User login
CF drug picks up indication for children as young as 6
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.
Cannabis vaping among teens tied to tobacco use
and that practice is associated with cigars, waterpipe and e-cigarette use, findings from a survey of nearly 3,000 adolescents have shown.
“Although the prevalence of e-cigarette use among youth has increased dramatically in the past decade, little epidemiologic data exist on the prevalence of using e-cigarette devices or other specialised devices to vaporise (‘vape’) cannabis in the form of hash oil, tetrahydrocannabinol (THC) wax or oil, or dried cannabis buds or leaves,” wrote Sarah D. Kowitt, PhD, of the University of North Carolina, Chapel Hill, and colleagues. “This is surprising given that (1) cannabis (also referred to as marijuana) and e-cigarettes are the most commonly used substances by adolescents in the USA, (2) evidence exists that adolescents dual use both tobacco e-cigarettes and cannabis, and (3) longitudinal research suggests that use of e-cigarettes is associated with progression to use of cannabis.”
In a study published in BMJ Open, the researchers used data from the 2017 North Carolina Youth Tobacco Survey, a school-based survey of students in grades 6-12. The study population included 2,835 adolescents in grades 9-12.
Overall, 9.6% of students reported ever vaping cannabis. In multivariate analysis, cannabis vaping was significantly more likely among adolescents who reported using e-cigarettes (adjusted odds ratio 3.18), cigars (aOR 3.76), or water pipes (aOR 2.32) in the past 30 days, compared with peers who didn’t use tobacco.
The researchers found no significant association between smokeless tobacco use or traditional cigarette use in the past 30 days and vaping cannabis.
In a bivariate analysis, vaping cannabis was significantly more common among males vs. females (11% vs. 8.2%) and among non-Hispanic white students (11.3%), Hispanic students (10.5%), and other non-Hispanic students (11.8%) compared with non-Hispanic black students (5.0%).
In addition, prevalence of cannabis vaping increased with grade level, from 4.7% of 9th graders to 15.5% of 12th graders.
The health impacts of vaping cannabis are not well researched, but the researchers note that among the potential safety issues are earlier initiation of tobacco or cannabis use, concomitant tobacco and cannabis use, increased frequency of use or misuse of tobacco or cannabis, or increased potency of cannabis.
The results of the study were limited by several factors including the use of data only from the state of North Carolina, the lack of data on frequency or current vaping cannabis behavior, lack of data on specific products, and lack of data on whether teens used specialized devices or e-cigarettes for cannabis vaping. However, the findings are consistent with studies on prevalence of cannabis vaping in other states such as Connecticut and California. “No studies to our knowledge have examined how adolescents who vape cannabis use other specific tobacco products (i.e., cigarettes, cigars, waterpipe, smokeless tobacco),” the researchers wrote.
The findings confirm that a large number of adolescents who use tobacco products have vaped cannabis as well, and this growing public health issue “is likely to affect and be affected by tobacco control and cannabis policies in states and at the federal level in the USA,” the researchers concluded.
“Increased research investigating how youth use e-cigarette devices for other purposes beyond vaping nicotine, like the current study, is needed,” they added.
The study was supported in part by the National Cancer Institute and the Food and Drug Administration’s Center for Tobacco Products. The researchers had no financial conflicts to disclose.
SOURCE: Kowitt SD et al. BMJ Open. 2019 Jun 13. doi: 10.1136/bmjopen-2018-028535.
and that practice is associated with cigars, waterpipe and e-cigarette use, findings from a survey of nearly 3,000 adolescents have shown.
“Although the prevalence of e-cigarette use among youth has increased dramatically in the past decade, little epidemiologic data exist on the prevalence of using e-cigarette devices or other specialised devices to vaporise (‘vape’) cannabis in the form of hash oil, tetrahydrocannabinol (THC) wax or oil, or dried cannabis buds or leaves,” wrote Sarah D. Kowitt, PhD, of the University of North Carolina, Chapel Hill, and colleagues. “This is surprising given that (1) cannabis (also referred to as marijuana) and e-cigarettes are the most commonly used substances by adolescents in the USA, (2) evidence exists that adolescents dual use both tobacco e-cigarettes and cannabis, and (3) longitudinal research suggests that use of e-cigarettes is associated with progression to use of cannabis.”
In a study published in BMJ Open, the researchers used data from the 2017 North Carolina Youth Tobacco Survey, a school-based survey of students in grades 6-12. The study population included 2,835 adolescents in grades 9-12.
Overall, 9.6% of students reported ever vaping cannabis. In multivariate analysis, cannabis vaping was significantly more likely among adolescents who reported using e-cigarettes (adjusted odds ratio 3.18), cigars (aOR 3.76), or water pipes (aOR 2.32) in the past 30 days, compared with peers who didn’t use tobacco.
The researchers found no significant association between smokeless tobacco use or traditional cigarette use in the past 30 days and vaping cannabis.
In a bivariate analysis, vaping cannabis was significantly more common among males vs. females (11% vs. 8.2%) and among non-Hispanic white students (11.3%), Hispanic students (10.5%), and other non-Hispanic students (11.8%) compared with non-Hispanic black students (5.0%).
In addition, prevalence of cannabis vaping increased with grade level, from 4.7% of 9th graders to 15.5% of 12th graders.
The health impacts of vaping cannabis are not well researched, but the researchers note that among the potential safety issues are earlier initiation of tobacco or cannabis use, concomitant tobacco and cannabis use, increased frequency of use or misuse of tobacco or cannabis, or increased potency of cannabis.
The results of the study were limited by several factors including the use of data only from the state of North Carolina, the lack of data on frequency or current vaping cannabis behavior, lack of data on specific products, and lack of data on whether teens used specialized devices or e-cigarettes for cannabis vaping. However, the findings are consistent with studies on prevalence of cannabis vaping in other states such as Connecticut and California. “No studies to our knowledge have examined how adolescents who vape cannabis use other specific tobacco products (i.e., cigarettes, cigars, waterpipe, smokeless tobacco),” the researchers wrote.
The findings confirm that a large number of adolescents who use tobacco products have vaped cannabis as well, and this growing public health issue “is likely to affect and be affected by tobacco control and cannabis policies in states and at the federal level in the USA,” the researchers concluded.
“Increased research investigating how youth use e-cigarette devices for other purposes beyond vaping nicotine, like the current study, is needed,” they added.
The study was supported in part by the National Cancer Institute and the Food and Drug Administration’s Center for Tobacco Products. The researchers had no financial conflicts to disclose.
SOURCE: Kowitt SD et al. BMJ Open. 2019 Jun 13. doi: 10.1136/bmjopen-2018-028535.
and that practice is associated with cigars, waterpipe and e-cigarette use, findings from a survey of nearly 3,000 adolescents have shown.
“Although the prevalence of e-cigarette use among youth has increased dramatically in the past decade, little epidemiologic data exist on the prevalence of using e-cigarette devices or other specialised devices to vaporise (‘vape’) cannabis in the form of hash oil, tetrahydrocannabinol (THC) wax or oil, or dried cannabis buds or leaves,” wrote Sarah D. Kowitt, PhD, of the University of North Carolina, Chapel Hill, and colleagues. “This is surprising given that (1) cannabis (also referred to as marijuana) and e-cigarettes are the most commonly used substances by adolescents in the USA, (2) evidence exists that adolescents dual use both tobacco e-cigarettes and cannabis, and (3) longitudinal research suggests that use of e-cigarettes is associated with progression to use of cannabis.”
In a study published in BMJ Open, the researchers used data from the 2017 North Carolina Youth Tobacco Survey, a school-based survey of students in grades 6-12. The study population included 2,835 adolescents in grades 9-12.
Overall, 9.6% of students reported ever vaping cannabis. In multivariate analysis, cannabis vaping was significantly more likely among adolescents who reported using e-cigarettes (adjusted odds ratio 3.18), cigars (aOR 3.76), or water pipes (aOR 2.32) in the past 30 days, compared with peers who didn’t use tobacco.
The researchers found no significant association between smokeless tobacco use or traditional cigarette use in the past 30 days and vaping cannabis.
In a bivariate analysis, vaping cannabis was significantly more common among males vs. females (11% vs. 8.2%) and among non-Hispanic white students (11.3%), Hispanic students (10.5%), and other non-Hispanic students (11.8%) compared with non-Hispanic black students (5.0%).
In addition, prevalence of cannabis vaping increased with grade level, from 4.7% of 9th graders to 15.5% of 12th graders.
The health impacts of vaping cannabis are not well researched, but the researchers note that among the potential safety issues are earlier initiation of tobacco or cannabis use, concomitant tobacco and cannabis use, increased frequency of use or misuse of tobacco or cannabis, or increased potency of cannabis.
The results of the study were limited by several factors including the use of data only from the state of North Carolina, the lack of data on frequency or current vaping cannabis behavior, lack of data on specific products, and lack of data on whether teens used specialized devices or e-cigarettes for cannabis vaping. However, the findings are consistent with studies on prevalence of cannabis vaping in other states such as Connecticut and California. “No studies to our knowledge have examined how adolescents who vape cannabis use other specific tobacco products (i.e., cigarettes, cigars, waterpipe, smokeless tobacco),” the researchers wrote.
The findings confirm that a large number of adolescents who use tobacco products have vaped cannabis as well, and this growing public health issue “is likely to affect and be affected by tobacco control and cannabis policies in states and at the federal level in the USA,” the researchers concluded.
“Increased research investigating how youth use e-cigarette devices for other purposes beyond vaping nicotine, like the current study, is needed,” they added.
The study was supported in part by the National Cancer Institute and the Food and Drug Administration’s Center for Tobacco Products. The researchers had no financial conflicts to disclose.
SOURCE: Kowitt SD et al. BMJ Open. 2019 Jun 13. doi: 10.1136/bmjopen-2018-028535.
FROM BMJ OPEN
Key clinical point: Use of tobacco products was significantly associated with cannabis vaping in teens.
Major finding: Approximately 10% of adolescents reported vaping cannabis.
Study details: The data come from a survey of 2,835 adolescents in North Carolina.
Disclosures: The study was supported in part by the National Cancer Institute and the FDA Center for Tobacco Products. The researchers had no financial conflicts to disclose.
Source: Kowitt SD et al. BMJ Open. 2019 Jun 13. doi: 10.1136/bmjopen-2018-028535.
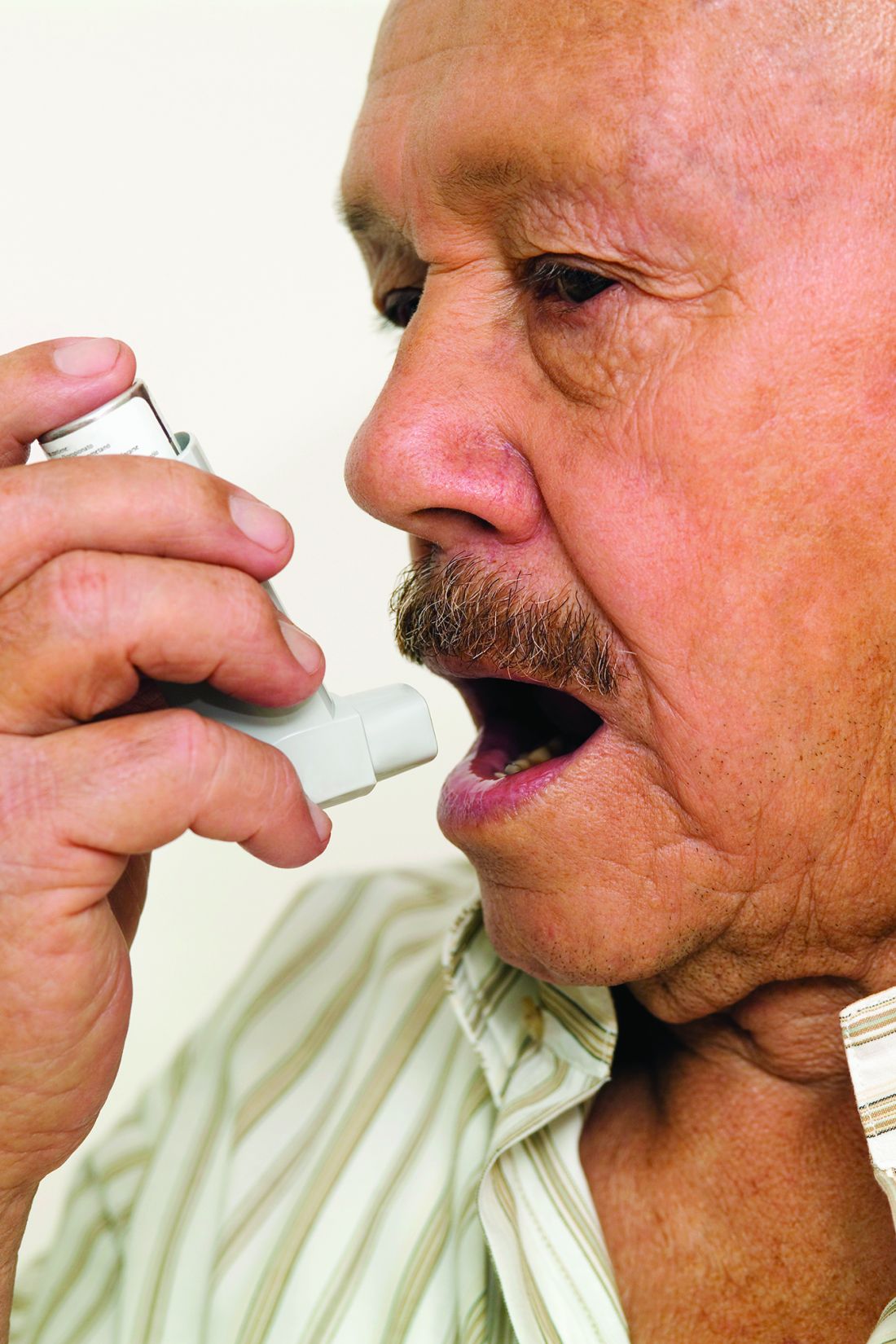
Inhaler technique not to blame for uncontrolled asthma in inner-city study
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
, a study has found.
“Incorrect inhaler technique cannot explain the poor disease control in our patient population,” wrote Patrick K. Gleeson, MD, of the University of Pennsylvania, Philadelphia, and coinvestigators. Their report is in the Journal of Allergy and Clinical Immunology: In Practice. “In individuals with poorly controlled asthma, other factors contributing to disease mortality must be considered.”
The 586 patients in the study were observed using their inhalers, and their technique was scored by way of a checklist developed for the study. Inhaler technique – widely regarded as a risk factor for poor disease control – was “better than expected,” the investigators reported, with 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers not making any errors.
“The seeming disassociation between subjects’ asthma control and inhaler technique is counterintuitive, and may be explained by important baseline characteristics in our patients,” they wrote. For instance, participants had suboptimal living conditions in lower income Philadelphia neighborhoods. Almost a quarter – 23% – were current smokers, and almost half were Medicaid recipients. In addition, their mean body mass index was 35.1 kg/m2.
The investigators hypothesized that patients with lower health literacy would have poorer technique but found instead that technique did not vary by reading comprehension or numeracy levels.
More than half of the adults in the study had uncontrolled asthma as defined by prednisone use, an emergency department visit, or a hospitalization for asthma in the past 12 months. A subset had moderate to severe disease per a physician’s diagnosis, forced expiratory volume in 1 second less than 80% predicted, and improvement with a bronchodilator. All patients, however, were considered to have uncontrolled asthma.
There is “uncertainty” in the field about how to measure inhaler technique, and the technique checklist used in the study “may have omitted potentially important errors,” the investigators noted. Still, “good technique predominated among our [population of vulnerable patients].”
The project was supported through awards from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute.
Coinvestigator Andrea J. Apter, MD, reported that she consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
SOURCE: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Factors other than inhaler technique should be considered to explain uncontrolled asthma in a low-income, inner-city population.
Major finding: In the study, 56% of patients using metered dose inhalers and 64% of those using dry powder inhalers were using their devices correctly.
Study details: In all, 586 patients were observed using their inhalers, and their technique was scored by way of a checklist developed for the study.
Disclosures: The National Institutes of Health/National Heart, Lung, and Blood Institute and the Patient-Centered Outcomes Research Institute supported the study. Coinvestigator Andrea J. Apter, MD, consults for UpToDate and is an associate editor for the journal. Coinvestigator Knashawn H. Morales, ScD, reported owning stock in Altria Group, British American Tobacco, and Philip Morris International. The other authors reported having no conflicts of interest.
Source: Gleeson PK. J Allergy Clin Immunol Pract. 2019 Jun 5. doi: 10.1016/j.jaip.2019.05.048.
Sleep quality linked to gut microbiome biodiversity
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
REPORTING FROM SLEEP 2019
Key clinical point: Better quality of sleep, but not duration of sleep, was associated with greater species richness and diversity of the gut microbiome.
Major finding: In fully adjusted models, greater daytime sleepiness was associated with lower richness and diversity of the gut microbiome on all indices (P = .01-.06).
Study details: An assessment of 482 individuals who participated in the Survey of the Health of Wisconsin.
Disclosures: The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
Source: Hagen EW et al. SLEEP 2019, Abstract 0106.
Mortality risk from mild to moderate OSA modified by age
SAN ANTONIO – , results from a large longitudinal analysis showed.
“The association between severe OSA and significant morbidity and mortality – particularly cardiovascular in nature – is well established,” the study’s first author, Alexandros N. Vgontzas, MD, said at the annual meeting of the Associated Professional Sleep Societies. “In contrast, mild to moderate OSA is highly prevalent in the general population but its association with morbidity and mortality is not well established.”
In an effort to examine the association between mild to moderate OSA and all-cause mortality, Dr. Vgontzas and colleagues drew from the Penn State Adult Cohort, a random general population sample of 1,741 men and women who were studied in the sleep lab with an 8-hour polysomnography at baseline and followed for a mean of 19.2 years for all-cause mortality.
The researchers retrieved mortality data from the Centers for Disease Control and Prevention’s National Death Index and defined mild OSA as an apnea/hypopnea index (AHI) of 5-14.9 events per hour, while moderate OSA was defined as an AHI of 15-29.9 events per hour. They used Cox proportional hazards regression to estimate all-cause mortality and adjusted for race, sex, body mass index, smoking, hypertension, diabetes, heart problems, and stroke.
Dr. Vgontzas, of the Sleep Research and Treatment Center at Penn State University, Hershey, Pa., reported that 596 individuals have died since the study began. On adjusted analysis, patients with an AHI between 5 and 29 were 1.28 times as likely to die overall (P = .019). The researchers found that the association with mortality was stronger among patients younger than age 60, compared with those aged 60 and older. The hazard ratio was 1.44 for study participants younger than age 60 (P = .027), and 1.14 for those aged 60 and older (P = .34).
“Mild to moderate sleep apnea is associated with significant all-cause mortality risk, but the strength of the association decreases markedly with age,” Dr. Vgontzas concluded. “These findings are in line with previous findings that the association of mild to moderate OSA with cardiometabolic risk is modified by age and suggests that OSA in older adults is a distinctly different phenotype than in the young and middle-aged.”
The explanation for the association remains unclear. “Is it because the people of older age have some kind of genetic protection, or is because their sleep apnea is milder?” he asked. “We don’t have the data to tell.”
Dr. Vgontzas reported having no financial disclosures.
SOURCE: Vgontzas A et al. SLEEP 2019, abstract 0504.
SAN ANTONIO – , results from a large longitudinal analysis showed.
“The association between severe OSA and significant morbidity and mortality – particularly cardiovascular in nature – is well established,” the study’s first author, Alexandros N. Vgontzas, MD, said at the annual meeting of the Associated Professional Sleep Societies. “In contrast, mild to moderate OSA is highly prevalent in the general population but its association with morbidity and mortality is not well established.”
In an effort to examine the association between mild to moderate OSA and all-cause mortality, Dr. Vgontzas and colleagues drew from the Penn State Adult Cohort, a random general population sample of 1,741 men and women who were studied in the sleep lab with an 8-hour polysomnography at baseline and followed for a mean of 19.2 years for all-cause mortality.
The researchers retrieved mortality data from the Centers for Disease Control and Prevention’s National Death Index and defined mild OSA as an apnea/hypopnea index (AHI) of 5-14.9 events per hour, while moderate OSA was defined as an AHI of 15-29.9 events per hour. They used Cox proportional hazards regression to estimate all-cause mortality and adjusted for race, sex, body mass index, smoking, hypertension, diabetes, heart problems, and stroke.
Dr. Vgontzas, of the Sleep Research and Treatment Center at Penn State University, Hershey, Pa., reported that 596 individuals have died since the study began. On adjusted analysis, patients with an AHI between 5 and 29 were 1.28 times as likely to die overall (P = .019). The researchers found that the association with mortality was stronger among patients younger than age 60, compared with those aged 60 and older. The hazard ratio was 1.44 for study participants younger than age 60 (P = .027), and 1.14 for those aged 60 and older (P = .34).
“Mild to moderate sleep apnea is associated with significant all-cause mortality risk, but the strength of the association decreases markedly with age,” Dr. Vgontzas concluded. “These findings are in line with previous findings that the association of mild to moderate OSA with cardiometabolic risk is modified by age and suggests that OSA in older adults is a distinctly different phenotype than in the young and middle-aged.”
The explanation for the association remains unclear. “Is it because the people of older age have some kind of genetic protection, or is because their sleep apnea is milder?” he asked. “We don’t have the data to tell.”
Dr. Vgontzas reported having no financial disclosures.
SOURCE: Vgontzas A et al. SLEEP 2019, abstract 0504.
SAN ANTONIO – , results from a large longitudinal analysis showed.
“The association between severe OSA and significant morbidity and mortality – particularly cardiovascular in nature – is well established,” the study’s first author, Alexandros N. Vgontzas, MD, said at the annual meeting of the Associated Professional Sleep Societies. “In contrast, mild to moderate OSA is highly prevalent in the general population but its association with morbidity and mortality is not well established.”
In an effort to examine the association between mild to moderate OSA and all-cause mortality, Dr. Vgontzas and colleagues drew from the Penn State Adult Cohort, a random general population sample of 1,741 men and women who were studied in the sleep lab with an 8-hour polysomnography at baseline and followed for a mean of 19.2 years for all-cause mortality.
The researchers retrieved mortality data from the Centers for Disease Control and Prevention’s National Death Index and defined mild OSA as an apnea/hypopnea index (AHI) of 5-14.9 events per hour, while moderate OSA was defined as an AHI of 15-29.9 events per hour. They used Cox proportional hazards regression to estimate all-cause mortality and adjusted for race, sex, body mass index, smoking, hypertension, diabetes, heart problems, and stroke.
Dr. Vgontzas, of the Sleep Research and Treatment Center at Penn State University, Hershey, Pa., reported that 596 individuals have died since the study began. On adjusted analysis, patients with an AHI between 5 and 29 were 1.28 times as likely to die overall (P = .019). The researchers found that the association with mortality was stronger among patients younger than age 60, compared with those aged 60 and older. The hazard ratio was 1.44 for study participants younger than age 60 (P = .027), and 1.14 for those aged 60 and older (P = .34).
“Mild to moderate sleep apnea is associated with significant all-cause mortality risk, but the strength of the association decreases markedly with age,” Dr. Vgontzas concluded. “These findings are in line with previous findings that the association of mild to moderate OSA with cardiometabolic risk is modified by age and suggests that OSA in older adults is a distinctly different phenotype than in the young and middle-aged.”
The explanation for the association remains unclear. “Is it because the people of older age have some kind of genetic protection, or is because their sleep apnea is milder?” he asked. “We don’t have the data to tell.”
Dr. Vgontzas reported having no financial disclosures.
SOURCE: Vgontzas A et al. SLEEP 2019, abstract 0504.
REPORTING FROM SLEEP 2019
Key clinical point: Among adults with mild to moderate obstructive sleep apnea, the risk of mortality is highest among those younger than age 60.
Major finding: The hazard ratio for mortality was 1.44 for study participants younger than age 60 (P = .027), and 1.14 for those aged 60 and older (P = .34).
Study details: An analysis of 1,741 men and women from the Penn State Adult Cohort.
Disclosures: Dr. Vgontzas reported having no financial disclosures.
Source: Vgontzas A et al. SLEEP 2019, Abstract 0504.
Daytime eating schedule found to help with weight management
SAN ANTONIO – In adults of normal weight, a small controlled study has shown that a , independent of caloric intake.
The findings come from an 8-week controlled trial presented at the annual meeting of the Associated Professional Sleep Societies, which set out to examine the impact of a daytime versus delayed eating schedule on body mass, adiposity, and energy homeostasis in adults of normal weight.
“It is best to stop eating as early as possible in the day, and to not eat late at night,” the study’s first author, Namni Goel, PhD, said in an interview at the meeting. “There’s an open question in our field: Should you stop eating at 7:00 p.m.? 8:00 p.m.? My own feeling is, the longer it is between when you stop eating and go to bed, the better off you are metabolically.”
Dr. Goel, associate professor in the division of sleep and chronobiology in the department of psychiatry at the University of Pennsylvania, Philadelphia, and colleagues enrolled 12 healthy adults to participate in a randomized cross-over study in free-living conditions. Three meals and two snacks consisting of comparable energy and macronutrient content were provided during two 8-week counterbalanced phases: 1) daytime eating (food consumed between 8:00 a.m. and 7:00 p.m, and 2) delayed eating (food consumed between 12:00 p.m. and 11:00 p.m. A 2-week washout period occurred between the conditions. “What we wanted to do is just manipulate the timing of eating and we provided all of the meals so we could control the caloric intake,” Dr. Goel said.
The researchers asked participants to maintain a sleep-wake cycle between 11:00 p.m. and 9:00 a.m. (verified by wrist actigraphy) and to limit physical activity. They assessed weight, adiposity, energy metabolism, and hormonal markers during four inpatient visits: 1) baseline; 2) after the first eating condition; 3) after the washout period, before the second eating condition began; and 4) after the second eating condition. They used two-way analysis of variance and Cohen’s d effect sizes to examine changes in anthropometrics and metabolic measures affected by eating schedule (daytime vs. delayed) and time (before vs. after each eating schedule).
The mean age of 12 study participants was 26 years; five were females. Their mean body mass index was 21.9 kg/m2. Dr. Goel reported that participants had excellent adherence to assigned eating schedules, with no differences between the conditions. Weight was decreased on the daytime vs. delayed eating schedule. Specifically, Cohen’s d effect sizes were 0.57 overall: 1.16 for females and 0.33 for males, all in the small to large range. Resting energy expenditure, respiratory quotient, and trunk fat percentage/leg fat percentage were decreased on the daytime vs. delayed eating condition, with Cohen’s d effect sizes of 0.45-1.02, all in the medium to large range. In addition, total cholesterol and insulin were decreased on the daytime eating condition (medium effect sizes of 0.60 and 0.57, respectively), while triglycerides and glucose were increased on the delayed condition (medium effect sizes of 0.46 and 0.52, respectively).
Weight, adiposity, energy metabolism, and hormonal measures did not differ significantly between the pre-daytime and pre-delayed eating conditions, suggesting that they returned to pre-condition levels after the washout period.
“One of the things we’re advocating is that with consistent daytime eating, you can lose weight and/or remain at weight maintenance,” Dr. Goel said. “Consistency is very important. Across 8 weeks, you’re becoming metabolically healthier because you’re not eating that late-night meal or snack. We had shown in previous sleep loss studies that people were eating 500 calories late in the evening on consecutive nights and gaining a substantial amount of weight.”
She and her colleagues are currently enrolling obese individuals into a similarly designed trial, “where we expect much bigger changes metabolically,” she said. The study was funded by a grant from the National Institutes of Health. Dr. Goel reported having no financial disclosures.
SOURCE: Goel N et al. SLEEP 2019, Abstract 0036.
SAN ANTONIO – In adults of normal weight, a small controlled study has shown that a , independent of caloric intake.
The findings come from an 8-week controlled trial presented at the annual meeting of the Associated Professional Sleep Societies, which set out to examine the impact of a daytime versus delayed eating schedule on body mass, adiposity, and energy homeostasis in adults of normal weight.
“It is best to stop eating as early as possible in the day, and to not eat late at night,” the study’s first author, Namni Goel, PhD, said in an interview at the meeting. “There’s an open question in our field: Should you stop eating at 7:00 p.m.? 8:00 p.m.? My own feeling is, the longer it is between when you stop eating and go to bed, the better off you are metabolically.”
Dr. Goel, associate professor in the division of sleep and chronobiology in the department of psychiatry at the University of Pennsylvania, Philadelphia, and colleagues enrolled 12 healthy adults to participate in a randomized cross-over study in free-living conditions. Three meals and two snacks consisting of comparable energy and macronutrient content were provided during two 8-week counterbalanced phases: 1) daytime eating (food consumed between 8:00 a.m. and 7:00 p.m, and 2) delayed eating (food consumed between 12:00 p.m. and 11:00 p.m. A 2-week washout period occurred between the conditions. “What we wanted to do is just manipulate the timing of eating and we provided all of the meals so we could control the caloric intake,” Dr. Goel said.
The researchers asked participants to maintain a sleep-wake cycle between 11:00 p.m. and 9:00 a.m. (verified by wrist actigraphy) and to limit physical activity. They assessed weight, adiposity, energy metabolism, and hormonal markers during four inpatient visits: 1) baseline; 2) after the first eating condition; 3) after the washout period, before the second eating condition began; and 4) after the second eating condition. They used two-way analysis of variance and Cohen’s d effect sizes to examine changes in anthropometrics and metabolic measures affected by eating schedule (daytime vs. delayed) and time (before vs. after each eating schedule).
The mean age of 12 study participants was 26 years; five were females. Their mean body mass index was 21.9 kg/m2. Dr. Goel reported that participants had excellent adherence to assigned eating schedules, with no differences between the conditions. Weight was decreased on the daytime vs. delayed eating schedule. Specifically, Cohen’s d effect sizes were 0.57 overall: 1.16 for females and 0.33 for males, all in the small to large range. Resting energy expenditure, respiratory quotient, and trunk fat percentage/leg fat percentage were decreased on the daytime vs. delayed eating condition, with Cohen’s d effect sizes of 0.45-1.02, all in the medium to large range. In addition, total cholesterol and insulin were decreased on the daytime eating condition (medium effect sizes of 0.60 and 0.57, respectively), while triglycerides and glucose were increased on the delayed condition (medium effect sizes of 0.46 and 0.52, respectively).
Weight, adiposity, energy metabolism, and hormonal measures did not differ significantly between the pre-daytime and pre-delayed eating conditions, suggesting that they returned to pre-condition levels after the washout period.
“One of the things we’re advocating is that with consistent daytime eating, you can lose weight and/or remain at weight maintenance,” Dr. Goel said. “Consistency is very important. Across 8 weeks, you’re becoming metabolically healthier because you’re not eating that late-night meal or snack. We had shown in previous sleep loss studies that people were eating 500 calories late in the evening on consecutive nights and gaining a substantial amount of weight.”
She and her colleagues are currently enrolling obese individuals into a similarly designed trial, “where we expect much bigger changes metabolically,” she said. The study was funded by a grant from the National Institutes of Health. Dr. Goel reported having no financial disclosures.
SOURCE: Goel N et al. SLEEP 2019, Abstract 0036.
SAN ANTONIO – In adults of normal weight, a small controlled study has shown that a , independent of caloric intake.
The findings come from an 8-week controlled trial presented at the annual meeting of the Associated Professional Sleep Societies, which set out to examine the impact of a daytime versus delayed eating schedule on body mass, adiposity, and energy homeostasis in adults of normal weight.
“It is best to stop eating as early as possible in the day, and to not eat late at night,” the study’s first author, Namni Goel, PhD, said in an interview at the meeting. “There’s an open question in our field: Should you stop eating at 7:00 p.m.? 8:00 p.m.? My own feeling is, the longer it is between when you stop eating and go to bed, the better off you are metabolically.”
Dr. Goel, associate professor in the division of sleep and chronobiology in the department of psychiatry at the University of Pennsylvania, Philadelphia, and colleagues enrolled 12 healthy adults to participate in a randomized cross-over study in free-living conditions. Three meals and two snacks consisting of comparable energy and macronutrient content were provided during two 8-week counterbalanced phases: 1) daytime eating (food consumed between 8:00 a.m. and 7:00 p.m, and 2) delayed eating (food consumed between 12:00 p.m. and 11:00 p.m. A 2-week washout period occurred between the conditions. “What we wanted to do is just manipulate the timing of eating and we provided all of the meals so we could control the caloric intake,” Dr. Goel said.
The researchers asked participants to maintain a sleep-wake cycle between 11:00 p.m. and 9:00 a.m. (verified by wrist actigraphy) and to limit physical activity. They assessed weight, adiposity, energy metabolism, and hormonal markers during four inpatient visits: 1) baseline; 2) after the first eating condition; 3) after the washout period, before the second eating condition began; and 4) after the second eating condition. They used two-way analysis of variance and Cohen’s d effect sizes to examine changes in anthropometrics and metabolic measures affected by eating schedule (daytime vs. delayed) and time (before vs. after each eating schedule).
The mean age of 12 study participants was 26 years; five were females. Their mean body mass index was 21.9 kg/m2. Dr. Goel reported that participants had excellent adherence to assigned eating schedules, with no differences between the conditions. Weight was decreased on the daytime vs. delayed eating schedule. Specifically, Cohen’s d effect sizes were 0.57 overall: 1.16 for females and 0.33 for males, all in the small to large range. Resting energy expenditure, respiratory quotient, and trunk fat percentage/leg fat percentage were decreased on the daytime vs. delayed eating condition, with Cohen’s d effect sizes of 0.45-1.02, all in the medium to large range. In addition, total cholesterol and insulin were decreased on the daytime eating condition (medium effect sizes of 0.60 and 0.57, respectively), while triglycerides and glucose were increased on the delayed condition (medium effect sizes of 0.46 and 0.52, respectively).
Weight, adiposity, energy metabolism, and hormonal measures did not differ significantly between the pre-daytime and pre-delayed eating conditions, suggesting that they returned to pre-condition levels after the washout period.
“One of the things we’re advocating is that with consistent daytime eating, you can lose weight and/or remain at weight maintenance,” Dr. Goel said. “Consistency is very important. Across 8 weeks, you’re becoming metabolically healthier because you’re not eating that late-night meal or snack. We had shown in previous sleep loss studies that people were eating 500 calories late in the evening on consecutive nights and gaining a substantial amount of weight.”
She and her colleagues are currently enrolling obese individuals into a similarly designed trial, “where we expect much bigger changes metabolically,” she said. The study was funded by a grant from the National Institutes of Health. Dr. Goel reported having no financial disclosures.
SOURCE: Goel N et al. SLEEP 2019, Abstract 0036.
REPORTING FROM SLEEP 2019
Key clinical point: A daytime eating schedule is likely beneficial for weight management and metabolic health.
Major finding: Weight was decreased on the daytime vs. delayed eating schedule with Cohen’s d effect of 0.57 overall: 1.16 for females and 0.33 for males, all in the small to large range.
Study details: A randomized trial of 12 healthy adults with normal body weight.
Disclosures: The study was funded by a grant from the National Institutes of Health. Dr. Goel reported having no financial disclosures.
Source: Goel N et al. SLEEP 2019, Abstract 0036.
Children with Down syndrome may need more screening for sleep-disordered breathing
because the condition frequently persists and recurs.
“Current screening recommendations to assess for SDB at a particular age may not be adequate in this population,” the authors of the study stated, adding that “persistence/recurrence of SDB is not easily predicted.”
The study, led by Joy Nehme, BSc, of Children’s Hospital of Eastern Ontario and the University of Ottawa, was published in Pediatric Pulmonology.
According to the study, research suggests that 43%-66% of children with Down syndrome have SDB, a category that encompasses sleep apnea (both obstructive and central) and hypoventilation. Those numbers are several times higher than the prevalence of SDB in children in the general population (1%-5%).
“Because SDB is associated with cardiometabolic and neurocognitive morbidity, its prompt and accurate diagnosis is important,” the researchers wrote. However, diagnosis requires a sleep study, which is not always performed although the American Academy of Pediatrics recommends children with Down syndrome undergo one by age 4.
Treatments include adenotonsillectomy (considered first-line), positive airway pressure, and lingual tonsillectomy.
The study aims to fill in gaps in knowledge about the condition over the long term since “there is little available literature on the trajectory of SDB in children and youth with Down syndrome over time.”
The researchers launched a retrospective study of 560 children with Down syndrome who were treated from 2004 to 2015 at Children’s Hospital of Eastern Ontario. Of those, 120 showed signs of SDB and underwent sleep studies (48% male, median age 6.6 years [range 4.5-10.5], median total apnea‐hypopnea index events per hour = 3.4 [1.6-10.8]).
Of the 120 children, 67 (56%) had obstructive-mixed SDB, 9 (8%) had central sleep apnea, and 5 (4%) had hypoventilation. The others (39, 32%) had no SDB.
Fifty-four children underwent at least two sleep studies during the period of the study, with at least one undergoing seven.
Researchers found weak, nonsignificant evidence that SDB persistence/occurrence varied by age (odds ratio per year = 1.15; 95% confidence interval, 0.96-1.41; P = .13).
As for treatment, adenotonsillectomy was most common, although “previous studies have ... shown that moderate to severe OSA in children with Down syndrome is likely to persist after a tonsillectomy.”
In regard to obstructive sleep apnea (OSA) specifically, the authors wrote, “our study ... showed that OSA‐SDB persisted or recurred in the vast majority of children. Further, persistence/recurrence could not be predicted by clinical features or SDB severity in our study. This, therefore, highlights the need for serial longitudinal screening for SDB in this population and for follow‐up PSG to ensure the success of treatment interventions.”
No study funding was reported. The study authors reported no disclosures.
SOURCE: Nehme J et al. Pediatr Pulmonol. 2019 Jun 6. doi: 10.1002/ppul.24380.
because the condition frequently persists and recurs.
“Current screening recommendations to assess for SDB at a particular age may not be adequate in this population,” the authors of the study stated, adding that “persistence/recurrence of SDB is not easily predicted.”
The study, led by Joy Nehme, BSc, of Children’s Hospital of Eastern Ontario and the University of Ottawa, was published in Pediatric Pulmonology.
According to the study, research suggests that 43%-66% of children with Down syndrome have SDB, a category that encompasses sleep apnea (both obstructive and central) and hypoventilation. Those numbers are several times higher than the prevalence of SDB in children in the general population (1%-5%).
“Because SDB is associated with cardiometabolic and neurocognitive morbidity, its prompt and accurate diagnosis is important,” the researchers wrote. However, diagnosis requires a sleep study, which is not always performed although the American Academy of Pediatrics recommends children with Down syndrome undergo one by age 4.
Treatments include adenotonsillectomy (considered first-line), positive airway pressure, and lingual tonsillectomy.
The study aims to fill in gaps in knowledge about the condition over the long term since “there is little available literature on the trajectory of SDB in children and youth with Down syndrome over time.”
The researchers launched a retrospective study of 560 children with Down syndrome who were treated from 2004 to 2015 at Children’s Hospital of Eastern Ontario. Of those, 120 showed signs of SDB and underwent sleep studies (48% male, median age 6.6 years [range 4.5-10.5], median total apnea‐hypopnea index events per hour = 3.4 [1.6-10.8]).
Of the 120 children, 67 (56%) had obstructive-mixed SDB, 9 (8%) had central sleep apnea, and 5 (4%) had hypoventilation. The others (39, 32%) had no SDB.
Fifty-four children underwent at least two sleep studies during the period of the study, with at least one undergoing seven.
Researchers found weak, nonsignificant evidence that SDB persistence/occurrence varied by age (odds ratio per year = 1.15; 95% confidence interval, 0.96-1.41; P = .13).
As for treatment, adenotonsillectomy was most common, although “previous studies have ... shown that moderate to severe OSA in children with Down syndrome is likely to persist after a tonsillectomy.”
In regard to obstructive sleep apnea (OSA) specifically, the authors wrote, “our study ... showed that OSA‐SDB persisted or recurred in the vast majority of children. Further, persistence/recurrence could not be predicted by clinical features or SDB severity in our study. This, therefore, highlights the need for serial longitudinal screening for SDB in this population and for follow‐up PSG to ensure the success of treatment interventions.”
No study funding was reported. The study authors reported no disclosures.
SOURCE: Nehme J et al. Pediatr Pulmonol. 2019 Jun 6. doi: 10.1002/ppul.24380.
because the condition frequently persists and recurs.
“Current screening recommendations to assess for SDB at a particular age may not be adequate in this population,” the authors of the study stated, adding that “persistence/recurrence of SDB is not easily predicted.”
The study, led by Joy Nehme, BSc, of Children’s Hospital of Eastern Ontario and the University of Ottawa, was published in Pediatric Pulmonology.
According to the study, research suggests that 43%-66% of children with Down syndrome have SDB, a category that encompasses sleep apnea (both obstructive and central) and hypoventilation. Those numbers are several times higher than the prevalence of SDB in children in the general population (1%-5%).
“Because SDB is associated with cardiometabolic and neurocognitive morbidity, its prompt and accurate diagnosis is important,” the researchers wrote. However, diagnosis requires a sleep study, which is not always performed although the American Academy of Pediatrics recommends children with Down syndrome undergo one by age 4.
Treatments include adenotonsillectomy (considered first-line), positive airway pressure, and lingual tonsillectomy.
The study aims to fill in gaps in knowledge about the condition over the long term since “there is little available literature on the trajectory of SDB in children and youth with Down syndrome over time.”
The researchers launched a retrospective study of 560 children with Down syndrome who were treated from 2004 to 2015 at Children’s Hospital of Eastern Ontario. Of those, 120 showed signs of SDB and underwent sleep studies (48% male, median age 6.6 years [range 4.5-10.5], median total apnea‐hypopnea index events per hour = 3.4 [1.6-10.8]).
Of the 120 children, 67 (56%) had obstructive-mixed SDB, 9 (8%) had central sleep apnea, and 5 (4%) had hypoventilation. The others (39, 32%) had no SDB.
Fifty-four children underwent at least two sleep studies during the period of the study, with at least one undergoing seven.
Researchers found weak, nonsignificant evidence that SDB persistence/occurrence varied by age (odds ratio per year = 1.15; 95% confidence interval, 0.96-1.41; P = .13).
As for treatment, adenotonsillectomy was most common, although “previous studies have ... shown that moderate to severe OSA in children with Down syndrome is likely to persist after a tonsillectomy.”
In regard to obstructive sleep apnea (OSA) specifically, the authors wrote, “our study ... showed that OSA‐SDB persisted or recurred in the vast majority of children. Further, persistence/recurrence could not be predicted by clinical features or SDB severity in our study. This, therefore, highlights the need for serial longitudinal screening for SDB in this population and for follow‐up PSG to ensure the success of treatment interventions.”
No study funding was reported. The study authors reported no disclosures.
SOURCE: Nehme J et al. Pediatr Pulmonol. 2019 Jun 6. doi: 10.1002/ppul.24380.
FROM PEDIATRIC PULMONOLOGY
Key clinical point: Sleep-disordered breathing (SDB) can be persistent and recurrent in children with Down syndrome, and long-term monitoring is warranted.
Major finding: SDB persistence/recurrence did not vary by age (odds ratio per year = 1.15; 95% confidence interval, 0.96-1.41; P = .13).
Study details: Retrospective cohort analysis of 120 children with Down syndrome tested via sleep study at least once for SDB (48% male, median age 6.6 years).
Disclosures: No study funding or author disclosures were reported.
Source: Nehme J et al. Pediatr Pulmonol. 2019 Jun 6. doi: 10.1002/ppul.24380.
FDA approves drug to treat low sexual desire in women
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
Genetics, neurobiology of borderline personality disorder remain uncertain
CRYSTAL CITY, VA. – Borderline personality disorder has a genetic and neurobiological component, but researchers remain unable to discern exactly why specific genetic markers are attributed to the disease, Emil F. Coccaro, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“The neurobiology at this point gives us clues that what’s going on with borderline personality disorder isn’t simply developmental or environmental. That’s all that it tells us,” said Dr. Coccaro, director of the Clinical Neuroscience & Psychopharmacology Research Unit at the University of Chicago.
Similarly, studies in twins that show heritability of borderline personal disorder at rates between 31% and 49% “only show there’s something in the DNA,” he added. Dr. Coccaro called the evidence for the neurobiology of borderline personality disorder “hazy.” said Dr. Coccaro, also chairman of the university’s department of psychiatry and behavioral neuroscience.
That is true of a lot of disorders, he said, so only the details explain why patients with borderline personality disorder look different from those who might have “similar types of circuitry abnormalities,” he said.
For example, genomewide association studies have found links between borderline personality disorder and the genes DPYD and PKP4, indicating problems with pyrimidine metabolism and myelin production. The study also found a strong association between borderline personality disorder, bipolar disorder, major depression, and schizophrenia (Transl Psychiatry. 2017 Jun. doi: 10.1038/tp.2017.115). DPYD has been associated with schizophrenia, but the relationship between DPYD and borderline personality disorder is unknown, Dr. Coccaro said.
“These [associations] are suggestive of what’s going on genetically, but it hardly makes a story that’s coherent enough to sink your teeth into,” he said.
The neuroscience behind borderline personality disorder, meanwhile, appears more promising, Dr. Coccaro noted. Studies of brain function have shown that negative emotions in patients with borderline personality disorder lead to increased amygdala reactivity. With regard to the neuroendocrinology of borderline personality disorder, trauma in those patients appears similar to what can be seen in patients with posttraumatic stress disorder (PTSD) with “increased central and decreased peripheral stress hormone response.” In fact, he said, 75% of people with borderline personality disorder experienced childhood physical, sexual, or emotional abuse (Curr Psychiatry Rep. 2005 Mar;7[1]:39).
Dr. Coccaro noted that, although the prevalence of borderline personality disorder is likely between 2% and 3%, the illness is encountered at a rate of 20% for patients in clinic and 40% for those in hospitals and emergency departments. Borderline personality disorder is more prevalent and more severe in women, but no gender differences are apparent in affective disturbance, impulsivity, or suicidality. Borderline personality disorder also is likely to be comorbid with at least two conditions: Men with borderline personality disorder tend to have narcissistic and antisocial personality disorders; women with borderline personality disorder have higher rates of major depression, anorexia and bulimia, and PTSD.
Borderline personality was traditionally associated with a “dismal prognosis,” but the lifetime course of the disorder appears to be more promising. In the Collaborative Longitudinal Personality Disorder Study (CLPS), 25% of 668 patients had achieved remission after 2 years, which was defined as having fewer than two symptoms for more than 2 months. After a decade, 85% of those patients had reached remission for at least 12 months (JAMA Psychiatry. 2011;68[8]:827-37). Another trial, the McLean Study of Adult Development, analyzed 290 patients who had a remission rate at 16 years of 78% that lasted for at least 8 years (J Pers Disord. 2005 Oct;19[5]:505-23).
However, Dr. Coccaro noted, patients with borderline personality disorder likely do not achieve true remission. Instead, he said, patients simply fail to meet all the criteria to be diagnosed with borderline personality disorder. “They still have some of the features, but they are less intense,” Dr. Coccaro said.
Dr. Coccaro reported serving as a consultant to Azevan, Avanir Pharma, and Brackett. He also reported receiving grants from the National Institute on Mental Illness and the National Institute on Alcoholic Abuse and Alcoholism, and receiving royalties from UpToDate.
The meeting was presented by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Borderline personality disorder has a genetic and neurobiological component, but researchers remain unable to discern exactly why specific genetic markers are attributed to the disease, Emil F. Coccaro, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“The neurobiology at this point gives us clues that what’s going on with borderline personality disorder isn’t simply developmental or environmental. That’s all that it tells us,” said Dr. Coccaro, director of the Clinical Neuroscience & Psychopharmacology Research Unit at the University of Chicago.
Similarly, studies in twins that show heritability of borderline personal disorder at rates between 31% and 49% “only show there’s something in the DNA,” he added. Dr. Coccaro called the evidence for the neurobiology of borderline personality disorder “hazy.” said Dr. Coccaro, also chairman of the university’s department of psychiatry and behavioral neuroscience.
That is true of a lot of disorders, he said, so only the details explain why patients with borderline personality disorder look different from those who might have “similar types of circuitry abnormalities,” he said.
For example, genomewide association studies have found links between borderline personality disorder and the genes DPYD and PKP4, indicating problems with pyrimidine metabolism and myelin production. The study also found a strong association between borderline personality disorder, bipolar disorder, major depression, and schizophrenia (Transl Psychiatry. 2017 Jun. doi: 10.1038/tp.2017.115). DPYD has been associated with schizophrenia, but the relationship between DPYD and borderline personality disorder is unknown, Dr. Coccaro said.
“These [associations] are suggestive of what’s going on genetically, but it hardly makes a story that’s coherent enough to sink your teeth into,” he said.
The neuroscience behind borderline personality disorder, meanwhile, appears more promising, Dr. Coccaro noted. Studies of brain function have shown that negative emotions in patients with borderline personality disorder lead to increased amygdala reactivity. With regard to the neuroendocrinology of borderline personality disorder, trauma in those patients appears similar to what can be seen in patients with posttraumatic stress disorder (PTSD) with “increased central and decreased peripheral stress hormone response.” In fact, he said, 75% of people with borderline personality disorder experienced childhood physical, sexual, or emotional abuse (Curr Psychiatry Rep. 2005 Mar;7[1]:39).
Dr. Coccaro noted that, although the prevalence of borderline personality disorder is likely between 2% and 3%, the illness is encountered at a rate of 20% for patients in clinic and 40% for those in hospitals and emergency departments. Borderline personality disorder is more prevalent and more severe in women, but no gender differences are apparent in affective disturbance, impulsivity, or suicidality. Borderline personality disorder also is likely to be comorbid with at least two conditions: Men with borderline personality disorder tend to have narcissistic and antisocial personality disorders; women with borderline personality disorder have higher rates of major depression, anorexia and bulimia, and PTSD.
Borderline personality was traditionally associated with a “dismal prognosis,” but the lifetime course of the disorder appears to be more promising. In the Collaborative Longitudinal Personality Disorder Study (CLPS), 25% of 668 patients had achieved remission after 2 years, which was defined as having fewer than two symptoms for more than 2 months. After a decade, 85% of those patients had reached remission for at least 12 months (JAMA Psychiatry. 2011;68[8]:827-37). Another trial, the McLean Study of Adult Development, analyzed 290 patients who had a remission rate at 16 years of 78% that lasted for at least 8 years (J Pers Disord. 2005 Oct;19[5]:505-23).
However, Dr. Coccaro noted, patients with borderline personality disorder likely do not achieve true remission. Instead, he said, patients simply fail to meet all the criteria to be diagnosed with borderline personality disorder. “They still have some of the features, but they are less intense,” Dr. Coccaro said.
Dr. Coccaro reported serving as a consultant to Azevan, Avanir Pharma, and Brackett. He also reported receiving grants from the National Institute on Mental Illness and the National Institute on Alcoholic Abuse and Alcoholism, and receiving royalties from UpToDate.
The meeting was presented by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Borderline personality disorder has a genetic and neurobiological component, but researchers remain unable to discern exactly why specific genetic markers are attributed to the disease, Emil F. Coccaro, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“The neurobiology at this point gives us clues that what’s going on with borderline personality disorder isn’t simply developmental or environmental. That’s all that it tells us,” said Dr. Coccaro, director of the Clinical Neuroscience & Psychopharmacology Research Unit at the University of Chicago.
Similarly, studies in twins that show heritability of borderline personal disorder at rates between 31% and 49% “only show there’s something in the DNA,” he added. Dr. Coccaro called the evidence for the neurobiology of borderline personality disorder “hazy.” said Dr. Coccaro, also chairman of the university’s department of psychiatry and behavioral neuroscience.
That is true of a lot of disorders, he said, so only the details explain why patients with borderline personality disorder look different from those who might have “similar types of circuitry abnormalities,” he said.
For example, genomewide association studies have found links between borderline personality disorder and the genes DPYD and PKP4, indicating problems with pyrimidine metabolism and myelin production. The study also found a strong association between borderline personality disorder, bipolar disorder, major depression, and schizophrenia (Transl Psychiatry. 2017 Jun. doi: 10.1038/tp.2017.115). DPYD has been associated with schizophrenia, but the relationship between DPYD and borderline personality disorder is unknown, Dr. Coccaro said.
“These [associations] are suggestive of what’s going on genetically, but it hardly makes a story that’s coherent enough to sink your teeth into,” he said.
The neuroscience behind borderline personality disorder, meanwhile, appears more promising, Dr. Coccaro noted. Studies of brain function have shown that negative emotions in patients with borderline personality disorder lead to increased amygdala reactivity. With regard to the neuroendocrinology of borderline personality disorder, trauma in those patients appears similar to what can be seen in patients with posttraumatic stress disorder (PTSD) with “increased central and decreased peripheral stress hormone response.” In fact, he said, 75% of people with borderline personality disorder experienced childhood physical, sexual, or emotional abuse (Curr Psychiatry Rep. 2005 Mar;7[1]:39).
Dr. Coccaro noted that, although the prevalence of borderline personality disorder is likely between 2% and 3%, the illness is encountered at a rate of 20% for patients in clinic and 40% for those in hospitals and emergency departments. Borderline personality disorder is more prevalent and more severe in women, but no gender differences are apparent in affective disturbance, impulsivity, or suicidality. Borderline personality disorder also is likely to be comorbid with at least two conditions: Men with borderline personality disorder tend to have narcissistic and antisocial personality disorders; women with borderline personality disorder have higher rates of major depression, anorexia and bulimia, and PTSD.
Borderline personality was traditionally associated with a “dismal prognosis,” but the lifetime course of the disorder appears to be more promising. In the Collaborative Longitudinal Personality Disorder Study (CLPS), 25% of 668 patients had achieved remission after 2 years, which was defined as having fewer than two symptoms for more than 2 months. After a decade, 85% of those patients had reached remission for at least 12 months (JAMA Psychiatry. 2011;68[8]:827-37). Another trial, the McLean Study of Adult Development, analyzed 290 patients who had a remission rate at 16 years of 78% that lasted for at least 8 years (J Pers Disord. 2005 Oct;19[5]:505-23).
However, Dr. Coccaro noted, patients with borderline personality disorder likely do not achieve true remission. Instead, he said, patients simply fail to meet all the criteria to be diagnosed with borderline personality disorder. “They still have some of the features, but they are less intense,” Dr. Coccaro said.
Dr. Coccaro reported serving as a consultant to Azevan, Avanir Pharma, and Brackett. He also reported receiving grants from the National Institute on Mental Illness and the National Institute on Alcoholic Abuse and Alcoholism, and receiving royalties from UpToDate.
The meeting was presented by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
REPORTING FROM FOCUS ON NEUROPSYCHIATRY 2019
Platelet glycoprotein genotypes predict responses to ITP therapy
AMSTERDAM – For patients with immune thrombocytopenia (ITP), platelet glycoprotein genotypes may predict effectiveness of specific therapies, according to investigators.
Significant relationships were detected between allelic polymorphisms of glycoprotein genes and durable responses to corticosteroids, thrombopoietin receptor agonists (aTPOr), and splenectomy, reported lead author Irina Zotova, MD, of the Russian Research Institute of Hematology and Transfusiology, Federal Biomedical Agency, in Saint Petersburg and colleagues.
These findings could guide clinical decision making in ITP, according to Dr. Zotova.
“Currently, the choice of therapeutic approach to ITP treatment, especially the second-line, is empirical, and often based on the experience of the clinician,” Dr. Zotova said during her presentation at the annual congress of the European Hematology Association. “Recent studies have shown that ... genes coding for glycoprotein are involved in platelet function and are associated with different responses to ITP treatment. This provides an opportunity for an individual approach and personalization of ITP therapy in accordance with the genetic status of the patient.”
In the present study, the investigators used PCR-RFLP (polymerase chain reaction–restriction fragment length polymorphism) to determine GPIa A1648G and GPIIb 2622TG gene status in 81 patients with primary ITP and compared these genotypes with clinical responses.
All patients received first-line corticosteroid therapy. If necessary, second-line treatment was delivered. In total, 37 patients (46%) received aTPOr, while 22 patients (27%) underwent splenectomy. Responses were classified as durable response, nondurable response, or no response.
Analysis showed that a significantly greater proportion of patients who had durable responses to corticosteroid therapy exhibited a heterozygous GPIIb 2622TG genotype, compared with patients who had nondurable responses (72.2% vs. 30.9%; odds ratio, 5.8; P = .005).
For second-line responses, patients who had durable response to aTPOr were much more likely to be homozygous for the GPIa A1648G than nonresponders (87.5% vs. 20.0%; OR, 28.0; P = .005). More strikingly, all patients who achieved durable responses to splenectomy had the GPIa A1648G genotype, compared with just 44% of nonresponders (OR, 33.0; P = .005).
“Gentoyping for the GPIIb T2622G and GPIa A1648G polymorphisms allows [clinicians] to predict the response to first-line and second-line treatment, giving the possibility to stratify patients to groups with favorable and unfavorable courses of the disease,” Dr. Zotova concluded.
Session moderator János Kappelmayer, MD, of the University of Debrecen (Hungary) highlighted a key opportunity presented by the study. “I think that one important aspect of these findings could be that splenectomy is an irreversible procedure, so one can exclude those patients who will not benefit from splenectomy,” he said. “I think this is a real advantage.”
The investigators reported no study funding or conflicts of interest.
SOURCE: Zotova I et al. EHA Congress, Abstract S848.
AMSTERDAM – For patients with immune thrombocytopenia (ITP), platelet glycoprotein genotypes may predict effectiveness of specific therapies, according to investigators.
Significant relationships were detected between allelic polymorphisms of glycoprotein genes and durable responses to corticosteroids, thrombopoietin receptor agonists (aTPOr), and splenectomy, reported lead author Irina Zotova, MD, of the Russian Research Institute of Hematology and Transfusiology, Federal Biomedical Agency, in Saint Petersburg and colleagues.
These findings could guide clinical decision making in ITP, according to Dr. Zotova.
“Currently, the choice of therapeutic approach to ITP treatment, especially the second-line, is empirical, and often based on the experience of the clinician,” Dr. Zotova said during her presentation at the annual congress of the European Hematology Association. “Recent studies have shown that ... genes coding for glycoprotein are involved in platelet function and are associated with different responses to ITP treatment. This provides an opportunity for an individual approach and personalization of ITP therapy in accordance with the genetic status of the patient.”
In the present study, the investigators used PCR-RFLP (polymerase chain reaction–restriction fragment length polymorphism) to determine GPIa A1648G and GPIIb 2622TG gene status in 81 patients with primary ITP and compared these genotypes with clinical responses.
All patients received first-line corticosteroid therapy. If necessary, second-line treatment was delivered. In total, 37 patients (46%) received aTPOr, while 22 patients (27%) underwent splenectomy. Responses were classified as durable response, nondurable response, or no response.
Analysis showed that a significantly greater proportion of patients who had durable responses to corticosteroid therapy exhibited a heterozygous GPIIb 2622TG genotype, compared with patients who had nondurable responses (72.2% vs. 30.9%; odds ratio, 5.8; P = .005).
For second-line responses, patients who had durable response to aTPOr were much more likely to be homozygous for the GPIa A1648G than nonresponders (87.5% vs. 20.0%; OR, 28.0; P = .005). More strikingly, all patients who achieved durable responses to splenectomy had the GPIa A1648G genotype, compared with just 44% of nonresponders (OR, 33.0; P = .005).
“Gentoyping for the GPIIb T2622G and GPIa A1648G polymorphisms allows [clinicians] to predict the response to first-line and second-line treatment, giving the possibility to stratify patients to groups with favorable and unfavorable courses of the disease,” Dr. Zotova concluded.
Session moderator János Kappelmayer, MD, of the University of Debrecen (Hungary) highlighted a key opportunity presented by the study. “I think that one important aspect of these findings could be that splenectomy is an irreversible procedure, so one can exclude those patients who will not benefit from splenectomy,” he said. “I think this is a real advantage.”
The investigators reported no study funding or conflicts of interest.
SOURCE: Zotova I et al. EHA Congress, Abstract S848.
AMSTERDAM – For patients with immune thrombocytopenia (ITP), platelet glycoprotein genotypes may predict effectiveness of specific therapies, according to investigators.
Significant relationships were detected between allelic polymorphisms of glycoprotein genes and durable responses to corticosteroids, thrombopoietin receptor agonists (aTPOr), and splenectomy, reported lead author Irina Zotova, MD, of the Russian Research Institute of Hematology and Transfusiology, Federal Biomedical Agency, in Saint Petersburg and colleagues.
These findings could guide clinical decision making in ITP, according to Dr. Zotova.
“Currently, the choice of therapeutic approach to ITP treatment, especially the second-line, is empirical, and often based on the experience of the clinician,” Dr. Zotova said during her presentation at the annual congress of the European Hematology Association. “Recent studies have shown that ... genes coding for glycoprotein are involved in platelet function and are associated with different responses to ITP treatment. This provides an opportunity for an individual approach and personalization of ITP therapy in accordance with the genetic status of the patient.”
In the present study, the investigators used PCR-RFLP (polymerase chain reaction–restriction fragment length polymorphism) to determine GPIa A1648G and GPIIb 2622TG gene status in 81 patients with primary ITP and compared these genotypes with clinical responses.
All patients received first-line corticosteroid therapy. If necessary, second-line treatment was delivered. In total, 37 patients (46%) received aTPOr, while 22 patients (27%) underwent splenectomy. Responses were classified as durable response, nondurable response, or no response.
Analysis showed that a significantly greater proportion of patients who had durable responses to corticosteroid therapy exhibited a heterozygous GPIIb 2622TG genotype, compared with patients who had nondurable responses (72.2% vs. 30.9%; odds ratio, 5.8; P = .005).
For second-line responses, patients who had durable response to aTPOr were much more likely to be homozygous for the GPIa A1648G than nonresponders (87.5% vs. 20.0%; OR, 28.0; P = .005). More strikingly, all patients who achieved durable responses to splenectomy had the GPIa A1648G genotype, compared with just 44% of nonresponders (OR, 33.0; P = .005).
“Gentoyping for the GPIIb T2622G and GPIa A1648G polymorphisms allows [clinicians] to predict the response to first-line and second-line treatment, giving the possibility to stratify patients to groups with favorable and unfavorable courses of the disease,” Dr. Zotova concluded.
Session moderator János Kappelmayer, MD, of the University of Debrecen (Hungary) highlighted a key opportunity presented by the study. “I think that one important aspect of these findings could be that splenectomy is an irreversible procedure, so one can exclude those patients who will not benefit from splenectomy,” he said. “I think this is a real advantage.”
The investigators reported no study funding or conflicts of interest.
SOURCE: Zotova I et al. EHA Congress, Abstract S848.
REPORTING FROM EHA CONGRESS