User login
ADHD symptoms are stable, then a sudden relapse
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
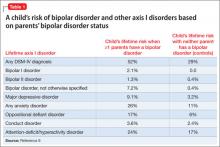
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
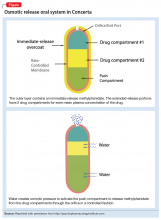
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
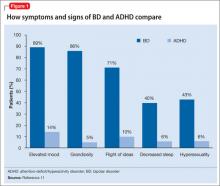
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
CASE
Sudden deterioration
R, age 11, has attention-deficit/hyperactivity disorder (ADHD), combined type, and oppositional defiant disorder, which has been stable for more than a year on extended-release (ER) methylphenidate (brand name: Concerta), 54 mg/d (1.2 mg/kg). With combined pharmacotherapy and behavioral management, his symptoms of hyperactivity, inattention, and impulsivity improved at school and at home. He shows some academic gains as evidenced by improved achievement at school.
Over 2 months, R experiences a substantial deterioration in behavioral and academic performance. Along with core symptoms of ADHD, he begins to exhibit physical and verbal aggression. A report from school states that R has been using obscene language and destroying property, and has had episodes of provoked aggression toward his peers. His grades drop and he receives 2 school suspensions because of aggressive behavior.
What could be causing R’s ADHD symptoms to reemerge?
a) nonadherence to treatment
b) substance abuse
c) medication change
d) all of the above
The authors’ observations
Worsening of psychiatric symptoms in a stable patient is relatively common. Many factors can contribute to patient destabilization. Treatment nonadherence is a leading cause, along with psychosocial stressors and substance use (Table).
EVALUATION
Adherence confirmed
R is hyperactive and distracted during his visit, a clear deterioration from his baseline status. R is oppositional and defiant toward his mother during the session, but shows good social skills when communicating with the physician.
R’s mother reports that her son seldom forgets to take his medication, and she ensures that he is swallowing the pill, rather than chewing it. Data from the prescription drug-monitoring program show that the family is filling the prescriptions regularly. The ER methylphenidate dosage is raised to 72 mg/d. The clinicians provide psychoeducation about adherence to a medication regimen to R and his family. Also, his parents and teachers receive Vanderbilt Assessment Scales for ADHD to assess the symptoms in different settings.
At a follow-up visit a week later, R’s mother reports that her son continues to have problems in school and at home. The Vanderbilt scales reveal that R is having clinically significant problems with attention, hyperactivity, impulse control, and oppositional behavior.
A urine drug screen is ordered to rule out the possibility of a sudden deterioration of ADHD symptoms secondary to substance use disorder. To ensure compliance, we recommend that R take his medication at the school nurse’s office in the morning.
A week later
Although R takes his medication at school, he continues to show core symptoms of ADHD without improvement. The urine drug screen is negative. A physical examination does not reveal any medical illness. The treatment team calls the pharmacist to obtain a complete list of medications R is taking, who confirms that he is only receiving ER methylphenidate, 72 mg/d. The pharmacist also notes that R’s medication was switched from the brand-name drug to a generic 3 months ago because of a change in insurance coverage. This change coincided with the reemergence of his ADHD symptoms.
R’s mother reports that the new pills do not look like the old ones even before the dosage was raised. A new brand-necessary prescription is sent to the pharmacy. With the brand-name medication, R’s symptoms quickly improve, and remain improved when the dosage is decreased to the previous dosage of 54 mg/d.
With osmotic-controlled release oral delivery system (OROS) and outer coating of ER methylphenidate, how much drug is released immediately vs slow release?
a) 22% immediate release and 78% slow release
b) 78% immediate release and 22% slow release
c) 50% immediate release and 50% slow release
The authors’ observations
Generic substitution of a brand medication can result in worsening of symptoms and increased adverse effects. Possible bioequivalence issues can lead to failure of drug therapy.1
In 2013, the FDA determined that 2 specific generic formulations of ER methylphenidate do not have therapeutic equivalency to the brand-name medication, Concerta. The FDA stated, “Based on an analysis of data, FDA has concerns about whether or not two approved generic versions of Concerta tablets (methylphenidate hydrochloride extended-release tablets), used to treat attention-deficit hyperactivity disorder in adults and children, are therapeutically equiv
In an apparent confirmation of the FDA’s concerns, a case series of children and adolescents with ADHD observed that almost all of the patients showed symptom improvement when they switched from a non-OROS formulation to an OROS preparation at the same dosage.3
The OROS preparation is thought to provide more predictable medication delivery over an extended period of time (Figure). A patient taking an ER formulation without OROS might lose this benefit, which could lead to symptom destabilization, even if the patient is taking the medication as instructed.
Brand vs generic
Under FDA regulations, companies seeking approval for generic formulations of approved drugs must demonstrate that their products are the same as the brand-name drug in terms of:
- active ingredients
- strength
- dosage form
- route of administration
- packaging label.
In addition, the pharmaceutical company must demonstrate that the generic form is absorbed and distributed to the part of the body at which it has its effect at acceptably similar levels to the brand-name drug. All medications—new or generic, in clinical trials or approved, prescription or over-the-counter—must be manufactured under controlled conditions that assure product quality.
However, some studies have disputed this equivalency. In 1 study, patients with schizophrenia receiving generic olanzapine had lower serum concentration than patients with schizophrenia taking equivalent dosages of brand-name olanzapine.4 Similarly, studies comparing generic and brand-name venlafaxine showed significant differences in peak plasma concentration (Cmax)between generic and brand-name compounds.5
The FDA has considered upgrading the manufacturers’ warnings about the risk of generic medications, but has delayed the decision to 2017.6
FDA’s approval process for generic drugs
To receive approval of a generic formulation in the United States, the FDA requires that the generic drug should be compared with the corresponding brand-name drug in small crossover trials involving at least 24 to 36 healthy volunteers.
Bioequivalence is then established based on assessments of the rate of absorption (Cmax and area under the plasma concentration-time curve [AUC]). The FDA’s criteria are designed to achieve 90% confidence that the ratios of the test-to-reference log-transformed mean values for AUC and Cmax are within the interval of 80% to 125%. The FDA accepts −20% to 25% variation in Cmax and AUC in products that are considered bioequivalent. This is much less stringent than its −5% to 5% standard used for brand-name products. The FDA publishes a list of generic drugs that have been certified as bioequivalent, known as the “Orange Book.”5
Considerations when substituting generic medication
Because of the growing number of generic formulations of the same medication, generic–generic switches are becoming more commonplace. Theoretically, any 2 generic versions of the same medication can have a variation of up to 40% in AUC and Cmax. Generic medications are tested in healthy human controls through single-dose studies, which raises concerns about their applicability to the entire patient population.
Bioequivalence. It is a matter of debate whether bioequivalence translates to therapeutic equivalency. For medications with a narrow therapeutic index, the FDA has accepted that these 2 phenomena are not necessarily linked. With the exception of a few medications, including lithium and some anticonvulsants such as divalproex sodium and carbamazepine, serum level of the medications usually does not predict clinical response.
Inert ingredients. Generic medications can include inert ingredients (excipients) that are different from those in their branded counterparts. Some of these inactive ingredients can cause adverse effects. A study comparing paroxetine mesylate and paroxetine hydrochloride showed differences in bioequivalence and clinical efficacy.7
In some cases, brand-to-generic substitution can thwart clinical progress in a stable patient. This small change in the medication could destabilize the patient’s condition, which, in turn, may lead to unnecessary and significant social and financial burdens on the patient’s family, school, community, and the health care system.
Recommendations
In the event of a change in clinical response, clinicians first should evaluate adherence and explore other factors, such as biological, psychological, medical, and social issues. Adherence can be adversely affected by a change in the physical characteristics of the pill. Prescribers should remain cognizant of brand–generic and generic–generic switches. It may be reasonable to adjust the dosage of the new generic medication to address changes in clinical effectiveness.
If these strategies are ineffective, consider switching to a brand-name medication. Write “Dispense As Written” on the prescription to ensure delivery of the branded medication or a specific generic version of the medication.
An insurance company might require prior authorization to approve payment for the brand medication. To save time, use electronic forms or fax for communicating with the insurance company. Adding references to FDA statements and research papers, along with the patient’s history and presentations, would be prudent to demonstrate doubts about efficacy of the generic medication.
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
1. Atif M, Azeem M, Sarwar MR. Potential problems and recommendations regarding substitution of generic antiepileptic drugs: a systematic review of literature. Springerplus. 2016;5:182. doi: 10.1186/s40064-016-1824-2.
2. U.S. Food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. http://www.fda.gov/Drugs/DrugSafety/ucm422568.htm. Updated November 13, 2014. Accessed August 29, 2016.
3. Lally MD, Kral MC, Boan AD. Not all generic Concerta is created equal: comparison of OROS versus non-OROS for the treatment of ADHD [published online October 14, 2015]. Clin Pediatr (Phila). doi:10.1177/0009922815611647.
4. Italiano DD, Bruno A, Santoro V, et al. Generic olanzapine substitution in patients with schizophrenia: assessment of serum concentrations and therapeutic response after switching. Ther Drug Monit. 2015;37(6):827-830.
5. Borgheini GG. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25(6):1578-1592.
6. Thomas K. F.D.A. delays rule on generic drug labels. http://www.nytimes.com/2016/05/20/business/fda-delays-rule-on-generic-drug-labels.html. Published May 19, 2016. Accessed August 29, 2016.
7. Pae CU, Misra A, Ham BJ, et al. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11(2):185-193
High-value intervention: Providing colorectal cancer screening
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
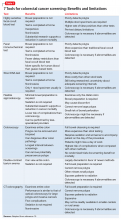
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
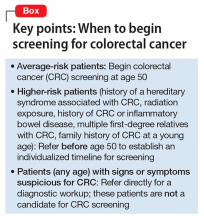
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
Hunting for ‘Woozles’ in the Hundred Acre Wood of ADHD
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
One fine winter’s day when Piglet was brushing away the snow
in front of his house he happened to look up, and there was
Winnie-the-Pooh. Pooh was walking round and round in a circle,
thinking of something else…
So begins the 1926 Winnie-the-Pooh story.1 In this chapter, the well-meaning yellow bear, Winnie-the-Pooh, has found strange tracks in the snow, which he believes belong to a “Woozle.” Pooh follows the tracks, not realizing that he’s walking in a circle. As such, he begins to notice that the tracks have multiplied, which he interprets as evidence of several Woozles.
This “Woozle Effect” has been well described in research settings and is believed to have resulted in conclusions that are not supported by or are inconsistent with the original data, which are then propagated through successive citations, resulting in a scientific “urban legend.”2
Throughout my training from medical school, through fellowship, and during my tenure as a faculty member, I have found myself, at times, searching for Woozles and often have joined my colleagues on these hunts. Herein, I would like to share with you 3 Woozles that have resulted in current false dogmas related to attention-deficit/hyperactivity disorder (ADHD) and stimulant psychopharmacology.
Stimulants worsen anxiety
FDA-required labeling for stimulants includes strong language noting that these drugs are “contraindicated in marked anxiety, tension, and agitation, since the drug may aggravate these symptoms.”3 However, data from randomized controlled trials and meta-analyses consistently have failed to demonstrate this effect. Moreover, sequenced treatment trials involving adolescents with anxiety disorders and co-occurring ADHD suggest that stimulants actually could reduce anxiety symptoms.
A recent meta-analysis4 that evaluated nearly 2 dozen studies involving approximately 3,000 pediatric patients with ADHD reported that stimulant treatment was associated with a decreased relative risk of anxiety (relative risk: 0.86). The study also observed a dose-response relationship between stimulant dosage and anxiety (Figure, page 6).4 Although the authors note that it is possible that some individuals might experience increased anxiety with stimulants, many patients could show improvement in anxiety symptoms when treated with stimulants, and the authors also advise us, as clinicians, to “consider re-challenging children with ADHD who report … anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.”4
More evidence of a lack of stimulant-induced anxiety comes from a large randomized controlled trial of pediatric patients (age 6 to 17) who met DSM-IV criteria for ADHD and a co-occurring anxiety disorder who were treated with methylphenidate (open-label) and then randomized to fluvoxamine or placebo for treatment of anxiety symptoms.5 However, in this trial >80% of the 32 medication-naïve youth improved after stimulant treatment to the point that they no longer had anxiety symptoms severe enough to be eligible for randomization to adjunctive fluvoxamine or placebo.
Stimulants are contraindicated in patients with tic disorders
The package inserts for most stimulant medications warn clinicians that stimulants are “contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome.” This is particularly concerning, especially because of the medicolegal implications of the term “contraindicated” and given that as many as 1 in 5 pediatric patients with ADHD have a tic disorder.6 Therefore, labels that list motor tics as a contraindication to stimulant use potentially eliminate the choice of stimulant pharmacotherapy—the most effective treatment for ADHD—for a large number of patients.
When hunting for the Woozle that linked stimulants and tics and led to this language in the package insert, it is worthwhile to review a recent meta-analysis of 22 studies (involving nearly 2,400 youths with ADHD) that suggested new-onset tics or worsening of tics to be present in 5.7% of patients receiving stimulants and in 6.5% of patients receiving placebo. In addition, in this meta-analysis the class of stimulant, dosage, treatment duration, or patient age did not seem to be associated with onset or worsening of tics.7
Polypharmacy represents a therapeutic failure and is not evidence-based
Although treatment guidelines generally have discouraged combination therapy for treating ADHD, there are—on the basis of efficacy—insufficient data to support this prohibition. Moreover, over the last decade, several studies have suggested benefits for combining ADHD medications that have complimentary mechanisms. In this regard, 2 extended-release formulations of α2 agonists have received FDA approval for as adjunctive treatments in pediatric patients with ADHD (extended-release guanfacine and extended-release clonidine). However, despite these FDA indications as adjunctive treatments, many clinicians remain concerned about combination therapy.
Several months ago, a large, 8-week, National Institutes of Health–sponsored trial shed more light on the use of α2agonist + stimulant combinations. Patients age 7 to 17 (N = 179) were randomized to (1) guanfacine + d-methylphenidate, (2) guanfacine monotherapy, or (3) d-methylphenidate monotherapy.8 In addition to clinical outcomes, the authors evaluated the effects of the medication on background cortical activity. Of interest, monotherapies differed between one another and the combination treatment in their effects on cortical activity. Guanfacine decreased alpha band power and methylphenidate administration was associated with an increase in frontal/central beta power, while combination treatment dampened theta band power and was associated with specific, focal increases in beta power.8 These results, although preliminary, suggest not only that medication results in changes in cortical activity that correlate with symptomatic improvement, but that combination treatment may be associated with a distinct cortical activity pattern that is more than the summation of the effects of the monotherapies. Moreover, these data raise the possibility that this synergistic effect on cortical activity may subtend—or at least—relate to the synergistic clinical effects of the 2 medications.
‘Think it over, think it under’
Having discussed several important Woozles that have inhabited the Hundred Acre Wood of ADHD for decades, it is important to remember there are countless Woozles in the larger “Thousand Acre Wood” of psychiatry and medicine. As we evaluate evidence for our interventions, whether psychopharmacologic or psychotherapeutic, we will do w
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
1. Milne AA. Winnie-the-Pooh. London, United Kingdom: Methuen & Co. Ltd.; 1926.
2. Strauss MA. Processes Explaining the concealment and distortion of evidence on gender symmetry in partner violence. Eur J Crim Pol Res. 1980;74:227-232.
3. Ritalin LA [package insert]. East Hanover, NJ: Novartis; 2015.
4. Coughlin CG, Cohen SC, Mulqueen JM, et al. Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25(8):611-617.
5. Abikoff H, McGough J, Vitiello B, et al; RUPP ADHD/Anxiety Study Group. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418-427.
6. Bloch MH, Panza KE, Landeros-Weisenberger A, et al. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884-893.
7. Cohen SC, Mulqueen JM, Ferracioli-Oda E, et al. Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54(9):728-736.
8. Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674-682.e1.
Where do you draw the line? Caveats for after-hours call coverage
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Handling patient emergencies is one of the most challenging parts of clinical care. Not only does the provider have to consider the best care for the patient, he (she) must think through medicolegal considerations, as well as what systems are sustainable in a practice, and then develop a plan that addresses all those interests. Being on-call for emergencies in a solo private practice can be especially complex, because the provider is always, and solely, responsible for handling or redirecting these calls, which is one reason some physicians choose to be part of a group practice or be an employee.
First, let’s define a few different types of “emergencies” that you might encounter:
- A genuine life or death situation. A patient calls during planning, or after attempting, suicide.
- An urgent matter. A patient has run out of medication or she (he) is having discontinuation symptoms or adverse effects. Although there is no imminent danger, the patient may be experiencing significant discomfort.
- A matter of high anxiety. The patient is experiencing situations that provoke high affect, and she needs attention at that moment to lessen the burden.
Of course, you might not know the true extent of the emergency until you talk to the patient, but being able to delineate different procedures for patients based on the types of emergency situations could streamline your workflow.
With this foundation in place, let’s discuss the most common practice policies for dealing with these emergencies.
Instructing patients to call 911 or go to the emergency room (ER)
The pros.
- Meets minimum standards without any additional work.
- Reinforces work-life boundaries.
- Makes private practice tolerable.
The cons.
- Patients might not feel properly cared for.
- The patient might not want to call 911 in some situations (eg, suicidality).
- You might not know if your patient went to the ER unless hospital staff or the patient contacts you afterwards.
Using an answering service
The pros.
- Patients feel reassured that they can get your attention after hours and get a call back from you.
- Patients are familiar with this practice because it is widely used in the medical field.
- Operators are trained to screen for emergencies and can be given a script of questions to ask, and given clear guidelines so they know whether to contact you immediately.
- Establishes a healthy boundary between work and personal life.
The cons.
- Cost.
- Patients still might be frustrated if they can’t directly connect with you.
- Requires training and trusting the answering service staff.
Giving your home or cell number to patients
The pros.
- Patients might feel cared for and reassured that they can reach you directly at any time, which may, itself, be calming and reduce their need to contact you.
- Providers can maintain complete control over their practice at all times.
- Providers can market the practice as a “concierge” service.
- You can give your personal phone number to certain patients at certain times, rather than making it a practice-wide policy.
The cons.
- Providers may feel like they are working all the time. What if you go out of the country, or find yourself in a cell phone dead zone? You’ll need to have a colleague cover for you or refer patients to 911 or the ER.
- Some patients could abuse the privilege.
- Boundaries between work and personal life can crumble.
- Being available 24/7 over a 30-year career could feel onerous.
Be sure to discuss your policies with your patient at the first visit. Choosing the best policies for your practice involves providing good patient care, meeting or exceeding the standard of care, and finding the right fit for you.
Rule out these causes of inattention before diagnosing ADHD
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
Inattention and distractibility are highly prevalent, and can exist secondary to a number of underlying causes. When a patient (or the patient’s family) asks whether he (she) might have attention-deficit/hyperactivity disorder (ADHD), you must perform a comprehensive assessment to rule out other medical and psychiatric disorders that might be manifesting as inattention. It is important not to miss a diagnosis of ADHD, and it is vital not to mistake another medical or psychiatric condition as ADHD.
Pay attention to components of the differential diagnosis while you are evaluating a patient with possible ADHD.
Medical conditions. Several disorders can present with cognitive, attentional, and executive functioning deficits that resemble the presentation of ADHD. These include absence seizures and other types of seizures, Lyme disease, HIV infection, and encephalopathy.1
People who have completed chemotherapy (particularly children) often exhibit attentional and executive functioning deficits similar to those found in ADHD.1
Anxiety disorders, the most prevalent of psychiatric disorders, correlate highly with difficulty concentrating. Chronic stress can have negative effects on hippocampus- and prefrontal cortical-based memory and cognitive functions.2 Be cautious, therefore, when diagnosing ADHD in a patient who suffers from significant, acute, or inadequately controlled anxiety—especially one who does not have a history of a childhood onset of attentional difficulties.
On the other hand, untreated ADHD can lead to anxiety symptoms.
Drugs. A number of substances of abuse—marijuana, cocaine, ecstasy, and caffeine—can produce symptoms of poor attention or impulsivity, similar to what is seen in ADHD, through their effects on the hippocampus and prefrontal cortex.3,4 MRI studies of the brains of 8-year-olds prenatally exposed to cocaine have found changes in frontal lobes suggesting potential long-term effects on attention and impulse control in these children.5,6
Use of certain medications, such as anticholinergics, also can contribute to attentional difficulties in some patient populations.
Abuse or trauma. Difficulty concentrating is one of the core symptoms of posttraumatic stress disorder (PTSD). Rule out PTSD and recent abuse or trauma when assessing for ADHD. Children with recent trauma often present with agitation, restlessness, and behavioral disturbance—symptoms that mimic ADHD.
Mood and adjustment disorders. Difficulty concentrating also is a criterion for major depressive disorder. On the other hand, untreated ADHD also can lead to, or contribute to, development of a depressive disorder. If a patient is experiencing a major depressive episode, obtain a thorough collateral history delineating a timeline of attention difficulties, which should allow for an accurate diagnosis.
In children, ADHD and bipolar disorder can have symptom overlap; both can present with distractibility, increased energy, and mood lability—therefore making a careful history a diagnostic necessity. Furthermore, ADHD and bipolar disorder can coexist in a small percentage of ADHD patients.
Hypothyroidism. Studies show a decrease in memory, attention, and concentration in patients with overt hypothyroidism, and at least a small decrease in these domains in patients with subclinical hypothyroidism.7 Decreased cerebral blood flow in brain regions that mediate attention and executive functioning, and decreased hippocampal volume, have been observed in patients with hypothyroidism.7 Therefore, the cognitive profile in these patients can look similar to, and can be confused with, ADHD, inattentive type.
Insomnia. Sleep plays a key role in memory consolidation and maintaining attention. Sleep disorders (eg, sleep apnea, restless legs syndrome, delayed sleep phase-onset disorder) can produce chronic tiredness and significantly affect attention, concentration, and cognitive functioning in children, adolescents, and adults.8
Studies in adults have shown that sleep deprivation is linked to attentional difficulty secondary to changes in prefrontal cortex activity.9 Other studies suggest that short sleep duration in healthy children is associated with inattention and poorer academic functioning, and also was found linked to teacher reports of inattention and a cognitive profile similar to what is seen in ADHD.8
Learning disorders and developmental disabilities. Children with an undiagnosed learning disorder often present with symptoms akin to those of ADHD.1 An undiagnosed reading or mathematics disorder, for example, can have a significant impact on academic functioning, in which the child might not be paying attention because of his (her) restricted ability to grasp the subject matter.
On the other hand, keep in mind that ADHD is highly comorbid with learning disorders.10
Last, children and adults with a developmental disability can present with signs and symptoms similar to those of ADHD.1
Summing up
Comprehensive assessment and management of any underlying condition is important to address the attention deficits you observe in a patient. A collateral history from parents and significant others, school reports, relevant laboratory tests, and a full physical examination are important tools for making an accurate diagnosis.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
1. Robb AS. Differential diagnosis of ADHD in school-age children. Medscape Psychiatry. http://www.medscape.com/viewarticle/544948. Published September 26, 2006. Accessed September 6, 2016.
2. Sandi C. Memory impairments associated with stress and aging. In: Bermúdez-Rattoni F, ed. Neural plasticity and memory: from genes to brain imaging. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:54-55,58-59.
3. Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725.
4. Hanson KL, Winward JL, Schweinsburg AD, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976.
5. Morrow CE, Culbertson JL, Accornero VH, et al. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30(3):905-931.
6. Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227-231.
7. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377-383.
8. Gruber R, Michaelsen S, Bergmame L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33-40.
9. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129.
10. Czamara D, Tiesler CM, Kohlböck G, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLoS One. 2013;8(5):e63859. doi: 10.1371/journal.pone.0063859.
‘They’re out to get me!’: Evaluating rational fears and bizarre delusions in paranoia
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.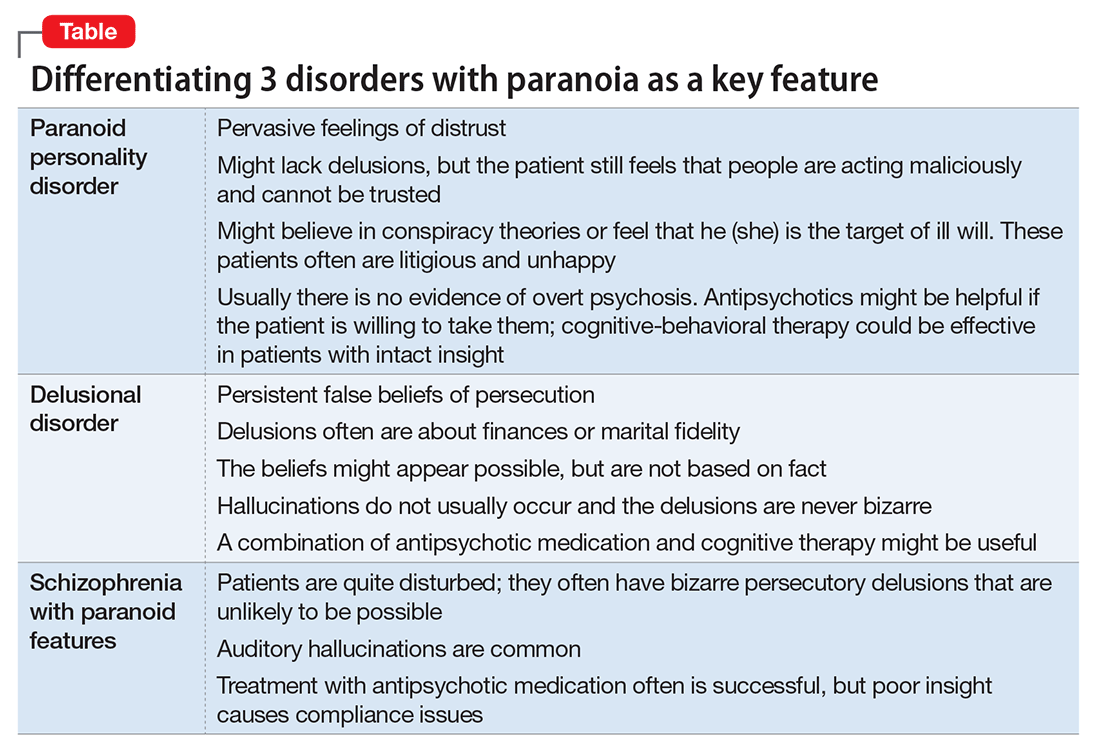
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
Even among healthy individuals, feelings of paranoia are not unusual. In modern psychiatry, we consider paranoia to be a pattern of unfounded thinking, centered on the fearful experience of perceived victimization or threat of intentional harm. This means that a patient with paranoia is, by nature, difficult to engage in treatment. A patient might perceive the clinician as attempting to mislead or manipulate him. A therapeutic alliance could require patience on the part of the clinician, creativity,1 and abandoning attempts at rational “therapeutic” persuasion. The severity of symptoms determines the approach.
In this article, we review the nature of paranoia and the continuum of syndromes to which it is a central feature, as well as treatment approaches.
Categorization and etiology
Until recently, clinicians considered “paranoid” to be a subtype of schizophrenia (Box2-7); in DSM-5 the limited diagnostic stability and reliability of the categorization rendered the distinction obsolete.8 There are several levels of severity of paranoia; this thought process can present in simple variations of normal fears and concerns or in severe forms, with highly organized delusional systems.
The etiology of paranoia is not clear. Over the years, it has been attributed to defense mechanisms of the ego, habitual fears from repetitive exposure, or irregular activity of the amygdala. It is possible that various types of paranoia could have different causes. Functional MRIs indicate that the amygdala is involved in anxiety and threat perception in both primates and humans.9
Rational fear vs paranoia
Under the right circumstances, anyone could sense that he (she) is being threatened. Such feelings are normal in occupied countries and nations at war, and are not pathologic in such contexts. Anxiety about potential danger and harassment under truly oppressive circumstances might be biologically ingrained and have value for survival. It is important to employ cultural sensitivity when distinguishing pathological and nonpathological paranoia because some immigrant populations might have increased prevalence rates but without a true mental illness.10
Perhaps the key to separating realistic fear from paranoia is the recognition of whether the environment is truly safe or hostile; sometimes this is not initially evident to the clinician. The first author (J.A.W.) experienced this when discovering that a patient who was thought to be paranoid was indeed being stalked by another patient.
Rapid social change makes sweeping explanations about the range of threats experienced by any one person of limited value. Persons living with serious and persistent mental illness experience stigma—harassment, abuse, disgrace—and, similar to victims of repeated sexual abuse and other violence, are not necessarily unreasonable in their inner experience of omnipresent threat. In addition, advances in surveillance technology, as well as the media proliferation of depictions of vulnerability and threat, can plant generalized doubt of historically trusted individuals and systems. Under conditions of severe social discrimination or life under a totalitarian regime, constant fear for safety and worry about the intentions of others is reasonable. We must remember that during the Cold War many people in Eastern Europe had legitimate concerns that their phones were tapped. There are still many places in the world where the fear of government or of one’s neighbors exists.
- paranoid personality disorder
- delusional disorder
- paranoia in schizophrenia (Table).
Paranoid personality disorder
The nature of any personality disorder is a long-standing psychological and behavioral pattern that differs significantly from the expectations of one’s culture. Such beliefs and behaviors typically are pervasive across most aspects of the individual’s interactions, and these enduring patterns of personality usually are evident by adolescence or young adulthood. Paranoid personality disorder is marked by pervasive distrust of others. Typical features include:
- suspicion about other people’s motives
- sensitivity to criticism
- keeping grudges against alleged offenders.8
The patient must have 4 of the following symptoms to confirm the diagnosis:
- suspicion of others and their motives
- reluctance to confide in others, due to lack of trust
- recurrent doubts about the fidelity of a significant other
- preoccupation with doubt regarding trusting others
- seeing threatening meanings behind benign remarks or events
- perception of attacks upon one’s character or reputation
- bears persistent grudges.8
Individuals with paranoid personality disorder tend to lead maladaptive lifestyles and might present as irritable, unpleasant, and emotionally guarded. Paranoid personality disorder is not a form of delusion, but is a pattern of habitual distrust of others.
The disorder generally is expressed verbally, and is seldom accompanied by hallucinations or unpredictable behavior. Distrust of others might result in social isolation and litigious behavior.8 Alternately, a patient with this disorder might not present for treatment until later in life after the loss of significant supporting factors, such as the death of parents or loss of steady employment. Examination of these older individuals is likely to reveal long-standing suspiciousness and distrust that previously was hidden by family members. For example, a 68-year-old woman might present saying that she can’t trust her daughter, but her recently deceased spouse would not let her discuss the topic outside of the home.
The etiology of paranoid personality disorder is unknown. Family studies suggest a possible a genetic connection to paranoia in schizophrenia.12 Others hypothesize that this dysfunction of personality might originate in early feelings of anxiety and low self-esteem, learned from a controlling, cruel, or sadistic parent; the patient then expects others to reject him (her) as the parent did.13,14 Such individuals might develop deep-seated distrust of others as a defense mechanism. Under stress, such as during a medical illness, patients could develop brief psychoses. Antipsychotic treatment might be useful in some cases of paranoid personality disorder, but should be limited.
Delusional disorder
Delusional disorder is a unique form of psychosis. Patients with delusional disorder might appear rational—as long as they are in independent roles—and their general functioning could go unnoticed. This could change when the delusions predominate their thoughts, or their delusional behavior is unacceptable in a structured environment. Such individuals often suffer from a highly specific delusion fixed on 1 topic. These delusions generally are the only psychotic feature. The most common theme is that of persecution. For example, a person firmly believes he is being followed by foreign agents or by a religious organization, which is blatantly untrue. Another common theme is infidelity.
Paranoia in delusional disorder is about something that is not actually occurring, but could.3 In other words, the delusion is not necessarily bizarre. The patient may have no evidence or could invent “evidence,” yet remain completely resistant to any logical argument against his belief system. In many situations, individuals with delusional disorder function normally in society, until the delusion becomes severe enough to prompt clinical attention.
Paranoia in schizophrenia
In patients with schizophrenia with paranoia, the typical symptoms of disorganization and disturbed affect are less prominent. The condition develops in young adulthood, but could start at any age. Its course typically is chronic and requires psychiatric treatment; the patient may require hospital care.
Although patients with delusional disorder and those with schizophrenia both have delusions, the delusions of the latter typically are bizarre and unlikely to be possible. For example, the patient might believe that her body has been replaced with the inner workings of an alien being or a robot. The paranoid delusions of persons with delusional disorder are much more mundane and could be plausible. Karl Jaspers, a clinician and researcher in the early 20th century, separated delusional disorder from paranoid schizophrenia by noting that the former could be “understandable, even if untrue” while the latter was “not within the realm of understandability.”5
A patient with schizophrenia with paranoid delusions usually experiences auditory hallucinations, such as voices threatening persecution or harm. When predominant, patients could be aroused by these fears and can be dangerous to others.2,4,5
Other presentations of paranoia
Paranoia can occur in affective disorders as well.13 Although the cause is only now being understood, clinicians have put forth theories for many years. A depressed person might suffer from excessive guilt and feel that he deserves to be persecuted, while a manic patient might think she is being persecuted for her greatness. In the past, response to electroconvulsive therapy was used to distinguish affective paranoia from other types.2
Paranoia in organic states
Substance use. Psychostimulants, which are known for their motor activity and arousal enhancing properties, as well as the potential for abuse and other negative consequences, could lead to acute paranoid states in susceptible individuals.15-17 In addition, tetrahydrocannabinol, the active chemical in Cannabis, can cause acute psychotic symptoms, such as paranoia,18,19 in a dose-dependent manner. A growing body of evidence suggests that a combination of Cannabis use with a genetic predisposition to psychosis may put some individuals at high risk of decompensation.19 Of growing concern is the evidence that synthetic cannabinoids, which are among the most commonly used new psychoactive substances, could be associated with psychosis, including paranoia.20
Dementia. Persons with dementia often are paranoid. In geriatric patients with dementia, a delusion of thievery is common. When a person has misplaced objects and can’t remember where, the “default” cognition is that someone has taken them. This confabulation may progress to a persistent paranoia and can be draining on caregivers.
Treating paranoia
A patient with paranoia usually has poor insight and cannot be reasoned with. Such individuals are quick to incorporate others into their delusional theories and easily develop notions of conspiracy. In acute psychosis, when the patient presents with fixed beliefs that are not amenable to reality orientation, and poses a threat to his well-being or that of others, alleviating underlying fear and anxiety is the first priority. Swift pharmacologic measures are required to decrease the patient’s underlying anxiety or anger, before you can try to earn his trust.
Psychopharmacologic interventions should be specific to the diagnosis. Antipsychotic medications generally will help decrease most paranoia, but affective syndromes usually require lithium or divalproex for best results.14,21
Develop a therapeutic relationship. The clinician must approach the patient in a practical and straightforward manner, and should not expect a quick therapeutic alliance. Transference and countertransference develop easily in the context of paranoia. Focus on behaviors that are problematic for the patient or the milieu, such as to ensure a safe environment. The patient needs to be aware of how he could come across to others. Clear feedback about behavior, such as “I cannot really listen to you when you’re yelling,” may be effective. It might be unwise to confront delusional paranoia in an agitated patient. Honesty and respect must continue in all communications to build trust. During assessment of a paranoid individual, evaluate the level of dangerousness. Ask your patient if he feels like acting on his beliefs or harming the people that are the targets of his paranoia.
As the patient begins to manage his anxiety and fear, you can develop a therapeutic alliance. The goals of treatment need be those of the patient—such as staying out of the hospital, or behaving in a manner that is required for employment. Over time, work toward growing the patient’s capacity for social interaction and productive activity. Insight might be elusive; however, some patients with paranoia can learn to take a detached view of their thoughts and emotions, and consider them impermanent events of the mind that make their lives difficult. Practice good judgment when aiming for recovery in a patient who does not have insight. For example, a patient can recognize that although there could be a microchip in his brain, he feels better when he takes medication.
In the case of paranoid personality disorder, treatment, as with most personality disorders, can be difficult. The patient might be unlikely to accept help and could distrust caregivers. Cognitive-behavioral therapy could be useful, if the patient can be engaged in the therapeutic process. Although it might be difficult to obtain enhanced insight, the patient could accept logical explanations for situations that provoke distrust. As long as anxiety and anger can be kept under control, the individual might learn the value of adopting the lessons of therapy. Pharmacological treatments are aimed at reducing the anxiety and anger experienced by the paranoid individual. Antipsychotics may be useful for short periods or during a crisis.14,21
The clinician must remain calm and reassuring when approaching an individual with paranoia, and not react to the projection of paranoid feelings from the patient. Respect for the patient can be conveyed without agreeing with delusions or bizarre thinking. The clinician must keep agreements and appointments with the client to prevent the erosion of trust. Paranoid conditions might respond slowly to pharmacological treatment, therefore establishing a consistent therapeutic relationship is essential.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
1. Frank C. Delirium, consent to treatment, and Shakespeare. A geriatric experience. Can Fam Physician. 1999;45:875-876.
2. Hamilton M. Fish’s schizophrenia. Bristol, United Kingdom: John Wright and Sons; 1962.
3. Munro A. Delusional disorder. New York, NY: Cambridge University Press; 2000.
4. Kahlbaum K. Die gruppierung de psychischen krankheiten. Danzig, Germany: Verlag von A. W. Kafemann; 1853.
5. Kraepelin E. Manic depressive insanity and paranoia. Barclay RM, trans. New York, NY: Arno Press; 1976.
6. Bleuler E. Dementia praecox or the group of schizophrenias. Ainkia J, trans. New York, NY: International University Press; 1950.
7. Mayer W. Uber paraphrene psychosen. Zeitschrift fur die gesamte. Neurology und Psychiatrie. 1921;71:187-206.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Pinkham AE, Liu P, Lu H, et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am J Psychiatry. 2015;172(8):784-792.
10. Sen P, Chowdhury AN. Culture, ethnicity and paranoia. Curr Psychiatry Rep. 2006;8(3):174-178.
11. Szasz TS. The manufacture of madness: a comparative study of the inquisition and the mental health movement. New York, NY: Harper and Row; 1970.
12. Schanda H, Berner P, Gabriel E, et al. The genetics of delusional psychosis. Schizophr Bull. 1983;9(4):563-570.
13. Levy B, Tsoy E, Brodt T, et al. Stigma, social anxiety and illness severity in bipolar disorder: implications for treatment. Ann Clin Psychiatry. 2015;27(1):55-64.
14. Benjamin LS. Interpersonal diagnosis and treatment of personality disorders. New York, NY: Gilford Press; 1993.
15. Busardo FP, Kyriakou C, Cipilloni L, et al. From clinical application to cognitive enhancement. Curr Neuropharmacol. 2015;13(2):281-295.
16. McKetin R, Gardner J, Baker AL, et al. Correlates of transient versus persistent psychotic symptoms among dependent methylamphetamine users. Psychiatry Res. 2016;238:166-171.
17. Djamshidian A. The neurobehavioral sequelae of psychostimulant abuse. Int Rev Neurobiol. 2015;120:161-177.
18. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? An oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401.
19. Bui QM, Simpson S, Nordstrom K. Psychiatric and medical management of marijuana intoxication in the emergency department. West J Emerg Med. 2015;16(3):414-417.
20. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234-243.
21. Lake CR. Hypothesis: grandiosity and guilt cause paranoia; paranoid schizophrenia is a psychotic mood disorder: a review. Schizophr Bull. 2008;34(6):1151-1162.
VIDEO: Transcriptomics link gastric cancer to RNA misediting
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Sequence alterations at the RNA level may contribute to the pathology and prognosis of gastric cancer.
Major finding: Compared with normal gastric tissue, almost all gastric cancer specimens exhibited an RNA misediting phenotype characterized by the respective gain and loss of the ADAR1 and ADAR2 genes.
Data source: High-throughput transcriptome sequencing (RNA-Seq) of gastric cancer and normal tissue, sequencing of gastric cancer cell lines, and analyses of The Cancer Genome Atlas.
Disclosures: The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The investigators had no disclosures.
Biologics did not up risk of serious infections in IBD
Treating inflammatory bowel disease with biologic therapies increases the risk of opportunistic infections but not the risk of serious infections, based on a systematic review and meta-analysis reported in the October issue of Clinical Gastroenterology and Hepatology.
Contrary to common belief, infection risk seemed similar with the integrin and anti–tumor necrosis factor classes, said Stefanos Bonovas, MD, MSc, PhD, of Humanitas Clinical and Research Center, Milan, with his associates. Clinicians and patients can use these findings to better weigh the risks and benefits of biologic therapies for IBD, although “studies in real-world settings, national and international registries, and clinical audits may serve as complementary data sources to further assess biologic treatments’ comparative and long-term safety profiles,” the researchers added.
Biologics can effectively manage IBD but raise concerns about infection and malignancy. To examine these risks in adults with IBD, the researchers systematically searched PubMed, Embase, Scopus, the Cochrane IBD Group Specialized Trials Register, the World Health Organization International Clinical Trials Registry Platform, and clinicaltrials.gov through March 2016 for randomized, placebo-controlled or head-to-head trials of approved IBD therapies, including adalimumab, certolizumab, golimumab, infliximab, natalizumab, and vedolizumab. After excluding systematic reviews, uncontrolled trials, and secondary analyses, 49 trials of 14,590 patients remained for meta-analysis, the investigators said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.039).
Biologic therapy conferred a “moderate increase” in the likelihood of any infection, with an odds ratio of 1.19 when compared with patients who did not receive biologics (95% confidence interval, 1.10-1.29), the researchers said. The risk of opportunistic infections was somewhat higher (OR, 1.90; 95% CI, 1.21-3.01). However, biologic therapy did not significantly heighten the risk of serious infections, which most studies defined as infections leading to hospitalization, intravenous antibiotic treatment, or death (OR, 0.89; 95% CI, 0.71-1.12). “On the contrary, biologics appeared to significantly reduce risk of serious infections in studies with low risk of bias (OR, 0.56; 95% CI, 0.35-0.90),” the investigators wrote. They did not find an increased risk of malignancy with biologic therapy (OR, 0.90; 95% CI, 0.54-1.50), “but [these] data were insufficient in terms of exposure and follow-up times,” they added.
Comparisons of individual agents and classes also did not reveal any significant associations with the risk of infection, the investigators noted. They did acknowledge several limitations. None of the trials were head-to-head comparisons between biologics, and many were industry funded. Estimates of comparative harm were based on indirect comparisons, and therefore merited cautious interpretation. Because the trials were carried out for regulatory purposes, they enrolled highly selected and homogeneous cohorts of IBD patients, leading to underrepresentation of high-risk and elderly individuals, the researchers noted. “Finally, the exposure and follow-up times were up to 24 months, a time period that is considered sufficient when analyzing infectious adverse effects but wholly insufficient for cancer outcomes. With this in mind, we must look to large register-based cohort studies to enhance our understanding of the biologics-cancer association, despite the biases inherent in observational study designs.”
Dr. Bonovas had no disclosures, while three coauthors reported relationships with a number of pharmaceutical companies.
Treating inflammatory bowel disease with biologic therapies increases the risk of opportunistic infections but not the risk of serious infections, based on a systematic review and meta-analysis reported in the October issue of Clinical Gastroenterology and Hepatology.
Contrary to common belief, infection risk seemed similar with the integrin and anti–tumor necrosis factor classes, said Stefanos Bonovas, MD, MSc, PhD, of Humanitas Clinical and Research Center, Milan, with his associates. Clinicians and patients can use these findings to better weigh the risks and benefits of biologic therapies for IBD, although “studies in real-world settings, national and international registries, and clinical audits may serve as complementary data sources to further assess biologic treatments’ comparative and long-term safety profiles,” the researchers added.
Biologics can effectively manage IBD but raise concerns about infection and malignancy. To examine these risks in adults with IBD, the researchers systematically searched PubMed, Embase, Scopus, the Cochrane IBD Group Specialized Trials Register, the World Health Organization International Clinical Trials Registry Platform, and clinicaltrials.gov through March 2016 for randomized, placebo-controlled or head-to-head trials of approved IBD therapies, including adalimumab, certolizumab, golimumab, infliximab, natalizumab, and vedolizumab. After excluding systematic reviews, uncontrolled trials, and secondary analyses, 49 trials of 14,590 patients remained for meta-analysis, the investigators said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.039).
Biologic therapy conferred a “moderate increase” in the likelihood of any infection, with an odds ratio of 1.19 when compared with patients who did not receive biologics (95% confidence interval, 1.10-1.29), the researchers said. The risk of opportunistic infections was somewhat higher (OR, 1.90; 95% CI, 1.21-3.01). However, biologic therapy did not significantly heighten the risk of serious infections, which most studies defined as infections leading to hospitalization, intravenous antibiotic treatment, or death (OR, 0.89; 95% CI, 0.71-1.12). “On the contrary, biologics appeared to significantly reduce risk of serious infections in studies with low risk of bias (OR, 0.56; 95% CI, 0.35-0.90),” the investigators wrote. They did not find an increased risk of malignancy with biologic therapy (OR, 0.90; 95% CI, 0.54-1.50), “but [these] data were insufficient in terms of exposure and follow-up times,” they added.
Comparisons of individual agents and classes also did not reveal any significant associations with the risk of infection, the investigators noted. They did acknowledge several limitations. None of the trials were head-to-head comparisons between biologics, and many were industry funded. Estimates of comparative harm were based on indirect comparisons, and therefore merited cautious interpretation. Because the trials were carried out for regulatory purposes, they enrolled highly selected and homogeneous cohorts of IBD patients, leading to underrepresentation of high-risk and elderly individuals, the researchers noted. “Finally, the exposure and follow-up times were up to 24 months, a time period that is considered sufficient when analyzing infectious adverse effects but wholly insufficient for cancer outcomes. With this in mind, we must look to large register-based cohort studies to enhance our understanding of the biologics-cancer association, despite the biases inherent in observational study designs.”
Dr. Bonovas had no disclosures, while three coauthors reported relationships with a number of pharmaceutical companies.
Treating inflammatory bowel disease with biologic therapies increases the risk of opportunistic infections but not the risk of serious infections, based on a systematic review and meta-analysis reported in the October issue of Clinical Gastroenterology and Hepatology.
Contrary to common belief, infection risk seemed similar with the integrin and anti–tumor necrosis factor classes, said Stefanos Bonovas, MD, MSc, PhD, of Humanitas Clinical and Research Center, Milan, with his associates. Clinicians and patients can use these findings to better weigh the risks and benefits of biologic therapies for IBD, although “studies in real-world settings, national and international registries, and clinical audits may serve as complementary data sources to further assess biologic treatments’ comparative and long-term safety profiles,” the researchers added.
Biologics can effectively manage IBD but raise concerns about infection and malignancy. To examine these risks in adults with IBD, the researchers systematically searched PubMed, Embase, Scopus, the Cochrane IBD Group Specialized Trials Register, the World Health Organization International Clinical Trials Registry Platform, and clinicaltrials.gov through March 2016 for randomized, placebo-controlled or head-to-head trials of approved IBD therapies, including adalimumab, certolizumab, golimumab, infliximab, natalizumab, and vedolizumab. After excluding systematic reviews, uncontrolled trials, and secondary analyses, 49 trials of 14,590 patients remained for meta-analysis, the investigators said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.039).
Biologic therapy conferred a “moderate increase” in the likelihood of any infection, with an odds ratio of 1.19 when compared with patients who did not receive biologics (95% confidence interval, 1.10-1.29), the researchers said. The risk of opportunistic infections was somewhat higher (OR, 1.90; 95% CI, 1.21-3.01). However, biologic therapy did not significantly heighten the risk of serious infections, which most studies defined as infections leading to hospitalization, intravenous antibiotic treatment, or death (OR, 0.89; 95% CI, 0.71-1.12). “On the contrary, biologics appeared to significantly reduce risk of serious infections in studies with low risk of bias (OR, 0.56; 95% CI, 0.35-0.90),” the investigators wrote. They did not find an increased risk of malignancy with biologic therapy (OR, 0.90; 95% CI, 0.54-1.50), “but [these] data were insufficient in terms of exposure and follow-up times,” they added.
Comparisons of individual agents and classes also did not reveal any significant associations with the risk of infection, the investigators noted. They did acknowledge several limitations. None of the trials were head-to-head comparisons between biologics, and many were industry funded. Estimates of comparative harm were based on indirect comparisons, and therefore merited cautious interpretation. Because the trials were carried out for regulatory purposes, they enrolled highly selected and homogeneous cohorts of IBD patients, leading to underrepresentation of high-risk and elderly individuals, the researchers noted. “Finally, the exposure and follow-up times were up to 24 months, a time period that is considered sufficient when analyzing infectious adverse effects but wholly insufficient for cancer outcomes. With this in mind, we must look to large register-based cohort studies to enhance our understanding of the biologics-cancer association, despite the biases inherent in observational study designs.”
Dr. Bonovas had no disclosures, while three coauthors reported relationships with a number of pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Biologic therapies for inflammatory bowel disease significantly increase the risk of opportunistic infections but not serious infections.
Major finding: Biologic therapy increased the odds of opportunistic infection about 1.9-fold, but did not significantly increase the odds of infections leading to hospitalization, intravenous antibiotic treatment, or death.
Data source: A systematic review and meta-analysis of 49 randomized controlled trials of 14,590 patients with inflammatory bowel disease.
Disclosures: The investigators did not report external funding sources. Dr. Bonovas had no disclosures, while three coauthors reported relationships with a number of pharmaceutical companies.
Analysis yields ‘strong evidence’ for benefit of physical activity in NAFLD
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Physical activity benefits measures of nonalcoholic fatty liver disease independently of diet.
Major finding: After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001).
Data source: A systematic review and meta-analysis of 28 randomized controlled trials comprising more than 16,000 patients.
Disclosures: The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The researchers had no disclosures.
An irritable, inattentive, and disruptive child: Is it ADHD or bipolar disorder?
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
Differentiating the irritable, oppositional child with attention-deficit/hyperactivity disorder (ADHD) from the child with bipolar disorder (BD) often is difficult. To make matters more complicated, 50% to 70% of patients with BD have comorbid ADHD.1,2 Accordingly, clinicians are often faced with the moody, irritable, disruptive child whose parents want to know if he (she) is “bipolar” to try to deal with oppositional and mood behaviors.
In this article, we present an approach that will help you distinguish these 2 disorders from each other.
Precision medicineThere is a lack of evidence-based methods for diagnosing psychiatric disorders in children and adolescents. DSM-5 provides clinicians with diagnostic checklists that rely on the clinician’s judgment and training in evaluating a patient.3 In The innovator’s prescription: a disruptive solution for health care, Christensen et al4 describe how medicine is moving from “intuitive medicine” to empirical medicine and toward “precision medicine.” Intuitive medicine depends on the clinician’s expertise, training, and exposure to different disorders, which is the traditional clinical model that predominates in child psychiatry. Empirical medicine relies on laboratory results, scans, scales, and other standardized tools.
Precision medicine occurs when a disorder can be precisely diagnosed and its cause understood, and when it can be treated with effective, evidence-based therapies. An example of this movement toward precision is Timothy syndrome (TS), a rare autosomal dominant disorder characterized by physical malformations, cardiac arrhythmias and structural heart defects, webbing of fingers and toes, and autism spectrum disorder. In the past, a child with TS would have been given a diagnosis of intellectual disability, or a specialist in developmental disorders might recognize the pattern of TS. It is now known that TS is caused by mutations in CACNA1C, the gene encoding the calcium channel Cav1.2α subunit, allowing precise diagnosis by genotyping.5
Although there are several tools that help clinicians assess symptoms of ADHD and BD, including rating scales such the Vanderbilt ADHD Diagnostic Rating Scale and Young Mania Rating Scale, none of these scales are diagnostic. Youngstrom et al6,7 have developed an evidence-based strategy to diagnose pediatric BD. This method uses a nomogram that takes into account the base rate of BD in a clinical setting and family history of BD.
We will describe and contrast the epidemiologic and clinical characteristics of pediatric BD from ADHD and use the Youngstrom nomogram to better define these patients. Although still far from precision medicine, the type of approach represents an ongoing effort in mental health care to increase diagnostic accuracy and improve treatment outcomes.
Pediatric bipolar disorder
Prevalence of pediatric BD is 1.8% (95% CI, 1.1% to 3.0%),8 which does not include sub-threshold cases of BD. ADHD and oppositional defiant disorder (ODD) are 8 to 10 times more prevalent. For the purposes of the nomogram, the “base rate” is the rate at which a disorder occurs in different clinical settings. In general outpatient clinics, BD might occur 6% to 8% of the time, whereas in a county-run child psychiatry inpatient facility the rate is 11%.6 A reasonable rate in an outpatient pediatric setting is 6%.
Family history. In the Bipolar Offspring Study,9 the rate of BD in children of parents with BD was 13 times greater than that of controls, and the rate of anxiety and behavior disorders was approximately twice that of children of parents without BD (Table 1).9 This study evaluated 388 children of 233 parents with BD and 251 children of 143 demographically matched controls.
Clinical characteristics. Children and adolescents with BD typically manifest with what can be described as a “mood cycle”—a pronounced shift in mood and energy from one extreme to another. An example would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours, then later becomes sad, depressed, and suicidal with no precipitant for either mood cycle.10 Pediatric patients with BD also exhibit other symptoms of mania during mood cycling periods.
Elevated or expansive mood. The child might have a mood that is inappropriately giddy, silly, elated, or euphoric. Often this mood will be present without reason and last for several hours. It may be distinguished from a transient cheerful mood by the intensity and duration of the episode. The child with BD may have little to no insight about the inappropriate nature of their elevated mood, when present.
Irritable mood. The child might become markedly belligerent or irritated with intense outbursts of anger, 2 to 3 times a day for several hours. An adolescent might appear extremely oppositional, belligerent, or hostile with parents and others.
Grandiosity or inflated self-esteem can be confused with brief childhood fantasies of increased capability. Typically, true grandiosity can manifest as assertion of great competency in all areas of life, which usually cannot be altered by contrary external evidence. Occasionally, this is bizarre and includes delusions of “super powers.” The child in a manic episode will not only assert that she can fly, but will jump off the garage roof to prove it.
Decreased need for sleep. The child may only require 4 to 5 hours of sleep a night during a manic episode without feeling fatigued or showing evidence of tiredness. Consider substance use in this differential diagnosis, especially in adolescents.
Increased talkativeness. Lack of inhibition to social norms may lead pediatric BD patients to blurt out answers during class or repeatedly be disciplined for talking to peers in class. Speech typically is rapid and pressured to the point where it might be continuous and seems to jump between loosely related subjects.
Flight of ideas or racing thoughts. The child or adolescent might report a subjective feeling that his thoughts are moving so rapidly that his speech cannot keep up. Often this is differentiated from rapid speech by the degree of rapidity the patient expresses loosely related topics that might seem completely unrelated to the listener.
Distractibility, short attention span. During a manic episode, the child or adolescent might report that it is impossible to pay attention to class or other outside events because of rapidly changing focus of their thoughts. This symptom must be carefully distinguished from the distractibility and inattention of ADHD, which typically is a more fixed and long-standing pattern rather than a brief episodic phenomenon in a manic or hypomanic episode.
Increase in goal-directed activity. During a mild manic episode, the child or adolescent may be capable of accomplishing a great deal of work. However, episodes that are more severe manifest as an individual starting numerous ambitious projects that she later is unable to complete.
Excessive risk-taking activities. The child or adolescent might become involved in forbidden, pleasurable activities that have a high risk of adverse consequences. This can manifest as hypersexual behavior, frequent fighting, increased recklessness, use of drugs and alcohol, shopping sprees, and reckless driving.
There are few studies comparing patients with comorbid BD and ADHD with patients with only ADHD. Geller et al11 compared 60 children with BD and ADHD (mean age, 10) to age- and sex-matched patients with ADHD and no mood disorder. Compared with children who had ADHD, those with BD exhibited significantly greater elevated mood, grandiosity, flight and/or racing of ideas, decreased need for sleep, and hypersexuality (Figure 1,11). Features common to both groups—and therefore not useful in differentiating the disorders—included irritability, hyperactivity, accelerated speech, and distractibility.
CASE REPORTIrritable and disruptiveBill, age 12, has been brought to see you by his mother because she is concerned about escalating behavior problems at home and school in the past several months. The school principal has called her about his obnoxious behavior with teachers and about other parents’ complaints that he has made unwanted sexual advances to girls who sit next to him in class.
Bill, who is in the 7th grade, is on the verge of being suspended for his inappropriate and disruptive behavior. His parents report that he is irritable around them and stays up all night, messaging his friends on the Internet from his iPad in his bedroom. They attribute his inappropriate sexual behavior to puberty and possibly to the Web sites he views.
Bill’s mother is concerned about his:
• increasing behavior problems during the last several months at home and school
• intensifying irritability and depressive symptoms
• staying up all night on the Internet, phoning friends, and doing projects
• frequent unprovoked, outbursts of rage occurring with increasing frequency and intensity (almost daily)
• moderate grandiosity, including telling the soccer coach and teachers how to do their jobs
• inappropriate sexual behavior, including kissing and touching female classmates.
During your history, you learn that Bill has been a bright and artistic child, with good academic performance. His peer relationships have been satisfactory, but not excellent—he tends to be “bossy” with his peers. He is medically healthy and not taking any medications. As part of your history, you also talk with Bill and his family about exposure to trauma or significant stressors, which they deny. You learn that Bill’s father was diagnosed with BD I at age 32.
Completing the nomogram developed by Youngstrom et al6,7 using these variables (see this article at CurrentPsychiatry.com for Figure 2)6,7 gives Bill a post-test probability of approximately 42%. The threshold for moving ahead with assessment and possible treatment, the “test-treatment threshold,” depends on your clinical setting.12,13 Our clinical experience is that, when the post-test probability exceeds 30%, further assessment for BD is warranted.
The next strategy is to look at Bill’s scores on externalizing behaviors using an instrument such as the Vanderbilt ADHD Diagnostic Parent Rating Scale. Few pediatric patients with BD will score low on externalizing behaviors.14 Bill scores in the clinically significant range for hyperactivity/impulsivity and positive on the screeners for ODD, conduct disorder (CD), and anxiety/depression.
You decide that Bill is at high risk of pediatric BD; he has a post-test probability of approximately 45%, and many externalizing behaviors on the Vanderbilt. You give Bill a diagnosis of BD I and ADHD and prescribe risperidone, 0.5 mg/d, which results in significant improvement in mood swings and other manic behaviors.
ADHD
Epidemiology. ADHD is one of the most common neurodevelopmental disorders in childhood, with prevalence estimates of 8% of U.S. children.15,16 Overall, boys are more likely to be assigned a diagnosis of ADHD than girls.15 Although ADHD often is diagnosed in early childhood, research is working to clarify the lifetime prevalence of ADHD into late adolescence and adulthood. Current estimates suggest that ADHD persists into adulthood in close to two-thirds of patients.17 However, the symptom presentation can change during adolescence and adulthood, with less overt hyperactivity and symptoms of impulsivity transitioning to risky behaviors involving trouble with the law, substance use, and sexual promiscuity.17
As in pediatric BD, comorbidity is common in ADHD, with uncomplicated ADHD being the exception rather than the rule. Recent studies have suggested that approximately two-thirds of children who have a diagnosis of ADHD have ≥1 comorbid diagnoses.15 Common comorbidities are similar to those seen in BD, including ODD, CD, anxiety disorders, depression, and learning disability. Several tools and resources are available to help clinicians navigate these issues within their practices.
Family history. Genetics appear to play a large role in ADHD, with twin studies suggesting inheritance of approximately 76%.18 Environmental factors contribute, either in the development of ADHD or in the exacerbation of an underlying familial predisposition. Interestingly, in children with BD, family history often is significant for several family members who have both ADHD and BD. However, in children with ADHD only, family history often reflects an absence of family members with BD.19 Although not diagnostic, this pattern can be helpful when considering a diagnosis of BD vs ADHD.
Clinical picture. ADHD often is recognized in childhood; DSM-5 criteria specify that symptoms be present before age 12 and persist for at least 6 months. This characterization of the timing of symptoms helps exclude behavioral disruptions related to external factors such as trauma (eg, death of a caregiver) or abuse. It also is important to note that symptoms might be present earlier but not come to attention clinically until a later age, perhaps because of increasing demands placed on the child by school, peer groups, and extracurricular activities. To make an ADHD diagnosis, symptoms must be present in >1 setting and interfere with functioning or development.
Core symptoms of ADHD include inattention, hyperactivity, and impulsivity that are out of proportion to the child’s developmental level (Table 2).20 When considering diagnosis of ADHD, 6 of 9 symptoms for inattention and/or hyperactivity-impulsivity must be present at a clinically significant level.
Three different ADHD presentations are recognized: combined, inattentive, and hyperactive impulsive. Children with predominant impulsive and hyperactive behaviors generally come to clinical attention at a younger age; inattentive symptoms often take longer to identify.
Children with ADHD have been noted to have lower tolerance for frustration, which might make anger outbursts and aggressive behavior more likely. Anger and aggression in ADHD often stem from impulsivity, rather than irritable mood seen with BD.18 Issues related to self-esteem, depression, substance use, and CD can contribute to symptoms of irritability, anger, and aggression that can occur in children with ADHD. Although these symptoms can overlap with those seen in children with BD, other core symptoms of ADHD will not be present.
ODD is one of the most common comorbidities among children with ADHD, and the combination of ODD and ADHD may be confused with BD. Children with ODD often are noted to exhibit a pattern of negative and defiant behavior that is out of proportion to what is seen in their peers and for their age and developmental level (Table 3).20 When considering an ODD diagnosis, 4 out of 8 symptoms must be present at a clinically significant level.
The following case highlights the potential similarities between ADHD/ODD and BD, with tips on how to distinguish them.
CASE REPORT
Angry and destructiveSam, age 7, has been given a diagnosis of ADHD, but his parents think that he isn’t improving with methylphenidate treatment. They are concerned that he has anger issues like his uncle, who has “bipolar disorder.”
Sam’s parents find that he gets frustrated easily and note that he has frequent short “meltdowns” and “mood swings.” During these episodes he yells, is aggressive towards others, and can be destructive. They are concerned because Sam will become angry quickly, then act as if nothing happened after the meltdown has blown over. Sam’s parents feel that he doesn’t listen to them and often argues when they make a request. His parents note that when they push harder, Sam digs in his heels, which can trigger his meltdowns.
Despite clearly disobeying his parents, Sam often says that things aren’t his fault and blames his parents or siblings instead. Sam seems to disagree with people often. His mother reports “if I say the water looks blue, he’ll say it’s green.” Often, Sam seems to argue or pester others to get a rise out of them. This is causing problems for Sam with his siblings and peers, and significant stress for his parents. Family history suggests that Sam’s uncle may have ADHD with CD or a substance use disorder, rather than true BD. Other than Sam’s uncle, there is no family history for BD.
Sam’s parents say that extended release methylphenidate, 20 mg/d, has helped with hyperactivity, but they are concerned that other symptoms have not improved. Aside from the symptoms listed above, Sam is described as a happy child. There is no history of trauma, and no symptoms of anxiety are noted. Sam sometimes gets “down” when things don’t go his way, but this lasts only for a few hours. Sam has a history of delayed sleep onset, which responded well to melatonin. No other symptoms that suggest mania are described.
You complete the pediatric bipolar nomogram (Figure 3)6,7 and Sam’s parents complete a Vanderbilt ADHD Diagnostic Parent Rating Scale. At first, Sam seems to have several factors that might indicate BD: aggressive behavior, mood swings, sleep problems, and, possibly, a family history of BD.
However, a careful history provides several clues that Sam has a comorbid diagnosis of ODD. Sam is exhibiting the classic pattern of negativist behavior seen in children with ODD. In contrast to the episodic pattern of BD, these symptoms are prevalent and persistent, and manifest as an overall pattern of functioning. Impulsivity seen in children with ADHD can complicate the picture, but again appears as a consistent pattern rather than bouts of irritability. Sam’s core symptoms of ADHD (hyperactivity) improved with methylphenidate, but the underlying symptoms of ODD persisted.
Sleep problems are common in children who have ADHD and BD, but Sam’s delayed sleep onset responded to melatonin, whereas the insomnia seen in BD often is refractory to lower-intensity interventions, such as melatonin. Taking a careful family history led you to believe that BD in the family is unlikely. Although this type of detail may not always be available, it can be helpful to ask about mental health symptoms that seem to “run in the family.”
Bottom Line
Distinguishing the child who has bipolar disorder from one who has attention-deficit/hyperactivity disorder can be challenging. A careful history helps ensure that you are on the path toward understanding the diagnostic possibilities. Tools such as the Vanderbilt Rating Scale can further clarify possible diagnoses, and the nomogram approach can provide even more predictive information when considering a diagnosis of bipolar disorder.
Related Resources
• Children and Adults with Attention Deficit/Hyperactivity Disorder (CHADD). www.chadd.org.
• American Academy of Child and Adolescent Psychiatry. Facts for Families. www.aacap.org/cs/root/facts_for_families/ facts_for_families.
• Froehlich TE, Delgado SV, Anixt JS. Expanding medication options for pediatric ADHD. Current Psychiatry. 2013;(12)12:20-29.
• Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic interventions in attention-deficit/hyperactivity disorder and pediatric bipolar disorder. Expert Rev Neurother. 2011;11(6):897-914.
Drug Brand Names
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Risperidone • Risperdal
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
1. Faraone SV, Biederman J, Wozniak J, et al. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36(8):1046-1055.
2. West SA, McElroy SL, Strakowski SM, et al. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995;152(2):271-273.
3. McHugh PR, Slavney PR. Mental illness–comprehensive evaluation or checklist? N Engl J Med. 2012;366(20): 1853-1855.
4. Christensen CM, Grossman JH, Hwang J. The innovator’s prescription: a disruptive solution for health care. New York, NY: McGraw-Hill; 2009.
5. Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230-234.
6. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7): 712-717.
7. Youngstrom EA, Jenkins MM, Doss AJ, et al. Evidence-based assessment strategies for pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49(1):15-27.
8. Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72(9):1250-1256.
9. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287-296.
10. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10 (1 pt 2):194-214.
11. Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL and TRF. J Affect Disord. 1998;51(2):93-100.
12. Richardson WS, Wilson MC, Guyatt GH, et al. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group. JAMA. 1999;281(13):1214-1219.
13. Nease RF Jr, Owens DK, Sox HC Jr. Threshold analysis using diagnostic tests with multiple results. Med Decis Making. 1989;9(2):91-103.
14. Youngstrom EA, Youngstrom JK. Evidence-based assessment of pediatric bipolar disorder, Part II: incorporating information from behavior checklists. J Am Acad Child Adolesc Psychiatry. 2005;44(8):823-828.
15. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
16. Larson K, Russ SA, Kahn RS, et al. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462-470.
17. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
18. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248.
19. Sood AB, Razdan A, Weller EB, et al. How to differentiate bipolar disorder from attention deficit hyperactivity disorder and other common psychiatric disorders: a guide for clinicians. Curr Psychiatry Rep. 2005;7(2): 98-103.
20. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.