User login
Financial Difficulties in Families of Hospitalized Children
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
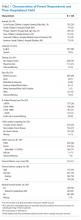
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.
Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
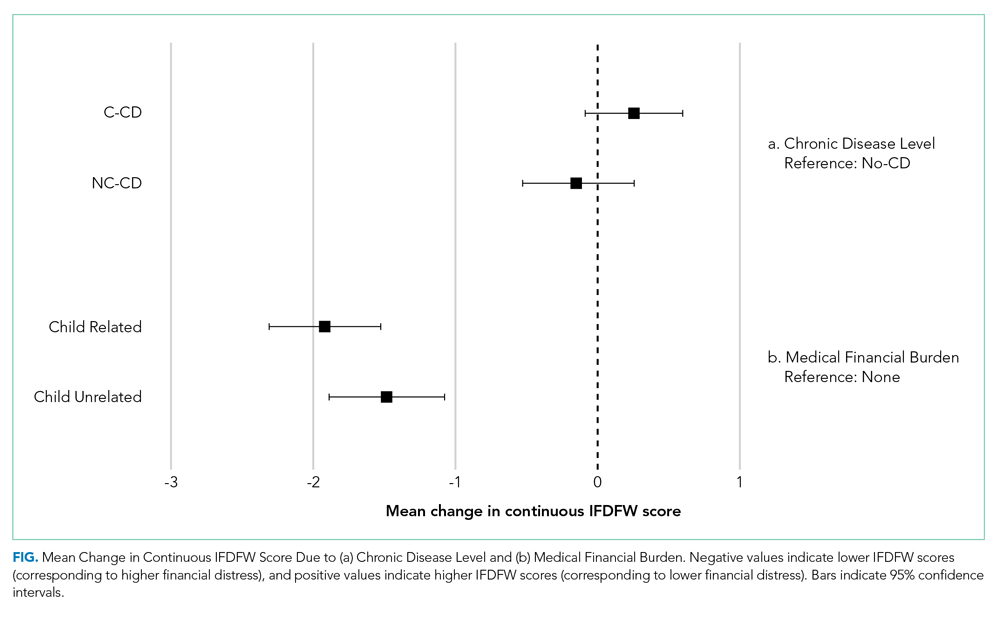
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.
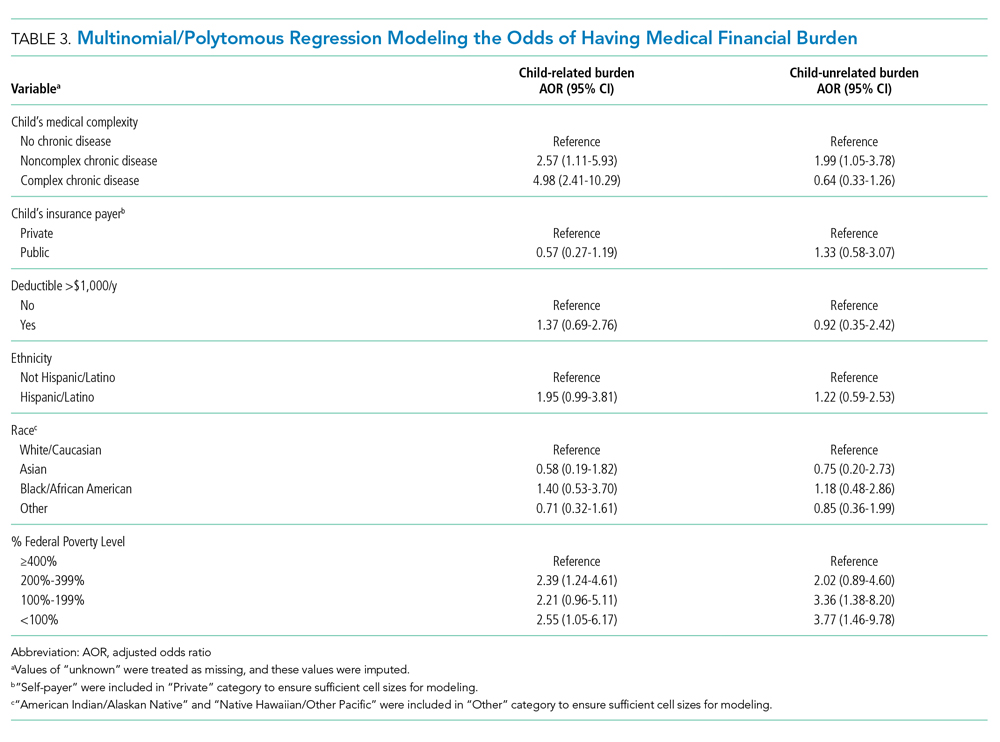
Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
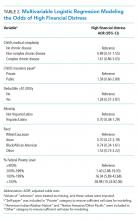
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.

Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.

Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.

Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.

Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
© 2020 Society of Hospital Medicine
Baricitinib reduces adult atopic dermatitis severity in phase 3 study
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
in the phase 3, double-blind, placebo-controlled, BREEZE-AD7 study.
The study enrolled patients with inadequate responses to topical corticosteroids, according to Kristian Reich, MD, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and his coauthors.
First test of baricitinib plus topical steroids
Baricitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, inhibits several cytokines in AD pathogenesis, and in two monotherapy studies (BREEZE-AD1 and BREEZE-AD2), it was superior to placebo for reducing several AD clinical signs and symptoms. The current BREEZE-AD7 study is the first to test baricitinib plus background topical corticosteroid therapy, more closely mirroring clinical practice, the authors noted.
BREEZE-AD7 was conducted at 68 centers in 10 countries in Asia, Australia, Europe, and South America. It included 329 adults with moderate to severe AD (mean age around 34 years, and around 34% were female) with inadequate responses to topical corticosteroids documented within the last 6 months. They were randomized 1:1:1 to daily baricitinib 4 mg, daily baricitinib 2 mg, or placebo for 16 weeks. All patients received moderate- and/or low-potency topical corticosteroids (such as 0.1%triamcinolone cream and 2.5% hydrocortisone ointment, respectively) for active lesions.
Significant benefit at 4 mg
At week 16, 31% of AD patients receiving baricitinib 4 mg achieved Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 (clear) or 1 (almost clear) versus 15% in the placebo group (odds ratio, 2.8; 95% confidence interval, 1.4-5.6; P = .004). Among patients receiving baricitinib 2 mg, 24% achieved vI-GA-AD scores of 0 or 1 (OR, 1.9; 95% CI, 0.9-3.9; P = .08).
The same pattern of improving scores from placebo to baricitinib 2 mg to baricitinib 4 mg persisted, as reflected with secondary endpoints at week 16. Among patients receiving baricitinib 4 mg, 48% achieved Eczema Area Severity Index (EASI) 75 responses, versus 43% and 23% in 2 mg and placebo groups, respectively. Percent changes from baseline in total EASI score were –67%, –58%, –45% for baricitinib 4 mg, baricitinib 2 mg, and placebo, respectively; the proportion of patients achieving 4-point or greater improvements in Itch Numeric Rating Scale (NRS) was 44%, 38%, and 20% for baricitinib 4 mg, baricitinib 2 mg and placebo, respectively.
Similarly, mean change from baseline on the Skin Pain numeric rating scale was –3.7, –3.2, and –2.1 for baricitinib 4 mg, baricitinib 2 mg and placebo. Nighttime itch awakenings were also reduced in a similar progression from placebo to the higher baricitinib dose.
Adverse events dose related
Treatment-related adverse events were reported more frequently in the baricitinib groups (58% baricitinib 4 mg, 56% baricitinib 2 mg) versus placebo 38%. Nasopharyngitis was most common, followed by oral herpes, upper respiratory tract infection, acne, diarrhea, and back pain. Serious adverse event rates were similar across treatment groups. Permanent discontinuation rates were low at 5% for baricitinib 4 mg, 0% for baricitinib 2 mg, and 1% for placebo. The side-effect profile for baricitinib was consistent with prior studies, Dr. Reich and his coauthors reported.
The authors noted further, “data in this study suggest that patients with AD treated with baricitinib may be able to reduce the frequency and total quantity of concomitant TCSs [topical corticosteroids] used, thus mitigating concerns associated with continual or sustained application of topical treatments.”
“Overall, this study provides further evidence to support the efficacy and safety profile of baricitinib for the treatment of moderate-severe AD,” commented one of the authors, Jonathan I. Silverberg, MD, PhD, MPH, of the department of dermatology at George Washington University in Washington.
“In particular, this study shows that adding topical corticosteroids to baricitinib increases the rate of treatment success compared with the efficacy seen in baricitinib monotherapy studies. These data will be important to guide the use of baricitinib with topical corticosteroids in clinical practice. I think these data are also important because they show that baricitinib 4 mg may be more effective than 2 mg in some patients,” he said in an interview.
In late September, the European Medicines Agency’s Committee for Medicinal Products for Human Use recommended approval of oral baricitinib for adults with moderate to severe AD who are candidates for systemic therapy. Baricitinib is approved in the European Union and the United States to treat moderate to severe active rheumatoid arthritis. If approved in Europe, it will be the first JAK inhibitor and first oral medication indicated to treat patients with AD.
The study was funded by Eli Lilly and Company under license from Incyte Corporation. Dr. Reich reported receiving fees to the institution for participation in clinical trials from Eli Lilly and Company during the conduct of the study and personal fees for lectures. Dr. Silverberg reported receiving fees from Eli Lilly and Company during the conduct of the study, and fees from companies outside of this work. Other authors also reported disclosures related to Eli Lilly and other pharmaceutical companies, and several authors were Eli Lilly employees.
SOURCE: Reich K et al. JAMA Dermatol. 2020 Sep 30. doi: 10.1001/jamadermatol.2020.3260.
FROM JAMA DERMATOLOGY
It’s not time to abandon routine screening mammography in average-risk women in their 40s
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.
Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
Can AML patients be too old for cell transplantation?
How old is too old for a patient to undergo hematopoietic cell transplantation (HCT)? That’s the wrong question to ask, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus. Instead, he said, look at other factors such as disease status and genetics.
“Transplantation for older patients, even beyond the age of 70, is acceptable, as long as it’s done with caution, care, and wisdom. So we’re all not too old for transplantation, at least not today,” said Daniel Weisdorf, MD, professor of medicine and deputy director of the University of Minnesota Clinical and Translational Science Institute.
As he noted, acute myeloid leukemia (AML) is often fatal. Among the general population, “the expected survival life expectancy at age 75 is 98% at 1 year, and most people living at 75 go on to live more than 10 years,” he said. “But if you have AML, at age 75, you have 20% survival at 1 year, 4% at 3 years. And since the median age of AML diagnosis is 68, and 75% of patients are diagnosed beyond the age of 55, this becomes relevant.”
Risk factors that affect survival after transplantation “certainly include age, but that interacts directly with the comorbidities people accumulate with age, their assessments of frailty, and their Karnofsky performance status, as well as the disease phenotype and molecular genetic markers,” Dr. Weisdorf said. “Perhaps most importantly, though not addressed very much, is patients’ willingness to undertake intensive therapy and their life outlook related to patient-reported outcomes when they get older.”
Despite the lack of indications that higher age by itself is an influential factor in survival after transplant, “we are generally reluctant to push the age of eligibility,” Dr. Weisdorf said. He noted that recently published American Society of Hematology guidelines for treatment of AML over the age of 55 “don’t discuss anything about transplantation fitness because they didn’t want to tackle that.”
Overall survival (OS) at 1 year after allogenic transplants only dipped slightly from ages 51-60 to 71 and above, according to Dr. Weisdorf’s analysis of U.S. data collected by the Center for International Blood and Marrow Transplant Research for the time period 2005-2019.
OS was 67.6% (66.8%-68.3%) for the 41-50 age group (n = 9,287) and 57.9% (56.1%-59.8%) for the 71 and older group, Dr. Weisdorf found. Overall, OS dropped by about 4 percentage points per decade of age, he said, revealing a “modest influence” of advancing years.
His analysis of autologous transplant data from the same source, also for 2005-2019, revealed “essentially no age influence.” OS was 90.8% (90.3%-91.2%) for the 41-50 age group (n = 15,075) and 86.6% (85.9%-87.3%) for the 71 and older group (n = 7,247).
Dr. Weisdorf also highlighted unpublished research that suggests that cord-blood transplant recipients older than 70 face a significantly higher risk of death than that of younger patients in the same category. Cord blood “may be option of last resort” because of a lack of other options, he explained. “And it may be part of the learning curve of cord blood transplantation, which grew a little bit in the early 2000s, and maybe past 2010, and then fell off as everybody got enamored with the haploidentical transplant option.”
How can physicians make decisions about transplants in older patients? “The transplant comorbidity index, the specific comorbidities themselves, performance score, and frailty are all measures of somebody’s fitness to be a good candidate for transplant, really at any age,” Dr. Weisdorf said. “But we also have to recognize that disease status, genetics, and the risk phenotype remain critical and should influence decision making.”
However, even as transplant survival improves overall, “very few people are incorporating any very specific biological markers” in decision-making, he said. “We’ve gotten to measures of frailty, but we haven’t gotten to any biologic measures of cytokines or other things that would predict poor chances for doing well. So I’m afraid we’re still standing at the foot of the bed saying: ‘You look okay.’ Or we’re measuring their comorbidity index. But it is disappointing that we’re using mostly very simple clinical measures to decide if somebody is sturdy enough to proceed, and we perhaps need something better. But I don’t have a great suggestion what it should be.”
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Weisdorf disclosed consulting fees from Fate Therapeutics and Incyte Corp.
SOURCE: “The Ever-Increasing Upper Age for Transplant: Is This Evidence-Based?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
How old is too old for a patient to undergo hematopoietic cell transplantation (HCT)? That’s the wrong question to ask, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus. Instead, he said, look at other factors such as disease status and genetics.
“Transplantation for older patients, even beyond the age of 70, is acceptable, as long as it’s done with caution, care, and wisdom. So we’re all not too old for transplantation, at least not today,” said Daniel Weisdorf, MD, professor of medicine and deputy director of the University of Minnesota Clinical and Translational Science Institute.
As he noted, acute myeloid leukemia (AML) is often fatal. Among the general population, “the expected survival life expectancy at age 75 is 98% at 1 year, and most people living at 75 go on to live more than 10 years,” he said. “But if you have AML, at age 75, you have 20% survival at 1 year, 4% at 3 years. And since the median age of AML diagnosis is 68, and 75% of patients are diagnosed beyond the age of 55, this becomes relevant.”
Risk factors that affect survival after transplantation “certainly include age, but that interacts directly with the comorbidities people accumulate with age, their assessments of frailty, and their Karnofsky performance status, as well as the disease phenotype and molecular genetic markers,” Dr. Weisdorf said. “Perhaps most importantly, though not addressed very much, is patients’ willingness to undertake intensive therapy and their life outlook related to patient-reported outcomes when they get older.”
Despite the lack of indications that higher age by itself is an influential factor in survival after transplant, “we are generally reluctant to push the age of eligibility,” Dr. Weisdorf said. He noted that recently published American Society of Hematology guidelines for treatment of AML over the age of 55 “don’t discuss anything about transplantation fitness because they didn’t want to tackle that.”
Overall survival (OS) at 1 year after allogenic transplants only dipped slightly from ages 51-60 to 71 and above, according to Dr. Weisdorf’s analysis of U.S. data collected by the Center for International Blood and Marrow Transplant Research for the time period 2005-2019.
OS was 67.6% (66.8%-68.3%) for the 41-50 age group (n = 9,287) and 57.9% (56.1%-59.8%) for the 71 and older group, Dr. Weisdorf found. Overall, OS dropped by about 4 percentage points per decade of age, he said, revealing a “modest influence” of advancing years.
His analysis of autologous transplant data from the same source, also for 2005-2019, revealed “essentially no age influence.” OS was 90.8% (90.3%-91.2%) for the 41-50 age group (n = 15,075) and 86.6% (85.9%-87.3%) for the 71 and older group (n = 7,247).
Dr. Weisdorf also highlighted unpublished research that suggests that cord-blood transplant recipients older than 70 face a significantly higher risk of death than that of younger patients in the same category. Cord blood “may be option of last resort” because of a lack of other options, he explained. “And it may be part of the learning curve of cord blood transplantation, which grew a little bit in the early 2000s, and maybe past 2010, and then fell off as everybody got enamored with the haploidentical transplant option.”
How can physicians make decisions about transplants in older patients? “The transplant comorbidity index, the specific comorbidities themselves, performance score, and frailty are all measures of somebody’s fitness to be a good candidate for transplant, really at any age,” Dr. Weisdorf said. “But we also have to recognize that disease status, genetics, and the risk phenotype remain critical and should influence decision making.”
However, even as transplant survival improves overall, “very few people are incorporating any very specific biological markers” in decision-making, he said. “We’ve gotten to measures of frailty, but we haven’t gotten to any biologic measures of cytokines or other things that would predict poor chances for doing well. So I’m afraid we’re still standing at the foot of the bed saying: ‘You look okay.’ Or we’re measuring their comorbidity index. But it is disappointing that we’re using mostly very simple clinical measures to decide if somebody is sturdy enough to proceed, and we perhaps need something better. But I don’t have a great suggestion what it should be.”
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Weisdorf disclosed consulting fees from Fate Therapeutics and Incyte Corp.
SOURCE: “The Ever-Increasing Upper Age for Transplant: Is This Evidence-Based?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
How old is too old for a patient to undergo hematopoietic cell transplantation (HCT)? That’s the wrong question to ask, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus. Instead, he said, look at other factors such as disease status and genetics.
“Transplantation for older patients, even beyond the age of 70, is acceptable, as long as it’s done with caution, care, and wisdom. So we’re all not too old for transplantation, at least not today,” said Daniel Weisdorf, MD, professor of medicine and deputy director of the University of Minnesota Clinical and Translational Science Institute.
As he noted, acute myeloid leukemia (AML) is often fatal. Among the general population, “the expected survival life expectancy at age 75 is 98% at 1 year, and most people living at 75 go on to live more than 10 years,” he said. “But if you have AML, at age 75, you have 20% survival at 1 year, 4% at 3 years. And since the median age of AML diagnosis is 68, and 75% of patients are diagnosed beyond the age of 55, this becomes relevant.”
Risk factors that affect survival after transplantation “certainly include age, but that interacts directly with the comorbidities people accumulate with age, their assessments of frailty, and their Karnofsky performance status, as well as the disease phenotype and molecular genetic markers,” Dr. Weisdorf said. “Perhaps most importantly, though not addressed very much, is patients’ willingness to undertake intensive therapy and their life outlook related to patient-reported outcomes when they get older.”
Despite the lack of indications that higher age by itself is an influential factor in survival after transplant, “we are generally reluctant to push the age of eligibility,” Dr. Weisdorf said. He noted that recently published American Society of Hematology guidelines for treatment of AML over the age of 55 “don’t discuss anything about transplantation fitness because they didn’t want to tackle that.”
Overall survival (OS) at 1 year after allogenic transplants only dipped slightly from ages 51-60 to 71 and above, according to Dr. Weisdorf’s analysis of U.S. data collected by the Center for International Blood and Marrow Transplant Research for the time period 2005-2019.
OS was 67.6% (66.8%-68.3%) for the 41-50 age group (n = 9,287) and 57.9% (56.1%-59.8%) for the 71 and older group, Dr. Weisdorf found. Overall, OS dropped by about 4 percentage points per decade of age, he said, revealing a “modest influence” of advancing years.
His analysis of autologous transplant data from the same source, also for 2005-2019, revealed “essentially no age influence.” OS was 90.8% (90.3%-91.2%) for the 41-50 age group (n = 15,075) and 86.6% (85.9%-87.3%) for the 71 and older group (n = 7,247).
Dr. Weisdorf also highlighted unpublished research that suggests that cord-blood transplant recipients older than 70 face a significantly higher risk of death than that of younger patients in the same category. Cord blood “may be option of last resort” because of a lack of other options, he explained. “And it may be part of the learning curve of cord blood transplantation, which grew a little bit in the early 2000s, and maybe past 2010, and then fell off as everybody got enamored with the haploidentical transplant option.”
How can physicians make decisions about transplants in older patients? “The transplant comorbidity index, the specific comorbidities themselves, performance score, and frailty are all measures of somebody’s fitness to be a good candidate for transplant, really at any age,” Dr. Weisdorf said. “But we also have to recognize that disease status, genetics, and the risk phenotype remain critical and should influence decision making.”
However, even as transplant survival improves overall, “very few people are incorporating any very specific biological markers” in decision-making, he said. “We’ve gotten to measures of frailty, but we haven’t gotten to any biologic measures of cytokines or other things that would predict poor chances for doing well. So I’m afraid we’re still standing at the foot of the bed saying: ‘You look okay.’ Or we’re measuring their comorbidity index. But it is disappointing that we’re using mostly very simple clinical measures to decide if somebody is sturdy enough to proceed, and we perhaps need something better. But I don’t have a great suggestion what it should be.”
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Weisdorf disclosed consulting fees from Fate Therapeutics and Incyte Corp.
SOURCE: “The Ever-Increasing Upper Age for Transplant: Is This Evidence-Based?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
FROM ALF 2020
Women make progress in pediatric dermatology leadership
Women account for approximately 78% of the pediatric dermatology workforce, and continue to gain influence through increased numbers of leadership positions and published research, based on data from a review of professional society leaders, grant recipients, and annual meeting presenters from 2010 to 2019.
“Despite extensive research on gender equality in general dermatology, studies have yet to explore the evolving representation of women as leaders and researchers in pediatric dermatology, a field where the majority of board-certified physicians are women,” wrote Catherine Baker, MD, and colleagues. Dr. Baker was a medical student at Geisel School of Medicine at Dartmouth, Hanover, N.H., at the time of the study and is now a resident physician at Brigham and Women’s Hospital, Boston.
In a study published in Pediatric Dermatology, the researchers reviewed data on society leadership, research grants, and annual meeting speakers in order to evaluate the impact of women in pediatric dermatology.
Overall, the Society for Pediatric Dermatology has had 20 women presidents since its founding in 1975 (45%), and 7 of the last 10 since 2011 have been women (70%). The Pediatric Dermatology Research Alliance, founded in 2013, has two cochairs each year, and 75% have been women.
The percentage of women as lead authors of published research in pediatric dermatology increased significantly from 1983 to 2019; 71% of first authors and 65% of senior authors of papers in the journal Pediatric Dermatology in 2019 were women.
In addition, 26 of the 31 physicians (84%) who received SPD/PeDRA pilot project awards between 2008 and 2018 were women, as were 88% of SPD/PeDRA team/collaborative grant winners from 2016 to 2018.
However, named lectures at annual meetings remain an area in which women are underrepresented, the researchers wrote. Although women have been well represented at PeDRA meetings, accounting for 65% of plenary speakers, but they accounted for less than half (44%) of Hurwitz and Founders’ lectures at SPD annual meetings from 2010 to 2019.
The study findings were limited by a lack of data on nonbinary genders and the possibility of error in assessing gender based on name and online profiles, the researchers noted. However, the results suggest that women have increased their influence in pediatric dermatology through leadership and research, although a gender gap persists in roles as senior authors and named lecturers at meetings, they wrote.
Overall, “we expect increasing gender equity in these positions as women continue to play important roles as leaders and researchers in pediatric dermatology,” the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Baker C et al. Pediatr Dermatol. 2020 Jul 9. doi: 10.1111/pde.14266.
Women account for approximately 78% of the pediatric dermatology workforce, and continue to gain influence through increased numbers of leadership positions and published research, based on data from a review of professional society leaders, grant recipients, and annual meeting presenters from 2010 to 2019.
“Despite extensive research on gender equality in general dermatology, studies have yet to explore the evolving representation of women as leaders and researchers in pediatric dermatology, a field where the majority of board-certified physicians are women,” wrote Catherine Baker, MD, and colleagues. Dr. Baker was a medical student at Geisel School of Medicine at Dartmouth, Hanover, N.H., at the time of the study and is now a resident physician at Brigham and Women’s Hospital, Boston.
In a study published in Pediatric Dermatology, the researchers reviewed data on society leadership, research grants, and annual meeting speakers in order to evaluate the impact of women in pediatric dermatology.
Overall, the Society for Pediatric Dermatology has had 20 women presidents since its founding in 1975 (45%), and 7 of the last 10 since 2011 have been women (70%). The Pediatric Dermatology Research Alliance, founded in 2013, has two cochairs each year, and 75% have been women.
The percentage of women as lead authors of published research in pediatric dermatology increased significantly from 1983 to 2019; 71% of first authors and 65% of senior authors of papers in the journal Pediatric Dermatology in 2019 were women.
In addition, 26 of the 31 physicians (84%) who received SPD/PeDRA pilot project awards between 2008 and 2018 were women, as were 88% of SPD/PeDRA team/collaborative grant winners from 2016 to 2018.
However, named lectures at annual meetings remain an area in which women are underrepresented, the researchers wrote. Although women have been well represented at PeDRA meetings, accounting for 65% of plenary speakers, but they accounted for less than half (44%) of Hurwitz and Founders’ lectures at SPD annual meetings from 2010 to 2019.
The study findings were limited by a lack of data on nonbinary genders and the possibility of error in assessing gender based on name and online profiles, the researchers noted. However, the results suggest that women have increased their influence in pediatric dermatology through leadership and research, although a gender gap persists in roles as senior authors and named lecturers at meetings, they wrote.
Overall, “we expect increasing gender equity in these positions as women continue to play important roles as leaders and researchers in pediatric dermatology,” the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Baker C et al. Pediatr Dermatol. 2020 Jul 9. doi: 10.1111/pde.14266.
Women account for approximately 78% of the pediatric dermatology workforce, and continue to gain influence through increased numbers of leadership positions and published research, based on data from a review of professional society leaders, grant recipients, and annual meeting presenters from 2010 to 2019.
“Despite extensive research on gender equality in general dermatology, studies have yet to explore the evolving representation of women as leaders and researchers in pediatric dermatology, a field where the majority of board-certified physicians are women,” wrote Catherine Baker, MD, and colleagues. Dr. Baker was a medical student at Geisel School of Medicine at Dartmouth, Hanover, N.H., at the time of the study and is now a resident physician at Brigham and Women’s Hospital, Boston.
In a study published in Pediatric Dermatology, the researchers reviewed data on society leadership, research grants, and annual meeting speakers in order to evaluate the impact of women in pediatric dermatology.
Overall, the Society for Pediatric Dermatology has had 20 women presidents since its founding in 1975 (45%), and 7 of the last 10 since 2011 have been women (70%). The Pediatric Dermatology Research Alliance, founded in 2013, has two cochairs each year, and 75% have been women.
The percentage of women as lead authors of published research in pediatric dermatology increased significantly from 1983 to 2019; 71% of first authors and 65% of senior authors of papers in the journal Pediatric Dermatology in 2019 were women.
In addition, 26 of the 31 physicians (84%) who received SPD/PeDRA pilot project awards between 2008 and 2018 were women, as were 88% of SPD/PeDRA team/collaborative grant winners from 2016 to 2018.
However, named lectures at annual meetings remain an area in which women are underrepresented, the researchers wrote. Although women have been well represented at PeDRA meetings, accounting for 65% of plenary speakers, but they accounted for less than half (44%) of Hurwitz and Founders’ lectures at SPD annual meetings from 2010 to 2019.
The study findings were limited by a lack of data on nonbinary genders and the possibility of error in assessing gender based on name and online profiles, the researchers noted. However, the results suggest that women have increased their influence in pediatric dermatology through leadership and research, although a gender gap persists in roles as senior authors and named lecturers at meetings, they wrote.
Overall, “we expect increasing gender equity in these positions as women continue to play important roles as leaders and researchers in pediatric dermatology,” the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Baker C et al. Pediatr Dermatol. 2020 Jul 9. doi: 10.1111/pde.14266.
FROM PEDIATRIC DERMATOLOGY
Experts tout immediate quadruple therapy for HFrEF patients
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
FROM HFSA 2020
‘Modest’ benefit for post-MI T2D glucose monitoring
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
FROM EASD 2020
COVID-19 antibody response not reduced with diabetes
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE
VOYAGER PAD: Paclitaxel-coated devices don’t increase mortality
a multithousand-patient randomized trial with long-term follow-up and ascertainment of vital status in 99.6% of participants.
Observers opined that the VOYAGER PAD findings effectively put to rest a nearly 2-year-old controversy over whether paclitaxel-coated devices for treatment of peripheral artery disease (PAD) carry an increased mortality risk. The imbroglio, which was ignited by a meta-analysis of clinical trials with substantial amounts of missing follow-up data, triggered an Food and Drug Administration warning letter to health care providers which threw the field of vascular medicine into disarray.
“Although as a community we’ve continued to struggle with this issue of paclitaxel and mortality, VOYAGER PAD does fill many of the gaps and addresses many of the limitations of currently available data,” Connie N. Hess, MD, said in reporting results of a prespecified analysis of the trial at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting. “I think these are the most definitive data to date supporting the safety of drug-coated device use.”
VOYAGER PAD was a double-blind, placebo-controlled clinical trial in which 6,564 patients undergoing lower-extremity revascularization for symptomatic PAD were randomized to rivaroxaban at 2.5 mg twice daily or placebo on top of background low-dose aspirin. In the previously reported primary outcome, the group on rivaroxaban plus aspirin had a significant 15% reduction in the risk of the composite endpoint of cardiovascular death, acute limb ischemia, MI, ischemic stroke, or major amputation for vascular causes.
Of the 4,316 patients included in the prespecified analysis by Dr. Hess, a cardiologist at the University of Colorado at Denver, Aurora, 31% received a paclitaxel-coated device. At 3.5 years of follow-up, they had a 10.2% all-cause mortality rate, significantly less than the 13.5% rate in patients who didn’t get a drug-coated device. But since study participants weren’t randomized for drug-coated device use, the investigators utilized a rigorous form of propensity adjustment called inverse probability treatment weighting to neutralize all between-group differences in potentially confounding baseline characteristics, including statin use, prevalence of claudication, and target lesion length.
In the weighted analysis, the all-cause mortality rate at 3.5 years was 12.1% in paclitaxel-coated device recipients and 12.6% in those who didn’t get such devices. The difference was not statistically significant, and the hazard ratio of 0.95 had tight confidence intervals.
“We don’t see a mortality benefit, but I think more importantly, we don’t see any risk for mortality,” the cardiologist observed at the meeting sponsored by the Cardiovascular Research Foundation.
There was no between-group difference in causes of mortality. Nor did all-cause mortality differ by device type, be it paclitaxel-coated balloon versus plain balloon angioplasty, or drug-eluting stent versus bare-metal stent.
Also, the benefit of rivaroxaban plus aspirin over aspirin alone in terms of cardiovascular and ischemic limb outcomes was consistent regardless of whether patients got a drug-coated device or not.
Discussant Robert Lookstein, MD, praised Dr. Hess for “a really enlightening presentation.”
“The entire vascular community has been waiting for a prospective, independently adjudicated trial to try to make determinations of whether we can put this issue behind us, and I think this trial is it,” said Dr. Lookstein, professor of interventional radiology and surgery at the Icahn School of Medicine at Mount Sinai, New York.
“Personally, I think this is probably the most impactful data seen regarding the paclitaxel issue in almost 2 years because it is randomized data, it’s prospectively collected data, and – most importantly from my perspective – they were able to collect vital statistics on more than 99.5% of the patients,” he added. “I think this is incredibly impactful to my practice.”
Frank Veith, MD, professor of surgery at New York University, concurred, declaring, “I think this study is a game changer. And I think the paclitaxel game is over.”
The VOYAGER PAD study was funded by institutional research grants from Bayer and Janssen.
a multithousand-patient randomized trial with long-term follow-up and ascertainment of vital status in 99.6% of participants.
Observers opined that the VOYAGER PAD findings effectively put to rest a nearly 2-year-old controversy over whether paclitaxel-coated devices for treatment of peripheral artery disease (PAD) carry an increased mortality risk. The imbroglio, which was ignited by a meta-analysis of clinical trials with substantial amounts of missing follow-up data, triggered an Food and Drug Administration warning letter to health care providers which threw the field of vascular medicine into disarray.
“Although as a community we’ve continued to struggle with this issue of paclitaxel and mortality, VOYAGER PAD does fill many of the gaps and addresses many of the limitations of currently available data,” Connie N. Hess, MD, said in reporting results of a prespecified analysis of the trial at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting. “I think these are the most definitive data to date supporting the safety of drug-coated device use.”
VOYAGER PAD was a double-blind, placebo-controlled clinical trial in which 6,564 patients undergoing lower-extremity revascularization for symptomatic PAD were randomized to rivaroxaban at 2.5 mg twice daily or placebo on top of background low-dose aspirin. In the previously reported primary outcome, the group on rivaroxaban plus aspirin had a significant 15% reduction in the risk of the composite endpoint of cardiovascular death, acute limb ischemia, MI, ischemic stroke, or major amputation for vascular causes.
Of the 4,316 patients included in the prespecified analysis by Dr. Hess, a cardiologist at the University of Colorado at Denver, Aurora, 31% received a paclitaxel-coated device. At 3.5 years of follow-up, they had a 10.2% all-cause mortality rate, significantly less than the 13.5% rate in patients who didn’t get a drug-coated device. But since study participants weren’t randomized for drug-coated device use, the investigators utilized a rigorous form of propensity adjustment called inverse probability treatment weighting to neutralize all between-group differences in potentially confounding baseline characteristics, including statin use, prevalence of claudication, and target lesion length.
In the weighted analysis, the all-cause mortality rate at 3.5 years was 12.1% in paclitaxel-coated device recipients and 12.6% in those who didn’t get such devices. The difference was not statistically significant, and the hazard ratio of 0.95 had tight confidence intervals.
“We don’t see a mortality benefit, but I think more importantly, we don’t see any risk for mortality,” the cardiologist observed at the meeting sponsored by the Cardiovascular Research Foundation.
There was no between-group difference in causes of mortality. Nor did all-cause mortality differ by device type, be it paclitaxel-coated balloon versus plain balloon angioplasty, or drug-eluting stent versus bare-metal stent.
Also, the benefit of rivaroxaban plus aspirin over aspirin alone in terms of cardiovascular and ischemic limb outcomes was consistent regardless of whether patients got a drug-coated device or not.
Discussant Robert Lookstein, MD, praised Dr. Hess for “a really enlightening presentation.”
“The entire vascular community has been waiting for a prospective, independently adjudicated trial to try to make determinations of whether we can put this issue behind us, and I think this trial is it,” said Dr. Lookstein, professor of interventional radiology and surgery at the Icahn School of Medicine at Mount Sinai, New York.
“Personally, I think this is probably the most impactful data seen regarding the paclitaxel issue in almost 2 years because it is randomized data, it’s prospectively collected data, and – most importantly from my perspective – they were able to collect vital statistics on more than 99.5% of the patients,” he added. “I think this is incredibly impactful to my practice.”
Frank Veith, MD, professor of surgery at New York University, concurred, declaring, “I think this study is a game changer. And I think the paclitaxel game is over.”
The VOYAGER PAD study was funded by institutional research grants from Bayer and Janssen.
a multithousand-patient randomized trial with long-term follow-up and ascertainment of vital status in 99.6% of participants.
Observers opined that the VOYAGER PAD findings effectively put to rest a nearly 2-year-old controversy over whether paclitaxel-coated devices for treatment of peripheral artery disease (PAD) carry an increased mortality risk. The imbroglio, which was ignited by a meta-analysis of clinical trials with substantial amounts of missing follow-up data, triggered an Food and Drug Administration warning letter to health care providers which threw the field of vascular medicine into disarray.
“Although as a community we’ve continued to struggle with this issue of paclitaxel and mortality, VOYAGER PAD does fill many of the gaps and addresses many of the limitations of currently available data,” Connie N. Hess, MD, said in reporting results of a prespecified analysis of the trial at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting. “I think these are the most definitive data to date supporting the safety of drug-coated device use.”
VOYAGER PAD was a double-blind, placebo-controlled clinical trial in which 6,564 patients undergoing lower-extremity revascularization for symptomatic PAD were randomized to rivaroxaban at 2.5 mg twice daily or placebo on top of background low-dose aspirin. In the previously reported primary outcome, the group on rivaroxaban plus aspirin had a significant 15% reduction in the risk of the composite endpoint of cardiovascular death, acute limb ischemia, MI, ischemic stroke, or major amputation for vascular causes.
Of the 4,316 patients included in the prespecified analysis by Dr. Hess, a cardiologist at the University of Colorado at Denver, Aurora, 31% received a paclitaxel-coated device. At 3.5 years of follow-up, they had a 10.2% all-cause mortality rate, significantly less than the 13.5% rate in patients who didn’t get a drug-coated device. But since study participants weren’t randomized for drug-coated device use, the investigators utilized a rigorous form of propensity adjustment called inverse probability treatment weighting to neutralize all between-group differences in potentially confounding baseline characteristics, including statin use, prevalence of claudication, and target lesion length.
In the weighted analysis, the all-cause mortality rate at 3.5 years was 12.1% in paclitaxel-coated device recipients and 12.6% in those who didn’t get such devices. The difference was not statistically significant, and the hazard ratio of 0.95 had tight confidence intervals.
“We don’t see a mortality benefit, but I think more importantly, we don’t see any risk for mortality,” the cardiologist observed at the meeting sponsored by the Cardiovascular Research Foundation.
There was no between-group difference in causes of mortality. Nor did all-cause mortality differ by device type, be it paclitaxel-coated balloon versus plain balloon angioplasty, or drug-eluting stent versus bare-metal stent.
Also, the benefit of rivaroxaban plus aspirin over aspirin alone in terms of cardiovascular and ischemic limb outcomes was consistent regardless of whether patients got a drug-coated device or not.
Discussant Robert Lookstein, MD, praised Dr. Hess for “a really enlightening presentation.”
“The entire vascular community has been waiting for a prospective, independently adjudicated trial to try to make determinations of whether we can put this issue behind us, and I think this trial is it,” said Dr. Lookstein, professor of interventional radiology and surgery at the Icahn School of Medicine at Mount Sinai, New York.
“Personally, I think this is probably the most impactful data seen regarding the paclitaxel issue in almost 2 years because it is randomized data, it’s prospectively collected data, and – most importantly from my perspective – they were able to collect vital statistics on more than 99.5% of the patients,” he added. “I think this is incredibly impactful to my practice.”
Frank Veith, MD, professor of surgery at New York University, concurred, declaring, “I think this study is a game changer. And I think the paclitaxel game is over.”
The VOYAGER PAD study was funded by institutional research grants from Bayer and Janssen.
FROM TCT 2020