User login
Part D proposal includes prior authorization, step therapy
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a Nov. 26 briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a Nov. 26 briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a Nov. 26 briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Skin rashes often accompany drug-induced liver injury
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Researchers hope the findings will shed light on the mechanism of injury.
Major finding: 28% of patients with DILI also had a skin rash.
Study details: Retrospective analysis of 921 DILI patients.
Disclosures: No source of funding was disclosed. Dr. Devarbhavi disclosed no relevant conflicts.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SABCS 2018: PHARE, KATHERINE, and KATE2 in HER2+ breast cancer
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Three drugs disappoint in SSc trials, but show some promise
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
REPORTING FROM THE ACR ANNUAL MEETING
Temixys plus other antiretrovirals approved for HIV-1
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
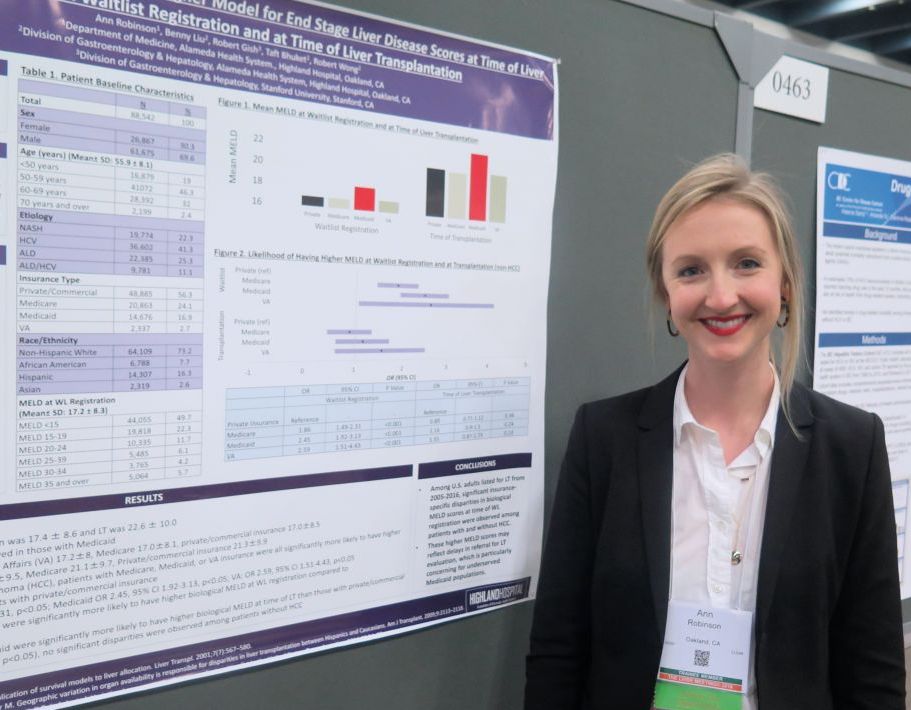
Medicaid patients have higher MELD scores at time of liver transplantation
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AT THE LIVER MEETING 2018
Key clinical point: Significant insurance-specific disparities in MELD scores at time of wait-list registration were observed among patients with and without hepatocellular carcinoma.
Major finding: Among patients without hepatocellular carcinoma, those with Medicaid coverage were 2.45 times more likely to have higher MELD scores at wait-list registration, compared with those covered by commercial or private insurance (P less than .01).
Study details: A retrospective analysis of 88,542 liver transplantation wait-list registrants.
Disclosures: Dr. Robinson reported having no disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AAP speaker emphasizes importance of understanding patients’ ‘lived experience’
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
REPORTING FROM AAP 2018
Use of smartphone app improves pain outcomes
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
REPORTING FROM PALLONC 2018
Key clinical point: Use of a smartphone app with artificial intelligence elements improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Major finding: Pain severity decreased over time from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, compared with baseline and 8-week values of 4.02 and 4.05 in the control group (P = .017).
Study details: A randomized study including 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were followed in a palliative care clinic.
Disclosures: The research was supported by the McKesson Foundation. The presenting author reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutic.
Source: Kamdar MM et al. PallOnc 2018, Abstract 76.
ACR CRISS: A way forward for scleroderma treatment trials?
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
REPORTING FROM THE ACR ANNUAL MEETING
The Liver Meeting 2018: Hepatitis B novel therapies debrief – key abstracts
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
REPORTING FROM THE LIVER MEETING 2018