User login
Point of Care Ultrasound (POCUS) for Small Bowel Obstruction in the ED
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
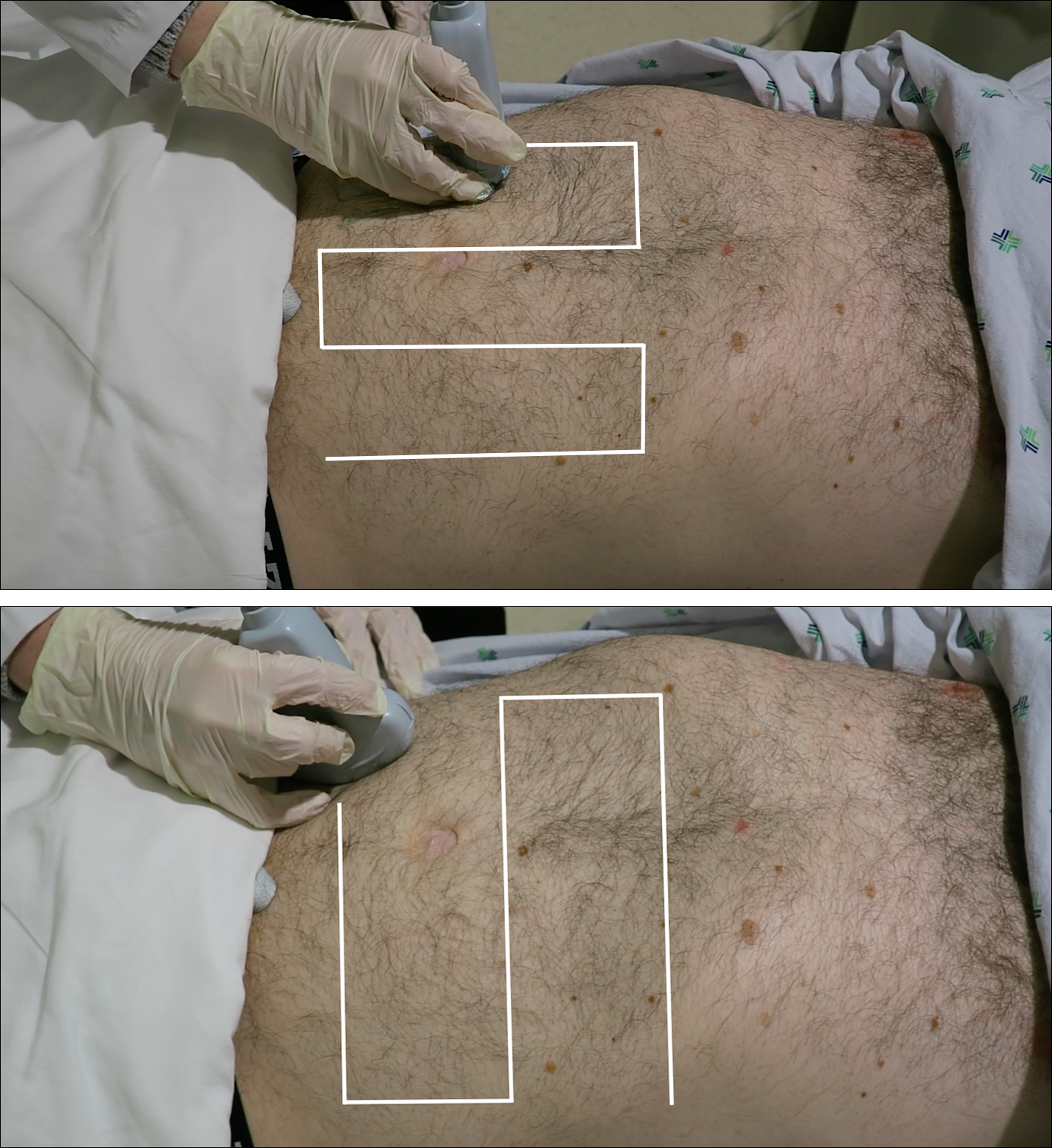
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.
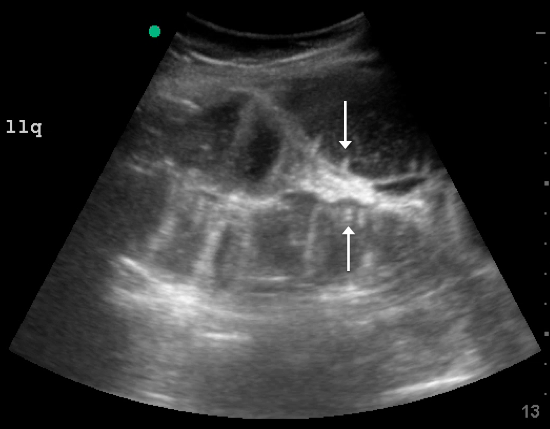
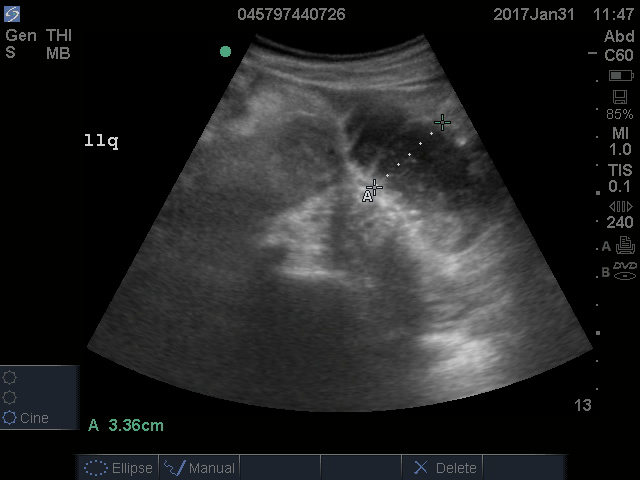
A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.
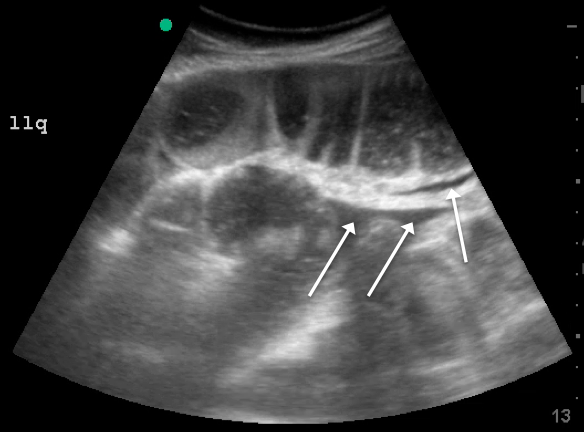
Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7
DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
A deep commitment to veterans’ medical needs
VA hospitalist Dr. Mel Anderson loves his work
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.
VA hospitalist Dr. Mel Anderson loves his work
VA hospitalist Dr. Mel Anderson loves his work
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.
Oops, Wrong Bottle
A 42-year-old man presented to the ED with a cut to his left forearm from a piece of metal. The patient only complained of pain at the site of injury; he had no numbness or weakness of the left hand. The patient was otherwise in good health, was taking no medications, and was current with his tetanus immunization.
On physical examination, the patient’s vital signs were normal. The emergency physician (EP) documented a vertical laceration of the mid-left forearm on the dorsal aspect, measuring 6 x 2 cm. The wound edges could be easily approximated. The distal motor and sensory exams were normal.
The EP anesthetized the area with local infiltration using 1% plain xylocaine. The EP then picked up a bottle of CaviCide that had been sitting on the counter and sprayed it on the patient’s wound. The patient immediately complained of burning pain, but the EP continued to spray the wound before suturing it closed with 4.0 nylon.
The patient, however, stated the pain was unbearable. He showed the ED manager the bottle of CaviCide and asked if it was an appropriate sterilizing solution for wounds. When informed it was not, the patient demanded the sutures be removed and the wound re-opened and irrigated with an appropriate solution. The EP re-opened the wound, irrigated it with sterile normal saline, and closed it once again using 4.0 nylon. The EP apologized to the patient, admitted that he made a mistake, and discharged the patient home with instructions to have the sutures removed in 10 days.
The patient developed severe pain at the site a few hours later, prompting him to go to a different ED. They applied lidocaine gel to the area and recommended ibuprofen by mouth for pain. The patient was discharged home.
The patient sued the EP, the nurse, and the hospital for negligence. The plaintiff alleged that under no circumstances should CaviCide be used on humans. The plaintiff’s EM expert testified that the error represented gross negligence. The hospital admitted the nurse violated the standard of care for not properly storing the CaviCide. The EP expert for the defense argued the patient did not suffer any new injury or pain, and that his symptoms were due to the laceration. A second defense expert (toxicology) explained that CaviCide is not toxic and that it would only cause short-term irritation. The plaintiff’s counsel asked for $172,800 in damages, explaining that he was requesting $1 per second for the time the patient experienced intense pain. After deliberating for five hours, the jury found in favor of the defense.
DISCUSSION
Over the years, I have seen variations of this case: hemoccult solution placed in the eye under the impression it was a topical anesthetic, and 1:1000 epinephrine given intravenously (IV) when it was thought to be 1:10,000 concentration.
The way to avoid this mistake is to force yourself to take a good look at whatever medication you are administering to a patient, be it by mouth or IV, on the eye or skin, in a muscle, or up the rectum. Read the name of the medication before giving it. It is fortunate for all involved in this case that no serious or permanent injury occurred.
According to the manufacturer of CaviCide (Metrex), it is a “convenient, ready-to-use, intermediate-level surface disinfectant which is effective against tuberculosis, HBV, HCV, viruses (hydrophilic and lipophilic), bacteria (including MRSA and VRE), and fungi. It is safe for use on non-porous surfaces, and for cleaning environmental and medical device surfaces.” While it sounds great for cleaning surfaces and objects, it is clearly not the right product to spray on a wound.
This accident falls under the general heading of a medication error. This category includes: selecting the wrong medication or dosage; giving the medication at the wrong frequency; administration to the wrong patient or via the wrong route; or failure to monitor the patients’ response to the medication. In the risk management world, it is recommended that providers consistently perform the “five rights” of medication administration: right patient; right drug; right dosage; right time; and right route. This case illustrates the problem of “right drug.” Clearly, CaviCide was not the right drug for this patient. Given different circumstances, the harm could have been significant.
SUMMARY
Fortunately, this is a relatively simple take-home message: know what drug you are giving your patient, always.
A 42-year-old man presented to the ED with a cut to his left forearm from a piece of metal. The patient only complained of pain at the site of injury; he had no numbness or weakness of the left hand. The patient was otherwise in good health, was taking no medications, and was current with his tetanus immunization.
On physical examination, the patient’s vital signs were normal. The emergency physician (EP) documented a vertical laceration of the mid-left forearm on the dorsal aspect, measuring 6 x 2 cm. The wound edges could be easily approximated. The distal motor and sensory exams were normal.
The EP anesthetized the area with local infiltration using 1% plain xylocaine. The EP then picked up a bottle of CaviCide that had been sitting on the counter and sprayed it on the patient’s wound. The patient immediately complained of burning pain, but the EP continued to spray the wound before suturing it closed with 4.0 nylon.
The patient, however, stated the pain was unbearable. He showed the ED manager the bottle of CaviCide and asked if it was an appropriate sterilizing solution for wounds. When informed it was not, the patient demanded the sutures be removed and the wound re-opened and irrigated with an appropriate solution. The EP re-opened the wound, irrigated it with sterile normal saline, and closed it once again using 4.0 nylon. The EP apologized to the patient, admitted that he made a mistake, and discharged the patient home with instructions to have the sutures removed in 10 days.
The patient developed severe pain at the site a few hours later, prompting him to go to a different ED. They applied lidocaine gel to the area and recommended ibuprofen by mouth for pain. The patient was discharged home.
The patient sued the EP, the nurse, and the hospital for negligence. The plaintiff alleged that under no circumstances should CaviCide be used on humans. The plaintiff’s EM expert testified that the error represented gross negligence. The hospital admitted the nurse violated the standard of care for not properly storing the CaviCide. The EP expert for the defense argued the patient did not suffer any new injury or pain, and that his symptoms were due to the laceration. A second defense expert (toxicology) explained that CaviCide is not toxic and that it would only cause short-term irritation. The plaintiff’s counsel asked for $172,800 in damages, explaining that he was requesting $1 per second for the time the patient experienced intense pain. After deliberating for five hours, the jury found in favor of the defense.
DISCUSSION
Over the years, I have seen variations of this case: hemoccult solution placed in the eye under the impression it was a topical anesthetic, and 1:1000 epinephrine given intravenously (IV) when it was thought to be 1:10,000 concentration.
The way to avoid this mistake is to force yourself to take a good look at whatever medication you are administering to a patient, be it by mouth or IV, on the eye or skin, in a muscle, or up the rectum. Read the name of the medication before giving it. It is fortunate for all involved in this case that no serious or permanent injury occurred.
According to the manufacturer of CaviCide (Metrex), it is a “convenient, ready-to-use, intermediate-level surface disinfectant which is effective against tuberculosis, HBV, HCV, viruses (hydrophilic and lipophilic), bacteria (including MRSA and VRE), and fungi. It is safe for use on non-porous surfaces, and for cleaning environmental and medical device surfaces.” While it sounds great for cleaning surfaces and objects, it is clearly not the right product to spray on a wound.
This accident falls under the general heading of a medication error. This category includes: selecting the wrong medication or dosage; giving the medication at the wrong frequency; administration to the wrong patient or via the wrong route; or failure to monitor the patients’ response to the medication. In the risk management world, it is recommended that providers consistently perform the “five rights” of medication administration: right patient; right drug; right dosage; right time; and right route. This case illustrates the problem of “right drug.” Clearly, CaviCide was not the right drug for this patient. Given different circumstances, the harm could have been significant.
SUMMARY
Fortunately, this is a relatively simple take-home message: know what drug you are giving your patient, always.
A 42-year-old man presented to the ED with a cut to his left forearm from a piece of metal. The patient only complained of pain at the site of injury; he had no numbness or weakness of the left hand. The patient was otherwise in good health, was taking no medications, and was current with his tetanus immunization.
On physical examination, the patient’s vital signs were normal. The emergency physician (EP) documented a vertical laceration of the mid-left forearm on the dorsal aspect, measuring 6 x 2 cm. The wound edges could be easily approximated. The distal motor and sensory exams were normal.
The EP anesthetized the area with local infiltration using 1% plain xylocaine. The EP then picked up a bottle of CaviCide that had been sitting on the counter and sprayed it on the patient’s wound. The patient immediately complained of burning pain, but the EP continued to spray the wound before suturing it closed with 4.0 nylon.
The patient, however, stated the pain was unbearable. He showed the ED manager the bottle of CaviCide and asked if it was an appropriate sterilizing solution for wounds. When informed it was not, the patient demanded the sutures be removed and the wound re-opened and irrigated with an appropriate solution. The EP re-opened the wound, irrigated it with sterile normal saline, and closed it once again using 4.0 nylon. The EP apologized to the patient, admitted that he made a mistake, and discharged the patient home with instructions to have the sutures removed in 10 days.
The patient developed severe pain at the site a few hours later, prompting him to go to a different ED. They applied lidocaine gel to the area and recommended ibuprofen by mouth for pain. The patient was discharged home.
The patient sued the EP, the nurse, and the hospital for negligence. The plaintiff alleged that under no circumstances should CaviCide be used on humans. The plaintiff’s EM expert testified that the error represented gross negligence. The hospital admitted the nurse violated the standard of care for not properly storing the CaviCide. The EP expert for the defense argued the patient did not suffer any new injury or pain, and that his symptoms were due to the laceration. A second defense expert (toxicology) explained that CaviCide is not toxic and that it would only cause short-term irritation. The plaintiff’s counsel asked for $172,800 in damages, explaining that he was requesting $1 per second for the time the patient experienced intense pain. After deliberating for five hours, the jury found in favor of the defense.
DISCUSSION
Over the years, I have seen variations of this case: hemoccult solution placed in the eye under the impression it was a topical anesthetic, and 1:1000 epinephrine given intravenously (IV) when it was thought to be 1:10,000 concentration.
The way to avoid this mistake is to force yourself to take a good look at whatever medication you are administering to a patient, be it by mouth or IV, on the eye or skin, in a muscle, or up the rectum. Read the name of the medication before giving it. It is fortunate for all involved in this case that no serious or permanent injury occurred.
According to the manufacturer of CaviCide (Metrex), it is a “convenient, ready-to-use, intermediate-level surface disinfectant which is effective against tuberculosis, HBV, HCV, viruses (hydrophilic and lipophilic), bacteria (including MRSA and VRE), and fungi. It is safe for use on non-porous surfaces, and for cleaning environmental and medical device surfaces.” While it sounds great for cleaning surfaces and objects, it is clearly not the right product to spray on a wound.
This accident falls under the general heading of a medication error. This category includes: selecting the wrong medication or dosage; giving the medication at the wrong frequency; administration to the wrong patient or via the wrong route; or failure to monitor the patients’ response to the medication. In the risk management world, it is recommended that providers consistently perform the “five rights” of medication administration: right patient; right drug; right dosage; right time; and right route. This case illustrates the problem of “right drug.” Clearly, CaviCide was not the right drug for this patient. Given different circumstances, the harm could have been significant.
SUMMARY
Fortunately, this is a relatively simple take-home message: know what drug you are giving your patient, always.
Review of pediatric data indicates link between vitamin D levels and atopic dermatitis severity
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
in the majority of studies, but evidence on whether supplementation can improve symptoms of the condition was inconsistent.
The data on the effect of vitamin D supplementation on AD severity “suggested potential benefit but were conflicting,” concluded Christina M. Huang, MD, of Queen’s University, Kingston, Ontario, and her coinvestigators from the department of dermatology, Hospital for Sick Children, Toronto. They reported the results of their systematic review of 21 studies published between 2008 and 2017, which included quantitative data on serum vitamin D levels or vitamin D supplementation and AD severity in patients aged 18 years or younger, in Pediatrics.
In the review, 16 studies explored the relationship between serum vitamin D status and disease severity (one was a randomized controlled trial; the rest were cohort, cross-sectional, or case control studies) in 1,847 children (average age, 5.6 years). Disease severity was measured with the SCORing Atopic Dermatitis (SCORAD) system. In 10 of the 16 studies, there was a significant inverse association between vitamin D levels and AD severity.
The studies that supported this association generally had larger sample sizes, which, the authors pointed out, suggested they were of higher quality and more reliable. However, the randomized controlled study of 89 children did not find a correlation, although in the study, vitamin D level and AD severity was a secondary outcome.
The randomized controlled trial of vitamin D supplementation used lower SCORAD cut-offs for the different severities of AD, which complicated interpretation the results, “as it may indicate that the severities reported in these articles were exaggerated as compared to other studies,” they wrote.
Six studies – four randomized controlled trials (including the study that was among the 16 studies on vitamin D and severity) and two cohort studies – with 354 participants (average age, 6.8 years) looked at the effects of oral vitamin D supplementation on the severity of AD, although dosage and duration of use varied across the studies. In four of the six studies, there were significant improvement in AD in patients given supplements, but the data were “conflicting,” partly because the largest study showing benefit used a different measure of disease severity, the Eczema Area and Severity Index (EASI), not SCORAD. “The inconsistency of tools used to measure outcomes makes it difficult to compare and understand results,” so the effects of vitamin D supplementation “are controversial and should be interpreted with caution, as certain patient populations may benefit more than others,” the authors wrote.
They also drew attention to previous research suggesting that vitamin D supplementation in the first year of life might actually increase the risk of AD in children. “Therefore, although there is a growing body of evidence supporting the beneficial effects of VD [vitamin D] supplementation, the age at which supplementation is given should be considered carefully.” The authors added that the inconclusive findings “may have been due to confounding factors that were not accounted for, such as age, season, latitude, dose, and duration. It is also possible that the lack of a true effect of VD may be contributing to the inconsistent results. Future large‐scale RCTs with consideration of these factors are needed.”
Funding and conflict of interest disclosures were not included in the study.
SOURCE: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: There is evidence that vitamin D levels in children affect atopic dermatitis severity, but further study is needed.
Major finding: Serum vitamin D levels were significantly inversely correlated with AD severity in children in 10 of 16 studies.
Study details: A systematic review of 21 pediatric studies looking at the association of vitamin D levels or supplementation on AD severity.
Disclosures: No funding or conflicts of declarations interest were available.
Source: Huang C et al. Pediatr Dermatol. 2018;35: 754-60. doi: 10.1111/pde.13639.
FDA approves larotrectinib for cancers with NTRK gene fusions
for adult and pediatric patients with solid tumors that harbor a genetic aberration known as an neurotrophic receptor tyrosine kinase (NTRK) fusion.
Specifically, the oral inhibitor of tropomyosin receptor kinase is approved for patients with solid tumors that have a NTRK gene fusion without a known acquired resistance mutation and have metastatic disease, are likely to experience severe morbidity from surgical resection, have no satisfactory alternative treatments, or have cancer that has progressed following treatment, the FDA said in a press release.
NTRK fusions are found in both children and adults with dozens of different cancer types. The genetic aberration tends to be rare in common cancers and nearly universal in certain uncommon cancers.
Approval of larotrectinib (Vitrakvi) was based on overall response rate and response duration in the first 55 patients with unresectable or metastatic solid tumors harboring an NTRK gene fusion enrolled across three multicenter, open-label, single-arm clinical trials. The ORR was 75% (95% confidence interval, 61%-85%), including 22% complete responses and 53% partial responses. At the time of database lock, median duration of response had not been reached, the FDA said.
The most common tumors were salivary gland tumors (22%), soft tissue sarcomas (20%), infantile fibrosarcomas (13%), and thyroid cancers (9%). Identification of positive NTRK gene fusion status was prospectively determined in local laboratories using next-generation sequencing or fluorescence in situ hybridization.
Results of the three trials, a phase 1 trial among 8 adult patients (LOXO-TRK-14001), a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 basket trial among 35 adult and adolescent patients (NAVIGATE), were presented at the annual meeting of the American Society of Clinical Oncology in 2017.
The safety of larotrectinib was evaluated in 176 patients enrolled across the three clinical trials, including 44 pediatric patients. The most common adverse reactions with larotrectinib were fatigue, nausea, dizziness, vomiting, increased AST, cough, increased ALT, constipation, and diarrhea, the FDA said.
The recommended larotrectinib doses are 100 mg orally twice daily for adults and 100 mg/m2 orally twice daily (maximum of 100 mg per dose) for pediatric patients.
The approval of larotrectinib follows the approval of pembrolizumab for certain solid tumors with the MSI-H biomarker, as the first approval for the treatment of cancer based on a biomarker rather than the particular body part or organ system affected by the tumor.
The FDA granted the accelerated approval of Vitrakvi to Loxo Oncology and Bayer.
for adult and pediatric patients with solid tumors that harbor a genetic aberration known as an neurotrophic receptor tyrosine kinase (NTRK) fusion.
Specifically, the oral inhibitor of tropomyosin receptor kinase is approved for patients with solid tumors that have a NTRK gene fusion without a known acquired resistance mutation and have metastatic disease, are likely to experience severe morbidity from surgical resection, have no satisfactory alternative treatments, or have cancer that has progressed following treatment, the FDA said in a press release.
NTRK fusions are found in both children and adults with dozens of different cancer types. The genetic aberration tends to be rare in common cancers and nearly universal in certain uncommon cancers.
Approval of larotrectinib (Vitrakvi) was based on overall response rate and response duration in the first 55 patients with unresectable or metastatic solid tumors harboring an NTRK gene fusion enrolled across three multicenter, open-label, single-arm clinical trials. The ORR was 75% (95% confidence interval, 61%-85%), including 22% complete responses and 53% partial responses. At the time of database lock, median duration of response had not been reached, the FDA said.
The most common tumors were salivary gland tumors (22%), soft tissue sarcomas (20%), infantile fibrosarcomas (13%), and thyroid cancers (9%). Identification of positive NTRK gene fusion status was prospectively determined in local laboratories using next-generation sequencing or fluorescence in situ hybridization.
Results of the three trials, a phase 1 trial among 8 adult patients (LOXO-TRK-14001), a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 basket trial among 35 adult and adolescent patients (NAVIGATE), were presented at the annual meeting of the American Society of Clinical Oncology in 2017.
The safety of larotrectinib was evaluated in 176 patients enrolled across the three clinical trials, including 44 pediatric patients. The most common adverse reactions with larotrectinib were fatigue, nausea, dizziness, vomiting, increased AST, cough, increased ALT, constipation, and diarrhea, the FDA said.
The recommended larotrectinib doses are 100 mg orally twice daily for adults and 100 mg/m2 orally twice daily (maximum of 100 mg per dose) for pediatric patients.
The approval of larotrectinib follows the approval of pembrolizumab for certain solid tumors with the MSI-H biomarker, as the first approval for the treatment of cancer based on a biomarker rather than the particular body part or organ system affected by the tumor.
The FDA granted the accelerated approval of Vitrakvi to Loxo Oncology and Bayer.
for adult and pediatric patients with solid tumors that harbor a genetic aberration known as an neurotrophic receptor tyrosine kinase (NTRK) fusion.
Specifically, the oral inhibitor of tropomyosin receptor kinase is approved for patients with solid tumors that have a NTRK gene fusion without a known acquired resistance mutation and have metastatic disease, are likely to experience severe morbidity from surgical resection, have no satisfactory alternative treatments, or have cancer that has progressed following treatment, the FDA said in a press release.
NTRK fusions are found in both children and adults with dozens of different cancer types. The genetic aberration tends to be rare in common cancers and nearly universal in certain uncommon cancers.
Approval of larotrectinib (Vitrakvi) was based on overall response rate and response duration in the first 55 patients with unresectable or metastatic solid tumors harboring an NTRK gene fusion enrolled across three multicenter, open-label, single-arm clinical trials. The ORR was 75% (95% confidence interval, 61%-85%), including 22% complete responses and 53% partial responses. At the time of database lock, median duration of response had not been reached, the FDA said.
The most common tumors were salivary gland tumors (22%), soft tissue sarcomas (20%), infantile fibrosarcomas (13%), and thyroid cancers (9%). Identification of positive NTRK gene fusion status was prospectively determined in local laboratories using next-generation sequencing or fluorescence in situ hybridization.
Results of the three trials, a phase 1 trial among 8 adult patients (LOXO-TRK-14001), a phase 1/2 trial among 12 pediatric patients (SCOUT), and a phase 2 basket trial among 35 adult and adolescent patients (NAVIGATE), were presented at the annual meeting of the American Society of Clinical Oncology in 2017.
The safety of larotrectinib was evaluated in 176 patients enrolled across the three clinical trials, including 44 pediatric patients. The most common adverse reactions with larotrectinib were fatigue, nausea, dizziness, vomiting, increased AST, cough, increased ALT, constipation, and diarrhea, the FDA said.
The recommended larotrectinib doses are 100 mg orally twice daily for adults and 100 mg/m2 orally twice daily (maximum of 100 mg per dose) for pediatric patients.
The approval of larotrectinib follows the approval of pembrolizumab for certain solid tumors with the MSI-H biomarker, as the first approval for the treatment of cancer based on a biomarker rather than the particular body part or organ system affected by the tumor.
The FDA granted the accelerated approval of Vitrakvi to Loxo Oncology and Bayer.
Substance use increases likelihood of psychiatric hold in pregnancy
AUSTIN, TEX. – Providers are no more likely to put an involuntary psychiatric hold on someone who is pregnant than not unless she is using substances, recent research shows.
“This raises a question regarding who psychiatrists consider to be their patients: the mother, the unborn child, or both?” Samuel J. House, MD, of the University of Arkansas, Little Rock, said at the annual meeting of the American Academy of Psychiatry and the Law (AAPL).
Dr. House sent out a survey to members of the AAPL to learn their attitudes toward involuntary psychiatric holds on pregnant women, with and without evidence of substance use, and he presented the results at the meeting.
“We know that the rates of involuntary hospitalizations very widely” across different jurisdictions and practice settings, Dr. House said, but research has shown that age, unmarried status, psychotic symptoms, aggression, and a low level of social function are associated with involuntary commitment. He wanted to explore where pregnancy fit.
Dr. House became interested in clinicians’ perspectives on this issue when he realized how few psychiatric holds he saw among pregnant women during the 4 years he spent at a university hospital’s level 1 trauma center. He included questions about substance use in his survey because of the “recent push to criminalize substance use during pregnancy, mainly in response to the significant impact substance use during pregnancy can have on the fetus or developing child,” he said.
Dr. House received 68 survey responses from AAPL members, most of whom were male with an average age of 47 years. The 7-question survey presented various clinical scenarios and asked what the respondent would do.
The first question concerned being called to the emergency department to evaluate a 28-year-old white woman with clinical signs of depression, history of a suicide attempt, and a mother who had committed suicide when the patient was 15. However, she states during evaluation: “I could never actually kill myself. My family would be too upset, and I would go to hell.”
Two-thirds of respondents (67.6%) said they would admit the woman to an inpatient unit for stabilization, and the others would discharge her with close follow-up.
The second question asked what the clinician would do if the patient declined admission: 41.2% would discharge, and 58.8% would place the woman on a psychiatric hold.
The third question introduced a positive pregnancy test for the woman, but none of the respondents said they would cancel the psychiatric hold. Most were split between proceeding with a hold (42.6%) or proceeding with a discharge (47.1%), though 10.3% would cancel the discharge and place the patient on a hold. Ultimately, respondents were no more likely to put the woman on a hold whether she was pregnant or not.
Then the survey repeated the scenario, but instead of a positive pregnancy test, the question asked what clinicians would do if her drug screen were positive after she had refused admission. In that scenario, the woman reported daily methamphetamine use to the emergency physician.
Among respondents, 48.5% would proceed with a psychiatric hold, 42.6% would proceed with a discharge, and 8.8% would cancel the discharge and put the patient on a hold.
The final question asks clinicians’ course of action if the woman’s pregnancy test were positive after the positive drug screen. Now, only a little over a quarter of respondents (26.5%) would proceed with a discharge and follow-up. More than half (57.4%) would proceed with a hold, and 16.2% would cancel the discharge and place a psychiatric hold.
Therefore, 73.6% of clinicians would place a pregnant woman with a history of substance use on a psychiatric hold, compared with 52.9% if the woman were pregnant but not using methamphetamine.
Laws on pregnancy, substance use
Dr. House considered those findings within the context of current laws governing substance use during pregnancy., with prosecution usually requiring detection of the substance at birth or during pregnancy, or evidence of risk to the child’s health.
Tennessee is unique in considering substance abuse in pregnancy assault if the child is born with dependence or other harm from the drug use. Women in Minnesota, South Dakota, and Wisconsin can be subject to civil commitment, including required inpatient drug treatment, for substance abuse during pregnancy (Am J Psychiatry. 2016 Nov 1;173[11]:1077-80).
Mandatory reporting laws for suspected substance abuse during pregnancy exist in 15 states, mostly in the Southwest, northern Midwest, and states around the District of Columbia. And four states – Kentucky, Iowa, Minnesota, and North Dakota – require pregnant women suspected of substance abuse to be tested.
Yet, most major relevant medical associations oppose criminalization of substance use during pregnancy, including the American Psychiatric Association, the American Academy of Addiction Psychiatry, the American Medical Association, and the American College of Obstetricians and Gynecologists.
“They are generally for increasing access for people, like voluntary screening, but against criminalization because it creates a barrier to accessing prenatal care,” Dr. House explained.
Aside from the question of whom psychiatrists consider their patients – the woman, her fetus, or both – the results raise another question, Dr. House said: “While studies have shown that criminalizing substance use during pregnancy discourages mothers from seeking prenatal care, does the threat of an involuntary psychiatric admission have a similar consequence?” That’s a question for further research.
No external funding was used. Dr. House was a clinical investigator without compensation for Janssen Pharmaceuticals from 2015 to 2017.
AUSTIN, TEX. – Providers are no more likely to put an involuntary psychiatric hold on someone who is pregnant than not unless she is using substances, recent research shows.
“This raises a question regarding who psychiatrists consider to be their patients: the mother, the unborn child, or both?” Samuel J. House, MD, of the University of Arkansas, Little Rock, said at the annual meeting of the American Academy of Psychiatry and the Law (AAPL).
Dr. House sent out a survey to members of the AAPL to learn their attitudes toward involuntary psychiatric holds on pregnant women, with and without evidence of substance use, and he presented the results at the meeting.
“We know that the rates of involuntary hospitalizations very widely” across different jurisdictions and practice settings, Dr. House said, but research has shown that age, unmarried status, psychotic symptoms, aggression, and a low level of social function are associated with involuntary commitment. He wanted to explore where pregnancy fit.
Dr. House became interested in clinicians’ perspectives on this issue when he realized how few psychiatric holds he saw among pregnant women during the 4 years he spent at a university hospital’s level 1 trauma center. He included questions about substance use in his survey because of the “recent push to criminalize substance use during pregnancy, mainly in response to the significant impact substance use during pregnancy can have on the fetus or developing child,” he said.
Dr. House received 68 survey responses from AAPL members, most of whom were male with an average age of 47 years. The 7-question survey presented various clinical scenarios and asked what the respondent would do.
The first question concerned being called to the emergency department to evaluate a 28-year-old white woman with clinical signs of depression, history of a suicide attempt, and a mother who had committed suicide when the patient was 15. However, she states during evaluation: “I could never actually kill myself. My family would be too upset, and I would go to hell.”
Two-thirds of respondents (67.6%) said they would admit the woman to an inpatient unit for stabilization, and the others would discharge her with close follow-up.
The second question asked what the clinician would do if the patient declined admission: 41.2% would discharge, and 58.8% would place the woman on a psychiatric hold.
The third question introduced a positive pregnancy test for the woman, but none of the respondents said they would cancel the psychiatric hold. Most were split between proceeding with a hold (42.6%) or proceeding with a discharge (47.1%), though 10.3% would cancel the discharge and place the patient on a hold. Ultimately, respondents were no more likely to put the woman on a hold whether she was pregnant or not.
Then the survey repeated the scenario, but instead of a positive pregnancy test, the question asked what clinicians would do if her drug screen were positive after she had refused admission. In that scenario, the woman reported daily methamphetamine use to the emergency physician.
Among respondents, 48.5% would proceed with a psychiatric hold, 42.6% would proceed with a discharge, and 8.8% would cancel the discharge and put the patient on a hold.
The final question asks clinicians’ course of action if the woman’s pregnancy test were positive after the positive drug screen. Now, only a little over a quarter of respondents (26.5%) would proceed with a discharge and follow-up. More than half (57.4%) would proceed with a hold, and 16.2% would cancel the discharge and place a psychiatric hold.
Therefore, 73.6% of clinicians would place a pregnant woman with a history of substance use on a psychiatric hold, compared with 52.9% if the woman were pregnant but not using methamphetamine.
Laws on pregnancy, substance use
Dr. House considered those findings within the context of current laws governing substance use during pregnancy., with prosecution usually requiring detection of the substance at birth or during pregnancy, or evidence of risk to the child’s health.
Tennessee is unique in considering substance abuse in pregnancy assault if the child is born with dependence or other harm from the drug use. Women in Minnesota, South Dakota, and Wisconsin can be subject to civil commitment, including required inpatient drug treatment, for substance abuse during pregnancy (Am J Psychiatry. 2016 Nov 1;173[11]:1077-80).
Mandatory reporting laws for suspected substance abuse during pregnancy exist in 15 states, mostly in the Southwest, northern Midwest, and states around the District of Columbia. And four states – Kentucky, Iowa, Minnesota, and North Dakota – require pregnant women suspected of substance abuse to be tested.
Yet, most major relevant medical associations oppose criminalization of substance use during pregnancy, including the American Psychiatric Association, the American Academy of Addiction Psychiatry, the American Medical Association, and the American College of Obstetricians and Gynecologists.
“They are generally for increasing access for people, like voluntary screening, but against criminalization because it creates a barrier to accessing prenatal care,” Dr. House explained.
Aside from the question of whom psychiatrists consider their patients – the woman, her fetus, or both – the results raise another question, Dr. House said: “While studies have shown that criminalizing substance use during pregnancy discourages mothers from seeking prenatal care, does the threat of an involuntary psychiatric admission have a similar consequence?” That’s a question for further research.
No external funding was used. Dr. House was a clinical investigator without compensation for Janssen Pharmaceuticals from 2015 to 2017.
AUSTIN, TEX. – Providers are no more likely to put an involuntary psychiatric hold on someone who is pregnant than not unless she is using substances, recent research shows.
“This raises a question regarding who psychiatrists consider to be their patients: the mother, the unborn child, or both?” Samuel J. House, MD, of the University of Arkansas, Little Rock, said at the annual meeting of the American Academy of Psychiatry and the Law (AAPL).
Dr. House sent out a survey to members of the AAPL to learn their attitudes toward involuntary psychiatric holds on pregnant women, with and without evidence of substance use, and he presented the results at the meeting.
“We know that the rates of involuntary hospitalizations very widely” across different jurisdictions and practice settings, Dr. House said, but research has shown that age, unmarried status, psychotic symptoms, aggression, and a low level of social function are associated with involuntary commitment. He wanted to explore where pregnancy fit.
Dr. House became interested in clinicians’ perspectives on this issue when he realized how few psychiatric holds he saw among pregnant women during the 4 years he spent at a university hospital’s level 1 trauma center. He included questions about substance use in his survey because of the “recent push to criminalize substance use during pregnancy, mainly in response to the significant impact substance use during pregnancy can have on the fetus or developing child,” he said.
Dr. House received 68 survey responses from AAPL members, most of whom were male with an average age of 47 years. The 7-question survey presented various clinical scenarios and asked what the respondent would do.
The first question concerned being called to the emergency department to evaluate a 28-year-old white woman with clinical signs of depression, history of a suicide attempt, and a mother who had committed suicide when the patient was 15. However, she states during evaluation: “I could never actually kill myself. My family would be too upset, and I would go to hell.”
Two-thirds of respondents (67.6%) said they would admit the woman to an inpatient unit for stabilization, and the others would discharge her with close follow-up.
The second question asked what the clinician would do if the patient declined admission: 41.2% would discharge, and 58.8% would place the woman on a psychiatric hold.
The third question introduced a positive pregnancy test for the woman, but none of the respondents said they would cancel the psychiatric hold. Most were split between proceeding with a hold (42.6%) or proceeding with a discharge (47.1%), though 10.3% would cancel the discharge and place the patient on a hold. Ultimately, respondents were no more likely to put the woman on a hold whether she was pregnant or not.
Then the survey repeated the scenario, but instead of a positive pregnancy test, the question asked what clinicians would do if her drug screen were positive after she had refused admission. In that scenario, the woman reported daily methamphetamine use to the emergency physician.
Among respondents, 48.5% would proceed with a psychiatric hold, 42.6% would proceed with a discharge, and 8.8% would cancel the discharge and put the patient on a hold.
The final question asks clinicians’ course of action if the woman’s pregnancy test were positive after the positive drug screen. Now, only a little over a quarter of respondents (26.5%) would proceed with a discharge and follow-up. More than half (57.4%) would proceed with a hold, and 16.2% would cancel the discharge and place a psychiatric hold.
Therefore, 73.6% of clinicians would place a pregnant woman with a history of substance use on a psychiatric hold, compared with 52.9% if the woman were pregnant but not using methamphetamine.
Laws on pregnancy, substance use
Dr. House considered those findings within the context of current laws governing substance use during pregnancy., with prosecution usually requiring detection of the substance at birth or during pregnancy, or evidence of risk to the child’s health.
Tennessee is unique in considering substance abuse in pregnancy assault if the child is born with dependence or other harm from the drug use. Women in Minnesota, South Dakota, and Wisconsin can be subject to civil commitment, including required inpatient drug treatment, for substance abuse during pregnancy (Am J Psychiatry. 2016 Nov 1;173[11]:1077-80).
Mandatory reporting laws for suspected substance abuse during pregnancy exist in 15 states, mostly in the Southwest, northern Midwest, and states around the District of Columbia. And four states – Kentucky, Iowa, Minnesota, and North Dakota – require pregnant women suspected of substance abuse to be tested.
Yet, most major relevant medical associations oppose criminalization of substance use during pregnancy, including the American Psychiatric Association, the American Academy of Addiction Psychiatry, the American Medical Association, and the American College of Obstetricians and Gynecologists.
“They are generally for increasing access for people, like voluntary screening, but against criminalization because it creates a barrier to accessing prenatal care,” Dr. House explained.
Aside from the question of whom psychiatrists consider their patients – the woman, her fetus, or both – the results raise another question, Dr. House said: “While studies have shown that criminalizing substance use during pregnancy discourages mothers from seeking prenatal care, does the threat of an involuntary psychiatric admission have a similar consequence?” That’s a question for further research.
No external funding was used. Dr. House was a clinical investigator without compensation for Janssen Pharmaceuticals from 2015 to 2017.
REPORTING FROM THE AAPL ANNUAL MEETING
Key clinical point: Women are more likely to receive a psychiatric hold if they are pregnant and using a substance.
Major finding: Almost 53% of clinicians would place a suicidal pregnant woman on a psychiatric hold, but 73.6% would do so if she were using methamphetamines.
Study details: The findings are based on an Internet survey of 68 members of the American Academy of Psychiatry and the Law.
Disclosures: No external funding was used. Dr. House was a clinical investigator without compensation for Janssen Pharmaceuticals from 2015 to 2017.
Glucocorticoid elimination works for 2/3 of RA patients on tocilizumab
CHICAGO – while remaining in at least low disease activity in a multicenter, randomized trial with 259 patients.
The results also showed that rheumatoid arthritis (RA) patients randomized to maintain prednisone treatment at 5 mg/day along with tocilizumab fared even better. After 24 weeks of the glucocorticoid-tapering regimen, by which time patients in the tapered arm had their prednisone dosage down to 0 mg, the average change from baseline in their disease activity score 28 with erythrocyte sedimentation rate (DAS28-ESR) was an increase of 0.538, compared with an average drop of 0.075 among untapered patients, a statistically significant difference for the study’s primary endpoint, Gerd R. Burmester, MD, said at the annual meeting of the American College of Rheumatology.
But while the results definitively showed that RA patients treated with the anti-interleukin 6 agent tocilizumab (Actemra) as their primary drug benefited from continued, additional treatment with low-dose prednisone, the results also showed that many patients could come off prednisone without immediate downside. Among the 131 randomized to a forced taper and eventual prednisone discontinuation, 85 (65%) went through this process without flaring and maintained at least low disease activity and sometimes remission, said Dr. Burmester, a professor and director of rheumatology and clinical immunology at Charité Hospital in Berlin. In contrast, 99 of 128 patients (77%) maintained on tocilizumab plus 5 mg/day of prednisone were free from any flares and had at least low disease activity after 24 weeks, a statistically significant between-group difference.
These findings “have potential to inform clinical practice and aid in conversations with patients. RA patients who achieve at least low disease activity while receiving tocilizumab and long-term glucocorticoid treatment at 5 mg/day should be considered for tapering of their glucocorticoid dosage, ideally targeting discontinuation,” Dr. Burmester said.
Addressing a comment from the audience that it’s common knowledge that patients who are well controlled on a potent immunomodulating drug like tocilizumab can often successfully wean off a glucocorticoid, Dr. Burmester insisted that it was important to show this in a randomized, blinded trial.
“When they discontinue [a glucocorticoid] patients can develop myalgias or other symptoms and we haven’t known whether that was real or psychological. In this study patients did not know whether they were being discontinued, so the psychological element was gone,” he said. The new findings confirm in a rigorous way that “we could discontinue a substantial number of patients who probably no longer need a glucocorticoid. It’s an important discussion” for clinicians to have with patients.
The SEMIRA (Steroid Elimination in RA) trial enrolled 68 patients who had already begun tocilizumab treatment and 191 patients new to the drug who had a 24-week run-in on tocilizumab, all of whom also received 5 mg prednisone daily. The trial included 46 sites in five European countries plus Russia and Tunisia. During the main 24-week phase of the study, 128 patients remained on their entry regimen of tocilizumab and prednisone, with 112 completers; 131 others went through a forced taper that culminated in no prednisone administered during the final 8 weeks, with 114 completers. Patients averaged about 54 years old, and just over three-quarters were women. Almost two-thirds of patients also received a nonbiologic disease-modifying antirheumatic drug.
A time-trend analysis of changes in DAS28-ERS showed that, while the difference in change from baseline in disease activity between the two treatment arms separated early in the taper process, the biggest uptick in DAS28-ESR in the patients coming off prednisone occurred once the final 1 mg/day dose was stopped.
The results also showed that tapering led to a statistically significant rise in the clinical disease activity index for RA, compared with that seen with not tapering, and tapering also was linked significantly with an increase in serum markers of bone turnover. The safety analysis showed no striking between-group differences in adverse events and in serious adverse events, and no patients developed confirmed adrenal insufficiency that required replacement therapy.
SOURCE: Burmester G et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract L18.
Tapering well-controlled rheumatoid arthritis patients off glucocorticoid treatment is often attempted in clinical practice, but until now no evidence base existed to justify this approach. While the results reported by Dr. Burmester from the SEMIRA study are not unexpected, it’s very helpful to see these data from a well-designed study.
What remains unclear is the generalizability of the findings: Do they only apply to patients receiving tocilizumab as their primary antirheumatic drug or does glucocorticoid tapering work the same way in RA patients on different drugs?
The results also confirm another impression that many clinicians have had but until now was unsubstantiated: Eliminating the final 1 mg of daily glucocorticoid treatment is the most difficult part of tapering patients down to complete corticosteroid withdrawal. What often happens in practice is that patients get down to the last 1 or 2 mg of daily glucocorticoid treatment but further reduction beyond that causes a flare or a manifestation of steroid-withdrawal syndrome.
The results from this study give clinicians a better context for discussing glucocorticoid tapering with patients. Even with a potent antirheumatic drug like tocilizumab, it’s hard for some patients to completely withdraw from a glucocorticoid. Even at very low dosages glucocorticoids still have a very meaningful effect on some patients.
David S. Pisetsky, MD , is a professor of medicine at Duke University in Durham, N.C. He has been a consultant to Celgene, Celltrion, GlaxoSmithKline, and Lilly, and he has received research support from Pfizer. He made these comments in an interview.
Tapering well-controlled rheumatoid arthritis patients off glucocorticoid treatment is often attempted in clinical practice, but until now no evidence base existed to justify this approach. While the results reported by Dr. Burmester from the SEMIRA study are not unexpected, it’s very helpful to see these data from a well-designed study.
What remains unclear is the generalizability of the findings: Do they only apply to patients receiving tocilizumab as their primary antirheumatic drug or does glucocorticoid tapering work the same way in RA patients on different drugs?
The results also confirm another impression that many clinicians have had but until now was unsubstantiated: Eliminating the final 1 mg of daily glucocorticoid treatment is the most difficult part of tapering patients down to complete corticosteroid withdrawal. What often happens in practice is that patients get down to the last 1 or 2 mg of daily glucocorticoid treatment but further reduction beyond that causes a flare or a manifestation of steroid-withdrawal syndrome.
The results from this study give clinicians a better context for discussing glucocorticoid tapering with patients. Even with a potent antirheumatic drug like tocilizumab, it’s hard for some patients to completely withdraw from a glucocorticoid. Even at very low dosages glucocorticoids still have a very meaningful effect on some patients.
David S. Pisetsky, MD , is a professor of medicine at Duke University in Durham, N.C. He has been a consultant to Celgene, Celltrion, GlaxoSmithKline, and Lilly, and he has received research support from Pfizer. He made these comments in an interview.
Tapering well-controlled rheumatoid arthritis patients off glucocorticoid treatment is often attempted in clinical practice, but until now no evidence base existed to justify this approach. While the results reported by Dr. Burmester from the SEMIRA study are not unexpected, it’s very helpful to see these data from a well-designed study.
What remains unclear is the generalizability of the findings: Do they only apply to patients receiving tocilizumab as their primary antirheumatic drug or does glucocorticoid tapering work the same way in RA patients on different drugs?
The results also confirm another impression that many clinicians have had but until now was unsubstantiated: Eliminating the final 1 mg of daily glucocorticoid treatment is the most difficult part of tapering patients down to complete corticosteroid withdrawal. What often happens in practice is that patients get down to the last 1 or 2 mg of daily glucocorticoid treatment but further reduction beyond that causes a flare or a manifestation of steroid-withdrawal syndrome.
The results from this study give clinicians a better context for discussing glucocorticoid tapering with patients. Even with a potent antirheumatic drug like tocilizumab, it’s hard for some patients to completely withdraw from a glucocorticoid. Even at very low dosages glucocorticoids still have a very meaningful effect on some patients.
David S. Pisetsky, MD , is a professor of medicine at Duke University in Durham, N.C. He has been a consultant to Celgene, Celltrion, GlaxoSmithKline, and Lilly, and he has received research support from Pfizer. He made these comments in an interview.
CHICAGO – while remaining in at least low disease activity in a multicenter, randomized trial with 259 patients.
The results also showed that rheumatoid arthritis (RA) patients randomized to maintain prednisone treatment at 5 mg/day along with tocilizumab fared even better. After 24 weeks of the glucocorticoid-tapering regimen, by which time patients in the tapered arm had their prednisone dosage down to 0 mg, the average change from baseline in their disease activity score 28 with erythrocyte sedimentation rate (DAS28-ESR) was an increase of 0.538, compared with an average drop of 0.075 among untapered patients, a statistically significant difference for the study’s primary endpoint, Gerd R. Burmester, MD, said at the annual meeting of the American College of Rheumatology.
But while the results definitively showed that RA patients treated with the anti-interleukin 6 agent tocilizumab (Actemra) as their primary drug benefited from continued, additional treatment with low-dose prednisone, the results also showed that many patients could come off prednisone without immediate downside. Among the 131 randomized to a forced taper and eventual prednisone discontinuation, 85 (65%) went through this process without flaring and maintained at least low disease activity and sometimes remission, said Dr. Burmester, a professor and director of rheumatology and clinical immunology at Charité Hospital in Berlin. In contrast, 99 of 128 patients (77%) maintained on tocilizumab plus 5 mg/day of prednisone were free from any flares and had at least low disease activity after 24 weeks, a statistically significant between-group difference.
These findings “have potential to inform clinical practice and aid in conversations with patients. RA patients who achieve at least low disease activity while receiving tocilizumab and long-term glucocorticoid treatment at 5 mg/day should be considered for tapering of their glucocorticoid dosage, ideally targeting discontinuation,” Dr. Burmester said.
Addressing a comment from the audience that it’s common knowledge that patients who are well controlled on a potent immunomodulating drug like tocilizumab can often successfully wean off a glucocorticoid, Dr. Burmester insisted that it was important to show this in a randomized, blinded trial.
“When they discontinue [a glucocorticoid] patients can develop myalgias or other symptoms and we haven’t known whether that was real or psychological. In this study patients did not know whether they were being discontinued, so the psychological element was gone,” he said. The new findings confirm in a rigorous way that “we could discontinue a substantial number of patients who probably no longer need a glucocorticoid. It’s an important discussion” for clinicians to have with patients.
The SEMIRA (Steroid Elimination in RA) trial enrolled 68 patients who had already begun tocilizumab treatment and 191 patients new to the drug who had a 24-week run-in on tocilizumab, all of whom also received 5 mg prednisone daily. The trial included 46 sites in five European countries plus Russia and Tunisia. During the main 24-week phase of the study, 128 patients remained on their entry regimen of tocilizumab and prednisone, with 112 completers; 131 others went through a forced taper that culminated in no prednisone administered during the final 8 weeks, with 114 completers. Patients averaged about 54 years old, and just over three-quarters were women. Almost two-thirds of patients also received a nonbiologic disease-modifying antirheumatic drug.
A time-trend analysis of changes in DAS28-ERS showed that, while the difference in change from baseline in disease activity between the two treatment arms separated early in the taper process, the biggest uptick in DAS28-ESR in the patients coming off prednisone occurred once the final 1 mg/day dose was stopped.
The results also showed that tapering led to a statistically significant rise in the clinical disease activity index for RA, compared with that seen with not tapering, and tapering also was linked significantly with an increase in serum markers of bone turnover. The safety analysis showed no striking between-group differences in adverse events and in serious adverse events, and no patients developed confirmed adrenal insufficiency that required replacement therapy.
SOURCE: Burmester G et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract L18.
CHICAGO – while remaining in at least low disease activity in a multicenter, randomized trial with 259 patients.
The results also showed that rheumatoid arthritis (RA) patients randomized to maintain prednisone treatment at 5 mg/day along with tocilizumab fared even better. After 24 weeks of the glucocorticoid-tapering regimen, by which time patients in the tapered arm had their prednisone dosage down to 0 mg, the average change from baseline in their disease activity score 28 with erythrocyte sedimentation rate (DAS28-ESR) was an increase of 0.538, compared with an average drop of 0.075 among untapered patients, a statistically significant difference for the study’s primary endpoint, Gerd R. Burmester, MD, said at the annual meeting of the American College of Rheumatology.
But while the results definitively showed that RA patients treated with the anti-interleukin 6 agent tocilizumab (Actemra) as their primary drug benefited from continued, additional treatment with low-dose prednisone, the results also showed that many patients could come off prednisone without immediate downside. Among the 131 randomized to a forced taper and eventual prednisone discontinuation, 85 (65%) went through this process without flaring and maintained at least low disease activity and sometimes remission, said Dr. Burmester, a professor and director of rheumatology and clinical immunology at Charité Hospital in Berlin. In contrast, 99 of 128 patients (77%) maintained on tocilizumab plus 5 mg/day of prednisone were free from any flares and had at least low disease activity after 24 weeks, a statistically significant between-group difference.
These findings “have potential to inform clinical practice and aid in conversations with patients. RA patients who achieve at least low disease activity while receiving tocilizumab and long-term glucocorticoid treatment at 5 mg/day should be considered for tapering of their glucocorticoid dosage, ideally targeting discontinuation,” Dr. Burmester said.
Addressing a comment from the audience that it’s common knowledge that patients who are well controlled on a potent immunomodulating drug like tocilizumab can often successfully wean off a glucocorticoid, Dr. Burmester insisted that it was important to show this in a randomized, blinded trial.
“When they discontinue [a glucocorticoid] patients can develop myalgias or other symptoms and we haven’t known whether that was real or psychological. In this study patients did not know whether they were being discontinued, so the psychological element was gone,” he said. The new findings confirm in a rigorous way that “we could discontinue a substantial number of patients who probably no longer need a glucocorticoid. It’s an important discussion” for clinicians to have with patients.
The SEMIRA (Steroid Elimination in RA) trial enrolled 68 patients who had already begun tocilizumab treatment and 191 patients new to the drug who had a 24-week run-in on tocilizumab, all of whom also received 5 mg prednisone daily. The trial included 46 sites in five European countries plus Russia and Tunisia. During the main 24-week phase of the study, 128 patients remained on their entry regimen of tocilizumab and prednisone, with 112 completers; 131 others went through a forced taper that culminated in no prednisone administered during the final 8 weeks, with 114 completers. Patients averaged about 54 years old, and just over three-quarters were women. Almost two-thirds of patients also received a nonbiologic disease-modifying antirheumatic drug.
A time-trend analysis of changes in DAS28-ERS showed that, while the difference in change from baseline in disease activity between the two treatment arms separated early in the taper process, the biggest uptick in DAS28-ESR in the patients coming off prednisone occurred once the final 1 mg/day dose was stopped.
The results also showed that tapering led to a statistically significant rise in the clinical disease activity index for RA, compared with that seen with not tapering, and tapering also was linked significantly with an increase in serum markers of bone turnover. The safety analysis showed no striking between-group differences in adverse events and in serious adverse events, and no patients developed confirmed adrenal insufficiency that required replacement therapy.
SOURCE: Burmester G et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract L18.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Many rheumatoid arthritis patients with low disease activity on tocilizumab plus a glucocorticoid can taper off the corticosteroid without flaring.
Major finding: In RA patients with low disease activity on tocilizumab plus prednisone, 65% came off the glucocorticoid without flaring or relapse.
Study details: SEMIRA, a multicenter, randomized study with 259 rheumatoid arthritis patients.
Disclosures: SEMIRA was funded by Hoffmann-La Roche. Dr. Burmester has been a consultant to and speaker on behalf of Roche and Sanofi-Genzyme.
Source: Burmester G et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract L18.
Overshadowed by opioids, meth is back and hospitalizations surge
The number of people hospitalized because of amphetamine use is skyrocketing in the United States, but the resurgence of the drug largely has been overshadowed by the nation’s intense focus on opioids.
Amphetamine-related hospitalizations jumped by about 245% during 2008-2015, according to a recent study in the Journal of the American Medical Association. That dwarfs the rise in hospitalizations from other drugs, such as opioids, which were up by about 46%. The most significant increases were in Western states.
The surge in hospitalizations and deaths from amphetamines “is just totally off the radar,” said Jane Maxwell, PhD, an researcher at the Addiction Research Institute at the University of Texas at Austin. “Nobody is paying attention.”
Doctors see evidence of the drug’s comeback in emergency departments, where patients arrive agitated, paranoid, and aggressive. Paramedics and police officers see it on the streets, where suspects’ heart rates are so high that they need to be taken to the hospital for medical clearance before being booked into jail. And medical examiners see it in the morgue, where in a few states, such as Texas and Colorado, overdoses from meth have surpassed those from the opioid heroin.
Amphetamines are stimulant drugs, which are legally prescribed to treat ADHD and produced illegally as methamphetamine. Most of the hospitalizations in the study are believed to be from methamphetamine use.
Commonly known as crystal meth, methamphetamine was popular in the 1990s before laws made it more difficult to access the pseudoephedrine, a common cold medicine, needed to produce it. In recent years, law enforcement officials said, there are fewer domestic meth labs and more meth is smuggled in from south of the border.
As opioids become harder to get, police said, more people have turned to meth, which is inexpensive and readily available.
Lupita Ruiz, 25, started using methamphetamine in her late teens but said she has been clean for about 2 years. When she was using, she said, her heart beat fast, she would stay up all night, and she would forget to eat.
Ms. Ruiz, who lives in Spokane, Wash., said she was taken to the hospital twice after having mental breakdowns related to methamphetamine use, including a month-long stay in the psychiatric ward in 2016. One time, Ms. Ruiz said, she yelled at and kicked police officers after they responded to a call to her apartment. Another time, she started walking on the freeway but doesn’t remember why.
“It just made me go crazy,” she said. “I was all messed up in my head.”
The federal government estimates that more than 10,000 people died of meth-related drug overdoses last year. Deaths from meth overdose generally result from multiple organ failure or heart attacks and strokes, caused by extraordinary pulse rates and skyrocketing blood pressure.
In California, the number of amphetamine-related overdose deaths rose by 127% from 456 in 2008 to 1,036 in 2013. During that same time, the number of opioid-related overdose deaths rose by 8.4% from 1,784 to 1,934, according to the most recent data from the state Department of Public Health.
“It taxes your first responders, your emergency rooms, your coroners,” said Robert Pennal, a retired supervisor with the California Department of Justice. “It’s an incredible burden on the health system.”
Costs also are rising. The JAMA study, based on hospital discharge data, found that the cost of amphetamine-related hospitalizations had jumped from $436 million in 2003 to nearly $2.2 billion by 2015. Medicaid was the primary payer.
“There is not a day that goes by that I don’t see someone acutely intoxicated on methamphetamine,” said Tarak Trivedi, MD,, an emergency room physician in Los Angeles and Santa Clara counties. “It’s a huge problem, and it is 100% spilling over into the emergency room.”
Dr. Trivedi said many psychiatric patients also are meth users. Some act so dangerously that they require sedation or restraints. He often sees people who have been using the drug for a long time and are dealing with the downstream consequences.
In the short term, the drug can cause a rapid heart rate and dangerously high blood pressure. In the long term, it can cause anxiety, dental problems and weight loss.
“You see people as young as their 30s with congestive heart failure as if they were in their 70s,” he said.
Jon E. Lopey, the sheriff-coroner of Siskiyou County in rural Northern California, said his officers frequently encounter meth users who are prone to violence and in the midst of what appear to be psychotic episodes. Many are emaciated and have missing teeth, dilated pupils, and a tendency to pick at their skin because of a sensation of something beneath it.
“Meth is very, very destructive,” said Sheriff Lopey, who also sits on the executive board of the California Peace Officers Association. “It is just so debilitating the way it ruins lives and health.”
Nationwide, amphetamine-related hospitalizations were primarily from mental health or cardiovascular complications of the drug use, the JAMA study found. About half of the amphetamine hospitalizations also involved at least one other drug.
Because there has been so much attention on opioids, “we have not been properly keeping tabs on other substance use trends as robustly as we should,” said study author Tyler Winkelman, MD, a physician at Hennepin Healthcare in Minneapolis.
Sometimes doctors have trouble distinguishing symptoms of methamphetamine intoxication and underlying mental health conditions, said Erik Anderson, MD, an ED physician at Highland Hospital in Oakland, Calif. Patients also may be homeless and using other drugs alongside the methamphetamine.
Unlike opioid addiction, meth addiction cannot be treated with medication. Rather, people addicted to the drug rely on counseling through outpatient and residential treatment centers.
The opioid epidemic, which resulted in about 49,000 overdose deaths last year, recently prompted bipartisan federal legislation to improve access to recovery, expand coverage to treatment, and combat drugs coming across the border.
There hasn’t been a similar recent legislative focus on methamphetamine or other drugs. And there simply aren’t enough resources devoted to amphetamine addiction to reduce the hospitalizations and deaths, said Dr. Maxwell. The number of residential treatment facilities, for example, has continued to decline, she said.
“We have really undercut treatment for methamphetamine,” Dr. Maxwell said. “Meth has been completely overshadowed by opioids.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage in California is supported in part by Blue Shield of California Foundation.
The number of people hospitalized because of amphetamine use is skyrocketing in the United States, but the resurgence of the drug largely has been overshadowed by the nation’s intense focus on opioids.
Amphetamine-related hospitalizations jumped by about 245% during 2008-2015, according to a recent study in the Journal of the American Medical Association. That dwarfs the rise in hospitalizations from other drugs, such as opioids, which were up by about 46%. The most significant increases were in Western states.
The surge in hospitalizations and deaths from amphetamines “is just totally off the radar,” said Jane Maxwell, PhD, an researcher at the Addiction Research Institute at the University of Texas at Austin. “Nobody is paying attention.”
Doctors see evidence of the drug’s comeback in emergency departments, where patients arrive agitated, paranoid, and aggressive. Paramedics and police officers see it on the streets, where suspects’ heart rates are so high that they need to be taken to the hospital for medical clearance before being booked into jail. And medical examiners see it in the morgue, where in a few states, such as Texas and Colorado, overdoses from meth have surpassed those from the opioid heroin.
Amphetamines are stimulant drugs, which are legally prescribed to treat ADHD and produced illegally as methamphetamine. Most of the hospitalizations in the study are believed to be from methamphetamine use.
Commonly known as crystal meth, methamphetamine was popular in the 1990s before laws made it more difficult to access the pseudoephedrine, a common cold medicine, needed to produce it. In recent years, law enforcement officials said, there are fewer domestic meth labs and more meth is smuggled in from south of the border.
As opioids become harder to get, police said, more people have turned to meth, which is inexpensive and readily available.
Lupita Ruiz, 25, started using methamphetamine in her late teens but said she has been clean for about 2 years. When she was using, she said, her heart beat fast, she would stay up all night, and she would forget to eat.
Ms. Ruiz, who lives in Spokane, Wash., said she was taken to the hospital twice after having mental breakdowns related to methamphetamine use, including a month-long stay in the psychiatric ward in 2016. One time, Ms. Ruiz said, she yelled at and kicked police officers after they responded to a call to her apartment. Another time, she started walking on the freeway but doesn’t remember why.
“It just made me go crazy,” she said. “I was all messed up in my head.”
The federal government estimates that more than 10,000 people died of meth-related drug overdoses last year. Deaths from meth overdose generally result from multiple organ failure or heart attacks and strokes, caused by extraordinary pulse rates and skyrocketing blood pressure.
In California, the number of amphetamine-related overdose deaths rose by 127% from 456 in 2008 to 1,036 in 2013. During that same time, the number of opioid-related overdose deaths rose by 8.4% from 1,784 to 1,934, according to the most recent data from the state Department of Public Health.
“It taxes your first responders, your emergency rooms, your coroners,” said Robert Pennal, a retired supervisor with the California Department of Justice. “It’s an incredible burden on the health system.”
Costs also are rising. The JAMA study, based on hospital discharge data, found that the cost of amphetamine-related hospitalizations had jumped from $436 million in 2003 to nearly $2.2 billion by 2015. Medicaid was the primary payer.
“There is not a day that goes by that I don’t see someone acutely intoxicated on methamphetamine,” said Tarak Trivedi, MD,, an emergency room physician in Los Angeles and Santa Clara counties. “It’s a huge problem, and it is 100% spilling over into the emergency room.”
Dr. Trivedi said many psychiatric patients also are meth users. Some act so dangerously that they require sedation or restraints. He often sees people who have been using the drug for a long time and are dealing with the downstream consequences.
In the short term, the drug can cause a rapid heart rate and dangerously high blood pressure. In the long term, it can cause anxiety, dental problems and weight loss.
“You see people as young as their 30s with congestive heart failure as if they were in their 70s,” he said.
Jon E. Lopey, the sheriff-coroner of Siskiyou County in rural Northern California, said his officers frequently encounter meth users who are prone to violence and in the midst of what appear to be psychotic episodes. Many are emaciated and have missing teeth, dilated pupils, and a tendency to pick at their skin because of a sensation of something beneath it.
“Meth is very, very destructive,” said Sheriff Lopey, who also sits on the executive board of the California Peace Officers Association. “It is just so debilitating the way it ruins lives and health.”
Nationwide, amphetamine-related hospitalizations were primarily from mental health or cardiovascular complications of the drug use, the JAMA study found. About half of the amphetamine hospitalizations also involved at least one other drug.
Because there has been so much attention on opioids, “we have not been properly keeping tabs on other substance use trends as robustly as we should,” said study author Tyler Winkelman, MD, a physician at Hennepin Healthcare in Minneapolis.
Sometimes doctors have trouble distinguishing symptoms of methamphetamine intoxication and underlying mental health conditions, said Erik Anderson, MD, an ED physician at Highland Hospital in Oakland, Calif. Patients also may be homeless and using other drugs alongside the methamphetamine.
Unlike opioid addiction, meth addiction cannot be treated with medication. Rather, people addicted to the drug rely on counseling through outpatient and residential treatment centers.
The opioid epidemic, which resulted in about 49,000 overdose deaths last year, recently prompted bipartisan federal legislation to improve access to recovery, expand coverage to treatment, and combat drugs coming across the border.
There hasn’t been a similar recent legislative focus on methamphetamine or other drugs. And there simply aren’t enough resources devoted to amphetamine addiction to reduce the hospitalizations and deaths, said Dr. Maxwell. The number of residential treatment facilities, for example, has continued to decline, she said.
“We have really undercut treatment for methamphetamine,” Dr. Maxwell said. “Meth has been completely overshadowed by opioids.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage in California is supported in part by Blue Shield of California Foundation.
The number of people hospitalized because of amphetamine use is skyrocketing in the United States, but the resurgence of the drug largely has been overshadowed by the nation’s intense focus on opioids.
Amphetamine-related hospitalizations jumped by about 245% during 2008-2015, according to a recent study in the Journal of the American Medical Association. That dwarfs the rise in hospitalizations from other drugs, such as opioids, which were up by about 46%. The most significant increases were in Western states.
The surge in hospitalizations and deaths from amphetamines “is just totally off the radar,” said Jane Maxwell, PhD, an researcher at the Addiction Research Institute at the University of Texas at Austin. “Nobody is paying attention.”
Doctors see evidence of the drug’s comeback in emergency departments, where patients arrive agitated, paranoid, and aggressive. Paramedics and police officers see it on the streets, where suspects’ heart rates are so high that they need to be taken to the hospital for medical clearance before being booked into jail. And medical examiners see it in the morgue, where in a few states, such as Texas and Colorado, overdoses from meth have surpassed those from the opioid heroin.
Amphetamines are stimulant drugs, which are legally prescribed to treat ADHD and produced illegally as methamphetamine. Most of the hospitalizations in the study are believed to be from methamphetamine use.
Commonly known as crystal meth, methamphetamine was popular in the 1990s before laws made it more difficult to access the pseudoephedrine, a common cold medicine, needed to produce it. In recent years, law enforcement officials said, there are fewer domestic meth labs and more meth is smuggled in from south of the border.
As opioids become harder to get, police said, more people have turned to meth, which is inexpensive and readily available.
Lupita Ruiz, 25, started using methamphetamine in her late teens but said she has been clean for about 2 years. When she was using, she said, her heart beat fast, she would stay up all night, and she would forget to eat.
Ms. Ruiz, who lives in Spokane, Wash., said she was taken to the hospital twice after having mental breakdowns related to methamphetamine use, including a month-long stay in the psychiatric ward in 2016. One time, Ms. Ruiz said, she yelled at and kicked police officers after they responded to a call to her apartment. Another time, she started walking on the freeway but doesn’t remember why.
“It just made me go crazy,” she said. “I was all messed up in my head.”
The federal government estimates that more than 10,000 people died of meth-related drug overdoses last year. Deaths from meth overdose generally result from multiple organ failure or heart attacks and strokes, caused by extraordinary pulse rates and skyrocketing blood pressure.
In California, the number of amphetamine-related overdose deaths rose by 127% from 456 in 2008 to 1,036 in 2013. During that same time, the number of opioid-related overdose deaths rose by 8.4% from 1,784 to 1,934, according to the most recent data from the state Department of Public Health.
“It taxes your first responders, your emergency rooms, your coroners,” said Robert Pennal, a retired supervisor with the California Department of Justice. “It’s an incredible burden on the health system.”
Costs also are rising. The JAMA study, based on hospital discharge data, found that the cost of amphetamine-related hospitalizations had jumped from $436 million in 2003 to nearly $2.2 billion by 2015. Medicaid was the primary payer.
“There is not a day that goes by that I don’t see someone acutely intoxicated on methamphetamine,” said Tarak Trivedi, MD,, an emergency room physician in Los Angeles and Santa Clara counties. “It’s a huge problem, and it is 100% spilling over into the emergency room.”
Dr. Trivedi said many psychiatric patients also are meth users. Some act so dangerously that they require sedation or restraints. He often sees people who have been using the drug for a long time and are dealing with the downstream consequences.
In the short term, the drug can cause a rapid heart rate and dangerously high blood pressure. In the long term, it can cause anxiety, dental problems and weight loss.
“You see people as young as their 30s with congestive heart failure as if they were in their 70s,” he said.
Jon E. Lopey, the sheriff-coroner of Siskiyou County in rural Northern California, said his officers frequently encounter meth users who are prone to violence and in the midst of what appear to be psychotic episodes. Many are emaciated and have missing teeth, dilated pupils, and a tendency to pick at their skin because of a sensation of something beneath it.
“Meth is very, very destructive,” said Sheriff Lopey, who also sits on the executive board of the California Peace Officers Association. “It is just so debilitating the way it ruins lives and health.”
Nationwide, amphetamine-related hospitalizations were primarily from mental health or cardiovascular complications of the drug use, the JAMA study found. About half of the amphetamine hospitalizations also involved at least one other drug.
Because there has been so much attention on opioids, “we have not been properly keeping tabs on other substance use trends as robustly as we should,” said study author Tyler Winkelman, MD, a physician at Hennepin Healthcare in Minneapolis.
Sometimes doctors have trouble distinguishing symptoms of methamphetamine intoxication and underlying mental health conditions, said Erik Anderson, MD, an ED physician at Highland Hospital in Oakland, Calif. Patients also may be homeless and using other drugs alongside the methamphetamine.
Unlike opioid addiction, meth addiction cannot be treated with medication. Rather, people addicted to the drug rely on counseling through outpatient and residential treatment centers.
The opioid epidemic, which resulted in about 49,000 overdose deaths last year, recently prompted bipartisan federal legislation to improve access to recovery, expand coverage to treatment, and combat drugs coming across the border.
There hasn’t been a similar recent legislative focus on methamphetamine or other drugs. And there simply aren’t enough resources devoted to amphetamine addiction to reduce the hospitalizations and deaths, said Dr. Maxwell. The number of residential treatment facilities, for example, has continued to decline, she said.
“We have really undercut treatment for methamphetamine,” Dr. Maxwell said. “Meth has been completely overshadowed by opioids.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage in California is supported in part by Blue Shield of California Foundation.
Vedolizumab, tofacitinib induced rapid improvements in IBD symptoms
Two recent studies highlight the ability of vedolizumab and tofacitinib to rapidly improve symptoms reported by patients with inflammatory bowel disease.
In a post hoc study of 1,758 patients with ulcerative colitis (UC) or Crohn’s disease (CD) in the phase 3 GEMINI trials, 2 weeks of vedolizumab (Entyvio) therapy effectively improved patient-reported outcomes, and these continued to improve through 6 weeks of treatment, wrote Brian G. Feagan, MD, and his associates in Clinical Gastroenterology and Hepatology.
Among UC patients who had not previously received tumor necrosis factor (TNF) antagonist therapy, 22% of vedolizumab recipients, compared with only 7% of placebo recipients, achieved complete resolution of rectal bleeding together with a meaningful reduction in stool frequency at treatment week 2, the investigators noted. Among CD patients who were naive to TNF antagonists, 15% reported meaningful decreases in abdominal pain and loose stools at treatment week 2, compared with only 8% of placebo recipients.
Although 2 weeks of vedolizumab also topped placebo for improving patient-reported outcomes among TNF antagonist–exposed patients, the effects were less pronounced, wrote Dr. Feagan, of the University of Western Ontario, London, and his associates. “These data add to the growing evidence that second-generation biologics, such as vedolizumab and ustekinumab, have higher efficacy in TNF antagonist–naive patients in both clinical trials and real-world settings. Recent trends in clinical practice are moving toward incorporating disease-modifying therapy earlier in the treatment of IBD to prevent disease progression and cumulative bowel damage; hence, the importance of evaluating the treatment effects of vedolizumab in biologic-naive patients.”
Patient-reported outcomes have become key during both clinical research and regulatory review of claims on proposed drug labels. In the second study, also published in Clinical Gastroenterology and Hepatology, Stephen B. Hanauer, MD, of Northwestern University, Chicago, and his associates performed a post hoc analysis of symptoms reported by 1,139 adults with UC who received the oral small-molecule Janus kinase inhibitor tofacitinib (10 g twice daily) or placebo during the 8-week OCTAVE Induction 1 and 2 trials. These were identical phase 3 studies of patients with moderate to severe UC who could not tolerate or had responded inadequately to TNF antagonists, corticosteroids, or thiopurines.
Compared with placebo, 3 days of tofacitinib therapy induced significantly greater reductions from baseline in patient-reported stool frequency and rectal bleeding (P less than .01 for each measure), Dr. Hanauer and his associates reported. The effect was independent of prior treatment for UC or baseline levels of C-reactive protein. These findings reflect the rapid onset of effect of tofacitinib therapy in patients with UC. In contrast, thiopurines (azathioprine and 6-mercaptopurine) can take at least 8 weeks to exhibit steroid-sparing effects.
While corticosteroids can induce UC remission within 5 days, their side effects tend to escalate over time and they “lack maintenance benefits,” the researchers wrote. “In these analyses, onset of tofacitinib efficacy occurred within 3 days, irrespective of concomitant corticosteroid use or prior anti-TNF treatment failure.”
Takeda funded the GEMINI studies. Dr. Feagan reported advisory relationships with Takeda, AbbVie, Amgen, AstraZeneca, and several other pharmaceutical companies. Dr. Hanauer also reported ties to numerous pharmaceutical companies, including Pfizer, which funded the OCTAVE trials.
SOURCES: Feagan BG et al. Clin Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.cgh.2018.05.026; Hanauer S et al. Clin Gastroenterol Hepatol. 2018 Jul 15. doi: 10.1016/j.cgh.2018.07.009.
Two recent studies highlight the ability of vedolizumab and tofacitinib to rapidly improve symptoms reported by patients with inflammatory bowel disease.
In a post hoc study of 1,758 patients with ulcerative colitis (UC) or Crohn’s disease (CD) in the phase 3 GEMINI trials, 2 weeks of vedolizumab (Entyvio) therapy effectively improved patient-reported outcomes, and these continued to improve through 6 weeks of treatment, wrote Brian G. Feagan, MD, and his associates in Clinical Gastroenterology and Hepatology.
Among UC patients who had not previously received tumor necrosis factor (TNF) antagonist therapy, 22% of vedolizumab recipients, compared with only 7% of placebo recipients, achieved complete resolution of rectal bleeding together with a meaningful reduction in stool frequency at treatment week 2, the investigators noted. Among CD patients who were naive to TNF antagonists, 15% reported meaningful decreases in abdominal pain and loose stools at treatment week 2, compared with only 8% of placebo recipients.
Although 2 weeks of vedolizumab also topped placebo for improving patient-reported outcomes among TNF antagonist–exposed patients, the effects were less pronounced, wrote Dr. Feagan, of the University of Western Ontario, London, and his associates. “These data add to the growing evidence that second-generation biologics, such as vedolizumab and ustekinumab, have higher efficacy in TNF antagonist–naive patients in both clinical trials and real-world settings. Recent trends in clinical practice are moving toward incorporating disease-modifying therapy earlier in the treatment of IBD to prevent disease progression and cumulative bowel damage; hence, the importance of evaluating the treatment effects of vedolizumab in biologic-naive patients.”
Patient-reported outcomes have become key during both clinical research and regulatory review of claims on proposed drug labels. In the second study, also published in Clinical Gastroenterology and Hepatology, Stephen B. Hanauer, MD, of Northwestern University, Chicago, and his associates performed a post hoc analysis of symptoms reported by 1,139 adults with UC who received the oral small-molecule Janus kinase inhibitor tofacitinib (10 g twice daily) or placebo during the 8-week OCTAVE Induction 1 and 2 trials. These were identical phase 3 studies of patients with moderate to severe UC who could not tolerate or had responded inadequately to TNF antagonists, corticosteroids, or thiopurines.
Compared with placebo, 3 days of tofacitinib therapy induced significantly greater reductions from baseline in patient-reported stool frequency and rectal bleeding (P less than .01 for each measure), Dr. Hanauer and his associates reported. The effect was independent of prior treatment for UC or baseline levels of C-reactive protein. These findings reflect the rapid onset of effect of tofacitinib therapy in patients with UC. In contrast, thiopurines (azathioprine and 6-mercaptopurine) can take at least 8 weeks to exhibit steroid-sparing effects.
While corticosteroids can induce UC remission within 5 days, their side effects tend to escalate over time and they “lack maintenance benefits,” the researchers wrote. “In these analyses, onset of tofacitinib efficacy occurred within 3 days, irrespective of concomitant corticosteroid use or prior anti-TNF treatment failure.”
Takeda funded the GEMINI studies. Dr. Feagan reported advisory relationships with Takeda, AbbVie, Amgen, AstraZeneca, and several other pharmaceutical companies. Dr. Hanauer also reported ties to numerous pharmaceutical companies, including Pfizer, which funded the OCTAVE trials.
SOURCES: Feagan BG et al. Clin Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.cgh.2018.05.026; Hanauer S et al. Clin Gastroenterol Hepatol. 2018 Jul 15. doi: 10.1016/j.cgh.2018.07.009.
Two recent studies highlight the ability of vedolizumab and tofacitinib to rapidly improve symptoms reported by patients with inflammatory bowel disease.
In a post hoc study of 1,758 patients with ulcerative colitis (UC) or Crohn’s disease (CD) in the phase 3 GEMINI trials, 2 weeks of vedolizumab (Entyvio) therapy effectively improved patient-reported outcomes, and these continued to improve through 6 weeks of treatment, wrote Brian G. Feagan, MD, and his associates in Clinical Gastroenterology and Hepatology.
Among UC patients who had not previously received tumor necrosis factor (TNF) antagonist therapy, 22% of vedolizumab recipients, compared with only 7% of placebo recipients, achieved complete resolution of rectal bleeding together with a meaningful reduction in stool frequency at treatment week 2, the investigators noted. Among CD patients who were naive to TNF antagonists, 15% reported meaningful decreases in abdominal pain and loose stools at treatment week 2, compared with only 8% of placebo recipients.
Although 2 weeks of vedolizumab also topped placebo for improving patient-reported outcomes among TNF antagonist–exposed patients, the effects were less pronounced, wrote Dr. Feagan, of the University of Western Ontario, London, and his associates. “These data add to the growing evidence that second-generation biologics, such as vedolizumab and ustekinumab, have higher efficacy in TNF antagonist–naive patients in both clinical trials and real-world settings. Recent trends in clinical practice are moving toward incorporating disease-modifying therapy earlier in the treatment of IBD to prevent disease progression and cumulative bowel damage; hence, the importance of evaluating the treatment effects of vedolizumab in biologic-naive patients.”
Patient-reported outcomes have become key during both clinical research and regulatory review of claims on proposed drug labels. In the second study, also published in Clinical Gastroenterology and Hepatology, Stephen B. Hanauer, MD, of Northwestern University, Chicago, and his associates performed a post hoc analysis of symptoms reported by 1,139 adults with UC who received the oral small-molecule Janus kinase inhibitor tofacitinib (10 g twice daily) or placebo during the 8-week OCTAVE Induction 1 and 2 trials. These were identical phase 3 studies of patients with moderate to severe UC who could not tolerate or had responded inadequately to TNF antagonists, corticosteroids, or thiopurines.
Compared with placebo, 3 days of tofacitinib therapy induced significantly greater reductions from baseline in patient-reported stool frequency and rectal bleeding (P less than .01 for each measure), Dr. Hanauer and his associates reported. The effect was independent of prior treatment for UC or baseline levels of C-reactive protein. These findings reflect the rapid onset of effect of tofacitinib therapy in patients with UC. In contrast, thiopurines (azathioprine and 6-mercaptopurine) can take at least 8 weeks to exhibit steroid-sparing effects.
While corticosteroids can induce UC remission within 5 days, their side effects tend to escalate over time and they “lack maintenance benefits,” the researchers wrote. “In these analyses, onset of tofacitinib efficacy occurred within 3 days, irrespective of concomitant corticosteroid use or prior anti-TNF treatment failure.”
Takeda funded the GEMINI studies. Dr. Feagan reported advisory relationships with Takeda, AbbVie, Amgen, AstraZeneca, and several other pharmaceutical companies. Dr. Hanauer also reported ties to numerous pharmaceutical companies, including Pfizer, which funded the OCTAVE trials.
SOURCES: Feagan BG et al. Clin Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.cgh.2018.05.026; Hanauer S et al. Clin Gastroenterol Hepatol. 2018 Jul 15. doi: 10.1016/j.cgh.2018.07.009.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The next-generation biologics vedolizumab and tofacitinib can rapidly improve clinical symptoms of inflammatory bowel disease.
Major finding: Both biologics induced clinically meaningful improvements in patient-reported outcomes early in the course of treatment: At 3 days, for tofacitinib in patients with ulcerative colitis, and by the second week of treatment, for vedolizumab in patients with ulcerative colitis or Crohn’s disease.
Study details: Post hoc analyses of symptom scores among patients with ulcerative colitis or Crohn’s disease from the placebo-controlled, phase 3 GEMINI and OCTAVE Induction 1 trials.
Disclosures: Takeda funded the GEMINI studies. Dr. Feagan reported advisory relationships with Takeda, AbbVie, Amgen, AstraZeneca, and several other pharmaceutical companies. Dr. Hanauer also reported ties to numerous pharmaceutical companies, including Pfizer, which funded the OCTAVE trials.
Sources: Feagan BG et al. Clin Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.cgh.2018.05.026; Hanauer S et al. Clin Gastroenterol Hepatol. 2018 Jul 15. doi: 10.1016/j.cgh.2018.07.009.
SABCS 2018: Further analysis on IMpassion130 for mTNBC
Investigators presented results of the phase 3 IMpassion130 earlier this year demonstrating, for the first time, that a combination of an immune checkpoint inhibitor and a taxane provided significant clinical benefit to patients with advanced triple-negative breast cancer. However, the benefit was seen only in patients positive for programmed death-ligand 1 (PD-L1), the investigators reported at the annual congress of the European Society for Medical Oncology.
In the trial, 902 patients with untreated metastatic triple-negative breast cancer (mTNBC) were randomly assigned to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-paclitaxel. In an interim analysis, although there was no significant difference in median overall survival among all participants, atezolizumab was associated with a 38% improvement in median overall survival among patients with PD-L1–positive disease.
Additional analyses of the efficacy within immune biomarker subgroups in IMpassion130, including efficacy by BRCA status, will be presented at the upcoming 2018 San Antonio Breast Cancer Symposium, to be held Dec. 4-8 in San Antonio.
Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology, co-leader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center, will present the additional analyses on Wednesday, Dec. 5th at 9:30 a.m. CST.
Investigators presented results of the phase 3 IMpassion130 earlier this year demonstrating, for the first time, that a combination of an immune checkpoint inhibitor and a taxane provided significant clinical benefit to patients with advanced triple-negative breast cancer. However, the benefit was seen only in patients positive for programmed death-ligand 1 (PD-L1), the investigators reported at the annual congress of the European Society for Medical Oncology.
In the trial, 902 patients with untreated metastatic triple-negative breast cancer (mTNBC) were randomly assigned to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-paclitaxel. In an interim analysis, although there was no significant difference in median overall survival among all participants, atezolizumab was associated with a 38% improvement in median overall survival among patients with PD-L1–positive disease.
Additional analyses of the efficacy within immune biomarker subgroups in IMpassion130, including efficacy by BRCA status, will be presented at the upcoming 2018 San Antonio Breast Cancer Symposium, to be held Dec. 4-8 in San Antonio.
Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology, co-leader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center, will present the additional analyses on Wednesday, Dec. 5th at 9:30 a.m. CST.
Investigators presented results of the phase 3 IMpassion130 earlier this year demonstrating, for the first time, that a combination of an immune checkpoint inhibitor and a taxane provided significant clinical benefit to patients with advanced triple-negative breast cancer. However, the benefit was seen only in patients positive for programmed death-ligand 1 (PD-L1), the investigators reported at the annual congress of the European Society for Medical Oncology.
In the trial, 902 patients with untreated metastatic triple-negative breast cancer (mTNBC) were randomly assigned to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-paclitaxel. In an interim analysis, although there was no significant difference in median overall survival among all participants, atezolizumab was associated with a 38% improvement in median overall survival among patients with PD-L1–positive disease.
Additional analyses of the efficacy within immune biomarker subgroups in IMpassion130, including efficacy by BRCA status, will be presented at the upcoming 2018 San Antonio Breast Cancer Symposium, to be held Dec. 4-8 in San Antonio.
Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology, co-leader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center, will present the additional analyses on Wednesday, Dec. 5th at 9:30 a.m. CST.






