User login
Donate Auction Items for Gala
The ‘Vascular Spectacular’ Gala has officially sold out, but it’s not too late to donate auction items for the live and silent auctions. So far donations consist of vacation stays, tickets to sporting events, entertainment, travel and time shares, chef and other classes, sports memorabilia, wine tastings and much more. All are welcome to participate as bidders in the silent auction. Get the full details here.
The ‘Vascular Spectacular’ Gala has officially sold out, but it’s not too late to donate auction items for the live and silent auctions. So far donations consist of vacation stays, tickets to sporting events, entertainment, travel and time shares, chef and other classes, sports memorabilia, wine tastings and much more. All are welcome to participate as bidders in the silent auction. Get the full details here.
The ‘Vascular Spectacular’ Gala has officially sold out, but it’s not too late to donate auction items for the live and silent auctions. So far donations consist of vacation stays, tickets to sporting events, entertainment, travel and time shares, chef and other classes, sports memorabilia, wine tastings and much more. All are welcome to participate as bidders in the silent auction. Get the full details here.
Programming for PAs Slated
The SVS will once again host an afternoon of education programming specifically for physician assistants. The afternoon session will be from 1 to 5 p.m. Thursday, June 13. Topics include discussions of PAs in different team settings, vascular diagnostics, venous disease and wound management, and dialysis access. Attendees can also earn additional credits. VAM is designated for 30 AAPA Category 1 CME credits. Register for VAM today.
The SVS will once again host an afternoon of education programming specifically for physician assistants. The afternoon session will be from 1 to 5 p.m. Thursday, June 13. Topics include discussions of PAs in different team settings, vascular diagnostics, venous disease and wound management, and dialysis access. Attendees can also earn additional credits. VAM is designated for 30 AAPA Category 1 CME credits. Register for VAM today.
The SVS will once again host an afternoon of education programming specifically for physician assistants. The afternoon session will be from 1 to 5 p.m. Thursday, June 13. Topics include discussions of PAs in different team settings, vascular diagnostics, venous disease and wound management, and dialysis access. Attendees can also earn additional credits. VAM is designated for 30 AAPA Category 1 CME credits. Register for VAM today.
Register for VAM by Tomorrow for a Chance to Win
The Society for Vascular Surgery will provide complimentary meeting registration to a lucky attendee. To be eligible, all you must do is register for the meeting before 5 p.m. CDT tomorrow, April 24. The winner will be selected at random. This year’s meeting will be June 12 to 15 at the Gaylord National Resort & Convention Center in National Harbor, Md., just outside Washington D.C. Read more about the VAM contest, and more, in the latest SVS VAMail.
The Society for Vascular Surgery will provide complimentary meeting registration to a lucky attendee. To be eligible, all you must do is register for the meeting before 5 p.m. CDT tomorrow, April 24. The winner will be selected at random. This year’s meeting will be June 12 to 15 at the Gaylord National Resort & Convention Center in National Harbor, Md., just outside Washington D.C. Read more about the VAM contest, and more, in the latest SVS VAMail.
The Society for Vascular Surgery will provide complimentary meeting registration to a lucky attendee. To be eligible, all you must do is register for the meeting before 5 p.m. CDT tomorrow, April 24. The winner will be selected at random. This year’s meeting will be June 12 to 15 at the Gaylord National Resort & Convention Center in National Harbor, Md., just outside Washington D.C. Read more about the VAM contest, and more, in the latest SVS VAMail.
Losing a patient to suicide

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.

Join us Wednesday, April 24, 2019, at 6:00 p.m. Eastern/5:00 p.m. Central as we open a lively Twitter discussion in psychiatry on the topic of losing a patient to suicide. Our special guests include physicians with expertise on the topic of patient suicide, Dinah Miller, MD, (@shrinkrapdinah) and Eric Plakun, MD, (@EricPlakunMD). We hope you join us April 24 at 6 p.m. ET. #MDedgeChats.
Suicides in the United States are on the rise; according to the Centers for Disease Control and Prevention, suicide was the cause of death of almost 45,000 people in 2016. Overall, it was the 10th leading cause of death, and the second leading cause of death among people aged 10-34 years. Twice as many people completed suicide as were victims of homicides.
Losing a patient to suicide is one of the most difficult and painful experiences a psychiatrist will face. In addition to concern for the patient and his or her family, psychiatrists may experience thoughts of responsibility and what they could have done differently to prevent the suicide. Although often trained in helping patients address grief, psychiatrists may not be as comfortable processing their own grief after the loss of a patient to suicide.
Topics of conversation
Q1: Have you ever lost a patient to suicide?
Q2: How do you think the loss of your patient changed your approach to psychiatry?
Q3: How did the loss change you?
Q4: If you did not discuss the suicide with your colleagues, what held you back?
Q5: How can you support medical professionals who lose patients to suicide?
Resources
Preventing suicide: What should clinicians do differently?
Individualized intervention key to reducing suicide attempts
Helping survivors in the aftermath of suicide loss
The blinding lies of depression
Suicide symposium: A multidisciplinary approach to risk assessment and the emotional aftermath of patient suicide
About Dr. Miller
Dr. Miller is the author of numerous books and articles, including “Committed: The Battle Over Involuntary Care” (Baltimore: Johns Hopkins University Press, 2016), which she wrote with Dr. Annette Hanson (@clinkshrink), and “When a Patient Dies by Suicide – The Physician’s Silent Sorrow” in the New England Journal of Medicine (@NEJM) (2019;380:311-13). She has a private practice in Baltimore and is affiliated with Johns Hopkins University (@HopkinsMedicine).
About Dr. Plakun
Dr. Plakun is the medical director and CEO of the Austen Riggs Center (@Austen Riggs), a “top 10” U.S. News and World Report “Best Hospital” in psychiatry based in Stockbridge, Mass. He also serves on the board of trustees of the American Psychiatric Association (@APA Psychiatric) representing New England and Eastern Canada, and was the founding leader of the APA Psychotherapy Caucus. Dr. Plakun is a board-certified psychiatrist, psychoanalyst, former member of the Harvard Medical School clinical faculty, and author of more than 50 publications.
Are you new to Twitter chats? We have included simple steps below to help you join and participate in the conversation.
Referral system aims to slash axial spondyloarthritis diagnostic delay
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Low back pain. A bane of human existence.
Almost everyone – 90% of us in fact – will have at least one bout of it. Snow shoveling, too much weight on the barbell, a strange twist while carrying in the groceries. A quick visit to a primary care doc, a prescription NSAID, a few days or weeks of rest, and a gradual resolution of symptoms is the usual course.
But in Toronto, a small group of clinicians aims to change this clinical picture. They’ve developed a secondary screening program to identify back pain patients at risk of axSpA, potentially bypassing the diagnostic merry-go-round, years of pain, and disease progression. Success relies on the alertness of primary care and the expertise of advanced practice physical therapists to make sure the right patients arrive in the rheumatologist’s office.
“We know the delay is on average 8-10 years, and often by the time a patient does show up in a rheumatology office, much damage has occurred,” Laura Passalent, a clinician researcher at University Health Network, Toronto, said in an interview. “But spondyloarthritis gets lost in the background noise of mechanical and musculoskeletal back pain, so it’s hard for primary care to accurately diagnose, and patients often bounce around the health care system for years before someone finally suspects. We are trying to change that paradigm, reduce the time to diagnosis, and identify patients earlier. If we can, we can treat earlier, and the evidence suggests that, like early treatment in RA, we can prevent disease progression.”
As in rheumatoid arthritis, getting patients on biologics sooner rather than later improves radiologic outcomes, daily function, and quality of life. Studies bear that out, including one by Ms. Passalent’s rheumatologist colleagues, Robert Inman, MD, and Nigel Haroon, MD, PhD, also with UHN. Their study of 334 patients with ankylosing spondylitis found that early treatment with a tumor necrosis factor (TNF) inhibitor reduced the odds of disease progression by up to 50% and was especially effective in those who got early treatment (Arthritis Rheum. 2013 Oct;65[10]:2645-54). Those who started at least 10 years after symptom onset were twice as likely to progress. Those who were on biologics for more than 50% of their disease duration were three times less likely to progress.
“It’s known that biologics improve the signs and symptoms of SpA, and the great majority of patients feel better on them,” Dr. Inman said in an interview. “But the really important outcomes are preventing structural damage, a finding already well established in RA. This study changed our thoughts on altering the natural history of this disease.”
Diagnostic delays worsen long-term outcomes in axSpA, just as in RA, but unlike RA, axSpA has no stepwise diagnostic algorithm, Dr. Inman said. “We had a real problem identifying a simple, reliable pathway for referrals. One of the strategies we investigated was this screening clinic model to facilitate appropriate and early referrals that are no longer dependent on primary care physicians.”
Community back pain clinics
Raja Rampersaud, MD, a spine surgeon at UHN, developed the first model – a community clinic that triages and treats people with low back pain. Primary care providers refer into the clinics, and advanced practice clinicians work with patients to create care plans. These might include low-level medical therapy, exercise, and other self-management techniques.
Ms. Passalent and her team partnered with these clinics in a pilot project to identify axSpA patients. The team provided clinician education and referral criteria for patients. These include back pain of more than 3 months’ duration in patients younger than 50 years who have other signs of inflammatory back pain. Primary care providers can refer such patients to a secondary screening program, run by an advanced care clinician, that further refines the diagnosis.
The clinic work-up includes the following:
- History, involving a description of back pain, peripheral joint involvement, and extra-articular manifestations.
- Physical exam looking at spinal mobility and vital signs, as well as tender/swollen joints, enthesitis, and dactylitis.
- Investigations that include pelvis and lateral lumbar and cervical spine radiographs, HLA-B27 testing, and measurements of C-reactive protein and erythrocyte sedimentation rate.
For those who don’t tick the axSpA boxes, the practitioner provides education on self-management, basic nonpharmacologic interventions, exercise guidance, and referrals back into primary care for their therapy.
But those who screen positive receive a direct rheumatology referral. This is an especially important component of the program because, like the United States, Canada has a chronic shortage of rheumatologists. However, in Canada there can be even greater distances than in the United States between a patient’s town and the closest rheumatology office. The back pain screening clinic reduced waiting time from up to 2 years to around 3 weeks – a notable accomplishment in a country with only about 500 rheumatologists – less than 1 per 75,000 residents.
First data
Ms. Passalent and the team presented their initial data from this model at recent annual meetings of the Canadian Rheumatology Association and the American College of Rheumatology (Arthritis Rheumatol. 2018;70[suppl 10]:Abstract 661).
During the first 3 years of the project, 410 patients were seen. Time from primary care referral to the secondary clinic appointment was roughly 22 days. These patients were young, with a mean age of about 37 years, and had experienced back pain for an average of 7 years. About 14% were positive for HLA-B27, but that characteristic signal actually performed poorly as an independent axSpA screen. It was highly specific (94%) but not very sensitive (28%), with a 71% positive and negative predictive value.
Assessment by the advanced care provider, on the other hand, had 90% specificity and 68% sensitivity. The negative and positive predictive values were 80% and 84%, respectively.
Among those who had a rheumatology consult, 18% received an axSpA diagnosis.
“We were very pleased to be able to decrease the time to diagnosis, from 9 years to 6 or 7,” Ms. Passalent said. “It’s still a long time, but you have to keep in mind this program is just getting started.”
Other benefits
It’s proven that early treatment prevents bone damage and improves spine-related function and quality of life for these patients. But if biologics help bone inflammation, could they also benefit the extra-articular manifestations that often accompany axSpA?
“The main comorbidities are anterior uveitis, inflammatory bowel diseases, and psoriasis,” Dr. Inman said. “In our cohort, 35% have uveitis, 12% have IBD, and 10% have psoriasis. Those are significant numbers, and the damage accrues over time. They are all inflammatory and maybe autoimmune.”
These extra-articular manifestations influence individual treatment plans, he said. “The presence of skin, eye, or joint inflammation does inform our selection. Generally, though, blocking TNF-alpha with a monoclonal antibody should also effectively treat these other issues in addition to SpA.”
A 2018 review touched on the uveitis/SpA treatment connection (Perm J. 2018;22:17-041. doi: 10.7812/TPP/17-041). Biologics – especially TNF blockers – are excellent choices for refractory uveitis and may confer a double benefit in patients with both diseases. Biologic choices for IBD and psoriasis also typically overlap those used in axSpA.
The literature is still evolving on this concept of cotreatment, Dr. Inman said, but it could represent an exciting option to prevent damage in multiple systems with one approach.
The future
Ms. Passalent, Dr. Inman, and Dr. Haroon see good things ahead for everyone involved in axSpA if the secondary screening clinic protocol expands throughout Canada.
“The thing that impresses me as a frontline worker, you can be an agent of change. If you’re surrounded by the right people and a supportive organization, you really can help to influence transformative change. It doesn’t happen overnight, but if you stick to it and work with the right champions, it’s amazing what influence on patient care you can have,” Ms. Passalent said.
Dr. Inman, Dr. Haroon, and Ms. Passalent have been consultants and received research funds from several pharmaceutical companies.
Does Residential Mobility Affect Childhood Leukemia?
Studies that look at the relationship between environment and childhood leukemia usually consider exposure at only a single residential address, such as the child’s home at birth or at time of diagnosis, say researchers from University of California and University of Southern California. But residential mobility, they contend, can have an impact on a number of relevant factors.
For instance, mobility can affect selection through the availability of data; cases are usually required to reside and be diagnosed in the same geographic area. It can affect exposure to electromagnetic fields and overhead power lines. Residential mobility can also function as a marker for other risk factors for childhood leukemia, such as maternal place of birth and younger maternal age at birth, as well as increased exposure to viruses or other infections potentially linked to higher leukemia risk. Finally, the type of dwelling can affect not only exposure but exposure assessment. Mobile homes and apartments, for instance, are more likely to lead to poor geographic information system (GIS) matching of the residential address.
The researchers hoped with their study to “disentangle the effect of mobility.” Using the California Power Lines Study, they analyzed data from 4,879 childhood leukemia patients born in California and diagnosed between 1988 and 2008.
Many childhood leukemia cases were mobile, the researchers found: 2,982 (61%) children changed residence between birth and diagnosis. Of those who moved, 618 stayed within 2 km of their birth home; 1,992 moved outside of their birth neighborhood. Children who moved tended to be older, lived in housing other than single-family homes, had younger mothers and fewer siblings, and were of lower socioeconomic status.
However, the effects of distance to power lines and magnetic field exposure on childhood leukemia were similar for a subset of residentially stable cases, and overall results were unchanged when the researchers controlled for proxies of mobility (except for dwelling). They found an OR for childhood leukemia of 1.44 for those whose birth residence was within 50 m of a 200+ kV line, and an OR of 1.50 for the highest exposure of calculated fields, compared with 1.62 and 1.71, respectively, among children who stayed in place.
While they believe their findings on mobility are relevant to other environmental exposures and other childhood outcome studies, the researchers conclude that confounding by mobility is an unlikely explanation for the associations observed between power lines exposure and childhood leukemia.
Studies that look at the relationship between environment and childhood leukemia usually consider exposure at only a single residential address, such as the child’s home at birth or at time of diagnosis, say researchers from University of California and University of Southern California. But residential mobility, they contend, can have an impact on a number of relevant factors.
For instance, mobility can affect selection through the availability of data; cases are usually required to reside and be diagnosed in the same geographic area. It can affect exposure to electromagnetic fields and overhead power lines. Residential mobility can also function as a marker for other risk factors for childhood leukemia, such as maternal place of birth and younger maternal age at birth, as well as increased exposure to viruses or other infections potentially linked to higher leukemia risk. Finally, the type of dwelling can affect not only exposure but exposure assessment. Mobile homes and apartments, for instance, are more likely to lead to poor geographic information system (GIS) matching of the residential address.
The researchers hoped with their study to “disentangle the effect of mobility.” Using the California Power Lines Study, they analyzed data from 4,879 childhood leukemia patients born in California and diagnosed between 1988 and 2008.
Many childhood leukemia cases were mobile, the researchers found: 2,982 (61%) children changed residence between birth and diagnosis. Of those who moved, 618 stayed within 2 km of their birth home; 1,992 moved outside of their birth neighborhood. Children who moved tended to be older, lived in housing other than single-family homes, had younger mothers and fewer siblings, and were of lower socioeconomic status.
However, the effects of distance to power lines and magnetic field exposure on childhood leukemia were similar for a subset of residentially stable cases, and overall results were unchanged when the researchers controlled for proxies of mobility (except for dwelling). They found an OR for childhood leukemia of 1.44 for those whose birth residence was within 50 m of a 200+ kV line, and an OR of 1.50 for the highest exposure of calculated fields, compared with 1.62 and 1.71, respectively, among children who stayed in place.
While they believe their findings on mobility are relevant to other environmental exposures and other childhood outcome studies, the researchers conclude that confounding by mobility is an unlikely explanation for the associations observed between power lines exposure and childhood leukemia.
Studies that look at the relationship between environment and childhood leukemia usually consider exposure at only a single residential address, such as the child’s home at birth or at time of diagnosis, say researchers from University of California and University of Southern California. But residential mobility, they contend, can have an impact on a number of relevant factors.
For instance, mobility can affect selection through the availability of data; cases are usually required to reside and be diagnosed in the same geographic area. It can affect exposure to electromagnetic fields and overhead power lines. Residential mobility can also function as a marker for other risk factors for childhood leukemia, such as maternal place of birth and younger maternal age at birth, as well as increased exposure to viruses or other infections potentially linked to higher leukemia risk. Finally, the type of dwelling can affect not only exposure but exposure assessment. Mobile homes and apartments, for instance, are more likely to lead to poor geographic information system (GIS) matching of the residential address.
The researchers hoped with their study to “disentangle the effect of mobility.” Using the California Power Lines Study, they analyzed data from 4,879 childhood leukemia patients born in California and diagnosed between 1988 and 2008.
Many childhood leukemia cases were mobile, the researchers found: 2,982 (61%) children changed residence between birth and diagnosis. Of those who moved, 618 stayed within 2 km of their birth home; 1,992 moved outside of their birth neighborhood. Children who moved tended to be older, lived in housing other than single-family homes, had younger mothers and fewer siblings, and were of lower socioeconomic status.
However, the effects of distance to power lines and magnetic field exposure on childhood leukemia were similar for a subset of residentially stable cases, and overall results were unchanged when the researchers controlled for proxies of mobility (except for dwelling). They found an OR for childhood leukemia of 1.44 for those whose birth residence was within 50 m of a 200+ kV line, and an OR of 1.50 for the highest exposure of calculated fields, compared with 1.62 and 1.71, respectively, among children who stayed in place.
While they believe their findings on mobility are relevant to other environmental exposures and other childhood outcome studies, the researchers conclude that confounding by mobility is an unlikely explanation for the associations observed between power lines exposure and childhood leukemia.
A primer on cannabis for cosmeceuticals: The endocannabinoid system
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
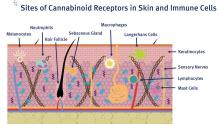
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
Ventricular Arrhythmia Due to MS Treatment
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
CMS plan will incentivize generic drug use
Patients will still be able to use drug manufacturer coupons and pay less at the pharmacy counter, but the money they spend won’t be applied to their out-of-pocket maximum if they choose the brand-name drug with coupon instead of the lower-cost generic.
The change will affect insurance plans offered in the individual market, small-group, large-group, and self-insured–group health plans.
The Centers for Medicare & Medicaid Services backed away from implementing two other generic drug provisions based on feedback on its draft proposal.
The provisions would have allowed insurers to remove brand-name drugs from a formulary midyear when a generic became available or move the drug to a higher tier, and allowed insurers to not count certain drug copayments toward the annual limits if the patient selected a brand-name drug when a generic was available.
“Based on issues raised by commenters, we are not finalizing these proposals,” CMS officials said.
Insurers “making mid-year formulary changes [should] only be permitted to move the brand-name drug to a different cost-sharing tier for the remainder of the plan year. [They] should not be allowed to remove a safe brand-name drug from the formulary until the time of plan renewal,” the American College of Physicians recommended in Feb. 12 comments on the proposal.
The final rule on Notice of Benefit and Payment Parameters for the 2020 benefit year also aims to provide more stability and lower premiums in the exchanges, according to CMS officials.
“The rule issued today will give consumers immediate premium relief by reducing the exchange user fees in the federally facilitated exchanges and state-based exchanges using the federal platform for 2020 thanks to successful efforts to improve the efficiency of the exchange,” CMS Administrator Seema Verma said in a statement.
CMS finalized a proposal to lower exchange user fees by 0.5%, fees that are generally passed on to consumers through premiums.
Other provisions cover exchange-related calculations, including how subsidies for exchange enrollees are calculated and how much enrollees are expected to contribute to benchmark plan premiums, which decreased slightly to 8.24%, down 0.07%, according to a CMS fact sheet.
For the out-of-pocket maximum, CMS said it “finalized a maximum annual limitation on cost sharing of $8,150 for self-only coverage and $16,300 for other than self-only coverage for the 2020 benefit year. This represents an approximately 3.16% increase above the 2019 parameters of $7,900 for self-only coverage and $15,800 for other than self-only coverage.”
Patients will still be able to use drug manufacturer coupons and pay less at the pharmacy counter, but the money they spend won’t be applied to their out-of-pocket maximum if they choose the brand-name drug with coupon instead of the lower-cost generic.
The change will affect insurance plans offered in the individual market, small-group, large-group, and self-insured–group health plans.
The Centers for Medicare & Medicaid Services backed away from implementing two other generic drug provisions based on feedback on its draft proposal.
The provisions would have allowed insurers to remove brand-name drugs from a formulary midyear when a generic became available or move the drug to a higher tier, and allowed insurers to not count certain drug copayments toward the annual limits if the patient selected a brand-name drug when a generic was available.
“Based on issues raised by commenters, we are not finalizing these proposals,” CMS officials said.
Insurers “making mid-year formulary changes [should] only be permitted to move the brand-name drug to a different cost-sharing tier for the remainder of the plan year. [They] should not be allowed to remove a safe brand-name drug from the formulary until the time of plan renewal,” the American College of Physicians recommended in Feb. 12 comments on the proposal.
The final rule on Notice of Benefit and Payment Parameters for the 2020 benefit year also aims to provide more stability and lower premiums in the exchanges, according to CMS officials.
“The rule issued today will give consumers immediate premium relief by reducing the exchange user fees in the federally facilitated exchanges and state-based exchanges using the federal platform for 2020 thanks to successful efforts to improve the efficiency of the exchange,” CMS Administrator Seema Verma said in a statement.
CMS finalized a proposal to lower exchange user fees by 0.5%, fees that are generally passed on to consumers through premiums.
Other provisions cover exchange-related calculations, including how subsidies for exchange enrollees are calculated and how much enrollees are expected to contribute to benchmark plan premiums, which decreased slightly to 8.24%, down 0.07%, according to a CMS fact sheet.
For the out-of-pocket maximum, CMS said it “finalized a maximum annual limitation on cost sharing of $8,150 for self-only coverage and $16,300 for other than self-only coverage for the 2020 benefit year. This represents an approximately 3.16% increase above the 2019 parameters of $7,900 for self-only coverage and $15,800 for other than self-only coverage.”
Patients will still be able to use drug manufacturer coupons and pay less at the pharmacy counter, but the money they spend won’t be applied to their out-of-pocket maximum if they choose the brand-name drug with coupon instead of the lower-cost generic.
The change will affect insurance plans offered in the individual market, small-group, large-group, and self-insured–group health plans.
The Centers for Medicare & Medicaid Services backed away from implementing two other generic drug provisions based on feedback on its draft proposal.
The provisions would have allowed insurers to remove brand-name drugs from a formulary midyear when a generic became available or move the drug to a higher tier, and allowed insurers to not count certain drug copayments toward the annual limits if the patient selected a brand-name drug when a generic was available.
“Based on issues raised by commenters, we are not finalizing these proposals,” CMS officials said.
Insurers “making mid-year formulary changes [should] only be permitted to move the brand-name drug to a different cost-sharing tier for the remainder of the plan year. [They] should not be allowed to remove a safe brand-name drug from the formulary until the time of plan renewal,” the American College of Physicians recommended in Feb. 12 comments on the proposal.
The final rule on Notice of Benefit and Payment Parameters for the 2020 benefit year also aims to provide more stability and lower premiums in the exchanges, according to CMS officials.
“The rule issued today will give consumers immediate premium relief by reducing the exchange user fees in the federally facilitated exchanges and state-based exchanges using the federal platform for 2020 thanks to successful efforts to improve the efficiency of the exchange,” CMS Administrator Seema Verma said in a statement.
CMS finalized a proposal to lower exchange user fees by 0.5%, fees that are generally passed on to consumers through premiums.
Other provisions cover exchange-related calculations, including how subsidies for exchange enrollees are calculated and how much enrollees are expected to contribute to benchmark plan premiums, which decreased slightly to 8.24%, down 0.07%, according to a CMS fact sheet.
For the out-of-pocket maximum, CMS said it “finalized a maximum annual limitation on cost sharing of $8,150 for self-only coverage and $16,300 for other than self-only coverage for the 2020 benefit year. This represents an approximately 3.16% increase above the 2019 parameters of $7,900 for self-only coverage and $15,800 for other than self-only coverage.”
Key clinical point: Patients will be able to use drug manufacturer coupons and pay less at the pharmacy counter, but the money they spend won’t be applied to their out-of-pocket maximum if they choose the brand-name drug with coupon instead of the lower-cost generic.
Major finding: The change will affect insurance plans offered in the individual market, small-group, large-group, and self-insured–group health plans.
Study details: Final rule for the Notice of Benefit and Payment Parameters for the 2020 benefit year
Disclosures: None.
Source: Patient Protection and Affordable Care Act; HHS Notice of Benefit and Payment Parameters for 2020. 45 CFR Parts 146, 147, 148, 153, 155, and 156.
Dr. Douglas Paauw gives updates on antihypertensives, statins, SGLT2 inhibitors
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
REPORTING FROM INTERNAL MEDICINE 2019