User login
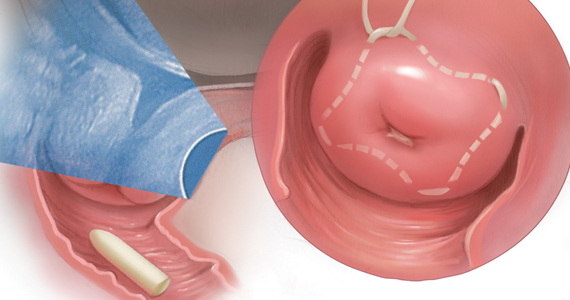
Bariatric surgery should be considered in individuals with class 1 obesity
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Mitchel L. Zoler’s article on Abstract A105, presented at Obesity Week 2019, addresses an important health concern and is timely.
Over the past 4 decades we have seen a rise in the prevalence of obesity and associated health complications, not just in the United States but across the world. The incidence of obesity (having a BMI greater than 30) was 35% for women and 31% for men in the United States, and associated deaths and disability were primarily attributed to diabetes and cardiovascular disease resulting from obesity.
This article references the benefits of bariatric/metabolic surgery in individuals with class 1 obesity. In the United States, more than half of those who meet the criteria for obesity come under the class 1 category (BMI, 30-34.9). Those in this class of obesity are at increased risk of developing diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease, obstructive sleep apnea, polycystic ovarian syndrome, and bone and joint disorders.
There are several studies that document the significant reduction in incidence of the above cardiometabolic risks with sustained weight loss. Nonsurgical interventions in individuals with class 1 obesity through lifestyle modifications and pharmacotherapy have not demonstrated success in providing persistent weight loss or metabolic benefits. The data presented in this article are of great significance to patients and physicians alike as they highlight the long-term benefits and reversal of metabolic disorders.
Current guidelines for bariatric surgery for individuals with a BMI greater than 35 were published in 1991. Since then several safe surgical options including laparoscopic procedures, sleeve gastrectomy, and adjustable gastric banding have been developed with decreased surgical risks, morbidity, and mortality.
The International Federation for the Surgery of Obesity and Metabolic Disorders, the International Diabetes Federation, and the National Institute for Health and Care Excellence of the United Kingdom, have supported the option of bariatric surgery in class 1 obese individuals with metabolic disorders.
While lifestyle modifications with medications should be the first-line treatment for class 1 obesity, as a primary care physician I believe that, given the major changes in the surgical options, the proven long-term benefits, and the rising incidences of obesity and metabolic syndrome, it is time for the health care community, insurers, patients, and all other stakeholders to consider bariatric surgery in class 1 obese individuals as a potential and viable option.
Noel N. Deep, MD, is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, Wausau, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News.
He made these comments in response to questions from MDedge and had no relevant disclosures.
Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
Preterm birth (PTB) remains a significant public health concern and a major cause of newborn morbidity and mortality. In the United States, 1 in 10 babies are born preterm (< 37 weeks), and this rate has changed little in 30 years.1
In 2011, the US Food and Drug Administration (FDA) approved progesterone supplementation—specifically, α-hydroxyprogesterone caproate (17P) injection (Makena)—to prevent recurrent PTB in women with a singleton pregnancy at high risk by virtue of a prior spontaneous PTB.2 This was the first-ever FDA-approved drug for PTB prevention, and it was the first drug approved by the FDA for use in pregnancy in more than 15 years. The approval of 17P utilized the FDA's Subpart H Accelerated Approval Pathway, which applies to therapies that: 1) treat serious conditions with unmet need, and 2) demonstrate safety and efficacy on surrogate end points reasonably likely to predict clinical benefit.3
By voting their approval of 17P in 2011, the FDA affirmed that PTB was a serious condition with unmet need, that birth < 37 weeks was an accepted surrogate end point, and that there was compelling evidence of safety and benefit. The compelling evidence presented was a single, randomized, vehicle-controlled clinical trial conducted by the Maternal-Fetal Medicine Units (MFMU) Network, which showed significant reduction in recurrent PTB < 37 weeks (from 54.9% in the placebo group to 36.3% in the 17P group; P<.001; relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81).4
In 2017, the Society for Maternal-Fetal Medicine (SMFM) reaffirmed the use of 17P to prevent recurrent PTB and, that same year, it was estimated that 75% of eligible patients received 17P.5,6 Importantly, Subpart H approval requires one or more follow-up clinical trials confirming safety and efficacy. And the FDA has the right—the responsibility—to revisit approval if such trials are either not performed or are unfavorable.
The recently published PROLONG study by Blackwell and colleagues is this required postapproval confirmatory trial conducted to verify the clinical benefit of 17P supplementation.7
Continue to: Study design, and stunning results...
Study design, and stunning results
PROLONG (Progestin's Role in Optimizing Neonatal Gestation) was a randomized (2:1), double-blind, vehicle-controlled, multicenter international trial (2009-2018) conducted to assess the safety and efficacy of 17P injection in 1,708 women with a singleton pregnancy and one or more prior spontaneous PTBs.7 Women in the active treatment group (n = 1,130) received weekly intramuscular injections of 17P, while those in the control group (n = 578) received weekly injections of inert oil vehicle.
Results of the trial showed no significant reduction in the co-primary end points, which were PTB < 35 weeks (11.0% in the 17P group vs 11.5% in the placebo group; RR, 0.95; 95% CI, 0.71-1.26) and neonatal morbidity index (5.6% in the 17P group vs 5.0% in the placebo group; RR, 1.12; 95% CI, 0.68-1.61). There was no evidence of benefit for any subpopulation (geographic region, race, or other PTB risk factor). Maternal outcomes also were similar between the groups. No significant safety concerns were identified.
Important differences between MFMU and PROLONG trials
Strengths of the PROLONG trial include its randomized, placebo-controlled design, excellent follow-up rate, and use of a protocol that mirrored that of the MFMU trial. The primary limitation of PROLONG is that participants experienced a lower rate of PTB compared with those in the MFMU trial. The rate of PTB < 37 weeks was 54.9% in the control group of the MFMU trial compared with 21.9% in PROLONG.
Given the low rate of PTB in PROLONG, the study was underpowered for the co-primary outcomes. In addition, lower rates of PTB in PROLONG compared with in the MFMU trial likely reflected different patient populations.8 Moreover, PROLONG was an international trial. Of the 1,708 participants, most were recruited in Russia (36%) and Ukraine (25%); only 23% were from the United States. By contrast, participants in the MFMU trial were recruited from US academic medical centers. Also, participants in the MFMU trial were significantly more likely to have a short cervix, to have a history of more than one PTB, and to be African American.
Discrepant trial results create clinical quandary
In October 2019, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17P. Committee members struggled with the conflicting data between the 2 trials and hesitated to remove a medication whose use has become standard practice. Ultimately, however, it was lack of substantial evidence of effectiveness of 17P that swayed the committee's vote. While the FDA generally follows the recommendation of an advisory committee, it is not bound to do so.
Societies' perspectives
So what are physicians and patients to do? It is possible that a small subgroup of women at extremely high risk for early PTB may benefit from 17P administration. SMFM stated: "...it is reasonable for providers to use 17-OHPC [17P] in women with a profile more representative of the very high-risk population reported in the Meis [MFMU] trial."8 Further, the American College of Obstetricians and Gynecologists (ACOG) stated in a Practice Advisory dated October 25, 2019, that "ACOG is not changing our clinical recommendations at this time... [We] will be reviewing subsequent forthcoming analyses and will issue updated clinical guidance as appropriate."9
Where we stand on 17P use going forward
17P should be available to women who previously may have benefited from its use. However, 17P should not be recommended routinely to prevent recurrent spontaneous PTB in women with one prior PTB and no other risk factors. Of note, the PROLONG trial does not change recommendations for cervical length screening. Women with a history of a prior spontaneous PTB should undergo cervical length screening to identify those individuals who may benefit from an ultrasound-indicated cerclage.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308-1317.
- Makena [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf. Accessed November 10, 2019.
- US Food and Drug Administration. Code of Federal Regulations Title 21. Subpart H--Acceleratedapproval of new drugs for serious or life-threatening illnesses. April 1, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed November 10, 2019.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Society for Maternal-Fetal Medicine Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am J Obstet Gynecol. 2017;216:B11-B13.
- Gallagher JR, Gudeman J, Heap K, et al. Understanding if, how, and why women with prior spontaneous preterm births are treated with progestogens: a national survey of obstetrician practice patterns. AJP Rep. 2018;8:e315-e324.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM statement: Use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://els-jbs-prod-cdn.literatumonline.com/pb/assets/raw/Health%20Advance/journals/ymob/SMFM_Statement_PRO LONG-1572023839767.pdf. Accessed November 10, 2019.
- American College of Obstetricians and Gynecologists. Practice advisory: Clinical guidance for integration of the findings of the PROLONG study: progestin's role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing?IsMobileSet=false. Accessed November 10, 2019.
SOX11 shows value as diagnostic marker in MCL
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
SOX11 may be an accurate diagnostic marker for mantle cell lymphoma (MCL), allowing clinicians to distinguish it from other lymphoproliferative disorders, according to findings from a meta-analysis of 14 case-control studies.
Woojoo Lee, PhD, of Inha University, Incheon, Republic of Korea, and coinvestigators, evaluated the diagnostic accuracy of SOX11 immunohistochemistry for the diagnosis of MCL. The results were published in PLoS One.
The researchers searched major databases for studies that evaluated the use of SOX11 immunohistochemistry in patients with MCL and other lymphoproliferative disorders. After applying the search parameters, the team identified 383 studies, 14 of which were included in the meta-analysis. Various data were extracted from eligible studies, including the type and clonality of anti-SOX11 antibody, number of SOX11-positive lymphomas (MCLs and other lymphoproliferative disorders), specificity, sensitivity, and others. After combining the data, the investigators calculated pooled sensitivity, specificity, and area under the curve. Among the included studies, hairy cell leukemia, Burkitt’s lymphoma, and lymphoblastic lymphoma were common among patient populations. In total, clone MRQ-58 mouse antibodies were used in five study populations.
The researchers found that the pooled specificity was 0.95 (95% CI, 0.9-0.97), and sensitivity was 0.9 (95% CI, 0.86-0.92). There was statistically significant substantial heterogeneity observed for specificity, but not for sensitivity, the investigators reported.
“The results demonstrated that SOX11 was a potential diagnostic marker for MCL,” they wrote. “Meta-regression revealed a significant inverse relationship between specificity and proportions of [Burkitt’s lymphoma, lymphoblastic lymphoma, and hairy cell leukemia].”
With respect to antibody type, the clone MRQ-58 mouse antibody showed consistently high specificity in the clinical setting, despite observed heterogeneity.
“Future studies using MRQ-58 are needed to improve our understanding of the diagnostic accuracy of SOX11 immunohistochemistry for MCL,” the investigators wrote.
The study was funded by the Next-Generation BioGreen 21 program (Republic of Korea). The authors reported having no conflicts of interest.
SOURCE: Lee W et al. PLoS One. 2019 Nov 12. doi: 10.1371/journal.pone.0225096.
FROM PLOS ONE
Acalabrutinib may outperform other targeted therapies in MCL
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
For patients with relapsed or refractory mantle cell lymphoma (MCL), second generation Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib may offer increased response rates and better tolerability compared with other single-agent targeted therapies, based on a recent analysis.
Improved safety could lead to long-term benefits resulting from extended treatment duration, according to lead author Claire Telford, PhD, of AstraZeneca in Gaithersburg, Md., and colleagues. AstraZeneca manufactures acalabrutinib (Calquence).
Currently, treatment for MCL is guided by a number of clinical considerations, the investigators explained in Clinical Therapeutics.
“The type of treatment recommended for relapsed/refractory MCL depends on multiple factors, namely time to the relapse, extent of disease, previous regimens, candidacy for allogeneic stem cell transplantation, and the patient’s overall health,” they wrote.
To determine how acalabrutinib stacks up with other options, the investigators drew data from the phase 2 ACE-LY-004 trial, which tested acalabrutinib in 124 patients with relapsed or refractory MCL. After matching, the investigators compared the ACE-LY-004 outcomes from 12 other trials, in which patients received different targeted therapies.
Results pointed to higher overall response and complete response rates for acalabrutinib, compared with other single-agent targeted therapy. Specifically, acalabrutinib had a higher overall response rate, compared with ibrutinib (9.3% higher), lenalidomide (38.1% higher), temsirolimus (40.7% higher), and bortezomib (50.6% higher). For each of these, complete responses also were higher.
There was no significant difference in overall response or complete response rates between acalabrutinib and rituximab combinations – bendamustine plus rituximab, ibrutinib plus rituximab, and lenalidomide plus rituximab.
The investigators also highlighted a number of safety advantages. Compared with ibrutinib, acalabrutinib was associated with significantly fewer instances of grade 3 or 4 atrial fibrillation. Risk of grade 3 or 4 thrombocytopenia was significantly lower with acalabrutinib than with ibrutinib, bortezomib, lenalidomide, and temsirolimus.
Still, in some instances, acalabrutinib was comparatively less tolerable. It was associated with a higher risk of grade 3 or 4 infections than bendamustine plus rituximab; and anemia was more common among patients receiving acalabrutinib than among those who had lenalidomide plus rituximab or ibrutinib plus rituximab.
“This comparison of targeted therapies used in the treatment of relapsed/refractory MCL has shown that acalabrutinib has the potential to provide higher response rates, with trends for longer [progression-free survival] and [overall survival], and an improved safety profile,” the investigators wrote.
The study was funded by AstraZeneca. Dr. Telford is an employee of AstraZeneca and other authors reported financial relationships with the company.
SOURCE: Telford C et al. Clin Ther. 2019 Nov 4. doi: 10.1016/j.clinthera.2019.09.012 .
FROM CLINICAL THERAPEUTICS
FDA approves atezolizumab combo as first line for advanced NSCLC
chemotherapy for first-line treatment of adults with metastatic, nonsquamous non–small cell lung cancer (NSCLC) with no EGFR or ALK genomic tumor aberrations.
Atezolizumab has been previously approved in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of adults with metastatic NSCLC with no EGFR or ALK genomic tumor aberrations. The monoclonal antibody is also approved to treat adults with metastatic NSCLC who have disease progression during or following chemotherapy, and for those with extensive-stage SCLC.
The current approval was based on a demonstrated improvement in overall survival in the phase 3 IMpower130 trial (NCT02367781). Median overall survival for advanced NSCLC patients who received atezolizumab in combination with chemotherapy was 18.6 months, compared with 13.9 months for patients who received chemotherapy alone (hazard ratio, 0.80; 95% confidence interval, 0.64-0.99; P = .0384) in the intention-to-treat wild-type population of 681 patients.
Grade 3-4 treatment-related adverse events were reported in 73.2% of people receiving atezolizumab plus chemotherapy, compared with 60.3% of people receiving chemotherapy alone, according to the company press release.
chemotherapy for first-line treatment of adults with metastatic, nonsquamous non–small cell lung cancer (NSCLC) with no EGFR or ALK genomic tumor aberrations.
Atezolizumab has been previously approved in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of adults with metastatic NSCLC with no EGFR or ALK genomic tumor aberrations. The monoclonal antibody is also approved to treat adults with metastatic NSCLC who have disease progression during or following chemotherapy, and for those with extensive-stage SCLC.
The current approval was based on a demonstrated improvement in overall survival in the phase 3 IMpower130 trial (NCT02367781). Median overall survival for advanced NSCLC patients who received atezolizumab in combination with chemotherapy was 18.6 months, compared with 13.9 months for patients who received chemotherapy alone (hazard ratio, 0.80; 95% confidence interval, 0.64-0.99; P = .0384) in the intention-to-treat wild-type population of 681 patients.
Grade 3-4 treatment-related adverse events were reported in 73.2% of people receiving atezolizumab plus chemotherapy, compared with 60.3% of people receiving chemotherapy alone, according to the company press release.
chemotherapy for first-line treatment of adults with metastatic, nonsquamous non–small cell lung cancer (NSCLC) with no EGFR or ALK genomic tumor aberrations.
Atezolizumab has been previously approved in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of adults with metastatic NSCLC with no EGFR or ALK genomic tumor aberrations. The monoclonal antibody is also approved to treat adults with metastatic NSCLC who have disease progression during or following chemotherapy, and for those with extensive-stage SCLC.
The current approval was based on a demonstrated improvement in overall survival in the phase 3 IMpower130 trial (NCT02367781). Median overall survival for advanced NSCLC patients who received atezolizumab in combination with chemotherapy was 18.6 months, compared with 13.9 months for patients who received chemotherapy alone (hazard ratio, 0.80; 95% confidence interval, 0.64-0.99; P = .0384) in the intention-to-treat wild-type population of 681 patients.
Grade 3-4 treatment-related adverse events were reported in 73.2% of people receiving atezolizumab plus chemotherapy, compared with 60.3% of people receiving chemotherapy alone, according to the company press release.
Rheumatology News Best of 2019 – The RA Report: Top News Highlights
The ideas and opinions expressed in Best of 2019 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
The ideas and opinions expressed in Best of 2019 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
The ideas and opinions expressed in Best of 2019 – The RA Report: Top News Highlights do not necessarily reflect those of the publisher. Frontline Medical Communications will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services mentioned herein.
Gunshot wound victims are at high risk for readmission
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
CHICAGO –
A study of individuals at a single institution who were hospitalized and had a prior history of gunshot wound found some patterns of injury that set patients up for a greater likelihood of readmission.
In particular, patients who sustained visceral gunshot wounds were over six times more likely to be readmitted to the hospital, Corbin Pomeranz, MD, a radiology resident at Thomas Jefferson University, Philadelphia, said in an interview at the annual meeting of the Radiological Society of North America. Dr. Pomeranz led the retrospective study that begins to fill a knowledge gap about what happens over the long term to those who sustain gunshot wounds.
“There continues to be profound lack of substantial information related to gun violence, particularly in predicting long-term outcomes,” Dr. Pomeranz and coauthors wrote in the abstract accompanying the presentation.
The researchers performed a single-site retrospective analysis over 3 months in 2018, tapping into an imaging database and looking for inpatient imaging exams that were nonacute, but related to gunshot wounds. From this information, the researchers went back to the original gunshot wound injury imaging, and recorded the pattern of injury, classifying wounds as neurologic, vascular, visceral, musculoskeletal, or involving multiple systems.
The investigators were able to glean additional information including the initial admitting hospital unit, information about interval admissions or surgeries, and demographic data. Regarding the nature of the gunshot injury itself, Dr. Pomeranz and coauthors went back to the earlier imaging studies to note bullet morphology, recording whether the bullet was intact, deformed, or had splintered into shrapnel within injured tissues.
In all, 174 imaging studies involving 110 patients were examined. Men made up 92% of the study population; the average age was 49.7 years. Neurologic and visceral gunshot wounds were moderately correlated with subsequent readmission (r = .436; P less than .001). However, some of this effect was blunted when patient age was controlled for in the statistical analysis.
Patients who were initially admitted to the intensive care unit, and who presumably had more severe injuries, were also more likely to be readmitted (r = .494, P less than .001). Here, “controlling for age had very little effect on the strength of the relationship between these two variables,” noted Dr. Pomeranz and coauthors.
A more elaborate statistical model incorporated several independent variables including age, type of injury, and body region involved, as well as bullet morphology. In this model, visceral injury was the strongest predictor for readmission, with an odds ratio of 6.44.
Dr. Pomeranz said that both the initial gunshot wound and subsequent gaps in care can contribute to readmissions. A patient who has a spinal cord injury may not be reimbursed adequately for supportive cushioning, or an appropriate wheelchair, and so may require admission for decubitus ulcers.
The number of admissions for osteomyelitis, which made up more than half of the subsequent admissions, initially surprised Dr. Pomeranz, until he realized that lack of mobility and sensory losses from gunshot-induced spinal cord injuries could easily lead to nonhealing lower extremity wounds, with osteomyelitis as a sequela.
Several patients were admitted for small bowel obstructions with no interval surgery since treatment for the gunshot wound. These readmissions, said Dr. Pomeranz, were assessed as related to the gunshot wound since it’s extremely rare for a patient with no history of abdominal surgery and no malignancy to have a small bowel obstruction. Exploratory laparotomies are common in the context of abdominal trauma caused by gunshot wounds, and either the gunshot itself or the laparotomy was the likely cause of adhesions.
Dr. Pomeranz acknowledged the many limitations of the study, but pointed out that some will be addressed when he and his coauthors conduct a larger study they have planned to look at readmissions from gunshot wounds at multiple hospitals in the Philadelphia area. The small sample size in the current study meant that the impact of socioeconomic status and other lifestyle and social variables and comorbidities couldn’t be adequately addressed in the statistical analysis. By casting a wider net within the greater Philadelphia area, the investigators should be able to track patients who receive care in more than one hospital system, increasing participant numbers, he said.
“Morbidity and outcomes from gun violence can only be assessed after a firm understanding of injury patterns on imaging,” noted Dr. Pomeranz. He said that interdisciplinary research investigating individual and societal short- and long-term costs of gun violence is sorely needed to inform public policy.
Dr. Pomeranz reported no outside sources of funding and reported that he had no conflicts of interest.
SOURCE: Pomeranz C et al. RSNA 2019, Presentation HP226-SD-THA3.
REPORTING FROM RSNA 2019
Effective NASH medications are coming ‘sooner than you think’
SAN ANTONIO – The therapeutic Dark Ages of nonalcoholic steatohepatitis (NASH) are finally drawing to a close.
“NASH-specific therapies are coming soon – sooner than you think,” Naim Alkhouri, MD, predicted at the annual meeting of the American College of Gastroenterology.
And that, he added, has important implications for clinical practice. Physicians are going to need to step up their game with regard to screening and staging patients with nonalcoholic fatty liver disease to identify the right candidates for the coming effective treatments.
The new treatment era in NASH could dawn as soon as the spring of 2020, by which time the Food and Drug Administration is expected to issue a decision on obeticholic acid, an oral FXR agonist for which the agency has granted breakthrough therapy status. Intercept Pharmaceuticals has filed for marketing approval of obeticholic acid for NASH on the strength of the positive 18-month histologic results of the pivotal phase 3 REGENERATE trial, the first-ever successful phase 3 study of a medication for NASH, noted Dr. Alkhouri, a gastroenterologist at the University of Texas, San Antonio, and director of the Metabolic Health Center at the Texas Liver Institute.
At present there are no FDA-approved pharmacotherapies for NASH. The unmet medical need is huge, since NASH is now recognized to be a full-blown, burgeoning epidemic. NASH will soon become the No. 1 indication for liver transplantation in the United States. A full-throttle race is on to find effective therapies targeting the various dimensions of NASH, with more than 70 drugs now in phase 2 studies. These drugs collectively address all four mechanisms of the disease’s development and progression: the metabolic targets, perturbations in the gut-liver axis, liver inflammation, and fibrosis.
Moreover, even as the FDA considers the application for approval of obeticholic acid in NASH, at least four other investigational drugs are in pivotal phase 3 clinical trials. These include elafibranor, aramchol, resmetirom, and cenicriviroc.
Cenicriviroc is a dual CCR 2/5 receptor antagonist that targets the hepatic inflammation and fibrosis dimensions of NASH. It is now being evaluated in the phase 3 AURORA trial on the strength of the earlier positive phase 2b Centaur study, in which patients randomized to cenicriviroc were twice as likely to experience significant improvement in fibrosis as were placebo-treated controls.
Elafibranor, aramchol, and resmetirom employ different mechanisms of action to address the metabolic derangements of NASH. What they share in common is their aim to reduce the influx of free fatty acids from adipose tissue to the liver, and/or to inhibit lipogenesis from carbohydrate building blocks. In so doing, these medications should result in reduced hepatocyte injury and liver inflammation.
Elafibranor is a peroxisome proliferator-activated receptor alpha/delta agonist that achieved significant biopsy-proven reversal of NASH in moderate- or severely affected patients in the phase 2 GOLDEN study. The phase 3 RESOLVE IT trial is underway.
Aramchol is a first-in-class synthetic fatty acid/bile acid conjugate that inhibits stearoyl-CoA desaturate activity. It’s designed to improve insulin resistance and curb accumulation of triglycerides in hepatocytes. In the 52-week, phase 2 ARREST trial, oral aramchol at 600 mg/day was 4.7-fold more likely than was placebo to achieve NASH resolution without worsening of fibrosis. The drug is now in phase 3 in the ARMOR study.
Resmetirom is a selective thyroid hormone receptor–beta agonist. Activation of the beta receptor lowers LDL cholesterol, triglycerides, and liver fat, whereas activation of the alpha receptor has the unwanted effects of promoting bone loss, thyrotoxicosis, and arrhythmias. In phase 2, 75% of patients on high-dose resmetirom achieved at least a 30% reduction in hepatic fat by MRI at week 12, compared with 18% of placebo-treated controls. And 39% of the high-dose resmetirom group showed histologic resolution of NASH on a week-36 liver biopsy, as did a mere 6% of controls. The phase 3 MAESTRO randomized trial is underway.
Obeticholic acid addresses the gut-liver axis abnormalities present in NASH, especially the exuberant bile acid circulation.
Clinical implications of the coming wave of medications
In Dr. Alkhouri’s view, .
“These are the patients with a high chance of progressing to cirrhosis and end-stage liver disease,” the gastroenterologist said.
Patients with earlier-stage nonalcoholic fatty liver disease are best managed via lifestyle changes, with particular emphasis upon 10% weight loss accompanied by exercise. And patients with more advanced disease – NASH with cirrhosis – appear thus far to be beyond the reach of the next-generation therapies.
None of the coming drugs is a cure-all. In the landmark phase 3 REGENERATE trial, for example, the rate of the primary outcome – fibrosis improvement of at least one stage plus no worsening of NASH at 18 months – was 23% in patients randomized to obeticholic acid at 25 mg/day, compared to 12% with placebo.
“These are not like hepatitis C medications, with 97% efficacy, so combination therapy targeting upstream and downstream for NASH is rational,” Dr. Alkhouri observed.
He reported serving on advisory boards for Allergan, Gilead, and Intercept, and receiving research grants from those companies as well as from Galmed, Genfit, and Madrigal.
*This story was updated on 12/5/2019.
SAN ANTONIO – The therapeutic Dark Ages of nonalcoholic steatohepatitis (NASH) are finally drawing to a close.
“NASH-specific therapies are coming soon – sooner than you think,” Naim Alkhouri, MD, predicted at the annual meeting of the American College of Gastroenterology.
And that, he added, has important implications for clinical practice. Physicians are going to need to step up their game with regard to screening and staging patients with nonalcoholic fatty liver disease to identify the right candidates for the coming effective treatments.
The new treatment era in NASH could dawn as soon as the spring of 2020, by which time the Food and Drug Administration is expected to issue a decision on obeticholic acid, an oral FXR agonist for which the agency has granted breakthrough therapy status. Intercept Pharmaceuticals has filed for marketing approval of obeticholic acid for NASH on the strength of the positive 18-month histologic results of the pivotal phase 3 REGENERATE trial, the first-ever successful phase 3 study of a medication for NASH, noted Dr. Alkhouri, a gastroenterologist at the University of Texas, San Antonio, and director of the Metabolic Health Center at the Texas Liver Institute.
At present there are no FDA-approved pharmacotherapies for NASH. The unmet medical need is huge, since NASH is now recognized to be a full-blown, burgeoning epidemic. NASH will soon become the No. 1 indication for liver transplantation in the United States. A full-throttle race is on to find effective therapies targeting the various dimensions of NASH, with more than 70 drugs now in phase 2 studies. These drugs collectively address all four mechanisms of the disease’s development and progression: the metabolic targets, perturbations in the gut-liver axis, liver inflammation, and fibrosis.
Moreover, even as the FDA considers the application for approval of obeticholic acid in NASH, at least four other investigational drugs are in pivotal phase 3 clinical trials. These include elafibranor, aramchol, resmetirom, and cenicriviroc.
Cenicriviroc is a dual CCR 2/5 receptor antagonist that targets the hepatic inflammation and fibrosis dimensions of NASH. It is now being evaluated in the phase 3 AURORA trial on the strength of the earlier positive phase 2b Centaur study, in which patients randomized to cenicriviroc were twice as likely to experience significant improvement in fibrosis as were placebo-treated controls.
Elafibranor, aramchol, and resmetirom employ different mechanisms of action to address the metabolic derangements of NASH. What they share in common is their aim to reduce the influx of free fatty acids from adipose tissue to the liver, and/or to inhibit lipogenesis from carbohydrate building blocks. In so doing, these medications should result in reduced hepatocyte injury and liver inflammation.
Elafibranor is a peroxisome proliferator-activated receptor alpha/delta agonist that achieved significant biopsy-proven reversal of NASH in moderate- or severely affected patients in the phase 2 GOLDEN study. The phase 3 RESOLVE IT trial is underway.
Aramchol is a first-in-class synthetic fatty acid/bile acid conjugate that inhibits stearoyl-CoA desaturate activity. It’s designed to improve insulin resistance and curb accumulation of triglycerides in hepatocytes. In the 52-week, phase 2 ARREST trial, oral aramchol at 600 mg/day was 4.7-fold more likely than was placebo to achieve NASH resolution without worsening of fibrosis. The drug is now in phase 3 in the ARMOR study.
Resmetirom is a selective thyroid hormone receptor–beta agonist. Activation of the beta receptor lowers LDL cholesterol, triglycerides, and liver fat, whereas activation of the alpha receptor has the unwanted effects of promoting bone loss, thyrotoxicosis, and arrhythmias. In phase 2, 75% of patients on high-dose resmetirom achieved at least a 30% reduction in hepatic fat by MRI at week 12, compared with 18% of placebo-treated controls. And 39% of the high-dose resmetirom group showed histologic resolution of NASH on a week-36 liver biopsy, as did a mere 6% of controls. The phase 3 MAESTRO randomized trial is underway.
Obeticholic acid addresses the gut-liver axis abnormalities present in NASH, especially the exuberant bile acid circulation.
Clinical implications of the coming wave of medications
In Dr. Alkhouri’s view, .
“These are the patients with a high chance of progressing to cirrhosis and end-stage liver disease,” the gastroenterologist said.
Patients with earlier-stage nonalcoholic fatty liver disease are best managed via lifestyle changes, with particular emphasis upon 10% weight loss accompanied by exercise. And patients with more advanced disease – NASH with cirrhosis – appear thus far to be beyond the reach of the next-generation therapies.
None of the coming drugs is a cure-all. In the landmark phase 3 REGENERATE trial, for example, the rate of the primary outcome – fibrosis improvement of at least one stage plus no worsening of NASH at 18 months – was 23% in patients randomized to obeticholic acid at 25 mg/day, compared to 12% with placebo.
“These are not like hepatitis C medications, with 97% efficacy, so combination therapy targeting upstream and downstream for NASH is rational,” Dr. Alkhouri observed.
He reported serving on advisory boards for Allergan, Gilead, and Intercept, and receiving research grants from those companies as well as from Galmed, Genfit, and Madrigal.
*This story was updated on 12/5/2019.
SAN ANTONIO – The therapeutic Dark Ages of nonalcoholic steatohepatitis (NASH) are finally drawing to a close.
“NASH-specific therapies are coming soon – sooner than you think,” Naim Alkhouri, MD, predicted at the annual meeting of the American College of Gastroenterology.
And that, he added, has important implications for clinical practice. Physicians are going to need to step up their game with regard to screening and staging patients with nonalcoholic fatty liver disease to identify the right candidates for the coming effective treatments.
The new treatment era in NASH could dawn as soon as the spring of 2020, by which time the Food and Drug Administration is expected to issue a decision on obeticholic acid, an oral FXR agonist for which the agency has granted breakthrough therapy status. Intercept Pharmaceuticals has filed for marketing approval of obeticholic acid for NASH on the strength of the positive 18-month histologic results of the pivotal phase 3 REGENERATE trial, the first-ever successful phase 3 study of a medication for NASH, noted Dr. Alkhouri, a gastroenterologist at the University of Texas, San Antonio, and director of the Metabolic Health Center at the Texas Liver Institute.
At present there are no FDA-approved pharmacotherapies for NASH. The unmet medical need is huge, since NASH is now recognized to be a full-blown, burgeoning epidemic. NASH will soon become the No. 1 indication for liver transplantation in the United States. A full-throttle race is on to find effective therapies targeting the various dimensions of NASH, with more than 70 drugs now in phase 2 studies. These drugs collectively address all four mechanisms of the disease’s development and progression: the metabolic targets, perturbations in the gut-liver axis, liver inflammation, and fibrosis.
Moreover, even as the FDA considers the application for approval of obeticholic acid in NASH, at least four other investigational drugs are in pivotal phase 3 clinical trials. These include elafibranor, aramchol, resmetirom, and cenicriviroc.
Cenicriviroc is a dual CCR 2/5 receptor antagonist that targets the hepatic inflammation and fibrosis dimensions of NASH. It is now being evaluated in the phase 3 AURORA trial on the strength of the earlier positive phase 2b Centaur study, in which patients randomized to cenicriviroc were twice as likely to experience significant improvement in fibrosis as were placebo-treated controls.
Elafibranor, aramchol, and resmetirom employ different mechanisms of action to address the metabolic derangements of NASH. What they share in common is their aim to reduce the influx of free fatty acids from adipose tissue to the liver, and/or to inhibit lipogenesis from carbohydrate building blocks. In so doing, these medications should result in reduced hepatocyte injury and liver inflammation.
Elafibranor is a peroxisome proliferator-activated receptor alpha/delta agonist that achieved significant biopsy-proven reversal of NASH in moderate- or severely affected patients in the phase 2 GOLDEN study. The phase 3 RESOLVE IT trial is underway.
Aramchol is a first-in-class synthetic fatty acid/bile acid conjugate that inhibits stearoyl-CoA desaturate activity. It’s designed to improve insulin resistance and curb accumulation of triglycerides in hepatocytes. In the 52-week, phase 2 ARREST trial, oral aramchol at 600 mg/day was 4.7-fold more likely than was placebo to achieve NASH resolution without worsening of fibrosis. The drug is now in phase 3 in the ARMOR study.
Resmetirom is a selective thyroid hormone receptor–beta agonist. Activation of the beta receptor lowers LDL cholesterol, triglycerides, and liver fat, whereas activation of the alpha receptor has the unwanted effects of promoting bone loss, thyrotoxicosis, and arrhythmias. In phase 2, 75% of patients on high-dose resmetirom achieved at least a 30% reduction in hepatic fat by MRI at week 12, compared with 18% of placebo-treated controls. And 39% of the high-dose resmetirom group showed histologic resolution of NASH on a week-36 liver biopsy, as did a mere 6% of controls. The phase 3 MAESTRO randomized trial is underway.
Obeticholic acid addresses the gut-liver axis abnormalities present in NASH, especially the exuberant bile acid circulation.
Clinical implications of the coming wave of medications
In Dr. Alkhouri’s view, .
“These are the patients with a high chance of progressing to cirrhosis and end-stage liver disease,” the gastroenterologist said.
Patients with earlier-stage nonalcoholic fatty liver disease are best managed via lifestyle changes, with particular emphasis upon 10% weight loss accompanied by exercise. And patients with more advanced disease – NASH with cirrhosis – appear thus far to be beyond the reach of the next-generation therapies.
None of the coming drugs is a cure-all. In the landmark phase 3 REGENERATE trial, for example, the rate of the primary outcome – fibrosis improvement of at least one stage plus no worsening of NASH at 18 months – was 23% in patients randomized to obeticholic acid at 25 mg/day, compared to 12% with placebo.
“These are not like hepatitis C medications, with 97% efficacy, so combination therapy targeting upstream and downstream for NASH is rational,” Dr. Alkhouri observed.
He reported serving on advisory boards for Allergan, Gilead, and Intercept, and receiving research grants from those companies as well as from Galmed, Genfit, and Madrigal.
*This story was updated on 12/5/2019.
REPORTING FROM ACG 2019
Geriatric Psychiatry Fellowship Position
Geriatric Psychiatry Fellowship Position Available effective July 1, 2020
Department of Psychiatry and Behavioral Neuroscience; University of Cincinnati Medical Center
Opening for 1-year fellowship in fully ACGME-accredited Geriatric Psychiatry Program. Dedicated Faculty with strong clinical focus. Experience a rich assortment of clinical environments within the University of Cincinnati Medical Center and Cincinnati VA Medical Center. Rotations include inpatient/outpatient, ECT, Consult/Liaison, Long-Term Care, Alois Alzheimer Center, Home-Based Primary Care, Hospice & Palliative Care & Cognitive Aging Program.
Interested applicants can contact Marcelle Shidler, Program Coordinator, at [email protected] or 513-558-2466, or go to http://med.uc.edu/psychiatry/fellowships/geriatric-psychiatry
Geriatric Psychiatry Fellowship Position Available effective July 1, 2020
Department of Psychiatry and Behavioral Neuroscience; University of Cincinnati Medical Center
Opening for 1-year fellowship in fully ACGME-accredited Geriatric Psychiatry Program. Dedicated Faculty with strong clinical focus. Experience a rich assortment of clinical environments within the University of Cincinnati Medical Center and Cincinnati VA Medical Center. Rotations include inpatient/outpatient, ECT, Consult/Liaison, Long-Term Care, Alois Alzheimer Center, Home-Based Primary Care, Hospice & Palliative Care & Cognitive Aging Program.
Interested applicants can contact Marcelle Shidler, Program Coordinator, at [email protected] or 513-558-2466, or go to http://med.uc.edu/psychiatry/fellowships/geriatric-psychiatry
Geriatric Psychiatry Fellowship Position Available effective July 1, 2020
Department of Psychiatry and Behavioral Neuroscience; University of Cincinnati Medical Center
Opening for 1-year fellowship in fully ACGME-accredited Geriatric Psychiatry Program. Dedicated Faculty with strong clinical focus. Experience a rich assortment of clinical environments within the University of Cincinnati Medical Center and Cincinnati VA Medical Center. Rotations include inpatient/outpatient, ECT, Consult/Liaison, Long-Term Care, Alois Alzheimer Center, Home-Based Primary Care, Hospice & Palliative Care & Cognitive Aging Program.
Interested applicants can contact Marcelle Shidler, Program Coordinator, at [email protected] or 513-558-2466, or go to http://med.uc.edu/psychiatry/fellowships/geriatric-psychiatry
FDA expands use of Toujeo to childhood type 1 diabetes
The Food and Drug Administration has expanded the indication for Toujeo (insulin glargine 300 units/mL injection; Sanofi) to include children as young as 6 years of age with type 1 diabetes.
The FDA first approved Toujeo in 2015 for adults with type 1 and type 2 diabetes, designed as a more potent follow-up to Sanofi’s top-selling insulin glargine (Lantus).
Last month, Sanofi reported positive results from the phase 3 EDITION JUNIOR trial of Toujeo in children and adolescents with type 1 diabetes. These were presented at the International Society for Pediatric and Adolescent Diabetes 45th Annual Conference in Boston.
In the trial, 463 children and adolescents (aged 6-17 years) treated for type 1 diabetes for at least 1 year and with A1c between 7.5% and 11.0% at screening were randomized to Toujeo or insulin glargine 100 units/mL (Gla-100); participants continued to take their existing mealtime insulin.
The primary endpoint was noninferior reduction in A1c after 26 weeks.
The study met its primary endpoint, confirming a noninferior reduction in A1c with Toujeo versus Gla-100 after 26 weeks (mean reduction, 0.4% vs. 0.4%; difference, 0.004%; 95% confidence interval, –0.17 to 0.18; upper bound was below the prespecified noninferiority margin of 0.3%).
Over 26 weeks, a comparable number of patients in each group experienced one or more hypoglycemic events documented at anytime over 24 hours. Numerically fewer patients taking Toujeo experienced severe hypoglycemia or experienced one or more episodes of hyperglycemia with ketosis compared with those taking Gla-100.
No unexpected safety concerns were reported based on the established profiles of both products, the company said.
In October 2019, the European Medicines Agency Committee for Medicinal Products for Human Use recommended approval of Toujeo for children age 6 years and older with diabetes.
For more diabetes and endocrinology news, follow us on Twitter and Facebook.
This story first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Toujeo (insulin glargine 300 units/mL injection; Sanofi) to include children as young as 6 years of age with type 1 diabetes.
The FDA first approved Toujeo in 2015 for adults with type 1 and type 2 diabetes, designed as a more potent follow-up to Sanofi’s top-selling insulin glargine (Lantus).
Last month, Sanofi reported positive results from the phase 3 EDITION JUNIOR trial of Toujeo in children and adolescents with type 1 diabetes. These were presented at the International Society for Pediatric and Adolescent Diabetes 45th Annual Conference in Boston.
In the trial, 463 children and adolescents (aged 6-17 years) treated for type 1 diabetes for at least 1 year and with A1c between 7.5% and 11.0% at screening were randomized to Toujeo or insulin glargine 100 units/mL (Gla-100); participants continued to take their existing mealtime insulin.
The primary endpoint was noninferior reduction in A1c after 26 weeks.
The study met its primary endpoint, confirming a noninferior reduction in A1c with Toujeo versus Gla-100 after 26 weeks (mean reduction, 0.4% vs. 0.4%; difference, 0.004%; 95% confidence interval, –0.17 to 0.18; upper bound was below the prespecified noninferiority margin of 0.3%).
Over 26 weeks, a comparable number of patients in each group experienced one or more hypoglycemic events documented at anytime over 24 hours. Numerically fewer patients taking Toujeo experienced severe hypoglycemia or experienced one or more episodes of hyperglycemia with ketosis compared with those taking Gla-100.
No unexpected safety concerns were reported based on the established profiles of both products, the company said.
In October 2019, the European Medicines Agency Committee for Medicinal Products for Human Use recommended approval of Toujeo for children age 6 years and older with diabetes.
For more diabetes and endocrinology news, follow us on Twitter and Facebook.
This story first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Toujeo (insulin glargine 300 units/mL injection; Sanofi) to include children as young as 6 years of age with type 1 diabetes.
The FDA first approved Toujeo in 2015 for adults with type 1 and type 2 diabetes, designed as a more potent follow-up to Sanofi’s top-selling insulin glargine (Lantus).
Last month, Sanofi reported positive results from the phase 3 EDITION JUNIOR trial of Toujeo in children and adolescents with type 1 diabetes. These were presented at the International Society for Pediatric and Adolescent Diabetes 45th Annual Conference in Boston.
In the trial, 463 children and adolescents (aged 6-17 years) treated for type 1 diabetes for at least 1 year and with A1c between 7.5% and 11.0% at screening were randomized to Toujeo or insulin glargine 100 units/mL (Gla-100); participants continued to take their existing mealtime insulin.
The primary endpoint was noninferior reduction in A1c after 26 weeks.
The study met its primary endpoint, confirming a noninferior reduction in A1c with Toujeo versus Gla-100 after 26 weeks (mean reduction, 0.4% vs. 0.4%; difference, 0.004%; 95% confidence interval, –0.17 to 0.18; upper bound was below the prespecified noninferiority margin of 0.3%).
Over 26 weeks, a comparable number of patients in each group experienced one or more hypoglycemic events documented at anytime over 24 hours. Numerically fewer patients taking Toujeo experienced severe hypoglycemia or experienced one or more episodes of hyperglycemia with ketosis compared with those taking Gla-100.
No unexpected safety concerns were reported based on the established profiles of both products, the company said.
In October 2019, the European Medicines Agency Committee for Medicinal Products for Human Use recommended approval of Toujeo for children age 6 years and older with diabetes.
For more diabetes and endocrinology news, follow us on Twitter and Facebook.
This story first appeared on Medscape.com.