User login
Sex differences provide insight into glioblastoma treatment
Sex-specific treatment strategies may prolong survival and improve outcomes in male and female patients with glioblastoma, investigators suggest after a retrospective analysis.
In an analysis of data from 63 men and women with glioblastoma, the researchers found that a significant reduction in tumor growth velocity during standard chemotherapeutic treatment was seen only for females patients with glioblastoma (P = .02569). In addition, they reported that for female patients, a reduction in tumor growth velocity was linked with significantly increased survival versus higher velocity growth (median survival, 3090 days vs. 681 days; P = .00817).
In contrast, the men in the study showed no statistically significant correlation between survival and velocity, the investigators reported.
The researchers analyzed data from 63 patients with glioblastoma using clinical information from a research database at the Mayo Clinic in Phoenix. They also incorporated transcriptome data, which was connected via an electronic algorithm, and were able to detect certain molecular variations of glioblastoma that are integral components of survival for individuals with the disease. From these findings, Wei Yang, PhD, of Washington University, St. Louis, along with his colleagues, were able to validate links between the effects of chemotherapy and gene expression.
“We measured sex differences in the in vitro cytotoxic effects of four common chemotherapeutics in a panel of nine (five male and four female) patient-derived glioblastoma cell isolates,” the investigators said in Science Translational Medicine.
The authors acknowledged a key limitation of the study was the retrospective nature of the imaging analysis, which has the potential to introduce interoperator differences in tumor growth velocity measurements. With these limitations, Dr. Yang and his colleagues highlighted the importance of ensuring imaging data is verified by trained professionals.
“Together, these results suggest that greater precision in glioblastoma molecular subtyping can be achieved through sex-specific analyses and that improved outcomes for all patients might be accomplished by tailoring treatment to sex differences in molecular mechanisms,” they concluded.
The study was supported by grant funding from the National Institutes of Health, Children’s Discovery Institute of Washington University, James S. McDonnell Foundation, Ivy Foundation, Ben & Catherine Ivy Foundation, and the Mayo Clinic. Albert H. Kim and Kristin R. Swanson reported financial affiliations with Monterris and the James S. McDonnell Foundation. Other authors reported no conflicts of interest related to the work.
SOURCE: Yang W et al. Sci Transl Med. 2019 Jan 02. doi: 10.1126/scitranslmed.aao5253.
Sex-specific treatment strategies may prolong survival and improve outcomes in male and female patients with glioblastoma, investigators suggest after a retrospective analysis.
In an analysis of data from 63 men and women with glioblastoma, the researchers found that a significant reduction in tumor growth velocity during standard chemotherapeutic treatment was seen only for females patients with glioblastoma (P = .02569). In addition, they reported that for female patients, a reduction in tumor growth velocity was linked with significantly increased survival versus higher velocity growth (median survival, 3090 days vs. 681 days; P = .00817).
In contrast, the men in the study showed no statistically significant correlation between survival and velocity, the investigators reported.
The researchers analyzed data from 63 patients with glioblastoma using clinical information from a research database at the Mayo Clinic in Phoenix. They also incorporated transcriptome data, which was connected via an electronic algorithm, and were able to detect certain molecular variations of glioblastoma that are integral components of survival for individuals with the disease. From these findings, Wei Yang, PhD, of Washington University, St. Louis, along with his colleagues, were able to validate links between the effects of chemotherapy and gene expression.
“We measured sex differences in the in vitro cytotoxic effects of four common chemotherapeutics in a panel of nine (five male and four female) patient-derived glioblastoma cell isolates,” the investigators said in Science Translational Medicine.
The authors acknowledged a key limitation of the study was the retrospective nature of the imaging analysis, which has the potential to introduce interoperator differences in tumor growth velocity measurements. With these limitations, Dr. Yang and his colleagues highlighted the importance of ensuring imaging data is verified by trained professionals.
“Together, these results suggest that greater precision in glioblastoma molecular subtyping can be achieved through sex-specific analyses and that improved outcomes for all patients might be accomplished by tailoring treatment to sex differences in molecular mechanisms,” they concluded.
The study was supported by grant funding from the National Institutes of Health, Children’s Discovery Institute of Washington University, James S. McDonnell Foundation, Ivy Foundation, Ben & Catherine Ivy Foundation, and the Mayo Clinic. Albert H. Kim and Kristin R. Swanson reported financial affiliations with Monterris and the James S. McDonnell Foundation. Other authors reported no conflicts of interest related to the work.
SOURCE: Yang W et al. Sci Transl Med. 2019 Jan 02. doi: 10.1126/scitranslmed.aao5253.
Sex-specific treatment strategies may prolong survival and improve outcomes in male and female patients with glioblastoma, investigators suggest after a retrospective analysis.
In an analysis of data from 63 men and women with glioblastoma, the researchers found that a significant reduction in tumor growth velocity during standard chemotherapeutic treatment was seen only for females patients with glioblastoma (P = .02569). In addition, they reported that for female patients, a reduction in tumor growth velocity was linked with significantly increased survival versus higher velocity growth (median survival, 3090 days vs. 681 days; P = .00817).
In contrast, the men in the study showed no statistically significant correlation between survival and velocity, the investigators reported.
The researchers analyzed data from 63 patients with glioblastoma using clinical information from a research database at the Mayo Clinic in Phoenix. They also incorporated transcriptome data, which was connected via an electronic algorithm, and were able to detect certain molecular variations of glioblastoma that are integral components of survival for individuals with the disease. From these findings, Wei Yang, PhD, of Washington University, St. Louis, along with his colleagues, were able to validate links between the effects of chemotherapy and gene expression.
“We measured sex differences in the in vitro cytotoxic effects of four common chemotherapeutics in a panel of nine (five male and four female) patient-derived glioblastoma cell isolates,” the investigators said in Science Translational Medicine.
The authors acknowledged a key limitation of the study was the retrospective nature of the imaging analysis, which has the potential to introduce interoperator differences in tumor growth velocity measurements. With these limitations, Dr. Yang and his colleagues highlighted the importance of ensuring imaging data is verified by trained professionals.
“Together, these results suggest that greater precision in glioblastoma molecular subtyping can be achieved through sex-specific analyses and that improved outcomes for all patients might be accomplished by tailoring treatment to sex differences in molecular mechanisms,” they concluded.
The study was supported by grant funding from the National Institutes of Health, Children’s Discovery Institute of Washington University, James S. McDonnell Foundation, Ivy Foundation, Ben & Catherine Ivy Foundation, and the Mayo Clinic. Albert H. Kim and Kristin R. Swanson reported financial affiliations with Monterris and the James S. McDonnell Foundation. Other authors reported no conflicts of interest related to the work.
SOURCE: Yang W et al. Sci Transl Med. 2019 Jan 02. doi: 10.1126/scitranslmed.aao5253.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: .
Major finding: In females, reduced tumor growth velocity was linked with significantly increased survival versus higher growth velocity (P = .00817).
Study details: Quantitative imaging and molecular analysis of 63 patients with glioblastoma.
Disclosures: The study was supported by grant funding from the National Institutes of Health, Children’s Discovery Institute of Washington University, James S. McDonnell Foundation, Ivy Foundation, Ben & Catherine Ivy Foundation, and the Mayo Clinic. Albert H. Kim and Kristin R. Swanson reported financial affiliations with Monterris and the James S. McDonnell Foundation. Other authors reported no conflicts of interest related to the work.
Source: Yang W et al. Sci Transl Med. 2019 Jan 02. doi: 10.1126/scitranslmed.aao5253.
Ascending Erythematous Nodules on the Arm
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.
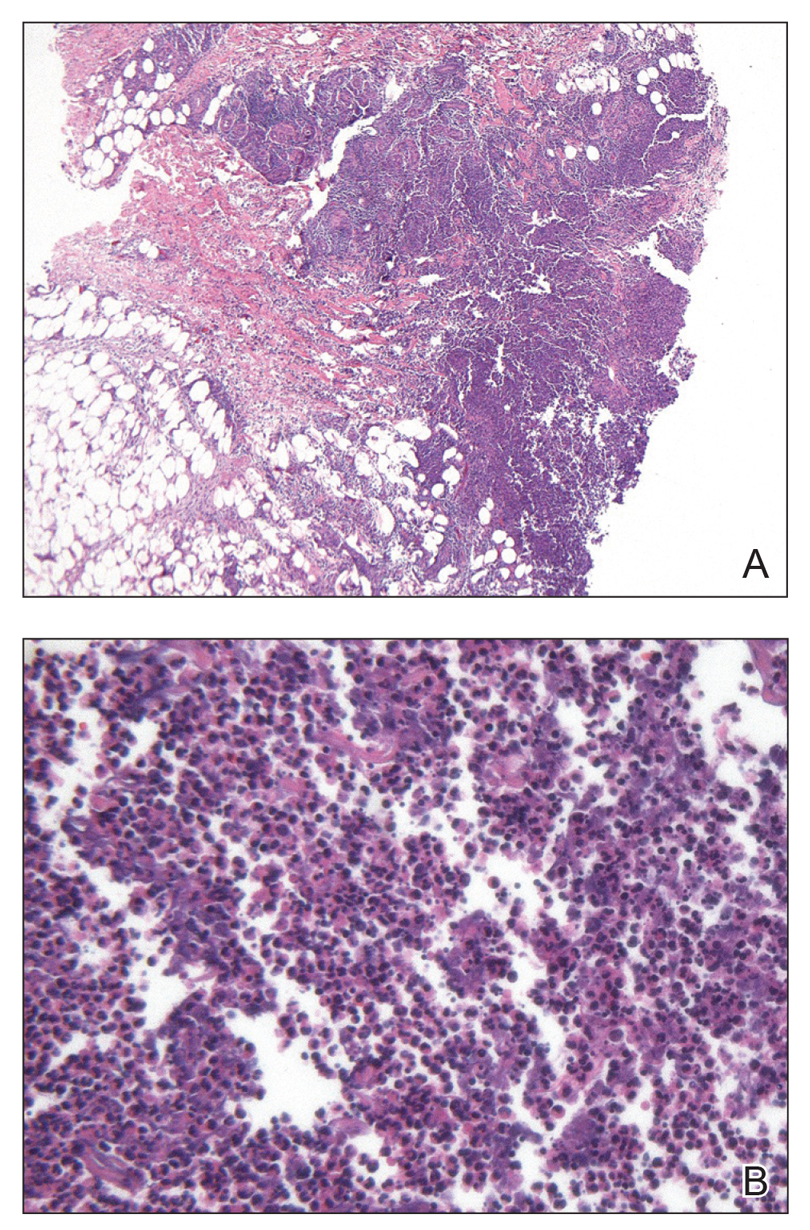
Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.
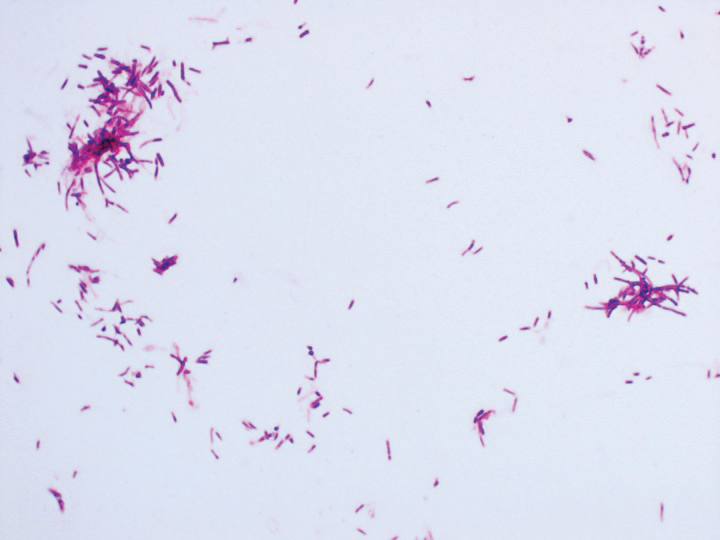
Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.

Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.

Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
The Diagnosis: Primary Cutaneous Nocardiosis
Comprehensive metabolic panel and complete blood cell count were unremarkable; human immunodeficiency virus screening was nonreactive. Punch biopsies were obtained for histopathology, as well as bacterial, fungal, and mycobacterial cultures. Histopathologic examination of a 4-mm punch biopsy of the forearm nodule showed a dermal abscess with neutrophilic infiltration in the dermis (Figure 1). No organisms were seen on Gram, methenamine-silver, periodic acid–Schiff, or acid-fast bacteria stains. Given the clinical suspicion for lymphocutaneous sporotrichosis, the patient was started on itraconazole. She reported modest improvement but subsequently developed a morbilliform eruption necessitating medication discontinuation.

Eighteen days after obtaining the tissue culture, acid-fast organisms grew in culture. These organisms were subcultured on Middlebrook 7H11 agar (Sigma-Aldrich) with growth noted at 30°C and 37°C. Gram stain revealed filamentous gram-variable bacteria (Figure 2) that were identified as Nocardia brasiliensis by 16S ribosomal DNA analysis. Given the patient’s sulfonamide allergy, she started oral minocycline 100 mg twice daily. She responded to the therapy and subsequent testing confirmed susceptibility.

Nocardia brasiliensis, isolated from subculture on Gram stain (original
magnification ×1000).
The genus Nocardia consists of more than 50 species of gram-positive, weakly acid-fast, aerobic actinomycetes that can cause primary cutaneous infection via percutaneous inoculation. Nocardia brasiliensis is the leading cause (approximately 80% of cases) of primary cutaneous or subcutaneous nocardiosis and is found ubiquitously in soil and decaying vegetation.1 The clinical presentation varies, rendering definitive diagnosis a challenge without histopathologic and microbiologic testing.2 Patients presenting with nocardial cellulitis often are suspected to have Streptococcus pyogenes or Staphylococcus aureus infections. The differential diagnosis for patients presenting with nocardial nodular lymphangitis, also known as lymphocutaneous syndrome, includes atypical mycobacterial infections, leishmaniasis, and lymphocutaneous sporotrichosis.2
Histologic examination of nocardial nodules typically shows granulomatous or neutrophilic inflammation, and organisms may appear in small collections resembling sulfur granules.2 The organism itself is weakly positive on acid-fast stain, and useful stains include acid-fast bacteria, methenamine silver, and periodic acid–Schiff.2 Tissue culture often provides the definitive diagnosis, as the histology is nonspecific and organisms may not be visualized.
Oral trimethoprim-sulfamethoxazole 2.5 to 10 mg/kg and 12.5 to 50 mg/kg, respectively, twice daily is the treatment of choice for primary cutaneous nocardiosis. Minocycline 100 to 200 mg twice daily is an accepted alternative in case of sulfonamide allergy, as in our patient. Antibiotics should be tailored according to the susceptibility profile of the isolated organism.3
This case highlights the importance of forming a broad differential diagnosis for patients presenting with lymphocutaneous syndrome. The incidence and prevalence of N brasiliensis infection is difficult to determine due to its nonspecific clinical presentation and a lack of recent epidemiologic studies. Although primary cutaneous nocardiosis in the United States often is diagnosed in the South or Southwest, cases have been reported in other regions.4-6 Traumatic inoculation of contaminated soil, plants, and other organic matter, a well-known method of Sporothrix schenckii transmission, also is a method of N brasiliensis transmission. Because this organism may not be detected on histologic examination, empiric treatment should be considered if the diagnosis is suspected.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
1. Brown-Eliot BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
2. Smego RA Jr, Castiglia M, Asperilla MO. Lymphocutaneous syndrome: a review of non-sporothrix causes. Medicine. 1999;78:38-63.
3. Lerner P. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
4. Smego RA Jr, Gallis HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6:164-180.
5. Fukuda H, Saotome A, Usami N, et al. Lymphocutaneous type of nocardiosis caused by Nocardia brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35:346-353.
6. Kil EH, Tsai CL, Kwark EH, et al. A case of nocardiosis with an uncharacteristically long incubation period. Cutis. 2005;76:33-36.
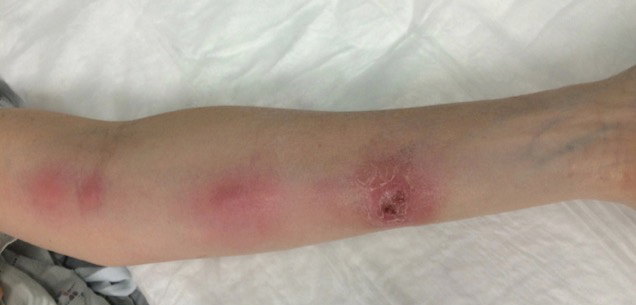
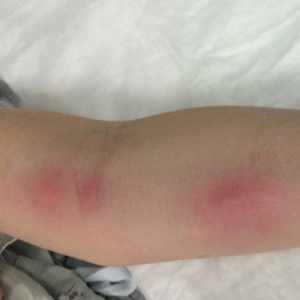
A 54-year-old woman called her primary care provider to report a painful pink nodule on the left wrist 1 week after sustaining thorn injuries while weeding in her garden. She started cephalexin and noted a pink streak with additional nodules extending up the arm over the next 2 days. She
was admitted to an outside hospital for incision and drainage of the wrist nodule and a 3-day course of intravenous vancomycin. Bacterial culture was negative, and she was discharged on oral clindamycin and doxycycline. Two days later, she presented to our emergency department with pain in the left axilla. Physical examination revealed 3 tender erythematous nodules in a linear distribution on the left arm with crusting at the incision and drainage site and painful left axillary lymphadenopathy. The patient was afebrile and otherwise asymptomatic.
Antidepressants tied to greater hip fracture incidence in older adults
Older patients in a Swedish registry who took antidepressants had a greater incidence of hip fracture the year before beginning antidepressant therapy and the year after starting therapy, compared with individuals in a matched control group.
The use of antidepressants is associated with adverse events such as a higher risk of falls, wrote Jon Brännström, MD, and his colleagues in JAMA Psychiatry. Some evidence also suggests that antidepressants “might affect bone metabolism, thereby increasing the risk of hip fracture.”
To examine the relationship between antidepressants and hip fracture, Dr. Brännström and his colleagues performed a nationwide cohort study of 204,072 individuals in the Prescribed Drug Register of Sweden’s National Board of Health and Welfare. All of the individuals were aged at least 65 years (mean age, 80.1 years; 63.1% women) and filled a prescription for an antidepressant between July 2006 and December 2011. Selective serotonin reuptake inhibitors made up 62.6% of the antidepressants used.
Patients who filled an antidepressant prescription during that time period were matched with a control group of individuals by birth year and gender and were studied the year before and after beginning antidepressant therapy.
In the year after initiating antidepressant therapy, there was a 3.5% incidence rate for hip fractures, compared with 1.3% in the control group.
After adjusting the results using a conditional logistic regression model, the highest rate of hip fracture among antidepressant users occurred between 16 days and 30 days prior to filling the prescription (odds ratio, 5.76; 95% confidence interval, 4.73-7.01); this association persisted in further subgroup analyses based on age, reported Dr. Brännström, who is affiliated with the department of community medicine and rehabilitation and geriatric medicine at Umeå University (Sweden), and his colleagues.
They noted that, although the study included all Swedish individuals who filled prescriptions for antidepressants during the study period, there is an absence of primary care comorbidity data and indications for antidepressant use. In addition, the definition of high- and low-medication doses does not always match what is considered high and low therapeutically and the information that can be gleaned from merging data from several different registries was limited.
“These findings raise questions about associations between antidepressant use and hip fracture seen in previous observational studies,” Dr. Brännström and his colleagues wrote. “Further analysis of this association in treatment studies and examination of the incidence of hip fracture before and after the discontinuation of treatment is required and may shed further light on the possible residual risk associated with treatment.”
This study was funded by the Swedish Research Council. The authors reported no relevant conflicts of interest.
SOURCE: Brännström J et al. JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3679.
In many cases where an adverse event is linked to a medication, such as in the case of gastrointestinal bleeds and blood thinners, the adverse event is not linked to the medication. However, this is not the case with antidepressants and hip fracture, Andrea Iaboni, MD, DPhil, and Donovan T. Maust, MD, wrote in a related editorial (JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3632).
“Patients are routinely prescribed antidepressants following a fracture,” the authors wrote, noting that depression can occur for patients who do not have a history of depression and can last as long as 1 year after hip fracture. The reasons for depression after hip fracture are possibly caused by the consequences of the event or a comorbid condition, such as cerebrovascular disease burden, cognitive impairment, frailty, and impaired functional status. In addition, new antidepressant prescriptions are 10 times the normal rate for older adults in the months after a hip fracture.
Many older users of antidepressants have a hip fracture event in their past, which could be caused by an untreated case of depression and an elevated risk of elevated fall or fracture, as suggested by Brännström et al., while other reasons could include off-label indications such as insomnia, poor motivation during rehabilitation therapy, pain, or hyperactive delirium.
“If individuals with untreated depression are at risk of falls and fractures, it follows that there would be an elevated rate of fractures before antidepressant use,” the authors wrote. “However, as discussed earlier, it is also important to recognize that, during the postfracture period, rightly or wrongly, antidepressants are prescribed at a high rate.”
Clinicians who treat these patients should not stop all antidepressant prescribing to this population. Instead, “a pragmatic preventive approach is warranted, starting with selecting the antidepressant, a cautious initial dose and dose-escalation schedule, a review of potentially interacting therapies ... and referral to fall prevention programs for patients with other risk factors for falls,” they wrote.
“For most older adults, the toll of untreated depression will likely outweigh the potential risks associated with antidepressant use.”
Dr. Iabroni is with the Toronto Rehabilitation Institute and the University of Toronto. He reported receiving fees from serving as a scientific adviser for Winterlight Labs. Dr. Maust is with the department of psychiatry at the University of Michigan, Ann Arbor. He reported no relevant conflicts of interest.
In many cases where an adverse event is linked to a medication, such as in the case of gastrointestinal bleeds and blood thinners, the adverse event is not linked to the medication. However, this is not the case with antidepressants and hip fracture, Andrea Iaboni, MD, DPhil, and Donovan T. Maust, MD, wrote in a related editorial (JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3632).
“Patients are routinely prescribed antidepressants following a fracture,” the authors wrote, noting that depression can occur for patients who do not have a history of depression and can last as long as 1 year after hip fracture. The reasons for depression after hip fracture are possibly caused by the consequences of the event or a comorbid condition, such as cerebrovascular disease burden, cognitive impairment, frailty, and impaired functional status. In addition, new antidepressant prescriptions are 10 times the normal rate for older adults in the months after a hip fracture.
Many older users of antidepressants have a hip fracture event in their past, which could be caused by an untreated case of depression and an elevated risk of elevated fall or fracture, as suggested by Brännström et al., while other reasons could include off-label indications such as insomnia, poor motivation during rehabilitation therapy, pain, or hyperactive delirium.
“If individuals with untreated depression are at risk of falls and fractures, it follows that there would be an elevated rate of fractures before antidepressant use,” the authors wrote. “However, as discussed earlier, it is also important to recognize that, during the postfracture period, rightly or wrongly, antidepressants are prescribed at a high rate.”
Clinicians who treat these patients should not stop all antidepressant prescribing to this population. Instead, “a pragmatic preventive approach is warranted, starting with selecting the antidepressant, a cautious initial dose and dose-escalation schedule, a review of potentially interacting therapies ... and referral to fall prevention programs for patients with other risk factors for falls,” they wrote.
“For most older adults, the toll of untreated depression will likely outweigh the potential risks associated with antidepressant use.”
Dr. Iabroni is with the Toronto Rehabilitation Institute and the University of Toronto. He reported receiving fees from serving as a scientific adviser for Winterlight Labs. Dr. Maust is with the department of psychiatry at the University of Michigan, Ann Arbor. He reported no relevant conflicts of interest.
In many cases where an adverse event is linked to a medication, such as in the case of gastrointestinal bleeds and blood thinners, the adverse event is not linked to the medication. However, this is not the case with antidepressants and hip fracture, Andrea Iaboni, MD, DPhil, and Donovan T. Maust, MD, wrote in a related editorial (JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3632).
“Patients are routinely prescribed antidepressants following a fracture,” the authors wrote, noting that depression can occur for patients who do not have a history of depression and can last as long as 1 year after hip fracture. The reasons for depression after hip fracture are possibly caused by the consequences of the event or a comorbid condition, such as cerebrovascular disease burden, cognitive impairment, frailty, and impaired functional status. In addition, new antidepressant prescriptions are 10 times the normal rate for older adults in the months after a hip fracture.
Many older users of antidepressants have a hip fracture event in their past, which could be caused by an untreated case of depression and an elevated risk of elevated fall or fracture, as suggested by Brännström et al., while other reasons could include off-label indications such as insomnia, poor motivation during rehabilitation therapy, pain, or hyperactive delirium.
“If individuals with untreated depression are at risk of falls and fractures, it follows that there would be an elevated rate of fractures before antidepressant use,” the authors wrote. “However, as discussed earlier, it is also important to recognize that, during the postfracture period, rightly or wrongly, antidepressants are prescribed at a high rate.”
Clinicians who treat these patients should not stop all antidepressant prescribing to this population. Instead, “a pragmatic preventive approach is warranted, starting with selecting the antidepressant, a cautious initial dose and dose-escalation schedule, a review of potentially interacting therapies ... and referral to fall prevention programs for patients with other risk factors for falls,” they wrote.
“For most older adults, the toll of untreated depression will likely outweigh the potential risks associated with antidepressant use.”
Dr. Iabroni is with the Toronto Rehabilitation Institute and the University of Toronto. He reported receiving fees from serving as a scientific adviser for Winterlight Labs. Dr. Maust is with the department of psychiatry at the University of Michigan, Ann Arbor. He reported no relevant conflicts of interest.
Older patients in a Swedish registry who took antidepressants had a greater incidence of hip fracture the year before beginning antidepressant therapy and the year after starting therapy, compared with individuals in a matched control group.
The use of antidepressants is associated with adverse events such as a higher risk of falls, wrote Jon Brännström, MD, and his colleagues in JAMA Psychiatry. Some evidence also suggests that antidepressants “might affect bone metabolism, thereby increasing the risk of hip fracture.”
To examine the relationship between antidepressants and hip fracture, Dr. Brännström and his colleagues performed a nationwide cohort study of 204,072 individuals in the Prescribed Drug Register of Sweden’s National Board of Health and Welfare. All of the individuals were aged at least 65 years (mean age, 80.1 years; 63.1% women) and filled a prescription for an antidepressant between July 2006 and December 2011. Selective serotonin reuptake inhibitors made up 62.6% of the antidepressants used.
Patients who filled an antidepressant prescription during that time period were matched with a control group of individuals by birth year and gender and were studied the year before and after beginning antidepressant therapy.
In the year after initiating antidepressant therapy, there was a 3.5% incidence rate for hip fractures, compared with 1.3% in the control group.
After adjusting the results using a conditional logistic regression model, the highest rate of hip fracture among antidepressant users occurred between 16 days and 30 days prior to filling the prescription (odds ratio, 5.76; 95% confidence interval, 4.73-7.01); this association persisted in further subgroup analyses based on age, reported Dr. Brännström, who is affiliated with the department of community medicine and rehabilitation and geriatric medicine at Umeå University (Sweden), and his colleagues.
They noted that, although the study included all Swedish individuals who filled prescriptions for antidepressants during the study period, there is an absence of primary care comorbidity data and indications for antidepressant use. In addition, the definition of high- and low-medication doses does not always match what is considered high and low therapeutically and the information that can be gleaned from merging data from several different registries was limited.
“These findings raise questions about associations between antidepressant use and hip fracture seen in previous observational studies,” Dr. Brännström and his colleagues wrote. “Further analysis of this association in treatment studies and examination of the incidence of hip fracture before and after the discontinuation of treatment is required and may shed further light on the possible residual risk associated with treatment.”
This study was funded by the Swedish Research Council. The authors reported no relevant conflicts of interest.
SOURCE: Brännström J et al. JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3679.
Older patients in a Swedish registry who took antidepressants had a greater incidence of hip fracture the year before beginning antidepressant therapy and the year after starting therapy, compared with individuals in a matched control group.
The use of antidepressants is associated with adverse events such as a higher risk of falls, wrote Jon Brännström, MD, and his colleagues in JAMA Psychiatry. Some evidence also suggests that antidepressants “might affect bone metabolism, thereby increasing the risk of hip fracture.”
To examine the relationship between antidepressants and hip fracture, Dr. Brännström and his colleagues performed a nationwide cohort study of 204,072 individuals in the Prescribed Drug Register of Sweden’s National Board of Health and Welfare. All of the individuals were aged at least 65 years (mean age, 80.1 years; 63.1% women) and filled a prescription for an antidepressant between July 2006 and December 2011. Selective serotonin reuptake inhibitors made up 62.6% of the antidepressants used.
Patients who filled an antidepressant prescription during that time period were matched with a control group of individuals by birth year and gender and were studied the year before and after beginning antidepressant therapy.
In the year after initiating antidepressant therapy, there was a 3.5% incidence rate for hip fractures, compared with 1.3% in the control group.
After adjusting the results using a conditional logistic regression model, the highest rate of hip fracture among antidepressant users occurred between 16 days and 30 days prior to filling the prescription (odds ratio, 5.76; 95% confidence interval, 4.73-7.01); this association persisted in further subgroup analyses based on age, reported Dr. Brännström, who is affiliated with the department of community medicine and rehabilitation and geriatric medicine at Umeå University (Sweden), and his colleagues.
They noted that, although the study included all Swedish individuals who filled prescriptions for antidepressants during the study period, there is an absence of primary care comorbidity data and indications for antidepressant use. In addition, the definition of high- and low-medication doses does not always match what is considered high and low therapeutically and the information that can be gleaned from merging data from several different registries was limited.
“These findings raise questions about associations between antidepressant use and hip fracture seen in previous observational studies,” Dr. Brännström and his colleagues wrote. “Further analysis of this association in treatment studies and examination of the incidence of hip fracture before and after the discontinuation of treatment is required and may shed further light on the possible residual risk associated with treatment.”
This study was funded by the Swedish Research Council. The authors reported no relevant conflicts of interest.
SOURCE: Brännström J et al. JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3679.
FROM JAMA PSYCHIATRY
Key clinical point: An association was found between greater hip fracture incidence for older individuals taking antidepressants in the year before beginning therapy and the year after starting therapy.
Major finding: Individuals who took antidepressants had a greater incidence of hip fractures in the year before (2.8% vs. 1.1%) and the year after (3.5% vs. 1.3%) beginning antidepressants, compared with individuals in a matched control group.
Study details: A nationwide cohort study of 408,144 individuals in the Prescribed Drugs Register of Sweden’s National Board of Health and Welfare who were aged 65 years or older.
Disclosures: This study was funded by the Swedish Research Council. The authors reported no relevant conflicts of interest.
Source: Brännström J et al. JAMA Psychiatry. 2019 Jan 2. doi: 10.1001/jamapsychiatry.2018.3679.
Cerebral small vessel disease progression linked to MCI in hypertensive patients
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
Patients with hypertension who show substantial progression of cerebral small vessel disease over time have sixfold higher odds of developing mild cognitive impairment than do those without signs of progression on brain MRI, new research has found.
The results, published online Jan. 4 in Hypertension, come from a longitudinal, population-based study of 976 patients with hypertension but with no history of dementia or clinical stroke. Participants underwent a vascular risk assessment, brain MRI, cognitive evaluation, and blood sampling at baseline, and 345 patients were also retested after a mean of nearly 4 years.
Researchers saw significant sixfold higher odds of developing incident mild cognitive impairment (MCI) among individuals who showed marked progression of periventricular white matter hyperintensities – an imaging hallmark of cerebral small vessel disease – compared with individuals who did not show any progression (odds ratio = 6.184; 95% confidence interval, 1.506-25.370; P = .011).
Patients with greater progression of periventricular white matter hyperintensities also showed significantly greater decreases in global cognition scores – both in total DRS-2 Z-score and executive function Z-score – when compared against individuals without white matter hyperintensity progression.
“As MCI is one of the most important risk factors in the development of dementia, future research should investigate the mechanisms by which PVH [periventricular white matter hyperintensities] trigger cognitive impairment and the clinical utility of its assessment,” wrote Joan Jiménez-Balado of Vall d’Hebron Research Institute, Barcelona, and his associates.
However, deep white matter hyperintensity progression – as opposed to periventricular – was not linked to cognitive changes, except in the case of bilateral occipital deep white matter hyperintensity changes, which were linked to a significant worsening in the attention Z-score.
The authors noted that the different impacts of periventricular versus deep white matter hyperintensities may relate to a number of factors. The first was that deep white matter hyperintensities disrupt cortico-cortical connections but periventricular ones are more likely to affect long cortico-subcortical association fibers, which “would be an important variable to determine the impaired networks involved in cognition.”
They also suggested that periventricular and deep white matter hyperintensities may affect different neuromodulator systems; the periventricular white matter could be closer to ascending cholinergic bundles that may play a role in vascular cognitive impairment.
Periventricular white matter hyperintensities may also accelerate the deposition of amyloid because of their association with venous collagenosis, which is linked to ischemia and disruptions of the interstitial fluid circulation.
“On the other hand, [deep white matter hyperintensity] may be more related to hypoperfusion, as deep areas are particularly vulnerable to low [blood pressure],” the authors wrote, while stressing that the pathophysiology of white matter hyperintensities is not fully understood, so further research is needed.
Overall, the 345 patients with follow-up data had a median age of 65 years at baseline and mean blood pressure of 143/78.2 mm Hg at baseline and 146.5/75 mm Hg at follow-up. White matter hyperintensity changes occurred periventricularly in 22% and in deep white matter in 48%. The researchers saw new infarcts in 6.1% of patients, and 5.5% had incident cerebral microbleeds. While incident cerebral microbleeds were significantly associated with declines in the attention Z-score, they did not affect other cognitive functions, and incidental infarcts were also not associated with cognitive changes.
Baseline blood pressure and average blood pressure during follow-up were not associated with changes in cardiac small vessel disease lesions. However, diastolic – but not systolic – blood pressure at baseline and follow-up was positively correlated with total, attention, and executive function DRS-2 Z-scores at follow-up.
Three-quarters of patients showed cognitive changes associated with normal aging both at baseline and follow-up, 9.1% had stable MCI, and 9.1% of patients had incident MCI. However, 6.6% of subjects reverted back to normal aging after having MCI at baseline.
The authors noted that they did not examine markers of neurodegeneration, such as tau or amyloid-beta, which could also be linked to hypertension and cerebral small vessel disease lesions.
The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
SOURCE: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090
FROM HYPERTENSION
Key clinical point: Cerebral small vessel disease changes are associated with the development of mild cognitive impairment in hypertensive patients.
Major finding: Periventricular white matter hyperintensities in patients with hypertension were associated with sixfold higher odds of mild cognitive impairment.
Study details: A longitudinal, population-based study of 345 patients with hypertension.
Disclosures: The study was supported by Instituto de Salud Carlos III, AGAUR (Agency for Management of University and Research Grants), the Secretary of Universities and Research of the Department of Economy and Knowledge, and the European Regional Development Fund. The authors said they have no conflicts of interest.
Source: Jiménez-Balado J et al. Hypertension. 2019 Jan 4. doi: 10.1161/HYPERTENSIONAHA.118.12090.
Aspirin and Omega-3 fatty acids fail
Also today, New data reveal that college students are at greater risk of meningococcal B infection, children who survive Hodgkin lymphoma face a massive increased risk for second cancers down the road, and the 2018/19 flu season shows high activity in nine states.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, New data reveal that college students are at greater risk of meningococcal B infection, children who survive Hodgkin lymphoma face a massive increased risk for second cancers down the road, and the 2018/19 flu season shows high activity in nine states.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, New data reveal that college students are at greater risk of meningococcal B infection, children who survive Hodgkin lymphoma face a massive increased risk for second cancers down the road, and the 2018/19 flu season shows high activity in nine states.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Raymond Barfield Part I
Now, he joins the Postcall Podcast to discuss why he’s back, what he’s working on to prevent burnout, and how he wants to remake pre-med education. You can read more from Dr. Barfield’s story here.
Apple Podcasts
Google Podcasts
Spotify
Now, he joins the Postcall Podcast to discuss why he’s back, what he’s working on to prevent burnout, and how he wants to remake pre-med education. You can read more from Dr. Barfield’s story here.
Apple Podcasts
Google Podcasts
Spotify
Now, he joins the Postcall Podcast to discuss why he’s back, what he’s working on to prevent burnout, and how he wants to remake pre-med education. You can read more from Dr. Barfield’s story here.
Apple Podcasts
Google Podcasts
Spotify
How Often Are Staring Spells Seizures?
Investigators review cases at a new-onset seizure clinic.
CHICAGO—About half of staring spells referred to a new-onset seizure (NOS) clinic turn out to be epileptic seizures, according to a retrospective chart review presented at the 47th Annual Meeting of the Child Neurology Society.
Children with nonepileptic staring were younger and more likely to have developmental delay, compared with children with epileptic staring, said Seunghyo Kim, MD, Visiting Associate Professor of Pediatrics at Emory University School of Medicine in Atlanta, and his colleagues
A Common Reason for Referral
Staring spells are common in children and a frequent reason for referral to NOS clinics, the investigators said. Staring spells may be generalized absence seizures, focal seizures, or nonepileptic events. Few studies, however, have examined patients who newly present to a neurology clinic with the chief complaint of staring spells.
To evaluate the clinical and demographic features of children who present with staring spells to a regional NOS clinic, the researchers reviewed charts from 2,818 patients who visited a children’s hospital between September 22, 2015, and March 19, 2018. The investigators identified 189 patients with staring spells.
They excluded 48 cases where staring was accompanied by or followed by generalized tonic-clonic seizures or other motor seizures. In addition, they excluded 16 cases of established epilepsy and four cases of provoked seizures, including febrile seizures.
The final study population included 121 cases. About 48% of these patients with staring spells were African American, and 38% were white. Patients’ mean age at first visit to the NOS clinic was 6, and mean age at staring spell onset was 5.2.
Fifty-nine patients (49%) had epileptic staring episodes, and 62 patients (51%) had nonepileptic events.
Continue to: Approximately 50% were referred by an emergency department...
Approximately 50% were referred by an emergency department, 48% by a primary care physician, and 2% by an urgent care clinic. Of the 62 cases that turned out to be nonepileptic events, about 61% were referred by primary care physicians.
MRI Findings in Patients With Focal Seizures
On average, patients with nonepileptic staring were younger at the initial clinic presentation and at staring spell onset. Patients with nonepileptic staring had an average age of 4.8 at their initial visit, whereas patients with focal seizures had an average age of 6.3. Patients with absence seizures had an average age of 8.3.
Patients with nonepileptic events were more likely to have developmental delay (approximately 35%) versus patients with focal seizures (23%) or absence seizures (8%).
Absence epilepsy was diagnosed in 24 patients (20%), and focal epilepsy in 35 patients (29%).
Of the 59 cases of epileptic staring, 46% (n = 27) were classified as syndromic; 36% (n = 21) had childhood absence epilepsy, and 5% (n = 3) had juvenile absence epilepsy. In addition, there was one case each of mesial temporal lobe epilepsy with hippocampal sclerosis, Panayiotopoulos syndrome, and generalized epilepsy with febrile seizures.
About one-third of patients received MRI, and seven patients had etiologically relevant findings (eg, brain tumor, malformation of cortical development, and periventricular leukomalacia). All of the patients with abnormal MRIs had focal seizures.
“An NOS clinic ... can provide rapid, accurate diagnoses for staring spells,” said Dr. Kim. “This is important, as children with nonepileptic events should not be given the diagnosis of epilepsy, and their events should not be treated with seizure medications. Similarly, children who have epileptic seizures require accurate diagnosis, as the treatment depends on the seizure type.”
Investigators review cases at a new-onset seizure clinic.
Investigators review cases at a new-onset seizure clinic.
CHICAGO—About half of staring spells referred to a new-onset seizure (NOS) clinic turn out to be epileptic seizures, according to a retrospective chart review presented at the 47th Annual Meeting of the Child Neurology Society.
Children with nonepileptic staring were younger and more likely to have developmental delay, compared with children with epileptic staring, said Seunghyo Kim, MD, Visiting Associate Professor of Pediatrics at Emory University School of Medicine in Atlanta, and his colleagues
A Common Reason for Referral
Staring spells are common in children and a frequent reason for referral to NOS clinics, the investigators said. Staring spells may be generalized absence seizures, focal seizures, or nonepileptic events. Few studies, however, have examined patients who newly present to a neurology clinic with the chief complaint of staring spells.
To evaluate the clinical and demographic features of children who present with staring spells to a regional NOS clinic, the researchers reviewed charts from 2,818 patients who visited a children’s hospital between September 22, 2015, and March 19, 2018. The investigators identified 189 patients with staring spells.
They excluded 48 cases where staring was accompanied by or followed by generalized tonic-clonic seizures or other motor seizures. In addition, they excluded 16 cases of established epilepsy and four cases of provoked seizures, including febrile seizures.
The final study population included 121 cases. About 48% of these patients with staring spells were African American, and 38% were white. Patients’ mean age at first visit to the NOS clinic was 6, and mean age at staring spell onset was 5.2.
Fifty-nine patients (49%) had epileptic staring episodes, and 62 patients (51%) had nonepileptic events.
Continue to: Approximately 50% were referred by an emergency department...
Approximately 50% were referred by an emergency department, 48% by a primary care physician, and 2% by an urgent care clinic. Of the 62 cases that turned out to be nonepileptic events, about 61% were referred by primary care physicians.
MRI Findings in Patients With Focal Seizures
On average, patients with nonepileptic staring were younger at the initial clinic presentation and at staring spell onset. Patients with nonepileptic staring had an average age of 4.8 at their initial visit, whereas patients with focal seizures had an average age of 6.3. Patients with absence seizures had an average age of 8.3.
Patients with nonepileptic events were more likely to have developmental delay (approximately 35%) versus patients with focal seizures (23%) or absence seizures (8%).
Absence epilepsy was diagnosed in 24 patients (20%), and focal epilepsy in 35 patients (29%).
Of the 59 cases of epileptic staring, 46% (n = 27) were classified as syndromic; 36% (n = 21) had childhood absence epilepsy, and 5% (n = 3) had juvenile absence epilepsy. In addition, there was one case each of mesial temporal lobe epilepsy with hippocampal sclerosis, Panayiotopoulos syndrome, and generalized epilepsy with febrile seizures.
About one-third of patients received MRI, and seven patients had etiologically relevant findings (eg, brain tumor, malformation of cortical development, and periventricular leukomalacia). All of the patients with abnormal MRIs had focal seizures.
“An NOS clinic ... can provide rapid, accurate diagnoses for staring spells,” said Dr. Kim. “This is important, as children with nonepileptic events should not be given the diagnosis of epilepsy, and their events should not be treated with seizure medications. Similarly, children who have epileptic seizures require accurate diagnosis, as the treatment depends on the seizure type.”
CHICAGO—About half of staring spells referred to a new-onset seizure (NOS) clinic turn out to be epileptic seizures, according to a retrospective chart review presented at the 47th Annual Meeting of the Child Neurology Society.
Children with nonepileptic staring were younger and more likely to have developmental delay, compared with children with epileptic staring, said Seunghyo Kim, MD, Visiting Associate Professor of Pediatrics at Emory University School of Medicine in Atlanta, and his colleagues
A Common Reason for Referral
Staring spells are common in children and a frequent reason for referral to NOS clinics, the investigators said. Staring spells may be generalized absence seizures, focal seizures, or nonepileptic events. Few studies, however, have examined patients who newly present to a neurology clinic with the chief complaint of staring spells.
To evaluate the clinical and demographic features of children who present with staring spells to a regional NOS clinic, the researchers reviewed charts from 2,818 patients who visited a children’s hospital between September 22, 2015, and March 19, 2018. The investigators identified 189 patients with staring spells.
They excluded 48 cases where staring was accompanied by or followed by generalized tonic-clonic seizures or other motor seizures. In addition, they excluded 16 cases of established epilepsy and four cases of provoked seizures, including febrile seizures.
The final study population included 121 cases. About 48% of these patients with staring spells were African American, and 38% were white. Patients’ mean age at first visit to the NOS clinic was 6, and mean age at staring spell onset was 5.2.
Fifty-nine patients (49%) had epileptic staring episodes, and 62 patients (51%) had nonepileptic events.
Continue to: Approximately 50% were referred by an emergency department...
Approximately 50% were referred by an emergency department, 48% by a primary care physician, and 2% by an urgent care clinic. Of the 62 cases that turned out to be nonepileptic events, about 61% were referred by primary care physicians.
MRI Findings in Patients With Focal Seizures
On average, patients with nonepileptic staring were younger at the initial clinic presentation and at staring spell onset. Patients with nonepileptic staring had an average age of 4.8 at their initial visit, whereas patients with focal seizures had an average age of 6.3. Patients with absence seizures had an average age of 8.3.
Patients with nonepileptic events were more likely to have developmental delay (approximately 35%) versus patients with focal seizures (23%) or absence seizures (8%).
Absence epilepsy was diagnosed in 24 patients (20%), and focal epilepsy in 35 patients (29%).
Of the 59 cases of epileptic staring, 46% (n = 27) were classified as syndromic; 36% (n = 21) had childhood absence epilepsy, and 5% (n = 3) had juvenile absence epilepsy. In addition, there was one case each of mesial temporal lobe epilepsy with hippocampal sclerosis, Panayiotopoulos syndrome, and generalized epilepsy with febrile seizures.
About one-third of patients received MRI, and seven patients had etiologically relevant findings (eg, brain tumor, malformation of cortical development, and periventricular leukomalacia). All of the patients with abnormal MRIs had focal seizures.
“An NOS clinic ... can provide rapid, accurate diagnoses for staring spells,” said Dr. Kim. “This is important, as children with nonepileptic events should not be given the diagnosis of epilepsy, and their events should not be treated with seizure medications. Similarly, children who have epileptic seizures require accurate diagnosis, as the treatment depends on the seizure type.”
Survey Identifies Variations in Management of Pediatric Posttraumatic Headache
Findings highlight a need to establish best evidence-based practices, researchers say.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.
Findings highlight a need to establish best evidence-based practices, researchers say.
Findings highlight a need to establish best evidence-based practices, researchers say.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.
CMT provides survival benefit in young HL patients
Combined modality therapy (CMT) can improve survival in young patients with early stage Hodgkin lymphoma (HL), according to research published in JAMA Oncology.
In a retrospective study, researchers compared chemotherapy followed by radiotherapy—CMT—to chemotherapy alone in more than 5,600 HL patients age 21 and younger.
There was a significant improvement in 5-year overall survival (OS) among patients who received CMT.
The treatment appeared particularly beneficial for adolescents and young adults as well as patients with low-risk disease.
However, the researchers observed a nearly 25% decrease in the use of CMT over the period studied.
“Nationwide, there has been a notable decrease in combined modality therapy, especially in clinical trials, many of which are designed to avoid this strategy,” said Rahul Parikh, MD, of Rutgers Cancer Institute of New Jersey in New Brunswick.
“This form of treatment has shown to be effective, with event-free survival rates greater than 80% and overall survival rates greater than 95%. The question then becomes, ‘does treatment benefit outweigh the risk of long-term side effects?”
With this in mind, Dr. Parikh and his colleagues compared CMT to chemotherapy alone using data from the National Cancer Database spanning the period from 2004 to 2015.
The researchers analyzed 5,657 patients with stage I/II classical HL who had a mean age of 17.1.
Roughly half of patients received CMT (50.3%, n=2845), and the other half received chemotherapy alone (49.7%, n=2812).
The median radiotherapy dose was 21.0 Gy, and the most common modality was photon therapy (59.0%).
Patients who received CMT were significantly more likely to be younger than 16 (P<0.001), be male (P<0.001), have stage II disease (P=0.02), and have private health insurance (P=0.002).
Results
The median follow-up was 5.1 years.
The 5-year OS was 94.5% for patients who received chemotherapy alone and 97.3% for patients treated with CMT.
CMT was significantly associated with improved OS in both univariate (hazard ratio [HR]=0.58, P<0.001) and multivariate analyses (HR=0.57, P<0.001).
In a sensitivity analysis, the researchers found the greatest benefits of CMT were in adolescents and young adults (age 14 and older, adjusted HR=0.47) and patients with low-risk disease (stage I-IIA, adjusted HR=0.59).
The researchers noted that this study was limited by their inability to control for unreported prognostic factors, such as the number of nodal sites and bulk of disease.
Another limitation was the duration of follow-up, which did not allow the researchers to fully assess secondary late effects of CMT and their potential impact on survival.
Still, Dr. Parikh said this study demonstrates a survival benefit for young HL patients treated with CMT.
“With that, physicians should be encouraged to discuss combined modality therapy as one of the many treatment options [for young HL patients],” he said.
“Investigators may also consider designing future clinical trials for this population to include combined modality therapy as a standard arm with the inclusion of interim treatment response assessment (PET scans, etc.). And as multiple disparities to the use of combined modality therapy have been identified through this work, future studies should address improving access to care for all pediatric patients.”
Dr. Parikh and his colleagues declared no conflicts of interest for the current study.
Combined modality therapy (CMT) can improve survival in young patients with early stage Hodgkin lymphoma (HL), according to research published in JAMA Oncology.
In a retrospective study, researchers compared chemotherapy followed by radiotherapy—CMT—to chemotherapy alone in more than 5,600 HL patients age 21 and younger.
There was a significant improvement in 5-year overall survival (OS) among patients who received CMT.
The treatment appeared particularly beneficial for adolescents and young adults as well as patients with low-risk disease.
However, the researchers observed a nearly 25% decrease in the use of CMT over the period studied.
“Nationwide, there has been a notable decrease in combined modality therapy, especially in clinical trials, many of which are designed to avoid this strategy,” said Rahul Parikh, MD, of Rutgers Cancer Institute of New Jersey in New Brunswick.
“This form of treatment has shown to be effective, with event-free survival rates greater than 80% and overall survival rates greater than 95%. The question then becomes, ‘does treatment benefit outweigh the risk of long-term side effects?”
With this in mind, Dr. Parikh and his colleagues compared CMT to chemotherapy alone using data from the National Cancer Database spanning the period from 2004 to 2015.
The researchers analyzed 5,657 patients with stage I/II classical HL who had a mean age of 17.1.
Roughly half of patients received CMT (50.3%, n=2845), and the other half received chemotherapy alone (49.7%, n=2812).
The median radiotherapy dose was 21.0 Gy, and the most common modality was photon therapy (59.0%).
Patients who received CMT were significantly more likely to be younger than 16 (P<0.001), be male (P<0.001), have stage II disease (P=0.02), and have private health insurance (P=0.002).
Results
The median follow-up was 5.1 years.
The 5-year OS was 94.5% for patients who received chemotherapy alone and 97.3% for patients treated with CMT.
CMT was significantly associated with improved OS in both univariate (hazard ratio [HR]=0.58, P<0.001) and multivariate analyses (HR=0.57, P<0.001).
In a sensitivity analysis, the researchers found the greatest benefits of CMT were in adolescents and young adults (age 14 and older, adjusted HR=0.47) and patients with low-risk disease (stage I-IIA, adjusted HR=0.59).
The researchers noted that this study was limited by their inability to control for unreported prognostic factors, such as the number of nodal sites and bulk of disease.
Another limitation was the duration of follow-up, which did not allow the researchers to fully assess secondary late effects of CMT and their potential impact on survival.
Still, Dr. Parikh said this study demonstrates a survival benefit for young HL patients treated with CMT.
“With that, physicians should be encouraged to discuss combined modality therapy as one of the many treatment options [for young HL patients],” he said.
“Investigators may also consider designing future clinical trials for this population to include combined modality therapy as a standard arm with the inclusion of interim treatment response assessment (PET scans, etc.). And as multiple disparities to the use of combined modality therapy have been identified through this work, future studies should address improving access to care for all pediatric patients.”
Dr. Parikh and his colleagues declared no conflicts of interest for the current study.
Combined modality therapy (CMT) can improve survival in young patients with early stage Hodgkin lymphoma (HL), according to research published in JAMA Oncology.
In a retrospective study, researchers compared chemotherapy followed by radiotherapy—CMT—to chemotherapy alone in more than 5,600 HL patients age 21 and younger.
There was a significant improvement in 5-year overall survival (OS) among patients who received CMT.
The treatment appeared particularly beneficial for adolescents and young adults as well as patients with low-risk disease.
However, the researchers observed a nearly 25% decrease in the use of CMT over the period studied.
“Nationwide, there has been a notable decrease in combined modality therapy, especially in clinical trials, many of which are designed to avoid this strategy,” said Rahul Parikh, MD, of Rutgers Cancer Institute of New Jersey in New Brunswick.
“This form of treatment has shown to be effective, with event-free survival rates greater than 80% and overall survival rates greater than 95%. The question then becomes, ‘does treatment benefit outweigh the risk of long-term side effects?”
With this in mind, Dr. Parikh and his colleagues compared CMT to chemotherapy alone using data from the National Cancer Database spanning the period from 2004 to 2015.
The researchers analyzed 5,657 patients with stage I/II classical HL who had a mean age of 17.1.
Roughly half of patients received CMT (50.3%, n=2845), and the other half received chemotherapy alone (49.7%, n=2812).
The median radiotherapy dose was 21.0 Gy, and the most common modality was photon therapy (59.0%).
Patients who received CMT were significantly more likely to be younger than 16 (P<0.001), be male (P<0.001), have stage II disease (P=0.02), and have private health insurance (P=0.002).
Results
The median follow-up was 5.1 years.
The 5-year OS was 94.5% for patients who received chemotherapy alone and 97.3% for patients treated with CMT.
CMT was significantly associated with improved OS in both univariate (hazard ratio [HR]=0.58, P<0.001) and multivariate analyses (HR=0.57, P<0.001).
In a sensitivity analysis, the researchers found the greatest benefits of CMT were in adolescents and young adults (age 14 and older, adjusted HR=0.47) and patients with low-risk disease (stage I-IIA, adjusted HR=0.59).
The researchers noted that this study was limited by their inability to control for unreported prognostic factors, such as the number of nodal sites and bulk of disease.
Another limitation was the duration of follow-up, which did not allow the researchers to fully assess secondary late effects of CMT and their potential impact on survival.
Still, Dr. Parikh said this study demonstrates a survival benefit for young HL patients treated with CMT.
“With that, physicians should be encouraged to discuss combined modality therapy as one of the many treatment options [for young HL patients],” he said.
“Investigators may also consider designing future clinical trials for this population to include combined modality therapy as a standard arm with the inclusion of interim treatment response assessment (PET scans, etc.). And as multiple disparities to the use of combined modality therapy have been identified through this work, future studies should address improving access to care for all pediatric patients.”
Dr. Parikh and his colleagues declared no conflicts of interest for the current study.
Chemo for solid tumors and risk of tMDS/AML
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.