User login
Making the Rounds to Diagnosis
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
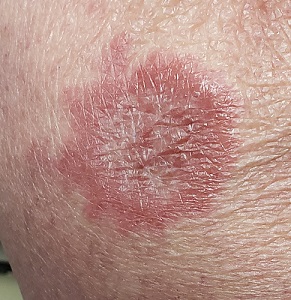
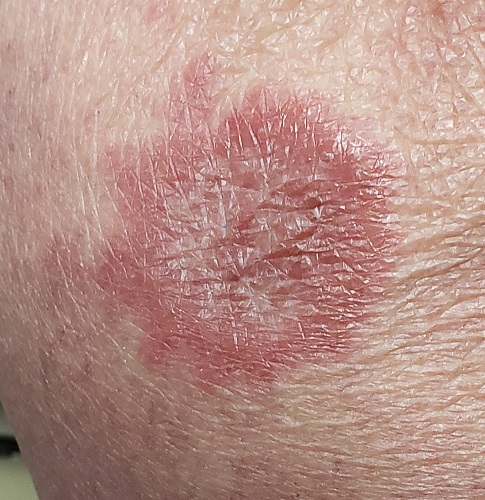
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
A 60-year-old woman is feeling fine—no fever, arthralgia, or malaise—but the sudden appearance of lesions on her extremities unsettles her to the point that she can think of little else. Visits to her primary care provider, the emergency department, several urgent care clinics, and a naturopath yield a consistent diagnosis: ringworm. Yet none of the topical or oral antifungal medications she is prescribed make the slightest difference. Thus, she finally agrees to consult a dermatologist.
The patient reports decent health, although she was a heavy smoker for years before quitting 2 years ago. She recently tested negative for diabetes.
History taking reveals no sources from which she could have acquired a fungal infection. No one else at home is similarly affected.
About 10 lesions, mostly located on the patient’s extremities, are examined. All are very similar in appearance: round, brownish-red intradermal plaques with no epidermal component (eg, scale, broken skin). The lesions vary from 6 mm to 4 cm in diameter. The centers of most lesions are slightly concave, with well-defined raised margins. On palpation, there is neither increased warmth nor tenderness. No nodes can be palpated in the groin, axillae, or epitrochlear locations.
A deep punch biopsy is performed on a thigh lesion, with the specimen submitted for pathologic examination.
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Suicide risk prediction tools fail people of color
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
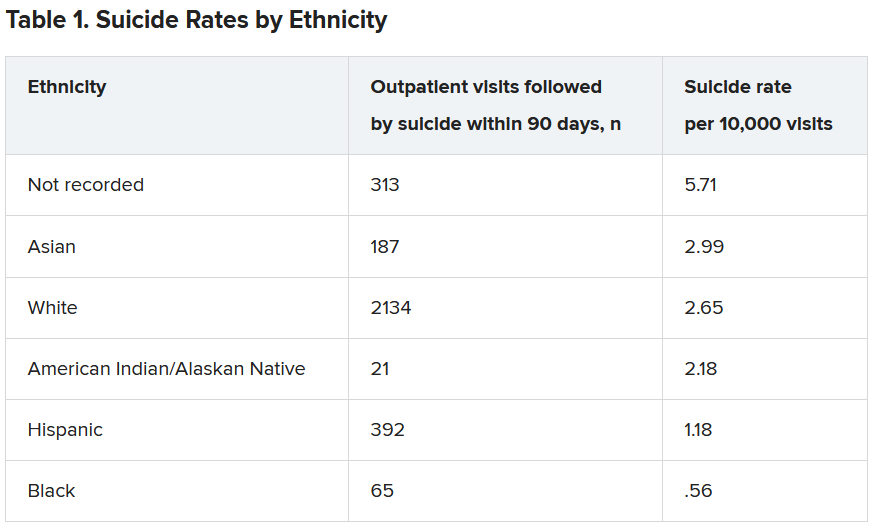
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current models used to predict suicide risk fall short for racialized populations including Black, Indigenous, and people of color (BIPOC), new research shows.
Investigators developed two suicide prediction models to examine whether these types of tools are accurate in their predictive abilities, or whether they are flawed.
They found both prediction models failed to identify high-risk BIPOC individuals. In the first model, nearly half of outpatient visits followed by suicide were identified in White patients versus only 7% of visits followed by suicide in BIPOC patients. The second model had a sensitivity of 41% for White patients, but just 3% for Black patients and 7% for American Indian/Alaskan Native patients.
“You don’t know whether a prediction model will be useful or harmful until it’s evaluated. The take-home message of our study is this: You have to look,” lead author Yates Coley, PhD, assistant investigator, Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The study was published online April 28, 2021, in JAMA Psychiatry.
Racial inequities
Suicide risk prediction models have been “developed and validated in several settings” and are now in regular use at the Veterans Health Administration, HealthPartners, and Kaiser Permanente, the authors wrote.
But the performance of suicide risk prediction models, while accurate in the overall population, “remains unexamined” in particular subpopulations, they noted.
“Health records data reflect existing racial and ethnic inequities in health care access, quality, and outcomes; and prediction models using health records data may perpetuate these disparities by presuming that past healthcare patterns accurately reflect actual needs,” Dr. Coley said.
Dr. Coley and associates “wanted to make sure that any suicide prediction model we implemented in clinical care reduced health disparities rather than exacerbated them.”
To investigate, researchers examined all outpatient mental health visits to seven large integrated health care systems by patients 13 years and older (n = 13,980,570 visits by 1,422,534 patients; 64% female, mean age, 42 years). The study spanned from Jan. 1, 2009, to Sept. 30, 2017, with follow-up through Dec. 31, 2017.
In particular, researchers looked at suicides that took place within 90 days following the outpatient visit.
Researchers used two prediction models: logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) variable selection and random forest technique, a “tree-based method that explores interactions between predictors (including those with race and ethnicity) in estimating probability of an outcome.”
The models considered prespecified interactions between predictors, including prior diagnoses, suicide attempts, and PHQ-9 [Patient Health Questionnaire–9] responses, and race and ethnicity data.
Researchers evaluated performance of the prediction models in the overall validation set and within subgroups defined by race/ethnicity.
The area under the curve measured model discrimination, and sensitivity was estimated for global and race/ethnicity-specific thresholds.
‘Unacceptable’ scenario
Within the total population, there were 768 deaths by suicide within 90 days of 3,143 visits. Suicide rates were highest for visits by patients with no recorded race/ethnicity, followed by visits by Asian, White, American Indian/Alaskan Native, Hispanic, and Black patients.
Both models showed “high” AUC sensitivity for White, Hispanic, and Asian patients but “poor” AUC sensitivity for BIPOC and patients without recorded race/ethnicity, the authors reported.
“Implementation of prediction models has to be considered in the broader context of unmet health care needs,” said Dr. Coley.
“In our specific example of suicide prediction, BIPOC populations already face substantial barriers in accessing quality mental health care and, as a result, have poorer outcomes, and using either of the suicide prediction models examined in our study will provide less benefit to already-underserved populations and widen existing care gaps,” a scenario Dr. Coley said is “unacceptable.”
“ she added.
Biased algorithms
Commenting on the study, Jonathan Singer, PhD, LCSW, associate professor at Loyola University, Chicago, described it as an “important contribution because it points to a systemic problem and also to the fact that the algorithms we create are biased, created by humans, and humans are biased.”
Although the study focused on the health care system, Dr. Singer believes the findings have implications for individual clinicians.
“If clinicians may be biased against identifying suicide risk in Black and Native American patients, they may attribute suicidal risk to something else. For example, we know that in Black Americans, expressions of intense emotions are oftentimes interpreted as aggression or being threatening, as opposed to indicators of sadness or fear,” noted Dr. Singer, who is also president of the American Academy of Suicidology and was not involved with the study,
“Clinicians who misinterpret these intense emotions are less likely to identify a Black client or patient who is suicidal,” Dr. Singer said.
The research was supported by the Mental Health Research Network from the National Institute of Mental Health. Dr. Coley has reported receiving support through a grant from the Agency for Healthcare Research and Quality. Dr. Singer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
Operational changes in primary care linked with improved smoking, blood pressure outcomes
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
FROM ANNALS OF FAMILY MEDICINE
Low-fat diet upped quality of life in ulcerative colitis
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
Diet plays an important role in Crohn’s disease and ulcerative colitis. Most patients with these diseases look to incorporate dietary modification as part of the treatment plan to achieve and maintain remission. With the development of tools that allow us to sequence the gut microbiome at high resolution, the role of dietary therapy for these diseases is being studied with increasing scientific rigor.
In a crossover study of 17 patients with ulcerative colitis in remission or with only mild disease, Fritsch and colleagues demonstrated that adherence to a low-fat, high-fiber diet was associated with an improvement in the health-related quality of life, a decrease in C-reactive protein, and beneficial changes in the gut bacteria including reduced abundance of Actinobacteria and an increase in organisms with anti-inflammatory potential such as Faecalibacterium prausnitzii. In conjunction with prior experimental studies that suggested an increase in risk of colitis with high fat intake, this study provides some evidence for recommending a lower fat intake in patients with established inflammatory bowel disease (IBD). Furthermore, an increase in fruits, vegetables and fiber intake even in those with a standard American diet was associated with a modest beneficial effect, challenging the longstanding unsupported dogma of broadly limiting all fiber intake in those with established IBD.
The much-needed progress in the scientific study of diet in IBD will provide us with the important answers that our patients are looking for.
Ashwin Ananthakrishnan, MD, MPH , is an associate professor of medicine at Massachusetts General Hospital and Harvard Medical School, both in Boston. He has no conflicts relevant to this commentary to declare.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
IL-6 trans-signaling targeted by olamkicept in IBD
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
Proinflammatory cytokine inhibition has revolutionized the care of patients with moderate to severe inflammatory bowel disease (IBD). However, some patients don’t respond, never gain remission, or lose response. Therefore, the search continues for more effective therapies. The study by Schreiber and colleagues highlights the importance of continued innovation surrounding inflammatory pathways.
The authors did extensive evaluation of the tissue and molecular effects and discovered possible differential target engagement with interleukin-6 transcriptional inhibition which is encouraging. Notably, however, there were a high number of reported adverse events. Per the authors, these were nonspecific and not indicative of severe immunosuppression. Importantly, there were no intestinal perforations.
Intense optimism for new mechanisms will remain tempered as we have seen other therapies hold promise but fail in larger randomized trials. However, it is encouraging to see how continued work on proinflammatory pathways into more targeted inhibitory approaches can lead to potential new therapies in IBD.
Sara Horst, MD, MPH, FACG, is an associate professor in the division of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center, Nashville, Tenn. She reports having been a consultant for Gilead, Takeda, and Janssen and receiving unrestricted grant funding from UCB.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Health costs over 25 times higher for hemophilia B patients than controls
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
FROM BLOOD ADVANCES
NSAIDs don’t make COVID-19 worse in hospitalized patients
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
FROM THE LANCET RHEUMATOLOGY
Recommendations for Pregnant Members of Dermatology Health Care Teams During the COVID-19 Pandemic
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
Practice Points
- Pregnant women are at an increased risk for severe illness due to COVID-19 compared with nonpregnant women; therefore, it is important to protect pregnant health care workers who are caring for patients during the current pandemic.
- Although currently available COVID-19 vaccines have not been tested in pregnant women, they should not be withheld from pregnant individuals.
- Pregnant attending physicians and residents in dermatology can continue to provide care through telemedicine; if they choose to, and if all recommended personal protective equipment (PPE) are available, they can continue to provide in-person care.
- Correct and comprehensive use of PPE by pregnant health care workers is paramount to minimizing exposure to SARS-CoV-2.