User login
Caught in the Hotbox
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
A 19-year-old woman presented to the emergency department (ED) with a 14-day history of progressive fevers, night sweats, abdominal pain, nonbloody and nonbilious vomiting, diarrhea, cough, and myalgia. The fever occurred daily with no noted temporal pattern, and she had no significant weight loss. The abdominal pain was diffuse and exacerbated by eating. She experienced multiple sporadic episodes of vomiting and diarrhea daily. She denied any rash or arthralgia.
She had no known medical history and took no medications. Family history was negative for autoinflammatory and autoimmune conditions. She had emigrated from Kenya to the United States 28 days ago. Her immunization status was unknown.
This patient has prolonged fevers and evidence of multisystem involvement. The most likely etiologic categories are infectious, inflammatory, rheumatologic, and neoplastic. For febrile patients who have recently emigrated to or travelled outside of the United States, it is important to consider common infections, as well as those endemic to the nation of exposure, which in this case includes malaria, typhoid fever, tuberculosis, cholera, acute viral hepatitis, chikungunya fever, dengue fever, yellow fever, and rickettsial disease. All of these, other than tuberculosis, commonly present with fever, vomiting, diarrhea, and myalgia. She may also have bacterial pneumonia or influenza given her fever and cough, although the chronicity and persistence of symptoms would be atypical. Acute infectious gastroenteritis is a common cause of fever, vomiting, and diarrhea. Most cases resolve in 7 to 10 days, so the duration raises suspicion for a nonviral etiology or an immunocompromised state.
Inflammatory causes could include the first presentation of inflammatory bowel disease (IBD), particularly if the patient develops weight loss or eye, skin, or joint manifestations. The lack of rash or arthralgia makes rheumatologic conditions less likely. Prolonged fevers and night sweats could indicate malignancy such as intra-abdominal lymphoma, although infectious etiologies should be ruled out first.
Previously, on day 9 of symptoms, the patient presented to an ED at another institution. Laboratory evaluation at that time demonstrated an elevated aspartate aminotransferase (AST) level of 229 IU/L (reference, 0-40 IU/L) and alanine aminotransferase (ALT) level of 60 IU/L (reference, 0-32 IU/L) with normal alkaline phosphatase and bilirubin levels, proteinuria to 3+ (normal, negative/trace), ketonuria to 2+ (normal, negative), and hematuria to 2+ (normal, negative). Complete blood count and electrolytes were normal. Computed tomography (CT) scans of the chest, abdomen, and pelvis with intravenous contrast were normal and without evidence of organomegaly.
AST and ALT elevations often indicate hepatocellular damage, although the normal bilirubin levels suggest normal hepatic function. Because CT may miss extrahepatic biliary pathology, a right upper quadrant ultrasound should be obtained to better evaluate patency of the biliary system and hepatic echotexture. Coagulation studies and viral hepatitis serology should be obtained. The disproportionate elevation of AST versus ALT can suggest alcohol use or nonhepatic etiologies such as myositis. Acute viral hepatitis is less likely given there is only mild to moderate elevation in aminotransferase levels. However, the remaining infectious etiologies can have this level of elevation and should still be considered.
Enteritis and IBD are still considerations despite the normal CT results. Transient asymptomatic hematuria or proteinuria can be seen in multiple conditions, particularly proteinuria with febrile illnesses. Urine microscopy to evaluate for casts could indicate a glomerular origin of the hematuria. First morning urine protein-to-creatinine ratio would help quantify the degree of proteinuria. Serum creatinine level should be measured to determine whether there is any renal dysfunction.
While early imaging can be falsely negative, the unremarkable chest CT makes pneumonia and active pulmonary tuberculosis less likely.
Vital signs during this presentation were: temperature, 39.7 °C; heart rate, 126 beats per minute; blood pressure, 109/64 mm Hg; respiratory rate, 20 breaths per minute; and oxygen saturation, 98% on room air. She was ill-appearing, with diffuse abdominal tenderness without peritoneal signs. Other than her tachycardia, findings from her cardiopulmonary, neurologic, and skin exams were normal.
Laboratory testing revealed a white blood cell count of 4,300/µL (reference range, 4,500-13,000/µL), a hemoglobin level of 10.9 g/dL (reference range, 11.7-15.7 g/dL) with a mean corpuscular volume of 77 fL (reference range, 80-96 fL) and reticulocyte percentage of 0.5% (reference range, 0.5%-1.5%), and a platelet count of 59,000/µL (reference range, 135,000-466,000/µL). Her prothrombin time was 13.5 seconds (reference range, 9.6-11.6 seconds) with an international normalized ratio of 1.3 (reference range, 0.8-1.1), erythrocyte sedimentation rate of 46 mm/h (reference range, 0-20 mm/h), C-reactive protein level of 7.49 mg/dL (reference range, <0.3 mg/dL), and AST level of 194 units/L (reference range, 9-35 units/L). ALT, total and direct bilirubin, lipase, electrolytes, and creatinine levels were normal. An abdominal x-ray showed scattered air-fluid levels in a nonobstructed pattern.
Her mildly elevated prothrombin time and international normalized ratio suggest a coagulopathy involving either her extrinsic or common coagulation pathway, with disseminated intravascular coagulation (DIC) being most likely given her new thrombocytopenia and anemia. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura should be considered but would not cause coagulopathy. A peripheral smear to evaluate for schistocytes associated with microangiopathic hemolysis and serum fibrinogen to distinguish between DIC (low) and thrombocytopenic purpura or hemolytic uremic syndrome (normal or elevated) should be obtained. A thick and thin smear for malaria should also be performed.
Her new pancytopenia suggests bone marrow suppression or infiltration with or without a concomitant consumptive process such as sepsis with resulting DIC. Given her clinical picture, marrow infiltrative processes might include tuberculosis or malignancy, and marrow suppression may be caused by HIV or other viral infection. If she is found to have HIV, disseminated fungal or mycobacterial infections would become more likely. She now has an isolated elevated AST level, which could be secondary to hemolysis rather than hepatocyte damage. Findings from her abdominal exam are nonfocal; this is consistent with her x-ray findings, which reflect possible enteritis or colitis.
The most likely diagnosis currently is an infectious enteritis with resulting hematologic and hepatic abnormalities. Given her recent emigration from Kenya, typhoid fever and cholera are both possible, although cholera typically does not present with prolonged fever or severe abdominal pain. The severity and duration of her illness, and the abnormalities of her laboratory findings, warrant empiric therapy with ceftriaxone to treat possible severe Salmonella enterica infection while awaiting blood and stool cultures.
The patient was admitted to the hospital and her symptoms continued. Results of serum HIV 1 and 2 polymerase chain reactions, herpes simplex virus 1 and 2 polymerase chain reactions, three malaria smears, human T-lymphotropic virus serologies, Toxoplasma serology, Bartonella serology, a stool culture, and multiple large volume blood cultures were negative. Serologic testing for hepatitis A, B, and C, Epstein-Barr virus, cytomegalovirus, and dengue virus were negative for acute infection. Results of an interferon-gamma release assay were indeterminate; results of purified protein derivative (PPD) and Candida antigen control testing were both negative. Ceruloplasmin and α1-antitrypsin levels were normal. An abdominal ultrasound showed central intrahepatic biliary duct dilatation, splenomegaly, and sluggish portal venous flow.
While central intrahepatic biliary ductal dilation could be caused by an obstructive lesion, none were seen on CT or ultrasound. Her normal alkaline phosphatase and bilirbuin levels also suggest functional patency of the biliary system. The thrombocytopenia, splenomegaly, and sluggish portal venous flow suggest possible portal hypertension, though no cirrhotic changes were noted on the ultrasound or abdominal CT. Her negative PPD and Candida antigen control results may suggest underlying immune dysregulation or suppression, though anergy could be secondary to sepsis.
Given her negative initial infectious evaluation, other etiologies such as atypical infections, rheumatologic disorders, and malignancies warrant consideration. She has no murmur; however, subacute bacterial endocarditis with a fastidious organism is possible, which could be investigated with a transthoracic echocardiogram. Other tests to consider include blood cultures for fungi and atypical mycobacterial species, and serology for Coxiella burnetii, chikungunya virus, and yellow fever. Rheumatologic conditions such as systemic lupus erythematosus, hemophagocytic lymphohistiocytosis (HLH), or adult Still’s disease should be considered. Complement levels and an antinuclear antibody panel, including those for dsDNA and Smith antigen, should be performed to evaluate for systemic lupus erythematosus. Serum ferritin, fibrinogen, and triglyceride levels should be measured to evaluate for HLH. Hematologic malignancy is also a consideration, particularly given her pancytopenia. Multicentric Castleman disease can cause prolonged fevers, pancytopenia, and elevated inflammatory markers, but is less likely without lymphadenopathy. A peripheral blood smear should be sent, and a bone marrow biopsy may be needed.
Empiric ciprofloxacin was initiated; however, the patient continued to have fevers up to 39.9 °C, abdominal pain, and myalgia. Ferritin level was over 3,000 ng/mL (reference range, 8-255 ng/mL), and a soluble interleukin-2 (IL-2) receptor level was 1,188 units/mL (reference range, 45-1,105 units/mL). Triglycerides were normal.
The elevated ferritin and soluble IL-2 levels raise concern for HLH. Hyperferritinemia is relatively nonspecific because extremely elevated ferritin can be seen with other conditions, such as renal failure, hepatocellular injury, infection, rheumatologic conditions, and hematologic malignancy. Soluble IL-2 receptor elevation is more specific for HLH than ferritin or triglycerides, but alone does not make the diagnosis because it can be elevated in other rheumatologic disorders and malignancy. The HLH-2004 criteria are commonly used and require either molecular diagnostic testing or meeting at least five out of eight clinical and lab criteria to make the diagnosis. Our patient currently meets three criteria (fever, splenomegaly, and elevated ferritin). Elevated soluble IL-2 is part of the HLH-2004 criteria, but her level of elevation does not meet the required threshold (≥2,400 units/mL). Her cytopenias have also not quite met the HLH-2004 thresholds (two of the following three: hemoglobin <9 g/dL, platelets <100,000/µL, and/or absolute neutrophil count <1,000/µL). Elevated aminotransferase levels and DIC are not part of the HLH-2004 criteria but are often seen with HLH.
Evaluation for an underlying infectious, rheumatologic, or malignant trigger should continue as previously discussed. If this patient does have HLH, it is most likely secondary to an infection, autoimmune disease, or malignancy rather than genetic HLH. HLH has a high mortality rate, but before beginning treatment with immunosuppressive agents, a peripheral smear and a bone marrow biopsy should be performed to evaluate for hematologic malignancy or signs of hemophagocytosis.
Empiric ciprofloxacin covers most bacterial etiologies of diarrhea, including those previously mentioned such as cholera and most strains of S enterica. Her symptoms and laboratory findings (including cytopenias, elevated aminotransferases, and coagulopathy) could suggest enteric fever due to S enterica serovar Typhi, which is endemic in Kenya. Results of blood and stool cultures, though negative, are relatively insensitive for this organism, particularly this far into the illness course. A bone marrow biopsy may also help with diagnosis of occult typhoid fever because marrow culture can be more sensitive than blood or stool culture.
A bone marrow aspiration revealed hemophagocytic histiocytes, no malignant cells, and negative bacterial (including anaerobic), fungal, and acid-fast bacilli cultures. Considering the high mortality rate of untreated HLH/macrophage activation syndrome (MAS), empiric glucocorticoid administration was considered. However, this was withheld due to concern for ongoing undetected infection given her persistent fever and systemic symptoms.
There should still be high suspicion for HLH. Further evaluation for other laboratory manifestations of HLH such as fibrinogen and natural killer cell activity should be considered, as well as repeating her complete blood count to see if her cytopenias have progressed. Her marrow shows no evidence of hematologic malignancy, so other triggers of possible HLH should be sought out by continuing the workup. Consulting specialists from rheumatology and infectious disease may help clarify possible underlying diagnoses and the best management plan. If she continues to have organ damage or clinically worsens, it may be prudent to empirically broaden her antibiotic coverage and begin antifungal agents while starting glucocorticoid therapy for suspected HLH.
A stool molecular screen from admission was returned positive for S enterica serovar Typhi. Ciprofloxacin was discontinued and ceftriaxone was started out of concern for antibiotic resistance. On hospital day 14, the patient’s brother presented to the ED with fever. His blood and stool cultures were positive for S enterica serovar Typhi with intermediate sensitivity to ciprofloxacin and sensitivity to ceftriaxone. With continued treatment with ceftriaxone, the patient improved significantly. Following discharge, she remained afebrile and asymptomatic. During outpatient follow up, a repeat PPD was positive and she was diagnosed with and treated for latent tuberculosis.
COMMENTARY
The evaluation of a patient who has recently emigrated from a foreign nation requires a broad differential diagnosis and a keen awareness of the clinical conditions present in the patient’s country of origin. This often involves knowledge of diseases infrequently encountered in daily practice, as well as awareness of the nuances of rare presentations and possible complications. When the presentation is not classic for a relevant infectious disease and clinical conditions from other diagnostic classes are considered, the evaluation and management of the patient is particularly challenging.
Typhoid fever is a severe systemic illness caused by the organism S enterica serovar Typhi. The organism is ingested, penetrates the small intestinal epithelium, enters the lymphoid tissue, and disseminates via the lymphatic and hematogenous routes. Onset of symptoms typically occurs 5 to 21 days after ingestion of contaminated food or water. Clinical features include fever, chills, relative bradycardia (pulse-temperature dissociation), abdominal pain, rose spots (salmon-colored macules) on the trunk and abdomen, and hepatosplenomegaly. Diarrhea is not a typical symptom of patients with typhoid fever, which can lead to a delayed or missed diagnosis. Life-threatening complications can be seen, including gastrointestinal bleeding, intestinal perforation, and meningitis.1 Typhoid fever is most prevalent in impoverished areas with poor access to sanitation. Regions with the highest incidence include south-central Asia, southeast Asia, and southern Africa.2-4 Approximately 200 to 300 cases are reported in the United States each year.5
Classically, the diagnosis is made by means of clinical symptoms and a positive culture from a sterile site. A recent study of 529 patients found that 61% had positive blood cultures and 96% had positive bone marrow cultures.6 Our patient’s diagnosis was significantly delayed by multiple negative cultures and failure to improve on first-line antibiotics, which initially suggested that the S enterica serovar Typhi stool molecular screen likely represented carriage caused by colonization. Chronic S enterica serovar Typhi carriage is defined as excretion of the organism in stool or urine 1 year or longer after acute infection. Rates of carriage range from 1% to 6%, and up to 25% of carriers have no history of typhoid fever.1,7,8 Carriage is more common in females and in those with biliary tract abnormalities.9,10
Once a presumptive diagnosis is made, antibiotic choice remains a challenge. Resistance to fluoroquinolones, the preferred drug for multidrug-resistant typhoid fever, is growing but remains rare, at approximately 5%.11,12 Ceftriaxone and azithromycin have been used successfully in areas with high resistance.13 Given the patient’s slow response to therapy even after transitioning from ciprofloxacin to ceftriaxone, her brother’s presentation and obtaining the antibiotic sensitivities for his organism were critical to confirming that our diagnosis and management decisions were correct.
One strongly considered diagnosis was HLH/MAS. MAS is an aggressive syndrome of excessive inflammation and tissue destruction caused by inappropriate immune system activation. It belongs to a group of histiocytic disorders collectively known as HLH. Aside from primary (genetic) forms, secondary forms exist that can be triggered by malignancy, infection, or rheumatologic disorders. In infection-associated HLH/MAS, viral infections are a common trigger, with Epstein-Barr virus being the most common. Association with bacterial infections, including tuberculosis and typhoid fever, has also been reported.14 Prompt therapy, often with immunosuppressive agents such as glucocorticoids, is essential for survival because there is a reported mortality rate of up to 50% when untreated.15 When infection-induced HLH/MAS occurs, treatment of the underlying infection is critical.14,15 The greatest barrier to a favorable outcome from HLH/MAS is often a delay in diagnosis because the rarity of this disease, the variable clinical presentation, and the lack of specificity of the clinical and laboratory findings make a conclusive diagnosis challenging.
In the presented case, diagnostic uncertainty challenged the decision to administer systemic glucocorticoids. Glucocorticoids conferred a risk of harm for multiple diagnoses that remained on the differential, including malignancy and infection. Her diagnostic evaluation made malignancy less likely, but because testing was unable to rule out tuberculosis as either the underlying cause or coinfection, the team opted to defer initiating glucocorticoids and instead closely monitor for a worsening inflammatory response. Following appropriate treatment of her systemic infection, her PPD was repeated and became positive. The negative PPD and Candida control obtained during her hospitalization were, therefore, likely caused by anergy in the setting of overwhelming systemic illness. Initiation of glucocorticoids prematurely in this case could have led to further harm because immunosuppression may have led to reactivation of latent tuberculosis or exacerbation of illness from an alternative but then undiagnosed infection.
The patient’s ultimate unifying diagnosis was typhoid fever; however, there are mixed expert opinions as to whether the systemic immune activation was significant enough to merit the diagnosis of infection-induced secondary HLH/MAS. Despite the high morbidity and mortality that can accompany HLH/MAS, it has been reported that a significant proportion of cases of secondary HLH/MAS can be managed effectively with treatment of the underlying etiology; this may have been the case for our patient.14,15 The clinicians in this case were caught between diagnoses, unable to safely reach either one—much like a baseball player stranded between bases. Fortunately for this patient, the diagnosis ultimately emerged after a careful and thorough workup, assisted by the more straightforward diagnosis of her brother with the same disease.
KEY TEACHING POINTS
- Salmonella enterica serovar Typhi has a high false-negative rate in blood and stool cultures; therefore, clinical suspicion should remain high in the setting of a high pre-test probability.
- Fluoroquinolones are traditionally first-line therapy for typhoid fever, but the use of ceftriaxone and azithromycin is increasing because of rising fluoroquinolone resistance.
- Hemophagocytic lymphohistiocytosis/macrophage activation syndrome is characterized by excessive inflammation and tissue destruction caused by inappropriate immune system activation. This syndrome can be fatal without appropriate immunosuppressive therapy; however, glucocorticoid administration must be pursued with caution when infection and malignancy are on the differential diagnosis.
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
1. Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347(22):1770-1782. https://doi.org/10.1056/nejmra020201
2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346-353.
3. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. https://doi.org/10.7189/jogh.02.010401
4. Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2(10):e570-e580. https://doi.org/10.1016/s2214-109x(14)70301-8
5. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999-2006. JAMA. 2009;302(8):859-865. https://doi.org/10.1001/jama.2009.1229
6. Mogasale V, Ramani E, Mogasale VV, Park J. What proportion of Salmonella typhi cases are detected by blood culture? a systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15(1):32. https://doi.org/10.1186/s12941-016-0147-z
7. Merselis JG Jr, Kaye D, Connolly CS, Hook EW. Quantitative bacteriology of the Typhoid carrier state. Am J Trop Med Hyg. 1964;13:425-429. https://doi.org/10.4269/ajtmh.1964.13.425
8. Lanata CF, Levine MM, Ristori C, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2(8347):441-443. https://doi.org/10.1016/s0140-6736(83)90401-4
9. Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87(9):1198-1199.
10. Hofmann E, Chianale J, Rollán A, Pereira J, Ferrecio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167(4):993-994. https://doi.org/10.1093/infdis/167.4.993
11. Steel AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AKM. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62 (Suppl 1):S4-S8. https://doi.org/10.1093/cid/civ976
12. Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, et al. Genomic signature of multidrug resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol. 2015;53(1):262-272. https://doi.org/10.1128/jcm.02026-14
13. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of Salmonella infections. Clin Microbiol Rev. 2015;28(4):901-936. https://doi.org/10.1128/cmr.00002-15
14. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7(12):814-822. https://doi.org/10.1016/s1473-3099(07)70290-6
15. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6(6):601-608. https://doi.org/10.3201/eid0606.000608
© 2021 Society of Hospital Medicine
Clinical Progress Note: Vascular Access Appropriateness Guidance for Pediatric Hospitalists
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table
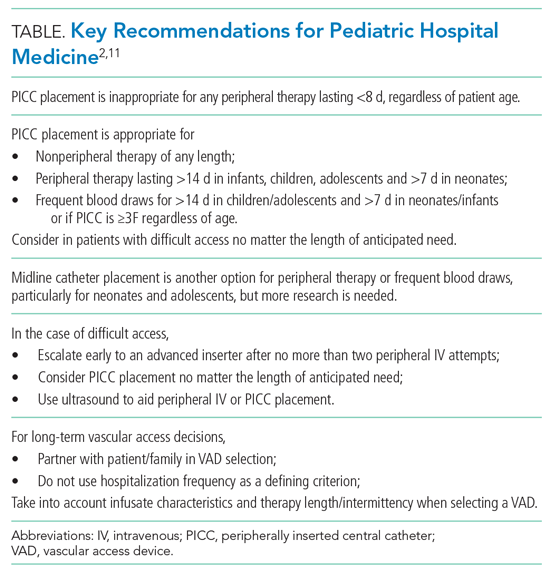
Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
© 2021 Society of Hospital Medicine
SARS-CoV-2 Seroprevalence Among Healthcare Workers by Job Function and Work Location in a New York Inner-City Hospital
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 
Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16
Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).
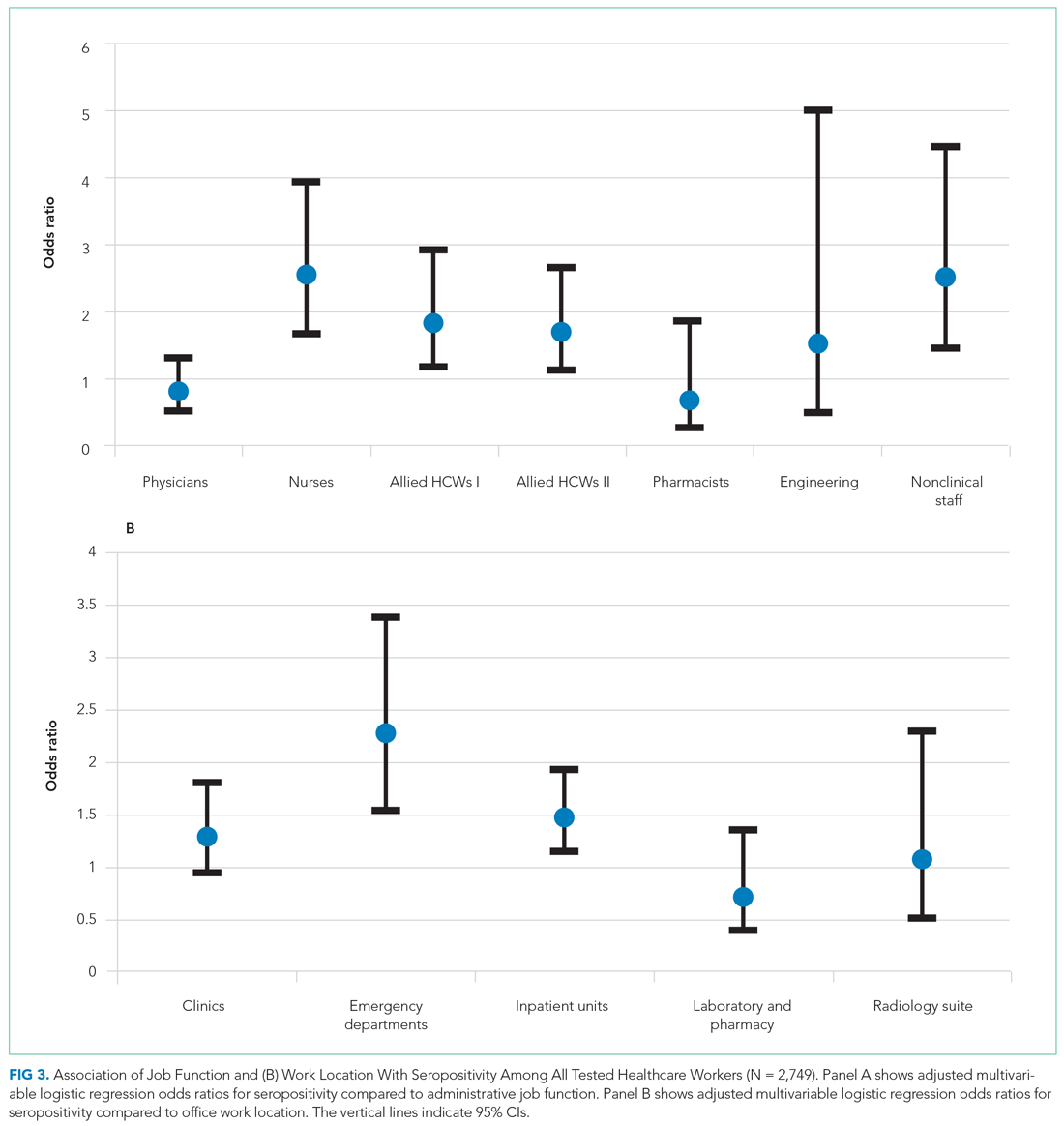
Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 

Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16

Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).

Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 

Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16

Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).

Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
© 2021 Society of Hospital Medicine
SARS-CoV-2 Seroprevalence Among Healthcare Workers by Workplace Exposure Risk in Kashmir, India
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 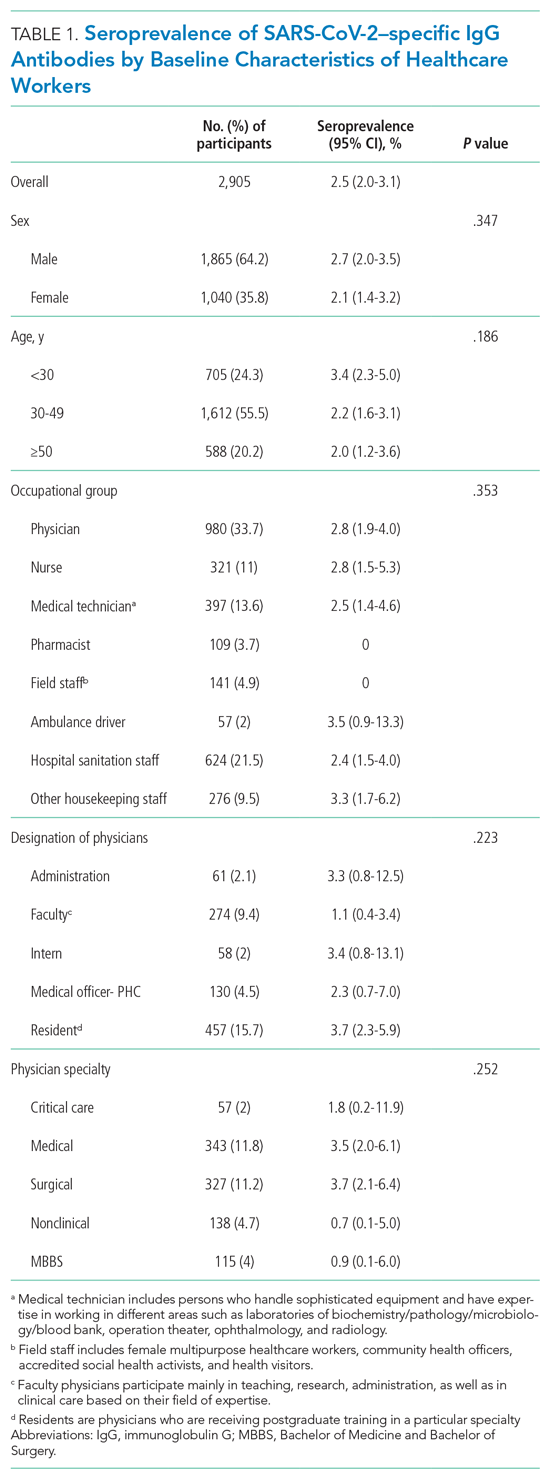

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).
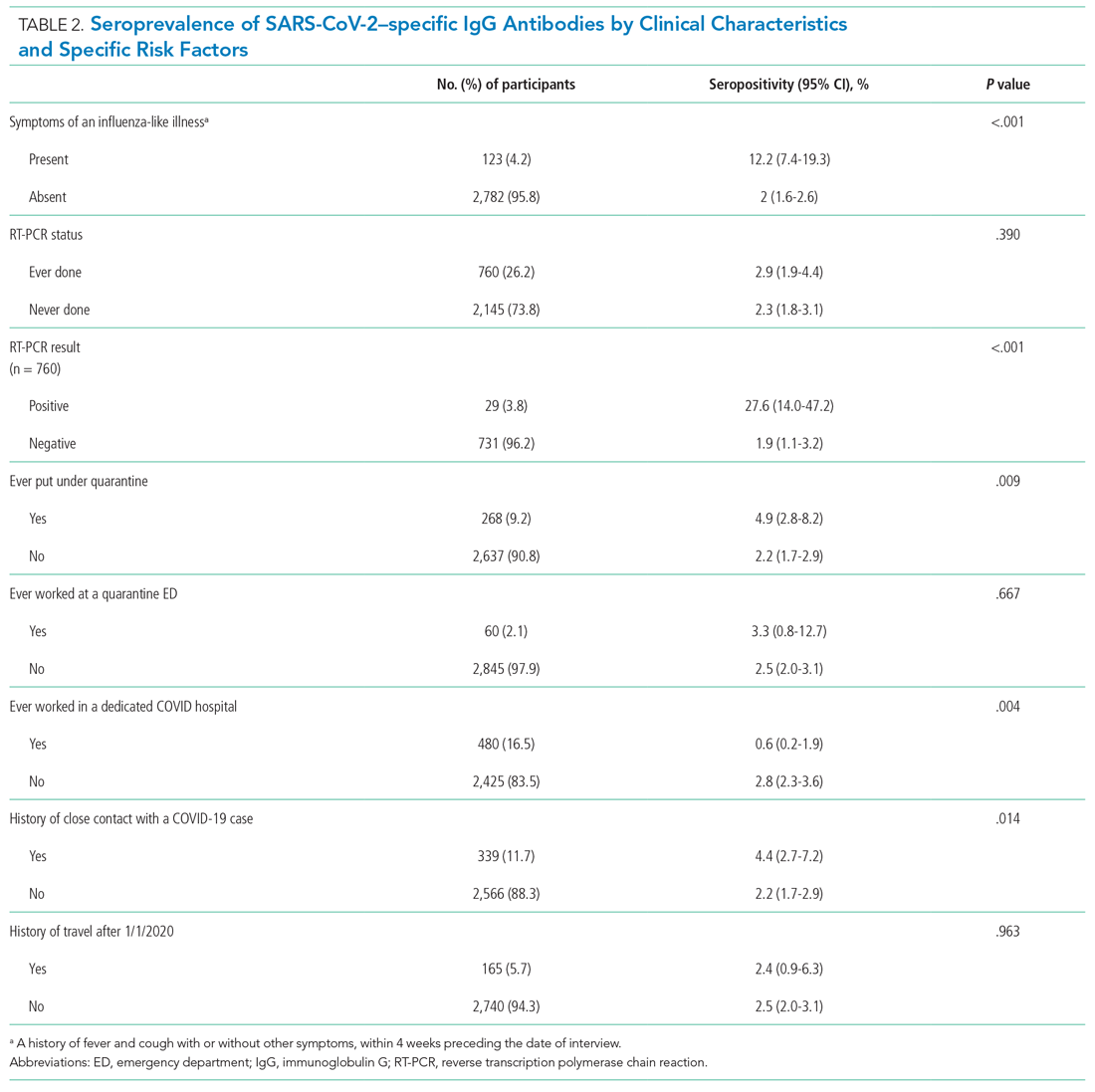
As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).

As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).

As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
©2021 Society of Hospital Medicine
Decreasing Hospital Observation Time for Febrile Infants
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
© 2021 Society of Hospital Medicine
Nine Seasons of a Bronchiolitis Observation Unit and Home Oxygen Therapy Protocol
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.
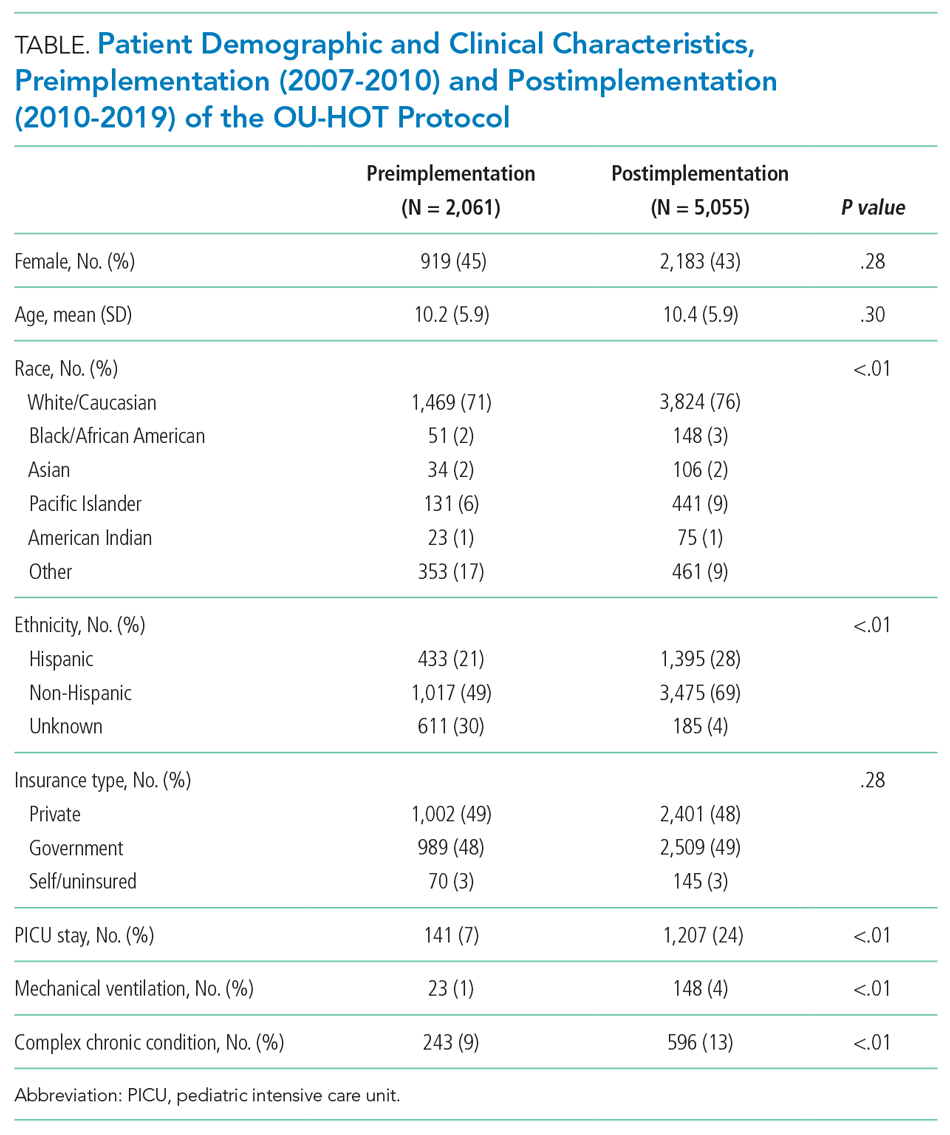
Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).
DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.

Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).

DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.

Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).

DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
© 2021 Society of Hospital Medicine
Epidural use shows no association with autism spectrum disorder in children
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
Exposure to epidural analgesia during labor did not show a link to a later diagnosis of autism spectrum disorder (ASD) in a population-based cohort study published April 19 in JAMA Pediatrics.
Though the initial analysis showed an association, adjustment for a wide range of demographic, medical, and birth factors eliminated the link. The authors note that their findings contrast with those of a cohort study in California published in the same journal last year.
“It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study,” write Elizabeth Wall-Wieler, PhD, of the University of Manitoba in Winnipeg and her colleagues. “To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.”
The researchers analyzed linked datasets from all singleton infants born in a hospital from 2005 to 2016 in Manitoba, Canada, to compare use of epidurals during birth with diagnoses of ASD before 18 months of age. The four data sources included the Statistics Canada, Manitoba Education, Manitoba Families, and Manitoba Health, Seniors and Active Living, which includes the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network. The researchers excluded women with cesarean deliveries because it was not possible to differentiate between scheduled and unscheduled cesarean deliveries.
Among 123,175 children born to mothers with an average age of 28 years, 38.2% had been exposed to epidural analgesia during their labor. Autism diagnoses occurred among 2.1% of those exposed to epidurals and 1.7% of those not exposed to epidurals. After the researchers controlled for a range of potential confounders, the difference became nonsignificant (hazard ratio, 1.08).
The adjusted analysis accounted for mother’s age; high school degree; marital status; neighborhood socioeconomic status; receipt of public assistance during pregnancy; and presence of diabetes, hypertension, anxiety, or depression in the year before the birth. Other covariates included in the adjustment included the following pregnancy factors: “parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use.” The researchers also included birth year, labor induction or augmentation, labor dystocia, fetal distress or macrosomia, gestational age at birth, the infant’s sex, and hospital type.
“There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs. nonexposed to epidural labor analgesia,” the authors report. “For example, births exposed to epidural labor analgesia were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.”
To take family history of ASD into account, the researchers also compared siblings who were and were not exposed to an epidural during labor: 80,459 children in the cohort had at least one sibling in it as well. The researchers still found no association between use of an epidural and a subsequent autism diagnosis (HR, 0.97). The authors conducted several sensitivity analyses for first-born children, those with two or more diagnostic codes for ASD on different days, and women with missing data on high school completion or marital status who delivered at 37 weeks of gestation or later; these results consistently showed no association between epidurals and ASD.
The findings are important but unsurprising, said Scott M. Myers, MD, a neurodevelopmental pediatrician and associate professor at the Geisinger Commonwealth School of Medicine’s Autism & Developmental Medicine Institute in Scranton, Pa. Dr. Myers, who was not involved in the study, said it was strengthened by the inclusion of a wide range of covariates and multiple sensitivity analyses.
“It confirms the suspicion of many experts who were skeptical of the association reported previously, that the small increase in ASD in offspring of mothers who had epidural labor analgesia was likely attributable to other factors that differed substantially between the exposed and unexposed groups,” Dr. Myers said in an interview. “The plausibility of exposure to epidural analgesia in labor having a large effect on ASD risk and accounting for changes in ASD prevalence over time is low.”
It’s possible to hypothesize about subgroups that are genetically susceptible to certain environmental risk factors, including epidurals, but such an association should show up in epidemiological research if the subgroup is large enough.
“For example, epidural labor analgesia can prolong labor, and if it were a significant risk factor for ASD, one might expect that longer labor would have been demonstrated to be associated with increased ASD risk, but this has been examined and is not the case,” he said. He also noted that other perinatal factors previously linked to ASD, such as cesarean delivery, may result from a shared factor that affects risk of both ASD and cesarean delivery.
“Although there haven’t been enough systematic postmortem brain studies to be certain that the findings are generalizable, the most consistent neuropathological findings associated with ASD clearly arise long before birth,”Dr. Myers said. “The information I would provide to a concerned pregnant mother is that the current weight of the evidence does not suggest an association between epidural analgesia in labor and increased likelihood of ASD in offspring, much less a causal association.”
Clay Jones, MD, a hospitalist specializing in neonatal-perinatal medicine at Newton (Mass.)–Wellesley Hospital, was not involved in the research and offered a similar assessment of the findings.
“Our understanding of autism is that it is more of a genetic condition which interferes with the organization of brain architecture, so the evidence for any environmental cause would need to be robust for it to change medical practice or our recommendations to the general public,” Dr. Jones said in an interview. Compared to the previous California study, “this new research is larger and better accounts for confounding variables that might increase the risk of a child eventually being diagnosed with autism,” he said.
While recognizing the value in conducting studies to uncover any potential environmental factors contributing to autism diagnoses, Dr. Jones also addressed science communication challenges related to this research.
“While many of these studies are valid early efforts at honing in on potential risk factors, they can be overhyped and lead to increased patient anxiety and potentially harmful changes in behavior,” Dr. Jones said. “There is already a significant amount of pressure for many women to avoid certain safe and effective pain reduction strategies during labor, such as epidural labor analgesia. This pressure is often based on misunderstandings of the risks, pseudoscientific beliefs regarding the benefits of so-called ‘natural childbirth,’ and blatant misogyny. I hope that this new study helps to reassure women that it is okay to request to be more comfortable during their labor experience with the help of epidural labor analgesia.”
The authors of the study also noted the benefits of epidural use during labor.
“It is recognized as the most effective method of providing labor analgesia,” they write. In addition, “the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.”
JAMA Pediatrics editor Dimitri A. Christakis, MD, MPH, wrote his second-ever Editor’s Note about this topic after the journal published two similar studies with different conclusions.
“Because there will never be experimental studies of environmental exposures, we are left with imperfect observational studies that are always at risk for residual confounding, especially when observed effect sizes are small,” Dr. Christakis writes. “Science is an imperfect and iterative process, and our responsibility as journal editors is to manage the process as best we can. Publishing two conflicting studies in such a short time frame serves as testament that we recognize the process for what it is.” His personal opinion is that any association has yet to be definitively established but that the journal will publish the study if a more definitive one is done.
In considering potentially contributing environmental risk factors to ASD, Gillian E. Hanley, PhD, of the University of British Columbia in Vancouver and two colleagues write that “meta-analyses have been unable to identify a single perinatal and neonatal factor associated with ASD risk, although some evidence suggested that exposure to a broad class of conditions such as fetal presentation, umbilical-cord complications, fetal distress, or multiple births, reflecting compromised neonatal health, may increase risk.”
Yet, they add, these studies are inconsistent in their effect size, likely because of differences in study methodology, comparison groups, sample size, diagnostic criteria, and exposure assessment.
“Thus, we continue to ask questions about whether biologically plausible associations exist or whether associations reflect residual confounding related to yet-to-be-determined maternal genetic or environmental factors,” Dr. Hanley and her colleagues write. They discuss precise differences between the California and Manitoba studies and the inevitability of selection bias since people who choose an epidural will differ in other ways from those who don’t.
“Epidural labor analgesia is an extremely effective approach to obstetric analgesia, and we have a collective responsibility to understand whether it is a safe option that sets a healthy developmental pathway well into childhood,” Dr. Hanley and her colleagues conclude. “Women have the right to make a truly informed choice about their pain relief during labor.”
The research was funded by the Canadian Institutes of Health. One author reported receiving personal fees or grants from Aetion, Alosa Foundation, Lilly, GSK, Pacira, and Takeda. No other authors had disclosures. Dr. Jones, Dr. Myers, and the editorial authors had no disclosures.
FROM JAMA PEDIATRICS
What COVID did to MD income in 2020
, according to the Medscape Physician Compensation Report 2021: The Recovery Begins.
Almost 18,000 physicians in more than 29 specialties told Medscape about their income, hours worked, greatest challenges, and the unexpected impact of COVID-19 on their compensation.
How many physicians avoided massive losses
When the pandemic started around March 2020, “a great many physicians saw reductions in volume at first,” says Robert Pearl, MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University.
Medscape’s survey report shows that a staggering 44% saw a 1%-25% reduction in patient volume, and 9% saw a 26%-50% decline. “That is indeed breathtaking,” Dr. Pearl says.
Several key factors saved many practices from hemorrhaging money, says Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas. “Many physicians used the federal Paycheck Protection Program [PPP] to help keep themselves afloat,” he says. “A large percentage reduced their staff, which reduced their expenses, and many got some of their volume back by transitioning to telemedicine.”
In a 2020 survey for the Physicians Foundation, conducted by Merritt Hawkins, 48% of physicians said their practice had received PPP support, and most of those said the support was enough to allow them to stay open without reducing staff. Only 6% of practices that received PPP support did not stay open.
Telemedicine helped many practices
Early in the pandemic, Medicare reimbursements for telemedicine were equal with those for face-to-face visits. “Since telemedicine takes a third less time than an inpatient visit, doctors could see more patients,” Dr. Pearl says.
The switch was almost instantaneous in some practices. Within 3 days, a 200-provider multispecialty practice in Wilmington, N.C., went from not using telehealth to its being used by all physicians, the Medical Group Management Association reported. By late April, the practice was already back up to about 70% of normal overall production.
However, telemedicine could not help every specialty equally. “Generally, allergists can’t do their allergy testing virtually, and patients with mild problems probably put off visits,” Dr. Pearl says. Allergists experienced a large percentage decline in compensation, according to Medscape’s survey. For some, income fell from $301,000 the prior year to $274,000 this year.
Primary care struggled
Primary care physicians posted lower compensation than they did the prior year, but most rebounded to some degree. A study released in June 2020 projected that, even with telemedicine, primary care physicians would lose an average of $67,774 for the year.
However, Medscape’s survey found that internists’ average compensation declined from $251,000 in the prior year to $248,000, and average family physicians’ compensation actually rose from $234,000.
Pediatricians had a harder slog. Their average compensation sank from $232,000 to $221,000, according to the report. Even with telemedicine, parents of young children were not contacting the doctor. In May 2020, visits by children aged 3-5 years were down by 56%.
Many proceduralists recovered
Procedure-oriented specialties were particularly hard-hit at first, because many hospitals and some states banned all elective surgeries at the beginning of the pandemic.
“In March and April, ophthalmology practices were virtually at a standstill,” says John B. Pinto, an ophthalmology practice management consultant in San Diego. “But by the fourth quarter, operations were back to normal. Practices were fully open, and patients were coming back in.”
Medscape’s survey shows that, by year’s end, compensation was about the same as the year before for orthopedic surgeons ($511,000 in both the 2020 and 2021 reports); cardiologists actually did better ($438,000 in our 2020 report and $459,000 in 2021); and ophthalmologists’ compensation was about the same ($378,000 in our prior report and $379,000 in 2021).
Some other proceduralists, however, did not do as well. Otolaryngologists’ compensation fell to $417,000, the second-biggest percentage drop. “This may be because otolaryngologists’ chief procedures are tonsillectomies, sinus surgery, and nasal surgery, which can be put off,” Dr. Pearl says.
Anesthesiologists, who depend on surgical volume, also did not earn as much in 2020. Their compensation declined from $398,000 in our 2020 report to $378,000 in Medscape’s 2021 report.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day,” an independent anesthesiology practice in Alabama told the MGMA early in the pandemic.
Plastic surgeons now the top earners
The biggest increase in compensation by far was made by plastic surgeons, whose income rose 9.8% over the year before, to $526,000. This put them at the top of the list
Dr. Pearl adds that plastic surgeons can perform their procedures in their offices, rather than in a hospital, where elective surgeries were often canceled.
Mr. Belkin says specialties other than plastic surgery had been offering more boutique cosmetic care even before the pandemic. In 2020, nonsurgical cosmetic procedures such as neurotoxin therapy, dermal filler procedures, chemical peels, and hair removal earned $3.1 billion in revenue, according to a survey by the Aesthetic Society.
Other specialties that earned more even during COVID
In Medscape’s survey, several specialties actually earned more during the pandemic than in 2019. Some specialties, such as critical care and public health, were integral in managing COVID patients and the pandemic.
However, some specialties involved in COVID care did not see an increase. Compensation for infectious disease specialists (at $245,000) and emergency medicine specialists (at $354,000) remained basically unchanged from the prior year, and for pulmonologists, it was slightly down.
Emergency departments reported decreases in volume of 40% or more early in the pandemic, according to the American College of Emergency Physicians. It was reported that patients were avoiding EDs for fear of contracting COVID, and car accidents were down because people ventured out less.
In this year’s report, psychiatrists saw a modest rise in compensation, to $275,000. “There has been an increase in mental health visits in the pandemic,” Dr. Pearl says. In 2020, about 4 in 10 adults in the United States reported symptoms of anxiety or depressive disorder, up from 1 in 10 adults the prior year. In addition, psychiatrists were third on the list of Merritt Hawkins’ most requested recruiting engagements.
Oncologists saw a rise in compensation, from $377,000 to $403,000. “Volume likely did not fall because cancer patients would go through with their chemotherapy in spite of the pandemic,” Dr. Pearl says. “The increase in income might have to do with the usual inflation in the cost of chemotherapy drugs.” Dr. Pinto saw the same trend for retinal surgeons, whose care also cannot be delayed.
Medscape’s survey also reports increases in compensation for rheumatologists, endocrinologists, and neurologists, but it reports small declines among dermatologists, radiologists, and gastroenterologists.
Gender-based pay gap remains in place
The gender-based pay gap in this year’s report is similar to that seen in Medscape’s report for the prior year. Men earned 27% more than women in 2021, compared with 25% more the year before. Some physicians commented that more women physicians maintained flexible or shorter work schedules to help with children who could not go into school.
“Having to be a full-time physician, full-time mom, and full-time teacher during our surge was unbelievable,” a primary care pediatrician in group practice and mother of two reported in November. “I felt pulled in all directions and didn’t do anything well.”
In addition, “men dominate some specialties that seem to have seen a smaller drop in volume in the pandemic, such as emergency medicine, infectious disease, pulmonology, and oncology,” says Halee Fischer-Wright, MD, CEO of MGMA.
Employed physicians shared their employers’ pain
Employed physicians, who typically work at hospitals, shared the financial pains of their institutions, particularly in the early stages of the pandemic. In April, hospital admissions were 34.1% below prepandemic levels, according to a study published in Health Affairs. That figure had risen by June, but it was still 8.3% below prepandemic volume.
By the end of the year, many hospitals and hospital systems were in the black, thanks in large part to generous federal subsidies, but actual operations still lost money for the year. Altogether, 42% of them posted an operational loss in 2020, up from the 23% in 2019, according to a survey by Moody’s Investors Service.
Medscape’s report shows that many employed physicians lost pay in 2020, and for many, pay had not returned to pre-COVID levels. Only 28% of primary care physicians and 32% of specialists who lost pay have seen it restored, according to the report. In addition, 15% of surveyed physicians did not receive an annual raise.
Many employed doctors are paid on the basis of relative value units (RVUs), which is a measure of the value of their work. In many cases, there was not enough work to reach RVU thresholds. Would hospitals and other employers lower RVU targets to meet the problem? “I haven’t seen our clients make concessions to providers along those lines,” Mr. Belkin says.
Physicians had to work longer hours
The Medscape report also found that in 2020, physicians saw fewer patients because each visit took longer.
“With the threat of COVID, in-person visits take more time than before,” Mr. Belkin says. “Physicians and staff have to prepare the exam room after each visit, and doctors must spend more time answering patients’ questions about COVID.”
“The new protocols to keep everyone safe add time between patients, and physicians have to answer patients’ questions about the pandemic and vaccines,” Dr. Fischer-Wright says. “You might see a 20% increase in time spent just on these non–revenue-generating COVID activities.”
Physicians still like their specialty
Although 2020 was a challenging year for physicians, the percentage of those who were satisfied with their specialty choice generally did not slip from the year before. It actually rose for several specialties – most notably, rheumatology, pulmonology, physical medicine and rehabilitation, and nephrology.
One specialty saw a decline in satisfaction with their specialty choice, and that was public health and preventive medicine, which plummeted 16 percentage points to 67% – putting it at the bottom of the list.
Even before the pandemic, many public health departments were chronically underfunded. This problem was possibly exacerbated by the pressures to keep up with COVID reporting and testing responsibilities.
Conclusion
Although 2020 was a wild ride for many physicians, many came out of it with only minor reductions in overall compensation, and some saw increases. Still, some specialties and many individuals experienced terrible financial stress and had to make changes in their lives and their spending in order to stay afloat.
“The biggest inhibitor to getting back to normal had to do with doctors who did not want to return because they did not want to risk getting COVID,” Dr. Pinto reports. But he notes that by February 2021 most doctors were completely vaccinated and could feel safe again.
A version of this article first appeared on Medscape.com.
, according to the Medscape Physician Compensation Report 2021: The Recovery Begins.
Almost 18,000 physicians in more than 29 specialties told Medscape about their income, hours worked, greatest challenges, and the unexpected impact of COVID-19 on their compensation.
How many physicians avoided massive losses
When the pandemic started around March 2020, “a great many physicians saw reductions in volume at first,” says Robert Pearl, MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University.
Medscape’s survey report shows that a staggering 44% saw a 1%-25% reduction in patient volume, and 9% saw a 26%-50% decline. “That is indeed breathtaking,” Dr. Pearl says.
Several key factors saved many practices from hemorrhaging money, says Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas. “Many physicians used the federal Paycheck Protection Program [PPP] to help keep themselves afloat,” he says. “A large percentage reduced their staff, which reduced their expenses, and many got some of their volume back by transitioning to telemedicine.”
In a 2020 survey for the Physicians Foundation, conducted by Merritt Hawkins, 48% of physicians said their practice had received PPP support, and most of those said the support was enough to allow them to stay open without reducing staff. Only 6% of practices that received PPP support did not stay open.
Telemedicine helped many practices
Early in the pandemic, Medicare reimbursements for telemedicine were equal with those for face-to-face visits. “Since telemedicine takes a third less time than an inpatient visit, doctors could see more patients,” Dr. Pearl says.
The switch was almost instantaneous in some practices. Within 3 days, a 200-provider multispecialty practice in Wilmington, N.C., went from not using telehealth to its being used by all physicians, the Medical Group Management Association reported. By late April, the practice was already back up to about 70% of normal overall production.
However, telemedicine could not help every specialty equally. “Generally, allergists can’t do their allergy testing virtually, and patients with mild problems probably put off visits,” Dr. Pearl says. Allergists experienced a large percentage decline in compensation, according to Medscape’s survey. For some, income fell from $301,000 the prior year to $274,000 this year.
Primary care struggled
Primary care physicians posted lower compensation than they did the prior year, but most rebounded to some degree. A study released in June 2020 projected that, even with telemedicine, primary care physicians would lose an average of $67,774 for the year.
However, Medscape’s survey found that internists’ average compensation declined from $251,000 in the prior year to $248,000, and average family physicians’ compensation actually rose from $234,000.
Pediatricians had a harder slog. Their average compensation sank from $232,000 to $221,000, according to the report. Even with telemedicine, parents of young children were not contacting the doctor. In May 2020, visits by children aged 3-5 years were down by 56%.
Many proceduralists recovered
Procedure-oriented specialties were particularly hard-hit at first, because many hospitals and some states banned all elective surgeries at the beginning of the pandemic.
“In March and April, ophthalmology practices were virtually at a standstill,” says John B. Pinto, an ophthalmology practice management consultant in San Diego. “But by the fourth quarter, operations were back to normal. Practices were fully open, and patients were coming back in.”
Medscape’s survey shows that, by year’s end, compensation was about the same as the year before for orthopedic surgeons ($511,000 in both the 2020 and 2021 reports); cardiologists actually did better ($438,000 in our 2020 report and $459,000 in 2021); and ophthalmologists’ compensation was about the same ($378,000 in our prior report and $379,000 in 2021).
Some other proceduralists, however, did not do as well. Otolaryngologists’ compensation fell to $417,000, the second-biggest percentage drop. “This may be because otolaryngologists’ chief procedures are tonsillectomies, sinus surgery, and nasal surgery, which can be put off,” Dr. Pearl says.
Anesthesiologists, who depend on surgical volume, also did not earn as much in 2020. Their compensation declined from $398,000 in our 2020 report to $378,000 in Medscape’s 2021 report.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day,” an independent anesthesiology practice in Alabama told the MGMA early in the pandemic.
Plastic surgeons now the top earners
The biggest increase in compensation by far was made by plastic surgeons, whose income rose 9.8% over the year before, to $526,000. This put them at the top of the list
Dr. Pearl adds that plastic surgeons can perform their procedures in their offices, rather than in a hospital, where elective surgeries were often canceled.
Mr. Belkin says specialties other than plastic surgery had been offering more boutique cosmetic care even before the pandemic. In 2020, nonsurgical cosmetic procedures such as neurotoxin therapy, dermal filler procedures, chemical peels, and hair removal earned $3.1 billion in revenue, according to a survey by the Aesthetic Society.
Other specialties that earned more even during COVID
In Medscape’s survey, several specialties actually earned more during the pandemic than in 2019. Some specialties, such as critical care and public health, were integral in managing COVID patients and the pandemic.
However, some specialties involved in COVID care did not see an increase. Compensation for infectious disease specialists (at $245,000) and emergency medicine specialists (at $354,000) remained basically unchanged from the prior year, and for pulmonologists, it was slightly down.
Emergency departments reported decreases in volume of 40% or more early in the pandemic, according to the American College of Emergency Physicians. It was reported that patients were avoiding EDs for fear of contracting COVID, and car accidents were down because people ventured out less.
In this year’s report, psychiatrists saw a modest rise in compensation, to $275,000. “There has been an increase in mental health visits in the pandemic,” Dr. Pearl says. In 2020, about 4 in 10 adults in the United States reported symptoms of anxiety or depressive disorder, up from 1 in 10 adults the prior year. In addition, psychiatrists were third on the list of Merritt Hawkins’ most requested recruiting engagements.
Oncologists saw a rise in compensation, from $377,000 to $403,000. “Volume likely did not fall because cancer patients would go through with their chemotherapy in spite of the pandemic,” Dr. Pearl says. “The increase in income might have to do with the usual inflation in the cost of chemotherapy drugs.” Dr. Pinto saw the same trend for retinal surgeons, whose care also cannot be delayed.
Medscape’s survey also reports increases in compensation for rheumatologists, endocrinologists, and neurologists, but it reports small declines among dermatologists, radiologists, and gastroenterologists.
Gender-based pay gap remains in place
The gender-based pay gap in this year’s report is similar to that seen in Medscape’s report for the prior year. Men earned 27% more than women in 2021, compared with 25% more the year before. Some physicians commented that more women physicians maintained flexible or shorter work schedules to help with children who could not go into school.
“Having to be a full-time physician, full-time mom, and full-time teacher during our surge was unbelievable,” a primary care pediatrician in group practice and mother of two reported in November. “I felt pulled in all directions and didn’t do anything well.”
In addition, “men dominate some specialties that seem to have seen a smaller drop in volume in the pandemic, such as emergency medicine, infectious disease, pulmonology, and oncology,” says Halee Fischer-Wright, MD, CEO of MGMA.
Employed physicians shared their employers’ pain
Employed physicians, who typically work at hospitals, shared the financial pains of their institutions, particularly in the early stages of the pandemic. In April, hospital admissions were 34.1% below prepandemic levels, according to a study published in Health Affairs. That figure had risen by June, but it was still 8.3% below prepandemic volume.
By the end of the year, many hospitals and hospital systems were in the black, thanks in large part to generous federal subsidies, but actual operations still lost money for the year. Altogether, 42% of them posted an operational loss in 2020, up from the 23% in 2019, according to a survey by Moody’s Investors Service.
Medscape’s report shows that many employed physicians lost pay in 2020, and for many, pay had not returned to pre-COVID levels. Only 28% of primary care physicians and 32% of specialists who lost pay have seen it restored, according to the report. In addition, 15% of surveyed physicians did not receive an annual raise.
Many employed doctors are paid on the basis of relative value units (RVUs), which is a measure of the value of their work. In many cases, there was not enough work to reach RVU thresholds. Would hospitals and other employers lower RVU targets to meet the problem? “I haven’t seen our clients make concessions to providers along those lines,” Mr. Belkin says.
Physicians had to work longer hours
The Medscape report also found that in 2020, physicians saw fewer patients because each visit took longer.
“With the threat of COVID, in-person visits take more time than before,” Mr. Belkin says. “Physicians and staff have to prepare the exam room after each visit, and doctors must spend more time answering patients’ questions about COVID.”
“The new protocols to keep everyone safe add time between patients, and physicians have to answer patients’ questions about the pandemic and vaccines,” Dr. Fischer-Wright says. “You might see a 20% increase in time spent just on these non–revenue-generating COVID activities.”
Physicians still like their specialty
Although 2020 was a challenging year for physicians, the percentage of those who were satisfied with their specialty choice generally did not slip from the year before. It actually rose for several specialties – most notably, rheumatology, pulmonology, physical medicine and rehabilitation, and nephrology.
One specialty saw a decline in satisfaction with their specialty choice, and that was public health and preventive medicine, which plummeted 16 percentage points to 67% – putting it at the bottom of the list.
Even before the pandemic, many public health departments were chronically underfunded. This problem was possibly exacerbated by the pressures to keep up with COVID reporting and testing responsibilities.
Conclusion
Although 2020 was a wild ride for many physicians, many came out of it with only minor reductions in overall compensation, and some saw increases. Still, some specialties and many individuals experienced terrible financial stress and had to make changes in their lives and their spending in order to stay afloat.
“The biggest inhibitor to getting back to normal had to do with doctors who did not want to return because they did not want to risk getting COVID,” Dr. Pinto reports. But he notes that by February 2021 most doctors were completely vaccinated and could feel safe again.
A version of this article first appeared on Medscape.com.
, according to the Medscape Physician Compensation Report 2021: The Recovery Begins.
Almost 18,000 physicians in more than 29 specialties told Medscape about their income, hours worked, greatest challenges, and the unexpected impact of COVID-19 on their compensation.
How many physicians avoided massive losses
When the pandemic started around March 2020, “a great many physicians saw reductions in volume at first,” says Robert Pearl, MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University.
Medscape’s survey report shows that a staggering 44% saw a 1%-25% reduction in patient volume, and 9% saw a 26%-50% decline. “That is indeed breathtaking,” Dr. Pearl says.
Several key factors saved many practices from hemorrhaging money, says Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas. “Many physicians used the federal Paycheck Protection Program [PPP] to help keep themselves afloat,” he says. “A large percentage reduced their staff, which reduced their expenses, and many got some of their volume back by transitioning to telemedicine.”
In a 2020 survey for the Physicians Foundation, conducted by Merritt Hawkins, 48% of physicians said their practice had received PPP support, and most of those said the support was enough to allow them to stay open without reducing staff. Only 6% of practices that received PPP support did not stay open.
Telemedicine helped many practices
Early in the pandemic, Medicare reimbursements for telemedicine were equal with those for face-to-face visits. “Since telemedicine takes a third less time than an inpatient visit, doctors could see more patients,” Dr. Pearl says.
The switch was almost instantaneous in some practices. Within 3 days, a 200-provider multispecialty practice in Wilmington, N.C., went from not using telehealth to its being used by all physicians, the Medical Group Management Association reported. By late April, the practice was already back up to about 70% of normal overall production.
However, telemedicine could not help every specialty equally. “Generally, allergists can’t do their allergy testing virtually, and patients with mild problems probably put off visits,” Dr. Pearl says. Allergists experienced a large percentage decline in compensation, according to Medscape’s survey. For some, income fell from $301,000 the prior year to $274,000 this year.
Primary care struggled
Primary care physicians posted lower compensation than they did the prior year, but most rebounded to some degree. A study released in June 2020 projected that, even with telemedicine, primary care physicians would lose an average of $67,774 for the year.
However, Medscape’s survey found that internists’ average compensation declined from $251,000 in the prior year to $248,000, and average family physicians’ compensation actually rose from $234,000.
Pediatricians had a harder slog. Their average compensation sank from $232,000 to $221,000, according to the report. Even with telemedicine, parents of young children were not contacting the doctor. In May 2020, visits by children aged 3-5 years were down by 56%.
Many proceduralists recovered
Procedure-oriented specialties were particularly hard-hit at first, because many hospitals and some states banned all elective surgeries at the beginning of the pandemic.
“In March and April, ophthalmology practices were virtually at a standstill,” says John B. Pinto, an ophthalmology practice management consultant in San Diego. “But by the fourth quarter, operations were back to normal. Practices were fully open, and patients were coming back in.”
Medscape’s survey shows that, by year’s end, compensation was about the same as the year before for orthopedic surgeons ($511,000 in both the 2020 and 2021 reports); cardiologists actually did better ($438,000 in our 2020 report and $459,000 in 2021); and ophthalmologists’ compensation was about the same ($378,000 in our prior report and $379,000 in 2021).
Some other proceduralists, however, did not do as well. Otolaryngologists’ compensation fell to $417,000, the second-biggest percentage drop. “This may be because otolaryngologists’ chief procedures are tonsillectomies, sinus surgery, and nasal surgery, which can be put off,” Dr. Pearl says.
Anesthesiologists, who depend on surgical volume, also did not earn as much in 2020. Their compensation declined from $398,000 in our 2020 report to $378,000 in Medscape’s 2021 report.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day,” an independent anesthesiology practice in Alabama told the MGMA early in the pandemic.
Plastic surgeons now the top earners
The biggest increase in compensation by far was made by plastic surgeons, whose income rose 9.8% over the year before, to $526,000. This put them at the top of the list
Dr. Pearl adds that plastic surgeons can perform their procedures in their offices, rather than in a hospital, where elective surgeries were often canceled.
Mr. Belkin says specialties other than plastic surgery had been offering more boutique cosmetic care even before the pandemic. In 2020, nonsurgical cosmetic procedures such as neurotoxin therapy, dermal filler procedures, chemical peels, and hair removal earned $3.1 billion in revenue, according to a survey by the Aesthetic Society.
Other specialties that earned more even during COVID
In Medscape’s survey, several specialties actually earned more during the pandemic than in 2019. Some specialties, such as critical care and public health, were integral in managing COVID patients and the pandemic.
However, some specialties involved in COVID care did not see an increase. Compensation for infectious disease specialists (at $245,000) and emergency medicine specialists (at $354,000) remained basically unchanged from the prior year, and for pulmonologists, it was slightly down.
Emergency departments reported decreases in volume of 40% or more early in the pandemic, according to the American College of Emergency Physicians. It was reported that patients were avoiding EDs for fear of contracting COVID, and car accidents were down because people ventured out less.
In this year’s report, psychiatrists saw a modest rise in compensation, to $275,000. “There has been an increase in mental health visits in the pandemic,” Dr. Pearl says. In 2020, about 4 in 10 adults in the United States reported symptoms of anxiety or depressive disorder, up from 1 in 10 adults the prior year. In addition, psychiatrists were third on the list of Merritt Hawkins’ most requested recruiting engagements.
Oncologists saw a rise in compensation, from $377,000 to $403,000. “Volume likely did not fall because cancer patients would go through with their chemotherapy in spite of the pandemic,” Dr. Pearl says. “The increase in income might have to do with the usual inflation in the cost of chemotherapy drugs.” Dr. Pinto saw the same trend for retinal surgeons, whose care also cannot be delayed.
Medscape’s survey also reports increases in compensation for rheumatologists, endocrinologists, and neurologists, but it reports small declines among dermatologists, radiologists, and gastroenterologists.
Gender-based pay gap remains in place
The gender-based pay gap in this year’s report is similar to that seen in Medscape’s report for the prior year. Men earned 27% more than women in 2021, compared with 25% more the year before. Some physicians commented that more women physicians maintained flexible or shorter work schedules to help with children who could not go into school.
“Having to be a full-time physician, full-time mom, and full-time teacher during our surge was unbelievable,” a primary care pediatrician in group practice and mother of two reported in November. “I felt pulled in all directions and didn’t do anything well.”
In addition, “men dominate some specialties that seem to have seen a smaller drop in volume in the pandemic, such as emergency medicine, infectious disease, pulmonology, and oncology,” says Halee Fischer-Wright, MD, CEO of MGMA.
Employed physicians shared their employers’ pain
Employed physicians, who typically work at hospitals, shared the financial pains of their institutions, particularly in the early stages of the pandemic. In April, hospital admissions were 34.1% below prepandemic levels, according to a study published in Health Affairs. That figure had risen by June, but it was still 8.3% below prepandemic volume.
By the end of the year, many hospitals and hospital systems were in the black, thanks in large part to generous federal subsidies, but actual operations still lost money for the year. Altogether, 42% of them posted an operational loss in 2020, up from the 23% in 2019, according to a survey by Moody’s Investors Service.
Medscape’s report shows that many employed physicians lost pay in 2020, and for many, pay had not returned to pre-COVID levels. Only 28% of primary care physicians and 32% of specialists who lost pay have seen it restored, according to the report. In addition, 15% of surveyed physicians did not receive an annual raise.
Many employed doctors are paid on the basis of relative value units (RVUs), which is a measure of the value of their work. In many cases, there was not enough work to reach RVU thresholds. Would hospitals and other employers lower RVU targets to meet the problem? “I haven’t seen our clients make concessions to providers along those lines,” Mr. Belkin says.
Physicians had to work longer hours
The Medscape report also found that in 2020, physicians saw fewer patients because each visit took longer.
“With the threat of COVID, in-person visits take more time than before,” Mr. Belkin says. “Physicians and staff have to prepare the exam room after each visit, and doctors must spend more time answering patients’ questions about COVID.”
“The new protocols to keep everyone safe add time between patients, and physicians have to answer patients’ questions about the pandemic and vaccines,” Dr. Fischer-Wright says. “You might see a 20% increase in time spent just on these non–revenue-generating COVID activities.”
Physicians still like their specialty
Although 2020 was a challenging year for physicians, the percentage of those who were satisfied with their specialty choice generally did not slip from the year before. It actually rose for several specialties – most notably, rheumatology, pulmonology, physical medicine and rehabilitation, and nephrology.
One specialty saw a decline in satisfaction with their specialty choice, and that was public health and preventive medicine, which plummeted 16 percentage points to 67% – putting it at the bottom of the list.
Even before the pandemic, many public health departments were chronically underfunded. This problem was possibly exacerbated by the pressures to keep up with COVID reporting and testing responsibilities.
Conclusion
Although 2020 was a wild ride for many physicians, many came out of it with only minor reductions in overall compensation, and some saw increases. Still, some specialties and many individuals experienced terrible financial stress and had to make changes in their lives and their spending in order to stay afloat.
“The biggest inhibitor to getting back to normal had to do with doctors who did not want to return because they did not want to risk getting COVID,” Dr. Pinto reports. But he notes that by February 2021 most doctors were completely vaccinated and could feel safe again.
A version of this article first appeared on Medscape.com.
Tic disorders proliferate in bipolar patients with OCD
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
Bipolar disorder patients with comorbid obsessive-compulsive disorder were significantly more likely to suffer from tic disorders, as well as hoarding, excoriation, and body dysmorphic disorder, than were those without comorbid OCD, data from 70 patients suggest.
Between 10% and 20% of patients with bipolar disorder (BD) also meet criteria for obsessive-compulsive disorder (OCD), and these patients are more likely to experience treatment resistance and poor prognosis than are BD patients without OCD. In addition, preliminary indications suggest a specific association between OCD and bipolar depression (BP-D) in particular, wrote Leonid Braverman, MD, of Ma’ale HaCarmel Mental Health Center, Tirat Carmel, Israel, and colleagues.
In addition, “there is compelling evidence indicating that OCD-spectrum and tic disorders share with OCD clinical characteristics, familial inheritance, neurobiological underpinnings and some aspects of pharmacotherapy,” and investigations into the clinical characteristics of OCD spectrum behaviors in BP-D patients with and without OCD are ongoing, they said.
In a study published in the Journal of Obsessive-Compulsive and Related Disorders (2021 Mar 21. doi: 10.1016/j.jocrd.2021.100643), the researchers reviewed data from 87 adults who met the DSM-5 criteria for BP-D. Of these, 27 also met criteria for OCD, 17 for subthreshold OCD, and 43 had neither OCD nor subthreshold OCD. The researchers compared the 27 OCD patients and the 43 non-OCD patients; the OCD patients had significantly higher rates overall of body dysmorphic disorder, hoarding disorder, excoriation disorder, and tic disorder, compared with non-OCD patients (P range from < .05-0.01 for all). No differences between the groups appeared for trichotillomania.
Also, the researchers found significant between-group differences in the number of patients with at least one OCD spectrum disorder and tic disorders (13 of 19 patients in the OCD group vs. 3 of 37 patients in the non-OCD group) and in the co-occurrence of two OCD-spectrum and tic disorders (3 of 19 patients in the OCD group vs. 1 patient in the non-OCD group).
The most common comorbid psychiatric disorders in both groups were substance use and combined anxiety disorders, followed by eating disorders, but no between-group differences were found in the frequencies of any of these conditions.
“From the clinical perspective, in BP-D patients,” the researchers noted.
The study findings were limited by several factors, including the small sample size, cross-sectional design, and exclusion of subsyndromic disorders, the researchers noted. However, the results support findings from previous studies, and the study emphasizes the clinical complexity and poor prognosis for these patients. Therefore, additional research is needed in patients with BP-D verse the manic/hypomanic phases of bipolar illness to determine similar patterns, they said. Medication trials are needed to address functional impairments in these patients, given the differences in treatment of BDD, hoarding, excoriation, and tic disorders, compared with “pure” OCD, they concluded.
The study received no outside funding. The researchers reported no financial conflicts.
FROM THE JOURNAL OF OBSESSIVE-COMPULSIVE AND RELATED DISORDERS
Open Notes
. While some clinicians consider it an unwelcome intrusion, advocates say it will improve communication and compliance.
Patient access to notes is not new. In many states, patients already have the ability to request copies of their charts, or to access truncated information via clinic websites. The difference is that most patients will now be able to click on a patient portal – such as MyChart, or other similar apps – and gain instantaneous, unfettered access to everything in their records.
Clinicians have traditionally thought of medical notes as private journal entries; but in the last few decades they have become an important component of the documentation necessary for billing, as well as evidence in the event of litigation. Now, with the implementation of the Cures Act, medical notes have evolved into a tool to communicate with the patient, rather than just among health care providers, lawyers, and billing departments.
Supporters contend that this change will make a big difference, because patients will be able to see exactly what their doctors have written, rather than just a list of confusing test results and diagnosis lists in “medicalese.”
OpenNotes, a think tank that has promoted the sharing of clinical notes with patients for years, calls the Cures Act legislation a “new world” where shared notes are valuable tools to improve communication between patients and physicians while strengthening their relationship. They cite evidence indicating that “when health professionals offer patients and families ready access to clinical notes, the quality and safety of care improves.”
Not all doctors are as enthusiastic. Many are concerned that patients might misinterpret what they see in their doctors’ notes, including complex descriptions of clinical assessments and decisions.
Others worry about patients having immediate access to their records, perhaps even before their physicians. The American Academy of Dermatology is working with the American Medical Association and other groups to gather real-world instances where the release of lab results, reports, or notes directly to patients before their physician could review the information with them caused emotional harm or other adverse consequences.
Undoubtedly, there are scenarios where unrestricted display of clinical notes could be problematic. One example is the issue of adolescents and reproductive health. Since parents now have access to their children’s records, some teenagers might hesitate to confide in their physicians and deny themselves important medical care.
The new rules permit blocking access to records if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or third parties. Psychotherapy counseling notes, for example, are completely exempt from the new requirements.
There are also state-level laws that can supersede the new federal law and block access to notes. For example, California law forbids providers from posting cancer test results without discussing them with the patient first.
Research indicates that shared notes have benefits that should outweigh the concerns of most physicians. One study showed that about 70% of patients said reviewing their notes helped them understand why medications were prescribed, which improved their compliance. This was particularly true for patients whose primary language is not English. A British study found that patients felt empowered by shared notes, and thought they improved their relationship with their physicians.
Other advantages of sharing notes include the ability of family members to review what happened at visits, which can be particularly important when dementia or other disabilities are involved. Patients will also be able to share their medical records with physicians outside of their health network, thus avoiding unnecessary or repetitious workups.
OpenNotes contends that when patients review their doctors’ notes, they gain “a newfound, deeper respect for what physicians have to understand to do their jobs.” Other predicted advantages include improved medical record accuracy and less miscommunication. In a study published in 2019 that evaluated experiences of patients who read ambulatory visit notes, only 5% were more worried after reading the notes and 3% were confused.
Alleviating worry among clinicians may be a bigger problem; but as a general principle, you should avoid judgmental language, and never write anything in a chart that you wouldn’t want your patients or their family members – or lawyers – to see.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
. While some clinicians consider it an unwelcome intrusion, advocates say it will improve communication and compliance.
Patient access to notes is not new. In many states, patients already have the ability to request copies of their charts, or to access truncated information via clinic websites. The difference is that most patients will now be able to click on a patient portal – such as MyChart, or other similar apps – and gain instantaneous, unfettered access to everything in their records.
Clinicians have traditionally thought of medical notes as private journal entries; but in the last few decades they have become an important component of the documentation necessary for billing, as well as evidence in the event of litigation. Now, with the implementation of the Cures Act, medical notes have evolved into a tool to communicate with the patient, rather than just among health care providers, lawyers, and billing departments.
Supporters contend that this change will make a big difference, because patients will be able to see exactly what their doctors have written, rather than just a list of confusing test results and diagnosis lists in “medicalese.”
OpenNotes, a think tank that has promoted the sharing of clinical notes with patients for years, calls the Cures Act legislation a “new world” where shared notes are valuable tools to improve communication between patients and physicians while strengthening their relationship. They cite evidence indicating that “when health professionals offer patients and families ready access to clinical notes, the quality and safety of care improves.”
Not all doctors are as enthusiastic. Many are concerned that patients might misinterpret what they see in their doctors’ notes, including complex descriptions of clinical assessments and decisions.
Others worry about patients having immediate access to their records, perhaps even before their physicians. The American Academy of Dermatology is working with the American Medical Association and other groups to gather real-world instances where the release of lab results, reports, or notes directly to patients before their physician could review the information with them caused emotional harm or other adverse consequences.
Undoubtedly, there are scenarios where unrestricted display of clinical notes could be problematic. One example is the issue of adolescents and reproductive health. Since parents now have access to their children’s records, some teenagers might hesitate to confide in their physicians and deny themselves important medical care.
The new rules permit blocking access to records if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or third parties. Psychotherapy counseling notes, for example, are completely exempt from the new requirements.
There are also state-level laws that can supersede the new federal law and block access to notes. For example, California law forbids providers from posting cancer test results without discussing them with the patient first.
Research indicates that shared notes have benefits that should outweigh the concerns of most physicians. One study showed that about 70% of patients said reviewing their notes helped them understand why medications were prescribed, which improved their compliance. This was particularly true for patients whose primary language is not English. A British study found that patients felt empowered by shared notes, and thought they improved their relationship with their physicians.
Other advantages of sharing notes include the ability of family members to review what happened at visits, which can be particularly important when dementia or other disabilities are involved. Patients will also be able to share their medical records with physicians outside of their health network, thus avoiding unnecessary or repetitious workups.
OpenNotes contends that when patients review their doctors’ notes, they gain “a newfound, deeper respect for what physicians have to understand to do their jobs.” Other predicted advantages include improved medical record accuracy and less miscommunication. In a study published in 2019 that evaluated experiences of patients who read ambulatory visit notes, only 5% were more worried after reading the notes and 3% were confused.
Alleviating worry among clinicians may be a bigger problem; but as a general principle, you should avoid judgmental language, and never write anything in a chart that you wouldn’t want your patients or their family members – or lawyers – to see.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
. While some clinicians consider it an unwelcome intrusion, advocates say it will improve communication and compliance.
Patient access to notes is not new. In many states, patients already have the ability to request copies of their charts, or to access truncated information via clinic websites. The difference is that most patients will now be able to click on a patient portal – such as MyChart, or other similar apps – and gain instantaneous, unfettered access to everything in their records.
Clinicians have traditionally thought of medical notes as private journal entries; but in the last few decades they have become an important component of the documentation necessary for billing, as well as evidence in the event of litigation. Now, with the implementation of the Cures Act, medical notes have evolved into a tool to communicate with the patient, rather than just among health care providers, lawyers, and billing departments.
Supporters contend that this change will make a big difference, because patients will be able to see exactly what their doctors have written, rather than just a list of confusing test results and diagnosis lists in “medicalese.”
OpenNotes, a think tank that has promoted the sharing of clinical notes with patients for years, calls the Cures Act legislation a “new world” where shared notes are valuable tools to improve communication between patients and physicians while strengthening their relationship. They cite evidence indicating that “when health professionals offer patients and families ready access to clinical notes, the quality and safety of care improves.”
Not all doctors are as enthusiastic. Many are concerned that patients might misinterpret what they see in their doctors’ notes, including complex descriptions of clinical assessments and decisions.
Others worry about patients having immediate access to their records, perhaps even before their physicians. The American Academy of Dermatology is working with the American Medical Association and other groups to gather real-world instances where the release of lab results, reports, or notes directly to patients before their physician could review the information with them caused emotional harm or other adverse consequences.
Undoubtedly, there are scenarios where unrestricted display of clinical notes could be problematic. One example is the issue of adolescents and reproductive health. Since parents now have access to their children’s records, some teenagers might hesitate to confide in their physicians and deny themselves important medical care.
The new rules permit blocking access to records if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or third parties. Psychotherapy counseling notes, for example, are completely exempt from the new requirements.
There are also state-level laws that can supersede the new federal law and block access to notes. For example, California law forbids providers from posting cancer test results without discussing them with the patient first.
Research indicates that shared notes have benefits that should outweigh the concerns of most physicians. One study showed that about 70% of patients said reviewing their notes helped them understand why medications were prescribed, which improved their compliance. This was particularly true for patients whose primary language is not English. A British study found that patients felt empowered by shared notes, and thought they improved their relationship with their physicians.
Other advantages of sharing notes include the ability of family members to review what happened at visits, which can be particularly important when dementia or other disabilities are involved. Patients will also be able to share their medical records with physicians outside of their health network, thus avoiding unnecessary or repetitious workups.
OpenNotes contends that when patients review their doctors’ notes, they gain “a newfound, deeper respect for what physicians have to understand to do their jobs.” Other predicted advantages include improved medical record accuracy and less miscommunication. In a study published in 2019 that evaluated experiences of patients who read ambulatory visit notes, only 5% were more worried after reading the notes and 3% were confused.
Alleviating worry among clinicians may be a bigger problem; but as a general principle, you should avoid judgmental language, and never write anything in a chart that you wouldn’t want your patients or their family members – or lawyers – to see.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].

