User login
Case study suggests maternal inheritance of PBC susceptibility
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
FROM THE WORLD JOURNAL OF GASTROENTEROLOGY
Does Anyone Really Understand Nutrition Labels?
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.
FDA approves first treatment for ECD
The US Food and Drug Administration (FDA) has expanded the approved use of vemurafenib (Zelboraf) to include the treatment of adults who have Erdheim-Chester disease (ECD) with BRAF V600 mutation.
Vemurafenib is a kinase inhibitor designed to inhibit some mutated forms of BRAF.
The drug was already approved by the FDA to treat patients with unresectable or metastatic melanoma with BRAF V600E mutation, as detected by an FDA-approved test.
Now, vemurafenib is the first FDA-approved treatment for ECD.
The FDA previously granted vemurafenib orphan drug and breakthrough therapy designations for this indication, and the supplemental new drug application for vemurafenib in ECD received priority review.
“Today’s approval of Zelboraf for patients with ECD demonstrates how we can apply knowledge of the underlying genetic characteristics of certain malignancies to other cancers,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“This product was first approved in 2011 to treat certain patients with melanoma that harbor the BRAF V600E mutation, and we are now bringing the therapy to patients with a rare cancer with no approved therapies.”
The application for vemurafenib in ECD was supported by data from the phase 2 VE-BASKET study. Initial results from this study were published in NEJM in August 2015.
VE-BASKET was designed to investigate the use of vemurafenib in patients with BRAF V600 mutation-positive diseases, including ECD.
In the 22 patients with ECD, the best overall response rate was 54.5%. Eleven patients experienced a partial response, and 1 patient achieved a complete response.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up of 26.6 months.
The most common adverse events (>50%) were joint pain, rash, hair loss, fatigue, change in heart rhythm, and skin tags. The most common grade 3 or higher adverse events (≥10%) were new skin cancers, high blood pressure, rash, and joint pain. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of vemurafenib (Zelboraf) to include the treatment of adults who have Erdheim-Chester disease (ECD) with BRAF V600 mutation.
Vemurafenib is a kinase inhibitor designed to inhibit some mutated forms of BRAF.
The drug was already approved by the FDA to treat patients with unresectable or metastatic melanoma with BRAF V600E mutation, as detected by an FDA-approved test.
Now, vemurafenib is the first FDA-approved treatment for ECD.
The FDA previously granted vemurafenib orphan drug and breakthrough therapy designations for this indication, and the supplemental new drug application for vemurafenib in ECD received priority review.
“Today’s approval of Zelboraf for patients with ECD demonstrates how we can apply knowledge of the underlying genetic characteristics of certain malignancies to other cancers,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“This product was first approved in 2011 to treat certain patients with melanoma that harbor the BRAF V600E mutation, and we are now bringing the therapy to patients with a rare cancer with no approved therapies.”
The application for vemurafenib in ECD was supported by data from the phase 2 VE-BASKET study. Initial results from this study were published in NEJM in August 2015.
VE-BASKET was designed to investigate the use of vemurafenib in patients with BRAF V600 mutation-positive diseases, including ECD.
In the 22 patients with ECD, the best overall response rate was 54.5%. Eleven patients experienced a partial response, and 1 patient achieved a complete response.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up of 26.6 months.
The most common adverse events (>50%) were joint pain, rash, hair loss, fatigue, change in heart rhythm, and skin tags. The most common grade 3 or higher adverse events (≥10%) were new skin cancers, high blood pressure, rash, and joint pain. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of vemurafenib (Zelboraf) to include the treatment of adults who have Erdheim-Chester disease (ECD) with BRAF V600 mutation.
Vemurafenib is a kinase inhibitor designed to inhibit some mutated forms of BRAF.
The drug was already approved by the FDA to treat patients with unresectable or metastatic melanoma with BRAF V600E mutation, as detected by an FDA-approved test.
Now, vemurafenib is the first FDA-approved treatment for ECD.
The FDA previously granted vemurafenib orphan drug and breakthrough therapy designations for this indication, and the supplemental new drug application for vemurafenib in ECD received priority review.
“Today’s approval of Zelboraf for patients with ECD demonstrates how we can apply knowledge of the underlying genetic characteristics of certain malignancies to other cancers,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“This product was first approved in 2011 to treat certain patients with melanoma that harbor the BRAF V600E mutation, and we are now bringing the therapy to patients with a rare cancer with no approved therapies.”
The application for vemurafenib in ECD was supported by data from the phase 2 VE-BASKET study. Initial results from this study were published in NEJM in August 2015.
VE-BASKET was designed to investigate the use of vemurafenib in patients with BRAF V600 mutation-positive diseases, including ECD.
In the 22 patients with ECD, the best overall response rate was 54.5%. Eleven patients experienced a partial response, and 1 patient achieved a complete response.
The median duration of response, progression-free survival, and overall survival were not reached at a median follow-up of 26.6 months.
The most common adverse events (>50%) were joint pain, rash, hair loss, fatigue, change in heart rhythm, and skin tags. The most common grade 3 or higher adverse events (≥10%) were new skin cancers, high blood pressure, rash, and joint pain. ![]()
Ibrutinib sustains efficacy in CLL at 4-year follow-up
NEW YORK, NY—The 4-year follow-up of the RESONATE trial suggests ibrutinib may provide long-term efficacy in previously treated patients with chronic lymphocytic leukemia (CLL).
The median progression-free survival (PFS) has not yet been reached in this trial, regardless of high-risk cytogenetics, according to Jennifer Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
She presented the update at Lymphoma & Myeloma 2017. The follow-up study was awarded the best clinical CLL abstract of the meeting.
In the phase 3 RESONATE study, investigators compared ibrutinib—the first-in-class, once-daily, oral inhibitor of Bruton tyrosine kinase—to ofatumumab in previously treated CLL/small lymphocytic lymphoma (SLL).
The primary analysis showed ibrutinib significantly improved survival, with a 78% reduction in the risk of progression and a 57% reduction in the risk of death.
The phase 3 trial randomized 195 CLL/SLL patients to oral ibrutinib at 420 mg once daily and 196 patients to intravenous ofatumumab at an initial dose of 300 mg followed by 2000 mg for 11 doses over 24 weeks.
One hundred thirty-three patients progressed on ofatumumab and crossed over to receive once-daily ibrutinib.
Patient characteristics
In each arm, the median patient age was 67, more than half of patients had an ECOG status of 1, and more than half had advanced-stage disease.
High-risk genetic abnormalities were common, Dr Brown said, with deletion 11q in a third of patients in the ibrutinib arm and 31% in the ofatumumab arm. Another third in each arm had deletion 17p, while 51% in the ibrutinib arm and 46% in the ofatumumab arm had TP53 mutation.
About a quarter of the patients in each arm had complex karyotype, and 73% and 63% in the ibrutinib and ofatumumab arms, respectively, were IGHV-unmutated.
Survival
Ibrutinib significantly extended PFS compared with ofatumumab. At a median follow-up for ibrutinib of 44 months (range, 0.33 – 53), ibrutinib led to an 87% reduction in the risk of progression or death. The 3-year PFS rate was 59% with ibrutinib and 3% with ofatumumab.
Ibrutinib conferred a benefit in PFS across all baseline patient characteristics.
Among ibrutinib-treated patients, the 3-year PFS was 53% for patients with deletion 17p, 66% for those with deletion 11q but not deletion 17p, and 58% for those with neither abnormality.
Dr Brown noted how closely complex karyotype associates with high-risk cytogenetics. Forty-two percent of patients with 17p deletion had a complex karyotype, as did 23% of patients with 11q deletion and 15% of patients with neither 17p nor 11q deletion.
For IGHV-mutation status, Dr Brown said there is no difference in PFS with this degree of follow-up.
In terms of TP53 mutation status, Dr Brown pointed out a trend toward a worse PFS in those patients with the mutation.
“We actually looked by individual p53 mutation versus 17p deletion, versus both, versus neither, in the 2-year follow-up paper and found that p53 with 17p, both abnormalities, did have worse PFS than neither,” she said.
“This may require further follow-up because we do know that most 17p patients also have a p53 mutation, particularly in the relapsed setting.”
As expected, Dr Brown said, those patients with more than 2 prior therapies had a worse PFS compared to patients with 2 or fewer prior therapies.
Multivariate analysis demonstrated that more than 2 prior lines of therapy or an elevated ß2 microglobulin were associated with decreased PFS with ibrutinib.
When the investigators adjusted the overall survival data for cross-over, ibrutinib was projected to continue the overall survival benefit compared with ofatumumab, with a hazard ratio of 0.37.
Response rates
Dr Brown noted that, early on, there’s quite a significant rate of partial response with lymphocytosis observed in patients on ibrutinib.
This “diminishes dramatically,” she said, but about 5% of patients at 3 and 4 years still have ongoing lymphocytosis.
“Similarly, initially, there’s a very low rate of complete remission, which has risen steadily to 9% at this follow-up,” she said.
And the overall response rate is 91%.
Treatment exposure and toxicity
The median duration of ibrutinib treatment is 41 months, and 46% of patients continue on treatment. Twenty-seven percent of patients discontinued due to progression, and 12% because of adverse events (AEs).
Of the 53 patients who discontinued therapy, 14 had transformation as their primary reason, 9 with diffuse large B-cell lymphoma, 3 with Hodgkin disease, and 2 with prolymphocytic lymphoma.
The most frequent AEs leading to discontinuation included pneumonia (n=3), anemia (n=2), thrombocytopenia (n=2), diarrhea (n=2), and anal incontinence (n=2).
AEs leading to discontinuation decreased over time—6% in year 0 to 1 and 4% in years 2 to 3.
“The most frequent cumulative AEs are similar to what we’ve seen in most prior studies,” Dr Brown said, including diarrhea, fatigue, and cough.
In terms of grade 3 or higher AEs, about a quarter of patients had neutropenia, 17% had pneumonia, and 8% had hypertension.
Six percent of patients had major hemorrhage, and all-grade atrial fibrillation occurred in 11% of patients.
“Now, many of the grade 3 and higher AEs did decline over time during the study,” Dr Brown noted. “You can see this is quite evident for neutropenia as well as pneumonia, and all infections declined from year 1 to subsequent years.”
Hypertension, in contrast, has been fairly steady over the later years, she said, and atrial fibrillation is highest in the first 6 months but then continues at a low rate thereafter.
The investigators believe these long-term results demonstrate that ibrutinib is tolerable and continues to show sustained efficacy in previously treated and high-genomic-risk patients with CLL. In addition, no long-term safety signals have emerged.
This study was sponsored by Pharmacyclics, LLC, an AbbVie company. ![]()
NEW YORK, NY—The 4-year follow-up of the RESONATE trial suggests ibrutinib may provide long-term efficacy in previously treated patients with chronic lymphocytic leukemia (CLL).
The median progression-free survival (PFS) has not yet been reached in this trial, regardless of high-risk cytogenetics, according to Jennifer Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
She presented the update at Lymphoma & Myeloma 2017. The follow-up study was awarded the best clinical CLL abstract of the meeting.
In the phase 3 RESONATE study, investigators compared ibrutinib—the first-in-class, once-daily, oral inhibitor of Bruton tyrosine kinase—to ofatumumab in previously treated CLL/small lymphocytic lymphoma (SLL).
The primary analysis showed ibrutinib significantly improved survival, with a 78% reduction in the risk of progression and a 57% reduction in the risk of death.
The phase 3 trial randomized 195 CLL/SLL patients to oral ibrutinib at 420 mg once daily and 196 patients to intravenous ofatumumab at an initial dose of 300 mg followed by 2000 mg for 11 doses over 24 weeks.
One hundred thirty-three patients progressed on ofatumumab and crossed over to receive once-daily ibrutinib.
Patient characteristics
In each arm, the median patient age was 67, more than half of patients had an ECOG status of 1, and more than half had advanced-stage disease.
High-risk genetic abnormalities were common, Dr Brown said, with deletion 11q in a third of patients in the ibrutinib arm and 31% in the ofatumumab arm. Another third in each arm had deletion 17p, while 51% in the ibrutinib arm and 46% in the ofatumumab arm had TP53 mutation.
About a quarter of the patients in each arm had complex karyotype, and 73% and 63% in the ibrutinib and ofatumumab arms, respectively, were IGHV-unmutated.
Survival
Ibrutinib significantly extended PFS compared with ofatumumab. At a median follow-up for ibrutinib of 44 months (range, 0.33 – 53), ibrutinib led to an 87% reduction in the risk of progression or death. The 3-year PFS rate was 59% with ibrutinib and 3% with ofatumumab.
Ibrutinib conferred a benefit in PFS across all baseline patient characteristics.
Among ibrutinib-treated patients, the 3-year PFS was 53% for patients with deletion 17p, 66% for those with deletion 11q but not deletion 17p, and 58% for those with neither abnormality.
Dr Brown noted how closely complex karyotype associates with high-risk cytogenetics. Forty-two percent of patients with 17p deletion had a complex karyotype, as did 23% of patients with 11q deletion and 15% of patients with neither 17p nor 11q deletion.
For IGHV-mutation status, Dr Brown said there is no difference in PFS with this degree of follow-up.
In terms of TP53 mutation status, Dr Brown pointed out a trend toward a worse PFS in those patients with the mutation.
“We actually looked by individual p53 mutation versus 17p deletion, versus both, versus neither, in the 2-year follow-up paper and found that p53 with 17p, both abnormalities, did have worse PFS than neither,” she said.
“This may require further follow-up because we do know that most 17p patients also have a p53 mutation, particularly in the relapsed setting.”
As expected, Dr Brown said, those patients with more than 2 prior therapies had a worse PFS compared to patients with 2 or fewer prior therapies.
Multivariate analysis demonstrated that more than 2 prior lines of therapy or an elevated ß2 microglobulin were associated with decreased PFS with ibrutinib.
When the investigators adjusted the overall survival data for cross-over, ibrutinib was projected to continue the overall survival benefit compared with ofatumumab, with a hazard ratio of 0.37.
Response rates
Dr Brown noted that, early on, there’s quite a significant rate of partial response with lymphocytosis observed in patients on ibrutinib.
This “diminishes dramatically,” she said, but about 5% of patients at 3 and 4 years still have ongoing lymphocytosis.
“Similarly, initially, there’s a very low rate of complete remission, which has risen steadily to 9% at this follow-up,” she said.
And the overall response rate is 91%.
Treatment exposure and toxicity
The median duration of ibrutinib treatment is 41 months, and 46% of patients continue on treatment. Twenty-seven percent of patients discontinued due to progression, and 12% because of adverse events (AEs).
Of the 53 patients who discontinued therapy, 14 had transformation as their primary reason, 9 with diffuse large B-cell lymphoma, 3 with Hodgkin disease, and 2 with prolymphocytic lymphoma.
The most frequent AEs leading to discontinuation included pneumonia (n=3), anemia (n=2), thrombocytopenia (n=2), diarrhea (n=2), and anal incontinence (n=2).
AEs leading to discontinuation decreased over time—6% in year 0 to 1 and 4% in years 2 to 3.
“The most frequent cumulative AEs are similar to what we’ve seen in most prior studies,” Dr Brown said, including diarrhea, fatigue, and cough.
In terms of grade 3 or higher AEs, about a quarter of patients had neutropenia, 17% had pneumonia, and 8% had hypertension.
Six percent of patients had major hemorrhage, and all-grade atrial fibrillation occurred in 11% of patients.
“Now, many of the grade 3 and higher AEs did decline over time during the study,” Dr Brown noted. “You can see this is quite evident for neutropenia as well as pneumonia, and all infections declined from year 1 to subsequent years.”
Hypertension, in contrast, has been fairly steady over the later years, she said, and atrial fibrillation is highest in the first 6 months but then continues at a low rate thereafter.
The investigators believe these long-term results demonstrate that ibrutinib is tolerable and continues to show sustained efficacy in previously treated and high-genomic-risk patients with CLL. In addition, no long-term safety signals have emerged.
This study was sponsored by Pharmacyclics, LLC, an AbbVie company. ![]()
NEW YORK, NY—The 4-year follow-up of the RESONATE trial suggests ibrutinib may provide long-term efficacy in previously treated patients with chronic lymphocytic leukemia (CLL).
The median progression-free survival (PFS) has not yet been reached in this trial, regardless of high-risk cytogenetics, according to Jennifer Brown, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
She presented the update at Lymphoma & Myeloma 2017. The follow-up study was awarded the best clinical CLL abstract of the meeting.
In the phase 3 RESONATE study, investigators compared ibrutinib—the first-in-class, once-daily, oral inhibitor of Bruton tyrosine kinase—to ofatumumab in previously treated CLL/small lymphocytic lymphoma (SLL).
The primary analysis showed ibrutinib significantly improved survival, with a 78% reduction in the risk of progression and a 57% reduction in the risk of death.
The phase 3 trial randomized 195 CLL/SLL patients to oral ibrutinib at 420 mg once daily and 196 patients to intravenous ofatumumab at an initial dose of 300 mg followed by 2000 mg for 11 doses over 24 weeks.
One hundred thirty-three patients progressed on ofatumumab and crossed over to receive once-daily ibrutinib.
Patient characteristics
In each arm, the median patient age was 67, more than half of patients had an ECOG status of 1, and more than half had advanced-stage disease.
High-risk genetic abnormalities were common, Dr Brown said, with deletion 11q in a third of patients in the ibrutinib arm and 31% in the ofatumumab arm. Another third in each arm had deletion 17p, while 51% in the ibrutinib arm and 46% in the ofatumumab arm had TP53 mutation.
About a quarter of the patients in each arm had complex karyotype, and 73% and 63% in the ibrutinib and ofatumumab arms, respectively, were IGHV-unmutated.
Survival
Ibrutinib significantly extended PFS compared with ofatumumab. At a median follow-up for ibrutinib of 44 months (range, 0.33 – 53), ibrutinib led to an 87% reduction in the risk of progression or death. The 3-year PFS rate was 59% with ibrutinib and 3% with ofatumumab.
Ibrutinib conferred a benefit in PFS across all baseline patient characteristics.
Among ibrutinib-treated patients, the 3-year PFS was 53% for patients with deletion 17p, 66% for those with deletion 11q but not deletion 17p, and 58% for those with neither abnormality.
Dr Brown noted how closely complex karyotype associates with high-risk cytogenetics. Forty-two percent of patients with 17p deletion had a complex karyotype, as did 23% of patients with 11q deletion and 15% of patients with neither 17p nor 11q deletion.
For IGHV-mutation status, Dr Brown said there is no difference in PFS with this degree of follow-up.
In terms of TP53 mutation status, Dr Brown pointed out a trend toward a worse PFS in those patients with the mutation.
“We actually looked by individual p53 mutation versus 17p deletion, versus both, versus neither, in the 2-year follow-up paper and found that p53 with 17p, both abnormalities, did have worse PFS than neither,” she said.
“This may require further follow-up because we do know that most 17p patients also have a p53 mutation, particularly in the relapsed setting.”
As expected, Dr Brown said, those patients with more than 2 prior therapies had a worse PFS compared to patients with 2 or fewer prior therapies.
Multivariate analysis demonstrated that more than 2 prior lines of therapy or an elevated ß2 microglobulin were associated with decreased PFS with ibrutinib.
When the investigators adjusted the overall survival data for cross-over, ibrutinib was projected to continue the overall survival benefit compared with ofatumumab, with a hazard ratio of 0.37.
Response rates
Dr Brown noted that, early on, there’s quite a significant rate of partial response with lymphocytosis observed in patients on ibrutinib.
This “diminishes dramatically,” she said, but about 5% of patients at 3 and 4 years still have ongoing lymphocytosis.
“Similarly, initially, there’s a very low rate of complete remission, which has risen steadily to 9% at this follow-up,” she said.
And the overall response rate is 91%.
Treatment exposure and toxicity
The median duration of ibrutinib treatment is 41 months, and 46% of patients continue on treatment. Twenty-seven percent of patients discontinued due to progression, and 12% because of adverse events (AEs).
Of the 53 patients who discontinued therapy, 14 had transformation as their primary reason, 9 with diffuse large B-cell lymphoma, 3 with Hodgkin disease, and 2 with prolymphocytic lymphoma.
The most frequent AEs leading to discontinuation included pneumonia (n=3), anemia (n=2), thrombocytopenia (n=2), diarrhea (n=2), and anal incontinence (n=2).
AEs leading to discontinuation decreased over time—6% in year 0 to 1 and 4% in years 2 to 3.
“The most frequent cumulative AEs are similar to what we’ve seen in most prior studies,” Dr Brown said, including diarrhea, fatigue, and cough.
In terms of grade 3 or higher AEs, about a quarter of patients had neutropenia, 17% had pneumonia, and 8% had hypertension.
Six percent of patients had major hemorrhage, and all-grade atrial fibrillation occurred in 11% of patients.
“Now, many of the grade 3 and higher AEs did decline over time during the study,” Dr Brown noted. “You can see this is quite evident for neutropenia as well as pneumonia, and all infections declined from year 1 to subsequent years.”
Hypertension, in contrast, has been fairly steady over the later years, she said, and atrial fibrillation is highest in the first 6 months but then continues at a low rate thereafter.
The investigators believe these long-term results demonstrate that ibrutinib is tolerable and continues to show sustained efficacy in previously treated and high-genomic-risk patients with CLL. In addition, no long-term safety signals have emerged.
This study was sponsored by Pharmacyclics, LLC, an AbbVie company. ![]()
FDA approves wider use of hematology analyzer
The US Food and Drug Administration (FDA) has expanded the approved use of the XW-100 Automated Hematology Analyzer.
The analyzer can now be used at non-traditional laboratory sites by non-medical personnel.
The XW-100 Automated Hematology Analyzer is intended for use in patients age 2 and older who require a whole blood cell count and white blood cell differential.
Test results can be used with other clinical and laboratory findings to provide early alerts of patients with serious conditions, such as severe anemia and agranulocytosis, who require additional testing.
The XW-100 Automated Hematology Analyzer is not intended to diagnose or monitor patients with primary and/or secondary hematologic diseases.
The device works by using a blood sample to classify and quantify 12 hematology parameters, which provides patients with a blood component profile as part of their overall health assessment.
Expanded clearance
The FDA granted the XW-100 Automated Hematology Analyzer a waiver under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The waiver allows the device to be used by a variety of non-traditional laboratory sites, including physicians’ offices, clinics, or other types of healthcare facilities with a CLIA Certificate of Waiver.
The XW-100 Automated Hematology Analyzer was reviewed through the dual submission pathway, a streamlined regulatory pathway for 510(k) marketing clearance and CLIA Waiver by Application.
A 510(k) notification is a premarket submission made by device manufacturers to the FDA to demonstrate that the new device is substantially equivalent to a legally marketed predicate device.
The XW-100 Automated Hematology Analyzer was originally cleared through the 510(k) pathway in 2015 for use at the patient’s point-of-care.
To support the use of this device in CLIA-waived settings with non-medical personnel, the analyzer is now accompanied by simple instructions for operator actions when results are flagged or outside of a specified range.
To further ensure accurate testing in this setting and to eliminate results that are most susceptible to inaccuracy or require additional testing, the number of hematology parameters has been reduced to 12.
The FDA found this modified version of the XW-100 Automated Hematology Analyzer to be substantially equivalent to the 2015 model.
In addition, data submitted by Sysmex America, Inc. (the company marketing the analyzer) demonstrated ease of use and a low risk of false results when the modified XW-100 Automated Hematology Analyzer was used by untrained operators.
The FDA reviewed data from a study conducted on 582 samples collected from patients ages 2 to 92.
In this study, researchers compared XW-100 Automated Hematology Analyzer results collected by non-medical personnel in CLIA-waived settings to results from a hematology analyzer in an accredited clinical laboratory.
Results showed that, by following the manufacturer’s instructions for use, accurate testing can be effectively conducted by untrained personnel. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of the XW-100 Automated Hematology Analyzer.
The analyzer can now be used at non-traditional laboratory sites by non-medical personnel.
The XW-100 Automated Hematology Analyzer is intended for use in patients age 2 and older who require a whole blood cell count and white blood cell differential.
Test results can be used with other clinical and laboratory findings to provide early alerts of patients with serious conditions, such as severe anemia and agranulocytosis, who require additional testing.
The XW-100 Automated Hematology Analyzer is not intended to diagnose or monitor patients with primary and/or secondary hematologic diseases.
The device works by using a blood sample to classify and quantify 12 hematology parameters, which provides patients with a blood component profile as part of their overall health assessment.
Expanded clearance
The FDA granted the XW-100 Automated Hematology Analyzer a waiver under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The waiver allows the device to be used by a variety of non-traditional laboratory sites, including physicians’ offices, clinics, or other types of healthcare facilities with a CLIA Certificate of Waiver.
The XW-100 Automated Hematology Analyzer was reviewed through the dual submission pathway, a streamlined regulatory pathway for 510(k) marketing clearance and CLIA Waiver by Application.
A 510(k) notification is a premarket submission made by device manufacturers to the FDA to demonstrate that the new device is substantially equivalent to a legally marketed predicate device.
The XW-100 Automated Hematology Analyzer was originally cleared through the 510(k) pathway in 2015 for use at the patient’s point-of-care.
To support the use of this device in CLIA-waived settings with non-medical personnel, the analyzer is now accompanied by simple instructions for operator actions when results are flagged or outside of a specified range.
To further ensure accurate testing in this setting and to eliminate results that are most susceptible to inaccuracy or require additional testing, the number of hematology parameters has been reduced to 12.
The FDA found this modified version of the XW-100 Automated Hematology Analyzer to be substantially equivalent to the 2015 model.
In addition, data submitted by Sysmex America, Inc. (the company marketing the analyzer) demonstrated ease of use and a low risk of false results when the modified XW-100 Automated Hematology Analyzer was used by untrained operators.
The FDA reviewed data from a study conducted on 582 samples collected from patients ages 2 to 92.
In this study, researchers compared XW-100 Automated Hematology Analyzer results collected by non-medical personnel in CLIA-waived settings to results from a hematology analyzer in an accredited clinical laboratory.
Results showed that, by following the manufacturer’s instructions for use, accurate testing can be effectively conducted by untrained personnel. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of the XW-100 Automated Hematology Analyzer.
The analyzer can now be used at non-traditional laboratory sites by non-medical personnel.
The XW-100 Automated Hematology Analyzer is intended for use in patients age 2 and older who require a whole blood cell count and white blood cell differential.
Test results can be used with other clinical and laboratory findings to provide early alerts of patients with serious conditions, such as severe anemia and agranulocytosis, who require additional testing.
The XW-100 Automated Hematology Analyzer is not intended to diagnose or monitor patients with primary and/or secondary hematologic diseases.
The device works by using a blood sample to classify and quantify 12 hematology parameters, which provides patients with a blood component profile as part of their overall health assessment.
Expanded clearance
The FDA granted the XW-100 Automated Hematology Analyzer a waiver under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The waiver allows the device to be used by a variety of non-traditional laboratory sites, including physicians’ offices, clinics, or other types of healthcare facilities with a CLIA Certificate of Waiver.
The XW-100 Automated Hematology Analyzer was reviewed through the dual submission pathway, a streamlined regulatory pathway for 510(k) marketing clearance and CLIA Waiver by Application.
A 510(k) notification is a premarket submission made by device manufacturers to the FDA to demonstrate that the new device is substantially equivalent to a legally marketed predicate device.
The XW-100 Automated Hematology Analyzer was originally cleared through the 510(k) pathway in 2015 for use at the patient’s point-of-care.
To support the use of this device in CLIA-waived settings with non-medical personnel, the analyzer is now accompanied by simple instructions for operator actions when results are flagged or outside of a specified range.
To further ensure accurate testing in this setting and to eliminate results that are most susceptible to inaccuracy or require additional testing, the number of hematology parameters has been reduced to 12.
The FDA found this modified version of the XW-100 Automated Hematology Analyzer to be substantially equivalent to the 2015 model.
In addition, data submitted by Sysmex America, Inc. (the company marketing the analyzer) demonstrated ease of use and a low risk of false results when the modified XW-100 Automated Hematology Analyzer was used by untrained operators.
The FDA reviewed data from a study conducted on 582 samples collected from patients ages 2 to 92.
In this study, researchers compared XW-100 Automated Hematology Analyzer results collected by non-medical personnel in CLIA-waived settings to results from a hematology analyzer in an accredited clinical laboratory.
Results showed that, by following the manufacturer’s instructions for use, accurate testing can be effectively conducted by untrained personnel. ![]()
Intervention improves well-being in AYAs with cancer
SAN DIEGO—New research suggests an intervention can improve psychosocial health in adolescents and young adults (AYAs) living with cancer.
The intervention, Promoting Resilience in Stress Management (PRISM), is designed to help patients manage stress, set goals, and change their perspective.
Overall, PRISM improved resilience, enhanced quality of life, increased hope, and lowered distress and depression in the patients studied.
Abby R. Rosenberg, MD, of Seattle Children’s Research Institute in Seattle, Washington, presented these results at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 176*).
“The experience of cancer is stressful in all realms, but we tend to focus more on physical symptoms than the equally important social and emotional challenges,” Dr Rosenberg said.
“This is particularly true for adolescents and young adults who already struggle with normal developmental changes. When you throw cancer into the mix, it can become much harder.”
With this in mind, Dr Rosenberg and her colleagues tested PRISM in AYAs with cancer. The trial included 99 English-speaking patients, ages 12 to 25, who were diagnosed with new or newly recurrent cancer.
The patients were randomized to receive PRISM (n=49) plus standard psychosocial supportive care or standard care alone (n=50). Standard care at Seattle Children’s Research Institute includes a dedicated social worker and access to psychologists, child-life specialists, and other experts in AYA oncology care, as needed.
PRISM targets 4 topics:
- Managing stress with skills based on mindfulness and relaxation
- Setting goals that are specific and realistic, as well as planning for roadblocks
- Positive reframing, or recognizing and replacing negative self-talk
- Making meaning, or identifying benefits, gratitude, purpose, and legacy.
Each of the 4 topics were discussed with patients in separate, one-on-one sessions with a trained research associate. The sessions lasted 30 minutes to an hour. Patients also received boosters and worksheets for practicing the skills discussed in the meetings.
After all 4 sessions had been completed, patients could participate in an optional family meeting. During this meeting, patients could discuss with their family members which aspects of PRISM worked.
Results
Patients completed surveys at study enrollment, 2 months, 4 months, and 6 months. There were 74 participants who were still alive and well enough to complete the 6-month survey—36 in the PRISM group and 38 in the control group.
At the 6-month mark, PRISM was associated with (sometimes significant) improvements in resilience (P=0.02), generic quality of life (P=0.08), cancer-specific quality of life (P=0.01), hope (P=0.34), and distress (P=0.03). (P values are for absolute difference from baseline to 6 months.)
In addition, the incidence of depression at 6 months was lower in the PRISM group than the control group—6% and 21%, respectively (odds ratio=0.09, 95% CI 0.01, 1.09).
All but 4 of the PRISM recipients chose to participate in the family meeting following their one-on-one sessions.
“We included the family meeting because teens told us they wanted to share with their parents, and parents told us they wanted to know what their children had learned,” Dr Rosenberg said. “While the specific impact of this meeting is yet to be determined, we hope it will guide families so that there is continued support of teen or young adult patients.”
Now, Dr Rosenberg and her colleagues would like to test PRISM in other patient populations.
“We need to include a much larger cultural demographic in future studies,” Dr Rosenberg noted. “Beyond that, we also need to determine if this type of intervention could translate to other centers where usual care may not be as comprehensive as what we have here.” ![]()
*Some data in the abstract differ from the presentation.
SAN DIEGO—New research suggests an intervention can improve psychosocial health in adolescents and young adults (AYAs) living with cancer.
The intervention, Promoting Resilience in Stress Management (PRISM), is designed to help patients manage stress, set goals, and change their perspective.
Overall, PRISM improved resilience, enhanced quality of life, increased hope, and lowered distress and depression in the patients studied.
Abby R. Rosenberg, MD, of Seattle Children’s Research Institute in Seattle, Washington, presented these results at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 176*).
“The experience of cancer is stressful in all realms, but we tend to focus more on physical symptoms than the equally important social and emotional challenges,” Dr Rosenberg said.
“This is particularly true for adolescents and young adults who already struggle with normal developmental changes. When you throw cancer into the mix, it can become much harder.”
With this in mind, Dr Rosenberg and her colleagues tested PRISM in AYAs with cancer. The trial included 99 English-speaking patients, ages 12 to 25, who were diagnosed with new or newly recurrent cancer.
The patients were randomized to receive PRISM (n=49) plus standard psychosocial supportive care or standard care alone (n=50). Standard care at Seattle Children’s Research Institute includes a dedicated social worker and access to psychologists, child-life specialists, and other experts in AYA oncology care, as needed.
PRISM targets 4 topics:
- Managing stress with skills based on mindfulness and relaxation
- Setting goals that are specific and realistic, as well as planning for roadblocks
- Positive reframing, or recognizing and replacing negative self-talk
- Making meaning, or identifying benefits, gratitude, purpose, and legacy.
Each of the 4 topics were discussed with patients in separate, one-on-one sessions with a trained research associate. The sessions lasted 30 minutes to an hour. Patients also received boosters and worksheets for practicing the skills discussed in the meetings.
After all 4 sessions had been completed, patients could participate in an optional family meeting. During this meeting, patients could discuss with their family members which aspects of PRISM worked.
Results
Patients completed surveys at study enrollment, 2 months, 4 months, and 6 months. There were 74 participants who were still alive and well enough to complete the 6-month survey—36 in the PRISM group and 38 in the control group.
At the 6-month mark, PRISM was associated with (sometimes significant) improvements in resilience (P=0.02), generic quality of life (P=0.08), cancer-specific quality of life (P=0.01), hope (P=0.34), and distress (P=0.03). (P values are for absolute difference from baseline to 6 months.)
In addition, the incidence of depression at 6 months was lower in the PRISM group than the control group—6% and 21%, respectively (odds ratio=0.09, 95% CI 0.01, 1.09).
All but 4 of the PRISM recipients chose to participate in the family meeting following their one-on-one sessions.
“We included the family meeting because teens told us they wanted to share with their parents, and parents told us they wanted to know what their children had learned,” Dr Rosenberg said. “While the specific impact of this meeting is yet to be determined, we hope it will guide families so that there is continued support of teen or young adult patients.”
Now, Dr Rosenberg and her colleagues would like to test PRISM in other patient populations.
“We need to include a much larger cultural demographic in future studies,” Dr Rosenberg noted. “Beyond that, we also need to determine if this type of intervention could translate to other centers where usual care may not be as comprehensive as what we have here.” ![]()
*Some data in the abstract differ from the presentation.
SAN DIEGO—New research suggests an intervention can improve psychosocial health in adolescents and young adults (AYAs) living with cancer.
The intervention, Promoting Resilience in Stress Management (PRISM), is designed to help patients manage stress, set goals, and change their perspective.
Overall, PRISM improved resilience, enhanced quality of life, increased hope, and lowered distress and depression in the patients studied.
Abby R. Rosenberg, MD, of Seattle Children’s Research Institute in Seattle, Washington, presented these results at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 176*).
“The experience of cancer is stressful in all realms, but we tend to focus more on physical symptoms than the equally important social and emotional challenges,” Dr Rosenberg said.
“This is particularly true for adolescents and young adults who already struggle with normal developmental changes. When you throw cancer into the mix, it can become much harder.”
With this in mind, Dr Rosenberg and her colleagues tested PRISM in AYAs with cancer. The trial included 99 English-speaking patients, ages 12 to 25, who were diagnosed with new or newly recurrent cancer.
The patients were randomized to receive PRISM (n=49) plus standard psychosocial supportive care or standard care alone (n=50). Standard care at Seattle Children’s Research Institute includes a dedicated social worker and access to psychologists, child-life specialists, and other experts in AYA oncology care, as needed.
PRISM targets 4 topics:
- Managing stress with skills based on mindfulness and relaxation
- Setting goals that are specific and realistic, as well as planning for roadblocks
- Positive reframing, or recognizing and replacing negative self-talk
- Making meaning, or identifying benefits, gratitude, purpose, and legacy.
Each of the 4 topics were discussed with patients in separate, one-on-one sessions with a trained research associate. The sessions lasted 30 minutes to an hour. Patients also received boosters and worksheets for practicing the skills discussed in the meetings.
After all 4 sessions had been completed, patients could participate in an optional family meeting. During this meeting, patients could discuss with their family members which aspects of PRISM worked.
Results
Patients completed surveys at study enrollment, 2 months, 4 months, and 6 months. There were 74 participants who were still alive and well enough to complete the 6-month survey—36 in the PRISM group and 38 in the control group.
At the 6-month mark, PRISM was associated with (sometimes significant) improvements in resilience (P=0.02), generic quality of life (P=0.08), cancer-specific quality of life (P=0.01), hope (P=0.34), and distress (P=0.03). (P values are for absolute difference from baseline to 6 months.)
In addition, the incidence of depression at 6 months was lower in the PRISM group than the control group—6% and 21%, respectively (odds ratio=0.09, 95% CI 0.01, 1.09).
All but 4 of the PRISM recipients chose to participate in the family meeting following their one-on-one sessions.
“We included the family meeting because teens told us they wanted to share with their parents, and parents told us they wanted to know what their children had learned,” Dr Rosenberg said. “While the specific impact of this meeting is yet to be determined, we hope it will guide families so that there is continued support of teen or young adult patients.”
Now, Dr Rosenberg and her colleagues would like to test PRISM in other patient populations.
“We need to include a much larger cultural demographic in future studies,” Dr Rosenberg noted. “Beyond that, we also need to determine if this type of intervention could translate to other centers where usual care may not be as comprehensive as what we have here.” ![]()
*Some data in the abstract differ from the presentation.
Rates, predictors, and variability of interhospital transfers
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Insulin Pump Therapy: Who, Why, and How
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
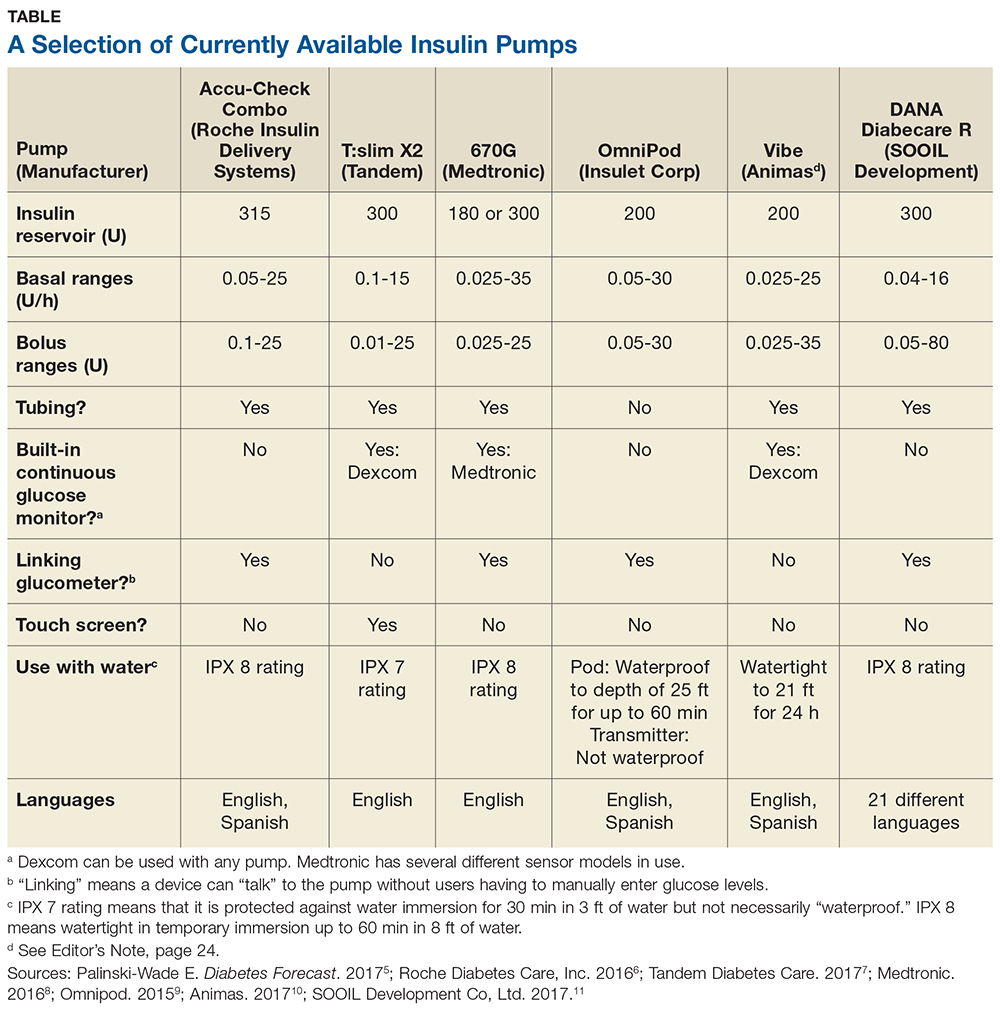
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12

Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12

Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
Moderate psoriasis: the new frontier for systemic therapies
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Roughly half of apremilast-treated patients with moderate psoriasis as defined by an involved body surface area of 5%-10% experienced a 50% reduction in a novel outcome metric: the product of the Physician’s Global Assessment plus the involved body surface area.
Data source: This 52-week study of 221 patients with truly moderate psoriasis featured a 16-week, double-blind, placebo-controlled phase, after which everyone continued on open-label apremilast out to 51 weeks.
Disclosures: The UNVEIL study was sponsored by Celgene. The presenter reported receiving research funding from and serving as a consultant to that company and numerous others.
Red Scaly Rash Following Tattoo Application
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.

A 26-year-old man presented with a mildly pruritic red scaly rash on the right arm of 3 weeks' duration. He reported having a tattoo placed on previously normal skin on the right lateral arm prior to the development of the rash. Two weeks after receiving the tattoo, he developed scaling and redness of the skin involved in the tattoo. He also had similar papules and plaques over the rest of his body. Physical examination showed well-demarcated, erythematous, scaly papules and plaques following the design of a black-pigmented tattoo on the lateral aspect of the right arm. There also were similar erythematous scaly plaques scattered over both arms and the trunk. He denied any pain or blister formation of the involved areas.