User login
CT simulation not needed in palliative radiotherapy planning
, according to a results from a randomized trial presented at the annual meeting of the American Society for Radiation Oncology.
The aim of this same-day CT scan, called a CT simulation scan, is to optimize radiation targeting by mimicking the conditions under which radiation is delivered using the latest information on the size and location of lesions.
But investigators reported that skipping the CT simulation scan saves patients hours in the clinic, allows patients to experience pain relief faster, and saves radiation oncologists time without compromising dosimetric coverage of cancerous lesions.
“This is huge in a symptomatic patient population,” said Melissa O’Neil, an advanced practice radiation therapist at the London, Ont., Health Sciences Centre and the lead investigator on the trial, dubbed DART (Diagnostic CT-Enabled Radiation Therapy).
“Diagnostic CT-based radiation planning substantially reduces time in the [treatment] center without a detriment in plan deliverability or quality,” Ms. O’Neil said.
In addition, patients are exposed to less radiation, and staff doesn’t have to spend as much time tending to symptomatic patients before treatment. Omitting this scan “should be considered for patients with a recent diagnostic CT scan who are undergoing simple palliative radiation,” Ms. O’Neil said.
CT simulation scans are standard of care in cases involving palliative radiation, but they create bottlenecks in the workflow. When a CT simulation is performed on the day of treatment, patients must wait hours as the results are translated into a treatment plan.
In the DART analysis, 33 patients with 42 treatment sites were randomly assigned to CT simulation planning or diagnostic CT planning.
Patients received up to 30 Gy in up to 10 fractions for bone or soft tissue metastases or primary tumor targets in the thorax, abdomen, pelvis, or proximal limbs. Single-fraction treatments were most common.
Three-quarters of the patients were men (median age, 72 years). Lung cancer was the most common type of primary tumor, followed by prostate and breast cancer.
The eight participants for whom the CT simulation approach was used waited 3-4 hours for treatment planning and overall spent a median of 4.8 hours in the cancer center on their day of treatment.
The 25 patients for whom diagnostic CT planning was used spent a median of 0.4 hours, or about 24 minutes, in the center on their day of treatment because radiation plans were completed before they arrived. The median time between their diagnostic CTs and radiation treatment was 13 days (range, 8-22 days).
Ms. O’Neil and her team found that if the original diagnostic CT was performed within 28 days, lesion anatomy would not have changed enough to warrant a new scan.
On the day of treatment, the study team used surface-guided radiation therapy techniques to ensure patients in the diagnostic CT planning group were positioned within 3 mm of where they were during their diagnostic scans, an essential step to ensure that radiation is delivered to the correct location. Ms. O’Neil noted that other investigators have used anatomic landmarks, a simpler approach, to achieve these results.
Overall, radiation oncologists rated radiation dose distribution as “acceptable” in about 80% of cases in both arms of DART and “acceptable with minor deviation” in the remaining 20% of cases.
Every radiation oncologist and medical physicists in the trial gave the workflow with diagnostic CT planning a 5 out of 5 rating for acceptability, and 90% of patients in this group rated the amount of time they spent for treatment as “acceptable.”
In contrast, only half of patients in the simulation arm said the amount of time spent was acceptable.
These findings align with several previous studies that support the diagnostic approach.
Jacob Scott, MD, a radiation oncologist at the Cleveland Clinic, said, “The comparable results using a recent diagnostic CT in place of a CT simulation for palliative radiation is an exciting step forward in radiation oncology. We may soon be in a world where we no longer need simulations.”
Dr. Scott also noted that combining diagnostic scans with cone beam or surface-guided positioning in lieu of CT simulations could further save “the health system and patients time and money.”
No external funding for the study was reported. The investigators, Ms. O’Neil, and Dr. Scott have disclosed no relevant financial relationships. One investigator reported receiving honoraria from Knight Therapeutics, AbbVie, Tersera, and Eisai and owns stock in Myovant.
A version of this article first appeared on Medscape.com.
, according to a results from a randomized trial presented at the annual meeting of the American Society for Radiation Oncology.
The aim of this same-day CT scan, called a CT simulation scan, is to optimize radiation targeting by mimicking the conditions under which radiation is delivered using the latest information on the size and location of lesions.
But investigators reported that skipping the CT simulation scan saves patients hours in the clinic, allows patients to experience pain relief faster, and saves radiation oncologists time without compromising dosimetric coverage of cancerous lesions.
“This is huge in a symptomatic patient population,” said Melissa O’Neil, an advanced practice radiation therapist at the London, Ont., Health Sciences Centre and the lead investigator on the trial, dubbed DART (Diagnostic CT-Enabled Radiation Therapy).
“Diagnostic CT-based radiation planning substantially reduces time in the [treatment] center without a detriment in plan deliverability or quality,” Ms. O’Neil said.
In addition, patients are exposed to less radiation, and staff doesn’t have to spend as much time tending to symptomatic patients before treatment. Omitting this scan “should be considered for patients with a recent diagnostic CT scan who are undergoing simple palliative radiation,” Ms. O’Neil said.
CT simulation scans are standard of care in cases involving palliative radiation, but they create bottlenecks in the workflow. When a CT simulation is performed on the day of treatment, patients must wait hours as the results are translated into a treatment plan.
In the DART analysis, 33 patients with 42 treatment sites were randomly assigned to CT simulation planning or diagnostic CT planning.
Patients received up to 30 Gy in up to 10 fractions for bone or soft tissue metastases or primary tumor targets in the thorax, abdomen, pelvis, or proximal limbs. Single-fraction treatments were most common.
Three-quarters of the patients were men (median age, 72 years). Lung cancer was the most common type of primary tumor, followed by prostate and breast cancer.
The eight participants for whom the CT simulation approach was used waited 3-4 hours for treatment planning and overall spent a median of 4.8 hours in the cancer center on their day of treatment.
The 25 patients for whom diagnostic CT planning was used spent a median of 0.4 hours, or about 24 minutes, in the center on their day of treatment because radiation plans were completed before they arrived. The median time between their diagnostic CTs and radiation treatment was 13 days (range, 8-22 days).
Ms. O’Neil and her team found that if the original diagnostic CT was performed within 28 days, lesion anatomy would not have changed enough to warrant a new scan.
On the day of treatment, the study team used surface-guided radiation therapy techniques to ensure patients in the diagnostic CT planning group were positioned within 3 mm of where they were during their diagnostic scans, an essential step to ensure that radiation is delivered to the correct location. Ms. O’Neil noted that other investigators have used anatomic landmarks, a simpler approach, to achieve these results.
Overall, radiation oncologists rated radiation dose distribution as “acceptable” in about 80% of cases in both arms of DART and “acceptable with minor deviation” in the remaining 20% of cases.
Every radiation oncologist and medical physicists in the trial gave the workflow with diagnostic CT planning a 5 out of 5 rating for acceptability, and 90% of patients in this group rated the amount of time they spent for treatment as “acceptable.”
In contrast, only half of patients in the simulation arm said the amount of time spent was acceptable.
These findings align with several previous studies that support the diagnostic approach.
Jacob Scott, MD, a radiation oncologist at the Cleveland Clinic, said, “The comparable results using a recent diagnostic CT in place of a CT simulation for palliative radiation is an exciting step forward in radiation oncology. We may soon be in a world where we no longer need simulations.”
Dr. Scott also noted that combining diagnostic scans with cone beam or surface-guided positioning in lieu of CT simulations could further save “the health system and patients time and money.”
No external funding for the study was reported. The investigators, Ms. O’Neil, and Dr. Scott have disclosed no relevant financial relationships. One investigator reported receiving honoraria from Knight Therapeutics, AbbVie, Tersera, and Eisai and owns stock in Myovant.
A version of this article first appeared on Medscape.com.
, according to a results from a randomized trial presented at the annual meeting of the American Society for Radiation Oncology.
The aim of this same-day CT scan, called a CT simulation scan, is to optimize radiation targeting by mimicking the conditions under which radiation is delivered using the latest information on the size and location of lesions.
But investigators reported that skipping the CT simulation scan saves patients hours in the clinic, allows patients to experience pain relief faster, and saves radiation oncologists time without compromising dosimetric coverage of cancerous lesions.
“This is huge in a symptomatic patient population,” said Melissa O’Neil, an advanced practice radiation therapist at the London, Ont., Health Sciences Centre and the lead investigator on the trial, dubbed DART (Diagnostic CT-Enabled Radiation Therapy).
“Diagnostic CT-based radiation planning substantially reduces time in the [treatment] center without a detriment in plan deliverability or quality,” Ms. O’Neil said.
In addition, patients are exposed to less radiation, and staff doesn’t have to spend as much time tending to symptomatic patients before treatment. Omitting this scan “should be considered for patients with a recent diagnostic CT scan who are undergoing simple palliative radiation,” Ms. O’Neil said.
CT simulation scans are standard of care in cases involving palliative radiation, but they create bottlenecks in the workflow. When a CT simulation is performed on the day of treatment, patients must wait hours as the results are translated into a treatment plan.
In the DART analysis, 33 patients with 42 treatment sites were randomly assigned to CT simulation planning or diagnostic CT planning.
Patients received up to 30 Gy in up to 10 fractions for bone or soft tissue metastases or primary tumor targets in the thorax, abdomen, pelvis, or proximal limbs. Single-fraction treatments were most common.
Three-quarters of the patients were men (median age, 72 years). Lung cancer was the most common type of primary tumor, followed by prostate and breast cancer.
The eight participants for whom the CT simulation approach was used waited 3-4 hours for treatment planning and overall spent a median of 4.8 hours in the cancer center on their day of treatment.
The 25 patients for whom diagnostic CT planning was used spent a median of 0.4 hours, or about 24 minutes, in the center on their day of treatment because radiation plans were completed before they arrived. The median time between their diagnostic CTs and radiation treatment was 13 days (range, 8-22 days).
Ms. O’Neil and her team found that if the original diagnostic CT was performed within 28 days, lesion anatomy would not have changed enough to warrant a new scan.
On the day of treatment, the study team used surface-guided radiation therapy techniques to ensure patients in the diagnostic CT planning group were positioned within 3 mm of where they were during their diagnostic scans, an essential step to ensure that radiation is delivered to the correct location. Ms. O’Neil noted that other investigators have used anatomic landmarks, a simpler approach, to achieve these results.
Overall, radiation oncologists rated radiation dose distribution as “acceptable” in about 80% of cases in both arms of DART and “acceptable with minor deviation” in the remaining 20% of cases.
Every radiation oncologist and medical physicists in the trial gave the workflow with diagnostic CT planning a 5 out of 5 rating for acceptability, and 90% of patients in this group rated the amount of time they spent for treatment as “acceptable.”
In contrast, only half of patients in the simulation arm said the amount of time spent was acceptable.
These findings align with several previous studies that support the diagnostic approach.
Jacob Scott, MD, a radiation oncologist at the Cleveland Clinic, said, “The comparable results using a recent diagnostic CT in place of a CT simulation for palliative radiation is an exciting step forward in radiation oncology. We may soon be in a world where we no longer need simulations.”
Dr. Scott also noted that combining diagnostic scans with cone beam or surface-guided positioning in lieu of CT simulations could further save “the health system and patients time and money.”
No external funding for the study was reported. The investigators, Ms. O’Neil, and Dr. Scott have disclosed no relevant financial relationships. One investigator reported receiving honoraria from Knight Therapeutics, AbbVie, Tersera, and Eisai and owns stock in Myovant.
A version of this article first appeared on Medscape.com.
FROM ASTRO 2023
Rheumatoid arthritis: Five things to know
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
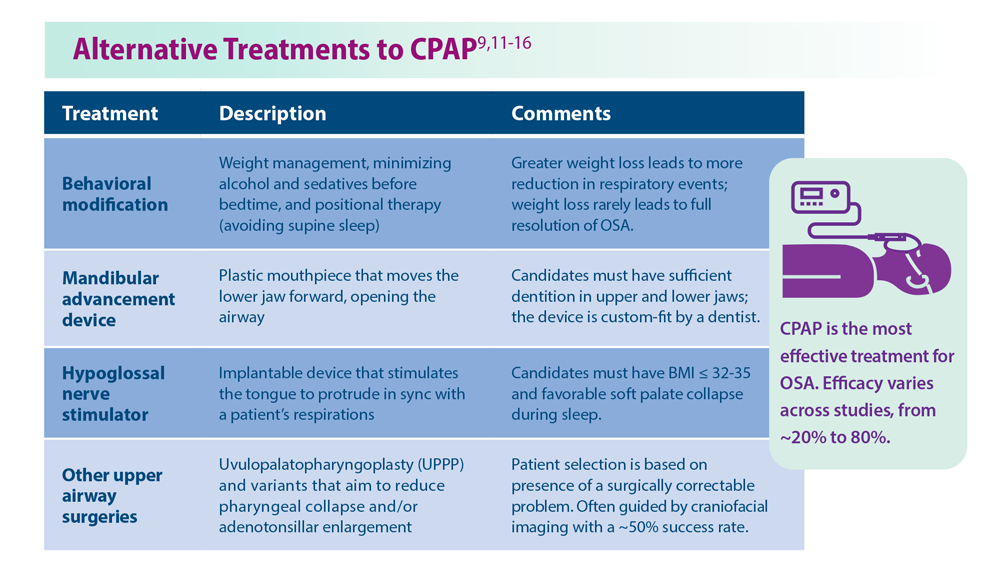
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
Early-onset NAFLD tied to higher cancer risk
TOPLINE:
New research shows that adults who develop nonalcoholic fatty liver disease (NAFLD) before age 45 are at increased risk of developing cancer, particularly digestive system and lung cancer.
METHODOLOGY:
- Researchers conducted a prospective age- and sex-matched cohort study of 63,696 adults (mean age, 51 years; 83% men) in China. The patients were followed for a median of 10 years; 31,848 had NAFLD, and 31,848 were control participants.
- Participants were grouped on the basis of age at the time of diagnosis of new-onset NAFLD: younger than 45, 45-54, 55-64, and 65 and older.
- Multivariable Cox models were used to analyze cancer risk by age at NAFLD onset. Population-attributable fractions were calculated to quantify cancer risk associated with age at NAFLD onset.
TAKEAWAY:
- During follow-up, 2,415 participants were diagnosed with cancer.
- NAFLD onset before age 45 was associated with highest cancer risk in comparison with the risk among control persons (average hazard ratio [AHR], 1.52). Cancer risk decreased as age at NAFLD onset increased (AHR, 1.50 for the 45-54 cohort, 1.13 for the 55-64 cohort, and 0.75 for the 65-and-older cohort).
- Among adults younger than 45 at NAFLD onset, cancers were mainly digestive and lung cancers (AHR, 2.00 and 2.14, respectively).
- Close to 18% of the cancer risk among adults younger than 45 at NAFLD onset was attributed to their fatty liver disease.
IN PRACTICE:
“The increasing incidence of NAFLD among younger populations highlights the underestimation of harmful outcomes associated with this condition,” the authors wrote. “Our findings suggest that early control and intervention against NAFLD progression may be crucial to reduce the occurrence of NAFLD-related cancers and lessen the burden on public health.”
SOURCE:
The study, with first author Chenan Liu, MD, PhD, Beijing Shijitan Hospital, Capital Medical University, was published online in JAMA Network Open.
LIMITATIONS:
The study population was predominantly male, and NAFLD diagnosis relied on ultrasound rather than liver biopsy, potentially missing mild cases. The study lacked data on liver fibrosis elastography measurement and blood biomarkers. For some cancers, incidence rates were low.
DISCLOSURES:
The study was funded by a grant from the National Key Research and Development Program of China. The authors reported no conflicts of interest.
TOPLINE:
New research shows that adults who develop nonalcoholic fatty liver disease (NAFLD) before age 45 are at increased risk of developing cancer, particularly digestive system and lung cancer.
METHODOLOGY:
- Researchers conducted a prospective age- and sex-matched cohort study of 63,696 adults (mean age, 51 years; 83% men) in China. The patients were followed for a median of 10 years; 31,848 had NAFLD, and 31,848 were control participants.
- Participants were grouped on the basis of age at the time of diagnosis of new-onset NAFLD: younger than 45, 45-54, 55-64, and 65 and older.
- Multivariable Cox models were used to analyze cancer risk by age at NAFLD onset. Population-attributable fractions were calculated to quantify cancer risk associated with age at NAFLD onset.
TAKEAWAY:
- During follow-up, 2,415 participants were diagnosed with cancer.
- NAFLD onset before age 45 was associated with highest cancer risk in comparison with the risk among control persons (average hazard ratio [AHR], 1.52). Cancer risk decreased as age at NAFLD onset increased (AHR, 1.50 for the 45-54 cohort, 1.13 for the 55-64 cohort, and 0.75 for the 65-and-older cohort).
- Among adults younger than 45 at NAFLD onset, cancers were mainly digestive and lung cancers (AHR, 2.00 and 2.14, respectively).
- Close to 18% of the cancer risk among adults younger than 45 at NAFLD onset was attributed to their fatty liver disease.
IN PRACTICE:
“The increasing incidence of NAFLD among younger populations highlights the underestimation of harmful outcomes associated with this condition,” the authors wrote. “Our findings suggest that early control and intervention against NAFLD progression may be crucial to reduce the occurrence of NAFLD-related cancers and lessen the burden on public health.”
SOURCE:
The study, with first author Chenan Liu, MD, PhD, Beijing Shijitan Hospital, Capital Medical University, was published online in JAMA Network Open.
LIMITATIONS:
The study population was predominantly male, and NAFLD diagnosis relied on ultrasound rather than liver biopsy, potentially missing mild cases. The study lacked data on liver fibrosis elastography measurement and blood biomarkers. For some cancers, incidence rates were low.
DISCLOSURES:
The study was funded by a grant from the National Key Research and Development Program of China. The authors reported no conflicts of interest.
TOPLINE:
New research shows that adults who develop nonalcoholic fatty liver disease (NAFLD) before age 45 are at increased risk of developing cancer, particularly digestive system and lung cancer.
METHODOLOGY:
- Researchers conducted a prospective age- and sex-matched cohort study of 63,696 adults (mean age, 51 years; 83% men) in China. The patients were followed for a median of 10 years; 31,848 had NAFLD, and 31,848 were control participants.
- Participants were grouped on the basis of age at the time of diagnosis of new-onset NAFLD: younger than 45, 45-54, 55-64, and 65 and older.
- Multivariable Cox models were used to analyze cancer risk by age at NAFLD onset. Population-attributable fractions were calculated to quantify cancer risk associated with age at NAFLD onset.
TAKEAWAY:
- During follow-up, 2,415 participants were diagnosed with cancer.
- NAFLD onset before age 45 was associated with highest cancer risk in comparison with the risk among control persons (average hazard ratio [AHR], 1.52). Cancer risk decreased as age at NAFLD onset increased (AHR, 1.50 for the 45-54 cohort, 1.13 for the 55-64 cohort, and 0.75 for the 65-and-older cohort).
- Among adults younger than 45 at NAFLD onset, cancers were mainly digestive and lung cancers (AHR, 2.00 and 2.14, respectively).
- Close to 18% of the cancer risk among adults younger than 45 at NAFLD onset was attributed to their fatty liver disease.
IN PRACTICE:
“The increasing incidence of NAFLD among younger populations highlights the underestimation of harmful outcomes associated with this condition,” the authors wrote. “Our findings suggest that early control and intervention against NAFLD progression may be crucial to reduce the occurrence of NAFLD-related cancers and lessen the burden on public health.”
SOURCE:
The study, with first author Chenan Liu, MD, PhD, Beijing Shijitan Hospital, Capital Medical University, was published online in JAMA Network Open.
LIMITATIONS:
The study population was predominantly male, and NAFLD diagnosis relied on ultrasound rather than liver biopsy, potentially missing mild cases. The study lacked data on liver fibrosis elastography measurement and blood biomarkers. For some cancers, incidence rates were low.
DISCLOSURES:
The study was funded by a grant from the National Key Research and Development Program of China. The authors reported no conflicts of interest.
New insight into genetic link between schizophrenia and CVD
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
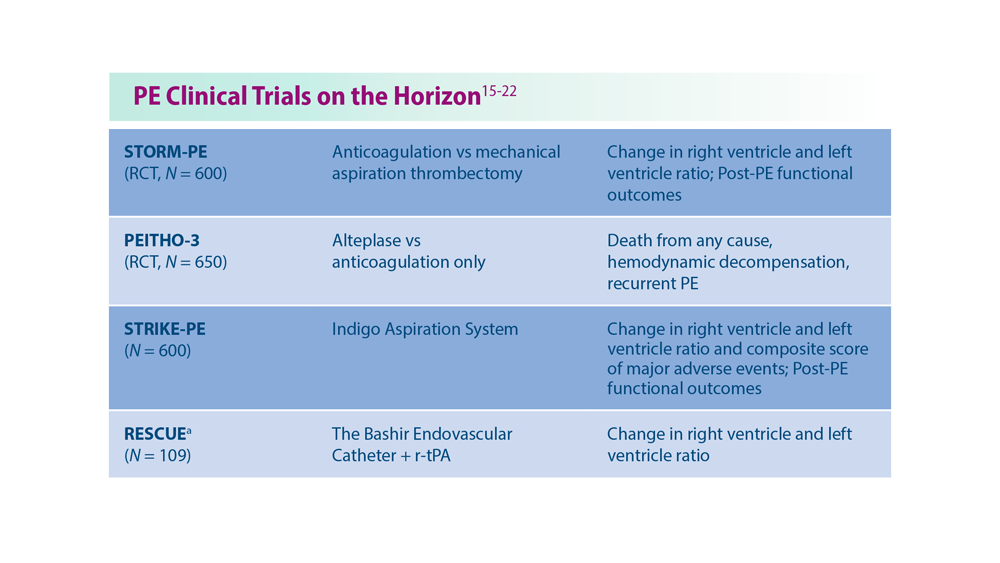
Decreasing Pulmonary Embolism-Related Mortality
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
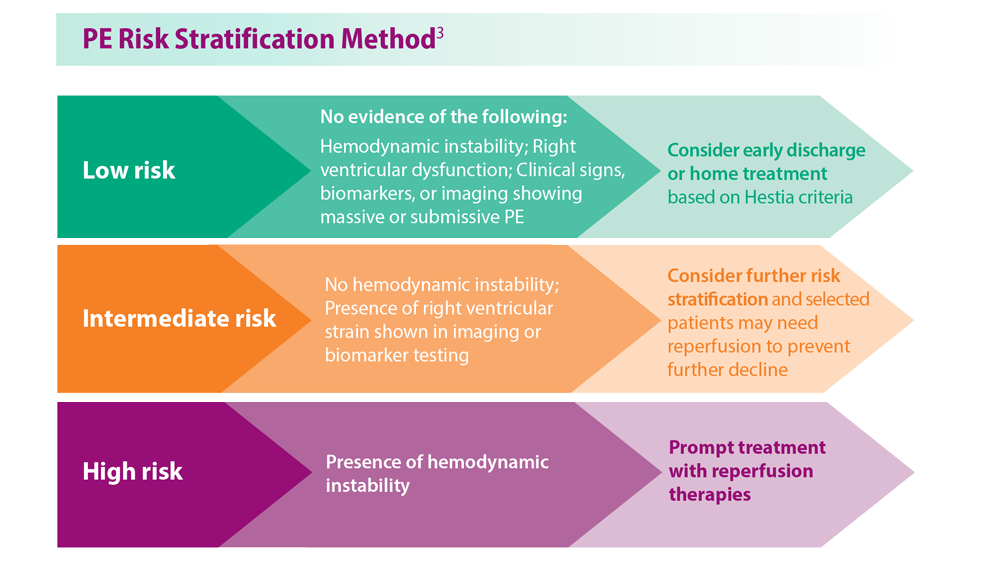
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
NCCN survey shows ongoing chemo drug shortages
Although access to carboplatin and cisplatin has improved slightly since June, when 93% and 70% of 27 NCCN member institutions reported shortages of the two agents, supplies remain limited and other anticancer drugs remain scarce, an NCCN follow-up survey shows.
Of 29 institutions surveyed last month, 86% reported having difficulty obtaining at least one anticancer drug, and 72% and 59% reported ongoing shortages of carboplatin and cisplatin, respectively – drugs recommended for treating patients involved in hundreds of different cancer scenarios, according to the NCCN.
“Drug shortages aren’t new, but the widespread impact makes this one particularly alarming,” NCCN’s chief executive officer, Robert W. Carlson, MD, said in a press statement. “It is extremely concerning that this situation continues despite significant attention and effort over the past few months.”
The latest survey, conducted between Sept. 6 and 27, was sent to the 33 NCCN member institutions. Overall, most respondents reported “being able to continue treating every patient who needs carboplatin or cisplatin, despite lowered supply, primarily by implementing strict waste management strategies,” the network noted, adding that “the responses may not reflect any additional challenges experienced by smaller community practices serving rural and marginalized patients.”
In addition to carboplatin and cisplatin shortages, the survey results also revealed that centers are experiencing shortages of a host of other drugs, including methotrexate (66%), 5-flourouracil (55%), fludarabine (45%), hydrocortisone (41%), and dacarbazine (28%), according to the press release.
“These drug shortages are the result of decades of systemic challenges,” noted Alyssa Schatz, senior director of policy and advocacy for NCCN, in a press release. “We recognize that comprehensive solutions take time, and we appreciate everyone who has put forth proposals to improve investment in generics and our data infrastructure. At the same time, we have to acknowledge that the cancer drug shortage has been ongoing for months, which is unacceptable for anyone impacted by cancer today.”
Following the June survey, the NCCN called for action from the federal government, the pharmaceutical industry, providers, and payers, encouraging them “to work together to ensure quality, effective, equitable, and accessible cancer care” and has since worked with multiple stakeholders and policymaking organizations to “advocate for short- and long-term fixes.”
“These new survey results remind us that we are still in an ongoing crisis and must respond with appropriate urgency,” Ms. Shatz added.
A version of this article first appeared on Medscape.com.
Although access to carboplatin and cisplatin has improved slightly since June, when 93% and 70% of 27 NCCN member institutions reported shortages of the two agents, supplies remain limited and other anticancer drugs remain scarce, an NCCN follow-up survey shows.
Of 29 institutions surveyed last month, 86% reported having difficulty obtaining at least one anticancer drug, and 72% and 59% reported ongoing shortages of carboplatin and cisplatin, respectively – drugs recommended for treating patients involved in hundreds of different cancer scenarios, according to the NCCN.
“Drug shortages aren’t new, but the widespread impact makes this one particularly alarming,” NCCN’s chief executive officer, Robert W. Carlson, MD, said in a press statement. “It is extremely concerning that this situation continues despite significant attention and effort over the past few months.”
The latest survey, conducted between Sept. 6 and 27, was sent to the 33 NCCN member institutions. Overall, most respondents reported “being able to continue treating every patient who needs carboplatin or cisplatin, despite lowered supply, primarily by implementing strict waste management strategies,” the network noted, adding that “the responses may not reflect any additional challenges experienced by smaller community practices serving rural and marginalized patients.”
In addition to carboplatin and cisplatin shortages, the survey results also revealed that centers are experiencing shortages of a host of other drugs, including methotrexate (66%), 5-flourouracil (55%), fludarabine (45%), hydrocortisone (41%), and dacarbazine (28%), according to the press release.
“These drug shortages are the result of decades of systemic challenges,” noted Alyssa Schatz, senior director of policy and advocacy for NCCN, in a press release. “We recognize that comprehensive solutions take time, and we appreciate everyone who has put forth proposals to improve investment in generics and our data infrastructure. At the same time, we have to acknowledge that the cancer drug shortage has been ongoing for months, which is unacceptable for anyone impacted by cancer today.”
Following the June survey, the NCCN called for action from the federal government, the pharmaceutical industry, providers, and payers, encouraging them “to work together to ensure quality, effective, equitable, and accessible cancer care” and has since worked with multiple stakeholders and policymaking organizations to “advocate for short- and long-term fixes.”
“These new survey results remind us that we are still in an ongoing crisis and must respond with appropriate urgency,” Ms. Shatz added.
A version of this article first appeared on Medscape.com.
Although access to carboplatin and cisplatin has improved slightly since June, when 93% and 70% of 27 NCCN member institutions reported shortages of the two agents, supplies remain limited and other anticancer drugs remain scarce, an NCCN follow-up survey shows.
Of 29 institutions surveyed last month, 86% reported having difficulty obtaining at least one anticancer drug, and 72% and 59% reported ongoing shortages of carboplatin and cisplatin, respectively – drugs recommended for treating patients involved in hundreds of different cancer scenarios, according to the NCCN.
“Drug shortages aren’t new, but the widespread impact makes this one particularly alarming,” NCCN’s chief executive officer, Robert W. Carlson, MD, said in a press statement. “It is extremely concerning that this situation continues despite significant attention and effort over the past few months.”
The latest survey, conducted between Sept. 6 and 27, was sent to the 33 NCCN member institutions. Overall, most respondents reported “being able to continue treating every patient who needs carboplatin or cisplatin, despite lowered supply, primarily by implementing strict waste management strategies,” the network noted, adding that “the responses may not reflect any additional challenges experienced by smaller community practices serving rural and marginalized patients.”
In addition to carboplatin and cisplatin shortages, the survey results also revealed that centers are experiencing shortages of a host of other drugs, including methotrexate (66%), 5-flourouracil (55%), fludarabine (45%), hydrocortisone (41%), and dacarbazine (28%), according to the press release.
“These drug shortages are the result of decades of systemic challenges,” noted Alyssa Schatz, senior director of policy and advocacy for NCCN, in a press release. “We recognize that comprehensive solutions take time, and we appreciate everyone who has put forth proposals to improve investment in generics and our data infrastructure. At the same time, we have to acknowledge that the cancer drug shortage has been ongoing for months, which is unacceptable for anyone impacted by cancer today.”
Following the June survey, the NCCN called for action from the federal government, the pharmaceutical industry, providers, and payers, encouraging them “to work together to ensure quality, effective, equitable, and accessible cancer care” and has since worked with multiple stakeholders and policymaking organizations to “advocate for short- and long-term fixes.”
“These new survey results remind us that we are still in an ongoing crisis and must respond with appropriate urgency,” Ms. Shatz added.
A version of this article first appeared on Medscape.com.
Training more doctors should be our first priority, says ethicist
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
Updated guidance from USPSTF on PrEP for HIV prevention
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.
Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
The new word in liver disease: The story behind NAFLD’s rebranding as MASLD
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.