User login
Self-compassion benefits psychiatrists, too
Congratulations to Ricks Warren, PhD, ABPP, Elke Smeets, PhD, and Kristen Neff, MD, the authors of “Self-criticism and self-compassion: Risk and resilience,” (Evidence-Based Reviews,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Warren responds
We couldn’t agree more with Dr. Moffic’s perspective that psychiatrists and other mental health clinicians likely would benefit from self-compassion during our clinical work in a complex, demanding, and rapidly changing mental health environment. Fortunately, attention to the importance of self-compassion for caregivers has been advocated, and recent studies of self-compassion in health care professionals have reported promising results. Because the neuroticism and self-criticism personality traits are most associated with depression and burnout in physicians, interventions that promote self-compassion are likely to lead to improved mental health in psychiatrists and other health care professionals. Recent research has found that self-compassion in health care providers is associated with less burnout and compassion fatigue, increased resilience, adaptive emotion regulation, and reduced sleep disturbance.1
The time is now right for clinical trials of self-compassion interventions in psychiatrists and other caregivers. Neff and Germer’s mindful self-compassion intervention,2 discussed in our article, could be easily adapted for psychiatrists and other mental health professionals. As Mills and Chapman,3 stated, “While being self-critical and perfectionistic may be common among doctors, being kind to oneself is not a luxury: it is a necessity. Self-care is, in a sense, a sine qua non for giving care for patients.”
Ricks Warren, PhD, ABPP
Clinical Associate Professor
Department of Psychiatry
University of Michigan Medical School
Ann Arbor, Michigan
1. Baker K, Warren R, Abelson J, et al. Physician mental health: depression and anxiety. In: Brower K, Riba M, eds. Physician mental health and well-being: research and practice. New York, NY: Springer. In press.
2. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013;69(1):28-44.
3. Mills J, Chapman M. Compassion and self-compassion in medicine: self-care for the caregiver. AMJ. 2016:9(5):87-91.
Congratulations to Ricks Warren, PhD, ABPP, Elke Smeets, PhD, and Kristen Neff, MD, the authors of “Self-criticism and self-compassion: Risk and resilience,” (Evidence-Based Reviews,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Warren responds
We couldn’t agree more with Dr. Moffic’s perspective that psychiatrists and other mental health clinicians likely would benefit from self-compassion during our clinical work in a complex, demanding, and rapidly changing mental health environment. Fortunately, attention to the importance of self-compassion for caregivers has been advocated, and recent studies of self-compassion in health care professionals have reported promising results. Because the neuroticism and self-criticism personality traits are most associated with depression and burnout in physicians, interventions that promote self-compassion are likely to lead to improved mental health in psychiatrists and other health care professionals. Recent research has found that self-compassion in health care providers is associated with less burnout and compassion fatigue, increased resilience, adaptive emotion regulation, and reduced sleep disturbance.1
The time is now right for clinical trials of self-compassion interventions in psychiatrists and other caregivers. Neff and Germer’s mindful self-compassion intervention,2 discussed in our article, could be easily adapted for psychiatrists and other mental health professionals. As Mills and Chapman,3 stated, “While being self-critical and perfectionistic may be common among doctors, being kind to oneself is not a luxury: it is a necessity. Self-care is, in a sense, a sine qua non for giving care for patients.”
Ricks Warren, PhD, ABPP
Clinical Associate Professor
Department of Psychiatry
University of Michigan Medical School
Ann Arbor, Michigan
Congratulations to Ricks Warren, PhD, ABPP, Elke Smeets, PhD, and Kristen Neff, MD, the authors of “Self-criticism and self-compassion: Risk and resilience,” (Evidence-Based Reviews,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Warren responds
We couldn’t agree more with Dr. Moffic’s perspective that psychiatrists and other mental health clinicians likely would benefit from self-compassion during our clinical work in a complex, demanding, and rapidly changing mental health environment. Fortunately, attention to the importance of self-compassion for caregivers has been advocated, and recent studies of self-compassion in health care professionals have reported promising results. Because the neuroticism and self-criticism personality traits are most associated with depression and burnout in physicians, interventions that promote self-compassion are likely to lead to improved mental health in psychiatrists and other health care professionals. Recent research has found that self-compassion in health care providers is associated with less burnout and compassion fatigue, increased resilience, adaptive emotion regulation, and reduced sleep disturbance.1
The time is now right for clinical trials of self-compassion interventions in psychiatrists and other caregivers. Neff and Germer’s mindful self-compassion intervention,2 discussed in our article, could be easily adapted for psychiatrists and other mental health professionals. As Mills and Chapman,3 stated, “While being self-critical and perfectionistic may be common among doctors, being kind to oneself is not a luxury: it is a necessity. Self-care is, in a sense, a sine qua non for giving care for patients.”
Ricks Warren, PhD, ABPP
Clinical Associate Professor
Department of Psychiatry
University of Michigan Medical School
Ann Arbor, Michigan
1. Baker K, Warren R, Abelson J, et al. Physician mental health: depression and anxiety. In: Brower K, Riba M, eds. Physician mental health and well-being: research and practice. New York, NY: Springer. In press.
2. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013;69(1):28-44.
3. Mills J, Chapman M. Compassion and self-compassion in medicine: self-care for the caregiver. AMJ. 2016:9(5):87-91.
1. Baker K, Warren R, Abelson J, et al. Physician mental health: depression and anxiety. In: Brower K, Riba M, eds. Physician mental health and well-being: research and practice. New York, NY: Springer. In press.
2. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013;69(1):28-44.
3. Mills J, Chapman M. Compassion and self-compassion in medicine: self-care for the caregiver. AMJ. 2016:9(5):87-91.
CORRECT: Insights into working at correctional facilities
Providing care in a correctional facility is inherent with danger, complexities, and risks. The mnemonic CORRECT strives to shed light on some of these factors and to provide a window of understanding on the needs and experiences of patients and staff in correctional facilities.
Challenges. The inherently coercive environment of a correctional facility affects all those confined within—staff and inmates. Staff members have varied background and experience (ie, custody, medical services, and mental health services). A large percentage of incarcerated individuals have been diagnosed with antisocial personality disorder, substance use disorder, psychosis, or medical illnesses. Many of these individuals have received little, if any, treatment, and are monitored most of the time by custody staff, who have limited training in mental health care.
Inmates also have considerable interaction with medical services. The goals of medical and psychiatric providers differ from that of corrections: to diagnose and treat vs to confine, deter, and punish.1 Disagreements and friction may be inevitable and require ongoing diplomacy.
Opportunity. Many inmates have a history of homelessness and arrive with untreated medical conditions; hypertension, impaired liver function, tuberculosis, and hepatitis C are common. Correctional facilities often become primary care providers for the physically and mentally ill. Inmates might have never received any form of patient education, and could respond well to patience, education, and compassion. Challenges can become opportunities to help this neglected, underserved, and underprivileged population.
Reflection. The need to continually assess a patient and provide a treatment plan is not unique to corrections. However, the patient caseload, the day-to-day continuum, and the need to complete patient care within time restrictions, can become a mundane process that could invite a sense of conditioned familiarity and boredom over the years, despite the predictable unpredictability of a correctional setting. The need to periodically stop and reflect is crucial, which can be done independently or with ongoing staff education.
Risks. A heightened level of risk starts from the time the incarcerated individual enters the correctional facility to the moment he (she) is released. This involves many facets, including physical, psychological, and medical exposure. Individuals could arrive in a state of drug withdrawal, and often in a state of delirium, which can complicate the presentation.
Potential inmate–inmate conflicts are a constant risk. Trading and swapping medications for sedative purposes or to get “high” is common in most correctional facilities, which has prompted many institutions to remove select medications from their formulary. Some individuals might prey on the novice, weak, or elderly inmates if they are taking sought-after medications. The suicide rate is high in correctional facilities. Because of these increased risks, the psychiatrist needs to be mindful of prescribing practices.
Experience. Despite years of education in medical school, residency, and fellowships, there is no substitute for clinical experience for novice correctional psychiatrists. Becoming competent can take years, and requires face-to-face evaluations, immersion, presence, and movement within a facility, and on-call responsibilities. Telepsychiatry is no replacement for the experience of being “in the trenches.” Despite a position of apparent power and superiority, physicians are human. Learning from mistakes is crucial to evolve and improve patient rapport.
Confidentiality. Lack of confidentiality often is the norm. Custody staff might be present during evaluations because of the potentially dangerous environment. Because certain areas of the facility require further caution, such as single cells or solitary confinement (as a result of unpredictability, dangerousness, specific charges, behavioral problems, etc.), the psychiatrist might be required to perform assessments at the front of the cell, in the presence of adjacent cells and other inmates and often an entire group. This might be unavoidable and requires a higher level of sensitivity. The need for correctional employees to maintain a sense of confidentiality has been well demonstrated in media events regarding serious boundary violations or sexual contact.
Treatment. Psychiatrists “confined” in corrections could feel isolated from the “outside” world and from their professional colleagues. Therefore, clinicians employed in corrections could develop a specific variety of burnout. Avoiding burnout requires a mindful discipline in self-care, efforts in healthy socialization, recreation, and outdoor activities. It’s crucial to maintain and update one’s knowledge base in order to provide treatment within the standard of care.
1. Dubler N. Ethical dilemmas in prison and jail health care. http://healthaffairs.org/blog/2014/03/10/ethical-dilemmas-in-prison-and-jail-health-care. Published March 10, 2014. Accessed December 14, 2016.
Providing care in a correctional facility is inherent with danger, complexities, and risks. The mnemonic CORRECT strives to shed light on some of these factors and to provide a window of understanding on the needs and experiences of patients and staff in correctional facilities.
Challenges. The inherently coercive environment of a correctional facility affects all those confined within—staff and inmates. Staff members have varied background and experience (ie, custody, medical services, and mental health services). A large percentage of incarcerated individuals have been diagnosed with antisocial personality disorder, substance use disorder, psychosis, or medical illnesses. Many of these individuals have received little, if any, treatment, and are monitored most of the time by custody staff, who have limited training in mental health care.
Inmates also have considerable interaction with medical services. The goals of medical and psychiatric providers differ from that of corrections: to diagnose and treat vs to confine, deter, and punish.1 Disagreements and friction may be inevitable and require ongoing diplomacy.
Opportunity. Many inmates have a history of homelessness and arrive with untreated medical conditions; hypertension, impaired liver function, tuberculosis, and hepatitis C are common. Correctional facilities often become primary care providers for the physically and mentally ill. Inmates might have never received any form of patient education, and could respond well to patience, education, and compassion. Challenges can become opportunities to help this neglected, underserved, and underprivileged population.
Reflection. The need to continually assess a patient and provide a treatment plan is not unique to corrections. However, the patient caseload, the day-to-day continuum, and the need to complete patient care within time restrictions, can become a mundane process that could invite a sense of conditioned familiarity and boredom over the years, despite the predictable unpredictability of a correctional setting. The need to periodically stop and reflect is crucial, which can be done independently or with ongoing staff education.
Risks. A heightened level of risk starts from the time the incarcerated individual enters the correctional facility to the moment he (she) is released. This involves many facets, including physical, psychological, and medical exposure. Individuals could arrive in a state of drug withdrawal, and often in a state of delirium, which can complicate the presentation.
Potential inmate–inmate conflicts are a constant risk. Trading and swapping medications for sedative purposes or to get “high” is common in most correctional facilities, which has prompted many institutions to remove select medications from their formulary. Some individuals might prey on the novice, weak, or elderly inmates if they are taking sought-after medications. The suicide rate is high in correctional facilities. Because of these increased risks, the psychiatrist needs to be mindful of prescribing practices.
Experience. Despite years of education in medical school, residency, and fellowships, there is no substitute for clinical experience for novice correctional psychiatrists. Becoming competent can take years, and requires face-to-face evaluations, immersion, presence, and movement within a facility, and on-call responsibilities. Telepsychiatry is no replacement for the experience of being “in the trenches.” Despite a position of apparent power and superiority, physicians are human. Learning from mistakes is crucial to evolve and improve patient rapport.
Confidentiality. Lack of confidentiality often is the norm. Custody staff might be present during evaluations because of the potentially dangerous environment. Because certain areas of the facility require further caution, such as single cells or solitary confinement (as a result of unpredictability, dangerousness, specific charges, behavioral problems, etc.), the psychiatrist might be required to perform assessments at the front of the cell, in the presence of adjacent cells and other inmates and often an entire group. This might be unavoidable and requires a higher level of sensitivity. The need for correctional employees to maintain a sense of confidentiality has been well demonstrated in media events regarding serious boundary violations or sexual contact.
Treatment. Psychiatrists “confined” in corrections could feel isolated from the “outside” world and from their professional colleagues. Therefore, clinicians employed in corrections could develop a specific variety of burnout. Avoiding burnout requires a mindful discipline in self-care, efforts in healthy socialization, recreation, and outdoor activities. It’s crucial to maintain and update one’s knowledge base in order to provide treatment within the standard of care.
Providing care in a correctional facility is inherent with danger, complexities, and risks. The mnemonic CORRECT strives to shed light on some of these factors and to provide a window of understanding on the needs and experiences of patients and staff in correctional facilities.
Challenges. The inherently coercive environment of a correctional facility affects all those confined within—staff and inmates. Staff members have varied background and experience (ie, custody, medical services, and mental health services). A large percentage of incarcerated individuals have been diagnosed with antisocial personality disorder, substance use disorder, psychosis, or medical illnesses. Many of these individuals have received little, if any, treatment, and are monitored most of the time by custody staff, who have limited training in mental health care.
Inmates also have considerable interaction with medical services. The goals of medical and psychiatric providers differ from that of corrections: to diagnose and treat vs to confine, deter, and punish.1 Disagreements and friction may be inevitable and require ongoing diplomacy.
Opportunity. Many inmates have a history of homelessness and arrive with untreated medical conditions; hypertension, impaired liver function, tuberculosis, and hepatitis C are common. Correctional facilities often become primary care providers for the physically and mentally ill. Inmates might have never received any form of patient education, and could respond well to patience, education, and compassion. Challenges can become opportunities to help this neglected, underserved, and underprivileged population.
Reflection. The need to continually assess a patient and provide a treatment plan is not unique to corrections. However, the patient caseload, the day-to-day continuum, and the need to complete patient care within time restrictions, can become a mundane process that could invite a sense of conditioned familiarity and boredom over the years, despite the predictable unpredictability of a correctional setting. The need to periodically stop and reflect is crucial, which can be done independently or with ongoing staff education.
Risks. A heightened level of risk starts from the time the incarcerated individual enters the correctional facility to the moment he (she) is released. This involves many facets, including physical, psychological, and medical exposure. Individuals could arrive in a state of drug withdrawal, and often in a state of delirium, which can complicate the presentation.
Potential inmate–inmate conflicts are a constant risk. Trading and swapping medications for sedative purposes or to get “high” is common in most correctional facilities, which has prompted many institutions to remove select medications from their formulary. Some individuals might prey on the novice, weak, or elderly inmates if they are taking sought-after medications. The suicide rate is high in correctional facilities. Because of these increased risks, the psychiatrist needs to be mindful of prescribing practices.
Experience. Despite years of education in medical school, residency, and fellowships, there is no substitute for clinical experience for novice correctional psychiatrists. Becoming competent can take years, and requires face-to-face evaluations, immersion, presence, and movement within a facility, and on-call responsibilities. Telepsychiatry is no replacement for the experience of being “in the trenches.” Despite a position of apparent power and superiority, physicians are human. Learning from mistakes is crucial to evolve and improve patient rapport.
Confidentiality. Lack of confidentiality often is the norm. Custody staff might be present during evaluations because of the potentially dangerous environment. Because certain areas of the facility require further caution, such as single cells or solitary confinement (as a result of unpredictability, dangerousness, specific charges, behavioral problems, etc.), the psychiatrist might be required to perform assessments at the front of the cell, in the presence of adjacent cells and other inmates and often an entire group. This might be unavoidable and requires a higher level of sensitivity. The need for correctional employees to maintain a sense of confidentiality has been well demonstrated in media events regarding serious boundary violations or sexual contact.
Treatment. Psychiatrists “confined” in corrections could feel isolated from the “outside” world and from their professional colleagues. Therefore, clinicians employed in corrections could develop a specific variety of burnout. Avoiding burnout requires a mindful discipline in self-care, efforts in healthy socialization, recreation, and outdoor activities. It’s crucial to maintain and update one’s knowledge base in order to provide treatment within the standard of care.
1. Dubler N. Ethical dilemmas in prison and jail health care. http://healthaffairs.org/blog/2014/03/10/ethical-dilemmas-in-prison-and-jail-health-care. Published March 10, 2014. Accessed December 14, 2016.
1. Dubler N. Ethical dilemmas in prison and jail health care. http://healthaffairs.org/blog/2014/03/10/ethical-dilemmas-in-prison-and-jail-health-care. Published March 10, 2014. Accessed December 14, 2016.
Ruling out delirium: Therapeutic principles of withdrawing and changing medications
Ms. M, age 71, was diagnosed with Alzheimer’s disease several months ago and her clinical presentation and Mini-Mental Status Exam score of 22 indicates mild dementia. In addition to chronic medications for hypertension, Ms. M has been taking lorazepam, 1 mg, 3 times daily, for >15 years for unspecified anxiety.
Ms. M becomes more confused at home over the course of a few days, and her daughter brings her to her primary care physician for evaluation. Recognizing that benzodiazepines can contribute to delirium, the physician discontinues lorazepam. Three days later, Ms. M’s confusion worsens, and she develops nausea and a tremor. She is taken to the local emergency department where she is admitted for benzodiazepine withdrawal and diagnosed with a urinary tract infection.
Because dementia is a strong risk factor for developing delirium,1 withdrawing or changing
Consider withdrawing or replacing medications that are strongly implicated in causing delirium with another medication for the same indication with a lower potential for
In general, there are no firm rules for how to taper and discontinue potentially deliriogenic medications, as both the need to taper and the best strategy for doing so depends on a number of factors and requires clinical judgement. When determining how quickly to withdraw a potentially offending medication in a patient with suspected delirium, clinicians should consider:
Dosage and duration of treatment. Consider tapering and discontinuing benzodiazepines in a patient who is taking more than the minimal scheduled dosages for ≥2 weeks, especially after 8 weeks of scheduled treatment. Consider tapering opioids in a patient taking more than the minimal scheduled dosage for more than a few days. When attempting to rule out delirium, taper opioids as quickly and as safely possible, with a recommended reduction of ≤20% per day to prevent withdrawal symptoms. In general, potentially deliriogenic medications can be discontinued without tapering if they are taken on a non-daily, as-needed basis.
The half-life of a medication determines both the onset and duration of withdrawal symptoms. Withdrawal occurs earlier when discontinuing medications with short
Nature of withdrawal symptoms. In patients with suspected delirium, tapering over weeks or
Care setting. When tapering and discontinuing a medication, regularly monitor patients for withdrawal symptoms; slow or temporarily stop the taper if withdrawal symptoms occur. Because close monitoring is easier in an inpatient vs an outpatient care setting, more aggressive tapering over 2 to 3 days generally can be considered, although more gradual tapering might be prudent to ensure safety of outpatients.
1. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
2. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
3. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Aging. 2010;40(1):23-29.
4. Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099-1105.
5. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481-1486.
6. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
Ms. M, age 71, was diagnosed with Alzheimer’s disease several months ago and her clinical presentation and Mini-Mental Status Exam score of 22 indicates mild dementia. In addition to chronic medications for hypertension, Ms. M has been taking lorazepam, 1 mg, 3 times daily, for >15 years for unspecified anxiety.
Ms. M becomes more confused at home over the course of a few days, and her daughter brings her to her primary care physician for evaluation. Recognizing that benzodiazepines can contribute to delirium, the physician discontinues lorazepam. Three days later, Ms. M’s confusion worsens, and she develops nausea and a tremor. She is taken to the local emergency department where she is admitted for benzodiazepine withdrawal and diagnosed with a urinary tract infection.
Because dementia is a strong risk factor for developing delirium,1 withdrawing or changing
Consider withdrawing or replacing medications that are strongly implicated in causing delirium with another medication for the same indication with a lower potential for
In general, there are no firm rules for how to taper and discontinue potentially deliriogenic medications, as both the need to taper and the best strategy for doing so depends on a number of factors and requires clinical judgement. When determining how quickly to withdraw a potentially offending medication in a patient with suspected delirium, clinicians should consider:
Dosage and duration of treatment. Consider tapering and discontinuing benzodiazepines in a patient who is taking more than the minimal scheduled dosages for ≥2 weeks, especially after 8 weeks of scheduled treatment. Consider tapering opioids in a patient taking more than the minimal scheduled dosage for more than a few days. When attempting to rule out delirium, taper opioids as quickly and as safely possible, with a recommended reduction of ≤20% per day to prevent withdrawal symptoms. In general, potentially deliriogenic medications can be discontinued without tapering if they are taken on a non-daily, as-needed basis.
The half-life of a medication determines both the onset and duration of withdrawal symptoms. Withdrawal occurs earlier when discontinuing medications with short
Nature of withdrawal symptoms. In patients with suspected delirium, tapering over weeks or
Care setting. When tapering and discontinuing a medication, regularly monitor patients for withdrawal symptoms; slow or temporarily stop the taper if withdrawal symptoms occur. Because close monitoring is easier in an inpatient vs an outpatient care setting, more aggressive tapering over 2 to 3 days generally can be considered, although more gradual tapering might be prudent to ensure safety of outpatients.
Ms. M, age 71, was diagnosed with Alzheimer’s disease several months ago and her clinical presentation and Mini-Mental Status Exam score of 22 indicates mild dementia. In addition to chronic medications for hypertension, Ms. M has been taking lorazepam, 1 mg, 3 times daily, for >15 years for unspecified anxiety.
Ms. M becomes more confused at home over the course of a few days, and her daughter brings her to her primary care physician for evaluation. Recognizing that benzodiazepines can contribute to delirium, the physician discontinues lorazepam. Three days later, Ms. M’s confusion worsens, and she develops nausea and a tremor. She is taken to the local emergency department where she is admitted for benzodiazepine withdrawal and diagnosed with a urinary tract infection.
Because dementia is a strong risk factor for developing delirium,1 withdrawing or changing
Consider withdrawing or replacing medications that are strongly implicated in causing delirium with another medication for the same indication with a lower potential for
In general, there are no firm rules for how to taper and discontinue potentially deliriogenic medications, as both the need to taper and the best strategy for doing so depends on a number of factors and requires clinical judgement. When determining how quickly to withdraw a potentially offending medication in a patient with suspected delirium, clinicians should consider:
Dosage and duration of treatment. Consider tapering and discontinuing benzodiazepines in a patient who is taking more than the minimal scheduled dosages for ≥2 weeks, especially after 8 weeks of scheduled treatment. Consider tapering opioids in a patient taking more than the minimal scheduled dosage for more than a few days. When attempting to rule out delirium, taper opioids as quickly and as safely possible, with a recommended reduction of ≤20% per day to prevent withdrawal symptoms. In general, potentially deliriogenic medications can be discontinued without tapering if they are taken on a non-daily, as-needed basis.
The half-life of a medication determines both the onset and duration of withdrawal symptoms. Withdrawal occurs earlier when discontinuing medications with short
Nature of withdrawal symptoms. In patients with suspected delirium, tapering over weeks or
Care setting. When tapering and discontinuing a medication, regularly monitor patients for withdrawal symptoms; slow or temporarily stop the taper if withdrawal symptoms occur. Because close monitoring is easier in an inpatient vs an outpatient care setting, more aggressive tapering over 2 to 3 days generally can be considered, although more gradual tapering might be prudent to ensure safety of outpatients.
1. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
2. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
3. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Aging. 2010;40(1):23-29.
4. Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099-1105.
5. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481-1486.
6. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
1. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
2. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
3. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Aging. 2010;40(1):23-29.
4. Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099-1105.
5. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481-1486.
6. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
Using rating scales in a clinical setting: A guide for psychiatrists
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
Confused with ataxia and urinary and fecal incontinence
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).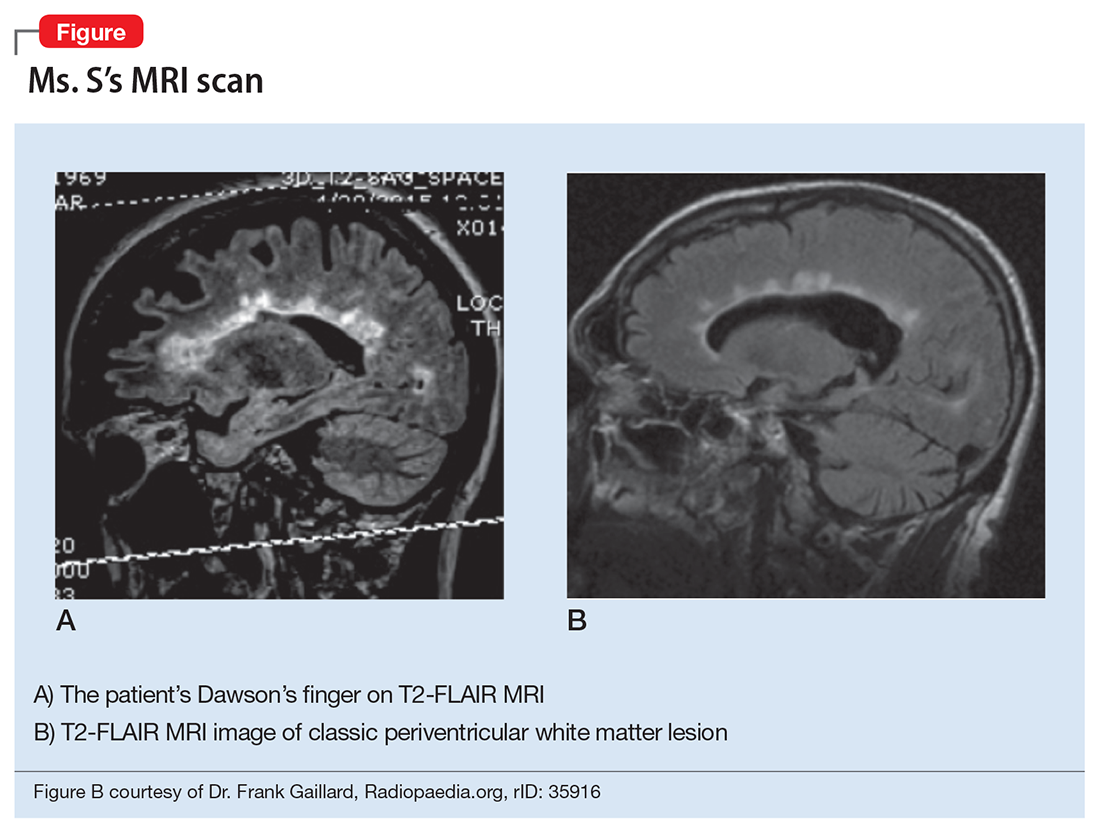
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
CASE Paranoia, ataxia
Ms. S, age 46, is admitted to the hospital for cellulitis and gait disturbance. She has been living in her car for the past week and presents to the local fire department to get help for housing. She is referred to this hospital where she was found to have cellulitis in her buttock secondary to urinary and fecal incontinence. She also was noted to have difficulty ambulating and a wide-based gait. Two weeks earlier, a hotel clerk found her on the floor, unable to get up. Ms. S was seen in a local emergency room (ER) and discharged after her glucose level was found to be normal.
At admission, she has an intact sensorium and is described as disheveled, illogical, rambling, and paranoid. Her mental status exam shows she is alert and oriented to person and time, with guarded and childlike behavior. Her affect/mood is irritable and oddly related, and her thought processes are concrete and simple with some thought-blocking and paranoid content. She denies thoughts of harming herself or others, and her insight is limited and judgment is poor.
Neurology is consulted to evaluate her gait disturbance. Ms. S has decreased muscle bulk in both calves, with brisk knee reflexes bilaterally. CT imaging shows nonspecific scattered periventricular white matter hypodensities consistent with microvascular ischemic diagnosis, but a demyelinating process could not be ruled out. Ms. S reports that the gait disturbance began in childhood, and that her grandmother had the same gait disturbance. Neurology recommends an electromyogram and MRI.
During her stay in the hospital, she is unwilling to cooperate with exams, declines to answer questions regarding her past, and appears suspicious of her acute care treatment team. The psychiatric team is consulted for evaluation of her paranoia and “seeming disorganization,” and she is transferred to the psychiatric unit. She appears to be repulsed by the fact that she was in a psychiatric ward stating, “I don’t belong here” and “I’m scared of the other people here.” She denies any psychiatric history, previous hospitalizations, or substance use, and no documentation of inpatient or outpatient care was found in the county’s computerized record system. Although she is willing to take a small dose of tranquilizer (eg, lorazepam) she refuses to take antipsychotic medications saying, “My mother told me not to take [antipsychotics]. I’m not psychotic.”
What is your diagnosis at this point?
a) normal pressure hydrocephalus
b) Charcot-Marie-Tooth disease
c) schizophrenia spectrum disorder
d) multiple sclerosis (MS)
e) vascular dementia
f) cord lesion compression
The authors’ observations
The neurology team initially suspected Charcot-Marie-Tooth disease because her clinical presentation included pes cavus, distal lower extremity weakness, and lower extremity muscle atrophy with a self-reported family history of similar gait disturbance, all of which are consistent with Charcot-Marie-Tooth disease.
Subcortical syndrome—a feature of vascular dementia—is characterized by focal motor deficits, gait disturbance, history of unsteadiness with frequent falls, urinary symptoms, personality and mood changes, and cognitive dysfunction.1-3 Subcortical syndrome is caused by chronic ischemia and lacunar infarctions that affect cerebral nuclei and white matter pathways.1 On imaging, subcortical vascular dementia is characterized by leukoaraiosis, which are hypointense spherical-like lesions on CT and hyperintense lesions in periventricular areas on T2 MRI.4
Although normal pressure hydrocephalus could be suspected given her clinical presentation of the Hakim-Adams triad (ie,“wacky, wobbly, and wet”), her head CT did not show any changes consistent with this condition.
Her clinical presentation does not align with schizophrenia spectrum disorder because of her history of higher functioning, acute later onset, and the absence of hallucinations, fixed delusions, or markedly disorganized speech. Although she is paranoid of her surroundings, her delusions were ill-formed. A cord lesion compression cannot be ruled out, and MRI is required urgently.
HISTORY High functioning
When asked, Ms. S states that she was admitted to the hospital because “someone who looked like a fake police officer [a member of the fire department] told me it was nice here.” She indicates that she initially thought it would be a nice place to live temporarily but later regretted coming after realizing that she was in a psychiatry unit. Available documentation from her recent hospitalization indicated that she was living in a motel on her own. Ms. S says that she works as an actress and has had minor roles in famous movies. She says that she studied at a well-known performance arts school and that her parents are famous musicians; however, she refuses to identify her parents or give permission to contact them—or any other collateral informant—because she is embarrassed about her current situation stating, “They would never believe it.”
During this interview, Ms. S appears confused as well as disorganized—which was a challenge to clearly delineate—disheveled, and guarded with hypoverbal and hypophonic speech. Her thought process is circumstantial, and she seems to be confabulating. She denies visual or auditory hallucinations but appears paranoid and states that she thinks we are experimenting on her. Except for the neurological exam, the rest of her physical exam is within normal limits. Urine toxicology screen and labs are negative except for a positive antinuclear antibody homogenous pattern with a titer of 1:640; B12 vitamin levels are not tested.
MRI is ordered, however, she does not consent to the scan saying, “It’s creepy, I don’t want people looking at my brain.” The team makes several attempts to encourage her for consent but she refuses. Because of the clinical urgency (ie, possible cord compression) and her refusal to provide a surrogate decision maker, the team felt the situation is urgent, confirmed by 2 physicians, which led them to perform the MRI on an emergent basis. The MRI reveals multiple periventricular, juxtacortical, infratentorial, and likely cervical spinal cord T2 hyperintense lesions (Figure).
What would be your differential diagnosis at this time?
a) acute disseminated encephalomyelitis (ADEM)
b) systemic lupus erythematous
c) multiple sclerosis
d) vascular dementia
e) vitamin B deficiency
The authors’ observations
Psychosis in the presence of white matter demyelination could be associated with autoimmune, vascular, or nutritional disturbances. Deficiencies in vitamins B6, 9, and 12 (pyridoxine, folate, cobalamin) have been shown to cause neuropsychiatric symptoms and white matter lesions.5 Low levels of vitamins B6, 9, and 12 are associated with elevated homocysteine, which can cause small vessel ischemia leading to white matter lesions similar to changes seen in vascular dementia.5 The exact pathophysiology of ADEM is unclear, however, it is thought that after an infection, antiviral antibodies cross react with autoantigens on myelin causing an autoimmune demyelinating disease. Another hypothesized mechanism is that circulating immune complexes and humoral factors increase vascular permeability and inflammation thereby opening the blood–brain barrier. Once it is open, cells such as lymphocytes, phagocytes, and microglia cause gliosis and demyelination. Case reports have described ADEM associated with psychotic features.6
Likewise, systemic lupus erythematous has been associated with psychosis and neuropsychiatric symptoms in 14% to 75% of patients. Of these patients, 40% will experience neuropsychiatric symptoms before onset of lupus symptoms.7 One study found the most common MRI finding in neuropsychiatric systemic lupus erythematous was leukoaraiosis, which appeared in 57.1% of patients.8 Ms. S’s MRI results strongly suggest a diagnosis of MS.
EVALUATION Questionable story
Ms. S appears delusional and grandiose when she meets with the psychiatry team. She states that before her hospitalization, she was an actress and could ambulate, rent a motel room, and drive a car without assistance. However, during the examination, she cannot walk without 2 staff members for support, and overall her self-reported history sounds questionable. There were several pieces of evidence that corroborate portions of her story: (1) a screen actors guild card was found among personal belongings; (2) she was transported to the ER from a local motel; (3) she had recently visited another hospital and, at that time, was deemed stable enough to be discharged.
On the Montreal Cognitive Assessment (MoCA) Ms. S scored 19/30, with deficits mainly in executive/visuospatial and delayed recall memory. An alternate form of the MoCA is administered 1 day later, and she scores 20/30 with similar deficits. After obtaining medication consent, she is given risperidone, up to 2 mg/d, and becomes more cooperative with the treatment team.
The authors’ observations
Approximately 40% to 65% of MS patients experience cognitive impairment.9 Cognitive dysfunction in a depressed patient with MS might appear as pseudo-dementia, but other possible diagnoses include:
- true dementia
- encephalitis or infection
- medication- or substance-induced.
White matter demyelination is associated with subcortical dementia, which is characterized by slowness of information processing, forgetfulness, apathy, depression, and impaired cognition. According to meta-analyses, the most prominent neuropsychological deficits in MS are found in the areas of verbal fluency, information processing speed, working memory, and long-term memory.10 Relapsing-remitting type MS patients generally have less cognitive impairment than those with the chronic progressive type of the disease.
EVALUATION Cognitive deficits
Because of her acute condition and resistance to the evaluation, a modified screening neuropsychological battery is used. During the evaluation Ms. S is guarded and demonstrates paucity of speech; her responses are odd at times or contain word-substitution errors. Hand stiffness, tremor, and imprecision are noted during writing and drawing. Results of testing indicate average-range premorbid intellectual ability, with impairments in memory and information processing speed and a mild weakness in phonemic verbal fluency. Ms. S endorses statements reflecting paranoia and hostility on a self-report measure of emotional and personality functioning, consistent with her behavioral presentation. However, her responses on other subscales, including depression and psychotic symptoms, are within normal limits. Her cognitive deficits would be unusual if she had a psychiatric illness alone and are likely associated with her positive neuroimaging findings that suggest a demyelinating process. Overall, the results of the evaluation support a MS diagnosis.
The authors’ observations
Psychosis is found at a higher rate among MS patients (2% to 3%) than the general population (0.5% to 1%).9 Although rare, psychosis often can cloud the diagnosis of MS. Psychiatric symptoms that can occur in MS include:
- hallucinations and delusions (>50%)
- irritability and agitation (20%)
- grandiosity (15%)
- confusion, blunted affect, flight of ideas, depression, reduced self-care, and pressured speech (10%).11
A review of 10 studies found that depression was the most prevalent symptom in MS, and that schizophrenia occurred in up to 7% of MS patients.12 There are currently 3 theories about the relationship between psychosis and MS:
- MS and psychosis are thought to share the same pathophysiological process.
- Psychotic symptoms arise from regional demyelination simultaneously with MS.
- Psychosis is caused by medical treatment of MS.9
Other causes of psychiatric symptoms in MS include:
- depression associated with brain atrophy and lesions
- depression and anxiety as a result of chronic illness
- depression resulting from inflammatory changes
- corticosteroid treatment causing depression, mania, or psychosis.12
The link between psychosis and MS is still poorly understood and further investigation is needed.
How would you treat Ms. S?
a) haloperidol
b) risperidone
c) corticosteroids
d) selective serotonin reuptake inhibitors
Treating psychiatric symptoms in the context of MS
The literature, mainly case reports, suggests several treatment modalities for psychosis with MS. Clozapine has been shown to be beneficial in several case reports, and risperidone9 and ziprasidone13 also have been effective. Other studies recommended low-dose chlorpromazine.9
For MS patients with cognitive impairment, one study showed that interferon beta-1b (IFN-1b) treatment resulted in significant improvement in concentration, attention, visual learning, and recall after 1 year compared with control patients.9 However, there are also case reports of IFN-1b and glucocorticoid-induced psychosis in patients, which resolved after discontinuing treatment.9
Psychotic symptoms have been shown to resolve after corticosteroid treatment of MS.14 In another case report, mania and delusions subsided 3 days after IV methylprednisolone, whereas risperidone had no effect on psychotic features. However, it was unclear whether risperidone was discontinued when methylprednisolone was administered, therefore the specific effect of methylprednisolone is difficult to discern.15 Finally, in a case of a patient who has chronic MS for 16 years and presented with acute onset paranoid psychosis, symptoms resolved with aripiprazole, 10 to 20 mg/d.16 Because of the limited utility of case reports, there is a need for further research in medical management of psychiatric symptoms in MS.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
1. de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145-151.
2. Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42(10):1966-1979.
3. Staekenborg SS, van der Flier WM, van Straaten EC, et al. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317-322.
4. Mortimer A, Likeman M, Lewis T. Neuroimaging in dementia: a practical guide. Pract Neurol. 2013;13(2):92-103.
5. Xiong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011:617927. doi:10.4061/2011/617927.
6. Habek M, Brinar M, Brinar VV, et al. Psychiatric manifestations of multiple sclerosis and acute disseminated encephalomyelitis. Clin Neurol Neurosug. 2006;108(3);290-294.
7. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune disease and psychosis. Biol Psychiatry. 2014;75(4);300-306.
8. Jeong HW, Her M, Bae JS, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. 2014;35(5):861-869.
9. Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2(1):13-29.
10. Thornton AE, DeFreitas VG. The neuropsychology of multiple sclerosis. In: Grant I, Adams KM, eds. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. New York, NY: Oxford University Press; 2009:280-305.
11. Kosmidis MH, Giannakou M, Messinis L, et al. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22(1):55-66.
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
13. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):734-744.
14. Thöne J, Kessler E. Improvement of neuropsychiatric symptoms in multiple sclerosis subsequent to high-dose corticosteroid treatment. Prim Care Companion J Clin Psychiatry. 2008;10(2):163-164.
15. Hoiter S, Maltete D, Bourre B, et al. A manic episode with psychotic features improved by methylprednisolone in a patient with multiple sclerosis. Gen Hosp Psychiatry. 2015;37(6):621.e1-621.e2.
16. Muzyk AJ, Christopher EJ, Gagliardi JP, et al. Use of aripiprazole in a patient with multiple sclerosis presenting with paranoid psychosis. J Psychiatr Pract. 2010;16(6):420-424.
Pills to powder: An updated clinician’s reference for crushable psychotropics
Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.
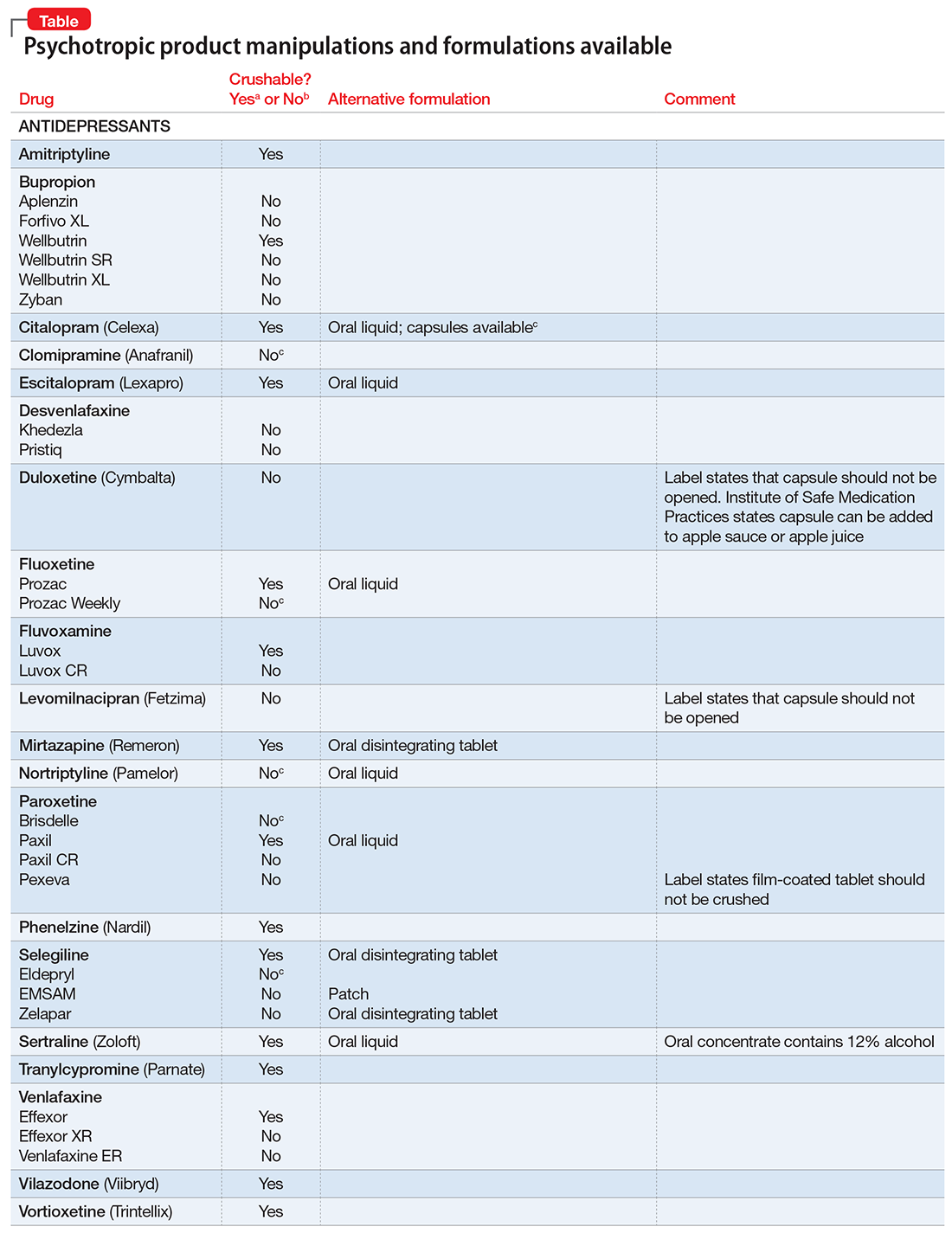
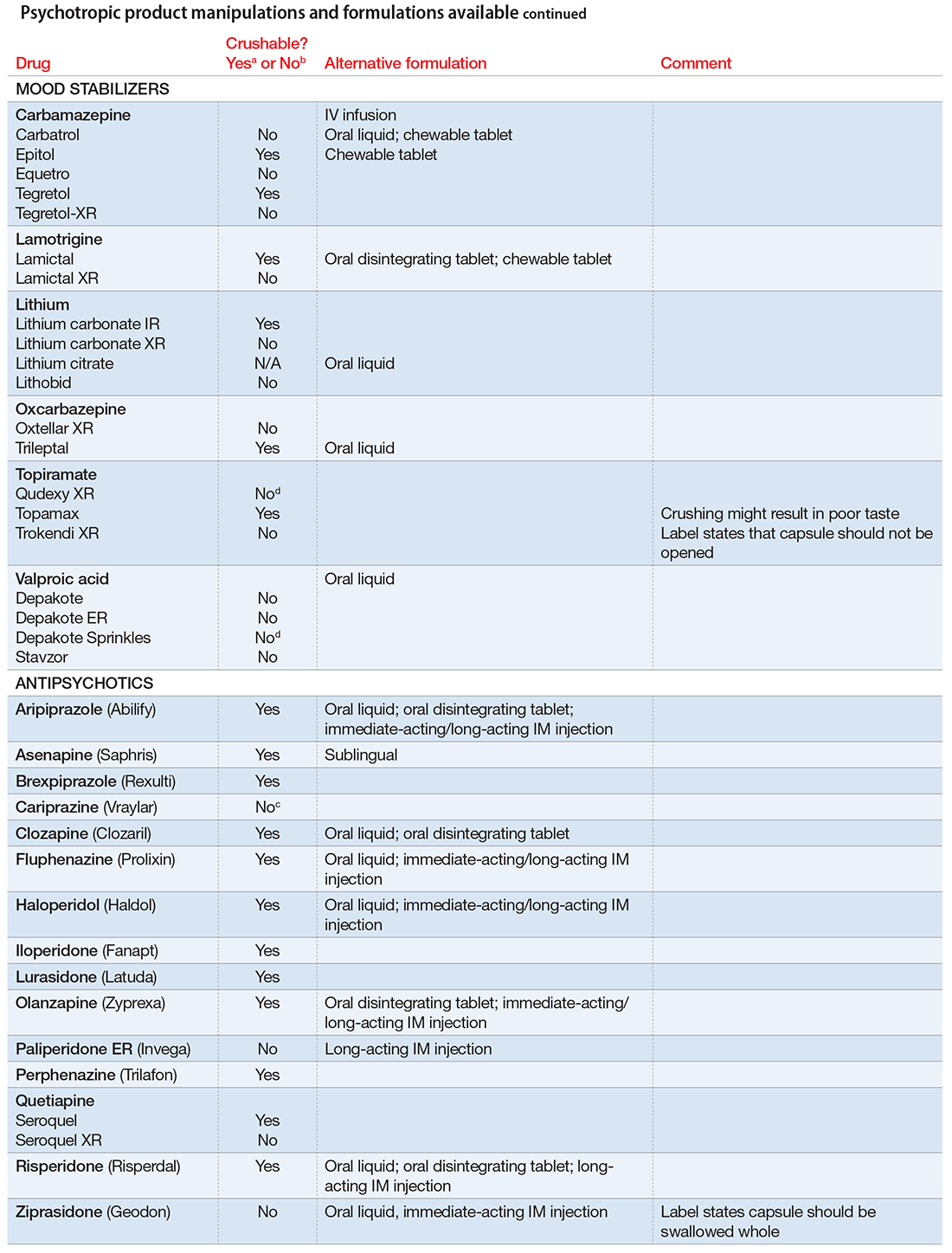
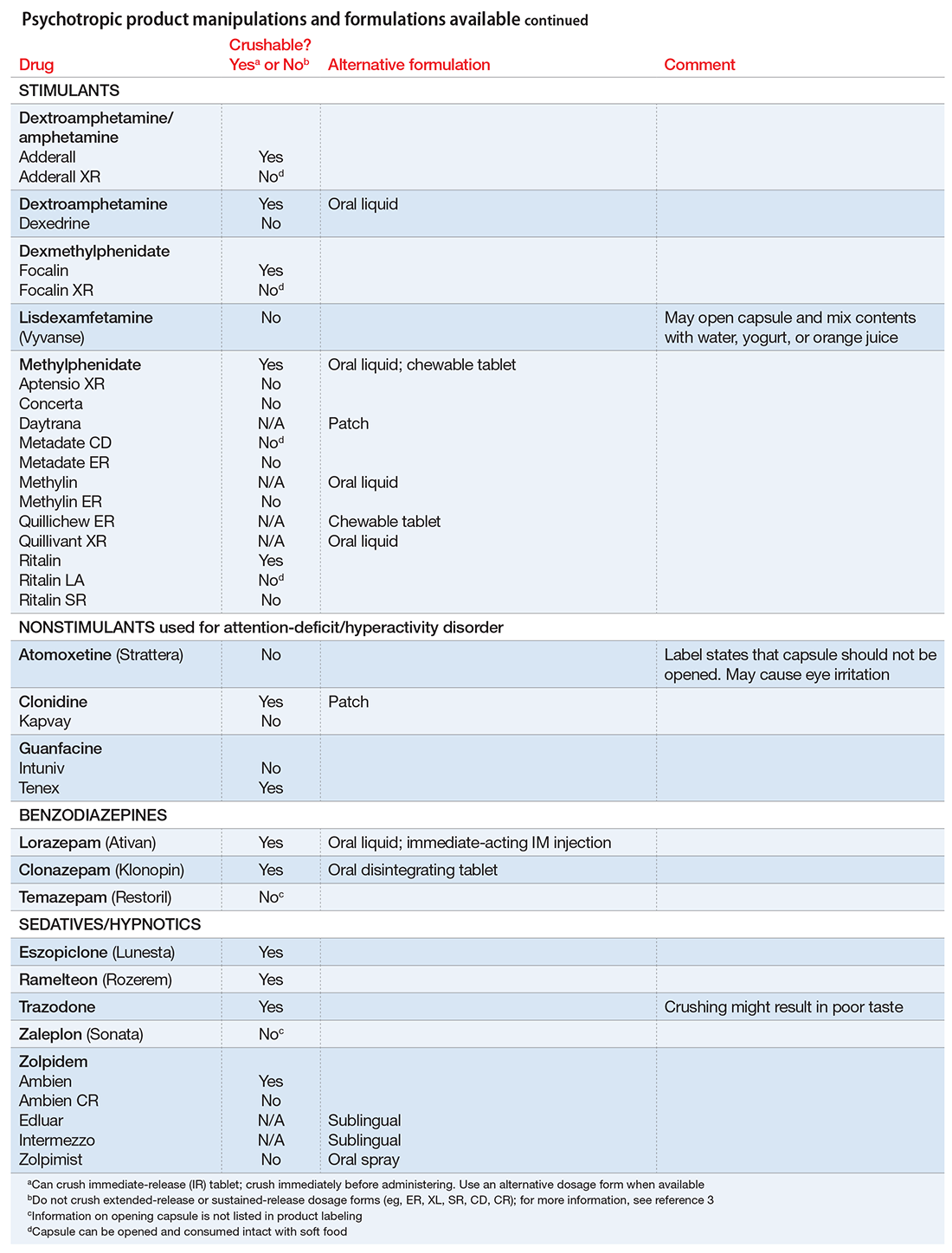
1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.



Many patients experience difficulty swallowing pills, for various reasons:
- discomfort (particularly pediatric and geriatric patients)
- postsurgical need for an alternate route of enteral intake (nasogastric tube, gastrostomy, jejunostomy)
- dysphagia due to a neurologic disorder (multiple sclerosis, impaired gag reflex, dementing processes)
- odynophagia (pain upon swallowing) due to gastroesophageal reflux or a structural abnormality
- a structural abnormality of the head or neck that impairs swallowing.1
If these difficulties are not addressed, they can interfere with medication adherence. In those instances, using an alternative dosage form or manipulating an available formulation might be required.
Crushing guidelines
There are limited data on crushed-form products and their impact on efficacy. Therefore, when patients have difficulty taking pills, switching to liquid solution or orally disintegrating forms is recommended. However, most psychotropics are available only as tablets or capsules. Patients can crush their pills immediately before administration for easier intake. The following are some general guidelines for doing so:2
- Scored tablets typically can be crushed.
- Crushing sublingual and buccal tablets can alter their effectiveness.
- Crushing sustained-release medications can eliminate the sustained-release action.3
- Enteric-coated medications should not be crushed, because this can alter drug absorption.
- Capsules generally can be opened to administer powdered contents, unless the capsule has time-release properties or an enteric coating.
The accompanying Table, organized by drug class, indicates whether a drug can be crushed to a powdered form, which usually is mixed with food or liquid for easier intake. The Table also lists liquid and orally disintegrating forms available, and other routes, including injectable immediate and long-acting formulations. Helping patients find a medication formulation that suits their needs strengthens adherence and the therapeutic relationship.



1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
1. Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4): 937-948.
2. PL Detail-Document, Meds That Should Not Be Crushed. Pharmacist’s Letter/Prescriber’sLetter. July 2012.
3. Mitchell JF. Oral dosage forms that should not be crushed. http://www.ismp.org/tools/donotcrush.pdf. Updated January 2015. Accessed January 17, 2017.
What family physicians can do to combat bullying
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9
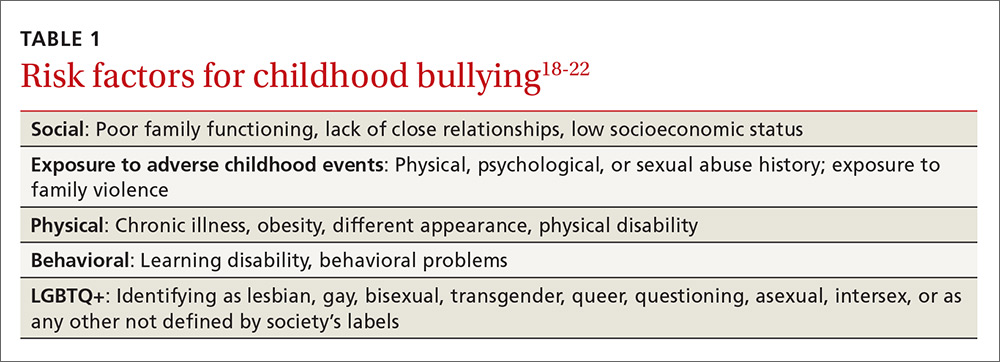
Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20
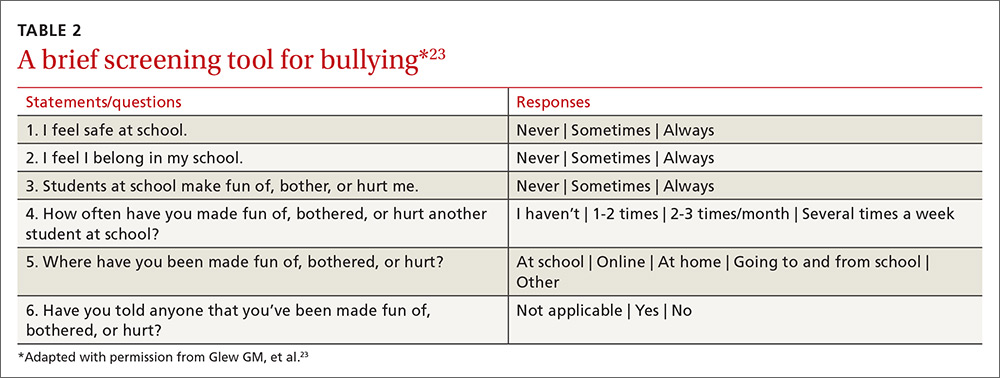
Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.
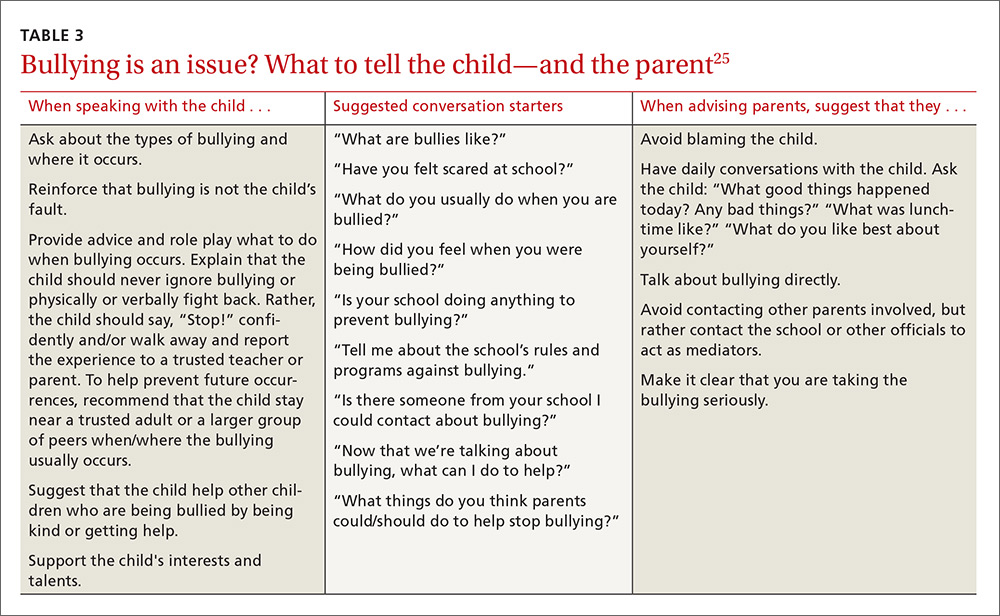
Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
CASE › Stacey, a 12-year-old girl with mild persistent asthma, presents to her family physician (FP) with her mother for her annual well visit. Stacey reports no complaints, but has visited twice recently for acute exacerbations of her asthma, which had previously been well-controlled. When reviewing her social history, Stacey reports that she started her second year of middle school 3 months ago. When asked if she enjoys school, Stacey looks down and says, “School is fine.” Her mother quickly adds that Stacey has quit the school cheerleading team—much to the coach’s dismay—and is having difficulty in her math class, a class in which she normally excels. Stacey appears embarrassed that her mother has brought these things up. Her mother says that at the beginning of the year, 2 girls began picking on Stacey, calling her names and making fun of her on social media and in front of other students.
For many years, bullying was trivialized. Some viewed it as a universal childhood experience; others considered it a rite of passage.1,2 It was not examined as a public health issue until the 1970s. In fact, no legislation addressing bullying or “peer abuse” existed in the United States until the mass shooting at Columbine High School in Littleton, Colo, in 1999. Within 3 years of the Columbine tragedy, the number of state laws that mentioned bullying went from zero to 15; within 10 years of Columbine, 41 states had laws addressing bullying,1 and by 2015, every state, the District of Columbia, and some territories had a bullying law in place.3
As research and advocacy regarding bullying has grown, its impact on the health of children, adolescents, and even adults has become more apparent. In a 2001 study of school-associated violent deaths in the United States between 1994 and 1999, the Centers for Disease Control and Prevention (CDC) found that among students, homicide perpetrators were more than twice as likely as homicide victims to have been bullied by peers.4 Given that homicide is the third leading cause of death in people ages 15 to 24,5 past exposure to bullying may be a significant contributing factor to mortality in this age group.4
In addition to a correlation with homicidal behavior, those involved in bullying—whether as the bully or victim—are at risk for a wide range of symptoms, conditions, and problems including poor psychosocial adjustment, depression, anxiety, suicide (the second leading cause of death in the 10-14 and 15-24 age groups5), academic decline, psychosomatic manifestations, fighting, alcohol use, smoking, and difficulty with the management of chronic diseases.6-10 Not only does being a victim of bullying have a direct impact on a child’s current mental and physical well-being, but it can have lasting psychological and behavioral effects that can follow children well into adulthood.7 The significant impact of bullying on individuals and society as a whole mandates a multifaceted approach that begins in your exam room. What follows is practical advice on screening, counseling, and working with schools and the community at large to curb the bullying epidemic.

Clarifying the problem: The CDC’s definition
Recognizing that varying definitions of bullying were being used in research studies that looked at violent or aggressive behaviors in youth, the CDC published a consensus statement in 2014 that proposed the following definition for bullying:11 any unwanted aggressive behavior by another youth or group of youths who are not siblings or current dating partners that involves an observed or perceived power imbalance and is repeated multiple times or is highly likely to be repeated. This expanded on an earlier definition by Olweus12,13 that also identified a longitudinal nature and power imbalance as key features.
Types of bullying. Direct bullying entails blatant attacks on a targeted young person, while indirect bullying involves communication with others about the targeted individual (eg, spreading harmful rumors). Bullying may be physical, verbal, or relational (eg, excluding someone from their usual social circle, denying friendship, the silent treatment, writing mean letters, eye rolling, etc.) and may involve damage to property. Boys tend toward more direct bullying behaviors, while girls more often engage in indirect bullying, which may be more challenging for both adults and other students to recognize.12,13 With increased use of technology and social media by adolescents, cyberbullying has become increasingly more prevalent, with its effects on adolescent health and academics being every bit as profound as those of traditional bullying.14
About 1 in 4/5 students suffer. The prevalence of bullying ranges by country and culture. The vast majority of early bullying research was conducted in Norway, which found that approximately 15% of students in elementary and secondary schools were involved in bullying in some capacity.12 In a study involving over 200,000 adolescents from 40 European countries, 26% of adolescents reported being involved in bullying, ranging from 8.6% to 45.2% for boys and 4.8% to 35.8% for girls.15 Variations in prevalence may be due to cultural differences in the acts of bullying or differences in interpretation of the term “bullying.”1,15
In the United States, a 2001 survey of more than 15,000 students in public and private schools (grades 6-10) asked the students about their involvement in bullying: 13% said they'd been a bully, 10.6% a victim, and 6.3% said they'd been both.6 There was no significant difference in the frequency of self-reported bullying among urban, suburban, or rural settings.
Despite efforts to educate the public about bullying and work with schools to intervene and prevent bullying, incidence remains largely unchanged. In 2013, the National Crime Victimization Survey reported that approximately 22% of adolescents ages 12 through 18 were victims of bullying.16 Similarly, the CDC's 2015 Youth Risk Behavior Surveillance System reported that 20.2% of high school students experienced bullying on school property.17
Screening: Best practices
The FP’s role begins with screening children at risk for bullying (TABLE 118-22) or those whose complaints suggest that they may be victims of bullying.
Start screening when children enter elementary school
Given that providers’ time is limited for every patient visit, it is important to address bullying at times that are most likely to yield impactful results. The American Academy of Pediatrics recommends that the topic of bullying be introduced at the 6-year-old well-child visit (a typical age for entry to elementary school).7 Views in the literature are inconsistent regarding when and how to address bullying at other time points. One approach is to pre-screen those with risk factors associated with bullying (TABLE 118-22), and to focus screening on those with warning signs of bullying, which include mood disorders, psychosomatic or behavioral symptoms, substance abuse, self-harm behaviors, suicidal ideation or a suicide attempt, a decline in academic performance, and reports of school truancy. Parental concerns, such as when a child suddenly needs more money for lunch, is having aggressive outbursts, or is exhibiting unexplained physical injuries, should also be regarded as cues to screen.9

Screen patients in high-risk groups

A number of groups of children are at high risk for bullying and warrant targeted screening efforts.
- Children with special health needs. Research has shown that children with special health needs are at increased risk for being bullied.18 In fact, the presence of a chronic disease may increase the risk for bullying, and bullying often negatively impacts chronic disease management. As a result, it’s important to have a high index of suspicion with patients who have a chronic disease and who are not responding as expected to medical management or who experience deterioration after being previously well-controlled.18
- Children who are under- or overweight. Similarly, bullying based on a child's weight is a phenomenon that has been recognized to have a significant impact on children’s emotional health.19
- Youth who identify as lesbian, gay, bisexual, transgender, or queer/questioning (LGBTQ+) are more likely than non-LGBTQ+ peers to attempt suicide when exposed to a hostile social environment, such as that created by bullying.20

Screening need not be complicated

One screening approach is simply to ask patients, “Are you being bullied?” followed by such questions as, “How often are you bullied?” or “How long have you been bullied?” Asking about the setting of the bullying (Does it happen at school? Traveling to/from school? Online?) and other details may help guide interventions and the provision of resources.9 Another approach is to provide patients with some type of written survey (see TABLE 223 for an example) to encourage responses that patients might be reluctant to disclose verbally.23,24 (See “Barriers to screening.")
SIDEBAR
Barriers to screeningScreening for any condition presupposes a response. Ideally, family physicians should be prepared to provide basic counseling, resources, and, if necessary, treatment, if a patient screens positive for bullying. But screening for violence or bullying can be difficult, and evidence-based guidelines for screening and intervention are lacking, leaving many primary care practitioners feeling ill-equipped to meaningfully respond.
One study of the use of a screening tool aimed at intimate partner violence (IPV) showed that even with the availability of a screening tool, health care providers’ use of the tool was inconsistent and referral practices were ineffective.1 Providers cited the following limiting factors in screening for IPV: 1) a lack of immediate referral availability, 2) a lack of time during the office visit, and 3) a lack of confidence in the ability to screen.1 These same issues may be barriers to screening for bullying.
1. Ramachandran DV, Covarrubias L, Watson C, et al. How you screen is as important as whether you screen: a qualitative analysis of violence screening practices in reproductive health clinics. J Community Health. 2013;38:856-863.
Provider and parental interventions
Interventions often entail counseling the patient and the family about bullying and its effects, empowering victims and their caregivers, and screening for bullying comorbidities and correlates.2 Refer patients to behavioral health specialists when there is evidence of pervasive effects on mood, behavior, or social development, but keep in mind that counseling can begin in your own exam room.
Effective discussion starters. Affirming the problem and its unacceptability, talking about the different types of bullying and where bullying may occur, and asking about patient perceptions of bullying can be effective discussion starters. FPs should help patients identify bullying, open lines of communication between children and their parents and between parents and other caregivers, and demonstrate respect and kindness in their approach to discussing the topic. Encourage children to speak with trusted adults when exposed to bullying. Talk to them about standing up to bullies (saying “stop” confidently or walking away from difficult situations) and staying safe by staying near adults or groups of peers when bullies are present (TABLE 325).
Empowering caregivers. Encourage parents to spend time each day talking with their child about the child’s time away from home (TABLE 325). Counsel parents/caregivers to expand their role. Knowing a child’s friends, encouraging the child academically, and increasing communication are all associated with lower risks of bullying.26 Similarly, parental oversight of Internet and social media use is associated with decreased participation in cyberbullying.27
In addition, the Positive Parenting telephone-based parenting education curriculum has been shown to decrease bullying, physical fighting, physical injuries, and victimization of children.28 The research-based, family strengthening program emphasizes 3 core elements of authoritative parenting: nurturance, discipline, and respect or granting of psychologic autonomy. The program entails 15- to 30-minute weekly phone conversations between parents and educators, as well as videos and a manual.

Are community programs in place—or are they needed?
Many schools have robust, state-mandated programs in place to identify bullying and provide support for students who are victims of bullying. (See “NJ’s harassment and bullying protocol: A case in point.”) Explaining this to victims and their families may help them come forward and seek assistance. FPs who want to advocate for their patients should start with local schools to support such programs and link students at risk with school counselors.
SIDEBAR
NJ's harassment and bullying protocol: A case in pointThere is no federal law that specifically applies to bullying, but all 50 states have some type of anti-bullying legislation on the books, and 40 of those states have additional detailed policies in place addressing the subject.1
New Jersey, for example, began enforcing one of the toughest harassment, intimidation, and bullying (HIB) protocols in the country back in September 20112 in the wake of the death of Rutgers University freshman Tyler Clementi, who committed suicide after his roommate allegedly shot a video of him with another man and posted it to the Internet.3 Among many other things, New Jersey’s legislation stipulates in its Anti-Bullying Bill of Rights4 that:
› Every school/district have plans in place that clearly define, prevent, prohibit, and promptly deal with acts of harassment, intimidation, or bullying, on school grounds, at school-sponsored functions, and on school buses.
› Plans must include a description of the type of behavior expected from each student and the consequences and remedial action for a person who commits an act of harassment, intimidation, or bullying. Student perpetrators may be suspended or expelled if convicted of any type of bullying, whether it be for teasing or something more severe.
› All school employees must act on any incidents of bullying reported to, or witnessed by, them and report such incidents on the same day to the school principal.
› Plans must include provisions and deadlines for investigating and resolving all matters in a timely fashion; investigations into allegations of bullying must be launched within one day.
› Every case of bullying must be reported to the state. Schools are graded by the state on their compliance with anti-bullying standards and policies and their handling of incidents.
› Schools must appoint safety teams made up of parents, teachers, and staff, and school personnel and students must receive extensive anti-bullying training.
1. US Department of Health and Human Services. Stopbullying.gov. Policies and laws. Available at: https://www.stopbullying.gov/laws/index.html.
Accessed January 5, 2017.
2. State of New Jersey Department of Education. An overview of amendments to laws on harassment, intimidation, and bullying. Available at: http://www.state.nj.us/education/students/safety/behavior/hib/overview.pdf. Accessed January 5, 2017.
3. Cohen A. Case study: Why New Jersey’s antibullying law should be a model for other states. Time. September 6, 2011. Available at: http://ideas.time.com/2011/09/06/why-new-jerseys-antibullying-law-should-be-a-model-for-other-states/. Accessed January 5, 2017.
4. New Jersey Legislature. Anti-bullying Bill of Rights Act. Available at: http://www.njleg.state.nj.us/2010/Bills/PL10/122.PDF. Accessed January 5, 2017.
If programs are lacking in your community, there is much you can do to educate yourself about successful programs and advise local community organizations and schools about them. Among the most successful and well-studied interventions for thwarting the bullying epidemic have been school-based community ones. The most studied of these is the Olweus Bullying Prevention Program (OBPP), which is based on 4 principles:1,29
- Adults both at home and at school should take a positive and encouraging interest in students.
- Unacceptable behavior should have strict and well-known limits.
- Sanctions should be applied consistently and should be non-hostile in nature.
- Adults both at home and in the educational environment should act as authorities.
In short, the program focuses on greater awareness and involvement on the part of adults, and employing measures at the school level (eg, surveys, better supervision during break and lunch times), the class level (eg, rules against bullying, regular class meetings with students), and the individual level (eg, serious talks with bullies, victims, parents of involved students).
Research has shown that the OBPP reduces bullying behaviors by as much as 50%, reduces vandalism and truancy, and reduces the number of new victims.12 Limits to the more widespread implementation of the OBPP have consisted mainly of the inability to appropriately train adults, including teachers and other school personnel in educational settings. Despite these limitations, the OBPP has been praised and endorsed by numerous groups, including the US Department of Justice.30
Other non-curricular, school-based programs exist, such as the School-Wide Positive Behavioral Interventions and Supports (SWPBIS). This program is a school-wide prevention strategy aimed at: 1) reducing behavior problems that lead to office discipline referrals and suspensions, and 2) changing perceptions of school safety. (For more information, see https://www.crimesolutions.gov/ProgramDetails.aspx?ID=385 and https://www.pbis.org/school/swpbis-for-beginners.)31
The research-based Second Step: Student Success Through Prevention (SS-SSTP) Middle School Program (http://www.cfchildren.org/second-step/middle-school)32 focuses on the often difficult middle school years. The program helps schools teach and model essential communication, coping, and decision-making skills to help adolescents navigate around common pitfalls such as peer pressure, substance abuse, and bullying (both in-person and online). The program aims to reduce aggression and provide support for a more inclusive environment that helps students stay in school, make good choices, and experience social and academic success.
The Positive Action Program (https://www.positiveaction.net/research/primer),33 which is predicated on the notion that we feel good about ourselves when we do positive things, features scripted lessons and kits of materials (eg, posters, games, worksheets, puzzles) appropriate for each grade level.
CASE › Stacey’s visit to her FP’s office has presented several clues that she may be a victim of bullying. Her mild persistent asthma appears to no longer be as well controlled as it was in the past. Direct questioning has revealed that 2 girls at school have been making fun of Stacey when she uses her inhaled corticosteroid in the morning before class, so she has stopped using it. These same students are on her cheerleading team, so she quit the team to avoid them. Her school-related anxiety is so great that she no longer pays attention in math class and is constantly worried that something is being posted about her online.
Stacy’s FP responds to this information with a multifaceted approach. In the exam room, he screens Stacy for depression. While she is negative and denies any suicidal ideation, Stacy is clearly having anxiety, so the FP refers Stacey to a counselor at a local mental health clinic. With Stacy’s permission, the FP discusses the issue with her mother and they decide together with Stacy that she should talk to a teacher at school about the ongoing bullying. Because this was not the first time that the FP has heard this from a child in the community, the FP plans to attend an upcoming school board meeting to advocate for an evidence-based bullying prevention program to help curb the ongoing problem facing his patients.
CORRESPONDENCE
Robert McClowry, MD, Department of Family and Community Medicine, Jefferson Family Medicine Associates, 833 Chestnut East, 3rd Floor, Suite 301, Philadelphia, PA, 19107-4414; [email protected].
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
1. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80:124-134.
2. Lyznicki JM, McCaffree MA, Robinowitz CB, et al. Childhood bullying: implications for physicians. Am Fam Physician. 2004;70:1723-1730.
3. Temkin D. All 50 states now have a bullying law. Now what? The Huffington Post. April 27, 2015. Available at: http://www.huffingtonpost.com/deborah-temkin/all-50-states-now-have-a_b_7153114.html. Accessed January 5, 2017.
4. Anderson M, Kaufman J, Simon TR, et al. School-associated violent deaths in the United States, 1994-1999. JAMA. 2001;286:2695-2702.
5. Centers for Disease Control and Prevention. Injury prevention and control: Data and statistics (WISQARS). Ten leading causes of death and injury. Available at: https://www.cdc.gov/injury/wisqars/leadingcauses.html. Accessed January 5, 2017.
6. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094-2100.
7. Committee on Injury, Violence, and Poison Prevention. Policy statement—Role of the pediatrician in youth violence prevention. Pediatrics. 2009;124:393-402.
8. Klein DA, Myhre KK, Ahrendt DM. Bullying among adolescents: a challenge in primary care. Am Fam Physician. 2013;88:87-92.
9. Lamb J, Pepler DJ, Craig W. Approach to bullying and victimization. Can Fam Physician. 2009;55:356-360.
10. Spector ND, Kelly SF. Pediatrician’s role in screening and treatment: bullying, prediabetes, oral health. Curr Opin Pediatr. 2006;18:661-670.
11. Gladden RM, Vivolo-Kantor AM, Hamburger ME, et al. Bullying surveillance among youths: uniform definitions for public health and recommended data elements. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention and US Department of Education; 2014. Available at: https://www.cdc.gov/violenceprevention/pdf/bullying-definitions-final-a.pdf. Accessed June 8, 2016.
12. Olweus D. Bullying at school: basic facts and effects of a school based intervention program. J Child Psychol Psychiatry. 1994;35:1171-1190.
13. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
14. Kowalski RM, Limber SP. Psychological, physical, and academic correlates of cyberbullying and traditional bullying. J Adolesc Health. 2013;53:S13-S20.
15. Craig W, Harel-Fisch Y, Fogel-Grinvald H, et al. A cross-national profile of bullying and victimization among adolescents in 40 countries. Int J Public Health. 2009;54(Suppl 2):216-224.
16. US Department of Education, National Center for Educational Statistics (2015). Student reports of bullying and cyberbullying: results from the 2013 School Crime Supplement to the National Victimization Survey. Available at: http://nces.ed.gov/pubsearch/pubsinfo.asp?pubid=2015056. Accessed November 9, 2016.
17. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1-174.
18. Van Cleave J, Davis MM. Bullying and peer victimization among children with special health care needs. Pediatrics. 2006;118:e1212-e1219.
19. Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733-738.
20. Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896-903.
21. Song LY, Singer MI, Anglin TM. Violence exposure and emotional trauma as contributors to adolescents’ violent behaviors. Arch Pediatr Adolesc Med. 1998;152:531-536.
22. Singer MI, Anglin TM, Song LY, et al. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477-482.
23. Glew GM, Fan MY, Katon W, et al. Bullying, psychosocial adjustment, and academic performance in elementary school. Arch Pediatr Adolesc Med. 2005;159:1026-1031.
24. Waseem M, Ryan M, Foster CB, et al. Assessment and management of bullied children in the emergency department. Pediatr Emerg Care. 2013;29:389-398.
25. US Department of Health and Human Services. stopbullying.gov. Available at: https//www.stopbullying.gov. Accessed January 5, 2017.
26. Shetgiri R, Lin H, Avila RM, et al. Parental characteristics associated with bullying perpetration in US children aged 10 to 17 years. Am J Public Health. 2012;102:2280-2286.
27. Hinduja S, Patchin JW. Social influences on cyberbullying behaviors among middle and high school students. J Youth Adolesc. 2013;42:711-722.
28. Borowsky IW, Mozayeny S, Stuenkel K, et al. Effects of a primary care-based intervention on violent behavior and injury in children. Pediatrics. 2004;114:e392-e399.
29. Olweus D. Bully/victim problems in school: Facts and intervention. Eur J Psychol Educ. 1997;12:495-510.
30. Mihalic SF. Blueprints for Violence Prevention: Report. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2004.
31. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med. 2012;166:149-156.
32. Espelage DL, Low S, Polanin JR, et al. The impact of a middle school program to reduce aggression, victimization, and sexual violence. J Adolesc Health. 2013;53:180-186.
33. Lewis KM, Schure MB, Bavarian N, et al. Problem behavior and urban, low-income youth: a randomized controlled trial of positive action in Chicago. Am J Prev Med. 2013;44:622-630.
PRACTICE RECOMMENDATIONS
› Suspect bullying when children with chronic conditions that were stable begin deteriorating for unexplained reasons or when children become non-adherent to medication regimens. C
› Empower not only patients, but also parents/caregivers, to take action and deter bullying behaviors. B
› Support school-based and community-oriented intervention programs, which have been shown to be among the most effective strategies for curbing bullying. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bipolar Disorder: Recognizing and Treating in Primary Care
CE/CME No: CR-1702
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define bipolar disorder according to the DSM-5 criteria.
• Recognize how patients with bipolar disorder can present to their primary care provider.
• Discuss how to perform a clinical and psychiatric evaluation on patients with suspected bipolar disorder.
• Describe the therapeutic options for a patient with bipolar disease in a primary care setting.
FACULTY
Jean Covino is a clinical professor at Pace University-Lenox Hill Hospital in New York City, and she practices at the Medemerge Family Practice Center in Green Brook, New Jersey. Jennifer Hofmann is an Associate Clinical Professor at Pace University-Lenox Hill Hospital in New York City.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2017.
Article begins on next page >>
Primary care clinicians are often the first point of contact for persons with bipolar disorder. Unfortunately, delays in diagnosis are common, as many of these patients are misdiagnosed with unipolar depression on initial presentation. Since an early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes, clinicians should be able to recognize its symptoms and initiate treatment of this deceptive disorder.
Bipolar disorder is a chronic mental illness characterized by fluctuations in mood and energy that manifests as recurrent episodes of manic or depressive symptoms. It is estimated that between 10% and 38% of patients with bipolar disorder receive all their mental health care in a primary care setting.1 Although patients with bipolar disorder often initially present to their primary care provider, they frequently go undiagnosed because of the complexity of the disorder’s symptomatology and a low index of suspicion among primary care providers.2 Comorbid medical conditions and psychiatric issues can also lead to misdiagnoses.
Because primary care providers are often the first point of contact for patients with bipolar disorder, they are well positioned to recognize bipolar symptoms early in the course of the illness. The pure subtypes of bipolar disorder include bipolar I and bipolar II. Clinicians who work in a primary care or emergency department setting should be able to recognize and initiate treatment for these two subtypes while the patient is waiting for a psychiatric evaluation. Accurate early diagnosis of this disabling disorder can reduce morbidity and improve outcomes by allowing for appropriate referral, pharmacotherapy, and psychotherapy.
DEFINITION
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5), bipolar disorder is a mood disorder defined by episodes of mania, hypomania, and major depression.3 Patients with bipolar I disorder experience manic episodes and almost always experience major depressive and hypomanic episodes. Bipolar II disorder is marked by at least one hypomanic episode, at least one major depressive episode (MDE), and the absence of manic episodes.3 A manic episode is at least one week of abnormally and continually elevated, expansive, or irritable mood and increased activity or energy accompanied by at least three of the following symptoms (or four if mood is only irritable): inflated self-esteem, decreased need for sleep, increased talkativeness, flight of ideas or racing thoughts, marked distractibility, increased goal-directed activity or agitation, and excessive involvement in dangerous or high-risk activities (eg, reckless spending or increased sexuality). To be considered a manic episode, the mood disturbance must cause marked impairment in social or occupational functioning, result in hospitalization, or involve psychotic features, and the symptoms cannot be attributable to the effects of drugs or medications or another medical condition.3
In a hypomanic episode, the period of elevated or irritable mood lasts for a shorter duration (at least four days); is associated with a clear, uncharacteristic change in functioning; and is observable by others but does not cause marked impairment, need for hospitalization, or psychosis. MDE is defined by the presence of at least five of nine symptoms for a minimum duration of two weeks and a change from previous functioning: depressed mood, markedly decreased interest or pleasure in activities, significant change in weight or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or excessive guilt, decreased ability to think or concentrate or indecisiveness, and recurrent thoughts of death or suicidality (at least one symptom must be depressed mood or loss of interest or pleasure).3
EPIDEMIOLOGY
Bipolar disorder affects men and women equally. It can occur at any age but is seen most commonly in persons younger than 25.4 The mean age at the first manic/hypomanic or major depressive episode was determined to be 18.2 in bipolar I and 20.3 in bipolar II.5 The lifetime prevalence of bipolar disorder in the United States is around 4%, with one study finding prevalence estimates of 1.0% for bipolar I disorder and 1.1% for bipolar II disorder.5
Bipolar disorder is common among primary care patients with depression. Two studies that explored the risk for bipolar disorder among depressed outpatients in primary care settings found that between 20% and 30% of these patients screened positive for bipolar disorder on the Mood Disorders Questionnaire (MDQ), indicating that a more thorough evaluation for bipolar disorder was needed.6,7 A systematic review of the literature found similar rates of positive results on the MDQ screening measure among primary care patients with depression, a trauma exposure, medically unexplained symptoms, or a psychiatric complaint; bipolar disorder was diagnosed with a structured clinical interview in 3% to 9% of these patients.8 Children of parents with bipolar disorder have a 4% to 15% risk for also being affected.4
CLINICAL PRESENTATION IN PRIMARY CARE
Bipolar patients are often depressed or euthymic for a majority of their lives but can also present in a manic or hypomanic state. In primary care settings, these patients often present with depression (including postpartum depression), which can obscure the diagnosis. Misdiagnosis of bipolar disorder as recurrent unipolar depression occurs in 60% of patients seeking treatment for depression.9
Patients with bipolar disorder who present to primary care usually demonstrate a wide range of mood symptomatology other than depression, including mood swings, anxiety, fatigue, sleep disturbances, and the inability to focus or concentrate. Patients can also present in mixed states. These are characterized by elements of irritability, increased energy, and sleeplessness with depressive features.
Several clues that can assist in detecting bipolar disorder relate to age at onset, family history, mood shifts, seasonality, and atypical depressive symptoms (eg, sleep dysregulation and appetite changes). Although the diagnosis of bipolar disorder is commonly delayed by many years, patients often report significant mood symptoms in their early 20s. In a study that used a self-administered questionnaire to assess the experience of persons living with bipolar disorder, 33% of the respondents were younger than 15 when their symptoms first started, 27% were between 15 and 19, and 39% were 20 or older.9 Parental and family history of bipolar disorder increases risk for the disorder in offspring, so a thorough family history is essential when the disorder is suspected.
Aside from the classic presentation defined by the DSM-5 criteria, patients with bipolar disorder can also exhibit other effects of their illness, such as alcohol-related problems and sexually transmitted or drug-related infections. In patients with bipolar disorder, rates of alcohol use range from 21.4% in adults to 54.5% in adolescents and young adults.10 Social history may reveal relationship and marital issues, financial problems, difficulties keeping a job, and legal problems.9,11 Suicide attempts and completed suicides are significantly more common among persons with bipolar disorder than among the general population.12,13
Comorbidity with at least one other disorder is common in bipolar disorder.5 The most common comorbid personality disorder associated with bipolar disorder is borderline personality disorder, which is characterized by ongoing instability in moods and behavior. Persons with this disorder can experience intense episodes of anger, depression, and anxiety that may last from hours to days. The high prevalence of persistent symptoms despite treatment in bipolar disorder and the unstable and partly remitting course of borderline personality make it difficult to distinguish between the two disorders.14 The frequent mood changes that occur with borderline personality disorder may appear to overlap with the mood swings characterizing bipolar disorder, but the mood episodes in borderline personality disorder are of shorter duration than those in bipolar disorder. Other common comorbid disorders seen in patients with bipolar disorder include substance abuse disorders, anxiety disorders (especially panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder), and attention-deficit/hyperactivity disorder.5
Primary care providers should be aware of other common comorbidities that may be present in patients with bipolar disorder. These patients commonly experience medical problems such as diabetes, obesity, and metabolic syndrome, which all lead to increased cardiovascular risk.15-17
CLINICAL EVALUATION
The initial clinical evaluation of the patient should include a thorough medical, social, family, and psychiatric history. Medical conditions that may mimic bipolar disorder include neurologic conditions (eg, partial seizures, neoplasm, strokes, dementia, delirium) and endocrine disorders (eg, Cushing disease, hyperthyroidism/hypothyroidism), as well as vitamin deficiencies (B12, folate, niacin, thiamine) and drug and substance use/misuse (alcohol, drugs including antidepressants and stimulants).4 All patients should have a baseline complete physical examination, including neurologic and mental status examinations. Diagnostic tests to assess for potential differential diagnoses and evaluate baseline levels include the following:
- Basic metabolic panel, including fasting glucose, to evaluate electrolytes and risk for diabetes or Cushing disease, and to assess baseline renal function
- Thyroid function tests
- Complete blood count to assess status prior to anticonvulsant treatment (eg, carbamazepine)
- Pregnancy test if applicable (prior to use of medications)
- Liver function tests to assess baseline measurements prior to use of medications
- Electrocardiography in patients older than 40 to establish baseline and assess QTc interval, especially with use of antipsychotics and carbamazepine
- Urine toxicology screen (to rule out substance abuse).
PSYCHIATRIC EVALUATION
Psychiatric evaluation should focus on age at onset of symptoms, the presence of hypomanic or manic symptoms, prior response to antidepressants, course of the disease including history and duration of depression or manic/hypomanic episodes, and sleep disturbances (increased during depressive episodes and significantly decreased during manic episodes). It is important to assess for a history of self-harm, suicidal ideation, suicide attempts, hospitalizations, legal issues, multiple career shifts, marriage and relationship issues, and smoking and alcohol/substance misuse. Patients with severe manic or depressive episodes may experience psychotic features such as grandiose or paranoid delusions and hallucinations. A history of symptoms from close family members or friends can assist in the diagnosis of bipolar patients.
The use of DSM-5 criteria, as summarized earlier, improves the accuracy of bipolar diagnosis.3 In addition, validated tools are available to help clinicians screen for bipolar disorder, although it is important to remember that a positive screening result is not sufficient to establish a bipolar disorder diagnosis. A widely used instrument that has been validated for screening for bipolar disorder is the MDQ (available at www.dbsalliance.org/pdfs/MDQ.pdf). This self-report questionnaire consists of 15 questions that assess hypomanic or manic symptoms and functional impairment. The first 13 questions of the MDQ screen for a lifetime history of DSM-based hypomanic or manic symptoms. The last two questions ask whether these symptoms occurred at the same time and whether they caused dysfunction in various domains, such as work and family life. The MDQ is considered positive if a patient endorses at least seven of the symptom items, indicates that symptoms have occurred at the same time, and rates their dysfunction in life domains as “moderate” or “serious.” As a screening tool, the MDQ has a reported sensitivity of 73% and a specificity of 90% for bipolar disorder.11 This questionnaire can and should be used by primary care providers to help determine if their patient is at risk and requires a comprehensive evaluation for bipolar disorder.
Notably, even after a clinician has properly diagnosed bipolar disorder, patients and family members are often reluctant to commence treatment due to the stigma associated with mental health disorders.18 To help offset the effects of stigma, patients should be referred for psychologic counseling, including family counseling.
MANAGEMENT
Management of bipolar disorder in the primary care setting includes psychiatric and psychologic counseling referrals. Primary care providers must know the medications used to treat bipolar disorder and their related adverse effects, toxicities, warnings, and drug interactions, as they may treat bipolar patients for other medical conditions. Early diagnosis and treatment/referral can improve prognosis and reduce the risk for relapse and subsequent disability.19 Inpatient management is generally recommended for severe manic episodes, psychotic episodes, patients who present a danger to themselves or others, and patients with suicidal or homicidal ideations/actions.
Medications are the primary treatment for all stages of bipolar disorder, and choice of medications is based on stage, previous response, and adverse effect profiles (see Table 1).2,4,20 Generally, antidepressants (serotonin and norepinephrine reuptake inhibitors [SNRIs] and selective serotonin reuptake inhibitors [SSRIs]) should be avoided or should be used with an effective antimanic/mood stabilizer. Many patients with severe bipolar symptoms require more than two medications, and it is imperative that all primary care providers understand that often one drug alone is not sufficient treatment for patients with bipolar disorder. For less severe manic or hypomanic states, monotherapy with antipsychotics may be effective.

Medications for severe acute manic episodes generally include the mood stabilizers lithium, valproate, or carbamazepine in conjunction with an antipsychotic, such as haloperidol, or an atypical antipsychotic, such as asenapine, aripiprazole, olanzapine, quetiapine, or risperidone.2 The goal of initial therapy in patients with acute mania is rapid resolution of symptoms and restoration of adequate sleep. Lithium has a slower onset of action than valproate and carbamazepine and requires titration and monitoring. Valproate and carbamazepine have a faster onset of action but are less effective than lithium.2 Atypical antipsychotics have a more rapid onset of action than mood stabilizers and are effective in controlling acute manic symptoms, psychosis, and sleep disturbances. Patients with severe acute mania may require hospital admission for stabilization, for their safety and the safety of others.
Acute bipolar depressive episodes can be treated with several different medication options, including combination olanzapine and fluoxetine; the atypical antipsychotic quetiapine; and recently lurasidone, alone or in combination with lithium or valproate. Lamotrigine is more effective for maintenance and prevention of depressive episodes than for treatment of acute episodes, and it is also indicated for treatment of bipolar II. Valproate is more effective than lithium for mixed states and can be titrated more rapidly for faster antimanic effects.4
Generally, due to the high rate of recurrence, maintenance medications should be continued indefinitely. Maintenance medications include the mood stabilizers, lamotrigine, and many of the antipsychotics, including olanzapine.4 Adherence to medications is essential in management of bipolar disorder and can decrease the risk for relapses and destabilization. Poor adherence to medications is common, however, with rates reported at approximately 50%.21 Patient and family education, as well as psychotherapy, can improve adherence rates.2 Primary care providers should educate patients and family members about medication options and adverse effects and must stress the need for adherence to prevent relapse. Providers should also understand the safety profile of mood stabilizers and antipsychotics and the required monitoring of laboratory tests for patients on these medications.
Psychosocial treatments are an elemental component of management. Patients should be referred early for psychologic treatments including, but not limited to, family therapy, group therapy, cognitive-behavioral therapy, and psychotherapy, which have been shown to improve daily functioning, recognition of recurrences, and medication adherence.2 The rate of relapse is significantly lower in patients receiving combination psychotherapy and pharmacotherapy.22
Clinical pearls that every primary care provider should know about bipolar disorder are summarized in Table 2.

CONCLUSION
Given the substantial impact of bipolar disorder on patients and the community, primary care clinicians must maintain a high index of suspicion for this disorder. An early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes. However, diagnosing and treating patients with bipolar disorder is challenging for primary care and specialty clinicians alike. In particular, establishing a diagnosis can be difficult, even for the most seasoned clinician, due to the diversity of symptoms. Nonetheless, diagnosing bipolar disorder, initiating treatment, and monitoring and referring patients when necessary are certainly within the purview of the primary care provider.
1. Kilbourne AM, Goodrich DE, O’Donnell AN, Miller CJ. Integrating bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
2. Culpepper L. The diagnosis and treatment of bipolar disorder: decision making in primary care. Prim Care Companion CNS Disord. 2014;16(3): doi 10.4088/PCC.13r01609.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
4. Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85:483-493.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Calabrese JR, Muzina DJ, Kemp DE, et al. Predictors of bipolar disorder risk among patients currently treated for major depression. MedGenMed. 2006;8(3):38.
7. Hirschfeld RM, Cass AR, Holt DC, Carlson CA. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
8. Cerimele JM, Chwastiak LA, Dodson S, Katon WJ. The prevalence of bipolar disorder in primary care patients with depression or other psychiatric complaints: a systematic review. Psychosomatics. 2013;54(6):515-524.
9. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003; 64:161-174.
10. Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol. 2005;15(4):425-434.
11. Piver A, Yatham LN, Lam RW. Bipolar spectrum disorders: new perspectives. Can Fam Physician. 2002;48:896-904.
12. Eroglu MZ, Karakus G, Tamam L. Bipolar disorder and suicide. J Psychiatry Neurol Sci. 2013;26:139-147.
13. Simon GE, Hunkeler E, Fireman B, Lee JY, Savarino J. Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007;9:526-530.
14. Marcinko D, Vuksan-Cusa B. Borderline personality disorder and bipolar disorder comorbidity in suicidal patients: diagnostic and therapeutic challenges. Psychiatr Danub. 2009;21:386-390.
15. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52:230-236.
16. Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013; 170:265-274.
17. Fiedorowicz JG, Solomon DA, Endicott J, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71(6):598-606.
18. Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: A review of the literature. J Affect Disord. 2013; 150:181-191.
19. Berk M, Brnabic A, Dodd S, et al. Does stage of illness impact treatment response in bipolar disorder? Empirical treatment data and their implication for the staging model and early intervention. Bipolar Disord. 2011;13(1):87-98.
20. Tegretol (carbamazepine) [package insert]. East Hanover, NJ: Novartis; 2015. www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf. Accessed November 18, 2016.
21. Arvilommi P, Suominen K, Mantere O, et al. Predictors of adherence to psychopharmacological and psychosocial treatment in bipolar I or II disorders—an 18-month prospective study. J Affect Disord. 2014;155:110-117.
22. Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord. 2013;149:247-252.
CE/CME No: CR-1702
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define bipolar disorder according to the DSM-5 criteria.
• Recognize how patients with bipolar disorder can present to their primary care provider.
• Discuss how to perform a clinical and psychiatric evaluation on patients with suspected bipolar disorder.
• Describe the therapeutic options for a patient with bipolar disease in a primary care setting.
FACULTY
Jean Covino is a clinical professor at Pace University-Lenox Hill Hospital in New York City, and she practices at the Medemerge Family Practice Center in Green Brook, New Jersey. Jennifer Hofmann is an Associate Clinical Professor at Pace University-Lenox Hill Hospital in New York City.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2017.
Article begins on next page >>
Primary care clinicians are often the first point of contact for persons with bipolar disorder. Unfortunately, delays in diagnosis are common, as many of these patients are misdiagnosed with unipolar depression on initial presentation. Since an early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes, clinicians should be able to recognize its symptoms and initiate treatment of this deceptive disorder.
Bipolar disorder is a chronic mental illness characterized by fluctuations in mood and energy that manifests as recurrent episodes of manic or depressive symptoms. It is estimated that between 10% and 38% of patients with bipolar disorder receive all their mental health care in a primary care setting.1 Although patients with bipolar disorder often initially present to their primary care provider, they frequently go undiagnosed because of the complexity of the disorder’s symptomatology and a low index of suspicion among primary care providers.2 Comorbid medical conditions and psychiatric issues can also lead to misdiagnoses.
Because primary care providers are often the first point of contact for patients with bipolar disorder, they are well positioned to recognize bipolar symptoms early in the course of the illness. The pure subtypes of bipolar disorder include bipolar I and bipolar II. Clinicians who work in a primary care or emergency department setting should be able to recognize and initiate treatment for these two subtypes while the patient is waiting for a psychiatric evaluation. Accurate early diagnosis of this disabling disorder can reduce morbidity and improve outcomes by allowing for appropriate referral, pharmacotherapy, and psychotherapy.
DEFINITION
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5), bipolar disorder is a mood disorder defined by episodes of mania, hypomania, and major depression.3 Patients with bipolar I disorder experience manic episodes and almost always experience major depressive and hypomanic episodes. Bipolar II disorder is marked by at least one hypomanic episode, at least one major depressive episode (MDE), and the absence of manic episodes.3 A manic episode is at least one week of abnormally and continually elevated, expansive, or irritable mood and increased activity or energy accompanied by at least three of the following symptoms (or four if mood is only irritable): inflated self-esteem, decreased need for sleep, increased talkativeness, flight of ideas or racing thoughts, marked distractibility, increased goal-directed activity or agitation, and excessive involvement in dangerous or high-risk activities (eg, reckless spending or increased sexuality). To be considered a manic episode, the mood disturbance must cause marked impairment in social or occupational functioning, result in hospitalization, or involve psychotic features, and the symptoms cannot be attributable to the effects of drugs or medications or another medical condition.3
In a hypomanic episode, the period of elevated or irritable mood lasts for a shorter duration (at least four days); is associated with a clear, uncharacteristic change in functioning; and is observable by others but does not cause marked impairment, need for hospitalization, or psychosis. MDE is defined by the presence of at least five of nine symptoms for a minimum duration of two weeks and a change from previous functioning: depressed mood, markedly decreased interest or pleasure in activities, significant change in weight or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or excessive guilt, decreased ability to think or concentrate or indecisiveness, and recurrent thoughts of death or suicidality (at least one symptom must be depressed mood or loss of interest or pleasure).3
EPIDEMIOLOGY
Bipolar disorder affects men and women equally. It can occur at any age but is seen most commonly in persons younger than 25.4 The mean age at the first manic/hypomanic or major depressive episode was determined to be 18.2 in bipolar I and 20.3 in bipolar II.5 The lifetime prevalence of bipolar disorder in the United States is around 4%, with one study finding prevalence estimates of 1.0% for bipolar I disorder and 1.1% for bipolar II disorder.5
Bipolar disorder is common among primary care patients with depression. Two studies that explored the risk for bipolar disorder among depressed outpatients in primary care settings found that between 20% and 30% of these patients screened positive for bipolar disorder on the Mood Disorders Questionnaire (MDQ), indicating that a more thorough evaluation for bipolar disorder was needed.6,7 A systematic review of the literature found similar rates of positive results on the MDQ screening measure among primary care patients with depression, a trauma exposure, medically unexplained symptoms, or a psychiatric complaint; bipolar disorder was diagnosed with a structured clinical interview in 3% to 9% of these patients.8 Children of parents with bipolar disorder have a 4% to 15% risk for also being affected.4
CLINICAL PRESENTATION IN PRIMARY CARE
Bipolar patients are often depressed or euthymic for a majority of their lives but can also present in a manic or hypomanic state. In primary care settings, these patients often present with depression (including postpartum depression), which can obscure the diagnosis. Misdiagnosis of bipolar disorder as recurrent unipolar depression occurs in 60% of patients seeking treatment for depression.9
Patients with bipolar disorder who present to primary care usually demonstrate a wide range of mood symptomatology other than depression, including mood swings, anxiety, fatigue, sleep disturbances, and the inability to focus or concentrate. Patients can also present in mixed states. These are characterized by elements of irritability, increased energy, and sleeplessness with depressive features.
Several clues that can assist in detecting bipolar disorder relate to age at onset, family history, mood shifts, seasonality, and atypical depressive symptoms (eg, sleep dysregulation and appetite changes). Although the diagnosis of bipolar disorder is commonly delayed by many years, patients often report significant mood symptoms in their early 20s. In a study that used a self-administered questionnaire to assess the experience of persons living with bipolar disorder, 33% of the respondents were younger than 15 when their symptoms first started, 27% were between 15 and 19, and 39% were 20 or older.9 Parental and family history of bipolar disorder increases risk for the disorder in offspring, so a thorough family history is essential when the disorder is suspected.
Aside from the classic presentation defined by the DSM-5 criteria, patients with bipolar disorder can also exhibit other effects of their illness, such as alcohol-related problems and sexually transmitted or drug-related infections. In patients with bipolar disorder, rates of alcohol use range from 21.4% in adults to 54.5% in adolescents and young adults.10 Social history may reveal relationship and marital issues, financial problems, difficulties keeping a job, and legal problems.9,11 Suicide attempts and completed suicides are significantly more common among persons with bipolar disorder than among the general population.12,13
Comorbidity with at least one other disorder is common in bipolar disorder.5 The most common comorbid personality disorder associated with bipolar disorder is borderline personality disorder, which is characterized by ongoing instability in moods and behavior. Persons with this disorder can experience intense episodes of anger, depression, and anxiety that may last from hours to days. The high prevalence of persistent symptoms despite treatment in bipolar disorder and the unstable and partly remitting course of borderline personality make it difficult to distinguish between the two disorders.14 The frequent mood changes that occur with borderline personality disorder may appear to overlap with the mood swings characterizing bipolar disorder, but the mood episodes in borderline personality disorder are of shorter duration than those in bipolar disorder. Other common comorbid disorders seen in patients with bipolar disorder include substance abuse disorders, anxiety disorders (especially panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder), and attention-deficit/hyperactivity disorder.5
Primary care providers should be aware of other common comorbidities that may be present in patients with bipolar disorder. These patients commonly experience medical problems such as diabetes, obesity, and metabolic syndrome, which all lead to increased cardiovascular risk.15-17
CLINICAL EVALUATION
The initial clinical evaluation of the patient should include a thorough medical, social, family, and psychiatric history. Medical conditions that may mimic bipolar disorder include neurologic conditions (eg, partial seizures, neoplasm, strokes, dementia, delirium) and endocrine disorders (eg, Cushing disease, hyperthyroidism/hypothyroidism), as well as vitamin deficiencies (B12, folate, niacin, thiamine) and drug and substance use/misuse (alcohol, drugs including antidepressants and stimulants).4 All patients should have a baseline complete physical examination, including neurologic and mental status examinations. Diagnostic tests to assess for potential differential diagnoses and evaluate baseline levels include the following:
- Basic metabolic panel, including fasting glucose, to evaluate electrolytes and risk for diabetes or Cushing disease, and to assess baseline renal function
- Thyroid function tests
- Complete blood count to assess status prior to anticonvulsant treatment (eg, carbamazepine)
- Pregnancy test if applicable (prior to use of medications)
- Liver function tests to assess baseline measurements prior to use of medications
- Electrocardiography in patients older than 40 to establish baseline and assess QTc interval, especially with use of antipsychotics and carbamazepine
- Urine toxicology screen (to rule out substance abuse).
PSYCHIATRIC EVALUATION
Psychiatric evaluation should focus on age at onset of symptoms, the presence of hypomanic or manic symptoms, prior response to antidepressants, course of the disease including history and duration of depression or manic/hypomanic episodes, and sleep disturbances (increased during depressive episodes and significantly decreased during manic episodes). It is important to assess for a history of self-harm, suicidal ideation, suicide attempts, hospitalizations, legal issues, multiple career shifts, marriage and relationship issues, and smoking and alcohol/substance misuse. Patients with severe manic or depressive episodes may experience psychotic features such as grandiose or paranoid delusions and hallucinations. A history of symptoms from close family members or friends can assist in the diagnosis of bipolar patients.
The use of DSM-5 criteria, as summarized earlier, improves the accuracy of bipolar diagnosis.3 In addition, validated tools are available to help clinicians screen for bipolar disorder, although it is important to remember that a positive screening result is not sufficient to establish a bipolar disorder diagnosis. A widely used instrument that has been validated for screening for bipolar disorder is the MDQ (available at www.dbsalliance.org/pdfs/MDQ.pdf). This self-report questionnaire consists of 15 questions that assess hypomanic or manic symptoms and functional impairment. The first 13 questions of the MDQ screen for a lifetime history of DSM-based hypomanic or manic symptoms. The last two questions ask whether these symptoms occurred at the same time and whether they caused dysfunction in various domains, such as work and family life. The MDQ is considered positive if a patient endorses at least seven of the symptom items, indicates that symptoms have occurred at the same time, and rates their dysfunction in life domains as “moderate” or “serious.” As a screening tool, the MDQ has a reported sensitivity of 73% and a specificity of 90% for bipolar disorder.11 This questionnaire can and should be used by primary care providers to help determine if their patient is at risk and requires a comprehensive evaluation for bipolar disorder.
Notably, even after a clinician has properly diagnosed bipolar disorder, patients and family members are often reluctant to commence treatment due to the stigma associated with mental health disorders.18 To help offset the effects of stigma, patients should be referred for psychologic counseling, including family counseling.
MANAGEMENT
Management of bipolar disorder in the primary care setting includes psychiatric and psychologic counseling referrals. Primary care providers must know the medications used to treat bipolar disorder and their related adverse effects, toxicities, warnings, and drug interactions, as they may treat bipolar patients for other medical conditions. Early diagnosis and treatment/referral can improve prognosis and reduce the risk for relapse and subsequent disability.19 Inpatient management is generally recommended for severe manic episodes, psychotic episodes, patients who present a danger to themselves or others, and patients with suicidal or homicidal ideations/actions.
Medications are the primary treatment for all stages of bipolar disorder, and choice of medications is based on stage, previous response, and adverse effect profiles (see Table 1).2,4,20 Generally, antidepressants (serotonin and norepinephrine reuptake inhibitors [SNRIs] and selective serotonin reuptake inhibitors [SSRIs]) should be avoided or should be used with an effective antimanic/mood stabilizer. Many patients with severe bipolar symptoms require more than two medications, and it is imperative that all primary care providers understand that often one drug alone is not sufficient treatment for patients with bipolar disorder. For less severe manic or hypomanic states, monotherapy with antipsychotics may be effective.

Medications for severe acute manic episodes generally include the mood stabilizers lithium, valproate, or carbamazepine in conjunction with an antipsychotic, such as haloperidol, or an atypical antipsychotic, such as asenapine, aripiprazole, olanzapine, quetiapine, or risperidone.2 The goal of initial therapy in patients with acute mania is rapid resolution of symptoms and restoration of adequate sleep. Lithium has a slower onset of action than valproate and carbamazepine and requires titration and monitoring. Valproate and carbamazepine have a faster onset of action but are less effective than lithium.2 Atypical antipsychotics have a more rapid onset of action than mood stabilizers and are effective in controlling acute manic symptoms, psychosis, and sleep disturbances. Patients with severe acute mania may require hospital admission for stabilization, for their safety and the safety of others.
Acute bipolar depressive episodes can be treated with several different medication options, including combination olanzapine and fluoxetine; the atypical antipsychotic quetiapine; and recently lurasidone, alone or in combination with lithium or valproate. Lamotrigine is more effective for maintenance and prevention of depressive episodes than for treatment of acute episodes, and it is also indicated for treatment of bipolar II. Valproate is more effective than lithium for mixed states and can be titrated more rapidly for faster antimanic effects.4
Generally, due to the high rate of recurrence, maintenance medications should be continued indefinitely. Maintenance medications include the mood stabilizers, lamotrigine, and many of the antipsychotics, including olanzapine.4 Adherence to medications is essential in management of bipolar disorder and can decrease the risk for relapses and destabilization. Poor adherence to medications is common, however, with rates reported at approximately 50%.21 Patient and family education, as well as psychotherapy, can improve adherence rates.2 Primary care providers should educate patients and family members about medication options and adverse effects and must stress the need for adherence to prevent relapse. Providers should also understand the safety profile of mood stabilizers and antipsychotics and the required monitoring of laboratory tests for patients on these medications.
Psychosocial treatments are an elemental component of management. Patients should be referred early for psychologic treatments including, but not limited to, family therapy, group therapy, cognitive-behavioral therapy, and psychotherapy, which have been shown to improve daily functioning, recognition of recurrences, and medication adherence.2 The rate of relapse is significantly lower in patients receiving combination psychotherapy and pharmacotherapy.22
Clinical pearls that every primary care provider should know about bipolar disorder are summarized in Table 2.

CONCLUSION
Given the substantial impact of bipolar disorder on patients and the community, primary care clinicians must maintain a high index of suspicion for this disorder. An early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes. However, diagnosing and treating patients with bipolar disorder is challenging for primary care and specialty clinicians alike. In particular, establishing a diagnosis can be difficult, even for the most seasoned clinician, due to the diversity of symptoms. Nonetheless, diagnosing bipolar disorder, initiating treatment, and monitoring and referring patients when necessary are certainly within the purview of the primary care provider.
CE/CME No: CR-1702
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define bipolar disorder according to the DSM-5 criteria.
• Recognize how patients with bipolar disorder can present to their primary care provider.
• Discuss how to perform a clinical and psychiatric evaluation on patients with suspected bipolar disorder.
• Describe the therapeutic options for a patient with bipolar disease in a primary care setting.
FACULTY
Jean Covino is a clinical professor at Pace University-Lenox Hill Hospital in New York City, and she practices at the Medemerge Family Practice Center in Green Brook, New Jersey. Jennifer Hofmann is an Associate Clinical Professor at Pace University-Lenox Hill Hospital in New York City.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2017.
Article begins on next page >>
Primary care clinicians are often the first point of contact for persons with bipolar disorder. Unfortunately, delays in diagnosis are common, as many of these patients are misdiagnosed with unipolar depression on initial presentation. Since an early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes, clinicians should be able to recognize its symptoms and initiate treatment of this deceptive disorder.
Bipolar disorder is a chronic mental illness characterized by fluctuations in mood and energy that manifests as recurrent episodes of manic or depressive symptoms. It is estimated that between 10% and 38% of patients with bipolar disorder receive all their mental health care in a primary care setting.1 Although patients with bipolar disorder often initially present to their primary care provider, they frequently go undiagnosed because of the complexity of the disorder’s symptomatology and a low index of suspicion among primary care providers.2 Comorbid medical conditions and psychiatric issues can also lead to misdiagnoses.
Because primary care providers are often the first point of contact for patients with bipolar disorder, they are well positioned to recognize bipolar symptoms early in the course of the illness. The pure subtypes of bipolar disorder include bipolar I and bipolar II. Clinicians who work in a primary care or emergency department setting should be able to recognize and initiate treatment for these two subtypes while the patient is waiting for a psychiatric evaluation. Accurate early diagnosis of this disabling disorder can reduce morbidity and improve outcomes by allowing for appropriate referral, pharmacotherapy, and psychotherapy.
DEFINITION
According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5), bipolar disorder is a mood disorder defined by episodes of mania, hypomania, and major depression.3 Patients with bipolar I disorder experience manic episodes and almost always experience major depressive and hypomanic episodes. Bipolar II disorder is marked by at least one hypomanic episode, at least one major depressive episode (MDE), and the absence of manic episodes.3 A manic episode is at least one week of abnormally and continually elevated, expansive, or irritable mood and increased activity or energy accompanied by at least three of the following symptoms (or four if mood is only irritable): inflated self-esteem, decreased need for sleep, increased talkativeness, flight of ideas or racing thoughts, marked distractibility, increased goal-directed activity or agitation, and excessive involvement in dangerous or high-risk activities (eg, reckless spending or increased sexuality). To be considered a manic episode, the mood disturbance must cause marked impairment in social or occupational functioning, result in hospitalization, or involve psychotic features, and the symptoms cannot be attributable to the effects of drugs or medications or another medical condition.3
In a hypomanic episode, the period of elevated or irritable mood lasts for a shorter duration (at least four days); is associated with a clear, uncharacteristic change in functioning; and is observable by others but does not cause marked impairment, need for hospitalization, or psychosis. MDE is defined by the presence of at least five of nine symptoms for a minimum duration of two weeks and a change from previous functioning: depressed mood, markedly decreased interest or pleasure in activities, significant change in weight or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or excessive guilt, decreased ability to think or concentrate or indecisiveness, and recurrent thoughts of death or suicidality (at least one symptom must be depressed mood or loss of interest or pleasure).3
EPIDEMIOLOGY
Bipolar disorder affects men and women equally. It can occur at any age but is seen most commonly in persons younger than 25.4 The mean age at the first manic/hypomanic or major depressive episode was determined to be 18.2 in bipolar I and 20.3 in bipolar II.5 The lifetime prevalence of bipolar disorder in the United States is around 4%, with one study finding prevalence estimates of 1.0% for bipolar I disorder and 1.1% for bipolar II disorder.5
Bipolar disorder is common among primary care patients with depression. Two studies that explored the risk for bipolar disorder among depressed outpatients in primary care settings found that between 20% and 30% of these patients screened positive for bipolar disorder on the Mood Disorders Questionnaire (MDQ), indicating that a more thorough evaluation for bipolar disorder was needed.6,7 A systematic review of the literature found similar rates of positive results on the MDQ screening measure among primary care patients with depression, a trauma exposure, medically unexplained symptoms, or a psychiatric complaint; bipolar disorder was diagnosed with a structured clinical interview in 3% to 9% of these patients.8 Children of parents with bipolar disorder have a 4% to 15% risk for also being affected.4
CLINICAL PRESENTATION IN PRIMARY CARE
Bipolar patients are often depressed or euthymic for a majority of their lives but can also present in a manic or hypomanic state. In primary care settings, these patients often present with depression (including postpartum depression), which can obscure the diagnosis. Misdiagnosis of bipolar disorder as recurrent unipolar depression occurs in 60% of patients seeking treatment for depression.9
Patients with bipolar disorder who present to primary care usually demonstrate a wide range of mood symptomatology other than depression, including mood swings, anxiety, fatigue, sleep disturbances, and the inability to focus or concentrate. Patients can also present in mixed states. These are characterized by elements of irritability, increased energy, and sleeplessness with depressive features.
Several clues that can assist in detecting bipolar disorder relate to age at onset, family history, mood shifts, seasonality, and atypical depressive symptoms (eg, sleep dysregulation and appetite changes). Although the diagnosis of bipolar disorder is commonly delayed by many years, patients often report significant mood symptoms in their early 20s. In a study that used a self-administered questionnaire to assess the experience of persons living with bipolar disorder, 33% of the respondents were younger than 15 when their symptoms first started, 27% were between 15 and 19, and 39% were 20 or older.9 Parental and family history of bipolar disorder increases risk for the disorder in offspring, so a thorough family history is essential when the disorder is suspected.
Aside from the classic presentation defined by the DSM-5 criteria, patients with bipolar disorder can also exhibit other effects of their illness, such as alcohol-related problems and sexually transmitted or drug-related infections. In patients with bipolar disorder, rates of alcohol use range from 21.4% in adults to 54.5% in adolescents and young adults.10 Social history may reveal relationship and marital issues, financial problems, difficulties keeping a job, and legal problems.9,11 Suicide attempts and completed suicides are significantly more common among persons with bipolar disorder than among the general population.12,13
Comorbidity with at least one other disorder is common in bipolar disorder.5 The most common comorbid personality disorder associated with bipolar disorder is borderline personality disorder, which is characterized by ongoing instability in moods and behavior. Persons with this disorder can experience intense episodes of anger, depression, and anxiety that may last from hours to days. The high prevalence of persistent symptoms despite treatment in bipolar disorder and the unstable and partly remitting course of borderline personality make it difficult to distinguish between the two disorders.14 The frequent mood changes that occur with borderline personality disorder may appear to overlap with the mood swings characterizing bipolar disorder, but the mood episodes in borderline personality disorder are of shorter duration than those in bipolar disorder. Other common comorbid disorders seen in patients with bipolar disorder include substance abuse disorders, anxiety disorders (especially panic disorder, generalized anxiety disorder, and obsessive-compulsive disorder), and attention-deficit/hyperactivity disorder.5
Primary care providers should be aware of other common comorbidities that may be present in patients with bipolar disorder. These patients commonly experience medical problems such as diabetes, obesity, and metabolic syndrome, which all lead to increased cardiovascular risk.15-17
CLINICAL EVALUATION
The initial clinical evaluation of the patient should include a thorough medical, social, family, and psychiatric history. Medical conditions that may mimic bipolar disorder include neurologic conditions (eg, partial seizures, neoplasm, strokes, dementia, delirium) and endocrine disorders (eg, Cushing disease, hyperthyroidism/hypothyroidism), as well as vitamin deficiencies (B12, folate, niacin, thiamine) and drug and substance use/misuse (alcohol, drugs including antidepressants and stimulants).4 All patients should have a baseline complete physical examination, including neurologic and mental status examinations. Diagnostic tests to assess for potential differential diagnoses and evaluate baseline levels include the following:
- Basic metabolic panel, including fasting glucose, to evaluate electrolytes and risk for diabetes or Cushing disease, and to assess baseline renal function
- Thyroid function tests
- Complete blood count to assess status prior to anticonvulsant treatment (eg, carbamazepine)
- Pregnancy test if applicable (prior to use of medications)
- Liver function tests to assess baseline measurements prior to use of medications
- Electrocardiography in patients older than 40 to establish baseline and assess QTc interval, especially with use of antipsychotics and carbamazepine
- Urine toxicology screen (to rule out substance abuse).
PSYCHIATRIC EVALUATION
Psychiatric evaluation should focus on age at onset of symptoms, the presence of hypomanic or manic symptoms, prior response to antidepressants, course of the disease including history and duration of depression or manic/hypomanic episodes, and sleep disturbances (increased during depressive episodes and significantly decreased during manic episodes). It is important to assess for a history of self-harm, suicidal ideation, suicide attempts, hospitalizations, legal issues, multiple career shifts, marriage and relationship issues, and smoking and alcohol/substance misuse. Patients with severe manic or depressive episodes may experience psychotic features such as grandiose or paranoid delusions and hallucinations. A history of symptoms from close family members or friends can assist in the diagnosis of bipolar patients.
The use of DSM-5 criteria, as summarized earlier, improves the accuracy of bipolar diagnosis.3 In addition, validated tools are available to help clinicians screen for bipolar disorder, although it is important to remember that a positive screening result is not sufficient to establish a bipolar disorder diagnosis. A widely used instrument that has been validated for screening for bipolar disorder is the MDQ (available at www.dbsalliance.org/pdfs/MDQ.pdf). This self-report questionnaire consists of 15 questions that assess hypomanic or manic symptoms and functional impairment. The first 13 questions of the MDQ screen for a lifetime history of DSM-based hypomanic or manic symptoms. The last two questions ask whether these symptoms occurred at the same time and whether they caused dysfunction in various domains, such as work and family life. The MDQ is considered positive if a patient endorses at least seven of the symptom items, indicates that symptoms have occurred at the same time, and rates their dysfunction in life domains as “moderate” or “serious.” As a screening tool, the MDQ has a reported sensitivity of 73% and a specificity of 90% for bipolar disorder.11 This questionnaire can and should be used by primary care providers to help determine if their patient is at risk and requires a comprehensive evaluation for bipolar disorder.
Notably, even after a clinician has properly diagnosed bipolar disorder, patients and family members are often reluctant to commence treatment due to the stigma associated with mental health disorders.18 To help offset the effects of stigma, patients should be referred for psychologic counseling, including family counseling.
MANAGEMENT
Management of bipolar disorder in the primary care setting includes psychiatric and psychologic counseling referrals. Primary care providers must know the medications used to treat bipolar disorder and their related adverse effects, toxicities, warnings, and drug interactions, as they may treat bipolar patients for other medical conditions. Early diagnosis and treatment/referral can improve prognosis and reduce the risk for relapse and subsequent disability.19 Inpatient management is generally recommended for severe manic episodes, psychotic episodes, patients who present a danger to themselves or others, and patients with suicidal or homicidal ideations/actions.
Medications are the primary treatment for all stages of bipolar disorder, and choice of medications is based on stage, previous response, and adverse effect profiles (see Table 1).2,4,20 Generally, antidepressants (serotonin and norepinephrine reuptake inhibitors [SNRIs] and selective serotonin reuptake inhibitors [SSRIs]) should be avoided or should be used with an effective antimanic/mood stabilizer. Many patients with severe bipolar symptoms require more than two medications, and it is imperative that all primary care providers understand that often one drug alone is not sufficient treatment for patients with bipolar disorder. For less severe manic or hypomanic states, monotherapy with antipsychotics may be effective.

Medications for severe acute manic episodes generally include the mood stabilizers lithium, valproate, or carbamazepine in conjunction with an antipsychotic, such as haloperidol, or an atypical antipsychotic, such as asenapine, aripiprazole, olanzapine, quetiapine, or risperidone.2 The goal of initial therapy in patients with acute mania is rapid resolution of symptoms and restoration of adequate sleep. Lithium has a slower onset of action than valproate and carbamazepine and requires titration and monitoring. Valproate and carbamazepine have a faster onset of action but are less effective than lithium.2 Atypical antipsychotics have a more rapid onset of action than mood stabilizers and are effective in controlling acute manic symptoms, psychosis, and sleep disturbances. Patients with severe acute mania may require hospital admission for stabilization, for their safety and the safety of others.
Acute bipolar depressive episodes can be treated with several different medication options, including combination olanzapine and fluoxetine; the atypical antipsychotic quetiapine; and recently lurasidone, alone or in combination with lithium or valproate. Lamotrigine is more effective for maintenance and prevention of depressive episodes than for treatment of acute episodes, and it is also indicated for treatment of bipolar II. Valproate is more effective than lithium for mixed states and can be titrated more rapidly for faster antimanic effects.4
Generally, due to the high rate of recurrence, maintenance medications should be continued indefinitely. Maintenance medications include the mood stabilizers, lamotrigine, and many of the antipsychotics, including olanzapine.4 Adherence to medications is essential in management of bipolar disorder and can decrease the risk for relapses and destabilization. Poor adherence to medications is common, however, with rates reported at approximately 50%.21 Patient and family education, as well as psychotherapy, can improve adherence rates.2 Primary care providers should educate patients and family members about medication options and adverse effects and must stress the need for adherence to prevent relapse. Providers should also understand the safety profile of mood stabilizers and antipsychotics and the required monitoring of laboratory tests for patients on these medications.
Psychosocial treatments are an elemental component of management. Patients should be referred early for psychologic treatments including, but not limited to, family therapy, group therapy, cognitive-behavioral therapy, and psychotherapy, which have been shown to improve daily functioning, recognition of recurrences, and medication adherence.2 The rate of relapse is significantly lower in patients receiving combination psychotherapy and pharmacotherapy.22
Clinical pearls that every primary care provider should know about bipolar disorder are summarized in Table 2.

CONCLUSION
Given the substantial impact of bipolar disorder on patients and the community, primary care clinicians must maintain a high index of suspicion for this disorder. An early and accurate diagnosis may reduce the burden of bipolar disorder and improve outcomes. However, diagnosing and treating patients with bipolar disorder is challenging for primary care and specialty clinicians alike. In particular, establishing a diagnosis can be difficult, even for the most seasoned clinician, due to the diversity of symptoms. Nonetheless, diagnosing bipolar disorder, initiating treatment, and monitoring and referring patients when necessary are certainly within the purview of the primary care provider.
1. Kilbourne AM, Goodrich DE, O’Donnell AN, Miller CJ. Integrating bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
2. Culpepper L. The diagnosis and treatment of bipolar disorder: decision making in primary care. Prim Care Companion CNS Disord. 2014;16(3): doi 10.4088/PCC.13r01609.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
4. Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85:483-493.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Calabrese JR, Muzina DJ, Kemp DE, et al. Predictors of bipolar disorder risk among patients currently treated for major depression. MedGenMed. 2006;8(3):38.
7. Hirschfeld RM, Cass AR, Holt DC, Carlson CA. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
8. Cerimele JM, Chwastiak LA, Dodson S, Katon WJ. The prevalence of bipolar disorder in primary care patients with depression or other psychiatric complaints: a systematic review. Psychosomatics. 2013;54(6):515-524.
9. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003; 64:161-174.
10. Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol. 2005;15(4):425-434.
11. Piver A, Yatham LN, Lam RW. Bipolar spectrum disorders: new perspectives. Can Fam Physician. 2002;48:896-904.
12. Eroglu MZ, Karakus G, Tamam L. Bipolar disorder and suicide. J Psychiatry Neurol Sci. 2013;26:139-147.
13. Simon GE, Hunkeler E, Fireman B, Lee JY, Savarino J. Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007;9:526-530.
14. Marcinko D, Vuksan-Cusa B. Borderline personality disorder and bipolar disorder comorbidity in suicidal patients: diagnostic and therapeutic challenges. Psychiatr Danub. 2009;21:386-390.
15. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52:230-236.
16. Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013; 170:265-274.
17. Fiedorowicz JG, Solomon DA, Endicott J, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71(6):598-606.
18. Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: A review of the literature. J Affect Disord. 2013; 150:181-191.
19. Berk M, Brnabic A, Dodd S, et al. Does stage of illness impact treatment response in bipolar disorder? Empirical treatment data and their implication for the staging model and early intervention. Bipolar Disord. 2011;13(1):87-98.
20. Tegretol (carbamazepine) [package insert]. East Hanover, NJ: Novartis; 2015. www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf. Accessed November 18, 2016.
21. Arvilommi P, Suominen K, Mantere O, et al. Predictors of adherence to psychopharmacological and psychosocial treatment in bipolar I or II disorders—an 18-month prospective study. J Affect Disord. 2014;155:110-117.
22. Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord. 2013;149:247-252.
1. Kilbourne AM, Goodrich DE, O’Donnell AN, Miller CJ. Integrating bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
2. Culpepper L. The diagnosis and treatment of bipolar disorder: decision making in primary care. Prim Care Companion CNS Disord. 2014;16(3): doi 10.4088/PCC.13r01609.
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
4. Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85:483-493.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Calabrese JR, Muzina DJ, Kemp DE, et al. Predictors of bipolar disorder risk among patients currently treated for major depression. MedGenMed. 2006;8(3):38.
7. Hirschfeld RM, Cass AR, Holt DC, Carlson CA. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18(4):233-239.
8. Cerimele JM, Chwastiak LA, Dodson S, Katon WJ. The prevalence of bipolar disorder in primary care patients with depression or other psychiatric complaints: a systematic review. Psychosomatics. 2013;54(6):515-524.
9. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003; 64:161-174.
10. Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol. 2005;15(4):425-434.
11. Piver A, Yatham LN, Lam RW. Bipolar spectrum disorders: new perspectives. Can Fam Physician. 2002;48:896-904.
12. Eroglu MZ, Karakus G, Tamam L. Bipolar disorder and suicide. J Psychiatry Neurol Sci. 2013;26:139-147.
13. Simon GE, Hunkeler E, Fireman B, Lee JY, Savarino J. Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007;9:526-530.
14. Marcinko D, Vuksan-Cusa B. Borderline personality disorder and bipolar disorder comorbidity in suicidal patients: diagnostic and therapeutic challenges. Psychiatr Danub. 2009;21:386-390.
15. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52:230-236.
16. Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013; 170:265-274.
17. Fiedorowicz JG, Solomon DA, Endicott J, et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med. 2009;71(6):598-606.
18. Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: A review of the literature. J Affect Disord. 2013; 150:181-191.
19. Berk M, Brnabic A, Dodd S, et al. Does stage of illness impact treatment response in bipolar disorder? Empirical treatment data and their implication for the staging model and early intervention. Bipolar Disord. 2011;13(1):87-98.
20. Tegretol (carbamazepine) [package insert]. East Hanover, NJ: Novartis; 2015. www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf. Accessed November 18, 2016.
21. Arvilommi P, Suominen K, Mantere O, et al. Predictors of adherence to psychopharmacological and psychosocial treatment in bipolar I or II disorders—an 18-month prospective study. J Affect Disord. 2014;155:110-117.
22. Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord. 2013;149:247-252.
Steroids during late preterm labor: Better later than never
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every 3 minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to providing corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor,2 and 8% are born in the late preterm period, defined as 34 to 36 weeks’ gestation.3 To reduce the risk of neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk of preterm delivery.2,4 Due to a lack of evidence from randomized controlled trials (RCTs) on the benefit of corticosteroids in late preterm labor, there have not been recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found newborns delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, of which 19,000 were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks; P<.001 for the trend).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This randomized placebo-controlled trial examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and 7 days after the planned randomization.
Patients were randomly assigned to receive either 2 intramuscular injections (12 mg each) of betamethasone or placebo, 24 hours apart. The 2 doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases where the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, which was defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least 2 consecutive hours, supplemental oxygen for at least 4 continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median time to delivery from enrollment was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR]=0.80; 95% CI, 0.66-0.97; P=.02; number needed to treat [NNT]=35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR=0.67; 95% CI, 0.53-0.84; P<.001; NNT=25). The betamethasone group also had a lower risk of transient tachypnea of the newborn (6.7% vs 9.9%; RR=0.68; 95% CI, 0.53-0.87; P=.002).
There were no significant differences in the occurrence of maternal chorioamnionitis (about 2%) or endometritis (about 1%) between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR=1.6; 95% CI, 1.37-1.87; P<.001; number needed to harm [NNH]=11). The betamethasone group had 2 neonatal deaths: one from septic shock and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone makes a difference even in the late, late preterm period
This study demonstrated clear benefit in neonatal respiratory outcomes when betamethasone vs placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT=37 to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis (including this trial) evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk of hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal intensive care unit stays that were 3 days or longer in the betamethasone group. Also, there was no difference in hospital length of stay between the 2 groups. In addition, it’s not clear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible since betamethasone is readily available
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Use steroids in women at risk of preterm delivery, even if they are 36 weeks, 6 days’ pregnant, because steroids may reduce respiratory complications in the newborn with minimal risk for neonatal or maternal complications.
Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.1
STRENGTH OF RECOMMENDATION
A: Based on a good quality randomized controlled trial and consistent with a meta-analysis.
Nausea/vomiting • tachycardia • unintentional weight loss • Dx?
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.