User login
Ob.Gyn. News welcomes Dr. Krishna to the board
Dr. Krishna is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta.
She has published articles on topics such as low fetal fraction in noninvasive prenatal screening, breast cancer in pregnancy, intrahepatic cholestasis of pregnancy, obesity, diabetes, and chronic hypertension. She is currently involved in research on chronic hypertension during pregnancy.
Dr. Krishna serves on numerous committees at Emory for the department of gynecology and obstetrics, including the resident clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee, ethics committee, and critical care committee.
Dr. Krishna received a Master of Public Health in epidemiology from Tulane University School of Public Health and Tropical Medicine in New Orleans, and a medical degree from Louisiana State University Health Sciences Center in Shreveport. She completed her residency training in obstetrics and gynecology and fellowship training in maternal-fetal medicine at Emory, where she received awards for her clinical skills in obstetrics and completed a thesis research project on prenatal genetic screening. She also was recognized by her staff and colleagues as Outstanding Emory Faculty for her contributions to teaching and clinical service. Dr. Krishna is board certified in obstetrics and gynecology and in maternal-fetal medicine. She is a member of the Society for Maternal-Fetal Medicine and a fellow of the American College of Obstetricians and Gynecologists.
Dr. Krishna is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta.
She has published articles on topics such as low fetal fraction in noninvasive prenatal screening, breast cancer in pregnancy, intrahepatic cholestasis of pregnancy, obesity, diabetes, and chronic hypertension. She is currently involved in research on chronic hypertension during pregnancy.
Dr. Krishna serves on numerous committees at Emory for the department of gynecology and obstetrics, including the resident clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee, ethics committee, and critical care committee.
Dr. Krishna received a Master of Public Health in epidemiology from Tulane University School of Public Health and Tropical Medicine in New Orleans, and a medical degree from Louisiana State University Health Sciences Center in Shreveport. She completed her residency training in obstetrics and gynecology and fellowship training in maternal-fetal medicine at Emory, where she received awards for her clinical skills in obstetrics and completed a thesis research project on prenatal genetic screening. She also was recognized by her staff and colleagues as Outstanding Emory Faculty for her contributions to teaching and clinical service. Dr. Krishna is board certified in obstetrics and gynecology and in maternal-fetal medicine. She is a member of the Society for Maternal-Fetal Medicine and a fellow of the American College of Obstetricians and Gynecologists.
Dr. Krishna is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta.
She has published articles on topics such as low fetal fraction in noninvasive prenatal screening, breast cancer in pregnancy, intrahepatic cholestasis of pregnancy, obesity, diabetes, and chronic hypertension. She is currently involved in research on chronic hypertension during pregnancy.
Dr. Krishna serves on numerous committees at Emory for the department of gynecology and obstetrics, including the resident clinical competency committee and program evaluation committee, as well as similar committees for the maternal-fetal medicine fellowship program. She also is a member of the Emory University Hospital Midtown’s quality enhancement committee, ethics committee, and critical care committee.
Dr. Krishna received a Master of Public Health in epidemiology from Tulane University School of Public Health and Tropical Medicine in New Orleans, and a medical degree from Louisiana State University Health Sciences Center in Shreveport. She completed her residency training in obstetrics and gynecology and fellowship training in maternal-fetal medicine at Emory, where she received awards for her clinical skills in obstetrics and completed a thesis research project on prenatal genetic screening. She also was recognized by her staff and colleagues as Outstanding Emory Faculty for her contributions to teaching and clinical service. Dr. Krishna is board certified in obstetrics and gynecology and in maternal-fetal medicine. She is a member of the Society for Maternal-Fetal Medicine and a fellow of the American College of Obstetricians and Gynecologists.
Does PTSD Get Passed Down?
Do parents pass along posttraumatic stress disorder (PTSD) to their children? Researchers from Universidade do Porto in Portugal, say although it seems a reasonable possibility, the “degree of controversy is high,” and studies have had conflicting results. For instance, some research has found that children of war veterans with PTSD have higher depression scores and higher rates of aggression and anxiety. While other research has shown no differences between veterans’ and nonveterans’ children.
The Universidade do Porto study involved 46 veterans of Portugal’s war with Angola, Mozambique, and Guinea from 1961 to 1974. The researchers studied the association of war veterans’ PTSD lifetime diagnosis and war exposure intensity with the self-reported psychopathology of their adult offspring, assessed 40 years after the end of the war. They also studied childhood adversities and attachment patterns, which have been implicated in intergenerational transmission of trauma and PTSD.
Both veterans and offspring were assessed via questionnaires, clinical interviews, and symptom scales, including the Brief Symptom Inventory (BSI). The veterans also answered the War Experiences Questionnaire. Offspring of fathers with PTSD were not different from offspring of fathers without PTSD, with respect to age, gender, socioeconomic status, and marital status.
The researchers found no association between the veterans’ lifetime PTSD and their children’s psychopathology, attachment dimensions, and self-reported overall childhood maltreatment. The fathers’ war experience carried more weight. It seemed, the researchers say, that the children were able to overcome living with a parent’s PTSD symptoms, but they were less resilient when it came to their fathers’ war experience.
Veterans’ war exposure was associated with BSI in the offspring with regard to somatization, phobic anxiety, Global Severity Index, and Positive Symptom Distress Index. It was also associated with offspring’s physical neglect as a childhood adversity.
These findings could have considerable social importance, the researchers say. They suggest that mental health support could benefit the children especially if provided early after highly traumatized veterans return from war, “not just later on—if and when they develop PTSD.”
Do parents pass along posttraumatic stress disorder (PTSD) to their children? Researchers from Universidade do Porto in Portugal, say although it seems a reasonable possibility, the “degree of controversy is high,” and studies have had conflicting results. For instance, some research has found that children of war veterans with PTSD have higher depression scores and higher rates of aggression and anxiety. While other research has shown no differences between veterans’ and nonveterans’ children.
The Universidade do Porto study involved 46 veterans of Portugal’s war with Angola, Mozambique, and Guinea from 1961 to 1974. The researchers studied the association of war veterans’ PTSD lifetime diagnosis and war exposure intensity with the self-reported psychopathology of their adult offspring, assessed 40 years after the end of the war. They also studied childhood adversities and attachment patterns, which have been implicated in intergenerational transmission of trauma and PTSD.
Both veterans and offspring were assessed via questionnaires, clinical interviews, and symptom scales, including the Brief Symptom Inventory (BSI). The veterans also answered the War Experiences Questionnaire. Offspring of fathers with PTSD were not different from offspring of fathers without PTSD, with respect to age, gender, socioeconomic status, and marital status.
The researchers found no association between the veterans’ lifetime PTSD and their children’s psychopathology, attachment dimensions, and self-reported overall childhood maltreatment. The fathers’ war experience carried more weight. It seemed, the researchers say, that the children were able to overcome living with a parent’s PTSD symptoms, but they were less resilient when it came to their fathers’ war experience.
Veterans’ war exposure was associated with BSI in the offspring with regard to somatization, phobic anxiety, Global Severity Index, and Positive Symptom Distress Index. It was also associated with offspring’s physical neglect as a childhood adversity.
These findings could have considerable social importance, the researchers say. They suggest that mental health support could benefit the children especially if provided early after highly traumatized veterans return from war, “not just later on—if and when they develop PTSD.”
Do parents pass along posttraumatic stress disorder (PTSD) to their children? Researchers from Universidade do Porto in Portugal, say although it seems a reasonable possibility, the “degree of controversy is high,” and studies have had conflicting results. For instance, some research has found that children of war veterans with PTSD have higher depression scores and higher rates of aggression and anxiety. While other research has shown no differences between veterans’ and nonveterans’ children.
The Universidade do Porto study involved 46 veterans of Portugal’s war with Angola, Mozambique, and Guinea from 1961 to 1974. The researchers studied the association of war veterans’ PTSD lifetime diagnosis and war exposure intensity with the self-reported psychopathology of their adult offspring, assessed 40 years after the end of the war. They also studied childhood adversities and attachment patterns, which have been implicated in intergenerational transmission of trauma and PTSD.
Both veterans and offspring were assessed via questionnaires, clinical interviews, and symptom scales, including the Brief Symptom Inventory (BSI). The veterans also answered the War Experiences Questionnaire. Offspring of fathers with PTSD were not different from offspring of fathers without PTSD, with respect to age, gender, socioeconomic status, and marital status.
The researchers found no association between the veterans’ lifetime PTSD and their children’s psychopathology, attachment dimensions, and self-reported overall childhood maltreatment. The fathers’ war experience carried more weight. It seemed, the researchers say, that the children were able to overcome living with a parent’s PTSD symptoms, but they were less resilient when it came to their fathers’ war experience.
Veterans’ war exposure was associated with BSI in the offspring with regard to somatization, phobic anxiety, Global Severity Index, and Positive Symptom Distress Index. It was also associated with offspring’s physical neglect as a childhood adversity.
These findings could have considerable social importance, the researchers say. They suggest that mental health support could benefit the children especially if provided early after highly traumatized veterans return from war, “not just later on—if and when they develop PTSD.”
Tender nodules after leprosy Tx
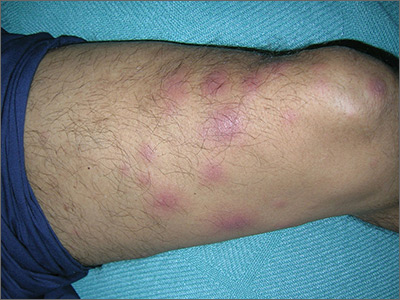
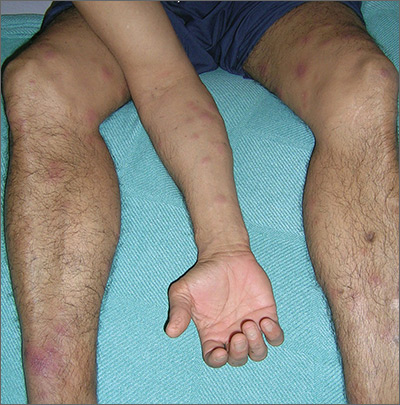
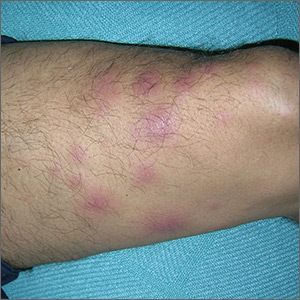
The treating physicians believed that the patient had developed erythema nodosum leprosum (ENL), based on the tender nodules that are characteristic of this treatment reaction. The CDC was again consulted and representatives there agreed.
ENL is a known as a Type 2 reaction to the treatment for leprosy. Management involves the use of oral prednisone, which may be needed for a prolonged time. The antibiotics used to treat leprosy should not be stopped, as ENL is not an allergic reaction. It is due to the destruction of bacilli and the immune response to the release of bacterial antigens.
The patient was transferred to a leprosy hospital in Baton Rouge, Louisiana for further management. The prednisone was not sufficient, and he required further treatment with thalidomide. After 2 years of treatment, he appeared to be free of leprosy and no longer had the ENL.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the third edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/


The treating physicians believed that the patient had developed erythema nodosum leprosum (ENL), based on the tender nodules that are characteristic of this treatment reaction. The CDC was again consulted and representatives there agreed.
ENL is a known as a Type 2 reaction to the treatment for leprosy. Management involves the use of oral prednisone, which may be needed for a prolonged time. The antibiotics used to treat leprosy should not be stopped, as ENL is not an allergic reaction. It is due to the destruction of bacilli and the immune response to the release of bacterial antigens.
The patient was transferred to a leprosy hospital in Baton Rouge, Louisiana for further management. The prednisone was not sufficient, and he required further treatment with thalidomide. After 2 years of treatment, he appeared to be free of leprosy and no longer had the ENL.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the third edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/


The treating physicians believed that the patient had developed erythema nodosum leprosum (ENL), based on the tender nodules that are characteristic of this treatment reaction. The CDC was again consulted and representatives there agreed.
ENL is a known as a Type 2 reaction to the treatment for leprosy. Management involves the use of oral prednisone, which may be needed for a prolonged time. The antibiotics used to treat leprosy should not be stopped, as ENL is not an allergic reaction. It is due to the destruction of bacilli and the immune response to the release of bacterial antigens.
The patient was transferred to a leprosy hospital in Baton Rouge, Louisiana for further management. The prednisone was not sufficient, and he required further treatment with thalidomide. After 2 years of treatment, he appeared to be free of leprosy and no longer had the ENL.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the third edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
An Atypical Problem for Atopical People
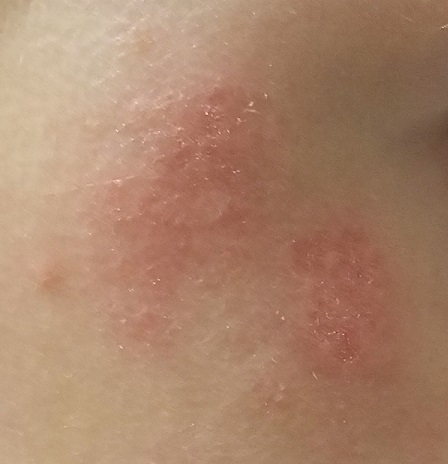
At age 1, a girl developed a blistery rash on the left side of her face. It was soon followed by a low-grade fever and modest malaise. All symptoms cleared within 2 weeks. Now, at age 4, she continues to experience similar, periodic outbreaks in the same location.
She has already been seen by various providers, including a dermatologist, and received several different diagnoses. The dermatologist scraped the rash and determined it to be a fungal infection. However, the recommended topical antifungal cream had no effect. At least 3 other providers (all nondermatology) called it cellulitis and treated with oral antibiotics, but these attempts also failed.
EXAMINATION
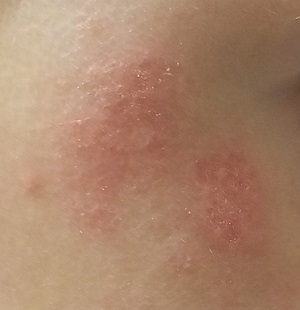
There are no active lesions at the time of this initial examination and no palpable adenopathy in the region. There is a large area of erythema in a macular pattern over the right cheek. No scarring is visible.
The patient later returns when a new outbreak occurs. This time, there are distinct blisters and reactive adenopathy in the adjacent nodal areas.
What’s the diagnosis?
DISCUSSION
Results of a viral culture indicate herpes simplex.
The recurrence of persistent, vesicular rashes in the same location signifies a herpetic nature. Herpes simplex virus (HSV) is easier to diagnose in an adult patient, due to the ability to elicit a reliable history of premonitory symptoms. Small children have difficulty verbalizing the distinction between a tingle, an itch, and mild pain, which herald the onset of an HSV outbreak.
An episode of HSV can be triggered by anything that raises the body temperature (eg, stress, sickness, or sun exposure). Also important to note, these kinds of outbreaks can occur almost anywhere on the body, including ears, fingers, nipples, noses, and eyelids.
In my experience, most patients with longstanding herpes outbreaks are atopic (ie, allergy prone) or come from families in which atopy is common. Atopic patients are well known to be susceptible to all manner of skin infections, but most especially to herpes. It’s as if their immune systems overreact to pollen, mold, dust, and other allergens, while viral, fungal, and bacterial antigens fly under their immune radar.
In this case, the child was treated with valacyclovir on a chronic, as opposed to episodic, basis. With a bit of luck, this treatment will help to diminish HSV attacks as she matures.
TAKE-HOME LEARNING POINTS
- Anything that raises body temperature (sun, colds, or even stress) can trigger an episode of herpes simplex virus (HSV).
- Vesicular rashes that recur in the same location should be presumed herpetic, until proven otherwise. Usually, viral cultures aren’t necessary since the differential is so narrow.
- Atopy can predispose one to all manner of skin infections, including viral, fungal, and bacterial.
- Treatment of chronic HSV can be episodic or preventive, depending on the frequency and severity of attacks.
At age 1, a girl developed a blistery rash on the left side of her face. It was soon followed by a low-grade fever and modest malaise. All symptoms cleared within 2 weeks. Now, at age 4, she continues to experience similar, periodic outbreaks in the same location.
She has already been seen by various providers, including a dermatologist, and received several different diagnoses. The dermatologist scraped the rash and determined it to be a fungal infection. However, the recommended topical antifungal cream had no effect. At least 3 other providers (all nondermatology) called it cellulitis and treated with oral antibiotics, but these attempts also failed.

EXAMINATION
There are no active lesions at the time of this initial examination and no palpable adenopathy in the region. There is a large area of erythema in a macular pattern over the right cheek. No scarring is visible.
The patient later returns when a new outbreak occurs. This time, there are distinct blisters and reactive adenopathy in the adjacent nodal areas.
What’s the diagnosis?
DISCUSSION
Results of a viral culture indicate herpes simplex.
The recurrence of persistent, vesicular rashes in the same location signifies a herpetic nature. Herpes simplex virus (HSV) is easier to diagnose in an adult patient, due to the ability to elicit a reliable history of premonitory symptoms. Small children have difficulty verbalizing the distinction between a tingle, an itch, and mild pain, which herald the onset of an HSV outbreak.
An episode of HSV can be triggered by anything that raises the body temperature (eg, stress, sickness, or sun exposure). Also important to note, these kinds of outbreaks can occur almost anywhere on the body, including ears, fingers, nipples, noses, and eyelids.
In my experience, most patients with longstanding herpes outbreaks are atopic (ie, allergy prone) or come from families in which atopy is common. Atopic patients are well known to be susceptible to all manner of skin infections, but most especially to herpes. It’s as if their immune systems overreact to pollen, mold, dust, and other allergens, while viral, fungal, and bacterial antigens fly under their immune radar.
In this case, the child was treated with valacyclovir on a chronic, as opposed to episodic, basis. With a bit of luck, this treatment will help to diminish HSV attacks as she matures.
TAKE-HOME LEARNING POINTS
- Anything that raises body temperature (sun, colds, or even stress) can trigger an episode of herpes simplex virus (HSV).
- Vesicular rashes that recur in the same location should be presumed herpetic, until proven otherwise. Usually, viral cultures aren’t necessary since the differential is so narrow.
- Atopy can predispose one to all manner of skin infections, including viral, fungal, and bacterial.
- Treatment of chronic HSV can be episodic or preventive, depending on the frequency and severity of attacks.
At age 1, a girl developed a blistery rash on the left side of her face. It was soon followed by a low-grade fever and modest malaise. All symptoms cleared within 2 weeks. Now, at age 4, she continues to experience similar, periodic outbreaks in the same location.
She has already been seen by various providers, including a dermatologist, and received several different diagnoses. The dermatologist scraped the rash and determined it to be a fungal infection. However, the recommended topical antifungal cream had no effect. At least 3 other providers (all nondermatology) called it cellulitis and treated with oral antibiotics, but these attempts also failed.

EXAMINATION
There are no active lesions at the time of this initial examination and no palpable adenopathy in the region. There is a large area of erythema in a macular pattern over the right cheek. No scarring is visible.
The patient later returns when a new outbreak occurs. This time, there are distinct blisters and reactive adenopathy in the adjacent nodal areas.
What’s the diagnosis?
DISCUSSION
Results of a viral culture indicate herpes simplex.
The recurrence of persistent, vesicular rashes in the same location signifies a herpetic nature. Herpes simplex virus (HSV) is easier to diagnose in an adult patient, due to the ability to elicit a reliable history of premonitory symptoms. Small children have difficulty verbalizing the distinction between a tingle, an itch, and mild pain, which herald the onset of an HSV outbreak.
An episode of HSV can be triggered by anything that raises the body temperature (eg, stress, sickness, or sun exposure). Also important to note, these kinds of outbreaks can occur almost anywhere on the body, including ears, fingers, nipples, noses, and eyelids.
In my experience, most patients with longstanding herpes outbreaks are atopic (ie, allergy prone) or come from families in which atopy is common. Atopic patients are well known to be susceptible to all manner of skin infections, but most especially to herpes. It’s as if their immune systems overreact to pollen, mold, dust, and other allergens, while viral, fungal, and bacterial antigens fly under their immune radar.
In this case, the child was treated with valacyclovir on a chronic, as opposed to episodic, basis. With a bit of luck, this treatment will help to diminish HSV attacks as she matures.
TAKE-HOME LEARNING POINTS
- Anything that raises body temperature (sun, colds, or even stress) can trigger an episode of herpes simplex virus (HSV).
- Vesicular rashes that recur in the same location should be presumed herpetic, until proven otherwise. Usually, viral cultures aren’t necessary since the differential is so narrow.
- Atopy can predispose one to all manner of skin infections, including viral, fungal, and bacterial.
- Treatment of chronic HSV can be episodic or preventive, depending on the frequency and severity of attacks.
MenB vaccination coverage higher in those receiving MenB-4C
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
FROM VACCINE
FDA approves istradefylline for Parkinson’s disease
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
Psoriasis Journal Scan: August 2019
Verrucous psoriasis: A rare variant of psoriasis masquerading as verrucous carcinoma.
Garvie K, McGinley Simpson M, Logemann N, Lackey J. JAAD Case Rep. 2019 Aug 5;5(8):723-725.
Verrucous psoriasis is a rare variant of psoriasis characterized by hyperkeratotic, papillomatous plaques that clinically resemble verrucous carcinoma in lesion appearance and distribution. It is amenable to medical treatments. Conversely, verrucous carcinoma, a rare subtype of well-differentiated squamous cell carcinoma, is treated with surgical excision. Histologically, they may be difficult to differentiate. This case report presents a patient with verrucous psoriasis of the heal that was initially diagnosed as verrucous carcinoma and excised.
Re-Categorization of Psoriasis Severity: Delphi Consensus from the International Psoriasis Council.
Strober B, Ryan C, van de Kerkhof P, et al. J Am Acad Dermatol. 2019 Aug 16.
This consensus statement on the classification of psoriasis severity preferentially ranked seven severity definitions. This most preferred statement rejects the mild, moderate and severe categories in favor of a dichotomous definition: Psoriasis patients should be classified as either candidates for topical therapy or candidates for systemic therapy; the latter are patients who meet at least one of the following criteria: 1) BSA > 10%, 2) Disease involving special areas, 3) Failure of topical therapy.
Gluten intake and risk of psoriasis, psoriatic arthritis and atopic dermatitis among US women.
Drucker AM, Qureshi AA, Thompson JM, Li T, Cho E. J Am Acad Dermatol. 2019 Aug 9.
Associations between gluten intake and psoriasis, psoriatic arthritis and atopic dermatitis are poorly understood. Gluten content of participants' diet was calculated every four years using food frequency questionnaires. Disease outcomes were assessed by self-report and subsequently validated.
Psoriasis and Mortality in the US: Data from the National Health and Nutrition Examination Survey.
Semenov YR, Herbosa CM, Rogers AT, et al. J Am Acad Dermatol. 2019 Aug 12.
In this retrospective population-based cohort study of adults and adolescents > 10 years (n=13,031) who participated in National Health and Nutrition Examination Surveys (2003-2006; 2009-2010), psoriasis was present in 2.7% of the study population. Over an average 52.3 months median follow-up, psoriasis was significantly associated with increased mortality risk. This relationship is partially mediated by an increased prevalence of cardiovascular, infectious, and neoplastic disorders seen among psoriatics.
Ostraceous Psoriasis Presenting as Koebner Phenomenon in a Tattoo.
Reinhart J, Willett M, Gibbs N. J Drugs Dermatol. 2019 Aug 1;18(8):825-826.
Psoriasis ostracea is defined as having pronounced adherent scales resembling an oyster shell. Many ostraceous cases occur as generalized outbreaks in patients with long-standing history of psoriasis. Rarely does this variant occur as a direct flare from a cutaneous insult. In these situations, when a pre-existing dermatosis appears in response to a traumatic insult to skin, the process is referred to as the Koebner phenomenon. In addition to lichen planus and vitiligo, psoriasis is a commonly known condition that can present as a Koebner reaction. In this atypical case, the authors present a 21-year-old male with remarkable ostraceous psoriatic lesions precipitated by an upper arm tattoo, demonstrating the Koebner phenomenon.
Verrucous psoriasis: A rare variant of psoriasis masquerading as verrucous carcinoma.
Garvie K, McGinley Simpson M, Logemann N, Lackey J. JAAD Case Rep. 2019 Aug 5;5(8):723-725.
Verrucous psoriasis is a rare variant of psoriasis characterized by hyperkeratotic, papillomatous plaques that clinically resemble verrucous carcinoma in lesion appearance and distribution. It is amenable to medical treatments. Conversely, verrucous carcinoma, a rare subtype of well-differentiated squamous cell carcinoma, is treated with surgical excision. Histologically, they may be difficult to differentiate. This case report presents a patient with verrucous psoriasis of the heal that was initially diagnosed as verrucous carcinoma and excised.
Re-Categorization of Psoriasis Severity: Delphi Consensus from the International Psoriasis Council.
Strober B, Ryan C, van de Kerkhof P, et al. J Am Acad Dermatol. 2019 Aug 16.
This consensus statement on the classification of psoriasis severity preferentially ranked seven severity definitions. This most preferred statement rejects the mild, moderate and severe categories in favor of a dichotomous definition: Psoriasis patients should be classified as either candidates for topical therapy or candidates for systemic therapy; the latter are patients who meet at least one of the following criteria: 1) BSA > 10%, 2) Disease involving special areas, 3) Failure of topical therapy.
Gluten intake and risk of psoriasis, psoriatic arthritis and atopic dermatitis among US women.
Drucker AM, Qureshi AA, Thompson JM, Li T, Cho E. J Am Acad Dermatol. 2019 Aug 9.
Associations between gluten intake and psoriasis, psoriatic arthritis and atopic dermatitis are poorly understood. Gluten content of participants' diet was calculated every four years using food frequency questionnaires. Disease outcomes were assessed by self-report and subsequently validated.
Psoriasis and Mortality in the US: Data from the National Health and Nutrition Examination Survey.
Semenov YR, Herbosa CM, Rogers AT, et al. J Am Acad Dermatol. 2019 Aug 12.
In this retrospective population-based cohort study of adults and adolescents > 10 years (n=13,031) who participated in National Health and Nutrition Examination Surveys (2003-2006; 2009-2010), psoriasis was present in 2.7% of the study population. Over an average 52.3 months median follow-up, psoriasis was significantly associated with increased mortality risk. This relationship is partially mediated by an increased prevalence of cardiovascular, infectious, and neoplastic disorders seen among psoriatics.
Ostraceous Psoriasis Presenting as Koebner Phenomenon in a Tattoo.
Reinhart J, Willett M, Gibbs N. J Drugs Dermatol. 2019 Aug 1;18(8):825-826.
Psoriasis ostracea is defined as having pronounced adherent scales resembling an oyster shell. Many ostraceous cases occur as generalized outbreaks in patients with long-standing history of psoriasis. Rarely does this variant occur as a direct flare from a cutaneous insult. In these situations, when a pre-existing dermatosis appears in response to a traumatic insult to skin, the process is referred to as the Koebner phenomenon. In addition to lichen planus and vitiligo, psoriasis is a commonly known condition that can present as a Koebner reaction. In this atypical case, the authors present a 21-year-old male with remarkable ostraceous psoriatic lesions precipitated by an upper arm tattoo, demonstrating the Koebner phenomenon.
Verrucous psoriasis: A rare variant of psoriasis masquerading as verrucous carcinoma.
Garvie K, McGinley Simpson M, Logemann N, Lackey J. JAAD Case Rep. 2019 Aug 5;5(8):723-725.
Verrucous psoriasis is a rare variant of psoriasis characterized by hyperkeratotic, papillomatous plaques that clinically resemble verrucous carcinoma in lesion appearance and distribution. It is amenable to medical treatments. Conversely, verrucous carcinoma, a rare subtype of well-differentiated squamous cell carcinoma, is treated with surgical excision. Histologically, they may be difficult to differentiate. This case report presents a patient with verrucous psoriasis of the heal that was initially diagnosed as verrucous carcinoma and excised.
Re-Categorization of Psoriasis Severity: Delphi Consensus from the International Psoriasis Council.
Strober B, Ryan C, van de Kerkhof P, et al. J Am Acad Dermatol. 2019 Aug 16.
This consensus statement on the classification of psoriasis severity preferentially ranked seven severity definitions. This most preferred statement rejects the mild, moderate and severe categories in favor of a dichotomous definition: Psoriasis patients should be classified as either candidates for topical therapy or candidates for systemic therapy; the latter are patients who meet at least one of the following criteria: 1) BSA > 10%, 2) Disease involving special areas, 3) Failure of topical therapy.
Gluten intake and risk of psoriasis, psoriatic arthritis and atopic dermatitis among US women.
Drucker AM, Qureshi AA, Thompson JM, Li T, Cho E. J Am Acad Dermatol. 2019 Aug 9.
Associations between gluten intake and psoriasis, psoriatic arthritis and atopic dermatitis are poorly understood. Gluten content of participants' diet was calculated every four years using food frequency questionnaires. Disease outcomes were assessed by self-report and subsequently validated.
Psoriasis and Mortality in the US: Data from the National Health and Nutrition Examination Survey.
Semenov YR, Herbosa CM, Rogers AT, et al. J Am Acad Dermatol. 2019 Aug 12.
In this retrospective population-based cohort study of adults and adolescents > 10 years (n=13,031) who participated in National Health and Nutrition Examination Surveys (2003-2006; 2009-2010), psoriasis was present in 2.7% of the study population. Over an average 52.3 months median follow-up, psoriasis was significantly associated with increased mortality risk. This relationship is partially mediated by an increased prevalence of cardiovascular, infectious, and neoplastic disorders seen among psoriatics.
Ostraceous Psoriasis Presenting as Koebner Phenomenon in a Tattoo.
Reinhart J, Willett M, Gibbs N. J Drugs Dermatol. 2019 Aug 1;18(8):825-826.
Psoriasis ostracea is defined as having pronounced adherent scales resembling an oyster shell. Many ostraceous cases occur as generalized outbreaks in patients with long-standing history of psoriasis. Rarely does this variant occur as a direct flare from a cutaneous insult. In these situations, when a pre-existing dermatosis appears in response to a traumatic insult to skin, the process is referred to as the Koebner phenomenon. In addition to lichen planus and vitiligo, psoriasis is a commonly known condition that can present as a Koebner reaction. In this atypical case, the authors present a 21-year-old male with remarkable ostraceous psoriatic lesions precipitated by an upper arm tattoo, demonstrating the Koebner phenomenon.
AAN guideline encourages vaccinations for MS patients
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
according to an American Academy of Neurology practice guideline.
A summary of the guideline on vaccine-preventable infections and immunization in MS was published online Aug. 28 in Neurology. The new effort updates a 2002 guideline on this topic and incorporates new evidence, vaccines, and disease-modifying therapies (DMTs). The guideline was endorsed by the Consortium of Multiple Sclerosis Centers and by the Multiple Sclerosis Association of America.
To create the guideline, lead author Mauricio F. Farez, MD, of the Raúl Carrea Institute for Neurological Research (FLENI) in Buenos Aires and colleagues on the 17-member guideline panel performed a systematic review of the evidence and reached consensus on recommendations using a modified Delphi voting process. The review included randomized, controlled trials; cohort studies; and case-control studies published between 1990 and March 2018.
“Immunosuppressive or immunomodulating agents used to treat MS may suppress or modulate normal immune function. These drugs may increase susceptibility to infections and may reduce vaccine effectiveness because of a decreased ability to mount an immune response,” the authors said.
Based on its review of the evidence, principles of care, and inferences, the authors made the following eight recommendations:
- Clinicians should discuss with patients the evidence regarding immunization in MS (Level B). In addition, clinicians should examine patients’ opinions, preferences, and questions regarding immunizations (Level B).
- Clinicians should recommend that patients with MS follow all local vaccine standards in the absence of specific contraindications (Level B).
- Clinicians should consider local risks of vaccine-preventable diseases when counseling patients (Level B).
- Clinicians should recommend that patients with MS receive the influenza vaccination if there is no specific contraindication, such as a previous severe reaction (Level B).
- When treatment with an immunosuppressive or immunomodulating agent is considered, clinicians should counsel patients about infection risks associated with the specific medication and the treatment-specific vaccination guidance in the medication’s prescribing instructions (Level B). In addition, physicians should assess patients’ vaccination status before prescribing immunosuppressive or immunomodulating therapy and vaccinate patients according to local regulatory standards and treatment-specific infectious risks at least 4-6 weeks before initiating therapy, as advised by the prescribing information (Level B). Furthermore, clinicians may discuss the advantages of vaccination soon after MS diagnosis, regardless of initial therapeutic plans, to prevent delays should immunosuppressive or immunomodulating therapies be initiated in the future (Level C, based on variation in patient preferences).
- Clinicians must screen for certain infections (such as hepatitis, tuberculosis, and varicella zoster virus) according to a medication’s prescribing information before starting immunosuppressive or immunomodulating treatment (Level A) and should treat patients who have latent infections before MS treatment according to the medication prescribing information (Level B, based on feasibility and cost relative to benefit). Further, in high-risk populations or in countries with a high burden of infectious disease, clinicians must screen for latent infections before starting immunosuppressive or immunomodulating medications, even when such screening is not specifically mentioned in the prescribing information (Level A). Clinicians should consult infectious disease or other specialists about treating patients with latent infection before starting immunosuppressive or immunomodulating medications (Level B).
- Clinicians should recommend against live-attenuated vaccines in people with MS who receive immunosuppressive or immunomodulating therapies or have recently discontinued these therapies (Level B, based on importance of outcomes). When the risk of infection is high, clinicians may recommend live-attenuated vaccines if killed vaccines are unavailable (Level C, based on variation in patient preferences, benefit relative to harm, and importance of outcomes).
- If a patient with MS is experiencing a relapse, clinicians should delay vaccination until the relapse has clinically resolved or is no longer active, often many weeks after relapse onset (Level B).
Personal and population-level benefits
“There is no evidence that vaccination increases the risk of MS exacerbation, although the literature is sparse,” the authors said. “In addition to conferring personal benefits, vaccination of the MS patient population contributes to the well-established phenomenon of herd immunity for the communities in which patients with MS live,” the authors wrote.
Because influenza infection has known risks of exacerbation and morbidity, whereas influenza vaccine has no identified risks of exacerbation, “benefits of influenza vaccination outweigh the risks in most scenarios, although patients with MS receiving some [immunosuppressive or immunomodulating] treatments (fingolimod [Gilenya], glatiramer acetate [Copaxone], and mitoxantrone) may have a reduced response to influenza vaccination,” the authors said. Studies in patients with diseases other than MS suggest that rituximab (Rituxan) also may be associated with reduced influenza vaccine responsiveness.
Immunosuppressive or immunomodulatory medications including alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod, mitoxantrone, natalizumab (Tysabri), ocrelizumab (Ocrevus), rituximab, and teriflunomide (Aubagio) have been associated with severe occurrences or recurrences of vaccine-preventable infections, and many package inserts approved by the Food and Drug Administration provide guidance regarding immunization with live vaccines and treatment.
Prescribing information for alemtuzumab, fingolimod, ocrelizumab, and teriflunomide recommends against the use of live vaccines during and immediately preceding treatment. Furthermore, the prescribing information recommends waiting 2-6 months after treatment to immunize with live vaccines, depending on the half-life of the specific therapy.
“The guideline panel identified no evidence that vaccines increase the risk of relapse or worsen relapse severity, but studies are limited,” Dr. Farez and colleagues wrote. “Experts remain concerned that vaccines may worsen relapse severity if given to patients who are actively experiencing an MS relapse.” In addition, use of glucocorticoids may raise concerns about the safety of live-virus vaccines. “Immunization is not typically an urgent need and, in most cases, can be temporarily delayed without a marked increase in infection risk,” the guideline says.
Few high-quality studies
Data were lacking or insufficient to assess whether most vaccine-preventable diseases increase the risk of MS exacerbations. “It is probable that individuals with active MS exacerbations have higher odds of varicella zoster virus viral DNA present in peripheral blood mononuclear cells than individuals with MS in remission,” the guideline says.
Human papillomavirus, pertussis, and tetanus toxoid vaccinations probably are associated with a lower likelihood of a subsequent MS diagnosis, and smallpox vaccination is possibly associated with a lower likelihood of a subsequent MS diagnosis, the review found.
Studies included in the systematic review did not address whether live-attenuated vaccines are as effective in patients with MS as they are in the general population. With regard to the effectiveness of inactivated vaccines, patients with MS possibly are less likely to have a sufficient response to influenza vaccination, compared with controls.
The systematic review “found few high-quality studies to inform recommendations,” the authors said. “As more [immunosuppressive or immunomodulating] agents are developed to manage chronic diseases such as MS, long-term prospective cohort studies are required to evaluate both the safety and effectiveness of immunizations in MS.”
Dr. Farez has received funding for travel from Teva Argentina, Novartis Argentina, and Merck Serono Argentina and has received research support from Biogen. Coauthors’ disclosures included financial ties to pharmaceutical companies.
SOURCE: Farez M et al. Neurology. 2019 Aug 28. doi: 10.1212/WNL.0000000000008157.
FROM NEUROLOGY
Nivolumab falls short in ccRCC patients with brain metastases
Nivolumab has “limited intracranial activity” in patients with clear cell renal cell carcinoma (ccRCC) and previously untreated brain metastases, according to researchers.
In a phase 2 trial, nivolumab produced an intracranial response rate of 12% in ccRCC patients with previously untreated brain metastases.
The median intracranial progression-free survival (PFS) was longer among patients who had received prior focal therapy than among those with previously untreated brain metastases.
These results suggest “brain imaging and focal therapy should be considered before immune checkpoint inhibitors in patients with metastatic ccRCC,” Ronan Flippot, MD, of Université Paris-Saclay in Villejuif, France, and colleagues wrote in the Journal of Clinical Oncology.
Dr. Flippot and colleagues conducted this analysis of patients from the phase 2 GETUG-AFU 26 NIVOREN trial (NCT03013335). The researchers looked at 73 ccRCC patients with asymptomatic brain metastases who had received at least one prior line of antiangiogenic treatment.
Patients were divided into two cohorts. Cohort A included patients with previously untreated brain metastases (n = 39), and cohort B included patients who had received focal therapy for brain metastases (n = 34).
Baseline characteristics were similar between the cohorts. The median ages were 61 years in cohort A (range, 39-77) and 58 years in cohort B (range, 33-78). Most patients had grade 3-4 tumors (64% in cohort A and 78% in cohort B), and most had one brain lesion (67% and 59%, respectively). The median sum of the diameters of target lesions was 11 mm in cohort A and 17 mm in cohort B.
All patients received intravenous nivolumab at 3 mg/kg every 2 weeks until they progressed, developed unacceptable toxicity, died, withdrew consent, or the investigator stopped treatment.
The median follow-up was 23.6 months in cohort A and 20.2 months in cohort B. The median duration of treatment was 4.9 months and 4.5 months, respectively. Five patients in cohort A and four in cohort B were still receiving nivolumab at the data cutoff.
Response
The primary endpoint was the intracranial response rate in cohort A, which was 12%. All four responders achieved a complete response. At baseline, all of them had grade 1-2 disease and a single brain lesion smaller than 1 cm.
Thirteen patients (38%) in cohort A had stable intracranial disease as their best response, and 17 (50%) had progressive intracranial disease. The remaining five patients could not be evaluated because they progressed and died before the first evaluation.
The extracranial response rate in cohort A was 21%, and all seven responders had partial responses. Ten patients had stable extracranial disease (30%), and 16 had extracranial progression (49%). The remaining six patients were not evaluable for extracranial response.
All four patients who achieved a complete intracranial response had a partial extracranial response. Six patients (18%) had discordant intracranial and extracranial responses.
Survival
The median intracranial PFS in cohort A was 2.7 months in cohort A versus 4.8 months in cohort B. When the researchers adjusted for baseline characteristics, they found that prior focal therapy decreased the risk of intracranial progression (hazard ratio, 0.49).
The median extracranial PFS was 2.8 months in cohort A versus 2.6 months in cohort B. The median global PFS was 2.4 months in cohort A versus 2.5 months in cohort B.
The overall survival rates at 12 months were 66.7% in cohort A and 58.8% in cohort B.
Safety
The most common treatment-related adverse events (in cohort A and B, respectively) were asthenia (21% and 24%) and rash (10% and 9%).
Grade 3/4 treatment-related adverse events occurred in four patients in cohort A and five in cohort B. In cohort A, these events were asthenia, elevated liver function tests, dyspnea, and atrioventricular block. In cohort B, the events were diarrhea, musculoskeletal pain, psoriasis, hypophosphatemia, and elevated creatinine (in two patients).
The patient who developed atrioventricular block permanently discontinued nivolumab. There were no other treatment-related adverse events that led to discontinuation.
This study was supported by Bristol-Myers Squibb. The researchers disclosed relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Flippot R et al. J Clin Oncol. 2019 Aug 10;37(23):2008-16.
Trial results have raised more questions about the efficacy of immune checkpoint inhibitors in patients with clear cell renal cell carcinoma (ccRCC) and brain metastases, according to Jarred P. Reed, MD, and colleagues.
In a phase 2 trial, half of ccRCC patients with previously untreated brain metastases had intracranial progression while receiving treatment with nivolumab. The risk of intracranial progression was higher among patients with previously untreated brain metastases than among those who had received prior focal therapy. However, global progression-free survival rates were similarly short in patients with previously treated and untreated brain metastases.
It isn’t clear why nivolumab exhibited such “disappointing activity” in this trial, Dr. Reed and colleagues wrote in an editorial, but the authors presented several possibilities.
The study’s results may be explained by “unique biologic mechanisms” associated with brain metastasis in ccRCC. The brain microenvironment may weaken the response to nivolumab, or patients with brain metastases may have tumor cells better able to evade the immune system. These patients may also have specific alterations in signaling pathways that are important for cell survival.
To gain more insight, future immunotherapy trials should include patients with brain metastases, Dr. Reed and coauthors wrote.
Jarred P. Reed, MD, and coauthors Edwin M. Posadas, MD, and Robert A. Figlin, MD, are from Cedars-Sinai Medical Center in Los Angeles. The authors disclosed relationships with Bristol-Myers Squibb, which markets nivolumab, and many other companies. These comments are adapted from their editorial (J Clin Oncol. 2019 Aug 10;37[23]:1987-9).
Trial results have raised more questions about the efficacy of immune checkpoint inhibitors in patients with clear cell renal cell carcinoma (ccRCC) and brain metastases, according to Jarred P. Reed, MD, and colleagues.
In a phase 2 trial, half of ccRCC patients with previously untreated brain metastases had intracranial progression while receiving treatment with nivolumab. The risk of intracranial progression was higher among patients with previously untreated brain metastases than among those who had received prior focal therapy. However, global progression-free survival rates were similarly short in patients with previously treated and untreated brain metastases.
It isn’t clear why nivolumab exhibited such “disappointing activity” in this trial, Dr. Reed and colleagues wrote in an editorial, but the authors presented several possibilities.
The study’s results may be explained by “unique biologic mechanisms” associated with brain metastasis in ccRCC. The brain microenvironment may weaken the response to nivolumab, or patients with brain metastases may have tumor cells better able to evade the immune system. These patients may also have specific alterations in signaling pathways that are important for cell survival.
To gain more insight, future immunotherapy trials should include patients with brain metastases, Dr. Reed and coauthors wrote.
Jarred P. Reed, MD, and coauthors Edwin M. Posadas, MD, and Robert A. Figlin, MD, are from Cedars-Sinai Medical Center in Los Angeles. The authors disclosed relationships with Bristol-Myers Squibb, which markets nivolumab, and many other companies. These comments are adapted from their editorial (J Clin Oncol. 2019 Aug 10;37[23]:1987-9).
Trial results have raised more questions about the efficacy of immune checkpoint inhibitors in patients with clear cell renal cell carcinoma (ccRCC) and brain metastases, according to Jarred P. Reed, MD, and colleagues.
In a phase 2 trial, half of ccRCC patients with previously untreated brain metastases had intracranial progression while receiving treatment with nivolumab. The risk of intracranial progression was higher among patients with previously untreated brain metastases than among those who had received prior focal therapy. However, global progression-free survival rates were similarly short in patients with previously treated and untreated brain metastases.
It isn’t clear why nivolumab exhibited such “disappointing activity” in this trial, Dr. Reed and colleagues wrote in an editorial, but the authors presented several possibilities.
The study’s results may be explained by “unique biologic mechanisms” associated with brain metastasis in ccRCC. The brain microenvironment may weaken the response to nivolumab, or patients with brain metastases may have tumor cells better able to evade the immune system. These patients may also have specific alterations in signaling pathways that are important for cell survival.
To gain more insight, future immunotherapy trials should include patients with brain metastases, Dr. Reed and coauthors wrote.
Jarred P. Reed, MD, and coauthors Edwin M. Posadas, MD, and Robert A. Figlin, MD, are from Cedars-Sinai Medical Center in Los Angeles. The authors disclosed relationships with Bristol-Myers Squibb, which markets nivolumab, and many other companies. These comments are adapted from their editorial (J Clin Oncol. 2019 Aug 10;37[23]:1987-9).
Nivolumab has “limited intracranial activity” in patients with clear cell renal cell carcinoma (ccRCC) and previously untreated brain metastases, according to researchers.
In a phase 2 trial, nivolumab produced an intracranial response rate of 12% in ccRCC patients with previously untreated brain metastases.
The median intracranial progression-free survival (PFS) was longer among patients who had received prior focal therapy than among those with previously untreated brain metastases.
These results suggest “brain imaging and focal therapy should be considered before immune checkpoint inhibitors in patients with metastatic ccRCC,” Ronan Flippot, MD, of Université Paris-Saclay in Villejuif, France, and colleagues wrote in the Journal of Clinical Oncology.
Dr. Flippot and colleagues conducted this analysis of patients from the phase 2 GETUG-AFU 26 NIVOREN trial (NCT03013335). The researchers looked at 73 ccRCC patients with asymptomatic brain metastases who had received at least one prior line of antiangiogenic treatment.
Patients were divided into two cohorts. Cohort A included patients with previously untreated brain metastases (n = 39), and cohort B included patients who had received focal therapy for brain metastases (n = 34).
Baseline characteristics were similar between the cohorts. The median ages were 61 years in cohort A (range, 39-77) and 58 years in cohort B (range, 33-78). Most patients had grade 3-4 tumors (64% in cohort A and 78% in cohort B), and most had one brain lesion (67% and 59%, respectively). The median sum of the diameters of target lesions was 11 mm in cohort A and 17 mm in cohort B.
All patients received intravenous nivolumab at 3 mg/kg every 2 weeks until they progressed, developed unacceptable toxicity, died, withdrew consent, or the investigator stopped treatment.
The median follow-up was 23.6 months in cohort A and 20.2 months in cohort B. The median duration of treatment was 4.9 months and 4.5 months, respectively. Five patients in cohort A and four in cohort B were still receiving nivolumab at the data cutoff.
Response
The primary endpoint was the intracranial response rate in cohort A, which was 12%. All four responders achieved a complete response. At baseline, all of them had grade 1-2 disease and a single brain lesion smaller than 1 cm.
Thirteen patients (38%) in cohort A had stable intracranial disease as their best response, and 17 (50%) had progressive intracranial disease. The remaining five patients could not be evaluated because they progressed and died before the first evaluation.
The extracranial response rate in cohort A was 21%, and all seven responders had partial responses. Ten patients had stable extracranial disease (30%), and 16 had extracranial progression (49%). The remaining six patients were not evaluable for extracranial response.
All four patients who achieved a complete intracranial response had a partial extracranial response. Six patients (18%) had discordant intracranial and extracranial responses.
Survival
The median intracranial PFS in cohort A was 2.7 months in cohort A versus 4.8 months in cohort B. When the researchers adjusted for baseline characteristics, they found that prior focal therapy decreased the risk of intracranial progression (hazard ratio, 0.49).
The median extracranial PFS was 2.8 months in cohort A versus 2.6 months in cohort B. The median global PFS was 2.4 months in cohort A versus 2.5 months in cohort B.
The overall survival rates at 12 months were 66.7% in cohort A and 58.8% in cohort B.
Safety
The most common treatment-related adverse events (in cohort A and B, respectively) were asthenia (21% and 24%) and rash (10% and 9%).
Grade 3/4 treatment-related adverse events occurred in four patients in cohort A and five in cohort B. In cohort A, these events were asthenia, elevated liver function tests, dyspnea, and atrioventricular block. In cohort B, the events were diarrhea, musculoskeletal pain, psoriasis, hypophosphatemia, and elevated creatinine (in two patients).
The patient who developed atrioventricular block permanently discontinued nivolumab. There were no other treatment-related adverse events that led to discontinuation.
This study was supported by Bristol-Myers Squibb. The researchers disclosed relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Flippot R et al. J Clin Oncol. 2019 Aug 10;37(23):2008-16.
Nivolumab has “limited intracranial activity” in patients with clear cell renal cell carcinoma (ccRCC) and previously untreated brain metastases, according to researchers.
In a phase 2 trial, nivolumab produced an intracranial response rate of 12% in ccRCC patients with previously untreated brain metastases.
The median intracranial progression-free survival (PFS) was longer among patients who had received prior focal therapy than among those with previously untreated brain metastases.
These results suggest “brain imaging and focal therapy should be considered before immune checkpoint inhibitors in patients with metastatic ccRCC,” Ronan Flippot, MD, of Université Paris-Saclay in Villejuif, France, and colleagues wrote in the Journal of Clinical Oncology.
Dr. Flippot and colleagues conducted this analysis of patients from the phase 2 GETUG-AFU 26 NIVOREN trial (NCT03013335). The researchers looked at 73 ccRCC patients with asymptomatic brain metastases who had received at least one prior line of antiangiogenic treatment.
Patients were divided into two cohorts. Cohort A included patients with previously untreated brain metastases (n = 39), and cohort B included patients who had received focal therapy for brain metastases (n = 34).
Baseline characteristics were similar between the cohorts. The median ages were 61 years in cohort A (range, 39-77) and 58 years in cohort B (range, 33-78). Most patients had grade 3-4 tumors (64% in cohort A and 78% in cohort B), and most had one brain lesion (67% and 59%, respectively). The median sum of the diameters of target lesions was 11 mm in cohort A and 17 mm in cohort B.
All patients received intravenous nivolumab at 3 mg/kg every 2 weeks until they progressed, developed unacceptable toxicity, died, withdrew consent, or the investigator stopped treatment.
The median follow-up was 23.6 months in cohort A and 20.2 months in cohort B. The median duration of treatment was 4.9 months and 4.5 months, respectively. Five patients in cohort A and four in cohort B were still receiving nivolumab at the data cutoff.
Response
The primary endpoint was the intracranial response rate in cohort A, which was 12%. All four responders achieved a complete response. At baseline, all of them had grade 1-2 disease and a single brain lesion smaller than 1 cm.
Thirteen patients (38%) in cohort A had stable intracranial disease as their best response, and 17 (50%) had progressive intracranial disease. The remaining five patients could not be evaluated because they progressed and died before the first evaluation.
The extracranial response rate in cohort A was 21%, and all seven responders had partial responses. Ten patients had stable extracranial disease (30%), and 16 had extracranial progression (49%). The remaining six patients were not evaluable for extracranial response.
All four patients who achieved a complete intracranial response had a partial extracranial response. Six patients (18%) had discordant intracranial and extracranial responses.
Survival
The median intracranial PFS in cohort A was 2.7 months in cohort A versus 4.8 months in cohort B. When the researchers adjusted for baseline characteristics, they found that prior focal therapy decreased the risk of intracranial progression (hazard ratio, 0.49).
The median extracranial PFS was 2.8 months in cohort A versus 2.6 months in cohort B. The median global PFS was 2.4 months in cohort A versus 2.5 months in cohort B.
The overall survival rates at 12 months were 66.7% in cohort A and 58.8% in cohort B.
Safety
The most common treatment-related adverse events (in cohort A and B, respectively) were asthenia (21% and 24%) and rash (10% and 9%).
Grade 3/4 treatment-related adverse events occurred in four patients in cohort A and five in cohort B. In cohort A, these events were asthenia, elevated liver function tests, dyspnea, and atrioventricular block. In cohort B, the events were diarrhea, musculoskeletal pain, psoriasis, hypophosphatemia, and elevated creatinine (in two patients).
The patient who developed atrioventricular block permanently discontinued nivolumab. There were no other treatment-related adverse events that led to discontinuation.
This study was supported by Bristol-Myers Squibb. The researchers disclosed relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Flippot R et al. J Clin Oncol. 2019 Aug 10;37(23):2008-16.
FROM JOURNAL OF CLINICAL ONCOLOGY
Extended half-life products cut infusions in Europe
Novel extended half-life factor VIII (FVIII) and factor IX (FIX) products appear to decrease the number of infusions and maintain higher trough levels, especially for patients with hemophilia B, according to recent survey findings.
Preliminary data from a European multinational survey suggest these benefits may help overcome current limitations with standard clotting factor products.
“We administered a survey to determine the efficacy of [extended half-life] products after they became available in several European countries,” wrote Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues. These results were published in Haemophilia.
The questionnaire, designed by the European Association for Haemophilia and Allied Disorders (EAHAD), was distributed to 48 hemophilia treatment centers in January 2018. In total, 33 centers completed the survey.
The survey explored the real-life clinical experiences of patients with hemophilia A and B using extended half-life FVIII and FIX products. At the time of the survey, pegylated factor products were not available for use. In particular, the survey collected general information related to the efficacy of prophylaxis after transitioning patients to novel extended half-life products.
After analysis, the researchers found that among responding centers, extended half-life FVIII products decreased the number of infusions by 30% or greater among hemophilia A patients and achieved trough levels of 3%-5% in 66%-67% of centers.
With respect to FIX products, all responding centers were able to reduce infusions by more than 30% among hemophilia B patients, with 67% maintaining a FIX trough level of no less than 5%-10%.
The researchers acknowledged that the findings are preliminary and should be confirmed by conducting a repeat survey.
“Evaluating the safety of these new drugs is of the utmost importance and should be monitored through careful, long‐term observation,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Alnylam, Grifols, Kedrion, Pfizer, Roche, Sanofi, Bayer, Shire, and several other companies.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Aug 16. doi: 10.1111/hae.13834.
Novel extended half-life factor VIII (FVIII) and factor IX (FIX) products appear to decrease the number of infusions and maintain higher trough levels, especially for patients with hemophilia B, according to recent survey findings.
Preliminary data from a European multinational survey suggest these benefits may help overcome current limitations with standard clotting factor products.
“We administered a survey to determine the efficacy of [extended half-life] products after they became available in several European countries,” wrote Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues. These results were published in Haemophilia.
The questionnaire, designed by the European Association for Haemophilia and Allied Disorders (EAHAD), was distributed to 48 hemophilia treatment centers in January 2018. In total, 33 centers completed the survey.
The survey explored the real-life clinical experiences of patients with hemophilia A and B using extended half-life FVIII and FIX products. At the time of the survey, pegylated factor products were not available for use. In particular, the survey collected general information related to the efficacy of prophylaxis after transitioning patients to novel extended half-life products.
After analysis, the researchers found that among responding centers, extended half-life FVIII products decreased the number of infusions by 30% or greater among hemophilia A patients and achieved trough levels of 3%-5% in 66%-67% of centers.
With respect to FIX products, all responding centers were able to reduce infusions by more than 30% among hemophilia B patients, with 67% maintaining a FIX trough level of no less than 5%-10%.
The researchers acknowledged that the findings are preliminary and should be confirmed by conducting a repeat survey.
“Evaluating the safety of these new drugs is of the utmost importance and should be monitored through careful, long‐term observation,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Alnylam, Grifols, Kedrion, Pfizer, Roche, Sanofi, Bayer, Shire, and several other companies.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Aug 16. doi: 10.1111/hae.13834.
Novel extended half-life factor VIII (FVIII) and factor IX (FIX) products appear to decrease the number of infusions and maintain higher trough levels, especially for patients with hemophilia B, according to recent survey findings.
Preliminary data from a European multinational survey suggest these benefits may help overcome current limitations with standard clotting factor products.
“We administered a survey to determine the efficacy of [extended half-life] products after they became available in several European countries,” wrote Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues. These results were published in Haemophilia.
The questionnaire, designed by the European Association for Haemophilia and Allied Disorders (EAHAD), was distributed to 48 hemophilia treatment centers in January 2018. In total, 33 centers completed the survey.
The survey explored the real-life clinical experiences of patients with hemophilia A and B using extended half-life FVIII and FIX products. At the time of the survey, pegylated factor products were not available for use. In particular, the survey collected general information related to the efficacy of prophylaxis after transitioning patients to novel extended half-life products.
After analysis, the researchers found that among responding centers, extended half-life FVIII products decreased the number of infusions by 30% or greater among hemophilia A patients and achieved trough levels of 3%-5% in 66%-67% of centers.
With respect to FIX products, all responding centers were able to reduce infusions by more than 30% among hemophilia B patients, with 67% maintaining a FIX trough level of no less than 5%-10%.
The researchers acknowledged that the findings are preliminary and should be confirmed by conducting a repeat survey.
“Evaluating the safety of these new drugs is of the utmost importance and should be monitored through careful, long‐term observation,” they concluded.
No funding sources were reported. The authors reported financial affiliations with Alnylam, Grifols, Kedrion, Pfizer, Roche, Sanofi, Bayer, Shire, and several other companies.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Aug 16. doi: 10.1111/hae.13834.
FROM HAEMOPHILIA