User login
HER+ early breast cancer: Fixed-dose SC pertuzumab-trastuzumab noninferior to IV dosing
Key clinical point: Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous (SC) injection was noninferior to intravenous (IV) dosing in patients with human epidermal growth factor receptor 2-positive (HER2+) early breast cancer.
Major finding: Geometric mean ratio of cycle 7 pertuzumab serum Ctrough of the SC group to the IV group was 1.22 (90% confidence interval, 1.14-1.31). Total pathologic complete response was achieved by 59.5% and 59.7% of patients in IV and SC groups, respectively. Common grade 3-4 adverse events were similar.
Study details: In the phase 3 FeDeriCa trial, patients with HER2+ early breast cancer were randomly assigned to receive either fixed-dose SC (n = 248) or IV (n = 252) pertuzumab and trastuzumab along with neoadjuvant chemotherapy.
Disclosures: This study was funded by F Hoffmann-La Roche and Genentech. The lead author AR Tan reported receving financial support from F Hoffmann-La Roche, Genentech, Pfizer, Merck, Tesaro, Novartis, Immunomedics, Celgene, and AbbVie. His coauthors also reported relationships with various pharmaceutical companies including F Hoffmann-La Roche and Genentech.
Source: Tan AR et al. Lancet Oncol. 2020 Dec 21. doi: 10.1016/S1470-2045(20)30536-2.
Key clinical point: Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous (SC) injection was noninferior to intravenous (IV) dosing in patients with human epidermal growth factor receptor 2-positive (HER2+) early breast cancer.
Major finding: Geometric mean ratio of cycle 7 pertuzumab serum Ctrough of the SC group to the IV group was 1.22 (90% confidence interval, 1.14-1.31). Total pathologic complete response was achieved by 59.5% and 59.7% of patients in IV and SC groups, respectively. Common grade 3-4 adverse events were similar.
Study details: In the phase 3 FeDeriCa trial, patients with HER2+ early breast cancer were randomly assigned to receive either fixed-dose SC (n = 248) or IV (n = 252) pertuzumab and trastuzumab along with neoadjuvant chemotherapy.
Disclosures: This study was funded by F Hoffmann-La Roche and Genentech. The lead author AR Tan reported receving financial support from F Hoffmann-La Roche, Genentech, Pfizer, Merck, Tesaro, Novartis, Immunomedics, Celgene, and AbbVie. His coauthors also reported relationships with various pharmaceutical companies including F Hoffmann-La Roche and Genentech.
Source: Tan AR et al. Lancet Oncol. 2020 Dec 21. doi: 10.1016/S1470-2045(20)30536-2.
Key clinical point: Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous (SC) injection was noninferior to intravenous (IV) dosing in patients with human epidermal growth factor receptor 2-positive (HER2+) early breast cancer.
Major finding: Geometric mean ratio of cycle 7 pertuzumab serum Ctrough of the SC group to the IV group was 1.22 (90% confidence interval, 1.14-1.31). Total pathologic complete response was achieved by 59.5% and 59.7% of patients in IV and SC groups, respectively. Common grade 3-4 adverse events were similar.
Study details: In the phase 3 FeDeriCa trial, patients with HER2+ early breast cancer were randomly assigned to receive either fixed-dose SC (n = 248) or IV (n = 252) pertuzumab and trastuzumab along with neoadjuvant chemotherapy.
Disclosures: This study was funded by F Hoffmann-La Roche and Genentech. The lead author AR Tan reported receving financial support from F Hoffmann-La Roche, Genentech, Pfizer, Merck, Tesaro, Novartis, Immunomedics, Celgene, and AbbVie. His coauthors also reported relationships with various pharmaceutical companies including F Hoffmann-La Roche and Genentech.
Source: Tan AR et al. Lancet Oncol. 2020 Dec 21. doi: 10.1016/S1470-2045(20)30536-2.
Adjuvant S-1 plus endocrine therapy improves invasive DFS in ER+/HER2− breast cancer
Key clinical point: In patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast cancer (ER+/HER2− BC), adjuvant S-1 plus standard endocrine therapy (ET) significantly reduced invasive disease-free survival (IDFS) events compared with ET alone.
Major finding: IDFS events were observed in 16% vs. 11% of patients in ET-only vs. ET+S-1 groups (hazard ratio [HR], 0.63; P = .0003), respectively. The estimated 5-year IDFS was 87% in the ET+S-1 group vs. 82% in the ET-only group. However, adverse events were higher in patients receiving ET+S-1 therapy (99%) vs. ET alone (79%) but were majorly manageable.
Study details: Phase 3 trial included 1,930 women with ER+/HER− BC with intermediate to high risk for recurrence who were randomly allocated to receive either ET alone (n=973) or ET+S-1 (n=957) for a median follow-up of 52.2 months.
Disclosures: This study was sponsored by the Public Health Research Foundation, Japan, and funded by Taiho Pharmaceuticals. Masakazu Toi and other study authors reported funding and ties with various pharmaceutical companies including Taiho during and outside the conduct of the study.
Source: Toi M et al. Lancet Oncol. 2021 Jan 1. doi: 10.1016/S1470-2045(20)30534-9.
Key clinical point: In patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast cancer (ER+/HER2− BC), adjuvant S-1 plus standard endocrine therapy (ET) significantly reduced invasive disease-free survival (IDFS) events compared with ET alone.
Major finding: IDFS events were observed in 16% vs. 11% of patients in ET-only vs. ET+S-1 groups (hazard ratio [HR], 0.63; P = .0003), respectively. The estimated 5-year IDFS was 87% in the ET+S-1 group vs. 82% in the ET-only group. However, adverse events were higher in patients receiving ET+S-1 therapy (99%) vs. ET alone (79%) but were majorly manageable.
Study details: Phase 3 trial included 1,930 women with ER+/HER− BC with intermediate to high risk for recurrence who were randomly allocated to receive either ET alone (n=973) or ET+S-1 (n=957) for a median follow-up of 52.2 months.
Disclosures: This study was sponsored by the Public Health Research Foundation, Japan, and funded by Taiho Pharmaceuticals. Masakazu Toi and other study authors reported funding and ties with various pharmaceutical companies including Taiho during and outside the conduct of the study.
Source: Toi M et al. Lancet Oncol. 2021 Jan 1. doi: 10.1016/S1470-2045(20)30534-9.
Key clinical point: In patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast cancer (ER+/HER2− BC), adjuvant S-1 plus standard endocrine therapy (ET) significantly reduced invasive disease-free survival (IDFS) events compared with ET alone.
Major finding: IDFS events were observed in 16% vs. 11% of patients in ET-only vs. ET+S-1 groups (hazard ratio [HR], 0.63; P = .0003), respectively. The estimated 5-year IDFS was 87% in the ET+S-1 group vs. 82% in the ET-only group. However, adverse events were higher in patients receiving ET+S-1 therapy (99%) vs. ET alone (79%) but were majorly manageable.
Study details: Phase 3 trial included 1,930 women with ER+/HER− BC with intermediate to high risk for recurrence who were randomly allocated to receive either ET alone (n=973) or ET+S-1 (n=957) for a median follow-up of 52.2 months.
Disclosures: This study was sponsored by the Public Health Research Foundation, Japan, and funded by Taiho Pharmaceuticals. Masakazu Toi and other study authors reported funding and ties with various pharmaceutical companies including Taiho during and outside the conduct of the study.
Source: Toi M et al. Lancet Oncol. 2021 Jan 1. doi: 10.1016/S1470-2045(20)30534-9.
Does metabolic syndrome influence outcomes in triple-negative breast cancer?
Key clinical point: Metabolic syndrome (MS) is associated with poorer disease-free survival (DFS), but not with overall survival (OS) in patients with triple-negative breast cancer (TNBC). Hypertension was the only MS component associated with poor DFS and OS.
Major finding: MS was significantly associated with poor DFS (adjusted HR [aHR], 2.24; P = .030), but not OS (aHR, 1.92; P = .103). Hypertension was significantly associated with worse DFS (aHR, 3.63; P = .006) and OS (aHR, 3.45; P = .035).
Study details: Retrospective study of 177 patients with TNBC with (n=48) or without (n=129) MS who were followed-up for at least 5 years.
Disclosures: The study was supported by the Sharpe Strumia Foundation. The authors declared no conflicts of interest.
Source: Kennard K et al. Breast Cancer Res Treat. 2021 Jan 3. doi: 10.1007/s10549-020-06034-1.
Key clinical point: Metabolic syndrome (MS) is associated with poorer disease-free survival (DFS), but not with overall survival (OS) in patients with triple-negative breast cancer (TNBC). Hypertension was the only MS component associated with poor DFS and OS.
Major finding: MS was significantly associated with poor DFS (adjusted HR [aHR], 2.24; P = .030), but not OS (aHR, 1.92; P = .103). Hypertension was significantly associated with worse DFS (aHR, 3.63; P = .006) and OS (aHR, 3.45; P = .035).
Study details: Retrospective study of 177 patients with TNBC with (n=48) or without (n=129) MS who were followed-up for at least 5 years.
Disclosures: The study was supported by the Sharpe Strumia Foundation. The authors declared no conflicts of interest.
Source: Kennard K et al. Breast Cancer Res Treat. 2021 Jan 3. doi: 10.1007/s10549-020-06034-1.
Key clinical point: Metabolic syndrome (MS) is associated with poorer disease-free survival (DFS), but not with overall survival (OS) in patients with triple-negative breast cancer (TNBC). Hypertension was the only MS component associated with poor DFS and OS.
Major finding: MS was significantly associated with poor DFS (adjusted HR [aHR], 2.24; P = .030), but not OS (aHR, 1.92; P = .103). Hypertension was significantly associated with worse DFS (aHR, 3.63; P = .006) and OS (aHR, 3.45; P = .035).
Study details: Retrospective study of 177 patients with TNBC with (n=48) or without (n=129) MS who were followed-up for at least 5 years.
Disclosures: The study was supported by the Sharpe Strumia Foundation. The authors declared no conflicts of interest.
Source: Kennard K et al. Breast Cancer Res Treat. 2021 Jan 3. doi: 10.1007/s10549-020-06034-1.
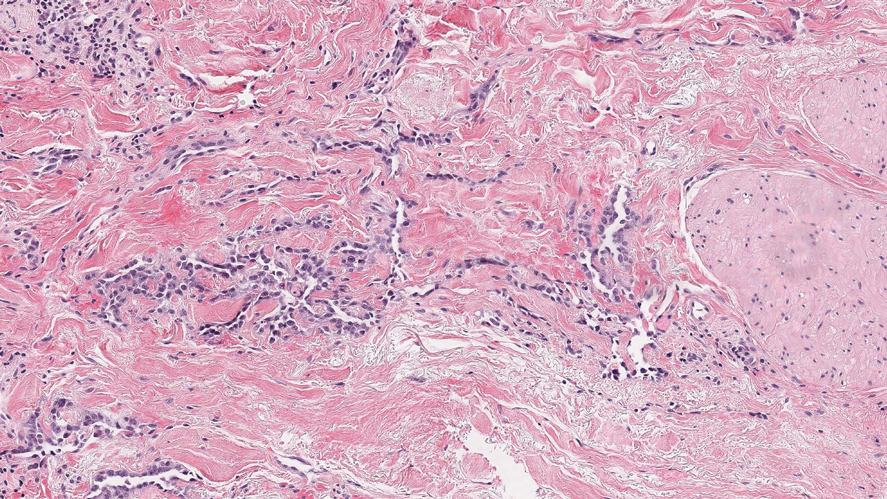
Violaceous Papule With an Erythematous Rim
The Diagnosis: Targetoid Hemosiderotic Hemangioma
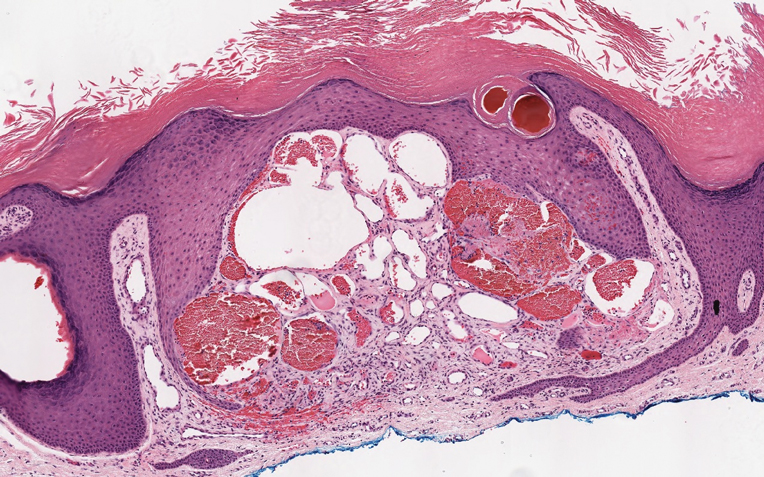
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
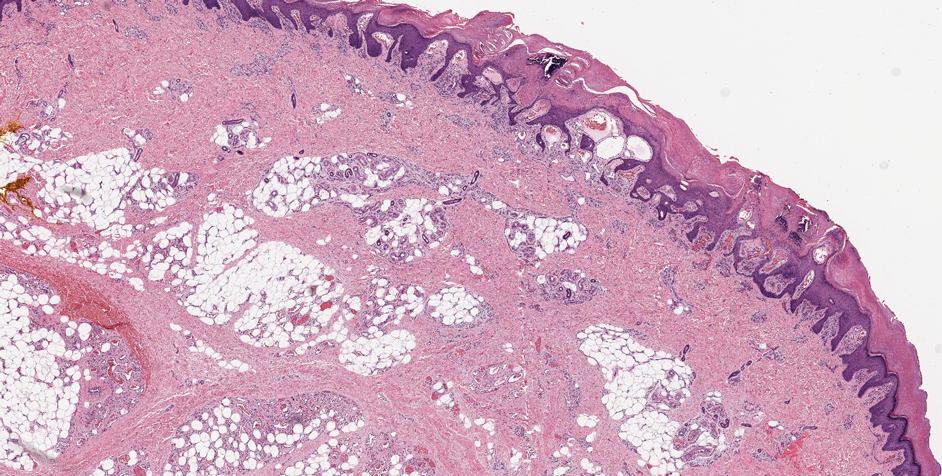
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6
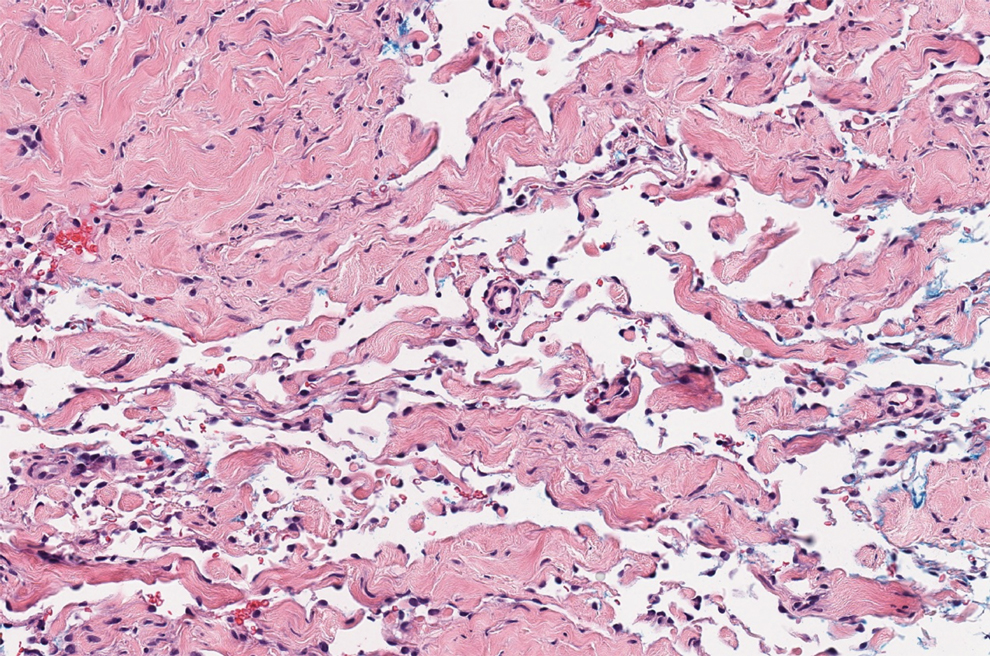
Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7
There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10
Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14
- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
The Diagnosis: Targetoid Hemosiderotic Hemangioma
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6

Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7

There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10

Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14

The Diagnosis: Targetoid Hemosiderotic Hemangioma
Targetoid hemosiderotic hemangioma (THH), also known as hobnail hemangioma, is a benign vascular tumor that usually occurs in young or middle-aged adults. It most commonly presents on the extremities or trunk as an isolated red-brown plaque or papule.1,2 Histologically, THH is characterized by superficial dilated ectatic vessels with underlying proliferating vascular channels lined by plump hobnail endothelial cells.1 Targetoid hemosiderotic hemangioma typically involves the dermis and spares the subcutis. The vascular channels may contain erythrocytes as well as pale eosinophilic lymph, as seen in our patient (quiz image). The deeper dermis contains vascular spaces that are more angulated and smaller and appear to be dissecting through the collagen bundles or collapsed.1,3 A variable amount of hemosiderin deposition and extravasated erythrocytes are seen.2,3 Histologic features evolve with the age of the lesion. Increasing amounts of hemosiderin deposition and erythrocyte extravasation may correspond histologically to the recent clinical color change reported by the patient.
Verrucous hemangioma is a rare congenital vascular abnormality that is characterized by dilated vessels in the papillary dermis along with acanthosis, hyperkeratosis, and irregular papillomatosis, as seen in angiokeratoma.4 However, the vascular proliferation composed of variably sized, thin-walled capillaries extends into the deep dermis as well as the subcutis (Figure 1). Verrucous hemangioma most commonly is reported on the legs and generally starts as a violaceous patch that progresses into a hyperkeratotic verrucous plaque or nodule.5,6

Angiokeratoma is characterized by superficial vascular ectasia of the papillary dermis in association with overlying acanthosis, hyperkeratosis, and rete elongation.7 The dilated vascular spaces appear encircled by the epidermis (Figure 2). Intravascular thrombosis can be seen within the ectatic vessels.7 In contrast to verrucous hemangioma, angiokeratoma is limited to the papillary dermis. Therefore, obtaining a biopsy of sufficient depth is necessary for differentiation.8 There are 5 clinical presentations of angiokeratoma: sporadic, angiokeratoma of Mibelli, angiokeratoma of Fordyce, angiokeratoma circumscriptum, and angiokeratoma corporis diffusum (Fabry disease). Angiokeratomas may present on the lower extremities, tongue, trunk, and scrotum as hyperkeratotic, dark red to purple or black papules.7

There are 3 clinical stages of Kaposi sarcoma: patch, plaque, and nodular stages. The patch stage is characterized histologically by vascular channels that dissect through the dermis and extend around native vessels (the promontory sign)(Figure 3).9,10 These features can show histologic overlap with THH. The plaque stage shows a more diffuse dermal vascular proliferation, increased cellularity of spindle cells, and possible extension into the subcutis.9,10 Focal plasma cells, hemosiderin, and extravasated red blood cells can be seen. The nodular stage is characterized by a proliferation of spindle cells with red blood cells squeezed between slitlike vascular spaces, hyaline globules, and scattered mitotic figures, but not atypical forms.10 In this stage, plasma cells and hemosiderin are more readily identifiable. A biopsy from the nodular stage is unlikely to enter the histologic differential diagnosis with THH. Clinically, there are 4 variants of Kaposi sarcoma: the classic or sporadic form, an endemic form, iatrogenic, and AIDS associated. Overall, it is more common in males and can occur at any age.10 Human herpesvirus 8 is seen in all forms, and infected cells can be highlighted by the immunohistochemical stain for latent nuclear antigen 1.9,10

Angiosarcoma is a malignant endothelial tumor of soft tissue, skin, bone, and visceral organs.11,12 Clinically, cutaneous angiosarcoma can present in a variety of ways, including single or multiple bluish red lesions that can ulcerate or bleed; violaceous nodules or plaques; and hematomalike lesions that can mimic epithelial neoplasms including squamous cell carcinoma, basal cell carcinoma, and malignant melanoma.11,13,14 The cutaneous lesions most commonly occur on sun-exposed skin, particularly on the face and scalp.12 Other clinical variants that are important to recognize are postradiation angiosarcoma, characterized by MYC gene amplification, and lymphedema-associated angiosarcoma (Stewart-Treves syndrome). Angiosarcoma can have a variety of morphologic features, ranging from well to poorly differentiated. Classically, angiosarcoma is characterized by infiltrating vascular spaces lined by atypical endothelial cells (Figure 4). Poorly differentiated angiosarcoma can demonstrate spindle, epithelioid, or polygonal cells with increased mitotic activity, pleomorphism, and irregular vascular spaces.11 Endothelial markers such as ERG (erythroblast transformation specific-related gene)(nuclear) and CD31 (membranous) can be used to aid in the diagnosis of a poorly differentiated lesion. Epithelioid angiosarcoma also occasionally stains with cytokeratins.13,14

- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Joyce JC, Keith PJ, Szabo S, et al. Superficial hemosiderotic lymphovascular malformation (hobnail hemangioma): a report of six cases. Pediatr Dermatol. 2014;31:281-285.
- Sahin MT, Demir MA, Gunduz K, et al. Targetoid haemosiderotic haemangioma: dermoscopic monitoring of three cases and review of the literature. Clin Exp Dermatol. 2005;30:672-676.
- Kakizaki P, Valente NY, Paiva DL, et al. Targetoid hemosiderotic hemangioma--case report. An Bras Dermatol. 2014;89:956-959.
- Oppermann K, Boff AL, Bonamigo RR. Verrucous hemangioma and histopathological differential diagnosis with angiokeratoma circumscriptum neviforme. An Bras Dermatol. 2018;93:712-715.
- Boccara, O, Ariche-Maman, S, Hadj-Rabia, S, et al. Verrucous hemangioma (also known as verrucous venous malformation): a vascular anomaly frequently misdiagnosed as a lymphatic malformation. Pediatr Dermatol. 2018;35:E378-E381.
- Mestre T, Amaro C, Freitas I. Verrucous haemangioma: a diagnosis to consider [published online June 4, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204612
- Ivy H, Julian CA. Angiokeratoma circumscriptum. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK549769/
- Shetty S, Geetha V, Rao R, et al. Verrucous hemangioma: importance of a deeper biopsy. Indian J Dermatopathol Diagn Dermatol. 2014;1:99-100.
- Bishop BN, Lynch DT. Cancer, Kaposi sarcoma. StatPearls. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK534839/
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17-28.
- Ambujam S, Audhya M, Reddy A, et al. Cutaneous angiosarcoma of the head, neck, and face of the elderly in type 5 skin. J Cutan Aesthet Surg. 2013;6:45-47.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.

A 35-year-old man presented with a reddish brown papule on the left upper chest of 1 year’s duration that had changed color to reddish purple. Physical examination revealed a 6-mm violaceous papule with an erythematous rim.
Palbociclib+ET fails to prolong PFS in AI-resistant metastatic breast cancer
Key clinical point: Palbociclib+endocrine therapy (ET) did not yield superior progression-free survival (PFS) vs. capecitabine in patients with aromatase inhibitor-resistant metastatic breast cancer (AIR-MBC) but was well tolerated and improved quality of life (QoL).
Major finding: Median PFS with palbociclib+fulvestrant vs. capecitabine was 7.5 vs. 10.0 months (adjusted hazard ratio [aHR], 1.13; P = .398). Palbociclib+ET did not show PFS superiority vs. capecitabine in patients with wild-type estrogen receptor-1 AIR-MBC (8.0 vs. 10.6 months; aHR, 1.11; P = .404). Palbociclib+ET was better tolerated and improved QoL (aHR, 0.67; P = .001).
Study details: Phase 3 PEARL trial randomly allocated postmenopausal women with AIR-MBC to receive either palbociclib+exemestane or capecitabine (n=296) or palbociclib+fulvestrant or capecitabine (n=305).
Disclosures: This study was supported by Pfizer and AstraZeneca and sponsored by GEICAM Spanish Breast Cancer Group. The lead author reported ties with AstraZeneca, Pfizer, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, and Daiichi-Sankyo. Other investigators reported owning stocks, being an employee, receiving support, and/or consulting for various pharmaceutical companies including Pfizer and AstraZeneca.
Source: Martin M et al. Ann Oncol. 2020 Dec 29. doi: 10.1016/j.annonc.2020.12.013.
Key clinical point: Palbociclib+endocrine therapy (ET) did not yield superior progression-free survival (PFS) vs. capecitabine in patients with aromatase inhibitor-resistant metastatic breast cancer (AIR-MBC) but was well tolerated and improved quality of life (QoL).
Major finding: Median PFS with palbociclib+fulvestrant vs. capecitabine was 7.5 vs. 10.0 months (adjusted hazard ratio [aHR], 1.13; P = .398). Palbociclib+ET did not show PFS superiority vs. capecitabine in patients with wild-type estrogen receptor-1 AIR-MBC (8.0 vs. 10.6 months; aHR, 1.11; P = .404). Palbociclib+ET was better tolerated and improved QoL (aHR, 0.67; P = .001).
Study details: Phase 3 PEARL trial randomly allocated postmenopausal women with AIR-MBC to receive either palbociclib+exemestane or capecitabine (n=296) or palbociclib+fulvestrant or capecitabine (n=305).
Disclosures: This study was supported by Pfizer and AstraZeneca and sponsored by GEICAM Spanish Breast Cancer Group. The lead author reported ties with AstraZeneca, Pfizer, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, and Daiichi-Sankyo. Other investigators reported owning stocks, being an employee, receiving support, and/or consulting for various pharmaceutical companies including Pfizer and AstraZeneca.
Source: Martin M et al. Ann Oncol. 2020 Dec 29. doi: 10.1016/j.annonc.2020.12.013.
Key clinical point: Palbociclib+endocrine therapy (ET) did not yield superior progression-free survival (PFS) vs. capecitabine in patients with aromatase inhibitor-resistant metastatic breast cancer (AIR-MBC) but was well tolerated and improved quality of life (QoL).
Major finding: Median PFS with palbociclib+fulvestrant vs. capecitabine was 7.5 vs. 10.0 months (adjusted hazard ratio [aHR], 1.13; P = .398). Palbociclib+ET did not show PFS superiority vs. capecitabine in patients with wild-type estrogen receptor-1 AIR-MBC (8.0 vs. 10.6 months; aHR, 1.11; P = .404). Palbociclib+ET was better tolerated and improved QoL (aHR, 0.67; P = .001).
Study details: Phase 3 PEARL trial randomly allocated postmenopausal women with AIR-MBC to receive either palbociclib+exemestane or capecitabine (n=296) or palbociclib+fulvestrant or capecitabine (n=305).
Disclosures: This study was supported by Pfizer and AstraZeneca and sponsored by GEICAM Spanish Breast Cancer Group. The lead author reported ties with AstraZeneca, Pfizer, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, and Daiichi-Sankyo. Other investigators reported owning stocks, being an employee, receiving support, and/or consulting for various pharmaceutical companies including Pfizer and AstraZeneca.
Source: Martin M et al. Ann Oncol. 2020 Dec 29. doi: 10.1016/j.annonc.2020.12.013.
One-third of HER2+ and triple-negative metastatic breast cancer patients develop brain metastases
Key clinical point: Incidence of brain metastases is high in patients with human epidermal growth factor receptor 2-positive (HER2+) and triple-negative metastatic breast cancer (MBC), highlighting the need for relevant screening in these high-risk populations.
Major finding: The pooled cumulative incidence of brain metastases in patients with HER2+ and triple-negative MBC was 31% (interquartile range [IQR], 24.0-34.0) and 32% (IQR,18.5-40.6) during a median follow-up of 30.7 and 32.8 months, respectively. The incidence of brain metastases per patient-year was 13% for HER2+ and triple negative MBC.
Study details: Meta-analysis of 25 studies including patients with HER2+ MBC (n=5,971), triple-negative (n=4,102), and hormone receptor+/HER2− MBC (n=14,656).
Disclosures: This study did not receive any funding. The lead author reported no disclosures. Some of his coinvestigators reported ties with various pharmaceutical companies.
Source: Kuksis M et al. Neuro Oncol. 2020 Dec 23. doi: 10.1093/neuonc/noaa285.
Key clinical point: Incidence of brain metastases is high in patients with human epidermal growth factor receptor 2-positive (HER2+) and triple-negative metastatic breast cancer (MBC), highlighting the need for relevant screening in these high-risk populations.
Major finding: The pooled cumulative incidence of brain metastases in patients with HER2+ and triple-negative MBC was 31% (interquartile range [IQR], 24.0-34.0) and 32% (IQR,18.5-40.6) during a median follow-up of 30.7 and 32.8 months, respectively. The incidence of brain metastases per patient-year was 13% for HER2+ and triple negative MBC.
Study details: Meta-analysis of 25 studies including patients with HER2+ MBC (n=5,971), triple-negative (n=4,102), and hormone receptor+/HER2− MBC (n=14,656).
Disclosures: This study did not receive any funding. The lead author reported no disclosures. Some of his coinvestigators reported ties with various pharmaceutical companies.
Source: Kuksis M et al. Neuro Oncol. 2020 Dec 23. doi: 10.1093/neuonc/noaa285.
Key clinical point: Incidence of brain metastases is high in patients with human epidermal growth factor receptor 2-positive (HER2+) and triple-negative metastatic breast cancer (MBC), highlighting the need for relevant screening in these high-risk populations.
Major finding: The pooled cumulative incidence of brain metastases in patients with HER2+ and triple-negative MBC was 31% (interquartile range [IQR], 24.0-34.0) and 32% (IQR,18.5-40.6) during a median follow-up of 30.7 and 32.8 months, respectively. The incidence of brain metastases per patient-year was 13% for HER2+ and triple negative MBC.
Study details: Meta-analysis of 25 studies including patients with HER2+ MBC (n=5,971), triple-negative (n=4,102), and hormone receptor+/HER2− MBC (n=14,656).
Disclosures: This study did not receive any funding. The lead author reported no disclosures. Some of his coinvestigators reported ties with various pharmaceutical companies.
Source: Kuksis M et al. Neuro Oncol. 2020 Dec 23. doi: 10.1093/neuonc/noaa285.
Partial breast irradiation vs. whole breast irradiation for early breast cancer
Key clinical point: Rates of ipsilateral breast tumor recurrence (IBTR) were similar between partial breast irradiation (PBI) and whole breast irradiation (WBI). Acute skin toxicities were lower with PBI.
Major finding: The rate of IBTR at 5 years was not different between PBI (1.8%; 95% highest posterior density [HPD], 0.68%-3.2%) and WBI (1.7%; 95% HPD, 0.92%-2.4%). Rates of grade 2 or greater acute skin toxicity were lower with PBI (7.1%; 95% HPD, 0%-63.4%) vs. WBI (47.5%; 95% HPD, 0%-93.4%).
Study details: Meta-analysis of 7 randomized trials including 9,758 patients receiving either WBI (n=4,840) or PBI (n=4,918).
Disclosures: No funding source was identified. The lead author reported ties with Impedimed, PreludeDX, Varian Medical Systems, and Vision RT. Some other coinvestigators reported receiving grants or consultation fees from various pharmaceutical companies.
Source: Shah C et al. Ann Surg Oncol. 2021 Jan 3. doi: 10.1245/s10434-020-09447-w.
Key clinical point: Rates of ipsilateral breast tumor recurrence (IBTR) were similar between partial breast irradiation (PBI) and whole breast irradiation (WBI). Acute skin toxicities were lower with PBI.
Major finding: The rate of IBTR at 5 years was not different between PBI (1.8%; 95% highest posterior density [HPD], 0.68%-3.2%) and WBI (1.7%; 95% HPD, 0.92%-2.4%). Rates of grade 2 or greater acute skin toxicity were lower with PBI (7.1%; 95% HPD, 0%-63.4%) vs. WBI (47.5%; 95% HPD, 0%-93.4%).
Study details: Meta-analysis of 7 randomized trials including 9,758 patients receiving either WBI (n=4,840) or PBI (n=4,918).
Disclosures: No funding source was identified. The lead author reported ties with Impedimed, PreludeDX, Varian Medical Systems, and Vision RT. Some other coinvestigators reported receiving grants or consultation fees from various pharmaceutical companies.
Source: Shah C et al. Ann Surg Oncol. 2021 Jan 3. doi: 10.1245/s10434-020-09447-w.
Key clinical point: Rates of ipsilateral breast tumor recurrence (IBTR) were similar between partial breast irradiation (PBI) and whole breast irradiation (WBI). Acute skin toxicities were lower with PBI.
Major finding: The rate of IBTR at 5 years was not different between PBI (1.8%; 95% highest posterior density [HPD], 0.68%-3.2%) and WBI (1.7%; 95% HPD, 0.92%-2.4%). Rates of grade 2 or greater acute skin toxicity were lower with PBI (7.1%; 95% HPD, 0%-63.4%) vs. WBI (47.5%; 95% HPD, 0%-93.4%).
Study details: Meta-analysis of 7 randomized trials including 9,758 patients receiving either WBI (n=4,840) or PBI (n=4,918).
Disclosures: No funding source was identified. The lead author reported ties with Impedimed, PreludeDX, Varian Medical Systems, and Vision RT. Some other coinvestigators reported receiving grants or consultation fees from various pharmaceutical companies.
Source: Shah C et al. Ann Surg Oncol. 2021 Jan 3. doi: 10.1245/s10434-020-09447-w.
Pediatric minority patients less likely to undergo ED imaging
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
FROM JAMA NETWORK OPEN
MS bears no effect on certain pregnancy complications, stillbirth, or congenital deformation
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
FROM NEUROLOGY CLINICAL PRACTICE
Believing in conspiracy theories is not delusional
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.