User login
Surgery vs radiofrequency ablation: Achieving better recurrence-free survival in small HCC
Key clinical point: Patients with small hepatocellular carcinoma (HCC) show a comparable improvement in recurrence-free survival (RFS) after undergoing surgery or radiofrequency ablation (RFA).
Main finding: The median RFS of patients who had undergone surgery was not significantly different from that of patients receiving RFA (3.46 years vs 3.04 years; hazard ratio, 0.92; P = .58).
Study details: Findings are from the phase 3 SURF-trial including 301 patients aged between 20 and 80 years with the largest HCC diameter ≤3 cm and ≤3 HCC nodules who were randomly assigned (1:1) to undergo either surgery (n=150) or RFA (n=151).
Disclosures: The study was sponsored by the Japanese Foundation for Multidisciplinary Treatment of Cancer and the Health and Labor Sciences Research Grant for Clinical Cancer Research. Some of the authors declared receiving lecture fees or research funds from or serving as an advisor for various companies. A few authors reported being on the editorial team/board of Liver Cancer.
Source: Takayama T et al. Liver Cancer. 2021 Dec 29. doi: 10.1159/000521665.
Key clinical point: Patients with small hepatocellular carcinoma (HCC) show a comparable improvement in recurrence-free survival (RFS) after undergoing surgery or radiofrequency ablation (RFA).
Main finding: The median RFS of patients who had undergone surgery was not significantly different from that of patients receiving RFA (3.46 years vs 3.04 years; hazard ratio, 0.92; P = .58).
Study details: Findings are from the phase 3 SURF-trial including 301 patients aged between 20 and 80 years with the largest HCC diameter ≤3 cm and ≤3 HCC nodules who were randomly assigned (1:1) to undergo either surgery (n=150) or RFA (n=151).
Disclosures: The study was sponsored by the Japanese Foundation for Multidisciplinary Treatment of Cancer and the Health and Labor Sciences Research Grant for Clinical Cancer Research. Some of the authors declared receiving lecture fees or research funds from or serving as an advisor for various companies. A few authors reported being on the editorial team/board of Liver Cancer.
Source: Takayama T et al. Liver Cancer. 2021 Dec 29. doi: 10.1159/000521665.
Key clinical point: Patients with small hepatocellular carcinoma (HCC) show a comparable improvement in recurrence-free survival (RFS) after undergoing surgery or radiofrequency ablation (RFA).
Main finding: The median RFS of patients who had undergone surgery was not significantly different from that of patients receiving RFA (3.46 years vs 3.04 years; hazard ratio, 0.92; P = .58).
Study details: Findings are from the phase 3 SURF-trial including 301 patients aged between 20 and 80 years with the largest HCC diameter ≤3 cm and ≤3 HCC nodules who were randomly assigned (1:1) to undergo either surgery (n=150) or RFA (n=151).
Disclosures: The study was sponsored by the Japanese Foundation for Multidisciplinary Treatment of Cancer and the Health and Labor Sciences Research Grant for Clinical Cancer Research. Some of the authors declared receiving lecture fees or research funds from or serving as an advisor for various companies. A few authors reported being on the editorial team/board of Liver Cancer.
Source: Takayama T et al. Liver Cancer. 2021 Dec 29. doi: 10.1159/000521665.
ABO blood group system may dictate the outcome of liver transplantation in HCC
Key clinical point: The oncological outcome in patients with hepatocellular carcinoma (HCC) who underwent liver transplantation (LT) is strongly affected by the ABO blood group system.
Main finding: Blood group A showed an independent association with increased tumor recurrence risk (adjusted hazard ratio [aHR], 1.574; P = .034) with group A vs non-A recipients having higher 5-year tumor recurrence rates (20.1% vs 13.2%; aHR, 1.66; P = .011) and lower 5-year recurrence-free survival rates (66.8% vs 71.3%; aHR, 1.38; P = .045).
Study details: The data are derived from a multicentric retrospective observational study including 925 adult patients with HCC who underwent LT, of whom 406, 94, 380, and 45 had blood group A, B, O, and AB, respectively.
Disclosures: The authors reported no funding source or conflict of interests.
Source: Kayvan M et al. Transplantation. 2021 Dec 27. doi: 10.1097/TP.0000000000004004.
Key clinical point: The oncological outcome in patients with hepatocellular carcinoma (HCC) who underwent liver transplantation (LT) is strongly affected by the ABO blood group system.
Main finding: Blood group A showed an independent association with increased tumor recurrence risk (adjusted hazard ratio [aHR], 1.574; P = .034) with group A vs non-A recipients having higher 5-year tumor recurrence rates (20.1% vs 13.2%; aHR, 1.66; P = .011) and lower 5-year recurrence-free survival rates (66.8% vs 71.3%; aHR, 1.38; P = .045).
Study details: The data are derived from a multicentric retrospective observational study including 925 adult patients with HCC who underwent LT, of whom 406, 94, 380, and 45 had blood group A, B, O, and AB, respectively.
Disclosures: The authors reported no funding source or conflict of interests.
Source: Kayvan M et al. Transplantation. 2021 Dec 27. doi: 10.1097/TP.0000000000004004.
Key clinical point: The oncological outcome in patients with hepatocellular carcinoma (HCC) who underwent liver transplantation (LT) is strongly affected by the ABO blood group system.
Main finding: Blood group A showed an independent association with increased tumor recurrence risk (adjusted hazard ratio [aHR], 1.574; P = .034) with group A vs non-A recipients having higher 5-year tumor recurrence rates (20.1% vs 13.2%; aHR, 1.66; P = .011) and lower 5-year recurrence-free survival rates (66.8% vs 71.3%; aHR, 1.38; P = .045).
Study details: The data are derived from a multicentric retrospective observational study including 925 adult patients with HCC who underwent LT, of whom 406, 94, 380, and 45 had blood group A, B, O, and AB, respectively.
Disclosures: The authors reported no funding source or conflict of interests.
Source: Kayvan M et al. Transplantation. 2021 Dec 27. doi: 10.1097/TP.0000000000004004.
Ketamine nasal spray provides slow-acting relief for cluster headache attacks
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
FROM HEADACHE
CVS Caremark formulary change freezes out apixaban
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Patients looking to refill a prescription for apixaban (Eliquis) through CVS Caremark may be in for a surprise following its decision to exclude the direct oral anticoagulant (DOAC) from its formulary starting Jan. 1.
The move leaves just one DOAC, rivaroxaban (Xarelto), on CVS’ commercial formulary and is being assailed as the latest example of “nonmedical switching” used by health insurers to control costs.
In a letter to CVS Caremark backed by 14 provider and patient organizations, the nonprofit Partnership to Advance Cardiovascular Health (PACH) calls on the pharmacy chain to reverse its “dangerously disruptive” decision to force stable patients at high risk of cardiovascular events to switch anticoagulation, without an apparent option to be grandfathered into the new plan.
PACH president Dharmesh Patel, MD, Stern Cardiovascular Center, Memphis, called the formulary change “reckless and irresponsible, especially because the decision is not based in science and evidence, but on budgets. Patients and their health care providers, not insurance companies, need to be trusted to determine what medication is best,” he said in a statement.
Craig Beavers, PharmD, vice president of Baptist Health Paducah, Kentucky, said that, as chair of the American College of Cardiology’s Cardiovascular Team Section, he and other organizations have met with CVS Caremark medical leadership to advocate for patients and to understand the company’s perspective.
“The underlying driver is cost,” he told this news organization.
Current guidelines recommend DOACs in general for a variety of indications, including to reduce the risk of stroke and embolism in nonvalvular atrial fibrillation and to prevent deep vein thrombosis, but there are select instances where a particular DOAC might be more appropriate, he observed.
“Apixaban may be better for a patient with a history of GI bleeding because there’s less GI bleeding, but the guidelines don’t necessarily spell those things out,” Dr. Beavers said. “That’s where the clinician should advocate for their patient and, unfortunately, they are making their decision strictly based off the guidelines.”
Requests to speak with medical officers at CVS Caremark went unanswered, but its executive director of communications, Christina Peaslee, told this news organization that the formulary decision “maintains clinically appropriate, cost-effective prescription coverage” for its clients and members.
“Both the American Heart Association/American College of Cardiology/Heart Rhythm Society and 2021 CHEST guidelines recommend DOACs over warfarin for treatment of various cardiology conditions such as atrial fibrillation, but neither list a specific agent as preferred – showing that consensus clinical guidelines do not favor one over the other,” she said in an email. “Further, Xarelto has more FDA-approved indications than Eliquis (e.g., Xarelto is approved for a reduction in risk of major CV events in patients with CAD or PAD) in addition to all the same FDA indications as Eliquis.”
Ms. Peaslee pointed out that all formulary changes are evaluated by an external medical expert specializing in the disease state, followed by review and approval by an independent national Pharmacy & Therapeutics Committee.
The decision to exclude apixaban is also limited to a “subset of commercial drug lists,” she said, although specifics on which plans and the number of affected patients were not forthcoming.
The choice of DOAC is a timely question in cardiology, with recent studies suggesting an advantage for apixaban over rivaroxaban in reducing the risk of recurrent venous thromboembolism, as well as reducing the risk of major ischemic or hemorrhagic events in atrial fibrillation.
Ms. Peaslee said CVS Caremark closely monitors medical literature for relevant clinical trial data and that most clients allow reasonable formulary exceptions when justified. “This formulary exceptions process has been successfully used for changes of this type and allows patients to get a medication that is safe and effective, as determined by their prescriber.”
The company will also continue to provide “robust, personalized outreach to the small number of members who will need to switch to an alternative medication,” she added.
Dr. Beavers said negotiations with CVS are still in the early stages, but, in the meantime, the ACC is providing health care providers with tools, such as drug copay cards and electronic prior authorizations, to help ensure patients don’t have gaps in coverage.
In a Jan. 14 news release addressing the formulary change, ACC notes that a patient’s pharmacy can also request a one-time override when trying to fill a nonpreferred DOAC in January to buy time if switching medications with their clinician or requesting a formulary exception.
During discussions with CVS Caremark, it says the ACC and the American Society of Hematology “underscored the negative impacts of this decision on patients currently taking one of the nonpreferred DOACs and on those who have previously tried rivaroxaban and changed medications.”
The groups also highlighted difficulties with other prior authorization programs in terms of the need for dedicated staff and time away from direct patient care.
“The ACC and ASH will continue discussions with CVS Caremark regarding the burden on clinicians and the effect of the formulary decision on patient access,” the release says.
In its letter to CVS, PACH argues that the apixaban exclusion will disproportionately affect historically disadvantaged patients, leaving those who can least afford the change with limited options. Notably, no generic is available for either apixaban or rivaroxaban.
The group also highlights a 2019 national poll, in which nearly 40% of patients who had their medication switched were so frustrated that they stopped their medication altogether.
PACH has an online petition against nonmedical switching, which at press time had garnered 2,126 signatures.
One signee, Jan Griffin, who survived bilateral pulmonary embolisms, writes that she has been on Eliquis [apixaban] successfully since her hospital discharge. “Now, as of midnight, Caremark apparently knows better than my hematologist as to what blood thinner is better for me and will no longer cover my Eliquis prescription. This is criminal, immoral, and unethical. #StopTheSwitch.”
A version of this article first appeared on Medscape.com.
Woman with throbbing unilateral headache
Migraine is a complex disorder characterized by recurrent episodes of headache, most often unilateral and in some cases associated with photophobia or phonophobia — a constellation known as aura — that usually arises before the head pain but may also occur during or afterward. Migraine is most common in women, and prevalence peaks between the ages of 25 and 55. In 2016, headache was the fifth most common reason for an ED visit and the third most common reason for an ED visit among female patients age 15-64.
Diagnosis of migraine is made on the basis of patient history. Examples of red flags in the differential would be the presence of neurologic symptoms, stiff neck, or fever, or history of head injury or major trauma. Migraine should also be distinguished from other common headaches. Tension-type headaches usually cause mild or moderate bilateral pain, with a deep, steady ache rather than the typical throbbing quality of migraine headache. In cluster headache, the patient experiences attacks of severe or very severe, strictly unilateral pain (orbital, supraorbital, or temporal pain), but the cadence of these headaches differs from that of migraines; these attacks last 15-180 minutes and occur from once every other day to eight times a day. Patients with basilar migraine, common among female patients, usually present with symptoms of vertebrobasilar insufficiency.
The American Headache Society defines migraine as when a patient reports at least five attacks. These episodes must last 4-72 hours and have at least two of these four characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity. In addition, during attacks, the patient must experience either nausea and/or vomiting or photophobia and phonophobia. Signs and symptoms cannot be accounted for by another diagnosis.
Treatment of migraines is often associated with a trial-and-error period. For mild to moderate migraines, these agents may be considered: NSAIDs, nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations. For moderate or severe attacks, or even mild to moderate attacks that do not respond well to therapy, migraine-specific agents are recommended: triptans, dihydroergotamine (DHE), small-molecule CGRP receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Menstrual migraines are treated via the same approaches as nonmenstrual migraines.
Many patients, like the one described here, experience severe nausea or vomiting with their migraine attacks. For these cases, nonoral agents may be considered (these agents are also an option for patients whose headaches do not respond well to traditional oral medication). Patients should be advised to limit medication use to an average of two headache days per week, and those who feel it necessary to exceed this limit should be offered a preventive treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
Migraine is a complex disorder characterized by recurrent episodes of headache, most often unilateral and in some cases associated with photophobia or phonophobia — a constellation known as aura — that usually arises before the head pain but may also occur during or afterward. Migraine is most common in women, and prevalence peaks between the ages of 25 and 55. In 2016, headache was the fifth most common reason for an ED visit and the third most common reason for an ED visit among female patients age 15-64.
Diagnosis of migraine is made on the basis of patient history. Examples of red flags in the differential would be the presence of neurologic symptoms, stiff neck, or fever, or history of head injury or major trauma. Migraine should also be distinguished from other common headaches. Tension-type headaches usually cause mild or moderate bilateral pain, with a deep, steady ache rather than the typical throbbing quality of migraine headache. In cluster headache, the patient experiences attacks of severe or very severe, strictly unilateral pain (orbital, supraorbital, or temporal pain), but the cadence of these headaches differs from that of migraines; these attacks last 15-180 minutes and occur from once every other day to eight times a day. Patients with basilar migraine, common among female patients, usually present with symptoms of vertebrobasilar insufficiency.
The American Headache Society defines migraine as when a patient reports at least five attacks. These episodes must last 4-72 hours and have at least two of these four characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity. In addition, during attacks, the patient must experience either nausea and/or vomiting or photophobia and phonophobia. Signs and symptoms cannot be accounted for by another diagnosis.
Treatment of migraines is often associated with a trial-and-error period. For mild to moderate migraines, these agents may be considered: NSAIDs, nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations. For moderate or severe attacks, or even mild to moderate attacks that do not respond well to therapy, migraine-specific agents are recommended: triptans, dihydroergotamine (DHE), small-molecule CGRP receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Menstrual migraines are treated via the same approaches as nonmenstrual migraines.
Many patients, like the one described here, experience severe nausea or vomiting with their migraine attacks. For these cases, nonoral agents may be considered (these agents are also an option for patients whose headaches do not respond well to traditional oral medication). Patients should be advised to limit medication use to an average of two headache days per week, and those who feel it necessary to exceed this limit should be offered a preventive treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
Migraine is a complex disorder characterized by recurrent episodes of headache, most often unilateral and in some cases associated with photophobia or phonophobia — a constellation known as aura — that usually arises before the head pain but may also occur during or afterward. Migraine is most common in women, and prevalence peaks between the ages of 25 and 55. In 2016, headache was the fifth most common reason for an ED visit and the third most common reason for an ED visit among female patients age 15-64.
Diagnosis of migraine is made on the basis of patient history. Examples of red flags in the differential would be the presence of neurologic symptoms, stiff neck, or fever, or history of head injury or major trauma. Migraine should also be distinguished from other common headaches. Tension-type headaches usually cause mild or moderate bilateral pain, with a deep, steady ache rather than the typical throbbing quality of migraine headache. In cluster headache, the patient experiences attacks of severe or very severe, strictly unilateral pain (orbital, supraorbital, or temporal pain), but the cadence of these headaches differs from that of migraines; these attacks last 15-180 minutes and occur from once every other day to eight times a day. Patients with basilar migraine, common among female patients, usually present with symptoms of vertebrobasilar insufficiency.
The American Headache Society defines migraine as when a patient reports at least five attacks. These episodes must last 4-72 hours and have at least two of these four characteristics: unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity. In addition, during attacks, the patient must experience either nausea and/or vomiting or photophobia and phonophobia. Signs and symptoms cannot be accounted for by another diagnosis.
Treatment of migraines is often associated with a trial-and-error period. For mild to moderate migraines, these agents may be considered: NSAIDs, nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations. For moderate or severe attacks, or even mild to moderate attacks that do not respond well to therapy, migraine-specific agents are recommended: triptans, dihydroergotamine (DHE), small-molecule CGRP receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Menstrual migraines are treated via the same approaches as nonmenstrual migraines.
Many patients, like the one described here, experience severe nausea or vomiting with their migraine attacks. For these cases, nonoral agents may be considered (these agents are also an option for patients whose headaches do not respond well to traditional oral medication). Patients should be advised to limit medication use to an average of two headache days per week, and those who feel it necessary to exceed this limit should be offered a preventive treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
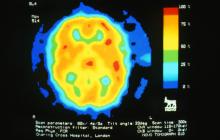
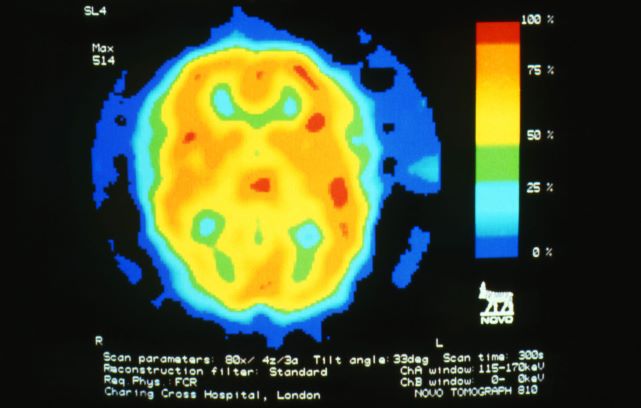
A 28-year-old woman presents with a throbbing unilateral headache (left side) and is very nauseated. She describes a white light in her line of vision. Her headaches are recurring, pulsating, and usually last for about 2 days without relief from nonsteroidal anti-inflammatory drugs (NSAIDs). She has been experiencing these episodes almost every month for the past 8 months and initially attributed them to her menstrual cycle, as she has always experienced moderate to severe headaches during this time. The patient is enrolled in a research trial in which single photon emission computed tomography (SPECT) imaging revealed low activity with reduced blood flow. The patient is nonfebrile.
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Appendectomy or antibiotics? Large trial helps decision-making
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
The presence of mineralized stool, known as appendicolith, was associated with a nearly twofold increased risk of undergoing appendectomy within 30 days of initiating antibiotics, write David Flum, MD, of the University of Washington, Seattle, and coauthors in a paper published in JAMA Surgery on Jan. 12, 2021.
But the surprise was the lack of an association between appendectomy and factors often presumed to be consistent with more severe appendicitis.
Physicians have had their own ideas about what factors make a patient more likely to need an appendectomy after an initial round of treatment with antibiotics, such as a high white blood cell count or a perforation seen on CT scan, Dr. Flum said in an interview. But the research didn’t support some of these theories.
“This is why we do the studies,” Dr. Flum said. “Sometimes we find out that our hunches were wrong.”
Dr. Flum and coauthors measured the association between different patient factors and disease severity and the need for appendectomy following a course of antibiotics. They used adjusted odds ratios to describe these relationships while accounting for other differences.
An OR of 1.0 – or when the confidence interval around an OR crosses 1 – signals that there is no association between that factor and appendectomy. Positive ORs with confidence intervals that exclude 1.0 suggest the factor was associated with appendectomy.
The OR was 1.99 for the presence of appendicolith, a finding with a 95% confidence interval of 1.28-3.10. The OR was 1.53 (95% CI, 1.01-2.31) for female sex.
But the OR was 1.14 (95% CI, 0.66-1.98) for perforation, abscess, or fat stranding.
The OR was 1.09 (95% CI, 1.00-1.18) for radiographic finding of a larger appendix, as measured by diameter.
And the OR was 1.03 (95% CI, 0.98-1.09) for having a higher white blood cell count, as measured by a 1,000-cells/mcL increase.
Appy or not?
This paper draws from the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial (NCT02800785), for which top-line results were published in 2020 in the New England Journal of Medicine. In that paper, Dr. Flum and colleagues reported on results for 1,552 adults (414 with an appendicolith) who were evenly randomized to either antibiotics treatment or appendectomy. After 30 days, antibiotics were found to be noninferior to appendectomy, as reported by this news organization.
The federal Patient-Centered Outcomes Research Institute funded the CODA research. Dr. Flum said the National Institutes of Health had not appeared interested in funding a look at the different options available to patients experiencing appendicitis. Congress created PCORI as part of the Affordable Care Act of 2010, seeking to encourage researchers to study which treatments best serve patients through direct comparisons. Its support was critical for Dr. Flum and colleagues in seeking to help people weigh their options for treating appendicitis.
The CODA study “models what the patient’s experience is like, and this has not been the focus of NIH as much,” Dr. Flum said.
The CODA team has sought to make it easy for patients to consider what its findings and other research on appendicitis mean for them. They created an online decision-making tool, available at the aptly named http://www.appyornot.org/ website, which has videos in English and Spanish explaining patients’ options in simple terms. The website also asks questions about personal preferences, priorities, and resources to help them choose a treatment based on their individual situation.
Shift away from ‘paternalistic framing’
In the past, surgeons focused on the risk for patients from procedures, making the decisions for them about whether or not to proceed. There’s now a drive to shift away from this “paternalistic framing” toward shared decision-making, Dr. Flum said.
Surgeons need to have conversations with their patients about what’s happening in their lives as well as to assess their fears and concerns about treatment options, he said. These are aspects of patient care that were not covered in medical school or surgical training, but they lead to “less paternalistic” treatment. A patient’s decision about whether to choose surgery or antibiotics for appendicitis may hinge on factors such as insurance coverage, access to childcare, and the ability to miss days of work.
Dr. Flum said his fellow surgeons by and large have reacted well to the CODA team’s work.
“To their credit, the surgical community has embraced a healthy skepticism about the role of surgery,” Dr. Flum said.
The guidelines of the American College of Surgeons state that there is “high-quality evidence” that most patients with appendicitis can be managed with antibiotics instead of appendectomy (69% overall avoid appendectomy by 90 days, 75% of those without appendicolith, and 59% of those with appendicolith).
“Based on the surgeon’s judgment, patient preferences, and local resources (e.g., hospital staff, bed, and PPE supply availability) antibiotics are an acceptable first-line treatment, with appendectomy offered for those with worsening or recurrent symptoms,” the ACS guidelines say.
In an interview, Samir M. Fakhry, MD, vice president of HCA Center for Trauma and Acute Care Surgery Research in Nashville, Tenn., agreed with Dr. Flum about the shift taking place in medicine.
The CODA research, including the new paper in JAMA Surgery, makes it easier for physicians to work with patients and their families to reach decisions about how to treat appendicitis, Dr. Fakhry said.
These important discussions take time, he said, and patients must be allowed that time. Patients might feel misled, for example, if a surgeon pressed for appendectomy without explaining that a course of antibiotics may have served them well. Other patients may opt for surgery right away, especially in cases with appendicoliths, to avoid the potential for repeat episodes of medical care.
“You’ve got people who just want to get it done and over with. You’ve got people who want to avoid surgery no matter what,” Dr. Fakhry said. “It’s not just about the science and the data.”
This study was supported by a grant from PCORI. The authors reported having served as consultants or reviewers or have received fees for work outside of this paper from Stryker, Kerecis, Acera, Medline, Shriner’s Research Fund, UpToDate, and Tetraphase Pharmaceuticals Stryker.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Dramatic increase in driving high after cannabis legislation
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Psychiatry resident’s viral posts reveal his own mental health battle
First-year psychiatry resident Jake Goodman, MD, knew he was taking a chance when he opened up on his popular social media platforms about his personal mental health battle. He mulled over the decision for several weeks before deciding to take the plunge.
As he voiced recently on his TikTok page, his biggest social media fanbase, with 1.3 million followers, it felt freeing to get his personal struggle off his chest.
“I’m a doctor in training, and most doctors would advise me not to post this,” the 29-year-old from Miami said in the video last month, which garnered 1.2 million views on TikTok alone. “They would say it’s risky for my career. But I didn’t join the medical field to continue the toxic status quo. I’m part of a new generation of health care professionals that are not afraid to be vulnerable and talk about mental health.”
“Dr. Jake,” as he calls himself on social media, admitted he was a physician who treats mental illness and also takes medication for it. “It felt good to say that. And by the way, I’m proud of it,” he said in the TikTok post.
A champion of mental health throughout the pandemic, Dr. Goodman called attention to the illness in the medical field. In a message on Instagram, he stated, “Opening up about your mental health as a medical professional, especially as a doctor who treats mental illness, can be taboo ... So here’s me leading by example.”
He also cited statistics on the challenge: “1 in 2 people will be diagnosed with a mental health illness at some point in their life. Yet many of us will never take medication that can help correct the chemical imbalance in our brains due to medication stigma: the fear that taking medications for our mental health somehow makes us weak.”
Mental health remains an issue among residents. Nearly 70% of residents polled by Medscape in its 2021 Residents Lifestyle & Happiness Report said they strongly or somewhat agree there’s a stigma against seeking mental health help. And nearly half, or 47% of those polled, said they sometimes (36%) or always/most of the time (11%) were depressed. The latter category rose in the past year.
Dr. Goodman told this news organization that he became passionate about mental health when he lost a college friend to suicide. “It really exposed the stigma” of mental health, he said. “I always knew it was there, but it took me seeing someone lose his life and [asking] why didn’t he feel comfortable talking to us, and why didn’t I feel comfortable talking to him?”
Stress of medical training
The decision to pursue psychiatry as his specialty came after a rotation in a clinic for people struggling with substance use disorders. “I was enthralled to see people change their life ... just by mental health care.” It’s why he went into medicine, he tells this news organization. “I always wanted to be in a field to help people [before they hit] rock bottom, when no one else could be there for them.”
Dr. Goodman’s personal battle with mental health didn’t arise until he started residency. “I was not really myself.” He said he felt numb and burned out. “I was not getting as much enjoyment out of things.” A friend pointed out that he might be depressed, so he went to see a therapist and then a psychiatrist and started on medication. “It had a profound impact on how I felt.”
Still, it took a while before Dr. Goodman was comfortable sharing his story with the 1.6 million followers he had already built across his social media platforms.
“I started on social media in 2020 with the goal of advocating for mental health and inspiring future doctors.” He said the message seemed to resonate with people struggling during the early part of the pandemic. On his social media accounts, he also talks about medical school, residency, and being a health care provider. His fiancé is also a resident doctor, in internal medicine.
Dr. Goodman is also trying to create a more realistic image of doctors than the superheroes he believed they were growing up. He wants those who grow up wanting to be doctors and who look up to him to see him as a human being with vulnerabilities, such as mental health.
“You can be a doctor and have mental health issues. Seeking treatment for mental health makes you a better doctor, and for other health care workers suffering in the midst of the pandemic, I want to let them know they are not alone.”
He pointed to the statistic that doctors have one of the highest suicide rates of any professions. “It’s better to talk about that in the early stages of training.”
Students, residents, or attending physicians who have mental health challenges shouldn’t allow their symptoms to go untreated, Dr. Goodman added. “Holding in all the stress and anxiety and feelings in a very traumatic field may be dangerous. ”
One of his goals is to campaign for the removal of a question on state medical licensing forms requiring doctors to report any mental health diagnosis. It’s why doctors may be afraid to admit that they are struggling. “I’m still here. It didn’t ruin my career.”
Doctors who seek treatment for mental health are theoretically protected under the Americans With Disabilities Act from being refused a license on the basis of that diagnosis. Dr. Goodman hopes to advocate at the state level to reduce discrimination and increase accessibility for doctors to seek mental health care.
Still, Dr. Goodman concedes he was initially fearful of the repercussions. “I opened up about it because this post could save lives. I was doing what I believed in.”
So if he runs into barriers to receive his medical license because of his admission, “that’s a serious problem,” he said. “There is already a shortage of doctors. We’ll see what happens in a few years. I am not the only one who will answer ‘yes’ to having sought treatment for a mental illness. The questions do not really need to be there.”
A version of this article first appeared on Medscape.com.
First-year psychiatry resident Jake Goodman, MD, knew he was taking a chance when he opened up on his popular social media platforms about his personal mental health battle. He mulled over the decision for several weeks before deciding to take the plunge.
As he voiced recently on his TikTok page, his biggest social media fanbase, with 1.3 million followers, it felt freeing to get his personal struggle off his chest.
“I’m a doctor in training, and most doctors would advise me not to post this,” the 29-year-old from Miami said in the video last month, which garnered 1.2 million views on TikTok alone. “They would say it’s risky for my career. But I didn’t join the medical field to continue the toxic status quo. I’m part of a new generation of health care professionals that are not afraid to be vulnerable and talk about mental health.”
“Dr. Jake,” as he calls himself on social media, admitted he was a physician who treats mental illness and also takes medication for it. “It felt good to say that. And by the way, I’m proud of it,” he said in the TikTok post.
A champion of mental health throughout the pandemic, Dr. Goodman called attention to the illness in the medical field. In a message on Instagram, he stated, “Opening up about your mental health as a medical professional, especially as a doctor who treats mental illness, can be taboo ... So here’s me leading by example.”
He also cited statistics on the challenge: “1 in 2 people will be diagnosed with a mental health illness at some point in their life. Yet many of us will never take medication that can help correct the chemical imbalance in our brains due to medication stigma: the fear that taking medications for our mental health somehow makes us weak.”
Mental health remains an issue among residents. Nearly 70% of residents polled by Medscape in its 2021 Residents Lifestyle & Happiness Report said they strongly or somewhat agree there’s a stigma against seeking mental health help. And nearly half, or 47% of those polled, said they sometimes (36%) or always/most of the time (11%) were depressed. The latter category rose in the past year.
Dr. Goodman told this news organization that he became passionate about mental health when he lost a college friend to suicide. “It really exposed the stigma” of mental health, he said. “I always knew it was there, but it took me seeing someone lose his life and [asking] why didn’t he feel comfortable talking to us, and why didn’t I feel comfortable talking to him?”
Stress of medical training
The decision to pursue psychiatry as his specialty came after a rotation in a clinic for people struggling with substance use disorders. “I was enthralled to see people change their life ... just by mental health care.” It’s why he went into medicine, he tells this news organization. “I always wanted to be in a field to help people [before they hit] rock bottom, when no one else could be there for them.”
Dr. Goodman’s personal battle with mental health didn’t arise until he started residency. “I was not really myself.” He said he felt numb and burned out. “I was not getting as much enjoyment out of things.” A friend pointed out that he might be depressed, so he went to see a therapist and then a psychiatrist and started on medication. “It had a profound impact on how I felt.”
Still, it took a while before Dr. Goodman was comfortable sharing his story with the 1.6 million followers he had already built across his social media platforms.
“I started on social media in 2020 with the goal of advocating for mental health and inspiring future doctors.” He said the message seemed to resonate with people struggling during the early part of the pandemic. On his social media accounts, he also talks about medical school, residency, and being a health care provider. His fiancé is also a resident doctor, in internal medicine.
Dr. Goodman is also trying to create a more realistic image of doctors than the superheroes he believed they were growing up. He wants those who grow up wanting to be doctors and who look up to him to see him as a human being with vulnerabilities, such as mental health.
“You can be a doctor and have mental health issues. Seeking treatment for mental health makes you a better doctor, and for other health care workers suffering in the midst of the pandemic, I want to let them know they are not alone.”
He pointed to the statistic that doctors have one of the highest suicide rates of any professions. “It’s better to talk about that in the early stages of training.”
Students, residents, or attending physicians who have mental health challenges shouldn’t allow their symptoms to go untreated, Dr. Goodman added. “Holding in all the stress and anxiety and feelings in a very traumatic field may be dangerous. ”
One of his goals is to campaign for the removal of a question on state medical licensing forms requiring doctors to report any mental health diagnosis. It’s why doctors may be afraid to admit that they are struggling. “I’m still here. It didn’t ruin my career.”
Doctors who seek treatment for mental health are theoretically protected under the Americans With Disabilities Act from being refused a license on the basis of that diagnosis. Dr. Goodman hopes to advocate at the state level to reduce discrimination and increase accessibility for doctors to seek mental health care.
Still, Dr. Goodman concedes he was initially fearful of the repercussions. “I opened up about it because this post could save lives. I was doing what I believed in.”
So if he runs into barriers to receive his medical license because of his admission, “that’s a serious problem,” he said. “There is already a shortage of doctors. We’ll see what happens in a few years. I am not the only one who will answer ‘yes’ to having sought treatment for a mental illness. The questions do not really need to be there.”
A version of this article first appeared on Medscape.com.
First-year psychiatry resident Jake Goodman, MD, knew he was taking a chance when he opened up on his popular social media platforms about his personal mental health battle. He mulled over the decision for several weeks before deciding to take the plunge.
As he voiced recently on his TikTok page, his biggest social media fanbase, with 1.3 million followers, it felt freeing to get his personal struggle off his chest.
“I’m a doctor in training, and most doctors would advise me not to post this,” the 29-year-old from Miami said in the video last month, which garnered 1.2 million views on TikTok alone. “They would say it’s risky for my career. But I didn’t join the medical field to continue the toxic status quo. I’m part of a new generation of health care professionals that are not afraid to be vulnerable and talk about mental health.”
“Dr. Jake,” as he calls himself on social media, admitted he was a physician who treats mental illness and also takes medication for it. “It felt good to say that. And by the way, I’m proud of it,” he said in the TikTok post.
A champion of mental health throughout the pandemic, Dr. Goodman called attention to the illness in the medical field. In a message on Instagram, he stated, “Opening up about your mental health as a medical professional, especially as a doctor who treats mental illness, can be taboo ... So here’s me leading by example.”
He also cited statistics on the challenge: “1 in 2 people will be diagnosed with a mental health illness at some point in their life. Yet many of us will never take medication that can help correct the chemical imbalance in our brains due to medication stigma: the fear that taking medications for our mental health somehow makes us weak.”
Mental health remains an issue among residents. Nearly 70% of residents polled by Medscape in its 2021 Residents Lifestyle & Happiness Report said they strongly or somewhat agree there’s a stigma against seeking mental health help. And nearly half, or 47% of those polled, said they sometimes (36%) or always/most of the time (11%) were depressed. The latter category rose in the past year.
Dr. Goodman told this news organization that he became passionate about mental health when he lost a college friend to suicide. “It really exposed the stigma” of mental health, he said. “I always knew it was there, but it took me seeing someone lose his life and [asking] why didn’t he feel comfortable talking to us, and why didn’t I feel comfortable talking to him?”
Stress of medical training
The decision to pursue psychiatry as his specialty came after a rotation in a clinic for people struggling with substance use disorders. “I was enthralled to see people change their life ... just by mental health care.” It’s why he went into medicine, he tells this news organization. “I always wanted to be in a field to help people [before they hit] rock bottom, when no one else could be there for them.”
Dr. Goodman’s personal battle with mental health didn’t arise until he started residency. “I was not really myself.” He said he felt numb and burned out. “I was not getting as much enjoyment out of things.” A friend pointed out that he might be depressed, so he went to see a therapist and then a psychiatrist and started on medication. “It had a profound impact on how I felt.”
Still, it took a while before Dr. Goodman was comfortable sharing his story with the 1.6 million followers he had already built across his social media platforms.
“I started on social media in 2020 with the goal of advocating for mental health and inspiring future doctors.” He said the message seemed to resonate with people struggling during the early part of the pandemic. On his social media accounts, he also talks about medical school, residency, and being a health care provider. His fiancé is also a resident doctor, in internal medicine.
Dr. Goodman is also trying to create a more realistic image of doctors than the superheroes he believed they were growing up. He wants those who grow up wanting to be doctors and who look up to him to see him as a human being with vulnerabilities, such as mental health.
“You can be a doctor and have mental health issues. Seeking treatment for mental health makes you a better doctor, and for other health care workers suffering in the midst of the pandemic, I want to let them know they are not alone.”
He pointed to the statistic that doctors have one of the highest suicide rates of any professions. “It’s better to talk about that in the early stages of training.”
Students, residents, or attending physicians who have mental health challenges shouldn’t allow their symptoms to go untreated, Dr. Goodman added. “Holding in all the stress and anxiety and feelings in a very traumatic field may be dangerous. ”
One of his goals is to campaign for the removal of a question on state medical licensing forms requiring doctors to report any mental health diagnosis. It’s why doctors may be afraid to admit that they are struggling. “I’m still here. It didn’t ruin my career.”
Doctors who seek treatment for mental health are theoretically protected under the Americans With Disabilities Act from being refused a license on the basis of that diagnosis. Dr. Goodman hopes to advocate at the state level to reduce discrimination and increase accessibility for doctors to seek mental health care.
Still, Dr. Goodman concedes he was initially fearful of the repercussions. “I opened up about it because this post could save lives. I was doing what I believed in.”
So if he runs into barriers to receive his medical license because of his admission, “that’s a serious problem,” he said. “There is already a shortage of doctors. We’ll see what happens in a few years. I am not the only one who will answer ‘yes’ to having sought treatment for a mental illness. The questions do not really need to be there.”
A version of this article first appeared on Medscape.com.
Could stopping ‘thousand cuts’ by insurers and PBMs help rheumatology’s workforce shortage?
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
I am hearing more and more often from colleagues about the number of rheumatologists taking early retirement because of the frustration of having doctor-patient shared decision-making taken out of their hands and given to the insurance companies and their pharmacy benefit managers (PBMs). Often the right medication for the patient is not available on the formulary, causing unnecessary administrative barriers to providing care. When you put that together with the decreased reimbursement and the many obstacles to the “buy-and-bill” model, many rheumatologists have just had enough and called it quits earlier than they thought they would. This is a significant contributor to the growing workforce problem in rheumatology.
Many of the issues affecting the availability of medications happen throughout the development and distribution of a drug treatment – regulatory approval and obstacles to commercial launch, such as patent thickets, “pay for delay,” and other anticompetitive tactics by the manufacturers. And once a medication is launched on to the marketplace, rheumatologists are at the mercy of the health plans and PBMs as to whether, when, and even where a medication can be used. Here is where much of the frustration begins, amplified by the knowledge that profit for the PBM is the driving force behind formulary construction.
To support rheumatologists in addressing these challenges, the Coalition of State Rheumatology Organizations started a “Reporting Insurance/Payer Issues” page. Here, rheumatologists can describe issues or complaints they have with payers regarding patient care. The responses we’ve received so far always have a sense of urgency and frustration in the description of whatever obstacle to care is being thrown up by an insurance company or PBM.
One of the recent issues that has arisen via the CSRO’s reporting form involves a new policy for an insurance plan that removes the availability of the intravenous formulation of a medication if it has a subcutaneous (sub Q) formulation. It is a commercial version of the Medicare self-administered drug list, but worse. At least Medicare takes the time to look at the actual usage of a formulation before moving it from Part B to Part D. This new policy flatly states that no patients will have access to the IV formulation until the sub Q formulation has been tried. This includes switching all stable IV patients over to the sub Q formulation. Because the IV formulation is weight based, switching patients from IV to sub Q can reduce their dosage by more than 50%. It appears that loss of disease control is a small price to pay for increased PBM profits (called “savings” by the PBM). Notably, IV medications through physician “buy and bill” offer no revenue to the insurance company, while sub Q medications increase profits through rebates, fees, and other price concessions.
The CSRO outlined these concerns in its Jan. 18, 2022, response to the insurance company’s reply to the coalition’s original letter, urging them to value patients over profits. In this response, the CSRO addressed nonmedical switching, site of care cost, outcome documentation, and grandfathering stable patients, and finished with a discussion on ERISA (Employee Retirement Income Security Act of 1974) protections.
While our Reporting Insurance/Payer Issues form cannot handle reimbursement issues, there needs to be a word about money and profit when it comes to physicians. Physicians whose specialties have few to no procedures, including rheumatologists, rely on office visits and ancillary services such as infusion suites for income. That income sustains their practice and maintains all their attendant expenses. Many of the recent policies put forth by health plans not only intrude on the doctor-patient relationship in treatment decisions, but also reduce reimbursements and place obstacles to “buy and bill,” shifting revenue from the physician to the insurance company.
All these insurance/payer issues boil down to a version of “death by a thousand cuts.” These cuts harm patients and impede rheumatologists’ ability to sustain their practices. They are a type of moral injury (among the many we see in health care providers) that are causing rheumatologists to retire early. Clearly, these issues ultimately affect the workforce. We need advocacy on many levels if we have any hope of dulling the knives that are delivering these “cuts.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].