User login
CMS finalizes measures to help combat opioid crisis
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
From weekend warriors to pros, athletes are plagued by skin disorders
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
EXPERT ANALYSIS FROM AAD 18
Ari Green, MD
Statins, ACE inhibitors linked to fetal cardiac anomalies
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
ORLANDO – Women exposed to statin treatment during the first trimester of pregnancy had a doubled rate of delivering neonates with a cardiac anomaly, and a nearly 400% increased rate of delivering a baby with a ventricular septal defect, compared with infants born to unexposed women in a case-control review of nearly 400,000 U.S. births during 2003-2014.
A similar, parallel analysis of the same cohort also showed that exposure to an ACE inhibitor at any time during pregnancy linked with roughly tripled rates of premature delivery, low birth weight, and neonatal cardiac anomaly, compared with unexposed women, Ming-Sum Lee, MD, and her associates reported in two posters presented at the annual meeting of the American College of Cardiology.
For the statin analysis the researchers reviewed data collected from 379,238 singleton pregnancies delivered during January 2003-December 2014 to women who received their health care from Kaiser Permanente of Southern California. The cohort included 280 women who filled at least one prescription for a statin during their first trimester of pregnancy. Half the women received simvastatin, and 37% received lovastatin. The researchers used propensity score matching to identify 1,160 women with no statin exposure who closely matched 279 of the women with statin exposure.
The review showed a 2.1% incidence of fetal cardiac anomalies in the infants born to the unexposed women and a 5.0% rate among the exposed women; the hazard ratio was 2.5, which was statistically significant. More detailed analysis showed that the increased incidence of cardiac anomalies was primarily caused by ventricular septal defects, which occurred at a 4.3% rate among the infants born to exposed mothers, a rate 370% higher than among the unexposed pregnancies. No other types of cardiac anomaly examined showed a significant increase among the exposed infants.
Assessment of links with ACE-inhibitor use focused on 404 women who had exposure to the drug class at any time during pregnancy. The most commonly used drug was lisinopril, by 98% of the women. The researchers compared these links against all the other women with exposure to an ACE inhibitor who delivered in the database without propensity score matching or in general any adjustment for clinical features or comorbidities. The analysis showed premature birth (less than 37 weeks’ gestational age) occurred at a 24% rate among the ACE inhibitor–exposed infants and 8% of the unexposed; low birth weight (less than 2,500 g) occurred in 15% of the exposed infants and in 5% of those not exposed, and any type of cardiac anomaly occurred in 4.5% of the exposed neonates and in 1.4% of the unexposed.
Dr. Lee and her associates reported the results of one adjusted analysis that factored maternal comorbidities into the calculation of the relative risk for delivering a neonate with any cardiac anomaly. After adjustment, the incremental risk linked with ACE inhibitor exposure any time during gestation was a statistically significant 80% increase.
Dr. Lee had no disclosures.
SOURCES: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
REPORTING FROM ACC 18
Key clinical point: Fetal exposures to statins or ACE inhibitors link to cardiac anomalies.
Major finding: Ventricular septal defects occurred 370% more often among infants born after first-trimester statin exposure.
Study details: A retrospective review of 379,238 singleton pregnancies delivered at Kaiser Permanente of Southern California during 2003-2014.
Disclosures: Dr. Lee had no disclosures.
Sources: Hekimian A et al. ACC 18, Poster 1124-366. Chintamaneni S et al. ACC 18, Poster 1124-365.
Open enrollment 2018: Plan selections down slightly
the Centers for Medicare & Medicaid Services reported.
That’s a drop of 3.3%, compared with the 12.2 million enrollees for 2017, and a drop of 7.1% from the peak year of 2016, according to the 2018 open enrollment final report.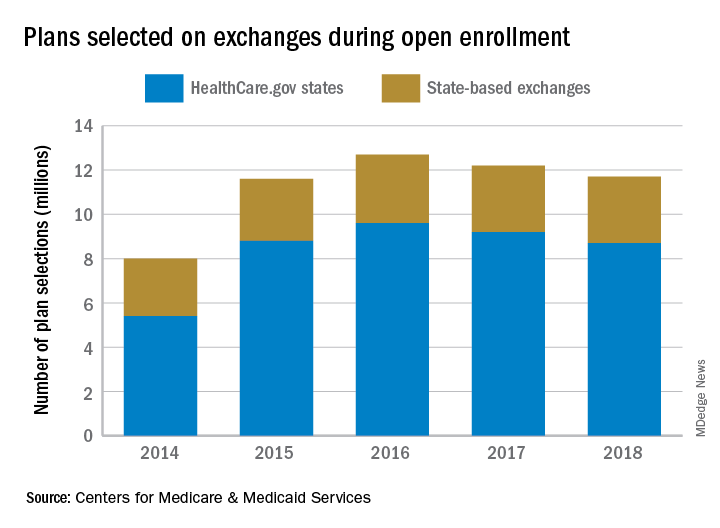
For 2018, the average premium before application of the premium tax credit was $621 in the HealthCare.gov states, which is an increase of 30% from the $476 average for 2017. After the tax credit, the average premium for 2018 was $153.
“Our primary goal this year was to ensure that Americans who wanted coverage through the exchange had a seamless experience,” CMS Administrator Seema Verma said in a statement. “We are pleased that consumer satisfaction was the highest it’s ever been during the 2018 open enrollment period.”
the Centers for Medicare & Medicaid Services reported.
That’s a drop of 3.3%, compared with the 12.2 million enrollees for 2017, and a drop of 7.1% from the peak year of 2016, according to the 2018 open enrollment final report.
For 2018, the average premium before application of the premium tax credit was $621 in the HealthCare.gov states, which is an increase of 30% from the $476 average for 2017. After the tax credit, the average premium for 2018 was $153.
“Our primary goal this year was to ensure that Americans who wanted coverage through the exchange had a seamless experience,” CMS Administrator Seema Verma said in a statement. “We are pleased that consumer satisfaction was the highest it’s ever been during the 2018 open enrollment period.”
the Centers for Medicare & Medicaid Services reported.
That’s a drop of 3.3%, compared with the 12.2 million enrollees for 2017, and a drop of 7.1% from the peak year of 2016, according to the 2018 open enrollment final report.
For 2018, the average premium before application of the premium tax credit was $621 in the HealthCare.gov states, which is an increase of 30% from the $476 average for 2017. After the tax credit, the average premium for 2018 was $153.
“Our primary goal this year was to ensure that Americans who wanted coverage through the exchange had a seamless experience,” CMS Administrator Seema Verma said in a statement. “We are pleased that consumer satisfaction was the highest it’s ever been during the 2018 open enrollment period.”
Clearer picture emerging of renal impact of SGLT2s
LOS ANGELES – Results from recent trials suggest that
“Despite optimal care around blood pressure control, glycemic control, and control of other risk factors, our patients still have a significant risk of both cardiovascular disease progression and renal disease progression,” David Cherney, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “In fact, when we have a narrow focus on glycemia, there is a lot of additional residual risk, and that A1c lowering by itself does not negate that risk and in fact has very little effect on clinical outcomes. That brings us to the newer hyperglycemic therapies, including the SGLT2 inhibitors. While these agents do indeed block the reabsorption of glucose in the kidney, they also have an effect on other nonglycemic risk factors.”
“Inside the kidney, there are direct effects on reducing intraglomerular hypertension, leading to reductions in proteinuria,” he said. “These agents are interesting because of the way that they influence how the kidney handles sodium. As a consequence, they impact on glomerular hypertension.”
Under normal physiological conditions, humans who become volume depleted or hypotensive experience a reduction in sodium delivery to the kidney by the afferent arteriole, he explained. If less sodium is delivered to the afferent arteriole, less is filtered and delivered to the macula densa, which is the sodium-sensing area of the kidney.
“If less sodium is delivered to the macula densa, less sodium will be reabsorbed, which is an energy-requiring process that leads to the breakdown of ATP [adenosine triphosphate],” Dr. Cherney said. “If less ATP is broken down to adenosine, then less adenosine is produced. Adenosine is a vasoconstrictor in this area. So, under conditions of hypervolemia or hypotension, that’s great, because we want to maintain blood flow to the kidney; that’s a protective autoregulatory response that all of us have called tubular glomerular feedback. It’s through sodium delivery to the macula densa.”
He went on to note that hyperglycemic patients who are not taking an SGLT2 inhibitor experience an increase in sodium absorption proximally, which decreases sodium delivery to the macula densa. As a result, this causes afferent dilation, which leads to a rise in glomerular pressure, glomerular hypertension, hyperfiltration, and an increased risk of renal disease progression.
“This leads to all the effects that we see clinically, including the GFR [glomerular filtration rate] dip and the reduction in proteinuria that these agents cause either when used alone or with an ACE or ARB [angiotensin II receptor blocker],” Dr. Cherney said. “SGLT2s constrict the afferent arterial and reduce glomerular hypertension and proteinuria, whereas ACE inhibitors dilate the efferent arterial, which also reduces glomerular hypertension and proteinuria.”
An analysis of renal data from the multicenter EMPA-REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) found that the use of empagliflozin was associated with slower progression of kidney disease than was placebo when added to standard care. Empagliflozin was also associated with a significantly lower risk of clinically relevant renal events, including a 40%-50% reduction in microalbuminuria in patients with micro- or macroalbuminuria (N Engl J Med. 2016 Jul 28;375:323-34).
In a recent study of EMPA-REG OUTCOME patients, Dr. Cherney and his associates examined the effects of empagliflozin on the urinary albumin to creatinine ratio in patients with type 2 diabetes and established cardiovascular disease (Lancet Diabetes Endocrinol. 2017 Aug;5[8]:610-21). They found that even in patients with normal albuminuria at baseline, by the end of the trial at about 3 years there was a modest but statistically significant 15% reduction in urinary albumin secretion. “That reduction was greater in patients with microalbuminuria at baseline,” Dr. Cherney said. “There was a more than 40% reduction in microalbuminuric patients, and almost a 50% in patients who had macroalbuminuria at baseline, suggesting that the effect is greater in patients with higher levels of albuminuria.”
Meanwhile, results from the CANVAS program, which integrated data from two trials of more than 10,000 patients with type 2 diabetes and high cardiovascular disease risk, showed that those who received canagliflozin had a 14% reduced risk of 3-point major adverse cardiovascular events (3P-MACE), compared with those who received placebo. (N Engl J Med. 2017 Aug;377:644-57). “There was a curious increased risk of amputation and fracture in the canagliflozin group, which has not been seen in other trials,” Dr. Cherney said. “That certainly merits further thought and investigation, to better understand how significant this risk is.”
Upcoming trials of renal endpoints to look out for, he said, include the CREDENCE study (results expected in 2019), DAPA-CKD, which is in the recruitment stage, and a new outcome study to evaluate the effect of empagliflozin for the treatment of people with chronic kidney disease. “This is an expanding area in the renal and cardiovascular world that we will hear a lot more about in the next 3-5 years,” he said.
Dr. Cherney reported consulting fees and/or honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe, and Sanofi.
LOS ANGELES – Results from recent trials suggest that
“Despite optimal care around blood pressure control, glycemic control, and control of other risk factors, our patients still have a significant risk of both cardiovascular disease progression and renal disease progression,” David Cherney, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “In fact, when we have a narrow focus on glycemia, there is a lot of additional residual risk, and that A1c lowering by itself does not negate that risk and in fact has very little effect on clinical outcomes. That brings us to the newer hyperglycemic therapies, including the SGLT2 inhibitors. While these agents do indeed block the reabsorption of glucose in the kidney, they also have an effect on other nonglycemic risk factors.”
“Inside the kidney, there are direct effects on reducing intraglomerular hypertension, leading to reductions in proteinuria,” he said. “These agents are interesting because of the way that they influence how the kidney handles sodium. As a consequence, they impact on glomerular hypertension.”
Under normal physiological conditions, humans who become volume depleted or hypotensive experience a reduction in sodium delivery to the kidney by the afferent arteriole, he explained. If less sodium is delivered to the afferent arteriole, less is filtered and delivered to the macula densa, which is the sodium-sensing area of the kidney.
“If less sodium is delivered to the macula densa, less sodium will be reabsorbed, which is an energy-requiring process that leads to the breakdown of ATP [adenosine triphosphate],” Dr. Cherney said. “If less ATP is broken down to adenosine, then less adenosine is produced. Adenosine is a vasoconstrictor in this area. So, under conditions of hypervolemia or hypotension, that’s great, because we want to maintain blood flow to the kidney; that’s a protective autoregulatory response that all of us have called tubular glomerular feedback. It’s through sodium delivery to the macula densa.”
He went on to note that hyperglycemic patients who are not taking an SGLT2 inhibitor experience an increase in sodium absorption proximally, which decreases sodium delivery to the macula densa. As a result, this causes afferent dilation, which leads to a rise in glomerular pressure, glomerular hypertension, hyperfiltration, and an increased risk of renal disease progression.
“This leads to all the effects that we see clinically, including the GFR [glomerular filtration rate] dip and the reduction in proteinuria that these agents cause either when used alone or with an ACE or ARB [angiotensin II receptor blocker],” Dr. Cherney said. “SGLT2s constrict the afferent arterial and reduce glomerular hypertension and proteinuria, whereas ACE inhibitors dilate the efferent arterial, which also reduces glomerular hypertension and proteinuria.”
An analysis of renal data from the multicenter EMPA-REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) found that the use of empagliflozin was associated with slower progression of kidney disease than was placebo when added to standard care. Empagliflozin was also associated with a significantly lower risk of clinically relevant renal events, including a 40%-50% reduction in microalbuminuria in patients with micro- or macroalbuminuria (N Engl J Med. 2016 Jul 28;375:323-34).
In a recent study of EMPA-REG OUTCOME patients, Dr. Cherney and his associates examined the effects of empagliflozin on the urinary albumin to creatinine ratio in patients with type 2 diabetes and established cardiovascular disease (Lancet Diabetes Endocrinol. 2017 Aug;5[8]:610-21). They found that even in patients with normal albuminuria at baseline, by the end of the trial at about 3 years there was a modest but statistically significant 15% reduction in urinary albumin secretion. “That reduction was greater in patients with microalbuminuria at baseline,” Dr. Cherney said. “There was a more than 40% reduction in microalbuminuric patients, and almost a 50% in patients who had macroalbuminuria at baseline, suggesting that the effect is greater in patients with higher levels of albuminuria.”
Meanwhile, results from the CANVAS program, which integrated data from two trials of more than 10,000 patients with type 2 diabetes and high cardiovascular disease risk, showed that those who received canagliflozin had a 14% reduced risk of 3-point major adverse cardiovascular events (3P-MACE), compared with those who received placebo. (N Engl J Med. 2017 Aug;377:644-57). “There was a curious increased risk of amputation and fracture in the canagliflozin group, which has not been seen in other trials,” Dr. Cherney said. “That certainly merits further thought and investigation, to better understand how significant this risk is.”
Upcoming trials of renal endpoints to look out for, he said, include the CREDENCE study (results expected in 2019), DAPA-CKD, which is in the recruitment stage, and a new outcome study to evaluate the effect of empagliflozin for the treatment of people with chronic kidney disease. “This is an expanding area in the renal and cardiovascular world that we will hear a lot more about in the next 3-5 years,” he said.
Dr. Cherney reported consulting fees and/or honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe, and Sanofi.
LOS ANGELES – Results from recent trials suggest that
“Despite optimal care around blood pressure control, glycemic control, and control of other risk factors, our patients still have a significant risk of both cardiovascular disease progression and renal disease progression,” David Cherney, MD, said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “In fact, when we have a narrow focus on glycemia, there is a lot of additional residual risk, and that A1c lowering by itself does not negate that risk and in fact has very little effect on clinical outcomes. That brings us to the newer hyperglycemic therapies, including the SGLT2 inhibitors. While these agents do indeed block the reabsorption of glucose in the kidney, they also have an effect on other nonglycemic risk factors.”
“Inside the kidney, there are direct effects on reducing intraglomerular hypertension, leading to reductions in proteinuria,” he said. “These agents are interesting because of the way that they influence how the kidney handles sodium. As a consequence, they impact on glomerular hypertension.”
Under normal physiological conditions, humans who become volume depleted or hypotensive experience a reduction in sodium delivery to the kidney by the afferent arteriole, he explained. If less sodium is delivered to the afferent arteriole, less is filtered and delivered to the macula densa, which is the sodium-sensing area of the kidney.
“If less sodium is delivered to the macula densa, less sodium will be reabsorbed, which is an energy-requiring process that leads to the breakdown of ATP [adenosine triphosphate],” Dr. Cherney said. “If less ATP is broken down to adenosine, then less adenosine is produced. Adenosine is a vasoconstrictor in this area. So, under conditions of hypervolemia or hypotension, that’s great, because we want to maintain blood flow to the kidney; that’s a protective autoregulatory response that all of us have called tubular glomerular feedback. It’s through sodium delivery to the macula densa.”
He went on to note that hyperglycemic patients who are not taking an SGLT2 inhibitor experience an increase in sodium absorption proximally, which decreases sodium delivery to the macula densa. As a result, this causes afferent dilation, which leads to a rise in glomerular pressure, glomerular hypertension, hyperfiltration, and an increased risk of renal disease progression.
“This leads to all the effects that we see clinically, including the GFR [glomerular filtration rate] dip and the reduction in proteinuria that these agents cause either when used alone or with an ACE or ARB [angiotensin II receptor blocker],” Dr. Cherney said. “SGLT2s constrict the afferent arterial and reduce glomerular hypertension and proteinuria, whereas ACE inhibitors dilate the efferent arterial, which also reduces glomerular hypertension and proteinuria.”
An analysis of renal data from the multicenter EMPA-REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) found that the use of empagliflozin was associated with slower progression of kidney disease than was placebo when added to standard care. Empagliflozin was also associated with a significantly lower risk of clinically relevant renal events, including a 40%-50% reduction in microalbuminuria in patients with micro- or macroalbuminuria (N Engl J Med. 2016 Jul 28;375:323-34).
In a recent study of EMPA-REG OUTCOME patients, Dr. Cherney and his associates examined the effects of empagliflozin on the urinary albumin to creatinine ratio in patients with type 2 diabetes and established cardiovascular disease (Lancet Diabetes Endocrinol. 2017 Aug;5[8]:610-21). They found that even in patients with normal albuminuria at baseline, by the end of the trial at about 3 years there was a modest but statistically significant 15% reduction in urinary albumin secretion. “That reduction was greater in patients with microalbuminuria at baseline,” Dr. Cherney said. “There was a more than 40% reduction in microalbuminuric patients, and almost a 50% in patients who had macroalbuminuria at baseline, suggesting that the effect is greater in patients with higher levels of albuminuria.”
Meanwhile, results from the CANVAS program, which integrated data from two trials of more than 10,000 patients with type 2 diabetes and high cardiovascular disease risk, showed that those who received canagliflozin had a 14% reduced risk of 3-point major adverse cardiovascular events (3P-MACE), compared with those who received placebo. (N Engl J Med. 2017 Aug;377:644-57). “There was a curious increased risk of amputation and fracture in the canagliflozin group, which has not been seen in other trials,” Dr. Cherney said. “That certainly merits further thought and investigation, to better understand how significant this risk is.”
Upcoming trials of renal endpoints to look out for, he said, include the CREDENCE study (results expected in 2019), DAPA-CKD, which is in the recruitment stage, and a new outcome study to evaluate the effect of empagliflozin for the treatment of people with chronic kidney disease. “This is an expanding area in the renal and cardiovascular world that we will hear a lot more about in the next 3-5 years,” he said.
Dr. Cherney reported consulting fees and/or honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe, and Sanofi.
EXPERT ANALYSIS FROM WCIRDC 2017
Fast-track catheter management offers little benefit after benign hysterectomy
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
REPORTING FROM SGS 2018
Key clinical point: Fast-track catheter management after surgery improves dwell time, but not other outcomes.
Major finding: Catheter dwell times were 650 versus 1,196 minutes with fast-track and conventional management, respectively.
Study details: A prospective, randomized study of 200 women.
Disclosures: Dr. Lang reported having no disclosures.
Source: Lang P et al. SGS 2018, Oral Poster 20.
Percutaneous coronary intervention (PCI) does not improve exercise time in patients with stable angina
Clinical question: Does PCI provide symptom relief in patients with stable angina?
Background: More than 500,000 PCIs are done yearly worldwide in patients with stable angina. Meta-analyses have demonstrated no impact of PCI on rates of death and myocardial infarction in patients with stable angina. Rather, relief of angina is the main reason for performance of PCI in patients with stable coronary artery disease, and this effect is frequently noted. However, there have been no data from double-blind, randomized, controlled trials to confirm the efficacy of PCI in relieving anginal pain.
Study design: Multicenter, double-blind, randomized, controlled trial.
Setting: Five sites in the United Kingdom.
Synopsis: The Objective Randomized Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial was designed to evaluate the effect of PCI, compared with placebo, on exercise time in patients with stable angina. The 230 patients who were enrolled in ORBITA had severe (70% or more), single-vessel stenosis. After enrolling, patients’ medication regimens were optimized so that almost all were taking aspirin, a second antiplatelet drug, and a statin. Beta-blockers and calcium channel blockers also were widely used by trial participants. Two hundred patients were randomized to either PCI (105 patients) or placebo procedure (95 patients). The primary endpoint was exercise time, and no difference was observed between the two groups. ORBITA’s results apply to patients with stable angina but not to those who undergo PCI for acute coronary syndrome (which includes ST-elevation MI); in the latter population, PCI has been demonstrated to reduce morbidity and mortality.
Bottom line: In patients with stable angina, PCI does not increase exercise time.
Citation: Al-Lamee R et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet. 2018 Jan;391(10115);31-40.
Dr. Clarke is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: Does PCI provide symptom relief in patients with stable angina?
Background: More than 500,000 PCIs are done yearly worldwide in patients with stable angina. Meta-analyses have demonstrated no impact of PCI on rates of death and myocardial infarction in patients with stable angina. Rather, relief of angina is the main reason for performance of PCI in patients with stable coronary artery disease, and this effect is frequently noted. However, there have been no data from double-blind, randomized, controlled trials to confirm the efficacy of PCI in relieving anginal pain.
Study design: Multicenter, double-blind, randomized, controlled trial.
Setting: Five sites in the United Kingdom.
Synopsis: The Objective Randomized Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial was designed to evaluate the effect of PCI, compared with placebo, on exercise time in patients with stable angina. The 230 patients who were enrolled in ORBITA had severe (70% or more), single-vessel stenosis. After enrolling, patients’ medication regimens were optimized so that almost all were taking aspirin, a second antiplatelet drug, and a statin. Beta-blockers and calcium channel blockers also were widely used by trial participants. Two hundred patients were randomized to either PCI (105 patients) or placebo procedure (95 patients). The primary endpoint was exercise time, and no difference was observed between the two groups. ORBITA’s results apply to patients with stable angina but not to those who undergo PCI for acute coronary syndrome (which includes ST-elevation MI); in the latter population, PCI has been demonstrated to reduce morbidity and mortality.
Bottom line: In patients with stable angina, PCI does not increase exercise time.
Citation: Al-Lamee R et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet. 2018 Jan;391(10115);31-40.
Dr. Clarke is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: Does PCI provide symptom relief in patients with stable angina?
Background: More than 500,000 PCIs are done yearly worldwide in patients with stable angina. Meta-analyses have demonstrated no impact of PCI on rates of death and myocardial infarction in patients with stable angina. Rather, relief of angina is the main reason for performance of PCI in patients with stable coronary artery disease, and this effect is frequently noted. However, there have been no data from double-blind, randomized, controlled trials to confirm the efficacy of PCI in relieving anginal pain.
Study design: Multicenter, double-blind, randomized, controlled trial.
Setting: Five sites in the United Kingdom.
Synopsis: The Objective Randomized Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial was designed to evaluate the effect of PCI, compared with placebo, on exercise time in patients with stable angina. The 230 patients who were enrolled in ORBITA had severe (70% or more), single-vessel stenosis. After enrolling, patients’ medication regimens were optimized so that almost all were taking aspirin, a second antiplatelet drug, and a statin. Beta-blockers and calcium channel blockers also were widely used by trial participants. Two hundred patients were randomized to either PCI (105 patients) or placebo procedure (95 patients). The primary endpoint was exercise time, and no difference was observed between the two groups. ORBITA’s results apply to patients with stable angina but not to those who undergo PCI for acute coronary syndrome (which includes ST-elevation MI); in the latter population, PCI has been demonstrated to reduce morbidity and mortality.
Bottom line: In patients with stable angina, PCI does not increase exercise time.
Citation: Al-Lamee R et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet. 2018 Jan;391(10115);31-40.
Dr. Clarke is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
VIDEO: Poorer cardiometabolic health seen in men with low sperm count
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
CHICAGO – Low testosterone levels alone didn’t account for the finding, said Alberto Ferlin, MD, PhD, professor of reproductive endocrinology at the University of Brescia, Italy.
“So at the end, we showed that, independent of testosterone, low sperm count could be a marker of general male health, in particular for cardiovascular risk factors or metabolic derangement,” said Dr. Ferlin in an interview following a press conference at the annual meeting of the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The Italian study, which Dr. Ferlin said was the largest of its kind to date, studied 5,177 males who were part of an infertile couple, comparing men with low sperm count (less than 39 million sperm per ejaculate) with those with normal sperm count (at least 39 million sperm per ejaculate). In all, 2,583 of the participants had low sperm counts.
“Our main aim was to understand if semen analysis and, in general, the reproductive function of a man, could be a marker of his general cardiovascular and metabolic health,” said Dr. Ferlin.
Only men with a comprehensive work-up were included, so all participants had a medical history and physical exam, and semen analysis and culture. Additional components of the evaluation included blood lipid and glucose metabolism testing, reproductive hormone levels, ultrasound of the testes and, for men diagnosed with hypogonadism, bone densitometry.
The study, said Dr. Ferlin, found that among men with a low total sperm count, there was a high prevalence of hypogonadism, defined as both low testosterone and elevated levels of luteinizing hormone. Additionally, these men had a high prevalence of elevated luteinizing hormones with normal testosterone – “so-called subclinical hypogonadism,” said Dr. Ferlin.
In men with a low sperm count – defined as fewer than 39 million sperm per ejaculate – the prevalence of biochemical hypogonadism was about 45%, compared with just 6% in men with normal sperm counts, said Dr. Ferlin. Men with infertility had an odds ratio for hypogonadism of 12.2, said Dr. Ferlin (95% confidence interval, 10.2-14.6).
Additionally, Dr. Ferlin reported that 35% of men with hypogonadism had osteopenia, and 17% met criteria for osteoporosis. The numbers surprised the investigators. “These are very young men – about 30 years old,” said Dr. Ferlin.
Dr. Ferlin and his collaborators also looked at the subset of eugonadal men in the study, comparing those with normal sperm counts (n = 2,431) to those who had low sperm counts, (n = 1,423). They found that men with low sperm counts had significantly higher body mass index, waist circumference, systolic blood pressure, hemoglobin A1c, and homeostatic model assessment of insulin resistance (HOMA-IR) levels (P less than .001 for all).
High density lipoprotein (HDL) cholesterol, testosterone, and follicle stimulating hormone levels were also significantly lower for men with low sperm count. “Men with oligozoospermia … have an increased risk of metabolic derangement – so, altered lipid profile with higher LDL cholesterol and lower HDL [cholesterol], higher triglycerides, higher insulin resistance,” said Dr. Ferlin.
The findings have implications for reproductive endocrinologists caring for couples with infertility, said Dr. Ferlin. “Infertile men should be studied comprehensively, and the diagnosis cannot be limited to just one semen analysis,” given the study’s findings, he said. “All these men should be counseled, should be treated … for worsening of these cardiovascular and metabolic risk factors that are present in such frequency in oligozoospermic men.”
Dr. Ferlin reported no conflicts of interest.
REPORTING FROM ENDO 2018
Spontaneous Regression of Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.

Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Practice Points
- Merkel cell carcinoma (MCC) is a rare malignancy with a high rate of metastasis and poor prognosis.
- T-cell mediated immunity appears to play an important role in tumor regression in MCC.
- Merkel cell polyomavirus appears to play a role in the pathogenesis of MCC and may be associated with a better prognosis.
- A better understanding of spontaneous regression of MCC could help in the development of new immunotherapeutic approaches to this malignancy.