User login
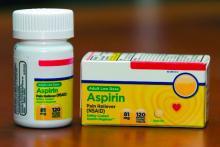
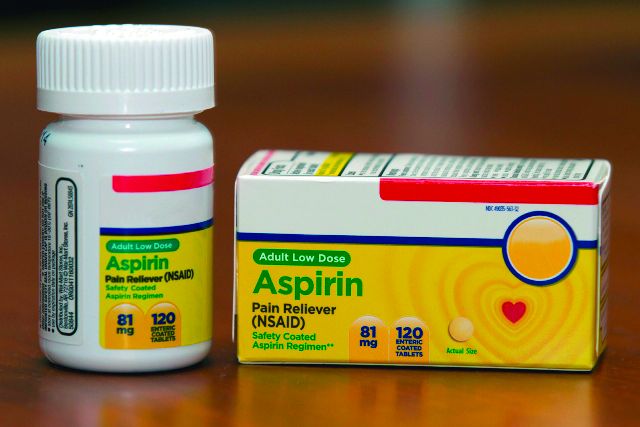
Expensive insulins, pen devices dominate U.S. diabetes care
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Dropping weight beneficial but some effects of obesity persist
It’s hard for people to completely escape a history of obesity, even when they later achieve a healthy weight.
American adults who once had obesity but later achieved and maintained a healthy body mass index (BMI) normalized some, but not all, of the excess clinical risk associated with obesity in a review of data collected from about 20,000 people during a series of eight NHANES surveys.
Maia P. Smith, MD, reported the findings at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
“For some conditions, such as hypertension and dyslipidemia, the recovery [following a sharp drop in BMI] appears to be total, while for other conditions, like diabetes, the recovery is probabilistic. Some recover, but some don’t,” explained Dr. Smith in an interview.
“Weight loss reverses all, or essentially all, of the damage done by obesity in some people, but does not cause full reversal of the harm and does not fully resolve [type 2] diabetes in many others,” added Dr. Smith, an epidemiologist in the Department of Public Health and Preventive Medicine at St. George’s University, Grenada.
“The fact that ... analyses comparing formerly obese people to normal weight populations demonstrated improvement in population mean levels of hypertension and dyslipidemia is remarkable,” commented Rebecca T. Emeny, PhD, an epidemiologist at the Dartmouth Institute of Health Policy and Clinical Practice in Lebanon, New Hampshire, who was not involved with Dr. Smith’s study.
“The observation that the individuals who were able to maintain normal weight after past obesity were still at greater risk for diabetes compared with the normal weight group speaks to the recent discussion of obesity as a metabolic disorder rather than a problem of calories in and calories out,” said Dr. Emeny in an interview.
She cited a recent article that proposed a carbohydrate-insulin model for obesity in place of an energy-balance model. This, however, is still somewhat contentious.
Dr. Emeny also cautioned that “the results of this study compare populations. The design and analysis do not allow for interpretation of individual risk resulting from changes in weight.”
Those who formerly had obesity can reverse hypertension, dyslipidemia
The study by Dr. Smith and associates used data collected in the National Health and Nutrition Examination Survey (NHANES), which is performed every 2 years by the U.S. Centers for Disease Control and Prevention.
They used data from eight consecutive surveys starting in 1999-2000 and continuing through 2013-2014, yielding data from nearly 40,000 adults who were at least 20 years old.
In addition to the 326 people who formerly had obesity at some time previously during their life (BMI ≥30 kg/m2) but now had a healthy BMI, and 6,235 who were consistently at a healthy BMI, they also included 13,710 people who currently had obesity. They dropped the remaining survey participants who did not fit into one of these three categories.
The participants who formerly had obesity averaged 54 years old, compared with a mean age of 48 years among those with current obesity and 41 years among those who currently had a healthy BMI (who had never had obesity). The results showed no differences by sex, but those who formerly had obesity had a much higher smoking prevalence.
The people who reported a healthy BMI (18.5-24.9 kg/m2) after previously having obesity had current prevalence rates of hypertension and dyslipidemia that were, respectively, 8% and 13% higher than the prevalence rates among adults who consistently maintained a healthy BMI – differences that were not significant.
In contrast, people who had current BMIs that indicated obesity had prevalence rates of hypertension and dyslipidemia that were each a significant threefold higher than those with a healthy BMI.
The 326 respondents who formerly had obesity but now were at a healthy BMI had a threefold higher prevalence of diabetes than did the 6,235 who consistently had maintained a healthy BMI. This was substantially less than the over sevenfold higher prevalence of diabetes among those who currently had obesity compared with those who always had a healthy BMI.
All these analyses were adjusted for the potential confounders of age, sex, smoking history, and ethnicity.
‘Quitting’ obesity better than current obesity
The finding that reaching a healthy BMI after a period of obesity could reverse some but not all risks associated with obesity is reminiscent of the effects of smoking, noted Dr. Smith.
“Never is better than ever, but quitting,” or dropping weight to reach a healthy BMI, “is better than current,” she concluded.
But Dr. Emeny said this interpretation, “while motivating and catchy, places emphasis on individual responsibility and choice rather than on social circumstances.”
Social effects “must be considered when evaluating population-level disparities in obesity-related cardiometabolic risk,” cautioned Dr. Emeny.
“’Quitting’ obesity is much more complicated than individual choice or ability.”
Dr. Smith also conceded that her analyses did not correct for the possible confounding effects that changes in diet or physical activity may have had on the observations.
“Neither diet nor physical activity has a well-known summary measure that we could have included as an adjuster,” she explained.
Dr. Smith and Dr. Emeny have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s hard for people to completely escape a history of obesity, even when they later achieve a healthy weight.
American adults who once had obesity but later achieved and maintained a healthy body mass index (BMI) normalized some, but not all, of the excess clinical risk associated with obesity in a review of data collected from about 20,000 people during a series of eight NHANES surveys.
Maia P. Smith, MD, reported the findings at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
“For some conditions, such as hypertension and dyslipidemia, the recovery [following a sharp drop in BMI] appears to be total, while for other conditions, like diabetes, the recovery is probabilistic. Some recover, but some don’t,” explained Dr. Smith in an interview.
“Weight loss reverses all, or essentially all, of the damage done by obesity in some people, but does not cause full reversal of the harm and does not fully resolve [type 2] diabetes in many others,” added Dr. Smith, an epidemiologist in the Department of Public Health and Preventive Medicine at St. George’s University, Grenada.
“The fact that ... analyses comparing formerly obese people to normal weight populations demonstrated improvement in population mean levels of hypertension and dyslipidemia is remarkable,” commented Rebecca T. Emeny, PhD, an epidemiologist at the Dartmouth Institute of Health Policy and Clinical Practice in Lebanon, New Hampshire, who was not involved with Dr. Smith’s study.
“The observation that the individuals who were able to maintain normal weight after past obesity were still at greater risk for diabetes compared with the normal weight group speaks to the recent discussion of obesity as a metabolic disorder rather than a problem of calories in and calories out,” said Dr. Emeny in an interview.
She cited a recent article that proposed a carbohydrate-insulin model for obesity in place of an energy-balance model. This, however, is still somewhat contentious.
Dr. Emeny also cautioned that “the results of this study compare populations. The design and analysis do not allow for interpretation of individual risk resulting from changes in weight.”
Those who formerly had obesity can reverse hypertension, dyslipidemia
The study by Dr. Smith and associates used data collected in the National Health and Nutrition Examination Survey (NHANES), which is performed every 2 years by the U.S. Centers for Disease Control and Prevention.
They used data from eight consecutive surveys starting in 1999-2000 and continuing through 2013-2014, yielding data from nearly 40,000 adults who were at least 20 years old.
In addition to the 326 people who formerly had obesity at some time previously during their life (BMI ≥30 kg/m2) but now had a healthy BMI, and 6,235 who were consistently at a healthy BMI, they also included 13,710 people who currently had obesity. They dropped the remaining survey participants who did not fit into one of these three categories.
The participants who formerly had obesity averaged 54 years old, compared with a mean age of 48 years among those with current obesity and 41 years among those who currently had a healthy BMI (who had never had obesity). The results showed no differences by sex, but those who formerly had obesity had a much higher smoking prevalence.
The people who reported a healthy BMI (18.5-24.9 kg/m2) after previously having obesity had current prevalence rates of hypertension and dyslipidemia that were, respectively, 8% and 13% higher than the prevalence rates among adults who consistently maintained a healthy BMI – differences that were not significant.
In contrast, people who had current BMIs that indicated obesity had prevalence rates of hypertension and dyslipidemia that were each a significant threefold higher than those with a healthy BMI.
The 326 respondents who formerly had obesity but now were at a healthy BMI had a threefold higher prevalence of diabetes than did the 6,235 who consistently had maintained a healthy BMI. This was substantially less than the over sevenfold higher prevalence of diabetes among those who currently had obesity compared with those who always had a healthy BMI.
All these analyses were adjusted for the potential confounders of age, sex, smoking history, and ethnicity.
‘Quitting’ obesity better than current obesity
The finding that reaching a healthy BMI after a period of obesity could reverse some but not all risks associated with obesity is reminiscent of the effects of smoking, noted Dr. Smith.
“Never is better than ever, but quitting,” or dropping weight to reach a healthy BMI, “is better than current,” she concluded.
But Dr. Emeny said this interpretation, “while motivating and catchy, places emphasis on individual responsibility and choice rather than on social circumstances.”
Social effects “must be considered when evaluating population-level disparities in obesity-related cardiometabolic risk,” cautioned Dr. Emeny.
“’Quitting’ obesity is much more complicated than individual choice or ability.”
Dr. Smith also conceded that her analyses did not correct for the possible confounding effects that changes in diet or physical activity may have had on the observations.
“Neither diet nor physical activity has a well-known summary measure that we could have included as an adjuster,” she explained.
Dr. Smith and Dr. Emeny have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s hard for people to completely escape a history of obesity, even when they later achieve a healthy weight.
American adults who once had obesity but later achieved and maintained a healthy body mass index (BMI) normalized some, but not all, of the excess clinical risk associated with obesity in a review of data collected from about 20,000 people during a series of eight NHANES surveys.
Maia P. Smith, MD, reported the findings at the virtual European Association for the Study of Diabetes (EASD) 2021 Annual Meeting.
“For some conditions, such as hypertension and dyslipidemia, the recovery [following a sharp drop in BMI] appears to be total, while for other conditions, like diabetes, the recovery is probabilistic. Some recover, but some don’t,” explained Dr. Smith in an interview.
“Weight loss reverses all, or essentially all, of the damage done by obesity in some people, but does not cause full reversal of the harm and does not fully resolve [type 2] diabetes in many others,” added Dr. Smith, an epidemiologist in the Department of Public Health and Preventive Medicine at St. George’s University, Grenada.
“The fact that ... analyses comparing formerly obese people to normal weight populations demonstrated improvement in population mean levels of hypertension and dyslipidemia is remarkable,” commented Rebecca T. Emeny, PhD, an epidemiologist at the Dartmouth Institute of Health Policy and Clinical Practice in Lebanon, New Hampshire, who was not involved with Dr. Smith’s study.
“The observation that the individuals who were able to maintain normal weight after past obesity were still at greater risk for diabetes compared with the normal weight group speaks to the recent discussion of obesity as a metabolic disorder rather than a problem of calories in and calories out,” said Dr. Emeny in an interview.
She cited a recent article that proposed a carbohydrate-insulin model for obesity in place of an energy-balance model. This, however, is still somewhat contentious.
Dr. Emeny also cautioned that “the results of this study compare populations. The design and analysis do not allow for interpretation of individual risk resulting from changes in weight.”
Those who formerly had obesity can reverse hypertension, dyslipidemia
The study by Dr. Smith and associates used data collected in the National Health and Nutrition Examination Survey (NHANES), which is performed every 2 years by the U.S. Centers for Disease Control and Prevention.
They used data from eight consecutive surveys starting in 1999-2000 and continuing through 2013-2014, yielding data from nearly 40,000 adults who were at least 20 years old.
In addition to the 326 people who formerly had obesity at some time previously during their life (BMI ≥30 kg/m2) but now had a healthy BMI, and 6,235 who were consistently at a healthy BMI, they also included 13,710 people who currently had obesity. They dropped the remaining survey participants who did not fit into one of these three categories.
The participants who formerly had obesity averaged 54 years old, compared with a mean age of 48 years among those with current obesity and 41 years among those who currently had a healthy BMI (who had never had obesity). The results showed no differences by sex, but those who formerly had obesity had a much higher smoking prevalence.
The people who reported a healthy BMI (18.5-24.9 kg/m2) after previously having obesity had current prevalence rates of hypertension and dyslipidemia that were, respectively, 8% and 13% higher than the prevalence rates among adults who consistently maintained a healthy BMI – differences that were not significant.
In contrast, people who had current BMIs that indicated obesity had prevalence rates of hypertension and dyslipidemia that were each a significant threefold higher than those with a healthy BMI.
The 326 respondents who formerly had obesity but now were at a healthy BMI had a threefold higher prevalence of diabetes than did the 6,235 who consistently had maintained a healthy BMI. This was substantially less than the over sevenfold higher prevalence of diabetes among those who currently had obesity compared with those who always had a healthy BMI.
All these analyses were adjusted for the potential confounders of age, sex, smoking history, and ethnicity.
‘Quitting’ obesity better than current obesity
The finding that reaching a healthy BMI after a period of obesity could reverse some but not all risks associated with obesity is reminiscent of the effects of smoking, noted Dr. Smith.
“Never is better than ever, but quitting,” or dropping weight to reach a healthy BMI, “is better than current,” she concluded.
But Dr. Emeny said this interpretation, “while motivating and catchy, places emphasis on individual responsibility and choice rather than on social circumstances.”
Social effects “must be considered when evaluating population-level disparities in obesity-related cardiometabolic risk,” cautioned Dr. Emeny.
“’Quitting’ obesity is much more complicated than individual choice or ability.”
Dr. Smith also conceded that her analyses did not correct for the possible confounding effects that changes in diet or physical activity may have had on the observations.
“Neither diet nor physical activity has a well-known summary measure that we could have included as an adjuster,” she explained.
Dr. Smith and Dr. Emeny have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
from easd 2021
Sleep apnea has many faces
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
5 years out, sleeve safer than gastric bypass
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
Five years out, sleeve gastrectomy had a lower risk of mortality, complications, and reinterventions than gastric bypass, but there was a higher risk of surgical revision, including conversion to another bariatric surgery, gastrectomy, or anastomotic revision, according to a new analysis.
Sleeve gastrectomy has gained rapid popularity, and now represents 60% of all bariatric procedures. It has demonstrated good efficacy and short-term safety, it is easier to perform than laparoscopic Roux-en-Y gastric bypass, and it is a safe option for high-risk patients, authors led by Ryan Howard, MD, of University of Michigan, Ann Arbor, wrote in JAMA Surgery.
Still, there are few comparative data on the long-term efficacy of the two procedures. Randomized, controlled trials have conducted long-term follow-up, but their small size has made it difficult to detect differences in rare outcomes. Observational studies are limited by the potential for bias. A novel approach to limiting bias is instrumental variables analysis, which controls for possible confounding using a factor that impacts treatment choice, but not patient outcome, to control for possible confounders. Studies using this approach confirmed the superior safety profile of sleeve gastrectomy in the short term.
The current study’s authors, used that method to examined 5-year outcomes in a Medicare population, in which obesity and its complications are especially frequent. Partly because of that lack of data, the Medical Evidence Development and Coverage Committee has called for more data in older patients and in patients with disabilities.
The researchers analyzed data from 95,405 Medicare claims between 2012 and 2018, using state-level variation in sleeve gastrectomy as the instrumental variable.
At 5 years, sleeve gastrectomy was associated with a lower cumulative frequency of mortality (4.27%; 95% confidence interval, 4.25%-4.30% vs. 5.67%; 95% CI, 5.63%-5.69%]), complications (22.10%; 95% CI, 22.06%-22.13% vs. 29.03%; 95% CI, 28.99%-29.08%), and reintervention (25.23%; 95% CI, 25.19%-25.27% vs. 33.57%; 95% CI, 33.52%-33.63%). At 5 years, surgical revision was more common in the sleeve gastrectomy group (2.91%; 95% CI, 2.90%-2.93% vs. 1.46%; 95% CI, 1.45%-1.47%).
The sleeve gastrectomy group had lower odds of all-cause hospitalization at 1 year (adjusted hazard ratio, 0.83; 95% CI, 0.80-0.86) and 3 years (aHR, 0.94; 95% CI, 0.90-0.98), as well as emergency department use at 1 year (aHR, 0.87; 95% CI, 0.84-0.90) and 3 years (aHR, 0.93; 95% CI, 0.90-0.97). There was no significant difference between the two groups at 5 years with respect to either outcome.
The effort to understand long-term outcomes of these two procedures has been challenging because follow-up is often incomplete, and because reporting isn’t always standardized, according Anita P. Courcoulas, MD, MPH, and Bestoun Ahmed, MD, in an accompanying editorial in JAMA Surgery. They noted that the differences in mortality is a new finding and the difference in surgical revisions confirmed something often seen in clinical practice. “Overall, these novel methods, which creatively balance unmeasured factors, have succeeded in providing important incremental findings about the long-term comparative safety outcomes between bariatric procedures that will be helpful in clinical practice,” the editorial authors wrote.
The complications discussed in the study are also difficult to interpret, according to Ali Aminian, MD, who is a professor of surgery and director of Bariatric and Metabolic Institute at Cleveland Clinic. They may be related to the surgery, or they may be complications that accrue as patients age. “So that doesn’t mean those were surgical complications, but [the findings are] in line with the other literature that [gastric sleeve] may be safer than gastric bypass, but in a different cohort of patients,” said Dr. Aminian, who was asked to comment.
“I thought it validated that which many of us in clinical practice see on a day to day basis,” said Shanu Kothari, MD, chair of surgery at Prisma Health, and the current president of American Society for Bariatric and Metabolic Surgery. He pointed out that the study was limited by its reliance on administrative claims, which makes it impossible to know the reduction in weight and obesity-related comorbid conditions following the procedures, as well as factors driving individual decisions: A surgeon might offer sleeve to a patient at higher risk of complications, but a gastric bypass to someone with more comorbidities. “What we don’t know is how to interpret this 35,000-foot view of Medicare data to that conversation with the patient sitting right in front of you,” said Dr. Kothari.
The authors similarly cited the “lack of clinical granularity in administrative claims data” among study limitations, as well as how the use of instrumental variables may leave the findings less applicable to patients more strongly indicated for one procedure over the other.
“Longer-term randomized clinical trials and observational studies are warranted to confirm these findings,” the study authors concluded. “Understanding the risk profile of various bariatric operations may further help patients and surgeons make the most appropriate decisions regarding plans of care.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Some study authors and editorialists reported funding from various groups and institutions, such as the National Institutes of Health and the VA Ann Arbor Health System. Dr. Kothari and Dr. Aminian have no relevant financial disclosures.
FROM JAMA SURGERY
What I will and won’t miss
Someday I plan to retire.
Hopefully it’s not coming up anytime soon, but I’m sure it’s sooner than I realize. I’ve been in practice for 23 years, so I’m well past the halfway point of an average medical career.
I will miss a lot. There will be many things I won’t miss, but the job has far more good than bad, even today.
I’ve spent a lot of time at this huge desk, which my dad bought for his solo law practice in 1967. Although I won’t miss the lack of sleep, I will miss the silence of getting to the office before first light, making tea, and getting started for the day. It’s a peaceful daily start in a less-then-predictable job.
I’ll miss my patients. Not all of them, but most. The majority are decent people, and it’s an honor to be able to help them. Doing that has been the driving force that started me on this path a long time ago and still keeps me moving forward.
In some respects I’ll feel bad about retiring and leaving them. Some have been with me since residency. It will bother me that they’ll have to start over with a new neurologist. Hopefully that person will give them care as good, if not better, than I have.
I’ll really miss my staff. I’ve been lucky. They’re awesome, and have stayed with me for this crazy ride. My MA has been here since 1999, my secretary since 2004. At work they’re my family. Away from work they’re a part of my family. The three of us have survived my hospital call, good economic times, bad economic times, moving the office, my MA moving to the boondocks, the antics and events of our kids, and, as of now, a pandemic. They make the day fun. I’ll feel bad that they’ll need to change jobs if they’re still working then.
What I won’t miss are more concrete things – the endless forms, time spent on the phone and online to get medications and tests approved, the difficult (personality wise) patients who think being nasty and mean is going to get them better care, and having to practice CYA defensive medicine.
It’s good to look back after 23 years, and still have, overall, no regrets about choosing this ride.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Someday I plan to retire.
Hopefully it’s not coming up anytime soon, but I’m sure it’s sooner than I realize. I’ve been in practice for 23 years, so I’m well past the halfway point of an average medical career.
I will miss a lot. There will be many things I won’t miss, but the job has far more good than bad, even today.
I’ve spent a lot of time at this huge desk, which my dad bought for his solo law practice in 1967. Although I won’t miss the lack of sleep, I will miss the silence of getting to the office before first light, making tea, and getting started for the day. It’s a peaceful daily start in a less-then-predictable job.
I’ll miss my patients. Not all of them, but most. The majority are decent people, and it’s an honor to be able to help them. Doing that has been the driving force that started me on this path a long time ago and still keeps me moving forward.
In some respects I’ll feel bad about retiring and leaving them. Some have been with me since residency. It will bother me that they’ll have to start over with a new neurologist. Hopefully that person will give them care as good, if not better, than I have.
I’ll really miss my staff. I’ve been lucky. They’re awesome, and have stayed with me for this crazy ride. My MA has been here since 1999, my secretary since 2004. At work they’re my family. Away from work they’re a part of my family. The three of us have survived my hospital call, good economic times, bad economic times, moving the office, my MA moving to the boondocks, the antics and events of our kids, and, as of now, a pandemic. They make the day fun. I’ll feel bad that they’ll need to change jobs if they’re still working then.
What I won’t miss are more concrete things – the endless forms, time spent on the phone and online to get medications and tests approved, the difficult (personality wise) patients who think being nasty and mean is going to get them better care, and having to practice CYA defensive medicine.
It’s good to look back after 23 years, and still have, overall, no regrets about choosing this ride.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Someday I plan to retire.
Hopefully it’s not coming up anytime soon, but I’m sure it’s sooner than I realize. I’ve been in practice for 23 years, so I’m well past the halfway point of an average medical career.
I will miss a lot. There will be many things I won’t miss, but the job has far more good than bad, even today.
I’ve spent a lot of time at this huge desk, which my dad bought for his solo law practice in 1967. Although I won’t miss the lack of sleep, I will miss the silence of getting to the office before first light, making tea, and getting started for the day. It’s a peaceful daily start in a less-then-predictable job.
I’ll miss my patients. Not all of them, but most. The majority are decent people, and it’s an honor to be able to help them. Doing that has been the driving force that started me on this path a long time ago and still keeps me moving forward.
In some respects I’ll feel bad about retiring and leaving them. Some have been with me since residency. It will bother me that they’ll have to start over with a new neurologist. Hopefully that person will give them care as good, if not better, than I have.
I’ll really miss my staff. I’ve been lucky. They’re awesome, and have stayed with me for this crazy ride. My MA has been here since 1999, my secretary since 2004. At work they’re my family. Away from work they’re a part of my family. The three of us have survived my hospital call, good economic times, bad economic times, moving the office, my MA moving to the boondocks, the antics and events of our kids, and, as of now, a pandemic. They make the day fun. I’ll feel bad that they’ll need to change jobs if they’re still working then.
What I won’t miss are more concrete things – the endless forms, time spent on the phone and online to get medications and tests approved, the difficult (personality wise) patients who think being nasty and mean is going to get them better care, and having to practice CYA defensive medicine.
It’s good to look back after 23 years, and still have, overall, no regrets about choosing this ride.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
Tramadol linked to higher risk of mortality, compared with codeine
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Few poorly prepped colonoscopies repeated within 1 year
Approximately one-third of colonoscopies with inadequate bowel preparation were repeated within 1 year despite current guidelines, according to data from a new study of more than 260,000 procedures.
Previous studies have shown that poor bowel prep, which occurs in approximately 25% of colonoscopies, can lead to lesion miss rates of as much as 42%-48%, Audrey H. Calderwood, MD, an associate professor of medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues wrote. However, factors affecting recommendations for repeat colonoscopies following poor bowel prep have not been examined.
In the study, published in Gastrointestinal Endoscopy, the researchers conducted a cross-sectional retrospective analysis of 260,314 colonoscopies reported to the GI Quality Improvement Consortium (GIQuIC) from 2011 to 2018. The review included adults aged 50-75 years in whom bowel preparation was deemed inadequate. The GIQuIC database defines adequate bowel preparation as “sufficient to accurately detect polyps greater than 5 mm in size,” the researchers noted. The procedures in this study were performed at 672 sites by 4,001 endoscopists, and the primary outcome was a recommendation for a repeat colonoscopy within 1 year.
In 31.9% of the procedures, the recommended follow-up interval for repeat colonoscopy was within 1 year, and there were no significant differences according to indication for the procedures (32.3% of screening and 31.2% of surveillance). Of these, 54.9% of patients received a follow-up interval of 1 year and 24.7% a follow-up interval within 3 months. Only 2.4% were advised they required no follow-up procedure.
The researchers found that patients with more severe disease had a higher likelihood of receiving a recommendation for follow-up colonoscopy within 1 year – 84% with adenocarcinoma, 51.8% with advanced lesions, and 23.2% with one to two small adenomas.
In the multivariate analysis, there were specific patient factors significantly associated with 1-year follow-up recommendations. The researchers found patients aged 70-75 years were less likely than younger patients (adjusted odds ratio, 0.96; 95% confidence interval, 0.93-0.98) to receive a 1-year follow-up recommendation; men were more likely than women (aOR, 1.04; 95% CI, 1.02-1.06) to receive a 1-year follow-up recommendation; and patients with adenocarcinoma findings more likely to receive a 1-year follow-up recommendation compared to those with no adenocarcinoma (aOR, 10.43; 95% CI, 7.77-13.98). In addition, they found patients residing in the Northeast and those whose procedure was performed by an endoscopist with an adenoma detection rate of at least25% were more likely to receive recommendations for a repeat colonoscopy within 1 year.
“The recommendation for repeat screening or surveillance colonoscopy within 1 year when the index colonoscopy has an inadequate bowel preparation is currently a quality measure in gastroenterology,” the researchers noted. “Although our study period started in 2011, when we looked at the time period of 2014 to 2018, which is after publication of guidelines of when to repeat colonoscopy after inadequate bowel preparation, there were still low rates of guideline-concordant recommendations.”
These overall low rates, which are consistent with other studies, may be due uncertainty on the part of the endoscopist in determining inadequate bowel prep based on evolving guidelines, the researchers noted. However, the higher frequency of recommendations for repeat procedures within 1 year for patients with advanced disease suggests that endoscopists are taking pathology into account.
The study findings were limited by several factors, including the lack of standardized assessment of bowel prep quality, variation in descriptions of bowel cleanliness, and lack of information on the primary factor in follow-up recommendations. However, the results were strengthened by the large sample size, the inclusion of multiple sites and providers, and the low volume of timely repeat procedures, which has clinical implications in terms of missed lesions, “including potential interval CRC [colorectal cancer],” the researchers said.
Get the word out on describing preps and planning follow-ups
The current study is important because it highlights that, even when endoscopists have a reasonable understanding on how to set follow-up intervals for polyp follow-up, what to do with a patient who comes in poorly prepped is more of a problem, Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
Dr Isaacs said she was not surprised by the study findings. “There are all gradations of inadequate preps that limit visualization in different ways, and there are many ways of recording this on procedure reports. The findings in the current study emphasize several points. The first is that the recommendation of following up an inadequate or poor prep in a year needs to be widely disseminated. The second is that there needs to be more education on standardization on how preps are described. In some poor preps, much of the colon can be seen, and clinicians can identify polyps 5-6 mm, so a 1-year follow-up may not be needed.” This type of research is challenging if the data are not standardized, she added.
Dr. Isaacs agreed with the authors’ description of repeat colonoscopies after poor bowel prep as a quality improvement area given the variability in following current recommendations, which leads into next steps for research.
“Understanding reasons for the recommendations that endoscopists made for follow-up would be the next step in this type of research,” Dr Isaacs noted. “After that, studies on the impact of an educational intervention, followed by repeating the initial assessment.”
The study received no outside funding. The researchers had no financial conflicts to disclose; however, lead author Dr. Calderwood disclosed support from the National Cancer Institute, the Dartmouth-Hitchcock Cancer Research Fellows Program, the Dartmouth-Hitchcock Norris Cotton Cancer Center, and the Dartmouth Clinical and Translational Science Institute. Dr Isaacs had no financial conflicts to disclose but has previously served on the editorial board of GI & Hepatology News.
Approximately one-third of colonoscopies with inadequate bowel preparation were repeated within 1 year despite current guidelines, according to data from a new study of more than 260,000 procedures.
Previous studies have shown that poor bowel prep, which occurs in approximately 25% of colonoscopies, can lead to lesion miss rates of as much as 42%-48%, Audrey H. Calderwood, MD, an associate professor of medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues wrote. However, factors affecting recommendations for repeat colonoscopies following poor bowel prep have not been examined.
In the study, published in Gastrointestinal Endoscopy, the researchers conducted a cross-sectional retrospective analysis of 260,314 colonoscopies reported to the GI Quality Improvement Consortium (GIQuIC) from 2011 to 2018. The review included adults aged 50-75 years in whom bowel preparation was deemed inadequate. The GIQuIC database defines adequate bowel preparation as “sufficient to accurately detect polyps greater than 5 mm in size,” the researchers noted. The procedures in this study were performed at 672 sites by 4,001 endoscopists, and the primary outcome was a recommendation for a repeat colonoscopy within 1 year.
In 31.9% of the procedures, the recommended follow-up interval for repeat colonoscopy was within 1 year, and there were no significant differences according to indication for the procedures (32.3% of screening and 31.2% of surveillance). Of these, 54.9% of patients received a follow-up interval of 1 year and 24.7% a follow-up interval within 3 months. Only 2.4% were advised they required no follow-up procedure.
The researchers found that patients with more severe disease had a higher likelihood of receiving a recommendation for follow-up colonoscopy within 1 year – 84% with adenocarcinoma, 51.8% with advanced lesions, and 23.2% with one to two small adenomas.
In the multivariate analysis, there were specific patient factors significantly associated with 1-year follow-up recommendations. The researchers found patients aged 70-75 years were less likely than younger patients (adjusted odds ratio, 0.96; 95% confidence interval, 0.93-0.98) to receive a 1-year follow-up recommendation; men were more likely than women (aOR, 1.04; 95% CI, 1.02-1.06) to receive a 1-year follow-up recommendation; and patients with adenocarcinoma findings more likely to receive a 1-year follow-up recommendation compared to those with no adenocarcinoma (aOR, 10.43; 95% CI, 7.77-13.98). In addition, they found patients residing in the Northeast and those whose procedure was performed by an endoscopist with an adenoma detection rate of at least25% were more likely to receive recommendations for a repeat colonoscopy within 1 year.
“The recommendation for repeat screening or surveillance colonoscopy within 1 year when the index colonoscopy has an inadequate bowel preparation is currently a quality measure in gastroenterology,” the researchers noted. “Although our study period started in 2011, when we looked at the time period of 2014 to 2018, which is after publication of guidelines of when to repeat colonoscopy after inadequate bowel preparation, there were still low rates of guideline-concordant recommendations.”
These overall low rates, which are consistent with other studies, may be due uncertainty on the part of the endoscopist in determining inadequate bowel prep based on evolving guidelines, the researchers noted. However, the higher frequency of recommendations for repeat procedures within 1 year for patients with advanced disease suggests that endoscopists are taking pathology into account.
The study findings were limited by several factors, including the lack of standardized assessment of bowel prep quality, variation in descriptions of bowel cleanliness, and lack of information on the primary factor in follow-up recommendations. However, the results were strengthened by the large sample size, the inclusion of multiple sites and providers, and the low volume of timely repeat procedures, which has clinical implications in terms of missed lesions, “including potential interval CRC [colorectal cancer],” the researchers said.
Get the word out on describing preps and planning follow-ups
The current study is important because it highlights that, even when endoscopists have a reasonable understanding on how to set follow-up intervals for polyp follow-up, what to do with a patient who comes in poorly prepped is more of a problem, Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
Dr Isaacs said she was not surprised by the study findings. “There are all gradations of inadequate preps that limit visualization in different ways, and there are many ways of recording this on procedure reports. The findings in the current study emphasize several points. The first is that the recommendation of following up an inadequate or poor prep in a year needs to be widely disseminated. The second is that there needs to be more education on standardization on how preps are described. In some poor preps, much of the colon can be seen, and clinicians can identify polyps 5-6 mm, so a 1-year follow-up may not be needed.” This type of research is challenging if the data are not standardized, she added.
Dr. Isaacs agreed with the authors’ description of repeat colonoscopies after poor bowel prep as a quality improvement area given the variability in following current recommendations, which leads into next steps for research.
“Understanding reasons for the recommendations that endoscopists made for follow-up would be the next step in this type of research,” Dr Isaacs noted. “After that, studies on the impact of an educational intervention, followed by repeating the initial assessment.”
The study received no outside funding. The researchers had no financial conflicts to disclose; however, lead author Dr. Calderwood disclosed support from the National Cancer Institute, the Dartmouth-Hitchcock Cancer Research Fellows Program, the Dartmouth-Hitchcock Norris Cotton Cancer Center, and the Dartmouth Clinical and Translational Science Institute. Dr Isaacs had no financial conflicts to disclose but has previously served on the editorial board of GI & Hepatology News.
Approximately one-third of colonoscopies with inadequate bowel preparation were repeated within 1 year despite current guidelines, according to data from a new study of more than 260,000 procedures.
Previous studies have shown that poor bowel prep, which occurs in approximately 25% of colonoscopies, can lead to lesion miss rates of as much as 42%-48%, Audrey H. Calderwood, MD, an associate professor of medicine at Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and colleagues wrote. However, factors affecting recommendations for repeat colonoscopies following poor bowel prep have not been examined.
In the study, published in Gastrointestinal Endoscopy, the researchers conducted a cross-sectional retrospective analysis of 260,314 colonoscopies reported to the GI Quality Improvement Consortium (GIQuIC) from 2011 to 2018. The review included adults aged 50-75 years in whom bowel preparation was deemed inadequate. The GIQuIC database defines adequate bowel preparation as “sufficient to accurately detect polyps greater than 5 mm in size,” the researchers noted. The procedures in this study were performed at 672 sites by 4,001 endoscopists, and the primary outcome was a recommendation for a repeat colonoscopy within 1 year.
In 31.9% of the procedures, the recommended follow-up interval for repeat colonoscopy was within 1 year, and there were no significant differences according to indication for the procedures (32.3% of screening and 31.2% of surveillance). Of these, 54.9% of patients received a follow-up interval of 1 year and 24.7% a follow-up interval within 3 months. Only 2.4% were advised they required no follow-up procedure.
The researchers found that patients with more severe disease had a higher likelihood of receiving a recommendation for follow-up colonoscopy within 1 year – 84% with adenocarcinoma, 51.8% with advanced lesions, and 23.2% with one to two small adenomas.
In the multivariate analysis, there were specific patient factors significantly associated with 1-year follow-up recommendations. The researchers found patients aged 70-75 years were less likely than younger patients (adjusted odds ratio, 0.96; 95% confidence interval, 0.93-0.98) to receive a 1-year follow-up recommendation; men were more likely than women (aOR, 1.04; 95% CI, 1.02-1.06) to receive a 1-year follow-up recommendation; and patients with adenocarcinoma findings more likely to receive a 1-year follow-up recommendation compared to those with no adenocarcinoma (aOR, 10.43; 95% CI, 7.77-13.98). In addition, they found patients residing in the Northeast and those whose procedure was performed by an endoscopist with an adenoma detection rate of at least25% were more likely to receive recommendations for a repeat colonoscopy within 1 year.
“The recommendation for repeat screening or surveillance colonoscopy within 1 year when the index colonoscopy has an inadequate bowel preparation is currently a quality measure in gastroenterology,” the researchers noted. “Although our study period started in 2011, when we looked at the time period of 2014 to 2018, which is after publication of guidelines of when to repeat colonoscopy after inadequate bowel preparation, there were still low rates of guideline-concordant recommendations.”
These overall low rates, which are consistent with other studies, may be due uncertainty on the part of the endoscopist in determining inadequate bowel prep based on evolving guidelines, the researchers noted. However, the higher frequency of recommendations for repeat procedures within 1 year for patients with advanced disease suggests that endoscopists are taking pathology into account.
The study findings were limited by several factors, including the lack of standardized assessment of bowel prep quality, variation in descriptions of bowel cleanliness, and lack of information on the primary factor in follow-up recommendations. However, the results were strengthened by the large sample size, the inclusion of multiple sites and providers, and the low volume of timely repeat procedures, which has clinical implications in terms of missed lesions, “including potential interval CRC [colorectal cancer],” the researchers said.
Get the word out on describing preps and planning follow-ups
The current study is important because it highlights that, even when endoscopists have a reasonable understanding on how to set follow-up intervals for polyp follow-up, what to do with a patient who comes in poorly prepped is more of a problem, Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
Dr Isaacs said she was not surprised by the study findings. “There are all gradations of inadequate preps that limit visualization in different ways, and there are many ways of recording this on procedure reports. The findings in the current study emphasize several points. The first is that the recommendation of following up an inadequate or poor prep in a year needs to be widely disseminated. The second is that there needs to be more education on standardization on how preps are described. In some poor preps, much of the colon can be seen, and clinicians can identify polyps 5-6 mm, so a 1-year follow-up may not be needed.” This type of research is challenging if the data are not standardized, she added.
Dr. Isaacs agreed with the authors’ description of repeat colonoscopies after poor bowel prep as a quality improvement area given the variability in following current recommendations, which leads into next steps for research.
“Understanding reasons for the recommendations that endoscopists made for follow-up would be the next step in this type of research,” Dr Isaacs noted. “After that, studies on the impact of an educational intervention, followed by repeating the initial assessment.”
The study received no outside funding. The researchers had no financial conflicts to disclose; however, lead author Dr. Calderwood disclosed support from the National Cancer Institute, the Dartmouth-Hitchcock Cancer Research Fellows Program, the Dartmouth-Hitchcock Norris Cotton Cancer Center, and the Dartmouth Clinical and Translational Science Institute. Dr Isaacs had no financial conflicts to disclose but has previously served on the editorial board of GI & Hepatology News.
FROM GASTROINTESTINAL ENDOSCOPY
Aspirin lowered preeclampsia risk in real-world lupus study
Women with systemic lupus erythematous (SLE) who are at risk for preeclampsia may benefit from timely treatment with low-dose aspirin and perhaps hydroxychloroquine, according to German researchers.
In a prospective, real-world study of 190 pregnancies in 148 women (average age, 30 years), the use of low-dose aspirin starting around the 16th week of gestation was associated with a lower risk for preeclampsia than was no aspirin use (adjusted odds ratio [aOR], 0.21; P < .05).
The use of hydroxychloroquine starting in the first trimester had a “moderating effect,” said Isabell Haase, MD, a senior clinician scientist in the department of rheumatology at Hiller-Research Unit, Düsseldorf, Germany. Although this was not a statistically significant effect (aOR, 0.47; P = .21), the association strengthened if only high-risk pregnancies were considered (aOR, 0.28; P = .075).
“I think this once more shows us that counseling and risk assessment in our lupus patients is very important to find out those with the highest risk and treat them as good as possible,” Dr. Haase said at an international congress on systemic lupus erythematosus.
Preeclampsia and lupus
“Women with SLE face a high risk of preeclampsia because of their autoimmune disease,” Dr. Haase explained. “This [risk] can be further increased if a woman carries additional risk factors, like hypertension or lupus nephritis.”
Low-dose aspirin is known to protect against the development of preeclampsia in women without autoimmune disease if started before the 16th gestational week of pregnancy, Dr. Haase added. That is why it’s recommended by both the American College of Rheumatology and the European Alliance of Associations for Rheumatology.
“For hydroxychloroquine, we only have some small studies and its mechanism of action that lead us to the idea that it could also have a beneficial effect on preeclampsia in lupus patients,” she said.
Study design and results
The aim of the study was to see in a real-world cohort whether there was any beneficial effect of either aspirin or hydroxychloroquine regarding the development of preeclampsia.
The researchers used prospectively collected data from pregnancies seen at an outpatient pregnancy clinic during 1995-2019. They used multiple logistic regression to determine whether there was any effect of four treatments on the development of preeclampsia: aspirin (n = 39 patients) or hydroxychloroquine (n = 39) alone, in combination (n = 43), or neither drug (n = 69).
Overall, 56% of the women had significant risk factors for preeclampsia, including a prior history, multifetal gestation, chronic hypertension, lupus nephritis, or antiphospholipid antibodies (aPL). A further 28% had moderate risk factors, including not having had children, a body mass index >30 kg/m2, and being older than 35 years.
The overall rate of preeclampsia in the study population was 13.2%, “which is in line with other studies in lupus pregnancies,” Dr. Haase said. Rates in each of the four treatment groups were 15.4% with aspirin alone, 7.7% with hydroxychloroquine alone, 14% with both drugs, and 14.5% with neither.
The odds of developing preeclampsia were lower with both aspirin and hydroxychloroquine. Factors that raised the odds were high disease activity in the first trimester (aOR, 4.55), a BMI of >30 kg/m2 (aOR, 6.14), having high-risk aPL or antiphospholipid syndrome (aOR, 8.02), and a history of preeclampsia (aOR, 9.78).
Only high disease activity in the first trimester and BMI >30 kg/m2 remained independent predictors of preeclampsia when the researchers considered only high-risk pregnancies (aOR, 7.74 for high disease activity in first trimester and 10.04 for a high BMI).
The results are “really impressive,” said Angela Tincani, MD, senior consultant at the Rheumatology and Clinical Immunology Unit of ASST–Spedali Civili di Brescia, in Italy.
Dr. Tincani observed that the study had covered a “large number of years” (1995-2020).
“I think that our attitude in looking after lupus patients [changed] during this time,” she said.
“As an example, I think that we probably use less corticosteroids now than in the 90s,” she said.
When asked whether changes in practices have influenced the findings, she acknowledged, “You can see that the prescription of the different medications has changed a lot. We also thought that we have to take into account the years as a confounder, but we haven’t statistically analyzed that, but it’s definitely something that we are going to do next.”
The study received no outside funding. Dr. Haase has received travel fees from AbbVie, Celgene, Chugai, Janssen-Cilag, Eli Lilly, and Medac. Dr. Tincani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with systemic lupus erythematous (SLE) who are at risk for preeclampsia may benefit from timely treatment with low-dose aspirin and perhaps hydroxychloroquine, according to German researchers.
In a prospective, real-world study of 190 pregnancies in 148 women (average age, 30 years), the use of low-dose aspirin starting around the 16th week of gestation was associated with a lower risk for preeclampsia than was no aspirin use (adjusted odds ratio [aOR], 0.21; P < .05).
The use of hydroxychloroquine starting in the first trimester had a “moderating effect,” said Isabell Haase, MD, a senior clinician scientist in the department of rheumatology at Hiller-Research Unit, Düsseldorf, Germany. Although this was not a statistically significant effect (aOR, 0.47; P = .21), the association strengthened if only high-risk pregnancies were considered (aOR, 0.28; P = .075).
“I think this once more shows us that counseling and risk assessment in our lupus patients is very important to find out those with the highest risk and treat them as good as possible,” Dr. Haase said at an international congress on systemic lupus erythematosus.
Preeclampsia and lupus
“Women with SLE face a high risk of preeclampsia because of their autoimmune disease,” Dr. Haase explained. “This [risk] can be further increased if a woman carries additional risk factors, like hypertension or lupus nephritis.”
Low-dose aspirin is known to protect against the development of preeclampsia in women without autoimmune disease if started before the 16th gestational week of pregnancy, Dr. Haase added. That is why it’s recommended by both the American College of Rheumatology and the European Alliance of Associations for Rheumatology.
“For hydroxychloroquine, we only have some small studies and its mechanism of action that lead us to the idea that it could also have a beneficial effect on preeclampsia in lupus patients,” she said.
Study design and results
The aim of the study was to see in a real-world cohort whether there was any beneficial effect of either aspirin or hydroxychloroquine regarding the development of preeclampsia.
The researchers used prospectively collected data from pregnancies seen at an outpatient pregnancy clinic during 1995-2019. They used multiple logistic regression to determine whether there was any effect of four treatments on the development of preeclampsia: aspirin (n = 39 patients) or hydroxychloroquine (n = 39) alone, in combination (n = 43), or neither drug (n = 69).
Overall, 56% of the women had significant risk factors for preeclampsia, including a prior history, multifetal gestation, chronic hypertension, lupus nephritis, or antiphospholipid antibodies (aPL). A further 28% had moderate risk factors, including not having had children, a body mass index >30 kg/m2, and being older than 35 years.
The overall rate of preeclampsia in the study population was 13.2%, “which is in line with other studies in lupus pregnancies,” Dr. Haase said. Rates in each of the four treatment groups were 15.4% with aspirin alone, 7.7% with hydroxychloroquine alone, 14% with both drugs, and 14.5% with neither.
The odds of developing preeclampsia were lower with both aspirin and hydroxychloroquine. Factors that raised the odds were high disease activity in the first trimester (aOR, 4.55), a BMI of >30 kg/m2 (aOR, 6.14), having high-risk aPL or antiphospholipid syndrome (aOR, 8.02), and a history of preeclampsia (aOR, 9.78).
Only high disease activity in the first trimester and BMI >30 kg/m2 remained independent predictors of preeclampsia when the researchers considered only high-risk pregnancies (aOR, 7.74 for high disease activity in first trimester and 10.04 for a high BMI).
The results are “really impressive,” said Angela Tincani, MD, senior consultant at the Rheumatology and Clinical Immunology Unit of ASST–Spedali Civili di Brescia, in Italy.
Dr. Tincani observed that the study had covered a “large number of years” (1995-2020).
“I think that our attitude in looking after lupus patients [changed] during this time,” she said.
“As an example, I think that we probably use less corticosteroids now than in the 90s,” she said.
When asked whether changes in practices have influenced the findings, she acknowledged, “You can see that the prescription of the different medications has changed a lot. We also thought that we have to take into account the years as a confounder, but we haven’t statistically analyzed that, but it’s definitely something that we are going to do next.”
The study received no outside funding. Dr. Haase has received travel fees from AbbVie, Celgene, Chugai, Janssen-Cilag, Eli Lilly, and Medac. Dr. Tincani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with systemic lupus erythematous (SLE) who are at risk for preeclampsia may benefit from timely treatment with low-dose aspirin and perhaps hydroxychloroquine, according to German researchers.
In a prospective, real-world study of 190 pregnancies in 148 women (average age, 30 years), the use of low-dose aspirin starting around the 16th week of gestation was associated with a lower risk for preeclampsia than was no aspirin use (adjusted odds ratio [aOR], 0.21; P < .05).
The use of hydroxychloroquine starting in the first trimester had a “moderating effect,” said Isabell Haase, MD, a senior clinician scientist in the department of rheumatology at Hiller-Research Unit, Düsseldorf, Germany. Although this was not a statistically significant effect (aOR, 0.47; P = .21), the association strengthened if only high-risk pregnancies were considered (aOR, 0.28; P = .075).
“I think this once more shows us that counseling and risk assessment in our lupus patients is very important to find out those with the highest risk and treat them as good as possible,” Dr. Haase said at an international congress on systemic lupus erythematosus.
Preeclampsia and lupus
“Women with SLE face a high risk of preeclampsia because of their autoimmune disease,” Dr. Haase explained. “This [risk] can be further increased if a woman carries additional risk factors, like hypertension or lupus nephritis.”
Low-dose aspirin is known to protect against the development of preeclampsia in women without autoimmune disease if started before the 16th gestational week of pregnancy, Dr. Haase added. That is why it’s recommended by both the American College of Rheumatology and the European Alliance of Associations for Rheumatology.
“For hydroxychloroquine, we only have some small studies and its mechanism of action that lead us to the idea that it could also have a beneficial effect on preeclampsia in lupus patients,” she said.
Study design and results
The aim of the study was to see in a real-world cohort whether there was any beneficial effect of either aspirin or hydroxychloroquine regarding the development of preeclampsia.
The researchers used prospectively collected data from pregnancies seen at an outpatient pregnancy clinic during 1995-2019. They used multiple logistic regression to determine whether there was any effect of four treatments on the development of preeclampsia: aspirin (n = 39 patients) or hydroxychloroquine (n = 39) alone, in combination (n = 43), or neither drug (n = 69).
Overall, 56% of the women had significant risk factors for preeclampsia, including a prior history, multifetal gestation, chronic hypertension, lupus nephritis, or antiphospholipid antibodies (aPL). A further 28% had moderate risk factors, including not having had children, a body mass index >30 kg/m2, and being older than 35 years.
The overall rate of preeclampsia in the study population was 13.2%, “which is in line with other studies in lupus pregnancies,” Dr. Haase said. Rates in each of the four treatment groups were 15.4% with aspirin alone, 7.7% with hydroxychloroquine alone, 14% with both drugs, and 14.5% with neither.
The odds of developing preeclampsia were lower with both aspirin and hydroxychloroquine. Factors that raised the odds were high disease activity in the first trimester (aOR, 4.55), a BMI of >30 kg/m2 (aOR, 6.14), having high-risk aPL or antiphospholipid syndrome (aOR, 8.02), and a history of preeclampsia (aOR, 9.78).
Only high disease activity in the first trimester and BMI >30 kg/m2 remained independent predictors of preeclampsia when the researchers considered only high-risk pregnancies (aOR, 7.74 for high disease activity in first trimester and 10.04 for a high BMI).
The results are “really impressive,” said Angela Tincani, MD, senior consultant at the Rheumatology and Clinical Immunology Unit of ASST–Spedali Civili di Brescia, in Italy.
Dr. Tincani observed that the study had covered a “large number of years” (1995-2020).
“I think that our attitude in looking after lupus patients [changed] during this time,” she said.
“As an example, I think that we probably use less corticosteroids now than in the 90s,” she said.
When asked whether changes in practices have influenced the findings, she acknowledged, “You can see that the prescription of the different medications has changed a lot. We also thought that we have to take into account the years as a confounder, but we haven’t statistically analyzed that, but it’s definitely something that we are going to do next.”
The study received no outside funding. Dr. Haase has received travel fees from AbbVie, Celgene, Chugai, Janssen-Cilag, Eli Lilly, and Medac. Dr. Tincani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM LUPUS 2021
Why toilet paper is the unofficial symbol of anxiety during COVID
How did toilet paper become the unofficial symbol of anxiety during the pandemic? Empty store shelves are a stark reminder of how COVID-19 has taken a toll on people.
At the beginning of the pandemic, stay-at-home orders drove people to buy large amounts of household goods, especially toilet paper. Demand grew to unforeseen heights in March 2020, with $1.45 billion in toilet paper sales in the 4-week period ending March 29, up 112% from the year before, according to IRI, a Chicago-based market research firm.
As the Delta variant drove a COVID-19 resurgence this summer, market research suggests that almost one in two Americans started stockpiling toilet paper again over fears that supply would run out. The higher demand causes ripples through the retail chain, and a growing number of stores are again facing challenges in stocking toilet paper.
Yet there is plenty for everyone if people don’t stockpile too much, according to paper industry market analyst Ronalds Gonzalez, PhD, an associate professor of conversion economics and sustainability at North Carolina State University, Raleigh.
“As long as people buy what they actually need and don’t get into a panic, there won’t be any issue with the supply of hygienic tissue,” he says, adding that “too much” would equate to stockpiling 6-8 months’ worth of toilet paper, as some people did early in the pandemic.
But retailers are worried that history will repeat itself. In late September 2021, warehouse retail giant Costco told Wall Street analysts that it decided to limit customer purchases of essential items like toilet paper and water. Another retailer, Sam’s Club, began limiting customer purchases of supplies like toilet paper at the end of July.
“We are wired to run with the herd,” says Bradley Klontz, PsyD, an associate professor of practice at Creighton University Heider College of Business, Omaha, N.E., who specializes in financial psychology.
“Quite literally, the last person to get to Costco doesn’t get the toilet paper, so when the herd is running in a certain direction, we feel a biological imperative to not be that last person. That fear of scarcity actually creates the experience of scarcity,” he explains.
The science behind the stockpile
People are collectively alerted by photos shared on social media showing store shelves stripped of toilet paper. Those images triggered consumers to rush out and buy bathroom tissue, even if they didn’t need it – and that herd behavior created toilet paper shortages.
Now, a year and half into the pandemic, people are hypervigilant to danger. Any hint of a possible toilet paper shortage can provoke anxiety and the desire to stockpile.
, says Dr. Klontz. He advises people to take a deep breath before buying extra toilet paper and then assess whether it is truly needed.
Deep in our brains is the limbic system, a group of structures that rules over emotions, motivation, reward, learning, memory, and the fight-or-flight response to stress and danger. When a person senses danger, the brain activates hormones to raise blood pressure and heart rate, increase blood flow, and boost the breath rate, making the body ready to fight or flee under threat.
Once everything settles, the body activates chemicals like dopamine that bring on positive feelings of well-being, rewarding that flight-or-fight response. In this way, the brain powerfully reinforces a key survival instinct.
This sequence of experiences and the brain chemistry behind them may explain why people panic-buy toilet paper.
“With toilet paper, my limbic system starts thinking about a perceived threat to safety,” says Julie Pike, PhD, a psychologist in Chapel Hill, N.C., who specializes in anxiety, hoarding, and posttraumatic stress disorder.
She notes that, in stockpiling toilet paper, “we avoid a perceived threat and then we are chemically rewarded” with dopamine. A storage closet full of toilet paper after a perceived threat of scarcity – no matter how unfounded – brings on that satisfied feeling.
When the market shifted
Paper producers make hygiene paper for two markets: the commercial (think: those big rolls of thin paper used in offices, schools, and restaurants) and the consumer (the soft paper you likely use at home). In the spring of 2020, the commercial market plummeted, and the consumer market skyrocketed.
Generally, the consumer toilet paper market is steady. The average American uses about 57 toilet sheets a day and about 50 pounds annually. Grocery stores and other retailers keep just enough toilet paper on hand to meet this steady demand, meaning panic buying at the start of the pandemic quickly depleted stocks. Paper makers had to change production to meet higher consumer demand and fewer commercial buyers.
By the end of the summer of 2020, toilet paper makers had adjusted for the market shift and caught up with demand, as consumers worked through their stockpiles of paper. But retail inventories remain lean because toilet paper doesn’t carry huge profit margins. For this reason, even healthy stocks remain sensitive to sudden shifts in consumer demand, Dr. Gonzalez says.
“If people buy more than they should, then they are just buying from other people,” creating an unnecessary scarcity of toilet paper, he says.
The supply chain
It is true that the supply chain is under unprecedented strain, leading to higher prices for many goods, says Katie Denis, vice president of research and industry narrative at the Consumer Brands Association, Washington, which represents toilet paper makers Georgia-Pacific and Procter & Gamble. Consumers should expect toilet paper to be available, but there may be fewer options for product sizes, she says.
Still, Dr. Gonzalez says consumers should not worry too much about the global supply chain affecting the domestic toilet paper supply. The raw material for toilet paper production is available domestically, and more than 97% of the supply on U.S. retailer shelves is made in the United States, he says.
In modern society, toilet paper is a primary link to civilization, health, and hygiene. While there is no easy substitute, alternatives do exist A bidet, for example, is a device that can spray water on the genital area. Other options are reusable cloths, sponges, baby wipes, napkins, towels, and washcloths.
Human health and hygiene
“Compared to many other items, toilet paper can’t really be replaced,” says Frank H. Farley, PhD, a professor of psychological studies in education at Temple University, who studies human motivation. “It is a unique consumer item that is perceived to be extremely necessary. In that way, it plays into that survivor mentality, that having it is necessary for survival.”
Being without it can truly seem like an existential threat.
New York City emergency planner Ira Tannenbaum advises families to assess their usage of essential household supplies like toilet paper (you can do so through this toilet paper calculator) and keep at least a 1-week supply on hand in case of emergency. New York City has posted recommendations to families for emergency planning, including the guidance to “avoid panic buying.”
Dr. Pike says she would stockpile a bit more, something that could be done gradually, before there’s a panic. She says that if people are tempted to buy more out of anxiety, they should remind themselves that shortages arise because of panicky purchasing.
“Leave some for other families – other people have children and partners and siblings just like us,” she says.
A version of this article first appeared on WebMD.com.
How did toilet paper become the unofficial symbol of anxiety during the pandemic? Empty store shelves are a stark reminder of how COVID-19 has taken a toll on people.
At the beginning of the pandemic, stay-at-home orders drove people to buy large amounts of household goods, especially toilet paper. Demand grew to unforeseen heights in March 2020, with $1.45 billion in toilet paper sales in the 4-week period ending March 29, up 112% from the year before, according to IRI, a Chicago-based market research firm.
As the Delta variant drove a COVID-19 resurgence this summer, market research suggests that almost one in two Americans started stockpiling toilet paper again over fears that supply would run out. The higher demand causes ripples through the retail chain, and a growing number of stores are again facing challenges in stocking toilet paper.
Yet there is plenty for everyone if people don’t stockpile too much, according to paper industry market analyst Ronalds Gonzalez, PhD, an associate professor of conversion economics and sustainability at North Carolina State University, Raleigh.
“As long as people buy what they actually need and don’t get into a panic, there won’t be any issue with the supply of hygienic tissue,” he says, adding that “too much” would equate to stockpiling 6-8 months’ worth of toilet paper, as some people did early in the pandemic.
But retailers are worried that history will repeat itself. In late September 2021, warehouse retail giant Costco told Wall Street analysts that it decided to limit customer purchases of essential items like toilet paper and water. Another retailer, Sam’s Club, began limiting customer purchases of supplies like toilet paper at the end of July.
“We are wired to run with the herd,” says Bradley Klontz, PsyD, an associate professor of practice at Creighton University Heider College of Business, Omaha, N.E., who specializes in financial psychology.
“Quite literally, the last person to get to Costco doesn’t get the toilet paper, so when the herd is running in a certain direction, we feel a biological imperative to not be that last person. That fear of scarcity actually creates the experience of scarcity,” he explains.
The science behind the stockpile
People are collectively alerted by photos shared on social media showing store shelves stripped of toilet paper. Those images triggered consumers to rush out and buy bathroom tissue, even if they didn’t need it – and that herd behavior created toilet paper shortages.
Now, a year and half into the pandemic, people are hypervigilant to danger. Any hint of a possible toilet paper shortage can provoke anxiety and the desire to stockpile.
, says Dr. Klontz. He advises people to take a deep breath before buying extra toilet paper and then assess whether it is truly needed.
Deep in our brains is the limbic system, a group of structures that rules over emotions, motivation, reward, learning, memory, and the fight-or-flight response to stress and danger. When a person senses danger, the brain activates hormones to raise blood pressure and heart rate, increase blood flow, and boost the breath rate, making the body ready to fight or flee under threat.
Once everything settles, the body activates chemicals like dopamine that bring on positive feelings of well-being, rewarding that flight-or-fight response. In this way, the brain powerfully reinforces a key survival instinct.
This sequence of experiences and the brain chemistry behind them may explain why people panic-buy toilet paper.
“With toilet paper, my limbic system starts thinking about a perceived threat to safety,” says Julie Pike, PhD, a psychologist in Chapel Hill, N.C., who specializes in anxiety, hoarding, and posttraumatic stress disorder.
She notes that, in stockpiling toilet paper, “we avoid a perceived threat and then we are chemically rewarded” with dopamine. A storage closet full of toilet paper after a perceived threat of scarcity – no matter how unfounded – brings on that satisfied feeling.
When the market shifted
Paper producers make hygiene paper for two markets: the commercial (think: those big rolls of thin paper used in offices, schools, and restaurants) and the consumer (the soft paper you likely use at home). In the spring of 2020, the commercial market plummeted, and the consumer market skyrocketed.
Generally, the consumer toilet paper market is steady. The average American uses about 57 toilet sheets a day and about 50 pounds annually. Grocery stores and other retailers keep just enough toilet paper on hand to meet this steady demand, meaning panic buying at the start of the pandemic quickly depleted stocks. Paper makers had to change production to meet higher consumer demand and fewer commercial buyers.
By the end of the summer of 2020, toilet paper makers had adjusted for the market shift and caught up with demand, as consumers worked through their stockpiles of paper. But retail inventories remain lean because toilet paper doesn’t carry huge profit margins. For this reason, even healthy stocks remain sensitive to sudden shifts in consumer demand, Dr. Gonzalez says.
“If people buy more than they should, then they are just buying from other people,” creating an unnecessary scarcity of toilet paper, he says.
The supply chain
It is true that the supply chain is under unprecedented strain, leading to higher prices for many goods, says Katie Denis, vice president of research and industry narrative at the Consumer Brands Association, Washington, which represents toilet paper makers Georgia-Pacific and Procter & Gamble. Consumers should expect toilet paper to be available, but there may be fewer options for product sizes, she says.
Still, Dr. Gonzalez says consumers should not worry too much about the global supply chain affecting the domestic toilet paper supply. The raw material for toilet paper production is available domestically, and more than 97% of the supply on U.S. retailer shelves is made in the United States, he says.
In modern society, toilet paper is a primary link to civilization, health, and hygiene. While there is no easy substitute, alternatives do exist A bidet, for example, is a device that can spray water on the genital area. Other options are reusable cloths, sponges, baby wipes, napkins, towels, and washcloths.
Human health and hygiene
“Compared to many other items, toilet paper can’t really be replaced,” says Frank H. Farley, PhD, a professor of psychological studies in education at Temple University, who studies human motivation. “It is a unique consumer item that is perceived to be extremely necessary. In that way, it plays into that survivor mentality, that having it is necessary for survival.”
Being without it can truly seem like an existential threat.
New York City emergency planner Ira Tannenbaum advises families to assess their usage of essential household supplies like toilet paper (you can do so through this toilet paper calculator) and keep at least a 1-week supply on hand in case of emergency. New York City has posted recommendations to families for emergency planning, including the guidance to “avoid panic buying.”
Dr. Pike says she would stockpile a bit more, something that could be done gradually, before there’s a panic. She says that if people are tempted to buy more out of anxiety, they should remind themselves that shortages arise because of panicky purchasing.
“Leave some for other families – other people have children and partners and siblings just like us,” she says.
A version of this article first appeared on WebMD.com.
How did toilet paper become the unofficial symbol of anxiety during the pandemic? Empty store shelves are a stark reminder of how COVID-19 has taken a toll on people.
At the beginning of the pandemic, stay-at-home orders drove people to buy large amounts of household goods, especially toilet paper. Demand grew to unforeseen heights in March 2020, with $1.45 billion in toilet paper sales in the 4-week period ending March 29, up 112% from the year before, according to IRI, a Chicago-based market research firm.
As the Delta variant drove a COVID-19 resurgence this summer, market research suggests that almost one in two Americans started stockpiling toilet paper again over fears that supply would run out. The higher demand causes ripples through the retail chain, and a growing number of stores are again facing challenges in stocking toilet paper.
Yet there is plenty for everyone if people don’t stockpile too much, according to paper industry market analyst Ronalds Gonzalez, PhD, an associate professor of conversion economics and sustainability at North Carolina State University, Raleigh.
“As long as people buy what they actually need and don’t get into a panic, there won’t be any issue with the supply of hygienic tissue,” he says, adding that “too much” would equate to stockpiling 6-8 months’ worth of toilet paper, as some people did early in the pandemic.
But retailers are worried that history will repeat itself. In late September 2021, warehouse retail giant Costco told Wall Street analysts that it decided to limit customer purchases of essential items like toilet paper and water. Another retailer, Sam’s Club, began limiting customer purchases of supplies like toilet paper at the end of July.
“We are wired to run with the herd,” says Bradley Klontz, PsyD, an associate professor of practice at Creighton University Heider College of Business, Omaha, N.E., who specializes in financial psychology.
“Quite literally, the last person to get to Costco doesn’t get the toilet paper, so when the herd is running in a certain direction, we feel a biological imperative to not be that last person. That fear of scarcity actually creates the experience of scarcity,” he explains.
The science behind the stockpile
People are collectively alerted by photos shared on social media showing store shelves stripped of toilet paper. Those images triggered consumers to rush out and buy bathroom tissue, even if they didn’t need it – and that herd behavior created toilet paper shortages.
Now, a year and half into the pandemic, people are hypervigilant to danger. Any hint of a possible toilet paper shortage can provoke anxiety and the desire to stockpile.
, says Dr. Klontz. He advises people to take a deep breath before buying extra toilet paper and then assess whether it is truly needed.
Deep in our brains is the limbic system, a group of structures that rules over emotions, motivation, reward, learning, memory, and the fight-or-flight response to stress and danger. When a person senses danger, the brain activates hormones to raise blood pressure and heart rate, increase blood flow, and boost the breath rate, making the body ready to fight or flee under threat.
Once everything settles, the body activates chemicals like dopamine that bring on positive feelings of well-being, rewarding that flight-or-fight response. In this way, the brain powerfully reinforces a key survival instinct.
This sequence of experiences and the brain chemistry behind them may explain why people panic-buy toilet paper.
“With toilet paper, my limbic system starts thinking about a perceived threat to safety,” says Julie Pike, PhD, a psychologist in Chapel Hill, N.C., who specializes in anxiety, hoarding, and posttraumatic stress disorder.
She notes that, in stockpiling toilet paper, “we avoid a perceived threat and then we are chemically rewarded” with dopamine. A storage closet full of toilet paper after a perceived threat of scarcity – no matter how unfounded – brings on that satisfied feeling.
When the market shifted
Paper producers make hygiene paper for two markets: the commercial (think: those big rolls of thin paper used in offices, schools, and restaurants) and the consumer (the soft paper you likely use at home). In the spring of 2020, the commercial market plummeted, and the consumer market skyrocketed.
Generally, the consumer toilet paper market is steady. The average American uses about 57 toilet sheets a day and about 50 pounds annually. Grocery stores and other retailers keep just enough toilet paper on hand to meet this steady demand, meaning panic buying at the start of the pandemic quickly depleted stocks. Paper makers had to change production to meet higher consumer demand and fewer commercial buyers.
By the end of the summer of 2020, toilet paper makers had adjusted for the market shift and caught up with demand, as consumers worked through their stockpiles of paper. But retail inventories remain lean because toilet paper doesn’t carry huge profit margins. For this reason, even healthy stocks remain sensitive to sudden shifts in consumer demand, Dr. Gonzalez says.
“If people buy more than they should, then they are just buying from other people,” creating an unnecessary scarcity of toilet paper, he says.
The supply chain
It is true that the supply chain is under unprecedented strain, leading to higher prices for many goods, says Katie Denis, vice president of research and industry narrative at the Consumer Brands Association, Washington, which represents toilet paper makers Georgia-Pacific and Procter & Gamble. Consumers should expect toilet paper to be available, but there may be fewer options for product sizes, she says.
Still, Dr. Gonzalez says consumers should not worry too much about the global supply chain affecting the domestic toilet paper supply. The raw material for toilet paper production is available domestically, and more than 97% of the supply on U.S. retailer shelves is made in the United States, he says.
In modern society, toilet paper is a primary link to civilization, health, and hygiene. While there is no easy substitute, alternatives do exist A bidet, for example, is a device that can spray water on the genital area. Other options are reusable cloths, sponges, baby wipes, napkins, towels, and washcloths.
Human health and hygiene
“Compared to many other items, toilet paper can’t really be replaced,” says Frank H. Farley, PhD, a professor of psychological studies in education at Temple University, who studies human motivation. “It is a unique consumer item that is perceived to be extremely necessary. In that way, it plays into that survivor mentality, that having it is necessary for survival.”
Being without it can truly seem like an existential threat.
New York City emergency planner Ira Tannenbaum advises families to assess their usage of essential household supplies like toilet paper (you can do so through this toilet paper calculator) and keep at least a 1-week supply on hand in case of emergency. New York City has posted recommendations to families for emergency planning, including the guidance to “avoid panic buying.”
Dr. Pike says she would stockpile a bit more, something that could be done gradually, before there’s a panic. She says that if people are tempted to buy more out of anxiety, they should remind themselves that shortages arise because of panicky purchasing.
“Leave some for other families – other people have children and partners and siblings just like us,” she says.
A version of this article first appeared on WebMD.com.