User login
Commentary: Examining Inpatient Admission, Hypothyroidism, and Vestibular Migraine, November 2023
Inpatient headache admissions are often considered a last-case scenario option for patients with chronic refractory migraine. Initially described by Raskin,1 the admission typically consists of repetitive infusions of dihydroergotamine (DHE) with a pretreatment of antihistamine, neuroleptic, and other antinausea medications in addition to anti-inflammatory or steroid medications, IV fluids, and magnesium. Often this is superimposed on a continuous infusion of lidocaine or ketamine. One common concern is whether the use of DHE is safe for patients at a higher risk for vascular disease. Wang and colleagues reported on the incidence of cardiovascular adverse events in this population receiving repetitive intravenous DHE.
They present findings based on the Jefferson Headache Center (Philadelphia, Pennsylvania) Inpatient Headache Protocol, looking at patients admitted from January through October 2019. Of the 347 patients admitted during this period, 64 were identified as having either an elevated or low risk for atherosclerotic vascular disease. The degree of vascular risk was determined on the basis of an atherosclerotic cardiovascular disease calculation, using body mass index values, cholesterol level, and mean arterial blood pressure readings, as well as smoking and diabetes history. A score < 5.0% was designated low risk, while elevated risk included scores up to 20%. DHE was not offered to patients with a history of moderate to severe ischemic heart disease, coronary vasospasm, peripheral artery disease, Raynaud phenomenon, or ischemic stroke.
The primary outcome was treatment effectiveness, determined by an 11-point pain scale; secondary outcomes included tolerability, as defined by change in the patient's QTc (corrected QT interval) based on their daily ECG monitoring, the incidence of chest pain or shortness of breath, and whether DHE needed to be tapered or discontinued. The researchers noted that the elevated-vascular-risk group had fewer patients receiving the maximum dose of DHE and receiving less DHE over the course of their admission as compared with the low-risk group. They also reported lower response rates and less freedom from pain after admission. No clinically significant adverse events were noted in either group, and only three patients had sustained ECG changes from baseline.
DHE remains an effective treatment for the most chronic and refractory migraine cases, and it can be provided safely and effectively in an appropriately monitored setting. Although there still are contraindications for receiving DHE, those who can receive it may benefit significantly. Ideally, this should be done with cardiac clearance if there is any doubt regarding the vascular risk for any individual patient.
The frequency and severity of migraine can fluctuate due to a myriad of factors. When faced with worsening migraine, most healthcare providers ask their patients about specific triggers or other potential causes that may have led to the recent subacute worsening. Many healthcare providers will also investigate further, and when appropriate, order serum lab testing to determine whether any potential metabolic derangement, vitamin deficiency, or other abnormality could be contributing. Subclinical hypothyroidism is a common finding when investigating for these potential causes of worsening migraine, and often our internal medicine or endocrinology colleagues will discount these findings as "borderline" or still within normal limits. Dev and colleagues sought to determine whether low-dose thyroid replacement was beneficial for migraine prevention in this situation.
This study defined subclinical hypothyroidism as a thyroid-stimulating hormone (TSH) level of 4.5-10.0 mIU/L with a normal free thyroxine (T4), measured twice within 6 weeks for confirmation. Patients with prior thyroid disease were excluded from the study. Participants were randomly assigned to take 25 μg levothyroxine supplementation or placebo and were allowed to continue their migraine treatments. The primary outcomes were reduction in headache duration, frequency, severity, and Migraine Disability Assessment (MIDAS) score after 3 months.
A total of 87 patients with migraine and subclinical hypothyroidism were recruited, and the investigators noted a statistically significant improvement in all parameters (migraine frequency, severity, duration, MIDAS score) after 3 months of low-dose thyroid supplementation. This was maintained at a 3-month follow-up visit as well. TSH levels normalized in 87% of participants after repeat testing 3 months after the intervention.
When evaluating patients with worsening migraine who had been stable, it is wise to consider subclinical hypothyroidism as a potential etiology. Low-dose thyroid hormone supplementation appears to be well tolerated and effective for normalizing both TSH levels and, importantly, the migrainous exacerbation. Clinicians often will start or add preventive medications, ignoring the reason that there was an exacerbation in the first place, even though many of our colleagues choose not to treat this degree of hypothyroidism.
Many variants of migraine are better recognized and understood now; chief among these is vestibular migraine (VM). VM is a migraine subtype characterized by frequent or near-constant vestibular symptoms (vertigo, lightheadedness, disequilibrium, or rocking) with superimposed headache symptoms with some migrainous features. VM is generally considered to be more difficult to treat and more treatment-refractory than episodic or chronic migraine without vestibular symptoms. There are few treatments that are specific for this variant of migraine. Chen and colleagues sought to better understand the evidence of specific treatments for VM via meta-analysis.
Only randomized controlled trials were included in this meta-analysis; seven studies that specifically recruited patients with vestibular migraine using International Classification of Headache Disorders (ICHD) criteria were included. The outcomes of the studies were changes in frequency or severity of vestibular migraine attacks. The studies that were included were published from 2014 to 2022 and comprised multiple treatment comparisons, including metoprolol, venlafaxine, valproic acid, propranolol, flunarizine, a probiotic, and relaxation techniques.
Three interventions were noted to be significantly beneficial for VM prevention, specifically a decrease in migraine frequency: valproic acid, propranolol, and venlafaxine. Valproic acid yielded the greatest decrease in VM frequency among all interventions. None of the interventions were associated with improvement in VM severity, and none of the treatments were associated with significantly different adverse-event and dropout rates.
VM is widely thought to be underdiagnosed and should be considered more frequently. This includes situations in which the headache component of the patient's complaints is relatively mild but still associated with features of migraines, such as sensitivities to light and sound, nausea, or unilateral presentations of pain. There remain very few high-quality VM studies, but this meta-analysis should highlight potential treatment options and raise the profile for this diagnosis in order for further trials to be performed.
Additional Reference
1. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995-997. doi: 10.1212/WNL.36.7.995
Inpatient headache admissions are often considered a last-case scenario option for patients with chronic refractory migraine. Initially described by Raskin,1 the admission typically consists of repetitive infusions of dihydroergotamine (DHE) with a pretreatment of antihistamine, neuroleptic, and other antinausea medications in addition to anti-inflammatory or steroid medications, IV fluids, and magnesium. Often this is superimposed on a continuous infusion of lidocaine or ketamine. One common concern is whether the use of DHE is safe for patients at a higher risk for vascular disease. Wang and colleagues reported on the incidence of cardiovascular adverse events in this population receiving repetitive intravenous DHE.
They present findings based on the Jefferson Headache Center (Philadelphia, Pennsylvania) Inpatient Headache Protocol, looking at patients admitted from January through October 2019. Of the 347 patients admitted during this period, 64 were identified as having either an elevated or low risk for atherosclerotic vascular disease. The degree of vascular risk was determined on the basis of an atherosclerotic cardiovascular disease calculation, using body mass index values, cholesterol level, and mean arterial blood pressure readings, as well as smoking and diabetes history. A score < 5.0% was designated low risk, while elevated risk included scores up to 20%. DHE was not offered to patients with a history of moderate to severe ischemic heart disease, coronary vasospasm, peripheral artery disease, Raynaud phenomenon, or ischemic stroke.
The primary outcome was treatment effectiveness, determined by an 11-point pain scale; secondary outcomes included tolerability, as defined by change in the patient's QTc (corrected QT interval) based on their daily ECG monitoring, the incidence of chest pain or shortness of breath, and whether DHE needed to be tapered or discontinued. The researchers noted that the elevated-vascular-risk group had fewer patients receiving the maximum dose of DHE and receiving less DHE over the course of their admission as compared with the low-risk group. They also reported lower response rates and less freedom from pain after admission. No clinically significant adverse events were noted in either group, and only three patients had sustained ECG changes from baseline.
DHE remains an effective treatment for the most chronic and refractory migraine cases, and it can be provided safely and effectively in an appropriately monitored setting. Although there still are contraindications for receiving DHE, those who can receive it may benefit significantly. Ideally, this should be done with cardiac clearance if there is any doubt regarding the vascular risk for any individual patient.
The frequency and severity of migraine can fluctuate due to a myriad of factors. When faced with worsening migraine, most healthcare providers ask their patients about specific triggers or other potential causes that may have led to the recent subacute worsening. Many healthcare providers will also investigate further, and when appropriate, order serum lab testing to determine whether any potential metabolic derangement, vitamin deficiency, or other abnormality could be contributing. Subclinical hypothyroidism is a common finding when investigating for these potential causes of worsening migraine, and often our internal medicine or endocrinology colleagues will discount these findings as "borderline" or still within normal limits. Dev and colleagues sought to determine whether low-dose thyroid replacement was beneficial for migraine prevention in this situation.
This study defined subclinical hypothyroidism as a thyroid-stimulating hormone (TSH) level of 4.5-10.0 mIU/L with a normal free thyroxine (T4), measured twice within 6 weeks for confirmation. Patients with prior thyroid disease were excluded from the study. Participants were randomly assigned to take 25 μg levothyroxine supplementation or placebo and were allowed to continue their migraine treatments. The primary outcomes were reduction in headache duration, frequency, severity, and Migraine Disability Assessment (MIDAS) score after 3 months.
A total of 87 patients with migraine and subclinical hypothyroidism were recruited, and the investigators noted a statistically significant improvement in all parameters (migraine frequency, severity, duration, MIDAS score) after 3 months of low-dose thyroid supplementation. This was maintained at a 3-month follow-up visit as well. TSH levels normalized in 87% of participants after repeat testing 3 months after the intervention.
When evaluating patients with worsening migraine who had been stable, it is wise to consider subclinical hypothyroidism as a potential etiology. Low-dose thyroid hormone supplementation appears to be well tolerated and effective for normalizing both TSH levels and, importantly, the migrainous exacerbation. Clinicians often will start or add preventive medications, ignoring the reason that there was an exacerbation in the first place, even though many of our colleagues choose not to treat this degree of hypothyroidism.
Many variants of migraine are better recognized and understood now; chief among these is vestibular migraine (VM). VM is a migraine subtype characterized by frequent or near-constant vestibular symptoms (vertigo, lightheadedness, disequilibrium, or rocking) with superimposed headache symptoms with some migrainous features. VM is generally considered to be more difficult to treat and more treatment-refractory than episodic or chronic migraine without vestibular symptoms. There are few treatments that are specific for this variant of migraine. Chen and colleagues sought to better understand the evidence of specific treatments for VM via meta-analysis.
Only randomized controlled trials were included in this meta-analysis; seven studies that specifically recruited patients with vestibular migraine using International Classification of Headache Disorders (ICHD) criteria were included. The outcomes of the studies were changes in frequency or severity of vestibular migraine attacks. The studies that were included were published from 2014 to 2022 and comprised multiple treatment comparisons, including metoprolol, venlafaxine, valproic acid, propranolol, flunarizine, a probiotic, and relaxation techniques.
Three interventions were noted to be significantly beneficial for VM prevention, specifically a decrease in migraine frequency: valproic acid, propranolol, and venlafaxine. Valproic acid yielded the greatest decrease in VM frequency among all interventions. None of the interventions were associated with improvement in VM severity, and none of the treatments were associated with significantly different adverse-event and dropout rates.
VM is widely thought to be underdiagnosed and should be considered more frequently. This includes situations in which the headache component of the patient's complaints is relatively mild but still associated with features of migraines, such as sensitivities to light and sound, nausea, or unilateral presentations of pain. There remain very few high-quality VM studies, but this meta-analysis should highlight potential treatment options and raise the profile for this diagnosis in order for further trials to be performed.
Additional Reference
1. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995-997. doi: 10.1212/WNL.36.7.995
Inpatient headache admissions are often considered a last-case scenario option for patients with chronic refractory migraine. Initially described by Raskin,1 the admission typically consists of repetitive infusions of dihydroergotamine (DHE) with a pretreatment of antihistamine, neuroleptic, and other antinausea medications in addition to anti-inflammatory or steroid medications, IV fluids, and magnesium. Often this is superimposed on a continuous infusion of lidocaine or ketamine. One common concern is whether the use of DHE is safe for patients at a higher risk for vascular disease. Wang and colleagues reported on the incidence of cardiovascular adverse events in this population receiving repetitive intravenous DHE.
They present findings based on the Jefferson Headache Center (Philadelphia, Pennsylvania) Inpatient Headache Protocol, looking at patients admitted from January through October 2019. Of the 347 patients admitted during this period, 64 were identified as having either an elevated or low risk for atherosclerotic vascular disease. The degree of vascular risk was determined on the basis of an atherosclerotic cardiovascular disease calculation, using body mass index values, cholesterol level, and mean arterial blood pressure readings, as well as smoking and diabetes history. A score < 5.0% was designated low risk, while elevated risk included scores up to 20%. DHE was not offered to patients with a history of moderate to severe ischemic heart disease, coronary vasospasm, peripheral artery disease, Raynaud phenomenon, or ischemic stroke.
The primary outcome was treatment effectiveness, determined by an 11-point pain scale; secondary outcomes included tolerability, as defined by change in the patient's QTc (corrected QT interval) based on their daily ECG monitoring, the incidence of chest pain or shortness of breath, and whether DHE needed to be tapered or discontinued. The researchers noted that the elevated-vascular-risk group had fewer patients receiving the maximum dose of DHE and receiving less DHE over the course of their admission as compared with the low-risk group. They also reported lower response rates and less freedom from pain after admission. No clinically significant adverse events were noted in either group, and only three patients had sustained ECG changes from baseline.
DHE remains an effective treatment for the most chronic and refractory migraine cases, and it can be provided safely and effectively in an appropriately monitored setting. Although there still are contraindications for receiving DHE, those who can receive it may benefit significantly. Ideally, this should be done with cardiac clearance if there is any doubt regarding the vascular risk for any individual patient.
The frequency and severity of migraine can fluctuate due to a myriad of factors. When faced with worsening migraine, most healthcare providers ask their patients about specific triggers or other potential causes that may have led to the recent subacute worsening. Many healthcare providers will also investigate further, and when appropriate, order serum lab testing to determine whether any potential metabolic derangement, vitamin deficiency, or other abnormality could be contributing. Subclinical hypothyroidism is a common finding when investigating for these potential causes of worsening migraine, and often our internal medicine or endocrinology colleagues will discount these findings as "borderline" or still within normal limits. Dev and colleagues sought to determine whether low-dose thyroid replacement was beneficial for migraine prevention in this situation.
This study defined subclinical hypothyroidism as a thyroid-stimulating hormone (TSH) level of 4.5-10.0 mIU/L with a normal free thyroxine (T4), measured twice within 6 weeks for confirmation. Patients with prior thyroid disease were excluded from the study. Participants were randomly assigned to take 25 μg levothyroxine supplementation or placebo and were allowed to continue their migraine treatments. The primary outcomes were reduction in headache duration, frequency, severity, and Migraine Disability Assessment (MIDAS) score after 3 months.
A total of 87 patients with migraine and subclinical hypothyroidism were recruited, and the investigators noted a statistically significant improvement in all parameters (migraine frequency, severity, duration, MIDAS score) after 3 months of low-dose thyroid supplementation. This was maintained at a 3-month follow-up visit as well. TSH levels normalized in 87% of participants after repeat testing 3 months after the intervention.
When evaluating patients with worsening migraine who had been stable, it is wise to consider subclinical hypothyroidism as a potential etiology. Low-dose thyroid hormone supplementation appears to be well tolerated and effective for normalizing both TSH levels and, importantly, the migrainous exacerbation. Clinicians often will start or add preventive medications, ignoring the reason that there was an exacerbation in the first place, even though many of our colleagues choose not to treat this degree of hypothyroidism.
Many variants of migraine are better recognized and understood now; chief among these is vestibular migraine (VM). VM is a migraine subtype characterized by frequent or near-constant vestibular symptoms (vertigo, lightheadedness, disequilibrium, or rocking) with superimposed headache symptoms with some migrainous features. VM is generally considered to be more difficult to treat and more treatment-refractory than episodic or chronic migraine without vestibular symptoms. There are few treatments that are specific for this variant of migraine. Chen and colleagues sought to better understand the evidence of specific treatments for VM via meta-analysis.
Only randomized controlled trials were included in this meta-analysis; seven studies that specifically recruited patients with vestibular migraine using International Classification of Headache Disorders (ICHD) criteria were included. The outcomes of the studies were changes in frequency or severity of vestibular migraine attacks. The studies that were included were published from 2014 to 2022 and comprised multiple treatment comparisons, including metoprolol, venlafaxine, valproic acid, propranolol, flunarizine, a probiotic, and relaxation techniques.
Three interventions were noted to be significantly beneficial for VM prevention, specifically a decrease in migraine frequency: valproic acid, propranolol, and venlafaxine. Valproic acid yielded the greatest decrease in VM frequency among all interventions. None of the interventions were associated with improvement in VM severity, and none of the treatments were associated with significantly different adverse-event and dropout rates.
VM is widely thought to be underdiagnosed and should be considered more frequently. This includes situations in which the headache component of the patient's complaints is relatively mild but still associated with features of migraines, such as sensitivities to light and sound, nausea, or unilateral presentations of pain. There remain very few high-quality VM studies, but this meta-analysis should highlight potential treatment options and raise the profile for this diagnosis in order for further trials to be performed.
Additional Reference
1. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995-997. doi: 10.1212/WNL.36.7.995
Breaking barriers to colorectal cancer care for Black patients
Black Americans are about 40% more likely to die from the disease than most other groups of patients. A recent study also found that 26% of Black Americans are diagnosed with CRC that has already metastasized, meaning the cancer has spread to other places in the body.
“The impact of social determinants of health on CRC diagnosis and treatment is clear to me as a practicing [cancer doctor] and person of color,” said Jason Willis, MD, PhD, a clinical investigator in the departments of Gastrointestinal Medical Oncology and Genomic Medicine at the University of Texas MD Anderson Cancer Center, Houston. “At a systemic level, we know that inequalities in health care access disproportionately impact many racial and ethnic minority groups. This is especially important when it comes to accessing preventative care and routine screening for common cancers, like CRC.”
The problem often exists throughout entire neighborhoods or cities.
“It may reflect a lack of access to primary care, inadequate referrals for screening, cultural barriers, and/or community-level factors,” Dr. Willis said. “Evidence has also suggested that some of the differences in CRC risk observed among various racial/ethnic communities may also be driven by differences in the prevalence of its underlying risk factors, such as tobacco use and type 2 diabetes.”
Black patients can also face information roadblocks when it comes to early CRC evaluation, said Christina M. Annunziata, MD, PhD, senior vice president of extramural discovery science at the American Cancer Society.
Other barriers may include a fear of the invasiveness of a colonoscopy, a lack of understanding of the benefits of screening, and lack of understanding of how family history with the disease plays a role, Dr. Annunziata said. “These apply across the U.S. population and are amplified with Black patients.”
Then there are disparities in treatment, which may come from a lack of health care access, including insurance coverage, transportation challenges, and the time required for treatment such as surgery, radiation, and chemotherapy, she said. “In addition, Black patients diagnosed with advanced-stage cancers require more intensive, expensive, and time-consuming treatment regimens that can be unattainable due to social and economic barriers,” she said.
Are there biological reasons Black people are more at risk for colorectal cancer?
Most likely, no. When Black patients received high-quality colonoscopies, there was no difference in the number of precancerous CRC polyps, or CRC tumors, when compared to White patients tested with the same equipment, according to data from Memorial Sloan Kettering Cancer Center. This further shows the importance of Black patients receiving early and effective screening for the disease.
But genetics may be one reason why CRC in Black patients can be difficult to treat. Additional research from Memorial Sloan Kettering found that colon cancer patients of African ancestry may have tumors that don’t respond well to immunotherapy and targeted cancer therapy.
The researchers found that these patients’ tumors were less likely to have the molecular profiles needed for these treatments to work.
But more research is needed. For now, researchers have very few clues as to why, when, and how these molecular and biological differences of CRC exist among various racial/ethnic and ancestral backgrounds, he said.
Black patients are also more likely to be diagnosed under the age of 50 as well. Researchers don’t know why this is exactly yet, but they think that poor diet, unhealthy bacteria in the gut, and inflammation may contribute to the cause. (Healthy eating and more exercise may lower a person’s risk.)
What are the symptoms of colorectal cancer?
Colorectal polyps, which are growths that can turn into colon cancer, and colon cancer itself can come without symptoms. If a person does have symptoms, they can include:
- Changes in bowel habits
- Blood in bowel movements
- Diarrhea
- Constipation
- The sensation that bowel movements aren’t complete
- Persistent stomachache, stomach pain, or cramps
- Weight loss without any explanation
Any or all of these symptoms warrant a trip to the doctor. These symptoms are the same for all racial and ethnic groups. Because CRC symptoms aren’t always obvious, this makes screening all the more important.
Where colon cancer spreads
Once cancerous cells break off from a tumor, what areas of the body does it spread to first?
What can Black patients do to lower their risk of getting colorectal cancer?
There are a number of solutions patients can pursue themselves.
Learn about CRC online
The untimely death of Oscar-nominated actor Chadwick Boseman from colon cancer at age 43 significantly boosted awareness of the disease, particularly for Black Americans. A study from the University of British Columbia and Simon Fraser University’s Beedie School of Business found there was an increase in internet searches about colon cancer in the months after Mr. Boseman’s August 2020 passing, particularly in areas where many Black Americans live. The study authors emphasized the importance of public health leaders discussing Mr. Mr. Boseman’s diagnosis with their Black patient population, so they will not only be inspired by his brave battle against the disease but will be proactive about getting tested for colon cancer themselves.
Reading about Mr. Boseman’s journey is an important start to patient education. It’s also key to learn about the disease itself, plus how colon cancer screening works specifically. Then, writing down questions to bring to the doctor before screening is an excellent way to feel empowered, and to understand what specific test results will mean.
Be proactive
Find out about family history.
“It’s challenging to determine the best age for screening if the patient doesn’t know their family history,” said Dr. Annunziata. Asking older members of the family whether CRC has affected previous generations is a helpful step.
If there is a strong family history, a patient will likely need earlier screening.
“[Doctors] should explain the benefits of colon cancer screening with colonoscopy starting at age 45 in the general population or earlier if the person has a family history of colon cancer,” Dr. Annunziata said. If a patient’s doctor doesn’t offer this information upfront, it’s definitely the right move to ask for the testing directly.
If a Black patient gets diagnosed with CRC, they should educate themselves about critical follow-up care after a diagnosis. Doctors should also be more proactive about enrolling patients in key clinical trials. According to additional data from the American Cancer Society, only 7% of patients enrolled in the FDA’s clinical cancer drug trials are Black. Doctors should also be more proactive about enrolling patients in these and other key clinical trials; it’s completely appropriate for a patient to search out trials on their own and bring them to their doctor’s attention too.
And attending all appointments and completing chemo or radiation treatment is vital.
“For patients undergoing treatment, physicians can ensure that the patients understand the importance of receiving the full recommended course of treatment and receive the support to tolerate the anticipated side effects,” Dr. Annunziata said.
Reach out for reassurance
Patients who are diagnosed with colorectal cancer have many resources for emotional support. The American Cancer Society offers support for all physical and emotional aspects of cancer 24 hours a day. The Colorectal Cancer Alliance offers comprehensive resource guides as well.
Support groups, through local hospitals or communities, can also be extremely helpful.
Reading the stories of Black patients who are surviving and thriving despite a colorectal cancer diagnosis can be incredibly inspiring as well.
It’s also very important for Black patients to let their doctors know if they don’t have the support they need. A doctor can help by navigating extra help within a patient’s community – an approach that is truly breaking down barriers to CRC care.
“What’s very encouraging is that meaningful improvements in CRC screening rates and early detection among Black communities have been seen through purposeful interventions and outreach,” Dr. Willis said. “In this way, all doctors can play a significant role at a broad and systemic level by advocating for and implementing interventions in their communities.”
A version of this article appeared on WebMD.com.
Black Americans are about 40% more likely to die from the disease than most other groups of patients. A recent study also found that 26% of Black Americans are diagnosed with CRC that has already metastasized, meaning the cancer has spread to other places in the body.
“The impact of social determinants of health on CRC diagnosis and treatment is clear to me as a practicing [cancer doctor] and person of color,” said Jason Willis, MD, PhD, a clinical investigator in the departments of Gastrointestinal Medical Oncology and Genomic Medicine at the University of Texas MD Anderson Cancer Center, Houston. “At a systemic level, we know that inequalities in health care access disproportionately impact many racial and ethnic minority groups. This is especially important when it comes to accessing preventative care and routine screening for common cancers, like CRC.”
The problem often exists throughout entire neighborhoods or cities.
“It may reflect a lack of access to primary care, inadequate referrals for screening, cultural barriers, and/or community-level factors,” Dr. Willis said. “Evidence has also suggested that some of the differences in CRC risk observed among various racial/ethnic communities may also be driven by differences in the prevalence of its underlying risk factors, such as tobacco use and type 2 diabetes.”
Black patients can also face information roadblocks when it comes to early CRC evaluation, said Christina M. Annunziata, MD, PhD, senior vice president of extramural discovery science at the American Cancer Society.
Other barriers may include a fear of the invasiveness of a colonoscopy, a lack of understanding of the benefits of screening, and lack of understanding of how family history with the disease plays a role, Dr. Annunziata said. “These apply across the U.S. population and are amplified with Black patients.”
Then there are disparities in treatment, which may come from a lack of health care access, including insurance coverage, transportation challenges, and the time required for treatment such as surgery, radiation, and chemotherapy, she said. “In addition, Black patients diagnosed with advanced-stage cancers require more intensive, expensive, and time-consuming treatment regimens that can be unattainable due to social and economic barriers,” she said.
Are there biological reasons Black people are more at risk for colorectal cancer?
Most likely, no. When Black patients received high-quality colonoscopies, there was no difference in the number of precancerous CRC polyps, or CRC tumors, when compared to White patients tested with the same equipment, according to data from Memorial Sloan Kettering Cancer Center. This further shows the importance of Black patients receiving early and effective screening for the disease.
But genetics may be one reason why CRC in Black patients can be difficult to treat. Additional research from Memorial Sloan Kettering found that colon cancer patients of African ancestry may have tumors that don’t respond well to immunotherapy and targeted cancer therapy.
The researchers found that these patients’ tumors were less likely to have the molecular profiles needed for these treatments to work.
But more research is needed. For now, researchers have very few clues as to why, when, and how these molecular and biological differences of CRC exist among various racial/ethnic and ancestral backgrounds, he said.
Black patients are also more likely to be diagnosed under the age of 50 as well. Researchers don’t know why this is exactly yet, but they think that poor diet, unhealthy bacteria in the gut, and inflammation may contribute to the cause. (Healthy eating and more exercise may lower a person’s risk.)
What are the symptoms of colorectal cancer?
Colorectal polyps, which are growths that can turn into colon cancer, and colon cancer itself can come without symptoms. If a person does have symptoms, they can include:
- Changes in bowel habits
- Blood in bowel movements
- Diarrhea
- Constipation
- The sensation that bowel movements aren’t complete
- Persistent stomachache, stomach pain, or cramps
- Weight loss without any explanation
Any or all of these symptoms warrant a trip to the doctor. These symptoms are the same for all racial and ethnic groups. Because CRC symptoms aren’t always obvious, this makes screening all the more important.
Where colon cancer spreads
Once cancerous cells break off from a tumor, what areas of the body does it spread to first?
What can Black patients do to lower their risk of getting colorectal cancer?
There are a number of solutions patients can pursue themselves.
Learn about CRC online
The untimely death of Oscar-nominated actor Chadwick Boseman from colon cancer at age 43 significantly boosted awareness of the disease, particularly for Black Americans. A study from the University of British Columbia and Simon Fraser University’s Beedie School of Business found there was an increase in internet searches about colon cancer in the months after Mr. Boseman’s August 2020 passing, particularly in areas where many Black Americans live. The study authors emphasized the importance of public health leaders discussing Mr. Mr. Boseman’s diagnosis with their Black patient population, so they will not only be inspired by his brave battle against the disease but will be proactive about getting tested for colon cancer themselves.
Reading about Mr. Boseman’s journey is an important start to patient education. It’s also key to learn about the disease itself, plus how colon cancer screening works specifically. Then, writing down questions to bring to the doctor before screening is an excellent way to feel empowered, and to understand what specific test results will mean.
Be proactive
Find out about family history.
“It’s challenging to determine the best age for screening if the patient doesn’t know their family history,” said Dr. Annunziata. Asking older members of the family whether CRC has affected previous generations is a helpful step.
If there is a strong family history, a patient will likely need earlier screening.
“[Doctors] should explain the benefits of colon cancer screening with colonoscopy starting at age 45 in the general population or earlier if the person has a family history of colon cancer,” Dr. Annunziata said. If a patient’s doctor doesn’t offer this information upfront, it’s definitely the right move to ask for the testing directly.
If a Black patient gets diagnosed with CRC, they should educate themselves about critical follow-up care after a diagnosis. Doctors should also be more proactive about enrolling patients in key clinical trials. According to additional data from the American Cancer Society, only 7% of patients enrolled in the FDA’s clinical cancer drug trials are Black. Doctors should also be more proactive about enrolling patients in these and other key clinical trials; it’s completely appropriate for a patient to search out trials on their own and bring them to their doctor’s attention too.
And attending all appointments and completing chemo or radiation treatment is vital.
“For patients undergoing treatment, physicians can ensure that the patients understand the importance of receiving the full recommended course of treatment and receive the support to tolerate the anticipated side effects,” Dr. Annunziata said.
Reach out for reassurance
Patients who are diagnosed with colorectal cancer have many resources for emotional support. The American Cancer Society offers support for all physical and emotional aspects of cancer 24 hours a day. The Colorectal Cancer Alliance offers comprehensive resource guides as well.
Support groups, through local hospitals or communities, can also be extremely helpful.
Reading the stories of Black patients who are surviving and thriving despite a colorectal cancer diagnosis can be incredibly inspiring as well.
It’s also very important for Black patients to let their doctors know if they don’t have the support they need. A doctor can help by navigating extra help within a patient’s community – an approach that is truly breaking down barriers to CRC care.
“What’s very encouraging is that meaningful improvements in CRC screening rates and early detection among Black communities have been seen through purposeful interventions and outreach,” Dr. Willis said. “In this way, all doctors can play a significant role at a broad and systemic level by advocating for and implementing interventions in their communities.”
A version of this article appeared on WebMD.com.
Black Americans are about 40% more likely to die from the disease than most other groups of patients. A recent study also found that 26% of Black Americans are diagnosed with CRC that has already metastasized, meaning the cancer has spread to other places in the body.
“The impact of social determinants of health on CRC diagnosis and treatment is clear to me as a practicing [cancer doctor] and person of color,” said Jason Willis, MD, PhD, a clinical investigator in the departments of Gastrointestinal Medical Oncology and Genomic Medicine at the University of Texas MD Anderson Cancer Center, Houston. “At a systemic level, we know that inequalities in health care access disproportionately impact many racial and ethnic minority groups. This is especially important when it comes to accessing preventative care and routine screening for common cancers, like CRC.”
The problem often exists throughout entire neighborhoods or cities.
“It may reflect a lack of access to primary care, inadequate referrals for screening, cultural barriers, and/or community-level factors,” Dr. Willis said. “Evidence has also suggested that some of the differences in CRC risk observed among various racial/ethnic communities may also be driven by differences in the prevalence of its underlying risk factors, such as tobacco use and type 2 diabetes.”
Black patients can also face information roadblocks when it comes to early CRC evaluation, said Christina M. Annunziata, MD, PhD, senior vice president of extramural discovery science at the American Cancer Society.
Other barriers may include a fear of the invasiveness of a colonoscopy, a lack of understanding of the benefits of screening, and lack of understanding of how family history with the disease plays a role, Dr. Annunziata said. “These apply across the U.S. population and are amplified with Black patients.”
Then there are disparities in treatment, which may come from a lack of health care access, including insurance coverage, transportation challenges, and the time required for treatment such as surgery, radiation, and chemotherapy, she said. “In addition, Black patients diagnosed with advanced-stage cancers require more intensive, expensive, and time-consuming treatment regimens that can be unattainable due to social and economic barriers,” she said.
Are there biological reasons Black people are more at risk for colorectal cancer?
Most likely, no. When Black patients received high-quality colonoscopies, there was no difference in the number of precancerous CRC polyps, or CRC tumors, when compared to White patients tested with the same equipment, according to data from Memorial Sloan Kettering Cancer Center. This further shows the importance of Black patients receiving early and effective screening for the disease.
But genetics may be one reason why CRC in Black patients can be difficult to treat. Additional research from Memorial Sloan Kettering found that colon cancer patients of African ancestry may have tumors that don’t respond well to immunotherapy and targeted cancer therapy.
The researchers found that these patients’ tumors were less likely to have the molecular profiles needed for these treatments to work.
But more research is needed. For now, researchers have very few clues as to why, when, and how these molecular and biological differences of CRC exist among various racial/ethnic and ancestral backgrounds, he said.
Black patients are also more likely to be diagnosed under the age of 50 as well. Researchers don’t know why this is exactly yet, but they think that poor diet, unhealthy bacteria in the gut, and inflammation may contribute to the cause. (Healthy eating and more exercise may lower a person’s risk.)
What are the symptoms of colorectal cancer?
Colorectal polyps, which are growths that can turn into colon cancer, and colon cancer itself can come without symptoms. If a person does have symptoms, they can include:
- Changes in bowel habits
- Blood in bowel movements
- Diarrhea
- Constipation
- The sensation that bowel movements aren’t complete
- Persistent stomachache, stomach pain, or cramps
- Weight loss without any explanation
Any or all of these symptoms warrant a trip to the doctor. These symptoms are the same for all racial and ethnic groups. Because CRC symptoms aren’t always obvious, this makes screening all the more important.
Where colon cancer spreads
Once cancerous cells break off from a tumor, what areas of the body does it spread to first?
What can Black patients do to lower their risk of getting colorectal cancer?
There are a number of solutions patients can pursue themselves.
Learn about CRC online
The untimely death of Oscar-nominated actor Chadwick Boseman from colon cancer at age 43 significantly boosted awareness of the disease, particularly for Black Americans. A study from the University of British Columbia and Simon Fraser University’s Beedie School of Business found there was an increase in internet searches about colon cancer in the months after Mr. Boseman’s August 2020 passing, particularly in areas where many Black Americans live. The study authors emphasized the importance of public health leaders discussing Mr. Mr. Boseman’s diagnosis with their Black patient population, so they will not only be inspired by his brave battle against the disease but will be proactive about getting tested for colon cancer themselves.
Reading about Mr. Boseman’s journey is an important start to patient education. It’s also key to learn about the disease itself, plus how colon cancer screening works specifically. Then, writing down questions to bring to the doctor before screening is an excellent way to feel empowered, and to understand what specific test results will mean.
Be proactive
Find out about family history.
“It’s challenging to determine the best age for screening if the patient doesn’t know their family history,” said Dr. Annunziata. Asking older members of the family whether CRC has affected previous generations is a helpful step.
If there is a strong family history, a patient will likely need earlier screening.
“[Doctors] should explain the benefits of colon cancer screening with colonoscopy starting at age 45 in the general population or earlier if the person has a family history of colon cancer,” Dr. Annunziata said. If a patient’s doctor doesn’t offer this information upfront, it’s definitely the right move to ask for the testing directly.
If a Black patient gets diagnosed with CRC, they should educate themselves about critical follow-up care after a diagnosis. Doctors should also be more proactive about enrolling patients in key clinical trials. According to additional data from the American Cancer Society, only 7% of patients enrolled in the FDA’s clinical cancer drug trials are Black. Doctors should also be more proactive about enrolling patients in these and other key clinical trials; it’s completely appropriate for a patient to search out trials on their own and bring them to their doctor’s attention too.
And attending all appointments and completing chemo or radiation treatment is vital.
“For patients undergoing treatment, physicians can ensure that the patients understand the importance of receiving the full recommended course of treatment and receive the support to tolerate the anticipated side effects,” Dr. Annunziata said.
Reach out for reassurance
Patients who are diagnosed with colorectal cancer have many resources for emotional support. The American Cancer Society offers support for all physical and emotional aspects of cancer 24 hours a day. The Colorectal Cancer Alliance offers comprehensive resource guides as well.
Support groups, through local hospitals or communities, can also be extremely helpful.
Reading the stories of Black patients who are surviving and thriving despite a colorectal cancer diagnosis can be incredibly inspiring as well.
It’s also very important for Black patients to let their doctors know if they don’t have the support they need. A doctor can help by navigating extra help within a patient’s community – an approach that is truly breaking down barriers to CRC care.
“What’s very encouraging is that meaningful improvements in CRC screening rates and early detection among Black communities have been seen through purposeful interventions and outreach,” Dr. Willis said. “In this way, all doctors can play a significant role at a broad and systemic level by advocating for and implementing interventions in their communities.”
A version of this article appeared on WebMD.com.
Experts offer guidance on GLP-1 receptor agonists prior to endoscopy
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Studies address primary care oral health screening and prevention for children
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
Both were published online in JAMA.
In one report, the United States Preventive Services Task Force (USPSTF) concludes that there is not enough evidence to assess harms versus benefits of routine screening or interventions for oral health conditions, including dental caries, in primary care for asymptomatic children and adolescents aged 5-17 years.
The evidence report on administering fluoride supplements, fluoride gels, sealants and varnish finds evidence that they improve outcomes. The report was done to inform the USPSTF for a new recommendation on primary care screening, dental referral, behavioral counseling, and preventive interventions for oral health in children and adolescents aged 5-17.
Primary care physicians’ role
One problem the USPSTF identified in its report was limited evidence on available clinical screening tools or assessments to identify which children have oral health conditions in the primary care setting.
The USPSTF’s team, led by Michael J. Barry, MD, of Harvard Medical School in Boston, calls for more research to fill in the gaps before it can reassess.
Michael S. Reddy, DMD, DMSc, with University of California San Francisco School of Dentistry, Oral Health Affairs, said in an accompanying editorial that the current lack of data should not keep primary care physicians from considering oral health during routine medical exams or keep dentists from finding ways to collaborate with primary care physicians. “Medical primary care must partner with dentistry,” they wrote.
Until there is enough evidence for a USPSTF reevaluation on the topic, primary care clinicians should ask patients about their oral hygiene routines, whether they have any dental symptoms, and when they last saw a dentist, as well as referring to a dentist as necessary, the editorialists wrote.
That works both ways, the editorialists added. “Equally important, oral health professionals are encouraged to collaborate and be a resource for their primary care colleagues. Prevention is one of the best tools clinicians have, and it is promoted by integrated, whole-person health effort, “ wrote Dr. Reddy and colleagues.
When oral health stays separate from medical care, patients are left vulnerable, and referrals between medical and dental offices should be a stronger two-way system, the editorialists said.
“[N]ot every primary care patient has access to a dentist,” they wrote. “Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health. Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Evidence that gels, varnish, sealants are effective
In a companion paper, done to inform the USPSTF, Roger Chou, MD, with Pacific Northwest Evidence-based Practice Center, Department of Medical Informatics and Clinical Epidemiology at Oregon Health & Science University in Portland, and colleagues found that when administered by a dental professional or in school settings, fluoride supplements, gels and varnish, and resin-based sealants improved health outcomes.
The findings were based on three systematic reviews (20,684 participants) and 19 randomized clinical trials; three nonrandomized trials; and one observational study (total 15,026 participants.)
With fluoride versus placebo or no intervention, researchers found a decrease from baseline in the number of decayed, missing, or filled permanent teeth (DMFT index) or decayed or filled permanent teeth (DFT index). The average difference was −0.73 [95% confidence interval [CI], −1.30 to −0.19]) at 1.5 to 3 years (six trials; n = 1,395).
Fluoride gels were associated with a DMFT- or DFT-prevented fraction of 0.18 (95% CI, 0.09-0.27) at outcomes closest to 3 years (four trials; n = 1,525).
Researchers found an association between fluoride varnish and a DMFT- or DFT-prevented fraction of 0.44 (95% CI, 0.11-0.76) at 1 to 4.5 years (five trials; n = 3,902). The sealants tested were associated with decreased risk of caries in first molars (odds ratio, 0.21 [95% CI, 0.16-0.28]) at 48-54 months (four trials; n = 440).
They noted that the feasibility of administering preventive measures in primary care is unknown; the effectiveness shown here was based on administration in dental and supervised school settings.
Barriers in primary care settings may include lack of training and equipment (particularly for sealants), uncertain reimbursement and lack of acceptance and uptake.
USPSTF working to close evidence gaps
Wanda Nicholson, MD, MPH, Prevention and Community Health, George Washington Milken Institute of Public Health in Washington, wrote in an accompanying editorial that to speed necessary research to facilitate recommendations, “the USPSTF and its stakeholders need a transparent, easily implementable communication tool that will systematically describe the research necessary to be directly responsive to the evidence gaps.”
The editorialists noted that the USPSTF in trying to update recommendations often has few, if any, high-quality additional studies to consider since its previous recommendation.
To address that, meetings were conducted in November of 2022 involving USPSTF members, Agency for Healthcare Research and Quality (AHRQ) staff, and leadership from the Office of Disease Prevention and the National Institutes of Health. Members formed a working group “to develop a standardized template for communicating research gaps” according to a framework developed by the National Academies of Sciences, Engineering, and Medicine.
Dr. Nicholson and colleagues wrote, “classifying evidence gaps and calling for specific research needs is a prudent, collaborative step in addressing missing evidence,” particularly for underserved populations.
The authors and editorialists declared no relevant conflicts of interest.
FROM JAMA
Suicide prevention and the pediatrician
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Even one night in the ED raises risk for death
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.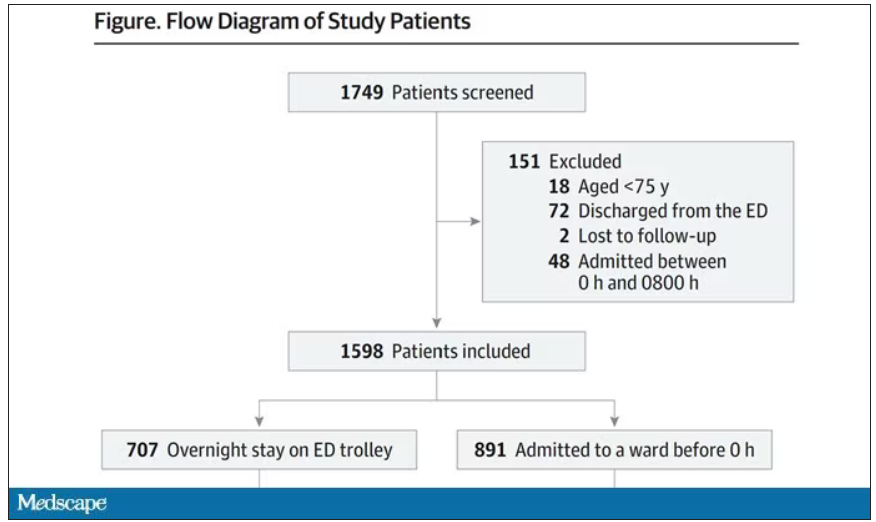
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.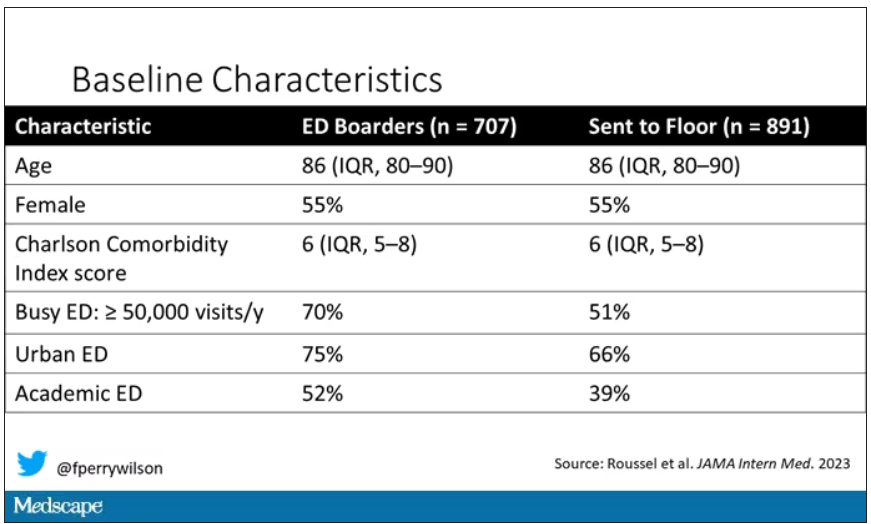
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Pervasive ‘forever chemicals’ linked to thyroid cancer?
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM EBIOMEDICINE
The placebo effect
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As I noted in my last column, I recently had a generic cold.
One of the more irritating aspects is that I usually get a cough that lasts a few weeks afterwards, and, like most people, I try to do something about it. So I load up on various over-the-counter remedies.
I have no idea if they work, or if I’m shelling out for a placebo. I’m not alone in buying these, or they wouldn’t be on the market, or making money, at all.
But the placebo effect is pretty strong. Phenylephrine has been around since 1938. It’s sold on its own and is an ingredient in almost every anti-cough/cold combination medication out there (NyQuil, DayQuil, Robitussin Multi-Symptom, and their many generic store brands). Millions of people use it every year.
Yet, after sifting through piles of accumulated data, the Food and Drug Administration announced earlier this year that phenylephrine ... doesn’t do anything. Zip. Zero. Nada. When compared with a placebo in controlled trials, you couldn’t tell the difference between them. So now the use of it is being questioned. CVS has started pulling it off their shelves, and I suspect other pharmacies will follow.
But back to my cough. A time-honored tradition in American childhood is having to cram down Robitussin and gagging from its nasty taste (the cherry and orange flavoring don’t make a difference, it tastes terrible no matter what you do). So that gets ingrained into us, and to this day I, and most adults, reach for a bottle of dextromethorphan when they have a cough.
But the evidence for that is spotty, too. Several studies have shown equivocal, if any, evidence to suggest it helps with coughs, though others have shown some. Nothing really amazing though.
But we still buy it by the gallon when we’re sick, because we want something, anything, that will make us better. Even if we’re doing so more from hope than conviction.
There’s also the old standby of cough drops, which have been used for more than 3,000 years. Ingredients vary, but menthol is probably the most common one. I go through those, too. I keep a bag in my desk at work. In medical school, during cold season, it was in my backpack. I remember sitting in the Creighton library to study, quietly sucking on a lozenge to keep my cough from disturbing other students.
But even then, the evidence is iffy as to whether they do anything. In fact, one interesting (though small) study in 2018 suggested they may actually prolong coughs.
The fact is that we are all susceptible to the placebo effect, regardless of how much we know about illness and medication. Maybe these things work, maybe they don’t, but it’s a valid question. How often do we let wishful thinking beat objective data?
Probably more often than we want to admit.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
No longer a death sentence, HIV diagnosis still hits hard
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.






