User login
Extent of insulin rationing in the U.S. is ‘shameful,’ say experts
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
DoD Explores Virtual Health for Traumatic Brain Injury
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
Improving Veteran Care With the Mission Act
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
NATIONAL HARBOR, MD–The US Department of Veterans Affairs (VA) is in the midst of a significant change in the way it will deliver care to veterans. Agency officials remain optimistic that the change will be for the better, and early indications are positive.
The change is being driven by the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (Mission) Act of 2018, a bill that opens health services options for veterans and integrates VA-administered care and care from community-based providers.
“This is change that is enhancing their experience in the system, and this is enhancing their options and the quality of the options in the system,” Jennifer MacDonald, MD, chief consultant to the principal deputy undersecretary for health at the VA, said during a December 3 session at the AMSUS 2019 annual meeting. “We need also for our workforce to understand how important they are to us across this degree of change.”
Dr. MacDonald highlighted integration with community-based care, including a community urgent care provision that allows veterans to access urgent care facilities and receive care without the need for prior authorization.
“The important piece about that is that we are also looking at the way this care has been accessed,” she said. “By and large, what we have seen from the data is that veterans are indeed seeking community urgent care at a site close to home. This may be CVS or Walgreens. It may be a stand-alone urgent care with a bit more functionality than those Minute Clinics tend to have. We are seeing veterans typically access care through those sites for those minor concerns and illnesses.”
However, she noted that this type of access does not alter the role the VA plays in administration of health care services.
“We are seeing them come back to VA for the majority of their care and for their core care–when there are serious issues, when insulin needs to be adjusted for diabetes, when there are heart disease medications that need to be refilled–we are seeing veterans not seek out urgent care, but come to us, and that is exactly what we want,” she said. “We want the continuity of care to continue and we want to help guide people to the right care, right place, right time.”
Dr. MacDonald also highlighted the expansion of a program that provides a stipend to caregivers that allows veterans to avoid institutionalization and remain within the community under that caregiver’s (a family or friend) supervision. This will expand by year’s end to Vietnam War-era veterans and within 2 years, to veterans that fall between the Vietnam War-era and the September 11, 2001, terrorist attacks.
“We wanted to do this equitably across all eras of veterans,” she said. “This now gives us that opportunity.”
Telehealth also plays a key role.
“For the first time ever, VA now has what we term ‘anywhere-to-anywhere’ telehealth under the Mission Act, an enormous opportunity for us,” she said. “Since we stretch … from New York City to Guam, we need the opportunity to provide care where it may be difficult to recruit and retain providers wherever veterans choose to live,” she said. “We believe that we should be able to meet people where they are regardless of where they choose to live. That’s an aspirational vision, but it is one we believe is exceptionally important and indeed we are moving toward that.”
These are just the beginning; the full implementation of the act goes out to 2034.
According to Dr. MacDonald, the agency is working hard to engage both veterans and the workforce to keep tabs on how the implementation is going.
“It’s a fundamental change in the day-to-day business that they’ve been doing, sometimes for years, and so extremely important across this change is that we have set up processes and now a joint operations center and a number of forums to hear directly from our front line and make sure that their issues are our issues in central office, in DC here, and that they feel heard and that they know that when they have needs, those needs are actioned,” she said.
The VA, under the Mission Act, is also working hard to engage health care providers in the community, including making VA training to community partners, including training on opioid use, suicide prevent and military culture.
However, all these change are for naught if the veterans are not on board. But so far, Dr. MacDonald said the early feedback is very positive.
She cited a VFW survey that asked a question about the Mission Act changes so far and whether they would recommend the VA to other veterans. Ninety percent of the respondents answered they would.
“That’s our marker that we are getting somewhere with these changes and the way we do business,” she said. “That is what we want to see continue to increase.”
Survival gains in HR+/HER2– MBC trials yet to be seen in real world
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
The introduction over the last decade of new systemic therapies for the treatment of hormone receptor positive, HER2-negative metastatic breast cancer has not translated into improved survival in a real-world setting, results of a retrospective study suggest.
Among 2,197 patients who received at least one line of systemic therapy for hormone receptor positive, HER2-negative metastatic breast cancer (HR+/HER2– MBC) from 2003 to 2013, there were no significant differences in median duration of hormonal therapy or median overall survival (OS) for patients treated in any of three time spans during that 10-year period, reported Dan Le, MD, MHA, of BC Cancer, Surrey, B.C., and colleagues.
“Despite the introduction of 9 new adjuvant therapies and 2 new metastatic treatments, survival in the metastatic setting for HR-positive, HER2-negative breast cancer did not improve between 2003 and 2013,” they wrote in a report published in Cancer.
Improvements in adjuvant therapy such as the introduction of cyclin-dependent kinase inhibitors (CDKI) may result in fewer relapses but may also affect the response of relapsed cancers to additional lines of therapy, the authors contended.
“Improved adjuvant therapy means that the cancers that do relapse may have more adverse biology, either intrinsically or because of selective pressure and clonal evolution from exposure to more and better drugs in the adjuvant setting. These factors could, in part, explain the lack of improved survival over time observed in this study,” they wrote.
To see whether significant increases in progression-free survival (PFS) in a clinical trial translated into improved outcomes – including OS – in population-based settings, the investigators identified 2,432 patients with HR+/HER2– MBC from data in the prospective Breast Cancer Outcomes Unit Database of BC Cancer. Of this group, 2,197 received at least one line of systemic therapy after an MBC diagnosis, and 1,752 received first and/or second-line hormonal therapy as well.
The patients were treated in one of three time cohorts: from 2003 through 2005, 2007 through 2009, or 2011 through 2013.
Nine new adjuvant systemic therapies with or without neoadjuvant therapy were approved by BC Cancer during the study period. For the entire decade of the study, the mean survival time was 3.1 years, and the median OS was 2.0 years.
The longest survival for patients diagnosed from 2003 through 2005 was 14.6 years, with 18.1% of these patients living at least 5 years after diagnosis. For patients diagnosed from 2007 through 2009, the longest survival was 10.6 years, with 17.7% of these patients living 5 years or longer post diagnosis. For patients in the most recent cohort (with patients diagnosed after August 2012 excluded), the longest survival was 6.6 years, with 17.3% living at least 5 years after diagnosis.
Overall, patients had a median of 9 months of first-line hormonal treatment, and 6.1 months of second-line hormonal therapy, with nearly identical duration across all three time cohorts.
“Ultimately, it seems likely that the greater the proportion of patients we cure with modern adjuvant therapy, the more challenging it will be to improve outcomes for patients with relapsed disease. This underscores the importance of 1) making continued progress in the adjuvant management of potentially curable breast cancer by first studying new therapeutic agents in the metastatic setting and 2) developing a better understanding of how selective pressure and clonal evolution may lead to more resistant biologic phenotypes in MBC,” the investigators wrote.
No specific study funding was disclosed. No authors disclosed potential conflicts of interest.
SOURCE: Le D et al. Cancer 2019 Nov 21. doi: 10.1002/cncr.32631.
FROM CANCER
Getting a Leg up on the Diagnosis
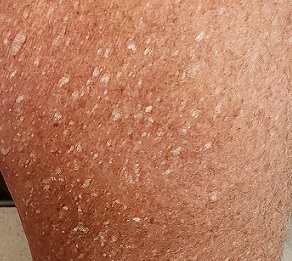
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.
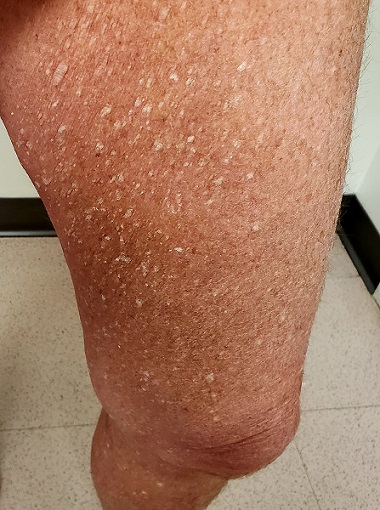
What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.

What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Three years ago, lesions began appearing on this now 68-year-old woman’s legs. They have grown in size and number, and their roughness disturbs the patient. She has been told the lesions are related to aging, but she has never seen anything like them on her friends or family—and she is worried about what they might mean for her health.
Her primary care provider diagnosed warts and performed cryotherapy on several of the lesions. However, the pain was intolerable and the treatment ineffective. To add insult to injury, each treated spot blistered and took more than a month to heal, leaving behind a pinkish brown blemish.
In all other respects, the patient’s health is excellent.
EXAMINATION
Both legs, from the upper thighs to the tops of the feet, are covered with thousands of uniformly distributed, tiny, keratotic, rough, dry papules. All the lesions are essentially identical: white, with no associated signs of inflammation. The patient’s skin is quite dry in general. Neither her palms nor soles are affected.

What’s the diagnosis?
DISCUSSION
The most common problem seen in dermatology offices worldwide is seborrheic keratosis (SK), a totally benign epidermal excrescence that appears to be related to aging and heredity. Most patients are in their 50s when they first notice an SK, and with a bit of luck, they will only see a few in their lifetime. But some patients develop hundreds of SKs, many of which become quite large (3-5 cm) and unsightly. In certain circumstances, SKs can herald the arrival of an occult carcinoma (the Leser-Trelat sign).
This patient has what some consider a variant of SK, called stucco keratosis. These lesions manifest almost exclusively on the lower legs and feet—perhaps due to the relative lack of sebaceous glands in those areas—and most often on men older than 60. Distressing as they are, stucco keratoses have no pathologic implications.
Grossly and histologically, stucco keratoses are different from ordinary SKs. Each stucco keratosis lesion is essentially identical to the others, with a spiculated surface, white color, and average diameter of 2 to 3 mm. Histologically, they demonstrate a thickened epidermis with focal exophytic upward projections that resemble church spires. The lesions do not extend into the dermis.
Treatment of stucco keratoses is, at best, tedious, painful, and futile. The modalities used are cryotherapy or electrodessication with curettage. For a degree of comfort, use of a loofah after bathing will remove or smooth down a few lesions, but this process must be followed by application of a heavy emollient. Alas, regrowth is a certainty.
TAKE-HOME LEARNING POINTS
- Stucco keratosis is considered a variant of seborrheic keratosis, although they differ in several significant ways.
- The lesions of stucco keratosis are fairly uniform in appearance: white, rough, spiculated, epidermal papules measuring 2 to 3 mm.
- Stucco keratoses affect about 10% of the population (men more often than women) and have no racial predilection or pathologic implications.
- The lesions are found almost exclusively on the legs, from the knees down to and including the dorsa of the feet.
- Treatment is far from satisfactory, for multiple reasons, including resultant pain and scarring.
Is it time for neurologists to manage high blood pressure?
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Nov. 1, 2019, issue of JAMA Neurology, an editorial argues that it’s time for neurologists to start managing high blood pressure.
It makes some very valid points: that targeting a systolic blood pressure of less than 120 mm Hg results in lower rates of cardiovascular events and all causes of mortality, that poorly controlled hypertension leads to debilitating neurologic conditions, and that high blood pressure is the most common modifiable risk factor for stroke.
All are strong points. I agree with them and definitely believe that more can and should be done to control hypertension.
The editorial then goes on to say that “first and foremost we are charging neurologists with actively diagnosing hypertension and prescribing medications when appropriate.”
Uh, no. I’m not going to be the one managing hypertension, nor should any outpatient neurologist.
Outpatient hypertension treatment has historically been, and should remain, the province of general practitioners, cardiologists, and nephrologists. Too many cooks, as they say, spoils the broth. I don’t want to be in a situation where two (or more) doctors are simultaneously trying to treat the same condition. On that path lies danger.
This doesn’t mean I ignore blood pressure. On the contrary, I take it (myself) at every patient visit, and put it in my note. In most cases I do nothing further, as nothing further needs to be done. On occasion, though, if it’s concerningly high, I’ll write it down for the patient and direct them to call the physician handling it. I also fax a note about it to that office, and if it’s dangerously high will call the doctor myself.
But try to manage it? No. Elevated readings definitely overlap with my world, but treating them shouldn’t.
The article says that, for some chronic patients, neurologists are their de facto internist. Perhaps for a few, but when a patient calls with concerns about a respiratory ailment, gastrointestinal problem, or other nonneurologic issue, I tell them to call their general practitioner. If they don’t have one I’m happy to give them the names and phone numbers of colleagues who practice that field, or even urgent care and emergency department information if needed. Just because I see them for their neurologic problems doesn’t qualify me to practice another branch of medicine.
Beyond the dangers of having more than one doctor involved, as a specialist it’s not practical for me to know the antihypertensive medications – possibly the largest group of agents on the market, – in detail, with their mechanisms of action, side effects, and contraindications. Yes, I do keep a handful in mind, since they’re needed off label for migraines and tremors, but not in the kind of detail a cardiologist would. I have to keep track of enough medications in my specialty as it is.
I wouldn’t try to handle blood pressure any more than I’d expect a nephrologist to treat epilepsy. It’s just looking for trouble.
Even when covering the hospital, I’ll stay out of that arena. This doesn’t mean I ignore blood pressure in such serious conditions as stroke or posterior reversible encephalopathy syndrome. I’m more than happy to provide guidelines and parameters. But as far as choosing the medications and doses? No.
Like driving, we all have to share the road. We may even be focused on the same journey (or patient). But part of practicing medicine and handling traffic is knowing when to stay in your lane.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Single-fraction radiation just misses mark for spinal compression relief
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
Single-fraction radiation could not be shown to be noninferior to multi-fraction radiation at improving walking function in patients with spinal compression from metastatic cancer, but the small differences seen in a noninferiority trial may not matter to patients, investigators suggest.
Among 686 patients with spinal compression from metastatic cancer randomly assigned in a clinical trial to receive either 8 Gy of radiation in a single fraction or 20 Gy delivered in 5 fractions over 5 consecutive days, 69.3% of patients in the single-fraction arm had good ambulatory status at 8 weeks, compared with 72.7% of patients in the multi-fraction arm (P for noninferiority = .06), reported Peter J Hoskin, BSc, MBBS, MD, of Mount Vernon Cancer Centre in Northwood, England, and colleagues.
The trial did not meet the endpoint of noninferiority of single-fraction radiation for improving ambulation at 8 weeks because the lower limit of the 95% confidence interval (CI) was –11.5%, overlapping the noninferiority margin of –11%.
“However, for all other time points, the CI limits were within the noninferiority margin, and the observed risk differences between single-fraction and multi-fraction radiotherapy groups in ambulatory status were small and unlikely to be of clinical importance,” the investigators wrote in JAMA.
The authors note that although radiotherapy is widely used as a palliative measure for patients with spinal canal compression caused my metastatic disease, there is no agreement on the optimum schedule, with some guidelines recommending higher doses in multiple fractions, and others recommending a single 8 Gy does for patients with painful spinal sites.
To see whether single-fraction radiation could be noninferior to multi-fraction, the investigators enrolled patients in 42 sites in the United Kingdom and 5 in Australia into the SCORAD trial, and randomly assigned them to either single-fraction (345 patients) or multi-fraction (341 patients) radiation. The median age of those enrolled was 70 years, and 44% had prostate cancer, 19% had lung cancer, and 12% had breast cancer.
As noted, the primary endpoint of noninferiority of single-fraction radiation at improving ambulatory status at week 8 was not met. Ambulatory status was based on a 4-point scale and was classified as either grade 1: ambulatory without the use of aids and grade 5 of 5 of muscle power, or grade 2: ambulatory with aids or grade 4 of 5 of muscle power.
An analysis of secondary endpoints showed that the difference in ambulatory status grade 1 or 2 in the single- vs. multi-fraction group at week 1 was −0.4% (P value for noninferiority = .004), at week 4 it was −0.7% (P value for noninferiority = .01), and at week 12 it was 4.1% (P value for noninferiority = .002).
Overall survival rates at 12 weeks were 50% in the single-fraction group vs. 55% in the multi-fraction group; this difference was not statistically significant.
Of 11 other secondary endpoints analyzed, including ambulatory and safety endpoints, the between-group differences were not statistically significant or did not meet noninferiority criteria, the authors noted.
They concluded that although the trial did not meet the primary endpoint, ”the extent to which the lower bound of the CI overlapped with the noninferiority margin should be taken into account when interpreting the clinical importance of these findings.”
Cancer Research UK and Cancer Council Queensland funded the trial. Dr. Hoskin reported being supported by the National Institute for Health Research Manchester Biomedical Research Centre.
SOURCE: Hoskin PJ et al. JAMA 2019 Dec 3. doi: 10.1001/jama.2019.17913.
FROM JAMA
Better Planning Needed to Help Veterans During, After Service
NATIONAL HARBOR, MD – More work and planning is needed for veterans after they serve in the Armed Forces, Rep. Brad Wenstrup, MD (R-Ohio), told attendees of the 2019 AMSUS annual meeting.
Rep. Wenstrup, in his December 3 keynote address, suggested that in addition to recruiters asking about what enlistees want to do in the military, they also should be asking what they want to do after military service.
“Let’s plan for that right now because it’s so much better, it’s so much healthier when you have a plan,” he said, noting that many military members struggle when integrating back into civilian life: They don’t have a plan, and they don’t feel “essential” and part of a team the way they did while serving. He pointed to graduating college students who have made plans for their next steps.
“We need to do the same thing for our troops who serve us [and] prepare them for what is next,” he said.
In addition to ensuring the health and well-being of service members when they come home, Rep. Wenstrup also discussed the need to ensure that medical staff are prepared for emergencies, identifying it as one of the challenges of military medicine right now.
“It’s a challenge for a lot in the active component when we are not at war … to sustain those skills, and it is very frustrating to the providers all the way around,” Rep. Wenstrup said. He noted that a lot of military medical staff help keep and refine those skills by working in the trauma department of major city hospitals.
“We are working together to try and bring our military assets, our civilian assets, DEA, whatever we can, together so that we make sure that we can provide and be ready,” he said. “And the importance of building these bridges is key to the future of medicine,” especially in a time of domestic crisis, so that all medical personnel, civilian and military, can work together for the common good of providing that service. “These are bridges we have to build.”
Rep. Wenstrup also talked about the need to do more to ensure a healthier population.
“The key is how do we live healthier, longer, because that is the real savings. A healthy nation is a stronger nation,” he said.
When comparing the amounts paid to providers for surgery with those paid for health and wellness, he contended that high payments for surgeons are due to the high skill set required for difficult procedures. However, health and wellness providers are paid poorly, as is payment for the management of chronic illness. “We don’t pay very well to the doctor who works with the patient and prevents the need to do open heart surgery,” he said. “Let’s start looking at that. Let’s start keeping people healthier longer.”
NATIONAL HARBOR, MD – More work and planning is needed for veterans after they serve in the Armed Forces, Rep. Brad Wenstrup, MD (R-Ohio), told attendees of the 2019 AMSUS annual meeting.
Rep. Wenstrup, in his December 3 keynote address, suggested that in addition to recruiters asking about what enlistees want to do in the military, they also should be asking what they want to do after military service.
“Let’s plan for that right now because it’s so much better, it’s so much healthier when you have a plan,” he said, noting that many military members struggle when integrating back into civilian life: They don’t have a plan, and they don’t feel “essential” and part of a team the way they did while serving. He pointed to graduating college students who have made plans for their next steps.
“We need to do the same thing for our troops who serve us [and] prepare them for what is next,” he said.
In addition to ensuring the health and well-being of service members when they come home, Rep. Wenstrup also discussed the need to ensure that medical staff are prepared for emergencies, identifying it as one of the challenges of military medicine right now.
“It’s a challenge for a lot in the active component when we are not at war … to sustain those skills, and it is very frustrating to the providers all the way around,” Rep. Wenstrup said. He noted that a lot of military medical staff help keep and refine those skills by working in the trauma department of major city hospitals.
“We are working together to try and bring our military assets, our civilian assets, DEA, whatever we can, together so that we make sure that we can provide and be ready,” he said. “And the importance of building these bridges is key to the future of medicine,” especially in a time of domestic crisis, so that all medical personnel, civilian and military, can work together for the common good of providing that service. “These are bridges we have to build.”
Rep. Wenstrup also talked about the need to do more to ensure a healthier population.
“The key is how do we live healthier, longer, because that is the real savings. A healthy nation is a stronger nation,” he said.
When comparing the amounts paid to providers for surgery with those paid for health and wellness, he contended that high payments for surgeons are due to the high skill set required for difficult procedures. However, health and wellness providers are paid poorly, as is payment for the management of chronic illness. “We don’t pay very well to the doctor who works with the patient and prevents the need to do open heart surgery,” he said. “Let’s start looking at that. Let’s start keeping people healthier longer.”
NATIONAL HARBOR, MD – More work and planning is needed for veterans after they serve in the Armed Forces, Rep. Brad Wenstrup, MD (R-Ohio), told attendees of the 2019 AMSUS annual meeting.
Rep. Wenstrup, in his December 3 keynote address, suggested that in addition to recruiters asking about what enlistees want to do in the military, they also should be asking what they want to do after military service.
“Let’s plan for that right now because it’s so much better, it’s so much healthier when you have a plan,” he said, noting that many military members struggle when integrating back into civilian life: They don’t have a plan, and they don’t feel “essential” and part of a team the way they did while serving. He pointed to graduating college students who have made plans for their next steps.
“We need to do the same thing for our troops who serve us [and] prepare them for what is next,” he said.
In addition to ensuring the health and well-being of service members when they come home, Rep. Wenstrup also discussed the need to ensure that medical staff are prepared for emergencies, identifying it as one of the challenges of military medicine right now.
“It’s a challenge for a lot in the active component when we are not at war … to sustain those skills, and it is very frustrating to the providers all the way around,” Rep. Wenstrup said. He noted that a lot of military medical staff help keep and refine those skills by working in the trauma department of major city hospitals.
“We are working together to try and bring our military assets, our civilian assets, DEA, whatever we can, together so that we make sure that we can provide and be ready,” he said. “And the importance of building these bridges is key to the future of medicine,” especially in a time of domestic crisis, so that all medical personnel, civilian and military, can work together for the common good of providing that service. “These are bridges we have to build.”
Rep. Wenstrup also talked about the need to do more to ensure a healthier population.
“The key is how do we live healthier, longer, because that is the real savings. A healthy nation is a stronger nation,” he said.
When comparing the amounts paid to providers for surgery with those paid for health and wellness, he contended that high payments for surgeons are due to the high skill set required for difficult procedures. However, health and wellness providers are paid poorly, as is payment for the management of chronic illness. “We don’t pay very well to the doctor who works with the patient and prevents the need to do open heart surgery,” he said. “Let’s start looking at that. Let’s start keeping people healthier longer.”
Dual vs Triple Therapy Following ACS or PCI in Patients with Atrial Fibrillation
Study Overview
Objective. To compare the benefit of apixaban with a vitamin K antagonist and compare aspirin with placebo in patients with atrial fibrillation who had acute coronary syndrome or underwent percutaneous coronary intervention (PCI) and were planning to take a P2Y12 inhibitor.
Design. Multicenter, international, open-label, prospective randomized controlled trial with a 2-by-2 factorial design.
Setting and participants. 4614 patients who had an acute coronary syndrome or had undergone PCI and were planning to take a P2Y12 inhibitor.
Intervention. Patients were assigned by means of an interactive voice-response system to receive apixaban or a vitamin K antagonist and to receive aspirin or matching placebo for 6 months.
Main outcome measures. The primary outcome was major or clinically relevant nonmajor bleeding. Secondary outcomes included death or hospitalization and a composite of ischemic events.
Main results. At 6 months, major or clinically relevant nonmajor bleeding had occurred in 10.5% of the patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.58-0.81, P < 0.001 for both noninferiority and superiority), and in 16.1% of the patients receiving aspirin, as compared with 9.0% of those receiving placebo (HR 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR 0.83; 95% CI, 0.74-0.93; P = 0.002) and similar incidence of ischemic events.
Conclusion. Among patients with atrial fibrillation and recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, an antithrombotic regimen that included apixaban without aspirin resulted in less bleeding and fewer hospitalizations without significant differences in the incidence of ischemic events than the regimens that included a vitamin K antagonist, aspirin, or both.
Commentary
PCI is performed in about 20% of patients with atrial fibrillation. These patients require dual antiplatelet therapy to prevent ischemic events, combined with long-term anticoagulation to prevent stroke due to atrial fibrillation. Because the combination of anticoagulation and antiplatelet therapy is associated with a higher risk of bleeding, balancing the risk and benefit of dual antiplatelet therapy and anticoagulation in this population is crucial.
Previous studies have assessed the risk and benefit associated with anticoagulation and antiplatelet therapy. When warfarin plus clopidogrel (double therapy) was compared with warfarin, aspirin, and clopidogrel (triple therapy) in patients with acute coronary syndromes and stable ischemic coronary disease undergoing PCI, use of clopidogrel without aspirin (double therapy) was associated with a significant reduction in bleeding complications (19.4% versus 44.4%, HR, 0.36; 95% CI, 0.26-0.20; P < 0.0001) without increasing thrombotic events.1 Recent studies have compared triple therapy with warfarin to double therapy using direct oral anticoagulants (DOAC). The PIONEER AF-PCI study, which compared low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor to vitamin K antagonist plus dual antiplatelet therapy, found that the rates of clinically significant bleeding were lower in the low-dose rivaroxaban group compared to the triple-therapy group with a vitamin K antagonist (16.8% versus 26.7%; HR, 0.59; 95% CI, 0.47-0.76; P < 0.001).2 Similarly, the RE-DUAL PCI studied dabigatran and showed that the dual therapy group with dabigatran had a lower incidence of major or clinically relevant nonmajor bleeding events during follow-up compared to triple therapy including a vitamin K antagonist (15.4% versus 26.9%; HR, 0.52; 95% CI, 0.42-0.63; P < 0.001).3
In this context, Lopes at al investigated the clinical question of dual therapy versus triple therapy by performing a well-designed randomized clinical trial. In this trial with a 2-by-2 factorial design, the authors studied the effect of apixaban compared to vitamin K antagonist and the effect of aspirin compared to placebo. Major or clinically relevant nonmajor bleeding occurred in 10.5% of patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (HR 0.69; 95% CI, 0.58-0.81; P < 0.001). The incidence of major or clinically relevant nonmajor bleeding was higher in patients receiving aspirin than in those receiving placebo (16.1% versus 9.0%; HR, 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR, 0.83; 95% CI, 0.74-0.93; P = 0.002). The incidence of ischemic events was similar between the apixaban group and vitamin K antagonist group and between the aspirin group and placebo group.
The strengths of this current study include the large number of patients it enrolled. Taking the results from the PIONEER-AF, RE-DUAL PCI, and AUGUSTUS trials, it is clear that DOAC reduces the risk of bleeding compared to vitamin K antagonist. In addition, the AUGUSTUS trial was the first that evaluated the effect of aspirin in patients treated with DOAC and antiplatelet therapy. Aspirin was associated with increased risk of bleeding, with a similar rate of ischemic events compared to placebo.
The AUGUSTUS trial has several limitations. Although the incidence of ischemic events was similar between the apixaban group and the vitamin K antagonist group, the study was not powered to evaluate for individual ischemic outcomes. However, there was no clear evidence of an increase in harm. Since more than 90% of P2Y12 inhibitors used were clopidogrel, the safety and efficacy of combining apixaban with ticagrelor or prasugrel will require further study.
Applications for Clinical Practice
In patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, dual therapy with a P2Y12 inhibitor and DOAC should be favored over a regimen that includes a vitamin K antagonist and/or aspirin.
—Taishi Hirai, MD, University of Missouri Medical Center, and John Blair, MD, University of Chicago Medical Center
1. Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115.
2. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-2434.
3. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
Study Overview
Objective. To compare the benefit of apixaban with a vitamin K antagonist and compare aspirin with placebo in patients with atrial fibrillation who had acute coronary syndrome or underwent percutaneous coronary intervention (PCI) and were planning to take a P2Y12 inhibitor.
Design. Multicenter, international, open-label, prospective randomized controlled trial with a 2-by-2 factorial design.
Setting and participants. 4614 patients who had an acute coronary syndrome or had undergone PCI and were planning to take a P2Y12 inhibitor.
Intervention. Patients were assigned by means of an interactive voice-response system to receive apixaban or a vitamin K antagonist and to receive aspirin or matching placebo for 6 months.
Main outcome measures. The primary outcome was major or clinically relevant nonmajor bleeding. Secondary outcomes included death or hospitalization and a composite of ischemic events.
Main results. At 6 months, major or clinically relevant nonmajor bleeding had occurred in 10.5% of the patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.58-0.81, P < 0.001 for both noninferiority and superiority), and in 16.1% of the patients receiving aspirin, as compared with 9.0% of those receiving placebo (HR 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR 0.83; 95% CI, 0.74-0.93; P = 0.002) and similar incidence of ischemic events.
Conclusion. Among patients with atrial fibrillation and recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, an antithrombotic regimen that included apixaban without aspirin resulted in less bleeding and fewer hospitalizations without significant differences in the incidence of ischemic events than the regimens that included a vitamin K antagonist, aspirin, or both.
Commentary
PCI is performed in about 20% of patients with atrial fibrillation. These patients require dual antiplatelet therapy to prevent ischemic events, combined with long-term anticoagulation to prevent stroke due to atrial fibrillation. Because the combination of anticoagulation and antiplatelet therapy is associated with a higher risk of bleeding, balancing the risk and benefit of dual antiplatelet therapy and anticoagulation in this population is crucial.
Previous studies have assessed the risk and benefit associated with anticoagulation and antiplatelet therapy. When warfarin plus clopidogrel (double therapy) was compared with warfarin, aspirin, and clopidogrel (triple therapy) in patients with acute coronary syndromes and stable ischemic coronary disease undergoing PCI, use of clopidogrel without aspirin (double therapy) was associated with a significant reduction in bleeding complications (19.4% versus 44.4%, HR, 0.36; 95% CI, 0.26-0.20; P < 0.0001) without increasing thrombotic events.1 Recent studies have compared triple therapy with warfarin to double therapy using direct oral anticoagulants (DOAC). The PIONEER AF-PCI study, which compared low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor to vitamin K antagonist plus dual antiplatelet therapy, found that the rates of clinically significant bleeding were lower in the low-dose rivaroxaban group compared to the triple-therapy group with a vitamin K antagonist (16.8% versus 26.7%; HR, 0.59; 95% CI, 0.47-0.76; P < 0.001).2 Similarly, the RE-DUAL PCI studied dabigatran and showed that the dual therapy group with dabigatran had a lower incidence of major or clinically relevant nonmajor bleeding events during follow-up compared to triple therapy including a vitamin K antagonist (15.4% versus 26.9%; HR, 0.52; 95% CI, 0.42-0.63; P < 0.001).3
In this context, Lopes at al investigated the clinical question of dual therapy versus triple therapy by performing a well-designed randomized clinical trial. In this trial with a 2-by-2 factorial design, the authors studied the effect of apixaban compared to vitamin K antagonist and the effect of aspirin compared to placebo. Major or clinically relevant nonmajor bleeding occurred in 10.5% of patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (HR 0.69; 95% CI, 0.58-0.81; P < 0.001). The incidence of major or clinically relevant nonmajor bleeding was higher in patients receiving aspirin than in those receiving placebo (16.1% versus 9.0%; HR, 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR, 0.83; 95% CI, 0.74-0.93; P = 0.002). The incidence of ischemic events was similar between the apixaban group and vitamin K antagonist group and between the aspirin group and placebo group.
The strengths of this current study include the large number of patients it enrolled. Taking the results from the PIONEER-AF, RE-DUAL PCI, and AUGUSTUS trials, it is clear that DOAC reduces the risk of bleeding compared to vitamin K antagonist. In addition, the AUGUSTUS trial was the first that evaluated the effect of aspirin in patients treated with DOAC and antiplatelet therapy. Aspirin was associated with increased risk of bleeding, with a similar rate of ischemic events compared to placebo.
The AUGUSTUS trial has several limitations. Although the incidence of ischemic events was similar between the apixaban group and the vitamin K antagonist group, the study was not powered to evaluate for individual ischemic outcomes. However, there was no clear evidence of an increase in harm. Since more than 90% of P2Y12 inhibitors used were clopidogrel, the safety and efficacy of combining apixaban with ticagrelor or prasugrel will require further study.
Applications for Clinical Practice
In patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, dual therapy with a P2Y12 inhibitor and DOAC should be favored over a regimen that includes a vitamin K antagonist and/or aspirin.
—Taishi Hirai, MD, University of Missouri Medical Center, and John Blair, MD, University of Chicago Medical Center
Study Overview
Objective. To compare the benefit of apixaban with a vitamin K antagonist and compare aspirin with placebo in patients with atrial fibrillation who had acute coronary syndrome or underwent percutaneous coronary intervention (PCI) and were planning to take a P2Y12 inhibitor.
Design. Multicenter, international, open-label, prospective randomized controlled trial with a 2-by-2 factorial design.
Setting and participants. 4614 patients who had an acute coronary syndrome or had undergone PCI and were planning to take a P2Y12 inhibitor.
Intervention. Patients were assigned by means of an interactive voice-response system to receive apixaban or a vitamin K antagonist and to receive aspirin or matching placebo for 6 months.
Main outcome measures. The primary outcome was major or clinically relevant nonmajor bleeding. Secondary outcomes included death or hospitalization and a composite of ischemic events.
Main results. At 6 months, major or clinically relevant nonmajor bleeding had occurred in 10.5% of the patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.58-0.81, P < 0.001 for both noninferiority and superiority), and in 16.1% of the patients receiving aspirin, as compared with 9.0% of those receiving placebo (HR 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR 0.83; 95% CI, 0.74-0.93; P = 0.002) and similar incidence of ischemic events.
Conclusion. Among patients with atrial fibrillation and recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, an antithrombotic regimen that included apixaban without aspirin resulted in less bleeding and fewer hospitalizations without significant differences in the incidence of ischemic events than the regimens that included a vitamin K antagonist, aspirin, or both.
Commentary
PCI is performed in about 20% of patients with atrial fibrillation. These patients require dual antiplatelet therapy to prevent ischemic events, combined with long-term anticoagulation to prevent stroke due to atrial fibrillation. Because the combination of anticoagulation and antiplatelet therapy is associated with a higher risk of bleeding, balancing the risk and benefit of dual antiplatelet therapy and anticoagulation in this population is crucial.
Previous studies have assessed the risk and benefit associated with anticoagulation and antiplatelet therapy. When warfarin plus clopidogrel (double therapy) was compared with warfarin, aspirin, and clopidogrel (triple therapy) in patients with acute coronary syndromes and stable ischemic coronary disease undergoing PCI, use of clopidogrel without aspirin (double therapy) was associated with a significant reduction in bleeding complications (19.4% versus 44.4%, HR, 0.36; 95% CI, 0.26-0.20; P < 0.0001) without increasing thrombotic events.1 Recent studies have compared triple therapy with warfarin to double therapy using direct oral anticoagulants (DOAC). The PIONEER AF-PCI study, which compared low-dose rivaroxaban (15 mg once daily) plus a P2Y12 inhibitor to vitamin K antagonist plus dual antiplatelet therapy, found that the rates of clinically significant bleeding were lower in the low-dose rivaroxaban group compared to the triple-therapy group with a vitamin K antagonist (16.8% versus 26.7%; HR, 0.59; 95% CI, 0.47-0.76; P < 0.001).2 Similarly, the RE-DUAL PCI studied dabigatran and showed that the dual therapy group with dabigatran had a lower incidence of major or clinically relevant nonmajor bleeding events during follow-up compared to triple therapy including a vitamin K antagonist (15.4% versus 26.9%; HR, 0.52; 95% CI, 0.42-0.63; P < 0.001).3
In this context, Lopes at al investigated the clinical question of dual therapy versus triple therapy by performing a well-designed randomized clinical trial. In this trial with a 2-by-2 factorial design, the authors studied the effect of apixaban compared to vitamin K antagonist and the effect of aspirin compared to placebo. Major or clinically relevant nonmajor bleeding occurred in 10.5% of patients receiving apixaban, as compared to 14.7% of those receiving a vitamin K antagonist (HR 0.69; 95% CI, 0.58-0.81; P < 0.001). The incidence of major or clinically relevant nonmajor bleeding was higher in patients receiving aspirin than in those receiving placebo (16.1% versus 9.0%; HR, 1.89; 95% CI, 1.59-2.24; P < 0.001). Patients in the apixaban group had a lower incidence of death or hospitalization than those in the vitamin K antagonist group (23.5% versus 27.4%; HR, 0.83; 95% CI, 0.74-0.93; P = 0.002). The incidence of ischemic events was similar between the apixaban group and vitamin K antagonist group and between the aspirin group and placebo group.
The strengths of this current study include the large number of patients it enrolled. Taking the results from the PIONEER-AF, RE-DUAL PCI, and AUGUSTUS trials, it is clear that DOAC reduces the risk of bleeding compared to vitamin K antagonist. In addition, the AUGUSTUS trial was the first that evaluated the effect of aspirin in patients treated with DOAC and antiplatelet therapy. Aspirin was associated with increased risk of bleeding, with a similar rate of ischemic events compared to placebo.
The AUGUSTUS trial has several limitations. Although the incidence of ischemic events was similar between the apixaban group and the vitamin K antagonist group, the study was not powered to evaluate for individual ischemic outcomes. However, there was no clear evidence of an increase in harm. Since more than 90% of P2Y12 inhibitors used were clopidogrel, the safety and efficacy of combining apixaban with ticagrelor or prasugrel will require further study.
Applications for Clinical Practice
In patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, dual therapy with a P2Y12 inhibitor and DOAC should be favored over a regimen that includes a vitamin K antagonist and/or aspirin.
—Taishi Hirai, MD, University of Missouri Medical Center, and John Blair, MD, University of Chicago Medical Center
1. Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115.
2. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-2434.
3. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
1. Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115.
2. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-2434.
3. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.