User login
The DNA Mismatch Repair System in Sebaceous Tumors: An Update on the Genetics and Workup of Muir-Torre Syndrome
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
Resident Pearls
- When patients present with a solitary sebaceous tumor, there is a high likelihood they have Muir-Torre syndrome (MTS) and thus are at a high risk to develop visceral malignancies.
- It is important to perform further testing using immunohistochemistry for DNA mismatch repair proteins and microsatellite instability gene analysis in some cases to confirm the diagnosis of MTS and to perform the appropriate cancer screening tests.
Update confirms survival benefit with trastuzumab in uterine serous carcinoma
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
Adding trastuzumab to carboplatin/paclitaxel improved survival in patients with advanced or recurrent HER2/Neu-positive uterine serous carcinoma (USC), according to an updated analysis from a phase 2 trial.
At a median follow-up of 25.9 months, the median progression-free survival (PFS) was 12.9 months in patients who received trastuzumab plus carboplatin/paclitaxel and 8.0 months in patients who received only carboplatin/paclitaxel. The median overall survival (OS) was 29.6 months and 24.4 months, respectively.
Amanda Nickles Fader, MD, of Johns Hopkins Medicine, Baltimore, and colleagues reported these findings in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Confirmed benefit
The phase 2 trial was designed to assess whether trastuzumab, a humanized monoclonal antibody that targets HER2/neu – a growth factor receptor found in almost all USC cases and overexpressed in 30% of cases – would improve survival in patients with USC, Dr. Nickles Fader explained in an interview. She noted that trastuzumab has been shown to provide benefit in breast cancer patients with HER2/neu overexpression.
“[U]terine serous carcinoma ... is a very aggressive high-grade endometrial cancer subtype that is associated with really poor clinical outcomes and significant mortality,” Dr. Nickles Fader said. “It represents fewer than 10% of all uterine cancer cases, but it actually accounts for a disproportionate 40% of all deaths from uterine cancer.”
The overall survival among USC patients is about 45%, compared with 91% for more common lower-grade types of uterine cancers, she added.
“The conventional treatments for uterine serous carcinoma include surgery and then chemotherapy, but we’ve only really gotten so far by using a sort of one-size-fits-all treatment philosophy,” Dr. Nickles Fader said.
Based on preliminary findings from the current trial (J Clin Oncol. 2018 Jul 10;36[20]:2044-51), trastuzumab plus carboplatin/paclitaxel is now recognized as an alternative standard in treating advanced or recurrent HER2/Neu-positive USC, and this updated analysis confirms the benefits of adding trastuzumab, she said.
PFS, OS, and toxicity
There were 58 evaluable patients with primary stage III-IV or recurrent USC who were randomized to receive six cycles of carboplatin/paclitaxel alone or in combination with intravenous trastuzumab given until toxicity or progression.
The median PFS at a median follow-up of 25.9 months “very significantly favored” the trastuzumab arm, Dr. Nickles Fader said. The median PFS was 12.9 months in the trastuzumab arm and 8.0 months in the carboplatin/paclitaxel arm (hazard ratio, 0.46; P = .005).
In the 41 patients undergoing primary treatment, the median PFS was 17.7 months in the trastuzumab arm and 9.3 months in the control arm (HR, 0.44; P = .015). In the 17 patients with recurrent disease, the median PFS was 9.2 months and 7.0 months, respectively (HR, 0.12; P = .004).
“We were very pleased to see that there was also an overall survival benefit of about 5 months in the trastuzumab arm, compared to the control arm,” Dr. Nickles Fader said. The median OS was 29.6 months and 24.4 months, respectively (HR, 0.58; P = .046).
The PFS and OS benefit was “particularly striking” in the stage III-IV patients, according to Dr. Nickles Fader and colleagues. In this subgroup, the median OS was not reached in the trastuzumab arm, and it was 25.4 months in the control arm (HR, 0.49; P = .041).
Long-term toxicity did not differ between the treatment arms.
Applications and next steps
“The take-home message here is women should be tested if they have this subtype,” Dr. Nickles Fader said. “If they’re newly diagnosed, they should be tested for the HER2/neu receptor, and if [it is overexpressed] and they have advanced disease, we do recommend treatment with not only the conventional treatment, but with trastuzumab added to that, because that’s where we saw the most benefit.”
This is the only trial that has ever shown a major PFS and OS difference with combination targeted therapy and conventional chemotherapy in USC, Dr. Nickles Fader noted.
“So it was really exciting to see that,” she said, adding that a “much larger cooperative group trial” is being designed by the National Cancer Institute and NRG Oncology Group to look at this approach in HER2-positive, advanced-stage uterine cancers. The trial will include patients with USC, but it will extend to other uterine cancer types as well.
“We’re looking at different combinations of anti-HER2 therapies to sort of validate the results of this trial, but also to study this in other tumors that are HER2 positive,” Dr. Nickles Fader explained.
She also stressed the importance of addressing racial disparities in survival among women with USC, as African American women have higher rates of USC and related mortality than do other groups.
“It’s going to be important to look at not only molecular targets and improving survival but also racial inequalities and potentially epigenetics to really improve survival across the board,” Dr. Nickles Fader said.
She reported having no disclosures. The trial was sponsored by Yale University in collaboration with Genentech.
SOURCE: Nickles Fader A et al. SGO 2020, Abstract 12.
FROM SGO 2020
Tuberous Sclerosis With Segmental Overgrowth
To the Editor:
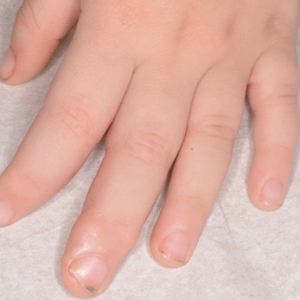
A 3-year-old boy with a history of tuberous sclerosis presented to our clinic for evaluation of bumps on the second and third fingers of the left hand. Physical examination revealed firm rubbery nodules on the palmar third metacarpophalangeal joint extending to the palm and the lateral aspect of the distal third dorsal finger. There also was asymmetric overgrowth of the left second and third digits consistent with bony segmental overgrowth (Figure).
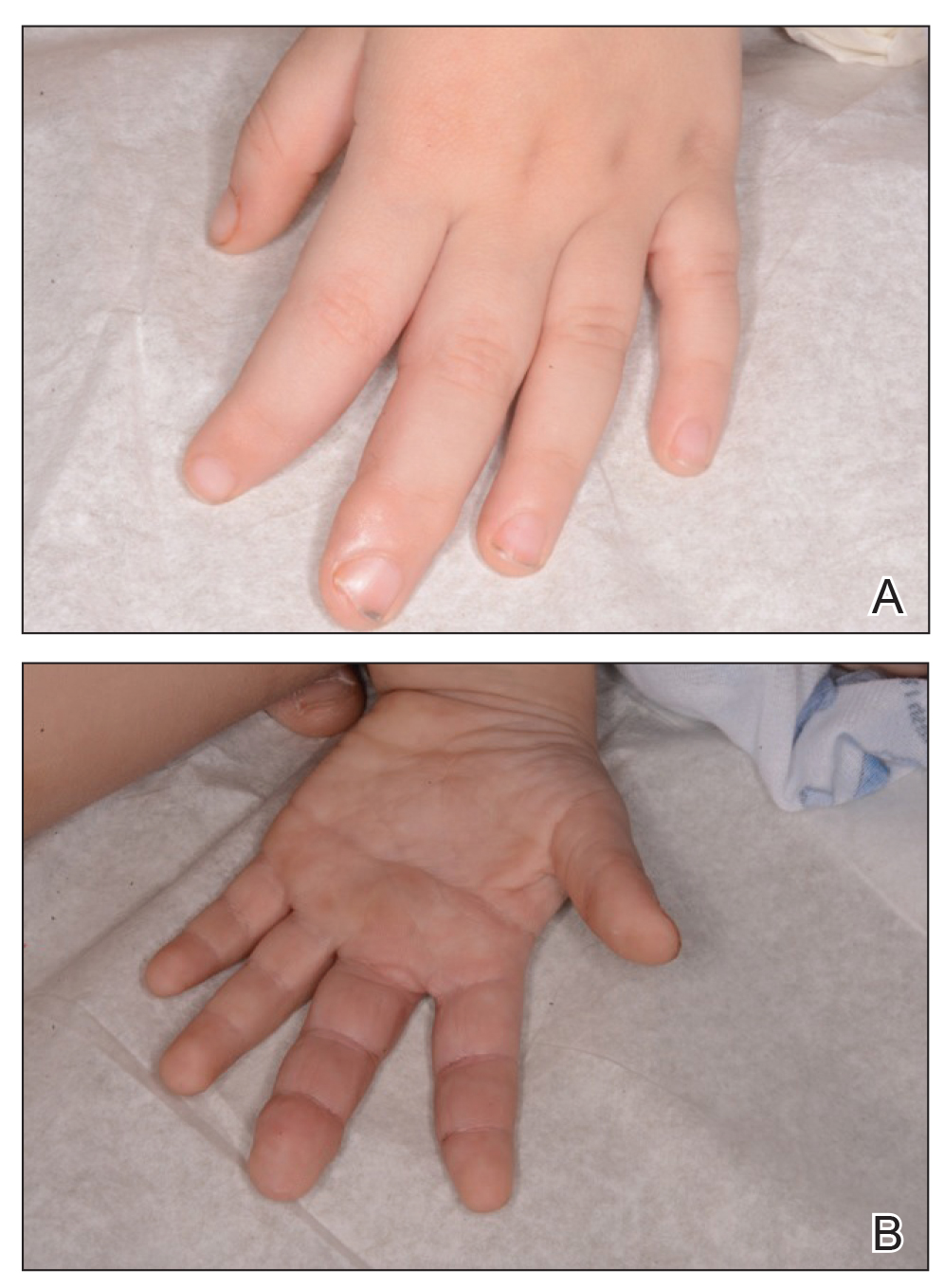
Tuberous sclerosis and overgrowth syndromes including Proteus syndrome have mutations that share a common pathway, namely the PI3K/AKT/mTOR (phosphoinositide 3-kinase/alpha serine/threonine-protein kinase/mammalian target of rapamycin) pathway.1 The mutations in tuberous sclerosis involve the loss of TSC1 (TSC complex subunit 1) on chromosome 9 or TSC2 (TSC complex subunit 2) on chromosome 16.2 The protein products of these genes, hamartin and tuberin, act together as a tumor suppressor complex.3 The inheritance pattern of tuberous sclerosis is autosomal dominant, though two-thirds of cases are due to de novo germline mutations.4 The second copy of the gene must be lost spontaneously in any particular cell for the deleterious effects of the disease to manifest. The mutation in overgrowth syndromes including Proteus syndrome involves the activation of AKT1 (AKT serine/threonine kinase 1) on chromosome 14. This mutation occurs in somatic cells as opposed to germ cells, as in tuberous sclerosis. This difference accounts for the mosaic expression of segmental overgrowth syndromes. This concept has been demonstrated in overgrowth syndromes such as Proteus syndrome, with cells from unaffected areas having different genetic makeup than those from affected tissues.5 These mutations, though different, result in the downstream effects of unchecked messenger RNA translation and dysregulated cellular growth.
In our patient, we hypothesized that a small proportion of his postfertilization somatic cells underwent a second de novo mutation in the AKT1 pathway, resulting in the bony overgrowth seen on the left hand. We suspected that this second mutation could be an activation of AKT1, the mutation seen in Proteus syndrome. Sequencing of the tissue may be performed in the future, especially if segmental overgrowth continues and necessitates therapy.
- Wu Y, Zhou BP. Kinases meet at TSC. Cell Res. 2007;17:971-973.
- Roach SE, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004;19:643-649.
- Barker KT, Houlston RS. Overgrowth syndromes: is dysfunctional PI3-kinase signaling a unifying mechanism? Eur J Hum Genet. 2003;11:665-670.
- Nothrup H, Koenig MK, Au KS. Tuberous sclerosis complex. GeneReviews. Seattle, WA: University of Washington; 1999.
- Lindhurst MJ, Parker VE, Payne F, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928-933.
To the Editor:
A 3-year-old boy with a history of tuberous sclerosis presented to our clinic for evaluation of bumps on the second and third fingers of the left hand. Physical examination revealed firm rubbery nodules on the palmar third metacarpophalangeal joint extending to the palm and the lateral aspect of the distal third dorsal finger. There also was asymmetric overgrowth of the left second and third digits consistent with bony segmental overgrowth (Figure).

Tuberous sclerosis and overgrowth syndromes including Proteus syndrome have mutations that share a common pathway, namely the PI3K/AKT/mTOR (phosphoinositide 3-kinase/alpha serine/threonine-protein kinase/mammalian target of rapamycin) pathway.1 The mutations in tuberous sclerosis involve the loss of TSC1 (TSC complex subunit 1) on chromosome 9 or TSC2 (TSC complex subunit 2) on chromosome 16.2 The protein products of these genes, hamartin and tuberin, act together as a tumor suppressor complex.3 The inheritance pattern of tuberous sclerosis is autosomal dominant, though two-thirds of cases are due to de novo germline mutations.4 The second copy of the gene must be lost spontaneously in any particular cell for the deleterious effects of the disease to manifest. The mutation in overgrowth syndromes including Proteus syndrome involves the activation of AKT1 (AKT serine/threonine kinase 1) on chromosome 14. This mutation occurs in somatic cells as opposed to germ cells, as in tuberous sclerosis. This difference accounts for the mosaic expression of segmental overgrowth syndromes. This concept has been demonstrated in overgrowth syndromes such as Proteus syndrome, with cells from unaffected areas having different genetic makeup than those from affected tissues.5 These mutations, though different, result in the downstream effects of unchecked messenger RNA translation and dysregulated cellular growth.
In our patient, we hypothesized that a small proportion of his postfertilization somatic cells underwent a second de novo mutation in the AKT1 pathway, resulting in the bony overgrowth seen on the left hand. We suspected that this second mutation could be an activation of AKT1, the mutation seen in Proteus syndrome. Sequencing of the tissue may be performed in the future, especially if segmental overgrowth continues and necessitates therapy.
To the Editor:
A 3-year-old boy with a history of tuberous sclerosis presented to our clinic for evaluation of bumps on the second and third fingers of the left hand. Physical examination revealed firm rubbery nodules on the palmar third metacarpophalangeal joint extending to the palm and the lateral aspect of the distal third dorsal finger. There also was asymmetric overgrowth of the left second and third digits consistent with bony segmental overgrowth (Figure).

Tuberous sclerosis and overgrowth syndromes including Proteus syndrome have mutations that share a common pathway, namely the PI3K/AKT/mTOR (phosphoinositide 3-kinase/alpha serine/threonine-protein kinase/mammalian target of rapamycin) pathway.1 The mutations in tuberous sclerosis involve the loss of TSC1 (TSC complex subunit 1) on chromosome 9 or TSC2 (TSC complex subunit 2) on chromosome 16.2 The protein products of these genes, hamartin and tuberin, act together as a tumor suppressor complex.3 The inheritance pattern of tuberous sclerosis is autosomal dominant, though two-thirds of cases are due to de novo germline mutations.4 The second copy of the gene must be lost spontaneously in any particular cell for the deleterious effects of the disease to manifest. The mutation in overgrowth syndromes including Proteus syndrome involves the activation of AKT1 (AKT serine/threonine kinase 1) on chromosome 14. This mutation occurs in somatic cells as opposed to germ cells, as in tuberous sclerosis. This difference accounts for the mosaic expression of segmental overgrowth syndromes. This concept has been demonstrated in overgrowth syndromes such as Proteus syndrome, with cells from unaffected areas having different genetic makeup than those from affected tissues.5 These mutations, though different, result in the downstream effects of unchecked messenger RNA translation and dysregulated cellular growth.
In our patient, we hypothesized that a small proportion of his postfertilization somatic cells underwent a second de novo mutation in the AKT1 pathway, resulting in the bony overgrowth seen on the left hand. We suspected that this second mutation could be an activation of AKT1, the mutation seen in Proteus syndrome. Sequencing of the tissue may be performed in the future, especially if segmental overgrowth continues and necessitates therapy.
- Wu Y, Zhou BP. Kinases meet at TSC. Cell Res. 2007;17:971-973.
- Roach SE, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004;19:643-649.
- Barker KT, Houlston RS. Overgrowth syndromes: is dysfunctional PI3-kinase signaling a unifying mechanism? Eur J Hum Genet. 2003;11:665-670.
- Nothrup H, Koenig MK, Au KS. Tuberous sclerosis complex. GeneReviews. Seattle, WA: University of Washington; 1999.
- Lindhurst MJ, Parker VE, Payne F, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928-933.
- Wu Y, Zhou BP. Kinases meet at TSC. Cell Res. 2007;17:971-973.
- Roach SE, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004;19:643-649.
- Barker KT, Houlston RS. Overgrowth syndromes: is dysfunctional PI3-kinase signaling a unifying mechanism? Eur J Hum Genet. 2003;11:665-670.
- Nothrup H, Koenig MK, Au KS. Tuberous sclerosis complex. GeneReviews. Seattle, WA: University of Washington; 1999.
- Lindhurst MJ, Parker VE, Payne F, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928-933.
Practice Points
- Tuberous sclerosis and Proteus syndrome share a common downstream effector pathway.
- For a patient to demonstrate features of both tuberous sclerosis and Proteus syndrome, he/she must have both a germline mutation (for tuberous sclerosis) as well as a postzygotic mutation (for Proteus syndrome) of this shared pathway.
Can convalescent plasma treat COVID-19 patients?
As an Episcopal priest, Father Robert Pace of Fort Worth, TX, is used to putting others first and reaching out to help. So when the pulmonologist who helped him through his ordeal with COVID-19 asked if he would like to donate blood to help other patients, he did not hesitate.
“I said, ‘Absolutely,’” Pace, 53, recalls. He says the idea was ‘very appealing.’ ” During his ordeal with COVID-19 in March, he had spent 3 days in the hospital, isolated and on IV fluids and oxygen. He was short of breath, with a heartbeat more rapid than usual.
Now, fully recovered, his blood was a precious commodity, antibody-rich and potentially life-saving.
As researchers scramble to test drugs to fight COVID-19, others are turning to an age-old treatment. They’re collecting the blood of survivors and giving it to patients in the throes of a severe infection, a treatment known as convalescent plasma therapy.
Doctors say the treatment will probably serve as a bridge until other drugs and a vaccine become available.
Although the FDA considers the treatment investigational, in late March, it eased access to it. Patients can get it as part of a clinical trial or through an expanded access program overseen by hospitals or universities. A doctor can also request permission to use the treatment for a single patient.
“It is considered an emergent, compassionate need,” says John Burk, MD, a pulmonologist at Texas Health Harris Methodist Hospital, Fort Worth, who treated Pace. “It is a way to bring it to the bedside.” And the approval can happen quickly. Burk says he got one from the FDA just 20 minutes after requesting it for a severely ill patient.
How it works
The premise of how it works is “quite straightforward,” says Michael Joyner, MD, a professor of anesthesiology at the Mayo Clinic, Rochester, MN. “When someone is recovered and no longer symptomatic, you can harvest those antibodies from their blood and give them to someone else, and hopefully alter the course of their disease.” Joyner is the principal investigator for the FDA’s national Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19, with 1,000 sites already signed on.
Convalescent therapy has been used to fight many other viruses, including Ebola, severe acute respiratory syndrome (SARS), the “bird” flu, H1N1 flu, and during the 1918 flu pandemic. Joyner says the strongest evidence for it comes from the 1950s, when it was used to treat a rodent-borne illness called Argentine hemorrhagic fever. Using convalescent plasma therapy for this infection reduced the death rate from nearly 43% before the treatment became common in the late 1950s to about 3% after it was widely used, one report found.
Data about convalescent therapy specifically for COVID-19 is limited. Chinese researchers reported on five critically ill patients, all on mechanical ventilation, treated with convalescent plasma after they had received antiviral and anti-inflammatory medicines. Three could leave the hospital after 51-55 days, and two were in stable condition in the hospital 37 days after the transfusion.
In another study of 10 severely ill patients, symptoms went away or improved in all 10 within 1 to 3 days after the transfusion. Two of the three on ventilators were weaned off and put on oxygen instead. None died.
Chinese researchers also reported three cases of patients with COVID-19 given the convalescent therapy who had a satisfactory recovery.
Researchers who reviewed the track record of convalescent therapy for other conditions recently concluded that the treatment doesn’t appear to cause severe side effects and it should be studied for COVID-19.
Although information on side effects specific to this treatment is evolving, Joyner says they are “very, very low.”
According to the FDA, allergic reactions can occur with plasma therapies. Because the treatment for COVID-19 is new, it is not known if patients might have other types of reactions.
Who can donate?
Blood bank officials and researchers running the convalescent plasma programs say the desire to help is widespread, and they’ve been deluged with offers to donate. But requirements are strict.
Donors must have evidence of COVID-19 infection, documented in a variety of ways, such as a diagnostic test by nasal swab or a blood test showing antibodies. And they must be symptom-free for 14 days, with test results, or 28 days without.
The treatment involves collecting plasma, not whole blood. Plasma, the liquid part of the blood, helps with clotting and supports immunity. During the collection, a donor’s blood is put through a machine that collects the plasma only and sends the red blood cells and platelets back to the donor.
Clinical trials
Requirements may be more stringent for donors joining a formal clinical trial rather than an expanded access program. For instance, potential donors in a randomized clinical trial underway at Stony Brook University must have higher antibody levels than required by the FDA, says study leader Elliott Bennett-Guerrero, MD, medical director of perioperative quality and patient safety and professor at the Renaissance School of Medicine.
He hopes to enroll up to 500 patients from the Long Island, NY, area. While clinical trials typically have a 50-50 split, with half of subjects getting a treatment and half a placebo, Bennett-Guerrero’s study will give 80% of patients the convalescent plasma and 20% standard plasma.
Julia Sabia Motley, 57, of Merrick, NY, is hoping to become a donor for the Stony Brook study. She and her husband, Sean Motley, 59, tested positive in late March. She has to pass one more test to join the trial. Her husband is also planning to try to donate. “I can finally do something,” Sabia Motley says. Her son is in the MD-PhD program at Stony Brook and told her about the study.
Many questions remain
The treatment for COVID-19 is in its infancy. Burk has given the convalescent plasma to two patients. One is now recovering at home, and the other is on a ventilator but improving, he says.
About 200 nationwide have received the therapy, Joyner says. He expects blood supplies to increase as more people are eligible to donate.
Questions remain about how effective the convalescent therapy will be. While experts know that the COVID-19 antibodies “can be helpful in fighting the virus, we don’t know how long the antibodies in the plasma would stay in place,” Bennett-Guerrero says.
Nor do doctors know who the therapy might work best for, beyond people with a severe or life-threatening illness. When it’s been used for other infections, it’s generally given in early stages once someone has symptoms, Joyner says.
Joyner says he sees the treatment as a stopgap ‘’until concentrated antibodies are available.” Several drug companies are working to retrieve antibodies from donors and make concentrated antibody drugs.
“Typically we would think convalescent plasma might be a helpful bridge until therapies that are safe and effective and can be mass-produced are available, such as a vaccine or a drug,” Bennett-Guerrero says.
Even so, he says that he doesn’t think he will have a problem attracting donors, and that he will have repeat donors eager to help.
More information for potential donors
Blood banks, the American Red Cross, and others involved in convalescent plasma therapy have posted information online for potential donors. People who don’t meet the qualifications for COVID-19 plasma donations are welcomed as regular blood donors if they meet those criteria
According to the FDA, a donation could potentially help save the lives of up to four COVID-19 patients.
Father Pace is already planning another visit to the blood bank. To pass the time last time, he says, he prayed for the person who would eventually get his blood.
This article first appeared on WebMD.com.
As an Episcopal priest, Father Robert Pace of Fort Worth, TX, is used to putting others first and reaching out to help. So when the pulmonologist who helped him through his ordeal with COVID-19 asked if he would like to donate blood to help other patients, he did not hesitate.
“I said, ‘Absolutely,’” Pace, 53, recalls. He says the idea was ‘very appealing.’ ” During his ordeal with COVID-19 in March, he had spent 3 days in the hospital, isolated and on IV fluids and oxygen. He was short of breath, with a heartbeat more rapid than usual.
Now, fully recovered, his blood was a precious commodity, antibody-rich and potentially life-saving.
As researchers scramble to test drugs to fight COVID-19, others are turning to an age-old treatment. They’re collecting the blood of survivors and giving it to patients in the throes of a severe infection, a treatment known as convalescent plasma therapy.
Doctors say the treatment will probably serve as a bridge until other drugs and a vaccine become available.
Although the FDA considers the treatment investigational, in late March, it eased access to it. Patients can get it as part of a clinical trial or through an expanded access program overseen by hospitals or universities. A doctor can also request permission to use the treatment for a single patient.
“It is considered an emergent, compassionate need,” says John Burk, MD, a pulmonologist at Texas Health Harris Methodist Hospital, Fort Worth, who treated Pace. “It is a way to bring it to the bedside.” And the approval can happen quickly. Burk says he got one from the FDA just 20 minutes after requesting it for a severely ill patient.
How it works
The premise of how it works is “quite straightforward,” says Michael Joyner, MD, a professor of anesthesiology at the Mayo Clinic, Rochester, MN. “When someone is recovered and no longer symptomatic, you can harvest those antibodies from their blood and give them to someone else, and hopefully alter the course of their disease.” Joyner is the principal investigator for the FDA’s national Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19, with 1,000 sites already signed on.
Convalescent therapy has been used to fight many other viruses, including Ebola, severe acute respiratory syndrome (SARS), the “bird” flu, H1N1 flu, and during the 1918 flu pandemic. Joyner says the strongest evidence for it comes from the 1950s, when it was used to treat a rodent-borne illness called Argentine hemorrhagic fever. Using convalescent plasma therapy for this infection reduced the death rate from nearly 43% before the treatment became common in the late 1950s to about 3% after it was widely used, one report found.
Data about convalescent therapy specifically for COVID-19 is limited. Chinese researchers reported on five critically ill patients, all on mechanical ventilation, treated with convalescent plasma after they had received antiviral and anti-inflammatory medicines. Three could leave the hospital after 51-55 days, and two were in stable condition in the hospital 37 days after the transfusion.
In another study of 10 severely ill patients, symptoms went away or improved in all 10 within 1 to 3 days after the transfusion. Two of the three on ventilators were weaned off and put on oxygen instead. None died.
Chinese researchers also reported three cases of patients with COVID-19 given the convalescent therapy who had a satisfactory recovery.
Researchers who reviewed the track record of convalescent therapy for other conditions recently concluded that the treatment doesn’t appear to cause severe side effects and it should be studied for COVID-19.
Although information on side effects specific to this treatment is evolving, Joyner says they are “very, very low.”
According to the FDA, allergic reactions can occur with plasma therapies. Because the treatment for COVID-19 is new, it is not known if patients might have other types of reactions.
Who can donate?
Blood bank officials and researchers running the convalescent plasma programs say the desire to help is widespread, and they’ve been deluged with offers to donate. But requirements are strict.
Donors must have evidence of COVID-19 infection, documented in a variety of ways, such as a diagnostic test by nasal swab or a blood test showing antibodies. And they must be symptom-free for 14 days, with test results, or 28 days without.
The treatment involves collecting plasma, not whole blood. Plasma, the liquid part of the blood, helps with clotting and supports immunity. During the collection, a donor’s blood is put through a machine that collects the plasma only and sends the red blood cells and platelets back to the donor.
Clinical trials
Requirements may be more stringent for donors joining a formal clinical trial rather than an expanded access program. For instance, potential donors in a randomized clinical trial underway at Stony Brook University must have higher antibody levels than required by the FDA, says study leader Elliott Bennett-Guerrero, MD, medical director of perioperative quality and patient safety and professor at the Renaissance School of Medicine.
He hopes to enroll up to 500 patients from the Long Island, NY, area. While clinical trials typically have a 50-50 split, with half of subjects getting a treatment and half a placebo, Bennett-Guerrero’s study will give 80% of patients the convalescent plasma and 20% standard plasma.
Julia Sabia Motley, 57, of Merrick, NY, is hoping to become a donor for the Stony Brook study. She and her husband, Sean Motley, 59, tested positive in late March. She has to pass one more test to join the trial. Her husband is also planning to try to donate. “I can finally do something,” Sabia Motley says. Her son is in the MD-PhD program at Stony Brook and told her about the study.
Many questions remain
The treatment for COVID-19 is in its infancy. Burk has given the convalescent plasma to two patients. One is now recovering at home, and the other is on a ventilator but improving, he says.
About 200 nationwide have received the therapy, Joyner says. He expects blood supplies to increase as more people are eligible to donate.
Questions remain about how effective the convalescent therapy will be. While experts know that the COVID-19 antibodies “can be helpful in fighting the virus, we don’t know how long the antibodies in the plasma would stay in place,” Bennett-Guerrero says.
Nor do doctors know who the therapy might work best for, beyond people with a severe or life-threatening illness. When it’s been used for other infections, it’s generally given in early stages once someone has symptoms, Joyner says.
Joyner says he sees the treatment as a stopgap ‘’until concentrated antibodies are available.” Several drug companies are working to retrieve antibodies from donors and make concentrated antibody drugs.
“Typically we would think convalescent plasma might be a helpful bridge until therapies that are safe and effective and can be mass-produced are available, such as a vaccine or a drug,” Bennett-Guerrero says.
Even so, he says that he doesn’t think he will have a problem attracting donors, and that he will have repeat donors eager to help.
More information for potential donors
Blood banks, the American Red Cross, and others involved in convalescent plasma therapy have posted information online for potential donors. People who don’t meet the qualifications for COVID-19 plasma donations are welcomed as regular blood donors if they meet those criteria
According to the FDA, a donation could potentially help save the lives of up to four COVID-19 patients.
Father Pace is already planning another visit to the blood bank. To pass the time last time, he says, he prayed for the person who would eventually get his blood.
This article first appeared on WebMD.com.
As an Episcopal priest, Father Robert Pace of Fort Worth, TX, is used to putting others first and reaching out to help. So when the pulmonologist who helped him through his ordeal with COVID-19 asked if he would like to donate blood to help other patients, he did not hesitate.
“I said, ‘Absolutely,’” Pace, 53, recalls. He says the idea was ‘very appealing.’ ” During his ordeal with COVID-19 in March, he had spent 3 days in the hospital, isolated and on IV fluids and oxygen. He was short of breath, with a heartbeat more rapid than usual.
Now, fully recovered, his blood was a precious commodity, antibody-rich and potentially life-saving.
As researchers scramble to test drugs to fight COVID-19, others are turning to an age-old treatment. They’re collecting the blood of survivors and giving it to patients in the throes of a severe infection, a treatment known as convalescent plasma therapy.
Doctors say the treatment will probably serve as a bridge until other drugs and a vaccine become available.
Although the FDA considers the treatment investigational, in late March, it eased access to it. Patients can get it as part of a clinical trial or through an expanded access program overseen by hospitals or universities. A doctor can also request permission to use the treatment for a single patient.
“It is considered an emergent, compassionate need,” says John Burk, MD, a pulmonologist at Texas Health Harris Methodist Hospital, Fort Worth, who treated Pace. “It is a way to bring it to the bedside.” And the approval can happen quickly. Burk says he got one from the FDA just 20 minutes after requesting it for a severely ill patient.
How it works
The premise of how it works is “quite straightforward,” says Michael Joyner, MD, a professor of anesthesiology at the Mayo Clinic, Rochester, MN. “When someone is recovered and no longer symptomatic, you can harvest those antibodies from their blood and give them to someone else, and hopefully alter the course of their disease.” Joyner is the principal investigator for the FDA’s national Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19, with 1,000 sites already signed on.
Convalescent therapy has been used to fight many other viruses, including Ebola, severe acute respiratory syndrome (SARS), the “bird” flu, H1N1 flu, and during the 1918 flu pandemic. Joyner says the strongest evidence for it comes from the 1950s, when it was used to treat a rodent-borne illness called Argentine hemorrhagic fever. Using convalescent plasma therapy for this infection reduced the death rate from nearly 43% before the treatment became common in the late 1950s to about 3% after it was widely used, one report found.
Data about convalescent therapy specifically for COVID-19 is limited. Chinese researchers reported on five critically ill patients, all on mechanical ventilation, treated with convalescent plasma after they had received antiviral and anti-inflammatory medicines. Three could leave the hospital after 51-55 days, and two were in stable condition in the hospital 37 days after the transfusion.
In another study of 10 severely ill patients, symptoms went away or improved in all 10 within 1 to 3 days after the transfusion. Two of the three on ventilators were weaned off and put on oxygen instead. None died.
Chinese researchers also reported three cases of patients with COVID-19 given the convalescent therapy who had a satisfactory recovery.
Researchers who reviewed the track record of convalescent therapy for other conditions recently concluded that the treatment doesn’t appear to cause severe side effects and it should be studied for COVID-19.
Although information on side effects specific to this treatment is evolving, Joyner says they are “very, very low.”
According to the FDA, allergic reactions can occur with plasma therapies. Because the treatment for COVID-19 is new, it is not known if patients might have other types of reactions.
Who can donate?
Blood bank officials and researchers running the convalescent plasma programs say the desire to help is widespread, and they’ve been deluged with offers to donate. But requirements are strict.
Donors must have evidence of COVID-19 infection, documented in a variety of ways, such as a diagnostic test by nasal swab or a blood test showing antibodies. And they must be symptom-free for 14 days, with test results, or 28 days without.
The treatment involves collecting plasma, not whole blood. Plasma, the liquid part of the blood, helps with clotting and supports immunity. During the collection, a donor’s blood is put through a machine that collects the plasma only and sends the red blood cells and platelets back to the donor.
Clinical trials
Requirements may be more stringent for donors joining a formal clinical trial rather than an expanded access program. For instance, potential donors in a randomized clinical trial underway at Stony Brook University must have higher antibody levels than required by the FDA, says study leader Elliott Bennett-Guerrero, MD, medical director of perioperative quality and patient safety and professor at the Renaissance School of Medicine.
He hopes to enroll up to 500 patients from the Long Island, NY, area. While clinical trials typically have a 50-50 split, with half of subjects getting a treatment and half a placebo, Bennett-Guerrero’s study will give 80% of patients the convalescent plasma and 20% standard plasma.
Julia Sabia Motley, 57, of Merrick, NY, is hoping to become a donor for the Stony Brook study. She and her husband, Sean Motley, 59, tested positive in late March. She has to pass one more test to join the trial. Her husband is also planning to try to donate. “I can finally do something,” Sabia Motley says. Her son is in the MD-PhD program at Stony Brook and told her about the study.
Many questions remain
The treatment for COVID-19 is in its infancy. Burk has given the convalescent plasma to two patients. One is now recovering at home, and the other is on a ventilator but improving, he says.
About 200 nationwide have received the therapy, Joyner says. He expects blood supplies to increase as more people are eligible to donate.
Questions remain about how effective the convalescent therapy will be. While experts know that the COVID-19 antibodies “can be helpful in fighting the virus, we don’t know how long the antibodies in the plasma would stay in place,” Bennett-Guerrero says.
Nor do doctors know who the therapy might work best for, beyond people with a severe or life-threatening illness. When it’s been used for other infections, it’s generally given in early stages once someone has symptoms, Joyner says.
Joyner says he sees the treatment as a stopgap ‘’until concentrated antibodies are available.” Several drug companies are working to retrieve antibodies from donors and make concentrated antibody drugs.
“Typically we would think convalescent plasma might be a helpful bridge until therapies that are safe and effective and can be mass-produced are available, such as a vaccine or a drug,” Bennett-Guerrero says.
Even so, he says that he doesn’t think he will have a problem attracting donors, and that he will have repeat donors eager to help.
More information for potential donors
Blood banks, the American Red Cross, and others involved in convalescent plasma therapy have posted information online for potential donors. People who don’t meet the qualifications for COVID-19 plasma donations are welcomed as regular blood donors if they meet those criteria
According to the FDA, a donation could potentially help save the lives of up to four COVID-19 patients.
Father Pace is already planning another visit to the blood bank. To pass the time last time, he says, he prayed for the person who would eventually get his blood.
This article first appeared on WebMD.com.
Protean manifestations of COVID-19: “Our ignorance is profound”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
The resurgence of Plaquenil (hydroxychloroquine)
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Hospitalist well-being during the COVID-19 crisis
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the spread of COVID-19, is overwhelming for many people. Health care workers in the United States and around the world are leading the battle on the front lines of the pandemic. Thus, they experience a higher level of stress, fear, and anxiety during this crisis.
Over the course of weeks, hospitalists have reviewed articles, attended webinars, and discussed institutional strategies to respond to COVID-19. They follow the most up-to-date clinical information about the approach to patient care, conserving personal protective equipment (PPE), and guidance on how to talk to patients and families during crisis situations. The safety of hospitalists has been underscored with persistent advocacy from multiple organizations, for PPE, access to testing supplies, and decreasing any unnecessary exposure.
While it is agreed that the safety and well-being of hospital medicine teams is crucial to our society’s victory over COVID-19, very little has been discussed with regards to the “hospitalist” well-being and wellness during this pandemic.
The well-being of providers is essential to the success of a health care system. Many hospitalists already experience moral injury and showed evidence of provider burnout before COVID-19. With the onset of the pandemic, this will only get worse and burnout will accelerate if nothing is done to stop it. We cannot wait for the dust to settle to help our colleagues, we must act now.
Many providers have expressed similar pandemic fears, including, uncertainty about screening and testing capability, fear of the PPE shortage, fear of being exposed and underprepared, and fear of bringing the virus home and making family members sick. This list is not exclusive, and there are so many other factors that providers are internally processing, all while continuing their commitment to patient care and safety.
Practicing medicine comes with the heaviest of responsibilities, including the defense of the health of humanity. Therefore, it is easy to understand that, while providers are on the battlefield of this pandemic as they defend the health of humanity, they are not thinking of their own wellness or well-being. Moral injury describes the mental, emotional, and spiritual distress people feel after “perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.” This is already happening, with many hospitals in various cities running out of ventilators, lacking basic supplies for provider safety and leaving providers in survival mode on the front lines without their “suits of armor.” However, many providers will never recognize moral injury or burnout because they are focused on saving as many lives as possible with very limited resources.
While many websites can aid patient and community members on wellness during COVID-19, there is no specific forum or outlet for providers. We must give all hospital medicine team members a multimedia platform to address the fear, anxiety, and uncertainty of COVID-19. We must also provide them with techniques for resilience, coping strategies, and develop a network of support as the situation evolves, in real time.
We must remind hospitalists, “You may be scared, you may feel anxious, and that is okay. It is normal to have these feelings and it is healthy to acknowledge them. Fear serves as an important role in keeping us safe, but if left unchecked it can be horrifying and crippling. However, to conquer it we must face our fears together, with strategy, knowledge, and advocacy. This is the way to rebuild the current health care climate with confidence and trust.”
Although the world may seem foreign and dangerous, it is in adversity that we will find our strength as a hospital medicine community. We go to work every day because that is what we do. Your courage to come to work every day, in spite of any danger that it may present to you, is an inspiration to the world. The battle is not lost, and as individuals and as a community we must build resilience, inspire hope, and empower each other. We are stronger together than we are alone. As hospitalists around the country, and throughout the world, we must agree to uphold the moral integrity of medicine without sacrificing ourselves.
Dr. Williams is the vice-president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as the vice-president of the Medical Executive Committee.
Resource
Dean, Wendy; Talbot, Simon; and Dean, Austin. Reframing clinician distress: Moral injury not burnout. Fed Pract. 2019 Sept;36(9):400-2.
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the spread of COVID-19, is overwhelming for many people. Health care workers in the United States and around the world are leading the battle on the front lines of the pandemic. Thus, they experience a higher level of stress, fear, and anxiety during this crisis.
Over the course of weeks, hospitalists have reviewed articles, attended webinars, and discussed institutional strategies to respond to COVID-19. They follow the most up-to-date clinical information about the approach to patient care, conserving personal protective equipment (PPE), and guidance on how to talk to patients and families during crisis situations. The safety of hospitalists has been underscored with persistent advocacy from multiple organizations, for PPE, access to testing supplies, and decreasing any unnecessary exposure.
While it is agreed that the safety and well-being of hospital medicine teams is crucial to our society’s victory over COVID-19, very little has been discussed with regards to the “hospitalist” well-being and wellness during this pandemic.
The well-being of providers is essential to the success of a health care system. Many hospitalists already experience moral injury and showed evidence of provider burnout before COVID-19. With the onset of the pandemic, this will only get worse and burnout will accelerate if nothing is done to stop it. We cannot wait for the dust to settle to help our colleagues, we must act now.
Many providers have expressed similar pandemic fears, including, uncertainty about screening and testing capability, fear of the PPE shortage, fear of being exposed and underprepared, and fear of bringing the virus home and making family members sick. This list is not exclusive, and there are so many other factors that providers are internally processing, all while continuing their commitment to patient care and safety.
Practicing medicine comes with the heaviest of responsibilities, including the defense of the health of humanity. Therefore, it is easy to understand that, while providers are on the battlefield of this pandemic as they defend the health of humanity, they are not thinking of their own wellness or well-being. Moral injury describes the mental, emotional, and spiritual distress people feel after “perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.” This is already happening, with many hospitals in various cities running out of ventilators, lacking basic supplies for provider safety and leaving providers in survival mode on the front lines without their “suits of armor.” However, many providers will never recognize moral injury or burnout because they are focused on saving as many lives as possible with very limited resources.
While many websites can aid patient and community members on wellness during COVID-19, there is no specific forum or outlet for providers. We must give all hospital medicine team members a multimedia platform to address the fear, anxiety, and uncertainty of COVID-19. We must also provide them with techniques for resilience, coping strategies, and develop a network of support as the situation evolves, in real time.
We must remind hospitalists, “You may be scared, you may feel anxious, and that is okay. It is normal to have these feelings and it is healthy to acknowledge them. Fear serves as an important role in keeping us safe, but if left unchecked it can be horrifying and crippling. However, to conquer it we must face our fears together, with strategy, knowledge, and advocacy. This is the way to rebuild the current health care climate with confidence and trust.”
Although the world may seem foreign and dangerous, it is in adversity that we will find our strength as a hospital medicine community. We go to work every day because that is what we do. Your courage to come to work every day, in spite of any danger that it may present to you, is an inspiration to the world. The battle is not lost, and as individuals and as a community we must build resilience, inspire hope, and empower each other. We are stronger together than we are alone. As hospitalists around the country, and throughout the world, we must agree to uphold the moral integrity of medicine without sacrificing ourselves.
Dr. Williams is the vice-president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as the vice-president of the Medical Executive Committee.
Resource
Dean, Wendy; Talbot, Simon; and Dean, Austin. Reframing clinician distress: Moral injury not burnout. Fed Pract. 2019 Sept;36(9):400-2.
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the spread of COVID-19, is overwhelming for many people. Health care workers in the United States and around the world are leading the battle on the front lines of the pandemic. Thus, they experience a higher level of stress, fear, and anxiety during this crisis.
Over the course of weeks, hospitalists have reviewed articles, attended webinars, and discussed institutional strategies to respond to COVID-19. They follow the most up-to-date clinical information about the approach to patient care, conserving personal protective equipment (PPE), and guidance on how to talk to patients and families during crisis situations. The safety of hospitalists has been underscored with persistent advocacy from multiple organizations, for PPE, access to testing supplies, and decreasing any unnecessary exposure.
While it is agreed that the safety and well-being of hospital medicine teams is crucial to our society’s victory over COVID-19, very little has been discussed with regards to the “hospitalist” well-being and wellness during this pandemic.
The well-being of providers is essential to the success of a health care system. Many hospitalists already experience moral injury and showed evidence of provider burnout before COVID-19. With the onset of the pandemic, this will only get worse and burnout will accelerate if nothing is done to stop it. We cannot wait for the dust to settle to help our colleagues, we must act now.
Many providers have expressed similar pandemic fears, including, uncertainty about screening and testing capability, fear of the PPE shortage, fear of being exposed and underprepared, and fear of bringing the virus home and making family members sick. This list is not exclusive, and there are so many other factors that providers are internally processing, all while continuing their commitment to patient care and safety.
Practicing medicine comes with the heaviest of responsibilities, including the defense of the health of humanity. Therefore, it is easy to understand that, while providers are on the battlefield of this pandemic as they defend the health of humanity, they are not thinking of their own wellness or well-being. Moral injury describes the mental, emotional, and spiritual distress people feel after “perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.” This is already happening, with many hospitals in various cities running out of ventilators, lacking basic supplies for provider safety and leaving providers in survival mode on the front lines without their “suits of armor.” However, many providers will never recognize moral injury or burnout because they are focused on saving as many lives as possible with very limited resources.
While many websites can aid patient and community members on wellness during COVID-19, there is no specific forum or outlet for providers. We must give all hospital medicine team members a multimedia platform to address the fear, anxiety, and uncertainty of COVID-19. We must also provide them with techniques for resilience, coping strategies, and develop a network of support as the situation evolves, in real time.
We must remind hospitalists, “You may be scared, you may feel anxious, and that is okay. It is normal to have these feelings and it is healthy to acknowledge them. Fear serves as an important role in keeping us safe, but if left unchecked it can be horrifying and crippling. However, to conquer it we must face our fears together, with strategy, knowledge, and advocacy. This is the way to rebuild the current health care climate with confidence and trust.”
Although the world may seem foreign and dangerous, it is in adversity that we will find our strength as a hospital medicine community. We go to work every day because that is what we do. Your courage to come to work every day, in spite of any danger that it may present to you, is an inspiration to the world. The battle is not lost, and as individuals and as a community we must build resilience, inspire hope, and empower each other. We are stronger together than we are alone. As hospitalists around the country, and throughout the world, we must agree to uphold the moral integrity of medicine without sacrificing ourselves.
Dr. Williams is the vice-president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as the vice-president of the Medical Executive Committee.
Resource
Dean, Wendy; Talbot, Simon; and Dean, Austin. Reframing clinician distress: Moral injury not burnout. Fed Pract. 2019 Sept;36(9):400-2.
COVID-19: Press pause on assisted reproduction?
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Cancer care ‘transformed in space of a month’ because of pandemic
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
COVID-19 pandemic spells trouble for children’s health
Although priority number one lies in controlling the spread of COVID-19, public health researchers are calling attention to the long-term repercussions of the pandemic on children’s health.
School closures could noticeably worsen the epidemic of childhood obesity that already threatens many children in the United States, say Paul Rundle, DrPH, and colleagues from Columbia University Mailman School of Public Health, New York City, in a perspective published online March 30 in Obesity.
“In part, we wrote the perspective to remind people that summer unhealthy weight gain seems to accumulate year to year,” he told Medscape Medical News in an email.
Rundle and colleagues estimate that time spent out of school will double this year because of school closures due to COVID-19. That, along with shelter-in-place orders, will pose challenges both for physical activity and healthy eating among children.
In addition, playgrounds have closed in many areas, and even where parks remain open, social distancing decreases opportunities for exercise. Team sports are on hold, and without physical education taught in schools, many children will not be getting as much active outdoor play as needed.
That’s especially true for children in urban areas, who may find it even more difficult to exercise inside cramped apartments, they add.
As a result, more and more children may turn to sedentary activities, and increased screen time goes hand in hand with childhood overweight and obesity, not just because of the lack of exercise but also because of snacking on unhealthy, empty-calorie foods while glued to the screen.
“We were hoping to get the word out on this issue, do some education or reminding, and at least let people know that this should be something to keep an eye on, among so many other things,” Rundle added.
Excess Eating Because of Stress and Boredom
Jessica Sparks Lilley, MD, director of the Pediatric Diabetes and Lipid Program at the Mississippi Center for Advanced Medicine in Madison, agrees that it is crucial to address these issues.
“Just like adults, children eat in response to emotions, including stress and boredom, and stress levels are high during these uncertain times,” she told Medscape Medical News.
Although both Rundle and Sparks Lilley acknowledged the challenges of finding good solutions at this time, they do offer some tips.
Schools should make physical education and at-home exercise a priority alongside other remote teaching. Physical education teachers could even stream exercise classes to children at home.
Even just walking in the park while maintaining social distancing could be better than nothing, and a brisk walk is probably even better.
Depending on the age of the child, online yoga may also be useful. Even though yoga burns relatively few calories, it incorporates mindfulness training that may be helpful.
“I think focusing on promoting mindful eating as compared to mindless or distracted eating is important. Even in the best of circumstances, it is hard to exercise enough to burn off high energy snacks,” Rundle said.
Additional Stressors From Poverty: Schools Can Help With Meals
Children living in poverty, already the most vulnerable to obesity and related health problems, have additional stressors, add the two experts.
“As more Americans are losing jobs, poverty is a real threat to many of the children I care for. Families living in poverty often rely on processed, high-calorie, low-nutrient foods for survival, because they are inexpensive and shelf-stable,” Sparks Lilley said.
Rundle and colleagues agree: “Our own experiences in supermarkets show...shelves that held...crackers, chips, ramen noodles, soda, sugary cereals, and processed ready-to-eat meals are quite empty. We anticipate that many children will experience higher calorie diets during the pandemic response.”
Similar to how they address food insecurity during summer holidays, school districts have responded by offering grab-and-go meals, Rundle and colleagues note.
To maintain social distancing for people with vulnerable family members, some school districts have also started delivering food using school buses that run along regularly scheduled routes.
Rundle also stresses that farmers’ markets, which often provide foods that appeal to immigrant and ethnic communities, should be considered part of essential food services.
As such, social distancing protocols should be established for them and they should be allowed to stay open, he argues.
“The safety of American children is at stake in many ways. The threat to themselves or their caregivers being infected with COVID-19 is rightly foremost in our concerns,” Sparks Lilley stressed.
“However, there is other fallout to consider. We’ve seen very clearly the need for public health and preventive medicine and can’t let vulnerable children fall through the cracks.”
Rundle agrees. Although it is a “priority” to mitigate the immediate impact of COVID-19, “it is important to consider ways to prevent its long-term effects, including new risks for childhood obesity.”
Rundle and coauthors, as well as Sparks Lilley, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Although priority number one lies in controlling the spread of COVID-19, public health researchers are calling attention to the long-term repercussions of the pandemic on children’s health.
School closures could noticeably worsen the epidemic of childhood obesity that already threatens many children in the United States, say Paul Rundle, DrPH, and colleagues from Columbia University Mailman School of Public Health, New York City, in a perspective published online March 30 in Obesity.
“In part, we wrote the perspective to remind people that summer unhealthy weight gain seems to accumulate year to year,” he told Medscape Medical News in an email.
Rundle and colleagues estimate that time spent out of school will double this year because of school closures due to COVID-19. That, along with shelter-in-place orders, will pose challenges both for physical activity and healthy eating among children.
In addition, playgrounds have closed in many areas, and even where parks remain open, social distancing decreases opportunities for exercise. Team sports are on hold, and without physical education taught in schools, many children will not be getting as much active outdoor play as needed.
That’s especially true for children in urban areas, who may find it even more difficult to exercise inside cramped apartments, they add.
As a result, more and more children may turn to sedentary activities, and increased screen time goes hand in hand with childhood overweight and obesity, not just because of the lack of exercise but also because of snacking on unhealthy, empty-calorie foods while glued to the screen.
“We were hoping to get the word out on this issue, do some education or reminding, and at least let people know that this should be something to keep an eye on, among so many other things,” Rundle added.
Excess Eating Because of Stress and Boredom
Jessica Sparks Lilley, MD, director of the Pediatric Diabetes and Lipid Program at the Mississippi Center for Advanced Medicine in Madison, agrees that it is crucial to address these issues.
“Just like adults, children eat in response to emotions, including stress and boredom, and stress levels are high during these uncertain times,” she told Medscape Medical News.
Although both Rundle and Sparks Lilley acknowledged the challenges of finding good solutions at this time, they do offer some tips.
Schools should make physical education and at-home exercise a priority alongside other remote teaching. Physical education teachers could even stream exercise classes to children at home.
Even just walking in the park while maintaining social distancing could be better than nothing, and a brisk walk is probably even better.
Depending on the age of the child, online yoga may also be useful. Even though yoga burns relatively few calories, it incorporates mindfulness training that may be helpful.
“I think focusing on promoting mindful eating as compared to mindless or distracted eating is important. Even in the best of circumstances, it is hard to exercise enough to burn off high energy snacks,” Rundle said.
Additional Stressors From Poverty: Schools Can Help With Meals
Children living in poverty, already the most vulnerable to obesity and related health problems, have additional stressors, add the two experts.
“As more Americans are losing jobs, poverty is a real threat to many of the children I care for. Families living in poverty often rely on processed, high-calorie, low-nutrient foods for survival, because they are inexpensive and shelf-stable,” Sparks Lilley said.
Rundle and colleagues agree: “Our own experiences in supermarkets show...shelves that held...crackers, chips, ramen noodles, soda, sugary cereals, and processed ready-to-eat meals are quite empty. We anticipate that many children will experience higher calorie diets during the pandemic response.”
Similar to how they address food insecurity during summer holidays, school districts have responded by offering grab-and-go meals, Rundle and colleagues note.
To maintain social distancing for people with vulnerable family members, some school districts have also started delivering food using school buses that run along regularly scheduled routes.
Rundle also stresses that farmers’ markets, which often provide foods that appeal to immigrant and ethnic communities, should be considered part of essential food services.
As such, social distancing protocols should be established for them and they should be allowed to stay open, he argues.
“The safety of American children is at stake in many ways. The threat to themselves or their caregivers being infected with COVID-19 is rightly foremost in our concerns,” Sparks Lilley stressed.
“However, there is other fallout to consider. We’ve seen very clearly the need for public health and preventive medicine and can’t let vulnerable children fall through the cracks.”
Rundle agrees. Although it is a “priority” to mitigate the immediate impact of COVID-19, “it is important to consider ways to prevent its long-term effects, including new risks for childhood obesity.”
Rundle and coauthors, as well as Sparks Lilley, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Although priority number one lies in controlling the spread of COVID-19, public health researchers are calling attention to the long-term repercussions of the pandemic on children’s health.
School closures could noticeably worsen the epidemic of childhood obesity that already threatens many children in the United States, say Paul Rundle, DrPH, and colleagues from Columbia University Mailman School of Public Health, New York City, in a perspective published online March 30 in Obesity.
“In part, we wrote the perspective to remind people that summer unhealthy weight gain seems to accumulate year to year,” he told Medscape Medical News in an email.
Rundle and colleagues estimate that time spent out of school will double this year because of school closures due to COVID-19. That, along with shelter-in-place orders, will pose challenges both for physical activity and healthy eating among children.
In addition, playgrounds have closed in many areas, and even where parks remain open, social distancing decreases opportunities for exercise. Team sports are on hold, and without physical education taught in schools, many children will not be getting as much active outdoor play as needed.
That’s especially true for children in urban areas, who may find it even more difficult to exercise inside cramped apartments, they add.
As a result, more and more children may turn to sedentary activities, and increased screen time goes hand in hand with childhood overweight and obesity, not just because of the lack of exercise but also because of snacking on unhealthy, empty-calorie foods while glued to the screen.
“We were hoping to get the word out on this issue, do some education or reminding, and at least let people know that this should be something to keep an eye on, among so many other things,” Rundle added.
Excess Eating Because of Stress and Boredom
Jessica Sparks Lilley, MD, director of the Pediatric Diabetes and Lipid Program at the Mississippi Center for Advanced Medicine in Madison, agrees that it is crucial to address these issues.
“Just like adults, children eat in response to emotions, including stress and boredom, and stress levels are high during these uncertain times,” she told Medscape Medical News.
Although both Rundle and Sparks Lilley acknowledged the challenges of finding good solutions at this time, they do offer some tips.
Schools should make physical education and at-home exercise a priority alongside other remote teaching. Physical education teachers could even stream exercise classes to children at home.
Even just walking in the park while maintaining social distancing could be better than nothing, and a brisk walk is probably even better.
Depending on the age of the child, online yoga may also be useful. Even though yoga burns relatively few calories, it incorporates mindfulness training that may be helpful.
“I think focusing on promoting mindful eating as compared to mindless or distracted eating is important. Even in the best of circumstances, it is hard to exercise enough to burn off high energy snacks,” Rundle said.
Additional Stressors From Poverty: Schools Can Help With Meals
Children living in poverty, already the most vulnerable to obesity and related health problems, have additional stressors, add the two experts.
“As more Americans are losing jobs, poverty is a real threat to many of the children I care for. Families living in poverty often rely on processed, high-calorie, low-nutrient foods for survival, because they are inexpensive and shelf-stable,” Sparks Lilley said.
Rundle and colleagues agree: “Our own experiences in supermarkets show...shelves that held...crackers, chips, ramen noodles, soda, sugary cereals, and processed ready-to-eat meals are quite empty. We anticipate that many children will experience higher calorie diets during the pandemic response.”
Similar to how they address food insecurity during summer holidays, school districts have responded by offering grab-and-go meals, Rundle and colleagues note.
To maintain social distancing for people with vulnerable family members, some school districts have also started delivering food using school buses that run along regularly scheduled routes.
Rundle also stresses that farmers’ markets, which often provide foods that appeal to immigrant and ethnic communities, should be considered part of essential food services.
As such, social distancing protocols should be established for them and they should be allowed to stay open, he argues.
“The safety of American children is at stake in many ways. The threat to themselves or their caregivers being infected with COVID-19 is rightly foremost in our concerns,” Sparks Lilley stressed.
“However, there is other fallout to consider. We’ve seen very clearly the need for public health and preventive medicine and can’t let vulnerable children fall through the cracks.”
Rundle agrees. Although it is a “priority” to mitigate the immediate impact of COVID-19, “it is important to consider ways to prevent its long-term effects, including new risks for childhood obesity.”
Rundle and coauthors, as well as Sparks Lilley, have reported no relevant financial relationships.
This article first appeared on Medscape.com.