User login
Pulmonary Perspectives® New Technology Enhances Electromagnetic Navigation Bronchoscopy
Following the National Lung Screening Trial (NLST), which showed at-risk patients screened with CT scans had reduced lung cancer-specific mortality, many institutions have incorporated lung cancer screening protocols into clinical practice (Aberle et al. N Engl J Med. 2011;365[5]:395). These protocols, along with new generation, high resolution multidetector CT scans, have increased the number of detected peripheral lung nodules, many smaller in size. It is estimated that over 150,000 solitary nodules are diagnosed each year in the United States (Herth et al. Expert Rev Respir Med. 2016; 0[8]:901) and, in keeping with the NLST, greater than 25% of subjects screened have lung nodules suspicious for lung cancer. As a result, many leading health practices have created specific lung nodule programs to handle the volume in an effort to deliver timely care in the evaluation of lung cancer.
Pulmonary specialists managing patients with lung nodules are faced with the difficult challenge of deciding if a patient with a nodule is a candidate for serial surveillance, tissue biopsy (transthoracic needle aspiration [TTNA] vs. bronchoscopic biopsy [TBX]), or surgical resection. Calculation of the probability of a nodule being malignant is most helpful in making these decisions for patients with low and high malignancy risk factors, as surveillance and resection are appropriate steps, respectively. However, for those with an intermediate (5%-65%) probability of having a malignant nodule, the diagnostic procedure risks, yields, and timing have to be considered because delayed sampling or false-negative results may negatively impact survival. Kanashiki et al (Oncol Rep. 2003;10[3]:649) showed that worse survival is associated with patients with imaging-to-diagnosis times of greater than 4 months. Over the past decade, image-guided bronchoscopy has been used to improve the yield for tissue sampling of smaller peripheral nodules in a timely fashion. The most common method of image-guided bronchoscopy today is electromagnetic navigation bronchoscopy (ENB).
Electromagnetic navigation bronchoscopy has shown promise for increasing diagnostic yields for peripheral nodules (PN) over conventional bronchoscopy. Over time, the improved yields have plateaued as ENB use in clinical practice increased and limitations of the early generation technology became apparent. Earlier ENB technology uses a single inspiratory CT scan of the chest to reconstruct a 3D virtual model of the airways and parenchyma. A tracked sensor is then used to navigate through the imaging reconstructed airways toward the targeted lesion, the sensor is then removed, and through a dedicated catheter instruments are used to obtain samples from the lesion. In a meta-analysis using this technology, lesions greater than 2 cm had a diagnostic yield ranging from 66.7% to 94.7%. However, as the PN size decreased to less than or equal to 2 cm, the diagnostic yield range dropped significantly with some yields reported as low as 18.2% (van ‘t Westeinde et al. Chest. 2012;142(2):377). More recently, Ost and colleagues performed a multicenter study of consecutive patients undergoing bronchoscopic sampling of PN (Ost et al. Am J Respir Crit Care Med. 2016;193[1]:68). Although it was not a randomized trial and each bronchoscopist influenced the selection of the sampling technique, the authors reported that the diagnostic yields for navigation-guided bronchoscopy were lower than conventional bronchoscopy, 38.5% and 63.7%, respectively. Taken on face value alone, one might conclude that ENB not be used to biopsy PNs. However, deeper analysis of the data showed that 97% of the ENB procedures were performed using the earlier technology described above, suggesting that the single inspiratory imaging CT scan and navigation procedure technique, which differs significantly from conventional bronchoscopy, may have some influence on the lower than expected yields.
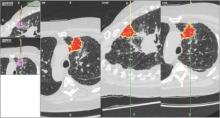
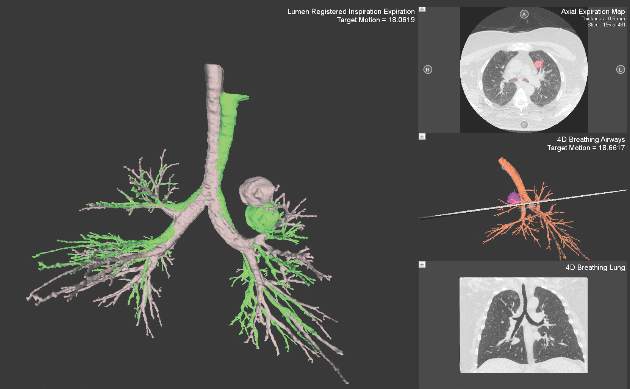
Despite increasing use and experience with ENB, diagnostic yields remain static. Chen and colleagues hypothesized that using a single inspiratory CT scan may not allow the endoscopist to make adjustments for PN movement as the lung moves during the respiratory cycle. Using different imaging protocol, the investigators assessed movement of 85 lung nodules during the respiratory cycle with paired-full inspiration and tidal-volume expiration, thin sliced (0.5-1.0 mm) CT scans. They found that the average motion of all lesions during respiration was 17.6 mm, 12.2 mm in the right-upper lobe, 10.6 mm in the left-upper lobe, and 25.3 mm and 23.8 mm in the right- and left- lower lobes, respectively (Chen et al. Chest. 2015;147[5];1275) (Fig. 1). They concluded that the location of targeted lesions on a single inspiration planning CT scan alone does not accurately represent the position of the lesion during bronchoscopy.
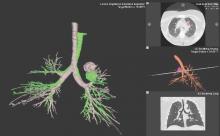
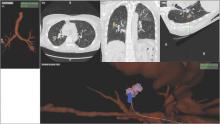
Although being able to correct for nodule movement throughout the respiratory cycle during the procedure is a significant improvement, it doesn’t guarantee that the tissue sample is obtained from the targeted lesion. To accomplish that, the system would have to be able to determine when the instrument being used to sample is in the target. The earlier ENB systems allowed for navigation to the target with a separate sensor through a steerable catheter. However, when the target was reached, the sensor had to be removed so that sampling instruments could be introduced into the catheter. Since the instruments are not tracked and the movement of the nodule is occurring, there is no guarantee that the instrument is in the target at the time of sampling. Advanced technology now allows for the tracking sensor to be placed in the tip of standard bronchoscopy instruments, making them “tip-tracked” and able to be used with standard bronchoscopes and equipment; thus, making the new ENB procedure similar to conventional bronchoscopy that was shown to have higher diagnostic yields (Figs. 2 and 3).
Our institution incorporated this technology (Veran Medical, St. Louis) into our advanced diagnostic and interventional pulmonary program for lung nodules and published our initial experience and results. During the initial 8 months of screening for lung cancer, we performed procedures on 44 patients with PNs suspicious for lung cancer. The rate for successful target sampling was 90.2% with a cancer diagnosis rate of 39%, which is similar to that found in the NLST. Those patients who had nonmalignant but abnormal pathologic findings (inflammation, granuloma, fibrosis, and so on) were monitored for a minimum of 12 months. Most of the lesions either remained stable or disappeared on follow-up imaging (Flenaugh et al. The Internet Journal of Pulmonary Medicine. 2016;18[1]). We concluded that (1) the combination of paired inspiratory and expiratory CT scan imaging accounts for nodule movement and (2) using tip-tracked conventional instruments to enter into the lesion at the time of biopsy contributes to improved yields.
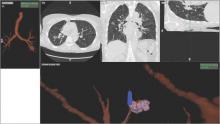
Newer ENB technology is not limited to transbronchial sampling. For PNs less than 2 cm and deep in the lung periphery, current recommendations prefer TTNA over bronchoscopic biopsy because of yield rates of 90% (Chest. 2007; 132(suppl 3):131S). Using the same paired CT scanning and tip-tracking method on transthoracic needles, the new systems allow pulmonologists to perform electromagnetic transthoracic needle aspiration (ETTNA) of PNs using the same basic equipment and during the same procedure visit (Fig. 4). This “one stop shopping” approach of bronchoscopy with the option of converting to ETTNA if the PN is not reachable endoscopically has proven to be cost efficient and allows for timely diagnosis and focused care (Yarmis et al. J Thorac Dis. 2016;8(1):186). In a prospective study designed specifically to assess feasibility, safety, and diagnostic yield of ETTNA in a single procedure, Yarmis and colleagues enrolled 24 patients to undergo endobronchial ultrasound for lung cancer staging followed by ENB and ETTNA. Ninety-six percent of the patients were candidates for ETTNA. The authors reported the yield for ETTNA was 83%, ETTNA plus ENB 87%, and ETTNA plus ENB plus endobronchial ultrasound for complete staging was 92%. Five pneumothoraces were reported; however, only two (8%) required a drainage intervention. This protocol is unique because it makes use of several advanced diagnostic procedures, including tip-tracked navigation technology, to localize, sample, diagnose, and stage during one patient procedure visit.
As lung cancer screening becomes commonplace in clinical practice and imaging technology improves, pulmonary specialists can expect to encounter and manage a greater number of pulmonary nodules. Advancements in technology now offer options for improving diagnostic accuracy while providing timely, safe, and cost effective care. While not all new technology will prove beneficial in disease management, those that improve the deficiencies of earlier technology offer us the best chance to improve practice. This perspective highlights such technology.
Dr. Flenaugh is Associate Professor, Director of Advanced Diagnostic & Interventional Pulmonary Service,Morehouse School of Medicine and Grady Hospital, Atlanta; Dr. Foreman is Professor of Medicine, Associate Chair for Research, Pulmonary & Critical Care Medicine, Morehouse School of Medicine.
Following the National Lung Screening Trial (NLST), which showed at-risk patients screened with CT scans had reduced lung cancer-specific mortality, many institutions have incorporated lung cancer screening protocols into clinical practice (Aberle et al. N Engl J Med. 2011;365[5]:395). These protocols, along with new generation, high resolution multidetector CT scans, have increased the number of detected peripheral lung nodules, many smaller in size. It is estimated that over 150,000 solitary nodules are diagnosed each year in the United States (Herth et al. Expert Rev Respir Med. 2016; 0[8]:901) and, in keeping with the NLST, greater than 25% of subjects screened have lung nodules suspicious for lung cancer. As a result, many leading health practices have created specific lung nodule programs to handle the volume in an effort to deliver timely care in the evaluation of lung cancer.
Pulmonary specialists managing patients with lung nodules are faced with the difficult challenge of deciding if a patient with a nodule is a candidate for serial surveillance, tissue biopsy (transthoracic needle aspiration [TTNA] vs. bronchoscopic biopsy [TBX]), or surgical resection. Calculation of the probability of a nodule being malignant is most helpful in making these decisions for patients with low and high malignancy risk factors, as surveillance and resection are appropriate steps, respectively. However, for those with an intermediate (5%-65%) probability of having a malignant nodule, the diagnostic procedure risks, yields, and timing have to be considered because delayed sampling or false-negative results may negatively impact survival. Kanashiki et al (Oncol Rep. 2003;10[3]:649) showed that worse survival is associated with patients with imaging-to-diagnosis times of greater than 4 months. Over the past decade, image-guided bronchoscopy has been used to improve the yield for tissue sampling of smaller peripheral nodules in a timely fashion. The most common method of image-guided bronchoscopy today is electromagnetic navigation bronchoscopy (ENB).
Electromagnetic navigation bronchoscopy has shown promise for increasing diagnostic yields for peripheral nodules (PN) over conventional bronchoscopy. Over time, the improved yields have plateaued as ENB use in clinical practice increased and limitations of the early generation technology became apparent. Earlier ENB technology uses a single inspiratory CT scan of the chest to reconstruct a 3D virtual model of the airways and parenchyma. A tracked sensor is then used to navigate through the imaging reconstructed airways toward the targeted lesion, the sensor is then removed, and through a dedicated catheter instruments are used to obtain samples from the lesion. In a meta-analysis using this technology, lesions greater than 2 cm had a diagnostic yield ranging from 66.7% to 94.7%. However, as the PN size decreased to less than or equal to 2 cm, the diagnostic yield range dropped significantly with some yields reported as low as 18.2% (van ‘t Westeinde et al. Chest. 2012;142(2):377). More recently, Ost and colleagues performed a multicenter study of consecutive patients undergoing bronchoscopic sampling of PN (Ost et al. Am J Respir Crit Care Med. 2016;193[1]:68). Although it was not a randomized trial and each bronchoscopist influenced the selection of the sampling technique, the authors reported that the diagnostic yields for navigation-guided bronchoscopy were lower than conventional bronchoscopy, 38.5% and 63.7%, respectively. Taken on face value alone, one might conclude that ENB not be used to biopsy PNs. However, deeper analysis of the data showed that 97% of the ENB procedures were performed using the earlier technology described above, suggesting that the single inspiratory imaging CT scan and navigation procedure technique, which differs significantly from conventional bronchoscopy, may have some influence on the lower than expected yields.
Despite increasing use and experience with ENB, diagnostic yields remain static. Chen and colleagues hypothesized that using a single inspiratory CT scan may not allow the endoscopist to make adjustments for PN movement as the lung moves during the respiratory cycle. Using different imaging protocol, the investigators assessed movement of 85 lung nodules during the respiratory cycle with paired-full inspiration and tidal-volume expiration, thin sliced (0.5-1.0 mm) CT scans. They found that the average motion of all lesions during respiration was 17.6 mm, 12.2 mm in the right-upper lobe, 10.6 mm in the left-upper lobe, and 25.3 mm and 23.8 mm in the right- and left- lower lobes, respectively (Chen et al. Chest. 2015;147[5];1275) (Fig. 1). They concluded that the location of targeted lesions on a single inspiration planning CT scan alone does not accurately represent the position of the lesion during bronchoscopy.
Although being able to correct for nodule movement throughout the respiratory cycle during the procedure is a significant improvement, it doesn’t guarantee that the tissue sample is obtained from the targeted lesion. To accomplish that, the system would have to be able to determine when the instrument being used to sample is in the target. The earlier ENB systems allowed for navigation to the target with a separate sensor through a steerable catheter. However, when the target was reached, the sensor had to be removed so that sampling instruments could be introduced into the catheter. Since the instruments are not tracked and the movement of the nodule is occurring, there is no guarantee that the instrument is in the target at the time of sampling. Advanced technology now allows for the tracking sensor to be placed in the tip of standard bronchoscopy instruments, making them “tip-tracked” and able to be used with standard bronchoscopes and equipment; thus, making the new ENB procedure similar to conventional bronchoscopy that was shown to have higher diagnostic yields (Figs. 2 and 3).
Our institution incorporated this technology (Veran Medical, St. Louis) into our advanced diagnostic and interventional pulmonary program for lung nodules and published our initial experience and results. During the initial 8 months of screening for lung cancer, we performed procedures on 44 patients with PNs suspicious for lung cancer. The rate for successful target sampling was 90.2% with a cancer diagnosis rate of 39%, which is similar to that found in the NLST. Those patients who had nonmalignant but abnormal pathologic findings (inflammation, granuloma, fibrosis, and so on) were monitored for a minimum of 12 months. Most of the lesions either remained stable or disappeared on follow-up imaging (Flenaugh et al. The Internet Journal of Pulmonary Medicine. 2016;18[1]). We concluded that (1) the combination of paired inspiratory and expiratory CT scan imaging accounts for nodule movement and (2) using tip-tracked conventional instruments to enter into the lesion at the time of biopsy contributes to improved yields.
Newer ENB technology is not limited to transbronchial sampling. For PNs less than 2 cm and deep in the lung periphery, current recommendations prefer TTNA over bronchoscopic biopsy because of yield rates of 90% (Chest. 2007; 132(suppl 3):131S). Using the same paired CT scanning and tip-tracking method on transthoracic needles, the new systems allow pulmonologists to perform electromagnetic transthoracic needle aspiration (ETTNA) of PNs using the same basic equipment and during the same procedure visit (Fig. 4). This “one stop shopping” approach of bronchoscopy with the option of converting to ETTNA if the PN is not reachable endoscopically has proven to be cost efficient and allows for timely diagnosis and focused care (Yarmis et al. J Thorac Dis. 2016;8(1):186). In a prospective study designed specifically to assess feasibility, safety, and diagnostic yield of ETTNA in a single procedure, Yarmis and colleagues enrolled 24 patients to undergo endobronchial ultrasound for lung cancer staging followed by ENB and ETTNA. Ninety-six percent of the patients were candidates for ETTNA. The authors reported the yield for ETTNA was 83%, ETTNA plus ENB 87%, and ETTNA plus ENB plus endobronchial ultrasound for complete staging was 92%. Five pneumothoraces were reported; however, only two (8%) required a drainage intervention. This protocol is unique because it makes use of several advanced diagnostic procedures, including tip-tracked navigation technology, to localize, sample, diagnose, and stage during one patient procedure visit.
As lung cancer screening becomes commonplace in clinical practice and imaging technology improves, pulmonary specialists can expect to encounter and manage a greater number of pulmonary nodules. Advancements in technology now offer options for improving diagnostic accuracy while providing timely, safe, and cost effective care. While not all new technology will prove beneficial in disease management, those that improve the deficiencies of earlier technology offer us the best chance to improve practice. This perspective highlights such technology.
Dr. Flenaugh is Associate Professor, Director of Advanced Diagnostic & Interventional Pulmonary Service,Morehouse School of Medicine and Grady Hospital, Atlanta; Dr. Foreman is Professor of Medicine, Associate Chair for Research, Pulmonary & Critical Care Medicine, Morehouse School of Medicine.
Following the National Lung Screening Trial (NLST), which showed at-risk patients screened with CT scans had reduced lung cancer-specific mortality, many institutions have incorporated lung cancer screening protocols into clinical practice (Aberle et al. N Engl J Med. 2011;365[5]:395). These protocols, along with new generation, high resolution multidetector CT scans, have increased the number of detected peripheral lung nodules, many smaller in size. It is estimated that over 150,000 solitary nodules are diagnosed each year in the United States (Herth et al. Expert Rev Respir Med. 2016; 0[8]:901) and, in keeping with the NLST, greater than 25% of subjects screened have lung nodules suspicious for lung cancer. As a result, many leading health practices have created specific lung nodule programs to handle the volume in an effort to deliver timely care in the evaluation of lung cancer.
Pulmonary specialists managing patients with lung nodules are faced with the difficult challenge of deciding if a patient with a nodule is a candidate for serial surveillance, tissue biopsy (transthoracic needle aspiration [TTNA] vs. bronchoscopic biopsy [TBX]), or surgical resection. Calculation of the probability of a nodule being malignant is most helpful in making these decisions for patients with low and high malignancy risk factors, as surveillance and resection are appropriate steps, respectively. However, for those with an intermediate (5%-65%) probability of having a malignant nodule, the diagnostic procedure risks, yields, and timing have to be considered because delayed sampling or false-negative results may negatively impact survival. Kanashiki et al (Oncol Rep. 2003;10[3]:649) showed that worse survival is associated with patients with imaging-to-diagnosis times of greater than 4 months. Over the past decade, image-guided bronchoscopy has been used to improve the yield for tissue sampling of smaller peripheral nodules in a timely fashion. The most common method of image-guided bronchoscopy today is electromagnetic navigation bronchoscopy (ENB).
Electromagnetic navigation bronchoscopy has shown promise for increasing diagnostic yields for peripheral nodules (PN) over conventional bronchoscopy. Over time, the improved yields have plateaued as ENB use in clinical practice increased and limitations of the early generation technology became apparent. Earlier ENB technology uses a single inspiratory CT scan of the chest to reconstruct a 3D virtual model of the airways and parenchyma. A tracked sensor is then used to navigate through the imaging reconstructed airways toward the targeted lesion, the sensor is then removed, and through a dedicated catheter instruments are used to obtain samples from the lesion. In a meta-analysis using this technology, lesions greater than 2 cm had a diagnostic yield ranging from 66.7% to 94.7%. However, as the PN size decreased to less than or equal to 2 cm, the diagnostic yield range dropped significantly with some yields reported as low as 18.2% (van ‘t Westeinde et al. Chest. 2012;142(2):377). More recently, Ost and colleagues performed a multicenter study of consecutive patients undergoing bronchoscopic sampling of PN (Ost et al. Am J Respir Crit Care Med. 2016;193[1]:68). Although it was not a randomized trial and each bronchoscopist influenced the selection of the sampling technique, the authors reported that the diagnostic yields for navigation-guided bronchoscopy were lower than conventional bronchoscopy, 38.5% and 63.7%, respectively. Taken on face value alone, one might conclude that ENB not be used to biopsy PNs. However, deeper analysis of the data showed that 97% of the ENB procedures were performed using the earlier technology described above, suggesting that the single inspiratory imaging CT scan and navigation procedure technique, which differs significantly from conventional bronchoscopy, may have some influence on the lower than expected yields.
Despite increasing use and experience with ENB, diagnostic yields remain static. Chen and colleagues hypothesized that using a single inspiratory CT scan may not allow the endoscopist to make adjustments for PN movement as the lung moves during the respiratory cycle. Using different imaging protocol, the investigators assessed movement of 85 lung nodules during the respiratory cycle with paired-full inspiration and tidal-volume expiration, thin sliced (0.5-1.0 mm) CT scans. They found that the average motion of all lesions during respiration was 17.6 mm, 12.2 mm in the right-upper lobe, 10.6 mm in the left-upper lobe, and 25.3 mm and 23.8 mm in the right- and left- lower lobes, respectively (Chen et al. Chest. 2015;147[5];1275) (Fig. 1). They concluded that the location of targeted lesions on a single inspiration planning CT scan alone does not accurately represent the position of the lesion during bronchoscopy.
Although being able to correct for nodule movement throughout the respiratory cycle during the procedure is a significant improvement, it doesn’t guarantee that the tissue sample is obtained from the targeted lesion. To accomplish that, the system would have to be able to determine when the instrument being used to sample is in the target. The earlier ENB systems allowed for navigation to the target with a separate sensor through a steerable catheter. However, when the target was reached, the sensor had to be removed so that sampling instruments could be introduced into the catheter. Since the instruments are not tracked and the movement of the nodule is occurring, there is no guarantee that the instrument is in the target at the time of sampling. Advanced technology now allows for the tracking sensor to be placed in the tip of standard bronchoscopy instruments, making them “tip-tracked” and able to be used with standard bronchoscopes and equipment; thus, making the new ENB procedure similar to conventional bronchoscopy that was shown to have higher diagnostic yields (Figs. 2 and 3).
Our institution incorporated this technology (Veran Medical, St. Louis) into our advanced diagnostic and interventional pulmonary program for lung nodules and published our initial experience and results. During the initial 8 months of screening for lung cancer, we performed procedures on 44 patients with PNs suspicious for lung cancer. The rate for successful target sampling was 90.2% with a cancer diagnosis rate of 39%, which is similar to that found in the NLST. Those patients who had nonmalignant but abnormal pathologic findings (inflammation, granuloma, fibrosis, and so on) were monitored for a minimum of 12 months. Most of the lesions either remained stable or disappeared on follow-up imaging (Flenaugh et al. The Internet Journal of Pulmonary Medicine. 2016;18[1]). We concluded that (1) the combination of paired inspiratory and expiratory CT scan imaging accounts for nodule movement and (2) using tip-tracked conventional instruments to enter into the lesion at the time of biopsy contributes to improved yields.
Newer ENB technology is not limited to transbronchial sampling. For PNs less than 2 cm and deep in the lung periphery, current recommendations prefer TTNA over bronchoscopic biopsy because of yield rates of 90% (Chest. 2007; 132(suppl 3):131S). Using the same paired CT scanning and tip-tracking method on transthoracic needles, the new systems allow pulmonologists to perform electromagnetic transthoracic needle aspiration (ETTNA) of PNs using the same basic equipment and during the same procedure visit (Fig. 4). This “one stop shopping” approach of bronchoscopy with the option of converting to ETTNA if the PN is not reachable endoscopically has proven to be cost efficient and allows for timely diagnosis and focused care (Yarmis et al. J Thorac Dis. 2016;8(1):186). In a prospective study designed specifically to assess feasibility, safety, and diagnostic yield of ETTNA in a single procedure, Yarmis and colleagues enrolled 24 patients to undergo endobronchial ultrasound for lung cancer staging followed by ENB and ETTNA. Ninety-six percent of the patients were candidates for ETTNA. The authors reported the yield for ETTNA was 83%, ETTNA plus ENB 87%, and ETTNA plus ENB plus endobronchial ultrasound for complete staging was 92%. Five pneumothoraces were reported; however, only two (8%) required a drainage intervention. This protocol is unique because it makes use of several advanced diagnostic procedures, including tip-tracked navigation technology, to localize, sample, diagnose, and stage during one patient procedure visit.
As lung cancer screening becomes commonplace in clinical practice and imaging technology improves, pulmonary specialists can expect to encounter and manage a greater number of pulmonary nodules. Advancements in technology now offer options for improving diagnostic accuracy while providing timely, safe, and cost effective care. While not all new technology will prove beneficial in disease management, those that improve the deficiencies of earlier technology offer us the best chance to improve practice. This perspective highlights such technology.
Dr. Flenaugh is Associate Professor, Director of Advanced Diagnostic & Interventional Pulmonary Service,Morehouse School of Medicine and Grady Hospital, Atlanta; Dr. Foreman is Professor of Medicine, Associate Chair for Research, Pulmonary & Critical Care Medicine, Morehouse School of Medicine.
From the Washington Office: Globals … again
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Apply by Sept. 1 for Resident Research Scholarships for 2017-2019
The American College of Surgeons (ACS) is offering 2-year Resident Research Scholarships to surgeons in training who are interested in pursuing careers in academic surgery. Eligibility for these scholarships is limited to the research projects of residents in general surgery or a surgical specialty. The closing date for receipt of the completed online application and all supporting documents is Sept. 1, 2016.
General policies covering the granting of the ACS Resident Research Scholarships are as follows:
The applicant must be a Resident Member of the College who has completed two postdoctoral years in an accredited surgical training program in the U.S. or Canada at the time the scholarship is awarded, July 1, 2017, and may not complete formal residency training before June 2019. Scholarships do not support research after completion of the chief residency year.
The scholarship is awarded for 2 years, and acceptance of it requires commitment for the 2-year period. The award is to support a research plan for the 2 years of the scholarship, July 2017 through June 2019. The projects of residents who are involved in full-time laboratory investigation will receive priority. Study outside the United States or Canada is permissible. Renewal of the scholarship for the 2nd year is required and is contingent upon the acceptance of a progress report and research study protocol for the 2nd year, as submitted to the Scholarships Section of the College by May 1, 2018.
Application for these scholarships may be submitted even if the resident has made a comparable application to other organizations. If the recipient is offered a scholarship, fellowship, or research award from another organization, it is the responsibility of the recipient to contact the ACS Scholarships Administrator to request approval of the additional award. The Scholarships Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The scholarship is $30,000 per year; the total amount is to support the research of the recipient and may be used for salary or stipend, research materials, and travel related to the research. Indirect costs are not paid to the recipient or the recipient’s institution.
The scholar must attend the ACS Clinical Congress in 2019 to present a report on the research as part of the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Approval of the application is required from the administration (dean or fiscal officer) of the institution. Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor who will be supervising the applicant’s research must be submitted. The College encourages diversity of applicants and institutions; only in exceptional circumstances will more than one scholarship be granted in a single year to applicants from the same institution.
For further information regarding this scholarship, click here, or contact the Scholarships Administrator at [email protected].
The American College of Surgeons (ACS) is offering 2-year Resident Research Scholarships to surgeons in training who are interested in pursuing careers in academic surgery. Eligibility for these scholarships is limited to the research projects of residents in general surgery or a surgical specialty. The closing date for receipt of the completed online application and all supporting documents is Sept. 1, 2016.
General policies covering the granting of the ACS Resident Research Scholarships are as follows:
The applicant must be a Resident Member of the College who has completed two postdoctoral years in an accredited surgical training program in the U.S. or Canada at the time the scholarship is awarded, July 1, 2017, and may not complete formal residency training before June 2019. Scholarships do not support research after completion of the chief residency year.
The scholarship is awarded for 2 years, and acceptance of it requires commitment for the 2-year period. The award is to support a research plan for the 2 years of the scholarship, July 2017 through June 2019. The projects of residents who are involved in full-time laboratory investigation will receive priority. Study outside the United States or Canada is permissible. Renewal of the scholarship for the 2nd year is required and is contingent upon the acceptance of a progress report and research study protocol for the 2nd year, as submitted to the Scholarships Section of the College by May 1, 2018.
Application for these scholarships may be submitted even if the resident has made a comparable application to other organizations. If the recipient is offered a scholarship, fellowship, or research award from another organization, it is the responsibility of the recipient to contact the ACS Scholarships Administrator to request approval of the additional award. The Scholarships Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The scholarship is $30,000 per year; the total amount is to support the research of the recipient and may be used for salary or stipend, research materials, and travel related to the research. Indirect costs are not paid to the recipient or the recipient’s institution.
The scholar must attend the ACS Clinical Congress in 2019 to present a report on the research as part of the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Approval of the application is required from the administration (dean or fiscal officer) of the institution. Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor who will be supervising the applicant’s research must be submitted. The College encourages diversity of applicants and institutions; only in exceptional circumstances will more than one scholarship be granted in a single year to applicants from the same institution.
For further information regarding this scholarship, click here, or contact the Scholarships Administrator at [email protected].
The American College of Surgeons (ACS) is offering 2-year Resident Research Scholarships to surgeons in training who are interested in pursuing careers in academic surgery. Eligibility for these scholarships is limited to the research projects of residents in general surgery or a surgical specialty. The closing date for receipt of the completed online application and all supporting documents is Sept. 1, 2016.
General policies covering the granting of the ACS Resident Research Scholarships are as follows:
The applicant must be a Resident Member of the College who has completed two postdoctoral years in an accredited surgical training program in the U.S. or Canada at the time the scholarship is awarded, July 1, 2017, and may not complete formal residency training before June 2019. Scholarships do not support research after completion of the chief residency year.
The scholarship is awarded for 2 years, and acceptance of it requires commitment for the 2-year period. The award is to support a research plan for the 2 years of the scholarship, July 2017 through June 2019. The projects of residents who are involved in full-time laboratory investigation will receive priority. Study outside the United States or Canada is permissible. Renewal of the scholarship for the 2nd year is required and is contingent upon the acceptance of a progress report and research study protocol for the 2nd year, as submitted to the Scholarships Section of the College by May 1, 2018.
Application for these scholarships may be submitted even if the resident has made a comparable application to other organizations. If the recipient is offered a scholarship, fellowship, or research award from another organization, it is the responsibility of the recipient to contact the ACS Scholarships Administrator to request approval of the additional award. The Scholarships Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The scholarship is $30,000 per year; the total amount is to support the research of the recipient and may be used for salary or stipend, research materials, and travel related to the research. Indirect costs are not paid to the recipient or the recipient’s institution.
The scholar must attend the ACS Clinical Congress in 2019 to present a report on the research as part of the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Approval of the application is required from the administration (dean or fiscal officer) of the institution. Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor who will be supervising the applicant’s research must be submitted. The College encourages diversity of applicants and institutions; only in exceptional circumstances will more than one scholarship be granted in a single year to applicants from the same institution.
For further information regarding this scholarship, click here, or contact the Scholarships Administrator at [email protected].
2016 International Exchange Travelers Announced
The International Relations Committee of the American College of Surgeons (ACS) sponsors three academic surgeon exchange programs to send a talented young U.S. or Canadian Fellow to the annual surgical meeting of each participating country – Australia-New Zealand (ANZ), Japan, and Germany. Afterward, the Fellows tour several sites tailored to their specific research interests. In exchange, the College accepts young academic surgeon-scholars from the participating societies to attend the annual Clinical Congress. This exchange is with the Royal Australasian College of Surgeons through the ACS Australia-New Zealand Chapter, the Japan Surgical Society through the ACS Japan Chapter, and the German Surgical Society through the ACS Germany Chapter.
The 2016 ANZ Exchange Fellow is Yi Chen, MB, BS, PhD, FRACS, a cardiothoracic surgery fellow at Monash Medical Centre, Melbourne, Australia. Dr. Chen is researching the role of Activin A, a novel cytokine in mouse models of atherosclerosis.
His U.S. counterpart, Sareh Parangi, MD, FACS, is an associate professor of surgery at Massachusetts General Hospital, Boston, specializing in endocrine surgery. She attended the Annual Scientific Congress of the Royal Australasian College of Surgeons held in Brisbane, Australia, in May 2016. Dr. Parangi’s report will be published in an upcoming issue of the Bulletin.
This October, the College will welcome Japan Exchange Fellow Takeo Toshima, MD, PhD, vice manager, hepatopancreatobiliary surgery, Matsuyama Red Cross Hospital. Dr. Toshima performs research on hepatocellular carcinoma and living donor liver transplants.
Daniel A. Anaya, MD, FACS, head, section of hepatobiliary tumors at H. Lee Moffitt Cancer Center, Tampa, attended the Japan Surgical Society meeting in Osaka in April 2016. Dr. Anaya’s report also will be published in the Bulletin.
The ACS Traveling Fellow to Germany, Perry Shen, MD, FACS, professor of surgery, Wake Forest Baptist Medical Center, Winston-Salem, N.C., attended the German Surgical Society’s annual meeting in Berlin in April 2016.
His German counterpart, Thilo Welsch, MD, PhD, head of surgical oncology at the University Cancer Center, Dresden, will attend Clinical Congress 2016 and visit several surgical sites under the guidance of his U.S. and German mentors. Dr. Welsch’s work centers on tumor metastasis and pancreatic surgery.
The International Relations Committee of the American College of Surgeons (ACS) sponsors three academic surgeon exchange programs to send a talented young U.S. or Canadian Fellow to the annual surgical meeting of each participating country – Australia-New Zealand (ANZ), Japan, and Germany. Afterward, the Fellows tour several sites tailored to their specific research interests. In exchange, the College accepts young academic surgeon-scholars from the participating societies to attend the annual Clinical Congress. This exchange is with the Royal Australasian College of Surgeons through the ACS Australia-New Zealand Chapter, the Japan Surgical Society through the ACS Japan Chapter, and the German Surgical Society through the ACS Germany Chapter.
The 2016 ANZ Exchange Fellow is Yi Chen, MB, BS, PhD, FRACS, a cardiothoracic surgery fellow at Monash Medical Centre, Melbourne, Australia. Dr. Chen is researching the role of Activin A, a novel cytokine in mouse models of atherosclerosis.
His U.S. counterpart, Sareh Parangi, MD, FACS, is an associate professor of surgery at Massachusetts General Hospital, Boston, specializing in endocrine surgery. She attended the Annual Scientific Congress of the Royal Australasian College of Surgeons held in Brisbane, Australia, in May 2016. Dr. Parangi’s report will be published in an upcoming issue of the Bulletin.
This October, the College will welcome Japan Exchange Fellow Takeo Toshima, MD, PhD, vice manager, hepatopancreatobiliary surgery, Matsuyama Red Cross Hospital. Dr. Toshima performs research on hepatocellular carcinoma and living donor liver transplants.
Daniel A. Anaya, MD, FACS, head, section of hepatobiliary tumors at H. Lee Moffitt Cancer Center, Tampa, attended the Japan Surgical Society meeting in Osaka in April 2016. Dr. Anaya’s report also will be published in the Bulletin.
The ACS Traveling Fellow to Germany, Perry Shen, MD, FACS, professor of surgery, Wake Forest Baptist Medical Center, Winston-Salem, N.C., attended the German Surgical Society’s annual meeting in Berlin in April 2016.
His German counterpart, Thilo Welsch, MD, PhD, head of surgical oncology at the University Cancer Center, Dresden, will attend Clinical Congress 2016 and visit several surgical sites under the guidance of his U.S. and German mentors. Dr. Welsch’s work centers on tumor metastasis and pancreatic surgery.
The International Relations Committee of the American College of Surgeons (ACS) sponsors three academic surgeon exchange programs to send a talented young U.S. or Canadian Fellow to the annual surgical meeting of each participating country – Australia-New Zealand (ANZ), Japan, and Germany. Afterward, the Fellows tour several sites tailored to their specific research interests. In exchange, the College accepts young academic surgeon-scholars from the participating societies to attend the annual Clinical Congress. This exchange is with the Royal Australasian College of Surgeons through the ACS Australia-New Zealand Chapter, the Japan Surgical Society through the ACS Japan Chapter, and the German Surgical Society through the ACS Germany Chapter.
The 2016 ANZ Exchange Fellow is Yi Chen, MB, BS, PhD, FRACS, a cardiothoracic surgery fellow at Monash Medical Centre, Melbourne, Australia. Dr. Chen is researching the role of Activin A, a novel cytokine in mouse models of atherosclerosis.
His U.S. counterpart, Sareh Parangi, MD, FACS, is an associate professor of surgery at Massachusetts General Hospital, Boston, specializing in endocrine surgery. She attended the Annual Scientific Congress of the Royal Australasian College of Surgeons held in Brisbane, Australia, in May 2016. Dr. Parangi’s report will be published in an upcoming issue of the Bulletin.
This October, the College will welcome Japan Exchange Fellow Takeo Toshima, MD, PhD, vice manager, hepatopancreatobiliary surgery, Matsuyama Red Cross Hospital. Dr. Toshima performs research on hepatocellular carcinoma and living donor liver transplants.
Daniel A. Anaya, MD, FACS, head, section of hepatobiliary tumors at H. Lee Moffitt Cancer Center, Tampa, attended the Japan Surgical Society meeting in Osaka in April 2016. Dr. Anaya’s report also will be published in the Bulletin.
The ACS Traveling Fellow to Germany, Perry Shen, MD, FACS, professor of surgery, Wake Forest Baptist Medical Center, Winston-Salem, N.C., attended the German Surgical Society’s annual meeting in Berlin in April 2016.
His German counterpart, Thilo Welsch, MD, PhD, head of surgical oncology at the University Cancer Center, Dresden, will attend Clinical Congress 2016 and visit several surgical sites under the guidance of his U.S. and German mentors. Dr. Welsch’s work centers on tumor metastasis and pancreatic surgery.
Applications being accepted for 2017-2019 Faculty Research Fellowships
The American College of Surgeons (ACS) is offering two-year Faculty Research Fellowships to surgeons entering academic careers in surgery or a surgical specialty. The fellowship is to assist a surgeon in the establishment of a new and independent research program. Applicants should have demonstrated their potential to work as independent investigators. The fellowship awards are $40,000 per year for each of two years – July 1, 2017 to June 30, 2019 – and are made possible through the generosity of Fellows, chapters, and friends of the College. The closing date for receipt of completed applications and all supporting documents is November 1, 2016.
The specific fellowships are as follows:
• The Franklin H. Martin, MD, FACS, Faculty Research Fellowship of the ACS honors Franklin H. Martin, MD, FACS, founder of the ACS.
• The C. James Carrico, MD, FACS, Faculty Research Fellowship for the Study of Trauma and Critical Care honors C. James Carrico, MD, FACS, ACS Past-President, and is designated for research in trauma and critical care.
• The Thomas R. Russell, MD, FACS, Faculty Research Fellowship honors Thomas R. Russell, MD, FACS, ACS Past-Executive Director, and is designated to support research into improving surgical outcomes.
Two additional undesignated Faculty Research Fellowships will be awarded.
General policies
The following policies cover the granting of the ACS Faculty Research Fellowships:
The fellowships are open to Fellows or Associate Fellows of the College who have: (1) completed the chief residency year or accredited fellowship training within the preceding five years, not including time off for maternity leave, military deployment, or medical leave; and (2) received a full-time faculty appointment in a department of surgery or a surgical specialty at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools in Canada. Applicants who directly enter academic surgery following residency or fellowship will receive preference.
Recipients may use this award to support their research or academic enrichment in any fashion that they deem maximally supportive of their investigations. Indirect costs are not paid to the recipient or to the recipient’s institution.
Application for this fellowship may be submitted even if a comparable application has been made to other entities such as the National Institutes of Health (NIH) or industry sources. If the recipient is offered a scholarship, fellowship, or research career development award from such an agency or organization, it is the responsibility of the recipient to contact the College’s Scholarships Administrator to request approval of the additional award. The Scholarship Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The ACS encourages applicants to leverage the funds provided by this fellowship with time and monies provided by their department. The College will look favorably upon formal statements of matching funds and time from the applicant’s department.
Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor supervising the applicant’s research effort must be submitted. This approval would involve a commitment to continuation of the academic position and of facilities for research. Only in exceptional circumstances will more than one fellowship be granted in a single year to applicants from the same institution.
The applicant must submit a research plan and budget for the two-year period of fellowship, even though renewed approval by the Scholarships Committee of the College is required for the second year.
A minimum of 50 percent of the fellow’s time must be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
The Faculty Research Fellows are expected to attend the ACS Clinical Congress in 2019 to present a report to the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Additional documents and questions are to be directed to the Scholarships Administrator: [email protected] or Scholarships Administrator, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL 60611-3211. Access the application at facs.org/member-services/scholarships/research/acsfaculty.
The American College of Surgeons (ACS) is offering two-year Faculty Research Fellowships to surgeons entering academic careers in surgery or a surgical specialty. The fellowship is to assist a surgeon in the establishment of a new and independent research program. Applicants should have demonstrated their potential to work as independent investigators. The fellowship awards are $40,000 per year for each of two years – July 1, 2017 to June 30, 2019 – and are made possible through the generosity of Fellows, chapters, and friends of the College. The closing date for receipt of completed applications and all supporting documents is November 1, 2016.
The specific fellowships are as follows:
• The Franklin H. Martin, MD, FACS, Faculty Research Fellowship of the ACS honors Franklin H. Martin, MD, FACS, founder of the ACS.
• The C. James Carrico, MD, FACS, Faculty Research Fellowship for the Study of Trauma and Critical Care honors C. James Carrico, MD, FACS, ACS Past-President, and is designated for research in trauma and critical care.
• The Thomas R. Russell, MD, FACS, Faculty Research Fellowship honors Thomas R. Russell, MD, FACS, ACS Past-Executive Director, and is designated to support research into improving surgical outcomes.
Two additional undesignated Faculty Research Fellowships will be awarded.
General policies
The following policies cover the granting of the ACS Faculty Research Fellowships:
The fellowships are open to Fellows or Associate Fellows of the College who have: (1) completed the chief residency year or accredited fellowship training within the preceding five years, not including time off for maternity leave, military deployment, or medical leave; and (2) received a full-time faculty appointment in a department of surgery or a surgical specialty at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools in Canada. Applicants who directly enter academic surgery following residency or fellowship will receive preference.
Recipients may use this award to support their research or academic enrichment in any fashion that they deem maximally supportive of their investigations. Indirect costs are not paid to the recipient or to the recipient’s institution.
Application for this fellowship may be submitted even if a comparable application has been made to other entities such as the National Institutes of Health (NIH) or industry sources. If the recipient is offered a scholarship, fellowship, or research career development award from such an agency or organization, it is the responsibility of the recipient to contact the College’s Scholarships Administrator to request approval of the additional award. The Scholarship Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The ACS encourages applicants to leverage the funds provided by this fellowship with time and monies provided by their department. The College will look favorably upon formal statements of matching funds and time from the applicant’s department.
Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor supervising the applicant’s research effort must be submitted. This approval would involve a commitment to continuation of the academic position and of facilities for research. Only in exceptional circumstances will more than one fellowship be granted in a single year to applicants from the same institution.
The applicant must submit a research plan and budget for the two-year period of fellowship, even though renewed approval by the Scholarships Committee of the College is required for the second year.
A minimum of 50 percent of the fellow’s time must be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
The Faculty Research Fellows are expected to attend the ACS Clinical Congress in 2019 to present a report to the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Additional documents and questions are to be directed to the Scholarships Administrator: [email protected] or Scholarships Administrator, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL 60611-3211. Access the application at facs.org/member-services/scholarships/research/acsfaculty.
The American College of Surgeons (ACS) is offering two-year Faculty Research Fellowships to surgeons entering academic careers in surgery or a surgical specialty. The fellowship is to assist a surgeon in the establishment of a new and independent research program. Applicants should have demonstrated their potential to work as independent investigators. The fellowship awards are $40,000 per year for each of two years – July 1, 2017 to June 30, 2019 – and are made possible through the generosity of Fellows, chapters, and friends of the College. The closing date for receipt of completed applications and all supporting documents is November 1, 2016.
The specific fellowships are as follows:
• The Franklin H. Martin, MD, FACS, Faculty Research Fellowship of the ACS honors Franklin H. Martin, MD, FACS, founder of the ACS.
• The C. James Carrico, MD, FACS, Faculty Research Fellowship for the Study of Trauma and Critical Care honors C. James Carrico, MD, FACS, ACS Past-President, and is designated for research in trauma and critical care.
• The Thomas R. Russell, MD, FACS, Faculty Research Fellowship honors Thomas R. Russell, MD, FACS, ACS Past-Executive Director, and is designated to support research into improving surgical outcomes.
Two additional undesignated Faculty Research Fellowships will be awarded.
General policies
The following policies cover the granting of the ACS Faculty Research Fellowships:
The fellowships are open to Fellows or Associate Fellows of the College who have: (1) completed the chief residency year or accredited fellowship training within the preceding five years, not including time off for maternity leave, military deployment, or medical leave; and (2) received a full-time faculty appointment in a department of surgery or a surgical specialty at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools in Canada. Applicants who directly enter academic surgery following residency or fellowship will receive preference.
Recipients may use this award to support their research or academic enrichment in any fashion that they deem maximally supportive of their investigations. Indirect costs are not paid to the recipient or to the recipient’s institution.
Application for this fellowship may be submitted even if a comparable application has been made to other entities such as the National Institutes of Health (NIH) or industry sources. If the recipient is offered a scholarship, fellowship, or research career development award from such an agency or organization, it is the responsibility of the recipient to contact the College’s Scholarships Administrator to request approval of the additional award. The Scholarship Committee reserves the right to review potentially overlapping awards and adjust its award accordingly.
The ACS encourages applicants to leverage the funds provided by this fellowship with time and monies provided by their department. The College will look favorably upon formal statements of matching funds and time from the applicant’s department.
Supporting letters from the head of the department of surgery (or the surgical specialty) and from the mentor supervising the applicant’s research effort must be submitted. This approval would involve a commitment to continuation of the academic position and of facilities for research. Only in exceptional circumstances will more than one fellowship be granted in a single year to applicants from the same institution.
The applicant must submit a research plan and budget for the two-year period of fellowship, even though renewed approval by the Scholarships Committee of the College is required for the second year.
A minimum of 50 percent of the fellow’s time must be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
The Faculty Research Fellows are expected to attend the ACS Clinical Congress in 2019 to present a report to the Scientific Forum and to receive a certificate at the annual meeting of the Scholarships Committee.
Additional documents and questions are to be directed to the Scholarships Administrator: [email protected] or Scholarships Administrator, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL 60611-3211. Access the application at facs.org/member-services/scholarships/research/acsfaculty.
New SoHM Report Brings Important Changes
It’s a little like giving birth: A nine-month-long process that started last January with the excitement and anticipation of launching the survey and encouraging as many hospital medicine groups (HMGs) as possible to participate. Then the long, drawn-out process of analyzing data, organizing everything into tables and charts, drafting the report, and reviewing it over and over until our eyes crossed. Watching it grow and take shape before our eyes, with a few small hiccups along the way. Then the agonizing process of copyediting, designing (both print and digital versions), and printing—a point at which, like all expectant parents, we said, “Enough already. When will this ever end?”
But we (that is, SHM’s Practice Analysis Committee) finally have a baby, and what proud parents we are! SHM’s 2016 State of Hospital Medicine Report (SoHM) should be available in early October, and the cover story for this issue of The Hospitalist previews some of the key findings.
I want to let you know what’s new and different about this year’s report and to explain why we made some of the changes we did. First, we had an opportunity this year to ask some questions that haven’t appeared in previous surveys, including:
- The percentage of the hospital’s total patient volume the HMG was responsible for caring for
- The presence of hospitalists within the HMG focusing their practice in a specific medical subspecialty, such as critical care, neurology, or oncology
- The annual dollar value of CME allowances for hospitalists
- The utilization of prolonged service codes by hospitalists
- Charge capture methodologies being used by HMGs
- For academic HMGs, the dollar amount of financial support provided for nonclinical work
- Questions regarding non-physician practice administrators that were asked in 2012 but not in 2014
One big change that users of the digital version will see is a much more user-friendly interface with vastly improved search and navigation features. This will be an enormous improvement over the essentially static PDF versions of previous years, and we’re very excited about it.
The other major change that all users will note is that beginning this year, SHM will no longer report findings broken out by employment model (e.g., hospital/IDS-employed versus management companies versus private local hospitalist groups, etc.). We know this will be a disappointment to some, but with the consolidation that has occurred in the management company space over the last couple of years, we found it would be difficult, if not impossible, to protect the confidentiality of information supplied by the largest management companies if the data are reported separately. Because of their sheer size, these mega-companies will be disproportionately represented in the survey results, and their identities and operational details might become apparent.
It’s crucial that management companies continue to be represented in SHM survey data because they represent an important and growing segment of the hospital medicine workforce. SoHM wouldn’t present a true picture of the hospital medicine field without them. We hope to continue to protect the confidentiality of all data and encourage more management companies to participate in future surveys while still providing meaningful information to our users. TH
Leslie Flores is a member of SHM’s Practice Analysis Committee and a partner in Nelson Flores Hospital Medicine Consultants.
It’s a little like giving birth: A nine-month-long process that started last January with the excitement and anticipation of launching the survey and encouraging as many hospital medicine groups (HMGs) as possible to participate. Then the long, drawn-out process of analyzing data, organizing everything into tables and charts, drafting the report, and reviewing it over and over until our eyes crossed. Watching it grow and take shape before our eyes, with a few small hiccups along the way. Then the agonizing process of copyediting, designing (both print and digital versions), and printing—a point at which, like all expectant parents, we said, “Enough already. When will this ever end?”
But we (that is, SHM’s Practice Analysis Committee) finally have a baby, and what proud parents we are! SHM’s 2016 State of Hospital Medicine Report (SoHM) should be available in early October, and the cover story for this issue of The Hospitalist previews some of the key findings.
I want to let you know what’s new and different about this year’s report and to explain why we made some of the changes we did. First, we had an opportunity this year to ask some questions that haven’t appeared in previous surveys, including:
- The percentage of the hospital’s total patient volume the HMG was responsible for caring for
- The presence of hospitalists within the HMG focusing their practice in a specific medical subspecialty, such as critical care, neurology, or oncology
- The annual dollar value of CME allowances for hospitalists
- The utilization of prolonged service codes by hospitalists
- Charge capture methodologies being used by HMGs
- For academic HMGs, the dollar amount of financial support provided for nonclinical work
- Questions regarding non-physician practice administrators that were asked in 2012 but not in 2014
One big change that users of the digital version will see is a much more user-friendly interface with vastly improved search and navigation features. This will be an enormous improvement over the essentially static PDF versions of previous years, and we’re very excited about it.
The other major change that all users will note is that beginning this year, SHM will no longer report findings broken out by employment model (e.g., hospital/IDS-employed versus management companies versus private local hospitalist groups, etc.). We know this will be a disappointment to some, but with the consolidation that has occurred in the management company space over the last couple of years, we found it would be difficult, if not impossible, to protect the confidentiality of information supplied by the largest management companies if the data are reported separately. Because of their sheer size, these mega-companies will be disproportionately represented in the survey results, and their identities and operational details might become apparent.
It’s crucial that management companies continue to be represented in SHM survey data because they represent an important and growing segment of the hospital medicine workforce. SoHM wouldn’t present a true picture of the hospital medicine field without them. We hope to continue to protect the confidentiality of all data and encourage more management companies to participate in future surveys while still providing meaningful information to our users. TH
Leslie Flores is a member of SHM’s Practice Analysis Committee and a partner in Nelson Flores Hospital Medicine Consultants.
It’s a little like giving birth: A nine-month-long process that started last January with the excitement and anticipation of launching the survey and encouraging as many hospital medicine groups (HMGs) as possible to participate. Then the long, drawn-out process of analyzing data, organizing everything into tables and charts, drafting the report, and reviewing it over and over until our eyes crossed. Watching it grow and take shape before our eyes, with a few small hiccups along the way. Then the agonizing process of copyediting, designing (both print and digital versions), and printing—a point at which, like all expectant parents, we said, “Enough already. When will this ever end?”
But we (that is, SHM’s Practice Analysis Committee) finally have a baby, and what proud parents we are! SHM’s 2016 State of Hospital Medicine Report (SoHM) should be available in early October, and the cover story for this issue of The Hospitalist previews some of the key findings.
I want to let you know what’s new and different about this year’s report and to explain why we made some of the changes we did. First, we had an opportunity this year to ask some questions that haven’t appeared in previous surveys, including:
- The percentage of the hospital’s total patient volume the HMG was responsible for caring for
- The presence of hospitalists within the HMG focusing their practice in a specific medical subspecialty, such as critical care, neurology, or oncology
- The annual dollar value of CME allowances for hospitalists
- The utilization of prolonged service codes by hospitalists
- Charge capture methodologies being used by HMGs
- For academic HMGs, the dollar amount of financial support provided for nonclinical work
- Questions regarding non-physician practice administrators that were asked in 2012 but not in 2014
One big change that users of the digital version will see is a much more user-friendly interface with vastly improved search and navigation features. This will be an enormous improvement over the essentially static PDF versions of previous years, and we’re very excited about it.
The other major change that all users will note is that beginning this year, SHM will no longer report findings broken out by employment model (e.g., hospital/IDS-employed versus management companies versus private local hospitalist groups, etc.). We know this will be a disappointment to some, but with the consolidation that has occurred in the management company space over the last couple of years, we found it would be difficult, if not impossible, to protect the confidentiality of information supplied by the largest management companies if the data are reported separately. Because of their sheer size, these mega-companies will be disproportionately represented in the survey results, and their identities and operational details might become apparent.
It’s crucial that management companies continue to be represented in SHM survey data because they represent an important and growing segment of the hospital medicine workforce. SoHM wouldn’t present a true picture of the hospital medicine field without them. We hope to continue to protect the confidentiality of all data and encourage more management companies to participate in future surveys while still providing meaningful information to our users. TH
Leslie Flores is a member of SHM’s Practice Analysis Committee and a partner in Nelson Flores Hospital Medicine Consultants.
VTE linked to permanent work-related disability

Image by Andre E.X. Brown
Unprovoked venous thromboembolism (VTE) may increase the risk of permanent work-related disability, according to research published in the Journal of Thrombosis and Haemostasis.
Researchers evaluated data from more than 60,000 people and found that individuals with unprovoked VTE had a 52% higher risk of work-related disability than those without VTE.
However, there was no association between provoked VTE and work-related disability.
In addition, only deep vein thrombosis (DVT)—not pulmonary embolism (PE)—was significantly associated with an increased risk of work-related disability.
For this study, the researchers analyzed data from the Tromsø Study and the Nord-Trøndelag Health Study, which enrolled 66,005 subjects from 1994 to 1997. The subjects’ mean age at inclusion was 41.3 (range, 20-65), and about half (51.2%, n=33,901) were women.
The subjects were followed through 2008. During the follow-up period, 384 individuals had a first VTE, and 9862 received a disability pension due to work-related disability.
Compared to individuals without VTE, those who had a VTE were slightly older and had a higher body mass index. VTE patients were more likely to have a history of cancer and cardiovascular disease, and they were more likely to have paying jobs.
In addition, subjects with VTE were more likely to have work-related disability. The crude incidence rate of disability was 37.5 per 1000 person-years among VTE patients and 13.5 per 1000 person-years among subjects without VTE.
VTE patients who received disability pension were slightly older than those who did not, and a higher proportion of the VTEs were DVTs rather than PEs. Half of the VTEs were unprovoked.
Multivariable analysis suggested that subjects with unprovoked VTE had a 52% higher risk of work-related disability than individuals without VTE (hazard ratio [HR]=1.52). However, there was no association between provoked VTE and work-related disability (HR=0.83).
When the researchers analyzed DVT and PE separately, they found that subjects with DVT had an 80% higher risk of work-related disability than those without DVT (HR=1.80). Subjects with PE had a moderately increased risk of work-related disability that was not statistically significant (HR=1.28).
When the researchers adjusted for baseline characteristics other than self-rated health, the association between work-related disability and DVT remained strong (HR=1.66), and there was no association between work-related disability and PE (HR=1.06).
The researchers said this study is the first of its kind to document a relationship between VTE and subsequent work-related disability. And the findings suggest the economic burden of VTE goes beyond costs to the healthcare system. The loss of economic output from people who are unable to work due to VTE-related complications is also a substantial economic cost of VTE. ![]()

Image by Andre E.X. Brown
Unprovoked venous thromboembolism (VTE) may increase the risk of permanent work-related disability, according to research published in the Journal of Thrombosis and Haemostasis.
Researchers evaluated data from more than 60,000 people and found that individuals with unprovoked VTE had a 52% higher risk of work-related disability than those without VTE.
However, there was no association between provoked VTE and work-related disability.
In addition, only deep vein thrombosis (DVT)—not pulmonary embolism (PE)—was significantly associated with an increased risk of work-related disability.
For this study, the researchers analyzed data from the Tromsø Study and the Nord-Trøndelag Health Study, which enrolled 66,005 subjects from 1994 to 1997. The subjects’ mean age at inclusion was 41.3 (range, 20-65), and about half (51.2%, n=33,901) were women.
The subjects were followed through 2008. During the follow-up period, 384 individuals had a first VTE, and 9862 received a disability pension due to work-related disability.
Compared to individuals without VTE, those who had a VTE were slightly older and had a higher body mass index. VTE patients were more likely to have a history of cancer and cardiovascular disease, and they were more likely to have paying jobs.
In addition, subjects with VTE were more likely to have work-related disability. The crude incidence rate of disability was 37.5 per 1000 person-years among VTE patients and 13.5 per 1000 person-years among subjects without VTE.
VTE patients who received disability pension were slightly older than those who did not, and a higher proportion of the VTEs were DVTs rather than PEs. Half of the VTEs were unprovoked.
Multivariable analysis suggested that subjects with unprovoked VTE had a 52% higher risk of work-related disability than individuals without VTE (hazard ratio [HR]=1.52). However, there was no association between provoked VTE and work-related disability (HR=0.83).
When the researchers analyzed DVT and PE separately, they found that subjects with DVT had an 80% higher risk of work-related disability than those without DVT (HR=1.80). Subjects with PE had a moderately increased risk of work-related disability that was not statistically significant (HR=1.28).
When the researchers adjusted for baseline characteristics other than self-rated health, the association between work-related disability and DVT remained strong (HR=1.66), and there was no association between work-related disability and PE (HR=1.06).
The researchers said this study is the first of its kind to document a relationship between VTE and subsequent work-related disability. And the findings suggest the economic burden of VTE goes beyond costs to the healthcare system. The loss of economic output from people who are unable to work due to VTE-related complications is also a substantial economic cost of VTE. ![]()

Image by Andre E.X. Brown
Unprovoked venous thromboembolism (VTE) may increase the risk of permanent work-related disability, according to research published in the Journal of Thrombosis and Haemostasis.
Researchers evaluated data from more than 60,000 people and found that individuals with unprovoked VTE had a 52% higher risk of work-related disability than those without VTE.
However, there was no association between provoked VTE and work-related disability.
In addition, only deep vein thrombosis (DVT)—not pulmonary embolism (PE)—was significantly associated with an increased risk of work-related disability.
For this study, the researchers analyzed data from the Tromsø Study and the Nord-Trøndelag Health Study, which enrolled 66,005 subjects from 1994 to 1997. The subjects’ mean age at inclusion was 41.3 (range, 20-65), and about half (51.2%, n=33,901) were women.
The subjects were followed through 2008. During the follow-up period, 384 individuals had a first VTE, and 9862 received a disability pension due to work-related disability.
Compared to individuals without VTE, those who had a VTE were slightly older and had a higher body mass index. VTE patients were more likely to have a history of cancer and cardiovascular disease, and they were more likely to have paying jobs.
In addition, subjects with VTE were more likely to have work-related disability. The crude incidence rate of disability was 37.5 per 1000 person-years among VTE patients and 13.5 per 1000 person-years among subjects without VTE.
VTE patients who received disability pension were slightly older than those who did not, and a higher proportion of the VTEs were DVTs rather than PEs. Half of the VTEs were unprovoked.
Multivariable analysis suggested that subjects with unprovoked VTE had a 52% higher risk of work-related disability than individuals without VTE (hazard ratio [HR]=1.52). However, there was no association between provoked VTE and work-related disability (HR=0.83).
When the researchers analyzed DVT and PE separately, they found that subjects with DVT had an 80% higher risk of work-related disability than those without DVT (HR=1.80). Subjects with PE had a moderately increased risk of work-related disability that was not statistically significant (HR=1.28).
When the researchers adjusted for baseline characteristics other than self-rated health, the association between work-related disability and DVT remained strong (HR=1.66), and there was no association between work-related disability and PE (HR=1.06).
The researchers said this study is the first of its kind to document a relationship between VTE and subsequent work-related disability. And the findings suggest the economic burden of VTE goes beyond costs to the healthcare system. The loss of economic output from people who are unable to work due to VTE-related complications is also a substantial economic cost of VTE. ![]()
What Hospitalists Can Learn from Basketball Coach Pat Summitt
I’m not exactly a devout follower of women’s college basketball. But having grown up in Knoxville, it was hard not to follow the University of Tennessee Lady Volunteers (“Lady Vols”) and the career of their longtime head coach, Pat Summitt. Summitt recently died from a swift and severe form of early-onset Alzheimer’s disease. In the wake of her death, many have analyzed the impact of her career and the legacy she has left from her lifetime of relentless coaching and developing of athletes. She was an incredible leader who should make us all reflect on the impact we are making in the lives of our patients and their families, our peers, and the next generation of hospitalists.
Early Career
Pat Summitt was born Patricia Sue Head in 1952 in Clarksville, Tenn., the daughter of Richard and Hazel Albright Head and the fourth of five children. When she was in high school, her family moved to another town so she could play basketball (as her local town did not have a girl’s team). Summitt attended the University of Tennessee at the Martin campus and played for its first women’s basketball coach. Although each of Summitt’s three brothers had received an athletic scholarship, at the time there were no athletic scholarships for women, so her parents supported her way through college.1
After college, Summitt started as a graduate assistant at the University of Tennessee. At the start of the 1974 basketball season, the head coach suddenly quit, and she was named the new head coach at the age of 22. (This was before women’s college basketball was even an NCAA-sanctioned sport.) Legend has it she was paid $250 a month and the team had almost no budget. She reportedly washed all the uniforms herself (which were purchased the year before from the proceeds of a doughnut sale) and drove the team van.1
Barely older than most of the players on the team, she coached her first game in December against Mercer University and lost 84–83. From then on, she racked up an incredible number of wins. In her second season, Summitt coached the team to a 16–11 record while working on her master’s degree in physical education.1
By 1978, Summitt recorded her 100th win and coached the Lady Vols in their first Association for Intercollegiate Athletics for Women Final Four. She ended the decade by winning their first-ever Southeastern Conference tournament. A few years later, in 1984, she coached the U.S. women’s team to an Olympic gold medal, becoming the first U.S. Olympian to win a basketball medal and coach a medal-winning team. There were countless other career milestones: She coached the Lady Vols in 16 SEC regular-season championships and 16 SEC tournament titles. She also coached the Lady Vols in 18 NCAA Final Fours.
Legacy
Summitt’s career-win total still stands as the most among NCAA Division I basketball coaches (men or women). Overall, Summitt finished her career with a record of 1,098-208 and a .841 winning percentage.
At the end of her career, there were 78 people mentored directly by her who were coaching basketball or working in administrative positions associated with the sport. Tennessee Athletic Director Dave Hart summarized her legacy:
“Pat Summitt is … truly is a global icon who transcended sports and spent her entire life making a difference in other peoples’ lives. … She was a genuine, humble leader who focused on helping people achieve more than they thought they were capable of accomplishing. … Her legacy will live on through the countless people she touched throughout her career.”2
Every player coached by Summitt finished her undergraduate degree, often with considerable prodding directly from her.
“Across the board with her kids, she also prepared them for life after basketball,” basketball coach Bob Knight said. “Not many people have prepared their players that well for life.”2
You don’t have to be a women’s basketball fan to understand and respect the impact that Summitt had on the lives she touched. She didn’t just win a lot of games—she changed the game. Think about how you will be remembered in your career as a hospitalist. Will you be remembered as someone clocking in and clocking out, just getting by for a paycheck? Or will you be remembered and revered as a “Summitt,” someone who always gave it their all and coached others to their best?
Hospital medicine is still in its relative infancy as a specialty. We all have the potential to pave a positive future for thousands more to come behind us; we all have the potential to be a Summitt. TH
References
1. Gregory S. Q&A: Tennessee Coach Pat Summitt. Time website. Accessed August 7, 2016.
2. Pat Summitt, winningest coach in Division I history, dies at 64. ESPN website. Accessed August 7, 2016.

I’m not exactly a devout follower of women’s college basketball. But having grown up in Knoxville, it was hard not to follow the University of Tennessee Lady Volunteers (“Lady Vols”) and the career of their longtime head coach, Pat Summitt. Summitt recently died from a swift and severe form of early-onset Alzheimer’s disease. In the wake of her death, many have analyzed the impact of her career and the legacy she has left from her lifetime of relentless coaching and developing of athletes. She was an incredible leader who should make us all reflect on the impact we are making in the lives of our patients and their families, our peers, and the next generation of hospitalists.
Early Career
Pat Summitt was born Patricia Sue Head in 1952 in Clarksville, Tenn., the daughter of Richard and Hazel Albright Head and the fourth of five children. When she was in high school, her family moved to another town so she could play basketball (as her local town did not have a girl’s team). Summitt attended the University of Tennessee at the Martin campus and played for its first women’s basketball coach. Although each of Summitt’s three brothers had received an athletic scholarship, at the time there were no athletic scholarships for women, so her parents supported her way through college.1
After college, Summitt started as a graduate assistant at the University of Tennessee. At the start of the 1974 basketball season, the head coach suddenly quit, and she was named the new head coach at the age of 22. (This was before women’s college basketball was even an NCAA-sanctioned sport.) Legend has it she was paid $250 a month and the team had almost no budget. She reportedly washed all the uniforms herself (which were purchased the year before from the proceeds of a doughnut sale) and drove the team van.1
Barely older than most of the players on the team, she coached her first game in December against Mercer University and lost 84–83. From then on, she racked up an incredible number of wins. In her second season, Summitt coached the team to a 16–11 record while working on her master’s degree in physical education.1
By 1978, Summitt recorded her 100th win and coached the Lady Vols in their first Association for Intercollegiate Athletics for Women Final Four. She ended the decade by winning their first-ever Southeastern Conference tournament. A few years later, in 1984, she coached the U.S. women’s team to an Olympic gold medal, becoming the first U.S. Olympian to win a basketball medal and coach a medal-winning team. There were countless other career milestones: She coached the Lady Vols in 16 SEC regular-season championships and 16 SEC tournament titles. She also coached the Lady Vols in 18 NCAA Final Fours.
Legacy
Summitt’s career-win total still stands as the most among NCAA Division I basketball coaches (men or women). Overall, Summitt finished her career with a record of 1,098-208 and a .841 winning percentage.
At the end of her career, there were 78 people mentored directly by her who were coaching basketball or working in administrative positions associated with the sport. Tennessee Athletic Director Dave Hart summarized her legacy:
“Pat Summitt is … truly is a global icon who transcended sports and spent her entire life making a difference in other peoples’ lives. … She was a genuine, humble leader who focused on helping people achieve more than they thought they were capable of accomplishing. … Her legacy will live on through the countless people she touched throughout her career.”2
Every player coached by Summitt finished her undergraduate degree, often with considerable prodding directly from her.
“Across the board with her kids, she also prepared them for life after basketball,” basketball coach Bob Knight said. “Not many people have prepared their players that well for life.”2
You don’t have to be a women’s basketball fan to understand and respect the impact that Summitt had on the lives she touched. She didn’t just win a lot of games—she changed the game. Think about how you will be remembered in your career as a hospitalist. Will you be remembered as someone clocking in and clocking out, just getting by for a paycheck? Or will you be remembered and revered as a “Summitt,” someone who always gave it their all and coached others to their best?
Hospital medicine is still in its relative infancy as a specialty. We all have the potential to pave a positive future for thousands more to come behind us; we all have the potential to be a Summitt. TH
References
1. Gregory S. Q&A: Tennessee Coach Pat Summitt. Time website. Accessed August 7, 2016.
2. Pat Summitt, winningest coach in Division I history, dies at 64. ESPN website. Accessed August 7, 2016.

I’m not exactly a devout follower of women’s college basketball. But having grown up in Knoxville, it was hard not to follow the University of Tennessee Lady Volunteers (“Lady Vols”) and the career of their longtime head coach, Pat Summitt. Summitt recently died from a swift and severe form of early-onset Alzheimer’s disease. In the wake of her death, many have analyzed the impact of her career and the legacy she has left from her lifetime of relentless coaching and developing of athletes. She was an incredible leader who should make us all reflect on the impact we are making in the lives of our patients and their families, our peers, and the next generation of hospitalists.
Early Career
Pat Summitt was born Patricia Sue Head in 1952 in Clarksville, Tenn., the daughter of Richard and Hazel Albright Head and the fourth of five children. When she was in high school, her family moved to another town so she could play basketball (as her local town did not have a girl’s team). Summitt attended the University of Tennessee at the Martin campus and played for its first women’s basketball coach. Although each of Summitt’s three brothers had received an athletic scholarship, at the time there were no athletic scholarships for women, so her parents supported her way through college.1
After college, Summitt started as a graduate assistant at the University of Tennessee. At the start of the 1974 basketball season, the head coach suddenly quit, and she was named the new head coach at the age of 22. (This was before women’s college basketball was even an NCAA-sanctioned sport.) Legend has it she was paid $250 a month and the team had almost no budget. She reportedly washed all the uniforms herself (which were purchased the year before from the proceeds of a doughnut sale) and drove the team van.1
Barely older than most of the players on the team, she coached her first game in December against Mercer University and lost 84–83. From then on, she racked up an incredible number of wins. In her second season, Summitt coached the team to a 16–11 record while working on her master’s degree in physical education.1
By 1978, Summitt recorded her 100th win and coached the Lady Vols in their first Association for Intercollegiate Athletics for Women Final Four. She ended the decade by winning their first-ever Southeastern Conference tournament. A few years later, in 1984, she coached the U.S. women’s team to an Olympic gold medal, becoming the first U.S. Olympian to win a basketball medal and coach a medal-winning team. There were countless other career milestones: She coached the Lady Vols in 16 SEC regular-season championships and 16 SEC tournament titles. She also coached the Lady Vols in 18 NCAA Final Fours.
Legacy
Summitt’s career-win total still stands as the most among NCAA Division I basketball coaches (men or women). Overall, Summitt finished her career with a record of 1,098-208 and a .841 winning percentage.
At the end of her career, there were 78 people mentored directly by her who were coaching basketball or working in administrative positions associated with the sport. Tennessee Athletic Director Dave Hart summarized her legacy:
“Pat Summitt is … truly is a global icon who transcended sports and spent her entire life making a difference in other peoples’ lives. … She was a genuine, humble leader who focused on helping people achieve more than they thought they were capable of accomplishing. … Her legacy will live on through the countless people she touched throughout her career.”2
Every player coached by Summitt finished her undergraduate degree, often with considerable prodding directly from her.
“Across the board with her kids, she also prepared them for life after basketball,” basketball coach Bob Knight said. “Not many people have prepared their players that well for life.”2
You don’t have to be a women’s basketball fan to understand and respect the impact that Summitt had on the lives she touched. She didn’t just win a lot of games—she changed the game. Think about how you will be remembered in your career as a hospitalist. Will you be remembered as someone clocking in and clocking out, just getting by for a paycheck? Or will you be remembered and revered as a “Summitt,” someone who always gave it their all and coached others to their best?
Hospital medicine is still in its relative infancy as a specialty. We all have the potential to pave a positive future for thousands more to come behind us; we all have the potential to be a Summitt. TH
References
1. Gregory S. Q&A: Tennessee Coach Pat Summitt. Time website. Accessed August 7, 2016.
2. Pat Summitt, winningest coach in Division I history, dies at 64. ESPN website. Accessed August 7, 2016.

Potential treatment strategy for dyskeratosis congenita

Preclinical research has revealed a potential treatment strategy for dyskeratosis congenita (DC).
Researchers found that DC is characterized by reductions in telomerase, telomere length, and telomere capping, which reduces Wnt pathway activity, resulting in intestinal stem cell failure.
However, treatment with Wnt agonists restored the Wnt-telomere feedback loop and reversed gastrointestinal DC phenotypes in vitro and in vivo.
Christopher J. Lengner, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues reported these discoveries in Cell Stem Cell.
“Right now, the main therapy for [DC] patients is a bone marrow transplant,” Dr Lengner said. “That can address the bone marrow failure but doesn’t fix other problems associated with the disease, and especially not the risk of cancer. This work suggests a way to address the underlying cause of the disease.”
Earlier research with mouse models of DC suggested there might be a connection between the Wnt pathway and telomerase. And a recent study in DC patients’ cells revealed a decrease in activity in the Wnt pathway.
So Dr Lengner and his colleagues wanted to explore whether activating Wnt could reverse the effects of the disease. To do so, the team used induced pluripotent stem cells (iPSCs), the CRISPR/Cas9 gene-editing system, and directed differentiation.
The researchers generated iPSCs from DKC1-mutant fibroblasts and from wild-type cells. The team also used CRISPR to introduce a DKC1 mutation into healthy human iPSCs and to correct the disease-causing mutation in iPSCs generated from DC patient samples.
The researchers then grew organoids through directed differentiation. iPSCs were coaxed to form a human intestinal organoid, which naturally forms a tube-like structure, recapitulating the tubes of the human gastrointestinal system.
When the researchers observed the development of intestinal organoids, they found that, initially, the DC cells seemed to form normally.
The original DKC1-mutant cells and the cells that had the DKC1 mutation introduced by CRISPR appeared to follow a normal course of development for several days. But by 2 weeks, they lacked the tube-like structure seen in the healthy samples and the disease-corrected samples.
The DKC1-mutant cells also had shorter telomeres, with the intestinal organoids from DC patients having the shortest of any cell type.
“We could see, at the molecular level, that this is accompanied by a failure to activate specific intestinal stem cell gene programs—specifically, genes in the Wnt pathway,” Dr Lengner said.
The next logical step was to activate Wnt to see if these defects could be reversed. The researchers treated organoids derived from DC patient iPSCs with a compound called CHIR that stimulates the Wnt pathway.
This restored the formation of the tube-like structure as well as intestinal stem cell gene expression. The treatment also increased telomerase activity and telomere length in the cells with mutant DKC1.
To assess this treatment approach in a more clinically relevant model, the researchers transplanted the human intestinal organoids into mice.
Mice that received a transplant containing the DKC1 mutation and received treatment with lithium, a stimulator of the Wnt pathway, maintained their intestinal tissue structure and had high expression of Wnt target genes.
In effect, these mice resembled the mice that received a transplant of an organoid derived from a healthy patient.
The researchers said this study offers proof of principle that activating the Wnt pathway can reverse at least the gastrointestinal phenotypes associated with DC. Looking ahead, the team would like to try accomplishing the same feat in other tissue types affected by the disease. ![]()

Preclinical research has revealed a potential treatment strategy for dyskeratosis congenita (DC).
Researchers found that DC is characterized by reductions in telomerase, telomere length, and telomere capping, which reduces Wnt pathway activity, resulting in intestinal stem cell failure.
However, treatment with Wnt agonists restored the Wnt-telomere feedback loop and reversed gastrointestinal DC phenotypes in vitro and in vivo.
Christopher J. Lengner, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues reported these discoveries in Cell Stem Cell.
“Right now, the main therapy for [DC] patients is a bone marrow transplant,” Dr Lengner said. “That can address the bone marrow failure but doesn’t fix other problems associated with the disease, and especially not the risk of cancer. This work suggests a way to address the underlying cause of the disease.”
Earlier research with mouse models of DC suggested there might be a connection between the Wnt pathway and telomerase. And a recent study in DC patients’ cells revealed a decrease in activity in the Wnt pathway.
So Dr Lengner and his colleagues wanted to explore whether activating Wnt could reverse the effects of the disease. To do so, the team used induced pluripotent stem cells (iPSCs), the CRISPR/Cas9 gene-editing system, and directed differentiation.
The researchers generated iPSCs from DKC1-mutant fibroblasts and from wild-type cells. The team also used CRISPR to introduce a DKC1 mutation into healthy human iPSCs and to correct the disease-causing mutation in iPSCs generated from DC patient samples.
The researchers then grew organoids through directed differentiation. iPSCs were coaxed to form a human intestinal organoid, which naturally forms a tube-like structure, recapitulating the tubes of the human gastrointestinal system.
When the researchers observed the development of intestinal organoids, they found that, initially, the DC cells seemed to form normally.
The original DKC1-mutant cells and the cells that had the DKC1 mutation introduced by CRISPR appeared to follow a normal course of development for several days. But by 2 weeks, they lacked the tube-like structure seen in the healthy samples and the disease-corrected samples.
The DKC1-mutant cells also had shorter telomeres, with the intestinal organoids from DC patients having the shortest of any cell type.
“We could see, at the molecular level, that this is accompanied by a failure to activate specific intestinal stem cell gene programs—specifically, genes in the Wnt pathway,” Dr Lengner said.
The next logical step was to activate Wnt to see if these defects could be reversed. The researchers treated organoids derived from DC patient iPSCs with a compound called CHIR that stimulates the Wnt pathway.
This restored the formation of the tube-like structure as well as intestinal stem cell gene expression. The treatment also increased telomerase activity and telomere length in the cells with mutant DKC1.
To assess this treatment approach in a more clinically relevant model, the researchers transplanted the human intestinal organoids into mice.
Mice that received a transplant containing the DKC1 mutation and received treatment with lithium, a stimulator of the Wnt pathway, maintained their intestinal tissue structure and had high expression of Wnt target genes.
In effect, these mice resembled the mice that received a transplant of an organoid derived from a healthy patient.
The researchers said this study offers proof of principle that activating the Wnt pathway can reverse at least the gastrointestinal phenotypes associated with DC. Looking ahead, the team would like to try accomplishing the same feat in other tissue types affected by the disease. ![]()

Preclinical research has revealed a potential treatment strategy for dyskeratosis congenita (DC).
Researchers found that DC is characterized by reductions in telomerase, telomere length, and telomere capping, which reduces Wnt pathway activity, resulting in intestinal stem cell failure.
However, treatment with Wnt agonists restored the Wnt-telomere feedback loop and reversed gastrointestinal DC phenotypes in vitro and in vivo.
Christopher J. Lengner, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues reported these discoveries in Cell Stem Cell.
“Right now, the main therapy for [DC] patients is a bone marrow transplant,” Dr Lengner said. “That can address the bone marrow failure but doesn’t fix other problems associated with the disease, and especially not the risk of cancer. This work suggests a way to address the underlying cause of the disease.”
Earlier research with mouse models of DC suggested there might be a connection between the Wnt pathway and telomerase. And a recent study in DC patients’ cells revealed a decrease in activity in the Wnt pathway.
So Dr Lengner and his colleagues wanted to explore whether activating Wnt could reverse the effects of the disease. To do so, the team used induced pluripotent stem cells (iPSCs), the CRISPR/Cas9 gene-editing system, and directed differentiation.
The researchers generated iPSCs from DKC1-mutant fibroblasts and from wild-type cells. The team also used CRISPR to introduce a DKC1 mutation into healthy human iPSCs and to correct the disease-causing mutation in iPSCs generated from DC patient samples.
The researchers then grew organoids through directed differentiation. iPSCs were coaxed to form a human intestinal organoid, which naturally forms a tube-like structure, recapitulating the tubes of the human gastrointestinal system.
When the researchers observed the development of intestinal organoids, they found that, initially, the DC cells seemed to form normally.
The original DKC1-mutant cells and the cells that had the DKC1 mutation introduced by CRISPR appeared to follow a normal course of development for several days. But by 2 weeks, they lacked the tube-like structure seen in the healthy samples and the disease-corrected samples.
The DKC1-mutant cells also had shorter telomeres, with the intestinal organoids from DC patients having the shortest of any cell type.
“We could see, at the molecular level, that this is accompanied by a failure to activate specific intestinal stem cell gene programs—specifically, genes in the Wnt pathway,” Dr Lengner said.
The next logical step was to activate Wnt to see if these defects could be reversed. The researchers treated organoids derived from DC patient iPSCs with a compound called CHIR that stimulates the Wnt pathway.
This restored the formation of the tube-like structure as well as intestinal stem cell gene expression. The treatment also increased telomerase activity and telomere length in the cells with mutant DKC1.
To assess this treatment approach in a more clinically relevant model, the researchers transplanted the human intestinal organoids into mice.
Mice that received a transplant containing the DKC1 mutation and received treatment with lithium, a stimulator of the Wnt pathway, maintained their intestinal tissue structure and had high expression of Wnt target genes.
In effect, these mice resembled the mice that received a transplant of an organoid derived from a healthy patient.
The researchers said this study offers proof of principle that activating the Wnt pathway can reverse at least the gastrointestinal phenotypes associated with DC. Looking ahead, the team would like to try accomplishing the same feat in other tissue types affected by the disease. ![]()
How to Nail the Diagnosis
A 33-year-old African-American man is referred to dermatology—somewhat reluctantly—by his primary care provider for evaluation. A “fungal infection” has affected his fingernail on and off for several years, persisting despite four months of terbinafine treatment (250 mg/d).
The patient denies any antecedent trauma to the finger. He does have a history of hand eczema, mostly affecting the palmar surface of his hand, and atopy, marked by seasonal allergies and sensitive skin.
EXAMINATION
The patient’s fourth fingernail on his right hand is significantly dystrophic, with transverse ridges and modest onychorrhexis. The cuticle is detached from the nail plate, but the distal nail plate appears normal.
There are signs of his dyshidrotic hand eczema, with several 2 to 3 mm intradermal vesicles in the distal palm. Some spill onto the sides of his fingers.
What is the diagnosis?
DISCUSSION
To the unwary provider, every fingernail problem is fungal in nature; they simply have no other items in their differential, which is why terbinafine is overprescribed for nonfungal conditions. Even when we suspect fungal infection, the appropriate course would be to sample the nail plate and send it for culture or pathologic examination—not just throw another prescription at it.
However, in cases such as this one, there are other explanations. This patient’s nail issues are secondary to his eczema and are likely related to the disconnect between the cuticle and the nail plate. This gap allows debris (bits of food, dirt, etc) access to the nail matrix, thereby causing a misshapen nail. The key to diagnosis is the transverse ridging and detached cuticles. A history of hand eczema bolsters this impression—infection is not involved.
Application of a mid-strength steroid ointment to the cuticle area encourages it to reattach to the nail. This gives the nail a chance to grow in normally.
TAKE-HOME LEARNING POINTS
• Fungal infections are significantly (18 times) more common on toenails than on fingernails.
• Eczema on the hand or body can also lead to transverse ridges in fingernails, especially when the cuticle detaches from the nail plate.
• Other items in the differential include psoriasis, lichen planus, and nail changes associated with alopecia areata.
A 33-year-old African-American man is referred to dermatology—somewhat reluctantly—by his primary care provider for evaluation. A “fungal infection” has affected his fingernail on and off for several years, persisting despite four months of terbinafine treatment (250 mg/d).
The patient denies any antecedent trauma to the finger. He does have a history of hand eczema, mostly affecting the palmar surface of his hand, and atopy, marked by seasonal allergies and sensitive skin.
EXAMINATION
The patient’s fourth fingernail on his right hand is significantly dystrophic, with transverse ridges and modest onychorrhexis. The cuticle is detached from the nail plate, but the distal nail plate appears normal.
There are signs of his dyshidrotic hand eczema, with several 2 to 3 mm intradermal vesicles in the distal palm. Some spill onto the sides of his fingers.
What is the diagnosis?
DISCUSSION
To the unwary provider, every fingernail problem is fungal in nature; they simply have no other items in their differential, which is why terbinafine is overprescribed for nonfungal conditions. Even when we suspect fungal infection, the appropriate course would be to sample the nail plate and send it for culture or pathologic examination—not just throw another prescription at it.
However, in cases such as this one, there are other explanations. This patient’s nail issues are secondary to his eczema and are likely related to the disconnect between the cuticle and the nail plate. This gap allows debris (bits of food, dirt, etc) access to the nail matrix, thereby causing a misshapen nail. The key to diagnosis is the transverse ridging and detached cuticles. A history of hand eczema bolsters this impression—infection is not involved.
Application of a mid-strength steroid ointment to the cuticle area encourages it to reattach to the nail. This gives the nail a chance to grow in normally.
TAKE-HOME LEARNING POINTS
• Fungal infections are significantly (18 times) more common on toenails than on fingernails.
• Eczema on the hand or body can also lead to transverse ridges in fingernails, especially when the cuticle detaches from the nail plate.
• Other items in the differential include psoriasis, lichen planus, and nail changes associated with alopecia areata.
A 33-year-old African-American man is referred to dermatology—somewhat reluctantly—by his primary care provider for evaluation. A “fungal infection” has affected his fingernail on and off for several years, persisting despite four months of terbinafine treatment (250 mg/d).
The patient denies any antecedent trauma to the finger. He does have a history of hand eczema, mostly affecting the palmar surface of his hand, and atopy, marked by seasonal allergies and sensitive skin.
EXAMINATION
The patient’s fourth fingernail on his right hand is significantly dystrophic, with transverse ridges and modest onychorrhexis. The cuticle is detached from the nail plate, but the distal nail plate appears normal.
There are signs of his dyshidrotic hand eczema, with several 2 to 3 mm intradermal vesicles in the distal palm. Some spill onto the sides of his fingers.
What is the diagnosis?
DISCUSSION
To the unwary provider, every fingernail problem is fungal in nature; they simply have no other items in their differential, which is why terbinafine is overprescribed for nonfungal conditions. Even when we suspect fungal infection, the appropriate course would be to sample the nail plate and send it for culture or pathologic examination—not just throw another prescription at it.
However, in cases such as this one, there are other explanations. This patient’s nail issues are secondary to his eczema and are likely related to the disconnect between the cuticle and the nail plate. This gap allows debris (bits of food, dirt, etc) access to the nail matrix, thereby causing a misshapen nail. The key to diagnosis is the transverse ridging and detached cuticles. A history of hand eczema bolsters this impression—infection is not involved.
Application of a mid-strength steroid ointment to the cuticle area encourages it to reattach to the nail. This gives the nail a chance to grow in normally.
TAKE-HOME LEARNING POINTS
• Fungal infections are significantly (18 times) more common on toenails than on fingernails.
• Eczema on the hand or body can also lead to transverse ridges in fingernails, especially when the cuticle detaches from the nail plate.
• Other items in the differential include psoriasis, lichen planus, and nail changes associated with alopecia areata.