User login
FDA approves caplacizumab for aTTP
The Food and Drug Administration has approved caplacizumab (Cablivi) in combination with plasma exchange and immunosuppressive therapy for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is an anti–von Willebrand factor nanobody designed to inhibit the interaction between von Willebrand factor and platelets. The injection previously received orphan drug designation from the FDA and was approved under priority review.
The FDA’s approval of caplacizumab was based on results from the phase 3 HERCULES trial (N Engl J Med 2019 Jan 24;380:335-46).
The trial (NCT02553317) included 145 adults with aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73), in addition to plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization), which was defined as a platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm – 2.69 days and 2.88 days, respectively. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12% in the caplacizumab arm and 49% in the placebo arm (P less than .001).
The most common treatment-emergent adverse events (occurring in at least 15% of patients in the caplacizumab and placebo arms, respectively) were epistaxis (32% and 3%), headache (23% and 8%), urticaria (17% and 7%), and hypokalemia (9% and 19%).
During the treatment period, there were no deaths in the caplacizumab arm and three deaths in the placebo arm. There was one death (from cerebral ischemia) in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
For more details on caplacizumab, see the full prescribing information.
The Food and Drug Administration has approved caplacizumab (Cablivi) in combination with plasma exchange and immunosuppressive therapy for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is an anti–von Willebrand factor nanobody designed to inhibit the interaction between von Willebrand factor and platelets. The injection previously received orphan drug designation from the FDA and was approved under priority review.
The FDA’s approval of caplacizumab was based on results from the phase 3 HERCULES trial (N Engl J Med 2019 Jan 24;380:335-46).
The trial (NCT02553317) included 145 adults with aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73), in addition to plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization), which was defined as a platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm – 2.69 days and 2.88 days, respectively. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12% in the caplacizumab arm and 49% in the placebo arm (P less than .001).
The most common treatment-emergent adverse events (occurring in at least 15% of patients in the caplacizumab and placebo arms, respectively) were epistaxis (32% and 3%), headache (23% and 8%), urticaria (17% and 7%), and hypokalemia (9% and 19%).
During the treatment period, there were no deaths in the caplacizumab arm and three deaths in the placebo arm. There was one death (from cerebral ischemia) in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
For more details on caplacizumab, see the full prescribing information.
The Food and Drug Administration has approved caplacizumab (Cablivi) in combination with plasma exchange and immunosuppressive therapy for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is an anti–von Willebrand factor nanobody designed to inhibit the interaction between von Willebrand factor and platelets. The injection previously received orphan drug designation from the FDA and was approved under priority review.
The FDA’s approval of caplacizumab was based on results from the phase 3 HERCULES trial (N Engl J Med 2019 Jan 24;380:335-46).
The trial (NCT02553317) included 145 adults with aTTP. They were randomized to receive caplacizumab (n = 72) or placebo (n = 73), in addition to plasma exchange and immunosuppression.
The study’s primary endpoint was the time to platelet count response (normalization), which was defined as a platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm, compared with the placebo arm – 2.69 days and 2.88 days, respectively. The platelet normalization rate ratio was 1.55 (P less than .01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least one major thromboembolic event during study treatment. The incidence of this combined endpoint was 12% in the caplacizumab arm and 49% in the placebo arm (P less than .001).
The most common treatment-emergent adverse events (occurring in at least 15% of patients in the caplacizumab and placebo arms, respectively) were epistaxis (32% and 3%), headache (23% and 8%), urticaria (17% and 7%), and hypokalemia (9% and 19%).
During the treatment period, there were no deaths in the caplacizumab arm and three deaths in the placebo arm. There was one death (from cerebral ischemia) in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
For more details on caplacizumab, see the full prescribing information.
Liver resection outcomes similar for robotic and open surgery
according to researchers who conducted a recent multi-institutional analysis.
The proportion of margin-negative resections was 92% in a series of 59 patients who underwent resection at one of three tertiary hospitals, the study authors reported.
The 3-year disease-free survival and overall survival rates were 41.9% and 66%, respectively, in the 44 patients for whom longer-term follow-up was available, according to the investigators, led by Francesco Guerra, MD, of Ospedali Riuniti Marche Nord Hospital in Pesaro, Italy.
“These findings are consistent with those of patients resected via conventional laparoscopy or open surgery in contemporary series,” Dr. Guerra and his coauthors wrote in a report published in Surgical Oncology.
It’s still a matter of debate, however, whether robotic surgery has clear advantages over conventional laparoscopy for liver surgery, according to the investigators. Direct comparisons of robotic versus conventional laparoscopic liver resection are scarce, with most available data stemming from often heterogeneous case-control series with no long-term oncologic outcome data.
“Most centers have consolidated experience either in laparoscopic or in robotic liver resection,” the authors wrote. “Hence, reliable comparative analyses are likely difficult to carry out.”
Because of the scarcity of results, Dr. Guerra and his colleagues sought to investigate short- and long-term outcomes for a consecutive series of 59 patients who underwent minimally invasive, ultrasound-guided robotic surgery between 2008 and 2018 at one of three tertiary facilities. Each procedure had been performed by surgeons experienced in both liver surgery and robotics. The median age of patients undergoing the procedure was 64 years, 63% were male, and the median body mass index was 26 kg/m2.
Almost half the patients (46%) had multiple lesions removed, while about one-quarter had concomitant procedures, mainly cholecystectomy, according to the report.
Robotic surgeries were converted to open procedures in seven patients, or about 12%, a statistic comparable with other reports of laparoscopic or robotic surgery, the investigators noted.
Postoperative complications were seen in 16 patients, or 27%. That included 13 patients with class I-II complications and 3 patients with class III-IV complications, including 2 cases of heart failure and 1 case of postoperative bile leak requiring radiologic and endoscopic therapy.
The reported 3-year disease-free and overall survival of 41.9% and 66.1% was based on a mean follow-up of 19.5 months in 44 patients for whom longer-term data were available. There were 16 cases of recurrent disease, including 10 patients with evidence of liver recurrence.
“Taken together, our data show that robotics is an effective option to resect colorectal liver metastases, providing an oncological outcome similar to that of laparoscopy and open surgery,” the investigators wrote.
Dr. Guerra and colleagues reported no disclosures related to their research.
SOURCE: Guerra F et al. Surg Oncol. 2018 Nov 1. doi: 10.1016/j.suronc.2018.10.011.
according to researchers who conducted a recent multi-institutional analysis.
The proportion of margin-negative resections was 92% in a series of 59 patients who underwent resection at one of three tertiary hospitals, the study authors reported.
The 3-year disease-free survival and overall survival rates were 41.9% and 66%, respectively, in the 44 patients for whom longer-term follow-up was available, according to the investigators, led by Francesco Guerra, MD, of Ospedali Riuniti Marche Nord Hospital in Pesaro, Italy.
“These findings are consistent with those of patients resected via conventional laparoscopy or open surgery in contemporary series,” Dr. Guerra and his coauthors wrote in a report published in Surgical Oncology.
It’s still a matter of debate, however, whether robotic surgery has clear advantages over conventional laparoscopy for liver surgery, according to the investigators. Direct comparisons of robotic versus conventional laparoscopic liver resection are scarce, with most available data stemming from often heterogeneous case-control series with no long-term oncologic outcome data.
“Most centers have consolidated experience either in laparoscopic or in robotic liver resection,” the authors wrote. “Hence, reliable comparative analyses are likely difficult to carry out.”
Because of the scarcity of results, Dr. Guerra and his colleagues sought to investigate short- and long-term outcomes for a consecutive series of 59 patients who underwent minimally invasive, ultrasound-guided robotic surgery between 2008 and 2018 at one of three tertiary facilities. Each procedure had been performed by surgeons experienced in both liver surgery and robotics. The median age of patients undergoing the procedure was 64 years, 63% were male, and the median body mass index was 26 kg/m2.
Almost half the patients (46%) had multiple lesions removed, while about one-quarter had concomitant procedures, mainly cholecystectomy, according to the report.
Robotic surgeries were converted to open procedures in seven patients, or about 12%, a statistic comparable with other reports of laparoscopic or robotic surgery, the investigators noted.
Postoperative complications were seen in 16 patients, or 27%. That included 13 patients with class I-II complications and 3 patients with class III-IV complications, including 2 cases of heart failure and 1 case of postoperative bile leak requiring radiologic and endoscopic therapy.
The reported 3-year disease-free and overall survival of 41.9% and 66.1% was based on a mean follow-up of 19.5 months in 44 patients for whom longer-term data were available. There were 16 cases of recurrent disease, including 10 patients with evidence of liver recurrence.
“Taken together, our data show that robotics is an effective option to resect colorectal liver metastases, providing an oncological outcome similar to that of laparoscopy and open surgery,” the investigators wrote.
Dr. Guerra and colleagues reported no disclosures related to their research.
SOURCE: Guerra F et al. Surg Oncol. 2018 Nov 1. doi: 10.1016/j.suronc.2018.10.011.
according to researchers who conducted a recent multi-institutional analysis.
The proportion of margin-negative resections was 92% in a series of 59 patients who underwent resection at one of three tertiary hospitals, the study authors reported.
The 3-year disease-free survival and overall survival rates were 41.9% and 66%, respectively, in the 44 patients for whom longer-term follow-up was available, according to the investigators, led by Francesco Guerra, MD, of Ospedali Riuniti Marche Nord Hospital in Pesaro, Italy.
“These findings are consistent with those of patients resected via conventional laparoscopy or open surgery in contemporary series,” Dr. Guerra and his coauthors wrote in a report published in Surgical Oncology.
It’s still a matter of debate, however, whether robotic surgery has clear advantages over conventional laparoscopy for liver surgery, according to the investigators. Direct comparisons of robotic versus conventional laparoscopic liver resection are scarce, with most available data stemming from often heterogeneous case-control series with no long-term oncologic outcome data.
“Most centers have consolidated experience either in laparoscopic or in robotic liver resection,” the authors wrote. “Hence, reliable comparative analyses are likely difficult to carry out.”
Because of the scarcity of results, Dr. Guerra and his colleagues sought to investigate short- and long-term outcomes for a consecutive series of 59 patients who underwent minimally invasive, ultrasound-guided robotic surgery between 2008 and 2018 at one of three tertiary facilities. Each procedure had been performed by surgeons experienced in both liver surgery and robotics. The median age of patients undergoing the procedure was 64 years, 63% were male, and the median body mass index was 26 kg/m2.
Almost half the patients (46%) had multiple lesions removed, while about one-quarter had concomitant procedures, mainly cholecystectomy, according to the report.
Robotic surgeries were converted to open procedures in seven patients, or about 12%, a statistic comparable with other reports of laparoscopic or robotic surgery, the investigators noted.
Postoperative complications were seen in 16 patients, or 27%. That included 13 patients with class I-II complications and 3 patients with class III-IV complications, including 2 cases of heart failure and 1 case of postoperative bile leak requiring radiologic and endoscopic therapy.
The reported 3-year disease-free and overall survival of 41.9% and 66.1% was based on a mean follow-up of 19.5 months in 44 patients for whom longer-term data were available. There were 16 cases of recurrent disease, including 10 patients with evidence of liver recurrence.
“Taken together, our data show that robotics is an effective option to resect colorectal liver metastases, providing an oncological outcome similar to that of laparoscopy and open surgery,” the investigators wrote.
Dr. Guerra and colleagues reported no disclosures related to their research.
SOURCE: Guerra F et al. Surg Oncol. 2018 Nov 1. doi: 10.1016/j.suronc.2018.10.011.
FROM SURGICAL ONCOLOGY
Key clinical point: Outcomes for robotic surgery for colorectal liver metastases are not inferior to laparoscopic and open surgical approaches.
Major finding: Negative margins were achieved in 92% of lesions, while 3-year disease-free and overall survival were 41.9% and 66.1%, respectively.
Study details: An analysis of 59 patients undergoing robotic surgery for colorectal liver metastases at one of three institutions between 2008 and 2018.
Disclosures: The study authors reported no disclosures.
Source: Guerra F et al. Surg Oncol. 2018 Nov 1. doi: 10.1016/j.suronc.2018.10.011.
Mood and behavior are different targets for irritability in children
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
BROOKLYN, N.Y. – As a target of therapy in children with a psychiatric disorder, irritability expressed as grumpy mood or anger should be uncoupled from irritability expressed as threatening behavior, according to an exploration of this common clinical issue at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Irritability is like fever,” reported Gabrielle A. Carlson, MD, professor of psychiatry and pediatrics, State University of New York at Stony Brook. “It is a nonspecific symptom that only tells you that something is wrong.”
Irritability might be nothing more than a negative mood, but it also can be the source of explosive aggression, leading to tantrums and destructive behaviors, according to Dr. Carlson. She placed them into two different categories when considering treatment. Irritability leading to annoyance, grumpiness, withdrawal, or persistent anger is characterized as the “internalizing” or “tonic” form of the symptom. As opposed to the aggressive subtype, the tonic form is more closely associated with depression or anxiety. Irritability leading to extreme verbal outbursts or physical violence is characterized as the “externalizing” or “phasic” form, Dr. Carlson said. This type of irritability, defined by behavior more than mood, might signal disruptive mood dysregulation disorder (DMDD). But it is important to recognize that DMDD can overlap with other conditions, such as attention-deficit/hyperactivity disorder (ADHD), bipolar disorder, oppositional defiant disorder (ODD), and autism spectrum disorders.
In defining the impact of treatments on tonic versus phasic symptoms of irritability within the context of the underlying diagnoses, studies have not done a good job in separating relative effects on the two key forms of irritability, Dr. Carlson said.
“Irritability needs to be measured not only by how one feels but what one does,” said Dr. Carlson, explaining that the impact of therapy has not always been adequately described in therapy studies.
For the tonic form, irritability is likely to improve or resolve with control of the underlying psychiatric condition. Although this might also be true of the phasic form, this type of irritability often accompanies conditions that are less readily controlled even through the threat of self-harm, harm to others, or other destructive behaviors invites intervention specifically targeted at this symptom.
Unfortunately, the best approach to irritability is unclear for many underling pathologies.
“Clinicians should recognize that empirical evidence is still lacking as to aggression-targeted treatments with favorable benefit-risk profiles for children and adolescents with ADHD and severe aggression,” said Dr. Carlson, providing ADHD as one of several examples.
Psychological interventions, such as dialectical behavior therapy in children (DBT-C), have been associated with control of both tonic and phasic forms of irritability, but Dr. Carlson cautioned that few studies have adequately differentiated improvement in irritability as measured by behavior relative to mood. In addition, the baseline severity and the degree to which improvement meant adequate control have been unclear.
“Many psychological treatments are school based or group delivered, making it likely that patients are less impaired than explosive kids in psychiatry clinics and hospitals,” Dr. Carlson said.
Providing some practical tips for addressing the phasic form of irritability, She advised clinicians to “maximize the treatment of the base condition” but to add pharmacologic therapies to psychological interventions if symptoms persist.
“Our pendulum has swung from dishing out atypicals to eschewing them completely,” Dr. Carlson noted. Although she agreed these are no longer appropriate as first-line therapies, she suggested they might be employed judiciously if weight gain is monitored carefully.
“If they don’t work, stop them. If they do work, try to limit the duration of use,” Dr. Carlson said.
She reported having no relevant financial relationships to disclose.
REPORTING FROM The PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Moment of truth approaches for low-risk TAVR
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
SNOWMASS, COLO. – There are now more transcatheter aortic valve replacements performed each year than surgical ones in the United States, a disparity that may grow vastly larger.
That’s if the results of the two pivotal randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in low-surgical-risk patients scheduled for presentation at the annual scientific session of the American College of Cardiology in March turn out to show TAVR outcomes are equivalent or superior to SAVR.
And that just might be the scenario, provided the eye-popping results already reported from another, much smaller study – the Low Risk TAVR study, a 200-patient, prospective, nonrandomized, observational study – are at all reflective of what’s to come when the pivotal PARTNER 3 and EVOLUT R trials are released at the ACC meeting in New Orleans, Michael J. Mack, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“The TAVR train has left the station on the way to low risk, and I don’t really see it coming back,” said Dr. Mack, medical director for cardiothoracic surgery at Baylor Scott & White Health in Dallas.
He wasn’t part of the Low Risk TAVR study, in which 200 low-surgical-risk patients with symptomatic severe aortic stenosis underwent TAVR with contemporary devices at 11 centers and were matched to 719 historical control SAVR patients at the same centers. But he called the study results “pretty spectacular”: zero 30-day all cause mortality in the TAVR group versus 1.7% with SAVR, no in-hospital strokes with TAVR versus a 0.6% rate with SAVR, and similar permanent pacemaker implantation rates of 5.0% with TAVR and 4.5% with SAVR.
Also, the TAVR group had a mere 3.0% rate of new-onset atrial fibrillation, a 2-day hospital length of stay, and a 0.5% incidence of greater-than-mild paravalvular leak at 30 days (J Am Coll Cardiol. 2018 Oct 30;72[18]:2095-105).
The two major trials due to report 1-year outcomes at the ACC meeting in March are similarly designed. The PARTNER 3 trial includes 1,000 low-surgical-risk patients with a mean age of 73 years and a predicted 30-day surgical mortality risk of 1.9%. Seventy-one percent of them were New York Heart Association (NYHA) Class II at enrollment. Participants were randomized to TAVR with the Edwards Lifesciences Sapien 3 valve or to SAVR, with the primary outcome being a composite of all-cause mortality, stroke, and rehospitalization 1 year post procedure. The EVOLUT R trial is similar, except the TAVR valve is the Medtronic CoreValve.
Both trials will continue to follow patients annually for 10 years in order to address the still-open issue of TAVR and SAVR valve durability. Also, the Food and Drug Administration has mandated that 4D CT imaging substudies be conducted in 800 of the combined 2,000 participants in the two trials in order to provide new insight into the issue of subclinical valve leaflet thrombosis, which was detected in 14% of participants in the Low Risk TAVR study 30 days post procedure.
“The clinical impact and need for anticoagulant therapy are currently unknown. However, clot anywhere else in the body doesn’t do good things, so it’s hard to imagine it’s helping here. Pretending it doesn’t exist isn’t going to make the problem go away,” Dr. Mack said.
The 4D CT imaging substudy results are expected to be presented later this year at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
In 2017, 51,064 TAVR procedures for symptomatic severe aortic stenosis were done in the United States, compared with 41,490 SAVRs. The past several years have seen a decreasing proportion of TAVRs being done in high-surgical-risk patients and a growing proportion in intermediate-risk patients.
Even if PARTNER 3 and EVOLUT R prove to be resoundingly positive for TAVR in low-risk patients, however, SAVR is not going to vanish, according to Dr. Mack. He cited four factors working against universal adoption of TAVR: the uncertainty surrounding valve durability, which will take years to resolve; the issue of TAVR valve leaflet thrombosis and the for-now theoretic possibility that all TAVR patients might need to receive postprocedure oral anticoagulation; the high rate of new permanent pacemaker implantation associated with TAVR, which Dr. Mack called the procedure’s Achilles heel; and the total absence of high-quality data on TAVR in patients with bicuspid aortic stenosis.
Even though TAVR for diseased bicuspid valves is not off-label therapy – the FDA’s indication for TAVR is for native valve aortic stenosis – patients with bicuspid valves weren’t included in any of the randomized trials, he explained.
Younger patients are likely to stick with SAVR for the foreseeable future, regardless of the outcomes of PARTNER 3 and EVOLUT R, according to the surgeon, because of the unresolved issue of valve durability, as well as TAVR’s greater associated need for a permanent pacemaker, both significant considerations in individuals with a life expectancy of another 20-30 years.
There are now roughly 600 TAVR centers and 1,150 SAVR centers nationally. One of the hot topics in the field stems from the fact that half of these TAVR centers do only one TAVR per week or less. That’s concerning in light of a recent New York State study showing a clear association between operator volume and outcomes.
“The more you do, the better your outcomes are, similar to many other procedures in medicine,” Dr. Mack commented.
On the other hand, it’s unlikely that patients who present to one of the roughly 550 SAVR-only centers are truly getting informed consent as to their options, he added.
TAVR timeline for 2019
March
PARTNER 3 and EVOLUT R primary outcomes to be presented at the American College of Cardiology annual scientific session.
Centers for Medicare & Medicaid Services to issue proposal for a revised National Coverage Determination for TAVR reimbursement.
June
Following a public comment period, CMS will release final revised criteria for TAVR reimbursement.
September
Results of the PARTNER 3 and EVOLUT R 4D CT imaging substudies will probably be presented late in the month at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Late 2019
If PARTNER 3 and EVOLUT R trials are positive, FDA approval of the TAVR valves in low-surgical-risk patients is expected.
Dr. Mack is coprincipal investigator of PARTNER 3, which was sponsored by Edwards Lifesciences, and of Abbott Vascular’s COAPT trial. He’s also on the executive committee of the INTREPID trial, sponsored by Medtronic.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Novel plasma biomarkers may predict preclinical Alzheimer’s disease
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
FROM SCIENCE ADVANCES
Key clinical point: Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management.
Major finding: A classifier developed using plasma proteomic analysis achieved an area under the receiver operator characteristic curve of 0.891.
Study details: An analysis of data from 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH).
Disclosures: The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
Source: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
Functional MRI detects consciousness after brain damage
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.
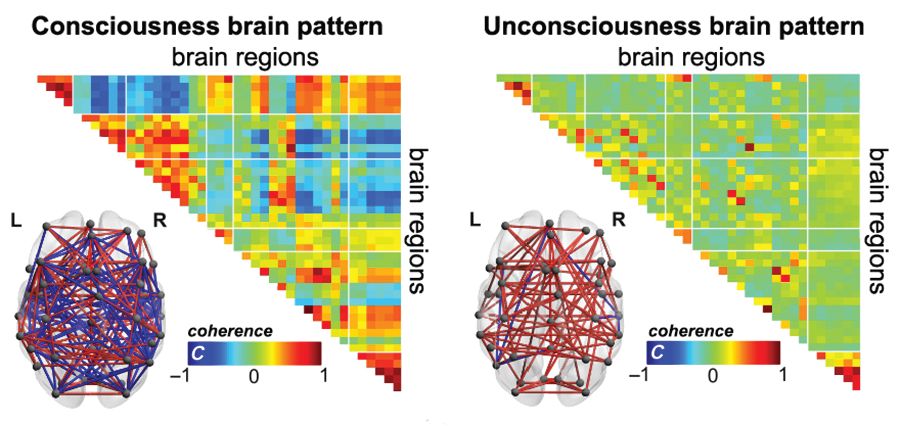
Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
FROM SCIENCE ADVANCES
Key clinical point:
Major finding: A brain-wide coordination pattern of high complexity became increasingly common when moving from patients with unresponsive wakefulness syndrome (UWS) to patients in a minimally conscious state (MCS) to healthy control individuals.
Study details: A study involving blood oxygen level–dependent (BOLD) fMRI scans at rest or under anesthesia in 159 participants at four independent research facilities.
Disclosures: The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
Source: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
American football and CTE: Is a racial divide inevitable?
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Public insurance income limits and hospitalizations for low-income children
Vulnerable populations at greater risk
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.
Vulnerable populations at greater risk
Vulnerable populations at greater risk
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.
Product Update: Bijuva; Liletta; Aegea Vapor System; Natural Cycles
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
Michigan Medicine launches effort to make wellness a cultural norm
CORONADO, CALIF. – Officials at staff, and learners.
“If you look long and hard at your hospitals, health centers, and medical schools, you would find incidences of depression, near-miss suicide, opioid addiction, substance abuse addictions, and suicide,” Carol R. Bradford, MD, said at the Triological Society’s Combined Sections Meeting. “Another component of this is that we all struggle with our work or learning communities where people don’t take care of each other. People don’t treat each other with respect and civility. Promoting a healthy and civil work environment are essential components of a supportive environment.”
According to Dr. Bradford, executive vice dean for academic affairs at the University of Michigan, Ann Arbor, the complexities and stress of the health care environment compromises the well-being of its workforce with a myriad of time-consuming tasks, including navigating electronic records and ever-populating email inboxes. “We are all connected to devices 24/7, and it has become more and more difficult to maintain a healthy work-life balance,” Dr. Bradford said. “The more accepted term now is integration, because it’s almost impossible to achieve balance. Burnout and other physical and health problems are the result of all of these challenges.”
In late 2017, she and her colleagues used two different validated survey questionnaires to assess the health of Michigan Medicine faculty physicians. They found that about 40% of faculty members in both clinical and basic science departments met criteria for burnout. The top 10 stressors based on the survey were email, clerical activity, time worked outside of regular hours, workload time pressure, work expectations, insufficient time for meaningful activities, in-basket messages, lack of decisional transparency, inadequate compensation, and too many work hours. The top 10 coping strategies were finding meaning in work, using all vacation time, paying attention to healthy/balanced eating, engaging in exercise, seek personal/professional balance, protecting time away from work, protecting sleep time, using a social support network, nurturing spiritual aspects, and engaging in recreation or hobbies.
Results of the survey prompted development of a task force to examine wellness and civility at Michigan Medicine, and to devise strategies and tactics to conquer these challenges. “The goal is to help all human beings who are suffering in our work environment,” said Dr. Bradford, who is also chief academic officer for Michigan Medicine. “What we learned initially is that there is a bit of an overlap. Some lack of wellness is due to a lack of civility, but there are wellness issues and civility issues that are independent of one another.”
Members of the task force formulated several recommendations, the first being to create a Michigan Medicine Wellness Office. Dr. Bradford is currently negotiating with a finalist to serve as its faculty director. She characterized the office as a “hub and spoke” model that will partner with existing entities, including human resources, the office of medical student education, the program in biological sciences, graduate medical education, the office of health equality and inclusion, the office of clinical affairs, and the office of counseling and workplace resilience. “The idea is to create a strategic wellness plan,” said Dr. Bradford, who is also a professor of otolaryngology–head and neck surgery. “One key strategy is to endorse the health and well-being of our faculty, staff, and learners as a core value and cultural norm of Michigan Medicine. In other words, the leadership has to make health and well-being a priority and a value.”
Another goal of the office is to improve the overall workplace environment and experience of Michigan Medicine’s faculty, staff, and learners. “You’re not going to have a well workplace if people are not treating each other with respect,” she said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “One of the many challenges is that there is great stigma in our profession for those who are suffering from mental health challenges such as stress, anxiety, depression, and perhaps substance abuse. We need to reduce the stigma, because it’s very dangerous if people who are struggling are unwilling to seek help. We don’t ask people that we supervise or work with how they’re doing, so we have adopted an optional wellness check-in that is incorporated into mid-year and annual evaluations for faculty, staff, and learners to enable leaders to address any challenges that may arise.” In addition, a group of residents is piloting the use of meditation and mindfulness applications such as MoodGym and Headspace to see if they affect resident wellness.
Ultimately, Dr. Bradford and her associates plan to use a standardized benchmark instrument to measure well-being, and include the measure in the institutional performance dashboard. “Administrative burden is a growing problem,” she said. “We’re going to address this for health care professionals, particularly as it relates to the electronic medical record. Our primary care colleagues sometimes spend as many hours outside of clinic documenting as they do in clinic. We want to develop and implement strategies to lessen or remove this burden in order to improve provider efficiency and satisfaction.”
In the course of helping to develop the wellness initiative, Dr. Bradford said that she learned the importance of addressing moral distress in the workplace. “We sort of lose our humanity if we don’t show emotion when tragedies happen. There is really good literature around terminal event debriefings, so if somebody dies unexpectedly in the operating room or in the CT scanner, rather than just walking away and pretending nothing happened, we’re supposed to pause and gather, and reflect on the sadness of the loss. Because if we don’t grieve our losses we become more like machines than human beings. It’s important to provide emotional support for all individuals involved.”
She reported having no relevant financial disclosures.
CORONADO, CALIF. – Officials at staff, and learners.
“If you look long and hard at your hospitals, health centers, and medical schools, you would find incidences of depression, near-miss suicide, opioid addiction, substance abuse addictions, and suicide,” Carol R. Bradford, MD, said at the Triological Society’s Combined Sections Meeting. “Another component of this is that we all struggle with our work or learning communities where people don’t take care of each other. People don’t treat each other with respect and civility. Promoting a healthy and civil work environment are essential components of a supportive environment.”
According to Dr. Bradford, executive vice dean for academic affairs at the University of Michigan, Ann Arbor, the complexities and stress of the health care environment compromises the well-being of its workforce with a myriad of time-consuming tasks, including navigating electronic records and ever-populating email inboxes. “We are all connected to devices 24/7, and it has become more and more difficult to maintain a healthy work-life balance,” Dr. Bradford said. “The more accepted term now is integration, because it’s almost impossible to achieve balance. Burnout and other physical and health problems are the result of all of these challenges.”
In late 2017, she and her colleagues used two different validated survey questionnaires to assess the health of Michigan Medicine faculty physicians. They found that about 40% of faculty members in both clinical and basic science departments met criteria for burnout. The top 10 stressors based on the survey were email, clerical activity, time worked outside of regular hours, workload time pressure, work expectations, insufficient time for meaningful activities, in-basket messages, lack of decisional transparency, inadequate compensation, and too many work hours. The top 10 coping strategies were finding meaning in work, using all vacation time, paying attention to healthy/balanced eating, engaging in exercise, seek personal/professional balance, protecting time away from work, protecting sleep time, using a social support network, nurturing spiritual aspects, and engaging in recreation or hobbies.
Results of the survey prompted development of a task force to examine wellness and civility at Michigan Medicine, and to devise strategies and tactics to conquer these challenges. “The goal is to help all human beings who are suffering in our work environment,” said Dr. Bradford, who is also chief academic officer for Michigan Medicine. “What we learned initially is that there is a bit of an overlap. Some lack of wellness is due to a lack of civility, but there are wellness issues and civility issues that are independent of one another.”
Members of the task force formulated several recommendations, the first being to create a Michigan Medicine Wellness Office. Dr. Bradford is currently negotiating with a finalist to serve as its faculty director. She characterized the office as a “hub and spoke” model that will partner with existing entities, including human resources, the office of medical student education, the program in biological sciences, graduate medical education, the office of health equality and inclusion, the office of clinical affairs, and the office of counseling and workplace resilience. “The idea is to create a strategic wellness plan,” said Dr. Bradford, who is also a professor of otolaryngology–head and neck surgery. “One key strategy is to endorse the health and well-being of our faculty, staff, and learners as a core value and cultural norm of Michigan Medicine. In other words, the leadership has to make health and well-being a priority and a value.”
Another goal of the office is to improve the overall workplace environment and experience of Michigan Medicine’s faculty, staff, and learners. “You’re not going to have a well workplace if people are not treating each other with respect,” she said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “One of the many challenges is that there is great stigma in our profession for those who are suffering from mental health challenges such as stress, anxiety, depression, and perhaps substance abuse. We need to reduce the stigma, because it’s very dangerous if people who are struggling are unwilling to seek help. We don’t ask people that we supervise or work with how they’re doing, so we have adopted an optional wellness check-in that is incorporated into mid-year and annual evaluations for faculty, staff, and learners to enable leaders to address any challenges that may arise.” In addition, a group of residents is piloting the use of meditation and mindfulness applications such as MoodGym and Headspace to see if they affect resident wellness.
Ultimately, Dr. Bradford and her associates plan to use a standardized benchmark instrument to measure well-being, and include the measure in the institutional performance dashboard. “Administrative burden is a growing problem,” she said. “We’re going to address this for health care professionals, particularly as it relates to the electronic medical record. Our primary care colleagues sometimes spend as many hours outside of clinic documenting as they do in clinic. We want to develop and implement strategies to lessen or remove this burden in order to improve provider efficiency and satisfaction.”
In the course of helping to develop the wellness initiative, Dr. Bradford said that she learned the importance of addressing moral distress in the workplace. “We sort of lose our humanity if we don’t show emotion when tragedies happen. There is really good literature around terminal event debriefings, so if somebody dies unexpectedly in the operating room or in the CT scanner, rather than just walking away and pretending nothing happened, we’re supposed to pause and gather, and reflect on the sadness of the loss. Because if we don’t grieve our losses we become more like machines than human beings. It’s important to provide emotional support for all individuals involved.”
She reported having no relevant financial disclosures.
CORONADO, CALIF. – Officials at staff, and learners.
“If you look long and hard at your hospitals, health centers, and medical schools, you would find incidences of depression, near-miss suicide, opioid addiction, substance abuse addictions, and suicide,” Carol R. Bradford, MD, said at the Triological Society’s Combined Sections Meeting. “Another component of this is that we all struggle with our work or learning communities where people don’t take care of each other. People don’t treat each other with respect and civility. Promoting a healthy and civil work environment are essential components of a supportive environment.”
According to Dr. Bradford, executive vice dean for academic affairs at the University of Michigan, Ann Arbor, the complexities and stress of the health care environment compromises the well-being of its workforce with a myriad of time-consuming tasks, including navigating electronic records and ever-populating email inboxes. “We are all connected to devices 24/7, and it has become more and more difficult to maintain a healthy work-life balance,” Dr. Bradford said. “The more accepted term now is integration, because it’s almost impossible to achieve balance. Burnout and other physical and health problems are the result of all of these challenges.”
In late 2017, she and her colleagues used two different validated survey questionnaires to assess the health of Michigan Medicine faculty physicians. They found that about 40% of faculty members in both clinical and basic science departments met criteria for burnout. The top 10 stressors based on the survey were email, clerical activity, time worked outside of regular hours, workload time pressure, work expectations, insufficient time for meaningful activities, in-basket messages, lack of decisional transparency, inadequate compensation, and too many work hours. The top 10 coping strategies were finding meaning in work, using all vacation time, paying attention to healthy/balanced eating, engaging in exercise, seek personal/professional balance, protecting time away from work, protecting sleep time, using a social support network, nurturing spiritual aspects, and engaging in recreation or hobbies.
Results of the survey prompted development of a task force to examine wellness and civility at Michigan Medicine, and to devise strategies and tactics to conquer these challenges. “The goal is to help all human beings who are suffering in our work environment,” said Dr. Bradford, who is also chief academic officer for Michigan Medicine. “What we learned initially is that there is a bit of an overlap. Some lack of wellness is due to a lack of civility, but there are wellness issues and civility issues that are independent of one another.”
Members of the task force formulated several recommendations, the first being to create a Michigan Medicine Wellness Office. Dr. Bradford is currently negotiating with a finalist to serve as its faculty director. She characterized the office as a “hub and spoke” model that will partner with existing entities, including human resources, the office of medical student education, the program in biological sciences, graduate medical education, the office of health equality and inclusion, the office of clinical affairs, and the office of counseling and workplace resilience. “The idea is to create a strategic wellness plan,” said Dr. Bradford, who is also a professor of otolaryngology–head and neck surgery. “One key strategy is to endorse the health and well-being of our faculty, staff, and learners as a core value and cultural norm of Michigan Medicine. In other words, the leadership has to make health and well-being a priority and a value.”
Another goal of the office is to improve the overall workplace environment and experience of Michigan Medicine’s faculty, staff, and learners. “You’re not going to have a well workplace if people are not treating each other with respect,” she said at the meeting, jointly sponsored by the Triological Society and the American College of Surgeons. “One of the many challenges is that there is great stigma in our profession for those who are suffering from mental health challenges such as stress, anxiety, depression, and perhaps substance abuse. We need to reduce the stigma, because it’s very dangerous if people who are struggling are unwilling to seek help. We don’t ask people that we supervise or work with how they’re doing, so we have adopted an optional wellness check-in that is incorporated into mid-year and annual evaluations for faculty, staff, and learners to enable leaders to address any challenges that may arise.” In addition, a group of residents is piloting the use of meditation and mindfulness applications such as MoodGym and Headspace to see if they affect resident wellness.
Ultimately, Dr. Bradford and her associates plan to use a standardized benchmark instrument to measure well-being, and include the measure in the institutional performance dashboard. “Administrative burden is a growing problem,” she said. “We’re going to address this for health care professionals, particularly as it relates to the electronic medical record. Our primary care colleagues sometimes spend as many hours outside of clinic documenting as they do in clinic. We want to develop and implement strategies to lessen or remove this burden in order to improve provider efficiency and satisfaction.”
In the course of helping to develop the wellness initiative, Dr. Bradford said that she learned the importance of addressing moral distress in the workplace. “We sort of lose our humanity if we don’t show emotion when tragedies happen. There is really good literature around terminal event debriefings, so if somebody dies unexpectedly in the operating room or in the CT scanner, rather than just walking away and pretending nothing happened, we’re supposed to pause and gather, and reflect on the sadness of the loss. Because if we don’t grieve our losses we become more like machines than human beings. It’s important to provide emotional support for all individuals involved.”
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE TRIOLOGICAL CSM