User login
Genetic analysis detects novel gene mutations in hemophilia A
A wide variety of genetic mutations, including two novel variants, were found in a cohort of Vietnamese patients with hemophilia A, according to a recent report.
Dung Vu Luu, MD, of the Hanoi (Vietnam) Medical University and his colleagues conducted a genetic analysis of 103 patients with hemophilia A. The results of the analysis were reported in a letter to the editor published in Haemophilia.
“In this letter, we report the spectrum of F8 mutations in Vietnamese patients with a well‐established clinical diagnosis of hemophilia A,” the researchers wrote.
Clinical assessment and validation of hemophilia was completed by hematologists from Vietnam’s National Institute of Hematology and Blood Transfusion. The team analyzed DNA that was extracted and isolated from blood samples collected from the patients.
After analysis, the researchers found that disease-causing genetic variants were present in 89% of patients. The intron 22 inversion mutation was detected in 34% of patients in the cohort.
Dr. Luu and his colleagues reported that two novel F8 mutations were also detected that resulted in a severe clinical phenotype: c.4550‐4551insA; p.His1518Serfs*13 and c.1268‐1269insG; p.Asp366GluFs*5.
“To our knowledge, and after an extensive search in many databases, these two variants have not been reported in any F8 mutation database or reported in any published articles,” they explained.
The researchers acknowledged that future studies need to be larger in size.
“The results of this study are adding to the global knowledge base for [hemophilia A] and thus will promote better awareness for molecular diagnosis and care for [hemophilia A] patients in Vietnam,” they wrote.
The study was supported by grant funding from the Ministry of Health of Vietnam. The authors reported having no conflicts of interest.
SOURCE: Luu DV et al. Haemophilia. 2019 Mar 26. doi: 10.1111/hae.13738.
A wide variety of genetic mutations, including two novel variants, were found in a cohort of Vietnamese patients with hemophilia A, according to a recent report.
Dung Vu Luu, MD, of the Hanoi (Vietnam) Medical University and his colleagues conducted a genetic analysis of 103 patients with hemophilia A. The results of the analysis were reported in a letter to the editor published in Haemophilia.
“In this letter, we report the spectrum of F8 mutations in Vietnamese patients with a well‐established clinical diagnosis of hemophilia A,” the researchers wrote.
Clinical assessment and validation of hemophilia was completed by hematologists from Vietnam’s National Institute of Hematology and Blood Transfusion. The team analyzed DNA that was extracted and isolated from blood samples collected from the patients.
After analysis, the researchers found that disease-causing genetic variants were present in 89% of patients. The intron 22 inversion mutation was detected in 34% of patients in the cohort.
Dr. Luu and his colleagues reported that two novel F8 mutations were also detected that resulted in a severe clinical phenotype: c.4550‐4551insA; p.His1518Serfs*13 and c.1268‐1269insG; p.Asp366GluFs*5.
“To our knowledge, and after an extensive search in many databases, these two variants have not been reported in any F8 mutation database or reported in any published articles,” they explained.
The researchers acknowledged that future studies need to be larger in size.
“The results of this study are adding to the global knowledge base for [hemophilia A] and thus will promote better awareness for molecular diagnosis and care for [hemophilia A] patients in Vietnam,” they wrote.
The study was supported by grant funding from the Ministry of Health of Vietnam. The authors reported having no conflicts of interest.
SOURCE: Luu DV et al. Haemophilia. 2019 Mar 26. doi: 10.1111/hae.13738.
A wide variety of genetic mutations, including two novel variants, were found in a cohort of Vietnamese patients with hemophilia A, according to a recent report.
Dung Vu Luu, MD, of the Hanoi (Vietnam) Medical University and his colleagues conducted a genetic analysis of 103 patients with hemophilia A. The results of the analysis were reported in a letter to the editor published in Haemophilia.
“In this letter, we report the spectrum of F8 mutations in Vietnamese patients with a well‐established clinical diagnosis of hemophilia A,” the researchers wrote.
Clinical assessment and validation of hemophilia was completed by hematologists from Vietnam’s National Institute of Hematology and Blood Transfusion. The team analyzed DNA that was extracted and isolated from blood samples collected from the patients.
After analysis, the researchers found that disease-causing genetic variants were present in 89% of patients. The intron 22 inversion mutation was detected in 34% of patients in the cohort.
Dr. Luu and his colleagues reported that two novel F8 mutations were also detected that resulted in a severe clinical phenotype: c.4550‐4551insA; p.His1518Serfs*13 and c.1268‐1269insG; p.Asp366GluFs*5.
“To our knowledge, and after an extensive search in many databases, these two variants have not been reported in any F8 mutation database or reported in any published articles,” they explained.
The researchers acknowledged that future studies need to be larger in size.
“The results of this study are adding to the global knowledge base for [hemophilia A] and thus will promote better awareness for molecular diagnosis and care for [hemophilia A] patients in Vietnam,” they wrote.
The study was supported by grant funding from the Ministry of Health of Vietnam. The authors reported having no conflicts of interest.
SOURCE: Luu DV et al. Haemophilia. 2019 Mar 26. doi: 10.1111/hae.13738.
FROM HAEMOPHILIA
Clinicians, CMS confer over heart failure–readmission penalty
NEW ORLEANS – Mounting evidence shows that heart failure patient mortality increased as an unintended consequence of a Medicare program that penalizes hospitals with too many 30-day readmissions of heart failure patients. This has prompted discussions among cardiologists, Medicare officials, and other stakeholders in an attempt to modify the penalty program so it no longer considers just readmissions but instead bases penalties on broader and more nuanced measures of patient outcomes.
Staffers at the Centers for Medicare & Medicaid Services, the federal agency that manages Medicare, “said that they take this seriously and will look into it, and they are interested in next-generation measures that are more patient centered” than simply the 30-day readmission rate, Gregg C. Fonarow, MD, said in an interview at the annual meeting of the American College of Cardiology. “This is a case where there is credible evidence of increased mortality that is consistent, reproducible, and strongly associated with the penalty and cannot be otherwise explained,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
He is among the most active researchers to document that, while CMS’s Hospital Readmissions Reduction Program (HRRP) led to significantly reduced readmission rates in patients with heart failure, this came at a cost of a significant increase in mortality among the same patients. For example, an article he published in 2018 that analyzed more than 115,000 Medicare beneficiaries during 2006-2014 showed that during the penalty phase, which began in 2012, readmissions fell after adjustment by a relative 8%, but adjusted mortality rose by a relative 10%, compared with how patients had fared prior to launching the HRRP (JAMA Cardiol. 2018 Jan;3[1]:44-53). Recent reports from other research groups have had similar findings, such as a study of more than 3 million Medicare beneficiaries with heart failure during 2005-2015 that also showed significantly increased mortality after the penalty phase for readmissions began (JAMA. 2018 Dec 25;320[24]:2542-52). In a commentary that accompanied this report, Dr. Fonarow cited the multiple analyses that show consistent findings and the need for CMS to “initiate an expeditious reconsideration and revision” of their current approach to penalizing hospitals for heart failure readmissions (JAMA. 2018 Dec 25;320[24]:2539-41).
The groups recently in discussion with CMS about this issue include the American College of Cardiology, the American Heart Association, the Heart Failure Society of America, the American College of Physicians, the American Hospital Association, and several other medical professional groups, said Biykem Bozkurt, MD, who has worked with Dr. Fonarow and representatives from these organizations in talks with CMS.
“We are trying to find a harmonized approach with patient-centric outcomes that reflect true improvements in quality of care,” she said in an interview. One possibility up for consideration is a combined measure of heart failure readmissions, mortality, and a patient-reported outcome. The measure would go to CMS directly from each patient’s electronic medical record, making data collection less burdensome to clinicians, said Dr. Bozkurt, professor of medicine at Baylor College of Medicine and cardiology section chief at the VA Medical Center in Houston. She expressed hope that a change in the CMS metric might happen later this year.
“CMS can’t simply stop the HRRP, so the discussion is on how to get a meaningful change. I’m increasingly optimistic, because the findings of harm [from current policies] are impossible to ignore,” Dr. Fonarow said. “There will be increasing pressure on CMS to develop a pathway to make modifications. It’s egregious to continue a policy that’s been associated with harm” to heart failure patients.
Dr. Fonarow and Dr. Bozkurt had no relevant commercial disclosures.
NEW ORLEANS – Mounting evidence shows that heart failure patient mortality increased as an unintended consequence of a Medicare program that penalizes hospitals with too many 30-day readmissions of heart failure patients. This has prompted discussions among cardiologists, Medicare officials, and other stakeholders in an attempt to modify the penalty program so it no longer considers just readmissions but instead bases penalties on broader and more nuanced measures of patient outcomes.
Staffers at the Centers for Medicare & Medicaid Services, the federal agency that manages Medicare, “said that they take this seriously and will look into it, and they are interested in next-generation measures that are more patient centered” than simply the 30-day readmission rate, Gregg C. Fonarow, MD, said in an interview at the annual meeting of the American College of Cardiology. “This is a case where there is credible evidence of increased mortality that is consistent, reproducible, and strongly associated with the penalty and cannot be otherwise explained,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
He is among the most active researchers to document that, while CMS’s Hospital Readmissions Reduction Program (HRRP) led to significantly reduced readmission rates in patients with heart failure, this came at a cost of a significant increase in mortality among the same patients. For example, an article he published in 2018 that analyzed more than 115,000 Medicare beneficiaries during 2006-2014 showed that during the penalty phase, which began in 2012, readmissions fell after adjustment by a relative 8%, but adjusted mortality rose by a relative 10%, compared with how patients had fared prior to launching the HRRP (JAMA Cardiol. 2018 Jan;3[1]:44-53). Recent reports from other research groups have had similar findings, such as a study of more than 3 million Medicare beneficiaries with heart failure during 2005-2015 that also showed significantly increased mortality after the penalty phase for readmissions began (JAMA. 2018 Dec 25;320[24]:2542-52). In a commentary that accompanied this report, Dr. Fonarow cited the multiple analyses that show consistent findings and the need for CMS to “initiate an expeditious reconsideration and revision” of their current approach to penalizing hospitals for heart failure readmissions (JAMA. 2018 Dec 25;320[24]:2539-41).
The groups recently in discussion with CMS about this issue include the American College of Cardiology, the American Heart Association, the Heart Failure Society of America, the American College of Physicians, the American Hospital Association, and several other medical professional groups, said Biykem Bozkurt, MD, who has worked with Dr. Fonarow and representatives from these organizations in talks with CMS.
“We are trying to find a harmonized approach with patient-centric outcomes that reflect true improvements in quality of care,” she said in an interview. One possibility up for consideration is a combined measure of heart failure readmissions, mortality, and a patient-reported outcome. The measure would go to CMS directly from each patient’s electronic medical record, making data collection less burdensome to clinicians, said Dr. Bozkurt, professor of medicine at Baylor College of Medicine and cardiology section chief at the VA Medical Center in Houston. She expressed hope that a change in the CMS metric might happen later this year.
“CMS can’t simply stop the HRRP, so the discussion is on how to get a meaningful change. I’m increasingly optimistic, because the findings of harm [from current policies] are impossible to ignore,” Dr. Fonarow said. “There will be increasing pressure on CMS to develop a pathway to make modifications. It’s egregious to continue a policy that’s been associated with harm” to heart failure patients.
Dr. Fonarow and Dr. Bozkurt had no relevant commercial disclosures.
NEW ORLEANS – Mounting evidence shows that heart failure patient mortality increased as an unintended consequence of a Medicare program that penalizes hospitals with too many 30-day readmissions of heart failure patients. This has prompted discussions among cardiologists, Medicare officials, and other stakeholders in an attempt to modify the penalty program so it no longer considers just readmissions but instead bases penalties on broader and more nuanced measures of patient outcomes.
Staffers at the Centers for Medicare & Medicaid Services, the federal agency that manages Medicare, “said that they take this seriously and will look into it, and they are interested in next-generation measures that are more patient centered” than simply the 30-day readmission rate, Gregg C. Fonarow, MD, said in an interview at the annual meeting of the American College of Cardiology. “This is a case where there is credible evidence of increased mortality that is consistent, reproducible, and strongly associated with the penalty and cannot be otherwise explained,” said Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
He is among the most active researchers to document that, while CMS’s Hospital Readmissions Reduction Program (HRRP) led to significantly reduced readmission rates in patients with heart failure, this came at a cost of a significant increase in mortality among the same patients. For example, an article he published in 2018 that analyzed more than 115,000 Medicare beneficiaries during 2006-2014 showed that during the penalty phase, which began in 2012, readmissions fell after adjustment by a relative 8%, but adjusted mortality rose by a relative 10%, compared with how patients had fared prior to launching the HRRP (JAMA Cardiol. 2018 Jan;3[1]:44-53). Recent reports from other research groups have had similar findings, such as a study of more than 3 million Medicare beneficiaries with heart failure during 2005-2015 that also showed significantly increased mortality after the penalty phase for readmissions began (JAMA. 2018 Dec 25;320[24]:2542-52). In a commentary that accompanied this report, Dr. Fonarow cited the multiple analyses that show consistent findings and the need for CMS to “initiate an expeditious reconsideration and revision” of their current approach to penalizing hospitals for heart failure readmissions (JAMA. 2018 Dec 25;320[24]:2539-41).
The groups recently in discussion with CMS about this issue include the American College of Cardiology, the American Heart Association, the Heart Failure Society of America, the American College of Physicians, the American Hospital Association, and several other medical professional groups, said Biykem Bozkurt, MD, who has worked with Dr. Fonarow and representatives from these organizations in talks with CMS.
“We are trying to find a harmonized approach with patient-centric outcomes that reflect true improvements in quality of care,” she said in an interview. One possibility up for consideration is a combined measure of heart failure readmissions, mortality, and a patient-reported outcome. The measure would go to CMS directly from each patient’s electronic medical record, making data collection less burdensome to clinicians, said Dr. Bozkurt, professor of medicine at Baylor College of Medicine and cardiology section chief at the VA Medical Center in Houston. She expressed hope that a change in the CMS metric might happen later this year.
“CMS can’t simply stop the HRRP, so the discussion is on how to get a meaningful change. I’m increasingly optimistic, because the findings of harm [from current policies] are impossible to ignore,” Dr. Fonarow said. “There will be increasing pressure on CMS to develop a pathway to make modifications. It’s egregious to continue a policy that’s been associated with harm” to heart failure patients.
Dr. Fonarow and Dr. Bozkurt had no relevant commercial disclosures.
REPORTING FROM ACC 19
Platelet-rich plasma shows promise in hemophilic arthropathy of the knee
A single intra-articular injection of platelet-rich plasma showed better pain and function scores at 6 months than did weekly injections of hyaluronic acid in patients with hemophilic arthropathy of the knee.
“In patients with chronic knee joint pain, our study shows that treatment with intra-articular [platelet-rich plasma] injection could reduce pain, improve hyperaemia and enhance knee joint function,” wrote Tsung-Ying Li, MD, of Tri-Service General Hospital in Taipei, Taiwan, and his colleagues. The results of the comparison study were published in Haemophilia.
Researchers conducted a nonrandomized, single-center, open-label comparison study of 22 patients with hemophilia A who had chronic hemophilic arthropathy of the knee. Patients were stratified into two treatment groups using a matched sampling method.
“Patients who could commit to hyaluronic acid treatment were allocated to the hyaluronic acid group, otherwise patients were allocated to the platelet‐rich plasma group,” the researchers wrote.
Participants in the hyaluronic acid arm were given five sequential weekly injections and those in the platelet-rich plasma arm received only a single injection. Outcomes were measured via the visual analogue scale (VAS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Chinese Version, and ultrasonography to determine synovial change.
Pain and function scores were significantly lower in patients administered intra-articular platelet-rich plasma versus hyaluronic acid at 6 months (P less than .05), the researchers reported. However, comparative analysis found no significant differences at earlier follow-up time points.
“Both treatments were found to be effective in reducing pain and improving functional status of the knee,” the researchers wrote.
They acknowledged a key limitation of the study was the short duration of follow-up, which was due to budget restrictions at the treatment facility.
“Further investigation using rigorous research methodology is warranted to establish the benefit of [platelet-rich plasma] on hemophilic arthropathy and standardized [platelet-rich plasma] preparation and dosing regimens,” they wrote.
The study was supported by grant funding from Tri-Service General Hospital in Taiwan. Three of the authors reported receiving research grants from Shire.
SOURCE: Li TY et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13711.
A single intra-articular injection of platelet-rich plasma showed better pain and function scores at 6 months than did weekly injections of hyaluronic acid in patients with hemophilic arthropathy of the knee.
“In patients with chronic knee joint pain, our study shows that treatment with intra-articular [platelet-rich plasma] injection could reduce pain, improve hyperaemia and enhance knee joint function,” wrote Tsung-Ying Li, MD, of Tri-Service General Hospital in Taipei, Taiwan, and his colleagues. The results of the comparison study were published in Haemophilia.
Researchers conducted a nonrandomized, single-center, open-label comparison study of 22 patients with hemophilia A who had chronic hemophilic arthropathy of the knee. Patients were stratified into two treatment groups using a matched sampling method.
“Patients who could commit to hyaluronic acid treatment were allocated to the hyaluronic acid group, otherwise patients were allocated to the platelet‐rich plasma group,” the researchers wrote.
Participants in the hyaluronic acid arm were given five sequential weekly injections and those in the platelet-rich plasma arm received only a single injection. Outcomes were measured via the visual analogue scale (VAS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Chinese Version, and ultrasonography to determine synovial change.
Pain and function scores were significantly lower in patients administered intra-articular platelet-rich plasma versus hyaluronic acid at 6 months (P less than .05), the researchers reported. However, comparative analysis found no significant differences at earlier follow-up time points.
“Both treatments were found to be effective in reducing pain and improving functional status of the knee,” the researchers wrote.
They acknowledged a key limitation of the study was the short duration of follow-up, which was due to budget restrictions at the treatment facility.
“Further investigation using rigorous research methodology is warranted to establish the benefit of [platelet-rich plasma] on hemophilic arthropathy and standardized [platelet-rich plasma] preparation and dosing regimens,” they wrote.
The study was supported by grant funding from Tri-Service General Hospital in Taiwan. Three of the authors reported receiving research grants from Shire.
SOURCE: Li TY et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13711.
A single intra-articular injection of platelet-rich plasma showed better pain and function scores at 6 months than did weekly injections of hyaluronic acid in patients with hemophilic arthropathy of the knee.
“In patients with chronic knee joint pain, our study shows that treatment with intra-articular [platelet-rich plasma] injection could reduce pain, improve hyperaemia and enhance knee joint function,” wrote Tsung-Ying Li, MD, of Tri-Service General Hospital in Taipei, Taiwan, and his colleagues. The results of the comparison study were published in Haemophilia.
Researchers conducted a nonrandomized, single-center, open-label comparison study of 22 patients with hemophilia A who had chronic hemophilic arthropathy of the knee. Patients were stratified into two treatment groups using a matched sampling method.
“Patients who could commit to hyaluronic acid treatment were allocated to the hyaluronic acid group, otherwise patients were allocated to the platelet‐rich plasma group,” the researchers wrote.
Participants in the hyaluronic acid arm were given five sequential weekly injections and those in the platelet-rich plasma arm received only a single injection. Outcomes were measured via the visual analogue scale (VAS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Chinese Version, and ultrasonography to determine synovial change.
Pain and function scores were significantly lower in patients administered intra-articular platelet-rich plasma versus hyaluronic acid at 6 months (P less than .05), the researchers reported. However, comparative analysis found no significant differences at earlier follow-up time points.
“Both treatments were found to be effective in reducing pain and improving functional status of the knee,” the researchers wrote.
They acknowledged a key limitation of the study was the short duration of follow-up, which was due to budget restrictions at the treatment facility.
“Further investigation using rigorous research methodology is warranted to establish the benefit of [platelet-rich plasma] on hemophilic arthropathy and standardized [platelet-rich plasma] preparation and dosing regimens,” they wrote.
The study was supported by grant funding from Tri-Service General Hospital in Taiwan. Three of the authors reported receiving research grants from Shire.
SOURCE: Li TY et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13711.
FROM HAEMOPHILIA
Novel percutaneous therapy for HOCM in development
SNOWMASS, COLO. – Percutaneous mitral valve plication shows early promise as a primary therapy for severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM), Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
He and his coworkers at the Minneapolis Heart Institute developed the novel procedure and published the first experience in the world with it. But further study in a much larger patient population is needed before percutaneous mitral plication is ready for prime time as a treatment for symptomatic HOCM, according to Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the institute.
Surgical myectomy is the guideline-recommended standard for treatment of patients with disabling symptoms caused by left ventricular outflow tract obstruction in the setting of hypertrophic cardiomyopathy. Alcohol septal ablation is a widely utilized alternative in patients who are frail and elderly or otherwise high-risk surgical candidates, or who lack ready access to an experienced surgical myectomy center, where the best outcomes are achieved. But roughly 20% of patients evaluated for alcohol septal ablation don’t have a septal artery anatomy amenable to the procedure. Other patients are put off by the 10%-50% risk that they will require a permanent pacemaker after alcohol septal ablation, depending upon their baseline ECG. And a small percentage of well-chosen patients – less than 10% – will experience inadequate gradient relief following alcohol septal ablation. So there is room for a novel therapeutic approach.
A percutaneous mitral clip–based solution, while technically challenging, offers several advantages over surgical myectomy and alcohol septal ablation. It’s less invasive, doesn’t create a potentially arrhythmogenic ablation scar requiring a permanent pacemaker, and it targets the mitral valve directly, addressing the mitral regurgitation that causes the left ventricular outflow tract obstruction. In contrast, both surgical myectomy and alcohol septal ablation target the ventricular septum.
Dr. Sorajja summarized his experience to date with percutaneous mitral valve plication for symptomatic HOCM, which encompasses the six patients in the published study (J Am Coll Cardiol. 2016 Jun 21;67[24]:2811-8) and the four others treated since then. The procedure was completed with placement of a single MitraClip in 9 of 10 patients. One patient experienced cardiac tamponade, resulting in a halt of the procedure; in the other nine, the intervention eliminated systolic anterior motion and markedly decreased the intraoperative left ventricular outflow tract gradient and mitral regurgitation.
At 2-5 years of follow-up, all patients have improved from New York Heart Association functional class III preprocedurally to class I or II. One patient required alcohol septal ablation. High systolic left ventricular outflow tract velocities in excess of 4 cm/s have been documented via follow-up echocardiography in some patients, the significance of which is under study.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Percutaneous mitral valve plication shows early promise as a primary therapy for severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM), Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
He and his coworkers at the Minneapolis Heart Institute developed the novel procedure and published the first experience in the world with it. But further study in a much larger patient population is needed before percutaneous mitral plication is ready for prime time as a treatment for symptomatic HOCM, according to Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the institute.
Surgical myectomy is the guideline-recommended standard for treatment of patients with disabling symptoms caused by left ventricular outflow tract obstruction in the setting of hypertrophic cardiomyopathy. Alcohol septal ablation is a widely utilized alternative in patients who are frail and elderly or otherwise high-risk surgical candidates, or who lack ready access to an experienced surgical myectomy center, where the best outcomes are achieved. But roughly 20% of patients evaluated for alcohol septal ablation don’t have a septal artery anatomy amenable to the procedure. Other patients are put off by the 10%-50% risk that they will require a permanent pacemaker after alcohol septal ablation, depending upon their baseline ECG. And a small percentage of well-chosen patients – less than 10% – will experience inadequate gradient relief following alcohol septal ablation. So there is room for a novel therapeutic approach.
A percutaneous mitral clip–based solution, while technically challenging, offers several advantages over surgical myectomy and alcohol septal ablation. It’s less invasive, doesn’t create a potentially arrhythmogenic ablation scar requiring a permanent pacemaker, and it targets the mitral valve directly, addressing the mitral regurgitation that causes the left ventricular outflow tract obstruction. In contrast, both surgical myectomy and alcohol septal ablation target the ventricular septum.
Dr. Sorajja summarized his experience to date with percutaneous mitral valve plication for symptomatic HOCM, which encompasses the six patients in the published study (J Am Coll Cardiol. 2016 Jun 21;67[24]:2811-8) and the four others treated since then. The procedure was completed with placement of a single MitraClip in 9 of 10 patients. One patient experienced cardiac tamponade, resulting in a halt of the procedure; in the other nine, the intervention eliminated systolic anterior motion and markedly decreased the intraoperative left ventricular outflow tract gradient and mitral regurgitation.
At 2-5 years of follow-up, all patients have improved from New York Heart Association functional class III preprocedurally to class I or II. One patient required alcohol septal ablation. High systolic left ventricular outflow tract velocities in excess of 4 cm/s have been documented via follow-up echocardiography in some patients, the significance of which is under study.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
SNOWMASS, COLO. – Percutaneous mitral valve plication shows early promise as a primary therapy for severely symptomatic, drug-refractory hypertrophic obstructive cardiomyopathy (HOCM), Paul Sorajja, MD, said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
He and his coworkers at the Minneapolis Heart Institute developed the novel procedure and published the first experience in the world with it. But further study in a much larger patient population is needed before percutaneous mitral plication is ready for prime time as a treatment for symptomatic HOCM, according to Dr. Sorajja, director of the Center of Valve and Structural Heart Disease at the institute.
Surgical myectomy is the guideline-recommended standard for treatment of patients with disabling symptoms caused by left ventricular outflow tract obstruction in the setting of hypertrophic cardiomyopathy. Alcohol septal ablation is a widely utilized alternative in patients who are frail and elderly or otherwise high-risk surgical candidates, or who lack ready access to an experienced surgical myectomy center, where the best outcomes are achieved. But roughly 20% of patients evaluated for alcohol septal ablation don’t have a septal artery anatomy amenable to the procedure. Other patients are put off by the 10%-50% risk that they will require a permanent pacemaker after alcohol septal ablation, depending upon their baseline ECG. And a small percentage of well-chosen patients – less than 10% – will experience inadequate gradient relief following alcohol septal ablation. So there is room for a novel therapeutic approach.
A percutaneous mitral clip–based solution, while technically challenging, offers several advantages over surgical myectomy and alcohol septal ablation. It’s less invasive, doesn’t create a potentially arrhythmogenic ablation scar requiring a permanent pacemaker, and it targets the mitral valve directly, addressing the mitral regurgitation that causes the left ventricular outflow tract obstruction. In contrast, both surgical myectomy and alcohol septal ablation target the ventricular septum.
Dr. Sorajja summarized his experience to date with percutaneous mitral valve plication for symptomatic HOCM, which encompasses the six patients in the published study (J Am Coll Cardiol. 2016 Jun 21;67[24]:2811-8) and the four others treated since then. The procedure was completed with placement of a single MitraClip in 9 of 10 patients. One patient experienced cardiac tamponade, resulting in a halt of the procedure; in the other nine, the intervention eliminated systolic anterior motion and markedly decreased the intraoperative left ventricular outflow tract gradient and mitral regurgitation.
At 2-5 years of follow-up, all patients have improved from New York Heart Association functional class III preprocedurally to class I or II. One patient required alcohol septal ablation. High systolic left ventricular outflow tract velocities in excess of 4 cm/s have been documented via follow-up echocardiography in some patients, the significance of which is under study.
Dr. Sorajja reported receiving research funding from Abbott Structural, Boston Scientific, Edwards Lifesciences, and Medtronic, and serving as a consultant to those companies and several others.
REPORTING FROM ACC SNOWMASS 2019
Eye bees, reversed innards, and the DNA van
Bees? Really?
This might be one of the weirdest thing we’ve ever seen. It’s definitely the weirdest thing this Taiwanese woman has ever seen, considering it’s BEES in her EYE.

One more time, for the people in the back: BEES. IN. HER. EYE.
The unfortunate 29-year-old, identified as He, reported to a hospital with sharp eye pain that she assumed was from dirt in her eyes after a vigorous afternoon of gardening near a grave site. Upon close inspection, the hospital’s head of ophthalmology noticed “something that looked like insect legs” floating in her eye and carefully pulled them out. (Pause to dry heave.)
The eyeball intruders turned out to be “sweat bees,” which nest in fallen trees and are attracted to human perspiration. The bees flew into He’s eye and started drinking from her tear duct, which was overproducing because of the immense pain she was in. Miraculously, He’s eyeball and sight are intact, and she’s expected to make a full recovery. The bees are fine, too.
Oh, and in case you’re wondering, sweat bees live all over the world. We’re wearing goggles outside from now on.
Flip it and reverse it
Imagine you’re back in your med school days, happily dissecting another cadaver. You pop the body open and … every organ is in the wrong place??

That’s what some students at Oregon Health & Science University (OHSU) experienced recently. They discovered their body donor had a condition called situs inversus with levocardia – meaning, her heart was in the right place (hah), but all her abdominal organs were transposed right to left. The deceased woman was Rose Marie Bentley, who had died at the ripe old age of 99 without ever knowing she had this condition.
What makes her remarkable is barely anyone with this condition lives to adulthood. Professor Cam Walker of OHSU estimates the odds are 1 in 50 million, and there are only two other known cases of older patients who lived with situs inversus. After taking a look at their mother’s medical records, Bentley’s children noted that the surgeon who removed her appendix recorded its strange location, but no one raised any alarm.
Ms. Bentley lived a full and happy life, despite her organs being a mirror image of virtually everyone else’s anatomy. Some people are just special.
Turn that smile upside down
Service with a smile. Put on a happy face. Let a smile be your umbrella. Seems like good advice, but don’t let these cliches fool you. They are all examples of surface acting – hiding feelings of annoyance behind a mask of forced pleasantness – and they’re not really good for you. At least not if you work with the public and are required to be nice.

Investigators at Penn State University and the University at Buffalo looked at survey data from nurses, teachers, and people in food service and found that they were more likely to drink after work than those who did not interact with the public on a regular basis. “In these jobs, there’s also often money tied to showing positive emotions and holding back negative feelings. Money gives you a motivation to override your natural tendencies, but doing it all day can be wearing,” lead investigator Alicia Grandey said in a written statement.
Personality also plays a part. The risk of increased drinking is greater for surface actors who are more impulsive. And it is stronger still for impulsive people who “also worked in jobs where employees have one-time service encounters with customers, like a call center or coffee shop, rather than relationships, like health care or education,” the investigators said.
They suggested that businesses could give their employees more autonomy, but we’ve got another idea. There are lots of awards for real actors, so how about one for surface actors? They could call it the Oscar … Mayer. Best performance in serving a drive-thru customer who smoked a little too much marijuana. Creative achievement while helping a credit card applicant who still doesn’t understand after being told the same thing five times. Stunt coordination while helping a patient use a bedpan (the “turn the other cheek” award). Choreography to get out of bed every day to face the blank stares of sullen teenagers (the “Lord, give me strength” award). The “okay, we get the picture, you can stop with the awards” award.
That’s not the ice cream truck
Any story that starts with strange men in white, unmarked vans is never going to end well. Especially when that strange man is offering $20 in exchange for DNA samples.

However, this is the situation that Louisville, Ky., finds itself in. According to WAVE3 News, the aforementioned men have been cruising the poorer neighborhoods of the city for a couple of months, offering money to anyone who belongs to Passport Health. Cheek swabs are collected, no questions are asked, and the person walks out with 20 extra dollars.
This is already shady territory, but to make things worse, Passport Health has denied any affiliation. And when asked by one of their customers, the men reported being with Freedom Health. The number the customer was given? Of course it didn’t work.
The cherry on top of the shady cake? According to a professor from the University of Louisville interviewed by WAVE3, the swabs can be used to test for only certain kinds of cancer, and those tests aren’t even commercially available yet. What, you mean this was all a scam? We’re shocked! SHOCKED!

Bees? Really?
This might be one of the weirdest thing we’ve ever seen. It’s definitely the weirdest thing this Taiwanese woman has ever seen, considering it’s BEES in her EYE.

One more time, for the people in the back: BEES. IN. HER. EYE.
The unfortunate 29-year-old, identified as He, reported to a hospital with sharp eye pain that she assumed was from dirt in her eyes after a vigorous afternoon of gardening near a grave site. Upon close inspection, the hospital’s head of ophthalmology noticed “something that looked like insect legs” floating in her eye and carefully pulled them out. (Pause to dry heave.)
The eyeball intruders turned out to be “sweat bees,” which nest in fallen trees and are attracted to human perspiration. The bees flew into He’s eye and started drinking from her tear duct, which was overproducing because of the immense pain she was in. Miraculously, He’s eyeball and sight are intact, and she’s expected to make a full recovery. The bees are fine, too.
Oh, and in case you’re wondering, sweat bees live all over the world. We’re wearing goggles outside from now on.
Flip it and reverse it
Imagine you’re back in your med school days, happily dissecting another cadaver. You pop the body open and … every organ is in the wrong place??

That’s what some students at Oregon Health & Science University (OHSU) experienced recently. They discovered their body donor had a condition called situs inversus with levocardia – meaning, her heart was in the right place (hah), but all her abdominal organs were transposed right to left. The deceased woman was Rose Marie Bentley, who had died at the ripe old age of 99 without ever knowing she had this condition.
What makes her remarkable is barely anyone with this condition lives to adulthood. Professor Cam Walker of OHSU estimates the odds are 1 in 50 million, and there are only two other known cases of older patients who lived with situs inversus. After taking a look at their mother’s medical records, Bentley’s children noted that the surgeon who removed her appendix recorded its strange location, but no one raised any alarm.
Ms. Bentley lived a full and happy life, despite her organs being a mirror image of virtually everyone else’s anatomy. Some people are just special.
Turn that smile upside down
Service with a smile. Put on a happy face. Let a smile be your umbrella. Seems like good advice, but don’t let these cliches fool you. They are all examples of surface acting – hiding feelings of annoyance behind a mask of forced pleasantness – and they’re not really good for you. At least not if you work with the public and are required to be nice.

Investigators at Penn State University and the University at Buffalo looked at survey data from nurses, teachers, and people in food service and found that they were more likely to drink after work than those who did not interact with the public on a regular basis. “In these jobs, there’s also often money tied to showing positive emotions and holding back negative feelings. Money gives you a motivation to override your natural tendencies, but doing it all day can be wearing,” lead investigator Alicia Grandey said in a written statement.
Personality also plays a part. The risk of increased drinking is greater for surface actors who are more impulsive. And it is stronger still for impulsive people who “also worked in jobs where employees have one-time service encounters with customers, like a call center or coffee shop, rather than relationships, like health care or education,” the investigators said.
They suggested that businesses could give their employees more autonomy, but we’ve got another idea. There are lots of awards for real actors, so how about one for surface actors? They could call it the Oscar … Mayer. Best performance in serving a drive-thru customer who smoked a little too much marijuana. Creative achievement while helping a credit card applicant who still doesn’t understand after being told the same thing five times. Stunt coordination while helping a patient use a bedpan (the “turn the other cheek” award). Choreography to get out of bed every day to face the blank stares of sullen teenagers (the “Lord, give me strength” award). The “okay, we get the picture, you can stop with the awards” award.
That’s not the ice cream truck
Any story that starts with strange men in white, unmarked vans is never going to end well. Especially when that strange man is offering $20 in exchange for DNA samples.

However, this is the situation that Louisville, Ky., finds itself in. According to WAVE3 News, the aforementioned men have been cruising the poorer neighborhoods of the city for a couple of months, offering money to anyone who belongs to Passport Health. Cheek swabs are collected, no questions are asked, and the person walks out with 20 extra dollars.
This is already shady territory, but to make things worse, Passport Health has denied any affiliation. And when asked by one of their customers, the men reported being with Freedom Health. The number the customer was given? Of course it didn’t work.
The cherry on top of the shady cake? According to a professor from the University of Louisville interviewed by WAVE3, the swabs can be used to test for only certain kinds of cancer, and those tests aren’t even commercially available yet. What, you mean this was all a scam? We’re shocked! SHOCKED!

Bees? Really?
This might be one of the weirdest thing we’ve ever seen. It’s definitely the weirdest thing this Taiwanese woman has ever seen, considering it’s BEES in her EYE.

One more time, for the people in the back: BEES. IN. HER. EYE.
The unfortunate 29-year-old, identified as He, reported to a hospital with sharp eye pain that she assumed was from dirt in her eyes after a vigorous afternoon of gardening near a grave site. Upon close inspection, the hospital’s head of ophthalmology noticed “something that looked like insect legs” floating in her eye and carefully pulled them out. (Pause to dry heave.)
The eyeball intruders turned out to be “sweat bees,” which nest in fallen trees and are attracted to human perspiration. The bees flew into He’s eye and started drinking from her tear duct, which was overproducing because of the immense pain she was in. Miraculously, He’s eyeball and sight are intact, and she’s expected to make a full recovery. The bees are fine, too.
Oh, and in case you’re wondering, sweat bees live all over the world. We’re wearing goggles outside from now on.
Flip it and reverse it
Imagine you’re back in your med school days, happily dissecting another cadaver. You pop the body open and … every organ is in the wrong place??

That’s what some students at Oregon Health & Science University (OHSU) experienced recently. They discovered their body donor had a condition called situs inversus with levocardia – meaning, her heart was in the right place (hah), but all her abdominal organs were transposed right to left. The deceased woman was Rose Marie Bentley, who had died at the ripe old age of 99 without ever knowing she had this condition.
What makes her remarkable is barely anyone with this condition lives to adulthood. Professor Cam Walker of OHSU estimates the odds are 1 in 50 million, and there are only two other known cases of older patients who lived with situs inversus. After taking a look at their mother’s medical records, Bentley’s children noted that the surgeon who removed her appendix recorded its strange location, but no one raised any alarm.
Ms. Bentley lived a full and happy life, despite her organs being a mirror image of virtually everyone else’s anatomy. Some people are just special.
Turn that smile upside down
Service with a smile. Put on a happy face. Let a smile be your umbrella. Seems like good advice, but don’t let these cliches fool you. They are all examples of surface acting – hiding feelings of annoyance behind a mask of forced pleasantness – and they’re not really good for you. At least not if you work with the public and are required to be nice.

Investigators at Penn State University and the University at Buffalo looked at survey data from nurses, teachers, and people in food service and found that they were more likely to drink after work than those who did not interact with the public on a regular basis. “In these jobs, there’s also often money tied to showing positive emotions and holding back negative feelings. Money gives you a motivation to override your natural tendencies, but doing it all day can be wearing,” lead investigator Alicia Grandey said in a written statement.
Personality also plays a part. The risk of increased drinking is greater for surface actors who are more impulsive. And it is stronger still for impulsive people who “also worked in jobs where employees have one-time service encounters with customers, like a call center or coffee shop, rather than relationships, like health care or education,” the investigators said.
They suggested that businesses could give their employees more autonomy, but we’ve got another idea. There are lots of awards for real actors, so how about one for surface actors? They could call it the Oscar … Mayer. Best performance in serving a drive-thru customer who smoked a little too much marijuana. Creative achievement while helping a credit card applicant who still doesn’t understand after being told the same thing five times. Stunt coordination while helping a patient use a bedpan (the “turn the other cheek” award). Choreography to get out of bed every day to face the blank stares of sullen teenagers (the “Lord, give me strength” award). The “okay, we get the picture, you can stop with the awards” award.
That’s not the ice cream truck
Any story that starts with strange men in white, unmarked vans is never going to end well. Especially when that strange man is offering $20 in exchange for DNA samples.

However, this is the situation that Louisville, Ky., finds itself in. According to WAVE3 News, the aforementioned men have been cruising the poorer neighborhoods of the city for a couple of months, offering money to anyone who belongs to Passport Health. Cheek swabs are collected, no questions are asked, and the person walks out with 20 extra dollars.
This is already shady territory, but to make things worse, Passport Health has denied any affiliation. And when asked by one of their customers, the men reported being with Freedom Health. The number the customer was given? Of course it didn’t work.
The cherry on top of the shady cake? According to a professor from the University of Louisville interviewed by WAVE3, the swabs can be used to test for only certain kinds of cancer, and those tests aren’t even commercially available yet. What, you mean this was all a scam? We’re shocked! SHOCKED!

Women with FXI deficiency face hemorrhage risk with gyn surgery
Women with factor XI (FXI) deficiency, particularly those with a history of bleeding and low plasmatic FXI levels, are at risk of experiencing obstetric and gynecologic hemorrhage, according to a retrospective analysis.
“[W]hile spontaneous bleeding episodes are unusual, FXI-deficient patients tend to bleed excessively after trauma or surgery, especially when highly fibrinolytic tissues are involved, such as rhino/oropharyngeal and genitourinary mucosa,” wrote Carlos Bravo-Perez, MD, of Universidad de Murcia in Spain, along with his colleagues. The report is in Medicina Clínica. “Menstruation, pregnancy and birth labour constitute intrinsic haemostatic challenges for FXI deficient women,” they noted.
The researchers conducted a retrospective analysis of 95 women with FXI deficiency. Cohort data was collected over a period of 20 years (1994 to 2014) from the city of Yecla, Spain.
Clinical information obtained included blood group, age, sex, aPTT ratio, and FXI coagulant levels, among others. The team completed a comprehensive molecular and biochemical analysis on all study patients.
“Surgical interventions and postoperative bleeding were recorded, as well as the use of antihemorrhagic prophylaxis or treatment,” the researchers wrote.
Bleeding occurred in 27.4% of women with FXI deficiency, with the majority of events being a single episode. The team reported that 52.5% of hemorrhagic events were provoked by surgical, obstetric, or dental procedures, while 47.5% were spontaneous bleeding events.
Among women who experienced bleeding events, 73.1% were gynecologic or obstetric hemorrhagic episodes. These included abnormal uterine bleeding, postpartum hemorrhage, excessive bleeding after a miscarriage, and bleeding after gynecologic surgery.
Overall, the proportion of abnormal uterine bleeding was 12.6%, the proportion of postpartum hemorrhage was 6.6%, and the proportion of gynecologic postoperative hemorrhage was 16%.
While the proportion of these bleeding events is higher than what has been observed in the general population, it is lower than what has been reported in other reviews for women with FXI deficiency, the researchers noted.
They also found that a positive history of bleeding and low plasmatic FXI levels were associated with a higher prevalence of gynecologic or obstetric bleeding.
The study was funded by Fundación Española de Trombosis y Hemostasia and Sociedad Española de Hematología y Hemoterapia in Spain. The authors reported having no conflicts of interest.
SOURCE: Bravo-Perez C et al. Med Clin (Barc). 2019 Mar 26. doi: 10.1016/j.medcli.2019.01.029.
Women with factor XI (FXI) deficiency, particularly those with a history of bleeding and low plasmatic FXI levels, are at risk of experiencing obstetric and gynecologic hemorrhage, according to a retrospective analysis.
“[W]hile spontaneous bleeding episodes are unusual, FXI-deficient patients tend to bleed excessively after trauma or surgery, especially when highly fibrinolytic tissues are involved, such as rhino/oropharyngeal and genitourinary mucosa,” wrote Carlos Bravo-Perez, MD, of Universidad de Murcia in Spain, along with his colleagues. The report is in Medicina Clínica. “Menstruation, pregnancy and birth labour constitute intrinsic haemostatic challenges for FXI deficient women,” they noted.
The researchers conducted a retrospective analysis of 95 women with FXI deficiency. Cohort data was collected over a period of 20 years (1994 to 2014) from the city of Yecla, Spain.
Clinical information obtained included blood group, age, sex, aPTT ratio, and FXI coagulant levels, among others. The team completed a comprehensive molecular and biochemical analysis on all study patients.
“Surgical interventions and postoperative bleeding were recorded, as well as the use of antihemorrhagic prophylaxis or treatment,” the researchers wrote.
Bleeding occurred in 27.4% of women with FXI deficiency, with the majority of events being a single episode. The team reported that 52.5% of hemorrhagic events were provoked by surgical, obstetric, or dental procedures, while 47.5% were spontaneous bleeding events.
Among women who experienced bleeding events, 73.1% were gynecologic or obstetric hemorrhagic episodes. These included abnormal uterine bleeding, postpartum hemorrhage, excessive bleeding after a miscarriage, and bleeding after gynecologic surgery.
Overall, the proportion of abnormal uterine bleeding was 12.6%, the proportion of postpartum hemorrhage was 6.6%, and the proportion of gynecologic postoperative hemorrhage was 16%.
While the proportion of these bleeding events is higher than what has been observed in the general population, it is lower than what has been reported in other reviews for women with FXI deficiency, the researchers noted.
They also found that a positive history of bleeding and low plasmatic FXI levels were associated with a higher prevalence of gynecologic or obstetric bleeding.
The study was funded by Fundación Española de Trombosis y Hemostasia and Sociedad Española de Hematología y Hemoterapia in Spain. The authors reported having no conflicts of interest.
SOURCE: Bravo-Perez C et al. Med Clin (Barc). 2019 Mar 26. doi: 10.1016/j.medcli.2019.01.029.
Women with factor XI (FXI) deficiency, particularly those with a history of bleeding and low plasmatic FXI levels, are at risk of experiencing obstetric and gynecologic hemorrhage, according to a retrospective analysis.
“[W]hile spontaneous bleeding episodes are unusual, FXI-deficient patients tend to bleed excessively after trauma or surgery, especially when highly fibrinolytic tissues are involved, such as rhino/oropharyngeal and genitourinary mucosa,” wrote Carlos Bravo-Perez, MD, of Universidad de Murcia in Spain, along with his colleagues. The report is in Medicina Clínica. “Menstruation, pregnancy and birth labour constitute intrinsic haemostatic challenges for FXI deficient women,” they noted.
The researchers conducted a retrospective analysis of 95 women with FXI deficiency. Cohort data was collected over a period of 20 years (1994 to 2014) from the city of Yecla, Spain.
Clinical information obtained included blood group, age, sex, aPTT ratio, and FXI coagulant levels, among others. The team completed a comprehensive molecular and biochemical analysis on all study patients.
“Surgical interventions and postoperative bleeding were recorded, as well as the use of antihemorrhagic prophylaxis or treatment,” the researchers wrote.
Bleeding occurred in 27.4% of women with FXI deficiency, with the majority of events being a single episode. The team reported that 52.5% of hemorrhagic events were provoked by surgical, obstetric, or dental procedures, while 47.5% were spontaneous bleeding events.
Among women who experienced bleeding events, 73.1% were gynecologic or obstetric hemorrhagic episodes. These included abnormal uterine bleeding, postpartum hemorrhage, excessive bleeding after a miscarriage, and bleeding after gynecologic surgery.
Overall, the proportion of abnormal uterine bleeding was 12.6%, the proportion of postpartum hemorrhage was 6.6%, and the proportion of gynecologic postoperative hemorrhage was 16%.
While the proportion of these bleeding events is higher than what has been observed in the general population, it is lower than what has been reported in other reviews for women with FXI deficiency, the researchers noted.
They also found that a positive history of bleeding and low plasmatic FXI levels were associated with a higher prevalence of gynecologic or obstetric bleeding.
The study was funded by Fundación Española de Trombosis y Hemostasia and Sociedad Española de Hematología y Hemoterapia in Spain. The authors reported having no conflicts of interest.
SOURCE: Bravo-Perez C et al. Med Clin (Barc). 2019 Mar 26. doi: 10.1016/j.medcli.2019.01.029.
FROM MEDICINA CLÍNICA
Survey finds psoriasis patients seek relief with alternative therapies
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
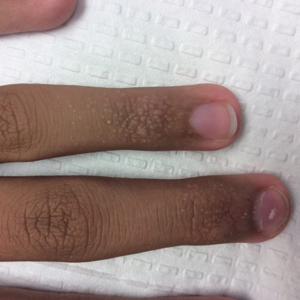
Acral Flesh-Colored Papules on the Fingers
The Diagnosis: Lichen Nitidus
Our patient represents a case of lichen nitidus (LN) that was diagnosed through clinicopathologic correlation, with the pathology results showing a lymphohistiocytic infiltrate in the papillary dermis enclosed by acanthotic rete ridges on either side. Lichen nitidus was first described by Pinkus in 1901 as a variant of lichen planus.1 It is a rare chronic inflammatory disease that is most prevalent in children and adolescents.2 Clinically, the lesions appear as 1- to 2-mm, shiny, flesh-colored papules with central umbilication.3 Typically, lesions are localized and discrete; however, vesicular, hemorrhagic, perforating, spinous follicular, linear, generalized, and actinic variants all have been reported in the literature. Lichen nitidus has a predilection for the lower abdomen, medial thighs, penis, forearms, ventral wrists, and hands.4 Cases of LN have been reported on the palms, soles, nails, and mucosa, presenting a diagnostic challenge.5 The pathogenesis of LN is unknown, and all races and sexes are affected equally.6
Histopathologically, LN has distinct findings including a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis embraced by elongated and acanthotic rete ridges.2 These histopathologic characteristics were seen in our patient's biopsy specimen (Figure) and have been described as the ball-and-claw configuration. Lichen nitidus may be pruritic but typically is asymptomatic.7 It often spontaneously regresses within months to years without any treatment7; however, successful outcomes have been seen with topical steroids, UVA/UVB phototherapy, and retinoids.2 Our patient was treated with topical steroids.

The differential diagnosis for LN includes verruca plana, dyshidrotic eczema, acral persistent papular mucinosis (APPM), and molluscum contagiosum. Verruca plana can occur as 1- to 5-mm, grouped, flesh-colored papules on the face, neck, dorsal hands, wrists, or knees.8 Most commonly, verruca plana occurs due to human papillomavirus type 3 and less commonly human papillomavirus types 10, 27, and 41. Verruca plana is easily differentiated from LN on pathology with findings of epidermal hyperkeratosis, irregular acanthosis, and koilocytic changes.8
Dyshidrotic eczema is a pruritic vesicular rash that is classically distributed symmetrically on the palmar aspects of the hands and lateral fingers.9 Histopathology of the lesions reveals spongiosis with an epidermal lymphocytic infiltrate. Exacerbating factors include exposure to allergens, stress, fungal infections, and genetic predisposition.9
Acral persistent papular mucinosis can present as multiple, 2- to 5-mm, flesh-colored papules on the dorsal aspects of the hands.10 However, the demographic is different from LN, as APPM most commonly affects middle-aged females versus adolescents. Lesions of APPM may multiply or spontaneously remit over time. Acral persistent papular mucinosis generally is asymptomatic but can be treated with cryotherapy, topical corticosteroids, electrodesiccation, or CO2 lasers for cosmetic purposes. Acral persistent papular mucinosis can be easily distinguished from LN on histology, as it will show areas of focal, well-circumscribed mucin in the papillary dermis and a spared Grenz zone.10
Molluscum contagiosum is a common viral skin infection caused by the poxvirus that affects children and adults.11 The skin lesions appear as 2- to 4-mm, dome-shaped, flesh-colored papules with central umbilication on the limbs, trunk, or face. Clinicians may choose to monitor lesions of molluscum contagiosum, as it is a self-limited condition, or it may be treated with cryotherapy, salicylic acid, imiquimod, curettage, laser, or cimetidine.11 On histology, epidermal budlike proliferations can be appreciated in the dermis, and characteristic large, eosinophilic, intracytoplasmic inclusion or molluscum bodies are found in the epidermis.12
- Barber HW. Case of lichen nitidus (Pinkus) or tuberculide lichéniforme et nitida (Chatellier). Proc R Soc Med. 1924;17:39.
- Frey MN, Luzzatto L, Seidel GB, et al. Case for diagnosis. An Bras Dermatol. 2010;85:561-563.
- Pielop JA, Hsu S. Tiny, skin-colored papules on the arms and hands. Am Fam Physician. 2005;72:343-344.
- Cho EB, Kim HY, Park EJ, et al. Three cases of lichen nitidus associated with various cutaneous diseases. Ann Dermatol. 2014;26:505-509.
- Podder I, Mohanty S, Chandra S, et al. Isolated palmar lichen nitidus--a diagnostic challenge: first case from Eastern India. Indian J Dermatol. 2015;60:308-309.
- Chen W, Schramm M, Zouboulis C. Generalized lichen nitidus. J Am Acad Dermatol. 1997;36:630-631.
- Rallis E, Verros C, Moussatou V, et al. Generalized purpuric lichen nitidus: a case report and review of the literature. Dermatol Online J. 2007;13:5.
- Pavithra S, Mallya H, Pai GS. Extensive presentation of verruca plana in a healthy individual. Indian J Dermatol. 2011;56:324-325.
- Paulsen L, Geller D, Guggenbiller M. Symmetrical vesicular eruption on the palms. Am Fam Physician. 2012;15:811-812.
- Alvarez-Garrido H, Najera L, Garrido-Rios A, et al. Acral persistent papular mucinosis: is it an under-diagnosed disease? Dermatol Online J. 2014;20:10
- Diaconu R, Oprea B, Vasilescu M, et al. Inflamed molluscum contagiosum in a 6-year-old boy: a case report. Rom J Morphol Embryol. 2015;56:843-845.
- Krishnamurthy J, Nagappa D. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74.
The Diagnosis: Lichen Nitidus
Our patient represents a case of lichen nitidus (LN) that was diagnosed through clinicopathologic correlation, with the pathology results showing a lymphohistiocytic infiltrate in the papillary dermis enclosed by acanthotic rete ridges on either side. Lichen nitidus was first described by Pinkus in 1901 as a variant of lichen planus.1 It is a rare chronic inflammatory disease that is most prevalent in children and adolescents.2 Clinically, the lesions appear as 1- to 2-mm, shiny, flesh-colored papules with central umbilication.3 Typically, lesions are localized and discrete; however, vesicular, hemorrhagic, perforating, spinous follicular, linear, generalized, and actinic variants all have been reported in the literature. Lichen nitidus has a predilection for the lower abdomen, medial thighs, penis, forearms, ventral wrists, and hands.4 Cases of LN have been reported on the palms, soles, nails, and mucosa, presenting a diagnostic challenge.5 The pathogenesis of LN is unknown, and all races and sexes are affected equally.6
Histopathologically, LN has distinct findings including a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis embraced by elongated and acanthotic rete ridges.2 These histopathologic characteristics were seen in our patient's biopsy specimen (Figure) and have been described as the ball-and-claw configuration. Lichen nitidus may be pruritic but typically is asymptomatic.7 It often spontaneously regresses within months to years without any treatment7; however, successful outcomes have been seen with topical steroids, UVA/UVB phototherapy, and retinoids.2 Our patient was treated with topical steroids.

The differential diagnosis for LN includes verruca plana, dyshidrotic eczema, acral persistent papular mucinosis (APPM), and molluscum contagiosum. Verruca plana can occur as 1- to 5-mm, grouped, flesh-colored papules on the face, neck, dorsal hands, wrists, or knees.8 Most commonly, verruca plana occurs due to human papillomavirus type 3 and less commonly human papillomavirus types 10, 27, and 41. Verruca plana is easily differentiated from LN on pathology with findings of epidermal hyperkeratosis, irregular acanthosis, and koilocytic changes.8
Dyshidrotic eczema is a pruritic vesicular rash that is classically distributed symmetrically on the palmar aspects of the hands and lateral fingers.9 Histopathology of the lesions reveals spongiosis with an epidermal lymphocytic infiltrate. Exacerbating factors include exposure to allergens, stress, fungal infections, and genetic predisposition.9
Acral persistent papular mucinosis can present as multiple, 2- to 5-mm, flesh-colored papules on the dorsal aspects of the hands.10 However, the demographic is different from LN, as APPM most commonly affects middle-aged females versus adolescents. Lesions of APPM may multiply or spontaneously remit over time. Acral persistent papular mucinosis generally is asymptomatic but can be treated with cryotherapy, topical corticosteroids, electrodesiccation, or CO2 lasers for cosmetic purposes. Acral persistent papular mucinosis can be easily distinguished from LN on histology, as it will show areas of focal, well-circumscribed mucin in the papillary dermis and a spared Grenz zone.10
Molluscum contagiosum is a common viral skin infection caused by the poxvirus that affects children and adults.11 The skin lesions appear as 2- to 4-mm, dome-shaped, flesh-colored papules with central umbilication on the limbs, trunk, or face. Clinicians may choose to monitor lesions of molluscum contagiosum, as it is a self-limited condition, or it may be treated with cryotherapy, salicylic acid, imiquimod, curettage, laser, or cimetidine.11 On histology, epidermal budlike proliferations can be appreciated in the dermis, and characteristic large, eosinophilic, intracytoplasmic inclusion or molluscum bodies are found in the epidermis.12
The Diagnosis: Lichen Nitidus
Our patient represents a case of lichen nitidus (LN) that was diagnosed through clinicopathologic correlation, with the pathology results showing a lymphohistiocytic infiltrate in the papillary dermis enclosed by acanthotic rete ridges on either side. Lichen nitidus was first described by Pinkus in 1901 as a variant of lichen planus.1 It is a rare chronic inflammatory disease that is most prevalent in children and adolescents.2 Clinically, the lesions appear as 1- to 2-mm, shiny, flesh-colored papules with central umbilication.3 Typically, lesions are localized and discrete; however, vesicular, hemorrhagic, perforating, spinous follicular, linear, generalized, and actinic variants all have been reported in the literature. Lichen nitidus has a predilection for the lower abdomen, medial thighs, penis, forearms, ventral wrists, and hands.4 Cases of LN have been reported on the palms, soles, nails, and mucosa, presenting a diagnostic challenge.5 The pathogenesis of LN is unknown, and all races and sexes are affected equally.6
Histopathologically, LN has distinct findings including a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis embraced by elongated and acanthotic rete ridges.2 These histopathologic characteristics were seen in our patient's biopsy specimen (Figure) and have been described as the ball-and-claw configuration. Lichen nitidus may be pruritic but typically is asymptomatic.7 It often spontaneously regresses within months to years without any treatment7; however, successful outcomes have been seen with topical steroids, UVA/UVB phototherapy, and retinoids.2 Our patient was treated with topical steroids.

The differential diagnosis for LN includes verruca plana, dyshidrotic eczema, acral persistent papular mucinosis (APPM), and molluscum contagiosum. Verruca plana can occur as 1- to 5-mm, grouped, flesh-colored papules on the face, neck, dorsal hands, wrists, or knees.8 Most commonly, verruca plana occurs due to human papillomavirus type 3 and less commonly human papillomavirus types 10, 27, and 41. Verruca plana is easily differentiated from LN on pathology with findings of epidermal hyperkeratosis, irregular acanthosis, and koilocytic changes.8
Dyshidrotic eczema is a pruritic vesicular rash that is classically distributed symmetrically on the palmar aspects of the hands and lateral fingers.9 Histopathology of the lesions reveals spongiosis with an epidermal lymphocytic infiltrate. Exacerbating factors include exposure to allergens, stress, fungal infections, and genetic predisposition.9
Acral persistent papular mucinosis can present as multiple, 2- to 5-mm, flesh-colored papules on the dorsal aspects of the hands.10 However, the demographic is different from LN, as APPM most commonly affects middle-aged females versus adolescents. Lesions of APPM may multiply or spontaneously remit over time. Acral persistent papular mucinosis generally is asymptomatic but can be treated with cryotherapy, topical corticosteroids, electrodesiccation, or CO2 lasers for cosmetic purposes. Acral persistent papular mucinosis can be easily distinguished from LN on histology, as it will show areas of focal, well-circumscribed mucin in the papillary dermis and a spared Grenz zone.10
Molluscum contagiosum is a common viral skin infection caused by the poxvirus that affects children and adults.11 The skin lesions appear as 2- to 4-mm, dome-shaped, flesh-colored papules with central umbilication on the limbs, trunk, or face. Clinicians may choose to monitor lesions of molluscum contagiosum, as it is a self-limited condition, or it may be treated with cryotherapy, salicylic acid, imiquimod, curettage, laser, or cimetidine.11 On histology, epidermal budlike proliferations can be appreciated in the dermis, and characteristic large, eosinophilic, intracytoplasmic inclusion or molluscum bodies are found in the epidermis.12
- Barber HW. Case of lichen nitidus (Pinkus) or tuberculide lichéniforme et nitida (Chatellier). Proc R Soc Med. 1924;17:39.
- Frey MN, Luzzatto L, Seidel GB, et al. Case for diagnosis. An Bras Dermatol. 2010;85:561-563.
- Pielop JA, Hsu S. Tiny, skin-colored papules on the arms and hands. Am Fam Physician. 2005;72:343-344.
- Cho EB, Kim HY, Park EJ, et al. Three cases of lichen nitidus associated with various cutaneous diseases. Ann Dermatol. 2014;26:505-509.
- Podder I, Mohanty S, Chandra S, et al. Isolated palmar lichen nitidus--a diagnostic challenge: first case from Eastern India. Indian J Dermatol. 2015;60:308-309.
- Chen W, Schramm M, Zouboulis C. Generalized lichen nitidus. J Am Acad Dermatol. 1997;36:630-631.
- Rallis E, Verros C, Moussatou V, et al. Generalized purpuric lichen nitidus: a case report and review of the literature. Dermatol Online J. 2007;13:5.
- Pavithra S, Mallya H, Pai GS. Extensive presentation of verruca plana in a healthy individual. Indian J Dermatol. 2011;56:324-325.
- Paulsen L, Geller D, Guggenbiller M. Symmetrical vesicular eruption on the palms. Am Fam Physician. 2012;15:811-812.
- Alvarez-Garrido H, Najera L, Garrido-Rios A, et al. Acral persistent papular mucinosis: is it an under-diagnosed disease? Dermatol Online J. 2014;20:10
- Diaconu R, Oprea B, Vasilescu M, et al. Inflamed molluscum contagiosum in a 6-year-old boy: a case report. Rom J Morphol Embryol. 2015;56:843-845.
- Krishnamurthy J, Nagappa D. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74.
- Barber HW. Case of lichen nitidus (Pinkus) or tuberculide lichéniforme et nitida (Chatellier). Proc R Soc Med. 1924;17:39.
- Frey MN, Luzzatto L, Seidel GB, et al. Case for diagnosis. An Bras Dermatol. 2010;85:561-563.
- Pielop JA, Hsu S. Tiny, skin-colored papules on the arms and hands. Am Fam Physician. 2005;72:343-344.
- Cho EB, Kim HY, Park EJ, et al. Three cases of lichen nitidus associated with various cutaneous diseases. Ann Dermatol. 2014;26:505-509.
- Podder I, Mohanty S, Chandra S, et al. Isolated palmar lichen nitidus--a diagnostic challenge: first case from Eastern India. Indian J Dermatol. 2015;60:308-309.
- Chen W, Schramm M, Zouboulis C. Generalized lichen nitidus. J Am Acad Dermatol. 1997;36:630-631.
- Rallis E, Verros C, Moussatou V, et al. Generalized purpuric lichen nitidus: a case report and review of the literature. Dermatol Online J. 2007;13:5.
- Pavithra S, Mallya H, Pai GS. Extensive presentation of verruca plana in a healthy individual. Indian J Dermatol. 2011;56:324-325.
- Paulsen L, Geller D, Guggenbiller M. Symmetrical vesicular eruption on the palms. Am Fam Physician. 2012;15:811-812.
- Alvarez-Garrido H, Najera L, Garrido-Rios A, et al. Acral persistent papular mucinosis: is it an under-diagnosed disease? Dermatol Online J. 2014;20:10
- Diaconu R, Oprea B, Vasilescu M, et al. Inflamed molluscum contagiosum in a 6-year-old boy: a case report. Rom J Morphol Embryol. 2015;56:843-845.
- Krishnamurthy J, Nagappa D. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74.
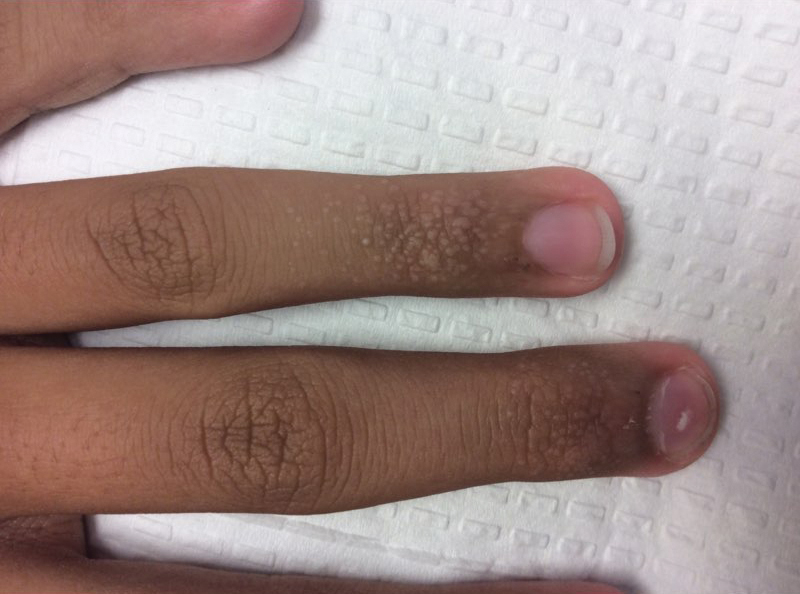
A 13-year-old otherwise healthy adolescent boy presented to the dermatology clinic for a rash on the bilateral dorsal hands of approximately 1 year’s duration. The rash was asymptomatic with no pain or pruritus reported. Physical examination revealed a well-nourished adolescent boy in no acute distress with 1- to 2-mm flesh-colored papules clustered on the bilateral dorsal fingers.
MET/MEK inhibitor duo shows activity in resistant NSCLC
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
ATLANTA – A combination of the epidermal growth factor receptor–targeted agent osimertinib (Tagrisso) with selumetinib, an investigational inhibitor of MEK1/2, was safe and associated with partial responses in about one-third of patients with non–small cell lung cancer in the phase 1b TATTON trial.
In the dose-finding phase of the trial, the objective response rate was 42% for 36 patients treated with the combination either as a second- or third-line therapy, or following prior therapy for the epidermal growth factor receptor (EGFR) T70M mutation. In the dose-expansion phase of the trial, the ORR was 34% for 47 patients treated regardless of mutational status, reported Suresh S. Ramalingam, MD, from the Winship Cancer Institute at Emory University, Atlanta.
“We conclude that combining osimertinib with intermittent selumetinib is feasible with manageable toxicity and has already demonstrated promising preliminary anticancer activity,” he said at the annual meeting of the American Association for Cancer Research.
The EGFR T790M mutation is the most common cause of resistance in patients with non–small cell lung cancer (NSCLC) bearing EGFR mutations who are treated with first- or second-generation, EGFR-targeted tyrosine kinase inhibitors (TKI). Up-regulation of the RAS/RAF/MEK/ERK signaling pathway has also been implicated in NSCLC resistance to EGFR-targeted TKIs.
Selumetinib is an oral, potent, and selective inhibitor of MEK1/2 with a short half-life.
In the phase 3 SELECT-1 trial, selumetinib in combination with docetaxel did not significantly improve progression-free survival, compared with docetaxel alone as second-line therapy for patients with KRAS-mutated NSCLC.
Therapeutic rationale
Invited discussant Roy S. Herbst, MD, PhD, chief of medical oncology at Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn., said that there is sound rationale for combining osimertinib and selumetinib in EGFR-mutant NSCLC.
In addition to the up-regulation of the RAS/RAF/MEK/ERK pathway noted before, resistance to osimertinib has been shown in models of EGFR-mutant NSCLC to develop from aberrant ERK signaling mediation in part by MEK1 amplification, and MEK kinase inhibitors can restore sensitivity to osimertinib in resistant cells, he said.
As previously reported, the TATTON investigators are evaluating in separate cohorts combinations of osimertinib with savolitinib, an investigational MET inhibitor for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents, or with selumetinib for patients with advanced EGFR-mutated NSCLC that had progressed on prior therapy, including EGFR-targeted TKIs, irrespective of T790M or KRAS status.
In part A, the dose-finding phase, patients received osimertinib 80 mg daily plus intermittent or continuous selumetinib. Asian patients received continuous selumetinib 25/50 mg twice daily, while other patients received continuous selumetinib 50/75 mg twice daily, or intermittent selumetinib 75 mg twice daily 4 days on, 3 days off or on days 1 and 4 of each week of treatment.
In part B, the dose-expansion phase, patients received osimertinib plus intermittent selumetinib 75 mg twice daily on the 4 days on/3 days off schedule.
An analysis of preliminary antitumor activity in part A showed an ORR in 15 of 36 patients (42%); all were partial responses (PR). In addition, 14 patients (39%) had stable disease at 6 weeks, 3 had progressive disease, 2 died, and 2 were not evaluable. The median duration of response was 16.6 months; 77% of the patients had responses lasting at least 12 months.
In part B, 16 of 47 patients enrolled (34%) had confirmed PR, and 16 had stable disease. Of the remaining patients, 11 had disease progression, 2 died, and 2 were not evaluable. The median duration of response in this group was 9.1 months, and 31% of patients remained in response at 12 months.
The most common treatment-related adverse events in the dose-finding phase were diarrhea in 75% of patients, nausea in 39% and fatigue in 33%. Dose-limiting toxicities occurred in six patients, all of whom had been treated with continuous selumetinib. These toxicities, all grade 3, include liver enzyme increases, diarrhea, asthenia, dizziness, nausea, and pneumonitis.
The most common treatment-related adverse events in the dose-expansion phase were diarrhea in 81%, stomatitis in 32%, and paronychia in 30% of patients.
Results ‘okay’
In his discussion, Dr. Herbst commented that the part A results “look okay, until you realize that the most of the activity is in those patients who are T790M-positive, who have not been exposed in this cohort to a third-generation T790M inhibitor.” Patients with the mutation who are treated with third-generation inhibitors would be expected to have a 78% response rate.
Part B included a few more patients with responses who were negative for T790M. “My thought here is that perhaps there is a biomarker” for selecting patients most likely to benefit from the combination, he said.
For MET-negative patients, the combination appears to have manageable toxicities with noncontinuous dosing of selumetinib, and there may be benefit to using it in the first-line setting in select patients, but that will require further trials and identification of suitable biomarkers, Dr. Herbst summarized.
TATTON was sponsored by AstraZeneca. Dr. Ramalingam reported receiving research support from the company and consulting/contracting with others. Dr. Herbst reported receiving research support from AstraZeneca, Eli Lilly, and Merck, and serving as a consultant for AstraZeneca, Eli Lilly, Genentech/Roche, Merck, NextCure, and Pfizer.
SOURCE: Ramalingam SS et al. AACR 2019, Abstract CT034.
REPORTING FROM AACR 2019
Experiences with the Best-CLI Trial
As the BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients With Critical Limb Ischemia) trial enters its last phase of new patient enrollment, I thought it was important to reflect on what this trial has meant for both the Vascular Surgery field and for me personally. This trial has been closely examining one of the most commonly treated conditions that we take care of - critical limb ischemia (more recently better described as chronic limb threatening ischemia (CLTI). BEST-CLI (ClinicalTrials.gov Identifier: NCT02060630) has the potential to be one of the most meaningful and impactful trials in the history of our profession, and that of our colleagues who also treat CLTI.
Unlike many of the industry-sponsored endovascular device trials, vascular surgeons are at the table and are key leaders and enrollers. The results will be quoted for decades and there will be many questions answered that we have not been able to answer before - including questions that were not even on people’s minds when the trial began - such as paclitaxel-related outcomes. This trial will also provide the long-term follow-up that has limited the impact of many other peripheral arterial disease trials.
From a personal point of view, I feel like the BEST trial as always been closely connected to my practice. I have been fortunate to be partners with one of the national principal investigators, Dr. Alik Farber. We enrolled the first patient in the trial in my second month as an attending in August of 2014. Since then, I have been able to operate on 30 patients that were randomized into the trial. It not only allowed me, as a junior attending, to get involved in a major trial, but also forced me to further develop both my open and endovascular skills so that I could provide the best care to each patient as needed.
This trial has also moved me to see things more objectively; I am now more aware of my personal treatment biases and try more consciously to suspend them when I have equipoise between treatment options. I also continue to follow patients that I enrolled and treated over 4 years ago. This trial will challenge many wide-spread beliefs, anecdotes, and urban legends in the field of peripheral arterial disease. The results will be scrutinized and analyzed and the results will be debated – particularly by some who do not find their preconceived biases confirmed.
A trial of this magnitude looking at limb threatening ischemia will most likely never happen again in this country. This is the one time for us as a group of professionals who care for patients with CLTI to do this correctly, rather than rely solely on data from single-arm studies, often industry-sponsored, that are typically focused on device approvals.
It is key, as we get close to the finish line, that we suspend our preconceived notions and finish enrollment. We need to ensure this trial has adequate power to give us the answers we need the most – how to best take care of the most vulnerable and ill patients that we treat; they will greatly benefit from a clear answer as to how best we should address their limb- and life-threatening problems.
Jeffrey J. Siracuse, MD
Associate Professor of Surgery
Division of Vascular and Endovascular Surgery
Boston University, School of Medicine
Boston Medical Center
As the BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients With Critical Limb Ischemia) trial enters its last phase of new patient enrollment, I thought it was important to reflect on what this trial has meant for both the Vascular Surgery field and for me personally. This trial has been closely examining one of the most commonly treated conditions that we take care of - critical limb ischemia (more recently better described as chronic limb threatening ischemia (CLTI). BEST-CLI (ClinicalTrials.gov Identifier: NCT02060630) has the potential to be one of the most meaningful and impactful trials in the history of our profession, and that of our colleagues who also treat CLTI.
Unlike many of the industry-sponsored endovascular device trials, vascular surgeons are at the table and are key leaders and enrollers. The results will be quoted for decades and there will be many questions answered that we have not been able to answer before - including questions that were not even on people’s minds when the trial began - such as paclitaxel-related outcomes. This trial will also provide the long-term follow-up that has limited the impact of many other peripheral arterial disease trials.
From a personal point of view, I feel like the BEST trial as always been closely connected to my practice. I have been fortunate to be partners with one of the national principal investigators, Dr. Alik Farber. We enrolled the first patient in the trial in my second month as an attending in August of 2014. Since then, I have been able to operate on 30 patients that were randomized into the trial. It not only allowed me, as a junior attending, to get involved in a major trial, but also forced me to further develop both my open and endovascular skills so that I could provide the best care to each patient as needed.
This trial has also moved me to see things more objectively; I am now more aware of my personal treatment biases and try more consciously to suspend them when I have equipoise between treatment options. I also continue to follow patients that I enrolled and treated over 4 years ago. This trial will challenge many wide-spread beliefs, anecdotes, and urban legends in the field of peripheral arterial disease. The results will be scrutinized and analyzed and the results will be debated – particularly by some who do not find their preconceived biases confirmed.
A trial of this magnitude looking at limb threatening ischemia will most likely never happen again in this country. This is the one time for us as a group of professionals who care for patients with CLTI to do this correctly, rather than rely solely on data from single-arm studies, often industry-sponsored, that are typically focused on device approvals.
It is key, as we get close to the finish line, that we suspend our preconceived notions and finish enrollment. We need to ensure this trial has adequate power to give us the answers we need the most – how to best take care of the most vulnerable and ill patients that we treat; they will greatly benefit from a clear answer as to how best we should address their limb- and life-threatening problems.
Jeffrey J. Siracuse, MD
Associate Professor of Surgery
Division of Vascular and Endovascular Surgery
Boston University, School of Medicine
Boston Medical Center
As the BEST-CLI (Best Endovascular vs. Best Surgical Therapy in Patients With Critical Limb Ischemia) trial enters its last phase of new patient enrollment, I thought it was important to reflect on what this trial has meant for both the Vascular Surgery field and for me personally. This trial has been closely examining one of the most commonly treated conditions that we take care of - critical limb ischemia (more recently better described as chronic limb threatening ischemia (CLTI). BEST-CLI (ClinicalTrials.gov Identifier: NCT02060630) has the potential to be one of the most meaningful and impactful trials in the history of our profession, and that of our colleagues who also treat CLTI.
Unlike many of the industry-sponsored endovascular device trials, vascular surgeons are at the table and are key leaders and enrollers. The results will be quoted for decades and there will be many questions answered that we have not been able to answer before - including questions that were not even on people’s minds when the trial began - such as paclitaxel-related outcomes. This trial will also provide the long-term follow-up that has limited the impact of many other peripheral arterial disease trials.
From a personal point of view, I feel like the BEST trial as always been closely connected to my practice. I have been fortunate to be partners with one of the national principal investigators, Dr. Alik Farber. We enrolled the first patient in the trial in my second month as an attending in August of 2014. Since then, I have been able to operate on 30 patients that were randomized into the trial. It not only allowed me, as a junior attending, to get involved in a major trial, but also forced me to further develop both my open and endovascular skills so that I could provide the best care to each patient as needed.
This trial has also moved me to see things more objectively; I am now more aware of my personal treatment biases and try more consciously to suspend them when I have equipoise between treatment options. I also continue to follow patients that I enrolled and treated over 4 years ago. This trial will challenge many wide-spread beliefs, anecdotes, and urban legends in the field of peripheral arterial disease. The results will be scrutinized and analyzed and the results will be debated – particularly by some who do not find their preconceived biases confirmed.
A trial of this magnitude looking at limb threatening ischemia will most likely never happen again in this country. This is the one time for us as a group of professionals who care for patients with CLTI to do this correctly, rather than rely solely on data from single-arm studies, often industry-sponsored, that are typically focused on device approvals.
It is key, as we get close to the finish line, that we suspend our preconceived notions and finish enrollment. We need to ensure this trial has adequate power to give us the answers we need the most – how to best take care of the most vulnerable and ill patients that we treat; they will greatly benefit from a clear answer as to how best we should address their limb- and life-threatening problems.
Jeffrey J. Siracuse, MD
Associate Professor of Surgery
Division of Vascular and Endovascular Surgery
Boston University, School of Medicine
Boston Medical Center