User login
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”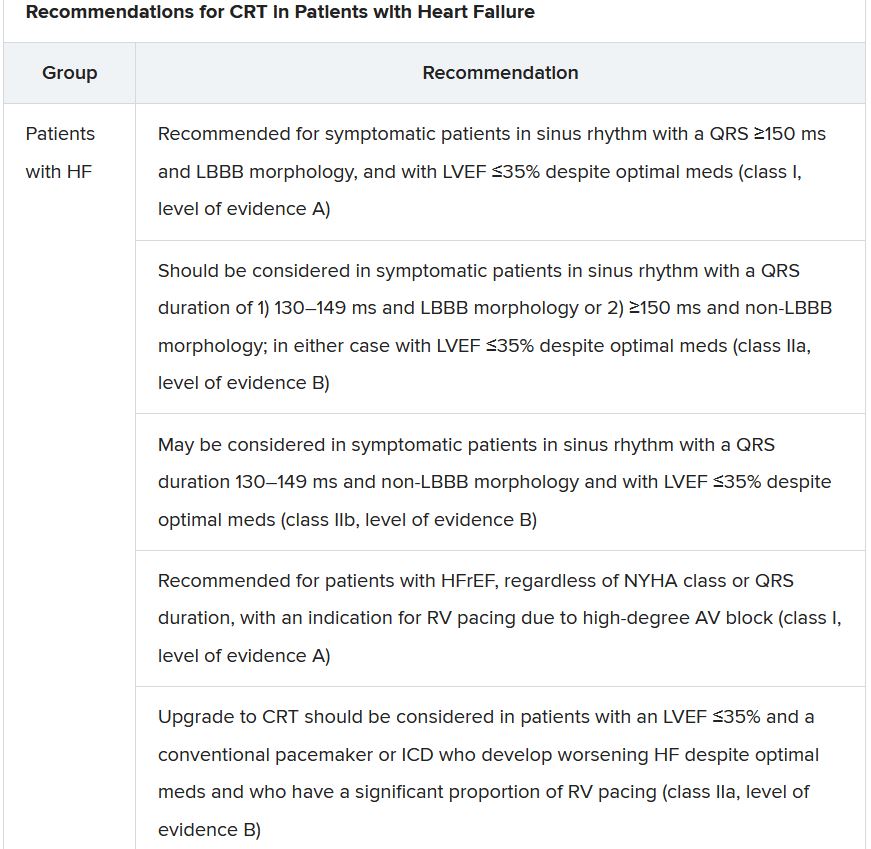
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.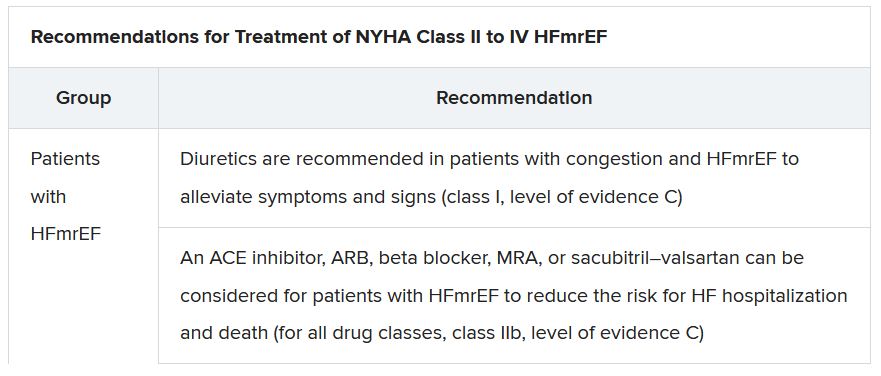
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
Study: Use urine sampling more broadly to rule out pediatric UTI
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
Study gives bleeding risk estimates for VTE patients on anticoagulants
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Pandemic strategies to boost trial enrollment should stay
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Novel diabetic foot ulcer cream shows promise in phase 3 trial
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Air pollution – second leading cause of lung cancer
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
The new data show that the rate of lung cancer deaths attributable to air pollution varies widely between countries. Serbia, Poland, China, Mongolia, and Turkey are among the worst affected. The analysis shows an association between deaths from lung cancer and the proportion of national energy that is produced from coal.
“Both smoking and air pollution are important causes of lung cancer,” said study presenter Christine D. Berg, MD, former codirector of the National Lung Screening Trial, and “both need to be eliminated to help prevent lung cancer and save lives.
“As lung cancer professionals, we can mitigate the effects of air pollution on causing lung cancer by speaking out for clean energy standards,” she said.
Dr. Berg presented the new analysis on Sept. 9 at the 2021 World Conference on Lung Cancer, which was organized by the International Association for the Study of Lung Cancer.
She welcomed the recent statement issued by the IASLC in support of the International Day of Clean Air for Blue Skies, which took place on Sept. 7. It was a call for action that emphasized the need for further efforts to improve air quality to protect human health.
The findings from the new analysis are “depressing,” commented Joachim G. J. V. Aerts, MD. PhD, department of pulmonary diseases, Erasmus University Medical Center, Rotterdam, the Netherlands.
It is now clear that air pollution has an impact not only on the incidence of lung cancer but also on its outcome, he added.
Indeed, previous research showed that each 10 mcg/m3 increase in particular matter of 2.5 mcg in size was associated with a 15%-27% increase in lung cancer mortality. There was no difference in rates between women and men.
A key question, Dr. Aerts said, is whether reducing air pollution would be beneficial.
Efforts to reduce air pollution over recent decades in the United Kingdom have not led to a reduction in lung cancer deaths. This is because of the increase in life expectancy – individuals have been exposed to pollution for longer, albeit at lower levels, he pointed out.
Because of lockdowns during the COVID pandemic, travel has been greatly reduced. This has resulted in a dramatic reduction in air pollution, “and this led to a decrease in the number of children born with low birth weight,” said Dr. Aerts.
Hopefully, that benefit will also be seen regarding other diseases, he added.
The call to action to reduce air pollution is of the “utmost importance,” he said. He noted that the focus should be on global, national, local, and personal preventive measures.
“It is time to join forces,” he added, “to ‘clean the air.’ ”
Dr. Berg’s presentation was warmly received on social media.
It was “fabulous,” commented Eric H. Bernicker, MD, director of medical thoracic oncology at Houston Methodist Cancer Center.
“Thoracic oncologists need to add air pollution to things they advocate about; we have an important voice here,” he added.
It is “so important to understand that air pollution is a human carcinogen,” commented Ivy Elkins, a lung cancer survivor and advocate and cofounder of the EGFR Resisters Lung Cancer Patient Group. “All you need are lungs to get lung cancer!”
Contribution of air pollution to lung cancer
In her presentation, Dr. Berg emphasized that lung cancer is the leading cause of cancer death worldwide, although the distribution between countries “depends on historical and current smoking patterns and the demographics of the population.”
Overall, data from GLOBOCAN 2018 indicate that annually there are approximately 2.1 million incident cases of lung cancer and almost 1.8 million lung cancer deaths around the globe.
A recent study estimated that, worldwide, 14.1% of all lung cancer deaths, including in never-smokers, are directly linked to air pollution.
Dr. Berg said that this makes it the “second-leading cause of lung cancer” behind smoking.
The figure is somewhat lower for the United States, where around 4.7% of lung cancer deaths each year are directly attributable to pollution. However, with “the wildfires out West, we’re going to be seeing more of a toll from air pollution,” she predicted.
She pointed out that the International Agency for Research on Cancer classifies outdoor air pollution, especially particulate matter, as a human carcinogen on the basis of evidence of an association with lung cancer.
It is thought that direct deposits and local effects of particulate matter lead to oxidative damage and low-grade chronic inflammation. These in turn result in molecular changes that affect DNA and gene transcription and inhibit apoptosis, all of which lead to the development of cancerous lesions, she explained.
Synthesizing various estimates on global burden of disease, Dr. Berg and colleagues calculated that in 2019 the rate of lung cancer deaths attributable to particular matter in people aged 50-69 years was highest in Serbia, at 36.88 attributable deaths per 100,000.
Next was Poland, with a rate of 27.97 per 100,000, followed by China at 24.63 per 100,000, Mongolia at 19.71 per 100,000, and Turkey at 19.2 per 100,000.
The major sources of air pollution in the most affected countries were transportation, indoor cooking, and energy sources, she said.
In Serbia, 70% of energy production was from coal. It was 74% in Poland, 65% in China, 80% in Mongolia, 35% in Turkey, and 19% in the United States.
At the time of the analysis, only 17.3% of U.S. adults were smokers, and the air concentration of particular matter of 2.5 mcm was 9.6% mcg/m3. Both of these rates are far below those seen in more severely affected countries.
“But 40% of our energy now comes from natural gas,” noted Dr. Berg, “which is still a pollutant and a source of methane. It’s a very potent greenhouse gas.”
No funding for the study has been reported. Dr. Berg has relationships with GRAIL and Mercy BioAnalytics. Dr. Aerts has relationships with Amphera, AstraZeneca, Bayer, BIOCAD, Bristol-Myers Squibb, Eli Lilly, and Roche.
A version of this article first appeared on Medscape.com.
At 18 months, much still unknown about diabetes and COVID-19
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA could authorize COVID-19 vaccine for ages 5-11 in October
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
Virtual Respiratory Urgent Clinics for COVID-19 Symptoms
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88