User login
Pediatric Hospital Medicine Core Competencies: 2020 Revision. Table of Contents
Authors and Editors.............................................................3
External Reviewers.............................................................11
ORIGINAL RESEARCH
The Pediatric Hospital Medicine Core Competencies:
2020 Revision—Introduction and Methodology.............................................................12
Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Sofia Teferi, MD, SFHM, FAAP; Erin Stucky Fisher, MD, MHM, FAAP
SECTION 1: COMMON CLINICAL DIAGNOSES AND CONDITIONS
1.01 Acute Abdominal Pain and Acute Abdomen.............................................................18
1.02 Acute Gastroenteritis.............................................................20
1.03 Acute Respiratory Failure.............................................................22
1.04 Altered Mental Status.............................................................24
1.05 Asthma.............................................................26
1.06 Bone and Joint Infections.............................................................28
1.07 Brief Resolved Unexplained Event.............................................................30
1.08 Bronchiolitis.............................................................32
1.09 Central Nervous System Infections.............................................................34
1.10 Constipation.............................................................36
1.11 Diabetes Mellitus.............................................................37
1.12 Failure to Thrive.............................................................39
1.13 Fever of Unknown Origin.............................................................41
1.14 Fluid and Electrolyte Management.............................................................43
1.15 Gastrointestinal and Digestive Disorders.............................................................45
1.16 Head and Neck Disorders.............................................................47
1.17 Kawasaki Disease.............................................................49
1.18 Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome.............................................................50
1.19 Neonatal Fever.............................................................52
1.20 Neonatal Jaundice.............................................................54
1.21 Pneumonia.............................................................56
1.22 Seizures.............................................................57
1.23 Sepsis and Shock.............................................................59
1.24 Sickle Cell Disease.............................................................61
1.25 Skin and Soft Tissue Infections.............................................................63
1.26 Toxin Ingestion and Exposure.............................................................65
1.27 Urinary Tract Infections.............................................................67
SECTION 2: CORE SKILLS
2.01 Bladder Catheterization and Interpretation
of Urinalysis.............................................................68
2.02 Communication.............................................................70
2.03 Diagnostic Imaging.............................................................72
2.04 Electrocardiogram Interpretation.............................................................74
2.05 Feeding Tubes.............................................................75
2.06 Intravenous Access and Phlebotomy.............................................................77
2.07 Lumbar Puncture.............................................................79
2.08 Non-invasive Monitoring.............................................................81
2.09 Nutrition.............................................................82
2.10 Oxygen Delivery and Airway Management.............................................................84
2.11 Pain Management.............................................................86
2.12 Pediatric Advanced Life Support.............................................................88
2.13 Peri-procedural Care.............................................................90
2.14 Preventive Care Services.............................................................92
2.15 Procedural Sedation.............................................................94
SECTION 3: SPECIALIZED SERVICES
3.01 Acute Behavioral and Psychiatric Conditions.............................................................96
3.02 Adolescent and Young Adult Medicine.............................................................98
3.03 Child Abuse and Neglect.............................................................100
3.04 Child with Medical Complexity.............................................................102
3.05 Chronic Behavioral and Psychiatric Conditions.............................................................104
3.06 Newborn Care and Delivery Room Management.............................................................106
3.07 Palliative Care and Hospice.............................................................108
3.08 Pediatric Interfacility Transport.............................................................110
SECTION 4: HEALTHCARE SYSTEMS: SUPPORTING AND ADVANCING CHILD HEALTH
4.01 Advocacy.............................................................112
4.02 Business Practices.............................................................114
4.03 Consultation and Co-management.............................................................116
4.04 Education.............................................................118
4.05 Ethics.............................................................120
4.06 Evidence-based Medicine.............................................................122
4.07 Family Centered Care.............................................................123
4.08 Handoffs and Transitions of Care.............................................................125
4.09 Health Information Technology.............................................................127
4.10 High Value Care.............................................................129
4.11 Infection Control and Antimicrobial Stewardship.............................................................131
4.12 Leadership in Healthcare.............................................................133
4.13 Legal Issues and Risk Management.............................................................134
4.14 Patient Safety.............................................................136
4.15 Quality Improvement.............................................................138
4.16 Research.............................................................140
APPENDIX
Chapter Links.............................................................142
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
Figure: Needs Assessment Survey.............................................................145
Dedication.............................................................153
To Michael Burke, our friend and colleague
Authors and Editors.............................................................3
External Reviewers.............................................................11
ORIGINAL RESEARCH
The Pediatric Hospital Medicine Core Competencies:
2020 Revision—Introduction and Methodology.............................................................12
Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Sofia Teferi, MD, SFHM, FAAP; Erin Stucky Fisher, MD, MHM, FAAP
SECTION 1: COMMON CLINICAL DIAGNOSES AND CONDITIONS
1.01 Acute Abdominal Pain and Acute Abdomen.............................................................18
1.02 Acute Gastroenteritis.............................................................20
1.03 Acute Respiratory Failure.............................................................22
1.04 Altered Mental Status.............................................................24
1.05 Asthma.............................................................26
1.06 Bone and Joint Infections.............................................................28
1.07 Brief Resolved Unexplained Event.............................................................30
1.08 Bronchiolitis.............................................................32
1.09 Central Nervous System Infections.............................................................34
1.10 Constipation.............................................................36
1.11 Diabetes Mellitus.............................................................37
1.12 Failure to Thrive.............................................................39
1.13 Fever of Unknown Origin.............................................................41
1.14 Fluid and Electrolyte Management.............................................................43
1.15 Gastrointestinal and Digestive Disorders.............................................................45
1.16 Head and Neck Disorders.............................................................47
1.17 Kawasaki Disease.............................................................49
1.18 Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome.............................................................50
1.19 Neonatal Fever.............................................................52
1.20 Neonatal Jaundice.............................................................54
1.21 Pneumonia.............................................................56
1.22 Seizures.............................................................57
1.23 Sepsis and Shock.............................................................59
1.24 Sickle Cell Disease.............................................................61
1.25 Skin and Soft Tissue Infections.............................................................63
1.26 Toxin Ingestion and Exposure.............................................................65
1.27 Urinary Tract Infections.............................................................67
SECTION 2: CORE SKILLS
2.01 Bladder Catheterization and Interpretation
of Urinalysis.............................................................68
2.02 Communication.............................................................70
2.03 Diagnostic Imaging.............................................................72
2.04 Electrocardiogram Interpretation.............................................................74
2.05 Feeding Tubes.............................................................75
2.06 Intravenous Access and Phlebotomy.............................................................77
2.07 Lumbar Puncture.............................................................79
2.08 Non-invasive Monitoring.............................................................81
2.09 Nutrition.............................................................82
2.10 Oxygen Delivery and Airway Management.............................................................84
2.11 Pain Management.............................................................86
2.12 Pediatric Advanced Life Support.............................................................88
2.13 Peri-procedural Care.............................................................90
2.14 Preventive Care Services.............................................................92
2.15 Procedural Sedation.............................................................94
SECTION 3: SPECIALIZED SERVICES
3.01 Acute Behavioral and Psychiatric Conditions.............................................................96
3.02 Adolescent and Young Adult Medicine.............................................................98
3.03 Child Abuse and Neglect.............................................................100
3.04 Child with Medical Complexity.............................................................102
3.05 Chronic Behavioral and Psychiatric Conditions.............................................................104
3.06 Newborn Care and Delivery Room Management.............................................................106
3.07 Palliative Care and Hospice.............................................................108
3.08 Pediatric Interfacility Transport.............................................................110
SECTION 4: HEALTHCARE SYSTEMS: SUPPORTING AND ADVANCING CHILD HEALTH
4.01 Advocacy.............................................................112
4.02 Business Practices.............................................................114
4.03 Consultation and Co-management.............................................................116
4.04 Education.............................................................118
4.05 Ethics.............................................................120
4.06 Evidence-based Medicine.............................................................122
4.07 Family Centered Care.............................................................123
4.08 Handoffs and Transitions of Care.............................................................125
4.09 Health Information Technology.............................................................127
4.10 High Value Care.............................................................129
4.11 Infection Control and Antimicrobial Stewardship.............................................................131
4.12 Leadership in Healthcare.............................................................133
4.13 Legal Issues and Risk Management.............................................................134
4.14 Patient Safety.............................................................136
4.15 Quality Improvement.............................................................138
4.16 Research.............................................................140
APPENDIX
Chapter Links.............................................................142
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
Figure: Needs Assessment Survey.............................................................145
Dedication.............................................................153
To Michael Burke, our friend and colleague
Authors and Editors.............................................................3
External Reviewers.............................................................11
ORIGINAL RESEARCH
The Pediatric Hospital Medicine Core Competencies:
2020 Revision—Introduction and Methodology.............................................................12
Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Sofia Teferi, MD, SFHM, FAAP; Erin Stucky Fisher, MD, MHM, FAAP
SECTION 1: COMMON CLINICAL DIAGNOSES AND CONDITIONS
1.01 Acute Abdominal Pain and Acute Abdomen.............................................................18
1.02 Acute Gastroenteritis.............................................................20
1.03 Acute Respiratory Failure.............................................................22
1.04 Altered Mental Status.............................................................24
1.05 Asthma.............................................................26
1.06 Bone and Joint Infections.............................................................28
1.07 Brief Resolved Unexplained Event.............................................................30
1.08 Bronchiolitis.............................................................32
1.09 Central Nervous System Infections.............................................................34
1.10 Constipation.............................................................36
1.11 Diabetes Mellitus.............................................................37
1.12 Failure to Thrive.............................................................39
1.13 Fever of Unknown Origin.............................................................41
1.14 Fluid and Electrolyte Management.............................................................43
1.15 Gastrointestinal and Digestive Disorders.............................................................45
1.16 Head and Neck Disorders.............................................................47
1.17 Kawasaki Disease.............................................................49
1.18 Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome.............................................................50
1.19 Neonatal Fever.............................................................52
1.20 Neonatal Jaundice.............................................................54
1.21 Pneumonia.............................................................56
1.22 Seizures.............................................................57
1.23 Sepsis and Shock.............................................................59
1.24 Sickle Cell Disease.............................................................61
1.25 Skin and Soft Tissue Infections.............................................................63
1.26 Toxin Ingestion and Exposure.............................................................65
1.27 Urinary Tract Infections.............................................................67
SECTION 2: CORE SKILLS
2.01 Bladder Catheterization and Interpretation
of Urinalysis.............................................................68
2.02 Communication.............................................................70
2.03 Diagnostic Imaging.............................................................72
2.04 Electrocardiogram Interpretation.............................................................74
2.05 Feeding Tubes.............................................................75
2.06 Intravenous Access and Phlebotomy.............................................................77
2.07 Lumbar Puncture.............................................................79
2.08 Non-invasive Monitoring.............................................................81
2.09 Nutrition.............................................................82
2.10 Oxygen Delivery and Airway Management.............................................................84
2.11 Pain Management.............................................................86
2.12 Pediatric Advanced Life Support.............................................................88
2.13 Peri-procedural Care.............................................................90
2.14 Preventive Care Services.............................................................92
2.15 Procedural Sedation.............................................................94
SECTION 3: SPECIALIZED SERVICES
3.01 Acute Behavioral and Psychiatric Conditions.............................................................96
3.02 Adolescent and Young Adult Medicine.............................................................98
3.03 Child Abuse and Neglect.............................................................100
3.04 Child with Medical Complexity.............................................................102
3.05 Chronic Behavioral and Psychiatric Conditions.............................................................104
3.06 Newborn Care and Delivery Room Management.............................................................106
3.07 Palliative Care and Hospice.............................................................108
3.08 Pediatric Interfacility Transport.............................................................110
SECTION 4: HEALTHCARE SYSTEMS: SUPPORTING AND ADVANCING CHILD HEALTH
4.01 Advocacy.............................................................112
4.02 Business Practices.............................................................114
4.03 Consultation and Co-management.............................................................116
4.04 Education.............................................................118
4.05 Ethics.............................................................120
4.06 Evidence-based Medicine.............................................................122
4.07 Family Centered Care.............................................................123
4.08 Handoffs and Transitions of Care.............................................................125
4.09 Health Information Technology.............................................................127
4.10 High Value Care.............................................................129
4.11 Infection Control and Antimicrobial Stewardship.............................................................131
4.12 Leadership in Healthcare.............................................................133
4.13 Legal Issues and Risk Management.............................................................134
4.14 Patient Safety.............................................................136
4.15 Quality Improvement.............................................................138
4.16 Research.............................................................140
APPENDIX
Chapter Links.............................................................142
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
Figure: Needs Assessment Survey.............................................................145
Dedication.............................................................153
To Michael Burke, our friend and colleague
The Pediatric Hospital Medicine Core Competencies: 2020 Revision Dedication
Thank you, Michael, for making us a stronger and more compassionate PHM Community.
The Editors and Associate Editors of The Pediatric Hospital Medicine Core Competencies: 2020 Revision:
Francisco Alvarez; Weijen Chang; Erin Fisher; Sandra Gage; Jennifer Maniscalco; Vineeta Mittal; Anand Sekaran; Amit Singh; Sofia Teferi
Thank you, Michael, for making us a stronger and more compassionate PHM Community.
The Editors and Associate Editors of The Pediatric Hospital Medicine Core Competencies: 2020 Revision:
Francisco Alvarez; Weijen Chang; Erin Fisher; Sandra Gage; Jennifer Maniscalco; Vineeta Mittal; Anand Sekaran; Amit Singh; Sofia Teferi
Thank you, Michael, for making us a stronger and more compassionate PHM Community.
The Editors and Associate Editors of The Pediatric Hospital Medicine Core Competencies: 2020 Revision:
Francisco Alvarez; Weijen Chang; Erin Fisher; Sandra Gage; Jennifer Maniscalco; Vineeta Mittal; Anand Sekaran; Amit Singh; Sofia Teferi
APPENDIX
NEEDS ASSESSMENT SURVEY
The editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council,and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
CHAPTER LINKS
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
NEEDS ASSESSMENT SURVEY
The editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council,and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
CHAPTER LINKS
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
NEEDS ASSESSMENT SURVEY
The editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council,and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
CHAPTER LINKS
These chapter links are guides to assist the reader in identifying chapters where some key relationships across knowledge, skills, attitudes, and systems organization and improvement may overlap. Chapter links are limited to 5 per chapter, are not comprehensive, and are intended as a general guide for the reader.
Research News: Neurologic Disorders (FULL)
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Modest Evidence for Benefit in Studies of Cannabis in MS
While several dozen studies have been conducted into cannabis-based treatments for symptoms of multiple sclerosis (MS), a new systematic review deems most to be of fair to poor quality. Reviewers found modest evidence of benefit and plenty of room for more research.
“Cannabis-based medicine may be useful for refractory MS symptoms, especially spasticity and pain, and side effects are usually well tolerated,” study lead author Natasha Breward, a graduate student at the College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, said in an interview. Breward spoke prior to the presentation of the study findings at the 2019 meeting of the Consortium of Multiple Sclerosis Centers.
For the review, Breward and colleagues focused on 60 studies—26 randomized controlled trials and 34 trials with other designs. Forty of the studies used nabiximols, an oromucosal spray that is derived from the cannabis sativa plant and approved for use in multiple countries but not yet in the US.
According to Breward, some of the other treatments included dried cannabis that is smoked or eaten and cannabidiol that’s typically delivered with tetrahydrocannabinol (THC) either oromucosally or as an oral capsule.
MS symptoms treated in the studies included spasticity (n = 29), pain (n = 8), and cognition (n = 6). The researchers considered 22 studies to be poor quality, 14 to be fair quality, and 24 to be good/excellent quality.
The researchers found that the cannabis-based medicine “significantly reduced spasticity and pain in several individual good-quality studies,” Breward said. The drugs seem to work by inhibiting neurotransmitter release via cannabinoids. “However, the variability in study quality—and in the products and regimens studied—make it hard to draw any conclusions about specific products and doses that may have the most potential benefit,” she added.
“Further research should focus on the use of different products and formulations of cannabis-based medicine such as cannabis oil and cannabidiol-prominent products, as no studies have focused on this area,” she said. “Research should also look at the potential of cannabis-based medicine for the treatment of disease progression, as cannabinoids are anti-inflammatory and immunomodulatory. Finally, more research regarding the potentially synergistic effects of cannabis-based medicine administered with current MS medications would also be useful.”
Randy Dotinga, MDedge.com/neurology
Brain Volumes After TBI Correlate With Clinical Features
Brain volumes of specific regions of interest can be used to classify traumatic brain injury subjects that fall into predetermined symptom categories, according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computed cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI.
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu), which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations. Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction.
In an abstract, the researchers noted that their study is ongoing. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc; BrainBox Solutions, Inc; and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc and has received research support from BrainBox Solutions. The other authors reported no other disclosures.
Glenn S. Williams, MDedge.com/neurology
What Other Drugs Do Patients Take When They Start MS Therapy?
Concomitant medication use is common when patients with multiple sclerosis (MS) start disease-modifying drugs (DMDs), according to research presented at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. The likelihood of particular comorbidities and concomitant medications varies by age and sex, researchers reported.
“This may have implications for MS treatment,” said study author Jacqueline Nicholas, MD, MPH, of Ohio Multiple Sclerosis Center in Columbus and colleagues. “A better understanding of the effects of comorbidities and concomitant medications on the effectiveness and safety of DMDs is needed to support clinical decision making.”
Researchers have examined comorbidities in patients with MS, but concomitant medication use among patients starting DMDs is poorly understood, the authors said.
To study this question, Dr. Nicholas and colleagues analyzed retrospective administrative claims data from IQVIA Real-World Data Adjudicated Claims–US database from Jan. 1, 2010, to June 30, 2017. Their analysis included patients with ≥ 2 MS diagnosis claims and at least 1 DMD claim between Jan. 1, 2011, and June 30, 2015. Eligible patients were aged 18 to 63 years and had continuous eligibility with commercial insurance 1 year before and 2 years after DMD initiation. In addition, patients had no evidence of DMD use during the 1-year baseline period.
The investigators used International Classification of Diseases, 9th and 10th revisions, Clinical Modification codes and claims to evaluate patients’ comorbidities and concomitant medications during the study period.
The researchers identified 8,251 eligible patients. Patients had a mean age of 43.2 years, and 75.5% were female. Average baseline Charlson Comorbidity Index was 0.41. In the 2 years after DMD initiation, common comorbid diagnoses were hyperlipidemia (30.0%), hypertension (28.2%), gastrointestinal disorders (26.2%), depression (25.5%), and anxiety (20.1%).
Common concomitant medications included antibiotics (70.6%); analgesics (57.0%); corticosteroids (52.0%); antidepressants (47.7%); anticonvulsants (46.7%); anxiolytics, sedatives, or hypnotics (43.2%); spasticity medications (36.2%); and muscle relaxants (35.4%).
Most comorbidities and many medications, including bladder and antifatigue medications, were more common among patients aged ≥ 55 years. Hyperlipidemia, hypertension, and diabetes mellitus were more likely in males than in females. Females were more likely to have gastrointestinal disease, depression, thyroid disease, anxiety, lung disease, and arthritis. In addition, females were more likely than males to use many of the concomitant medications.
Dr. Nicholas disclosed grant support from EMD Serono. A coauthor is an employee of Health Services Consulting Corporation and received funding from EMD Serono to conduct the study. Other coauthors are employees of EMD Serono.
Jake Rem
Depression, Fatigue, Pain, and Anxiety Are Common in the Year After MS Diagnosis
In the 12 months after diagnosis, pain, fatigue, depression, and anxiety are common among patients with multiple sclerosis (MS), researchers reported at the 2019 meeting of the Consortium of Multiple Sclerosis Centers. In a novel study, about half of patients with MS reported clinically significant symptoms of depression or pain, and about 60% reported fatigue during that time.
Pain, fatigue, depression, and anxiety are common in MS, but their prevalence in the first year after diagnosis is not well understood. To examine the rates of these conditions and how often they co-occur during that period, Anna L. Kratz, PhD, associate professor of physical medicine and rehabilitation at the University of Michigan in Ann Arbor, and her research colleagues had 231 adults with MS complete validated surveys at 1, 2, 3, 6, 9, and 12 months after diagnosis to assess symptoms of these conditions.
Overall, 47.2% of patients reported clinically significant levels of depression, 38.5% reported clinically significant levels of anxiety, 50.4% reported clinically significant pain, and 62.2% reported clinically significant fatigue at any point during the year after diagnosis. “Of those who did not have clinically significant symptoms at time of diagnosis, 21.3% went on to develop clinically significant depression, 17.0% anxiety, 30.9% pain, and 34.1% fatigue,” the authors reported.
About 23% of patients did not have clinically significant symptoms for any condition, while 20% had clinically significant symptoms for 1 condition, 21% for 2, 19% for 3, and 17% for all 4. Depression and fatigue had the highest rate of comorbidity, whereas pain and anxiety had the lowest rate of comorbidity.
“Important clinical symptoms associated with MS are present at high levels in the first year post diagnosis,” Dr. Kratz and colleagues concluded. “While the rates and severity are marginally lower than have been identified in studies of individuals farther into the MS disease course, this study is a reminder that early MS intervention should incorporate interventions for these symptoms that are known to have strong associations with quality of life.”
The researchers had no disclosures.
Jake Remaly, MDedge.com/neurology
Experts Propose New Definition and Recommendations for Alzheimer-like Disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain. “As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer, are seen in > 20% of individuals aged > 80 years, according to large, community-based autopsy series. It coexists with Alzheimer disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and colleagues noted.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer disease neuropathologic changes.
The authors proposed a 3-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus. The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E ∑ 4 allele, known to be a risk factor for Alzheimer disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report. LATE also could obscure the effects of potentially disease-modifying agents being tested in Alzheimer disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote. Dr. Nelson and coauthors reported no author disclosures.
Source: Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527.
Andrew D. Bowser, MDedge.com/neurology
Proton Pump Inhibitor Use and Risk of Dementia in the Veteran Population (FULL)
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
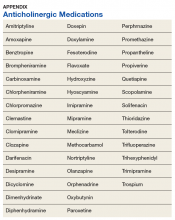
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
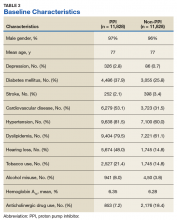
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
Proton pump inhibitors (PPIs) have become the mainstay of therapy in the treatment of acid-related disorders since their introduction in 1989. Due to their high potency, excellent tolerability, and generic availability, PPIs have largely replaced histamine-2 receptor antagonists for gastric problems. Since they were first released on the market, the use of PPIs has continued to rise in both the hospital and primary care settings.1 However, this rapid growth has led to the concern of overutilization. A study conducted at the Department of Veterans Affairs (VA) Ann Arbor Health Care System found that out of 946 patients in the ambulatory care setting taking PPIs, only 35% were appropriately prescribed PPIs.2
Although the short-term adverse effects of PPI use seem minimal, chronic PPI use consequences are a growing concern. Chronic PPI use is associated with increased risks of osteoporosis, pneumonia, and Clostridium difficile infections.3 Another long-term risk that has been associated with chronic PPI use is dementia. Dementia is a cognitive syndrome that is characterized by a progressive decline beyond what is expected in normal aging in 1 or more of the cognitive domains of memory, language, orientation, learning capacity, executive function, or social cognition.4 Because it interferes with activities of daily living, dementia is a major cause of disability in the elderly and is an immense burden for caregivers. Currently, about 47 million people globally live with dementia.5 This number is projected to nearly triple by 2050 to 132 million.5 With no cure, identification of risk factors and creation of protective measures are critical in decreasing the prevalence of dementia.
Although the exact pathophysiology behind the link between PPIs and dementia is unknown, several theories exist. One such theory is that PPI-induced vitamin B12 deficiency leads to cognitive decline.6,7 Another theory suggests that PPIs can directly cause dementia by inhibiting enzymes that normally degrade β amyloid.8 This leads to increased levels of β-amyloid plaques, which is a known characteristic of dementia patients. This theory is derived from animal studies that have shown increased amyloid levels in the brains of mice given PPIs.8
Current studies are conflicting regarding the association between PPIs and dementia. Two German prospective, cohort studies found statistically significant increased risks of dementia in patients taking PPIs with hazard ratios (HR) of 1.38 (95% CI, 1.04-1.83) and 1.44 (95% CI, 1.36-1.52), respectively.9,10 A study conducted in Taiwan also found an increased risk of dementia among PPI users with a HR of 1.22 (95% CI, 1.05-1.42).11 On the contrary, other studies have failed to show an increased risk of dementia with PPI use. In fact, Goldstein and colleagues found a decreased risk of dementia in PPI users with a HR of 0.78 (95% CI, 0.76-0.93).12 This study was an observational study conducted in the US using data from the National Alzheimer’s Coordinating Center database.12 Another recent retrospective study conducted in Finland showed that PPI use was not associated with a significantly increased risk of Alzheimer disease.13
Much is unknown about the cause of dementia, and no curative treatment exists. Investigation into potential risk factors for dementia can lead to the development of preventative measures, which can lead to significant improvement in quality of life for both patients and caregivers. Current studies regarding the association between PPIs and dementia are conflicting, and to our knowledge, no study analyzing the effects of PPIs and dementia has been conducted within the veteran population specifically. The objective of the current study is to investigate the association between PPI use and dementia in the veteran population.
Methods
This study is a retrospective, cohort, single-center, chart review study conducted at the Sioux Falls Veteran Affairs Health Care System (SFVAHCS). Data were extracted from the VA electronic health record (EHR) from January 1, 2005 through December 31, 2015. The study included both currently living and deceased veterans who received ≥ 2 documented outpatient visits at the SFVAHCS during the study time frame. Patients also had to be aged ≥ 60 years at the start of the study period. Patients were excluded if they received only a ≤ 30-day PPI prescription. Patients with dementia related to head trauma, acute intoxication, or other known diseases were excluded.
To analyze the primary endpoint of association between PPI use and dementia, the study compared the rate of dementia in a cohort of veterans who had received an outpatient prescription for a PPI within the study time frame vs the rate of dementia in a random, equal number of veterans who had never been prescribed PPIs within the study time frame. In this study, veterans were classified as having dementia if they had a diagnosis of dementia based on ICD-9 or ICD-10 codes (Table 1), or if they had been prescribed medications used to treat dementia (donepezil, ergoloid mesylates, galantamine, memantine, and rivastigmine).
Secondary endpoints included analysis of the effects of PPI agent, PPI dose, and PPI duration on the risk of dementia. For the PPI dose analysis, cumulative doses were converted into defined daily doses (DDDs) using the World Health Organization calculation to equalize the different potencies of PPI agents (Table 2).14 In addition, the effect of PPI use on vitamin B12 levels was analyzed as an exploratory endpoint to investigate the hypothesis that PPI may be associated with vitamin B12 deficiency, which in turn may be associated with dementia.6,7
Baseline characteristics were collected to determine the variability between the treatment and control group. Data collected included age, gender, past medical history of diseases that may increase risk of dementia, and anticholinergic drug use. Anticholinergic drugs were included if they were classified as having “definite anticholinergic effects” based on the Aging Brain Care Anticholinergic Burden Scale (Appendix).15
Statistical Analysis
The primary endpoint was analyzed using a χ2 for association test. For the secondary endpoints, a χ2 for association test was used for endpoints with nominal data, and the Mood median test was used for endpoints with continuous data. The exploratory endpoint analyzing vitamin B12 levels was analyzed with the Mood median test. A P value of < .05 was defined as being statistically significant. Power analysis was not performed since all veterans who met the criteria were included in the study.
Results
Records of 23,656 veterans were included in the study with 11,828 veterans in both the PPI cohort and the non-PPI cohort (Table 3).
Primary Endpoint
Within the PPI group, 1,119 (9.5%) veterans had dementia compared with only 740 (6.3%) veterans in the non-PPI group. There was a statistically significant association between PPI use and dementia (P < .001). These results yielded an odds ratio of 1.55 for dementia risk in PPI users vs nonusers and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use.
Secondary Endpoints
Users of rabeprazole had the highest rate of dementia (12.8%), followed by lansoprazole (10.9%), omeprazole (9.7%), esomeprazole (7.7%), and pantoprazole (7.0%). The rate of dementia for non-PPI users was 6.3% (P < .001). The median cumulative doses of PPIs were not significant: 597 DDDs (95% CI, 540-630) in the dementia group vs 570 DDDs (95% CI, 540-624) in the nondementia group (P = .79). The median cumulative duration of PPI use in the dementia group was 4.6 years (95% CI, 4.25-4.92) vs 5.3 years (95% CI, 5.08-5.42) in the nondementia group (P < .001).
Exploratory Endpoint
The median B12 level in the PPI group was 521 pg/mL (95% CI, 509-533) compared with 480 pg/mL (95% CI, 465-496) in the non-PPI group (P < .001). However, both groups fell within the normal range for vitamin B12 (200-900 pg/mL).16
Discussion
The aim of this study was to determine whether an association existed between PPI use and dementia. This study showed a statistically significant association between PPI use and dementia within the veteran population. This study also showed a significant association between specific PPI agents and dementia. When analyzing the individual PPI agents, the rabeprazole group yielded the strongest relationship. However, this study was not powered to evaluate and compare risks of dementia between individual PPI agents. More data are needed to determine statistical and clinical significance of associations between individual PPI agents and risk of dementia.
The veterans with dementia had a higher median cumulative PPI dose than did the veterans without dementia; however, the results were not statistically significant. Therefore, the data cannot correlate higher doses of PPI use to increased risk of dementia.
The cumulative duration of PPI use was statistically significant but opposite of the expected outcome. The dementia group had a lower median lifetime duration of PPI use compared with that of the nondementia group. It is difficult to determine the reason for this outcome, but it seems that for this study population, a longer duration of PPI use was not associated with an increased risk of dementia.
Finally, the exploratory endpoint analyzed vitamin B12 levels, since it has been shown that PPI use can lead to vitamin B12 deficiency and that B12 deficiency can lead to dementia.6-8 This study found that the dementia group had significantly higher vitamin B12 levels than the nondementia group. These data suggest that PPI use may not be associated with vitamin B12 deficiency. However, it is important to note that this study was unable to collect data on the use of vitamin B12 supplementation due to the unreliability of over-the-counter (OTC) and non-VA medication use records. Therefore, it is possible that the PPI group had higher rates of B12 deficiency but were effectively treated with B12 supplementation. More research is needed to determine the exact relationship between PPI use, vitamin B12 deficiency, and dementia risk.
Strengths/Limitations
Strengths of this study that support its findings include the large population size. Additionally, the use of the VA EHR allowed for a complete drug dispensing history to be collected, which improves reliability of the data.
This study also had some limitations. First, the causal relationship of PPI use and dementia cannot be proven using a retrospective cohort design. This study’s design can show association, but it cannot prove causation. Also, due to the retrospective design, exposure to PPI use could not be randomized; thus, correlation between PPI use and dementia may be explained by confounding variables that are not captured within this study. This is especially true since the baseline characteristics were not equally distributed between the 2 groups. In fact, the PPI group had higher rates of many clinical comorbidities. This imbalance may have skewed the results of the primary endpoint. Lastly, OTC PPI use and non-VA PPI prescriptions were not available. Therefore, some of the patients included in the non-PPI group may have been PPI users if they received PPIs from OTC or non-VA sources, which could skew the results.
Conclusion
This study showed a significant association between PPI use and dementia within the veteran study population. The study also showed a significant association between PPI use and dementia within the secondary endpoint of individual PPI agent. Higher cumulative dose and duration of PPI use did not seem to increase risk of dementia. Finally, PPI use was not associated with significantly low vitamin B12 levels. More studies are needed to determine causation of dementia and its risk factors.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
1. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19-24.
2. Heidelbaugh J, Goldberg K, Inadomi J. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234.
3. Heidelbaugh JJ, Kim AH, Chang R. Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). American Psychiatric Association: Washington, DC; 2013.
5. World Health Organization. Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Published December 12, 2017. Accessed March 10, 2019.
6. Vogiatzoglou A, Smith AD, Nurk E, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29.
7. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442.
8. Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837.
9. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
10. Gomm W, von Holt K, Thomé F, et al. Association between proton pump inhibitors with risk of dementia. A pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
11. Tai SY, Chien CY, Wu DC, et al. Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006.
12. Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1674.
13. Taipale H, Tolppanen AM, Tiihonen M. Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112(12):1801-1808.
14. World Health Organization Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Updated February 7, 2018. Accessed March 13, 2019.
15. Indiana University Center for Aging Research, Aging Brain Program. Anticholinergic cognitive burden scale. http://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf. Updated 2012. Accessed March 10, 2019.
16. US National Library of Medicine, MedlinePlus. Vitamin B12 level. https://medlineplus.gov/ency/article/003705.htm. Updated March 7, 2019. Accessed March 13, 2019.
Understanding Psychosis in a Veteran With a History of Combat and Multiple Sclerosis (FULL)
A patient with significant combat history and previous diagnoses of multiple sclerosis and unspecified schizophrenia spectrum and other psychotic disorder was admitted with acute psychosis inconsistent with expected clinical presentations.
Multiple sclerosis (MS) is an immune-mediated neurodegenerative disease that affects > 700,000 people in the US.1 The hallmarks of MS pathology are axonal or neuronal loss, demyelination, and astrocytic gliosis. Of these, axonal or neuronal loss is the main underlying mechanism of permanent clinical disability.
MS also has been associated with an increased prevalence of psychiatric illnesses, with mood disorders affecting up to 40% to 60% of the population, and psychosis being reported in 2% to 4% of patients.2 The link between MS and mood disorders, including bipolar disorder and depression, was documented as early as 1926,with mood disorders hypothesized to be manifestations of central nervous system (CNS) inflammation.3 More recently, inflammation-driven microglia have been hypothesized to impair hippocampal connectivity and activate glucocorticoid-insensitive inflammatory cells that then overstimulate the hypothalamic-pituitary-adrenal axis.4,5
Although the prevalence of psychosis in patients with MS is significantly rarer, averaging between 2% and 4%.6 A Canadian study by Patten and colleagues reviewed data from 2.45 million residents of Alberta and found that those who identified as having MS had a 2% to 3% prevalence of psychosis compared with 0.5% to 1% in the general population.7 The connection between psychosis and MS, similar to that between mood disorders and MS, has been described as a common regional demyelination process. Supporting this, MS manifesting as psychosis has been found to present with distinct magnetic resonance imaging (MRI) findings, such as diffuse periventricular lesions.8 Still, no conclusive criteria have been developed to distinguish MS presenting as psychosis from a primary psychiatric illness, such as schizophrenia.
In patients with combat history, it is possible that both neurodegenerative and psychotic symptoms can be explained by autoantibody formation in response to toxin exposure. When soldiers were deployed to Iraq and Afghanistan, they may have been exposed to multiple toxicities, including depleted uranium, dust and fumes, and numerous infectious diseases.9 Gulf War illness (GWI) or chronic multisymptom illness (CMI) encompass a cluster of symptoms, such as chronic pain, chronic fatigue, irritable bowel syndrome, dermatitis, and seizures, as well as mental health issues such as depression and anxiety experienced following exposure to these combat environments.10,11
In light of this diagnostic uncertainty, the authors detail a case of a patient with significant combat history previously diagnosed with MS and unspecified schizophrenia spectrum and other psychotic disorder (USS & OPD) presenting with acute psychosis.
Case Presentation
A 35-year-old male veteran, with a history of MS, USS & OPD, posttraumatic stress disorder, and traumatic brain injuries (TBIs) was admitted to the psychiatric unit after being found by the police lying in the middle of a busy intersection, internally preoccupied. On admission, he reported a week of auditory hallucinations from birds with whom he had been communicating telepathically, and a recurrent visual hallucination of a tall man in white and purple robes. He had discontinued his antipsychotic medication, aripiprazole 10 mg, a few weeks prior for unknown reasons. He was brought to the hospital by ambulance, where he presented with disorganized thinking, tangential thought process, and active auditory and visual hallucinations. The differential diagnoses included USS & OPD, schizophrenia, schizoaffective disorder and ruled out substance-induced psychotic disorder, and psychosis as a manifestation of MS.
The patient had 2 psychotic episodes prior to this presentation. He was hospitalized for his first psychotic break in 2015 at age 32, when he had tailed another car “to come back to reality” and ended up in a motor vehicle accident. During that admission, he reported weeks of thought broadcasting, conspiratorial delusions, and racing thoughts. Two years later, he was admitted to a psychiatric intensive care unit for his second episode of severe psychosis. After several trials of different antipsychotic medications, his most recent pharmacologic regimen was aripiprazole 10 mg once daily.
His medical history was complicated by 2 TBIs, in November 2014 and January 2015, with normal computed tomography (CT) scans. He was diagnosed with MS in December 2017, when he presented with intractable emesis, left facial numbness, right upper extremity ataxia, nystagmus, and imbalance. An MRI scan revealed multifocal bilateral hypodensities in his periventricular, subcortical, and brain stem white matter. Multiple areas of hyperintensity were visualized, including in the right periatrial region and left brachium pontis. More than 5 oligoclonal bands on lumbar puncture confirmed the diagnosis.
He was treated with IV methylprednisolone followed by a 2-week prednisone taper. Within 1 week, he returned to the psychiatric unit with worsening symptoms and received a second dose of IV steroids and plasma exchange treatment. In the following months, he completed a course of rituximab infusions and physical therapy for his dysarthria, gait abnormality, and vision impairment.
His social history was notable for multiple first-degree relatives with schizophrenia. He reported a history of sexual and verbal abuse and attempted suicide once at age 13 years by hanging himself with a bathrobe. He left home at age 18 years to serve in the Marine Corps (2001-2006). His service included deployment to Afghanistan, where he received a purple heart. Upon his return, he received BA and MS degrees. He married and had 2 daughters but became estranged from his wife. By his most recent admission, he was unemployed and living with his half-sister.
On the first day of this most recent psychiatric hospitalization, he was restarted on aripiprazole 10 mg daily, and a medicine consult was sought to evaluate the progression of his MS. No new onset neurologic symptoms were noted, but he had possible residual lower extremity hyperreflexia and tandem gait incoordination. The episodes of psychotic and neurologic symptoms appeared independent, given that his psychiatric history preceded the onset of his MS.
The patient reported no visual hallucinations starting day 2, and he no longer endorsed auditory hallucinations by day 3. However, he continued to appear internally preoccupied and was noticed to be pacing around the unit. On day 4 he presented with newly pressured speech and flights of ideas, while his affect remained euthymic and his sleep stayed consistent. In combination with his ongoing pacing, his newfound symptoms were hypothesized to be possibly akathisia, an adverse effect (AE) of aripiprazole. As such, on day 5 his dose was lowered to 5 mg daily. He continued to report no hallucinations and demonstrated progressively increased emotional range. A MRI scan was done on day 6 in case a new lesion could be identified, suggesting a primary MS flare-up; however, the scan identified no enhancing lesions, indicating no ongoing demyelination. After a neurology consult corroborated this conclusion, he was discharged in stable condition on day 7.
As is the case with the majority of patients with MS-induced psychosis, he continued to have relapsing psychiatric disease even after MS treatment had been started. Unfortunately, because this patient had stopped taking his atypical antipsychotic medication several weeks prior to his hospitalization, we cannot clarify whether his psychosis stems from a primary psychiatric vs MS process.
Discussion
Presently, treatment preferences for MS-related psychosis are divided between atypical antipsychotics and glucocorticoids. Some suggest that the treatment remains similar between MS-related psychosis and primary psychotic disorders in that atypical antipsychotics are the standard of care.12 A variety of atypical antipsychotics have been used successfully in case reports, including zipradisone, risperidone, olanzapine, quetiapine, and aripiprazole.13,14 First-generation antipsychotics and other psychotropic drugs that can precipitate extra-pyramidal AEs are not recommended given their potential additive effect to motor deficits associated with MS.12 Alternatively, several case reports have found that MS-related psychotic symptoms respond to glucocorticoids more effectively, while cautioning that glucocorticoids can precipitate psychosis and depression.15,16 One review article found that 90% of patients who received corticosteroids saw an improvement in their psychotic symptoms.2
Finally, it is possible that our patient’s neuropsychiatric symptoms can be explained by autoantibody formation in response to toxin exposure during his time in Afghanistan. In a pilot study of veterans with GWI, Abou-Donia and colleagues found 2-to-9 fold increase in autoantibody reactivity levels of the following neuronal and glial-specific proteins relative to healthy controls: neurofilament triplet proteins, tubulin, microtubule-associated tau proteins, microtubule-associated protein-2, myelin basic protein, myelin-associated glycoprotein, glial fibrillary acidic protein, and calcium-calmodulin kinase II.17,18 Many of these autoantibodies are longstanding explicit markers for neurodegenerative disorders, given that they target proteins and antigens that support axonal transport and myelination. Still Gulf War veteran status has yet to be explicitly linked to an increased risk of MS,19 making this hypothesis less likely for our patient. Future research should address the clinical and therapeutic implications of different autoantibody levels in combat veterans with psychosis.
Conclusion
For patients with MS, mood disorder and psychotic symptoms should warrant a MRI given the possibility of a psychiatric manifestation of MS relapse. Ultimately, our patient’s presentation was inconsistent with the expected clinical presentations of both a primary psychotic disorder and psychosis as a manifestation of MS. His late age at his first psychotic break is atypical for primary psychotic disease, and the lack of MRI imaging done at his initial psychotic episodes cannot exclude a primary MS diagnosis. Still, his lack of MRI findings at his most recent hospitalization, negative symptomatology, and strong history of schizophrenia make a primary psychotic disorder likely.
Following his future clinical course will be necessary to determine the etiology of his psychotic episodes. Future episodes of psychosis with neurologic symptoms would suggest a primary MS diagnosis and potential benefit of immunosuppressant treatment, whereas repeated psychotic breaks with minimal temporal lobe involvement or demyelination as seen on MRI would be suspicious for separate MS and psychotic disease processes. Further research on treatment regimens for patients experiencing psychosis as a manifestation of MS is still necessary.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Camara-Lemarroy CR, Ibarra-Yruegas BE, Rodriguez-Gutierrez R, Berrios-Morales I, Ionete C, Riskind P. The varieties of psychosis in multiple sclerosis: a systematic review of cases. Mult Scler Relat Disord. 2017;12:9-14.
3. Cottrel SS, Wilson SA. The affective symptomatology of disseminated sclerosis: a study of 100 cases. J Neurol Psychopathology. 1926;7(25):1-30.
4. Johansson V, Lundholm C, Hillert J, et al. Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler. 2014;20(14):1881-1891.
5. Rossi S, Studer V, Motta C, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. 2017;89(13):1338-1347.
6. Gilberthorpe TG, O’Connell KE, Carolan A, et al. The spectrum of psychosis in multiple sclerosis: a clinical case series. Neuropsychiatric disease and treatment. 2017;13:303.
7. Patten SB, Svenson LW, Metz LM. Psychotic disorders in MS: population-based evidence of an association. Neurology 2005;65(7):1123-1125.
8. Kosmidis MH, Giannakou M, Messinis L, Papathanasopoulos P. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010; 22(1):55-66.
9. US Department of Veterans Affairs. Public health: military exposures. https://www.publichealth.va.gov/exposures/. Updated April 16, 2019. Accessed May 13, 2019.
10. DeBeer BB, Davidson D, Meyer EC, Kimbrel NA, Gulliver SB, Morissette SB. The association between toxic exposures and chronic multisymptom illness in veterans of the wars of Iraq and Afghanistan. J Occup Environ Med. 2017;59(1):54-60.
11. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410.
12. Murphy R, O’Donoghue S, Counihan T, et al. Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(8):697-708.
13. Davids E, Hartwig U, Gastpar, M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuro Psychopharmacol Biol Psychiatry. 2004;28(4):743-744.
14. Lo Fermo S, Barone R, Patti F, et al. Outcome of psychiatric symptoms presenting at onset of multiple sclerosis: a retrospective study. Mult Scler. 2010;16(6):742-748.
15. Enderami A, Fouladi R, Hosseini HS. First-episode psychosis as the initial presentation of multiple sclerosis: a case report. Int Medical Case Rep J. 2018;11:73-76.
16. Fragoso YD, Frota ER, Lopes JS, et al. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33(6):312-316.
17. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol Teratol. 2017;61:36-46.
18. Abou-Donia MB, Lieberman A, Curtis L. Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures. Toxicol Ind Health. 2018;34(1):44-53.
19. Wallin MT, Kurtzke JF, Culpepper WJ, et al. Multiple sclerosis in Gulf War era veterans. 2. Military deployment and risk of multiple sclerosis in the first Gulf War. Neuroepidemiology. 2014;42(4):226-234.
A patient with significant combat history and previous diagnoses of multiple sclerosis and unspecified schizophrenia spectrum and other psychotic disorder was admitted with acute psychosis inconsistent with expected clinical presentations.
A patient with significant combat history and previous diagnoses of multiple sclerosis and unspecified schizophrenia spectrum and other psychotic disorder was admitted with acute psychosis inconsistent with expected clinical presentations.
Multiple sclerosis (MS) is an immune-mediated neurodegenerative disease that affects > 700,000 people in the US.1 The hallmarks of MS pathology are axonal or neuronal loss, demyelination, and astrocytic gliosis. Of these, axonal or neuronal loss is the main underlying mechanism of permanent clinical disability.
MS also has been associated with an increased prevalence of psychiatric illnesses, with mood disorders affecting up to 40% to 60% of the population, and psychosis being reported in 2% to 4% of patients.2 The link between MS and mood disorders, including bipolar disorder and depression, was documented as early as 1926,with mood disorders hypothesized to be manifestations of central nervous system (CNS) inflammation.3 More recently, inflammation-driven microglia have been hypothesized to impair hippocampal connectivity and activate glucocorticoid-insensitive inflammatory cells that then overstimulate the hypothalamic-pituitary-adrenal axis.4,5
Although the prevalence of psychosis in patients with MS is significantly rarer, averaging between 2% and 4%.6 A Canadian study by Patten and colleagues reviewed data from 2.45 million residents of Alberta and found that those who identified as having MS had a 2% to 3% prevalence of psychosis compared with 0.5% to 1% in the general population.7 The connection between psychosis and MS, similar to that between mood disorders and MS, has been described as a common regional demyelination process. Supporting this, MS manifesting as psychosis has been found to present with distinct magnetic resonance imaging (MRI) findings, such as diffuse periventricular lesions.8 Still, no conclusive criteria have been developed to distinguish MS presenting as psychosis from a primary psychiatric illness, such as schizophrenia.
In patients with combat history, it is possible that both neurodegenerative and psychotic symptoms can be explained by autoantibody formation in response to toxin exposure. When soldiers were deployed to Iraq and Afghanistan, they may have been exposed to multiple toxicities, including depleted uranium, dust and fumes, and numerous infectious diseases.9 Gulf War illness (GWI) or chronic multisymptom illness (CMI) encompass a cluster of symptoms, such as chronic pain, chronic fatigue, irritable bowel syndrome, dermatitis, and seizures, as well as mental health issues such as depression and anxiety experienced following exposure to these combat environments.10,11
In light of this diagnostic uncertainty, the authors detail a case of a patient with significant combat history previously diagnosed with MS and unspecified schizophrenia spectrum and other psychotic disorder (USS & OPD) presenting with acute psychosis.
Case Presentation
A 35-year-old male veteran, with a history of MS, USS & OPD, posttraumatic stress disorder, and traumatic brain injuries (TBIs) was admitted to the psychiatric unit after being found by the police lying in the middle of a busy intersection, internally preoccupied. On admission, he reported a week of auditory hallucinations from birds with whom he had been communicating telepathically, and a recurrent visual hallucination of a tall man in white and purple robes. He had discontinued his antipsychotic medication, aripiprazole 10 mg, a few weeks prior for unknown reasons. He was brought to the hospital by ambulance, where he presented with disorganized thinking, tangential thought process, and active auditory and visual hallucinations. The differential diagnoses included USS & OPD, schizophrenia, schizoaffective disorder and ruled out substance-induced psychotic disorder, and psychosis as a manifestation of MS.
The patient had 2 psychotic episodes prior to this presentation. He was hospitalized for his first psychotic break in 2015 at age 32, when he had tailed another car “to come back to reality” and ended up in a motor vehicle accident. During that admission, he reported weeks of thought broadcasting, conspiratorial delusions, and racing thoughts. Two years later, he was admitted to a psychiatric intensive care unit for his second episode of severe psychosis. After several trials of different antipsychotic medications, his most recent pharmacologic regimen was aripiprazole 10 mg once daily.
His medical history was complicated by 2 TBIs, in November 2014 and January 2015, with normal computed tomography (CT) scans. He was diagnosed with MS in December 2017, when he presented with intractable emesis, left facial numbness, right upper extremity ataxia, nystagmus, and imbalance. An MRI scan revealed multifocal bilateral hypodensities in his periventricular, subcortical, and brain stem white matter. Multiple areas of hyperintensity were visualized, including in the right periatrial region and left brachium pontis. More than 5 oligoclonal bands on lumbar puncture confirmed the diagnosis.
He was treated with IV methylprednisolone followed by a 2-week prednisone taper. Within 1 week, he returned to the psychiatric unit with worsening symptoms and received a second dose of IV steroids and plasma exchange treatment. In the following months, he completed a course of rituximab infusions and physical therapy for his dysarthria, gait abnormality, and vision impairment.
His social history was notable for multiple first-degree relatives with schizophrenia. He reported a history of sexual and verbal abuse and attempted suicide once at age 13 years by hanging himself with a bathrobe. He left home at age 18 years to serve in the Marine Corps (2001-2006). His service included deployment to Afghanistan, where he received a purple heart. Upon his return, he received BA and MS degrees. He married and had 2 daughters but became estranged from his wife. By his most recent admission, he was unemployed and living with his half-sister.
On the first day of this most recent psychiatric hospitalization, he was restarted on aripiprazole 10 mg daily, and a medicine consult was sought to evaluate the progression of his MS. No new onset neurologic symptoms were noted, but he had possible residual lower extremity hyperreflexia and tandem gait incoordination. The episodes of psychotic and neurologic symptoms appeared independent, given that his psychiatric history preceded the onset of his MS.
The patient reported no visual hallucinations starting day 2, and he no longer endorsed auditory hallucinations by day 3. However, he continued to appear internally preoccupied and was noticed to be pacing around the unit. On day 4 he presented with newly pressured speech and flights of ideas, while his affect remained euthymic and his sleep stayed consistent. In combination with his ongoing pacing, his newfound symptoms were hypothesized to be possibly akathisia, an adverse effect (AE) of aripiprazole. As such, on day 5 his dose was lowered to 5 mg daily. He continued to report no hallucinations and demonstrated progressively increased emotional range. A MRI scan was done on day 6 in case a new lesion could be identified, suggesting a primary MS flare-up; however, the scan identified no enhancing lesions, indicating no ongoing demyelination. After a neurology consult corroborated this conclusion, he was discharged in stable condition on day 7.
As is the case with the majority of patients with MS-induced psychosis, he continued to have relapsing psychiatric disease even after MS treatment had been started. Unfortunately, because this patient had stopped taking his atypical antipsychotic medication several weeks prior to his hospitalization, we cannot clarify whether his psychosis stems from a primary psychiatric vs MS process.
Discussion
Presently, treatment preferences for MS-related psychosis are divided between atypical antipsychotics and glucocorticoids. Some suggest that the treatment remains similar between MS-related psychosis and primary psychotic disorders in that atypical antipsychotics are the standard of care.12 A variety of atypical antipsychotics have been used successfully in case reports, including zipradisone, risperidone, olanzapine, quetiapine, and aripiprazole.13,14 First-generation antipsychotics and other psychotropic drugs that can precipitate extra-pyramidal AEs are not recommended given their potential additive effect to motor deficits associated with MS.12 Alternatively, several case reports have found that MS-related psychotic symptoms respond to glucocorticoids more effectively, while cautioning that glucocorticoids can precipitate psychosis and depression.15,16 One review article found that 90% of patients who received corticosteroids saw an improvement in their psychotic symptoms.2
Finally, it is possible that our patient’s neuropsychiatric symptoms can be explained by autoantibody formation in response to toxin exposure during his time in Afghanistan. In a pilot study of veterans with GWI, Abou-Donia and colleagues found 2-to-9 fold increase in autoantibody reactivity levels of the following neuronal and glial-specific proteins relative to healthy controls: neurofilament triplet proteins, tubulin, microtubule-associated tau proteins, microtubule-associated protein-2, myelin basic protein, myelin-associated glycoprotein, glial fibrillary acidic protein, and calcium-calmodulin kinase II.17,18 Many of these autoantibodies are longstanding explicit markers for neurodegenerative disorders, given that they target proteins and antigens that support axonal transport and myelination. Still Gulf War veteran status has yet to be explicitly linked to an increased risk of MS,19 making this hypothesis less likely for our patient. Future research should address the clinical and therapeutic implications of different autoantibody levels in combat veterans with psychosis.
Conclusion
For patients with MS, mood disorder and psychotic symptoms should warrant a MRI given the possibility of a psychiatric manifestation of MS relapse. Ultimately, our patient’s presentation was inconsistent with the expected clinical presentations of both a primary psychotic disorder and psychosis as a manifestation of MS. His late age at his first psychotic break is atypical for primary psychotic disease, and the lack of MRI imaging done at his initial psychotic episodes cannot exclude a primary MS diagnosis. Still, his lack of MRI findings at his most recent hospitalization, negative symptomatology, and strong history of schizophrenia make a primary psychotic disorder likely.
Following his future clinical course will be necessary to determine the etiology of his psychotic episodes. Future episodes of psychosis with neurologic symptoms would suggest a primary MS diagnosis and potential benefit of immunosuppressant treatment, whereas repeated psychotic breaks with minimal temporal lobe involvement or demyelination as seen on MRI would be suspicious for separate MS and psychotic disease processes. Further research on treatment regimens for patients experiencing psychosis as a manifestation of MS is still necessary.
Multiple sclerosis (MS) is an immune-mediated neurodegenerative disease that affects > 700,000 people in the US.1 The hallmarks of MS pathology are axonal or neuronal loss, demyelination, and astrocytic gliosis. Of these, axonal or neuronal loss is the main underlying mechanism of permanent clinical disability.
MS also has been associated with an increased prevalence of psychiatric illnesses, with mood disorders affecting up to 40% to 60% of the population, and psychosis being reported in 2% to 4% of patients.2 The link between MS and mood disorders, including bipolar disorder and depression, was documented as early as 1926,with mood disorders hypothesized to be manifestations of central nervous system (CNS) inflammation.3 More recently, inflammation-driven microglia have been hypothesized to impair hippocampal connectivity and activate glucocorticoid-insensitive inflammatory cells that then overstimulate the hypothalamic-pituitary-adrenal axis.4,5
Although the prevalence of psychosis in patients with MS is significantly rarer, averaging between 2% and 4%.6 A Canadian study by Patten and colleagues reviewed data from 2.45 million residents of Alberta and found that those who identified as having MS had a 2% to 3% prevalence of psychosis compared with 0.5% to 1% in the general population.7 The connection between psychosis and MS, similar to that between mood disorders and MS, has been described as a common regional demyelination process. Supporting this, MS manifesting as psychosis has been found to present with distinct magnetic resonance imaging (MRI) findings, such as diffuse periventricular lesions.8 Still, no conclusive criteria have been developed to distinguish MS presenting as psychosis from a primary psychiatric illness, such as schizophrenia.
In patients with combat history, it is possible that both neurodegenerative and psychotic symptoms can be explained by autoantibody formation in response to toxin exposure. When soldiers were deployed to Iraq and Afghanistan, they may have been exposed to multiple toxicities, including depleted uranium, dust and fumes, and numerous infectious diseases.9 Gulf War illness (GWI) or chronic multisymptom illness (CMI) encompass a cluster of symptoms, such as chronic pain, chronic fatigue, irritable bowel syndrome, dermatitis, and seizures, as well as mental health issues such as depression and anxiety experienced following exposure to these combat environments.10,11
In light of this diagnostic uncertainty, the authors detail a case of a patient with significant combat history previously diagnosed with MS and unspecified schizophrenia spectrum and other psychotic disorder (USS & OPD) presenting with acute psychosis.
Case Presentation
A 35-year-old male veteran, with a history of MS, USS & OPD, posttraumatic stress disorder, and traumatic brain injuries (TBIs) was admitted to the psychiatric unit after being found by the police lying in the middle of a busy intersection, internally preoccupied. On admission, he reported a week of auditory hallucinations from birds with whom he had been communicating telepathically, and a recurrent visual hallucination of a tall man in white and purple robes. He had discontinued his antipsychotic medication, aripiprazole 10 mg, a few weeks prior for unknown reasons. He was brought to the hospital by ambulance, where he presented with disorganized thinking, tangential thought process, and active auditory and visual hallucinations. The differential diagnoses included USS & OPD, schizophrenia, schizoaffective disorder and ruled out substance-induced psychotic disorder, and psychosis as a manifestation of MS.
The patient had 2 psychotic episodes prior to this presentation. He was hospitalized for his first psychotic break in 2015 at age 32, when he had tailed another car “to come back to reality” and ended up in a motor vehicle accident. During that admission, he reported weeks of thought broadcasting, conspiratorial delusions, and racing thoughts. Two years later, he was admitted to a psychiatric intensive care unit for his second episode of severe psychosis. After several trials of different antipsychotic medications, his most recent pharmacologic regimen was aripiprazole 10 mg once daily.
His medical history was complicated by 2 TBIs, in November 2014 and January 2015, with normal computed tomography (CT) scans. He was diagnosed with MS in December 2017, when he presented with intractable emesis, left facial numbness, right upper extremity ataxia, nystagmus, and imbalance. An MRI scan revealed multifocal bilateral hypodensities in his periventricular, subcortical, and brain stem white matter. Multiple areas of hyperintensity were visualized, including in the right periatrial region and left brachium pontis. More than 5 oligoclonal bands on lumbar puncture confirmed the diagnosis.
He was treated with IV methylprednisolone followed by a 2-week prednisone taper. Within 1 week, he returned to the psychiatric unit with worsening symptoms and received a second dose of IV steroids and plasma exchange treatment. In the following months, he completed a course of rituximab infusions and physical therapy for his dysarthria, gait abnormality, and vision impairment.
His social history was notable for multiple first-degree relatives with schizophrenia. He reported a history of sexual and verbal abuse and attempted suicide once at age 13 years by hanging himself with a bathrobe. He left home at age 18 years to serve in the Marine Corps (2001-2006). His service included deployment to Afghanistan, where he received a purple heart. Upon his return, he received BA and MS degrees. He married and had 2 daughters but became estranged from his wife. By his most recent admission, he was unemployed and living with his half-sister.
On the first day of this most recent psychiatric hospitalization, he was restarted on aripiprazole 10 mg daily, and a medicine consult was sought to evaluate the progression of his MS. No new onset neurologic symptoms were noted, but he had possible residual lower extremity hyperreflexia and tandem gait incoordination. The episodes of psychotic and neurologic symptoms appeared independent, given that his psychiatric history preceded the onset of his MS.
The patient reported no visual hallucinations starting day 2, and he no longer endorsed auditory hallucinations by day 3. However, he continued to appear internally preoccupied and was noticed to be pacing around the unit. On day 4 he presented with newly pressured speech and flights of ideas, while his affect remained euthymic and his sleep stayed consistent. In combination with his ongoing pacing, his newfound symptoms were hypothesized to be possibly akathisia, an adverse effect (AE) of aripiprazole. As such, on day 5 his dose was lowered to 5 mg daily. He continued to report no hallucinations and demonstrated progressively increased emotional range. A MRI scan was done on day 6 in case a new lesion could be identified, suggesting a primary MS flare-up; however, the scan identified no enhancing lesions, indicating no ongoing demyelination. After a neurology consult corroborated this conclusion, he was discharged in stable condition on day 7.
As is the case with the majority of patients with MS-induced psychosis, he continued to have relapsing psychiatric disease even after MS treatment had been started. Unfortunately, because this patient had stopped taking his atypical antipsychotic medication several weeks prior to his hospitalization, we cannot clarify whether his psychosis stems from a primary psychiatric vs MS process.
Discussion
Presently, treatment preferences for MS-related psychosis are divided between atypical antipsychotics and glucocorticoids. Some suggest that the treatment remains similar between MS-related psychosis and primary psychotic disorders in that atypical antipsychotics are the standard of care.12 A variety of atypical antipsychotics have been used successfully in case reports, including zipradisone, risperidone, olanzapine, quetiapine, and aripiprazole.13,14 First-generation antipsychotics and other psychotropic drugs that can precipitate extra-pyramidal AEs are not recommended given their potential additive effect to motor deficits associated with MS.12 Alternatively, several case reports have found that MS-related psychotic symptoms respond to glucocorticoids more effectively, while cautioning that glucocorticoids can precipitate psychosis and depression.15,16 One review article found that 90% of patients who received corticosteroids saw an improvement in their psychotic symptoms.2
Finally, it is possible that our patient’s neuropsychiatric symptoms can be explained by autoantibody formation in response to toxin exposure during his time in Afghanistan. In a pilot study of veterans with GWI, Abou-Donia and colleagues found 2-to-9 fold increase in autoantibody reactivity levels of the following neuronal and glial-specific proteins relative to healthy controls: neurofilament triplet proteins, tubulin, microtubule-associated tau proteins, microtubule-associated protein-2, myelin basic protein, myelin-associated glycoprotein, glial fibrillary acidic protein, and calcium-calmodulin kinase II.17,18 Many of these autoantibodies are longstanding explicit markers for neurodegenerative disorders, given that they target proteins and antigens that support axonal transport and myelination. Still Gulf War veteran status has yet to be explicitly linked to an increased risk of MS,19 making this hypothesis less likely for our patient. Future research should address the clinical and therapeutic implications of different autoantibody levels in combat veterans with psychosis.
Conclusion
For patients with MS, mood disorder and psychotic symptoms should warrant a MRI given the possibility of a psychiatric manifestation of MS relapse. Ultimately, our patient’s presentation was inconsistent with the expected clinical presentations of both a primary psychotic disorder and psychosis as a manifestation of MS. His late age at his first psychotic break is atypical for primary psychotic disease, and the lack of MRI imaging done at his initial psychotic episodes cannot exclude a primary MS diagnosis. Still, his lack of MRI findings at his most recent hospitalization, negative symptomatology, and strong history of schizophrenia make a primary psychotic disorder likely.
Following his future clinical course will be necessary to determine the etiology of his psychotic episodes. Future episodes of psychosis with neurologic symptoms would suggest a primary MS diagnosis and potential benefit of immunosuppressant treatment, whereas repeated psychotic breaks with minimal temporal lobe involvement or demyelination as seen on MRI would be suspicious for separate MS and psychotic disease processes. Further research on treatment regimens for patients experiencing psychosis as a manifestation of MS is still necessary.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Camara-Lemarroy CR, Ibarra-Yruegas BE, Rodriguez-Gutierrez R, Berrios-Morales I, Ionete C, Riskind P. The varieties of psychosis in multiple sclerosis: a systematic review of cases. Mult Scler Relat Disord. 2017;12:9-14.
3. Cottrel SS, Wilson SA. The affective symptomatology of disseminated sclerosis: a study of 100 cases. J Neurol Psychopathology. 1926;7(25):1-30.
4. Johansson V, Lundholm C, Hillert J, et al. Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler. 2014;20(14):1881-1891.
5. Rossi S, Studer V, Motta C, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. 2017;89(13):1338-1347.
6. Gilberthorpe TG, O’Connell KE, Carolan A, et al. The spectrum of psychosis in multiple sclerosis: a clinical case series. Neuropsychiatric disease and treatment. 2017;13:303.
7. Patten SB, Svenson LW, Metz LM. Psychotic disorders in MS: population-based evidence of an association. Neurology 2005;65(7):1123-1125.
8. Kosmidis MH, Giannakou M, Messinis L, Papathanasopoulos P. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010; 22(1):55-66.
9. US Department of Veterans Affairs. Public health: military exposures. https://www.publichealth.va.gov/exposures/. Updated April 16, 2019. Accessed May 13, 2019.
10. DeBeer BB, Davidson D, Meyer EC, Kimbrel NA, Gulliver SB, Morissette SB. The association between toxic exposures and chronic multisymptom illness in veterans of the wars of Iraq and Afghanistan. J Occup Environ Med. 2017;59(1):54-60.
11. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410.
12. Murphy R, O’Donoghue S, Counihan T, et al. Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(8):697-708.
13. Davids E, Hartwig U, Gastpar, M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuro Psychopharmacol Biol Psychiatry. 2004;28(4):743-744.
14. Lo Fermo S, Barone R, Patti F, et al. Outcome of psychiatric symptoms presenting at onset of multiple sclerosis: a retrospective study. Mult Scler. 2010;16(6):742-748.
15. Enderami A, Fouladi R, Hosseini HS. First-episode psychosis as the initial presentation of multiple sclerosis: a case report. Int Medical Case Rep J. 2018;11:73-76.
16. Fragoso YD, Frota ER, Lopes JS, et al. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33(6):312-316.
17. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol Teratol. 2017;61:36-46.
18. Abou-Donia MB, Lieberman A, Curtis L. Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures. Toxicol Ind Health. 2018;34(1):44-53.
19. Wallin MT, Kurtzke JF, Culpepper WJ, et al. Multiple sclerosis in Gulf War era veterans. 2. Military deployment and risk of multiple sclerosis in the first Gulf War. Neuroepidemiology. 2014;42(4):226-234.
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Camara-Lemarroy CR, Ibarra-Yruegas BE, Rodriguez-Gutierrez R, Berrios-Morales I, Ionete C, Riskind P. The varieties of psychosis in multiple sclerosis: a systematic review of cases. Mult Scler Relat Disord. 2017;12:9-14.
3. Cottrel SS, Wilson SA. The affective symptomatology of disseminated sclerosis: a study of 100 cases. J Neurol Psychopathology. 1926;7(25):1-30.
4. Johansson V, Lundholm C, Hillert J, et al. Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler. 2014;20(14):1881-1891.
5. Rossi S, Studer V, Motta C, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. 2017;89(13):1338-1347.
6. Gilberthorpe TG, O’Connell KE, Carolan A, et al. The spectrum of psychosis in multiple sclerosis: a clinical case series. Neuropsychiatric disease and treatment. 2017;13:303.
7. Patten SB, Svenson LW, Metz LM. Psychotic disorders in MS: population-based evidence of an association. Neurology 2005;65(7):1123-1125.
8. Kosmidis MH, Giannakou M, Messinis L, Papathanasopoulos P. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010; 22(1):55-66.
9. US Department of Veterans Affairs. Public health: military exposures. https://www.publichealth.va.gov/exposures/. Updated April 16, 2019. Accessed May 13, 2019.
10. DeBeer BB, Davidson D, Meyer EC, Kimbrel NA, Gulliver SB, Morissette SB. The association between toxic exposures and chronic multisymptom illness in veterans of the wars of Iraq and Afghanistan. J Occup Environ Med. 2017;59(1):54-60.
11. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51(4):401-410.
12. Murphy R, O’Donoghue S, Counihan T, et al. Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(8):697-708.
13. Davids E, Hartwig U, Gastpar, M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuro Psychopharmacol Biol Psychiatry. 2004;28(4):743-744.
14. Lo Fermo S, Barone R, Patti F, et al. Outcome of psychiatric symptoms presenting at onset of multiple sclerosis: a retrospective study. Mult Scler. 2010;16(6):742-748.
15. Enderami A, Fouladi R, Hosseini HS. First-episode psychosis as the initial presentation of multiple sclerosis: a case report. Int Medical Case Rep J. 2018;11:73-76.
16. Fragoso YD, Frota ER, Lopes JS, et al. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33(6):312-316.
17. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol Teratol. 2017;61:36-46.
18. Abou-Donia MB, Lieberman A, Curtis L. Neural autoantibodies in patients with neurological symptoms and histories of chemical/mold exposures. Toxicol Ind Health. 2018;34(1):44-53.
19. Wallin MT, Kurtzke JF, Culpepper WJ, et al. Multiple sclerosis in Gulf War era veterans. 2. Military deployment and risk of multiple sclerosis in the first Gulf War. Neuroepidemiology. 2014;42(4):226-234.
Early and Accurate Identification of Parkinson Disease Among US Veterans (FULL)
Parkinson disease (PD) affects about 680,000 in the US, including > 110,000 veterans (Caroline Tanner, MD, PhD, unpublished data).1 In the next 10 years, this number is expected to double, in part because of the aging of the US population.1 Although the classic diagnostic criteria emphasize motor symptoms that include tremor, gait disturbance, and paucity of movement, there is increasing recognition that disease pathology begins decades before the development of motor impairment.2
Pathologic studies confirm that by the onset of motor symptoms, at least 30% of nigrostriatal neurons are lost or dysfunctional.3-5 Similarly, the Braak staging hypothesis posits initial deposition of Lewy bodies in the olfactory bulb and the dorsal motor nucleus of the vagus nerve, followed by prion-like spread through the brain stem into the midbrain/substantia nigra, and finally into the cortex (Figure 1).6
The decades-long prodromal or preclinical phase represents a unique opportunity for early identification of those at highest risk for developing the motor symptoms of Parkinson disease.7 Accurate identification, ideally before the onset of manifest motor disability, would not only improve prognostic counseling of veterans and families, but also could allow for early enrollment into trials of potentially disease-modifying therapeutic agents. Thus, early and accurate identification of PD is an important goal of the care of veterans with potential PD.
Prodromal Symptoms
Prodromal PD, as defined by the International Parkinson Disease and Movement Disorders Society (MDS), focuses on nonmotor symptoms that herald the onset of manifest motor PD.8 The most commonly assessed nonmotor features include olfaction, constipation, sleep disturbance, and mood disorders.
Olfaction is impaired in > 90% of patients with motor PD at the time of diagnosis; by contrast, the prevalence of hyposmia in the general population ranges from 20% to 50%, with higher rates in older adults and in smokers.9-11 Thus, olfaction appears to be a relatively sensitive, though nonspecific, prodromal feature. Importantly, subjective report of hyposmia is poorly reliable, so a number of different tests have been developed for objective assessment of olfactory dysfunction.12 The 12-item Brief Smell Identification Test (B-SIT), derived from the longer University of Pennsylvania Smell Identification Test, is a “scratch-and-sniff” forced multiple choice test that can be self-administered by cooperative patients.13,14 The B-SIT has been validated in multiple ethnic and cultural groups and shows high discrimination between PD subjects and controls.13,15 Of note, olfactory impairment appears to be associated with risk of cognitive decline in PD, further emphasizing the need for accurate assessment to guide prognosis.16
Like hyposmia, constipation can be noted long before the diagnosis of manifest motor PD.17 After adjustment for lifestyle factors, constipated individuals have up to 4.5-fold increased odds of developing PD, and those with constipation suffer worsened disease outcomes and health-related quality of life.17-20 Some groups have demonstrated alterations in gut microbiota of those with prodromal PD, which suggests local inflammatory processes and intestinal permeability may contribute to protein misfolding and disease development.21,22 This also raises the intriguing possibility that dietary alterations may be neuroprotective or neurorestorative, although this has yet to be tested in humans.23,24
Like constipation, mood changes can precede the appearance of manifest motor PD.25,26 Case control studies suggest a higher risk of developing PD among individuals who were previously diagnosed with depression or anxiety, particularly in the 1 to 2 years prior to PD diagnosis.27-29 Both apathy and anxiety are associated with striatal dopamine dysfunction, particularly in the right caudate nucleus, which suggests that mood changes are directly related to disease pathology.30,31
Of the prodromal features, rapid eye movement sleep behavior disorder (RBD) is associated with the highest risk of conversion to motor PD.8 Up to 80% of older men with socalled idiopathic RBD develop a parkinsonian syndrome within 20 years; risk is divided about equally between idiopathic PD and dementia with Lewy bodies (DLB).32 Collateral history from a bed-partner is usually sufficient to make the diagnosis, although, this is often confounded by the prevalence of nightmares in those with posttraumatic stress disorder in the veteran population.32 Thus, in suspected cases, obtaining a polysomnogram can aid in distinguishing between idiopathic PC and DLB.33 Given the specificity of RBD as a marker of synuclein deposition and the high risk of progression to a degenerative syndrome, accurate diagnosis and counseling is imperative.
Each of the prodromal nonmotor features of PD are at best moderately sensitive or specific in isolation, but in concert, they can be used to develop a Parkinson risk score. For instance, the MDS prodromal criteria combine individual likelihood ratios into Bayesian analysis to determine a combined probability of PD, which can be further stratified to probable or possible prodromal PD (probability > 80%, > 50%, respectively).8 These criteria have been applied to several independent cohorts and demonstrate high sensitivity and specificity, especially over time.34,35 Applicability in a veteran population has yet to be determined.
Use of Imaging in Diagnosis
Although clinical diagnostic criteria and prodromal features can improve diagnostic accuracy, it can be extremely challenging to distinguish idiopathic PD from nondegenerative parkinsonism or atypical syndromes (see below). Compared with the gold standard of pathologic assessment, the clinical diagnostic accuracy for PD ranges from 73% for nonexperts to 80% for fellowship-trained movement disorders specialists.36 Thus, objective biomarkers are sought to improve diagnostic accuracy both for clinical care as well as for research purposes, such as enrollment into clinical trials.
Multiple potential imaging biomarkers for preclinical PD can aid in early diagnosis and help differentiate PD from related but distinct disorders. While beyond the scope of this review, these techniques have recently been reviewed.7 Of these, the most widely available and accurate is dopamine transporter (DAT) imaging, which uses a radioiodinated ligand that binds to DAT on striatal dopaminergic terminals; binding is detected through single photon emission computed tomography (SPECT) scanning. Thus, a SPECT DaTscan (GE Healthcare Bio-Sciences, Little Chalfont, England) directly assesses the integrity of the presynaptic nigrostriatal system and is well correlated with severity of motor and nonmotor parkinsonism.37,38
In individuals with suspected prodromal PD, abnormal DaTscans are associated with faster progression to manifest motor PD.39 However, it should be noted that a number of medications, several of which are commonly utilized in the veteran population, can affect the outcome of a DaTscan.40 Some of these medications only mildly affect the outcome, so the physician interpreting the scan should be made aware of their use, while others need to be held for days to weeks so as not to invalidate the DaTscan. DaTscan also do not differentiate between PD and atypical degenerative parkinsonisms such as multiple system atrophy (MSA), DLB, progressive supranuclear palsy (PSP), or corticobasal syndrome (CBS). Nevertheless, these scans can be used to distinguish degenerative parkinsonisms from other conditions that can be difficult to distinguish clinically from PD, including essential tremor, normal pressure hydrocephalus, vascular parkinsonism, or druginduced parkinsonism (DIP).
DIP usually is caused by blockade of postsynaptic dopamine receptors by antipsychotic medications, which are prescribed to as many as 1 in 4 older veterans; antiemetic agents such as metoclopramide are also potential offenders if used chronically.41 The risk of DIP appears to be associated with the D2 binding affinity of the drug. Thus, of the newer atypical antipsychotics, clozapine and quetiapine appear to have the lowest risk, while ziprasidone and aripiprazole have the highest binding affinity and therefore the highest risk.42 In many patients, parkinsonism persists even after discontinuation of the offending agent, suggesting that in at least a subset of patients, DIP may be an “unmasking” of latent PD rather than a true adverse effect of the medication. The prodromal features discussed above can be used to distinguish isolated DIP from unmasked latent PD.43 In a study we conducted in veterans at the Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, hyposmia in particular was shown to be highly predictive of an underlying dopaminergic deficit with an odds ratio of 63.44
Other important considerations in the differential diagnosis of PD are the atypical degenerative parkinsonian syndromes, formerly called Parkinson plus syndromes. These may be further divided into the synucleinopathies (MSA, DLB) or the tauopathies (PSP, CBS), depending on the predominant amyloidogenic protein. Early in the disease, the atypical syndromes and idiopathic PD may be clinically indistinguishable, although the atypical syndromes tend to progress more rapidly and often have a less robust response to levodopa.
Radiologic and fluid biomarkers for the atypical syndromes are under active investigation; at present the most accessible study is magnetic resonance imaging (MRI), which may show characteristic features such as degeneration of the pontocerebellar fibers in MSA or midbrain atrophy in PSP.45,46 By contrast, standard MRI sequences in idiopathic PD are usually normal, although high-resolution (7 tesla) imaging can reveal loss of neuromelanin in the substantia nigra.47 MRI also can be useful in the workup of suspected normal pressure hydrocephalus or vascular parkinsonism, which would show disproportionate ventriculomegaly with transependymal flow, or white matter lesions in the basal ganglia, respectively.
Data-Based Identification of Preclinical PD
The integration of clinical motor or prodromal features with biomarker data has led to the development of several large-scale clinical and administrative databases to identify PD. The Parkinson Progression Markers Initiative initially enrolled only de novo clinically identified people with PD, but it expanded to include a prodromal cohort who are being assessed for rates of conversion to PD.48 Similarly, metabolic imaging can be combined with prodromal symptoms, such as hyposmia or RBD, to predict risk for phenoconversion into manifest motor PD.49
The PREDICT-PD study synthesizes mood symptoms, RBD, smell testing, genotyping, and keyboard-tapping tasks to divide individuals into high-, middle-, and low-risk groups; interim analysis at 3 years of follow-up (N = 842) demonstrated a hazard ratio of 4.39 (95% CI, 1.03-18.68) for the diagnosis of PD in the highrisk group compared with the low-risk group.50 Lastly, administrative claims data for prodromal features, such as constipation, RBD, and mood symptoms, is highly predictive of eventual PD diagnosis.51 VA databases accessed through the Corporate Data Warehouse are complementary sources of information to nonveteranspecific Medicare databases; to our knowledge there has not yet been a comprehensive search of VA databases to identify veterans with preclinical PD.
Risk Factors Associated With Military Service
A number of potential environmental risk factors may increase the risk of developing Parkinson disease for veterans. Perhaps the most commonly recognized is pesticide exposure, particularly given the presumptive service connections established by the VA for Parkinson disease and exposure to Agent Orange or contaminated water at Camp Lejeune.52,53 Both dioxin, the toxic ingredient in Agent Orange, and the solvents trichloroethylene and perchloroethylene, found in the water supply at Camp Lejeune, interfere with mitochondrial function leading to oxidative stress and apoptosis of nigrostriatal neurons.54,55 Other potential exposures, which are not necessarily limited to the veteran population, include rotenone, a phytochemical used to kill fish in reservoirs, and paraquat, an herbicide that may directly promote synuclein aggregation.56,57 Veterans who have reported exposure to these or other environmental chemicals in civilian life should be carefully assessed for the presence of motor PD or prodromal features.
Traumatic brain injury (TBI) also may be a risk factor for PD, which may be particularly relevant for veterans who had served in Iraq or Afghanistan. Retrospective claims data suggest a strong association between PD and recent TBI in the 5 to 10 years prior to motor PD diagnosis.58,59 A recent assessment of combat veterans with TBI found that even mild TBI was associated with a 56% increased risk of PD, while moderate-to-severe TBI was associated with an 83% higher risk of PD.60 The pathologic mechanism for this link is unclear, but post-TBI inflammatory processes may lead to the formation of reactive oxygen species and/or glutamatergic excitotoxicity, thus leading to secondary injury in the nigrostriatal pathway.61 As with prodromal symptoms, the risk of PD related to environmental risk factors may be synergistic; repetitive TBI may be more damaging than a single injury, and a combination of TBI and pesticide exposure markedly increases PD risk beyond the risk of TBI or the risk of pesticides alone.62 Recently, parkinsonism, including Parkinson disease, was recognized as a service connected condition for veterans with a servicerelated moderate or severe TBI.63
Conclusion
Because of the substantial impact on quality of life and disability-adjusted life years, early and accurate identification and management of veterans at risk for PD is an important priority area for the VA. The 10-year cost of PD-related benefits through the VA was estimated at $3.5 billion in fiscal year 2010, and that number is likely to rise in coming years, due to the aging population as well as synergistic effects of independent risk factors described above.64 In response, the VA has created a network of specialty care sites, known as Parkinson Disease Research, Education, and Clinical Centers (PADRECCs) located in Philadelphia, Pennsylvania; Richmond, Virginia; Houston, Texas; West Los Angeles and San Francisco, California; and Seattle, Washington/ Portland, Oregon (www.parkinsons.va.gov).
The PADRECCs are supplemented by a National VA PD Consortium network of VA physicians trained in PD management (Figure 2). Studies, including one investigating care of veterans with PD, have demonstrated that involvement of specialty care services early in the course of PD leads to improved patient outcomes.65,66 In addition to patient-facing resources such as support groups and specialized physical/occupational/speech therapy, PADRECCs and the consortium sites are national leaders in PD education and clinical trials and provide high-quality, multidisciplinary care for veterans with PD.67 Thus, veterans with significant risk factors or prodromal symptoms of PD should be referred into the PADRECC/Consortium network in order to maximize their quality of care and quality of life.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601.
3. Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(pt 5):2283-2301.
4. Greffard S, Verny M, Bonnet A-M, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63(4):584-588.
5. Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378-382.
6. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211.
7. Mantri S, Morley JF, Siderowf AD. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat Disord. 2018;pii:S1353-8020(18)30396-1. [Epub ahead of print]
8. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611.
9. Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson’s disease – a multicenter study. Parkinsonism Relat Disord. 2009;15(7):490-494.
10. Mullol J, Alobid I, Mariño-Sánchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6).pii:e001256.
11. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441-1443.
12. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329-339.
13. Double KL, Rowe DB, Hayes M, et al. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol. 2003;60(4):545-549.
14. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502.
15. Morley JF, Cohen A, Silveira-Moriyama L, et al. Optimizing olfactory testing for the diagnosis of Parkinson’s disease: item analysis of the University of Pennsylvania smell identification test. NPJ Parkinsons Dis. 2018;4:2.
16. Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
17. Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752-1758.
18. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456-462.
19. Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811-826.
20. Yu QJ, Yu SY, Zuo LJ, et al. Parkinson disease with constipation: clinical features and relevant factors. Sci Rep. 2018;8(1):567.
21. Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739-749.
22. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37): 10609-10620.
23. Shah SP, Duda JE. Dietary modifications in Parkinson’s disease: a neuroprotective intervention? Med Hypotheses. 2015;85(6):1002-1005.
24. Perez-Pardo P, de Jong EM, Broersen LM, et al. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front Aging Neurosci. 2017;9:57.
25. Fang F, Xu Q, Park Y, et al. Depression and the subsequent risk of Parkinson’s disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25(9):1157-1162.
26. Leentjens AFG, Van den Akker M, Metsemakers JFM, Lousberg R, Verhey FRJ. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord. 2003;18(4):414-418.
27. Alonso A, Rodriguez LAG, Logroscino G, Hernán MA. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry. 2009;80(6):671-674.
28. Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I, Ascherio A. Prospective study of phobic anxiety and risk of Parkinson’s disease. Mov Disord. 2003;18(6):646-651.
29. Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140(2):429-441.
30. Santangelo G, Vitale C, Picillo M, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2015;21(5):489-493.
31. Erro R, Pappatà S, Amboni M, et al. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(9):1034-1038.
32. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):
744-748.
33. Melendez J, Hesselbacher S, Sharafkhaneh A, Hirshkowitz M. Assessment of REM sleep behavior disorder in veterans with posttraumatic stress disorder. Chest. 2011;140(4):967A.
34. Pilotto A, Heinzel S, Suenkel U, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;32(7):1025-1034.
35. Fereshtehnejad S-M, Montplaisir JY, Pelletier A, Gagnon J-F, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord. 2017;32(6):865-873.
36. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566-576.
37. Moccia M, Pappatà S, Picillo M, et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson’s disease. J Neurol. 2014;261(11):2112-2118.
38. Siepel FJ, Brønnick KS, Booij J, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord. 2014;29(14):1802-1808.
39. Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797-805.
40. Booij J, Kemp P. Dopamine transporter imaging with [(123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging. 2008;35(2):424-438.
41. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
42. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-1191.
43. Morley JF, Duda JE. Use of hyposmia and other non-motor symptoms to distinguish between drug-induced parkinsonism and Parkinson’s disease. J Parkinsons Dis. 2014;4(2):169-173.
44. Morley JF, Cheng G, Dubroff JG, Wood S, Wilkinson JR, Duda JE. Olfactory impairment predicts underlying dopaminergic deficit in presumed drug-induced parkinsonism. Mov Disord Clin Pract. 2017;4(4):603-606.
45. Whitwell JL, Höglinger GU, Antonini A, et al; Movement Disorder Society-endorsed PSP Study Group. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord. 2017;32(7):955-971.
46. Laurens B, Constantinescu R, Freeman R, et al. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiol Dis. 2015;80:29-41.
47. Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017;15:215-227.
48. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629-635.
49. Meles SK, Vadasz D, Renken RJ, et al. FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov Disord. 2017;32(10):1482-1486.
50. Noyce AJ, R’Bibo L, Peress L, et al. PREDICT‐PD: an online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Feb; 32(2): 219–226.
51. Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017;89(14):1448-1456.
52. Institute of Medicine. Veterans and Agent Orange: Update 2012. National Academies Press: Washington, DC; 2013.
53. Department of Veterans Affairs. Diseases associated with exposure to Contaminants in the Water Supply at Camp Lejeune. Final rule. Fed Regist. 2017;82(9):4173-4185.
54. Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776-784.
55. Liu M, Shin EJ, Dang DK, et al. Trichloroethylene and Parkinson’s disease: risk assessment. Mol Neurobiol. 2018;55(7):6201-6214.
56. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301-1306.
57. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Monte DAD. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641-1644.
58. Camacho-Soto A, Warden MN, Searles Nielsen S, et al. Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using medicare data. Ann Neurol. 2017;82(5):744-754.
59. Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987-995.
60. Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K. Mild TBI and risk of Parkinson disease: a Chronic Effects of Neurotrauma Consortium Study. Neurology. 2018;90(20):e1771-e1779.
61. Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017;6:20.
62. Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012;79(20):2061-2066.
63. Disabilities that are proximately due to, or aggravated by, service-connected disease or injury. 38 CFR §3.310.
64. Diseases Associated With Exposure to Certain Herbicide Agents (Hairy Cell Leukemia and Other Chronic B-Cell Leukemias, Parkinson’s Disease and Ischemic Heart Disease). Federal Regist. 2010;75(173):53202-53216. To be codified at 38 CFR §3.
65. Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson’s disease. Mov Disord. 2007;22(4):515-522.
66. Qamar MA, Harington G, Trump S, Johnson J, Roberts F, Frost E. Multidisciplinary care in Parkinson’s disease. Int Rev Neurobiol. 2017;132:511-523.
67. Pogoda TK, Cramer IE, Meterko M, et al. Patient and organizational factors related to education and support use by veterans with Parkinson’s disease. Mov Disord. 2009;24(13):1916-1924.
Parkinson disease (PD) affects about 680,000 in the US, including > 110,000 veterans (Caroline Tanner, MD, PhD, unpublished data).1 In the next 10 years, this number is expected to double, in part because of the aging of the US population.1 Although the classic diagnostic criteria emphasize motor symptoms that include tremor, gait disturbance, and paucity of movement, there is increasing recognition that disease pathology begins decades before the development of motor impairment.2
Pathologic studies confirm that by the onset of motor symptoms, at least 30% of nigrostriatal neurons are lost or dysfunctional.3-5 Similarly, the Braak staging hypothesis posits initial deposition of Lewy bodies in the olfactory bulb and the dorsal motor nucleus of the vagus nerve, followed by prion-like spread through the brain stem into the midbrain/substantia nigra, and finally into the cortex (Figure 1).6
The decades-long prodromal or preclinical phase represents a unique opportunity for early identification of those at highest risk for developing the motor symptoms of Parkinson disease.7 Accurate identification, ideally before the onset of manifest motor disability, would not only improve prognostic counseling of veterans and families, but also could allow for early enrollment into trials of potentially disease-modifying therapeutic agents. Thus, early and accurate identification of PD is an important goal of the care of veterans with potential PD.
Prodromal Symptoms
Prodromal PD, as defined by the International Parkinson Disease and Movement Disorders Society (MDS), focuses on nonmotor symptoms that herald the onset of manifest motor PD.8 The most commonly assessed nonmotor features include olfaction, constipation, sleep disturbance, and mood disorders.
Olfaction is impaired in > 90% of patients with motor PD at the time of diagnosis; by contrast, the prevalence of hyposmia in the general population ranges from 20% to 50%, with higher rates in older adults and in smokers.9-11 Thus, olfaction appears to be a relatively sensitive, though nonspecific, prodromal feature. Importantly, subjective report of hyposmia is poorly reliable, so a number of different tests have been developed for objective assessment of olfactory dysfunction.12 The 12-item Brief Smell Identification Test (B-SIT), derived from the longer University of Pennsylvania Smell Identification Test, is a “scratch-and-sniff” forced multiple choice test that can be self-administered by cooperative patients.13,14 The B-SIT has been validated in multiple ethnic and cultural groups and shows high discrimination between PD subjects and controls.13,15 Of note, olfactory impairment appears to be associated with risk of cognitive decline in PD, further emphasizing the need for accurate assessment to guide prognosis.16
Like hyposmia, constipation can be noted long before the diagnosis of manifest motor PD.17 After adjustment for lifestyle factors, constipated individuals have up to 4.5-fold increased odds of developing PD, and those with constipation suffer worsened disease outcomes and health-related quality of life.17-20 Some groups have demonstrated alterations in gut microbiota of those with prodromal PD, which suggests local inflammatory processes and intestinal permeability may contribute to protein misfolding and disease development.21,22 This also raises the intriguing possibility that dietary alterations may be neuroprotective or neurorestorative, although this has yet to be tested in humans.23,24
Like constipation, mood changes can precede the appearance of manifest motor PD.25,26 Case control studies suggest a higher risk of developing PD among individuals who were previously diagnosed with depression or anxiety, particularly in the 1 to 2 years prior to PD diagnosis.27-29 Both apathy and anxiety are associated with striatal dopamine dysfunction, particularly in the right caudate nucleus, which suggests that mood changes are directly related to disease pathology.30,31
Of the prodromal features, rapid eye movement sleep behavior disorder (RBD) is associated with the highest risk of conversion to motor PD.8 Up to 80% of older men with socalled idiopathic RBD develop a parkinsonian syndrome within 20 years; risk is divided about equally between idiopathic PD and dementia with Lewy bodies (DLB).32 Collateral history from a bed-partner is usually sufficient to make the diagnosis, although, this is often confounded by the prevalence of nightmares in those with posttraumatic stress disorder in the veteran population.32 Thus, in suspected cases, obtaining a polysomnogram can aid in distinguishing between idiopathic PC and DLB.33 Given the specificity of RBD as a marker of synuclein deposition and the high risk of progression to a degenerative syndrome, accurate diagnosis and counseling is imperative.
Each of the prodromal nonmotor features of PD are at best moderately sensitive or specific in isolation, but in concert, they can be used to develop a Parkinson risk score. For instance, the MDS prodromal criteria combine individual likelihood ratios into Bayesian analysis to determine a combined probability of PD, which can be further stratified to probable or possible prodromal PD (probability > 80%, > 50%, respectively).8 These criteria have been applied to several independent cohorts and demonstrate high sensitivity and specificity, especially over time.34,35 Applicability in a veteran population has yet to be determined.
Use of Imaging in Diagnosis
Although clinical diagnostic criteria and prodromal features can improve diagnostic accuracy, it can be extremely challenging to distinguish idiopathic PD from nondegenerative parkinsonism or atypical syndromes (see below). Compared with the gold standard of pathologic assessment, the clinical diagnostic accuracy for PD ranges from 73% for nonexperts to 80% for fellowship-trained movement disorders specialists.36 Thus, objective biomarkers are sought to improve diagnostic accuracy both for clinical care as well as for research purposes, such as enrollment into clinical trials.
Multiple potential imaging biomarkers for preclinical PD can aid in early diagnosis and help differentiate PD from related but distinct disorders. While beyond the scope of this review, these techniques have recently been reviewed.7 Of these, the most widely available and accurate is dopamine transporter (DAT) imaging, which uses a radioiodinated ligand that binds to DAT on striatal dopaminergic terminals; binding is detected through single photon emission computed tomography (SPECT) scanning. Thus, a SPECT DaTscan (GE Healthcare Bio-Sciences, Little Chalfont, England) directly assesses the integrity of the presynaptic nigrostriatal system and is well correlated with severity of motor and nonmotor parkinsonism.37,38
In individuals with suspected prodromal PD, abnormal DaTscans are associated with faster progression to manifest motor PD.39 However, it should be noted that a number of medications, several of which are commonly utilized in the veteran population, can affect the outcome of a DaTscan.40 Some of these medications only mildly affect the outcome, so the physician interpreting the scan should be made aware of their use, while others need to be held for days to weeks so as not to invalidate the DaTscan. DaTscan also do not differentiate between PD and atypical degenerative parkinsonisms such as multiple system atrophy (MSA), DLB, progressive supranuclear palsy (PSP), or corticobasal syndrome (CBS). Nevertheless, these scans can be used to distinguish degenerative parkinsonisms from other conditions that can be difficult to distinguish clinically from PD, including essential tremor, normal pressure hydrocephalus, vascular parkinsonism, or druginduced parkinsonism (DIP).
DIP usually is caused by blockade of postsynaptic dopamine receptors by antipsychotic medications, which are prescribed to as many as 1 in 4 older veterans; antiemetic agents such as metoclopramide are also potential offenders if used chronically.41 The risk of DIP appears to be associated with the D2 binding affinity of the drug. Thus, of the newer atypical antipsychotics, clozapine and quetiapine appear to have the lowest risk, while ziprasidone and aripiprazole have the highest binding affinity and therefore the highest risk.42 In many patients, parkinsonism persists even after discontinuation of the offending agent, suggesting that in at least a subset of patients, DIP may be an “unmasking” of latent PD rather than a true adverse effect of the medication. The prodromal features discussed above can be used to distinguish isolated DIP from unmasked latent PD.43 In a study we conducted in veterans at the Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, hyposmia in particular was shown to be highly predictive of an underlying dopaminergic deficit with an odds ratio of 63.44
Other important considerations in the differential diagnosis of PD are the atypical degenerative parkinsonian syndromes, formerly called Parkinson plus syndromes. These may be further divided into the synucleinopathies (MSA, DLB) or the tauopathies (PSP, CBS), depending on the predominant amyloidogenic protein. Early in the disease, the atypical syndromes and idiopathic PD may be clinically indistinguishable, although the atypical syndromes tend to progress more rapidly and often have a less robust response to levodopa.
Radiologic and fluid biomarkers for the atypical syndromes are under active investigation; at present the most accessible study is magnetic resonance imaging (MRI), which may show characteristic features such as degeneration of the pontocerebellar fibers in MSA or midbrain atrophy in PSP.45,46 By contrast, standard MRI sequences in idiopathic PD are usually normal, although high-resolution (7 tesla) imaging can reveal loss of neuromelanin in the substantia nigra.47 MRI also can be useful in the workup of suspected normal pressure hydrocephalus or vascular parkinsonism, which would show disproportionate ventriculomegaly with transependymal flow, or white matter lesions in the basal ganglia, respectively.
Data-Based Identification of Preclinical PD
The integration of clinical motor or prodromal features with biomarker data has led to the development of several large-scale clinical and administrative databases to identify PD. The Parkinson Progression Markers Initiative initially enrolled only de novo clinically identified people with PD, but it expanded to include a prodromal cohort who are being assessed for rates of conversion to PD.48 Similarly, metabolic imaging can be combined with prodromal symptoms, such as hyposmia or RBD, to predict risk for phenoconversion into manifest motor PD.49
The PREDICT-PD study synthesizes mood symptoms, RBD, smell testing, genotyping, and keyboard-tapping tasks to divide individuals into high-, middle-, and low-risk groups; interim analysis at 3 years of follow-up (N = 842) demonstrated a hazard ratio of 4.39 (95% CI, 1.03-18.68) for the diagnosis of PD in the highrisk group compared with the low-risk group.50 Lastly, administrative claims data for prodromal features, such as constipation, RBD, and mood symptoms, is highly predictive of eventual PD diagnosis.51 VA databases accessed through the Corporate Data Warehouse are complementary sources of information to nonveteranspecific Medicare databases; to our knowledge there has not yet been a comprehensive search of VA databases to identify veterans with preclinical PD.
Risk Factors Associated With Military Service
A number of potential environmental risk factors may increase the risk of developing Parkinson disease for veterans. Perhaps the most commonly recognized is pesticide exposure, particularly given the presumptive service connections established by the VA for Parkinson disease and exposure to Agent Orange or contaminated water at Camp Lejeune.52,53 Both dioxin, the toxic ingredient in Agent Orange, and the solvents trichloroethylene and perchloroethylene, found in the water supply at Camp Lejeune, interfere with mitochondrial function leading to oxidative stress and apoptosis of nigrostriatal neurons.54,55 Other potential exposures, which are not necessarily limited to the veteran population, include rotenone, a phytochemical used to kill fish in reservoirs, and paraquat, an herbicide that may directly promote synuclein aggregation.56,57 Veterans who have reported exposure to these or other environmental chemicals in civilian life should be carefully assessed for the presence of motor PD or prodromal features.
Traumatic brain injury (TBI) also may be a risk factor for PD, which may be particularly relevant for veterans who had served in Iraq or Afghanistan. Retrospective claims data suggest a strong association between PD and recent TBI in the 5 to 10 years prior to motor PD diagnosis.58,59 A recent assessment of combat veterans with TBI found that even mild TBI was associated with a 56% increased risk of PD, while moderate-to-severe TBI was associated with an 83% higher risk of PD.60 The pathologic mechanism for this link is unclear, but post-TBI inflammatory processes may lead to the formation of reactive oxygen species and/or glutamatergic excitotoxicity, thus leading to secondary injury in the nigrostriatal pathway.61 As with prodromal symptoms, the risk of PD related to environmental risk factors may be synergistic; repetitive TBI may be more damaging than a single injury, and a combination of TBI and pesticide exposure markedly increases PD risk beyond the risk of TBI or the risk of pesticides alone.62 Recently, parkinsonism, including Parkinson disease, was recognized as a service connected condition for veterans with a servicerelated moderate or severe TBI.63
Conclusion
Because of the substantial impact on quality of life and disability-adjusted life years, early and accurate identification and management of veterans at risk for PD is an important priority area for the VA. The 10-year cost of PD-related benefits through the VA was estimated at $3.5 billion in fiscal year 2010, and that number is likely to rise in coming years, due to the aging population as well as synergistic effects of independent risk factors described above.64 In response, the VA has created a network of specialty care sites, known as Parkinson Disease Research, Education, and Clinical Centers (PADRECCs) located in Philadelphia, Pennsylvania; Richmond, Virginia; Houston, Texas; West Los Angeles and San Francisco, California; and Seattle, Washington/ Portland, Oregon (www.parkinsons.va.gov).
The PADRECCs are supplemented by a National VA PD Consortium network of VA physicians trained in PD management (Figure 2). Studies, including one investigating care of veterans with PD, have demonstrated that involvement of specialty care services early in the course of PD leads to improved patient outcomes.65,66 In addition to patient-facing resources such as support groups and specialized physical/occupational/speech therapy, PADRECCs and the consortium sites are national leaders in PD education and clinical trials and provide high-quality, multidisciplinary care for veterans with PD.67 Thus, veterans with significant risk factors or prodromal symptoms of PD should be referred into the PADRECC/Consortium network in order to maximize their quality of care and quality of life.
Parkinson disease (PD) affects about 680,000 in the US, including > 110,000 veterans (Caroline Tanner, MD, PhD, unpublished data).1 In the next 10 years, this number is expected to double, in part because of the aging of the US population.1 Although the classic diagnostic criteria emphasize motor symptoms that include tremor, gait disturbance, and paucity of movement, there is increasing recognition that disease pathology begins decades before the development of motor impairment.2
Pathologic studies confirm that by the onset of motor symptoms, at least 30% of nigrostriatal neurons are lost or dysfunctional.3-5 Similarly, the Braak staging hypothesis posits initial deposition of Lewy bodies in the olfactory bulb and the dorsal motor nucleus of the vagus nerve, followed by prion-like spread through the brain stem into the midbrain/substantia nigra, and finally into the cortex (Figure 1).6
The decades-long prodromal or preclinical phase represents a unique opportunity for early identification of those at highest risk for developing the motor symptoms of Parkinson disease.7 Accurate identification, ideally before the onset of manifest motor disability, would not only improve prognostic counseling of veterans and families, but also could allow for early enrollment into trials of potentially disease-modifying therapeutic agents. Thus, early and accurate identification of PD is an important goal of the care of veterans with potential PD.
Prodromal Symptoms
Prodromal PD, as defined by the International Parkinson Disease and Movement Disorders Society (MDS), focuses on nonmotor symptoms that herald the onset of manifest motor PD.8 The most commonly assessed nonmotor features include olfaction, constipation, sleep disturbance, and mood disorders.
Olfaction is impaired in > 90% of patients with motor PD at the time of diagnosis; by contrast, the prevalence of hyposmia in the general population ranges from 20% to 50%, with higher rates in older adults and in smokers.9-11 Thus, olfaction appears to be a relatively sensitive, though nonspecific, prodromal feature. Importantly, subjective report of hyposmia is poorly reliable, so a number of different tests have been developed for objective assessment of olfactory dysfunction.12 The 12-item Brief Smell Identification Test (B-SIT), derived from the longer University of Pennsylvania Smell Identification Test, is a “scratch-and-sniff” forced multiple choice test that can be self-administered by cooperative patients.13,14 The B-SIT has been validated in multiple ethnic and cultural groups and shows high discrimination between PD subjects and controls.13,15 Of note, olfactory impairment appears to be associated with risk of cognitive decline in PD, further emphasizing the need for accurate assessment to guide prognosis.16
Like hyposmia, constipation can be noted long before the diagnosis of manifest motor PD.17 After adjustment for lifestyle factors, constipated individuals have up to 4.5-fold increased odds of developing PD, and those with constipation suffer worsened disease outcomes and health-related quality of life.17-20 Some groups have demonstrated alterations in gut microbiota of those with prodromal PD, which suggests local inflammatory processes and intestinal permeability may contribute to protein misfolding and disease development.21,22 This also raises the intriguing possibility that dietary alterations may be neuroprotective or neurorestorative, although this has yet to be tested in humans.23,24
Like constipation, mood changes can precede the appearance of manifest motor PD.25,26 Case control studies suggest a higher risk of developing PD among individuals who were previously diagnosed with depression or anxiety, particularly in the 1 to 2 years prior to PD diagnosis.27-29 Both apathy and anxiety are associated with striatal dopamine dysfunction, particularly in the right caudate nucleus, which suggests that mood changes are directly related to disease pathology.30,31
Of the prodromal features, rapid eye movement sleep behavior disorder (RBD) is associated with the highest risk of conversion to motor PD.8 Up to 80% of older men with socalled idiopathic RBD develop a parkinsonian syndrome within 20 years; risk is divided about equally between idiopathic PD and dementia with Lewy bodies (DLB).32 Collateral history from a bed-partner is usually sufficient to make the diagnosis, although, this is often confounded by the prevalence of nightmares in those with posttraumatic stress disorder in the veteran population.32 Thus, in suspected cases, obtaining a polysomnogram can aid in distinguishing between idiopathic PC and DLB.33 Given the specificity of RBD as a marker of synuclein deposition and the high risk of progression to a degenerative syndrome, accurate diagnosis and counseling is imperative.
Each of the prodromal nonmotor features of PD are at best moderately sensitive or specific in isolation, but in concert, they can be used to develop a Parkinson risk score. For instance, the MDS prodromal criteria combine individual likelihood ratios into Bayesian analysis to determine a combined probability of PD, which can be further stratified to probable or possible prodromal PD (probability > 80%, > 50%, respectively).8 These criteria have been applied to several independent cohorts and demonstrate high sensitivity and specificity, especially over time.34,35 Applicability in a veteran population has yet to be determined.
Use of Imaging in Diagnosis
Although clinical diagnostic criteria and prodromal features can improve diagnostic accuracy, it can be extremely challenging to distinguish idiopathic PD from nondegenerative parkinsonism or atypical syndromes (see below). Compared with the gold standard of pathologic assessment, the clinical diagnostic accuracy for PD ranges from 73% for nonexperts to 80% for fellowship-trained movement disorders specialists.36 Thus, objective biomarkers are sought to improve diagnostic accuracy both for clinical care as well as for research purposes, such as enrollment into clinical trials.
Multiple potential imaging biomarkers for preclinical PD can aid in early diagnosis and help differentiate PD from related but distinct disorders. While beyond the scope of this review, these techniques have recently been reviewed.7 Of these, the most widely available and accurate is dopamine transporter (DAT) imaging, which uses a radioiodinated ligand that binds to DAT on striatal dopaminergic terminals; binding is detected through single photon emission computed tomography (SPECT) scanning. Thus, a SPECT DaTscan (GE Healthcare Bio-Sciences, Little Chalfont, England) directly assesses the integrity of the presynaptic nigrostriatal system and is well correlated with severity of motor and nonmotor parkinsonism.37,38
In individuals with suspected prodromal PD, abnormal DaTscans are associated with faster progression to manifest motor PD.39 However, it should be noted that a number of medications, several of which are commonly utilized in the veteran population, can affect the outcome of a DaTscan.40 Some of these medications only mildly affect the outcome, so the physician interpreting the scan should be made aware of their use, while others need to be held for days to weeks so as not to invalidate the DaTscan. DaTscan also do not differentiate between PD and atypical degenerative parkinsonisms such as multiple system atrophy (MSA), DLB, progressive supranuclear palsy (PSP), or corticobasal syndrome (CBS). Nevertheless, these scans can be used to distinguish degenerative parkinsonisms from other conditions that can be difficult to distinguish clinically from PD, including essential tremor, normal pressure hydrocephalus, vascular parkinsonism, or druginduced parkinsonism (DIP).
DIP usually is caused by blockade of postsynaptic dopamine receptors by antipsychotic medications, which are prescribed to as many as 1 in 4 older veterans; antiemetic agents such as metoclopramide are also potential offenders if used chronically.41 The risk of DIP appears to be associated with the D2 binding affinity of the drug. Thus, of the newer atypical antipsychotics, clozapine and quetiapine appear to have the lowest risk, while ziprasidone and aripiprazole have the highest binding affinity and therefore the highest risk.42 In many patients, parkinsonism persists even after discontinuation of the offending agent, suggesting that in at least a subset of patients, DIP may be an “unmasking” of latent PD rather than a true adverse effect of the medication. The prodromal features discussed above can be used to distinguish isolated DIP from unmasked latent PD.43 In a study we conducted in veterans at the Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, hyposmia in particular was shown to be highly predictive of an underlying dopaminergic deficit with an odds ratio of 63.44
Other important considerations in the differential diagnosis of PD are the atypical degenerative parkinsonian syndromes, formerly called Parkinson plus syndromes. These may be further divided into the synucleinopathies (MSA, DLB) or the tauopathies (PSP, CBS), depending on the predominant amyloidogenic protein. Early in the disease, the atypical syndromes and idiopathic PD may be clinically indistinguishable, although the atypical syndromes tend to progress more rapidly and often have a less robust response to levodopa.
Radiologic and fluid biomarkers for the atypical syndromes are under active investigation; at present the most accessible study is magnetic resonance imaging (MRI), which may show characteristic features such as degeneration of the pontocerebellar fibers in MSA or midbrain atrophy in PSP.45,46 By contrast, standard MRI sequences in idiopathic PD are usually normal, although high-resolution (7 tesla) imaging can reveal loss of neuromelanin in the substantia nigra.47 MRI also can be useful in the workup of suspected normal pressure hydrocephalus or vascular parkinsonism, which would show disproportionate ventriculomegaly with transependymal flow, or white matter lesions in the basal ganglia, respectively.
Data-Based Identification of Preclinical PD
The integration of clinical motor or prodromal features with biomarker data has led to the development of several large-scale clinical and administrative databases to identify PD. The Parkinson Progression Markers Initiative initially enrolled only de novo clinically identified people with PD, but it expanded to include a prodromal cohort who are being assessed for rates of conversion to PD.48 Similarly, metabolic imaging can be combined with prodromal symptoms, such as hyposmia or RBD, to predict risk for phenoconversion into manifest motor PD.49
The PREDICT-PD study synthesizes mood symptoms, RBD, smell testing, genotyping, and keyboard-tapping tasks to divide individuals into high-, middle-, and low-risk groups; interim analysis at 3 years of follow-up (N = 842) demonstrated a hazard ratio of 4.39 (95% CI, 1.03-18.68) for the diagnosis of PD in the highrisk group compared with the low-risk group.50 Lastly, administrative claims data for prodromal features, such as constipation, RBD, and mood symptoms, is highly predictive of eventual PD diagnosis.51 VA databases accessed through the Corporate Data Warehouse are complementary sources of information to nonveteranspecific Medicare databases; to our knowledge there has not yet been a comprehensive search of VA databases to identify veterans with preclinical PD.
Risk Factors Associated With Military Service
A number of potential environmental risk factors may increase the risk of developing Parkinson disease for veterans. Perhaps the most commonly recognized is pesticide exposure, particularly given the presumptive service connections established by the VA for Parkinson disease and exposure to Agent Orange or contaminated water at Camp Lejeune.52,53 Both dioxin, the toxic ingredient in Agent Orange, and the solvents trichloroethylene and perchloroethylene, found in the water supply at Camp Lejeune, interfere with mitochondrial function leading to oxidative stress and apoptosis of nigrostriatal neurons.54,55 Other potential exposures, which are not necessarily limited to the veteran population, include rotenone, a phytochemical used to kill fish in reservoirs, and paraquat, an herbicide that may directly promote synuclein aggregation.56,57 Veterans who have reported exposure to these or other environmental chemicals in civilian life should be carefully assessed for the presence of motor PD or prodromal features.
Traumatic brain injury (TBI) also may be a risk factor for PD, which may be particularly relevant for veterans who had served in Iraq or Afghanistan. Retrospective claims data suggest a strong association between PD and recent TBI in the 5 to 10 years prior to motor PD diagnosis.58,59 A recent assessment of combat veterans with TBI found that even mild TBI was associated with a 56% increased risk of PD, while moderate-to-severe TBI was associated with an 83% higher risk of PD.60 The pathologic mechanism for this link is unclear, but post-TBI inflammatory processes may lead to the formation of reactive oxygen species and/or glutamatergic excitotoxicity, thus leading to secondary injury in the nigrostriatal pathway.61 As with prodromal symptoms, the risk of PD related to environmental risk factors may be synergistic; repetitive TBI may be more damaging than a single injury, and a combination of TBI and pesticide exposure markedly increases PD risk beyond the risk of TBI or the risk of pesticides alone.62 Recently, parkinsonism, including Parkinson disease, was recognized as a service connected condition for veterans with a servicerelated moderate or severe TBI.63
Conclusion
Because of the substantial impact on quality of life and disability-adjusted life years, early and accurate identification and management of veterans at risk for PD is an important priority area for the VA. The 10-year cost of PD-related benefits through the VA was estimated at $3.5 billion in fiscal year 2010, and that number is likely to rise in coming years, due to the aging population as well as synergistic effects of independent risk factors described above.64 In response, the VA has created a network of specialty care sites, known as Parkinson Disease Research, Education, and Clinical Centers (PADRECCs) located in Philadelphia, Pennsylvania; Richmond, Virginia; Houston, Texas; West Los Angeles and San Francisco, California; and Seattle, Washington/ Portland, Oregon (www.parkinsons.va.gov).
The PADRECCs are supplemented by a National VA PD Consortium network of VA physicians trained in PD management (Figure 2). Studies, including one investigating care of veterans with PD, have demonstrated that involvement of specialty care services early in the course of PD leads to improved patient outcomes.65,66 In addition to patient-facing resources such as support groups and specialized physical/occupational/speech therapy, PADRECCs and the consortium sites are national leaders in PD education and clinical trials and provide high-quality, multidisciplinary care for veterans with PD.67 Thus, veterans with significant risk factors or prodromal symptoms of PD should be referred into the PADRECC/Consortium network in order to maximize their quality of care and quality of life.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601.
3. Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(pt 5):2283-2301.
4. Greffard S, Verny M, Bonnet A-M, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63(4):584-588.
5. Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378-382.
6. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211.
7. Mantri S, Morley JF, Siderowf AD. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat Disord. 2018;pii:S1353-8020(18)30396-1. [Epub ahead of print]
8. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611.
9. Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson’s disease – a multicenter study. Parkinsonism Relat Disord. 2009;15(7):490-494.
10. Mullol J, Alobid I, Mariño-Sánchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6).pii:e001256.
11. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441-1443.
12. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329-339.
13. Double KL, Rowe DB, Hayes M, et al. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol. 2003;60(4):545-549.
14. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502.
15. Morley JF, Cohen A, Silveira-Moriyama L, et al. Optimizing olfactory testing for the diagnosis of Parkinson’s disease: item analysis of the University of Pennsylvania smell identification test. NPJ Parkinsons Dis. 2018;4:2.
16. Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
17. Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752-1758.
18. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456-462.
19. Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811-826.
20. Yu QJ, Yu SY, Zuo LJ, et al. Parkinson disease with constipation: clinical features and relevant factors. Sci Rep. 2018;8(1):567.
21. Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739-749.
22. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37): 10609-10620.
23. Shah SP, Duda JE. Dietary modifications in Parkinson’s disease: a neuroprotective intervention? Med Hypotheses. 2015;85(6):1002-1005.
24. Perez-Pardo P, de Jong EM, Broersen LM, et al. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front Aging Neurosci. 2017;9:57.
25. Fang F, Xu Q, Park Y, et al. Depression and the subsequent risk of Parkinson’s disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25(9):1157-1162.
26. Leentjens AFG, Van den Akker M, Metsemakers JFM, Lousberg R, Verhey FRJ. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord. 2003;18(4):414-418.
27. Alonso A, Rodriguez LAG, Logroscino G, Hernán MA. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry. 2009;80(6):671-674.
28. Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I, Ascherio A. Prospective study of phobic anxiety and risk of Parkinson’s disease. Mov Disord. 2003;18(6):646-651.
29. Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140(2):429-441.
30. Santangelo G, Vitale C, Picillo M, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2015;21(5):489-493.
31. Erro R, Pappatà S, Amboni M, et al. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(9):1034-1038.
32. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):
744-748.
33. Melendez J, Hesselbacher S, Sharafkhaneh A, Hirshkowitz M. Assessment of REM sleep behavior disorder in veterans with posttraumatic stress disorder. Chest. 2011;140(4):967A.
34. Pilotto A, Heinzel S, Suenkel U, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;32(7):1025-1034.
35. Fereshtehnejad S-M, Montplaisir JY, Pelletier A, Gagnon J-F, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord. 2017;32(6):865-873.
36. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566-576.
37. Moccia M, Pappatà S, Picillo M, et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson’s disease. J Neurol. 2014;261(11):2112-2118.
38. Siepel FJ, Brønnick KS, Booij J, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord. 2014;29(14):1802-1808.
39. Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797-805.
40. Booij J, Kemp P. Dopamine transporter imaging with [(123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging. 2008;35(2):424-438.
41. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
42. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-1191.
43. Morley JF, Duda JE. Use of hyposmia and other non-motor symptoms to distinguish between drug-induced parkinsonism and Parkinson’s disease. J Parkinsons Dis. 2014;4(2):169-173.
44. Morley JF, Cheng G, Dubroff JG, Wood S, Wilkinson JR, Duda JE. Olfactory impairment predicts underlying dopaminergic deficit in presumed drug-induced parkinsonism. Mov Disord Clin Pract. 2017;4(4):603-606.
45. Whitwell JL, Höglinger GU, Antonini A, et al; Movement Disorder Society-endorsed PSP Study Group. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord. 2017;32(7):955-971.
46. Laurens B, Constantinescu R, Freeman R, et al. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiol Dis. 2015;80:29-41.
47. Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017;15:215-227.
48. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629-635.
49. Meles SK, Vadasz D, Renken RJ, et al. FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov Disord. 2017;32(10):1482-1486.
50. Noyce AJ, R’Bibo L, Peress L, et al. PREDICT‐PD: an online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Feb; 32(2): 219–226.
51. Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017;89(14):1448-1456.
52. Institute of Medicine. Veterans and Agent Orange: Update 2012. National Academies Press: Washington, DC; 2013.
53. Department of Veterans Affairs. Diseases associated with exposure to Contaminants in the Water Supply at Camp Lejeune. Final rule. Fed Regist. 2017;82(9):4173-4185.
54. Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776-784.
55. Liu M, Shin EJ, Dang DK, et al. Trichloroethylene and Parkinson’s disease: risk assessment. Mol Neurobiol. 2018;55(7):6201-6214.
56. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301-1306.
57. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Monte DAD. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641-1644.
58. Camacho-Soto A, Warden MN, Searles Nielsen S, et al. Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using medicare data. Ann Neurol. 2017;82(5):744-754.
59. Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987-995.
60. Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K. Mild TBI and risk of Parkinson disease: a Chronic Effects of Neurotrauma Consortium Study. Neurology. 2018;90(20):e1771-e1779.
61. Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017;6:20.
62. Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012;79(20):2061-2066.
63. Disabilities that are proximately due to, or aggravated by, service-connected disease or injury. 38 CFR §3.310.
64. Diseases Associated With Exposure to Certain Herbicide Agents (Hairy Cell Leukemia and Other Chronic B-Cell Leukemias, Parkinson’s Disease and Ischemic Heart Disease). Federal Regist. 2010;75(173):53202-53216. To be codified at 38 CFR §3.
65. Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson’s disease. Mov Disord. 2007;22(4):515-522.
66. Qamar MA, Harington G, Trump S, Johnson J, Roberts F, Frost E. Multidisciplinary care in Parkinson’s disease. Int Rev Neurobiol. 2017;132:511-523.
67. Pogoda TK, Cramer IE, Meterko M, et al. Patient and organizational factors related to education and support use by veterans with Parkinson’s disease. Mov Disord. 2009;24(13):1916-1924.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601.
3. Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(pt 5):2283-2301.
4. Greffard S, Verny M, Bonnet A-M, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63(4):584-588.
5. Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005;62(3):378-382.
6. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211.
7. Mantri S, Morley JF, Siderowf AD. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat Disord. 2018;pii:S1353-8020(18)30396-1. [Epub ahead of print]
8. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611.
9. Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson’s disease – a multicenter study. Parkinsonism Relat Disord. 2009;15(7):490-494.
10. Mullol J, Alobid I, Mariño-Sánchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6).pii:e001256.
11. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441-1443.
12. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329-339.
13. Double KL, Rowe DB, Hayes M, et al. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol. 2003;60(4):545-549.
14. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502.
15. Morley JF, Cohen A, Silveira-Moriyama L, et al. Optimizing olfactory testing for the diagnosis of Parkinson’s disease: item analysis of the University of Pennsylvania smell identification test. NPJ Parkinsons Dis. 2018;4:2.
16. Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45-51.
17. Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73(21):1752-1758.
18. Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456-462.
19. Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811-826.
20. Yu QJ, Yu SY, Zuo LJ, et al. Parkinson disease with constipation: clinical features and relevant factors. Sci Rep. 2018;8(1):567.
21. Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739-749.
22. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21(37): 10609-10620.
23. Shah SP, Duda JE. Dietary modifications in Parkinson’s disease: a neuroprotective intervention? Med Hypotheses. 2015;85(6):1002-1005.
24. Perez-Pardo P, de Jong EM, Broersen LM, et al. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front Aging Neurosci. 2017;9:57.
25. Fang F, Xu Q, Park Y, et al. Depression and the subsequent risk of Parkinson’s disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25(9):1157-1162.
26. Leentjens AFG, Van den Akker M, Metsemakers JFM, Lousberg R, Verhey FRJ. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord. 2003;18(4):414-418.
27. Alonso A, Rodriguez LAG, Logroscino G, Hernán MA. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry. 2009;80(6):671-674.
28. Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I, Ascherio A. Prospective study of phobic anxiety and risk of Parkinson’s disease. Mov Disord. 2003;18(6):646-651.
29. Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140(2):429-441.
30. Santangelo G, Vitale C, Picillo M, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2015;21(5):489-493.
31. Erro R, Pappatà S, Amboni M, et al. Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(9):1034-1038.
32. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14(8):
744-748.
33. Melendez J, Hesselbacher S, Sharafkhaneh A, Hirshkowitz M. Assessment of REM sleep behavior disorder in veterans with posttraumatic stress disorder. Chest. 2011;140(4):967A.
34. Pilotto A, Heinzel S, Suenkel U, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;32(7):1025-1034.
35. Fereshtehnejad S-M, Montplaisir JY, Pelletier A, Gagnon J-F, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord. 2017;32(6):865-873.
36. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566-576.
37. Moccia M, Pappatà S, Picillo M, et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson’s disease. J Neurol. 2014;261(11):2112-2118.
38. Siepel FJ, Brønnick KS, Booij J, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord. 2014;29(14):1802-1808.
39. Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797-805.
40. Booij J, Kemp P. Dopamine transporter imaging with [(123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging. 2008;35(2):424-438.
41. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
42. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-1191.
43. Morley JF, Duda JE. Use of hyposmia and other non-motor symptoms to distinguish between drug-induced parkinsonism and Parkinson’s disease. J Parkinsons Dis. 2014;4(2):169-173.
44. Morley JF, Cheng G, Dubroff JG, Wood S, Wilkinson JR, Duda JE. Olfactory impairment predicts underlying dopaminergic deficit in presumed drug-induced parkinsonism. Mov Disord Clin Pract. 2017;4(4):603-606.
45. Whitwell JL, Höglinger GU, Antonini A, et al; Movement Disorder Society-endorsed PSP Study Group. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord. 2017;32(7):955-971.
46. Laurens B, Constantinescu R, Freeman R, et al. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiol Dis. 2015;80:29-41.
47. Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017;15:215-227.
48. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629-635.
49. Meles SK, Vadasz D, Renken RJ, et al. FDG PET, dopamine transporter SPECT, and olfaction: combining biomarkers in REM sleep behavior disorder. Mov Disord. 2017;32(10):1482-1486.
50. Noyce AJ, R’Bibo L, Peress L, et al. PREDICT‐PD: an online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Feb; 32(2): 219–226.
51. Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017;89(14):1448-1456.
52. Institute of Medicine. Veterans and Agent Orange: Update 2012. National Academies Press: Washington, DC; 2013.
53. Department of Veterans Affairs. Diseases associated with exposure to Contaminants in the Water Supply at Camp Lejeune. Final rule. Fed Regist. 2017;82(9):4173-4185.
54. Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776-784.
55. Liu M, Shin EJ, Dang DK, et al. Trichloroethylene and Parkinson’s disease: risk assessment. Mol Neurobiol. 2018;55(7):6201-6214.
56. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301-1306.
57. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Monte DAD. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641-1644.
58. Camacho-Soto A, Warden MN, Searles Nielsen S, et al. Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using medicare data. Ann Neurol. 2017;82(5):744-754.
59. Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987-995.
60. Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K. Mild TBI and risk of Parkinson disease: a Chronic Effects of Neurotrauma Consortium Study. Neurology. 2018;90(20):e1771-e1779.
61. Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R. Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodegener. 2017;6:20.
62. Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012;79(20):2061-2066.
63. Disabilities that are proximately due to, or aggravated by, service-connected disease or injury. 38 CFR §3.310.
64. Diseases Associated With Exposure to Certain Herbicide Agents (Hairy Cell Leukemia and Other Chronic B-Cell Leukemias, Parkinson’s Disease and Ischemic Heart Disease). Federal Regist. 2010;75(173):53202-53216. To be codified at 38 CFR §3.
65. Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson’s disease. Mov Disord. 2007;22(4):515-522.
66. Qamar MA, Harington G, Trump S, Johnson J, Roberts F, Frost E. Multidisciplinary care in Parkinson’s disease. Int Rev Neurobiol. 2017;132:511-523.
67. Pogoda TK, Cramer IE, Meterko M, et al. Patient and organizational factors related to education and support use by veterans with Parkinson’s disease. Mov Disord. 2009;24(13):1916-1924.
Hemolytic Uremic Syndrome With Severe Neurologic Complications in an Adult (FULL)
The case of a female presenting with Shiga toxin-producing Escherichia coli and hemolytic uremic syndrome highlights a severe neurologic complication that canbe associated with these conditions.
Hemolytic uremic syndrome (HUS) is a rare illness that can be acquired through the consumption of food products contaminated with strains of Shiga toxin-producing Escherichia coli (E coli; STEC).1 Between 6% and 15% of individuals infected with STEC develop HUS, with children affected more frequently than adults.2,3 This strain of E coli releases Shiga toxin into the systemic circulation, which causes a thrombotic microangiopathy resulting in the characteristic HUS triad of symptoms: acute renal insufficiency, thrombocytopenia, and hemolytic anemia.4-6
Although neurologic features are common in HUS, they have not been extensively studied, particularly in adults. We report a case of STEC 0157:H7 subtype HUS in an adult with severe neurologic complications. This case highlights the neurological sequelae in an adult with typical STEC-HUS. The use of treatment modalities, such as plasmapheresis and eculizumab, and their use in adult typical STEC-HUS also is explored.
Case
A 53-year-old white woman with no pertinent past medical history presented to the Bay Pines Veterans Affairs Healthcare System Emergency Department with a 2-day history of abdominal pain, vomiting, nausea, diarrhea, and bright bloody stools. She returned from a cruise to the Bahamas 3 days prior, where she ate local foods, including salads. She reported no fever, shortness of breath, chest pain, headache, and cognitive difficulties. She presented with a normal mental status and neurologic exam. Apart from leukocytosis and elevated glucose level, her laboratory results at initial presentation were normal, (Table). A stool sample showed occult blood with white blood cell counts (WBCs) but was negative for Clostridium difficile. She was started on ciprofloxacin 400 mg and metronidazole 500 mg on the day of admission.
Hematuria was found on hospital day 2. On hospital day 4, the patient exhibited word finding difficulties. Blood studies revealed anemia, thrombocytopenia, leukocytosis, and increasing blood urea nitrogen (BUN) and creatinine. A computed tomography scan of the head was normal. Laboratory analysis showed schistocytes in the peripheral blood smear.
The patient’s cognitive functioning deteriorated on hospital day 5. She was not oriented to time or place. Her laboratory results showed complement level C3 at 70 mg/dL (ref: 83-193 mg/dL) complement C4 at 12 mg/dL (ref: 15-57mg/dL). The patient exhibited oliguria and hyponatremia, as well as both metabolic and respiratory acidosis; dialysis was then initiated. Results from the stool sample that was collected on hospital day 1 were received and tested positive for Shiga toxin.
At this point, the patient’s presentation of hemolytic anemia and thrombocytopenia in the setting of acute bloody diarrheal illness with known Shiga toxin, schistocytes on blood smear, and lack of pertinent medical history for other causes of this presentation made STEC-HUS the leading differential diagnosis. Plasmapheresis was ordered and performed on hospital day 6 and 7. Shiga toxin was no longer detected in the stool and antibiotics were stopped on hospital day 7.
The patient’s progressive deterioration in mental status continued on hospital day 8. She was not oriented to time or place, unable to perform simple calculations, and could not spell the word “hand” backwards. Physicians observed repetitive jerking motions of the upper extremities that were worse on the left side. An electroencephalogram (EEG) revealed right hemispheric sharp waves that were thought to be epileptiform (Figure 1). The patient began taking levetiracetam 1500 mg IV with 750 mg bid maintenance for seizure control. Plasmapheresis was discontinued due to her continued neurologic deterioration on this therapy. Consequently, eculizumab 900 mg IV was given along with the Neisseria meningitidis (N meningitidis) vaccine and a 19-day course of azithromycin 250 mg po as prophylaxis for encapsulated bacteria.
The patient continued to seize on hospital days 10 through 13. Oculocephalic maneuvers showed a tendency to keep her eyes deviated to the right. Her pupils continued to react to light. A repeat EEG showed diffuse slowing (5-6 Hz) with no epileptic activity seen (Figure 2). A second dose of eculizumab 900 mg IV was administered on hospital day 15. The patient experienced cardiac arrest on hospital day 16 and was successfully resuscitated. On hospital day 25 (10 days after receiving her second dose of eculizumab), the patient was able to speak and follow simple commands but exhibited difficulty concentrating and poor impulse control.
The patient was alert and oriented to person, place, time, and situation on hospital day 28 (6 days after the third and final dose of eculizumab). A neurologic exam was significant only for a slight intention tremor. She was continued on levetiracetam with a plan to be maintained on the medication for the next 6 months for seizure control. She was discharged on hospital day 30.
Twenty-eight days postdischarge (57 days postadmission), the patient showed marked recovery. She had returned to her previous employment as a business administrator on a part-time basis and exhibited no deficiencies in executive functioning or handling activities of daily living. Although she had been very active prior to this illness, she now experienced decreased physical and mental endurance; however, this gradually improved with physical therapy. She planned on returning to work on a full-time basis when she had regained her stamina. She also noticed difficulties in retaining short term memory since her discharge but believed that these symptoms were remitting. On examination her mental status and neurologic exam was significant for inability to continue serial 7s, left sided 4/5 muscle strength in quadriceps and thumb to 5th metacarpal adduction, bilateral 1+ reflexes in muscle groups tested (triceps, biceps, brachioradialis, patellar, and Achilles), loss of dull pinprick sensation bilaterally at web of hands, deficit in tandem gait while looking away, and slight intention tremor on finger to nose testing bilaterally (with left hand tremor more pronounced than right). Her complete blood count was normal. Her recovery continues to be monitored in an outpatient setting.
Discussion
HUS is characterized by 3 core clinical features: microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury.4 Schistocytes are seen on peripheral blood smear and occur due to the passage of red blood cells over the microvascular thrombi induced by the disease. HUS can be classified as typical, atypical, or occurring with a coexisting disease. Typical HUS is associated with STEC 0157:H7 subtype, a bacterium known to be acquired through contaminated food and via human-to-human transmission.6-8 In the case of typical STEC 0157:H7, the bacterium releases a verotoxin that damages the vascular endothelium, thereby leading to activation of the coagulation cascade and eventually the formation of thrombi.4 It has been hypothesized that the Shiga toxin also activates the alternative complement pathway directly, which could contribute to thrombosis.9 This would explain the findings of low complement levels in our patient. Atypical HUS is primarily attributable to mutations in the alternative complement pathway. Causes for the third type of HUS can include Streptococcus pneumoniae, HIV, drug toxicity, and alterations in the metabolism of cobalamin C.
Epidemiologically, 15.3% of children aged < 5 years develop typical HUS after exposure to STEC compared with 1.2% of adults aged 18 to 59 years. The median age of patients who developed HUS from STEC exposure was 4 years compared with 16 years for those who did not develop HUS.2
Neurologic manifestations increase mortality for HUS patients.10 These have been described in the pediatric population as alteration in consciousness (85%), seizures (71%), pyramidal syndrome (52%), and extrapyramidal syndrome with hypertonia (42%).11 Brain imaging in children has demonstrated hemorrhagic lesions involving the pons, basal ganglia, and occipital cortex.11 Blood flow to areas such as the cerebellum, brainstem, and orbitofrontal area can be compromised.10 Adult patients with HUS can present without lesions on cranial magnetic resonance imaging (MRI), but instead with transient symmetric vasogenic edema of the central brain stem.12 Unfortunately in this case, MRI was not performed because it was thought to provide limited aid in diagnosis and to avoid unnecessary testing for the acutely ill patient.
The underlying pathophysiology of neurologic manifestations in patients may be due to a metabolic disturbance, toxin-mediated damage of the vascular endothelium, or toxin-induced cytokine release resulting in death of neural cells and subsequent neuroinflammation. However, the most likely mechanism is parenchymal ischemic changes related to microangiopathy.11,13 Pediatric patients often experience seizures and altered mental status, and their EEGs display delta waves.13 This patient’s diffuse slowing on her second EEG and altered mental status suggests that the neuropathologic mechanisms for typical HUS in adults may be similar to those in children.
HUS Treatment
The treatment and management of adults with typical STEC-HUS is evolving. The patient was first suspected to have an infectious colitis and empiric antibiotics were initiated. Some studies suggest that antibiotic administration may worsen the course of HUS in children as it may lead to release and subsequent absorption of Shiga toxin in the intestine.9,14 However, there is little evidence to suggest harm or efficacy of administration in adults. It is unclear what role antibiotic administration played in the recovery time of HUS given the co-administration of other treatments such as eculizumab and plasmapheresis, but it does appear to have helped with the initial E coli infection.
Plasmapheresis was subsequently administered, due to its documented benefit in the treatment of HUS.15 However, it should be noted that even though plasmapheresis is currently used in patients with CNS involvement, it remains unproven with conflicting information on its efficacy.3,16 The mechanism of action is unclear, but it has been hypothesized that plasmapheresis prevents microangiopathy caused by microthrombi.3,16 For this reason, eculizumab is becoming the mainstay for treatment of STEC-HUS with neurologic complications given the lack of well researched alternative treatments. In this case study, the use of plasmapheresis did not result in clinical improvement, and was abandoned after 2 days of treatment.
Eculizumab is a humanized, recombinant monoclonal IgG antibody that is a terminal complement inhibitor of the alternative complement system at the final step to cleave C5.17 The Shiga toxin may directly activate the complement system via the alternative pathway, which can result in uncontrolled platelet and white blood cell activation and depletion, endothelial cell damage, and hemolysis. The galvanized complement system leads to a series of cascading events that contribute to organ damage and death.9 Eculizumab is FDA approved for use in atypical HUS.18 It also can be used off-label to treat typical-HUS in adults with neurologic complications.
Eculizumab interferes with the immune response against encapsulated bacteria because it inhibits the alternative complement pathway. Thus, vaccination against N meningitides is recommended 2 weeks prior to the administration of eculizumab. However, in situations where the risks of delaying eculizumab for 2 weeks are greater than the risk of developing an N meningitides infection, eculizumab may be given without delay.18 Given the rapid deterioration of our patient’s condition, the vaccine and eculizumab were given together with prophylactic azithromycin. Although penicillin is the standard for prophylaxis in this situation, the patient’s penicillin allergy led to the use of azithromycin 250 mg po once a day. Literature also suggests azithromycin reduces the carriage duration of E coli-induced colitis.19 As such, it is possible that some improvement in the patient’s condition could be attributed to the elimination of the pathogen and toxin.
Conclusion
Three doses of eculizumab were administered at weekly intervals, with the first dose on hospital day 8 and the final dose on hospital day 22. Prior to the first dose, the patient displayed significant decline in mental status with EEG findings of right hemisphere epileptogenic discharges. After her third dose, she was found to have a drastically improved mental status exam and a normal EEG. One week later, she was discharged home. At the time of her 1-month follow-up, she was independent in all activities of daily living and had returned to part-time work. Apart from subtle cognitive changes, the remainder of her neurologic exam was normal.
There is evidence that supports the efficacy of eculizumab in children with HUS with neurologic symptoms on dialysis.20 However, its use in adults is not well established.21 This patient required dialysis and had neurologic symptoms similar to pediatric patients described in the literature, and responded similarly to the eculizumab. The rationale for the use of eculizumab in STEC-HUS also is evidenced by in vitro demonstrations of complement activation in STEC-HUS.22-25 This case report adds to the literature supporting the use of eculizumab in adult patients with typical HUS with neurological complications. Further research is necessary to develop guidelines in the treatment of adult STEC-HUS with regards to neurologic complications.
Acknowledgments
The authors would like to thank Pete DiStaso, REEGT for his work on obtaining the electroencephalograms and Anthony Rinaldi, PsyD; Julie Cessnapalas, PsyD; and Syed Faizan Sagheer for proof-reading the article.
1. Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-1086.
2. Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clin Infect Dis. 2009;49(10):1480-1485.
3. Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333(6):364-368.
4. Rondeau E, Peraldi MN. Escherichia coli and the hemolytic-uremic syndrome. N Engl J Med. 1996;335(9):660-662.
5. Te Loo DM, van Hinsbergh VW, van den Heuvel LP, Monnens LA. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J Am Soc Nephrol. 2001;12(4):800-806.
6. Tran SL, Jenkins C, Livrelli V, Schüller S. Shiga toxin 2 translocation across intestinal epithelium is linked to virulence of Shiga toxin-producing Escherichia coli in humans. Microbiology. 2018;164(4):509-516.
7. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856.
8. Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis. 2011;8(4):465-487.
9. Percheron L, Gramada R, Tellier S, et al. Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol. 2018;33(8):1385-1394.
10. Hosaka T, Nakamagoe K, Tamaoka A. Hemolytic uremic syndrome-associated encephalopathy successfully treated with corticosteroids. Intern Med. 2017;56(21):2937-2941.
11. Nathanson S, Kwon T, Elmaleh M, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5(7):1218-1228.
12. Wengenroth M, Hoeltje J, Repenthin J, et al. Central nervous system involvement in adults with epidemic hemolytic uremic syndrome. AJNR Am J Neuroradiol. 2013;34(5):1016-1021, S1.
13. Eriksson KJ, Boyd SG, Tasker RC. Acute neurology and neurophysiology of haemolytic-uraemic syndrome. Arch Dis Child. 2001;84(5):434-435.
14. Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342(26):1930-1936.
15. Nguyen TC, Kiss JE, Goldman JR, Carcillo JA. The role of plasmapheresis in critical illness. Crit Care Clin. 2012;28(3):453-468, vii.
16. Loos S, Ahlenstiel T, Kranz B, et al. An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis. 2012;55(6):753-759.
17. Hossain MA, Cheema A, Kalathil S, et al. Atypical hemolytic uremic syndrome: Laboratory characteristics, complement-amplifying conditions, renal biopsy, and genetic mutations. Saudi J Kidney Dis Transpl. 2018;29(2):276-283.
18. Soliris (eculizumab) [package insert]. Cheshire, CT: Alexion Pharmaceuticals, Inc; 2011.
19. Keenswijk W, Raes A, Vande Walle J. Is eculizumab efficacious in Shigatoxin-associated hemolytic uremic syndrome? A narrative review of current evidence. Eur J Pediatr. 2018;177(3):311-318.
20. Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364(26):2561-2563.
21. Pape L, Hartmann H, Bange FC, Suerbaum S, Bueltmann E, Ahlenstiel-Grunow T. Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine (Baltimore). 2015;94(24):e1000.
22. Kim Y, Miller K, Michael AF. Breakdown products of C3 and factor B in hemolytic-uremic syndrome. J Lab Clin Med. 1977;89(4):845-850.
23. Monnens L, Molenaar J, Lambert PH, Proesmans W, van Munster P. The complement system in hemolytic-uremic syndrome in childhood. Clin Nephrol. 1980;13(4):168-171.
24. Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4(12):1920-1924.
25. Ståhl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117(20):5503-5513.
The case of a female presenting with Shiga toxin-producing Escherichia coli and hemolytic uremic syndrome highlights a severe neurologic complication that canbe associated with these conditions.
The case of a female presenting with Shiga toxin-producing Escherichia coli and hemolytic uremic syndrome highlights a severe neurologic complication that canbe associated with these conditions.
Hemolytic uremic syndrome (HUS) is a rare illness that can be acquired through the consumption of food products contaminated with strains of Shiga toxin-producing Escherichia coli (E coli; STEC).1 Between 6% and 15% of individuals infected with STEC develop HUS, with children affected more frequently than adults.2,3 This strain of E coli releases Shiga toxin into the systemic circulation, which causes a thrombotic microangiopathy resulting in the characteristic HUS triad of symptoms: acute renal insufficiency, thrombocytopenia, and hemolytic anemia.4-6
Although neurologic features are common in HUS, they have not been extensively studied, particularly in adults. We report a case of STEC 0157:H7 subtype HUS in an adult with severe neurologic complications. This case highlights the neurological sequelae in an adult with typical STEC-HUS. The use of treatment modalities, such as plasmapheresis and eculizumab, and their use in adult typical STEC-HUS also is explored.
Case
A 53-year-old white woman with no pertinent past medical history presented to the Bay Pines Veterans Affairs Healthcare System Emergency Department with a 2-day history of abdominal pain, vomiting, nausea, diarrhea, and bright bloody stools. She returned from a cruise to the Bahamas 3 days prior, where she ate local foods, including salads. She reported no fever, shortness of breath, chest pain, headache, and cognitive difficulties. She presented with a normal mental status and neurologic exam. Apart from leukocytosis and elevated glucose level, her laboratory results at initial presentation were normal, (Table). A stool sample showed occult blood with white blood cell counts (WBCs) but was negative for Clostridium difficile. She was started on ciprofloxacin 400 mg and metronidazole 500 mg on the day of admission.
Hematuria was found on hospital day 2. On hospital day 4, the patient exhibited word finding difficulties. Blood studies revealed anemia, thrombocytopenia, leukocytosis, and increasing blood urea nitrogen (BUN) and creatinine. A computed tomography scan of the head was normal. Laboratory analysis showed schistocytes in the peripheral blood smear.
The patient’s cognitive functioning deteriorated on hospital day 5. She was not oriented to time or place. Her laboratory results showed complement level C3 at 70 mg/dL (ref: 83-193 mg/dL) complement C4 at 12 mg/dL (ref: 15-57mg/dL). The patient exhibited oliguria and hyponatremia, as well as both metabolic and respiratory acidosis; dialysis was then initiated. Results from the stool sample that was collected on hospital day 1 were received and tested positive for Shiga toxin.
At this point, the patient’s presentation of hemolytic anemia and thrombocytopenia in the setting of acute bloody diarrheal illness with known Shiga toxin, schistocytes on blood smear, and lack of pertinent medical history for other causes of this presentation made STEC-HUS the leading differential diagnosis. Plasmapheresis was ordered and performed on hospital day 6 and 7. Shiga toxin was no longer detected in the stool and antibiotics were stopped on hospital day 7.
The patient’s progressive deterioration in mental status continued on hospital day 8. She was not oriented to time or place, unable to perform simple calculations, and could not spell the word “hand” backwards. Physicians observed repetitive jerking motions of the upper extremities that were worse on the left side. An electroencephalogram (EEG) revealed right hemispheric sharp waves that were thought to be epileptiform (Figure 1). The patient began taking levetiracetam 1500 mg IV with 750 mg bid maintenance for seizure control. Plasmapheresis was discontinued due to her continued neurologic deterioration on this therapy. Consequently, eculizumab 900 mg IV was given along with the Neisseria meningitidis (N meningitidis) vaccine and a 19-day course of azithromycin 250 mg po as prophylaxis for encapsulated bacteria.
The patient continued to seize on hospital days 10 through 13. Oculocephalic maneuvers showed a tendency to keep her eyes deviated to the right. Her pupils continued to react to light. A repeat EEG showed diffuse slowing (5-6 Hz) with no epileptic activity seen (Figure 2). A second dose of eculizumab 900 mg IV was administered on hospital day 15. The patient experienced cardiac arrest on hospital day 16 and was successfully resuscitated. On hospital day 25 (10 days after receiving her second dose of eculizumab), the patient was able to speak and follow simple commands but exhibited difficulty concentrating and poor impulse control.
The patient was alert and oriented to person, place, time, and situation on hospital day 28 (6 days after the third and final dose of eculizumab). A neurologic exam was significant only for a slight intention tremor. She was continued on levetiracetam with a plan to be maintained on the medication for the next 6 months for seizure control. She was discharged on hospital day 30.
Twenty-eight days postdischarge (57 days postadmission), the patient showed marked recovery. She had returned to her previous employment as a business administrator on a part-time basis and exhibited no deficiencies in executive functioning or handling activities of daily living. Although she had been very active prior to this illness, she now experienced decreased physical and mental endurance; however, this gradually improved with physical therapy. She planned on returning to work on a full-time basis when she had regained her stamina. She also noticed difficulties in retaining short term memory since her discharge but believed that these symptoms were remitting. On examination her mental status and neurologic exam was significant for inability to continue serial 7s, left sided 4/5 muscle strength in quadriceps and thumb to 5th metacarpal adduction, bilateral 1+ reflexes in muscle groups tested (triceps, biceps, brachioradialis, patellar, and Achilles), loss of dull pinprick sensation bilaterally at web of hands, deficit in tandem gait while looking away, and slight intention tremor on finger to nose testing bilaterally (with left hand tremor more pronounced than right). Her complete blood count was normal. Her recovery continues to be monitored in an outpatient setting.
Discussion
HUS is characterized by 3 core clinical features: microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury.4 Schistocytes are seen on peripheral blood smear and occur due to the passage of red blood cells over the microvascular thrombi induced by the disease. HUS can be classified as typical, atypical, or occurring with a coexisting disease. Typical HUS is associated with STEC 0157:H7 subtype, a bacterium known to be acquired through contaminated food and via human-to-human transmission.6-8 In the case of typical STEC 0157:H7, the bacterium releases a verotoxin that damages the vascular endothelium, thereby leading to activation of the coagulation cascade and eventually the formation of thrombi.4 It has been hypothesized that the Shiga toxin also activates the alternative complement pathway directly, which could contribute to thrombosis.9 This would explain the findings of low complement levels in our patient. Atypical HUS is primarily attributable to mutations in the alternative complement pathway. Causes for the third type of HUS can include Streptococcus pneumoniae, HIV, drug toxicity, and alterations in the metabolism of cobalamin C.
Epidemiologically, 15.3% of children aged < 5 years develop typical HUS after exposure to STEC compared with 1.2% of adults aged 18 to 59 years. The median age of patients who developed HUS from STEC exposure was 4 years compared with 16 years for those who did not develop HUS.2
Neurologic manifestations increase mortality for HUS patients.10 These have been described in the pediatric population as alteration in consciousness (85%), seizures (71%), pyramidal syndrome (52%), and extrapyramidal syndrome with hypertonia (42%).11 Brain imaging in children has demonstrated hemorrhagic lesions involving the pons, basal ganglia, and occipital cortex.11 Blood flow to areas such as the cerebellum, brainstem, and orbitofrontal area can be compromised.10 Adult patients with HUS can present without lesions on cranial magnetic resonance imaging (MRI), but instead with transient symmetric vasogenic edema of the central brain stem.12 Unfortunately in this case, MRI was not performed because it was thought to provide limited aid in diagnosis and to avoid unnecessary testing for the acutely ill patient.
The underlying pathophysiology of neurologic manifestations in patients may be due to a metabolic disturbance, toxin-mediated damage of the vascular endothelium, or toxin-induced cytokine release resulting in death of neural cells and subsequent neuroinflammation. However, the most likely mechanism is parenchymal ischemic changes related to microangiopathy.11,13 Pediatric patients often experience seizures and altered mental status, and their EEGs display delta waves.13 This patient’s diffuse slowing on her second EEG and altered mental status suggests that the neuropathologic mechanisms for typical HUS in adults may be similar to those in children.
HUS Treatment
The treatment and management of adults with typical STEC-HUS is evolving. The patient was first suspected to have an infectious colitis and empiric antibiotics were initiated. Some studies suggest that antibiotic administration may worsen the course of HUS in children as it may lead to release and subsequent absorption of Shiga toxin in the intestine.9,14 However, there is little evidence to suggest harm or efficacy of administration in adults. It is unclear what role antibiotic administration played in the recovery time of HUS given the co-administration of other treatments such as eculizumab and plasmapheresis, but it does appear to have helped with the initial E coli infection.
Plasmapheresis was subsequently administered, due to its documented benefit in the treatment of HUS.15 However, it should be noted that even though plasmapheresis is currently used in patients with CNS involvement, it remains unproven with conflicting information on its efficacy.3,16 The mechanism of action is unclear, but it has been hypothesized that plasmapheresis prevents microangiopathy caused by microthrombi.3,16 For this reason, eculizumab is becoming the mainstay for treatment of STEC-HUS with neurologic complications given the lack of well researched alternative treatments. In this case study, the use of plasmapheresis did not result in clinical improvement, and was abandoned after 2 days of treatment.
Eculizumab is a humanized, recombinant monoclonal IgG antibody that is a terminal complement inhibitor of the alternative complement system at the final step to cleave C5.17 The Shiga toxin may directly activate the complement system via the alternative pathway, which can result in uncontrolled platelet and white blood cell activation and depletion, endothelial cell damage, and hemolysis. The galvanized complement system leads to a series of cascading events that contribute to organ damage and death.9 Eculizumab is FDA approved for use in atypical HUS.18 It also can be used off-label to treat typical-HUS in adults with neurologic complications.
Eculizumab interferes with the immune response against encapsulated bacteria because it inhibits the alternative complement pathway. Thus, vaccination against N meningitides is recommended 2 weeks prior to the administration of eculizumab. However, in situations where the risks of delaying eculizumab for 2 weeks are greater than the risk of developing an N meningitides infection, eculizumab may be given without delay.18 Given the rapid deterioration of our patient’s condition, the vaccine and eculizumab were given together with prophylactic azithromycin. Although penicillin is the standard for prophylaxis in this situation, the patient’s penicillin allergy led to the use of azithromycin 250 mg po once a day. Literature also suggests azithromycin reduces the carriage duration of E coli-induced colitis.19 As such, it is possible that some improvement in the patient’s condition could be attributed to the elimination of the pathogen and toxin.
Conclusion
Three doses of eculizumab were administered at weekly intervals, with the first dose on hospital day 8 and the final dose on hospital day 22. Prior to the first dose, the patient displayed significant decline in mental status with EEG findings of right hemisphere epileptogenic discharges. After her third dose, she was found to have a drastically improved mental status exam and a normal EEG. One week later, she was discharged home. At the time of her 1-month follow-up, she was independent in all activities of daily living and had returned to part-time work. Apart from subtle cognitive changes, the remainder of her neurologic exam was normal.
There is evidence that supports the efficacy of eculizumab in children with HUS with neurologic symptoms on dialysis.20 However, its use in adults is not well established.21 This patient required dialysis and had neurologic symptoms similar to pediatric patients described in the literature, and responded similarly to the eculizumab. The rationale for the use of eculizumab in STEC-HUS also is evidenced by in vitro demonstrations of complement activation in STEC-HUS.22-25 This case report adds to the literature supporting the use of eculizumab in adult patients with typical HUS with neurological complications. Further research is necessary to develop guidelines in the treatment of adult STEC-HUS with regards to neurologic complications.
Acknowledgments
The authors would like to thank Pete DiStaso, REEGT for his work on obtaining the electroencephalograms and Anthony Rinaldi, PsyD; Julie Cessnapalas, PsyD; and Syed Faizan Sagheer for proof-reading the article.
Hemolytic uremic syndrome (HUS) is a rare illness that can be acquired through the consumption of food products contaminated with strains of Shiga toxin-producing Escherichia coli (E coli; STEC).1 Between 6% and 15% of individuals infected with STEC develop HUS, with children affected more frequently than adults.2,3 This strain of E coli releases Shiga toxin into the systemic circulation, which causes a thrombotic microangiopathy resulting in the characteristic HUS triad of symptoms: acute renal insufficiency, thrombocytopenia, and hemolytic anemia.4-6
Although neurologic features are common in HUS, they have not been extensively studied, particularly in adults. We report a case of STEC 0157:H7 subtype HUS in an adult with severe neurologic complications. This case highlights the neurological sequelae in an adult with typical STEC-HUS. The use of treatment modalities, such as plasmapheresis and eculizumab, and their use in adult typical STEC-HUS also is explored.
Case
A 53-year-old white woman with no pertinent past medical history presented to the Bay Pines Veterans Affairs Healthcare System Emergency Department with a 2-day history of abdominal pain, vomiting, nausea, diarrhea, and bright bloody stools. She returned from a cruise to the Bahamas 3 days prior, where she ate local foods, including salads. She reported no fever, shortness of breath, chest pain, headache, and cognitive difficulties. She presented with a normal mental status and neurologic exam. Apart from leukocytosis and elevated glucose level, her laboratory results at initial presentation were normal, (Table). A stool sample showed occult blood with white blood cell counts (WBCs) but was negative for Clostridium difficile. She was started on ciprofloxacin 400 mg and metronidazole 500 mg on the day of admission.
Hematuria was found on hospital day 2. On hospital day 4, the patient exhibited word finding difficulties. Blood studies revealed anemia, thrombocytopenia, leukocytosis, and increasing blood urea nitrogen (BUN) and creatinine. A computed tomography scan of the head was normal. Laboratory analysis showed schistocytes in the peripheral blood smear.
The patient’s cognitive functioning deteriorated on hospital day 5. She was not oriented to time or place. Her laboratory results showed complement level C3 at 70 mg/dL (ref: 83-193 mg/dL) complement C4 at 12 mg/dL (ref: 15-57mg/dL). The patient exhibited oliguria and hyponatremia, as well as both metabolic and respiratory acidosis; dialysis was then initiated. Results from the stool sample that was collected on hospital day 1 were received and tested positive for Shiga toxin.
At this point, the patient’s presentation of hemolytic anemia and thrombocytopenia in the setting of acute bloody diarrheal illness with known Shiga toxin, schistocytes on blood smear, and lack of pertinent medical history for other causes of this presentation made STEC-HUS the leading differential diagnosis. Plasmapheresis was ordered and performed on hospital day 6 and 7. Shiga toxin was no longer detected in the stool and antibiotics were stopped on hospital day 7.
The patient’s progressive deterioration in mental status continued on hospital day 8. She was not oriented to time or place, unable to perform simple calculations, and could not spell the word “hand” backwards. Physicians observed repetitive jerking motions of the upper extremities that were worse on the left side. An electroencephalogram (EEG) revealed right hemispheric sharp waves that were thought to be epileptiform (Figure 1). The patient began taking levetiracetam 1500 mg IV with 750 mg bid maintenance for seizure control. Plasmapheresis was discontinued due to her continued neurologic deterioration on this therapy. Consequently, eculizumab 900 mg IV was given along with the Neisseria meningitidis (N meningitidis) vaccine and a 19-day course of azithromycin 250 mg po as prophylaxis for encapsulated bacteria.
The patient continued to seize on hospital days 10 through 13. Oculocephalic maneuvers showed a tendency to keep her eyes deviated to the right. Her pupils continued to react to light. A repeat EEG showed diffuse slowing (5-6 Hz) with no epileptic activity seen (Figure 2). A second dose of eculizumab 900 mg IV was administered on hospital day 15. The patient experienced cardiac arrest on hospital day 16 and was successfully resuscitated. On hospital day 25 (10 days after receiving her second dose of eculizumab), the patient was able to speak and follow simple commands but exhibited difficulty concentrating and poor impulse control.
The patient was alert and oriented to person, place, time, and situation on hospital day 28 (6 days after the third and final dose of eculizumab). A neurologic exam was significant only for a slight intention tremor. She was continued on levetiracetam with a plan to be maintained on the medication for the next 6 months for seizure control. She was discharged on hospital day 30.
Twenty-eight days postdischarge (57 days postadmission), the patient showed marked recovery. She had returned to her previous employment as a business administrator on a part-time basis and exhibited no deficiencies in executive functioning or handling activities of daily living. Although she had been very active prior to this illness, she now experienced decreased physical and mental endurance; however, this gradually improved with physical therapy. She planned on returning to work on a full-time basis when she had regained her stamina. She also noticed difficulties in retaining short term memory since her discharge but believed that these symptoms were remitting. On examination her mental status and neurologic exam was significant for inability to continue serial 7s, left sided 4/5 muscle strength in quadriceps and thumb to 5th metacarpal adduction, bilateral 1+ reflexes in muscle groups tested (triceps, biceps, brachioradialis, patellar, and Achilles), loss of dull pinprick sensation bilaterally at web of hands, deficit in tandem gait while looking away, and slight intention tremor on finger to nose testing bilaterally (with left hand tremor more pronounced than right). Her complete blood count was normal. Her recovery continues to be monitored in an outpatient setting.
Discussion
HUS is characterized by 3 core clinical features: microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury.4 Schistocytes are seen on peripheral blood smear and occur due to the passage of red blood cells over the microvascular thrombi induced by the disease. HUS can be classified as typical, atypical, or occurring with a coexisting disease. Typical HUS is associated with STEC 0157:H7 subtype, a bacterium known to be acquired through contaminated food and via human-to-human transmission.6-8 In the case of typical STEC 0157:H7, the bacterium releases a verotoxin that damages the vascular endothelium, thereby leading to activation of the coagulation cascade and eventually the formation of thrombi.4 It has been hypothesized that the Shiga toxin also activates the alternative complement pathway directly, which could contribute to thrombosis.9 This would explain the findings of low complement levels in our patient. Atypical HUS is primarily attributable to mutations in the alternative complement pathway. Causes for the third type of HUS can include Streptococcus pneumoniae, HIV, drug toxicity, and alterations in the metabolism of cobalamin C.
Epidemiologically, 15.3% of children aged < 5 years develop typical HUS after exposure to STEC compared with 1.2% of adults aged 18 to 59 years. The median age of patients who developed HUS from STEC exposure was 4 years compared with 16 years for those who did not develop HUS.2
Neurologic manifestations increase mortality for HUS patients.10 These have been described in the pediatric population as alteration in consciousness (85%), seizures (71%), pyramidal syndrome (52%), and extrapyramidal syndrome with hypertonia (42%).11 Brain imaging in children has demonstrated hemorrhagic lesions involving the pons, basal ganglia, and occipital cortex.11 Blood flow to areas such as the cerebellum, brainstem, and orbitofrontal area can be compromised.10 Adult patients with HUS can present without lesions on cranial magnetic resonance imaging (MRI), but instead with transient symmetric vasogenic edema of the central brain stem.12 Unfortunately in this case, MRI was not performed because it was thought to provide limited aid in diagnosis and to avoid unnecessary testing for the acutely ill patient.
The underlying pathophysiology of neurologic manifestations in patients may be due to a metabolic disturbance, toxin-mediated damage of the vascular endothelium, or toxin-induced cytokine release resulting in death of neural cells and subsequent neuroinflammation. However, the most likely mechanism is parenchymal ischemic changes related to microangiopathy.11,13 Pediatric patients often experience seizures and altered mental status, and their EEGs display delta waves.13 This patient’s diffuse slowing on her second EEG and altered mental status suggests that the neuropathologic mechanisms for typical HUS in adults may be similar to those in children.
HUS Treatment
The treatment and management of adults with typical STEC-HUS is evolving. The patient was first suspected to have an infectious colitis and empiric antibiotics were initiated. Some studies suggest that antibiotic administration may worsen the course of HUS in children as it may lead to release and subsequent absorption of Shiga toxin in the intestine.9,14 However, there is little evidence to suggest harm or efficacy of administration in adults. It is unclear what role antibiotic administration played in the recovery time of HUS given the co-administration of other treatments such as eculizumab and plasmapheresis, but it does appear to have helped with the initial E coli infection.
Plasmapheresis was subsequently administered, due to its documented benefit in the treatment of HUS.15 However, it should be noted that even though plasmapheresis is currently used in patients with CNS involvement, it remains unproven with conflicting information on its efficacy.3,16 The mechanism of action is unclear, but it has been hypothesized that plasmapheresis prevents microangiopathy caused by microthrombi.3,16 For this reason, eculizumab is becoming the mainstay for treatment of STEC-HUS with neurologic complications given the lack of well researched alternative treatments. In this case study, the use of plasmapheresis did not result in clinical improvement, and was abandoned after 2 days of treatment.
Eculizumab is a humanized, recombinant monoclonal IgG antibody that is a terminal complement inhibitor of the alternative complement system at the final step to cleave C5.17 The Shiga toxin may directly activate the complement system via the alternative pathway, which can result in uncontrolled platelet and white blood cell activation and depletion, endothelial cell damage, and hemolysis. The galvanized complement system leads to a series of cascading events that contribute to organ damage and death.9 Eculizumab is FDA approved for use in atypical HUS.18 It also can be used off-label to treat typical-HUS in adults with neurologic complications.
Eculizumab interferes with the immune response against encapsulated bacteria because it inhibits the alternative complement pathway. Thus, vaccination against N meningitides is recommended 2 weeks prior to the administration of eculizumab. However, in situations where the risks of delaying eculizumab for 2 weeks are greater than the risk of developing an N meningitides infection, eculizumab may be given without delay.18 Given the rapid deterioration of our patient’s condition, the vaccine and eculizumab were given together with prophylactic azithromycin. Although penicillin is the standard for prophylaxis in this situation, the patient’s penicillin allergy led to the use of azithromycin 250 mg po once a day. Literature also suggests azithromycin reduces the carriage duration of E coli-induced colitis.19 As such, it is possible that some improvement in the patient’s condition could be attributed to the elimination of the pathogen and toxin.
Conclusion
Three doses of eculizumab were administered at weekly intervals, with the first dose on hospital day 8 and the final dose on hospital day 22. Prior to the first dose, the patient displayed significant decline in mental status with EEG findings of right hemisphere epileptogenic discharges. After her third dose, she was found to have a drastically improved mental status exam and a normal EEG. One week later, she was discharged home. At the time of her 1-month follow-up, she was independent in all activities of daily living and had returned to part-time work. Apart from subtle cognitive changes, the remainder of her neurologic exam was normal.
There is evidence that supports the efficacy of eculizumab in children with HUS with neurologic symptoms on dialysis.20 However, its use in adults is not well established.21 This patient required dialysis and had neurologic symptoms similar to pediatric patients described in the literature, and responded similarly to the eculizumab. The rationale for the use of eculizumab in STEC-HUS also is evidenced by in vitro demonstrations of complement activation in STEC-HUS.22-25 This case report adds to the literature supporting the use of eculizumab in adult patients with typical HUS with neurological complications. Further research is necessary to develop guidelines in the treatment of adult STEC-HUS with regards to neurologic complications.
Acknowledgments
The authors would like to thank Pete DiStaso, REEGT for his work on obtaining the electroencephalograms and Anthony Rinaldi, PsyD; Julie Cessnapalas, PsyD; and Syed Faizan Sagheer for proof-reading the article.
1. Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-1086.
2. Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clin Infect Dis. 2009;49(10):1480-1485.
3. Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333(6):364-368.
4. Rondeau E, Peraldi MN. Escherichia coli and the hemolytic-uremic syndrome. N Engl J Med. 1996;335(9):660-662.
5. Te Loo DM, van Hinsbergh VW, van den Heuvel LP, Monnens LA. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J Am Soc Nephrol. 2001;12(4):800-806.
6. Tran SL, Jenkins C, Livrelli V, Schüller S. Shiga toxin 2 translocation across intestinal epithelium is linked to virulence of Shiga toxin-producing Escherichia coli in humans. Microbiology. 2018;164(4):509-516.
7. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856.
8. Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis. 2011;8(4):465-487.
9. Percheron L, Gramada R, Tellier S, et al. Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol. 2018;33(8):1385-1394.
10. Hosaka T, Nakamagoe K, Tamaoka A. Hemolytic uremic syndrome-associated encephalopathy successfully treated with corticosteroids. Intern Med. 2017;56(21):2937-2941.
11. Nathanson S, Kwon T, Elmaleh M, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5(7):1218-1228.
12. Wengenroth M, Hoeltje J, Repenthin J, et al. Central nervous system involvement in adults with epidemic hemolytic uremic syndrome. AJNR Am J Neuroradiol. 2013;34(5):1016-1021, S1.
13. Eriksson KJ, Boyd SG, Tasker RC. Acute neurology and neurophysiology of haemolytic-uraemic syndrome. Arch Dis Child. 2001;84(5):434-435.
14. Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342(26):1930-1936.
15. Nguyen TC, Kiss JE, Goldman JR, Carcillo JA. The role of plasmapheresis in critical illness. Crit Care Clin. 2012;28(3):453-468, vii.
16. Loos S, Ahlenstiel T, Kranz B, et al. An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis. 2012;55(6):753-759.
17. Hossain MA, Cheema A, Kalathil S, et al. Atypical hemolytic uremic syndrome: Laboratory characteristics, complement-amplifying conditions, renal biopsy, and genetic mutations. Saudi J Kidney Dis Transpl. 2018;29(2):276-283.
18. Soliris (eculizumab) [package insert]. Cheshire, CT: Alexion Pharmaceuticals, Inc; 2011.
19. Keenswijk W, Raes A, Vande Walle J. Is eculizumab efficacious in Shigatoxin-associated hemolytic uremic syndrome? A narrative review of current evidence. Eur J Pediatr. 2018;177(3):311-318.
20. Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364(26):2561-2563.
21. Pape L, Hartmann H, Bange FC, Suerbaum S, Bueltmann E, Ahlenstiel-Grunow T. Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine (Baltimore). 2015;94(24):e1000.
22. Kim Y, Miller K, Michael AF. Breakdown products of C3 and factor B in hemolytic-uremic syndrome. J Lab Clin Med. 1977;89(4):845-850.
23. Monnens L, Molenaar J, Lambert PH, Proesmans W, van Munster P. The complement system in hemolytic-uremic syndrome in childhood. Clin Nephrol. 1980;13(4):168-171.
24. Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4(12):1920-1924.
25. Ståhl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117(20):5503-5513.
1. Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-1086.
2. Gould LH, Demma L, Jones TF, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clin Infect Dis. 2009;49(10):1480-1485.
3. Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333(6):364-368.
4. Rondeau E, Peraldi MN. Escherichia coli and the hemolytic-uremic syndrome. N Engl J Med. 1996;335(9):660-662.
5. Te Loo DM, van Hinsbergh VW, van den Heuvel LP, Monnens LA. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J Am Soc Nephrol. 2001;12(4):800-806.
6. Tran SL, Jenkins C, Livrelli V, Schüller S. Shiga toxin 2 translocation across intestinal epithelium is linked to virulence of Shiga toxin-producing Escherichia coli in humans. Microbiology. 2018;164(4):509-516.
7. Jokiranta TS. HUS and atypical HUS. Blood. 2017;129(21):2847-2856.
8. Ferens WA, Hovde CJ. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis. 2011;8(4):465-487.
9. Percheron L, Gramada R, Tellier S, et al. Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol. 2018;33(8):1385-1394.
10. Hosaka T, Nakamagoe K, Tamaoka A. Hemolytic uremic syndrome-associated encephalopathy successfully treated with corticosteroids. Intern Med. 2017;56(21):2937-2941.
11. Nathanson S, Kwon T, Elmaleh M, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5(7):1218-1228.
12. Wengenroth M, Hoeltje J, Repenthin J, et al. Central nervous system involvement in adults with epidemic hemolytic uremic syndrome. AJNR Am J Neuroradiol. 2013;34(5):1016-1021, S1.
13. Eriksson KJ, Boyd SG, Tasker RC. Acute neurology and neurophysiology of haemolytic-uraemic syndrome. Arch Dis Child. 2001;84(5):434-435.
14. Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342(26):1930-1936.
15. Nguyen TC, Kiss JE, Goldman JR, Carcillo JA. The role of plasmapheresis in critical illness. Crit Care Clin. 2012;28(3):453-468, vii.
16. Loos S, Ahlenstiel T, Kranz B, et al. An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis. 2012;55(6):753-759.
17. Hossain MA, Cheema A, Kalathil S, et al. Atypical hemolytic uremic syndrome: Laboratory characteristics, complement-amplifying conditions, renal biopsy, and genetic mutations. Saudi J Kidney Dis Transpl. 2018;29(2):276-283.
18. Soliris (eculizumab) [package insert]. Cheshire, CT: Alexion Pharmaceuticals, Inc; 2011.
19. Keenswijk W, Raes A, Vande Walle J. Is eculizumab efficacious in Shigatoxin-associated hemolytic uremic syndrome? A narrative review of current evidence. Eur J Pediatr. 2018;177(3):311-318.
20. Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364(26):2561-2563.
21. Pape L, Hartmann H, Bange FC, Suerbaum S, Bueltmann E, Ahlenstiel-Grunow T. Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine (Baltimore). 2015;94(24):e1000.
22. Kim Y, Miller K, Michael AF. Breakdown products of C3 and factor B in hemolytic-uremic syndrome. J Lab Clin Med. 1977;89(4):845-850.
23. Monnens L, Molenaar J, Lambert PH, Proesmans W, van Munster P. The complement system in hemolytic-uremic syndrome in childhood. Clin Nephrol. 1980;13(4):168-171.
24. Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4(12):1920-1924.
25. Ståhl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117(20):5503-5513.
Starting to assess the toll
This morning, Megan A. Adams (a GI & Hepatology News Associate Editor and a Michigan faculty member) and I held an hour-long video conference with all of our Michigan GI fellows. Our four third-year fellows talked about their job search and employment plans for July. Three will join academic centers (UNC, University of Wisconsin, Henry Ford) and one will enter private practice (Atlanta Gastroenterology). I was glad to hear that all had been reassured that their positions were secure despite the COVID-19 impact. As I speak with colleagues across the country, all (whether health system physicians, academic faculty, or community gastroenterologists) are experiencing the financial, emotional, and operational effects of this pandemic. This is an experience that will define our professional careers.
As one of three chief clinical officers at Michigan Medicine, I am part of a four-person team that leads the faculty medical group and the ambulatory portion of our health system. Each of our segments (ambulatory, adult hospital, children’s hospital, and medical school) have targets for sustained cost reductions that total $400 million and Michigan Medicine (as published in the news) plans to reduce our workforce (nonfaculty) by 1,400. We have a hiring freeze, leaders are taking salary reductions, and we have instituted other painful, cost-saving measures. The physician leaders we hired just 12 months ago to oversee a new faculty group structure were thrust into a firestorm. Department chairs, division chiefs, nursing and administrative leaders all are having to make heart-wrenching cost-cutting decisions. Together, we have to make individual reductions in force or retain decisions about people we work with daily. This emotional toll will never truly heal for anyone involved.
There will be little time to recover. We are scrambling to reopen safely, with a planned process. We have a backlog of 12,000 surgeries and 8,000 endoscopy procedures that have been deferred. Eight-hundred children are behind in their well-child medical care, frightened patients are sitting home with critical aortic stenosis, dangerous hypertension, growing cancers, and other urgent medical needs. Private practices are facing the same issues, financial pressures, and emotional toll.
Anna Quindlen once said, “Grief is a whisper in the world, but a clamor within.” Recognize the toll this is taking and don’t be alone with your grief.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This morning, Megan A. Adams (a GI & Hepatology News Associate Editor and a Michigan faculty member) and I held an hour-long video conference with all of our Michigan GI fellows. Our four third-year fellows talked about their job search and employment plans for July. Three will join academic centers (UNC, University of Wisconsin, Henry Ford) and one will enter private practice (Atlanta Gastroenterology). I was glad to hear that all had been reassured that their positions were secure despite the COVID-19 impact. As I speak with colleagues across the country, all (whether health system physicians, academic faculty, or community gastroenterologists) are experiencing the financial, emotional, and operational effects of this pandemic. This is an experience that will define our professional careers.
As one of three chief clinical officers at Michigan Medicine, I am part of a four-person team that leads the faculty medical group and the ambulatory portion of our health system. Each of our segments (ambulatory, adult hospital, children’s hospital, and medical school) have targets for sustained cost reductions that total $400 million and Michigan Medicine (as published in the news) plans to reduce our workforce (nonfaculty) by 1,400. We have a hiring freeze, leaders are taking salary reductions, and we have instituted other painful, cost-saving measures. The physician leaders we hired just 12 months ago to oversee a new faculty group structure were thrust into a firestorm. Department chairs, division chiefs, nursing and administrative leaders all are having to make heart-wrenching cost-cutting decisions. Together, we have to make individual reductions in force or retain decisions about people we work with daily. This emotional toll will never truly heal for anyone involved.
There will be little time to recover. We are scrambling to reopen safely, with a planned process. We have a backlog of 12,000 surgeries and 8,000 endoscopy procedures that have been deferred. Eight-hundred children are behind in their well-child medical care, frightened patients are sitting home with critical aortic stenosis, dangerous hypertension, growing cancers, and other urgent medical needs. Private practices are facing the same issues, financial pressures, and emotional toll.
Anna Quindlen once said, “Grief is a whisper in the world, but a clamor within.” Recognize the toll this is taking and don’t be alone with your grief.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This morning, Megan A. Adams (a GI & Hepatology News Associate Editor and a Michigan faculty member) and I held an hour-long video conference with all of our Michigan GI fellows. Our four third-year fellows talked about their job search and employment plans for July. Three will join academic centers (UNC, University of Wisconsin, Henry Ford) and one will enter private practice (Atlanta Gastroenterology). I was glad to hear that all had been reassured that their positions were secure despite the COVID-19 impact. As I speak with colleagues across the country, all (whether health system physicians, academic faculty, or community gastroenterologists) are experiencing the financial, emotional, and operational effects of this pandemic. This is an experience that will define our professional careers.
As one of three chief clinical officers at Michigan Medicine, I am part of a four-person team that leads the faculty medical group and the ambulatory portion of our health system. Each of our segments (ambulatory, adult hospital, children’s hospital, and medical school) have targets for sustained cost reductions that total $400 million and Michigan Medicine (as published in the news) plans to reduce our workforce (nonfaculty) by 1,400. We have a hiring freeze, leaders are taking salary reductions, and we have instituted other painful, cost-saving measures. The physician leaders we hired just 12 months ago to oversee a new faculty group structure were thrust into a firestorm. Department chairs, division chiefs, nursing and administrative leaders all are having to make heart-wrenching cost-cutting decisions. Together, we have to make individual reductions in force or retain decisions about people we work with daily. This emotional toll will never truly heal for anyone involved.
There will be little time to recover. We are scrambling to reopen safely, with a planned process. We have a backlog of 12,000 surgeries and 8,000 endoscopy procedures that have been deferred. Eight-hundred children are behind in their well-child medical care, frightened patients are sitting home with critical aortic stenosis, dangerous hypertension, growing cancers, and other urgent medical needs. Private practices are facing the same issues, financial pressures, and emotional toll.
Anna Quindlen once said, “Grief is a whisper in the world, but a clamor within.” Recognize the toll this is taking and don’t be alone with your grief.
John I. Allen, MD, MBA, AGAF
Editor in Chief
The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Authors, Editors, and Reviewers
AUTHORS
Francisco Alvarez, MD
Associate Chief, Regional Pediatric Hospitalist Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Brian Alverson, MD
Director, Division of Pediatric Hospital Medicine
Hasbro Children’s Hospital
Professor of Pediatrics
Alpert School of Medicine, Bro
Providence, RI
Pneumonia
Eric Balighian, MD
Director, Pediatric Emergency Department
St. Agnes Hospital
Asistant Professor, Department of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Acute Abdominal Pain and Acute Abdomen
Julia Beauchamp-Walters, MD
Medical Director, Helen Bernardy Center for Medically Fragile Children
Medical Director, Home Care
Co-Medical Director, Emergency Transport Program
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Feeding Tubes
Pediatric Interfacility Transport
Eric Biondi, MD, MS
Director, Pediatric Hospital Medicine Division
Johns Hopkins Children’s Center
Associate Professor of Pediatrics
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Neonatal Fever
Rebecca Blankenberg, MD, MPH
Associate Chair of Education
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics and Emergency Medicine
Stanford University School of Medicine
Stanford, CA
Education
Colin Bridgeman, MD
Penn State Children’s Hospital
Assistant Professor of Pediatrics
Division of General Inpatient Pediatrics
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Jeffrey Brown, MD, MPH, CAP, FAAP
Texas Newborn Services/Pediatrix Medical Group
Clinical Professor of Pediatrics
University of Colorado School of Medicine
Fort Worth, TX
Business Practices
April O. Buchanan, MD
Associate Dean for Curriculum
Prisma Health Children’s Hospital at Greenville
Associate Professor of Clinical Pediatrics
University of South Carolina School of Medicine
Greenville, SC
Sepsis and Shock
Douglas Carlson, MD
Medical Director
HSHS St. John’s Children’s Hospital
Professor and Chair of Pediatrics
Southern Illinois University School of Medicine
Springfield, MO
Procedural Sedation
Pearl Chang, MD
Seattle Children’s Hospital
Assistant Professor
Department of Pediatrics, University of Washington
Seattle, WA
Neonatal Jaundice
Eric Coon, MD, MS
Co-Director, Pediatric Hospital Medicine Fellowship
Primary Children’s Medical Center
Assistant Professor of Pediatrics
University of Utah Health Science
Salt Lake City, UT
Research
Yasmeen N. Daud, MD
St. Louis Children’s Hospital
Associate Professor of Pediatrics
Washington University School of Medicine
St. Louise, MO
Oxygen Delivery and Airway Management
Sarah Denniston, MD, FAAP
Fellowship Director, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center
Assistant Professor of Pediatrics
Tufts University School of Medicine
Associate DIO for Quality and Safety
Tufts Medical Center
Boston, MA
Peri-procedural Care
Craig C. DeWolfe, MD, MEd, FAAP
Children’s National Health System
Director of Medical Student Education in Pediatrics
Associate Professor of Pediatrics,
George Washington University School of Medicine
Washington, DC
Brief Resolved Unexplained Event
Stephanie Anne Deutsch, MD, MS, FAAP
Section Chief, Child Abuse Pediatrics
Nemours/Alfred I. duPont Hospital for Children
Co-medical Director, CARE (Children at Risk Evaluation) Program
Assistant Clinical Professor of Pediatrics
Sidney Kimmel Medical College at Thomas Jefferson University
Wilmington, Delaware
Child Abuse and Neglect
Ami Doshi, MD
Medical Director, Inpatient Palliative Care Program
Rady Children’s Hospital San Diego
Clinical Associate Professor of Pediatrics
University of California San Diego School of Medicine
San Diego, CA
Palliative Care and Hospice
Erin Fisher, MD, FAAP, MHM
Medical Director, Quality Improvement
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Director, Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
San Diego, CA
Quality Improvement
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Mary Pat Gallagher, MD
Director, Pediatric Diabetes Center
Division of Pediatric Endocrinology, Hassenfeld Children’s Hospital
Assistant Professor
Department of Pediatrics
NYU Langone
New York, NY
Diabetes Mellitus
Amrit Gill, MD
Cleveland Clinic Children’s Hospital
Clinical Assistant Professor of Pediatrics
Case Western Reserve University School of Medicine
Cleveland, OH
Patient Safety
Veena Goel Jones, MD, FAAP
Medical Director, Digital Patient Experience, Sutter Health
Sutter Palo Alto Medical Foundation
Palo Alto, CA
Health Information Technology
Jeffrey Grill, MD
Vice Chair, Community Relations and Outreach
Chief, Division of Pediatric Hospital Medicine
Director, Just for Kids Hospitalist Service
Norton Children’s Hospital
Professor, Department of Pediatrics
University of Louisville School of Medicine
Louisville, KY
Constipation
Arun Gupta, MD
Director, Neonatal Hospitalist Program
Lucile Packard Children’s Hospital Stanford
Clinical Associate Professor, Pediatrics
Stanford University School of Medicine
Stanford, CA
Newborn Care and Delivery Room Management
Brian F Herbst Jr, MD
Medical Director, Hospital Medicine Adult Care
Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Internal Medicine and Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Daniel Hershey, MD, SFHM
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Non-invasive Monitoring
Kim Hoang, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Education
Alison Volpe Holmes, MD, MPH
Children’s Hospital at Dartmouth-Hitchcock
Associate Dean for Student Affairs, Career Advising
Vice-Chair for Education, Department of Pediatrics
Associate Professor of Pediatrics and of The Dartmouth Institute
Geisel School of Medicine at Dartmouth
Hanover, NH
Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome
Akshata Hopkins, MD, FAAP, FHM
Director, Pediatric Residency Program
Johns Hopkins All Children’s Hospital
Assistant Professor of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
High Value Care
Yemisi Jones, MD, FAAP, FHM
Co-Medical Director, Continuing Medical Education
Co-Director Liberty Simulation Education
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Clinical Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Intravenous Access and Phlebotomy
Alisa Khan, MD, MPH
Health Services Researcher
Division of General Pediatrics, Boston Children’s Hospital
Clinical Instructor in Pediatrics
Harvard Medical School
Boston, MA
Family Centered Care
Vivian Lee, MD
Children’s Hospital Los Angeles
Clinical Assistant Professor of Pediatrics
University of Southern California Keck School of Medicine
Los Angeles, CA
Altered Mental Status
Su-Ting T. Li, MD, MPH
Associate Vice Chair of Education
Pediatric Residency Program Director
University of California Davis Children’s Hospital
Professor of Pediatrics
University of California, Davis
Sacramento, CA
Skin and Soft Tissue Infections
Patricia S. Lye, MD, MEd, FAAP
Children’s Hospital of Wisconsin
Professor of Pediatrics, Retired
Medical College of Wisconsin
Milwaukee, WI
Handoffs and Transitions of Care
Tamara Maginot, PhD
Pediatric Psychologist
Program Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Assistant Professor, Department of Psychiatry
UC San Diego Eating Disorders Center for Treatment and Research Behavioral Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Christopher Maloney, MD, PhD, FAAP
Chief Medical Officer and Senior Vice President
Children’s Hospital & Medical Center
Professor of Pediatrics and Pediatric Critical Care
Department of Pediatrics
University of Nebraska Medical Center College of Medicine
Omaha, NE
Pediatric Advanced Life Support
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Failure to Thrive
Elizabeth Mannino Avila, MD
Rady Children’s Hospital
Assistant Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Kawasaki Disease
Alison Markowsky, MD, MSHS, FAAP
Medical Director
Children’s National Pediatric Hospitalist Program at Mary Washington Healthcare
Children’s National Health System
Assistant Professor of Pediatrics
The George Washington University School of Medicine & Health Sciences
Washington, DC
Newborn Care and Delivery Room Management
Michelle Marks, DO, FAAP, SFHM
Chair, Pediatric Hospital Medicine
Cleveland Clinic Children’s Hospital
Clinical Associate Professor
Cleveland Clinic Lerner College of Medicine, Case Western Reserve University
Cleveland, OH
Nutrition
Armand H. Matheny Antommaria, MD, PhD, FAAP
Lee Ault Carter Chair Pediatric Ethics and Pediatric Hospitalist
Cincinnati Children’s Hospital
Associate Professor of Clinical-Affiliated
University of Cincinnati School of Medicine
Cincinnati, OH
Ethics
Erich Maul, MD
Division Chief, Hospital Medicine
Medical Director, Acute Care and Progressive Care
Kentucky Children’s Hospital
Professor of Pediatrics
University of Kentucky School of Medicine
Lexington, KY
Electrocardiogram Interpretation
Rusty McCulloh, MD
Chief, Division of Hospital Medicine
Children’s Hospital & Medical Center
Associate Professor, Division of Hospital Medicine
University of Nebraska College of Medicine
Omaha, NE
Infection Control and Antimicrobial Stewardship
Anjna Melwani, MD
Director, Preoperative Care Clinic
Children’s National Medical Center
Associate Professor of Pediatrics
George Washington University School of Medicine and Health Sciences
Washington, DC
Consultation and Co-management
Christopher Miller, MD
Pediatric Allergist
Children’s Mercy Hospitals and Clinics
Assistant Professor of Pediatrics
Section of Allergy and Immunology
University of Missouri-Kansas City School of Medicine
Kansas City, MO
Asthma
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System Dallas
Dallas, TX
Acute Respiratory Failure
Beth Natt, MD, MPH, FAAP, SFHM
Director, Pediatric Hospital Medicine, Regional Programs
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Farmington, CT
Bladder Catheterization and Interpretation of Urinalysis
Jennifer O’Toole, MD, MEd, FAAP, SFHM
Program Director, Internal Medicine – Pediatrics Residency
Director of Education, Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Associate Professor of Pediatrics and Internal Medicine
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Mary Ottolini, MD, MPH, MEd, FAAP
George W. Hallett Chair of Pediatrics
Barbara Bush Children’s Hospital at Maine Medical Center
Professor of Pediatrics
Tufts University School of Medicine
Portland, ME
Fluid and Electrolyte Management
Jack Percelay, MD, MPH, FAAP, MHM
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics
Stanford University School of Medicine
Stanford, CA
Advocacy
Seizures
Shannon Phillips, MD, MPH
Chief Patient Safety and Experience Officer
Primary Children’s Medical Center
Intermountain Healthcare, Inc.
Adjunct Associate Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Patient Safety
David Pressel, MD, PhD, FAAP, FHM
Medical Director, Pediatric Hospitalist Program
Capital Health Medical Center- Hopewell
Pennington, NJ
Acute Behavioral and Psychiatric Conditions
Child Abuse and Neglect
Ricardo Quinonez, MD, FAAP
Chief, Pediatric Hospital Medicine
Texas Children’s Hospital
Associate Professor of Pediatrics
Baylor College of Medicine
Houston, TX
High Value Care
Shawn Ralston, MA, MD, MS
Johns Hopkins Children’s Center
Editor, Hospital Pediatrics, American Academy of Pediatrics
Associate Professor of Pediatrics
Division of Pediatric Quality and Safety
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Evidence Based Medicine
David I. Rappaport, MD, FAAP, FHM
Associate Residency Program Director
Division of General Pediatrics
Nemours/AI duPont Hospital for Children
Wilmington, DE
Associate Professor of Pediatrics
Sidney Kimmel Medical College at Jefferson
Philadelphia, PA
Consultation and Co-management
Daniel Rauch, MD, FAAP, SFHM
Chief, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center.
Professor of Pediatrics
Tufts University School of Medicine
Boston, MA
Preventive Care Services
Kyung (Kay) Rhee, MD, MSc, MA
Director of Research, Division of Pediatric Hospital Medicine
Medical Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Department of Pediatrics, Division of General Academic Pediatrics, Developmental Pediatrics, and Center for Community Health
University of San Diego School of Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Jeffrey Riese, MD
Associate Pediatric Residency Program Director
Hasbro Children’s Hospital
Associate Professor of Pediatrics
Warren Alpert School of Medicine at Brown University
Providence, RI
Neonatal Fever
Ken Roberts, MD, FAAP
Professor Emeritus of Pediatrics
University of North Carolina School of Medicine
Chapel Hill, NC
Urinary Tract Infections
Amanda Rogers, MD
Associate Pediatric Residency Program Director
Children’s Hospital of Wisconsin
Assistant Professor, Section of Hospital Medicine
Medical College of Wisconsin
Milwaukee, WI
Lumbar Puncture
Rebecca E. Rosenberg, MD, MPH
Chief, Section of Hospital Medicine, Division of General Pediatrics
Hassenfeld Children’s Hospital at NYU Langone Health
Associate Professor of Pediatrics
NYU School of Medicine
New York, NY
Peri-procedural Care
Michael Ruhlen, MD, MHCM, FHM, FACHE
Vice President, Division of Medical Education
Vice Chair, RRC ACGME
Atrium Health System
Charlotte, NC
Legal Issues and Risk Management
Christopher J. Russell, MD, MS, FAAP
Research Director, Division of Hospital Medicine
Children’s Hospital Los Angeles
Assistant Professor of Clinical Pediatrics
Keck School of Medicine, University of Southern California
Los Angeles, CA
Child with Medical Complexity
Christopher Russo, MD
Director of Pediatrics
Central Lynchburg General Hospital
Assistant Professor of Pediatrics
Liberty University College of Osteopathic Medicine
Lynchburg, VA
Advocacy
Klint M. Schwenk, MD, MBA, FAAP, FHM
Associate Division Chief, Pediatric Hospital Medicine
Norton Children’s Hospital
Associate Professor of Pediatrics
University of Louisville
Louisville, KY
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Anand Sekaran, MD, FAAP
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Diagnostic Imaging
Kristin A. Shadman, MD, FAAP
American Family Children’s Hospital
Associate Professor of Pediatrics
Division of Hospital Medicine
University of Wisconsin School of Medicine and Public Health
Madison, WI
Oxygen Delivery and Airway Management
Samir S. Shah, MD, MSCE
Director, Division of Hospital Medicine
James M. Ewell Endowed Chair
Attending Physician in Hospital Medicine & Infectious Diseases
Chief Metrics Officer
Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Bone and Joint Infections
Mark Shen, MD, MBA, FAAP, SFHM
Associate Professor of Pediatrics
Dell Medical School at the University of Texas at Austin
Austin, TX
Leadership in Healthcare
Tamara Simon, MD, MSPH, FAAP
Principal Investigator, Center for Clinical and Translational Research
Seattle Children’s Research Institute
Associate Professor of Pediatrics
Divisions of Hospital Medicine and General Pediatrics, Department of Pediatrics
University of Washington
Seattle, WA
Child with Medical Complexity
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine, Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
Communication
Karen Smith, MD, MEd, SFHM, FAAP
Chief, Division of Pediatric Hospital Medicine
Children’s National Medical Center
Associate Professor of Pediatrics
The George Washington School of Medicine and Health Sciences
Washington, DC
Business Practices
Nita Srinivas, MD
Pediatric Hospitalist and Infectious Disease Specialist
Fellowship Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Rajendu Srivastava, MD, FRCP(C), MPH
Primary Children’s Medical Center
Assistant Vice President of Research and Medical Director of the Office of Research
Intermountain Healthcare Inc.
Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Research
Lynne Sterni, MD
Pediatric Anesthesiology and Pain Medicine
Naval Medical Center San Diego
Assistant Professor
Uniformed Services University School of Health Sciences
San Diego, CA
Pain Management
E. Douglas Thompson Jr, MD, FAAP
Chief, Section of Hospital Medicine
Associate Chair, Access and Partnerships
St. Christopher’s Hospital for Children
Associate Professor of Pediatrics
Drexel University School of Medicine and Health Sciences
Philadelphia, PA
Sickle Cell Disease
Joanna Thomson, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor, Department of Pediatrics
University of Cincinnati School of Medicine
Cincinnati, OH
Acute Respiratory Failure
Joel Tieder, MD, MPH
Seattle Children’s Hospital
Associate Professor of Pediatrics, Division of Hospital Medicine
University of Washington School of Medicine
Seattle, WA
Brief Resolved Unexplained Event
Adriana Tremoulet, MD, MAS
Associate Director, Kawasaki Disease Research Center
Division of Host-Microbe Systems and Therapeutics
Pediatric Infectious Diseases and Kawasaki Disease
Associate Professor of Pediatrics, University of California San Diego
San Diego, CA
Kawasaki Disease
Marie E. Wang, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Central Nervous System Infections
Ronald Williams, MD, FAAP, FACP
Director, Combined Internal Medicine/Pediatrics Residency Program
Penn State Hershey Children’s Hospital
Professor of Pediatrics and Medicine
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Susan Wu, MD, FAAP
Children’s Hospital Los Angeles
Associate Professor of Clinical Pediatrics
Division of Hospital Medicine, Department of Pediatrics
USC Keck School of Medicine
Los Angeles, CA
Bronchiolitis
EDITORS
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Erin Fisher, MD, MHM, FAAP
Medical Director Quality Improvement
Rady Children’s Hospital
Professor of Clinical Pediatrics
Director of Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
Department of Pediatrics
San Diego, CA
CONTRIBUTING EDITOR, COMMUNITY PERSPECTIVE EXPERTISE
Sofia Teferi, MD, FAAP, SFHM
Physician Executive
Richmond, VA
ASSOCIATE EDITORS
Francisco Alvarez, MD, FAAP
Associate Chief, Regional Pediatric Hospital Medicine Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford School of Medicine
Stanford, CA
Michael Burke, MD (1957 – 2019)
In memory: Chairman of Pediatrics
Saint Agnes Hospital
Associate Professor of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Weijen Chang, MD
Division Chief, Pediatric Hospital Medicine
Vice Chair for Clinical Affairs, Department of Pediatrics
Baystate Children’s Hospital
Associate Professor of Pediatrics
University of Massachusetts Medical School-Baystate
Springfield, MA
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System, Dallas
Dallas, TX
Anand Sekaran, MD
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine
Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
EXTERNAL REVIEWERS
Academic Pediatric Association Hospital Medicine Special Interest Group
American Academy of Pediatrics
- Committee on Psychological Aspects of Child and Family Health
- Council on Children with Disabilities
- Council on Community Pediatrics
- Disaster Preparedness Advisory Council
- Family Partnerships Network
- Section on Anesthesiology and Pain Medicine
- Section on Breastfeeding
- Section on Cardiology and Cardiac Surgery
- Section on Critical Care
- Section on Hematology/Oncology
- Section on Hospice and Palliative Medicine
- Section on Hospital Medicine
- Section on LGBT Health and Wellness
- Section on Medicine-Pediatrics
- Section on Nephrology
- Section on Neurology
- Section on Pediatric Trainees
- Section on Surgery
- Section on Transport Medicine
- Section on Urology
Association of Pediatric Program Directors Curriculum Committee
Society of Hospital Medicine Pediatrics Special Interest Group
Society of Hospital Medicine Medicine-Pediatrics Special Interest Group
AUTHORS
Francisco Alvarez, MD
Associate Chief, Regional Pediatric Hospitalist Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Brian Alverson, MD
Director, Division of Pediatric Hospital Medicine
Hasbro Children’s Hospital
Professor of Pediatrics
Alpert School of Medicine, Bro
Providence, RI
Pneumonia
Eric Balighian, MD
Director, Pediatric Emergency Department
St. Agnes Hospital
Asistant Professor, Department of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Acute Abdominal Pain and Acute Abdomen
Julia Beauchamp-Walters, MD
Medical Director, Helen Bernardy Center for Medically Fragile Children
Medical Director, Home Care
Co-Medical Director, Emergency Transport Program
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Feeding Tubes
Pediatric Interfacility Transport
Eric Biondi, MD, MS
Director, Pediatric Hospital Medicine Division
Johns Hopkins Children’s Center
Associate Professor of Pediatrics
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Neonatal Fever
Rebecca Blankenberg, MD, MPH
Associate Chair of Education
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics and Emergency Medicine
Stanford University School of Medicine
Stanford, CA
Education
Colin Bridgeman, MD
Penn State Children’s Hospital
Assistant Professor of Pediatrics
Division of General Inpatient Pediatrics
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Jeffrey Brown, MD, MPH, CAP, FAAP
Texas Newborn Services/Pediatrix Medical Group
Clinical Professor of Pediatrics
University of Colorado School of Medicine
Fort Worth, TX
Business Practices
April O. Buchanan, MD
Associate Dean for Curriculum
Prisma Health Children’s Hospital at Greenville
Associate Professor of Clinical Pediatrics
University of South Carolina School of Medicine
Greenville, SC
Sepsis and Shock
Douglas Carlson, MD
Medical Director
HSHS St. John’s Children’s Hospital
Professor and Chair of Pediatrics
Southern Illinois University School of Medicine
Springfield, MO
Procedural Sedation
Pearl Chang, MD
Seattle Children’s Hospital
Assistant Professor
Department of Pediatrics, University of Washington
Seattle, WA
Neonatal Jaundice
Eric Coon, MD, MS
Co-Director, Pediatric Hospital Medicine Fellowship
Primary Children’s Medical Center
Assistant Professor of Pediatrics
University of Utah Health Science
Salt Lake City, UT
Research
Yasmeen N. Daud, MD
St. Louis Children’s Hospital
Associate Professor of Pediatrics
Washington University School of Medicine
St. Louise, MO
Oxygen Delivery and Airway Management
Sarah Denniston, MD, FAAP
Fellowship Director, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center
Assistant Professor of Pediatrics
Tufts University School of Medicine
Associate DIO for Quality and Safety
Tufts Medical Center
Boston, MA
Peri-procedural Care
Craig C. DeWolfe, MD, MEd, FAAP
Children’s National Health System
Director of Medical Student Education in Pediatrics
Associate Professor of Pediatrics,
George Washington University School of Medicine
Washington, DC
Brief Resolved Unexplained Event
Stephanie Anne Deutsch, MD, MS, FAAP
Section Chief, Child Abuse Pediatrics
Nemours/Alfred I. duPont Hospital for Children
Co-medical Director, CARE (Children at Risk Evaluation) Program
Assistant Clinical Professor of Pediatrics
Sidney Kimmel Medical College at Thomas Jefferson University
Wilmington, Delaware
Child Abuse and Neglect
Ami Doshi, MD
Medical Director, Inpatient Palliative Care Program
Rady Children’s Hospital San Diego
Clinical Associate Professor of Pediatrics
University of California San Diego School of Medicine
San Diego, CA
Palliative Care and Hospice
Erin Fisher, MD, FAAP, MHM
Medical Director, Quality Improvement
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Director, Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
San Diego, CA
Quality Improvement
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Mary Pat Gallagher, MD
Director, Pediatric Diabetes Center
Division of Pediatric Endocrinology, Hassenfeld Children’s Hospital
Assistant Professor
Department of Pediatrics
NYU Langone
New York, NY
Diabetes Mellitus
Amrit Gill, MD
Cleveland Clinic Children’s Hospital
Clinical Assistant Professor of Pediatrics
Case Western Reserve University School of Medicine
Cleveland, OH
Patient Safety
Veena Goel Jones, MD, FAAP
Medical Director, Digital Patient Experience, Sutter Health
Sutter Palo Alto Medical Foundation
Palo Alto, CA
Health Information Technology
Jeffrey Grill, MD
Vice Chair, Community Relations and Outreach
Chief, Division of Pediatric Hospital Medicine
Director, Just for Kids Hospitalist Service
Norton Children’s Hospital
Professor, Department of Pediatrics
University of Louisville School of Medicine
Louisville, KY
Constipation
Arun Gupta, MD
Director, Neonatal Hospitalist Program
Lucile Packard Children’s Hospital Stanford
Clinical Associate Professor, Pediatrics
Stanford University School of Medicine
Stanford, CA
Newborn Care and Delivery Room Management
Brian F Herbst Jr, MD
Medical Director, Hospital Medicine Adult Care
Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Internal Medicine and Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Daniel Hershey, MD, SFHM
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Non-invasive Monitoring
Kim Hoang, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Education
Alison Volpe Holmes, MD, MPH
Children’s Hospital at Dartmouth-Hitchcock
Associate Dean for Student Affairs, Career Advising
Vice-Chair for Education, Department of Pediatrics
Associate Professor of Pediatrics and of The Dartmouth Institute
Geisel School of Medicine at Dartmouth
Hanover, NH
Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome
Akshata Hopkins, MD, FAAP, FHM
Director, Pediatric Residency Program
Johns Hopkins All Children’s Hospital
Assistant Professor of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
High Value Care
Yemisi Jones, MD, FAAP, FHM
Co-Medical Director, Continuing Medical Education
Co-Director Liberty Simulation Education
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Clinical Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Intravenous Access and Phlebotomy
Alisa Khan, MD, MPH
Health Services Researcher
Division of General Pediatrics, Boston Children’s Hospital
Clinical Instructor in Pediatrics
Harvard Medical School
Boston, MA
Family Centered Care
Vivian Lee, MD
Children’s Hospital Los Angeles
Clinical Assistant Professor of Pediatrics
University of Southern California Keck School of Medicine
Los Angeles, CA
Altered Mental Status
Su-Ting T. Li, MD, MPH
Associate Vice Chair of Education
Pediatric Residency Program Director
University of California Davis Children’s Hospital
Professor of Pediatrics
University of California, Davis
Sacramento, CA
Skin and Soft Tissue Infections
Patricia S. Lye, MD, MEd, FAAP
Children’s Hospital of Wisconsin
Professor of Pediatrics, Retired
Medical College of Wisconsin
Milwaukee, WI
Handoffs and Transitions of Care
Tamara Maginot, PhD
Pediatric Psychologist
Program Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Assistant Professor, Department of Psychiatry
UC San Diego Eating Disorders Center for Treatment and Research Behavioral Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Christopher Maloney, MD, PhD, FAAP
Chief Medical Officer and Senior Vice President
Children’s Hospital & Medical Center
Professor of Pediatrics and Pediatric Critical Care
Department of Pediatrics
University of Nebraska Medical Center College of Medicine
Omaha, NE
Pediatric Advanced Life Support
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Failure to Thrive
Elizabeth Mannino Avila, MD
Rady Children’s Hospital
Assistant Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Kawasaki Disease
Alison Markowsky, MD, MSHS, FAAP
Medical Director
Children’s National Pediatric Hospitalist Program at Mary Washington Healthcare
Children’s National Health System
Assistant Professor of Pediatrics
The George Washington University School of Medicine & Health Sciences
Washington, DC
Newborn Care and Delivery Room Management
Michelle Marks, DO, FAAP, SFHM
Chair, Pediatric Hospital Medicine
Cleveland Clinic Children’s Hospital
Clinical Associate Professor
Cleveland Clinic Lerner College of Medicine, Case Western Reserve University
Cleveland, OH
Nutrition
Armand H. Matheny Antommaria, MD, PhD, FAAP
Lee Ault Carter Chair Pediatric Ethics and Pediatric Hospitalist
Cincinnati Children’s Hospital
Associate Professor of Clinical-Affiliated
University of Cincinnati School of Medicine
Cincinnati, OH
Ethics
Erich Maul, MD
Division Chief, Hospital Medicine
Medical Director, Acute Care and Progressive Care
Kentucky Children’s Hospital
Professor of Pediatrics
University of Kentucky School of Medicine
Lexington, KY
Electrocardiogram Interpretation
Rusty McCulloh, MD
Chief, Division of Hospital Medicine
Children’s Hospital & Medical Center
Associate Professor, Division of Hospital Medicine
University of Nebraska College of Medicine
Omaha, NE
Infection Control and Antimicrobial Stewardship
Anjna Melwani, MD
Director, Preoperative Care Clinic
Children’s National Medical Center
Associate Professor of Pediatrics
George Washington University School of Medicine and Health Sciences
Washington, DC
Consultation and Co-management
Christopher Miller, MD
Pediatric Allergist
Children’s Mercy Hospitals and Clinics
Assistant Professor of Pediatrics
Section of Allergy and Immunology
University of Missouri-Kansas City School of Medicine
Kansas City, MO
Asthma
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System Dallas
Dallas, TX
Acute Respiratory Failure
Beth Natt, MD, MPH, FAAP, SFHM
Director, Pediatric Hospital Medicine, Regional Programs
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Farmington, CT
Bladder Catheterization and Interpretation of Urinalysis
Jennifer O’Toole, MD, MEd, FAAP, SFHM
Program Director, Internal Medicine – Pediatrics Residency
Director of Education, Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Associate Professor of Pediatrics and Internal Medicine
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Mary Ottolini, MD, MPH, MEd, FAAP
George W. Hallett Chair of Pediatrics
Barbara Bush Children’s Hospital at Maine Medical Center
Professor of Pediatrics
Tufts University School of Medicine
Portland, ME
Fluid and Electrolyte Management
Jack Percelay, MD, MPH, FAAP, MHM
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics
Stanford University School of Medicine
Stanford, CA
Advocacy
Seizures
Shannon Phillips, MD, MPH
Chief Patient Safety and Experience Officer
Primary Children’s Medical Center
Intermountain Healthcare, Inc.
Adjunct Associate Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Patient Safety
David Pressel, MD, PhD, FAAP, FHM
Medical Director, Pediatric Hospitalist Program
Capital Health Medical Center- Hopewell
Pennington, NJ
Acute Behavioral and Psychiatric Conditions
Child Abuse and Neglect
Ricardo Quinonez, MD, FAAP
Chief, Pediatric Hospital Medicine
Texas Children’s Hospital
Associate Professor of Pediatrics
Baylor College of Medicine
Houston, TX
High Value Care
Shawn Ralston, MA, MD, MS
Johns Hopkins Children’s Center
Editor, Hospital Pediatrics, American Academy of Pediatrics
Associate Professor of Pediatrics
Division of Pediatric Quality and Safety
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Evidence Based Medicine
David I. Rappaport, MD, FAAP, FHM
Associate Residency Program Director
Division of General Pediatrics
Nemours/AI duPont Hospital for Children
Wilmington, DE
Associate Professor of Pediatrics
Sidney Kimmel Medical College at Jefferson
Philadelphia, PA
Consultation and Co-management
Daniel Rauch, MD, FAAP, SFHM
Chief, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center.
Professor of Pediatrics
Tufts University School of Medicine
Boston, MA
Preventive Care Services
Kyung (Kay) Rhee, MD, MSc, MA
Director of Research, Division of Pediatric Hospital Medicine
Medical Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Department of Pediatrics, Division of General Academic Pediatrics, Developmental Pediatrics, and Center for Community Health
University of San Diego School of Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Jeffrey Riese, MD
Associate Pediatric Residency Program Director
Hasbro Children’s Hospital
Associate Professor of Pediatrics
Warren Alpert School of Medicine at Brown University
Providence, RI
Neonatal Fever
Ken Roberts, MD, FAAP
Professor Emeritus of Pediatrics
University of North Carolina School of Medicine
Chapel Hill, NC
Urinary Tract Infections
Amanda Rogers, MD
Associate Pediatric Residency Program Director
Children’s Hospital of Wisconsin
Assistant Professor, Section of Hospital Medicine
Medical College of Wisconsin
Milwaukee, WI
Lumbar Puncture
Rebecca E. Rosenberg, MD, MPH
Chief, Section of Hospital Medicine, Division of General Pediatrics
Hassenfeld Children’s Hospital at NYU Langone Health
Associate Professor of Pediatrics
NYU School of Medicine
New York, NY
Peri-procedural Care
Michael Ruhlen, MD, MHCM, FHM, FACHE
Vice President, Division of Medical Education
Vice Chair, RRC ACGME
Atrium Health System
Charlotte, NC
Legal Issues and Risk Management
Christopher J. Russell, MD, MS, FAAP
Research Director, Division of Hospital Medicine
Children’s Hospital Los Angeles
Assistant Professor of Clinical Pediatrics
Keck School of Medicine, University of Southern California
Los Angeles, CA
Child with Medical Complexity
Christopher Russo, MD
Director of Pediatrics
Central Lynchburg General Hospital
Assistant Professor of Pediatrics
Liberty University College of Osteopathic Medicine
Lynchburg, VA
Advocacy
Klint M. Schwenk, MD, MBA, FAAP, FHM
Associate Division Chief, Pediatric Hospital Medicine
Norton Children’s Hospital
Associate Professor of Pediatrics
University of Louisville
Louisville, KY
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Anand Sekaran, MD, FAAP
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Diagnostic Imaging
Kristin A. Shadman, MD, FAAP
American Family Children’s Hospital
Associate Professor of Pediatrics
Division of Hospital Medicine
University of Wisconsin School of Medicine and Public Health
Madison, WI
Oxygen Delivery and Airway Management
Samir S. Shah, MD, MSCE
Director, Division of Hospital Medicine
James M. Ewell Endowed Chair
Attending Physician in Hospital Medicine & Infectious Diseases
Chief Metrics Officer
Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Bone and Joint Infections
Mark Shen, MD, MBA, FAAP, SFHM
Associate Professor of Pediatrics
Dell Medical School at the University of Texas at Austin
Austin, TX
Leadership in Healthcare
Tamara Simon, MD, MSPH, FAAP
Principal Investigator, Center for Clinical and Translational Research
Seattle Children’s Research Institute
Associate Professor of Pediatrics
Divisions of Hospital Medicine and General Pediatrics, Department of Pediatrics
University of Washington
Seattle, WA
Child with Medical Complexity
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine, Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
Communication
Karen Smith, MD, MEd, SFHM, FAAP
Chief, Division of Pediatric Hospital Medicine
Children’s National Medical Center
Associate Professor of Pediatrics
The George Washington School of Medicine and Health Sciences
Washington, DC
Business Practices
Nita Srinivas, MD
Pediatric Hospitalist and Infectious Disease Specialist
Fellowship Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Rajendu Srivastava, MD, FRCP(C), MPH
Primary Children’s Medical Center
Assistant Vice President of Research and Medical Director of the Office of Research
Intermountain Healthcare Inc.
Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Research
Lynne Sterni, MD
Pediatric Anesthesiology and Pain Medicine
Naval Medical Center San Diego
Assistant Professor
Uniformed Services University School of Health Sciences
San Diego, CA
Pain Management
E. Douglas Thompson Jr, MD, FAAP
Chief, Section of Hospital Medicine
Associate Chair, Access and Partnerships
St. Christopher’s Hospital for Children
Associate Professor of Pediatrics
Drexel University School of Medicine and Health Sciences
Philadelphia, PA
Sickle Cell Disease
Joanna Thomson, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor, Department of Pediatrics
University of Cincinnati School of Medicine
Cincinnati, OH
Acute Respiratory Failure
Joel Tieder, MD, MPH
Seattle Children’s Hospital
Associate Professor of Pediatrics, Division of Hospital Medicine
University of Washington School of Medicine
Seattle, WA
Brief Resolved Unexplained Event
Adriana Tremoulet, MD, MAS
Associate Director, Kawasaki Disease Research Center
Division of Host-Microbe Systems and Therapeutics
Pediatric Infectious Diseases and Kawasaki Disease
Associate Professor of Pediatrics, University of California San Diego
San Diego, CA
Kawasaki Disease
Marie E. Wang, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Central Nervous System Infections
Ronald Williams, MD, FAAP, FACP
Director, Combined Internal Medicine/Pediatrics Residency Program
Penn State Hershey Children’s Hospital
Professor of Pediatrics and Medicine
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Susan Wu, MD, FAAP
Children’s Hospital Los Angeles
Associate Professor of Clinical Pediatrics
Division of Hospital Medicine, Department of Pediatrics
USC Keck School of Medicine
Los Angeles, CA
Bronchiolitis
EDITORS
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Erin Fisher, MD, MHM, FAAP
Medical Director Quality Improvement
Rady Children’s Hospital
Professor of Clinical Pediatrics
Director of Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
Department of Pediatrics
San Diego, CA
CONTRIBUTING EDITOR, COMMUNITY PERSPECTIVE EXPERTISE
Sofia Teferi, MD, FAAP, SFHM
Physician Executive
Richmond, VA
ASSOCIATE EDITORS
Francisco Alvarez, MD, FAAP
Associate Chief, Regional Pediatric Hospital Medicine Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford School of Medicine
Stanford, CA
Michael Burke, MD (1957 – 2019)
In memory: Chairman of Pediatrics
Saint Agnes Hospital
Associate Professor of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Weijen Chang, MD
Division Chief, Pediatric Hospital Medicine
Vice Chair for Clinical Affairs, Department of Pediatrics
Baystate Children’s Hospital
Associate Professor of Pediatrics
University of Massachusetts Medical School-Baystate
Springfield, MA
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System, Dallas
Dallas, TX
Anand Sekaran, MD
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine
Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
EXTERNAL REVIEWERS
Academic Pediatric Association Hospital Medicine Special Interest Group
American Academy of Pediatrics
- Committee on Psychological Aspects of Child and Family Health
- Council on Children with Disabilities
- Council on Community Pediatrics
- Disaster Preparedness Advisory Council
- Family Partnerships Network
- Section on Anesthesiology and Pain Medicine
- Section on Breastfeeding
- Section on Cardiology and Cardiac Surgery
- Section on Critical Care
- Section on Hematology/Oncology
- Section on Hospice and Palliative Medicine
- Section on Hospital Medicine
- Section on LGBT Health and Wellness
- Section on Medicine-Pediatrics
- Section on Nephrology
- Section on Neurology
- Section on Pediatric Trainees
- Section on Surgery
- Section on Transport Medicine
- Section on Urology
Association of Pediatric Program Directors Curriculum Committee
Society of Hospital Medicine Pediatrics Special Interest Group
Society of Hospital Medicine Medicine-Pediatrics Special Interest Group
AUTHORS
Francisco Alvarez, MD
Associate Chief, Regional Pediatric Hospitalist Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Brian Alverson, MD
Director, Division of Pediatric Hospital Medicine
Hasbro Children’s Hospital
Professor of Pediatrics
Alpert School of Medicine, Bro
Providence, RI
Pneumonia
Eric Balighian, MD
Director, Pediatric Emergency Department
St. Agnes Hospital
Asistant Professor, Department of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Acute Abdominal Pain and Acute Abdomen
Julia Beauchamp-Walters, MD
Medical Director, Helen Bernardy Center for Medically Fragile Children
Medical Director, Home Care
Co-Medical Director, Emergency Transport Program
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Feeding Tubes
Pediatric Interfacility Transport
Eric Biondi, MD, MS
Director, Pediatric Hospital Medicine Division
Johns Hopkins Children’s Center
Associate Professor of Pediatrics
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Neonatal Fever
Rebecca Blankenberg, MD, MPH
Associate Chair of Education
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics and Emergency Medicine
Stanford University School of Medicine
Stanford, CA
Education
Colin Bridgeman, MD
Penn State Children’s Hospital
Assistant Professor of Pediatrics
Division of General Inpatient Pediatrics
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Jeffrey Brown, MD, MPH, CAP, FAAP
Texas Newborn Services/Pediatrix Medical Group
Clinical Professor of Pediatrics
University of Colorado School of Medicine
Fort Worth, TX
Business Practices
April O. Buchanan, MD
Associate Dean for Curriculum
Prisma Health Children’s Hospital at Greenville
Associate Professor of Clinical Pediatrics
University of South Carolina School of Medicine
Greenville, SC
Sepsis and Shock
Douglas Carlson, MD
Medical Director
HSHS St. John’s Children’s Hospital
Professor and Chair of Pediatrics
Southern Illinois University School of Medicine
Springfield, MO
Procedural Sedation
Pearl Chang, MD
Seattle Children’s Hospital
Assistant Professor
Department of Pediatrics, University of Washington
Seattle, WA
Neonatal Jaundice
Eric Coon, MD, MS
Co-Director, Pediatric Hospital Medicine Fellowship
Primary Children’s Medical Center
Assistant Professor of Pediatrics
University of Utah Health Science
Salt Lake City, UT
Research
Yasmeen N. Daud, MD
St. Louis Children’s Hospital
Associate Professor of Pediatrics
Washington University School of Medicine
St. Louise, MO
Oxygen Delivery and Airway Management
Sarah Denniston, MD, FAAP
Fellowship Director, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center
Assistant Professor of Pediatrics
Tufts University School of Medicine
Associate DIO for Quality and Safety
Tufts Medical Center
Boston, MA
Peri-procedural Care
Craig C. DeWolfe, MD, MEd, FAAP
Children’s National Health System
Director of Medical Student Education in Pediatrics
Associate Professor of Pediatrics,
George Washington University School of Medicine
Washington, DC
Brief Resolved Unexplained Event
Stephanie Anne Deutsch, MD, MS, FAAP
Section Chief, Child Abuse Pediatrics
Nemours/Alfred I. duPont Hospital for Children
Co-medical Director, CARE (Children at Risk Evaluation) Program
Assistant Clinical Professor of Pediatrics
Sidney Kimmel Medical College at Thomas Jefferson University
Wilmington, Delaware
Child Abuse and Neglect
Ami Doshi, MD
Medical Director, Inpatient Palliative Care Program
Rady Children’s Hospital San Diego
Clinical Associate Professor of Pediatrics
University of California San Diego School of Medicine
San Diego, CA
Palliative Care and Hospice
Erin Fisher, MD, FAAP, MHM
Medical Director, Quality Improvement
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Director, Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
San Diego, CA
Quality Improvement
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Mary Pat Gallagher, MD
Director, Pediatric Diabetes Center
Division of Pediatric Endocrinology, Hassenfeld Children’s Hospital
Assistant Professor
Department of Pediatrics
NYU Langone
New York, NY
Diabetes Mellitus
Amrit Gill, MD
Cleveland Clinic Children’s Hospital
Clinical Assistant Professor of Pediatrics
Case Western Reserve University School of Medicine
Cleveland, OH
Patient Safety
Veena Goel Jones, MD, FAAP
Medical Director, Digital Patient Experience, Sutter Health
Sutter Palo Alto Medical Foundation
Palo Alto, CA
Health Information Technology
Jeffrey Grill, MD
Vice Chair, Community Relations and Outreach
Chief, Division of Pediatric Hospital Medicine
Director, Just for Kids Hospitalist Service
Norton Children’s Hospital
Professor, Department of Pediatrics
University of Louisville School of Medicine
Louisville, KY
Constipation
Arun Gupta, MD
Director, Neonatal Hospitalist Program
Lucile Packard Children’s Hospital Stanford
Clinical Associate Professor, Pediatrics
Stanford University School of Medicine
Stanford, CA
Newborn Care and Delivery Room Management
Brian F Herbst Jr, MD
Medical Director, Hospital Medicine Adult Care
Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Internal Medicine and Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Daniel Hershey, MD, SFHM
Rady Children’s Hospital
Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Non-invasive Monitoring
Kim Hoang, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Education
Alison Volpe Holmes, MD, MPH
Children’s Hospital at Dartmouth-Hitchcock
Associate Dean for Student Affairs, Career Advising
Vice-Chair for Education, Department of Pediatrics
Associate Professor of Pediatrics and of The Dartmouth Institute
Geisel School of Medicine at Dartmouth
Hanover, NH
Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome
Akshata Hopkins, MD, FAAP, FHM
Director, Pediatric Residency Program
Johns Hopkins All Children’s Hospital
Assistant Professor of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
High Value Care
Yemisi Jones, MD, FAAP, FHM
Co-Medical Director, Continuing Medical Education
Co-Director Liberty Simulation Education
Cincinnati Children’s Hospital Medical Center
Assistant Professor of Clinical Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Intravenous Access and Phlebotomy
Alisa Khan, MD, MPH
Health Services Researcher
Division of General Pediatrics, Boston Children’s Hospital
Clinical Instructor in Pediatrics
Harvard Medical School
Boston, MA
Family Centered Care
Vivian Lee, MD
Children’s Hospital Los Angeles
Clinical Assistant Professor of Pediatrics
University of Southern California Keck School of Medicine
Los Angeles, CA
Altered Mental Status
Su-Ting T. Li, MD, MPH
Associate Vice Chair of Education
Pediatric Residency Program Director
University of California Davis Children’s Hospital
Professor of Pediatrics
University of California, Davis
Sacramento, CA
Skin and Soft Tissue Infections
Patricia S. Lye, MD, MEd, FAAP
Children’s Hospital of Wisconsin
Professor of Pediatrics, Retired
Medical College of Wisconsin
Milwaukee, WI
Handoffs and Transitions of Care
Tamara Maginot, PhD
Pediatric Psychologist
Program Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Assistant Professor, Department of Psychiatry
UC San Diego Eating Disorders Center for Treatment and Research Behavioral Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Christopher Maloney, MD, PhD, FAAP
Chief Medical Officer and Senior Vice President
Children’s Hospital & Medical Center
Professor of Pediatrics and Pediatric Critical Care
Department of Pediatrics
University of Nebraska Medical Center College of Medicine
Omaha, NE
Pediatric Advanced Life Support
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Failure to Thrive
Elizabeth Mannino Avila, MD
Rady Children’s Hospital
Assistant Clinical Professor of Pediatrics
University of California, San Diego
San Diego, CA
Kawasaki Disease
Alison Markowsky, MD, MSHS, FAAP
Medical Director
Children’s National Pediatric Hospitalist Program at Mary Washington Healthcare
Children’s National Health System
Assistant Professor of Pediatrics
The George Washington University School of Medicine & Health Sciences
Washington, DC
Newborn Care and Delivery Room Management
Michelle Marks, DO, FAAP, SFHM
Chair, Pediatric Hospital Medicine
Cleveland Clinic Children’s Hospital
Clinical Associate Professor
Cleveland Clinic Lerner College of Medicine, Case Western Reserve University
Cleveland, OH
Nutrition
Armand H. Matheny Antommaria, MD, PhD, FAAP
Lee Ault Carter Chair Pediatric Ethics and Pediatric Hospitalist
Cincinnati Children’s Hospital
Associate Professor of Clinical-Affiliated
University of Cincinnati School of Medicine
Cincinnati, OH
Ethics
Erich Maul, MD
Division Chief, Hospital Medicine
Medical Director, Acute Care and Progressive Care
Kentucky Children’s Hospital
Professor of Pediatrics
University of Kentucky School of Medicine
Lexington, KY
Electrocardiogram Interpretation
Rusty McCulloh, MD
Chief, Division of Hospital Medicine
Children’s Hospital & Medical Center
Associate Professor, Division of Hospital Medicine
University of Nebraska College of Medicine
Omaha, NE
Infection Control and Antimicrobial Stewardship
Anjna Melwani, MD
Director, Preoperative Care Clinic
Children’s National Medical Center
Associate Professor of Pediatrics
George Washington University School of Medicine and Health Sciences
Washington, DC
Consultation and Co-management
Christopher Miller, MD
Pediatric Allergist
Children’s Mercy Hospitals and Clinics
Assistant Professor of Pediatrics
Section of Allergy and Immunology
University of Missouri-Kansas City School of Medicine
Kansas City, MO
Asthma
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System Dallas
Dallas, TX
Acute Respiratory Failure
Beth Natt, MD, MPH, FAAP, SFHM
Director, Pediatric Hospital Medicine, Regional Programs
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Farmington, CT
Bladder Catheterization and Interpretation of Urinalysis
Jennifer O’Toole, MD, MEd, FAAP, SFHM
Program Director, Internal Medicine – Pediatrics Residency
Director of Education, Division of Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Associate Professor of Pediatrics and Internal Medicine
University of Cincinnati College of Medicine
Cincinnati, OH
Adolescent and Young Adult Medicine
Mary Ottolini, MD, MPH, MEd, FAAP
George W. Hallett Chair of Pediatrics
Barbara Bush Children’s Hospital at Maine Medical Center
Professor of Pediatrics
Tufts University School of Medicine
Portland, ME
Fluid and Electrolyte Management
Jack Percelay, MD, MPH, FAAP, MHM
Stanford Lucile Packard Children’s Hospital
Clinical Associate Professor of Pediatrics
Stanford University School of Medicine
Stanford, CA
Advocacy
Seizures
Shannon Phillips, MD, MPH
Chief Patient Safety and Experience Officer
Primary Children’s Medical Center
Intermountain Healthcare, Inc.
Adjunct Associate Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Patient Safety
David Pressel, MD, PhD, FAAP, FHM
Medical Director, Pediatric Hospitalist Program
Capital Health Medical Center- Hopewell
Pennington, NJ
Acute Behavioral and Psychiatric Conditions
Child Abuse and Neglect
Ricardo Quinonez, MD, FAAP
Chief, Pediatric Hospital Medicine
Texas Children’s Hospital
Associate Professor of Pediatrics
Baylor College of Medicine
Houston, TX
High Value Care
Shawn Ralston, MA, MD, MS
Johns Hopkins Children’s Center
Editor, Hospital Pediatrics, American Academy of Pediatrics
Associate Professor of Pediatrics
Division of Pediatric Quality and Safety
The Johns Hopkins Hospital University School of Medicine
Baltimore, MD
Evidence Based Medicine
David I. Rappaport, MD, FAAP, FHM
Associate Residency Program Director
Division of General Pediatrics
Nemours/AI duPont Hospital for Children
Wilmington, DE
Associate Professor of Pediatrics
Sidney Kimmel Medical College at Jefferson
Philadelphia, PA
Consultation and Co-management
Daniel Rauch, MD, FAAP, SFHM
Chief, Pediatric Hospital Medicine
The Floating Hospital for Children at Tufts Medical Center.
Professor of Pediatrics
Tufts University School of Medicine
Boston, MA
Preventive Care Services
Kyung (Kay) Rhee, MD, MSc, MA
Director of Research, Division of Pediatric Hospital Medicine
Medical Director, Medical Behavioral Unit
Rady Children’s Hospital San Diego
Professor of Clinical Pediatrics
Department of Pediatrics, Division of General Academic Pediatrics, Developmental Pediatrics, and Center for Community Health
University of San Diego School of Medicine
San Diego, CA
Chronic Behavioral and Psychiatric Conditions
Jeffrey Riese, MD
Associate Pediatric Residency Program Director
Hasbro Children’s Hospital
Associate Professor of Pediatrics
Warren Alpert School of Medicine at Brown University
Providence, RI
Neonatal Fever
Ken Roberts, MD, FAAP
Professor Emeritus of Pediatrics
University of North Carolina School of Medicine
Chapel Hill, NC
Urinary Tract Infections
Amanda Rogers, MD
Associate Pediatric Residency Program Director
Children’s Hospital of Wisconsin
Assistant Professor, Section of Hospital Medicine
Medical College of Wisconsin
Milwaukee, WI
Lumbar Puncture
Rebecca E. Rosenberg, MD, MPH
Chief, Section of Hospital Medicine, Division of General Pediatrics
Hassenfeld Children’s Hospital at NYU Langone Health
Associate Professor of Pediatrics
NYU School of Medicine
New York, NY
Peri-procedural Care
Michael Ruhlen, MD, MHCM, FHM, FACHE
Vice President, Division of Medical Education
Vice Chair, RRC ACGME
Atrium Health System
Charlotte, NC
Legal Issues and Risk Management
Christopher J. Russell, MD, MS, FAAP
Research Director, Division of Hospital Medicine
Children’s Hospital Los Angeles
Assistant Professor of Clinical Pediatrics
Keck School of Medicine, University of Southern California
Los Angeles, CA
Child with Medical Complexity
Christopher Russo, MD
Director of Pediatrics
Central Lynchburg General Hospital
Assistant Professor of Pediatrics
Liberty University College of Osteopathic Medicine
Lynchburg, VA
Advocacy
Klint M. Schwenk, MD, MBA, FAAP, FHM
Associate Division Chief, Pediatric Hospital Medicine
Norton Children’s Hospital
Associate Professor of Pediatrics
University of Louisville
Louisville, KY
Acute Gastroenteritis
Gastrointestinal and Digestive Disorders
Anand Sekaran, MD, FAAP
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Diagnostic Imaging
Kristin A. Shadman, MD, FAAP
American Family Children’s Hospital
Associate Professor of Pediatrics
Division of Hospital Medicine
University of Wisconsin School of Medicine and Public Health
Madison, WI
Oxygen Delivery and Airway Management
Samir S. Shah, MD, MSCE
Director, Division of Hospital Medicine
James M. Ewell Endowed Chair
Attending Physician in Hospital Medicine & Infectious Diseases
Chief Metrics Officer
Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics
University of Cincinnati College of Medicine
Cincinnati, OH
Bone and Joint Infections
Mark Shen, MD, MBA, FAAP, SFHM
Associate Professor of Pediatrics
Dell Medical School at the University of Texas at Austin
Austin, TX
Leadership in Healthcare
Tamara Simon, MD, MSPH, FAAP
Principal Investigator, Center for Clinical and Translational Research
Seattle Children’s Research Institute
Associate Professor of Pediatrics
Divisions of Hospital Medicine and General Pediatrics, Department of Pediatrics
University of Washington
Seattle, WA
Child with Medical Complexity
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine, Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
Communication
Karen Smith, MD, MEd, SFHM, FAAP
Chief, Division of Pediatric Hospital Medicine
Children’s National Medical Center
Associate Professor of Pediatrics
The George Washington School of Medicine and Health Sciences
Washington, DC
Business Practices
Nita Srinivas, MD
Pediatric Hospitalist and Infectious Disease Specialist
Fellowship Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Fever of Unknown Origin
Rajendu Srivastava, MD, FRCP(C), MPH
Primary Children’s Medical Center
Assistant Vice President of Research and Medical Director of the Office of Research
Intermountain Healthcare Inc.
Professor of Pediatrics
University of Utah Health Sciences
Salt Lake City, UT
Research
Lynne Sterni, MD
Pediatric Anesthesiology and Pain Medicine
Naval Medical Center San Diego
Assistant Professor
Uniformed Services University School of Health Sciences
San Diego, CA
Pain Management
E. Douglas Thompson Jr, MD, FAAP
Chief, Section of Hospital Medicine
Associate Chair, Access and Partnerships
St. Christopher’s Hospital for Children
Associate Professor of Pediatrics
Drexel University School of Medicine and Health Sciences
Philadelphia, PA
Sickle Cell Disease
Joanna Thomson, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Cincinnati Children’s Hospital Medical Center
Assistant Professor, Department of Pediatrics
University of Cincinnati School of Medicine
Cincinnati, OH
Acute Respiratory Failure
Joel Tieder, MD, MPH
Seattle Children’s Hospital
Associate Professor of Pediatrics, Division of Hospital Medicine
University of Washington School of Medicine
Seattle, WA
Brief Resolved Unexplained Event
Adriana Tremoulet, MD, MAS
Associate Director, Kawasaki Disease Research Center
Division of Host-Microbe Systems and Therapeutics
Pediatric Infectious Diseases and Kawasaki Disease
Associate Professor of Pediatrics, University of California San Diego
San Diego, CA
Kawasaki Disease
Marie E. Wang, MD, MPH, FAAP
Associate Fellowship Program Director, Pediatric Hospital Medicine
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Stanford University School of Medicine
Stanford, CA
Central Nervous System Infections
Ronald Williams, MD, FAAP, FACP
Director, Combined Internal Medicine/Pediatrics Residency Program
Penn State Hershey Children’s Hospital
Professor of Pediatrics and Medicine
Penn State College of Medicine
Hershey, PA
Head and Neck Disorders
Susan Wu, MD, FAAP
Children’s Hospital Los Angeles
Associate Professor of Clinical Pediatrics
Division of Hospital Medicine, Department of Pediatrics
USC Keck School of Medicine
Los Angeles, CA
Bronchiolitis
EDITORS
Sandra Gage, MD, PhD, FAAP, SFHM
Associate Division Chief and Associate Fellowship Director
Division of Hospital Medicine
Phoenix Children’s Hospital
Clinical Associate Professor
University of Arizona College of Medicine – Phoenix
Department of Child Health
Phoenix, AZ
Jennifer Maniscalco, MD, MPH, MAcM, FAAP
Designated Institutional Official
Johns Hopkins All Children’s Hospital
Assistant Professor
Department of Pediatrics
Johns Hopkins University School of Medicine
St. Petersburg, FL
Erin Fisher, MD, MHM, FAAP
Medical Director Quality Improvement
Rady Children’s Hospital
Professor of Clinical Pediatrics
Director of Pediatric Quality and Safety Graduate Medical Education
Fellowship Director and Division Director, Pediatric Hospital Medicine
University of California San Diego School of Medicine
Department of Pediatrics
San Diego, CA
CONTRIBUTING EDITOR, COMMUNITY PERSPECTIVE EXPERTISE
Sofia Teferi, MD, FAAP, SFHM
Physician Executive
Richmond, VA
ASSOCIATE EDITORS
Francisco Alvarez, MD, FAAP
Associate Chief, Regional Pediatric Hospital Medicine Programs
Lucile Packard Children’s Hospital
Clinical Associate Professor
Stanford School of Medicine
Stanford, CA
Michael Burke, MD (1957 – 2019)
In memory: Chairman of Pediatrics
Saint Agnes Hospital
Associate Professor of Pediatrics
Johns Hopkins University School of Medicine
Baltimore, MD
Weijen Chang, MD
Division Chief, Pediatric Hospital Medicine
Vice Chair for Clinical Affairs, Department of Pediatrics
Baystate Children’s Hospital
Associate Professor of Pediatrics
University of Massachusetts Medical School-Baystate
Springfield, MA
Vineeta Mittal, MD, MBA
Imm. Past President of the Medical/Dental Staff
Children’s Medical Center
Associate Professor of Pediatrics
Director of Pediatric Hospital Medicine
Department of Pediatrics
UT Southwestern Medical Center & Children’s Health System, Dallas
Dallas, TX
Anand Sekaran, MD
Associate Chair of Pediatrics, Clinical Affairs
Division Chief, Hospital Medicine
Connecticut Children’s Medical Center
Associate Professor of Pediatrics
University of Connecticut School of Medicine
Hartford, CT
Amit Singh, MD, FAAP
Lucile Packard Children’s Hospital
Clinical Assistant Professor
Division of Pediatric Hospital Medicine
Department of Pediatrics
Stanford University School of Medicine
Stanford, CA
EXTERNAL REVIEWERS
Academic Pediatric Association Hospital Medicine Special Interest Group
American Academy of Pediatrics
- Committee on Psychological Aspects of Child and Family Health
- Council on Children with Disabilities
- Council on Community Pediatrics
- Disaster Preparedness Advisory Council
- Family Partnerships Network
- Section on Anesthesiology and Pain Medicine
- Section on Breastfeeding
- Section on Cardiology and Cardiac Surgery
- Section on Critical Care
- Section on Hematology/Oncology
- Section on Hospice and Palliative Medicine
- Section on Hospital Medicine
- Section on LGBT Health and Wellness
- Section on Medicine-Pediatrics
- Section on Nephrology
- Section on Neurology
- Section on Pediatric Trainees
- Section on Surgery
- Section on Transport Medicine
- Section on Urology
Association of Pediatric Program Directors Curriculum Committee
Society of Hospital Medicine Pediatrics Special Interest Group
Society of Hospital Medicine Medicine-Pediatrics Special Interest Group