User login
Eosinophilic Pustular Folliculitis in the Setting of Untreated Chronic Lymphocytic Leukemia
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
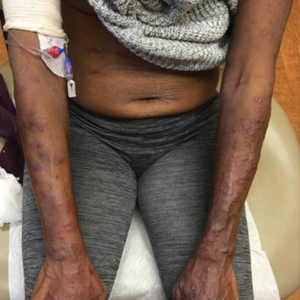
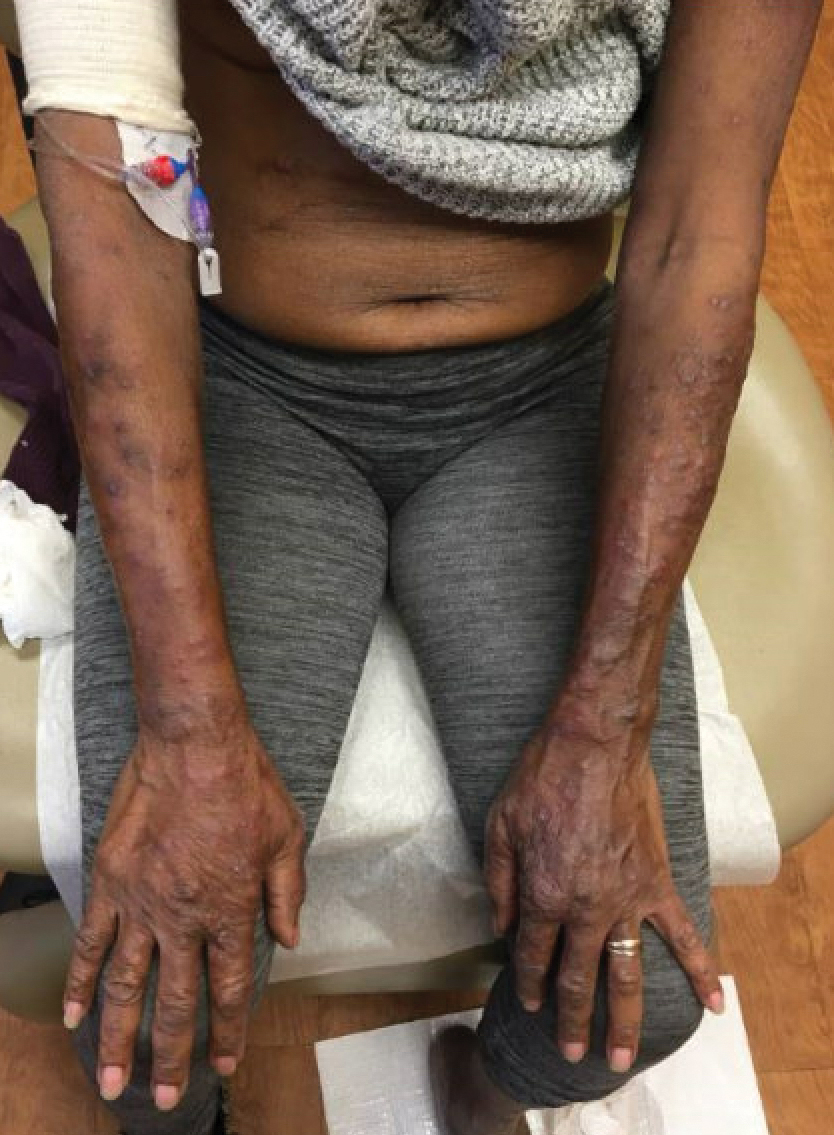
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.
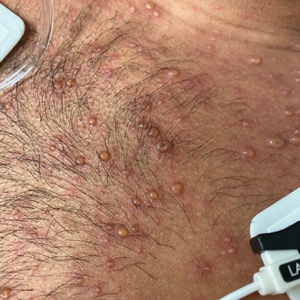
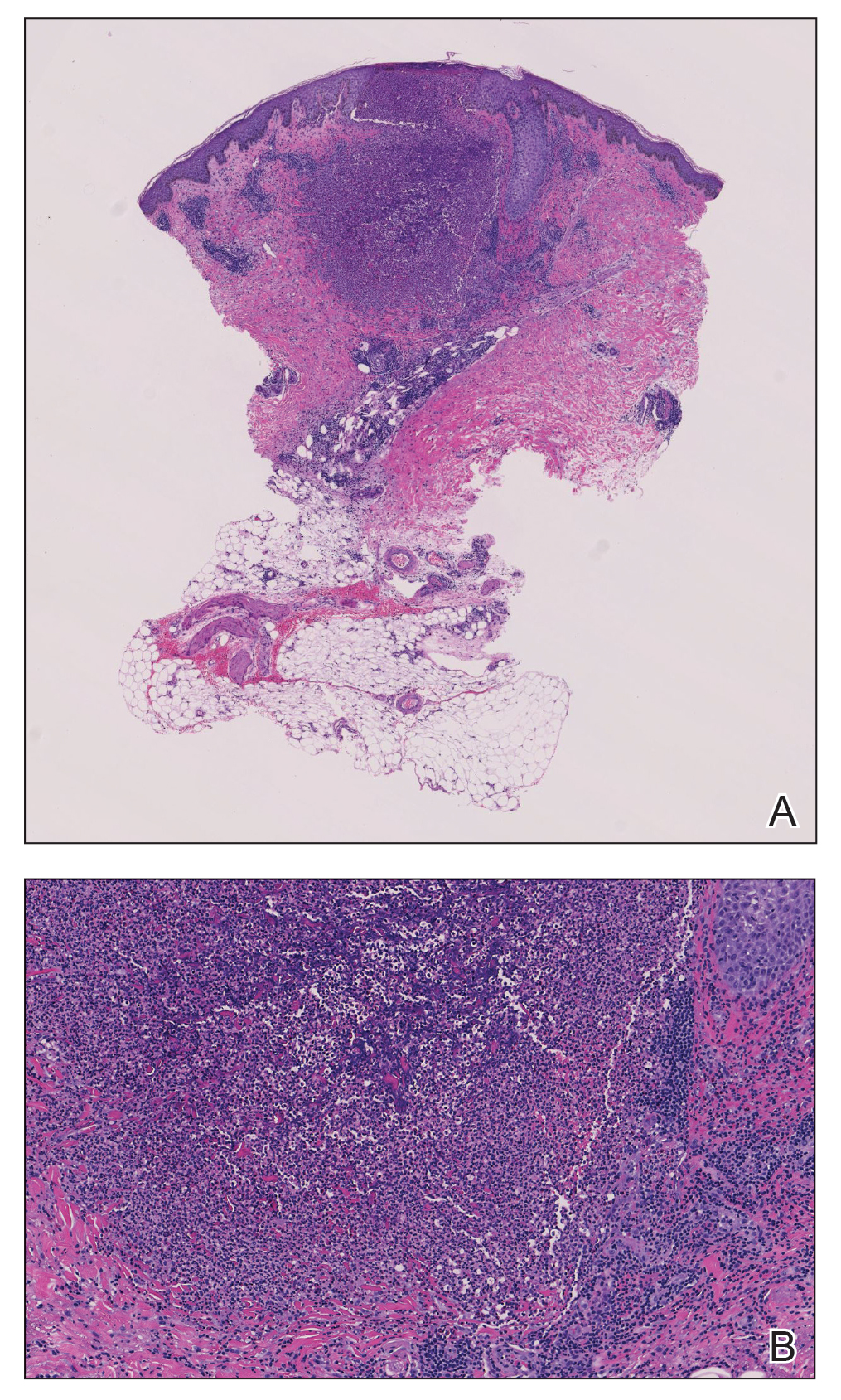
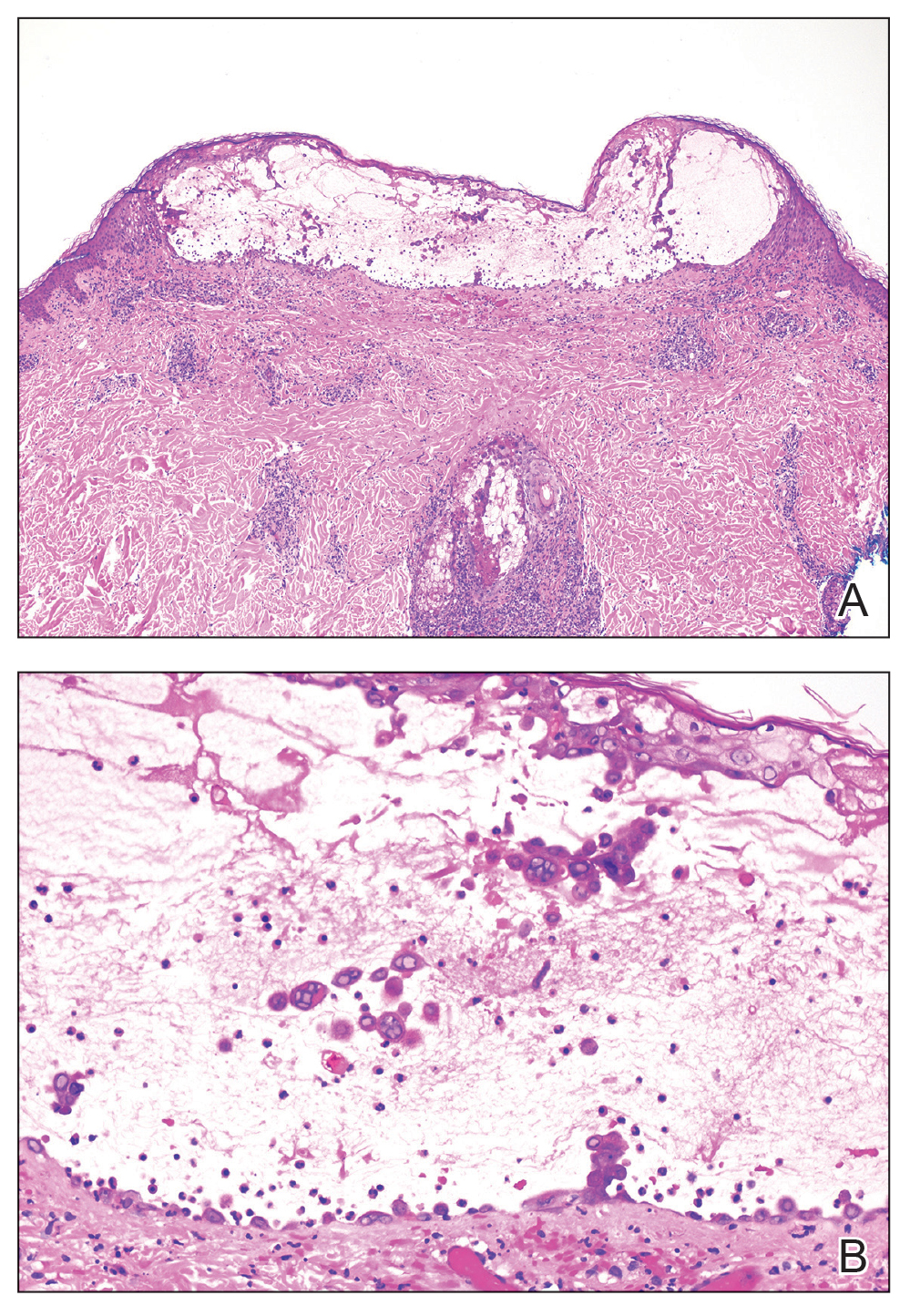
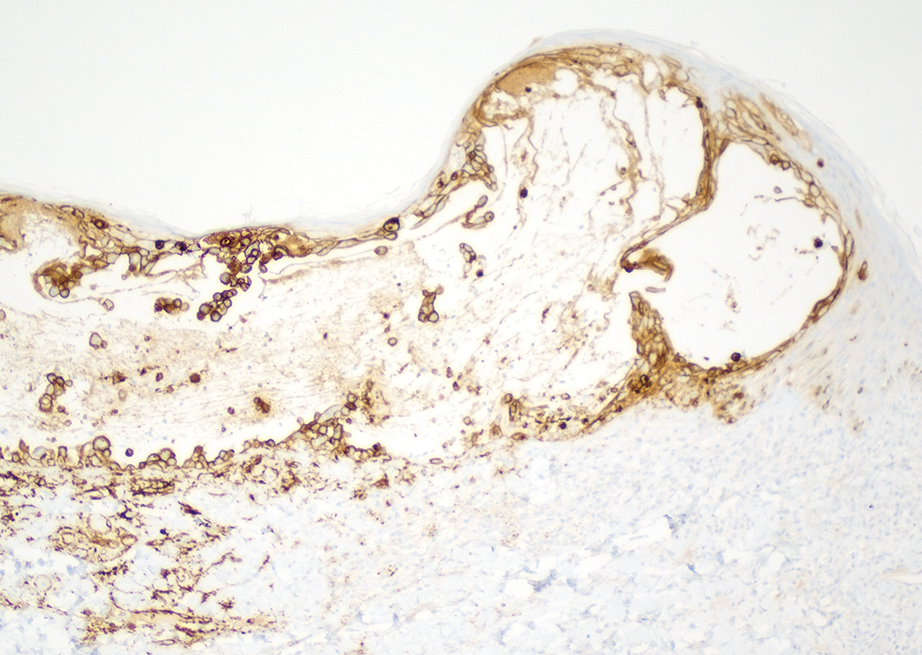
A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.
Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
Practice Points
- Eosinophilic pustular folliculitis (EPF) is associated with an immunosuppressed state, as in patients with underlying hematologic malignancy.
- Topical corticosteroids remain the first-line treatment for EPF; however, antimicrobial agents have been used with moderate success when topical therapies have failed.
Papulosquamous Dermatophytid Reaction in a Child With Tinea Capitis
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
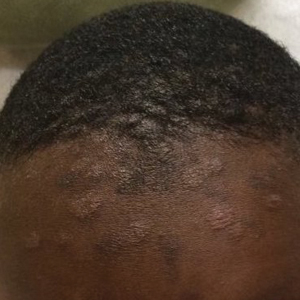
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.
Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
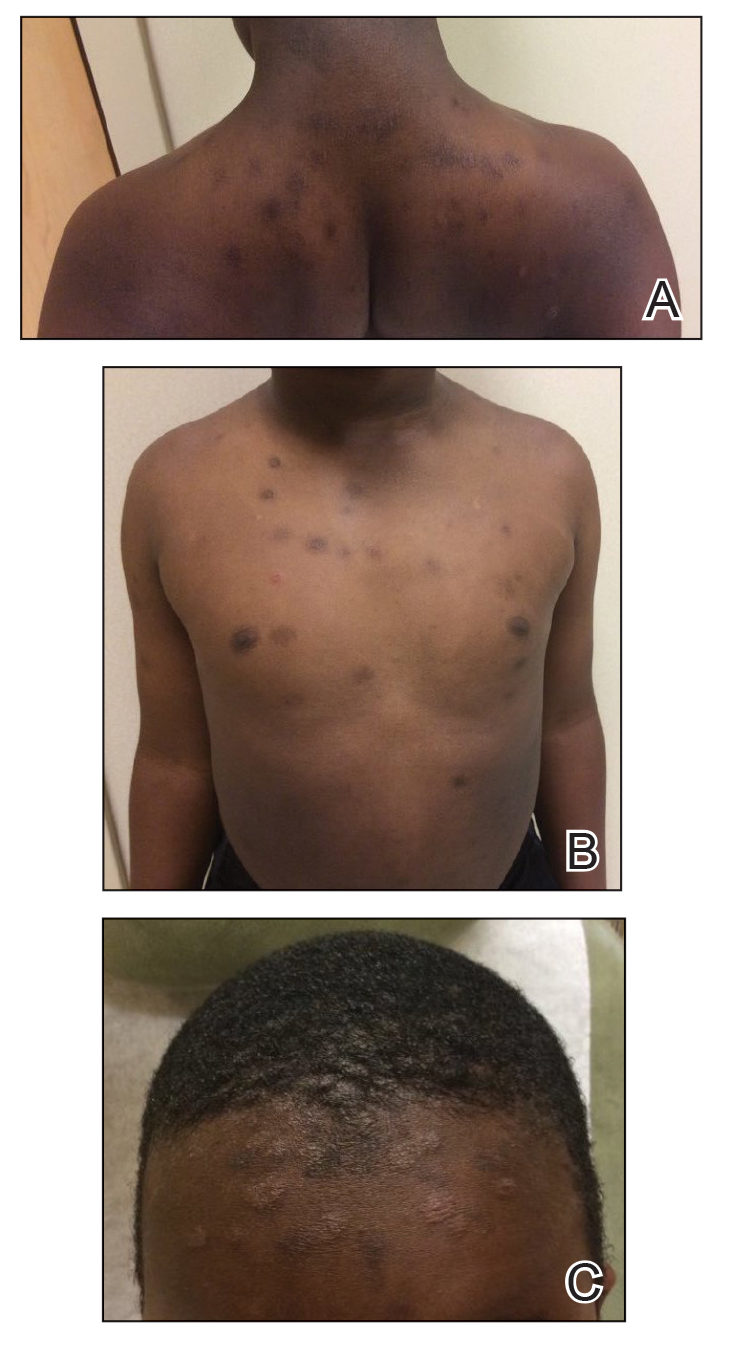
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
Practice Points
- Dermatophytid (id) reactions can manifest as papulosquamous eruptions after cutaneous infections or inflammatory skin conditions.
- High clinical suspicion for id reaction in patients of the appropriate age group and demographic—pediatric patients of African descent—is imperative for reaching the correct diagnosis.
- Repeat cultures of the scalp may be indicated in patients with high clinical probability for an id reaction despite a negative fungal culture or empiric systemic treatment.
The Role of Dermatology in Identifying and Reporting a Primary Varicella Outbreak
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
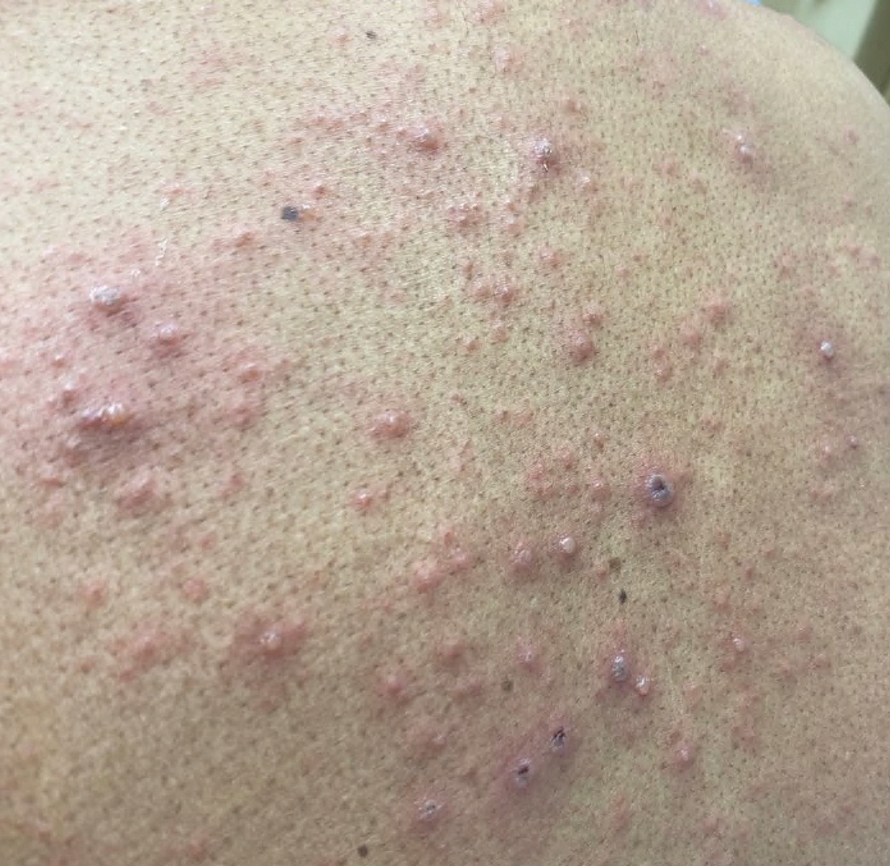
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).
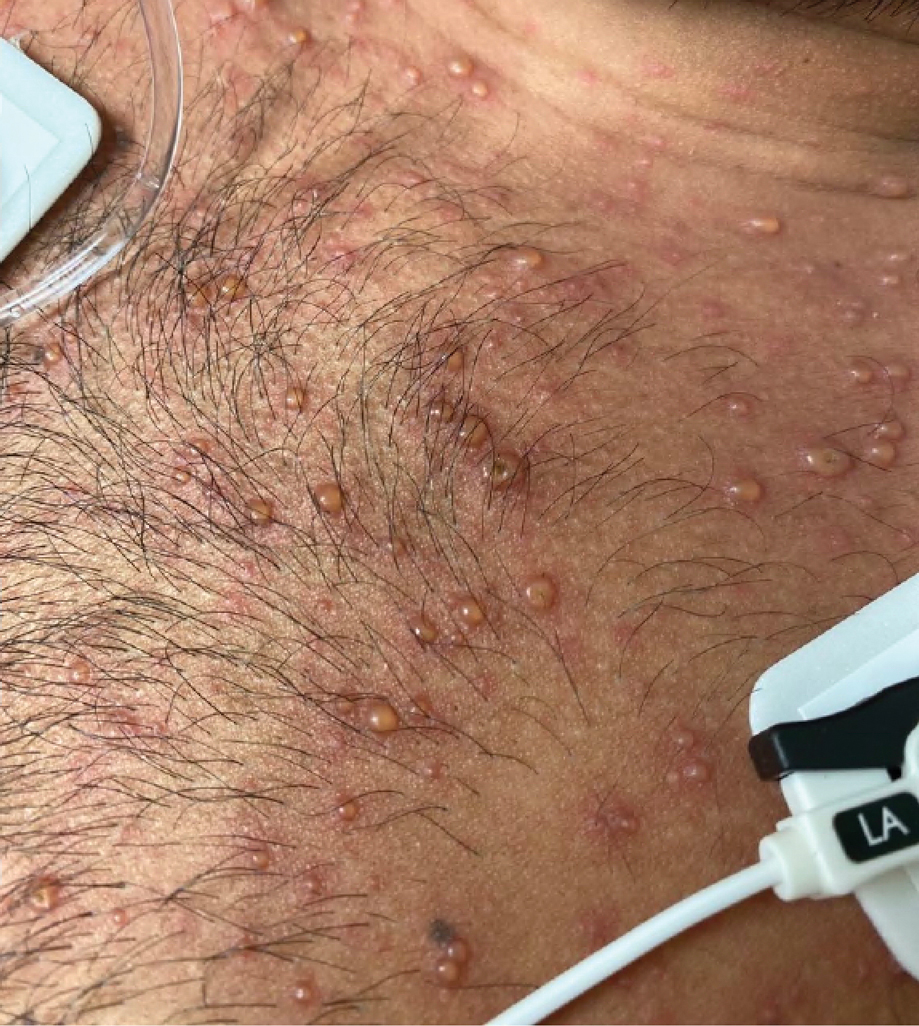
Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).
Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).
Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).
The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
Practice Points
- Primary varicella is a relatively infrequent occurrence since the introduction of vaccination, creating the need for a reminder on the importance of including it in the differential when clinically appropriate.
- When outbreaks do happen, typically among unvaccinated communities, swift identification via physical examination and histology is imperative to allow infection control teams and public health officials to quickly take action.
Getting ready for Boston
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2024 is on track to offer the highest tier of pulmonary, critical care, and sleep medicine education that attendees have come to expect from the CHEST Annual Meeting.
While planning for the meeting started with the open call for 2024 sessions at the conclusion of the CHEST Annual Meeting 2023, CHEST 2024 began to take shape when the schedule—and the curriculum chairs—came together. In mid-February, members of the Scientific Program Committee gathered in person at CHEST headquarters in Glenview, Illinois, to review submissions and solidify the schedule for the upcoming CHEST 2024 meeting, taking place in Boston, October 6 to 9.
Following CHEST 2023 in Honolulu, those planning for Boston were brimming with excitement to start planning a meeting closer to home. One event in particular that committee members are excited for will be a session dedicated to the “Black Angels,” the nurses who helped cure TB, featuring surviving member, Virginia Allen, and book (The Black Angels: The Untold Story of the Nurses Who Helped Cure Tuberculosis) author, Maria Smilios. Because of the location, both Allen and Smilios will be able to join on-site in Boston and will bring with them, for the first time on public display, a curated selection of papers from Edward Robitzek, MD, courtesy of the Robitzek family. This collection will include records of TB treatment trials that forever changed the course of the disease in 1952.
In addition to this look into the history of chest medicine, the CHEST Annual Meeting 2024 will also feature the latest advancements in the field, including the anticipated hot topic of the meeting, the use of artificial intelligence (AI) in medicine.
“There [are] going to be a lot of hot topics covered at CHEST 2024, like bronchoscopy approaches, treatments for COPD,” said Gabe Bosslet, MD, FCCP, Chair of the Scientific Program Committee. “But if there was one that sort of was the outlier this year, I think it’s artificial intelligence and its use in pulmonary and critical care medicine.”
The sessions covering AI include its presence in medical education, as well as treating interstitial lung disease, chest infections, and more.
Beyond the latest in artificial technology, the CHEST Annual Meeting 2024 will feature more than 200 sessions covering eight curriculum groups with something for everyone in chest medicine:
- Airways Disorders
- Critical Care
- Cardiovascular/Pulmonary Vascular Disease
- Chest Infections/Disaster Medicine/Systemic Disease
- Interstitial Lung Disease/Transplant
- Interdisciplinary/Practice Operations/Education
- Lung Cancer/Interventional Pulmonology/Bronchoscopy/Radiology
- Sleep Medicine
The meeting will host topics for a wide range of experience levels (from those still in training to those who are years or decades into their careers) and welcomes all members of the care team. “These are not physician-centric issues, topics, or sessions. These are sessions that if you’re working around patients with pulmonary or critical care diseases, these are definitively for you,” Dr. Bosslet said.
With something for everyone—and for the first time ever in Boston—CHEST 2024 will not be a meeting to miss. Keep an eye out for registration to open in May, as early bird pricing will be available for a short time.
Dr. Danckers’ social media takeover
For an inside look into what happens during a meeting of the Scientific Program Committee, we invited member of the committee, Mauricio Danckers, MD, FCCP, to take the reins of CHEST social media to share his experience.
Dr. Danckers posted behind-the-scenes pictures of each of the curriculum chairs and teased a picture of the completed session schedule.
To see the takeover posts, visit the CHEST Instagram (@accpCHEST) and view the CHEST 2024 story pinned to the top of the profile.


A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2024 is on track to offer the highest tier of pulmonary, critical care, and sleep medicine education that attendees have come to expect from the CHEST Annual Meeting.
While planning for the meeting started with the open call for 2024 sessions at the conclusion of the CHEST Annual Meeting 2023, CHEST 2024 began to take shape when the schedule—and the curriculum chairs—came together. In mid-February, members of the Scientific Program Committee gathered in person at CHEST headquarters in Glenview, Illinois, to review submissions and solidify the schedule for the upcoming CHEST 2024 meeting, taking place in Boston, October 6 to 9.
Following CHEST 2023 in Honolulu, those planning for Boston were brimming with excitement to start planning a meeting closer to home. One event in particular that committee members are excited for will be a session dedicated to the “Black Angels,” the nurses who helped cure TB, featuring surviving member, Virginia Allen, and book (The Black Angels: The Untold Story of the Nurses Who Helped Cure Tuberculosis) author, Maria Smilios. Because of the location, both Allen and Smilios will be able to join on-site in Boston and will bring with them, for the first time on public display, a curated selection of papers from Edward Robitzek, MD, courtesy of the Robitzek family. This collection will include records of TB treatment trials that forever changed the course of the disease in 1952.
In addition to this look into the history of chest medicine, the CHEST Annual Meeting 2024 will also feature the latest advancements in the field, including the anticipated hot topic of the meeting, the use of artificial intelligence (AI) in medicine.
“There [are] going to be a lot of hot topics covered at CHEST 2024, like bronchoscopy approaches, treatments for COPD,” said Gabe Bosslet, MD, FCCP, Chair of the Scientific Program Committee. “But if there was one that sort of was the outlier this year, I think it’s artificial intelligence and its use in pulmonary and critical care medicine.”
The sessions covering AI include its presence in medical education, as well as treating interstitial lung disease, chest infections, and more.
Beyond the latest in artificial technology, the CHEST Annual Meeting 2024 will feature more than 200 sessions covering eight curriculum groups with something for everyone in chest medicine:
- Airways Disorders
- Critical Care
- Cardiovascular/Pulmonary Vascular Disease
- Chest Infections/Disaster Medicine/Systemic Disease
- Interstitial Lung Disease/Transplant
- Interdisciplinary/Practice Operations/Education
- Lung Cancer/Interventional Pulmonology/Bronchoscopy/Radiology
- Sleep Medicine
The meeting will host topics for a wide range of experience levels (from those still in training to those who are years or decades into their careers) and welcomes all members of the care team. “These are not physician-centric issues, topics, or sessions. These are sessions that if you’re working around patients with pulmonary or critical care diseases, these are definitively for you,” Dr. Bosslet said.
With something for everyone—and for the first time ever in Boston—CHEST 2024 will not be a meeting to miss. Keep an eye out for registration to open in May, as early bird pricing will be available for a short time.
Dr. Danckers’ social media takeover
For an inside look into what happens during a meeting of the Scientific Program Committee, we invited member of the committee, Mauricio Danckers, MD, FCCP, to take the reins of CHEST social media to share his experience.
Dr. Danckers posted behind-the-scenes pictures of each of the curriculum chairs and teased a picture of the completed session schedule.
To see the takeover posts, visit the CHEST Instagram (@accpCHEST) and view the CHEST 2024 story pinned to the top of the profile.


A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2024 is on track to offer the highest tier of pulmonary, critical care, and sleep medicine education that attendees have come to expect from the CHEST Annual Meeting.
While planning for the meeting started with the open call for 2024 sessions at the conclusion of the CHEST Annual Meeting 2023, CHEST 2024 began to take shape when the schedule—and the curriculum chairs—came together. In mid-February, members of the Scientific Program Committee gathered in person at CHEST headquarters in Glenview, Illinois, to review submissions and solidify the schedule for the upcoming CHEST 2024 meeting, taking place in Boston, October 6 to 9.
Following CHEST 2023 in Honolulu, those planning for Boston were brimming with excitement to start planning a meeting closer to home. One event in particular that committee members are excited for will be a session dedicated to the “Black Angels,” the nurses who helped cure TB, featuring surviving member, Virginia Allen, and book (The Black Angels: The Untold Story of the Nurses Who Helped Cure Tuberculosis) author, Maria Smilios. Because of the location, both Allen and Smilios will be able to join on-site in Boston and will bring with them, for the first time on public display, a curated selection of papers from Edward Robitzek, MD, courtesy of the Robitzek family. This collection will include records of TB treatment trials that forever changed the course of the disease in 1952.
In addition to this look into the history of chest medicine, the CHEST Annual Meeting 2024 will also feature the latest advancements in the field, including the anticipated hot topic of the meeting, the use of artificial intelligence (AI) in medicine.
“There [are] going to be a lot of hot topics covered at CHEST 2024, like bronchoscopy approaches, treatments for COPD,” said Gabe Bosslet, MD, FCCP, Chair of the Scientific Program Committee. “But if there was one that sort of was the outlier this year, I think it’s artificial intelligence and its use in pulmonary and critical care medicine.”
The sessions covering AI include its presence in medical education, as well as treating interstitial lung disease, chest infections, and more.
Beyond the latest in artificial technology, the CHEST Annual Meeting 2024 will feature more than 200 sessions covering eight curriculum groups with something for everyone in chest medicine:
- Airways Disorders
- Critical Care
- Cardiovascular/Pulmonary Vascular Disease
- Chest Infections/Disaster Medicine/Systemic Disease
- Interstitial Lung Disease/Transplant
- Interdisciplinary/Practice Operations/Education
- Lung Cancer/Interventional Pulmonology/Bronchoscopy/Radiology
- Sleep Medicine
The meeting will host topics for a wide range of experience levels (from those still in training to those who are years or decades into their careers) and welcomes all members of the care team. “These are not physician-centric issues, topics, or sessions. These are sessions that if you’re working around patients with pulmonary or critical care diseases, these are definitively for you,” Dr. Bosslet said.
With something for everyone—and for the first time ever in Boston—CHEST 2024 will not be a meeting to miss. Keep an eye out for registration to open in May, as early bird pricing will be available for a short time.
Dr. Danckers’ social media takeover
For an inside look into what happens during a meeting of the Scientific Program Committee, we invited member of the committee, Mauricio Danckers, MD, FCCP, to take the reins of CHEST social media to share his experience.
Dr. Danckers posted behind-the-scenes pictures of each of the curriculum chairs and teased a picture of the completed session schedule.
To see the takeover posts, visit the CHEST Instagram (@accpCHEST) and view the CHEST 2024 story pinned to the top of the profile.


Certain Pesticides Linked With Risk for Pancreatic Cancer
One of them, a case-control study, showed an elevated risk in individuals whose adipose tissue contained substances that are now banned.
“The association between pesticides and pancreatic cancer exists. It is of low magnitude but robust, concerning cumulative pesticides and three substances: Mancozeb, glyphosate, and sulfur in spray form,” said Mathias Brugel, MD, hospital practitioner at Basque Coast Hospital Center in Bayonne, France, during his presentation.
Regarding the four other liposoluble substances associated with an increased risk for pancreatic cancer in the second study, “their use has been banned since the 1990s, but they are still present in soils and in the air,” Dr. Brugel told this news organization.
For example, in Reims, France, the assessment of air quality by ATMO Grand Est revealed the presence of banned pesticides in the air, he added. However, Dr. Brugel stressed that a cause-effect relationship between pesticide exposure and the risk for pancreatic cancer cannot be established with these studies.
Incidence Rising Constantly
The incidence of pancreatic adenocarcinoma has been increasing steadily for more than 30 years. In France, nearly 16,000 new cases were reported in 2023, which represented an annual increase of about 2%. According to the National Cancer Institute, “pancreatic adenocarcinoma could become the second leading cause of cancer mortality by 2030.”
“This increase in incidence is particularly strong in France compared with other Western countries. The causes are still poorly understood. One might wonder whether environmental factors like pesticides are involved,” said Dr. Brugel.
Known to have a mechanism of action favoring oncogenesis, pesticides are suspected of being responsible for the rise in certain cancers, especially given their extensive use in France. In total, around 300 substances are authorized, and 65,000 tons are applied each year, making France the largest consumer of pesticides in Europe.
“Contamination is ubiquitous, meaning they are found in soil, water, air, and in individuals,” said Dr. Brugel. According to a study by the Institute for Scientific Expertise Research, pesticide residues were detected in 64% of hair samples taken from French volunteers.
The literature increasingly reported data suggesting a link between pesticide exposure and the development of certain diseases like cancer. A 2021 document by Inserm notably confirmed the strong presumption of a link between occupational pesticide exposure and pathologies such as non-Hodgkin’s lymphoma and prostate cancer.
High-Incidence Zones
To explore the link between pesticide exposure and pancreatic cancer, Dr. Brugel and his colleagues conducted the EcoPESTIPAC and PESTIPAC studies, the results of which were presented at this year’s conference.
In EcoPESTIPAC, researchers conducted a national ecological regression by dividing the entire French territory into 5529 spatial units. The number of pancreatic cancer cases per spatial unit per year (disease-mapping) was determined using the National Health Data System.
Nine chemicals, including glyphosate, were included, thus covering half of pesticide purchases in France. The cumulative quantity of pesticides, regardless of molecule, was also examined. Pesticide exposure was estimated by the median ratio between pesticide purchase and agricultural area per spatial unit over an 11-year period from early 2011 to the end of 2021.
Mor than 134,000 cases of pancreatic cancer were reported during this period. The analysis revealed three high-incidence zones located around Paris, in central France, and in the Mediterranean basin, while spatial units in the western region showed the lowest incidences.
The heterogeneous distribution of the disease suggests the involvement of risk factors, said Dr. Brugel. After adjusting for confounding factors such as smoking, the study showed an increased risk for pancreatic cancer associated with the cumulative quantity of pesticides and three specific substances: Sulfur in spray form, mancozeb, and glyphosate.
Risk Increases
A dose-response relationship was evident. For an increase in pesticide use of 2.5 kg/hectare over 11 years, the risk for pancreatic adenocarcinoma increased from 0.9% to 1.4%. “The increase is relatively small, but one must not forget that this risk applies to all of France,” said Dr. Brugel. Indeed, the risk appeared homogeneous across the entire territory.
This was the first study to explore this link at the national level. Although the association between the four identified factors and pancreatic risk was robust, the study had some limitations. It relied on the quantities of pesticides purchased to estimate the quantities used, Dr. Brugel pointed out.
The second study, PESTIPAC, was a case-control study conducted at the Reims University Hospital to explore the association between pancreatic adenocarcinoma and concentrations of organochlorine pesticides in fat and urine.
The study included 26 patients with pancreatic cancer who had abdominal surgery that allowed for adipose tissue sampling (minimum 10 g). Urine was collected in the morning on an empty stomach.
A control group was formed by including 26 other patients who underwent surgery for a benign abdominal condition such as gallstones or hernia, thus allowing for the same sampling. Individuals in both groups were matched for age and body mass index, two risk factors for pancreatic cancer.
Banned Substances
In total, 345 substances were searched for using chromatography and mass spectrometry. Analyses revealed the presence of five banned substances in all patients, while nine substances were found in half of the samples.
“Contamination is very widespread, both in patients with pancreatic cancer and in the controls,” said Dr. Brugel. Consequently, for this study, between-group comparisons of substances present in all individuals could not be performed.
After adjustment, an association with an increased risk for pancreatic cancer was nonetheless observed with four liposoluble substances: 4,4-DDE, mirex or perchlordecone, trans-nonachlor, and cis-nonachlor. All four substances are herbicides that have been banned for at least 30 years.
The study also aimed to assess the effect of pesticide presence in the body on survival after pancreatic cancer. The results showed no significant difference for overall survival or progression-free survival.
“Pesticides are a credible candidate to explain the increase in the incidence of pancreatic adenocarcinoma,” said Dr. Brugel. However, “if associations between pancreatic cancer and pesticides exist, they remain poorly understood, and it is difficult to establish clear causality.”
Further large-scale studies will be needed to confirm these associations. An evaluation of the general population’s exposure to banned substances also appears justified, according to the researchers.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
One of them, a case-control study, showed an elevated risk in individuals whose adipose tissue contained substances that are now banned.
“The association between pesticides and pancreatic cancer exists. It is of low magnitude but robust, concerning cumulative pesticides and three substances: Mancozeb, glyphosate, and sulfur in spray form,” said Mathias Brugel, MD, hospital practitioner at Basque Coast Hospital Center in Bayonne, France, during his presentation.
Regarding the four other liposoluble substances associated with an increased risk for pancreatic cancer in the second study, “their use has been banned since the 1990s, but they are still present in soils and in the air,” Dr. Brugel told this news organization.
For example, in Reims, France, the assessment of air quality by ATMO Grand Est revealed the presence of banned pesticides in the air, he added. However, Dr. Brugel stressed that a cause-effect relationship between pesticide exposure and the risk for pancreatic cancer cannot be established with these studies.
Incidence Rising Constantly
The incidence of pancreatic adenocarcinoma has been increasing steadily for more than 30 years. In France, nearly 16,000 new cases were reported in 2023, which represented an annual increase of about 2%. According to the National Cancer Institute, “pancreatic adenocarcinoma could become the second leading cause of cancer mortality by 2030.”
“This increase in incidence is particularly strong in France compared with other Western countries. The causes are still poorly understood. One might wonder whether environmental factors like pesticides are involved,” said Dr. Brugel.
Known to have a mechanism of action favoring oncogenesis, pesticides are suspected of being responsible for the rise in certain cancers, especially given their extensive use in France. In total, around 300 substances are authorized, and 65,000 tons are applied each year, making France the largest consumer of pesticides in Europe.
“Contamination is ubiquitous, meaning they are found in soil, water, air, and in individuals,” said Dr. Brugel. According to a study by the Institute for Scientific Expertise Research, pesticide residues were detected in 64% of hair samples taken from French volunteers.
The literature increasingly reported data suggesting a link between pesticide exposure and the development of certain diseases like cancer. A 2021 document by Inserm notably confirmed the strong presumption of a link between occupational pesticide exposure and pathologies such as non-Hodgkin’s lymphoma and prostate cancer.
High-Incidence Zones
To explore the link between pesticide exposure and pancreatic cancer, Dr. Brugel and his colleagues conducted the EcoPESTIPAC and PESTIPAC studies, the results of which were presented at this year’s conference.
In EcoPESTIPAC, researchers conducted a national ecological regression by dividing the entire French territory into 5529 spatial units. The number of pancreatic cancer cases per spatial unit per year (disease-mapping) was determined using the National Health Data System.
Nine chemicals, including glyphosate, were included, thus covering half of pesticide purchases in France. The cumulative quantity of pesticides, regardless of molecule, was also examined. Pesticide exposure was estimated by the median ratio between pesticide purchase and agricultural area per spatial unit over an 11-year period from early 2011 to the end of 2021.
Mor than 134,000 cases of pancreatic cancer were reported during this period. The analysis revealed three high-incidence zones located around Paris, in central France, and in the Mediterranean basin, while spatial units in the western region showed the lowest incidences.
The heterogeneous distribution of the disease suggests the involvement of risk factors, said Dr. Brugel. After adjusting for confounding factors such as smoking, the study showed an increased risk for pancreatic cancer associated with the cumulative quantity of pesticides and three specific substances: Sulfur in spray form, mancozeb, and glyphosate.
Risk Increases
A dose-response relationship was evident. For an increase in pesticide use of 2.5 kg/hectare over 11 years, the risk for pancreatic adenocarcinoma increased from 0.9% to 1.4%. “The increase is relatively small, but one must not forget that this risk applies to all of France,” said Dr. Brugel. Indeed, the risk appeared homogeneous across the entire territory.
This was the first study to explore this link at the national level. Although the association between the four identified factors and pancreatic risk was robust, the study had some limitations. It relied on the quantities of pesticides purchased to estimate the quantities used, Dr. Brugel pointed out.
The second study, PESTIPAC, was a case-control study conducted at the Reims University Hospital to explore the association between pancreatic adenocarcinoma and concentrations of organochlorine pesticides in fat and urine.
The study included 26 patients with pancreatic cancer who had abdominal surgery that allowed for adipose tissue sampling (minimum 10 g). Urine was collected in the morning on an empty stomach.
A control group was formed by including 26 other patients who underwent surgery for a benign abdominal condition such as gallstones or hernia, thus allowing for the same sampling. Individuals in both groups were matched for age and body mass index, two risk factors for pancreatic cancer.
Banned Substances
In total, 345 substances were searched for using chromatography and mass spectrometry. Analyses revealed the presence of five banned substances in all patients, while nine substances were found in half of the samples.
“Contamination is very widespread, both in patients with pancreatic cancer and in the controls,” said Dr. Brugel. Consequently, for this study, between-group comparisons of substances present in all individuals could not be performed.
After adjustment, an association with an increased risk for pancreatic cancer was nonetheless observed with four liposoluble substances: 4,4-DDE, mirex or perchlordecone, trans-nonachlor, and cis-nonachlor. All four substances are herbicides that have been banned for at least 30 years.
The study also aimed to assess the effect of pesticide presence in the body on survival after pancreatic cancer. The results showed no significant difference for overall survival or progression-free survival.
“Pesticides are a credible candidate to explain the increase in the incidence of pancreatic adenocarcinoma,” said Dr. Brugel. However, “if associations between pancreatic cancer and pesticides exist, they remain poorly understood, and it is difficult to establish clear causality.”
Further large-scale studies will be needed to confirm these associations. An evaluation of the general population’s exposure to banned substances also appears justified, according to the researchers.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
One of them, a case-control study, showed an elevated risk in individuals whose adipose tissue contained substances that are now banned.
“The association between pesticides and pancreatic cancer exists. It is of low magnitude but robust, concerning cumulative pesticides and three substances: Mancozeb, glyphosate, and sulfur in spray form,” said Mathias Brugel, MD, hospital practitioner at Basque Coast Hospital Center in Bayonne, France, during his presentation.
Regarding the four other liposoluble substances associated with an increased risk for pancreatic cancer in the second study, “their use has been banned since the 1990s, but they are still present in soils and in the air,” Dr. Brugel told this news organization.
For example, in Reims, France, the assessment of air quality by ATMO Grand Est revealed the presence of banned pesticides in the air, he added. However, Dr. Brugel stressed that a cause-effect relationship between pesticide exposure and the risk for pancreatic cancer cannot be established with these studies.
Incidence Rising Constantly
The incidence of pancreatic adenocarcinoma has been increasing steadily for more than 30 years. In France, nearly 16,000 new cases were reported in 2023, which represented an annual increase of about 2%. According to the National Cancer Institute, “pancreatic adenocarcinoma could become the second leading cause of cancer mortality by 2030.”
“This increase in incidence is particularly strong in France compared with other Western countries. The causes are still poorly understood. One might wonder whether environmental factors like pesticides are involved,” said Dr. Brugel.
Known to have a mechanism of action favoring oncogenesis, pesticides are suspected of being responsible for the rise in certain cancers, especially given their extensive use in France. In total, around 300 substances are authorized, and 65,000 tons are applied each year, making France the largest consumer of pesticides in Europe.
“Contamination is ubiquitous, meaning they are found in soil, water, air, and in individuals,” said Dr. Brugel. According to a study by the Institute for Scientific Expertise Research, pesticide residues were detected in 64% of hair samples taken from French volunteers.
The literature increasingly reported data suggesting a link between pesticide exposure and the development of certain diseases like cancer. A 2021 document by Inserm notably confirmed the strong presumption of a link between occupational pesticide exposure and pathologies such as non-Hodgkin’s lymphoma and prostate cancer.
High-Incidence Zones
To explore the link between pesticide exposure and pancreatic cancer, Dr. Brugel and his colleagues conducted the EcoPESTIPAC and PESTIPAC studies, the results of which were presented at this year’s conference.
In EcoPESTIPAC, researchers conducted a national ecological regression by dividing the entire French territory into 5529 spatial units. The number of pancreatic cancer cases per spatial unit per year (disease-mapping) was determined using the National Health Data System.
Nine chemicals, including glyphosate, were included, thus covering half of pesticide purchases in France. The cumulative quantity of pesticides, regardless of molecule, was also examined. Pesticide exposure was estimated by the median ratio between pesticide purchase and agricultural area per spatial unit over an 11-year period from early 2011 to the end of 2021.
Mor than 134,000 cases of pancreatic cancer were reported during this period. The analysis revealed three high-incidence zones located around Paris, in central France, and in the Mediterranean basin, while spatial units in the western region showed the lowest incidences.
The heterogeneous distribution of the disease suggests the involvement of risk factors, said Dr. Brugel. After adjusting for confounding factors such as smoking, the study showed an increased risk for pancreatic cancer associated with the cumulative quantity of pesticides and three specific substances: Sulfur in spray form, mancozeb, and glyphosate.
Risk Increases
A dose-response relationship was evident. For an increase in pesticide use of 2.5 kg/hectare over 11 years, the risk for pancreatic adenocarcinoma increased from 0.9% to 1.4%. “The increase is relatively small, but one must not forget that this risk applies to all of France,” said Dr. Brugel. Indeed, the risk appeared homogeneous across the entire territory.
This was the first study to explore this link at the national level. Although the association between the four identified factors and pancreatic risk was robust, the study had some limitations. It relied on the quantities of pesticides purchased to estimate the quantities used, Dr. Brugel pointed out.
The second study, PESTIPAC, was a case-control study conducted at the Reims University Hospital to explore the association between pancreatic adenocarcinoma and concentrations of organochlorine pesticides in fat and urine.
The study included 26 patients with pancreatic cancer who had abdominal surgery that allowed for adipose tissue sampling (minimum 10 g). Urine was collected in the morning on an empty stomach.
A control group was formed by including 26 other patients who underwent surgery for a benign abdominal condition such as gallstones or hernia, thus allowing for the same sampling. Individuals in both groups were matched for age and body mass index, two risk factors for pancreatic cancer.
Banned Substances
In total, 345 substances were searched for using chromatography and mass spectrometry. Analyses revealed the presence of five banned substances in all patients, while nine substances were found in half of the samples.
“Contamination is very widespread, both in patients with pancreatic cancer and in the controls,” said Dr. Brugel. Consequently, for this study, between-group comparisons of substances present in all individuals could not be performed.
After adjustment, an association with an increased risk for pancreatic cancer was nonetheless observed with four liposoluble substances: 4,4-DDE, mirex or perchlordecone, trans-nonachlor, and cis-nonachlor. All four substances are herbicides that have been banned for at least 30 years.
The study also aimed to assess the effect of pesticide presence in the body on survival after pancreatic cancer. The results showed no significant difference for overall survival or progression-free survival.
“Pesticides are a credible candidate to explain the increase in the incidence of pancreatic adenocarcinoma,” said Dr. Brugel. However, “if associations between pancreatic cancer and pesticides exist, they remain poorly understood, and it is difficult to establish clear causality.”
Further large-scale studies will be needed to confirm these associations. An evaluation of the general population’s exposure to banned substances also appears justified, according to the researchers.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Do New Antiobesity Meds Still Require Lifestyle Management?
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Is lifestyle counseling needed with the more effective second-generation nutrient-stimulated, hormone-based medications like semaglutide and tirzepatide?
If so, how intensive does the counseling need to be, and what components should be emphasized?
These are the clinical practice questions at the top of mind for healthcare professionals and researchers who provide care to patients who have overweight and/or obesity.
This is what we know. Lifestyle management is considered foundational in the care of patients with obesity.
Because obesity is fundamentally a disease of energy dysregulation, counseling has traditionally focused on dietary caloric reduction, increased physical activity, and strategies to adapt new cognitive and lifestyle behaviors.
On the basis of trial results from the Diabetes Prevention Program and the Look AHEAD studies, provision of intensive behavioral therapy (IBT) is recommended for treatment of obesity by the Centers for Medicare & Medicaid Services and by the US Preventive Services Task Force (Moyer VA; US Preventive Services Task Force).
IBT is commonly defined as consisting of 12-26 comprehensive and multicomponent sessions over the course of a year.
Reaffirming the primacy of lifestyle management, all antiobesity medications are approved by the US Food and Drug Administration as an adjunct to a reduced-calorie diet and increased physical activity.
The beneficial effect of combining IBT with earlier-generation medications like naltrexone/bupropion or liraglutide demonstrated that more participants in the trials achieved ≥ 10% weight loss with IBT compared with those taking the medication without IBT: 38.4% vs 20% for naltrexone/bupropion and 46% vs 33% for liraglutide.
Although there aren’t trial data for other first-generation medications like phentermine, orlistat, or phentermine/topiramate, it is assumed that patients taking these medications would also achieve greater weight loss when combined with IBT.
The obesity pharmacotherapy landscape was upended, however, with the approval of semaglutide (Wegovy), a glucagon-like peptide-1 (GLP-1) receptor agonist, in 2021; and tirzepatide (Zepbound), a GLP-1 and glucose-dependent insulinotropic polypeptide dual receptor agonist, in 2023.
These highly effective medications harness the effect of naturally occurring incretin hormones that reduce appetite through direct and indirect effects on the brain. Although the study designs differed between the STEP 1 and STEP 3 trials, the addition of IBT to semaglutide increased mean percent weight loss from 15% to 16% after 68 weeks of treatment (Wilding JPH et al; Wadden TA).
Comparable benefits from the STEP 3 and SURMOUNT-1 trials of adding IBT to tirzepatide at the maximal tolerated dose increased mean percent weight loss from 21% to 24% after 72 weeks (Wadden TA; Jastreboff AM). Though multicomponent IBT appears to provide greater weight loss when used with nutrient-stimulated hormone-based therapeutics, the additional benefit may be less when compared with first-generation medications.
So, how should we view the role and importance of lifestyle management when a patient is taking a second-generation medication? We need to shift the focus from prescribing a calorie-reduced diet to counseling for healthy eating patterns.
Because the second-generation drugs are more biologically effective in suppressing appetite (ie, reducing hunger, food noise, and cravings, and increasing satiation and satiety), it is easier for patients to reduce their food intake without a sense of deprivation. Furthermore, many patients express less desire to consume savory, sweet, and other enticing foods.
Patients should be encouraged to optimize the quality of their diet, prioritizing lean protein sources with meals and snacks; increasing fruits, vegetables, fiber, and complex carbohydrates; and keeping well hydrated. Because of the risk of developing micronutrient deficiencies while consuming a low-calorie diet — most notably calcium, iron, and vitamin D — patients may be advised to take a daily multivitamin supplement. Dietary counseling should be introduced when patients start pharmacotherapy, and if needed, referral to a registered dietitian nutritionist may be helpful in making these changes.
Additional counseling tips to mitigate the gastrointestinal side effects of these drugs that most commonly occur during the early dose-escalation phase include eating slowly; choosing smaller portion sizes; stopping eating when full; not skipping meals; and avoiding fatty, fried, and greasy foods. These dietary changes are particularly important over the first days after patients take the injection.
The increased weight loss achieved also raises concerns about the need to maintain lean body mass and the importance of physical activity and exercise counseling. All weight loss interventions, including dietary restriction, pharmacotherapy, or bariatric surgery, result in loss of fat mass and lean body mass.
The goal of lifestyle counseling is to minimize and preserve muscle mass (a component of lean body mass) which is needed for optimal health, mobility, daily function, and quality of life. Counseling should incorporate both aerobic and resistance training. Aerobic exercise (eg, brisk walking, jogging, dancing, elliptical machine, and cycling) improves cardiovascular fitness, metabolic health, and energy expenditure. Resistance (strength) training (eg, weightlifting, resistance bands, and circuit training) lessens the loss of muscle mass, enhances functional strength and mobility, and improves bone density (Gorgojo-Martinez JJ et al; Oppert JM et al).
Robust physical activity has also been shown to be a predictor of weight loss maintenance. A recently published randomized placebo-controlled trial demonstrated the benefit of supervised exercise in maintaining body weight and lean body mass after discontinuing 52 weeks of liraglutide treatment compared with no exercise.
Rather than minimizing the provision of lifestyle management, using highly effective second-generation therapeutics redirects the focus on how patients with obesity can strive to achieve a healthy and productive life.
A version of this article first appeared on Medscape.com.
Why Do So Many Doctors Embrace Superstitions and Rituals?
The second-floor operating rooms at Lehigh Valley Hospital in Allentown, Pennsylvania, are numbered sequentially — except when you get to what should be operation room (OR) 13. It’s OR M. The M doesn’t stand for Maternity or any other specialty. Rather in this high-tech, state-of-the art healthcare center, it’s there to ward off bad juju and evil spirits.
“Just as taller buildings usually don’t have a 13th floor or hotels don’t have a room 13, it revolves around the common superstition of the unlucky nature of number 13,” said a hospital spokesperson.
During the pandemic, the public was told repeatedly that modern medicine is science-based. But when I started talking to surgeons and other physicians for this article, I uncovered something decidedly unscientific.
In ORs and emergency rooms (ERs), small-town doctor’s offices, and mega hospitals, there’s a measure of dread before full moons and Friday the 13th, and no one dares utter the Q word (as in, “It sure is quiet today.”) That would risk bringing the wrath of the medical gods, and you’d earn the reputation of being a jinx or “black cloud.” Likewise, the songs “Stairway to Heaven” or “Another One Bites the Dust” will never be heard in any waiting room, elevator, or OR.
Indeed, when it comes to superstitions and rituals in medicine, it seems everyone has a story or a belief. …
A 2-Hour Ritual
Carmen Fong, MD, a colorectal surgeon in New York City, had a presurgical ritual that took her nearly 2 hours to complete. “I’d wake up at the same time every day, pack two hard-boiled eggs and a thermos of coffee in my small leather bag, walk to work via the same route, and swipe into the preop area while waving hi to the front desk,” she recounted. “I’d talk to the patient, sign the consent with the same ballpoint pen, go upstairs to my office, change into my scrubs [same cap and Danskos], then turn on my computer, and take a sip of coffee before heading back down to the OR. I’d always remove my badge and place it near the nurses’ workstation, then put on the patient’s SCDs [sequential compression devices] myself. I’d hold the oxygen mask while telling the patient, ‘See you later.’ Never ‘It will be okay’ or ‘Have a good sleep.’ Always ‘See you later.’ ”
Dr. Fong did this for 5 years prior to more than a thousand surgeries. She did it because it made her feel calm and in control, which translated to more successful operations. “It never failed me.”
Wonder Woman Clogs
Anureet Bajaj, MD, a plastic surgeon in Oklahoma City, wore Wonder Woman clogs in the OR for years because “they made me feel stronger, and my surgeries went better.” She’s also very specific about her OR playlist; “it must be ‘80s music.” And for a time, she wore a friendship bracelet that one of her employees made to commemorate getting through a particularly hard day. “If I forgot it, my heart sank, and my anxiety rose,” she said. “Wearing it gave me security and confidence that the day would go well.”
A Moment of Silence
Juliet Emamaullee, MD, PhD, is a liver and kidney transplant surgeon at Keck Hospital and Children’s Hospital Los Angeles. Because of the complexity of her operations, she must know every aspect of her patients’ medical history. This leads to a level of intimacy that most people never have with their doctors. “Transplant surgeons are playing god in many ways,” she said. “During procurement, after we prep and drape the donor and right before I make the incision, everyone in the OR has a moment of silence to acknowledge the donation. If the organ has been transported, then I’ll say a prayer to myself that I do good work with this generous gift of life.”
Magical Thinking
Before we go any further, I should clarify that there’s a difference between rituals and superstitions like the ones just shared and routines and practices such as handwashing or doublechecking that it’s the right hip and not the left. All pilots have a preflight checklist that’s necessary for safety, but some might also make the sign of the cross.
Lester Gottesman, MD, has been a surgeon at the Icahn School of Medicine at Mount Sinai Hospital in New York City for nearly 50 years. He believes rituals and superstitions are more prevalent in medicine than in any other profession, despite there being no definitive research confirming their effectiveness.
In fact, it’s the opposite.
One of the few studies to examine superstitions among physicians was published in the Annals of Surgery in 2021. Researchers analyzed the operational records of 27,914 consecutive patients who underwent general, visceral, or vascular surgery. They found no association of moon phases, zodiac signs, or Friday the 13th with poor outcomes. Having acute coronary syndrome on Friday the 13th also did not influence the 13-year mortality rate compared to other dates in the year. And although 70% of physicians believe that some colleagues are “black clouds,” an analysis of 96 physicians and 6149 admissions found no such pattern.
Granted, this is just one analysis, but the results aren’t surprising. No one really believes in this stuff. So, why does it persist?
Dr. Gottesman cited an episode from the popular medical TV show Grey’s Anatomy, in which chief surgeon Meredith Grey puts it this way: “Superstition lies in the space between what we can control and what we can’t. …We rely on superstitions because we are smart enough to know we don’t have all the answers and that life works in mysterious ways. Don’t diss the juju from wherever it comes.”
“Superstition and science both start at the same place — to explain an unexplainable event,” said Dr. Gottesman, who always checks his suture lines at the end of a surgery in the order in which he did them. “If science provides a coherent answer, so be it. If not, the human’s need for order will assign causality to otherwise inanimate objects, noncausal events, or divine influence.”
In other words, the more unknowns and trepidation, the greater the tendency toward what Dr. Gottesman called “magical thinking.” And when you consider healing’s long history, you realize that ritual and superstition defined medicine for centuries. Gottesman pointed out that it wasn’t until Hippocrates separated religion and superstition from disease around 430 BC that modern medicine was born. But because doctors still don’t know everything, an element of magic endures.
The question is, in this high-tech age, do these stubborn beliefs still have a place? Do they help or hinder doctors, and, most important, do they have any effect on patient outcomes?
Five Benefits
To reiterate, there are no studies showing that Wonder Woman clogs convey surgical superpowers or that eating two hard-boiled eggs boosts OR performance. But anecdotally, many doctors admit to experiencing noticeable perks from their quirks. Let’s start with the supposed benefits:
- Less stress: A quarter of US clinicians are considering switching careers, primarily due to burnout, according to a 2022 Bain survey. “The fact that [rituals and superstitions] are so prevalent in such a high-stress field can’t be coincidence,” said Dr. Fong. “Offloading some of the responsibility to whatever gods there may be is a way of taming our anxieties so we can function better.”
- Hyperfocus: Dr. Emamaullee played volleyball in high school and college. She suggested that her presurgical routine isn’t all that different from her warmup before a championship match. It’s habitual behavior that helps induce a state of heightened concentration, confidence, and immersion. Athletes call it being “in the zone” or in a “state of flow,” and Dr. Emamaullee said she experiences the same thing in the OR.
- More control: Remember those horrific images of patients with COVID-19 overwhelming ERs in Brooklyn and Queens during the pandemic? Dr. Fong was in the middle of that. “In crisis situations where there are more unknowns, rituals and superstitions become even more important,” she said. “I may not be able to control what’s happening, but I can control myself. Rituals help restore some normalcy and organization, and they give me a sense of calm.”
- Better performance: A series of general-population experiments published in the journal Psychological Science in 2010 concluded that “good-luck–related superstitions” boosted self-confidence in mastering upcoming tasks and improved motor dexterity, memory, and overall performance.
- Placebo effect: This phenomenon is well-established in medicine. Give someone a special pill or treatment, and a significant portion will claim benefit. “Placebo is magical thinking,” said Dr. Gottesman. “It has identifiable and quantifiable effects on human disease.” And perhaps on medical practitioners, too. If a doctor believes her friendship bracelet has special powers and helps her be a better physician, then it just might.
Four Drawbacks
- Compulsive behavior: When superstitious beliefs or repetitive behaviors begin causing personal distress, interfering with daily duties, or negatively affecting patient outcomes, then there’s a problem. There’s a story on Quora about a neurosurgeon who always ate two Hostess Ho Hos chocolate cakes before operations. When he forgot to do so one day, he supposedly left his patient on the table and ran off to eat them. Even if it’s urban legend, it’s a useful illustration of quirk disrupting work.
- Less flexibility: Every human body and every surgery is different. “When ritualistic behaviors or habits become so rigid that you lose the ability to adapt, then that becomes dangerous for the patient,” said Dr. Fong. “The art of medicine, not unlike jazz, often comes from the improvisation.”
- Self-fulfilling: Just as rituals and superstitions can empower and provide a sense of control, they can quickly turn on physicians who forget a part of their routine or leave their talisman on the bureau. Instead of confidence, they supply doubt. The karma becomes kryptonite.
- Avoiding responsibility: After years of friendship bracelets and Wonder Woman clogs, Dr. Bajaj is making a deliberate effort to excise magical thinking from her practice. “It can hold you back if you’re not careful,” she said. “If you start using it as a crutch when something goes wrong — like ‘Oh, I wasn’t wearing my clogs today and that’s why my flap failed’ — then you’re not doing your due diligence and figuring out what really happened.” Rather than placing the responsibility for her day going well on superstition, she’s trying to own it herself by living with more intent.
The Diagnosis
Most of the medical experts I spoke with didn’t think there was anything wrong with rituals or superstitions as long as they didn’t become compulsive or a convenient repository of blame.
“Rituals and superstitions are an acknowledgment that forces external to ourselves exist,” concluded Dr. Fong. “They’re like tiny offerings to whatever gods are out there to please be on our side. And we keep doing them because there’s a reward — better patient outcomes, which is all we want to achieve in the end. I say embrace them.”
A version of this article first appeared on Medscape.com.
The second-floor operating rooms at Lehigh Valley Hospital in Allentown, Pennsylvania, are numbered sequentially — except when you get to what should be operation room (OR) 13. It’s OR M. The M doesn’t stand for Maternity or any other specialty. Rather in this high-tech, state-of-the art healthcare center, it’s there to ward off bad juju and evil spirits.
“Just as taller buildings usually don’t have a 13th floor or hotels don’t have a room 13, it revolves around the common superstition of the unlucky nature of number 13,” said a hospital spokesperson.
During the pandemic, the public was told repeatedly that modern medicine is science-based. But when I started talking to surgeons and other physicians for this article, I uncovered something decidedly unscientific.
In ORs and emergency rooms (ERs), small-town doctor’s offices, and mega hospitals, there’s a measure of dread before full moons and Friday the 13th, and no one dares utter the Q word (as in, “It sure is quiet today.”) That would risk bringing the wrath of the medical gods, and you’d earn the reputation of being a jinx or “black cloud.” Likewise, the songs “Stairway to Heaven” or “Another One Bites the Dust” will never be heard in any waiting room, elevator, or OR.
Indeed, when it comes to superstitions and rituals in medicine, it seems everyone has a story or a belief. …
A 2-Hour Ritual
Carmen Fong, MD, a colorectal surgeon in New York City, had a presurgical ritual that took her nearly 2 hours to complete. “I’d wake up at the same time every day, pack two hard-boiled eggs and a thermos of coffee in my small leather bag, walk to work via the same route, and swipe into the preop area while waving hi to the front desk,” she recounted. “I’d talk to the patient, sign the consent with the same ballpoint pen, go upstairs to my office, change into my scrubs [same cap and Danskos], then turn on my computer, and take a sip of coffee before heading back down to the OR. I’d always remove my badge and place it near the nurses’ workstation, then put on the patient’s SCDs [sequential compression devices] myself. I’d hold the oxygen mask while telling the patient, ‘See you later.’ Never ‘It will be okay’ or ‘Have a good sleep.’ Always ‘See you later.’ ”
Dr. Fong did this for 5 years prior to more than a thousand surgeries. She did it because it made her feel calm and in control, which translated to more successful operations. “It never failed me.”
Wonder Woman Clogs
Anureet Bajaj, MD, a plastic surgeon in Oklahoma City, wore Wonder Woman clogs in the OR for years because “they made me feel stronger, and my surgeries went better.” She’s also very specific about her OR playlist; “it must be ‘80s music.” And for a time, she wore a friendship bracelet that one of her employees made to commemorate getting through a particularly hard day. “If I forgot it, my heart sank, and my anxiety rose,” she said. “Wearing it gave me security and confidence that the day would go well.”
A Moment of Silence
Juliet Emamaullee, MD, PhD, is a liver and kidney transplant surgeon at Keck Hospital and Children’s Hospital Los Angeles. Because of the complexity of her operations, she must know every aspect of her patients’ medical history. This leads to a level of intimacy that most people never have with their doctors. “Transplant surgeons are playing god in many ways,” she said. “During procurement, after we prep and drape the donor and right before I make the incision, everyone in the OR has a moment of silence to acknowledge the donation. If the organ has been transported, then I’ll say a prayer to myself that I do good work with this generous gift of life.”
Magical Thinking
Before we go any further, I should clarify that there’s a difference between rituals and superstitions like the ones just shared and routines and practices such as handwashing or doublechecking that it’s the right hip and not the left. All pilots have a preflight checklist that’s necessary for safety, but some might also make the sign of the cross.
Lester Gottesman, MD, has been a surgeon at the Icahn School of Medicine at Mount Sinai Hospital in New York City for nearly 50 years. He believes rituals and superstitions are more prevalent in medicine than in any other profession, despite there being no definitive research confirming their effectiveness.
In fact, it’s the opposite.
One of the few studies to examine superstitions among physicians was published in the Annals of Surgery in 2021. Researchers analyzed the operational records of 27,914 consecutive patients who underwent general, visceral, or vascular surgery. They found no association of moon phases, zodiac signs, or Friday the 13th with poor outcomes. Having acute coronary syndrome on Friday the 13th also did not influence the 13-year mortality rate compared to other dates in the year. And although 70% of physicians believe that some colleagues are “black clouds,” an analysis of 96 physicians and 6149 admissions found no such pattern.
Granted, this is just one analysis, but the results aren’t surprising. No one really believes in this stuff. So, why does it persist?
Dr. Gottesman cited an episode from the popular medical TV show Grey’s Anatomy, in which chief surgeon Meredith Grey puts it this way: “Superstition lies in the space between what we can control and what we can’t. …We rely on superstitions because we are smart enough to know we don’t have all the answers and that life works in mysterious ways. Don’t diss the juju from wherever it comes.”
“Superstition and science both start at the same place — to explain an unexplainable event,” said Dr. Gottesman, who always checks his suture lines at the end of a surgery in the order in which he did them. “If science provides a coherent answer, so be it. If not, the human’s need for order will assign causality to otherwise inanimate objects, noncausal events, or divine influence.”
In other words, the more unknowns and trepidation, the greater the tendency toward what Dr. Gottesman called “magical thinking.” And when you consider healing’s long history, you realize that ritual and superstition defined medicine for centuries. Gottesman pointed out that it wasn’t until Hippocrates separated religion and superstition from disease around 430 BC that modern medicine was born. But because doctors still don’t know everything, an element of magic endures.
The question is, in this high-tech age, do these stubborn beliefs still have a place? Do they help or hinder doctors, and, most important, do they have any effect on patient outcomes?
Five Benefits
To reiterate, there are no studies showing that Wonder Woman clogs convey surgical superpowers or that eating two hard-boiled eggs boosts OR performance. But anecdotally, many doctors admit to experiencing noticeable perks from their quirks. Let’s start with the supposed benefits:
- Less stress: A quarter of US clinicians are considering switching careers, primarily due to burnout, according to a 2022 Bain survey. “The fact that [rituals and superstitions] are so prevalent in such a high-stress field can’t be coincidence,” said Dr. Fong. “Offloading some of the responsibility to whatever gods there may be is a way of taming our anxieties so we can function better.”
- Hyperfocus: Dr. Emamaullee played volleyball in high school and college. She suggested that her presurgical routine isn’t all that different from her warmup before a championship match. It’s habitual behavior that helps induce a state of heightened concentration, confidence, and immersion. Athletes call it being “in the zone” or in a “state of flow,” and Dr. Emamaullee said she experiences the same thing in the OR.
- More control: Remember those horrific images of patients with COVID-19 overwhelming ERs in Brooklyn and Queens during the pandemic? Dr. Fong was in the middle of that. “In crisis situations where there are more unknowns, rituals and superstitions become even more important,” she said. “I may not be able to control what’s happening, but I can control myself. Rituals help restore some normalcy and organization, and they give me a sense of calm.”
- Better performance: A series of general-population experiments published in the journal Psychological Science in 2010 concluded that “good-luck–related superstitions” boosted self-confidence in mastering upcoming tasks and improved motor dexterity, memory, and overall performance.
- Placebo effect: This phenomenon is well-established in medicine. Give someone a special pill or treatment, and a significant portion will claim benefit. “Placebo is magical thinking,” said Dr. Gottesman. “It has identifiable and quantifiable effects on human disease.” And perhaps on medical practitioners, too. If a doctor believes her friendship bracelet has special powers and helps her be a better physician, then it just might.
Four Drawbacks
- Compulsive behavior: When superstitious beliefs or repetitive behaviors begin causing personal distress, interfering with daily duties, or negatively affecting patient outcomes, then there’s a problem. There’s a story on Quora about a neurosurgeon who always ate two Hostess Ho Hos chocolate cakes before operations. When he forgot to do so one day, he supposedly left his patient on the table and ran off to eat them. Even if it’s urban legend, it’s a useful illustration of quirk disrupting work.
- Less flexibility: Every human body and every surgery is different. “When ritualistic behaviors or habits become so rigid that you lose the ability to adapt, then that becomes dangerous for the patient,” said Dr. Fong. “The art of medicine, not unlike jazz, often comes from the improvisation.”
- Self-fulfilling: Just as rituals and superstitions can empower and provide a sense of control, they can quickly turn on physicians who forget a part of their routine or leave their talisman on the bureau. Instead of confidence, they supply doubt. The karma becomes kryptonite.
- Avoiding responsibility: After years of friendship bracelets and Wonder Woman clogs, Dr. Bajaj is making a deliberate effort to excise magical thinking from her practice. “It can hold you back if you’re not careful,” she said. “If you start using it as a crutch when something goes wrong — like ‘Oh, I wasn’t wearing my clogs today and that’s why my flap failed’ — then you’re not doing your due diligence and figuring out what really happened.” Rather than placing the responsibility for her day going well on superstition, she’s trying to own it herself by living with more intent.
The Diagnosis
Most of the medical experts I spoke with didn’t think there was anything wrong with rituals or superstitions as long as they didn’t become compulsive or a convenient repository of blame.
“Rituals and superstitions are an acknowledgment that forces external to ourselves exist,” concluded Dr. Fong. “They’re like tiny offerings to whatever gods are out there to please be on our side. And we keep doing them because there’s a reward — better patient outcomes, which is all we want to achieve in the end. I say embrace them.”
A version of this article first appeared on Medscape.com.
The second-floor operating rooms at Lehigh Valley Hospital in Allentown, Pennsylvania, are numbered sequentially — except when you get to what should be operation room (OR) 13. It’s OR M. The M doesn’t stand for Maternity or any other specialty. Rather in this high-tech, state-of-the art healthcare center, it’s there to ward off bad juju and evil spirits.
“Just as taller buildings usually don’t have a 13th floor or hotels don’t have a room 13, it revolves around the common superstition of the unlucky nature of number 13,” said a hospital spokesperson.
During the pandemic, the public was told repeatedly that modern medicine is science-based. But when I started talking to surgeons and other physicians for this article, I uncovered something decidedly unscientific.
In ORs and emergency rooms (ERs), small-town doctor’s offices, and mega hospitals, there’s a measure of dread before full moons and Friday the 13th, and no one dares utter the Q word (as in, “It sure is quiet today.”) That would risk bringing the wrath of the medical gods, and you’d earn the reputation of being a jinx or “black cloud.” Likewise, the songs “Stairway to Heaven” or “Another One Bites the Dust” will never be heard in any waiting room, elevator, or OR.
Indeed, when it comes to superstitions and rituals in medicine, it seems everyone has a story or a belief. …
A 2-Hour Ritual
Carmen Fong, MD, a colorectal surgeon in New York City, had a presurgical ritual that took her nearly 2 hours to complete. “I’d wake up at the same time every day, pack two hard-boiled eggs and a thermos of coffee in my small leather bag, walk to work via the same route, and swipe into the preop area while waving hi to the front desk,” she recounted. “I’d talk to the patient, sign the consent with the same ballpoint pen, go upstairs to my office, change into my scrubs [same cap and Danskos], then turn on my computer, and take a sip of coffee before heading back down to the OR. I’d always remove my badge and place it near the nurses’ workstation, then put on the patient’s SCDs [sequential compression devices] myself. I’d hold the oxygen mask while telling the patient, ‘See you later.’ Never ‘It will be okay’ or ‘Have a good sleep.’ Always ‘See you later.’ ”
Dr. Fong did this for 5 years prior to more than a thousand surgeries. She did it because it made her feel calm and in control, which translated to more successful operations. “It never failed me.”
Wonder Woman Clogs
Anureet Bajaj, MD, a plastic surgeon in Oklahoma City, wore Wonder Woman clogs in the OR for years because “they made me feel stronger, and my surgeries went better.” She’s also very specific about her OR playlist; “it must be ‘80s music.” And for a time, she wore a friendship bracelet that one of her employees made to commemorate getting through a particularly hard day. “If I forgot it, my heart sank, and my anxiety rose,” she said. “Wearing it gave me security and confidence that the day would go well.”
A Moment of Silence
Juliet Emamaullee, MD, PhD, is a liver and kidney transplant surgeon at Keck Hospital and Children’s Hospital Los Angeles. Because of the complexity of her operations, she must know every aspect of her patients’ medical history. This leads to a level of intimacy that most people never have with their doctors. “Transplant surgeons are playing god in many ways,” she said. “During procurement, after we prep and drape the donor and right before I make the incision, everyone in the OR has a moment of silence to acknowledge the donation. If the organ has been transported, then I’ll say a prayer to myself that I do good work with this generous gift of life.”
Magical Thinking
Before we go any further, I should clarify that there’s a difference between rituals and superstitions like the ones just shared and routines and practices such as handwashing or doublechecking that it’s the right hip and not the left. All pilots have a preflight checklist that’s necessary for safety, but some might also make the sign of the cross.
Lester Gottesman, MD, has been a surgeon at the Icahn School of Medicine at Mount Sinai Hospital in New York City for nearly 50 years. He believes rituals and superstitions are more prevalent in medicine than in any other profession, despite there being no definitive research confirming their effectiveness.
In fact, it’s the opposite.
One of the few studies to examine superstitions among physicians was published in the Annals of Surgery in 2021. Researchers analyzed the operational records of 27,914 consecutive patients who underwent general, visceral, or vascular surgery. They found no association of moon phases, zodiac signs, or Friday the 13th with poor outcomes. Having acute coronary syndrome on Friday the 13th also did not influence the 13-year mortality rate compared to other dates in the year. And although 70% of physicians believe that some colleagues are “black clouds,” an analysis of 96 physicians and 6149 admissions found no such pattern.
Granted, this is just one analysis, but the results aren’t surprising. No one really believes in this stuff. So, why does it persist?
Dr. Gottesman cited an episode from the popular medical TV show Grey’s Anatomy, in which chief surgeon Meredith Grey puts it this way: “Superstition lies in the space between what we can control and what we can’t. …We rely on superstitions because we are smart enough to know we don’t have all the answers and that life works in mysterious ways. Don’t diss the juju from wherever it comes.”
“Superstition and science both start at the same place — to explain an unexplainable event,” said Dr. Gottesman, who always checks his suture lines at the end of a surgery in the order in which he did them. “If science provides a coherent answer, so be it. If not, the human’s need for order will assign causality to otherwise inanimate objects, noncausal events, or divine influence.”
In other words, the more unknowns and trepidation, the greater the tendency toward what Dr. Gottesman called “magical thinking.” And when you consider healing’s long history, you realize that ritual and superstition defined medicine for centuries. Gottesman pointed out that it wasn’t until Hippocrates separated religion and superstition from disease around 430 BC that modern medicine was born. But because doctors still don’t know everything, an element of magic endures.
The question is, in this high-tech age, do these stubborn beliefs still have a place? Do they help or hinder doctors, and, most important, do they have any effect on patient outcomes?
Five Benefits
To reiterate, there are no studies showing that Wonder Woman clogs convey surgical superpowers or that eating two hard-boiled eggs boosts OR performance. But anecdotally, many doctors admit to experiencing noticeable perks from their quirks. Let’s start with the supposed benefits:
- Less stress: A quarter of US clinicians are considering switching careers, primarily due to burnout, according to a 2022 Bain survey. “The fact that [rituals and superstitions] are so prevalent in such a high-stress field can’t be coincidence,” said Dr. Fong. “Offloading some of the responsibility to whatever gods there may be is a way of taming our anxieties so we can function better.”
- Hyperfocus: Dr. Emamaullee played volleyball in high school and college. She suggested that her presurgical routine isn’t all that different from her warmup before a championship match. It’s habitual behavior that helps induce a state of heightened concentration, confidence, and immersion. Athletes call it being “in the zone” or in a “state of flow,” and Dr. Emamaullee said she experiences the same thing in the OR.
- More control: Remember those horrific images of patients with COVID-19 overwhelming ERs in Brooklyn and Queens during the pandemic? Dr. Fong was in the middle of that. “In crisis situations where there are more unknowns, rituals and superstitions become even more important,” she said. “I may not be able to control what’s happening, but I can control myself. Rituals help restore some normalcy and organization, and they give me a sense of calm.”
- Better performance: A series of general-population experiments published in the journal Psychological Science in 2010 concluded that “good-luck–related superstitions” boosted self-confidence in mastering upcoming tasks and improved motor dexterity, memory, and overall performance.
- Placebo effect: This phenomenon is well-established in medicine. Give someone a special pill or treatment, and a significant portion will claim benefit. “Placebo is magical thinking,” said Dr. Gottesman. “It has identifiable and quantifiable effects on human disease.” And perhaps on medical practitioners, too. If a doctor believes her friendship bracelet has special powers and helps her be a better physician, then it just might.
Four Drawbacks
- Compulsive behavior: When superstitious beliefs or repetitive behaviors begin causing personal distress, interfering with daily duties, or negatively affecting patient outcomes, then there’s a problem. There’s a story on Quora about a neurosurgeon who always ate two Hostess Ho Hos chocolate cakes before operations. When he forgot to do so one day, he supposedly left his patient on the table and ran off to eat them. Even if it’s urban legend, it’s a useful illustration of quirk disrupting work.
- Less flexibility: Every human body and every surgery is different. “When ritualistic behaviors or habits become so rigid that you lose the ability to adapt, then that becomes dangerous for the patient,” said Dr. Fong. “The art of medicine, not unlike jazz, often comes from the improvisation.”
- Self-fulfilling: Just as rituals and superstitions can empower and provide a sense of control, they can quickly turn on physicians who forget a part of their routine or leave their talisman on the bureau. Instead of confidence, they supply doubt. The karma becomes kryptonite.
- Avoiding responsibility: After years of friendship bracelets and Wonder Woman clogs, Dr. Bajaj is making a deliberate effort to excise magical thinking from her practice. “It can hold you back if you’re not careful,” she said. “If you start using it as a crutch when something goes wrong — like ‘Oh, I wasn’t wearing my clogs today and that’s why my flap failed’ — then you’re not doing your due diligence and figuring out what really happened.” Rather than placing the responsibility for her day going well on superstition, she’s trying to own it herself by living with more intent.
The Diagnosis
Most of the medical experts I spoke with didn’t think there was anything wrong with rituals or superstitions as long as they didn’t become compulsive or a convenient repository of blame.
“Rituals and superstitions are an acknowledgment that forces external to ourselves exist,” concluded Dr. Fong. “They’re like tiny offerings to whatever gods are out there to please be on our side. And we keep doing them because there’s a reward — better patient outcomes, which is all we want to achieve in the end. I say embrace them.”
A version of this article first appeared on Medscape.com.
How Abdominal Fibrogenesis Affects Adolescents With Obesity
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Insulin resistance and obesity in adolescents may lead to increased abdominal fibrogenesis, impairing the capacity of the abdominal subcutaneous adipose tissue (SAT) to store lipids, which may cause fat accumulation in the visceral adipose tissue (VAT) depot and in other organs such as the liver.
METHODOLOGY:
- Abdominal fibrogenesis, but not adipose tissue expandability, is known to increase in adults with obesity and reduce insulin sensitivity; however, little is known about fibrogenesis in adolescents with obesity.
- In this study, researchers investigated if lipid dynamics, fibrogenesis, and abdominal and gluteal adipocyte turnover show dysregulation to a greater extent in insulin-resistant adolescents with obesity than in insulin-sensitive adolescents with obesity.
- They recruited 14 individuals between 12 and 20 years with a body mass index over 30 from the Yale Clinic, of whom seven participants were classified as insulin resistant.
- Deuterated water methodologies were used to study the indices of adipocyte turnover, lipid dynamics, and fibrogenesis in abdominal and gluteal fat deposits.
- A 3-hour oral glucose tolerance test and multisection MRI scan of the abdominal region were used to assess the indices of glucose metabolism, abdominal fat distribution patterns, and liver fat content.
TAKEAWAY:
- The abdominal and gluteal SAT turnover rate of lipid components (triglyceride production and breakdown as well as de novo lipogenesis contribution) was similar in insulin-resistant and insulin-sensitive adolescents with obesity.
- The insoluble collagen (type I, subunit alpha2) level was higher in the abdominal adipose tissue of insulin-resistant adolescents than in insulin-sensitive adolescents (difference in fractional synthesis rate, 0.611; P < .001), indicating increased abdominal fibrogenesis.
- Abdominal insoluble collagen I alpha2 was associated with higher fasting plasma insulin levels (correlation [r], 0.579; P = .015), a higher visceral to total adipose tissue ratio (r, 0.643; P = .007), and a lower whole-body insulin sensitivity index (r, -0.540; P = .023).
- There was no evidence of increased collagen production in the gluteal adipose tissue, and as a result, fibrogenesis was observed.
IN PRACTICE:
“The increased formation of insoluble collagen observed in insulin-resistant compared with insulin-sensitive individuals contributes to lipid spillover from SAT to VAT and, in turn, serves as a critically important mechanism involved in the complex sequelae of obesity-related metabolic and liver disease pathology,” the authors wrote.
SOURCE:
This study, led by Aaron L. Slusher, Department of Pediatrics, Yale School of Medicine, New Haven, Connecticut, was published online in Obesity.
LIMITATIONS:
The researchers did not measure hepatic collagen synthesis rates. The analysis was performed on a small study population. The authors were also unable to assess potential sex differences.
DISCLOSURES:
The study was funded by the Foundation for the National Institutes of Health and Clara Guthrie Patterson Trust Mentored Research Award. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Pessary or Progesterone for Preterm Birth? Advantage Med
TOPLINE:
A study comparing cervical pessary and vaginal progesterone for the prevention of preterm birth in women with a short cervix of ≤ 35 mm found no significant difference between the interventions for perinatal complications. Among women with a cervical length of ≤ 25 mm, however, pessaries appeared to be less effective at preventing spontaneous preterm birth and adverse outcomes, according to the researchers.
METHODOLOGY:
- Researchers conducted an open-label, randomized controlled trial at 20 hospitals and five obstetric ultrasound practices in the Netherlands.
- The study included 635 women with healthy singleton pregnancies between 18 and 22 weeks’ gestation and an asymptomatic short cervix of ≤ 35 mm. Participants had no history of spontaneous preterm birth.
- Women were randomly assigned to receive either an Arabin cervical pessary or 200 mg/d vaginal progesterone for ≤ 36 weeks of gestation.
- The investigators examined a composite measure of adverse perinatal outcomes, including (grade, > 1), chronic lung disease, (grade, III or IV), (stage, > 1), , stillbirth, and death of the baby.
TAKEAWAY:
- Adverse perinatal outcomes occurred in 6% of each treatment group, and the rate of spontaneous preterm birth did not differ significantly between the groups.
- In a subgroup analysis of 131 patients with a cervical length of ≤ 25 mm, spontaneous preterm birth at < 28 weeks occurred more often in the pessary group (16% vs 4%).
- Adverse perinatal outcomes also seemed to occur more frequently in the pessary group (24% vs 12%; relative risk, 2.1 [95% CI, 0.95-4.60]), in the subgroup analysis, according to the researchers.
IN PRACTICE:
“Even though the study was not powered for the subgroup with a short cervix of ≤ 25 mm, results suggest that a cervical pessary should not be used as preventive treatment in this group,” the researchers wrote.
SOURCE:
The study was led by Charlotte E. van Dijk, MD, with Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands. It was published online in The BMJ.
LIMITATIONS:
The researchers were unable to mask the treatment groups, which could introduce bias. The study’s reliance on self-reported medication adherence in the progesterone group and a lack of extra training for pessary placement might have influenced the outcomes, the researchers noted.
DISCLOSURES:
The study was supported by Stichting Stoptevroegbevallen, a nonprofit research foundation. An author disclosed financial ties with Merck.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A study comparing cervical pessary and vaginal progesterone for the prevention of preterm birth in women with a short cervix of ≤ 35 mm found no significant difference between the interventions for perinatal complications. Among women with a cervical length of ≤ 25 mm, however, pessaries appeared to be less effective at preventing spontaneous preterm birth and adverse outcomes, according to the researchers.
METHODOLOGY:
- Researchers conducted an open-label, randomized controlled trial at 20 hospitals and five obstetric ultrasound practices in the Netherlands.
- The study included 635 women with healthy singleton pregnancies between 18 and 22 weeks’ gestation and an asymptomatic short cervix of ≤ 35 mm. Participants had no history of spontaneous preterm birth.
- Women were randomly assigned to receive either an Arabin cervical pessary or 200 mg/d vaginal progesterone for ≤ 36 weeks of gestation.
- The investigators examined a composite measure of adverse perinatal outcomes, including (grade, > 1), chronic lung disease, (grade, III or IV), (stage, > 1), , stillbirth, and death of the baby.
TAKEAWAY:
- Adverse perinatal outcomes occurred in 6% of each treatment group, and the rate of spontaneous preterm birth did not differ significantly between the groups.
- In a subgroup analysis of 131 patients with a cervical length of ≤ 25 mm, spontaneous preterm birth at < 28 weeks occurred more often in the pessary group (16% vs 4%).
- Adverse perinatal outcomes also seemed to occur more frequently in the pessary group (24% vs 12%; relative risk, 2.1 [95% CI, 0.95-4.60]), in the subgroup analysis, according to the researchers.
IN PRACTICE:
“Even though the study was not powered for the subgroup with a short cervix of ≤ 25 mm, results suggest that a cervical pessary should not be used as preventive treatment in this group,” the researchers wrote.
SOURCE:
The study was led by Charlotte E. van Dijk, MD, with Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands. It was published online in The BMJ.
LIMITATIONS:
The researchers were unable to mask the treatment groups, which could introduce bias. The study’s reliance on self-reported medication adherence in the progesterone group and a lack of extra training for pessary placement might have influenced the outcomes, the researchers noted.
DISCLOSURES:
The study was supported by Stichting Stoptevroegbevallen, a nonprofit research foundation. An author disclosed financial ties with Merck.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A study comparing cervical pessary and vaginal progesterone for the prevention of preterm birth in women with a short cervix of ≤ 35 mm found no significant difference between the interventions for perinatal complications. Among women with a cervical length of ≤ 25 mm, however, pessaries appeared to be less effective at preventing spontaneous preterm birth and adverse outcomes, according to the researchers.
METHODOLOGY:
- Researchers conducted an open-label, randomized controlled trial at 20 hospitals and five obstetric ultrasound practices in the Netherlands.
- The study included 635 women with healthy singleton pregnancies between 18 and 22 weeks’ gestation and an asymptomatic short cervix of ≤ 35 mm. Participants had no history of spontaneous preterm birth.
- Women were randomly assigned to receive either an Arabin cervical pessary or 200 mg/d vaginal progesterone for ≤ 36 weeks of gestation.
- The investigators examined a composite measure of adverse perinatal outcomes, including (grade, > 1), chronic lung disease, (grade, III or IV), (stage, > 1), , stillbirth, and death of the baby.
TAKEAWAY:
- Adverse perinatal outcomes occurred in 6% of each treatment group, and the rate of spontaneous preterm birth did not differ significantly between the groups.
- In a subgroup analysis of 131 patients with a cervical length of ≤ 25 mm, spontaneous preterm birth at < 28 weeks occurred more often in the pessary group (16% vs 4%).
- Adverse perinatal outcomes also seemed to occur more frequently in the pessary group (24% vs 12%; relative risk, 2.1 [95% CI, 0.95-4.60]), in the subgroup analysis, according to the researchers.
IN PRACTICE:
“Even though the study was not powered for the subgroup with a short cervix of ≤ 25 mm, results suggest that a cervical pessary should not be used as preventive treatment in this group,” the researchers wrote.
SOURCE:
The study was led by Charlotte E. van Dijk, MD, with Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands. It was published online in The BMJ.
LIMITATIONS:
The researchers were unable to mask the treatment groups, which could introduce bias. The study’s reliance on self-reported medication adherence in the progesterone group and a lack of extra training for pessary placement might have influenced the outcomes, the researchers noted.
DISCLOSURES:
The study was supported by Stichting Stoptevroegbevallen, a nonprofit research foundation. An author disclosed financial ties with Merck.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Polygenic Risk Scores Improve Breast Cancer Screening
TOPLINE:
METHODOLOGY:
A polygenic risk score — a measure of an individual’s risk for a disease based on the estimated effects of many genetic variants — is not typically included alongside family histories and pathogenic variants of genes, such as BRCA1 and PALB2, when assessing a woman’s risk for breast cancer and the need for earlier or more frequent screening.
To assess the potential for a polygenic risk score to improve breast cancer risk stratification, investigators in Finland used a nationwide genetic database to calculate polygenic risk score scores for 117,252 women and then linked the scores to their breast cancer outcomes, using the country’s nationwide mammography screening program, which screens women, ages 50-69 years, every 2 years.
The researchers evaluated the use of polygenic risk scores both alone and in combination with family histories and pathogenic variants — specifically, CHEK2 and PALB2 variants common in Finland.
The researchers also looked at how well polygenic risk scores predicted a person’s risk for any breast cancer as well as invasive, in situ, and bilateral at three timepoints: before, during, and after screening age.
TAKEAWAY:
Compared with a lower polygenic risk score (below 90%), a high polygenic risk score — a score in the top 10% — was associated with more than a twofold higher risk for any breast cancer before, during, and after screening age (hazard ratio [HR], 2.50, 2.38, and 2.11, respectively). Pathogenic variants and family histories led to similar risk assessments (HR, 3.13, 2.30, and 1.95, respectively, for pathogenic variants; HR, 1.97, 1.96, and 1.68, respectively, for family history).
A high polygenic risk score had a positive predictive value of 39.5% for a breast cancer diagnosis after a positive screening mammography, about the same as positive family history (35.5%) and pathogenic variants (35.9%). Combining a high polygenic risk score with a positive family history increased the positive predictive value to 44.6% and with pathogenic variant carriers increased the positive predictive value to 50.6%.
A high polygenic risk score was also associated with a twofold higher risk for interval breast cancer — a cancer diagnosed between screenings — and a higher risk for bilateral breast cancer during screening ages (HR, 4.71), suggesting that women with high scores may benefit from a shorter time interval between screenings or earlier screening, the researchers said.
Women with scores in the bottom 10% had a very low risk for both interval and screen-detected cancers. Those with negative family histories and no pathogenic variants did not reach the 2% cumulative incidence threshold for breast cancer screening until age 62 years, “suggesting opportunities for less frequent screens,” the researchers noted.
IN PRACTICE:
This study demonstrates the effectiveness of using a breast cancer polygenic risk score for risk stratification, “with optimal stratification reached through combining” this information with family history and pathogenic variants, the researchers concluded.
SOURCE:
The study, led by Nina Mars, MD, PhD, of the University of Helsinki, Helsinki, Finland, was published in the Journal of Clinical Oncology.
LIMITATIONS:
The work was limited largely to people with Finnish genetic ancestry. The benefits of including polygenic risk scores in screening programs need to be confirmed in clinical trials in areas with broader genetic ancestry; several trials are underway in the United States and elsewhere.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 research and innovation program, the Cancer Foundation Finland, and others. The investigators didn’t have any relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
A polygenic risk score — a measure of an individual’s risk for a disease based on the estimated effects of many genetic variants — is not typically included alongside family histories and pathogenic variants of genes, such as BRCA1 and PALB2, when assessing a woman’s risk for breast cancer and the need for earlier or more frequent screening.
To assess the potential for a polygenic risk score to improve breast cancer risk stratification, investigators in Finland used a nationwide genetic database to calculate polygenic risk score scores for 117,252 women and then linked the scores to their breast cancer outcomes, using the country’s nationwide mammography screening program, which screens women, ages 50-69 years, every 2 years.
The researchers evaluated the use of polygenic risk scores both alone and in combination with family histories and pathogenic variants — specifically, CHEK2 and PALB2 variants common in Finland.
The researchers also looked at how well polygenic risk scores predicted a person’s risk for any breast cancer as well as invasive, in situ, and bilateral at three timepoints: before, during, and after screening age.
TAKEAWAY:
Compared with a lower polygenic risk score (below 90%), a high polygenic risk score — a score in the top 10% — was associated with more than a twofold higher risk for any breast cancer before, during, and after screening age (hazard ratio [HR], 2.50, 2.38, and 2.11, respectively). Pathogenic variants and family histories led to similar risk assessments (HR, 3.13, 2.30, and 1.95, respectively, for pathogenic variants; HR, 1.97, 1.96, and 1.68, respectively, for family history).
A high polygenic risk score had a positive predictive value of 39.5% for a breast cancer diagnosis after a positive screening mammography, about the same as positive family history (35.5%) and pathogenic variants (35.9%). Combining a high polygenic risk score with a positive family history increased the positive predictive value to 44.6% and with pathogenic variant carriers increased the positive predictive value to 50.6%.
A high polygenic risk score was also associated with a twofold higher risk for interval breast cancer — a cancer diagnosed between screenings — and a higher risk for bilateral breast cancer during screening ages (HR, 4.71), suggesting that women with high scores may benefit from a shorter time interval between screenings or earlier screening, the researchers said.
Women with scores in the bottom 10% had a very low risk for both interval and screen-detected cancers. Those with negative family histories and no pathogenic variants did not reach the 2% cumulative incidence threshold for breast cancer screening until age 62 years, “suggesting opportunities for less frequent screens,” the researchers noted.
IN PRACTICE:
This study demonstrates the effectiveness of using a breast cancer polygenic risk score for risk stratification, “with optimal stratification reached through combining” this information with family history and pathogenic variants, the researchers concluded.
SOURCE:
The study, led by Nina Mars, MD, PhD, of the University of Helsinki, Helsinki, Finland, was published in the Journal of Clinical Oncology.
LIMITATIONS:
The work was limited largely to people with Finnish genetic ancestry. The benefits of including polygenic risk scores in screening programs need to be confirmed in clinical trials in areas with broader genetic ancestry; several trials are underway in the United States and elsewhere.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 research and innovation program, the Cancer Foundation Finland, and others. The investigators didn’t have any relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
A polygenic risk score — a measure of an individual’s risk for a disease based on the estimated effects of many genetic variants — is not typically included alongside family histories and pathogenic variants of genes, such as BRCA1 and PALB2, when assessing a woman’s risk for breast cancer and the need for earlier or more frequent screening.
To assess the potential for a polygenic risk score to improve breast cancer risk stratification, investigators in Finland used a nationwide genetic database to calculate polygenic risk score scores for 117,252 women and then linked the scores to their breast cancer outcomes, using the country’s nationwide mammography screening program, which screens women, ages 50-69 years, every 2 years.
The researchers evaluated the use of polygenic risk scores both alone and in combination with family histories and pathogenic variants — specifically, CHEK2 and PALB2 variants common in Finland.
The researchers also looked at how well polygenic risk scores predicted a person’s risk for any breast cancer as well as invasive, in situ, and bilateral at three timepoints: before, during, and after screening age.
TAKEAWAY:
Compared with a lower polygenic risk score (below 90%), a high polygenic risk score — a score in the top 10% — was associated with more than a twofold higher risk for any breast cancer before, during, and after screening age (hazard ratio [HR], 2.50, 2.38, and 2.11, respectively). Pathogenic variants and family histories led to similar risk assessments (HR, 3.13, 2.30, and 1.95, respectively, for pathogenic variants; HR, 1.97, 1.96, and 1.68, respectively, for family history).
A high polygenic risk score had a positive predictive value of 39.5% for a breast cancer diagnosis after a positive screening mammography, about the same as positive family history (35.5%) and pathogenic variants (35.9%). Combining a high polygenic risk score with a positive family history increased the positive predictive value to 44.6% and with pathogenic variant carriers increased the positive predictive value to 50.6%.
A high polygenic risk score was also associated with a twofold higher risk for interval breast cancer — a cancer diagnosed between screenings — and a higher risk for bilateral breast cancer during screening ages (HR, 4.71), suggesting that women with high scores may benefit from a shorter time interval between screenings or earlier screening, the researchers said.
Women with scores in the bottom 10% had a very low risk for both interval and screen-detected cancers. Those with negative family histories and no pathogenic variants did not reach the 2% cumulative incidence threshold for breast cancer screening until age 62 years, “suggesting opportunities for less frequent screens,” the researchers noted.
IN PRACTICE:
This study demonstrates the effectiveness of using a breast cancer polygenic risk score for risk stratification, “with optimal stratification reached through combining” this information with family history and pathogenic variants, the researchers concluded.
SOURCE:
The study, led by Nina Mars, MD, PhD, of the University of Helsinki, Helsinki, Finland, was published in the Journal of Clinical Oncology.
LIMITATIONS:
The work was limited largely to people with Finnish genetic ancestry. The benefits of including polygenic risk scores in screening programs need to be confirmed in clinical trials in areas with broader genetic ancestry; several trials are underway in the United States and elsewhere.
DISCLOSURES:
The study was funded by the European Union’s Horizon 2020 research and innovation program, the Cancer Foundation Finland, and others. The investigators didn’t have any relevant disclosures.
A version of this article appeared on Medscape.com.