User login
Distress and Factors Associated with Suicidal Ideation in Veterans Living with Cancer (FULL)
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
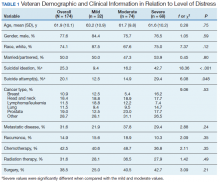
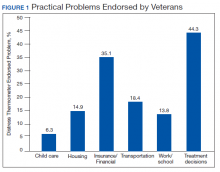
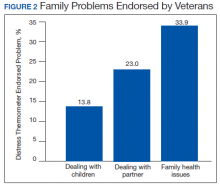
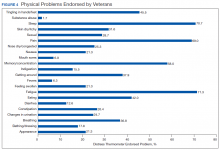
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
It was estimated that physicians would diagnose a form of invasive cancer > 1.7 million times in 2019. As the second most common cause of death in the US, > 600,000 people were projected to die from cancer in 2019.1 Many individuals with cancer endure distress, which the National Comprehensive Cancer Network (NCCN) defines as a “multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”2,3 Distress in people living with cancer has been attributed to various psychosocial concerns, such as family problems, whichinclude dealing with partners and children; emotional problems, such as depression and anxiety; and physical symptoms, such as pain and fatigue.4-9 Certain factors associated with distress may increase a patient’s risk for suicide.4
Veterans are at particularly high risk for suicide.10 In 2014, veterans accounted for 18% of completed suicides in the US but only were 8.5% of the total population that same year.10 Yet, little research has been done on the relationship between distress and suicide in veterans living with cancer. Aboumrad and colleagues found that 45% of veterans with cancer who completed suicide reported family issues and 41% endorsed chronic pain.11 This study recommended continued efforts to assess and treat distress to lessen risk of suicide in veterans living with cancer; however, to date, only 1 study has specifically evaluated distress and problems endorsed among veterans living with cancer.7
Suicide prevention is of the highest priority to the US Department of Veterans Affairs (VA).12 Consistent with the VA mission to end veteran suicide, the current study aimed to better understand the relationship between distress and suicide within a sample of veterans living with cancer. Findings would additionally be used to tailor clinical assessments and interventions for veterans living with cancer.
This study had 3 primary goals. First, we sought to understand demographic and clinical factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. Second, the study investigated the most commonly endorsed problems by veterans living with cancer. Finally, we examined which problems were related to suicidal ideation (SI). It was hypothesized that veterans who reported severe distress would be significantly more likely to endorse SI when compared with veterans who reported mild or moderate distress. Based on existing literature, it was further hypothesized that family, emotional, and physical problems would be significantly associated with SI.7,11
Methods
The current study was conducted at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida. Inclusion criteria included veterans who were diagnosed with cancer, attended an outpatient psychology-oncology evaluation, and completed mental health screening measures provided during their evaluation. Exclusion criteria included veterans who: were seen in response to an inpatient consult, were seen solely for a stem cell transplant evaluation, or did not complete the screening measures.
Measures
A veteran’s demographic (eg, age, sex, ethnicity) and clinical (eg, cancer type, stage of disease, recurrence, cancer treatments received) information was abstracted from their VA medical records. Marital status was assessed during a clinical interview and documented as part of the standardized suicide risk assessment.
The Distress Thermometer (DT) is a subjective measure developed by the NCCN.2 The DT provides a visual representation of a thermometer and asks patients to rate their level of distress over the past week with 0 indicating no distress and 10 indicating extreme distress.
The measurement additionally lists 39 problems nested within 5 domains: practical, family, emotional, spiritual/religious, and physical. Patients may endorse listed items under each problem domain by indicating yes or no. Endorsement of various items are intended to provide more detailed information about sources of distress. Due to the predominantly male and mostly older population included in this study the ability to have children measure was removed from the family problem domain.
SI was assessed in 2 ways. First, by patients’ self-report through item-9 of the Patient Health Questionnaire-9 (PHQ-9).14 Item-9 asks “over the last 2 weeks, how often have you been bothered by thoughts that you would be better off dead or of hurting yourself in some way?” Responses range from 0 (not at all) to 3 (nearly every day).14 Responses > 0 were considered a positive screen for SI.
Procedure
Participants were a sample of veterans who were referred for psychology-oncology services. The NCCN DT and Problems List were administered prior to the start of clinical interviews, which followed a checklist and included standardized assessments of SI and history of a suicide attempt(s). A licensed clinical psychologist or a postdoctoral resident conducted these assessments under the supervision of a licensed psychologist. Data gathered during the clinical interview and from the DT and problems list were documented in health records, which were retrospectively reviewed for relevant information (eg, cancer diagnosis, SI). Therefore, informed consent was waived. This study was approved by the JAHVH Institutional Review Board.
Analysis
Data were analyzed using SPSS Version 25. Data analysis proceeded in 3 steps. First, descriptive statistics included the demographic and clinical factors present in the current sample. Difference between those with and without suicidal ideation were compared using F-statistic for continuous variables and χ2 analyses for categorical variables. Second, to examine relationships between each DT problem domain and SI, χ2 analyses were conducted. Third, DT problem domains that had a significant relationship with SI were entered in a logistic regression. This analysis determined which items, if any, from a DT problem domain predicted SI. In the logistic regression model, history of suicide attempts was entered into the first block, as history of suicide attempts is a well-established risk factor for subsequent suicidal ideation. In the second block, other variables that were significantly related to suicidal ideation in the second step of analyses were included. Before interpreting the results of the logistic regression, model fit was tested using the Hosmer-Lemeshow test. Significance of each individual predictor variable in the model is reported using the Wald χ2 statistic; each Wald statistic is compared with a χ2 distribution with 1 degree of freedom (df). Results of logistic regression models also provide information about the effect of each predictor variable in the regression equation (beta weight), odds a veteran who endorsed each predictor variable in the model would also endorse SI (as indicated by the odds ratio), and an estimate of the amount of variance accounted for by each predictor variable (using Nagelkerke’s pseudo R2, which ranges in value from 0 to 1 with higher values indicating more variance explained). For all analyses, P value of .05 (2-tailed) was used for statistical significance.
Results
The sample consisted of 174 veterans (Table 1). The majority (77.6%) were male with a mean age of nearly 62 years (range, 29-87). Most identified as white (74.1%) with half reporting they were either married or living with a partner.
Prostate cancer (19.0%) was the most common type of cancer among study participants followed by head and neck (18.4%), lymphoma/leukemia (11.5%), lung (11.5%), and breast (10.9%); 31.6% had metastatic disease and 14.9% had recurrent disease. Chemotherapy (42.5%) was the most common treatment modality, followed by surgery (38.5%) and radiation (31.6%). The sample was distributed among the 3 distress DT categories: mild (18.4%), moderate (42.5%), and severe (39.1%).
Problems Endorsed
Treatment decisions (44.3%) and insurance/financial concerns (35.1%) were the most frequently endorsed practical problems (Figure 1). Family health issues (33.9%) and dealing with partner (23.0%) were the most frequently endorsed family problems (Figure 2). Worry (73.0%) and depression (69.5%) were the most frequent emotional problems; of note, all emotional problems were endorsed by at least 50% of veterans (Figure 3). Fatigue (71.3%), sleep (70.7%), and pain (69%), were the most frequently endorsed physical problems (Figure 4). Spiritual/religious problems were endorsed by 15% of veterans.
Suicidal Ideation
Overall, 25.3% of veterans endorsed SI. About 20% of veterans reported a history of ≥ 1 suicide attempts in their lifetime. A significant relationship among distress categories and SI was found (χ2 = 18.36, P < .001). Veterans with severe distress were more likely to endorse SI (42.7%) when compared with veterans with mild (9.4%) or moderate (16.2%) distress.
Similarly, a significant relationship among distress categories and a history of a suicide attempt(s) was found (χ2 = 6.08, P = .048). Veterans with severe distress were more likely to have attempted suicide (29.4%) when compared with veterans with mild (12.5%) or moderate (14.9%) distress.
χ2 analyses were conducted to examine the relationships between DT problem domains and SI. A significant relationship was found between family problems and SI (
Logistic regression analyses determined whether items representative of the family problems domain were predictive of SI. Suicide attempt(s) were entered in the first step of the model to evaluate risk factors for SI over this already established risk factor. The assumptions of logistic regression were met.
The Hosmer-Lemeshow test (χ2 = 3.66, df = 5, P = .56) demonstrated that the model fit was good. The group of predictors used in the model differentiate between people who were experiencing SI and those who were not experiencing SI at the time of evaluation. A history of a suicide attempt(s) predicted SI, as expected (Wald = 6.821, df = 1, P = .01). The odds that a veteran with a history of a suicide attempt(s) would endorse SI at the time of the evaluation was nearly 3 times greater than that of veterans without a history of a suicide attempt(s). Over and above suicide attempts, problems dealing with partner (Wald = 15.142; df = 1, P < .001) was a second significant predictor of current SI. The odds that a veteran who endorsed problems dealing with partner would also endorse SI was > 5 times higher than that of veterans who did not endorse problems dealing with partner. This finding represents a significant risk factor for SI, over and above a history of a suicide attempt(s). The other items from the family problems domains were not significant (P > .05) (Table 3).
Discussion
This study aimed to understand factors associated with low, moderate, and severe levels of distress in veterans living with cancer who were referred for psychology services. As hypothesized, veterans who endorsed severe distress were significantly more likely to endorse SI. They also were more likely to have a history of a suicide attempt(s) when compared with those with mild or moderate distress.
A second aim of this study was to understand the most commonly endorsed problems. Consistent with prior literature, treatment decisions were the most commonly endorsed practical problem; worry and depression were the most common emotional problems; and fatigue, sleep, and pain were the most common physical problems.7
A finding unique to the current study is that family health issues and dealing with partner were specified as the most common family problems. However, a study by Smith and colleagues did not provide information about the rank of most frequently reported problems within this domain.7
The third aim was to understand which problems were related to SI. It was hypothesized that family, emotional, and physical problems would be related to SI. However, results indicated that only family problems (specifically, problems dealing with a partner) were significantly associated with SI among veterans living with cancer.
Contrary to expectations, emotional and physical problems were not found to have a significant relationship with SI. This is likely because veterans endorsed items nested within these problem domains with similar frequency. The lack of significant findings does not suggest that emotional and physical problems are not significant predictors of SI for veterans living with cancer, but that no specific emotional or physical symptom stood out as a predictor of suicidal ideation above the others.
The finding of a significant relationship between family problems (specifically, problems dealing with a partner) and SI in this study is consistent with findings of Aboumrad and colleagues in a study that examined root-cause analyses of completed suicides by veterans living with cancer.11 They found that nearly half the sample endorsed family problems prior to their death, and a small but notable percentage of veterans who completed suicide reported divorce as a stressor prior to their death.
This finding may be explained by Thomas Joiner's interpersonal-psychological theory of suicidal behavior (IPT), which suggests that completed suicide may result from a thwarted sense of belonging, perceived burdensomeness, and acquired capability for suicide.16 Problems dealing with a partner may impact a veteran’s sense of belonging or social connectedness. Problems dealing with a partner also may be attributed to perceived burdens due to limitations imposed by living with cancer and/or undergoing treatment. In both circumstances, the veteran’s social support system may be negatively impacted, and perceived social support is a well-established protective factor against suicide.17
Partner distress is a second consideration. It is likely that veterans’ partners experienced their own distress in response to the veteran’s cancer diagnosis and/or treatment. The partner’s cause, severity, and expression of distress may contribute to problems for the couple.
Finally, the latter point of the IPT refers to acquired capability, or the ability to inflict deadly harm to oneself.18 A military sample was found to have more acquired capability for suicide when compared with a college undergraduate sample.19 A history of a suicide attempt(s) and male gender have been found to significantly predict acquired capability to complete suicide.18 Furthermore, because veterans living with cancer often are in pain, fear of pain associated with suicide may be reduced and, therefore, acquired capability increased. This suggests that male veterans living with cancer who are in pain, have a history of a suicide attempt(s), and current problems with their partner may be an extremely vulnerable population at-risk for suicide. Results from the current study emphasize the importance of veterans having access to mental health and crisis resources for problems dealing with their partner. Partner problems may foreshadow a potentially lethal type of distress.
Strengths
This study’s aims are consistent with the VA’s mission to end veteran suicide and contributes to literature in several important ways.12 First, veterans living with cancer are an understudied population. The current study addresses a gap in existing literature by researching veterans living with cancer and aims to better understand the relationship between cancer-related distress and SI. Second, to the best of the authors’ knowledge, this study is the first to find that problems dealing with a partner significantly increases a veteran’s risk for SI above a history of a suicide attempt(s). Risk assessments now may be more comprehensive through inclusion of this distress factor.
It is recommended that future research use IPT to further investigate the relationship between problems dealing with a partner and SI.16 Future research may do so by including specific measures to assess for the tenants of the theory, including measurements of burdensomeness and belongingness. An expanded knowledge base about what makes problems dealing with a partner a significant suicide risk factor (eg, increased conflict, lack of support, etc.) would better enable clinicians to intervene effectively. Effective intervention may lessen suicidal behaviors or deaths from suicides within the Veteran population.
Limitations
One limitation is the focus on patients who accepted a mental health referral. This study design may limit the generalizability of results to veterans who would not accept mental health treatment. The homogenous sample of veterans is a second limitation. Most participants were male, white, and had a mean age of 62 years. These demographics are representative of the veterans that most typically utilize VA services; however, more research is needed on veterans living with cancer who are female and of diverse racial and ethnic backgrounds. There are likely differences in problems endorsed and factors associated with SI based on age, race, sex, and other socioeconomic factors. A third limitation is the cross-sectional, retrospective nature of this study. Future studies are advised to assess for distress at multiple time points. This is consistent with NCCN Standards of Care for Distress Management.2 Longitudinal data would enable more findings about distress and SI throughout the course of cancer diagnosis and treatment, therefore enhancing clinical implications and informing future research.
Conclusion
This is among the first of studies to investigate distress and factors associated with SI in veterans living with cancer who were referred for psychology services. The prevalence of distress caused by psychosocial factors (including treatment decisions, worry, and depression) highlights the importance of including mental health services as part of comprehensive cancer treatment.
Distress due to treatment decisions may be attributed to a litany of factors such as a veteran’s consideration of adverse effects, effectiveness of treatments, changes to quality of life or functioning, and inclusion of alternative or complimentary treatments. These types of decisions often are reported to be difficult conversations to have with family members or loved ones, who are likely experiencing distress of their own. The role of a mental health provider to assist veterans in exploring their treatment decisions and the implications of such decisions appears important to lessening distress.
Early intervention for emotional symptoms would likely benefit veterans’ management of distress and may lessen suicide risk as depression is known to place veterans at-risk for SI.20 This underscores the importance of timely distress assessment to prevent mild emotional distress from progressing to potentially severe or life-threatening emotional distress. For veterans with a psychiatric history, timely assessment and intervention is essential because psychiatric history is an established suicide risk factor that may be exacerbated by cancer-related distress.12
Furthermore, management of intolerable physical symptoms may lessen risk for suicide.4 Under medical guidance, fatigue may be improved using exercise.21 Behavioral intervention is commonly used as first-line treatment for sleep problems.22 While pain may be lessened through medication or nonpharmacological interventions.23
Considering the numerous ways that distress may present itself (eg, practical, emotional, or physical) and increase risk for SI, it is essential that all veterans living with cancer are assessed for distress and SI, regardless of their presentation. Although veterans may not outwardly express distress, this does not indicate the absence of either distress or risk for suicide. For example, a veteran may be distressed due to financial concerns, transportation issues, and the health of his/her partner or spouse. This veteran may not exhibit visible symptoms of distress, as would be expected when the source of distress is emotional (eg, depression, anxiety). However, this veteran is equally vulnerable to impairing distress and SI as someone who exhibits emotional distress. Distress assessments should be further developed to capture both the visible and less apparent sources of distress, while also serving the imperative function of screening for suicide. Other researchers also have noted the necessity of this development.24 Currently, the NCCN DT and Problems List does not include any assessment of SI or behavior.
Finally, this study identified a potentially critical factor to include in distress assessment: problems dealing with a partner. Problems dealing with a partner have been noted as a source of distress in existing literature, but this is the first study to find problems dealing with a partner to be a predictor of SI in veterans living with cancer.4-6
Because partners often attend appointments with veterans, it is not surprising that problems dealing with their partner are not disclosed more readily. It is recommended that clinicians ask veterans about potential problems with their partner when they are alone. Directly gathering information about such problems while assessing for distress may assist health care workers in providing the most effective, accurate type of intervention in a timely manner, and potentially mitigate risk for suicide.
As recommended by the NCCN and numerous researchers, findings from the current study underscore the importance of accurate, timely assessment of distress.2,4,8 This study makes several important recommendations about how distress assessment may be strengthened and further developed, specifically for the veteran population. This study also expands the current knowledge base of what is known about veterans living with cancer, and has begun to fill a gap in the existing literature. Consistent with the VA mission to end veteran suicide, results suggest that veterans living with cancer should be regularly screened for distress, asked about distress related to their partner, and assessed for SI. Continued efforts to enhance assessment of and response to distress may lessen suicide risk in veterans with cancer.11
Acknowledgements
This study is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Riba MB, Donovan, KA, Andersen, B. National Comprehensive Cancer Network clinical practice guidelines in oncology. Distress management (Version 3.2019). J Natl Compr Can Net, 2019;17(10):1229-1249.
3. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Pianta dosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
4. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8(1):4-12.
5. Bulli F, Miccinesi G, Maruelli A, Katz M, Paci E. The measure of psychological distress in cancer patients: the use of distress thermometer in the oncological rehabilitation center of Florence. Support Care Cancer. 2009;17(7):771–779.
6. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
7. Smith J, Berman S, Dimick J, et al. Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory. Fed Pract. 2017;34(Suppl 1):43S–50S.
8. Carlson LE, Waller A, Groff SL, Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems--secondary outcomes of a randomized controlled trial. Psychooncology. 2013;22(8):1880-1888.
9. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12(7):694-708.
10. US Department of Veterans Affairs Office of Suicide Prevention. Suicide among veterans and other Americans 2001-2014. https://www.mentalhealth.va.gov/docs/2016suicidedatareport.pdf. Published August 3, 2016. Accessed April 13, 2020.
11. Aboumrad M, Shiner B, Riblet N, Mills, PD, Watts BV. Factors contributing to cancer-related suicide: a study of root-cause-analysis reports. Psychooncology. 2018;27(9):2237-2244.
12. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. National Strategy for Preventing Veteran Suicide 2018–2028. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf Published 2018. Accessed April 13, 2020.
13. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613.
15. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28(1):71-77.
16. Joiner TE. Why People Die by Suicide. Cambridge, MA: Harvard University Press, 2005.
17. Kleiman EM, Riskind JH, Schaefer KE. Social support and positive events as suicide resiliency factors: examination of synergistic buffering effects. Arch Suicide Res. 2014;18(2):144-155.
18. Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE Jr. Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76(1):72–83.
19. Bryan CJ, Morrow CE, Anestis MD, Joiner TE. A preliminary test of the interpersonal -psychological theory of suicidal behavior in a military sample. Personal Individual Differ. 2010;48(3):347-350.
20. Miller SN, Monahan CJ, Phillips KM, Agliata D, Gironda RJ. Mental health utilization among veterans at risk for suicide: Data from a post-deployment clinic [published online ahead of print, 2018 Oct 8]. Psychol Serv. 2018;10.1037/ser0000311.
21. Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899-909.
22. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
23. Ngamkham S, Holden JE, Smith EL. A systematic review: Mindfulness intervention for cancer-related pain. Asia Pac J Oncol Nurs. 2019;6(2):161-169.
24. Granek L, Nakash O, Ben-David M, Shapira S, Ariad S. Oncologists’, nurses’, and social workers’ strategies and barriers to identifying suicide risk in cancer patients. Psychooncology. 2018;27(1):148-154.
Atrial Fibrillation and Bleeding in Patients With Chronic Lymphocytic Leukemia Treated with Ibrutinib in the Veterans Health Administration (FULL)
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
Chronic lymphocytic leukemia (CLL) is the most common leukemia diagnosed in developed countries, with an estimated 21,040 new diagnoses of CLL expected in the US in 2020. 1-3 CLL is an indolent cancer characterized by the accumulation of B-lymphocytes in the blood, marrow, and lymphoid tissues. 4 It has a heterogeneous clinical course; the majority of patients are observed or receive delayed treatment following diagnosis, while a minority of patients require immediate treatment. After first-line treatment, some patients experience prolonged remissions while others require retreatment within 1 or 2 years. Fortunately, advances in cancer biology and therapeutics in the last decade have increased the number of treatment options available for patients with CLL.
Until recently, most CLL treatments relied on a chemotherapy or a chemoimmunotherapy backbone; however, the last few years have seen novel therapies introduced, such as small molecule inhibitors to target molecular pathways that promote the normal development, expansion, and survival of B-cells.5 One such therapy is ibrutinib, a targeted Bruton tyrosine kinase inhibitor that received accelerated approval by the US Food and Drug Administration (FDA) in February 2014 for patients with CLL who received at least 1 prior therapy. The FDA later expanded this approval to include use of ibrutinib in patients with CLL with relapsed or refractory disease, with or without chromosome 17p deletion. In 2016, based on data from the RESONATE-17 study, the FDA approved ibrutinib for first-line therapy in patients with CLL.6
Ibrutinib’s efficacy, ease of administration and dosing (all doses are oral and fixed, rather than based on weight or body surface area), and relatively favorable safety profile have resulted in a rapid growth in its adoption.7 Since its adverse event (AE) profile is generally more tolerable than that of a typical chemoimmunotherapy, its use in older patients with CLL and patients with significant comorbidities is particularly appealing.8
However, the results of some clinical trials suggest an association between treatment with ibrutinib and an increased risk of bleeding-related events of any grade (44%) and major bleeding events (4%).7,8 The incidence of major bleeding events was reported to be higher (9%) in one clinical trial and at 5-year follow-up, although this trial did not exclude patients receiving concomitant oral anticoagulation with warfarin.6,9
Heterogeneity in clinical trials’ definitions of major bleeding confounded the ability to calculate bleeding risk in patients treated with ibrutinib in a systematic review and meta-analysis that called for more data.10 Additionally, patients with factors that might increase the risk of major bleeding with ibrutinib treatment were likely underrepresented in clinical trials, given the carefully selected nature of clinical trial subjects. These factors include renal or hepatic disease, gastrointestinal disease, and use of a number of concomitant medications such as antiplatelets or anticoagulant medications. Accounting for use of the latter is particularly important because patients who develop atrial fibrillation (Afib), one of the recognized AEs of treatment with ibrutinib, often are treated with anticoagulant medications in order to decrease the risk of stroke or other thromboembolic complications.
A single-site observational study of patients treated with ibrutinib reported a high utilization rate of antiplatelet medications (70%), anticoagulant medications (17%), or both (13%) with a concomitant major bleeding rate of 18% of patients.11 Prevalence of bleeding events seemed to be highly affected by the presence of concomitant medications: 78% of patients treated with ibrutinib while concurrently receiving both antiplatelet and anticoagulant medications developed a major bleeding event, while none of the patients who were not receiving antiplatelets, anticoagulants, or medications that interact with cytochrome P450 (an enzyme that metabolized chemotherapeutic agents used to treat cancer) experienced a major bleeding event.11
The prevalence of major bleeding events, comorbidities, and utilization of medications that could increase the risk of major bleeding in patients with CLL on ibrutinib in the Veterans Health Administration (VHA) is not known. The VHA is the largest integrated health care system in the US. To address these knowledge gaps, a retrospective observational study was conducted using data on demographics, comorbidities that could affect bleeding, use of anticoagulant and antiplatelet medications, and bleeding events in patients with CLL who were treated in the first year of ibrutinib availability from the VHA.
The first year of ibrutinib availability was chosen for this study since we anticipated that many health care providers would be unfamiliar with ibrutinib during that time given its novelty, and therefore more likely to codispense ibrutinib with medications that could increase the risk of a bleeding event. Since Afib is both an AE associated with ibrutinib treatment and a condition that often is treated with anticoagulants, the prevalence of Afib in this population was also included. For context, the incidence of bleeding and Afib and use of anticoagulant and antiplatelet medications during treatment in a cohort of patients with CLL treated with bendamustine + rituximab (BR) also was reported.
Methods
The VHA maintains the centralized US Department of Veterans Affairs Cancer Registry System (VACRS), with electronic medical record data and other sources captured in its Corporate Data Warehouse (CDW). The VHA CDW is a national repository comprising data from several VHA clinical and administrative systems. The CDW includes patient identifiers; demographics; vital status; lab information; administrative information (such as diagnostic International Statistical Classification of Diseases and Related Health Problems [ICD-9] codes); medication dispensation tables (such as outpatient fill); IV package information; and notes from radiology, pathology, outpatient and inpatient admission, discharge, and daily progress.
Registrars abstract all cancer cases within the VHA system (or diagnosed outside the VHA, if patients subsequently receive treatment in the VHA). It is estimated that VACRS captures 3% of cancer cases in the US.12 Like most registries, VACRS captures data such as diagnosis, age, gender, race, and vital status.
The study received approval from the University of Utah Institutional Review Board and used individual patient-level historical administrative, cancer registry, and electronic health care record data. Patients diagnosed and treated for CLL at the VHA from 2010 to 2014 were identified through the VACRS and CDW; patients with a prior malignancy were excluded. Patients who received ibrutinib or BR based on pharmacy dispensation information were selected. Patients were followed until December 31, 2016 or death; patients with documentation of another cancer or lack of utilization of the VHA hematology or oncology services (defined as absence of any hematology and/or oncology clinic visits for ≥ 18 months) were omitted from the final analysis (Figure).
Previous and concomitant utilization of antiplatelet (aspirin, clopidogrel) or anticoagulant (dalteparin, enoxaparin, fondaparinux, heparin, rivaroxaban, and warfarin) medications was extracted 6 months before and after the first dispensation of ibrutinib or BR using pharmacy dispensation records.
Study Definitions
Prevalence of comorbidities that could increase bleeding risk was determined using administrative ICD-9-CM codes. Liver disease was identified by presence of cirrhosis, hepatitis C virus, or alcoholic liver disease using administrative codes validated by Kramer and colleagues, who reported positive and negative predictive values of 90% and 87% for cirrhosis, 93% and 92% for hepatitis C virus, and 71% and 98% for alcoholic liver disease.13 Similarly, end-stage liver disease was identified using a validated coding algorithm developed by Goldberg and colleagues, with a positive predictive value of 89.3%.14 The presence of controlled or uncontrolled diabetes mellitus (DM) was identified using the procedure described by Guzman and colleagues.15 Quan’s algorithm was used to calculate Charlson Comorbidity Index (CCI) based on ICD-9-CM codes for inpatient and outpatient visits within a 6-month lookback period prior to treatment initiation.16
A major bleeding event was defined as a hospitalization with an ICD-9-CM code suggestive of major bleeding as the primary reason, as defined by Lane and colleagues in their study of major bleeding related to warfarin in a cohort of patients treated within the VHA.17 Incidence rates of major bleeding events were identified during the first 6 months of treatment. Incidence of Afib—defined as an inpatient or outpatient encounter with the 427.31 ICD-9-CM code—also was examined within the first 6 months after starting treatment. The period of 6 months was chosen because bendamustine must be discontinued after 6 months.
Study Analysis
Descriptive statistics were used to examine patient demographics, disease characteristics, and treatment history from initial CLL diagnosis through end of study observation period. Categorical variables were summarized using frequencies and accompanying proportions, while a mean and standard deviation were used to summarize continuous variables. For the means of continuous variables and of categorical data, 95% CIs were used. Proportions and accompanying 95% CIs characterized treatment patterns, including line of therapy, comorbidities, and bleeding events. Treatment duration was described using mean and accompanying 95% CI. Statistical tests were not conducted for comparisons among treatment groups. Patients were censored at the end of follow-up, defined as the earliest of the following scenarios: (1) end of study observation period (December 31, 2016); (2) development of a secondary cancer; or (3) last day of contact given absence of care within the VHA for ≥ 18 months (with care defined as oncology and/or oncology/hematology visit with an associated note). Analysis was performed using R 3.4.0.
Results
Between 2010 and 2014, 2,796 patients were diagnosed and received care for CLL within the VHA. Overall, all 172 patients who were treated with ibrutinib during our inclusion period were selected. These patients were treated between January 1, 2014 and December 31, 2016, following ibrutinib’s approval in early 2014. An additional 291 patients were selected who received BR (Table). Reflecting the predominantly male population of the VHA, 282 (97%) BR patients and 167 (97%) ibrutinib patients were male. The median age at diagnosis was 67 years for BR patients and 69 years for ibrutinib patients. About 76% of patients who received ibrutinib and 82% of patients who received BR were non-Hispanic white; 17% and 14% were African American, respectively.
Less than 10% of patients receiving either ibrutinib or BR had liver disease per criteria used by Kramer and colleagues, or end-stage liver disease using criteria developed by Goldberg and colleagues.12,13 About 5% of patients had a history of previous bleeding in the 6-month period prior to initiating either therapy. Mean CCI (excluding malignancy) score was 1.5 (range, 0-11) for the ibrutinib group, and 2.1 (range, 0-9) for the BR group. About 16% of the ibrutinib group had controlled DM and fewer than 10% had uncontrolled DM, while 4% of patients in the BR group met the criteria for controlled DM and another 4% met the criteria for uncontrolled DM.
There was very low utilization of anticoagulant or antiplatelet medication prior to initiation of ibrutinib (2.9% and 2.3%, respectively) or BR (< 1% each). In the first 6 months after treatment initiation, about 8% of patients in both ibrutinib and BR cohorts received anticoagulant medication while antiplatelet utilization was < 5% in either group.
In the BR group, 8 patients (2.7%) experienced a major bleeding event, while 14 patients (8.1%) in the ibrutinib group experienced a bleeding event (P = .008). While these numbers were too low to perform a formal statistical analysis of the association between clinical covariates and bleeding in either group, there did not seem to be an association between bleeding and liver disease or DM. Of patients who experienced a bleeding event, about 1 in 4 patients had had a prior bleeding event in both the ibrutinib and the BR groups. Interestingly, while none of the patients who experienced a bleeding event while receiving BR were taking concomitant anticoagulant medication, 3 of the 14 patients who experienced a bleeding event in the ibrutinib group showed evidence of anticoagulant utilization. Finally, the incidence of Afib (defined as patients with no evidence of Afib in the 6 months prior to treatment but with evidence of Afib in the 6 months following treatment initiation) was 4% in the BR group, and about 8% in the ibrutinib group (P = .003).
Discussion
To the authors’ knowledge, this study is the first to examine the real-world incidence of bleeding and Afib in veterans who received ibrutinib for CLL in the first year of its availability. The study found minimal use of anticoagulants and/or antiplatelet agents prior to receiving first-line ibrutinib or BR, and very low use of these agents in the first 6 months following the initiation of first-line treatment. This finding suggests a high awareness among VA providers of potential adverse effects (AEs) of ibrutinib and chemotherapy, and a careful selection of patients that lack risk factors for AEs.
In patients treated with first-line ibrutinib when compared with patients treated with first-line BR, moderate increases in bleeding (2.7% vs 8.1%, P = .008) and Afib (10.5% vs 3%, P = .003) also were observed. These results are concordant with previous findings examining the use of ibrutinib in patients with CLL.18-20
Limitations
The results of this study should be interpreted with caution, as some limitations must be considered. The study was conducted in the early days of ibrutinib adoption. Since then, more patients have been treated with ibrutinib and for longer durations. As clinicians gain more familiarity and with ibrutinib, and as additional novel therapeutics emerge, it is possible that the initial awareness about risks for possible AEs may diminish; patients with high comorbidity burdens and concomitant medications would be especially vulnerable in cases of reduced physician vigilance.
Another limitation of this study stems from the potential for dual system use among patients treated in the VHA. Concurrent or alternating use of multiple health care systems (use of VHA and private-sector facilities) may present gaps in the reconstruction of patient histories, resulting in missing data as patients transition between commercial, the Centers for Medicare and Medicaid Services, and VHA care. As a result, the results presented here do not reflect instances where a patient experienced a bleeding event treated outside the VA.
Problems with missing data also may occur due to incomplete extraction from the electronic health record; these issues were addressed by leveraging an understanding of the multiple data marts within the CDW environment to harmonize missing and/or erroneous information through use of other data marts when possible. Lastly, this research represents a population-level study of the VHA, thus all findings are directly relevant to the VHA. The generalizability of the findings outside the VHA would depend on the characteristics of the external population.
Conclusion
Real-world evidence from a nationwide cohort of veteran patients with CLL treated with ibrutinib suggest that, while there is an association of increased bleeding-related events and Afib, the risk is comparable to those reported in previous studies.18-20 These findings suggest that patients in real-world clinical care settings with higher levels of comorbidities may be at a slight increased risk for bleeding events and Afib.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
1. Scarfò L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169-182.
2. Devereux S, Cuthill K. Chronic lymphocytic leukaemia. Medicine (Baltimore). 2017;45(5):292-296.
3. American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed April 24, 2020.
4. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096.
5. Owen C, Assouline S, Kuruvilla J, Uchida C, Bellingham C, Sehn L. Novel therapies for chronic lymphocytic leukemia: a Canadian perspective. Clin Lymphoma Myeloma Leuk. 2015;15(11):627-634.e5.
6. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418.
7. Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437.
8. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
9. O’Brien S, Furman R, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919.
10. Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772-778.
11. Kunk PR, Mock J, Devitt ME, Palkimas S, et al. Major bleeding with ibrutinib: more than expected. Blood. 2016;128(22):3229.
12. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
13. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282.
14. Goldberg D, Lewis JD, Halpern SD, Weiner M, Lo Re V 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21(7):765-769.
15. Guzman JZ, Iatridis JC, Skovrlj B, et al. Outcomes and complications of diabetes mellitus on patients undergoing degenerative lumbar spine surgery. Spine (Phila Pa 1976). 2014;39(19):1596-1604.
16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
17. Lane MA, Zeringue A, McDonald JR. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127(7):657–663.e2.
18. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140.
19. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571-1578.
20. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796-1805.
Radiotherapeutic Care of Patients With Stage IV Lung Cancer with Thoracic Symptoms in the Veterans Health Administration (FULL)
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
Lung cancer is the leading cause of cancer mortality both in the US and worldwide.1 Many patients diagnosed with lung cancer present with advanced disease with thoracic symptoms such as cough, hemoptysis, dyspnea, and chest pain.2-4 Palliative radiotherapy is routinely used in patients with locally advanced and metastatic lung cancer with the goal of relieving these symptoms and improving quality of life. Guidelines published by the American Society for Radiation Oncology (ASTRO) in 2011, and updated in 2018, provide recommendations on palliation of lung cancer with external beam radiotherapy (EBRT) and clarify the roles of concurrent chemotherapy and endobronchial brachytherapy (EBB) for palliation.5,6
After prostate cancer, lung cancer is the second most frequently diagnosed cancer in the Veterans Health Administration (VHA).7 The VHA consists of 172 medical centers and is the largest integrated health care system in the US. At the time of this study, 40 of these centers had onsite radiation facilities. The VHA Palliative Radiation Taskforce has conducted a series of surveys to evaluate use of palliative radiotherapy in the VHA, determine VHA practice concordance with ASTRO and American College of Radiology (ACR) guidelines, and direct educational efforts towards addressing gaps in knowledge. These efforts are directed at ensuring best practices throughout this large and heterogeneous healthcare system. In 2016 a survey was conducted to evaluate concordance of VHA radiation oncologist (RO) practice with the 2011 ASTRO guidelines on palliative thoracic radiotherapy for non-small cell lung cancer (NSCLC).
Methods
A survey instrument was generated by VHA National Palliative Radiotherapy Taskforce members. It was reviewed and approved for use by the VHA Patient Care Services office. In May of 2016, the online survey was sent to the 88 VHA ROs practicing at the 40 sites with onsite radiation facilities. The survey aimed to determine patterns of practice for palliation of thoracic symptoms secondary to lung cancer.
Demographic information obtained included years in practice, employment status, academic appointment, board certification, and familiarity with ASTRO lung cancer guidelines. Two clinical scenarios were presented to glean opinions on dose/fractionation schemes preferred, use of concurrent chemotherapy, and use of EBB and/or yttrium aluminum garnet (YAG) laser technology. Survey questions also assessed use of EBRT for palliation of hemoptysis, chest wall pain, and/or stridor as well as use of stereotactic body radiotherapy (SBRT) for palliation.
Survey results were assessed for concordance with published ASTRO guidelines. χ2 tests were run to test for associations between demographic factors such as academic appointment, years of practice, full time vs part time employment, and familiarity with ASTRO palliative lung cancer guidelines, with use of EBRT for palliation, dose and fractionation preference, use of concurrent chemotherapy, and strategy for management of endobronchial lesions.
Results
Of the 88 physicians surveyed, 54 responded for a response rate of 61%. Respondents represented 37 of the 40 (93%) VHA radiation oncology departments (Table 1). Among respondents, most were board certified (96%), held academic appointments (91%), and were full-time employees (85%). Forty-four percent of respondents were in practice for > 20 years, 19% for 11 to 20 years, 20% for 6 to 10 years, and 17% for < 6 years. A majority reported familiarity with the ASTRO guidelines (64%), while just 11% reported no familiarity with the guidelines.
When asked about use of SBRT for palliation of hemoptysis, stridor, and/or chest pain, the majority (87%) preferred conventional EBRT. Of the 13% who reported use of SBRT, most (11%) performed it onsite, with 2% of respondents referring offsite to non-VHA centers for the service. When asked about use of EBB for palliation, only 2% reported use of that procedure at their facilities, while 26% reported referral to non-VHA facilities for EBB. The remaining 72% of respondents favor use of conventional EBRT.
Respondents were presented with a case of a male patient aged 70 years who smoked and had widely metastatic NSCLC, a life expectancy of about 3 months, and 10/10 chest wall pain from direct tumor invasion. All respondents recommended palliative radiotherapy. The preferred fractionation was 20 Gray (Gy) in 5 fractions, which was recommended by 69% of respondents. The remainder recommended 30 Gy in 10 fractions (22%) or a single fraction of 10 Gy (9%). No respondent recommended the longer fractionation options of 60 Gy in 30 fractions, 45 Gy in 15 fractions, or 40 Gy in 20 fractions. The majority (98%) did not recommend concurrent chemotherapy.
When the above case was modified for an endobronchial lesion requiring palliation with associated lung collapse, rather than chest wall invasion, 20 respondents (38%) reported they would refer for EBB, and 20 respondents reported they would refer for YAG laser. As > 1 answer could be selected for this question, there were 12 respondents who selected both EBB and YAG laser; 8 selected only EBB, and 8 selected only YAG laser. Many respondents added comments about treating with EBRT, which had not been presented as an answer choice. Nearly half of respondents (49%) were amenable to referral for the use of EBB or YAG laser for lung reexpansion prior to radiotherapy. Three respondents mentioned referral for an endobronchial stent prior to palliative radiotherapy to address this question.
χ2 tests were used to evaluate for significant associations between demographic factors, such as number of years in practice, academic appointment, full-time vs part-time status, and familiarity with ASTRO guidelines with clinical management choices (Table 2). The χ2 analysis revealed that these demographic factors were not significantly associated with familiarity with ASTRO guidelines, offering SBRT for palliation, EBRT fractionation scheme preferred, use of concurrent chemotherapy, or use of EBB or YAG laser.
Discussion
This survey was conducted to evaluate concordance of management of metastatic lung cancer in the VHA with ASTRO guidelines. The relationship between respondents’ familiarity with the guidelines and responses also was evaluated to determine the impact such guidelines have on decision-making. The ASTRO guidelines for palliative thoracic radiation make recommendations regarding 3 issues: (1) radiation doses and fractionations for palliation; (2) the role of EBB; and (3) the use of concurrent chemotherapy.5,6
Radiation Dose and Fractionation for Palliation
A variety of dose/fractionation schemes are considered appropriate in the ASTRO guideline statement, including more prolonged courses such as 30 Gy/10 fractions as well as more hypofractionated regimens (ie, 20 Gy/5 fractions, 17 Gy/2 fractions, and a single fraction of 10 Gy). Higher dose regimens, such as 30 Gy/10 fractions, have been associated with prolonged survival, as well as increased toxicities such as radiation esophagitis.8 Therefore, the guidelines support use of 30 Gy/10 fractions for patients with good performance status while encouraging use of more hypofractionated regimens for patients with poor performance status. In considering more hypofractionated regimens, one must consider the possibility of adverse effects that can be associated with higher dose per fraction. For instance, 17 Gy/2 fractions has been associated with myelopathy; therefore it should be used with caution and careful treatment planning.9
For the survey case example (a male aged 70 years with a 3-month life expectancy who required palliation for chest wall pain), all respondents selected hypofractionated regimens; with no respondent selected the more prolonged fractionations of 60 Gy/30 fractions, 45 Gy/15 fractions, or 40 Gy/20 fractions. These more prolonged fractionations are not endorsed by the guidelines in general, and particularly not for a patient with poor life expectancy. All responses for this case selected by survey respondents are considered appropriate per the consensus guideline statement.
Role of Concurrent Chemotherapy
The ASTRO guidelines do not support use of concurrent chemotherapy for palliation of stage IV NSCLC.5,6 The 2018 updated guidelines established a role for concurrent chemotherapy for patients with stage III NSCLC with good performance status and life expectancy of > 3 months. This updated recommendation is based on data from 2 randomized trials demonstrating improvement in overall survival with the addition of chemotherapy for patients with stage III NSCLC undergoing palliative radiotherapy.10-12
These newer studies are in contrast to an older randomized study by Ball and colleagues that demonstrated greater toxicity from concurrent chemotherapy, with no improvement in outcomes such as palliation of symptoms, overall survival, or progression free survival.13 In contrast to the newer studies that included only patients with stage III NSCLC, about half of the patients in the Ball and colleagues study had known metastatic disease.10-13 Of note, staging for metastatic disease was not carried out routinely, so it is possible that a greater proportion of patients had metastatic disease that would have been seen on imaging. In concordance with the guidelines, 98% of the survey respondents did not recommend concurrent chemotherapy for palliation of intrathoracic symptom; only 1 respondent recommended use of chemotherapy for palliation.
Role of Endobronchial Brachytherapy
EBB involves implantation of radioactive sources for treatment of endobronchial lesions causing obstructive symptoms.14 Given the lack of randomized data that demonstrate a benefit of EBB over EBRT, the ASTRO guidelines do not endorse routine use of EBB for initial palliative management.15,16 The ASTRO guidelines reference a Cochrane Review of 13 trials that concluded that EBRT alone is superior to EBB alone for initial palliation of symptoms from endobronchial NSCLC.17
Of respondents surveyed, only 1 facility offered onsite EBB. The majority of respondents (72%) preferred the use of conventional EBRT techniques, while 26% refer to non-VHA centers for EBB. Lack of incorporation of EBB into routine VHA practice likely is a reflection of the unclear role of this technology based on the available literature and ASTRO guidelines. In the setting of a right lower lung collapse, more respondents (49%) would consider use of EBB or YAG laser technology for lung reexpansion prior to EBRT.
The ASTRO guidelines recommend that initial EBB in conjunction with EBRT be considered based on randomized data demonstrating significant improvement in lung reexpansion and in patient reported dyspnea with addition of EBB to EBRT over EBRT alone.18 However, the guidelines do not mandate the use of EBB in this situation. It is possible that targeted education regarding the role of EBB would improve knowledge of the potential benefit in the setting of lung collapse and increase the percentage of VHA ROs who would recommend this procedure.
Limitations
The study is limited by lack of generalizability of these findings to all ROs in the country. It is also possible that physician responses do not represent practice patterns with complete accuracy. The use of EBB varied among practitioners. Further study of this technology is necessary to clarify its role in the management of endobronchial obstructive symptoms and to determine whether efforts should be made to increase access to EBB within the VHA.
Conclusions
Most of the ROs who responded to our survey were cognizant and compliant with current ASTRO guidelines on management of lung cancer. Furthermore, familiarity with ASTRO guidelines and management choices were not associated with the respondents’ years in practice, academic appointment, full-time vs part-time status, or familiarity with ASTRO guidelines. This study is a nationwide survey of ROs in the VHA system that reflects the radiation-related care received by veterans with metastatic lung cancer. Responses were obtained from 93% of the 40 radiation oncology centers, so it is likely that the survey accurately represents the decision-making process at the majority of centers. It is possible that those who did not respond to the survey do not treat thoracic cases.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 65(2):87-108.
2. Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Chute CG, Greenberg ER, Baron J, Korson R, Baker J, Yates J. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107-2111.
4. Hyde L, Hyde Cl. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306.
5. Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1(2):60-71.
6. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
7. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the United States Veterans Affairs (VA) healthcare system. Mil Med. 2012;177(6):693-701.
8. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001-4011.
9. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65(6):934-941.
10. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255-1262.
11. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
12. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Aasebø U. Poor prognosis patients with inoperable locally advanced NSCLC and large tumors benefit from palliative chemoradiotherapy: a subset analysis from a randomized clinical phase III trial. J Thorac Oncol. 2014;9(6):825-833.
13. Ball D, Smith J, Bishop J, et al. A phase III study of radiotherapy with and without continuous-infusion fluorouracil as palliation for non-small-cell lung cancer. Br J Cancer. 1997;75(5):690-697.
14. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1-11.
15. Sur R, Ahmed SN, Donde B, Morar R, Mohamed G, Sur M, Pacella JA, Van der Merwe E, Feldman C. Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: preliminary analysis of a randomized prospective study. J Brachytherapy Int. 2001;17(4):309-315.
16. Sur R, Donde B, Mohuiddin M, et al. Randomized prospective study on the role of high dose rate intraluminal brachytherapy (HDRILBT) in palliation of symptoms in advanced non-small cell lung cancer (NSCLC) treated with radiation alone. Int J Radiat Oncol Biol Phys. 2004;60(1):S205.
17. Ung YC, Yu E, Falkson C, et al. The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small cell lung cancer: a systematic review. Brachytherapy. 2006;5:189-202.
18. Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol. 2001;58(3):257-268.
Success in LGBTQ+ medicine requires awareness of risk
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
Most labeled penicillin-allergic are no longer intolerant
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Corticosteroid bursts may increase risk of sepsis, GI bleeding in children
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
FROM JAMA PEDIATRICS
Nonpharma approach a potential ‘game changer’ in schizophrenia?
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cognitive remediation (CR), a therapy that encompasses nonpharmacologic approaches to improving cognitive function for patients with severe mental illness, may lead to significant improvement for patients with schizophrenia, new research suggests.
A systematic review of 130 worldwide studies that included almost 9,000 participants showed that CR significantly improved global cognition and global functioning. In addition, investigators identified key patient characteristics that flagged ideal candidates for the therapy.
“Because pharmacological treatment exerts limited effects on cognitive deficits, and clinical remission does not necessarily result in functional recovery, widespread implementation of CR could be a game-changer for achieving the patient’s personal recovery goals,” the researchers wrote.
“We hope that this systematic review could help clinicians understand how to make CR even more effective and even more personalized,” lead author Antonio Vita, MD, PhD, department of clinical and experimental sciences, University of Brescia, Italy, said in an interview.
Dr. Vita noted that he would also encourage clinicians to consider “proposing it for clinical practice.”
The findings were presented at the virtual congress of the Schizophrenia International Research Society (SIRS 2021) and were published simultaneously in JAMA Psychiatry.
Resistance continues
Cognition “should be a focus of treatment because most of the disability and functional consequences of the disease are related to ... neurocognitive impairment and impairment of social cognition,” Dr. Vita said.
He noted that treatments that focus on cognition are crucial for the recovery of patients with schizophrenia.
However, despite a “solid body of evidence” supporting the efficacy of CR and guideline recommendations that CR be included in psychiatric services, reluctance remains, the investigators noted.
The study’s goal was to determine optimal candidates for CR and to assess outcomes of the therapy and its four core elements:
- The presence of an active and trained therapist.
- Repeated practice of cognitive exercises.
- Structured development of cognitive strategies.
- Techniques to improve the transfer of cognitive gains to the real world, such as integrated psychosocial rehabilitation.
The investigators conducted a systematic literature search of the PubMed, Scopus, and PsychInfo databases to find relevant studies of CR published between January 2011 and February 2020. They also “hand-searched” meta-analyses, reviews, and reference lists.
Ultimately, the analysis included 130 randomized clinical trials comparing CR with a control condition in 8,851 patients with schizophrenia spectrum disorders.
Of these studies, 57 were conducted in Europe, 38 in the United States, 22 in Asia, 4 in Canada, 4 in Middle Eastern countries, 3 in Australia, and 2 in Brazil.
The mean age of the participants was 36.7 years, and 68% were men. The average age at the time of schizophrenia onset was 23.3 years, and the mean duration of illness was 13.8 years.
The average duration of CR treatment was 15.2 weeks. The four elements were well represented; each was offered to at least 71% of patients.
The comparator therapy was treatment as usual (TAU), in 34.3% of cases, or active TAU with multidisciplinary rehabilitation, in 15.2% of cases. The remaining interventions were either nonspecific (30.8%) or were devised specifically for the study (19.9%).
Results showed that CR had a significant, albeit moderate, effect on global cognition (Cohen’s d effect size, 0.29; P < .001) and global functioning (effect size, 0.22; P < .01).
Having an active and trained therapist had a significant impact on cognition and functioning (P = .04 for both), as did the structured development of cognitive strategies (P = .002 for cognition; P = .004 for functioning).
The integration of psychosocial rehabilitation also had a significant effect on functioning (P = .003).
Interventions that included all of the core elements had a “highly significant” association with global cognition (P = .02) and global functioning (P < .001), the investigators reported. Longer treatments were significantly associated with greater functional improvement (P = .006).
The investigators found that improvements were greater among patients who had fewer years of education (P = .03 for cognition; P = .02 for functioning), lower premorbid IQ scores (P = .04 for functioning), and more severe symptoms at baseline (P = .005 for cognition).
The researchers noted that CR should become more widely available because it has the “potential to be an element of standard care rather than an optional intervention targeting selected individuals.”
An overlooked treatment option
Commenting on the findings for this news organization, Alice Medalia, PhD, director of the Lieber Recovery Clinic at Columbia University Irving Medical Center, New York, noted that this study is the second large-scale analysis of the use of CR for patients with schizophrenia to come out this year. The other was published in Schizophrenia Bulletin.
“So this is a banner year for large reviews,” she said. “It’s great to have two studies like this [that] tell a very consistent story.”
Dr. Medalia, who was not involved with the research, said individuals “don’t really talk about cognition very much.”
CR, she added, is “one of an array of services that one should be providing, and the bigger picture is that every single person should have their cognitive health needs addressed.
“If someone is having problems, and it’s getting in the way of them being the kind of person they want to be and doing want they want to do, we need to intervene. How we intervene should always be in the least disruptive and intense way,” she said.
These measures could include examining sleep hygiene, adjusting medications, or introducing exercise.
“But there really does come a time for some people where cognitive remediation is going to be helpful, so it should be more available,” Dr. Medalia said.
Although increased availability is partially dependent on having enough trained therapists, the main reason CR is not more widely available is because “people just don’t think about cognition and they don’t know how to talk about it,” she noted. In addition, she said, even when it is available, clinicians don’t refer patients.
“That tells you something. The solution here is not to put a cognitive remediation program everywhere but ... to get people comfortable talking about cognition and identifying when an intervention is needed,” said Dr. Medalia.
One study author received grants from the National Institute for Health Research during the conduct of the study and is the creator of CIRCuiTs, a cognitive remediation software program. The other investigators and Dr. Medalia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Pivotal Moment in Cancer Surgery, Captured on Film
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Few ever see this side of cancer care. Our cameras go behind the scenes as a surgical oncologist faces a crucial moment in the OR in this first episode of a new video series, The Oncologists.
Medscape Oncology © 2021 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: A Pivotal Moment in Cancer Surgery, Captured on Film - Medscape - Feb 18, 2021.
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
COVID lockdowns linked to PTSD in patients with eating disorders
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 and its resulting lockdowns are linked to posttraumatic stress disorder symptoms and other adverse outcomes among patients with eating disorders (EDs), two new studies show.
Results of the first study show that patients with EDs had more stress, anxiety, depression, and PTSD-related symptoms during the lockdowns than their mentally healthy peers.
In the second study, treatment-related symptom improvement among patients with bulimia nervosa (BN) slowed following lockdown. In addition, patients with BN or anorexia nervosa (AN) experienced significant worsening of disorder-specific behaviors, including binge eating and overexercising.
Because of the strict lockdown measures introduced by the Italian government to contain the COVID-19 pandemic, “everyday life of all citizens was disrupted,” Veronica Nisticò, MS, Università Degli Studi Di Milano, who led the first study, told delegates attending the virtual European Psychiatric Association 2021 Congress.
In addition to difficulties in accessing health care, “it became difficult to go to the supermarket, to the gym, and to have the social support we were all used to,” all of which had a well-documented impact on mental health, added Ms. Nisticò, who is also affiliated with Aldo Ravelli Research Center for Neurotechnology and Experimental Brain Therapeutics.
Loss of control
Previous research suggests that individuals with EDs experience high levels of anxiety and an increase in binge eating, exercise, and purging behaviors, said Ms. Nisticò.
To investigate further, the researchers conducted a longitudinal study of the changes in prevalence of adverse outcomes. In the study, two assessments were conducted.
The second group served as the control group.
Participants completed an online survey that included several standardized depression and anxiety scales, as well as an ad hoc survey adapted from the Eating Disorder Examination Questionnaire. This assessed changes in restrictive dieting, control over food, body image, and psychological well-being in comparison with prepandemic levels.
The results, which were also recently published online in Eating and Weight Disorders – Studies on Anorexia, Bulimia and Obesity, showed that patients with EDs experienced significantly more stress, anxiety, depression, and PTSD-related symptoms in comparison with control persons (P < .05 for all).
In addition, the investigators found that those with EDs were more fearful of losing control over their eating behavior, spent more time thinking about food and their body, and became more uncomfortable seeing their body than before the lockdown in comparison with those without EDs (P < .05).
Clinical implications
A second assessment, which occurred in June 2020, after lockdown restrictions were lifted, included 40 patients with EDs who had taken part in the first assessment. This time, participants were asked to compare their current eating behavior with their eating behavior during the lockdown.
Although the lifting of lockdown restrictions was associated with significant improvement in PTSD-related symptoms, the impact on stress, anxiety, and depression persisted.
These findings, said Ms. Nisticó, support the hypothesis that specific conditions that occurred during the lockdown had a direct effect on specific ED symptoms.
These findings, she added, should be considered when developing interventions for EDs in the context of individual psychotherapy and when designing large, preventive interventions.
In the second study, Eleonora Rossi, MD, psychiatric unit, department of health sciences, University of Florence (Italy), and colleagues examined the longitudinal impact of the pandemic on individuals with EDs.
They examined 74 patients with AN or BN who had undergone baseline assessments and had completed a number of questionnaires in the first months of 2019 in conjunction with being enrolled in another study.
Participants were treated with enhanced cognitive-behavioral therapy and were reevaluated between November 2019 and January 2020. They were then compared with 97 healthy individuals.
Bulimia patients more vulnerable
After the outbreak of the pandemic, most treatment was administered online, so patients were able to continue therapy, Dr. Rossi said during her presentation.
All participants were assessed again in April 2020, 6 weeks after the start of Italy’s lockdown.
The results, which were published in the International Journal of Eating Disorders, show that the patients with EDs “underwent a significant improvement in terms of general and eating disorder specific psychopathology” during the first treatment period, Dr. Rossi reported. In addition, among those with AN, body mass index increased significantly (P < .05 for all).
Patients with AN continued to improve during the lockdown when therapy was administered online. However, improvements that had occurred among those with BN slowed, Dr. Rossi noted.
In addition, both groups of patients with EDs experienced a worsening of their pathological eating behaviors during the lockdown, in particular, objective binge eating and compensatory physical exercise (P < .05).
“Indeed, the positive trajectory of improvement observed before lockdown was clearly interrupted during the pandemic period,” Dr. Rossi said. This could “represent a possible hint of an imminent exacerbation of the disease.”
The results also suggest that the occurrence of arguments within the household and fear regarding the safety of loved ones predicted an increase in symptoms during the lockdown, she added.
In addition, patients with BN reported more severe COVID-related PTSD symptoms than did those with AN and the control group. This increase in severity of symptoms was more prevalent among patients who had a history of childhood trauma and among those with insecure attachment, suggesting that such patients may be more vulnerable.
Evidence of recovery
Commenting on the studies, David Spiegel, MD, associate chair of psychiatry, Stanford (Calif.) University, noted that EDs commonly occur after physical or sexual trauma earlier in life.
“It’s a standard thing with trauma-related disorders that any other, even relatively minor, traumatic experience can exacerbate PTSD symptoms,” said Dr. Spiegel, who was not involved in the studies. In addition, the trauma of the COVID pandemic “was not minor.
“The relative isolation and the lack of outside contact may focus many people with eating disorders even more on their struggles with how they are taking care of their bodies,” said Dr. Spiegel.
“It struck me that the anorexia nervosa group were more impervious than the bulimia nervosa group, but I think that’s the case with the disorder. In some ways it’s more severe, obviously a more life-threatening disorder,” he added.
The “hopeful thing is that there seemed to be some evidence of recovery and improvement, particularly with the posttraumatic stress exacerbation, as time went on,” Dr. Spiegel said, “and that’s a good thing.”
The study authors and Dr. Spiegel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.