diabetes. Prior studies elsewhere worldwide have used mostly medical records or administrative data, have small sample sizes, and include youth with variable disease duration.”
The aim of the current study was to assess and compare the prevalence of several complications in youth with either type 1 or type 2 diabetes of similar age and disease (short) duration, and to explore the potential risk factors for observed differences by diabetes type. Dr. Dabelea of the department of epidemiology at the University of Colorado School of Public Health in Denver, and her colleagues from the SEARCH for Diabetes in Youth Study, developed a cohort of 1,746 individuals with type 1 and 272 with type 2 diabetes, diagnosed when younger than age 20, assembled from the population-based SEARCH Registry in five U.S. sites, and registered upon diagnosis between 2002 and 2008. The most recent visit was between 2011 and 2015 when participants were at least 10 years of age.
Outcomes, measured once at the cohort visit between 2010 and 2015, included diabetic kidney disease, diabetic retinopathy, peripheral neuropathy, cardiovascular autonomic neuropathy, arterial stiffness, and hypertension.
Dr. Dabelea reported that 32% of youth with type 1 diabetes and 72% of those with type 2 diabetes had at least one early complication. For all complications, except cardiovascular autonomic neuropathy, the prevalence was significantly higher in those with type 2 diabetes, compared with those who had type 1 disease, with a similar pattern by race, especially for minority youth. Estimates were also usually higher within type among minority vs. non-white Hispanic youth, especially for type 2 diabetes. The findings “indicate a need for heightened clinical suspicion and detection of early complications, together with aggressive risk factor control, especially in type 2 diabetes and minority youth,” she concluded.
The Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Dabelea reported having no financial disclosures.
NEW ORLEANS – There is a high burden of early complications among young adults with type 1 or type 2 diabetes, results from an ongoing study demonstrated.
“We are witnessing a recent increase in type 2 diabetes in the pediatric U.S. population, paralleling a somewhat more established rise in type 1 diabetes among youth worldwide,” Dana Dabelea, MD, PhD, said at the annual scientific sessions of the American Diabetes Association. “In the face of this increasing disease burden, we are left with limited information on the consequences of having diabetes on these youth, specifically the burden of diabetes-related chronic complications. No study exists in the U.S. that is able to compare this burden in youth with type 2 versus those with type 1 diabetes. Prior studies elsewhere worldwide have used mostly medical records or administrative data, have small sample sizes, and include youth with variable disease duration.”
The aim of the current study was to assess and compare the prevalence of several complications in youth with either type 1 or type 2 diabetes of similar age and disease (short) duration, and to explore the potential risk factors for observed differences by diabetes type. Dr. Dabelea of the department of epidemiology at the University of Colorado School of Public Health in Denver, and her colleagues from the SEARCH for Diabetes in Youth Study, developed a cohort of 1,746 individuals with type 1 and 272 with type 2 diabetes, diagnosed when younger than age 20, assembled from the population-based SEARCH Registry in five U.S. sites, and registered upon diagnosis between 2002 and 2008. The most recent visit was between 2011 and 2015 when participants were at least 10 years of age.
Outcomes, measured once at the cohort visit between 2010 and 2015, included diabetic kidney disease, diabetic retinopathy, peripheral neuropathy, cardiovascular autonomic neuropathy, arterial stiffness, and hypertension.
Dr. Dabelea reported that 32% of youth with type 1 diabetes and 72% of those with type 2 diabetes had at least one early complication. For all complications, except cardiovascular autonomic neuropathy, the prevalence was significantly higher in those with type 2 diabetes, compared with those who had type 1 disease, with a similar pattern by race, especially for minority youth. Estimates were also usually higher within type among minority vs. non-white Hispanic youth, especially for type 2 diabetes. The findings “indicate a need for heightened clinical suspicion and detection of early complications, together with aggressive risk factor control, especially in type 2 diabetes and minority youth,” she concluded.
The Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Dabelea reported having no financial disclosures.
NEW ORLEANS – There is a high burden of early complications among young adults with type 1 or type 2 diabetes, results from an ongoing study demonstrated.
“We are witnessing a recent increase in type 2 diabetes in the pediatric U.S. population, paralleling a somewhat more established rise in type 1 diabetes among youth worldwide,” Dana Dabelea, MD, PhD, said at the annual scientific sessions of the American Diabetes Association. “In the face of this increasing disease burden, we are left with limited information on the consequences of having diabetes on these youth, specifically the burden of diabetes-related chronic complications. No study exists in the U.S. that is able to compare this burden in youth with type 2 versus those with type 1 diabetes. Prior studies elsewhere worldwide have used mostly medical records or administrative data, have small sample sizes, and include youth with variable disease duration.”
The aim of the current study was to assess and compare the prevalence of several complications in youth with either type 1 or type 2 diabetes of similar age and disease (short) duration, and to explore the potential risk factors for observed differences by diabetes type. Dr. Dabelea of the department of epidemiology at the University of Colorado School of Public Health in Denver, and her colleagues from the SEARCH for Diabetes in Youth Study, developed a cohort of 1,746 individuals with type 1 and 272 with type 2 diabetes, diagnosed when younger than age 20, assembled from the population-based SEARCH Registry in five U.S. sites, and registered upon diagnosis between 2002 and 2008. The most recent visit was between 2011 and 2015 when participants were at least 10 years of age.
Outcomes, measured once at the cohort visit between 2010 and 2015, included diabetic kidney disease, diabetic retinopathy, peripheral neuropathy, cardiovascular autonomic neuropathy, arterial stiffness, and hypertension.
Dr. Dabelea reported that 32% of youth with type 1 diabetes and 72% of those with type 2 diabetes had at least one early complication. For all complications, except cardiovascular autonomic neuropathy, the prevalence was significantly higher in those with type 2 diabetes, compared with those who had type 1 disease, with a similar pattern by race, especially for minority youth. Estimates were also usually higher within type among minority vs. non-white Hispanic youth, especially for type 2 diabetes. The findings “indicate a need for heightened clinical suspicion and detection of early complications, together with aggressive risk factor control, especially in type 2 diabetes and minority youth,” she concluded.
The Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Dabelea reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: There is a high prevalence of early complications in youth with either type of diabetes.
Major finding: About 32% of youths with type 1 diabetes and 72% of those with type 2 diabetes had at least one early complication.
Data source: A cohort of 1,746 individuals with type 1 and 272 with type 2 diabetes who were diagnosed before 20 years of age assembled from the population-based SEARCH Registry in five U.S. sites, and who were registered upon diagnosis between 2002 and 2008.
Disclosures: The Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. Dabelea reported having no financial disclosures.
Make the Diagnosis - July 2016
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
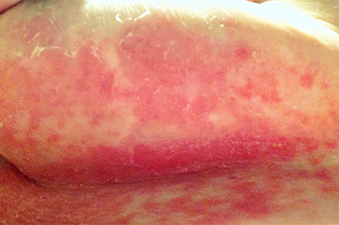
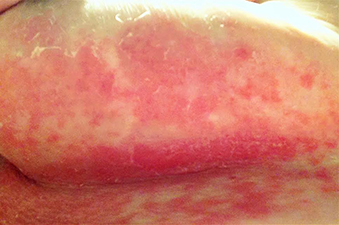
A 63-year-old woman presented with extremely painful plaques on the breasts, inframammary folds, and chest, and under the abdomen, for 3 years. The lesions had progressively increased in size with worsening pain over the past several months. She also reported painful raw areas on her genitals. Physical examination revealed multiple well-demarcated oval and geographic plaques with central white induration with areas of superficial erosion and pink peripheral borders on the breasts, inframammary folds, midchest, and abdomen that were exquisitely tender to light palpation. Genital examination revealed a vulvar white plaque with superficial erosions and an atrophic labia minora.
Cognitive Biases in Dermatology Training
As young physicians, we are taught to be as objective as possible when evaluating a patient; however, cognitive biases are regularly encountered in day-to-day patient experiences and can unfortunately influence our clinical decision-making skills to be subpar.
Consider the following case: An overweight 74-year-old man with diabetes mellitus and a nonhealing ulceration on the left lower extremity presented to the emergency department for repeat evaluation. He previously had been treated by an outside dermatologist for stasis dermatitis and was being managed with compression, elevation, and lubrication of both lower extremities. Often, the initial reaction is to conclude that the patient does in fact have an ulceration associated with stasis dermatitis and changing the management strategy or performing a biopsy would not change the outcome. However, this response limits the potential to provide the patient with a thorough examination. If the patient is treated with the same management strategies that previously failed rather than delving into all the causes for nonhealing ulceration on the left lower extremity, a vital diagnosis could be missed. In this scenario, when the patient was ultimately biopsied, the diagnosis was an ulcerative squamous cell carcinoma.
These subconscious predetermined decisions regarding difficult patient encounters come from the physician’s heuristics, a process of decision-making wherein a snap judgment about a patient occurs because it is similar to prior patient encounters or a set of views from prior knowledge of the disease.1,2
A recent article by Cohen and Burgin3 elucidated a set of cognitive biases that often are encountered in dermatology practices, including affective, anchoring, availability, and confirmation biases; zebra retreat; and Sutton’s slip.
Affective Bias
Affective bias is a process in which emotions regarding a patient interaction alter the objective prospective and reasoning of a patient. For example, consider the case of a pemphigus vulgaris patient who does not want to be on prednisone due to weight gain and persistently presents to the dermatology clinic insisting that the physician taper the dosage. To avoid the constant frustration and upsetting the patient further, the dermatologist tapers the dosage of prednisone prematurely and the patient has a flare.
Anchoring Bias
Anchoring bias occurs when initial information regarding a patient causes one to jump to a conclusion rather than developing a thorough history. An example may be if an infant presents with a mole on the nasal dorsum that the patient’s father reports has only been present for a short while. Without performing imaging studies or asking for further history, the physician decides to biopsy the lesion. The biopsy results show a neural mass, such that a nasal glioma cannot be ruled out. In this bias, magnetic resonance imaging would have been prudent prior to biopsy and premature action.
Availability Bias
Availability bias refers to a diagnosis that immediately comes to mind, as it is common or recently encountered, such as in the example presented at the beginning of this column about the patient with squamous cell carcinoma.
Confirmation Bias
Confirmation bias caters to elucidating information that confirms your own clinical suspicion as opposed to determining the true cause of the disease etiology. Consider the following example: An obese patient presents with a history of painful sores on the bilateral lower extremities. The physician asks specifically about diabetes mellitus and mobility. When the patient answers yes to poor mobility and diabetes mellitus, the physician asks questions confirming an initial suspected diagnosis of stasis dermatitis. Unfortunately, as the patient continues to get worse, it is revealed that his medication history indicates he has been taking sulfasalazine for several years, and it is eventually determined that the patient has cutaneous Crohn disease.
Zebra Retreat
This bias describes a physician’s unwillingness to consider a diagnosis because it is very obscure, even if it is correct. For example, the case of the patient described in the previous example with a diagnosis of cutaneous Crohn disease also can be considered as an example of zebra retreat. Because the clinician may rarely think of this diagnosis due to its infrequent presentation, he/she may not consider doing a biopsy or investigate further.
Sutton’s Slip
This bias describes a situation in which a physician disregards a problem because a thorough examination is not performed, which is classically noted when physicians treat their family and friends. If asked about a mole or lesion regarding its questionable nature, a dermatologist may either disregard it or not evaluate it carefully, as the person is in a casual setting.
Final Thoughts
Although there are several other types of cognitive biases, those described here show that on several occasions, dermatologists can be swayed toward an incorrect diagnosis simply because of a subconscious thought process. Often times, such as in multiple-choice examinations, initial guesses are usually the best answers, but care has to be taken when in a clinical setting. Our patients rarely are good historians and do not present in well-written question stems. The biases emphasize that dermatologists in training should keep their minds open, focus on getting a clear and concise history, and use their knowledge as a tool to derive a well thought-out answer.
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Hicks EP, Kluemper GT. Heuristic reasoning and cognitive biases: are they hindrances to judgments and decision making in orthodontics? Am J Orthod Dentofacial Orthop. 2011;139:297-304.
3. Cohen JM, Burgin S. Cognitive biases in clinical decision making: a primer for the practicing dermatologist. JAMA Dermatol. 2016;152:253-254.
As young physicians, we are taught to be as objective as possible when evaluating a patient; however, cognitive biases are regularly encountered in day-to-day patient experiences and can unfortunately influence our clinical decision-making skills to be subpar.
Consider the following case: An overweight 74-year-old man with diabetes mellitus and a nonhealing ulceration on the left lower extremity presented to the emergency department for repeat evaluation. He previously had been treated by an outside dermatologist for stasis dermatitis and was being managed with compression, elevation, and lubrication of both lower extremities. Often, the initial reaction is to conclude that the patient does in fact have an ulceration associated with stasis dermatitis and changing the management strategy or performing a biopsy would not change the outcome. However, this response limits the potential to provide the patient with a thorough examination. If the patient is treated with the same management strategies that previously failed rather than delving into all the causes for nonhealing ulceration on the left lower extremity, a vital diagnosis could be missed. In this scenario, when the patient was ultimately biopsied, the diagnosis was an ulcerative squamous cell carcinoma.
These subconscious predetermined decisions regarding difficult patient encounters come from the physician’s heuristics, a process of decision-making wherein a snap judgment about a patient occurs because it is similar to prior patient encounters or a set of views from prior knowledge of the disease.1,2
A recent article by Cohen and Burgin3 elucidated a set of cognitive biases that often are encountered in dermatology practices, including affective, anchoring, availability, and confirmation biases; zebra retreat; and Sutton’s slip.
Affective Bias
Affective bias is a process in which emotions regarding a patient interaction alter the objective prospective and reasoning of a patient. For example, consider the case of a pemphigus vulgaris patient who does not want to be on prednisone due to weight gain and persistently presents to the dermatology clinic insisting that the physician taper the dosage. To avoid the constant frustration and upsetting the patient further, the dermatologist tapers the dosage of prednisone prematurely and the patient has a flare.
Anchoring Bias
Anchoring bias occurs when initial information regarding a patient causes one to jump to a conclusion rather than developing a thorough history. An example may be if an infant presents with a mole on the nasal dorsum that the patient’s father reports has only been present for a short while. Without performing imaging studies or asking for further history, the physician decides to biopsy the lesion. The biopsy results show a neural mass, such that a nasal glioma cannot be ruled out. In this bias, magnetic resonance imaging would have been prudent prior to biopsy and premature action.
Availability Bias
Availability bias refers to a diagnosis that immediately comes to mind, as it is common or recently encountered, such as in the example presented at the beginning of this column about the patient with squamous cell carcinoma.
Confirmation Bias
Confirmation bias caters to elucidating information that confirms your own clinical suspicion as opposed to determining the true cause of the disease etiology. Consider the following example: An obese patient presents with a history of painful sores on the bilateral lower extremities. The physician asks specifically about diabetes mellitus and mobility. When the patient answers yes to poor mobility and diabetes mellitus, the physician asks questions confirming an initial suspected diagnosis of stasis dermatitis. Unfortunately, as the patient continues to get worse, it is revealed that his medication history indicates he has been taking sulfasalazine for several years, and it is eventually determined that the patient has cutaneous Crohn disease.
Zebra Retreat
This bias describes a physician’s unwillingness to consider a diagnosis because it is very obscure, even if it is correct. For example, the case of the patient described in the previous example with a diagnosis of cutaneous Crohn disease also can be considered as an example of zebra retreat. Because the clinician may rarely think of this diagnosis due to its infrequent presentation, he/she may not consider doing a biopsy or investigate further.
Sutton’s Slip
This bias describes a situation in which a physician disregards a problem because a thorough examination is not performed, which is classically noted when physicians treat their family and friends. If asked about a mole or lesion regarding its questionable nature, a dermatologist may either disregard it or not evaluate it carefully, as the person is in a casual setting.
Final Thoughts
Although there are several other types of cognitive biases, those described here show that on several occasions, dermatologists can be swayed toward an incorrect diagnosis simply because of a subconscious thought process. Often times, such as in multiple-choice examinations, initial guesses are usually the best answers, but care has to be taken when in a clinical setting. Our patients rarely are good historians and do not present in well-written question stems. The biases emphasize that dermatologists in training should keep their minds open, focus on getting a clear and concise history, and use their knowledge as a tool to derive a well thought-out answer.
As young physicians, we are taught to be as objective as possible when evaluating a patient; however, cognitive biases are regularly encountered in day-to-day patient experiences and can unfortunately influence our clinical decision-making skills to be subpar.
Consider the following case: An overweight 74-year-old man with diabetes mellitus and a nonhealing ulceration on the left lower extremity presented to the emergency department for repeat evaluation. He previously had been treated by an outside dermatologist for stasis dermatitis and was being managed with compression, elevation, and lubrication of both lower extremities. Often, the initial reaction is to conclude that the patient does in fact have an ulceration associated with stasis dermatitis and changing the management strategy or performing a biopsy would not change the outcome. However, this response limits the potential to provide the patient with a thorough examination. If the patient is treated with the same management strategies that previously failed rather than delving into all the causes for nonhealing ulceration on the left lower extremity, a vital diagnosis could be missed. In this scenario, when the patient was ultimately biopsied, the diagnosis was an ulcerative squamous cell carcinoma.
These subconscious predetermined decisions regarding difficult patient encounters come from the physician’s heuristics, a process of decision-making wherein a snap judgment about a patient occurs because it is similar to prior patient encounters or a set of views from prior knowledge of the disease.1,2
A recent article by Cohen and Burgin3 elucidated a set of cognitive biases that often are encountered in dermatology practices, including affective, anchoring, availability, and confirmation biases; zebra retreat; and Sutton’s slip.
Affective Bias
Affective bias is a process in which emotions regarding a patient interaction alter the objective prospective and reasoning of a patient. For example, consider the case of a pemphigus vulgaris patient who does not want to be on prednisone due to weight gain and persistently presents to the dermatology clinic insisting that the physician taper the dosage. To avoid the constant frustration and upsetting the patient further, the dermatologist tapers the dosage of prednisone prematurely and the patient has a flare.
Anchoring Bias
Anchoring bias occurs when initial information regarding a patient causes one to jump to a conclusion rather than developing a thorough history. An example may be if an infant presents with a mole on the nasal dorsum that the patient’s father reports has only been present for a short while. Without performing imaging studies or asking for further history, the physician decides to biopsy the lesion. The biopsy results show a neural mass, such that a nasal glioma cannot be ruled out. In this bias, magnetic resonance imaging would have been prudent prior to biopsy and premature action.
Availability Bias
Availability bias refers to a diagnosis that immediately comes to mind, as it is common or recently encountered, such as in the example presented at the beginning of this column about the patient with squamous cell carcinoma.
Confirmation Bias
Confirmation bias caters to elucidating information that confirms your own clinical suspicion as opposed to determining the true cause of the disease etiology. Consider the following example: An obese patient presents with a history of painful sores on the bilateral lower extremities. The physician asks specifically about diabetes mellitus and mobility. When the patient answers yes to poor mobility and diabetes mellitus, the physician asks questions confirming an initial suspected diagnosis of stasis dermatitis. Unfortunately, as the patient continues to get worse, it is revealed that his medication history indicates he has been taking sulfasalazine for several years, and it is eventually determined that the patient has cutaneous Crohn disease.
Zebra Retreat
This bias describes a physician’s unwillingness to consider a diagnosis because it is very obscure, even if it is correct. For example, the case of the patient described in the previous example with a diagnosis of cutaneous Crohn disease also can be considered as an example of zebra retreat. Because the clinician may rarely think of this diagnosis due to its infrequent presentation, he/she may not consider doing a biopsy or investigate further.
Sutton’s Slip
This bias describes a situation in which a physician disregards a problem because a thorough examination is not performed, which is classically noted when physicians treat their family and friends. If asked about a mole or lesion regarding its questionable nature, a dermatologist may either disregard it or not evaluate it carefully, as the person is in a casual setting.
Final Thoughts
Although there are several other types of cognitive biases, those described here show that on several occasions, dermatologists can be swayed toward an incorrect diagnosis simply because of a subconscious thought process. Often times, such as in multiple-choice examinations, initial guesses are usually the best answers, but care has to be taken when in a clinical setting. Our patients rarely are good historians and do not present in well-written question stems. The biases emphasize that dermatologists in training should keep their minds open, focus on getting a clear and concise history, and use their knowledge as a tool to derive a well thought-out answer.
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Hicks EP, Kluemper GT. Heuristic reasoning and cognitive biases: are they hindrances to judgments and decision making in orthodontics? Am J Orthod Dentofacial Orthop. 2011;139:297-304.
3. Cohen JM, Burgin S. Cognitive biases in clinical decision making: a primer for the practicing dermatologist. JAMA Dermatol. 2016;152:253-254.
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Hicks EP, Kluemper GT. Heuristic reasoning and cognitive biases: are they hindrances to judgments and decision making in orthodontics? Am J Orthod Dentofacial Orthop. 2011;139:297-304.
3. Cohen JM, Burgin S. Cognitive biases in clinical decision making: a primer for the practicing dermatologist. JAMA Dermatol. 2016;152:253-254.
Register for October Coding Course
Registration is open for the Coding and Reimbursement Workshop, to be held Oct. 21-22, 2016, at the Millennium Knickerbocker Hotel in Chicago.
The workshop addresses the 2016 updates, including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
The course also will focus on reporting standards for interventional and open surgical procedures, plus includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement.
Registration is open for the Coding and Reimbursement Workshop, to be held Oct. 21-22, 2016, at the Millennium Knickerbocker Hotel in Chicago.
The workshop addresses the 2016 updates, including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
The course also will focus on reporting standards for interventional and open surgical procedures, plus includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement.
Registration is open for the Coding and Reimbursement Workshop, to be held Oct. 21-22, 2016, at the Millennium Knickerbocker Hotel in Chicago.
The workshop addresses the 2016 updates, including changes to endovascular stent placement outside the lower extremity and PQRS as well as coding for intravascular embolization and retrograde intrathoracic carotid stenting.
The course also will focus on reporting standards for interventional and open surgical procedures, plus includes information about the global surgical package and how it impacts billing and reimbursement, along with the application of modifiers for streamlined reimbursement.
New PAD Reporting Standards Announced
Endovascular treatments are the focus
The Society for Vascular Surgery has released new reporting standards focused on endovascular treatment of chronic lower extremity peripheral artery disease.
Recommended reporting standards for lower extremity ischemia were last published by the SVS in 1997.
The variety of endovascular devices and techniques to treat occlusive disease has exploded over the past 10 years and critical evaluation of the reported results may be problematic.
This document clarifies and updates the 1997 standards, specifically for reports on endovascular treatment. The document is divided into sections: Claudication Reporting, Critical Limb Ischemia Reporting, Preintervention Assessment and Nonanatomic Treatment, Intervention, Outcome Measures – Procedural, Outcome Measures – Disease Specific and Complications.
The linked document provides a summary for reporting endovascular revascularization techniques in the setting of chronic disease, with much of it based on prior publications and SVS-proposed standards.
The document also should:
• Serve as a guide for the design of clinical trials
• Be a reference for journal editors and reviewers when considering scientific work pertaining to endovascular therapy for chronic lower extremity arterial disease.
Endovascular treatments are the focus
The Society for Vascular Surgery has released new reporting standards focused on endovascular treatment of chronic lower extremity peripheral artery disease.
Recommended reporting standards for lower extremity ischemia were last published by the SVS in 1997.
The variety of endovascular devices and techniques to treat occlusive disease has exploded over the past 10 years and critical evaluation of the reported results may be problematic.
This document clarifies and updates the 1997 standards, specifically for reports on endovascular treatment. The document is divided into sections: Claudication Reporting, Critical Limb Ischemia Reporting, Preintervention Assessment and Nonanatomic Treatment, Intervention, Outcome Measures – Procedural, Outcome Measures – Disease Specific and Complications.
The linked document provides a summary for reporting endovascular revascularization techniques in the setting of chronic disease, with much of it based on prior publications and SVS-proposed standards.
The document also should:
• Serve as a guide for the design of clinical trials
• Be a reference for journal editors and reviewers when considering scientific work pertaining to endovascular therapy for chronic lower extremity arterial disease.
Endovascular treatments are the focus
The Society for Vascular Surgery has released new reporting standards focused on endovascular treatment of chronic lower extremity peripheral artery disease.
Recommended reporting standards for lower extremity ischemia were last published by the SVS in 1997.
The variety of endovascular devices and techniques to treat occlusive disease has exploded over the past 10 years and critical evaluation of the reported results may be problematic.
This document clarifies and updates the 1997 standards, specifically for reports on endovascular treatment. The document is divided into sections: Claudication Reporting, Critical Limb Ischemia Reporting, Preintervention Assessment and Nonanatomic Treatment, Intervention, Outcome Measures – Procedural, Outcome Measures – Disease Specific and Complications.
The linked document provides a summary for reporting endovascular revascularization techniques in the setting of chronic disease, with much of it based on prior publications and SVS-proposed standards.
The document also should:
• Serve as a guide for the design of clinical trials
• Be a reference for journal editors and reviewers when considering scientific work pertaining to endovascular therapy for chronic lower extremity arterial disease.
Colorectal cancer screening: The USPSTF’s final word
EMA reviewing hemophilia A products

The European Medicines Agency (EMA) has started a review of medicines containing factor VIII (FVIII) to assess the risk of inhibitor development among patients starting treatment for hemophilia A.
The agency is conducting this review because results of the SIPPET study suggested that patients are more likely to develop inhibitors if they receive FVIII products made by DNA recombinant technology rather than FVIII products derived from blood.
The EMA is evaluating data from the SIPPET study as well as all other relevant data on blood-derived and recombinant FVIII products.
The agency said it will consider the implications of these data for previously untreated patients with hemophilia A and whether there is a need for risk minimization measures or other changes to the marketing authorizations of these products.
The review will cover all medicines containing FVIII that are authorized for use within the European Union. For details on the products to be reviewed, including the different product names used in each country, visit the EMA website (Factor VIII Article-31 referral - Annex I).
The EMA’s review has been initiated at the request of the Paul-Ehrlich-Institute, under Article 31 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be sent to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt the EMA’s opinion.
Finally, the European Commission will adopt a legally binding decision applicable in all member states of the European Union. ![]()

The European Medicines Agency (EMA) has started a review of medicines containing factor VIII (FVIII) to assess the risk of inhibitor development among patients starting treatment for hemophilia A.
The agency is conducting this review because results of the SIPPET study suggested that patients are more likely to develop inhibitors if they receive FVIII products made by DNA recombinant technology rather than FVIII products derived from blood.
The EMA is evaluating data from the SIPPET study as well as all other relevant data on blood-derived and recombinant FVIII products.
The agency said it will consider the implications of these data for previously untreated patients with hemophilia A and whether there is a need for risk minimization measures or other changes to the marketing authorizations of these products.
The review will cover all medicines containing FVIII that are authorized for use within the European Union. For details on the products to be reviewed, including the different product names used in each country, visit the EMA website (Factor VIII Article-31 referral - Annex I).
The EMA’s review has been initiated at the request of the Paul-Ehrlich-Institute, under Article 31 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be sent to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt the EMA’s opinion.
Finally, the European Commission will adopt a legally binding decision applicable in all member states of the European Union. ![]()

The European Medicines Agency (EMA) has started a review of medicines containing factor VIII (FVIII) to assess the risk of inhibitor development among patients starting treatment for hemophilia A.
The agency is conducting this review because results of the SIPPET study suggested that patients are more likely to develop inhibitors if they receive FVIII products made by DNA recombinant technology rather than FVIII products derived from blood.
The EMA is evaluating data from the SIPPET study as well as all other relevant data on blood-derived and recombinant FVIII products.
The agency said it will consider the implications of these data for previously untreated patients with hemophilia A and whether there is a need for risk minimization measures or other changes to the marketing authorizations of these products.
The review will cover all medicines containing FVIII that are authorized for use within the European Union. For details on the products to be reviewed, including the different product names used in each country, visit the EMA website (Factor VIII Article-31 referral - Annex I).
The EMA’s review has been initiated at the request of the Paul-Ehrlich-Institute, under Article 31 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be sent to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt the EMA’s opinion.
Finally, the European Commission will adopt a legally binding decision applicable in all member states of the European Union. ![]()
New insights into infant leukemia
Photo by Matthias Zepper
Researchers may have identified the cells responsible for MLL-AF4+ infant B-cell acute lymphoblastic leukemia, according to a paper published in Cell Reports.
The team analyzed mouse embryos and discovered a “window of opportunity” during which a pre-leukemic state can take hold.
They also found evidence to suggest this pre-leukemic state is driven by lymphoid-primed multipotent progenitor cells.
“One of the most common and aggressive types of infant blood cancer is associated with the MLL-AF4 fusion gene, which arises during pregnancy,” said study author Katrin Ottersbach, PhD, of the University of Edinburgh in the UK.
“One of the main impediments to improving the survival rates in infants is the lack of knowledge on where and when during development this mutation arises and how it affects the developing blood system of the baby.”
To gain some insight, Dr Ottersbach and her colleagues bred mice where one parent carries an inactive form of the MLL-AF4 fusion gene and the other parent expresses a gene for an enzyme that activates the fusion gene.
The embryos from this pairing entered a pre-leukemic state between days 12 and 14. The researchers found that MLL-AF4 imparted enhanced B lymphoid potential and increased repopulation and self-renewal capacity during this time.
Further investigation suggested that lymphoid-primed multipotent progenitor cells were the major contributors to the enhanced B lymphoid output and may therefore be the cells of origin.
The researchers noted that the mice did not actually develop infant leukemia, so more research is needed to identify the events required for progression to MLL-AF4+ infant B-cell acute lymphoblastic leukemia.
“Our findings reveal the first changes that take place in blood development caused by the MLL-AF4 mutation during a pre-cancerous state,” Dr Ottersbach said. “This has increased our knowledge on how this aggressive disease develops and will help identify early signs of disease and points for therapeutic intervention.” ![]()

Photo by Matthias Zepper
Researchers may have identified the cells responsible for MLL-AF4+ infant B-cell acute lymphoblastic leukemia, according to a paper published in Cell Reports.
The team analyzed mouse embryos and discovered a “window of opportunity” during which a pre-leukemic state can take hold.
They also found evidence to suggest this pre-leukemic state is driven by lymphoid-primed multipotent progenitor cells.
“One of the most common and aggressive types of infant blood cancer is associated with the MLL-AF4 fusion gene, which arises during pregnancy,” said study author Katrin Ottersbach, PhD, of the University of Edinburgh in the UK.
“One of the main impediments to improving the survival rates in infants is the lack of knowledge on where and when during development this mutation arises and how it affects the developing blood system of the baby.”
To gain some insight, Dr Ottersbach and her colleagues bred mice where one parent carries an inactive form of the MLL-AF4 fusion gene and the other parent expresses a gene for an enzyme that activates the fusion gene.
The embryos from this pairing entered a pre-leukemic state between days 12 and 14. The researchers found that MLL-AF4 imparted enhanced B lymphoid potential and increased repopulation and self-renewal capacity during this time.
Further investigation suggested that lymphoid-primed multipotent progenitor cells were the major contributors to the enhanced B lymphoid output and may therefore be the cells of origin.
The researchers noted that the mice did not actually develop infant leukemia, so more research is needed to identify the events required for progression to MLL-AF4+ infant B-cell acute lymphoblastic leukemia.
“Our findings reveal the first changes that take place in blood development caused by the MLL-AF4 mutation during a pre-cancerous state,” Dr Ottersbach said. “This has increased our knowledge on how this aggressive disease develops and will help identify early signs of disease and points for therapeutic intervention.” ![]()

Photo by Matthias Zepper
Researchers may have identified the cells responsible for MLL-AF4+ infant B-cell acute lymphoblastic leukemia, according to a paper published in Cell Reports.
The team analyzed mouse embryos and discovered a “window of opportunity” during which a pre-leukemic state can take hold.
They also found evidence to suggest this pre-leukemic state is driven by lymphoid-primed multipotent progenitor cells.
“One of the most common and aggressive types of infant blood cancer is associated with the MLL-AF4 fusion gene, which arises during pregnancy,” said study author Katrin Ottersbach, PhD, of the University of Edinburgh in the UK.
“One of the main impediments to improving the survival rates in infants is the lack of knowledge on where and when during development this mutation arises and how it affects the developing blood system of the baby.”
To gain some insight, Dr Ottersbach and her colleagues bred mice where one parent carries an inactive form of the MLL-AF4 fusion gene and the other parent expresses a gene for an enzyme that activates the fusion gene.
The embryos from this pairing entered a pre-leukemic state between days 12 and 14. The researchers found that MLL-AF4 imparted enhanced B lymphoid potential and increased repopulation and self-renewal capacity during this time.
Further investigation suggested that lymphoid-primed multipotent progenitor cells were the major contributors to the enhanced B lymphoid output and may therefore be the cells of origin.
The researchers noted that the mice did not actually develop infant leukemia, so more research is needed to identify the events required for progression to MLL-AF4+ infant B-cell acute lymphoblastic leukemia.
“Our findings reveal the first changes that take place in blood development caused by the MLL-AF4 mutation during a pre-cancerous state,” Dr Ottersbach said. “This has increased our knowledge on how this aggressive disease develops and will help identify early signs of disease and points for therapeutic intervention.” ![]()